Abstract
Background
Primary care is an important setting in which to treat tobacco addiction. However, the rates at which providers address smoking cessation and the success of that support vary. Strategies can be implemented to improve and increase the delivery of smoking cessation support (e.g. through provider training), and to increase the amount and breadth of support given to people who smoke (e.g. through additional counseling or tailored printed materials).
Objectives
To assess the effectiveness of strategies intended to increase the success of smoking cessation interventions in primary care settings.
To assess whether any effect that these interventions have on smoking cessation may be due to increased implementation by healthcare providers.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and trial registries to 10 September 2020.
Selection criteria
We included randomized controlled trials (RCTs) and cluster‐RCTs (cRCTs) carried out in primary care, including non‐pregnant adults. Studies investigated a strategy or strategies to improve the implementation or success of smoking cessation treatment in primary care. These strategies could include interventions designed to increase or enhance the quality of existing support, or smoking cessation interventions offered in addition to standard care (adjunctive interventions). Intervention strategies had to be tested in addition to and in comparison with standard care, or in addition to other active intervention strategies if the effect of an individual strategy could be isolated. Standard care typically incorporates physician‐delivered brief behavioral support, and an offer of smoking cessation medication, but differs across studies. Studies had to measure smoking abstinence at six months' follow‐up or longer.
Data collection and analysis
We followed standard Cochrane methods. Our primary outcome ‐ smoking abstinence ‐ was measured using the most rigorous intention‐to‐treat definition available. We also extracted outcome data for quit attempts, and the following markers of healthcare provider performance: asking about smoking status; advising on cessation; assessment of participant readiness to quit; assisting with cessation; arranging follow‐up for smoking participants. Where more than one study investigated the same strategy or set of strategies, and measured the same outcome, we conducted meta‐analyses using Mantel‐Haenszel random‐effects methods to generate pooled risk ratios (RRs) and 95% confidence intervals (CIs).
Main results
We included 81 RCTs and cRCTs, involving 112,159 participants. Fourteen were rated at low risk of bias, 44 at high risk, and the remainder at unclear risk.
We identified moderate‐certainty evidence, limited by inconsistency, that the provision of adjunctive counseling by a health professional other than the physician (RR 1.31, 95% CI 1.10 to 1.55; I2 = 44%; 22 studies, 18,150 participants), and provision of cost‐free medications (RR 1.36, 95% CI 1.05 to 1.76; I2 = 63%; 10 studies,7560 participants) increased smoking quit rates in primary care. There was also moderate‐certainty evidence, limited by risk of bias, that the addition of tailored print materials to standard smoking cessation treatment increased the number of people who had successfully stopped smoking at six months' follow‐up or more (RR 1.29, 95% CI 1.04 to 1.59; I2 = 37%; 6 studies, 15,978 participants).
There was no clear evidence that providing participants who smoked with biomedical risk feedback increased their likelihood of quitting (RR 1.07, 95% CI 0.81 to 1.41; I2 = 40%; 7 studies, 3491 participants), or that provider smoking cessation training (RR 1.10, 95% CI 0.85 to 1.41; I2 = 66%; 7 studies, 13,685 participants) or provider incentives (RR 1.14, 95% CI 0.97 to 1.34; I2 = 0%; 2 studies, 2454 participants) increased smoking abstinence rates. However, in assessing the former two strategies we judged the evidence to be of low certainty and in assessing the latter strategies it was of very low certainty. We downgraded the evidence due to imprecision, inconsistency and risk of bias across these comparisons. There was some indication that provider training increased the delivery of smoking cessation support, along with the provision of adjunctive counseling and cost‐free medications. However, our secondary outcomes were not measured consistently, and in many cases analyses were subject to substantial statistical heterogeneity, imprecision, or both, making it difficult to draw conclusions.
Thirty‐four studies investigated multicomponent interventions to improve smoking cessation rates. There was substantial variation in the combinations of strategies tested, and the resulting individual study effect estimates, precluding meta‐analyses in most cases. Meta‐analyses provided some evidence that adjunctive counseling combined with either cost‐free medications or provider training enhanced quit rates when compared with standard care alone. However, analyses were limited by small numbers of events, high statistical heterogeneity, and studies at high risk of bias. Analyses looking at the effects of combining provider training with flow sheets to aid physician decision‐making, and with outreach facilitation, found no clear evidence that these combinations increased quit rates; however, analyses were limited by imprecision, and there was some indication that these approaches did improve some forms of provider implementation.
Authors' conclusions
There is moderate‐certainty evidence that providing adjunctive counseling by an allied health professional, cost‐free smoking cessation medications, and tailored printed materials as part of smoking cessation support in primary care can increase the number of people who achieve smoking cessation. There is no clear evidence that providing participants with biomedical risk feedback, or primary care providers with training or incentives to provide smoking cessation support enhance quit rates. However, we rated this evidence as of low or very low certainty, and so conclusions are likely to change as further evidence becomes available. Most of the studies in this review evaluated smoking cessation interventions that had already been extensively tested in the general population. Further studies should assess strategies designed to optimize the delivery of those interventions already known to be effective within the primary care setting. Such studies should be cluster‐randomized to account for the implications of implementation in this particular setting. Due to substantial variation between studies in this review, identifying optimal characteristics of multicomponent interventions to improve the delivery of smoking cessation treatment was challenging. Future research could use component network meta‐analysis to investigate this further.
Plain language summary
Are there ways to improve stop‐smoking treatment in primary care to help more people to quit smoking?
What is stop‐smoking treatment in primary care?
Primary care, also known as family medicine or general practice, is where people go to see a health professional for mostly day‐to‐day health issues. It is one of the best places for people who smoke tobacco to get help to quit. When people visit primary care they may be asked if they smoke. If they do, they may then be helped to quit, typically through counseling and medications.
Why we did this Cochrane Review
Support to stop smoking in primary care is not always delivered well or consistently. Health providers may be unsure how best to deliver treatment, may have limited time to deliver it, or lack the resources needed. Ways to improve the delivery and success of stop‐smoking support in primary care have been suggested. Some of these are designed to make sure the treatment already available is delivered often and well, e.g. training providers on how best to help people quit, and some are designed to increase the support available for participants, e.g. providing additional counseling and printed materials. Our aim was to look at which of these approaches works best on their own or together.
What did we do?
We searched for studies that looked at ways to improve standard stop‐smoking support within primary care, and where the treatments people received were decided at random.
We wanted to find out:
‐ how many people were asked about their smoking and provided with advice and support;
‐ how many people tried to quit smoking; and
‐ how many people stopped smoking for at least six months.
We included evidence published to 10th September 2020.
What we found
We found 81 studies including 112,159 smokers in primary care patients. Studies looked at many ways to improve the delivery and success of stop‐smoking support in primary care. Some looked at just one strategy, and some looked at two or more in combination. More than one study looked at each of the following individual strategies: additional counseling; free medications; feedback to participants on markers of their individual health risk linked to smoking; printed materials tailored to participants; health provider training; and rewards to health providers for providing support.
Most studies took place in Europe (39 studies) and the USA (26 studies).
What are the results of our review?
More people probably stop smoking for at least six months when they are given additional counseling (22 studies, 18,150 people), free stop‐smoking medications (10 studies, 7560 people), or printed materials tailored to them (6 studies, 15,978 people), as part of stop‐smoking support in primary care. We are uncertain whether providing people with feedback on markers of their individual health risk, providing healthcare providers with training, or with rewards for providing stop‐smoking support, help more people to quit.
Thirty‐four studies looked at more than one strategy to improve stop‐smoking treatment in primary care. Combinations differed greatly across studies, with different levels of success, and it was not possible to draw conclusions on what worked best.
There was not enough information to help us clearly understand whether there were increases in the amount of stop‐smoking support provided or increases in the numbers of people making a quit attempt.
How reliable are these results?
For some of our results the data varied widely, for some there was not enough data, and in some cases there were quality issues with included studies.
We are moderately confident that people are more likely to quit smoking if someone in addition to the primary care doctor also provides stop‐smoking counseling, if free stop‐smoking medications are provided, or if printed materials tailored to the participant are provided as part of stop‐smoking support offered in primary care. However, results might change as further evidence becomes available.
We are less confident about the effectiveness of providing people with feedback on markers of their individual health risk, giving healthcare providers training on stop‐smoking treatments, or giving healthcare providers rewards for giving stop‐smoking support. These results are likely to change when more evidence becomes available.
Summary of findings
Background
Description of the condition
Tobacco use is the leading cause of premature death worldwide (World Health Organization 2017). From a chronic illness perspective, people who smoke have a 50% to 70% greater chance of dying from stroke or coronary heart disease than people who do not, and 85% of cancers of the trachea, bronchus, and lung are directly attributable to tobacco use (U.S. Department of Health and Human Services 2014). Tobacco use is also a leading risk factor for other major causes of death, including 16 types of cancer, chronic obstructive pulmonary disease, and lower respiratory tract infections (U.S. Department of Health and Human Services 2014; World Health Organization 2017).
There is overwhelming evidence to support both the health and economic benefits of smoking cessation. If a person smokes, supporting them with quitting is the single most effective intervention a clinician can provide to reduce the risk of premature disease, disability and death (Fiore 2008; Royal College of Physicians 2018; Tengs 1995). Quitting smoking reduces the excess risk of smoking‐related coronary heart disease by approximately 50% within one year, and to normal levels within five years (U.S. Department of Health and Human Services 2014). Smoking cessation is also considered to be among the most cost‐effective preventive interventions available to clinicians and health systems (Tengs 1995; Cromwell 1997; Roncker 2005; Franco 2007; Gaziano 2007; Royal College of Physicians 2018; U.S. Department of Health and Human Services 2020).
Description of the intervention
Primary care practice, also known as family medicine or general practice, has been identified as an important setting for intervening with tobacco users because of the large reach of primary care settings, the long‐term relationships with patients and their role in addressing disease prevention (U.S. Department of Health and Human Services 2020; World Health Organization 2020).
Evidence‐based guidelines for the delivery of tobacco treatment emphasize the important role of primary care clinicians in tobacco treatment delivery (Verbiest 2017). The World Health Organization (WHO) and other international authorities have called for smoking cessation to be integrated into primary health care globally, as it is seen as the most suitable health system 'environment' for providing advice and support on smoking cessation (Fiore 2008; World Health Organization 2008; World Health Organization 2020; U.S. Department of Health and Human Services 2020). Specifically, the combination of behavioral support and stop‐smoking pharmacotherapy have been shown to significantly enhance long‐term cessation rates; it follows that increasing the use of these evidence‐based treatments is an important target (Stead 2016). While models of delivery differ across international settings, clinical practice guidelines recommend that primary care providers support people who smoke with quitting by: asking them about their smoking status, providing advice on quitting to those identified as smoking, and supporting cessation by offering behavioral counseling and/or pharmaceutical treatment or both when smokers identify themselves as ready to quit (Verbiest 2017). See Secondary outcomes below for more information.
However, there is a well‐documented ‘practice gap’ in the rates at which smoking cessation is addressed by practitioners in clinical settings. International studies have documented that between 40% and 70% of people who smoke report having received cessation advice from their physician (Bartsch 2016; Papadakis 2014; Reid 2019; World Health Organization 2020). While practitioners tend to deliver advice to quit at moderate rates, studies have shown that the rates of providing specific assistance (i.e. behavioral counseling, printed self‐help materials, stop‐smoking medications, or follow‐up support) are much lower (Bartsch 2016; Papadakis 2014). When it is offered, the amount and breadth of assistance is also likely to differ considerably across practices, which may have an effect on rates of smoking cessation.
How the intervention might work
Several barriers to optimal cessation practice in primary care have been identified at the patient, provider, and practice levels (Martin‐Cantera 2020; Van Rossem 2015; Vogt 2005; Young 2001). Identified barriers include a lack of knowledge and skills among providers, provider attitudes and perceptions, lack of time and organizational supports, and a lack of patient motivation and other patient‐level factors. Interventions which address these barriers are expected to enhance rates of tobacco treatment delivery by primary care providers, increase the use of evidence‐based stop smoking treatment by patients, and subsequently lead to enhanced quit rates among patients identified in primary care (Van Rossem 2015; Martin‐Cantera 2020; Vogt 2005; Young 2001).
Strategies to improve the delivery of standard smoking cessation support in primary care could include the provision of provider training, real‐time counseling prompts, and provider performance feedback. These examples represent strategies that span practice‐ and provider‐implementation levels. Another way to boost smoking quit rates in primary care could be to incorporate additional intervention components alongside those already commonly delivered as part of standard care, e.g. provision of tailored print materials, adjunctive counseling provided by allied health professionals and providing people with specific feedback about their smoking‐related health risks. These strategies could be implemented either individually or as part of a multicomponent intervention (combining more than one strategy). While there is a lack of implementation knowledge to inform the design and delivery of tobacco treatment interventions in primary care practice, multicomponent interventions have previously been shown to be the most effective method for increasing both provider performance in the delivery of smoking cessation treatment and improving cessation rates among participants (Anderson 2004; Fiore 2008; Grimshaw 2001; Martin‐Cantera 2015; Papadakis 2010). They are designed to address several barriers to treatment delivery in a synergistic manner, acknowledging the need for more complex or sophisticated intervention models, or both, to bring about changes in healthcare practice and behavior.
Why it is important to do this review
Reflecting the challenges surrounding the effective implementation of smoking cessation treatment in primary care, much research has been carried out investigating how to improve both the implementation and success of these interventions. Some have focused on practice‐level interventions (such as electronic medical record prompts or outreach facilitation (Cummings 1989a; Verbiest 2014); some have focused on provider‐level interventions, such as provider training and incentives (Lennox 1998; Olano Espinosa 2013; Roski 2003), and some have focused on patient‐level interventions (over and above the standard advice delivered by primary care physicians; such as adjunctive counseling, cost‐free medications, biomedical feedback, and tailored printed materials; An 2006; Meyer 2008; Minué‐Lorenzo 2019; Ronaldson 2018). Others have tested a combination of these approaches in multicomponent interventions (e.g. Katz 2004; Twardella 2007; Unrod 2007). Bringing this evidence together allows us to summarize the research methodologies used and to synthesize the evidence in support of specific strategies, or the combination of strategies, that are effective in increasing rates of smoking cessation in the primary care setting. This can be used to inform both clinical practice and the implementation of health policy. Several published meta‐analyses have examined the effect of physician advice and other provider interventions on smoking cessation, but many of these reviews have not been specific to the primary care setting (Boyle 2014; Carson 2012; Clair 2019; Fiore 2008; Rice 2017; Stead 2013; Van den Brand 2017). These previous reviews have also focused on the effect of providing advice on smoking abstinence only; they have not examined improvements in provider performance in the delivery of evidence‐based smoking cessation treatments that may have ultimately led to any increase in effectiveness. Two published meta‐analyses have aimed to do this: Anderson 2004 reviewed the literature published up to 2001, and Papadakis 2010 published an update which examined the literature prior to 2009. Additionally, Martin‐Cantera 2015 narratively reviewed the literature examining multicomponent interventions in primary care, published up to 2014. This review provides an up‐to‐date synthesis of the literature in this field.
Objectives
To assess the effectiveness of strategies intended to increase the success of smoking cessation interventions in primary care settings.
To assess whether any effect that these interventions have on smoking cessation may be due to increased implementation by healthcare providers.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), cluster‐RCTs (cRCTs).
Types of participants
Participants include adult primary healthcare patients. For the purposes of this review, we defined primary care as family medicine or general medical practice. We did not include public health or community interventions in our definition of primary care, nor did we assess interventions delivered in dental offices or pharmacies. We included trials which covered the whole practice population, as well as those which included specific subpopulations recruited from primary care settings (e.g. people with chronic obstructive pulmonary disease (COPD), or people with diabetes). We did not include studies that solely addressed the behavior of pregnant women or adolescents, as they are addressed by other Cochrane Reviews (Chamberlain 2017; Claire 2020; Fanshawe 2017).
For our primary outcome, and most of our secondary outcomes, all participants were required to be people who used tobacco at study baseline. However, for our secondary outcome, 'Number of patients asked whether they smoke', participants could include the general population of primary care patients (i.e. both people who used tobacco and people who did not use tobacco at baseline).
Types of interventions
To be included in this review, studies must have investigated an intervention strategy or strategies designed to improve the implementation or success of smoking cessation treatment in primary care. The interventions under investigation in this review were therefore not standard smoking cessation support incorporating brief advice delivered by a primary care physician, or the standard provision of smoking cessation medications in primary care. Interventions of interest could include any strategy or strategies designed to increase or enhance the quality of the support offered, or an adjunctive smoking cessation intervention offered in addition to standard care. Interventions could be implemented at any level (i.e. practice, provider or participant) and the patient‐level components could be delivered by any health professional within a primary care practice setting. Examples of patient‐level interventions investigated in this review included adjunctive counseling delivered by a health professional other than the physician, cost‐free smoking cessation medications and the provision of tailored print materials. Examples of provider‐level interventions included provider training and provider incentives. Examples of practice‐level interventions were outreach facilitation and electronic medical record (EMR) prompts. The categorization of these interventions is subjective, and some interventions may fit equally well at more than one level. For example, we considered in detail whether cost‐free medications should be categorized as a patient‐level, practice‐level, or system‐level intervention (e.g. where medication costs are government‐subsidized), and decided that it could be categorized as all of these. We decided on patient‐level in this instance, as the participant is the beneficiary of the cost savings, which have the potential to increase medication use.
Valid intervention groups were tested as an adjunct to and in comparison with 'standard' smoking cessation support or 'usual care', in order to test the effect of the additional implementation strategy over and above standard care. Standard care is defined differently within and across different communities and studies; however, it typically involves brief behavioral support from the primary care physician, alongside an offer of smoking cessation medication. We also included studies with head‐to‐head comparisons of two or more active interventions, but only if it was possible to isolate the effects of a single strategy or component designed to enhance the delivery of tobacco cessation treatment in primary care.
We did not include studies which covered interventions to enhance tobacco treatment delivery as part of a multifactorial lifestyle intervention.
Types of outcome measures
Primary outcomes
The primary outcome measure was smoking abstinence at long‐term follow‐up in participants who reported smoking at baseline. To be eligible for inclusion, studies had to measure smoking status at least six months from the start of the intervention. We excluded studies with abstinence measured at less than six months' follow‐up.
In trials with more than one measure of abstinence, we preferred the measure using the longest follow‐up and the strictest criteria, in line with the Russell Standard (West 2005). We used sustained or continuous abstinence over point prevalence abstinence, and biochemically‐validated abstinence, such as exhaled carbon monoxide (CO), over self‐report. We favored biochemically‐validated point prevalence abstinence over self‐reported continuous or prolonged abstinence. We considered participants lost to follow‐up to be still smoking, in line with the practice of the Cochrane Tobacco Addiction Group.
We chose smoking abstinence as the primary outcome, as this is the most clinically relevant outcome; an increase in the number of people quitting is the ultimate goal of any attempt to increase the implementation of smoking cessation treatment.
Secondary outcomes
Our secondary outcomes are deemed to be process outcomes. We therefore did not include studies that only reported on our secondary outcomes and did not investigate our primary outcome.
-
Provider performance in tobacco treatment delivery (these outcomes were informed by the 5As; a sequence of actions proposed by US smoking cessation guidelines that can be applied in primary care settings; Fiore 2008)
Number of participants asked whether they smoke (the denominator for this also includes participants who were not smoking at baseline in studies that enrolled people who smoked and people that did not);
Number of participants identified as smoking who were advised to quit;
Number of participants identified as smoking whose readiness to quit was assessed;
Number of participants identified as smoking who were assisted to quit (further divided into general assistance, medications prescribed, quit date set, counseling provided, self‐help materials provided);
Number of participants identified as smoking who had follow‐up appointments arranged to address smoking.
Participant quit attempts, as defined by individual studies.
Search methods for identification of studies
Electronic searches
We searched the following databases to 10th September 2020:
Cochrane Tobacco Addiction Group Specialized Register;
Cochrane Central Register of Controlled Trials (CENTRAL);
MEDLINE (via PubMed);
Embase.
The search strategy used the following keyword terms: ('smoking' or 'smoking cessation' or 'tobacco‐use cessation', or 'tobacco‐use‐disorder) AND ('primary health care' or 'physicians' or 'family practice' or 'general practice' or 'general practitioners' or 'physicians, family'). We used standard search strings, using the Cochrane Highly Sensitive Search Strategy for identifying randomized controlled trials, as well as 'controlled trials' or 'evaluation studies'. We applied no restrictions by language or publication status. See Appendix 1 and Appendix 2 for the example PubMed and Specialized Register search strategies respectively.
Searching other resources
We searched the following trial registers: www.clinicaltrials.gov and the International Clinical Trials Registration Platform (WHO ICTRP), and reference lists of eligible studies. We also contacted study authors for unpublished results of completed studies.
Data collection and analysis
Selection of studies
SP, GP and BH independently reviewed titles and abstracts of reports for possible inclusion. We reviewed the full text of any reports which could not be fully assessed using the title and abstract, along with any reports that appeared to be eligible based on the available information. Two review authors (from SP, GP and BH) then independently assessed all of the full‐text articles retrieved, and resolved discrepancies by discussion with a third review author (AP or NL), who acted as an arbiter. We then linked multiple reports of the same eligible study. We recorded all reports of studies excluded at the full‐text screening phase, together with the reason for exclusion.
Data extraction and management
One review author (from SP, GP, BH) extracted data on study characteristics of eligible studies. Two review authors (from SP, GP, BH) independently extracted data on outcomes, and categorized studies according to the type and level of intervention. We extracted the following information from each of the included studies:
lead author and year of publication;
country in which intervention was delivered;
methods of recruiting healthcare practices and participants within practices;
inclusion criteria, including subpopulations;
type of study design (RCT, cluster‐RCT);
target of intervention (participant, provider, practice);
data collection method (interview, telephone, mail survey);
characteristics of study participants (age, sex, comorbidities, readiness to quit);
duration of intervention (in weeks);
details of the intervention;
description of the comparator intervention;
outcomes measured, including definitions used and time point at which they were assessed (in weeks);
use of biochemical validation and participant response rate;
methods used to manage missing data;
for each outcome: number of participants in each arm; loss to follow‐up rate; number of events in each arm; intra‐class correlation coefficient (ICC) (cluster‐RCTs only);
study funding source;
authors' declarations of interest.
Methods for categorizing details of intervention
We categorized intervention strategies into three groups, based on the level at which they were designed to intervene (i.e. participant, provider, practice). We further categorized interventions as either a single or a multicomponent intervention. For the purposes of this review, we defined single‐component interventions as those which included only one intervention strategy. We defined multicomponent interventions as interventions which included two or more intervention strategies, at any level. We used a preliminary list of intervention strategies based on previous systematic reviews (Anderson 2004; Fiore 2008; Papadakis 2010) with further categories added as appropriate to describe other intervention modalities identified in the literature.
Assessment of risk of bias in included studies
Two review authors (from SP, GP, BH, NL) independently assessed the risk of bias of the included studies, using Cochrane's RoB1 (Higgins 2011).
We assessed the following domains:
sequence generation (as an indicator of selection bias)
allocation concealment (as an indicator of selection bias)
blinding of outcome assessors (as an indicator of detection bias)
incomplete outcome data (as an indicator of attrition bias)
We did not assess any indicators of performance bias, as all of the studies were assessing a behavioral strategy, and therefore it would have been impossible to blind research staff and participants.
We also assessed the following other sources of bias for c‐RCTs only:
recruitment bias due to recruitment of participants to clusters after allocation;
unbalanced baseline characteristics;
whether statistical adjustment had been made to the analysis to account for the potential correlation of effects within clusters.
Measures of treatment effect
For each study and outcome (smoking abstinence, physician performance outcomes, quit attempts) we calculated the risk ratio (RR) and 95% confidence interval (CI) for each relevant comparison investigated (intervention group versus control group). For smoking abstinence and quit attempts the denominators were the number of people randomized to each study arm, assuming that any participants lost to follow‐up were continuing to smoke or had not made a quit attempt. For the physician performance outcomes we carried out a complete case analysis where possible.
Unit of analysis issues
All analyses contain participant‐level data from both RCTs and c‐RCTs. We investigated the effect of adjusting for clustering in c‐RCTs by inflating the standard error of the log RR using the design effect calculated from the estimated ICC that was reported in the study. If no ICC was reported, we assumed a typical ICC value for smoking cessation trials, based on Baskerville 2001. See below (Sensitivity analysis) for more details.
Dealing with missing data
We recorded the proportions of participants lost to follow‐up in each relevant arm of included studies and used this information in our risk of bias assessments. Any participants with missing smoking or quit attempts data at follow‐up were deemed to have returned to active smoking or to have not made a quit attempt respectively, and were included in the denominator for calculating the risk ratio. We did not impute missing data for physician performance outcomes.
Assessment of heterogeneity
We assessed statistical heterogeneity within meta‐analyses and between subgroups using the I2 statistic (Higgins 2003). We considered an I2 value greater than 50% to indicate moderate to substantial heterogeneity. Where an I2 of greater than 75% was recorded for the pooled result of a meta‐analysis, and this remained unexplained by subgroup analyses, we judged whether it was appropriate to present the pooled estimate.
Assessment of reporting biases
Where analyses included 10 or more studies we generated funnel plots to investigate potential publication bias.
Data synthesis
We grouped studies by intervention type. Where there was more than one study testing an intervention type(s), and where appropriate, we performed meta‐analyses using Mantel‐Haenszel random‐effect models for each outcome. In studies that tested a single intervention component, we did not calculate a pooled estimate across intervention types for each level of intervention (e.g. participant, provider, practice) due to clinical heterogeneity. We were able to carry out meta‐analyses of our primary outcome for comparisons investigating the following singular intervention types:
Participant‐level:
adjunctive counseling
cost‐free medications
biomedical feedback (e.g. spirometry, CO monitoring)
tailored print materials
Provider‐level:
provider training
provider incentives
Some of the studies included in the analyses tested the intervention components above alongside standard care and also used standard care as the comparator, whereas other studies tested the intervention component as part of a multicomponent intervention with a comparator that received the same multicomponent intervention minus the intervention component of interest. These two types of studies were combined in the same meta‐analysis with subgrouping to investigate whether the study design had an impact on study findings.
We were also able to meta‐analyze the following multicomponent interventions versus standard care alone, where more than one study tested the same combination of components:
adjunctive counseling and cost‐free medications
adjunctive counseling and provider training
provider training and flow sheet
provider training and outreach facilitation
Again we carried out meta‐analyses using random‐effects Mantel‐Haenszel methods to calculate RRs and 95% CIs. Where it was not possible to conduct meta‐analyses, i.e. there was only one study investigating the comparison, we summarized studies narratively, calculating and presenting their individual RRs and 95% CIs.
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses to investigate any differences in the effects observed between studies for the primary smoking abstinence outcome, where possible:
for all comparisons: studies that tested the intervention component(s) of interest alongside and in comparison with standard care only versus studies that tested the intervention component(s) of interest alongside and in comparison with other intervention component(s) of interest;
for all comparisons: participants with chronic disease versus those without chronic disease, e.g. diabetes, COPD;
for adjunctive counseling comparisons: provider type; intensity; mode;
for biomedical feedback: type of biomedical feedback, e.g. spirometry, CO monitoring;
for tailored print materials: theoretical basis of tailoring.
Sensitivity analysis
We used sensitivity analyses to examine the effects of excluding studies with the following characteristics for the primary smoking abstinence outcome:
studies deemed to be at high risk of bias (i.e. judged to be at high risk for at least one risk of bias domain);
individually randomized studies (as opposed to c‐RCTs). c‐RCTs provide the best evidence for the effects of the interventions tested when implemented in primary care, as they would be in the 'real world'.
We also carried out two sensitivity analyses adjusting for appropriate estimates of ICCs in those c‐RCTs that did not report controlling for the clustered nature of the design, or those in which the ICC was not reported. Separate sensitivity analyses used estimated ICCs of 0.01 and 0.05 respectively (Baskerville 2001).
Summary of findings and assessment of the certainty of the evidence
We used GRADEpro GDT to import data from Review Manager 5 in order to create summary of findings tables (GRADEpro GDT; Review Manager 2020) for comparisons investigating the following single intervention components:
adjunctive counseling;
cost‐free medications;
biomedical feedback;
tailored print materials;
provider training;
provider incentives.
A summary of the intervention effect for the primary smoking abstinence outcome was produced for each comparison, and we used the GRADE approach to assess the certainty of the body of evidence (Schünemann 2013), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for our primary outcome ‐ smoking abstinence, downgrading by one level for serious or by two levels for very serious limitations for each consideration.
Results
Description of studies
Results of the search
Our searches identified 4518 non‐duplicate records. We screened all records and retrieved the full‐text papers of 824 potentially relevant articles. After screening the full texts we included 81 studies (see Characteristics of included studies), and identified 18 ongoing studies (see Characteristics of ongoing studies) and four studies awaiting classification (Studies awaiting classification). Figure 1 presents the PRISMA flow chart for this review.
1.
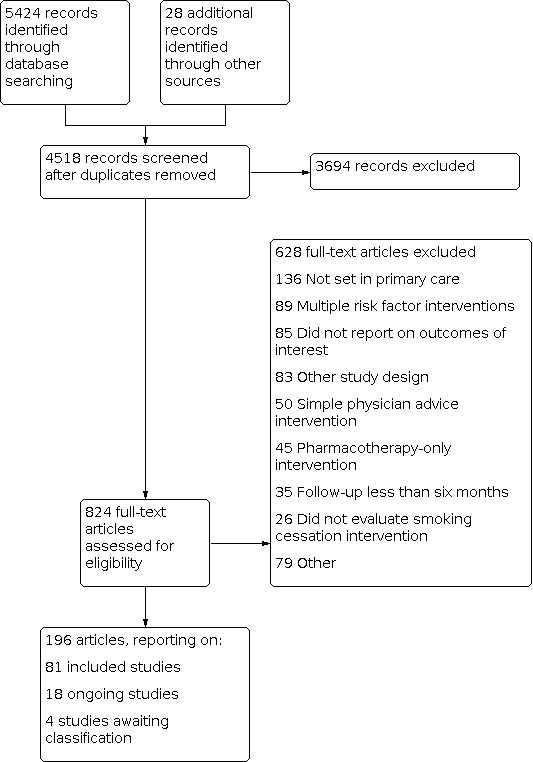
Study flow diagram.
Included studies
Of the 81 included studies 43 were individually‐randomized RCTs, 37 were c‐RCTs, and one was a factorial trial. Thirty‐nine studies were conducted in Europe, 26 in the USA, seven in Australia, two each in South America, South Korea and Canada, and one each in China, Pakistan and Thailand. All studies were conducted in primary care settings.
Participants
The 81 included studies represented 112,159 participants. Individual study sample sizes ranged from 48 to 6856. For all but one of the outcomes, all participants were people who attended the practices as patients and smoked tobacco at study baseline. However, where studies measured the number of patients who were asked whether they smoked, participants contributing to the outcome included anyone attending the primary care practice. These non‐smoking participants are included in the total number of participants specified above, but very few studies assessed this outcome.
Five studies recruited from specific population groups; two of these specifically recruited participants with COPD, one recruited participants with diabetes and hypertension, another participants with diabetes only, and the final study recruited participants with a low or moderate household income. The average age of participants ranged from 33 to 64 across studies, and average cigarettes per day ranged from 14 to 26.
Interventions and comparators
We classified study arms according to whether they offered any interventions designed to improve the delivery or success of smoking cessation treatment, over and above standard care. Standard smoking cessation support typically involves brief advice from a physician with a potential offer of medication, and generic printed self‐help materials. Some study arms offered multiple additional components (multicomponent interventions), whereas others offered a single additional component. We classified these components as either patient‐level, provider‐level or practice‐level. Within these classifications we identified the intervention types listed in the table below (for more detailed definitions of the strategies listed see Appendix 3):
| PATIENT‐LEVEL | PROVIDER‐LEVEL | PRACTICE‐LEVEL |
| Adjunctive counseling (offered by a health professional other than the primary care physician, i.e. via a practice nurse, counselor, or smoking quitline) | Provider training | Modified vital sign stamp |
| Tailored printed materials | Provider performance audit and feedback | Treatment flow sheets/consult forms |
| Biomedical feedback (including CO monitoring, gene testing for lung cancer, spirometry and a combination of CO monitoring and spirometry) | Provider incentives | Electronic medical record (EMR) and decision support |
| Medication prompts | ‐ | Outreach facilitation |
| Patient incentives | ‐ | ‐ |
| SMS and Internet cessation programs | ‐ | ‐ |
| Information videos | ‐ | ‐ |
| Access to cost‐free medications (as opposed to medications with a fee, which would be considered standard care) | ‐ | ‐ |
| Proactive outreach | ‐ | ‐ |
Most of the included studies tested smoking cessation intervention components that were provided at the participant level, in addition to standard care (e.g. adjunctive behavioral support, tailored printed materials), rather than testing interventions that aimed to improve the implementation of an existing intervention (e.g. training health providers or EMR prompts), which were provided at the provider or practice level. The latter intervention types are highlighted in bold in the table above.
In order to be included in the review the intervention arm needed to include one or more of the components in the table above in addition to standard smoking cessation care, and in comparison with standard care, in order to isolate the effect of one or more intervention components designed to improve the delivery of smoking cessation treatment in primary care. Studies were also included if they compared an intervention made up of a number of the components above, plus standard care, with the same multicomponent intervention minus one of the components. Again this allowed us to isolate the effect of a single intervention component of interest.
Outcomes
In order to be included in the review, studies had to measure smoking abstinence at six‐month follow‐up or longer, so all 81 studies measured this primary outcome. Most studies had a longest follow‐up of 12 months (42 studies) and 30 studies had a follow‐up of six months. The maximum length of follow‐up was 24 months, measured by five studies. Most studies measured point prevalence abstinence (35 studies), 20 studies measured continuous abstinence and 17 studies measured abstinence that was sustained for a period of time between two time points e.g. between three and six months follow‐up. In nine cases the definition of abstinence used was unclear. Around half of the studies (42 studies) used biochemical validation, such as carbon monoxide monitoring or cotinine levels, to confirm smoking abstinence.
Twenty‐five of the 81 included studies reported on the number of quit attempts made by study participants split by study arm, and 21 of the studies reported on one of the provider performance outcomes in a way that allowed between‐group comparison.
Excluded studies
We list 155 studies excluded at full‐text stage, along with reasons for exclusion, in the Characteristics of excluded studies table. Common reasons for exclusion were that studies were not conducted in a primary care setting, that participant care was focused on multiple risk factors as opposed to just smoking cessation, that follow‐up was less than six months and that the study investigated standard smoking cessation support, such as brief physician advice.
Risk of bias in included studies
Overall, we judged 14 studies to be at low risk of bias, 23 to be at unclear risk, and the remaining 44 at high risk of bias.
Details of 'Risk of bias' judgments for each domain of each included study can be found in the Characteristics of included studies table. Figure 2 illustrates judgments for each included study.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
When judging sequence generation 10 of the included studies were judged to be at high risk of bias, 32 at unclear risk and 39 at low risk. When judging allocation concealment 12 studies were judged to be at high risk, 37 at unclear risk and 32 at low risk. As is common in many older trials, in many cases sequence generation and allocation concealment were not well described in study reports, hence the high numbers of unclear judgments. This does not necessarily mean that bias was present, but that we were unable to make a judgement based on the information available.
Blinding (detection bias)
When judging the quality of outcome assessment for the primary outcome (smoking abstinence), 23 studies were deemed to be at high risk of bias, seven studies were deemed to be at unclear risk and 51 studies at low risk. Those studies at high risk of bias did not biochemically confirm abstinence and the level of participant contact varied between arms. This means that misreporting of abstinence may have been higher in those study arms with higher contact levels due to social pressures.
Incomplete outcome data
Ten studies were judged to be at high risk of attrition bias, 21 studies were judged to be at unclear risk and 50 studies were judged to be at low risk. Studies at low risk had attrition rates of less than 50% overall and had a less than 20% difference in attrition rates between study arms. Studies in which this domain was judged to be unclear either did not report overall attrition, did not report attrition by study arm, or both.
Recruitment bias due to recruitment of participants to clusters after allocation (cluster‐RCTs only)
Of the 37 cRCTs, 32 were judged to be at low risk of bias for this domain, as participants were already patients at the primary care sites (clusters) before randomization of clusters took place. Five studies were deemed to be at unclear risk of bias.
Unbalanced baseline characteristics (cluster‐RCTs only)
Of the 37 cRCTs, five studies reported unbalanced baseline characteristics between study arms and were therefore deemed to be at high risk of bias. Twenty‐nine studies were judged to be at low risk of bias and three at unclear risk.
Statistical adjustment to account for potential correlation effects within clusters (cluster‐RCTs only)
Twenty‐nine of the 37 cRCTs were judged to be at low risk of bias for this domain, as they reported an attempt to test for, or control for the effects of clustering on the analysis. Two studies were judged to be at unclear risk of bias and six studies were deemed to be at high risk.
Other potential sources of bias
One study (Ronaldson 2018) was deemed to be at high risk of 'other' bias due to it using a wait‐list control design. It appeared that participants in the control group knew that they were on a waiting‐list, meaning they may have postponed their quit attempt until after the trial when they knew that they would receive treatment.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Adjunctive counseling in addition to standard smoking cessation care in primary care.
| Adjunctive counseling in addition to standard smoking cessation care in primary care | ||||||
| Patient or population: people who attend primary care and smoke tobacco Setting: primary care (Australia, Europe, South Korea, United States) Intervention: adjunctive counseling plus standard or multicomponent smoking cessation support Comparison: standard or multicomponent smoking cessation support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with control | Risk with adjunctive counseling | |||||
| Smoking abstinence at 6‐month follow‐up or more. All studies | Study population | RR 1.31 (1.10 to 1.55) | 18,150 (22 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 7 per 100 | 9 per 100 (8 to 11) | |||||
| Smoking abstinence at 6‐month follow‐up or more. Subgroup comparator: standard care | Study population | RR 1.43 (1.15 to 1.78) | 12,852 (17 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ | |
| 4 per 100 | 6 per 100 (5 to 8) | |||||
| Smoking abstinence at 6‐month follow‐up or more. Subgroup comparator: multicomponent intervention | Study population | RR 1.04 (0.87 to 1.23) | 5298 (5 RCTs) | ⊕⊕⊝⊝ LOWc | ‐ | |
| 14 per 100 | 14 per 100 (12 to 17) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: Randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to inconsistency. A subgroup analysis subgrouping by the nature of the comparator resulted in substantial subgroup differences (I2 = 80%). bDowngraded one level due to risk of bias. Removing the studies at high risk of bias shifted the confidence intervals so that they incorporated the potential for no benefit of adjunctive counseling. cDowngraded two levels due to imprecision. CI encompassed both potential benefit and harm.
Summary of findings 2. Cost‐free medications used in addition to standard care in primary care.
| Cost‐free medications used in addition to standard care in primary care | ||||||
| Patient or population: people who attend primary care and smoke tobacco Setting: primary care (Australia, Europe, Pakistan, United States) Intervention: cost‐free medications plus standard or multicomponent smoking cessation support Comparison: standard or multicomponent smoking cessation support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with cost‐free medications | |||||
| Smoking abstinence at 6‐month follow‐up or more | Study population | RR 1.36 (1.05 to 1.76) | 7560 (10 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | ‐ | |
| 12 per 100 | 17 per 100 (13 to 22) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: Randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to inconsistency. I2 = 63%. bThe funnel plot highlighted one outlier (the smallest study showed a large positive effect of the intervention). However, when this outlier was removed from the analysis the interpretation of the result remained consistent.
Summary of findings 3. Biomedical feedback in addition to standard smoking cessation treatment in primary care.
| Biomedical feedback in addition to standard smoking cessation treatment in primary care | ||||||
| Patient or population: people who attend primary care and smoke tobacco Setting: primary care (Europe, USA) Intervention: biomedical feedback plus standard smoking cessation support Comparison: standard smoking cessation support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with biomedical feedback | |||||
| Smoking abstinence at 6‐month follow‐up or more | Study population | RR 1.07 (0.81 to 1.41) | 3491 (7 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 10 per 100 | 11 per 100 (8 to 14) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: Randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels due to imprecision. CI encompassed the potential for both benefit and harm.
Summary of findings 4. Tailored print materials in addition to standard smoking cessation treatment in primary care.
| Tailored print materials in addition to standard smoking cessation treatment in primary care | ||||||
| Patient or population: people who attend primary care and smoke tobacco Setting: primary care (Europe) Intervention: tailored print materials plus standard smoking cessation support Comparison: standard smoking cessation support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with tailored print materials | |||||
| Smoking abstinence at 6‐month follow‐up or more | Study population | RR 1.29 (1.04 to 1.59) | 15,978 (6 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 3 per 100 | 4 per 100 (4 to 5) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias. Removing the two studies judged to be at high risk of bias shifted the CI so that it incorporated the potential for no difference in cessation rates between intervention and comparator groups.
Summary of findings 5. Provider training in addition to standard smoking cessation treatment in primary care.
| Provider training in addition to standard smoking cessation treatment in primary care | ||||||
| Patient or population: people who attend primary care and smoke tobacco Setting: primary care (Argentina, Canada, Europe, USA) Intervention: provider training plus standard or multicomponent smoking cessation support Comparison: standard or multicomponent smoking cessation support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with provider training | |||||
| Smoking abstinence at 6‐month follow‐up or more | Study population | RR 1.10 (0.85 to 1.41) | 13,685 (7 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ | |
| 5 per 100 | 6 per 100 (5 to 8) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RCT: Randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to inconsistency. I2 = 66%. bDowngraded one level due to imprecision. CI incorporated the potential for both benefit of the intervention and no difference between intervention and control (taking into account the anticipated absolute effects).
Summary of findings 6. Provider incentives in addition to standard smoking cessation treatment in primary care.
| Provider incentives in addition to standard smoking cessation treatment in primary care | ||||||
| Patient or population: people who attend primary care and smoke tobacco Setting: primary care (Germany, USA) Intervention: provider incentives plus standard or multicomponent smoking cessation support Comparison: standard or multicomponent smoking cessation support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with provider incentives (provider‐level) | |||||
| Smoking abstinence at 6‐month follow‐up or more | Study population | RR 1.14 (0.97 to 1.34) | 2454 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | ‐ | |
| 18 per 100 | 21 per 100 (17 to 24) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to risk of bias: both included studies were judged to be at high risk of bias. bDowngraded two levels due to imprecision: CIs incorporate the potential of both benefit and harm.
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6 for summaries of effect estimates and GRADE ratings. See: Supplementary file 1; Supplementary file 2 for the results of analyses controlling for the effects of clustering in both individual studies and meta‐analyses.
Studies were meta‐analyzed where there was more than one study providing data for an outcome for a comparison. The first three comparisons (adjunctive counseling, cost‐free medications, biomedical feedback) investigate patient‐level interventions intended to directly improve smoking quit rates, whereas the fourth and fifth comparisons investigate provider‐level interventions which are designed to boost provider implementation of smoking cessation support, in order to ultimately improve quit rates.
Adjunctive counseling (patient‐level strategy)
We found 22 studies that looked at the effect of adding counseling (delivered by an allied health professional rather than the primary care physician) to standard care or a multicomponent smoking cessation intervention. Pooling these studies provided evidence that additional counseling resulted in more favorable smoking quit rates (RR 1.31, 95% CI 1.10 to 1.55; I2 = 44%; 18,150 participants; Analysis 1.1). The interpretation of the result remained the same when 12 studies judged to be at high risk of bias were removed from the analysis, when 15 individually‐randomized studies were removed from the analysis, and when sensitivity analyses adjusting for clustering were carried out.
1.1. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 1: Long‐term abstinence (subgrouped by single vs. multicomponent intervention type)
However, a subgroup analysis grouping the studies by whether counseling was provided as an adjunct to standard care alone or as an adjunct to an intervention that also included other strategies designed to improve smoking cessation treatment, found evidence of a subgroup difference (I2 = 80%). Where the counseling was used as an add‐on to standard care the RR was 1.43 (95% CI 1.15 to 1.78; I2 = 39%; 18 studies, 12,852 participants), suggesting a beneficial effect of counseling. However, where the counseling was added to standard care with other potential improvement strategies the RR for counseling was 1.04, with CIs suggesting that the addition of counseling could provide no benefit or could potentially enhance or decrease the quit rate (95% CI 0.87 to 1.23; I2 = 9%; 7 studies, 5298 participants). Further subgrouping by provider, mode of delivery and intensity of counseling did not provide evidence that the effect of adjunctive counseling was influenced by these factors (Analysis 1.2; Analysis 1.3; Analysis 1.4).
1.2. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 2: Long‐term abstinence (subgrouped by provider)
1.3. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 3: Long‐term abstinence (subgrouped by mode)
1.4. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 4: Long‐term abstinence (subgrouped by intensity)
More than one of the included studies investigating the effects of adjunctive counseling measured each of the following process measures: advice rates; assistance rates; arrangement of follow‐up; quit attempts. The evidence was inconclusive on whether adjunctive counseling improved rates of smoking cessation advice, the provision of self‐help materials or counselling, or assistance to set a quit date (Analysis 1.5; Analysis 1.6); however, there was some evidence that adjunctive counseling may have a beneficial impact on the provision of smoking cessation medications (Analysis 1.6), and the number of people who made a quit attempt (Analysis 1.8). There was also some evidence, limited by imprecision, that adjunctive counseling may increase the arrangement of patient follow‐up by physicians (Analysis 1.7). When pooling the relevant data statistical heterogeneity was high (I2 = 83%), but we decided not to suppress the pooled effect estimate as all of the point estimates demonstrated a beneficial effect of adjunctive counseling.
1.5. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 5: Advise rates
1.6. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 6: Assistance rates
1.8. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 8: Quit attempts
1.7. Analysis.

Comparison 1: Adjunctive counseling (patient‐level), Outcome 7: Arrange follow‐up support rates
Cost‐free medications (patient‐level strategy)
We pooled 10 RCTs looking at the effect of adding cost‐free medications to standard smoking cessation care or including cost‐free medications as part of a multicomponent smoking cessation intervention. There was evidence that providing cost‐free medication increased the number of people who successfully quit smoking (RR 1.36, 95% CI 1.05 to 1.76; I2 = 63%; 7560 participants; Analysis 2.1). Although moderate statistical heterogeneity was detected, subgrouping by whether cost‐free medications were added to standard care alone or were delivered as part of a multicomponent intervention did not result in a meaningful subgroup effect (I2 = 0%). We judged seven of the studies included in this analysis to be at high risk of bias, but a sensitivity analysis removing these did not result in a meaningful change to the result. Likewise, sensitivity analyses removing the five studies individually randomized and investigating the potential impact of clustering on the effect estimate did not result in meaningful changes to the result.
2.1. Analysis.

Comparison 2: Cost‐free medications (patient‐level), Outcome 1: Long‐term abstinence (subgrouped by single vs. multicomponent intervention type)
Three of the studies that investigated cost‐free medications also investigated their effect on participant quit attempts. There was evidence that their provision resulted in a higher number of quit attempts made (RR 1.21, 95% CI 1.02 to 1.43; 3 studies, 2669 participants; Analysis 2.2). Heterogeneity was substantial (I2 = 72%), but in all cases the study effect estimates favored the intervention arm.
2.2. Analysis.

Comparison 2: Cost‐free medications (patient‐level), Outcome 2: Quit attempts
Biomedical feedback (patient‐level strategy)
We identified seven trials looking at the effects of adding biomedical feedback to smoking cessation treatment in primary care. Four of these studies investigated spirometry (Irizar Aramburu 2013; Parkes 2008; Ronaldson 2018; Segnan 1991), one study investigated CO monitoring (Jamrozik 1984), one study CO monitoring and spirometry (Sippel 1999), and one study looked at the effect of gene testing for lung cancer risk (Nichols 2017). We pooled the seven studies and subgrouped according to feedback type. There was no clear evidence of a beneficial effect of biomedical feedback on smoking cessation rates (RR 1.07, 95% CI 0.81 to 1.41; I2 = 40%; 3491 participants; Analysis 3.1). The result was imprecise, with a CI encompassing the potential for both an increase and a decrease in quit rates. There was no evidence of a difference in effect depending on the type of biomedical feedback used (I2 = 0%), and a sensitivity analysis removing the three studies at high risk of bias did not change the interpretation of the results. None of the studies included in this meta‐analysis were cluster‐RCTs, so we did not conduct a sensitivity analysis removing individually‐randomized studies or adjusting for clustering.
3.1. Analysis.

Comparison 3: Biomedical feedback (patient‐level), Outcome 1: Long‐term abstinence (subgrouped by type)
Tailored print materials (patient‐level strategy)
We pooled six studies assessing the addition of tailored printed materials to standard smoking cessation support, subgrouped based on the theoretical basis of the tailoring (Analysis 4.1). Two of the studies were based on the transtheoretical model, but four studies did not have a clear theoretical basis. Overall, there was evidence that providing participants with tailored printed materials increased their smoking cessation rates (RR 1.29, 95% CI 1.04 to 1.59; I2 = 37%; 15,978 participants), and there was no evidence that the effect was moderated by the theoretical basis of the material. However, a sensitivity analysis removing two studies at high risk of bias resulted in increased imprecision, so that the resulting CIs encompassed the possibility of no effect of tailored printed materials on smoking cessation rates, as well as a potential positive impact (RR 1.25, 95% CI 0.92 to 1.70). This analysis contained no c‐RCTs and therefore sensitivity analyses were not required to assess the potential effects of removing individually‐randomized studies or adjusting for clustering.
4.1. Analysis.

Comparison 4: Tailored print materials (patient‐level), Outcome 1: Long‐term abstinence (subgrouped by theoretical basis)
Three of the studies that assessed the effects of tailored printed materials also looked at quit attempts as an outcome (Gilbert 2013; Gilbert 2017; Hoving 2010). The pooled effect estimates and 95% CIs incorporated the possibility that providing tailored printed materials led to no increase in attempts to quit smoking, as well as the possibility of an increase in quit attempts (RR 1.08, 95% CI 1.00 to 1.17; I2 = 17%; 11,122 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: Tailored print materials (patient‐level), Outcome 2: Quit attempts
Provider training (provider‐level strategy)
Seven RCTs looked at the effects of adding provider smoking cessation training to other smoking cessation strategies or standard treatment. Pooling these studies resulted in an RR of 1.10 (95% CI 0.85 to 1.41; 13,685 participants; Analysis 5.1). There was no evidence of a clear benefit of provider training, but there was evidence of both substantial imprecision and moderate statistical heterogeneity (I2 = 66%). There was no evidence that the effect differed depending on whether provider training was offered in addition to and compared with standard care, or whether provider training was offered alongside other strategies to improve the delivery of smoking cessation and compared with those multicomponent interventions minus provider training (I2 = 0%). Sensitivity analyses removing two studies judged to be at high risk of bias and adjusting for the effect of clustering had no appreciable impact on the result or its interpretation. As none of the studies was individually randomized a sensitivity analysis removing this study type was not required.
5.1. Analysis.

Comparison 5: Provider training (provider‐level), Outcome 1: Long‐term abstinence
A number of studies examined the effect of training on provider performance and participant quit attempts outcomes. Evidence from meta‐analyses suggested that provider training increased the amount that physicians asked participants whether they smoked tobacco, increased the number of people physicians advised about their smoking, and increased the amount of assistance given in the form of providing printed self‐help materials and providing counseling (Analysis 5.2; Analysis 5.3; Analysis 5.4). In some of these cases statistical heterogeneity was high, but point estimates always favored the intervention, and so we deemed it appropriate to present a pooled estimate. In four cases (aiding the participant in setting a quit date; provision of smoking cessation medication, participant quit attempts and the arrangement of follow‐up support), the point estimates favored provider training, but there was imprecision, so that the CIs incorporated the possibility of no effect of provider training, as well as a potential positive effect (Analysis 5.4; Analysis 5.5; Analysis 5.6).
5.2. Analysis.

Comparison 5: Provider training (provider‐level), Outcome 2: Asking rates
5.3. Analysis.

Comparison 5: Provider training (provider‐level), Outcome 3: Advise rates
5.4. Analysis.

Comparison 5: Provider training (provider‐level), Outcome 4: Assistance rates
5.5. Analysis.

Comparison 5: Provider training (provider‐level), Outcome 5: Arrange follow‐up support rates
5.6. Analysis.

Comparison 5: Provider training (provider‐level), Outcome 6: Quit attempts
Provider incentives (provider‐level strategy)
Two studies looked at the effects of the addition of provider incentives to standard smoking cessation care or as part of a multicomponent smoking cessation intervention. When pooled these studies resulted in an RR of 1.14 (95% CI 0.97 to 1.34; I2 = 0%; 2454 participants; Analysis 6.1). There was imprecision, with CIs incorporating the potential for a reduction in quit rates as well as the potential for an increase, when provider incentives were implemented. Subgrouping by whether the intervention was provided alongside standard care alone or other delivery improvement strategies provided no evidence of effect moderation, and a sensitivity analysis adjusting for clustering did not affect our interpretation of the result. Neither of the studies was individually randomized and so a sensitivity analysis to remove this type of study was not required. However, we rated both studies at high risk of bias and so results should be interpreted with caution.
6.1. Analysis.

Comparison 6: Provider incentives (provider‐level), Outcome 1: Long‐term abstinence
Other single strategies
In addition, to the studies described as part of the comparisons above, Supplementary file 3 narratively summarizes six additional included studies that investigated a single novel strategy. These strategies were reinforcement text messages (Cobos‐Campos 2016), proactive patient outreach by mailings and telephone (Fu 2014), a smoking cessation video (Lee 2016), an internet smoking cessation program (Pisinger 2010), tailored letters to participants and a provider desktop resource with treatment advice (Meyer 2012). There was also a randomized factorial trial which investigated a number of different strategies (Piper 2016).
Multi‐component interventions
Thirty‐four included studies compared the combination of two or more strategies to improve the delivery of smoking cessation treatment in primary care (multicomponent interventions) in addition to standard smoking cessation, in comparison with a control arm of standard care. These studies are summarized narratively in Supplementary file 4 with RRs and their corresponding 95% confidence intervals. Twenty‐seven (77%) of the multicomponent studies investigated patient‐level strategies, 25 (71%) provider‐level strategies, and 12 (34%) practice‐level strategies. Some studies incorporated strategies from all three levels, with a maximum of five different strategies used in some studies. Where more than one of these studies investigated the same combination of strategies we conducted meta‐analyses.
Three studies looked at the effect of adjunctive counseling and cost‐free medications (both patient‐level strategies designed to directly increase quit rates) on smoking abstinence rates. The pooled estimate suggested a benefit of providing these two intervention components in addition to standard support (RR 3.09, 95% CI 1.13 to 8.44; 1066 participants; Analysis 7.1). However, this result should be treated with caution, as we judged all of the studies to be at high risk of bias. There was also substantial statistical heterogeneity (I2 = 75%), but as the point estimates all indicated benefit we deemed it appropriate to present a pooled estimate. None of the three studies in this analysis was a cRCT and so sensitivity analyses removing individually‐randomized studies and adjusting for clustering were not necessary.
7.1. Analysis.

Comparison 7: Adjunctive counseling + cost‐free meds versus standard care, Outcome 1: Long‐term abstinence
Six studies investigated the effects of both adjunctive counseling and provider training in addition to standard care (combining patient‐ and provider‐level strategies intended to directly boost quit rates and increase implementation respectively). The pooled analysis suggested a benefit of these components on smoking cessation rates (RR 2.66, 95% CI 1.27 to 5.57; 11,310 participants; Analysis 8.1). Again, statistical heterogeneity was high (I2 = 96%), but in all cases the point estimates of individual studies indicated a benefit of the interventions. A sensitivity analysis removing two studies at high risk of bias found that there was a marked impact on the interpretation of the results, with the CIs indicating that the interventions had the potential to decrease as well as the potential to increase quit rates (RR 2.88, 95% CI 0.89 to 9.28). Sensitivity analyses removing the one individually‐randomized controlled trial and adjusting for clustering did not change the interpretation of the results.
8.1. Analysis.

Comparison 8: Adjunctive counseling + provider training versus standard care, Outcome 1: Long‐term abstinence
We pooled three studies that looked at the effect of both provider training and treatment flow sheets to aid provider decision‐making (provider‐ and practice‐level strategies, both aimed at increasing provider implementation of smoking cessation support). The overall result suggested that these combined interventions boosted participant quit rates (RR 1.70, 95% CI 1.27 to 2.27; I2 = 0%; 2651 participants; Analysis 9.1). The interpretation of this result remained the same when we removed the single study deemed to be at high risk of bias from the analysis, when the one study that was individually randomized was removed, and when adjustments were made to control for any clustering effects. Two of the studies in this analysis also examined some of our secondary process outcomes (in this case rates physicians asked about smoking, rates physicians assisted participants to quit by supplying medications and rates physicians arranged follow‐ups for participants). In all cases statistical heterogeneity was high (I2 > 90%). For the arrangement of follow‐up we still present the pooled estimate, as for both studies the point estimate was in favor of the intervention (Analysis 9.4). However, for the asking and assistance rates we have suppressed the pooled estimates, as in both cases the point estimates of one of the studies indicated a benefit of the intervention, whilst the other indicated harm (Analysis 9.2; Analysis 9.3). All of the outcomes for this comparison were subject to considerable imprecision due to low event rates (< 300 participants for all analyses).
9.1. Analysis.

Comparison 9: Provider training + flow sheet versus standard care, Outcome 1: Long‐term abstinence
9.4. Analysis.

Comparison 9: Provider training + flow sheet versus standard care, Outcome 4: Arrange follow‐up support rates
9.2. Analysis.

Comparison 9: Provider training + flow sheet versus standard care, Outcome 2: Asking rates
9.3. Analysis.

Comparison 9: Provider training + flow sheet versus standard care, Outcome 3: Assistance rates
Two studies investigated the effect of a combination of provider training and outreach facilitation (provider‐ and practice‐level strategies, both aimed at increasing provider implementation of smoking cessation support) in addition to and in comparison with standard smoking cessation care. For the smoking abstinence outcome the pooled RR was 1.55 (95% CI 0.95 to 2.52; I2 = 0%; 2972 participants; Analysis 10.1). Neither of the studies was judged to be at high risk of bias, or was individually randomized, and adjustments to account for clustering did not affect the interpretation of the result. However the result was imprecise with the confidence intervals indicating the potential for the intervention to result in no improvement in cessation rates, as well as a substantial improvement. The two studies in this analysis also measured a number of the provider performance outcomes, as well as the participant quit attempts outcome. These offered some evidence that the combination of provider training and outreach facilitation strategies resulted in providers assisting more patients to stop smoking by helping them to set a quit date, providing more patients with self‐help materials (Analysis 10.3), and making arrangements to follow up more participants (Analysis 10.4). The point estimate for the rates providers asked their patients about their smoking was also in favor of provider training plus outreach facilitation, however, there was imprecision, with CIs incorporating the possibility of no effect of the intervention, as well as a potential positive effect (Analysis 10.2). There was no evidence that provider training and outreach facilitation increased the rate that physicians assisted patients by providing smoking cessation medication (Analysis 10.3), or increased the number of participants who made a smoking quit attempt (Analysis 10.5).
10.1. Analysis.

Comparison 10: Provider training + outreach facilitation versus standard care, Outcome 1: Long‐term abstinence
10.3. Analysis.

Comparison 10: Provider training + outreach facilitation versus standard care, Outcome 3: Assistance rates
10.4. Analysis.

Comparison 10: Provider training + outreach facilitation versus standard care, Outcome 4: Arrange follow‐up support rates
10.2. Analysis.

Comparison 10: Provider training + outreach facilitation versus standard care, Outcome 2: Asking rates
10.5. Analysis.

Comparison 10: Provider training + outreach facilitation versus standard care, Outcome 5: Quit attempts
Studies without data
There were five studies that we were unable to include in our syntheses as the relevant data were not presented, or they were not presented in a form that we could use (Buffels 2006; Canga 2000; RBR‐7yx9hd; Salkeld 1997; Sherman 2008). In all cases we tried to contact the authors, but without success. Further information on these studies is available in the Characteristics of included studies tables.
Discussion
Summary of main results
This review includes 81 studies investigating the effectiveness of interventions designed to improve the delivery, success, or both, of smoking cessation treatment in primary care. The strategies tested across studies varied widely, with some focusing on additional components, such as more intensive counseling or tailored printed materials, to standard support, and some focusing on improving the implementation of standard care, by training providers or offering provider incentives. Most looked at the former rather than the latter. Some studies looked into singular strategies and others looked at the effects of multicomponent interventions. We were able to carry out analyses of studies investigating the following singular strategies when offered in addition to standard or other smoking cessation support: adjunctive counseling; cost‐free medications; biomedical feedback; tailored print materials; provider training; provider incentives. We found moderate‐certainty evidence that adjunctive counseling (delivered by a health professional other than the primary care physician), cost‐free medications and tailored print materials all had a beneficial impact on smoking quit rates. However, there was some evidence that the beneficial impact of adjunctive counseling was only evident when offered in addition to standard smoking cessation care. When adjunctive counseling was offered as part of a multicomponent intervention to increase the delivery of smoking cessation treatment, there was no clear evidence that the isolated effect of adjunctive counseling was favorable. This could be because the multicomponent interventions in the comparison arms raised quit rates substantially on their own, and the relative utility of additional intervention support declines once a certain level of support is already available. There was some limited evidence that providing adjunctive counseling led to a higher provider implementation level, i.e. adjunctive counseling was associated with an increase in the provision of smoking cessation medications and in the arrangement of participant follow‐up, and that both adjunctive counseling and cost‐free medications increased the likelihood of participants making a quit attempt. There was also low‐certainty evidence that biomedical feedback and provider training, and very low‐certainty evidence that provider incentives did not have a clear beneficial impact on smoking cessation rates when used as strategies to improve the delivery of tobacco use treatment in primary care. However, there was some evidence of an improvement in some of the markers of physician performance in response to physician training, although not in patient quit attempts.
Among the multicomponent interventions assessed, a wide variety of individual strategies were tested, leading to considerable heterogeneity in our cessation outcomes across studies. However, where more than one study examined the effects of the same combination of strategies in comparison to standard care we were able to conduct meta‐analyses. There was some evidence that adjunctive counseling combined with either cost‐free medications or provider training, enhanced quit rates when compared to standard care alone. However, these results were limited by small numbers of events and high statistical heterogeneity, and the analyses included a large proportion of studies judged to be at high risk of bias. Two analyses looking at the effect of combining provider training with flow sheets to aid physician decision‐making, and with outreach facilitation found no clear evidence that these combinations of strategies increased participant quit rates, but these analyses were again limited by imprecision. There was some limited evidence that these two latter combinations may have a positive impact on the number of patients assisted to make a quit attempt.
A number of studies investigated unique singular strategies or combinations of strategies. We reported on these narratively, with effects differing considerably across intervention types.
Overall completeness and applicability of evidence
The searches conducted for this review were broad, in our attempt to find any smoking cessation study that took place in primary care. As well as medical databases, we also searched trial registers to identify any ongoing or completed but unpublished registered studies. We therefore feel confident in our search approach. Most of the studies identified in this review were conducted in Europe and the USA, and therefore are specific to these settings. As primary care and standard smoking cessation support differ globally this may affect the applicability of the evidence outside of these settings and may also have contributed to some of the heterogeneity in results.
Most studies were carried out in primary care patients in general, rather than in specific patient groups and so the results should be relevant to the former population; however, the characteristics of these groups are likely to differ across countries due to a variety of factors, including access to treatment services through public or private health care.
More than half of the studies included in this review individually randomized participants rather than being cluster‐randomized. As such, these trials test whether interventions or strategies could be effective, for example showing evidence that adjunctive counseling is an effective intervention when delivered by primary care staff to patients in primary care willing to attempt to quit smoking with behavioral support. Individually, randomized studies can tell us very little in addition to the existing reviews of smoking cessation intervention components in the general population (Boyle 2014; Carson 2012; Clair 2019; Fiore 2008; Rice 2017; Stead 2013; Van den Brand 2017).The key issue that needs addressing, however, is interventions to increase the engagement of clinicians in primary care in proactively raising the topic of smoking and delivering effective support when they do so. Making adjunctive counseling available could do this because clinicians may feel that they have something active to offer their patients, motivating them to intervene. Only cluster‐randomized trials could address this question, but none have done so. Arguably, the biggest gains in smoking cessation would come from interventions to increase the uptake of effective aids to cessation (pharmacotherapy and behavioral support), and interventions that achieve this through engaging primary care staff are needed. Cluster‐randomized trials will be needed to test these interventions.
Studies had to assess long‐term smoking abstinence as a criterion for inclusion; most studies were therefore able to contribute cessation data to the relevant comparisons. However, due to the wide range of relevant comparisons the data for each of these were sparse in places. In addition, some studies investigated our secondary process outcomes looking at provider performance and participant quit attempts. However, they were not measured as consistently as smoking abstinence, and in many cases were subject to substantial statistical heterogeneity, imprecision, or both. No clear conclusions could therefore be drawn from these secondary outcomes and their interpretation is likely to change as more evidence becomes available. As we were interested in our secondary outcomes as process outcomes, there were studies that could have contributed to these outcomes that were not included, as they did not measure cessation.
Due to the marked clinical variance in the nature of the strategies assessed in studies comparing multicomponent interventions to standard care in this review, we did not attempt to pool these studies, and we have drawn only a few tentative conclusions on the efficacy of interventions that used a number of different strategies to improve the delivery of smoking cessation treatment. Component network meta‐analysis could be used in the future, to investigate the relative effectiveness of relevant intervention strategies and how they could be combined to build effective multicomponent strategies in primary care. This was unfortunately out of scope for this review.
Quality of the evidence
Of the 81 studies included in this review, we judged 14 to be at low risk of bias for all domains, 44 to be at high risk in one or more domains, and the remainder to be at unclear risk. In many cases, studies were rated at an unclear risk because they did not report key information. In these cases, it is impossible to know whether these studies were at any risk of bias or whether the information was simply not reported. To investigate the potential impact on results of studies that we judged to be at high risk of bias, we removed these studies in sensitivity analyses. This only affected the interpretation of the overall pooled result in a single case; removing the two studies judged to be at high risk of bias in the tailored print materials analysis shifted the CI so that it incorporated the potential for no difference in cessation rates between the intervention and control group, as well as a beneficial effect of tailored print materials.
We assessed the certainty of the evidence by creating summary of findings tables for analyses investigating each of the singular strategies to improve the delivery of smoking cessation care: adjunctive counseling (Table 1); cost‐free medications (Table 2); biomedical feedback (Table 3); tailored print materials (Table 4); provider training (Table 5); and provider incentives (Table 6). We carried out GRADE ratings for the smoking cessation outcome for each one.
The certainty of the evidence that adjunctive counseling, cost‐free medications and tailored print materials resulted in an increase in long‐term smoking quit rates was judged to be moderate. All of these comparisons were downgraded once; for adjunctive counseling and cost‐free medications this was due to inconsistency. In the former case, a subgroup analysis resulted in substantial subgroup differences (I2 = 80%), suggesting that there was only clear evidence of a beneficial effect of adjunctive counseling when it was offered alongside standard care alone, and not when it was offered alongside other strategies to increase the delivery of smoking cessation care. In the latter case, the pooling of eligible studies resulted in moderate unexplained statistical heterogeneity (I2 = 63%). The evidence for tailored print materials was downgraded once due to risk of bias. Removing the two studies judged to be at high risk of bias from the analysis shifted the CI so that it incorporated the potential for no difference in cessation rates between the intervention and comparator groups.
We judged the evidence that biomedical feedback and provider training did not have a clear benefit for cessation rates to be of low certainty in both cases. In the case of biomedical feedback, we downgraded by two levels due to imprecision. The CIs accompanying the pooled estimate incorporated the possibility of both an increase and a decrease in quit success rates as a result of providing biomedical feedback in addition to other smoking cessation treatment in primary care. We downgraded the evidence on provider training once due to imprecision and once due to inconsistency. The CI incorporated the potential for both benefit of the intervention and no difference between intervention and control, and unexplained statistical heterogeneity was identified between studies (I2 = 66%).
Finally, we rated the evidence indicating no clear benefit of provider incentives to improve smoking quit rates in primary care as of very low certainty. The CIs incorporated the potential for the intervention to cause both benefit and harm, and so we downgraded the evidence twice for imprecision. In addition, both of the two included studies were judged to be at high risk of bias, and so we downgraded a third time due to risk of bias. As a result, we have very little confidence in the effect estimate, and the true effect of provider incentives is likely to be substantially different from our estimate of effect.
Potential biases in the review process
To conduct this review we followed standard Cochrane methods and consider the review process used to be robust. For risk of bias outcome assessment, we followed the standard methods used for all Cochrane Tobacco Addiction Group cessation reviews. We also considered participants lost to follow‐up as continuing to smoke, which is standard practice in this field (West 2005). Our search strategy included the Cochrane Tobacco Addiction Group Specialized Register, which incorporates results from trial registers, and we were able to identify a number of ongoing studies. However, there may be unpublished data that we did not uncover.
Behavioral smoking cessation interventions are not always well described, and it is possible that we may have misclassified interventions by misinterpreting them, or not identifying all of the strategies used within a study. In addition, comparator groups are often sparsely described in smoking cessation studies, making it difficult to be certain what is meant by the terms 'usual' or 'standard' care. This means that in some cases it was hard to be completely sure what usual or standard care entailed, and this may have differed substantially across studies (Black 2020). As studies took place over multiple different countries, where primary care practices differ, this seems particularly likely to be the case in this instance. However, we categorized the studies as rigorously as possible, given the information provided.
Two of our analyses included 10 or more studies, so we generated two funnel plots to investigate the potential for publication bias. Figure 3 illustrates the funnel plot for the adjunctive counseling comparison and found no evidence of publication bias. Figure 4 illustrates the funnel plot for the analysis of cost‐free medications. In this case there was one outlier in the analysis, which resulted in a skewed funnel plot. However, when this study was removed from the analysis it had no impact on the interpretation of the result, and so is not biasing the result.
3.

Funnel plot of comparison: 1 Adjunctive counseling (patient‐level), outcome: 1.1 Long‐term abstinence (subgrouped by single vs. multicomponent intervention type).
4.

Funnel plot of comparison: 2 Cost‐free medications (patient‐level), outcome: 2.1 Long‐term abstinence.
Where authors of this review were authors of included studies eligibility decisions, data extraction and risk of bias assessments were made independently by members of the team who were not authors of those studies.
Agreements and disagreements with other studies or reviews
This review follows two previous non‐Cochrane reviews, examining strategies to influence provider smoking cessation support behaviors in the primary care setting. Anderson 2004 reviewed the literature published up to 2001 and Papadakis 2010 published an update which covered the literature prior to 2009. Anderson 2004 focused solely on educational or practice‐level interventions, and found evidence that educational programs for providers did increase smoking quit rates. This impact was higher for practitioners who were still in training than for established practitioners, and could help to explain the difference in our results. A Cochrane Review specifically looking at the effects of smoking cessation training for any health professional also found that quit rates were improved when professionals were trained in providing smoking cessation support (Carson 2012). It is worth noting that the certainty of the evidence on provider training in this review was low, so there is a possibility that the true effect is substantially different from our estimate of the effect. In addition, our provider implementation outcomes gave some indication that provider training did increase the amount of advice and support that physicians provided, bearing in mind that even brief smoking cessation interventions are effective in primary care and an increase in smoking quit rates would be expected to follow (Aveyard 2012). Further research in this area would be beneficial and could also benefit from identifying those physicians that may gain the most from training.
In line with the findings of this review, Papadakis 2010 found that adjunctive counseling significantly increased rates of smoking abstinence. Papadakis 2010 also concluded that multicomponent interventions appeared to be particularly promising. In our review we took the decision not to pool all multicomponent interventions across strategy types, due to considerable clinical variation between studies, and so we are unable to draw conclusions on the effectiveness of multicomponent interventions as a whole. However, identifying all of the relevant studies and summarizing them narratively confirmed substantial heterogeneity in treatment effects, as was found in a similar review by Martin‐Cantera 2015. This unexplained variation identifies this as an important area in which to conduct future research to inform primary care practice. Although we could not explore all of the apparent complexity using standard meta‐analysis methods, a component network meta‐analysis would allow consideration of all the different strategies used, and look at the effects of both combining and comparing different approaches.
As well as Carson 2012, a number of other Cochrane Reviews have looked at the effects of the strategies tested in this review in the wider population. A review that looked at the effect of adding or increasing the intensity of behavioral support for people using smoking cessation medications also found a benefit of adjunctive counseling (RR 1.15, 95% CI 1.08 to 1.22, I2 = 8%; 65 studies, 23,331 participants; Hartmann‐Boyce 2019). Van den Brand 2017 investigated healthcare financing systems for increasing the use of tobacco dependence treatment, incorporating the use of both cost‐free medications and provider incentives. Like us, they found evidence that financial interventions directed at people who smoked had a favorable effect on abstinence at six months or longer, and no clear effect of provider incentives on smoking quit rates. A review of biomedical risk assessment for smoking cessation (Clair 2019) and a review of print‐based self‐help interventions (Livingstone‐Banks 2019) also found similar results to those found in this review, i.e. no clear evidence of a benefit from a variety of biomedical feedback interventions, but a benefit of tailored print materials when provided as an adjunct to brief advice (RR 1.72, 95% CI 1.17 to 2.53; I2 = 10%; 1839 participants).
Authors' conclusions
Implications for practice.
There is moderate‐certainty evidence that the following patient‐level strategies: counseling (provided by health professionals other than the primary care physician); cost‐free medications; tailored print materials, may increase smoking quit rates when provided in addition to standard smoking cessation care in primary care practice.
There is no clear evidence of increased long‐term smoking quit rates when biomedical feedback is provided to patients, or when providers receive training or incentives to provide smoking cessation support, in addition to standard care. However this evidence was of low or very low certainty, and there was some evidence that provider training may increase provider implementation of smoking cessation support. Further evidence is therefore likely to change our conclusions.
Research studies have tested a wide range of strategies and combinations of strategies designed to aid the delivery of tobacco use treatment in primary care. Effects differ substantially across studies and very few studies have investigated the same combinations of interventions; we were therefore unable to draw conclusions on the most effective multicomponent interventions based on the findings of this review.
Implications for research.
Most studies in this review assessed smoking cessation interventions that have already been tested in the wider population, with similar results. Fewer studies assessed interventions to improve or increase the implementation of these techniques. It is likely that the most effective approach to increasing smoking quit rates in primary care would be to combine the smoking cessation interventions demonstrated to be effective in this review with effective strategies to improve the implementation of these interventions. Future studies should consider testing this hypothesis.
Trials are needed to test the effectiveness of interventions to increase the motivation and capacity of primary care staff to support people who smoke to stop. Necessarily, such trials will be cluster‐randomized.
Future studies, examining strategies to improve the delivery of tobacco use over and above standard care should clearly define what standard care incorporates, and provide clear descriptions of all intervention components, as well as the health professionals receiving or delivering the interventions.
There is contradictory evidence on the effectiveness of physician training on smoking cessation to enhance smoking quit rates. Further research should investigate the circumstances under which this training is most beneficial and how it can be designed to maximize success.
Researchers planning further evidence synthesis should take into account the substantial variation in the interventions used across the evidence base and consider using component network meta‐analysis to assess the effects of individual strategies alone, in comparison with one another, and in combination. This would be particularly useful when trying to inform the content of multicomponent interventions to maximize effectiveness.
History
Protocol first published: Issue 3, 2015
Acknowledgements
We would like to acknowledge the support of Lindsay Stead and Jonathan Livingstone‐Banks from the Tobacco Addiction Group for their consultation and support in the preparation of this review. We would also like to thank Tobias Raupach, Institute of Medical Education, University Hospital Bonn, and Daniel Kotz, Institute of General Practice (ifam), Addiction Research and Clinical Epidemiology Unit, Centre for Health and Society (chs), Medical Faculty of the Heinrich‐Heine‐University Düsseldorf for their peer review, Sandra Wilcox for consumer review and Paul Aveyard, University of Oxford for editorial review.
NL's salary is funded through core infrastructure funding for the Cochrane Tobacco Addiction Group from the National Institute for Health Research (NIHR). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Thank you to Hilde Engjom, Marilyn Nicolay for screening non‐English articles. We would also like to thank Jong‐Wook Ban and Lindsay Stead, for their assistance with extracting data from Korean and Spanish articles.
Appendices
Appendix 1. Appendix: PubMed search strategy
| Search | Query |
| #28 | (#23 AND #24 AND #27) (smoking terms, primary care terms, study terms (no animals)) |
| #27 | (#26 NOT #20) (All study terms NOT animals) |
| #26 | (#25 OR #21 OR #22) (Cochrane with eval and clinical) |
| #25 | (#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) (Cochrane Search) |
| #24 | (#8 OR #9 OR #10 OR #11 OR #12) (Primary Care Terms) |
| #23 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) (Smoking Terms) |
| #22 | clinical trial |
| #21 | evaluation studies |
| #20 | (animals [mh] NOT humans [mh]) |
| #19 | trial [ti] |
| #18 | randomly [tiab] |
| #17 | clinical trials as topic [mesh: noexp] |
| #16 | placebo [tiab] |
| #15 | randomized [tiab] |
| #14 | controlled clinical trial [pt] |
| #13 | randomized controlled trial [pt] |
| #12 | general practitioner* |
| #11 | general practice* |
| #10 | family physician* |
| #9 | primary care |
| #8 | primary health care |
| #7 | tobacco use disorder |
| #6 | tobacco use cessation |
| #5 | smoking/therapy |
| #4 | smoking/prevention and control |
| #3 | smoking cessation |
| #2 | nicotine |
| #1 | tobacco |
Appendix 2. Specialised Register search strategy
Searched 02/04/2015 in Cochrane Register of Studies, Tobacco Addiction Group segment
#1 general practitioner*:TI,AB,XKY,MH,EMT,KY,KW
#2 general practice*:TI,AB,XKY,MH,EMT,KY,KW
#3 family physician*:TI,AB,XKY,MH,EMT,KY,KW
#4 primary care:TI,AB,XKY,MH,EMT,KY,KW
#5 primary health care:TI,AB,XKY,MH,EMT,KY,KW
#6 family medicine:TI,AB,XKY,MH,EMT,KY,KW
#7 family practice*:TI,AB,XKY,MH,EMT,KY,KW
#8 physicians, family*:XKY,MH,EMT,KY,KW
#9 physicians, primary care*:XKY,MH,EMT,KY,KW
#10 MeSH DESCRIPTOR Physicians, Family
#11 MeSH DESCRIPTOR Physicians, Primary Care
#12 MeSH DESCRIPTOR Primary Health Care Explode All
#13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #10 OR #11 OR #12
Appendix 3. Glossary of strategies used to improve the delivery of smoking cessation treatment in primary care
Patient‐level
Adjunctive counseling: counseling offered over and above standard care, i.e. brief advice, and provided by a health professional other than the primary care physician ‐ this could be via a practice nurse or counselor, or through a smoking quitline.
Biomedical feedback: measurements are taken from the body, for example exhaled carbon monoxide levels, genetic predisposition to lung cancer, lung function through spirometry testing. This is then fed back to the patient in the context of their smoking behavior
Cost‐free medications: the provision of smoking cessation medications at no cost to the participants (as opposed to medications with a charge, which is considered standard care). We considered in detail whether this intervention type should be categorized as a patient‐level or practice‐level intervention, and decided that it could be categorized as either. We decided on patient‐level in this instance as the patient is the beneficiary of the lack of cost, which has the potential to increase medication use.
Information videos: smoking cessation information provided by a video
Medication prompts: participants are provided with prompts to take their medications, i.e. through automated phone calls or text messages.
Patient incentives: rewards provided to participants for successful smoking cessation.
SMS and internet cessation programs: smoking cessation programs offered in addition to standard smoking cessation support and delivered via text message or the internet.
Proactive outreach: primary care staff proactively contact practice patients via the mail or telephone to raise the issue of their smoking and encourage them to quit and access support.
Tailored print materials: printed self‐help materials tailored to the individual, for example, based on their readiness to quit smoking
Provider‐level
Performance audit and feedback: primary care providers are assessed on their performance of smoking cessation actions and care, e.g. asking patients about whether they smoke and providing smoking cessation medications and counseling. The results of this audit are then fed back to providers.
Provider incentives: primary care providers are provided with financial incentives to meet key smoking cessation‐related performance targets, e.g. assisting patients to quit smoking, patient quit rates.
Provider training: additional training given to primary care smoking cessation support providers on the topic of smoking cessation (this did not include study specific training).
Practice‐level
Electronic medical record (EMR) and decision support: encouragement to record patients smoking status in electronic medical records and to use linked system features such as treatment prompts.
Modified vital sign stamps: an ink stamp used to imprint information on to a patient’s medical record, which, as well as including traditional information on vital signs, also includes information on a patient's smoking status. This was designed to prompt adherence to smoking cessation guidelines.
Outreach facilitation: external facilitators assist primary care physicians with the implementation and quality improvement of smoking cessation care within their practice.
Treatment flow sheets/Consult forms: a document supplied to providers with details of how to provide smoking cessation care that the provider can use to prompt them during a consultation.
Data and analyses
Comparison 1. Adjunctive counseling (patient‐level).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Long‐term abstinence (subgrouped by single vs. multicomponent intervention type) | 22 | 18150 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.10, 1.55] |
| 1.1.1 Adjunctive counseling + standard care vs standard care | 17 | 12852 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.15, 1.78] |
| 1.1.2 Adjunctive counseling + multicomponent int vs multicomponent int | 5 | 5298 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.23] |
| 1.2 Long‐term abstinence (subgrouped by provider) | 22 | 18150 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.10, 1.55] |
| 1.2.1 Nurses | 11 | 3214 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.97, 1.50] |
| 1.2.2 Psychologists & counselors | 12 | 14835 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.03, 1.66] |
| 1.2.3 Pharmacists | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.99, 5.70] |
| 1.3 Long‐term abstinence (subgrouped by mode) | 22 | 18150 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.10, 1.55] |
| 1.3.1 Face to face | 14 | 11753 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.06, 1.61] |
| 1.3.2 Telephone | 10 | 6397 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.98, 1.75] |
| 1.4 Long‐term abstinence (subgrouped by intensity) | 22 | 18150 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.10, 1.55] |
| 1.4.1 Brief/minimal | 6 | 2533 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.07, 1.88] |
| 1.4.2 More substantial | 18 | 15617 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.04, 1.57] |
| 1.5 Advise rates | 2 | 724 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.26] |
| 1.5.1 Adjunctive counseling + standard care vs standard care | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.94, 1.54] |
| 1.5.2 Adjunctive counseling + multicomponent int vs multicomponent int | 1 | 534 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 1.6 Assistance rates | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 Medication | 3 | 1094 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.20, 2.15] |
| 1.6.2 Counseling | 3 | 1460 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.94, 2.88] |
| 1.6.3 Quit date set | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.58, 3.44] |
| 1.6.4 Self‐help materials | 1 | 190 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.70, 1.42] |
| 1.7 Arrange follow‐up support rates | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 Adjunctive counseling + standard care vs standard care | 3 | 1718 | Risk Ratio (M‐H, Random, 95% CI) | 4.65 [1.67, 12.90] |
| 1.8 Quit attempts | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.8.1 Adjunctive counseling + standard care vs standard care | 3 | 1764 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.02, 1.49] |
Comparison 2. Cost‐free medications (patient‐level).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Long‐term abstinence (subgrouped by single vs. multicomponent intervention type) | 10 | 7560 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.05, 1.76] |
| 2.1.1 Cost‐free meds + standard care vs standard care | 6 | 4975 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.05, 2.03] |
| 2.1.2 Cost‐free meds + multicomponent int vs multicomponent int | 4 | 2585 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.81, 1.82] |
| 2.2 Quit attempts | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.2.1 Cost‐free meds + standard care vs standard care | 3 | 2669 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.02, 1.43] |
Comparison 3. Biomedical feedback (patient‐level).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Long‐term abstinence (subgrouped by type) | 7 | 3491 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.81, 1.41] |
| 3.1.1 Spirometry | 4 | 2137 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.72, 1.86] |
| 3.1.2 CO monitoring | 1 | 1040 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.87, 1.51] |
| 3.1.3 Gene testing for lung cancer risk | 1 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.40, 1.80] |
| 3.1.4 CO monitoring & spirometry | 1 | 205 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.29, 1.40] |
Comparison 4. Tailored print materials (patient‐level).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Long‐term abstinence (subgrouped by theoretical basis) | 6 | 15978 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.04, 1.59] |
| 4.1.1 Based on transtheoretical model | 2 | 2470 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.07, 2.17] |
| 4.1.2 No clear theoretical basis | 4 | 13508 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.90, 1.61] |
| 4.2 Quit attempts | 3 | 11122 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.00, 1.17] |
Comparison 5. Provider training (provider‐level).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Long‐term abstinence | 7 | 13685 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.85, 1.41] |
| 5.1.1 Provider training + standard care vs standard care | 5 | 12011 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.81, 1.53] |
| 5.1.2 Provider training + multicomponent int vs multicomponent int | 2 | 1674 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.56, 1.93] |
| 5.2 Asking rates | 4 | 3591 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.05, 1.13] |
| 5.2.1 Provider training + standard care vs standard care | 3 | 2724 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.03, 1.12] |
| 5.2.2 Provider training + multicomponent int vs multicomponent int | 1 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.06, 1.31] |
| 5.3 Advise rates | 4 | 4112 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.02, 1.24] |
| 5.3.1 Provider training + standard care vs standard care | 2 | 2438 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.95, 1.54] |
| 5.3.2 Provider training + multicomponent int vs multicomponent int | 2 | 1674 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.98, 1.12] |
| 5.4 Assistance rates | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.4.1 Quit date set | 3 | 3305 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.86, 3.14] |
| 5.4.2 Self‐help materials | 4 | 4380 | Risk Ratio (M‐H, Random, 95% CI) | 2.32 [1.16, 4.62] |
| 5.4.3 Medication | 2 | 1674 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.90, 1.47] |
| 5.4.4 Counseling | 1 | 867 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.09, 1.45] |
| 5.5 Arrange follow‐up support rates | 3 | 2674 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.95, 2.69] |
| 5.6 Quit attempts | 5 | 6700 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.98, 1.10] |
| 5.6.1 Provider training + standard care vs standard care | 3 | 5026 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.94, 1.10] |
| 5.6.2 Provider training + multicomponent int vs multicomponent int | 2 | 1674 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.98, 1.19] |
Comparison 6. Provider incentives (provider‐level).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Long‐term abstinence | 2 | 2454 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.97, 1.34] |
| 6.1.1 Provider incentives + standard care vs standard care | 1 | 2089 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.98, 1.37] |
| 6.1.2 Provider incentives + multicomponent int vs multicomponent int | 1 | 365 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.43, 1.70] |
Comparison 7. Adjunctive counseling + cost‐free meds versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Long‐term abstinence | 3 | 1066 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [1.13, 8.44] |
Comparison 8. Adjunctive counseling + provider training versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Long‐term abstinence | 6 | 11310 | Risk Ratio (M‐H, Random, 95% CI) | 2.66 [1.27, 5.57] |
Comparison 9. Provider training + flow sheet versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Long‐term abstinence | 3 | 2651 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [1.27, 2.27] |
| 9.2 Asking rates | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.3 Assistance rates | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.3.1 Medication | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9.4 Arrange follow‐up support rates | 2 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 5.53 [0.41, 73.81] |
Comparison 10. Provider training + outreach facilitation versus standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Long‐term abstinence | 2 | 2972 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.95, 2.52] |
| 10.2 Asking rates | 2 | 2700 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.98, 1.64] |
| 10.3 Assistance rates | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.3.1 Medication | 2 | 1321 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.57, 1.22] |
| 10.3.2 Quit date set | 2 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 5.73 [4.19, 7.83] |
| 10.3.3 Self‐help materials | 2 | 2700 | Risk Ratio (M‐H, Random, 95% CI) | 3.34 [2.54, 4.38] |
| 10.4 Arrange follow‐up support rates | 2 | 1321 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.15, 2.03] |
| 10.5 Quit attempts | 2 | 2972 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.96, 1.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aleixandre 1998.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primary care clinic, Spain Recruitment: Clinic & community volunteers |
|
| Participants | 48 people who smoked (excludes 6 dropouts) 65% female, av. age 36, av. cpd 24 ‐ 27 Therapist: unclear, primary care clinic staff | |
| Interventions | Intervention: participants received 4 x 30‐minute sessions of counseling over 4 weeks, which consisted of video, cognitive therapy, social influences and relapse prevention Control: participants received 3 minutes of advice immediately after randomization | |
| Outcomes | Abstinence at 12m (abstinence not defined) Validation: None | |
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Participants self‐reported smoking status in person or by telephone. Intervention group received greater face‐to‐face contact |
| Incomplete outcome data All outcomes | Low risk | Loss to follow‐up low and similar between arms: 4/25 control, 2/29 intervention |
An 2006.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primary care clinics of Veterans Affairs Medical Centers, USA Recruitment: Invitation letter to primary care patients |
|
| Participants | 837 people who smoked daily, 26 cpd, av.age 57, 10% F | |
| Interventions | Intervention: participants received behavioral counseling via telephone (7 calls over 2 months) with mailing of smoking cessation medications as clinically indicated. Additional calls were placed over a 12‐month period at the discretion of the counselor Control: participants were mailed self‐help materials and had continued access to clinical smoking cessation services through their Veterans Affairs Medical Center. All of these had referral‐based smoking cessation programs. Program structure varied by site (e.g., number of sessions and group or individual therapy). However, nicotine patches, nicotine gum, and slow‐release bupropion were available at all sites |
|
| Outcomes | 6m sustained abstinence at 12m Validation: None Measures of provider implementation: Assist‐Meds, Arrange |
|
| Funding Source | Department of Veterans Affairs Health Services Research and Development Service grant SUI 99101‐1 | |
| Author's declarations of interest | Authors declared that they had no financial conflict of interest. | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated randomization scheme stratified by primary care facility and blocked within sites |
| Allocation concealment | Low risk | Computer‐generated |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported. Intervention group had greater face‐to‐face contact. |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 14.6% (n = 122/838); 13.9% (n = 58/418) in the intervention group and 15.2% (n = 64/420) in the control group were lost to follow‐up at 12 months |
Aung 2019.
| Study characteristics | ||
| Methods | Design: randomized controlled trial Setting: 7 primary healthcare settings in rural districts where people often grow tobacco in their gardens and consume home‐made hand‐rolled cigarettes, Northern Thailand Recruitment: QUOTE: "Recruitment for the study started simultaneously at seven primary health care units within the mobile non‐communicable diseases clinic network of Maetha district, Lampang province, in June 2012" |
|
| Participants | 319 people who smoked, aged between 35 and 80 years and have diabetes and/or hypertension; who had never succeeded in quitting smoking; 28.9% female; median age 64 years | |
| Interventions | Intervention: Participants received: 1) adjunctive counseling; 2) carbon monoxide testing; 3) NRT gum; 4) a family‐assisted smoking cessation diary Nurses attended 2 pre‐intervention training workshops to deliver the intervention service package Control: participants received brief advice and a reminder to quit by a healthcare worker on subsequent visits to the hospital. Participants were requested to inform the healthcare worker when and if they quit smoking |
|
| Outcomes | CO‐validated smoking abstinence at 6m (self‐reported rates were also collected at 12m, but are not used in our analysis) Validation: CO |
|
| Funding Source | Ministry of Education, Japan | |
| Author's declarations of interest | Authors declared that they had no competing interests. | |
| Notes | Strategy: multicomponent (adjunctive counseling, CO monitoring, cost‐free medications, provider training) Level: patient, Provider Comparison type: multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "Random sequences are generated by the statistician on the basis of blocks of 24" |
| Allocation concealment | Low risk | QUOTE: "The allocated arm for each participant will be provided to the study sites PCUs in opaque, sealed envelopes" |
| Blinding of outcome assessors All outcomes | Low risk | Abstinence was biochemically verified |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 3.8% (n = 12/319); 1.9% (n = 3/160) in the intervention group and 5.7% (n = 9/159) in the control group were lost to follow‐up at 12 months |
Aveyard 2003.
| Study characteristics | ||
| Methods | Design: 4‐group randomized controlled trial Setting: General practices in West Midlands, UK Recruitment: Mailed invitations to patients of general practices. |
|
| Participants | 65 practices 2471 people who smoked, 55% F, av.age 41, 20 cpd |
|
| Interventions | Intervention 1: participants received self help workbook and three tailored letters Intervention 2: patients received self‐help workbook, three tailored letters, and three telephone calls Intervention 3: patients received self‐help workbook, three tailored letters, and three appointments with a nurse Control: patients received four standard items of self‐help materials |
|
| Outcomes | 6m sustained abstinence at 12m Validation: Salivary cotinine <14.2 ng/ml |
|
| Funding Source | The health authorities of the West Midlands | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategy: Adjunctive counseling + Tailored print materials Level: Patient Comparison type: 1) Single component vs. standard care; 2) multicomponent vs. standard care; 3) active vs. active |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Low risk | QUOTE: "Questionnaires were read optically and the data transferred automatically to the Access database that performed the minimization and controlled the contacts. There was no reason and no way that the clerical assistant running the database could alter the questionnaire reading schedule, which would have altered the allocation of particular individuals" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking abstinence was biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 39.7% (n = 981/2471); 40.0% (n = 273/683) in the manual group, 43.6% (n = 299/685) in the phone group, 49.2% (n = 203/413) in the nurse group, and 29.9% (n = 206/690) in the control group were lost to follow‐up at 12 months |
Bock 2014.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Inner‐city hospital‐based primary care clinics, UK Recruitment: During routine healthcare visits in primary care clinics |
|
| Participants | 846 adults who smoked randomized to intervention (n = 406) and control (n = 440), 68.7% female, average age 39, cpd of at least 10 | |
| Interventions | Intervention: participants received a 45‐minute counseling session with health educators and follow‐up calls at quit date and 2 weeks, in addition to the standard care (described below) Control: Healthcare professionals received training on smoking cessation guidelines and applying the 5 As Participants received brief advice from their physician and 8 weeks of NRT |
|
| Outcomes | 7‐day PPA at 12m Validation: Expired CO ≤ 5 ppm | |
| Funding Source | National Institutes of Health, National Institute on Drug Abuse (R01DA010860) | |
| Author's declarations of interest | Authors declared that they had no conflict of interest. | |
| Notes | Strategy: Provider training, adjunctive counseling, cost‐free medication Level: Patient, provider Comparison type: Active vs. active |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | The computer used a random number program to assign participants at random to one of two treatment conditions |
| Allocation concealment | Low risk | Computerised system |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was validated by carbon monoxide |
| Incomplete outcome data All outcomes | High risk | Follow‐up rate was low overall (< 50%) : Intervention: 47.3%, Control: 41.4% |
Borland 2008.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial with 2 active comparators Setting: Primary care practices, Australia Recruitment: Conducted in clinic, by GP or practice staff |
|
| Participants | 1039 adults who smoked, 55.4% female, 17 cpd 45 providers |
|
| Interventions | Common components in both groups: GPs were instructed to adhere to the National Guidelines, including a brief assessment of readiness to quit and if relevant, to deal with use of pharmacotherapy Intervention 1: in‐practice management GPs were encouraged to provide people who smoked with additional information and help to stop smoking. GPs were not precluded from recommending external assistance or from referring participants to the quitline if this was their clinical practice Intervention 2: referral GPs were encouraged to offer people who smoked with any interest in quitting referral to the quitline and were provided with a brochure on quitline services. Participants received an introductory call/letter from quitline, followed by up to 2 pre‐quitting and 4 post‐quitting telephone counseling sessions |
|
| Outcomes | ≥10m sustained abstinence at 12m Validation: None Quit attempts Measures of provider implementation: Assist, Arrange |
|
| Funding Source | National Health and Medical Research Council (284346) | |
| Author's declarations of interest | QUOTE: "RB, JB and NB are employees of The Cancer Council Victoria that runs the quitline service used in this study. None are involved in day to day operations of the service" | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "GPs were randomised by computer prior to their attendance at an education session" |
| Allocation concealment | Low risk | Computerised system |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported and not validated biochemically. The amount of contact differed between trial arms |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and similar across the groups. The overall loss to follow‐up was 33.6% (n = 349/1039); 32.0% (n = 233/728) in the referral group and 37.3% (n = 116/311) in the in‐practice group were lost to follow up at 12 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization. QUOTE: "Patients who presented for any reason who were current smokers...were eligible for recruitment"; "Method of patient recruitment did not differ by condition (P=0.79)" |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | No significant differences between groups |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | Statistical analyses were performed with Stata, controlling for practice as a clustering variable QUOTE: "In order to take into account the correlated nature of the data and repeated measures over time, generalized estimating equations were used for a final analysis of outcomes" |
Buffels 2006.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: General practices, Belgium Recruitment: All patients screened for tobacco use |
|
| Participants | 16 providers 1206 adults who smoked. Characteristics not described |
|
| Interventions | Common components in both groups: general practitioners received a 4‐hour training in giving advice to quit smoking Intervention: • General practitioners received training in performance and interpretation of spirometry, using a microspirometer • Participants received the minimal intervention strategy during a 12‐week period; those in a motivation stage 3 or 4 were asked to set a quit day and were offered a follow‐up contact as well as NRT and/or bupropion • Participaents also underwent spirometry and were provided with lung function measurement values and their flow/volume curve Control: participants received the minimal intervention strategy as described above but no spirometry |
|
| Outcomes | Smoking abstinence (undefined) at 24m Validation: Urinary cotinine (completed by 24.2% of self‐reported quitters) (cut‐off not reported) |
|
| Funding Source | Unconditional grant by Voorzorgskas voor Geneesheren, Brussels, Belgium | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training, spirometry Level: Provider, patient Type: Active vs. active (isolates spirometry) Unable to extract abstinence data for intervention group from full‐text. Attempt was made to contact authors unsuccessfully ‐ unable to include in meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Coin toss |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Unclear risk | Smoking status was validated by urinary cotinine, but response rate was very low. |
| Incomplete outcome data All outcomes | Unclear risk | No details reported |
Cabezas 2011.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Primary care practices, Spain Recruitment: Patients who attended primary care practice for any reason and who answered ‘yes’ to the question: ‘Do you currently smoke cigarettes?’. |
|
| Participants | 176 basic care units within 82 primary care centers 2827 people who currently smoked, aged 14 – 85 years. Randomized to intervention (1482) or control (1345). 50% F, average age 43, 20 cpd |
|
| Interventions | Common components in both groups: brief advice for patients and training (of different breadth of content for each group) for healthcare professionals Intervention: • Healthcare professionals received a 20‐hour workshop on smoking cessation interventions • Participants received intervention tailored to TTM stage: ‐ Precontemplation or contemplation stage: brief motivational interview and leaflet ‐ Preparation or action who preferred no specific help: brief advice, leaflet, an offer of NRT and 1 follow‐up contact ‐ Preparation or action stage requesting specific help: 9 scheduled follow‐up visits over 6 months that included behavioral interventions and pharmacological agents Control: • Healthcare professionals only received the training session in the practical aspects of the protocol • Participants received usual care that included brief smoking cessation advice for diseases related to tobacco consumption |
|
| Outcomes | 12m continuous abstinence at 24m follow‐up Validation: Expired CO < 10 ppm |
|
| Funding Source | Spanish Preventive Services Network (Red de Actividades Preventivas y Promoción de la Salud en Atención Primaria) granted by the Carlos III Health Institute (Instituto de Salud Carlos III) (G03/170 y RD06/0018) and from another project grant (PI021471) in 2002 also from the Carlos III Health Institute | |
| Author's declarations of interest | Authors declared that they had no conflict of interest. | |
| Notes | Strategy: Provider training + adjunctive counseling Level: Patient + Provider Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "the random sequence was generated by an independent statistician who used a computer program and who was blinded to the basic care unit identities" |
| Allocation concealment | Low risk | QUOTE: "Basic care unit were informed about their allocation after giving final consent to participation" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was validated by carbon monoxide levels |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and similar between groups. The overall loss to follow‐up was 44.0% (n = 1244/2827); 43.3% (n = 641/1482) in the intervention group and 44.8% (n = 603/1345) in the control group were lost to follow‐up at 2 years |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization. QUOTE: "...recruited from 2003 to 2005 who consulted a primary care centre for any reason and who answered 'yes' to the question: 'do you currently smoke cigarettes?" |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | Statistically significant differences between the study groups were found in the following variables: stage of change (precontemplation, contemplation, preparation, action), Richmond test, confidence in quitting and readiness to quit (P = 0.001); however, these differences were small and clinically irrelevant |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "...a multi‐level analysis was conducted initially. Because no significant variation was found between basic care units, a logistic regression analysis of individual level data using methods for clustered data (adjusting the standard errors for the design effect) was used in order to analyse the intervention as a predictor of smoking cessation" |
Canga 2000.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: 15 primary care centres, 2 hospitals, Spain Recruitment: Identified through practice records |
|
| Participants | 280 people who smoked with diabetes (incl 16 recent quitters) aged 17 ‐ 84 (133 control, 147 intervention), average age 40.7, 19 cpd, 15% female, did not need to be motivated to quit | |
| Interventions | Intervention: participants received a 40‐minute, face‐to‐face interview on smoking cessation with a nurse and set a quit date. Participants also received self‐help materials. All of those who smoked heavily received nicotine patches unless contraindicated. In addition, participants were provided with a follow‐up program consisting of 5 contacts: a telephone call the day before the quit date, a follow‐up visit 2 weeks after the quit date, a letter 3 weeks after the quit day, a second follow‐up visit 2 months after the quit date, and a final evaluation after 6 months. Control: participants received usual care, established in the Navarre diabetes care program, including advice to quit smoking. No further details reported |
|
| Outcomes | > 5m sustained abstinence at 6m Quit attempts Validation: Urine cotinine < 20 ng/ml |
|
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care It is not possible to separate the primary care settings' data from the secondary care settings' data, and so this study is not included in any meta‐analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated allocation method |
| Allocation concealment | Low risk | Sealed envelopes |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was validated by urinary cotinine |
| Incomplete outcome data All outcomes | Low risk | Overall, 0.7% of participants were lost to follow‐up |
Carpenter 2020.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized trial Setting: 22 primary care clinics in South Carolina, USA Recruitment: patients identified at routine visits |
|
| Participants | 1245 adults who smoked, 61% female, average age 50.7, average cpd 15 | |
| Interventions | Intervention: cessation advice and brochure with information on quitline, plus a 2‐week supply of both nicotine patch and lozenge, with minimal instructions on use
Control: cessation advice and brochure with information on quitline Training given to providers was based on study procedures and standard care |
|
| Outcomes | 7‐day PPA at 6m Validation: none Quit attempts |
|
| Funding Source | National Institute on Drug Abuse (R01 DA 021619), with additional research support through NIH UL1 TR001450 and K23 DA 045766 | |
| Author's declarations of interest | Some authors have received consulting honoraria from Pfizer (does not produce NRT) | |
| Notes | Strategy: Cost‐free medication Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Stratified randomization lists created at the outset of the study |
| Allocation concealment | Low risk | Accessible only to the study statistician |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was not validated, but both groups had minimal contact, with no difference in the face‐to‐face contact between the arms |
| Incomplete outcome data All outcomes | Low risk | At patient level, the number of participants lost was 41.1% (512/1245); 40.3% (263/652) in the standard care group and 42.0% (249/593) in the intervention group, no significant differences between groups, intention‐to‐ treat used to analyze data |
| Recruitment bias (cluster RCTs only) | Low risk | 22/24 clinics approached agreed to participants. Patient participants were affiliated with the practice before randomization. 6 eligible patients did not enrol |
| Balanced baseline characteristics? (cluster RCTs only) | High risk | Quote: "several baseline variables differed significantly between groups…" and adjusted for in the analysis |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | adjustment for clustering was conducted |
Cobos‐Campos 2016.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: 2 health centres in the Basque Health Service, Spain Recruitment: Identified through EMR and sent invitation letter |
|
| Participants | 320 adults who smoked, 44% female, average age 45 | |
| Interventions | Intervention: participants received health advice from a doctor or nurse, as in the other group, plus reinforcement text messages to their mobile phones Control: participants received health advice provided by a doctor or a nurse |
|
| Outcomes | Continuous abstinence at 12m Validation: Expired CO < 7 ppm |
|
| Funding Source | This study was funded by the Departamento de Industria del Gobierno Vasco of the Basque Country under the 2012 Saiotek funding round (reference number SAIO12‐OA12BF001). This research was also supported by Departamento de Educación, Política Lingüística y Cultura del Gobierno Vasco (IT620‐13) | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: SMS messages Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated sequence |
| Allocation concealment | Low risk | QUOTE: "the Bioaraba Research Institute assigned patients to one of the two arms of the trial...after receiving the patient randomization form, and hence research nurse did not know about the treatment group until patient allocation" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was validated by carbon monoxide |
| Incomplete outcome data All outcomes | High risk | The overall loss to follow‐up was 87.5% (n = 280/320); 80.6% (n = 129/160) in the text messaging+health advice group and 94.4% (n = 151/160) in the health advice‐only group were lost to follow‐up at 12 months |
Cummings 1989a.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Private practices of internal medicine and family practice, USA Recruitment: Patients were recruited by primary care staff, or by research staff in clinics |
|
| Participants | 916 adult who smoked (446 control, 470 intervention). 56% F, 20 cpd, av.age 44 | |
| Interventions | Intervention: • Physicians received training on smoking cessation counseling in 3 x 1‐hour seminars • Practices were provided with free self‐help materials, stickers, quit date prescription pads, and posters. Nurses and office staff were coached on the program and supporting materials by a member of the research staff Control: usual care. No further description on what the usual care entailed was reported |
|
| Outcomes | Continuous abstinence at 12m Validation: CO levels (cut‐off not defined), salivary cotinine < 30 ng/ml Quit attempts Measures of provider implementation: Ask, Assist‐Self‐help, Assist‐Quit date, Assist‐Meds, Arrange |
|
| Funding Source | Grant # CA38337 from the National Cancer Institute and by the Henry J. Kaiser Foundation Faculty Fellowship in General Internal Medicine (SRC). | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategy: Provider training + Outreach facilitation Level: Provider + Practice Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was biochemically validated |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and the difference between groups was less than 20%. The overall loss to follow‐up was 22.7% (n = 208/916 survivors); 24.9% (n = 117/470 survivors) in the intervention group and 20.4% (n = 91/446) in the control group were lost to follow‐up at 1 year |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | Balanced between arms |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Individual patients were the units of analysis for the results we are presenting. A few physicians were clustered by offices and patients were clustered by physician. We tested the effect of this clustering in other analyses in which the sampling variances were adjusted for cluster sampling. These adjustments had no discernible effect on significance levels and did not alter our conclusions. We also analysed our results using the physician as the unit of analysis... These results were similar to the results of the analyses in which patients were the units of analysis. Thus, we omitted them to simplify the presentation" |
Cummings 1989b.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 4 health maintenance organization medical centres, USA Recruitment: Conducted in clinic waiting rooms |
|
| Participants | 81 providers 2056 English‐speaking people who made a visit to any doctor participating in the study were eligible for inclusion. (1032 control, 1024 intervention), av.age 45, 55% F, 17 cpd |
|
| Interventions | Intervention: • Physicians received training on smoking cessation counseling in 3 x 1‐hour seminars • Practices were provided with free self‐help materials, stickers, quit date prescription pads, and posters. Nurses and office staff were coached on the program and supporting materials by a member of the research staff Control: usual care. No further description on what the usual care entailed was reported |
|
| Outcomes | Continuous 9m abstinence at 12m follow‐up Validation: CO levels (cut‐off not defined), salivary cotinine < 30 ng/ml Quit attempts Measures of provider implementation: Ask, Assist‐Self‐help, Assist‐Quit date, Assist‐Prescribe, Arrange |
|
| Funding Source | Partial support by grant CA38337 from the National Cancer Institute. Dr. Cummings' work was supported in part by the Henry J. Kaiser Foundation Faculty Fellowship in General Internal Medicine | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training + Outreach facilitation Level: Provider + Practice Comparison type: Multi‐component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "A computer randomly assigned the units to either the experimental or control group" |
| Allocation concealment | Low risk | QUOTE: "A computer randomly assigned the units to either the experimental or control group" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was biochemically validated |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and similar between groups. The overall loss to follow‐up was 24.7% (n = 507/2056); 23.5% (n = 241/1024) in the intervention group and 25.8% (n = 266/1032) in the control group were lost to follow‐up at 1 year |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | Similar between experimental and control groups |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "We tested the effect of this two‐stage cluster sampling design by estimating logistic regression models with random effects terms representing the groupings by physician. These adjustments had no substantial effect on comparisons between the experimental and control groups. Therefore, for simplicity, we present the results with the patient as the unit of analysis" |
Dent 2009.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: A Veterans Health Administration, community‐based outpatient clinic in the Rocky Mountain region, USA Recruitment: Patients identified through EMR‐generated list. Called by pharmacist and invited to participate |
|
| Participants | 101 adults who smoked 1 or more cigarettes daily for 7 days, were at least somewhat ready to quit in the next 2 weeks (≥ 4 on a 10‐point motivational scale), were willing and capable of attending 3 scheduled sessions at the clinic, and were interested in participating in the study. av.age 56, 19 cpd, 93% M | |
| Interventions | Common components in both groups: all people who smoked were referred to a clinical pharmacist via the electronic computerized patient record system. They were offered their choice of immediate‐release bupropion tablets or nicotine patch at no cost. Intervention: participants who smoked participated in a face‐to‐face 3‐session group program at the clinic, delivered by the pharmacist and pharmacy students. For follow‐up, all participants were instructed to call the clinic for questions or to receive additional support as needed. Control: The pharmacist or pharmacy student used a structured script and delivered 1 timed 5‐ to‐10 minute session to the participants over the telephone that included all the components of standard care recommended by the Clinical Practice Guidelines |
|
| Outcomes | Continuous abstinence at 6m Validation: Urinary cotinine < 0.3 ug/ml |
|
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "Randomization codes assigned to each participant were computer generated by the study statistician and stratified by sex in blocks of 6" |
| Allocation concealment | Low risk | QUOTE: "Randomisation codes assigned to each participant were computer generated by the study statistician and stratified by sex in blocks of 6" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was validated by urinary cotinine |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 4.0% (n = 4/101); 2.0% (n = 1/50) in the intervention group and 5.9% (3/51) in the control group were lost to follow‐up at 6 months. Therefore, dropout was low and balanced between arms |
Ellerbeck 2009.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial with 3 active trial arms Setting: Rural primary care practices, Kansas, USA | |
| Participants | 726 adults who smoked >10 cpd, randomized to intervention (n = 482) and control (n = 244), 41.5% M; av.age 47.2, 24 cpd | |
| Interventions | Common component in all groups: offer of free pharmacotherapy Intervention 1: pharmacotherapy only At baseline and 6, 12,and 18 months, participants received a mailed offer of free pharmacotherapy that consisted of either 6‐weeks of nicotine patch (21 mg/d) or 7‐weeks of sustained‐release bupropion (150 mg twice daily) Intervention 2: moderate‐intensity disease management Participants received an offer of free pharmacotherapy (as above) with educational support and 2 telephone‐based counseling sessions every 6 months Intervention 3: high‐intensity disease management Participants received an offer of free pharmacotherapy (as above) with educational support and 6 telephone‐based counseling sessions every 6 months |
|
| Outcomes | 7‐day PPA at 24m Validation: Salivary cotinine level < 15 ng/mL in a mailed saliva sample. Because of resistance by participants to providing salivary samples at month 12, validation by proxy report from a significant other at month 24 was used for quitters who did not return a salivary sample. The validated quit rate at 24m is a mixture of the 2 approaches |
|
| Funding Source | National Cancer Institute (grant R01‐101963). Study medication was provided by GlaxoSmithKline | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Adjunctive counseling + cost‐free medication Level: Patient Comparison type: Active vs. active (isolating adjunctive counseling) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "randomization occurred at the participant level. A computer‐generated random‐number table was used to generate allocation cards in blocks of 24, with allocation equally distributed across treatment groups" |
| Allocation concealment | Low risk | QUOTE: "to conceal allocation, we placed these cards in sequentially numbered, opaque, sealed envelopes" |
| Blinding of outcome assessors All outcomes | Unclear risk | Smoking status was biochemically validated, but there was a low return rate and so proxy report was also used |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 17.3% (n = 130/750); 13.2% (n = 33/250) in the PM group, 20.0% (n = 50/249) in the MDM group and 18.7% (n = 47/251) in the HDM group were lost to follow‐up at 24 months |
Fu 2014.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Veterans Affairs primary care sites, USA Recruitment: Identified through VA's EMR Health Factors Dataset at each participating site |
|
| Participants | 6400 veterans who were currently smoking, average age 56, average cpd 18 | |
| Interventions | Intervention: participants received proactive outreach (mailed invitation materials followed by telephone outreach) and offer of choice of smoking cessation services (telephone care or in‐person care) Control: participants had access to tobacco treatment services from their VA hospital |
|
| Outcomes | 6m prolonged abstinence at 12m Validation: None |
|
| Funding Source | Funded by the Department of Veterans Affairs (VA) Health Services Research and Development (HSR&D) | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Proactive mailings Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Smoking abstinence was self‐report and contact with the study team was differential across study arms |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 34.0% (n = 1741/5123); 35.9% (n = 905/2519) in the intervention group and 32.1% (n = 836/2604) in the control group were lost to follow‐up at 12 months |
Gilbert 2013.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: General practices from the MRC General Practice Research Framework, UK Recruitment: People who smoked, identified using the computer system in participating practice |
|
| Participants | 6697 adults who smoked, 56% F, av.age 44.6, 17.8 cpd | |
| Interventions | Intervention: participants received non‐tailored information plus a computer‐tailored advice report based on the information obtained in the baseline assessment questionnaire, accompanied by a letter from the GP endorsing the information contained in the report. Participants were sent a follow‐up assessment 1 month after baseline, and received a tailored progress report generated from these additional data Control: participants received standard, non‐tailored information (the NHS ‘Stop Smoking Start Living’ booklet) |
|
| Outcomes | 3m prolonged abstinence at 6m Validation: None Quit attempts |
|
| Funding Source | The trial was supported by funding from Cancer Research UK | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Tailored print materials Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated. |
| Allocation concealment | Low risk | QUOTE: "These blocked randomisation codes were generated externally and given to an independent administrator in sealed envelopes upon receipt of completed questionnaires" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking abstinence was self‐reported, but person‐to‐person contact was similar between groups |
| Incomplete outcome data All outcomes | Low risk | The follow‐up response rate, based on the analyzed sample (n = 6697), was 78.8% (2644) and 75.7% (2530) in the control and intervention groups respectively |
Gilbert 2017.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: General practices in England, UK Recruitment: People who were currently smoking were identified from medical records in participating practices and sent an invitation letter |
|
| Participants | 4384 adults who smoked, 50% M, av. age 49, av. cpd 16 99 general practices in 18 Stop Smoking Service areas |
|
| Interventions | Intervention: participants received a brief personalized and tailored letter sent from the GP that included information specific to the participant and a personal invitation to attend a “come and try it” taster session for cessation services Control: participants received a standard generic letter from the GP practice, which advertised the local SSS and asked the participant to contact the service to make an appointment to see an adviser |
|
| Outcomes | 3m prolonged abstinence at 6m Validation: Salivary cotinine level < 12 ng/mL Quit attempts |
|
| Funding Source | This study was funded by the National Institute for Health Research Health Technology Assessment Programme (project number 08/58/02). | |
| Author's declarations of interest | QUOTE: "Irwin Nazareth is a member of the National Institute for Health Research Health Technology Assessment Funding Commissioning Panel. | |
| Notes | Strategy: Tailored materials Level: Patients Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated permutated block randomization |
| Allocation concealment | Low risk | Allocated by computer after participant consented |
| Blinding of outcome assessors All outcomes | Low risk | Abstinence was biochemically validated. Participant contact was minimal in both groups. |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 23.1% (n = 1012/4384); 23.4% (n = 616/2636) in the intervention group and 22.7% (n = 396/1748) in the control group were lost to follow‐up at 6 months |
Girgis 2011.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: General practices in South West Sydney, Australia Recruitment: Asked by practice receptionists to complete a health questionnaire |
|
| Participants | 213 adults who smoked of Arabic background, av.age 38, 48% M, 18 cpd | |
| Interventions | Intervention: participants received an offer from their GP of free referral to telephone‐based counseling by bilingual Arabic‐speaking registered psychologists in the language of choice (Arabic or English) at times convenient to the participant. Within 2 weeks, 1 of the psychologists telephoned participants and offered counseling based on the ‘5As’ approach Control: participants received the GP’s usual smoking cessation care. No further details on the usual care reported |
|
| Outcomes | 24h PPA at 12m Validation: None Secondary outcomes: Quit attempts |
|
| Funding Source | National Health and Medical Research Council project grant awarded to SG, NAZ and JEW (grant number 295000) | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | QUOTE: "...an unobtrusive mark visible only to the general practitioners, to convey group randomisation. General practitioners scanned the questionnaire to determine smoking status and group allocation". No further details reported. |
| Blinding of outcome assessors All outcomes | Unclear risk | Smoking abstinence was self‐report. It is unclear whether 1 group had greater number of person‐to‐person contact than the other |
| Incomplete outcome data All outcomes | High risk | The overall loss to follow‐up was 39.6% (n = 161/407); 44.6% (n = 95/213) in the intervention group and 34.0% (n = 66/194) in the control group were lost to follow‐up at 12 months. But 52.6% (n = 112/213) of the participants who were allocated to intervention did not consent to the intervention. Also, of the 101 participants who consented, 54.5% (n = 55/101) did not receive counseling as they either refused, had already quit smoking, or could not be contacted. Therefore, 46 people in the intervention group actually received counseling and only 8 of those completed the 6 telephone calls |
Haas 2015.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: General practices in greater Boston, USA Recruitment: Eligible patients identified through electronic medical record and received a mailed invitation letter |
|
| Participants | 707 adults who smoked, living in a low or moderate household income census tract. (399 intervention, 308 control) av.age 50, 68% F, 15 cpd | |
| Interventions | Intervention: participants received up to 4 counseling calls, a 6‐week supply of NRT patch, access to community‐based referrals to address sociocontextual mediators of tobacco use, and integration of all components into their normal health care through the electronic health records system Control: participants received usual care. No further details on the usual care reported |
|
| Outcomes | 7d PPA at 9m Validation: None |
|
| Funding Source | Lung Cancer Disparities Center at the Harvard School of Public Health (funded by National Cancer Institute grant P50 CA148596) and the Harvard Catalyst and from the Harvard Clinicaland Translational Science Center (funded by National Institutes of Health [NIH] grant 1 UL1 RR025758‐01 and financial contributions from participating institutions) | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Adjunctive counseling + cost‐free medications Level: Patient Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | QUOTE: "Randomisation was performed in batches based on the date of the clinic visit..." |
| Allocation concealment | High risk | QUOTE: "...The first patient randomised in each batch was randomised to intervention status; batches with an odd number of participants therefore resulted in an imbalance in the size of the intervention and control groups" |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐report and there was variable contact between groups |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 34.2% (n = 242/707); 36.1% (n = 144/399) in the intervention group and 31.8% (n = 98/308) in the control group were lost to follow‐up at 9 months |
Hilberink 2011.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: Primary care, The Netherlands Recruitment: Patients with COPD identified from medical records in participating practices |
|
| Participants | 667 patients with COPD (148 control, 243 intervention 1, 276 intervention 2), age > 35 years, av.age 60, 16 cpd, 49% M | |
| Interventions |
Intervention 1: counseling strategy, recommendation of NRT • The general practice team received a 4‐hour group training session about chronic obstructive pulmonary disease and smoking cessation, and 3 visits by an outreach visitor for additional individual support • Participants received a leaflet especially developed for people with chronic obstructive pulmonary disease who smoked, a videotape, self‐efficacy enhancing information, information about NRT, proactive telephone calls Intervention 2: counseling strategy, recommendation of NRT, advice to use bupropion‐SR • The general practice team received a 4‐hour group training session about chronic obstructive pulmonary disease and smoking cessation, and 3 visits by an outreach visitor for additional individual support • Participants received a leaflet especially developed for people with chronic obstructive pulmonary disease who smoked, a videotape, self‐efficacy enhancing information, information about NRT, proactive telephone call, and advice to use bupropion‐SR Control: usual care consisting of periodic regular check‐ups and chronic obstructive pulmonary disease information |
|
| Outcomes | PPA at 12m Validation: Urinary cotinine < 50 ng/mL |
|
| Funding Source | Financed by the Dutch Asthma Foundation, Netherlands Organization for Health Research and Development (ZonMW), and Pharmacia | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Provider training, outreach facilitation, adjunctive counseling Level: Patient + Provider + Practice Comparison type: Multicomponent vs. SC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was biochemically validated |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and similar between groups. The overall loss to follow‐up was 18.7% (n = 130/697); 3.6% (n = 9/252) in 'counseling + NRT' group, 5.2% (n = 15/291) in 'counseling+NRT+prescription of bupropion' group, 3.9% (n = 6/154) in the control group were lost to follow‐up at 1 year. |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization. |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | Groups were balanced |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "We used multilevel analyses to test treatment effects because of the study's hierarchical structure..." |
Hollis 1993.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial with 4 active trial arms Setting: 2 large primary care clinics, USA Recruitment: Recruited in practice by receptionists |
|
| Participants | 2707 adults who smoked, av. age 40, 57% F, 18 cpd | |
| Interventions |
Intervention 1: participants received physician advice, carbon monoxide assessment, a quit‐smoking video, a quit kit (gum, toothpicks and cinnamon sticks), self‐help materials, mailed newsletters and a follow‐up call at 2 ‐ 4 weeks from a counselor. They were encouraged to set a quit date Intervention 2: participants received physician advice, carbon monoxide assessment, a video encouraging them to attend a smoking cessation support group, self‐help materials, coupon for quit‐smoking group, postcards reminding them of group meeting times Intervention 3: participants received the support offered to the control group and intervention 2 group combined Control: participants received a 30‐second advice message and a pamphlet |
|
| Outcomes | 7d PPA at 3m and 12m Validation: Salivary cotinine |
|
| Funding Source | Public Health Service Grant 1P01‐CA44648 from the National Cancer Institute. | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategy: Adjunctive counseling + CO monitoring Level: Patient Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | QUOTE: "two random digits contained in the patient's health record number were used to assign patients..." |
| Allocation concealment | High risk | QUOTE: "two random digits contained in the patient's health record number were used to assign patients..." |
| Blinding of outcome assessors All outcomes | Low risk | Smoking abstinence was biochemically validated |
| Incomplete outcome data All outcomes | Unclear risk | Response rates did not differ between groups. Rates not reported. |
Hoving 2010.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Dutch pharmacies and primary care clinics, The Netherlands Recruitment: Passively recruited through baseline questionnaires in waiting rooms |
|
| Participants | 474 adults who smoked (from GP sample) motivated to quit smoking within 6 months. 59% F, av.age 42, av. cpd 22 | |
| Interventions | Intervention: participants received a tailored letter based on responses to a questionnaire. Messages addressed perceived advantages and disadvantages of smoking cessation and anticipated difficult situations to refrain from smoking. Additionally, the tailored letter was personalized by including individual information. All personally relevant messages were then combined into a 5 ‐ 7 page letter Control: participants received a thank‐you letter after completing a questionnaire |
|
| Outcomes | Continuous abstinence at 6m Validation: None Quit attempts |
|
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategy: Tailored print material Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | QUOTE: "Smokers were randomised based on the colour coding on their questionnaire..." |
| Allocation concealment | High risk | QUOTE: "Smokers were randomised based on the colour coding on their questionnaire..." |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was self‐report, but contact was low, reducing potential risk of bias |
| Incomplete outcome data All outcomes | Unclear risk | No details on the number of participants lost to follow‐up in the control group reported |
Hughes 1991.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial with 3 active trial arms Setting: 2 rural family practices, USA Recruitment: Conducted in practice waiting room |
|
| Participants | 106 adults who smoked who had never used NRT gum, did not need to be motivated to quit, 62% M, av.age 37, 26 cpd | |
| Interventions |
Intervention 1: participants received 10 minutes of brief advice from physician, instruction to use free nicotine gum and a stop‐smoking booklet Intervention 2: participants received 10 minutes of brief advice from physician, instruction to use cost‐reduced NRT gum (USD 6/box) and a stop‐smoking booklet. Intervention 3: participants received 10 minutes of brief advice from physician, instruction to use nicotine gum (to purchase at a full price of USD 20/box) and a stop‐smoking booklet |
|
| Outcomes | Sustained abstinence at 12m Validation: No biochemical validation. Observers were used to verify cessation Quit attempts |
|
| Funding Source | Grant (DA‐04066) and Research Scientist Development Award (DA‐00109) from the National Institute on Drug Abuse. Merreil‐Dow Research Institute provided nicotine gum | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Cost‐free medications Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Low risk | QUOTE: "After the advice had been given, the physician opened a sealed envelope and signed a prescription that indicated the price group to which the smoker had been assigned" |
| Blinding of outcome assessors All outcomes | Low risk | Observers were used to verify cessation and contact matched between trial arms |
| Incomplete outcome data All outcomes | Unclear risk | No details reported |
Irizar Aramburu 2013.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primary care practices in Gipuzkoa, Spain Recruitment: Randomly selected from electronic medical record |
|
| Participants | 335 adults who smoked, 52% F, av.age 53.6, av. cpd not reported | |
| Interventions | Intervention: participants received a spirometry test delivered by a nurse and the same brief anti‐smoking intervention as the control group Participant also received a short explanation of the spirometry results Control: participants received brief anti‐smoking intervention |
|
| Outcomes | 7‐day PPA at 12m Validation: Expired CO < 10 ppm |
|
| Funding Source | International Centre of Research Excellence in Chronicity, Kronikgune and the Department of Health of the Basque Government | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Spirometry Level: Patient Comparison type: Single component Some information obtained from study author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | The randomization sequence was generated by computer and kept in the research unit |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was validated by carbon monoxide |
| Incomplete outcome data All outcomes | Unclear risk | No details reported |
Jamrozik 1984.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial with 3 active trial arms Recruitment: Conducted in clinic waiting room Setting: 6 primary care practices, UK |
|
| Participants | 2110 people who smoked, over the age of 16, being seen for a medical appointment, av. age not reported, av. cpd not reported | |
| Interventions |
Intervention 1: participants received verbal advice from their doctor and a self‐help booklet Intervention 2: participants received verbal advice from their doctor, a self‐help booklet and demonstration of carbon monoxide levels Intervention 3: participants received verbal advice from their doctor, a self‐help booklet and a card with information on how to contact a health visitor for further help with quitting smoking Control: no intervention |
|
| Outcomes | 7‐day PPA at 12m Validation: Urinary cotinine < 100 ng/ml in a subsample of participants (Results reported are for self‐report) Quit attempts ‐ but unable to calculate data needed for analysis from paper |
|
| Funding Source | Health Education Council | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: CO monitoring Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | According to day of attendance |
| Allocation concealment | High risk | Based on day of attendance, could have been foreseen |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was only validated in a subsample of participants by urinary cotinine; but contact was matched between trial arms, thereby minimizing risk of bias |
| Incomplete outcome data All outcomes | Unclear risk | 72% returned 1‐year follow‐up questionnaire. No further details reported |
Joseph 2004.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 20 Veterans Affairs Medical Centers, USA Recruitment: Calls to patients who visited primary care provider in the past 6 weeks |
|
| Participants | Pre‐intervention 4254 adults who smoked who had visited their primary care provider in the past 6 weeks. av.age 64, 95% M, av. cpd not reported Post‐intervention 1424 adults who smoked who had visited their primary care provider in the past 6 weeks. av.age 64, 97% M, av. cpd not reported 575 (280 in the intervention group and 295 in the control) participants made up the cohort of people who smoked and who were contacted both pre‐and post‐intervention |
|
| Interventions | Intervention: • Providers (the site‐based principal investigator and 1 other key advocate from each site) received a 2‐day training meeting ‐ Emphasis on options for increasing identification of people who smoked in the computerized patient record system ‐ Promotion of treatment in the primary care setting rather than use of referral‐based care ‐ Encouragement of removal of formulary restrictions to prescription of smoking‐cessation aids and provision of materials to address Pharmacy and Therapeutics Committees • Sites were visited by the interventionist for 2 or 3 days to provide academic detailing of the implementation strategies that was sensitive to local hurdles Control: no information provided on the care this group received or did not receive |
|
| Outcomes | PPA at 12m Validation: None Quit attempts Measures of provider implementation: Ask, Advise, Assist and Assist‐Meds |
|
| Funding Source | Grant from the Veterans Administration Health Services Research and Development Service: CPG 97‐039 | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategy: Provider training + EMR prompts + outreach facilitation Level: Provider + Practice Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported. |
| Allocation concealment | Unclear risk | No details reported. |
| Blinding of outcome assessors All outcomes | Unclear risk | Smoking status was self‐reported and the number of contacts in the control group is not reported |
| Incomplete outcome data All outcomes | Low risk | At site level, there was no loss to follow‐up (n = 0/20) at 1 year. At patient level, it was not the intention of this study to follow up the same participants from the outset. Patients were randomly selected at baseline and at 1 year and surveyed. |
| Recruitment bias (cluster RCTs only) | Unclear risk | Participants were affiliated with the practice before randomization. QUOTE: "...among a sample randomly selected from patients who had seen their primary care provider within 6 weeks..." |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "There were no significant differences between subject characteristics in the 2 treatment groups" |
| Adjustment for clustering in analysis? (cluster RCTs only) | High risk | No adjustment for cluster nature of data reported |
Juarranz 1998.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primary care centre, Spain Recruitment: By telephone from healthcare centre lists |
|
| Participants | 195 adults who smoked (aged 16 ‐ 65), 48% female, av.age 37, 23 cpd | |
| Interventions | Intervention: participants received the following from their doctor and nurse: ‐ Brief standardized advice (3 ‐ 5 minutes) about smoking cessation ‐ An instruction booklet ‐ Nicotine patches ‐ A follow‐up phone call 2 days after the quit date ‐ Additional visits at 2 weeks, 3 months and 6 months Control: participants received usual care. No further details reported |
|
| Outcomes | Continuous abstinence at 6m Validation: Expired CO < 8 ppm |
|
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling + cost‐free medications Level: Patient Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | Potential participants were ranked randomly in a list, then assigned alternately from list |
| Allocation concealment | High risk | Assigned from open list |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was validated by carbon monoxide |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 4.9% (n = 10/205); 5.9% (n=6/102) in the intervention group and 3.9% (n = 4/103) in the control group were lost to follow‐up at 6 months. |
Kalkhoran 2018.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primary care network, USA Recruitment: interactive voice response technology was used for participant recruitment; contact information was identified from electronic health record |
|
| Participants | 233 people who smoked, av. age 53, av. cpd 15 | |
| Interventions |
Intervention 1: participants received brief counseling provided by a health center‐based Tobacco Care Coordinator, coordinated medications with primary care physicians, and a referral to additional care (in‐person, phone call or text) Intervention 2: participants were transferred directly to a community‐based Quitline for counseling and a free sample of nicotine replacement therapy Control: participants were given the state quitline number and advised to contact their primary care physician for assistance to quit smoking. Each practice had a certified tobacco treatment specialist available 1 day per week to provide free in‐person individual cessation support. Primary care physicians could also fax a referral to the quitline |
|
| Outcomes | 30‐day PPA at 6m
Validation: None Measures of provider implementation: Assist‐counselling, Assist‐medications |
|
| Funding Source | Pfizer Independent Grants for Learning and Change | |
| Author's declarations of interest | QUOTE: "Drs. Rigotti and Kalkhoran receive royalties from UpToDate, Inc. Dr. Rigotti has been an unpaid consultant to Pfizer, Inc. and a paid consultant to Achieve Life Sciences. No other authors have any conflicts of interest to disclose" | |
| Notes | Strategy: Adjunctive counseling, cost‐free medications Level: Patient Comparison types: Multicomponent vs. standard care; single component (adjunctive counseling) vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Random‐number generator |
| Allocation concealment | Low risk | Implemented by interactive voice response |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐report, and contact was differential between arms |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 45.4% (n = 106/233); 48.1% (n = 38/79) in the internal care coordination group, 47.4% (n = 37/78) in the external community referral group and 40.8% (n = 31/76) in the usual care group were lost to follow‐up at 6 months |
Katz 2004.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Community‐based primary care clinics in southern Wisconsin, USA Recruitment: Patients willing to complete exit interviews |
|
| Participants | Pre‐intervention: 1022 adults who smoked (> 10 cpd) (509 control, 513 intervention) av.age 42, 46% M, 17 cpd Post‐intervention: 1141 adults who smoked (> 10 cpd) (499 control, 642 intervention) av.age 40, 45% M, 17 cpd |
|
| Interventions | Intervention: • Clinicians received training tutorial on smoking cessation, and group and confidential individual feedback on whether they had assessed smoking status and whether they had provided cessation counseling • A modified vital signs stamp was imprinted on each patient's encounter form for the clinical visit • Participants were offered free NRT patches and/or proactive telephone counseling Control: usual care • Physicians were provided with general information about the Agency for Healthcare Research Quality guideline evaluation trial • Participants who smoked were identified and counseled at the discretion of the clinical staff; neither intake clinicians nor primary care clinicians were instructed to provide (or to not provide) smoking cessation counseling |
|
| Outcomes | Repeated PPA at 2m and 6m Validation: None (salivary cotinine validation was attempted but abandoned due to distribution and response issues) Quit attempts Measures of provider implementation: Ask, Advise, Assess, Assist‐Self‐help, Assist‐Quit date, Assist‐Meds |
|
| Funding Source | Funded by a Preventive Oncology Academic Award (K07‐CA78540) from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, with supplemental support from the University of Wisconsin Comprehensive Cancer Center and the University of Wisconsin Medical School. GlaxoSmithKline donated transdermal nicotine patches for use in this trial | |
| Author's declarations of interest | QUOTE: "M.C.Fiore has served as a consultant for, has given lectures sponsored by, or has conducted research sponsored by GlaxoSmithKline (Research Triangle Park, NC) and was appointed by the University of Wisconsin to a named Chair made possible by an unrestricted gift to the university from GlaxoSmithKline" | |
| Notes | Strategy: Provider training + Vital signs stamp + Cost‐free medications + Adjunctive counseling + Audit & feedback Level: Patient + Provider + Practice Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "...the project statistician used a random number generator to randomly assign each clinic to receive either the intervention or usual care" |
| Allocation concealment | Unclear risk | QUOTE: "...we enrolled 2163 consecutive adult patients who smoked...". No further details reported. |
| Blinding of outcome assessors All outcomes | High risk | Smoking abstinence rates were not biochemically validated and contact with patients varied between arms |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and similar across the groups. The overall loss to follow‐up was 9.6% (n = 208/2163); 10.2% (n = 118/1155) in the intervention group and 8.9% (n = 90/1008) in the control group were lost to follow‐up at 6 months. |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "No statistically significant differences in sociodemographic characteristics (except educational level), self‐rated health status, and cigarette or alcohol use" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | Constructed 3‐level hierarchical logistic regression models of performance and cessation outcomes across the test and control sites combined |
Kim 2003.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: 1 family practice housed in a tertiary care hospital, South Korea Recruitment: Participsnts were recruited from outpatient clinic of family medicine department |
|
| Participants | 152 male adults who smoked (76 intervention, 76 control), av.age 46, av. cpd not reported | |
| Interventions | Intervention: participants received telephone counseling (for 5 – 10 minutes using stage of change model and motivational interviewing techniques) delivered by a trained nurse at 8 weeks and 17 weeks. Participants also received educational material about smoking cessation provided to the control group Control: participants received educational material about smoking cessation |
|
| Outcomes | Abstinence (undefined) at 25 wks | |
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Used a random‐number table |
| Allocation concealment | Unclear risk | Not reported |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported and the intervention group received additional face‐to‐face contact |
| Incomplete outcome data All outcomes | Low risk | 19/76 (25%) in the control and 21/76 (28%) in the intervention group were lost. Loss to follow‐up was therefore less than 50% overall and similar between groups |
Kottke 1989.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: Primary care, USA Recruitment: Providers were recruited through mailing with brochure. Participants were recruited in practice |
|
| Participants | 66 providers, 15% F, av.age 40, av. cpd 19 1653 patients smoked |
|
| Interventions |
Intervention 1: physicians received a 6‐hour workshop on smoking cessation and smoking cessation manuals Intervention 2: physicians received smoking cessation manuals (same as the one given for those in intervention 1) to hand out to people who smoked 3. Control: no assistance. No further details reported |
|
| Outcomes | Abstinence (undefined) at 12m Validation: Blood cotinine levels (cut‐off not reported) Quit attempts Measures of provider implementation: Ask, Advise, Assist‐Quit date, Assist‐Self‐help, Arrange |
|
| Funding Source | This study was supported in part by National Institutes of Health grant CA38361, National Institute of Drug Abuse grant DA04066, and a National Institute of Drug Abuse Research Scientist Award, DA00109 | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training Level: Provider Comparison type: Single component versus standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | High risk | QUOTE: "After the randomization had been initiated, it became apparent that some physicians had given home addresses while others had given work addresses. This had prevented the investigators from recognizing all cases in which multiple physicians from the same group had responded to the recruitment letter. To prevent contamination from having physicians of the same practice in different trial groups, all physicians in the same practice were either moved to the most intense level of intervention to which any of them had been originally randomized or, if not yet randomized at the time this problem was discovered, added to the group to which their partner(s) had been randomized" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and similar across the groups. At physician level, there was no loss to follow‐up (n = 0/66) at 1 year. At participant level, the overall loss to follow‐up was 13.0% (n = 215/1653); 13.2% (n = 87/660) in the workshop intervention group, 12.5% (n = 74/593) in the materials group, and 13.3% (n = 53/400) in the control group were lost to follow‐up at 1 year. |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "Neither the mean age of the physicians, nor the patient load on the physician differed significantly among the three groups"; "While a higher proportion of the patients of physicians in the no‐assistance group had at least some education beyond high school (51.8% vs 42.1% for patients of physicians in the workshop group and 42.9% for patients of physicians in the materials group [P<.001]), the distributions for the other variables did not differ significantly among the patients in the three groups" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "The physician was the unit of analysis...multivariate regression was used to adjust for potentially confounding effects of differences among the groups of doctors and their patients" |
Lancaster 1999.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: 6 general practices in Oxfordshire, Buckinghamshire, and Berkshire, UK Recruitment: Opportunistic recruitment of people who smoked visiting clinic, mailed invitation letters to those identified through practice records |
|
| Participants | 497 adults who smoked, (249 intervention, 248 control) av.age 43, 52% F, 17 cpd | |
| Interventions | Intervention: participants received smoking cessation counseling from a nurse, a carbon monoxide test and up to 5 follow‐up visits Control: participants received verbal or written advice from their physician to quit smoking and self‐help materials |
|
| Outcomes | Sustained abstinence at 12m Validation: Salivary cotinine < 113.5 mmol/l Quit attempts |
|
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "an independent statistical adviser performed randomisation from computer‐generated random numbers" |
| Allocation concealment | Low risk | QUOTE: "the allocations, in blocks of 20, were in sequential sealed, opaque envelopes opened by the research nurse at the time of recruitment" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking abstinence biochemically validated |
| Incomplete outcome data All outcomes | Unclear risk | The overall loss to follow‐up was 24.7% (n = 123/497) at 12 months. No further details on the number lost to follow‐up by group were reported |
Lasser 2017.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Boston Medical Center’s adult primary care, USA Recruitment: Calls and letters to potentially eligible identified from electronic medical record, and recruited from waiting rooms |
|
| Participants | 352 participants, av.age 50, 54% F, 15 cpd | |
| Interventions | Intervention: participants received a low literacy smoking cessation brochure and a list of hospital and community resources for smoking cessation. In addition, they received up to 4 hours of patient navigation delivered over 6 months, and financial incentives for biochemically‐confirmed smoking cessation at 6 and 12 months following enrolment Control: participants received assessment of their smoking status, brief cessation counseling, a low‐literacy smoking cessation brochure and a list of hospital and community resources for smoking cessation |
|
| Outcomes | 7‐day PPA at 12m Validation: Salivary cotinine > 10 ng/mL or urinary anabasine > 3 ng/mL |
|
| Funding Source | This study was supported by American Cancer Society (grant No. 125785‐RSG‐14‐034‐01CPPB) | |
| Author's declarations of interest | QUOTE: "Dr Quintiliani was a consultant on a research grant to Partners HealthCare Inc. unrelated to the work presented in this article. No other conflicts are reported" | |
| Notes | Strategy: Adjunctive counseling + Financial incentive Level: Patient Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Random‐number generator |
| Allocation concealment | Low risk | Sealed envelopes |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 28.7% (n = 101/352); 27.1% (n = 48/177) in the intervention group and 30.3% (n = 53/175) in the control group were lost to follow‐up at 12 months |
Lee 2016.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Outpatient clinic of the Department of Family Medicine and the Health Screening Center of Seoul National University Hospital, South Korea Recruitment: Opportunitistically in practice |
|
| Participants | 414 adults who smoked, av. age 48, 92% M, av. cpd 17 | |
| Interventions | Intervention: a 7‐minute long animated video containing information and options about smoking cessation. Following this, physicians gave a brief consultation about smoking problems or prescribed medications if participants asked for them Control: routine medical care only. The participants were not provided with the decision aid, any proactive smoking cessation counseling or prescription |
|
| Outcomes | PPA (undefined) at 6m Validation: None Measures of provider implementation: Assist‐Meds |
|
| Funding Source | This work was supported by a grant for investigator‐initiated research from Pfizer (Pfizer Reference #WS2033889). None of the sponsors had a role in any aspect of the present study, including design and conduct of study; collection, management, analysis, and interpretation of the data, and preparation, review, or approval of the manuscript | |
| Author's declarations of interest | The authors declared that they had no conflict of interest and that none of the sponsors had a role in any aspect of the study | |
| Notes | Strategy: Video Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | Based on the month. Exam rooms were randomized based on their number and the month (i.e. odd numbered exam rooms were intervention rooms) |
| Allocation concealment | High risk | Could have been foreseen as randomization was based on the month |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was self‐reported. The intervention was a decision aid video so there was no person‐to‐person contact in either group |
| Incomplete outcome data All outcomes | High risk | Attrition rates were under 50% but the difference between groups was greater than 20%. The overall loss to follow‐up was 20.5% (n = 85/414); 33.8% (n = 66/195) in the intervention group and 8.7% (n = 19/219) in the control group were lost to follow‐up at 6 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the Department of Family Medicine and the Health Screening Center of Seoul National University Hospital before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "None of the characteristics was significantly different between the control and intervention groups" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "To investigate the impact of the decision aid on the outcomes, univariate and multivariate logistic regression tests were used, with accounting for the clustering effect of nesting physicians"; "The intracluster correlation coefficient values were 0.21 for the primary outcome variable and 0.10 for the secondary outcome variable..." |
Lennox 1998.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: General practices in Aberdeen, UK Recruitment: Mailing of questionnaire to adults from practice list |
|
| Participants | 16 providers (8 intervention, 8 control). 2588 people who smoked (aged 16 ‐ 65) identified through questionnaires, av. age not reported, av. cpd not reported |
|
| Interventions | Intervention:1‐day training for providers on the stages of change for smoking cessation Control: usual care. No further details reported |
|
| Outcomes | Continuous abstinence from 8m to 14m Validation: None Secondary outcomes: Quit attempts Measures of provider implementation: Ask |
|
| Funding Source | Funded by the Chief Scientist Office. Scottish Office Department of Health. Grampian Health Board funded the running of the workshops | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training Level: Provider Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "A computer‐generated random sample" |
| Allocation concealment | Low risk | QUOTE: "A computer‐generated random sample" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was self‐reported. The intervention was a 1‐day training workshop aimed at staff so the number of face‐to‐face contacts differed between arms at practice level, but not at participant level |
| Incomplete outcome data All outcomes | Low risk | At practice level, no practices were lost to follow‐up (n = 0/16). At participant level, attrition rates were under 50% and similar between groups. The overall loss to follow‐up was 24.1% (n = 408/1693); 24.9% (n = 224/898) in the intervention group and 23.1% (n = 184/795) in the control group were lost to follow‐up at 14 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "There was no significant difference between the two arms of the study in response rate, age, sex, addiction score or readiness to change smoking behaviour. Intervention subjects were less affluent than control subjects, and regression techniques were therefore used to adjust for deprivation" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "A generalised linear mixed model (GLMM) approach used regression techniques which added the general practice, as a random factor nested within the treatment groups, to the other fixed‐effect factors" |
Lennox 2001.
| Study characteristics | ||
| Methods | Design: 3‐group randomized controlled trial Setting: 6 general practices in Aberdeen, UK Recruitment: Mailed lettered to patients identified in EMR |
|
| Participants | 2553 people who smoked aged 17 ‐ 65, av. age not reported, av. cpd not reported | |
| Interventions |
Intervention 1: participants received an untailored letter on smoking cessation Intervention 2: participants received a tailored letter on smoking cessation Control: participants received a letter thanking them for participation and informing them that they would receive material at the end of the study (either a tailored or a non‐tailored letter) |
|
| Outcomes | 7‐day PPA at 6m Validation: Salivary cotinine, cut‐off not reported (only completed in 3.5% of participants) |
|
| Funding Source | The Chief Scientist Office, Scottish Executive Health Department, with additional funding from the Engineering and Physical Sciences Research Council. The Health Economics Research Unit is funded by the Chief Scientist Office | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Tailored print materials Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated random numbers were used |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Salivary cotinine, cut‐off not reported (only completed in 3.5% of participants). Face‐to‐face contact was minimal in all groups |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 23.6% (n = 615/2610); 24.5% (n = 213/870) in the tailored letter group, 27.2% (n = 236/869) in the standard letter group and 19.1% (n = 166/871) in the control group were lost to follow‐up at 6 months |
Leppänen 2019.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Primary healthcare centres, Sweden Recruitment: QUOTE: "Eligibility was assessed using a short screening questionnaire before patients were invited to participate. The patients were recruited by one to three appointed PHC providers at each PHC centre that were responsible for the treatment of patients in the study" |
|
| Participants | 250 adults who smoked from 18 primary healthcare centres in Sweden. Participants had a mean age of 54.4 years, av. cpd not reported, most had chronic disease (70%) | |
| Interventions | Intervention: Tobacco Cessation on Prescription (TCP) consisting of 1) person‐centered tobacco cessation counseling from a qualified healthcare professional for at least 10 minutes; 2) an individualized prescription of tobacco cessation treatment; 3) follow‐up on at least 1 occasion; 4) providers received 3 hours of training. Healthcare providers could use the prescription form as a basis for tobacco cessation counseling with the patient, discussing available treatment options and deciding together what option(s) would suit the participant best Control: participants received standard treatment (brief advice consisting of < 5 minutes of tobacco cessation counseling, but providers were free to offer whatever treatment they wanted as long as this was documented). Providers also received a written manual and 3 hours of training in tobacco cessation treatment |
|
| Outcomes | 7‐day PPA at 6m and 12m Validation: none Quit attempts (however, result only reported narratively and unable to extract data for analysis) |
|
| Funding Source | The study is funded by grants from the Stockholm County Council (grant no: HSN 1309‐1029), The Public Health Agency of Sweden (grant no: 03074‐2015‐6.2) and Livförsäkringsbolaget Skandia. | |
| Author's declarations of interest | None | |
| Notes | Strategy: Adjunctive counseling, provider training Level: Patient & provider Comparison type: Active vs. active (isolates adjunctive counseling) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated |
| Allocation concealment | Low risk | Quote: "A computer generated random allocation sequence will be applied to randomize the PHC centers to either intervention or control conditions" |
| Blinding of outcome assessors All outcomes | High risk | Self‐reported outcomes and more contact in the intervention group |
| Incomplete outcome data All outcomes | High risk | 56% participants responded to 6‐month follow‐up questionnaire. Imputation used for missing data |
| Recruitment bias (cluster RCTs only) | Unclear risk | 77 PHC centers invited and 17 agreed |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | Quote: "The patients were similar in the treatment groups but patients in the intervention group were more often female, born in Sweden, had more previous quit attempts, experience of pharmacotherapy and lower prevalence of chronic disease compared to the control group." |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | Adjustment for clustering conducted |
Lindsay 1989.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: Primary care practices, Canada Recruitment: Receptionists identified people who smoked while visiting provider for routine appointment |
|
| Participants | 83 providers 1942 people who smoked aged > 16 years, 64% smoked at least 20 cpd, av. age not reported, av. cpd not reported |
|
| Interventions |
Intervention 1: Gum only • Physicians were cued by a project document indicating the participant's agreement to participate. Physicians in this group were instructed to advise the participant to quit smoking • Participants were advised to use nicotine gum (at their own cost) by their physician Intervention 2: Gum plus • Physicians attended a training session on smoking cessation. Flow sheet provided to them to help them deliver intervention. Physicians in this group were instructed to advise the participant to quit smoking • Participants received self‐help materials and were advised to use nicotine gum (at their own cost) by their physician Control: usual care. QUOTE: "If it was part of their usual practice to address the smoking issue with patients, this occurred. We gave no instructions to patients about whether they should mention their agreement to participate to their physician, and we had no way of assessing whether this, in fact, occurred" |
|
| Outcomes | 3m continuous abstinence measured at 12 months Validation: Salivary cotinine < 0.057 umol/L Quit attempts Measures of provider implementation: Ask, Advise, Assist, Assist‐Meds, Assist‐Self‐help, Assist‐Quit Date, Arrange |
|
| Funding Source | National Institute of Health (USA) and Canadian National Research and Development Program | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training + flow sheet Level: Provider + Practice Comparison type: Multi‐component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking abstinence was validated by cotinine |
| Incomplete outcome data All outcomes | Unclear risk | Authors reported that 21.3% (n = 129/606) of participants in the gum‐plus group attended 4 or 5 follow‐up visits and that no data were available for gum‐only group. No further details reported |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization. QUOTE: "patients entered the study when they visited their physician for a routine office appointment" |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "we observed no differences on physician characteristics among experimental groups"; "we observed some difference in motivation levels among groups and the main analyses of outcome adjusted for these differences" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "treatment effect was assessed using analysis of covariance; the unit of analysis being the practice" |
Lou 2013.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 14 healthcare units in rural area of Xuzhou city, China Recruitment: Physicians recruited their patients |
|
| Participants | 136 providers, 14 practices 3562 participants diagnosed with chronic obstructive pulmonary disease, aged 35 or older, smoked 1 cpd or no quit attempts longer than 3m, , av. age not reported, av. cpd not reported |
|
| Interventions | Intervention: • Healthcare professionals received a 6‐hour training in behavioral interventions for quitting smoking • Participants received a brief smoking cessation advice after the baseline interview, were provided with a plan to quit smoking (e.g. setting a quit date). Other measures to encourage smoking cessation included home visit by the providers at least once a week). They were followed up by the providers once a week in the first month and thereafter once a month until the end of study. Participants in healthcare centres were visited by 'the professional group' (e.g. respiratory, rehabilitation, nutrition, sports and psychology specialists) every 2 months and were provided with smoking‐related and obesity‐related psychological support Control: usual care. QUOTE: "The content and number of usual care services were not standardized. Participants were followed up every two months and asked whether the symptoms aggravated, what medication they used, etc." No further details reported |
|
| Outcomes | Continuous abstinence at 6m Validation: Expired CO ≤ 10 ppm |
|
| Funding Source | Science and Technology Projects of Xuzhou City | |
| Author's declarations of interest | The authors declared that they had no competing interests | |
| Notes | Strategy: Adjunctive counseling & provider training Level: Patient & Provider Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | QUOTE: "The healthcare centres were classified in two classes: with high or low task delegation from general practitioners to nurses. The healthcare centres in the classes were then randomly allocated to the groups". No further information. |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 24.5% (n = 873/3562); 21.5% (n = 390/1814) in the intervention group and 27.6% (n = 483/1748) in the control group were lost to follow‐up at 48 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with their family physicians before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | No statistically significant differences were found between groups |
| Adjustment for clustering in analysis? (cluster RCTs only) | High risk | None apparent |
Marley 2014.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: 2 Aboriginal Controlled Community Health Services, Australia Recruitment: Passive recruitment through visits to primary care clinics and active recruitment by researchers through community and family links |
|
| Participants | 163 Aboriginal and/or Torres Strait Islanders, ≥ 16 years of age, reporting current smoking or quitting within 2 weeks of recruitment, thinking about cutting down or quitting smoking, regular client of 1 of 2 Aboriginal Health Services. av. age 39, 54% F, av. cpd 15 | |
| Interventions | Intervention: in addition to usual care, participants received in‐person smoking cessation counseling scheduled weekly for the first 4 weeks, monthly to 6 months and 2‐monthly to 12 months (12 sessions). Delivered by Aboriginal researchers Control: participants received usual care ‐ smoking cessation support at their local primary health care service, including advice regarding quitting, pharmacotherapy, and self‐initiated follow‐up |
|
| Outcomes | 7‐day PPA at 12m Validation: Urinary cotinine < 50 ng/mL |
|
| Funding Source | National Health and Medical Research Council of Australia (NHMRC, project grant number 513818) | |
| Author's declarations of interest | Authors declared that they had no competing interest. | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "A computer generated random allocation sequence was used" |
| Allocation concealment | Low risk | QUOTE: "Sealed envelopes containing the allocation were kept at the centralised coordinating site. Allocation occurred via telephone with envelopes being opened in sequential order" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 14.3% (n = 24/168); 15.5% (n = 9/58) in the intervention group and 13.6% (n = 15/110) in the control group were lost to follow‐up at 12 months |
Mejia 2015.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 6 clinical systems in the cities of Buenos Aires, La Plata and Olavarria, Argentina Recruitment: Physicians were recruited from 6 clinical systems. All patients who saw their physician within 30 days of the intervention were included |
|
| Participants | 254 physicians, 52.4% F 1378 patients (750 intervention, 628 control) 80.9% F, av. age not reported, av. cpd not reported |
|
| Interventions | Intervention: 2 x 3‐hour training session on tobacco cessation. Physicians also received monthly emails as reminders with useful tips to help patients stop smoking or manage withdrawal Control: usual care. No further details reported |
|
| Outcomes | 7‐day PPA at 12m Validation: None Quit attempts Measures of provider implementation: Ask, Advise, Assist‐Quit date, Assist‐Self‐help |
|
| Funding Source | Funded by grant No.TW05935 from the Tobacco ResearchNetwork Program, Fogarty International Center, National Cancer Institute, NationalInstitute of Drug Abuse, National Institutes of Health, the National Cancer Institute for Redes en Acción (U01CA86117 and U54CA153511) and by grant No. 001726‐037 from Research on International Tobacco Control, International Development Research Center, Canada | |
| Author's declarations of interest | Authored declared that they had no conflict of interest | |
| Notes | Strategy: Provider training Level: Provider Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Unclear risk | Smoking status self‐report. Amount of face‐to‐face contact unclear |
| Incomplete outcome data All outcomes | Low risk | At physician level, the overall loss to follow‐up was 30.0% (n = 76/254); 25.8% (n = 32/124) in the intervention group and 33.8% (n = 44/130) in the control group were lost to follow‐up at 12 months. At participant level, the overall loss to follow‐up was 32.3% (n = 445/1378) at 12 months and the split of this between the groups was not reported |
| Recruitment bias (cluster RCTs only) | Unclear risk | QUOTE: "Lists of patients seen within 30 days after the study physicians were randomized (control) or had completed the smoking cessation course (intervention) were obtained" |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | Balanced between trial arms |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "P values for group by time interaction are from generalised linear mixed model analysis accounting for clustering of observations by physician and repeated measures per patient" |
Meyer 2008.
| Study characteristics | ||
| Methods | Design: 3‐group randomized controlled trial Setting: Primary care in Germany Recruitment: For a period of 3 weeks all consecutive patients were screened for smoking status by a research nurse covering complete office hours |
|
| Participants | 1499 adults who smoked, 48% F, av.age 33, 16 cpd, 64% unmotivated | |
| Interventions |
Intervention 1: participants received by post up to 3 computer‐generated tailored letters, accompanied by a series of self‐help manuals Intervention 2: • Pratitioners received 2‐hour training on smoking cessation. The practitioners received a summary sheet of basic information about their patients' smoking‐related variables, as a prompt to offer counseling • Participants received brief advice, lasting 10 minutes, from their practitioner and self‐help manuals. The intervention was delivered within the regular consultation Control: QUOTE: "no intervention beside usual practice routine was provided for the control group. No information about the participants was given to the practice team or the practitioner and no self‐help manuals have been provided" |
|
| Outcomes | 6m sustained abstinence 24m Validation: None |
|
| Funding Source | Funded by the German Federal Ministry of Research and Education (grant no.01EB0120, 01EB0420), the Social Ministry of the Stateof Mecklenburg‐Vorpommern (grant no. IX311a406.68.43.05) and the German Research Foundation (Deutsche Forschungsgemeinschaft, grant no. JO150/6‐1) | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Tailored materials, flow sheet, provider training Level: Patient, provider, practice Comparison types: Single component vs. standard care; multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | Assigned based on the week they were seen at the practice |
| Allocation concealment | High risk | Assigned based on the week they were seen at the practice |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported and participants in the brief advice group had additional 10 minutes in their consultation to listen to the advice |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 37.2% (n = 558/1499);42.8 % (n = 209/488) in the tailored letter group, 33.8% (n = 136/402) in the brief advice group and 35.0% (n = 213/609) in the control group were lost to follow‐up at 24 months |
Meyer 2012.
| Study characteristics | ||
| Methods | Design: Clustered randomized controlled trial with 3 active comparators Setting: Primary care in North‐Eastern Germany Recruitment: Practices contacted by research team and invited to participate |
|
| Participants | 151 practices 3086 participants, 43% F, av.age 40, av. cpd not reported |
|
| Interventions |
Intervention 1: brief advice with desktop resource Participants received a 10‐minute brief advice which incorporated elements of health behavior change counseling and were provided with self‐help materials. Physicians received a summary sheet of smoking‐related characteristics to prompt counseling and also a desktop resource with a flow chart illustrating the elements of counseling and general communication strategies Intervention 2: tailored letters Participants received 2 tailored letters, based on their answers to 2 questionnaires. Letters were given while participant was in the practice Intervention 3: combination Participants received both brief advice from the physician and a tailored letter. The same self‐help manuals used in the other conditions were provided to the participants |
|
| Outcomes | 6m prolonged abstinence at 12m Validation: None Provided some data on intervention 'reach' but it was not possible to classify this into our provider implementation outcomes |
|
| Funding Source | The German Federal Ministry of Research and EducationThe Social Ministry of the State of Mecklenburg‐Vorpommern The German Research Foundation | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Tailored materials, flow sheet Level: Patient, practice Comparison type: Active vs. active (2 comparisons: int 1 vs. int 3 & int 2 vs. intervention 3) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Smoking status self‐report, and contact differed between trial arms |
| Incomplete outcome data All outcomes | Low risk | Attrition rates were under 50% and the difference among groups was less than 20%. At practice level, no practice was reported to have been lost to follow up (n = 0/151), At participant level, the overall loss to follow‐up was 26.8% (n = 863/3215); 30.6% (n = 189/618) in the brief advice group, 22.3% (n = 331/1484) in the tailored letters group, and 30.8% (n = 343/1113) in the combination group were lost to follow‐up at 12 months |
| Recruitment bias (cluster RCTs only) | Unclear risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "There were no significant differences in the characteristics of the participating practices and practitioners between study groups" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "For all analyses at the patient level, we took clustering within practices into account using sample survey methods in STATA 10.1" |
Minué‐Lorenzo 2019.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Primary care practice, Spain Recruitment: Patients who attended the healthcare centre for any reason were approached by a general practitioner or a nurse |
|
| Participants | 1154 adults who attended the primary healthcare centre for any reason between June and December 2009, smoking ≥ 10 cigarettes/day, at any stage of the smoking cessation process, av. age 46, av. cpd 22 | |
| Interventions | Intervention: participants received first‐line quit‐smoking medication (varenicline, bupropion or NRT) free of cost. Type of pharmacotherapy was chosen by the physician in accordance with participant preference. NRT provided for 8 weeks and dose based on CPD; Varenicline or bupropion standard doses for 12 weeks. Participants also received usual care as defined below Control: usual care described as behavioral treatment and recommendation for using pharmacological treatment in accordance with standard health services offered in primary care (prescribed pharmacological treatment but had to purchase it). No further details on the behavioral treatment reported |
|
| Outcomes | CO‐confirmed continuous abstinence at 12m (self‐reported 12m rates also reported) Validation: CO |
|
| Funding Source | Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (ISCIII), the European Regional Development Fund (ERDF) | |
| Author's declarations of interest | QUOTE: "The authors declare that they have no competing interests, financial or otherwise, related to the current work. C.Minue‐Lorenzo reports grants from Fondo de Investigaciones Sanitarias (FIS) del Instituto de Salud Carlos III (ISCIII), European Regional Development Fund (ERDF), grants from Fundacion para la Investigacion e Innovacion Biosanitaria en Atencion Primaria (FIIBAP), during the conduct of the study. The rest of the authors have also completed and submitted an ICMJE form for disclosure of potential conflicts of interest" | |
| Notes | Strategy: Cost‐free pharmacotherapy Level: Patient Comparison type: Single‐component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "computer‐generated random sequence" |
| Allocation concealment | Low risk | QUOTE: "Randomization was performed centrally by a researcher not involved in the study, and who was blind to the identity of the HCCs" |
| Blinding of outcome assessors All outcomes | Low risk | Abstinence was biochemically validated |
| Incomplete outcome data All outcomes | Low risk | 32/387 (8.3%) in the usual care arm and 53/767 (6.9%) in the intervention arm were lost to follow‐up |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were members of the practices before they were randomized |
| Balanced baseline characteristics? (cluster RCTs only) | Unclear risk | QUOTE: "the intervention group comprised a larger percentage of men, smoked more cigarettes per day, and showed higher scores in the FTND (Table 1). Additionally, the rate of patients at the preparation and action stages of the cessation process was significantly higher in the intervention group" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | A multilevel logistic regression model was built and significant variables tested as covariates, taking into consideration sampling by clusters |
Morgan 1996.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Primary care practices in suburban Philadelphia and eastern Pennsylvania, USA Recruitment: Conducted in practice |
|
| Participants | 49 practices without a formalized smoking intervention program 1318 people who currently smoked aged 50 ‐ 74 years, presenting for a non‐emergency visit to the practice. 56% F, 20 cpd, av. age 60 |
|
| Interventions | Intervention: • Practices received on‐site training to implement a modified National Cancer Institute (NCI) smoking cessation intervention based on the 4 A's. Physicians were trained to praise participants for previous quit efforts, provide personalized feedback, discuss the health benefits of quitting for older people who are smoking, and give a clear message to quit smoking • Participants received a smoking cessation guide tailored to older people who smoke and were offered help with quitting. They were also sent a follow‐up letter drafted by the Clear Horizons office from their physician within 1 week of their visit, a brief follow‐up quitline counseling call from the project staff within 2 ‐ 4 weeks of the intervention visit. They were also provided with a medical record flowchart specifically made for smoking cessation ‐ people who smoked, in the precontemplation stage, who declined help: received brief guide‐based counseling to overcome quitting barriers ‐ people who smoked, in the contemplation stage received brief guide‐based counseling to set up a quit plan and quit date and a prescription for nicotine gum (free 1‐week samples) Control (usual care): practices in this group were instructed to provide usual care to older people who smoked over the accrual and follow‐up period. No further details reported |
|
| Outcomes | 7‐day PPA at 6m Validation: None Provider implementation outcomes were only measured in the intervention group |
|
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training + cost‐free medications + adjunctive counseling + flowchart Level: Patient, Provider, Practice Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status self‐report. At participant level, there was no variation in contact |
| Incomplete outcome data All outcomes | Unclear risk | QUOTE: "Of the 659 patients who completed the baseline questionnaire, 573 (87%) were contacted for a telephone interview at the 6‐month follow‐up". Follow‐up rates by group were not reported |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | High risk | QUOTE: "Immediate and delayed intervention practices did not differ significantly in the mean number of patients enrolled, gender of patients enrolled, or reporting of quit attempts lasting 24 hr or more in the previous year... patients in the two conditions did differ in age, average number of cigarettes smoked daily, time elapsed until first cigarette of the morning, and contemplation status" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "A correlated logistic regression model that accounted for dependencies among respondents within a given practice was utilized" |
Murray 2008.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 3 Nottingham Primary Care Trust areas, UK Recruitment: Proactive identification of people who smoked via a letter offering smoking cessation support through the National Health Smoking Cessation Service |
|
| Participants | 6856 adults who smoked, av.age 45, 51% M, av. cpd not reported | |
| Interventions | Intervention: participants received brief advice on smoking cessation and information about their local NHS Stop Smoking Service by the research team via telephone • If participants wished, an initial consultation with the NHS stop smoking service was booked. Paarticipants who attended this were offered the option of one‐to‐one or group behavioral support lasting an average of 8 weeks, and NRT or bupropion therapy, and set a quit date • If participants declined an appointment or were uncontactable, an information pack was sent. The pack included an information leaflet from the service, encouragement to use the service, and contact details for the research team and the local service Control: QUOTE: "for six months from baseline, smokers in the control practices received no further intervention other than that provided by usual care. Previous studies suggest that, in most cases, little or no advice or support would have been given". No further details reported |
|
| Outcomes | 7‐day PPA at 6m Validation: Salivary cotinine < 15 ng/ml or exhaled CO < 10 ppm |
|
| Funding Source | Funded by the British Heart Foundation. The study was designed, conducted, analyzed and interpreted independently of all funding sources | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | At participant level, the overall loss to follow‐up was 48.8% (n = 3344/6856). The mean response was 47.9% (range 28.8 ‐ 55.6) in the intervention group and 53.7% (range 39.6 ‐ 63.3) in the control group at 6 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "The distribution of gender and age was similar for participants in intervention and control practices" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "We used a two‐level hierarchical model with subjects nested within practices, a random effect of practice, intervention fitted at the practice level, and age, sex, Townsend score and amount smoked per day at the subject level"; "...assuming an intracluster correlation coefficient of not more than 0.007..." |
Nebot 1992.
| Study characteristics | ||
| Methods | Design: cluster‐randomized controlled trial Setting: 3 urban reformed primary care centres in Barcelona, Spain Recruitment: All people who smoked (> 1 cpd) visiting physician for any reason |
|
| Participants | 15 primary care teams within 3 primary care centres 425 adults who smoked, 30% F, av. age not reported, av. cpd not reported |
|
| Interventions |
Intervention 1: physician counseling Participants received standard physician advice operatively defined as a personalized firm counsel to stop smoking, lasting 3 ‐ 5 minutes Intervention 2: physician counseling + nicotine gum Participants received standard physician advice plus a free supply of nicotine gum sufficient to last 2 ‐ 4 weeks Intervention 3: nurse counseling Participants received up to 15 minutes of nurse advice |
|
| Outcomes | Abstinence (undefined) at 12m follow‐up Validation: Expired CO < 8 ppm |
|
| Funding Source | Grant from the Fondo de Investigaciones Sanitarias de la Seguridad Social | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Cost‐free medication + Adjunctive counseling Level: Patient Comparison types: Single component vs. standard care (testing cost‐free medications and adjunct counseling individually in separate trial arms) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status validated by carbon monoxide levels |
| Incomplete outcome data All outcomes | Unclear risk | Authors reported that 82% were followed up at 2 months, but they did not report the follow‐up rate at 12 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "The three groups had no significant differences in these characteristics except for the proportion of smokers having tried to quit before (higher among the B group patients)" |
| Adjustment for clustering in analysis? (cluster RCTs only) | High risk | None apparent |
Nichols 2017.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primark care clinics, UK Recruitment: Mailed letters to patients in GP database |
|
| Participants | 109 adults who smoked. 55.6% F; mean age 49 years; mean Fagerström score 4.9, av. cpd 18 | |
| Interventions | Intervention: participants received an 8‐week smoking cessation program, where a participant is offered a fact sheet on the health risks of smoking (including lung cancer) and the option of the gene‐based test for calculation of lung cancer susceptibility Control: participants received a smoking cessation program without option of gene‐based test |
|
| Outcomes | Continuous abstinence at 6m Validation: Expired CO and salivary cotinine, cut‐offs not reported |
|
| Funding Source | JN and PG are in receipt of research grants from Lab 21, Cambridge who are marketing the Respiragene test in the UK and Synergenz Bioscience Ltd. who financed the development of the test from its origins in New Zealand | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Gene‐based test Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 43.1% (n = 47/109); 37.0% (n=20/54) in the intervention group and 49.0% (n = 27/55) in the control group were lost to follow‐up at 6 months |
Ockene 1994.
| Study characteristics | ||
| Methods | Design: 3‐group randomized controlled trial Setting: Primary care, USA Recruitment: Opportunistic recruitment from practice |
|
| Participants | 1499 adults who smoked aged 18 ‐ 75 years, 57% F, av.age 35.3, 23 cpd | |
| Interventions |
Intervention 1: participants received simple, individualized advice to stop smoking from their physician Intervention 2: participants received counseling with a patient‐centered approach, consisting of questions addressing motivation and a written plan for change. Participants also received a self‐help booklet and a list of local smoking cessation programs and scheduling of a follow‐up visit or telephone call Intervention 3: participants received the same counseling as in intervention, plus a prescription of free 2 mg nicotine gum if agreed to set a quit date |
|
| Outcomes | Maintained 7‐day PPA at 12m Validation: None |
|
| Funding Source | National Cancer Institute Grant | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Cost‐free medications Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Self‐reported smoking cessation plus varying contact between groups |
| Incomplete outcome data All outcomes | Unclear risk | The overall loss to follow‐up was 15.9% (n = 238/1499) at 12 months. No further details on the number lost to follow‐up by group were reported |
Olano Espinosa 2013.
| Study characteristics | ||
| Methods | Design: Cluster‐ randomized controlled trial Setting: Healthcare centres in Area 11 of the Spanish Madrid Health System, Spain Recruitment: Participants selected from computerized clinic records |
|
| Participants | 405 nurses and 425 doctors from the 35 clinics 5910 adults who smoked. av.age 43, 53% F, av. cpd not reported |
|
| Interventions | Intervention: professionals received 4 x 90‐minute training sessions on smoking cessation Control: professionals were offered the training after finishing the follow‐up period |
|
| Outcomes | Continuous abstinence at 6m Validation: Salivary cotinine < 13 ng/ml |
|
| Funding Source | Spanish Public Health Investigations Fund, with no role in the study | |
| Author's declarations of interest | QUOTE: "Two authors (FJA and EOE) work as directors of Cantabria University Tobacco Control Master" | |
| Notes | Strategy: Provider training Level: Provider Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "...randomly selected 15 patients for each quota by using number combinations generated with EPIDAT 2.0 software" |
| Allocation concealment | Low risk | QUOTE: "An independent research assistant assigned the 35 health care centers randomly using SPSS v.12 software...". No further detail provided. |
| Blinding of outcome assessors All outcomes | Low risk | Abstinence biochemically validated |
| Incomplete outcome data All outcomes | Unclear risk | Not reported |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "No significant differences were found between both groups with respect to profession, age, sex, professional experience, percentage of smokers, and previous training in treating tobacco addiction" |
| Adjustment for clustering in analysis? (cluster RCTs only) | High risk | Not apparent |
Papadakis 2018.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Family medicine practices in Ontario, Canada Recruitment: Invitation letters sent to practices. Participants recruited in the waiting room of the clinics |
|
| Participants | 15 practices 867 adults who smoked completed post‐intervention exit survey, av. age not reported, av. cpd not reported | |
| Interventions |
Intervention 1: all teams received the Ottawa Model for Smoking Cessation program which included outreach facilitation, provider training, real‐time prompts, and an automated follow‐up program Intervention 2: all teams received the Ottawa Model for Smoking Cessation program (as described above). In addition, general practitioners and nurse practitioners received a supplemental 1½‐hour coaching session for providers 4 weeks following the program launch at their clinic Providers were given a report of their performance in delivering tobacco use treatment interventions |
|
| Outcomes | 12w prolonged abstinence at 6m Validation: None Measures of provider implementation: Ask, Advise, Assist‐Meds, Assist‐Quit date, Assist‐Self‐help, Arrange Secondary outcome: Quit attempts |
|
| Funding Source | This study was funded through a Grant‐in‐aid from the Heart and Stroke Foundation of Canada (Grant # NA7193) | |
| Author's declarations of interest | QUOTE: "R.D.R. has received speaker and consulting fees from Pfizer and Johnson & Johnson, K‐A.M. has received speaker fees from Pfizer, A.L.P. has received speaker and consulting fees from Pfizer and Johnson & Johnson that are not related to this study." All others report none | |
| Notes | Strategy: Adjunctive counseling, Provider training, Audit & feedback, Vital Sign Stamp, Consult Form, EMR prompts, Outreach facilitation, Performance coaching Level: Patient, Provider, Practice Comparison type: Active vs. active (isolates performance coaching) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | QUOTE: "A simple blocked randomization scheme was used in which blocks of four clinics were randomized". No further detail given |
| Allocation concealment | Unclear risk | QUOTE: "The Methods Centre provided the principal investigator with the list of practice assignments". No further detail given |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was self‐report; but contact with participants was balanced between arms |
| Incomplete outcome data All outcomes | Low risk | At participant level, 21.3% (n = 85/399) in the OMSC group and 18.1% (n = 86/475) in OMSC+ group were lost to follow‐up at 6 months post‐intervention |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "There were no differences in practice and clinician characteristics between intervention groups" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Multilevel models account for the clustered design. A 3‐level generalised linear mixed model estimated the effect of the intervention for each outcome measure..." |
Parkes 2008.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: 5 general practices in Hertfordshire, UK Recruitment: Identified potentially eligible participants by searching computerized patient records from the practices. A letter of invitation was sent to the identified patients. 2 weeks later, those who had not already responded were telephoned and offered an invitation to participate. Those who could not be contacted by telephone were sent a second letter |
|
| Participants | 561 adults who smoked (> 35 years). av.age 53, 46% M, 17 cpd | |
| Interventions | Intervention: Participant performed spirometry then was given their results verbally in the form of “lung age” with a graphic display. Within 4 weeks of data collection the research doctor sent all participants an individualized letter. Written results were given to the intervention group as “lung age.” Control: Participants were not told their spirometry results, but informed that they would be invited for a second test after 12 months to “see if there had been any change in lung function.” Within 4 weeks of data collection the research doctor sent all participants an individualized letter. Written results were given to the control group as simple FEV1 (liters per second) with no further explanation |
|
| Outcomes | 24‐hour PPA at 12m Validation: Salivary cotinine < 14.2 ng/ml |
|
| Funding Source | Funding: Leading practice through research award from the Health Foundation | |
| Author's declarations of interest | Authors declared that they had no competing interest | |
| Notes | Strategy: Spirometry (Lung age monitoring) Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Clerk prepared 600 sequentially‐numbered opaque sealed envelopes, each containing a card with allocation group determined by computer‐generated random number |
| Allocation concealment | Low risk | Opaque sequentially‐numbered sealed envelopes |
| Blinding of outcome assessors All outcomes | Low risk | Smoking abstinence was biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 11.2% (n = 63/561); 11.1% (n = 31/280) in the intervention group and 11.4% (n = 32/281) in the control group were lost to follow‐up at 12 months |
Pereira 2006.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: General practitioners in the Languedoc‐Roussillon region of France Recruitment: Practices were sent a letter of invitation, participants recruited in practice |
|
| Participants | 1075 adults who smoked, 52% F, av.age 41, av. cpd not reported | |
| Interventions | 1. Intervention: 3‐day training program for GPs, consisted of concrete steps in creating and installing routine cessation interventions in general practice, taught by 8 professionals, whose expertise lay in cessation programs, teaching methods or patient education.Trained GPs offered 8 special consultations for smoking cessation 2. Control: Usual care. No further detail |
|
| Outcomes | Abstinence (undefined) at 12m Validation: None Measures of provider implementation: Assist‐Self‐help, Assist‐Prescribe |
|
| Funding Source | All sources were either non governmental associations, either government and health department or public health assurance | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training Level: Provider Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Unclear risk | Abstinence was self‐report; hard to tell with contact different between arms |
| Incomplete outcome data All outcomes | Unclear risk | Follow‐up rate: 68.5%. No further information |
| Recruitment bias (cluster RCTs only) | Unclear risk | No details reported |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | No statistical differences in GPs by group. Participant differences were noted and controlled for in analysis |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Marginal models, estimated by GEE and mixed generalised linear models are used for this type of design" |
Pérez Tortosa 2015.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Primary care teams from Barcelona, Spain Recruitment: Opportunistically in practice |
|
| Participants | 948 people with diabetes who smoked, aged 14 or older, that receive routine diabetes care in the participating practice (456 intervention, 492 control). 73% M, av.age 58, 17cpd. | |
| Interventions | Intervention: • Doctors and nurses received a full‐day specific training workshop on motivational interview and pharmacological treatment. Workshops were focused on people with diabetes who smoked and were taught by trained experts. They also were trained in the dynamics of the follow‐up visits according to the stages of change and in how to use the electronic data collection systems • Participants received adjunctive counseling. Control: providers attended a practical training session that covered the methodology of the study and the electronic data collection system |
|
| Outcomes | 6m continuous abstinence at 12m Validation: Expired CO < 6 ppm |
|
| Funding Source | Financial help from an Evaluation of SanitaryTechnologies and Health Services grant (Evaluación de Tecnologías Sanitariasy Servicios de Salud), given by the Carlos III Health Institute (PI08/90345) in 2008 | |
| Author's declarations of interest | QUOTE: "the authors declare that they have no conflicts of interest in relation to this study" | |
| Notes | Strategy: Provider training + Adjunctive counseling Level: Provider + Patient Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "...using a centralized, computerized randomisation system" |
| Allocation concealment | Low risk | QUOTE: "...using a centralized, computerized randomisation system" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | At participant level, the overall loss to follow‐up was 23.8% (n = 226/948); 24.3% (n = 111/456) in the intervention group and 23.4% (n = 115/492) in the control group at 12 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "Patients in the intervention arm as compared with controls showed significantly higher scores in the Richmond test". Statistically significant differences in baseline TTM stages, with a lower percentage of participants in the pre‐contemplation stage (27.8% vs. 49.6%) and a higher percentage in the preparation/action stage in the intervention group than in controls. Adjusted for differences. |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "A multilevel mixed‐effects logistic regression with random effect estimates for primary care team clusters was performed to assess the effect of intervention on smoking abstinence adjusted by TTM stage at inclusion in the study" |
Piper 2016.
| Study characteristics | ||
| Methods | Design: Fractional factorial screening experiment Setting: Primary care clinics in southern Wisconsin, USA Recruitment: participants were recruited from 11 primary care clinics. During clinic visits, clinical care staff were prompted by electronic health record technology to invite people identified as smoking to participate in the study |
|
| Participants | 637 participants, 55% F, av. age 45.8, average cpd not reported | |
| Interventions |
Intervention 1. Pre‐quit nicotine patch Half the participants were assigned to the active condition and received 14 mg patches for the 3 weeks prior to the TQD, while the other half did not receive prequit patches Intervention 2. Prequit nicotine gum Participants in the active condition received 2 mg nicotine gum for the 3 weeks prior to the TQD (≥ 9 pieces of gum/day, 1 piece/1 – 2 hours); the other half did not. Participants who received both Prequit Patch and Gum were told to use at least 5 pieces/day of gum, unless such use produced adverse effects Intervention 3. Preparation counseling Participants in the active condition received 3 x 20‐min counseling sessions prior to the TQD, focused on coping skills, reduction, and making practice quit attempts,while the other half of participants did not. The sessions 3 weeks and 1 week before the TQD were in‐person, and the week‐2 session was over the phone Intervention 4. In‐person counseling Participants in the intensive condition received 3 x 20‐min face‐to‐face counseling sessions: 1 week pre‐TQD, on the TQD, and at week 1. Sessions focused on skill building and intra‐treatment social support. Participants assigned to the minimal level received 1 x 3‐min in‐person session at Week‐1 Intervention 5. Phone counseling Participants in the intensive condition received 3 x 15‐min phone sessions (TQD, Days 2 and 10), focused on coping skills, avoiding smoking cues, and intra‐treatment social support. Participants assigned to the minimal condition received 1 x 10‐min session on the TQD. Thus, all participants received someTQD phone counseling Intervention 6. Extended medication All participants received combination NRT (nicotine patch + nicotine gum) starting on their TQD. Half were assigned to receive 8 weeks of patches and 8 weeks of nicotine gum. The other half received 16 weeks of patches and 16 weeks of gum |
|
| Outcomes | 7‐day PPA at 6m Validation: None Quit attempts |
|
| Funding Source | Grants from the National Cancer Institute to the University of Wisconsin Center for Tobacco Research and Intervention and by the Wisconsin Partnership Program. Dr. Collins is also supported by NIH grants | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Adjunctive counseling (preparation phase and cessation phase, in person and via telephone), cost‐free medications (pre‐ and post‐quit, gum and patch) Level: Patient Comparison types: Active vs. active Due to the complexity of the intervention components and combinations of intervention components tested, it was impossible to include this study in any of our meta‐analyses. Instead we describe the authors' conclusions narratively, in supplementary table 3. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Database that used stratified permuted block randomization |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Smoking status self‐report. Different contact between trial arms |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 41.3% (n = 263/637); the number lost to follow‐up ranged from 36% to 46% across the 6 factors at 6 months |
Piper 2018.
| Study characteristics | ||
| Methods | Design: randomized controlled trial Setting: 7 primary care clinics within 2 Wisconsin healthcare systems, USA Recruitment: Mailings to patients identified from electronic health record |
|
| Participants | 623 people who smoked 57.3% F, av. age 49.7, av. cpd 16.8 | |
| Interventions | Common components in both groups: counseling and nicotine patches (duration different between the groups) Intervention: participants received 3 weeks of prequit mini‐lozenges, 26 weeks of nicotine patch + mini‐lozenges, 3 in‐person and 8 phone counseling sessions, and 7 – 11 automated prompts to use medication Control: participants received 10 minutes of in‐person counseling, 8 weeks of nicotine patch, and referral to quitline services |
|
| Outcomes | PPA at 12m Validation: CO < 6 ppm | |
| Funding Source | National Institutes of Health | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Adjunctive counseling, cost‐free medications, medication prompts Level: Patient Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "Computer‐based randomization used a 1:1 randomization within blocks of six participants, stratified by gender" |
| Allocation concealment | Low risk | QUOTE: "Computer‐based randomization used a 1:1 randomization within blocks of six participants, stratified by gender" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 45.1% (n = 281/623); 48.1% (n = 148/308) in the intervention group and 42.2% (n = 133/315) in the control group were lost to follow‐up at 12 months |
Pisinger 2010.
| Study characteristics | ||
| Methods | Design: 3‐group cluster randomized controlled trial Setting: Primary care, Denmark Recruitment: Practices recruited by mailed invitation letter. All patients seen by GP were registered |
|
| Participants | 1518 adults who smoked, 62.6% F, av.age 48, 17 cpd | |
| Interventions |
Intervention 1: GPs were instructed to briefly and freely talk about smoking with all people who smoked and refer those people who were motivated to quit to a group‐based smoking cessation counseling. Intervention 2: GPs were instructed to briefly and freely talk about smoking with all people who smoked and refer all those motivated to quit to an Internet‐based smoking cessation program Control: GPs were instructed to give smoking cessation advice and assistance to quit as they used to (not necessarily to all people who were smoking). The control group did not have any special program, beyond what is known from a national survey on Danish GPs ultimo 2004. In this study, the control group only registered whether they discussed smoking with the participant or not and the time consumed by counseling |
|
| Outcomes | 7‐day PPA at 12m Validation: Urinary cotinine < 200 ng/ml |
|
| Funding Source | Danish Centre for Evaluation and Health and Technology Assessment | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling + Internet program Level: Patient Component type: Single component vs. standard care (different single components tested in different intervention arms) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "The GPs were pre‐randomised at the Research Centre by a computer generated list to one of the three groups" |
| Allocation concealment | Low risk | QUOTE: "The GPs were pre‐randomised at the Research Centre by a computer generated list to one of the three groups" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | High risk | Overall, 50.2% (n = 758/1518) of participants were lost to follow‐up at 12 months. No further details by group were reported |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "There were no significant differences between the groups in terms of the number of patients seen or smokers included" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | No additional effect on self‐reported 1‐year abstinence rates of either referral to group‐ based SC counseling was found in cluster analyses |
Ramos 2010.
| Study characteristics | ||
| Methods | Design: 3‐group randomized controlled trial Setting; A primary healthcare setting in Mallorca, Spain Recruitment: Patients who met inclusion criteria were invited to participate |
|
| Participants | 287 adults who smoked, who are preparing to quit. (81 in individual intervention, 111 in group intervention, 95 control), av. age 44, av. cpd 20 | |
| Interventions |
Intervention 1: As control (below), plus individual counseling on pharmacological treatment • Participants attended 6 visits during which the following were provided: counseling, pharmacotherapy, psychological support and standard follow‐up • Physicians and nurses received training on how to implement intensive interventions Intervention 2: As control below, plus group counseling on pharmacological treatment • Participants attended 6 visits during which the following were provided: counseling, pharmacotherapy, psychological support and standard follow‐up • Physicians and nurses received training on how to implement intensive interventions Control: • Participants received pharmacotherapy (nicotine derivatives or bupropion) • Physician and nurse received basic training on how to diagnose smoking addiction and provide brief counseling • Support provided by microteam, composed of 1 physician and 1 nurse |
|
| Outcomes | Continuous abstinence at 12m Validation: Expired CO < 6 ppm |
|
| Funding Source | Health Research Fund of Spain's Ministry of Health and Consumer Affairs, Health Promotion and Preventive Activities in Primary Health Care Research Network | |
| Author's declarations of interest | Authors declared that they had no competing interest | |
| Notes | Strategy: Adjunctive counseling + Provider training Level: Patient + Provider Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported. |
| Allocation concealment | Low risk | QUOTE: "an allocation concealment method based on the use of sequentially‐numbered, opaque, sealed envelopes was used" |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated. |
| Incomplete outcome data All outcomes | High risk | Follow‐up at 12 months was very low and completed in 31% of cases in intervention 1 (individual), 28% in the intervention 2 (group) and 24% in the control group (minimal intervention). |
RBR‐7yx9hd.
| Study characteristics | ||
| Methods | Design: randomized controlled trial Setting: primary healthcare unit, Brazil Recruitment: no details provided |
|
| Participants | Target was 80 adults who smoked (final recruitment not confirmed). Eligible participants were adult daily cigarettes users for at least 1 year, av. age not reported, av. cpd not reported | |
| Interventions | Intervention: participants received a 40‐minute counseling session on cessation using brief intervention with motivational interviewing by a trained interviewer with a degree in psychology or medicine Control: participants received 1 session per week of standard treatment for smoking with a cognitive‐behavioral approach for 8 weeks |
|
| Outcomes | 7‐day PPA at 3m and 6m Validation: none Quit attempts |
|
| Funding Source | Universidade Federal de Juiz de Fora ‐ Juiz de Fora, MG, Brazil | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care Trial ID: RBR‐7yx9hd. The study lead investigator was contacted by email and she confirmed that they do not intend to analyse and publish study results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Not reported |
| Allocation concealment | Unclear risk | Not reported |
| Blinding of outcome assessors All outcomes | Unclear risk | Not reported |
| Incomplete outcome data All outcomes | Unclear risk | Not reported |
Richmond 1993.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial with 3 active trial arms Setting: GPs in Sydney, Australia Recruitment: GPs invited their patients to participate. |
|
| Participants | 450 adults who smoked (16 ‐ 65 years) free from any condition which was contraindicated for the use of nicotine gum. 60% F, av.age 35, 22 cpd | |
| Interventions |
Intervention 1: Participant saw GP at baseline, 1w, 3w, 3m, 6m and received comprehensive counseling from GP on how to quit smoking Intervention 2: Participant saw GP at baseline, 1w, 3w, 3m, 6m and received comprehensive counseling from GP on how to quit smoking Participants were given a supply of nicotine gum, an explanation of its use, and an instruction booklet Intervention 3: Participants attended a baseline visit and follow‐up visits at 3m and 6m. At at baseline participants were advised to quit by GP and given a supply of nicotine gum, an explanation of its use, and an instruction booklet |
|
| Outcomes | PPA at 12m Continuous abstinence at 12m Validation: None |
|
| Funding Source | Funded by the Department of Health, Housing and Community Services, Community Health Anti‐Tuberculosis Association, Glaxo Australia, and the Drug and Alcohol Directorate, NSW Department of Health | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Cost‐free medications, adjunctive counseling Level: Patient Type: Active vs. active (isolates cost‐free medications) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | All participants were allocated according to random weekly assignment to 1 of 3 intervention groups |
| Allocation concealment | High risk | Weekly allocation. Could have been foreseen |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported, and different contact between groups |
| Incomplete outcome data All outcomes | Unclear risk | QUOTE: "a number of patients who were out of contact were eliminated from the predictor analyses...and 12 month follow‐ups (n = 59, 13%)"; "After the initial consultation, 15‐25% of the patients on each occasion did not attend the session and could not be contacted". No further details reported |
Ronaldson 2018.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primary care practice, UK Recruitment: QUOTE: "patients at participating practices who met eligibility criteria were sent a recruitment pack through the post), and opportunistic recruitment by general practitioners (GPs) and nurses at face‐to‐face consultations" |
|
| Participants | 674 adults older than 35 years; 49% F; mean age 53 years; 16 cpd on average | |
| Interventions | The smoking cessation advice for both groups typically involved participants being offered a stop smoking program lasting 6 to 8 weeks, either on a one‐to‐one basis or with group support, with or without medication, which could have comprised nicotine or non‐nicotine products. Intervention: participants received lung function tests (spirometry, microspirometry, peak flow meter measurement, and a WheezoMeter) and case finding questionnaires Control: participants were on waiting list for the intervention, as well as receiving usual care (above). Participants received the spirometry intervention after the final trial follow‐up |
|
| Outcomes | Self‐reported PPA at 6m Validation: None |
|
| Funding Source | Department of Health Respiratory Programme | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Spirometry Level: patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "the sequence generated by an independent data manager" |
| Allocation concealment | Low risk | QUOTE: "The randomisation sequence was concealed using York Trials Unit's secure randomised system, which was accessed by computer" |
| Blinding of outcome assessors All outcomes | High risk | Abstinence was self‐reported and there was different contact between trial arms |
| Incomplete outcome data All outcomes | Low risk | 32/387 (8.3%) in the usual care arm and 53/767 (6.9%) in the intervention arm were lost to follow‐up |
| Other bias | High risk | Waitlist control study. It appears that participants knew they were on a waiting list, based on the following statement: QUOTE: "Participants, clinicians, investigators, and evaluators were not blind to the participants' group allocation because of the nature of the trial design and analysis". This means participants in the control arm may have postponed their quit attempt until after the trial, when they received treatment |
Roski 2003.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: 40 clinics of a large multispecialty medical group practice providing primary care services, USA Recruitment: Exit interviews with patients in the clinic |
|
| Participants | 4813 patients (873 people who smoked) at baseline survey. 4734 patients (863 people who smoked) at follow‐up. Patients were 18 years or older who had visited their provider in the past 30 days, av.age not reported, av. cpd not reported | |
| Interventions | Common component in all groups: printed versions of the smoking cessation guidelines distributed to the practices. Intervention 1: practices received printed versions of the smoking cessation guidelines, financial incentives for reaching preset clinical performance targets Intervention 2: practices received printed versions of the smoking cessation guidelines, financial incentives for reaching preset clinical performance targets combined with access to a centralized registry of people who smoked and intervention system which delivered telephone counseling Control: distribution of printed versions of the smoking cessation guidelines only |
|
| Outcomes | 7‐day PPA at 6m Validation: None Measures of provider implementation: Ask, Advise, Assist |
|
| Funding Source | Supported in part by a grant from the Robert Wood Johnson Foundation (Grant 036023) | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling + Provider incentive Level: Patient + Practice + System Comparison type: Single component (provider incentives vs. standard care), active vs. active (isolating adjunctive counseling) & multicomponent vs. SC (provider incentives & adjunctive counseling) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Randomly allocated by block randomization. No further details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Smoking status self‐report. Contact between arms was different |
| Incomplete outcome data All outcomes | Unclear risk | No details reported |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "At baseline no differences were found between the experimental conditions with respect to identification of tobacco use, provision of advice to quit, and assistance in quitting at the most recent clinic visit" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Analyses of practice pattern changes (identification, offer of advice to quit) and patient outcomes (quitting) made use of clustered logistic regression" |
Russell 1983.
| Study characteristics | ||
| Methods | Design: 3‐group randomized controlled trial Setting: 6 group practices in London and Kent, UK Recruitment: Completed in practice |
|
| Participants | 2106 adults who smoked aged 16 years or more; 57% F; mean age 40.5 years; 17.5 cigarettes per day on average | |
| Interventions |
Intervention 1: participants were advised to quit smoking and given a booklet Intervention 2: participants were advised to quit smoking, given a booklet and a prescription for free NRT gum Control: no intervention, no advice. No further details reported |
|
| Outcomes | Abstinence (undefined) at 12m Validation: Expired CO < 8 ppm Quit attempts |
|
| Funding Source | Financial support was provided by the Medical Research Council and the AB Leo Research Foundation, Sweden | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Cost‐free medications Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | Participants were assigned to groups according to their week of attendance |
| Allocation concealment | High risk | Not concealed |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported. Authors state that the control group had no advice, which implies that face‐to‐face contact was greater in the intervention groups |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 8.0% (n = 168/2106); 8.8% (n = 65/740) in the 'advice+booklet' group, 6.9% (n = 50/729) in the 'advice+booklet+prescription' group and 8.3% (n = 53/637) in the control group were lost to follow‐up at 12 months |
Salkeld 1997.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: GPs practising in the Western Metropolitan Region of Sydney, Australia Recruitment: GPs recruited patients in practice |
|
| Participants | 75 practices 82 providers 755 patients (255 people who currently smoked): 49% F, av.age 52; av. cpd not reported |
|
| Interventions |
Intervention 1: • General practitioners received an education guide and a video to help them assess individual patient risk factors and plan a program for risk factor behavior change • Participants received a risk factor assessment, education materials, a series of videos to watch on lifestyle behaviors Intervention 2: as per Intervention 1. In addition, participants received a self‐help booklet (not relevant to this review) Control: GP training and standard care. No further details reported |
|
| Outcomes | Undefined abstinence at 12m Validation: None |
|
| Funding Source | This work was funded by the General Practice Evaluation Program, Commonwealth Department of Human Services and Health, Australia | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Provider training & video education Level: Patient and provider Type: Active vs. active (isolates video education) Multirisk factor study Data subgrouped and not unable for the whole sample. Attempts to contact authors unsuccessful, so data are not presented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was self‐reported. The interventions were in the form of a video or a combination of a video and written material so face‐to‐face contact was similar in the routine care group and 2 intervention groups |
| Incomplete outcome data All outcomes | High risk | At participant level, the overall loss to follow‐up was 36.1% (n = 273/757); 49.0% (n = 125/255) in the routine group, 26.3% (n = 71/270) in the video group and 33.2% (n = 77/232) in the video and self‐help group. Altough the number lost to follow‐up was less than 50%, losses were different between groups and some clusters were lost in all groups (5 GPs in the routine group and 4 GPs in the video+self help group) |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Unclear risk | No details reported |
| Adjustment for clustering in analysis? (cluster RCTs only) | High risk | QUOTE: "...No adjustment was made for clustering effects" |
Sanz‐Pozo 2006.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial
Setting: Primary care clinic, Spain Recruitment: GPs recruited patients in practice |
|
| Participants | 125 people who smoked daily, attending clinic, motivated to make a quit attempt but not interested in using pharmacotherapy, av.age ~ 40, av.cpd 19, 52% F (intervention), 62% F (control) | |
| Interventions | Intervention: participants received brief advice from their doctor at recruitment, an appointment with clinic nurse 7 days before TQD, on TQD, 1w, 1m, 2m, 3m. Control: participants received brief advice only | |
| Outcomes | 12m sustained abstinence at 24m Validation: Expired CO < 8 ppm | |
| Funding Source | Not reported | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | QUOTE: "the patients recruited were randomised, according to the clinic from which they came, to the group that received...". No further detail. |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking abstinence was biochemically validated |
| Incomplete outcome data All outcomes | High risk | The overall loss to follow‐up was 42.4% (n = 53/125); 75% (n = 45/60) in the intervention group and 12.3% (n = 8/65) in the control group were lost to follow‐up at 2 years |
Secades Villa 2009.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: 3 primary care centers in Asturias, Spain Recruitment: Opportunistic recruitment of people who smoked attending the practice |
|
| Participants | 89 adults who smoked (> 10 cpd) ready to quit. 61% F, 22 cpd, av.age 43 | |
| Interventions |
Intervention 1: participants received a 7‐minute brief counseling in which they set a quit date, self‐help materials and 4 follow‐up telephone calls from a general practitioner or a primary care nurse Intervention 2: participants received 1 x 20‐min counseling session weekly for 5 weeks, delivered by a clinical psychologist Control: participants received a 7‐minute brief counseling and self‐help materials |
|
| Outcomes | Continuous abstinence at 12m Validation: Expired CO ≤ 4 ppm |
|
| Funding Source | Supported by research grant no. MB‐02‐506‐2 from the University of Oviedo (Spain) | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | At 6‐month follow‐up, 100% of participants were located |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | High risk | There were statistically significant differences among the groups (P < .05) on 2 characteristics: age and years smoking |
| Adjustment for clustering in analysis? (cluster RCTs only) | Unclear risk | No details reported |
Segnan 1991.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial with 4 active comparators Setting: Primary care practices in Turin, Italy Recruitment: GPs recruited patients opportunistically in practice |
|
| Participants | 44 providers: GPs with at least 400 individuals on patient list 923 patients: 20 ‐ 60 years of age, currently smoking, no life‐threatening disease. 32.3% F, av. age not reported, av. cpd not reported |
|
| Interventions | Common components in all groups: • Physicians attended 2 x 3‐hour training sessions on counseling techniques and organizational aspects of the study Intervention 1: minimal intervention group Participants received 1 session of face‐to‐face counseling and an explanatory brochure Intervention 2: repeated counseling group Participants received 1 session of face‐to‐face counseling, an explanatory brochure and follow‐up appointments at months 1, 3, 6, and 9 (non‐relevant intervention group as GPs provided adjunctive counseling) Intervention 3: nicotine gum group Participants received 1 session of face‐to‐face counseling, an explanatory brochure, follow‐up appointments at months 1, 3, 6, and 9, plus nicotine gum to last until the first follow‐up visit Intervention 4: spirometry group Participants received 1 session of face‐to‐face counseling, an explanatory brochure, follow‐up appointments at months 1, 3, 6, and 9, plus a prescription for spirometry test. Report showed results in the form of lung age |
|
| Outcomes | 3m prolonged abstinence at 12m Validation: Urinary cotinine < 100 ng/mg Measures of provider implementation: Advise, Arrange |
|
| Funding Source | Piedmont Health Authority | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Spirometry + Cost‐free medications + Provider training Level: Patient + Provider Type: Active vs active |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | Authors state that a predetermined randomized sequence was used. No further detail provided |
| Allocation concealment | Low risk | Opaque envelopes were used |
| Blinding of outcome assessors All outcomes | Low risk | Abstinence was biochemically validated |
| Incomplete outcome data All outcomes | Unclear risk | The overall loss to follow‐up was 13% at 12 months. No details on the number lost to follow‐up by group were reported |
Sherman 2007.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 2 primary care teams at the Sepulveda VA Ambulatory Care Center, USA Recruitment: All patients with at least 3 primary care visits in the past year were invited to participate through a computer‐assisted telephone interview |
|
| Participants | 482 adults who smoked within the Sepulveda Ambulatory Care Centre, av. age not reported, av. cpd not reported | |
| Interventions | Intervention: • Providers had access to an on‐call counselor who could be paged to provide 10 ‐ 15 minutes of counseling and make a referral to a smoking cessation program or a quitline as required. The counselors provided case management for all participants for 2 months, making follow‐up calls to them each lasting 5 ‐ 15 minutes Each provider received monthly educational outreach visits from the counselors or the opinion leader for the first 3 months. In addition, providers were posted profiling data. The provider who referred the most patients was presented with financial incentives (USD 25 gift certificate) at the end of each month. Participants received case management by the counselor and also medications. Control: usual care. No further details reported |
|
| Outcomes | 30‐day PPA at 6m Validation: None Quit attempts Measures of provider implementation: Ask, Assist‐Prescribe (NRT), Assist‐Prescribe Bupropion, Arrange‐Quitline referral, Arrange‐Cessation program |
|
| Funding Source | This work was funded by a grant from the California Tobacco‐Related Disease Research Program (#10RT‐0023) | |
| Author's declarations of interest | Authors declared that they had no conflict of interest | |
| Notes | Strategy: Adjunctive counseling & cost‐free medications + academic detailing + financial incentives + audit & feedback Level: Patient, Provider, Practice Comparison type: Multicomponent vs standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | 1 primary care team was randomly assigned by coin flip |
| Allocation concealment | Unclear risk | Coin flip to assign 1 team to the intervention and the other team to usual care |
| Blinding of outcome assessors All outcomes | High risk | Self‐report. Different contact between groups |
| Incomplete outcome data All outcomes | High risk | At participant level, the overall loss to follow‐up was 47.9% (n = 231/482); 50.9% (n = 108/212) in the intervention group and 45.6% (n = 123/270) in the usual care group at post‐intervention follow‐up |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the primary care clinic before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | High risk | Participants on the intervention team were more likely to have ever tried to quit smoking (OR (95% CI): 2.4 (1.4 ‐ 4.2)) and to have quit for at least 1 day in the last year (OR (95% CI): 1.5 (1.1 ‐ 2.2)). They were less likely than participants on the control team to have chronic obstructive pulmonary disease |
| Adjustment for clustering in analysis? (cluster RCTs only) | High risk | QUOTE: "Multilevel modeling could not be used to account for clustering at the team level, as there were only 2 teams" |
Sherman 2008.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 18 Veterans Health Administration (VA) sites in California, USA Recruitment: Proactive calls to patients |
|
| Participants | 2965 patients referred for smoking cessation telephone counseling. av.age 57, 93% M, av. cpd not reported | |
| Interventions | Intervention: • Practices received telephone care coordination program which allowed providers to be able to make a simple 2‐click referral. Practices were also provided with proactive care coordination • Participants, once connected to the quitline, were scheduled to receive a single 30 ‐ 45‐minute counseling sessions within 7 days. A Veterans Health Administration care coordinator monitored medications (nicotine patches or bupropion) prescribed by a designated smoking cessation clinician. The care coordinators also offered follow‐up counseling telephone calls at 2, 4, 6 and 8 weeks after the quit date and at 6 months. Control: usual care comprising direct treatment by a primary care provider, referral to a Veterans Health Administration smoking clinic, or informal referral to an outside resource such as a quitline |
|
| Outcomes | 30‐day PPA at 6m Valdiation: None Providers were asked to approximate the following provider implementation outcomes:Assist, Arrange; |
|
| Funding Source | Grant SUDCC 3.10 from the Veterans Affairs Substance Use Disorders Quality Enhancement Research Initiative and by grant HFP 94‐028 from the Veterans Affairs Health Services Research and Development Center of Excellence for the Study of Healthcare Provider Behavior | |
| Author's declarations of interest | QUOTE: "the authors (SES, NT, PK, EG, JWF, JC, JFK, GJJ, WK) report no relationship or financial interest with any entry that would pose a conflict of interest with the subject matter of this article" | |
| Notes | Strategy: Adjunctive counseling + EMR prompts Level: Patient, practice Comparison type: Multicomponent vs. standard care Abstinence is only reported for the intervention arm and not the standard‐care arm. Attempts to contact the authors were unsuccessful. Data are therefore not analyzed for any of the outcomes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Smoking status self‐report. Person‐to‐person contact was different between groups |
| Incomplete outcome data All outcomes | Unclear risk | No details on loss to follow‐up at participant level were reported |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the sites before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Unclear risk | No details reported |
| Adjustment for clustering in analysis? (cluster RCTs only) | Unclear risk | No details reported |
Siddiqi 2013.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: Health centers in Pakistan Recruitment: Opportunistic in practice |
|
| Participants | 1955 patients aged 18 years or older with suspected pulmonary tuberculosis (cough for 3 weeks without any other cause) who also regularly smoked tobacco (≥ 1 cpd), av. age 41, 95% M, av. cpd 16 | |
| Interventions |
Intervention 1: Behavioral support sessions (BSS) • Participants received 2 structured sessions delivered by directly‐observed therapy facilitators using an educational flipbook (session 1: 30 minutes; session 2: 10 minutes on the quit day) • Directly‐observed therapy facilitators received a 1‐day training program delivered by the research team • Other healthcare professionals received briefing about BSS Intervention 2: BSS+ • Participants received a free 7‐week course of sustained‐release bupropion in addition to BSS • Physicians received training and written guidance on prescribing bupropion Control: • Participants received usual care and a self‐help leaflet on smoking cessation. No further details on the usual care reported • Directly‐observed therapy facilitators received information on trial procedures only |
|
| Outcomes | Continuous abstinence at 6m Valiation: Expired CO ≤ 9 ppm |
|
| Funding Source | International Development and Research Centre, Canada | |
| Author's declarations of interest | Unable to access this information through the link provided in the paper | |
| Notes | Strategy: Adjunctive counseling + Cost‐free medications + Provider training Level: Patient + Provider Comparison type: Multicomponent vs. standard care; active vs active (isolating effect of cost‐free medications) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "...computer generated random‐number lists to generate the allocation sequence" |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | High risk | Smoking status is self‐report and there was different contact between the control arm and the intervention arms |
| Incomplete outcome data All outcomes | Low risk | At participant level, the overall loss to follow‐up was 5.8% (n = 114/1955 survivors); 8.0% (n = 53/659) in the BSS+ group, 3.1% (n = 20/640) in the BSS group, and 6.3% (n = 41/656) in the control group at 6‐month follow‐up |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the health centers before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "The 3 groups were generally similar with respect to the baseline characteristics, although mean age, sex, and smoking type differed slightly" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "...adjusting for cluster effect using an intraclass correlation coefficient of 0.036" |
Sippel 1999.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: 2 university‐affiliated primary care clinics, USA Recruitment: Patients approached by research staff in practice |
|
| Participants | 205 adults who smoked, average age 38 years, 62% F, 20 cpd | |
| Interventions | Intervention: participants received advice and cessation information. Additionally, they spent 10 ‐ 15 minutes receiving spirometry, carbon monoxide analysis, interpretation and education Control: participants received advice and cessation information |
|
| Outcomes | Continuous abstinence at 6m Validation: None Secondary outcomes: Quit attempts > 24 hours |
|
| Funding Source | Funded by the American Lung Association of Oregon and the American Academy of Family Practice | |
| Author's declarations of interest | Not reported. | |
| Notes | Strategies: Spirometry + CO monitoring Level: Patient Comparison type: Multi‐component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | High risk | QUOTE: "questionnaires were numbered consecutively at each clinic throughout the study period. Subjects receiving odd‐numbered questionnaires were selected as the intervention group and those receiving even‐numbered questionnaires were selected as the control group..." |
| Allocation concealment | High risk | QUOTE: "the nurses performing patient check‐in were blinded to the questionnaire numbers. As four to six nurses conducted patient check‐ins independently and simultaneously at each clinic, it is unlikely that any given patient would be preferentially enrolled into either study arm". It is unclear how the nurses were blinded to the questionnaire numbers. |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported and face‐to‐face was different between the groups |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 15.6% (n = 32/205); 12.6% (n = 13/103) in the intervention group and 18.6% (n = 19/102) were lost to follow‐up at 6 months |
Swartz 2006.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: 50 primary care practices, USA Recruitment: Practices were recruited by telephone invitations |
|
| Participants | 1892 adults who smoked (807 eligible for abstinence analysis: av.age 42, 25% M, 15 cpd) | |
| Interventions | Common components in both groups: detailing sheet summarizing effective treatment, profiling data feedback (by mail in the control group) and a Treating Tobacco Together pen Intervention: • Providers received the same intervention as the control arm, plus: A 20 ‐ 30 minute educational session on evidence‐based tobacco treatment in their practice and a second educational session 5 ‐ 6 months later. Providers were encouraged to use the ICD‐9 diagnosis code 205.1 and given information about the Maine Tobacco HelpLine which offers counseling Control: providers received the detailing sheet and all profiling data feedback graphs with a summary of findings and a Treating Tobacco Together pen by mail |
|
| Outcomes | 7‐day PPA at 15 ‐ 18 m Validation: None Measures of provider implementation: Advise, Assess, Assist‐Self‐help, Assist‐Meds, Arrange Quit attempts |
|
| Funding Source | Agency of Research and Healthcare Quality | |
| Author's declarations of interest | QUOTE: "Dr Swartz has received honoraria and research support from Pfizer. At the time of the study, Dr Goldstein was employee of Bayer Pharmaceutical Corporation. After the study was conducted, Mr Cowan became an employee of Health Dialog Analytic Solutions. No conflicts: Mooney‐Murray, Haskins, DePue, Thompson, Leighton, Salem‐Schatz" | |
| Notes | Strategy: Outreach facilitation, Audit & feedback, Provider training Level: Provider + Practice Comparison type: Active vs. active (isolates provider training) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status self‐report. However, contact with participants did not differ |
| Incomplete outcome data All outcomes | Unclear risk | QUOTE: "Of 1,892 patients who smoked at baseline, 1,238 were contacted at follow‐up (65.4% response)". No further details by group were reported |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practices before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | No significant differences between the clusters, except for more participants in the control group practices were Medicaid enrollees |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Models were adjusted for the clustering effect of patients within practices using the survey logistic procedure" |
Twardella 2007.
| Study characteristics | ||
| Methods | Design: 4‐group cluster‐randomized controlled trial Setting: Primary care in the Rhine–Neckar region, Germany Recruitment: Providers recruited patients in practice |
|
| Participants | 587 adults aged 36 – 75 years who smoked at least 10 cigarettes/day, av. age not reported, av. cpd not reported | |
| Interventions |
Intervention 1: • General medical practitioners received a 2‐hour cost‐free group tutorial in methods of promoting smoking cessation. The general medical practitioners were assured a financial remuneration of EUR 130 after study completion for each study participant they recruited who was abstinent at 12 months follow‐up Intervention 2: • General medical practitioners received a 2‐hour cost‐free group tutorial in methods of promoting smoking cessation • Participants were reimbursed up to EUR 130 for the purchase of nicotine replacement therapy or bupropion for up to 12 months. Intervention 3: • General medical practitioners received a 2‐hour cost‐free group tutorial in methods of promoting smoking cessation. General practitioners were assured a financial remuneration of EUR 130 after study completion for each study participant they recruited who was abstinent at 12 months follow‐up • Participants were reimbursed up to EUR 130 for the purchase of nicotine replacement therapy or bupropion for up to 12 months Control: usual care. No further details reported |
|
| Outcomes | 6m sustained at 12m Validation: Salivary cotinine <15 ng/ml |
|
| Funding Source | Funded by the German Ministry of Education andResearch (Bundesministerium für Bildung und Forschung), project number01EB0113, within the context of the Baden–Wurttemberg Research Network on Addiction (project 01EB0113) | |
| Author's declarations of interest | Authors declared that they had no competing interests. | |
| Notes | Strategy: Provider training + Provider incentive + Cost‐free medication Level: Provider + Patient Comparison type: Active vs. active (isolating cost‐free medications & provider incentive) & multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated random sequence used |
| Allocation concealment | Low risk | QUOTE: "Randomisation was performed centrally at the German Center for Research on Ageing, Heidelberg, Germany...". Computer‐generated random sequence used |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status validated biochemically |
| Incomplete outcome data All outcomes | Low risk | At participant level, the overall loss to follow‐up was 15.0% (n = 88/588); 19.7% (n = 15/76) in the usual care group, 15.1% (n = 22/146) in the TI group, 15.9% (n = 23/145) in the TM group, 12.7% (n = 28/221) in the TM+TI group were lost at 12‐month follow‐up |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practice before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | High risk | In arms TM and TI+TM, the proportion of participants in the pre‐contemplation stage was lower, and the proportion of participants in both the contemplation and preparation stages were higher than in the usual care and TI arms (P < 0.001) |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "The effect of both interventions on smoking abstinence at 12 months follow‐up was assessed simultaneously in a mixed logistic regression model accounting for cluster randomisation ‐ that is, including a random effect for medical practice in the model..." |
Unrod 2007.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Physicians located in the 4 largest metropolitan boroughs of New York City; Bronx, Brooklyn, Manhattan, and Queens, USA Recruitment: Facsimile invitation to physicians followed by telephone calls from a physician recruiter. Participants were recruited in physician waiting rooms |
|
| Participants | 518 adults who smoked, who had smoked more than 100 cigarettes in their lifetime. av. age 43, 60% M, 14 cpd | |
| Interventions | Intervention: • Physicians received a 40‐minute training on brief smoking cessation counseling which followed an academic detailing approach. Physicians also received a copy of a 1‐page computerized report that characterized the participants’ smoking habit and history and offered tailored recommendations • Participants received a copy of the same computerized report that their physicians received Control: physicians were not given any training and were instructed to continue their usual smoking cessation practice. No further details reported |
|
| Outcomes | 7‐day PPA at 6m Validation: Salivary cotinine < 25 ng/ml Secondary outcomes: Quit attempts Measures of provider implementation: Ask, Advise, Assess, Assist‐Self‐help, Assist‐Prescribe, Arrange |
|
| Funding Source | The Agency for Healthcare Research and Quality | |
| Author's declarations of interest | Authors declared that they had no financial conflicts of interest | |
| Notes | Strategy: Provider training + Tailored print materials Level: Patient & Provider Comparison type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Random‐number generator used |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status validated biochemically |
| Incomplete outcome data All outcomes | Low risk | At physician level, there was no loss to follow‐up at 6‐month follow‐up (0% (n = 0/70)). At participant level, the overall loss to follow‐up was 10.2% (n = 465/518); 12.2% (n = 33/270) in the intervention arm and 8.1% (n = 20/248) in the control group were lost to follow‐up at 6 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practices before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "Intervention and control groups did not differ on any demographic variables" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Patient 7‐day point‐prevalence abstinence was analyzed via a generalized linear model using Logit Link function, with physician as clustering variable. Mixed linear modeling, with physician as clustering variable, was used to examine longest quit attempt, number of quit attempts, and stage‐of‐change progression" |
Van Rossem 2017.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: Primary healthcare center in The Netherlands Recruitment: Participants were recruited by practice assistants, general practitioners (GPs) and practice nurses (PNs) and via a brief and easily written leaflet displayed in the waiting room |
|
| Participants | 295 participants, 19 cpd, av.age 48, 53% F | |
| Interventions | PN Group: Participants were offered 3 face‐to‐face and 7 telephone sessions, starting 1 week prior to the quit attempt until 1 year after the quit attempt. Participants also received a prescription for varenicline GP Group: Participants received a minimum of 1 visit in which they received a prescription for varenicline. Participants were free to contact their GP in case of questions or side‐effects | |
| Outcomes | Prolonged abstinence from 9w to 26w Validation: Expired CO < 10 ppm |
|
| Funding Source | This was an investigator‐initiated trial, funded by a collaboration of Eindhoven Corporation of Primary Health Care Centres (SGE), Pfizer (grant number GPIHP_RG_2010014T1330) and Research School CAPHRI | |
| Author's declarations of interest | QUOTE: "D.K. received an unrestricted grant from Pfizer Inc. and The Eindhoven Corporation of Primary Health Care Centers for this investigator‐initiated smoking cessation trial. C.S. received funding for research proposals from GlaxoSmithKline and Pfizer. A.L. was a general practitioner at The Eindhoven Corporation of Primary Health Care Centers during the research. All other authors declare that they have no competing interests in relation to this paper" | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | Computer‐generated random‐number sequence |
| Allocation concealment | Low risk | Computer‐generated random‐number sequence, allocation disclosed by phone |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status biochemically validated |
| Incomplete outcome data All outcomes | Low risk | The overall loss to follow‐up was 18.0% (n = 53/295); 14.8% (n = 22/149) in the PN group and 20.0% (n = 31/146) in the GP group were lost to follow‐up at 6 months |
Verbiest 2014.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: General practices, The Netherlands Recruitment: Physicans were recruited by letter and a follow‐up telephone call. During the study period (January – August 2011), adult patients visiting participating GPs in both conditions were asked to complete a questionnaire after consultation |
|
| Participants | 49 providers, 57.1% M, av. age 52 2068 patients at baseline, including 433 adults who smoked. No further demographic details specifically on those who smoked reported |
|
| Interventions | Intervention: general practitioners attended a single, 1‐hour training session based on the 5‐As behavior change model. In addition all general practitioners received a toolkit, which contained a smoking cessation care flowchart, a summary of pharmacological support, leaflets for patients, and an opportunity to receive additional feedback support Control: usual care defined as QUOTE: "the smoking cessation care that is usually provided by the general practitioner when not being trained, which is likely to vary between the general practitioners". No further details reported |
|
| Outcomes | Continuous abstinence at 9m Validation: None Measures of provider implementation: Ask, Advise, Assist–Prescribe, Arrange |
|
| Funding Source | Unrestricted grant from Pfizer and CAPHRI | |
| Author's declarations of interest | Authored declared that they had no conflict of interest | |
| Notes | Strategy: Provider Training + Flow sheet Level: Provider + Practice Type: Multicomponent vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "...using a simple randomization procedure (coin tossing) by an independent researcher not involved in the recruitment of the GPs" |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status was self‐reported. At participant level, person‐to‐person contact did not differ |
| Incomplete outcome data All outcomes | Low risk | At participant level, including only those who reported smoking at baseline, the overall loss to follow‐up was 48.0% (n = 208/433); 42.6% (n = 83/195) in the intervention group and 52.5% (n = 125/238) in the control group at 9‐month follow‐up |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practices before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "At baseline, more patients in the control group reported a chronic airway disease compared to the intervention group (15.4% vs. 12.4%; p=0.03)". Authors report using generalized estimating equations to adjust for participant characteristics |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Generalized estimating equations adjusted for clustering and patient characteristics" |
Vetter 1990.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: A health center in a small town in the countryside, UK Recruitment: Postal questionnaire to practice patients |
|
| Participants | 471 people who smoked aged 60 years and older, registered as a patient with the group practice; 48.0% F, av. age not reported, av. cpd not reported | |
| Interventions | Intervention: Participants received physician advice and additional smoking cessation counseling by a practice nurse Control: Participants received physician advice only |
|
| Outcomes | PPA at 6m Validation: Expired CO, cut‐off not reported |
|
| Funding Source | The Grand Charity | |
| Author's declarations of interest | Not reported | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Abstinence was biochemically validated by carbon monoxide levels |
| Incomplete outcome data All outcomes | Unclear risk | QUOTE: "approximately 10% of subjects failed to respond to the second questionnaire". No further details were reported. |
Yano 2008.
| Study characteristics | ||
| Methods | Design: Cluster‐randomized controlled trial Setting: Veterans Health Administration (VA) primary care practices across 5 southwestern states, USA Recruitment: All eligible practices within the Veterans Health Administation were approached |
|
| Participants | 1941 primary care patients who were currently smoking, av.age 57, 94% M, av. cpd not reported | |
| Interventions | Intervention: Each intervention practice received the following: ‐ 30‐minute didactic sessions on population‐based smoking cessation ‐ Implementation planning ‐ Evidence summaries ‐ Recommendations for minimum protocols and implementation strategies ‐ Smoking cessation resource materials and tools for participants and providers ‐ Quality improvement manual outlining intervention processes and linking sites with research team assistance ‐ Monthly audio or video conferences with site leadership to facilitate ongoing local adaptation of the prioritized interventions ‐ Bimonthly newsletters highlighting practice successes and challenges among participating sites ‐ Quarterly audit‐and‐feedback progress reports Control: sites received guideline copies and audit‐feedback reports from externally‐audited random patient records |
|
| Outcomes | 30 day PPA at 12m Validation: None Measures of provider implementation: Advise, Arrange |
|
| Funding Source | Funded by the VAHSR&D Service | |
| Author's declarations of interest | QUOTE: "The authors have no relevant financial interests or advocacy positions pertaining to this manuscript. VA policy requires submission of a copy of manuscripts on acceptance for internal preparation of briefings and/or press release as needed in anticipation of publication, but they do not undergo or require internal peer review or comment periods..." | |
| Notes | Strategy: Outreach facilitation + Audit & feedback + Provider training Level: Provider + Practice Comparison type: Multi‐component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | No details reported |
| Blinding of outcome assessors All outcomes | Low risk | Smoking status self‐report, but contact did not differ between arms |
| Incomplete outcome data All outcomes | Low risk | At participant level, the overall loss to follow‐up was 44.4% (n = 861/1941); 44.3% (n = 410/925) in the intervention group and 44.4% (n = 451/1016) in the control group were lost to follow‐up at 12 months |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practices before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "We found no baseline differences in sociodemographics, health habits, readiness to change, or primary care visits. Control site patients were more likely to smoke everyday (p<0.01), wake up to smoke (p<0.05), and to have tried nicotine patches (p<0.01), attended a smoking cessation program (p<0.0001), and tried other ways to quit preintervention (p<0.05)...". Authors report adjusting for baseline differences in their analyses. |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "We assessed the intraclass correlation coefficient to determine the need for cluster adjustment; because the intraclass correlation coefficient was not statistically significant from zero, an unadjusted analytic approach was used" |
Young 2008.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Setting: General practices in South Western Sydney, Australia Recruitment: Eligible GPs were approached by research staff and invited to participate. All eligible patients were approached in the waiting room and were given an information letter and self‐administered questionnaire to complete before seeing their GP |
|
| Participants | 318 adults who smoked (169 intervention, 149 control) av.age 38, 46% M | |
| Interventions | Intervention: participants received a phone call from a nurse who delivered intervention based on the 5As. Participants were mailed a quit kit, encouraged to use NRT and set a quit date. Those that set a quit date were called on the specified quit day, then 1 week and 3 weeks after the quit date. During these 3 calls, participants were congratulated if they had quit, were encouraged to maintain quitting and assisted in resolving any problems arising. People who relapsed to smoking received motivation advice and were encouraged to 'reframe' relapse as a learning experience for future cessation Control: QUOTE: "control group smokers received the GP's usual care. We also provided GPs with free copies of government‐sponsored quit kits to distribute to smokers in this group". No further details reported |
|
| Outcomes | Undefined PPA at 12m Validation: None Quit attempts Measures of provider implementation: Advise, Arrange, Assist‐Quit date, Assist‐Self‐help, Assist‐Medication |
|
| Funding Source | Project Grant G00S0686 from the National Heart Foundation of Australia. At the time of the study, JY was supported by a National Health and Medical Research Foundation Public Health (Australia) Fellowship (No 007024) | |
| Author's declarations of interest | Authors declared that they had no competing interest | |
| Notes | Strategy: Adjunctive counseling Level: Patient Comparison type: Single component vs. standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Low risk | QUOTE: "Questionnaires were randomly ordered and coded prior to delivery to the practice by selecting sequential numbers from a computer generated random number list" |
| Allocation concealment | High risk | QUOTE: "Pre‐randomised questionnaires and allocated unobtrusive marks that were meaningful only to the GPs in order to convey group allocation". Does not specify that this was concealed |
| Blinding of outcome assessors All outcomes | High risk | Smoking status was self‐reported and person‐to‐person contact differed between the groups |
| Incomplete outcome data All outcomes | Unclear risk | The response rate was 69% in the intervention group and 59% in the control group at 12 months ‐ not reported by group |
Zwar 2015.
| Study characteristics | ||
| Methods | Design: 3‐group cluster‐randomized controlled trial Setting: General practices in Sydney and Melbourne, Australia Recruitment: Practices were invited to participate during visits with study staff. Participants were recruited in waiting rooms by study staff |
|
| Participants | 2390 adults who smoked (daily or weekly). av.age 42, 45% M, 17cpd | |
| Interventions |
Intervention 1: quit with practice nurse (PN) • Nurses attended a 1‐day training program on 5A approach to smoking cessation counseling. The nurses were provided with checklists for use at each patient visit, a printed resource for distribution to patients and support from 3 proactive mentoring telephone calls from an experienced smoking cessation counselor • General practitioners encouraged all patients who smoked to see the practice nurse • Participants were assisted by a practice nurse to develop a quit plan and were offered a flexible package of ongoing support with a further 3 face‐to‐face visits to the practice nurse. Participants who were unable to attend face‐to‐face consultations or preferred other modes were offered telephone support from the nurse or the quitline Intervention 2: quitline • General practitioners were asked to assess the patients' willingness to quit and to offer brief advice. The general practitioners were provided brief feedback from the quitline on uptake and outcome of services offered to their patients • Patients interested in quitting were offered referral to the quitline. If agreed with patients, the quitline offered counseling service and a series of free evidence‐based proactive call‐back counseling/advice sessions Control: QUOTE: "GPs were asked to assess patients' willingness to quit and offer assistance in accordance with their usual practice. This could include advice within the practice, referral to quitline or both, but no provision was made to facilitate either". No further details reported |
|
| Outcomes | > 10 m sustained abstinence at 12m Validation: None |
|
| Funding Source | Australian National Health and Medical Research CouncilProject Grant (568617) | |
| Author's declarations of interest | Authors declared no conflict of interest | |
| Notes | Strategy: Provider training + Adjunctive counseling Level: Patient + Provider Comparison type: Multicomponent vs standard care |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Sequence Generation | Unclear risk | No details reported |
| Allocation concealment | Unclear risk | QUOTE: "Randomization of practices was performed after practice recruitment but prior to patient recruitment with allocation concealment by a researcher who took no further part in the study" |
| Blinding of outcome assessors All outcomes | High risk | Smoking status self‐report. Person‐to‐person contact differed across the groups |
| Incomplete outcome data All outcomes | Low risk | At patient level, the overall loss to follow‐up was 17.6% (n = 421/2390); 18.3% (n = 160/876) in the 'quit with PN' group, 16.9% (n = 141/836) in the quitline referral group, 17.7% (n = 120/678) in the usual care group at 12‐month follow‐up |
| Recruitment bias (cluster RCTs only) | Low risk | Participants were affiliated with the practices before randomization |
| Balanced baseline characteristics? (cluster RCTs only) | Low risk | QUOTE: "Groups were very similar on demographics and smoking behaviour at baseline" |
| Adjustment for clustering in analysis? (cluster RCTs only) | Low risk | QUOTE: "Adjustment for clustering was made on the basis of the intraclass correlation coefficient of 0.013 observed by Lennox et al. in a smoking cessation trial in general practice"; "...multilevel logistic regression models were used with two dichotomous dependent variables adjusted for clustering of three occasions at level 1, patients at level 2 and practices at level 3" |
BSS: behavioral support sessions; CA: continuous abstinence; CO: carbon monoxide; cpd: cigarettes per day; EMR: electronic medical record; F: female; NRT: nicotine replacement therapy; PPA: point prevalence abstinence; ppm: parts per million; SSS: Stop‐smoking service; TTM: transtheoretical model [stages of change]
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adair 2013 | Addressed multiple risk factors. No specific intervention strategy for smoking cessation |
| Adam 2019 | Outcomes: Cessation not reported |
| Agarwal 2018 | Cessation outcome not reported. (Main outcome: quit attempts) |
| An 2008 | Follow‐up < 6 months |
| Andrews 2001 | Cessation outcome not reported. |
| Aveyard 2007 | Compares 2 active interventions ‐ unable to isolate a single component |
| Bachmann 2019 | Outcomes: Cessation not reported |
| Bakkevig 2000 | Intervention not conducted in primary care |
| Bentz 2007 | Follow‐up < 6 months |
| Bosworth 2008 | Outcomes of interest not reported (Main outcome: Medication adherence and improvement of hypertension‐related health behaviors) |
| Burke 1993 | Only evaluated the efficacy of pharmacotherapy |
| Butler 1999 | Counseling performed by general practitioners |
| Carey 2016 | Follow‐up < 6 months |
| Cheung 2019 | Intervention not conducted in primary care |
| Cockburn 1992 | Intervention relates to marketing strategies for smoking cessation programs |
| Cohen 2011 | Outcomes of interest not reported (Main outcome: Change in proportion of participants achieving target glycemic and cardiac risk factor goals) |
| Coma 2019 | Outcomes: Cessation not reported |
| Conger 1987 | Only intervention was advice from GPs |
| de Ruijter 2018 | Did not assess smoking cessation |
| Dey 1999 | Follow‐up < 6 months |
| Dickinson 2013 | Addressed multiple risk factors. Tobacco users could choose to not participate in the smoking cessation part of the intervention |
| Dignan 2019 | Intervention not conducted in primary care |
| Drexel 2011 | Outcomes of interest not reported (Main outcome: Provision of evidence based COPD care) |
| Dubey 2006 | Follow‐up < 6 months |
| Efraimsson 2008 | Follow‐up l< 6 months |
| Eikelenboom 2013 | Addressed multiple risk factors. No specific intervention strategy for smoking cessation |
| Emery 2019 | No specific intervention strategy for smoking cessation |
| Emmons 2014 | Outcomes of interest not reported (Main outcome: Multiple risk behavior score) |
| Engle 2019 | Intervention tests only simple counseling and medication |
| Escortell‐Mayor 2020 | Multiple risk factor study |
| Etter 2000 | Follow‐up < 6 months |
| Felton 2019 | Outcomes: Cessation not reported |
| Ferketich 2014 | Follow‐up < 6 months |
| Ferrer 2009 | Multiple risk factor study |
| Fiore 2019 | Does not report on a cessation outcome. |
| Flocke 2014 | Follow‐up < 6 months |
| Folz 2016 | Smoking was a secondary outcome and very few people who smoked were involved ‐ 9 in intervention group and 1 in control group |
| Frank 2004 | Follow‐up < 6 months |
| Fu 2015 | Outcomes of interest not reported (Main outcome: Perceived skill in use of 5As and confidence in addressing smoking cessation) |
| Fulton 2019 | Intervention not conducted in primary care |
| Gerbert 2003 | Outcomes of interest not reported (Main outcome: Acceptability of video‐doctor program) |
| Gilbert 1989 | GP delivered counseling. NRT was not cost‐free. |
| Gilbert 1992 | GP delivered follow‐up counseling compared to no follow‐up does not meet our criteria for adjunctive counseling |
| Gilbert 2007 | Follow‐up < 6 months |
| Gilbody 2019 | Intervention tests simple counseling and medication. |
| Godycki‐Cwirko 2014 | Addressed multiple risk factors. No specific intervention strategy for smoking cessation |
| Green 2020 | Does not report on a cessation outcome |
| Grischott 2019 | Tests simple counseling intervention |
| Groner 2000 | The participants of the study were not patients of the general practitioner, but rather the people accompanying the patient |
| Hall 2003 | Outcomes of interest not reported (Main outcome: Readiness to quit) |
| Harding 2019 | Does not report on a cessation outcome |
| Haug 1994 | Only brief advice intervention |
| Houston 2015 | Compares 2 active interventions ‐ unable to isolate a single component |
| Hughes 1981 | Not conducted in primary care |
| Humphris 2004 | Outcomes of interest not reported (Main outcome: Knowledge of oral cancer) |
| Imperial Cancer Research Fund GP Research Group | Only evaluated the efficacy of pharmacotherapy |
| Javitz 2004 | Compares 2 active interventions ‐ unable to isolate a single component |
| Jennings 2014 | Follow‐up < 6 months |
| Jolly 2017 | Not a smoking cessation intervention |
| Kalkhoran 2016 | Cessation outcome not reported |
| Kalkhoran 2019 | Counseling intervention tested, but no cluster randomization |
| Kamstrup‐Larsen 2019 | Tests simple counseling intervention only. |
| Karner 2012 | No smoking cessation intervention tested |
| Kastaun 2021 | Compared 2 types of health provider training head to head. Did not allow for separation of effect of provider training |
| Kennedy 2019 | Not a smoking cessation intervention |
| Kim 2020 | Does not report on a cessation outcome |
| Kirkman 1994 | Multiple risk factor study |
| Knight 1989 | Outcomes of interest not reported (Main outcome: Predictors of quitting smoking) |
| Krones 2010 | Outcomes of interest not reported (Main outcome: Validity of the Theory of Planned Behavior in a decision aid) |
| Kruse 2020 | Follow‐up < 6 months |
| Lasser 2013 | Outcomes of interest not reported (Main outcome: Engagement in smoking cessation treatment) |
| Leung 2019 | Tests simple pharmacotherapy only. |
| Liang 2019 | Tests simple counseling intervention only |
| Liebmann 2019 | Adjunctive counseling intervention tests but not cluster‐randomized |
| Linder 2009 | Follow‐up < 6 months |
| Lycett 2010 | All participants had identical treatment to stop smoking |
| Machline‐Carrion 2019 | Does not include a cessation outcome. |
| Mahapatra 2019 | Not an RCT |
| Markun 2018 | Smoking cessation was never an intended outcome |
| McAlister 2009 | Multiple risk factor study |
| McEwen 2002 | Follow‐up < 6 months |
| McGrath 2014 | Follow‐up < 6 months |
| McPhee 1991 | Cessation outcome not reported. |
| McRee 2005 | Cessation outcome not reported. Main outcome: |
| McRobbie 2008 | Follow‐up < 6 months |
| Mehring 2014 | Follow‐up < 6 months |
| Minian 2019 | Not a smoking cessation intervention |
| Muckelbauer 2015 | Multiple risk factor study |
| Naughton 2014 | Compares 2 active interventions ‐ unable to isolate a single component |
| NCT01072422 | follow‐up < 6 months |
| NCT03221010 | follow‐up < 6 months |
| NCT04200534 | Cessation measured < 6 months |
| NCT04316260 | Cessation measured < 6 months |
| Neuner‐Jehle 2013 | Cessation outcome not reported. |
| Nilsson 1996 | Follow‐up < 6 months |
| Ojedokun 2013 | Follow‐up < 6 months |
| Papadakis 2013 | Follow‐up < 6 months |
| Parchman 2019 | Multiple risk factor study |
| Peckham 2019 | Not conducted in primary care |
| Peprah 2019 | Multiple risk factor study |
| Persai 2020 | Does not report on a cessation outcome |
| Persell 2013 | Outcomes of interest not reported (Main outcome: LDL Cholesterol levels) |
| Pieterse 2001 | GP delivered counseling |
| Piper 2003 | Pre‐post evaluation, not an RCT study design |
| Prabhakaran 2019 | No smoking cessation intervention tested |
| Prochaska 2005 | Multiple risk factor study |
| Prokhorov 2010 | Follow‐up < 6 months |
| Redfern 2014a | Addressed multiple risk factors. No specific intervention strategy for smoking cessation |
| Richmond 1985 | GP delivered counseling. |
| Richmond 1998 | Cessation outcome not reported. |
| Richter 2015 | Compares 2 active interventions ‐ unable to isolate a single component |
| Rigotti 2011 | Follow‐up < 6 months |
| Robson 1989 | Follow‐up < 6 months |
| Rodriguez Alverez 2008 | Compares 2 active interventions ‐ unable to isolate a single component |
| Roski 1998 | Cessation outcome not reported. |
| Rosser 1992 | Follow‐up < 6 months |
| Rothemich 2008 | Follow‐up < 6 months |
| Rothemich 2010 | Follow‐up < 6 months |
| Sanders 1989 | Only brief advice intervention |
| Satterfield 2018 | Follow‐up < 6 months, cessation outcome not reported |
| Schwartz 2015 | Multiple risk factor study |
| Sejourne 2010 | Outcomes of interest not reported (Main outcome: Readiness to quit) |
| Senesael 2013 | Addressed multiple risk factors. No specific intervention strategy for smoking cessation |
| Shaughnessy 1987 | Couseling only on use of medications |
| Sheffer 2012 | Cessation outcome not reported. |
| Shelley 2016 | Did not set out to measure smoking abstinence |
| Sherman 2017 | Compares 2 active interventions ‐ unable to isolate a single component |
| Silveira 2019 | Simple counseling intervention tested only |
| Silverman 2004 | Cessation outcome not reported. |
| Slama 1990 | GP delivered counseling |
| Smit 2010 | Deviated from protocol, not set in primary care |
| Sperl‐Hillen 2018 | Multiple risk factor study |
| Stratelis 2006 | Compares 2 active interventions ‐ unable to isolate a single component |
| Strecher 1994 | Follow‐up < 6 months |
| Taylor 2019 | Did not occur in primary care |
| Thompson 1988 | GP delivered counseling |
| Thompson 2015 | Follow‐up < 6 months |
| Valdivieso‐Lopez 2013 | Study population is pregnant women |
| Velasquez 2014 | Study population is pregnant women |
| Vidrine 2013 | Follow‐up < 6 months |
| Vogt 2009 | Does not report on abstinence outcome. |
| Voogdt‐Pruis 2011 | Outcomes of interest not reported (Main outcome: Number of lifestyle and medical interventions) |
| Vorderstrasse 2013 | Not a smoking cessation intervention |
| Waage 1997 | No smoking cessation strategy conducted in primary care |
| Wadland 2007 | Does not report on abstinence outcome. |
| Weingarten 1989 | Follow‐up < 6 months |
| West 1998 | Follow‐up < 6 months |
| Wilson 1982 | GP delivered follow‐up counseling |
| Woollard 1995 | Outcomes of interest not reported (Main outcome: Blood pressure) |
| Yalcin 2014 | Only evaluating different intensities of counseling |
| Yingst 2018 | Follow‐up < 6 months |
| Young 2002a | Follow‐up < 6 months |
| Young 2002b | Follow‐up < 6 months |
| Ziyash 2019 | Tests simple counseling intervention only |
| Zwar 2016 | Multiple risk factor study |
Characteristics of studies awaiting classification [ordered by study ID]
Martin‐Lujan 2011.
| Methods | Multicenter randomized clinical trial |
| Participants | Target: 600 people who smoked with a cumulative habit of more than 10 packs of cigarettes per year |
| Interventions | Intervention: participants will receive usual advice to quit by a general practitioner as well as a 20‐minute personalized visit to provide detailed information about spirometry results Control: participants will receive usual care |
| Outcomes | Smoking abstinence |
| Notes | NCT01194596 |
Martin‐Lujan 2016.
| Methods | Multicenter randomized clinical trial |
| Participants | Target: 1000 adults who smoke |
| Interventions | Intervention: patrticipats will receive brief, 5‐minute health counseling plus detailed personalized information about the results of a spirometry test Control: participants will receive brief, 5‐minute health counseling |
| Outcomes | Point‐prevalence abstinence, prolonged abstinence |
| Notes | NCT02153047 |
Ripoll 2012.
| Methods | Parallel randomized controlled trial with blind evaluation |
| Participants | 942 adults who smoke |
| Interventions | Intervention: brief advice plus exhaled carbon monoxide measure Control: brief face‐to‐face anti‐smoking advice from the physician during patient consultation |
| Outcomes | Sustained abstinence (at 6 and 12 months) validated by urine cotinine test |
| Notes | ISRCTN67499921 |
Smith 2003.
| Methods | Randomized controlled trial |
| Participants | Target: 2850 participants who smoke, 42 primary care providers |
| Interventions | Intervention 1: brief clinical intervention Intervention 2: enhanced clinical intervention control: usual care |
| Outcomes | Not reported |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Avila‐Tomas 2019.
| Study name | Effectiveness of a chat‐bot for the adult population to quit smoking: protocol of a pragmatic clinical trial in primary care |
| Methods | Randomized, controlled, multicentric, pragmatic clinical trial |
| Participants | Target: 460 people who smoke > 18 years of age who attend a healthcare center and accept help to quit smoking in the following month |
| Interventions | Use of a chat‐bot with evidence‐based contents to help quit smoking |
| Outcomes | Continuous abstinence at 6m |
| Starting date | 07 October 2018 |
| Contact information | joseavil@gmail.com |
| Notes | NCT03445507 |
Bendtsen 2020.
| Study name | Effects of a text messaging smoking cessation intervention among online help seekers and primary healthcare visitors in Sweden: a randomized controlled trial |
| Methods | 2‐arm parallel‐group randomized controlled trial |
| Participants | People who smoke, aged 18 years or older |
| Interventions | 12‐week text message program with messages sent to participants' mobile phones on a daily basis |
| Outcomes | Prolonged abstinence at 3m and 6m 4‐week point‐prevalence abstinence at 3m and 6m |
| Starting date | 01 January 2020 |
| Contact information | marcus.bendtsen@liu.se |
| Notes | ISRCTN13455271 |
Diaz‐Gete 2013.
| Study name | Effectiveness of an e‐mail tracking intervention among the continued abstinence of tobacco consumption |
| Methods | Randomized controlled multicentric trial |
| Participants | Target: 1060 people who smoke who regularly check their email |
| Interventions | 2 face‐to‐face interviews and 4 emails |
| Outcomes | Point prevalence abstinence and continuous abstinence at 6m & 12m |
| Starting date | December 2012 |
| Contact information | Carlos Martin Cantera, cardiocat@gmail.com |
| Notes | NCT01494246 |
Gerber 2017.
| Study name | Patient navigation for lung cancer screening in an urban safety‐net system: protocol for a pragmatic randomized clinical trial |
| Methods | Randomized controlled trial |
| Participants | Target: 340 participants eligible for lung cancer screening |
| Interventions | Patient navigation |
| Outcomes | Self‐reported abstinence at 6m and 18m |
| Starting date | June 2017 |
| Contact information | David E. Gerber, david.gerber@UTSouthwestern.edu |
| Notes | NCT02758054 |
ISRCTN38129107.
| Study name | The coaching for smokers trial |
| Methods | Single‐center double‐blind cluster‐randomized parallel controlled clinical trial |
| Participants | Target: 60 general practitioners and 200 patients with a target cluster size of four |
| Interventions | Intervention: general practitioners will be trained in coaching to promote a change in modifiable health risk factors including smoking, in group training sessions of 4 hours. Participants will receive individual coaching or counseling sessions from their general practitioners. The coaching will follow the principles of "Gesundheitscoaching‐KHM" Control: participants will receive state‐of‐the‐art smoking cessation counseling |
| Outcomes | Smoking cessation rates self‐reported at 1, 6 and 12 months and verified by saliva cotinine at 12 months |
| Starting date | January 2017 |
| Contact information | Stefan Neuner‐Jehle, stefan.neuner‐jehle@usz.ch |
| Notes |
ISRCTN44559004.
| Study name | Improving quit rates among smokers in primary care: pragmatic trial of effectiveness and cost effectiveness of a tailored web‐ and text message‐based intervention for smoking cessation (iQuit in Practice) |
| Methods | Randomized controlled trial |
| Participants | Target: 1452 adults who smoke |
| Interventions | Tailored print report and supportive SMS messages |
| Outcomes | Continuous abstinence at 6m |
| Starting date | July 2016 |
| Contact information | Joanna Mitchell, jm294@medschl.cam.ac.uk |
| Notes | ISRCTN44559004 |
ISRCTN54228638.
| Study name | Lung age or exhaled carbon monoxide feedback combined with very brief advice and support for smoking cessation in Medical Faculty Skopje Macedonia |
| Methods | Multicenter non‐blinded 3‐armed randomized controlled trial |
| Participants | Target: 885 people who currently smoke, smoking at least 10 cigarettes per day and aged ≥ 35 years |
| Interventions | Intervention 1: participants will receive lung age with very brief advice and support to quit smoking Intervention 2: participants will receive feedback on exhaled carbon monoxide levels with very brief advice and support to quit smoking Intervention 3: participants will receive very brief advice alone and support to quit smoking |
| Outcomes | 7‐day point prevalence abstinence, prolonged abstinence with smoking induction period of 3 weeks post‐randomization, confirmed with salivary cotinine at 4, 12 and 26 weeks |
| Starting date | June 2017 |
| Contact information | Radmila Ristovska |
| Notes |
Mak 2015.
| Study name | The acceptance and commitment therapy for smoking cessation in the primary health care setting |
| Methods | Prospective randomized controlled trial |
| Participants | 142 adults who smoke |
| Interventions | Intervention: participants will receive self‐help materials, face‐to‐face session, 2 telephone acceptance and commitment therapy sessions at 1 week and 1 month following the first session Control: participants will receive self‐help materials |
| Outcomes | 7‐day point prevalence abstinence at 6 months |
| Starting date | 2012 |
| Contact information | Yim Wah Mak, yw.mak@polyu.edu.hk |
| Notes | NCT01652508 |
NCT02500589.
| Study name | Telephone‐based smoking cessation |
| Methods | Randomized comparative effectiveness trial |
| Participants | 350 adults who smoke with significant depressive symptoms |
| Interventions | Intervention: participants receive telephone counseling on smoking cessation and mood management Control: participants receive telephone counseling on smoking cessation |
| Outcomes | Prolonged abstinence at 6m and 12m |
| Starting date | February 2016 |
| Contact information | Jennifer M Gierisch, jennifer.gierisch@va.gov |
| Notes | NCT02500589 |
NCT03612804.
| Study name | Promoting smoking cessation in lung cancer screening through proactive treatment |
| Methods | Cluster‐randomized controlled trial |
| Participants | Target: 540 primary care patients |
| Interventions | Intervention: patients of providers assigned to the proactive study group will be contacted by specially trained counselors at the Veterans Affairs Quitline. Counselors will attempt to provide 2 sessions of proactive telephone support Control: usual care |
| Outcomes | Self‐reported abstinence from smoking for 7 days, biochemically confirmed with saliva cotinine 12 months after lung cancer screening |
| Starting date | 2019 |
| Contact information | Steven B Zeliadt, Steven.Zeliadt@va.gov |
| Notes | NCT03612804 |
NCT04188873.
| Study name | Cessation Screening Project |
| Methods | Randomized factorial experiment |
| Participants | 608 adults who smoke |
| Interventions | Fully crossed, 2x2x2x2 factorial experiment that evaluates 4 different factors:
1. Medication type (Varenicline vs. Combination NRT) 2. Preparation Medication (4 Weeks vs. Standard) 3. Medication Duration (Extended [24 weeks] vs. Standard [12 weeks]) 4. Counseling (Intensive vs. Minimal) |
| Outcomes | 7‐day point‐prevalence abstinence at 12m |
| Starting date | November 2020 |
| Contact information | University of Wisconsin, Madison |
| Notes | NCT04188873 |
NCT04199117.
| Study name | Centralized health system interventions to enhance reach: a factorial screening experiment |
| Methods | Randomized factorial experiment |
| Participants | Adult primary care patients smoked at least 5 cigarettes per day for at least 6 months at enrolment. Able to speak and read English |
| Interventions | 2x2x2x2 factorial experiment: 1. Modest financial incentives (USD 40) for completing an initial counseling session in a smoking cessation treatment (vs. none) 2. Automated semi‐annual outreach materials sent via participants' preferred communication modality using data in the electronic health record to tailor and personalize invitations to use available treatments to quit smoking (vs. untailored letters) 3. Direct, proactive telephone outreach from a tobacco care manager who will promote treatment use and deliver motivational intervention twice per year (vs. none) 4. Access to 3 no‐cost telephone smoking cessation counseling calls with combination nicotine replacement therapy (C‐NRT) or varenicline (vs. state tobacco quitline and primary care provider referral) |
| Outcomes | 7‐day point‐prevalence abstinence at 3w, 3m, 6m, 2y |
| Starting date | 11 March 2020 |
| Contact information | Michael C Fiore mcf@ctri.wisc.edu |
| Notes | NCT04199117 |
NCT04223336.
| Study name | A web‐enabled integrated care pathway (ICP) for addressing multiple modifiable risk factors as a part of smoking cessation treatment in primary care |
| Methods | Randomized controlled trial |
| Participants | 5000 adults enrolled in the STOP program with at least 1 of the following 2 modifiable risk factors: low levels of physical activity and/or low levels of fruits/vegetable consumption |
| Interventions | Integrated care pathway for physical activity and fruits/vegetable consumption |
| Outcomes | 7‐day point‐prevalence abstinence at 6m |
| Starting date | 30 November 2019 |
| Contact information | Peter Selby peter.selby@camh.ca |
| Notes | NCT04223336 |
NCT04276116.
| Study name | Effectiveness of the evaluation and communication of "Pulmonary Age" as help for smoking cessation: a cluster randomized essay |
| Methods | Cluster‐randomized trial in 2 parallel groups |
| Participants | Adults who smoke |
| Interventions | Communication of pulmonary age |
| Outcomes | Undefined abstinence at 12m |
| Starting date | March 2020 |
| Contact information | Nicolas Roche nicolas.roche@htd.aphp.fr |
| Notes | NCT04276116 |
Olano 2018.
| Study name | Effectiveness of a chat‐bot for the adult population to quit smoking: protocol of a pragmatic clinical trial in primary care (Dejal@) |
| Methods | Multicenter, pragmatic randomized controlled trial |
| Participants | Target: 460 people aged over 18 years and smoking |
| Interventions | Intervention: participants will use a chat‐bot with evidence‐based contents to help quit smoking Control: participants will receive usual treatment |
| Outcomes | Smoking cessation rate, biochemically validated at 6 months |
| Starting date | October 2018 |
| Contact information | Jose Avila‐Thomas, joseavil@gmail.com |
| Notes | NCT03445507 |
Parker 2013.
| Study name | Translating the GOLD COPD guidelines into primary care practice |
| Methods | Cluster‐randomized trial |
| Participants | 3593 patients aged 40 years or older and had been seen at least once in the past 2 years by their primary care provider |
| Interventions | Intervention: • Practices will receive portable spirometer • Medical staff will receive: ‐ Training on spiromatory and how to use the tools and integrate them into workflow ‐ Web‐based COPD guideline tool, patient activation tool, COPD patient education toolkit ‐ 2 academic detailing visits ‐ Baseline and post‐intervention chart audits ‐ Exit interviews Usual care: • Practices will receive portable spirometer • Medical staff will receive: ‐ Spirometry training ‐ 2 non‐academic detailing visits ‐ Baseline and post‐intervention chart audits ‐ Exit interviews |
| Outcomes | Adherence to COPD guidelines at 12 months |
| Starting date | October 2010 |
| Contact information | Donna Parker, Donna_Parker@Brown.edu |
| Notes | NCT01237561 |
Proctor 2020.
| Study name | Assessment of the effectiveness and cost‐effectiveness of tailored web‐ and text‐based smoking cessation support in primary care (iQuit in Practice II): protocol for a randomized cControlled Trial |
| Methods | Two‐arm, parallel‐group, randomized controlled trial |
| Participants | Adults who smoke and have a mobile phone |
| Interventions | Tailored smoking cessation system designed for use by healthcare practitioners during the delivery of routine cessation support |
| Outcomes | Prolonged abstinence at 6m |
| Starting date | 2020 |
| Contact information | Stephen Sutton srs34@medschl.cam.ac.uk. |
| Notes | PMID: 32673255 |
Sanchez‐Aguadero 2017.
| Study name | Effectiveness of an intensive intervention to improve lifestyles in people with intermediate cardiovascular risk (DATE study): Study protocol for a randomized controlled trial |
| Methods | Randomized controlled trial |
| Participants | 208 participants with intermediate cardiovascular risk |
| Interventions | Intervention: participants will receive individual standardized counseling on lifestyles plus 4 weekly group sessions focusing on cardiovascular risk, healthy diet, moderation in alcohol consumption, daily physical activity, stress management and smoking cessation and 2 motivational follow‐up calls Control: participants will receive individual standardized counseling on lifestyles |
| Outcomes | Abstinence at 3m and 12m |
| Starting date | June 2017 |
| Contact information | Natalia Sanchez‐Aguadero, natalia.san.ag@gmail.com |
| Notes | NCT03164499 |
Differences between protocol and review
The following revisions were made to the published protocol for the present review:
The title of the review was changed from 'Strategies to improve the delivery of tobacco use treatment in primary care practice' to 'Strategies to improve smoking cessation rates in primary care'. This was in response to peer review comments, which suggested that the title did not accurately reflect the inclusion criteria for the studies included in the review.
Due to the volume of relevant studies, we excluded non‐randomized studies (before‐after controlled trials).
We had originally planned to include studies which tested interventions to enhance tobacco treatment delivery as part of a multifactorial lifestyle intervention; however, due to the extensive literature identified and the high clinical and methodological heterogeneity between studies purely focusing on smoking and those looking at multiple risk factors we ultimately excluded them.
One review author extracted data for study characteristics, due to the high number of included studies.
We excluded studies with short‐term follow‐up (less than six months). We had originally planned to include studies with a shorter follow‐up, as we expected to find limited studies; but, based on the considerable body of evidence identified through our searches we deferred to the usual guidance of the Cochrane Tobacco Addiction Group.
We did not assess performance bias in line with the guidance provided by the Cochrane Tobacco Addiction Group on studies of behavioral interventions.
We did not assess funding source as a source of bias in line with Cochrane recommendations.
We assessed detection bias for the primary outcome only and considered biochemical validation of quitting in our judgment of this domain rather than as a separate domain.
We generated funnel plots for any outcomes with 10 or more studies contributing to the analysis.
Analyses were carried out using a random‐effects model rather than a fixed‐effect model, in line with the most recent guidance provided by the Cochrane Tobacco Addiction Group.
The protocol had assumed that studies would be grouped more broadly in analyses, but this was deemed inappropriate due to substantial clinical variance in the studies identified. Analyses were structured based on the strategies identified through the searches, and as such we chose appropriate subgroup analyses based on this restructuring.
Studies at high risk of bias only (i.e. not unclear risk) were removed in sensitivity analyses, in line with the common practice of the Cochrane Tobacco Addiction Group.
We carried out sensitivity analyses removing individually‐randomized studies based on the comments of the Co‐ordinating editor. c‐RCTs are the most appropriate study type to test the interventions eligible for this review in primary care specifically.
Contributions of authors
| Roles and responsibilities* | |
| Task | Who carried out this task? |
| Drafting of protocol | Sophia Papadakis, George Wells, Andrew Pipe |
| Development of search strategy | Sophia Papadakis, Gillian Pritchard, Lindsay Stead (editorial) |
| Searched for trials | Sophia Papadakis, Gillian Pritchard |
| Obtained copies of trials | Gillian Pritchard |
| Eligibility assessment | Sophia Papadakis, Gillian Pritchard, Andrew Pipe, Bosun Hong, Nicola Lindson |
| Extracted data | Sophia Papadakis, Gillian Pritchard, Nicola Lindson, Bosun Hong |
| Entered data into Review Manager 5 | Sophia Papadakis, Gillian Pritchard, Nicola Lindson, Bosun Hong |
| Carried out analysis | Sophia Papadakis, Nicola Lindson, Thomas Fanshawe |
| Interpreted analyses | Nicola Lindson |
| Drafting the final review | Sophia Papadakis, Nicola Lindson, Thomas Fanshawe; Bosun Hong |
| Review of draft | All authors |
Sources of support
Internal sources
-
Nicola Lindson, UK
NL Is employed by the University of Oxford
The University of Ottawa Heart Institute, Ottawa, Canada provides salary, office space and library resources for SP, GP, AP, GW, Canada
External sources
-
Nicola Lindson, UK
NL's salary is funded through infrastructure funding for the Cochrane Tobacco Addiction Group from the NIHR. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Declarations of interest
NL: none known
TF: none known
BH: none known
GP: none known
AP is employed by the University of Ottawa Heart Institute, which has received educational and research grants from Pfizer Canada, the Heart and Stroke Foundation of Ontario, Public Health Agency of Canada, Ontario Ministry of Health and Long Term Care. AP has received consulting fees and speaker honoraria from Pfizer, Johnson and Johnson, Merck, Glaxo‐Smith Kline. AP is an inventor of the Ottawa Model for Smoking Cessation. A commercial organization uses the Ottawa Model for Smoking Cessation program, and the inventors have received royalty payments in the past, through the University of Ottawa Heart Institute.
GW: none known
SP is an inventor of the Ottawa Model for Smoking Cessation. A commercial organization uses strategies informed by the Ottawa Model for Smoking Cessation program, and SP has received royalty payments in the past, through the University of Ottawa Heart Institute.
New
References
References to studies included in this review
Aleixandre 1998 {published data only}
- Aleixandre E, Casanova Matutano MA, Mitjans Lafont J, Sanchez Monfort J, Sanmartin Almenar A. Clinical trial of two tobacco use cessation interventions in primary care [Ensayo clinico de dos intervenciones de deshabituacion tabaquica en atencion primaria]. Atencion Primaria 1998;22(7):424-8. [PubMed] [Google Scholar]
An 2006 {published data only}
- An LC, Zhu SH, Arikian NJ, Nelson DB, Nugent SM, Partin M, et al. Telestop: a randomized trial of increased access to behavioral and pharmacological therapy for smoking cessation. Nicotine and Tobacco Research 2005;7(4):689. [Google Scholar]
- An LC, Zhu SH, Nelson DB, Arikian NJ, Nugent S, Partin MR, et al. Benefits of telephone care over primary care for smoking cessation: a randomized trial. Archives of Internal Medicine 2006;166(5):536-42. [DOI: 10.1001/archinte.166.5.536] [DOI] [PubMed] [Google Scholar]
Aung 2019 {published data only}
- Aung MN, Yuasa M, Moolphate S, Lorga T, Yokokawa H, Fukuda H, et al. Effectiveness of a new multi-component smoking cessation service package for patients with hypertension and diabetes in northern Thailand: a randomized controlled trial. Substance Abuse Treatment, Prevention, and Policy 2019;14(1):10. [DOI: 10.1186/s13011-019-0197-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aveyard 2003 {published data only}
- Aveyard P, Griffin C, Lawrence T, Cheng KK. A controlled trial of an expert system and self-help manual intervention based on the stages of change versus standard self-help materials in smoking cessation. Addiction 2003;98(3):345-54. [DOI: 10.1046/j.1360-0443.2003.00302.x.] [DOI] [PubMed] [Google Scholar]
Bock 2014 {published data only}
- Bock BC, Papandonatos GD, Dios MA, Abrams DB, Azam MM, Fagan M, et al. Tobacco cessation among low-income smokers: motivational enhancement and nicotine patch treatment. Nicotine and Tobacco Research 2014;16(4):413-22. [DOI: 10.1093/ntr/ntt166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borland 2008 {published data only}
- Borland R, Balmford J, Bishop N, Segan C, Piterman L, McKay-Brown L, et al. In-practice management versus quitline referral for enhancing smoking cessation in general practice: a cluster randomized trial. Family Practice 2008;25(5):382-9. [DOI: 10.1093/fampra/cmn046] [DOI] [PubMed] [Google Scholar]
Buffels 2006 {published data only}
- Buffels J, Degryse J, Decramer M, Heyrman J. Can spirometry improve the success rate of smoking cessation in general practice. European Respiratory Journal 2005;26:670. [Google Scholar]
- Buffels J, Degryse J, Decramer M, Heyrman J. Spirometry and smoking cessation advice in general practice: a randomised clinical trial. Respiratory Medicine 2006;100(11):2012-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cabezas 2011 {published data only}
- Cabezas C, Advani M, Puente D, Rodriguez-Blanco T, Martin C. Effectiveness of a stepped primary care smoking cessation intervention: cluster randomized clinical trial (ISTAPS study). Addiction 2011;106(9):1696-706. [DOI: 10.1111/j.1360-0443.2011.03491.x] [DOI] [PubMed] [Google Scholar]
- Cabezas C, Martin C, Granollers S, Morera C, Ballve JL, Zarza E, et al. Effectiveness of a stepped primary care smoking cessation intervention (ISTAPS study): design of a cluster randomized trial. BMC Public Health 2009;9:48. [DOI: 10.1186/1471-2458-9-48] [DOI] [PMC free article] [PubMed]
- Codern-Bove N, Pujol-Ribera E, Pla M, Gonzalez-Bonilla J, Granollers S, Ballve JL, et al. Motivational interviewing interactions and the primary health care challenges presented by smokers with low motivation to stop smoking: a conversation analysis. BMC Public Health 2014;14:1225. [DOI] [PMC free article] [PubMed]
- Puente D, Cabezas C, Gonzalez FJ, Camarelles F, Torrecilla M, Dominguez J. Primary care smoking cessation intervention conducted in a sample of Spanish smokers: predictors of smoking cessation. Journal of Epidemiology and Community Health 2007;61:A5-A6. [Google Scholar]
Canga 2000 {published data only}
Carpenter 2020 {published data only}
- Carpenter MJ, Wahlquist AE, Dahne J, Gray KM, Garrett-Mayer E, Cummings KM, et al. Nicotine replacement therapy sampling for smoking cessation within primary care: results from a pragmatic cluster randomized clinical trial. Addiction 2020;115(7):1358-67. [DOI: 10.1111/add.14953] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahne J, Wahlquist AE, Boatright AS, Garrett-Mayer E, Fleming DO, Davis R, et al. Nicotine replacement therapy sampling via primary care: methods from a pragmatic cluster randomized clinical trial. Contemporary Clinical Trials 2018;72:1-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahne J, Wahlquist AE, Smith TT, Carpenter MJ. The differential impact of nicotine replacement therapy sampling on cessation outcomes across established tobacco disparities groups. Preventive Medicine 2020;136:106096. [DOI: 10.1016/j.ypmed.2020.106096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cobos‐Campos 2016 {published data only}
- Cobos-Campos R, Apinaniz Fernandez de Larrinoa A, Saez de Lafuente Morinigo A, Parraza Diez N, Aizpuru Barandiaran F. Effectiveness of text messaging as an adjuvant to health advice in smoking cessation programs in primary care. A randomized clinical trial. Nicotine and Tobacco Research 2016;19(8):901-7. [DOI: 10.1093/ntr/ntw300] [DOI] [PubMed]
- NCT01746069. Evaluation of a reinforcement program using text messages through mobile phone in smoking cessation programs in primary care. Clinicaltrials.gov/ct2/show/NCT01746069 (first received 10 December 2012).
Cummings 1989a {published data only}
- Cummings SR, Richard RJ, Duncan CL, Hansen B, Vander Martin R, Gerbert B, et al. Training physicians about smoking cessation: a controlled trial in private practice. Journal of General Internal Medicine 1989;4(6):482-9. [DOI: 10.1007/BF02599545] [DOI] [PubMed] [Google Scholar]
Cummings 1989b {published data only}
- Cummings SR, Coater TJ, Richard RJ, Hansen B, Zahnd EG, Vander Martin R, et al. Training physicians in counseling about smoking cessation: a randomized trial of the 'Quit for Life' program. Annals of Internal Medicine 1989;110(8):640-7. [DOI: 10.7326/0003-4819-110-8-640] [DOI] [PubMed] [Google Scholar]
Dent 2009 {published data only}
- Dent LA, Harris KJ, Noonan CW. Randomized trial assessing the effectiveness of a pharmacist-delivered program for smoking cessation. Annals of Pharmacotherapy 2009;43(2):194-201. [DOI: 10.1345/aph.1L556] [DOI] [PubMed] [Google Scholar]
Ellerbeck 2009 {published data only}
- Cox LS, Cupertino AP, Mussulman LM, Nazir N, Greiner KA, Mahnken JD, et al. Design and baseline characteristics from the KAN-QUIT disease management intervention for rural smokers in primary care. Preventive Medicine 2008;47(2):200-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbeck EF, Mahnken JD, Cupertino AP, Cox LS, Greiner KA, Mussulman LM, et al. Effect of varying levels of disease management on smoking cessation: a randomized trial. Annals of Internal Medicine 2009;150(7):437-46. [DOI: 10.7326/0003-4819-150-7-200904070-00003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 2014 {published data only}
- Fu S, Burgess D, Van Ryn M, Sherman S, Noorbaloochi S, Clothier B, et al. Using electronic health record registries to increase use of telephone quitline services among vulnerable priority populations. Journal of General Internal Medicine 2011;26(Suppl 1):S297-8. [Google Scholar]
- Fu SS, Van Ryn M, Sherman SE, Burgess DJ, Noorbaloochi S, Clothier B, et al. Population-based tobacco treatment: study design of a randomized controlled trial. BMC Public Health 2012;12:159. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SS, Van Ryn M, Sherman SE, Burgess DJ, Noorbaloochi S, Clothier B, et al. Proactive tobacco treatment and population-level cessation: a pragmatic randomized clinical trial. Journal of American Medical Association Internal Medicine 2014;174(5):671-7. [DOI: 10.1001/jamainternmed.2014.177] [DOI] [PubMed] [Google Scholar]
- Melzer AC, Clothier B, Japuntich SJ, Noorbaloochi S, Hammett P, Burgess DJ, et al. Comparative effectiveness of proactive tobacco treatment among smokers with and without chronic lower respiratory disease. Annals of the American Thoracic Society 2018;15(3):341-7. [DOI: 10.1513/AnnalsATS.201707-582OC] [DOI] [PubMed] [Google Scholar]
Gilbert 2013 {published data only}
- Bennett K, Gilbert H, Sutton S. Computer-tailored smoking cessation advice matched to reading ability: perceptions of participants from the ESCAPE trial. Patient Education and Counseling 2015;98:1577-84. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H, Leurent B, Sutton S, Morris R, Alexis-Garsee C, Nazareth I. An exploration of general practice factors predicting recruitment to a UK wide primary care smoking cessation study (the ESCAPE trial). Family Practice 2011;29(1):110-7. [DOI: 10.1093/fampra/cmr030] [DOI] [PubMed] [Google Scholar]
- Gilbert H, Leurent B, Sutton S, Morris R, Alexis-Garsee C, Nazareth I. An exploration of general practice factors predicting recruitment to a UK wide primary care smoking cessation study (The ESCAPE trial). Family Practice 2012;29:110-7. [DOI: 10.1093/fampra/cmr030] [DOI] [PubMed] [Google Scholar]
- Gilbert H, Nazareth I, Sutton S, Morris R, Godfrey C. Effectiveness of computer-tailored Smoking Cessation Advice in Primary Care (ESCAPE): a randomised trial. Trials 2008;9:23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert HM, Leurent B, Sutton S, Alexis-Garsee C, Morris RW, Nazareth I. ESCAPE: a randomised controlled trial of computer-tailored smoking cessation advice in primary care. Addiction 2013;108(4):811-9. [DOI: 10.1111/add.12005] [DOI] [PubMed] [Google Scholar]
- Gilbert HM, Sutton SR, Leurent B, Alexis-Garsee C, Morris RW, Nazareth I. Characteristics of a population-wide sample of smokers recruited proactively for the ESCAPE trial. Public Health 2012;126(4):308-16. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gilbert 2017 {published data only}
- Gardner L, Gilbert H, Nazareth I. Recruitment to the pilot phase of Start2quit - lessons learned. Trials 2011;12(Suppl 1):A120. [DOI: 10.1186/1745-6215-12-s1-a120] [DOI] [Google Scholar]
- Gilbert H, Sutton S, Morris R, Parrot S, Galton S, Nazareth I. Evaluating the effectiveness of using personal tailored risk information and taster sessions to increase the uptake of smoking cessation services: study protocol for a randomised controlled trial. Trials 2012;13:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H, Sutton S, Morris R, Petersen I, Galton S, Wu Q, et al. Effectiveness of personalised risk information and taster sessions to increase the uptake of smoking cessation services (Start2quit): a randomised controlled trial. Lancet 2017;389(10071):823-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H, Sutton S, Morris R, Petersen I, Wu Q, Parrott S, et al. Start2quit: A randomised clinical controlled trial to evaluate the effectiveness and cost-effectiveness of using personal tailored risk information and taster sessions to increase the uptake of the NHS stop smoking services. Health Technology Assessment 2017;21(3):1-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Gilbert H, Nazareth I, Sutton S, Morris R, Petersen I, et al. Cost-effectiveness of personal tailored risk information and taster sessions to increase the uptake of the NHS Stop Smoking Services: the Start2quit randomised controlled trial. Addiction 2017;113(4):708-718. [DOI: 10.1111/add.14086] [DOI] [PMC free article] [PubMed] [Google Scholar]
Girgis 2011 {published data only}
- Girgis S, Adily A, Velasco MJ, Garden FL, Zwar NA, Jalaludin BB, et al. Smoking patterns and readiness to quit--a study of the Australian Arabic community. Australian Family Physician 2009;38(3):154-61. [PMID: ] [PubMed] [Google Scholar]
- Girgis S, Adily A, Velasco MJ, Zwar N, Jalaludin BB, Ward JE. Feasibility, acceptability and impact of a telephone support service initiated in primary medical care to help Arabic smokers quit. Australian Journal of Primary Health 2011;17(3):274-81. [DOI: 10.1071/PY10066] [DOI] [PubMed] [Google Scholar]
Haas 2015 {published data only}
- Haas JS, Linder JA, Park ER, Gonzalez I, Rigotti NA, Klinger EV. Proactive tobacco cessation outreach to smokers of low socioeconomic status: a randomized clinical trial. Journal of American Medical Assocation Internal Medicine 2015;175(2):218-26. [DOI: 10.1001/jamainternmed.2014.6674] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Klinger EV, Linder JA, Fleegler EW, Rigotti NA, Park ER, et al. Cost-effectiveness of a health system-based smoking cessation program. Nicotine and Tobacco Research 2017;19(12):1508-15. [DOI: 10.1093/ntr/ntw243] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JA, Haas J, Rigotti NA, Park ER, Kontos E, Gonzalez I, et al. Proactive outreach of tobacco cessation treatment to disadvantaged smokers after a primary care visit: a randomized controlled trial. Journal of General Internal Medicine 2014;29(Suppl 1):S184. [Google Scholar]
Hilberink 2011 {published data only}
- Hilberink SR, Jacobs JE, Bottema BJ, Vries H, Grol RP. Smoking cessation in patients with COPD in daily general practice (SMOCC): six months' results. Preventive Medicine 2005;41(5-6):822-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hilberink SR, Jacobs JE, Breteler MH, Vries H, Grol RP. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: impact after 1 year of two complex interventions. Patient Education and Counseling 2011;83(1):120-4. [DOI: 10.1016/j.pec.2010.04.009] [DOI] [PubMed] [Google Scholar]
- Jacobs JE, Hilberink SR, Keegstra J, Straver M. Smoking cessation counseling for patients with COPD in general practice (SMOCC): screening method and characteristics of included patients. Primary Care Respiratory Journal 2002;11(2):62. [Google Scholar]
Hollis 1993 {published data only}
- Hollis JF, Lichtenstein E, Mount K, Vogt TM, Stevens VJ. Nurse-assisted smoking counseling in medical settings: minimizing demands on physicians. Preventive Medicine 1991;20(4):497-507. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse-assisted counseling for smokers in primary care. Annals of Internal Medicine 1993;118(7):521-5. [DOI: 10.7326/0003-4819-118-7-199304010-00006] [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Hollis J. Patient referral to a smoking cessation program: who follows through? Journal of Family Practice 1992;34(6):739-44. [PMID: ] [PubMed] [Google Scholar]
Hoving 2010 {published data only}
- Hoving C, Mudde AN, Dijk F, Vries H. Effectiveness of a smoking cessation intervention in Dutch pharmacies and general practices. Health Education 2010;110(1):17-29. [DOI: 10.1108/09654281011008726] [DOI] [Google Scholar]
Hughes 1991 {published data only}
- Hughes JR, Wadland WC, Fenwick JW, Lewis J, Bickel WK. Effect of cost on the self-administration and efficacy of nicotine gum: a preliminary study. Preventive Medicine 1991;20(4):486-96. [DOI: 10.1016/0091-7435(91)990046-7] [DOI] [PubMed] [Google Scholar]
Irizar Aramburu 2013 {published data only}
- Irizar-Aramburu MI, Martínez-Eizaguirre JM, Pacheco-Bravo P, Diaz-Atienza M, Aguirre-Arratibel I, Peña-Peña MI, et al. Effectiveness of spirometry as a motivational tool for smoking cessation: a clinical trial, the ESPIMOAT study. BMC Family Practice 2013;14:185. [DOI: 10.1186/1471-2296-14-185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jamrozik 1984 {published data only}
- Jamrozik K, Vessey M, Fowler G, Wald N, Parker G, Vunakis H. Controlled trial of three different antismoking interventions in general practice. British Medical Journal (Clinical Research Edition) 1984;288(6429):1499-503. [DOI: 10.1136/bmj.288.6429.1499] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamrozik KD, Wald NJ. A randomised trial of three different anti-smoking interventions in general practice. Proceedings from the 5th World Conference on Smoking and Health 1983;1:371-82. [Google Scholar]
Joseph 2004 {published data only}
- Joseph AM, Arikian NJ, An LC, Nugent SM, Sloan RJ, Pieper CF. Results of a randomized controlled trial of intervention to implement smoking guidelines in Veterans Affairs medical centers: increased use of medications without cessation benefit. Medical Care 2004;42(11):1100-10. [DOI: 10.1097/00005650-200411000-00009] [DOI] [PubMed] [Google Scholar]
Juarranz 1998 {published data only}
- Juarranz Sanz M, Soriano Llora T, Reyes Martin G, Sanchez Sanchez MI. Efficacy of the treatment with substitute of nicotine in the smoking cessation from the primary health care. Revista de Medicina Familiar y Comunitaria 1998;8(6):376-83.
Kalkhoran 2018 {published data only}
- Kalkhoran S, Inman E, Kulesa Kelley JH, Ashburner JM, Rigotti NA. Proactive population health strategy to offer tobacco dependence treatment to smokers in a primary care practice network. Journal of General Internal Medicine 2018;33(2 Suppl 1):312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2004 {published data only}
- Katz DA, Muehlenbruch DR, Brown RL, Fiore MC, Baker TB. Effectiveness of implementing the agency for healthcare research and quality smoking cessation clinical practice guideline: a randomized, controlled trial. Journal of the National Cancer Institute 2004;96(8):594-603. [PMID: 15100337] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2003 {published data only}
- Kim YE, Song YM, Lee JK, Jung HS, Kang SC. A study evaluating the effect of telephone counselling on smoking performed by a nurse cessation: a preliminary, randomized, controlled trial. Journal of the Korean Academy of Family Medicine 2003;24(7):634-41. [Google Scholar]
Kottke 1989 {published data only}
- Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomized trial to increase smoking intervention by physicians. Doctors Helping Smokers, Round I. The Journal of the Americal Medical Association 1989;261(14):2101-6. [PMID: ] [PubMed] [Google Scholar]
Lancaster 1999 {published data only}
- Lancaster T, Dobbie W, Vos K, Yudkin P, Murphy M, Fowler G. Randomized trial of nurse-assisted strategies for smoking cessation in primary care. British Journal of General Practice 1999;49(440):191-4. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Lasser 2017 {published data only}
- Lasser KE, Quintiliani LM, Truong V, Xuan Z, Murillo J, Jean C, et al. Effect of patient navigation and financial incentives on smoking cessation among primary care patients at an urban safety-net hospital: a randomized clinical trial. Journal of American Medical Association Internal Medicine 2017;177(12):1798-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser KE, Quintiliani LM, Truong V, Xuan Z, Pbert L. Patient navigation to promote smoking cessation in primary care: preliminary findings from an ongoing randomized controlled trial. Journal of General Internal Medicine 2017;32:266. [Google Scholar]
- Quintiliani LM, Russinova ZL, Bloch PP, Truong V, Xuan Z, Pbert L, et al. Patient navigation and financial incentives to promote smoking cessation in an underserved primary care population: a randomized controlled trial protocol. Contemporary Clinical Trials 2015;45(Part B):449-57. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee JE, Shin DW, Suh B, Chun S, Nam Y-S, Cho B. Development and application of culturally appropriate decision aids for smoking cessation in Korea: a pragmatic clustered randomization crossover trial. Patient Preference and Adherence 2016;10:1929-36. [DOI: 10.2147/ppa.s114387] [DOI] [PMC free article] [PubMed]
Lennox 1998 {published data only}
- Lennox AS, Bain N, Taylor RJ, McKie L, Donnan PT, Groves J. Stages of change training for opportunistic smoking intervention by the primary health care team. Part 1: randomized controlled trial of the effect of training on patient smoking outcomes and health professional behaviour as recalled by patients. Health Education Journal 1998;57(2):140. [DOI: 10.1177/001789699805700206] [DOI] [Google Scholar]
Lennox 2001 {published data only}
- Lennox AS, Osman LM, Reiter E, Robertson R, Friend J, McCann I, et al. Cost effectiveness of computer tailored and non-tailored smoking cessation letters in general practice: randomised controlled trial. BMJ 2001;322(7299):1396. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leppänen 2019 {published data only}ISRCTN11498135
- Leppänen A, Ekblad S, Tomson. Tobacco Cessation on Prescription as a primary health care intervention targeting a context with socioeconomically disadvantaged groups in Sweden: a qualitative study of perceived implementation barriers and facilitators among providers. PLOS One 2019;14(2):e0212641. [DOI: 10.1371/journal.pone.0212641] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen A, Lindgren P, Sundberg CJ. A cluster-randomized controlled trial evaluating the effectiveness and cost-effectiveness of tobacco cessation on prescription in Swedish primary health care: a protocol of the Motivation 2 Quit (M2Q) Study. JMIR Research Protocols 2016;5(3):e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen A. Tobacco Cessation on Prescription: A Primary Healthcare Intervention Targeting Socioeconomically Disadvantaged Areas in Stockholm [thesis]. Stockholm: Karolinska Institutet, 2019. [ISBN 978-91-7831-567-3] [Google Scholar]
Lindsay 1989 {published data only}
- Lindsay EA, Wilson DM, Best JA, Willms DG, Singer J, Gilbert JR, et al. A randomized trial of physician training for smoking cessation. American Journal of Health Promotion 1989;3(3):11-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wilson DM, Lindsay EA, Best JA, Gilbert JR, Willms DG, Singer J. A smoking cessation intervention program for family physicians. Canadian Medical Association Journal 1987;137(7):613-9. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Taylor W Gilbert JR, Best A, Lindsay EA, Willms DG, et al. A randomized trial of family physician training for smoking cessation. JAMA 1988;260(11):1570-4. [PMID: ] [PubMed] [Google Scholar]
Lou 2013 {published data only}
- Lou P, Chen P, Zhang P, Yu J, Wang Y, Chen N, et al. A COPD health management program in a community-based primary care setting: a randomized controlled trial. Respiratory Care 2015;60(1):102-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lou P, Zhu Y, Chen P, Zhang P, Yu J, Zhang N, et al. Supporting smoking cessation in chronic obstructive pulmonary disease with behavioral intervention: a randomized controlled trial. BMC Family Practice 2013;14:91. [DOI: 10.1186/1471-2296-14-91] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marley 2014 {published data only}
- Marley JV, Atkinson D, Kitaura T, Nelson C, Gray D, Metcalf S, et al. The Be Our Ally Beat Smoking (BOABS) study, a randomised controlled trial of an intensive smoking cessation intervention in a remote aboriginal Australian health care setting. BMC Public Health 2014;14:32. [DOI: 10.1186/1471-2458-14-32] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley JV, Atkinson D, Nelson C, Kitaura T, Gray D, Metcalf S, et al. The protocol for the Be Our Ally Beat Smoking (BOABS) study, a randomised controlled trial of an intensive smoking cessation intervention in a remote Aboriginal Australian health care setting. BMC Public Health 2012;12:232. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mejia 2015 {published data only}
- Mejia R, Kaplan CP, Alderete M, Pena L, Gregorich S, Schoj V, et al. Effectiveness of an intervention to teach physicians in a middle-income country how to help their patients quit smoking. Journal of General Internal Medicine 2013;28(Suppl):S69. [Google Scholar]
- Mejia R, Perez Stable EJ, Kaplan CP, Gregorich SE, Livaudais-Toman J, Pena L, et al. Effectiveness of an intervention to teach physicians how to assist patients to quit smoking in Argentina. Nicotine and Tobacco Research 2015;18(5):1101-9. [DOI: 10.1093/ntr/ntv153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 2008 {published data only}
- Haug S, Meyer C, Ulbricht S, Schorr G, Ruge J, Rumpf HJ, et al. Predictors and moderators of outcome in different brief interventions for smoking cessation in general medical practice. Patient Education and Counseling 2010;78(1):57-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Klein G, Ulbricht S, Haug S, Gross B, Rumpf HJ, John U, et al. Effects of practitioner-delivered brief counseling and computer-generated tailored letters on cigarettes per day among smokers who do not quit - a quasi-randomized controlled trial. Drug and Alcohol Dependence 2010;112(1-2):81-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Meyer C, Ulbricht S, Baumeister SE, Schumann A, Rüge J, Bischof G, et al. Proactive interventions for smoking cessation in general medical practice: a quasi-randomized controlled trial to examine the efficacy of computer-tailored letters and physician-delivered brief advice. Addiction 2008;103(2):294-304. [DOI: 10.1111/j.1360-0443.2007.02031.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Ulbricht S, Schumann A, Hannover W, Hapke U, Rumpf H-J, et al. Inteventions fostering the motivation to quit for smokers in general practice. Suchtmedizin in Forschung und Praxis 2003;5(2):134-6.
- Ulrich John U, Ulbricht S, Goeze C, Meyer C. Brief intervention to motivate smokers to quit in primary medical care. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(1 Suppl 1):S40. [Google Scholar]
Meyer 2012 {published data only}
- Meyer C, Ulbricht S, Gross B, Kästel L, Wittrien S, Klein G, et al. Adoption, reach and effectiveness of computer-based, practitioner delivered and combined smoking interventions in general medical practices: a three-arm cluster randomized trial. Drug and Alcohol Dependence 2012;121(1-2):124-32. [DOI: 10.1016/j.drugalcdep.2011.08.019] [DOI] [PubMed] [Google Scholar]
Minué‐Lorenzo 2019 {published data only}
- Minué-Lorenzo C, Olano-Espinosa E, Cura-González I, Vizcaíno-Sánchez JM, Camerelles-Guillem F, Granados-Garrido JA, et al. Subsidized pharmacological treatment for smoking cessation by the Spanish public health system: a randomized, pragmatic, clinical trial by clusters. Tobacco Induced Diseases 2019;17:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 1996 {published data only}
- Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers: tailored interventions for routine medical care. Preventative Medicine 1996;25(3):346-54. [DOI: 10.1006/pmed.1996.0065] [DOI] [PubMed] [Google Scholar]
Murray 2008 {published data only}
- Murray RL, Coleman T, Antoniak M, Fergus A, Britton J, Lewis SA. Promoting smoking cessation in primary care: a cluster-randomised controlled intervention trial of pro-actively identifying smokers and offering evidence-based support to stop smoking. American Journal of Respiratory and Critical Care Medicine 2007;175:A600. [Google Scholar]
- Murray RL, Coleman T, Antoniak M, Stocks J, Fergus A, Britton J, et al. The effect of proactively identifying smokers and offering smoking cessation support in primary care populations: a cluster-randomized trial. Addiction 2008;103(6):998-1006. [DOI: 10.1111/j.1360-0443.2008.02206.x] [DOI] [PubMed] [Google Scholar]
Nebot 1992 {published data only}
- Nebot M, Cabezas C, Oller M, Moreno F, Rodrigo J, Sarda T, et al. Medical counseling, nursing counseling, and nicotine chewing gum for smoking cessation in primary care [Consejo medico, consejo de enfermeria y chicle de nicotina para dejar de fumar en atencion primaria]. Medicina Clinica 1990;95(2):57-61. [PubMed] [Google Scholar]
- Nebot M, Cabezas C. Does nurse counseling or offer of nicotine gum improve the effectiveness of physician smoking-cessation advice? Family Practice Research Journal 1992;12(3):263-70. [PubMed] [Google Scholar]
Nichols 2017 {published data only}
- Hopkins RJ, Nichols JA, Grob P, Kite W, Williams P, Young RP. Gene-based risk test of lung cancer risk shows a dose-response effect in smoking cessation programme in the Surrey primary care study. American Journal of Respiratory and Critical Care Medicine 2014;189(Suppl):A1084. [Google Scholar]
- Nichols JA, Grob P, Lusignan S, Kite W, Williams P. Genetic test to stop smoking (GeTSS) trial protocol: randomised controlled trial of a genetic test (Respiragene) and Auckland formula to assess lung cancer risk. BMC Pulmonary Medicine 2014;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols JAA, Grob P, Kite W, Williams P, Lusigan S. Using a genetic/clinical risk score to stop smoking (GeTSS): randomised controlled trial. BMC Research Notes 2017;10(507):1-9. [DOI: 10.1186/s13104-017-2831-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ockene 1994 {published data only}
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician-delivered smoking interventions: a randomized clinical trial. Journal of General Internal Medicine 1991;6(1):1-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Pbert L, Hebert JR, Luippold R, Goldberg RJ, et al. The physician-delivered smoking intervention project: can short-term interventions produce long-term effects for a general outpatient population? Health Psychology 1994;13(3):278-81. [DOI: 10.1037//0278-6133.13.3.278] [DOI] [PubMed] [Google Scholar]
Olano Espinosa 2013 {published data only}
- Olano-Espinosa E, Matilla-Pardo B, Minué C, Antón E, Gómez-Gascón T, Ayesta FJ. Effectiveness of a health professional training program for treatment of tobacco addiction.. Nicotine and Tobacco Research 2013;15(10):1682-9. [DOI: 10.1093/ntr/ntt040] [DOI] [PubMed] [Google Scholar]
Papadakis 2018 {published data only}
- Papadakis S, Cole AG, Reid RD, Assi R, Gharib M, Tulloch HE, et al. From good to great: the role of performance coaching in enhancing tobacco-dependence treatment rates. Annals of Family Medicine 2018;16(6):498-506. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadakis S, Pipe AL, Reid RD, Tulloch H, Mullen KA, Assi R, et al. Effectiveness of performance coaching for enhancing rates of smoking cessation treatment delivery by primary care providers: study protocol for a cluster randomized controlled trial. Contemporary Clinical Trials 2015;45(Pt B):184-90. [DOI] [PubMed] [Google Scholar]
- Papadakis S, Reid RD, Cole AG, Assi R, Tulloch H, Gharib M, et al. Effectiveness of two multi-component interventions to increase tobacco dependence treatment rates in primary care: a cluster randomized controlled trial (as supplied 4 May 2017). Data on file.
Parkes 2008 {published data only}
- Parkes G, Greenhalgh T, Griffin, M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008;336(7644):598-600. [DOI: 10.1136/bmj.39503.582396.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pereira 2006 {published data only}
- Pereira BC, Stoebner-Delbarre A. A cluster randomised controlled trial of a continuing education programme - smoking cessation intervention. European Journal of Epidemiology 2006;21(Supplement 1):99. [DOI: 10.1007/s10654-006-9021-1] [DOI] [Google Scholar]
- Stoebner-Delbarre A, Pereira B, Slama K, Gourgou S, Rijnoveanu A, Pujol H, et al. A cluster randomised controlled trial of a continuing education programme to increase general practitioner provision of smoking cessation interventions (as supplied 22 October 2015). Data on file.
Pérez Tortosa 2015 {published data only}
- Pérez-Tortosa S, Roig L, Manresa JM, Martin-Cantera C, Puigdomènech E, Roura P, et al. Continued smoking abstinence in diabetic patients in primary care: a cluster randomized controlled multicenter study. Diabetes Research and Clinical Practice 2015;107(1):94-103. [DOI: 10.1016/j.diabres.2014.09.009] [DOI] [PubMed] [Google Scholar]
- Roig L, Perez S, Prieto G, Martin C, Advani M, Armengol A, et al. Cluster randomized trial in smoking cessation with intensive advice in diabetic patients in primary care. ITADI Study. BMC Public Health 2010;10:58. [DOI] [PMC free article] [PubMed]
Piper 2016 {published data only}
- Piper ME, Cook JW, Schlam TR, Smith SS, Bolt DM, Collins LM, et al. Toward precision smoking cessation treatment I: Moderator results from a factorial experiment. Drug and Alcohol Dependence 2017;171:59-65. [DOI: 10.1016/j.drugalcdep.2016.11.027] [DOI] [PMC free article] [PubMed]
- Piper ME , Cook JW , Schlam TR , Smith SS , Bolt DM , Collins LM, et al. Toward precision smoking cessation treatment II: Proximal effects of smoking cessation intervention components on putative mechanisms of action. Drug and Alcohol Dependence 2017;171:50-8. [DOI: 10.1016/j.drugalcdep.2016.11.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Fiore MC, Smith SS, Fraser D, Bolt DM, Collins LM, et al. Identifying effective intervention components for smoking cessation: a factorial screening experiment. Addiction 2016;111(1):129-41. [DOI: 10.1111/add.13162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piper 2018 {published data only}
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Collins LM, et al. A randomized controlled trial of an optimized smoking treatment delivered in primary care. Annals of Behavioral Medicine 2018;52(10):854-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pisinger 2010 {published data only}
- Pisinger C, Jørgensen MM, Møller NE, Døssing M, Jørgensen T. A cluster randomized trial in general practice with referral to a group-based or an Internet-based smoking cessation programme. Journal of Public Health 2010;32(1):62-70. [DOI: 10.1093/pubmed/fdp072] [DOI] [PubMed] [Google Scholar]
Ramos 2010 {published data only}
- Montserrat MR. Effectiveness of advanced group and individual interventions in tackling tobacco dependency in primary care [Efectividad de las intervenciones grupal e individual avanzadas para el abordaje del tabaquismo.]. Atencion Primaria 2005;36(8):462-5. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos M, Ripoll J, Estrades T, Socias I, Fe A, Duro R, et al. Effectiveness of intensive group and individual interventions for smoking cessation in primary health care settings: a randomized trial. BMC Public Health 2010;10:89. [DOI: 10.1186/1471-2458-10-89] [DOI] [PMC free article] [PubMed] [Google Scholar]
RBR‐7yx9hd {published data only}
- RBR-7yx9hd. Cost-effectiveness evaluation between two approaches to treatment of smoking in the SUS (Unified Health System): brief intervention and intensive approach to the smoker [Avaliação de Custoefetividade entre duas abordagens para o tratamento do Tabagismo no SUS: Intervenção Breve e abordagem intensiva do fumante]. www.ensaiosclinicos.gov.br/rg/RBR-7yx9hd/ (first received 6 January 2015).
Richmond 1993 {published data only}
- Richmond RL, Kehoe LA, Webster IW. Multivariate models for predicting abstention following intervention to stop smoking by general practitioners. Addiction 1993;88(8):1127-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Richmond RL, Makinson RJ, Kehoe LA, Giugni AA, Webster IW. One-year evaluation of three smoking cessation interventions administered by general practitioners. Addictive Behaviors 1993;18(2):187-99. [DOI: 10.1016/0306-4603(93)90049-f] [DOI] [PubMed] [Google Scholar]
Ronaldson 2018 {published data only}
- Ronaldson SJ, Dyson L, Clark L, Hewitt CE, Torgerson DJ, Cooper BG, et al. The impact of lung function case-finding tests on smoking behaviour: a nested randomised trial within a case-finding cohort. Health Science Reports 2018;1(6):e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roski 2003 {published data only}
- Roski J, Jeddeloh R, An L, Lando H, Hannan P, Hall C, et al. The impact of financial incentives and a patient registry on preventive care quality: increasing provider adherence to evidence-based smoking cessation practice guidelines. Preventive Medicine 2003;36(3):291-9. [DOI: 10.1016/s0091-7435(02)00052-x] [DOI] [PubMed] [Google Scholar]
Russell 1983 {published data only}
- Russell MA, Merriman R, Stapleton J, Taylor W. Effect of nicotine chewing gum as an adjunct to general practitioner's advice against smoking. British Meical Journal (Clinical Research Edition) 1983;287(6407):1782-5. [DOI: 10.1136/bmj.287.6407.1782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salkeld 1997 {published data only}
- Salkeld G, Phongsavan P, Oldenburg B, Johannesson M, Convery P, Graham-Clarke P, et al. The cost-effectiveness of a cardiovascular risk reduction program in general practice. Health Policy 1997;41(2):105-19. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sanz‐Pozo 2006 {published data only}
- Sanz-Pozo B, Miguel-Diaz J, Aragon-Blanco M, Garcia-Caballo M, Gomez-Suarez E, Fernandez-Dominguez JF. Effectiveness of a programme of intensive tobacco counselling by nursing professionals [Efectividad de un programa de consejo antitabaco intensivo realizado por profesionales de enfermería]. Atencion Primaria 2006;37:266-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Pozo B, Miguel Diaz J, Aragon Blanco M, Gonzalez Gonzalez AI, Cortes Catalan M, Vazquez I. Effectiveness of non-pharmacological primary care methods for giving up tobacco dependency [Efectividad de los métodos no farmacológicos para la deshabituación tabáquica en atención primaria]. Atencion Primaria 2003;32(6):366-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Secades Villa 2009 {published data only}
- Alonso-Perez F, Secades-Villa R, Duarte Climent G. Are psychological treatments effective to stop smoking? [?Son eficientes los tratamientos psicologicos para dejar de fumar?]. Trastornos Adictivos 2007;9(1):21-30. [Google Scholar]
- Secades-Villa R, Alonso-Pérez F, García-Rodríguez O, Fernández-Hermida JR. Effectiveness of three intensities of smoking cessation treatment in primary care. Psychological Reports 2009;105(3 Part 1):747-58. [DOI: 10.2466/PR0.105.3.747-758] [DOI] [PubMed] [Google Scholar]
Segnan 1991 {published data only}
- Segnan N, Ponti A, Battista RN, Senore C, Rosso S, Shapiro SH, et al. A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes and Control 1991;2(4):239-46. [DOI: 10.1007/BF00052140] [DOI] [PubMed] [Google Scholar]
Sherman 2007 {published data only}
- Sherman SE, Estrada M, Lanto AB, Farmer MM, Aldana I. Effectiveness of an on-call counselor at increasing smoking treatment. Journal of General Internal Medicine 2007;22(8):1125-31. [DOI: 10.1007/s11606-007-0232-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2008 {published data only}
- Sherman SE, Takahashi N, Kalra P, Gifford E, Finney JW, Canfield J, et al. Care coordination to increase referrals to smoking cessation telephone counseling: a demonstration project. American Journal of Managed Care 2008;14(3):141-8. [PMID: ] [PubMed] [Google Scholar]
Siddiqi 2013 {published data only}
- Dogar O, Jawad M, Shah SK, Newell JN, Kanaan M, Khan MA, et al. Effect of cessation interventions on hookah smoking: post-hoc analysis of a cluster-randomized controlled trial. Nicotine and Tobacco Research 2014;16(6):682-8. [DOI] [PubMed] [Google Scholar]
- Siddiqi K, Khan A, Ahmad M, Dogar O, Kanaan M, Newell JN, et al. Action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trial. Annals of Internal Medicine 2013;158(9):667-75. [DOI: 10.7326/0003-4819-158-9-201305070-00006] [DOI] [PubMed] [Google Scholar]
- Siddiqi K, Khan A, Ahmand M, Rehman SU. An intervention to stop smoking among patients suspected of TB - evaluation of an integrated approach. BMC Public Health 2010;10:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sippel 1999 {published data only}
- Sippel JM, Osborne ML, Bjornson W, Goldberg B, Buist AS. Smoking cessation in primary care clinics. Journal of General Internal Medicine 1999;14(11):670-6. [DOI: 10.1046/j.1525-1497.1999.11088.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swartz 2006 {published data only}
- Swartz S, Cowan T, DePue J, Mooney-Murray K, Haskins AE, Leighton AR, et al. Academic detailing and data feedback to increase tobacco treatment in primary care: a randomized trial (as supplied 21 October 2015). Data on file.
- Swartz S, Cowan T. A randomized trial of academic profiling increases provider tobacco intervention. Nicotine and Tobacco Research 2006;9(1 Supplement 2):S135. [DOI: 10.1080/14622200601038651] [DOI] [Google Scholar]
Twardella 2007 {published data only}
- Brenner H, Twardella D. Best practice for smoking cessation in general practices - a RCT. Suchtkongress 2008;54(4):250-4. [Google Scholar]
- Brenner H, Twardella D. Giving up smoking with a general practitioner - results of a cluster-randomised study [Raucherentwohnung beim Hausarzt - Ergebnisse einer Cluster-randominisierten Studie]. Sucht 2006;52(1):63. [Google Scholar]
- Salize HJ, Merkel S, Reinhard I, Twardella D, Mann K, Brenner H. Cost-effective primary care-based strategies to improve smoking cessation: more value for money. Archives of Internal Medicine 2009;169(3):230-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Salize HJ, Reinhard I, Mann K, Twardella D, Brenner H. Cost-effectiveness of smoking cessation treatment in primary care. Suchtkongress 2008;54(4):250-4. [Google Scholar]
- Twardella D, Brenner H. Effects of practitioner education, practitioner payment and reimbursement of patients' drug costs on smoking cessation in primary care: a cluster randomised trial. Tobacco Control 2007;16(1):15-21. [DOI: 10.1136/tc.2006.016253] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardella D, Brenner H. New strategies for the promotion of smoking cessation in general practice: a cluster-randomised trial. Suchtmedizin in Forschung und Praxis 2003;5(2):142-4.
- Twardella D, Brenner H. Strategies to enhance smoking cessation in general practice: results of a cluster-randomized trial. European Journal of Epidemiology 2006;21:1-17. [DOI: 10.1007/s10654-006-9021-1] [DOI] [Google Scholar]
- Twardella D, Brenner H. Strategies to promote smoking cessation in general practice: results of a cluster-randomized trial. American Journal of Epidemiology 2005;161(Suppl):S105. [Google Scholar]
- Twardella D, Brenner H. Strategies to promote smoking cessation in general practise: results of a cluster-randomized trial. In: Weltkongress für Biomedizin. 2004:46A.
Unrod 2007 {published data only}
- Smith MY, Cromwell J, DePue J, Spring B, Redd W, Unrod M. Determining the cost-effectiveness of a computer-based smoking cessation intervention in primary care. Managed Care 2007;16(7):48-55. [PubMed]
- Unrod M, Smith M, Spring B, DePue J, Redd W, Winkel G. Randomized controlled trial of a computer-based, tailored intervention to increase smoking cessation counseling by primary care physicians. Journal of General Internal Medicine 2007;22(4):478-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rossem 2017 {published data only}
- Van Rossem C, Spigt M, Smit ES, Viechtbauer W, Mijnheer KK, Schayck CP, et al. Combining intensive practice nurse counselling or brief general practitioner advice with varenicline for smoking cessation in primary care: study protocol of a pragmatic randomized controlled trial. Contemporary Clinical Trials 2015;41:298-312. [PMID: 25657051] [DOI] [PubMed] [Google Scholar]
- Rossem C, Spigt M, Viechtbauer W, Lucas AEM, Schayck OCP, Kotz D. Effectiveness of intensive practice nurse counselling versus brief general practitioner advice, both combined with varenicline, for smoking cessation: a randomized pragmatic trial in primary care. Addiction 2017;112(12):2237-47. [DOI: 10.1111/add.13927] [DOI] [PubMed] [Google Scholar]
Verbiest 2014 {published data only}
- Verbiest ME, Crone MR, Scharloo M, Chavannes NH, Van der Meer V, Kaptein AA, et al. One-hour training for general practitioners in reducing the implementation gap of smoking cessation care: a cluster-randomized controlled trial. Nicotine and Tobacco Research 2014;16(1):1-10. [DOI: 10.1093/ntr/ntt100] [DOI] [PubMed] [Google Scholar]
- Verbiest ME, Presseau J, Chavannes NH, Scharloo M, Kaptein AA, Assendelft WJ, et al. Use of action planning to increase provision of smoking cessation care by general practitioners: role of plan specificity and enactment. Implementation Science 2014;9:180. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vetter 1990 {published data only}
- Vetter NJ, Ford D. Smoking prevention among people aged 60 and over: a randomized controlled trial. Age and Ageing 1990;19(3):164-8. [DOI] [PubMed] [Google Scholar]
Yano 2008 {published data only}
- Yano EM, Rubenstein LV, Farmer MM, Chernof BA, Mittman BS, Lanto AB, et al. Targeting primary care referrals to smoking cessation clinics does not improve quit rates: Implementing evidence-based interventions into practice. Health Research and Educational Trust 2008;43(5):1637-61. [DOI: 10.1111/j.1475-6773.2008.00865.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2008 {published data only}
- Young JM, Girgis S, Bruce TA, Hobbs M, Ward JE. Acceptability and effectiveness of opportunistic referral of smokers to telephone cessation advice from a nurse: a randomised trial in Australian general practice. BMC Family Practice 2008;9:16. [DOI: 10.1186/1471-2296-9-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwar 2015 {published data only}
- Zwar N, Richmond R, Halcomb E, Furler J, Smith J, Hermiz O, et al. Quit in general practice: a cluster randomised trial of enhanced in-practice support for smoking cessation. BMC Family Practice 2010;11:59. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwar N, Richmond R, Halcomb E, Furler J, Smith J, Hermiz O, et al. Supporting smoking cessation in primary care: results of the quit in general practice study. Respirology 2014;19:23.
- Zwar N, Richmond RL, Halcomb E, Furler J, Smith J, Hermiz O, et al. Supporting smoking cessation in Australian primary care: results of the quit in general practice study. Global Heart 2014;9(1 Supplement 1):e232.
- Zwar NA, Richmond RL, Halcomb EJ, Furler JS, Smith JP, Hermize O, et al. Quit in general practice: a cluster randomized trial of enhanced in-practice support for smoking cessationQuit in general practice: a cluster randomized trial of enhanced in-practice support for smoking cessation. Family Practice 2015;32(2):173-80. [DOI: 10.1093/fampra/cmu089] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adair 2013 {published data only}
- Adair R, Wholey DR, Christianson J, White KM, Britt H, Lee S. Improving chronic disease care by adding laypersons to the primary care team: a parallel randomized trial. Annals of Internal Medicine 2013;159(3):176-84. [DOI] [PubMed] [Google Scholar]
Adam 2019 {published data only}
- Adam A, Schwartz RP, Wu LT, Subramaniam G, Laska E, Sharma G, et al. Electronic self-administered screening for substance use in adult primary care patients: feasibility and acceptability of the tobacco, alcohol, prescription medication, and other substance use (myTAPS) screening tool. Addiction Science & Clinical Practice 2019;14(1):39. [DOI: 10.1186/s13722-019-0167-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agarwal 2018 {published data only}
- Agarwal SD, Kerwin M, Meindertsma J, Wolf AM. A novel decision aid to encourage smoking cessation among patients at an urban safety net clinic. Preventing Chronic Disease 2018;15:E124. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
An 2008 {published data only}
- An LC, Bluhm JH, Foldes SS, Alesci NL, Klatt CM, Center BA , et al. A randomized trial of a pay-for-performance program targeting clinician referral to a state tobacco quitline. Archives of Internal Medicine 2008;168(18):1993-9. [DOI: 10.1001/archinte.168.18.1993] [DOI] [PubMed] [Google Scholar]
Andrews 2001 {published data only}
- Andrews JO, Tingen MS, Waller JL, Harper RJ. Provider feedback improves adherence with AHCPR Smoking Cessation Guideline. Preventative Medicine 2001;33(5):415-21. [DOI] [PubMed] [Google Scholar]
Aveyard 2007 {published data only}
- Aveyard P, Brown K, Saunders C, Alexander A, Johnstone E, Munafò MR, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax 2007;62(10):898-903. [DOI: 10.1136/thx.2006.071837] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachmann 2019 {published data only}
- Bachmann MO, Bateman ED, Stelmach R, Cruz AA, Pacheco de Andrade M, et al. Effects of PACK guide training on the management of asthma and chronic obstructive pulmonary disease by primary care clinicians: a pragmatic cluster randomised controlled trial in Florianópolis, Brazil.. BMJ Global Health 2019;4(6):e001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakkevig 2000 {published data only}
- Bakkevig O, Steine S, Von Hafenbradl K, Laerum E. Smoking cessation: a comparative, randomised study between management in general practice and the behavioural programme SmokEnders. Scandinavian Journal of Primary Health Care 2000;18(4):247-51. [DOI] [PubMed] [Google Scholar]
Bentz 2007 {published data only}
- Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, Hunt JS, et al. Provider feedback to improve 5A's tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine and Tobacco Research 2007;9(3):341-9. [DOI: 10.1080/14622200701188828] [DOI] [PubMed] [Google Scholar]
Bosworth 2008 {published data only}
- Bosworth HB, Olsen MK, Neary A, Orr M, Grubber J, Svetkey L, et al. Take Control of Your Blood Pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Education and Counseling 2008;70(3):338-47. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burke 1993 {published data only}
- Burke P, Chivers A, Clements J, Dawes M, Eastwood I, Ebbs D, et al. Effectiveness of a nicotine patch in helping people stop smoking: results of a randomised trial in general practice. BMJ 1993;306(6888):1304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 1999 {published data only}
- Butler CC, Rollnick S, Cohen D, Bachmann M, Russell I, Stott N. Motivational consulting versus brief advice for smokers in general practice: a randomized trial. British Journal of General Practice 1999;49(445):611-6. [Google Scholar]
Carey 2016 {published data only}
- ACTRN12616001443482. Testing the effectiveness of point-of-care touchscreen computer assessment and printed feedback for improving self-management of health risks among general practice patients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616001443482 (first submitted 29 September 2016).
- Carey M, Sanson-Fisher R, Oldmeadow C, Mansfield E, Walsh J. Improving self-management of cancer risk factors, under screening for cancer and depression among general practice patients: study protocol of a randomised controlled trial. BMJ Open 2016;6:e014782. [DOI: 10.1136/bmjopen-2016-014782] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2019 {published data only}
- Cheung NW, Redfern J, Thiagalingam A, Hng TM, Islam SM, Haider R, et al. Text messaging support for patients with diabetes or coronary artery disease (SupportMe): protocol for a pragmatic randomised controlled trial. BMJ Open 2019;9:e025923. [DOI: 10.1136/bmjopen-2018-025923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cockburn 1992 {published data only}
- Cockburn J, Ruth D, Silagy C, Dobbin M, Reid Y, Scollo M, et al. Randomised trial of three approaches for marketing smoking cessation programmes to Australian general practitioners. BMJ 1992;304(6828):691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2011 {published data only}
- Cohen LB, Taveira TH, Khatana SA, Dooley AG, Pirraglia PA, Wu WC. Pharmacist-led shared medical appointments for multiple cardiovascular risk reduction in patients with type 2 diabetes. Diabetes Educator 2011;37(6):801-12. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coma 2019 {published data only}
- Coma E, Medina M, Méndez L, Hermosilla E, Iglesias M, Olmos C, et al. Effectiveness of electronic point-of-care reminders versus monthly feedback to improve adherence to 10 clinical recommendations in primary care: a cluster randomized clinical trial. BMC Medical Informatics and Decision Making 2019;19(1):245. [DOI: 10.1186/s12911-019-0976-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conger 1987 {published data only}
- Conger B, Nelson EC, Dietrich AJ. Effectiveness of physician antismoking advice. American Journal of Preventive Medicine 1987;3(4):223-6. [PubMed] [Google Scholar]
de Ruijter 2018 {published data only}
- Ruijter D, Candel M, Smit ES, Vries H, Hoving C. The effectiveness of a computer-tailored e-learning program for practice nurses to improve their adherence to smoking cessation counseling guidelines: randomized controlled trial. Journal of Medical Internet Research 2018;20(5):e193. [DOI: 10.2196/jmir.9276] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijter D, Hoving C, Evers S, Hudales R, Vries H, Smit E. An economic evaluation of a computer-tailored e-learning program to promote smoking cessation counseling guideline adherence among practice nurses. Patient Education and Counseling 2019;102(10):1802-11. [DOI: 10.1016/j.pec.2019.07.015] [DOI] [PubMed] [Google Scholar]
- Ruijter D, Smit ES, Vries H, Hoving C. Web-based computer-tailoring for practice nurses aimed to improve smoking cessation guideline adherence: a study protocol for a randomized controlled effectiveness trial. Contemporary Clinical Trials 2016;48:125-32. [DOI] [PubMed] [Google Scholar]
Dey 1999 {published data only}
- Dey P, Foy R, Woodman M, Fullard, B, Gibbs A. Should smoking cessation cost a packet? A pilot randomized controlled trial of the cost-effectiveness of distributing nicotine therapy free of charge. British Journal of General Practice 1999;49(439):127-8. [PMC free article] [PubMed] [Google Scholar]
Dickinson 2013 {published data only}
- Dickinson WP, Glasgow RE, Fisher L, Dickinson LM, Christensen SM, Estabrooks PA, et al. Use of a website to accomplish health behavior change: if you build it, will they come? And will it work if they do? Journal of the American Board of Family Medicine 2013;26(2):168-76. [DOI] [PubMed] [Google Scholar]
Dignan 2019 {published data only}
- Dignan MB, Jones K, Burhansstipanov L, Ahamed SI, Krebs LU, Williams D, et al. A randomized trial to reduce smoking among American Indians in South Dakota: the walking forward study. Contemporary Clinical Trials 2019;81:28-33. [DOI: 10.1016/j.cct.2019.04.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drexel 2011 {published data only}
- Drexel C, Jacobson A, Hanania NA, Whitfield B, Katz J, Sullivan T. Measuring the impact of a live, case-based, multiformat, interactive continuing medical education program on improving clinician knowledge and competency in evidence-based COPD care. International Journal of Chronic Obstructive Pulmonary Disease 2011;6:297-307. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubey 2006 {published data only}
- Dubey V, Mathew R, Iglar K, Moineddin R, Glazier R. Improving preventive service delivery at adult complete health check-ups: the Preventive Health Evidence-based Recommendation Form (PERFORM) cluster randomized controlled trial. BMC Family Practice 2006;7:44. [PMID: 16836761] [DOI] [PMC free article] [PubMed] [Google Scholar]
Efraimsson 2008 {published data only}
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self-care management education at a nurse-led primary health care clinic. Scandinavian Journal of Caring Sciences 2008;22(2):178-85. [DOI: 10.1111/j.1471-6712.2007.00510.x] [DOI] [PubMed] [Google Scholar]
Eikelenboom 2013 {published data only}
- Eikelenboom N, Van Lieshout J, Wensing M, Smeele I, Jacobs AE. Implementation of personalized self-management support using the self-management screening questionnaire SeMaS; a study protocol for a cluster randomized trial. Trials 2013;14:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lieshout J, Huntlink E, Koetsenruijter J, Wensing M. Tailored implementation of cardiovascular risk management in general practice: a cluster randomized trial. Implementation Science 2016;11:115. [DOI: 10.1186/s13012-016-0460-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Emery 2019 {published data only}
- Emery JD, Murray SR, Walter FM, Martin A, Goodall S, Mazza D, et al. The Chest Australia Trial: a randomised controlled trial of an intervention to increase consultation rates in smokers at risk of lung cancer. Thorax 2019;74(4):362-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Emmons 2014 {published data only}
- Emmons KM, Puleo E, Greaney ML, Gillman MW, Bennett GG, Haines J, et al. A randomized comparative effectiveness study of Healthy Directions 2-a multiple risk behavior intervention for primary care. Preventive Medicine 2014;64:96-102. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engle 2019 {published data only}
- Engle JL, Mermelstein R, Baker TB, Smith SS, Schlam TR, Piper ME, et al. Effects of motivation phase intervention components on quit attempts in smokers unwilling to quit: a factorial experiment. Drug and Alcohol Dependence 2019;197:149-57. [DOI: 10.1016/j.drugalcdep.2019.01.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Escortell‐Mayor 2020 {published data only}
- Escortell-Mayor E, Cura-González I, Ojeda-Ruiz E, Sanz-Cuesta T, Rodríguez-Salceda I, García-Soltero J, et al. A primary healthcare information intervention for communicating cardiovascular risk to patients with poorly controlled hypertension: the Education and Coronary Risk Evaluation (Educore) study - a pragmatic, cluster-randomized trial. PlOS One 2020;15(1):e0226398. [DOI: 10.1371/journal.pone.0226398] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Salceda I, Escortell-Mayor E, Rico-Blázquez M, Riesgo-Fuertes R, Asúnsolo-del Barco A, Valdivia-Pérez A, et al. EDUCORE project: a clinical trial, randomised by clusters, to assess the effectof a visual learning method on blood pressure control in the primary healthcare setting. BMC Public Health 2010;10:449. [DOI: 10.1186/1471-2458-10-449] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2000 {published data only}
- Etter JF, Rielle JC, Perneger TV. Labeling smokers' charts with a "smoker" sticker: results of a randomized controlled trial among private practitioners. Journal of General Internal Medicine 2000;15(6):421-4. [DOI: jgi04119 [pii]] [DOI] [PMC free article] [PubMed] [Google Scholar]
Felton 2019 {published data only}
- Felton WL, Kornstein SG, Huynh C, Masho SW, Gondwe T, Wallenborn JT. A stroke and cardiovascular disease risk alert in the electronic health record increased prescriptions for smoking cessation in women. Stroke 2019;50(Suppl 1):WP377. [DOI: 10.1161/str.50.suppl_1.WP377] [DOI] [Google Scholar]
Ferketich 2014 {published data only}
- Ferketich AK, Pennell M, Seiber EE, Wang L, Farietta T, Jin Y, et al. Provider-delivered tobacco dependence treatment to Medicaid smokers. Nicotine and Tobacco Research 2014;16(6):786-93. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrer 2009 {published data only}
- Ferrer RL, Mody-Bailey P, Jaen CR, Gott S, Araujo S. A medical assistant-based program to promote healthy behaviors in primary care. Annals of Family Medicine 2009;7(6):504-12. [DOI: 10.1370/afm.1059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2019 {published data only}
- Fiore M, Adsit R, Zehner M, McCarthy D, Lundsten S, Hartlaub P, et al. An electronic health record-based interoperable eReferral system to enhance smoking Quitline treatment in primary care. Journal of the American Medical Informatics Assiciation 2019;26(8-9):778-86. [DOI: 10.1093/jamia/ocz044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flocke 2014 {published data only}
- Flocke SA, Antognoli E, Step MM, Marsh S, Parran T, Mason MJ. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Services Research 2012;12:109. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flocke SA, Step MM, Antognoli E, Lawson, PJ, Smith S, Jackson B, et al. A randomized trial to evaluate primary care clinician training to use the teachable moment communication process for smoking cessation counseling. Preventive Medicine 2014;69:267-73. [DOI: 10.1016/j.ypmed.2014.10.020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Folz 2016 {published data only}
- Folz H, Murphy B, Byrd J, Day M. Assessing the impact of pharmacist interventions versus standard care in the outpatient management of chronic obstructive pulmonary disease. Journal of the American Pharmacists Association 2015;55(2):e200-1.
- Folz HN, Murphy BL. Implementation of an outpatient, pharmacist-directed clinic for chronic obstructive pulmonary disease. Excerpts in Pharmacy Research Journal 2016;2(1):1-5. [Google Scholar]
Frank 2004 {published data only}
- Frank O, Litt J, Beilby J. Opportunistic electronic reminders. Improving performance of preventive care in general practice. Australian Family Physician 2004;33(1-2):87-90. [PMID: ] [PubMed] [Google Scholar]
Fu 2015 {published data only}
- Fu SS, Roth C, Battaglia CT, Nelson DB, Farmer MM, Do T, et al. Training primary care clinicians in motivational interviewing: a comparison of two models. Patient Education and Counseling 2015;98(1):61-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fulton 2019 {published data only}
- Fulton E, Newby K, Gokal K, Kwah K, Schumacher L, Jackson LJ, et al. Tailored digital behaviour change intervention with e-referral system to increase attendance at NHS stop smoking services (the MyWay project): study protocol for a randomised controlled feasibility trial. BMJ Open 2019;9:e028721. [DOI: 10.1136/bmjopen-2018-028721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerbert 2003 {published data only}
- Gerbert B, Berg-Smith S, Mancuso M, Caspers N, McPhee S, Null D, et al. Using innovative video doctor technology in primary care to deliver brief smoking and alcohol intervention. Health Promotion Practice 2003;4(3):249-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gilbert 1989 {published data only}
- Gilbert JR, Wilson DM, Best JA, Taylor DW, Lindsay EA, Singer J, et al. Smoking cessation in primary care. A randomized controlled trial of nicotine-bearing chewing gum. Journal of Family Practice 1989;28(1):49-55. [PubMed] [Google Scholar]
Gilbert 1992 {published data only}
- Gilbert JR, Wilson DM, Singer J, Lindsay EA, Willms DG, Best JA, et al. A family physician smoking cessation program: an evaluation of the role of follow-up visits. American Journal of Preventive Medicine 1992;8(2):91-5. [PubMed] [Google Scholar]
Gilbert 2007 {published data only}
- Gilbert H, Nazareth I, Sutton S. Assessing the feasibility of proactive recruitment of smokers to an intervention in general practice for smoking cessation using computer-tailored feedback reports. Family Practice 2007;24(4):395-400. [DOI: 10.1093/fampra/cmm028] [DOI] [PubMed] [Google Scholar]
Gilbody 2019 {published data only}
- Gilbody S, Peckham E, Bailey D, Arundel C, Heron P, Crosland S, et al. Smoking cessation for people with severe mental illness (SCIMITAR+): a pragmatic randomised controlled trial. Lancet Psychiatry 2019;6(5):379-90. [DOI: 10.1016/S2215-0366(19)30047-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Godycki‐Cwirko 2014 {published data only}
- Godycki-Cwirko M, Zakowska I, Kosiek K, Wensing M, Krawczyk J, Kowalczyk A. Evaluation of a tailored implementation strategy to improve the management of patients with chronic obstructive pulmonary disease in primary care: a study protocol of a cluster randomized trial. Trials 2014;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2020 {published data only}
- Green E, Peterson KS, Markiewicz K, O'Brien J, Arring NM. Cautionary study on the effects of pay for performance on quality of care: a pilot randomised controlled trial using standardised patients. BMJ Quality and Safety 2020;29(8):664-71. [DOI: 10.1136/bmjqs-2019-010260] [DOI] [PubMed] [Google Scholar]
Grischott 2019 {published data only}
- Grischott T, Senn O, Rosemann T, Frei A, Cornuz J, Martin-Diener E, et al. Efficacy of motivating short interventions for smokers in primary care (COSMOS trial): study protocol for a cluster-RCT. Trials 2019;20:81. [DOI: 10.1186/s13063-018-3071-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Groner 2000 {published data only}
- Groner JA, Ahijevych K, Grossman LK, Rich LN. The impact of a brief intervention on maternal smoking behavior. Pediatrics 2000;105(1):267-71. [PubMed] [Google Scholar]
Hall 2003 {published data only}
- Hall S, Bishop AJ, Marteau TM. Increasing readiness to stop smoking in women undergoing cervical screening: evaluation of two leaflets. Nicotine and Tobacco Research 2003;5(6):821-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Harding 2019 {published data only}
- Harding R. A novel integrated person-centred palliative care intervention in primary care for patients with chronic lung disease and their families: a cluster stepped wedge feasibility hybrid type 2 RCT. www.isrctn.com/ISRCTN44976471 (date first received 27 March 2020).
Haug 1994 {published data only}
- Haug K, Fugelli P, Aaro LE, Foss OP. Is smoking intervention in general practice more successful among pregnant than non-pregnant women? Family Practice 1994;11(2):111-6. [DOI] [PubMed] [Google Scholar]
Houston 2015 {published data only}
- Houston TK, Sadasivam RS, Allison JJ, Ash AS, Ray MN, English TM, et al. Evaluating the QUIT-PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study. Implementation Science 2015;10:154. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston TK, Sadasivam RS, Ford DE, Richman J, Ray MN, Allison JJ. The QUIT-PRIMO provider-patient Internet-delivered smoking cessation referral intervention: a cluster-randomized comparative effectiveness trial: study protocol. Implementation Science 2010;5:87. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam RS, Delaughter K, Crenshaw K, Sobko HJ, Williams JH, Coley HL, et al. Development of an interactive, web-delivered system to increase provider-patient engagement in smoking cessation. Journal of Medical Internet Research 2011;13(4):e87. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivam RS, Hogan TP, Volkman JE, Smith BM, Coley HL, Williams JH, et al. Implementing point of care "e-referrals" in 137 clinics to increase access to a quit smoking internet system: the Quit-Primo and National Dental PBRN HI-QUIT Studies. Translational Behavioral Medicine 2013;3(4):370-8. [DOI: 10.1007/s13142-013-0230-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 1981 {published data only}
- Hughes GH, Hymowitz N, Ockene JK, Simon N, Vogt TM. The multiple risk factor intervention trial (MRFIT). V. Intervention on smoking. Preventive Medicine 1981;10(4):476-500. [DOI] [PubMed] [Google Scholar]
- Multiple Risk Factor Intervention Trial Research Group. Mortality after 10 1/2 years for hypertensive participants in the Multiple Risk Factor Intervention Trial. Circulation 1990;82(5):1616-28. [DOI] [PubMed] [Google Scholar]
Humphris 2004 {published data only}
- Humphris GM, Field EA. An oral cancer information leaflet for smokers in primary care: results from two randomised controlled trials. Community Dentistry and Oral Epidemiology 2004;32(2):143-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Imperial Cancer Research Fund GP Research Group {published data only}
- Imperial Cancer Research Fund General Practice Research Group. Effectiveness of a nicotine patch in helping people to stop smoking: results of a randomised trial in general practice. BMJ 1993;306(6888):1304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Randomised trial of nicotine patches in general practice: results at one year. BMJ 1994;308(6942):1476-7. [PMC free article] [PubMed] [Google Scholar]
Javitz 2004 {published data only}
- Jack LM, Swan GE, Thompson E, Curry SJ, McAfee T, Dacey S, et al. Bupropion SR and smoking cessation in actual practice: methods for recruitment, screening, and exclusion for a field trial in a managed-care setting. Preventive Medicine 2003;36(5):585-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker D, et al. Return on investment of different combinations of bupropion SR dose and behavioral treatment for smoking cessation in a health care setting: an employer's perspective. Value in Health 2004;7(5):535-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Javitz HS, Swan GE, Zbikowski SM, Curry SJ, McAfee TA, Decker DL, et al. Cost-effectiveness of different combinations of bupropion SR dose and behavioral treatment for smoking cessation: a societal perspective. American Journal of Managed Care 2004;10(3):217-26. [PMID: ] [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Archives of Internal Medicine 2003;163(19):2337-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jennings 2014 {published data only}
- Jennings C, Kotseva K, De Bacquer D, Hoes A, De Velasco J, Brusaferro S, et al. Effectiveness of a preventive cardiology programme for high CVD risk persistent smokers: the EUROACTION PLUS varenicline trial. European Heart Journal 2014;35(21):1411-20. [DOI: 10.1093/eurheartj/ehu051] [DOI] [PubMed] [Google Scholar]
- Jennings CS, Kotseva K, De Bacquer D, Hoes A, De Velasco J, Brusaferro S, et al. Nurses meet the challenge of helping high CVD risk smokers to quit with the help of varenicline in a preventive cardiology programme. Heart 2012;98:A78. [Google Scholar]
- Kotseva K, Jennings C, De Bacquer D, Hoes A, De Velasco J, Brusaferro S, et al. Euroaction Plus: a randomised controlled trial on preventive cardiology programme plus intensive smoking cessation with varenicline for vascular and high CVD risk smokers and their partners: principal results. Heart 2012;98(Suppl 1):A80-1. [DOI: 10.1136/heartjnl-2012-301877b.145] [DOI] [Google Scholar]
- Kotseva K, Jennings C, De Bacquer D, Hoes A, Velasco J, Brusaferro S, et al. EUROACTION PLUS: A randomized controlled trial on preventive cardiology programme plus intensive smoking cessation with Varenicline for vascular and high cardiovascular disease risk smokers and their partners. European Journal of Preventive Cardiology 2012;19(1 Suppl 1):S103. [Google Scholar]
- Thompson E, Jennings C, Kotseva K, De Bacquer D, Hoes A, De Velasco J, et al. Effectiveness of the EUROACTION PLUS (EA+) preventive cardiology programme for high CVD risk smokers in modifying dietary habits and anthropometric indices. European Heart Journal 2015;36(Suppl 1):475. [Google Scholar]
Jolly 2017 {published data only}
- Jolly K, Sidhu M, Hewitt C, Daley A, Jordan R, Coventry P, et al. Telephone health coaching in primary care patients with MRC I/II COPD: randomised controlled trial. European Respiratory Journal 2017;50(61):OA2914. [Google Scholar]
Kalkhoran 2016 {published data only}
- Kalkhoran S, Appelle NA, Napoles AM, Munoz RF, Lum PL, Alvarado N, et al. Beyond the ask and advise: implementation of a computer tablet Intervention to enhance provider adherence to the 5As for smoking cessation. Journal of Substance Abuse Treatment 2016;60:91-100. [DOI: 10.1016/j.jsat.2015.05.009] [DOI] [PMC free article] [PubMed]
- NCT02046408. Computer-facilitated 5A's for smoking cessation in primary care. clinicaltrials.gov/ct2/show/NCT02046408 (first received 23 January 2014).
Kalkhoran 2019 {published data only}
- Kalkhoran S, Inman EM, Kelley JHK, Ashburner JM, Rigotti NA. Proactive population health strategy to offer tobacco dependence treatment to smokers in a primary care practice network. Journal of General Internal Medicine 2019;34(8):1571-7. [DOI: 10.1007/s11606-019-05079-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamstrup‐Larsen 2019 {published data only}
- Kamstrup-Larsen N, Dalton SO, Grønbæk M, Broholm-Jørgensen M, Thomsen JL, Larsen LB, et al. The effectiveness of general practice-based health checks on health behaviour and incidence on non-communicable diseases in individuals with low socioeconomic position: a randomised controlled trial in Denmark. BMJ Open 2019;9(9):e029180. [DOI: 10.1136/bmjopen-2019-029180] [DOI] [PMC free article] [PubMed] [Google Scholar]
Karner 2012 {published data only}
- Karner A, Nilsson S, Jaarsma T, Andersson A, Wirehn AB, Wodlin P, et al. The effect of problem-based learning in patient education after an event of CORONARY heart disease--a randomised study in PRIMARY health care: design and methodology of the COR-PRIM study. BMC Family Practice 2012;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kastaun 2021 {published data only}
- Kastaun S, Leve V, Hildebrandt J, Funke C, Becker S, Lubisch D, et al. Effectiveness of training general practitioners to improve the implementation of brief stop-smoking advice in German primary care: study protocol of a pragmatic, 2-arm cluster randomised controlled trial (the ABCII trial). BMC Family Practice 2019;20:107. [DOI: 10.1186/s12875-019-0986-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastaun S, Leve V, Hildebrandt J, Funke C, Klosterhalfen, Lubisch D, et al. Training general practitioners in the ABC versus 5As method of delivering stop-smoking advice: a pragmatic, two-arm cluster randomised controlled trial. ERJ Open Research 2021;7(3):00621-2020. [DOI: 10.1183/23120541.00621-2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kennedy 2019 {published data only}
- Kennedy O, Su F, Pears R, Walmsley E, Roderick P. Evaluating the effectiveness of the NHS Health Check programme in South England: a quasi-randomised controlled trial. BMJ Open 2019;9(9):e029420. [DOI: 10.1136/bmjopen-2019-029420] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2020 {published data only}
- Kim N, McCarthy DE, Cook JW, Piper ME, Schlam TR, Baker TB. Time-varying effects of 'optimized smoking treatment' on craving, negative affect, and anhedonia. Addiction 2020;116(3):608-17. [DOI: 10.1111/add.15232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkman 1994 {published data only}
- Kirkman MS, Weinberger M, Landsman PB, Samsa GP, Shortliffe EA, Simel DL, et al. A telephone-delivered intervention for patients with NIDDM. Effect on coronary risk factors. Diabetes Care 1994;17(8):840-6. [DOI: 10.2337/diacare.17.8.840] [DOI] [PubMed] [Google Scholar]
Knight 1989 {published data only}
- Knight RA, Hay DA. The relevance of the health belief model to Australian smokers. Social Science and Medicine 1989;28(12):1311-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Krones 2010 {published data only}
- Krones T, Keller H, Becker A, Sonnichsen A, Baum E, Donner-Banzhoff N. The theory of planned behaviour in a randomized trial of a decision aid on cardiovascular risk prevention. Patient Education and Counseling 2010;78(2):169-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kruse 2020 {published data only}
- Kruse GR, Park E, Haberer JE, Abroms L, Shahid NN, Howard SE, et al. Proactive text messaging (GetReady2Quit) and nicotine replacement therapy to promote smoking cessation among smokers in primary care: a pilot randomized trial protocol. Contemporary Clinical Trials 2019;80:48-54. [DOI: 10.1016/j.cct.2019.03.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse GR, Park ER, Chang Y, Haberer JE, Abroms LC, Shahid NN, et al. Proactively offered text messages and mailed nicotine replacement therapy for smokers in primary care practices: a pilot randomized trial. Nicotine and Tobacco Research 2020;22(9):1509-14. [DOI: 10.1093/ntr/ntaa050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lasser 2013 {published data only}
- Lasser KE, Kenst KS, Quintiliani LM, Wiener RS, Murillo J, Pbert L, et al. Patient navigation to promote smoking cessation among low-income primary care patients: a pilot randomized controlled trial. Journal of Ethnicity in Substance Abuse 2013;12(4):374-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2019 {published data only}
- Leung MK, Bai D, Yip BH, Fong MY, Lai PM, Lai P, et al. Combined nicotine patch with gum versus nicotine patch alone in smoking cessation in Hong Kong primary care clinics: a randomised controlled trial. BMC Public Health 2019;19:1302. [DOI: 10.1186/s12889-019-7634-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2019 {published data only}
- Liang J, Abramson MJ, Russell G, Holland AE, Zwar NA, Bonevski B, et al. Interdisciplinary COPD intervention in primary care: a cluster randomised controlled trial. European Respiratory Journal 2019;53(4):1801530. [DOI: 10.1183/13993003.01530-2018] [DOI] [PubMed] [Google Scholar]
Liebmann 2019 {published data only}
- Liebmann EP, Preacher KJ, Richter KP, Cupertino AP, Catley D. Identifying pathways to quitting smoking via telemedicine-delivered care. Health Psychology 2019;38(7):638-47. [DOI: 10.1037/hea0000740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linder 2009 {published data only}
- Linder JA, Rigotti NA, Schneider LI, Kelley JH, Brawarsky P, Haas JS. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Archives of Internal Medicine 2009;169(8):781-7. [DOI: 10.1001/archinternmed.2009.53] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lycett 2010 {published data only}
- Lycett D, Hajek P, Aveyard P. Trial protocol: randomised controlled trial of the effects of very low calorie diet, modest dietary restriction, and sequential behavioural programme on hunger, urges to smoke, abstinence and weight gain in overweight smokers stopping smoking. Trials 2010;11:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett D. Weight Gain Associated with Smoking Cessation: A Cohort Analysis and Feasibility Trial for Dietary Management [thesis]. University of Birmingham, 2011. [ISRCTN: 83865809] [Google Scholar]
Machline‐Carrion 2019 {published data only}
- Machline-Carrion MJ, Soares RM, Damiani LP, Campos VB, Sampaio B, Fonseca FH, et al. Effect of a multifaceted quality improvement intervention on the prescription of evidence-based treatment in patients at high cardiovascular risk in Brazil: the BRIDGE cardiovascular prevention cluster randomized clinical trial. JAMA Cardiology 2019;4(5):408-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahapatra 2019 {published data only}
- Mahapatra S, Panda R. Effectiveness of face-to-face counseling with follow-up in primary care settings in India. Tobacco Induced Diseases 2018;16(1):843. [DOI: 10.18332/tid/84368] [DOI] [Google Scholar]
Markun 2018 {published data only}
- Markun S, Rosemann T, Dalla-Lana K, Steurer-Stey C. Care in Chronic Obstructive Lung Disease (CAROL): a randomised trial in general practice. European Respiratory Journal 2018;51(5):1701873. [DOI: 10.1183/13993003.01873-2017] [DOI] [PubMed] [Google Scholar]
- Steurer-Stey C, Markun S, Lana KD, Frei A, Held U, Wensing M, et al. The improving care in chronic obstructive lung disease study: CAROL improving processes of care and quality of life of COPD patients in primary care: study protocol for a randomized controlled trial. Trials 2014;15:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
McAlister 2009 {published data only}
- McAlister FA, Fradette M, Graham M, Majumdar SR, Ghali WA, Williams R, et al. A randomized trial to assess the impact of opinion leader endorsed evidence summaries on the use of secondary prevention strategies in patients with coronary artery disease: the ESP-CAD trial protocol. Implementation Science 2006;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister FA, Fradette M, Majumdar SR, Williams R, Graham M, McMeekin J, et al. The enhancing secondary prevention in coronary artery disease trial. Canadian Medical Association Journal 2009;181(12):897-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
McEwen 2002 {published data only}
- McEwen A, Preston A, West R. Effect of a GP desktop resource on smoking cessation activities of general practitioners. Addiction 2002;97(5):595-7. [DOI: 10.1046/j.1360-0443.2002.00113.x] [DOI] [PubMed] [Google Scholar]
McGrath 2014 {published data only}
McPhee 1991 {published data only}
- McPhee SJ, Bird JA, Fordham D, Rodnick JE, Osborn EH. Promoting cancer prevention activities by primary care physicians: results of a randomized, controlled trial. JAMA 1991;266(4):538-44. [PubMed] [Google Scholar]
McRee 2005 {published data only}
- McRee B, Granger J, Babor T, Feder I, Horn A, Steinberg J, et al. Reducing tobacco use and risky drinking in underserved populations: the need for better implementation models. Annals of Family Medicine 2005;3(Supplement 2):S58–60. [DOI: 10.1370/afm.362] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRee B, Granger J, Babor T, Feder I, Horn A Jr, Steinberg J, et al. Implementing screening and brief intervention models to reduce tobacco use and at-risk drinking in primary care clinics in the USA. Annals of Family Medicine 2005;3(2):S58-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McRobbie 2008 {published data only}
- McRobbie H, Hajek P, Feder G, Eldridge S. A cluster-randomised controlled trial of a brief training session to facilitate general practitioner referral to smoking cessation treatment. Tobacco Control 2008;17(3):173-6. [DOI: 10.1136/tc.2008.024802] [DOI] [PubMed] [Google Scholar]
Mehring 2014 {published data only}
- Mehring M, Haag M, Linde K, Wagenpfeil S, Schneider A. Effects of a guided web-based smoking cessation program with telephone counseling: a cluster randomized controlled trial. Journal of Medical Internet Research 2014;16(9):e218. [DOI: 10.2196/jmir.3536] [DOI] [PMC free article] [PubMed] [Google Scholar]
Minian 2019 {published data only}
- Minian N, Baliunas D, Noormohamed A, Zawertailo L, Giesbrecht N, Hendershot CS, et al. The effect of a clinical decision support system on prompting an intervention for risky alcohol use in a primary care smoking cessation program: a cluster randomized trial. Implementation Science 2019;14:85. [DOI: 10.1186/s13012-019-0935-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muckelbauer 2015 {published data only}
- Muckelbauer R, Englert H, Rieckmann N, Chen CM, Wegscheider K, Voller H, et al. Long-term effect of a low-intensity smoking intervention embedded in an adherence program for patients with hypercholesterolemia: randomized controlled trial. Preventive Medicine 2015;77:155-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Muller-Nordhorn J, Muckelbauer R, Englert H, Grittner U, Berger H, Sonntag F, et al. Longitudinal association between body mass index and health-related quality of life. PLOS One 2014;9(3):e93071. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willich SN, Englert H, Sonntag F, Voller H, Meyer-Sabellek W, Wegscheider K, et al. Impact of a compliance program on cholesterol control: results of the randomized ORBITAL study in 8108 patients treated with rosuvastatin. European Journal of Cardiovascular Prevention and Rehabilitation 2009;16(2):180-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Willich SN, Muller-Nordhorn J, Sonntag F, Voller H, Meyer-Sabellek W, Wegscheider K, et al. Economic evaluation of a compliance-enhancing intervention in patients with hypercholesterolemia: design and baseline results of the open label primary care study: rosuvastatin based compliance initiatives to achievements of LDL Goals (ORBITAL) study. American Heart Journal 2004;148(6):1060-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Naughton 2014 {published data only}
- Faulkner K, Sutton S, Jamison J, Sloan M, Boase S, Naughton F. Are nurses and auxiliary healthcare workers equally effective in delivering smoking cessation support in primary care? Nicotine and Tobacco Research 2016;18(5):1054-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton F, Jamison J, Boase S, Sloan M, Gilbert, H, Prevost AT, et al. Randomized controlled trial to assess the short-term effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care (iQuit in Practice). Addiction 2014;109(7):1184-93. [DOI: 10.1111/add.12556] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton S, Smith S, Jamison J, Boase S, Mason D, Prevost AT, et al. Study protocol for iQuit in Practice: a randomised controlled trial to assess the feasibility, acceptability and effectiveness of tailored web- and text-based facilitation of smoking cessation in primary care. BMC Public Health 2013;13:324. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01072422 {published data only}
- NCT01072422. Smoking cessation in primary health care patients with obstructive pulmonary disease. clinicaltrials.gov/ct2/show/NCT01072422 (first received 19 February 2010).
NCT03221010 {published data only}
- NCT03221010. Use of the motivational interviewing in the treatment of smokers in groups in primary health care. clinicaltrials.gov/ct2/show/NCT03221010 (first received 18 July 2017).
NCT04200534 {published data only}
- NCT04200534. Centralized Lung Cancer EARly Detection Among Smokers (CLEAR Study). clinicaltrials.gov/ct2/show/NCT04200534 (first received 16 December 2019).
NCT04316260 {published data only}
- NCT04316260. Development and testing of a smoking cessation e-visit for implementation in primary care. clinicaltrials.gov/ct2/show/NCT04316260 (first received 1 May 2019).
Neuner‐Jehle 2013 {published data only}
- Neuner-Jehle S, Knecht MI, Stey-Steurer C, Senn O. Acceptance and practicability of a visual communication tool in smoking cessation counselling: a randomised controlled trial. Primary Care Respiratory Journal 2013;22(4):412-6. [DOI: 10.4104/pcrj.2013.00086] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nilsson 1996 {published data only}
- Nilsson P, Lundgren H, Söderström M, Fagerström KO, Nilsson-Ehle P. Effects of smoking cessation on insulin and cardiovascular risk factors - a controlled study of 4 months' duration. Journal of Internal Medicine 1996;240(4):189-94. [DOI: 10.1046/j.1365-2796.1996.16844000.x.] [DOI] [PubMed] [Google Scholar]
Ojedokun 2013 {published data only}
- Ojedokun J. Lung age bio-feedback using a portable lung age meter with brief advice during routine consultations promote smoking cessation - Know2quit multicenter randomized control trial. Journal of General Practice 2013;1(3):1000123. [DOI: 10.4172/2329-9126.1000123] [DOI] [Google Scholar]
- Ojedokun S, Keane KO. The effect of lung age feedback with brief smoking cessation advice during routine consultations on smoking habit- Know2quit multicenter randomized control trial. European Journal of General Practice 2013;19:29-51. [Google Scholar]
Papadakis 2013 {published data only}
- Papadakis S, McDonald PW, Pipe AL, Letherdale ST, Reid RD, Brown KS. Effectiveness of telephone-based follow-up support delivered in combination with a multi-component smoking cessation intervention in family practice: a cluster-randomized trial. Preventive Medicine 2013;56(6):390-7. [DOI] [PubMed] [Google Scholar]
Parchman 2019 {published data only}
- Parchman M, Fagnan L, Dorr D, Evans P, Cook AJ, Penfold RB, et al. Study protocol for “Healthy Hearts Northwest”: a 2 × 2 randomized factorial trial to build quality improvement capacity in primary care. Implementation Science 2016;11:138. [DOI: 10.1186/s13012-016-0502-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchman ML, Anderson ML, Dorr DA, Fagnan LJ, O'Meara ES, Tuzzio L, et al. A randomized trial of external practice support to improve cardiovascular risk factors in primary care. Annals of Family Medicine 2019;17(1):S40-9. [DOI: 10.1370/afm.2407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peckham 2019 {published data only}
- Peckham E, Arundel C, Bailey D, Crosland S, Fairhurst C, Heron P, et al. A bespoke smoking cessation service compared with treatment as usual for people with severe mental ill health: the SCIMITAR+ RCT. Health Technology Assessment 2019;23(50):1-116. [DOI: 10.3310/hta23500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peprah 2019 {published data only}
- Ciolino JD, Jackson KL, Liss DT, Brown T, Walunas TL, Murakami L, et al. Design of healthy hearts in the heartland (H3): a practice-randomized, comparative effectiveness study. Contemporary Clinical Trials 2018;71:47-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Peprah YA, Brown T, Ciolino J, Hountz R, Iversen K, Murakami L, et al. Effect of practice facilitation on preventive cardiology in primary care: a practice-randomized, comparative effectiveness study. Journal of General Internal Medicine. Annual Meeting of the Society of General Internal Medicine. United States 2019;34(2 Suppl):S200-1. [Google Scholar]
- Persell SD, Liss DT, Walunas TL, Ciolino JD, Ahmad FS, Brown T, et al. Effects of 2 forms of practice facilitation on cardiovascular prevention in primary care: a practice-randomized, comparative effectiveness trial. Medical Care 2020;58(4):344-51. [DOI] [PubMed] [Google Scholar]
Persai 2020 {published data only}
- Persai D, Karan A, Panda R. Incremental benefits of multiple tobacco control interventions: a factorial randomized control trial. Asian Pacific Journal of Cancer Prevention 2020;21(7):1905-11. [DOI: 10.31557/APJCP.2020.21.7.1905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Persell 2013 {published data only}
- Persell SD, Lloyd-Jones DM, Friesema EM, Cooper AJ, Baker DW. Electronic health record-based patient identification and individualized mailed outreach for primary cardiovascular disease prevention: a cluster randomized trial. Journal of General Internal Medicine 2013;28(4):554-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pieterse 2001 {published data only}
- Pieterse ME, Seydel ER, DeVries H, Mudde AN, Kok GJ. Effectiveness of a minimal contact smoking cessation program for Dutch general practitioners: a randomized controlled trial. Preventative Medicine 2001;32(2):182-90. [DOI] [PubMed] [Google Scholar]
- Pieterse ME, Seydel ER, De Vries H. Diffusion of a minimal contact smoking cessation program for Dutch general practitioners. Psycho-oncology 1996;5:143-211. [Google Scholar]
Piper 2003 {published data only}
- Piper ME, Fiore MC, Smith SS, Jorenby DE, Wilson JR, Zehner ME, et al. Use of the vital sign stamp as a systematic screening tool to promote smoking cessation. Mayo Clinic Proceedings 2003;78(6):716-22. [DOI] [PubMed] [Google Scholar]
Prabhakaran 2019 {published data only}
- Prabhakaran D, Jha D, Prieto-Merino D, Roy A, Singh K, Ajay VS, et al. Effectiveness of an mHealth-based electronic decision support system for integrated management of chronic conditions in primary care: the mWellcare cluster-randomized controlled trial. Circulation 2019;139(3):380-91. [DOI: 10.1161/CIRCULATIONAHA.118.038192] [DOI] [PubMed] [Google Scholar]
Prochaska 2005 {published data only}
- Prochaska JO, Velicer WF, Redding C, Rossi JS, Goldstein M, DePu J, et al. Stage-based expert systems to guide a population of primary care patients to quit smoking, eat healthier, prevent skin cancer, and receive regular mammograms. Preventative Medicine 2005;41(2):406-16. [DOI: 10.1016/j.ypmed.2004.09.050] [DOI] [PubMed] [Google Scholar]
Prokhorov 2010 {published data only}
- Prokhorov AV, Hudmon KS, Marani S, Foxhall L, Ford KH, Luca NS, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Archives of Internal Medicine 2010;170(18):1640-6. [DOI: 10.1001/archinternmed.2010.344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Redfern 2014a {published data only}
- Redfern J, Usherwood T, Harris MF, Rodgers A, Hayman N, Panaretto K, et al. A randomised controlled trial of a consumer-focused e-health strategy for cardiovascular risk management in primary care: the Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) study protocol. BMJ Open 2014;4(2):e004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richmond 1985 {published data only}
- Richmond R, Webster I. Evaluation of general practitioners' use of a smoking intervention programme. International Journal of Epidemiology 1985;14(3):396-401. [DOI] [PubMed] [Google Scholar]
Richmond 1998 {published data only}
- Richmond C, Mendelsohn L, Kehoe L. Family physicians’ utilization of a brief smoking cessation program following reinforcement contact aftertraining: a randomized trial. Preventive Medicine 1998;27(1):77-83. [DOI: 10.1006/pmed.1997.0240] [DOI] [PubMed] [Google Scholar]
Richter 2015 {published data only}
- Mussulman L, Ellerbeck EF, Cupertino AP, Preacher KJ, Spaulding R, Catley D, et al. Design and participant characteristics of a randomized-controlled trial of telemedicine for smoking cessation among rural smokers. Contemporary Clinical Trials 2014;38(2):173-81. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, Shireman TI, Ellerbeck EF, Cupertino AP, Cately D, Cox LS, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. Journal of Medical Internet Research 2015;17(5):e113. [DOI: 10.2196/jmir.3975] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rigotti 2011 {published data only}
- Rigotti NA, Bitton A, Kelley JK, Hoeppner BB, Levy DE, Mort E. Offering population-based tobacco treatment in a healthcare setting: a randomized controlled trial. American Journal of Preventive Medicine 2011;41(5):498-503. [DOI: 10.1016/j.amepre.2011.07.022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Robson 1989 {published data only}
- Robson J, Boomla K, Fitzpatrick S, Jewell AJ, Taylor J, Self J, et al. Using nurses for preventive activities with computer assisted follow up: a randomised controlled trial. BMJ 1989;298(6671):433-6. [DOI: 10.1136/bmj.298.6671.433] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez Alverez 2008 {published data only}
- Rodriguez-Alvarez M, Torán-Monserrat P, Muñoz-Ortiz L, Negrete-Palma A, Montero-Alia JJ, Jiménez-González M, et al. Effectiveness of regular reporting of spirometric results combined with a smoking cessation advice by a primary care physician on smoking quit rate in adult smokers: a randomized controlled trial. ESPIROTAB study. BMC Family Practice 2011;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Alvarez MM, Zurilla LE, Muñoz O, I, Martinez GS, Montero Alia JJ, Negrete Palma A, et al. Role of the spirometry in the decrease of smoking habit. Preliminary results. Primary Care Respiratory Journal 2008;17(2):123. [Google Scholar]
Roski 1998 {published data only}
- Roski J, Jeddeloh R, Roemhild HF, Werb P, Koele R, Sherwood N. Implementing smoking cessation practice guidelines in primary care. Abstract Book Association for Health Services Research 1998;15:206. [Google Scholar]
Rosser 1992 {published data only}
- Rosser W, McDowell I, Newell C. Documenting smoking status: trial of three strategies. Canadian Family Physician 1992;38:1623-8. [PMC free article] [PubMed] [Google Scholar]
Rothemich 2008 {published data only}
- Rothemich SF, Woolf SH, Johnson RE, Burgett AE, Flores SK, Marsland DW, et al. Effect on cessation counseling of documenting smoking status as a routine vital sign: an ACORN study. Annals of Family Medicine 2008;6(1):60-8. [DOI: 10.1370/afm.750] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothemich 2010 {published data only}
- Rothemich SF, Woolf SH, Johnson RE, Devers KJ, Flores SK, Villars P, et al. Promoting primary care smoking-cessation support with quitlines: the QuitLink randomized controlled trial. American Journal of Preventive Medicine 2010;38(4):367-74. [DOI: 10.1016/j.amepre.2010.01.008] [DOI] [PubMed] [Google Scholar]
Sanders 1989 {published data only}
- Sanders D, Fowler G, Mant D, Fuller A, Jones L, Marzillier J. Randomized controlled trial of anti-smoking advice by nurses in general practice. Journal of the Royal College of General Practitioners 1989;39(324):273-6. [PMC free article] [PubMed] [Google Scholar]
Satterfield 2018 {published data only}
- Satterfield JM, Gregorich SE, Kalkhoran S, Lum PJ, Bloome J, Alvarado N, et al. Computer-facilitated 5A's for smoking cessation: a randomized trial of technology to promote provider adherence. American Journal of Preventive Medicine 2018;55(1):35-43. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwartz 2015 {published data only}
- Schwartz MD, Jensen A, Wang B, Bennett K, Dembitzer A, Strauss S, et al. Panel management to improve smoking and hypertension outcomes by VA primary care teams: a cluster-randomized controlled trial. Journal of General Internal Medicine 2015;30(7):916-23. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Jensen AE, Wang B, Bennett K, Dembitzer A, Strauss S, et al. The use of panel management assistants to improve smoking cessation and hypertension management by VA primary care teams: a cluster randomized controlled trial. Abstracts from the 37th Annual Meeting of the Society of General Internal Medicine. Journal of General Internal Medicine 2014;29(Suppl):S234. [DOI: 10.1007/s11606-014-2834-9] [DOI] [Google Scholar]
Sejourne 2010 {published data only}
- Sejourne C, Parot-Schinckel E, Rouquette A, Pare F, Delcroix M, Fanello S. Impact of exhaled CO measurement. A randomised study among 578 smoking patients in general practice [Evaluation de l'impact de la mesure du monoxyde de carbone (CO) dans l'air expire. Etude randomisee effectuee aupres de 578 fumeurs consultant en medecine generale]. Revue des maladies respiratoires 2010;27(3):213-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Senesael 2013 {published data only}
- Senesael E, Borgermans L, Van De Vijver E, Devroey D. Effectiveness of a quality improvement intervention targeting cardiovascular risk factors: are patients responsive to information and encouragement by mail or post? Journal of Vascular Health and Risk Management 2013;9:13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaughnessy 1987 {published data only}
- Shaughnessy AF, Davis RE, Reeder CE. Nicotine chewing gum: effectiveness and the influence of patient education in a family practice. Journal of Family Practice 1987;25(3):266-9. [PubMed] [Google Scholar]
Sheffer 2012 {published data only}
- Sheffer MA, Baker TB, Fraser DL, Adsit RT, McAfee TA, Fiore MC. Fax referrals, academic detailing, and tobacco quitline use: a randomized trial. American Journal of Preventative Medicine 2012;42(1):21-8. [DOI: 10.1016/j.amepre.2011.08.028] [DOI] [PubMed] [Google Scholar]
Shelley 2016 {published data only}
- Shelley DR, Ogedegbe G, Anane S, Wu WY, Goldfield K, Gold HT, et al. Testing the use of practice facilitation in a cluster randomized stepped-wedge design trial to improve adherence to cardiovascular disease prevention guidelines: HealthyHearts NYC. Implementation Science 2016;11:88. [DOI: 10.1186/s13012-016-0450-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherman 2017 {published data only}
- Sherman SE, Krebs P, York LS, Cummins SE, Kuschner W, Guvenc-Tuncturk S, et al. Telephone care co-ordination for tobacco cessation: randomised trials testing proactive versus reactive models. Tobacco Control 2017;27(1):78-82. [DOI: 10.1136/tobaccocontrol-2016-053327] [DOI] [PubMed] [Google Scholar]
Silveira 2019 {published data only}
- Silveira LC, Aliti GB, Da Silva EM, Pimentel RP, Gus M, Rabelo-Silva ER. Effect of motivational interviewing in hypertensive patients (MIdNIgHT): study protocol for a randomized controlled trial. Trials 2019;20(1):414. [DOI: 10.1186/s13063-019-3486-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 2004 {published data only}
- Silverman C, Majchrzak NE, Regan S, Conroy M, Chang Y, Schneider LI, et al. The effects of feedback and reimbursement on physicians rates od counseling about smoking: a controlled trial. Nicotine and Tobacco Research 2004;6(4):712. [Google Scholar]
Slama 1990 {published data only}
- Slama K, Redman S, Perkins J, Reid AL, Sanson-Fisher RW. The effectiveness of two smoking cessation programmes for use in general practice: a randomised clinical trial. BMJ 1990;300(6741):1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smit 2010 {published data only}
- Smit ES, Vries H, Hoving C. Effectiveness of a web-based multiple tailored smoking cessation program: a randomized controlled trial among Dutch adult smokers. Journal of Medical Internet Research 2012;14(3):e82. [DOI: 10.2196/jmir.1812] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit ES, Vries H, Hoving C. The PAS study: a randomized controlled trial evaluating the effectiveness of a web-based multiple tailored smoking cessation programme and tailored counselling by practice nurses. Contemporary Clinical Trials 2010;31(3):251-8. [NTR: 1351] [DOI] [PubMed] [Google Scholar]
Sperl‐Hillen 2018 {published data only}
- Sperl-Hillen JM, Crain AL, Margolis KL, Ekstrom HL, Appana D, Amundson G, et al. Clinical decision support directed to primary care patients and providers reduces cardiovascular risk: a randomized trial. Journal of the American Medical Informatics Association 2018;25(9):1137-46. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratelis 2006 {published data only}
- Stratelis G, Mölstad S, Jakobsson P, Zetterström O. Diagnosis of COPD combined with brief smoking cessation advice increases smoking cessation rate in comparison with those with normal lung function. Primary Care Respiratory Journal 2006;15:210. [DOI: 10.1016/j.pcrj.2006.04.180] [DOI] [Google Scholar]
- Stratelis G, Mölstad S, Jakobsson P, Zetterström O. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scandinavian Journal of Primary Health Care 2006;24(3):133-9. [DOI: 10.1080/02813430600819751] [DOI] [PubMed] [Google Scholar]
Strecher 1994 {published data only}
- Strecher VJ, Kreuter M, Den Boer DJ, Kobrin S, Hospers HJ, Skinner CS. The effects of computer-tailored smoking cessation messages in family practice settings. Journal of Family Practice 1994;39(3):262-70. [PubMed] [Google Scholar]
Taylor 2019 {published data only}
- Taylor G, Aveyard P, Bartlem K, Shaw A, Player J, Metcalfe C, et al. IntEgrating Smoking Cessation treatment As part of usual Psychological care for dEpression and anxiety (ESCAPE): protocol for a randomised and controlled, multicentre, acceptability, feasibility and implementation trial. Pilot Feasibility Studies 2019;5:16. [DOI: 10.1186/s40814-018-0385-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 1988 {published data only}
- Thompson RS, Michnich ME, Friedlander L, Gilson B, Grothaus LC, Storer B. Effectiveness of smoking cessation interventions integrated into primary care practice. Medical Care 1988;26(1):62-76. [DOI] [PubMed] [Google Scholar]
Thompson 2015 {published data only}
- Taylor AH, Thompson TP, Greaves CJ, Taylor RS, Green C, Warren FC, et al. A pilot randomised trial to assess the methods and procedures for evaluating the clinical effectiveness and cost-effectiveness of Exercise Assisted Reduction then Stop (EARS) among disadvantaged smokers. Health Technology Assessment 2014;18(4):1-324. [DOI: 10.3310/hta18040] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CJ, Greaves R, Ayres P, Aveyard FC, Warren R, Byng RS, et al. Lessons learned from recruiting socioeconomically disadvantaged smokers into a pilot randomized controlled trial to explore the role of Exercise Assisted Reduction then Stop (EARS) smoking. Trials 2015;16:1. [DOI: 10.1186/1745-6215-16-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson TP, Greaves CJ, Ayres R, Aveyard P, Warren FC, Byng R, et al. An exploratory analysis of the smoking and physical activity outcomes from a pilot randomized controlled trial of an exercise assisted reduction to stop smoking intervention in disadvantaged groups. Nicotine and Tobacco Research 2015;18(3):1-11. [DOI: 10.1093/ntr/ntv099] [DOI] [PubMed] [Google Scholar]
Valdivieso‐Lopez 2013 {published data only}
- Valdivieso-Lopez E, Flores-Mateo G, Molina-Gomez JD, Rey-Renones C, Barrera Uriarte ML, Duch J, et al. Efficacy of a mobile application for smoking cessation in young people: study protocol for a clustered, randomized trial. BMC Public Health 2013;13:704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velasquez 2014 {published data only}
- Velasquez MM, Von Sternberg K. Preventing alcohol and tobacco-exposed pregnancies in primary care settings: results of a randomized controlled trial. In: Alcoholism: Clinical and Experimental Research. 2014:343A.
Vidrine 2013 {published data only}
- Vidrine JI, Shete S, Cao Y, Greisinger A, Harmonson P, Sharp B, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Internal Medicine 2013;173(6):458-64. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Shete S, Li Y, Cao Y, Alford MH, Galindo-Talton M, et al. The Ask-Advise-Connect approach for smokers in a safety net healthcare system: a group-randomized trial. American Journal of Preventive Medicine 2013;45(6):737-41. [DOI: 10.1016/j.amepre.2013.07.011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogt 2009 {published data only}
- Vogt S, Hall M, Hankins M, Marteau TM. Evaluating three theory-based interventions to increase physicians recommendations of smoking cessation services. Health Psychology 2009;28(2):174-82. [DOI] [PubMed] [Google Scholar]
Voogdt‐Pruis 2011 {published data only}
- Voogdt-Pruis HR, Van Ree JW, Gorgels AP, Beusmans GH. Adherence to a guideline on cardiovascular prevention: a comparison between general practitioners and practice nurses. International Journal of Nursing Studies 2011;48(7):798-807. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vorderstrasse 2013 {published data only}
- Vorderstrasse AA, Ginsburg GS, Kraus WE, Maldonado MC, Wolever RQ. Health coaching and genomics-potential avenues to elicit behavior change in those at risk for chronic disease: protocol for personalized medicine effectiveness study in air force primary care. Global Advances in Health and Medicine 2013;2(3):26-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waage 1997 {published data only}
- Waage HP, Vatten LJ, Opedal E, Hilt B. Smoking intervention in subjects at risk of asbestos-related lung cancer. American Journal of Industrial Medicine 1997;31(6):705-12. [DOI] [PubMed] [Google Scholar]
Wadland 2007 {published data only}
- Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J. Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders. Annals of Family Medicine 2007;5(2):135-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weingarten 1989 {published data only}
- Weingarten MA, Bazel D, Shannon HS. Computerized protocol for preventive medicine: a controlled self-audit in family practice. Family Practice 1989;6(2):120-4. [DOI: 10.1093/fampra/6.2.120] [DOI] [PubMed] [Google Scholar]
West 1998 {published data only}
- West R, Edwards M, Hajek P. A randomized controlled trial of a "buddy" systems to improve success at giving up smoking in general practice. Addiction 1998;93(7):1007-11. [DOI] [PubMed] [Google Scholar]
Wilson 1982 {published data only}
- Wilson D, Wood G, Johnston N, Sicurella J. Randomized clinical trial of supportive follow-up for cigarette smokers in a family practice. Canadian Medical Association Journal 1982;126(2):127-9. [PMC free article] [PubMed] [Google Scholar]
Woollard 1995 {published data only}
- Woollard J, Beilin L, Lord T, Puddey I, MacAdam D, Rouse I. A controlled trial of nurse counselling on lifestyle change for hypertensives treated in general practice: preliminary results. Clinical and Experimental Pharmacology and Physiology 1995;22(6-7):466-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yalcin 2014 {published data only}
- Yalcin BM, Unal M, Pirdal H, Karahan TF. Effects of an anger management and stress control program on smoking cessation: a randomized controlled trial. Jounral of the American Board of Family Physicians 2014;27(5):645-60. [DOI] [PubMed] [Google Scholar]
Yingst 2018 {published data only}
- Yingst JM, Veldheer S, Hrabovsky S, Hammett E, Nicholson J, Berg A, et al. Pilot randomized trial of an automated smoking cessation intervention via mobile phone text messages as an adjunct to varenicline in primary care. Journal of Health Communication 2018;23(4):370-8. [DOI: 10.1080/10810730.2018.1453890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2002a {published data only}
- Young JM, Ward J. Can distance learning improve smoking cessation advice in family practice? A randomized trial. Journal of Continuing Education in the Health Professions 2002;22(2):84-93. [DOI: 10.1002/chp.1340220204] [DOI] [PubMed] [Google Scholar]
Young 2002b {published data only}
- Young JM, D'Este C, Ward JE. Improving family physicians' use of evidence-based smoking cessation strategies: a cluster randomization trial. Preventive Medicine 2002;35(6):572-83. [DOI: 10.1006/pmed.2002.1111] [DOI] [PubMed] [Google Scholar]
Ziyash 2019 {published data only}
- Ziyash T, Alima K. Studying the efficiency of motivating interview during the treatment of patients with cardiovascular diseases. Minerva Medica. Conference: 3rd International Scientific and Educational Conference "The Internationalization of Continuing Medical Education. Prospection". Kazakhstan 2019;110(1):49-50. [Google Scholar]
Zwar 2016 {published data only}
- Bunker JM, Reddel JK, Dennis SM, Middleton S, Van Schayck CP, Crockett AJ, et al. A pragmatic cluster randomized controlled trial of early intervention for chronic obstructive pulmonary disease by practice nurse-general practitioner teams: study protocol. Implementation Science 2012;7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwar NA, Bunker JM, Reddel HK, Dennis SM, Middleton S, Van Schayck OC, et al. Early intervention for chronic obstructive pulmonary disease by practice nurse and GP teams: a cluster randomized trial. Family Practice 2016;33(6):663-70. [DOI: 10.1093/fampra/cmw077] [DOI] [PubMed]
References to studies awaiting assessment
Martin‐Lujan 2011 {published data only}
- Martin-Lujan F, Pinol-Moreso JL, Martin-Vergara N, Basora-Gallisa J, Pascual-Palacios I, Sagarra-Alamo R, et al. Effectiveness of a structured motivational intervention including smoking cessation advice and spirometry information in the primary care setting: the ESPITAP study. BMC Public Health 2011;11:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Lujan 2016 {published data only}
- Martin-Lujan F, Santigosa-Ayala A, Pinol-Moreso JL, Sorli-Aguilar M, Flores-Mateo G, Bladé-Creixent J, et al. Multicentric randomized clinical trial to evaluate the long-term effectiveness of a motivational intervention against smoking, based on the information obtained from spirometry in primary care: the RESET study protocol. BMC Family Practice 2016;17:15. [DOI: 10.1186/s12875-016-0415-1] [DOI] [PMC free article] [PubMed]
- NCT02153047. Multicentric randomized clinical trial to evaluate the long-term effectiveness of a motivational intervention against smoking, based on the information obtained from spirometry in primary care. (RESET-ESPITAP2). clinicaltrials.gov/ct2/show/NCT02153047 (first received 28 May 2014).
Ripoll 2012 {published data only}
- Ripoll J, Girauta H, Ramos M, Medina-Bombardo D, Pastor A, Alvarez-Ossoria C, et al. Clinical trial on the efficacy of exhaled carbon monoxide measurement in smoking cessation in primary health care. BMC Public Health 2012;12:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2003 {published data only}
- Smith PO, Sheffer CE, Payne TJ, Applegate BW, Crews KM. Smoking cessation research in primary care treatment centers: the SCRIPT-MS project. American Journal of Medical Sciences 2003;326(4):238-41. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Avila‐Tomas 2019 {published data only}
- Avila-Tomas JF, Olano-Espinosa E, Minué-Lorenzo C, Martinez-Suberbiola FJ, Matilla-Pardo B, Serrano-Serrano ME, et al. Effectiveness of a chat-bot for the adult population to quit smoking: protocol of a pragmatic clinical trial in primary care (Dejal@). BMC Medical Informatics and Decision Making 2019;19(1):249. [DOI: 10.1186/s12911-019-0972-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bendtsen 2020 {published data only}
- Bendtsen M. Effects of a text messaging smoking cessation intervention among online help seekers and primary healthcare visitors in Sweden. //www.isrctn.com/ISRCTN13455271 (first received 27 July 2020).
Diaz‐Gete 2013 {published data only}
- Diaz-Gete L, Puigdomenech E, Briones EM, Fabregas-Escurriola M, Fernandez S, Val JL, et al. Effectiveness of an intensive E-mail based intervention in smoking cessation (TABATIC study): study protocol for a randomized controlled trial. BMC Public Health 2013;13:364. [DOI: 10.1186/1471-2458-13-364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerber 2017 {published data only}
- Gerber DE, Hamann HA, Santini NO, Abbara S, Chiu H, McGuire M, et al. Patient navigation for lung cancer screening in an urban safety-net system: protocol for a pragmatic randomized clinical trial. Contemporary Clinical Trials 2017;60:78-85. [DOI: 10.1016/j.cct.2017.07.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN38129107 {published data only}
- ISRCTN38129107. The coaching for smokers trial. www.isrctn.com/ISRCTN38129107 (first received 2 October 2017).
ISRCTN44559004 {published data only}
- ISRCTN44559004. iQuit in practice. www.isrctn.com/ISRCTN44559004 (first received 13 July 2016).
ISRCTN54228638 {published data only}
- ISRCTN54228638. Lung age or exhaled CO feedback combined with very brief advice and support for smoking cessation in FYR Macedonia. www.isrctn.com/ISRCTN54228638 (first received 7 September 2018).
Mak 2015 {published data only}
- Mak YW, Loke AY. The acceptance and commitment therapy for smoking cessation in the primary health care setting: a study protocol. BMC Public Health 2015;15:105. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02500589 {published data only}
- NCT02500589. Telephone-based smoking cessation. clinicaltrials.gov/ct2/show/NCT02500589 (first received 24 June 2015).
NCT03612804 {published data only}
- NCT03612804. Promoting smoking cessation in lung cancer screening through proactive treatment. clinicaltrials.gov/ct2/show/NCT03612804 (first received 2 August 2018).
NCT04188873 {published data only}
- NCT04188873. Cessation screening project. clinicaltrials.gov/ct2/show/NCT04188873 (first received 6 December 2019).
NCT04199117 {published data only}
- NCT04199117. Health systems reach interventions project. clinicaltrials.gov/ct2/show/NCT04199117 (first received 3 December 2020).
NCT04223336 {published data only}
- NCT04223336. A web-enabled integrated care pathway (ICP) for addressing multiple modifiable risk factors as a part of smoking cessation treatment in primary care settings. clinicaltrials.gov/ct2/show/NCT04223336 (first received 10 January 2020).
NCT04276116 {published data only}
- NCT04276116. Effectiveness of the evaluation and communication of "pulmonary age" as help for smoking cessation (CAPAST). clinicaltrials.gov/ct2/show/NCT04276116 (first received 19 February 2020).
Olano 2018 {published data only}
- Olano-Espinosa E, Minué-Lorenzo C, Martínez-Suverbiola J, Ávila-Tomás J. Effectiveness of a chat bot for smoking cessation: a pragmatic trial in primary care. Tobacco Prevention and Cessation 2018;4:A148. [DOI: 10.18332/tpc/90314] [DOI] [Google Scholar]
Parker 2013 {published data only}
- Parker DR, Eaton CB, Ahern DK, Roberts MB, Rafferty C Goldman RE, et al. The study design and rationale of the randomized controlled trial: translating COPD guidelines into primary care practice. BMC Family Practice 2013;14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Proctor 2020 {published data only}
- Proctor J, Naughton F, Sloan M, Hopewell S, Brimicombe J, Prevost AT, et al. Assessment of the effectiveness and cost-effectiveness of tailored web- and text-based smoking cessation support in primary care (iQuit in Practice II): protocol for a randomized controlled trial. JMIR Research Protocols 2020;9(7):e17160. [DOI: 10.2196/17160] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanchez‐Aguadero 2017 {published data only}
- Sanchez-Aguadero N, Mora-Simon S, Recio-Rodriguez JI, Alonso-Dominguez R, Gonzalez-Sanchez J, Martin-Martin C, et al. Effectiveness of an intensive intervention to improve lifestyles in people with intermediate cardiovascular risk (DATE study): study protocol for a randomized controlled trial. Journal of Advanced Nursing 2017;74(4):957-67. [DOI: 10.1111/jan.13503] [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 2004
- Anderson P, Jane-Llopis E. How can we increase the involvement of primary health care in the treatment of tobacco dependence? A meta-analysis. Addiction 2004;99(3):299-312. [DOI] [PubMed] [Google Scholar]
Aveyard 2012
- Aveyard P, Begh R, Parsons A, West R. Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance. Addiction 2012;107(6):1066-73. [DOI: ] [DOI] [PubMed] [Google Scholar]
Bartsch 2016
- Bartsch AL, Härter M, Niedrich J, Brutt AL, Buchholz A. A systematic literature review of self-reported smoking cessation counseling by primary care physicians. PLOS One 2016;11(12):e0168482. [DOI: 10.1371/journal.pone.0168482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baskerville 2001
- Baskerville NB, Hogg W, Lemelin J. The effect of cluster randomization on sample size in prevention research. Journal of Family Practice 2001;50(3):W241-6. [PubMed] [Google Scholar]
Black 2020
- Black N, Eisma MC, Viechtbauer W, Johnston M, West R, Hartmann-Boyce J, et al. Variability and effectiveness of comparator group interventions in smoking cessation trials: a systematic review and meta-analysis. Addiction 2020;115(9):1607-17. [DOI: 10.1111/add.14969] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyle 2014
- Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD008743. [DOI: 10.1002/14651858.CD008743.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2012
- Carson KV, Verbiest ME, Crone MR, Brinn MP, Esterman AJ, Assendelft WJJ, et al. Training health professionals in smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 5. Art. No: CD000214. [DOI: 10.1002/14651858.CD000214.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 2017
- Chamberlain C, O'Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No: CD001055. [DOI: 10.1002/14651858.CD001055.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clair 2019
- Clair C, Mueller Y, Livingstone‐Banks J, Burnand B, Camain JY, Cornuz J, et al. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD004705. [DOI: 10.1002/14651858.CD004705.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Claire 2020
- Claire R, Chamberlain C, Davey MA, Cooper SE, Berlin I, Leonardi-Bee J, et al. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD010078. [DOI: 10.1002/14651858.CD010078.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cromwell 1997
- Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. JAMA 1997;278(21):1759-66. [PubMed] [Google Scholar]
Fanshawe 2017
- Fanshawe TR, Halliwell W, Lindson N, Aveyard P, Livingstone-Banks J, Hartmann-Boyce J. Tobacco cessation interventions for young people. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD003289. [DOI: 10.1002/14651858.CD003289.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. U.S. Department of Health and Human Services 2008.
Franco 2007
- Franco OH, Der Kinderen AJ, De Laet C, Peeters A, Bonneux L. Primary prevention of cardiovascular disease: cost-effectiveness comparison. International Journal of Technology Assessment in Health Care 2007;23(1):71-9. [DOI] [PubMed] [Google Scholar]
Gaziano 2007
- Gaziano TA, Galea G, Reddy KS. Chronic diseases 2—scaling up interventions for chronic disease prevention: the evidence. Lancet 2007;370(9603):1939-46. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEproGDT. Version accessed 13 January 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Grimshaw 2001
- Grimshaw JM, Shirran L, Thomas R, Mowatt G, Fraser C, Bero L, et al. Changing provider behavior: an overview of systematic reviews of interventions. Medical Care 2001;39(8 Suppl 2):II2-45. [PubMed] [Google Scholar]
Hartmann‐Boyce 2019
- Hartmann‐Boyce J, Hong B, Livingstone‐Banks J, Wheat H, Fanshawe TR. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD009670. [DOI: 10.1002/14651858.CD009670.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Juni P, Moher D, Oxman AD, et al, Cochrane Bias Methods Group, Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Livingstone‐Banks 2019
- Livingstone-Banks J, Ordóñez-Mena JM, Hartmann-Boyce J. Print-based self-help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD001118. [DOI: 10.1002/14651858.CD001118.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Cantera 2015
- Martin-Cantera C, Puigdomènech E, Ballvé JL, Arias OL, Clemente L, Casas R, et al. Effectiveness of multicomponent interventions in primary healthcare settings to promote continuous smoking cessation in adults: a systematic review. BMJ Open 2015;5(10):e008807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Cantera 2020
- Martin-Cantera C, Iglesias Sanmartín JM, Furió Martínez A, Minué Lorenzo C, Barchilón Cohen V, Clemente Jiménez ML. Good practice regarding smoking cessation management in Spain: challenges and opportunities for primary care physicians and nurses. Tobacco Prevention and Cessation 2020;6:55. [DOI: 10.18332/tpc/126630] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papadakis 2010
- Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Preventive Medicine 2010;51(3-4):199-213. [DOI] [PubMed] [Google Scholar]
Papadakis 2014
- Papadakis S, Gharib M, Hambleton J, Reid RD, Assi R, Pipe AL. Delivering evidence-based smoking cessation treatment: the experience of Ontario Family Health Teams. Canadian Family Physician 2014;60:e362-71. [PMID: PMCID: PMC4096282] [PMC free article] [PubMed] [Google Scholar]
Reid 2019
- Reid JL, Hammond D, Tariq U, Burkhalter R, Rynard VL, Douglas O. Tobacco Use in Canada: Patterns and Trend. 2019 edition. Waterloo, ON: University of Waterloo, 2019. [Google Scholar]
Review Manager 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rice 2017
- Rice VH, Health L , Livingstone-Banks J, Hartmann-Boyce J. Nursing interventions for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 12. Art. No: CD001188. [DOI: 10.1002/14651858.CD001188.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roncker 2005
- Ronckers ET, Groot W, Ament AJ. Systematic review of economic evaluations of smoking cessation: standardizing the cost-effectiveness. Medical Decision Making 2005;25(4):437-48. [DOI: 10.1177/0272989X05278431] [DOI] [PubMed] [Google Scholar]
Royal College of Physicians 2018
- Royal College of Physicians. Hiding in plain sight: treating tobacco dependency in the NHS: a report by the Tobacco Advisory Group of the Royal College of Physicians. London: RCP 2018. [ISBN 978-1-86016-731-7]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (Editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Stead 2013
- Stead L, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD000165. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2016
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD008286. [DOI: 10.1002/14651858.CD008286.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tengs 1995
- Tengs TO, Adams ME, Pliskin JS, Safran DG, Siegel JE, Weinstein MC, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Analysis 1995;15(3):369-90. [DOI] [PubMed] [Google Scholar]
U.S. Department of Health and Human Services 2014
- US Department of Health and Human Services. 2014 Surgeon General's Report: the Health Consequences of Smoking—50 Years of Progress. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
U.S. Department of Health and Human Services 2020
- US Department of Health and Human Services. Smoking cessation: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2020. [Google Scholar]
Van den Brand 2017
- Van den Brand FA, Nagelhout GE, Reda AA, Winkens B, Evers SM, Kotz D, et al. Healthcare financing systems for increasing the use of tobacco dependence treatment. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD004305. [DOI: 10.1002/14651858.CD004305.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Rossem 2015
- Van Rossem C, Spigt MG, Kleijsen JR, Hendricx M, Van Schayck CP, Kotz D. Smoking cessation in primary care: exploration of barriers and solutions in current daily practice from the perspective of smokers and healthcare professionals. European Journal of General Practice 2015;21(2):111-7. [DOI: 10.3109/13814788.2014.990881] [DOI] [PubMed] [Google Scholar]
Verbiest 2017
- Verbiest M, Brakema E, Van der Kleij R, Sheals K, Allistone G, Williams S, et al. National guidelines for smoking cessation in primary care: a literature review and evidence analysis. NPJ Primary Care Respiratory Medicine 2017;27:2. [DOI: 10.1038/s41533-016-0004-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogt 2005
- Vogt F, Hall S, Marteau TM. General practitioners' and family physicians' negative beliefs and attitudes towards discussing smoking cessation with patients: a systematic review. Addiction 2005;100(10):1423-31. [DOI] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299-303. [DOI: 10.1111/j.1360-0443.2004.00995.x] [DOI] [PubMed] [Google Scholar]
World Health Organization 2008
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER package. Geneva: World Health Organization, 2008. [Google Scholar]
World Health Organization 2017
- World Health Organisation. WHO report on the global tobacco epidemic, 2017: monitoring tobacco use and prevention policies. World Health Organization: Geneva 2017. [ISBN 978-92-4-151282-4]
World Health Organization 2020
- World Health Organization. WHO report on the global tobacco epidemic 2019: offer help to quit tobacco use. Geneva: WHO, 2019. [ISBN: 978 92 4 151620 4] [Google Scholar]
Young 2001
- Young JM, Ward JE. Implementing guidelines for smoking cessation advice in Australian general practice: opinions, current practices, readiness to change and perceived barriers. Family Practice 2001;18(1):14-20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Papadakis 2015
- Papadakis S, Pipe A, Kelly S, Pritchard G, Wells GA. Strategies to improve the delivery of tobacco use treatment in primary care practice. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD011556. [DOI: 10.1002/14651858.CD011556] [DOI] [Google Scholar]


