Abstract
Background
Hypertension is a major public health problem that increases the risk of cardiovascular and kidney diseases. Several studies have shown an inverse association between calcium intake and blood pressure, as small reductions in blood pressure have been shown to produce rapid reductions in vascular disease risk even in individuals with normal blood pressure ranges. This is the first update of the review to evaluate the effect of calcium supplementation in normotensive individuals as a preventive health measure.
Objectives
To assess the efficacy and safety of calcium supplementation versus placebo or control for reducing blood pressure in normotensive people and for the prevention of primary hypertension.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials up to September 2020: the Cochrane Hypertension Specialised Register, CENTRAL (2020, Issue 9), Ovid MEDLINE, Ovid Embase, the WHO International Clinical Trials Registry Platform, and the US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov. We also contacted authors of relevant papers regarding further published and unpublished work. The searches had no language restrictions.
Selection criteria
We selected trials that randomised normotensive people to dietary calcium interventions such as supplementation or food fortification versus placebo or control. We excluded quasi‐random designs. The primary outcomes were hypertension (defined as blood pressure ≥ 140/90 mmHg) and blood pressure measures.
Data collection and analysis
Two review authors independently selected trials for inclusion, abstracted the data and assessed the risks of bias. We used the GRADE approach to assess the certainty of evidence.
Main results
The 2020 updated search identified four new trials. We included a total of 20 trials with 3512 participants, however we only included 18 for the meta‐analysis with 3140 participants. None of the studies reported hypertension as a dichotomous outcome. The effect on systolic and diastolic blood pressure was: mean difference (MD) ‐1.37 mmHg, 95% confidence interval (CI) ‐2.08, ‐0.66; 3140 participants; 18 studies; I2 = 0%, high‐certainty evidence; and MD ‐1.45, 95% CI ‐2.23, ‐0.67; 3039 participants; 17 studies; I2 = 45%, high‐certainty evidence, respectively. The effect on systolic and diastolic blood pressure for those younger than 35 years was: MD ‐1.86, 95% CI ‐3.45, ‐0.27; 452 participants; eight studies; I2 = 19%, moderate‐certainty evidence; MD ‐2.50, 95% CI ‐4.22, ‐0.79; 351 participants; seven studies ; I2 = 54%, moderate‐certainty evidence, respectively. The effect on systolic and diastolic blood pressure for those 35 years or older was: MD ‐0.97, 95% CI ‐1.83, ‐0.10; 2688 participants; 10 studies; I2 = 0%, high‐certainty evidence; MD ‐0.59, 95% CI ‐1.13, ‐0.06; 2688 participants; 10 studies; I2 = 0%, high‐certainty evidence, respectively. The effect on systolic and diastolic blood pressure for women was: MD ‐1.25, 95% CI ‐2.53, 0.03; 1915 participants; eight studies; I2 = 0%, high‐certainty evidence; MD ‐1.04, 95% CI ‐1.86, ‐0.22; 1915 participants; eight studies; I2 = 4%, high‐certainty evidence, respectively. The effect on systolic and diastolic blood pressure for men was MD ‐2.14, 95% CI ‐3.71, ‐0.59; 507 participants; five studies; I2 = 8%, moderate‐certainty evidence; MD ‐1.99, 95% CI ‐3.25, ‐0.74; 507 participants; five studies; I2 = 41%, moderate‐certainty evidence, respectively. The effect was consistent in both genders regardless of baseline calcium intake.
The effect on systolic blood pressure was: MD ‐0.02, 95% CI ‐2.23, 2.20; 302 participants; 3 studies; I2 = 0%, moderate‐certainty evidence with doses less than 1000 mg; MD ‐1.05, 95% CI ‐1.91, ‐0.19; 2488 participants; 9 studies; I2 = 0%, high‐certainty evidence with doses 1000 to 1500 mg; and MD ‐2.79, 95% CI ‐4.71, 0.86; 350 participants; 7 studies = 8; I2 = 0%, moderate‐certainty evidence with doses more than 1500 mg. The effect on diastolic blood pressure was: MD ‐0.41, 95% CI ‐2.07, 1.25; 201 participants; 2 studies; I2 = 0, moderate‐certainty evidence; MD ‐2.03, 95% CI ‐3.44, ‐0.62 ; 1017 participants; 8 studies; and MD ‐1.35, 95% CI ‐2.75, ‐0.05; 1821 participants; 8 studies; I2 = 51%, high‐certainty evidence, respectively.
None of the studies reported adverse events.
Authors' conclusions
An increase in calcium intake slightly reduces both systolic and diastolic blood pressure in normotensive people, particularly in young people, suggesting a role in the prevention of hypertension. The effect across multiple prespecified subgroups and a possible dose response effect reinforce this conclusion. Even small reductions in blood pressure could have important health implications for reducing vascular disease. A 2 mmHg lower systolic blood pressure is predicted to produce about 10% lower stroke mortality and about 7% lower mortality from ischaemic heart disease.
There is a great need for adequately‐powered clinical trials randomising young people. Subgroup analysis should involve basal calcium intake, age, sex, basal blood pressure, and body mass index. We also require assessment of side effects, optimal doses and the best strategy to improve calcium intake.
Plain language summary
Extra calcium to prevent high blood pressure
Review question
We wanted to find out the effects of calcium intake on blood pressure in people with normal blood pressure.
Background
Hypertension is a serious health problem that increases the risk of heart and kidney diseases. Several studies have shown that increasing calcium intake lowers blood pressure even in individuals within a normal blood pressure range. Increasing calcium intake also has benefits for pregnancy outcomes, effects which are thought to be mediated also by blood pressure reduction. High blood pressure has been identified as a major risk factor for mortality and even small reductions in blood pressure can decrease the occurrence of coronary artery disease, stroke and death.
Study characteristics
We selected studies that assessed the effect of dietary calcium interventions such as supplementation or food fortification on blood pressure in normotensive people of all ages. Searches were last run in September 2020.
Key findings
This review analysed information from 20 trials of which 18 trials (3140participants) provided date for the effect of the intervention. We found that an increase in calcium intake slightly reduces both systolic and diastolic blood pressure by 1.37 mmHg lower and by 1.45 mmHg lower, respectively. This effect was higher with doses of calcium above 1000 mg/day. Systolic blood pressure was reduced by 1.05 mmHg with doses of calcium 1000 to 1500 mg/day and by 2.79 mmHg with doses of calcium equal to or over 1500 mg/day.
We noted a reduction in blood pressure in both men and women and at ages from 11 to 82 years old, but the reduction was greater among younger people. Systolic blood pressure was reduced by 1.86 mmHg among those less than 35 years and by 0.97 mmHg among those 35 years or older.
None of the studies reported adverse events. We need further research to determine the ideal dosage of supplementation and whether it is more effective and safer as part of the diet or as a supplement.
Quality of the evidence
We found high quality of evidence for systolic and diastolic blood pressure in both men and women. The quality of evidence was also high for participants 35 years or older and moderate for younger people.
The quality of evidence was high for doses of calcium of 1000 to 1500 mg/day and was moderate for lower or higher doses.
Five of the 18 trials were industry funded.
Summary of findings
Summary of findings 1. Calcium supplementation/fortification compared to control for prevention of primary hypertension.
| Calcium supplementation/fortification compared to control for prevention of primary hypertension | |||||
| Patient or population: People who may be at risk for primary hypertension Settings: US (8), New Zealand (3), and one each in The Netherlands, Belgium and Denmark, Guatemala and Iran Intervention: Calcium supplementation/fortification Comparison: Placebo | |||||
| Outcomes | Illustrative blood pressure in control group b | Mean difference in mmHg (95% CIa) | No of participants (studies) | Quality of the evidence (GRADE)c | Comments |
| Systolic blood pressure (range of follow‐up from 4 weeks to 4 years) | 115.62 | 1.37 lower (2.08 lower to 0.66 lower) | 3140 (18 studies) | ⊕⊕⊕⊕ high | Women: ‐1.25 mmHg [95% CI: ‐2.53, 0.03; 8 studies] Men: ‐2.07 mmHg [95% CI: ‐3.56, ‐0.59; 5 studies] Both: ‐1.11 mmHg [95% CI: ‐2.15, ‐0.08; 6 studies] |
| Diastolic blood pressure (range of follow‐up from 4 weeks to 4 years) | 78.17 |
1.45 lower (2.23 lower to 0.67 lower) |
3039 (17 studies) | ⊕⊕⊕⊕ high | Women: ‐1.03 mmHg [95% CI: ‐1.80, ‐0.26; 8 studies] Men: ‐1.91 mmHg [95% CI: ‐2.80, ‐1.02; 5 studies] Both: ‐0.25 mmHg [95% CI: ‐1.08, 0.57; 5 studies] |
| Systolic blood pressure. Dose less than 1000 mg a day (range of follow‐up from 12 weeks to 2 years) | 103.74 | 0.02 lower (2.23 lower to 2.20 higher) | 302 (3 studies) | ⊕⊕⊕⊝ moderate1 | Subgroup analysis by dose |
| Systolic blood pressure. Dose between 1000 mg a day and less than 1500 mg a day (range from 4 weeks to 2 years) | 116.29 | 1.05 lower (1.91 lower to 0.19 lower) | 2488 (9 studies) | ⊕⊕⊕⊕ high | Subgroup analysis by dose |
| Systolic blood pressure. Dose 1500 mg a day or more (range of follow‐up from 4 weeks to 4 years) | 112.85 | 2.79 lower (4.71 lower to 0.86 lower) | 350 (7 studies) | ⊕⊕⊕⊝ moderate1 | Subgroup analysis by dose |
| Systolic blood pressure. Less than 35 years of age (range of follow‐up from 4 weeks to 22 weeks) | 113.23 | 1.79 lower (3.20 lower to 0.38 lower) | 452 (9 studies) | ⊕⊕⊕⊝ moderate1 | Subgroup analysis by age |
| Systolic blood pressure. 35 years or older (range of follow‐up from 4 weeks to 4 years) | 124.20 | 0.97 lower (1.83 lower to 0.10 lower) | 2688 (10 studies) | ⊕⊕⊕⊕ high | Subgroup analysis by age |
| Adverse events (secondary outcome) | One study evaluated side effects, but none were reported. A further two studies mentioned that the supplements were well tolerated. No trials reported any incidence of kidney stone formation, iron deficiency anaemia, anaemia, cardiovascular events, myocardial infarction, stroke or mortality. | ||||
| aCI: Confidence interval; bEstimated using Comprehensive Meta‐Analysis Software Software; cGRADE Working Group grades of evidence | |||||
| High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. |
|||||
1. Downgraded one level for imprecision due to small number of participants.
Background
Description of the condition
The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure defines "hypertension" as blood pressure above 139 mmHg systolic or diastolic above 89 mmHg, or both. It also defines blood pressure ranging from 120–139 mmHg systolic or 80–89 mmHg diastolic, or both, as “prehypertension” in order to identify those individuals in whom early intervention by adoption of healthy lifestyles could reduce blood pressure, decrease the rate of progression of blood pressure to hypertensive levels with age, or prevent hypertension entirely (Chobanian 2003).
Primary hypertension may develop as a result of environmental or genetic causes. Secondary hypertension has multiple aetiologies, such as renal, vascular, and endocrine causes. Primary or essential hypertension accounts for 90‐95% of adult cases and secondary hypertension accounts for 2‐10% of cases (Carretero 2000).
Hypertension is a major public health problem that increases the risk of cardiovascular and kidney diseases in both the developed and the developing world. The global prevalence of hypertension and high blood pressure is estimated to be 30% and 26%, respectively (Kearney 2004), and high blood pressure has been estimated to increase to 29% by the year 2025 (Kearney 2005).
High blood pressure has been identified as the leading risk factor for mortality and the third leading risk factor for disease burden globally (Ezzati 2002). In the year 2001, 7.6 million (13.5%) of all deaths were attributable to high blood pressure (Lawes 2008).
While the prevalence of hypertension seems to be stabilising or decreasing in the developed world, it is increasing in developing countries (Kearney 2004). Low‐income and middle‐income regions contribute up to 80% of the attributable burden of disease, affecting the younger age groups more than in high‐income countries (Lawes 2008). While chronic diseases have increased in these countries, problems related to undernutrition such as micronutrient deficiencies persist, causing a double burden of disease (Llanos 2008). These present a challenge to developing interventions, as excess and deficit nutritional problems have to be tackled within the same population and frequently within the same home (Garrett 2005).
Description of the intervention
Several studies have shown an inverse association between calcium intake and blood pressure or hypertension. The hypothesis originated with the observation that indigenous Guatemalan women have a low incidence of oedema‐, proteinuria‐, and hypertension‐gestosis associated with a high calcium intake due to the Mayan habit of treating corn with lime water (Belizan 1980). Based on this hypothesis, a series of studies has been conducted mainly in pregnant women, but also in children, as well as in young and older adults (Belizan 1980; Belizan 1983).
A recent World Health Organization (WHO) review of observational epidemiological and ecological studies found an inverse (protective) association between cardiovascular disease mortality and increased water hardness (measured by calcium carbonate or another hardness parameter and/or the calcium and magnesium content of water) (WHO 2009).
A Cochrane review in 2006 found that calcium supplementation in hypertensive people elicited a small but statistically significant reduction in systolic blood pressure (SBP) (mean difference: ‐2.5 mmHg, 95% confidence interval (CI) ‐4.5 to ‐0.6), but not in diastolic blood pressure (DBP) (mean difference: ‐0.8 mmHg, 95% CI ‐2.1 to 0.4) (Dickinson 2006).
Several reviews have shown an association between calcium intake and blood pressure (Allender 1996; Griffith 1999; Van Mierlo 2006). A review in 2006 found that calcium supplementation (mean daily dose: 1200 mg) reduced SBP by 1.86 mmHg (95% CI 2.91 to 0.81) and DBP by 0.99 mmHg (95% CI 1.61 to 0.37) (Van Mierlo 2006). In people with a relatively low calcium intake (less than 800 mg per day), higher blood pressure reduction was obtained, a mean of 2.63 (95% CI 4.03 to 1.24) for SBP and 1.30 (95% CI 2.13 to 0.47) for DBP.
Furthermore, a Cochrane review has shown that calcium supplementation has an effect on reducing pregnancy hypertensive diseases (Hofmeyr 2018).
How the intervention might work
Calcium intake may regulate blood pressure by modifying intracellular calcium in vascular smooth muscle cells and by varying vascular volume through the renin–angiotensin–aldosterone system. Low calcium intake produces a rise of parathyroid gland activity (Villa‐Etchegoyen 2019). The parathyroid hormone (PTH) increases intracellular calcium in vascular smooth muscles resulting in vasoconstriction. Parathyroidectomised animals did not show an increase in blood pressure when fed a low calcium diet as did sham‐operated animals (Belizan 1984). Low calcium intake also increases the synthesis of calcitriol in a direct manner or mediated by PTH. Calcitriol increases intracellular calcium in vascular smooth muscle cells. Both low calcium intake and PTH may stimulate renin release and consequently angiotensin II and aldosterone synthesis (Villa‐Etchegoyen 2019).
Why it is important to do this review
Small reductions in blood pressure have been predicted to have important health implications, as they have been shown to produce rapid reductions in vascular disease risk even in individuals with normal blood pressure ranges (Lewington 2002). A 2 mmHg‐lower systolic blood pressure is predicted to produce about 10% lower stroke mortality and about 7% lower mortality from ischaemic heart disease, while a 5 mmHg reduction in SBP at the population level is predicted to result in a 14% reduction in stroke death, a 9% reduction in coronary artery disease‐related death and a 7% reduction in total mortality (Whelton 2002). In the same way, a 2 mmHg reduction in SBP in adults is estimated to have the potential to save about 12,000 lives a year in the United States (Stamler 1991).
Due to the high frequency of hypertension, population‐based strategies to reduce blood pressure are more cost‐effective than individual strategies (Kearney 2005).
Calcium supplementation or food fortification are affordable interventions that, if proven effective in reducing blood pressure even by small levels, could have considerable impact at a population level (Cormick 2021). The effects on children and young people are of particular importance, as blood pressure tends to track into adulthood (Williams 2011).
This review explores the efficacy and safety of calcium supplementation or food fortification in preventing hypertensive‐related problems in normotensive people of different ages. It looks at the effect of reducing blood pressure in each population group and of preventing, rather than treating, hypertensive‐related problems. It also provides more information on the effect of increasing calcium intake on blood pressure in non‐pregnant women of reproductive age. Reviewing the effect of calcium in a normotensive population is valuable for assessing whether it could allow women to reach pregnancy with a lower range of blood pressure and a lower risk of developing pre‐eclampsia or eclampsia.
As there have been some concerns about adverse events of calcium supplementation (Bolland 2008; Curhan 2004; Harris 2002), there is a need to assess adverse events such as renal tract stone formation, impaired absorption of other minerals and increased cardiovascular events.
Excess calcium in the body had been implicated as a risk factor for kidney stone formation; however, data suggest that free calcium in the body does not increase the risk and that high calcium intake may actually be a protective factor against the formation of kidney stones (Curhan 2004; Heaney 2006; Jackson 2006; Williams 2001; Cormick 2019a).
The effect of calcium supplementation on cardiovascular events is unclear, as there are currently conflicting data, studies have not been powered to significantly detect cardiac events, and the methodology does not allow the results to be generalisable to a broader population. Two studies that were conducted in cohorts of older women have reported a higher incidence of cardiovascular events such as myocardial infarction and the composite end point of myocardial infarction, stroke, or sudden death in the experimental groups, however, these differences were not statistically significant (Bolland 2008; Sabbagh 2009). More recent meta‐analyses have questioned this evidence (Lewis 2012; Lewis 2015).
Calcium has been shown to interfere with iron absorption in the short term; however, research has also shown that prolonged calcium supplementation has no effect on iron absorption over time (Harris 2002; Ilich‐Ernst 1998; Kalkwarf 1998; Palacios 2021; Sokoll 1992).
It is important to update this review as new evidence has been published since the last publication of our review in 2015.
Objectives
To assess the efficacy and safety of calcium supplementation versus placebo or control for reducing blood pressure in normotensive people and for the prevention of primary hypertension.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing trials with random allocation to dietary calcium intervention such as supplementation or food fortification versus placebo or control. We excluded quasi‐random designs and the second phase of cross‐over trials from the analysis.
Types of participants
Participants included normotensive people of different ages, but excluding pregnant women.
Types of interventions
We included calcium interventions such as supplementation using pills, tablets or sprinkle powder, or any food or beverage fortification, compared to placebo or control.
Calcium fortification could include salt of calcium carbonate, sulphate, citrate, citrate malate, chloride, hydroxyapatite, phosphate, acetate, lactate, glycerophosphate, gluconate, oxide, or hydroxide. Calcium content in these salts varies from 9% to 70% (Allen 2006).
We excluded studies with no placebo or control. We also excluded interventions where calcium was combined with other macro‐ or micronutrients to assess the effects of both.
Types of outcome measures
We selected the following outcomes. Minimum follow‐up time was two weeks. For multiple time points, we analysed the longest intervention period.
Primary outcomes
Hypertension, defined as blood pressure ≥ 140/90 mmHg
Systolic and diastolic blood pressure
Secondary outcomes
Any adverse event
Withdrawals due to adverse events
Kidney stone formation
Iron deficiency anaemia
Anaemia
Total mortality
Cardiovascular events
Myocardial infarction
Stroke
Sudden death
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist designed strategies for and searched the following databases without language, publication year or publication status restrictions:
the Cochrane Hypertension Specialised Register via the Cochrane Register of Studies (to 30 September 2020);
the Cochrane Central Register of Controlled Trials (CENTRAL, 2020 Issue 9) via the Cochrane Register of Studies (to 29 September 2020);
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) (1946 to 29 September 2020);
Embase Ovid (1974 to 29 September 2020);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) (to 29 September 2020);
World Health Organization International Clinical Trials Registry Platform via the Cochrane Register of Studies (to 30 September 2020).
The Information Specialist modelled subject strategies for databases in the search strategy designed for MEDLINE. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive‐ and precision‐maximising search strategy designed by Cochrane for identifying randomised controlled (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.d. (Higgins 2011)). We present search strategies for major databases in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Hypertension Specialised Register segment (which includes searches of MEDLINE, Embase, and Epistemonikos for systematic reviews) to retrieve existing reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Specialised Register also includes searches for controlled trials in CAB Abstracts & Global Health, CINAHL, ProQuest Dissertations & Theses and Web of Science.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
Where necessary, we contacted authors of key papers and abstracts to request additional information about their trials.
Data collection and analysis
Pairs of review authors (GC, MSC and MLC) independently assessed the methodological quality and other inclusion criteria of the identified trials, resolving disagreements by consensus.
Selection of studies
We imported references and abstracts of searched results to Early Reviewer Organizing Software (EROS) (Ciapponi 2011; Glujovsky 2010), basing selection of studies on the criteria listed above.
Data extraction and management
Pairs of review authors (GC, MSC and MLC) independently extracted data, using a standard form, and then cross‐checked them. A third person (AC) confirmed all numeric calculations and graphic interpolations.
Descriptive data included authors, year of publication, country, time span of the trial, gender, type of placebo, baseline dietary calcium intake, type, dose and duration of calcium‐related intervention, compliance, co‐interventions, trial quality assessments, and numbers randomised and analysed.
The position of the participant during blood pressure measurement may affect the blood pressure‐lowering effect. However, in order to not lose valuable data if only one position was reported, we collected data from that position. When blood pressure measurement data were available in more than one position, sitting blood pressure was the first preference. If both standing and supine measurements were available, we used standing blood pressure.
Assessment of risk of bias in included studies
GC, MSC and MLC independently assessed risks of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement through discussion with the whole team. We made explicit judgements about whether studies had high risk of bias, according to the criteria described below. We assessed the magnitude and direction of the bias and whether we considered it was likely to impact on the findings through sensitivity analysis. See Sensitivity analysis below.
(1) Sequence generation (checking for possible selection bias)
We described the method used to generate the allocation sequence for each included study in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
| • | low risk of bias (any truly random process, e.g. random number table; computer random number generator) |
| • | high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number) |
| • | unclear risk of bias |
(2) Allocation concealment (checking for possible selection bias)
We described the method used to conceal the allocation sequence for each included study and determined whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We assessed the methods as:
| • | low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered sealed opaque envelopes) |
| • | high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth) |
| • | unclear risk of bias |
(3) Blinding (checking for possible performance and detection bias)
We described for each included study the methods used, if any, to blind study participants and personnel and outcome assesors from knowledge of which intervention a participant received. We considered studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for participants and personnel and for outcome assessores and for different outcomes .
We assessed the methods as:
| • | low, high or unclear risk of bias for participants (performance bias |
| • | low, high or unclear risk of bias for personnel (performance bias) |
| • | low, high or unclear risk of bias for outcome assessors (detection bias) |
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the re‐analyses.
We assessed methods as:
| • | low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups) |
| • | high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation) |
| • | unclear risk of bias |
(5) Selective reporting bias
We described for the included study how we investigated the possibility of selective outcome reporting bias and our findings.
We assessed the methods as:
| • | low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported) |
| • | high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported) |
| • | unclear risk of bias |
(6) Other sources of bias
We described any important concerns we had about other possible sources of bias for each included study.
We assessed whether the study was free of other problems that could put it at risk of bias and recorded our judgement as:
| • | low risk of bias (the study appears to be free of other sources of bias) |
| • | high risk of bias (potential source of bias related to the specific study design used; or has been claimed to have been fraudulent; or had some other problem) |
| • | unclear risk of bias |
Measures of treatment effect
For continuous data, we used the mean difference (MD) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome but used different methods. For dichotomous data, we planned to calculate risk ratios (RR) with 95% confidence intervals (CI). None of the studies reported hypertension as a dichotomous outcome.
Unit of analysis issues
In the case of studies with more than one treatment comparison, we divided the control groups by the number of subgroups.
Dealing with missing data
In the case of missing information in the included studies, we contacted investigators (using email, letter and/or fax) to obtain the missing information. In the case of missing standard deviations of blood pressure change, we imputed the standard deviation based on the information in the same trial or from other trials which assessed calcium‐related interventions. We used the following hierarchy (listed from high to low preference) to impute standard deviation values:
| 1. | standard deviation of change in blood pressure taken in a different position from that of the blood pressure data used |
| 2. | standard deviation of blood pressure at the end of treatment |
| 3. | standard deviation of blood pressure at the end of treatment measured in a different position from that of the blood pressure data used |
| 4. | standard deviation of blood pressure at baseline (except if this measure was used as an entry criterion) |
| 5. | mean standard deviation of change in blood pressure from other trials assessing calcium‐related interventions |
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics (Higgins 2003; Higgins 2011). We regarded heterogeneity as moderate if T² was greater than zero and either I² was greater than 30% or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. I² values greater than 50% indicate high levels of heterogeneity.
Assessment of reporting biases
We investigated reporting biases (such as publication bias) by producing funnel plots if at least 10 studies were included in the analysis. We assessed funnel plot asymmetry visually. In case of asymmetry suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2014). For continuous data, we used the mean difference (MD) and its 95% confidence interval (CI) if outcomes were measured in the same way between trials. We used the standardised mean difference (SMD) to combine trials that measured the same outcome but using different methods. We compared categorical data using risk ratios (RRs) and their 95% CIs. We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect, i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods to be sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used random‐effects meta‐analysis to produce an overall summary where we considered an average treatment effect across trials was clinically meaningful. We treated the random‐effects summary as the average range of possible treatment effects, and we discussed the clinical implications of treatment effects differing between trials.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses:
We analysed sex and age using recommended nutrient intake age groups (1 to less than 4 years; 4 to less than 6 years; 6 to less than 10 years; 10 to less than 19 years; 19 to less than 50 years; 50 years and over), for men and women.
Ethnicity
Duration of calcium intervention
Dose received
Intake of other minerals: where possible we analysed groups according to intakes of minerals involved in blood pressure regulation such as sodium, magnesium, potassium
Fat intake
Baseline calcium intake: we divided population groups into low or adequate calcium intake, according to WHO Food and Agriculture Organization (FAO) recommendations by age group
Baseline blood pressure: blood pressure as defined by trial authors. Ideally, we analysed pre‐hypertension defined as diastolic blood pressure ≥ 80 mmHg (or systolic blood pressure ≥ 120 mmHg).CDC 2021
Sensitivity analysis
We planned sensitivity analyses to explore the effect of risk of bias assessed by concealment of allocation, high attrition rates, or both, excluding the studies with high RoB in theses domains from the analyses in order to assess whether this made any difference to the overall result. We tested the robustness of the results using several sensitivity analyses, including:
| 1. | Trials that were industry‐sponsored versus non‐industry sponsored |
| 2. | Trials with blood pressure data measured in the sitting position versus other measurement positions |
| 3. | Trials with reported standard deviations of blood pressure change versus imputed standard deviations |
| 4. | Risk of bias items |
In order to explore the robustness of the results, we performed four post hoc sensitivity analyses. The first sensitivity analysis was by mean difference and standardised mean difference in those cases when the results came from a combination of final blood pressure values and blood pressure change from baseline. We decided to present the results as mean differences, as they are easier to interpret. However, in order to be more accurate, we compared the mean difference results with the standardised mean differences. We based the other analyses on duration of intervention, on blood pressure methodology (auscultatory and oscillometric method) and on clinic blood pressure measurements and automated ambulatory blood pressure.
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables using GRADEpro and Cochrane methods (GRADEpro GDT 2015; Higgins 2011). These tables evaluated the overall quality of the body of evidence for the main review outcomes and main review comparison . Additional summary of findings tables were also prepared for the main review outcomes for other important comparisons. We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias). Judgements about the quality of the evidence (high, moderate, low or very low) were made by two review authors (GC, AC) working independently, with disagreements resolved by discussion. Judgements were justified, documented, and incorporated into reporting of results for each outcome. We extracted study data, formatted our comparisons in data tables and prepared summary of findings tables before writing the results and conclusions of our review.
Results
Description of studies
See tables 'Characteristics of included studies' and 'Characteristics of excluded studies' for details of individual studies.
Results of the search
We retrieved 1627 references from the electronic searches in 2015. As a result of the updated search, the total number of references retrieved was 6990 (3840 after de‐duplication). Out of the 154 references selected by full text, we finally included 20 studies. See the flow diagram in Figure 1.
1.
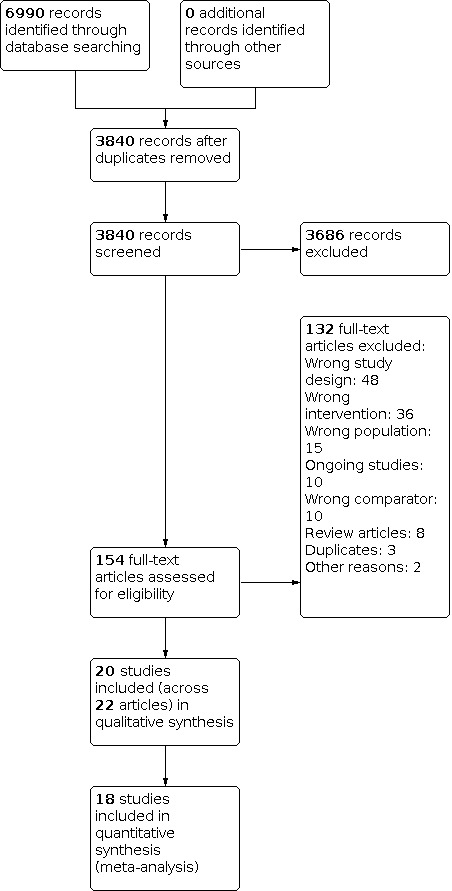
Study flow diagram.
Included studies
In this update, we included four new randomised trials (Entezari 2015; Karanja 1987; Yanovski 2009; Yosephin 2015) to the 16 randomised trials identified in 2015, coming from 22 articles (there were two secondary references for Lyle 1987 and Lijnen 1995). See Characteristics of included studies. We also identified 10 ongoing trials (Ongoing studies).
Participants
Most of the studies were performed in adults; five studies were performed in older men and women (Reid 2005; Reid 2010; Thomsen 1987;Yanovski 2009; Van Beresteyn 1986), one study in teenagers (Davis 1996) and one in 11‐year‐old children (Gillman 1995).
We found 14 studies (Belizan 1983; Cutler 1992; Entezari 2015; Gillman 1995; Hilary Green 2000; Johnson 1985; Karanja 1987 Lyle 1992; Reid 2005; Reid 2010; Sacks 1998; Shidfar 2010;Yanovski 2009; Van Beresteyn 1986) reporting baseline mean calcium intake with values ranging from around 400 mg to 1120 mg a day in adult groups. Using this range, we organised the studies into three categories: less than 600 mg a day, 600 to less than 800 mg a day, and 800 mg a day or more for people between 19 and 50 years of age.
We found seven studies that only included women (Entezari 2015; Johnson 1985; Reid 2005; Sacks 1998; Thomsen 1987; Van Beresteyn 1986; Yosephin 2015) and four studies that only included men (Lijnen 1995; Lyle 1987; Reid 2010; Shidfar 2010).
Sample sizes
For most studies, the sample size was fewer than 100 participants; three studies had a sample size between 100 and 200 participants; and the three largest studies had 340 participants (Yanovski 2009) 471 participants (Cutler 1992) and 1471 participants (Reid 2005).
Settings
Most studies were performed in higher‐income countries, with ten set in the USA (Cutler 1992; Davis 1996; Gillman 1995; Johnson 1985; Karanja 1987; Lyle 1987; Lyle 1992; McCarron 1985; Sacks 1998; Yanovski 2009 ), three in New Zeland (Hilary Green 2000; Reid 2005; Reid 2010), and three in Europe (Lijnen 1995 in Belgium; Thomsen 1987 in Denmark; Van Beresteyn 1986 in the Netherlands). Four studies were set in low‐ and middle‐income countries: Belizan 1983 in Guatemala; Yosephin 2015 in Indonesia, and Entezari 2015 and Shidfar 2010 in Iran.
Interventions
The intervention consisted of a supplement tablet in 17 studies, while one study (Hilary Green 2000) evaluated the effect of two servings per day of high‐calcium skim milk versus ordinary skim milk (control), and two studies used a fortified juice (Gillman 1995; Van Beresteyn 1986).
For most studies, the intervention was 1000 to 2000 mg of elemental calcium per day. The intervention in one study was 500 mg of calcium a day (Yosephin 2015); two studies had an intervention group with 600 mg of calcium a day (Gillman 1995; Reid 2010) and another study compared a high‐calcium skim milk containing 1075 mg to 720 mg of the non‐fortified skim milk (Hilary Green 2000).
Nine studies used calcium carbonate for the intervention (Cutler 1992; Johnson 1985; Lyle 1992; Lyle 1987; Shidfar 2010; Sacks 1998; Van Beresteyn 1986; Entezari 2015; Yanovski 2009); three studies used calcium citrate (Gillman 1995; McCarron 1985; Reid 2005), one study used gluconate (Lijnen 1995) and two studies used a combination of calcium salts (Belizan 1983; Thomsen 1987). Five did not report the salt used (Davis 1996; Hilary Green 2000; Karanja 1987; Reid 2010; Yosephin 2015).
We did not specify a minimum intervention time for inclusion of studies. However, the included studies had a median follow‐up intervention period of 3.5 months. After initiation of calcium supplementation, blood pressure seemed to stabilise at between 1.5 and 2.5 months (Belizan 1983). Five studies had interventions that lasted a year or more: Thomsen 1987 one year, Reid 2010 and Yanovski 2009 two years, Reid 2005 two and a half years and Johnson 1985 four years.
Excluded studies
Seventeen studies were first included and then excluded. Four studies were excluded for not having a randomised controlled trial (RCT) design (Luft 1986; Ong 2016; Rahman 2003; Smith 1987), three studies for not reporting the number of participants (Dwyer 1998; Morris 1988; Weinberge 1993), two studies had a co‐intervention that could affect the blood pressure result (Eftekhari 2009; Shalileh 2010), three studies included hypertensive people (Bostick 2000; Hofmeyr 2015; Pan 1993), four studies had a wrong comparator (Das 2017, Ferreira 2016, Sakai 2017, Zhang 2009) and we could not extract data from one study in Chinese (Pan 2000). See Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2; Figure 3. Some information to assess risk of bias was not available for 10 published papers. We found contact details for eight of those studies and obtained the required information from five (Cutler 1992; Gillman 1995; Lyle 1987; Lyle 1992; Sacks 1998).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Risk of bias for allocation concealment was low for eight of the 20 included studies, and unclear or not described or the remaining 12 studies. For the eight studies classified as low risk, allocation was made by a centralised unit or packets were of identical appearance and were numbered at randomisation.
Blinding
Risk of performance bias from blinding bias was low for 13 of the 20 studies and unclear or not described for the remaining seven studies. For the 13 studies classified as having low risk of performance bias, blinding of participants and personnelwas ensured by a double‐blind design and identical appearance of the food or supplement provided.
Ten studies (Cutler 1992; Davis 1996; Gillman 1995; Hilary Green 2000; Lyle 1987; McCarron 1985; Reid 2005; Reid 2010; Sacks 1998; Van Beresteyn 1986) were at low risk of detection bias as they used a random‐baseline sphygmomanometer, a blood pressure machine that automatically entered the blood pressure data on computer tape, an ambulatory blood pressure monitor, or trained personnel who were blinded to the allocation groups. Detection bias was uncertain for a three studies (Entezari 2015; Lyle 1992; Yosephin 2015) that did not specified the methodology and for five that used a mercury sphygmomanometer (Belizan 1983; Johnson 1985; Lijnen 1995; Shidfar 2010; Thomsen 1987).
Incomplete outcome data
Attrition bias was low for 12 of the 20 studies, while we classified three studies at high risk (Belizan 1983; Entezari 2015; Johnson 1985), as they had more than 10% dropouts. For the remaining four studies, the information was unclear or not described.
Selective reporting
We classified all studies but one (Karanja 1987) at low risk of reporting bias, as all primary outcomes were addressed or there was no evidence of selective reporting bias. Karanja 1987 described blood pressure as an outcome, however, the study does not report results.
Other potential sources of bias
We detected no other bias for 13 of the 20 studies. Davis 1996 did not present baseline characteristics of the population so we rated it as being at unclear risk. We rated four studies as having high risk of bias: baseline characteristics of intervention and placebo groups presented small differences (in different directions) in Hilary Green 2000; in Lyle 1992, the treatment group presented at baseline more men than in the placebo group, although blood pressure values showed no difference; Yosephin 2015 had 79% of women with high Body Mass Index (BMI) in the calcium group while, in the control group, 55% of women had high BMI; finally, Thomsen 1987 placebo participants had higher initial weight and lower systolic blood pressure than in the intervention group.
Effects of interventions
See: Table 1
Primary outcomes
Hypertension was defined as blood pressure ≥ 140/90 mmHg. None of the studies reported hypertension as a dichotomous outcome. Out of the 20 included studies 18 were considered in the meta‐analysis as Yanovski 2009 did not report baseline blood pressure and the results were adjusted imputed and Karanja 1987 did not report blood pressure.
Systolic and diastolic blood pressure
Effect considering all the studies reporting change or final value of blood pressure
There was a reduction in blood pressure with calcium supplementation/fortification compared with control. The overall effect on systolic blood pressure was a mean difference (MD) of ‐1.37 mmHg (95% confidence interval (CI) ‐2.08 to ‐0.66) reported in 18 trials (N = 3140) with low heterogeneity (P = 0.68; I² = 0%) (Analysis 1.1); the effect on diastolic blood pressure was ‐1.45 mmHg (95% CI ‐2.23 to ‐0.67) in 17 trials (N = 3039) with moderate heterogeneity (P = 0.01; I² = 45%) (Analysis 1.2).
1.1. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 1: Mean difference in systolic blood pressure
1.2. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 2: Mean difference in diastolic blood pressure
Effect considering only the studies reporting change in blood pressure
The estimated effect on change in systolic pressure was ‐1.27 mmHg (95% CI ‐2.02 to ‐0.52), reported in eleven trials (N = 2786) (Analysis 1.3). The estimated effect on change in diastolic pressure was ‐1.62 (95% CI ‐2.61 to ‐0.63 ) reported in ten trials (N = 2685) (Analysis 1.4). Heterogeneity was low for systolic blood pressure (P = 0.67; I² = 0%) and high for diastolic (P = 0.001; I² = 64%).
1.3. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 3: Change data: systolic blood pressure
1.4. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 4: Change data: diastolic blood pressure
Effect considering only the studies reporting final values of blood pressure
The estimated effect on final systolic blood pressure was ‐1.93 mmHg (95% CI ‐3.72 to ‐0.14), reported in 12 trials (N = 630) (Analysis 1.5) and on diastolic blood pressure ‐1.46 mmHg (95% CI ‐2.82 to ‐0.11), reported in eleven trials (N = 529) (Analysis 1.6). Heterogeneity was low for both systolic (P = 0.26; I² = 18%) and diastolic blood pressure (P = 0.28; I² = 16%).
1.5. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 5: Final value: systolic blood pressure
1.6. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 6: Final value: diastolic blood pressure
Subgroup analyses
We reported tests for subgroup differences only when P values were less than 0.1.
Analysis by sex
Of the 18 studies included, 12 studies (Belizan 1983; Johnson 1985; Lijnen 1995; Lyle 1987; Reid 2005; Reid 2010; Sacks 1998; Shidfar 2010; Thomsen 1987; Van Beresteyn 1986) presented the results by sex.
Effect considering all the studies reporting change or final value of blood pressure
The overall effect on systolic blood pressure was ‐1.25 mmHg (95% CI ‐2.53 to 0.03) for women, eight studies (N = 1915) with low heterogeneity (P = 0.85; I² = 0%) and ‐2.14 mmHg (95% CI ‐3.71 to ‐0.57) for men, five studies (N = 507) with low heterogeneity (P = 0.37; I² = 8%) (Analysis 1.1). The effect on diastolic blood pressure was ‐1.04, mmHg (95% CI ‐1.86 to ‐0.22) for women, eight studies (N = 1915) with low heterogeneity (P = 0.40; I² = 4%) and ‐1.99 mmHg (95% CI ‐3.25 to ‐0.74) in men, five studies (N = 507) with moderate heterogeneity (P = 0.12; I² = 41%) (Analysis 1.2) (test for subgroup differences: Chi² = 7.15, df = 2 (P = 0.03), I² = 72.0%).
Effect considering only the studies reporting change in blood pressure
For those studies showing change in systolic blood pressure, the effect was ‐1.47 mmHg (95% CI ‐2.87 to ‐0.08) for women, five studies (N = 1748) with low heterogeneity (P = 0.84; I² = 0%) and ‐2.01 mmHg (95% CI ‐3.95 to ‐0.08) for men, four studies (N = 432) with low heterogeneity (P = 0.23; I² = 29%) (Analysis 1.3). The effect on diastolic blood pressure was ‐1.87 mmHg (95% CI ‐3.62 to ‐0.12) for women, five studies (N = 1748) with moderate heterogeneity (P = 0.05; I² = 58%) and ‐2.24 mmHg (95% CI ‐3.75 to ‐0.73) for men, four studies (N = 432) with high heterogeneity (P = 0.06; I² = 57%) (Analysis 1.4).
Effect considering only the studies reporting final values of blood pressure
In those studies reporting final values, the effect on systolic blood pressure was ‐0.20 mmHg (95% CI ‐3.00 to 2.60) for women, five studies (N = 259) with low heterogeneity (P = 0.86; I² = 0%) and ‐5.36 mmHg (95% CI ‐9.03 to ‐1.70) for men, two studies (N = 124) with low heterogeneity (P = 0.30; I² = 17%) (Analysis 1.5). For diastolic blood pressure, the effect was ‐0.52 mmHg (95% CI ‐2.38 to 1.34) in women, five studies (N = 259) with low heterogeneity (P = 0.43; I² = 0%) and ‐1.88 mmHg (95% CI ‐4.26 to 0.50) in men, two studies (N = 124) with low heterogeneity (P = 0.46; I² = 0%) (Analysis 1.6).
Analysis by age
Although all studies reported the age groups of the population, most of them did not present their results by age group, so it was not possible to do the analysis using the groups originally planned. We divided studies into those that presented a mean age of less than 35 years and those with a mean age of 35 years or more.
Effect considering all the studies reporting change or final value of blood pressure
The overall effect on systolic blood pressure was ‐1.86 mmHg (95% CI ‐3.45 to ‐0.27) for those younger than 35 years, eight studies (N = 452) with moderate heterogeneity (P = 0.27; I² = 19%) and ‐0.97 mmHg (95% CI ‐1.83 to ‐0.10) for those aged 35 years or more, ten studies (N = 2688) with low heterogeneity (P = 0.86; I² = 0%) (Analysis 1.7). The overall effect on diastolic blood pressure was ‐2.50 mmHg (95% CI ‐4.22 to ‐0.79) for those younger than 35 years, seven studies (N = 351) with high heterogeneity (P = 0.03; I² = 54%) and ‐0.59 mmHg (95% CI ‐1.13 to ‐0.06) for those aged 35 years or more, ten studies (N = 2688) with low heterogeneity (P = 0.78; I² = 0%) (Analysis 1.8) (test for subgroup differences: Chi² = 11.59, df = 1; P = 0.0007, I² = 91.4%).
1.7. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 7: Mean difference in systolic blood pressure by age
1.8. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 8: Mean difference in diastolic blood pressure by age
Effect considering only the studies reporting change in blood pressure
For those studies showing change in systolic blood pressure, the effect was ‐2.34 mmHg (95% CI ‐4.55 to ‐0.13) for those younger than 35 years, three studies (N = 142) with low heterogeneity (P = 0.44; I² = 0%) and ‐0.98 mmHg (95% CI ‐1.87 to ‐0.10) for those aged 35 years or more, six studies (N = 2509) with low heterogeneity (P = 0.453; I² = 0%) (Analysis 1.9). The effect on diastolic blood pressure was ‐4.22 mmHg (95% CI ‐5.68 to ‐2.76) for those younger than 35 years, three studies (N = 142) with low heterogeneity (P = 0.44; I² = 0%) and ‐0.60 mmHg (95% CI ‐1.19 to ‐0.02) for those aged 35 years or more, six studies (N = 2509;) with low heterogeneity (P = 0.37; I² = 7%) (Analysis 1.10).
1.9. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 9: Change in systolic blood pressure by age
1.10. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 10: Change in diastolic blood pressure by age
Effect considering all the studies reporting final value of blood pressure
In those studies reporting final values, the effect on systolic blood pressure was ‐1.48 mmHg (95% CI (‐3.57 to 0.62) for those younger than 35 years, six studies (N = 363) with low heterogeneity (P = 0.24; I² = 25%) and ‐3.28 mmHg (95% CI ‐6.77 to ‐0.21) for those aged 35 years or more, six studies (N = 367) with low heterogeneity (P = 0.37; I² = 8%) (Analysis 1.11); diastolic blood pressure was ‐1.39 mmHg (95% CI ‐3.67 to 0.89) in those younger than 35 years, five studies (N = 262) with high heterogeneity (P = 0.05; I² = 54%) and ‐1.52 mmHg (95% CI ‐3.52 to 0.48) in those aged 35 years or more, six studies (N = 267) with low heterogeneity (P = 0.82; I² = 0%) (Analysis 1.12).
1.11. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 11: Final value in systolic blood pressure by age
1.12. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 12: Final value in diastolic blood pressure by age
Analysis by basal calcium intake
Of the 18 studies included and pooled in meta‐analysis, 11 studies presented results by basal calcium intake. See Description of studies. However, one study (Gillman 1995) was carried out in children, so we excluded it from the analysis as the nutrient recommendations for children are different, and another study (Lyle 1992) gave a range of intakes and could not be classified for this analysis.
Effect considering all the studies reporting change or final value of blood pressure
The effect on systolic blood pressure was ‐1.70 mmHg (95% CI ‐6.33 to 2.33) for those that were consuming on average less than 600 mg, one study (N = 58); ‐0.76 mmHg (‐1.75 to 0.22) for those that consumed between 600 and 800 mg of calcium per day, six studies (N = 839) without heterogeneity (P = 0.43; I² = 0%); and ‐1.34 mmHg (95% CI ‐2.80 to 0.13) for those consuming more than 800 mg of calcium per day, four studies (N = 1860) with low heterogeneity (P = 0.78); I² = 0% (Analysis 1.13). The overall effect on diastolic blood pressure was 1.40 mmHg (95% CI ‐1.90 to 4.70) for those that were consuming on average less than 600 mg of calcium per day, one study (N = 58); ‐1.19 mmHg (95% CI ‐2.49 to 0.11) for those that consumed between 600 and 800 mg of calcium per day, six studies (N = 839) with high heterogeneity (P = 0.06; I² = 53%); and ‐1.24 mmHg (95% CI ‐2.29 to ‐0.19) for those consuming more than 800 mg of calcium per day, four studies (N = 1860) with low heterogeneity (P = 0.25; I² = 25%) (Analysis 1.14).
1.13. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 13: Mean difference in systolic blood pressure by basal calcium intake
1.14. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 14: Mean difference in diastolic blood pressure by basal calcium intake
Effect considering only the studies reporting change in blood pressure
None of the studies showing basal calcium intake and reporting change in blood pressure had a group with calcium intake less than 600 mg/day. For those studies showing change in systolic blood pressure, the effect was ‐0.89 mmHg (95% CI ‐1.90 to 0.12) for those who consumed between 600 and 800 mg of calcium per day, five studies (N = 758) with low heterogeneity (P = 0.45; I² = 0%) and ‐1.37 mmHg (95% CI ‐2.86 to 0.12) for those consuming more than 800 mg of calcium per day, three studies (N = 1822) with low heterogeneity (P = 0.64; I² = 0%) (Analysis 1.15). The effect on diastolic blood pressure was ‐1.86 mmHg (95% CI ‐3.68 to 0.03) for those who consumed between 600 and 800 mg of calcium per day, five studies (N = 758) with high heterogeneity (P = 0.05; I² = 73%) and ‐1.32 mmHg (95% CI ‐2.54 to ‐0.10) for those consuming more than 800 mg of calcium per day, three studies (N = 1822) with moderate heterogeneity (P = 0.15; I² = 44%) (Analysis 1.16).
1.15. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 15: Change in systolic blood pressure by basal calcium intake
1.16. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 16: Change in diastolic blood pressure by basal calcium intake
Effect considering all the studies reporting final value of blood pressure
In those studies reporting final values, the effect on systolic blood pressure was ‐1.70 mmHg (95% CI ‐6.33 to 2.93) for those consuming less than 600 mg a day, one study (N = 58); ‐2.17 mmHg (95% CI ‐8.54 to 4.20) for those who consumed between 600 and 800 mg of calcium per day, three studies (N = 183) with high heterogeneity (P = 0.02; I² = 75%); and 0.00 mmHg (95% CI ‐8.93 to 8.93) for those consuming more than 800 mg of calcium per day, one study (N = 38) (Analysis 1.17). The effect on diastolic blood pressure was 1.40 mmHg (95% CI ‐1.90 to 4.70) for those consuming less than 600 mg a day, one study (N = 58); ‐2.18 mmHg (95% CI ‐4.60 to ‐0.25) for those who consumed between 600 and 800 mg of calcium per day, three studies (N = 183) with low heterogeneity (P = 0.26; I² = 25%); and ‐1.00 mmHg (95% CI ‐6.72 to 4.72) for those consuming more than 800 mg of calcium per day, one study (N = 38) (Analysis 1.18).
1.17. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 17: Final value in systolic blood pressure by basal calcium intake
1.18. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 18: Final value in diastolic blood pressure by basal calcium intake
Analysis by dose
Effect considering all the studies reporting change or final value of blood pressure
The overall effect on systolic blood pressure was ‐0.02 mmHg (95% CI ‐2.23 to 2.20) for the group with doses less than 1000 mg, three studies (N = 302) with low heterogeneity (P = 0.88; I² = 0%); ‐1.05 mmHg (95% CI ‐1.91 to ‐0.19) with doses between 1000 and 1500 mg, nine studies (N = 2488) with low heterogeneity (P = 0.69; I² = 0%); and ‐2.79 mmHg (95% CI ‐4.71 to ‐0.86) with doses more than 1500 mg, seven studies (N = 350) with low heterogeneity (P = 0.45; I² = 0%) (Analysis 1.19).
1.19. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 19: Mean difference in systolic blood pressure by dose
The overall effect on diastolic blood pressure was ‐0.41 mmHg (95% CI ‐2.07 to 1.25) for the group with doses less than 1000 mg, two studies (N = 162) without heterogeneity (P = 0.39; I² = 0%); ‐2.03 mmHg (95% CI ‐3.44 to ‐0.62) with doses between 1000 and 1500 mg, eight studies (N = 964) with high heterogeneity (P = 0.006; I² = 63%); and ‐1.35 mmHg (95% CI ‐2.75 to ‐0.05) with doses more than 1500 mg, eight studies (N = 1821) with high heterogeneity (P = 0.04; I² = 51%) (Analysis 1.20).
1.20. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 20: Mean difference in diastolic blood pressure by dose
Effect considering all the studies reporting change value of blood pressure
For those studies showing change in systolic blood pressure, the effect was ‐0.00 (95% CI ‐2.87 to 2.87) with less than 1000 mg of calcium intake, two studies (N = 162) without heterogeneity (P = 0.87; I² = 0%); ‐1.14 (95% CI ‐2.01 to ‐0.27) with 1000‐1500 of calcium intake, eight studies (N = 2365) without heterogeneity (P = 0.65; I² = 0%); and ‐5.70 (95% CI ‐10.58 to ‐0.82) with 1500 mg or more of calcium intake, one study (N = 32) (Analysis 1.21).
1.21. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 21: Change in systolic blood pressure by dose
For those studies showing change in diastolic blood pressure, the effect was ‐0.41 (95% CI ‐2.07 to 1.25) with less than 1000 mg of calcium intake, two studies (N = 162) without heterogeneity (P = 0.39; I² = 0%); ‐2.11 (95% CI ‐3.67 to ‐0.56) with 1000‐1500 of calcium intake, six studies (N = 947) with high heterogeneity (P = 0.002; I² = 71%); and ‐2.15 (95% CI ‐4.59 to ‐0.29) with 1500 mg or more of calcium intake, two studies (N = 1503) (Analysis 1.22).
1.22. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 22: Change in diastolic blood pressure by dose
Effect considering all the studies reporting final value of blood pressure
For those studies reporting final values in systolic blood pressure, the effect was ‐0.11 (95% CI ‐3.44 to 3.21) with less than 1000 mg of calcium intake, two studies (N = 140) without heterogeneity (P = 0.62; I² = 0%); 1.05 (95% CI ‐3.06 to 5.16) with 1000‐1500 of calcium intake, three studies (N = 123) without heterogeneity (P = 0.82; I² = 0%); and ‐2.25 (95% CI ‐4.34 to ‐0.16) with 1500 mg or more of calcium intake, six studies (N = 318) (Analysis 1.23).
1.23. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 23: Final value in systolic blood pressure by dose
For those studies reporting final values in diastolic blood pressure, the effect was ‐3.50 (95% CI ‐7.28, 0.28) with less than 1000 mg of calcium intake, one study (N = 53); ‐1.65 (95% CI ‐5.37 to 2.07) with 1000‐1500 of calcium intake, three studies (N = 109) without heterogeneity (P = 0.87 ; I² = 0%); and ‐0.82 (95% CI ‐2.73 to 1.10) with 1500 mg or more of calcium intake, six studies (N = 318) (Analysis 1.24).
1.24. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 24: Final value in diastolic blood pressure by dose
Analysis by intervention duration
The overall effect on systolic blood pressure was ‐1.63 mmHg (95% CI ‐2.72 to ‐0.53) where the intervention lasted less than six months, 13 studies (N = 766) with low heterogeneity (P = 0.47; I² = 0%); and ‐0.83 mmHg (95% CI ‐1.83 to 0.17) where the intervention lasted six months or more, five studies (N = 2374) with low heterogeneity (P = 0.76; I² = 0%) (Analysis 1.25). The overall effect on diastolic blood pressure was ‐2.16 mmHg (95% CI ‐3.34 to ‐0.98) where the intervention lasted less than six months, 12 studies (N = 665) with moderate heterogeneity (P = 0.06; I² = 40%); and ‐0.43 mmHg (95% CI ‐1.03 to 0.17) where the intervention lasted six months or more, five studies (N = 2374) with low heterogeneity (P = 0.54; I² = 0%) (Analysis 1.26) (test for subgroup differences: Chi² = 8.65, df = 1, P = 0.002, I² = 89.6%).
1.25. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 25: Mean difference in systolic blood pressure by duration
1.26. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 26: Mean difference in diastolic blood pressure by duration
Analysis by intervention type (fortification and supplementation)
The overall effect on systolic blood pressure was ‐1.26 mmHg (95% CI ‐2.02 to ‐0.50) where the intervention was food supplementation, 16 studies (N = 3001) with low heterogeneity (P = 0.54; I² = 0%); and 0.09 mmHg (95% CI ‐3.11 to 3.29) where the intervention was food fortification, two studies (N = 139) with low heterogeneity (P = 0.98; I² = 0%) (Analysis 1.27). The overall effect on diastolic blood pressure was ‐1.45 mmHg (95% CI ‐2.27 to ‐0.43) where the intervention was food supplementation, 17 studies (N = 3039) with high heterogeneity (P = 0.008; I² = 49%); and ‐1.00 mmHg (95% CI ‐6.72 to 4.72) where the intervention was food fortification, one study (N = 38) (Analysis 1.28).
1.27. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 27: Mean difference in systolic blood pressure by intervention type
1.28. Analysis.

Comparison 1: Calcium supplementation/fortification vs control, Outcome 28: Mean difference in diastolic blood pressure by intervention type
Analysis by ethnicity, fat intake, other minerals
It was not possible to do these analyses, as presented in the protocol, as the information was not available.
Planned sensitivity analysis results
1 Sensitivity analysis according to risk of bias
Figure 2 shows risk of bias classification of studies.
Mean effect on systolic blood pressure in 18 studies (N = 3140) (mean difference in all cases) was ‐1.37 mmHg (‐2.08 to ‐0.66). When we restricted the analyses to only those studies with low risk of bias, the results still showed a significant effect:
Random sequence: ‐1.26 mmHg (‐2.04 to ‐0.49) in 10 studies (N = 1730)
Allocation concealment: ‐1.20 mmHg (‐2.09 to ‐0.31) in 7 studies (N = 1193)
Blinding of participants: ‐1.36 mmHg (‐2.11 to ‐0.59) in 13 studies (N = 2827)
Blinding of outcome assessment: ‐1.26 mmHg (‐2.01 to ‐0.50) in 11 studies (N = 2800)
Incomplete outcome data: ‐1.68 mmHg (‐2.69 to ‐0.67) in 11 studies (N = 1250)
Mean effect on diastolic blood pressure in 17 studies (N = 3039) was ‐1.45 mmHg (‐2.23 to ‐0.67). When we restricted the analyses to only those studies with low risk of bias, the results still showed a significant effect:
Random sequence: ‐1.52 mmHg (‐2.49 to ‐0.55) in 9 studies (N = 1629)
Allocation concealment: ‐1.91 mmHg (‐3.38 to ‐0.45) in 6 studies (N = 1092)
Blinding of participants: ‐1.67 mmHg (‐2.63 to ‐0.62) in 12 studies (N = 2726)
Blinding of outcome assessment: ‐1.27 mmHg (‐2.13 to ‐0.41) in 10 studies (N = 2799)
Incomplete outcome data: ‐1.39 mmHg (‐2.55 to ‐0.24) in 10 studies (N = 1149)
2 Sensitivy analysis for industry‐funded studies
We performed a sensitivity analysis excluding five studies that we believed to be industry‐funded (Gillman 1995; Hilary Green 2000; Johnson 1985; Lijnen 1995; Reid 2010).
Mean difference of the effect on systolic blood pressure excluding industry‐funded studies was ‐1.31 [‐2.14, ‐0.49 13 studies (N = 2565), whereas for the industry‐funded studies, the mean difference was ‐1.54 mmHg (95% CI ‐2.94 to ‐0.15) 5 studies (N = 575).Analysis 1.1
Mean difference of the effect on diastolic blood pressure excluding industry‐funded studies was ‐1.45 mmHg (95% CI ‐2.42 to ‐0.49) 12 studies (N = 1257), whereas, for the industry‐funded studies, the mean difference was ‐1.52 mmHg (95% CI ‐2.88 to ‐0.17) 4 studies (N = 474).
3 Sensitivity analysis by position of the participant during blood pressure measurement
| Systolic blood pressure | |
| Sitting position (Belizan 1983; Gillman 1995; Johnson 1985) | ‐1.60 mmHg (95% CI ‐3.23 to 0.03), 3 studies (N = 299) |
| Standing (Lijnen 1995) | ‐5.70 mmHg (95% CI ‐10.58 to ‐0.82), 1 study (N = 32) |
| Supine (McCarron 1985; Thomsen 1987; Entezari 2015) | 1.09 mmHg (95% CI ‐3.23 to 5.42), 3 studies (N = 113) |
| Diastolic blood pressure | |
| Sitting (Belizan 1983; Johnson 1985) | ‐3.30 mmHg (95% CI ‐6.99 to ‐0.40), 2 studies (N = 138) |
| Standing (Lijnen 1995) | ‐3.50 mmHg (95% CI ‐5.29 to ‐1.71), 1 study (N = 32) |
| Supine (McCarron 1985; Thomsen 1987; Entezari 2015 | ‐3.04 mmHg (95% CI ‐6.00 to ‐0.07), 3 studies (N = 113) |
4 Sensitivity analysis for trials with imputed standard deviations
We did not impute any standard deviations for the data from these 16 trials.
Post hoc sensitivity analyses
Sensitivity analysis comparing mean difference and standardised mean difference results
We did a sensitivity analysis comparing mean difference (MD) and standardised mean difference (SMD) results for all 28 outcomes reported in data analysis. Even though the mean difference results were in the same direction, of the 28 analyses performed, eight presented confidence intervals with different statistical significance between MD and SMD results, suggesting that we should be more cautious in interpreting these results. The following list shows cases where the confidence interval crossed the line of no effect on one measurement method but not on the other:
1. The mean difference effect on systolic blood pressure for women was ‐1.25 mmHg (95% CI ‐2.53 to ‐0.03), whereas the standardised mean difference was ‐0.10 mmHg (95% CI ‐0.19 to 0.01) (Analysis 1.1).
2. The mean difference effect on change of systolic blood pressure for men was ‐2.01 mmHg (95% CI ‐3.95 to ‐0.08), whereas the standardised mean difference was ‐0.23 mmHg (95% CI ‐0.49 to 0.02) (Analysis 1.3).
3. The mean difference effect on change of diastolic blood pressure for women was ‐1.87 mmHg (95% CI ‐3.62 to ‐0.12), whereas the standardised mean difference was ‐0.25 mmHg (95% CI ‐0.53 to 0.03) (Analysis 1.4).
4. The mean difference effect on the final value of diastolic blood pressure for both genders was ‐2.33 mmHg (95% CI ‐4.50 to ‐0.17), whereas the standardised mean difference was ‐0.32 mmHg (95% CI ‐0.65 to 0.01) (Analysis 1.6).
5. The mean difference effect on change in systolic blood pressure in those less than 35 years was ‐2.34 (95% CI ‐4.55 to ‐0.13), whereas the standardised mean difference was (SMD ‐0.31, 95% CI ‐0.64 to 0.03) (Analysis 1.9).
6. The mean difference effect on systolic blood pressure in the group with intakes higher than 800 mg a day was ‐1.34 mmHg (95% CI ‐2.80 to 0.13), whereas the standardised mean difference was (SMD ‐0.09 (‐0.19, ‐0.00)(Analysis 1.13).
7. The mean difference effect on diastolic blood pressure in the group with intakes higher than 800 mg a day was ‐1.24 mmHg (95% CI ‐2.29 to 0.19), whereas the standardised mean difference was (SMD ‐0.14 (‐0.30, 0.01) (Analysis 1.14).
8. The mean difference effect on change of diastolic blood pressure in the group with intakes higher than 800 mg a day was ‐1.32 mmHg (95% CI ‐2.54 to 0.10), whereas the standardised mean difference was (SMD ‐0.17 (‐0.36, 0.03) (Analysis 1.16).
When we analysed the results in units of standard deviation (SMDs), each study weight was modified; if the weight increased in those studies showing more effect, the final result using this method showed a higher effect. Correspondingly, when the weights were increased in the studies with no effect, the final result tended to show a weaker global effect.
Sensitivity analysis excluding studies with less than 3.5 months of intervention
Of the 18 studies included in the meta‐analysis, eight (Belizan 1983; Cutler 1992; Johnson 1985; Lijnen 1995; Reid 2005; Reid 2010; Sacks 1998; Thomsen 1987) presented interventions lasting more than 3.5 months (N = 2619).
The mean effect in systolic blood pressure was ‐1.37 mmHg (‐2.08 to ‐0.66) (Analysis 1.1). When we performed a sensitivity analysis only including the studies with interventions lasting more than 3.5 months, the results were still significant: ‐1.03 mmHg (‐1.87 to ‐0.19).
The mean effect in diastolic blood pressure was ‐1.45 mmHg (‐2.23 to ‐0.67) (Analysis 1.2). When we performed a sensitivity analysis only including the studies with interventions lasting more than 3.5 months, the results were still significant: ‐1.38 mmHg (95% CI ‐2.435to ‐0.41).
Sensitivity analysis by blood pressure methodology
Blood pressure was measured using an auscultatory method in seven studies Belizan 1983; Cutler 1992; Johnson 1985; Lyle 1992; Lyle 1987; McCarron 1985; Thomsen 1987; (N = 786), and using an oscillometric method in six studies Davis 1996; Gillman 1995 (only systolic blood pressure (N = 101); Hilary Green 2000; Reid 2005; Reid 2010; Sacks 1998 (N = 2123).
| Systolic blood pressure | |
| Auscultatory | ‐1.12 mmHg (95% CI ‐2.19 to ‐0.04) |
| Oscillometric | ‐1.34 mmHg (95% CI ‐2.38 to ‐0.31) |
| Diastolic blood pressure | |
| Auscultatory | ‐2.19 mmHg (95% CI ‐4.12 to ‐0.25) |
| Oscillometric | ‐0.85 mmHg (95% CI ‐1.54 to ‐0.16) |
Sensitivity analysis by studies reporting clinic blood pressure measurements and automated ambulatory blood pressure
Blood pressure was measured at a clinic in eight studies (Belizan 1983; Cutler 1992; Entezari 2015; Gillman 1995; Johnson 1985; Lyle 1987; Lyle 1992; McCarron 1985; Thomsen 1987; Yosephin 2015 (N = 887)); and using automated ambulatory measurements in three studies (Davis 1996; Hilary Green 2000; Sacks 1998 (N = 228)).
We did not find any study using ambulatory measurements reported by the participant. Those studies reporting ambulatory measurement were conducted with automated devices.
| Systolic blood pleasure | |
| Clinic measurements | ‐1.15 mmHg (95% CI ‐2.09 to 0.20) |
| Automated ambulatory measurements | ‐0.92 mmHg (95% CI ‐2.63 to 0.78) |
| Diastolic blood pressure | |
| Clinic | ‐2.24 mmHg (95% CI ‐3.496to 0.52) |
| Automated ambulatory measurements | ‐0.83 mmHg (95% CI ‐2.05 to 0.39) |
Assessment of potential reporting biases (such as publication bias)
Funnel plot visual analysis revealed no asymmetry (Figure 4; Figure 5).
4.

Funnel plot of comparison: 1. Calcium supplementation/fortification vs control. Outcome 1.1: Mean difference in systolic blood pressure
5.

Funnel plot of comparison: 1. Calcium supplementation/fortification vs control. Outcome 1.2: Mean difference in diastolic blood pressure
Secondary Outcomes
Cutler 1992 was the only article evaluating side effects, but reported none. A further two study reports (Lyle 1987; McCarron 1985) mentioned that the supplements were well tolerated and that no participants required withdrawal from the trial after randomisation.
No trials reported any incidence of kidney stone formation, iron deficiency anaemia, anaemia, cardiovascular events, myocardial infarction, stroke or mortality.
Discussion
The aim of this review was to evaluate the effectiveness of calcium supplementation, as a single nutrient, for the prevention of primary hypertension. We analysed the effect of calcium according to sex, intervention dose, intervention duration, age of participants and basal calcium intake.
Summary of main results
There was a small reduction in both systolic and diastolic blood pressure in the groups receiving calcium compared to those receiving placebo or control. We found a lower effect in those studies that did not discriminate between the results by sex and, in at least one of those studies (Lyle 1992), a sex imbalance at randomisation was reported as a possible explanation.
The effect was confirmed in multiple prespecified subgroups. We detected a dose‐response effect trend, both in systolic and in diastolic blood pressure, that could reinforce the efficacy of the intervention. Those studies with interventions of 1500 mg of calcium a day or higher showed a higher decrease in systolic and diastolic blood pressure than those studies with interventions less than 1000 mg a day. For those studies with interventions of less than 1000 mg, we found no effect, although in this last group there were very few studies from which to draw any conclusion.
When we evaluated the overall effect and change of blood pressure before and after the intervention with calcium, those studies that were performed in younger people tended to show higher reductions in systolic and diastolic blood pressure than those in older people.
There was no difference in the effect by baseline calcium intake, reported in ten of the 18 studies included in the meta‐analysis. This could be due to different methods used in assessing calcium intake among the studies. The information provided in this review, therefore, does not contradict the possibility of a higher effect in populations with low calcium intake, as has been suggested before (Belizan 1980; Belizan 1983; WHO 2009). Only two of the selected studies were performed in low‐ or middle‐income countries.
It is difficult to assess the effect of differences in the forms of calcium interventions, such as diet, fortification or supplements, since 14 of the 18 studies included in the meta‐analysis used supplementation as the intervention.
Our data show a greater effect in those studies lasting less than six months. There is some suggestion that the effect might be lost over time in populations with adequate calcium intake, as some studies showed no effect after 30 months (Reid 2005) and one year (Thomsen 1987).
None of the secondary outcomes were reported in the included studies, therefore, we found no evidence of adverse effects.
Overall completeness and applicability of evidence
We found a substantial number of studies to address the objectives of the review, with no evidence of publication bias, although some population groups such as children and teenagers might not be well represented. Only one study was performed in children (Gillman 1995), and one in teenagers (Davis 1996).
The effect was higher in four studies from low‐ and middle‐income countries (Belizan 1983; Entezari 2015; Shidfar 2010; Yosephin 2015) (MD ‐1.73 mmHG, 95% CI ‐3.76 to ‐0.29); however, we also found an effect on blood pressure reduction in high‐income countries, 14 studies (‐1.32 mmHg, 95% CI ‐2.08 to ‐0.57).
The effect on diastolic blood pressure was higher in men, in those younger than 35 years and in those receiving the intervention for less than six months (test for subgroup differences: P = 0.03, P = 0.004 and P = 0.001, respectively).
The other subgroup analyses look underpowered and, therefore, need to be interpreted cautiously. For example, we observed a trend to higher effect with increasing doses; however, the test for subgroup differences indicated P values that were not statistically significant (0.14 and 0.34 for systolic and diastolic blood pressure, respectively).
The findings of this review support the importance of an adequate calcium intake for the prevention of high blood pressure and the need to explore interventions to increase calcium intake in both men and women. For cardiovascular risk prevention, a small decrease in blood pressure outweighs a larger decrease only among hypertensive groups (Gillman 1995). Additionally, small reductions in blood pressure of the general population are predicted to have important health implications, as they are shown to produce rapid reductions in vascular disease risk even in individuals with normal blood pressure ranges (Lewington 2002). Population‐wide decreases in blood pressure of 2–3 mmHg could decrease the prevalence of hypertension by 17%, the risk of coronary artery disease by 6% and the risk of stroke by 15% (Cook 1995). A 2 mmHg lower systolic blood pressure is predicted to produce about 10% lower stroke mortality and about 7% lower mortality from ischaemic heart disease, and a 5 mmHg reduction in systolic blood pressure at the population level is predicted to result in a 14% reduction in stroke death, 9% reduction in coronary artery disease‐related death and a 7% reduction in total mortality (Whelton 2002). In the same way, a 2 mmHg reduction in systolic blood pressure in adults is estimated to have the potential to save about 12,000 lives a year in the United States (Stamler 1991) and to generate an increase in life expectancy of 1.8 months in men and 1.4 months in women (Selmer 2000).
Globally, around 3.5 billion people are at risk of calcium deficiency (Kumssa 2015). A fortification strategy may be the most appropriate strategy to target countries with general low calcium intake (Cormick 2019a; Cormick 2020).
Quality of the evidence
We included 20 trials, of which 18 were included in the meta‐analysis with 3140 participants, providing high‐quality evidence (Guyatt 2011) of the effect of calcium supplementation on systolic and diastolic blood pressure (Table 1). The quality of some outcomes was rated as being at moderate certainty due to imprecision (small sample size).
Risks of bias for random sequence generation and incomplete outcome data were low for 55% of the studies; allocation concealment risk of bias was low for 40% of the studies and unclear for the remainder; blinding of participants and personnel was low for 65% of the studies and risk of detection bias and attrition bias was low for 60% of the studies. We rated 85% of the studies as being at low risk of reporting bias.
Potential biases in the review process
We restricted this review to clinical trials in which the intervention was calcium supplementation as a single ingredient, which limited the number of studies we could include. On the other hand, we used an exhaustive search strategy to avoid publication selection bias. Two review authors independently assessed the articles and double‐checked data extraction to minimise errors.
Many of the studies were old and, even though in these cases published information was not enough to assess risk of bias, we attempted to contact authors, although the response was limited. Nevertheless, there was generally a low risk of bias.
Agreements and disagreements with other studies or reviews
Our results are in line with the most recent review by Van Mierlo 2006 that included a meta‐analysis of 40 randomised controlled trials (RCTs) with normotensive and hypertensive people, showing that supplementation with around 1 gm of calcium per day significantly reduced systolic blood pressure by 1.9 mmHg and diastolic blood pressure by 1.0 mmHg. This review also found a higher effect in populations with low basal calcium intake. In a previous meta‐analysis involving 42 trials in normotensive and hypertensive people, the pooled analysis showed a reduction in systolic blood pressure of ‐1.44 mmHg (95% CI ‐2.20 to ‐0.68; P < 0.001) and in diastolic blood pressure of ‐0.84 mmHg (95% CI ‐1.44 to ‐0.24; P < 0.001) (Griffith 1999).
Our results are in the same direction as the Dickinson 2006 review in hypertensive people. Although this showed a statistically significantly larger reduction in blood pressure in the calcium group, the authors interpret this as more likely reflecting a bias due to poor‐quality trials than a real effect. We performed a sensitivity analysis excluding studies classified at high and moderate risk of bias. All studies were classified at low risk of selective reporting bias, so we could conduct no analysis for this domain. For the remaining five domains evaluated, the effect persisted after removing studies classified as being at high or moderate risk. The Evidence Analysis Library (EAL) guideline encourages adults with hypertension to consume adequate amounts of dietary calcium to meet the Dietary Reference Intakes (DRI). The authors stated that dietary calcium intake of 800 mg or more per day reduced systolic blood pressure up to 4 mm Hg and diastolic blood pressure up to 2 mm Hg in adults with hypertension. If an adult is unable to meet the DRI for calcium with diet alone, they consider calcium supplementation of 1,000 to 1,500 mg/day to aid in blood pressure control. A strong body of research indicated that calcium supplementation of 1,000 to 1,500 mg/d reduced systolic blood pressure up to 3.0 mm Hg and diastolic blood pressure up to 2.5 mm Hg in adults with hypertension (Lennon 2017).
Calcium intake also showed effects on different populations. A Cochrane review (Hofmeyr 2018) showed that a good calcium intake has benefits for pregnancy outcomes, effects which are thought to be mediated by blood pressure reduction. Preliminary observations showed that calcium supplementation during pregnancy could also have effects on reducing the blood pressure of the progeny (Belizan 1988, Hatton 2003). Consequently, calcium intake could play a role in the prevention of hypertension, particularly at a young age where small changes in blood pressure could have a higher effect. It has been shown that lowering blood pressure at younger ages is relevant, since the relative risk of cardiovascular diseases with blood pressure decreases with age and no significant deviations from linearity occurred in the associations of either systolic or diastolic blood pressure (Rapsomaniki 2014).
A recent systematic review and meta‐analysis investigated the effect of calcium and vitamin D co‐supplementation on blood pressure (Morvaridzadeh 2020). The meta‐analysis of the eight trials included showed a reduction in blood pressure in the intervention group compared with control (standardised mean difference (SMD) −0.23; 95% CI, −0.52 to 0.06; SMD −0.29; 95% CI, −0.55 to −0.02, respectively), with a greater diastolic blood pressure reduction in young adults than other age groups. Our findings go in line with this study, although with lower reduction in both systolic and diastolic blood pressure (SMD ‐0.10 (95% CI ‐0.19 to ‐0.01); SMD ‐0.24 (95% CI ‐0.43 to ‐0.04), respectively) and higher blood pressure reductions in young adults. Such findings could suggest a major effect of vitamin D co‐supplementation.
Authors' conclusions
Implications for practice.
An increase in calcium intake slightly reduces both systolic and diastolic blood pressure in normotensive people. The effect was confirmed in multiple prespecified subgroups, including a possible dose‐response effect, reinforcing the efficacy of the intervention. The effects can be observed after only 3.5 months of intervention. Although the effect is small, an adequate calcium intake should be an objective to be reached in the general population.
Implications for research.
Randomised controlled trials (RCT) are needed with high power in the early stages of life for a long period of time (at least one year), randomising young people of both sexes to attain a daily calcium intake of at least 1 gm in comparison with a control group. Subgroup analyses should be prespecified and powered to assess outcomes on systolic and diastolic blood pressure related to basal calcium intake, age, sex, basal blood pressure, and body mass index (BMI).
There is a need for clinical and basic studies designed to confirm the mechanisms proposed about the effect of calcium intake on blood pressure (Villa‐Etchegoyen 2019). This will allow the identification of early markers of individuals that could be more susceptible to calcium intake.
More research is needed to assess the dose required and the best strategy to improve calcium intake, comparing the effect of dietary calcium with a supplemental version. Furthermore, if the effect of calcium intake on blood pressure is confirmed, it will be desirable that studies of calcium fortification include populations with low calcium intake to assess a universal effect on blood pressure.
Any future research on calcium intake must report adverse events, particularly in older people.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2021 | New citation required but conclusions have not changed | 2 additional studies identified |
| 27 November 2020 | Amended | Addition of the methods section "Summary of findings and assessment of the certainty of the evidence" |
| 25 November 2020 | New search has been performed | New search run. Two new studies included. |
History
Protocol first published: Issue 8, 2012 Review first published: Issue 6, 2015
Acknowledgements
The authors would like to acknowledge the contribution of Daniel Comandé to the search strategy.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Daily and Versions(R) <1946 to September 28, 2020> Search Search Date: 29 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 dietary supplements/ 2 calcium, dietary/ 3 calcium carbonate/ 4 *calcium/ad, th, tu 5 (calcium adj4 (add$ or boost$ or consum$ or daily or day or diet$ or enrich$ or extra or fed or feed$ or fortif$ or intake$ or suppl$)).tw,kf. 6 (calcium adj4 (beverag$ or capsul$ or compound$ or food$ or liquid$ or oral$ or pill$ or powder$ or sprinkl$ or tab$)).tw,kf. 7 (calcium adj3 (acetate$ or carbonate$ or chloride$ or citrate$ or gluconate$ or glycerophosphate$ or hydroxide$ or hydroxyapatite$ or lactate$ or oxide$ or phosphate$ or sulfate$)).tw,kf. 8 (apocal or "apo‐cal" or aragonite or biocal or "bo‐ne‐ca" or cacit or cal sup or "cal‐sup" or calcanate or calcefor or calci aid or calci chew or calcichew or calcigamma or calcigaurol or calcilos or calcimax or calcimix or calcioral or calciprat or calcite or calcitridin or calcuren or caldoral or "calmate 500" or calperos or calsan or calsup or caltab or caltrate or cantacid or "cc‐nefro 500" or fixical or maalox or mastical or maxicalc or maxi kalz or maxicalc or mega cal or netra or noacid or orocal or "os cal" or "os‐cal" or oscal or "ospur ca 500" or "osteocal 500" or osteomin or pluscal or renacal or "tums ultra" or "tzarevet x" or vaterite).tw,kf. 9 or/1‐8 10 (antihypertens$ or hypertens$ or prehypertens$).tw,kf. 11 exp blood pressure/ 12 (blood pressur$ or bloodpressur$).tw,kf. 13 ((arterial or diastolic or systolic) adj2 pressur$).tw,kf. 14 .tw,kf. 15 or/10‐14 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomi?ed.ab. 19 placebo.ab. ) 20 clinical trials as topic/ 21 randomly.ab. 22 trial.ti. 23 or/16‐22 24 animals/ not (humans/ and animals/) 25 (eclampsia or preeclampsia).ti. 26 23 not (24 or 25) 27 9 and 15 and 26
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Hypertension Specialised Register via Cochrane Register of Studies (CRS‐Web)
Search Date: 30 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 (calcium NEAR4 (add* OR boost* OR consum* OR daily OR day OR diet* OR enrich* OR extra OR fed OR feed* OR fortif* OR intake* OR suppl*)) AND INSEGMENT #2 (calcium NEAR4 (beverag* OR capsul* OR compound* OR food* OR liquid* OR oral* OR pill* OR powder* OR sprinkl* OR tab*)) AND INSEGMENT #3 (calcium NEAR3 (acetate* OR carbonate* OR chloride* OR citrate* OR gluconate* OR glycerophosphate* OR hydroxide* OR hydroxyapatite* OR lactate* OR oxide* OR phosphate* OR sulfate*)) AND INSEGMENT #4 (apocal OR "apo‐cal" OR aragonite OR biocal OR "bo‐ne‐ca" OR cacit OR cal sup OR "cal‐sup" OR calcanate OR calcefor OR calci aid OR calci chew OR calcichew OR calcigamma OR calcigaurol OR calcilos OR calcimax OR calcimix OR calcioral OR calciprat OR calcite OR calcitridin OR calcuren OR caldoral OR "calmate 500" OR calperos OR calsan OR calsup OR caltab OR caltrate OR cantacid OR "cc‐nefro 500" OR fixical OR maalox OR mastical OR maxicalc OR maxi kalz OR maxicalc OR mega cal OR netra OR noacid OR orocal OR "os cal" OR "os‐cal" OR oscal OR "ospur ca 500" OR "osteocal 500" OR osteomin OR pluscal OR renacal OR "tums ultra" OR "tzarevet x" OR vaterite) AND INSEGMENT #5 (#1 OR #2 OR #3 OR #4) AND INSEGMENT #6 RCT:DE AND INSEGMENT #7 Review:ODE AND INSEGMENT #8 (#6 OR #7) AND INSEGMENT #9 #5 AND #8 AND INSEGMENT
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Cochrane Central Register of Controlled Trials (Issue 8, 2020) via Cochrane Register of Studies (CRS‐Web)
Search Date: 29 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Dietary Supplements AND CENTRAL:TARGET #2 MESH DESCRIPTOR Calcium, Dietary AND CENTRAL:TARGET #3 MESH DESCRIPTOR Calcium Carbonate AND CENTRAL:TARGET #4 MESH DESCRIPTOR Calcium WITH QUALIFIER AD TU AND CENTRAL:TARGET #5 (calcium NEAR4 (add* OR boost* OR consum* OR daily OR day OR diet* OR enrich* OR extra OR fed OR feed* OR fortif* OR intake* OR suppl*)) AND CENTRAL:TARGET #6 (calcium NEAR4 (beverag* OR capsul* OR compound* OR food* OR liquid* OR oral* OR pill* OR powder* OR sprinkl* OR tab*)) AND CENTRAL:TARGET #7 (calcium NEAR3 (acetate* OR carbonate* OR chloride* OR citrate* OR gluconate* OR glycerophosphate* OR hydroxide* OR hydroxyapatite* OR lactate* OR oxide* OR phosphate* OR sulfate*)) AND CENTRAL:TARGET #8 (apocal OR "apo‐cal" OR aragonite OR biocal OR "bo‐ne‐ca" OR cacit OR cal sup OR "cal‐sup" OR calcanate OR calcefor OR calci aid OR calci chew OR calcichew OR calcigamma OR calcigaurol OR calcilos OR calcimax OR calcimix OR calcioral OR calciprat OR calcite OR calcitridin OR calcuren OR caldoral OR "calmate 500" OR calperos OR calsan OR calsup OR caltab OR caltrate OR cantacid OR "cc‐nefro 500" OR fixical OR maalox OR mastical OR maxicalc OR maxi kalz OR maxicalc OR mega cal OR netra OR noacid OR orocal OR "os cal" OR "os‐cal" OR oscal OR "ospur ca 500" OR "osteocal 500" OR osteomin OR pluscal OR renacal OR "tums ultra" OR "tzarevet x" OR vaterite) AND CENTRAL:TARGET #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) AND CENTRAL:TARGET #10 (antihypertens* OR hypertens* OR prehypertens*):TI,AB AND CENTRAL:TARGET #11 MESH DESCRIPTOR Blood Pressure EXPLODE ALL AND CENTRAL:TARGET #12 (blood pressure* OR bloodpressur*) AND CENTRAL:TARGET #13 ((arterial OR diastolic OR systolic) NEAR2 pressur*) AND CENTRAL:TARGET #14 (bp OR dbp OR hbp OR sbp) AND CENTRAL:TARGET #15 (#10 OR #11 OR #12 OR #13 OR #14) AND CENTRAL:TARGET #16 #9 AND #15 AND CENTRAL:TARGET
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: Embase <1974 to 2020 September 28> Search Date: 29 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 diet supplementation/ 2 calcium intake/ 3 calcium carbonate/ 4 (calcium adj4 (add$ or boost$ or consum$ or daily or day or diet$ or enrich$ or extra or fed or feed$ or fortif$ or intake$ or suppl$)).tw. 5 (calcium adj4 (beverag$ or capsul$ or compound$ or food$ or liquid$ or oral$ or pill$ or powder$ or sprinkl$ or tab$)).tw. 6 (calcium adj3 (acetate$ or carbonate$ or chloride$ or citrate$ or gluconate$ or glycerophosphate$ or hydroxide$ or hydroxyapatite$ or lactate$ or oxide$ or phosphate$ or sulfate$)).tw. 7 (apocal or "apo‐cal" or aragonite or biocal or "bo‐ne‐ca" or cacit or cal sup or "cal‐sup" or calcanate or calcefor or calci aid or calci chew or calcichew or calcigamma or calcigaurol or calcilos or calcimax or calcimix or calcioral or calciprat or calcite or calcitridin or calcuren or caldoral or "calmate 500" or calperos or calsan or calsup or caltab or caltrate or cantacid or "cc‐nefro 500" or fixical or maalox or mastical or maxicalc or maxi kalz or maxicalc or mega cal or netra or noacid or orocal or "os cal" or "os‐cal" or oscal or "ospur ca 500" or "osteocal 500" or osteomin or pluscal or renacal or "tums ultra" or "tzarevet x" or vaterite).tw. 8 or/1‐7 9 (antihypertens$ or hypertens$ or prehypertens$).tw. 10 exp blood pressure/ 11 (blood pressur$ or bloodpressur$).tw. 12 ((arterial or diastolic or systolic) adj2 pressur$).tw. 13 (bp or dbp or hbp or sbp).tw. 14 or/9‐13 15 randomized controlled trial/ 16 crossover procedure/ 17 double‐blind procedure/ 18 (randomi$ed or randomly).tw. 19 (crossover$ or cross‐over$).tw. 20 placebo.ab. 21 (doubl$ adj blind$).tw. 22 assign$.ab. 23 allocat$.ab. 24 or/15‐23 25 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 26 (eclampsia or preeclampsia).ti. 27 24 not (25 or 26) 28 8 and 14 and 27
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: ClinicalTrials.gov Search Date: 29 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Other terms: randomized Study type: Interventional Studies (Clinical Trials) Interventions: Dietary Calcium Supplement OR calcium supplement OR calcium carbonate Outcome Measures: blood pressure
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Database: WHO International Clinical Trials Registry Platform (ICTRP) via Cochrane Register of Studies (CRS‐Web) Search Date: 30 September 2020 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1 MESH DESCRIPTOR Calcium, Dietary AND CENTRAL:TARGET #2 MESH DESCRIPTOR Calcium Carbonate AND CENTRAL:TARGET #3 MESH DESCRIPTOR Calcium WITH QUALIFIER AD TU TO AND CENTRAL:TARGET #4 (calcium NEAR4 (add* OR boost* OR consum* OR daily OR day OR diet* OR enrich* OR extra OR fed OR feed* OR fortif* OR intake* OR suppl*)) AND CENTRAL:TARGET #5 (calcium NEAR4 (beverag* OR capsule* OR compound* OR food* OR liquid* OR oral* OR pill* OR powder* OR sprinkl* OR tab*)) AND CENTRAL:TARGET #6 (apocal OR "apo‐cal" OR aragonite OR biocal OR "bo‐ne‐ca" OR cacit OR cal sup OR "cal‐sup" OR calcanate OR calcefor OR calci aid OR calci chew OR calcichew OR calcigamma OR calcigaurol OR calcilos OR calcimax OR calcimix OR calcioral OR calciprat OR calcite OR calcitridin OR calcuren OR caldoral OR "calmate 500" OR calperos OR calsan OR calsup OR caltab OR caltrate OR cantacid OR "cc‐nefro 500" OR fixical OR maalox OR mastical OR maxicalc OR maxi kalz OR maxicalc OR mega cal OR netra OR noacid OR orocal OR "os cal" OR "os‐cal" OR oscal OR "ospur ca 500" OR "osteocal 500" OR osteomin OR pluscal OR renacal OR "tums ultra" OR "tzarevet x" OR vaterite) AND CENTRAL:TARGET #7 (calcium NEAR3 (acetate* OR carbonate* OR chloride* OR citrate* OR gluconate* OR glycerophosphate* OR hydroxide* OR hydroxyapatite* OR lactate* OR oxide* OR phosphate* OR sulfate*)) AND CENTRAL:TARGET #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) AND CENTRAL:TARGET #9 (antihypertens* OR hypertens* OR prehypertens*) AND CENTRAL:TARGET #10 MESH DESCRIPTOR Blood pressure EXPLODE ALL AND CENTRAL:TARGET #11 (blood pressur* OR bloodpressur*) AND CENTRAL:TARGET #12 (bp OR dbp OR hbp OR sbp) AND CENTRAL:TARGET #13 (#9 OR #10 OR #11 OR #12) AND CENTRAL:TARGET #14 (NCT0* or ACTRN* or ChiCTR* or DRKS* or EUCTR* or eudract* or IRCT* or ISRCTN* or JapicCTI* or JPRN* or NTR0* or NTR1* or NTR2* or NTR3* or NTR4* or NTR5* or NTR6* or NTR7* or NTR8* or NTR9* or SRCTN* or UMIN0*):AU AND CENTRAL:TARGET #15 http*:SO AND CENTRAL:TARGET #16 (#14 OR #15) AND CENTRAL:TARGET #17 #8 AND #13 AND #16 AND CENTRAL:TARGET
Data and analyses
Comparison 1. Calcium supplementation/fortification vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean difference in systolic blood pressure | 18 | 3140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.20, ‐0.06] |
| 1.1.1 Women | 8 | 1915 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.19, ‐0.01] |
| 1.1.2 Men | 5 | 507 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.43, ‐0.04] |
| 1.1.3 Both genders | 6 | 718 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.34, 0.00] |
| 1.2 Mean difference in diastolic blood pressure | 17 | 3039 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐2.23, ‐0.67] |
| 1.2.1 Women | 8 | 1915 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.86, ‐0.22] |
| 1.2.2 Men | 5 | 507 | Mean Difference (IV, Random, 95% CI) | ‐1.99 [‐3.25, ‐0.74] |
| 1.2.3 Both genders | 5 | 617 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐3.83, 0.74] |
| 1.3 Change data: systolic blood pressure | 11 | 2786 | Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.02, ‐0.52] |
| 1.3.1 Women | 5 | 1748 | Mean Difference (IV, Random, 95% CI) | ‐1.47 [‐2.87, ‐0.08] |
| 1.3.2 Men | 4 | 432 | Mean Difference (IV, Random, 95% CI) | ‐2.01 [‐3.95, ‐0.08] |
| 1.3.3 Both genders | 3 | 606 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.96, 0.18] |
| 1.4 Change data: diastolic blood pressure | 10 | 2685 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.61, ‐0.63] |
| 1.4.1 Women | 5 | 1748 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐3.62, ‐0.12] |
| 1.4.2 Men | 4 | 432 | Mean Difference (IV, Random, 95% CI) | ‐2.24 [‐3.75, ‐0.73] |
| 1.4.3 Both genders | 2 | 505 | Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.73, 1.01] |
| 1.5 Final value: systolic blood pressure | 12 | 630 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.72, ‐0.14] |
| 1.5.1 Women | 5 | 259 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐3.00, 2.60] |
| 1.5.2 Men | 2 | 124 | Mean Difference (IV, Random, 95% CI) | ‐5.36 [‐9.03, ‐1.70] |
| 1.5.3 Both genders | 5 | 247 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐4.07, 1.41] |
| 1.6 Final value: diastolic blood pressure | 11 | 529 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐2.82, ‐0.11] |
| 1.6.1 Women | 5 | 259 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐2.38, 1.34] |
| 1.6.2 Men | 2 | 124 | Mean Difference (IV, Random, 95% CI) | ‐1.88 [‐4.26, 0.50] |
| 1.6.3 Both genders | 4 | 146 | Mean Difference (IV, Random, 95% CI) | ‐2.25 [‐5.49, 0.99] |
| 1.7 Mean difference in systolic blood pressure by age | 18 | 3140 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.18, ‐0.04] |
| 1.7.1 Less than 35 years of age | 8 | 452 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.43, ‐0.02] |
| 1.7.2 35 years and older | 10 | 2688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.17, ‐0.02] |
| 1.8 Mean difference in diastolic blood pressure by age | 17 | 3039 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.33, ‐0.07] |
| 1.8.1 Less than 35 years of age | 7 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.81, ‐0.10] |
| 1.8.2 35 years and older | 10 | 2688 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.16, ‐0.01] |
| 1.9 Change in systolic blood pressure by age | 9 | 2651 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.99, ‐0.35] |
| 1.9.1 Less than 35 years of age | 3 | 142 | Mean Difference (IV, Random, 95% CI) | ‐2.34 [‐4.55, ‐0.13] |
| 1.9.2 35 years and older | 6 | 2509 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.87, ‐0.10] |
| 1.10 Change in diastolic blood pressure by age | 9 | 2651 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.79, ‐0.67] |
| 1.10.1 Less than 35 years of age | 3 | 142 | Mean Difference (IV, Random, 95% CI) | ‐4.22 [‐5.68, ‐2.76] |
| 1.10.2 35 years and older | 6 | 2509 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.19, ‐0.02] |
| 1.11 Final value in systolic blood pressure by age | 12 | 630 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.72, ‐0.14] |
| 1.11.1 Less than 35 years of age | 6 | 363 | Mean Difference (IV, Random, 95% CI) | ‐1.48 [‐3.57, 0.62] |
| 1.11.2 35 years and older | 6 | 267 | Mean Difference (IV, Random, 95% CI) | ‐3.28 [‐6.77, 0.21] |
| 1.12 Final value in diastolic blood pressure by age | 11 | 529 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐2.82, ‐0.11] |
| 1.12.1 Less than 35 years of age | 5 | 262 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.67, 0.89] |
| 1.12.2 35 years and older | 6 | 267 | Mean Difference (IV, Random, 95% CI) | ‐1.52 [‐3.52, 0.48] |
| 1.13 Mean difference in systolic blood pressure by basal calcium intake | 10 | 2757 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.77, ‐0.16] |
| 1.13.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐6.33, 2.93] |
| 1.13.2 Calcium Intake from 600 to less than 800 mg a day | 6 | 839 | Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.75, 0.22] |
| 1.13.3 Calcium intake above 800 mg a day | 4 | 1860 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐2.80, 0.13] |
| 1.14 Mean difference in diastolic blood pressure by basal calcium intake | 10 | 2757 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.84, ‐0.23] |
| 1.14.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐1.90, 4.70] |
| 1.14.2 Calcium Intake from 600 to less than 800 mg a day | 6 | 839 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.49, 0.11] |
| 1.14.3 Calcium intake above 800 mg a day | 4 | 1860 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.29, ‐0.19] |
| 1.15 Change in systolic blood pressure by basal calcium intake | 7 | 2580 | Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.88, ‐0.21] |
| 1.15.1 Calcium Intake from 600 to less than 800 mg a day | 5 | 758 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.90, 0.12] |
| 1.15.2 Calcium intake above 800 mg a day | 3 | 1822 | Mean Difference (IV, Random, 95% CI) | ‐1.37 [‐2.86, 0.12] |
| 1.16 Change in diastolic blood pressure by basal calcium intake | 7 | 2580 | Mean Difference (IV, Random, 95% CI) | ‐1.46 [‐2.49, ‐0.43] |
| 1.16.1 Calcium Intake from 600 to less than 800 mg a day | 5 | 758 | Mean Difference (IV, Random, 95% CI) | ‐1.86 [‐3.68, ‐0.03] |
| 1.16.2 Calcium intake above 800 mg a day | 3 | 1822 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐2.54, ‐0.10] |
| 1.17 Final value in systolic blood pressure by basal calcium intake | 5 | 279 | Mean Difference (IV, Random, 95% CI) | ‐1.77 [‐5.48, 1.93] |
| 1.17.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐6.33, 2.93] |
| 1.17.2 Calcium Intake from 600 to less than 800 mg a day | 3 | 183 | Mean Difference (IV, Random, 95% CI) | ‐2.17 [‐8.54, 4.20] |
| 1.17.3 Calcium intake above 800 mg a day | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐8.93, 8.93] |
| 1.18 Final value in diastolic blood pressure by basal calcium intake | 5 | 279 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐3.25, 0.87] |
| 1.18.1 Calcium Intake below 600 mg a day | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐1.90, 4.70] |
| 1.18.2 Calcium Intake from 600 to less than 800 mg a day | 3 | 183 | Mean Difference (IV, Random, 95% CI) | ‐2.18 [‐4.60, 0.25] |
| 1.18.3 Calcium intake above 800 mg a day | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐6.72, 4.72] |
| 1.19 Mean difference in systolic blood pressure by dose | 18 | 3140 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐1.93, ‐0.45] |
| 1.19.1 Less than 1000 mg of calcium intake | 3 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐2.23, 2.20] |
| 1.19.2 1000 ‐ 1500 of calcium intake | 9 | 2488 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.91, ‐0.19] |
| 1.19.3 1500 mg or more of calcium intake | 7 | 350 | Mean Difference (IV, Random, 95% CI) | ‐2.79 [‐4.71, ‐0.86] |
| 1.20 Mean difference in diastolic blood pressure by dose | 17 | 3039 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐2.35, ‐0.63] |
| 1.20.1 Diary calcium intake less than 1000 mg | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐2.07, 1.25] |
| 1.20.2 Diary calcium intake 1000‐1250 mg | 8 | 1017 | Mean Difference (IV, Random, 95% CI) | ‐2.03 [‐3.44, ‐0.62] |
| 1.20.3 Diary calcium intake 1500 mg or more | 8 | 1821 | Mean Difference (IV, Random, 95% CI) | ‐1.35 [‐2.75, 0.05] |
| 1.21 Change in systolic blood pressure by dose | 9 | 2651 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.99, ‐0.35] |
| 1.21.1 Less than 1000 mg of calcium intake | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐2.87, 2.87] |
| 1.21.2 1000‐1500 of calcium intake | 7 | 2418 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.01, ‐0.27] |
| 1.21.3 1500 mg or more of calcium intake | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐10.58, ‐0.82] |
| 1.22 Change in diastolic blood pressure by dose | 9 | 2651 | Mean Difference (IV, Random, 95% CI) | ‐1.73 [‐2.79, ‐0.67] |
| 1.22.1 Diary calcium intake less than 1000 mg | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐2.07, 1.25] |
| 1.22.2 Diary calcium intake 1000‐1500 mg | 6 | 947 | Mean Difference (IV, Random, 95% CI) | ‐2.11 [‐3.67, ‐0.56] |
| 1.22.3 Diary calcium intake 1500 mg or more | 2 | 1503 | Mean Difference (IV, Random, 95% CI) | ‐2.15 [‐4.59, 0.29] |
| 1.23 Final value in systolic blood pressure by dose | 11 | 581 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.85, 0.40] |
| 1.23.1 Less than 1000 mg of calcium intake | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐3.44, 3.21] |
| 1.23.2 1000‐1500 of calcium intake | 3 | 123 | Mean Difference (IV, Random, 95% CI) | 1.05 [‐3.06, 5.16] |
| 1.23.3 1500 mg or more of calcium intake | 6 | 318 | Mean Difference (IV, Random, 95% CI) | ‐2.25 [‐4.34, ‐0.16] |
| 1.24 Final value in diastolic blood pressure by dose | 10 | 480 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.65, 0.19] |
| 1.24.1 Diary calcium intake less than 1000 mg | 1 | 53 | Mean Difference (IV, Random, 95% CI) | ‐3.50 [‐7.28, 0.28] |
| 1.24.2 Diary calcium intake 1000‐1500 mg | 3 | 109 | Mean Difference (IV, Random, 95% CI) | ‐1.65 [‐5.37, 2.07] |
| 1.24.3 Diary calcium intake 1500 mg or more | 6 | 318 | Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐2.73, 1.10] |
| 1.25 Mean difference in systolic blood pressure by duration | 18 | 3140 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐1.93, ‐0.45] |
| 1.25.1 Less than 6 months | 13 | 766 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.72, ‐0.53] |
| 1.25.2 6 months or more | 5 | 2374 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.83, 0.17] |
| 1.26 Mean difference in diastolic blood pressure by duration | 17 | 3039 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.23, ‐0.63] |
| 1.26.1 Less than 6 months | 12 | 665 | Mean Difference (IV, Random, 95% CI) | ‐2.16 [‐3.34, ‐0.98] |
| 1.26.2 6 months or more | 5 | 2374 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐1.03, 0.17] |
| 1.27 Mean difference in systolic blood pressure by intervention type | 18 | 3140 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐1.93, ‐0.45] |
| 1.27.1 Supplementation | 16 | 3001 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐2.02, ‐0.50] |
| 1.27.2 Fortification | 2 | 139 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐3.11, 3.29] |
| 1.28 Mean difference in diastolic blood pressure by intervention type | 17 | 3039 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐2.23, ‐0.63] |
| 1.28.1 Supplementation | 16 | 3001 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐2.27, ‐0.63] |
| 1.28.2 Fortification | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐6.72, 4.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belizan 1983.
| Study characteristics | ||
| Methods | Randomised double‐blind clinical trial The trial was conducted in Guatemala. |
|
| Participants | 57 subjects (28 men and 29 women) Age:18 and 35 years Healthy subjects not receiving medical treatment, women were not using hormonal contraceptives. Subjects .. "were free of diseases as assessed by a comprehensive clinical examination and blood and urine tests". |
|
| Interventions | Calcium supplementation vs placebo tablets Intervention group: daily oral tablet containing 0.8 gm of calcium carbonate and 5.23 gm of calcium lactate gluconate (Calcium‐Sandoz, 1000 mg), representing 1 gm of elemental calcium Placebo group: daily oral tablet of the same weight, size, and organoleptic characteristics as the calcium tablet Trial duration: 22 weeks |
|
| Outcomes | Systolic blood pressure: read when the appearance of the first Korotkoff's sound occurred Diastolic blood pressure: taken at the disappearance of the fifth Korotkoff's sound The final value and SD were calculated from the reported basal blood pressure values and the percent changes between basal values and stable period (weeks 9 through 23) reported in the article. Blood levels of total calcium and magnesium by atomic absorption spectrophotometry Blood levels of inorganic phosphate by spectrophotometry Blood levels of albumin by dye‐binding bromocresol purpose Total calcium intake: basal dietary intake measured by 24‐hr food record plus compliance with supplementation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer random number generator was used. Participants were randomly assigned to 2 treatment groups. "Separate randomisation schedules were used for sex and age groups (18 ‐ 23 years and 24 ‐ 35 years)". |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered containers were similar for both types of tablets, and a key number indicated the composition. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo group received a daily tablet of the same weight, size, and organoleptic characteristics as the calcium tablet". The treatment assignment was made double‐blind. The composition of the tablet was unknown to participants or to the professional in charge of the examinations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Composition of the tablet was unknown to participants or to the professional in charge of the examinations or BP measurements. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28 men and 29 women were randomised to the study groups and 23 men and 20 women completed the study. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes addressed |
| Other bias | Low risk | No differences between groups were found in the variables collected during the baseline period except for systolic BP in the dorsal position among the men. |
Cutler 1992.
| Study characteristics | ||
| Methods | Randomised double‐blind clinical trial | |
| Participants | Healthy subjects. with high‐normal diastolic blood pressure, not taking antihypertensive drugs, not grossly obese (BMI < 36.15 kg/m2*), and not consuming more than 21 alcohol‐containing drinks weekly Intervention group: 237 participants assigned to receive calcium Control group: 234 participants assigned to receive placebo Gender: Men and women Age: 30 to 54 years Exclusion criteria included pre‐existing cardiovascular or life‐threatening conditions, conditions requiring or contraindicating any of the study interventions, and intent to become pregnant during the study period. Age average: 43 years; 69% were men, 86% were white, and 51% had completed college. Baseline blood pressures averaged 125/84 mm Hg and BMI averaged 27.3 kg/m2. Dietary calcium intake: average 970 mg |
|
| Interventions | Calcium supplementation vs placebo tablets Intervention group: calcium carbonate representing calcium, 25 mmol or 1.0 g (2 pills per day) Control group: placebo tablet Trial duration: 6 months |
|
| Outcomes | Primary: "change in diastolic blood pressure from baseline to final follow‐up" Secondary: "changes in systolic blood pressure and intervention compliance measures" |
|
| Notes | Dietary calcium intakes according to the food frequency questionnaire data averaged 970 mg. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation lists were computer‐generated at the TOHP Data Coordinating Center. |
| Allocation concealment (selection bias) | Low risk | Randomisation assignments were obtained from the co‐ordinating centre by telephone when possible, otherwise sealed opaque envelopes were used to convey the treatment assignment. Adherence to the appropriate assignment sequence was monitored by the coordinating centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind fashion, with placebo controls |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Trained, certified observers who were blinded to participants' treatments. Blood pressure was measured with a Hawksley random‐zero sphygmomanometer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Blood pressure data were complete for 95% of participants at 3 months and 93% at 6 months. Pill counts were obtained for 91% at 6 weeks, 90% at 3 months, and 84% at 6 months. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | Baseline characteristics were similar. |
Davis 1996.
| Study characteristics | ||
| Methods | Randomised controlled trial The trial was conducted in the United States of America. |
|
| Participants | 34 healthy, normotensive adolescents Ethnicity: African‐American Age:14‐19 years |
|
| Interventions | Intervention: 1.5 grams of calcium per day Control group: daily placebo tablets Trial duration: 4 weeks |
|
| Outcomes | Ambulatory systolic blood pressure and diastolic blood pressure | |
| Notes | There was no information on calcium intake reported. Participants were recruited from a high school in Los Angeles. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors reported that participants were randomly assigned to the treatment or control group. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The ambulatory blood pressure unit measured the blood pressure every 30 minutes during the day. "Unit was placed on each participant for 24 hours". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported how many participants gave data for the results |
| Selective reporting (reporting bias) | Low risk | The planned outcome was reported. |
| Other bias | Unclear risk | No information on baseline characteristics was reported. |
Entezari 2015.
| Study characteristics | ||
| Methods | Double‐blinded placebo‐controlled clinical trial | |
| Participants | Normotensive females Age: 18 to 30 years Setting: Shahid Beheshti University of Medical Sciences in Tehran, Iran |
|
| Interventions | Intervention: four capsules of supplementary calcium daily; each capsule contained 625 mg calcium carbonate, which is equal to 250 mg of calcium element. Control: 1000 mg dextrose capsules instead of calcium carbonate Trial duration: 1 month |
|
| Outcomes | Systolic and diastolic blood pressure in supine position after 10 min of rest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned: "Eligible cases were randomly divided into two groups (treatment and control); treatment group received four capsules of supplementary calcium daily". |
| Allocation concealment (selection bias) | Unclear risk | The method was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This double‐blinded placebo‐controlled clinical trial was carried out in normotensive females. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 75 started; the authors did not mention how many in each group. More than "15 withdrew"; 22 in total (29.3%) finished "27 in calcium and 26 in control". |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | This study was done in a population who were calcium deficient, and therefore the effect of calcium supplement should be shown more prominently. |
Gillman 1995.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial The trial was conducted in the United States of America. |
|
| Participants | 101 5th‐grade students Gender: 50 girls and 51 boys Ethnicity: 61 were black. Setting: inner city school |
|
| Interventions | Intervention: 480 mL of juice containing 600 mg calcium (as calcium citrate malate) daily Control: Same juice with no calcium Trial duration: 12 weeks |
|
| Outcomes | "Blood pressure 4 times on each of 3 weekly sittings at baseline and at follow‐up" | |
| Notes | Nutrient data from 3 sets of 2‐day food records on each participant Funding: Procter and Gamble Co |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was performed by a centralised unit with the ID numbers that researchers provided. ID labels were affixed to each 'juice box', and sent to researchers who were completely blinded to treatment assignment. |
| Allocation concealment (selection bias) | Low risk | Random allocation was performed by a centralised unit with the ID numbers that researchers provided. ID labels were affixed to each 'juice box' and sent to researchers who were completely blinded to treatment assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and participants were masked to treatment assignment throughout the intervention period. "The intervention and placebo beverages were formulated to look and taste the same". "Single‐serving containers ("juice boxes") and labelled with the subject's name and study identification number" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Automated device (Dinamap Vital Signs Monitor model 845‐A, Critikon, Inc., Tampa, Fla.)". "Blood pressure data were automatically recorded on a floppy disk; investigators and participants were masked to these data until the end of the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 106 participants randomised, 5 moved from the school and the analyses included 101 participants. Age, sex, and race of non‐participants and those who dropped out before intervention were similar. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were small differences (in different directions) between intervention and placebo participants in baseline systolic blood pressure, hours of television watched, and amount of dietary calcium. |
Hilary Green 2000.
| Study characteristics | ||
| Methods | Double‐blind, randomised, controlled cross‐over study The trial was conducted in New Zealand. |
|
| Participants | 38 healthy volunteers Age: over 40 years |
|
| Interventions | Intervention: high‐calcium skim powder milk Control: replacement of usual liquid milk with 2 servings a day of skim non‐fortified powder milk Trial duration: 4 weeks, with a minimum of 4 weeks of wash‐out between interventions |
|
| Outcomes | Systolic blood pressure and ambulatory blood pressure | |
| Notes | "For many people in the trial, the control skim milk provided additional calcium to the diet. This may explain the small reduction in office".. standing systolic blood pressure observed in the control group. Calcium intake was calculated using 24‐hour food recalls. This study was supported by The New Zealand Dairy Board. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated: "Randomized double‐blind controlled trial'. Double‐blind, randomised, controlled cross‐over study. "Each volunteer consumed each of the milks in randomised order". "The milk was provided to the volunteers as a dry powder". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Ambulatory blood pressure monitor. Automated oscillometric blood pressure monitor (A&D, Model UA‐751; A&D Medical Division, Milpitas, California, USA)" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported. |
| Other bias | High risk | There were small differences (in different directions) between intervention and placebo participants in baseline office and ambulatory blood pressure, except for baseline systolic blood pressure: Skim milk 121 ± 14 and high‐calcium skim milk 125 ± 19. Controls may have accidentally received a calcium boost from the placebo milk that should be treated as a potential bias. |
Johnson 1985.
| Study characteristics | ||
| Methods | Randomised double‐blind clinical trial. Women were divided into a control and an experimental group in a double blind design. The trial was conducted in the United States of America. |
|
| Participants | 81 normotensive and 34 medicated hypertensive women Age: between 35 and 65 years |
|
| Interventions | Intervention group: 3 daily tablets of a calcium carbonate supplement containing 500 mg calcium per tablet Control group: placebo tablets Trial duration: 4 years |
|
| Outcomes | Bone mineral content and blood pressure | |
| Notes | Most of the women were using thiazides. "Dietary calcium of all women was determined using a precoded food record form, which had been tested for validity against weighed food intakes". This study was supported by Wisconsin Milk Marketing Board, Inc, Marion Laboratories, Kansas City, MO. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The original group of women, including the hypertensives, was divided into a control and an experimental group in a double‐blind design". However, methods were not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Blood pressure was measured from the right arm of seated participants using a standard mercury sphygmomanometer". Not reported if outcome assessors were blinded to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 44 participants were randomised to the intervention and 41 were analysed. 51 participants were randomised to placebo and 40 were analysed. |
| Selective reporting (reporting bias) | Low risk | All primary outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between the groups. |
Karanja 1987.
| Study characteristics | ||
| Methods | Subjects were assigned randomly to one of two treatment regimens: 1) 8 wk of calcium (phase I) followed by a washout period of 4 wk on placebo and then 8 wk of placebo (phase II); or 2) 8 wk of placebo (phase I) followed by a 4‐wk washout period on placebo and then 8 wk of calcium (phase II) |
|
| Participants | 32 normotensive subjects recruited from the community and from the Outpatient Clinic of the Oregon Health Sciences University Aged 21‐70 yrs |
|
| Interventions | Intervention group: One gram elemental calcium was supplied in two tablets of calcium carbonate (BioCal) or effervescent calcium (mono‐calcium citrate). Control group: placebo tablets Trial duration: 8 weeks |
|
| Outcomes | Total Cholesterol Triglycerides HDL‐Cholesterol LDL‐Cholesterol | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjects were assigned randomly to one of two treatment regimens. |
| Allocation concealment (selection bias) | Unclear risk | Medication was dispensed every 2 wk with placebo or calcium taken at bedtime. One gram elemental calcium was supplied in two tablets of calcium carbonate (BioCal) or effervescent calcium (mono‐calcium citrate). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subjects were assigned randomly to one of two treatment regimens. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blood samples were collected at the end of the baseline evaluation and again at the end of phases I and II. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 participants were randomised and 27 reported in the results. |
| Selective reporting (reporting bias) | High risk | Blood pressure was not reported. |
| Other bias | Unclear risk | No other bias reported |
Lijnen 1995.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled parallel‐group cross‐over study The trial was conducted in Belgium. |
|
| Participants | 32 male participants Age: 24 ± 1 (range 20 ‐ 44 years) and weight 75.9 ± 1.3 kg |
|
| Interventions | Intervention group: 1 g elemental calcium as calcium gluconate powder twice a day (morning and evening) Control group: placebo with the same orange flavour as intervention Trial duration: 16 weeks |
|
| Outcomes | Blood pressure recorded in standing position Intracellular cationic concentrations Transmembrane cation transport systems Plasma total and ionised calcium Calciotropic hormones |
|
| Notes | This study was supported by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind placebo‐control parallel‐group. The calcium supplement and placebo were both orange flavour. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were included in the results. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes results were reported. |
| Other bias | Low risk | Baseline characteristics were similar between calcium and placebo groups. |
Lyle 1987.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial The trial was conducted in the United States of America. |
|
| Participants | Normotensive male participants Ethnicity: Black (n = 21) and white (n = 54) Age: 19 to 52 years |
|
| Interventions | Internvention group: calcium, 1500 mg a day Control group: placebo "Participants were randomly assigned within racial groups to either treatment". Trial duration: 12‐week period |
|
| Outcomes | Blood pressure Serum levels of total and ionised calcium Total inorganic phosphorus Parathyroid hormone Overnight urinary electrolyte values |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | The supplements were strip‐wrapped individually and coded by someone not involved in the research study. The participants did not know which group they were assigned to, and the researcher(s) who collected other information also were not aware of the group assignment. Early analyses were completed prior to revealing the assigned groups as well. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Assignment was double‐blind. "Indistinguishable placebo tablets were composed of microcrystalline methylcellulose and starch". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Use of a random‐baseline sphygmomanometer and blinded observers to eliminate bias during blood pressure measurement, documentation of nutrient intake other than the supplement, and control for body weight and other possible confounders" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants randomised were included in the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | The groups were similar at baseline. |
Lyle 1992.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial The trial was conducted in the United States of America. |
|
| Participants | 42 adults Gender: men and women High normal or mildly hypertensive levels of blood pressure |
|
| Interventions | Intervention group: 500 mg of elemental calcium as calcium carbonate tablets Control group: placebo tablets Trial duration: 8 weeks |
|
| Outcomes | Blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were given a random number of calcium or placebo tablets. |
| Allocation concealment (selection bias) | Low risk | The supplements were strip‐wrapped individually and coded by someone not involved in the research study. The participants did not know which group they were assigned to, and the researcher(s) who collected other information also were not aware of the group assignment. Early analyses were completed prior to revealing the assigned groups as well. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Experimental group assessment was double‐blind. Tablets contained 500 mg of elemental calcium in the form of calcium carbonate. Indistinguishable placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measurements were taken with a random‐zero sphygmomanometer at least 1 minute apart. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 44 men and women participants were randomised, 2 participants withdrew due to appointment conflicts and 42 participants completed the study. Missing outcome data balanced in numbers across intervention groups |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | High risk | In the treatment group, there were more men than in the placebo group. 8 of the 10 women were allocated to the placebo group. However, blood pressure measurements showed no statistically significant differences between groups. |
McCarron 1985.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over trial The trial was conducted in the United States of America. |
|
| Participants | 32 normotensive subjects Healthy volunteers with no signs of secondary hypertension Age: between 21 and 70 years |
|
| Interventions | Intervention group: 1000 mg a day of elemental calcium as carbonate or citrate salt Control group: placebo tablets Trial duration: 8 weeks |
|
| Outcomes | Change in blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment of participants was done separately in blocks by computer. |
| Allocation concealment (selection bias) | Low risk | Medications were pre‐packaged by randomisation number for each participant and dispensed every 2 weeks. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Placebo tablets consisted of microcrystalline cellulose and starch and were identical in taste and appearance to the calcium carbonate tablets. Subjects and members of the investigative staff were blinded". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "A Hawksley random‐zero sphygmomanometer (Hawksley & Sons, Ltd., Lancing, England) was used for measurement of blood pressure after the participant was supine for 5 minutes and after standing for 2 minutes". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 32 normotensive participants were reported in the results. |
| Selective reporting (reporting bias) | Low risk | Primary outcome result reported |
| Other bias | Low risk | The baseline characteristics were similar between the groups. |
Reid 2005.
| Study characteristics | ||
| Methods | Double‐blind, randomised, controlled trial The trial was conducted in New Zealand. |
|
| Participants | Healthy postmenopausal women more than 5 years from postmenopause Age: more than 55 years (mean age, 74 years) Mean baseline weight: 67 kg Mean baseline blood pressure:134/70 mmHg Exclusion criteria: participants receiving therapy for osteoporosis or taking calcium supplements, major ongoing disease including serum creatinine greater than 1.8 mg/dL (0.2 mmol/litre), untreated hypo‐ or hyperthyroidism, liver disease, serum 25‐hydroxyvitamin D below 10 g/litre (25 nmol/litre), malignancy, or metabolic bone disease, users of hormone replacement therapy, anabolic steroids, glucocorticoids, or bisphosphonate in the previous 1 year |
|
| Interventions | Intervention group: calcium as calcium citrate (1 gm of elemental calcium a day; n = 732) Control group: identical placebo (n = 739) Trial duration 30 months |
|
| Outcomes | Primary outcome: fracture incidence Secondary analysis: ‐ Body weight ‐ Blood pressure |
|
| Notes | Dietary calcium intake was assessed using a validated food frequency questionnaire. Calcium was provided by Citracal, Mission Pharmacal, San Antonio TX. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatments were allocated randomly using a minimisation algorithm balancing for current thiazide use, age, and the occurrence of fractures resulting from minimal trauma after the age of 40 years". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study. "Subjects received 1 g elemental calcium daily as citrate (Citracal, Mission Pharmacal, San Antonio TX) or an identical placebo". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blood pressure was measured using a Dinamap automatic monitor (Johnson & Johnson, Tampa, FL) at each visit". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were not reported. |
| Selective reporting (reporting bias) | Low risk | A variety of preplanned models were run: an intention‐to‐treat analysis, with and without imputation (maximum likelihood) of missing values, and with and without adjustment for compliance; a per protocol analysis; and an analysis of the change in blood pressure, excluding those taking blood pressure‐lowering medication. |
| Other bias | Low risk | Baseline characteristics were similar between groups. |
Reid 2010.
| Study characteristics | ||
| Methods | Randomised controlled trial The trial was conducted in New Zealand. |
|
| Participants | 323 healthy men Age: over 40 years |
|
| Interventions | Intervention groups: group 1: 600 mg calcium a day or group 2: 1200 mg calcium a day as calcium citrate Control group: placebo Trial duration: 2 years |
|
| Outcomes | Primary endpoint: change in the ratio of HDL to LDL cholesterol Secondary endpoints: changes in cholesterol fractions, triglycerides, blood pressure, and body composition |
|
| Notes | This study was supported by Mission Pharmacal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatments were allocated randomly by using computer‐generated random numbers (Microsoft Excel 2003; Microsoft, Redmond, WA) within blocks of random sizes in multiples of 3". |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed before the study began by the study statistician and was conveyed to a staff member who dispensed the study medication into numbered containers. This individual had no direct contact with other study staff nor with trial participants. Subjects were allocated a study number according to the sequence of their enrolment". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study staff were blinded to treatment allocation throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blood pressure was measured by using a Dinamap automatic monitor (Johnson & Johnson, Tampa, FL)". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Complete follow‐up was achieved in 96% of the participants, and the proportions of those randomly assigned still receiving the trial medication at study end were as follows: 93% in the placebo group, 91% in the Ca600 group, and 86%in the Ca1200 group (P = 0.19 for between‐group comparisons)". |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | There were small differences (in different directions) between intervention and placebo participants. |
Sacks 1998.
| Study characteristics | ||
| Methods | Randomised, double‐blind parallel‐group trial The trial was conducted in the United States of America. |
|
| Participants | 321 participants: 93% completed baseline and midpoint measurements. "Exclusion criteria included reported diastolic blood pressure 65 mm Hg; hypertension; BMI > 32 kg/m²; insulin‐dependent diabetes; cardiovascular disease; renal failure; medications that affect blood pressure, weight loss diets, use of nutritional supplements of calcium, magnesium, or potassium (including antacid preparations)". |
|
| Interventions | Intervention group: calcium carbonate 1200 mg daily (caltrate 600 mg twice daily, Lederle Laboratories) Control group:identical placebo Trial duration: 16 weeks "The placebo group received twice the number of participants as the four treatment groups to improve statistical power". |
|
| Outcomes | Ambulatory 24‐hour blood pressure 24‐hour urine Body weight Health and side effects questionnaire Pill counts |
|
| Notes | Participants who had baseline systolic blood pressure above 160 mmHg or diastolic blood pressure above 95 mm Hg were excluded and advised to see their physicians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed by a computer program directed by the statistician on the project. The statistician had no contact with the data collectors or the participants (information provided by the author). |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were under double‐blind conditions for 16 weeks but methods not described. The participants were not informed about their specific supplement group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The blood pressure machine automatically entered the blood pressure data on computer tape that was later converted to an ASCII file at the study office. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 321 participants were randomised. 300 participants were available for follow‐up measurements and 290 completed the study measurements. |
| Selective reporting (reporting bias) | Low risk | Primary outcome reported |
| Other bias | Low risk | Baseline characteristics were similar. |
Shidfar 2010.
| Study characteristics | ||
| Methods | Randomised, double‐blind clinical trial The trial was conducted in Iran. | |
| Participants | 49 overweight men (BMI > 25 kg/m², BMI = 27.5 ± 1.7) Age: 34.4 ± 4.8 years |
|
| Interventions | Intervention group: carbonate calcium (1250 mg elemental calcium daily) Control group: placebo Trial duration: 8 weeks |
|
| Outcomes | Blood pressure Serum lipid profile |
|
| Notes | Diet was assessed with a 24‐hour dietary recall questionnaires at baseline, 4th week, and end of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were divided randomly (by random number tables) into case and placebo groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind clinical trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants used low‐calorie diets and we had to exclude them from the study (fewer than 10%). |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between the 2 groups |
Thomsen 1987.
| Study characteristics | ||
| Methods | Double‐blind randomised placebo‐controlled trial The trial was conducted in Denmark. |
|
| Participants | 28 healthy women with early menopause (6 months to 3 years earlier). Overweight was not an exclusion criterion. | |
| Interventions | Intervention group: 2000 mg calcium per day (14 participants) Control group: identical‐looking placebo tablets (14 participants) Trial duration: 1 year |
|
| Outcomes | Blood pressure. BP was measured by mercury manometer after 10 min of supine rest. | |
| Notes | Tablets were provided by Sandoz. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated into 2 groups according to random sampling numbers. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial. Participants received identical‐looking tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were evaluated at the end of the study. |
| Selective reporting (reporting bias) | Low risk | The primary outcome was reported. |
| Other bias | High risk | Placebo participants had higher initial weight and lower systolic blood pressure. |
Van Beresteyn 1986.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled trial Participants were assigned to 2 groups according to a randomised block design that accounted for habitual calcium intake and BMI. The trial was conducted in the Netherlands. |
|
| Participants | 58 normotensive healthy female dietetic students, not receiving any medical treatment at the time of recruitment Age: 20‐23 years Weight: 49‐76 kg |
|
| Interventions | Intervention group: Daily lemonade or apple juice with powder containing 1500 mg calcium ‐ calcium carbonate (1.251 g), citric acid (2.168 g), sodium‐hydrogen carbonate (0.5 g), and dextrose (2.88 g) Control group: Daily lemonade or apple juice placebo powder with citric acid (0.85 g), sodium‐hydrogen carbonate (0.5 g), dextrose (4.5 g), and corn‐flour (0. 1 g) Both groups received a low‐calcium diet (500 mg calcium a day) restricting intake of dairy products. Trial duration: 6 weeks |
|
| Outcomes | Difference for each individual between baseline blood pressure and final blood pressure Individual change in blood pressure during the experiment as indicated by the regression coefficient (slope) obtained from linear regression analysis of blood pressure versus time during the experimental period |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to 2 groups according to a randomised block design that accounted for habitual calcium intake and body mass index. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, placebo‐controlled trial but methods not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported in the results. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar between the 2 groups. |
Yanovski 2009.
| Study characteristics | ||
| Methods | Randomised controlled trial | |
| Participants | Men and women 18 years or older. The trial was conducted in the United States of America. | |
| Interventions | Intervention: Calcium supplement (calcium carbonate 1500 mg/day) Comparator: Placebo capsules with no calcium |
|
| Outcomes | Primary: Body weight Secondary: triceps skinfold fold thickness, body circumferences and DXA percentage fat. |
|
| Notes | 23% reported dietary calcium intake less than 600 mg/d and 75% reported dietary calcium intake less than the U.S. dietary reference intake for persons age 51 to 70 years (1200 mg/d). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The National Institutes of Health Clinical Center Pharmaceutical Development Section used permuted blocks with stratification to generate the alloca‐ tions that translated code numbers into study group assign‐ ments by using a pseudo‐random number program. |
| Allocation concealment (selection bias) | Low risk | The Pharmaceutical Development Section prepared placebo and calcium capsules to appear identical. Pharmacy personnel, not otherwise involved with the conduct of the study, dispensed study capsules with medication placed in con‐ tainers that appeared identical and differed only by the individual participant code number. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments for the duration of the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No participant, investigator, or other medical or nursing staff interacting with participants was aware of study group assignments for the duration of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information presented in the article |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | No baseline or crude data reported |
Yosephin 2015.
| Study characteristics | ||
| Methods | Randomised controlled trial Parallel‐group | |
| Participants | Female workers aged 30–45 years, healthy, married, not pregnant and breastfeeding, not a smoker, not an alcohol drinker, not on a diet and willing to sign ethical informed consent form. The study was conducted in Indonesia. | |
| Interventions | Intervention: 400 IU of vitamin D and 500 mg of calcium Control: 400 IU of vitamin D Trial duration: 12 weeks |
|
| Outcomes | Serum 25(OH)D and blood pressure | |
| Notes | More than half of the VDC group subjects (55.0%) had unusual BMI: 15.0% overweight and 40.0% obese. More than two‐thirds of the VD group subjects (78.9%) had unusual BMI; 10.5% overweight and 68.4% obese. The average levels of serum 25(OH)D in the VDC group was 16.7 ng/dL with the highest subject’s serum at 24.9 ng/dL and the lowest at 8.7 ng/dL. The average levels of serum 25 OH)D in the VD group was 14.9 ng/dL with the highest subject’s serum at 22.20 ng/dL and the lowest at 3.5 ng/dL. When the average levels of serum 25(OH)D in both groups were being compared, the difference was not significant (P > 0.05). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double‐blind randomised controlled trial |
| Allocation concealment (selection bias) | Unclear risk | The research subjects were randomly divided into two treatments in which each treatment consisted of 21 subjects. VDC formulation consisted of 400 IU of vitamin D and 500 mg of calcium, while VD formulation consisted of 400 IU of vitamin D. Each week, both capsule formulae (7 capsules) were transferred into sealed small plastic bags. On each plastic bag, respondents’ names and the type of formula that respondents received were randomised at the beginning of the treatment. Each small plastic bag was delivered to the distribution crew, namely, 2 labour union staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Type of supplement and differences in the composition contained in capsules provided to each woman worker were not known to researchers and distribution crew. Each week, both capsule formulae (7 capsules) were transferred into sealed small plastic bags. On each plastic bag, respondents’ names and the type of formula that respondents received were randomised at the beginning of the treatment. Each small plastic bag was delivered to the distribution crew, namely, 2 labour union staff. Type of supplement and differences in the composition contained in capsules provided to each female worker were not known to researchers and distribution crew. Subjects took the supplement capsules by using drinking water in front of the labour union staff and capsule strip tears were collected in the plastic bags. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blood pressure measurement was also conducted twice before and after 12‐week supplementation by a physician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 42 research subjects were selected. Each treatment group had 21 subjects, but one of them was pregnant on the VDC group while on the VD group there were 2 subjects who could not complete the intervention due to resigning from the garment factory. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | More than half of the VDC group subjects (55.0%) had unusual BMI; 15.0% overweight and 40.0% obese. More than two‐thirds of the VD group subjects (78.9%) had unusual BMI; 10.5% overweight and 68.4% obese. |
ABP: ambulatory blood pressure BMI: body mass index BP: blood pressure DBP: diastolic blood pressure HDL: high‐density lipids ID: Identification LDL: low‐density lipids SBP: systolic blood pressure SD: standard deviation VD: Vitamin D VDC:Vitamin D and Calciium
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bostick 2000 | There was no discrimination in the outcomes for hypertensive or normotensive participants. "Persons with or without hypertension or hypercholesterolaemia, or taking or not taking antihypertensive or cholesterol‐lowering medications were eligible to participate except as specified below". |
| Das 2017 | Wrong comparator |
| Dwyer 1998 | Number of cases in each cross‐over step were not reported. |
| Eftekhari 2009 | Low fat, high fibre diet was a co‐intervention. |
| Ferreira 2016 | Wrong comparator |
| Hofmeyr 2015 | Wrong patient population |
| Luft 1986 | Quasi‐randomised trial |
| Morris 1988 | No details of number of participants in calcium placebo groups |
| Ong 2016 | Wrong study design |
| Pan 1993 | Most participants (63%) were taking antihypertensive drugs. |
| Pan 2000 | Blood pressure is not an outcome of the study. |
| Rahman 2003 | Wrong study design |
| Sakai 2017 | Wrong comparator |
| Shalileh 2010 | Energy‐restricted diet was a co‐intervention. |
| Smith 1987 | Quasi‐randomised study. It used even and odd numbers from a table of random numbers. |
| Weinberge 1993 | Salt‐sensitive or salt‐resistant participants. No details of number of participants in calcium placebo groups |
| Zhang 2009 | Wrong comparator |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617000697381.
| Study name | Calcium supplements and 24‐hour blood pressure: a randomised, cross‐over, placebo‐controlled trial in postmenopausal women |
| Methods | Randomised, cross‐over, placebo‐controlled trial |
| Participants | Females at least 5 years postmenopause. Age > 55 years if age at menopause unknown |
| Interventions | 0.5 grams of calcium tablets as citrate salt to be taken twice a day (once in the morning and once in the evening, 12 hours apart) |
| Outcomes | Difference in the change from baseline in diastolic blood pressure between day one of calcium supplementation and day one of placebo Difference in the change from baseline in systolic blood pressure between day 1 of calcium supplementation and day 1 of placebo |
| Starting date | 17/01/2018 |
| Contact information | Dr Sarah Bristow Faculty of Medical and Health Sciences, The University of Auckland Private Bag 92019 Auckland 1142, New Zealand +6499233773 s.bristow@auckland.ac.nz |
| Notes | https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372481&isReview=true |
ChiCTR‐IOR‐15006495.
| Study name | The influence of different sources of calcium on metabolism in postmenopausal women: a randomized intervention study |
| Methods | Randomised intervention study |
| Participants | Postmenopausal women with dyslipidaemia and change in glucose metabolism. Subjects were considered to have dyslipidaemia and change in glucose metabolism if they met >= 1 of the following criteria: 1. TC >= 5.18 mmol/L; 2. TG >= 1.7 mmol/L; 3. LDL‐C >= 3.37 mmol/L; 4. HDL < 1.04 mmol/L; 5. FBG >= 5.6 mmol/L |
| Interventions | Control group: normal diet and supplemented with 400 IU vitamin D per day Calcium supplements group: supplemented with 430 mg calcium from calcium supplements per day + 400 IU vitamin D per day Milk group: supplemented with 430 mg calcium from milk per day + 400 IU vitamin D |
| Outcomes | Blood lipids; blood glucose; serum calcium and phosphorus; serum insulin; physical activity level; intake of energy and nutrients; RMR; BMI; blood pressure; serum estradiol concentration; serum 25(OH)D3; liver function; renal function |
| Starting date | 01/06/2015 |
| Contact information | Lixin Na 157 Baojian Road, Nangang District, Harbin +86 0451 87502730 nalixin2003@163.com Harbin Medical University |
| Notes | http://www.chictr.org.cn/showproj.aspx?proj=11096 |
ChiCTR‐TRC‐12002806.
| Study name | The preventive effect of calcium supplementation on the incidence of chronic non‐communicable diseases in healthy women |
| Methods | Randomised controlled parallel‐group trial |
| Participants | Females, in good general health, premenopausal women aged 30‐40 y and postmenopausal women aged 50‐60 years |
| Interventions | Calcium supplementation: supplemented with 600 mg calcium per day; placebo group: placebo |
| Outcomes | Blood glucose; blood lipids; BMI; intima‐media thickness of carotid; blood pressure |
| Starting date | 01/07/2008 |
| Contact information | Lixin Na, nalixin2003@163.com 157 Baojian Road, Nangang District, Harbin Harbin Medical University |
| Notes | http://www.chictr.org.cn/showprojen.aspx?proj=6748 |
Irct2014021116555N.
| Study name | Comparison of the effects of calcium, vitamin D, and calcium plus vitamin D on anthropometric indices, body composition, lipid profile, blood pressure, and blood glucose in overweight or obese premenopausal women |
| Methods | Randomised controlled trial |
| Participants | Premenopausal women; aged between 20‐50 years; BMI between 25‐40 kg/m2; not having any cancer or severe endocrine, mental, hepatic, renal, gastrointestinal, cardiovascular, neurologic, rheumatologic, haematologic, skeletal, and eating disorders; not taking any medication, nutritional supplements, or herbal preparations that could affect calcium and vitamin D status, anthropometric indices, body composition, lipid profile, blood pressure, and blood glucose during the last 12 weeks; not having any history of drug intolerance or adverse reaction to the study supplements; dairy product consumption of 3 servings/d; consumption of 5 cups of coffee/d; not being pregnant or lactating; not being a smoker or taking alcohol; not being a participant in other trials over the last 6 months; stable body weight (body weight change <3 kg for 3 months before intervention); providing written informed consent Exclusion criteria: development of serious adverse events |
| Interventions | Intervention 1: 2 tablets of placebo with low calorie diet Intervention 2: 2 tablets of calcium (500 mg calcium carbonate/tablet) with low calorie diet Intervention 3: 2 tablets of calcium plus vitamin D (500 mg calcium carbonate and 200 IU vitamin D/tablet) with low calorie diet Intervention 4: 2 tablets of vitamin D (200 IU vitamin D/tablet) with low calorie diet Placebo treatment |
| Outcomes | Blood glucose Blood pressure Body fat mass Body mass index(BMI) lipid profile Waist circumference |
| Starting date | 18/05/2014 |
| Contact information | Hamide Rajaie, School of Nutrition and Food Science Shiraz, Iran |
| Notes | http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014021116555N1 |
Irct2016111016123N.
| Study name | Effect of vitamin D and calcium supplementation on serum levels of hormones affecting postpartum depression |
| Methods | Randomised controlled double‐blind trial |
| Participants | Body mass index (BMI) less than 35; age 18 to 45 years; earning a score of 12 and above in Edinburgh questionnaire; time period ranging from 1 month until 6 months after childbirth Exclusion criteria: normal or above normal range for vitamin D levels; chronic diseases such as diabetes; renal failure; gastrointestinal diseases; liver problems; thyroid problems; kidney stones; cancer in the past 5 years; birth abnormalities; taking contraceptive drugs; endocrine disorders; severe depression and other mental disorders; using antidepressants |
| Interventions | Intervention 1: 50,000 IU pearl vitamin D3, every 14 days + 500 milligram calcium carbonate tablet, daily, for 8 weeks Intervention 2: 50,000 IU pearl vitamin D3, every 14 days + calcium carbonate placebo, daily, for 8 weeks Intervention 3: pearl paraffin as vitamin D placebo, every 14 days + calcium carbonate placebo, daily, for 8 weeks |
| Outcomes | Cortisol (time point: before and after 8 weeks of intervention). Method of measurement: serum level SECONDARY OUTCOMES: blood pressure, body mass index, depression severity, serum calcium (time point: before the intervention, end of the fourth week and end of the eighth week) |
| Starting date | 28/08/2017 |
| Contact information | Sima Jafarirad, Ahvaz Jundishapur University of Medical Science, Golestan Blv. Ahvaz, Iran. sjafarirad@gmail.com |
| Notes | http://en.irct.ir/trial/15190 |
NCT01561131.
| Study name | The effect of protein and calcium on weight change and blood lipid profile |
| Methods | Randomised controlled trial |
| Participants | |
| Interventions | Group 1: Whey protein enriched with calcium Group 2: Whey protein with no calcium Group 3: Soy protein Contol: Maltodextrin |
| Outcomes | Difference in body weight and composition during the weight maintenance period Difference in fasting blood lipid profile during the weight maintenance period Difference in fasting insulin, glucose, C‐peptide, glucagon, insulin‐like growth factor 1 (IGF‐1), ionised calcium, parathyroideahormone (PTH), and angiopoietin‐like protein 4 (Angpt14) during the weight maintenance period Difference in resting blood pressure and pulse during the weight maintenance period |
| Starting date | 22/03/2012 |
| Contact information | Arne Asrup, Department of Nutrition, University of Copenhagen |
| Notes | https://ClinicalTrials.gov/show/NCT01561131 2012 University of Copenhagen|Arla Foods|Nupo A/S, Denmark 2012 |
NCT02534064.
| Study name | Effect of consumption of yogurt fortified in calcium and vit. D on circulating levels of 25OHD in postmenopausal women |
| Methods | Randomised controlled trial |
| Participants | Women aged 55 to 75 years |
| Interventions | 2 yogurts |
| Outcomes | Serum vitamin D concentration Secondary outcomes: parathyroid hormone level, blood pressure, weight |
| Starting date | 27/08/2015 |
| Contact information | Mathilde Latreille Barbier, Eurofins Optimed, Yoplait France SAS |
| Notes |
NCT03878667.
| Study name | Effects of calcium supplementation on women in the Curves for Women program |
| Methods | Randomised controlled trial |
| Participants | Women aged 45 to 65 years, overweight (BMI > 27) and postmenopausal, sedentary |
| Interventions | Dietary supplement: curves calcium Dietary supplement: calcium carbonate Dietary supplement: placebo |
| Outcomes | Body composition: body fat Body composition: fat free mass Body composition: bone mass Body composition: fat mass Body composition: lean mass Body composition: body weight Energy homeostasis: resting energy expenditure (REE) Energy homeostasis: respiratory exchange ratio (RER) Muscular strength: 1 repetition maximum bench press Muscular Strength: 1 repetition maximum leg press Muscular endurance: 80% of 1 repetition maximum bench press endurance test Muscular endurance: 80% of 1 repetition maximum leg press endurance test Body composition: body water Lipid panel: cholesterol Lipid panel: HDL cholesterol Lipid panel: LDL Cholesterol Lipid panel: triglycerides Comprehensive metabolic panel ‐ glucose Comprehensive metabolic panel ‐ calcium Comprehensive metabolic panel ‐ albumin Comprehensive metabolic panel ‐ total protein Comprehensive metabolic panel ‐ sodium Comprehensive metabolic panel ‐ potassium Comprehensive metabolic panel ‐ carbon dioxide (CO2) Comprehensive metabolic panel ‐ chloride Comprehensive metabolic panel ‐ blood urea nitrogen Comprehensive metabolic panel ‐ creatinine Comprehensive metabolic panel ‐ alkaline phosphatase (ALP) Comprehensive metabolic panel ‐ amino transferase (ALT) Comprehensive metabolic panel ‐ bilirubin Complete blood count ‐ CBC: white blood cell (WBC) count Complete blood count ‐ CBC: white blood cell differential Complete blood count ‐ CBC: red blood cell (RBC) count Complete blood count ‐ CBC: red cell distribution width (RDW) Complete blood count ‐ CBC: haemoglobin Complete blood count ‐ CBC: haematocrit Complete blood count ‐ CBC: platelet count Complete blood count ‐ CBC: mean platelet volume (MPV) Complete blood count ‐ CBC: neutrophils Complete blood count ‐ CBC: lymphocytes Complete blood count ‐ CBC: basophils Complete blood count ‐ CBC: eosinophils Complete blood count ‐ CBC: monocytes Complete blood count ‐ CBC: mean corpuscular volume (MCV) Complete blood count ‐ CBC: mean corpuscular haemoglobin (MCH) Complete blood count ‐ CBC: mean corpuscular haemoglobin concentration (MCHC) Hormones: insulin Hormones: leptin Haemodynamic variable: resting heart rate (HR) Haemodynamic variable: resting systolic blood pressure (SBP) Haemodynamic variable: resting diastolic blood pressure (DBP) Anthropometric measures: waist circumference Anthropometric measures: hip circumference Anthropometric measures: waist to hip ratio Anthropometric measures: body mass index Cardiac variable: resting electrocardiogram (ECG) Exercise capacity: graded exercise test (GXT) Subjective rating of quality of life Subjective rating of appetite Subjective rating of hunger Subjective rating of satisfaction from food Subjective rating of feelings of fullness Subjective rating of amount of energy Subjective rating of overall quality of diet Dietary energy intake ‐ total caloric intake Dietary energy intake ‐ carbohydrate Dietary energy intake ‐ fat Dietary energy intake |
| Starting date | 01/01/2004 |
| Contact information | Richard B. Kreider, Executive Director, Human Clinical Research Facility, Texas A&M University |
| Notes | https://ClinicalTrials.gov/show/NCT03878667 2004 |
UMIN000001176.
| Study name | Randomised controlled trial of calcium supplementation |
| Methods | Randomised controlled trial |
| Participants | Healthy women aged 50‐75 years |
| Interventions | Calcium carbonate tablets (calcium 500 mg/day) vs placebo |
| Outcomes | Two‐year changes in bone mineral density of hip and vertebra SECONDARY OUTCOME: blood pressure, blood biochemical tests |
| Starting date | 01/11/2008 |
| Contact information | Kazutoshi Nakamura 1‐757 Asahimachi‐dori, Chuo‐ku, Niigata city, 951‐8510, JAPAN Japan 025‐227‐2125 kazun@med.niigata‐u.ac.jp NIIGATA UNIVERSITY GRADUATE SCHOOL OF MEDICAL AND DENTAL SCIENCES Department of Community Preventive Medicine, Division of Social and Environmental Medicine |
| Notes | http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000001176 |
UMIN000018952.
| Study name | Effect of calcium combination lemon drink absorption on lifestyle‐related disease |
| Methods | Randomised controlled trial |
| Participants | Healthy women |
| Interventions | Calcium combination lemon drink, period: 11 months, volume: 30 mL of lemon fruit juice and calcium 35 mg per bottle, frequency: 1 bottle/day Lemon drink, period: 11 months, volume: 30 mL of lemon fruit juice per bottle, frequency: 1 bottle/day No drink |
| Outcomes | 1. Subjective‐objective symptoms by a questionnaire 2. Somatometry 3. Blood pressure 4. Bone mineral density 5. Bone metabolism marker 6. Oxidation stress marker |
| Starting date | 12/10/2015 |
| Contact information | Toshihide Harada, 1‐1 gakuen‐machi Mihara, Hiroshima, Japan |
| Notes |
ALP:alkaline phosphatase ALT: amino transferase Angpt14: angiopoietin‐like protein 4 BMI: Body Mass Index CBC: Complete blood count CO2: carbon dioxide DBP: Dyastilic blood pressure ECG:electrocardiogram FBG: Fasting blood glucose GXT: graded exercise test HDL: High densituy lipoprotein HR: Heart rate IGF‐1: insulin‐like growth factor LDL: Low density lopoprotein MCH: mean corpuscular haemoglobin MCHC: mean corpuscular haemoglobin concentration MCV: mean corpuscular volume MPV: mean platelet volume PTH: parathyroideahormone RBC: blood cell count RDW: red cell distribution width REE: resting energy expenditure RER: respiratory exchange ratio RMR: Resting Metabolic rate SBP: Systolic blood pressure TC: Total Colesterol TG: Triglycerides WBC: white blood cell
Differences between protocol and review
Agustina Mazzoni was listed in the published protocol as having the role of extracting data but María Luisa Cafferata, Maria Sol Cormick and Gabriela Cormick did this work.
We planned to include cluster‐randomised trials in the analyses along with individually randomised trials. We planned to adjust their sample sizes using the methods described in the Cochrane Handbook (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomised trials and individually randomised trials, we planned to synthesise the relevant information. We considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and if the choice of randomisation unit was considered to be unlikely. We planned also to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit. We did not find any cluster‐randomised trials that met our eligibility criteria.
For dichotomous data, we planned to calculate risk ratios (RR) with 95% confidence intervals (CI). None of the studies reported hypertension as a dichotomous outcome.
We included all trials with random allocation to dietary calcium intervention such as supplementation or food fortification versus placebo or control, but we excluded the second phase of cross‐over trials from the analysis.
We investigated reporting biases (such as publication bias) by producing funnel plots if at least 10 studies were included in the analysis.
We added the four post hoc analyses.
In order to explore the robustness of the results, we performed four post hoc sensitivity analyses. The first sensitivity analysis was the comparisons of results from mean differences and standardised mean differences in those cases when the result combined final blood pressure values and blood pressure change from baseline. We decided to present the results as mean differences, as they are easier to interpret; however, in order to be more accurate, we compared the mean difference results with the standardised mean difference results. We based the other analyses on duration of intervention, on blood pressure methodology (auscultatory and oscillometric method) and on clinic blood pressure measurements and automated ambulatory blood pressure.
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO HAS UNDERTAKEN THE TASK |
| Drafted the protocol | Gabriela Cormick/Agustín Ciapponi/José M Belizán |
| Selected which trials to include (2 people + 1 arbiter in the event of dispute) | Gabriela Cormick/María Luisa Cafferata/Agustín Ciapponi |
| Extracted data from trials (3 people) | Gabriela Cormick/María Luisa Cafferata/Agustín Ciapponi |
| Entered data into RevMan (Cochrane software) | Gabriela Cormick/Agustín Ciapponi |
| Carried out the analysis | Gabriela Cormick/Agustín Ciapponi |
| Interpreted the analysis | Gabriela Cormick/Agustín Ciapponi/ María Luisa Cafferata/José M Belizán |
| Drafted the final review | Gabriela Cormick/Agustín Ciapponi/María Luisa Cafferata/José M Belizán |
| Responsible to keep the review up to date | Gabriela Cormick/Agustín Ciapponi/ María Luisa Cafferata |
Sources of support
Internal sources
-
Institute for Clinical Effectiveness and Health Policy, Argentina
www.iecs.org.ar
External sources
-
New source of support, UK
No sources of support supplied
Declarations of interest
Gabriela Cormick: Nothing to declare.
Agustín Ciapponi: Nothing to declare.
María Luisa Cafferata: Nothing to declare.
José M Belizán: Nothing to declare.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Belizan 1983 {published data only}
- Belizan JM, Villar J, Pineda O, Gonzalez AE, Sainz E, Garrera G, et al. Reduction of blood pressure with calcium supplementation in young adults. JAMA 1983;249(9):1161-5. [PubMed] [Google Scholar]
Cutler 1992 {published data only}
- Cutler JA, Whelton PK, Appel L, Charleston J, Dalcin AT, Ewart C, et al. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the trials of hypertension prevention, phase I. JAMA 1992;4(267):1213-20. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME, Applegate WB, Klag MJ, Borhani NO, Cohen JD, Kirchner KA, et al, Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Lack of blood pressure effect with calcium and magnesium supplementation in adults with high-normal blood pressure. Results from Phase I of the Trials of Hypertension Prevention (TOHP). Annals of Epidemiology 1995;5(2):96-107. [DOI] [PubMed] [Google Scholar]
Davis 1996 {published data only}
- Davis IJ, Grim C, Dwyer K, Nicholson L, Dwyer J. The effects of calcium supplementation on ambulatory blood pressure in African-American adolescents. Journal of the National Medical Association 1996;88(12):774-8. [PMC free article] [PubMed] [Google Scholar]
Entezari 2015 {published data only}
- Entezari MH. The effect of supplementary calcium on blood pressure in healthy adult women aged 18-30 years in Tehran, Iran. Journal of Education and Health Promotion 2015;4:67. [DOI: 10.4103/2277-9531.162388] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillman 1995 {published data only}
- Gillman MW, Hood MY, Moore LL, Nguyen US, Singer MR, Andon MB. Effect of calcium supplementation on blood pressure in children. Journal of Pediatrics 1995;127(2):186-92. [DOI] [PubMed] [Google Scholar]
Hilary Green 2000 {published data only}
- Hilary Green J, Richards JK, Bunning RL. Blood pressure responses to high-calcium skim milk and potassium-enriched high-calcium skim milk. Journal of Hypertension 2000;18(9):1331-9. [DOI] [PubMed] [Google Scholar]
Johnson 1985 {published data only}
- Johnson NE, Smith EL, Freudenheim JL. Effects on blood pressure of calcium supplementation of women. American Journal of Clinical Nutrition 1985;42(1):12-7. [DOI] [PubMed] [Google Scholar]
Karanja 1987 {published data only}
- Karanja N, Morris CD, Illingworth DR, McCarron DA. Plasma lipids and hypertension: response to calcium supplementation. American Journal of Clinical Nutrition 1987;45(1):60-5. [DOI] [PubMed] [Google Scholar]
Lijnen 1995 {published data only}
- Lijnen P, Petrov V. Dietary calcium, blood pressure and cell membrane cation transport systems in males. Journal of Hypertension 1995;13(8):875-82. [DOI] [PubMed] [Google Scholar]
- Petrov V, Lijnen P. Modification of intracellular calcium and plasma renin by dietary calcium in men. American Journal of Hypertension 1999;12(Pt 1-2):1217-24. [DOI] [PubMed] [Google Scholar]
Lyle 1987 {published data only}
- Lyle RM, Melby CL, Hyner GC, Edmondson JW, Miller JZ, Weinberger MH. Blood pressure and metabolic effects of calcium supplementation in normotensive white and black men. JAMA 1987;257(13):1772-6. [PubMed] [Google Scholar]
- Lyle RM, Melby CL, Hyner GC. Metabolic differences between subjects whose blood pressure did or did not respond to oral calcium supplementation. American Journal of Clinical Nutrition 1988;47(6):1030-5. [DOI] [PubMed] [Google Scholar]
Lyle 1992 {published data only}
- Lyle RM. Does baseline serum total calcium level influence the blood pressure response to calcium supplementation? A double-blind study. Netherlands Journal of Medicine 1992;41(1-2):48-55. [PubMed] [Google Scholar]
McCarron 1985 {published data only}
- McCarron DA, Morris CD. Blood pressure response to oral calcium in persons with mild to moderate hypertension. A randomized, double-blind, placebo-controlled, crossover trial. Annals of Internal Medicine 1985;103(6 ( Pt 1)):825-31. [DOI] [PubMed] [Google Scholar]
Reid 2005 {published data only}
- Reid IR, Horne A, Mason B, Ames R, Bava U, Gamble GD. Effects of calcium supplementation on body weight and blood pressure in normal older women: a randomized controlled trial. Journal of Clinical Endocrinology & Metabolism 2005;90(7):3824-9. [DOI] [PubMed] [Google Scholar]
Reid 2010 {published data only}
- Reid IR, Ames R, Mason B, Bolland MJ, Bacon CJ, Reid HE, et al. Effects of calcium supplementation on lipids, blood pressure, and body composition in healthy older men: a randomized controlled trial. American Journal of Clinical Nutrition 2010;91(1):131-9. [DOI] [PubMed] [Google Scholar]
Sacks 1998 {published data only}
- Sacks FM, Willett WC, Smith A, Brown LE, Rosner B, Moore TJ. Effect on blood pressure of potassium, calcium, and magnesium in women with low habitual intake. Hypertension 1998;31(1):131-8. [DOI] [PubMed] [Google Scholar]
Shidfar 2010 {published data only}
- Shidfar F, Moghayedi M, Kerman SR, Hosseini S, Shidfar S. Effects of a calcium supplement on serum lipoproteins, apolipoprotein B, and blood pressure in overweight men. International Journal of Endocrinology and Metabolism 2010;8(4):194-200. [Google Scholar]
Thomsen 1987 {published data only}
- Thomsen K, Nilas L, Christiansen C. Dietary calcium intake and blood pressure in normotensive subjects. Acta Medica Scandinavica 1987;222(1):51-6. [DOI] [PubMed] [Google Scholar]
Van Beresteyn 1986 {published data only}
- Van Beresteyn EC, Schaafsma G, De Waard H. Oral calcium and blood pressure: a controlled intervention trial. American Journal of Clinical Nutrition 1986;44(6):883-8. [DOI] [PubMed] [Google Scholar]
Yanovski 2009 {published data only}
- JA Yanovski, SJ Parikh, LB Yanoff, BI Denkinger, KA Calis, JC Reynolds, NG Sebring, McHugh T. Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial.. Annals of Internal Medicine 2009/06//;150(12):821-6 9p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00030238. Supplemental Calcium in Overweight People. https://clinicaltrials.gov/ct2/show/NCT00030238?cond=NCT00030238&draw=2&rank=1 (date received 12 Feb 2002).
Yosephin 2015 {published data only}
- Yosephin B, Khomsan A, Briawan D, Rimbawan R. Vitamin D plus calcium supplementation increased serum 25(OH)D on reproductive age women workers. Asian Pacific Journal of Tropical Disease 2015;5(11):877-80. [DOI: 10.1016/S2222-1808(15)60948-3] [DOI] [Google Scholar]
References to studies excluded from this review
Bostick 2000 {published data only}
- Bostick RM, Fosdick L, Grandits GA, Grambsch P, Gross M, Louis TA. Effect of calcium supplementation on serum cholesterol and blood pressure. A randomized, double-blind, placebo-controlled, clinical trial. Archives of Family Medicine 2000;9(1):31-8; Discussion 39. [DOI] [PubMed] [Google Scholar]
Das 2017 {published data only}
- Das S, Goltzman D, Ong AM, Gorgui J, Wall M, Morin SN, et al. Dietary calcium intake and cardiovascular health: is there any relationship? Journal of Bone and Mineral Research (2016 Annual Meeting of the American Society for Bone and Mineral Research, ASBMR 2016. United States) 2017;31(Suppl 1):97-98. [Google Scholar]
Dwyer 1998 {published data only}
- Dwyer JH, Dwyer KM, Scribner RA, Sun P, Li L, Nicholson LM, et al. Dietary calcium, calcium supplementation, and blood pressure in African American adolescents. American Journal of Clinical Nutrition 1998;68(3):648-55. [DOI] [PubMed] [Google Scholar]
Eftekhari 2009 {published data only}
- Eftekhari MH, Rajaeifard AR, Ahmadi A, Kashfi SM, Khajeh Rahim AA. Effect of two isocaloric diets, low fat-high calcium and low fat-high fiber on weight reduction, lipid profile, and blood pressure. International Cardiovascular Research Journal 2009;3(4):200-6. [Google Scholar]
Ferreira 2016 {published data only}
- Ferreira TD, Leal PM, Antunes VP, Sanjuliani AF, Klein MR. No difference in acute effects of supplemental v. dietary calcium on blood pressure and microvascular function in obese women challenged with a high-fat meal: a cross-over randomised study. British Journal of Nutrition 2016;116(9):1564-72. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2015 {published data only}
- Hofmeyr GJ, Seuc AH, Betran AP, Purnat TD, Ciganda A, Munjanja SP, et al. The effect of calcium supplementation on blood pressure in non-pregnant women with previous pre-eclampsia: an exploratory, randomized placebo controlled study. Pregnancy Hypertension 2015;5(4):273-9. [DOI] [PubMed] [Google Scholar]
Luft 1986 {published data only}
- Luft FC, Aronoff GR, Sloan RS, Fineberg NS, Weinberger MH. Short-term augmented calcium intake has no effect on sodium homeostasis. Clinical Pharmacology and Therapeutics 1986;39(4):414-9. [DOI] [PubMed] [Google Scholar]
Morris 1988 {published data only}
- Morris CD, Karaja N, McCarron DA. 42 Dietary versus supplemental calcium to reduce blood pressure. Journal of Hypertension 1988;6(4):139A. [Google Scholar]
Ong 2016 {published data only}
- Ong AM, Weiler HA, Wall M, Haddad R, Gorgui J, Daskalopoulou SS, et al. Feasibility of a clinical trial to assess the effect of dietary calcium v. supplemental calcium on vascular and bone markers in healthy postmenopausal women. British Journal of Nutrition 2016;116(1):104-14. [DOI] [PubMed] [Google Scholar]
Pan 1993 {published data only}
- Pan WH, Wang CY, Li LA, Kao LS, Yeh SH. No significant effect of calcium and vitamin D supplementation on blood pressure and calcium metabolism in elderly Chinese. Chinese Journal of Physiology 1993;36(2):85-94. [PubMed] [Google Scholar]
Pan 2000 {published data only}
- Pan Z, Zhao L, Guo D, Yang R, Xu C, Wu X. Effects of oral calcium supplementation on blood pressure in population. Zhonghua Yu Fang Yi Xue Za Zhi 2000;34(2):109-12. [PubMed] [Google Scholar]
Rahman 2003 {published data only}
- Rahman M, Lyle RM, Teegarden D. Dairy calcium supplementation and its effects on the blood pressure of normotensive adult females. Research Quarterly for Exercise and Sport 2003;75(1 suppl):A-30. [Google Scholar]
Sakai 2017 {published data only}
- Sakai S, Hien VTT, Tuyen LD, Duc HA, Masuda Y, Yamamoto S. Effects of eggshell calcium supplementation on bone mass in postmenopausal Vietnamese women. Journal of Nutritional Science and Vitaminology 2017;63(2):120-4. [DOI] [PubMed] [Google Scholar]
Shalileh 2010 {published data only}
- Shalileh M, Shidfar F, Haghani H, Eghtesadi S, Heydari I. The influence of calcium supplement on body composition, weight loss and insulin resistance in obese adults receiving low calorie diet. Journal of Research in Medical Sciences 2010;15(4):191-201. [PMC free article] [PubMed] [Google Scholar]
Smith 1987 {published data only}
- Smith AN. The Effect of Calcium Supplementation on Blood Pressure of Black Women. Chappel Hill: University of North Carolina, 1987. [Google Scholar]
Weinberge 1993 {published data only}
- Weinberger MH, Wagner UL, Fineberg NS. The blood pressure effects of calcium supplementation in humans of known sodium responsiveness. American Journal of Hypertension 1993;6(9):799-805. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang J, Marsili E. Effects of nutritional supplements on blood pressure. Journal of Chiropractic Education (ACC Conference Proceedings) 2009;23(1):102. [Google Scholar]
References to ongoing studies
ACTRN12617000697381 {published data only}
- ACTRN12617000697381. Calcium supplements and 24-hour blood pressure. who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617000697381 (date received 16 May 2017).
ChiCTR‐IOR‐15006495 {published data only}
- ChiCTR-IOR-15006495. The influence of different sources of calcium on metabolism in postmenopausal women: a Randomized Intervention Study. www.chictr.org.cn/showproj.aspx?proj=11096 (date received 06 Feb 2015).
ChiCTR‐TRC‐12002806 {published data only}
- ChiCTR-TRC-12002806. The preventive effect of calcium supplementation on the incidence of chronic non-communicable diseases in healthy women. http://www.chictr.org.cn/historyversionpub.aspx?regno=ChiCTR-TRC-12002806 (date received 21 May 2008).
Irct2014021116555N {published data only}
- IRCT2014021116555N. Comparison of the Effects of Calcium, Vitamin D, and Calcium plus Vitamin D On Anthropometric Indices, Body Composition, Lipid Profile, Blood Pressure, and Blood Glucose in Overweight or Obese Premenopausal women. en.irct.ir/trial/15465 (date received 18 May 2014).
Irct2016111016123N {published data only}
- IRCT2016111016123N. Effect of vitamin D and calcium supplementation on serum levels of hormones affecting postpartum depression. https://www.irct.ir/trial/15190 (date received 28 Sep 2017).
NCT01561131 {published data only}
- NCT01561131. The Effect of Protein and Calcium on Weight Change and Blood Lipid Profile. ClinicalTrials.gov/show/NCT01561131 (date received 22 March 2012).
NCT02534064 {published data only}
- NCT02534064. Effect of Consumption of Yogurt Fortified in Calcium and Vit. D on Circulating Levels of 25OHD in Postmenopausal Women. https://ClinicalTrials.gov/show/NCT02534064 (date received 27 Aug 2015).
NCT03878667 {published data only}
- NCT03878667. Effects of Calcium Supplementation on Women in the Curves for Women Program. ClinicalTrials.gov/show/NCT03878667 (date received 18 March 2019).
UMIN000001176 {published data only}
- UMIN000001176. Randomized controlled trial of calcium supplementation. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000001440 (date received 6 June 2008).
UMIN000018952 {published data only}
- UMIN000018952. Effect of calcium combination lemon drink absorption on lifestyle-related disease. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000021926 (date received 31 March 2017).
Additional references
Allen 2006
- Allen L, De Benoist B, Dary O, Hurrel R. Guidelines on food fortification with micronutrients (WHO/FAO). who.int/nutrition/publications/micronutrients/9241594012/en/ (accessed 16th May 2015).
Allender 1996
- Allender PS, Cutler JA, Follmann D, Cappuccio FP, Pryer J, Elliott P. Dietary calcium and blood pressure: a meta-analysis of randomized clinical trials. Annals of Internal Medicine 1996;124(9):825-31. [DOI] [PubMed] [Google Scholar]
Belizan 1980
- Belizan JM, Villar J. The relationship between calcium intake and edema-, proteinuria-, and hypertension-gestosis: an hypothesis. American Journal of Clinical Nutrition 1980;33(10):2202-10. [DOI] [PubMed] [Google Scholar]
Belizan 1984
- Belizan JM, Villar J, Self S, Pineda O, González I, Sainz E. The mediating role of the parathyroid gland in the effect of low calcium intake on blood pressure in the rat. Archivos Latinoamericanos de Nutricion 1984;34(4):666-75. [PubMed] [Google Scholar]
Belizan 1988
- Belizan JM, Villar J, Repke J. The relationship between calcium intake and pregnancy-induced hypertension: up-to-date evidence. American Journal of Obstetrics and Gynecology 1988;158(4):898-902. [DOI] [PubMed] [Google Scholar]
Bolland 2008
- Bolland MJ, Barber PA, Doughty RN, Mason B, Horne A, Ames R, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ 2008;336(7638):262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carretero 2000
- Carretero OA, Oparil S. Essential hypertension: part I: definition and etiology. Circulation 2000;101:329-35. [DOI] [PubMed] [Google Scholar]
CDC 2021
- Centers for Disease Control and Prevention (CDC). High Blood pressure. www.cdc.gov/bloodpressure/about.htm (accessed 18 May 2021).
Chobanian 2003
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003;42(6):1206-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ciapponi 2011
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: a new software for early stage of systematic reviews. In: Health Technology Assessment International Conference; 2011; Rio de Janeiro, Brazil. 2011.
Cook 1995
- Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Archives of Internal Medicine 1995;155(7):701-9. [PubMed] [Google Scholar]
Cormick 2019a
- Cormick G, Belizán JM. Calcium intake and health. Nutrients 2019;11(7):1606. [DOI] [PMC free article] [PubMed]
Cormick 2020
- Cormick G, Betrán AP, Metz F, Palacios C, Beltrán-Velazquez F, De Las Nieves García-Casal M, et al. Regulatory and policy-related aspects of calcium fortification of foods: implications for implementing national strategies of calcium fortification. Nutrients 2020;12(4):1022. [DOI] [PMC free article] [PubMed]
Cormick 2021
- Cormick G, Betran AP, Romero IB, Cormick MS, Belizán JM, Bardach A, et al. Effect of calcium fortified foods on health outcomes: a systematic review and meta-analysis. Nutrients 2021;13(2):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curhan 2004
- Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses' Health Study II. Archives of Internal Medicine 2004;164(8):885-91. [DOI] [PubMed] [Google Scholar]
Dickinson 2006
- Dickinson HO, Nicolson D, Cook JV, Campbell F, Beyer FR, Ford GA, et al. Calcium supplementation for the management of primary hypertension in adults. Cochrane Database of Systematic Reviews 2006, Issue 2. Art. No: CD004639. [DOI: 10.1002/14651858.CD004639.pub2] [DOI] [PubMed] [Google Scholar]
Ezzati 2002
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet 2002;360(9343):1347-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garrett 2005
- Garrett J, Ruel MT. The coexistence of child undernutrition and maternal overweight: prevalence, hypotheses, and programme and policy implications. Maternal and Child Nutrition 2005;1(3):185-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glujovsky 2010
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. New software for early stage of systematic reviews. In: XVIII Cochrane Colloquium (joint Colloquium of the Cochrane & Campbell Collaborations); 2010; Keystone Resort, Colorado, USA. 2010.
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University GRADEpro. Version accessed prior to 9 June 2021. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Griffith 1999
- Griffith LE, Guyatt GH, Cook RJ, Bucher HC, Cook DJ. The influence of dietary and nondietary calcium supplementation on blood pressure. American Journal of Hypertension 1999;12(1):92. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
Harris 2002
- Harris SS. The effect of calcium consumption on iron absorption and iron status. Nutrition in Clinical Care 2002;5(5):231-5. [DOI] [PubMed] [Google Scholar]
Hatton 2003
- Hatton DC, Harrison-Hohner J, Coste S, Reller M, McCarron D. Gestational calcium supplementation and blood pressure in the offspring. American Journal of Hypertension 2003;16(10):801-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heaney 2006
- Heaney RP. Calcium intake and disease prevention. Arquivos Brasileiros de Endocrinologia e Metabologia 2006;50(4):685-93. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1.
Hofmeyr 2018
- Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD001059. [DOI: 10.1002/14651858.CD001059.pub5] [CD001059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ilich‐Ernst 1998
Jackson 2006
Kalkwarf 1998
- Kalkwarf HJ, Harrast SD. Effects of calcium supplementation and lactation on iron status. American Journal of Clinical Nutrition 1998 ;67(6):1244-9. [DOI] [PubMed] [Google Scholar]
Kearney 2004
- Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. Journal of Hypertension 2004 ;22(1):11-9. [DOI] [PubMed] [Google Scholar]
Kearney 2005
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet 2005;365(9455):217-23. [DOI] [PubMed] [Google Scholar]
Kumssa 2015
- Kumssa DB, Joy EJM, Ander EL, Watts MJ, Young SD, Walker S, et al. Dietary calcium and zinc deficiency risks are decreasing but remain prevalent [Dietary calcium and zinc deficiency risks are decreasing but remain prevalent]. Scientific Reports 2015;5:10974. [DOI: 10.1038/srep10974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawes 2008
- Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease 2001. Lancet 2008;371(9623):1513-8. [DOI] [PubMed] [Google Scholar]
Lennon 2017
- Lennon SL, DellaValle DM, Rodder SG, Prest M, Sinley RC, Hoy MK, et al. 2015 evidence analysis library evidence-based Nutrition Practice Guideline for the management of hypertension in adults. Journal of the Academy of Nutrition and Dietetics 2017;117(9):1445-1458 e17. [DOI] [PubMed] [Google Scholar]
Lewington 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360(9349):1903-13. [DOI] [PubMed] [Google Scholar]
Lewis 2012
- Lewis JR, Zhu K, Prince RL. Adverse events from calcium supplementation: relationship to errors in myocardial infarction self-reporting in randomized controlled trials of calcium supplementation. Journal of Bone and Mineral Research 2012;27(3):719-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lewis 2015
- Lewis JR, Radavelli-Bagatini S, Rejnmark L, Chen JS, Simpson JM, Lappe JM, et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. Journal of Bone and Mineral Research 2015;30(1):165-75. [PMID: ] [DOI] [PubMed] [Google Scholar]
Llanos 2008
- Llanos A, Oyarzún MT, Bonvecchio A, Rivera JA, Uauy R. Are research priorities in Latin America in line with the nutritional problems of the population? Public Health Nutrition 2008;11(5):466-77. [DOI] [PubMed] [Google Scholar]
Morvaridzadeh 2020
- Morvaridzadeh M, Sepidarkish M, Fazelian S, Rahimlou M, Omidi A, Ardehali SH, et al. Effect of calcium and vitamin D co-supplementation on blood pressure: a systematic review and meta-analysis. Clinical Therapeutics 2020;42(3):e45-63. [DOI: 10.1016/j.clinthera.2020.01.005] [DOI] [PubMed] [Google Scholar]
Palacios 2021
- Palacios C, Cormick G, Hofmeyr GJ, Garcia-Casal MN, Peña-Rosas JP, Betrán AP. Calcium-fortified foods in public health programs: considerations for implementation. Annals of the New York Academy of Sciences 2021;1485(1):3-21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rapsomaniki 2014
- Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet 2014;383(9932):1899-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sabbagh 2009
- Sabbagh Z, Vatanparast H. Is calcium supplementation a risk factor for cardiovascular diseases in older women? Nutrition Reviews 2009;67(2):105-8. [DOI] [PubMed] [Google Scholar]
Selmer 2000
- Selmer RM, Kristiansen IS, Haglerod A, Graff-Iversen S, Larsen HK, Meyer HE, et al. Cost and health consequences of reducing the population intake of salt. Journal of Epidemiology and Community Health 2000;54(9):697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sokoll 1992
- Sokoll LJ, Dawson-Hughes B. Calcium supplementation and plasma ferritin concentrations in premenopausal women. American Journal of Clinical Nutrition 1992;56(6):1045-8. [DOI] [PubMed] [Google Scholar]
Stamler 1991
- Stamler J. Blood pressure and high blood pressure. Aspects of risk. Hypertension 1991;18(3 Suppl):I95-107. [DOI] [PubMed] [Google Scholar]
Van Mierlo 2006
- Van Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, et al. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. Journal of Human Hypertension 2006;20(8):571-80. [DOI] [PubMed] [Google Scholar]
Villa‐Etchegoyen 2019
- Villa-Etchegoyen C, Lombarte M, Matamoros N, Belizán JM, Cormick G. Mechanisms involved in the relationship between low calcium intake and high blood pressure. Nutrients 2019;11(5):1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whelton 2002
- Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al. Primary prevention of hypertension: clinical and public health advisory from The National High Blood Pressure Education Program. JAMA 2002;288(15):1882-8. [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization. Calcium and Magnesium in Drinking Water. Geneva: World Health Organization, 2009. [Google Scholar]
Williams 2001
- Williams CP, Child DF, Hudson PR, Davies GK, Davies MG, John R, et al. Why oral calcium supplements may reduce renal stone disease: report of a clinical pilot study. Journal of Clinical Pathology 2001 ;54(1):54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2011
- Williams B. High blood pressure in young people and premature death. BMJ 2011;22:342:d1104. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cormick 2012
- Cormick G, Ciapponi A, Mazzoni A, Belizán JM, Cafferata ML. Calcium supplementation for prevention of primary hypertension. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No: CD010037. [DOI: 10.1002/14651858.CD010037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cormick 2015
- Cormick G, Ciapponi A, Cafferata ML, Belizan JM. Calcium supplementation for prevention of primary hypertension. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD010037. [DOI: 10.1002/14651858.CD010037.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


