Abstract
Objective
Isoniazid preventive therapy initiation and completion rates are suboptimal among children. Shorter tuberculosis (TB) preventive treatment (TPT) regimens have demonstrated safety and efficacy in children and may improve adherence but are not widely used in high TB burden countries. Understanding preferences regarding TPT regimens’ characteristics and service delivery models is key to designing services to improve TPT initiation and completion rates. We examined paediatric TPT preferences in Eswatini, a high TB burden country.
Design
We conducted a sequential mixed-methods study utilising qualitative methods to inform the design of a discrete choice experiment (DCE) among HIV-positive children, caregivers and healthcare providers (HCP). Drug regimen and service delivery characteristics included pill size and formulation, dosing frequency, medication taste, treatment duration and visit frequency, visit cost, clinic wait time, and clinic operating hours. An unlabelled, binary choice design was used; data were analysed using fixed and mixed effects logistic regression models, with stratified models for children, caregivers and HCP.
Setting
The study was conducted in 20 healthcare facilities providing TB/HIV care in Manzini, Eswatini, from November 2018 to December 2019.
Participants
Ninety-one stakeholders completed in-depth interviews to inform the DCE design; 150 children 10–14 years, 150 caregivers and 150 HCP completed the DCE.
Results
Despite some heterogeneity, the results were fairly consistent among participants, with palatability of medications viewed as the most important TPT attribute; fewer and smaller pills were also preferred. Additionally, shorter waiting times and cost of visit were found to be significant drivers of choices.
Conclusion
Palatable medication, smaller/fewer pills, low visit costs and shorter clinic wait times are important factors when designing TPT services for children and should be considered as new paediatric TPT regimens in Eswatini are rolled out. More research is needed to determine the extent to which preferences drive TPT initiation, adherence and completion rates.
Keywords: health economics, public health, tuberculosis
Strengths and limitations of this study.
Strengths of this study include its use of qualitative and quantitative methods, inclusion of the perspectives of healthcare providers (HCP), children and caregivers, and the rigour of the discrete choice experiment (DCE) approach.
While the sample size was robust, participants were not randomly selected, and therefore may not be representative of all tuberculosis (TB) preventive treatment (TPT)-eligible children, caregivers or HCP.
Participants described their preferences for hypothetical attributes of TPT that they (children) or their children (caregivers) had not yet received and therefore may not reflect actual experiences.
This study was conducted in the Manzini region of Eswatini, which is the most populous region in the country, with the largest proportion of TB cases and a mix of urban and rural clinics. However, findings may not be generalisable to other regions of the country.
This study used a DCE design with no opt-out option for each hypothetical scenario, which maximises the amount of information but means there is no reliable measure for actual uptake of and adherence to TPT. This study aimed to assess preference structures rather than estimate actual uptake. Therefore, this design choice is appropriate as it maximises the amount of information about how participants make trade-offs between attributes.
Background
In 2019, there were 10 million tuberculosis (TB) cases globally, of which approximately 12% occurred in children <15 years, resulting in at least 200 000 deaths.1 Given the significant TB burden in young children, who are at great risk of rapidly progressing to severe TB disease and death,2 3 implementation of effective TB preventive treatment (TPT) is paramount and recommended by the WHO for all HIV-positive children <15 years and for HIV-negative children <5 years following close contact with an adult TB case.4 TPT with 6–9 months of daily isoniazid, or isoniazid preventive therapy (IPT), has been found to be effective in children5 and highly cost-effective.6 However, a systematic review of child contact management practices in high TB burden countries found poor IPT initiation and completion rates.7
Recent global efforts in pharmaceutical and medical research and development have resulted in the availability of a range of alternative shorter TPT regimens recommended by WHO8; these have been shown to be efficacious and safe in children, as well as have a positive influence on TPT uptake and completion.9 These shorter regimens include: (1) 3 months of once weekly rifapentine plus isoniazid, (2) 3 months of once daily rifampin plus isoniazid, (3) 4 months of once daily rifampin and (4) 1 month of daily rifapentine plus isoniazid. In low TB burden countries, shorter regimens were found to be associated with higher TPT initiation and completion rates.10 However, these shorter TPT regimens are not yet widely used in high TB burden countries. While child-friendly, dispersible WHO-prequalified fixed dose combination tablets of rifampin/isoniazid are widely available in high burden countries for the treatment of TB disease, their use for TPT has been limited. A paediatric formulation of isoniazid/rifapentine will not be licensed or available in high TB burden countries for several years. As countries tackle the challenge of implementing TPT among at-risk children and consider the use of newer TPT regimens, it is important to determine the TPT regimen and service delivery model preferences and reasons underlying these preferences among HIV-positive children and HIV-negative child contacts, their caregivers and healthcare providers (HCP).
Patient involvement in healthcare choices has been limited, at the macro level in terms of informing the planning and development of healthcare services, as well as at the micro level in terms of one-on-one patient-provider consultations and elicitation of patients’ preferences.11 This is particularly the case in low-income and middle-income countries. Recent interest in providing patient-centred care has sparked increased emphasis on patient participation in shared decision-making,12–15 particularly when multiple treatment options are available and a clearly superior one is not evident. Despite recent interest in patient-centred models of TB care, which include addressing individual patient needs and preferences, there are few published reports of patients being asked about their TPT preferences.16–19 Children’s caregivers are acknowledged to play an important role in determining uptake and outcomes of various child-targeted interventions. Thus, exploring TPT preferences among caregivers of children who are potential candidates for TPT is important as they may influence children’s treatment outcomes. Similarly, since HCP are credible sources of information and gatekeepers for treatment options, it is important to understand their preferences for children’s TPT regimens. A discrete choice experiment (DCE) is a quantitative behavioural economics method used to understand the relative importance of preferences for different characteristics of health services, the trade-offs people are willing to make, and the total benefit and satisfaction derived from different combinations of these characteristics. They are ideal for estimating an individual’s preferences for attributes of treatment regimens and service delivery models and can shed light on their relative value,20 quantifying trade-offs and predicting uptake to inform policy and programme design.
The PRovide Options for Treatment of Exposed Children against TB (PROTECT) Study was a mixed-methods study conducted in Eswatini to examine preferences among key stakeholders (HIV-positive children, caregivers and HCP) regarding TPT regimens and service delivery models offered to HIV-positive children and HIV-negative child contacts using in-depth interviews (IDI), a DCE and a quantitative survey.
Methods
Study setting
Eswatini has one of the world’s highest rates of new TB cases, estimated at 363/100 000, with 66% of patients with TB living with HIV.1 Manzini, the most populous and industrialised region of Eswatini, has the country’s largest proportion of TB cases (45%) and an HIV prevalence of 27.3%.21 Study participants were recruited from all 20 public health facilities providing HIV and TB services in Manzini. As per national guidelines, HIV-negative child contacts aged <5 years and all HIV-positive children aged <15 years are recommended to receive a course of IPT.
Study design
Understanding the choice context, conceptualising the choice process, selecting attributes and levels, and choice of experimental design are the foundation for designing a good DCE instrument.22 23 We therefore conducted a sequential mixed-methods study utilising qualitative methods to inform the design of the DCE. We developed semistructured interview guides to lead the discussion with key stakeholders. IDI with HIV-positive children, caregivers, HCP and key informants (policy makers and implementers) were initially used to explore preferences among key stakeholders regarding TPT regimens and service delivery models. The IDI were conducted between November 2018 and February 2019 and provided contextual information as well as feedback on attributes and images to inform the design of the DCE. Subsequently, a combined DCE and quantitative survey was conducted between August and December 2019 among three subgroups: HIV-positive children, caregivers and HCP, to document preferred characteristics of TPT regimens and service delivery models. The DCE offered participants a choice between hypothetical scenarios of different attributes and the survey explored participants’ socioeconomic characteristics, health literacy, child autonomy, interest in shared decision-making, TB-related attitudes (including risk perception and stigma), social support, alcohol and drug use, depression and barriers to healthcare.
The exploratory IDI were conducted with 91 individuals, including 40 caregivers, 20 HIV-positive children, 20 HCP and 11 key informants. We had enrolment targets for each IDI group and reached saturation in all groups. Nearly all (90%) of the caregivers were female, mean age was 38 (range 21–64) years, 35% were employed and mean number of children living with them was 3 (range 1–9). Among the children, 45% were female and mean age was 12.4 (range 10–14) years; HCP were 75% female with mean age of 34.8 (range 24–46) years. We used purposive sampling within each stakeholder group to achieve a heterogeneous sample, which helped capture a wide range of perspectives about preferences and patterns of care. Based on literature reviews, we derived a list of 13 attributes and levels for the DCE that were further refined based on the IDI. IDI participants were asked about TB preventive services for children and the importance of each of the 13 TPT attributes and reasons behind their determination. The IDI guides are included as online supplemental file 1. At the end of each IDI, participants were asked to select the three most important TPT attributes. The attributes were ranked in order of importance among each group and then rankings were compared across the groups to ascertain agreement and divergence of opinions. The final attributes included in the DCE were pill size and formulation, medication taste, dosing frequency, visit cost, clinic wait time, TPT duration and visit frequency, and clinic hours. Table 1 shows the final list of treatment-related, health system and structural attributes and levels that were included in the DCE design.
Table 1.
Final list of attributes and levels included in the DCE design
| Attribute | Level 1 | Level 2 | Level 3 | Level 4 | |
| Treatment-related | 1. Duration of treatment and visit frequency | 3 months of treatment, 1 visit | 6 months of treatment, 1 visit | 3 months of treatment, 3 visits | 6 months of treatment, 6 visits |
| 2. Dosing frequency | Once a day | Once every 2 days | Once a week | Once ever | |
| 3. Formulation/pill size | Dissolvable | 2 small pills | 6 small pills | 2 medium pills | |
| 4. Taste | Bitter | Not bitter | – | – | |
| Health system | 5. Wait time in the clinic | 15 min | 45 min | 1 hour 30 min | 3 hours |
| 6. Times of operation | Regular operating hours | Regular operating hours plus extended morning hours | Regular operating hours plus extended evening hours | Regular operating hours 7 days a week | |
| Structural | 7. Cost of visit, including travel to health facility | Free | SZL 10.00 (approx. US$0.7535) | SZL 40.00 (approx. US$3.0035) | SZL 80.00 (approx. US$6.0035) |
DCE, discrete choice experiment.
bmjopen-2020-048443supp001.pdf (7.2MB, pdf)
DCE design
The DCE design used a binary choice, main effects, fractional factorial design, following a method for generating statistically optimal designs24 and the principles of efficient designs.25 An orthogonal main effects plan was generated and used as the first alternative in each choice set. The second alternative was then generated by using a set of systematic level changes to the levels in the first alternative in each set. The final design, which included 32 choice sets, was organised into four survey versions using a blocking variable as part of the design, such that each participant responded only to a subset consisting of eight choice sets to reduce cognitive burden and improve data quality. Participants were randomly allocated to one of the four survey versions and asked to select their most preferred scenario in each choice set as the design did not include an opt-out option. Binary designs are common in healthcare research and are cognitively less burdensome for participants.20 26 Excluding an opt-out option increases the amount of information obtained about preference structures, as the instrument forces trade-offs between attributes and levels, even in cases where neither scenario is particularly favourable or attractive to them. Research has shown that the choice to opt-out increases as trade-offs become more difficult, and individuals often opt-out to prevent themselves from making ‘poor choices’.27–30 In this study, using an opt-out design was not necessary because the DCE did not aim to estimate demand/uptake directly but rather to understand preference structures. Therefore, we employed a design with no opt-out alternative to reduce the chance of participants opting out to minimise their effort in making difficult trade-offs or reduce internal conflicts generated by making ‘poor choices’. Finally, we used an unlabelled design (ie, the different alternatives in each choice set had generic labels such as ‘Option A’ or ‘Option B’) given that we did not expect alternative specific constants for any of the attributes offered.31
Once the DCE design was finalised, the choice sets were developed into booklets, which presented the choices using images and descriptors in English. The DCE was administered one-on-one by trained interviewers who used a set of scripted instructions to introduce the DCE task and captured participant choices on an electronic tablet. Figure 1 shows an example of a choice set.
Figure 1.
PROTECT Study discrete choice experiment (DCE) choice set example.
Piloting
Once the DCE instrument had been designed, the choice sets were piloted among study staff and among a small number of children to ensure that the tools had face validity and were easy to understand, especially by children. Feedback from fieldworkers and the children indicated that the DCE instrument was well understood and intuitive to use; no changes were made following the pilot. Throughout data collection during the pilot and the main study, fieldworkers consistently affirmed the children’s ability to understand and engage with the DCE. As an additional validation exercise, the first question in each version was repeated later in the DCE to check whether participants were consistent when faced with the same choice. Most participants were consistent in their choices, and there were no significant differences in the preference structures of participants who were consistent and those who were not (data not shown). The direction and relative strength of preference structures remained consistent in the analysis even when participants who did not answer the repeated question consistently were removed from the analysis.
Study participants
Because HIV-positive children aged <15 years are specifically identified as targets for TPT as part of the WHO’s comprehensive package of HIV services, we focused this research on HIV-positive children aged 10–14 years as they were deemed able to respond to the DCE. HIV-negative children are currently not eligible for TPT in these settings unless they are household contacts of an adult with TB and are aged <5 years. To broaden our understanding of preferences, we also included caregivers of both HIV-positive and HIV-negative children aged <15 years to understand what additional factors were important to caregivers of potential child TB contacts. We did not exclude caregivers of children who are contacts of pulmonary TB cases; rather, we did not specify that as an inclusion criterion. HCP preferences were also examined as providers’ preferences matter when they give advice and make recommendations to their patients.
Caregivers and children were enrolled in the study at facilities with the assistance of facility staff who referred them to study staff during routine visits. Study flyers were given by clinic staff to potentially eligible HIV-positive children to inform their caregivers about the study so that they could accompany their children and provide parental consent. Eligibility criteria for caregivers were: caregiver of HIV-negative and/or HIV-positive child; aged ≥18 years; siSwati-speaking or English-speaking; receiving health services at a study site; and capacity for consent. Eligibility criteria for children were: HIV-positive; aged 10–14 years; siSwati-speaking or English-speaking; receiving health services at a study site; not currently taking TPT; and capacity for assent. HCP, including nurses, physicians and community workers, were recruited with the help of facility managers. Eligibility criteria were: providing care at a study site; aged ≥18 years; siSwati-speaking or English-speaking; and capacity for consent. Participation in the qualitative component of the study was not an exclusion criterion for participating in the DCE.
Sampling
Given the design of the DCE, we estimated a minimum sample size of 125 participants per subpopulation, based on the following rule:
where l is the maximum number of levels for any attribute (4), J is the number of alternatives in each choice task (2) and S is the number of choice sets presented to any participant (8). Factoring in a margin of error of 20%, 150 participants were recruited from each subpopulation. Although the sample for the DCE was not selected strictly randomly from the full population, given the challenges experienced working with children in a low-resource settings and the size distribution and nature of the population of caregivers, fieldworkers aimed to reduce any biases in sampling by recruiting at different facilities on different days and times during the week, and by using different strategies for recruitment, including recruitment on weekends at Teen Clubs, where HIV-positive teens participate in adherence support groups at the facility. Participants were randomly assigned to one of the four versions of the DCE survey, which helped minimise group differences in bias since bias was experienced by participants in each of the four surveys, thus improving the robustness of the results.
Data analysis
As is common in DCE studies, we started by running a simple fixed effects logit mode (Model 1) and then ran random effects logit model (Model 2) for the main effects, using dummy coding of attribute levels.26 Results from these models were compared for consistency and a Hausmann test was conducted to test for violations of the assumption of independence of irrelevant alternatives (IIA) underlying the fixed effects logit model. The Hausmann test returned a negative value, indicating that a fixed effects model is more appropriate, although the direction of effects and levels of significance were similar. Following common practice, we then moved to more complicated models which allow for the investigation of preference heterogeneity.32–34 We ran a mixed effects logit model (Model 3) using Halton draws with 1000 replications to estimate the relative utility of the main effects of each of the attribute levels. Mixed effects models allow for relaxing the IIA assumption and an assessment of heterogeneity in preferences across attributes from the SD estimates for each attribute level. To better understand potential sources of preference heterogeneity, we ran two interaction models and stratified analyses by sample subgroups. The first interaction model was a caregiver/child interaction, which used a dummy variable (children=0; caregivers=1), multiplied by each attribute level and a fixed effects logit model run on the original attribute levels and the new interaction attribute levels (Model 4). The second interaction compared the preferences of children and caregivers as one group (children=caregivers=0) and HCP (HCP=1) (Model 5). Finally, stratified fixed effects logit models were run for children (Model 6), caregivers (Model 7) and HCP (Model 8).
Patient and public involvement
The study was reviewed initially and then quarterly at Stakeholders Advisory Group meetings, where community stakeholders advised on study design, implementation, challenges and preliminary findings. Stakeholders included implementing partners, representatives from non-governmental organisations and community representatives; no patients were represented in these meetings.
Results
Participant characteristics
A total of 450 individuals were approached and all agreed to participate and were enrolled in the study. All participants completed the DCE and quantitative survey, including 150 children, 150 caregivers and 150 HCP. The median age was 36 years among caregivers and HCP; 41% of children were aged 10–11 years and 59% were 12–14 years. Half (49%) of the children were female; caregivers and HCP were primarily female (93% and 81%, respectively). Most children (77%) reported they had someone reminding them to take medicines, generally a parent or caregiver (89%), which provides some context to children’s reliance on caregivers for support in taking medication.
Main effects
The fixed effects logit (Model 1) and mixed effects logit (Model 3) models produced similar results, with the direction and significance of effects largely consistent (see online supplemental file 2). Figure 2 shows the odds ratios (ORs) of the main effects means for the full sample from the mixed logistic regression model (Model 3), as well as the p values and confidence intervals (CIs), with the estimates of the standard devations (SDs) (ORs, p values and CIs) shown in the table below (figure 2).
Figure 2.
Mixed effects logit model (Model 3) main effects (above) and SDs (table below).
bmjopen-2020-048443supp002.pdf (53.6KB, pdf)
Among treatment regimen attributes, taste was found to be the most significant driver of preferences overall, with participants more than three times as likely to choose a treatment alternative if the medication was palatable compared with an alternative with bitter medication if all other attributes were held constant (OR=3.51, 95% CI 2.81 to 4.38). We also found that the duration of treatment and number of clinic visits had a small and mostly non-significant effect on preferences. There was no significant difference between 6 months of treatment with monthly visits and 3 months of treatment with monthly visits. No difference was found between 3-month treatment regimens that require monthly visits or a once-off visit. There was a significant but relatively small preference not to have 6 months of treatment with six clinic visits compared with 3 months of treatment with only one clinic visit (OR=0.75, 95% CI 0.60 to 0.95).
Overall, participants preferred less frequent dosing schedules, with a bi-weekly, weekly and monthly dose preferred to daily dosing (OR=1.78, 95% CI 1.43 to 2.22; OR=1.99, 95% CI 1.57 to 2.53; and OR=2.34, 95% CI 1.80 to 3.05, respectively). We found no significant difference in preferences between a dissolvable formulation of the medication and two small pills. However, participants were significantly less likely to choose a treatment regimen when the dose was six small pills or two large pills (OR=0.62, 95% CI 0.49 to 0.79 and OR=0.65 95% CI 0.48 to 0.90, respectively) compared with a dose of two small pills if all the other attributes remained constant.
Within health system attributes, shorter waiting times were preferred, although the size of the effect was relatively small. Participants preferred a 15 min waiting time compared with a 45 min waiting time (OR=1.36, 95% CI 1.10 to 1.68) and preferred not to have a 3-hour waiting time (OR=0.59, 95% CI 0.47 to 0.74) compared with a 45 min waiting time. We found no significant preferences regarding clinic operating hours.
The structural attribute, cost, was found to be a significant driver of preferences. Free services were significantly preferred to services costing US$0.75 (OR=1.37, 95% CI 1.10 to 1.72), and participants preferred not to pay a fee of US$3.00 or US$6.00 (OR=0.60, 95% CI 0. 0.48 to 0.75 and OR=0.31, 95% CI 0.24 to 0.40, respectively) compared with a cost of US$0.75.
Divergence in preferences among children, caregivers and HCP
As shown in figure 2, the SD, ORs and p values from the mixed effects logistic regression model indicate some preference heterogeneity, especially for cost, dosing frequency and taste. Interaction models were used to understand where preferences diverged between key subgroups in the study sample. Table 2 presents the results of the interaction analysis, first showing differences in preferences of children versus caregivers (Model 4) and then between children and caregivers versus HCP (Model 5).
Table 2.
Analysis of interaction between groups
| Attribute (reference level) | Level | Model 4 | Model 5 | ||||
| Children | Children and caregivers | ||||||
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Duration of treatment and visit frequency (3 months of treatment, 1 clinic visit) | 6 months 1 visit | 1.077 | 0.866 to 1.339 | 0.505 | 0.982 | 0.845 to 1.141 | 0.810 |
| 3 months 3 visits | 1.154 | 0.893 to 1.491 | 0.273 | 1.120 | 0.939 to 1.336 | 0.208 | |
| 6 months 6 visits | 0.975 | 0.780 to 1.220 | 0.828 | 0.899 | 0.771 to 1.049 | 0.178 | |
| Dosing frequency (daily) | Bi-weekly | 1.128 | 0.903 to 1.410 | 0.288 | 1.171* | 1.005 to 1.364 | 0.043 |
| Weekly | 1.014 | 0.785 to 1.310 | 0.914 | 1.197* | 1.003 to 1.427 | 0.046 | |
| Monthly | 0.912 | 0.737 to 1.129 | 0.399 | 1.175* | 1.013 to 1.363 | 0.033 | |
| Pill size and formulation (2 small pills) | 6 small | 0.828 | 0.665 to 1.030 | 0.090 | 0.826* | 0.710 to 0.961 | 0.013 |
| 2 large | 0.886 | 0.689 to 1.138 | 0.343 | 0.812* | 0.682 to 0.967 | 0.019 | |
| Dissolvable | 1.022 | 0.821 to 1.272 | 0.847 | 1.001 | 0.859 to 1.165 | 0.994 | |
| Taste (bitter) | Not bitter | 2.195† | 1.932 to 2.495 | <0.001 | 1.960† | 1.794 to 2.140 | <0.001 |
| Waiting time (45 min) | 15 min | 1.294* | 1.039 to 1.613 | 0.022 | 1.183* | 1.015 to 1.378 | 0.032 |
| 90 min | 1.073 | 0.832 to 1.385 | 0.587 | 1.003 | 0.841 to 1.196 | 0.973 | |
| 3 hours | 0.800* | 0.642 to 0.995 | 0.045 | 0.758† | 0.651 to 0.883 | <0.001 | |
| Operating hours (regular clinic hours) | Including early mornings | 1.007 | 0.796 to 1.274 | 0.953 | 1.011 | 0.862 to 1.187 | 0.889 |
| Including evenings | 0.961 | 0.748 to 1.236 | 0.759 | 0.946 | 0.795 to 1.126 | 0.535 | |
| Including weekends | 0.966 | 0.786 to 1.188 | 0.745 | 0.981 | 0.848 to 1.135 | 0.796 | |
| Cost (US$0.75) | Free | 1.451† | 1.164 to 1.808 | 0.001 | 1.243† | 1.070 to 1.444 | 0.004 |
| US$3.00 | 0.824 | 0.641 to 1.059 | 0.130 | 0.781† | 0.656 to 0.930 | 0.006 | |
| US$6.00 | 0.535† | 0.429 to 0.666 | <0.001 | 0.523† | 0.448 to 0.610 | <0.001 | |
| Caregivers | Healthcare providers | ||||||
| Duration of treatment and visit frequency (3 months of treatment, 1 clinic visit) | 6 months 1 visit | 0.836 | 0.618 to 1.132 | 0.247 | 0.825 | 0.622 to 1.094 | 0.181 |
| 3 months 3 visits | 0.942 | 0.660 to 1.344 | 0.741 | 0.794 | 0.565 to 1.115 | 0.183 | |
| 6 months 6 visits | 0.846 | 0.619 to 1.155 | 0.293 | 0.862 | 0.640 to 1.160 | 0.328 | |
| Dosing frequency (daily) | Bi-weekly | 1.090 | 0.801 to 1.483 | 0.585 | 1.919† | 1.432 to 2.571 | <0.001 |
| Weekly | 1.394 | 0.977 to 1.989 | 0.067 | 2.138† | 1.528 to 2.991 | <0.001 | |
| Monthly | 1.645† | 1.219 to 2.219 | 0.001 | 3.026* | 2.249 to 4.071 | <0.001 | |
| Pill size and formulation (2 small pills) | 6 small | 0.996 | 0.734 to 1.351 | 0.980 | 0.739* | 0.550 to 0.993 | 0.045 |
| 2 large | 0.847 | 0.596 to 1.204 | 0.355 | 0.824 | 0.591 to 1.150 | 0.255 | |
| Dissolvable | 0.964 | 0.709 to 1.310 | 0.814 | 0.885 | 0.660 to 1.185 | 0.411 | |
| Taste (bitter) | Not bitter | 0.809* | 0.677 to 0.967 | 0.020 | 1.337† | 1.128 to 1.585 | 0.001 |
| Waiting time (45 min) | 15 min | 0.844 | 0.621 to 1.149 | 0.281 | 0.966 | 0.715 to 1.306 | 0.824 |
| 90 min | 0.884 | 0.620 to 1.259 | 0.494 | 0.782 | 0.560 to 1.093 | 0.150 | |
| 3 hours | 0.903 | 0.664 to 1.227 | 0.513 | 0.828 | 0.612 to 1.120 | 0.220 | |
| Operating hours (regular clinic hours) | Including early mornings | 1.012 | 0.732 to 1.398 | 0.944 | 1.217 | 0.900 to 1.647 | 0.202 |
| Including evenings | 0.969 | 0.682 to 1.376 | 0.859 | 1.364 | 0.979 to 1.901 | 0.066 | |
| Including weekends | 1.028 | 0.767 to 1.378 | 0.851 | 1.320* | 1.003 to 1.736 | 0.047 | |
| Cost (US$0.75) | Free | 0.751 | 0.554 to 1.017 | 0.064 | 1.024 | 0.773 to 1.357 | 0.867 |
| US$3.00 | 0.903 | 0.636 to 1.283 | 0.569 | 0.892 | 0.637 to 1.248 | 0.504 | |
| US$6.00 | 0.957 | 0.703 to 1.303 | 0.781 | 0.788 | 0.581 to 1.070 | 0.127 | |
*Significant at 95%.
†Significant at 99%.
Preferences of children and caregivers were largely similar, although they differed significantly regarding dosing frequency and taste. Among children, there was no significant preference for less frequent dosing schedules, while caregivers were found to have a significantly stronger preference for monthly dosing than daily dosing compared with children (OR=1.65, 95% CI 1.22 to 2.22). Children had a strong and significant preference for medication formulations that are not bitter (OR=2.20, 95% CI 1.93 to 2.50). Taste was less of a concern for caregivers, who were less likely than children to choose a treatment alternative because the taste was not bitter (OR=0.81, 95% CI 0.68 to 0.97).
Given that preferences of children and caregivers were mostly consistent, in the next model we examined the interaction combining children and caregivers as one group compared with HCP (table 2, Model 5). HCP’s preferences diverged significantly on several attributes, most importantly in terms of dosing frequency. Although there was a significant preference for less frequent dosing among children and caregivers, the effect was relatively small (bi-weekly OR=1.17, 95% CI 1.01 to 1.36; weekly: OR=1.20, 95% CI 1.00 to 1.43; monthly: OR=1.18, 95% CI 1.01 to 1.36 compared with daily dosing). HCP were significantly more likely to choose treatment regimens with less frequent dosing schedules than children and caregivers (bi-weekly: OR=1.92, 95% CI 1.43 to 2.57; weekly: OR=2.14, 95% CI 1.53 to 2.99; monthly: OR=3.03, 95% CI 2.25 to 4.07 compared with daily dosing). HCP were also significantly more likely than children and caregivers to prioritise formulations that are not bitter (OR=1.34, 95% CI 1.13 to 1.59). HCP were more likely than caregivers and children to prefer expanded clinic operating hours that include weekend hours (OR=1.32, 95% CI 1.00 to 1.74).
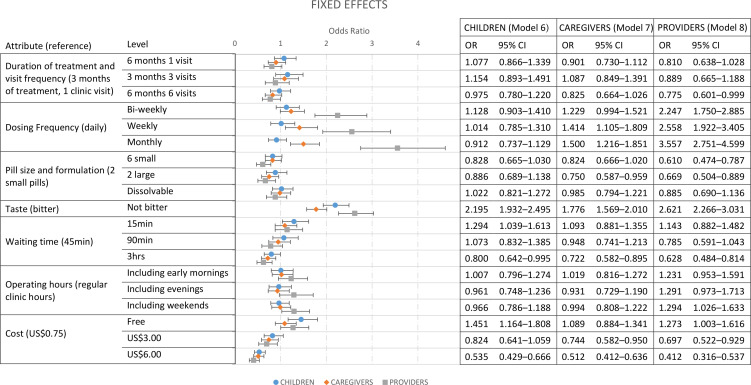
To explore the preferences of each subpopulation in more detail, stratified models were run. Figure 3 shows the results of the three stratified models for children (Model 6), caregivers (Model 7) and HCP (Model 8). The stratified analysis similarly shows that preferences of caregivers and children are largely consistent—effects are in the same direction, although children have fewer preferences that are significant. These preference structures of caregivers and children are also generally consistent with the main effects results from the full sample presented above, which provides a good anchor for understanding some of the nuances in the attributes and levels where preferences diverge. However, while children were found to have no significant preference regarding dosing frequency, caregivers preferred weekly and monthly dosing (OR=1.41, 95% CI 1.11 to 1.81 and OR=1.50, 95% CI 1.22 to 1.85, respectively). While the preference of HCP is also comparable with the main effects results from the full sample, we found that they made more complex trade-offs between attributes and had more attribute levels that were significant in driving preferences than children and caregivers. While children and caregivers were indifferent between six small pills and two small pills per dose, HCP were significantly less likely to choose an alternative with six small pills compared with two small pills (OR=0.61, 95% CI 0.47 to 0.79). HCP also had much stronger preferences for less frequent dosing schedules and were more than twice as likely to choose alternatives with bi-weekly and weekly dosing schedules, and more than three times as likely to choose monthly dosing compared with alternatives with daily doses if all other attributes were held constant. HCP had a small but significant preference for clinic operating hours that included weekend hours (OR=1.29, 95% CI 1.03 to 1.63). Finally, treatment duration and visit frequency was only found to be significant for HCP, and only for a large difference in both treatment duration and number of visits (6 months of treatment with six clinic visits (OR=0.78, 95% CI 0.60 to 0.99) compared with 3 months of treatment with just one clinic visit).
Figure 3.
Stratified fixed effects logit models for children (Model 6), caregivers (Model 7) and HCP (Model 8).
Discussion
The scale-up of TPT for children in high TB and HIV prevalence settings is a key component of an effective healthcare response. The development of new drug regimens and improvement of existing drug formulations along with refining service delivery models are important for the provision of patient-centred services that will encourage uptake and improve adherence. Traditionally, a regimen of TPT consisted of 6–9 months of daily isoniazid. New drug regimens approved and recommended by the WHO show that progress is indeed being made in developing better drug formulations that allow for shorter treatment duration.1 8 However, our results indicate that overall drug regimen formulations were more important when considering the design of TPT services for children; these attributes had a greater effect on choices than the duration of treatment or dosing frequency. Specifically, our results reveal that palatability of medication was the most important attribute of TPT among all study groups, with participants more than three times as likely to choose a treatment alternative if the medication was palatable compared with a bitter alternative. Although there may be other factors that drive uptake and adherence which were not included in the design of this DCE, our results suggest that shorter regimens may not have a substantial effect on TPT uptake and adherence in children unless they have a palatable formulation. While a dissolvable formulation of the medication was not viewed as important, participants were significantly less likely to choose a treatment regimen when the dose was six small pills or two large pills compared with a dose of two small pills if all other attributes remained constant.
In this study, we found that shorter treatment duration did not emerge as an important attribute overall. In qualitative interviews conducted prior to the DCE, we included treatment duration in the list of attributes we explored but it did not emerge as an important attribute. However, because treatment duration is a defining feature of newer TPT regimens and has been shown to influence TPT uptake and completion,9 we included this attribute in our DCE. The results of the DCE analysis were consistent with this attribute not emerging as one of the important attributes in the preliminary qualitative work, and we found that the duration of treatment and number of clinic visits had a small and primarily insignificant effect on preferences. In terms of the treatment regimens, the participants were found to prefer less frequent dosing schedules. The finding that treatment duration is not a central driver of choice is likely to be partly due to other treatment and service delivery characteristics being more important, but also to the study context.
All of the children in our sample were HIV-positive and on daily antiretroviral therapy (ART), so it should be expected that they indicate that treatment duration is less important than other attributes. Our findings may not be generalisable to the entire population of children eligible for TPT, and specifically to HIV-negative children and those aged <5 years who are prescribed TPT following contact with a TB case. Given the WHO guidance to specifically target HIV-positive children for TPT as part of a comprehensive package of HIV care,8 the preferences of the children included in our study are of utmost importance. In this study, given that preferences of caregivers (half of whom had HIV-negative children) and HCP were largely consistent, it is possible that the finding that shorter treatment regimens do not have a strong effect on preferences is not generalisable. However, the effectiveness of shorter treatment regimens in improving uptake and adherence, which was found in previous studies, could be greater and requires further evaluation, particularly among HIV-negative children and children younger than 5 years who were excluded in this study. For example, in a pilot study in Lesotho, caregivers of children who completed TPT were interviewed about TPT preferences and identified pill burden, treatment duration and related frequency of dosing as important TPT attributes.17 However, the children represented in the latter study were all HIV-negative child contacts and not on any other treatment. A small exploratory study from Peru found that among caregivers of children exposed to TB in the household, having a child-friendly formulation was more important than regimen duration.19 A DCE conducted among TPT-eligible adults in Canada found a preference for shorter duration of treatment,16 but two-thirds of participants were not on another treatment at the time of the study and therefore were unaccustomed to taking medications. Duration of treatment was not explored in a recent study from South Africa that examined prioritisation of attributes for TPT among people living with HIV.18
Our analysis found some evidence of preference heterogeneity, which was in part explained by differences in preferences of each subgroup—children, caregivers and HCP. Children’s trade-offs were less complex than those of caregivers and HCP but overall, children and caregivers had similar preferences. Caregivers are a key group as they represent the preferences of younger children who did not participate in the DCE. One area where children’s and caregivers’ preferences diverged from those of HCP related to treatment duration and dosing frequency, possibly because HCP prioritised alleviating the burden on the health system, thereby reducing congestion in health facilities. The perceptions of HCP are important because they are sources of information and gatekeepers for TPT options. HCP considered treatment duration and dosing/visit frequency to be more important than caregivers or children. Preferences regarding health system attributes were mostly consistent across the three groups, with shorter waiting times preferred but with no significant preferences regarding extended clinic operating hours. Because most of the children who participated in study attended Teen Clubs, it is possible that they did not consider weekend hours to be extended clinic hours. The structural attribute, cost of visit, which included travel to the health facility and represents a potential barrier to access, was found to be a significant driver of preferences even for small changes in the cost of services. TPT is provided free of charge in public health facilities in Eswatini, but there are often other costs that children and caregivers face in accessing services. Using more diverse strategies for targeting and identifying potential children for TPT initiation and continuation, such as community outreach models rather than clinic-based models and multi-month dispensing of medications, could help to reduce these costs for some patients and help to improve patient engagement with TPT services.
To our knowledge, this is the first study to examine TPT preferences among children in a high TB burden country. The only other DCE exploring TPT preferences was conducted in adults in a low TB burden country and only included patients.16 Strengths of this study are its use of qualitative and quantitative methods, inclusion of the perspectives of both HCP and patients (represented by children and caregivers), and the rigour of the DCE approach. While the sample size was robust, participants were not randomly selected and therefore may not be representative of all TPT-eligible children, including younger children with HIV (aged <10 years) and HIV-negative child contacts. In addition, participants described their preferences for hypothetical attributes that they had not yet experienced and therefore some concerns might be addressed once individuals actually received TPT. However, the children participating in this study were aged 10–14 years and living with HIV; given the children’s ages, they were most likely taking daily ART since birth and therefore experienced in taking medication. A dissolvable formulation of TPT might not have been viewed as important because the children participating in the study were between 10 and 14 years old. The difficulty in swallowing pills may be a concern for younger children, especially children aged <5 years who are contacts of patients with pulmonary TB and therefore a priority for TPT. In this study, we included caregivers of children to try to understand their perspective as those who would be administering the treatment to younger children, as well as the preferences of HCP. While the results of this study suggest that both caregivers and HCP were indifferent between dissolvable formulations or two small pills (preferring these alternatives to large pill formulations), more research is needed to understand whether this finding holds, especially for caregivers of children aged <5 years. This study was limited to the Manzini region in Eswatini; thus, the results may not be generalisable to patients in other regions. However, we purposively selected Manzini as it is the most populous region of Eswatini, with the country’s largest proportion of TB cases and a mix of urban and rural clinics. In addition, we did not have a random sample as it is difficult to get a true random sample in this context in terms of the size and spread of the caregiver population. We therefore randomly assigned participants to different versions of the DCE and sent fieldworkers out on different days and at different times in an effort to mitigate the non-randomness of the sample. Lastly, this study used a DCE design with no opt-out option. However, given that this study aimed to assess preference structures rather than estimate actual uptake, this design choice is appropriate as it maximises the amount of information about trade-offs, but means there is no reliable anchor for TPT. Therefore, the results of this DCE should be viewed for understanding overall preference structures and willingness to trade-off different regimen and service delivery model characteristics.
Conclusion
Understanding preferences of key stakeholders regarding TPT regimens and service delivery models offered to HIV-positive children and HIV-negative child contacts in Eswatini will enable the Eswatini National TB Control Programme to prioritise and allocate limited resources more efficiently. More research is needed to understand how TPT preferences translate into uptake and adherence, as well as how HIV-negative children may perceive TPT.
Supplementary Material
Acknowledgments
We are grateful to the study participants for partaking in the study. We appreciate the invaluable support for this study from staff at the study sites, the Manzini Region Health Management Team, the Stakeholders Advisory Group and the Eswatini Ministry of Health.
Footnotes
Contributors: YH-M, MS, GG, ArM, WME-S, JEM and AAH contributed to the conceptualisation and design of the study including the development of the study tools. YH-M and MS collected the data and did initial analysis and drafts of the manuscript. GG, ArM, AnM, SS, GSD, WME-S, JEM and AAH contributed to the data analysis, manuscript writing and editing. YH-M is the guarantor. All authors read and approved the final version of the manuscript.
Funding: The PROTECT study was supported by the National Institute of Allergy and Infectious Diseases (NIAID) R21AI138807 (Principal Investigator: Yael Hirsch-Moverman, PhD, MPH, MS). Joanne Mantell was also supported by a NIMH Center Grant P30-MH43520 (Principal Investigator: Robert H Remien, PhD).
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of NIAID.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. De-identified data can be made available upon request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The protocol was approved by the Columbia University Irving Medical Center Institutional Review Board (Ref AAAR8178) and the Eswatini Health and Human Research Review Board (Ref SHR026/1018). All participants provided written informed consent (or in the case of child participants, parental consent and child assent) prior to participation. Consent/assent was facilitated by providing potential study participants with a detailed study description and an adequate opportunity to ask questions.
References
- 1.World Health Organization . Global tuberculosis control: WHO report 2020. Available: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf [Accessed 18 Oct 2020].
- 2.Zar HJ, Pai M. Childhood tuberculosis - a new era. Paediatr Respir Rev 2011;12:1–2. 10.1016/j.prrv.2010.09.004 [DOI] [PubMed] [Google Scholar]
- 3.Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8:392–402. [PubMed] [Google Scholar]
- 4.World Health Organization . Guidance for national tuberculosis programmes on the management of tuberculosis in children second edition, 2014. Available: http://apps.who.int/medicinedocs/documents/s21535en/s21535en.pdf [Accessed 3 Dec 2016]. [PubMed]
- 5.Ayieko J, Abuogi L, Simchowitz B, et al. Efficacy of isoniazid prophylactic therapy in prevention of tuberculosis in children: a meta-analysis. BMC Infect Dis 2014;14:91. 10.1186/1471-2334-14-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandalakas AM, Hesseling AC, Gie RP, et al. Modelling the cost-effectiveness of strategies to prevent tuberculosis in child contacts in a high-burden setting. Thorax 2013;68:247–55. 10.1136/thoraxjnl-2011-200933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szkwarko D, Hirsch-Moverman Y, Du Plessis L, et al. Child contact management in high tuberculosis burden countries: a mixed-methods systematic review. PLoS One 2017;12:e0182185. 10.1371/journal.pone.0182185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . WHO operational Handbook on tuberculosis. module 1: prevention – tuberculosis preventive treatment. Licence: CC BY-NC-SA 3.0 IGO. Geneva: WHO; 2020. [Google Scholar]
- 9.Pradipta IS, Houtsma D, van Boven JFM, et al. Interventions to improve medication adherence in tuberculosis patients: a systematic review of randomized controlled studies. NPJ Prim Care Respir Med 2020;30:21. 10.1038/s41533-020-0179-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandul AL, Nwana N, Holcombe JM, et al. High rate of treatment completion in program settings with 12-Dose Weekly isoniazid and rifapentine for latent Mycobacterium tuberculosis infection. Clin Infect Dis 2017;65:1085–93. 10.1093/cid/cix505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ 2000;320:1530–3. 10.1136/bmj.320.7248.1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44:681–92. 10.1016/S0277-9536(96)00221-3 [DOI] [PubMed] [Google Scholar]
- 13.Charles C, Gafni A, Whelan T. Decision-Making in the physician-patient encounter: revisiting the shared treatment decision-making model. Soc Sci Med 1999;49:651–61. 10.1016/S0277-9536(99)00145-8 [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld BD, White M, Passik SD. Making treatment decisions with HIV infection: a pilot study of patient preferences. Med Decis Making 1997;17:307–14. 10.1177/0272989X9701700307 [DOI] [PubMed] [Google Scholar]
- 15.Swift JK, Callahan JL. The impact of client treatment preferences on outcome: a meta-analysis. J Clin Psychol 2009;65:368–81. 10.1002/jclp.20553 [DOI] [PubMed] [Google Scholar]
- 16.Guo N, Marra CA, FitzGerald JM, et al. Patient preference for latent tuberculosis infection preventive treatment: a discrete choice experiment. Value Health 2011;14:937–43. 10.1016/j.jval.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 17.Hirsch-Moverman Y, Mantell JE, Lebelo L, et al. Tuberculosis preventive treatment preferences among care givers of children in Lesotho: a pilot study. Int J Tuberc Lung Dis 2018;22:858–62. 10.5588/ijtld.17.0809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim H-Y, Hanrahan CF, Dowdy DW, et al. Priorities among HIV-positive individuals for tuberculosis preventive therapies. Int J Tuberc Lung Dis 2020;24:396–402. 10.5588/ijtld.18.0740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuen CM, Millones AK, Galea JT, et al. Toward patient-centered tuberculosis preventive treatment: preferences for regimens and formulations in Lima, Peru. BMC Public Health 2021;21:121. 10.1186/s12889-020-10098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan M, Gerard K. Using discrete choice experiments to value health care programmes: current practice and future research reflections. Appl Health Econ Health Policy 2003;2:55–64. [PubMed] [Google Scholar]
- 21.Government of the Kingdom of Eswatini . Swaziland HIV incidence measurement survey 2 (SHIMS2) 2016-2017. final report. Mbabane: Government of the Kingdom of Eswatini, 2019. https://phia.icap.columbia.edu/wp-content/uploads/2019/05/SHIMS2_Final-Report_05.03.2019_forWEB.pdf [Google Scholar]
- 22.Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user's guide. Pharmacoeconomics 2008;26:661–77. 10.2165/00019053-200826080-00004 [DOI] [PubMed] [Google Scholar]
- 23.Louviere J, Hensher D, Swait J. Stated choice methods: analysis and applications. Cambridge: Cambridge University Press, 2000. [Google Scholar]
- 24.Street DJ, Burgess L, Louviere JJ. Quick and easy choice sets: constructing optimal and nearly optimal stated choice experiments. Int J Res Mark 2005;22:459–70. 10.1016/j.ijresmar.2005.09.003 [DOI] [Google Scholar]
- 25.Huber J, Zwerina K. The importance of utility balance in efficient choice designs. J Marketing Res 1996;33:307–17. 10.1177/002224379603300305 [DOI] [Google Scholar]
- 26.de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145–72. 10.1002/hec.1697 [DOI] [PubMed] [Google Scholar]
- 27.Luce MF. Choosing to avoid: coping with negatively Emotion‐Laden consumer decisions. J Consum Res 1998;24:409–33. 10.1086/209518 [DOI] [Google Scholar]
- 28.Luce MF, Payne JW, Bettman JR. Emotional trade-off difficulty and choice. J Marketing Res 1999;36:143–59. 10.1177/002224379903600201 [DOI] [Google Scholar]
- 29.Ritov I, Baron J. Status-quo and omission biases. J Risk Uncertain 1992;5:49–61. 10.1007/BF00208786 [DOI] [Google Scholar]
- 30.Veldwijk J, Lambooij MS, de Bekker-Grob EW, et al. The effect of including an opt-out option in discrete choice experiments. PLoS One 2014;9:e111805. 10.1371/journal.pone.0111805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Bekker-Grob EW, Hol L, Donkers B, et al. Labeled versus unlabeled discrete choice experiments in health economics: an application to colorectal cancer screening. Value Health 2010;13:315–23. 10.1111/j.1524-4733.2009.00670.x [DOI] [PubMed] [Google Scholar]
- 32.de Bekker-Grob EW, Swait JD, Kassahun HT, et al. Are healthcare choices predictable? the impact of discrete choice experiment designs and models. Value Health 2019;22:1050–62. 10.1016/j.jval.2019.04.1924 [DOI] [PubMed] [Google Scholar]
- 33.Lancsar E, Fiebig DG, Hole AR. Discrete choice experiments: a guide to model specification, estimation and software. Pharmacoeconomics 2017;35:697–716. 10.1007/s40273-017-0506-4 [DOI] [PubMed] [Google Scholar]
- 34.Quaife M, Vickerman P, Manian S, et al. The effect of HIV prevention products on incentives to supply condomless commercial sex among female sex workers in South Africa. Health Econ 2018;27:1550–66. 10.1002/hec.3784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apicella M, Campopiano MC, Mantuano M, et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol 2020;8:782–92. 10.1016/S2213-8587(20)30238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-048443supp001.pdf (7.2MB, pdf)
bmjopen-2020-048443supp002.pdf (53.6KB, pdf)
Data Availability Statement
Data are available upon reasonable request. De-identified data can be made available upon request from the corresponding author.