Abstract
In Canada, piglets receive analgesia to control pain after surgical castration. There is interest in examining the potential to mix non-steroidal anti-inflammatory drugs with iron dextran prior to injection to minimize piglet handling and labor. The objective of this study was to compare pharmacokinetics and the relative bioavailability of ketoprofen given alone (3.0 mg/kg IM) versus the same dose of ketoprofen mixed with iron dextran (52.8 mg/kg IM) (ketoprofen + iron dextran) before injection in piglets. Piglets 8 to 11 d old were allocated into 2 treatment groups (n = 8/group). Plasma drug concentrations were measured using mass spectrometry at 13 time points after injection. No significant differences were detected between the 2 groups when examining pharmacokinetic parameters (e.g., Cmax, Tmax, AUC) or relative bioavailability for either S- or R-ketoprofen enantiomers (P > 0.05). However, pain control efficacy and food safety studies of these formulations are required to further examine this practice.
Résumé
Pharmacocinétique et biodisponibilité du kétoprofène lorsque mélangé avec du fer dextran pour utilisation chez les porcelets allaitants. Au Canada, les porcelets reçoivent une analgésie pour diminuer la douleur après une castration chirurgicale. Il y a un intérêt à examiner la possibilité de mélanger des anti-inflammatoires non stéroïdiens avec du fer dextran avant l’injection afin de minimiser la manipulation des porcelets et le travail. L’objectif de cette étude était de comparer la pharmacocinétique et la biodisponibilité relative du kétoprofène administré seul (3,0 mg/kg IM) par rapport à la même dose de kétoprofène mélangé à du fer dextran (52,8 mg/kg IM) (kétoprofène + fer dextran) avant l’injection des porcelets. Des porcelets âgés de 8 à 11 jours ont été répartis en deux groupes de traitement (n = 8/groupe). Les concentrations plasmatiques de médicament ont été mesurées par spectrométrie de masse à 13 moments dans le temps après l’injection. Aucune différence significative n’a été détectée entre les deux groupes lors de l’examen des paramètres pharmacocinétiques (par ex., Cmax, Tmax, AUC) ou de la biodisponibilité relative pour les énantiomères S- ou R-kétoprofène (P > 0,05). Cependant, des études sur l’efficacité de la diminution de la douleur et la sécurité alimentaire de ces formulations sont nécessaires pour examiner de manière plus approfondie cette pratique.
(Traduit par Dr Serge Messier)
Introduction
Piglet processing procedures, including castration and tail docking, are common husbandry practices in commercial swine production causing acute and chronic pain (1,2). The Canadian Code of Practice for Care and Handling of Pigs states that, at any age, piglets undergoing castration or tail docking are to receive analgesia to control post-procedural pain (3).
Non-steroidal anti-inflammatory drugs (NSAIDs) approved for use in swine in Canada include ketoprofen, meloxicam, and flunixin meglumine. In Canada, meloxicam is currently the only NSAID labeled for use in piglets to control castration pain, as well as to reduce inflammation and lameness. Ketoprofen and flunixin meglumine are both labeled in Canada to aid in reducing pyrexia associated with swine respiratory disease (4).
Piglets receive iron dextran as a common practice in North America to prevent iron deficiency anemia. There is interest within the swine industry about the possibility of mixing medications on-farm to minimize labor inputs, as well as to minimize additional discomfort and handling stress on piglets by combining iron dextran with NSAIDs in the same bottle, and subsequently giving a single injection at processing (5). Mixing any NSAID with iron dextran before injection is considered compounding, formally defined as the customization of any prescription medication by a veterinarian or pharmacist (6). Although benefits may exist with this practice, concerns also exist regarding pharmaceutical interactions that may result in altered bioavailability and/or pharmacokinetics of the drug intended to produce analgesia (7). The compounding of prescription drugs for use in food-producing animals is discouraged. A position statement issued by the Canadian Veterinary Medical Association (CVMA) on the topic discourages compounding due to concerns associated with altered drug withdrawal times and violative drug residues that may occur in edible products intended for human or animal consumption (8).
Previous evaluation of the NSAIDs meloxicam (Metacam; Boehringer Ingelheim Canada, Burlington, Ontario), and flunixin meglumine (Banamine; Merck Animal Health Canada, Kirkland, Quebec), when mixed with iron dextran before injection in piglets indicated a decreased relative bioavailability of both NSAIDs in the compounded formulations compared to the NSAIDs alone (9). Whereas this raises possible concerns of this practice and the level of analgesia provided to the piglet, other research has suggested that meloxicam or ketoprofen, when mixed with iron dextran, can reduce post-castration pain equivalent to when these drugs are given alone (10,11).
Evaluation of ketoprofen-specific pharmacokinetics and relative bioavailability when mixed with iron dextran for administration to piglets has not been investigated. Ketoprofen products licensed for veterinary use in Canada are formulated as a racemic mixture, existing as 1:1 mixtures of 2 enantiomers i.e., the S- and R-isoforms, each of which warrants evaluation separately, as there are indications that each has an important role in analgesia (12–14). The objective of this study was to compare the pharmacokinetics and relative bioavailability of the S- and R-enantiomers of racemic ketoprofen administered alone or in combination (compounded) with iron dextran to piglets.
Materials and methods
This project was conducted following the guidelines of the Canadian Council on Animal Care, and the animal use protocol (AUP #3030) was reviewed and approved by the University of Guelph Animal Care Committee.
Animals and husbandry
Eighteen piglets (Landrace x Duroc x Yorkshire; 9 males, 9 females) sourced from a commercial farm were selected for study. Litters with piglets of target age were evaluated for general health, and appropriate weight range, to ensure uniformity among piglets selected from those litters for treatment. The first male and female piglet identified (1 of each sex, to control for litter effect) that met these criteria were enrolled in the study. Piglets were equally dispersed by sex in each treatment group.
Piglets arrived 5 d prior to injection of the test article at the research facility (University of Guelph Animal Bioscience animal facilities). Piglets were studied in 2 separate batches (n = 9 for each batch) due to logistics of sampling time design, availability of housing, and technical support. Room temperature was controlled, and heat lamps were provided for each piglet as a supplemental heat source. At arrival, piglets were a mean age of 4.4 d (range: 3 to 6 d), and a mean weight of 2.6 kg (range: 2.3 to 2.9 kg). Piglets were acclimated to housing and individual feeding for 3 d prior to jugular catheter placement. Piglets were housed individually with perforated pen dividers to allow nose-to-nose contact. Pens were washed and disinfected prior to the arrival of each batch of piglets to the facility. Piglets were trained to self-feed a supplemental milk powder (Supp-Le-Milk; Soppe Systems, Manchester, Iowa, USA) mixed with water from individual bowls, and were fed 4 times daily.
Monitoring of individual piglets throughout the trial was conducted by research team members and facility staff. Body weight, body temperature, feed intake, general demeanor, health measures, and catheter site assessment were evaluated once or twice daily.
Jugular vein catheterization
Piglets had indwelling catheters surgically placed in the right jugular vein under general anesthesia to facilitate repeated blood sample collection. Piglets were given buprenorphine hydrochloride (Temgesic 0.3 mg/mL; Invidior, Slough, Berkshire, UK) at 0.01 mg/kg by IM injection for post-operative analgesia. There was a 2-day washout period between the surgical catheter placement and test article administration, to allow for clearance of drugs associated with the surgical procedure. Catheters were regularly inspected for damage or evidence of infection and flushed 4 times daily with heparinized physiological saline (10 IU/mL; Ontario Veterinary College Pharmacy) to ensure catheters remained patent.
Treatment groups and test article administration
At the time of treatment, mean piglet age was 9.4 d (range: 8 to 11 d), and the mean piglet weight was 3.1 kg (range: 2.5 to 3.4 kg). A random number generator (www.random.org) was used to allocate piglets into 1 of 3 treatment groups: a – ketoprofen (K), b – iron dextran, or c – ketoprofen mixed (compounded) with iron dextran. In total, 8 piglets (4 males, 4 females) were assigned to each treatment group, and 2 piglets (1 male, 1 female) were assigned, 1 per batch, to the iron dextran treatment group as iron dextran controls. Sample size considerations for this study were informed from similar studies of NSAID pharmacokinetics in pigs using parallel study designs (14,15).
The target dose of ketoprofen for each piglet in both treatment groups was 3.0 mg/kg. To ensure that the administered dosage of ketoprofen in the compounded formulation would be the same as the administered dosage of ketoprofen when administered alone, the compounded formulation was dosed on a mg/kg basis for the ketoprofen component of the final compounded formulation. The compounded formulation containing ketoprofen (racemic formulation; Anafen, 100 mg/mL; Merial Canada, Baie d’Urfé, Quebec) and iron dextran (Dexafer-200, 200 mg/mL; Vétoquinol, Lavaltrie, Quebec) was prepared fresh daily in a sterile 100 mL injection vial by adding 10.2 mL of ketoprofen to 89.8 mL of iron dextran. The combined products were then gently agitated by hand for 15 s to mix the products and achieve a uniform mixture of a final theoretical concentration of 10.2 mg ketoprofen and 179.6 mg iron dextran, per mL of solution. This compounded formulation of ketoprofen and iron dextran was administered to piglets within 10 min after preparation, and additionally, at the time of dosing for each piglet the vial was gently agitated before drawing up the dose to ensure thorough mixing. The final concentration of ketoprofen in the compounded formulation was based on the administration of a maximum volume of 1.0 mL of the final compounded formulation via tuberculin syringe and 22-gauge needle to a 3.4 kg piglet with 1 injection. This ensured accurate dosing of ketoprofen at 3.0 mg/kg for the weight range of enrolled piglets (2.5 to 3.4 kg). As a result, piglets in the ketoprofen + iron dextran treatment group received iron dextran in amounts proportional to their body weight, i.e., 52.8 mg/kg iron dextran (dose range: 148 to 200 mg/piglet). The piglets in the iron dextran treatment group were also dosed on a proportional weight basis at 52.8 mg/kg iron dextran.
Day 0 of the trial was considered the day the test article was administered to piglets. Individual piglets were assigned to a test article that was administered by IM injection on the side of the neck contralateral to the site of jugular catheterization.
Blood collection
Blood sampling for plasma drug concentrations of ketoprofen in the ketoprofen and ketoprofen + iron dextran treatment groups was done pre-dosing, and at 10, 20, 30, and 45 min, and 1, 2, 4, 8, 12, 24, 36, 48, and 72 h after test article administration. Blood for plasma ketoprofen determination from the iron dextran treatment group was taken pre-dosing and at 1 h after test article administration. Blood was collected using a 2-stage drawing technique, to remove catheter dead space (0.6 mL) and obtain 1.5 mL fresh whole blood with a new syringe and 22-gauge needle, which was then placed in heparinized tubes. Catheters were then flushed with heparinized saline (10 IU/mL) to the limit of the catheter dead space to ensure catheter patency. Blood samples were kept on ice until centrifugation (Centra, CL3R; Thermo IEC, Waltham, Massachusetts, USA) for 20 min at 5°C and 390 g. Plasma obtained was aliquoted into 2.0 mL cryovials and stored at −80°C until analysis. On day 3, at study completion, piglets were euthanized with sodium pentobarbital (Euthansol 340 mg/mL; Merck Animal Health, Kirkland, Quebec).
Quantitation of (S)-ketoprofen and (R)-ketoprofen using liquid chromatography-tandem mass spectrometry (LC-MS/MS)
A 0.5-mL aliquot of each plasma sample was extracted using Oasis Max SPE cartridges (1 mL, 30 mg, Waters, Mississauga, Ontario). An internal standard, ketoprofen-d4 (100 ng/mL), was added and samples were treated with an equal volume of 4% ammonia solution, then passed through the SPE cartridges. Ketoprofen was eluted with 1 mL of 2% formic acid in methanol/water (90:10, v:v). The elutes were then dried under a gentle stream of nitrogen and reconstituted in 250 μL of mobile phase for LC–MS/MS analysis.
Liquid chromatography was performed with an Agilent 1100 LC system including an Agilent 1200 autosampler (Agilent Technologies, Santa Clara, California, USA). S- and R-ketoprofen enantiomers were separated at 35°C on an Astec Chirobiotic R chiral column (150 × 2.1 mm ID, 5 μm, Supelco, Bellefonte, Pennsylvania, USA), which was protected by a Phenomenex Security Guard (C18, 4 × 2.0 mm, California, USA). The mobile phase consisted of a 20 mM aqueous ammonium acetate solution with methanol (70:30, v/v) and an isocratic flow rate set at 0.2 mL/min. The sample injection volume was 10 μL and the run time was 10 min. S- and R-ketoprofen were eluted at 4.90 to 5.15 min and 5.60 to 5.75 min, respectively. A hybrid triple quadrupole/linear ion trap mass spectrometer (QTRAP 4000; AB Sciex, Concord, Ontario) equipped with a Turbo-Ion Spray ion source was used for MS analysis. Using the MRM mode, the target ion transitions monitored for ketoprofen and ketoprofen-d4 were m/z 253.0 to m/z 208.9 and m/z 257.0 to m/z 213.0, respectively. Product ion of m/z 196.8 (collision energy 10eV) was also monitored for ketoprofen as the confirming ion. Signal output was captured and processed with Analyst software v1.6.3 (AB Sciex).
Validation of quantitation method
Standard calibration curves were generated with racemic ketoprofen (Sigma Aldrich, Oakville, Ontario). (S)-(+) ketoprofen (Sigma Aldrich) was used to identify the S-ketoprofen peak. Ketoprofen-d4 was used as internal standard (CDN Isotopes, Pointe-Claire, Quebec). Eight-point standard calibration curves in blank plasma matrix were generated on 3 separate days and were run at the beginning and end of each sample batch. Following the US Environmental Protection Agency (US EPA) guidelines, limit of detection (LOD) was determined as 8 ng/mL, and the limit of quantitation (LOQ) was 25 ng/mL for both S- and R-ketoprofen (16). For each curve, the peak-area ratios of the analyte to the internal standard were calculated and plotted against the analyte concentration. Calibration curves were generated by weighted (1/×) linear regression analysis. The linear range was 25 to 10 000 ng/mL for both S- and R-ketoprofen enantiomers, with R2 values > 0.99 for all calibration curves. Coefficients of variation (CV) of inter-day precision were < 15% for all standards. Accuracy ranged from 93.8 to 114.0% for S-ketoprofen and from 89.8 to 111.0% for R-ketoprofen.
Pharmacokinetic analysis
Pharmacokinetic analysis and generation of parameters was done using Phoenix, WinNonlin (Certara USA, Princeton, New Jersey, USA). A non-compartmental analysis of the plasma concentration-time profile for each piglet was performed. The pharmacokinetic parameters evaluated for the ketoprofen and ketoprofen + iron dextran treatment groups for each of S- and R-ketoprofen included peak plasma drug concentration (Cmax, ng/mL) and time of peak plasma drug concentration (Tmax, h), both read directly from the data; slope of the terminal phase (λ, 1/h) determined by linear regression of the data plotted on a log-linear scale; terminal plasma half-life (T1/2, h) calculated as ; area under the concentration-time curve from time 0 to last measured concentration (AUC0–last, h × ng/mL) calculated using the trapezoidal rule (linear up/log down); area under the curve extrapolated to infinity (AUC0–∞, h × ng/mL) calculated by adding the term ; the ratio of the area under the first moment curve (AUMC) and AUC, both extrapolated to infinity, to calculate the mean residence time from time 0 to infinity (MRT, h); total body clearance confounded by unknown absolute bioavailability (Cl/F, mL/h/kg) calculated as ; and the apparent volume of distribution confounded by unknown absolute bioavailability (Vd/F, mL/kg) calculated as .
Statistical analysis
Comparisons of pharmacokinetic parameters between treatment groups were performed using SAS software (Proc mixed, SAS 9.4; SAS, Cary, North Carolina, USA). Assumptions of analysis of variance (ANOVA) were assessed via residual analyses. Residuals were formally tested for normality (using 4 tests offered by SAS: Shapiro-Wilk, Kolmogorov-Smirnov, Cramer-von Mises, and Anderson-Darling). In addition, residuals were plotted against predicted values to determine if transformation of the data was needed, any outliers, or unequal variance between treatments. Where necessary, the data were transformed using either a log or reciprocal transform (footnotes in Tables 1 and 2 indicate parameters that were data transformed). In the case of normal data in which no transformation was indicated, but unequal variance was suggested by visual inspection, AIC values were used to decide if there was evidence of this that would necessitate accommodation by the statistical software (Proc MIXED accommodates unequal variance when conducting tests and computing estimates). Comparisons of parameters between treatment groups were done using 2-sample Student’s t-tests, and although considerations were made to accommodate for unequal variance with an accommodating Student’s t-test, it was not required for any of our parameters evaluated.
Table 1.
Comparison of pharmacokinetic parameters of S-ketoprofen in plasma from pigletsa treated with ketoprofen ketoprofen mixed with iron dextran before injection.
| Parameterb (units) | Treatment | Averagec | 90% Confidence interval | P-valued | |
|---|---|---|---|---|---|
|
| |||||
| LL | UL | ||||
| Cmax (μg/mL) | ketoprofen | 7.63e | 7.06 | 8.20 | 0.054 |
| ketoprofen + iron dextran | 6.67e | 6.09 | 7.33 | ||
| AUC0–last (h × μg/mL) | ketoprofen | 32.12f | 25.36 | 40.68 | 0.196 |
| ketoprofen + iron dextran | 24.77f | 19.56 | 31.38 | ||
| AUC0–∞ (h × μg/mL) | ketoprofen | 32.66f | 25.87 | 41.23 | 0.194 |
| ketoprofen + iron dextran | 23.31f | 20.05 | 31.95 | ||
| λ (L/h) | ketoprofen | 0.22g | 0.19 | 0.27 | 0.206 |
| ketoprofen + iron dextran | 0.28g | 0.23 | 0.35 | ||
| T1/2 (h) | ketoprofen | 3.10e | 2.55 | 3.66 | 0.206 |
| ketoprofen + iron dextran | 2.52e | 1.96 | 3.07 | ||
| Tmax (h) | ketoprofen | 0.51e | 0.38 | 0.64 | 0.320 |
| ketoprofen + iron dextran | 0.40e | 0.28 | 0.53 | ||
| MRT0–∞ (h) | ketoprofen | 4.46e | 3.64 | 5.27 | 0.281 |
| ketoprofen + iron dextran | 3.73f | 2.91 | 4.54 | ||
| Vd/F (mL/kg) | ketoprofen | 191.74g | 177.31 | 208.72 | 0.271 |
| ketoprofen + iron dextran | 207.27g | 190.51 | 227.26 | ||
| Cl/F (mL/h/kg) | ketoprofen | 45.93f | 36.38 | 57.97 | 0.194 |
| ketoprofen + iron dextran | 59.27f | 46.96 | 74.82 | ||
Piglets: n = 8 per treatment group (4 male, 4 female).
Cmax — maximal concentration; AUC — area under the curve; λ — elimination half-life; T1/2— elimination rate constant; Tmax — maximum time; MRT — mean residence time; Vd/F — apparent volume of distribution; Cl/F — clearance; LL — lower limit; UL — upper limit.
Calculation of averages for each parameter based on optimal normality and variance equality.
Based on a standard 2-sample Student’s t-test.
Arithmetic mean.
Geometric mean.
Harmonic mean.
Table 2.
Comparison of pharmacokinetic parameters of R-ketoprofen in plasma from pigletsa treated with ketoprofen versus ketoprofen mixed with iron dextran before injection.
| Parameterb (units) | Treatment | Averagec | 90% Confidence interval | P-valued | |
|---|---|---|---|---|---|
|
| |||||
| LL | UL | ||||
| Cmax (μg/mL) | ketoprofen | 3.97e | 3.60 | 4.33 | 0.449 |
| ketoprofen + iron dextran | 3.74e | 3.38 | 4.10 | ||
| AUC0–last (h × μg/mL) | ketoprofen | 2.30f | 1.97 | 2.69 | 0.600 |
| ketoprofen + iron dextran | 2.46f | 2.10 | 2.87 | ||
| AUC0–∞ (h × μg/mL) | ketoprofen | 2.58f | 2.22 | 3.00 | 0.717 |
| ketoprofen + iron dextran | 2.69g | 2.32 | 3.13 | ||
| λ (1/h) | ketoprofen | 1.78g | 1.44 | 2.31 | 0.304 |
| ketoprofen + iron dextran | 1.48g | 1.24 | 1.84 | ||
| T1/2 (h) | ketoprofen | 0.34g | 0.29 | 0.42 | 0.198 |
| ketoprofen + iron dextran | 0.43g | 0.35 | 0.57 | ||
| Tmax (h) | ketoprofen | 0.16h | — | — | — |
| ketoprofen + iron dextran | 0.16h | — | — | ||
| MRT0–∞ (h) | ketoprofen | 0.65e | 0.55 | 0.75 | 0.366 |
| ketoprofen + iron dextran | 0.73e | 0.62 | 0.83 | ||
| Vd/F (mL/kg) | ketoprofen | 299.51g | 266.66 | 341.60 | 0.143 |
| ketoprofen + iron dextran | 353.92g | 308.94 | 414.22 | ||
| Cl/F (mL/h/kg) | ketoprofen | 582.02f | 500.75 | 676.48 | 0.884 |
| ketoprofen + iron dextran | 556.62f | 478.90 | 646.96 | ||
Piglets: n = 8 per treatment group (4 male, 4 female).
Cmax — maximal concentration; AUC — area under the curve; λ — elimination half-life; T1/2— elimination rate constant; MRT — mean residence time; Vd/F — apparent volume of distribution; Cl/F — clearance; LL — lower limit; UL — upper limit.
Calculation of averages for each parameter based on optimal normality and variance equality.
Based on a standard 2-sample Student’s t-test.
Arithmetic mean.
Geometric mean.
Harmonic mean.
Tmax values for all study subjects were the same (Tmax = 0.16 h) regardless of treatment; group averages were unable to be compared statistically due to lack of variance.
Relative ketoprofen bioavailability was determined by comparing the geometric means of treatment groups for AUC0–last and AUC0–∞ by examining ratios of the compounded formulation (ketoprofen + iron dextran) to the reference formulation (ketoprofen). The significance of the ratio differences from 1.0 was determined with 2-sample Student’s t-tests to compare treatment group means.
Results
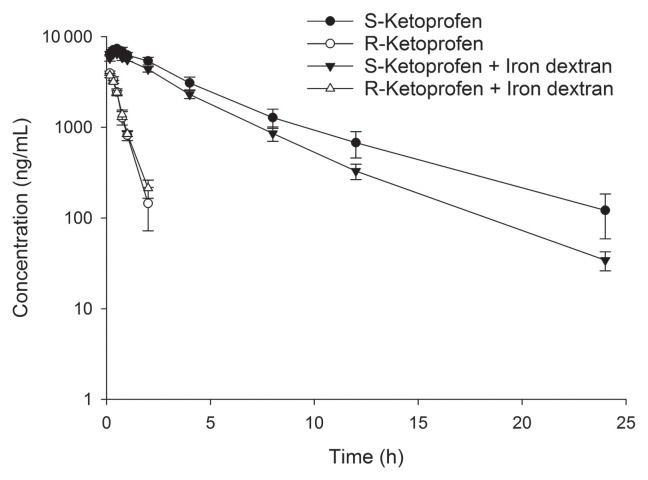
Plasma drug concentrations over time were plotted for both S-ketoprofen and R-ketoprofen (Figure 1). Visual evaluation of the S-ketoprofen plots demonstrated higher average plasma drug concentrations over time in the ketoprofen treatment group compared to the ketoprofen + iron dextran treatment group. No visible difference was apparent between treatment groups for the R-ketoprofen plots. S-ketoprofen concentrations were higher than R-ketoprofen concentrations at each timepoint. In both treatment groups, R-ketoprofen was below the assay LOQ by 4 h after injection, whereas S-ketoprofen remained above the assay LOQ for 12 h after injection, except for 2 piglets: 1 piglet in each of the ketoprofen and ketoprofen + iron dextran treatment groups. By 24 h, 8 piglets were < LOQ for S-ketoprofen: 3 piglets in the ketoprofen group and 5 piglets in the ketoprofen and iron dextran treatment group.
Figure 1.
Plasma drug concentration (± standard error) versus time curve for S- and R-ketoprofen enantiomers evaluated for pigs treated with either ketoprofen alone, or ketoprofen mixed with iron dextran before injection.
Statistical analyses of pharmacokinetic parameters for the S-ketoprofen enantiomer detected no significant difference between the ketoprofen and ketoprofen + iron dextran treatment groups (Table 1). Similarly, there were also no significant differences with pharmacokinetic parameters for R-ketoprofen between the ketoprofen and ketoprofen + iron dextran treatment groups (Table 2). For both S- and R-ketoprofen, the percentage of the AUC0–∞ that was extrapolated from the last measurable concentration was < 20%.
No significant differences in the relative bioavailability of the ketoprofen and ketoprofen + iron dextran treatment group (the compounded formulation) compared to the ketoprofen treatment group (ketoprofen alone) for the parameters AUC0–last and AUC0–∞ for both the S- and R-ketoprofen enantiomers were detected (ratios not different from 1, P > 0.05) (Table 3). All baseline (pre-dosing) blood samples and blood samples taken from piglets in the iron dextran group recorded no detectable concentrations of ketoprofen.
Table 3.
Relative bioavailability of S-ketoprofen and R-ketoprofen in pigletsa treated with ketoprofen versus ketoprofen mixed with iron dextran before injection.
| Parameter | Units | Ketoprofen | Ketoprofen + iron dextran | Relative bioavailability | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Averageb | 90% CI | Averageb | 90% CI | Ratio (ketoprofen + iron dextran/ketoprofen) | 90% CI | |||||
|
|
|
|
||||||||
| LL | UL | LL | UL | LL | UL | |||||
| S-ketoprofen | ||||||||||
| AUC0–last | h × μg/mL | 32.12 | 25.36 | 40.68 | 24.77 | 19.56 | 31.38 | 0.77 | 0.55 | 1.08 |
| AUC0–∞ | h × μg/mL | 32.66 | 25.87 | 41.23 | 25.31 | 20.05 | 31.95 | 0.77 | 0.56 | 1.08 |
| R-ketoprofen | ||||||||||
| AUC0–last | h × μg/mL | 2.30 | 1.97 | 2.69 | 2.46 | 2.10 | 2.87 | 1.07 | 0.86 | 1.33 |
| AUC0–∞ | h × μg/mL | 2.58 | 2.22 | 3.00 | 2.69 | 2.32 | 3.13 | 1.06 | 0.85 | 1.29 |
Piglets: n = 8 per treatment group (4 males, 4 females).
Treatment group averages are the estimate of the geometric mean (log transformed data) based on best fit of the data for normality and equal variance.
AUC — area under the curve; CI — confidence interval; LL — lower limit; UL — upper limit.
Discussion
There were no significant differences between the PK parameters or relative bioavailability for both S- and R-enantiomers when comparing the ketoprofen and ketoprofen + iron dextran treatment groups. However, some important considerations must be made when interpreting this statistical finding and extrapolating it to a clinical context. First, it is prudent to recognize that the lack of statistical difference could be attributed to insufficient statistical power to detect a difference. However, if a statistical difference was detected, that result could not be extrapolated and interpreted as differences in the clinical efficacy of the compounded products used in this study. For example, plasma concentrations of NSAIDs were not well-correlated with pharmacodynamic effects, likely owing to the accumulation of the NSAID at the target site and continued efficacy even though plasma NSAID concentrations have significantly decreased (17,18). Recently, it was demonstrated that in 6-day-old piglets given 3 mg/kg ketoprofen IM, the T1/2 of S-ketoprofen in plasma was ~63% that of the T1/2 of S-ketoprofen in interstitial fluid (19). Ketoprofen is 1 of the most potent inhibitors of COX-1, with moderate inhibition of COX-2 activity (20), although species differences in COX selectivity have been noted (17). The COX-2 activity of ketoprofen is primarily due to the S-enantiomer (13,21). However, ketoprofen’s efficacy is dependent on contributions from both S-ketoprofen and R-ketoprofen enantioselective pharmacodynamics, with the latter providing analgesia by mechanisms other than COX inhibition that are as yet unknown in pigs (14). It is also possible that these differences in analgesic mechanisms contribute to differing conclusions when comparing pain control studies. For example, where different outcome measures are used to assess pain, such as that seen between nociceptive threshold testing (14) and chute navigation time (11) to assess ketoprofen analgesia. Although a limitation of the study may be lower animal numbers in the treatment groups, further support for our findings comes from results of a recently published efficacy study by our group with no statistical differences between ketoprofen and ketoprofen + iron dextran treated piglets of the same age as this study, in a castration model, using chute navigation times as a measurement of efficacy (11).
In the present study, both treatment groups had enantioselective pharmacokinetics. This has been noted in other studies conducted in nursing piglets receiving racemic ketoprofen (14,19,22). The S-ketoprofen concentrations were higher than R-ketoprofen concentrations for both treatment groups at all sampling times, consistent with previous pharmacokinetic research with ketoprofen in piglets of a similar age (14,19,22) and at 9- to 13-wk of age (23). The concentrations of R-ketoprofen for all piglets were below the assay LOQ by 4 h post-dosing, which is also consistent with previous studies conducted in nursing piglets (14,19,22) 9- to 13-wk (23). Differences in S and R enantiomer concentrations and their respective pharmacokinetics may, in part, be the result of chiral inversion of the R enantiomer to the S enantiomer. The administration of the R enantiomer of ketoprofen to 9-week-old pigs resulted in a predominance of S-ketoprofen versus R-ketoprofen by 1 h post-dosing that appeared to be due to chiral inversion that occurred rapidly, reaching a maximum rate of 70% inversion (24).
Pharmacokinetic parameters obtained with the ketoprofen treatment group in our study were similar to results from other studies using ketoprofen in pigs (14,22,23), including older growing pigs in which age-related changes in physiology could have altered pharmacokinetics (16,22). Although not significant, the differences in pharmacokinetic parameters between ketoprofen and ketoprofen + iron dextran treatment groups implied a possible effect of iron dextran on ketoprofen pharmacokinetics. In previous studies, chiral inversion of R- to S-ketoprofen was responsible for at least some of the disappearance of R-ketoprofen from plasma (24). Stabilization and or inhibition of the chiral inversion of R- to S-ketoprofen could result in a longer T1/2 of the former and a smaller AUC and Cmax of the latter as observed in the ketoprofen + iron dextran treatment group compared to the ketoprofen treatment group, albeit not significant. A larger Cl/F value and shorter MRT for S-ketoprofen in the ketoprofen + iron dextran treatment group would coincide with the observed decrease in AUC, which affects these parameters mathematically. Although Cl/F and MRT may be affected by increased metabolism rates in some cases, the pattern in pharmacokinetic parameters with S-ketoprofen were opposite compared to R-ketoprofen in this study. In particular, R-ketoprofen for the ketoprofen + iron dextran group had a longer T1/2, smaller Cl/F, longer MRT, and increased AUC values compared to the ketoprofen group. Based on these results, combined with those for S-ketoprofen, we inferred that altered chiral inversion may be a more feasible explanation, as opposed to alterations in drug metabolism or excretion. Although it appears that the compounding of ketoprofen with iron dextran may result in an alteration to chiral inversion, the absolute change noted was small, and it is uncertain whether this would constitute a clinically relevant difference in pain control efficacy. Additional studies would be needed to test the hypothesis that iron dextran decreases the R- to S-ketoprofen conversion.
Injectable iron supplemented to pigs is combined with dextran, a weakly negatively charged bacterial polysaccharide (25). Perhaps an interaction between charges on iron dextran and ketoprofen occurred after they were compounded. A ketoprofen-dextran ester prodrug was a viable option for colon site-specific drug delivery after oral administration in pigs (26). This type of ester bonding may be occurring when iron dextran is mixed with ketoprofen resulting in alterations with chiral inversion, drug absorption, clearance, or distribution and drug availability at the target site of action. Koncic et al (27) demonstrated significant in vitro iron chelation of ketoprofen by examining the activity of its hydroxamic acid derivative, suggesting iron interactions with ketoprofen could also modify ketoprofen pharmacokinetics. Perhaps drug-drug interactions between iron dextran and ketoprofen discussed earlier rendered a proportion of the administered dose unabsorbable from the injection site. The current study did not measure injection-site ketoprofen drug concentrations.
Interestingly, although most pharmacokinetic parameters reported in the current study were very similar to those reported in similar aged nursing piglets also given ketoprofen at 3 mg/kg IM, AUC0–∞ and AUC0–last values for S-ketoprofen for the ketoprofen treatment group in the current study were ~60% of those reported in the other study (19). However, the AUC0–∞ values in the current study were in line with those reported in other studies using the same route of administration and dose of ketoprofen (3 mg/kg IM) in both older piglets (23) and 52-kg pigs (28). The AUC provided a measure of total systemic exposure to ketoprofen and is pivotal to the determination of relative bioavailability. Based on a lack of significant difference with AUC values, and relative bioavailability ratios between ketoprofen and ketoprofen + iron dextran treatment groups, we inferred that piglets would receive similar systemic exposure to ketoprofen and potentially similar pain control efficacy.
One concern with the clinical use of a compounded formulation of ketoprofen and iron dextran is the potential for subtherapeutic ketoprofen concentrations and inadequate efficacy. Pharmacokinetic/pharmacodynamic modeling following IM administration of racemic ketoprofen at 6 mg/kg in young piglets subjected to kaolin-induced inflammation revealed an ED50 of 2.5 mg/kg for racemic ketoprofen and median IC50 values for the R-ketoprofen and S-ketoprofen enantiomers of 1.6 and 26.7 μg/mL, respectively (14). In the current study, plasma concentrations of S-ketoprofen did not reach the median IC50 value at any measured timepoint, although R-ketoprofen had some piglets retain plasma concentrations above the IC50 up to 45 min post-dosing. It is important to note however that the pharmacodynamic modeling described above using kaolin-induced inflammation may not accurately predict the pain of other procedures, such as castration.
The compounding of drugs for use in food-producing animals in Canada is discouraged by regulatory authorities and the Canadian Veterinary Medical Association. Potency and shelf-life of drugs compounded for therapeutic use are among the greatest concerns. In addition, use of compounded drugs in food-producing animals raises concerns about possible increased risks of violative drug residues that might affect human food safety. The results of this study, at the dosages used for the compounded ketoprofen formulation and ketoprofen administered alone implied minimal risks of violative residues with the compounded formulation. However, tissue depletion data with the compounded ketoprofen formulation would provide the most convincing data and should be determined. Furthermore, differing combinations of ketoprofen and iron dextran or differing dosage regimens may yield differing results, warranting caution regarding human food safety.
Acknowledgments
This project was supported by Ontario Pork, University of Guelph — OMAFRA Research Partnership (Ontario Agri-Food Innovation Alliance), and the Ontario Veterinary College scholarship program.
The authors acknowledge Dr. Julia Zhu for her assistance with jugular catheter construction and surgical placement. Help of staff and students in the Department of Population Medicine Swine Research group, was greatly appreciated.
The authors also acknowledge Yu Gu for assistance in describing mass spectrometry methods, and to William Sears for assistance with statistical methodology.
The authors also acknowledge Certara for providing the software program Phoenix WinNonlin through their Centers of Excellence program. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Hansson M, Lundeheim N, Nyman G, Johansson G. Effect of local anesthesia and/or analgesia on pain responses induced by piglet castration. Acta Vet Scand. 2011;53:34. doi: 10.1186/1751-0147-53-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay M, Vulin A, Génin S, Sales P, Prunier B. Assessment of pain induced by castration in piglets: Behavioural and physiological responses over the subsequent 5 days. Appl Anim Behav Sci. 2003;82:201–218. [Google Scholar]
- 3.Code of Practice for the Care and Handling of Pigs. National Farm Animal Care Council[ www.nfacc.com] [Last accessed September 8, 2021];Canadian Pork Council/National Farm Animal Care Council. c-2014 :33. [updated 2020]. Available from: http://www.nfacc.com/pdfs/codes/pig_code_of_practice.pdf.
- 4.BioAgriMix [ www.bioagrimix.com] Compendium of Veterinary Products — Canada edition. Animalytix LLC; c-2020. [Last accessed September 8, 2021]. Available from: https://bam.cvpservice.com/ [updated March 2, 2020] [Google Scholar]
- 5.Perri AM, Friendship RM, Harding JCS, O’Sullivan TI. An investigation of iron deficiency and anemia in piglets and the effect of iron status at weaning on post-weaning performance. J Swine Health Prod. 2016;24:10–20. [Google Scholar]
- 6.Policy on Extra-Label Drug Use (ELDU) in food producing animals[ https://www.canada.ca/en/health-canada] Health Canada; c2008. [Last accessed September 8, 2021]. Available from: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/hpfb-dgpsa/pdf/vet/pol_eldu-umdde-eng.pdf. [Google Scholar]
- 7.Cribb AE, Peyrou M. Adverse drug reactions. In: Talcott P, Peterson M, editors. Small Animal Toxicology. 3rd ed. St. Louis, Missouri: Elsevier Saunders; 2006. pp. 199–222. [Google Scholar]
- 8.Canadian Veterinary Medical Association Guidelines for the Legitimate Use of Compounded Drugs in Veterinary Practice[ www.canadianveterinarians.net] Canadian Veterinary Medical Association; c2006. [Last accessed January 30, 2020]. Available to CVMA members only from: http://www.canadianveterinarians.net/cvma-guidelines-for-legitimate-use-of-compounded-drugs-in-veterinary-practice-2006. [Google Scholar]
- 9.Ramkissoon S, O’Sullivan T, DeLay J, Enouri S, Friendship R, Johnson R. Mixing (compounding) iron dextran with NSAIDs for use in piglets. [Last accessed September 8, 2021];Proc 34th Centralia Swine Res Update. 2015 :II–17. Available from: https://www.uoguelph.ca/osrn/sites/default/files/Centralia%202015.pdf.
- 10.Barz A, Ritzmann M, Breitinger I, et al. Examination of different options for combined administration of an NSAID (meloxicam) and iron for piglets being castrated. Tierärztl Prax Groβtiere. 2010;1:23–30. [Google Scholar]
- 11.Reynolds K, Johnson R, Friendship R, Brown J, O’Sullivan TL. Assessing pain control efficacy of meloxicam and ketoprofen when compounded with iron dextran in nursing piglets using a navigation chute. Animals. 2020;10:1237. doi: 10.3390/ani10071237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamali F, Brocks DR. Clinical pharmacokinetics of ketoprofen and its enantiomers. Clin Pharmacokinet. 1990;19:197–217. doi: 10.2165/00003088-199019030-00004. [DOI] [PubMed] [Google Scholar]
- 13.Suesa N, Fernandez MF, Gutierrez M, et al. Stereoselective cyclooxegenase inhibition in cellular models by the enantiomers of ketoprofen. Chirality. 1993;5:589–595. doi: 10.1002/chir.530050805. [DOI] [PubMed] [Google Scholar]
- 14.Fosse TK, Toutain PL, Spadavecchia C, Haga HA, Horsberg TE, Ranheim B. Ketoprofen in piglets: Enantioselective pharmacokinetics, pharmacodynamics and PK / PD modelling. J Vet Pharmacol Ther. 2010;34:338–349. doi: 10.1111/j.1365-2885.2010.01236.x. [DOI] [PubMed] [Google Scholar]
- 15.Fosse TK, Spadavecchia C, Horsberg TE, Haga HA, Ranheim B. Pharmacokinetics and pharmacodynamic effects of meloxicam in piglets subjected to a kaolin inflammation model. J Vet Pharmacol Ther. 2010;34:367–375. doi: 10.1111/j.1365-2885.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- 16. [Last accessed September 10, 2021];EPA 40 CFR Part 136, Appendix B, Revision 1.11 [page on the Internet] Available from: https://www.ecfr.gov/current/title-40/chapter-I/subchapter-D/part-136.
- 17.Lees P, Landoni M, Giraudel J, Toutain P. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J Vet Pharmacol Ther. 2004;27:479–490. doi: 10.1111/j.1365-2885.2004.00617.x. [DOI] [PubMed] [Google Scholar]
- 18.Lees P, Giraudel J, Landoni M, Toutain P. PK-PD integration and PK-PD modelling of non-steroidal anti-inflammatory drugs: Principles and applications in veterinary pharmacology. J Vet Pharmacol Ther. 2004;27:491–502. doi: 10.1111/j.1365-2885.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 19.Nixon E, Almond GW, Baynes RE, Messenger KM. Comparative plasma and interstitial fluid pharmacokinetics of meloxicam, flunixin, and ketoprofen in neonatal piglets. Front Vet Sci. 2020;7:82. doi: 10.3389/fvets.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used monsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 21.Hayball PJ, Nation RL, Bochner F. Enantioselective pharmacodynamics of the nonsteroidal antiinflammatory drug ketoprofen: In vitro inhibition of human platelet cyclooxygenase activity. Chirality. 1992;4:484–487. doi: 10.1002/chir.530040805. [DOI] [PubMed] [Google Scholar]
- 22.Fosse TK, Horsberg TE, Haga HA, Hormazabal V, Ranheim B. Enantioselective pharmacokinetics of ketoprofen in piglets: The significance of neonatal age. J Vet Pharmacol Ther. 2010;34:153–159. doi: 10.1111/j.1365-2885.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 23.Mustonen K, Niemi A, Raekallio M, et al. Enantiospecific ketoprofen concentrations in plasma after oral and intramuscular administration in growing pigs. Acta Vet Scand. 2012;54:55. doi: 10.1186/1751-0147-54-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neirinckx E, Croubels S, Remon JP, Devreese M, De Backer P, Vervaet C. Chiral inversion of R(−) to S(+) ketoprofen in pigs. Vet J. 2011;190:290–292. doi: 10.1016/j.tvjl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Martin LE, Bates CM, Beresford CR, et al. The pharmacology of an iron-dextran intramuscular haematinic. Brit J Pharmacol. 1955;10:375. doi: 10.1111/j.1476-5381.1955.tb00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen F, Jensen BH, Olesen HP, Larsen C. Multiple oral administration of a ketoprofen-dextran ester prodrug in pigs: Assessment of gastrointestinal bioavailability by deconvolution. Pharm Res. 1992;9:915–919. doi: 10.1023/a:1015805000595. [DOI] [PubMed] [Google Scholar]
- 27.Koncic MZ, Rajić Z, Petric N, Zorc B. Antioxidant activity of NSAID hydroxamic acids. Acta Pharm. 2009;59:235–242. doi: 10.2478/v10007-009-0017-8. [DOI] [PubMed] [Google Scholar]
- 28.Raekallio M, Mustonen KM, Heinonen ML, et al. Evaluation of bioequivalence after oral, intramuscular, and intravenous administration of racemic ketoprofen in pigs. Am J Vet Res. 2008;69:108–113. doi: 10.2460/ajvr.69.1.108. [DOI] [PubMed] [Google Scholar]