Abstract
Barrett’s esophagus is the condition in which a metaplastic columnar epithelium replaces the stratified squamous epithelium that normally lines the distal esophagus. The condition develops as a consequence of chronic gastroesophageal reflux disease and predisposes the patient to the development of esophageal adenocarcinoma. The diagnosis and management of Barrett’s esophagus have undergone dramatic changes over the years and continue to evolve today. Endoscopic eradication therapy has revolutionized the management of dysplastic Barrett’s esophagus and early esophageal adenocarcinoma by significantly reducing the morbidity and mortality associated with the prior gold standard of therapy, esophagectomy. The purpose of this review is to highlight current principles in the management and endoscopic treatment of this disease.
Keywords: Barrett’s esophagus, cryotherapy, dysplasia, early esophageal adenocarcinoma, endoscopic eradication therapy, radiofrequency ablation
Introduction
Barrett’s esophagus (BE) is defined as the replacement of normal squamous epithelium in the tubular esophagus by metaplastic columnar epithelium for at least 1 cm above the gastroesophageal junction (GEJ). 1 BE affects approximately 1–2% of the entire population and is thought to be caused by chronic gastroesophageal reflux (GERD). 1 Patients with BE can develop dysplasia and eventual stepwise progression to esophageal adenocarcinoma (EAC), making early detection, treatment, and surveillance crucial in the management of this condition. Our understanding of each of these components has dramatically changed over the past two decades. The purpose of this article is to review the history of BE and highlight current principles in the management and treatment of this disease.
Diagnostic criteria for BE
The 2017 American College of Gastroenterology (ACG) guidelines for the diagnosis and management of BE outline clear diagnostic criteria for BE based on the risk of progression to dysplasia and EAC. Diagnosis of BE requires the presence of at least 1 cm of metaplastic columnar epithelium above the GEJ in the tubular esophagus. 1 Over the years, several problems with this simple definition have been addressed. Identification of the GEJ itself is unreliable as the location may be affected by respiration, gut motor activity, and the degree of distention of the esophagus and stomach. Nonetheless, based on the majority of published literature on BE, the proximal extent of the gastric folds is used as a surrogate landmark for the GEJ. 2 A threshold of 1 cm of metaplastic epithelium is used because of its clinical significance in that the presence of shorter segments of abnormal mucosa have low rates of prevalent and incident dysplasia and EAC.3,4 While there is some debate regarding the necessity of intestinal metaplasia (IM) on endoscopic biopsy for diagnosis of BE, large studies have shown that patients with columnar metaplasia without IM have a significantly lower risk of EAC than those with IM. 5 In addition, the lack of IM in visually aberrant mucosa may reflect inadequate sampling, given that the yield for IM directly correlates with the number of endoscopic biopsies obtained. 6 As such, American guidelines recommend assigning a diagnosis of BE only in patients with IM on biopsy as this specific group is primarily at increased risk of developing EAC. If BE is present, reporting the extent of metaplastic change using the Prague C&M classification as well as documentation of the location of the diaphragmatic pinch, GEJ, and squamocolumnar junction are recommended. Subsequently, biopsies should be obtained using the Seattle protocol: a systematic four-quadrant biopsy sampling technique, obtaining specimens at intervals of every 2 cm in patients without dysplasia and every 1 cm in patients with prior dysplasia. 7 Not only do employing these standardized sampling and reporting techniques increase inter-observer agreement among endoscopists for accurate diagnosis of BE, 8 they also carry clinical significance. The presence of IM and the risk of developing EAC directly correlate with the extent of the metaplastic change. 8 In addition, in patients with dysplasia, the extent of metaplasia may influence the choice among the therapeutic options. 2
Surveillance recommendations for BE
The primary aim of surveillance in patients with BE is to identify dysplasia and EAC before progression to distant disease occurs. While there is some controversy regarding the true mortality benefit of endoscopic surveillance, especially in patients with non-dysplastic Barrett’s esophagus (NDBE), 9 multiple studies have demonstrated that if EAC is detected as part of endoscopic surveillance instead of based on symptoms, morbidity and mortality is improved due to diagnosis and treatment of earlier stage disease.10–15 Systematic biopsies using the Seattle protocol as described above have been proven to detect more Barrett’s dysplasia than nonsystematic biopsies. 16 Traditionally, surveillance intervals have depended on the grade of dysplasia found in the metaplastic epithelium as follows: 3–5 years for patients without dysplasia, 6–12 months for those with low-grade dysplasia (LGD), and every 3 months for patients with high-grade dysplasia (HGD) that are not undergoing invasive therapy. 2 However, as will be discussed below, the current standard of care is that nearly all patients with confirmed dysplasia undergo endoscopic eradication therapy (EET).
Advances in non-invasive screening for BE
Screening for BE relies on the assumption that early detection of BE and treatment of dysplasia or EAC will reduce the incidence, morbidity, and mortality associated with EAC. However, given the number of patients involved, screening of the general population with endoscopy would result in increased resource use and costs for patients and the healthcare system as well as increased potential harm from endoscopy. 17 As such, screening for BE is currently recommended only in patients with multiple risk factors for adenocarcinoma. These factors include age ⩾50, male sex, chronic GERD (>5-year duration), white race, central obesity, cigarette smoking, and a confirmed history of BE or EAC in a first-degree relative.18,19 Recent progress in minimally invasive and less expensive modalities for BE screening may influence future recommendations for screening. The ACG has noted that unsedated transnasal endoscopy may be used as an alternative to traditional endoscopy for screening in BE 20 with few procedure-related complications 21 as well as 91% sensitivity for detection of IM compared to standard EGD. 22 However, this is still considered to be an invasive procedure, and patient enthusiasm for unsedated endoscopy has been modest. Esophageal capsule endoscopy (ECE) is a non-invasive unsedated imaging technique that aims to visualize the esophagus using a wireless camera contained within a capsule. Although a meta-analysis including 618 patients showed a moderate pooled sensitivity of 77% and specificity of 86% for diagnosis of BE, major limitations of this technique include lack of ability for tissue acquisition, large variability in esophageal transit time, number of frames of GEJ obtained, and interference by secretions or bubbles. ECE is not useful in patients undergoing surveillance for BE because of the need to obtain biopsies. 23 Recently, focus has shifted to the development of biomarkers coupled with non-invasive methods of tissue acquisition for BE screening such as tethered sponges or inflatable balloons (Cytosponge and EsophaCap).24–26 These methods aim to collect esophageal tissue using a sponge that is wrapped in a soluble capsule and attached to a catheter or string. The capsule is swallowed while the endoscopist holds the catheter or string outside the mouth. In the stomach, the outer capsule dissolves allowing the sponge to expand so that cytologic materials attach to the sponge during its exit as it is pulled up through the esophagus and out of the mouth. The collected cells are then analyzed for molecular biomarkers that have been associated with BE and EAC. Studies evaluating these techniques have shown a patient preference for the non-endoscopic procedures, which have also been associated with high completion rates. However, given the small sample sizes and diversity in tested biomarkers, the performance between the different modes of sampling and the specific diagnostic value of individual biomarkers or panels cannot be ascertained at present. 27
Advances in optical technologies for management of BE
High-definition white light endoscopy (HD-WLE) has rapidly replaced standard definition endoscopy as the gold standard in screening for BE due to its use of more than 1 million pixels allowing for detection of subtle mucosal changes and more accurate biopsies of areas concerning for dysplastic BE. 28 Despite being the gold standard, limitations of HD-WLE include prohibitive costs associated with population-based screening to detect BE and high dysplasia miss rates based on operator experience and variable compliance with surveillance biopsy protocols, with some rates as low as 51.2%. 29 As such, there has been increasing interest in developing advanced imaging technologies to enhance the screening, surveillance, and treatment of patients with BE. The American Society for Gastrointestinal Endoscopy (ASGE) has developed criteria for the “preservation and incorporation of valuable endoscopic innovations” (PIVI) for advanced imaging techniques. An imaging technique can replace random four-quadrant biopsies if their per-patient sensitivity is greater than or equal to 90%, specificity is greater than or equal to 80%, and negative predictive value (NPV) is greater than or equal to 98% for detecting HGD or EAC. Several advanced imaging technologies have been measured against these standards. 30 Of these, targeted biopsies with acetic acid chromoendoscopy, narrow band imaging (NBI), and endoscope-based confocal laser endomicroscopy (CLE) meet these thresholds.30,31 Dye-based chromoendoscopy is a method in which topical agents are applied during endoscopy to enhance detection of aberrant mucosal changes thereby increasing the accuracy of targeted biopsies. 32 Acetic acid breaks down the superficial mucus layer by disrupting glycoprotein disulfide bonds, which then allows the acid to cause reversible denaturation of proteins. This results in an acetowhitening reaction that enhances the structural surface pattern. 33 A meta-analysis on acetic acid chromoendoscopy showed high sensitivity 92% and specificity 96% for HGD and EAC. 34 Methylene blue (MB) is selectively absorbed by small intestinal and colonic epithelium but not by squamous mucosa or gastric epithelium, allowing for targeted biopsies of the stained area. 33 Indigo carmine is a contrast dye that pools in the mucosal grooves, allowing better topographic definition of the mucosa. 33 Neither MB or indigo carmine are recommended for routine use currently due to mixed results in efficacy and a theoretical increased risk of carcinogenesis associated with MB.30,31,35 Although inexpensive and widely available, dye-based chromoendoscopy is highly operator-dependent due to the lack of a standardized classification system and paucity of experience among general providers in using this technique. 31 Virtual chromoendoscopy enhances the mucosal surface and vascular pattern through electronic contrast enhancement rather than topical dye application and is most widely used in the form of NBI. NBI uses filtered light favoring short wavelength green and blue light leading to better visualization of the mucosal surface pattern. 35 A meta-analysis of nine studies evaluating surveillance of non-dysplastic BE with NBI showed a pooled sensitivity, NPV, and specificity of greater than 94% each, 30 and a separate study indicated an overall 86% reduction in need for biopsies while detecting all HGD and early adenocarcinoma. 36 The Barrett’s International NBI Group (BING) developed a simple classification system to predict the presence or absence of dysplasia in patients with BE based on the esophageal mucosal and vascular pattern under NBI examination. The BING classification system was found to be able to classify BE with a >90% sensitivity and specificity. 37
Other proprietary virtual chromoendoscopy techniques such as i-Scan (Pentax), flexible spectral imaging color enhancement (FICE, Fujinon), and blue light imaging (BLI, Fujinon) accentuate the mucosa by filtering different wavelengths of light and have been shown to improve the detection of dysplasia in BE. 31 CLE, available in either endoscope-based (eCLE) or more commonly in probe-based (pCLE) form, is a technique that scans light emitted by a highly focused laser beam over a plane of interest allowing for a 1000-fold magnification and generation of high-resolution microscopy images that approximate histologic evaluation. 38 The major potential advantage of using CLE is to eliminate the need for biopsy and tissue processing, thereby facilitating the use of endoscopic therapy concurrently with the CLE-procedure. CLE is used in conjunction with application of a fluorescent contrast agent applied intravenously or topically to enhance visualization of cells. Criteria for distinguishing dysplastic from non-dysplastic cells in BE using this technique, called the Miami criteria, have been shown to have sensitivity and specificity of 88% and 96%, respectively, with high inter-observer agreement (kappa 0.72) for the detection of dysplasia. 39 However, Bajbouj et al. evaluated in vivo detection of dysplasia using pCLE and found a specificity of 95% and sensitivity of 12% with an NPV of 92% and positive predictive value of 18%. The authors concluded that pCLE is non-inferior to standard biopsy in excluding neoplasia but recommend against completely replacing standard biopsy acquisition with endomicroscopy imaging due to its poor sensitivity. 40 While promising for short segment BE, limitations of this technique include long procedure times especially with longer-segment BE and technical expertise that is primarily limited to tertiary care centers. Similar to CLE, endocytoscopy (ECS) is a technique that attempts to obtain a real-time, in vivo, histological diagnosis by capturing highly magnified images of the epithelial surface and analyzing cellular and subcellular features. Although it has been reported to have a specificity over 80%, the technology requires an alternate primary surveillance technique to identify suspicious areas, and there is currently a lack of prospective data evaluating its efficacy.41,42 Optical coherence tomography (OCT) and volumetric laser endomicroscopy (VLE) are new technologies that use infrared light, rather than ultrasound, to produce high-resolution microscopic images without need for contrast. OCT is able to acquire images with 10-fold higher resolution compared with high-frequency ultrasound at a rapid acquisition speed up to 400 frames per second. 43 VLE is a second-generation, advanced OCT that uses near-infrared light and provides up to 1200 cross-sectional images over a 6-cm VLE scan allowing larger BE segments to be evaluated in a shorter time. 44 At the present time, there are no commercially available OCT devices. In addition, overall data are limited to support the routine use of VLE. 43
EET and patient selection
Advances in endoscopic treatment modalities have revolutionized the treatment of dysplastic BE and early EAC by effectively preventing progression to invasive cancer while avoiding the morbidity and mortality associated with the prior standard of care, esophagectomy.1,45 EET is defined as the combination of resection and ablative techniques used to completely eradicate all BE-associated dysplasia and intestinal metaplasia. Societal guidelines recommend performing EET for BE with a confirmed diagnosis of HGD or early (T1a) EAC.1,2,46 EET is not routinely recommended for patients with NDBE because of their low risk of progression to EAC. The role of EET in patients with BE with low-grade dysplasia (BE-LGD) is evolving. The diagnosis of BE-LGD has a high inter-observer variability; therefore, the diagnosis should be confirmed by two pathologists, at least one of whom should have expertise in BE. The overall annual rate of progression of all patients from LGD to EAC has been reported to be as low as 0.5%, suggesting that surveillance without EET may be a reasonable management strategy for BE-LGD.46,47 However, several studies have shown that when diagnosis of LGD is confirmed by more than one pathologist or persistence of LGD is seen on consecutive endoscopies (vs down-staging of likely inflammatory changes to NDBE on consecutive endoscopies), the annual rate of progression is much higher, approximately 7–13%.48–50 When comparing surveillance versus EET, a meta-analysis by Qumseya et al. 51 showed that treatment of BE-LGD with radiofrequency ablation (RFA) significantly reduced the risk of disease progression compared to surveillance alone (RR: 0.14%; 95% confidence interval (CI): 0.04–0.45; p = 0.001). The Surveillance versus Radiofrequency Ablation (SURF) trial showed similar results in reducing the risk of disease progression with EET in patients with confirmed BE-LGD. 52 Given the availability of extensive high-quality evidence, patients with BE-LGD that is confirmed by two expert pathologists, especially if persistent on consecutive endoscopies, should undergo EET. As noted above, EET is now a universally accepted management strategy for T1a cancers with data showing a greater than 91% 5-year survival rate.53,54 The role of EET in T1b adenocarcinoma is controversial, but it may be appropriate in select patients with a low risk of nodal involvement. Histologic characteristics of poor differentiation, increased depth of submucosal invasion, and lymphovascular invasion (LVI) have all been associated with an increased risk of lymph node metastases, with the likelihood of lymphatic spread reaching 50% when all three factors were present.55–57 While there is need for further long-term prospective data evaluating the role of EET in T1b adenocarcinoma, it may be considered an alternative to esophagectomy in patients who are poor surgical candidates with well-differentiated, superficial tumors (sm1) without LVI.
As noted earlier, EET aims to eliminate Barrett’s epithelium either by resection or ablation of the aberrant tissue with the goal of achieving complete eradication of all intestinal metaplasia (CE-IM). 58 Patients who only achieve eradication of dysplasia (CE-D) but have persistent IM carry an increased risk of dysplasia recurrence and progression to HGD or EAC and thus establishing CE-IM is the goal-standard for EET. 59 Over the past two decades, several methods of endoscopic therapy have been evaluated for the treatment of BE (Table 1). The first treatments in the early 2000s consisted of focal ablation techniques in the form of argon plasma coagulation (APC) and multipolar electrocoagulation (MPEC). APC uses ionized argon gas, applied via a probe passed through the endoscope, to convey electrical energy to the tissue, resulting in thermal destruction. While there is a great deal of heterogeneity regarding the efficacy and durability in achieving CE-D with APC, overall it has been shown to have high initial eradication rates >95%.60–63 Unfortunately, BE treated with APC has been shown to result in a high rate of recurrent metaplasia and dysplasia in up to 35% of patients at long-term follow-up, and a risk of progression to EAC similar to patients who do not undergo ablative therapy at all (3% annual risk of progression), which may be due to buried glands under the neo-squamous epithelium that persist even after achieving complete eradication.60,64,65 MPEC is another ablative therapy which requires direct contact between an endoscopic heater probe and the tissue being treated. Electricity passes between alternating arrays of positive and the negative electrodes located at the tip of the probe resulting in thermal destruction of the tissue, similar to APC. While there are no significant differences in achieving CE-IM or CE-D or differences in adverse effects (esophageal stricture, perforation, or gastrointestinal bleeding) with either method, APC offers the advantage of non-contact coagulation and therefore has been more widely used.66,67 The major limitation of “point and shoot” technologies, such as APC and MPEC, is that they do not guarantee that energy is distributed evenly over the entire Barrett’s segment. Depending on the endoscopist’s technique and experience, energy delivery tends to be highest at the site of treatment initiation but decreases as the probe is moved further along the BE segment. Repeated treatment of the initial site might induce deep tissue injury (which increases the risk of stricturing) or transmural necrosis/perforation while undertreatment may result in incomplete eradication and the development of buried intestinal metaplasia. 68 Of note, the rate of stricture formation with these modalities is not insignificant, between 4% and 9%, and increases the overall number of endoscopic procedures.69–72 As such, experts typically limit the use of these modalities to patients with a small burden of BE, typically in the form of widely scattered islands. 68
Table 1.
Comparison of endoscopic therapies for treatment of BE.
| Treatment | Pros | Cons |
|---|---|---|
| Photodynamic therapy | • Reasonably good efficacy for achieving CE-D
(77–100%) • Low risk of perforation |
• High photosensitivity, often limiting quality of
life • High rate of stricture formation requiring multiple dilations • High cost |
| Radiofrequency ablation | • Excellent efficacy for achieving CE-IM (88–91%) and CE-D (up
to 99%) • Very low risk of bleeding and perforation; low risk of stricture • Abundance of literature supporting use • Readily available |
• Limited depth of penetration • Post-procedure discomfort |
| Endoscopic mucosal resection | • Excellent efficacy for achieving CE-IM (~85%) and CE-D
(~96%) • Relatively low risk of perforation (although higher than ablative methods) • Ability to obtain specimen for histologic diagnosis |
• Higher recurrences rates of IM than with combination
therapy • Risk of metachronous lesions with focal EMR • High risk of bleeding and stricture formation depending on size of treatment area • Chest pain |
| Endoscopic submucosal dissection | • Excellent efficacy for achieving CE-neoplasia
(~98%) • En bloc resection with well-defined margins • Possible role in EAC with submucosal invasion |
• High risk of bleeding and stricture formation depending on
size of treatment area • Relatively higher risk of perforation • No clear oncologic/recurrence benefit over piecemeal EMR • Chest pain |
| Cryospray (liquid nitrogen) | • Good efficacy for achieving CE-D (81–97%) • Low post-procedure discomfort • Low risk of bleeding, perforation, and stricture formation |
• Paucity of data for first-line use and lower CE-IM
rates • Needs intraprocedural decompression tube |
| Cryoballoon (nitrous oxide) | • Excellent efficacy for achieving CE-IM (~84%) and CE-D
(~95%) • Low post-procedure discomfort • Low risk of bleeding and perforation • No console/hardware needed |
• Moderate rate of stricture formation • New technology; limited prospective data on use |
| Endoscopic resection + ablation | • Supported by large and growing body of
evidence • Relatively low risk of bleeding and perforation • High efficacy for CE-D ~92% |
• Recurrence rate ~13% • Moderate to high risk of stricture formation (mostly dependent on extent of EMR) |
BE, Barrett’s esophagus; CE-D, complete eradiation of dysplasia; CE-IM, complete eradication of intestinal metaplasia; EAC, esophageal adenocarcinoma; EMR, endoscopic mucosal resection.
Along with the thermal ablative techniques described above, photodynamic therapy (PDT) was one of the earliest non-thermal ablation methods proposed for the treatment of BE. PDT relies on the principle that metaplastic and neoplastic cells have a greater affinity for uptake of photosensitizing compounds than normal squamous epithelium, and when these compounds are activated by light, they generate superoxide free radicals that cause selective apoptosis of those cells. In the United States, intravenously administered porfimer sodium is used as the photosensitizer, whereas an orally administered 5-aminolevulinic acid is predominantly used in Europe. 73 About 48 hours after photosensitizer administration, upper endoscopy is performed, and red light (wavelength of 630 nm) is transmitted through the endoscope, which activates porfimer sodium, thereby causing localized destruction of the targeted cells. 73 In patients with BE and HGD, CE-D is achieved at a rate of 77–100%73–75 but remains limited by its high procedural and drug costs, photosensitivity (69%) and a greater than 35% chance of developing a post-treatment esophageal stricture.73,75 In one study, the majority of these strictures required over six dilations to achieve resolution. 75
In the early to mid-2000s, around the same time as the aforementioned ablative methods were being studied, cryotherapy emerged as another non-thermal ablative technique. It causes rapid freezing and slow thawing of aberrant tissue in multiple cycles leading to cellular injury, ischemia, and eventually apoptosis. 73 The modalities of cryotherapy differ with respect to the gas used, temperatures achieved, delivery methods, and dosimetry. The older technique is liquid nitrogen-based spray cryotherapy delivered by a non-contact method at −196°C through the endoscope. The site is frozen for 20–30 seconds followed by cooling for at least 45–60 seconds, repeated over several (usually three) freeze-thaw cycles. 73 A decompression tube is required to vent the esophagus and the stomach to reduce the risk of perforation due to the rapid expansion of nitrogen gas. 76 Today, due to the wide acceptance of RFA as the first-line ablative therapy for treatment of BE due to its large body of evidence regarding efficacy and safety, cryotherapy has been primarily used as salvage therapy for patients refractory to RFA. 77 However, due to its low rate of post-procedure discomfort, it is increasingly being evaluated as a potential first-line therapy for treatment of BE. A meta-analysis by Tariq et al. 77 evaluating cryotherapy as a primary treatment for BE showed a pooled rate of CE-D of 84% and CE-IM of 64% using spray cryotherapy. Similar results were shown by Hamade et al. 76 with the pooled rate of CE-D of 90.6% and CE-IM of 69.35%.
Since its introduction as a treatment for BE in the mid-2000s, robust, long-term evidence have emerged to support RFA as a safe and effective first-line treatment for flat dysplastic BE. 78 RFA uses radiofrequency energy to destroy aberrant esophageal tissue, which is in turn replaced with neo-squamous epithelium. Historically, circumferential RFA required a two-step procedure to first determine esophageal size with a sizing catheter, followed by use of an ablation catheter with an appropriate diameter for ablation therapy. Technologic advancements over the years have now resulted in a bipolar electrode wrapped around an inflatable self-sizing balloon that automatically inflates to 3 psi, a pressure that corresponds to an appropriate diameter based on the patient’s esophageal anatomy. 79 This has eliminated the need for a sizing process, thereby decreasing procedure time by 20%.58,79 Radiofrequency energy is delivered through the electrodes at a preset energy density of 10 J/cm2, which results in circumferential mucosal ablation. 79 Focal ablation (usually at 12 J/cm2) is used for more limited areas, either as an initial treatment or during follow-up after circumferential RFA, can be performed using a multitude of over-the-scope or through the scope catheters, depending on the length and distribution of the abnormal mucosa. 79 The ablation of intestinal metaplasia (AIM) dysplasia trial, a landmark, randomized, multi-center controlled trial to evaluate RFA as a treatment for BE, showed that in patients with LGD or HGD, RFA significantly reduced the progression to EAC compared with sham treatment in patients with HGD (2% vs 19%, respectively). In patients with LGD, the risk of progression to HGD was reduced from 13.6% to 4.8% following RFA treatment. 69 A follow-up durability analysis from this cohort of patients showed that recurrence of BE after CE-IM occurred in up to one-third of patients; however, after allowing for intervening treatment of recurrences, 99% achieved CE-D and 90% achieved CE-IM at 5 years of surveillance. 80 While RFA is not without procedure-related risks, the adverse event rate is modest at 8.8% with the most common side effects being stricture formation at 5.6% (typically occurring 3 weeks after treatment), bleeding in 1%, and a very low rate of perforation at 0.6% (not-related to thermal injury). 81
Early studies with RFA showed that some patients readily achieve CE-IM, while others require numerous repeat ablations or fail to achieve complete eradication of BE with RFA. A meta-analysis aimed at determining the durability of RFA showed a pooled IM recurrence rate of 13% after eradication with RFA. 82 Simialrly, Fujii-Lau et al. 83 showed a pooled incidence of IM recurrence rate of 5.8 per 100 patient years and reinforced the need for ongoing surveillance after achieving CE-IM. It is now understood that ongoing reflux exposure despite twice-daily proton pump inhibitor (PPI) therapy is associated with persistent IM after RFA.84–86 In a large prospective study that aimed to assess outcomes and durability of RFA for BE, it was found that patients treated with a structured reflux management protocol had a significantly lower recurrence of CE-IM after RFA (4.8%) compared with patients without optimized reflux management (10.9%). 87
Around the same time that RFA was becoming widely studied and accepted for the treatment of BE in the mid 2000s, stepwise radical endoscopic resection (SRER) was also emerging as a potential treatment modality for dysplastic BE and early neoplasia. The most significant advantage of endoscopic resection (ER) over ablation techniques is the ability to obtain a specimen for histologic evaluation, which may dictate further management. ER, usually by endoscopic mucosal resection (EMR), is a well-validated method for successful eradication of visible or raised lesions associated with dysplastic BE or early EAC with a 5-year survival rate up to 95% (See Figure 1 for results of EMR). 88 Two EMR techniques are employed in current practice. The first is the cap-assisted mucosectomy. In this method, submucosal injection of saline is used to lift the lesion away from the deeper wall layers followed by application of a snare fitted around the rim of a specialized transparent cap. The lesion is suctioned into the cap, the snare is tightened, and the subsequent pseudopolyp is resected using electrocautery. 88 The second and more commonly used method is multiband ligation in which a cap with several bands is used to suction the lesion/tissue into the cap after which the band is deployed to create a pseudopolyp that isolates the lesion from the surrounding tissue of the esophageal wall. A snare is placed below the band, and the targeted lesion is resected. 88 Multiband ligation has been shown to be more cost-effective with fewer complications than cap-assisted mucosectomy. 88 While EMR is efficacious for achieving eradication of BE and EAC (CE-IM: 85%, CE-neoplasia: 95%),89,90 long-term data on durability has shown high recurrences rates of IM between 15.7–39.5% and neoplasia between 5.8% and 6.2% with EMR alone.58,90–92 In addition, focal EMR can lead to the development of metachronous lesions in the residual BE segment during follow-up in over 30% of patients.93–96 SRER is a technique in which the entire BE segment is removed in consecutive ER sessions. In one of the largest American studies evaluating this technique, Chennat et al. 93 showed that CE-IM was achieved in 96.9% of patients with remission maintained for a mean of 22.9 months during endoscopic surveillance. Similarly, a multi-center randomized control trial in Europe comparing SRER and RFA showed that eradication of neoplasia was achieved in 100% of patients and CE-IM in 92% with remission maintained over a median of 25 months (1 out of 25 patients developed recurrent EAC). 96 Despite the high efficacy, the major limitation of SRER is a significant rate of stricture formation at 37% in the American study and up to 88% in the European study.93,96 Although most were successfully treated by esophageal dilation, 5 of 22 SRER stenoses in the European study were resistant to treatment, requiring >5 dilations and combination treatment. Subsequently, treatment of SRER-induced stenoses doubled the total number of endoscopic procedures in the SRER group compared with the RFA group. 96
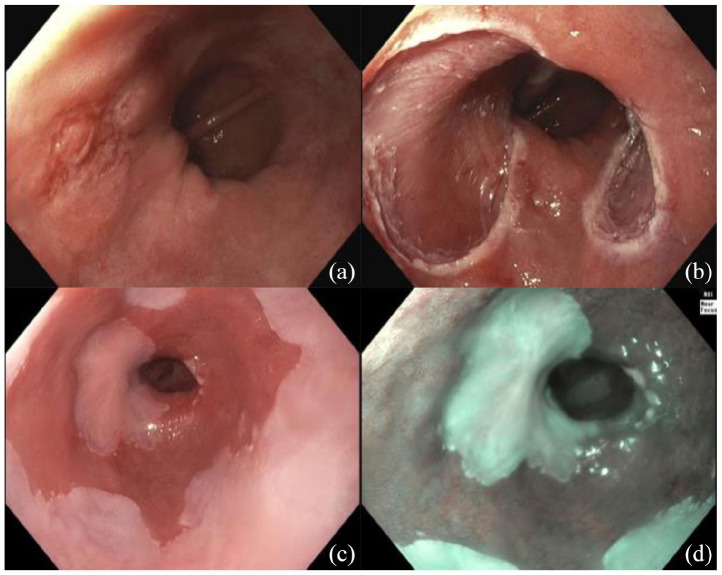
Figure 1.
(a) Focus of EAC in BE before EMR. (b) immediately after EMR. (c) At two-month follow-up EGD in HD-WLE. (d) NBI.
BE, Barrett’s esophagus; EAC, esophageal adenocarcinoma; EGD, esophagogastroduodenoscopy; EMR, endoscopic mucosal resection; HD-WLE, high-definition white; NBI, narrow band imaging.
Taking these data into account, a combination of ER of all visible neoplastic lesions followed by ablation (typically with RFA) of the remaining Barrett’s epithelium emerged as the ideal treatment strategy, combining the positive effect of tissue acquisition/resection with a low complication rate (especially for strictures) associated with ablation. A study by Phoa et al. showed that multimodal therapy, together referred to as EET, achieves CE-D in 92% of patients and CE-IM in 87% of patients with 4% recurrence of neoplasia and 8% recurrence of metaplasia at 27 months.58,97 EMR of all visible lesions followed by RFA of residual flat lesions is the current gold standard approach for the management of dysplastic BE and early EAC (see Figure 2 for results of EET).
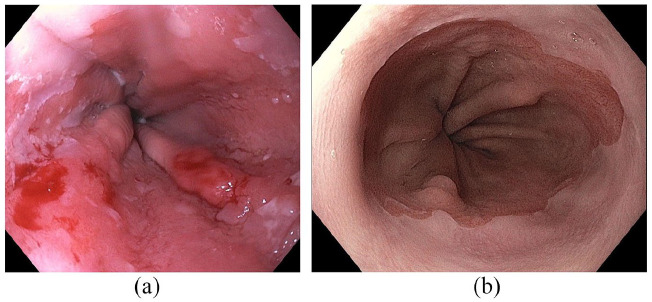
Figure 2.
(a) Segment of BE with T1a cancer prior to EET. (b) The same patient after EMR + RFA without evidence of residual disease on HD-WLE.
BE, Barrett’s esophagus; EET, endoscopic eradication therapy; EMR, endoscopic mucosal resection; HD-WLE, high-definition white light endoscopy; RFA, radiofrequency ablation.
New EET techniques
EMR is limited in the size of specimen it can remove in one piece, preventing en bloc resection of lesions that are more than 2 cm. Piecemeal resection can be performed of larger lesions, but is associated with a higher incidence of recurrence compared with en bloc resection and may impair histological diagnosis due to risk of missing neoplastic areas and impairs staging when a malignancy is present. In contrast to EMR, endoscopic submucosal dissection (ESD) allows for en bloc resection of >2 cm lesions and has been shown to be superior to EMR for curative resection in other gastrointestinal (GI) tumors such as gastric cancer. 98 In ESD, cautery is used to mark the area of resection, approximately 2–3 mm outside the margins of the lesion. Injection of a glycerin or hyaluronic acid solution is used to create submucosal lift so that the mucosa surrounding the lesion can be safely incised, and the underlying submucosal layer can be subsequently dissected until the lesion is completely removed. 99 A randomized trial that compared EMR and ESD for early EAC showed that while R0 resection (defined as margins free of neoplasia) was achieved more frequently with ESD than EMR (58.8% vs 11.7%, respectively), there was no difference in complete remission from neoplasia at 3 months. 98 While there were no significant difference in rates of overall adverse events between the two groups (including temporary chest pain and intraprocedural bleeding), 98 severe adverse events including esophageal perforation were only seen in the ESD group, and procedural time was significantly higher in ESD compared to EMR. 98 The average rate of stricture formation with ESD is less than 12%, (comparable to EMR); however, the risk significantly increases with defect size. Post-ESD strictures occur in 90% of patients with defects involving more than 75% of the esophageal circumference and 100% in those undergoing circumferential ESD. Other risk factors associated with stricture formation include tumor invasion into the muscularis mucosa (m3) or into the submucosa to a depth of less than 1/3 (sm1).100–102 ESD may have a greater role in cases where there is concern for submucosal involvement of EAC where histological details of the resected tissue such as the maximum depth of invasion will greatly influence the decision between pursuing ongoing endoscopic management or recommending surgical resection. 98
As discussed above, endoscopic spray cryotherapy has been shown to achieve CE-D at a rate of 84–90% and CE-IM rate of 64–69%.76,77 However, due to the overwhelming data in support of RFA, its use has been largely limited to patients with BE refractory to RFA. Due to its low rate of post-procedure discomfort, cryotherapy is increasingly being re-evaluated as a first-line therapy for treatment of BE. The cryoballoon, a newer technique that has been introduced in the last decade, uses a balloon catheter that is passed through the endoscope and attached to a handle that contains a cartridge with liquid nitrous oxide. The balloon is inflated using an external trigger and liquid nitrogen is delivered to the ablation site for 10 seconds, cooling the tissue to −85°C. 73 Cryoballoon has shown high rates of CE-D and CE-IM at 95% and 88%, respectively. Notably, CE-D rate was significantly lower at 67% in those with ultra-long BE compared with those with <8 cm. 103 A recent meta-analysis showed that the safety profile of cryoballoon is also relatively favorable with a post-ablation stricture formation of 5.8% (comparable to RFA), mucosal laceration at 0.7%, perforation at 0.4%, and gastrointestinal bleeding at 0.4%. 104
As previously discussed, APC was shown to have high initial eradication rates >95%60–63 but a significant stricture formation rate in up to 4–9% of patients. Studies have shown that the combination of APC with prior submucosal injection, or hybrid-APC, can lower the depth of tissue damage,105,106 possibly by up to 50% in comparison with standard APC. 107 In a recent study evaluating hybrid-APC, Manner et al. 71 showed a macroscopic ablation success of 96% and a very low rate of stricture formation at 2%, making this technique a promising new treatment approach.
Algorithmic approach to EET and best practices
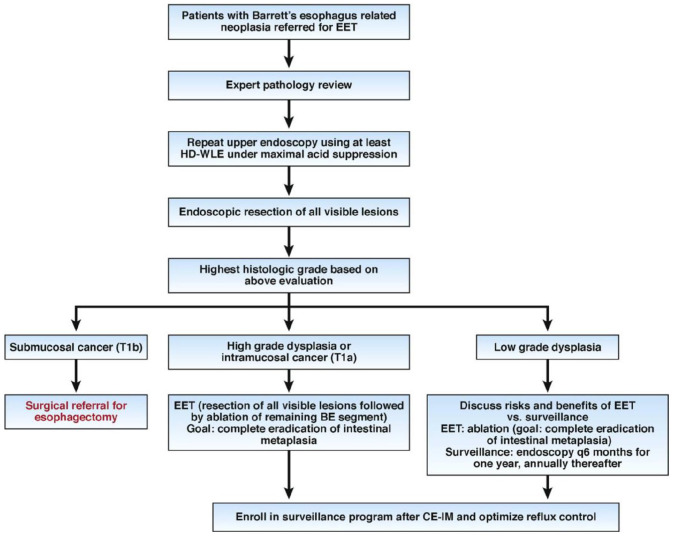
Given the vast amount of data and rapidly developing technologic advancements regarding EET of BE, an algorithmic approach for performing EET in BE patients is critical and a recommended approach is outlined in Figure 3. 108 Furthermore, quality indicators in EET for the management of BE-related neoplasia have been developed and endorsed by the ASGE and the ACG (Table 2).45,108 Adherence to evidence-based algorithms and these quality indicators will hopefully improve the quality of care in the management of BE-related neoplasia.
Figure 3.
Decision tool with an algorithmic approach to management of BE patients referred for EET.
Source: Adapted and reprinted with permission from Komanduri et al. 87
BE, Barrett’s esophagus; CE-IM, complete eradication of intestinal metaplasia; EET, endoscopic eradication therapy; HD-WLE, high-definition white light endoscopy.
Table 2.
Quality indicators for EET.
| Quality indicator | Performance target | |
|---|---|---|
| Pre-procedure | The rate at which the reading is made by a GI pathologist or confirmed by a second pathologist before EET is begun for patients in whom a diagnosis of dysplasia has been made | 90% |
| Centers in which EET is performed showed have available HD-WLE and expertise in mucosal ablation and EMR techniques | N/a | |
| The rate at which documentation of a discussion of the risks, benefits, and alternatives to EET is obtained from the patient prior to treatment | >98% | |
| Intra-procedure | The rate at which landmarks and length of BE is documented (e.g., Prague grading system) in patients with BE before EET | 90% |
| The rate at which the presence or absence of visible lesions is reported in patients with BE referred for EET | 90% | |
| The rate at which the BE segment is inspected using HD-WLE | 95% | |
| The rate at which complete endoscopic resection (en bloc or piece-meal is performed in patients with BE with visible lesions | 90% | |
| The rate at which a defined interval for subsequent EET is documented for patients undergoing EET who have not yet achieved complete eradication of intestinal metaplasia | 90% | |
| The rate at which complete eradication of dysplasia is achieved by 18 months in patient with BE-related dysplasia or intramucosal cancer referred for EET | 80% | |
| The rate at which complete eradication of intestinal metaplasia is achieved by 18 months in patient with BE-related dysplasia or intramucosal cancer referred for EET | 70% | |
| Post-procedure | The rate at which a recommendation is documented for endoscopic surveillance at a defined interval for patients who achieve complete eradication of intestinal metaplasia | 90% |
| The rate at which biopsies of any visible mucosal abnormalities are performed during endoscopic surveillance after EET | 95% | |
| The rate at which an anti-reflux regimen is recommended after EET | 90% | |
| The rate at which adverse events are being tracked and documented in individuals after EET | 90% |
Source: Adapted from Wani et al. 45
BE, Barrett’s esophagus; EET, endoscopic eradication therapy; EMR, endoscopic mucosal resection; HD-WLE, high-definition white light endoscopy.
Conclusion
Since its initial identification in the early 1900s, the diagnosis and management of BE have undergone dramatic changes and continue to evolve today. The increasing incidence of EAC and high rates of EAC-related mortality necessitate safe, effective, and cost-effective screening, diagnostic and treatment modalities for BE, the precursor lesion to EAC. Currently, BE is defined as at least 1 cm of metaplastic columnar epithelium that replaces the normal stratified squamous epithelial lining of the distal tubular esophagus above the GEJ. 1 Societal guidelines recommend endoscopic surveillance of NDBE using Seattle protocol, 4-quadrant biopsies every 2 cm in patients without dysplasia. 7 Currently, endoscopic screening for BE is recommended only in patients with multiple risk factors for BE due to high costs and procedure-related risks associated with screening the general population. However, advances in less invasive and expensive modalities, such as non-invasive esophageal cell collection devices in conjunction with molecular biomarkers may change the approach to screening in BE.24–26 Similarly, advances in imaging technology have the potential to enhance our ability to detect dysplastic BE. PIVI criteria established by the ASGE currently support the use of acetic acid chromoendoscopy, NBI, and endoscope-based CLE as modalities that may replace four-quadrant random biopsies for detecting HGD and EAC.30,31 EET has revolutionized the management of dysplastic BE and EAC by dramatically reducing the morbidity and mortality associated with the prior gold standard of therapy, esophagectomy. Currently, EET is generally recommended for all BE patients with confirmed dysplasia and early esophageal (up to T1a) cancer. There is a large body of evidence supporting the use of EET via ER of visible lesions followed by ablative therapies for residual flat lesions. While most of the available literature has evaluated EMR and RFA as first-line modalities of treatment, new technologies with better side effect profiles, increased efficacy or utility in refractory cases are being developed and studied as novel EET techniques for BE.
Footnotes
Author contributions: A.C. performed literature review and drafted the manuscript; R.M. performed critical revisions of the manuscript. All authors read and approved the final manuscipt.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VRM: consultant and research support for Boston Scientific and Medtronic; consultant for Medivators and Interpace Diagnostics; stockholder in Capsovision; Honoraria member in Torax Medical/Ethicon. AC reports no relevant disclosures.
ORCID iD: V. Raman Muthusamy  https://orcid.org/0000-0001-5703-5309
https://orcid.org/0000-0001-5703-5309
Contributor Information
Ashwinee Condon, Vatche & Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, Los Angeles, CA, USA.
V. Raman Muthusamy, Vatche & Tamar Manoukian Division of Digestive Diseases, David Geffen School of Medicine, UCLA, 200 UCLA Medical Plaza, Room 330-37, Los Angeles, CA 90095, USA.
References
- 1. Shaheen NJ, Falk GW, Iyer PG, et al. ACG clinical guideline: diagnosis and management of Barrett’s esophagus. Am J Gastroenterol 2016; 111: 30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology 2011; 140: e18–e52; quiz e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol 2011; 106: 1447–1455; quiz 1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wani S, Williams JL, Falk GW, et al. An analysis of the GIQuIC Nationwide Quality Registry reveals unnecessary surveillance endoscopies in patients with normal and irregular Z-lines. Am J Gastroenterol 2020; 115: 1869–1878. [DOI] [PubMed] [Google Scholar]
- 5. Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst 2011; 103: 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrison R, Perry I, Haddadin W, et al. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol 2007; 102: 1154–1161. [DOI] [PubMed] [Google Scholar]
- 7. Gross SA, Kingsbery J, Jang J, et al. Evaluation of dysplasia in Barrett esophagus. Gastroenterol Hepatol 2018; 14: 233–239. [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology 2006; 131: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 9. Rubenstein JH, Sonnenberg A, Davis J, et al. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc 2008; 68: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Streitz JM, Jr, Andrews CW, Jr, Ellis FH., Jr. Endoscopic surveillance of Barrett’s esophagus. Does it help? J Thorac Cardiovasc Surg 1993; 105: 383–387; discussion 387–388. [PubMed] [Google Scholar]
- 11. Peters JH, Clark GW, Ireland AP, et al. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg 1994; 108: 813–821; discussion 821–822. [PubMed] [Google Scholar]
- 12. Fountoulakis A, Zafirellis KD, Dolan K, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg 2004; 91: 997–1003. [DOI] [PubMed] [Google Scholar]
- 13. Cooper GS, Yuan Z, Chak A, et al. Association of prediagnosis endoscopy with stage and survival in adenocarcinoma of the esophagus and gastric cardia. Cancer 2002; 95: 32–38. [DOI] [PubMed] [Google Scholar]
- 14. Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol 2009; 104: 1356–1362. [DOI] [PubMed] [Google Scholar]
- 15. Kearney DJ, Crump C, Maynard C, et al. A case-control study of endoscopy and mortality from adenocarcinoma of the esophagus or gastric cardia in persons with GERD. Gastrointest Endosc 2003; 57: 823–829. [DOI] [PubMed] [Google Scholar]
- 16. Levine DS, Blount PL, Rudolph RE, et al. Safety of a systematic endoscopic biopsy protocol in patients with Barrett’s esophagus. Am J Gastroenterol 2000; 95: 1152–1157. [DOI] [PubMed] [Google Scholar]
- 17. ASGE Standards of Practice Committee, Qumseya B, Sultan S, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc 2019; 90: 335–359.e2. [DOI] [PubMed] [Google Scholar]
- 18. Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011; 140: 1084–1091. [DOI] [PubMed] [Google Scholar]
- 19. Rubenstein JH, Scheiman JM, Sadeghi S, et al. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: synthesis and estimates from population studies. Am J Gastroenterol 2011; 106: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maitra I, Date RS, Martin FL. Towards screening Barrett’s oesophagus: current guidelines, imaging modalities and future developments. Clin J Gastroenterol 2020; 13: 635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shariff MK, Bird-Lieberman EL, O’Donovan M, et al. Randomized crossover study comparing efficacy of transnasal endoscopy with that of standard endoscopy to detect Barrett’s esophagus. Gastrointest Endosc 2012; 75: 954–961. [DOI] [PubMed] [Google Scholar]
- 22. Jobe BA, Hunter JG, Chang EY, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol 2006; 101: 2693–2703. [DOI] [PubMed] [Google Scholar]
- 23. Bhardwaj A, Hollenbeak CS, Pooran N, et al. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol 2009; 104: 1533–1539. [DOI] [PubMed] [Google Scholar]
- 24. Moinova HR, LaFramboise T, Lutterbaugh JD, et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci Transl Med 2018; 10: eaao5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multi-center case-control study. PLoS Med 2015; 12: e1001780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Z, Kambhampati S, Cheng Y, et al. Methylation biomarker panel performance in EsophaCap cytology samples for diagnosing Barrett’s esophagus: a prospective validation study. Clin Cancer Res 2019; 25: 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah AK, Joshi V, Hartel G, et al. To BE or not to BE: non-invasive screening for Barrett’s esophagus, dysplasia and adenocarcinoma. Transl Gastroenterol Hepatol 2019; 4: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Steele D, Baig KKK, Peter S. Evolving screening and surveillance techniques for Barrett’s esophagus. World J Gastroenterol 2019; 25: 2045–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Beg S, Mensa M, Fullard M, et al. Impact of advanced endoscopic imaging on Barrett’s esophagus in daily clinical practice. Gastrointest Endosc 2018; 87: 1189–1194. [DOI] [PubMed] [Google Scholar]
- 30. ASGE Technology Committee, Thosani N, Abu Dayyeh BK, et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE Preservation and Incorporation of Valuable Endoscopic Innovations thresholds for adopting real-time imaging-assisted endoscopic targeted biopsy during endoscopic surveillance of Barrett’s esophagus. Gastrointest Endosc 2016; 83: 684–698.e7. [DOI] [PubMed] [Google Scholar]
- 31. Sanghi V, Thota PN. Barrett’s esophagus: novel strategies for screening and surveillance. Ther Adv Chronic Dis 2019; 10: 2040622319837851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maes S, Sharma P, Bisschops R. Review: surveillance of patients with Barrett oesophagus. Best Pract Res Clin Gastroenterol 2016; 30: 901–912. [DOI] [PubMed] [Google Scholar]
- 33. Trivedi PJ, Braden B. Indications, stains and techniques in chromoendoscopy. QJM 2013; 106: 117–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coletta M, Sami SS, Nachiappan A, et al. Acetic acid chromoendoscopy for the diagnosis of early neoplasia and specialized intestinal metaplasia in Barrett’s esophagus: a meta-analysis. Gastrointest Endosc 2016; 83: 57–67.e1. [DOI] [PubMed] [Google Scholar]
- 35. Gill RS, Singh R. Endoscopic imaging in Barrett’s esophagus: current practice and future applications. Ann Gastroenterol 2012; 25: 89–95. [PMC free article] [PubMed] [Google Scholar]
- 36. Singh R, Shahzad MA, Tam W, et al. Preliminary feasibility study using a novel narrow-band imaging system with dual focus magnification capability in Barrett’s esophagus: is the time ripe to abandon random biopsies? Dig Endosc 2013; 25(Suppl. 2): 151–156. [DOI] [PubMed] [Google Scholar]
- 37. Sharma P, Bergman JJ, Goda K, et al. Development and validation of a classification system to identify high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus using narrow-band imaging. Gastroenterology 2016; 150: 591–598. [DOI] [PubMed] [Google Scholar]
- 38. Leggett CL, Gorospe EC. Application of confocal laser endomicroscopy in the diagnosis and management of Barrett’s esophagus. Ann Gastroenterol 2014; 27: 193–199. [PMC free article] [PubMed] [Google Scholar]
- 39. Wallace M, Lauwers GY, Chen Y, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy 2011; 43: 882–891. [DOI] [PubMed] [Google Scholar]
- 40. Bajbouj M, Vieth M, Rösch T, et al. Probe-based confocal laser endomicroscopy compared with standard four-quadrant biopsy for evaluation of neoplasia in Barrett’s esophagus. Endoscopy 2010; 42: 435–440. [DOI] [PubMed] [Google Scholar]
- 41. Thekkek N, Anandasabapathy S, Richards-Kortum R. Optical molecular imaging for detection of Barrett’s-associated neoplasia. World J Gastroenterol 2011; 17: 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pohl H, Koch M, Khalifa A, et al. Evaluation of endocytoscopy in the surveillance of patients with Barrett’s esophagus. Endoscopy 2007; 39: 492–496. [DOI] [PubMed] [Google Scholar]
- 43. Rodriguez MAC, de Moura DTH, Ribeiro IB, et al. Volumetric laser endomicroscopy and optical coherence tomography in Barrett’s esophagus: a systematic review and meta-analysis. Endosc Int Open 2019; 7: E1078–E1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wolfsen HC. Volumetric laser endomicroscopy in patients with Barrett esophagus. Gastroenterol Hepatol 2016; 12: 719–722. [PMC free article] [PubMed] [Google Scholar]
- 45. Wani S, Muthusamy VR, Shaheen NJ, et al. Development of quality indicators for endoscopic eradication therapies in Barrett’s esophagus: the TREAT-BE (Treatment with Resection and Endoscopic Ablation Techniques for Barrett’s Esophagus) Consortium. Gastrointest Endosc 2017; 86: 1–17.e3. [DOI] [PubMed] [Google Scholar]
- 46. Sharma P, Shaheen NJ, Katzka D, et al. AGA clinical practice update on endoscopic treatment of Barrett’s esophagus with dysplasia and/or early cancer: expert review. Gastroenterology 2020; 158: 760–769. [DOI] [PubMed] [Google Scholar]
- 47. Singh S, Manickam P, Amin AV, et al. Incidence of esophageal adenocarcinoma in Barrett’s esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc 2014; 79: 897–909.e4; quiz 983.e1, 983.e3. [DOI] [PubMed] [Google Scholar]
- 48. Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol 2010; 105: 1523–1530. [DOI] [PubMed] [Google Scholar]
- 49. Duits LC, Phoa KN, Curvers WL, et al. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut 2015; 64: 700–706, http://group.bmj.com/group/rights-licensing/permissions [DOI] [PubMed] [Google Scholar]
- 50. Kestens C, Offerhaus GJ, van Baal JW, et al. Patients with Barrett’s esophagus and persistent low-grade dysplasia have an increased risk for high-grade dysplasia and cancer. Clin Gastroenterol Hepatol 2016; 14: 956–962.e1. [DOI] [PubMed] [Google Scholar]
- 51. Qumseya BJ, Wani S, Gendy S, et al. Disease progression in Barrett’s low-grade dysplasia with radiofrequency ablation compared with surveillance: systematic review and meta-analysis. Am J Gastroenterol 2017; 112: 849–865. [DOI] [PubMed] [Google Scholar]
- 52. Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014; 311: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 53. Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer). Gastrointest Endosc 2007; 65: 3–10. [DOI] [PubMed] [Google Scholar]
- 54. Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology 2014; 146: 652–660.e1. [DOI] [PubMed] [Google Scholar]
- 55. Cen P, Hofstetter WL, Correa AM, et al. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer 2008; 112: 1020–1027. [DOI] [PubMed] [Google Scholar]
- 56. Ancona E, Rampado S, Cassaro M, et al. Prediction of lymph node status in superficial esophageal carcinoma. Ann Surg Oncol 2008; 15: 3278–3288. [DOI] [PubMed] [Google Scholar]
- 57. Boys JA, Worrell SG, Chandrasoma P, et al. Can the risk of lymph node metastases be gauged in endoscopically resected submucosal esophageal adenocarcinomas? A multi-center study. J Gastrointest Surg 2016; 20: 6–12; discussion 12. [DOI] [PubMed] [Google Scholar]
- 58. Hamade N, Sharma P. Ablation therapy for Barrett’s esophagus: new rules for changing times. Curr Gastroenterol Rep 2017; 19: 48. [DOI] [PubMed] [Google Scholar]
- 59. Sawas T, Alsawas M, Bazerbachi F, et al. Persistent intestinal metaplasia after endoscopic eradication therapy of neoplastic Barrett’s esophagus increases the risk of dysplasia recurrence: meta-analysis. Gastrointest Endosc 2019; 89: 913–925.e6. [DOI] [PubMed] [Google Scholar]
- 60. Sie C, Bright T, Schoeman M, et al. Argon plasma coagulation ablation versus endoscopic surveillance of Barrett’s esophagus: late outcomes from two randomized trials. Endoscopy 2013; 45: 859–865. [DOI] [PubMed] [Google Scholar]
- 61. Van Laethem JL, Cremer M, Peny MO, et al. Eradication of Barrett’s mucosa with argon plasma coagulation and acid suppression: immediate and mid term results. Gut 1998; 43: 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schulz H, Miehlke S, Antos D, et al. Ablation of Barrett’s epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc 2000; 51: 659–663. [PubMed] [Google Scholar]
- 63. Wronska E, Polkowski M, Orlowska J, et al. Argon plasma coagulation for Barrett’s esophagus with low-grade dysplasia: a randomized trial with long-term follow-up on the impact of power setting and proton pump inhibitor dose. Endoscopy 2021; 53: 123–132. [DOI] [PubMed] [Google Scholar]
- 64. Milashka M, Calomme A, Van Laethem JL, et al. Sixteen-year follow-up of Barrett’s esophagus, endoscopically treated with argon plasma coagulation. United European Gastroenterol J 2014; 2: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Madisch A, Miehlke S, Bayerdorffer E, et al. Long-term follow-up after complete ablation of Barrett’s esophagus with argon plasma coagulation. World J Gastroenterol 2005; 11: 1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dulai GS, Jensen DM, Cortina G, et al. Randomized trial of argon plasma coagulation vs. multipolar electrocoagulation for ablation of Barrett’s esophagus. Gastrointest Endosc 2005; 61: 232–240. [DOI] [PubMed] [Google Scholar]
- 67. Sharma P, Jaffe PE, Bhattacharyya A, et al. Laser and multipolar electrocoagulation ablation of early Barrett’s adenocarcinoma: long-term follow-up. Gastrointest Endosc 1999; 49: 442–446. [DOI] [PubMed] [Google Scholar]
- 68. Shaheen NJ. New data on an old weapon: is argon plasma coagulation adequate treatment for dysplastic Barrett’s esophagus? Endoscopy 2021; 53: 133–135. [DOI] [PubMed] [Google Scholar]
- 69. Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med 2009; 360: 2277–2288. [DOI] [PubMed] [Google Scholar]
- 70. Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barrett’s mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol 2006; 101: 1762–1769. [DOI] [PubMed] [Google Scholar]
- 71. Manner H, May A, Kouti I, et al. Efficacy and safety of hybrid-APC for the ablation of Barrett’s esophagus. Surg Endosc 2016; 30: 1364–1370. [DOI] [PubMed] [Google Scholar]
- 72. Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic Barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology 2013; 145: 87–95. [DOI] [PubMed] [Google Scholar]
- 73. Singh T, Sanaka MR, Thota PN. Endoscopic therapy for Barrett’s esophagus and early esophageal cancer: where do we go from here? World J Gastrointest Endosc 2018; 10: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sánchez A, Reza M, Blasco JA, et al. Effectiveness, safety, and cost-effectiveness of photodynamic therapy in Barrett’s esophagus: a systematic review. Dis Esophagus 2010; 23: 633–640. [DOI] [PubMed] [Google Scholar]
- 75. Overholt BF, Lightdale CJ, Wang KK, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc 2005; 62; 488–498. [DOI] [PubMed] [Google Scholar]
- 76. Hamade N, Desai M, Thoguluva Chandrasekar V, et al. Efficacy of cryotherapy as first line therapy in patients with Barrett’s neoplasia: a systematic review and pooled analysis. Dis Esophagus 2019; 32: doz040. [DOI] [PubMed] [Google Scholar]
- 77. Tariq R, Enslin S, Hayat M, et al. Efficacy of cryotherapy as a primary endoscopic ablation modality for dysplastic Barrett’s esophagus and early esophageal neoplasia: a systematic review and meta-analysis. Cancer Control 2020; 27: 1073274820976668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Watts AE, Cotton CC, Shaheen NJ. Radiofrequency ablation of Barrett’s esophagus: have we gone too far, or not far enough? Curr Gastroenterol Rep 2020; 22: 29. [DOI] [PubMed] [Google Scholar]
- 79. Navaneethan U, Thosani N, Goodman A, et al. Radiofrequency ablation devices. VideoGIE 2017; 2: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cotton CC, Wolf WA, Overholt BF, et al. Late recurrence of Barrett’s esophagus after complete eradication of intestinal metaplasia is rare: final report from ablation in intestinal metaplasia containing dysplasia trial. Gastroenterology 2017; 153: 681–688.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Qumseya BJ, Wani S, Desai M, et al. Adverse events after radiofrequency ablation in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2016; 14: 1086–1095.e6. [DOI] [PubMed] [Google Scholar]
- 82. Orman ES, Li N, Shaheen NJ. Efficacy and durability of radiofrequency ablation for Barrett’s esophagus: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2013; 11: 1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fujii-Lau LL, Cinnor B, Shaheen N, et al. Recurrence of intestinal metaplasia and early neoplasia after endoscopic eradication therapy for Barrett’s esophagus: a systematic review and meta-analysis. Endosc Int Open 2017; 5: E430–E449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012; 143: 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. van Vilsteren FG, Alvarez Herrero L, Pouw RE, et al. Predictive factors for initial treatment response after circumferential radiofrequency ablation for Barrett’s esophagus with early neoplasia: a prospective multicenter study. Endoscopy 2013; 45: 516–525. [DOI] [PubMed] [Google Scholar]
- 86. Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett’s esophagus. Dig Dis Sci 2012; 57: 2625–2632. [DOI] [PubMed] [Google Scholar]
- 87. Komanduri S, Kahrilas PJ, Krishnan K, et al. Recurrence of Barrett’s esophagus is rare following endoscopic eradication therapy coupled with effective reflux control. Am J Gastroenterol 2017; 112: 556–566. [DOI] [PubMed] [Google Scholar]
- 88. Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett’s neoplasia. Gastrointest Endosc 2011; 74: 35–43. [DOI] [PubMed] [Google Scholar]
- 89. Konda VJ, Gonzalez Haba Ruiz M, Koons A, et al. Complete endoscopic mucosal resection is effective and durable treatment for Barrett’s-associated neoplasia. Clin Gastroenterol Hepatol 2014; 12: 2002–2010.e1–e2. [DOI] [PubMed] [Google Scholar]
- 90. Tomizawa Y, Konda VJA, Coronel E, et al. Efficacy, durability, and safety of complete endoscopic mucosal resection of Barrett esophagus: a systematic review and meta-analysis. J Clin Gastroenterol 2018; 52: 210–216. [DOI] [PubMed] [Google Scholar]
- 91. Tomizawa Y, Iyer PG, Wong Kee Song LM, et al. Safety of endoscopic mucosal resection for Barrett’s esophagus. Am J Gastroenterol 2013; 108: 1440–1447; quiz 1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Anders M, Bähr C, El-Masry MA, et al. Long-term recurrence of neoplasia and Barrett’s epithelium after complete endoscopic resection. Gut 2014; 63: 1535–1543, http://group.bmj.com/group/rights-licensing/permissions [DOI] [PubMed] [Google Scholar]
- 93. Chennat J, Konda VJ, Ross AS, et al. Complete Barrett’s eradication endoscopic mucosal resection: an effective treatment modality for high-grade dysplasia and intramucosal carcinoma—an American single-center experience. Am J Gastroenterol 2009; 104: 2684–2692. [DOI] [PubMed] [Google Scholar]
- 94. Larghi A, Lightdale CJ, Ross AS, et al. Long-term follow-up of complete Barrett’s eradication endoscopic mucosal resection (CBE-EMR) for the treatment of high grade dysplasia and intramucosal carcinoma. Endoscopy 2007; 39: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 95. May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett’s oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002; 14: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 96. van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett’s oesophagus with high-grade dysplasia or early cancer: a multicentre randomised trial. Gut 2011; 60: 765–773. [DOI] [PubMed] [Google Scholar]
- 97. Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: results of an European multicentre study (EURO-II). Gut 2016; 65: 555–562, http://www.bmj.com/company/products-services/rights-and-licensing/ [DOI] [PubMed] [Google Scholar]
- 98. Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut 2017; 66: 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ishihara R, Yamamoto S, Hanaoka N, et al. Endoscopic submucosal dissection for superficial Barrett’s esophageal cancer in the Japanese state and perspective. Ann Transl Med 2014; 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009; 41: 661–665. [DOI] [PubMed] [Google Scholar]
- 101. Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70: 860–866. [DOI] [PubMed] [Google Scholar]
- 102. Shi Q, Ju H, Yao LQ, et al. Risk factors for postoperative stricture after endoscopic submucosal dissection for superficial esophageal carcinoma. Endoscopy 2014; 46: 640–644. [DOI] [PubMed] [Google Scholar]
- 103. Canto MI, Shaheen NJ, Almario JA, et al. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrett’s esophagus (with video). Gastrointest Endosc 2018; 88: 438–446.e2. [DOI] [PubMed] [Google Scholar]
- 104. Westerveld DR, Nguyen K, Banerjee D, et al. Safety and effectiveness of balloon cryoablation for treatment of Barrett’s associated neoplasia: systematic review and meta-analysis. Endosc Int Open 2020; 8: E172–E178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Norton ID, Wang L, Levine SA, et al. Efficacy of colonic submucosal saline solution injection for the reduction of iatrogenic thermal injury. Gastrointest Endosc 2002; 56: 95–99. [DOI] [PubMed] [Google Scholar]
- 106. Fujishiro M, Kodashima S, Ono S, et al. Submucosal injection of normal saline can prevent unexpected deep thermal injury of argon plasma coagulation in the in vivo porcine stomach. Gut Liver 2008; 2: 95–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Manner H, Neugebauer A, Scharpf M, et al. The tissue effect of argon-plasma coagulation with prior submucosal injection (hybrid-APC) versus standard APC: a randomized ex-vivo study. United European Gastroenterol J 2014; 2: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Komanduri S, Muthusamy VR, Wani S. Controversies in endoscopic eradication therapy for Barrett’s esophagus. Gastroenterology 2018; 154: 1861–1875.e1. [DOI] [PubMed] [Google Scholar]