Abstract
Background
Chronic obstructive pulmonary disease (COPD, including bronchitis and emphysema) is a chronic condition causing shortness of breath, cough, and exacerbations leading to poor health outcomes. Face‐to‐face visits with health professionals can be hindered by severity of COPD or frailty, and by people living at a distance from their healthcare provider and having limited access to services. Telehealth technologies aimed at providing health care remotely through monitoring and consultations could help to improve health outcomes of people with COPD.
Objectives
To assess the effectiveness of telehealth interventions that allow remote monitoring and consultation and multi‐component interventions for reducing exacerbations and improving quality of life, while reducing dyspnoea symptoms, hospital service utilisation, and death among people with COPD.
Search methods
We identified studies from the Cochrane Airways Trials Register. Additional sources searched included the US National Institutes of Health Ongoing Trials Register, the World Health Organization International Clinical Trials Registry Platform, and the IEEEX Xplore Digital Library. The latest search was conducted in April 2020. We used the GRADE approach to judge the certainty of evidence for outcomes.
Selection criteria
Eligible randomised controlled trials (RCTs) included adults with diagnosed COPD. Asthma, cystic fibrosis, bronchiectasis, and other respiratory conditions were excluded. Interventions included remote monitoring or consultation plus usual care, remote monitoring or consultation alone, and mult‐component interventions from all care settings. Quality of life scales included St George's Respiratory Questionnaire (SGRQ) and the COPD Assessment Test (CAT). The dyspnoea symptom scale used was the Chronic Respiratory Disease Questionnaire Self‐Administered Standardized Scale (CRQ‐SAS).
Data collection and analysis
We used standard Cochrane methodological procedures. We assessed confidence in the evidence for each primary outcome using the GRADE method. Primary outcomes were exacerbations, quality of life, dyspnoea symptoms, hospital service utilisation, and mortality; a secondary outcome consisted of adverse events.
Main results
We included 29 studies in the review (5654 participants; male proportion 36% to 96%; female proportion 4% to 61%). Most remote monitoring interventions required participants to transfer measurements using a remote device and later health professional review (asynchronous). Only five interventions transferred data and allowed review by health professionals in real time (synchronous). Studies were at high risk of bias due to lack of blinding, and certainty of evidence ranged from moderate to very low. We found no evidence on comparison of remote consultations with or without usual care.
Remote monitoring plus usual care (8 studies, 1033 participants)
Very uncertain evidence suggests that remote monitoring plus usual care may have little to no effect on the number of people experiencing exacerbations at 26 weeks or 52 weeks. There may be little to no difference in effect on quality of life (SGRQ) at 26 weeks (very low to low certainty) or on hospitalisation (all‐cause or COPD‐related; very low certainty). COPD‐related hospital re‐admissions are probably reduced at 26 weeks (hazard ratio 0.42, 95% confidence interval (CI) 0.19 to 0.93; 106 participants; moderate certainty). There may be little to no difference in deaths between intervention and usual care (very low certainty). We found no evidence for dyspnoea symptoms or adverse events.
Remote monitoring alone (10 studies, 2456 participants)
Very uncertain evidence suggests that remote monitoring may result in little to no effect on the number of people experiencing exacerbations at 41 weeks (odds ratio 1.02, 95% CI 0.67 to 1.55). There may be little to no effect on quality of life (SGRQ total at 17 weeks, or CAT at 38 and 52 weeks; very low certainty). There may be little to no effect on dyspnoea symptoms on the CRQ‐SAS at 26 weeks (low certainty). There may be no difference in effects on the number of people admitted to hospital (very low certainty) or on deaths (very low certainty). We found no evidence for adverse events.
Multi‐component interventions with remote monitoring or consultation component (11 studies, 2165 participants)
Very uncertain evidence suggests that multi‐component interventions may have little to no effect on the number of people experiencing exacerbations at 52 weeks. Quality of life at 13 weeks may improve as seen in SGRQ total score (mean difference ‐9.70, 95% CI ‐18.32 to ‐1.08; 38 participants; low certainty) but not at 26 or 52 weeks (very low certainty). COPD assessment test (CAT) scores may improve at a mean of 38 weeks, but evidence is very uncertain and interventions are varied.
There may be little to no effect on the number of people admitted to hospital at 33 weeks (low certainty). Multi‐component interventions are likely to result in fewer people re‐admitted to hospital at a mean of 39 weeks (OR 0.50, 95% CI 0.31 to 0.81; 344 participants, 3 studies; moderate certainty). There may be little to no difference in death at a mean of 40 weeks (very low certainty). There may be little to no effect on people experiencing adverse events (very low certainty). We found no evidence for dyspnoea symptoms.
Authors' conclusions
Remote monitoring plus usual care provided asynchronously may not be beneficial overall compared to usual care alone. Some benefit is seen in reduction of COPD‐related hospital re‐admissions, but moderate‐certainty evidence is based on one study. We have not found any evidence for dyspnoea symptoms nor harms, and there is no difference in fatalities when remote monitoring is provided in addition to usual care.
Remote monitoring interventions alone are no better than usual care overall for health outcomes.
Multi‐component interventions with asynchronous remote monitoring are no better than usual care but may provide short‐term benefit for quality of life and may result in fewer re‐admissions to hospital for any cause. We are uncertain whether remote monitoring is responsible for the positive impact on re‐admissions, and we are unable to discern the long‐term benefits of receiving remote monitoring as part of patient care.
Owing to paucity of evidence, it is unclear which COPD severity subgroups would benefit from telehealth interventions. Given there is no evidence of harm, telehealth interventions may be beneficial as an additional health resource depending on individual needs based on professional assessment. Larger studies can determine long‐term effects of these interventions.
Plain language summary
Telehealth technologies for people with chronic obstructive pulmonary disease (COPD)
Review question
Do telehealth technologies help improve the health of people who have COPD?
Background
Chronic obstructive pulmonary disease (COPD) includes a group of lung conditions that cause breathing difficulties. Symptoms include shortness of breath (dyspnoea), coughing, and increased mucus. COPD causes limited airflow in the lungs when breathing out; this can be measured by spirometry (a measure to assess how well the lungs function). The spirometer takes two measurements: volume of air when breathing out forcefully in one second, and total amount of air breathed out. When COPD gets worse over time, this leads to greater symptom severity and can reduce quality of life. Disease progression and sudden flare‐ups (exacerbations) of symptoms can increase someone's risks of hospitalisation and death. Telehealth technologies could improve delivery of health care for people with COPD, which could reduce exacerbations, improve quality of life, and lower rates of hospitalisation. However, it is unclear whether providing telehealth care improves health‐related outcomes for people with COPD. We wanted to explore whether telehealth technologies were helpful for people with COPD.
What are telehealth technologies?
Study investigators used a range of telehealth technologies. Some included remote monitoring technology, which requires daily use of a laptop or a tablet with monitoring equipment, with results received by the healthcare professional. Typical monitoring equipment included a stethoscope (to measure blood pressure and heart rate), a pulse oximeter (to measure oxygen levels in the blood), a spirometer (to measure lung function), a thermometer, and other devices. Interventions involved regular phone calls with healthcare professionals for patients to talk about their symptoms and completion of health questionnaires.
Identifying and selecting studies
We searched online databases up until April 2020. We searched for studies published worldwide, in any language, at any time. Two review authors looked at lists of studies separately, then agreed on which studies should be included.
To find the best answer to our question, we looked for studies that recruited people with COPD of any severity. To make the comparison fair, we looked for studies in which investigators compared remote monitoring, remote monitoring plus usual care, and multi‐component treatments. People included in these studies had to have the same random chance (like the flip of a coin) to receive one of these teleheath technologies or usual care.
Key results
We found 29 studies (5654 people with moderate to very severe COPD) that were suitable for inclusion in our review. Duration of these studies ranged from 3 to 12 months.
We did not find any important benefits or harms for patients who were monitored with any of the telehealth technologies when we looked at number of exacerbations, improvement in quality of life, and reduction in breathing distress symptoms, hospitalisations, or death. However, people who were monitored through telehealth technology plus usual care had some reduction in risk of hospital re‐admission. Thus, telehealth technologies that were part of a care package reduced COPD‐related hospital re‐admissions.
We could not be certain of any harms of stand‐alone remote monitoring. We are also uncertain of any benefits or harms of stand‐alone remote monitoring of patient experiences or reports of breathing distress.
Quality of evidence
Currently, no good quality evidence is available. We are very uncertain about evidence for exacerbations, quality of life, dyspnoea symptoms, hospitalisations, deaths, and side effects. However, we are moderately certain about our findings for hospital re‐admissions.
Conclusion
We are not clear whether telehealth technologies for monitoring or consultation provide benefit, but we have not found any information on harms. Telehealth could play a role in the care and management of people with COPD. Telehealth as part of multi‐component care packages may provide short‐term benefit for quality of life and hospital re‐admissions. Telehealth in the form of remote monitoring in addition to usual care may reduce the risk of hospital re‐admission. There is little impact on exacerbations, quality of life, and death. Owing to limited information, the findings of this review should be interpreted with caution. More studies are needed to determine whether telehealth provides any long‐term benefits for people with COPD of varying severity.
Summary of findings
Background
Description of the condition
The Global Burden of Disease (GBD) analysis from 1990 to 2017 shows that more than 500 million people worldwide are living with a chronic respiratory condition that is a large contributor to premature death (GBD 2015; Soriano 2020). Moreover, the World Health Organization has predicted that chronic obstructive pulmonary disease (COPD) will be among the top causes of death by the year 2030 (WHO 2018). Although most information about COPD death comes from high‐income countries, it is known that 90% of COPD deaths occur in low‐ to middle‐income countries (WHO 2018). COPD represents 3.9% of the entire global burden of disease (Soriano 2020); it is a growing global public health problem that remains under‐recognised, under‐diagnosed, and under‐treated (Quaderi 2018).
Although the burden of COPD in high‐income countries is significant, this is compounded in low‐ to middle‐income countries by poverty and greater exposure to smoking and environmental factors such as outside and household air pollution (Quaderi 2018). It is expected that continued exposure to risk factors, population growth, and ageing will further increase the burden of this disease (Lopez‐Campos 2016). Disease severity, symptoms (e.g. frequent exacerbations leading to hospitalisation), and common comorbidities (e.g. cardiovascular disease) (in approximately 30% to 57% of people with COPD) increase the burden for patients and their carers, while exerting an economic burden for healthcare systems (Udsen 2017a). Respiratory diseases account for approximately 6% of the total healthcare budget in the EU, and more than half of this cost is attributed to COPD (ATS 2014). There is a direct correlation between severity of COPD, number of coexisting conditions, and increasing costs of care (GOLD 2021a).
COPD is a chronic lung disease that is characterised by persistent respiratory symptoms and limited airflow due to airway or alveolar abnormalities (or both) resulting from significant exposure to noxious particles or gases (including tobacco smoking and environmental factors such as exposure to biomass fuel and air pollution) (WHO 2018). Diagnosis of COPD is considered when a person has symptoms such as dyspnoea, cough, sputum production, or a combination of these, and when spirometry (presence of post‐bronchodilator forced expiratory volume in one second (FEV₁)/forced vital capacity (FVC) < 70%) confirms the presence of persistent airflow limitation (GOLD 2021). Exacerbations occur with increasing frequency as the disease progresses, leading to increased risk of hospitalisation or mortality (or both) (BLF 2018a; GOLD 2021a). Despite optimised treatment, people with COPD experience debilitating symptoms (e.g. frequent exacerbations, lung infection, reduced self‐care capability, limited physical function, anxiety, depression, cognitive deterioration), which can have an impact on their functional status, access to health services, and quality of life. 'Informal' carers play a key role in supporting people with COPD, particularly as the disease progresses. Physical, emotional, and financial impact on carers can be substantial (Andrianopoulos 2017; Farquhar 2018).
Description of the intervention
Telehealth is a broad term referring to "delivery of health care services where patients and providers are separated by distance" (WHO 2010).
Health care delivered through telehealth technologies can be received remotely by patients in many ways, including telephone, email, computer, monitoring, or video consultation.
Remote monitoring can facilitate the timely transfer of patient data, such as physiological parameters (e.g. oxygen saturation, blood pressure), through digital devices (e.g. telephone line, web‐based devices) to health professionals (Annandale 2011).
Remote monitoring has the potential to alert healthcare professionals to changes in a person's symptoms early in deterioration (McLean 2011), allowing the best opportunity for early intervention. Early intervention is known to decrease exacerbation severity, hospitalisation frequency, and disease progression in COPD (GOLD 2021a). Additionally, continuous monitoring can provide a more robust picture of a person's condition when compared with the single snapshot or retrospective symptoms recalled by the patient (or both), which clinicians commonly rely on in traditional face‐to‐face consultations (Breen 2015; Tomasic 2018).
Remote monitoring can be asynchronous or synchronous. Asynchronous technologies (e.g. store and forward technology) do not require live interaction with the person when data are collected. Data are collected in a file format that is sent to the necessary healthcare professional via a secured encrypted Internet connection, allowing healthcare professionals to receive and analyse these data as they would if the data were collected from the person in a usual clinic setting (McLean 2011). 'Synchronous' refers to real‐time technology that facilitates monitoring of physiological parameters, live‐streaming of medical images, and video consultations (AMD Global Telemedicine 2015; McLean 2011).
Real‐time remote consultation consists of live interaction between patient and healthcare professional by video, telephone, or web‐based application (e.g. Skype, text messaging). Remote consultations can be provided when patients are not able to have face‐to‐face consultation, or they can be given in addition to face‐to‐face home visits or clinic visits (Hernandez 2014).
Remote monitoring or remote consultation (or both) can be provided as part of an integrated package of care, which we refer to in this review as "multi‐component" interventions.
How the intervention might work
Hospital admissions and re‐admissions pose a significant burden for healthcare services, with respiratory disease contributing as the second most common cause of emergency hospital admissions in the UK (BLF 2018b). As populations age, and as people live longer with chronic conditions, there is a need to explore more efficient approaches to healthcare delivery that are flexible and tailored (McLean 2011), while supporting people's acquisition and strengthening of their own resources in self‐management of their day‐to‐day activities (Luhr 2018). Remote monitoring and remote consultation (with a health professional), in addition to usual care, provide closer and more timely monitoring of patients in their own home, along with early intervention for fluctuations and exacerbations of COPD. Ongoing monitoring and management based on ongoing fluctuations in disease and symptoms are needed for people with COPD, who often have difficulty accessing face‐to‐face services at their time of need. Remote monitoring and consultation may allow serial collection of data over a longer period ‐ a benefit over traditional face‐to‐face healthcare settings, where the clinician often relies on a clinical snapshot provided by the patient at the time of the face‐to‐face consultation. Ultimately these types of interventions have the potential to optimise COPD management, consequently reducing hospitalisation and improving quality of life for people with COPD.
Why it is important to do this review
Although it may be appropriate for healthcare providers to promote remote monitoring or consultation, it is not clear whether these technologies improve outcomes for people with COPD. Mixed evidence of effectiveness is derived from published systematic reviews, and some report potential for improving health‐related outcomes.
Two systematic reviews have addressed this topic (Lundell 2015; McLean 2011). Our current scoping searches suggest that more than 50 new publications of potentially relevant studies have become available since the last Cochrane Review was published.
Similarly, evidence for cost‐effectiveness of telemonitoring or consultations is limited and unclear, with one such trial showing that remote monitoring plus usual care resulted in similar quality‐adjusted life‐years (QALYs) as usual care alone and was not cost‐effective when provided with standard support and treatment (Henderson 2013).
Therefore, it is essential to determine which interventions (i.e. remote interventions for monitoring or remote consultations) are clinically effective and safe for people with COPD who are unable to have face‐to‐face contact with health professionals, or may live a considerable distance from healthcare facilities.
Objectives
To assess the effectiveness of telehealth interventions that allow remote monitoring and consultation and multi‐component interventions for reducing exacerbations and improving quality of life, while reducing dyspnoea symptoms, hospital service utilisation, and death among people with COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only. We included cluster‐randomised trials but meta‐analysed data from such trials only if they were adjusted to account for clustering. We included cross‐over trials but meta‐analysed data from such trials only if outcome data from the pre‐cross‐over phase were obtainable, as the carry‐over effect could not be excluded. We included studies that reported in full text, those published in abstract format only, and unpublished data. We included studies from primary care and hospital settings.
Types of participants
We included adults (aged 18 years and over) who had a diagnosis of COPD according to established criteria (e.g. Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging, European Respiratory Society (ERS) or American Thoracic Society (ATS) criteria), including adults with any comorbidities. We excluded adults with diagnosed asthma, cystic fibrosis, bronchiectasis, or other respiratory conditions.
Types of interventions
We included studies that explored the following telehealth interventions and comparators.
Remote monitoring (linked to a healthcare professional) plus usual care versus usual care alone (as reported by trialists).
Remote consultation (e.g. real‐time contact with a healthcare professional) plus usual care versus usual care alone (e.g. face‐to‐face visit for a check‐up with a health professional in a health service, or as reported by trialists).
Remote monitoring or remote consultation versus usual care (e.g. when telehealth care has replaced an element of usual face‐to‐face care).
We analysed data from the above three groups separately.
We included the following telehealthcare intervention categories.
Wired or wireless telehealthcare systems to monitor physiological parameters that are processed or authorised by a healthcare professional with feedback provided to the patient via telephone or video.
Store and forward telehealthcare systems to transfer data to healthcare professionals regarding the condition of the patient for offline assessment.
Internet‐based telecommunication with healthcare professionals via methods such as video or telephone (e.g. Skype, text messaging, email).
We excluded interventions that delivered or monitored pulmonary rehabilitation remotely.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Exacerbations (as defined by trialists; depending on available data, we extracted numbers of participants experiencing one or more exacerbation, exacerbation rate, or both)
Quality of life (validated scales, such as St George's Respiratory Questionnaire (SGRQ))
Dyspnoea symptoms (validated scales)
Hospital service utilisation (e.g. emergency department presentation, hospitalisation, re‐admission, length of stay, as defined by trialists; depending on available data; we extracted numbers of participants who require hospitalisation, hospitalisation utilisation rate, or both)
Mortality (all‐cause)
We reported outcomes using the following time points.
Three months or longer to less than six months.
Six months or longer to less than 12 months.
12 months or longer.
Secondary outcomes
Adverse effects (i.e. numbers of participants with adverse effects)
Anxiety and depression (validated scales, e.g. Hospital Anxiety and Depression Scale)
Self‐efficacy (as defined by trialists, depending on available data)
Participant satisfaction (as defined by trialists, depending on available data)
Reporting one or more of the outcomes listed here was not an inclusion criterion for studies for this review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register on 28 April 2020, which was maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE OvidSP from 1946.
Weekly searches of Embase OvidSP from 1974.
Monthly searches of PsycINFO OvidSP from 1967.
Monthly searches of the Cumulcative Index to Nursing and Allied Health Literature (CINAHL) EBSCO from 1937.
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO.
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register were identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, along with a list of handsearched conference proceedings, are provided in Appendix 1. See Appendix 2 for the search terms we used to identify studies for this review.
We searched the following additional sources with appropriately adapted search terms.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
IEEE Xplore Digital Library (https://ieeexplore.ieee.org/Xplore/home.jsp).
We searched the Cochrane Airways Trials Register and additional sources from inception to 28 April 2020, with no restriction on language of publication. We searched grey literature such as conference abstracts through the Cochrane Airways Trials Register.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for study information.
We searched on 17 March 2021 for errata or retractions from included studies published in full text on PubMed.
Data collection and analysis
Selection of studies
Three review authors (SJ, CT, DC) screened titles and abstracts of search results independently and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports of all potentially eligible studies, and three review authors (SJ, CT, DC) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. We resolved any disagreements through discussion; if required, we consulted a fourth review author (RD). We identified and excluded duplicates, and we collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and the Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a Microsoft Excel spreadsheet piloted on at least one study in the review to collect data for study characteristics, interventions, and outcomes. Two review authors (SJ, DC) extracted the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study settings, withdrawals, and dates of study.
Participants: number, mean age, age range, numbers of males and females recruited, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention and comparison.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for studies and notable conflicts of interest of trial authors.
Three review authors (SJ, CT, DC) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table when outcome data were not reported in a usable way. We resolved disagreements by consensus or by consultation with a fourth review author (RD). One review author (SJ) transferred data into Review Manager 5 (Review Manager 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review with information provided in study reports. A second review author (DC) spot‐checked study characteristics for accuracy against the study report.
We produced a table summarising the key characteristics of each study, including region, baseline characteristics of participants, study size, interventions investigated, and effects reported in each study.
Assessment of risk of bias in included studies
Three review authors (SJ, CT, DC) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We resolved any disagreements by discussion or by consultation with another review author (RD). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We judged each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each listed domain. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a participant‐reported pain scale). It is unlikely that participants were blinded to the intervention. We took this into account in risk of bias and GRADE assessments, and we considered the potential impact of lack of blinding on a case‐by‐case basis (e.g. subjective outcomes were likely to be more at risk than objective outcomes). When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted this systematic review according to the published protocol and justified any deviations from it in the Differences between protocol and review section of the review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) when studies used the same scale, and as standardised mean differences (SMDs) when studies used different scales. For SMD analyses in which duration of treatment was varied, we calculated and reported absolute effects with 95% confidence intervals (CIs). When data from rating scales were combined in a meta‐analysis, we ensured they were entered with a consistent direction of effect (e.g. lower scores always indicating improvement).
We undertook meta‐analyses only when this was meaningful, that is, when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
We presented data as forest plots when it was possible to show size and direction of effect for treatment with 95% CIs using Review Manager 5 (Review Manager 2014).
We described skewed data narratively (e.g. medians and interquartile ranges for each group).
When a single study reported multiple trial arms, we included only relevant arms. We reported details of additional arms in the Characteristics of included studies table; when two comparisons (e.g. intervention A versus usual care, intervention B versus usual care) were combined in the same meta‐analysis, we combined the active arms or halved the control group to avoid double‐counting.
When available, we used adjusted analyses (ANOVA or ANCOVA) as a preference in our meta‐analyses. When both change from baseline and endpoint scores were available for continuous data, we used change from baseline unless there was low correlation between measurements among participants. When a study reported outcomes at multiple time points, we used the latest time point. When studies reported post‐treatment follow‐up, we extracted this information and reported it narratively.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses when they were reported (i.e. when data were imputed for participants who were randomly assigned but did not complete the study) instead of completer or per‐protocol analyses.
Unit of analysis issues
For dichotomous outcomes, we used participants, rather than events, as the unit of analysis (e.g. number of participants admitted to hospital rather than number of admissions per participant). However, when a study reported rate ratios, we analysed them on this basis. We meta‐analysed data from cluster‐RCTs only when available data were adjusted (or could be adjusted) to account for clustering.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was published as an abstract only). When this was not possible, and missing data were thought to introduce serious bias, we took this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among studies in each analysis. When we identified substantial heterogeneity (I² ≥ 40%), we reported this and explored possible causes by pre‐specified subgroup analysis.
Assessment of reporting biases
We were unable to pool more than 10 studies to create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used a random‐effects model, with the assumption that included studies may have heterogeneous, but related, intervention effect estimates (due to the clinical nature of the intervention). We performed a sensitivity analysis by using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Recent hospitalisation (within six months) versus no hospitalisation.
Cognitive function (presence or absence, e.g. Mini‐Mental State Examination score < 26).
Mean number of comorbidities (≤ 1 versus > 1; e.g. Charleston index).
We planned to include the following outcomes in subgroup analyses.
Exacerbations.
Quality of life.
Hospitalisation utilisation.
Mortality.
We planned to use the formal test for subgroup interactions in Review Manager 5 (Review Manager 2014).
Sensitivity analysis
We planned to carry out the following sensitivity analyses, removing the following from the primary analyses.
Studies with high risk of bias in one or more domains.
We compared results obtained with a fixed‐effect model versus results obtained with a random‐effects model when possible.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table using the following outcomes: exacerbations, quality of life, dyspnoea symptoms, hospital utilisation, mortality, and adverse effects. We presented effect size with 95% CI for each outcome, as well as absolute effects (generated by GRADEpro GDT software). We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the overall certainty of a body of evidence (low, moderate, or high certainty) as it relates to studies that contributed data for pre‐specified outcomes. We used the methods and recommendations provided in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019), using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies using footnotes, and we provided comments to aid the reader's understanding of the review when necessary. We applied the clinical importance of results using the published minimally important difference (MID) when available (e.g. SGRQ has well‐established MIDs in the literature).
Results
Description of studies
Details of the 29 studies are described in the Characteristics of included studies tables. Among included studies, interventions included remote monitoring in addition to usual care (Antoniades 2012; Berkhof 2015; Ho 2016; Lewis 2010; McDowell 2015; Pinnock 2013; Shany 2016; Vianello 2016), remote monitoring only compared with usual care (Calvo 2014; De San Miguel 2013; Jódar‐Sanchez 2013; Minguez 2017; Pedone 2013; Sink 2020; Soriano 2018; Stamenova 2020; Udsen 2017; Walker 2018), or multi‐component interventions compared with usual care (Bourbeau 2016; Casas 2006; Farmer 2017; Koff 2009; Ringbaek 2015; Ritchie 2016; Rose 2018; Jakobsen 2015; Sorknaes 2013; Tabak 2014; Yan 2018). Intervention comparisons and classifications are listed in Table 4.
1. Study classifications according to intervention type.
| Interventions | Remote monitoring (linked to healthcare professional) plus usual care vs usual care alone | Remote consultation (with health professional) plus usual care vs usual care alone (face‐to‐face) | Remote monitoring versus usual care (where telehealth replaces an element of usual care) | Remote consultation vs usual care (where telehealth replaces an element of usual care) | Integrated intervention vs usual care or interventions that include both monitoring and video consultations |
| Wired telehealth system to monitor physiological parameters processed or authorised by HCP with feedback to patient via telephone or video |
Antoniades 2012 Lewis 2010 McDowell 2015 Pinnock 2013 |
No studies |
Calvo 2014 De San Miguel 2013 Jódar‐Sanchez 2013 Minguez 2017 Soriano 2018 |
No studies | Koff 2009 |
| Wireless telehealth system to monitor physiological parameters that are processed or authorised by HCP with feedback to patient via telephone or video |
Berkhof 2015 Ho 2016 Shany 2016 Vianello 2016 |
No studies |
Pedone 2013 Sink 2020 Walker 2018 Stamenova 2020 Udsen 2017 |
No studies |
Bourbeau 2016 Farmer 2017 Ringbaek 2015 Jakobsen 2015 Yan 2018 Sorknaes 2013 |
| Store and forward telehealth system to transfer data regarding condition of patient to HCP for assessment offline | No studies | No studies | No studies | No studies | No studies |
| Internet‐based telecommunication such as video or telephone links with HCP (Skype, text, email) | No studies | No studies | No studies | No studies |
Casas 2006 Ritchie 2016 Rose 2018 Tabak 2014 |
HCP: healthcare professional.
Results of the search
We conducted database searches in 2018 and 2020. Through these searches we retrieved 1440 records after removing duplicates. Of the 1440 references screened, we excluded 1317 based on titles and abstracts. We assessed full texts for 123 relevant references for inclusion. Of these, we identified 55 studies that met the inclusion criteria. We included 29 studies in the quantitative analysis (Figure 1). We placed 21 studies under awaiting classification for further assessment, as we could not find information about these studies, and five were ongoing. GRADE certainty ratings of the evidence for primary outcomes are presented in Table 1, Table 2, and Table 3.
1.
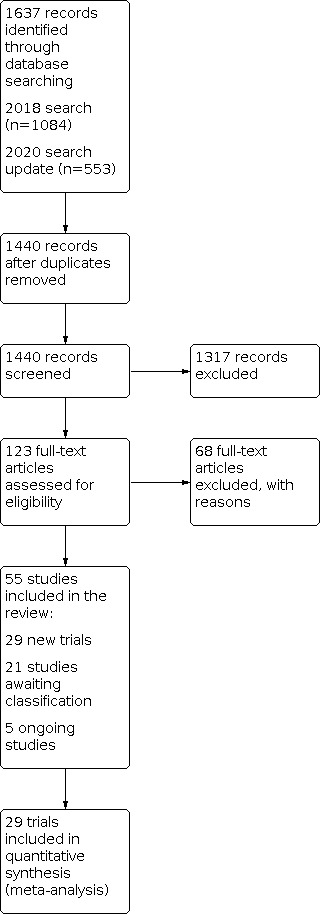
Study flow diagram.
Summary of findings 1. Remote monitoring plus usual care compared to usual care.
| Remote monitoring plus usual care compared to usual care | ||||||
| Patient or population: people with chronic obstructive pulmonary disease Setting: primary, secondary, tertiary care; general hospital, specialist respiratory service, hospital‐based respiratory care; single‐centre or multi‐centre Intervention: remote monitoring plus usual care Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with remote monitoring plus usual care | |||||
| Exacerbations | ||||||
| Number of people experiencing 1 or more exacerbations Follow‐up: 26 weeks Asynchronous remote monitoring |
469 per 1000 | 525 per 1000 (343 to 703) | OR 1.25 (0.59 to 2.67) | 108 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | Imprecision: does not meet OIS of 200 participants |
| Quality of life | ||||||
| SGRQ total score Follow‐up: 26 weeks Scale: 0 to 100 Lower score is better Asynchronous or synchronous remote monitoring |
Mean SGRQ total was 66.8 | MD 1.49 lower (9.43 lower to 6.44 higher) | ‐ | 204 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWb, c, d | MID: 4 points (Jones 2005) Control arm MD was taken from McDowell 2015 |
| SGRQ total score Follow‐up: 52 weeks Scale: 0 to 100 Lower score is better |
Mean SGRQ total was 67.3 | MD 0.9 higher (3.71 lower to 5.51 higher) | ‐ | 205 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb, e | MID: 4 points (Jones 2005) |
| Dyspnoea symptoms | ||||||
| No evidence identified | ||||||
| Hospital service utilisation | ||||||
| Time to first hospitalisation after starting intervention Follow‐up: 52 weeks Asynchronous remote monitoring |
HR 1.08 (0.80 to 1.46) | 256 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb, e | |||
| Time to first COPD‐related re‐admission Follow‐up: 26 weeks Asynchronous remote monitoring |
HR 0.42 (0.19 to 0.93) | 106 (1 RCT) | ⊕⊕⊕⊝ MODERATE f | Imprecision: does not meet OIS of 200 participants | ||
| Mortality | ||||||
| Mortality (all‐cause) Follow‐up: 44 weeks** Asynchronous or synchronous remote monitoring |
93 per 1000 | 92 per 1000 (60 to 139) | OR 0.99 (0.62 to 1.58) | 927 (7 RCTs) | ⊕⊝⊝⊝ VERY LOWb, g | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Weighted mean duration. CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; MD: mean difference; MID: minimally important difference; OIS: optimal information size; OR: odds ratio; RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence for this outcome was downgraded by 2 due to performance, detection, and selective reporting bias. Allocation concealment was unclear.
bEvidence for this outcome was downgraded by 1 due to wide confidence intervals.
c Evidence for this outcome was downgraded by 2 due to performance and detection bias. One study was at high risk of selective reporting.
d Evidence for this outcome was downgraded by 2 due to very high heterogeneity.
e Evidence was downgraded by 2 due to performance and detection bias.
f Evidence for this outcome was downgraded by 1 due to performance bias. Allocation concealment was unclear.
g Evidence for this outcome was downgraded by 2 due to allocation concealment and performance, detection, and attrition bias in one or more studies.
Summary of findings 2. Remote monitoring compared to usual care.
| Remote monitoring compared to usual care | ||||||
| Patient or population: people with chronic obstructive pulmonary disease Setting: regional, international (university hospital; specialist respiratory outpatient clinics; community‐based primary care clinics and health services), single‐centre or multi‐centre Intervention: remote monitoring Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with remote monitoring | |||||
| Exacerbations | ||||||
| Number of people experiencing 1 or more exacerbations Follow‐up: 41 weeks** Asynchronous or synchronous remote monitoring |
370 per 1000 | 375 per 1000 (283 to 477) | OR 1.02 (0.67 to 1.55) | 424 (4 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | |
| Quality of life | ||||||
| SGRQ total score Follow‐up: 17 weeks Scale: 0 to 100 Lower score is better Asychronous remote monitoring |
Mean SGRQ total score was ‐4.5 | MD 6.4 lower (18.56 lower to 5.76 higher) | ‐ | 45 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,c | MID: 4 points (Jones 2005) Imprecision: does not meet OIS |
| CAT score Follow‐up: 38 weeks** Scale: 0 to 40 Lower score is better Asynchronous remote monitoring |
Mean CAT total score was 17.2 | MD 0.06 higher (1.34 lower to 1.45 higher) | ‐ | 405 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWb,d | MID: 2 points (Kon 2014) MD in control arm taken from the study of longer duration (Walker 2018) |
| CAT total score Follow‐up: 52 weeks Scale: 0 to 40 Lower score is better Asynchronous remote monitoring |
Mean CAT total score was 21.4 | MD 0.1 higher (1.42 lower to 1.62 higher) | ‐ | 229 (1 RCT) | ⊕⊝⊝⊝ VERY LOWb,e | MID: 2 points (Kon 2014) |
| Symptoms of dyspnoea | ||||||
| CRQ‐SAS dyspnoea symptoms score Follow‐up: 26 weeks Scale: 0 to 100 Higher score is better Asychronous remote monitoring |
Mean dyspnoea symptoms score on the CRQ‐SAS was 4.16 | MD 0.44 lower (1.04 lower to 0.16 higher) | ‐ | 70 (1 RCT) | ⊕⊕⊝⊝ LOWb,f | MID: 0.5 reflects a small change. A change of 1.0 reflects a moderate change, and a difference of 1.5 reflects a large change (Schünemann 2003) Imprecision: does not meet OIS |
| Hospital service utilisation | ||||||
| Number of people admitted to hospital Follow‐up: 36 weeks** Asynchronous remote monitoring |
246 per 1000 | 283 per 1000 (196 to 387) | OR 1.21 (0.75 to 1.94) | 357 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWb,g | |
| Mortality | ||||||
| Mortality (all‐cause) Follow‐up: 38 weeks** Asynchronous remote monitoring |
73 per 1000 | 51 per 1000 (28 to 89) | OR 0.68 (0.37 to 1.25) | 798 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWb,e | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Weighted mean duration. CAT: COPD assessment test; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CRQ‐SAS: chronic respiratory disease questionnaire self‐administered; MD: mean difference; MID: minimally important difference; OIS: optimal information size; OR: odds ratio; RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence for this outcome was downgraded by 2 due to allocation concealment and performance, detection, attrition, and selective reporting bias.
bEvidence for this outcome was downgraded by 1 due to wide confidence intervals.
3Evidence was downgraded by 2 due to performance and detection bias. Selection bias (randomisation and allocation concealment) and selective reporting were unclear.
dEvidence for this outcome was downgraded by 2 due to performance and detection bias. One study was at high risk of selective reporting.
eEvidence for this outcome was downgraded by 2 due to allocation concealment and performance and detection bias.
fEvidence for this outcome was downgraded by 1 due to performance bias. Detection, attrition, and selective reporting were unclear.
gEvidence was downgraded by 2 due to performance and detection bias.
Summary of findings 3. Multi‐component interventions (with telehealth as a component of care) compared to usual care.
| Multi‐component interventions (with telehealth as a component of care) compared to usual care | ||||||
| Patient or population: people with chronic obstructive pulmonary disease Setting: primary, secondary, tertiary care; community teaching hospitals, outpatient clinics (COPD‐specific (university‐based), general respiratory, or physiotherapy practice); single‐centre or multi‐centre Intervention: multi‐component interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with multi‐component interventions | |||||
| Exacerbations | ||||||
| Number of people experiencing at least 1 exacerbation/moderate to severe exacerbation Follow‐up: 52 weeks Asynchronous or synchronous remote monitoring |
347 per 1000 | 343 per 1000 (283 to 405) | OR 0.98 (0.74 to 1.28) | 955 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | |
| Time to first exacerbation Follow‐up: 52 weeks Asynchronous remote monitoring |
HR 1.05 (0.67 to 1.65) | 166 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd,e | Does not meet OIS | ||
| Quality of life | ||||||
| SGRQ total score Follow‐up: 13 weeks Scale: 0 to 100 Lower score is better Asynchronous remote monitoring |
Mean SGRQ total score was ‐0.6 | MD 9.7 lower (18.32 lower to 1.08 lower) | ‐ | 38 (1 RCT) | ⊕⊕⊝⊝ LOWf | MID: 4 points (Jones 2005) Imprecision: does not meet OIS |
| SGRQ total score Follow‐up: 26 weeks Scale: 0 to 100 Lower score is better Asynchronous remote monitoring and synchronous video conference |
Mean SGRQ total score was 48 | MD 7 higher (4.79 lower to 18.79 higher) | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,g | MID: 4 points (Jones 2005) Imprecision: does not meet OIS |
| SGRQ total score Follow‐up: 52 weeks Scale: 0 to 100 Lower score is better Asynchronous remote monitoring |
Mean SGRQ total score was 56.8 | MD 1.09 lower (6.24 lower to 4.05 higher) | ‐ | 203 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWc,h | MID: 4 points (Jones 2005) MD in control arm taken from Farmer 2017 |
| CAT score Follow‐up: mean 38 weeks Scale 0 to 40 Lower score is better Asynchronous remote monitoring and synchronous video consultation |
Mean CAT score was 18.6 | MD 3.93 lower (7.75 lower to 0.12 lower) | 521 (2 RCTs) |
⊕⊝⊝⊝ VERY LOWi,j | MID: 2 points (Kon 2014) | |
| Dyspnoea symptoms | ||||||
| No evidence identified | ||||||
| Hospital service utilisation | ||||||
| Number of people who had at least 1 hospital admission Follow‐up: 33 weeks** Asynchronous remote monitoring alone or additional synchronous video consultation |
485 per 1000 | 432 per 1000 (341 to 526) | OR 0.81 (0.55 to 1.18) | 447 (2 RCTs) | ⊕⊕⊝⊝ LOWc,j | |
| Number of people re‐admitted (all‐cause) Follow‐up: 39 weeks** Asynchronous remote monitoring alone or additional video conference or telephone calls |
476 per 1000 | 312 per 1000 (220 to 424) | OR 0.50 (0.31 to 0.81) | 344 (3 RCTs) | ⊕⊕⊕⊝ MODERATEj | |
| Mortality | ||||||
| Mortality (all‐cause) overall analysis Follow‐up: 40 weeks** Asynchronous remote monitoring alone or additional video conference, or synchronous telephone consultations |
113 per 1000 | 73 per 1000 (47 to 114) | OR 0.62 (0.39 to 1.01) | 1886 (9 RCTs) | ⊕⊝⊝⊝ VERY LOWg,k | |
| Adverse events: number of people with 1 or more (all‐cause) Follow‐up: 52 weeks Asynchronous remote monitoring |
528 per 1000 | 504 per 1000 (409 to 598) | OR 0.91 (0.62 to 1.33) | 485 (2 RCTs) |
⊕⊝⊝⊝ VERY LOWa,c | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **Weighted mean duration of follow‐up. CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; MD: mean difference; MID: minimally important difference; OIS: optimal information size; OR: odds ratio; RCT: randomised controlled trial; SGRQ: St George's Respiratory Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence for this outcome was downgraded by 2 due to performance, detection, and attrition bias.
bEvidence for this outcome was downgraded by 1 due to differences in multi‐component interventions.
cEvidence for this outcome was downgraded by 1 due to wide confidence intervals.
dEvidence for this outcome was downgraded by 2 due to performance and detection bias. Allocation concealment and attrition were unclear.
eThere was no difference between intervention and control. Confidence intervals crossed the line of no effect.
fEvidence for this outcome was downgraded by 2 due to performance and detection bias. Randomisation method and selective reporting were unclear.
gEvidence was downgraded by 2 due to performance and detection bias.
hEvidence for this outcome was downgraded by 2 due to performance and detection bias. Randomisation method, detection, attrition, and selective reporting were unclear in one or more studies.
iEvidence for this outcome was downgraded by 2 due to very high heterogeneity.
jEvidence was downgraded by 1 due to performance bias.
kEvidence for this outcome was downgraded by 1 due to moderate heterogeneity.
Included studies
Setting, design, and duration
Fourteen studies were single‐centre, and fifteen were multi‐centre, parallel‐assignment randomised trials. Four studies were conducted in Denmark (Jakobsen 2015; Ringbaek 2015; Sorknaes 2013; Udsen 2017), four in Spain (Calvo 2014; Jódar‐Sanchez 2013; Minguez 2017; Soriano 2018); three each in the UK (Farmer 2017; Lewis 2010; Pinnock 2013), Australia (Antoniades 2012; De San Miguel 2013; Shany 2016), and the USA (Koff 2009; Ritchie 2016; Sink 2020); and two each in the Netherlands (Berkhof 2015; Tabak 2014), Canada (Rose 2018; Stamenova 2020), and Italy (Pedone 2013; Vianello 2016). One study each was conducted in China (Yan 2018), Ireland (McDowell 2015), and Taiwan (Ho 2016). Three were multi‐national studies (Bourbeau 2016; Casas 2006; Walker 2018). The duration of interventions ranged from 12 weeks to 52 weeks' follow‐up, and settings included primary, secondary, and tertiary care.
Baseline participant characteristics
Participant characteristics at baseline are presented in Table 5. The mean age of participants ranged from 63 to 79 years. The proportion of males recruited in these studies ranged from 36% to 96%, and the proportion of females ranged from 4% to 61% (Table 5). COPD severity ranged from mild to very severe, as diagnosed by GOLD staging criteria, and concomitant medications included long‐acting beta‐agonists (LABAs), long‐acting muscarinic agonists (LAMAs), inhaled corticosteroids (ICSs), theophylline, phosphodiesterase‐4 inhibitors (PDE‐4s), and short‐acting beta‐agonists (SABAs). Participants in three studies were receiving home oxygen (Berkhof 2015; De San Miguel 2013), or were given long‐term oxygen therapy (Jódar‐Sanchez 2013); however some studies also reported participants who had received influenza or pneumonia vaccines (Casas 2006Koff 2009McDowell 2015Rose 2018). Most studies did not report exacerbations in the previous 12 months; however, mean exacerbations among three studies ranged from 1 to 19 (Bourbeau 2016; Ho 2016; Stamenova 2020). Hospitalisations in the previous 12 months ranged from mean 0.55 to 2.75 across 14 studies. Comorbidities were reported by most studies (except for Antoniades 2012Berkhof 2015De San Miguel 2013Jakobsen 2015Koff 2009Pedone 2013Ritchie 2016Sink 2020Tabak 2014 and Yan 2018); these are presented in Table 5. Anxiety and depression, hypertension, cardiovascular disease, infection, and diabetes were among the comorbidities more commonly reported by studies, ranging from mean 1.9 to 3.5 comorbidities per person (Bourbeau 2016; Casas 2006; Table 5).
2. Baseline characteristics of study participants.
| Study ID | Concomitant treatments |
COPD severity |
Comorbidities, percentage, mean (SD), or median (IQR) |
Mean age, years |
Male or female |
Exacerbations in the last 12 months, mean |
Hospital admissions in the last 12 months, mean (SD) or median (IQR) |
| Remote monitoring plus usual care | |||||||
| Antoniades 2012 | NR | Moderate /severe |
NR | RM + UC = 68 UC = 70 |
males: 20/44 (45%); females: 24/44 (54%) | NR | RM + UC = median 2 (1 to 4); UC = median 1 (1 to 2) |
| Berkhof 2015 | Home oxygen | Severe | NR | 68 | males: 68/91 (75%); females: 23/91 (25%) | NR | NR |
| Ho 2016 | SABA LABA Anticholinergic ICS |
Mild /moderate |
RM + UC: CHD (23%), HF (26%), hypertension (53%), diabetes (21%) UC: CHD (17%), HF (25%), hypertension (62%), diabetes (19%) |
RM + UC = 84 UC = 79 |
males: 81/106 (76%); females: 25/106 (24%) | RM + UC = 19 UC = 17 |
RM + UC = 16 UC = 19 |
| Lewis 2010 | NR | Moderate/very severe | Known comorbidity: RM: 92%, UC: 88% | RM = 70 UC = 73 |
males: 20/40 (50%); females 20/40 (50%) | NR | RM = median 0 (0 to 1.0) UC = median 0 (0 to 0.8) |
| McDowell 2015 | Flu vaccine | GOLD stage II/III | HADS total Anxiety: RM: 8.3 ± 5.2; UC: 7.9 ± 4.3 Depression: RM: 6.8 ± 3.8; UC: 7.9 ± 3.9 |
69.8 RM and 70.2 UC | males: 48/110 (44%); females: 62/110 (56%) | NR | RM: 0.82 UC: 1.05 |
| Pinnock 2013 | NR | GOLD stage mild/moderate, severe, very severe | 1 or more comorbidities: RM: 61%; UC: 71%; HADS total Anxiety: RM: 9.8 ± 5.2; UC: 9.6 ± 4.6 Depression: RM: 8.9 ± 4.4; UC: 8.2 ± 4.1 |
69.4 RM and 68.4 UC | males: 116/256 (45%); females: 140/256 (55%) | NR | RM+UC = 2.3 UC = 2.5 |
| Shany 2016 | NR | GOLD stage severe | HADS total Anxiety: RM + UC: 7.8 ± 4.7; UC: 6.2 ± 4.0 Depression: RM + UC:6.0 ± 3.0; UC: 6.4 ± 4.5 |
RM + UC 72.1 UC = 74.2 |
males: 19/42 (45%); females: 23/42 (55%) | NR | RM+UC = 3 UC = 2.5 |
| Udsen 2017 | NR | GOLD stage I, II, III, IV | Diabetes: RM: 10%; UC: 9.8% CHD: RM: 33%; UC: 31% Mental health problems: RM: 4.8%; UC: 4.79% Musculoskeletal disorder: RM: 24.9%; UC: 29% Cancer: RM: 6%; UC: 4.79% |
RM = 69.6 UC = 70.3 |
males: 562/1225 (46%); females: 663/1225 (54%) | NR | NR |
| Vianello 2016 | LABA: RM 97.8% and UC 94.1% LAMA: RM 87.2% and UC 86.3% ICS: RM 83.5% and UC 76.9% Systemic steroid: RM: 6.5% and UC: 4.8% |
GOLD stage III, IV | HADS total: Anxiety: RM + UC: 4.68 ± 3.45; UC: 5.4 ± 3.35 Depression: RM + UC: 5.1 ± 4.42; UC: 5.48 ± 4.49 Hypertension: RM + UC: 61%; UC: 64% IHD: RM + UC:38.9%; UC: 35% |
RM + UC = 75.96 UC = 76.48 |
males: 240/334 (72%); females: 94/334 (28%) |
NR | NR |
| Walker 2018 | NR | GOLD stage I, II, III, IV | CHF: RM + UC: 12%; UC: 8% IHD: RM + UC: 25%; UC: 23% CHF + IHD: RM + UC: 12%; UC: 13% Hypertension: RM + UC: 72%; UC: 68% Osteoporosis: RM + UC: 17%; UC:15% Hyperlipidaemia: RM + UC: 53%; UC: 58% Number of comorbidities per person, median (IQR): RM + UC: 2.0 (1.0 to 3.0); UC: 2.0 (1.0 to 3.0) |
71 | males: 206/312 (62%); females: 106/312 (34%) | More than 1 exacerbation: RM + UC = 59% UC = 63% |
RM = 42% UC = 41% |
| Remote monitoring alone | |||||||
| Calvo 2014 | LAMA + LABA + ICS PDE4 inhibitors Mucolytics Theophylline Oral steroids |
Severe /very severe |
Charlson comorbidity index score: RM: 3.7 ± 1.4; UC:3.4 ± 2.1 | RM = 75 UC = 72.7 |
males: 44/59 (75%); 15/59 females (25%) | NR | RM = 1.7 UC = 1.9 |
| De San Miguel 2013 | Oxygen | NR | NR | RM = 71 UC = 74 |
males: 37/71 (52%); females: 34/71 (48%) | NR | NR |
| Jódar‐Sanchez 2013 | LTOT | Very severe | Adjusted Charlson comorbidity index score: RM: 6.6 ± 2.8; UC: 5.1 ± 2 10% in each group had anxiety/depression |
RM = 74 UC = 71 |
males: 43/45 (96%); females: 2/45 (4%) | NR | NR |
| Minguez 2017 | NR | NR (FEV₁ % = 50 and 51.1) | Charlson comorbidity index score: (median (IQR): RM: 4 (3 to 5); UC: 4.45 (3.6 to 6.2) |
RM = 68 UC = 70 | males: 77/111 (69%); females: 34/111 (31%) | NR | NR |
| Pedone 2013 | NR | GOLD stage II/III | NR | 74.1 RM and 75.4 UC | males: 36/50 and 31/49 (68%); females: 32/99 (32%) | NR | NR |
| Sink 2020 | NR | GOLD stage mild to very severe | NR | RM = 59.8 UC = 61.9 |
males: 61/168 (36%); females: 107/168 (64%) | NR | NR |
| Soriano 2018 | LABA (98%), LAMA (98%), ICS (94%), SAA (57%), PDE4 inhibitors (16%), theophylline (14%), oral steroids (4%), β2‐adrenergic receptor agonists (5%) | GOLD stage severe (stable) |
Charlson comorbidity index score: RM: 2.4 ± 1.5; UC: 2.4 ± 1.5 Goldberg anxiety: RM: 1.5 ± 2.3; UC: 1.8 ± 2.5 Goldberg depression: RM: 2.5 ± 2.4; UC: 2.9 ± 2.5 |
RM = 71.5 UC = 71.3 |
males: 184/229 (80%); females: 45/229 (20%) | NR | RM = 2 UC = 2 |
| Stamenova 2020 | NR | NR (FEV₁ % 50 and 45) |
RM group had lower rates of osteoporosis (P = 0.02), pulmonary hypertension compared to UC group (P = 0.04) | RM = 71.98 UC = 72.78 |
males: 44/81 (54%); females: 37/81 (46%) | MC = 2 UC = 1 |
MC = 0 UC = 0 |
| Multi‐component or integrated care (where remote monitoring, consultation, or both are components of care) | |||||||
| Bourbeau 2016 | Long‐acting anticholinergics LABA Long‐acting ICS |
GOLD stage III/IV |
Overall: severe anxiety (26.7%), severe depression (78.6%) (HADS); age‐adjusted Charlson comorbidity index score (4.2 ± 1.8); number of concomitant diseases: 3.5 ± 2.0 |
MC = 67.3 UC = 66.6 |
males: 222/319 (70%); females: 97/319 (30%) | 1.3 | MC = 20 UC = 19 |
| Casas 2006 | Influenza and Pneumococcal vaccination |
NR (FEV₁ % = 42) |
Goldberg score: MC: 8.5 ± 5.6; UC: 8.2 ± 5.9 Mean comorbidities: MC: 1.9 ± 1.4; UC: 1.8 ± 1.5 |
MC = 70 UC = 72 |
males: 129/155 (83%); females: 26/155 (17%) | NR | MC = 1 UC = 0.6 |
| Farmer 2017 | COPD medication (not described) |
Moderate /severe/very severe |
IG: 80.9%; SC: 83.9% had comorbidities including high blood pressure, osteoporosis, high cholesterol, diabetes, heart disease, depression |
69.8 | males: 102/166 (61%); females: 64/166 (39%) | NR | NR |
| Jakobsen 2015 | Corticosteroid (prednisone) Antibiotics (amoxicillin, clavulanic acid) β₂‐agonists Anticholinergics Fenoterol Ipratropium bromide nebuliser O₂ therapy as needed Sedative levomepromazine as needed |
GOLD stage III/IV | NR | NR | males: 22/57 (39%); females: 35/57 (61%) | NR | NR |
| Koff 2009 | Flu vaccine | GOLD stage III/IV | NR | RM = 66.6 UC = 65 |
males: 19/40 (47%); females: 21/40 (53% | NR | RM = 0.55 UC = 0.6 |
| Ringbaek 2015 | Oral prednisolone Roflumilast ICS LAMA LABA |
GOLD stage severe and very severe | Charlson comorbidity index score: MC: 1.7 ± 1.49, UC: 1.96 ± 1.51 | MC = 69.8 UC = 69.4 |
males 130/281 (46%); females: 151/281 (54%) | NR | MC = 0.91 UC =. 1.22 |
| Ritchie 2016 | NR | NR | NR | MC = 63.8 UC = 63.4 |
males: 73/132 (55%); females: 59/132 (45%) | NR | NR |
| Rose 2018 | Inhaled bronchodilator Inhaled steroid Antihypertensive Influenza vaccine Pneumonia vaccine |
NR (FEV₁ % 43 and 45) | CVD: MC: 75%; UC: 76% Diabetes: MC: 18%; UC: 22% Depression: MC: 17%; UC: 20% Osteopenia and osteoporosis: MC: 30%; UC: 29% GORD: MC: 14%; UC: 12% Hypothyroidism: MC: 9%; UC: 9% Osteoarthritis: MC: 9%; UC: 9% CKD: MC: 7%; UC: 7% Anxiety: MC: 7%; UC: 7% OSA: MC: 5%; UC: 6% Lung cancer: MC: 6%; UC: 6% |
71 in both groups | males: 220/470 (47%); females: 250/470 (53%) | NR | MC = 1.3 UC = 1.4 |
| Sorknaes 2013 | NR | GOLD stage severe | Infection: MC: 52%; UC: 55%; HD: MC: 35%; UC: 36%; CVD: MC: 9%; UC: 8%; Depression: MC: 2%; UC: 2% Diabetes: MC: 1% to 4%; UC: 11% Osteoporosis: MC: 17%; UC: 19% Cancer: MC: 0%; UC: 1% |
MC = 71 UC = 72 | males: 104/266 (39%); females: 162/266 (61%) |
NR | MC = 2.75 UC = 2.64 |
| Tabak 2014 | NR | NR (FEV₁ % 50 and 36) |
NR | MC = 64.1 UC = 62.8 |
males: 12/24 (50%); females: 12/24 (50%) | NR | NR |
| Yan 2018 | NR | GOLD stage I, II, III, IV | NR | RM = 65.4 UC = 64.6 |
males: 152/240 (63%); females: 88/240 (37) | NR | NR |
CHF: chronic heart failure; COPD: chronic obstructive pulmonary disease; CVD: cerebrovascular disease; FEV₁: forced expiratory volume in one second; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GORD: gastro‐oesophageal reflux disease; HD: heart disease; HF: heart failure ICS: inhaled corticosteroid; IG: intervention group; IHD: Ischaemic heart disease; LABA: long‐acting beta‐agonist; LAMA: long‐acting muscarinic antagonist; LTOT: long‐term oxygen therapy; MC: multi‐component; NR: not reported; PDE4: phosphodiesterase 4; RM: remote monitoring; SABA: short‐acting beta‐agonist; SC: standard care; UC: usual care.
Description of interventions
All descriptions of interventions are presented in Table 6.
3. Details of interventions.
| &&Study details | Intervention and description |
| Remote monitoring plus usual care | |
|
Antoniades 2012 52 weeks |
INTERVENTION: remote monitoring using the TeleMedCare system (laptop computer with digital measurement capabilities) and standard best practice Participant data entry: automatic
Study administrator: study nurse, nursing informatics project manager, outreach nurse, study doctor Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professional: yes
|
|
Berkhof 2015 26 weeks |
INTERVENTION: remote in‐home monitoring via telephone and usual care practices Participant data entry: based on telephone calls
Study administrator: nurse, pulmonologist, pulmonary nurse practitioner Data transmission: via telephone call Data acquisition: synchronous Clinical alert: none Feedback from health professional: yes
|
|
Ho 2016 26 weeks |
INTERVENTION: self‐monitoring of COPD using a telehealth electronic diary on a website Participant data entry: manual
Study administrator: primary care physicians, study nurses, study team, attending pulmonologist Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm dependent
Feedback from health professional: yes
|
|
Lewis 2010 26 weeks |
INTERVENTION: remote in‐home monitoring intervention Participant data entry: manual
Study administrator: TM training team, chronic disease management team, hospital respiratory nurse Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
McDowell 2015 26 weeks |
INTERVENTION: remote in‐home monitoring intervention Participant data entry: manual
Study administrator: community respiratory team, telemonitoring technician, telemonitoring nurse, general practitioner Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Pinnock 2013 52 weeks |
INTERVENTION: remote in‐home monitoring intervention Participant data entry: manual
Study administrator: specialist respiratory team in Edinburgh, nurse specialist in Midlothian, trained call handler in East/West Lothian, GP Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Shany 2016 52 weeks |
INTERVENTION: remote monitoring intervention RACS‐Plus care (home visits, respiratory rehab, telephone) Participant data entry: automatic
Study administrator: respiratory community nurse, nurse at respiratory ambulatory care services Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: unclear
|
|
Vianello 2016 52 weeks |
INTERVENTION: remote monitoring with self‐management education and call centre Participant data entry: manual
Study administrator: operators, nurse, clinical staff, pulmonary specialist Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: symptom dependent
Feedback from health professionals: yes
|
| Remote monitoring alone | |
|
Calvo 2014 30 weeks |
INTERVENTION: remote in‐home monitoring using telephone line to submit data through a modem (Tele‐ModemTM, Aerotel Medical Systems) Participant data entry: manual
Study administrator: nurse, pneumologist, nursing staff, primary care physician Data transmission: automatic Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professional: yes
|
|
De San Miguel 2013 26 weeks |
INTERVENTION: remote in‐home monitoring and disease education using Docobo HealthHub Participant data entry: manual
Study administrator: telehealth nurse, general practitioner Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Jódar‐Sanchez 2013 17 weeks |
INTERVENTION: remote in‐home monitoring via a hub (Tele‐Modem, Aerotel Medical Systems) Participant data entry: manual
Study administrator: nurses, clinical call centre team (case manager, specialist in respiratory medicine, nurses) Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Minguez 2017 26 weeks |
INTERVENTION: remote in‐home monitoring intervention Participant data entry: manual
Study administrator: pulmonologist, specialist nurses Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: unclear
|
|
Pedone 2013 39 weeks |
INTERVENTION: remote monitoring via Bluetooth using the “SweetAge” monitoring system, which was web‐based Participant data entry: automatic
Study administrator: physician (skilled in care of respiratory patients) Data transmission: automatic
Data acquisition: synchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Sink 2020 34 weeks |
INTERVENTION: remote monitoring via EpxCOPD system via messaging Participant data entry: based on text message or automated telephone call via monitoring system
Study administrator: clinic medical residents Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: based on symptoms worsening
Feedback from health professionals: yes
|
|
Soriano 2018 52 weeks |
INTERVENTION: remote monitoring via Hub Internet connection Participant data entry: manual/automatic
Study administrator: nurse, healthcare personnel Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Stamenova 2020 26 weeks |
INTERVENTION: remote in‐home monitoring intervention Participant data entry: automatic
Study adminstrator: clinical project specialist (who was a respiratory therapist) Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: symptom‐dependent
Feedback from health professionals: yes
|
|
Udsen 2017 52 weeks |
INTERVENTION: remote monitoring via wireless transmission Participant data entry: automatic
Study administrator: nurses, health assistants, GP Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Walker 2018 39 weeks |
INTERVENTION: remote monitoring intervention with set phone calls Participant data entry: automatic
Study administrator: study nurse, physician Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
| Multi‐component or integrated care (where remote monitoring, consultation, or both, were components of care) | |
|
Bourbeau 2016 52 weeks |
INTERVENTION: remote in‐ home monitoring via telephone/web platform and self‐management education Participant data entry: manual
Study administrator: case managers (healthcare professionals), investigator, hospital physician Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professional: yes
|
|
Casas 2006 52 weeks |
INTERVENTION: integrative care intervention with individualized care plan via an ICT platform and web‐based call centre Participant data entry: based on telephone calls
Study administrator: specialised nurse case manager, primary care team (physician, nurse, and social worker), specialised respiratory nurse Data transmission:
Data acquisition: asynchronous
Clinical alert: none Feedback from health professional: yes
|
|
Farmer 2017 52 weeks |
INTERVENTION: integrated care intervention with individualised self‐management: EDGE platform on a tablet computer Participant data entry: automatic
Study administrator: research nurse, nurse, physiotherapist, doctor Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: physiological parameter‐dependent
Feedback from health professional: yes
|
|
Koff 2009 13 weeks |
INTERVENTION: integrated care intervention with disease‐specific education, teaching of self‐management techniques, and remote home monitoring Participant data entry: manual
Study adminstrator: study co‐ordinator, respiratory therapist, primary care physician Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Jakobsen 2015 26 weeks |
INTERVENTION: integrated care intervention, remote telemonitoring with a touch screen with a webcam Participant data entry: manual
Study administrator: nurses, research staff, physicians Data transmission: automatic
Data acquisition: asynchronous and synchronous
Clinical alert: none Feedback from health professionals: yes
|
|
Ringbaek 2015 26 weeks |
INTERVENTION: integrated care intervention with remote monitoring, pulmonary rehab, and support discharge Participant data entry: manual
Study administrator: respiratory nurses, respiratory specialist Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Ritchie 2016 12 weeks |
INTERVENTION: E‐Coach web‐based platform Interactive voice response monitoring system with self‐management and education of the disease Participant data entry: manual
Study administrator: care transition nurse Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: algorithm‐dependent
Feedback from health professionals: yes
|
|
Rose 2018 52 weeks |
INTERVENTION: integrated care intervention with education on living with COPD and individualised care, action plan for self‐management, and telephone consultation Participant data entry: based on telephone calls
Study administrator: case managers, GP Data transmission: not applicable Data acquisition: synchronous
Clinical alert: not applicable Feedback from health professionals: yes
|
|
Sorknaes 2013 26 weeks |
INTERVENTION: integrated care intervention with exacerbation prevention education and remote in‐home monitoring with face‐to‐face video Participant data entry: automatic
Study administrator: nurse, respiratory physician, general practitioner Data transmission: automatic
Data acquisition: synchronous
Clinical alert: none Feedback from health professionals: yes
|
|
Tabak 2014 39 weeks |
INTERVENTION: integrated care intervention using a mobile phone and web portal for exacerbation self‐management, web‐based exercise programme and activity coaching, tele‐ consultation via web portal Participant data entry: based on consultation via web portal
Study administrator: nurse practitioner, chest physician, physiotherapist Data transmission: automatic
Data acquisition: asynchronous
Clinical alert: none Feedback from health professionals: yes
|
|
Yan 2018 52 weeks |
INTERVENTION: remote consultation via mobile telephone with doctor Participant data entry: manual
Study administrator: doctor Data transmission: automatic
Data acquisition: synchronous
Clinical alert: none Feedback from health professionals: yes
|
6MWD: 6‐min walking distance; B2‐agonist: beta2‐agonist; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; CCC: clinical call centre; CCQ: clinical chronic obstructive pulmonary disease questionnaire; CDMT: chronic disease management team; CHF: congestive heart failure; CHROMED: Telemonitoring in Chronic Obstructive Pulmonary Disease in five countries; CMC: Clinical Monitoring Centre; COPD: chronic obstructive pulmonary disease; CRT: community respiratory team; ECG: electrocardiogram; E‐Coach: an in‐hospital assessment and discretionary post‐discharge support by a care transition nurse; ED: emergency department; EDGE: sElf‐management anD support proGrammE; e‐Health: location to central data management, Location Regional e‐Health Centre; EpxCOPD: Epharmix chronic obstructive pulmonary disease system; EQ‐5D: EuroQoL 5 Dimensions questionnaire; FEV1: forced expiratory volume in 1 second; GP(s): general practitioner(s); HCP(s): healthcare practitioner(s); ICT: information and communication technologies; IVR: interactive voice response; LTOT: long term oxygen therapy; MCID: minimal clinical important difference; MLHFQ: Minnesota Living with Heart Failure questionnaire; MRC: Medical Research Council dyspnoea score; NHS: National Health Service; NOWOX: wearable device that records time of oxygen use and respiration rate; PC: personal computer; PEF: peak expiratory flow; PHQ‐9: Patient Health Questionnaire‐9; RACS‐Plus: Respiratory Ambulatory Care Service‐Plus; RM: remote monitoring; TM: telemonitoring; TVC: telemedicine video consultation
Definitions: synchronous: data acquired in real‐time; asynchronous: data acquired once transmitted by participant
Remote monitoring plus usual care
Four studies reported interventions that consisted of a remote home monitoring system that was wired to a telephone or assessed physiological parameters (e.g. blood pressure, oxygen saturation) that were processed or authorised by a health professional, with feedback provided to the participant in addition to usual or standard care (Antoniades 2012; Lewis 2010; McDowell 2015; Pinnock 2013). Berkhof 2015, Ho 2016, Shany 2016, and Vianello 2016 used a wireless home remote monitoring system to monitor physiological parameters that were processed by a health professional, with feedback provided to participants in addition to usual care.
Participant data transfer process
Participants in four studies entered physiological parameters manually into the remote monitoring system (Ho 2016; Lewis 2010; McDowell 2015; Vianello 2016), whereas in two studies (Antoniades 2012; Shany 2016), the apparatus was connected to the remote monitoring system, allowing automatic transfer of data. Participant data in Berkhof 2015 were obtained through telephone calls made by the nurse.
Data were transmitted automatically via the linked remote system (computer‐based device or device connected to a telephone line) to secure servers and were acquired by study administrators asynchronously (i.e. once the data had been transmitted) in seven studies (Antoniades 2012; Ho 2016; Lewis 2010; McDowell 2015; Pinnock 2013; Shany 2016; Vianello 2016). Participant data in Berkhof 2015 were obtained synchronously (i.e. in real time) through telephone calls.
In seven studies, symptom‐ or algorithm‐based clinical alerts or 'red flags' were generated when readings were outside pre‐set parameters on the monitoring system. At the first instance, participants were contacted by the person monitoring the alerts to either take another reading or confirm health status, and to then escalate to specialists who could decide on further intervention. No clinical alert was generated in Berkhof 2015, as the intervention was based on telephone calls.
Remote monitoring only
All ten studies consisted of a remote monitoring setup that included apparatus to measure physiological parameters at home. Five studies consisted of a wired remote monitoring device set up at home that included apparatus for participants to measure, for example, blood pressure, oxygen saturation, and heart rate (Calvo 2014; De San Miguel 2013; Jódar‐Sanchez 2013; Minguez 2017; Soriano 2018). The remaining five studies included a wireless remote monitoring system with apparatus to measure physiological parameters via Bluetooth connection (Pedone 2013; Stamenova 2020; Udsen 2017), by automated telephone calls or text messaging (Sink 2020), or by a touch‐screen computer (Walker 2018).
Participant data transfer process
Participants in four studies entered their physiological data manually using apparatus provided with the remote monitoring system; data were then transmitted automatically to a secure website or to a clinical health centre connected by telephone and modem or via Internet (Calvo 2014; De San Miguel 2013; Jódar‐Sanchez 2013). In five studies, participants measured physiological parameters via Bluetooth equipment (Pedone 2013; Stamenova 2020), wireless equipment (Udsen 2017; Walker 2018), and automated telephone calls and texts (Sink 2020), which allowed data to be transmitted automatically. Participants in Soriano 2018 entered physiological parameters manually, but respiratory rate and oxygen use adherence data were collected automatically by a device attached to the oxygen feed from participants' main oxygen source.
In nine studies, administrators reviewed the data asynchronously once transmitted (Calvo 2014; De San Miguel 2013; Jódar‐Sanchez 2013; Minguez 2017; Sink 2020; Soriano 2018; Stamenova 2020; Udsen 2017; Walker 2018). Participant data were acquired synchronously in Pedone 2013.
Data were triaged based on whether readings were within pre‐set parameters (green), or were not provided (yellow). A red alert was created if readings were outside the pre‐set parameters, after which the administrator contacted the participant, or escalated to clinical staff for further intervention (Calvo 2014; Jódar‐Sanchez 2013; Soriano 2018; Udsen 2017). In De San Miguel 2013; Minguez 2017, Pedone 2013, Sink 2020, Stamenova 2020, and Walker 2018, participants were contacted when a clinical alert was created because readings were outside the parameters, and were escalated to clinical staff for further investigation.
Multi‐component intervention (with remote monitoring, consultation, or both, as a component of the intervention)
Eight studies were described as integrated care interventions with a remote monitoring or consultation platform set up in participants’ homes (Casas 2006; Farmer 2017; Jakobsen 2015; Koff 2009; Ringbaek 2015; Rose 2018; Sorknaes 2013; Tabak 2014). Bourbeau 2016 used a telephone‐based remote monitoring system, whereas Ritchie 2016 provided remote monitoring via a web‐based interactive voice response system. Yan 2018 provided remote consultation via a mobile phone.
One study included a wired remote monitoring system that allowed monitoring of physiological parameters (e.g. FEV₁, oxygen saturation, steps in the 6‐minute walk distance (6MWD)) transmitted by participants using apparatus provided (Koff 2009). Three studies used wireless systems (Bourbeau 2016; Farmer 2017; Ringbaek 2015). Bourbeau 2016 included a wireless remote monitoring system (web and telephone) to monitor physiological parameters and long‐term oxygen therapy, whereas Farmer 2017 included a wireless tablet computer for participants to measure physiological parameters via Bluetooth connection. Ringbaek 2015 provided equipment for remote monitoring and for measurement of physiological parameters that could be transferred by the participant via a wireless tablet computer with a webcam and a microphone. Casas 2006 consisted of monitoring via an integrated platform including a web‐based call centre and telephone calls from the call centre. In Ritchie 2016, participants used a web‐based platform and telephone calls for remote monitoring of physiological parameters. Rose 2018 included telephone consultation for monitoring and assessment of symptoms. Similarly, Tabak 2014 provided web‐based consultations and telephone calls. Jakobsen 2015 consisted of both remote monitoring and a consultation platform via a touch screen and a web cam. Sorknaes 2013 included video consultations, remote monitoring of physiological parameters, and follow‐up telephone calls. Yan 2018 was based on a remote consultation mobile platform that provided care by text, voice, photo, or video.
Participant data transfer process
Data entry was manual in six studies and required participants to measure and record physiological parameters on the remote system set up at home (Bourbeau 2016; Jakobsen 2015; Koff 2009; Ringbaek 2015; Ritchie 2016; Yan 2018). In Farmer 2017, data were transmitted automatically through Bluetooth‐connected apparatus, and in Sorknaes 2013, data were automatically collected through video consultations with the nurse. In Casas 2006, an integrated web‐based call centre was available for participants. Participants in Rose 2018 had telephone consultations with a health professional that included monitoring and assessment of symptoms. Tabak 2014 provided remote consultations via a web portal whereby participants could communicate with health professionals about their digital diary.
Participant data were acquired asynchronously by the administrator in seven studies (Bourbeau 2016; Casas 2006; Farmer 2017; Koff 2009; Ringbaek 2015; Ritchie 2016; Tabak 2014), and they were acquired synchronously in three studies (Rose 2018; Sorknaes 2013; Yan 2018). Jakobsen 2015 used asynchronous acquisition of participant data for hospital rounds and synchronous acquisition for real‐time video consultations for data review.
Clinical alerts were created by the telehealth system based on scores or symptoms (Bourbeau 2016; Yan 2018), physiological parameter thresholds (Farmer 2017; Jakobsen 2015), or algorithms based on participant data, and were then triaged via a colour code system: green for normal readings, yellow for warning or no reading, and red for readings outside pre‐set thresholds (Koff 2009; Ringbaek 2015; Ritchie 2016). Clinical alerts were not generated in six studies (Casas 2006; Jakobsen 2015; Rose 2018; Sorknaes 2013; Tabak 2014; Yan 2018).
Administrators contacted participants if a red flag or clinical alert was created on the system (Bourbeau 2016; Casas 2006; Farmer 2017; Koff 2009; Ringbaek 2015; Ritchie 2016). In Rose 2018, telephone consultations included ‘teach‐back’ sessions.
Excluded studies
We excluded 68 trials from the review, along with 19 additional references to these trials. Details of the excluded studies can be found under Characteristics of excluded studies along with exclusion reasons.
Risk of bias in included studies
An overview of the risk of bias in individual studies is provided in Figure 2; support for judgements in individual studies is shown in risk of bias tables under Characteristics of included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We evaluated 21 studies as having low risk of bias for random sequence generation, and 11 studies as having low risk and 4 studies as having high risk of bias for allocation concealment. Limited information is available from publications reviewed for sequence generation and allocation concealment, but we have considered this to be a low source of bias, as studies used standard methods to minimise the risk of selection bias. We therefore determined the risk of selection bias to be low, although sequence generation and allocation concealment are unclear in several studies (Figure 2).
Blinding
Many studies reviewed were reported as open‐label studies. The overall risk of performance and detection bias evaluated was high. We evaluated 29 studies as having high risk of performance bias. The structure of the study design and the nature of the intervention made it difficult to blind participants and personnel. We judged overall risk of performance and detection bias as high.
We assessed 19 studies as having high risk of bias for outcome assessment; personnel knew which participants were receiving treatment because the nature of the intervention provided in the studies made it difficult to blind.
Incomplete outcome data
The overall rate of withdrawal was similar in each study arm and was generally less than 20%; 17 arms had attrition greater than 20%, resulting in some concerns. However three studies ‐ Shany 2016 (47.6% telehealth and 14.3% control), Tabak 2014 (33.3% telehealth and 85.7% control), and Udsen 2017 (55.4% telehealth and 51.2% control) ‐ reported higher rates of attrition bias overall than were reported in other studies. Shany 2016 and Tabak 2014 included small sample sizes, and Udsen 2017 was lost to follow‐up, so we judged these three studies to be at high risk of attrition bias. Rose 2018 had low attrition overall; however, data related to secondary outcome measures assessed by questionnaires were missing, which could have led to bias in the results.
Selective reporting
We assessed 14 studies as having low risk and 6 as having high risk of reporting bias. We found limited information available for the remaining nine studies, classified as having unclear risk of reporting bias due to no registry information found to verify outcomes reported as planned. We therefore had some concern regarding reporting bias.
Other potential sources of bias
Most of the included studies (29 studies) were assessed as having low risk for other potential source of bias. However, Minguez 2017 and Sink 2020 reported information resulting in a judgement for other potential sources of bias as high risk for these studies. Minguez 2017 reported that the selection process could not be generalised to the whole COPD population and patients were selected due to intellect and cognitive capacity. Sink 2020 reported adding 17 participants to the control group without randomisation, and differences in FEV₁/FVC values among randomised and non‐randomised participants in the control group.
Effects of interventions
See: Table 1; Table 2; Table 3
Interventions were classified according to comparisons outlined in the Methods. Interventions with more than two components were classed as multi‐component interventions. Classification of studies according to intervention type is shown in Table 4, and details of baseline characteristics and individual interventions are listed in Table 5 and Table 6.
No studies were identified for remote consultation plus usual care or remote consultation alone versus usual care comparisons. Data for outcomes not included in the analyses are presented in Table 7 and Table 8 and are briefly described in the relevant comparison section.
4. Remote monitoring plus usual care or remote monitoring alone: data not included in analyses.
| Outcome | Duration and effect estimate (95% CI) | Studies |
| Remote monitoring plus usual care vs usual care | ||
| Quality of life: CRDQ | 26 weeks: MD ‐1.00 (95% CI ‐13.47 to 11.47) 52 weeks: MD ‐5.00 (95% CI ‐16.71 to 6.71) |
1 study (Antoniades 2012) |
| Quality of life: CCQ* | 26 weeks: MD 0.17 (95% CI ‐0.20 to 0.54) | 1 study (Berkhof 2015) |
| Quality of life: EQ‐5D* | 26 weeks: MD 0.08 (95% CI ‐0.04 to 0.20) | 1 study (McDowell 2015) |
| Quality of life: SF‐36* | 26 weeks: MD ‐0.04 (95% CI ‐15.83 to 7.83) | 1 study (Antoniades 2012) |
| Quality of life: SF‐36* | 52 weeks: MD ‐0.04 (95% CI ‐16.15 to 8.15) | 1 study (Antoniades 2012) |
| Quality of life: SF‐36 mental composite* | Mean 46 weeks: MD 0.44 (95% CI ‐2.20 to 3.08) | 2 studies (Berkhof 2015; Vianello 2016) |
| Quality of life: SF‐36 physical composite* | Mean 46 years: MD ‐0.69 (95% CI ‐2.74 to 1.35) | 2 studies (Berkhof 2015; Vianello 2016) |
| Quality of life: SF‐36 general subscale* | Mean 46 weeks: MD 0.03 (95% CI ‐2.29 to 2.34) | 2 studies (Berkhof 2015; Vianello 2016) |
| Hospital admission: COPD‐related hospital admission | 52 weeks: rate ratio: 0.89 (95% CI 0.79 to 1.00) | 1 study (Vianello 2016) |
| Remote monitoring vs usual care | ||
| Quality of life: SF‐36 mental composite* | 52 weeks: MD 0.00 (95% CI ‐1.50 to 1.50) | 1 study (Udsen 2017) |
| Quality of life: EQ‐5D* | 17 weeks: MD 0.03 (95% CI ‐0.13 to 0.18) | 1 study (Jódar‐Sanchez 2013) |
| Anxiety/depression: Goldberg anxiety and depression subscales | 52 weeks: anxiety: MD ‐0.10 (95% CI ‐0.61 to 0.41) 52 weeks: depression: MD ‐0.40 (95% CI ‐1.02 to 0.22) |
1 study (Soriano 2018) |
| Quality of life: MLHFQ score* | 39 weeks: MD 0.80 (95% CI ‐3.27 to 4.87) | 1 study (Walker 2018) |
*Higher = better.
CCQ: Clinical Chronic Obstructive Pulmonary Disease Questionnaire; CI: confidence interval; CRDQ: Chronic Respiratory Disease Questionnaire; EQ‐5D: EuroQoL 5 Dimensions Questionnaire; GIV: generic inverse variance; MD: mean difference; MLHFQ: Minnesota Living With Heart Failure Questionnaire; SF‐36: Short Form 36 quality of life questionnaire.
5. Multi‐component interventions: data not included in analyses.
| Outcome | Effect (95% CI) | Studies |
| Exacerbations: mean number of days to first exacerbation | MD 0.90 (95% CI ‐25.65 to 27.45) | 1 study (Bourbeau 2016) |
| Exacerbations: mean number of ED presentations (all‐cause) | MD ‐0.40 (95% CI ‐0.89 to 0.09) | 1 study (Rose 2018) |
| HA: mean number of hospital admissions (all‐cause) | MD ‐0.11 (95% CI ‐0.36 to 0.14) | 2 studies (Ringbaek 2015; Rose 2018) |
| HA: mean number of hospital admissions (COPD) | MD 0.01 (95% CI ‐0.24 to 026) | 1 study (Ringbaek 2015) |
| HA: mean number of re‐admissions (all cause) | MD ‐0.32 (95% CI ‐0.68 to 0.05) | 2 studies (Casas 2006; Sorknaes 2013) |
| HA: mean number of re‐admissions (COPD) | MD ‐0.06 (95% CI ‐0.57 to 0.45) | 1 study (Sorknaes 2013) |
CI: confidence interval; COPD: chronic obstructive pulmonary disease; ED: emergency department; HA: hospital admission; MD: mean difference.
Remote monitoring plus usual care versus usual care
We identified eight studies that compared a remote monitoring intervention in addition to usual care versus usual care and included them in the analyses (Antoniades 2012; Berkhof 2015; Ho 2016; Lewis 2010; McDowell 2015; Pinnock 2013; Shany 2016; Vianello 2016). We reported the main outcomes in Table 1. Outcomes that were not analysed are reported in Table 7.
Primary outcome: exacerbations
Number of people experiencing one or more exacerbations (follow‐up 26 weeks)
One included study compared an asynchronous remote in‐home telemonitoring intervention plus usual care versus regular outpatient visits (Berkhof 2015). Evidence is very uncertain and suggests that in‐home telemonitoring plus usual care may result in little to no difference in the number of people experiencing one or more exacerbations compared to regular outpatient visits at 26 weeks (odds ratio (OR) 1.25, 95% confidence interval (CI) 0.59 to 2.67; 108 participants, 1 study; very low‐certainty evidence; Analysis 1.1; Table 1).
1.1. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 1: RM + UC: exacerbations: number of people experiencing 1 or more exacerbations
Mean exacerbations (follow‐up 26 or 52 weeks)
Two included studies compared asynchronous home remote monitoring interventions plus usual care versus control (usual clinical care) (McDowell 2015; Pinnock 2013). Evidence suggests that a home remote monitoring intervention plus usual care may result in little to no difference in mean exacerbations compared to usual care at either 26 weeks (mean difference (MD) ‐0.46, 95% CI ‐1.19 to 0.27; 100 participants, 1 study; Analysis 1.2) or 52 weeks (MD 0.10, 95% CI ‐0.40 to 0.60; 189 participants, 1 study; Analysis 1.2).
1.2. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 2: RM + UC: exacerbations: mean number of exacerbations (subgroup duration)
Primary outcome: quality of life
St George's Respiratory Questionnaire (SGRQ total) (follow‐up 26 or 52 weeks)
Included studies compared asynchronous and synchronous home remote monitoring interventions plus usual care versus usual care (Berkhof 2015; McDowell 2015; Pinnock 2013). Each study measured quality of life using St George's Respiratory Questionnaire (SGRQ), which consists of 50 items from three domains (symptoms, activities, and impact). Total scores range from 0 (no limitations) to 100 (increased limitations). Only Berkhof 2015 reported that the tool was self‐reported. Evidence is very uncertain and suggests that asynchronous or synchronous home remote monitoring intervention plus usual care may result in little to no difference in quality of life improvement at 26 weeks compared to usual care (MD ‐1.49, 95% CI ‐9.43 to 6.44; 204 participants, 2 studies; I² = 75%; very low‐certainty evidence; Analysis 1.3; Table 1). Similarly, evidence is uncertain at 52 weeks and suggests that an asynchronous home telemonitoring plus usual care intervention may result in little to no difference in quality of life improvement at 52 weeks (MD 0.90, 95% CI ‐3.71 to 5.51; 205 participants, 1 study; Analysis 1.3; Table 1).
1.3. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 3: RM + UC: quality of life: SGRQ total (subgroup duration)
Explanation of heterogeneity in SGRQ at 26 weeks
At 26 weeks, heterogeneity in the meta‐analysis was very high (Analysis 1.3). We used a random‐effects model based on the assumption that intervention effect estimates are different, which cannot be explained by other factors, that is, differences observed are random. Although heterogeneity is not a concern in this model, we explored the differences between Berkhof 2015 and McDowell 2015. Berkhof 2015 was a single‐centre study in which participants in the remote monitoring group had worse health outcomes (Clinical Chronic Obstructive Pulmonary Disease Questionnaire (CCQ), symptoms) at baseline and increased use of home oxygen, as well as hospitalisations, compared to the control group. McDowell 2015 was a two‐centre study that included participants with moderate to severe COPD. Participants in the remote monitoring group received increased ambulatory oxygen therapy compared to those in the usual care group (40% versus 33%), although long‐term oxygen therapy was similar in both groups (27% versus 25%). At baseline, study participants had similar health status, which was measured by the EuroQoL Group Quality of Life Questionnaire based on 5 dimensions (EQ‐5D), the EuroQoL Group Visual Analogue Scale (EQ‐VAS), and SGRQ total scores. Interventions in both studies were home‐based monitoring systems, but monitoring mechanisms were different. Participants in Berkhof 2015 were remotely monitored by fortnightly telephone calls with a call centre nurse, whereas those in McDowell 2015 were monitored via a home remote monitoring system that was connected to a telephone line. Measurements (heart rate, oxygen saturation, blood pressure) and symptoms (tiredness, sputum, difficulty breathing, cough) were monitored regularly through the system. If an alert was triggered, the nurse called the patient to obtain more information, to repeat monitoring, or to escalate to the community respiratory team for further advice on what action should be taken. It may be likely that differences in these intervention processes may result in the variation observed in the analysis.
Quality of life measures not included in main analyses
Quality of life measures not included in the main analyses are listed in Table 7. Antoniades 2012 reported results from the Chronic Respiratory Disease Questionnaire (CRDQ) at 26 weeks and 52 weeks. At both time points, there may be little to no effect on quality of life. The CCQ was measured in Berkhof 2015 at 26 weeks; there may be little to no effect on quality of life with a remote monitoring intervention plus usual care compared to usual care alone. The Short Form Health Survey (SF‐36) was reported by Antoniades 2012. At 26 weeks and at 52 weeks, there was little to no improvement in quality of life with remote monitoring in addition to usual care compared to usual care alone. Little to no effect was seen in the SF‐36 mental, physical, or general subscales (Berkhof 2015; Vianello 2016).
Primary outcome: dyspnoea symptoms
We identified no studies that reported dyspnoea symptoms.
Primary outcome: hospital service utilisation
Six studies reported data for hospital service utilisation (Antoniades 2012; Ho 2016; McDowell 2015; Pinnock 2013; Shany 2016; Vianello 2016).
Mean hospital admissions (all‐cause) (follow‐up 52 weeks)
Three included studies compared asynchronous remote monitoring intervention plus standard best practice (SBP) or usual care versus standard best practice or usual care alone (Antoniades 2012; Pinnock 2013; Shany 2016). Each study measured mean hospital admissions (all‐cause). Evidence is very uncertain and suggests that a remote monitoring plus usual care intervention may result in little to no difference in mean hospital admissions at 52 weeks compared to usual care alone (MD 0.09, 95% CI ‐0.43 to 0.60; 342 participants, 3 studies; I² = 0%; Analysis 1.4).
1.4. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 4: RM + UC: hospital service utilisation: mean hospital admissions (all‐cause) (single)
Mean hospital admissions (COPD‐related) (follow‐up mean 45 weeks)
Three included studies compared asynchronous remote monitoring interventions plus standard best practice or usual care with standard best practice or usual care alone (Antoniades 2012; McDowell 2015; Pinnock 2013). One study had follow‐up of 26 weeks (McDowell 2015), and two studies had follow‐up of 52 weeks (Antoniades 2012; Pinnock 2013). We converted the analysis to standardised mean differences (SMDs) and 95% CIs to account for different follow‐up times, and we assessed imprecision by calculating the absolute effect estimate. Evidence is very uncertain and suggests that a remote monitoring intervention plus standard best practice (SBP) or usual care had little to no effect on mean hospital admissions compared to SBP or usual care alone at a mean of 45 weeks (SMD ‐0.01, 95% CI ‐0.21 to 0.18; 400 participants, 3 studies; I² = 0%; Analysis 1.5). The absolute effect estimate for COPD‐related hospital admissions per year was ‐0.016 (95% CI ‐0.336 to 0.288).
1.5. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 5: RM + UC: hospital service utilisation: hospital admissions (COPD‐related)
Hospital admission rate (follow‐up 52 weeks)
One included study compared an asynchronous remote monitoring intervention plus usual care versus usual care alone (Vianello 2016). Evidence is very uncertain and suggests that a remote monitoring intervention plus usual care may result in little to no difference in hospital admission rate per year compared to usual care alone (rate ratio 0.84, 95% CI 0.66 to 1.07; 334 participants; Analysis 1.6).
1.6. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 6: RM + UC: hospital service utilisation: hospital admission rate ratio (GIV)
Time to first hospitalisation after start of intervention (all‐cause or COPD‐related) (follow‐up 52 weeks)
One included study compared an asynchronous remote monitoring intervention plus usual care versus usual care alone (Pinnock 2013). Evidence is very uncertain and suggests that a remote monitoring intervention plus usual care may result in little to no difference in mean time to first hospitalisation compared to usual care alone at 52 weeks (hazard ratio (HR) 1.08, 95% CI 0.80 to 1.46; 256 participants; Table 1; Analysis 1.7).
1.7. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 7: RM + UC: hospital service utilisation: HR: time to first hospitalisation after start of intervention
In the same study (Pinnock 2013), evidence is very uncertain for risk of COPD‐related hospitalisation and suggests that a remote monitoring intervention plus usual care may result in little to no difference in the risk of COPD‐related hospitalisation compared to usual care alone at 52 weeks (HR 1.10, 95% CI 0.78 to 1.55; 256 participants; Analysis 1.8; Table 1).
1.8. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 8: RM + UC: hospital service utilisation: hospital admissions (COPD‐related) (hazard ratio)
Time to first COPD‐related hospital re‐admission (follow‐up 26 weeks)
One included study compared an asynchronous remote monitoring intervention plus usual care versus usual care alone (Ho 2016). Moderately certain evidence shows that a remote monitoring intervention plus usual care likely results in reduced risk of COPD‐related hospital re‐admission at 26 weeks (HR 0.42, 95% CI 0.19 to 0.93; 106 participants; Analysis 1.9; Table 1).
1.9. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 9: RM + UC vs UC: hospital use: time to first COPD‐related re‐admission
Time to first COPD‐related emergency department visit (follow‐up 26 weeks)
One included study compared an asynchronous remote monitoring intervention plus usual care versus usual care alone (Ho 2016). Evidence is uncertain and suggests that a remote monitoring intervention plus usual care may result in little to no difference in the risk of a COPD‐related emergency department visit at 26 weeks (HR 0.50, 95% CI 0.24 to 1.04; 106 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 10: RM + UC: hospital use: time to first COPD‐related ED visit
Length of stay (days, all‐cause) (follow‐up 52 weeks)
Four included studies compared asynchronous remote monitoring interventions plus usual care versus usual care alone (Antoniades 2012; Pinnock 2013; Shany 2016; Vianello 2016). Evidence suggests that a remote monitoring intervention plus usual care may result in little to no effect on all‐cause length of stay in hospital compared to usual care alone at 52 weeks (MD ‐0.81 days, 95% CI ‐4.83 to 3.22; 604 participants, 4 studies; I² = 0%; Analysis 1.11).
1.11. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 11: RM + UC: hospital service utilisation: length of stay (all‐cause)
One included study compared an asynchronous remote monitoring intervention plus usual care versus usual care alone (Pinnock 2013). Evidence is very uncertain and suggests that a remote monitoring intervention plus usual care may result in little to no effect on the risk of all‐cause duration of stay in hospital (HR 1.05 days, 95% CI 0.75 to 1.47; 256 participants; Analysis 1.12).
1.12. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 12: RM + UC: hospital service utilisation: length of stay (all‐cause) (hazard ratio)
Length of stay (days, COPD‐related) (follow‐up mean 47 weeks)
Three included studies compared asynchronous remote monitoring interventions plus usual care (McDowell 2015; Pinnock 2013; Vianello 2016). One study had follow‐up of 26 weeks (McDowell 2015), and two studies had follow‐up of 52 weeks (Pinnock 2013; Vianello 2016). We converted the analysis to standardised mean differences (SMDs) and 95% CIs to account for different follow‐up times, and we assessed imprecision by calculating the absolute effect estimate. Evidence is very uncertain and suggests that a remote monitoring intervention plus usual care may result in little to no effect on COPD‐related length of hospital stay compared to usual care alone at a mean of 47 weeks (SMD ‐0.11 days, 95% CI ‐0.30 to 0.09; 618 participants, 3 studies; I² = 28%; Analysis 1.13). This is also observed at 52 weeks, with little to no effect of asynchronous remote monitoring plus usual care compared to usual care alone on risk of length of stay (COPD‐related), as evidence is very uncertain (HR 1.03 days, 95% CI 0.70 to 1.52; 256 participants; Analysis 1.14).
1.13. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 13: RM + UC: hospital service utilisation: length of stay (COPD‐related)
1.14. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 14: RM + UC: hospital service utilisation: length of stay (COPD‐related) (hazard ratio)
On further investigation of variation observed in the analysis (I² = 28%), by taking Vianello 2016 out of the analysis, we no longer noted any variation. This could have been due to a number of reasons. First, over time, the number of actual hospitalisations may vary across studies, and mean length of stay data may be skewed. Vianello 2016 was conducted in Italy. McDowell 2015 and Pinnock 2013 were conducted in the UK and in Northern Ireland, respectively. McDowell 2015 was the only 26‐week study included in the analysis, whereas both Pinnock 2013 and Vianello 2016 were 52‐week studies. Both McDowell 2015 and Vianello 2016 included participants with moderate to severe COPD, whereas Pinnock 2013 included participants with mild to very severe COPD. Intervention processes were similar across all three studies, but uptake and behaviour of the intervention could have contributed to differences observed. We were unable to perform a subgroup analysis based on our pre‐specified criteria, as they were not reported by all three studies. Only two studies reported previous hospitalisations (McDowell 2015; Pinnock 2013), and participants in Pinnock 2013 had approximately two hospitalisations in the last 12 months. Participants in McDowell 2015 had approximately one hospitalisation in the last year. Cognitive impairment could not investigated, as it was not reported in any study. McDowell 2015 did not report comorbidities, but Pinnock 2013 reported that more than 70% of participants who had at least one comorbidity, and Vianello 2016 reported that more than 60% had hypertension or Ischaemic heart disease (or both).
Hospital admission measures not included in main analyses
Vianello 2016 reported COPD‐related hospital admissions. At 52 weeks, remote monitoring plus usual care had little to no effect on the rate of COPD‐related hospital admissions compared to usual care alone (Table 7).
Primary outcome: mortality
Seven included studies compared six asynchronous and one synchronous remote monitoring intervention plus SBP or usual care versus SBP or usual care alone (Antoniades 2012; Berkhof 2015; Lewis 2010; McDowell 2015; Pinnock 2013; Shany 2016; Vianello 2016). Evidence is very uncertain and suggests that a remote monitoring intervention plus SBP or usual care may result in little to no difference in the number of deaths compared to SBP or usual care alone at a mean of 44 weeks (OR 0.99, 95% CI 0.62 to 1.58; 927 participants, 7 studies; I² = 0%; Analysis 1.15; Table 1).
1.15. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 15: RM + UC: mortality (all‐cause)
Secondary outcome: adverse events
We identified no studies that reported adverse events.
Secondary outcome: anxiety and depression
Hospital Anxiety & Depression Scale (HADS) anxiety score (follow‐up 26 or 52 weeks)
Four included studies compared asynchronous remote monitoring interventions plus SBP or usual care versus SBP or usual care alone (Lewis 2010; McDowell 2015; Pinnock 2013; Vianello 2016). Evidence suggests that a remote monitoring intervention plus SBP or usual care does not reduce anxiety measured by the HADS‐anxiety scale at 26 weeks (Analysis 1.16). At 52 weeks, evidence suggests that a remote monitoring intervention plus SBP or usual care may result in little to no effect on the HADS‐anxiety score (Analysis 1.16).
1.16. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 16: RM + UC: A/D: HADS anxiety (change from baseline, mean difference between groups)
HADS depression score (follow‐up 26 or 52 weeks)
Three included studies compared asynchronous remote monitoring interventions plus usual care versus usual care alone (McDowell 2015; Pinnock 2013; Vianello 2016). Evidence suggests that a remote monitoring intervention plus usual care may result in little to no effect on the HADS‐depression score at 26 weeks (Analysis 1.17) or at 52 weeks (Analysis 1.17).
1.17. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 17: RM + UC: A/D: HADS depression (change from baseline, mean difference between groups) (single)
Secondary outcome: self‐efficacy
One included study compared an asynchronous remote monitoring intervention plus usual care versus usual care alone (Pinnock 2013). Evidence suggests that a remote monitoring intervention plus usual care may result in little to no effect on self‐efficacy on the Self‐Efficacy for Managing Chronic Disease‐6 (SEMCD‐6) Scale at 52 weeks compared to usual care alone (Analysis 1.18).
1.18. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 18: RM + UC: self‐efficacy: self‐efficacy for managing chronic disease (6‐item scale)
Secondary outcome: participant satisfaction
We identified no studies that reported participant satisfaction.
Remote monitoring versus usual care
We identified ten studies that compared a remote monitoring intervention versus usual care and were included in the analyses (Calvo 2014; De San Miguel 2013; Jódar‐Sanchez 2013; Minguez 2017; Pedone 2013; Sink 2020; Soriano 2018; Stamenova 2020; Udsen 2017; Walker 2018).
Primary outcome: exacerbations
Number of people experiencing one or more exacerbations (follow‐up mean 41 weeks)
Four included studies compared three asynchronous and one synchronous remote monitoring interventions versus usual care (Jódar‐Sanchez 2013; Minguez 2017; Pedone 2013; Soriano 2018). Evidence is very uncertain and suggests that a remote monitoring intervention may have little to no effect on the number of people experiencing one or more exacerbations compared to usual care at a mean follow‐up of 41 weeks (OR 1.02, 95% CI 0.67 to 1.55; 424 participants, 4 studies; I² = 0%; Analysis 2.1; Table 2).
2.1. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 1: RM vs UC: exacerbations: number of people experiencing 1 or more exacerbations
Mean exacerbations (follow‐up mean 46 weeks)
Two included studies compared asynchronous remote monitoring interventions versus usual care (Soriano 2018; Stamenova 2020). The analysis was converted to standardised mean differences (SMDs) and 95% CIs to account for different follow‐up times, and we assessed imprecision by calculating the absolute effect estimate. Evidence suggests that a remote monitoring intervention may have little to no effect on mean exacerbations compared to usual care at a mean follow‐up of 46 weeks (SMD 0.22, 95% CI ‐0.01 to 0.44; 297 participants, 2 studies; I² = 0%; Analysis 2.2). The absolute effect estimate was 0.22 (95% CI ‐0.01 to 0.45) exacerbations per year.
2.2. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 2: RM vs UC: exacerbations: mean number of exacerbations (subgroup duration)
Time to first exacerbation (follow‐up 26 weeks)
One included study compared an asynchronous remote monitoring intervention versus usual care (Minguez 2017). Evidence is very uncertain and suggests that a remote monitoring intervention may have little to no effect on risk of an exacerbation compared to usual care at 26 weeks (HR 1.29, 95% CI 0.72 to 2.31; 1 study, 116 participants; Analysis 2.3; Table 2).
2.3. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 3: RM vs UC: time to first exacerbation
Primary outcome: quality of life
SGRQ total score (follow‐up 17 weeks)
One included study compared an asynchronous remote monitoring intervention versus usual care (Jódar‐Sanchez 2013). Evidence is very uncertain and suggests that a remote monitoring intervention may have little to no effect on quality of life compared to usual care at 17 weeks (MD ‐6.40, 95% CI ‐18.56 to 5.76; 45 participants; Analysis 2.4; Table 2).
2.4. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 4: RM vs UC: quality of life: SGRQ total (duration of treatment)
CAT score (follow‐up mean 38 weeks or 52 weeks)
Three included studies compared effects of asynchronous remote monitoring interventions versus usual care on quality of life as measured by the CAT score (score range 0 to 40; lower scores represent better outcomes) (Minguez 2017; Soriano 2018; Walker 2018). Included studies did not report whether the tool was self‐reported) (Minguez 2017; Walker 2018). Evidence is very uncertain and suggests that a remote monitoring intervention may have little to no effect on quality of life compared to usual care at a mean of 38 weeks (MD 0.06, 95% CI ‐1.34 to 1.45; 405 participants, 2 studies; I² = 0%; Analysis 2.5; Table 2). Similarly, very uncertain evidence based on one study suggests that a remote monitoring intervention may have little to no effect on quality of life compared with usual care at 52 weeks (MD 0.10, 95% CI ‐1.42 to 1.62; 229 participants, 1 study; Analysis 2.5; Table 2) (Soriano 2018).
2.5. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 5: RM vs UC: quality of life: CAT total score
Quality of life measures not included in the main analyses
Udsen 2017 showed little to no difference in effects of a remote monitoring intervention compared to usual care on the SF‐36 mental composite score at 52 weeks (Table 7). Jódar‐Sanchez 2013 measured quality of life using the EQ‐5D scale at 17 weeks, which showed little to no difference in effects between a remote monitoring intervention and usual care (Table 7). There was no difference in quality of life improvement as measured by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) at 39 weeks (Walker 2018; Table 7).
Primary outcome: dyspnoea symptoms
Chronic Respiratory Disease Questionnaire Self‐Administered Standardized Scale (CRQ‐SAS) (follow‐up 26 weeks)
One included study compared an asynchronous remote monitoring intervention versus usual care (De San Miguel 2013). Evidence is uncertain and suggests that a remote monitoring intervention may have little to no effect in reducing dyspnoea symptoms compared to usual care at 26 weeks (MD ‐0.44, 95% CI ‐1.04 to 0.16; 70 participants; Analysis 2.6; Table 2).
2.6. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 6: RM vs UC: dyspnoea symptoms: CRQ‐SAS
Primary outcome: hospital service utilisation
Number of people admitted to hospital (all‐cause) (follow‐up mean 36 weeks)
Two included studies compared asynchronous remote monitoring interventions versus usual care (Jódar‐Sanchez 2013; Walker 2018). Evidence is very uncertain and suggests that a remote monitoring intervention may have little to no effect in reducing the number of people admitted to hospital compared to usual care at a mean of 36 weeks (OR 1.21, 95% CI 0.75 to 1.94; 357 participants, 2 studies; I² = 0%; Analysis 2.7; Table 2).
2.7. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 7: RM vs UC: hospital service utilisation: number of people admitted to hospital
Hospital admissions (all‐cause) (follow‐up mean 48 weeks)
Four included studies compared asynchronous remote monitoring interventions versus usual care (De San Miguel 2013; Jódar‐Sanchez 2013; Stamenova 2020; Udsen 2017). The analysis was converted to standardised mean differences (SMDs) and 95% CIs to account for different follow‐up times. We assessed imprecision by calculating the absolute effect estimate of ‐0.02 hospital admissions (95% CI ‐0.27 to 0.23) per year. Evidence is very uncertain and suggests that a remote monitoring intervention may have little to no effect in reducing mean hospital admissions compared to usual care at a mean of 48 weeks (SMD ‐0.02, 95% CI ‐0.22 to 0.19; 1409 participants, 4 studies; I² = 29%; Analysis 2.8).
2.8. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 8: RM vs UC: hospital service utilisation: mean hospital admissions (all‐cause) (single)
Note:forUdsen 2017, the standard error (SE) for the control arm was reported as 0.49, which was calculated as a standard deviation (SD) of 12.4 with the RevMan calculator. Upon further discussion, we concluded that the reported SE should be 0.049, not 0.49, due to an error in the publication. For an SE of 0.049, this would give a pooled SD of approximately 1, which fits the standardised difference. The mean difference is 0.046, and when divided by the pooled SD from both arms, this becomes 3%, which is 0.03, so the pooled SD should be roughly 1.5.
Hospital admissions (COPD‐related) (follow‐up 26 weeks)
Two included studies compared asynchronous remote monitoring interventions versus usual care (De San Miguel 2013; Stamenova 2020). Evidence is very uncertain and suggests that a remote monitoring intervention may have little to no effect in reducing COPD‐related hospital admissions compared to usual care at 26 weeks (MD ‐0.19, 95% CI ‐0.41 to 0.02; 129 participants, 2 studies; I² = 0%; Analysis 2.9).
2.9. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 9: RM vs UC: hospital service utilisation: hospital admissions (COPD‐related)
Time to first hospitalisation (follow‐up 34 weeks)
One included study compared an asynchronous remote monitoring intervention versus an active control (reported as usual care) (Sink 2020). Evidence is uncertain and suggests that a remote monitoring intervention may result in a slight reduction in the risk of hospitalisation compared to usual care at 34 weeks (HR 2.36, 95% CI 1.02 to 5.46; 168 participants; Analysis 2.10; Table 2).
2.10. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 10: RM + fb vs RM: hospital service utilisation: HR: time to first hospitalisation after start of intervention
Hospital re‐admissions
Walker 2018 compared an asynchronous remote monitoring intervention versus usual care at 39 weeks. Hospital re‐admission was reported as the incidence rate ratio (IRR 0.46, 95% CI 0.24 to 0.87). Among participants who were previously hospitalised due to a COPD exacerbation, a 53% reduction in the re‐hospitalisation rate was noted in the remote monitoring group compared to the usual care group (P = 0.017).
Length of stay (all‐cause) (follow‐up mean 49 weeks)
Five included studies compared asynchronous remote monitoring interventions versus usual care (De San Miguel 2013; Jódar‐Sanchez 2013; Soriano 2018; Stamenova 2020; Udsen 2017). The analysis was converted to standardised mean differences (SMDs) and 95% CIs to account for different follow‐up times. We assessed imprecision by calculating the absolute effect estimate (MD ‐0.39 days, 95% CI ‐1.50 to 0.63). Evidence suggests that a remote monitoring intervention may have little to no effect in reducing all‐cause length of stay at a mean of 49 weeks (SMD ‐0.05 days, 95% CI ‐0.19 to 0.08; 1638 participants, 5 studies; I² = 17%; Analysis 2.11).
2.11. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 11: RM vs UC: hospital service utilisation: length of stay (all‐cause)
Length of stay (COPD‐related) (follow‐up 26 weeks)
One included study compared an asynchronous remote monitoring intervention versus usual care (De San Miguel 2013). Evidence suggests that a remote monitoring intervention may result in little to no difference in COPD‐related length of stay compared to usual care at 26 weeks (MD ‐2.20 days, 95% CI ‐6.02 to 1.62; 71 participants; Analysis 2.12).
2.12. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 12: RM vs UC: hospital service utilisation: length of stay (COPD‐related)
Primary outcome: mortality
Six included studies compared asynchronous remote monitoring interventions versus usual care (Calvo 2014; De San Miguel 2013; Jódar‐Sanchez 2013; Soriano 2018; Stamenova 2020; Walker 2018). Evidence is very uncertain and suggests neither benefit nor harm compared to usual care at a mean of 38 weeks (OR 0.68, 95% CI 0.37 to 1.25; 798 participants, 6 studies; I² = 0%; Analysis 2.13; Table 2).
2.13. Analysis.

Comparison 2: Remote monitoring vs usual care, Outcome 13: RM vs UC: mortality (all‐cause)
Secondary outcome: adverse events
We identified no studies that reported adverse events.
Secondary outcome: anxiety and depression
Anxiety or depression measures not included in the main analyses
One included study compared an asynchronous remote monitoring intervention versus usual care (Soriano 2018). Evidence suggests that a remote monitoring intervention may result in little to no effect in reducing Goldberg anxiety or depression subscale scores compared to usual care at 52 weeks (Table 7).
Secondary outcome: self‐efficacy
We identified no studies that reported self‐efficacy.
Secondary outcome: participant satisfaction
We identified no studies that reported participant satisfaction.
Multi‐component or integrated care (when remote monitoring, remote consultations, or both, are components of care) versus usual care
We identified 11 studies that compared a multi‐component intervention versus usual care and were included in the analyses (Bourbeau 2016; Casas 2006; Farmer 2017; Jakobsen 2015; Koff 2009; Ringbaek 2015; Ritchie 2016; Rose 2018; Sorknaes 2013; Tabak 2014; Yan 2018).
Primary outcome: exacerbations
Number of participants experiencing at least one exacerbation or moderate to severe exacerbations (follow‐up 52 weeks)
Three included studies compared multi‐component interventions versus usual care (Bourbeau 2016; Farmer 2017; Rose 2018). Evidence is very uncertain and suggests that multi‐component interventions with asynchronous or synchronous remote monitoring may result in little to no effect in reducing the number of people experiencing at least one exacerbation or moderate to severe exacerbations compared to usual care at 52 weeks (OR 0.98, 95% CI 0.74 to 1.28; 955 participants, 3 studies; I² = 0%; Analysis 3.1; Table 3).
3.1. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 1: Multi: exacerbations: number of people experiencing at least 1 exacerbation/moderate to severe exacerbation (52 weeks)
Mean time to first exacerbation (days) (follow‐up 52 weeks)
One included study compared a multi‐component intervention versus usual care (Farmer 2017). Evidence is very uncertain and suggests that a multi‐component intervention with asynchronous remote monitoring may result in little to no effect on risk of time to a first exacerbation compared to usual care at 52 weeks (HR 1.05, 95% CI 0.67 to 1.65; 166 participants; Analysis 3.2; Table 3).
3.2. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 2: Multi: exacerbations: time to first exacerbation (hazard ratio)
Exacerbation measures not included in the main analyses
Rose 2018 reported mean exacerbations per person, noting little to no difference in effects of a multi‐component intervention on mean exacerbations at 52 weeks (Table 8). Bourbeau 2016 reported the mean number of days to a first exacerbation, which showed little to no difference in effects on the outcome with a multi‐component intervention compared to usual care (Table 8).
Primary outcome: quality of life
SGRQ total
Five included studies compared a multi‐component intervention (asynchronous remote monitoring or both asynchronous and synchronous monitoring and video conferencing) versus usual care (Casas 2006; Farmer 2017; Jakobsen 2015; Koff 2009; Rose 2018). Data from these studies were not pooled and were separated by follow‐up duration. At 13 weeks, one included study comparing a multi‐component intervention versus usual care showed that evidence was uncertain and suggested that a multi‐component intervention may result in improved quality of life (MD ‐9.70, 95% CI ‐18.32 to ‐1.08; 38 participants; Analysis 3.3; Table 3) (Koff 2009). However, this effect is not seen at 26 weeks (MD 7.00, 95% CI ‐4.79 to 18.79; 40 participants, 1 study; Analysis 3.3; Table 3) nor at 52 weeks (MD ‐1.09, 95% CI ‐6.24 to 4.05; 203 participants, 2 studies; I² = 0%; Analysis 3.3; Table 3). Evidence was very uncertain at 26 and 52 weeks. Rose 2018 was not pooled in the main analysis; however, there is little to no difference in effects between a multi‐component intervention and usual care (Analysis 3.4).
3.3. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 3: Multi: quality of life: SGRQ total
3.4. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 4: Multi: quality of life: SGRQ total (GIV)
COPD Assessment Test (CAT) score (follow‐up mean 38 weeks)
Two included studies compared effects of a multi‐component intervention (asynchronous or synchronous remote monitoring and remote or video consultation) versus usual care on quality of life as measured by the CAT tool (scale range 0 to 40; lower scores represent better outcomes). Only Ringbaek 2015 reported the tool as a patient‐reported measure) (Ringbaek 2015; Yan 2018). Multi‐component interventions may result in improved quality of life on the CAT score compared to usual care at a mean of 38 weeks; however evidence is very uncertain (MD ‐3.93, 95% CI ‐7.75 to ‐0.12; 521 participants, 2 studies; I² = 95%; Analysis 3.5; Table 3).
3.5. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 5: Multi: quality of life: CAT
It should be noted that although a random‐effects model was applied, a very high level of heterogeneity suggests fundamental differences between the two studies. First, Ringbaek 2015 was a 26‐week study conducted in Denmark, whereas Yan 2018, a Chinese study, reported a longer duration of 52 weeks. Interventions from both studies were integrated; Ringbaek 2015 included a computer tablet for remote monitoring, whereas Yan 2018 provided a mobile platform doctor or network consultancy and change to medications through consultation with the participant if needed. As the number of studies was limited, we were unable to perform subgroup analyses.
Primary outcome: dyspnoea symptoms
We identified no studies that reported dyspnoea symptoms.
Primary outcome: hospital service utilisation
Number of people who had at least one hospitalisation (follow‐up mean 33 weeks)
Two included studies compared a multi‐component intervention (with asynchronous remote monitoring or additional video consultation) versus usual care (Farmer 2017; Ringbaek 2015). Evidence is uncertain and suggests that a multi‐component intervention may result in little to no difference in the number of people experiencing hospitalisation compared to usual care at a mean of 33 weeks (OR 0.81, 95% CI 0.55 to 1.18; 447 participants, 2 studies; I² = 0%; Analysis 3.6; Table 3).
3.6. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 6: Multi: hospital use: number of people who had at least 1 hospital admission (26 or 52 weeks)
Length of stay (days) all‐cause or COPD‐related (follow‐up 26 weeks)
Two included studies compared a multi‐component intervention (with asynchronous remote monitoring or additional video consultation) versus usual care (Ringbaek 2015; Sorknaes 2013 ). Evidence was uncertain and suggests that a multi‐component intervention may result in little to no difference in length of stay compared to usual care at 26 weeks (MD ‐0.66 days, 95% CI ‐2.40 to 1.08; 523 participants, 2 studies; I² = 0%; Analysis 3.7). Evidence about a multi‐component intervention is uncertain (with asynchronous remote monitoring or additional video consultation) and suggests that it may have little to no effect on COPD‐related length of stay compared to usual care at 26 weeks (MD ‐0.47 days, 95% CI ‐1.49 to 0.55; 523 participants, 2 studies; I² = 0%; Analysis 3.8).
3.7. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 7: Multi: hospital use: length of stay (mean days)
3.8. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 8: Multi: hospital use: COPD‐related length of stay (days) (26 weeks)
Number of people who had a re‐admission (all‐cause) (follow‐up mean 39 weeks)
Three included studies compared a multi‐component intervention (with asynchronous remote monitoring and synchronous video conference, or telephone calls) versus usual care (Casas 2006; Jakobsen 2015; Ritchie 2016). Overall evidence is of moderate certainty and suggests that multi‐component interventions likely result in a reduction in the number of people re‐admitted (all‐cause) compared to usual care at a mean of 39 weeks (OR 0.50, 95% CI 0.31 to 0.81; 344 participants, 3 studies; I² = 0%; Analysis 3.9; Table 3). On further investigation, a greater reduction was noted in the number of people re‐admitted at 52 weeks compared to 12 or 26 weeks (Analysis 3.9).
3.9. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 9: Multi: hospital use: number of people re‐admitted (all‐cause)
Hospital re‐admissions (follow‐up mean 39 weeks)
Three included studies compared a multi‐component intervention (with asynchronous remote monitoring or additional video conference) versus usual care (Casas 2006; Jakobsen 2015; Ritchie 2016). Overall evidence is very uncertain and suggests that multi‐component interventions may result in little to no effect in reducing risk of hospital re‐admissions compared to usual care at a mean of 39 weeks (HR 0.77, 95% CI 0.38 to 1.57; 349 participants, 3 studies; Analysis 3.10; Table 3). On further investigation, a greater reduction was noted in the risk of hospital re‐admissions at 52 weeks, but not at 12 or 26 weeks (Analysis 3.10).
3.10. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 10: Multi: hospital use: hospital re‐admission (hazard ratio)
Hospital admission measures not included in the main analyses
There was little to no difference in mean all‐cause or COPD‐related hospital admissions and re‐admissions (Table 8). There was little to no difference in mean all‐cause emergency department presentations (Table 8).
Primary outcome: mortality
Nine included studies compared a multi‐component intervention (with asynchronous remote monitoring and synchronous video consultation) versus usual care (Bourbeau 2016; Casas 2006; Farmer 2017; Jakobsen 2015; Koff 2009; Ringbaek 2015; Ritchie 2016; Rose 2018; Sorknaes 2013). Overall evidence is very uncertain and suggests that multi‐component interventions may result in little to no effect in reducing all‐cause deaths compared to usual care at a mean of 40 weeks (OR 0.62, 95% CI 0.39 to 1.01; 1886 participants, 9 studies; I² = 40%; Analysis 3.11; Table 3). Intervention duration did not appear to affect all‐cause deaths overall, but deaths at 52 weeks were reduced in Rose 2018.
3.11. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 11: Multi: mortality (all‐cause)
Bourbeau 2016 reported considerably more deaths in the usual care group group compared to the multi‐component group, and compared to other studies of this duration. Further investigation of Bourbeau 2016 revealed that the multi‐component intervention (disease management programme) included a self‐management and e‐health telephone/web platform, as well as a home monitoring component (requiring daily and weekly symptom reporting; FEV₁, spirometry, and heart rate tests; oxygen saturation; diary card/symptom scoring; and monitoring and feedback regarding alerts on worsening symptoms). Among study participants, 74% receiving long‐term oxygen therapy and 80% had GOLD stage C disease (high risk with fewer symptoms). Deaths in the usual care group resulted from COPD exacerbations. Reduced deaths observed in the multi‐component intervention group may have occurred due to optimisation of self‐management of exacerbations and home monitoring by case managers, resulting in timely treatment and prevention of complications and death.
Secondary outcome: adverse events
Two included studies compared a multi‐component intervention (with asynchronous remote monitoring) versus usual care (Bourbeau 2016; Farmer 2017). Evidence suggests that a multi‐component intervention may result in little to no effect on the numbers of people experiencing adverse events compared to usual care (Analysis 3.12; Table 3).
3.12. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 12: Multi: AE: number of people who had an adverse event (52 weeks) (add to SOF table)
Secondary outcome: anxiety and depression
Two included studies compared a multi‐component intervention (with asynchronous remote monitoring or additional synchronous video conference) versus usual care (Bourbeau 2016; Jakobsen 2015). Evidence suggests that a multi‐component intervention may result in little to no effect on anxiety or depression (HADS total) at 26 or 52 weeks (Analysis 3.13). Rose 2018 reported both HADS anxiety and HADS depression scores. At 52 weeks, results show little to no difference in effects of a multi‐component intervention (with synchronous telephone consultations) on HADS depression compared to usual care but a reduction in HADS anxiety scale scores (Analysis 3.14). These results should be interpreted with caution due to missing data at 52 weeks that may lead to bias in the results.
3.13. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 13: Multi: A/D: HADS total
3.14. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 14: HADS‐A and HADS‐D
Secondary outcome: self‐efficacy
We identified no studies that reported self‐efficacy.
Secondary outcome: participant satisfaction
One included study compared a multi‐component intervention versus usual care (Tabak 2014). Evidence suggests that a multi‐component intervention (with asynchronous telephone and synchronous remote consultation) may result in little to no effect on participant satisfaction compared to usual care at 39 weeks (Analysis 3.15).
3.15. Analysis.

Comparison 3: Multi‐component vs usual care, Outcome 15: Multi: satisfaction: client satisfaction questionnaire
Discussion
Summary of main results
The review question was a topic prioritised by our patient advisory group; we evaluated randomised trials that assessed the effectiveness of remote monitoring technologies in addition to usual care, remote monitoring technologies alone, and multi‐component interventions, of which telehealth technology was a part. Primary health outcomes investigated include exacerbations, quality of life, dyspnoea symptoms, hospitalisation, and mortality.
Remote monitoring plus usual care
Based on one study (108 participants), we found that an asynchronous remote monitoring intervention in addition to usual care was no better than usual care at 26 weeks' follow‐up. Similarly, additional asynchronous remote monitoring interventions were of no benefit for mean exacerbations over the short or long term.
Overall, we found no benefit of asynchronous or synchronous remote monitoring in addition to usual care for improving quality of life compared to usual care, as measured by St George's Respiratory Questionnaire (SGRQ) total score at 26 weeks (2 studies, 204 participants) and at 52 weeks (1 study, 205 participants).
We found no evidence for dyspnoea symptoms.
Remote monitoring in addition to usual care interventions was no better than usual care in reducing mean all‐cause or chronic obstructive pulmonary disease (COPD)‐related hospital admissions at 52 weeks and at 45 weeks, respectively. However, additional asynchronous remote monitoring interventions likely reduced the number of people re‐admitted to hospital at 26 weeks.
We did not find differences in mortality rates between remote monitoring in addition to usual care versus usual care alone.
Remote monitoring interventions only
Based on four studies (424 participants), asynchronous or synchronous remote monitoring interventions alone were no better than usual care in terms of numbers of people experiencing exacerbations at a mean of 41 weeks.
Asynchronous remote monitoring was no better than usual care for improving quality of life at 17 weeks as seen in SGRQ total score (1 study, 45 participants), nor on COPD Assessment Test (CAT) score at a mean of 38 weeks (2 studies, 413 participants) or 52 weeks (1 study, 229 participants). Asynchronous remote monitoring interventions were no better than usual care for improving dyspnoea symptoms at 26 weeks.
Asynchronous remote monitoring interventions were no better than usual care for reducing the number people admitted to hospital at 36 weeks (2 studies, 357 participants). Risk of hospitalisation may be reduced at 34 weeks, but this result is based on 1 study of 168 participants, comparing an active (usual care) control group (Analysis 2.11).
We identified 6 studies with 798 participants reporting deaths at a mean of 38 weeks. We could not determine whether asynchronous remote monitoring interventions were beneficial in reducing deaths compared to usual care. A total of 22 fewer deaths were reported in the remote monitoring group, but due to very wide upper and lower confidence intervals (28 to 89) of the absolute risk, we are very uncertain about the effects observed.
Multi‐component interventions (telehealth as a component of care)
Most studies included asynchronous or synchronous remote monitoring and remote or video consultation components of multi‐component care provision.
Based on two studies, we could not determine whether multi‐component interventions were beneficial in terms of numbers of people experiencing moderate or severe exacerbations, or in terms of risk of exacerbation (1 study) at 52 weeks.
We found that quality of life (as measured by SGRQ total score) may improve with a multi‐component intervention at 13 weeks, but this small benefit was not observed at 26 weeks nor at 52 weeks. Similarly, quality of life based on two studies may improve at a mean of 38 weeks (CAT score); however, the studies were different geographically (China and Denmark) and the care package was varied, as one intervention included a computer tablet for remote monitoring, whereas the other consisted of a mobile platform doctor or network consultancy that allowed patient and doctor to have consultations about medications (Analysis 3.5). Behaviour of patients and ease of use may contribute to uptake of these interventions.
We did not find evidence for dyspnoea symptoms.
Evidence (2 studies, 447 participants) for effects of multi‐component interventions on numbers of people admitted to hospital was uncertain; these may have little to no effect compared to usual care at a mean of 33 weeks. However, we are moderately certain that multi‐component interventions are likely to result in fewer people re‐admitted to hospital at a mean of 39 weeks, with greater reduction at 52 weeks. In addition, the risk of hospital re‐admissions is reduced at 52 weeks, but not at 12 or 26 weeks (3 studies, 349 participants; Analysis 3.10).
Among nine studies (1886 participants), multi‐component interventions were no better than usual care in reducing deaths compared to usual care at a mean of 40 weeks' duration. Only one study at 52 weeks had fewer deaths compared to other studies of the same duration, probably because of the nature of the intervention and because self‐management of exacerbations and monitoring were optimised by case managers. In Bourbeau 2016, more deaths occurred in the control group than in the telehealth group, probably due to a high BODE index (integrates body mass index, airflow limitation (forced expiratory volume in 1 second), dyspnoea, and 6‐minute walk distance) at the end of 1‐year follow‐up, and due to the fact that large numbers of hospitalisation days were reported during the study (as a result of COPD exacerbations). It is possible that the multi‐component intervention was successful in reducing deaths, but it is not clear which component of the intervention (self‐management, home monitoring, early and prompt treatment) could have prevented deaths.
Overall completeness and applicability of evidence
We did not include digital interventions for supported self‐management, as this is covered in a linked Cochrane Review (Janjua 2021). The focus of this review was to explore the effectiveness of asynchronous or synchronous interventions including remote monitoring or remote consultation interventions, in addition to usual care (with health professional involvement), remote monitoring or remote consultations alone, or multi‐component interventions (of which remote monitoring or remote consultations were component(s)) compared to usual care.
Our search of the evidence led to the inclusion of 29 relevant studies. Despite the large number of studies included in our review, we could not clearly demonstrate benefit or harm of these interventions for most health outcomes among study populations, except for hospital re‐admissions. We are moderately certain that a remote monitoring intervention in addition to usual care may confer some benefit for risk of re‐admission at 26 weeks; however, this result was based on the findings of one study (106 participants) (Analysis 1.9). Similarly, a multi‐component intervention resulted in fewer people re‐admitted to hospital at 39 weeks' follow‐up (344 participants, 3 studies; moderate‐certainty evidence) (Analysis 3.9). We did not find any data for remote consultations in addition to usual care nor for remote consultations alone compared to usual care, and data for our primary outcomes are limited due to small numbers of study participants.
Severity of COPD among study populations ranged from mild to very severe. When conducting the review, we were interested to find out whether these interventions might help people with more severe COPD who are unable to have face‐to‐face appointments. People with severe COPD are often frail (Marengoni 2018), and they may have one or more long‐term comorbidities such as cardiovascular disease, diabetes, and depression (Anecchino 2007; Hillas 2015; Vanfleteren 2013), and their mobility can be compromised by COPD. On this basis, the healthcare professional may advise patients against exposure to hospital‐induced exposure risk. Conversely, face‐to‐face appointments may be of particular benefit for this demographic because such appointments provide an opportunity for clinicians to assess people holistically: reviewing their general health, their symptom burden, and how they are managing at home. Face‐to‐face reviews have the potential to help people better manage their long‐term conditions while maintaining their independence. Non‐pharmacological interventions such as education, pulmonary rehabilitation, and smoking cessation face‐to‐face may be easier to deliver remotely. Unfortunately, study results were not disaggregated according to severity type, and we could not determine whether any COPD severity group would receive particular benefit from remote interventions.
Several factors may contribute to lack of effectiveness of these interventions over usual care. No model for remote monitoring of people with COPD has been established, and interventions in included studies were highly heterogeneous. Interventions varied by technological method of monitoring (e.g. telephone calls, remote monitoring systems), by health professional monitoring (e.g. nurse, respiratory therapist), and by parameters monitored (e.g. symptoms, oxygen saturation, forced expiratory volume in 1 second, and steps in 6‐minute walk distance (6MWD). Such variations could impact the effectiveness of interventions.
We did not measure individual physiological parameters; however, participant ethnicity was not always well reported in trials and may be of relevance when one considers that a commonly used remote monitoring intervention ‐ pulse oximetry ‐ may not be as accurate for participants with darker skin, potentially leading to poorer outcomes and widened healthcare inequality (Sjoding 2020).
Of importance, we found no evidence to indicate that remote monitoring interventions are worse than usual care; such interventions may be a valid replacement for usual care for some people with COPD. This has particular relevance during the current SARS‐CoV‐2 pandemic, when many people with COPD may want to limit unnecessary contact to reduce their risk of contracting COViD‐19. From this review, we are unable to determine which patients may be best suited to or may prefer this approach, but we have shown that most interventions follow an asynchronous approach to monitoring people's physiological parameters rather than using a continuous or real‐time approach. Continuous remote care, with real‐time monitoring, in which the individual does not have to enter data manually for example, may be helpful for early detection in people who have more severe COPD and may help to reduce exacerbations, hospitalisations, and deaths. The asynchronous approach may be better suited for people who have stable but less severe COPD. Nevertheless, decisions on which type of remote care should be given are likely to be dependent on the health professional's assessment of the individual and his or her needs, as well as on funding provided for the healthcare provider to run the service.
Levels of health literacy and technological literacy and beliefs about the value of an intervention can affect uptake and adherence (Hoass 2016). Individuals may have anxiety about the technology itself (preferring face‐to‐face interaction, forgetting to use technology, needing technical support, or finding health care to be a repetitive process) (Gorst 2014). Whilst several studies included participant satisfaction with remote monitoring as an outcome measure, we could not find any studies that compared satisfaction between remote monitoring and usual care groups. This is an important measure for inclusion in future research because satisfaction and compliance data can reveal more information on whether or not an intervention is working. Indeed, a major drawback of the included studies is lack of a patient voice. The current COVID‐19 pandemic is likely to have increased the use of telehealth technologies (in single‐component or multi‐component format), and more data will enable investigation of their effectiveness in the future. Further quantitative research would provide valuable data on patients' thoughts about remote monitoring and the impact of disease severity, health beliefs, and technological literacy on effectiveness of these interventions. In addition, qualitative information would shed light on the issues (and benefits) that patients with COPD experience when using telehealth interventions.
Quality of the evidence
Studies that contributed evidence for key outcomes including exacerbations, quality of life, hospital service utilisation, mortality, and adverse events have high risk of bias due to lack of blinding (performance bias) overall; we judged the evidence for these outcomes to be of moderate to very low certainty (as assessed by GRADE). The GRADE assessment incorporated risk of bias assessments for outcomes, which reduced our certainty in the evidence for exacerbations and quality of life measures. Inconsistency was observed in some analyses, and this could have resulted in differences in COPD severity among populations, as well as in interventions, processes of care, uptake of interventions, settings, and countries where trials were conducted. We could not determine what may contribute to differences observed, but it is likely that collectively all factors play a role in the effectiveness of the intervention.
For most outcomes, we downgraded the certainty of evidence due to imprecision and small participant numbers; this resulted in analyses showing little to no difference in effects between intervention and usual care groups. Therefore, we could not determine benefit or harm of interventions for our pre‐specified outcomes. Only evidence for hospital re‐admissions is of moderate certainty, as we noted no issues with imprecision (Analysis 1.9; Table 1; Analysis 3.9; Table 3). We are unable to to investigate publication bias for each outcome because of the small number of included studies.
We noted no issues of indirectness for participants or interventions.
For mean hospitalisations (number of admissions and length of stay (LOS)), some analyses show that duration of follow‐up varied among studies. To overcome issues of skewed data, we converted meta‐analyses to standardised mean differences; however, we could make no robust conclusions based on these analyses.
Potential biases in the review process
We noted any deviations from the published protocol under Differences between protocol and review, and we provided reasons for the changes made. Due to heterogeneity of interventions and their components, it was difficult to categorise interventions according to inclusion criteria; however, we kept to categorisation as stated in the protocol as best as we could. This could have introduced some subjectivity in decisions about multi‐component interventions (interventions with two or more components). We could not determine effects of telehealth interventions as a component of a multi‐component intervention due to the pairwise nature of the data analysis. Heterogeneity and the large numbers of tools used to assess outcomes made it difficult to compare many studies. We did not analyse data nor interpret results while taking into consideration the superiority of interventions among trials.
Screening of studies was difficult due to the complexity of interventions, which led to re‐checking of studies that we had initially included. We did contact study authors directly for any information about studies that needed further clarification. We did not include data from some studies, as no further information was provided by study authors, or only data for the intervention group were available. Any non‐English language papers were translated by volunteers, who used a structured table to ascertain relevance to the review.
Agreements and disagreements with other studies or reviews
In this review, we cannot clearly demonstrate that telehealth interventions overall improve exacerbations, quality of life, or deaths. This is in consensus with another Cochrane Review (McLean 2012).
McLean 2012 investigated the effectiveness of ten telehealth interventions for people with COPD in improving clinical and process outcomes. Review authors found that telehealth care did not improve quality of life but did reduce hospital emergency department admissions and hospitalisations. In our review, we included 29 studies of varying telehealth interventions and found some very limited evidence for improvement of quality of life on SGRQ and for reduced hospital re‐admissions, which McLean did not report. We did not find reductions in hospital admissions in general; this does not reflect findings of the McLean review. In terms of fatalities, our review is in agreement with McLean 2012, in that mortality rates did not differ between comparison groups.
We found that remote monitoring interventions alone and multi‐component interventions are likely to reduce the number of people with COPD re‐admitted to hospital; however, the evidence base is small for both intervention types, and studies have limitations due to lack of blinding. Given differences in usual care setup across studies, our results suggest that telehealth interventions may be similar in effectiveness to usual care for health outcomes, and they may be acceptable as part of a management service, for example, for re‐admissions. We have not investigated further the cost‐effectiveness of telehealth interventions; however, one study suggests that reduced re‐admissions outweigh the costs of managing telehealth system alerts (Walker 2018).
Our findings are consistent with guidance from the National Institute for Health and Care Excellence (NICE), which recommends that telehealth interventions "should not be offered as part of COPD management", specifically routine monitoring, because of lack of improvement in quality of life and lack of reduction in hospital admissions (NICE 2018). However, NICE recommend that use of telehealth monitoring for specific reasons such as short‐term monitoring following discharge from hospital should not be avoided.
Guidance on telehealth interventions suggests that although no clear evidence for effectiveness of telehealth monitoring is available, these interventions are increasingly utilised and may have a role in healthcare services. Current lack of clear evidence should not change or prevent use of these interventions for the COPD population, if required for a specific reason (e.g. home monitoring after discharge from hospital) (NICE 2018). Lenferink 2017 suggests that telemedicine may be better placed as an adjunct to COPD management; however, uncertainty among studies about its effectiveness is ongoing (Ancochea 2018). Emerging evidence from pulmonary rehabilitation studies on patient preferences and barriers to implementation of virtual or digital approaches to care may shed some light on issues surrounding uptake of telehealth monitoring interventions (Bryant 2019).
Authors' conclusions
Implications for practice.
Evidence of low to very low quality suggests that asynchronous or synchronous telehealth interventions in addition to usual care or provided alone, or as part of a multi‐component intervention, may have little to no effect on exacerbations, quality of life, hospitalisation, or death, and may be no different from usual care. We are moderately certain that stand‐alone and multi‐component interventions are likely to reduce the risk of hospital re‐admission (COPD‐related or all‐cause), but more research is required to test whether these effects are seen in larger studies examining these interventions. We cannot determine which COPD severity subgroup would benefit from telehealth due to lack of disaggregated data in studies. Outcome data from separate COPD severity groups would provide more information on effectiveness of interventions. Experiences of people with COPD and of health professionals could also provide more information on perceptions of telehealth and reasons why these interventions may or may not work in certain COPD severity groups. Training for staff and patients could facilitate use of technology associated with telehealth interventions.
Although the findings of this review do not show benefit, they also do not show harm. These interventions cannot be dismissed, particularly in light of challenges involving access to services for many individuals with COPD. It is possible that with careful consideration by the health professional, an individualised approach that involves discussion with individuals around remote monitoring or consultation as part of their management, along with support from informal carers, may be crucial for the effectiveness of remote management. Further research is warranted.
Implications for research.
This Cochrane Review has highlighted the following areas for further research.
Further investigation is needed for enhanced understanding of results of this review.
A qualitative Cochrane Review investigating why there is variation in effects observed that cannot be determined from quantitative data. Qualitative information can enhance understanding of barriers and facilitators that people with COPD may experience when using telehealth interventions, for example, participants with sensory or physical impairment may struggle to fully access telehealthcare interventions.
Investigation of safety related to accuracy of pulse oximetry, blood pressure measurement, and spirometry in remote monitoring interventions.
Subgroup analysis of those living alone compared to those receiving some support from informal carers, or from adult social care service workers.
Investigation of telehealth interventions for the COPD population post COVID‐19.
Future trials should include the following.
Clear reporting of outcome data and information about protocols in trial registries.
Participant and carer assessments of understanding of digital interventions through a teach‐back technique, including technology literacy nested in the randomised trial.
Outcomes that measure a person's behaviour towards telehealth interventions.
Disaggreggated COPD severity group data, to gain an understanding of which group(s) would benefit from telehealth interventions.
Reporting of hospital admission rates per year as a more accurate measure of the outcome, as mean hospitalisation data may be skewed due to variable duration.
Well‐reported standardised or validated scales, for example, for patient satisfaction. Standardised assessment mechanisms in telehealth monitoring in general so that efficacy and overall benefit can be more easily established in the future. Researchers should also include data for the control group for comparison.
Hospital admission rates per year, as a more accurate measure of the outcome, as mean hospitalisation data may be skewed due to variable duration.
Standardisation of terminology for telehealth interventions.
Comparison of preference for remote consultations compared to face‐to‐face visits.
Comparison of continuous and non‐continuous remote monitoring, to investigate whether continuous monitoring has a greater impact on acute events such as exacerbations.
History
Protocol first published: Issue 11, 2018
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
The review authors would like to thank Katja Boehm and Anja Lieder for translation assistance. The review authors and the Airways Editorial team would like to thank Linzy Houchen‐Wolloff (UK), Ivan Tomasic (Sweden), and Stella Maria O'Brien (UK) for peer and consumer comments on this review.
This project was funded by the National Institute for Health Research Systematic Reviews Programme (project number 16/114/21). This project was also supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Health Research Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
Condition search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
17. exp Aspergillosis, Allergic Bronchopulmonary/
18. lung diseases, fungal/
19. aspergillosis/
20. 18 and 19
21. (bronchopulmonar$ adj3 aspergillosis).mp.
22. 17 or 20 or 21
23. 16 or 22
24. Lung Diseases, Obstructive/
25. exp Pulmonary Disease, Chronic Obstructive/
26. emphysema$.mp.
27. (chronic$ adj3 bronchiti$).mp.
28. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
29. COPD.mp.
30. COAD.mp.
31. COBD.mp.
32. AECB.mp.
33. or/24‐32
34. exp Bronchiectasis/
35. bronchiect$.mp.
36. bronchoect$.mp.
37. kartagener$.mp.
38. (ciliary adj3 dyskinesia).mp.
39. (bronchial$ adj3 dilat$).mp.
40. or/34‐39
41. exp Sleep Apnea Syndromes/
42. (sleep$ adj3 (apnoea$ or apnoea$)).mp.
43. (hypopnoea$ or hypopnoea$).mp.
44. OSA.mp.
45. SHS.mp.
46. OSAHS.mp.
47. or/41‐46
48. Lung Diseases, Interstitial/
49. Pulmonary Fibrosis/
50. Sarcoidosis, Pulmonary/
51. (interstitial$ adj3 (lung$ or disease$ or pneumon$)).mp.
52. ((pulmonary$ or lung$ or alveoli$) adj3 (fibros$ or fibrot$)).mp.
53. ((pulmonary$ or lung$) adj3 (sarcoid$ or granulom$)).mp.
54. or/48‐53
55. 23 or 33 or 40 or 47 or 54
Filter to identify randomised controlled trials
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify studies in other electronic databases.
Appendix 2. Search strategies
| Source and date of the last search | Search strategy | Results |
|
Cochrane Airways Trials Register (via Cochrane Register of Studies) Date of most recent search=28 April 2020 |
#1 MESH DESCRIPTOR Pulmonary Disease, Chronic Obstructive EXPLODE ALL AND INSEGMENT #2 MeSH DESCRIPTOR Bronchitis, Chronic AND INSEGMENT #3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) AND INSEGMENT #4 COPD:MISC1 AND INSEGMENT #5 (COPD OR COAD OR COBD OR AECOPD):TI,AB,KW AND INSEGMENT #6 #1 OR #2 OR #3 OR #4 OR #5 #7 MESH DESCRIPTOR Telemedicine EXPLODE ALL AND INSEGMENT #8 telehealth* or tele‐health* AND INSEGMENT #9 telemedicine* or tele‐medicine* AND INSEGMENT #10 telemanagement or tele‐management AND INSEGMENT #11 telecare* or tele‐care* AND INSEGMENT #12 telematic* AND INSEGMENT #13 telepharmacy or tele‐pharmacy AND INSEGMENT #14 telenurs* or tele‐nurs* AND INSEGMENT #15 tele‐homecare or telehomecare AND INSEGMENT #16 teleconsultation or tele‐consultation AND INSEGMENT #17 (remote* or distant or distance) NEAR (consult* or monitor* or care or treat* or therap*) AND INSEGMENT #18 (mobile* or digital*) NEXT health* AND INSEGMENT #19 ehealth or e‐health AND INSEGMENT #20 mhealth or m‐health AND INSEGMENT #21 MESH DESCRIPTOR Technology EXPLODE ALL AND INSEGMENT #22 MESH DESCRIPTOR Telephone EXPLODE ALL AND INSEGMENT #23 MESH DESCRIPTOR Videoconferencing EXPLODE ALL AND INSEGMENT #24 MESH DESCRIPTOR Electronic Mail EXPLODE ALL AND INSEGMENT #25 MESH DESCRIPTOR Text Messaging EXPLODE ALL AND INSEGMENT #26 MESH DESCRIPTOR Software EXPLODE ALL AND INSEGMENT #27 MESH DESCRIPTOR Software EXPLODE ALL AND INSEGMENT #28 MESH DESCRIPTOR Computers, Handheld EXPLODE ALL AND INSEGMENT #29 MESH DESCRIPTOR Computer‐Assisted Instruction AND INSEGMENT #30 MESH DESCRIPTOR Decision Making, Computer‐Assisted EXPLODE ALL AND INSEGMENT #31 MESH DESCRIPTOR Wireless Technology AND INSEGMENT #32 MESH DESCRIPTOR Internet EXPLODE ALL AND INSEGMENT #33 (internet* or computer* or web* or online*):ti,ab,kw AND INSEGMENT #34 (telephone or phone*):ti,ab,kw AND INSEGMENT #35 (sms or mms or texting or text messag*):ti,ab,kw AND INSEGMENT #36 (video* or skype*):ti,ab,kw AND INSEGMENT #37 (email or e‐mail or electronic mail):ti,ab,kw AND INSEGMENT #38 interactive* or telecommunication* AND INSEGMENT #39 wireless* or bluetooth* AND INSEGMENT #40 smartphone* or cellphone* AND INSEGMENT #41 (iphone* or ipod* or podcast* or ipad* or android* or blackberr* or palm pilot*):ti,ab,kw AND INSEGMENT #42 (pda* or personal digital assistant*):ti,ab,kw AND INSEGMENT #43 (tablet* or hand‐held*) near3 (device or computer) AND INSEGMENT #44 social* near3 (media* or network*) AND INSEGMENT #45 smart watch or smartwatch AND INSEGMENT #46 wearable*:ti,ab,kw AND INSEGMENT #47 #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 #48 #47 AND #6 | November 2018=1084 April 2020=553 |
|
IEEE Xplore Digital Library (https://ieeexplore.ieee.org/Xplore/home.jsp) Date of most recent search=28 April 2020 |
((COPD OR “chronic obstructive pulmonary disease” OR “chronic obstructive lung disease” OR “chronic obstructive airways disease” OR emphysema OR “chronic bronchitis” OR AECOPD)) | November 2018=105 April 2020=25 |
|
ClinicalTrials.gov (https://www.clinicaltrials.gov/) Date of most recent search=28 April 2020 |
Condition: COPD Study type: Interventional: Intervention: telehealth OR telemedicine OR telemanagement OR telecare OR telematic OR telepharmacy OR telenursing OR telehomecare OR teleconsultation OR telemonitoring OR remote OR distant OR mobile OR digital OR mhealth OR ehealth OR internet OR web OR online OR video OR skype OR text OR SMS OR email OR smartphone OR cellphone OR ipad OR social media OR smartwatch OR wearable |
November 2018=132 November 2020=26 |
|
WHO ICTRP (https://www.who.int/clinical‐trials‐registry‐platform) Date of most recent search: 21 November 2018 |
Condition: COPD Intervention: telehealth OR telemedicine OR telemanagement OR telecare OR telematic OR telepharmacy OR telenursing OR telehomecare OR teleconsultation OR telemonitoring OR remote OR distant OR mobile OR digital OR mhealth OR ehealth OR internet OR web OR online OR video OR skype OR text OR SMS OR email OR smartphone OR cellphone OR ipad OR social media OR smartwatch OR wearable |
November 2018=51 April 2020: not searched (inaccessible) |
Data and analyses
Comparison 1. Remote monitoring plus usual care vs usual care alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 RM + UC: exacerbations: number of people experiencing 1 or more exacerbations | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 6 to < 12 months | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 RM + UC: exacerbations: mean number of exacerbations (subgroup duration) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.1 6 to < 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2.2 ≥ 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.3 RM + UC: quality of life: SGRQ total (subgroup duration) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 6 to < 12 months | 2 | 204 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐9.43, 6.44] |
| 1.3.2 ≥ 12 months | 1 | 205 | Mean Difference (IV, Random, 95% CI) | 0.90 [‐3.71, 5.51] |
| 1.4 RM + UC: hospital service utilisation: mean hospital admissions (all‐cause) (single) | 3 | 342 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.43, 0.60] |
| 1.5 RM + UC: hospital service utilisation: hospital admissions (COPD‐related) | 3 | 400 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.18] |
| 1.6 RM + UC: hospital service utilisation: hospital admission rate ratio (GIV) | 1 | Rate Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.7 RM + UC: hospital service utilisation: HR: time to first hospitalisation after start of intervention | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.8 RM + UC: hospital service utilisation: hospital admissions (COPD‐related) (hazard ratio) | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.9 RM + UC vs UC: hospital use: time to first COPD‐related re‐admission | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.10 RM + UC: hospital use: time to first COPD‐related ED visit | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.11 RM + UC: hospital service utilisation: length of stay (all‐cause) | 4 | 604 | Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐4.83, 3.22] |
| 1.12 RM + UC: hospital service utilisation: length of stay (all‐cause) (hazard ratio) | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.13 RM + UC: hospital service utilisation: length of stay (COPD‐related) | 3 | 618 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.09] |
| 1.14 RM + UC: hospital service utilisation: length of stay (COPD‐related) (hazard ratio) | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.15 RM + UC: mortality (all‐cause) | 7 | 927 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.58] |
| 1.16 RM + UC: A/D: HADS anxiety (change from baseline, mean difference between groups) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.16.1 26 weeks | 2 | Mean Difference (IV, Random, 95% CI) | 1.86 [0.68, 3.04] | |
| 1.16.2 52 weeks | 2 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.47, 1.10] | |
| 1.17 RM + UC: A/D: HADS depression (change from baseline, mean difference between groups) (single) | 3 | 577 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.76, 0.76] |
| 1.17.1 26 weeks | 1 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐2.05, 0.79] |
| 1.17.2 52 weeks | 2 | 467 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.68, 1.13] |
| 1.18 RM + UC: self‐efficacy: self‐efficacy for managing chronic disease (6‐item scale) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.19 RM + UC: hospital service utilisation: length of stay (COPD‐related) (subgroup duration) | 3 | 618 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.09] |
1.19. Analysis.

Comparison 1: Remote monitoring plus usual care vs usual care alone, Outcome 19: RM + UC: hospital service utilisation: length of stay (COPD‐related) (subgroup duration)
Comparison 2. Remote monitoring vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 RM vs UC: exacerbations: number of people experiencing 1 or more exacerbations | 4 | 424 | Odds Ratio (M‐H, Random, 95% CI) | 1.02 [0.67, 1.55] |
| 2.1.1 3 to < 6 months | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [0.33, 7.59] |
| 2.1.2 6 to < 12 months | 2 | 210 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.34, 1.93] |
| 2.1.3 ≥ 12 months | 1 | 169 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.64, 2.14] |
| 2.2 RM vs UC: exacerbations: mean number of exacerbations (subgroup duration) | 2 | 297 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.01, 0.44] |
| 2.2.1 6 to < 12 months | 1 | 68 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.15, 0.81] |
| 2.2.2 ≥ 12 months | 1 | 229 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.08, 0.44] |
| 2.3 RM vs UC: time to first exacerbation | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.4 RM vs UC: quality of life: SGRQ total (duration of treatment) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4.1 3 to < 6 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.5 RM vs UC: quality of life: CAT total score | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.5.1 6 to < 12 months | 2 | 405 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐1.34, 1.45] |
| 2.5.2 ≥ 12 months | 1 | 229 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐1.42, 1.62] |
| 2.6 RM vs UC: dyspnoea symptoms: CRQ‐SAS | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.7 RM vs UC: hospital service utilisation: number of people admitted to hospital | 2 | 357 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.75, 1.94] |
| 2.7.1 3 to < 6 months | 1 | 45 | Odds Ratio (M‐H, Random, 95% CI) | 1.58 [0.33, 7.59] |
| 2.7.2 6 to < 12 months | 1 | 312 | Odds Ratio (M‐H, Random, 95% CI) | 1.18 [0.72, 1.94] |
| 2.8 RM vs UC: hospital service utilisation: mean hospital admissions (all‐cause) (single) | 4 | 1409 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.19] |
| 2.9 RM vs UC: hospital service utilisation: hospital admissions (COPD‐related) | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.41, 0.02] |
| 2.10 RM + fb vs RM: hospital service utilisation: HR: time to first hospitalisation after start of intervention | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.11 RM vs UC: hospital service utilisation: length of stay (all‐cause) | 5 | 1638 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.19, 0.08] |
| 2.12 RM vs UC: hospital service utilisation: length of stay (COPD‐related) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.13 RM vs UC: mortality (all‐cause) | 6 | 798 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.37, 1.25] |
Comparison 3. Multi‐component vs usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Multi: exacerbations: number of people experiencing at least 1 exacerbation/moderate to severe exacerbation (52 weeks) | 3 | 955 | Odds Ratio (M‐H, Random, 95% CI) | 0.98 [0.74, 1.28] |
| 3.2 Multi: exacerbations: time to first exacerbation (hazard ratio) | 1 | Hazard Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Multi: quality of life: SGRQ total | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3.1 3 to < 6 months | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐9.70 [‐18.32, ‐1.08] |
| 3.3.2 6 to < 12 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 7.00 [‐4.79, 18.79] |
| 3.3.3 ≥ 12 months | 2 | 203 | Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐6.24, 4.05] |
| 3.4 Multi: quality of life: SGRQ total (GIV) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.4.1 ≥ 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.5 Multi: quality of life: CAT | 2 | 521 | Mean Difference (IV, Random, 95% CI) | ‐3.93 [‐7.75, ‐0.12] |
| 3.6 Multi: hospital use: number of people who had at least 1 hospital admission (26 or 52 weeks) | 2 | 447 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.55, 1.18] |
| 3.7 Multi: hospital use: length of stay (mean days) | 2 | 523 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐2.40, 1.08] |
| 3.7.1 6 to < 12 months | 2 | 523 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐2.40, 1.08] |
| 3.8 Multi: hospital use: COPD‐related length of stay (days) (26 weeks) | 2 | 523 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.49, 0.55] |
| 3.9 Multi: hospital use: number of people re‐admitted (all‐cause) | 3 | 344 | Odds Ratio (M‐H, Random, 95% CI) | 0.50 [0.31, 0.81] |
| 3.9.1 3 to < 6 months | 1 | 132 | Odds Ratio (M‐H, Random, 95% CI) | 0.53 [0.21, 1.37] |
| 3.9.2 6 to < 12 months | 1 | 57 | Odds Ratio (M‐H, Random, 95% CI) | 0.81 [0.29, 2.30] |
| 3.9.3 ≥ 12 months | 1 | 155 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.78] |
| 3.10 Multi: hospital use: hospital re‐admission (hazard ratio) | 3 | Hazard Ratio (IV, Random, 95% CI) | 0.77 [0.38, 1.57] | |
| 3.10.1 3 to < 6 months | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.56 [0.23, 1.36] | |
| 3.10.2 6 to < 12 months | 1 | Hazard Ratio (IV, Random, 95% CI) | 2.01 [0.71, 5.69] | |
| 3.10.3 ≥ 12 months | 1 | Hazard Ratio (IV, Random, 95% CI) | 0.55 [0.35, 0.86] | |
| 3.11 Multi: mortality (all‐cause) | 9 | 1886 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.39, 1.01] |
| 3.11.1 3 to < 6 months | 2 | 172 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.25] |
| 3.11.2 6 to < 12 months | 3 | 604 | Odds Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.51] |
| 3.11.3 ≥ 12 months | 4 | 1110 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.22, 1.22] |
| 3.12 Multi: AE: number of people who had an adverse event (52 weeks) (add to SOF table) | 2 | 485 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.62, 1.33] |
| 3.13 Multi: A/D: HADS total | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.14 HADS‐A and HADS‐D | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.14.1 HADS‐A | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.14.2 HADS‐D | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.15 Multi: satisfaction: client satisfaction questionnaire | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antoniades 2012.
| Study characteristics | ||
| Methods |
Study design: single‐centre, single‐blinded, parallel individual randomised controlled trial in Australia Duration: 52 weeks Setting: tertiary care hospital |
|
| Participants |
Population: 44 adults recruited from a metropolitan tertiary care hospital Baseline characteristics: % Male: 45 RM + SBP and 45 SBP, Mean age: 68 RM + SBP and 70 SBP, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): RM + SBP 0.91, SBP 0.66, FVC (% mean): RM + SBP 2.13, SBP 1.98, FEV₁/FVC (% mean): RM + SBP 39.9, SBP 32, Current smokers (n): SBP + RM 0/22 and SBP 6/22, GOLD stage: moderate to severe on COPD criteria, COPD exacerbations lasting 12 months: not reported, Hospitalisations in past 12 months: RM + SBP: 2 (1 to 4) and SBP: 1 (1 to 2) Inclusion criteria: moderate to severe COPD diagnosed by COPD criteria, at least 1 hospitalisation in previous 12 months, fluent English, able to use keyboard and mouse, willing to use computer in self‐management, ambulant, living independently Exclusion criteria: significant comorbidities including cancer, renal failure, and cognitive impairment |
|
| Interventions |
Run‐in: initial home training was provided to all participants by a nursing informatics project manager; measurements taken at baseline, 6 months, and 12 months Treatment arms
|
|
| Outcomes |
Primary outcomes: hospital admissions (COPD‐related or non‐COPD‐related), inpatient bed‐days, quality of life (SF‐36 form and CRDQ form completed at 6 and 12 months) Secondary outcomes: 6‐minute walk distance (6MWD) measured at baseline and 12 months, adherence to daily monitoring, reproducibility of physiological measurements, patient acceptance of remote monitoring |
|
| Notes |
Funding: Department of Human Services; Victoria, Australia Other identifier: ACTRN12611000112965 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised; designations were randomly generated and sequentially numbered, but it is unclear how the sequence was generated |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated to either group, using a set of sequentially numbered, opaque, sealed envelopes containing randomly generated designations |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind patients and personnel due to nature of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assumed that outcome assessors were not blinded because study was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27% vs 9% withdrawals in TM group vs standard best practice group, respectively |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned; trial registered in Australian registry website |
| Other bias | Low risk | None |
Berkhof 2015.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in the Netherlands Duration: 26 weeks Setting: Hospital Isala in Zwolle |
|
| Participants |
Population: 101 adults recruited from 1 hospital, Isala, in Zwolle, Netherlands Baseline characteristics: % Male: 65 TM and 69 UC, Mean age: 68 TM in past 12 months: TM: 23 (44.2) and UC: 17 (34.7). Participants in TM group were more likely to have worse CCQ total and symptom scores, increased home oxygen and hospitalisations Inclusion criteria: smoking history > 10 pack‐years, diagnosis of severe COPD, post‐bronchodilator FEV₁ < 50%, FEV₁/FVC < 70%, written informed consent Exclusion criteria: history of asthma, unable to answer phone, life expectancy < 6 months |
|
| Interventions | Measurements taken at baseline and 6 months Treatment arms
|
|
| Outcomes |
Primary outcomes: COPD‐specific health status using clinical COPD questionnaire Secondary outcomes: SGRQ and SF‐36 questionnaires, resource use in primary and secondary care |
|
| Notes |
Funding: Isala hospital Other identifier: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed with a computer minimisation programme to achieve balanced groups for gender, age (< 65 years or ≥ 65 years), predicted forced expiratory volume in 1 second (FEV₁ < 35% or ≥ 35%), body mass index (< 21 or ≥ 21 kg/m²) |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind patients and personnel due to nature of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although no further information was provided, it was probably not possible to blind due to nature of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although attrition was slightly higher in control group, it was still below 10% |
| Selective reporting (reporting bias) | High risk | No protocol found on registry websites, so unclear whether outcomes were reported as planned. Hospitalisation outcomes reported as median and IQR, suggesting that data are not normally distributed. Contacted study authors, no response |
| Other bias | Low risk | None |
Bourbeau 2016.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐blinded, parallel individual randomised controlled trial in France, Germany, Italy, and Spain Duration: 52 weeks Setting: 33 investigative centres: 12 in France, 8 in Germany, 6 in Italy, 7 in Spain |
|
| Participants |
Population: 319 adults recruited from 33 investigative centres in 4 countries (12 centres in France, 8 in Germany, 6 in Italy, 7 in Spain) Baseline characteristics: % Male: 69.4 TH and 69.8 UC, Mean age: 67.3 TH and 66.6 UC, % White: not reported, % African: not reported, % LTOT: 75.8 TH and 72.8 UC, % Home oxygen: not reported, % Anxiety or depression: TH: moderate to severe anxiety 22.8 and moderate to severe depression 77.8, UC: moderate to severe anxiety 30.5 and moderate to severe depression 79.3, Baseline medications: long‐acting anticholinergics, long‐acting beta2‐agonist, long‐acting inhaled corticosteroids, FEV₁ (% mean): TH 37.8 and UC 36.4, FVC (% mean): not reported, FEV₁/FVC (% mean): TH 45.7 and UC 43.7, Current smokers (n): TH 34 and UC 34, GOLD stage III/IV, COPD exacerbations last 12 months: TH: 1.3 ± 0.7 and UC: 1.3 ± 0.8, Hospitalisation in past 12 months: TH: 20 (12.7) and UC: 19 (11.7) Inclusion criteria: COPD patients aged ≥ 35 years with post‐bronchodilator FEV₁/FVC ratio ≤ 70%; FEV₁ < 50% of predicted value; ≥ 10 pack‐year smoking history; at least 1 severe exacerbation in previous year Exclusion criteria: not expected to survive longer than 6 months; unable to read or speak the country language or having cognitive/psychiatric disease; on continuous treatment of > 10 mg per day prednisone or equivalent for longer than 6 weeks; living in a nursing home |
|
| Interventions |
Run‐in: each patient received multi‐component home‐based disease management or usual management care training and education, and was assessed for respiratory and global health status during a 3‐ to 5‐week run‐in period Treatment arms
|
|
| Outcomes |
Primary outcomes: number of unscheduled all‐cause hospitalisation days, normalised to 1 year of follow‐up Secondary outcomes: number of COPD exacerbations (mild, moderate, or severe to require hospitalisation and/or death), 6‐minute walk distance (6MWD), BODE, anxiety and depression (HADS), health status using SGRQ‐C |
|
| Notes |
Funding: Air Liquide Healthcare Other identifier: NCT01241526 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A pre‐specified randomised list was generated prior to the study by a partial minimisation computer algorithm. Participants were randomised via a dedicated interactive voice response system |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study design; neither study investigators nor patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open study design; neither study investigators nor outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20/157 participants in intervention arm did not complete the study, with 34/162 participants in UC arm; 23/34 of this group resulted in deaths compared to 3/157 deaths in intervention arm. Overall attrition in total randomised group was 15% |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported according to protocol |
| Other bias | Low risk | None |
Calvo 2014.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel cluster‐randomised controlled trial in Spain Duration: 30 weeks Setting: pneumology services and primary care centres |
|
| Participants |
Population: 60 adults recruited from pneumology services at Hospital University La Princesa, Primary Care Centres, in its area including Goya, Montesa, Lagasca, and Castello, and other primary care centres in the district of Salamanca in Madrid but not identified Baseline characteristics: % Male: 75.9 TH and 73.3 UC, Mean age: 75 TH and 72.7 UC, % White: not reported, % African: not reported, % LTOT: 100 TH and 100 UC, % Home oxygen: not reported, % Anxiety or depression: TH 3.70 anxiety and 3.80 depression, UC 3.0 anxiety and 3.5 depression, Baseline medications: 83% LAMA + LABA + ICS; 13% PDE4 inhibitors; 39% mucolytics; 8% theophyllines; 8% oral steroids, FEV₁ (% mean): TH 38.3 and UC 37.1, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): none for last 6 months, GOLD stage: severe to very severe, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: TH: 1.7 ± 1.0 and UC: 1.9 ± 1.4 Inclusion criteria: prior COPD diagnosis according to GOLD criteria 2011, severe/very severe FEV₁/FVC < 0.70 and % FEV₁ < 50, age ≥ 50 years, long‐term home oxygen therapy, not a current smoker for at least 6 months Exclusion criteria: did not meet at least 1 of the inclusion criteria, enrolled in palliative care programme for lung or other disease, at risk for social exclusion or institutionalised, deemed unable to understand all procedures |
|
| Interventions |
Run‐in: patients entering study had to be in stable situation and 15 days free of COPD exacerbation; initial clinic visit Treatment arms
|
|
| Outcomes |
Primary outcomes: numbers of emergency room visits, hospitalisations; length of hospital stay; mortality Secondary outcomes: none listed |
|
| Notes |
Funding: Linde Healthcare Other identifier: NCT02499068 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised by a 2‐colour code, either individually randomised or cluster‐randomised (depending on location of referral); not enough information |
| Allocation concealment (selection bias) | Low risk | Allocation was achieved by using coloured envelopes selected at chance |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study; neither study investigators nor patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study; neither study investigators nor patients were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low withdrawal rates: 2/30 patients withdrew from treatment arm, none from conventional care arm |
| Selective reporting (reporting bias) | Unclear risk | No further information about trial registration or whether outcomes reported were planned |
| Other bias | Low risk | None |
Casas 2006.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, single‐blinded, parallel individual randomised controlled trial in Spain and Belgium Duration: 52 weeks Setting: tertiary care hospitals |
|
| Participants |
Population: 155 adults recruited from 2 tertiary hospitals, Hospital Clinic Barcelona and University Hospital Gathuisberg, UZ‐Leuven Baseline characteristics: % Male: 77 IC and 88 UC, Mean age: 70 IC and 72 UC, % White: not reported, % African: not reported, % LTOT: 25 IC and 23 UC, % Home oxygen: not reported, % Anxiety or depression: IC 8.5 and UC 8.2, Baseline medications: influenza and pneumococcal vaccination, FEV₁ (% mean): IC 43 and UC 41, FVC (% mean): IC 64 and UC 63, FEV₁/FVC (% mean): IC 48 and UC 48, Current smokers (n): IC 21 and UC 19, GOLD stage: not reported, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: IC: 1.0 ± 1.3 and UC 0.6 ± 1.2 Inclusion criteria: COPD patients discharged from hospital from previous episode of exacerbation requiring hospitalisation for > 48 hours Exclusion criteria: not living in healthcare area, severe comorbidity (lung cancer, extremely severe neurological/cardiovascular condition), admitted to nursing home, unable to participate because not literate or no phone access at home |
|
| Interventions |
Run‐in: during hospitalisation, 2 hours before discharge, participants received a 2‐hour comprehensive education on disease and disease management; at Barcelona only, participant received 1 visit 72 hours after discharge; in Leuven, general practitioners made regular planned home visits Treatment arms
|
|
| Outcomes |
Primary outcomes: re‐hospitalisation rate during follow‐up Secondary outcomes: not reported |
|
| Notes |
Funding: CHRONIC project (IST‐1999/12158) from European Union Other identifier: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by using computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Participants were blindly allocated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study design; neither study investigators nor patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar percentage of withdrawals in each group: 74% intervention and 80% usual care patients at end of follow‐up; majority of dropouts due to death/palliative care |
| Selective reporting (reporting bias) | Unclear risk | No registry information found; unclear whether outcomes reported as planned |
| Other bias | Low risk | None |
De San Miguel 2013.
| Study characteristics | ||
| Methods |
Study design: single‐centre, single‐blinded, parallel individual randomised controlled trial in Western Australia Duration: 26 weeks Setting: health and community care organisation based in Western Australia |
|
| Participants |
Population: 80 adults recruited from Western region of Australia Baseline characteristics: % Male: 38.9 RM and 57 UC, Mean age: 71 RM and 74 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: oxygen, FEV₁ (% mean): not reported, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): not reported, GOLD stage: not reported, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: COPD diagnosis, receiving domiciliary oxygen, English speaking, living in metropolitan area Exclusion criteria: dementia, receiving palliative care, no telephone land line, unable to use telehealth equipment due to cognitive impairment/physical impairment |
|
| Interventions | Measurements taken at baseline, monthly, and at end of study Treatment arms
|
|
| Outcomes |
Primary outcomes: health services usage, annual cost savings, quality of life, participant satisfaction Secondary outcomes: none |
|
| Notes |
Funding: Australian Department of Health and Ageing Other identifier: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before recruitment, random number generator in STATA version 9 was used to randomly allocate 80 study numbers to intervention or control group (40 in each) |
| Allocation concealment (selection bias) | Low risk | Envelopes were made up with study number written on the outside and group assignment on the inside |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No further information provided, but not possible to blind patients or personnel due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9/80 patients withdrew from the study (11.25%) (7 deceased, 2 withdrawn). Of 2 participants who withdrew, 1 was unable to manage the equipment, and 1 was no longer interested in taking part. Unclear which allocations patients who withdrew came from |
| Selective reporting (reporting bias) | Unclear risk | No registry information found; unclear if outcomes reported as planned |
| Other bias | Low risk | None |
Farmer 2017.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised trial in United Kingdom Duration: 52 weeks Setting: primary and secondary care clinics |
|
| Participants |
Population: 166 adults recruited from primary and secondary care, respiratory hospital outpatient clinics, pulmonary rehab courses in adjacent counties of Oxfordshire and Berkshire, UK Baseline characteristics: % Male: 61.8 RM and 60.7 UC, Mean age: 69.8 RM and 69.8 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: RM group: median 5 COPD medications and UC group median 5 COPD medications; RM group took median 4 other medications and UC group took 5 other medications, FEV₁ (% mean): RM 47.4 and UC 50.1, FVC (% mean): RM 47.6 and UC 49.8, FEV₁/FVC (% mean): not reported, Current smokers (n): RM 23 and UC 13, GOLD stage: RM: 37.3% moderate, 62.7% severe/very severe; UC: 41.1% moderate, 58.9% were severe/very severe, COPD exacerbations in last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: COPD diagnosis FEV₁, post bronchodilation < 80% and predicted FEV₁:FVC ratio < 0.70. Smoking > 10 pack‐years, MRC dyspnoea ≥ 2, registered with GP and COPD exacerbation in last 12 months, or referred to PR Exclusion criteria: other significant lung disease, chronic heart failure, life expectancy < 3 months, cognitive impairment, no Internet‐enabled mobile phone network |
|
| Interventions |
Run‐in: initial 6‐week period of EDGE platform, symptom diary, and physiological measurements done daily; measurements taken at baseline and at 3, 6, and 12 months Treatment arms
|
|
| Outcomes |
Primary outcomes: quality of life scales: SGRQ‐C Secondary outcomes: hospital admissions, length of stay, deaths, number of recorded exacerbations, antibiotic/oral steroid use, presenting at ED or admitted to hospital due to acute change in respiratory condition, time to first exacerbation, EQ‐5D, Anxiety (SCL‐10A), depression (SCL‐20) |
|
| Notes |
Funding: Health Innovation Challenge fund (Wellcome Trust, Dept of Health). Trial was sponsored by University of Oxford. Study authors received funding from NIHR and Biomedical Research Centre (BRC) Other identifier: ISRCTN 40367841 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme (Sortition V1.2) was used to randomise participants. Research nurse carried out randomisation by accessing Sortition using Web browser on a tablet computer at assessment visit only after completion of consent procedures and baseline measurements |
| Allocation concealment (selection bias) | Unclear risk | Allocation of participants was carried out in 2:1 ratio of intervention and usual care. However, it is unclear whether allocation was concealed. Research nurse carried out randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study; neither study investigators nor patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study; neither study investigators nor patients were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was similar in each treatment group, with similar numbers, although more deaths occurred in the intervention group. 14/110 (12.7%) in intervention arm withdrew, 7/56 (12.5%) from control arm withdrew. An additional 5 allocated to intervention did not receive the intervention; this is unclear |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned; study authors provided data on request |
| Other bias | Low risk | None |
Ho 2016.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in Taiwan Duration: 26 weeks Setting: tertiary care |
|
| Participants |
Population: 106 adults recruited from 1 hospital (National Taiwan University Hospital, a tertiary care referral centre) Baseline characteristics: % Male: 81 TM and 72 UC, Mean age: 81.4 TM and 79.0 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: SABA: TM group: 89%; UC group: 85%; LABA: TM group: 60%, UC group: 66%; anticholinergic: TM group: 68%, UC group: 64%; ICS: TM group: 62%, UC group: 70%, FEV₁ (% mean): TM 62 and UC 62, FVC (% mean): not reported, FEV₁/FVC (% mean): TM 0.53 and UC 0.55, Current smokers (n): not reported, GOLD stage: Mild/moderate: TM group: 66%; UC group: 64%. Severe/very severe: TM group:34%, UC group:36%, COPD exacerbations last 12 months: TM: 19 (36) and UC: 17 (32), Hospitalisations in past 12 months: TM: 16 (30) and UC: 19 (36) Inclusion criteria: COPD exacerbation as main diagnosis, current or former smoker, spirometry‐confirmed airflow limitation (value of forced expiratory volume in 1 second divided by forced vital capacity < 0.71), discharge to home, accessibility to Internet and phone Exclusion criteria: consent not provided, unable to access study website, enrolled in other trials |
|
| Interventions |
Run‐in: prior to hospital discharge, patients were trained in use of equipment (pulse oximeter, thermometer, sphygmomanometer) and online diary by study nurse Treatment arms
|
|
| Outcomes |
Primary outcomes: frequency of re‐admission, time to first hospital re‐admission due to COPD exacerbation Secondary outcomes: time to first ED visit due to COPD, all‐cause hospital re‐admissions, number of all‐cause ED visits |
|
| Notes |
Funding: National Taiwan University Other identifier: NCT01724684 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised via a computer‐generated programme |
| Allocation concealment (selection bias) | Unclear risk | It is unclear whether allocation concealment was achieved |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants or investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants finished study |
| Selective reporting (reporting bias) | Low risk | Outcomes reported as planned; trial registered at clinical.trials.gov |
| Other bias | Low risk | None |
Jakobsen 2015.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial in Denmark Duration: 26 weeks Setting: university hospitals |
|
| Participants |
Population: 57 adults recruited from 2 hospital in Copenhagen, Denmark: Frederiksberg University Hospital and Herlev University Hospital Baseline characteristics: % Male: 37.9 RM and 39.3 UC, Mean age: not reported, % White: not reported, % African: not reported, % LTOT: 3.4 RM and 7.1 UC, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: Corticosteroids (prednisone), antibiotics (amoxicillin, clavulanic acid, beta2‐agonists and anticholinergics, fenoterol, ipratropium bromide nebuliser, 02 therapy as needed, sedative levomepromazine as needed, FEV₁ (% mean): RM 0.7 (0.4 to 2.1) and UC 0.7 (0.4 to 1.8), FVC (% mean): RM 1.5 (0.5 to 3.4) and UC 1.6 (0.7 to 3.4), FEV₁/FVC (% mean): not reported, Current smokers (n): RM 16 and UC 14, GOLD stage: III/IV, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: GOLD stage III or IV, able to follow instructions, admission > 2 days, ≥ 45 years of age Exclusion criteria: need for NIV/ventilator at time of baseline, severely overweight, serious comorbidity (cancer, unstable heart disease, diabetes, any condition that prevents participation), unable to follow instructions, temperature above 38 degrees requiring antibiotics, in another trial within 30 days of current trial, MMSE score < 24, not literate, unable to understand Danish, not able to complete follow‐up, severe psychiatric disorder, neuropsychological testing in last year, severe vision or hearing disorder |
|
| Interventions |
Run‐in: within 24 hours after hospitalisation, patient was trained with telehealth equipment; re‐test of equipment was done when patient got home within first 24 hours of admission; measurements taken at baseline, during intervention, and at 30, 60, 90, and 180 days after discharge Treatment arms
|
|
| Outcomes |
Primary outcomes: re‐admission due to COPD Secondary outcomes: mortality, NIV, hospitalisation days, QOL, adverse events, patient satisfaction, healthcare costs, physiological measures |
|
| Notes |
Funding: The Philanthropic Foundation TrygFonden, The Health Insurance Foundation, The Danish Lung Association, The Toyota Foundation, The Frederiksberg Foundation, and Lykfeldt’s grant Other identifier: NCT01155856 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were externally randomised 1:1 in fixed blocks of 4; the sequence was computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in sequentially numbered sealed opaque envelopes delivered to hospitals in batches of 10. The envelope was opened by participant only after written consent |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was reported as open‐label at clinical trials website |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was reported as open‐label at clinical trials website |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar discontinuation numbers in each group; similar numbers of deaths in each group: IC 10/29 (24%), UC 8/28 (29%) |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned in the protocol; study was registered at trial registry |
| Other bias | Low risk | None |
Jódar‐Sanchez 2013.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in Spain Duration: 17 weeks Setting: hospital care |
|
| Participants |
Population: 45 adults recruited from hospital in Madrid, Spain Baseline characteristics: % Male: 95 RM and 95 UC, Mean age: 74 RM and 71 UC, % White: not reported, % African: not reported, % LTOT: 100 RM and 100 UC, % Home oxygen: not reported, % Anxiety or depression: RM 10 and UC 10, Baseline medications: not reported, FEV₁ (% mean): RM 38 and UC 37, FVC (% mean): RM 59 and UC 63, FEV₁/FVC (% mean): not reported, Current smokers (n): not reported, GOLD stage: very severe, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: adult diagnosis of COPD and chronic respiratory failure with LTOT indication according to GOLD, at least 1 hospitalisation in the last year, clinically stable in the last 3 months Exclusion criteria: not following LTOT at enrolment, no home telephone line, not given informed consent |
|
| Interventions |
Run‐in: measurements taken at baseline and at end of study Treatment arms
|
|
| Outcomes |
Primary outcomes: exacerbations, A&E department visits, hospital admissions Secondary outcomes: SGRQ, EQ‐5D, patient satisfaction |
|
| Notes |
Funding: Spanish Ministry of Science and Innovation Other identifier: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial was reported as randomised, but randomisation process not described |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study; neither study investigators nor patients were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study; no measures were reported to show that outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 death and 1 withdrawal in each group were observed; all analysed |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were reported as planned; however, no trial registration details were found |
| Other bias | Low risk | None |
Koff 2009.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in the United States Duration: 13 weeks Setting: clinics in a university hospital |
|
| Participants |
Population: 40 adults recruited from COPD clinic and general pulmonary clinic at University of Colorado Hospital, in Aurora, Colorado Baseline characteristics: % Male: 45 IC and 50 UC, Mean age: 66.6 IC and 65.0 UC, % White: 85 IC and 95 UC, % African: 10 IC and 5 UC, % LTOT: 95 IC and 95 UC, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: flu vaccine, FEV₁ (% mean): IC 33.6 and UC 31.1, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): IC 3 and UC 4, GOLD stage: III/IV, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: IC: 0.55 ± 0.21 and UC: 0.6 ± 0.21 Inclusion criteria: COPD GOLD stage III/IV, phone land line Exclusion criteria: non‐literate, active treatment for lung cancer, not able to speak English, not able to complete a 6‐minute walk test |
|
| Interventions | Measurements made at baseline and 3 months Treatment arms
|
|
| Outcomes |
Primary outcomes: quality of life measured by SGRQ Secondary outcomes: healthcare costs, COPD exacerbations, equipment satisfaction |
|
| Notes |
Funding: University of Colorado Hospital Other identifier:NCT01044927 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial was reported as randomised, but randomisation process was not described |
| Allocation concealment (selection bias) | Low risk | Participants chose a "blinded envelope that contained a group indicator" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants or personnel not possible due to nature of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors not possible due to nature of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in each arm; 5%; those who withdrew were accounted for |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether outcomes were reported as planned. Could not find a protocol nor registration at trial website. Number of people who had an exacerbation in the UC group was reported as unknown |
| Other bias | Low risk | None |
Lewis 2010.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in the United Kingdom Duration: 26 weeks Setting: general hospital |
|
| Participants |
Population: 40 adults recruited from a general hospital in Wales, UK Baseline characteristics: % Male: 50 RM and 50 UC, Mean age: 70 RM and 73 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: RM: HADSA: 5.6 ± 3.5, HADSD 6.3 ± 3.5 and UC: HADSA: 6.3 ± 3.5, HADSD 5.9 ± 2.8, Baseline medications: not reported, FEV₁ (% mean): RM 38 and UC 40, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): RM 1 and UC 1, GOLD stage: moderate/severe, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: RM: 0 (0, 1.0) and UC: 0 (0, 0.8) Inclusion criteria: COPD (GOLD stage moderate/severe), completed 12 to 18 sessions of PR programme, maximal respiratory medication, standard telephone line installed at home, willing to have TM equipment installed at home, willing to provide consent Exclusion criteria: chronic asthma and ILD, went to < 12 sessions of PR programme, not living at home |
|
| Interventions |
Run‐in: measurements were taken at baseline, 4 weeks, 25 weeks, 30 weeks, and 52 weeks Treatment arms
|
|
| Outcomes |
Primary outcomes: SGRQ Secondary outcomes: EQ‐5D, HADS, mortality, patient satisfaction |
|
| Notes |
Funding: EU grant Other identifier: ISRCTN 41424840 Other: study was planned for 26 weeks, but usual care continued for 52 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme was used to generate random numbers into 2 groups |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes were used to conceal randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It would not be possible to blind participants to the intervention. Clinical staff (hospital doctors and general practitioners) were not aware of telemonitoring allocation; however, it is unclear whether Chronic Disease Management Team was aware of allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 withdrawals (including 2 deaths) occurred in the RM group (15%); unclear how many deaths/withdrawals occurred in SC group |
| Selective reporting (reporting bias) | High risk | Data reported as medians and IQRs; means given for hospitalisations, but no SDs. Trial was registered |
| Other bias | Low risk | None |
McDowell 2015.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial in Ireland Duration: 26 weeks Setting: specialist respiratory service |
|
| Participants |
Population: 110 adults recruited from a specialist respiratory service in Northern Ireland Baseline characteristics: % Male: 41.8 RM and 45.5 UC, Mean age: 69.8 RM and 70.2 UC, % White: not reported, % African: not reported, % LTOT: 27.3 RM and 25.5 UC, % Home oxygen: not reported, % Anxiety or depression: RM: HADSA: 8.3 (5.2); HADSD: 6.8 (3.8) UC: HADSA: 7.9 (4.3); HADSD: 7.9 (3.9), Baseline medications: flu vaccine, FEV₁ (% mean): RM 45.5 and UC 43.4, FVC (% mean): RM 71.7 and UC 70.4, FEV₁/FVC (% mean): not reported, Current smokers (n): RM 21 and UC 18, GOLD stage: II/III, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: RM: 0.82 (0.9) and UC: 1.05 (0.9) Inclusion criteria: COPD diagnosis GOLD II or III, at least 2 of ED admissions, hospital admissions, or emergency GP contacts in last year before the study Exclusion criteria: other respiratory disease, cognitively impaired/unable to learn about telemonitoring intervention |
|
| Interventions |
Run‐in: 5 consecutive days (mornings) of clinical and symptom observations reported by participant prior to study for trending Treatment arms
|
|
| Outcomes |
Primary outcomes: health‐related quality of life: SGRQ‐C Secondary outcomes: EQ‐5D, HADSA HADSD, health care utilisation, number of exacerbations, satisfaction, cost‐effectiveness |
|
| Notes |
Funding: European Centre for Connected Health Other identifier: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation procedure was used to generate the sequence, which was prepared by a researcher who was not involved in the trial |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was concealed in sequentially numbered envelopes and was consecutively opened on receipt of informed consent from the patient |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary outcome assessors were not blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of attrition in each group; however, more withdrawals from trial in the RM group than in the usual care group |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether trial was registered; therefore, unclear if all outcomes were reported as planned |
| Other bias | Low risk | None |
Minguez 2017.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in Spain Duration: 26 weeks Setting: university hospital |
|
| Participants |
Population: 116 adults recruited from Pneumology Department of Puerta de Hierro University Hospital, in Majadahonda, Spain Baseline characteristics: % Male: 76 RM and 62.5 UC, Mean age: 68 RM and 70 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: 36 RM and 32 UC, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): RM 50 and UC 51.5, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): RM 23 and UC 18, GOLD stage: not reported, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: COPD diagnosis, admission due to exacerbation, no severe coexisting condition, no fever for 48 hours, aerosol treatment at most every 6 hours, IV glucocorticoid < 40 mg twice daily, thoracic radiography without new disease, subjective improvement in patient, familiar suitable environment Exclusion criteria: terminal conditions including neoplasia, alcoholism, IV medication, not able to understand and take part in programme, ICU or NIV during exacerbation, institutionalised, hemodynamic instability |
|
| Interventions |
Run‐in: early assisted discharge from hospital; measurements taken at baseline, 30 days, and 6 months Treatment arms
|
|
| Outcomes |
Primary outcomes: time to first exacerbation Secondary outcomes: satisfaction, anxiety, QOL, adherence to treatment, monitoring compliance, use of health resources |
|
| Notes |
Funding: Strategic Health Action, PITES‐ISA research projects Other identifier:NCT01951261 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomised, but randomisation process was not described |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label; due to nature of intervention, participants or personnel could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study; investigators were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in both groups: 5/56 in control group, 6/55 in TM group |
| Selective reporting (reporting bias) | Unclear risk | Not all information was provided in publication; continuous outcomes were reported as medians and IQRs. However, upon contact with study authors, we were able to obtain results as means and SDs. Number of participants completing protocol follow‐up was different in the publication from numbers provided by study authors |
| Other bias | High risk | Study authors stated that due to selection process, results cannot be generalised to the whole COPD population; patients were selected due to intellect and cognitive capacity |
Pedone 2013.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel block and stratified randomised controlled trial in Italy Duration: 39 weeks Setting: university pulmonary medicine outpatient clinic |
|
| Participants |
Population: 99 adults recruited from 1 university pulmonary medicine outpatient facility, in Rome, Italy Baseline characteristics: % Male: 72 RM and 63 UC, Mean age: 74.1 RM and 75.4 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): RM 52.5 and UC 55.4, FVC (% mean): RM 78.8 and UC 78.5, FEV₁/FVC (% mean): not reported, Current smokers (n): not reported, GOLD stage: II/III, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: COPD GOLD II and III Exclusion criteria: cognitive impairment preventing use of experimental intervention |
|
| Interventions | Measurements were taken at baseline and daily Treatment arms
|
|
| Outcomes |
Primary outcomes: number of exacerbations, number of hospitalisations Secondary outcomes: not reported |
|
| Notes |
Funding: Lazio Region through FILAS Other identifier: NCT01481506 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated number list was used to randomise participants |
| Allocation concealment (selection bias) | Unclear risk | No further information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No participants in usual care group dropped out, whereas 11/50 in RM group did (22%) |
| Selective reporting (reporting bias) | Low risk | Study authors reported outcomes as planned in trials registry, but SDs for length of stay were incomplete. Contacted study authors, who provided data for SDs. Trial was registered |
| Other bias | Low risk | None |
Pinnock 2013.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial in the United Kingdom Duration: 52 weeks Setting: primary care |
|
| Participants |
Population: 256 adults randomised from 96 primary care practices Baseline characteristics: % Male: 41 RM and 49 UC, Mean age: 69.4 RM and 68.4 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: HADS: RM 9.8 (5.2) and UC 9.6 (4.6), Depression: RM 8.9 (4.4) and UC 8.2 (4.1), Baseline medications: not reported, FEV₁ (% mean): RM 44 and UC 40, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): RM 37 and UC 30, GOLD stage: Mild/moderate: RM 46 and UC 42, Severe: RM 45 and UC 42, Very severe: RM 37 and UC 44, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: RM 2.3 (2.1) and UC 2.5 (2.6) Inclusion criteria: patient registered with GP practice in Lothian and admitted to 1 of 3 acute hospitals with a primary diagnosis of COPD exacerbation in the last 12 months Exclusion criteria: other significant lung disease, unable to consent, unable to use the intervention, other significant medical or social reasons at GP discretion |
|
| Interventions | Measurements taken at baseline and at 3, 6, 9, and 12 months Treatment arms
|
|
| Outcomes |
Primary outcomes: time to first hospital admission with exacerbation of COPD Secondary outcomes: frequency of admissions, time to first hospitalisation due to COPD exacerbation, number of deaths, number and duration of admissions (all cause), number of exacerbations, SGRQ, HADS, self‐efficacy scale SECD6, number and duration of contacts with community services, LINQ, MARS |
|
| Notes |
Funding: Chief Scientist Office, NHS Applied Research Programme Grant Other identifier: ISRCTN96634935 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a stratified approach according to clinical service providing COPD care, and were centrally randomised 1:1 via randomised blocks of 2 or 4. All eligible participants will be randomised by randomised blocks of varying size, stratified by the service that will providing clinical care (i.e. Edinburgh Respiratory Physiotherapy Service, Mid‐Lothian Chronic Disease Nursing Team) to control or intervention. This will be managed by the telephone randomisation service of the Edinburgh Clinical Trials Unit, which will generate the randomisation sequence |
| Allocation concealment (selection bias) | High risk | "It is not possible to blind clinicians or patients to allocation thus potentially introducing bias in subsequent care" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "It is not possible to blind clinicians or patients to allocation thus potentially introducing bias in subsequent care" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Trial administrators entering data were blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers of participants in each group did not complete questionnaires at endpoint: 23/128 in RM group, 28/128 in control group |
| Selective reporting (reporting bias) | Low risk | Study authors reported outcomes as planned; their protocol was registered at ISRCTN Registry |
| Other bias | Low risk | None |
Ringbaek 2015.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel block randomised controlled trial in Denmark Duration: 26 weeks Setting: respirator outpatient clinics |
|
| Participants |
Population: 281 adults recruited from pulmonary wards at 4 hospitals: Hvidovre, Bispedjerg, Herlev, Amager Hospitals Baseline characteristics: % Male: 39 TM and 55 UC, Mean age: 69.8 TM and 69.4 UC, % White: not reported, % African: not reported, % LTOT: 26 TM and 27 UC, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: Oral prednisone (8.5%), Roflumilast (4.6%), ICS (91%), LAMA (89%), LABA (96%), FEV₁ (% mean): TM 34.9 and UC 33.8, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): TM 35 and UC 47, GOLD stage: severe and very severe, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: TM 0.91 (0 to 7) and UC 1.22 (0 to 23) Inclusion criteria: stable severe to very severe COPD as measured by GOLD, at high risk of exacerbations and hospitalisations, FEV₁ < 0.7, post‐bronchodilator FEV₁ < 60% predicted, hospitalisation in last 3 years due to exacerbation, LTOT for at least 3 months, regular respiratory outpatient clinic visits, COPD as main cause of disability, living in 1 of 6 municipalities of Copenhagen, living within area of recruiting hospital Exclusion criteria: COPD exacerbation 3 weeks before trial, not giving informed consent, unable to use tablet computer, not able to participate/living outside catchment area 2 weeks or more during study period, language barrier or cognitive disorder, no telephone line |
|
| Interventions | Measurements taken at baseline and at 6‐month follow‐up Treatment arms
|
|
| Outcomes |
Primary outcomes: health‐related QOL by 15D questionnaire Secondary outcomes: CAT |
|
| Notes |
Funding: not reported Other identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "1:1 allocation using randomised blocks of four (via numbered envelopes) for 6 months" |
| Allocation concealment (selection bias) | Low risk | "1:1 allocation using randomised blocks of four (via numbered envelopes) for 6 months" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It would not be possible to blind patients in the telehealth care arm to treatment, nor people who are administering the intervention due to the nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar numbers of deaths were observed in both treatment arms (8 vs 9). Two people in the intervention arm withdrew for technical reasons (although the technical reasons are not explained further). Similar attrition overall in both treatment groups |
| Selective reporting (reporting bias) | Unclear risk | No protocol was registered; it is not clear whether outcomes were reported as planned |
| Other bias | Low risk | There was good compliance with the TM intervention: "100 (82.6%) patients participated in at least six consultations" |
Ritchie 2016.
| Study characteristics | ||
| Methods |
Study design: single‐centre, single‐blinded, parallel individual randomised controlled trial in the United States Duration: 12 weeks Setting: urban academic hospital |
|
| Participants |
Population: 137 adults recruited from an urban academic hospital in Alabama that serves central and northern regions Baseline characteristics: % Male: 41.5 IC and 68.7 UC, Mean age: 63.8 IC and 63.4 UC, % White: 67.7 IC and 67.2 UC, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): not reported, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): IC 18 and UC 21, GOLD stage: not reported, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: English‐speaking, admitted to hospital from home, > 6 months' prognosis of COPD or CHF, access to telephone, expected to be discharged to home, impaired cognition (on validated scale) 6+ (eligible to participate, with caregiver willing to act as proxy), Medicare beneficiary Exclusion criteria: prognosis < 6 months, cognitive impairment without proxy/caregiver, heart and lung transplants, dialysis, already in CF programme/receiving intensive monitored care, ventricular assist device, use of pre‐planned phone service |
|
| Interventions |
Run‐in: 1 visit by care transition nurse prior to discharge; measurements at baseline and 30 days Treatment arms
|
|
| Outcomes |
Primary outcomes: re‐hospitalisation in 30 days Secondary outcomes: mortality, number of patient days in hospital vs at home at 30 days |
|
| Notes |
Funding: Agency for HealthCare Research and Quality of Care of Complex Patients Grant Other identifier: NCT01135381 Note: randomisation was stratified by condition; COPD only participants were the only group studied |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted stratified according to disease group in two independent trials (permuted block design), through a computer based random number generator. "For patients randomised to the intervention, a computer‐generated alert was sent to the CTNs, who then met with the patient prior to discharge" |
| Allocation concealment (selection bias) | Unclear risk | Research personnel recruiting participants were blinded to group assignment, but no description of how this was achieved is provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, care transition nurses or participants could not be blinded to the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was low % withdrawal in each group; reasons for withdrawal in COPD subgroups remain unclear |
| Selective reporting (reporting bias) | High risk | Outcomes were reported according to the protocol; however in the publication, study authors stated that 2 deaths occurred in the usual care group at 30 days, but this is not reported at clinicaltrials.gov and is not clearly explained in the publication |
| Other bias | Low risk | Although not reported in the publication, AEs and SAEs were reported at clinicaltrials.gov |
Rose 2018.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel stratified randomised controlled trial in Canada Duration: 52 weeks Setting: large community teaching hospitals |
|
| Participants |
Population: 475 adults recruited from large community teaching hospitals in Canada Baseline characteristics: % Male: 50 IC and 44 UC, Mean age: 71 IC and 71 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: 33 IC and 27 UC, % Anxiety or depression: not reported, Baseline medications: inhaled bronchodilator (95%), inhaled steroid (91%), antihypertensive (65%), influenza vaccine, pneumonia vaccine, FEV₁ (% mean): IC 43 and UC 45, FVC (% mean): not reported, FEV₁/FVC (% mean): IC 50 and UC 52, Current smokers (n): IC 53 and UC 59, GOLD stage: not reported, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: IC 1.3 ± 1.3 and UC 1.4 ± 1.3 Inclusion criteria: COPD diagnosis, FEV₁ < 70%, 2 or more comorbidities as identified by Canadian Thoracic Society COPD Guidelines, CVD, osteopenia/osteoporosis, glaucoma/cataract, cachexia/malnutrition, peripheral muscle dysfunction, lung cancer, diabetes, chronic kidney disease/other primary admitting/presenting diagnosis + COPD as a significant morbidity + ≥ 1 other morbidity, admission to hospital or presenting at a participating ED, first referral to respiratory centre/respirology team with 1 or more ED presentations or hospital admissions in the last 12 months Exclusion criteria: no access to primary care physician, asthma, terminal disease with ≤ 6 months' life expectancy, dementia/no caregiver, uncontrolled psychiatric disorder, cognitive dysfunction, no phone, not able to attend follow‐up visit at participating hospital |
|
| Interventions | Measurements taken at baseline and at 3, 6, and 12 months Treatment arms
|
|
| Outcomes |
Primary outcomes: number of emergency department visits Secondary outcomes: number of hospital admissions, number of hospitalisation days, mortality, time to first emergency department presentation, change in BODE index, EQ‐5D‐3L, SGRQ, HADS, COPD‐SES, CSQ8, Caregiver Impact Scale, adherence to chronic disease management measures, smoking cessation, vaccination status ‐ all at 52 weeks |
|
| Notes |
Funding: not reported Other identifier: NCT01648621 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed 1:1 via a centralised computer‐generated schedule stratified by study site |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Premature terminations low in intervention (N = 8) and control (N = 4) groups. 3 people in control group withdrew It should be noted that study authors stated that for secondary outcomes measured at 12 months by questionnaire (e.g. SGRQ, HADS), data were missing, and results should be interpreted with caution, as this would likely have introduced bias in the results |
| Selective reporting (reporting bias) | High risk | HRQOL data were not reported sufficiently; requested further information."Most outcomes mentioned were reported, though we do not have sight of a published protocol". Study authors were "unable to compare the frequency of exacerbation that did not result in an emergency department visit or hospitalisation in the control arm as these participants were not contacted weekly or monthly to collect these data". On contact with study author, we were unable to obtain disaggregated data for each treatment arm, as data analysis was combined |
| Other bias | Low risk | None |
Shany 2016.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in Sydney, Australia Duration: 52 weeks Setting: hospital‐based respiratory care |
|
| Participants |
Population: 42 adults recruited from a hospital‐based respiratory Ambulatory Care Service‐Plus in the suburbs of Sydney Baseline characteristics: % Male: 48 RM and 43 UC, Mean age: 72.1 RM and 74.2 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: RM Anxiety: 7.8 ± 4.7 RM Depression 6.0 ± 3.0 and UC Anxiety: 6.2 ± 4.0 UC Depression 6.4 ± 4.5, Baseline medications: not reported, FEV₁ (% mean): RM 32.1 and UC 39.7, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): not reported, GOLD stage: severe, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: RM 3.0 ± 2.0 and UC 2.5 ± 0.9 Inclusion criteria: at least 1 hospital admission for COPD exacerbation in preceding year Exclusion criteria: not fluent in English, cognitive impairment, motor deficit, part of another trial, no land line connection at home |
|
| Interventions | Measurements taken at baseline and at end of study Treatment arms
|
|
| Outcomes |
Primary outcomes: ED visits, hospital admissions, hospital LOS Secondary outcomes: QOL measures, anxiety and depression, costs for hospital admissions |
|
| Notes |
Funding: Department of State and Regional Development of NSW Government, TelemedCare, Australian Research Council, Sydney West Area Health Service, University of NSW Other identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study authors reported in their additional document that randomisation was performed according to a computerised randomisation programme in which participants were stratified according to how long they had been on the RACS‐plus programme |
| Allocation concealment (selection bias) | Unclear risk | Concealment of the allocation process was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind patients or personnel because the intervention was delivered differently to each group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding was reported in the study of outcome assessors. The only blinding that took place involved assessment of the duration of ED presentation and hospitalisation, and COPD categorisation. "The duration of ED presentation and hospital admissions as well as their categorisation as a result of COPD were blinded assessments of the Health Information Records Service in the hospital. This was compared to an independent, un‐blinded search of electronic patient records and discharge diagnoses in the electronic medical record" Comment: mixed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The percentage of attrition was higher in the intervention group (47%) than in the control group (14%) due to premature termination of the intervention. This occurred because participants were unwell, refused to consent to the intervention, or were in a nursing home |
| Selective reporting (reporting bias) | High risk | Registration of the trial was not found. Study authors reported to measure SGRQ and HADS, but results reported only at baseline, not at end of treatment |
| Other bias | Low risk | None |
Sink 2020.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial in the United States Duration: 34 weeks Setting: primary care clinic |
|
| Participants |
Population: 168 adults recruited from 2 hospitals in Missouri Baseline characteristics: % Male: 35 RM and 38 UC, Mean age: 59.8 RM and 61.9 UC, % White: 29 RM and 28 UC, % African: 66 RM and 65 UC, % LTOT: % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): RM 0.65 and UC 0.63, FVC (% mean): not reported, FEV₁/FVC (% mean): RM 0.64 and UC 0.61, Current smokers (n): RM 41 and UC 32, GOLD stage: mild (22%), moderate (54%), severe (17%) very severe (7%), COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: COPD diagnosis, > 18 years of age, consent to provide telephone number to receive text or voice messages, able to complete enrolment process, able to understand voice calls in English Exclusion criteria: intending to move away from clinic during the study period |
|
| Interventions | Measurements taken at baseline, daily or twice a week, and at end of study Treatment arms
|
|
| Outcomes |
Primary outcomes: time to hospitalisation Secondary outcomes: engagement with Epharmix Telemed System |
|
| Notes |
Funding: none Other identifier: NCT03002311 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed using the Excel random number generator function in a 1:1 ratio. Randomisation was carried out by independent researchers. 17 participants in the control group were included without randomisation |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although similar withdrawals, percentage of withdrawals was > 20% in each arm |
| Selective reporting (reporting bias) | Low risk | Trial was registered at the trials registry, but this publication seems to be just about COPD subgroups, so not all outcomes have been reported in the publication |
| Other bias | High risk | 17 people were included in the trial, even though they were not assigned to 1 of the residents at the time of enrolment because these patients had been seen in previous years by resident physicians who had graduated at the time of the study. They were included in the control group without randomisation, so 68/85 were randomised in the control group. FEV₁/FVC was different between randomised and non‐randomised participants in the control group |
Soriano 2018.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel block randomised controlled trial in Spain Duration: 52 weeks Setting: hospitals and primary care centres |
|
| Participants |
Population: 229 adults recruited from 5 Madrid hospitals Baseline characteristics: % Male: 78.3 RM and 82.5 UC, Mean age: 71.5 RM and 71.3 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: 100 RM and 100 UC, % Anxiety or depression: RM Goldberg anxiety 1.5 ± 2.3 and Goldberg depression 2.5 ± 2.4 and UC Goldberg anxiety 1.8 ± 2.5 and Goldberg depression 2.9 ± 2.5, Baseline medication: LABA (98%), LAMA (98%), ICS (94%), SAA (57%), PDE4 inhibitor (16%), theophylline (14%), oral steroid (4%), b2‐adrenergic receptor agonists (5%), FEV₁ (% mean): RM 34.2 and UC 32.2, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): not reported, GOLD stage: stable and severe, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: RM 2.0 ± 1.3 and UC 2.0 ± 1.2 Inclusion criteria: 50 to 90 years of age, COPD diagnosis (GesEPOC criteria), FEV₁ < 50%, 2+ moderate/severe exacerbations per year, clinically stable, home O₂ therapy, signed informed consent Exclusion criteria: unable to understand TM programme, < 12 months' life expectancy, terminal heart failure, advanced renal insufficiency/dialysis, residential hospice or institutionalised, MM test score < 24 (dementia), recommended as not complying with treatment/monitoring required by lung disease, failure to complete inclusion criteria |
|
| Interventions |
Run‐in: initial home visit to install equipment and train patient or caregiver and 4 days of physiological measurements Treatment arms
|
|
| Outcomes |
Primary outcomes: severe exacerbations resulting in emergency department visit or hospitalisation Secondary outcomes: quality of life, costs, patient/clinician satisfaction |
|
| Notes |
Funding: Fundación Teófilo Hernando, Universidad Autónoma de Madrid, with support of Linde Healthcare Other identifier: NCT02499068 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised (block randomisation), no further information, contacted study author |
| Allocation concealment (selection bias) | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar attrition in both groups, with 28/115 in TM group and 32/114 in RCP group |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned; trial was registered at clinicaltrials.gov |
| Other bias | Low risk | None |
Sorknaes 2013.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, single‐blinded, parallel individual randomised controlled trial in Denmark Duration: 26 weeks Setting: hospital (2 hospital sites) |
|
| Participants |
Population: 266 adults recruited from acute medicine unit and respiratory medicine unit at 2 hospital sites in Funen, Denmark Baseline characteristics: % Male: 40 RM and 38 UC, Mean age: 71 RM and 72 UC, % White: not reported, % African: not reported, % LTOT: 9 RM and 12 UC, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): RM 33 and UC 37, FVC (% mean): not reported, FEV₁/FVC (% mean): RM 48 and UC 47, Current smokers (n): RM 48 and UC 46, GOLD stage: severe, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: RM 2.75 (2.32) and UC 2.64 (2.5) Inclusion criteria: 40+ years, COPD diagnosis by spirometry, COPD exacerbations (defined as increased need for medication, increased dyspnoea, increased expectorate, increased coughing), resident in Funen and islands, written consent Exclusion criteria: unable to communicate via phone and/or computer screen, previous participant in protocol or received COPD suitcase, systolic BP < 100 mmHg, saturation < 90, malignancy or lobar pneumonia, cancer/recurrence of cancer in last 5 years, septic shock, AMI/renal disease/or other serious disease, diagnosed HF (EF < 30%), refused to participate |
|
| Interventions | Measurements taken at baseline and at 4, 8, 12, and 26 weeks Treatment arms
|
|
| Outcomes |
Primary outcomes: hospital admission Secondary outcomes: mortality, time before first re‐admission, hospital admissions, hospital days |
|
| Notes |
Funding: partial funding from European Commission, Danish Health Foundation, Danish Nurses' Organisation, University of Southern Denmark, OUH‐Odense University Hospital, Svenborg Hospital Other identifier: NCT01178879 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A central telephone voice response service from a computer‐generated system was used for block randomisation of 10 and 14. 1:1 allocation was done, and randomisation was stratified by smoking status and trial site |
| Allocation concealment (selection bias) | Unclear risk | Reported allocation in 1:1 ratio; allocation concealment of outcome assessors not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was single‐blind; assumed patients and personnel were not blinded to treatment allocation, although not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to treatment allocation as reported on the NCT website |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of patient deaths was similar in each group at 26 weeks; overall attrition in each group < 10% |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were reported as planned; trial was registered at clinicaltrials.gov. Study authors mentioned time‐to‐event data as survival analyses, but there was no access to the data. Study authors reported as days without standard deviations |
| Other bias | Low risk | None |
Stamenova 2020.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in Ontario, Canada Duration: 26 weeks Setting: community‐based hospital outpatient clinic |
|
| Participants |
Population: 122 adults recruited from an outpatient COPD clinic (and from respirologist practices) who worked at the clinic and from an outpatient COPD rehab programme affiliated with the community‐based hospital Baseline characteristics: % Male: 56 RM and 52 SC, Mean age: 71.98 RM and 72.78 SC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): RM 0.50 and SC 0.45, FVC (% mean): not reported, FEV₁/FVC (% mean): RM 0.54 and SC 0.56, Current smokers (n): RM 10 and SC 9, GOLD stage: not reported, COPD exacerbations last 12 months: RM 2 and SC 1, Hospitalisations in past 12 months: RM 0 and SC 0 Inclusion criteria: diagnosis of COPD defined by respirologist as per clinical guidelines, > 18 years old Exclusion criteria: diagnosis of ILL, patients without Wi‐Fi access at home, non‐English‐speaking, taking part in other RM programmes, not able to use technology due to physical/cognitive impairment |
|
| Interventions | Measurements taken at baseline (in person) and at 3 months and 6 months (in person or remotely) Treatment arms
|
|
| Outcomes |
Primary outcomes: Partners in Health Scale (knowledge and skills to monitor disease) Secondary outcomes: SGRQ, Bristol COPD Knowledge Questionnaire, patient self‐report (COPD ED visits, hospital admissions, length of hospital stay, number of exacerbations, COPD‐related visits to GP, COPD‐related RN contacts, use of medication, smoking cessation) |
|
| Notes |
Funding: Ontario Centres of Excellence Health Technologies Fund, grant 27009 Other identifier: NCT03741855 Other: 3‐arm study; each arm was separate; self‐monitoring (41), remote monitoring (41), standard care (40) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | People were randomised 1:1:1 using a web‐based random number generator |
| Allocation concealment (selection bias) | High risk | Participants were allocated using sealed envelopes to conceal allocation from the clinical study specialist; however, the specialist opened the envelopes so participants and specialist were aware of the assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was similar in each group at 3 and 6 months |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned, a protocol was published, and the trial was registered. SGRQ was reported in graph format; study authors were contacted for response |
| Other bias | Low risk | None |
Tabak 2014.
| Study characteristics | ||
| Methods |
Study design: single‐centre, single‐blind, parallel individual randomised controlled trial in the Netherlands Duration: 39 weeks Setting: hospital and primary care physiotherapy practice |
|
| Participants |
Population: 29 adults recruited from a hospital and from primary care physiotherapy practices in Enschede, Netherlands Baseline characteristics: % Male: 50 IC and 50 UC, Mean age: 64.1 IC and 62.8 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): IC 50.0 and UC 36.0, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): IC 4 and UC 4, GOLD stage: not reported, COPD exacerbations last 12 months: not reported, Hospitalisations in last 12 months: not reported Inclusion criteria: clinical diagnosis of COPD according to GOLD guidelines, not exacerbation‐free in the month prior to enrolment, ≥ 3 exacerbations or hospitalisations (respiratory related) in the previous 2 years, ex/current smoker, 40+ years, FEV₁ 25% to 80% predicted, Dutch‐speaking and understanding Dutch, Internet at home Exclusion criteria: other serious illness, short life expectancy, other condition affecting bronchial symptoms/lung function, severe mental illness, uncontrolled diabetes during COPD exacerbation in past, hospitalisation due to diabetes in previous 2 years, regular oxygen therapy, maintenance antibiotic therapy, alpha‐1‐antitrypsin deficiency, disorder/condition seriously affecting daily activities, hand impairment/unable to use app |
|
| Interventions |
Run‐in: two 90‐minute sessions with the nurse practitioner for disease self‐management; measurements taken at baseline and at 1, 3, 6, and 9 months Treatment arms
|
|
| Outcomes |
Primary outcomes: number of hospitalisations, length of stay, number of emergency department visits Secondary outcomes: 6MWT, EuroQoL‐5D, Multidimensional Fatigue Inventory 20, Clinical COPD Questionnaire, dyspnoea |
|
| Notes |
Funding: NL Agency, a division of the Dutch Ministry of Economic Affairs Other identifier: Netherlands Trial register (NTR3072) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a computer‐generated randomisation list (Blocked Stratified Randomisation version 5; Steven Piantadosi) |
| Allocation concealment (selection bias) | Low risk | Participants were allocated by a data manager in order of inclusion following the randomisations list, which was placed in a sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 85% in the usual care group withdrew, and 33% in the telehealth group "The study showed a high attrition rate. The strict criteria in relation to exacerbations/hospitalisations meant that the participants in general had a poor and unstable health status, especially in the control group, who had significantly worse dyspnoea levels" |
| Selective reporting (reporting bias) | Low risk | Contacted study authors regarding a few of the outcomes, as they were not reported in a format that could be used. The trial was registered, and all outcomes were reported as planned |
| Other bias | Low risk | None |
Udsen 2017.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel cluster randomised controlled trial in Denmark Duration: 52 weeks Setting: primary care |
|
| Participants |
Population: 1225 adults recruited from 26 municipal districts in the North Denmark region Baseline characteristics: % Male: 48.3 RM and 43.7 UC, Mean age: 69.6 RM and 70.3 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medications: not reported, FEV₁ (% mean): RM 47.7 and UC 48.4, FVC (% mean): RM 70.4 and UC 73.3, FEV₁/FVC (% mean): not reported, Current smokers (n): RM 196 and UC 189, GOLD stage: I, II, III, IV COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: COPD diagnosis by spirometry, treated according to GOLD guidelines, wanting to get COPD treatment, COPD the primary condition, residing permanently in the North Denmark region, MRC modified ≥ 2 or MRC ≥ 3 or CAT ≥ 10, at least 2 exacerbations in the last year Exclusion criteria: no phone line or GSM coverage, unable to speak or understand Danish to complete questionnaires, cognitive impairment |
|
| Interventions | Measurements taken at baseline and at 12 months Treatment arms
|
|
| Outcomes |
Primary outcomes: quality of life (SF‐36 physical and mental composite subscale scores) Secondary outcomes: incremental cost‐effectiveness ratio |
|
| Notes |
Funding: North Denmark Region, 11 municipalities in the North Denmark Region; Obel Family Foundation; Danish Agency for Digitalization Policy Strategy; European Social Fund Other identifier: NCT01984840 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Districts were distributed randomly by a blinded volunteer with no relation to the trial, who performed randomisation by throwing a dice |
| Allocation concealment (selection bias) | Low risk | Randomisation of clusters was done by sealed envelopes overseen by a person not affiliated with the trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was similar in both groups; however, more people in the THC group withdrew consent to the intervention compared to the UC group. Overall, attrition was high, with 50% of participants dropping out of the study. 61% of participants at all cost categories and EQ‐5D summary scores had completed registration |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported as planned; trial was registered at clinicaltrials.gov |
| Other bias | Low risk | None |
Vianello 2016.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised control trial in Italy Duration: 52 weeks Setting: primary and secondary clinics |
|
| Participants |
Population: 334 adults recruited from a hospital or from outpatient pulmonary clinics in Padova, Treviso, Venice, and Verona, Italy Baseline characteristics: % Male: 71 RM and 73 UC, Mean age: 75.96 RM and 76.48 UC, % White: not reported, % African: not reported, % LTOT: 41.30 RM and 39.42 UC, % Home oxygen: not reported, % Anxiety or depression: HADSA: RM 4.68 (3.45) and UC 5.4 (3.35), HADSD: RM 5.1 (4.42) and UC 5.48 (4.49), Baseline medications: LABA: RM 97% and UC 94%, LAMA: RM 87.17% and UC 86.27%, Inhaled ICS: RM 83.48% and UC 76.92%, Systemic steroid: RM 6.52% and UC 4.81%, FEV₁ (% mean): RM 41.90 and UC 41.87, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): RM 10 and UC 3, GOLD stage: III/IV, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: 18+ years, COPD GOLD class III and IV, life expectancy > 12 months, able to use telemonitoring equipment (assisted or alone) Exclusion criteria: unwilling to use telemonitoring equipment, significant lung disease, not willing to provide consent, serious social problems, negative feedback from GP |
|
| Interventions | Measurements at baseline and at 12 months Treatment arms
|
|
| Outcomes |
Primary outcomes: HRQL, SF‐36v2 (Italian version) Secondary outcomes: HADS, number and duration of hospitalisations, AECOPD, number and duration of any cause hospitalisations, number of re‐admissions due to exacerbations, number of any cause re‐admissions, number of appointments with pulmonary specialist, number of ED visits, number of deaths |
|
| Notes |
Funding: part of the Renewing Health Project founded by the European Commission Other identifier: NCT01513980 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme was used for randomisation of participants and allowed check of any inequality of characteristics by age and gender. Patients were randomised in a 2:1 allocation |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed, but participants were allocated in a 2:1 ratio for TM and control groups, respectively |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was similar in each treatment group, although it was > 20% in each group and overall |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned; study was registered at clinicaltrials.gov |
| Other bias | Low risk | None |
Walker 2018.
| Study characteristics | ||
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial in Spain, United Kingdom, Slovenia, Estonia, and Sweden Duration: 39 weeks Setting: clinics, hospitals, and community health services |
|
| Participants |
Population: 312 adults recruited from 6 sites in 5 countries (United Kingdom 75, Sweden 63, Estonia 80, Spain 61, Slovenia 33) Baseline characteristics: % Male: 66 RM and 65 UC, Mean age: 71 RM and 71 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: Mean depression PHQ‐9 score RM 6.27 (5.69) and UC 5.97 (5.79), Baseline medications: not reported, FEV₁ (% mean): RM 49.4 and UC 50.4, FVC (% mean): RM 73.8 and UC 75.8, FEV₁/FVC (% mean): RM 0.53 and UC 0.53, Current smokers (n): not reported, GOLD stage: RM: I (3%), II (47%), III (36%), IV (15%) and UC: I (2%), II (48%), III (39%), IV (11%), COPD exacerbations last 12 months: 1 exacerbation: RM 63 (41%) and UC 59 (37%); More than 1 exacerbation: RM 91 (59%) and UC 99 (63%), Hospitalisations in past 12 months: RM 64 (42%) and UC 65 (41%) Inclusion criteria: GOLD grade II or higher, exacerbations or hospitalisation or both in the last year, comorbidities such as CHF, SDB, smoking pack‐years > 10 years, able to provide written consent, able to use TM equipment at home, reliable mobile phone coverage at home, > 60 years Exclusion criteria: any condition likely to put patient at risk, significant visual or mental condition, planned long‐time absence from home |
|
| Interventions | Measurements taken at baseline, every 2 months (CAT, PHQ‐9, MLHFQ), every 3 months (EQ‐5D, exacerbations, medication use, use of GP), and at end of study Treatment arms
|
|
| Outcomes |
Primary outcomes: time to first hospitalisation, quality of life (change in EQ‐5D utility index score) Secondary outcomes: moderate exacerbation rate, hospitalisation, CAT, PHQ‐9, MLHFQ questionnaires, cost utility analysis |
|
| Notes |
Funding: European commission grant Other identifier: NCT01960907 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisation with 4‐element block design stratified by centre was used |
| Allocation concealment (selection bias) | Unclear risk | Randomisation sequence was concealed, but it is unclear how allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Trial was open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was similar in TM (29%) and control groups (22%) |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported as planned. Study authors were contacted about time to first hospitalisation to see if they could provide HR and 95% CI, which they provided on request. Trial was registered at clnicaltrials.gov |
| Other bias | Low risk | None |
Yan 2018.
| Study characteristics | ||
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in China Duration: 52 weeks Setting: respiratory and nosocomial infection departments at a hospital |
|
| Participants |
Population: 240 adults recruited from the Respiratory and Nosocomial Infection Departments at a hospital in Wuhan, China Baseline characteristics: % Male: 60 RM and 66 UC, Mean age: 65.4 RM and 64.6 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, baseline medications: not reported, FEV₁ (% mean): RM 40.98 and UC 41.08, FVC (% mean): not reported, FEV₁/FVC (% mean): RM 54.08 and UC 53.47, Current smokers (n): RM 108 and UC 104, GOLD stage: RM: I (12), II (27), III (67), IV (14) and UC: I (10), II (25), III (70), IV (15), COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
|
| Interventions | Measurements at baseline and at 1 year Treatment arms
|
|
| Outcomes |
Primary outcomes: pulmonary function tests, quality of life (CAT) assessments, hospitalisations Secondary outcomes: not reported |
|
| Notes |
Funding: China Medical Board Other identifier: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors reported that participants were randomly assigned but provided no further information |
| Allocation concealment (selection bias) | Unclear risk | No further information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants and personnel due to nature of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No further information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were included, but it is unclear whether any withdrew |
| Selective reporting (reporting bias) | High risk | No Prisma diagram provided, data in Tables 3 and 4 (continuous data) not clear. Unclear whether SDs or SEs were reported. Trial not registered at trials website; unable to contact study author as email provided was incorrect. Contacted one of the other study authors; awaiting response |
| Other bias | Low risk | None |
6MWD: 6‐minute walking distance; 6MWT: 6‐minute walk test; A&E: accident and emergency visits; AECOPD: acute exacerbations of chronic obstructive pulmonary disease; AEs: adverse events; AMI: acute myocardial infarction; β₂‐agonist: beta2‐agonist; BODE: body mass index, airflow obstruction, dyspnoea, and exercise capacity index; BP: blood pressure; BRC: Biomedical Research Centre; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; CCQ: Clinical Chronic Obstructive Pulmonary Disease Questionnaire; CF: cystic fibrosis; CHF: congestive heart failure; CHROMED: Telemonitoring in Chronic Obstructive Pulmonary Disease in five countries; CHRONIC: an information Capture and Processing Environment for Chronic Patients in the Information Society project; CI: confidence interval; COPD: chronic obstructive pulmonary disease; COPD‐SES: Chronic Obstructive Pulmonary Disease Self‐Efficacy Scale; CRDQ: Chronic Respiratory Disease Questionnaire; CSQ‐8: Client Satisfaction Questionnaire‐8; CTN: care transition nurse; CVD: cardiovascular disease; ED: emergency department; EDGE: sElf‐management anD support proGrammE; EF: ejection fraction; EpxCOPD: Epharmix chronic obstructive pulmonary disease system; EQ‐5D: EuroQoL 5 Dimensions Questionnaire; EQ‐5D‐3L: EuroQoL 5 Dimensions 3‐Level Version Questionnaire; EU: European Union; EuroQoL‐5D: European Quality of Life 5 Dimension Questionnaire; FEV₁: forced expiratory volume in one second; FEV₁/FVC: forced expiratory volume in one second/forced vital capacity ratio; FILAS: locations and financial instruments for producers in Rome and Lazio; FVC: forced vital capacity; GesEPOC: Spanish National Guidelines for Chronic Obstructive Disease Care; GOLD: Global Initiative for Obstructive Lung Disease; GOLD I: Global Initiative for Obstructive Lung Disease stage 1; GOLD II: Global Initiative for Obstructive Lung Disease stage 2; GOLD III: Global Initiative for Obstructive Lung Disease stage 3; GOLD IV: Global Initiative for Obstructive Lung Disease stage 4; GP: general practitioner; GSM: Group Special Mobile; HADS: Hospital Anxiety and Depression Scale; HADS‐A: Hospital Anxiety and Depression Scale ‐ Anxiety; HADS‐D: Hospital Anxiety and Depression Scale ‐ Depression; HCP: healthcare provider; HF: heart failure; HR: hazard ratio; HRQOL: health‐related quality of life; IC: integrated care; ICS: inhaled corticosteroid; ICT: information and communication technologies; ICU: intensive care unit; ILD: interstitial lung disease; ILL: interstitial lung disease; IQR: interquartile range; ISRCTN: primary clinical trial registry recognised by World Health Organization and International Committee of Medical Journal Editors; IST: Information Sciences and Technology; IV: intravenous; LABA: long‐acting beta‐adrenergic agonist; LAMA: long‐acting muscarinic antagonist; LINQ: Lung Information Needs Questionnaire; LOS: length of stay; LTOT: long‐term oxygen therapy; MARS: Medication Adherence Report Scale; MLHFQ: Minnesota Living With Heart Failure Questionnaire; MM: Mini Mental Test; MMSE: Mini Mental State Examination; MRC: Medical Research Council; (n): number; NCT: ClinicalTrials.gov identifier; NHS: National Health Service; NIHR: National Institute for Health Research; NIV: non‐invasive ventilation; NL Agency: division of the Dutch Ministry of Economic Affairs; NSW: New South Wales; O₂: oxygen; PDE4: phosphodiesterase 4 inhibitors; PHQ‐9: Patient Health Questionnaire‐9; PITES‐ISA: Strategic Health Action research projects; PR: pulmonary rehabilitation; QOL: quality of life; RACS‐Plus: Respiratory Ambulatory Care Service‐Plus; RCP: routine clinical practice; RM: remote in‐home telemonitoring; RN: registered nurse; SAA: short‐acting adrenergic; SABA: short‐acting beta2‐agonist; SAE: serious adverse event; SBP: standard best practice care; SC: standard care; SCL‐10A: Standard Checklist 10‐Item Anxiety Measure; SCL‐20: Standard Checklist 20‐Item Questionnaire; SD: standard deviation; SDB: sleep‐disordered breathing; SECD6: Self‐Efficacy for Managing Chronic Disease 6‐Item Scale; SE: standard error; SF‐36: Short Form 36 questionnaire; SF36v2: Short Form 36 questionnaire Italian version; SGRQ: St George’s Respiratory Questionnaire; SGRQ‐C: chronic obstructive pulmonary disease‐specific version of St George’s Respiratory Questionnaire; STATA: Software for Statistics and Data Science; TH: telehealth; THC: telehealthcare; TM: telemonitoring; UC: usual care; UK: United Kingdom.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12614000296639 | Wrong intervention |
| Alonso 2004 | Wrong study design |
| Bentley 2014 | Intervention duration < 3 months |
| Bernocchi 2016 | Wrong intervention |
| Bischoff 2012 | Wrong intervention |
| Chau 2012 | Intervention duration < 3 months |
| Cooper 2019 | Wrong study design |
| Cordova 2007 | Wrong population |
| Dinesen 2012 | Wrong intervention/comparator: telerehabilitation intervention compared to exercise |
| Emme 2014 | Wrong population |
| Finkelstein 2004 | COPD population < 50% |
| Fors 2018 | Wrong intervention |
| Gaeckle 2016 | Wrong study design |
| Gellis 2014 | COPD population < 50% |
| Grabenhorst 2013 | Wrong intervention |
| Henderson 2013 | COPD population < 50% |
| ISRCTN13081008 | Wrong study design |
| ISRCTN34235668 | Wrong study design |
| ISRCTN34252610 | Wrong study design |
| ISRCTN41238563 | COPD population < 50% |
| Jehn 2013 | Wrong intervention |
| Johnston 2000 | COPD population < 50% |
| Juan 2011 | Wrong study design |
| Kamei 2011 | Wrong study design |
| Kamei 2018 | Wrong study design |
| Kenealy 2015 | COPD population < 50% |
| Ko 2015 | Wrong intervention |
| Lavensen 2012_2016 | Intervention duration < 3 months |
| Levine 2018 | COPD population < 50% |
| Mair 2002 | Wrong population |
| Mudiyanselage 2018 | COPD population < 50% |
| NCT00916799 | Wrong study design |
| NCT01044927 | Wrong study design |
| NCT01495780 | Wrong study design |
| NCT01644045 | Wrong study design |
| NCT01892566 | Wrong population |
| NCT02085187 | Wrong intervention |
| NCT02269618 | Mixed population |
| NCT02528370 | Wrong study design |
| NCT02706600 | Wrong intervention |
| NCT02791451 | Wrong study design |
| NCT02803489 | Wrong study design |
| NCT03127852 | Wrong population |
| NCT03129477 | Wrong study design |
| NCT03131622 | Wrong intervention |
| NCT03353064 | Wrong population |
| NCT03640260 | Wrong intervention |
| NCT03739957 | Wrong study design |
| NCT03837847 | Wrong intervention |
| NCT04108143 | Wrong study design |
| Nohra 2020 | Wrong study design |
| Norgaard 2014 | Wrong population |
| Paquin 2014 | Unclear population |
| Pare 2006 | Wrong study design |
| Pinnock 2012 | Wrong study design |
| Reinius 2013 | COPD population < 50% |
| Shah 2017 | Wrong study design |
| Sirichana 2013 | Wrong study design |
| Sorknaes 2011 | Intervention duration < 3 months |
| Sridhar 2008 | Wrong intervention |
| Tong 2012 | Wrong study design |
| Troosters 2003 | Wrong intervention |
| Vega 2008 | Wrong study design |
| Vitacca 2008 | COPD population < 50% |
| Walters 2013 | Wrong intervention |
| Whitten 2007 | COPD population < 50% |
| Wolpin 2011 | Wrong intervention |
| Wong 2005 | Wrong intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Cartwright 2013.
| Methods |
Study design: multi‐centre, unknown blinding, parallel, cluster‐randomised controlled trial in United Kingdom Duration: 52 weeks Setting: GP practices |
| Participants |
Population: 3230 adults recruited from 238 practices in Cornwall, Kent, and Newham, United Kingdom Baseline characteristics: unknown Inclusion criteria: ≥ 18 years, diagnosis of primary or secondary care of COPD, diabetes or HF (no formal clinical assessment of severity of disease but inclusion based on relevant Quality Outcomes Framework register in primary care, confirmed medical diagnosis in primary or secondary care medical records: general practice read codes or ICD‐10 classification, or confirmed by local clinician or patient's hospital consultant, patients were not excluded on basis of physical comorbidities) Exclusion criteria: does not understand English, not able to complete questionnaires, does not have appropriate power supply or telephone line, previous telehealth study with telehealth equipment |
| Interventions | Measurements at baseline and at 3 and 6 months Treatment arms
|
| Outcomes |
Primary outcomes: quality of life (SF‐12, EQ‐5D, generic COPD QOL questionnaire, depression (CES‐D), anxiety (STAI‐6) Secondary outcomes: none listed |
| Notes |
Funding: Department of Health, England Other identifier: ISRCTN43002091 |
Chatwin 2016.
| Methods |
Study design: multi‐centre, single‐blinded, stratified randomised controlled trial in United Kingdom Duration: 52 weeks Setting: primary and secondary clinics |
| Participants |
Population: 68 adults recruited from outpatient and inpatient clinics at Royal Brompton & Harefield NHS Foundation Trust, West Middlesex University Hospital, and St George’s University Hospital Baseline characteristics: % Male: 63 all, Mean age: 65.3 all, % White: not reported, % African: not reported, % LTOT: 59 all, % Home oxygen: not reported, % Anxiety and depression 8 (4) all and 7 (4) all, Baseline medications: not reported, FEV₁: all 0.9 (0.5), FVC: all 2.1 (0.9), FEV₁/FVC: not reported, Current smokers (n): not reported, GOLD stage: all 3 (1), COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: ≥ 18 years old, COPD or chronic respiratory failure due to another chronic respiratory disease, admitted exacerbation in previous 6 months, met criteria for LTOT or had oxygen saturation level ≤ 90% on air for past admission Exclusion criteria: cognitive impairment that impairs understanding of the trial or use of telemonitoring, age < 18 years |
| Interventions | Measurements taken at baseline and at 3, 6, and 12 months Treatment arms
|
| Outcomes |
Primary outcomes: time to first hospital admission for exacerbation Secondary outcomes: hospital admissions, general practitioner (GP) consultations and home visits by nurses, quality of life measured by EuroQoL‐5D and hospital anxiety and depression (HAD) scale, self‐efficacy score |
| Notes |
Funding: National Institute for Health Research (NIHR) under the Collaborations for Leadership in Applied Health Research and Care (CLAHRC) programme for North West London Other identifier:NCT02180919 |
Martin‐Lesende 2013.
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial Duration: 52 weeks Setting: primary care health centres in Spain |
| Participants |
Population: 58 adults recruited from 20 health centres in Bilbao, Spain Baseline characteristics: % Male: 50 RM and 66.7 UC, 57.1 RM and 46.7 UC, % Anxiety and depression: not reported, Baseline meds: not reported, FEV₁: not reported, FVC: not reported, FEV₁/FVC: not reported, Current smokers (n): not reported, GOLD stage: moderate (17.4%), severe (21.7%), and very severe (60.9%), COPD exacerbations last 12 months: not reported, Hospitalisation in past 12 months: not reported Inclusion criteria: home care adult patients, diagnosis of heart failure and/or chronic lung disease 14+ years, history of 2+ hospital admissions in last year with at least 1 admission associated with at least 1 of said conditions for study Exclusion criteria: in residential care, receiving regular monitoring or treatment by specialist or hospitalist services, life expectancy < 6 months due to other illness, known cognitive impairment, not willing to participate |
| Interventions | Measurements at baseline and at 3, 6, and 12 months Treatment arms
|
| Outcomes |
Primary outcomes: number of hospital admissions Secondary outcomes: length of hospital stay, mortality, use of other healthcare resources (ED visits, home visits, health centres, specialists, telephone calls), number of alerts by telemonitoring system in 5 days leading up to admission |
| Notes |
Funding: Spanish Ministry of Health Other identifier: ISRCTN89041993 |
NCT00752531.
| Methods |
Study design: single‐blinded, parallel individual randomised controlled trial Duration: 78 weeks Setting: unknown |
| Participants |
Population: 280 adult participants to be recruited in the United States Baseline characteristics: unknown Inclusion criteria: ≥ 21 years, understands English, has working telephone/cable, with diagnosed COPD, stage II/III COPD Exclusion criteria: moving from study area before study complete, health condition causing participant to not carry out study expectations, not able to use telephone and without assistance to do so |
| Interventions | Measurements at baseline and at 3, 6, 9, 12, 15, and 18 months Treatment arms
|
| Outcomes |
Primary outcomes: lung function, respiratory symptoms Secondary outcomes: quality of life, use of health care, activities of daily living, self‐efficacy, exercise tolerance |
| Notes |
Funding: John Hopkins University Other identifier:NCT00752531 |
NCT00893685.
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial Duration: 130 weeks Setting: healthcare systems |
| Participants |
Population: 300 adults recruited from Denmark, Estonia, Germany, Italy, Spain, Sweden Baseline characteristics: unknown Inclusion criteria: < 65 years; diagnosis of CHF, DM, or COPD Exclusion criteria: unable to use study equipment, dependent on others for daily living, diagnosis of dementia, impaired language, no signed informed consent, no access to ISDN or DSL service |
| Interventions | Measurements taken at baseline and at 15 and 30 months Treatment arms
|
| Outcomes |
Primary outcomes: SF‐36 questionnaire Secondary outcomes: hospital length of stay, transfer to elderly home, number of hospitalisations, HADS, death, injury, ambulance transport, emergency department visits, home visits by nurses, consults with GP or specialists |
| Notes |
Funding: Health Information Management, Belgium Other identifier:NCT00893685 |
NCT01342263.
| Methods |
Study design: mult‐ centre, single‐blinded, parallel individual randomised controlled trial in Canada Duration: 104 weeks Setting: primary and secondary clinics |
| Participants |
Population: 234 adults recruited from Northern Health, Fraser Health, Interior Health, Vancouver Island Health, and Vancouver Coastal Health, Canada Baseline characteristics: unknown Inclusion criteria: ≥ 19 years of age; ≥ 2 of the following chronic diseases: CHF, DM, COPD, kidney disease, heart disease; Internet access; able to read, write, understand English Exclusion criteria: scheduled surgical procedures, not able to give informed consent, comorbidities interfering with management |
| Interventions | Measurements taken at baseline and at 24 months Treatment arms
|
| Outcomes |
Primary outcomes: hospital admissions, emergency room visits, hospital length of stay, physician visits, procedures (diagnostic and lab) Secondary outcomes: SF‐36, heiQ, satisfaction (participants and providers), social support, EQ‐5D‐5L, adhering to intervention |
| Notes |
Funding: Simon Fraser University Other identifier:NCT01342263 |
NCT01489241.
| Methods |
Study design: unknown centres, single‐blinded, parallel individual randomised controlled trial in Greece Duration: 12 weeks Setting: hospitals |
| Participants |
Population: 155 adults recruited from Central Greece Baseline characteristics: unknown Inclusion criteria: ≥ 40 years, able to use devices for study, willing to participate, COPD per GOLD guidelines Exclusion criteria: involved in previous COPD monitoring study |
| Interventions | Measurements taken at baseline and at 3 months Treatment arms
|
| Outcomes |
Primary outcomes: hospital readmissions Secondary outcomes: QOL SF‐36, HADS, SGRQ, FEV₁, mortality, patient satisfaction survey |
| Notes |
Funding: Regional Health Authority of Sterea & Thessaly Other identifier:NCT01489241 |
NCT01512992.
| Methods |
Study design: unknown centres, single‐blinded, parallel individual randomised controlled trial in Spain Duration: 12 weeks Setting: clinic, hospital |
| Participants |
Population: 380 adults recruited from clinics/hospitals in Spain Baseline characteristics: unknown Inclusion criteria: ≥ 40 years of age, COPD exacerbation, willing to participate in study, able to use devices for study Exclusion criteria: participated in previous COPD home telehealth study |
| Interventions | Measurements taken at baseline and at 3 months Treatment arms
|
| Outcomes |
Primary outcomes: hospital re‐admissions Secondary outcomes: HQOL by SF‐36, FEV₁, CAT, HADS, mortality, time to first re‐admission, emergency department visits, length of stay for re‐admission, patient satisfaction |
| Notes |
Funding: Catalan Agency for Health Information, Assessment, and Quality Other identifier:NCT01512992 |
NCT01560741.
| Methods |
Study design: unknown centres; double‐blinded, parallel individual randomised controlled trial in Portugal Duration: 36 weeks Setting: hospital |
| Participants |
Population: 128 adults recruited from Portugal Baseline characteristics: unknown Inclusion criteria: < 80 years of age, PaCO₂ > 45 mmHg, IMC > 40 kg/m², LTOT for at least 3 months, 1 exacerbation in last year, FEV₁ < 50%predicted, FEV₁/FVC < 60%, TLC > 90% predicted, GOLD guidelines therapy, pH > 7.35, free of exacerbations 4 weeks before recruitment Exclusion criteria: OHS: COPD, NMD; COPD: 15% increase in FEV₁ after inhaled salbutamol (200 μg), actively smoking, history of OSA; NMD and CWD: COPD; OHS; PCF < 270; MIC/VC = 1, severe bulbar weakness |
| Interventions | Measurements taken at baseline, at 12 weeks, and at end of study Treatment arms
|
| Outcomes |
Primary outcomes: difference of 1 hour in the mean of nightly hours of use Secondary outcomes: QOL, health economics, arterial blood gases |
| Notes |
Funding: Hospital Sao Joao Other identifier:NCT01560741; TeleMotiNIV2012 |
NCT01580072 .
| Methods |
Study design: unknown centres; open‐label, parallel individual randomised controlled trial in Austria Duration: 52 weeks Setting: unknown |
| Participants |
Population: 65 adults recruited from Carinthia, Austria Baseline characteristics: unknown Inclusion criteria: III/IV GOLD COPD, life expectancy > 12 months, able to use system Exclusion criteria: unknown |
| Interventions | Measurements taken at baseline and at 12 months Treatment arms
|
| Outcomes |
Primary outcomes: QOL SF‐36, inpatient stays Secondary outcomes: number of bed days for hospitalised patients, number of PC visits, number of specialist visits, number of emergency department visits, mortality, CAT, SGRQ |
| Notes |
Funding: Ladeskrankenanstalten‐Betriebsgesellschaft Other identifier: NCT01580072; C250487 |
NCT01744028.
| Methods |
Study design: unknown centres; open‐label, parallel individual randomised controlled trial in Spain and Sweden Duration: 52 weeks Setting: centres |
| Participants |
Population: 200 adults recruited from centres in Spain and Sweden Baseline characteristics: unknown Inclusion criteria: diagnosis of COPD GOLD II or higher, current or ex‐smoker with 10 pack‐years nt, post‐bronchodilator FEV₁ < 80% of predicted within 12 months prior, post‐bronchodilator FEV₁/FVC < 70% within 12 months prior to/at screening, documented COPD exacerbations ≥ 2 in previous 12 months Exclusion criteria: use of investigative drugs at time of enrolment/within 30 days of 5 half‐lives of enrolment, history of asthma prior to age 40 years, COPD exacerbation not resolved within 30 days prior to screening |
| Interventions | Measurements taken at baseline and at 12 months Treatment arms
|
| Outcomes |
Primary outcomes: number of hospitalisations for COPD, number of emergency department visits for COPD Secondary Outcomes: length of hospitalisation, time to first hospitalisation, used of healthcare resources (hospital, office, telephone), number of medical visits all |
| Notes |
Funding: Novartis Pharmaceuticals Other identifier:NCT01744028; CIDD001D2401 |
NCT01951261.
| Methods |
Study design: unknown centres; open‐label, parallel individual randomised controlled trial in Spain Duration: 24 weeks Setting: hospital |
| Participants |
Population: 116 adults recruited from Spain Baseline characteristics: unknown Inclusion criteria: admitted to hospital with COPD exacerbation, w/o fever 48 hours, aerosol treatment every 6 hours, no other serious unstable disease, chest X‐ray without new disease, suitable environment for treatment with glucocorticoid intravenous < 40 mg twice daily Exclusion criteria: alcoholism, institutionalisation, not stable haemodynamics, ICU or on invasive mechanical ventilation during exacerbation, intravenous medicine, neoplasia or other chronic disease in terminal situation, inability to understand or participate in study |
| Interventions | Measurements taken at baseline and at 1, 4, and 24 weeks Treatment arms
|
| Outcomes |
Primary outcomes: time until first exacerbation Secondary outcomes: STAI, SATISFAD 10, medication adherence, telemonitoring compliance, number of home visits, CAT |
| Notes |
Funding: unknown Other identifier:NCT01951261; TELEMEDCOPD |
NCT02180919.
| Methods |
Study design: unknown centres; open‐label, cross‐over randomised controlled trial Duration: 52 weeks Setting: unknown |
| Participants |
Population: 85 adults recruited from United Kingdom Baseline characteristics: unknown Inclusion criteria: heart failure patients: ≥ 18 years of age in New York Heart Association Class II to IV at time of discharge; respiratory patients: > 18 years with diagnosis of COPD or respiratory insufficiency due to chronic respiratory disease diagnosed by a respiratory physician; arterial oxygen saturation ≤ 90%, LTOT Exclusion criteria: < 18 years of age, cognitive impairment to interfere with study |
| Interventions | Measurements taken at baseline and at 3, 6, 9, and 12 months Treatment arms
|
| Outcomes |
Primary outcomes: time to first exacerbation Secondary outcomes: compliance with telemonitoring, self‐efficacy, contact with GP, emergency department visits, HADS, Minnesota Living With Heart Failure Questionnaire, EQ‐5D, CRQ |
| Notes |
Funding: Royal Brompton & Harefield NHS Foundation Trust Other identifier:NCT02180919; 10/H0704/19 |
NCT02615795.
| Methods |
Study design: unknown centres; open‐label, parallel individual randomised controlled trial in Denmark Duration: 26 weeks Setting: hospital |
| Participants |
Population: 160 adults recruited from Denmark Baseline characteristics: unknown Inclusion criteria: COPD with FEV₁/FVC < 70% at all times during study, FEV₁ < 51% during inclusion and during further study, included into study during hospitalisation with exacerbation in pulmonary symptoms Exclusion criteria: alcohol or drug abuse, not able to use equipment or with language barrier, asthma, psychiatric issues causing disability, unstable heart disease, terminal disease, not able to given written or verbal consent |
| Interventions | Measurements taken at baseline and at 12 months Treatment arms
|
| Outcomes |
Primary outcomes: hospitalisation days Secondary outcomes: mortality, contact with GP, QOL: SGRQ, HADS, SF‐36, physiological measurements detecting COPD exacerbation, number of self‐addressed COPD exacerbations, emergency room visits COPD‐related, number of hospitalisations for COPD exacerbations, length of hospital days for COPD exacerbations |
| Notes |
Funding: University of Aarhus Other identifier:NCT02615795; UAarhusFA |
NCT02901535 .
| Methods |
Study design: unknown centres; single‐blinded, parallel individual randomised controlled trial in Brazil Duration: 20 weeks Setting: primary care |
| Participants |
Population: 240 adults recruited from Brazil Baseline characteristics: unknown Inclusion criteria: Modified Medical Research Council dyspnoea > 0, spirometry from Telessaude RS‐Universidad Federal do Rio Grande do Sul Exclusion criteria: normal spirometry, inadequate spirometry |
| Interventions | Measurements taken at baseline and at 20 weeks Treatment arms
|
| Outcomes |
Primary outcomes: mMRC Secondary outcomes: FEV₁, FVC |
| Notes |
Funding: unknown Other identifier: NCT02901535 |
NCT03183817 .
| Methods |
Study design: unknown centres; open‐label, parallel individual randomised controlled trial in Sweden Duration: 104 weeks Setting: hospital |
| Participants |
Population: 224 adults recruited from hospital in Sweden Baseline characteristics: unknown Inclusion criteria: diagnosis COPD and/or CHF, listed at a primary care centre in Narhalsan, understands written and spoken Swedish Exclusion criteria: no registered address, impairment preventing use of eHealth support, SPMSQ score > 6, expected survival < 12 months from disease, alcohol or drug abuse, other disease interfering with follow‐up, participating in a conflicting randomised study |
| Interventions | Measurements taken at baseline and at 3, 6, 12, and 24 months Treatment arms
|
| Outcomes |
Primary outcomes: change in self‐efficacy Secondary outcomes: number of admissions, health care use, self‐efficacy scale, incremental cost‐utility ratio, EQ‐5D, HADS, shortness of breath in heart failure questionnaire, CAT, MRC |
| Notes |
Funding: Goteborg University Other identifier: NCT03183817; PROTECT |
NCT03505138.
| Methods |
Study design: unknown centres; open‐label, parallel individual randomised controlled trial Duration: 52 weeks Setting: unknown |
| Participants |
Population: 120 adults recruited from Spain; baseline characteristics: unknown Inclusion criteria: diagnosis of COPD, re‐admission (2+) in last year, stable 6 weeks before study, ≥ 18 years of age, able to use a tablet to track and monitor for the study Exclusion criteria: does not give consent, inadequate social/family support, phone coverage issues, severe comorbidities |
| Interventions | Measurements taken at baseline and at 12 months Treatment arms
|
| Outcomes |
Primary outcomes: re‐admission in patients with COPD Secondary outcomes: ICER, CAT, lung function (FEV₁, FVC, FEV₁/FVC), mortality, biomarker predictor of exacerbation severity, medication compliance, patient and caregiver satisfaction, EQ‐5D |
| Notes |
Funding: Sociedad Espanola de Neumologia y Circugia Toracica Other identifier:NCT03505138; CRONEX3.0 |
Ramos 2018.
| Methods |
Study design: multi‐centre, unknown blinding, parallel individual randomised controlled trial in unknown country Duration: 12 weeks Setting: not reported |
| Participants |
Population: 20 adults recruited Baseline characteristics: % Male: 100 RM and 100 UC, Mean age: 77.0 RM and 76.63 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline meds: not reported, FEV₁ (% mean): RM 48.75 and UC 42.81, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): not reported, GOLD stage: not reported, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: not reported Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Measurements taken at baseline and at end of study Treatment arms
|
| Outcomes |
Primary outcomes: exacerbation rate, hospital admission, mortality Secondary outcomes: not reported |
| Notes |
Funding: not reported Other identifier: not reported Other: only conference abstract available; pilot project |
Tivota 2015.
| Methods |
Study design: single‐centre, open‐label, parallel individual randomised controlled trial in Norway Duration: 104 weeks Setting: hospital ‐ Trondheim University Hospital |
| Participants |
Population: 172 adults recruited from Department of Thoracic Medicine or Observation Unit at Trondheim University Hospital in Norway Baseline characteristics: % Male: 43 IC and 45 UC, Mean age: 72.5 IC and 73.1 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety or depression: not reported, Baseline medication: Inhaled LAMA: IC group: 39%; UC group 51%. LABA + ICS: IC group: 70%; UC group: 71%, FEV₁ (% mean): IC 34.9 and UC 33.4, FVC (% mean): not reported, FEV₁/FVC (% mean): not reported, Current smokers (n): IC 18 and UC 15, GOLD stage: III/IV, COPD exacerbations last 12 months: not reported, Hospitalisations in past 12 months: IC: 1.0 (1,1) and UC: 1.0 (1,2) Inclusion criteria: admission due to AECOPD, GOLD III/IV diagnosis, residing in Trondheim area, Norwegian‐speaking, able to sign consent form Exclusion criteria: short life span due to serious disease (< 6 months' survival) |
| Interventions | Routine calls per month; home visits at days 3 and 14, then at 6, 12, 24 months post discharge Treatment arms
|
| Outcomes |
Primary outcomes: number of hospital admissions (AECOPD), number of in‐hospital days (AECOPD), QOL (SGRQ), HADS Secondary outcomes: mortality, Charlson Co‐morbidity Index |
| Notes |
Funding: Central Norway Regional Health Authority and The Research Council of Norway Other identifier: NCT00702078 |
Venter 2012.
| Methods |
Study design: multi‐centre, unknown blinding, parallel individual randomised controlled trial in New Zealand Duration: 52 weeks Setting: hospital |
| Participants |
Population: 20 patients recruited from Turangi or Taupo area in New Zealand Baseline characteristics: unknown Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions | Measurements taken at baseline and at 6 and 12 months Treatment arms
|
| Outcomes |
Primary outcomes: usefulness of telehealth technology, effects of health outcomes, effects of telehealth monitoring and early intervention Secondary outcomes: unknown |
| Notes |
Funding: Lakes District Health Board, Lake Taupo Primary Health Organisation, Healthcare of New Zealand Other identifier: unknown |
Walker 2017.
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial in Estonia, Slovenia, Spain, Sweden, United Kingdom Duration: 39 weeks Setting: hospital, clinic, community health service |
| Participants |
Population: 312 adults recruited from 6 sites (hospital, clinic, community health service) in 5 countries (Estonia, Slovenia, Spain, Sweden, UK) Baseline characteristics: % Male: 66 IC and 65 UC, Mean age: 71 IC and 71 UC, % White: not reported, % African: not reported, % LTOT: not reported, % Home oxygen: not reported, % Anxiety and depression: Mean depression PHQ9 score was 6.27 (IC) and Mean depression PHQ9 score was 5.97 (UC), Baseline meds: not reported, FEV₁: IC 49.4 and UC 50.4, FVC: IC 73.8 and UC 75.8, FEV₁/FVC: IC: 0.53 and UC: 0.53, Current smokers (n): not reported, GOLD stage: IC: I (3%), II (47%), III (36%), IV (15%) and UC: I (2%), II (48%), III (39%), IV (11%), COPD exacerbations last 12 months: 1 exacerbation: IC 63 (41%) and UC 59 (37%); more than 1 exacerbation: IC 91 (59%) and UC 99 (63%), Hospitalisation in past 12 months: IC 64 (42%) and UC 65 (41%) Inclusion criteria: GOLD grade II or higher, exacerbations or hospitalisation or both in the last year, comorbidities such as CHF, SDB, smoking pack‐years > 10 years, able to provide written consent, able to use TM equipment at home, reliable mobile phone coverage at home, > 60 years of age Exclusion criteria: any condition likely to put patient at risk, significant visual or mental condition, planned long‐time absence from home |
| Interventions | Measurements taken at baseline and at end of study Treatment arms
|
| Outcomes |
Primary outcomes: time to first hospitalisation, quality of life (change in EQ‐5D utility index score) Secondary outcomes: moderate exacerbation rate, hospitalisation, CAT, PHQ‐9, MLHFQ questionnaires, cost‐utility analysis |
| Notes |
Funding: European Commission grant Other identifier: NCT01960907 |
AECOPD: acute exacerbations of chronic obstructive pulmonary disease; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; CE: Conformity European (marked Philips Motiva System); CES‐D: Centre for Epidemiologic Studies Depression Scale; CHF: congestive heart failure; CLAHRC: Collaborations for Leadership in Applied Health Research and Care; COPD: chronic obstructive pulmonary disease; CRQ: Chronic Respiratory Disease Questionnaire; CWD: chest wall disease; DM: diabetes mellitus; DSL: digital subscriber line; ED: emergency department; EQ‐5D: EuroQoL 5 Dimensions Questionnaire; EQ‐5D‐5L: EuroQoL 5 Dimensions 5‐Level Version Questionnaire; EuroQoL‐5D: European Quality of Life 5 Dimension Questionnaire; EXACT: Exacerbations of Chronic Pulmonary Disease Tool; FEV₁: forced expiratory volume in one second; FEV₁/FVC: forced expiratory volume in one second/forced vital capacity ratio; FVC: forced vital capacity; GOLD: Global Initiative for Obstructive Lung Disease; GOLD I: Global Initiative for Obstructive Lung Disease stage 1; GOLD II: Global Initiative for Obstructive Lung Disease stage 2; GOLD III: Global Initiative for Obstructive Lung Disease stage 3; GOLD IV: Global Initiative for Obstructive Lung Disease stage 4; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; heiQ: Health Education Impact Questionnaire; HF: heart failure; HRQOL: health‐related quality of life; IC: integrated care; ICD10: International Statistical Classification of Diseases and Related Health Problems, Tenth Edition; ICER: incremental cost‐effectiveness ratio; ICU: intensive care unit; IMC: equivalent to body mass index; ISDN: Integrated Services Digital Network; LABA + ICS: long‐acting beta‐adrenergic agonist + inhaled corticosteroid; LAMA: long‐acting muscarinic antagonist; LTOT: long‐term oxygen therapy; MIC/VC: maximal insufflation capacity/vital capacity ratio; MLHFQ: Minnesota Living With Heart Failure Questionnaire; mMRC: Modified Medical Research Council; MRC: Medical Research Council; (n): number; NIHR: National Institute for Health Research; NMD: neuromuscular disease; nt: number of packages of cigarettes smoked daily, number of years of smoking; OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnoea; PaCO₂: partial pressure of carbon dioxide; PC: primary care; PCF: peak cough flow; PDA: personal digital assistant; pH: power of hydrogen (acidity or basicity of aqueous solution); PHQ‐9: Patient Health Questionnaire‐9; QOL: quality of life; QOL SF‐36: Health‐Related Quality of Life as Measured by Short Form 36 Version 2 Questionnaire; RM: remote in‐home telemonitoring; SATISFAD 10: instrument that evaluates satisfaction with home care services, self‐administered; SDB: sleep‐disordered breathing; SF‐12: Short Form 12 Questionnaire; SF‐36: Short Form 36 Questionnaire; SGRQ: St George’s Respiratory Questionnaire; SPMSQ: Short Portable Mental Status Questionnaire; STAI: State‐Trait Anxiety Inventory; STAI‐6: State Trait Anxiety Inventory ‐ 6 anxiety scores; TLC: total lung capacity; TM: telemonitoring; TV: television; UC: usual care; UK: United Kingdom; w/o: without.
Characteristics of ongoing studies [ordered by study ID]
NCT02756533.
| Study name | Impact of a telemonitoring program on the rate of hospitalizations for worsening of cardio‐respiratory symptoms in COPD patients treated at home by long‐term non‐invasive ventilation (NIV) |
| Methods |
Study design: multi‐centre, double‐blinding, parallel individual randomised controlled trial in France Duration: 52 weeks Setting: clinic or hospital |
| Participants |
Population: 140 patients recruited from hospitals and clinics in France Baseline characteristics: unknown Inclusion criteria: ≥ 18 years of age, COPD diagnosis, Social Security coverage, hospitalised ≥ 1 time in last year for exacerbation, treated by long‐term NIV Exclusion criteria: major protected by law, pregnant, deprived of liberty, GP or pulmonologist of patient not willing to participate, disease causing a threat to life excluding COPD |
| Interventions | Measurements taken unknown Treatment arms:
|
| Outcomes |
Primary outcomes: number of hospitalisations for cardio‐respiratory symptoms Secondary outcomes: number of hospitalisations, mortality, detection of COPD exacerbation, length of hospitalisation, medical cost, QOL by SRI |
| Starting date | 08.01.2016 |
| Contact information | Jean‐Christian Borel, PhD, +33762707821, j.borel@agiradom.com; Renaud Tamisier, Pr MD PhD, +33476768732, rtamisier@chu‐grenoble.fr |
| Notes |
Funding: University Hospital, Grenoble Other identifier:NCT02756533; 38RC15.179 |
NCT03396172.
| Study name | FreeDom: innovative strategy for the management of COPD exacerbations combining early hospitalisation discharge, automated oxygen weaning at home, telemedicine, and telerehabilitation |
| Methods |
Study design: unknown centres, open‐blinding, parallel individual randomised controlled trial in Canada Duration: 12 weeks Setting: hospital |
| Participants |
Population: 100 patients recruited from hospitals in Canada Baseline characteristics: unknown Inclusion criteria: diagnosis of COPD, ex‐smoker (10 pack‐year history), acute exacerbation (< 15 days), oxygen therapy need (rate < 6 L/min for SpO₂ > 90%), ≥ 40 years of age Exclusion criteria: no consent, imminent intubation per pulmonologists, sleep apnoea, NIV used at home, non‐autonomous and alone at home, lives > 50 km from hospital, already in the study within 3 months, lack of free O₂ system at time of study |
| Interventions |
Run‐in: at hospital before discharge. Measurements taken at baseline and at 1 and 3 months Treatment arms
|
| Outcomes |
Primary outcomes: number of hospital days during COPD exacerbation Secondary outcomes: emergency department visits, hospital re‐admissions, HRQL, costs of care, oxygenation, number of consultations (phone, video, rehab, home) |
| Starting date | 05.24.2018 |
| Contact information | Francois Lellouche, 418‐656‐8711 ext 3572, francois.lellouche@criucpq.ulaval.ca; Pierre‐alexandre Bouchard, 418‐656‐8711 ext 2712, pierre‐alexandre.bouchard@criucpq.ulaval.ca |
| Notes |
Funding: Laval University Other identifier:NCT03396172; 21419 |
NCT03558763 .
| Study name | Remote monitoring of patients with chronic obstructive pulmonary disease using a tablet system. A randomised cross‐over pilot study of feasibility evaluation and quality of life measurements |
| Methods |
Study design: unknown centres; open‐label blinding, cross‐over individual randomised controlled trial in Sweden Duration: 56 weeks Setting: unknown |
| Participants |
Population: 70 patients recruited from Sweden Baseline characteristics: unknown Inclusion criteria: diagnosis of COPD, GOLD grade D, FEV < 80% predicted, cognitive ability for study judged by investigator, living at home and able to manage daily living activities, gives informed consent and willing to participate, FEV₁/FVC (post bronchodilator) < 0.7 Exclusion criteria: COPD exacerbation during 1 month before study, severe disease other than COPD affecting HRQL as judged by investigator, long‐term stay away from home (> 2 weeks) w/o Internet connectivity |
| Interventions | Measurements taken at baseline and at 26 weeks, 30 weeks, and 56 weeks Treatment arms
|
| Outcomes |
Primary outcomes: SF‐12 Secondary outcomes: cost‐utility evaluation |
| Starting date | 06.07.2018 |
| Contact information | None listed |
| Notes |
Funding: Vastra Gotaland Region Other identifier: NCT03558763 |
NCT04080570.
| Study name | Remote physician care for home hospital patients |
| Methods |
Study design: multi‐centre, open‐label, parallel individual randomised controlled trial in the United States Duration: 4 weeks Setting: 2 hospitals |
| Participants |
Population: estimated 260 adults to be recruited from 2 hospitals in Massachusetts Baseline characteristics: unknown which characteristics Inclusion criteria: within 5 miles of ED, able to consent, has caregiver who can stay with the participant for the first 24 hours, primary diagnosis of COPD Exclusion criteria: undomiciled, on methadone, police custody, in nursing facility, domestic violence, acute delirium, end‐stage kidney disease, AMI, acute cerebral vascular accident, acute haemorrhage, primary diagnosis requiring multiple/routine administration of IV narcotics for pain control, unable to walk to bedside toilet unless help at home, CT, MRI, endoscopic procedure, blood transfusion, cardiac stress test, surgery, high risk of clinical decline |
| Interventions |
Run in: initial in‐home visit by a physician Treatment arms
|
| Outcomes |
Primary outcomes: adverse events Secondary outcomes: unplanned re‐admissions after first admission, Picker Experience Questionnaire score, global experience score |
| Starting date | |
| Contact information | |
| Notes |
Funding: Brigham and Women's Hospital Other identifier:NCT04080570 |
Rassouli 2018.
| Study name | Telehealth vs standard care COPD ‐ an international randomised controlled trial |
| Methods |
Study design: multi‐centre, unknown blinding, cross‐over randomised controlled trial in Switzerland Duration: 52 weeks Setting: 6 centres in Switzerland |
| Participants |
Population: projected number of participants 175 from 6 centres (Cantonal Hospital St Gallen, University Hospital Basel, Fachkliniken Wangen, University Hospital Zurich, Cantonal Hospital Glarus, Cantonal Hospital Munsterlingen) Baseline characteristics: unknown which characteristics Inclusion criteria: COPD GOLD diagnosis B‐D, ≥ 40 years old Exclusion criteria: unable to consent, unable to follow trial procedures |
| Interventions | Measurements taken at baseline and at 6 months (at cross‐over) and 12 months; CAT scores done weekly Treatment arms
|
| Outcomes |
Primary outcomes: change in CAT at 6 months Secondary outcomes: change in SF‐36, change in SGRQ, patient satisfaction |
| Starting date | 01.05.2016 |
| Contact information | Prof Dr Martin Brutsche, Klinik fur Pneumologie and Schlafmedizin, Kantonsspital St. Gallen, Rorschacher Strasse 95, 9007 St. Gallen, Phone: +41 71 494 11 11, Fax: +41 71 494 61 18, E‐mail: martin.brutsche@kssg.ch |
| Notes |
Funding: provided in future protocol Other identifier: EKSG‐Nr: 15/184 |
AMI: acute myocardial infarction; CAT: Chronic Obstructive Pulmonary Disease Assessment Test; COPD: chronic obstructive pulmonary disease; CT: computed tomography; ED: emergency department; FEV₁: forced expiratory volume in one second; FEV₁/FVC: forced expiratory volume in one second/forced vital capacity ratio; GOLD: Global Initiative for Obstructive Lung Disease; GOLD B: Global Initiative for Obstructive Lung Disease ‐ moderate; GOLD C: Global Initiative for Obstructive Lung Disease ‐ severe; GOLD D: Global Initiative for Obstructive Lung Disease ‐ very severe; GP: general practitioner; HRQOL: health‐related quality of life; IV: intravenous; NIV: non‐invasive ventilation; pk/yr: pack per year; QOL: quality of life; SF‐12: Short Form 12‐Item Questionnaire; SF‐36: Short Form 36‐Item Questionnaire; SGRQ: St George’s Respiratory Questionnaire; SpO₂: oxygen saturation; SRI: severe respiratory insufficiency score; w/o: without.
Differences between protocol and review
Under Types of interventions, we included studies in which the intervention was part of a complex multi‐component integration care intervention, but we did not include these studies in meta‐analyses for the above pre‐specified comparisons.
Under Types of participants, we excluded mixed population studies in which the COPD population was less than 50%. If the COPD population was 50% to 80%, we contacted study authors for disaggregated COPD data, if these were not already reported in the publication. If we did not hear from the study authors, we excluded the study. If the COPD population was 80%, we included the study.
Under Methods, we included dyspnoea symptoms, as this was considered an important primary outcome by co‐authors of this review.
Owing to the large volume of references, another co‐author of this review (DC) helped to screen references, extract data from studies, and perform risk of bias assessments.
We excluded studies of less than 3 months' duration, as effects of interventions would not be observed below this time point.
Contributions of authors
SJ: drafting of background and methods of protocol. Sifting, data extraction, risk of bias assessment, and write‐up of full review.
CT: drafting of background and methods of protocol. Sifting, data extraction, risk of bias assessment, and write‐up of full review.
SP: critical review of protocol, analysis and interpretation, approval of final draft of full review.
RD: conceptual and clinical advice, drafting of background and methods of protocol. Arbitration of conflicts, analysis and interpretation, and approval of final draft of full review.
DC: sifting, data extraction, risk of bias assessment, and write‐up of full review.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor) edited the protocol; advised on methods; approved the protocol prior to publication.
Milo Puhan (Contact Editor): edited the review; advised on methods, interpretation, and content.
Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review.
Emma Jackson (Assistant Managing Editor): conducted peer review; obtained translations; edited reference sections and other sections of the protocol and the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Lucy Goldsmith: checked data entry prior to full write‐up of the review.
Sources of support
Internal sources
-
All, Other
The review authors declare that no such funding was received for this systematic review.
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Programme Grant 16/114/21: NHS priorities in the management of chronic respiratory disease
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to the Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Declarations of interest
SJ: was employed full‐time as a systematic reviewer by a National Institute for Health Research (NIHR) Programme Grant to complete work on this review.
DC: has no conflicts of interest related to the review
CT: was employed part‐time in 2017‐18 by an NIHR Programme Grant to complete work on this Cochrane Review. He is currently a Specialty Registrar in Clinical Pharmacology and Therapeutics and General Internal Medicine.
SP: has received payment for lectures including speaking services from Boehringer Ingelheim, NAPP, Novartis, Pfizer, Nutricia, AstraZeneca, and TEVA, and travel expenses from Nutricia, AstraZeneca, and TEVA. SP has no conflicts of interest related to the review.
RD: has no conflicts of interest. She is supported by an Australian Research Council DECRA Fellowship and by the Australian Government Department of Health Rural Health Multidisciplinary programme as a Senior Research Fellow within an academic unit.
New
References
References to studies included in this review
Antoniades 2012 {published data only}
- Antoniades NC, Rochford PD, Pretto JJ, Pierce RJ, Gogler J, Steinkrug J, et al. Pilot study of remote telemonitoring in COPD. Telemedicine Journal and E-health 2012;18(8):634-40. [DOI: 10.1089/tmj.2011.0231] [DOI] [PubMed] [Google Scholar]
- Antoniades NC, Rochford PD, Pretto JJ, Pierce RJ, Gogler J, Steinkrug J, et al. Remote monitoring in chronic obstructive pulmonary disease (COPD) does not reduce hospital admissions or improve quality of life when compared to standard best practice care [Abstract]. Respirology 2009;14:A54. [Google Scholar]
- Rochford PD, Antoniades NC, Pretto JJ, Peirce RJ, Gogler J, Steinkrug J, et al. Remote monitoring in chronic obstructive pulmonary disease (COPD) does not reduce hospital admissions or improve quality of life when compared to standard best practice care. Respirology 2009;14:A3. [Google Scholar]
Berkhof 2015 {published data only}
- Berkhof FF, den Berg JWK, Uil SM, Kerstjens HAM. Telemedicine, the effect of nurse-initiated telephone follow up, on health status and health-care utilization in COPD patients: a randomized trial. Respirology 2015;20(2):279-85. [DOI: 10.1111/resp.12437] [DOI] [PubMed] [Google Scholar]
Bourbeau 2016 {published data only}
- Bourbeau J, Casan P, Tongella S, Haidl P, Texereau JB, Kessler R. An international randomised study of a home-based self-management programme for severe COPD: the COMET. International Journal of COPD 2016;11:1147-51. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbeau J, Viejo JL, Koehler D, Dal Negro R, Casan P, Tognella S, et al. Geographic differences in patients enrolled in the COPD patient management European trial (COMET). American Journal of Respiratory and Critical Care Medicine 2015;191:A1117. [Google Scholar]
- Kessler R, Casan-Clara P, Koehler D, Tognella S, Viejo JL, Dal Negro RW, et al. COMET: a multicomponent home-based disease-management programme versus routine care in severe COPD. European Respiratory Journal 2018;51(1):1701612. [DOI: ] [DOI] [PubMed] [Google Scholar]
Calvo 2014 {published data only}
- Calvo GS, Gomez-Suarez C, Soriano JB, Zamora E, Gonzalez-Gamarra A, Gonzalez-Bejar M, et al. A home telehealth programme for patients with severe COPD: the PROMETE study. Respiratory Medicine 2014;108(3):453-62. [DOI: ] [DOI] [PubMed] [Google Scholar]
- NCT02499068. Madrid project on the management of chronic obstructive pulmonary disease with home telemonitoring [Proyecto Madrileño sobre el manejo de la enfermedad pulmonar obstructiva crónica con telemonitorización a domicilio. (Multicentre project on the home telemonitoring of patients with severe chronic obstructive pulmonary disease.]. clinicaltrials.gov/ct2/show/nct02499068 (first received 15 July 2015).
- Segrelles G, Gomez-Suarez C, Zamora E, Gonzalez-Gamarra A, Gonzalez-Bejar M, Jordan A, et al. A home telehealth service for patients with severe COPD. The PROMETE study [Abstract]. European Respiratory Journal 2012;40:99s [P633]. [DOI] [PubMed] [Google Scholar]
Casas 2006 {published data only}
- Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernandez C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. European Respiratory Journal 2006;28:123-30. [DOI: 10.1183/09031936.06.00063205] [DOI] [PubMed] [Google Scholar]
- Garcia-Aymerich J, Hernandez C, Alonso A, Casas A, Rodriguez-Roisin R, Anto JM, et al. Effects of an integrated care intervention on risk factors of COPD readmission. Respiratory Medicine 2007;101(7):1462-9. [DOI] [PubMed] [Google Scholar]
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig-Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. European Respiratory Journal 2003;21:58-67. [DOI] [PubMed] [Google Scholar]
- Hernandez C. Enhanced integrated care management of COPD patients using a wireless point-of-care application [Abstract]. Proceedings of the American Thoracic Society 2006;3:A810 [Poster 902]. [Google Scholar]
De San Miguel 2013 {published data only}
- De San Miguel K, Smith J, Lewin G. Telehealth remote monitoring for community dwelling older adults with chronic obstructive pulmonary disease. Telemedicine Journal and E-Health 2013;19(9):652-7. [DOI: 10.1089/tmj.2012.0244] [DOI] [PubMed] [Google Scholar]
Farmer 2017 {published data only}
- Farmer A, Williams V, Velardo C, Shah SA, Yu L-M, Rutter H, et al. Self-management support using a digital health system compared with usual care for chronic obstructive pulmonary disease: a randomised controlled trial. Journal of Medical Internet Research 2017;19(5):e144. [DOI: 10.2196/jmir.7116] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velardo C, Shah SA, Gibson O, Clifford G, Heneghan C, Rutter H, et al. Digital health system for personalised COPD long-term management. BMC Medical Informatics and Decision Making 2017;17(1):19. [DOI: 10.1186/x12911-017-0414-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2016 {published data only}
- Ho T-W, Huang C-T, Chiu H-C, Ruan S-Y, Tsai Y-J, Yu C-J, et al. Effectiveness of telemonitoring in patients with chronic obstructive pulmonary disease in Taiwan - a randomised controlled trial. Scientific Reports 2016;6:23797. [DOI: 10.1038/srep23797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01724684. Feasibility and effectiveness of telehealth in patients with chronic obstructive pulmonary disease in Taiwan. clinicaltrials.gov/ct2/show/nct01724684 (first received 12 November 2012).
Jakobsen 2015 {published data only}
- Jakobsen AS, Chrisitan L, Ostergaard B, Rydahl-Hansen S, Emme C, Schou L, et al. Hospital admitted COPD patients treated at home using telemedicine technology: a randomised, multi-centre trial. European Respiratory Journal 2012;40:531s [P2902]. [Google Scholar]
- Jakobsen AS, Laursen LC, Rydahl-Hansen S, Ostergaard B, Gerds TA, Emme C, et al. Home-based telehealth hospitalisation for exacerbation of chronic obstructive pulmonary disease: findings from the "virtual hospital" trial. Telemedicine and E-health 2015;21(5):364-73. [DOI: 10.1089/tmj.2014.0098] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsen SV, Laursen LC, Ostergaard B, Rydahl-Hansen S, Phanareth KV. Hospital-admitted COPD patients treated at home using telemedicine technology in The Virtual Hospital Trial: methods of a randomized effectiveness trial. Trials 2013;14(1):280. [http://www.trialjournal.com/content/14/1/280] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schou L, Ostergaard B, Rasmussen L, Rydahl-Hansen S, Jakobsen AS, Emme C, et al. Cognitive function of patients with COPD after virtual admission: a randomized clinical trial. European Respiratory Journal 2012;40:81s [P549]. [Google Scholar]
- Schou L, Ostergaard B, Rasmussen L, Rydahl-Hansen S, Jakobsen AS, Emme C, et al. Telemedicine-based treatment versus hospitalization in patients with severe chronic obstructive pulmonary disease and exacerbation: effect on cognitive function. A randomized clinical trial. Telemedicine Journal and E-health 2014;20(7):640-6. [DOI] [PubMed] [Google Scholar]
- Schou L, Ostergaard B, Rydahl-Hansen S, Rasmussen LS, Emme C, Jakobsen AS, et al. A randomised trial of telemedicine-based treatment versus conventional hospitalisation in patients with severe COPD and exacerbation - effect on self-reported outcome. Journal of Telemedicine and Telecare 2013;19(3):160-5. [DOI: 10.1177/1357633X13483255] [DOI] [PubMed] [Google Scholar]
Jódar‐Sanchez 2013 {published data only}
- Jódar-Sánchez F, Ortega F, Parra C, Gómez-Suárez C, Jordán A, Pérez P, et al. Implementation of a telehealth programme for patients with severe chronic obstructive pulmonary disease treated with long-term oxygen therapy. Journal of Telemedicine and Telecare 2013;19(1):11-7. [DOI: 10.1177/1357633X12473909] [DOI] [PubMed] [Google Scholar]
Koff 2009 {published data only}
- Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. European Respiratory Journal 2009;33(5):1031-8. [DOI: 10.1183/09031936.00063108] [DOI] [PubMed] [Google Scholar]
- Koff PB, Stevens CC, Cashman J, Greene KE, Jones RH, Vandivier RW, et al. Telemonitoring/ehealth management improves quality of life and healthcare expenditures in COPD [Abstract]. Proceedings of the American Thoracic Society 2006;4:A123 [Poster J87]. [Google Scholar]
Lewis 2010 {published data only}
- Lewis KE, Annandale JA, Warm DL, Hurlin C, Lewis MJ, Lewis L. Home telemonitoring and quality of life in stable, optimised chronic obstructive pulmonary disease. Journal of Telemedicine and Telecare 2010;16(5):253-9. [DOI: 10.1258/jtt.2009.090907] [DOI] [PubMed] [Google Scholar]
- Lewis KE, Annandale JA, Warm DL, Rees SE, Hurlin S, Blyth H, et al. Does home telemonitoring after pulmonary rehabilitation reduce healthcare use in optimized COPD? A pilot randomized trial. COPD 2010;7(1):44-55. [DOI: 10.3109/15412550903499555] [DOI] [PubMed] [Google Scholar]
McDowell 2015 {published data only}
- McDowell JE, McClean S, FitzGibbon F, Tate F. A randomised clinical trial of the effectiveness of home-based health care with telemonitoring in patients with COPD. Journal of Telemedicine and Telecare 2015;21(2):80-7. [DOI: 10.1177/1357633X14566575] [DOI] [PubMed] [Google Scholar]
- McDowell JE, McKeown G, Hanna B, Sloan H, Howard J, Jackson E, et al. A model of home based healthcare with telehealth monitoring improves quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2012;185:A5787. [Google Scholar]
- NCT01522859. Telehealth monitoring in chronic obstructive pulmonary disease [The efficacy of telehealth monitoring in the management of patients with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/nct01522859 2012;(first received 1 February 2012).
Minguez 2017 {published data only}
- Minguez P, Cadavid B, Mata C, Malo R, Aguilar M, Valle M, et al. Early assisted discharge with generic telemedicine for chronic obstructive pulmonary disease exacerbations: results of a randomized controlled trial. Chest 2014;145(3):198A. [DOI: 10.1378/chest.1793561] [DOI] [Google Scholar]
- Minguez P, Pascual M, Mata C, Malo R, Carmona M, Lopez F, et al. Chapter 2. Implementation of an early detection service for COPD exacerbations: experimental evaluation for an early discharge hospital-at-home programme. In: Carrasco MP, Carrero AM, editors(s). PITES-ISA: New Services Based on Telemedicine and e-Health Aimed at Interoperability, Patient Safety and Decision Support. Madrid: Instituto de salud Carlos III, 2017:24-41. [Google Scholar]
Pedone 2013 {published data only}
- NCT01481506. Multiparametric telemonitoring in elderly people with chronic obstructive pulmonary disease (SweetAge) [Efficacy of multiparametric telemonitoring on respiratory outcomes in elderly people with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/nct01481506 (first received 29 November 2011).
- Pedone C, Chiurco D, Scarlata S, Incalzi RA. Efficacy of multiparametric telemonitoring on respiratory outcomes in elderly people with COPD: a randomised controlled trial. BMC Health Services Research 2013;13:82. [DOI: 10.1186/1472-6963-13-82] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinnock 2013 {published data only}
- Pinnock H, Hanley J, Lewis S, MacNee W, Pagliari C, Pol M, et al. The impact of a telemetric chronic obstructive pulmonary disease monitoring service: randomised controlled trial with economic evaluation and nested qualitative study. Primary Care Respiratory Journal 2009;18(3):233-5. [DOI: 10.4104/pcrj.2009.00040] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock H, Hanley J, McCloughan L, Todd A, Krishan A, Lewis S, et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multi-centre, randomised controlled trial. BMJ 2013;347:f6070. [DOI: 10.1136/bmj.f6070] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart A, va de Poi M, Pinnock H, Hanley J, McCloughan L, Todd A, et al. Telemonitoring for chronic obstructive pulmonary disease: a cost and cost-utility analysis of a randomised controlled trial. Journal of Telemedicine and Telecare 2015;21(2):108-18. [DOI: 10.1177/1357633X14566574] [DOI] [PubMed] [Google Scholar]
Ringbaek 2015 {published data only}
- Ringbaek T, Green A, Laursen LC, Frausing E, Brondum E, Ulrik CS. Effect of tele health care on exacerbations and hospital admissions in patients with chronic obstructive pulmonary disease: a randomised clinical trial. International Journal of COPD 2015;10:1801-8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper O, Gregersen TL, Ringbaek T, Brondum E, Frausing E, Green A, et al. Effect of tele healthcare on quality of life in patients with severe COPD: a randomized clinical trial. European Respiratory Journal 2017;50:A3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper OD, Gregersen TL, Ringbaek T, Brondum E, Frausing E, Green A, et al. Effect of tele-health care on quality of life in patients with severe COPD: a randomized clinical trial. International Journal of COPD 2018;13:2657-62. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ritchie 2016 {published data only}
- Ritchie C, Richman J, Sobko H, Bodner E, Phillips B, Houston T. The E-coach transition support computer telephony implementation study: protocol of a randomized trial. Contemporary Clinical Trials 2012;33(6):1172-9. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Ritchie CS, Houston TK, Richman JS, Sobko HJ, Berner ES, Taylor BB, et al. The E-Coach technology-assisted care transition system: a pragmatic randomised trial. Translational Behavioral Medicine 2016;6:428-37. [DOI: 10.1007/s13142-016-0422-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2018 {published data only}
- Rose L, Istanboulian L, Carriere L, Thomas A, Lee HB, Rezaie S, et al. Programme of integrated care for patients with chronic obstructive pulmonary disease and multiple comorbidities (PIC COPD +): a randomised controlled trial. European Respiratory Journal 2018;51(1):1701567. [DOI: ] [DOI] [PubMed] [Google Scholar]
Shany 2016 {published data only}
- Roberts M, Robinson T. Telemed: bringing technology to the homes of patients with chronic obstructive pulmonary disease - lessons learnt [Abstract]. Respirology 2011;16:P9 [TO 001]. [Google Scholar]
- Shany T, Hession M, Pryce D, Roberts M, Basilakis J, Redmond S, et al. A small-scale randomised controlled trial of home telemonitoring in patients with severe chronic obstructive pulmonary disease. Journal of Telemedicine and Telecare 2016;23(7):650-6. [DOI: 10.1177/1357633X16659410] [DOI] [PubMed] [Google Scholar]
Sink 2020 {published data only}
- Sink E, Patel K, Groenendyk J, Peters R, Som A, Kim E, et al. Effectiveness of a novel, automated telephone intervention on time to hospitalisation in patients with COPD: a randomised controlled trial. Journal of Telemedicine and Telecare 2018;0(0):1-8. [DOI: 10.1177/1357633X18800211] [DOI] [PubMed] [Google Scholar]
- Sink E, Patel K, Groenendyk J, Peters R, Som A, Kim E, et al. Effectiveness of a novel, automated telephone intervention on time to hospitalisation in patients with COPD: a randomised controlled trial. Journal of Telemedicine and Telecare 2020;26(3):132-9. [DOI: 10.1177/1357633X18800211] [DOI] [PubMed] [Google Scholar]
Soriano 2018 {published data only}
- Soriano JB, Garcia-Rio F, Vazquez-Espinosa E, Conforto JI, Hernando-Sanz A, Lopez-Yepes L, et al. A multi-centre, randomised controlled trial of telehealth for the management of COPD. Respiratory Medicine 2018;144:74-81. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Soriano JB, Garcia-Rio F, Vazquez-Espinosa E, Atauri JD, Yepes LL, Martinez RG, et al. Efficacy and costs of telehealth for the management of COPD: a multicentre, randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2018;197:A4546. [Google Scholar]
- Soriano JB, Garcia-Rio F, Vazquez-Espinosa E, Diaz de Atauri J, Lopez Yepes L, Galera Martinez R, et al. Efficacy and costs of telehealth for the management of COPD: PROMOTE II: a multicentre, randomized controlled trial. European Respiratory Journal 2017;50:Oa4665. [DOI: 10.1183/1303000.congress-2017.OA4665] [DOI] [PubMed] [Google Scholar]
Sorknaes 2013 {published data only}
- NCT01178879. Randomised trial of telehealth consultations for nursing care of chronic obstructive pulmonary disease (COPD) patients [Effectiveness of nurse led telehealth consultations in patients with chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/ct2/show/NCT01178879 (first received 10 August 2010).
- Sorknaes AD, Bech M, Madsen H, Titlestad IL, Hounsgaard L, Hansen-Nord M, et al. The effect of real-time tele-consultations between hospital-based nurses and patients with severe COPD discharged after an exacerbation. Journal of Telemedicine and Telecare 2013;19(8):466-74. [DOI: 10.1177/1357633X13512067] [DOI] [PubMed] [Google Scholar]
- Sorknaes AD, Bech M, Madsen H, Titlestad IL, Hounsgaard L, Hansen-Nord M, et al. The effects of real-time telemedicine consultations between hospital-based nurses and severe COPD patients discharged after exacerbation admissions [Abstract]. In: European Respiratory Society 23rd Annual Congress; 2013 Sep 7-11; Barcelona. 2013.
- Sorknaes AD. The effect of tele-consultation between a hospital-based nurse and a COPD patient. Studies in Health Technology and Informatics 2016;225:883-4. [DOI: 10.3233/978-1-61499-658-3-883] [DOI] [PubMed] [Google Scholar]
Stamenova 2020 {published data only}
- Stamenova V, Yang R, Engel K, Liang K, Lieshout F, Lalingo E, et al. Technology-enabled self-monitoring of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: a randomised controlled trial. Journal of Medical Internet Research 2020;22(7):e18598. [DOI: doi: 10.2196/18598] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenova V, Yang R, Engel K, Liang K, Lieshout F, Lalingo E, et al. Technology-enabled self-monitoring of chronic obstructive pulmonary disease with or without asynchronous remote monitoring: protocol for a randomized controlled trial. JMIR Research Protocols 2019;8(8):e13920. [DOI: 10.2196/13920] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tabak 2014 {published data only}
- Tabak M, Brusse-Keizer M, Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomised controlled trial. International Journal of COPD 2014;9:935-44. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak M, Brusse-Keizer M, Ommeren C, Kotte H, Weltevreden P, Hermens H, et al. A telecare programme for self-management of COPD exacerbations and promotion of an active lifestyle [Abstract]. In: European Respiratory Society 23rd Annual Congress; 2013 Sep 7-11; Barcelona. Vol. 42. 2013:1041s [P4911].
Udsen 2017 {published data only}
- Haesum LKE, Ehlers L, Hejlesen OK. Interaction between functional health literacy and tele home care: short-term effects from a randomised trial. Nursing and Health Sciences 2016;18(3):328-33. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Lilholt PH, Udsen FW, Ehlers L, Hejlesen OK. Telehealthcare for patients suffering from chronic obstructive pulmonary disease: effects on health-related quality of life: results from the Danish 'TeleCare North' cluster-randomised trial. BMJ Open 2017;7(5):e014587. [DOI: 10.1136/bmjopen-2016-014587] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01984840. Telemedicine for patients suffering from COPD (Danish Telecare North Trial) (TCN) [Effectiveness and cost-effectiveness of telemedicine for chronic obstructive pulmonary disease: the Danish "TeleCare North" pragmatic cluster-randomized trial]. clinicaltrials.gov/show/nct01984840 (first received 15 November 2013).
- Udsen FW, Lilholt PH, Hejlesen O, Ehlers L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish 'TeleCare North' cluster-randomised trial. BMJ Open 2017;7(5):e014616. [DOI: 10.1136/bmjopen-2016-014616] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udsen FW, Lilholt PH, Hejlesen O, Ehlers L. Effectiveness and cost-effectiveness of telehealthcare for chronic obstructive pulmonary disease: study protocol for a cluster randomized controlled trial. Trials 2014;15(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udsen FW, Lilholt PH, Hejlesen O, Ehlers LH. Subgroup analysis of telehealthcare for patients with chronic obstructive pulmonary disease: the cluster-randomized Danish Telecare North Trial. ClinicoEconomics and Outcomes Research 2017;9:391-401. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vianello 2016 {published data only}
- NCT01513980. Life-long monitoring of COPD in Veneto region [RENEWING HEALTH - large scale pilot in Veneto region: life-long monitoring in COPD]. clinicaltrials.gov/show/nct01513980 (first received 20 January 2012).
- Vianello A, Fusello M, Gubian L, Rinaldo C, Dario C, Concas A, et al. Home telemonitoring for patients with acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled trial. BMC Pulmonary Medicine 2016;16(1):157. [DOI: 10.1186/s12890-016-0321-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2018 {published data only}
- Walker PP, Pompilio PP, Zanaboni P, Bergmo TS, Prikk K, Malinovschi A, et al. Telemonitoring in chronic obstructive pulmonary disease (CHROMED): a randomized clinical trial. American Journal of Respiratory and Critical Care Medicine 2018;198(5):620-8. [DOI: 10.1164/rccm.201712-2404OC] [DOI] [PubMed] [Google Scholar]
Yan 2018 {published data only}
- Yan Y, Liu L, Zeng J, Zhang L. Evaluation and exploration on the effect of the management of chronic obstructive pulmonary disease in rural areas through an internet-based network consulting room. Medical Principles and Practice 2018;27(3):222-6. [DOI: 10.1159/000488591] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ACTRN12614000296639 {published data only}
- ACTRN12614000296639. Electronic snapshot for outpatient management of chronic obstructive pulmonary disease (COPD). www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000296639 (first received 16 March 2014).
Alonso 2004 {published data only}
- Alonso A. A new model for home care for COPD. Studies in Health Technology and Informatics 2004;103:368-73. [PubMed] [Google Scholar]
Bentley 2014 {published data only}
- Bentley CL, Mountain GA, Thompson J, Fitzsimmons DA, Lowrie K, Parker SG, et al. A pilot randomised controlled trial of a telehealth intervention in patients with chronic obstructive pulmonary disease: challenges of clinician-led data collection. Trials 2014;15(1):313. [DOI: 10.1186/1745-6215-15-313] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimmons DA, Thompson J, Hawley M, Mountain GA. Preventative tele-health supported services for early stage chronic obstructive pulmonary disease: a protocol for a pragmatic randomized controlled trial pilot. Trials 2011;12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernocchi 2016 {published data only}
- Bernocchi P, Scalvini S, Galli T, Paneroni M, Baratti D, Turla O, et al. A multidisciplinary telehealth program in patients with combined chronic obstructive pulmonary disease and chronic heart failure: study protocol for a randomized controlled trial. Trials 2016;17(1):462. [DOI: 10.1186/s13063-016-1584-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalvini S, Bernocchi P, Baratti D, Gatti T, Paneroni M, La Rovere MT, et al. Multidisciplinary telehealth program for patients affected by chronic heart failure and chronic obstructive pulmonary disease. European Journal of Heart Failure 2016;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bischoff 2012 {published data only}
- Bischoff E, Akkermans R, Bourbeau J, Vercoulen J, Weel C, Schermer T. Comprehensive self management and routine monitoring in chronic obstructive pulmonary disease patients in general practice: randomised controlled trial. BMJ 2012;345(7885):e7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chau 2012 {published data only}
- Chau JP-C, Lee DT-F, Yu DS-F, Chow AY-M, Yu W-C, Chair S-Y, et al. A feasibility study to investigate the accessibility and potential effectiveness of a telecare service for older people with chronic obstructive pulmonary disease. International Journal of Medical Informatics 2012;81(10):674-82. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cooper 2019 {published data only}
- Cooper CB, Sirichana W, Arnold MT, Neufeld EV, Taylor M, Wang X, et al. Remote patient monitoring for the detection of COPD exacerbations. International Journal of COPD 2020;15:2005-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CB, Sirichana W, Neufeld EV, Taylor M, Wang X, Dolezal BA. Statistical process control improves the feasibility of remote physiological monitoring in patients with COPD. International Journal of COPD 2019;14:2485-96. [DOI: 10.214/COPD.S207626] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cordova 2007 {published data only}
- Ciccolella DE, Cordova F, Grabianowski C, Gaughan J, Criner GJ. Effect of a telemedicine-based treatment program for acute COPD exacerbation (AECOPD) on quality of life (QOL) - Pennsylvania study of chronic obstructive pulmonary exacerbations (Pa-Scope) [Abstract]. In: American Thoracic Society International Conference; 2009 May 15-20 San Diego. A2930 [Poster #G27] edition. 2009.
- Cordova FC, Ciccolella D, Grabianowski C, Gaughan J, Brennan K, Goldstein F, et al. A telemedicine-based intervention reduces the frequency and severity of COPD exacerbation symptoms: a randomized, controlled trial. Telemedicine Journal and E-health 2016;22(2):114-22. [DOI: 10.1089/tmj.2015.0035] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova FC, Kerper MM, Grabianowski C, McClelland R, Gaughan J, Lando S, et al. Use of a telemedicine based treatment program prevents acute COPD exacerbations (AECOPD) - the Pennsylvania study of chronic obstructive pulmonary exacerbations (PA-SCOPE) [Abstract]. In: American Thoracic Society International Conference; 2007 May 18-23; San Francisco. 2007:A284 [Poster #410].
- Cordova FC. Impact of telemedicine based daily monitoring and early intervention on clinical symptoms in AECOPD - the Pennsylvania study of chronic obstructive pulmonary exacerbation [Abstract]. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. 2008:Poster #D43.
Dinesen 2012 {published data only}
- Dinesen B, Haesum LKE, Soerensen N, Nielsen C, Grann O, Hejlesen O, et al. Using preventive home monitoring to reduce hospital admission rates and reduce cost: a case study of telehealth among chronic obstructive pulmonary disease patients. Journal of Telemedicine and Telecare 2012;18(4):221-5. [DOI: 10.1258/jtt.2012.110704] [DOI] [PubMed] [Google Scholar]
Emme 2014 {published data only}
- Emme C, Mortensen EL, Rydahl-Hansen S, Ostergaard B, Jakobsen AS, Schou L, et al. The impact of virtual admission on self-efficacy in patients with chronic obstructive pulmonary disease - a randomised clinical trial. Journal of Clinical Nursing 2014;23(21):3124-37. [DOI: 10.1111/jocn.12553] [DOI] [PubMed] [Google Scholar]
Finkelstein 2004 {published data only}
- Finkelstein SM, Speedie SM, Demiris G, Veen M, Lundgren JM, Potthoff S. Telehomecare: quality, perception, satisfaction. Telemedicine Journal and E-health 2004;10(2):122-8. [DOI] [PubMed] [Google Scholar]
- Finkelstein SM, Speedie SM, Potthoff S. Home telehealth improves clinical outcomes at lower cost for home healthcare. Telemedicine Journal and E-health 2006;12(2):128-36. [DOI] [PubMed] [Google Scholar]
Fors 2018 {published data only}
- Fors A, Blanck E, Ali L, Ekberg-Jansson A, Fu M, Lindström Kjellberg I, et al. Effects of a person-centred telephone support in patients with OCPD and/or CHF - a randomised controlled trial. PLOS Online 2018;13(8):e0203031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fors A, Blanck E, Ali L, Swedberg K, Ekman I. Person-centred telephone-support is effective in patients with chronic obstructive pulmonary disease and/or chronic heart failure: six-month follow-up of a randomized controlled trial. European Journal of Heart Failure 2018;20:Suppl S1. [Google Scholar]
- ISRCTN55562827. Care 4 Ourselves - person-centered support for people with chronic heart failure and / or obstructive pulmonary disease. [Care 4 Ourselves - Person-centered information and communication technology (ICT) support interventions in persons suffering from chronic heart failure and / or obstructive pulmonary disease.]. https://www.isrctn.com/ISRCTN55562827?q=&filters=conditionCategory:Circulatory%20System,recruitmentCountry:Sweden&sort=&offset=8&totalResults=58&page=1&pageSize=100&searchType=basic-search (first received 14 January 2015).
Gaeckle 2016 {published data only}
- Gaeckle N, Ciccolella D, Criner A, Criner G. Participation in a telemedicine program for chronic obstructive pulmonary disease improves daily symptoms. American Journal of Respiratory and Critical Care Medicine 2016;193:A1688. [Google Scholar]
Gellis 2014 {published data only}
- Gellis ZD, Kenaley BL, Have TT. Integrated telehealth care for chronic illness and depression in geriatric home care patients: the integrated telehealth education and activation of mood (I-TEAM) study. Journal of the American Geriatrics Society 2014;62(5):88995. [DOI: 10.1111/jags.12776] [DOI] [PubMed] [Google Scholar]
Grabenhorst 2013 {published data only}
- Grabenhorst M, Jehn M, Maldener N, Liebers U, Kohler F, Witt C. Telemedicine in patients with COPD: feasibility of regular exercise testing via remote patient monitoring. Pneumologie 2013;67:P377. [Google Scholar]
Henderson 2013 {published data only}
- Henderson C, Knapp M, Fernandez JL, Beecham J, Hirani SP, Cartwright M, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346(7902):f1035. [DOI: 10.1136/bmj.f1035] [DOI] [PubMed] [Google Scholar]
- Henderson C, Knapp M, Fernandez JL, Beecham J, Hirani SP, Cartwright M, et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 2013;346(7902):f2065 Correction. [DOI: doi: 10.1136/bmj.f2065] [DOI] [PubMed] [Google Scholar]
ISRCTN13081008 {published data only}
- ISRCTN13081008. InterSPACE: feasibility of an integrated telehealth and self-management programme for individuals hospitalised with an exacerbation of COPD. isrctn.com/ISRCTN13081008 (first received 17 November 2014).
ISRCTN34235668 {published data only}
- ISRCTN34235668. ADAPT (After DischArge Pulmonary Telehealth): home telemonitoring follow-up for chronic obstructive pulmonary disease (COPD) patients post hospital discharge [Does flexible home telemonitoring (Tm) after hospital discharges for chronic obstructive pulmonary disease patients reduce hospital re-admission? A clustered, interventional trial]. isrctn.com/ISRCTN34235668 (first received 1 October 2013).
ISRCTN34252610 {published data only}
- ISRCTN34252610. The eHealth Diary: digital pen telemonitoring of patients with advanced chronic obstructive pulmonary disease (COPD) and heart failure within specialised home care [Telemonitoring of patients with advanced COPD and heart failure within specialised home care - based on digital pen technology and a Health Diary form]. https://www.isrctn.com/ISRCTN34252610q=&filters=conditionCategory:Circulatory%20System,recruitmentCountry:Sweden,trialStatus:Ongoing&sort=&offset=6&totalResults=16&page=1&pageSize=10&searchType=basic-search (first received 31 October 2013).
ISRCTN41238563 {published data only}
- ISRCTN41238563. Effects of remote patient monitoring on chronic disease management [Randomized controlled trial of a mobile phone-based telemonitoring application for self-management and clinical decision support for patients with complex chronic conditions]. www.isrctn.com/ISRCTN41238563 (first received 5 August 2016).
Jehn 2013 {published data only}
- Jehn M, Donaldson G, Kiran B, Liebers U, Mueller K, Scherer D, et al. Telemonitoring reduces exacerbation of COPD in the context of climate change - a randomised controlled trial. Environmental Health 2013;12(99):1-8. [DOI: 10.1186/1476-069X-12-99] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehn M, Witt C. Telemedicine as a tool for assessing the influence of climate change in patients with chronic lung diseases. Pneumonologie 2014;11(3):204-13. [Google Scholar]
Johnston 2000 {published data only}
- Johnston B, Wheeler L, Deuser J, Sousa KH. Outcomes of the Kaiser Permanente tele-home health research project. Archives of Family Medicine 2000;9(1):40-5. [DOI] [PubMed] [Google Scholar]
Juan 2011 {published data only}
- Juan C, Sanjoaquin AC, Lopez M, Romero D, Pinilla R, Ochoa P. Presentation of a frail elderly telemonitoring service: lessons learned in the Barbarstro health care area. In: European Geriatric Medicine Conference (7th Congress of the EUGMS); 2011 September 28-30; Malaga. Vol. 2. 2011:S128.
Kamei 2011 {published data only}
- Kamei T, Yamamoto Y, Kajii F, Nakayama Y, Kamei N. Preventing acute respiratory exacerbation and readmission of chronic obstructive pulmonary disease (COPD) patients with home oxygen therapy: evaluation of home-monitoring based telenursing practice by a randomized-controlled trial. Journal of Japan Academy of Nursing Science 2011;31(2):24-33. [Google Scholar]
Kamei 2018 {published data only}
- Kamei T, Yamamoto Y, Kanamori T, Nakayama Y, Porter SE. Detection of early-stage changes in people with chronic diseases: a telehome monitoring-based telenursing feasibility study. Nursing and Health Sciences 2018;20(3):313-22. [DOI: 10.1111/nhs.12563] [DOI] [PubMed] [Google Scholar]
Kenealy 2015 {published data only}
- Kenealy TW, Parsons MJG, Rouse APB, Doughty RN, Sheridan NF, Hindmarsh JKH, et al. Telecare for diabetes, CHF or COPD: effect on quality of life, hospital use and costs. A randomised controlled trial and qualitative evaluation. PLOS One 2015;10(3):e0116188. [DOI: 10.1371/journal.pone.0116188] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2015 {published data only}
- Ko FWS, Cheung N-K, Rainer T, Lum CCM, Hui D. Integrated care programme for patients with chronic obstructive pulmonary disease (COPD) - a randomized controlled trial. Respirology 2015;20(Suppl 3):38. [Google Scholar]
- Ko FWS, Cheung N-K, Rainer T, Lum CCM, Hui DSC. Comprehensive care programme for patients with chronic obstructive pulmonary disease (COPD) - a randomized controlled trial (RCT). European Respiratory Journal 2015;46:OA272. [Google Scholar]
- Ko FWS, Cheung N-K, Rainer TH, Lum C, Wong I, Hui DSC. Comprehensive care programme for patients with chronic obstructive pulmonary disease (COPD) - a randomized controlled trial (RCT). Thorax 2015;72:122-8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Lavensen 2012_2016 {published data only}
- Lavensen M, Ladelund S, Frederiksen AJ, Lindhardt BO, Overgaard D. Nurse-initiated telephone follow-up on patients with chronic obstructive pulmonary disease improves patient empowerment, but cannot prevent readmissions. Danish Medical Journal 2016;63(10):A5276. [PubMed] [Google Scholar]
- Lavensen M, Overgaard R, Mazurek S, Just A, Overgaard D. Do telephone interventions of patients with COPD prevent readmission? [Abstract]. European Respiratory Journal 2012;40:220s [P1203]. [Google Scholar]
Levine 2018 {published data only}
- Levine DM, Burke KP, Paz M, Schnipper JL. Predictors and reasons why patients decline to participate in high tech and novel sites of care: a home hospital experience. Journal of General Internal Medicine 2018;33(2):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DM, Ouchi K, Blanchfield B, Diamond K, Licurse A, Pu CT, et al. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. Journal of General Internal Medicine 2018;33(5):729-36. [DOI: 10.1007/s11606-018-4307-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DM, Ouchi K, Blanchfield BB, Pu CT, Schnipper JL. Hospital-level care at home for acutely ill adults: a pilot randomized controlled trial. Journal of General Internal Medicine 2018;32(2):S209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mair 2002 {published data only}
- Mair F, Boland A, Angus R, Haycox A, May C, Hibbert D, et al. A randomised controlled trial of home telecare. Journal of Telemedicine and Telecare 2002;8(Suppl 2):58-60. [DOI] [PubMed] [Google Scholar]
- Mair FS, Goldstein P, May C, Angus R, Shiels C, Hibbert D, et al. Patient and provider perspectives on home telecare: preliminary results from a randomised controlled trial. Journal of Telemedicine and Telecare 2005;11(Suppl 1):95-7. [DOI] [PubMed] [Google Scholar]
Mudiyanselage 2018 {published data only}
- Mudiyanselage SB, Stevens J, Watts JJ, Toscano J, Kotowicz MA, Steinfort CL, et al. Personalised telehealth intervention for chronic disease management: a pilot randomised controlled trial. Journal of Telemedicine and Telecare 2019;25(6):343-52. [DOI: 10.1177/1357633X18775850] [DOI] [PubMed] [Google Scholar]
NCT00916799 {published data only}
- NCT00916799. VISN 23 - chronic obstructive pulmonary disease (COPD) case management using home telehealth equipment. clinicaltrials.gov/ct2/show/NCT00916799 (first received 10 June 2009).
NCT01044927 {published data only}
- NCT01044927. Advanced eHealth for chronic obstructive pulmonary disease (COPD) in Colorado [Phase 3 clinical trial studying the efficacy of a proactive integrated approach to care in patients with advanced COPD]. clinicaltrials.gov/show/nct01044927 (first received 8 January 2010).
NCT01495780 {published data only}
- NCT01495780. eRT remote health monitoring [Feasibility and cost effectiveness of physiological monitoring at home in COPD patients]. clinicaltrials.gov/show/nct01495780 (first received 20 December 2011).
NCT01644045 {published data only}
- NCT01644045. Prehospital emergency care of obstructive respiratory emergencies with the use of teleconsultation. clinicaltrials.gov/show/nct01644045 (first received 18 July 2012).
NCT01892566 {published data only}
- NCT01892566. Using mobile health to respond early to acute exacerbations of COPD in HIV (mReach). clinicaltrials.gov/show/nct01892566 (first received 4 July 2013).
NCT02085187 {published data only}
- NCT02085187. Early telemedicine training in patients with COPD [Early telemedicine training and counselling after hospitalization in patients with severe chronic obstructive pulmonary disease: a feasibility study]. clinicaltrials.gov/show/nct02085187 (first received 12 March 2014).
NCT02269618 {published data only}
- NCT02269618. Telehealth program in chronic patients [Innovative multidisciplinary telehealth program in COPD and CHF patients: a randomized control trial]. clinicaltrials.gov/show/nct02269618 (first received 21 October 2014).
NCT02528370 {published data only}
- NCT02528370. Evaluation of the effectiveness of a telemonitoring program in a cohort of COPD patients with frequent readmissions. clinicaltrials.gov/show/nct02528370 (first received 19 August 2015).
NCT02706600 {published data only}
- NCT02706600. Trial of e-health platform supported care vs usual care after exacerbation of COPD (RESCUE) [RESCUE-COPD 1 randomised trial of e-health platform supported care vs usual care after exacerbation of COPD]. clinicaltrials.gov/show/nct02706600 (first received 11 March 2016).
NCT02791451 {published data only}
- NCT02791451. Predicting exacerbation of COPD with wireless telemonitoring [Open-label prospective study of predicting exacerbation of COPD with wireless telemonitoring of respiratory rate, heart rate and sleep]. clinicaltrials.gov/show/nct02791451 (first received 6 June 2016).
NCT02803489 {published data only}
- NCT02803489. Evaluation of the performance of an e-health system [Evaluation of the performance of an e-health system comprising tele-monitoring /tele-notification and tele-coaching in ambulatory multi-morbid adult patients]. clinicaltrials.gov/show/nct02803489 (first received 17 June 2016).
NCT03127852 {published data only}
- NCT03127852. Effects of remote patient monitoring on chronic disease management [Randomized controlled trial of a mobile phone-based telemonitoring application for self-management and clinical decision support for patients with complex chronic conditions]. clinicaltrials.gov/show/nct03127852 (first received 25 April 2017).
NCT03129477 {published data only}
- NCT03129477. TELE-monitoring in chronic obstructive pulmonary disease (TELECOPD). clinicaltrials.gov/show/nct03129477 (first received 26 April 2017).
NCT03131622 {published data only}
- NCT03131622. Impact of Ibis on patients with advanced COPD [Impact of Ibis, a digital health solution for patient activation and early intervention, on acute care utilization by patients with advanced COPD]. clinicaltrials.gov/show/nct03131622 ( first received 27 April 2017).
NCT03353064 {published data only}
- NCT03353064. Telemedicine for improving outcome in inner city patient population with hypercapnic respiratory failure (ETOUCH) [Telemedicine as a proposed solution towards efficiency of healthcare delivery for Einstein pulmonary patients on PAP/NIPPV for hypercapnia]. clinicaltrials.gov/show/nct03353064 (first received 27 November 2017).
NCT03640260 {published data only}
- NCT03640260. Respiratory regulation with biofeedback in COPD (RRBCOPD) [Effects of respiratory regulation with biofeedback in patients with chronic obstructive pulmonary disease]. clinicaltrials.gov/show/nct03640260 (first received 21 August 2018).
NCT03739957 {published data only}
- NCT03739957. Telemonitoring system for early diagnosis of COPD exacerbations. clinicaltrials.gov/show/nct03739957 (first received 14 November 2018).
NCT03837847 {published data only}
- NCT03837847. Mechanistic effects of health coaching to reduce COPD hospitalisations. https://clinicaltrials.gov/show/nct03837847 (first received 12 February 2019).
NCT04108143 {published data only}
- NCT04108143. Use of MonitorMe in COPD [Use of MonitorMe in COPD: a mixed-methods feasibility study]. ClinicalTrials.gov/show/NCT04108143 (27 September 2019).
Nohra 2020 {published data only}
- NCT04196699. Evaluating the feasibility, acceptability and pre testing the impact of a self-management and telemonitoring program for chronic obstructive pulmonary disease patients in Lebanon: protocol for a feasibility study. ClinicalTrials.gov/show/NCT04196699 (first received 12 December 2019).
- Nohra RG, Sacre H, Pascal S, Rothan-Tondeur M. Evaluating the feasibility, acceptability and pre testing the impact of a self-management and telemonitoring program for chronic obstructive pulmonary disease patients in Lebanon: protocol for a feasibility study. Medicine 2020;99(6):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norgaard 2014 {published data only}
- Norgaard B, Ersgard K, Hansen MS. Horsens ahead. European Journal of Cardiovascular Nursing 2014;13:S85 [P214]. [Google Scholar]
Paquin 2014 {published data only}
- Paquin S, Landry L, Nault D, Dagenais J, Lefrancois E, St-Jules D, et al. Telehome care for patients with chronic pulmonary disease: the experience of a Canadian second line respiratory speciality care service (Abstract). American Journal of Respiratory and Critical Care Medicine 2014;189:A1395. [Google Scholar]
Pare 2006 {published data only}
- Pare G, Poba-Nzaou P, Sicottee C, Beaupre A, Lefrancois E, Nault D, et al. Comparing the costs of home telemonitoring and usual care of chronic obstructive pulmonary disease patients: a randomized controlled trial. European Research in Telemedicine 2013;2(2):35-47. [DOI: ] [Google Scholar]
- Pare G, Sicottee C, St-Jules D, Gauthier R. Cost-minimisation analysis of a telehome care program for patients with chronic obstructive pulmonary disease. Telemedicine Journal and E-health 2006;12(2):114-21. [DOI] [PubMed] [Google Scholar]
Pinnock 2012 {published data only}
- Pinnock H, Fairbrother P, McCloughlan L, Todd A, McKinstry B. Perspectives of patient and professional participants on telehealthcare and the impact on self-management: qualitative study nested in the TELESCOT COPD trial [Abstract]. Thorax 2012;67(Suppl 2):A145 [P186]. [DOI: 10.1136/thoraxjnl-2012-202678.247] [DOI] [Google Scholar]
Reinius 2013 {published data only}
- Reinius P, Johansson M, Fjellner A, Werr J, Ohlen G, Edgren G. A telephone-based case-management intervention reduces healthcare utilization for frequent emergency department visitors. European Journal of Emergency Medicine 2013;20(5):327-34. [DOI] [PubMed] [Google Scholar]
Shah 2017 {published data only}
- Shah Sa, Barber V, Kim B, Eysenbach G. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. Journal of Medical Internet Research 2017;19(3):e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sirichana 2013 {published data only}
- Sirichana W, Moore-Gillon CE, Patel MH, Abrazado M, Tseng CH, Taylor M, et al. Feasibility of remote monitoring of physiology and symptoms in COPD patients. American Journal of Respiratory and Critical Care Medicine 2013;187:A4392. [Google Scholar]
Sorknaes 2011 {published data only}
- NCT00918905. Nurse teleconsultations with discharged COPD patients reduce the numbers of readmissions [KOL-Fyn - an explorative study with telemedicine in COPD patients at home]. clinicaltrials.gov/ct2/show/NCT00918905 (first received 11 June 2009).
- Sorknaes AD, Madsen H, Hallas J, Jest P, Nord-Hansen M. Nurse teleconsultations with discharged COPD patients reduce early readmissions - an international study. Clinical Respiratory Journal 2011;5(1):26-34. [DOI: 10.1111/j.1752-699X.2010.00187.x] [DOI] [PubMed] [Google Scholar]
Sridhar 2008 {published data only}
- Sridhar M, Taylor R, Dawson S, Roberts NJ, Patridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63(3):194-200. [DOI: 10.1136/thx.2007.077578] [DOI] [PubMed] [Google Scholar]
Tong 2012 {published data only}
- Tong C, Hart D, Corna N, Forbes-Faulkner L, Goodman M, Masson S, et al. Application of self-management systems evaluation trial (asset) for COPD patients in counties Manukau (funded by the primary health care innovations fund). Respirology 2012;17:87. [Google Scholar]
Troosters 2003 {published data only}
- Troosters T, Celis G, Deprez S, Spruit MA, Gosselink R, Decramer M. Telephone supported discharge reduces readmission after acute exacerbations in COPD [abstract]. In: American Thoracic Society 99th International Conference; 2003 May 16-21; Seattle. Vol. A108. 2003:Poster C72.
Vega 2008 {published data only}
- Vega Me, Ciccolella D, Cordova FC, Gaughan J, Grabianowski C, Criner GJ, et al. Telemedicine directed COPD outpatient management reduces AECOPD hospitalisation duration. In: American Thoracic Society International Conference; 2008 May 16-21; Toronto. 2008:A138 [#D41].
Vitacca 2008 {published data only}
- NCT00563745. Telemedicine for patients with chronic respiratory insufficiency [Randomised trial on telemedicine to save health care requests for patients with severe chronic respiratory failure]. clinicaltrials.gov/show/nct00563745 (first received 27 November 2007).
- Vitacca M, Bianchi L, Guerra A, Fracchia C, Spanevello A, Balbi B, et al. Tele-assistance in chronic respiratory failure patients: a randomised clinical trial. European Respiratory Journal 2009;33:411-8. [DOI] [PubMed] [Google Scholar]
- Vitacca M, Blanchi L, Guerra C, Fracchia C, Balbi B, Scalvini S. Tele-assistance in chronic respiratory failure patients: a randomised clinical trial [Abstract]. In: European Respiratory Society 18th Annual Congress; 2008 Oct 3-7; Berlin. 2008:E2802. [DOI] [PubMed]
Walters 2013 {published data only}
- Walters J, Cameron-Tucker H, Wills K, Schuz N, Scott J, Robinson A, et al. Effects of telephone health mentoring in community-recruited chronic obstructive pulmonary disease on self-management capacity, quality of life and psychological morbidity: a randomised controlled trial. BMJ Open 2013;3(9):e003097. [DOI: 10.1136/bmjopen-2013-003097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitten 2007 {published data only}
- Whitten P, Mickus M. Home telecare for COPD/CHF patients: outcomes and perceptions. Journal of Telemedicine and Telecare 2007;13(2):69-73. [DOI] [PubMed] [Google Scholar]
Wolpin 2011 {published data only}
- Wolpin S, Nguyen HQ, Donesky-Cuenco D, Carrieri-Kohlman V, Doorenbos A. Effects of automated prompts for logging symptom and exercise data on mobile devices in patients with chronic obstructive pulmonary disease. Computers, Informatics, Nursing 2011;29(2):75-80. [DOI] [PubMed] [Google Scholar]
Wong 2005 {published data only}
- Wong KW, Wong FKY, Chan MF. Effects of nurse-initiated telephone follow-up on self-efficacy among patients with chronic obstructive pulmonary disease. Journal of Advanced Nursing 2005;49(2):210-22. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cartwright 2013 {published data only}
- Bardsley M, Steventon A, Doll H. Impact of telehealth on general practice contacts: findings from the whole systems demonstrator cluster randomised trial. BMC Health Services Research 2013;13:395. [CENTRAL: http://www.biomedcentral.com/1472-6963/13/395] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower P, Cartwright M, Hirani SP, Barlow J, Hendy J, Knapp M, et al. A comprehensive evaluation of the impact of telemonitoring in patients with long-term conditions and social care needs: protocol for the whole systems demonstrator cluster randomised trial. BMC Health Services Research 2011;11:184. [CENTRAL: http://www.biomedcentral.com/1472-6963/11/184] [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright M, Hirani SP, Rixon L, Beynon M, Doll H, Bower P, et al. Effect of telehealth on quality of life and psychological outcomes over 12 months (Whole Systems Demonstrator telehealth questionnaire study): nested study of patient reported outcomes in a pragmatic, cluster randomised controlled trial. BMJ 2013;346(7897):f365. [DOI: 10.1136/bmj.f653] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon A, Bardsley M, Billings J, Dixon J, Doll H, Hirani S, et al. Effect of telehealth on use of secondary care and mortality: findings from the Whole System Demonstrator cluster randomised trial. BMJ 2012;344:e3874. [DOI: 10.1136/bmj.e3874] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatwin 2016 {published data only}
- Chatwin M, Hawkins G, Panicchia L, Woods A, Hanak A, Lucas R, et al. Randomised crossover trial of telemonitoring in chronic respiratory patients (TeleCRAFT trial). Thorax 2016;71(4):305-11. [DOI: 10.113/thoraxjnl-2015-207045] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Lesende 2013 {published data only}
- Entesari-Tatafi D, Stevens J, Hayles R, Bell J, Steinfort C. Telemedicine to deliver personalised health care in chronic obstructive pulmonary disease may reduce hospital admissions. Respirology 2017;22:128. [Google Scholar]
- Martin-Lesende I, Orruno E, Bilbao A, Vergara I, Cairo C, Bayon JC, et al. Impact of telemonitoring home care patients with heart failure or chronic lung disease from primary care on healthcare resource use (the TELBIL study randomised controlled trial). BMC Health Services Research 2013;13:118. [http://www.biomedcentral.com/1472-6963/13/118] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00752531 {published data only}
- NCT00752531. Effectiveness of home automated tele management in chronic obstructive pulmonary disorder. https://clinicaltrials.gov/show/nct00752531 (first received 15 September 2008).
NCT00893685 {published data only}
- NCT00893685. Randomised controlled trial of home telemonitoring for elderly people (DREAMING) [Elderly friendly alarm handling and monitoring (DREAMING)]. clinicaltrials.gov/show/nct00893685 (first received 6 May 2009).
NCT01342263 {published data only}
- NCT01342263. Trial of an Internet-based platform for managing chronic diseases at a distance (iCDM) [Utilization of an interactive internet-based platform for managing chronic diseases at a distance]. clinicaltrials.gov/show/nct01342263 (first received 27 April 2011).
NCT01489241 {published data only}
- NCT01489241. Short-term telehealth follow up after hospital discharge for chronic obstructive pulmonary disease exacerbation (RHCluster4GR) [Renewing health RCT in central Greece for the evaluation of short-term telehealth follow up after hospital discharge for COPD exacerbation]. clinicaltrials.gov/show/nct01489241 (first received 9 December 2011).
NCT01512992 {published data only}
- NCT01512992. Home telehealth follow-up after hospital discharge for chronic obstructive pulmonary disease (COPD) patients [Renewing health RCT in Catalonia for the evaluation of home telehealth follow-up after hospital discharge for COPD patients]. clinicaltrials.gov/show/nct01512992 (first received 20 January 2012).
NCT01560741 {published data only}
- NCT01560741. Telemedicine and ventilator titration in chronic respiratory patients initiating non-invasive ventilation (TeleMotiNIV) [Randomized trial comparing telemedicine monitoring and titration in patients initiating non-invasive ventilation with usual care (TELEMOTINIV study)]. clinicaltrials.gov/show/nct01560741 (first received 22 March 2012).
NCT01580072 {published data only}
- NCT01580072. Telemonitoring of patients with COPD in Carinthia (RenewingHealth) [REgions of Europe workiNg toGether for HEALTH (Renewing Health)]. clinicaltrials.gov/show/nct01580072 (first received 18 April 2012).
NCT01744028 {published data only}
- NCT01744028. Compare the effect of a remote monitoring system using the EXACT tool to reduce hospitalizations due to chronic obstructive pulmonary disease (COPD) exacerbations. EXACT = Exacerbations of Chronic Pulmonary Disease Tool. (PREMIERE) [A 52-week multi-center randomized trial to evaluate remote patient monitoring using the EXACT patient-reported outcome tool on reduction of hospitalizations from exacerbations in patients with chronic obstructive pulmonary disease as compared to those managed by usual care]. clinicaltrials.gov/show/nct01744028 (first received 6 December 2012).
NCT01951261 {published data only}
- NCT01951261. Early assisted discharge for COPD exacerbations with telemonitoring [Early assisted discharge with generic community nursing and pulmonary physicians vs telemonitoring at home for chronic obstructive pulmonary disease exacerbations]. clinicaltrials.gov/show/nct01951261 (first received 26 September 2013).
NCT02180919 {published data only}
- NCT02180919. Implementation of telemonitoring in chronic heart or lung failure (TELECRAFT) [Implementation of telemonitoring in the management of acute exacerbations of chronic heart failure and respiratory failure/chronic obstructive pulmonary disease]. clinicaltrials.gov/show/nct02180919 (first received 3 July 2014).
NCT02615795 {published data only}
- NCT02615795. Telehealth monitoring service for patients with chronic obstructive pulmonary disease [Tele health monitoring service for patients with chronic obstructive pulmonary disease. A clinical, randomized, controlled study for evaluation of clinical and economic consequences of monitoring service]. clinicaltrials.gov/show/nct02615795 (first received 26 November 2015).
NCT02901535 {published data only}
- NCT02901535. Tele-spirometry in primary care - randomized clinical trial cluster: telemedicine in chronic obstructive pulmonary disease (RESPIRANET-C) [Tele-spirometry in primary care - randomized clinical trial cluster: the effectiveness of multifaceted intervention in symptoms patients with respiratory illness]. clinicaltrials.gov/show/nct02901535 (first received 15 September 2016).
NCT03183817 {published data only}
- NCT03183817. Person-centred care at distance (PROTECT) [Person-centred care at distance for persons with chronic heart failure (CHF) and/or chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/nct03183817 (first received 12 June 2017).
NCT03505138 {published data only}
- NCT03505138. Impact of telemedicine in the rate of readmission for COPD. Project CRONEX 3.0 [Impact of telemedicine in the rate of readmission for COPD and cost-effectiveness analysis (e-pneumo): project CRONEX 3.0]. clinicaltrials.gov/show/nct03505138 (first received 23 April 2018).
Ramos 2018 {published data only}
- Ramos AO, Tinedo JRT, Gonzalez VG, Castillejo EO, Espinal JCV, Maestu LP, et al. Chronic obstructive pulmonary disease patients: pilot project of 90-day monitoring. European Respiratory Journal 2018;52:PA725. [DOI: 10.1183/13993003.congress-2018.PA725] [DOI] [Google Scholar]
Tivota 2015 {published data only}
- Tivota E, Salvesen O, Bentsen SB, Sunde S, Steinshamn S, Henriksen AH. Does an integrated care intervention for COPD patients have long-term effects on quality of life and patient activation? A prospective, open, controlled single-centre intervention study. PLOS One 2017;12(1):e0167887. [DOI: 10.1371/journal.pone.0167887] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivota E, Steinshamn S, Indredavik B, Henriksen AH. Long term effects of an integrated care intervention on hospital utilization in patients with severe COPD: a single centre controlled study. Respiratory Research 2015;16(1):8. [DOI: 10.1186/s12931-015-0170-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venter 2012 {published data only}
- Venter A, Burns R, Hefford M, Ehrenberg N. Results of a telehealth-enabled chronic care management service to support people with long-term conditions at home. Journal of Telemedicine and Telecare 2012;18(3):172-5. [DOI: 10.1258/jtt.2012.SFR112] [DOI] [PubMed] [Google Scholar]
Walker 2017 {published data only}
- Walker KA, Graham N, Brennan D, Groninger H, Holder RM, Malotte K, et al. PATCH2 program: the creation of a virtual palliative care clinic. Journal of Palliative Medicine 2017;20:4. [Google Scholar]
References to ongoing studies
NCT02756533 {published data only}
- NCT02756533. Impact of a telemonitoring program on the rate of hospitalizations for worsening of cardio-respiratory symptoms in COPD patients treated at home by long-term non-invasive ventilation (NIV) (EXA-VNI2) [Impact of a telemonitoring program on the rate of hospitalizations for worsening of cardio-respiratory symptoms in COPD patients treated at home by long-term non-invasive ventilation (NIV): a randomized controlled trial]. clinicaltrials.gov/show/nct02756533 (first received 29 April 2016).
NCT03396172 {published data only}
- NCT03396172. FreeDom: innovative strategy for the management of COPD exacerbations [FreeDom: innovative strategy for the management of COPD exacerbations combining early hospitalisation discharge, automated oxygen weaning at home, telemedicine and tele-rehabilitation]. clinicaltrials.gov/show/nct03396172 (first received 10 January 2018).
NCT03558763 {published data only}
- NCT03558763. Remote monitoring of patients with COPD [Remote MONITORing of patients with chronic obstructive pulmonary disease using a tablet system. A randomized cross over pilot study of feasibility evaluation and quality of life measurements]. clinicaltrials.gov/show/nct03558763 (first received 15 June 2018).
NCT04080570 {published data only}
- NCT04080570. Remote physician care for home hospital patients [Remote physician care for home hospital patients: a randomized controlled trial]. clinicaltrials.gov/ct2/show/NCT04080570?term=nct04080570&draw=2&rank=1 (first received 6 September 2019).
Rassouli 2018 {published data only}
- Rassouli F, Baty F, Stolz D, Kohler M, Thurnheer R, Brack T, et al. Telehealth care vs. standard care in COPD. Respiration 2018;95(6):208. [https://erj.ersjournals.com/content/52/suppl_62/PA699] [Google Scholar]
- Rassouli F. Telehealth vs. standard care in COPD - an international randomised controlled trial. Project ID: EKSG 15/184, BASEC Nr. 2015-00065. https://www.researchgate.net/publication/326560796_Title_Telehealthcare_vs_standard_care_in_COPD-an_international_randomised_controlled_trial (accessed prior to 16 March 2021).
Additional references
AMD Global Telemedicine 2015
- AMD Global Telemedicine. Telemedicine technologies: real-time versus store and forward. www.amdtelemedicine.com/blog/article/telemedicine-technologies-real-time-versus-store-and-forward (accessed 23 May 2018).
Ancochea 2018
- Ancochea J, García-Río F, Vázquez-Espinosa E, Hernando-Sanz A, López-Yepes L, Galera-Martínez R, et al. Efficacy and costs of telehealth for the management of COPD: the PROMETE II trial. European Respiratory Journal 2018;51:1800354. [DOI] [PubMed] [Google Scholar]
Andrianopoulos 2017
- Andrianopoulos V, Gloeckl R, Vogiatzis I, Kenn K. Cognitive impairment in COPD: should cognitive evaluation be part of respiratory assessment? Breathe 2017;13:e1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anecchino 2007
- Anecchino C, Rossi E, Fanizza C, De Rosa M, Tognoni G, Romero M, et al. Prevalence of chronic obstructive pulmonary disease and pattern of co morbidities in a general population. International Journal of Chronic Obstructive Pulmonary Disease 2007;2(4):567-74. [PMC free article] [PubMed] [Google Scholar]
Annandale 2011
- Annandale J, Lewis KE. Can telehealth help patients with COPD? Nursing Times 2011;107(15-16):12-4. [PubMed] [Google Scholar]
ATS 2014
- American Thoracic Society. The global burden of lung disease, 2014. foundation.thoracic.org/news/global-burden.php (accessed 23 September 2018).
BLF 2018a
- British Lung Foundation. COPD – how can I manage my COPD better? www.blf.org.uk/support-for-you/copd/treatment/managing-my-copd (accessed 24 September 2018).
BLF 2018b
- British Lung Foundation. Lung disease in the UK – big picture statistics. statistics.blf.org.uk/lung-disease-uk-big-picture#hospital-admissions-uk (accessed 21 June 2018).
Breen 2015
- Breen S, Ritchie D, Schofield P, Hsueh YS, Gough K, Santamaria N. The Patient Remote Intervention and Symptom Management System (PRISMS) – a telehealth-mediated intervention enabling real-time monitoring of chemotherapy side-effects in patients with haematological malignancies: study protocol for a randomised controlled trial. Trials 2015;16:472. [DOI: 10.1186/s13063-015-0970-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bryant 2019
- Bryant MS, Bandi VD, Nguyen C, Lan C, Henson HK, Sharafkhaneh A. Telehealth pulmonary rehabilitation for patients with severe chronic obstructive pulmonary disease. Federal Practitioner 2019;36(9):430-5. [PMC free article] [PubMed] [Google Scholar]
Farquhar 2018
- Farquhar M. Assessing carer needs in chronic obstructive pulmonary disease. Chronic Respiratory Disease 2018;15(1):26-35. [DOI: 10.1177/1479972317719086] [DOI] [PMC free article] [PubMed] [Google Scholar]
GBD 2015
- Global Burden of Disease 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with a disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2021
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD 2021. goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (accessed prior to 16 March 2021).
GOLD 2021a
- Global Initiative for chronic obstructive lung Disease (GOLD). Pocket guide to COPD diagnosis, management, and prevention (a guide for health care professionals), 2021. goldcopd.org/wp-content/uploads/2020/12/GOLD-2021-POCKET-GUIDE-v2.0-14Dec20_WMV.pdf (accessed prior to 16 March 2021).
Gorst 2014
- Gorst SL, Armitage CJ, Brownsell S, Hawley MS. Home telehealth uptake and continued use among heart failure and chronic obstructive pulmonary disease patients: a systematic review. Annals of Behavioural Medicine 2014;48(3):323-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) (accessed prior to 21 June 2018) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime) (accessed prior to 21 June 2018), 2015. Available at gradepro.org.
Hernandez 2014
- Hernandez C, Mallow J, Narsavage GL. Delivering telemedicine interventions in chronic respiratory disease. Breathe 2014;10(3):198-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester, UK: John Wiley & Sons, 2019. [Google Scholar]
Hillas 2015
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing co morbidities in COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:95-109. [DOI: 10.2147/COPD.S54473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoass 2016
- Hoass H, Andreassen HK, Lien LA, Hjalmarsen A, Zanaboni P. Adherence and factors affecting satisfaction in long-term tele rehabilitation for patients with chronic obstructive pulmonary disease: a mixed methods study. BMC Medical Informatics and Decision Making 2016;16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janjua 2021
- Janjua S, Banchoff E, Fletcher J, Threapleton CJD, Prigmore S, Disler RT. Digital interventions for the management of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No: CD013246. [DOI: 10.1002/14651858.CD013246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2:75-9. [DOI] [PubMed] [Google Scholar]
Kon 2014
- Kon SSc, Canavan JL, Jones SE, Nolan CM. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respiratory Medicine 2014;2(3):P135-203. [DOI] [PubMed] [Google Scholar]
Lenferink 2017
- Lenferink A, Brusse-Keiser M, Valk PDLM, Frith PA, Zwerink M, Monninkhof EM, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD011682. [DOI: 10.1002/14651858.CD011682.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez‐Campos 2016
- Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016;21:14-23. [DOI: 10.1111/resp.12660] [DOI] [PubMed] [Google Scholar]
Luhr 2018
- Luhr K, Holmefur M, Theander K, Eldh AC. Patient participation during and after self-management programme in primary healthcare – the experience of patients with chronic obstructive pulmonary disease or chronic heart failure. Patient Education and Counselling 2018;101:1137-42. [DOI: 10.1016/j.pec.2017.12.020] [DOI] [PubMed] [Google Scholar]
Lundell 2015
- Lundell S, Holmner Å, Rehn B, Nyberg A, Wadell K. Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnoea. Respiratory Medicine 2015;109:11-26. [DOI: 10.1016/j.rmed.2014.10.008] [DOI] [PubMed] [Google Scholar]
Marengoni 2018
- Marengoni A, Vetrano DL, Manes-Gravina E, Bernabei R, Onder G, Palmer K. The relationship between COPD and frailty: a systematic review and meta-analysis of observational studies. Chest 2018;154(1):21-40. [DOI: 10.1016/j.chest.2018.02.014] [DOI] [PubMed] [Google Scholar]
McLean 2011
- McLean S, Nurmatov U, Liu JL, Pagliari C, Car J, Sheikh A. Telehealthcare for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 7. Art. No: CD007718. [DOI: 10.1002/14651858.CD007718.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 2012
- McLean S, Nurmatov U, Lui JLY, Pagliari C, Car J and Sheikh A. Telehealthcare for chronic obstructive pulmonary disease: Cochrane review and meta-analysis. British Journal of General Practice 2012;62(604):e739-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. www.nice.org.uk/guidance/ng115/evidence/c-selfmanagement-interventions-education-and-telehealth-monitoring-pdf-6602768752 (accessed prior to 16 March 2021). [PubMed]
Quaderi 2018
- Quaderi SA, Hurst JR. The unmet global burden of COPD. Global Health, Epidemiology and Genomics 2018;3:e4. [DOI: 10.1017/gheg.2018.1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2003
- Schünemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Austin P, Guyatt GH. A comparison of the original chronic respiratory questionnaire with a standardised version. Chest 2003;124(4):1421-9. [DOI] [PubMed] [Google Scholar]
Sjoding 2020
- Sjoding MW, Dickson RP, Iwashyna TJ, Gay SE, Valley TS. Racial bias in pulse oximetry measurement. New England Journal of Medicine 2020;383(25):2477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soriano 2020
- Soriano JB, Kendrick PJ, Paulson KR, Gupta V, Abrams EM, Adedoyin RF, et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Respiratory Medicine 2020;8(6):585-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomasic 2018
- Tomasic I, Tomasic N, Trobec R, Krpan M, Kevala T. Continuous remote monitoring of COPD patients - justification and explanation of the requirements and a survey of the available technologies. Medical and Biological Engineering and Computing 2018;56:547-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Udsen 2017a
- Udsen FW, Lilholt PH, Hejlesen O, Ehlers L. Cost-effectiveness of telehealthcare to patients with chronic obstructive pulmonary disease: results from the Danish 'Telecare north' cluster randomised trial. BMJ Open 2017;7:e014616. [DOI: 10.1136/bmjopen-2016-014616] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanfleteren 2013
- Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, Empel VP, Bruijnzeel PL, et al. Clusters of co morbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2013;187(7):728-35. [DOI: 10.1164/rccm.201209-1665OC] [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. Telemedicine: opportunities and developments in member states: report on the Second Global Survey on eHealth, 2010. www.who.int/goe/publications/goe_telemedicine_2010.pdf (accessed prior to 21 June 2018).
WHO 2018
- World Health Organization. Causes of COPD, 2018. www.who.int/respiratory/copd/causes/en/ (accessed prior to 13 November 2018).


