Abstract
Background:
Older patients from nursing homes are commonly exposed to polypharmacy before a hospital admission. Deprescribing has been promoted as a solution to this problem, though systematic reviews have not found benefit. The aim of this study was to understand if in-hospital deprescribing of certain classes of medications is associated with certain benefits or risks.
Methods:
We conducted a prospective, multicentre, cohort study in 239 medical inpatients ⩾75 years (mean age 87.4 years) who were exposed to polypharmacy (⩾5 medications) prior to admission and discharged to a nursing home for permanent placement. Patients were categorised by whether deprescribing occurred, mortality and readmissions were assessed 30 and 90 days after hospital discharge. The EQ-5D-5 L health survey assessed changes in health-related quality of life (HRQOL) at 90 days, with comparison to EQ-5D-5 L results at day 30. Latent class analysis (LCA) was used to investigate associations between patterns of prescribed and deprescribed medications and mortality.
Results:
Patients for whom deprescribing occurred had a higher Charlson Index; there were no differences between the groups in principal diagnosis, total or Beers list number of medications on admission. The number of Beers list medications increased in both groups before discharge. Patients who had medications deprescribed had nonsignificantly greater odds of dying within 90 days [odds ration (OR) = 3.23 (95% confidence interval (CI): 0.68, 14.92; p = 0.136]. Deprescribing of certain classes was associated with higher 90-day mortality: antihypertensives (OR = 2.27, 95% CI: 1.004, 5; p = 0.049) and statins (OR = 5, 95% CI: 1.61, 14.28; p = 0.005). Readmissions and 1-year mortality rates were similar. There was no deterioration in HRQOL when medications were deprescribed. LCA showed that patients with the least medication changes had the lowest mortality.
Conclusion:
Deprescribing certain classes of medications during hospitalisation was associated with worse mortality, but not readmissions or overall HRQOL. Larger controlled deprescribing studies targeting specific medications are warranted to further investigate these findings.
This study was registered with the Australian and New Zealand Clinical Trials Registry, ACTRN1 2616001336471.
Plain language summary
Background: When an older person living in a nursing home is admitted to hospital, does stopping long-term medications help them?
Many older people from nursing homes take a large number of medications each day to treat symptoms and prevent adverse events. “Polypharmacy” is a term used to describe taking multiple long-term medications, and it is associated with many negative outcomes such as increased number of falls, cognitive decline, hospital readmission, even death. Deprescribing of nonessential medications – whether stopping or reducing the dose – is promoted as good hospital practice and is assumed to help older frail people live longer and feel better. However, we often don’t fully understand what is and is not essential.
We wanted to better understand the effect of deprescribing long-term medications for older frail patients during an unplanned hospital admission as they were going to a nursing home to live.
Methods: While admitted to hospital, medications are often reviewed by a clinical pharmacist and specialist physician. Sometimes medications are ceased; sometimes they are not. This gave us the opportunity to study two groups of older frail people from nursing homes: those who had regular, long-term medications ceased or reduced and those who did not. We wanted to see if one group did better. For example, did they feel worse if we stopped certain medications? Did they suffer other bad events compared with those patients for whom no medications were ceased? Were they readmitted to hospital earlier or more often?
Results and conclusion: Despite the assumption that stopping medications for this type of patient is good practice, we found no benefit. We were also surprised to find stopping or reducing certain drug classes (e.g. antihypertensives and cholesterol-lowering drugs) was associated with greater mortality. Larger, randomised studies will better answer these important questions.
Keywords: aged care, deprescribing, elderly, EQ-5D-5 L, health-related quality of life, hospital medicine, mortality, nursing homes, prescribing
Introduction/background
Older people are frequent consumers of hospital and pharmacy resources. In 2016, 42% of Australian hospital discharges were for people 65 years of age and older; 1 this age group is the largest per capita user of prescription medications. 2 An unplanned hospitalisation becomes a conduit of new prescriptions to treat symptoms and prevent major clinical events. 3 Therefore, it is no surprise that the hospital is a common source of potentially inappropriate medications (PIM) and can exacerbate, rather than reduce exposure to polypharmacy. 2 Deprescribing, whether inpatient or outpatient, has been promoted as a solution to the vexing problem of polypharmacy and a way to improve the negative outcomes attending polypharmacy, 4 but systematic reviews of this approach have been unable to support a clear and consistent benefit.5,6 These reviews were highly heterogeneous in part due to inconsistent definitions of ‘older’. Indeed, early studies were very promising, with some suggesting a 50% reduction in mortality, 7 and improvement in quality of life, 8 following a reduction of medication burden. However, when systematic reviews of randomised trials began to accumulate, the results were less encouraging. A systematic review of deprescribing in a hospital setting was also hampered by the heterogeneity of included studies. 9 Some studies measured mortality, some the number of PIM, and some emergency department presentations or readmissions to hospital. Results were limited to improvements that did not reach statistical significance, with no effect on readmissions. Health-related quality of life (HRQOL), too, has been evaluated by systematic review, finding 10 studies that fulfilled criteria, but failed to show a significant effect on HRQOL. 10 Studies in this review which did find an improvement in HRQOL were limited by heterogeneous patient populations, nonspecific medication targets and differing outcome measures, dimming a clear signal of benefit. Other systematic reviews of deprescribing, with similar conclusions, focused on different groups of participants, such as patients with frailty, 11 community-dwelling older people, 12 or those with limited life-expectancy. 13
It remains unclear when, in an older person’s life, the time-to-benefit of preventive medications is too long to continue exposure, even when the risk is slight. Furthermore, an individual’s prognosis can change, adding complexity to the already difficult task of prescribing responsibly.
In this context, nursing home residents are an important population to study for several reasons: their presence on an inpatient hospital ward is common; polypharmacy is highly prevalent; compliance with medications is generally accepted as 100%; mortality is increased compared with community-dwelling older people; 14 and the burden of medications might be greater both in sheer number and in the prevalence of adverse drug events. Older people who live permanently in nursing homes carry the greatest medication burden 15 and medication risk because half of these vulnerable people are on PIM. 16 An unplanned hospital admission in this group is an opportunity to review medications – indications and doses – as well as monitor for withdrawal syndromes that deprescribing could precipitate. However, this opportunity is often lost amid increasing pressures to discharge patients.
We recently published a small observational study evaluating the outcomes of a group of older medical inpatients, comparing those who had medications ceased during their hospital stay to those who did not. After controlling for various confounders, including the number of PIM taken, there was no difference in mortality or readmissions. Albeit our sample size was relatively small (n = 100), there was a signal of improved HRQOL, measured with Short Form-8, in those patients who had medications deprescribed. 17 As HRQOL is an important patient-centred endpoint, we addressed this issue in a larger patient population, using a different HRQOL measure, the EQ-5D-5 L, for ease of administration without loss of performance. We speculated that any improvement between 30 and 90 days post discharge would better reflect long-term medication changes made during the admission. Furthermore, we sought to determine whether deprescribing, as well as prescribing, certain classes of medications is associated with specific benefits or risks. This would be useful to target specific classes for future deprescribing studies.
Methods
Our study was registered with the Australian and New Zealand Clinical Trials Registry, ACTRN12616001336471. 18 This was a prospective cohort study of older medical inpatients (>75 years) on five or more regular medications, a commonly accepted definition of polypharmacy, before an unplanned admission to five metropolitan hospitals in Adelaide, South Australia, between June 2018 and April 2019. The study is reported according to the STROBE checklist for observational studies. 19 All patients were discharged to permanent placement in a nursing home. Ethics approval was obtained for the study from the Royal Adelaide Hospital Ethics Committee (HREC/15/RAH/302) with mutual recognition at participating sites. We investigated the associations between deprescribing and mortality, readmission rate, and HRQOL. Patients consented to ongoing follow-up; deprescribing that occurred while in hospital was not on a voluntary basis, but in line with best practice that medications be reviewed and nonbeneficial medications ceased. Patients were categorised and analysed according to whether they had medication deprescribed or not. Since medication reconciliation can sometimes be delayed, patients were excluded if admitted for less than 48 hours; we also excluded patients expected to die within 30 days.
All patients received routine care consisting of a multidisciplinary assessment of medications involving a clinical pharmacist and a specialist physician. The multidisciplinary team used the patient’s medication list at the time of admission provided by the nursing home or confirmed with the community pharmacist. Decisions about medication changes were at the discretion of the treating team and involved the patient and the carer, as well as the patient’s general practitioner whenever possible. Investigators did not engage collectively in prescribing or deprescribing actions. All deprescribing decisions were made by the multidisciplinary team members involved in the care of that patient. In addition, the team could employ any available deprescribing guideline to identify potentially inappropriate medications. Teams were not required to adhere to a specific guideline for deprescribing. After enrolment, clinical pharmacists entered each patient’s medication list from admission and again at discharge into a secure web application REDCap (Research Electronic Data Capture). Medications were categorised as ceased, unchanged, or total daily dose increased or decreased. The indications for medication changes were not recorded. A medication was considered deprescribed if it was either ceased or its dose reduced. Only medications given regularly were counted; pro re nata (PRN) medications, topical creams, and eye drops were excluded from analysis.20,21 However, PRN medications were considered regularly scheduled if given more than once weekly, as defined in Thillainadesan et al.’s 2018 study. 21 Short-term antibiotics and glucocorticoids were excluded if ceased before the 30-day follow-up. Combination medications (e.g. amlodipine/atorvastatin) were counted as two medications if one of the medications was likely to be altered independent of the other; medications given regularly, but at long intervals (e.g. 6-monthly denosumab) were counted as regularly scheduled, unless ceased; a change of drug class with a similar dose (e.g. pantoprazole to omeprazole; aspirin to clopidogrel) was not considered deprescribing. Consistent definitions were kept for admission medication list and discharge medication list to enable consistent counting of medications for participants.
Important sources of bias might be sheer number of medications, number of potentially inappropriate medications, and comorbidities. To address possible selection bias (where a group of older patients on inappropriate medications have medications deprescribed and are compared to another similar group of older patients who were not on inappropriate medications), we used the Beers 2015 list of potentially inappropriate medications to compare the number of potentially inappropriate medications taken before admission and at the time of discharge. 22 The participant’s age, gender, and Charlson Comorbidity Index (CCI) 23 were also recorded. CCI was based on clinical coding at the end of a patient’s hospital stay; ICD-10 codes are generated by clinical coders by reviewing the discharge summary and the medical record of a hospital stay. ICD-10 codes are used to generate CCI for each hospital stay, 24 but only the index admission was used for our study.
Mortality and readmissions were assessed 30 and 90 days after hospital discharge using hospital administrative databases. Nursing staff at the nursing home were contacted by phone at 30 and 90 days to review the participant’s current medication list. Limitations in death certification at nursing homes meant that only all-cause mortality could be reported. Readmission data were extracted from the hospital database. Mortality was assessed again at 1 year using hospital administrative databases.
We assessed self-reported HRQOL at 30 and 90 days. For HRQOL, patients served as their own controls. The EQ-5D-5 L health survey was administered (with permission from the EuroQol Research Foundation) 30 days after discharge to establish a baseline after recovery from acute hospitalisation followed by comparison with the same survey administered 90 days after discharge. The investigators were concerned that an earlier baseline measurement would be confounded by recovery from an acute illness. The EQ-5D-5 L includes five levels within five domains, as well as an index score and a visual analogue scale (VAS) measuring self-rated health on a scale ranging from 0 (worst imaginable health state) to 100 (best imaginable health state). If the participant was unable to answer, a substitute was sought from the nursing home staff or family. Informed consent was obtained from all participants, or next of kin in the case of cognitive impairment or language barrier.
Statistical analysis
The statistical software used was SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were calculated for variables across deprescribed and nondeprescribed groups; comparisons were made using chi-square tests and Fisher’s exact tests (categorical variables), independent t-tests (normally distributed continuous variables) and Wilcoxon rank sum tests (nonnormally distributed continuous variable). Assessment of the association between HRQOL at 90 days and deprescribing was investigated using linear and ordinal logistic regression models with various quality of life outcomes regressed against having any medication deprescribed. This model allowed for clustering within hospitals (five centres) with the site included as a fixed effect. Adjusted models were then performed, including the confounders: age, sex, diabetic, CCI, length of stay (LOS), discharge Beer score and site. Any patient who died within 90 days after discharge was excluded from the HRQOL analysis, as they were censored by death; these patients were included in mortality analysis.
We followed all participants until death or 1 year. We categorised and analysed medications by Anatomical Therapeutic Chemical (ATC) class: acid suppression, antihypertensive, antiplatelet, antipsychotic, antiresorptive, benzodiazepine, diuretics, long-acting nitrates, statins, or other. Multivariable binary logistic, ordinal logistic and linear regression models were then used to investigate associations between mortality, readmission and HRQOL outcomes versus all deprescribed discharged medication classes, adjusted by the site as a covariate.
Furthermore, after completion of the study, we found that a significant number of new, long-term medications were started during admission and continued after discharge. We grouped and analysed these by the most common: cardiovascular medications, vitamins, cathartics, analgesics, anti-thrombotics, sedative/antipsychotics, inhalants, gastrointestinal, and antidiabetic medications. We evaluated these classes using multivariable binary logistic, ordinal logistic and linear regression models to investigate the association between HRQOL, mortality and readmission outcomes, versus all newly prescribed discharge medication classes, adjusted by site as a covariate.
Finally, to search for any hidden clusters of participants within the entire cohort that might be at greater risk, but who otherwise appear the same, we performed a latent class analysis (LCA). 25 LCA is a post hoc grouping method. It uses machine learning to identify patient profiles (classes) amid complex patient data, 26 such as prescription drug use, that might not be visible (i.e. that might be ‘latent’) using conventional analytical statistical methods; the approach relates these categories to an outcome of interest (e.g. mortality) to generate new hypotheses. LCA uses binary indicators (‘yes/no’ for deprescribed medication, and ‘yes/no’ for new medication added, along with ‘yes/no’ for mortality).
Results
A total of 267 eligible patients were approached for enrolment; 239 (90%) consented; 146 (61%) of these required third party consent. Cluster sizes ranged from five patients to 126 patients. Fifty-nine participants were excluded: 20 withdrew at some point in the study; 21 were invalid entries; 14 died during admission; and four had incomplete data, leaving 180 participants with complete data. There were no patients lost to follow-up. Medications were deprescribed in 118 (66%) of the 180 participants while an inpatient. The remaining 62 (34%) participants did not have any medications deprescribed during their inpatient stay. Table 1 gives descriptive statistics for relevant variables across deprescribed and nondeprescribed groups.
Table 1.
Patient demographics, Beers list, EQ-5D-5 L, readmission, and mortality results.
| Variable | Value | All Data (N = 180) | Deprescribed (N = 118) | Not deprescribed (N = 62) |
p-value |
|---|---|---|---|---|---|
| Age, years – mean (SD) | 87.4 (5.3) | 87.6 (4.9) | 86.9 (5.9) | 0.45 a | |
| Gender – N (%) | male | 72 (40) | 45 (38) | 27 (44) | 0.48 b |
| female | 108 (60) | 73 (62) | 35 (56) | ||
| Charlson Index – median (IQR) | 1 (0, 2) | 2 (1,3) | 1 (0,1) | <0.0001 c | |
| Length of stay, days – median (IQR) | 6 (3, 11) | 6 (3, 11) | 6 (3,9) | 0.36 c | |
| Days until first Readmission (excluding 30d deaths) – median (IQR) | 82 (23, 186) | 65 (22,186) | 94 (56, 187) | <0.0001 c | |
| Number of medications on admission – median (IQR) | 10 (8, 13) | 10 (8, 13) | 10 (7, 12) | 0.14 | |
| Number of medications on discharge – median (IQR) | 9 (7, 12) | 9 (7, 11) | 10 (7, 13) | 0.04 | |
| Beer score admission – mean (SD) | 2 (1,3) | 1.9 (1.1) | 1.7 (1.5) | 0.35 a | |
| Beer score discharge – mean (SD) | 2 (1.4) | 2.1 (1.2) | 1.8 (1.5) | 0.17 a | |
| Eq5d index at 90d – mean (SD) | 0.38 (0.33) | 0.35 (0.32) | 0.42 (0.33) | 0.34 a | |
| Mobility at 90d – median (IQR) | 3 (2,5) | 3 (3,5) | 3 (2,4) | 0.22 c | |
| Self-care at 90d – median (IQR) | 3 (3,5) | 3 (3,5) | 3 (2,5) | 0.36 c | |
| Usual activities at 90d – median (IQR) | 3 (2,4) | 3 (2,4) | 3(2,4) | 0.77 c | |
| Pain at 90d – median (IQR) | 2 (1,3) | 2 (1,3) | 1 (1,3) | 0.13 c | |
| Anxiety at 90d – median (IQR) | 1 (1,3) | 1 (1,3) | 2 (1,3) | 0.68 c | |
| VAS at 90d – mean (SD) | 53.5 (21.9) | 52.3 (22.3) | 55.2 (21.4) | 0.54 c | |
| Any readmission? (excluding deaths < 30d) – N (%) | 93 (62) | 57 (60) | 36 (67) | 0.42 b | |
| Diabetic – N (%) | 39 (22) | 29 (25) | 10 (16) | 0.19 b | |
| Meds deprescribed | 118 (66) | 118(100) | 0 | ||
| Death before 30d – N (%) | 28 (16) | 22 (19) | 6 (10) | 0.16 b | |
| Death before 90d (excluding deaths < 30d) – N (%) | 23 (15) | 19 (20) | 4 (7) | 0.14 d | |
| Death before 1yr (no 30d or 90d deaths) – N (%) | 25 (20) | 16 (22) | 9 (18) | 0.62 b |
IQR, inter-quartile range; VAS, visual analogue scale.
t-test p value.
Chi-square p value.
Wilcoxon rank sum test p value.
Fisher’s exact test p value.
Mortality by group
Patients who had medications deprescribed had odds of dying within 90 days that was 3.23 times higher than those in the nondeprescribed group [odds ratio (OR) = 3.23]; however, this difference was not significant in the adjusted model. The overall 1-year mortality was 42%, with no significant differences between the two groups. Deprescribing certain classes of medications was associated with increased mortality at 90 days: antihypertensives (where survival was worse) and statins (survival was also worse). Deprescribing long-acting nitrates was associated with improved 1-year survival, while 1-year mortality was worse in patients whose diuretics were deprescribed. These findings are displayed in Table 4.
Table 4.
Associations with mortality.
| Medication class (ATC code) deprescribed before discharge | Number of patients with this medication change | Adjusted OR (95% CI) and p value for 90-day mortality with deprescribing versus not deprescribing | Adjusted OR (95% CI) and p value for 1-year mortality with deprescribing versus not deprescribing |
|---|---|---|---|
| Any medication | 118 | 3.2, (0.69, 15.04), p = 0.14 | 1.33 (0.43, 4.12), p = 0.62 |
| Antihypertensives (C02, C07, C08, C09) | 44 | 2.27 (1.00, 5.12), p = 0.05 | 1.49 (0.68, 3.22) p = 0.31 |
| Statins (C10) | 20 | 4.95 (1.63,15.09), p = 0.005 | 4.0 (1.25, 12.5), p = 0.019 |
| Long-acting nitrates (C01) | 13 | 0.33 (0.07, 1.52), p = 0.16 |
0.17 (0.04, 0.79), p = 0.02 |
| Diuretics (C03) | 38 | 2.32 (1.00, 5.40), p = .05 |
2.22 (1.0, 5.0), p = 0.05 |
ATC, Anatomical Therapeutic Chemical; CI, confidence interval; OR, odds ratio.
Readmission
The readmission rate was lower for those patients in the deprescribed group [OR = 0.58, 95% confidence interval (CI): 0.22, 1.55; p = 0.2782]; however, this was not statistically significant. After controlling for all other visible factors, however, there was a decrease in time to readmission for those people who had analgesics initiated. Patients who did not have analgesic medications initiated stayed out of hospital 146 days longer than those whose discharge medication list included a new prescription for analgesics (p = 0.0199). There was also a statistically significant higher readmission proportion by 1 year in those who continued statins compared with those who were deprescribed statins (53% versus 25%) (p = 0.0190). There were no significant differences in readmission rate at 30 days or 90 days.
HRQOL
There was no statistically significant deterioration in HRQOL when medications were deprescribed. Results of EQ-5D-5 L at 90 days are presented in Table 1.
There were no significant differences in the specific domains – mobility, self-care, usual activities, pain, anxiety/depression – between those patients for whom deprescribing occurred and those for whom no deprescribing occurred. There were also no differences found in the EQ-5D-5 L index or VAS. However, a new prescription for gastrointestinal agents was associated with a decline in self-reported mobility at 90 days (OR = 0.12, CI: 0.02, 0.68; p = 0.016) but an improvement in EQ-5D Index at 90 days (OR = 3.03, CI: −1.6, −16.66; p = 0.018).
Medication burden
Table 2 lists the most commonly deprescribed medications. There was no statistically significant difference between the two groups in the mean number of potentially inappropriate medications taken either before admission or at discharge. There was a statistically significant difference between the two groups in mean number of medications taken at discharge. In addition, in the deprescribed group, the mean number of Beers list medications was 0.22 greater at the point of discharge than on admission (estimate = 0.22, CI: 0.14, 0.30; p < 0.0001). Similarly, in the nondeprescribed group, the mean number of Beers list medications at the point of discharge was greater than at the point of admission (estimate = 0.13, CI: 0.01, 0.24; p = 0.03).
Table 2.
Medications deprescribed.
| Drug Class (Anatomical Therapeutic Chemical code) |
Total number of medications in this class prescribed before admission | Meds deprescribed during admission (% of prescribed) |
|---|---|---|
| Acid suppression meds (A02) | 119 | 25 (21) |
| Antihypertensives (C02, C07, C08, C09) | 189 | 52 (28) |
| Diuretics (C03) | 130 | 49 (38) |
| Antiplatelet agents (B01) | 85 | 23 (27) |
| Benzodiazepines (N05 C, N05B) | 61 | 15 (25) |
| Antipsychotics (N05AH, N05AX) | 37 | 9 (24) |
| Antiresorptive treatment (M05B) | 27 | 4 (15) |
| Statins (C10) | 72 | 21 (29) |
| Long-acting nitrates (C01) | 57 | 14 (25) |
| Other | 1153 | 254 (22) |
| Total | 1930 | 466 (24) |
New medications were started while in hospital. The most common of these were cardiovascular medications (17%) – including diuretics, antihypertensive agents, and digoxin; but also vitamins (16%); cathartics (15%); analgesic medications (13%, opioid and nonopioid); and gastrointestinal agents (6%) – mostly proton pump inhibitors.
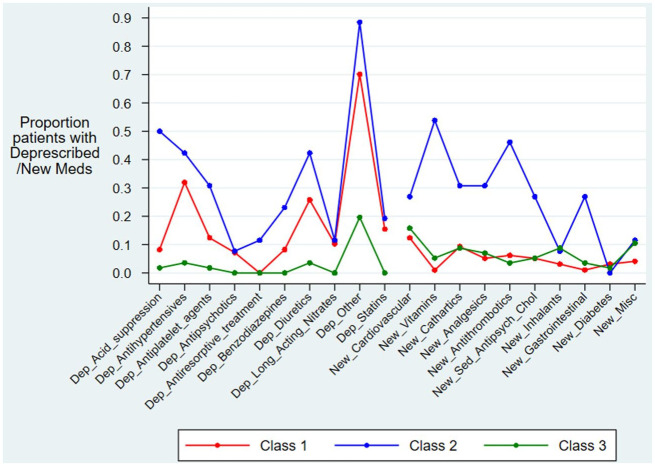
Latent class analysis (LCA)
Table 4 and Figure 1 show results for LCA. We identified three mortality groups (classes) that fit the mortality modelling best, a solution based on whether old medications were deprescribed and if new ones were started. Class 3 had no changes made to long-term medications; class 1 had only modest changes to the long-term medication lists; while class 2 had the most medication changes, both medications deprescribed and medications started.
Figure 1.
Classes of deprescribed medicines and new medication usage identified from the latent class analysis.
Class 1: Moderate deprescribing and a low number of new medications.
Class 2: High deprescribing and a high number of new medications.
Class 3: Low deprescribing and a low number of new medications.
Class 3 had the lowest odds of mortality and shortest length of stay; these patients also had the fewest medication changes (mostly none); this class was therefore used as the reference group. Class 1 had a moderate amount of deprescribing, but few new medications and a slightly worse 30-day mortality than the reference class (multivariate OR = 1.4, CI: 0.5, 4.1); this was not statistically significant (p = 0.582). Class 2 had the greatest number of medications changed (deprescribed and new medications started) and suffered the worst 30-day mortality (multivariate OR = 6.5, CI: 1.6, 25.5; p = 0.008) and had the longest LOS. Similar mortality was found at 90 days for the three classes.
Discussion
Much of the benefit of an unplanned hospital admission for older people from permanent residence in a nursing home might be paradoxically diminished by the addition of potentially inappropriate long-term medications.27–29 Despite the negative associations with polypharmacy, some of them serious, obvious, and preventable, this vexing, modern-day problem persists. Starting medications comes with the expectation of benefit from both prescriber and consumer; yet for an individual patient, the true benefit of a medication can be difficult to know. Similarly, deprescribing comes with a hope of benefit, but one that has not been made clear. Several systematic reviews of deprescribing have not demonstrated a benefit associated with reducing medication burden.3,4 Our study found no deterioration in HRQOL for the group who had medications deprescribed, but a faster readmission. Furthermore, we found worse mortality in those patients ceasing antihypertensives and statins. Several points arising from this study require further discussion; see Table 3 for Association between latent class and 30-day, 90-day, and 12-month mortality (n = 180).
Table 3.
Association between latent class and 30-day, 90-day, and 12-month mortality (n = 180).
| Alive, N (%) | Died, N (%) | Univariate OR (95% CI) |
p value | Overall p value a |
Multivariate
b
OR (95% CI) |
p value | Overall p value a |
|
|---|---|---|---|---|---|---|---|---|
| 30-day mortality | ||||||||
| Class 1 | 84 (86.6) | 13 (13.4) | 1.3 (0.5, 3.7) | 0.60 | 0.02 | 1.4 (0.5, 4.3) | 0.58 | 0.01 |
| Class 2 | 17 (65.4) | 9 (34.6) | 4.5 (1.4, 14.5) | 0.01 | 6.5 (1.6, 25.6) | 0.008 | ||
| Class 3 | 51 (89.5) | 6 (10.5) | Reference | — | Reference | — | ||
| 90-day mortality | ||||||||
| Class 1 | 65 (67.0) | 32 (33.0) | 2.6 (1.1, 6.0) | 0.02 | 0.04 | 2.8 (1.2, 6.9) | 0.02 | 0.02 |
| Class 2 | 16 (61.5) | 10 (38.5) | 3.3 (1.2, 9.7) | 0.03 | 4.6 (1.4, 15.4) | 0.01 | ||
| Class 3 | 48 (84.2) | 9 (15.8) | Reference | — | Reference | — | ||
| 12-month mortality | ||||||||
| Class 1 | 50 (51.6) | 47 (48.5) | 2.0 (1.0, 4.0) | 0.04 | 0.13 | 2.2 (1.0, 4.6) | 0.05 | 0.14 |
| Class 2 | 15 (57.7) | 11 (42.3) | 1.6 (0.6, 4.1) | 0.34 | 1.8 (0.6, 5.2) | 0.29 | ||
| Class 3 | 39 (68.4) | 18 (31.6) | Reference | — | Reference | — | ||
Class 1: Moderate deprescribing and low number of new medications. Class 2: High deprescribing and high number of new medications. Class 3: Low deprescribing and low number of new medications. OR, odds ratio; CI, confidence interval.
Overall association for latent class analysis.
Using binary logistic regression with adjustment for age, gender, hospital, diabetic status and Charlson comorbidity score.
First, a significant number of participants died within 30 and 90 days despite excluding any patient expected to die within 30 days. This high baseline mortality rate should be kept in mind when reviewing medications at the point of discharge from hospital to identify opportunities to reduce medication burden. Despite this complexity, we attempted to ascertain the signal of benefit, be it mortality (important to doctors), readmission (important to administrators), or HRQOL (important to consumers). We looked for differences associated with deprescribing certain classes of medications and found some – ceasing antihypertensives was associated with worse mortality. This could be due to by indication: older people with low blood pressure are likely to have antihypertensive medications ceased or reduced; these patients are also known to have a worse mortality than other older people with normal or high-normal blood pressure. 30 For example, the PARTAGE study, published in 2015, enrolled over 1,100 patients older than 80 years (mean age = 87.6 years) from nursing homes in France and Italy. This study found a startling association between lower blood pressure (systolic blood pressure < 130 mmHg) and worse mortality (two times worse) in those patients on two or more antihypertensive medications, compared with those with similar blood pressure taking only one medication or none, extending out to a 2-year follow-up. 31 As deprescribing while in hospital is common in this patient group, randomised studies are warranted to further investigate this association. 32 It is not clear if stopping blood pressure lowering medications helps or harms when the blood pressure is considered normal.
Second, using LCA, we identified a subgroup of patients with more inpatient medication changes and whose mortality was worse. While it is difficult to identify a cause–effect relationship, this class might have been more acutely unwell than others, a cohort that could be identified more easily with a simple disease severity score (e.g. APACHE), rather than LCA. But starting and stopping medications in a short period of time might be too much for a person with limited resilience and nearing the end of life. Environmental factors – doctors prescribing behaviours – might influence outcomes more than we appreciate. The class who enjoyed the best mortality had the fewest changes to their medications. Despite excluding from this study patients expected to die within 30 days, unseen bias could be at play. Impressions of futility so visible at the bedside of the frail elderly might have influenced clinicians to deprescribe and thus skew the results towards worse outcomes for those whose death was inevitable. Only randomised studies where patients are stratified according to risk of death will begin to unravel this complex association.
Third, our results also suggest clinicians should pause before adding gastric acid suppression medications before hospital discharge. While many studies have shown an association between proton pump inhibitors (PPIs) and mortality, 33 or even improved quality of life, 34 ours found mixed results for quality of life when they are prescribed: decline in mobility, but improved associations with overall quality of life (EQ-5D Index). Our results should be considered with caution, noting the wide confidence intervals. A larger study that focuses only on deprescribing PPIs is required to investigate further.
Fourth, we found no statistically significant associations between deprescribing and HRQOL. In each of the five domains of the EQ-5D, there was no significant difference between the deprescribed and nondeprescribed group’s change in self-reported preferences in the five domains between 30 and 90 days, as well as the EQ-5D Index and VAS. A systematic review of the impact of deprescribing on quality of life was published in 2019. 10 It included 12 heterogeneous studies and found no impact in all but two. A nonrandomised study from 2011 separated patients older than 75 years into three groups based on the number of medications and found a significant association between appropriate medication prescribing and quality of life using EQ-5D. 35 Our study demonstrates no broad signal of benefit on quality of life. It could be that substituting new medications for the ones ceased explains very simply the lack of difference in HRQOL. Most adverse drug events are not detected by explicit criteria like Beers list medications because they are the result of more commonly prescribed medications such as anti-thrombotics, hypoglycaemics, and antihypertensive agents. Similar to mortality and readmissions rate, these results highlight the need for randomisation and specificity.
Finally, our study highlights the inherent complexities of polypharmacy. For those people in the deprescribing group, we added more medications at discharge than we added to the lists of those people categorised in the nondeprescribing group. The hospital has long been a source of potentially inappropriate prescribing. 36 Although at first glance one might surmise that we selected patients with higher Charlson Index to stop medications, we also seemed to select the same patients to add medications. This is reinforced by the findings in the LCA, which identified a subgroup of participants who experienced different outcomes (worse 30- and 90-day mortality) from other participants (Table 4) with similar demographics. We did not measure acuity or disease severity. There could be variables more visible at the bedside that cannot be detected in routinely collected administrative data, an intuition that a patient’s prognosis is worse than a Charlson Index might predict and one that prompts more dramatic changes in medication lists. What is clear, though, is the central role the hospital plays in maintaining the problem of polypharmacy. Even clinicians thoughtful about deprescribing seemed to add medications before discharge. This seems to be a problem many clinicians encounter, but of which few take ownership.
We felt the two groups in our cohort were well-matched for age, gender, and hospital LOS, as well as number of medications, and number of PIM. But there were several limitations of our study worthy of mention, the most important of which was the observational method. Also, 30-day and 90-day follow-up medication lists were snapshots; there could have been medications intended for long-term use which were started, then stopped (for example, at the 60 day mark). We considered this exposure minimal, since a single practitioner is less likely to re-start, cease, and then re-start again, long-term medications in this cohort. Furthermore, we could not account for medications that might be given from the ward stock at a nursing home. Some nursing homes do not have a strict culture of recording medications given on an as-needed, occasional basis. Also, it was not possible to determine why medications were ceased: some patients might have a medication ceased because their prognosis is clearly poor and a shift to comfort as a dominant guiding principle is clear; some patients have a medication ceased because their prognosis is clearly good and the desire to maximise function is a dominant guiding principle (e.g. stopping anticholinergic medications to preserve cognitive function). Our greatest limitation, though, was the convenience sampling, which reduces the generalisability of our findings. 37 Although our two groups appeared the same in many ways, there were clear differences in the Charlson Index and nonrandom sampling creates more opportunity for bias.
This is a foundational study, helping assess the local feasibility of recruitment, consent, digital data collection, and follow-up for this complex group of patients with special needs. Improved design and power for a study of deprescribing likely requires greater focus on specific medications in as narrow a group of patients as possible at similar risk of the outcome of interest. Stopping a medication that might be causing harm could require a different study design from one where the deprescribed medication is simply nonbeneficial. Our study will inform the design and conduct of larger, more precise randomised trials of deintensification of medications as people approach the last chapter of life.
In conclusion, deprescribing was not associated with a worsening of HRQOL, but deprescribing certain classes (antihypertensives or statins) might be associated with worse mortality. Deprescribing medications for older medical inpatients in the last phase of life remains an important, but moving target. Long-term regular medications are stopped, other long-term regular medications are started. In future work, more rigorous methodology is needed, similar to that used to demonstrate the benefit of prescribing a specific medication in the first place. Nonspecific deprescribing might be no more beneficial than nonspecific prescribing. Studies are required to investigate the specific benefits of rigorous deprescribing interventions in order to better justify this practice in clinical care.
Acknowledgments
We would like to acknowledge the support of the Healthy Ageing Research Consortium Investigator Team and the Registry of Senior Australians’ (ROSA) South Australian Health and Medical Research Institute Research Team including their funding support for this study from the South Australian Government Department for Innovation and Skills. Also Suzanne Edwards, senior statistician from the University of Adelaide, for her persistent help with statistics; Matthew Horsfall, Flinders Medical Centre, for providing administrative data; Euro-Quol, for use of EQ-5D-5 L; Shalem Leemaqz, programmer from Flinders University, for his help building the pharmacy data structure; and Professor Claire Roberts, Flinders University, for her encouragement.
Footnotes
Editor’s note: The Editor-in-Chief of Therapeutic Advances in Drug Safety is an author of this paper, therefore, the peer-review process was managed by alternative members of the Board and the submitting Editor had no involvement in the decision-making process.
Author contributions: Made a substantial contribution to the concept or design of the work; or acquisition, analysis or interpretation of data (PTR, UH, CHT, RW, AAM). Drafted the article or revised it critically for important intellectual content (PTR, UH, CHT, RW, AAM). Approved the version to be published (PTR, UH, CHT, RW, AAM). All authors have participated sufficiently in the work and take public responsibility for the content (PTR, UH, CHT, RW, AAM). The following are Investigators in the South Australian Deprescribing Initiative:
Tim Bangsund (Central Adelaide Local Health Network), Gregory Crawford (Northern Adelaide Local Health Network), Miriam Cursaro (Central Adelaide Local Health Network), Debajyoti Chaudhuri (Northern Adelaide Local Health Network), Lucia Caretti (Central Adelaide Local Health Network), Maneesha Dedigama (Southern Adelaide Local Health Network), Ciaran Flood (Central Adelaide Local Health Network), Alex Fong (Northern Adelaide Local Health Network), Jessica Gehlert (Southern Adelaide Local Health Network), Nicky Gordon, (Southern Adelaide Local Health Network), Travis Green (Southern Adelaide Local Health Network), Dirk Hofmann (Southern Adelaide Local Health Network), Jyoti Khadka (Flinders University), Hun Khaw (Central Adelaide Local Health Network), Ivanka Koeper (Southern Adelaide Local Health Network), Keth Lyn Koo (Central Adelaide Local Health Network), Sara Laubscher (Southern Adelaide Local Health Network), Emily Lawton (Central Adelaide Local Health Network), Jason Lim (Central Adelaide Local Health Network), Elena Logvinskaia (Central Adelaide Local Health Network), Karen Macolino (Central Adelaide Local Health Network), Julia New-Tolley (Central Adelaide Local Health Network), Linda Nguyen (Central Adelaide Local Health Network), Margaret Owade (Central Adelaide Local Health Network), Greg Roberts (Southern Adelaide Local Health Network), Shasti Smith (Northern Adelaide Local Health Network), Lucy Sadecki (Central Adelaide Local Health Network), Sepehr Shakib, (Central Adelaide Local Health Network), Valerie Teo (Central Adelaide Local Health Network), Daniella Tocchetti (Central Adelaide Local Health Network), Kylie Tram (Central Adelaide Local Health Network), Winnie Tran (Central Adelaide Local Health Network), Shin Loong Yap (Central Adelaide Local Health Network).
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the Registry of Senior Australians based at the South Australian Health and Medical Research Institute.
Ethical approval/patient consent: Written or oral consent was obtained from all patients or their surrogate decision makers.
ORCID iD: Patrick Russell  https://orcid.org/0000-0002-2534-3371
https://orcid.org/0000-0002-2534-3371
Contributor Information
Patrick Russell, Internal Medicine, Royal Adelaide Hospital, Adelaide, SA 5000, Australia.
Udul Hewage, Internal Medicine, Southern Adelaide Local Health Network, Bedford Park, SA, Australia.
Cameron McDonald, Department of Pharmacy, Central Adelaide Local Health Network, Adelaide, SA, Australia.
Campbell Thompson, General Medicine, Faculty of Health and Medical Sciences, The University of Adelaide, Adelaide, SA, Australia.
Richard Woodman, College of Medicine and Public Health, Flinders University, Adelaide SA, Australia.
Arduino A. Mangoni, Discipline of Clinical Pharmacology, Flinders University and Flinders Medical Centre, Bedford Park, SA, Australia
References
- 1. https://www.gen-agedcaredata.gov.au/www_aihwgen/media/Interfaces-reports/Interfaces-between-the-aged-care-and-health-systems-in-Australia-Hospital.pdf
- 2. Page RL, II, Linnebur SA, Bryant LL, et al. Inappropriate prescribing in the hospitalized elderly patient: defining the problem, evaluation tools, and possible solutions. Clin Interv Aging 2010; 5: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts G, Pegoli M, Grzeskowiak L, et al. Hospital admission as a deprescribing triage point for patients discharged to Residential Aged Care Facilities. Age Ageing 2021; 50: 1600–1606. [DOI] [PubMed] [Google Scholar]
- 4. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175: 827–834. [DOI] [PubMed] [Google Scholar]
- 5. Page AT, Clifford RM, Potter K. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol 2016; 82: 583–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thillainadesan J, Gnjidic D, Green S, et al. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: a systematic review of randomised trials. Drugs Aging 2018; 35: 303–319. [DOI] [PubMed] [Google Scholar]
- 7. Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J 2007; 9: 430–434. [PubMed] [Google Scholar]
- 8. Kutner JS, Blatchford PJ, Taylor DH, Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: a randomized clinical trial. JAMA Intern Med 2015; 175: 691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thillainadesan J, Gnjidic D, Green S, et al. Impact of deprescribing interventions in older hospitalised patients on prescribing and clinical outcomes: a systematic review of randomised trials. Drugs Aging 2018; 35: 303–319. [DOI] [PubMed] [Google Scholar]
- 10. Pruskowski JA, Springer S, Thorpe CT, et al. Does deprescribing improve quality of life? A systematic review of the literature. Drugs Aging 2019; 36: 1097–1110. [DOI] [PubMed] [Google Scholar]
- 11. Ibrahim K, Cox NJ, Stevenson JM, et al. A systematic review of the evidence for deprescribing interventions among older people living with frailty. BMC Geriatr 2021; 21: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bloomfield HE, Greer N, Linsky AM, et al. Deprescribing for community-dwelling older adults: a systematic review and meta-analysis. J Gen Intern Med 2020; 35: 3323–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thompson W, Lundby C, Graabaek T, et al. Tools for deprescribing in frail older persons and those with limited life expectancy: a systematic review. J Am Geriatr Soc 2019; 67: 172–180. [DOI] [PubMed] [Google Scholar]
- 14. Inacio MC, Lang CE, Khadka J, et al. Mortality in the first year of aged care services in Australia. Australas J Ageing 2020; 39: e537–e544. [DOI] [PubMed] [Google Scholar]
- 15. Sommers M, Rose E, Simmonds A, et al. Quality use of medicines in residential aged care. Aust Fam Physician 2010; 39: 413–416. [PubMed] [Google Scholar]
- 16. Morin L, Laroche ML, Texier G, et al. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc 2016; 17: 862.e1-e9. [DOI] [PubMed] [Google Scholar]
- 17. Russell P, Laubscher S, Roberts GW, et al. A pilot cohort study of deprescribing for nursing home patients acutely admitted to hospital. Ther Adv Drug Saf. Epub ahead of print 4 June 2019. DOI: 10.1177/2042098619854876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.www.ANZCTR.org.au
- 19. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 20. Reeve E, Gnjidic D, Long J, et al. A systematic review of the emerging definition of ‘deprescribing’ with network analysis: implications for future research and clinical practice. Br J Clin Pharmacol 2015; 80: 1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Potter K, Flicker L, Page A, et al. Deprescribing in frail older people: a randomised controlled trial. PLoS ONE 2016; 11: e0149984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The American Geriatrics Society 2015. Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. JAGS 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 23. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 24. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 25. Kongsted A, Nielsen AM. Latent class analysis in health research. J Physiother 2017; 63: 55–58. [DOI] [PubMed] [Google Scholar]
- 26. Juul-Larsen HG, Christensen LD, Bandholm T, et al. Patterns of multimorbidity and differences in healthcare utilization and complexity among acutely hospitalized medical patients (⩾65 years) – a latent class approach. Clin Epidemiol 2020; 12: 245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Edwards RF, Harrison TM, Davis SM. Potentially inappropriate prescribing for geriatric inpatients: an acute care of the elderly unit compared to a general medicine service. Consult Pharm 2003; 18: 37–42. [PubMed] [Google Scholar]
- 28. Hanlon JT, Artz MB, Pieper CF, et al. Inappropriate medication use among frail elderly inpatients. Ann Pharmacother 2004; 38: 9–14. [DOI] [PubMed] [Google Scholar]
- 29. Onder G, Landi F, Cesari M, et al. Inappropriate medication use among hospitalized older adults in Italy: results from the Italian Group of Pharmacoepidemiology in the Elderly. Eur J Clin Pharmacol 2003; 59: 157–162. [DOI] [PubMed] [Google Scholar]
- 30. Bangalore S, Messerli FH, Wun CC, et al. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J 2010; 31: 2897–2908. [DOI] [PubMed] [Google Scholar]
- 31. Benetos A, Labat C, Rossignol P, et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: the PARTAGE Study. JAMA Intern Med 2015; 175: 989–995. [DOI] [PubMed] [Google Scholar]
- 32. Scott IA, Hilmer SN, Le Couteur DG. Going beyond the guidelines in individualising the use of antihypertensive drugs in older patients. Drugs Aging 2019; 36: 675–685. [DOI] [PubMed] [Google Scholar]
- 33. Bell JS, Strandberg TE, Teramura-Gronblad M, et al. Use of proton pump inhibitors and mortality among institutionalized older people. Arch Intern Med 2010; 170: 1604–1605. [DOI] [PubMed] [Google Scholar]
- 34. Scarpignato C. Effective and safe proton pump inhibitor therapy in acid-related diseases: a position paper addressing benefits and potential harms of acid suppression. BMC Med 2016; 14: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olsson IN, Runnamo R, Engfeldt P. Medication quality and quality of life in the elderly, a cohort study. Health Qual Life Outcomes 2011; 9: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perez T, Moriarty F, Wallace E, et al. Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. BMJ 2018; 363: k4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elfil M, Negida A. Sampling methods in clinical research; an educational review. Emerg (Tehran) 2017; 5: e52. [PMC free article] [PubMed] [Google Scholar]