Abstract
Background
A variety of minimally invasive treatments are available as an alternative to transurethral resection of the prostate (TURP) for management of lower urinary tract symptoms (LUTS) in men with benign prostatic hyperplasia (BPH). However, it is unclear which treatments provide better results.
Objectives
Our primary objective was to assess the comparative effectiveness of minimally invasive treatments for lower urinary tract symptoms in men with BPH through a network meta‐analysis. Our secondary objective was to obtain an estimate of relative ranking of these minimally invasive treatments, according to their effects.
Search methods
We performed a comprehensive search of multiple databases (CENTRAL, MEDLINE, Embase, Scopus, Web of Science and LILACS), trials registries, other sources of grey literature, and conference proceedings, up to 24 February 2021. We had no restrictions on language of publication or publication status.
Selection criteria
We included parallel‐group randomized controlled trials assessing the effects of the following minimally invasive treatments, compared to TURP or sham treatment, on men with moderate to severe LUTS due to BPH: convective radiofrequency water vapor therapy (CRFWVT); prostatic arterial embolization (PAE); prostatic urethral lift (PUL); temporary implantable nitinol device (TIND); and transurethral microwave thermotherapy (TUMT).
Data collection and analysis
Two review authors independently screened the literature, extracted data, and assessed risk of bias. We performed statistical analyses using a random‐effects model for pair‐wise comparisons and a frequentist network meta‐analysis for combined estimates. We interpreted them according to Cochrane methods. We considered a minimally important difference of three points for the International Prostate Symptoms Score[IPSS]. We used the GRADE approach to rate the certainty of evidence.
Main results
We included 27 trials involving 3017 men, mostly over age 50, with severe LUTS due to BPH. The overall certainty of evidence was low to very low due to concerns regarding bias, imprecision, inconsistency (heterogeneity), and incoherence. Based on the network meta‐analysis, results for our main outcomes were as follows.
Urologic symptoms (19 studies, 1847 participants): PUL and PAE may result in little to no difference in urologic symptoms scores compared to TURP (3 to 12 months; MD of IPSS range 0 to 35; higher scores indicate worse symptoms; PUL: 1.47, 95% CI ‐4.00 to 6.93; PAE: 1.55, 95% CI ‐1.23 to 4.33; low‐certainty evidence). CRFWVT, TUMT, and TIND may result in worse urologic symptoms scores compared to TURP at short‐term follow‐up, but the CIs include little to no difference (CRFWVT: 3.6, 95% CI ‐4.25 to 11.46; TUMT: 3.98, 95% CI 0.85 to 7.10; TIND: 7.5, 95% CI ‐0.68 to 15.69; low‐certainty evidence).
Quality of life (QoL) (13 studies, 1459 participants): All interventions may result in little to no difference in the QoL scores, compared to TURP (3 to 12 months; MD of IPSS‐QoL score; MD range 0 to 6; higher scores indicate worse symptoms; PUL: 0.06, 95% CI ‐1.17 to 1.30; PAE: 0.09, 95% CI ‐0.57 to 0.75; CRFWVT: 0.37, 95% CI ‐1.45 to 2.20; TUMT: 0.65, 95% CI ‐0.48 to 1.78; TIND: 0.87, 95% CI ‐1.04 to 2.79; low‐certainty evidence).
Major adverse events (15 studies, 1573 participants): TUMT probably results in a large reduction of major adverse events compared to TURP (RR 0.20, 95% CI 0.09 to 0.43; moderate‐certainty evidence). PUL, CRFWVT, TIND and PAE may also result in a large reduction in major adverse events, but CIs include substantial benefits and harms at three months to 36 months; PUL: RR 0.30, 95% CI 0.04 to 2.22; CRFWVT: RR 0.37, 95% CI 0.01 to 18.62; TIND: RR 0.52, 95% CI 0.01 to 24.46; PAE: RR 0.65, 95% CI 0.25 to 1.68; low‐certainty evidence).
Retreatment (10 studies, 799 participants): We are uncertain about the effects of PAE and PUL on retreatment compared to TURP (12 to 60 months; PUL: RR 2.39, 95% CI 0.51 to 11.1; PAE: RR 4.39, 95% CI 1.25 to 15.44; very low‐certainty evidence). TUMT may result in higher retreatment rates (RR 9.71, 95% CI 2.35 to 40.13; low‐certainty evidence). There was insufficient data to include data on CRFWVT and TIND in this analysis.
Erectile function (six studies, 640 participants): We are very uncertain of the effects of minimally invasive treatments on erectile function (MD of International Index of Erectile Function [IIEF‐5]; range 5 to 25; higher scores indicates better function; CRFWVT: 6.49, 95% CI ‐8.13 to 21.12; TIND: 5.19, 95% CI ‐9.36 to 19.74; PUL: 3.00, 95% CI ‐5.45 to 11.44; PAE: ‐0.03, 95% CI ‐6.38, 6.32; very low‐certainty evidence).
Ejaculatory dysfunction (eight studies, 461 participants): We are uncertain of the effects of PUL, PAE and TUMT on ejaculatory dysfunction compared to TURP (3 to 12 months; PUL: RR 0.05, 95 % CI 0.00 to 1.06; PAE: RR 0.35, 95% CI 0.13 to 0.92; TUMT: RR 0.34, 95% CI 0.17 to 0.68; low‐certainty evidence). There was insufficient data to include data on CRFWVT and TIND in this analysis.
TURP is the reference treatment with the highest likelihood of being the most efficacious for urinary symptoms, QoL and retreatment, but the least favorable in terms of major adverse events, erectile function and ejaculatory function. Among minimally invasive procedures with sufficient data for analysis, PUL and PAE have the highest likelihood of being the most efficacious for urinary symptoms and QoL, TUMT for major adverse events, PUL for retreatment, CRFWVT and TIND for erectile function and PUL for ejaculatory function.
Authors' conclusions
Minimally invasive treatments may result in similar or worse effects concerning urinary symptoms and QoL compared to TURP at short‐term follow‐up. They may also result in fewer major adverse events. PUL and PAE resulted in better rankings for symptoms scores and PUL may result in fewer retreatments, especially compared to TUMT, which had the highest retreatment rates. We are very uncertain about the effects of these interventions on erectile and ejaculatory function. There was limited long‐term data, especially for CRFWVT and TIND. Future high‐quality studies with more extended follow‐up, comparing different, active treatment modalities, and adequately reporting critical outcomes relevant to patients, including those related to sexual function, could provide more information on the relative effectiveness of these interventions.
Plain language summary
How do minimally invasive treatments compare to traditional surgery for treating lower urinary tract symptoms in men?
Background
Older men often suffer from urinary complaints such as frequent urination or a weak urine stream. If these symptoms can be blamed on an enlarged prostate gland and lifestyle changes and medications don't help enough, there are surgical procedures that may help. One such procedure is called transurethral resection of the prostate (traditional surgery). This traditional surgery has been widely used for a long time, and is known to work well, but it does require anesthesia and has several unwanted effects. Other 'minimally invasive' surgical procedures have become available. These procedures are said to work similarly well, but with fewer unwanted effects. The five minimally invasive procedures are 'prostatic urethral lift', 'convective radiofrequency water vapor therapy', 'transurethral microwave thermotherapy', 'prostatic arterial embolization', and 'temporary implantable nitinol device'.
Review question
We performed this review to compare five newer treatment forms for men with lower urinary tract symptoms to traditional surgery or 'sham surgery'. In sham surgery, men thought they were getting surgery but really did not have anything done.
Methods
We used recommended Cochrane methods and GRADE to rate the certainty of evidence. We also used a special statistical method called network meta‐analysis to compare different treatments.
Search date
The findings of our study are up‐to‐date until February 2021.
Included studies
We included 27 randomized controlled trials. In this type of study, random 'chance' determined whether men were assigned to receive one of the newer surgical procedures, or traditional surgery (or sham surgery). This method of assigning participants to 'intervention' or 'control' groups helps to reduce bias in research studies.
Men were mostly over 50 years of age and had severe urinary symptoms. Most studies (16 studies) used transurethral microwave thermotherapy. Eleven studies followed men for less than one year and nine studies followed men for one year. Only seven studies followed men for two years or longer.
Funding
Most studies did not report their funding sources, while others reported that those who paid for the study received at least some money for the company that made the device that was used.
Key results
We only report the results for what we thought were the three most important outcomes: urinary symptoms, urinary quality of life, and unwanted effects, comparing these treatments to traditional surgery. The review also includes information on several other outcomes and how they compared to sham surgery.
Prostatic urethral lift and arterial embolization may result in little to no difference in men's symptoms than traditional surgery in the short term (up to 12 months). The other minimally invasive interventions may result in worse symptom scores than traditional surgery at short‐term follow‐up, but there may be no difference. All treatments may result in little to no difference in the quality of life compared to traditional surgery at short‐term follow‐up. Transurethral microwave thermotherapy probably results in a large reduction in major adverse events compared to traditional surgery, whereas the other minimally invasive treatments may result in a large reduction in major adverse events. Transurethral microwave thermotherapy may result in higher retreatment rates, but we are uncertain about the other minimally invasive procedures. We are also uncertain of the effects of these interventions on erectile function and ejaculation.
Certainty of evidence
Our level of certainty about the evidence was different for each of the outcomes, but was mostly low or very low. This means that we cannot be sure that the results of this review are accurate. A common reason for grading down the certainty of evidence included flaws in the ways the studies were planned and conducted. Also, the results differed a lot among studies, and the results of studies were often imprecise.
Summary of findings
Background
Description of the condition
The prostate gland is an organ in males. It is approximately the size of a walnut, and is located below the urinary bladder encircling the urethra (Leissner 1979). Benign prostatic obstruction (BPO) is a form of bladder outlet obstruction and may be diagnosed when the cause of outlet obstruction is known to be benign prostatic enlargement (BPE) due to benign prostatic hyperplasia (BPH); however, the latter is restricted to the histological diagnosis, defined as increased numbers of epithelial and stromal cells in the prostate (Abrams 2003). BPH may or may not cause lower urinary tract symptoms (LUTS), characterized by urination frequency, hesitancy, and a weak stream, mainly in men over the age of 40, and receives clinical relevance when associated with perceived bother (Dunphy 2015). Symptom bother typically correlates with increased number and severity of symptoms, which are related to both the impairment in the quality of life and treatment‐seeking (Agarwal 2014). Although we understand that LUTS is a functional unit with a multi‐factorial etiology of associated symptoms, we considered the term BPH for this Cochrane Review due to its familiarity with the general public (EAU 2021).
The degree of bother across all LUTS can be assessed through self‐administered questionnaires, namely, the International Prostate Symptom Score (IPSS; also known as the American Urological Association [AUA] Symptom Index), which includes the quality of life domain (Barry 1995). Chapple 2017 reported that increasing LUTS severity was associated with worsening men's overall distress through the patient perception of the bladder condition, which is a single‐item global question (with responses ranging from 1 (causes no problems at all) to 6 (causes severe problems)).
Progression of LUTS has been observed in up to 31% of men with BPH at seven‐year follow‐up (Emberton 2008). Progression to acute urinary retention is less frequent, and in men with moderate symptoms can range from 3.0 per 1000 person‐years in those aged 40 to 49 years to 34.7 per 1000 person‐years in those aged 70 to 79 years (Emberton 2008). BPH also has a negative impact on public health and reduces a person's quality of life (Kozminski 2015; Martin 2014). In Europe, 30% of men over 50 years of age, equivalent to 26 million men, are affected by bothersome LUTS, including storage symptoms (such as urinary frequency, urgency, and nocturia) or voiding symptoms (such as urinary hesitancy, weak urinary stream, straining to void, and prolonged voiding), or both. The yearly reported associated number of medical prescriptions was estimated to be around 11.6 million for 74 million people at risk from 2004 to 2008 (Cornu 2010). According to an international study involving 7588 men, the prevalence of LUTS was 18% during their 40s, 29% in their 50s, 40% in their 60s, and 56% in their 70s (Homma 1997). More recent data show the lifetime prevalence of BPH as 26.2% (95% confidence interval (CI) 22.8% to 29.6%) (Lee 2017).
Diagnosis
Initial evaluation of LUTS suggestive of BPH includes patient history, physical examination including a digital rectal examination (DRE), urinalysis, a prostate‐specific antigen (PSA) blood test if a diagnosis of prostate cancer changes management, use of a voiding diary, and IPSS (EAU 2021; McVary 2011). A DRE is performed to assess both nodules suspicious for cancer and prostate size; recently, additional imaging studies have been recommended for patients considering surgical intervention (Foster 2019).
PSA is secreted by the prostate gland and is found to be abnormally elevated in conditions such as prostate cancer, BPH, infection, or inflammation of the prostate (EAU 2021; McVary 2011). The IPSS is used to assess urinary symptom severity and quality of life. It is also used to document subjective responses to treatment (Barry 1992; EAU 2021; McVary 2011). Measurement of maximum flow rate (Qmax) and postvoid residual (PVR) is often used in diagnosis and treatment decisions (EAU 2021; McVary 2011). A low Qmax and a large PVR predict an increased risk of symptom progression (Crawford 2006). Other tests such as radiological imaging, urodynamic evaluation, and cystoscopy can help the clinician determine appropriate treatment and predict treatment response (Egan 2016; McVary 2011).
Treatment
Treatment decisions are based on symptoms, and the degree of symptom bother noted by the patient. Initial treatment options for BPH include conservative management (watchful waiting and lifestyle modification) and the use of medications (alpha‐blockers, 5‐alpha reductase inhibitors, and, recently, phosphodiesterase inhibitors) (EAU 2021; McVary 2011). When patients have been refractory to conservative and medical treatment, or if BPH causes subsequent complications, such as acute urinary retention, recurrent urinary tract infection, bladder stones, haematuria, or renal insufficiency, surgical options are considered (EAU 2021; McVary 2011).
Until the 1970s, the only option available to treat this condition and relieve LUTS was open simple prostatectomy (in very large prostates) or endoscopic surgery in the form of transurethral prostatectomy, with the aim of removing or resecting prostatic tissue to open up the blocked urethra (Pariser 2015). Clinical guidelines continue to recommend monopolar or bipolar transurethral resection of the prostate (TURP) as a ('gold') reference standard treatment to provide subjective symptom relief while attaining objective improvement in urinary flow (Alexander 2019; EAU 2021; McVary 2011), but this procedure is associated with some morbidity and long‐term complications, including hematuria, possibly requiring a blood transfusion, urethral stricture, urinary tract infection, and incontinence, and it usually requires at least overnight hospitalisation. Moreover, men may experience ejaculatory (65%) and erectile dysfunction (10%) related to TURP (Roehrborn 2003). Furthermore, BPH is a disease that is common among elderly men, who have increased preoperative risk for complications of general anesthesia and surgery in general (Dunphy 2015; Yoo 2012).
Recently, several other minimally invasive treatments (MITs) that can be performed in an office setting and do not require general anesthesia have been developed as alternatives to TURP (EAU 2021; McVary 2011) to provide therapeutic alternatives involving lower morbidity. However, most men who consider surgical intervention do so with the expectation that this is a more definitive therapy for LUTS that will preclude the need for additional medical or surgical therapy. Given the relatively high rate of reoperation or continued use of medical therapy after surgical treatment (or both), concern has been raised about the durability of newly launched minimal invasive surgeries (NICE 2015; Strope 2015).
Description of the intervention
Minimally invasive treatments that can be performed in an office setting and do not require general anesthesia include convective radiofrequency water vapor therapy (CRFWVT), prostatic arterial embolization (PAE), prostatic urethral lift (PUL), a temporary implantable nitinol device (TIND), and transurethral microwave thermotherapy (TUMT).
Convective radiofrequency water vapor therapy
The Rezūm system (NxThera Inc., Maple Grove, MN, USA) uses radiofrequency to create thermal energy in the form of water vapor to ablate prostatic tissue (Woo 2017). This system consists of two main components: a radiofrequency power supply generator and a single‐use transurethral delivery device that incorporates a standard rigid cystoscope lens, which allows the procedure to be performed under direct visualization. Water vapor thermal energy is generated by applying a radiofrequency current against an inductive coil heater. The handheld control delivers water vapor, providing a consistent energy dose of ~ 208 calories into the prostate tissue through a retractable needle (Woo 2017). CRFWVT is performed with the person in the dorsal lithotomy position, using conscious sedation. A cystoscopic examination is performed to confirm the contours of the prostate and the planned distribution of thermal lesions (Darson 2017; Dixon 2015; Woo 2017). The treatment needle is positioned for starting approximately one centimeter distal from the bladder neck and targeting the transition and central prostate adenoma by eye. Each injection of water vapor lasts approximately nine seconds. Additional injections of vapor are delivered every one centimeter from the initial injection site of the prostatic urethra to the proximal edge of the verumontanum. The total number of injections in each lobe of the prostate is determined by the length of the prostatic urethra and the configuration of the prostate gland (Dixon 2015; Woo 2017). Saline flush irrigation is used to enhance visualization and to cool the urethral surface (Woo 2017). Although most adverse events are transient and are classified as Clavien‐Dindo Grade I or II, a non‐randomized pilot study has reported 125 adverse events in 45 of 64 participants (69.2%) (Dixon 2015). The most common adverse events are postoperative urinary retention (33.8%), dysuria (21.5%), urinary urgency (20%), and suspected urinary tract infection (20%). Twelve serious adverse events were reported in 10 participants, one of which was suspected to be a procedure‐ or device‐related adverse event (Clavien‐Dindo Grade IIIb urinary retention) (Dixon 2015).
Prostatic arterial embolization
Embolization of the prostatic arteries has historically been used to control persistent or massive prostatic bleeding not otherwise amenable for treatment, with typical causes being BPH and locally advanced prostate cancer, or to treat hemorrhage occurring after TURP (Mitchell 1976). DeMeritt 2000 reported a case in which PAE was performed with polyvinyl alcohol particles for BPH‐induced hematuria; hematuria was immediately stopped, and the patient reported symptomatic improvement of his BPH symptoms. These researchers also found that prostate size was reduced by 52% and 62% of the initial size at five‐month and 12‐month follow‐up, respectively. Carnevale 2010 reported positive preliminary results of PAE procedures with microspheres as a primary treatment in two patients with acute urinary retention due to BPH. For elderly patients with symptomatic BPH, PAE can be an alternative treatment performed by a femoral or radial artery puncture using conscious sedation instead of general anesthesia. This procedure is typically performed on an outpatient basis and usually does not require catheterization unless the patient is experiencing urinary retention (Wang 2015). In preparation for PAE, preoperative computed tomography or magnetic resonance angiography is typically performed to evaluate the pelvic artery anatomy. Digital subtraction angiography of the right and left internal iliac arteries is performed to assess the prostatic blood supply (Martins Pisco 2012). Super‐selective microcatheterization and embolization are then performed on the prostatic arteries. Embolization is typically performed to complete stasis (Carnevale 2010; Martins Pisco 2012; Wang 2015). Cone‐beam computed tomography can be used not only to help identify all prostatic arteries but also to identify and avoid embolization of vessels feeding adjacent pelvic structures (Wang 2015). Particle embolics are used almost exclusively, with wide variation in the type and size of particles (Carnevale 2010; DeMeritt 2000). Vasodilators to mitigate vasospasm once the prostatic artery is catheterized are also recommended by some researchers to avoid premature stasis (Martins Pisco 2012). Although the major complication rate is low (less than 1%) (Pisco 2016), perineal pain (9.4%), hematuria (9%), and acute urinary retention (7%) are commonly reported as complications of PAE (Feng 2017). The highest prevalence of acute urinary retention amongst the included studies was 28.4% (Wang 2015). Minor complications, such as hematospermia, rectal bleeding, urinary tract infection, inguinal hematoma, and transient urinary frequency are also reported (Feng 2017; Kuang 2017; Pyo 2017; Shim 2017). However, there is inconsistency in the reporting or classification of adverse events.
Prostatic urethral lift
Prostatic urethral lift (PUL), marketed commercially as UroLift (Teleflex Inc., Pleasanton, CA, USA), has recently become available in several countries and can be performed under local anesthesia with oral or intravenous sedation; it can also be performed in men with blood clotting disorders or in men receiving anticoagulant therapy. It is therefore being proposed and marketed for men at high risk of general anesthesia (Chin 2012; Woo 2012). Typical inclusion criteria for PUL include prostate volume between 20 mL and 70 mL, IPSS of 12 or greater, measured Qmax of 15 mL/s or less, and PVR of less than 350 mL (McNicholas 2016). The PUL system consists of two single‐use components (a delivery device and an implant). The delivery device consists of a handheld pistol grip to which a needle‐shaped probe is attached. Each PUL implant consists of a super‐elastic nitinol capsular tab, a polyethylene terephthalate monofilament, and a stainless steel urethral end piece. The surgeon inserts the probe into the urethra until it reaches the widest part of the prostatic urethra; a fine needle at the end of the probe then is deployed to secure an implant in a lobe of the prostate (McNicholas 2016). One end of the implant is anchored in the urethra, and the other is attached to the firm outer surface of the prostatic capsule, thus pulling the prostatic lobe away from the urethra. This is repeated on the other lobe of the prostate. Systematically, four implants for PUL are delivered — two each to the right and left lateral lobes of the prostate (at the 2 o'clock and 10 o'clock positions, distally, from approximately 1.5 cm distal to the bladder neck). PUL generally is not used to treat a hypertrophied median lobe of the prostate, which causes obstructive intravesical protrusion of the prostate (McNicholas 2016); however, a recent small observational study indicated that this might be feasible and effective (Rukstalis 2019). Mild adverse events, such as transient dysuria and haematuria, are commonly reported with PUL (Chin 2012; Woo 2012). Incontinence may be less prevalent with PUL (5%) than with TURP (11%) (NICE 2015). However, reoperation rates appear to be higher with PUL (8%) than with TURP (6%) (NICE 2015). In one feasibility study, implant encrustation occurred when PUL implants were placed too close to the bladder and were exposed to static urine (Chin 2012; Woo 2012).
Temporary implantable nitinol device
The temporary implantable nitinol device (TIND), commercially marketed as Medi‐Tate (Medi‐Tate Ltd., Hadera, Israel), is a novel device that aims to provide prostatic patency. This new minimally invasive procedure can be performed in an outpatient setting under light sedation. The device is placed inside the prostatic urethra via cystoscopy and is expanded upon release (Porpiglia 2015), reshaping the bladder neck and the prostatic urethra. No catheterization is required. The 50‐mm‐long, 33‐mm‐diameter device comprises three elongated struts and an anchoring leaflet ‐ all made of nitinol, a biocompatible super‐elastic shape memory alloy (Porpiglia 2015). The device is removed 5 days after placement in an outpatient setting under local anesthesia (lidocaine gel) with retraction via a cytoscope.
A single‐arm multi‐center observational study with 32 participants indicated that median IPSS scores decreased from 19 at baseline to 10 at three‐week follow‐up and to 9 at 12‐month follow‐up. Four patients suffered short‐term complications (urinary incontinence, urinary retention, urinary tract infection, and prostatic abscess) (Porpiglia 2015). A three‐year follow‐up indicated that IPSS scores reached a median of 12, and no further complications were reported (Porpiglia 2018).
A second‐generation TIND device (iTIND) with structural differences is currently available. Only three struts are used, and the upper part of the device allows action exerted on the urethral mucosa at the level of the bladder neck, with potential avoidance of bladder mucosal injury (Bertolo 2018). A single‐arm multi‐center observational study evaluating iTIND on 81 participants indicated that mean IPSS scores decreased from 22.5 ± 5.6 at baseline to 11.7 ± 8.0 at 1‐month follow‐up and to 8.8 ± 6.4 at 12‐month follow‐up. Only mild complications were reported: haematuria (12.3%), micturition urgency (11.1%), pain (9.9%), dysuria (7.4%), urinary tract infection (6.2%), and urinary retention (9.9%). Only one participant required re‐intervention in the form of TURP (Porpiglia 2019). At least two ongoing randomized controlled trials are evaluating this treatment (Bertolo 2018). Newer devices, such as the XFLO Expander system, have been tested in pilot studies, with promising results (Woo 2020).
Transurethral microwave thermotherapy
Transurethral microwave thermotherapy (TUMT) uses microwave‐induced heat to ablate prostatic tissue and is designed to have fewer major complications than TURP (Walmsley 2004). The patient is treated in an outpatient setting. Once the patient's bladder is emptied by straight catheterization, a local lidocaine gel is inserted for local anesthesia. The treatment catheter is then placed within the urethra, and this is confirmed by return of the sterile water and by transabdominal or transrectal ultrasound; then, the balloon is inflated. The catheter is composed of a curved tip, a temperature sensor, and a microwave unit. The distal port contains the bladder balloon, allowing for urine drainage and cooling. A rectal probe may be inserted and can be used to monitor rectal temperature (Rubeinstein 2003).
TUMT has evolved over the past decades. The first systems worked at lower energy or heat settings, and treatment would take around an hour with minimal discomfort; however, results were disappointing. Subsequent systems incorporated catheters that provided urethral cooling, thus allowing higher energy delivery. These advancements reduced the procedure time to around 30 minutes and improved outcomes. However, higher energy leads to greater discomfort during the procedure, for which patients often require sedation and analgesia and presents a risk for urinary retention (EAU 2021; Walmsley 2004).
How the intervention might work
Convective radiofrequency water vapor therapy
The Rezūm system directly transfers targeted and controlled convective thermal energy doses to the transition zone of the prostate gland to treat BPH by using sterile water vapor through tissue interstitial spaces between cells releases its stored thermal energy to create apoptosis and necrosis when in contact with hyperplastic prostatic tissue (Aoun 2015). Reportedly, no thermal effects are seen beyond the confines of the prostate, thereby leaving the urethra, bladder neck, and external sphincter unaffected (Aoun 2015; Woo 2017). In comparison, conductive ablation therapy can cause necrosis of surrounding tissues as higher temperatures and longer heating periods are required to achieve therapeutic effects (Woo 2017).
Prostatic arterial embolization
The underlying mechanism of PAE is the ischemia or hypoxia that induces apoptosis, necrosis, sclerosis, and prostatic shrinkage with cystic transformation of part, or all, of the gland, resulting in a softer gland with reduced compression of the urethra (DeMeritt 2000; Sun 2008). In addition, PAE may decrease the plasma concentration of free testosterone that enters prostate cells, thereby lowering dihydrotestosterone levels in the prostate. This may result in the secondary inhibition of prostate growth (Sun 2008). Ischemia or hypoxia may induce prostate cell death and necrosis with a decreased number of some receptors, such as alpha‐adrenergic receptors. Therefore, the neuromuscular tone may decrease, resulting in improved clinical symptoms associated with the dynamic pathological component of BPH (Zlotta 1997).
Prostatic urethral lift
The fundamental idea of PUL consists of the separation and distraction of enlarged prostatic tissue by a series of implants. The PUL system uses adjustable, permanent implants to hold excess prostatic tissue out of the way, thereby opening the narrowed urethra without cutting or removing enlarged prostatic tissue (McNicholas 2016). These implants are shaped as a double‐ended hook and aim to expand the opening of the urethra (McNicholas 2016).
Temporary implantable nitinol device
The fundamental principle of the TIND device involves 'reshaping' the prostatic urethra and bladder neck, thereby reducing urinary flow obstruction (Porpiglia 2015). This may be caused by the radial force of sustained expansion of the TIND device, causing ischemic necrosis of the tissue and leading to incision to the bladder neck and prostatic urethra.
Transurethral microwave thermotherapy
TUMT uses a special transurethral catheter that transmits heat into the prostate via electromagnetic radiation of microwaves, penetrating water‐rich tissue. Energy transferred by the microwave to the tissue in the form of heat induces coagulation necrosis, reducing prostatic volume. This mechanism may also cause denervation of receptors, decreasing the smooth muscle tone of the prostatic urethra (Walmsley 2004). Temperatures lower than 45º C seem ineffective in causing this effect; therefore, higher‐energy devices were developed to reach temperatures greater than 70º C, causing thermoablation of the prostatic tissue (Aoun 2015).
Why it is important to do this review
The Cochrane Urology Group has developed four reviews of studies comparing each MIT to TURP and other therapies (Franco 2021; Jung 2017; Jung 2019; Kang 2020); however, these reviews found few head‐to‐head comparisons. A recent systematic review and network meta‐analysis evaluated surgical therapies for BPH, but it covered only invasive therapies such as different forms of TURP and laser ablation (Huang 2019). We found no systematic review and network meta‐analysis to date that has used the same rigorous methods used in a Cochrane Review, which includes applying the GRADE approach and focusing on patient‐important outcomes (Guyatt 2008). A network meta‐analysis could improve the precision of estimates for each pair‐wise comparison, create estimates for which no head‐to‐head trial was found, and provide a ranking of available interventions (Chaimani 2021). In contemporary practice, with the availability of numerous MITs to treat BPH, the findings of this Cochrane Review are expected to be relevant to policymakers, healthcare providers, and patients.
Objectives
Primary
Our primary objective was to assess the comparative effectiveness of minimally invasive treatments for lower urinary tract symptoms in men with benign prostatic hyperplasia through a network meta‐analysis.
Secondary
To obtain an estimate of relative ranking of these minimally invasive treatments according to their effects.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomized controlled trials (RCTs) only to avoid threatening the transitivity assumption. We excluded cross‐over and cluster trials, as these study designs are not relevant in this setting. We excluded single‐armed studies, quasi‐randomized trials, and observational studies. We included RCTs regardless of their publication status or the language of publication.
Types of participants
We defined the eligible patient population as men over the age of 40 years with a prostate volume of 20 mL or greater (as assessed by DRE, ultrasound, and/or cross‐sectional imaging) with LUTS (determined by an IPSS of 8 or over), and a maximal urinary flow rate (Qmax) less than 15 mL/s (as measured by non‐invasive uroflowmetry, invasive pressure flow studies, or both) (Dunphy 2015; EAU 2021; McNicholas 2016; McVary 2011). The age limitation for this review was based on the observation that the prevalence of BPH is increased in middle‐aged and older men and that BPH is infrequent in younger men (Barry 1997; EAU 2021; Egan 2016). If these inclusion criteria had not been fully described, we would have performed a sensitivity analysis (see Sensitivity analysis).
We excluded trials of men with active urinary tract infection; bacterial prostatitis; chronic renal failure; untreated bladder calculi or large diverticula; prostate cancer; urethral stricture disease; or prior prostate, bladder neck, or urethral surgery. We excluded studies of men with other conditions that affect urinary symptoms, such as neurogenic bladder due to spinal cord injury, multiple sclerosis, or central nervous system disease.
We assessed the transitivity assumption by comparing the characteristics of participants and the distribution of potential effect modifiers, including age, prostate volume, and severity of LUTS.
Types of interventions
We included the following interventions.
Experimental interventions (decision set)
CRFWVT
PAE
PUL
TIND
TUMT
Comparator interventions (supplementary set)
Sham control (or no intervention)
TURP (monopolar or bipolar)
Comparisons
We predefined the structure of the network and its nodes in our protocol (Franco 2020). We included trials comparing experimental interventions versus comparator interventions or performing head‐to‐head comparisons between experimental interventions (the representation of each network is embedded in the figure accompanying the main outcomes of the review in the section Effects of interventions). We did not include the comparison of TURP versus sham control because our primary interest is the comparative effectiveness of minimally invasive treatments compared to TURP. Participants in the network could in principle be randomized to any of the methods being compared, and we verified this by comparing characteristics of study design, participants, interventions, and comparisons (Salanti 2012) while considering potential sources of clinical heterogeneity and effect modification (see Subgroup analysis and investigation of heterogeneity).
Types of outcome measures
We did not use measurement of the outcomes assessed in this review as an eligibility criterion.
Primary outcomes
Urological symptom scores
Quality of life
Major adverse events
Secondary outcomes
Retreatment
Erectile function
Ejaculatory function
Minor adverse events
Acute urinary retention
Indwelling urinary catheter
Method and timing of outcome measurement
We considered clinically important differences for all outcomes as the basis for rating the certainty of the evidence for imprecision in the 'Summary of findings' tables (Jaeschke 1989; Johnston 2013).
Urological symptom scores
Mean change measured as IPSS (also known as the AUA Symptom Index) or other validated scores (such as Madsen‐Iversen symptom scores). The latter would not be included in a network meta‐analysis (see Measures of treatment effect).
We considered an improvement in IPSS score of 3 points as a minimal clinically important difference (MCID) to assess the efficacy and comparative effectiveness (Barry 1995). If possible, we used different thresholds of MCID based on the severity of IPSS, with a threshold of 3 for mild LUTS, 5 for moderate LUTS, and 8 for severe LUTS (Barry 1995).
Quality of life
Mean change measured as IPSS‐quality of life.
No formal threshold was established for IPSS‐quality of life. We used an MCID of 1 to assess the efficacy and comparative effectiveness (Brasure 2016; Rees 2015).
Major adverse events
Examples include postoperative hemorrhage requiring admission or intervention.
We used the Clavien‐Dindo classification system to assess surgical complications and categorized Grade III, IV, and V complications as major (Dindo 2004).
Based on Guyatt 2011a, we considered a 25% relative change as the threshold for a clinically important difference.
Retreatment
Events requiring other surgical treatment modalities (e.g. TURP) after an intervention. We considered the first retreatment and accounted for repetitive events in a narrative synthesis.
Based on Guyatt 2011a, we considered a 25% relative change as the threshold for a clinically important difference.
Erectile function
Mean change, measured as the total score on the International Index of Erectile Function (IIEF)‐5 questionnaire (also known as the Sexual Health Inventory for Men) (Rosen 1997).
We considered a difference in IIEF‐5 over 5 points as the MCID (Spaliviero 2010).
Ejaculatory function
Mean change, measured on the Male Sexual Health Questionnaire for Ejaculatory Dysfunction (MSHQ‐EjD) (Rosen 2007).
We used an MCID of 25% improvement from baseline on the MSHQ‐EjD for ejaculatory function (Nickel 2015).
Minor adverse events
Examples include postoperative fever or pain requiring medication.
We used the Clavien–Dindo classification system to assess surgical complications and categorized Grade I and II complications as minor (Dindo 2004).
Based on Guyatt 2011a, we considered a 25% relative change as the threshold for a clinically important difference.
Acute urinary retention
Events requiring catheterization after intervention.
Based on Guyatt 2011a, we considered a 25% relative change as the threshold for a clinically important difference.
Indwelling urinary catheter
Proportion of participants with an indwelling catheter at postoperative 24 hours.
Based on Guyatt 2011a, we considered a 25% relative change as the threshold for a clinically important difference.
We considered outcomes measured up to 12 months after randomisation as short‐term and those later than 12 months as long‐term, for urological symptom scores, quality of life, retreatment, erectile function, ejaculatory function, minor adverse events, and acute urinary retention. We assessed major adverse events including short‐term and long‐term data and indwelling urinary catheter over the short term only.
The selection of patient‐important outcomes was based on the input of the clinical authors and their day‐to‐day practice; we did not formally involve men with BPH symptoms.
Main outcomes for 'Summary of findings' tables
We presented 'Summary of findings' tables reporting the following outcomes listed according to priority.
Urological symptom scores
Quality of life
Major adverse events
Retreatment
Erectile function
Ejaculatory function
Search methods for identification of studies
We performed a comprehensive search with no restrictions on language of publication or publication status.
Electronic searches
We retrieved relevant studies from existing Cochrane Reviews for each individual treatment (Franco 2021; Jung 2017; Jung 2019; Kang 2020). We updated searches for each of the individual Cochrane Reviews assessing each minimally invasive treatment. We performed a comprehensive search for TIND from the inception of each of the following databases (see Appendix 1).
-
Cochrane Library via Wiley (from inception until 24 February 2021)
Cochrane Database of Systematic Reviews
Cochrane Central Register of Controlled Trials
Database of Abstracts of Reviews of Effects
Health Technology Assessment Database
MEDLINE via Ovid (from 1946 until 24 February 2021)
Embase via Elsevier (from 1974 until 24 February 2021)
Scopus (from 1966 until 24 February 2021)
Web of Science (from 1900 until 24 February 2021)
Latin American and the Caribbean Health Sciences Literature (LILACS; www.bireme.br/, from 1982 until 24 February 2021)
We also searched the following on 24 February 24 2021.
ClinicalTrials.gov at the US National Institutes of Health (www.clinicaltrials.gov/)
World Health Organization (WHO) International Clinical Trials Registry Platform search portal (apps.who.int/trialsearch/)
Grey literature repository from the current Grey Literature Report (www.greylit.org/)
Searching other resources
We tried to identify other potentially eligible trials and ancillary publications by searching the reference lists of retrieved included trials, reviews, meta‐analyses, and health technology assessment reports. We contacted the study authors of included trials to identify further studies that we may have missed. We contacted drug/device manufacturers for ongoing or unpublished trials. We searched abstract proceedings of relevant meetings of the American Urological Association, the European Association of Urology, and the International Continence Society for 2018 to 2020 for unpublished studies (see Appendix 2).
Data collection and analysis
Selection of studies
We used Covidence to identify and remove potential duplicate records. Two review authors (JVAF, LG) scanned abstracts, titles, or both to determine which studies should be assessed further using the same software. Two review authors (JVAF, LG) investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies following the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). We resolved any discrepancies through consensus or recourse to a third review author (PD). We documented the reasons for exclusion. We presented a PRISMA flow diagram showing the process of study selection (Page 2021).
Data extraction and management
We developed a dedicated data abstraction form that we pilot‐tested ahead of time. Because we retrieved relevant studies from existing Cochrane Reviews for each individual treatment for which study characteristics, outcome data, and risk of bias assessments were done by members of our review team (Franco 2021; Jung 2017; Jung 2019; Kang 2020), the following sections apply only to new studies identified by our search methods.
For studies that fulfilled inclusion criteria, two review authors (of JVAF, LG, and JHJ) independently abstracted the following information.
Study design
Study dates
Study settings and country
Participant inclusion and exclusion criteria (e.g. age, baseline IPSS)
Participant details, baseline demographics (e.g. age, prostate size, IPSS)
Numbers of participants by study and by study arm
Details of relevant experimental intervention (e.g. size of the cystoscope, energy‐generating device, embolization agent, delivery device) and comparator intervention (e.g. monopolar versus bipolar energy, specifications of the sham procedure)
Definitions of relevant outcomes and methods (e.g. type of instrument, such as IPSS) and timing of outcome measurement (e.g. in months), as well as relevant subgroups (e.g. based on age, prostate volume, the severity of LUTS)
Study funding sources
Declarations of interest by primary investigators
We extracted outcome data relevant to this Cochrane Review as needed for the calculation of summary statistics and measures of variance. For dichotomous outcomes, we presented numbers of events and totals for populations in a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we obtained the means and standard deviations or data necessary to calculate this information.
We resolved any disagreements by discussion or, if required, by consultation with a third review author (PD).
In tables, we provided information about potentially relevant studies, including the trial identifiers.
We contacted the authors of included studies to obtain key missing data as needed.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (JVAF and LG) independently assessed the risk of bias of each included study. We resolved disagreements by consensus or by consultation with a third review author (PD). We presented a 'Risk of bias' summary figure to illustrate these findings. We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains, for each outcome in accordance with the approach for summary assessments of risk of bias presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of risk of bias in randomized controlled trials
We assessed the risk of bias using Cochrane's 'Risk of bias' assessment tool (Higgins 2011). We assessed the following domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias
We judged the risk of bias domains as 'low risk', 'high risk', or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook (Higgins 2011).
For selection bias (random sequence generation and allocation concealment), we evaluated the risk of bias at the trial level. For performance bias (blinding of participants and personnel), we considered all outcomes similarly susceptible to performance bias. For detection bias (blinding of outcome assessment), we grouped outcomes as susceptible to detection bias (subjective) or not susceptible to detection bias (objective) outcomes.
We defined the following endpoints as subjective outcomes.
Urological symptom scores
Quality of life
Major adverse events
Erectile function
Ejaculatory function
Minor adverse events
We defined the following endpoints as objective outcomes.
Retreatment
Acute urinary retention
Indwelling urinary catheter
We considered studies that compared MITs to TURP to be unblinded (at high risk of performance bias and detection bias for subjective outcomes). Studies that compared MITs to sham treatments and aimed to blind participants were considered at low risk of detection bias and also performance bias if personnel were also blinded. We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and we presented the judgement for each outcome separately when reporting our findings in 'Risk of bias' tables.
For reporting bias (selective reporting), we evaluated the risk of bias at a trial level.
Measures of treatment effect
Relative treatment effect
We expressed dichotomous data as risk ratios (RRs) with 95% confidence interval (CIs) to enhance the interpretability of results. We expressed continuous data as mean differences (MDs) with 95% CIs. We prioritized post‐intervention over change from baseline measurements. We anticipated that different scales might be used for urological symptom scores (e.g. Madsen symptom score in few older studies), in which case we included outcome data using the preferred scale for this outcome (i.e. IPSS) in order to preserve the transitivity of the network. In the presence of binary and continuous data for the same outcome, we performed analysis for continuous data. If this was not possible due to network geometry, we performed analysis for binary data.
Relative treatment ranking
We obtained a treatment hierarchy using P scores for all outcomes of the review (Rücker 2015). P scores allow describing the mean extent of certainty that the underlying treatment effect is larger than that of any other intervention.
Unit of analysis issues
The unit of analysis was the individual participant. When multiple trial arms are reported in a single trial, we included only the arms with comparisons relevant to prespecified nodes in our network.
Dealing with missing data
We obtained missing data (e.g. missing standard deviations) from study authors and performed intention‐to‐treat analyses if data were available. We investigated attrition rates (e.g. dropouts, losses to follow‐up, withdrawals) and critically appraised issues of missing data. We did not impute missing data.
Assessment of heterogeneity
Network meta‐analysis
Assessment of the transitivity assumption
Before conducting a network meta‐analysis, we assessed the transitivity assumption. Network meta‐analysis rests on the assumption of transitivity, that is, that effect modifiers have a comparable distribution across treatment comparisons in a network (Cipriani 2013; Jansen 2013). To assess the plausibility of this assumption, we visually inspected the comparability of distributions of age, prostate volume, and urological symptom score severity (IPSS), the time point of outcome assessment, and risk of bias (randomization, allocation concealment, and blinding to the risk of bias) as potential treatment effect modifiers across comparisons (Salanti 2014). We assessed the similarity of inclusion and exclusion criteria of all studies, including participants, treatments, and outcomes, to evaluate whether they impacted treatment effects.
Assessment of statistical consistency
Lack of transitivity in a network can threaten the validity of the consistency assumption, that is, the statistical agreement between direct and indirect evidence (Caldwell 2005; Lu 2004). Results can be misleading in the presence of inconsistency in the network. We evaluated the presence of inconsistency both locally and globally. We evaluated each network locally using the loop‐specific method by generating an inconsistency factor along with a 95% CI for each closed‐loop (Veroniki 2013). This way, we identified which piece of evidence would be responsible for inconsistency, and we explored this further. We also applied a global assessment for consistency in each network by applying the design‐by‐treatment interaction model (White 2012a). It has been shown that inconsistency tests have low power to detect true inconsistency (Song 2012; Veroniki 2014). Hence, we assessed transitivity even in the absence of evidence for inconsistency. If inconsistency was found, we followed the guidance provided in the Cochrane Handbook (Section 11.4.4.4; Chaimani 2021).
Pair‐wise meta‐analysis
We identified heterogeneity through visual inspection of forest plots to assess the overlap of CIs and the I² statistic, which quantifies between‐study variation across studies, to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003). We interpreted the I² statistic as follows (Deeks 2021).
0% to 40%: may not be important.
30% to 60%: may indicate moderate heterogeneity.
50% to 90%: may indicate substantial heterogeneity.
75% to 100%: considerable heterogeneity.
We also used Cochran’s Q test to assess for heterogeneity of estimated effect sizes from individual studies. However, we cautiously interpreted these results considering both the low power to detect true heterogeneity when the number of studies is small and the excessive power needed to detect negligible heterogeneity when the number of studies is high (Huedo‐Medina 2006; Pereira 2010).
Assessment of reporting biases
We attempted to obtain study protocols to assess for selective outcome reporting.
We used comparison‐adjusted funnel plots to assess small‐study effects (Chaimani 2013). Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials), and publication bias. We, therefore, interpreted these results carefully.
Data synthesis
Methods for indirect and network comparisons
We fitted a random‐effects network meta‐analysis model because we anticipated methodological and clinical heterogeneity across studies. We assumed a common within‐network heterogeneity estimate across comparisons, and we estimated this using the restricted maximum likelihood (REML) method (Veroniki 2016). This is a reasonable assumption, given that all treatments included in the network are of the same nature. An advantage of this approach is that treatment comparisons informed by a single study can borrow strength from the rest of the studies in the network (Higgins 1996; Salanti 2008). Each network meta‐analysis treatment effect estimate was presented along with a 95% CI and a 95% predictive interval (PrI) with reference to the standard treatment (TURP). A PrI is an interval within which the treatment effect estimate of a future study is expected to lie, accounting for both the uncertainty of the treatment effect and between‐study variance estimates (Higgins 2009; Riley 2011). We conducted a network meta‐analysis using the network suite of commands in Stata (STATA 2019; White 2012; White 2015).
Relative treatment ranking
We estimated the ranking probabilities that all treatments would be at each possible rank for each intervention. We used the surface under the cumulative ranking curve (SUCRA) to rank the effectiveness and safety of minimally invasive interventions (Salanti 2011). SUCRA accounts for both effect size magnitude and uncertainty around the underlying effect size. We displayed results (network plot, SUCRA plots and league table) using the 'network graph package' in Stata (STATA 2019; Chaimani 2015).
Methods for direct treatment comparisons
We performed analyses according to recommendations provided in Chapter 9 of the Cochrane Handbook (Deeks 2021), and we used Cochrane's statistical software, Review Manager 5 (Review Manager 2014), for analysis. When possible, we performed these standard pair‐wise meta‐analyses using a random‐effects model because we anticipated methodological and clinical heterogeneity across studies. We calculated corresponding 95% CIs for all analyses, and we graphically presented the results using forest plots. When trials were clinically too heterogeneous to be combined, we performed only subgroup analyses without calculating an overall estimate. In order to avoid duplication with the supporting reviews of this network meta‐analysis, we described only the pairwise comparisons for the data that could not be included in the network due to concerns about transitivity.
Subgroup analysis and investigation of heterogeneity
When we find important heterogeneity and/or inconsistency, we explored possible sources for primary outcomes. When sufficient studies are available, we performed subgroup analysis by using the following potential effect modifiers as possible sources of inconsistency and/or heterogeneity.
Patient age (younger than 65 years versus 65 years and older).
Prostate volume (≤ 40 mL or > 40 mL).
Severity of LUTS based on IPSS (score ≤ 19 (moderately symptomatic) versus > 19 (severely symptomatic)).
These subgroup analyses are based on the following observations.
Age is a well‐known risk factor for BPH surgery. Older people have a higher rate of postoperative complications compared with younger people (Bhojani 2014; Pariser 2015). The age cut‐off is based on the WHO definition of old age (WHO 2002).
Outcomes and complications of minimally invasive procedures, such as TURP, correlate with prostate volume (Reich 2008). Prostate volume cut‐off greater than 40 mL is based on this being the most commonly used threshold to distinguish 'small' from 'large' for the indication of treatment with a 5‐alpha reductase inhibitor (EAU 2021).
The relationship between changes in IPSS scores and patient global ratings of improvement is influenced by baseline scores (Barry 1995).
We planned to perform subgroup analyses limited to the primary outcomes.
Sensitivity analysis
We planned to perform sensitivity analyses limited to the primary outcomes to explore the influence of the following factors (when applicable) on effect size.
Restricting the analysis in RCTs by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk' (studies with at least one domain at 'high risk' or 'unclear risk' of bias for the analyzed outcome).
Restricting the analysis to RCTs with adequately described inclusion criteria (prostate size, age, IPSS value, and Qmax).
Summary of findings and assessment of the certainty of the evidence
We used 'Summary of findings' tables to summarize key results of the review, using the Confidence in Network Meta‐analysis (CINeMA) framework and software (Chaimani 2021; CINeMA 2017; Salanti 2014). We included the following outcomes.
Urological symptom scores
Quality of life
Major adverse events
Retreatment
Erectile function
Ejaculatory function
Our reference for the network meta‐analysis was TURP, considering that it is the reference treatment for all minimally invasive procedures. We used the five GRADE criteria (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to evaluate the quality of the body of evidence as it relates to studies that contributed data to the meta‐analysis for each pre‐specified outcome (Guyatt 2008). Two review authors (JVAF and LG) independently made judgments about the certainty of the evidence (high, moderate, low, or very low) and resolved disagreements by discussion or consultation with a third review author (PD). We created a 'Summary of findings' table for each outcome, using the approach presented by Yepes‐Nuñez 2019.
Results
Description of studies
Details of the included studies are presented in Characteristics of included studies and Table 7.
1. Baseline characteristics of included studies.
| Study name | Trial period | Country | Description of participants | Intervention(s) and comparator(s) |
Duration of follow‐up |
Age* | IPSS* | Prostate volume* |
| Convective radiofrequency water vapor therapy (CRFWVT) | ||||||||
| McVary 2016 | 2013‐2014 | USA | Men ≥ 50 years; symptomatic BPH with IPSS ≥ 13; Qmax 5‐15 mL/s voided volume ≥ 125 mL; prostate volume 30‐80 g | CRFWVT | 3 months | 63 ± 7.1 | 22 ± 4.8 | 45.8 ± 13.0 |
| Sham | 62.9 ± 7.0 | 21.9 ± 4.7 | 44.5 ± 13.3 | |||||
| Prostatic arterial embolization (PAE) | ||||||||
| Abt 2018 | 2014‐2017 | Switzerland | Men ≥ 40 years, refractory symptoms, prostate 25‐80 mL, with IPSS ≥ 8, IPSS‐QoL ≥ 3, with Qmax < 12 mL/s or urinary retention | PAE | 24 months | 65.7 ± 9.3 | 19.38 ± 6.37 | 52.8 ± 32.0 |
| TURP | 66.1 ± 9.8 | 17.59 ± 6.17 | 56.5 ± 31.1 | |||||
| Carnevale 2016 | 2010‐2012 | Brazil | Men > 45 years; IPSS > 19; refractory symptoms > 6 months; prostate 30‐90 mL; bladder outlet obstruction (urodynamic examination) | PAE | 12 months | 63.5 ± 8.7 | 25.3 ± 3.6 | 63.0 ± 17.8 |
| TURP | 66.4 ± 5.6 | 27.6 ± 3.2 | 56.6 ± 21.5 | |||||
| Gao 2014 | 2007‐2012 | China | Men with IPSS > 7 after failed medical therapy, prostate volume 20‐100 mL, Qmax < 15 mL/sec | PAE | 24 months | 67.7 ± 8.7 | 22.8 ± 5.9 | 64.7 ± 19.7 |
| TURP | 66.4 ± 7.8 | 23.1 ± 5.8 | 63.5 ± 18.6 | |||||
| Insausti 2020 | 2014‐2017 | Spain | Men > 60 years; LUTS refractory to medical treatment >6 months; IPSS ≥ 8; IPSS‐QoL ≥ 3; Qmax ≤ 10 mL/s or urinary retention | PAE |
12 months | 72.4 ± 6.2 |
25.8 ± 4.64 |
60.0 ± 21.6 |
| TURP | 71.8 ± 5.5 |
26.0 ± 7.29 |
62.8 ± 23.8 | |||||
| Pisco 2020 | 2014‐2018 | Portugal | Men > 45 years; severe LUTS; IPSS ≥ 20 and IPSS‐QoL ≥ 3 > 6 months' treatment with alpha‐blockers; Qmax < 12 mL/s; prostate volume 40 mL | PAE | 6 months | 64 | 25.5 | 63.5 |
| Sham | 64 | 27.5 | 66 | |||||
| Radwan 2020 | 2016‐2018 | Egypt | Men with LUTS with an IPSS score of 8 to 35, Qmax ≤ 10 mL/s; prostate volume < 100 mL | PAE | 6 months | 63.0 ± 7.2 | 27.0 ± 5.0 | 58.7 ± 23.4 |
| TURP | 62.0 ± 9.0 | 26.5 ± 4.0 | 60.1 ± 21.5 | |||||
| Zhu 2018 | 2016 | China | Men with comprehensive diagnosis of BPH through ultrasound prostate examination, digital rectal examination, IPSS, etc.; no absolute contraindication for surgery; no previous history of surgery; not taking 5‐alpha reductase inhibitors | PAE | 12 months | 61.1 ± 4.4 | 25.63 ± 4.28 | 81.21 ± 6.34 |
| TURP | 62.4 ± 4.9 | 26.22 ± 4.35 | 82.09 ± 6.47 | |||||
| Prostatic urethral lift (PUL) | ||||||||
| Gratzke 2017 | 2012‐2013 | Europe | Men ≥ 50 years with IPSS > 12, Qmax ≤ 15 mL/second for 125 mL voided volume, PRV < 350 mL, prostate volume ≤ 60 mL, sexually active, Incontinence Severity Index score ≤ 4 | PUL | 24 months | 63 ± 6.8 | 22 ± 5.7 | 38 ± 12 mL |
| TURP | 65 ± 6.4 | 23 ± 5.9 | 41 ± 13 mL | |||||
| Roehrborn 2013 | 2011 | 19 centres/US, Canada, and Australia | Men ≥ 50 years, AUASI ≥ 13, Qmax ≤ 12 mL/second with a 125 mL voided volume and a 30‐80 mL prostate volume | PUL | 3 months | 67 ± 8.6 | 22.2 ± 5.48 | 44.5 ± 12.4 mL |
| Sham | 65 ± 8.0 | 24.4 ± 5.75 | 40.9 ± 10.8 mL | |||||
| Temporary implantable nitinol device (TIND) | ||||||||
| Chughtai 2020 | 2015‐2018 | USA/Canada | Men ≥ 50 years; symptomatic BPH. IPSS ≥ 10, Qmax < 12 ml/sec; voided volume > 125 mL; prostate volume 25‐75 ml |
TIND | 3 months | 61.5 ± 6.5 | 22.1 ± 6.8 | 43.4 ± 15.5 |
| Sham | 60.1 ± 6.3 | 22.8 ± 6.2 | 43.8 ± 13.3 | |||||
| Transurethral microwave thermotherapy (TUMT) | ||||||||
| Abbou 1995 | N/A | France | Men ≥ 50 years with symptoms > 3 months, prostate 30‐80 g, Qmax < 15 mL/s, PVR < 300 mL | TUMT | 12 months | 65 ± 8 | N/A | 45 ± 15 |
| Sham | 66 ± 7 | N/A | 44 ± 11 | |||||
| Ahmed 1997 | N/A | UK | Men ≥ 55 years with AUA score >12 > 1‐year, prostate 25‐100 mL, Qmax < 15 mL/s and a PVR < 300 mL | TUMT | 6 months | 69.36 | 18.5 | 36.6 |
| TURP | 69.45 | 18.4 | 46.1 | |||||
| Albala 2002 | N/A | USA | Men 50‐80 years, AUA index > 13 and a bother score >11, Qmax < 12 mL/sec and PVR > 125 mL; prostate 30‐100 mL without a significant intravesical middle lobe | TUMT | 12 months | 65.2 ± 7.3 | 22.2 ± 5.0 | 50.5 ± 18.6 |
| Sham | 64.6 ± 7.1 | 22.7 ± 5.7 | 47.1 ± 17.9 | |||||
| Bdesha 1994 | N/A | UK | Men with prostatism (WHO score > 14), PVR > 50 mL, Qmax < 15 ml/s | TUMT | 3 months | 63.7 | 19.2 | N/A |
| Sham | 62.6 | 18.8 | N/A | |||||
| Blute 1996 | N/A | USA | Men suffering from urinary symptoms (Madsen Symptom score >8), PVR 10000 mL, Qmax < 10 mL/s, prostate length 30 – 50 mm | TUMT | 12 months | 66.9 ± 7.8 | 19.9 ± 7.2 | 37.4 ± 14.2 |
| Sham | 66.9 ± 7.1 | 20.8 ± 6.7 | 36.1 ± 13.4 | |||||
| Brehmer 1999 | N/A | Sweden | Men suffering from lower urinary tract symptoms and with an enlarged prostate | TUMT | 12 months | 70.4 | N/A | N/A |
| Sham | ||||||||
| D'Ancona 1998 | 1994‐1995 | Netherlands | Men ≥ 45 years with Madsen score > 8 months, prostate 2.5‐5 cm/30‐100 mL, Qmax < 15 mL/s PRV < 350 mL | TUMT | 24 months | 69.6 ± 8.5 | 16.7 ± 5.6 | 45 ± 15 |
| TURP | 69.3 ± 5.9 | 18.3 ± 6.3 | 43 ± 12 | |||||
| Dahlstrand 1995 | N/A | Sweden | Men ≥ 45 years with Madsen score > 8 months, prostate 3.5‐5 cm, Qmax < 15 mL/s PRV > 150 mL | TUMT | 24 months | 68 | N/A | 33 |
| TURP | 79 | N/A | 37 | |||||
| De Wildt 1996 | 1991‐1992 | Netherlands/UK | Men ≥ 45 years with Madsen score > 8 months, Qmax < 15 mL/s PRV > 150 mL | TUMT | 12 months | 63.3 ± 8.1 | N/A | 48.6 ± 16.6 |
| Sham | 66.9 ± 6.0 | N/A | 49.0 ± 20.0 | |||||
| Floratos 2001 | 1996‐1997 | Netherlands | Men ≥ 45 years, prostate ≥ 30 cm3, prostatic urethral length ≥ 25 mm, a Madsen symptom score ≥ 8, Qmax ≤ 15 ml/s, PVR ≤ 350 ml | TUMT | 36 months | 68 | 21 | 42 |
| TURP | 66 | 20 | 48 | |||||
| Larson 1998 | 1994‐1996 | USA | Men ≥ 45 years with AUA score > 9, enlarged prostate (3‐5 cm TRUS), Qmax < 12 mL/s without a significantly enlarged middle lobe | TUMT | 12 months | 66 | 20.8 | 38.1 |
| Sham | 65.9 | 21.3 | 44.7 | |||||
| Nawrocki 1997 | N/A | UK | Men with a Madsen symptom score ≥ 8, Qmax ≤ 15 ml/s, PVR > 150 ml, detrussor pressure > 70 cm H2O | TUMT | 6 months | 70 | 19 | 41.2 ± 14.6 |
| Sham | 17.5 | 46.7 ± 16.8 | ||||||
| Norby 2002 | 1996‐1997 | Denmark | Men ≥ 50 years, IPSS ≥ 7, Qmax ≤ 12 ml/s | TUMT | 6 months | 66 ± 7 | 20.5 ± 5.7 | 43 |
| TURP/TUIP | 68 ± 7 | 21.3 ± 6.6 | 44 | |||||
| Roehrborn 1998 | N/A | United States | Men ≥ 55 years, AUA‐SI ≥ 13, Qmax ≤ 12 ml/s, prostate volume 25‐100 mL | TUMT | 6 months | 66.3 ± 6.5 | 23.6 ± 5.6 | 48.1 ± 16.2 |
| Sham | 66.0 ± 5.8 | 23.9 ± 5.6 | 50.5 ± 18.1 | |||||
| Venn 1995 | N/A | UK | Men with a Madsen symptom score ≥ 8, PVR < 250 ml | TUMT | 6 months | 70.5 | 19.2 | 40.4 |
| Sham | 68 | 20.1 | 40.6 | |||||
| Wagrell 2002 | 1998‐1999 | Scandinavia/USA | Men IPSS ≥ 13, Qmax ≤ 13 ml/s, prostate volume 30‐100 mL | TUMT | 5 years | 67 ± 8 | 21.0 ± 5.4 | 48.9 ± 15.8 |
| TURP | 69 ± 8 | 20.4 ± 5.9 | 52.7 ± 17.3 | |||||
(*) mean/median, ± standard deviation when available. AUA‐SI/IPSS score: American Urological Association Symptom Index/International Prostate Symptom Score; BPH: benign prostatic hyperplasia; CRFWVT: convective radiofrequency water vapor therapy; LUTS: lower urinary tract symptoms; PAE: prostatic arterial embolization; PSA: prostate‐specific antigen; PUL: prostatic urethral lift; PVR: postvoid residual; Qmax: maximum flow rate; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
Results of the search
We retrieved 26 studies from the previous Cochrane reviews.
Seven studies (18 reports) from the PAE review (Jung 2020) — last updated on 28 September 2020
One study (17 reports) from the CRFWVT (Rezūm) review (Kang 2020) — last updated on 30 October 2020
Two studies (28 reports) from the PUL (UroLift) review (Jung 2019) — last updated on 28 October 2020
16 studies (37 reports) from the TUMT review (Franco 2021) — last updated on 31 May 2021
For the TIND search, we identified 469 records from electronic databases. We found no relevant records in the grey literature repository. After removing duplicates, we screened the titles and abstracts of the remaining 339 records, 331 of which we excluded. We assessed eight full‐text articles, and we excluded six records for various reasons. Finally, we included one study (two reports) in this review for this intervention. There were no ongoing studies for this intervention that met the inclusion criteria or were relevant to the review question. We have shown the flow of literature through the assessment process in the PRISMA flowchart (Figure 1).
1.
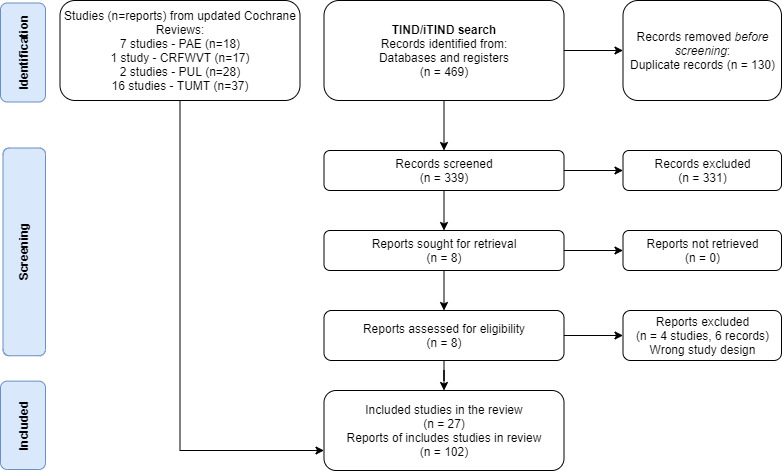
PRISMA 2020 flow diagram
Included studies
Study design and sample size
We included 27 trials with 3017 randomized participants. Their median sample size was 103 (interquartile range 61‐155).
Setting
The studies were conducted usually in tertiary hospitals, mostly in Europe, the USA and Canada, except for four PAE trials in China, Brazil, and Egypt. Most of the TUMT trials were conducted between 1991 and 1999, whereas the other interventions (CRFWVT, PUL, PAE, and TIND) took place between 2007 and 2018.
Participants
Most studies included men over 45 to 50 years old with moderate LUTS refractory to medical treatment; with a Qmax < 12/15 mL/s, a voided volume ≥ 125 mL and a prostate volume between 30/100 g to 60/100 g. Participants were usually screened for prostate cancer and infection, among other comorbidities, before inclusion.
Interventions and comparisons
We included trials with the following interventions and comparisons.
CRFWVT versus sham treatment (McVary 2016)
PAE versus sham treatment (Pisco 2020)
PAE versus TURP (Abt 2018; Carnevale 2016; Gao 2014; Insausti 2020; Radwan 2020; Zhu 2018)
PUL versus sham treatment (Gratzke 2017)
PUL versus TURP (Roehrborn 2013)
TIND versus sham treatment (Chughtai 2020)
TUMT versus sham treatment (Abbou 1995; Albala 2002; Bdesha 1994; Blute 1996; Brehmer 1999; De Wildt 1996; Larson 1998; Nawrocki 1997; Roehrborn 1998; Venn 1995)
TUMT versus TURP (Ahmed 1997; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Norby 2002; Wagrell 2002)
Outcomes
Most trials reported the primary outcomes of our review: urologic symptoms scores and quality of life (measured by IPSS and IPSS‐QoL) and major adverse events. Older trials assessing TUMT included other scales such as the Madsen‐Iversen symptom score, which is thoroughly described in one of our supporting reviews (Franco 2021). Retreatment rates were mostly reported narratively, and we had to analyze which ones constituted retreatment as defined in our review or retreatment as a major adverse event (i.e. retreatment due to a complication). Ejaculatory function and erectile function were usually reported in a subset of sexually active participants, contributing to the risk of bias due to attrition. We extracted both the IIEF‐5/IIEF scale and the MSHQ‐EjD scale, but since they were not consistently reported across studies, we also extracted data on the incidence of sexual dysfunction (i.e. erectile dysfunction and ejaculatory problems), for which we present the analysis using the continuous and dichotomous data. Other outcomes such as minor adverse events and acute urinary retention were also poorly reported across studies. The duration of indwelling urinary catheterization was only reported in two studies and described narratively as subsidiary to acute urinary retention.
Funding
Fourteen studies did not state their funding sources (Ahmed 1997; Albala 2002; Bdesha 1994; Blute 1996; Brehmer 1999; Carnevale 2016; D'Ancona 1998; Dahlstrand 1995; De Wildt 1996; Floratos 2001; Gao 2014; Radwan 2020; Venn 1995; Zhu 2018), nine studies were funded by the manufacturers or sponsors of the procedure (Chughtai 2020; Gratzke 2017; Insausti 2020; Larson 1998; McVary 2016; Pisco 2020; Roehrborn 1998; Roehrborn 2013; Wagrell 2002) and four studies were funded by public institutions or hospitals (Nawrocki 1997; Norby 2002; Abbou 1995; Abt 2018).
Excluded studies
For TIND we excluded two single‐arm studies (Porpiglia 2015; Porpiglia 2019), one case series (Lim 2011), and one study assessing the wrong intervention (Yachia 1996). For PUL we excluded a single‐arm study (Gratzke 2018). For PAE we excluded five studies due to a wrong study design (Bagla 2017; Brown 2018; NCT01835860; Pereira 2018; Qiu 2017). Another study was excluded due to wrong comparison (PAE versus simple prostatectomy, Russo 2015). Another report was a letter to the editor (Bilhim 2015). For CRFWVT we excluded one educational lecture from a conference (Woo 2018). For TUMT, we excluded 22 studies for the following reasons: two studies addressed transrectal thermotherapy (Zerbib 1992; Zerbib 1994; Albala 2000), three studies provided economic data on published trials (Kobelt 2004; Norby 2002b; Waldén 1998), two were cross‐over studies with insufficient data (Albala 2000; Tan 2005), nine were observational studies and other non‐randomized comparisons (Arai 2000; D'Ancona 1997; Hahn 2000; Hansen 1998; Mulvin 1994; Ohigashi 2007; Servadio 1987; Trock 2004; Vesely 2006), two were review articles identified through full‐text assessment (Dahlstrand 2003; Nørby 2004), three had an ineligible comparison (Djavan 1999; Schelin 2006; Shore 2010) and one was a terminated study (ISRCTN23921450).
Ongoing trials
We have identified six ongoing trials assessing the effects of PAE (ACTRN12617001235392; NCT02006303; NCT02566551; NCT04236687) and PUL (NCT04178811; NCT04338776).
Risk of bias in included studies
See Characteristics of included studies for a full description of the risk of bias assessment by study and outcome.
Allocation
Random sequence generation
We identified 14 studies that adequately described the random sequence generation (mostly using electronic systems, random numbers tables, random permuted blocks) and were rated as having a low risk of bias (Abbou 1995; Abt 2018; Blute 1996; Chughtai 2020; Gao 2014; Gratzke 2017; Insausti 2020; McVary 2016; Nawrocki 1997; Pisco 2020; Roehrborn 1998; Roehrborn 2013; Venn 1995; Zhu 2018). The remaining studies were rated as unclear risk of bias as they did not provide sufficient information for judgement.
Allocation concealment
We rated eight studies as having a low risk of bias, mostly by using a centralized allocation using software (Abt 2018; Blute 1996; Chughtai 2020; Gratzke 2017; McVary 2016; Pisco 2020; Roehrborn 1998; Roehrborn 2013). Two studies used inadequate methods to conceal allocation or had evidence of possible tampering of the process (Ahmed 1997; Nawrocki 1997). The remaining studies were rated as having an unclear risk of bias due to a lack of information on the allocation method.
Blinding
Blinding of participants and personnel
Minimally invasive treatments versus sham treatment
While the eight studies were rated as low risk of bias due to blinding of participants and personnel (Blute 1996; Nawrocki 1997; Roehrborn 1998; Abbou 1995; Bdesha 1994; Chughtai 2020; De Wildt 1996; Larson 1998), three studies were rated as high risk of bias due to lack of blinding of study personnel (McVary 2016; Pisco 2020; Roehrborn 2013). Three studies did not adequately describe blinding methods (Albala 2002; Brehmer 1999; Venn 1995).
Minimally invasive treatments versus TURP
All 13 studies were judged as having a high risk of bias given lack of assurance of appropriate methods of blinding of participants and personnel considering the nature of the comparison (Abt 2018; Ahmed 1997; Carnevale 2016; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Gao 2014; Gratzke 2017; Insausti 2020; Norby 2002; Radwan 2020; Wagrell 2002; Zhu 2018).
Blinding of outcome assessment
Minimally invasive treatments versus sham treatment
Subjective outcomes (urologic symptom scores, quality of life, major adverse events, erectile function, ejaculatory disorders, and minor adverse events): All 14 studies were considered to be at low risk of bias since participants were blinded (Abbou 1995; Albala 2002; Bdesha 1994; Blute 1996; Brehmer 1999; Chughtai 2020; De Wildt 1996; Larson 1998; McVary 2016; Nawrocki 1997; Pisco 2020; Roehrborn 1998; Roehrborn 2013; Venn 1995)
Objective outcomes (re‐treatment, acute urinary retention, indwelling urinary catheter, and hospital stay): we rated all studies as having a low risk of bias for these outcomes as they were unlikely to be affected by lack of blinding (ascertaining this does not involve judgement)
Minimally invasive treatments versus TURP
Subjective outcomes (urologic symptom scores, quality of life, major adverse events, erectile function, ejaculatory disorders, and minor adverse events): we judged all 13 studies as having a high risk of bias given lack of assurance of appropriate methods of blinding considering the nature of the comparison (Abt 2018; Ahmed 1997; Carnevale 2016; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Gao 2014; Gratzke 2017; Insausti 2020; Norby 2002; Radwan 2020; Wagrell 2002; Zhu 2018).
Objective outcomes (retreatment, acute urinary retention, indwelling urinary catheter, and hospital stay): we rated all studies as having a low risk of bias for these outcomes as they were unlikely to be affected by lack of blinding (ascertaining this does not involve judgement).
Incomplete outcome data
Urologic symptoms score/quality of life
Short‐term follow‐up: Six studies were rated as having a high risk of bias due to substantial or unbalanced attrition (Abbou 1995; Blute 1996; Chughtai 2020; D'Ancona 1998; Insausti 2020; Larson 1998), four studies were rated as unclear risk of bias due to insufficient information or moderate attrition (Ahmed 1997; Gao 2014; Gratzke 2017; Roehrborn 1998) and the rest of the studies were rated as low risk of bias.
Long‐term follow‐up: three studies with a low risk of bias at short‐term follow‐up suffered important attrition in the long term and were rated as high risk of bias (Abt 2018; Dahlstrand 1995; Wagrell 2002).
Major/minor adverse events
Four studies were rated as having a high risk of bias due to substantial or unbalanced attrition (Abbou 1995; Chughtai 2020; D'Ancona 1998; Larson 1998), five studies were rated as unclear risk of bias due to insufficient information or moderate attrition (Ahmed 1997; Blute 1996; Brehmer 1999; Radwan 2020; Roehrborn 1998), and the rest of the studies were rated as low risk of bias.
Retreatment
Six studies were rated as having a high risk of bias (Abbou 1995; Chughtai 2020; Dahlstrand 1995; D'Ancona 1998; Larson 1998; Wagrell 2002), and one study was rated as having an unclear risk of bias (Brehmer 1999), and the rest of the studies were rated as low risk of bias.
Erectile function
We rated four studies as having a high risk of bias (Chughtai 2020; Floratos 2001; Gratzke 2017; McVary 2016) primarily due to the measurement of the outcome in a subgroup of sexually active participants. Three studies were rated as unclear risk of bias (Ahmed 1997; Blute 1996; Roehrborn 1998) and the rest as unclear risk of bias.
Ejaculatory function
We rated six studies as having a high risk of bias (Chughtai 2020; Floratos 2001; Gratzke 2017; Larson 1998; McVary 2016; Roehrborn 2013) primarily due to the measurement of the outcome in a subgroup of sexually active participants. Three studies were rated as unclear risk of bias (Ahmed 1997; Blute 1996; Roehrborn 1998) and the rest as unclear risk of bias.
Acute urinary retention
We rated three studies as having a high risk of bias (Abbou 1995; Chughtai 2020; Larson 1998), three studies with an unclear risk of bias (Albala 2002; Blute 1996; Roehrborn 1998) the rest of the studies as low risk of bias.
Indwelling urinary catheter
We rated one study as having a high risk of bias (Abbou 1995). Except for three studies that adequately reported this outcome for nearly all participants (Abt 2018; Gao 2014; McVary 2016), the rest of the studies only included a narrative statement, not fully reporting this outcome.
Selective reporting
Three studies were rated as high risk of bias due to the selective presentation of data for a single group (active treatment) or for only certain time points, and the definitions of outcomes that did not match the protocol (Albala 2002; Blute 1996; Insausti 2020). Four studies reported their results according to a pre‐specified plan and were rated as having a low risk of bias (Gratzke 2017; McVary 2016; Pisco 2020; Roehrborn 2013). The rest of the studies did not provide sufficient information for judgement, mostly due to the lack of a pre‐registered or published protocol.
Other potential sources of bias
We rated all studies as having low risk of bias as we identified no other sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings 1. Urologic symptoms scores ‐ short term.
| Minimally invasive treatments versus transurethral resection of the prostate | ||||
|
Patient or population: men with moderate to severe lower urinary symptoms due to benign prostatic hyperplasia Interventions: minimally invasive treatments Comparator (reference): transurethral resection of the prostate Setting: hospital procedure – outpatient follow‐up | ||||
|
Outcome: urinary symptoms scores Measured by: IPSS range 0‐35 (lower scores indicate fewer symptoms) Follow‐up: 3 to 12 months (most of the data is at 3 months follow‐up) | ||||
|
19 studies 1847 participants |
Anticipated absolute effect (95% CI) * | Certainty of the evidence | Ranking (SUCRA) ** | |
| With TURP | With a minimally invasive procedure | |||
|
PUL (UroLift) (mixed estimate) |
Mean score in the included studies: 6.82 (range 5.1 to 12.6)a | 1.47 higher (4.00 lower to 6.93 higher) |
⊕⊕⊝⊝ LOW b c |
2.8 (70.5%) |
|
PAE (mixed estimate) |
1.55 higher (1.23 lower to 4.33 higher) |
⊕⊕⊝⊝ LOW b d |
2.9 (69.2%) |
|
|
CRFWVT (Rezūm) (indirect estimate) |
3.60 higher (4.25 lower to 11.46 higher) |
⊕⊕⊝⊝ LOW b c |
3.9 (52.4%) |
|
|
TUMT (mixed estimate) |
3.98 higher (0.85 higher to 7.10 higher) |
⊕⊕⊝⊝ LOW b e |
4.4 (43.0%) |
|
|
TIND (indirect estimate) |
7.50 higher (0.68 lower to 15.69 higher) |
⊕⊕⊝⊝ LOW b e |
5.5 (21.5%) |
|
|
CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IPSS: International Prostate Symptom Score; MD: mean difference; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; SUCRA: surface under the cumulative ranking curve; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of prostate. Network meta‐analysis summary of findings table definitions: * Estimates are reported as mean difference and CI. ** Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. Between brackets are the surface under the curve (SUCRA) estimates. | ||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aTURP was the highest‐ranked intervention for this outcome with a mean rank of 1.7 (SUCRA 88.9%)
bDowngraded by one level due to major concerns on within‐study bias: nearly all studies contributing to this estimate had an overall high risk of bias.
cDowngraded by one level due to major concerns on imprecision: the estimate crosses the threshold for minimally important difference (three points for IPSS) and the line of no effect.
dDowngraded by one level due to some concerns on imprecision and inconsistency (heterogeneity): the estimate and prediction interval cross one threshold for minimally important difference (three points for IPSS)
eDowngraded by one level due to some concerns regarding inconsistency (heterogeneity): the prediction interval crosses one threshold for minimally important difference (three points for IPSS).
Summary of findings 2. Quality of life ‐ short term.
| Minimally invasive treatments versus transurethral resection of the prostate | ||||
|
Patient or population: men with moderate to severe lower urinary symptoms due to benign prostatic hyperplasia Interventions: minimally invasive treatments Comparator (reference): transurethral resection of the prostate Setting: hospital procedure – outpatient follow‐up | ||||
|
Outcome: Quality of life Measured by: IPSS QoL range 0‐6 (lower scores indicate a fewer impact on the quality of life) Follow‐up: 3 to 12 months | ||||
|
13 studies 1469 participants |
Anticipated absolute effect (95% CI) * | Certainty of the evidence | Ranking (SUCRA) ** | |
| With TURP | With a minimally invasive procedure | |||
|
PUL (UroLift) (mixed estimate) |
Mean score in the included studies: 2.09 (range 0.9 to 3.26)a | 0.06 higher (1.17 lower to 1.30 higher) |
⊕⊕⊝⊝ LOW b c |
2.8 (70.3%) |
|
PAE (mixed estimate) |
0.09 higher (0.57 lower to 0.75 higher) |
⊕⊕⊝⊝ LOW b d |
2.9 (68.1%) |
|
|
CRFWVT (Rezūm) (indirect estimate) |
0.37 higher (1.45 lower to 2.20 higher) |
⊕⊕⊝⊝ LOW b c |
3.6 (56.3%) |
|
|
TUMT (mixed estimate) |
0.65 higher (0.48 lower to 1.78 higher) |
⊕⊕⊝⊝ LOW b e |
4.5 (42.2%) |
|
|
TIND (indirect estimate) |
0.87 higher (1.04 lower to 2.79 higher) |
⊕⊕⊝⊝ LOW b c |
5.0 (33.4%) |
|
|
CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IPSS: International Prostate Symptom Score; MD: mean difference; QoL: quality of life; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; SUCRA: surface under the cumulative ranking curve; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of prostate. Network meta‐analysis summary of findings table definitions: * Estimates are reported as mean difference and CI. ** Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. Between brackets are the surface under the curve (SUCRA) estimates. | ||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aTURP was the highest‐ranked intervention for this outcome with a mean rank of 2.5 (SUCRA 75.7%)
bDowngraded by one level due to major concerns on within‐study bias: nearly all studies contributing to this estimate had an overall high risk of bias.
cDowngraded by one level due to major concerns on imprecision: the estimate crosses the threshold for minimally important difference (one point for IPSS‐QoL) and the line of no effect.
dDowngraded by one level due to major concerns on inconsistency (heterogeneity): the prediction interval crosses the threshold for minimally important difference (one point for IPSS‐QoL) and the line of no effect.
eDowngraded by one level due to some concerns regarding inconsistency (heterogeneity) and imprecision: the estimate and the prediction interval crosses the threshold for minimally important difference (one point for IPSS‐QoL)
Summary of findings 3. Major adverse events.
| Minimally invasive treatments versus transurethral resection of the prostate | |||||
|
Patient or population: men with moderate to severe lower urinary symptoms due to benign prostatic hyperplasia Interventions: minimally invasive treatments Comparator (reference): transurethral resection of the prostate Setting: hospital procedure – outpatient follow‐up | |||||
|
Outcome: major adverse events Defined as: Clavien‐Dindo Grade III, IV, and V, including hospitalizations and procedures to treat complications related to the initial intervention. Follow‐up: 3‐36 months | |||||
|
15 studies 1573 participants |
Anticipated absolute effect (95% CI) * |
Relative effect (95% CI) |
Certainty of the evidence | Ranking (SUCRA) ** | |
| With TURP | With a minimally invasive procedure | ||||
|
TUMT (mixed estimate) |
Median rate of major adverse events: 130 per 1000a | 104 fewer per 1000 (118 fewer to 74 fewer) |
RR 0.20 (0.09 to 0.43) |
⊕⊕⊕⊝ MODERATE b |
2.7 (72.1%) |
|
PUL (UroLift) (mixed estimate) |
90 fewer per 1000 (125 fewer to 159 more) | RR 0.30 (0.04 to 2.22) |
⊕⊕⊝⊝ LOW b c |
3.6 (56.9%) |
|
|
CRFWVT (Rezūm) (indirect estimate) |
81 fewer per 1000 (129 fewer to 870 more) | RR 0.37 (0.01 to 18.68) |
⊕⊕⊝⊝ LOW b c |
4.0 (50.0%) |
|
|
TIND (indirect estimate) |
63 fewer per 1000 (129 fewer to 870 more) | RR 0.52 (0.01 to 24.46) |
⊕⊕⊝⊝ LOW b c |
4.3 (44.7%) |
|
|
PAE (mixed estimate) |
45 fewer per 1000 (97 to 89 more) | RR 0.65 (0.25 to 1.68) |
⊕⊕⊝⊝ LOW b c |
5.0 (33.6%) |
|
|
CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IPSS: International Prostate Symptom Score; MD: mean difference; QoL: quality of life; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; RR: risk ratio; SUCRA: surface under the cumulative ranking curve; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of prostate. Network meta‐analysis summary of findings table definitions. * Estimates are reported as risk difference and confidence interval (CI). ** Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. Between brackets are the surface under the curve (SUCRA) estimates. | |||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aAverage rate of retreatment in the control group (13%) or 130 per 1000. TURP was the lowest‐ranked intervention for this outcome with a mean rank of 5.9 (SUCRA 17.9%)
bDowngraded by one level due to major concerns on within‐study bias: nearly all studies contributing to this estimate had an overall high risk of bias.
cDowngraded by one level due to major concerns on imprecision: wide confidence interval.
Summary of findings 4. Retreatment ‐ long term.
| Minimally invasive treatments versus transurethral resection of the prostate | |||||
|
Patient or population: men with moderate to severe lower urinary symptoms due to benign prostatic hyperplasia Interventions: minimally invasive treatments. Comparator (reference): transurethral resection of the prostate Setting: hospital procedure – outpatient follow‐up | |||||
|
Outcome: retreatment Defined as: number of participants requiring a follow‐up procedure for lower urinary tract symptoms including another minimally invasive treatment or TURP (this does not include procedures to treat complications ‐ these are included under major adverse events) Follow‐up: 12 ‐ 60 months | |||||
|
10 studies 799 participants |
Anticipated absolute effect (95% CI) * |
Relative effect (95% CI) |
Certainty of the evidence | Ranking (SUCRA) ** | |
| With TURP | With a minimally invasive procedure | ||||
|
PUL (UroLift) (mixed estimate) |
Median rate of retreatment: 12 per 1000a | 17 more per 1000 (6 fewer to 121 more) |
RR 2.39 (0.51 to 11.10) |
⊕⊝⊝⊝ VERY LOW b c d |
2.2 (68.8%) |
|
PAE (mixed estimate) |
41 more per 1000 (3 more to 173 more) | RR 4.39 (1.25 to 15.44) |
⊕⊝⊝⊝ VERY LOW b d e |
3.0 (50.8%) |
|
|
TUMT (mixed estimate) |
104 more per 1000 (16 more to 470 more) | RR 9.71 (2.35 to 40.13) |
⊕⊕⊕⊝ LOW b d |
3.7 (32.1%) |
|
|
CRFWVT (Rezūm) (pairwise) |
Based on one study with 197 participants, we are very uncertain about the effects of CRFWVT on retreatment compared to sham at three months follow‐up (RR 1.36, 95% CI 0.06 to 32.86). |
⊕⊝⊝⊝ VERY LOW f |
Data could not be included in NMA to preserve the transitivity of each network | ||
|
TIND (pairwise) |
Based on one study with 185 participants, we are very uncertain about the effects of TIND on retreatment compared to sham at three‐month follow‐up (RR 0.67, 95% CI 0.11 to 3.89). |
⊕⊝⊝⊝ VERY LOW f |
Data could not be included in NMA to preserve the transitivity of each network | ||
|
CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IPSS: International Prostate Symptom Score; NMA: network meta‐analysis; QoL: quality of life; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; RR: risk ratio; SUCRA: surface under the cumulative ranking curve; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of prostate. Network meta‐analysis summary of findings table definitions. * Estimates are reported as risk difference and confidence interval (CI). ** Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. Between brackets are the surface under the curve (SUCRA) estimates. | |||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aAverage rate of retreatment in the control group (1.15%) or 12 per 1000. TURP was the highest rank intervention for this outcome with a mean rank of 1.1 (SUCRA 96.4%)
bDowngraded by one level due to major concerns on within‐study bias: nearly all studies contributing to this estimate had an overall high risk of bias.
cDowngraded by one level due to major concerns on imprecision: wide confidence interval.
dDowngraded by one level due to major concerns on incoherence: the network does not present close loops to assess incoherence.
eDowngraded by one level due to some concerns on imprecision and inconsistency (heterogeneity): wide confidence interval and prediction interval.
fDowngraded by three levels due to concerns on within‐study bias (single study at high risk of bias) and severe imprecision (wide confidence interval).
Summary of findings 5. Erectile function ‐ short term.
| Minimally invasive treatments versus transurethral resection of the prostate | ||||
|
Patient or population: men with moderate to severe lower urinary symptoms due to benign prostatic hyperplasia Interventions: minimally invasive treatments. Comparator (reference): sham procedure or transurethral resection of the prostate Setting: hospital procedure – outpatient follow‐up | ||||
|
Outcome: erectile function Measured by: IIEF scores range 5‐25 (higher scores indicate better function). Follow‐up 3 to 12 months | ||||
|
6 studies 640 participants |
Anticipated absolute effect (95% CI) * | Certainty of the evidence | Ranking (SUCRA) ** | |
| With TURP | With a minimally invasive procedure | |||
|
CRFWVT (Rezūm) (indirect estimate) |
Mean score in the included studies: 15.16 (range 11.67 to 17.70)a | 6.49 higher (8.13 lower to 21.12 higher) |
⊕⊝⊝⊝ VERY LOW b c d |
2.5 (70.7%) |
|
TIND (indirect estimate) |
5.19 higher (9.36 lower to 19.74 higher) |
⊕⊝⊝⊝ VERY LOW b c d |
2.9 (61.7%) |
|
|
PUL (UroLift) (mixed estimate) |
3.00 higher (5.45 lower to 11.44 higher) |
⊕⊝⊝⊝ VERY LOW b c d |
3.5 (49.5%) |
|
|
PAE (mixed estimate) |
0.03 lower (6.38 lower to 6.32 higher) |
⊕⊝⊝⊝ VERY LOW b c d |
4.4 (31.1%) |
|
| TUMT | Not reported | |||
|
CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; MD: mean difference; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; SUCRA: surface under the cumulative ranking curve; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of prostate. Network meta‐analysis summary of findings table definitions: * Estimates are reported as mean difference and confidence interval (CI). ** Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. Between brackets are the surface under the curve (SUCRA) estimates. | ||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aTURP was the lowest‐ranked intervention for this outcome with a mean rank of 4.6 (SUCRA 27.2%)
bDowngraded by one level due to major concerns on within‐study bias: nearly all studies contributing to this estimate had an overall high risk of bias.
cDowngraded by one level due to major concerns on imprecision: the estimate crosses the threshold for minimally important difference (five points for IIEF‐5) including substantial benefits and harms.
dDowngraded by one level due to major concerns on incoherence: the network does not present close loops to assess incoherence.
Summary of findings 6. Ejaculatory function ‐ short term.
| Minimally invasive treatments versus transurethral resection of the prostate | |||||
|
Patient or population: men with moderate to severe lower urinary symptoms due to benign prostatic hyperplasia Interventions: minimally invasive treatments Comparator (reference): transurethral resection of the prostate Setting: hospital procedure – outpatient follow‐up | |||||
|
Outcome: ejaculatory function Defined as: men with ejaculatory dysfunction ‐ loss or substantial reduction in ejaculation (as an indication of retrograde ejaculation) Follow‐up: 3 to 12 months | |||||
|
8 studies 461 participants |
Anticipated absolute effect (95% CI) * |
Relative effect (95% CI) |
Certainty of the evidence | Ranking (SUCRA) ** | |
| With TURP | With a minimally invasive procedure | ||||
|
PUL (UroLift) (mixed estimate) |
Median rate of ejaculatory dysfunction: 550 per 1000a | 521 fewer per 1000 (549 fewer to 32 more) | RR 0.05 (0.01 to 1.06) |
⊕⊝⊝⊝ VERY LOW b c d |
1.2 (92.1%) |
|
TUMT (mixed estimate) |
364 fewer per 1000 (458 fewer to 173 fewer) | RR 0.34 (0.17 to 0.68) |
⊕⊝⊝⊝ VERY LOW b c d |
2.3 (55.1%) |
|
|
PAE (mixed estimate) |
356 fewer per 1000 (476 fewer to 42 fewer) | RR 0.35 (0.13 to 0.92) |
⊕⊝⊝⊝ VERY LOW b c d |
2.5 (51.1%) |
|
|
CRFWVT (Rezūm) (pairwise) |
Based on one study with 131 participants, CRFWVT may result in little to no difference in events of ejaculatory dysfunction compared to sham at short‐term follow‐up (RR 4.01, 95% CI 0.22 to 72.78). |
⊕⊝⊝⊝ VERY LOW e |
Data could not be included in NMA to preserve the transitivity of each network | ||
|
TIND (pairwise) |
The study assessing TIND compared to sham reported no events of ejaculatory dysfunction. |
⊕⊝⊝⊝ VERY LOW e |
Data could not be included in NMA to preserve the transitivity of each network | ||
|
CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IPSS: International Prostate Symptom Score; NMA: network meta‐analysis; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; RR: risk ratio; SUCRA: surface under the cumulative ranking curve; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of prostate. Network meta‐analysis summary of findings table definitions. * Estimates are reported as risk difference and confidence interval (CI). ** Rank statistics is defined as the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. Between brackets the surface under the curve (SUCRA) estimates. | |||||
|
GRADE Working Group grades of evidence (or certainty of the evidence). High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aAverage rate of retreatment in the control group (55%) or 550 per 1000. TURP was the lowest‐ranked intervention for this outcome with a mean rank of 4 (SUCRA 1.4%)
bDowngraded by one level due to major concerns on within‐study bias: nearly all studies contributing to this estimate had an overall high risk of bias.
cDowngraded by one level due to concerns on inconsistency (heterogeneity): predictive intervals include substantial benefits and harms.
dDowngraded by one level due to major concerns on incoherence: the network does not present close loops to assess incoherence.
eDowngraded by two levels due to concerns on within‐study bias (single study at high risk of bias) and imprecision (wide confidence interval crossing the minimally importance difference).
1. Network meta‐analysis: Minimally invasive treatments versus TURP
The geometry of the networks is presented in each of the figures (Figure 2; Figure 3; Figure 4; Figure 5; Figure 6; Figure 7). Considering that the majority of trials assessed the effect of TUMT and PAE, the networks were not densely connected, and in some cases, they were star‐shaped with no closed loops (this is discussed in the section Quality of the evidence). The following analyses present data from networks with no concerns on transitivity or global consistency (except in those networks in which it was not possible to assess it due to the lack of closed loops).
2.
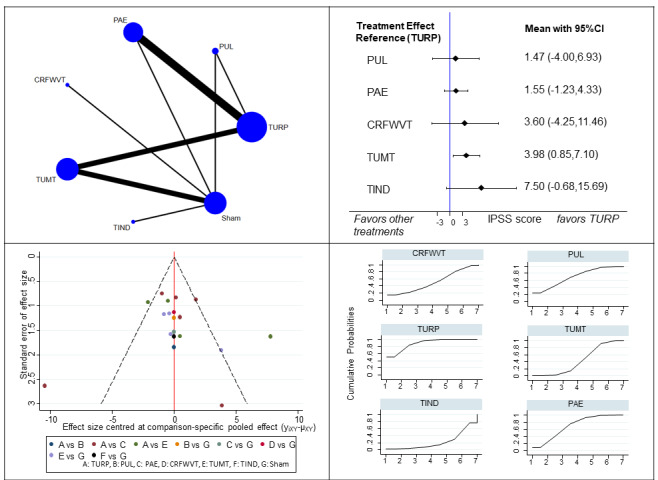
Urologic symptoms scores (IPSS). Top left: visual representation of the network. Bottom left: comparison‐adjusted funnel plot for the detection of publication bias. We found no important asymmetry in this plot; therefore publication bias is not strongly suspected.Top right panel: forest plot representing the estimates from the network meta‐analysis. Bottom right: The surface under the curve (SUCRA) of each of these plots defines the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. CRFWVT: convective radiofrequency water vapor therapy; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
3.
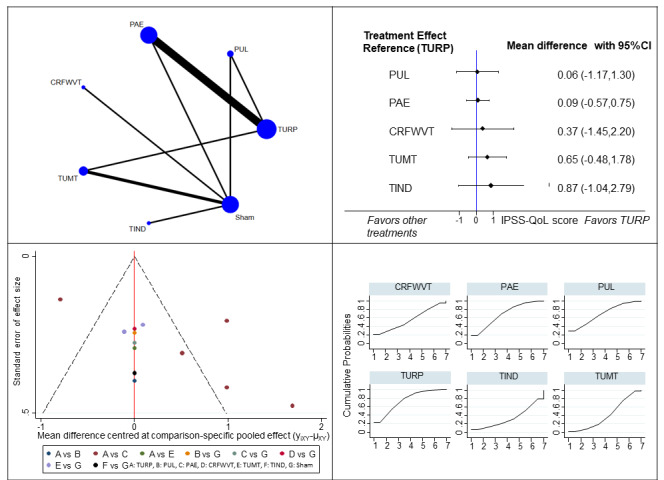
Quality of life (IPSS‐QoL). Top left: visual representation of the network. Bottom left: comparison‐adjusted funnel plot for the detection of publication bias. We found no important asymmetry in this plot, therefore publication bias is not strongly suspected.Top right panel: forest plot representing the estimates from the network meta‐analysis. Bottom right: The surface under the curve (SUCRA) of each of these plots defines the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. CRFWVT: convective radiofrequency water vapor therapy; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
4.
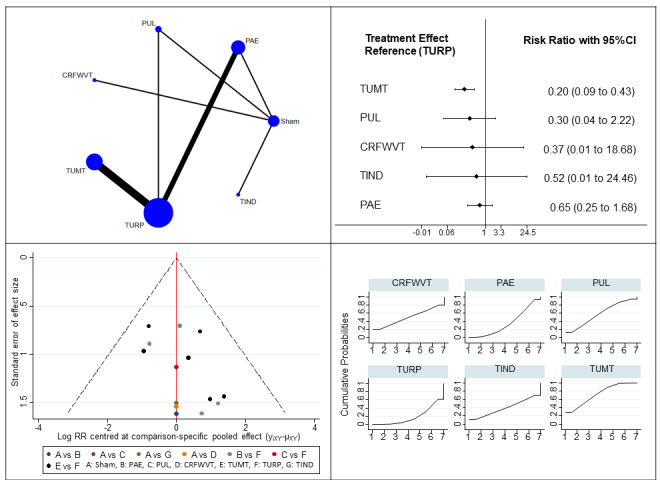
Major adverse events. Top left: visual representation of the network. Bottom left: comparison‐adjusted funnel plot for the detection of publication bias. We found no important asymmetry in this plot, therefore publication bias is not strongly suspected.Top right panel: forest plot representing the estimates from the network meta‐analysis, log scale. Bottom right: The surface under the curve (SUCRA) of each of these plots defines the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. CRFWVT: convective radiofrequency water vapor therapy; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
5.
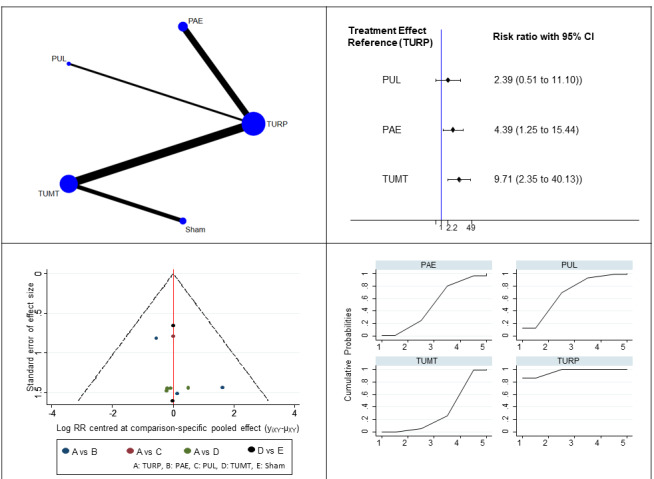
Retreatment. Top left: visual representation of the network. Bottom left: comparison‐adjusted funnel plot for the detection of publication bias. We found no important asymmetry in this plot, therefore publication bias is not strongly suspected. Top right panel: forest plot representing the estimates from the network meta‐analysis, log scale. Bottom right: The surface under the curve (SUCRA) of each of these plots defines the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. PAE: prostatic arterial embolization; PUL: prostatic urethral lift; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
6.
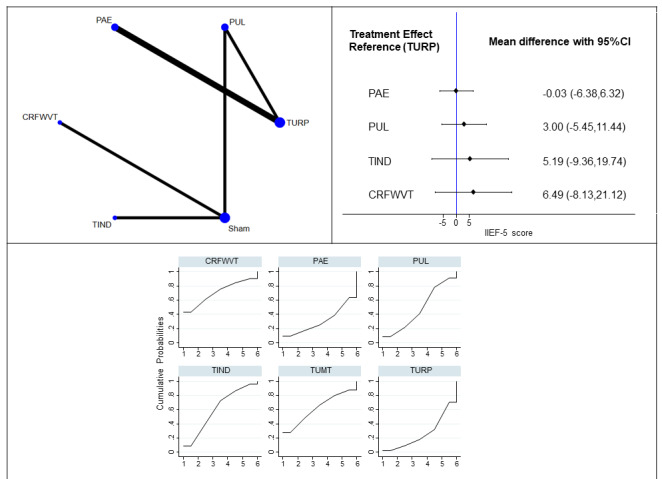
Erectile function (IIEF‐5). Top left: visual representation of the network.Top right panel: forest plot representing the estimates from the network meta‐analysis. Bottom: The surface under the curve (SUCRA) of each of these plots defines the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. A funnel plot is not available (few trials). CRFWVT: convective radiofrequency water vapor therapy; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
7.
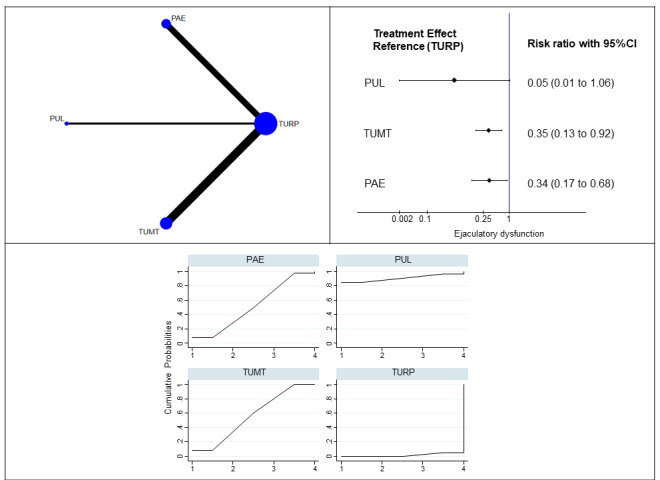
Erectile function (IIEF‐5). Top left: visual representation of the network.Top right panel: forest plot representing the estimates from the network meta‐analysis, log scale. Bottom: The surface under the curve (SUCRA) of each of these plots defines the probability that a treatment out of n treatments in a network meta‐analysis is the best, the second, the third, and so on until the least effective treatment. A funnel plot is not available (few trials). PAE: prostatic arterial embolization; PUL: prostatic urethral lift; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
1.1. Urologic symptoms scores
See Table 1, Table 8 (league table with the effect estimates) and Figure 2 (forest plot and SUCRA).
2. League table ‐ Network meta‐analysis.
| IPSS scores ‐ short term (mean difference in IPSS scores and 95% CI) | |||||||
| TURP | PUL | PAE | REZUM | TUMT | iTIND | ||
| TURP | 1.47 (‐4.00,6.93) | 1.55 (‐1.23,4.33) | 3.60 (‐4.25,11.46) | 3.98 (0.85,7.10) | 7.50 (‐0.68,15.69) | ||
| PUL | ‐1.47 (‐6.93,4.00) | 0.09 (‐5.85,6.02) | 2.14 (‐6.56,10.84) | 2.51 (‐3.13,8.15) | 6.04 (‐2.96,15.03) | ||
| PAE | ‐1.55 (‐4.33,1.23) | ‐0.09 (‐6.02,5.85) | 2.05 (‐6.02,10.13) | 2.43 (‐1.50,6.35) | 5.95 (‐2.44,14.34) | ||
| REZUM | ‐3.60 (‐11.46,4.25) | ‐2.14 (‐10.84,6.56) | ‐2.05 (‐10.13,6.02) | 0.37 (‐7.17,7.91) | 3.90 (‐6.05,13.84) | ||
| TUMT | ‐3.98 (‐7.10,‐0.85) | ‐2.51 (‐8.15,3.13) | ‐2.43 (‐6.35,1.50) | ‐0.37 (‐7.91,7.17) | 3.53 (‐4.35,11.41) | ||
| iTIND | ‐7.50 (‐15.69,0.68) | ‐6.04 (‐15.03,2.96) | ‐5.95 (‐14.34,2.44) | ‐3.90 (‐13.84,6.05) | ‐3.53 (‐11.41,4.35) | ||
| IPSS QOL scores ‐ short term (mean difference in IPSS scores and 95% CI) | |||||||
| TURP | PUL | PAE | REZUM | TUMT | iTIND | ||
| TURP | 0.06 (‐1.17,1.30) | 0.09 (‐0.57,0.75) | 0.37 (‐1.45,2.20) | 0.65 (‐0.48,1.78) | 0.87 (‐1.04,2.79) | ||
| PUL | ‐0.06 (‐1.30,1.17) | 0.03 (‐1.29,1.35) | 0.31 (‐1.59,2.21) | 0.59 (‐0.81,1.99) | 0.81 (‐1.18,2.80) | ||
| PAE | ‐0.09 (‐0.75,0.57) | ‐0.03 (‐1.35,1.29) | 0.28 (‐1.55,2.12) | 0.56 (‐0.63,1.76) | 0.78 (‐1.14,2.70) | ||
| REZUM | ‐0.37 (‐2.20,1.45) | ‐0.31 (‐2.21,1.59) | ‐0.28 (‐2.12,1.55) | 0.28 (‐1.46,2.02) | 0.50 (‐1.67,2.67) | ||
| TUMT | ‐0.65 (‐1.78,0.48) | ‐0.59 (‐1.99,0.81) | ‐0.56 (‐1.76,0.63) | ‐0.28 (‐2.02,1.46) | 0.22 (‐1.62,2.06) | ||
| iTIND | ‐0.87 (‐2.79,1.04) | ‐0.81 (‐2.80,1.18) | ‐0.78 (‐2.70,1.14) | ‐0.50 (‐2.67,1.67) | ‐0.22 (‐2.06,1.62) | ||
| Sham | ‐1.57 (‐2.65,‐0.50) | ‐1.51 (‐2.71,‐0.31) | ‐1.48 (‐2.57,‐0.40) | ‐1.20 (‐2.68,0.28) | ‐0.92 (‐1.85,0.01) | ‐0.70 (‐2.28,0.88) | |
| Major adverse events ‐ risk ratio and 95% CI | |||||||
| TURP | TUMT | PUL | CRFWVT | TIND | PAE | ||
| TURP | 0.20 (0.09,0.43) | 0.30 (0.04,2.22) | 0.37 (0.01,18.62) | 0.52 (0.01,24.46) | 0.65 (0.25,1.68) | ||
| TUMT | 4.95 (2.32,10.57) | 1.50 (0.18,12.64) | 1.85 (0.03,99.13) | 2.57 (0.05,130.34) | 3.23 (0.96,10.88) | ||
| PUL | 3.29 (0.45,24.04) | 0.66 (0.08,5.59) | 1.23 (0.03,58.48) | 1.71 (0.04,76.76) | 2.14 (0.26,17.85) | ||
| CRFWVT | 2.68 (0.05,133.78) | 0.54 (0.01,29.08) | 0.81 (0.02,38.83) | 1.39 (0.02,94.69) | 1.75 (0.04,85.57) | ||
| TIND | 1.93 (0.04,90.82) | 0.39 (0.01,19.76) | 0.59 (0.01,26.34) | 0.72 (0.01,48.95) | 1.26 (0.03,58.08) | ||
| PAE | 1.53 (0.59,3.96) | 0.31 (0.09,1.05) | 0.47 (0.06,3.88) | 0.57 (0.01,28.02) | 0.80 (0.02,36.80) | ||
| Retreatment ‐ long term ‐ risk ratio and 95% CI | |||||||
| TURP | PUL | PAE | TUMT | ||||
| TURP | 2.39 (0.51,11.10) | 4.39 (1.25,15.44) | 9.71 (2.35,40.13) | ||||
| PUL | 0.42 (0.09,1.95) | 1.84 (0.25,13.41) | 4.07 (0.50,32.97) | ||||
| PAE | 0.23 (0.06,0.80) | 0.54 (0.07,3.96) | 2.21 (0.33,14.72) | ||||
| TUMT | 0.10 (0.02,0.43) | 0.25 (0.03,1.99) | 0.45 (0.07,3.01) | ||||
| Erectile function ‐ short term (mean difference in IIEF scores and 95% CI) | |||||||
| TURP | CRFWVT | TIND | PUL | PAE | |||
| TURP | 6.49 (‐8.13,21.12) | 5.19 (‐9.36,19.74) | 3.00 (‐5.45,11.44) | ‐0.03 (‐6.38,6.32) | |||
| CRFWVT | ‐6.49 (‐21.12,8.13) | ‐1.30 (‐13.33,10.73) | ‐3.50 (‐15.44,8.45) | ‐6.52 (‐22.47,9.42) | |||
| TIND | ‐5.19 (‐19.74,9.36) | 1.30 (‐10.73,13.33) | ‐2.20 (‐14.05,9.66) | ‐5.22 (‐21.10,10.65) | |||
| PUL | ‐3.00 (‐11.44,5.45) | 3.50 (‐8.45,15.44) | 2.20 (‐9.66,14.05) | ‐3.03 (‐13.59,7.54) | |||
| PAE | 0.03 (‐6.32,6.38) | 6.52 (‐9.42,22.47) | 5.22 (‐10.65,21.10) | 3.03 (‐7.54,13.59) | |||
| Ejaculatory function ‐ risk ratio and 95% CI | |||||||
| TURP | PUL | PAE | TUMT | ||||
| TURP | 0.05 (0.00,1.06) | 0.35 (0.13,0.92) | 0.34 (0.17,0.68) | ||||
| PUL | 18.75 (0.94,372.21) | 6.61 (0.29,152.77) | 6.35 (0.29,136.77) | ||||
| PAE | 2.83 (1.08,7.43) | 0.15 (0.01,3.49) | 0.96 (0.31,2.98) | ||||
| TUMT | 2.95 (1.46,5.98) | 0.16 (0.01,3.39) | 1.04 (0.34,3.23) | ||||
| Minor adverse events ‐ risk ratio and 95% CI | |||||||
| TURP | TUMT | CRFWVT | TIND | PAE | Rank (SUCRA) | ||
| TURP | 1.43 (0.74,2.75) | 1.78 (0.51,6.21) | 3.35 (0.74,15.26) | 1.06 (0.57,1.99) | 2.4 (72.4%) | ||
| TUMT | 0.70 (0.36,1.35) | 1.24 (0.40,3.91) | 2.35 (0.56,9.81) | 0.74 (0.35,1.60) | 4.0 (39.6%) | ||
| CRFWVT | 0.56 (0.16,1.96) | 0.80 (0.26,2.53) | 1.88 (0.36,9.79) | 0.60 (0.17,2.13) | 4.3 (32.0%) | ||
| TIND | 0.30 (0.07,1.36) | 0.43 (0.10,1.78) | 0.53 (0.10,2.76) | 0.32 (0.07,1.47) | 5.5 (10.6%) | ||
| PAE | 0.94 (0.50,1.76) | 1.34 (0.62,2.89) | 1.67 (0.47,5.95) | 3.15 (0.68,14.57) | 2.7 (66.2%) | ||
| Acute urinary retention ‐ risk ratio and 95% CI | |||||||
| TURP | TUMT | PUL | CRFWVT | TIND | PAE | Rank (SUCRA) | |
| TURP | 2.93 (1.19,7.22) | 1.09 (0.12,10.03) | 2.02 (0.07,55.79) | 2.73 (0.10,73.42) | 1.82 (0.75,4.41) | 3.1 (65.5%) | |
| TUMT | 0.34 (0.14,0.84) | 0.37 (0.04,3.43) | 0.69 (0.03,17.12) | 0.93 (0.04,22.51) | 0.62 (0.17,2.26) | 5.7 (22.1%) | |
| PUL | 0.92 (0.10,8.49) | 2.69 (0.29,24.92) | 1.86 (0.04,78.93) | 2.51 (0.06,104.22) | 1.68 (0.15,18.46) | 3.6 (56.8%) | |
| CRFWVT | 0.50 (0.02,13.71) | 1.45 (0.06,36.08) | 0.54 (0.01,22.91) | 1.35 (0.02,96.96) | 0.90 (0.03,28.27) | 4.5 (42.0%) | |
| TIND | 0.37 (0.01,9.83) | 1.07 (0.04,25.85) | 0.40 (0.01,16.48) | 0.74 (0.01,52.83) | 0.67 (0.02,20.29) | 5.0 (33.9%) | |
| PAE | 0.55 (0.23,1.33) | 1.61 (0.44,5.85) | 0.60 (0.05,6.58) | 1.11 (0.04,34.71) | 1.50 (0.05,45.73) | 4.7 (38.8%) | |
Each cell represents the effect of the intervention in the column versus the intervention in the row. CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IPSS: International Prostate Symptom Score; PAE: prostatic arterial embolization; PUL: prostatic urethral lift; QoL: quality of life; SUCRA: surface under the cumulative ranking curve; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
Based on 19 studies with 1847 participants (Abt 2018; Ahmed 1997; Bdesha 1994; Blute 1996; Carnevale 2016; Chughtai 2020; D'Ancona 1998; Gao 2014; Gratzke 2017; Insausti 2020; Larson 1998; McVary 2016; Norby 2002; Pisco 2020; Radwan 2020; Roehrborn 1998; Roehrborn 2013; Wagrell 2002; Zhu 2018) PUL and PAE may result in little to no difference in urologic symptoms scores compared to TURP at short‐term follow‐up (3 to 12 months, MD of IPSS score, range 0 to 35, higher scores indicate worse symptoms; PUL: 1.47, 95% CI ‐4.00 to 6.93; PAE: 1.55, 95% CI ‐1.23 to 4.33). CRFWVT, TUMT, and TIND may result in worse urologic symptoms scores compared to TURP at short‐term follow‐up, but the confidence intervals include little to no difference (CRFWVT: 3.6, 95% CI ‐4.25 to 11.46; TUMT: 3.98, 95% CI 0.85 to 7.10; TIND: 7.5, 95% CI ‐0.68 to 15.69). TURP had the highest likelihood of being the most efficacious for this outcome, however, among minimally invasive procedures PUL and PAE were the highest‐ranked interventions (See SUCRA plot in Figure 2). The certainty of the evidence is low due to major concerns about within‐study bias, imprecision and inconsistency (heterogeneity, see Table 9).
3. Confidence intervals and predictive intervals ‐ Considerations on inconsistency (heterogeneity).
| Urinary symptoms score | MD | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| PUL | 1.47 | (‐4.00, 6.93) | (‐7.88, 10.81) | no concerns |
| PAE | 1.55 | (‐1.23, 4.33) | (‐6.22, 9.32) | some concerns |
| CRFWVT (Rezūm) | 3.6 | (‐4.25, 11.46) | (‐7.62, 14.83) | no concerns |
| TUMT | 3.98 | (0.85, 7.10) | (‐3.95, 11.91) | some concerns |
| TIND | 7.5 | (‐0.68, 15.69) | (‐4.00, 19.01) | some concerns |
| Quality of life | MD | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| PUL | 0.06 | (‐1.17, 1.30) | (‐2.20, 2.32) | no concerns |
| PAE | 0.09 | (‐0.57, 0.75) | (‐1.78, 1.96) | major concerns |
| CRFWVT (Rezūm) | 0.37 | (‐1.45, 2.20) | (‐2.41, 3.15) | no concerns |
| TUMT | 0.65 | (‐0.48, 1.78) | (‐1.52, 2.83) | some concerns |
| TIND | 0.87 | (‐1.04, 2.79) | (‐1.99, 3.74) | no concerns |
| Major adverse events | RR | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| TUMT | 0.20 | (0.09, 0.43) | (0.08, 0.49) | no concerns |
| PUL | 0.30 | (0.04, 2.22) | (0.03, 3.02) | no concerns |
| CRFWVT (Rezūm) | 0.37 | (0.01, 18.62) | (0.00, 34.03) | no concerns |
| TIND | 0.52 | (0.01, 24.46) | (0.01, 44.30) | no concerns |
| PAE | 0.65 | (0.25, 1.68) | (0.22, 1.95) | no concerns |
| Retreatment | RR | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| PUL | 2.39 | (0.51, 11.1) | (0.35, 16.27) | no concerns |
| PAE | 4.39 | (1.25, 15.44) | (0.91, 21.10) | some concerns |
| TUMT | 9.71 | (2.35, 40.13) | (1.65, 57.09) | no concerns |
| Erectile function | MD | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| CRFWVT (Rezūm) | 6.49 | (‐8.13, 21.12) | (‐101.30, 114.29) | no concerns |
| TIND | 5.19 | (‐9.36, 19.74) | (‐102.18, 112.56) | no concerns |
| PUL | 3.00 | (‐5.45, 11.44) | (‐72.02, 78.02) | no concerns |
| PAE | ‐0.03 | (‐6.38, 6.32) | (‐65.78, 65.72) | no concerns |
| Ejaculatory function | RR | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| PUL | 0.05 | (0.00, 1.06) | (0.00, 3.28) | some concerns |
| PAE | 0.35 | (0.13, 0.92) | (0.07, 1.62) | major concerns |
| TUMT | 0.34 | (0.17, 0.68) | (0.06, 2.10) | major concerns |
| Minor adverse events | RR | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| PAE | 1.06 | (0.57, 1.99) | (0.30, 3.72) | no concerns |
| TUMT | 1.43 | (0.74, 2.75) | (0.40, 5.11) | no concerns |
| CRFWVT | 1.78 | (0.51, 6.21) | (0.30, 10.61) | no concerns |
| TIND | 3.35 | (0.74, 15.26) | (0.43, 26.07) | no concerns |
| Urinary retention | RR | 95% CI | 95% PrI | Inconsistency (heterogeneity) |
| PAE | 1.82 | (0.75, 4.41) | (0.43, 7.69) | no concerns |
| PUL | 1.09 | (0.12, 10.03) | (0.08, 15.67) | no concerns |
| TUMT | 2.93 | (1.19, 7.22) | (0.68, 12.53) | major concerns |
| CRFWVT | 2.02 | (0.07, 55.79) | (0.04, 91.05) | no concerns |
| TIND | 2.73 | (0.1, 73.42) | (0.06, 119.61) | no concerns |
The reference for these estimates is TURP. CI: confidence interval; CRFWVT: convective radiofrequency water vapor therapy; IPSS: International Prostate Symptom Score; MD: mean difference; PAE: prostatic arterial embolization; Pri: predictive interval; PUL: prostatic urethral lift; QoL: quality of life; RR: risk ratio; TIND: temporary implantable nitinol device; TUMT: transurethral microwave thermotherapy; TURP: transurethral resection of the prostate.
1.2. Quality of life
See Table 2, Table 8 (league table with the effect estimates) and Figure 3 (forest plot and SUCRA).
Based on 13 studies with 1469 participants (Abt 2018; Carnevale 2016; Chughtai 2020; Gao 2014; Gratzke 2017; Insausti 2020; Larson 1998; McVary 2016; Pisco 2020; Roehrborn 1998; Roehrborn 2013; Wagrell 2002; Zhu 2018), all interventions (PUL, PAE, CRFWVT, TUMT, TIND) may result in little to no difference in the quality of life scores compared to TURP at short‐term follow‐up (3 to 12 months; MD of IPSS‐QoL score, range 0‐6, higher scores indicate worse symptoms; PUL: 0.06, 95% CI ‐1.17 to 1.30; PAE: 0.09, 95% CI ‐0.57 to 0.75; CRFWVT: 0.37, 95% CI ‐1.45 to 2.20; TUMT: 0.65, 95% CI ‐0.48 to 1.78; TIND: 0.87, 95% CI ‐1.04 to 2.79). TURP had the highest likelihood of being the most efficacious for this outcome, however, among minimally invasive procedures PUL and PAE were the highest‐ranked interventions (See SUCRA plot in Figure 3). The certainty of the evidence is low due to major concerns on within‐study bias, imprecision and inconsistency (heterogeneity, see Table 9).
1.3. Major adverse events
See Table 3, Table 8 (league table with the effect estimates) and Figure 4 (forest plot and SUCRA).
Based on 15 studies with 1573 participants (Abt 2018; Ahmed 1997; Carnevale 2016; Chughtai 2020; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Gao 2014; Gratzke 2017; Insausti 2020; McVary 2016; Norby 2002; Pisco 2020; Roehrborn 2013; Wagrell 2002) TUMT probably results in a large reduction in major adverse events compared to TURP (RR 0.20, 95% CI 0.09 to 0.43). PUL, CRFWVT, TIND, and PAE may also result in a large reduction in major adverse events, but the confidence interval includes substantial benefits and harms (at 3 to 36 months; PUL: RR 0.30, 95% CI 0.04 to 2.22; CRFWVT: RR 0.37, 95% CI 0.01 to 18.62; TIND: 0.52, 95% CI 0.01 to 24.46; PAE: 0.65, 95% CI 0.25 to 1.68). Furthermore, TUMT has the highest likelihood of being the most efficacious for this outcome while TURP was the lowest‐ranked intervention (See SUCRA plot in Figure 4). The certainty of the evidence is low for CRFWVT, TIND, PUL, and PAE due to major concerns on the within‐study bias and severe imprecision. The certainty of the evidence for TUMT is moderate due to major concerns on the within‐study bias.
The most commonly reported major adverse events included hematuria with blood clots requiring evacuation or transfusion and severe infection. Less frequently and with a delayed presentation, some patients developed meatal/urethral stenosis, which usually required additional procedures for resolution (bladder neck incision/urethrotomy).
1.4. Retreatment
See Table 4, Table 8 (league table with the effect estimates) and Figure 5 (forest plot and SUCRA).
Based on 10 studies with 799 participants (Abt 2018; Bdesha 1994; Brehmer 1999; Carnevale 2016; D'Ancona 1998; Dahlstrand 1995; Floratos 2001; Gao 2014; Gratzke 2017; Wagrell 2002), we are uncertain about the effects of PAE and PUL on retreatment compared to TURP at long‐term follow‐up (12 to 60 months; PUL: RR 2.39, 95% CI 0.51 to 11.1; PAE: RR 4.39, 95% CI 1.25 to 15.44). TUMT may result in a higher increase in retreatment rates (RR 9.71, 95% CI 2.35 to 40.13). TURP had the highest likelihood of being the most efficacious for this outcome; however, among minimally invasive procedures, PUL was the highest‐ranked intervention (See SUCRA plot in Figure 5). The certainty of the evidence is very low for PUL and PAE due to major concerns about the within‐study bias, imprecision, inconsistency (heterogeneity, see Table 9) and incoherence. The certainty of the evidence for TUMT is low due to major concerns about within‐study bias and incoherence.
These results do not include CRFWVT or TIND because of short‐term follow‐up (these results are displayed separately below, under pairwise comparisons).
1.5. Erectile function
See Table 5, Table 8 (league table with the effect estimates) and Figure 6 (forest plot and SUCRA).
Based on six studies with 640 participants (Abt 2018; Carnevale 2016; Chughtai 2020; Gratzke 2017; McVary 2016; Roehrborn 2013), we are very uncertain of the effects of minimally invasive treatments on erectile function (MD of IIEF‐5, range 5 to 25, higher scores indicates better function; CRFWVT: 6.49, 95% CI ‐8.13 to 21.12; TIND: 5.19, 95% CI ‐9.36 to 19.74; PUL: 3.00, 95% CI ‐5.45 to 11.44; PAE: ‐0.03, 95% CI ‐6.38, 6.32). CRFWVT and TIND have the highest likelihood of being the most efficacious for this outcome, while TURP was the lowest‐ranked intervention (See SUCRA plot in Figure 6); the certainty of the evidence is very low due to major concerns about the within‐study bias, incoherence and severe imprecision.
Studies related to TUMT did not report this outcome as defined in this analysis (these results are displayed separately below in pairwise comparisons).
1.6. Ejaculatory function
See Table 6, Table 8 (league table with the effect estimates) and Figure 7 (forest plot and SUCRA).
Based on eight studies with 461 participants (Abt 2018; Ahmed 1997; Carnevale 2016; Dahlstrand 1995; Floratos 2001; Gratzke 2017; Insausti 2020; Norby 2002), we are uncertain of the effects of PUL, PAE, and TUMT on ejaculatory dysfunction compared to TURP (at 3 to 12 months; PUL: RR 0.05, 95 % CI 0.00 to 1.06; PAE: RR 0.35, 95% CI 0.13 to 0.92; TUMT: RR 0.34, 95% CI 0.17 to 0.68). PUL has the highest likelihood of being the most efficacious for this outcome, while TURP was the lowest‐ranked intervention (See SUCRA plot in Figure 7). The certainty of the evidence is very low due to major concerns about the within‐study bias, inconsistency (heterogeneity, see Table 9), and incoherence.
CRFWVT was not included in this section because these studies were disconnected from the network (see description below). The study assessing TIND reported no events of ejaculatory dysfunction.
1.7. Minor adverse events
Based on 13 studies with 1374 participants (Abbou 1995; Blute 1996; Carnevale 2016; Chughtai 2020; D'Ancona 1998; Dahlstrand 1995; Gao 2014; Larson 1998; McVary 2016; Norby 2002; Pisco 2020; Radwan 2020; Wagrell 2002), TUMT, PAE, CRFWVT, and TIND may result in a greater incidence of minor adverse events compared to TURP, but the confidence interval includes substantial benefits and harms (TUMT: RR 1.43, 95% CI 0.74 to 2.75; CRFWVT: RR 1.78, 95% CI 0.51 to 6.21; TIND: RR 3.35, 95% CI 0.74 to 15.26; PAE: RR 1.06, 95% CI 0.57 to 1.99). TURP had the highest likelihood of being the most efficacious for this outcome; however, among minimally invasive procedures PAE was the highest‐ranked intervention (see data in Table 8). The certainty of the evidence is low due to major concerns about within‐study bias and severe imprecision.
The most commonly reported minor adverse events included: urinary tract infection, hematuria, dysuria, hematospermia, and pain. For PAE, a “post‐embolization syndrome” was described, consisting primarily of pain, malaise, and frequent urination.
PUL was not included in this analysis since the contributing studies reported minor adverse events in greater detail and incidence, which contributed to significant incoherence in the network (these results are displayed separately below in pairwise comparisons).
1.8. Acute urinary retention
Based on 19 studies with 2235 participants (Abt 2018; Ahmed 1997; Albala 2002; Blute 1996; Chughtai 2020; Dahlstrand 1995; De Wildt 1996; Gao 2014; Gratzke 2017; Insausti 2020; Larson 1998; McVary 2016; Nawrocki 1997; Norby 2002; Radwan 2020; Roehrborn 1998; Roehrborn 2013; Wagrell 2002; Zhu 2018), CRFWFT, TIND, and PAE may result in a greater incidence of acute urinary retention compared to TURP, but the confidence interval includes substantial benefits and harms (CRFWVT: RR 2.02, 95% CI 0.07 to 55.79; TIND: RR 2.73, 95% CI 0.1 73.42; PAE: RR 1.82, 95% CI 0.75 to 4.41). PUL may result in little to no difference in the incidence of acute urinary retention compared to TURP, but the confidence interval includes substantial benefits and harms (RR 1.09, 95% CI 0.12 to 10.03). The certainty of the evidence for these estimates is low due to major concerns about within‐study bias and imprecision. TUMT may result in a greater incidence of acute urinary retention compared to TURP (RR 2.93, 95% CI 1.19 to 7.22). The certainty of the evidence is low due to major concerns on within‐study bias and inconsistency (heterogeneity, see Table 9). Furthermore, TURP and PUL had the highest likelihood of being the most efficacious for this outcome (see data in Table 8).
1.9. Indwelling urinary catheter
Most of the included studies did not adequately report this outcome since they usually only mention catheterization as an event related to acute urinary retention. Therefore, there was insufficient information to perform a network meta‐analysis.
2. Pairwise comparisons
The supporting data from the pairwise comparisons are available in the analyses Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13; Analysis 1.14; Analysis 1.15; Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19; Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9; Analysis 2.10; Analysis 2.11; Analysis 2.12; Analysis 2.13; Analysis 2.14. The full descriptions of these results are available in our supporting reviews (Franco 2021; Jung 2017; Jung 2019; Kang 2020). We describe here some key information that we were unable to include in our network meta‐analysis, to preserve the transitivity of each network.
1.1. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 1: Urologic symptom scores
1.2. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 2: Urologic symptoms score (long term)
1.3. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 3: Quality of life
1.4. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 4: Quality of life (long term)
1.5. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 5: Major adverse events
1.6. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 6: Retreatment (short term)
1.7. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 7: Retreatment (long term)
1.8. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 8: Erectile function (short term)
1.9. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 9: Erectile function (long term)
1.10. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 10: Erectile function (short term)
1.11. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 11: Erectile function (long term)
1.12. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 12: Ejaculatory function
1.13. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 13: Ejaculatory function (short term)
1.14. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 14: Ejaculatory function (long term)
1.15. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 15: Minor adverse events
1.16. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 16: Acute urinary retention
1.17. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 17: Indwelling urinary catheter
1.18. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 18: Urologic symptom scores (subgroup: age)
1.19. Analysis.

Comparison 1: Minimally invasive treatment versus TURP, Outcome 19: Urologic symptom scores (subgroup: severity)
2.1. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 1: Urologic symptom scores
2.2. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 2: Quality of life
2.3. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 3: Major adverse events
2.4. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 4: Retreatment (short term)
2.5. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 5: Retreatment (long term)
2.6. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 6: Erectile function (IIEF‐5)
2.7. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 7: Erectile function (IIEF)
2.8. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 8: Ejaculatory function
2.9. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 9: Ejaculatory function
2.10. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 10: Minor adverse events
2.11. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 11: Acute urinary retention
2.12. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 12: Indwelling urinary catheter
2.13. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 13: Urologic symptom scores (subgroup: age)
2.14. Analysis.

Comparison 2: Miminally invasive treatment versus sham, Outcome 14: Quality of life (subgroup: age)
2.1. Retreatment: CRFWVT and TIND
Based on one study with 197 participants (McVary 2016), we are very uncertain about the effects of CRFWVT on retreatment compared to sham treatment at three months follow‐up (RR 1.36, 95% CI 0.06 to 32.86; Analysis 2.4). Based on another study with 185 participants (Chughtai 2020), we are very uncertain about the effects of TIND on retreatment compared to sham treatment at three‐month follow‐up (RR 0.67, 95% CI 0.11 to 3.89; Analysis 2.4). The certainty of the evidence is very low due to concerns about the risk of bias and severe imprecision. These results could not be included in the network due to their short‐term follow‐up.
2.2. Erectile function: TUMT
Based on four studies with 278 participants (Ahmed 1997; Floratos 2001; Norby 2002; Wagrell 2002), TUMT may result in little to no difference in erectile function (defined as an event of erectile dysfunction) compared to TURP at short‐term follow‐up (RR 0.79, 95% CI 0.40 to 1.55; I² = 0%, Analysis 1.10). One study (Wagrell 2002) found a similar result at long‐term follow‐up (RR 0.49, 95% CI 0.17 to 1.41, Analysis 1.11). The certainty of the evidence is low due to concerns about the risk of bias and imprecision. These results could not be included in the network because they were assessed as binary data and not IIEF scores.
2.3. Ejaculatory function: CRFWVT
Based on one study with 131 participants (McVary 2016), CRFWVT may result in little to no difference in events of ejaculatory dysfunction compared to sham treatment at short‐term follow‐up (RR 4.01, 95% CI 0.22 to 72.78, Analysis 2.9). The certainty of the evidence is low due to concerns about the risk of bias and imprecision. These results could not be included in the network because they were disconnected from all nodes.
2.4. Minor adverse events: PUL
Based on one study with 79 participants (Gratzke 2017), PUL may result in little to no difference on minor adverse events compared to TURP (RR 0.88, 95% CI 0.70 to 1.09; Analysis 1.15). The certainty of the evidence is low due to concerns about the risk of bias and imprecision. These results could not be included in the network because they introduced incoherence, probably related to a different pattern in the report of adverse events (they reported a higher incidence, and reported in greater detail).
3. Subgroup analysis
We investigated the sources of heterogeneity for urologic symptoms scores and quality of life. We did not identify heterogeneity for major adverse events. Some of the subgroup analyses were not possible to perform due to the scarcity of data (see Differences between protocol and review).
3.1. Urologic symptoms scores
We were unable to identify subgroup differences due to age or symptom severity for the comparisons to TURP (Test for subgroup differences: Chi² = 0.01, degrees of freedom [df] = 1 [P = 0.93], I² = 0%, see Analysis 1.18; Test for subgroup differences: Chi² = 0.31, df = 1 [P = 0.58], I² = 0%, see Analysis 1.19) or due to age for the comparisons to sham treatment (test for subgroup differences: Chi² = 0.99, df = 1 [P = 0.32], I² = 0%, see Analysis 2.13).
3.2. Quality of life
We were unable to find subgroup differences due to age for the comparisons to sham treatment (Analysis 2.14).
Discussion
Summary of main results
We included 27 trials with 3017 randomized participants, assessing the effects of minimally invasive treatments, compared to TURP or sham treatment. The main findings of our network meta‐analysis are the following.
Urologic symptoms scores: At short‐term follow‐up, PUL and PAE may result in little to no difference in urologic symptoms scores compared to TURP at short‐term follow‐up. CRFWVT, TUMT, and TIND may result in worse urologic symptoms scores compared to TURP, but the confidence intervals include little to no difference.
Quality of life: At short‐term follow‐up, all interventions may result in little to no difference in the quality of life, compared to TURP.
Major adverse events: TUMT probably results in a large reduction in major adverse events compared to TURP, whereas the other treatment modalities (PUL, CRFWVT, TIND, and PAE) may result in a large reduction in major adverse events.
Retreatment: We are very uncertain of the effects of PUL and PAE on retreatment when compared to TURP. TUMT may result in a substantial increase in retreatment rates.
Erectile function: We are very uncertain of the effects of CRFWVT, TIND, PUL, and PAE on erectile function.
Ejaculatory function: We are very uncertain of the effects of PUL, PAE, and TUMT on ejaculatory dysfunction compared to TURP.
Minor adverse events: TUMT, PAE, CRFWVT, and TIND may result in a greater incidence of minor adverse events compared to TURP. PAE had a higher probability of being the best intervention, compared to others.
Acute urinary retention: TUMT, CRFWFT, TIND, and PAE may result in a greater incidence of acute urinary retention compared to TURP, and PUL may result in little to no difference in this outcome.
Indwelling urinary catheter: There was insufficient information to perform a network meta‐analysis for this outcome.
TURP is the reference treatment with the highest likelihood of being the most efficacious for urinary symptoms, quality of life, retreatment, minor adverse events, and acute urinary retention, but the least favorable in terms of major adverse events, erectile function, and ejaculatory function. Among minimally invasive procedures, PUL and PAE have the highest likelihood of being the most efficacious for urinary symptoms and quality of life; TUMT for major adverse events; PUL for retreatment, ejaculatory function, and acute urinary retention; CRFWVT and TIND for erectile function; and PAE for minor adverse events.
Overall completeness and applicability of evidence
The largest limitation of this study relates to issues related to the underlying body of evidence (see below), in particular, the lack of head‐to‐head trials for MITs against TURP. For example, RCTs for CRFWVT (McVary 2016) and TIND (Chughtai 2020) were limited to comparisons against sham treatment that were unblinded after three months and in many cases had short‐term follow‐up. The latter issues are underscored by the fact that the AUA guideline panel on the surgical management of LUTS had determined it required a minimum follow‐up of greater than 12 months to supports its recommendations (Foster 2019, Parsons 2020), as reflected in the underlying systematic review (Dahm 2021a). Since longer‐term RCT data is so limited, observational data may provide complementary information. For example, a systematic review of such studies found that the rate of retreatment may be higher for PUL than assessed here, close to 6% per year (Miller 2020a). Meanwhile, another systematic review has suggested that the long‐term effects of CRFWVT may be sustained with a relatively low retreatment rate (Miller 2020b).
The reporting of adverse events was not uniform across studies, especially those that might be different across procedures, such as the 'post‐embolization syndrome' in PAE. This was also highlighted in a recent review of observational data in which over a quarter of patients suffered this syndrome, but it was not uniformly characterized (Svarc 2020). Whereas the Clavien‐Dindo (Dindo 2004) system provides a well‐established system to grade the severity of surgical complications, it may be less than ideal to characterize, for example, the adverse event profile for such different MITs as PUL and PAE.
A recent systematic review on men's values and preferences highlighted that they expect a high success rate with low remission and complication rates, which minimally invasive treatments may provide compared to TURP (Malde 2021). However, men also value the preservation of their sexual function, for which we have greater uncertainties. It is therefore important that clinicians engage in shared‐decision making with their patients when discussing the available options (Dahm 2021b).
Quality of the evidence
The certainty of the evidence was mostly low to very low due to the following considerations:
Within‐study bias: All of the included studies were rated as having a high or unclear risk of bias across outcomes. While in the comparisons to TURP it was mostly due to the lack of blinding of participants and personnel, there were also significant problems related to missing outcome data and an inadequate report of randomisation and allocation methods.
Imprecision: Most of our combined estimates in the network meta‐analysis and many in our pairwise analysis had substantial imprecision, including substantial benefits and harms. This was primarily due to a low number of participants in each comparison and, for dichotomous outcomes, few events.
Inconsistency (heterogeneity): we found substantial unexplained heterogeneity in our estimates, although it was not a major concern in most cases.
Incoherence: We drew our networks and compiled our data with careful consideration of transitivity by inspecting the distribution of effect modifiers to reduce the probability of finding global and local incoherence (see below). Nevertheless, some of our networks were loosely connected. Due to the lack of closed loops, we were unable to assess incoherence adequately. Therefore, following the current guidance, we rated down the certainty of the evidence.
There is also the possibility of novelty bias, which refers to the mere appearance that a new treatment is better when it is new (Salanti 2010; Salanti 2014). This type of bias can be assessed by the visual inspection of funnel plots (see Figure 3) where newer treatments such as PAE produce asymmetries with relation to older treatments in the distribution of effect sizes, related to the quality of life.
Potential biases in the review process
We made minor modifications from our protocol regarding the reporting of additional data available in each supporting review (especially pairwise comparisons), and the display of the ranking results both graphically and in the 'Summary of findings' tables. These changes were documented in Differences between protocol and review.
Due to the adjustment in the outcome data that was required for our network meta‐analysis (see above), there are minor differences with the estimates presented in the supporting reviews (Franco 2021; Jung 2017; Jung 2019; Kang 2020), with no substantial changes in direction and magnitude of effects.
The most important specification that we made throughout the conduct of our review was to restrict our network meta‐analysis to the comparison of minimally invasive treatments versus TURP. This limited the presentation of multiple head‐to‐head comparisons between minimally invasive treatments. Therefore, we prioritized this main comparison, which would be most relevant to clinicians deciding between alternatives to TURP. Furthermore, considering the scarcity of data, we would have had an extremely low certainty of the evidence for these indirect estimates.
For our main analysis (Urologic symptoms scores ‐ short‐term), we found substantial incoherence based on the data of our supporting reviews. We then identified as a possible cause the different time points in which the outcomes were assessed (12, 24, and 52 weeks). Therefore, we extracted the data, when possible, for nearly all our results to the time point of 12 weeks, and incoherence was not subsequently identified. Additionally, we reclassified some of the events extracted as 'retreatment' within 'major adverse events', considering that our definition of retreatment was restricted to other interventions aimed at treating lower urinary tract symptoms and not including complications of the first procedure (which would be a major adverse event). Due to this, the pairwise comparisons do not exactly match those of our supporting reviews, although, in general, they present similar estimates. We had defined at the protocol stage the timing of each outcome as short‐term and long‐term, but for adverse events, this was not clear from the report; therefore, we conducted a single analysis considering that most of these events (hematuria and clotting) were in the short term.
We were unable to include all available trials and interventions in all networks, primarily due to the lack of reporting of the outcomes in the desired format or definition. For the outcome 'retreatment', we were unable to include CRFWVT or TIND because of short‐term follow‐up; for erectile function, ejaculatory function, CRFWVT was not included because the study was disconnected from the network, and the study related to TIND reported no events. For minor adverse events, PUL was not included in this analysis since the contributing studies reported minor adverse events in greater detail and incidence, which contributed to significant incoherence in the network. Moreover, long‐term data was insufficient to build networks for some critical outcomes. Nevertheless, we included all available data in pairwise comparisons.
Finally, we were unable to perform subgroup and sensibility analysis due to the limited representation of subgroups in trials. Moreover, sensitivity analyses were not possible, considering that most of the studies were at a high or unclear risk of bias.
Agreements and disagreements with other studies or reviews
We identified several systematic reviews focusing on minimally invasive treatments, reporting similar findings with regard to the efficacy of TIND, PUL, PAE, and CRFWVT, and highlighting that these are relatively effective treatments, with a lower incidence of adverse events and sexual dysfunction, compared to TURP (Amparore 2019; Jing 2020; Knight 2021; Tallman 2021; Tzeng 2021; Xiang 2021). While some of these findings are similar to our review, we highlight the uncertainty surrounding some of these outcomes, especially those related to sexual function, in which the data are sparse and usually available for only a subset of participants in each study, as was highlighted by one review (Lokeshwar 2020). Furthermore, many of these reviews included evidence from non‐randomized studies and had an overall low quality (Malling 2019; Tanneru 2020). In some cases, the evidence was synthesized by the authors of the primary studies (Amparore 2019; Zumstein 2019). There is a paucity of reviews focusing on TUMT in the last few years, considering that no trials are available since the previous version of the Cochrane Review (Hoffman 2012).
Authors' conclusions
Implications for practice.
Minimally invasive treatments may result in similar or worse effects concerning urinary symptoms and quality of life, compared to the standard treatment (transurethral resection of the prostate) at short‐term follow‐up. They may result in a large reduction of major adverse events, especially in the use of prostatic urethral lift and prostatic arterial embolization, which resulted in better rankings for symptomatic symptoms scores. Prostatic urethral lift may result in fewer retreatments compared to other interventions, especially transurethral microwave thermotherapy, which has the highest retreatment rates at long‐term follow‐up. We are very uncertain about the effects of these interventions on erectile function; however, these treatments may result in fewer cases of ejaculatory dysfunction. Considering that patients value the effects of these treatments on urinary symptoms, retreatment rates, and adverse events, including sexual function, it becomes necessary to engage in shared decision‐making when discussing their different treatment options, highlighting the existing uncertainties and eliciting their preferences.
Implications for research.
There needs to be a better reporting of basic trial methodology, such as methods of randomisation and allocation concealment, as well as a greater emphasis on patient‐reported outcomes, especially those related to sexual function. These were usually described poorly in the included studies. Many studies broke the blinding period after three months, and patients crossed to the active treatment group, which prevented us from knowing the long‐term effects of these interventions. This is particularly relevant for convective radiofrequency water vapor therapy and temporary implantable nitinol device, both of which are supported only by single trials that compared the new therapeutic approach to a sham control, with a three‐month time horizon. Given the existence of a well‐established and effective standard of care, and the availability of multiple other active treatment modalities, sham‐controlled trials provide only limited and indirect evidence to inform decision‐making (Dahm 2021a). Future research should be conducted in accordance with the 'Idea, Development, Exploration, Assessment, Long‐term study' (IDEAL) principles, with the 'Assessment Stage' (corresponding to Phase III trials in drug development) centered around an active comparison of active treatment and a focus on patient‐important outcomes (Tradewell 2019). Also, as reflected in a priori determinations by the American Urological Association guideline panel (Foster 2019; Parsons 2020), decision‐making about surgical treatment options should be based on follow‐up data of greater than 12 months. A core outcome set, as it is available for a few other urological disease entities (Duffy 2021; Foust‐Wright 2017; MacLennan 2017), should establish which outcomes should be collected, and how and when they should be collected.
What's new
| Date | Event | Description |
|---|---|---|
| 10 November 2021 | Amended | We ammended the abstract with post‐publication feedback and we added information on the funding sources. |
History
Protocol first published: Issue 6, 2020 Review first published: Issue 7, 2021
Notes
We based portions of the Methods section of this review on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which was modified and adapted for use by Cochrane Urology. General concepts of benign prostatic hyperplasia and review methods have been adapted from one of the reviews from the suite on this topic (Franco 2021; Jung 2017; Jung 2019; Kang 2020).
Acknowledgements
We are very grateful to Cochrane Urology, especially Assistant Managing Editor Jenn Mariano, as well as Cochrane Urology Korea, for supporting this review. We are also grateful for the constructive feedback from the Cancer Network and the Methods Support Unit.
We also thank the following individuals:
Gretchen Kuntz for revising and providing feedback on the search strategies
Marco Blanker, Sevann Helo, and Murad Mohammad for their peer review input of the protocol.
Dominik Abt, Bilal Chughtai, and Ahmed Higazy for providing details on the outcomes of their trials, for them to be incorporated accurately in our review.
Marc Sapoval, Deepak Agarwal, Cameron Alexander, Harris Foster, and Mitchell Humphreys for their peer review input of the review.
Juan Víctor Ariel Franco is a PhD candidate in the Programme of Methodology of Biomedical Research and Public Health, Universitat Autònoma de Barcelona (Spain).
This project is funded by the National Institute for Health Research (NIHR) [Cochrane Incentive Award (NIHR130819)]. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Appendices
Appendix 1. Search strategy
| Cochrane Library (via Wiley) |
| #1 MeSH descriptor: [Prostatic Hyperplasia] explode all tree #2 MeSH descriptor: [Prostatism] explode all trees #3 MeSH descriptor: [Urinary Bladder Neck Obstruction] explode all trees #4 (Prostat* near/3 hyperplasia*):ti,ab,kw #5 (Prostat* near/3 hypertroph*):ti,ab,kw #6 (Prostat* near/3 adenoma*):ti,ab,kw #7 (BPH OR BPO OR BPE):ti,ab,kw #8 (prostat* near/3 enlarg*):ti,ab,kw #9 (Prostatism):ti,ab,kw #10 (Bladder* near/3 obstruct*):ti,ab,kw #11 (BOO):ti,ab,kw #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 (Nitinol):ti,ab,kw #14 (TIND):ti,ab,kw #15 (iTIND):ti,ab,kw #16 #13 OR #14 OR #15 #17 #12 AND #16 |
| MEDLINE (via Ovid) |
| #1 exp Prostatic Hyperplasia/ #2 exp Prostatism/ #3 exp Urinary Bladder Neck Obstruction/ #4 (Prostat* adj3 hyperplasia*).tw. #5 (Prostat* adj3 hypertroph*).tw. #6 (Prostat* adj3 adenoma*).tw. #7 (BPH or BPO or BPE).tw. #8 (prostat* adj3 enlarg*).tw. #9 Prostatism.tw.(590) #10 (Bladder* adj3 obstruct*).tw. #11 BOO.tw. #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13 Nitinol.tw. #14 TIND.tw. #15 iTIND.tw. #16 #13 OR #14 OR #15 #17 #12 AND #16 |
| Embase (via Elsevier) |
| #1. 'prostate hypertrophy'/exp #2. 'prostatism'/exp #3. 'bladder obstruction'/exp #4. (prostat* NEAR/3 hyperplasia*):ti,ab,kw #5. (prostat* NEAR/3 hypertroph*):ti,ab,kw #6. (prostat* NEAR/3 adenoma*):ti,ab,kw #7. bph:ti,ab,kw OR bpo:ti,ab,kw OR bpe:ti,ab,kw #8. (prostat* NEAR/3 enlarg*):ti,ab,kw #9. prostatism:kw,ti,ab #10. (bladder* NEAR/3 obstruct*):ti,ab,kw #11. boo:ti,ab,kw #12. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #13. 'nitinol'/exp #14. nitinol:ta,ab,kw #15. tind:ta,ab,kw #16. itind:ta,ab,kw #17. #13 OR #14 OR #15 OR #16 #18. #12 AND #17 |
| LILACS |
| tw:(“prostatic hyperplasia” OR “hiperplasia prostática” OR prostat* OR “urinary bladder neck obstruction” OR “obstrucción del cuello de la vejiga urinaria” OR “obstrução do colo da bexiga urinária” OR bph OR bpo OR bpe) AND tw:(Nitinol OR TIND OR DNIT) |
| Scopus |
| TITLE‐ABS‐KEY ( "Prostatic Hyperplasia" OR prostat* OR "Urinary Bladder Neck Obstruction" ) AND TITLE‐ABS‐KEY ( nitinol OR tind OR itind ) |
| Web of Science |
| #1 TI=("Prostatic Hyperplasia" OR Prostat* OR "Urinary Bladder Neck Obstruction") #2 TS=("Prostatic Hyperplasia" OR Prostat* OR "Urinary Bladder Neck Obstruction") #3 #1 OR #2 #4 TS=(nitinol OR tind OR itind ) OR TI=( nitinol OR tind OR itind) #5 #4 AND #3 |
Appendix 2. Searches in conference proceedings
| Conference | Website (last access April 2021) |
| American Urology Association 2020 | https://www.aua2020.org/abstracts |
| American Urology Association 2019 | http://www.aua2019.org/abstracts |
| American Urology Association 2018 | http://www.aua2018.org/abstracts |
| International Continence Society 2020 | https://www.ics.org/2020/ |
| International Continence Society 2019 | https://www.ics.org/2019/ |
| International Continence Society 2018 | https://www.ics.org/2018/ |
| European Association of Urology 2020 | https://resource‐centre.uroweb.org/resource‐centre/eau20v |
| European Association of Urology 2019 | https://urosource.uroweb.org/resource‐centre/eau19 |
| European Association of Urology 2018 | https://urosource.uroweb.org/resource‐centre/eau18 |
Data and analyses
Comparison 1. Minimally invasive treatment versus TURP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Urologic symptom scores | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 PUL | 1 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐3.90, 3.30] |
| 1.1.2 TUMT | 4 | 304 | Mean Difference (IV, Random, 95% CI) | 3.44 [‐0.16, 7.05] |
| 1.1.3 PAE | 6 | 369 | Mean Difference (IV, Random, 95% CI) | 2.42 [0.37, 4.47] |
| 1.2 Urologic symptoms score (long term) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 TUMT | 2 | 126 | Mean Difference (IV, Random, 95% CI) | 1.45 [‐0.54, 3.44] |
| 1.2.2 PUL | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 4.80 [1.11, 8.49] |
| 1.2.3 PAE | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐1.54, 6.71] |
| 1.3 Quality of life | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 PUL | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.17, 0.97] |
| 1.3.2 TUMT | 1 | 125 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.17, 0.97] |
| 1.3.3 PAE | 5 | 309 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.75, 1.25] |
| 1.4 Quality of life (long term) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 PAE | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.11, 0.95] |
| 1.4.2 PUL | 1 | 69 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.07, 1.53] |
| 1.4.3 TUMT | 1 | 97 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.46, 0.46] |
| 1.5 Major adverse events | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5.1 PUL | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.03, 2.44] |
| 1.5.2 TUMT | 6 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.43] |
| 1.5.3 PAE | 4 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.25, 1.76] |
| 1.6 Retreatment (short term) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.6.1 TUMT | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.06, 34.66] |
| 1.6.2 PAE | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 9.76 [0.49, 194.21] |
| 1.7 Retreatment (long term) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.7.1 PUL | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [0.51, 11.10] |
| 1.7.2 TUMT | 4 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 9.71 [2.35, 40.15] |
| 1.7.3 PAE | 3 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 4.44 [1.24, 15.93] |
| 1.8 Erectile function (short term) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 PUL | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 3.00 [0.02, 5.98] |
| 1.8.2 PAE | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐6.35, 6.29] |
| 1.9 Erectile function (long term) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 PUL | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐0.80, 4.00] |
| 1.10 Erectile function (short term) | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.10.1 TUMT | 4 | 278 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.40, 1.55] |
| 1.10.2 PUL | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 2.14] |
| 1.10.3 PAE | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.02, 1.56] |
| 1.11 Erectile function (long term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.11.1 TUMT | 1 | 119 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.17, 1.41] |
| 1.12 Ejaculatory function | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.12.1 PUL (short term) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 6.30 [4.47, 8.13] |
| 1.12.2 PUL (long term) | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 6.00 [3.89, 8.11] |
| 1.13 Ejaculatory function (short term) | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.13.1 TUMT | 4 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.53] |
| 1.13.2 PAE | 3 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.06, 1.19] |
| 1.13.3 PUL | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.90] |
| 1.14 Ejaculatory function (long term) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.14.1 TUMT | 1 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.85] |
| 1.14.2 PAE | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 0.98] |
| 1.15 Minor adverse events | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.15.1 TUMT | 4 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.75, 2.15] |
| 1.15.2 PAE | 3 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.40, 4.02] |
| 1.15.3 PUL | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.70, 1.09] |
| 1.16 Acute urinary retention | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.16.1 TUMT | 4 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [1.05, 6.47] |
| 1.16.2 PAE | 5 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.67, 4.77] |
| 1.16.3 PUL | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 7.20 [0.40, 129.38] |
| 1.17 Indwelling urinary catheter | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.17.1 PAE | 1 | 99 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐2.55, ‐1.45] |
| 1.18 Urologic symptom scores (subgroup: age) | 11 | 749 | Mean Difference (IV, Random, 95% CI) | 2.51 [0.85, 4.18] |
| 1.18.1 Average age < 65 | 2 | 100 | Mean Difference (IV, Random, 95% CI) | 2.37 [1.25, 3.49] |
| 1.18.2 Average age > 65 | 9 | 649 | Mean Difference (IV, Random, 95% CI) | 2.49 [0.19, 4.80] |
| 1.19 Urologic symptom scores (subgroup: severity) | 11 | 749 | Mean Difference (IV, Random, 95% CI) | 2.51 [0.85, 4.18] |
| 1.19.1 IPSS < 19 | 2 | 112 | Mean Difference (IV, Random, 95% CI) | 4.96 [‐4.74, 14.66] |
| 1.19.2 IPSS > 19 | 9 | 637 | Mean Difference (IV, Random, 95% CI) | 2.17 [0.70, 3.64] |
Comparison 2. Miminally invasive treatment versus sham.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Urologic symptom scores | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1.1 PAE | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐12.70 [‐15.69, ‐9.71] |
| 2.1.2 CRFWVT | 1 | 197 | Mean Difference (IV, Random, 95% CI) | ‐6.70 [‐8.90, ‐4.50] |
| 2.1.3 PUL | 1 | 206 | Mean Difference (IV, Random, 95% CI) | ‐7.30 [‐9.73, ‐4.87] |
| 2.1.4 TUMT | 4 | 491 | Mean Difference (IV, Random, 95% CI) | ‐5.47 [‐7.17, ‐3.77] |
| 2.1.5 iTIND | 1 | 124 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐5.98, 0.38] |
| 2.2 Quality of life | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 PAE | 1 | 80 | Mean Difference (IV, Random, 95% CI) | ‐2.05 [‐2.59, ‐1.51] |
| 2.2.2 CRFWVT | 1 | 197 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.65, ‐0.75] |
| 2.2.3 PUL | 1 | 206 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.68, ‐0.72] |
| 2.2.4 TUMT | 2 | 347 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.13, ‐0.49] |
| 2.2.5 iTIND | 1 | 185 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.31, ‐0.09] |
| 2.3 Major adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 PAE | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 71.51] |
| 2.3.2 CRFWVT | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.11, 46.44] |
| 2.3.3 PUL | 1 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.09, 10.21] |
| 2.3.4 TIND | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 3.15 [0.17, 59.95] |
| 2.4 Retreatment (short term) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4.1 CRFWVT | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.06, 32.86] |
| 2.4.2 iTIND | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.11, 3.89] |
| 2.5 Retreatment (long term) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.5.1 TUMT | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.08, 0.88] |
| 2.6 Erectile function (IIEF‐5) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 Rezum | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐1.61, 5.01] |
| 2.6.2 PUL | 1 | 197 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐4.39, 0.79] |
| 2.6.3 TIND | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐2.56, 3.36] |
| 2.7 Erectile function (IIEF) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.7.1 PAE | 1 | 80 | Mean Difference (IV, Random, 95% CI) | 5.70 [‐2.83, 14.23] |
| 2.8 Ejaculatory function | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.8.1 CRFWVT | 1 | 130 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.52, 1.72] |
| 2.8.2 PUL | 1 | 144 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.77, 1.57] |
| 2.9 Ejaculatory function | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.9.1 CRFWVT | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 4.01 [0.22, 72.78] |
| 2.10 Minor adverse events | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.10.1 PAE | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.58, 1.99] |
| 2.10.2 TUMT | 3 | 378 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.00, 2.01] |
| 2.10.3 PUL | 1 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.33, 2.16] |
| 2.10.4 CRFWVT | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.15, 3.11] |
| 2.10.5 iTIND | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 3.56 [1.32, 9.60] |
| 2.11 Acute urinary retention | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.11.1 TUMT | 6 | 858 | Risk Ratio (M‐H, Random, 95% CI) | 9.02 [3.31, 24.63] |
| 2.11.2 iTIND | 1 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 6.74 [0.39, 116.11] |
| 2.11.3 CRFWVT | 1 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 4.98 [0.28, 88.63] |
| 2.11.4 PUL | 1 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.03, 7.42] |
| 2.12 Indwelling urinary catheter | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.12.1 CRFWVT | 1 | 134 | Mean Difference (IV, Random, 95% CI) | 2.50 [1.77, 3.23] |
| 2.13 Urologic symptom scores (subgroup: age) | 8 | 1098 | Mean Difference (IV, Random, 95% CI) | ‐6.55 [‐8.51, ‐4.60] |
| 2.13.1 Average age > 65 | 4 | 657 | Mean Difference (IV, Random, 95% CI) | ‐5.42 [‐6.69, ‐4.14] |
| 2.13.2 Average age < 65 | 4 | 441 | Mean Difference (IV, Random, 95% CI) | ‐7.80 [‐11.74, ‐3.87] |
| 2.14 Quality of life (subgroup: age) | 6 | 1015 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐1.50, ‐0.75] |
| 2.14.1 Average age > 65 | 3 | 553 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐1.20, ‐0.65] |
| 2.14.2 Average age < 65 | 3 | 462 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐2.05, ‐0.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abbou 1995.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study. Study dates: study dates not available Setting: outpatient, multicenter centre, national Country: France |
|
| Participants |
Inclusion criteria: male participants:
Exclusion criteria: male participants:
Total number of participants randomized: 200 Group 1: n = 66 Transurethral route hyperthermia
Group 2: n = 31 transurethral sham
Group 3: n = 65 Transrectal route hyperthermia
Group 4: n = 38 transrectal sham
|
|
| Interventions |
Group 1 (n = 66) TUMT Three devices were used for transurethral treatment (Thermex II, Technorex, Israel: Prostcare, Brucker Spectrospin, France; BSD‐50. BSD Medical Corp, USA). Prostate temperature was monitored by an integrated microwave generator and controlled in each device through a fiber optic temperature monitor. All devices were used according to the manufacturer's instructions to deliver a temperature compatible with hyperthermia treatment (45 °C). Treatment was delivered in one session of 1 to 3 hs (depending on the device used). Group 2 (n = 31) Sham TUMT: Sham treatment consisted of a single session with the temperature maintained at 37 °C. Group 3 (n = 65) Transrectal route hyperthermia: Three devices were used for transrectal treatment (Prostathermer system, Biodan Medical Systems, Israel: Prostcare, Brucker Spectrospin, France: Primus, Tecnomatix Medical, Belgium). Prostate temperature was monitored by an integrated microwave generator and controlled in each device through a fiber‐optic temperature monitor. All devices were used according to the manufacturer's instructions to deliver a temperature compatible with hyperthermia treatment (45 °C). Treatment was delivered in six sessions of 1 to 3hs (depending on the device used) for each session over 3 weeks. Group 4 (n = 38) transrectal sham: sham treatment consisted of a single session with the temperature maintained at 37 °C. Co‐interventions: not reported |
|
| Outcomes |
Urologic symptom scores How measured: Madsen score. Additionally, responders were participants showing excellent, good or moderate responses according to each of the criteria analyzed separately (Madsen score decrease >30%; a PFR >10 mL/s with a PFR increase > 30%). Time points measured: baseline, 3, 6, and 12 months Time points reported: baseline and 12 months Subgroups: none Retreatment How measured: number of participants with medical or surgical procedure (reported the numbers separately for each) Time points measured: during treatment and 1 to 4 weeks after treatment (early post treatment complications) Time points reported: during treatment and 1 to 4 weeks after treatment (early post treatment complications) Subgroups: none Major and minor adverse event/acute urinary retention How measured: number of patients with urethral bleeding, pain and urinary tract infection, acute urinary retention Time points measured: during treatment and 1 to 4 weeks after treatment (early post treatment complications) Time points reported: during treatment and 1 to 4 weeks after treatment (early post treatment complications) Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | This study was supported by a grant from the Comite d’Evaluation et de Diffusion des Innovations Technologiques (CEDIT), Assistance Publique‐Hopitaux de Paris. Devices were lent by the following companies: Biodan, Brucker, BSD, Direx, and Tecnomatix. | |
| Declarations of interest | Not available | |
| Notes | We only included transurethral active and sham groups for the purpose of this review. No contact information available. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was stratified by the investigating centre and by approach (transrectal or transurethral), and was performed using permutation tables such that equal sample sizes were obtained for each type of approach, device and sham group.” The investigators describe a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomly allocated to a treatment in a single treatment centre after verification of the inclusion criteria.” Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: “Patients were not informed of their treatment, nor was the investigator who enrolled the patients.” Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “Patients were not informed of their treatment, nor was the investigator who enrolled the patients.” Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “Patients were not informed of their treatment, nor was the investigator who enrolled the patients.” Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | High risk | There is an imbalance in numbers or reasons for missing data across intervention groups and potentially inappropriate application of simple imputation. Quote: “Patients lost to follow‐up were classified according to maximum bias (in the sham groups as 'responders' and in the hyperthermia groups as 'non‐responders').” Missing data only in group 2. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | High risk | There is an imbalance in numbers or reasons for missing data across intervention groups and potentially inappropriate application of simple imputation. Quote: “Patients lost to follow‐up were classified according to maximum bias (in the sham groups as 'responders' and in the hyperthermia groups as 'non‐responders').” Missing data only in group 2. |
| Incomplete outcome data (attrition bias) Retreatment | High risk | There is an imbalance in numbers or reasons for missing data across intervention groups and potentially inappropriate application of simple imputation. Quote: “Patients lost to follow‐up were classified according to maximum bias (in the sham groups as 'responders' and in the hyperthermia groups as 'non‐responders').” Missing data only in group 2. |
| Incomplete outcome data (attrition bias) Acute urinary retention | High risk | There is an imbalance in numbers or reasons for missing data across intervention groups and potentially inappropriate application of simple imputation. Quote: “Patients lost to follow‐up were classified according to maximum bias (in the sham groups as 'responders' and in the hyperthermia groups as 'non‐responders').” Missing data only in group 2. |
| Incomplete outcome data (attrition bias) Indwelling catheter | High risk | There is an imbalance in numbers or reasons for missing data across intervention groups and potentially inappropriate application of simple imputation. Quote: “Patients lost to follow‐up were classified according to maximum bias (in the sham groups as 'responders' and in the hyperthermia groups as 'non‐responders').” Missing data only in group 2. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Abt 2018.
| Study characteristics | ||
| Methods |
Study design: open label, randomized controlled trial (non‐inferiority trial) Study dates: February 2014 to May 2017 Setting: single centre, national, outpatient/inpatient Country: Sweden |
|
| Participants |
Inclusion criteria: men aged at least 40 years, TURP indicated, refractory to medical treatment or not willing to undergo or continue medical treatment, with a prostate size 25‐80 mL as measured by transabdominal ultrasound, with an IPSS of at least 8, with an IPSS related QoL of at least 3 points, with a maximum urinary flow rate of less than 12 mL/s or urinary retention, and who provided written informed consent. Exclusion criteria: men with severe atherosclerosis, aneurysmatic changes or severe tortuosity in the aortic bifurcation or internal iliac arteries, a contractile detrusor, neurogenic lower urinary tract dysfunction, urethral stenosis, bladder diverticulum, bladder stone, allergy to intravenous contrast media, contraindication for magnetic resonance imaging, pre‐interventionally proven carcinoma of the prostate, and renal failure (glomerular filtration rate < 60 mL/min). Total number of participants randomly assigned: 103 Group A(PAE)
Group B(TURP)
|
|
| Interventions |
Group A: PAE Group B: monopolar TURP Follow‐up: 12 weeks |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: at the baseline, 1, 6, and 12 weeks, 6, 12, and 24 months. Time points reported: at the baseline, 1, 6, and 12 weeks, 12 and 24 months. Subgroups: none Quality of life How measured: IPSS QOL Time points measured: at the baseline, 1, 6, and 12 weeks, 6, 12, and 24 months. Time points reported: at the baseline, 1, 6, and 12 weeks, 6, 12, and 24 months. Subgroups: none Erectile function How measured: IPSS QOL Time points measured: at the baseline, 1, 6, and 12 weeks, 6, 12, and 24 months. Time points reported: at the baseline, 1, 6, and 12 weeks, 6, 12, and 24 months. Subgroups: none Ejaculatory disorder/Acute urinary retention/Indwelling urinary catheter How measured: Narratively Time points measured: at the baseline, 1, 6, and 12 weeks, 6, 12, and 24 months. Time points reported: likely cumulative incidence Subgroups: none Retreatment How measured: Number of participants receiving TURP Time points measured: not specified Time points reported: at 24 months Subgroups: none Major/Minor adverse events How measured: How measured: modified Clavien system and common terminology criteria for adverse events. Time points measured: at the baseline, 1, 6, and 12 weeks, 6, 12, and 24 months. Time points reported: likely cumulative incidence Subgroups: none |
|
| Funding sources | Grant from the research committee of St Gallen Cantonal Hospital | |
| Declarations of interest | None | |
| Notes |
Protocol: NCT02054013 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “using the data management software SecuTrial, stratifying for patient age (< 70 or ≥ 70 years) and prostate volume (< 50 or ≥ 50 mL) through minimization. SecuTrial was programmed by the clinical trials unit’s data manager, and automatic treatment allocation by SecuTrial was determined for individual patients without a predefined sequence after inclusion and entry of baseline characteristics by the investigators.” |
| Allocation concealment (selection bias) | Low risk | Quote: “using the data management software SecuTrial, stratifying for patient age (< 70 or ≥ 70 years) and prostate volume (< 50 or ≥ 50 mL) through minimization. SecuTrial was programmed by the clinical trials unit’s data manager, and automatic treatment allocation by SecuTrial was determined for individual patients without a predefined sequence after inclusion and entry of baseline characteristics by the investigators”. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “Masking: None (Open Label)” in protocol. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: “Masking: None (Open Label)” in protocol. Judgement: subjective outcomes are likely to be affected by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes are likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Judgement: Short term: 48/51 (92.3%) and 47/52 (90.3%) participants randomized in PAE and TURP were included in the analysis, respectively (low risk of bias) Long term: 34/51 (66.7%) and 47/52 (90.3%) were included at 24‐month follow‐up (high risk of bias) |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: 48/51 (92.3%) and 51/52 (98.0%) participants randomized in PAE and TURP were included in the analysis, respectively (short term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: 48/51 (92.3%) and 51/52 (98.0%) participants randomized in PAE and TURP were included in the analysis, respectively (long‐term — attrition was due to retreatment). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgement: Short term: 48/51 (92.3%) and 47/52 (90.3%) participants randomized in PAE and TURP were included in the analysis, respectively (low risk of bias). Long term: 34/51 (66.7%) and 47/52 (90.3%) were included at 24‐month follow‐up (high risk of bias). |
| Incomplete outcome data (attrition bias) Ejaculatory function | Low risk | Judgement: Short term: 48/51 (92.3%) and 47/52 (90.3%) participants randomized in PAE and TURP were included in the analysis, respectively (low risk of bias). Long term: 34/51 (66.7%) and 47/52 (90.3%) were included at 24‐month follow‐up (high risk of bias). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: 48/51 (92.3%) and 51/52 (98.0%) participants randomized in PAE and TURP were included in the analysis, respectively (short term). |
| Incomplete outcome data (attrition bias) Indwelling catheter | Low risk | Judgement: 48/51 (92.3%) and 51/52 (98.0%) participants randomized in PAE and TURP were included in the analysis, respectively (short term). |
| Selective reporting (reporting bias) | Unclear risk | Judgement: protocol was published and author shared the data (not shown in the article). Results that were not predefined in the protocol were reported. Data from bladder diary was not described in method section while they were described in protocol. |
| Other bias | Low risk | Judgement: not detected. |
Ahmed 1997.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study. Study dates: study dates not available Setting: outpatient, single‐centre, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men with:
Total number of participantsrandomized: 60 Group 1: n = 30 transurethral microwave thermotherapy (TUMT)
Group 2: n = 30 transurethral resection of the prostate (TURP)
|
|
| Interventions |
Group 1 (n = 30): TUMT Done by a single operator using the Prostatron treatment catheter using the Prostasoft software (TechnoMed, Lyon, France) in a single 60‐min session under topical anesthesia with Instillagel(r) (FarcoPharma GmBH, Cologne, Germany). Group 2 (n = 30): TURP Performed on the routine operating lists by a surgeon of Senior Registrar grade or above using a standard technique. No post‐operative irrigation was used and all the resected tissue was submitted for histological examination. The urethral catheter was removed 3 or 4 days after surgery. Co‐interventions: “Intramuscular gentamicin (80 mg) was given before the treatment and oral trimethoprim (200 mg twice daily) was continued for 5 days. The patients were followed up at 6 weeks, 3 and 6 months, with a detailed evaluation performed at the last assessment.” |
|
| Outcomes |
Urologic symptom scores How measured: AUA symptom score Time points measured: baseline, 6 weeks, 3 and 6 months Time points reported: not reported (probably 6 months) Subgroups: none Indwelling urinary catheter/acute urinary retention How measured: number of patients requiring an indwelling catheter after treatment due to acute urinary retention Time points measured: 6 weeks, 3 and 6 months Time points reported: not reported Subgroups: none Major adverse event How measured: number of patients requiring blood transfusions after treatment. Time points measured: not reported Time points reported: not reported Subgroups: none Minor adverse event / Erectile function / Ejaculatory function How measured: number of patients developing urinary tract infections or meatal narrowing that required dilatation. Furthermore, adverse events related to erectile function and ejaculation are described under adverse events. Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | No contact information available. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “[...] patients were randomized to each treatment by selecting a sealed envelope. [...] Patients failing to complete treatment or return for follow‐up were substituted.” Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | High risk | Quote: “[...] patients were randomized to each treatment by selecting a sealed envelope. [...] Patients failing to complete treatment or return for follow‐up were substituted.” Whereas envelopes might be sealed, substitution might indicate tampering of allocation. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The objective outcomes were unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Unclear risk | Due to “substitution” noted above, the number of participants with missing outcome data was not provided. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Unclear risk | Due to “substitution” noted above, the number of participants with missing outcome data was not provided. |
| Incomplete outcome data (attrition bias) Erectile function | Unclear risk | Due to “substitution” noted above, the number of participants with missing outcome data was not provided. |
| Incomplete outcome data (attrition bias) Ejaculatory function | Unclear risk | Due to “substitution” noted above, the number of participants with missing outcome data was not provided. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Unclear risk | Due to “substitution” noted above, the number of participants with missing outcome data was not provided. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Due to “substitution” noted above, the number of participants with missing outcome data was not provided. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were detected. |
Albala 2002.
| Study characteristics | ||
| Methods |
Study design: parallel group randomized trial Study dates: study dates not available Setting: outpatient/inpatient – national/multicenter Country: USA |
|
| Participants |
Inclusion criteria:
Exclusion criteria: none described Total number of participants randomly assigned: 190 Group 1: 125 (TUMT)
Group 2: 65 (Sham)
All participants were men |
|
| Interventions |
Group 1 (n = 125): TUMT The TherMatrx TMx‐2000 device with the RX‐200 prostate applicator was used for heating and monitoring (with two thermo‐sensor tracks on the surface of the catheter). The RX‐200 was inserted, balloon inflated, and a drainage lumen connected to a collection bag. The length from the bladder neck to the verumontanum was measured by ultrasound. Temperature reached a peak of 50º to 55 ºC with a monitoring of rectal temperature (< 42.5 ºC). A Foley catheter inserted into the bladder was left in place from 2 to 4 days. Group 2 (n = 65): Sham Patients underwent placement of the microwave catheter for the treatment period without energy delivery and received the same post‐treatment care as the active‐treatment patients. Co‐interventions: ketorolac 10 mg, narcotic agents, lorazepam 2 mg before treatment. Lidocaine jelly was applied to the urethra for 15 minutes. Alpha‐blockers were not permitted. |
|
| Outcomes |
Urologic symptoms score How measured: AUA‐SI score Time points measured: baseline, 1, 3, 6, 9, and 12 months Time points reported: baseline, 3, 6, 12 months (for Group 1), baseline and 3 months (for Group 2) Subgroups: none Quality of life How measured: AUA‐SI score Time points measured: baseline, 1, 3, 6, 9, and 12 months Time points reported: baseline, 3, 6, 12 months (only for Group 1) Subgroups: none Major and minor adverse event / ejaculatory function / acute urinary retention How measured: major and minor adverse events, including ejaculatory adverse events and recatheterization Time points measured: not reported Time points reported: at 3 months Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | 2:1 randomization “All patients were unblinded after the 3‐month follow‐up visit, and the sham‐treated patients were given the opportunity to receive active treatment.” “The treatment arm contains only those patients originally randomized to receive an active treatment, and not any patients who crossed over from the sham arm.” The 5‐year follow‐up study (presented at a conference) only included data on the active treatment arm. Contact information Dr. Albala: albaloo2@mc.duke.edu Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Allocation concealment (selection bias) | Unclear risk | No information available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Quote: “All patients were blinded as to their group assignment, and outcome analysis was performed by individuals blinded to the randomization.” Judgement: it is unclear whether personnel was blinded. We wrote to study authors. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “All patients were blinded as to their group assignment, and outcome analysis was performed by individuals blinded to the randomization.” Judgement: participants (outcome assessors of subjective outcomes) were blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “All patients were blinded as to their group assignment, and outcome analysis was performed by individuals blinded to the randomization.” Judgement: it is unclear whether personnel was blinded however the outcomes are unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Urologic symptom score: outcome data available for 125/125 participants in Group 1 and 63/65 participants in Group 2. Presumably similar attrition in other outcomes. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Urologic symptom score: outcome data available for 125/125 participants in Group 1 and 63/65 participants in Group 2. Presumably similar attrition in other outcomes. |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Urologic symptom score: outcome data available for 125/125 participants in Group 1 and 63/65 participants in Group 2. Presumably similar attrition in other outcomes. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Urologic symptom score: outcome data available for 125/125 participants in Group 1 and 63/65 participants in Group 2. Presumably similar attrition in other outcomes. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not measured (not fully applicable ‐ see narrative description of this outcome). |
| Selective reporting (reporting bias) | High risk | Protocol not available ‐ outcome data (urologic symptom score) was not available for Group 2 at time points beyond three months. Quality of life data was not available for Group 2. We wrote to study authors. |
| Other bias | Low risk | No other sources of bias were identified. |
Bdesha 1994.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study. Study dates: study dates not available Setting: outpatient, single center, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men with:
Total number of participantsrandomized: 40 Group 1: n = 22 microwave treatment
Group 2: n = 18 sham treatment
|
|
| Interventions |
Group 1 (n = 22):TUMT LEO Microthermer was used in all participants in a single active 90‐minute treatment using a LEO Microthermer. This machine delivers a maximum power output of 20 watts at 915 MHz and incorporates an automatic power cutoff, which operates if the rectal temperature increases to greater than 42.5C. Group 2 (n = 18) sham: Same procedure, however participants received 90‐min sham treatment with no power delivered. Participants received a heating pad to simulate hyperthermia. Co‐interventions: topical lidocaine gel was used alongside flexible cystoscopy to exclude a coexisting lower urinary tract pathological condition and to measure the prostate. |
|
| Outcomes |
Urologic symptom scores How measured: AUA symptom score and WHO symptom score. Time points measured: baseline and 3 months Time points reported: baseline and 3 months Subgroups: none Minor and major adverse events / Erectile function / Ejaculatory function How measured: Narratively (including sexual adverse events) Time points measured: not reported Time points reported: not reported Subgroups: none Acute urinary retention How measured: not reported Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: narratively (TURP after sham) Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Study unblinded with cross‐over at 3 months and follow‐up to 1 year. No contact information available. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | The study describes only “sealed envelope.” Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: “The patients were also asked which treatment they thought they had received: 19 of those who had received microwave treatment answered correctly, while half the patients who had received sham treatment thought they had received a real treatment.” Judgement: Participants and personnel administrating the questionnaires were blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Double‐blind study. Participants and study personnel were blinded (see above). |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Double‐blind study. Participants and study personnel were blinded (see above). |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Only two participants (10%) in the sham group were lost at follow‐up. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Only two participants (10%) in the sham group were lost at follow‐up. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Only two participants (10%) in the sham group were lost at follow‐up. |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Only two participants (10%) in the sham group were lost at follow‐up. |
| Incomplete outcome data (attrition bias) Ejaculatory function | Low risk | Only two participants (10%) in the sham group were lost at follow‐up. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Only two participants (10%) in the sham group were lost at follow‐up. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Only two participants (10%) in the sham group were lost at follow‐up. Not fully measured (narrative statement). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Blute 1996.
| Study characteristics | ||
| Methods |
Study design: parallel group randomized trial Study dates: study dates not available Setting: outpatient Country: USA |
|
| Participants |
Inclusion criteria: men suffering from urinary symptoms (Madsen Symptom score > 8), PVR between 100 and 200 mL, PFR < 10 mL/s, prostate length between 35 and 50 mm on ultrasound examination. Exclusion criteria: men receiving medication for said symptoms, metallic implants, conditions suggesting neuropathic bladder, evidence of prostate cancer previous surgery (rectal or transurethral), antiandrogen therapy, serum creatinine > 2 mg/dL, urinary retention, bladder stones, uncontrolled dysrhythmias or cardiac pacemakers, and asymmetric median lobe enlargement. Total number of participantsrandomized: 115 Group 1 (n = 78) TUMT
Group 2 (n = 37) sham
|
|
| Interventions |
Group 1 (n = 78): TUMT Prostatron device is inserted by a 20F transurethral applicator (with 2 cooling channels) catheter and a rectal probe confirmed by ultrasonography. The specially designed transurethral catheter is comprised of a microwave antenna that allows. The treatment catheter emits a radiofrequency of 1,296 MHz. The treatment consists of three stages: 1) cooling (to 27 ºC), 2) microwave emission to a threshold of 42.5 ºC rectal temperature, 3) progressive cooling. (Details provided in the report of a previous non‐randomized study Blute 1996) Group 2 (n = 37): Sham This consisted of circulation of urethral coolant without application of microwave power while a sham treatment was displayed on the computer monitor and the program run for 60 minutes. Co‐interventions: Patients were given anti‐inflammatory agents and prophylactic antibiotics before and after (7 days) the procedure. If the patient experiences difficulties, a Foley catheter is inserted. Sedation was used at discretion in (no sedation in 89% of TUMT sessions, and 100% of sham sessions). |
|
| Outcomes |
Urologic symptom scores How measured: Madsen Symptom score / AUA symptom score Time points measured: baseline, 6 weeks, 3, 6, and 12 months Time points reported: baseline, 6 weeks, 3, 6, and 12 months (mostly graphically; comparative outcome data was only available at 3 months) Minor adverse events (including erectile/ejaculatory function) How measured: narratively including sexual adverse events Time points measured: at complete follow‐up (12 months) Time points reported: at complete follow‐up (12 months) Acute urinary retention How measured: narratively Time points measured: at complete follow‐up (12 months) Time points reported: at complete follow‐up (12 months) Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Randomization ratio 2:1 Whereas the blinding lasted for 3 months, the follow‐up time was 12 months. The reporting of outcomes was not disaggregated by group (intervention vs. sham, but for the entire population) for most outcomes and time points. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The patients were randomized to TUMT or sham treatment in a 2:1 ratio based on a permuted‐blocks procedure.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization assignments were distributed in sealed envelopes identified only by a unique patient number. The treating physician opened the envelope after completing all screening tests just prior to treatment.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: “The evaluating physician was not the treating physician and was not allowed to enter the room. The study nurse who administered symptom score tests and supervised uroflowmetry was also blinded to the randomization scheme” There was also “blinding verification” at 1 week after procedure: “When patients were queried about the treatment they had received, only half of the TUMT patients (51.3%; 40 of 78) guessed correctly, and in the sham‐treatment group, less than half of the patients (44.4%; 16 of 36) guessed correctly (Table 8).” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Double blind study ‐ see above. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Double blind study ‐ see above. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | High risk | “Of the 150 patients treated 118 had Madsen symptom score data at 12 months, since 11 discontinued the study or were lost to follow‐up, 16 were re‐treated with the Prostatron unit, 4 received alternative therapy (3 underwent transurethral procedures, and 1 received terazosin) and 1 was missing a Madsen score at follow‐up.” |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Unclear risk | No information on missing data for other outcomes beyond urinary symptoms. |
| Incomplete outcome data (attrition bias) Erectile function | Unclear risk | No information on missing data for other outcomes beyond urinary symptoms. |
| Incomplete outcome data (attrition bias) Ejaculatory function | Unclear risk | No information on missing data for other outcomes beyond urinary symptoms. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Unclear risk | No information on missing data for other outcomes beyond urinary symptoms. |
| Selective reporting (reporting bias) | High risk | No protocol available. Data was presented graphically for most time points. Comparative outcome data was only available at 3 month‐follow‐up for some outcomes. |
| Other bias | Low risk | No other sources of bias were detected. |
Brehmer 1999.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study. Study dates: study dates not available Setting: outpatient, single center, national Country: Sweden |
|
| Participants |
Inclusion criteria: men with low urinary tract symptoms dominated by
Exclusion criteria: men with:
Total number of participantsrandomized: 44 Age, mean (Range): 70.4 (53‐83) years. (No disaggregated data by group reported) Other baseline characteristics: Group 1: n = 16: 60 min TUMT
Group 2: n = 14: 30 min TUMT
Group 3: n = 14: Sham
|
|
| Interventions |
Group 1 (n = 16): 60 min TUMT ECP system (Comair, Sweden) equipped with a 22 F catheter with a microwave antenna (915 MHz), a fibre‐optic system for measuring the temperature in the urethra and, by a rectal probe, in the rectum. The two‐way urethral catheter has a circulating cooling system that reduces the heat delivered to the urethral wall. Maximum heating is achieved within 30 s and the temperature limit is 46 °C in the urethra and 43 °C in the rectum. After treatment, the patients were asked to remain in the department to attempt to void; if difficulties arose, a urethral catheter was inserted and left in place for 3 days. All the patients were given antibiotics (norfloxacin) for 5 days.” Group 2 (n = 14): Similar intervention as group 1, except that the duration of the session was 30 min. Group 3 (n = 14): “only water at 20 °C was circulated in the treatment catheter and a computer monitor, visible to the patient, showed a simulated heat‐treatment curve, similar to that produced during TUMT.” Co‐interventions: not reported |
|
| Outcomes |
Urologic symptom scores How measured: ICS questionnaires A and B (see notes) Time points measured: baseline and 3 to 6 months Time points reported: baseline and 4 months Subgroups: none Indwelling urinary catheter How measured: number of patients requiring an indwelling catheter after treatment. Time points measured: not reported Time points reported: not reported Subgroups: none Minor and major adverse event How measured: number of patients suffering a bacterial cystitis despite antibiotic treatment. Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: number of patients requiring other treatment within the follow‐up year. Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | ICS questionnaire consists of 32 questions, most of which comprise an ‘A’ question about the actual symptom and a ‘B’ question about the bother related to the symptom. The questionnaire also includes several questions about sexual function (nos 24‐27); these were all excluded from the instrument used in the present study. The maximum A and B scores are 124 and 92, respectively; a high score indicates worse symptoms. Two patients withdrew during the 1‐year study period, leaving 42 patients for the final evaluation. No contact information available. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “The patients were randomized to undergo 30 or 60 min of TUMT, or to sham treatment (14, 16 and 14 men, respectively).” Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | The participants were blinded: “study where the patients were unaware of the type of treatment given.” No information about blinding of personnel. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | The participants were blinded: “study where the patients were unaware of the type of treatment given.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information about blinding however the outcomes are unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. |
| Incomplete outcome data (attrition bias) Retreatment | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Carnevale 2016.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized, controlled study Study dates: November 2010 to December 2012 Setting: single center, national, outpatient/inpatient Country: Brazil |
|
| Participants |
Inclusion criteria: men aged > 45 years; IPSS > 19; symptoms refractory to medical treatment for at least 6 months; negative screening for prostate cancer; prostate volume between 30 and 90 mL on magnetic resonance imaging; and bladder outlet obstruction confirmed by urodynamic examination. Exclusion criteria: men with renal failure, bladder calculi or diverticula, suspected prostate cancer, urethral stenosis, or neurogenic bladder disorders. Total number of participants randomly assigned:30 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: monopolar TURP Follow‐up: 12 months |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: baseline and 1 year Time points reported: baseline and 1 year Subgroups: none Quality of life How measured: IPSS QoL Time points measured: baseline and 1 year Time points reported: baseline and 1 year Subgroups: none Erectile function How measured: IIEF‐5 Time points measured: baseline and 1 year Time points reported: baseline and 1 year Subgroups: none Retreatment How measured: Number of participants that received TURP Time points measured: baseline and 1 year Time points reported: baseline and 1 year Subgroups: none Minor and major adverse event (including ejaculatory function) How measured: National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0 Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | No financial disclosure | |
| Declarations of interest | None | |
| Notes |
Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: not described. |
| Allocation concealment (selection bias) | Unclear risk | Judgement: not described. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes are likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Ejaculatory function | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Selective reporting (reporting bias) | Unclear risk | Judgement: study outcomes were well pre‐defined and described, but protocol was not found. |
| Other bias | Low risk | Judgement: statistical differences in baseline IIEF and Qmax, but those likely underestimates the effect size of PAE (more conservative). |
Chughtai 2020.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study. Study dates: July 2015 and October 2018 Setting: outpatient, multicenter, international Country: United States and Canada |
|
| Participants |
Inclusion criteria: men with:
Exclusion Criteria:
Total number of participantsrandomized: 185 Group 1: n = 128 temporarily implanted nitinol device (iTIND)
Group 2: n = 57 Sham control
|
|
| Interventions |
Group 1 (n = 128): “the iTind device is comprised of three elongated, intertwined nitinol struts at the 12, 5, and 7 o’clock positions, an anti‐migration anchoring leaflet at 6 o’clock, and a polyester retrieval suture for easy device removal. The device is implanted for 5‐7 days, during which it expands and exerts radial force, creating deep ischemic incisions, and a remodeling on the prostate tissue at the bladder neck and anterior prostatic fossa. The iTind is deployed under direct visualization in an ambulatory procedure using a rigid cystoscopy. The device is removed through either a rigid cystoscope or an open ended 22F Foley catheter with topical anaesthesia. Both implantation and removal can be done under local, IV, or general anaesthesia at the discretion of the performing physician. Catheterisation is not required following either implantation or removal.” Group 2 (n = 57): “The sham control was the insertion and removal of an 18F silicon Foley catheter in order to simulate both the implantation and retrieval procedures. Throughout the procedure, the surgeon gave verbal description as if deploying the iTind device, after which the catheter was removed. A similar protocol was followed for the removal. Although the iTind device is deployed through a rigid cystoscope, a Foley catheter was used to minimize the risk of procedure‐related morbidity.” Co‐interventions: Subjects in both the device and control groups were draped to prevent them from seeing the treating physician and the device. |
|
| Outcomes |
Urologic symptom scores How measured: IPSS score change from baseline Time points measured: baseline, 1.5 and 3 months Time points reported: baseline, 1.5, 3 (blinded) and 12 months (unblinded) Subgroups: none Quality of life How measured: not reported Time points measured: baseline, 1.5 and 3 months Time points reported: baseline, 1.5, 3, and 12 months (unblinded) Subgroups: none Acute urinary retention How measured: number of patients developing acute urinary retention Time points measured: not reported Time points reported: not reported Subgroups: none Erectile function How measured: IIEF and SHIM score Time points measured: baseline, 1.5 and 3 months Time points reported: not reported. Subgroups: none Retreatment How measured: Number of participants that received additional treatment Time points measured: baseline and 1 year Time points reported: baseline and 1 year (global, not by group) Subgroups: none Minor and major adverse event (including ejaculatory/erectile dysfunction/urinary retention) How measured: National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0 Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Medi‐Tate Ltd. sponsored this study. | |
| Declarations of interest | Bilal Chughtai, MD is a consultant for Medi‐Tate Ltd, Olympus, Boston Scientific, and Medeon Bio. | |
| Notes | The study was unblinded at three months follow‐up. Contact info: Bilal Chughtai, E‐mail: bic9008@med.cornell.edu Protocol: trial registry (NCT02506465) Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Subjects were randomized in 2:1 ratio to either iTind or control groups using permuted blocks stratified by center by using a central electronic data program.” The investigators describe a random component in the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | Quote: “Subjects were randomized in 2:1 ratio to either iTind or control groups using permuted blocks stratified by center by using a central electronic data program.” Participants and investigators enrolling participants could not foresee assignment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Subjective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Participants were blinded. Quote: “This prospective, randomized, controlled, single blinded study of the second‐generation iTind procedure...” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Participants were blinded. These outcomes are unlikely to be affected by blinding. Quote: “This prospective, randomized, controlled, single blinded study of the second‐generation iTind procedure...” |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | High risk | Quote: Outcome data provided by the authors at 3 months: 84/128 intervention group and 40/57 in the sham group. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | High risk | The authors state that 13/118 (11%) and 10/57 (17%) were lost to follow‐up at 3 months. Unbalanced attrition. This analysis was not imputed. |
| Incomplete outcome data (attrition bias) Retreatment | High risk | The authors state that 13/118 (11%) and 10/57 (17%) were lost to follow‐up at 3 months. Unbalanced attrition. This analysis was not imputed. |
| Incomplete outcome data (attrition bias) Erectile function | High risk | The authors state that 13/118 (11%) and 10/57 (17%) were lost to follow‐up at 3 months. Unbalanced attrition. This analysis was not imputed. |
| Incomplete outcome data (attrition bias) Ejaculatory function | High risk | The authors state that 13/118 (11%) and 10/57 (17%) were lost to follow‐up at 3 months. Unbalanced attrition. This analysis was not imputed. |
| Incomplete outcome data (attrition bias) Acute urinary retention | High risk | The authors state that 13/118 (11%) and 10/57 (17%) were lost to follow‐up at 3 months. Unbalanced attrition. This analysis was not imputed. |
| Selective reporting (reporting bias) | Unclear risk | The study registry only specified two outcomes at three months (IPSS and “secondary safety”). We wrote to the study author for more information. |
| Other bias | Low risk | No other sources of bias were detected. |
D'Ancona 1998.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized trial Study dates: January 1994 to August 1995 Setting: outpatient Country: Netherlands |
|
| Participants |
Inclusion criteria: men
Exclusion criteria:
Sample size: 52 patients were randomized Group 1: n = 125 transurethral microwave thermotherapy (TUMT)
Group 1: n = 125 transurethral resection of the prostate (TURP)
|
|
| Interventions |
Group 1 (n = 31): TUMT Delivered using Prostatron device with software version 2.5, for 60 minutes increasing thermal dose up to 70 watts. Urethral and rectal thermal sensors provided feedback to prevent harms. Preparation included 100 mg diclofenac suppository and 2 mg of midazolam intramuscularly. If necessary, further intravenous sedation was administered. All participants left with an indwelling urinary catheter. Group 2 (n = 21): TURP Performed by two experienced urologists with use of spinal anaesthesia. The surgical capsule was reached circumferentially from the bladder neck to the verumontanum using 24 Ch. Resectoscopes. Co‐interventions: not described |
|
| Outcomes |
Urologic symptom scores How measured: Madsen symptom score and IPSS Time points measured: 1, 3, 6,and 12 months Time points reported: 3, 6, 12 months Subgroups: none Major and minor adverse events How measured: episodes of urinary tract infection, haematuria Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: “repeat treatment” Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | No contact information available. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were randomized.” Judgement: No information available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants were randomized.” Judgement: No information available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Participants and personnel were not blinded. Outcomes are unlikely to be affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | High risk | Outcome data was available for 44/52 participants at 1 year follow‐up, 2 were lost in the TURP group (bladder cancer and bladder neck sclerosis) and 6 in the TUMT group (1 underwent TURP, 1 died, 1 lost to follow‐up, 3 refused follow‐up). Unbalanced attrition. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | High risk | Outcome data was available for 44/52 participants at 1 year follow‐up, 2 were lost in the TURP group (bladder cancer and bladder neck sclerosis) and 6 in the TUMT group (1 underwent TURP, 1 died, 1 lost to follow‐up, 3 refused follow‐up). Unbalanced attrition. |
| Incomplete outcome data (attrition bias) Retreatment | High risk | Outcome data was available for 44/52 participants at 1 year follow‐up, 2 were lost in the TURP group (bladder cancer and bladder neck sclerosis) and 6 in the TUMT group (1 underwent TURP, 1 died, 1 lost to follow‐up, 3 refused follow‐up). Unbalanced attrition. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Narrative description (insufficient information). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were detected. |
Dahlstrand 1995.
| Study characteristics | ||
| Methods |
Study design: parallel group randomized trial Study dates: study dates not available Setting: outpatient (TUMT), inpatient (TURP), single‐center, national Country: Sweden |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Total number of participantsrandomized: 93 Group 1 (n = 46) TUMT
Group 2 (n = 47) TURP
|
|
| Interventions |
Group 1 (n = 39): TUMT One‐hour treatment in a single session performed by a single physician using the Prostatron (Technomed International, France) only with topical anaesthesia and oral analgesia. The urethral catheter delivered up to 60 W of microwave energy and monitored temperature (as well as the rectal probe) through a software. The urethral temperature could reach a maximum temperature of 44.5 °C and the rectal temperature could reach a maximum temperature of 42.5 °C. Postoperatively oral norfloxacin 400 mg twice a day, was administered for 5 days. An indwelling urethral catheter was placed and left in place for 3‐5 days if the patient was unable to void after treatment. Group 2 (n = 44): TURP Urologists who were at the level of senior registrar or above resected the prostate, using resectoscopes with a Charrière of 24‐28, down to the surgical capsule circumferentially and extended from the bladder neck to the verumontanum. Co‐interventions: not reported. |
|
| Outcomes |
Urologic symptom scores How measured: Madsen symptom score Time points measured: baseline, 2‐3‐6‐12 months, 2 years Time points reported: baseline, 2‐3‐6‐12 months, 2 years Subgroups: none Major and minor adverse events (including erectile and ejaculatory dysfunction) How measured: not reported Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: number of participants that required another session of TUMT or TURP Time points measured: not reported Time points reported: not reported Subgroups: none Indwelling urinary catheter/Acute urinary retention How measured: number of patients that required catheterization after the procedure. Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | There are two reports of this study by the same authors. In the first report there are 83 randomized participants, whereas in the second report there are 72. We accounted this as attrition. Email for the contact author was not available so we wrote to his coauthor Dr. Fall (magnus.fall@urology.gu.se) for details and he did not have this information. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “patients were recruited for the study and blindly randomized.” Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “patients were recruited for the study and blindly randomized.” Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The objective outcomes were unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | 12‐month follow‐up, 78 participants (93%) had available data (first report) Quote: “Four patients were excluded; 1 patient because he contracted severe hepatitis while abroad precluding follow‐up; 2 patients because cancer was discovered at the time of histological examination of the TUR specimen requiring orchiectomy, and 1 patient who refused randomisation to TURP.” Judgement (12 months): low risk of bias (main judgement). 2‐year follow‐up, 61 participants (73%) had available data (second report). Quote: “All patients were followed for 2 years but in 10 patients the follow‐up was incomplete. In the TURP group, one patient died from a brain tumour after his 6‐month follow‐up. At the 2‐year follow‐up, one patient underwent an operation for a lumbar disc hernia and was unavailable. In the TUMT group, one patient was abroad at the 3‐month follow‐up and after the 6‐month follow‐up, two patients had a TURP and were excluded from the study, one patient refused further follow‐up and another suffered severe pancreatitis which precluded that visit. Two patients who had undergone a second TUMT after the 6‐month follow‐up took part in the 1‐year follow‐up but had not improved and, after undergoing TURP, they were excluded before the 2‐year follow‐up. One patient was disabled due to severe neurological disease after the 1‐year follow‐up.” Judgement (2 years): high risk of bias (long‐term data). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | See above (main judgement). |
| Incomplete outcome data (attrition bias) Retreatment | High risk | See above (long‐term judgement). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | See above (main judgement). |
| Incomplete outcome data (attrition bias) Ejaculatory function | Low risk | See above (main judgement). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | See above (main judgement). |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not fully measured (narrative statement). |
| Selective reporting (reporting bias) | Low risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. We wrote to study authors. |
| Other bias | Low risk | No other sources of bias were identified. |
De Wildt 1996.
| Study characteristics | ||
| Methods |
Study design: parallel group randomized trial Study dates: Start date June 1991 – End date December 1992 Setting: outpatient, multicenter, international Country: Netherlands and the United Kingdom |
|
| Participants |
Inclusion criteria: men aged > 45 years complaining of symptoms of bladder outlet obstruction for > 3 months, with a Madsen symptom score of > 8 and urinary free‐flow rate estimates of < 15 mL/s during two voids of >150 mL. Prostatic enlargement was confirmed by transrectal ultrasonography, PSA or prostatic biopsy if necessary. Exclusion criteria: prostate cancer, prostatitis, urethral stricture, intravesical pathology (stones, neoplasm), neurogenic bladder dysfunction, urinary tract infection, isolated enlargement of the middle lobe, a residual urine volume of >300 mL, use of drugs influencing bladder or prostate function, previous transurethral resection of the prostate or transurethral incision, a metallic pelvic implant, disorders of blood flow or coagulation, diabetes mellitus and mental incapacity or inability to give informed consent. Total number of participantsrandomized: 93 men recruited but 90 were randomized (there is no further detail on the report) Group 1: n = 46 TUMT
Group 2: n = 47 Sham
|
|
| Interventions |
Group 1 (n = 46): TUMT A single session of Prostatron treatment unit which consisted of a microwave generator, urethral applicator/cooler, fiber optic temperature‐monitor, and couch. This study used the lower energy thermotherapy protocol (Prostasoft 2.0). Group 2 (n = 47): Sham Same procedure as in TUMT with a simulated program. Co‐interventions: Not described |
|
| Outcomes |
Urologic symptoms score How measured: Madsen symptom score Time points measured: baseline, 6, 12, 26, 52 weeks Time points reported: baseline, 6, 12, 26, 52 weeks Subgroups: none Quality of life How measured: ad‐hoc questionnaire (not validated) Time points measured: baseline, 12 and 26 weeks Time points reported: baseline, 12 and 26 weeks (this questionnaire includes questions of sexual function) Major and minor adverse event How measured: major and minor adverse events Time points measured: not reported Time points reported: at 3 months Indwelling urinary catheter/acute urinary retention How measured: number of participants that required a catheter after the procedure due to urinary retention Time points measured: not reported Time points reported: at 3 months Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | This study reports the trial by location and globally. The quality of life results are only available for the Netherlands report. After three months patients were offered TUMT. 27 participants in the Sham group and 4 participants in the TUMT group received a verum procedure, thus the results of this trial beyond three months are not included in this review. No contact information available. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized after informed consent was obtained.” Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomized after informed consent was obtained.” Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: “As far as possible, the patient and the investigator were kept unaware as to the treatment administered.” (first three months) Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “As far as possible, the patient and the investigator were kept unaware as to the treatment administered.” (first three months) Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “As far as possible, the patient and the investigator were kept unaware as to the treatment administered.” (first three months) Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Data was not available at three months for 3 participants in the Sham group (2 losses at follow‐up and 1 technical failure) and 2 participants in the TUMT group (underwent TURP). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Data was not available at three months for 3 participants in the Sham group (2 losses at follow‐up and 1 technical failure) and 2 participants in the TUMT group (underwent TURP). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Data was not available at three months for 3 participants in the Sham group (2 losses at follow‐up and 1 technical failure) and 2 participants in the TUMT group (underwent TURP). |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Data was not available at three months for 3 participants in the Sham group (2 losses at follow‐up and 1 technical failure) and 2 participants in the TUMT group (underwent TURP). Not fully measured (narrative statement). |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Floratos 2001.
| Study characteristics | ||
| Methods |
Study design: parallel group randomized trial Study dates: start date January 1996 – end date March 1997 Setting: outpatient/inpatient, national, single‐center Country: The Netherlands |
|
| Participants |
Inclusion criteria: Male participants aged 45 years and older with a prostate volume ≥ 30 cm3, prostatic urethral length ≥ 25 mm, a Madsen symptom score ≥ 8, maximum peak flow rate ≤ 15 mL/s and a postvoid residual ≤ 350 mL. Exclusion criteria: men with acute prostatitis or urinary tract infection, evidence of prostate carcinoma, an isolated obstructed prostatic middle lobe, diabetes mellitus, intravesical pathology, neurological disorders, or current treatment with drugs that may influence the bladder function. Total number of participants randomly assigned: 155 Group 1 (n = 82) TUMT
Group 2 (n = 73) TURP
|
|
| Interventions |
Group 1 (n = 74): TUMT A one‐hour session was administered by the Prostatron device (EDAP Technomed, Lyon, France) with a second‐generation, high‐energy protocol (Prostasoft 2.5) with a maximum power of 70 W and a rectal threshold set at 43.5 °C. Patients were administered 40 mg of morphine sulfate orally 2 hours before treatment. All participants received an indwelling Foley catheter following an outpatient voiding trial. Patients also received co‐trimoxazole 960 mg twice a day for 5 days after treatment as prophylaxis. Group 2 (n = 73): TURP It was performed under spinal anaesthesia and intended to remove as much prostate tissue as possible and all patients received an indwelling Foley catheter, which was removed when hematuria decreased sufficiently, and the participant completed a successful voiding trial. Co‐interventions: not described |
|
| Outcomes |
Urologic symptoms score How measured: IPSS score and Madsen score Time points measured: baseline, 3, 6, 12, 18, 24, and 36 months Time points reported: baseline, 12, 24, and 36 months Subgroups: none Quality of life How measured: 41‐item questionnaire designed for BPH patients Time points measured: baseline, 1, 3, 6, and 12 months Time points reported: baseline, 12, and 52 weeks Subgroups: none Retreatment How measured: narratively Time points measured: baseline, 3, 6, 12, 18, 24,and 36 months Time points reported: 6, 12, 18, 24, 30, and 26 months Major and minor adverse events How measured: major and minor adverse events Time points measured: not reported Time points reported: at 3 months Erectile function/Ejaculatory function (“Sexual function”) How measured: ad‐hoc questionnaire that assessed erections, sexual activities, orgasms, and satisfactions, among other aspects. Time points measured: baseline, 3 months and 1 year Time points reported: baseline, 3 months and 1 year Relevant outcomes not reported in this study:
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | No contact information available. We found a secondary report on sexual function with a greater attrition of data and with a slightly lower number of randomized individuals (147 participants versus 155 in the original report). Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “All patients were randomized after informed consent had been obtained.” Judgement: Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “All patients were randomized after informed consent had been obtained.” Judgement: Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Open label study. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Open label study. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Open label study. However, the outcomes are unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Quote: “Although […] 155 patients initially randomized, unfortunately because of the 10 who skipped the assigned treatment and 1 who died before the scheduled treatment, we have no follow‐up information.” Attrition was documented and was balanced (7 in the thermotherapy group and 11 in the TURP group). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Quote: “Although […] 155 patients initially randomized, unfortunately because of the 10 who skipped the assigned treatment and 1 who died before the scheduled treatment, we have no follow‐up information.” Attrition was documented and was balanced (7 in the thermotherapy group and 11 in the TURP group). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Quote: “Although […] 155 patients initially randomized, unfortunately because of the 10 who skipped the assigned treatment and 1 who died before the scheduled treatment, we have no follow‐up information.” Attrition was documented and was balanced (7 in the thermotherapy group and 11 in the TURP group). |
| Incomplete outcome data (attrition bias) Erectile function | High risk | Sexual function report. Quote: “A total of 66 patients undergoing transurethral microwave thermotherapy and 56 undergoing transurethral prostatic resection were evaluated.” (subset of participants) |
| Incomplete outcome data (attrition bias) Ejaculatory function | High risk | Sexual function report. Quote: “A total of 66 patients undergoing transurethral microwave thermotherapy and 56 undergoing transurethral prostatic resection were evaluated.” (subset of participants) |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not applicable (see comment on characteristics of included studies). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Gao 2014.
| Study characteristics | ||
| Methods |
Study design: prospective, parallel randomized controlled study Dates when study was conducted: January 2007 to January 2012 Setting: not defined Country: China |
|
| Participants |
Inclusion criteria: men with IPSS greater than 7 after failed medical therapy with a washout period of 2 or more weeks, prostate volume of 20 ‐100 mL on transrectal ultrasonographic or magnetic resonance imaging, Qmax of less than 15 mL/sec, and negative prostate biopsy if PSA > 4 ng/mL or abnormal digital rectal examination. Exclusion criteria: men with detrusor hyperactivity or hypocontractility at urodynamic study, urethral stricture, prostate cancer, diabetes mellitus, and previous prostate, bladder neck, urethral surgery, or positive prostate biopsy. Total number of participants randomly assigned: 114 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: bipolar TURP Follow‐up: 24 months |
|
| Outcomes |
Urologic symptoms score How measured: IPSS score Time points measured: at baseline, 1 month, 3 months, 6 months, 1 year, and 2 years Time points reported: at baseline, 1 month, 3 months, 6 months, 1 year, and 2 years Subgroups: none Quality of life How measured: IPSS QoL Time points measured: at baseline, 1 month, 3 months, 6 months, 1 year, and 2 years Time points reported: at baseline, 1 month, 3 months, 6 months, 1 year, and 2 years Subgroups: none Acute urinary retention/Indwelling urinary catheter How measured: Narratively Time points measured: not reported Time points reported: early (< 30 days), late (≤ 2 years) Subgroups: none Major and minor adverse events How measured: modified Clavien Classification system Time points measured: not reported Time points reported: early (< 30 days), late (≤ 2 years) Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computer‐generated simple random tables.” |
| Allocation concealment (selection bias) | Unclear risk | Judgement: not described. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes are likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Unclear risk | Judgement: 47/57 (82.5%) and 48/57 (84.3%) randomized participants in PAE and TURP were included in the analysis, respectively (short and long term). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: 54/57 (94.8%) and 53/57 (93.0%) randomized participants in PAE and TURP were included in the analysis, respectively (short and long term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: 54/57 (94.8%) and 53/57 (93.0%) randomized participants in PAE and TURP were included in the analysis, respectively (long term). |
| Incomplete outcome data (attrition bias) Indwelling catheter | Low risk | Judgement: 54/57 (94.8%) and 53/57 (93.0%) randomized participants in PAE and TURP were included in the analysis, respectively (short term). |
| Selective reporting (reporting bias) | Unclear risk | Judgement: study outcomes were well pre‐defined and described, but protocol was not found. |
| Other bias | Low risk | Judgement: not detected. |
Gratzke 2017.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized, controlled, non‐blinded study Dates when study was conducted: February 2012 to October 2013 Setting: multicentre / international / outpatient/inpatient / 10 centres in Europe Countries: Denmark, the UK, Germany |
|
| Participants |
Inclusion criteria: men aged ≥ 50 years with IPSS > 12, Qmax ≤ 15 mL/second for 125 mL voided volume, postvoid residual volume < 350 mL, prostate volume ≤ 60 mL on ultrasound, sexually active within 6 months before the index procedure, Sexual Health Inventory for Men score > 6, positive response to MSHQ‐EjD (excluding the response “Could not ejaculate”), Incontinence Severity Index score ≤ 4 Exclusion criteria: active urinary tract infection at time of treatment, bacterial prostatitis within 1 year of the index procedure, cystolithiasis within 3 months of the index procedure, obstructive median lobe as assessed via ultrasound and cystoscopy, current urinary retention, urethral conditions that may prevent insertion of a rigid 20 F cystoscope, previous TURP or laser procedure, pelvic surgery or irradiation, PSA ≥ 10 ng/L, history of prostate or bladder cancer, severe cardiac comorbidities, anticoagulants within 3 days of the index procedure (excluding up to 100 mg aspirin (acetylsalicylic acid), other medical condition or comorbidity contraindicative for TURP or PUL, unwilling to report sexual function Total number of participants randomly assigned: 91 Group A (PUL)
Group B (TURP)
|
|
| Interventions |
Group A: PUL PUL involved transurethral placement of small, permanent UroLift implants to retract the lateral lobes of the prostate and reduce obstruction. Typically, multiple implants are placed to deobstruct the prostatic urethra. Surgeons' experiences with PUL varied from 0 to 20 procedures before enrollment. Group B: TURP Licensed urologists trained and experienced in TURP conducted procedures in accordance with their own normal standards and practices. Follow‐up: 24 months |
|
| Outcomes |
Urologic symptoms score How measured: IPSS score Time points measured: at baseline, 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years Time points reported: at baseline, 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years Subgroups: none Quality of life How measured: IPSS QoL, SF‐12, Derivative single‐index SF‐6D utility score Time points measured: at baseline, 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years Time points reported: at baseline, 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years Subgroups: none Minor and major adverse events (including indwelling urinary catheter and acute urinary retention) How measured: Clavien‐Dindo classification of adverse events Time points measured: not reported Time points reported: at 1 year Subgroups: none Erectile function and ejaculatory function How measured: Sexual Health Inventory for Men, MSHQ‐EjD Time points measured: at baseline, 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years Time points reported: at baseline, 2 weeks, 1 month, 3 months, 6 months, 1 year, and 2 years Subgroups: none Retreatment How measured: secondary treatment Time points measured: not reported Time points reported: at 2 years Subgroups: none |
|
| Funding sources | Drs Speakman, Berges, Sievert, and Sønksen reported grants from NeoTract, Inc. | |
| Declarations of interest | Dr Gratzke reported honoraria from Astellas, Lilly, Janssen, and Amgen. Dr Barber reported support from NeoTract, Inc., Olympus, Boston Scientific, and Intuitive Surgical for proctoring and lecturing. Dr Chapple reported personal fees and non‐financial support from Allergan, grants, personal fees and non‐financial support from Astellas, personal fees and non‐financial support from Boston, personal fees and non‐financial support from Medtronic, personal fees from Pfizer, personal fees and non‐financial support from Recordati, and grants from NeoTract, Inc. during the conduct of the study. Dr Sonksen reported support from NeoTract, Inc. for proctoring and lecturing. | |
| Notes |
Protocol: NCT01533038 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Parallel 1:1 randomisation was performed using permuted blocks of random sizes, stratified by study site.” |
| Allocation concealment (selection bias) | Low risk | Quote: “concealed through a password‐protected computer system,” “random sequence revealed at the time of the procedure.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Judgement: non‐blinded study. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgement: non‐blinded study. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes were not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Unclear risk | Judgement: 40/45 (88.8%) of randomized participants in PUL and 32/35 (91.4%) in TURP groups were included in analysis (short term)/ 37/45 (82.2%) of randomized participants in PUL and 32/35 (91.4%) in TURP groups were included in analysis (long term). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: all participants who were randomized were included in analyses. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: 44/45 (97.7%) of randomized participants in PUL and 35/35 (100%) in TURP groups were included in analysis. |
| Incomplete outcome data (attrition bias) Erectile function | High risk | Judgement: Short term: 32/45 (71.1%) of randomized participants in PUL and 27/35 (77.1%) in TURP were included in analysis. Long term: 29/45 (64.4%) of randomized participants in PUL and 28/35 (80.0%) in TURP were included in analysis. |
| Incomplete outcome data (attrition bias) Ejaculatory function | High risk | Judgement: Short term: 32/45 (71.1%) of randomized participants in PUL and 27/35 (77.1%) in TURP were included in analysis. Long term: 29/45 (64.4%) of randomized participants in PUL and 27/35 (77.1%) in TURP were included in analysis. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: 44/45 (97.7%) of randomized participants in PUL and 35/35 (100%) in TURP were included in analysis. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Judgement: not described in the study or protocol (not adequately described). |
| Selective reporting (reporting bias) | Low risk | Judgement: review outcomes were prespecified in the protocol (NCT01533038) and were analyzed as planned. |
| Other bias | Low risk | Judgement: not detected. |
Insausti 2020.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized, non‐inferiority clinical trial Dates when study was conducted: November 2014 and January 2017 Setting: single center Country: Spain |
|
| Participants |
Inclusion criteria: men over 60 years; BPH‐related LUTS refractory to medical treatment for at least 6 months or the patient could not tolerate medical treatment; TURP was indicated; the IPSS was ≥ 8; QoL related to LUTS was ≥ 3; and Qmax was ≤ 10 mL/s or urinary retention. Exclusion criteria: men with advanced atherosclerosis and tortuosity of the iliac arteries, non‐visualization of the prostatic artery or other accessory arteries supplying the prostate on computed tomography angiography, urethral stenosis, detrusor failure or neurogenic bladder, glomerular filtration rate of less than 30 mL/min, and the presence of prostate cancer. Total number of participants randomly assigned: 61 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: bipolar TURP Follow‐up: 12 months |
|
| Outcomes |
Urologic symptoms score How measured: IPSS score Time points measured: at baseline, 3, 6, and 12 months Time points reported: at baseline, 3, 6, and 12 months Subgroups: none Quality of life How measured: IPSS QoL Time points measured: at baseline, 3, 6, and 12 months Time points reported: at baseline, 3, 6, and 12 months Subgroups: none Erectile function How measured: IIEF‐5 Time points measured: at baseline, 3, 6, and 12 months Time points reported: (planned but not reported because there were few participants with sexual relationships) Subgroups: none Minor and major adverse events (including ejaculatory function and urinary retention) How measured: Clavien‐Dindo classification of adverse events Time points measured: at all follow‐up visit Time points reported: likely cumulative incidence Subgroups: none Relevant outcomes not reported in this study:
|
|
| Funding sources | Biocompatibles UK Ltd | |
| Declarations of interest | Biocompatibles UK Ltd | |
| Notes |
Protocol: NCT01963312 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Principal Investigator randomly selected a number from a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Quote: “the individual enrolling participants were unaware of the allocation of the next participants.” Judgement: the method was not described. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “There was no blinding of clinicians or patients due to the nature of the trial.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Quote: “There was no blinding of clinicians or patients due to the nature of the trial.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes are likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | High risk | Judgement: 23/31 (74.1%) and 22/30 (73.3%) participants randomized in PAE and TURP were included in the analysis, respectively (short term). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Ejaculatory function | Low risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Judgement: no information given (not measured, narrative statement) |
| Selective reporting (reporting bias) | High risk | Judgement: protocol was published, but study outcomes were not identical with the outcomes pre‐specified in protocol. |
| Other bias | Low risk | Judgement: BPH medication was prescribed for a longer time in the PAE group, however, it may not have affected results at 12 months after treatment. |
Larson 1998.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study. Study dates: September 1994 to June 1996 Setting: outpatient, multicenter, national Country: United States |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: men with:
Total number of participantsrandomized: 169 Group 1: n = 125 transurethral microwave thermotherapy (TUMT)
Group 2: n = 44 Sham
|
|
| Interventions |
Group 1 (n = 125): Transurethral Microwave Thermotherapy (TUMT) power was applied in increments to achieve a target urethral temperature of 40 ± 1 °C with measurement by the catheter’s fiberoptic thermosensor. Microwave treatment was administered continuously for 1 hour, with the circulation of coolant at 8 °C. Group 2 (n = 44): The same procedure as TUMT group, with the exception that microwave power was not applied, and coolant temperature was increased in increments from 8 to 20 °C over the same time period as microwave power was increased in the microwave group. It was not feasible to increase the urethral temperature further in the sham group because the Targis cooling system is not designed or equipped to provide active heating of coolant other than that occurring as the result of the application of microwave energy. The sham‐group patients experienced rising urethral temperatures rather than unchanging low temperatures. Co‐interventions: All participants underwent insertion of a Targis (formerly T3) transurethral thermoablation system treatment catheter (Urologix, Inc., Minneapolis, Minn). It is a compact and portable unit equipped with a 21F silicone treatment catheter containing a helical dipole microwave antenna operating in the range 902 to 928 MHz. This provides urethral cooling via circumferential cooling compartments and also includes a urine drainage canal and a fiberoptic thermosensor for monitoring urethral catheter interface temperatures. The thermoablation system automatically interrupts microwave power if urethral temperatures reach 44.5 °C or higher or rectal temperatures reach 42.5 °C or higher. Catheterization was carried out under topical lidocaine anaesthesia. The positioning of the catheter balloon and antenna was confirmed by TRUS. The catheter was then secured in the proper spatial orientation with respect to the posteroanterior prostatic axis. A rectal thermal unit equipped with five thermocouples was used to monitor rectal temperatures. All participants received a 3‐day prescription of prophylactic oral antibiotics and catheterization for 36 to 60 hours. |
|
| Outcomes |
Urologic symptom scores How measured: AUA score Time points measured: baseline, 6 weeks, 3 months, and 6 months Time points reported: baseline, 6 weeks, 3 months, and 6 months Subgroups: none Quality of Life How measured: QOL score was evaluated by patient responses to the question of how they would feel if their current urinary symptoms were to continue indefinitely. Time points measured: Baseline and 6 months Time points reported: baseline, 6, 9, and 12 months follow‐up (these last two time points were not reported In group 2) Subgroups: none Minor and major adverse event How measured: number of patients with UTI confirmed by urine culture and resolved with antibiotics, among other adverse events. Time points measured: not reported Time points reported: not reported Subgroups: none Retreatment How measured: number of patients requiring other treatment within the 6 months follow‐up. Time points measured: 6 months Time points reported: 6 months Subgroups: none Ejaculatory function How measured: number of patients with loss of ejaculate Time points measured: not reported Time points reported: not reported Subgroups: none Acute urinary retention How measured: number of patients with urinary retention > 1 week after the procedure Time points measured: >1 week Time points reported: > 1 week Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | This study was supported by a grant from Urologix Inc. | |
| Declarations of interest | Not available | |
| Notes | Randomization 3:1 ratio. Blinding was broken after 6 months. Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomized in a 3:1 target ratio to the microwave (n = 125) or sham (n = 44) group.” Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were randomized in a 3:1 target ratio to the microwave (n = 125) or sham (n = 44) group.” Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote: “The study was double‐blind: Neither the patients nor any of the investigators and support staff involved in carrying out the study procedures had knowledge of group assignment (microwave versus sham).” Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “The study was double‐blind: Neither the patients nor any of the investigators and support staff involved in carrying out the study procedures had knowledge of group assignment (microwave versus sham).” Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: “The study was double‐blind: Neither the patients nor any of the investigators and support staff involved in carrying out the study procedures had knowledge of group assignment (microwave versus sham).” Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | High risk | Quote: “Of the 169 patients enrolled, 155 were evaluable at the conclusion of the 6‐month blinded phase of the study (Table III) and 114 at the end of the full 12‐month follow‐up period. Analyses of efficacy results are presented for the 155 subjects evaluable at the conclusion of the blinded phase.” Unbalanced attrition at six months (20% vs 4%). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | High risk | Quote: “Of the 169 patients enrolled, 155 were evaluable at the conclusion of the 6‐month blinded phase of the study (Table III) and 114 at the end of the full 12‐month follow‐up period. Analyses of efficacy results are presented for the 155 subjects evaluable at the conclusion of the blinded phase.” Unbalanced attrition at six months (20% vs 4%). |
| Incomplete outcome data (attrition bias) Retreatment | High risk | Quote: “Of the 169 patients enrolled, 155 were evaluable at the conclusion of the 6‐month blinded phase of the study (Table III) and 114 at the end of the full 12‐month follow‐up period. Analyses of efficacy results are presented for the 155 subjects evaluable at the conclusion of the blinded phase.” Unbalanced attrition at six months (20% vs 4%). |
| Incomplete outcome data (attrition bias) Ejaculatory function | High risk | Quote: “Of the 169 patients enrolled, 155 were evaluable at the conclusion of the 6‐month blinded phase of the study (Table III) and 114 at the end of the full 12‐month follow‐up period. Analyses of efficacy results are presented for the 155 subjects evaluable at the conclusion of the blinded phase.” Unbalanced attrition at six months (20% vs 4%). |
| Incomplete outcome data (attrition bias) Acute urinary retention | High risk | Quote: “Of the 169 patients enrolled, 155 were evaluable at the conclusion of the 6‐month blinded phase of the study (Table III) and 114 at the end of the full 12‐month follow‐up period. Analyses of efficacy results are presented for the 155 subjects evaluable at the conclusion of the blinded phase.” Unbalanced attrition at six months (20% vs 4%). |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not reported (narrative statement). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
McVary 2016.
| Study characteristics | ||
| Methods |
Study design: prospective, multicentre, double‐blinded study Study dates: September 2013 to August 2014 Setting: multicenter (15) / outpatient / national Country: USA |
|
| Participants |
Inclusion criteria
Exclusion criteria
Total number of menrandomized: 197 Group A (convective radiofrequency water vapor thermal therapy)
Group B (sham)
|
|
| Interventions |
Group A: Rezūm Thermal treatment procedure was performed using the Rezūm system, including a generator containing an RF power supply, system controls and a single‐use transurethral delivery device that incorporates a standard 4 mm, 30 degree cytoscopy lens. Group B:Sham procedure Insertion of a rigid cystoscope and the Rezūm System generator. The device was activated by the investigator’s staff to generate similar sensations to the participant's body. |
|
| Outcomes |
Urologic symptom scores How measured: IPSS score Time points measured: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Time points reported: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Subgroups: IPSS severity Quality of life How measured: IPSS‐QoL / BPH Impact Index II Time points measured: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Time points reported: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Subgroups: IPSS severity Erectile function How measured: IIEF‐15 Time points measured: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Time points reported: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Subgroups: IPSS severity Ejaculatory function How measured: MSHQ‐EjD Time points measured: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Time points reported: before the procedure and at follow‐up visits (at 2 weeks, and 1, 3, 6, 12, 24, 36 months) Subgroups: IPSS severity Retreatment How measured: participants with a surgical procedure at follow‐up Time of measurement: NR but likely for follow‐up period Time of reporting: likely cumulative incidence Major and minor adverse events (including acute urinary retention and indwelling catheter) How measured: adjudicated by independent evaluation committee Time of measurement: NR but likely for follow‐up period Time of reporting: likely cumulative incidence |
|
| Funding sources | NxThera Inc., Maple Grove, Minnesota | |
| Declarations of interest | Several co‐authors had direct financial interest or relationships described as 'other' with NeoTract and NxThera as the device manufacturer. | |
| Notes | The study was unblinded at three months and patients crossed‐over (we did not include data after unblinding). Protocol:ClinicalTrial.gov (NCT01912339) Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomized with an electronic program before treatment using permuted blocks of random sizes, stratified by investigational site”. Judgement: appropriate method of sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: “randomized with an electronic program before treatment using permuted blocks of random sizes, stratified by investigational site”. Judgement: not explicitly described, but likely central randomization with allocation concealment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “Study participants and study personnel administering questionnaires were double‐blinded until the 3‐month follow‐up... The treating physician was not blinded in order to perform the treatments but did not participate in the follow‐up or the administration of outcomes questionnaires.” Judgement: personnel were not blinded (surgeon: could not feasibly be). |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “Study participants and study personnel administering questionnaires were double‐blinded until the 3‐month follow‐up... An independent data monitoring committee reviewed safety. All AEs reviewed were adjudicated by an independent clinical evaluation committee.” Judgement: the outcomes grouped here are either self‐assessed by the participant or refer to adverse event assessment. For both types of outcomes, the study provides assurance of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: blinding deemed not relevant to these outcomes. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Judgement: almost all randomized men were included in the analyses for all reported outcomes. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: almost all randomized men were included in the analyses for all reported outcomes. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: almost all randomized men were included in the analyses for all reported outcomes. |
| Incomplete outcome data (attrition bias) Erectile function | High risk | Quote: “At baseline, 32% (43 of 134) of the observed treatment subjects and 33% (20 of 61) of control subjects were not sexually active (reported “did not attempt intercourse”) within the past 4 weeks and were eliminated from the primary sexual function analyses.” Judgement: 90/136 (66.1%) and 40/61 (65.5%) men in experimental and control group were included in the analysis (subjects who reported no sexual intercourse were excluded from the analysis for sexual function: concern over prognostic imbalance). Comment: analyses of these outcomes were based on a non‐random subset of men. |
| Incomplete outcome data (attrition bias) Ejaculatory function | High risk | Quote: “At baseline, 32% (43 of 134) of the observed treatment subjects and 33% (20 of 61) of control subjects were not sexually active (reported “did not attempt intercourse”) within the past 4 weeks and were eliminated from the primary sexual function analyses.” Judgement: 90/136 (66.1%) and 40/61 (65.5%) men in experimental and control group were included in the analysis (subjects who reported no sexual intercourse were excluded from the analysis for sexual function: concern over prognostic imbalance). Comment: analyses of these outcomes were based on a non‐random subset of men. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: almost all randomized men were included in the analyses for all reported outcomes. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Low risk | Judgement: almost all randomized men were included in the analyses for all reported outcomes. |
| Selective reporting (reporting bias) | Low risk | Judgement: all outcomes prespecified in the protocol (NCT01912339) were reported and analyzed as planned. |
| Other bias | Low risk | Judgement: not detected. |
Nawrocki 1997.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized parallel study. Study dates: not reported Setting: outpatient, single center, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with symptoms of lower urinary tract dysfunction due to benign enlargement of the prostate meriting surgical treatment Qmax < 15 mL/s and voided volume ≥ 150 mL and a maximum detrusor pressure ≥ 70 cm H2O. Exclusion criteria: men with:
Total number of participants randomized: 120 Age, median (range): 70 (56‐80) years (no disaggregated data by group available) Group 1: n = 38 transurethral microwave thermotherapy (TUMT) AUA score, median(range): 19 (7‐31) Qmax, mean (SD): 8.83 (2.32) mL/s Prostate volume, mean (SD): 41.2 (14.6) mL Group 2: n = 40 sham transurethral microwave thermotherapy (TUMT) AUA score, median(range): 17.5 (7‐28) Qmax, mean (SD): 9.44 (2.78) mL/s Prostate volume, mean (SD): 46.7 (16.8) mL Group 3: n = 42 no treatment AUA score, median(range): 18 (10‐29) Qmax, mean (SD): 8.79 (2.66) mL/s Prostate volume, mean (SD): 46.4 (19.9) mL |
|
| Interventions |
Group 1 (n = 38): TUMT was delivered for an hour under local anaesthesia, through a urethral catheter. The temperature was measured through the catheter and a rectal probe and guided the cooling of the urethra through a software (Prostasoft v2.0) which was not under the control of the operator. Group 2 (n = 40): A technically identical procedure to standard TUMT with no microwaves, with similar noise and appearance with simulated heat using a heat pad. Group 3 (n = 42): No treatment (they received treatment after completion of the study). Co‐interventions: not reported |
|
| Outcomes |
Urologic symptom scores How measured: AUA score Time points measured: baseline and 6 months Time points reported: baseline and 6 months Subgroups: none Major and minor adverse events How measured: not reported Time points measured: not reported Time points reported: not reported Subgroups: none Acute urinary retention How measured: number of patients developing acute urinary retention in the first 24hs after treatment. Time points measured: not reported Time points reported: not reported Subgroups: none Indwelling urinary catheter How measured: number of patients developing acute urinary retention in the first 24hs after treatment which required catheterization for up to one week. Time points measured: not reported Time points reported: not reported Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | LORS grant from the South East Thames Regional Research Committee. | |
| Declarations of interest | Not available | |
| Notes | We included that TUMT and sham arm of these studies in our review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process. Quote: “Randomization was carried out by selecting one of three differently numbered but otherwise identical balls from a sealed bag.” |
| Allocation concealment (selection bias) | High risk | The allocation could be tampered considering that the balls could be re‐inserted to the bag and pulled out again. Quote: “Randomization was carried out by selecting one of three differently numbered but otherwise identical balls from a sealed bag.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. Quote: “The treatment of the standard and simulated TUMT groups was designed and carried out as a double‐blind, so that neither the operator nor the patient was aware of which treatment was being per‐ formed. Patients randomized to group 3 were treated after completion of the study” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | No apparent missing outcome data. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | No apparent missing outcome data. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | No apparent missing outcome data. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not fully measured (narrative statement). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. There is a trial registry (ISRCTN24866285), however it was retrospectively registered and there is no information regarding the outcomes. |
| Other bias | Low risk | No other sources of bias were identified. |
Norby 2002.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study. Study dates: May 1996 and November 1999 Setting: outpatient, multicenter, national Country: Denmark |
|
| Participants |
Inclusion criteria: symptomatic benign prostatic hyperplasia (BPH) and
Exclusion criteria: men with:
Total number of participantsrandomized: 118 Group 1: 48 Interstitial laser coagulation (ILC)
Group 2: 46 transurethral microwave thermotherapy (TUMT)
Group 3: 24 (control: TURP or TUIP)
|
|
| Interventions |
Group 1 (n = 48): “ILC was delivered by a MediLas 4100 Fibertom (Dornier, Germany), a Nd‐YAG laser with a wavelength of 1064 nm. The energy was delivered using an applicator with a quartz glass tip (length 20 mm, diameter 1.9 mm). The 3‐min radiation was used, thus applying 20 W for 30 s, 15 W for 30 s, 10 W for 30 s and 7 W for 90 s. Treatments were undertaken with a laser cystoscope (18 F) using saline as the irrigant. The fibre was placed deep within the lateral lobes at an angle in the plane of the urethra of a 30° (to avoid heating the urethral mucosa). If a median lobe was present it was treated with one or two punctures in the direction of the bladder. Initially the intent was to apply one puncture per 10 mL of prostate tissue, but later the regimen became more aggressive, aiming at one puncture per 5 mL. All patients had a suprapubic tube placed at the start of the procedure and most also had a transurethral catheter for 12‐24 h to reduce prostatic oedema. All patients received prophylactic antibiotics. Patients were discharged after removing the urethral catheter and scheduled to visit the outpatient clinic for removal of the suprapubic tube, generally at fixed intervals of 1‐2 weeks.” Group 2 (n = 46): “TUMT was administered using the Prostatron® system; before treatment cystoscopy was used to exclude bladder pathology. Prostasoft v2.0 was chosen when the prostatic volume was < 30 mL and v2.5 in larger prostates. Treatment comprised 1 h sessions under local anaesthesia with Installagel® (Farco‐Pharma GmbH, Cologne, Germany); 1 h beforehand, 100 mg of diclofenac and 500 mg ciprofloxacin was administered. During treatment pethidine was given if necessary. If patients developed urinary retention after treatment a suprapubic or a transurethral catheter was inserted and the patient seen at weekly intervals until spontaneous voiding with an acceptable PVR (in general < 100 mL) was achieved.” Group 3 (n = 24): “Patients underwent TUIP or TURP according to the surgeons’ decision. The prostate was resected using a 26 F Iglesias resectoscope with a standard resection loop and 1.5% glycine for irrigation. TUIP comprised a unilateral incision in the 7 o’clock position starting proximal to the bladder neck and extending distally to the verumontanum. After surgery a three‐way irrigation catheter was inserted and first removed when bleeding had stopped. Prophylactic antibiotics were given according to the routine of the department.” Co‐interventions: “All treatments were administered by one of the two consultants or the senior registrar. Patients were treated under spinal or general anaesthesia.” |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: baseline, 1,3, and 6 months Time points reported: baseline and 6 months Subgroups: none Quality of life How measured: not reported Time points measured: baseline, 1,3, and 6 months Time points reported: baseline and 6 months Subgroups: none Major and minor adverse event How measured: number of patients with bleeding necessitating transfusion Time points measured: 6 months Time points reported: 6 months Subgroups: none Retreatment How measured: number of patients undergoing TURP or other treatment Time points measured: 6 months Time points reported: 6 months Subgroups: none Erectile function How measured: To evaluate erectile function patients scoring 0 or 1 (i.e. normal or slightly reduced erectile capacity) were defined as ‘normal’, whereas patients scoring 2 or 3 (i.e. greatly reduced or no erectile function) were defined having decreased erectile capacity. Time points measured: 6 months Time points reported: 6 months Subgroups: none Ejaculatory function How measured: number of patients with retrograde ejaculation Time points measured: 6 months Time points reported: 6 months Subgroups: none Acute urinary retention How measured: number of patients with persistent retention after treatment Time points measured: 6 months Time points reported: 6 months Subgroups: none Indwelling urinary catheter How measured: not reported Time points measured: 6 months Time points reported: 6 months Subgroups: none |
|
| Funding sources | The study was supported by a grant from Vejle County, Denmark. | |
| Declarations of interest | Not available | |
| Notes | 2:1:1: Randomization ‐ ILC group data is not included in this review. Antibiotic regimen in ILC group was changed during the study because there was a high rate of UTI. “The study had to be stopped at the final date because of financial restrictions.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “A weighted randomisation was therefore chosen as the object was to gain maximum information about the new treatments.” Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Patients were recruited from two centres and randomized at a 2 : 2: 1 to TUMT, ILC or the control group.” Method of allocation concealment is not described in sufficient detail to allow a definite judgement. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | No blinding, and the outcomes are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding, and the outcomes are likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No blinding, but the outcomes ar not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Quote: “Analyses are presented on an intention‐to‐treat basis”. Group 1: “Before ILC but after randomisation two patients had prostate cancer diagnosed and one had a urethral stricture. A further two patients declined surgery. One of these patients completed the IPSS at 6 months by mail contact. Thus, 44 patients were available for evaluation at 6 months.” Group 2: “All patients were followed at 6 months except one who developed an apoplexy at 4 months. One patient had TURP.” Group 3: “23 of 24 patients were treated according to the randomisation. One patient declined surgery. Two patients were excluded as the pathology revealed T1 prostate cancer.” |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Quote: “Analyses are presented on an intention‐to‐treat basis”. Group 1: “Before ILC but after randomisation two patients had prostate cancer diagnosed and one had a urethral stricture. A further two patients declined surgery. One of these patients completed the IPSS at 6 months by mail contact. Thus, 44 patients were available for evaluation at 6 months.” Group 2: “All patients were followed at 6 months except one who developed an apoplexy at 4 months. One patient had TURP.” Group 3: “23 of 24 patients were treated according to the randomisation. One patient declined surgery. Two patients were excluded as the pathology revealed T1 prostate cancer.” |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Quote: “Analyses are presented on an intention‐to‐treat basis”. Group 1: “Before ILC but after randomisation two patients had prostate cancer diagnosed and one had a urethral stricture. A further two patients declined surgery. One of these patients completed the IPSS at 6 months by mail contact. Thus, 44 patients were available for evaluation at 6 months.” Group 2: “All patients were followed at 6 months except one who developed an apoplexy at 4 months. One patient had TURP.” Group 3: “23 of 24 patients were treated according to the randomisation. One patient declined surgery. Two patients were excluded as the pathology revealed T1 prostate cancer.” |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Quote: “Analyses are presented on an intention‐to‐treat basis”. Group 1: “Before ILC but after randomisation two patients had prostate cancer diagnosed and one had a urethral stricture. A further two patients declined surgery. One of these patients completed the IPSS at 6 months by mail contact. Thus, 44 patients were available for evaluation at 6 months.” Group 2: “All patients were followed at 6 months except one who developed an apoplexy at 4 months. One patient had TURP.” Group 3: “23 of 24 patients were treated according to the randomisation. One patient declined surgery. Two patients were excluded as the pathology revealed T1 prostate cancer.” |
| Incomplete outcome data (attrition bias) Ejaculatory function | Low risk | Quote: “Analyses are presented on an intention‐to‐treat basis”. Group 1: “Before ILC but after randomisation two patients had prostate cancer diagnosed and one had a urethral stricture. A further two patients declined surgery. One of these patients completed the IPSS at 6 months by mail contact. Thus, 44 patients were available for evaluation at 6 months.” Group 2: “All patients were followed at 6 months except one who developed an apoplexy at 4 months. One patient had TURP.” Group 3: “23 of 24 patients were treated according to the randomisation. One patient declined surgery. Two patients were excluded as the pathology revealed T1 prostate cancer.” |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Quote: “Analyses are presented on an intention‐to‐treat basis”. Group 1: “Before ILC but after randomisation two patients had prostate cancer diagnosed and one had a urethral stricture. A further two patients declined surgery. One of these patients completed the IPSS at 6 months by mail contact. Thus, 44 patients were available for evaluation at 6 months.” Group 2: “All patients were followed at 6 months except one who developed an apoplexy at 4 months. One patient had TURP.” Group 3: “23 of 24 patients were treated according to the randomisation. One patient declined surgery. Two patients were excluded as the pathology revealed T1 prostate cancer.” |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not fully measured (narrative statement). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Pisco 2020.
| Study characteristics | ||
| Methods |
Study design:parallel randomized controlled study Dates when study was conducted: September 2014 to March 2018 Setting: single center/ National Country: Portugal |
|
| Participants |
Inclusion criteria:men over 45 years old; diagnosis of LUTS/BPH based on clinical history, digital rectal examination, urinalysis, transrectal ultrasound, and PSA; severe LUTS defined, in a screening and in a baseline visit two weeks apart, by an IPSS of 20 and a QoL score of 3 after a minimum of six months treatment with alpha‐blockers for LUTS/BPH; Qmax < 12 mL/s; prostate volume 40 mL. Exclusion criteria:men with computed tomography angiography showing that prostatic arteries were not feasible for PAE; previous surgical or invasive prostate treatments such as TURP, transurethral microwave therapy, transurethral needle ablation, laser, or any other minimally invasive treatment; acute or chronic prostatitis or suspected prostatitis including chronic pain, intermittent pain, or abnormal sensation in the penis, testis, anal, or pelvic area in the previous 12 months; history of prostate or bladder cancer or pelvic irradiation; active or recurrent urinary tract infections (more than one episode in the previous 12 months); history of neurogenic bladder or LUTS secondary to neurologic disease; advanced atherosclerosis and tortuosity of iliac and prostatic arteries; secondary renal insufficiency (due to prostatic obstruction); large bladder diverticula or stones; detrusor failure; previous history of acute urinary retention; current severe, significant, or uncontrolled disease; bleeding disorder such as hemophilia, clotting factor deficiency, anticoagulation, or bleeding diathesis; hypersensitivity or contraindication to tamsulosin use; mental condition or disorder that would interfere with the patient’s ability to provide informed consent; participation in a study of any investigational drug or device in the previous three months; and administration of the 5‐alpha reductase inhibitors, finasteride and dutasteride, in the previous six and three months, respectively. The latter criterion was changed by a protocol amendment to the administration of the 5‐alpha reductase inhibitors, finasteride and dutasteride, in the previous two weeks and four months, respectively (these patients may be included if they stop those medications and replace them for tamsulosin, alfuzosin, or silodosin for at least two weeks and four months, respectively). Total number of participants randomly assigned: 80 Group A (PAE)
Group B (Sham)
|
|
| Interventions |
Group A:PAE Group B:sham (after catheterization of one prostatic artery, the catheter was removed and no particles were injected) Follow‐up: six months |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: baseline, 1,3, and 6 months Time points reported: baseline and 6 months Subgroups: none Quality of life How measured: IPSS QoL / BPH II Time points measured: baseline, 1,3, and 6 months Time points reported: baseline and 6 months Subgroups: none Erectile function How measured: IIEF‐15 Time points measured: baseline, 1,3, and 6 months Time points reported: baseline and 6 months Subgroups: none Major and minor adverse events (including acute urinary retention and ejaculatory disorders) How measured:Clavien‐Dindo classification Time points measured: at baseline, 1, 3, and 6 months Time points reported: likely cumulative incidence Subgroup: none Retreatment How measured: participants with a surgical procedure at follow‐up Time of measurement: NR but likely for follow‐up period Time of reporting: likely cumulative incidence Relevant outcomes not reported in this study
|
|
| Funding sources | Partially funded by an unrestricted grant from BTG plc (London, UK). | |
| Declarations of interest | None | |
| Notes |
Protocol: NCT02074644 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A randomisation list consisting of permuted blocks of size varying between 4 and 8 was prepared by the trial biostatistician.” |
| Allocation concealment (selection bias) | Low risk | Quote: “the allocation sequence was concealed using opaque envelopes numbered sequentially.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Quote: “Patients were blinded to the intervention received until the end of single‐blind period.” Judgement: single blinded study (participants). |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Judgement: Participants (for patient‐reported outcomes) were blinded. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes are likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: no information (not reported): author reply — all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Ejaculatory function | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Selective reporting (reporting bias) | Low risk | Judgement: protocol was published and study outcomes were well pre‐defined and described. |
| Other bias | Low risk | Judgement: Tamsulosin was prescribed for a longer time in the sham group; however, this may not have affected results. |
Radwan 2020.
| Study characteristics | ||
| Methods |
Study design: parallel randomized controlled study Dates when study was conducted: January 2016 to January 2018 Setting: single center/national Country: Egypt |
|
| Participants |
Inclusion criteria:men complained of LUTS with an IPSS score of 8 to 35 (8 being moderate and 35 being severe), uroflowmetry with an average flow ≤ 10 mL/s, and a prostate volume less than 100 mL by TRUS Exclusion criteria: men with elevated kidney functions (1.5 mg/dL), with allergy to intravenous contrast media, unfit for surgery, with prostatic adenocarcinoma, with previous history of prostatic or urethral operations, with signs of the decompensated bladder (e.g., bladder diverticulum), with signs of upper urinary tract infection revealed by pelvic abdominal ultrasound were excluded Total number of participants randomly assigned: 60 Group A (PAE)
Group B (TURP)
|
|
| Interventions |
Group A: PAE Group B: TURP (monopolar or bipolar) Follow‐up: 6 months |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: baseline, 1 and 6 months Time points reported: baseline, 1 and 6 months Subgroups: none Retreatment How measured: participants with a surgical procedure at follow‐up Time of measurement: NR but likely for follow‐up period Time of reporting: likely cumulative incidence Major and minor adverse events (including acute urinary retention) How measured:Clavien‐Dindo classification Time points measured: at baseline, 1, 3, and 6 months Time points reported: likely cumulative incidence Subgroup: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not reported | |
| Declarations of interest | None | |
| Notes |
Protocol: not available Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement: not described. |
| Allocation concealment (selection bias) | Unclear risk | Judgement: not described. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Unclear risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: all randomized participants were included in the analysis. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Judgement: all randomized participants were included in the analysis (catheter removal time: TURP [third postoperative day], PAE [fifth postoperative day]). |
| Selective reporting (reporting bias) | Unclear risk | Judgement: protocol was not found, the outcomes at prespecified time point (likely 1 month) were omitted. |
| Other bias | Low risk | Judgement: not detected. |
Roehrborn 1998.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study. Study dates: not reported Setting: outpatient, multicenter center, national Country: United States of America |
|
| Participants |
Inclusion criteria: men with
Exclusion criteria: not reported Total number of participantsrandomized: 220 Group 1 (n = 147) TUMT
Group 2 (n = 73) Sham
|
|
| Interventions |
Group 1 (n = 147) TUMT The Dornier Urowave (second‐generation microwave therapy device), can deliver up to 90 W of power and has an integrated water‐cooling circuit. The safety threshold was set at 50 °C in the urethra and at 42.5 °C in the rectum. Group 2 (n = 73) Sham: sham‐treated patients received a 60‐minute, preprogrammed sham treatment cycle with the catheter in place. Co‐interventions: All patients had negative urine cultures before treatment and were given peritreatment antibiotic prophylaxis (investigators’ choice). After treatment, an indwelling Foley catheter was inserted and left in place for 2 to 5 days, depending on logistics. |
|
| Outcomes |
Urologic symptom scores How measured: AUA‐SI (0 to 35 points) Time points measured: baseline, 1, 3, and 6 months. Time points reported: baseline, 1, 3, and 6 months. Subgroups: none Quality of Life How measured: AUA‐SI subscore (0 to 6 points) Time points measured: baseline, 1, 3, and 6 months. Time points reported: baseline, 1, 3, and 6 months. Subgroups: none Major and minor adverse events (including ejaculatory and erectile function) How measured: Adverse events were solicited from patients during and after treatment as well as at each follow‐up visit. Adverse events were designated as treatment related or unrelated to treatment by the investigator. Time points measured: during treatment, 72 h after treatment and up to 6 months Time points reported: during treatment, 72 h after treatment and up to 6 months Subgroups: none Acute urinary retention How measured: not reported Time points measured: baseline, 1, 3, and 6 months. Time points reported: 6 months. Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Funded by Dornier MedTech, Atlanta, Georgia | |
| Declarations of interest | Not available | |
| Notes | A secondary report states that quality of life was also measured by another scale (0‐21), however, it is not clear which scale was used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process. Quote: “The physician administering the treatment opened the centrally provided randomization envelope immediately before treatment.” |
| Allocation concealment (selection bias) | Low risk | Participants and investigators enrolling participants could not foresee assignment. Quote: “The physician administering the treatment opened the centrally provided randomization envelope immediately before treatment.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken. Quote: “They were made aware that in this trial there would be an active/sham randomization at a ratio of 2:1. Furthermore, patients were made aware that a ‘‘subset’’ of patients would have interstitial temperature monitoring by way of inserting a needle through the perineum into the prostate. However, for ethical reasons, only actively treated patients received such monitoring. Thus, the patients were effectively blinded as to whether or not they underwent active or sham treatment despite the fact that only the actively treated patients had interstitial temperature monitoring.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken. Quote: “The treating physician and assistant were excluded from the follow‐up evaluation of the patient. The physician and/or nurse involved in the follow‐up evaluation was not present in the room during treatment.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Blinding of participants and key study personnel was ensured, and it was unlikely that the blinding could have been broken. Quote: “The treating physician and assistant were excluded from the follow‐up evaluation of the patient. The physician and/or nurse involved in the follow‐up evaluation was not present in the room during treatment.” |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. Quote: “For the various parameters, between 124 and 130 of the actively treated patients (86% to 88%) were available for 6‐month follow‐up; in the sham‐treated group, between 65 and 67 (89% to 92%) of patients were available for 6‐month follow‐up.” |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. Quote: “For the various parameters, between 124 and 130 of the actively treated patients (86% to 88%) were available for 6‐month follow‐up; in the sham‐treated group, between 65 and 67 (89% to 92%) of patients were available for 6‐month follow‐up.” |
| Incomplete outcome data (attrition bias) Erectile function | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. Quote: “For the various parameters, between 124 and 130 of the actively treated patients (86% to 88%) were available for 6‐month follow‐up; in the sham‐treated group, between 65 and 67 (89% to 92%) of patients were available for 6‐month follow‐up.” |
| Incomplete outcome data (attrition bias) Ejaculatory function | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. Quote: “For the various parameters, between 124 and 130 of the actively treated patients (86% to 88%) were available for 6‐month follow‐up; in the sham‐treated group, between 65 and 67 (89% to 92%) of patients were available for 6‐month follow‐up.” |
| Incomplete outcome data (attrition bias) Acute urinary retention | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk Quote: “For the various parameters, between 124 and 130 of the actively treated patients (86% to 88%) were available for 6‐month follow‐up; in the sham‐treated group, between 65 and 67 (89% to 92%) of patients were available for 6‐month follow‐up.” |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not applicable (pre‐defined by protocol — only narrative statement). |
| Selective reporting (reporting bias) | Low risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Roehrborn 2013.
| Study characteristics | ||
| Methods |
Study design: multicentre randomized blinded trial Dates when study was conducted:February to December 2011 Setting: multicentre / International / outpatient Countries: 19 centres in US 14, Canada 2, Australia 3 |
|
| Participants |
Inclusion criteria: men aged ≥ 50 years, provided informed consent, had no prior surgical treatment for BPH, and were required to undergo washouts of 2 weeks for alpha‐blocker, 3 months for 5a‐reductase inhibitor, and 3 days for anticoagulants. Admission to the study required ≥ IPSS 13, Qmax ≤ 12 mL/second with a 125 mL voided volume and a 30‐ to 80‐mL prostate volume Exclusion criteria: median lobe obstruction, retention, postvoid residual volume > 250 mL, active infection, PSA > 10 ng/mL (unless negative biopsy), cystolithiasis within 3 months, and bacterial prostatitis within 1 year Total number of participants randomly assigned: 206 Group A (PUL)
Group B (Sham)
|
|
| Interventions |
Group A: PUL Transprostatic adjustable UroLift implants are permanently implanted to retract obstructing lateral lobes and expand the urethral lumen. After rigid cystoscopy is performed, the implant delivery device is inserted into the 20‐F sheath. Under cystoscopic visualization using a 2.9 mm 0‐degree lens, the delivery device is angled anterolaterally to compress the obstructive lobe. A 19‐gauge needle, housing a monofilament with metallic tab, is then deployed through the prostate lobe. As the needle is retracted, the tab engages the prostate capsule and the monofilament is tensioned. Finally, the urethral end‐piece is attached to the monofilament, which is then cut, delivering the in situ‐sized implant. Group B: sham Conducted with as similar an experience as possible to PUL. Follow‐up: 3 months |
|
| Outcomes |
Urologic symptom scores How measured: Reduction in IPSS at 3 months after the PUL procedure was ≥ 25% greater than that of sham Time points measured: at baseline, 2 weeks, 1 month, and 3 months Time points reported: at baseline, 2 weeks, 1 month, and 3 months Subgroups: none Quality of Life How measured: IPSS‐QoL BPH II Time points measured: at baseline, 2 weeks, 1 month, and 3 months Time points reported: at baseline, 2 weeks, 1 month, and 3 months Subgroups: none Erectile function How measured: IIEF Time points measured: at baseline, 2 weeks, 1 month, and 3 months Time points reported: at baseline, 2 weeks, 1 month, and 3 months Subgroups: none Ejaculatory function How measured: MSHQ‐EjD Time points measured: at baseline, 2 weeks, 1 month, and 3 months Time points reported: at baseline, 2 weeks, 1 month, and 3 months Subgroups: none Retreatment How measured: number of participants requiring surgery Time points measured: not reported Time points reported: likely cumulative incidence Subgroups: none Major and minor adverse events (including acute urinary retention) How measured: adverse events Time points measured: not reported Time points reported: 3 months Subgroup: none Relevant outcomes not reported in this study
|
|
| Funding sources | NeoTract, Fe/Male Health Centre | |
| Declarations of interest | NeoTract, Fe/Male Health Centre | |
| Notes |
Protocol: NCT01294150 Language of publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was conducted just before treatment using permuted blocks of various sizes chosen at random through a central electronic data program.” |
| Allocation concealment (selection bias) | Low risk | Quote: “concealed through password protected electronic database program.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Judgement: we contacted with author, and they clarified the blinding of participants and outcome assessor. The personnel were not blinded. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: “An independent data monitoring committee assessed safety, and all AEs were adjudicated and assessed by an independent clinical events committee… A double‐blind was maintained through the 3‐month end point with the patient and questionnaire administrator blinded to randomisation. Blinding of participants was tested upon discharge and at each follow‐up to 3 months.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes were not likely affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Judgement: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | Judgement: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) Retreatment | Low risk | Judgement: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | Judgement: 132/140 (94.2%) of randomized participants in PUL and 65/66 (98.4%) in sham groups were included in the analysis. |
| Incomplete outcome data (attrition bias) Ejaculatory function | High risk | Judgement: 94/140 (67.1%) of randomized participants in PUL and 50/66 (75.7%) in sham groups were included in analysis. |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: all participants who were randomized were included in analysis. |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Judgement: not described in the study or protocol (described in a narrative statement). |
| Selective reporting (reporting bias) | Low risk | Judgement: review outcomes were prespecified in the protocol (NCT01294150) and were analyzed as planned. |
| Other bias | Low risk | Judgement: not detected. |
Venn 1995.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study. Study dates: not reported Setting: outpatient, multicenter center, national Country: United Kingdom |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: not reported Total number of participantsrandomized: 96 Group 1: n = 48 Transurethral microwave hyperthermia
* no SD or 95% CI reported Group 2: n = 48 transurethral sham
* no SD or 95% CI reported |
|
| Interventions |
Group 1 (n = 48) TUMT Patients in the treated group underwent 1 h of microwave hyperthermia, with a maximum urethral temperature of 46 °C or a maximum rectal temperature of 42.5 °C. The machine was designed and constructed in conjunction with Microwave Engineering Designs, Newport, Isle of Wight, UK (434MHz, maximum power of 50 W). The antenna was a helical coil, loaded in a modified eyeless 22F Foley Simplastic catheter fitted with water cooling. Group 2 (n = 48) Sham Treated with the same procedure but without the use of heat. Co‐interventions: After selection for inclusion in the trial a treatment catheter was inserted under antibiotic cover (gentamicin 80 mg). |
|
| Outcomes |
Urologic symptom scores How measured: Madsen score. AUA score and AUA bothersome score. Time points measured: baseline, 3 and 6 months Time points reported: baseline, 3 and 6 months Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes | Patients were selected from waiting lists for transurethral resection of the prostate (TURP) at St Thomas's Hospital and Worthing Hospital, or by direct referral. Cross‐over: after 3 months, 47 patients in the treated group and 46 of the controls were assessed. After 6 months, 42 treated patients and 20 control patients were assessed, because 24 patients in the control group had been made aware of the sham treatment and so were not included in the analysis. Protocol: not available. Language of publication: English. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process. Quote: “patients were then randomly assigned to either a treated or control group by selection of sealed envelopes prepared before the trial.” |
| Allocation concealment (selection bias) | Unclear risk | Participants and investigators enrolling participants could not foresee assignment, although it is not clear if the envelopes were opaque. Quote: “patients were then randomly assigned to either a treated or control group by selection of sealed envelopes prepared before the trial.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | It is unclear if personnel was blinded (first three months). Quote: “The patients were not aware of the group to which they were assigned.” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | These outcomes are likely to be affected by blinding. Quote: “The patients were not aware of the group to which they were assigned.” |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | These outcomes are unlikely to be affected by blinding. Quote: “The patients were not aware of the group to which they were assigned.” |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | All outcomes: outcome data was available for nearly all participants. After 3 months, 47/48 patients in the treated group and 46/48 of the controls were assessed (6 month data not included in this review, see “notes”). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Wagrell 2002.
| Study characteristics | ||
| Methods |
Study design: prospective, randomized study Study dates: October 1998 to November 1999 Setting: outpatient, multicenter center, international Country: Scandinavia and United States of America |
|
| Participants |
Inclusion criteria: men with:
Exclusion criteria: not reported Total number of participantsrandomized: 154 Group 1: n = 103 Microwave Treatment
Group 2: n = 51 Transurethral resection of the prostate
|
|
| Interventions |
Group 1 (n = 103) TUMT ProstaLund Feedback measured temperatures and were continuously displayed on the device computer. Using the heat equation, the device also calculates the extent of the coagulation necrosis continuously during the treatment, stopping at 55 °C. Group 2 (n = 51): TURP TURP was performed as a clinical standard inpatient procedure according to the routines at each center. Co‐interventions: A washout period of at least 6 weeks preceded the treatment for patients who had been using any alpha‐receptor blocker or finasteride. |
|
| Outcomes |
Urologic symptom scores How measured: International Prostate Symptom Score (IPSS) Time points measured: baseline, 3, 6, 12, 24, 36, 48, and 60 months. Time points reported: baseline, 3, 6, 12, 24, 36, 48, and 60 months. Subgroups: none Quality of Life How measured: QoL domain of IPSS score Time points measured: baseline, 3, 6, 12, 24, 36, 48, and 60 months. Time points reported: baseline, 3, 6, 12, 24, 36, 48, and 60 months. Subgroups: none Mayor adverse events How measured: All adverse events occurring during the entire study period were reported. A serious adverse event was defined according to International Congress on Harmonization as any untoward medical event that resulted in death, was life‐threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, was cancer, or required intervention to prevent permanent damage to body functions or structure. Time points measured: during treatment and up to 12 months. Time points reported: during treatment and up to 12 months. Subgroups: none Minor adverse events (includes acute urinary retention and erectile dysfunction) How measured: not reported Time points measured: during treatment or up to 12 months, and from 12 to 60 months. Time points reported: during treatment or up to 12 months, and from 12 to 60 months. Subgroups: none Indwelling urinary catheter How measured: time with the catheter Time points measured: after the procedure Time points reported: after the procedure Subgroups: none Retreatment How measured: number of participants with additional medical or surgical treatment Time points measured: after the procedure Time points reported: after the procedure Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Funded by ProstaLund. | |
| Declarations of interest | Wagrell L, Schelin S, Larson TR, and Mattiasson A were paid consultants to the sponsor of this study. | |
| Notes | A total of 154 patients were included on an intention‐to‐treat basis. Eight patients (5 in the TURP and 3 in the PLFT group) were withdrawn before treatment, resulting in a total of 146 treated patients; 100 in the PLFT arm and 46 in the TURP arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. Quote: “The randomisation ratio between PLFT and TURP was 2:1.” |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The subjective outcomes were likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Whereas blinding was not mentioned, the interventions were visibly different (surgery versus outpatient procedure). The objective outcomes were unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | 12 months: balanced attrition, and outcome data was available for 133/154 (86%). Judgement: low risk of bias (short term). 24 months: outcome data was available for 79/103 in the TUMT group and 39/51 in the TURP group (76%). 36 months: outcome data was available for 69/103 in the TUMT group and 35/51 in the TURP group. 60 months: outcome data was available for 62/103 in the TUMT group and 34/51 in the TURP group. Judgement: high risk of bias (long term). |
| Incomplete outcome data (attrition bias) Major adverse events/minor adverse events | Low risk | 12 months: balanced attrition, and outcome data was available for 133/154 (86%). Judgement: low risk of bias (short term data only). |
| Incomplete outcome data (attrition bias) Retreatment | High risk |
24 months: outcome data was available for 79/103 in the TUMT group and 39/51 in the TURP group (76%). 36 months: outcome data was available for 69/103 in the TUMT group and 35/51 in the TURP group. 60 months: outcome data was available for 62/103 in the TUMT group and 34/51 in the TURP group. Judgement: high risk of bias (long term). |
| Incomplete outcome data (attrition bias) Erectile function | Low risk | 12 months: balanced attrition, and outcome data was available for 133/154 (86%). Judgement: low risk of bias (short term). 24 months: outcome data was available for 79/103 in the TUMT group and 39/51 in the TURP group (76%). 36 months: outcome data was available for 69/103 in the TUMT group and 35/51 in the TURP group. 60 months: outcome data was available for 62/103 in the TUMT group and 34/51 in the TURP group. Judgement: high risk of bias (long term). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | 12 months: balanced attrition, and outcome data was available for 133/154 (86%). Judgement: low risk of bias (short term data only). |
| Incomplete outcome data (attrition bias) Indwelling catheter | Unclear risk | Not fully measured (narrative statement). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | No other sources of bias were identified. |
Zhu 2018.
| Study characteristics | ||
| Methods |
Study design: parallel randomized controlled study Dates when study was conducted: January to October 2016 Setting: single center Country:China |
|
| Participants |
Inclusion criteria:men with:
Exclusion criteria: men with:
Total number of participants randomly assigned: 40 Group A (PAE)
Group B (Sham)
|
|
| Interventions |
Group A: PAE Group B: TURP (not defined) Follow‐up: 12 months |
|
| Outcomes |
Urologic symptom scores How measured: IPSS Time points measured: at baseline, 3, 6, and 12 months Time points reported: at baseline, 3, 6, and 12 months Subgroups: none Quality of Life How measured: IPSS‐QoL Time points measured: at baseline, 3, 6, and 12 months Time points reported: at baseline, 3, 6, and 12 months Subgroups: none Acute urinary retention How measured: not reported Time points measured: within 12 months Time points reported: likely cumulative incidence. Subgroups: none Relevant outcomes not reported in this study
|
|
| Funding sources | Not available | |
| Declarations of interest | Not available | |
| Notes |
Protocol: not available Language of publication: Chinese |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement: random number table method. |
| Allocation concealment (selection bias) | Unclear risk | Judgement: not described. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Judgement: not described; blinding highly unlikely to have taken place. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Judgement: objective outcomes are likely not affected by lack of blinding. |
| Incomplete outcome data (attrition bias) Urologic symptom scores/Quality of life | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Incomplete outcome data (attrition bias) Acute urinary retention | Low risk | Judgement: all randomized participants were included in the analysis (short term). |
| Selective reporting (reporting bias) | Unclear risk | Judgement: study outcomes were well pre‐defined and described, but protocol was not found. |
| Other bias | Low risk | Judgement: not detected. |
BPH: benign prostatic hyperplasia; ICS male IS‐SF: International Continence Society short‐form male questionnaire; IIEF‐15: International index of erectile function; IPSS: International Prostate Symptom Score; MSHQ‐EJD; Male sexual health questionnaire for ejaculatory dysfunction; NA: not available; NR: not reported; OAB‐q SF: Overactive bladder questionnaire short form; PGI‐I: Patient Global Impression of Improvement; PSA: prostate specific antigen; PUL: prostatic urethral lift; PVR: post‐void residual volume; Qmax: maximum flow rate; QoL: quality of life; SD: standard deviation; SF‐6D: Short‐Form Six‐Dimension; SF‐12: 12‐item Short‐Form Health Survey; TURP: transurethral resection of prostate; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albala 2000 | Ineligible intervention (Variant technique: periurethral); cross‐over at 3 months with no interpretable outcome data. |
| Arai 2000 | Prospective observational study comparing TUMT with other modalities. |
| Bagla 2017 | Irrelevant study design (retrospective chart review for cost analysis). |
| Bilhim 2015 | Letter to editor. |
| Brown 2018 | Irrelevant study design (retrospective comparative study). |
| D'Ancona 1997 | Observational non‐comparative study. |
| Dahlstrand 2003 | Review article (full‐text assessment). |
| Djavan 1999 | Ineligible comparison: TUMT ± neoadjuvant alpha‐blocker. |
| Gratzke 2018 | Wrong study design: single arm study for prostatic urethral lift. |
| Hahn 2000 | Observational study on cardiovascular complications of TUMT. |
| Hansen 1998 | Methods paper on the symptoms scores. The TUMT data come from an observational study. |
| ISRCTN23921450 | “Please note that this trial was terminated due to poor recruitment.” |
| Kobelt 2004 | Economic data only from the Wagrell 2002 trial. |
| Lim 2011 | Case series of a temporary nitinol device. |
| Mulvin 1994 | Non‐randomized comparative study of TUMT and transurethral catheter therapy. |
| NCT01835860 | Irrelevant study design (single group assignment). |
| Norby 2002b | Economic data only of the Nørby 2002a study. |
| Nørby 2004 | Review article (full‐text assessment). |
| Ohigashi 2007 | Prospective observational study comparing TUMT with other modalities. |
| Pereira 2018 | Irrelevant study design (retrospective comparative study). |
| Porpiglia 2015 | Single‐arm study of the first‐generation TIND device. |
| Porpiglia 2019 | Single‐arm study of the second‐generation TIND device. |
| Qiu 2017 | Irrelevant study design (retrospective comparative study). |
| Russo 2015 | Irrelevant comparator (open simple prostatectomy). |
| Schelin 2006 | Ineligible comparison: Compares TUMT to a group of participants that underwent TURP and enucleation surgery (no disaggregated data available). |
| Servadio 1987 | Observational study of the use of TUMT for various diseases of the prostate. |
| Shore 2010 | Ineligible comparison: Compared 2 similar energy TUMT systems that differed only by an adjunct balloon dilator. |
| Tan 2005 | Long‐term follow‐up of the sham crossed‐over group. Ten out of 12 participants in the sham group had crossed over to the active treatment group and no disaggregated data were available for this group before crossing over. |
| Trock 2004 | Pooled observational with previously extracted RCT data. |
| Vesely 2006 | Non‐randomized comparative study: participants were assigned by severity to TUMT or TURP. |
| Waldén 1998 | Economic data only on the Dahlstrand 1995 study. |
| Woo 2018 | Wrong study design (educational lecture). |
| Yachia 1996 | Non‐randomized comparison of two types of prostatic stents. |
| Zerbib 1992 | Ineligible intervention: Transrectal hyperthermia. |
| Zerbib 1994 | Ineligible intervention: Transrectal hyperthermia. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617001235392.
| Study name | PAE for patients with LUTS due to BPH |
| Methods |
Study design: parallel randomized controlled trial (open label) Setting/Country: single center / New Zealand |
| Participants |
Inclusion criteria
|
| Interventions |
Group A: PAE Group B: TURP |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | August 2017 |
| Contact information | martin.krauss@cdhb.health.nz |
| Notes | Sponsor: Christchurch hospital |
NCT02006303.
| Study name | Prostatic artery embolization versus 532 nm green light PVP for catheterized patients |
| Methods |
Study design: parallel randomized controlled trial (open label) Setting/Country: multicenter / Canada |
| Participants |
Inclusion criteria:
|
| Interventions |
Group A: PAE Group B: Green light PVP |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | December 2013 |
| Contact information | mostafa.elhilali@muhc.mcgill.ca |
| Notes | The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years. Sponsor: Royal Victoria Hospital, Canada |
NCT02566551.
| Study name | Prospective controlled randomized study of PAE vs TURP for BPH treatment |
| Methods |
Study design: single (outcome assessor) blinded parallel randomized controlled trial Setting/Country: single center / Spain |
| Participants |
Inclusion criteria: Patients evaluated in the Urology Service because of BPH, candidate to TURP.
|
| Interventions |
Group A: PAE Group B: TURP |
| Outcomes |
Primary outcome
Secondary outcomes
Other outcomes
|
| Starting date | October 2015 |
| Contact information | mgregori@unizar.es |
| Notes | This study is currently recruiting participants. Sponsor: Group of Research in Minimally Invasive Techniques Hospital Clínico Universitario Lozano Blesa Universidad de Zaragoza |
NCT04178811.
| Study name | Comparison between Holmium laser enucleation and prostatic urethral lift in management of BPH |
| Methods |
Study design: single (outcome assessor) blinded parallel randomized controlled trial Setting/Country: likely single center / Egypt |
| Participants |
Inclusion criteria:
|
| Interventions |
Group A: Holmium laser enucleation Group B: prostatic urethral lift |
| Outcomes |
Primary outcome
|
| Starting date | November 2020 |
| Contact information | mostafamostafa@aun.edu.eg |
| Notes | Sponsor: Assiut University |
NCT04236687.
| Study name | PAE compared to Holmium laser enucleation of the prostate for BPH |
| Methods |
Study design: parallel randomized controlled trial (open label) Setting/Country: single center / Spain |
| Participants |
Inclusion criteria Patients evaluated in the urology department and candidates to surgical treatment
|
| Interventions |
Group A: PAE Group B: Holmium laser enucleation of the prostate |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | February 2020 |
| Contact information | fagreda.germanstrias@gencat.cat |
| Notes | Sponsor: Hospital Universitari Germans Trias i Pujol |
NCT04338776.
| Study name | Comparing UroLift experience against Rezūm (CLEAR) |
| Methods |
Study design: parallel randomized controlled trial (open label) Setting/Country: not reported |
| Participants |
Inclusion criteria
|
| Interventions |
Group A: UroLift (prostatic urethral lift) Group B: Rezūm (convective radiofrequency water vapor thermal therapy) |
| Outcomes |
Primary outcome
|
| Starting date | August 2020 |
| Contact information | emily.friedland@teleflex.com |
| Notes | Sponsor: NeoTract, Inc. |
BPH: benign prostatic hyperplasia; IIEF: International Index of Erectile Function; IPSS: International Prostate Symptom Score; LUTS: lower urinary tract symptoms; MRI: magnetic resonance imaging; PAE: prostatic arterial embolization; PSA: prostate specific antigen; PVR: post void residual; Qmax: maximum flow rate; QoL: quality of life; TURP: transurethral resection of prostate.
Differences between protocol and review
Outcomes
We analyzed the data for major adverse events using short‐term and long‐term data (i.e. studies with short‐term and long‐term follow‐up) since most studies do not differentiate the timing for this outcome. With the consultation of experts, we inferred that most major adverse events occur at short‐term follow‐up. We did not add “short term” to the outcome because some adverse events (e.g. urethral stricture) occurred at long‐term follow‐up.
Selection of studies
We used Covidence for de‐duplicating results instead of EndNote 2016.
Measurement of treatment effect
We had specified that we “will report outcome data from other scales separately in a narrative synthesis of quantitative data.” Considering that outcome data in other scales (Madsen scores) were only available for one intervention and were fully reported in a supporting review (Franco 2021), we did not include the narrative synthesis in this review.
We had specified that we “will use the rank‐heat plot to present SUCRA values for all outcomes in a single plot (Veroniki 2016)”; however, we decided to display them in the traditional format, using the package in Stata.
Data synthesis
While in our protocol we specified methods for pairwise comparison, in order to avoid duplication with the supporting reviews of this network meta‐analysis, we described only the pairwise comparisons for the data that could not be included in the network due to concerns about transitivity.
'Summary of findings' tables
We had planned to include a confidence interval for ranking, but we considered it inadequate, as we are using a frequentist approach that accounts for uncertainty in the ranking (Veroniki 2018). Therefore, we reported the 'probability of being the best' instead.
We had not specified in the protocol which would be the reference treatment when displaying effect estimates in 'Summary of findings' tables and for our main network meta‐analyses. With the input of experts, we decided to display results regarding TURP, considering that this is the standard treatment for the condition.
Methods not implemented
We could not perform network meta‐analysis for all outcomes and time points due to the scarcity of data, especially long term results.
We did not perform sensitivity analysis considering the lack of studies at low risk of bias for our outcomes.
We were unable to perform subgroup analysis for prostate size since only one study included participants with prostate size < 40 mL. Furthermore, we were unable to perform other subgroup analyses based on age and symptoms severity due to the scarcity of information (few trials included participants < 65 years and IPSS scores < 19; see Table 7).
Contributions of authors
JVAF: conception and study design and drafting the protocol, data extraction and analysis, writing the full review.
JHJ: drafting the protocol, data extraction and analysis, writing the full review.
MI: drafting the protocol, providing clinical input and approving the final draft.
MB: drafting the protocol, providing clinical input and approving the final draft.
SY: revising the protocol, providing clinical input and approving the final draft.
MIO: drafting the protocol, providing clinical input and approving the final draft.
JG: providing clinical input and approving the final draft.
CMEL: creating search strategies and searching for trials, writing the methods and results section related to the searches and approving the final draft.
AAV: drafting the protocol, providing supervision on the statistics and approving the final draft.
LG: drafting the protocol, data extraction and analysis, writing the full review.
PD: conception and study design, providing clinical and methodological advice on the protocol.
Sources of support
Internal sources
-
Instituto Universitario Hospital Italiano, Argentina
Salary support for Juan Franco, Luis Garegnani, Camila Micalea Escobar Liquitay
-
Department of Urology, Yonsei University Wonju College of Medicine, Korea, South
Salary support for Jae Hung Jung
-
Minneapolis VA Health Care System, USA
Salary support for Philipp Dahm
-
Department of Urology, University of Minnesota, USA
Support in kind for Philipp Dahm
External sources
-
None, Argentina
N/A
-
National Institute for Health Research (NIHR), UK
Cochrane Incentive Award 2019: NIHR130819
Declarations of interest
JVAF: none known.
JHJ: none known.
MI: none known.
SY: Boston Scientific (speaker), Galvanize (consultant)
JG: none known.
MB: Boston Scientific (consultant for endourology and stone management), Auris Health (consultant for robotic surgery and endourology).
MIO: none known.
CMEL: none known.
AAV: none known.
LG: none known.
PD: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Abbou 1995 {published data only}
- Abbou CC, Payan C, Viens-Bitker C, Richard F, Boccon-Gibod L, Jardin A, et al. Transrectal and transurethral hyperthermia versus sham treatment in benign prostatic hyperplasia: a double-blind randomized multicentre clinical trial. British Journal of Urology 1995;76:619-24. [DOI] [PubMed] [Google Scholar]
Abt 2018 {published data only}
- Abt D, Hechelhammer L, Müllhaupt G, Kessler T, Schmid H P, Engeler DS, et al. Prostatic artery embolization vs conventional TUR-P in the treatment of benign prostatic hyperplasia: First results of a prospective, randomized non-inferiority trial. European Urology, Supplements 2016;15(3):e1080. [DOI: 10.1016/S1569-9056(16)61081-3] [DOI] [Google Scholar]
- Abt D, Hechelhammer L, Müllhaupt G, Markart S, Güsewell S, Kessler TM, et al. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ 2018;361:k2338. [DOI: 10.1136/bmj.k2338] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt D, Müllhaupt G, Hechelhammer L, Markart S, Güsewell S, Schmid HP, et al. Prostatic artery mmbolisation versus transurethral resection of the prostate for penign prostatic hyperplasia: 2-yr outcomes of a randomised, open-label, single-centre trial. European Urology 18 February 2021 [Online ahead of print]. [DOI: 10.1016/j.eururo.2021.02.008] [DOI] [PubMed]
- Abt D, Mordasini L, Hechelhammer L, Kessler TM, Schmid HP, Engeler DS. Prostatic artery embolization versus conventional TUR-P in the treatment of benign prostatic hyperplasia: protocol for a prospective randomized non-inferiority trial. BMC Urology 2014;14:94. [DOI: 10.1186/1471-2490-14-94] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02054013. Prostatic artery embolization vs. conventional transurethral prostatectomy in the treatment of benign prostatic hyperplasia. Available from clinicaltrials.gov/ct2/show/NCT02054013 (accessed 15 March 2020).
- NCT03521648. Database for the assessment of efficacy and safety of BPH treatment. Available from clinicaltrials.gov/show/NCT03521648 (accessed 15 March 2020).
Ahmed 1997 {published data only}
- Ahmed M, Bell T, Lawrence WT, Ward JP, Watson GM. Transurethral microwave thermotherapy (Prostatron version 2.5) compared with transurethral resection of the prostate for the treatment of benign prostatic hyperplasia: a randomized, controlled, parallel study. British Journal of Urology 1997;79:181-5. [DOI] [PubMed] [Google Scholar]
Albala 2002 {published data only}
- Albala DM, Fulmer BR, Turk TMT, Koleski F, Andriole G, Davis BE, et al. Office-based transurethral microwave thermotherapy using the TherMatrx TMx-2000. Journal of Endourology 2002;16:57-61. [DOI] [PubMed] [Google Scholar]
Bdesha 1994 {published data only}
- Bdesha AS, Bunce CJ, Kelleher JP, Snell ME, Vukusic J, Witherow RO. Transurethral microwave treatment for benign prostatic hypertrophy: a randomised controlled clinical trial. BMJ 1993;306:1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bdesha AS, Bunce CJ, Snell ME, Witherow RO. A sham controlled trial of transurethral microwave therapy with subsequent treatment of the control group. Journal of Urology 1994;152:453-8. [DOI] [PubMed] [Google Scholar]
Blute 1996 {published data only}
- Blute ML, Patterson DE, Segura JW, Tomera KM, Hellerstein DK. Transurethral microwave thermotherapy v sham treatment: double-blind randomized study. Journal of Endourology 1996;10:565-73. [DOI] [PubMed] [Google Scholar]
Brehmer 1999 {published data only}
- Brehmer M, Wiksell H, Kinn A. Sham treatment compared with 30 or 60 min of thermotherapy for benign prostatic hyperplasia: a randomized study. BJU International 1999;84:292-6. [DOI] [PubMed] [Google Scholar]
Carnevale 2016 {published data only}
- Carnevale FC, Iscaife A, Yoshinaga EM, Moreira AM, Antunes AA, Srougi M. Transurethral resection of the prostate (TURP) versus original and perfected prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovascular and interventional radiology 2016;39(1):44-52. [DOI: 10.1007/s00270-015-1202-4] [DOI] [PubMed]
- Yoshinaga EM, Nakano E, Marchini GS, Galvao O, Baroni R, Carnevale FC, et al. A prospective and randomized trial comparing transurethral resection of the prostate (TURP) to prostate artery embolization (PAE) for treatment of bladder outlet obstruction due to benign prostatic hyperplasia (BPH). Journal of Urology 2014;191(4 Suppl):e793. [DOI: 10.1016/j.juro.2014.02.2168] [DOI]
Chughtai 2020 {published data only}
- Chughtai B, Elterman D, Shore N, Gittleman M, Motola J, Pike S et al. The iTind Temporarily Implanted Nitinol Device for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia: A Multicenter, Randomized, Controlled Trial. Urology 2020;Online ahead of print:1-7. [DOI: 10.1016/j.urology.2020.12.022] [DOI] [PubMed] [Google Scholar]
- NCT02506465. Pivotal study to assess the safety and effectiveness of the iTIND device. Available from clinicaltrials.gov/ct2/show/NCT02506465 (First posted: 23 July 2015).
D'Ancona 1998 {published data only}
- D'Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, la Rosette JJ. High energy thermotherapy versus transurethral resection in the treatment of benign prostatic hyperplasia: results of a prospective randomized study with 1 year of followup. Journal of Urology 1997;158:120-5. [DOI] [PubMed] [Google Scholar]
- D'Ancona FC, Francisca EA, Witjes WP, Welling L, Debruyne FM, De La Rosette JJ. Transurethral resection of the prostate vs high-energy thermotherapy of the prostate in patients with benign prostatic hyperplasia: long-term results. British Journal of Urology 1998;81:259-64. [DOI] [PubMed] [Google Scholar]
Dahlstrand 1995 {published data only}
- Dahlstrand C, Geirsson G, Fall M, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for benign prostatic hyperplasia: preliminary results of a randomized study. European Urology 1993;23:292-8. [DOI] [PubMed] [Google Scholar]
- Dahlstrand C, Walden M, Geirsson G, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for symptomatic benign prostatic obstruction: a prospective randomized study with a 2-year follow-up. British Journal of Urology 1995;76:614-8. [DOI] [PubMed] [Google Scholar]
- Dahlstrand C, Walden M, Geirsson G, Sommar S, Pettersson S. Transurethral microwave thermotherapy versus transurethral resection for BPH. Transurethral microwave thermotherapy versus transurethral resection for BPH. Progress in Clinical and Biological Research 1994;386:455-61. [PubMed] [Google Scholar]
De Wildt 1996 {published data only}
- De La Rosette JJMCH, De Wilt MJAM, Alivizatos G, Froeling FMJA, Debruyne FMJ. Transurethral microwave thermotherapy (TUMT) in benign prostatic hyperplasia: placebo versus TUMT. Urology 1994;44:58-63. [DOI] [PubMed] [Google Scholar]
- De Wildt MJ, Hubregtse M, Ogden C, Carter SS, Debruyne FM, De la Rosette JJ. A 12-month study of the placebo effect in transurethral microwave thermotherapy. British Journal of Urology 1996;77:221-7. [DOI] [PubMed] [Google Scholar]
- Francisca EA, d Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, la Rosette JJ. Quality of life assessment in patients treated with lower energy thermotherapy (Prostasoft 2.0): results of a randomized transurethral microwave thermotherapy versus sham study. Journal of Urology 1997;158:1839-44. [DOI] [PubMed] [Google Scholar]
- Ogden CW, Reddy P, Johnson H, Ramsay JW, Carter SS. Sham versus transurethral microwave thermotherapy in patients with symptoms of benign prostatic bladder outflow obstruction. Lancet 1993;341:14-7. [DOI] [PubMed] [Google Scholar]
Floratos 2001 {published data only}
- Floratos DL, Kiemeney LA, Rossi C, Kortmann BB, Debruyne FM, La Rosette JJ. Long-term followup of randomized transurethral microwave thermotherapy versus transurethral prostatic resection study. Journal of Urology 2001;165:1533-8. [PubMed] [Google Scholar]
- Francisca EA, d Ancona FC, Hendriks JC, Kiemeney LA, Debruyne FM, La Rosette JJ. A randomized study comparing high-energy TUMT to TURP: quality-of-life results. European Urology 2000;38:569-75. [DOI] [PubMed] [Google Scholar]
Gao 2014 {published data only}
- Gao Ya, Huang Y, Zhang R, Yang Yd, Zhang Q, Hou M, et al. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate--a prospective, randomized, and controlled clinical trial. Radiology 2014;270(3):920-8. [DOI: 10.1148/radiol.13122803] [DOI] [PubMed]
Gratzke 2017 {published data only}
- Barber N, Sønsken J, Gratze C, Speakman M, Berges R, Wetterauer U, et al. BPH6 randomized study of prostatic urethral lift (PUL) vs transurethral resection of the prostate (TURP): outcomes and patient satisfaction. Journal of Urology 2015;193(Suppl 4):e19. [DOI: 10.1016/j.juro.2015.02.104] [DOI] [Google Scholar]
- Chin P, Sønsken J, Barber N, Gratzke C, Speakman M, Berges R, et al. BPH6 trial: a multi-centre, prospective, randomised study of the prostatic urethral lift vs. transurethral resection of the prostate (TURP). BJU International 2015;115(Suppl 4):10. [DOI: 10.1111/bju.13072] [DOI] [Google Scholar]
- Chin P, Woo H, Speakman M, Sønksen J, Gratzke C. Improved sleep after TURP and prostatic urethral lift (PUL): prospective, randomized study. BJU International 2017;119(Suppl 2):82. [DOI: 10.1111/bju.13752] [DOI] [PubMed] [Google Scholar]
- Gratzke C, Barber N, Speakman MJ, Berges R, Wetterauer U, Greene D, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU International 2017;119(5):767-75. [DOI: 10.1111/bju.13714] [DOI] [PubMed] [Google Scholar]
- Gratzke C, Barber NJ, Speakman MJ, Berges R, Wetterauer U, Greene D, et al. Two year results of the BPH6 trial: a multi-center, prospective, randomized study of the prostatic urethral lift (PUL) vs transurethral resection of the prostate (TURP). European Urology Supplements 2016;15(3):e1076-a. [DOI: 10.1016/S1569-9056(16)61077-1] [DOI]
- Gratzke C, Chin P, Barber N, Speakman M, Berges R, Wetterauer U, et al. BPH6 trial two year results: the multi-national, prospective, randomised study of the prostatic urethral lift (PUL) compared to transurethral resection of the prostate (TURP). BJU International 2016;117(Suppl 3):17-8. [DOI: 10.1111/bju.13452] [DOI]
- NCT01533038. BPH-6: comparison of the UroLift System to TURP for benign prostatic hyperplasia. Available from clinicaltrials.gov/ct2/show/NCT01533038 (first received 15 February 2012). [NCT01533038]
- Sønksen J, Barber NJ, Speakman MJ, Berges R, Wetterauer U, Greene D, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. European Urology 2015;68(4):643-52. [DOI: 10.1016/j.eururo.2015.04.024] [DOI] [PubMed]
- Sønsken J, Barber N, Speakman M, Berges R, Wetterauer U, Greene D, et al. Multi-national, prospective, randomized study of the prostatic urethral lift (PUL) vs. transurethral resection of the prostate (TURP): two year results. Journal of Urology 2016;195(Suppl 4):e456. [DOI: 10.1016/j.juro.2016.02.1468] [DOI] [Google Scholar]
Insausti 2020 {published data only}
- Giral Villalta PJ, Aguilar Guevara JF, Lopez Ubillos G, Lacarra Fernandez S, Zabalo San Juan A, Asiáin Urmeneta M, et al. Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: 12 month results of a clinical trial. European Urology, Supplements 2019;18(1):e1494-5. [DOI: 10.1016/S1569-9056(19)31075-9] [DOI]
- Insausti I, Sáez de Ocáriz García A, Galbete A, Capdevila F, Solchaga S, Giral P, et al. Randomized comparison of prostatic arterial embolization versus transurethral resection of the prostate for treatment of benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology 2020 Apr 2 [Epub ahead of print]. [DOI: 10.1016/j.jvir.2019.12.810] [DOI] [PubMed]
- Napal Lecumberri S, Insausti Gorbea I, Sáez de Ocáriz García A, Solchaga Álvarez S, Cebrián Lostal JL, Monreal Beortegui R, et al. Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: protocol for a non-inferiority clinical trial. Research and Reports in Urology 2018;10:17-22. [DOI: 10.2147/RRU.S139086] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01963312. Clinical trial to evaluate the efficacy and safety of the transarterial supraselective embolization of the prostate to treat the urinary symptoms. Available from clinicaltrials.gov/ct2/show/NCT01963312 (accessed 15 March 2020).
- Saez De Ocariz Garcia A, Insausti Gorbea I, Solchaga Alvarez S, Monreal Beortegui R, Giral Villalta PJ, Napal Lecumberri S, et al. Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: 6-month results of a clinical trial. Cardiovascular and Interventional Radiology 2017;40(2):S117-8. [Google Scholar]
Larson 1998 {published data only}
- Larson TR, Blute ML, Bruskewitz RC, Mayer RD, Ugarte RR, Utz WJ. A high-efficiency microwave thermoablation system for the treatment of benign prostatic hyperplasia: results of a randomized, sham-controlled, prospective, double-blind, multicenter clinical trial. Urology 1998;51:731-42. [DOI] [PubMed] [Google Scholar]
McVary 2016 {published data only}
- Albala DM, McVary K, Roehrborn C. Convective water vapor thermal therapy: 3-year durable outcomes of a randomized controlled study for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Canadian Urological Association Journal 2018;12(9):S197. [DOI: 10.5489/cuaj.5656] [DOI] [Google Scholar]
- Albala DM, McVary KT, Roehrborn CG, Ulchaker JC. Transurethral convective radiofrequency water vapor thermal therapy for symptomatic benign prostatic hyperplasia: two year outcomes of a randomized, controlled, and prospective crossover study. Canadian Urological Association Journal 2017;11(9):S326-7. [DOI: 10.5489/cuaj.4896] [DOI] [Google Scholar]
- Gupta N, Köhler T, McVary K. Convective radiofrequency water vapor energy ablation (Rezūm®) effectively treats lower urinary tract symptoms due to benign prostatic enlargement regardless of obesity while preserving erectile and ejaculatory function. Journal of Urology 2017;197(4):e609. [Google Scholar]
- Gupta N, Köhler T, McVary K. Convective radiofrequency water vapor energy ablation effectively treats lower urinary tract symptoms due to benign prostatic enlargement regardless of obesity while preserving erectile and ejaculatory function: results of a multicenter, randomized, controlled trial. European Urology 2017;16(3 (Supplement)):e521-2. [DOI: 10.1016/S1569-9056(17)30366-4] [DOI] [Google Scholar]
- Gupta N, Köhler TS, McVary KT. Convective water vapor energy ablation effectively treats lower urinary tract symptoms due to benign prostatic enlargement while preserving erectile and ejaculatory function: results of a multicenter, randomized, controlled trial. Journal of Sexual Medicine 2017;14(2):e33. [Google Scholar]
- Helo S, Tadros N, Gupta N, Köhler TS, McVary KT. Convective radiofrequency thermal therapy (Rezūm®) effectively treats lower urinary tract symptoms due to benign prostatic hyperplasia regardless of obesity while preserving erectile and ejaculatory function. Journal of Sexual Medicine 2018;15(2):S18. [Google Scholar]
- McVary K, Gange S, Gittelman M, Goldberg K, Patel K, Shore N, et al. Treatment of lower urinary tract symptoms due to benign prostatic hyperplasia with convective water vapor energy ablation: Preserved erectile and ejaculatory function. Journal of Urology 2016;195(4):e457-8. [Google Scholar]
- McVary K, Mynderse L, Gange S, Gittelman M, Goldberg K, Patel K, et al. Using the thermal energy of convectively delivered water vapor for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: the Rezūm II study. Journal of Urology 2015;193(4):e410. [Google Scholar]
- McVary K, Roehrborn C. Five year results of the prospective, randomized controlled trial of water vapor thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Journal of Urology 2020;203:e1021. [DOI: 10.1097/JU.0000000000000946.06] [DOI] [Google Scholar]
- McVary K, Roehrborn C. Water vapor thermal therapy with Rezūm system: 3-year results of prospective crossover trial replicate durable outcomes of phase III randomized controlled study for treatment of lower urinary tract symptoms/benign prostatic hyperplasia. Journal of Urology 2018;199(4):e989. [DOI: 10.1016/j.juro.2018.03.109] [DOI] [Google Scholar]
- McVary KT, Gange SN, Gittelman MC, Goldberg KA, Patel K, Shore ND, et al. Erectile and ejaculatory function preserved with convective water vapor energy treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: randomized controlled study. Journal of Sexual Medicine 2016;13(6):924-33. [DOI: 10.1016/j.jsxm.2016.03.372] [DOI] [PubMed]
- McVary KT, Gange SN, Gittelman MC, Goldberg KA, Patel K, Shore ND, et al. Minimally invasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. Journal of Urology 2016;195(5):1529-37. [DOI: 10.1016/j.juro.2015.10.181] [DOI] [PubMed]
- McVary KT, Roehrborn CG. Three-year outcomes of the prospective, randomized controlled Rezūm system study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology 2018;111:1-9. [DOI: 10.1016/j.urology.2017.10.023] [DOI] [PubMed] [Google Scholar]
- McVary KT, Rogers T, Roehrborn CG. Rezūm water vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology 2019;126:171-9. [DOI: 10.1016/j.urology.2018.12.041] [DOI] [PubMed] [Google Scholar]
- NCT01912339. Safety and efficacy study for the treatment of BPH (enlarged prostate). Available from clinicaltrials.gov/show/NCT01912339 (first posted 31 July 2013).
- Roehrborn C, Gange S, Gittelman M, Goldberg K, Patel K, Shore N, et al. Convective radiofrequency thermal therapy: durable two-year outcomes of a randomized controlled and prospective crossover study to relieve lower urinary tract symptoms due to benign prostatic hyperplasia. Journal of Urology 2017;197(4):e450-1. [DOI: 10.1016/j.juro.2017.02.1074] [DOI] [PubMed] [Google Scholar]
- Roehrborn CG, Gange SN, Gittelman MC, Goldberg KA, Patel K, Shore ND, et al. Convective thermal therapy: durable 2-year results of randomized controlled and prospective crossover studies for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Journal of Urology 2017;197(6):1507-16. [DOI: 10.1016/j.juro.2016.12.045] [DOI] [PubMed]
Nawrocki 1997 {published data only}
- Nawrocki JD, Bell TJ, Lawrence WT, Ward JP. A randomized controlled trial of transurethral microwave thermotherapy. British Journal of Urology 1997;79:389-93. [DOI] [PubMed] [Google Scholar]
Norby 2002 {published data only}
- Norby B, Nielsen HV, Frimodt-Moller PC. Transurethral interstitial laser coagulation of the prostate and transurethral microwave thermotherapy vs transurethral resection or incision of the prostate: results of a randomized, controlled study in patients with symptomatic benign prostatic hyperplasia. BJU International 2002;90:853-62. [DOI] [PubMed] [Google Scholar]
Pisco 2020 {published data only}
- NCT02074644. Clinical trial of prostatic arterial embolization versus a sham procedure to treat benign prostatic hyperplasia. Available from clinicaltrials.gov/ct2/show/NCT02074644 (accessed 13 March 2020).
- Pisco JM, Bilhim T, Costa NV, Torres D, Pisco J, Pinheiro LC, et al. Randomised clinical trial of prostatic artery embolisation versus a sham procedure for benign prostatic hyperplasia. European Urology 2020;77(3):354-62. [DOI: 10.1016/j.eururo.2019.11.010] [DOI] [PubMed] [Google Scholar]
Radwan 2020 {published data only}
- Radwan A, Farouk A, Higazy A, Samir YR, Tawfeek AM, Gamal MA. Prostatic artery embolization versus transurethral resection of the prostate in management of benign prostatic hyperplasia. Prostate International 2020 April 23 [Epub ahead of print]. [DOI: 10.1016/j.prnil.2020.04.001] [DOI] [PMC free article] [PubMed]
Roehrborn 1998 {published data only}
- Roehrborn CG, Preminger G, Newhall P, Denstedt J, Razvi H, Chin LJ, et al. Microwave thermotherapy for benign prostatic hyperplasia with the Dornier Urowave: results of a randomized, double-blind, multicenter, sham-controlled trial. Urology 1998;51:19-28. [DOI] [PubMed] [Google Scholar]
- Trachtenberg J, Roehborn CG. Updated results of a randomized, double-blind, multicenter sham-controlled trial of microwave thermotherapy with the Dornier Urowave in patients with symptomatic benign prostatic hyperplasia. World Journal of Urology 1998;16:102-8. [DOI] [PubMed] [Google Scholar]
Roehrborn 2013 {published data only}
- Barkin J. Prospective, randomized, controlled study of five-year results on prostatic urethral lift (PUL). Canadian Urological Association Journal 2017;11(9):S326. [DOI: 10.5489/cuaj.4896] [DOI] [Google Scholar]
- McVary KT, Gange SN, Shore ND, Bolton DM, Cowan BE, Brown BT, et al. Treatment of LUTS secondary to BPH while preserving sexual function: randomized controlled study of prostatic urethral lift. Journal of Sexual Medicine 2014;11(1):279-87. [DOI: 10.1111/jsm.12333] [DOI] [PubMed]
- NCT01294150. The safety and effectiveness of UroLift: LIFT Pivotal Study (LIFT). Available from clinicaltrials.gov/ct2/show/NCT01294150 (first received 11 February 2011).
- Rane A, McNicholas T, Woo H, Roehrborn C. 4 year results of the randomized, controlled, blinded, multi-center study for the prostatic urethral lift: the L.I.F.T. study. Journal of Endourology 2017;30(Suppl 2):A105-6.
- Rashid P, Chin P, Bolton D, Roehrborn C, McVary K. Prospective, randomised study of prostatic urethral lift: five year results. BJU International 2017;119(Suppl 2):45. [DOI: 10.1111/jsm.12333] [DOI] [Google Scholar]
- Rashid P, Chin P, Bolton D, Rukstalis D, Giddens J, Gange S, et al. Prospective, randomised study of prostatic urethral lift (PUL) with three year results. BJU international 2015;115(Suppl 4):10-1. [DOI: 10.1111/bju.13072] [DOI]
- Rashid P. Multi-center prospective study of the prostatic urethral lift with two year durability. BJU International 2014;113(Suppl 4):14-5. [DOI: 10.1111/bju.12618] [DOI] [Google Scholar]
- Roehrborn C, Gange S, Shore N, Giddens J, Bolton D, Cowan B, et al. 5 year prospective, randomized, controlled study results on the minimally invasive prostatic urethral lift (PUL). Journal of Urology 2017;197(Suppl 4):e511. [10.1016/j.juro.2017.02.1222]
- Roehrborn C, Gange S, Shore N, Giddens J, Bolton D, Cowan B, et al. Four year results from the largest, prospective, randomized study of prostatic urethral lift (PUL). European Urology Supplements 2016;15(3):e1077-a. [DOI: 10.1016/S1569-9056(16)61078-3] [DOI] [Google Scholar]
- Roehrborn C, Gange S, Shore N, Giddens J, Bolton D, Cowan B, et al. Long term (5 year) results from the largest, prospective, randomized, controlled study of the minimally invasive prostatic urethral lift (PUL). European Urology Supplements 2017;16(3):e334-5. [DOI: 10.1016/S1569-9056(17)30258-0] [DOI] [Google Scholar]
- Roehrborn C, Gange S, Shore N, Giddens J, Bolton D, Cowan B, et al. Prospective, randomised, blinded study of prostatic urethral lift (PUL): four year results. BJU International 2017;117(Suppl 3):19-20. [DOI: 10.1111/bju.13452] [DOI]
- Roehrborn C, Gange S, Shore N, Giddens J, Bolton D, Cowan B, et al. Prospective, randomized, blinded study of prostatic urethral lift (PUL): four year results. Journal of Urology 2016;195(Suppl 4):e456-7. [DOI: 10.1016/j.juro.2016.02.1469] [DOI] [Google Scholar]
- Roehrborn C, Gange S, Shore N, Giddens J, Bolton D, Cowan B, et al. Three year durability of the prostatic urethral lift for BPH: Results of a prospective, multi-center, randomized study. Journal of Urology 2015;193(Suppl 4):e92. [DOI: 10.1016/j.juro.2015.02.300] [DOI]
- Roehrborn CG, Barkin J, Gange SN, Shore ND, Giddens JL, Bolton DM, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Canadian Journal of Urology 2017;24(3):8802-13. [PMID: ] [PubMed] [Google Scholar]
- Roehrborn CG, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, et al. Durability of the prostatic urethral lift: 2-year results of the L.I.F.T. study. Urology Practice 2015;2(1):26-32. [DOI: 10.1016/j.urpr.2014.08.001] [DOI] [PubMed]
- Roehrborn CG, Gange SN, Shore ND, Giddens JL, Bolton DM, Cowan BE, et al. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. study. Journal of Urology 2013;190(6):2161-7. [DOI: 10.1016/j.juro.2013.05.116] [DOI] [PubMed]
- Roehrborn CG, Rukstalis DB, Barkin J, Gange SN, Shore ND, Giddens JL, et al. Three year results of the prostatic urethral L.I.F.T. study. Canadian Journal of Urology 2015;22(3):7772-82. [PMID: ] [PubMed]
- Roehrborn CG, Rukstalis DB, Giddens JL, Gange SN, Shore ND, Bolton DM, et al. Two year durability of the prostatic urethral lift: multi-center prospective study. Journal of Urology 2014;191(Suppl 4):e792-3. [DOI: 10.1016/j.juro.2014.02.2167] [DOI]
- Woo H. Prostatic urethral lift treats LUTS while preserving sexual function: results of a randomized controlled trial. BJU International 2014;113(Suppl 4):16-7. [DOI: 10.1111/bju.12618] [DOI] [Google Scholar]
Venn 1995 {published data only}
- Venn SN, Montgomery BS, Sheppard SA, Hughes SW, Beard RC, Bultitiude MI, et al. Microwave hyperthermia in benign prostatic hypertrophy: a controlled clinical trial. British Journal of Urology 1995;76:73-6. [DOI] [PubMed] [Google Scholar]
Wagrell 2002 {published data only}
- Mattiasson A, Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, et al. Five-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology 2007;69:91-7. [DOI] [PubMed] [Google Scholar]
- Wagrell L, Schelin S, Nordling J, Richthoff J, Magnusson B, Schain M, et al. Three-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology 2004;64:698-702. [DOI] [PubMed] [Google Scholar]
- Wagrell L, Schelin S, Nordling J, Richthoff J, Mangnusson B, Schain M, et al. Feedback microwave thermotherapy versus TURP for clinical BPH − a randomized controlled multicenter study. Urology 2002;60:292-9. [DOI] [PubMed] [Google Scholar]
Zhu 2018 {published data only}
- Zhu C, Lin W, Huang Z, CAI J. Prostate artery embolization and transurethral resection of prostate for benign prostatic hyperplasia: a prospective randomized controlled trial. Chinese Journal of Interventional Imaging and Therapy 2018;15(3):134-8. [DOI: 10.13929/j.1672-8475.201711043] [DOI] [Google Scholar]
References to studies excluded from this review
Albala 2000 {published data only}
- Albala DM, Turk TM, Fulmer BR, Koleski F, Andriole G, Davis BE, et al. Periurethral transurethral microwave thermotherapy for the treatment of benign prostatic hyperplasia: an interim 1-year safety and efficacy analysis using the thermatrx TMx-2000. Techniques in Urology 2000;6(4):288-93. [PubMed] [Google Scholar]
Arai 2000 {published data only}
- Arai Y, Aoki Y, Okubo K, Maeda H, Terada N, Matsuta Y, et al. Impact of interventional therapy for benign prostatic hyperplasia on quality of life and sexual function: A prospective study. Journal of Urology 2000;164(4):1206-11. [PubMed] [Google Scholar]
Bagla 2017 {published data only}
- Bagla S, Smirniotopoulos J, Orlando J, Piechowiak R. Cost analysis of prostate artery embolization (PAE) and transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2017;40(11):1694-7. [DOI: 10.1007/s00270-017-1700-7] [DOI] [PubMed] [Google Scholar]
- Bagla S, Vadlamudi V, Orlando J, Smirniotopoulos J. Cost analysis of prostate artery embolization (PAE) and transurethral resection of the prostate (TURP) in the treatment of benign prostatic hyperplasia. Journal of Vascular and Interventional Radiology 2016;27(3):S56. [DOI: 10.1016/j.jvir.2015.12.154] [DOI] [PubMed] [Google Scholar]
Bilhim 2015 {published data only}
- Bilhim T, Bagla S, Sapoval M, Carnevale FC, Salem R, Golzarian J. Prostatic arterial embolization versus transurethral resection of the prostate for benign prostatic hyperplasia. Radiology 2015;276(1):310-1. [DOI] [PubMed] [Google Scholar]
Brown 2018 {published data only}
D'Ancona 1997 {published data only}
- D'Ancona FC, Francisca EA, Debruyne FM, De la Rosette JJ. High-energy transurethral microwave thermotherapy in men with lower urinary tract symptoms. Journal of Endourology 1997;11(4):285-9. [DOI] [PubMed] [Google Scholar]
Dahlstrand 2003 {published data only}
- Dahlstrand C. High-energy microwave therapy with benign prostatic hyperplasia. A good and safe therapeutic choice--for both the patient and the health care. Läkartidningen 2003;100(35):2678-83. [PubMed] [Google Scholar]
Djavan 1999 {published data only}
- Djavan B, Seitz C, Roehrborn CG, Remzi M, Fakhari M, Waldert M, et al. Targeted transurethral microwave thermotherapy versus alpha-blockade in benign prostatic hyperplasia: outcomes at 18 months. Urology 2001;57(1):66-70. [DOI] [PubMed] [Google Scholar]
- Djavan B, Shariat S, Fakhari M, Ghawidel K, Seitz C, Partin AW, et al. Neoadjuvant and adjuvant alpha-blockade improves early results of high-energy transurethral microwave thermotherapy for lower urinary tract symptoms of benign prostatic hyperplasia: a randomized, prospective clinical trial. Urology 1999;53(2):251-9. [DOI] [PubMed] [Google Scholar]
Gratzke 2018 {published data only}
- Gratzke C, Barkin J, Roehrborn C. Predictors of response to the prostatic urethral lift ( (PUL) treatment. European Urology Supplements 2018;17(2):e1041. [DOI: 10.1016/S1569-9056(18)31556-2] [DOI] [Google Scholar]
Hahn 2000 {published data only}
- Hahn RG, Farahmand BY, Hallin A, Hannar N, Persson P-G. Incidence of acute myocardial infarction and cause-specific mortality after transurethral treatments of prostatic hypertrophy. Urology 2000;55(2):236-40. [DOI] [PubMed] [Google Scholar]
Hansen 1998 {published data only}
- Hansen BJ, Mortensen S, Mensink HJ, Flyger H, Riehmann M, Hendolin N, et al. Comparison of the Danish Prostatic Symptom Score with the international Prostatic Symptom Score, the Madsen-Iversen and Boyarsky symptom indexes. British Journal of Urology 1998;81(1):36-41. [DOI] [PubMed] [Google Scholar]
ISRCTN23921450 {published data only}
- ISRCTN23921450. A randomised controlled trial comparing the efficacy, safety and cost-effectiveness of transurethral resection (TURP), laser vaporisation (LVAP), transurethral needle ablation (TUNA) and microwave thermoablation (MTA) of the prostate. www.isrctn.com/ISRCTN23921450 (first received 25 April 2003).
Kobelt 2004 {published data only}
- Kobelt G, Spangberg A, Mattiasson A. The cost of feedback microwave thermotherapy compared with transurethral resection of the prostate for treating benign prostatic hyperplasia. BJU International 2004;93(4):543-8. [DOI] [PubMed] [Google Scholar]
Lim 2011 {published data only}
- Kim CS, Song HY, Jeong IG, Yeo HJ, Kim EY, Park JH, et al. Temporary placement of covered retrievable expandable nitinol stents with barbs in high-risk surgical patients with benign prostatic hyperplasia: work in progress. Journal of Vascular Interventional Radiology 2011;22(10):1420-6. [DOI] [PubMed] [Google Scholar]
Mulvin 1994 {published data only}
- Mulvin D, Creagh T, Kelly D, Smith J, Quinlan D, Fitzpatrick J. Transurethral microwave thermotherapy versus transurethral catheter therapy for benign prostatic hyperplasia. European Urology 1994;26(1):6-9. [DOI] [PubMed] [Google Scholar]
NCT01835860 {unpublished data only}
- NCT01835860. Prostatic artery embolization for benign prostatic hyperplasia. Available from clinicaltrials.gov/ct2/show/NCT01835860 (accessed 15 March 2020).
Norby 2002b {published data only}
- Nørby B, Nielsen HV, Frimodt-Møller PC. Cost-effectiveness of new treatments for benign prostatic hyperplasia: results of a randomized trial comparing the short-term cost-effectiveness of transurethral interstitial laser coagulation of the prostate, transurethral microwave thermotherapy and standard transurethral resection or incision of the prostate. Scandinavian Journal of Urology and Nephrology 2002;36(4):286-95. [DOI] [PubMed] [Google Scholar]
Nørby 2004 {published data only}
- Nørby B. Minimally invasive treatment of benign prostatic hyperplasia. Ugeskrift for laeger 2004;166(8):688-90. [PubMed] [Google Scholar]
Ohigashi 2007 {published data only}
- Ohigashi T, Nakamura K, Nakashima J, Baba S, Murai M. Long-term results of three different minimally invasive therapies for lower urinary tract symptoms due to benign prostatic hyperplasia: Comparison at a single institute. International Journal of Urology 2007;14(4):326-30. [DOI] [PubMed] [Google Scholar]
Pereira 2018 {published data only}
- NCT03043222. Innovative minimally invasive options in treatment of urinary problems related to prostate enlargement (BPH) in men. Available from clinicaltrials.gov/show/NCT03043222 (accessed 6 November 2018).
- Pereira K, Ford-Glanton S, Johar R, Xu P, Pham K, Gadani S, et al. Prostatic artery embolization (PAE) and prostatic urethral lift (PUL) procedures for symptomatic benign prostatic enlargement (BPH): a retrospective, single-center comparison of outcomes. Journal of Vascular and Interventional Radiology 2018;29(4 Suppl 1):S6. [DOI: 10.1016/j.jvir.2018.01.010] [DOI]
Porpiglia 2015 {published data only}
- Fiori C, Amparore D, Checcucci E, Ottaviano G, De Cillis S, Di Stasio A, et al. Medi-Tate temporary implantable nitinol device (TIND) in the treatment of benign prostatic obstruction: two tears of follow-up results. Journal of Urology 2016;195(4, S):E459. [Google Scholar]
- Porpiglia F, Fiori C, Bertolo R, Garrou D, Cattaneo G, Amparore D. Temporary implantable nitinol device (TIND): A novel, minimally invasive treatment for relief of lower urinary tract symptoms (LUTS) related to benign prostatic hyperplasia (BPH): Feasibility, safety and functional results at 1 year of follow-up. BJU International 2015;116(2):278-87. [DOI] [PubMed] [Google Scholar]
Porpiglia 2019 {published data only}
- Porpiglia F, Fiori C, Amparore D, Kadner G, Manit A, Valerio M, et al. Second-generation of temporary implantable nitinol device for the relief of lower urinary tract symptoms due to benign prostatic hyperplasia: results of a prospective, multicentre study at 1 year of follow-up. BJU International 2019;123(6):1061-9. [DOI] [PubMed] [Google Scholar]
- Porpiglia F, Fiori C, Amparore D, Volpi G, Kadner G, Manit A, et al. MP01-05: The results of one-arm multicenter prospective study on an innovative minimally invasive surgical technique for LUTS management. The Journal of Urology 2019;201:e2. [Google Scholar]
Qiu 2017 {published data only}
- Qiu ZL, Zhang CC, Wang XS, Cheng K, Liang X, Wang DW, et al. Clinical evaluation of embolization of the superior vesical prostatic artery for treatment of benign prostatic hyperplasia: a single-center retrospective study. Wideochir Inne Tech Maloinwazyjne 2017;12(4):409-16. [DOI: 10.5114/wiitm.2017.72324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russo 2015 {published data only}
- Russo GI, Kurbatov D, Sansalone S, Lepetukhin A, Dubsky S, Sitkin I, et al. Prostatic arterial embolization vs open prostatectomy: a 1-year matched-pair analysis of functional outcomes and morbidities. Urology 2015;86(2):343-8. [DOI] [PubMed] [Google Scholar]
- Russo GI, Kurbatov D, Sansalone S, Lepetukhin A, Dubsky S, Sitkin I, et al. Prostatic arterial embolization vs open prostatectomy: a matched-pair analysis of functional outcomes and morbidities after 1 year of follow-up. European Urology Supplement 2015;14(2):e570. [DOI] [PubMed] [Google Scholar]
Schelin 2006 {published data only}
- Schelin S, Geertsen U, Walter S, Spångberg A, Duelund-Jacobsen J, Krøyer K, et al. Feedback microwave thermotherapy versus TURP/prostate enucleation surgery in patients with benign prostatic hyperplasia and persistent urinary retention: a prospective, randomized, controlled, multicenter study. Urology 2006;68(4):795-9. [DOI] [PubMed] [Google Scholar]
Servadio 1987 {published data only}
- Servadio C, Leib Z, Lev A. Diseases of prostate treated by local microwave hyperthermia. Urology 1987;30(2):97-9. [DOI] [PubMed] [Google Scholar]
Shore 2010 {published data only}
- Shore ND, Sethi PS. A controlled, randomized, head-to-head comparison of the Prolieve Thermodilation System versus the Targis System for benign prostatic hyperplasia: safety, procedural tolerability, and clinical results. Journal of Endourology 2010;24(9):2469-75. [DOI] [PubMed] [Google Scholar]
Tan 2005 {published data only}
- Tan AH, Nott L, Hardie WR, Chin JL, Denstedt JD, Razvi H. Long-term results of microwave thermotherapy for symptomatic benign prostatic hyperplasia. Journal of Endourology 2005;19(10):1191-5. [DOI] [PubMed] [Google Scholar]
Trock 2004 {published data only}
- Trock BJ, Brotzman M, Utz WJ, Ugarte RR, Kaplan SA, Larson TR, et al. Long-term pooled analysis of multicenter studies of cooled thermotherapy for benign prostatic hyperplasia: results at three months through four years. Urology 2004;63(4):716-21. [DOI] [PubMed] [Google Scholar]
Vesely 2006 {published data only}
- Vesely S, Knutson T, Damber J-E, Dicuio M, Dahlstrand C. TURP and low-energy TUMT treatment in men with LUTS suggestive of bladder outlet obstruction selected by means of pressure-flow studies: 8-Year follow-up. Neurourology and Urodynamics 2006;25(7):770-5. [DOI] [PubMed] [Google Scholar]
Waldén 1998 {published data only}
- Waldén M, Acosta S, Carlsson P, Pettersson S, Dahlstrand C. A cost-effectiveness analysis of transurethral resection of the prostate and transurethral microwave thermotherapy for treatment of benign prostatic hyperplasia: two-year follow-up. Scandinavian Journal of Urology and Nephrology 1998;32(3):204-10. [DOI] [PubMed] [Google Scholar]
Woo 2018 {published data only}
- Woo H. Convective radiofrequency water vapor thermal therapy. In: International Journal of Urology. Vol. 25. 2018:139.
Yachia 1996 {published data only}
- Yachia D, Aridogan A. Comparison between first-generation (fixed-caliber) and second-generation (self-expanding, large caliber) temporary prostatic stents. Urol Int 1996;57(3):165-9. [DOI] [PubMed] [Google Scholar]
Zerbib 1992 {published data only}
- Zerbib M, Steg A, Conquy S, Martinache PR, Flam TA, Debre B. Localized hyperthermia versus the sham procedure in obstructive benign hyperplasia of the prostate: a prospective randomized study. Journal of Urology 1992;147(4):1048-52. [DOI] [PubMed] [Google Scholar]
Zerbib 1994 {published data only}
- Zerbib M, Steg A, Conquy S, Debre B. Hyperthermia: a randomized prospective study applying hyperthermia or a sham procedure in obstructive benign hyperplasia of the prostate. Progress in Clinical and Biological Research 1994;386:439-48. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12617001235392 {unpublished data only}
- ACTRN12617001235392. Prostate artery embolization for patients with lower urinary tract symptoms due to benign prostate hyperplasia. Available from anzctr.org.au/Trial/Registration/TrialReview.aspx?id=373427 (first posted 24 August 2017).
NCT02006303 {unpublished data only}
- NCT02006303. Prostatic artery embolization versus 532 nm Green Light PVP for catheterized patients. Available from clinicaltrials.gov/ct2/show/NCT02006303 (first posted 10 December 2013).
NCT02566551 {unpublished data only}
- NCT02566551. Prospective controlled randomized study of PAE vs TURP for BPH treatment. Available from clinicaltrials.gov/ct2/show/NCT02566551 (first posted 2 October 2015).
NCT04178811 {published data only}
- NCT04178811. Comparison between HoLEP and PUL in management of BPH. Available from clinicaltrials.gov/ct2/show/NCT04178811 (first received 26 November 2019). [NCT04178811]
NCT04236687 {unpublished data only}
- NCT04236687. Prostate artery embolization compared to holmium laser enucleation of the prostate for benign prostatic hyperplasia. Available from clinicaltrials.gov/ct2/show/NCT04236687 (first posted 22 January 2020).
NCT04338776 {published data only}
- NCT04338776. Comparing UroLift Experience Against Rezūm (CLEAR). Available from clinicaltrials.gov/ct2/show/NCT04338776 (first received 8 April 2020). [NCT04338776]
Additional references
Abrams 2003
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61(1):37-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Agarwal 2014
- Agarwal A, Eryuzlu LN, Cartwright R, Thorlund K, Tammela TL, Guyatt GH, et al. What is the most bothersome lower urinary tract symptom? Individual- and population-level perspectives for both men and women. European Urology 2014;65(6):1211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 2019
- Alexander CE, Scullion MMF, Omar MI, Yuan Y, Mamoulakis C, N'Dow JMO, et al. Bipolar versus monopolar transurethral resection of the prostate for lower urinary tract symptoms secondary to benign prostatic obstruction. Cochrane Database of Systematic Reviews 2019, Issue 12. Art. No: CD009629. [DOI: 10.1002/14651858.CD009629.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amparore 2019
- Amparore D, De Cillis S, Volpi G, Checcucci E, Manfredi M, Morra I, et al. First- and Second-Generation Temporary Implantable Nitinol Devices As Minimally Invasive Treatments for BPH-Related LUTS: Systematic Review of the Literature. Current urology reports 2019;20(8):47. [PMID: ] [DOI] [PubMed] [Google Scholar]
Aoun 2015
- Aoun F, Marcelis Q, Roumeguère T. Minimally invasive devices for treating lower urinary tract symptoms in benign prostate hyperplasia: technology update. Research and Reports in Urology 2015;7:125-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barry 1992
- Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. Journal of Urology 1992;148(5):1549-57; discussion 1564. [DOI] [PubMed] [Google Scholar]
Barry 1995
- Barry MJ, Williford WO, Chang Y, Machi M, Jones K, Walker-Corkery E, et al. Benign prostatic hyperplasia-specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? Journal of Urology 1995;154(5):1770-4. [DOI] [PubMed] [Google Scholar]
Barry 1997
- Barry MJ, Fowler FJ Jr, Bin L, Pitts JC 3rd, Harris CJ, Mulley AG Jr. The natural history of patients with benign prostatic hyperplasia as diagnosed by North American urologists. Journal of Urology 1997;157(1):10-4; discussion 14-5. [PubMed] [Google Scholar]
Bertolo 2018
Bhojani 2014
- Bhojani N, Gandaglia G, Sood A, Rai A, Pucheril D, Chang SL, et al. Morbidity and mortality after benign prostatic hyperplasia surgery: data from the American College of Surgeons national surgical quality improvement program. Journal of Endourology 2014;28(7):831-40. [DOI] [PubMed] [Google Scholar]
Blute 1996
- Blute ML, Tornera KM, Hellerstein DK, McKiel CF Jr, Lynch JH, Regan JB, Sankey NE. Transurethral microwave thermotherapy for management of benign prostatic hyperplasia: results of the United States Prostatron Cooperative Study. The Journal of urology 1993;150:1591. [DOI] [PubMed] [Google Scholar]
Brasure 2016
- Brasure M, MacDonald R, Dahm P, Olson CM, Nelson VA, Fink HA, et al. AHRQ comparative effectiveness reviews. In: Newer Medications for Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia: A Review. Rockville (MD): Agency for Healthcare Research and Quality (US), 2016. [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. British Medical Journal 2005;331(7521):897-900. [DOI] [PMC free article] [PubMed]
Carnevale 2010
- Carnevale FC, Antunes AA, da Motta Leal Filho JM, Oliveira Cerri LM, Baroni RH, Marcelino AS, et al. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovascular and Interventional Radiology 2010;33(2):355-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015;15(4):905-50.
Chaimani 2021
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11. Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from www.training.cochrane.org/handbook. Cochrane, 2021. [Google Scholar]
Chapple 2017
- Chapple C, Castro-Diaz D, Chuang YC, Lee KS, Liao L, Liu SP, et al. Prevalence of lower urinary tract symptoms in China, Taiwan, and South Korea: results from a cross-sectional, population-based study. Advances in Therapy 2017;34(8):1953-65. [DOI] [PMC free article] [PubMed]
Chin 2012
- Chin PT, Bolton DM, Jack G, Rashid P, Thavaseelan J, Yu RJ, et al. Prostatic urethral lift: two-year results after treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Urology 2012;79(1):5-11. [DOI] [PubMed] [Google Scholar]
CINeMA 2017 [Computer program]
- Institute of Social and Preventive Medicine, University of Bern CINeMA: Confidence in Network Meta-Analysis. Institute of Social and Preventive Medicine, University of Bern, 2017. Available from cinema.ispm.unibe.ch.
Cipriani 2013
- Cipriani A, Higgins JPT, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI] [PubMed] [Google Scholar]
Cornu 2010
- Cornu JN, Cussenot O, Haab F, Lukacs B. A widespread population study of actual medical management of lower urinary tract symptoms related to benign prostatic hyperplasia across Europe and beyond official clinical guidelines. European Urology 2010;58(3):450-6. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 16 August 2017. Melbourne, Australia: Veritas Health Innovation, 2013. Available at www.covidence.org.
Crawford 2006
- Crawford ED, Wilson SS, McConnell JD, Slawin KM, Lieber MC, Smith JA, et al. Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. Journal of Urology 2006;175(4):1422-6; discussion 1426-7. [DOI] [PubMed] [Google Scholar]
Dahm 2021a
- Dahm P, MacDonald R, McKenzie L, Jung JH, Greer N, Wilt T. Newer minimally Invasive treatment modalities to treat lower urinary tract symptoms attributed to benign prostatic hyperplasia. European Urology Open Science 2021;26:72-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahm 2021b
- Dahm P, Franco J. Re: A systematic review of patients' values, preferences, and expectations for the diagnosis and treatment of male lower urinary tract symptoms. European Urology 2021;Epub ahead of print:S0302-2838(21)00249-9.. [DOI] [PubMed] [Google Scholar]
Darson 2017
- Darson MF, Alexander EE, Schiffman ZJ, Lewitton M, Light RA, Sutton MA, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezūm system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Research and Reports in Urology 2017;9:159-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses.. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Available from www.training.cochrane.org/handbook. Cochrane, 2021. [Google Scholar]
DeMeritt 2000
- DeMeritt JS, Elmasri FF, Esposito MP, Rosenberg GS. Relief of benign prostatic hyperplasia-related bladder outlet obstruction after transarterial polyvinyl alcohol prostate embolization. Journal of Vascular and Interventional Radiology 2000;11(6):767-70. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dixon 2015
- Dixon CM, Cedano ER, Pacik D, Vit V, Varga G, Wagrell L, et al. Two-year results after convective radiofrequency water vapor thermal therapy of symptomatic benign prostatic hyperplasia. Research and Reports in Urology 2016;8:207-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2021
- Duffy JMN, Al-Ahwany H, Bhattacharya S, Collura B, Curtis C, Evers JLH, et al, Core Outcome Measure for Infertility Trials initiative. Developing a core outcome set for future infertility research: an international consensus development study. Fertility and Sterility 2021;115(1):191-200. [DOI] [PubMed] [Google Scholar]
Dunphy 2015
- Dunphy C, Laor L, Te A, Kaplan S, Chughtai B. Relationship between depression and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Reviews in Urology 2015;17(2):51-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
EAU 2021
- European Association of Urology. Management of non-neurogenic male lower urinary tract symptoms (LUTS), including benign prostatic obstruction (BPO). Available from uroweb.org/guideline/treatment-of-non-neurogenic-male-luts/ç (accessed 21 April 2021).
Egan 2016
- Egan KB. The epidemiology of benign prostatic hyperplasia associated with lower urinary tract symptoms: prevalence and incident rates. Urologic Clinics of North America 2016;43(3):289-97. [DOI] [PubMed] [Google Scholar]
Emberton 2008
- Emberton M, Cornel EB, Bassi PF, Fourcade RO, Gómez JMF, Castro R. Benign prostatic hyperplasia as a progressive disease: a guide to the risk factors and options for medical management. International Journal of Clinical Practice 2008;62(7):1076-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote 2016 [Computer program]
- EndNote. Version 7.5. Philadelphia (PA): Clarivate Analytics, 2016. Available at endnote.com/.
Feng 2017
- Feng S, Tian Y, Liu W, Li Z, Deng T, Li H, et al. Prostatic arterial embolization treating moderate-to-severe lower urinary tract symptoms related to benign prostate hyperplasia: a meta-analysis. Cardiovascular and Interventional Radiology 2017;40(1):22-32. [DOI] [PubMed] [Google Scholar]
Foster 2019
- Foster HE, Dahm P, Kohler TS, Lerner LB, Parsons JK, Wilt TJ, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2019. Journal of Urology 2019;202(3):592-8. [DOI] [PubMed] [Google Scholar]
Foust‐Wright 2017
- Foust-Wright C, Wissig S, Stowell C, Olson E, Anderson A, Anger J, et al. Development of a core set of outcome measures for OAB treatment. International Urogynecology Journal 2017;28(12):1785-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franco 2021
- Franco JVA, Garegnani L, Escobar Liquitay CM, Borofsky M, Dahm P. Transurethral microwave thermotherapy for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2021, Issue 6. Art. No: CD004135. [DOI: 10.1002/14651858.CD004135.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed) 2008;336(7651):995-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64(12):1283-93. [DOI] [PubMed] [Google Scholar]
Higgins 1996
- Higgins JPT, Whitehead A. Borrowing strength from external trials in a meta-analysis. Statistics in Medicine 1996;15:2733-49. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed) 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. Journal of the Royal Statistical Society. Series A: Statistics in Society 2009;172(1):137-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA. Chapter 8. Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2021
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Available from www.training.cochrane.org/handbook. Cochrane, 2021. [Google Scholar]
Hoffman 2012
- Hoffman RM, Monga M, Elliott SP, MacDonald R, Langsjoen J, Tacklind J, et al. Microwave thermotherapy for benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2012, Issue 9. Art. No: CD004135. [DOI: 10.1002/14651858.CD004135.pub3] [DOI] [PubMed] [Google Scholar]
Homma 1997
- Homma Y, Kawabe K, Tsukamoto T, Yamanaka H, Okada K, Okajima E, et al. Epidemiologic survey of lower urinary tract symptoms in Asia and Australia using the international prostate symptom score. International Journal of Urology 1997;4(1):40-6. [DOI] [PubMed] [Google Scholar]
Huang 2019
- Huang SW, Tsai CY, Tseng CS, Shih MC, Yeh YC, Chien KL, et al. Comparative efficacy and safety of new surgical treatments for benign prostatic hyperplasia: systematic review and network meta-analysis. BMJ (Clinical Research Ed.) 2019;367:l5919. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huedo‐Medina 2006
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychological Methods 2006/06;11(2):193-206. [DOI] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989;10(4):407-15. [DOI] [PubMed] [Google Scholar]
Jansen 2013
- Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Medicine 2013;11(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jing 2020
- Jing J, Wu Y, Du M, Zhang N, Wang M, Xu B, et al. Urethral Lift as a Safe and Effective Procedure for Prostatic Hyplasia Population: A Systematic Review and Meta-Analysis. Frontiers in surgery 2020;7:598728. [PMID: ] [DOI] [PMC free article] [PubMed]
Johnston 2013
- Johnston BC, Patrick DL, Busse JW, Schünemann HJ, Agarwal A, Guyatt GH. Patient-reported outcomes in meta-analyses – Part 1: assessing risk of bias and combining outcomes. Health and Quality of Life Outcomes 2013;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2017
- Jung JH, Shin TY, McCutcheon KA, Borofsky M, Narayan V, Young S, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD012867. [DOI: 10.1002/14651858.CD012867] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2019
- Jung JH, Reddy B, McCutcheon KA, Borofsky M, Narayan V, Kim MH, et al. Prostatic urethral lift for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2019, Issue 5. Art. No: CD012832. [DOI: 10.1002/14651858.CD012832.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jung 2020
- Jung JH, McCutcheon KA, Borofsky M, Young S, Golzarian J, Reddy B et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD012867. [DOI: 10.1002/14651858.CD012867.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2020
- Kang TW, Jung JH, Hwang EC, Borofsky M, Kim MH, Dahm P. Convective radiofrequency water vapour thermal therapy for lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD013251. [DOI: 10.1002/14651858.CD013251.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2021
- Knight GM, Talwar A, Salem R, Mouli S. Systematic review and meta-analysis comparing prostatic artery embolization to gold-standard transurethral resection of the prostate for benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2021;44(2):183-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kozminski 2015
- Kozminski MA, Wei JT, Nelson J, Kent DM. Baseline characteristics predict risk of progression and response to combined medical therapy for benign prostatic hyperplasia (BPH). BJU International 2015;115(2):308-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuang 2017
- Kuang M, Vu A, Athreya S. A systematic review of prostatic artery embolization in the treatment of symptomatic benign prostatic hyperplasia. Cardiovascular and Interventional Radiology 2017;40(5):655-63. [DOI] [PubMed] [Google Scholar]
Lee 2017
- Lee SWH, Chan EMC, Lai YK. The global burden of lower urinary tract symptoms suggestive of benign prostatic hyperplasia: a systematic review and meta-analysis. Scientific Reports 2017;7(1):7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leissner 1979
- Leissner KH, Tisell LE. The weight of the human prostate. Scandinavian Journal of Urology 1979;13(2):137-42. [DOI] [PubMed] [Google Scholar]
Lokeshwar 2020
- Lokeshwar SD, Valancy D, Lima TFN, Blachman-Braun R, Ramasamy R. A Systematic Review of Reported Ejaculatory Dysfunction in Clinical Trials Evaluating Minimally Invasive Treatment Modalities for BPH. Current urology reports 2020;21(12):54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lu 2004
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23(20):3105-24. [DOI] [PubMed] [Google Scholar]
MacLennan 2017
- MacLennan S, Williamson PR, Bekema H, Campbell M, Ramsay C, N'Dow J, et al, Group Compacters Study. A core outcome set for localised prostate cancer effectiveness trials. BJU International 2017;120(5B):E64-E79. [DOI] [PubMed] [Google Scholar]
Malde 2021
- Malde S, Umbach R, Wheeler JR, Lytvyn L, Cornu JN, Gacci M, et al. A systematic review of patients' values, preferences, and expectations for the diagnosis and treatment of male lower urinary tract symptoms. European Urology 2021;79(6):796-809. [DOI] [PubMed] [Google Scholar]
Malling 2019
- Malling B, Roder MA, Brasso K, Forman J, Taudorf M, Lonn L. Prostate artery embolisation for benign prostatic hyperplasia: a systematic review and meta-analysis. European Radiology 2019;29(1):287-98. [PMID: ] [DOI] [PubMed] [Google Scholar]
Martin 2014
- Martin S, Lange K, Haren MT, Taylor AW, Wittert G. Risk factors for progression or improvement of lower urinary tract symptoms in a prospective cohort of men. Journal of Urology 2014;191(1):130-7. [DOI] [PubMed] [Google Scholar]
Martins Pisco 2012
- Martins Pisco J, Pereira J, Rio Tinto H, Fernandes L, Bilhim T. How to perform prostatic arterial embolization. Techniques in Vascular and Interventional Radiology 2012;15(4):286-9. [DOI] [PubMed] [Google Scholar]
McNicholas 2016
- McNicholas TA. Benign prostatic hyperplasia and new treatment options – a critical appraisal of the UroLift system. Medical Devices 2016;9:115-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
McVary 2011
- McVary KT, Roehrborn CG, Avins AL, Barry MJ, Bruskewitz RC, Donnell RF, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. Journal of Urology 2011;185(5):1793-803. [DOI] [PubMed] [Google Scholar]
Miller 2020a
- Miller LE, Chughtai B, Dornbier RA, McVary KT. Surgical Reintervention Rate after Prostatic Urethral Lift: Systematic Review and Meta-Analysis Involving over 2,000 Patients. The Journal of urology 2020;204(5):1019-26. [PMID: ] [DOI] [PubMed] [Google Scholar]
Miller 2020b
- Miller LE, Chughtai B, McVary K, Gonzalez RR, Rojanasarot S, DeRouen K, et al. Water vapor thermal therapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia: Systematic review and meta-analysis. Medicine 2020;99(30):e21365. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 1976
- Mitchell ME, Waltman AC, Athanasoulis CA, Kerr WS Jr, Dretler SP. Control of massive prostatic bleeding with angiographic techniques. Journal of Urology 1976;115(6):692-5. [DOI] [PubMed] [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. UroLift for treating lower urinary tract symptoms of benign prostatic hyperplasia. www.nice.org.uk/guidance/mtg26 (accessed 28 September 2017).
Nickel 2015
- Nickel JC, Brock GB, Herschorn S, Dickson R, Henneges C, Viktrup L. Proportion of tadalafil-treated patients with clinically meaningful improvement in lower urinary tract symptoms associated with benign prostatic hyperplasia – integrated data from 1,499 study participants. BJU International 2015;115(5):815-21. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. [DOI: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pariser 2015
- Pariser JJ, Pearce SM, Patel SG, Bales GT. National trends of simple prostatectomy for benign prostatic hyperplasia with an analysis of risk factors for adverse perioperative outcomes. Urology 2015;86(4):721-5. [DOI] [PubMed] [Google Scholar]
Parsons 2020
- Parsons JK, Dahm P, Köhler TS, Lerner LB, Wilt TJ. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment, 2020. Journal of Urology 2020;204(4):799-804. [DOI] [PubMed] [Google Scholar]
Pereira 2010
- Pereira TV, Patsopoulos NA, Salanti G, Ioannidis JPA. Critical interpretation of Cochran's Q test depends on power and prior assumptions about heterogeneity. Research Synthesis Methods 2010/04;1(2):149-61. [DOI] [PubMed] [Google Scholar]
Pisco 2016
- Pisco JM, Bilhim T, Pinheiro LC, Fernandes L, Pereira J, Costa NV, et al. Medium- and long-term outcome of prostate artery embolization for patients with benign prostatic hyperplasia: results in 630 patients. Journal of Vascular and Interventional Radiology 2016;27(8):1115-22. [DOI] [PubMed] [Google Scholar]
Porpiglia 2018
- Porpiglia F, Fiori C, Bertolo R, Giordano A, Checcucci E, Garrou D, et al. 3-year follow-up of temporary implantable nitinol device implantation for the treatment of benign prostatic obstruction. BJU International 2018;122(1):106-12. [DOI: 10.1111/bju.14141] [DOI] [PubMed] [Google Scholar]
Pyo 2017
- Pyo JS, Cho WJ. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clinical Radiology 2017;72(1):16-22. [DOI] [PubMed] [Google Scholar]
Rees 2015
- Rees J. Patients not P values. BJU international 2015;115(5):678-9. [DOI] [PubMed] [Google Scholar]
Reich 2008
- Reich O, Gratzke C, Bachmann A, Seitz M, Schlenker B, Hermanek P, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. Journal of Urology 2008;180(1):246-9. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342(7804):964-7. [DOI] [PubMed] [Google Scholar]
Roehrborn 2003
- Roehrborn C, McConnell J, Barry M, Benaim E, Bruskewitz R, Blute ML, et al. American Urological Association guideline: management of benign prostatic hyperplasia (BPH). Available from www.auanet.org/documents/education/clinical-guidance/Benign-Prostatic-Hyperplasia.pdf (accessed 28 September 2017).
Rosen 1997
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49(6):822-30. [DOI] [PubMed] [Google Scholar]
Rosen 2007
- Rosen RC, Catania JA, Althof SE, Pollack LM, O'Leary M, Seftel AD, et al. Development and validation of four-item version of Male Sexual Health Questionnaire to assess ejaculatory dysfunction. Urology 2007;69(5):805-9. [DOI] [PubMed] [Google Scholar]
Rubeinstein 2003
Rukstalis 2019
- Rukstalis D, Grier D, Stroup SP, Tutrone R, deSouza E, Freedman S, et al. Prostatic urethral lift (PUL) for obstructive median lobes: 12 month results of the MedLift study. Prostate Cancer and Prostatic Diseases 2019;22(3):411-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JPT, Ades AE, Ioannidis JPA. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279-301. [DOI] [PubMed] [Google Scholar]
Salanti 2010
- Salanti Georgia, Dias Sofia, Welton Nicky J, Ades A E, Golfinopoulos Vassilis, Kyrgiou Maria, Mauri Davide, Ioannidis John P A. Evaluating novel agent effects in multiple-treatments meta-regression. Statistics in Medicine 2010;29(23):2369-2383. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2012
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80-97. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JPT. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shim 2017
- Shim SR, Kanhai KJ, Ko YM, Kim JH. Efficacy and safety of prostatic arterial embolization: systematic review with meta-analysis and meta-regression. Journal of Urology 2017;197(2):465-79. [DOI] [PubMed] [Google Scholar]
Song 2012
- Song F, Clark A, Bachmann MO, Maas J. Simulation evaluation of statistical properties of methods for indirect and mixed treatment comparisons. BMC Medical Research Methodology 2012;12(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spaliviero 2010
- Spaliviero M, Strom KH, Gu X, Araki M, Culkin DJ, Wong C. Does Greenlight HPS laser photoselective vaporization prostatectomy affect sexual function? Journal of Endourology 2010;24(12):2051-7. [DOI] [PubMed] [Google Scholar]
STATA 2019 [Computer program]
- Stata Statistical Software: Release 16.. College Station, TX: StataCorp LLC., 2019.
Strope 2015
- Strope SA, Vetter J, Elliott S, Andriole GL, Olsen MA. Use of medical therapy and success of laser surgery and transurethral resection of the prostate for benign prostatic hyperplasia. Urology 2015;86(6):1115-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2008
- Sun F, Sanchez FM, Crisostomo V, Lima JR, Luis L, Garcia-Martinez V, et al. Benign prostatic hyperplasia: transcatheter arterial embolization as potential treatment - preliminary study in pigs. Radiology 2008;246(3):783-9. [DOI] [PubMed] [Google Scholar]
Svarc 2020
- Svarc P, Taudorf M, Nielsen MB, Stroomberg HV, Roder MA, Lönn L. Postembolization syndrome after prostatic artery embolization: a systematic review. Diagnostics (Basel, Switzerland) 2020;10(9):659. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tallman 2021
- Tallman CT, Zantek PF, Hernandez N, Morton RA Jr, Qi D, Gonzalez RR. Effectiveness of convective water vapor energy therapy versus prostatic urethral lift for symptomatic benign prostatic hyperplasia: a systematic review and indirect comparison. World journal of urology 2021;.:Online ahead of print. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tanneru 2020
- Tanneru K, Gautam S, Norez D, Kumar J, Alam MU, Koocheckpour S, et al. Meta-analysis and systematic review of intermediate-term follow-up of prostatic urethral lift for benign prostatic hyperplasia. International urology and nephrology 2020;52(6):999-1008. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tradewell 2019
- Tradewell M B, Albersheim J, Dahm P. Use of the IDEAL framework in the urological literature: where are we in 2018? BJU Int 2019;123(6):1078-1085. [DOI] [PubMed] [Google Scholar]
Tzeng 2021
- Tzeng M, Basourakos SP, Lewicki PJ, Hu JC, Lee RK. New endoscopic In-office surgical therapies for benign prostatic hyperplasia: a systematic review. European Urology Focus 2021;S2405-4569(21):00056-0. [PMID: ] [DOI] [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Higgins Haris SV, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42(1):332-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veroniki 2014
- Veroniki AA, Mavridis D, Higgins JPT, Salanti G. Characteristics of a loop of evidence that affect detection and estimation of inconsistency: a simulation study. BMC Medical Research Methodology 2014;14(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Veroniki 2016
- Veroniki AA, Straus SE, Fyraridis A, Tricco AC. The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes. Journal of Clinical Epidemiology 2016;76:193-9. [DOI] [PubMed] [Google Scholar]
Veroniki 2018
- Veroniki AA, Straus SE, Rücker G, Tricco AC. Is providing uncertainty intervals in treatment ranking helpful in a network meta-analysis? Journal of clinical epidemiology 2018;100:122-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walmsley 2004
- Walmsley K, Kaplan SA. Transurethral microwave thermotherapy for benign prostate hyperplasia: separating truth from marketing hype. Journal of Urology 2004;172:1249-55. [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang MQ, Guo LP, Zhang GD, Yuan K, Li K, Duan F, et al. Prostatic arterial embolization for the treatment of lower urinary tract symptoms due to large (> 80 mL) benign prostatic hyperplasia: results of midterm follow-up from Chinese population. BMC Urology 2015;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012;3(2):111-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2012a
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Research Synthesis Methods 2012;3(2):111-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2015
- White IR. Network meta-analysis. Stata Journal 2015;15(4):951-85.
WHO 2002
- World Health Organization. Proposed working definition of an older person in Africa for the MDS project. www.who.int/healthinfo/survey/ageingdefnolder/en (accessed 17 August 2017).
Woo 2012
- Woo HH, Bolton DM, Laborde E, Jack G, Chin PT, Rashid P, et al. Preservation of sexual function with the prostatic urethral lift: a novel treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. Journal of Sexual Medicine 2012;9(2):568-75. [DOI] [PubMed] [Google Scholar]
Woo 2017
- Woo HH, Gonzalez RR. Perspective on the Rezūm® System: a minimally invasive treatment strategy for benign prostatic hyperplasia using convective radiofrequency water vapor thermal therapy. Medical Devices 2017;10:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woo 2020
- Woo H, Huang CP, Lien CS, Chkhotua A. MP25-08: First-in-human clinical experience with the XFLO expander system to treat lower urinary tract symptoms secondary to benign prostatic hyperplasia. Journal of Urology 1 April 2020;203(4S):e393-4. [DOI: 10.1097/JU.0000000000000864.08] [DOI] [Google Scholar]
Xiang 2021
- Xiang P, Guan D, Du Z, Hao Y, Yan W, Wang Y, et al. Efficacy and safety of prostatic artery embolization for benign prostatic hyperplasia: a systematic review and meta-analysis of randomized controlled trials. European radiology 2021;.:Online ahead of print. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yepes‐Nuñez 2019
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of clinical epidemiology 2019;115:1-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yoo 2012
- Yoo TK, Cho HJ. Benign prostatic hyperplasia: from bench to clinic. Korean Journal of Urology 2012;53(3):139-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zlotta 1997
- Zlotta AR, Raviv G, Peny MO, Noel JC, Haot J, Schulman CC. Possible mechanisms of action of transurethral needle ablation of the prostate on benign prostatic hyperplasia symptoms: a neurohistochemical study. Journal of Urology 1997;157(3):894-9. [PubMed] [Google Scholar]
Zumstein 2019
- Zumstein V, Betschart P, Vetterlein MW, Kluth LA, Hechelhammer L, Mordasini L, et al. Prostatic artery embolization versus standard surgical treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. European Urology Focus 2019;5(6):1091-100. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Franco 2020
- Franco JVA, Jung JH, Imamura M, Borofsky M, Omar MI, Escobar Liquitay CM, et al. Minimally invasive treatments for lower urinary tract symptoms in men with benign prostatic hyperplasia: a network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 6. Art. No: CD013656. [DOI: 10.1002/14651858.CD013656] [DOI] [PMC free article] [PubMed] [Google Scholar]


