SUMMARY
Aberrant hedgehog (Hh) signaling contributes to the pathogenesis of multiple cancers. Available inhibitors target Smoothened (Smo), which can acquire mutations causing drug resistance. Thus, compounds that inhibit Hh signaling downstream of Smo are urgently needed. We identified dynarrestin, a novel inhibitor of cytoplasmic dyneins 1 and 2. Dynarrestin acts reversibly to inhibit cytoplasmic dynein 1-dependent microtubule binding and motility in vitro without affecting ATP hydrolysis. It rapidly and reversibly inhibits endosome movement in living cells and perturbs mitosis by inducing spindle misorientation and pseudoprometaphase delay. Dynarrestin reversibly inhibits cytoplasmic dynein 2-dependent intraflagellar transport (IFT) of the cargo IFT88 and flux of Smo within cilia without interfering with ciliogenesis and suppresses Hh-dependent proliferation of neuronal precursors and tumor cells. As such, dynarrestin is a valuable tool for probing cytoplasmic dynein-dependent cellular processes and a promising compound for medicinal chemistry programs aimed at development of anti-cancer drugs.
Graphical Abstract
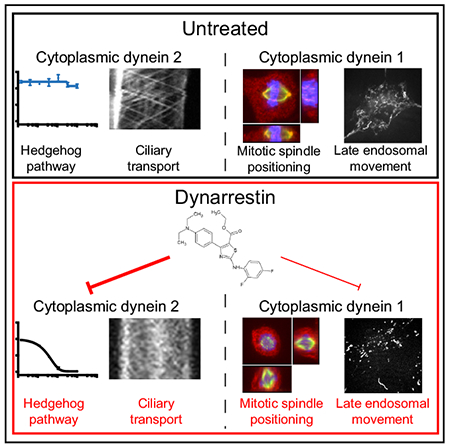
In Brief
Höing, Yeh et al. identify dynarrestin, a novel inhibitor of the Hedgehog-signaling pathway. Dynarrestin specifically inhibits dynein in a reversible and novel manner.
INTRODUCTION
The hedgehog (Hh)-signaling pathway is a critical regulator of differentiation and proliferation. Hh signaling is required for specification of motor neurons (MNs) (Marti et al., 1995; Roelink et al., 1995) and osteoblasts (Long et al., 2004), and stimulates the proliferation of undifferentiated cells (Lai et al., 2003). Hh pathway defects lead to developmental disorders. Aberrant activation of the Hh pathway through genetic mutation contributes to oncogenesis, including medulloblastoma (Pomeroy et al., 2002), basal cell (Johnson et al., 1996), and breast cancers (Souzaki et al., 2011). Due to the link between aberrant Hh signaling and carcinogenesis, Hh pathway inhibition is a potential therapeutic strategy for cancer.
The transmembrane receptor Smoothened (Smo) mediates signaling downstream of the Hh receptor, Patched (Alcedo et al., 1996), and multiple small-molecule Smo inhibitors are in development and clinical use (Lin and Matsui, 2012). However, cancers arising from mutations in Hh pathway components downstream of Smo are not expected to be sensitive to these drugs (Lee et al., 2007b), and it is known that cancer cells in patients treated with a Smo inhibitor can acquire mutations that lead to drug resistance (Yauch et al., 2009). Drugs inhibiting the Hh signaling pathway at a point downstream of Smo are needed.
The microtubule-based motor cytoplasmic dynein is an attractive target for inhibiting Hh-dependent cancers. Dyneins are multimeric protein complexes that convert energy from ATP hydrolysis into mechanical work to drive movement toward microtubule minus ends. Dyneins are of two classes: axonemal dyneins, which power the beating movement of cilia and eukaryotic flagella, and cytoplasmic dyneins (hereafter dyneins), which drive movements within cells. Dynein 1 is essential for proper mitotic spindle function (Pfarr et al., 1990; Steuer et al., 1990), translocation of membranous organelles and other subcellular components (Schnapp and Reese, 1989; Schroer et al., 1989), and cell viability. Dynein 2 drives transport of molecules within eukaryotic cilia and flagella (Hou and Witman, 2015). This process, known as intraflagellar transport (IFT), is essential for activation of Hh signaling in vertebrates (Huangfu and Anderson, 2005; May et al., 2005). Since IFT depends upon dynein 2, dynein 2 inhibitors are attractive drug targets for Hh-dependent cancers.
Few tools exist to interfere with the activity of either dynein 1 or dynein 2. Genetic perturbations of dynein 1 have the drawback that they create a new steady-state condition in which both plus and minus end-directed microtubule-based organelle transports are suppressed (Gross et al., 2002; Martin et al., 1999). Many studies aimed at understanding dynein 1 function relied on perturbation of the dynein cofactor, dynactin, by protein overexpression or depletion, but this approach yields bidirectional motility impairment (Valetti et al., 1999; Yeh et al., 2012), making it difficult to interpret experimental results and leading to the widely accepted model that the activities of dynein 1 and kinesin motors are coupled (Fu and Holzbaur, 2014). Selective interference with dynein 2 activity in IFT also presents a challenge, because ciliogenesis, a process closely linked to IFT, is almost invariably affected, preventing incisive analysis of the role of dynein 2 in key events. The lack of genetic tools that selectively impair dynein 1- and 2-driven movement underscores the need for acutely, reversibly acting small-molecule inhibitors.
The only small-molecule inhibitors of dynein available are the ciliobrevins, which inhibit dynein 1 ATPase activity (Firestone et al., 2012; See et al., 2016). Unfortunately, the ciliobrevins present problems due to their lack of potency; over 100 μM ciliobrevin is required to inhibit dynein 1 (See et al., 2016). Some investigators have reported a lack of efficacy (Clift and Schuh, 2015). Additional development identified ciliobrevins specific for dynein 2, but even the most potent variants had half-maximal inhibitory concentration (IC50) values <10 μM (See et al., 2016). Recently, isosteres of ciliobrevins were identified with significantly lower IC50 values, but considerable toxicity was observed at 20 μM, which is only 2-fold above the concentration used for efficient dynein inhibition (Steinman et al., 2017). Thus, small-molecule inhibitors that target dynein 1 and 2 more potently and exhibit reliable performance would greatly benefit the field. In addition, ciliobrevins inhibit cilium formation. Aberrant ciliogenesis is associated with diseases such as polycystic kidney disease, nephronophthisis, liver disease, and pathologies collectively known as ciliopathies (Brown and Witman, 2014). For clinical use, dynein inhibitors are needed that inhibit the Hh pathway downstream of Smo without blocking ciliogenesis.
In this study, we describe dynarrestin, a novel aminothiazole inhibitor of dyneins 1 and 2 that we identified using a high-throughput screen for Hh pathway antagonists. Pull-down experiments revealed dynein to be the target of dynarrestin. Dynarrestin rapidly and reversibly inhibits dynein 1-driven microtubule gliding in vitro plus a range of dynein 1- and 2-dependent processes in cells including endosome translocation, mitotic spindle morphogenesis and positioning, and Smo flux and IFT particle movement in primary cilia. Dynarrestin acts reversibly to inhibit cytoplasmic dynein 1-dependent microtubule binding and motility in vitro without affecting ATP hydrolysis, making dynarrestin the first-known small-molecule dynein inhibitor that decouples ATP hydrolysis from motor activity. Dynarrestin inhibits Hh-dependent signaling in mouse and human cells and blocks Hh-dependent proliferation of primary mouse medulloblastoma cells at a step downstream of the Smo receptor. Importantly, dynarrestin inhibits dynein 2 but does not impair ciliogenesis. Dynarrestin is a valuable tool for studying dynein-based phenomena and has great potential as a lead compound for medicinal chemistry programs targeting cancer.
RESULTS
Identification of a Small-Molecule Antagonist of Hh Activity
Two assays were used to screen a chemical library containing 337,119 compounds for novel Hh pathway inhibitors. One assay evaluated differentiation of MNs from mouse embryonic stem cells (mESCs) that express an MN-specific Hb9-GFP reporter. Since MN specification requires Hh signaling (Marti et al., 1995; Roelink et al., 1995), Hh pathway inhibition blocks green fluorescent protein (GFP) production (Höing et al., 2012; Wichterle et al., 2002). mESCs were differentiated as embryoid bodies in the presence of retinoic acid (RA; 1 μM) and the Smo agonist purmorphamine (PMA; 1 μg/mL) for 6 days (Figure 1A). To validate the assay, we treated ESCs with 10 ng/mL bone morphogenetic protein 4 (BMP4), which is known to inhibit Hh-mediated MN specification (Liem et al., 2000), or 10 μM forskolin, which activates protein kinase A (PKA) and inhibits the Hh pathway by triggering degradation of the Gli effector proteins (Wang et al., 2000). The treatments yielded approximately 26% and 4%, respectively, of the GFP intensity seen for RA/PMA-treated controls (Figure 1B). Our chemical library screen yielded dynarrestin (Figure 1C) as a candidate that reduced Hb9:GFP expression by about 98% (Figure 1D). Compounds L455 and L557, which are structurally similar to dynarrestin (Figure 1E), were less active (Figure 1D). Quantitative RT-PCR confirmed that dynarrestin reduced expression of the endogenous MN progenitor marker Olig2 and post-mitotic MN markers Hb9 and Isl1 (Supplemental Information, Figures S1A–S1C). In parallel, increased levels of dorsal interneuron progenitor marker Pax7 were detected (Figure S1D), fully consistent with Hh pathway inhibition.
Figure 1. Identification of Dynarrestin.
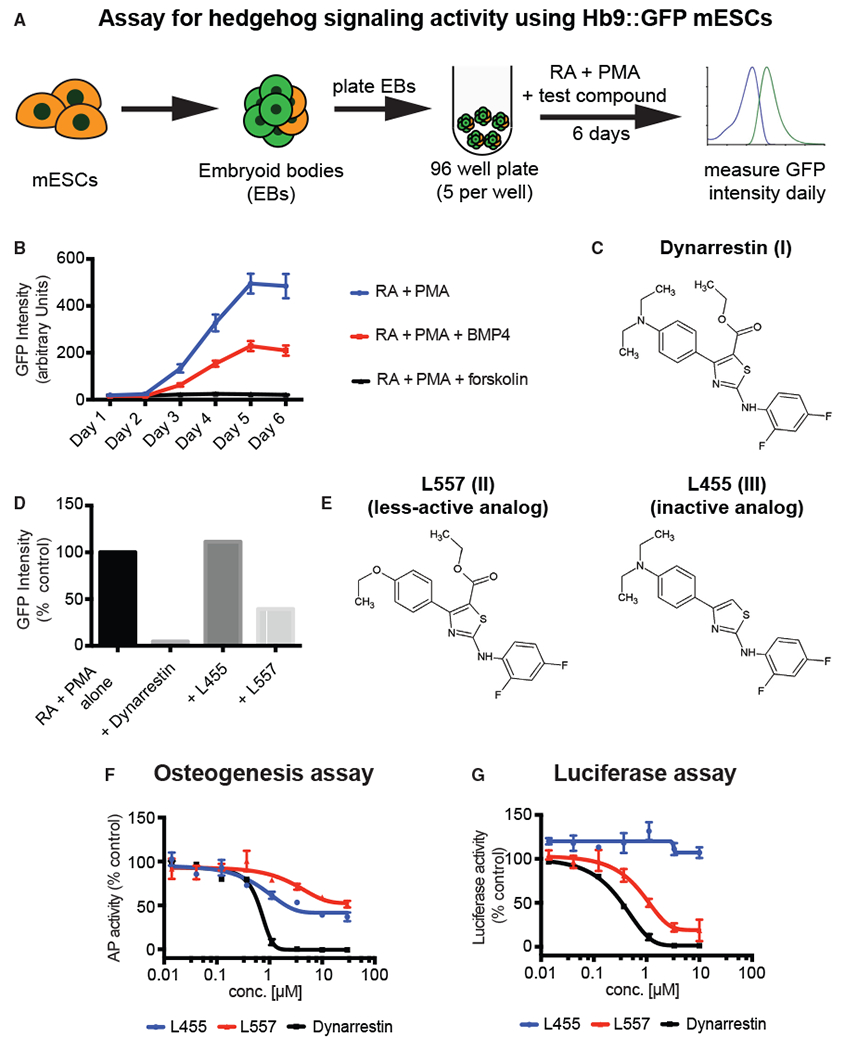
(A) Schematic of the assay for embryoid body (EB)-based differentiation of mouse embryonic stem cells (mESCs) into motor neurons in an Hh-dependent manner.
(B) Time course of Hb9:GFP expression under the indicated conditions.
(C) Chemical structure of dynarrestin.
(D) Effect of the indicated compound on Hb9:GFP intensity in treated EBs (Day 5).
(E–G) Chemical structure of dynarrestin analogs (E). Effects of the indicated compounds in assays of (F) Hh-dependent osteogenesis of C3H10T1/2 cells and (G) luciferase activity using Shh-LIGHT2 cells.
Error bars indicate data range. See also Figures S1 and S2.
We confirmed that dynarrestin inhibits the Hh pathway using an assay for differentiation of C3H10T1/2 cells into osteoblasts (Kinto et al., 1997), which requires Hh signaling. Osteoblasts express alkaline phosphatase (AP), and differentiation requires Hh signaling (Long et al., 2004). C3H10T1/2 cells were cultured for 4 days in the presence of 1 μM PMA and then assayed using CDP-Star, a chemiluminescent AP substrate (Figure S1E). Vismodegib, a Smo inhibitor (a positive control for Hh pathway inhibition) approved by the US Food and Drug Administration, decreased AP activity by about 98% (Figure S1F). Dynarrestin also dramatically decreased AP activity with a IC50 of 0.3 μM, as compared with the less-active control compounds L557 and L445 (Figure 1F).
We evaluated the effect of dynarrestin on Hh-dependent luciferase expression using LIGHT2 cells, an NIH3T3 cell derivative with a stably integrated luciferase reporter (Taipale et al., 2000). Consistent with our other results, dynarrestin inhibited Hh-Luc activity with an IC50 of 0.22 μM, whereas the L455 and L557 control compounds showed significantly less inhibition (Figure 1G). To rule out the possibility that dynarrestin reduced Hh-Luc activity owing to a cytotoxic effect, we measured LIGHT2 viability using the chemiluminescent reporter, CellTiter Glo. The dynarrestin concentration yielding half-maximal cytotoxicity after 72 hr treatment was approximately two orders of magnitude higher than for Hh inhibition (Figures S1G–S1J), indicating that dynarrestin inhibits Hh signaling without causing cytotoxicity.
Dynarrestin Binds Dynein 1
To identify the cellular target of dynarrestin, we used an unbiased approach in which dynarrestin was conjugated to a biotin tag and used to pull down interacting proteins for identification via liquid chromatography and label-free quantitative (LFQ) tandem mass spectrometry (LC-MS/MS). We synthesized a number of dynarrestin analogs to identify where a biotin tag could be added without disrupting Hh inhibition. Compound L655, which contained a methoxy substitution in the para-position in the phenyl ring of R1 that was accessible to linker conjugation, retained the ability to inhibit Hh signaling in both the luciferase and osteogenesis assays (Figures S2A–S2C). Compound L626, which was modified at position R2, did not inhibit Hh signaling (Figures S2A–S2C), suggesting that R2 might be required for target binding. Using this information, we synthesized two biotinylated analogs of dynarrestin: L568, which was conjugated at position R1 and expected to bind the target, and L527, which was conjugated at R2 and expected not to bind the target (Figure 2A). We synthesized analogs of compound XIb, another aminothiazole Hh pathway inhibitor (Oinas et al., 2006) (Figures S2D–S2F). Compound XIb is similar in structure to dynarrestin but lacks the ethyl ester group at the 5 position of the aminothiazole ring (R2) required for Hh pathway inhibition by dynarrestin. A dynarrestin/XIb hybrid compound was inactive, suggesting that compound XIb and dynarrestin bind different cellular targets (Figures S2D–S2F). We found that the 6 position in the phenyl ring was accessible to linker conjugation, so we synthesized L357, a derivative of XIb and the XIb/dynarrestin hybrid compound with a linker and biotin tag at that position (Figure 2A). Because the hybrid compound was inactive, we expected the L357 probe would not pull down the same target as dynarrestin (i.e., L568), providing an additional control to L527.
Figure 2. Pull-Down Analysis Identified Dynein as a Target of Dynarrestin.

(A–C) Chemical structure of analogs of dynarrestin (A). Box-and-whisker, which indicate minimum to maximum, plots for label-free quantitative (LFQ) LC-MS/MS results for (B) DYNC1I2 as well as (C) DYNC1LI1, which are both part of the cytoplasmic type-1 dynein protein complex. *p < 0.05, **p < 0.01 according to one-way ANOVA.
(D) Semi-quantitative LC-MS/MS results using fractional intensity-based absolute quantification (iBAQ) scores for the indicated dynein complex component proteins.
(E and F) Validation of the LC-MS/MS data using western blot analysis for dynein intermediate chain (IC74) after pull-down using the indicated probe as well as positive and negative controls. (E) Extracts from C3H10T1/2 and Shh-Light 2 cells were used, as well as (F) purified bovine dynein 1 as indicated.
The biotinylated compounds (Figure 2A) were adsorbed to streptavidin-coated beads and then incubated with extract prepared from C3H10T1/2 cells. After washing, the beads were boiled and the samples analyzed using LFQ LC-MS/MS. Principal component analysis demonstrated that the samples clustered separately (data not shown). Proteins were filtered for significant differences in binding to R1-linked dynarrestin (i.e., L568) compared with the other two compounds (Table S1). We found that the LFQ values for two dynein 1 components, the intermediate chain (DYNC1I2) and light intermediate chain (DYNC1LI1), were significantly higher in the samples pulled down with dynarrestin compared with L527 and L357 (Figures 2B and 2C). We analyzed the pull-down data semi-quantitatively using intensity-based absolute quantification (iBAQ) values for each protein as a fractional value of the sum of all iBAQ values for the sample. Consistent with the LFQ values, significantly higher fractional iBAQ values were obtained for dynein 1 components in the samples pulled down with dynarrestin compared with L527 and L357 (Figure 2D). This analysis revealed higher fractional iBAQ values for the dynein heavy chain (DYNC1H1) and light chain Tctex-1 (DYNLT1). To confirm that dynarrestin binds dynein 1, we repeated the pull-down using extracts from both C3H10T1/2 and LIGHT2 cells and then probed the sample for dynein 1 intermediate chain via immunoblot. As per the LC-MS/MS data, the active compound demonstrated marked enrichment for dynein compared with the inactive control (Figure 2E). A pull-down using purified native dynein 1 was used to verify that dynarrestin binds dynein directly (Figure 2F). These results demonstrate that dynein 1 is a molecular target of dynarrestin.
Off-target in vivo effects are a concern for small-molecule inhibitors. Aminothiazoles are known ATP-competitive kinase inhibitor scaffolds (Das et al., 2006), raising the possibility that dynarrestin might impact Hh pathway signaling in vivo by targeting a kinase. To rule out this possibility, we performed a kinome-wide ATP-competition assay (Figure S3A). Although insulin receptor, cyclin-dependent kinase 2, and casein kinase 1δ emerged as possible targets (Figure S3A), activity assays revealed that none were significantly inhibited by dynarrestin (Figure S3B). This analysis demonstrated that dynarrestin does not inhibit Hh pathway signaling by targeting a kinase. Another aminothiazole, JK184 (Cupido et al., 2009), antagonizes Hh signaling by inhibiting alcohol dehydrogenase 7 (ADH7) (Lee et al., 2007a), but a surface plasmon resonance assay demonstrated that dynarrestin does not bind ADH7 (data not shown).
Dynarrestin Inhibits Dynein 1 Activity In Vitro and In Vivo
To verify that dynarrestin can act as a direct inhibitor of dynein, we performed in vitro microtubule gliding assays using dynein 1 purified from bovine brain (Bingham et al., 1998). Dynarrestin profoundly suppressed gliding in a dose-dependent manner (IC50 = 5 μM) by impairing the ability of microtubules to remain associated with dynein adsorbed to the coverslip surface (Figures 3A–3C). Microtubule binding and movement were completely restored following drug washout, indicating that the effects of dynarrestin on dynein are reversible. For the few microtubules that remained associated with the coverslip surface and exhibited movement in the presence of dynarrestin, we observed a dose-dependent reduction in gliding velocity (6.25 μM dynarrestin: 0.82 ± 0.13 μm/s, p < 10−8; 12.5 μM: 0.76 ± 0.14 μm/s, p < 10−11, 25 μM dynarrestin: 0.41 ± 0.05 μm/s, p < 10−15, versus 1.04 ± 0.07 μm/s in the DMSO control and 1.04 ± 0.07 μm/s in 12.5 μM L557; Figures 3D and 3E). Normal microtubule gliding speeds were restored following dynarrestin washout (0.96 ± 0.08 μm/s) (Figure 3D). Dynarrestin had no effect on kinesin-1-dependent microtubule gliding (Figure 3E). Dynein 1-driven microtubule gliding was not affected by 50 μM ciliobrevin A or D (velocity: 1.02 ± 0.06 μm/s and 1.01 ± 0.08 μm/s; see also Figures S4A and S4B). These results demonstrate that dynarrestin is a direct and specific inhibitor of cytoplasmic dynein 1.
Figure 3. Dynarrestin Inhibits Dynein-1-Dependent Microtubule Gliding and Late Endosome Movement.
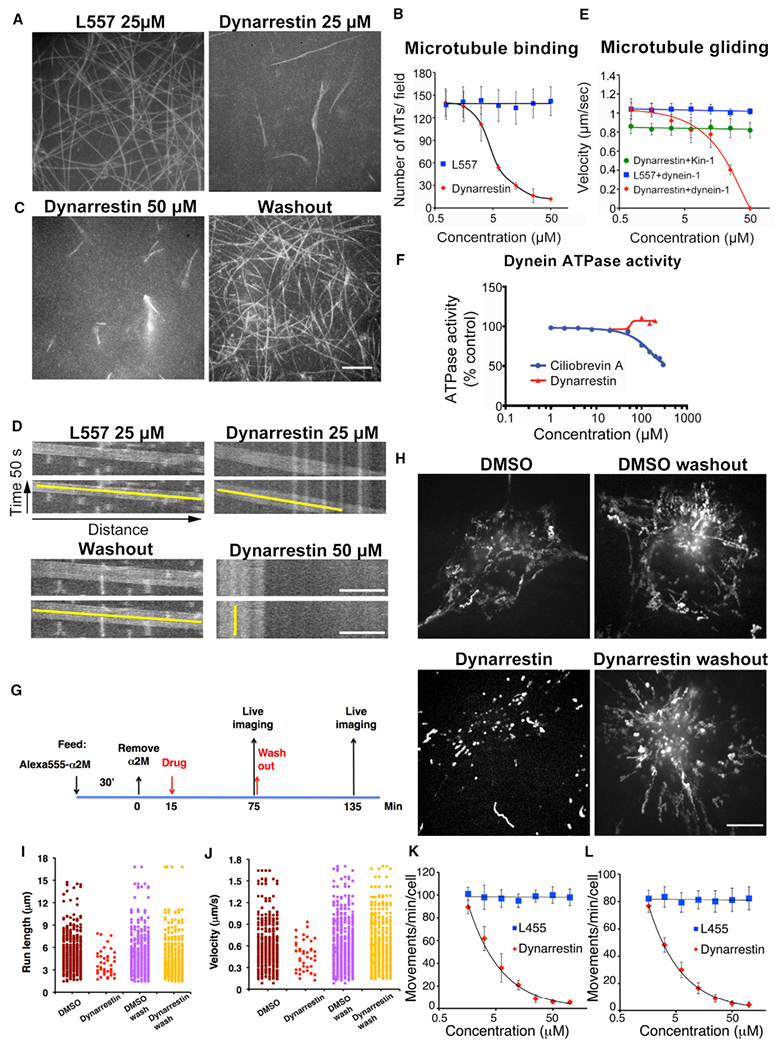
(A–E) The effects of dynarrestin on dynein-dependent microtubule (MT) gliding assay. (A) Maximal projection of 50 s movies illustrating movements of rhodamine-MTs driven by purified bovine dynein in samples incubated for 30 min in 25 μM L557 and dynarrestin. (B) Average number of MTs bound per field (mean ± SD, 15 fields in three experiments). (C) Maximal projection of 50 s movies as in (A) in 50 μM dynarrestin (left) or after drug washout (right). (D) Kymographs illustrating MT movements in (A) and (C). Scale bar, 5 μm. Yellow lines indicate slope. (E) Average gliding velocities by dynein-1 in various concentrations of dynarrestin (red) or L557 (blue) treatment, or by kinesin-1 (green) in dynarrestin treatment (mean ± SD, three experiments; n > 150 MTs).
(F) Dynein ATPase activity of purified porcine brain dynein in the presence of MTs.
(G) Method for labeling and assessing motility of late endosomes (loaded with Alexa 555-α2-macroglobulin; α2M) in Cos7 cells.
(H) Images showing the maximal projection of particle movements in 50 s. Scale bar, 5 μm.
(I–L) Dot plots of particle (I) run lengths and (J) velocities in 500 s observation (50 s/cell, 10 cells/condition). Each dot corresponds to a moving particle that translocated >1.5 μm. Dynarrestin dose-response curves for late endosome motility are shown for (K) Cos7 cells and (L) IMCD3 cells in 720 s observation (mean ± SEM, three experiments, n = 12 cells).
See also Figure S4.
Ciliobrevins A and D antagonize dynein motor activity by inhibiting ATPase activity. We confirmed that ciliobrevin A inhibits microtubule-stimulated ATP hydrolysis by dynein 1 with an IC50 of about 84 μM (Figure 3F). ATP hydrolysis by dynein 1 was almost unchanged (93% of control) in the presence of 50 μM ciliobrevin A, explaining why we saw no effect on microtubule translocation. Dynarrestin did not affect dynein-dependent ATP hydrolysis at concentrations >100 μM. This suggests that dynarrestin inhibits dynein by decoupling ATP hydrolysis from the microtubule binding cycle, an effect that is distinct from that of the ciliobrevins, which directly compete for ATP binding. Precedent for this mode of inhibition is seen in the case of the physiological dynein regulator, Lis1, which allows ATP hydrolysis to persist in the absence of microtubule binding and release (Huang et al., 2012).
Dynein 1 contributes to many types of subcellular motility, so we conducted a series of experiments to evaluate dynarrestin effects in vivo. To evaluate endomembrane translocation, we labeled Cos7 cells with the endocytic tracer Alexa 555-α2-macroglobulin, treated them with 25 μM dynarrestin for 60 min, and then subjected them to live cell imaging (Figure 3G). Long-range, bidirectional movement of α2-macroglobulin-loaded late endosomes was profoundly inhibited (Figures 3G–3J). Late endosome motility in Cos-7 and IMCD3 cells responded to dynarrestin in a dose-dependent manner (IC50 = 4.2 μM and 3.7 μM, respectively) (Figures 3K and 3L). In a time-course experiment, movement ceased after ≈20 min and at all time points, both inward (dynein-based) and outward (kinesin-based), translocations were suppressed. This is consistent with extensive previous work showing that the activities of dynein 1 and kinesin family motors are functionally coupled on endosomes (Granger et al., 2014; Yeh et al., 2012). Bidirectional motility was fully restored after drug washout, indicating that the effects of dynarrestin are reversible in vivo. Consistent with this, dynarrestin did not affect dynein abundance, composition, or structural integrity (Figure S4C). Suppressed endosome motility was not due to a change in the underlying microtubule cytoskeleton, as microtubule abundance and radial organization were normal (Figures S4D and S4E). In keeping with our in vitro findings, 50 μM ciliobrevin A or D had no effect on endosome movement (Figures S4F–S4H), demonstrating that dynarrestin inhibits dynein 1 in vivo more potently than ciliobrevin.
Dynein 1 also plays important roles in mitosis. Although we observed approximately normal numbers of mitotic spindles (using microtubule staining) in asynchronous Cos7 cells treated with dynarrestin for 1 hr (mitotic index = 6.0% ± 0.5% versus 5.7% ± 0.4% for controls), analysis of mitotic stage based on chromosome configuration revealed a significant, dose-dependent reduction (IC50 = 6.25 μM) in the number of cells in metaphase, anaphase, or telophase (Figure 4A). This suggests that kinetochore-associated dynein, which contributes to chromosome alignment and the spindle assembly checkpoint, is affected. Spindle orientation was profoundly altered, as we observed a significantly higher number of spindles oriented at >15° relative to the substrate. Pole-to-pole distance was reduced (Figures 4B–4F). These results agree with reports that spindle anchoring to the cortex is dynein 1-dependent (Kiyomitsu and Cheeseman, 2013). Changes in spindle pole integrity were detected, but only after prolonged (>2 hr) dynarrestin treatment (Figures S5A and S5B). No effects on spindle morphology or mitotic progression were seen with 50 μM ciliobrevin A or D (Figures S5C and S5D). These results indicate that the mitotic pools of dynein 1 at the cell cortex and kinetochore are highly sensitive to dynarrestin.
Figure 4. Mitotic Effects of Dynarrestin.
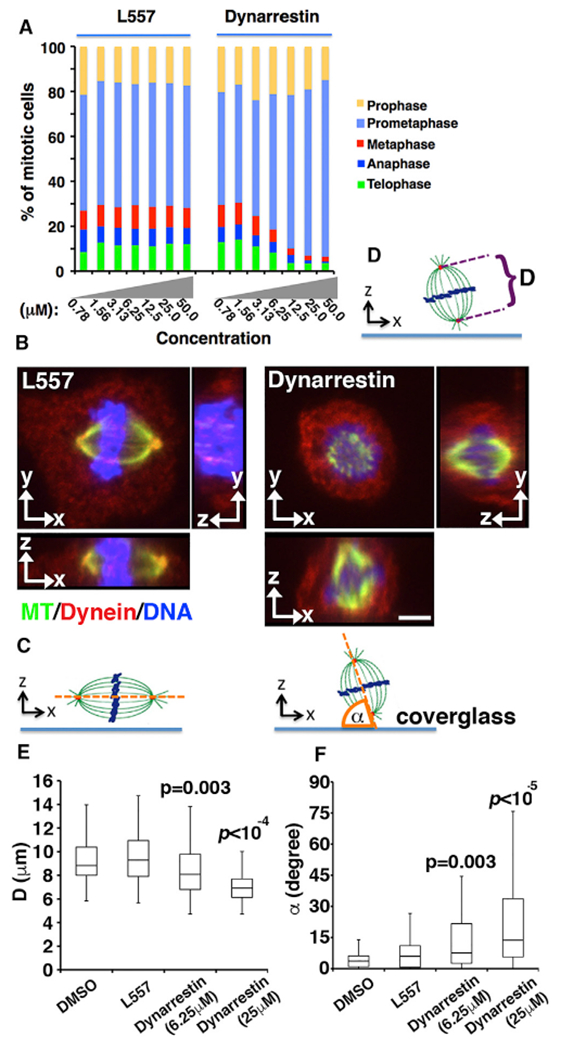
(A) Asynchronous Cos7 populations were treated with dynarrestin for 1 hr at 37°C, then assigned on the basis of chromosome and microtubule configuration (three experiments, n > 600 mitotic cells).
(B–D) Effects on spindle orientation and pole-to-pole distance. (B) Cos7 cells treated as above were stained for MTs (green), dynein (intermediate chain; red) and DAPI (blue). Typical images in x-y, x-z, and y-z planes are shown. Bar, 4 μm. (C and D) Schematics illustrating pole-to-pole distances (“D”) measured from the confocal images (D) and α, the angle between the spindle axis (orange line) and the coverslip (C).
(E) Box-and-whisker plots of pole-to-pole distances as in (D) measured in 50 cells (two independent experiments).
(F) Box-and-whisker plots of spindle orientation (n = 50 cells in two independent experiments).
p Values were calculated using the Mann-Whitney U test. See also Figures S5 and S6.
Cellular toxicity can complicate the use of small-molecule inhibitors. For this reason, we assessed the effects of ciliobrevin A and dynarrestin on mitochondrial activity, which is a sensitive readout of cellular toxicity. Cos7 cells were treated for 1 hr with 25 μM dynarrestin, ciliobrevin A, or ciliobrevin D, and assayed for their mitochondrial membrane potential using a pulse of 200 nM tetramethylrhodamine, ethyl ester (TMRE). Ciliobrevins A and D showed significant cytoplasmic staining and altered mitochondrial morphology compared with DMSO (Figure S6). This suggests that using ciliobrevins to inhibit dynein in vivo is complicated because their IC50 for dynein 1 inhibition is greater than the concentration that yields mitochondrial toxicity. By contrast, morphology and membrane potential of mitochondria in Cos7 cells treated with dynarrestin were indistinguishable from DMSO controls (Figure S6). Thus, dynarrestin shows greater potency for dynein inhibition and decreased toxicity, possibly resulting from its ATPase-independent mechanism for dynein inhibition.
Dynarrestin Inhibits Dynein 2-Mediated Translocation of IFT Cargoes
The inhibitory effects of ciliobrevins on Hh signaling verify the importance of dynein 2 in the Hh pathway. Primary cilia are essential for proper Hh pathway function in vertebrates. The Hh receptor, Patched (Ptch), normally resides in cilia (Rohatgi et al., 2007). Smo, by contrast, diffuses between the cell body and the cilium, and only accumulates in the cilium when Hh binds Ptch (Corbit et al., 2005). Smo flux is dependent upon dynein 2. We had observed that low dynarrestin concentrations (i.e., <1 μM) are sufficient to significantly inhibit Hh signaling (Figures 1F and 1G), whereas dynein 1 inhibition requires a higher concentration (>5 μM) both in vitro and in vivo (Figures 3B, 3I, 3J, 4E, and 4F). These findings are consistent with the hypothesis that dynarrestin inhibits Hh signaling by altering dynein 2-dependent trafficking of critical Hh pathway effectors and not by suppressing dynein 1-dependent events. These findings further suggest that dynein 2 is more sensitive than dynein 1 to dynarrestin, a phenomenon also observed for ciliobrevins (Firestone et al., 2012; See et al., 2016).
To explore whether Smo flux is suppressed by dynarrestin, we used NIH3T3 cells, which can be induced to form primary cilia by overnight serum starvation. Ciliated cells were treated with dynarrestin, then fixed and co-immunostained for Smo and the primary cilium marker acetylated tubulin. Ciliobrevins A and D were used as controls. Ciliobrevins A and D caused Smo to accumulate in cilia in the absence of Hh signaling (Figure 5A). Dynarrestin also caused Smo to accumulate in cilia in the absence of Hh signaling, sometimes to an even greater extent than ciliobrevin, and at significantly lower concentrations (Figure 5B). Furthermore, dynarrestin inhibited Smo flux out of the cilium at the lowest concentration tested (1 μM), similar to the concentration that inhibited Hh signaling in the assays we used in our library screen (Figures 1E and 1F). Unlike the ciliobrevins, dynarrestin did not prevent cilium formation when it was included during overnight serum starvation (Figures 5C and 5D). These data strongly suggest that dynarrestin inhibits dynein 2, and re-inforces the hypothesis that dynarrestin and ciliobrevins inhibit dynein activity in distinct ways.
Figure 5. Dynarrestin Inhibits Smoothened Trafficking.
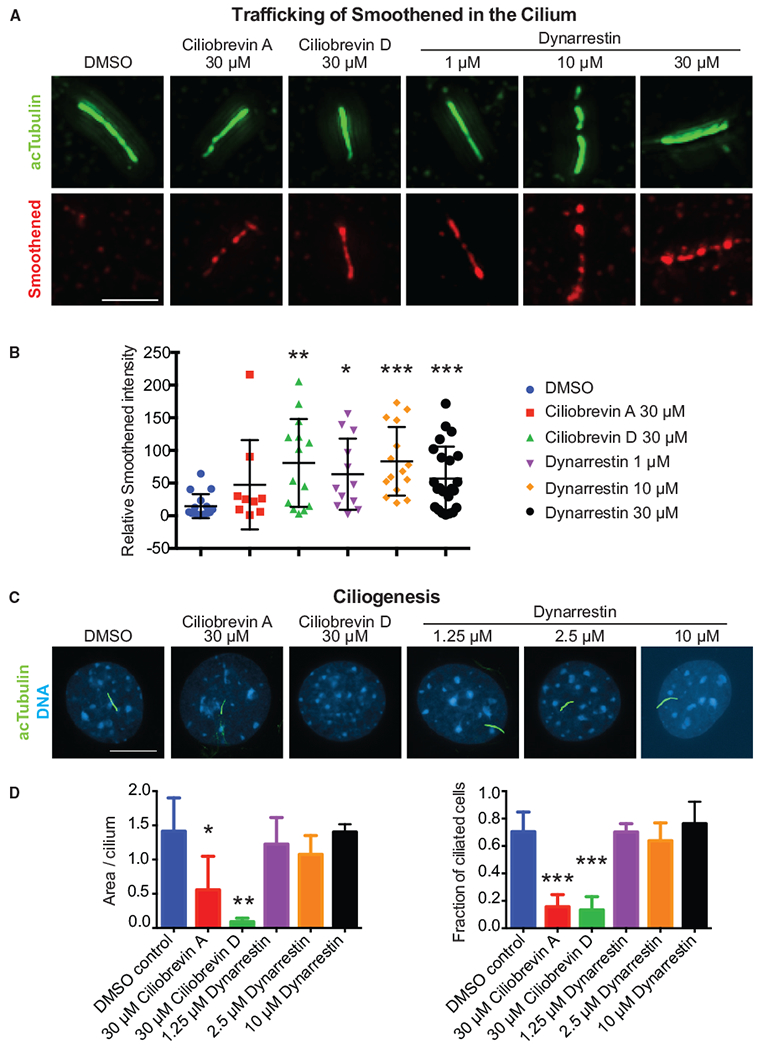
(A) Smoothened and acetylated tubulin (acTubulin) in primary cilia treated for 8 hr with the indicated conditions. Scale bar, 2.4 μm.
(B) Scatter dot plot of Smoothened intensity with bars indicating mean with SD.
(C) Immunostaining for acTubulin of cells induced to form primary cilia under the indicated conditions. Scale bar, 10 μm.
(D) Quantification of ciliogenesis.
Error bars indicate SD. *p < 0.05; **p < 0.01; ***p < 0.001 according to t tests with Welch’s correction.
We used live imaging to directly evaluate the effects of dynarrestin on dynein 2-dependent IFT. We used IMCD3 cells that can form cilia on their basal surfaces, which facilitated imaging (Breslow et al., 2013). Cells were transfected with the primary cilium markers IFT88-GFP and Arl13b-mCherry (He et al., 2014), then serum starved for 24 hr to induce ciliogenesis. Dynarrestin treatment (1 hr, 125 nM, or 1.25 μM) yielded a conspicuous change in localization of both markers, which were lost from the basal body and accumulated in the cilium (Figures 6A and 6B). Kymograph analysis revealed that dynarrestin significantly reduced the movement of IFT88-GFP puncta in both directions (Figures 6C and 6D). Bidirectional motility and basal body accumulation were restored after dynarrestin washout. These results verify that dynarrestin inhibits dynein 2-dependent IFT. Significantly, dynarrestin inhibits IFT at considerably lower concentrations than those required to inhibit dynein 1. Furthermore, IFT was inhibited by 125 nM dynarrestin, indicating that it is considerably more potent than the recently developed dynein 2-selective ciliobrevins 37 and 47 whose IC50 values are 11 and 16 μM, respectively (See et al., 2016).
Figure 6. Dynarrestin Inhibits Intraflagellar Transport.
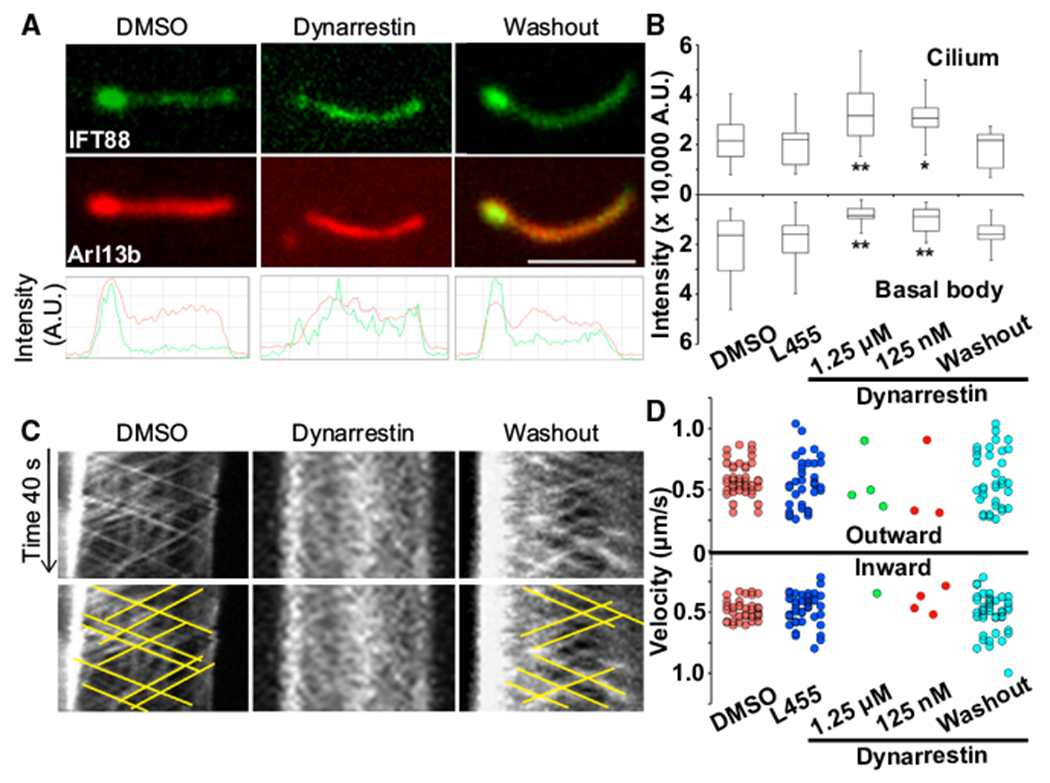
(A) IFT88-GFP (green) and Arl13b-mCherry (red) in primary cilia of serum-starved IMCD3 cells after treatment with 1 % DMSO, 1.25 μM dynarrestin for 1 hr, or 1 hr after dynarrestin washout.
(B) Box-and-whisker plots of the fluorescence intensity of IFT88-GFP in the basal body (lower) and cilia (upper) after 1 hr treatment with 1% DMSO, 1.25 μM L455, 1.25 μM or 125 nM dynarrestin, or after dynarrestin washout (n = 30 cells in two independent experiments). *p < 0.05, **p < 0.01 determined using the Mann-Whitney U test compared with L455 control.
(C) Kymographs illustrating IFT88-GFP particle movements in (A). Yellow lines highlight the moving particles.
(D) Dot plots of IFT88-GFP particle velocities in 600 s observation (60 s/cilium, 10 cilia/condition). Scale bar, 5 μm.
Dynarrestin Blocks Tumor Cell Proliferation Downstream of Smo
Inhibition of the Hh pathway is a clinically validated strategy for combating cancer, but compounds currently in the clinic target Smo, which can acquire mutations that confer drug resistance. Since the Hh pathway in vertebrates depends upon localization and trafficking of Smo and Gli family proteins in cilia, agents that interfere with dynein 2-based protein trafficking are predicted to suppress Hh-dependent cell proliferation at a step downstream of Smo. As proof that dynarrestin can block Hh pathway function in a physiological and clinically relevant context, we evaluated its effects in tumor cells.
We used a mouse medulloblastoma (MB) model in which Hh signaling is upregulated via heterozygous knockout of patched (Ptch−/+), triggering MB (Goodrich et al., 1997). Dynarrestin was tested for the ability to suppress proliferation of primary MB cells derived from these mice (Figure 7A). The Smo inhibitor, vismodegib, inhibited cell proliferation with an IC50 of 0.022 μM. Dynarrestin inhibited proliferation with an IC50 of 0.068 μM. Next, we treated Ptc−/+ MB cells with SAG, a direct activator of Smo. The Smo agonist vismodegib did not inhibit cell proliferation in the presence of SAG (Figure 7A), since vismodegib directly competes with SAG for binding to Smo (Robarge et al., 2009). In contrast, dynarrestin inhibited SAG-induced cell proliferation with an IC50 of 0.35 μM (Figure 7A), demonstrating that dynarrestin inhibits Hh-dependent tumor cell proliferation even when Smo is being directly activated. We performed a binding assay to rule out the possibility that dynarrestin inhibits Smo directly. Membrane preparations from cells expressing Smo were incubated to equilibrium with radiolabeled cyclopamine. Increasing concentrations of a test compound were added. Afterward, the bound and free fractions were separated, and the radioactivity quantified. Vismodegib bound to Smo with an inhibitory constant (Ki) of 17 nM (Figure 7B), but neither ciliobrevin A nor dynarrestin exhibited an interaction (Figure 7B). These results verify that dynarrestin inhibits the Hh pathway without binding to Smo.
Figure 7. Dynarrestin Inhibits Tumor Cell Proliferation Independently of Smoothened Binding.
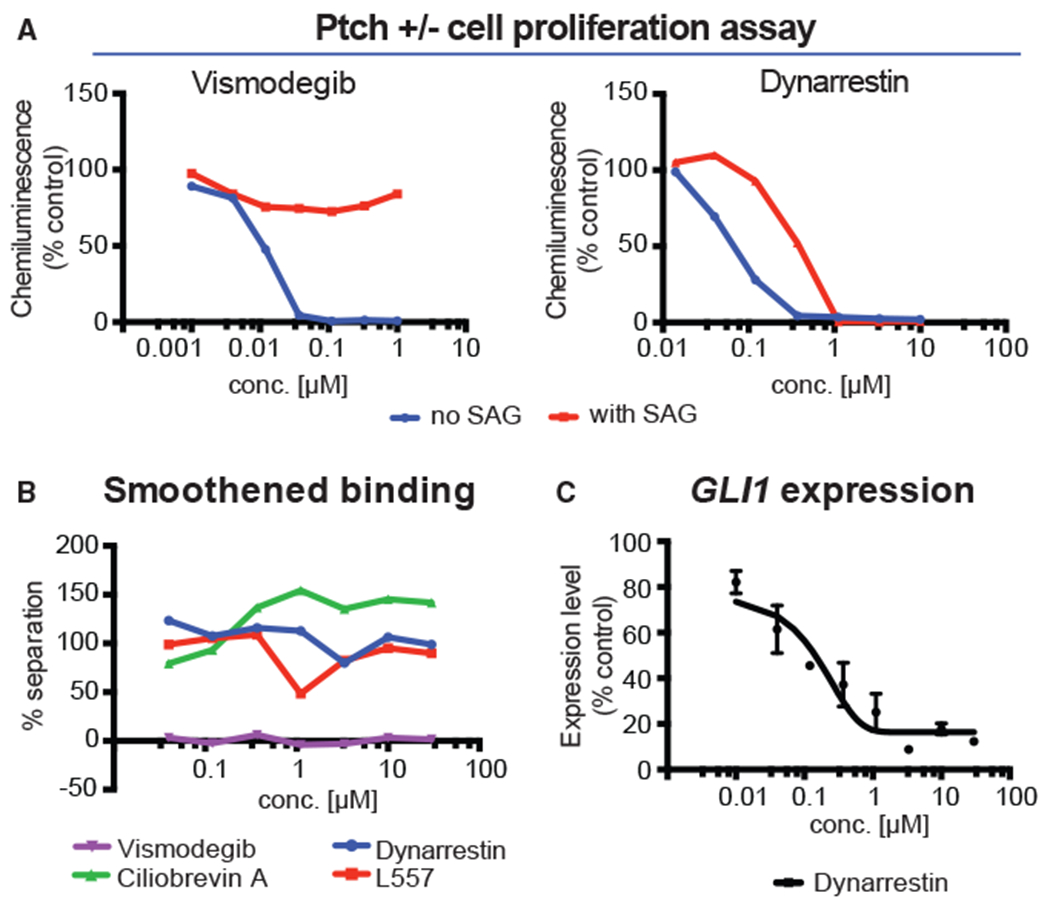
(A) Ptch−/+ medulloblastoma cells were treated as indicated and measured for proliferation.
(B) Smoothened binding assay results for the indicated compounds.
(C) GLI1 expression in KYSE180 cells with error bars indicating SD.
See also Figure S7.
As an additional test of dynarrestin effects on the Hh pathway in cancer cells, we used the human esophageal squamous cell carcinoma cell line KYSE180 that expresses GLI1 in a Hh-dependent manner. GLI1 expression is triggered by the Smo agonist, PMA (Figure 7C). As a control, we treated cells with the Smo inhibitor, vismodegib (Figure S7A). Dynarrestin reduced PMA-stimulated GLI1 expression by up to 90% with an IC50 of 0.21 μM, consistent with the hypothesis that dynarrestin suppresses dynein 2-dependent Hh signaling.
To gain insight into how dynarrestin affects the Hh pathway in human cells, we used a model of human neural development to test if dynarrestin acts downstream of Suppressor of fused (SUFU). Smo activation releases Gli proteins from the repressor SUFU. The activated Gli proteins must then be transported out of the cilium in a dynein 2-dependent process in order to activate Hh-dependent transcription (Dorn et al., 2012). Neuroepithelial stem cells (hNESCs) derived from induced pluripotent stem cells (Reinhardt et al., 2013) exhibit Hh-dependent expression of NKX2.1, an endogenous marker of ventral neural tube progenitor cells. Dynarrestin decreased NKX2.1 expression by approximately 80% compared with cells treated with the Smo agonist PMA alone (Figure S7B). We depleted SUFU, using a lentiviral shRNA vector (vector F12; Figure S7C). Cells depleted of SUFU expressed NKX2.1 in the absence of Hh pathway stimulation (Figure S7D). Dynarrestin and ciliobrevin A both reduced NKX2.1 expression in this context, whereas vismodegib did not, verifying that SUFU blocks the Hh pathway at a step downstream of Smo. To interrogate the pathway further, we determined the ratio of NKX2.1 expression in control versus SUFU-depleted cells treated with dynein inhibitors or vismodegib (Figure S7E). Vismodegib treatment yielded an almost 50-fold increase in NKX2.1 levels in the absence of SUFU, whereas dynarrestin and ciliobrevin A showed increases of only about 3- and 10-fold, respectively. These data demonstrate that dynarrestin acts at a similar step as ciliobrevin A in the Hh pathway, downstream of both Smo and SUFU, most likely in dynein 2-dependent transport of Gli from the tip of the cilium toward the cell body.
DISCUSSION
Here, we report the identification of dynarrestin, an aminothiazole that antagonizes dynein activity and Hh signaling downstream of Smo. The dynein motor domain is a pseudohexameric ring of six divergent AAA+ modules, four of which (AAA1–4) can bind adenine nucleotide (Kon et al., 2004). AAA1 is the major site for ATP hydrolysis, which triggers the major conformational changes associated with the dynein “step.” One other AAA module, AAA3, can hydrolyze ATP, but at about an order of magnitude more slowly than AAA1 (DeWitt et al., 2015). It has been suggested that ciliobrevins inhibit dynein by competing for ATP binding, which would be most consistent with binding to AAA1 (Firestone et al., 2012). Unlike the ciliobrevins, dynarrestin does not inhibit dynein ATPase, but instead permits ATP hydrolysis, suggesting that dynarrestin inhibits dynein via a different mechanism.
Inhibition of dynein 2 has great potential as a therapeutic strategy for patients with diverse cancers. Ciliobrevins provide critical proof of principle that inhibition of dynein is an effective method for inhibiting the Hh signaling cascade, but more specific compounds are needed, particularly compounds that do not disturb ciliogenesis. To our knowledge, this is the first report of a small molecule that inhibits dynein 2-dependent Hh signaling and Smo trafficking without disturbing ciliogenesis, making dynarrestin a powerful tool for probing dynein 2 function that should enable the generation of new drugs targeting Hh signaling-dependent cancers.
It is worth noting that dynarrestin and ciliobrevins have different effects on IFT88 localization; ciliobrevin causes accumulation at the apical tip (See et al., 2016), but dynarrestin results in a more diffuse pattern. Unlike ciliobrevin, dynarrestin may inhibit dynein motor activity by binding outside the motor domain. Our pulldown results suggest that dynarrestin could interact with multiple subunits of the holodynein complex and not just the heavy chain. One of these, the light intermediate chain (LIC; DYNC1LI1) contains a GDP-binding region (Schroeder et al., 2014), raising the possibility that dynarrestin directly interacts with and modulates DYNC1LI1. Like dynein 1, dynein 2 contains LICs (DYNC2LI1) having a nucleoside triphosphate hydrolase domain (Kessler et al., 2015). Primary cilia form in cells with mutations in the DYNC2LI1, but show increased variability in length as well as disrupted Smo trafficking and altered GLI3 processing (Taylor et al., 2015). By contrast, depletion of DYNC2LI1 by siRNA resulted in altered ciliary morphology and a slightly reduced length without altering the number of ciliated cells (Kessler et al., 2015).
That different alterations to the dynein motor yield distinct cellular outcomes opens the possibility for developing a battery of differentially acting dynein-selective compounds in medicinal chemistry programs.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jared Sterneckert (jared.sterneckert@tu-dresden.de).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Lines
All mammalian cell lines were maintained in a humidified incubator at 37°C and with 5% CO2. Hb9::GFP ESCs were maintained feeder-free in ESC medium on gelatin-coated plates. ESC medium consisted of 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 100 μM β-mercaptoethanol (β-ME), 1% nonessential amino acids (NEAA), 5% fetal bovine serum gold (FBS; all PAA Laboratories), and 15% Knockout-Serum Replacement (KO-SR), in Knockout-DMEM (all Invitrogen) supplemented with 2,000 units/ml leukemia inhibitory factor (LIF; Millipore or own production). The sex of Hb9::GFP ESCs is not known and, consequently, not available. Shh-light 2 cells were cultured in DMEM high-glucose containing 10% FCS, 1% Gln, 400μg/ml G418 and 150 μg/ml zeocin (Invitrogen). Shh-Light 2 cells are derived from NIH 3T3 cells, which are male. C3H/10T1/2 cells were cultivated in DMEM high glucose containing 10% heat-inactivated FCS and 1% glutamine. The sex of C3H/10T1/2 ESCs is not known and, consequently, not available.
METHOD DETAILS
Synthesis of Dynarrestin
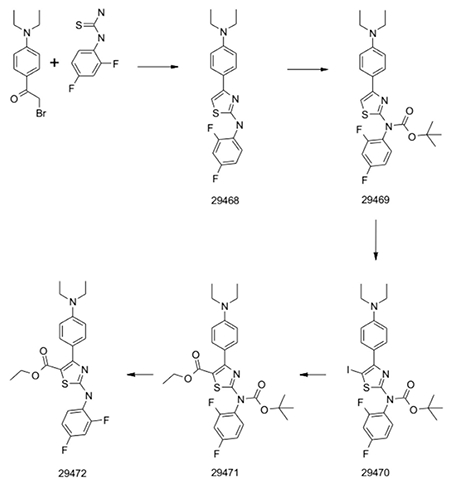
29468
A classical Hantzsch condensation of commercially available building blocks in ethanol at reflux yielded the aminothiazole compound. >90% purity and quantitative yield without the need of any purification.
29469
Boc protection was carried out using an excess of Boc2O, Et3N and catalytic DMAP. After careful work up, the product was isolated with 80% yield and >95% purity.
29470
Iodination with NIS in DMF at 0°C showed full consumption of the starting material within 2 hours. After work up, a crude was isolated with ~90% purity and directly used in the carbonylation.
29471
Carbonylation was achieved using PdCl2dppf (10 mol%), EtOH and Et3N in DMF. All the material was put in a microwave vial that was closed and cooled down with liquid nitrogen under a nitrogen atmosphere. CO gas was introduced via a needle and the mixture stirred at room temperature overnight. A second CO addition and reaction over night was necessary to reach >90% conversion of the starting material. After workup and purification by chromatography on silica yield was 55%.
29472
For Boc deprotection the starting material was heated in 1.25M HCl/EtOH for 1h. Solvent was removed under reduced pressure. The crude product was distributed between ethyl acetate and saturated NaHCO3 solution. The organic phase was separated, dried and concentrated. Purification by chromatography on silica yield the final product which was recrystallized from water/acetonitrile.
Handling Dynarrestin
We recommend that researchers store dynarrestin as a powder at 4°C in the dark and prepare fresh DMSO stocks for single use. Because of stability problems, dynarrestin tends to go bad when stored at −20. It tends to become discolored and generate high fluorescent background signal, which can be convenient indicators. In addition, due to solubility issues, dynarrestin can sometimes form crystal-like structures when visualized through a microscope. For this reason, it is recommended that serial dilutions be performed in DMSO.
Motor Neuron Differentiation Assay
Hb9::GFP ESCs were differentiated into MNs as described in Wichterle et al. (2002). To form embryoid bodies (EBs), single-cell ESCs were plated on petri dishes at a density of 2x105 cells/ml in DK10 medium. DK10 medium consisted of DMEM/F-12 (1:1), 10% KO-SR, 100 μM β-ME, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% NEAA. On day 2, 5 EBs were manually plated in 1 well of a 96 well plate in DK10 medium containing 1 μM retinoic acid (Sigma-Aldrich) and 1 μg/ml purmorphamine (Alexis Biochemicals). GFP intensity was measured daily for 6 days using a plate reader.
Shh-Light 2 Assay
For the 384 well assay 8000 Shh-Light 2 cells per well were seeded in white 384 well plates in a total volume of 25μl DMEM high glucose containing 10% FCS, 1% glutamine and 5mM Hepes pH7.4. After culturing the cells for 24h at 37°C, compounds were added automatically by Echo Liquid Handler (Labcyte). After a further 1h incubation at 37°C 10 μl of purmorphamine with a final concentration of 1 μM were added to stimulate the hedgehog-pathway. The plates were incubated for 48h at 37°C and assayed for luciferase activity using the One Glo reporter kit (Promega). Wells containing 10 μM vismodegib were used as a positive control.
Cell Titer Glo Assay (Shh-Light 2 Cells)
To assay the toxicity of the compounds on shh-light 2 cells, 600 shh-light 2 cells per 384- well were seeded in 25 μl DMEM high glucose containing 10% FCS, 1% glutamine and 5 mM Hepes pH 7.4. After culturing the cells for 24h at 37°C, the compounds were added automatically by Echo Liquid Handler (Labcyte). 1h later 10μl purmorphamine was added with a final concentration of 1 μM. The cells were incubated for further 48h at 37°C and finally assayed for viability using the Cell Titer Glo Assay (Promega). Wells containing 10 μM staurosporine were used as a positive control.
Osteogenesis Assay
The murine stem cell line C3H/10T1/2 (ATCC CCL-226) was cultivated in DMEM high glucose containing 10% heat-inactivated FCS and 1% glutamine. The assay was performed with C3H/10T1/2 cells from the passage numbers 15 up to 20 as follows: 800 cells per well were seeded in white 384 well plates in cell culture medium and incubated for 24h at 37°C. The compounds were added automatically by Echo Liquid Handler (Labcyte) and – after 1 h incubation – cells were stimulated for differentiation by 10μl 1μM purmorphamine (final concentration 1 μM). Cells were incubated for 96 h at 37°C to differentiate into osteoblasts. To measure the alkaline phosphatase activity of the cells as differentiation rate, supernatant was removed and cells were lysed with 35 μl lysis buffer per well (100 mM Tris pH 9.5, 250 mM NaCl, 25 mM MgCl2, 1% Triton X-100) containing the alkaline phosphatase substrate “CDP-Star” (Roche; final conc. 250 μM). After 1h incubation in the dark luminescence was measured to determine the differentiation rate of the cells.
Cell Titer Glo Assay (C3H/10T1/2 Cells)
To assay the toxicity of the compounds on C3H/10T1/2 cells, 150 cells per 384 well were seeded in 25 μl of DMEM high glucose containing 10% FCS, 1% glutamine. After cultivating the cells for 24 h at 37°C, the compounds were added automatically by Echo Liquid Handler (Labcyte). 1 h later 10 μl purmorphamine was added with a final concentration of 1 μM. The cells were incubated for further 48 h at 37°C and finally assayed for viability using the Cell Titer Glo Assay (Promega). Wells containing 10 μM staurosporine were used as a positive control.
Quantitative RT-PCR (KYSE180 Cells)
Kyse180 cells, which are male, were seeded into 6-well plates (160,000 cells/well) and cultured for 24 h in RPMI containing 10% FCS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. After 24 h the cells were cultured further in RPMI containing 0.5% FCS, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.4 μM purmorphamine or 0.2 μM Smo agonist (SAG) together with various concentrations of dynarrestin or vismodegib. Related 8 point 1:3 serial dilutions starting from 30 μM or 10 μM were prepared by diluting 100 mM DMSO stock solutions (final DMSO concentration 0.1%). Each concentration was tested in triplicate. In order to control for the effects of DMSO, DMSO was added to all control wells. After 96 h cells were harvested in 300 μL RNAprotect cell reagent (Qiagen) and RNA was extracted and cDNA synthesized according to manufacturer’s protocols (RNeasy Mini Kit with DnaseI on column digestion, Qiagen and High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor, Applied Biosystems). Gli1 and the endogenous control TBP transcript levels were quantified with Taqman probes (Hs01110766_m1 and Hs00427620_m1) and TaqMan Fast Advanced Master Mix on a StepOne Plus Real-Time PCR system (all Applied Biosystems). Data was analyzed using the ΔΔCt method to determine fold-changes for each compound condition relative to the maximum pathway activation associated with purmorphamine or SAG treatment alone normalized with respect to TBP. IC50 values were calculated using the 4 parameter logistic model (ExcelFit).
Viability Assay of Ptch1+/− Medulloblastoma Cells
All animal studies were performed by Exiris using their mice and protocols. Medulloblastoma tumors, derived from cerebella of post-natal irradiated Ptch1+/− mice, were serially passaged in vivo subcutaneously. Information regarding sex of the mice and animals is not available. When tumor volumes reached between 400 and 1000 mm3, mice were sacrificed and tumors explanted to obtain single cell suspensions by mechanical and enzymatic dissociation treatment with Collagenase XI (Sigma) and Hyaluronidase (Sigma). Single cells were resuspended in NPMM medium (Neural Progenitor Basal Medium) supplemented with growth factors (Lonza). Viable cells were counted and diluted with complete NPMM medium in order to plate 10000 viable cells per well in a 96 well plate. Cells were incubated in the presence or in the absence of 0.3 μM of SAG. After 72 hrs of incubation, BrdU was added for incorporation for additional 24 h. At the end of the incubation time the number of viable cells was determined using the Cell Proliferation ELISA BrdU chemiluminescent assay, essentially by following the manufacturer’s recommendations. Cell proliferation was determined as percentage of residual BrdU incorporation of compound-treated cells vs. DMSO-treated cells and IC50 values were computed by fitting the experimental data with a three-parameter logistic equation using KaleidaGraph 3.5.
Smo Receptor Binding Assay
Radioligand binding assays were performed in polypropylene 96 well plates by SB Drug Discovery. Compounds were tested at 30 μM, 10 μM, 3.33 μM, 1.11 μM, 0.37 μM, 0.12 μM, and 0.04 μM with a single well tested for each concentration. Membrane preparations from Smo cell line (Multispan Inc) were added to plates at a concentration of 14 μg per well and incubated with 7 nM [3H] Cyclopamine until equilibrium was reached. The separation of bound from free radioligand was carried out using a Packard Filtermate Harvester and glass filter plates. Radioactivity was quantified using a Packard Topcount.
Affinity-based Chemical Proteomics
C3H10T1/2 cells were lysed in a lysis buffer containing 50 mM PIPES (pH 7.4), 50 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 0.1% NP40, 0.1% Triton X-100,0.1% Tween 20, protease and phosphatase inhibitors and 1mM DTT. Streptavidin magnetic beads (New England BioLabs) were washed with PBS and then incubated with 10 μM of the biotinylated compounds in PBS for 30 min at room temperature. Immobilized probes were washed with PBS prior to incubation with the cell lysate (total protein amount 1.25 mg) for 1 h at 4°C. The beads were washed twice with lysis buffer that was supplemented with 75 mM MgCl2 followed by washing two times with PBS. Enriched proteins were leased from the matrix by boiling the samples in SDS sample buffer. Supernatants were loaded on a 4-20% SDS-polyacrylamide gel for protein separation. Gels were stained Colloidal Coomassie, cut into 10-15 subsequent slices per lane, and subjected to in-gel digestion (Shevchenko et al., 2006). Extracted peptides were then desalted using Empore-C18 StageTips (Rappsilber et al., 2003). For LC-MSMS analysis peptides were eluted twice with 20μl of 80% ACN, 0.5% acetic acid each, dried in an Eppendorf concentrator to a volume of about 2μl and resuspended in 10 μl Buffer A (0.5% acetic acid). Peptides present in 6 μl of this solution were subsequently separated by reversed-phase chromatography using an EASY nLC HPLC system and in-house packed fused silica emitter columns (columns 15 cm × 75 μm ID by New Objective, Woburn, MA, USA; 3μm C18 particles by Dr. Maisch, Ammerbuch-Entringen, Germany) that were online coupled via a NanoFlex ESI source (Thermo Scientific) to a Orbitrap Velos mass spectrometer (Thermo Scientific). Peptides were separated at a flow rate of 250 nl/min using a 2-segment gradient from 0-40% B (0.5% acetic acid in 80% acetonitril) in 70min and from 40 – 98% B in 5 min). The mass spectrometer was operated in data-dependent mode, acquiring full scan spectra at a resolution of 60.000 and an AGC target value of 3e6 (scan range 300 - 1650 m/z). The 15 most intense ions were chosen for collisional induced dissociation (CID). Dynamic exclusion was allowed and set to 30 sec (list size 500; duration 90 sec), uncharged as well as singly charged compounds were excluded from the analysis. Data were recorded with Xcalibur software (Thermo Scientific).
MS raw files were processed using the MaxQuant computional platform (version 1.5.2.8) (Cox and Mann, 2008). Identification of peptides and proteins was enabled by the built-in Andromeda search engine by querying the concatenated forward and reverse HUMAN Uniprot database (version from November 2012), including common contaminants. The allowed initial mass deviation was set to 7ppm and 20ppm in the search for precursor and fragment ions, respectively. Trypsin with full enzyme specificity and only peptides with a minimum length of 7 amino acids were selected. A maximum of two missed cleavages was allowed. Carbamidomethylation (Cys) was set as fixed modification, while Oxidation (Met) and N - acetylation were defined as variable modifications. For protein and peptide identification a minimum false discovery rate (FDR) of 1% was required.
Relative label free quantification was based on the measurements of 4 independent pulldown experiments with each compound (2 replicates for L357) and was performed using the LFQ algorithm of MaxQuant with the ‘match between runs’ option turned on. Data processing was performed using the Perseus module of MaxQuant version 1.5.1.6. The output file (proteinGroups.txt) was filtered for reverse and contaminant hits as well as proteins that were identified by a modified site only. Proteins included in the analysis had to be identified with at least 2 different peptide sequences, one of these being unique for the protein group. Proteins that could not be discrimiminated by the presence of unique peptides were grouped together. Intensity values were logarithmized and missing values (NaN) were replaced by imputation, simulating signals of very low abundant proteins within the distribution of measured values. A width of 0.3 SD and a downshift of 1.8 SD was used for this purpose(Hubner et al., 2010). To identify proteins that significantly stand out in a groupwise comparison the ANOVA function of Perseus was applied (p = 0.05). Principal component analysis was performed on the list of significantly changed proteins to project the proteome measurements of the different compounds onto a two-dimensional data space. Hierarchical clustering (rows: proteins; columns: compounds) was performed using default values of Perseus (Euclidian distance; average linkage; pre-processing with K-means) subsequent to normalization of the matrix of significantly changed proteins by Z-transformation.
Pull Down of Purified Dynein Complex
Active (L568) and inactive (L527) pulldown probe were immobilized on streptavidin magnetic beads (Dynabeads M-280 Streptavidin) and incubated with purified bovine dynein (45 nM) in buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100). After extensive washing, beads were boiled in 2x Lämmli buffer and analyzed using SDS-PAGE and Immunoblotting. Bound dynein complex was identified using a specific antibody against dynein intermediate chain (IC74, Abcam MAB1618; 1:2000).
Quantification of Ciliogenesis
NIH/3T3 cells were maintained in standard DMEM containing 10% fetal calf serum, sodium pyruvate and penicillin/streptomycin. For induction of ciliogenesis cells were incubated under low serum conditions (fully supplemented DMEM with 0.5% FCS) in the presence of dynarrestin or controls. After 24 h cells were fixed with 4% Paraformaldehyde in PBS, permeabilised with 0.1% Triton X-100 and stained for acetylated-alpha-Tubulin (Sigma T6793, 1:5000) and DNA (DAPI 1μg/ml). Samples were analyzed on a Zeiss Observer Z1 (Carl Zeiss, Germany) and a Plan Apochromat 63x/1.40 Oil DIC M27 objective. For the quantification, random pictures at 63x magnification were acquired and afterwards analyzed for ciliary area/ cell or ciliated cells/ all cells using ImageJ.
For live imaging of IFT movement in primary cilia, 107 mouse IMCD3 cells were resuspended in 0.5 ml OPTI-MEM and electroporated with 10 μg plasmid DNAs (IFT88–GFP and ARL13b–mCherry in a pCAG-based expression vector, kindly provided by Kathryn Anderson (He et al., 2014) at 240 V using a BTX electro cell manipulator 600. Three h after transfection, 1.5×106 cells were replated onto an alcian blue-coated glass-bottom dish (MatTek) and grown for 18 h, then serum starved (0.2% FBS) for an additional 24 h to induce ciliogenesis. Images were acquired at the speed of 0.25 s per frame for 60 s using a Plan Apochromat 100× NA 1.4 DIC objective on a Yokogawa CSU-X1 spinning-disk confocal microscope (AxioObserver; Zeiss) with 488 diode lasers and an Evolve EMCCD camera (Photometrics) in a 37°C environmental chamber. Movies were generated in Zen2 and kymographs were produced and analyzed using ImageJ.
Smo Trafficking Assay
NIH-3T3 cells (DSMZ, ACC 59) were cultured in DMEM supplemented with 10 % fetal calf serum (heat-inactivated), 2 mM L-glutamine and 1 mM sodium pyruvate. To detect trafficking of Smo, cells were seeded on cover slips (Thermo Scientific, 12 mm) placed in 24-well plates (Sarstedt). On the next day, the medium was exchanged for assay medium (DMEM, high glucose, supplemented with 0.5 % heat-inactivated fetal calf serum) to induce ciliogenesis. Cells were treated with the compounds, which were diluted in assay medium, for 2, 4 and 8 h. After a total incubation time of 24 h in the assay medium, cells were fixed in buffer (4 % ice-cold paraformaldehyde in PBS) for 10 min, washed three times with ice-cold PBS and incubated in blocking solution (1 % heat-inactivated horse serum, 0.1 % Triton-X-100 in PBS) for 30 min at room temperature. Cells were incubated with the primary antibodies diluted in blocking solution overnight at 4°C. Anti-Smo antibody (abcam, ab38686), and anti-acTubulin antibody (Sigma, T6793) were used to visualize the Smo receptor and the primary cilia, respectively. Cells were then washed three times with 0.1 % Triton-X-100 in PBS and incubated with secondary antibodies and DAPI in blocking solution for 45 min at room temperature. A donkey anti-mouse Alexa488-conjugated antibody (Invitrogen, A-21202) and a goat anti-rabbit Alexa594-conjugated antibody (Invitrogen, A-11012) were used. Afterwards, cells were washed two times in 0.1 % Triton X100 in PBS and once in PBS and mounted on glass slides. Imaging was performed with a Deltavision Elite System (GE Healthcare, UK) equipped with an IX-71 inverted microscope (Olympus, Japan), UPLanSApo 100x/1.4 NA (Olympus) and a pco.edge sCMOS camera (PCO-TECH Inc., USA). Images were acquired as Z-sections (using the softWoRx software from Deltavision) and converted into maximal intensity projections TIFF files for illustrative purposes. A Zeiss Observer Z1 (Carl Zeiss, Germany) with a 63x objective (LD Plan-Neofluar 63x/0.75 Corr Ph2 M27) was used for image acquisition as well. The staining protocol was adopted (with modifications) from Rohatgi et al. (Rohatgi et al., 2007). Using Adobe Photoshop, the average Smo immunostaining intensity was quantified for manually selected acTubulin-positive cilia.
Immunofluorescence Microscopy and Image Analysis of Mitotic Cells
Cell culture and immunostaining of fixed Cos7 cells were performed as described (Yeh et al., 2012). Cells were fixed with −20°C methanol and stained for microtubules (YL1/2, Serotec) and dynein intermediate chain (DIC74.1, Millipore) or microtubules (DM1A, Sigma) and pericentrin (Abcam) with DAPI. Fixed samples on glass slides were imaged using a 100×, NA 1.4 oil immersion objective on a Yokogawa CSU-X1 spinning-disk confocal microscope (AxioObserver; Zeiss) with 405, 488, and 561 diode lasers, and an Evolve EMCCD camera (Photometrics). 0.27 μm image planes were collected then image stacks were processed to measure α (the angle between the spindle axis and the coverslip) and D (pole-to-pole distance) using Zen2 imaging software before export to Image J. Mitotic stages were determined on the basis of chromosome configuration as described previously (Yeh et al., 2013).
In Vitro Motility Assay
Porcine brain tubulin and TAMRA rhodamine-labeled tubulin were purchased from Cytoskeleton, Inc. Bovine brain dynein was purified as described (Bingham et al., 1998). Rhodamine-labeled microtubules (10% TAMRA rhodamine-labeled) were polymerized at 37° C for 20 min. Each motility assay sample contained rhodamine-labeled microtubules (10 μg/ml) and dynein (40 μg/ml) in motility buffer (25 mM imidazole, pH 7.4, 50 mM KCl, 1 mM EGTA, 4 mM MgCl2, 2 mM β-mercaptoethanol, 1 mM GTP, 20 μM taxol, 2 mM ATP) and an oxygen scavenging mixture (50 μg/ml catalase, 126 μg/ml glucose oxidase, 3 mg/ml D-glucose), with dynarrestin or 2.5% DMSO as a control. Motility assays were performed in glass slide–coverslip chambers made by placing a #0 coverslip (Gold Seal cover glass, Clay Adams) on two strips of double stick tape set about 5 mm apart on a slide to make a perfusion chamber with a 70-μm-thick channel. 20 μL of reaction mixture was loaded in the chamber for each assay. The chambers were kept at room temperature for 20 min, then moved to a cage incubator at 37° C for 10 min prior to imaging. Images were acquired at the speed of 0.5 s per frame for 50 s using a Zeiss alpha Plan Fluar 100× NA 1.45 oil objective on a total internal reflection fluorescence (TIRF) microscope (3i Marianas) with 561 nm laser and Cascade II 512 EMCCD camera (Photometrics). All motility assays were performed within 30-60 min after sample preparation.
Live Cell Imaging of α2M-Labeled Endosome Movement
Human α2-macroglobulin (α2M) (Calbiochem, San Diego, CA) was conjugated with Alexa Fluor 555 using an Invitrogen protein-labeling kit according to the manufacturer’s instructions. Cos7 and IMCD3 cells were fed 100 μg/ml Alexa555-α2M for 30 min and chased for 15 min before adding 25 μM dynarrestin or 1% DMSO. Time-lapse movies were imaged in a 37° C cage incubator between 60 and 90 min after drug addition. Images were acquired at the speed of 0.5 s per frame for 50 s using a 100× NA 1.4 oil objective on a Yokogawa CSU-X1 spinning-disk confocal microscope (AxioObserver; Zeiss) with 561 diode lasers and an Evolve EMCCD camera (Photometrics). To determine dynarrestin reversibility, cells were treated as above for 1 h, then the cells were rinsed, the medium was replaced and live cell imaging was performed starting 1 h later. Displacements and velocities of moving α2M particles were analyzed as described previously (Yeh et al., 2012). All motile particles in 10 independent drug-treated or control cells were analyzed.
TMRE Assay
Cos7 cells were fed 25 μM dynarrestin, ciliobrevin A, ciliobrevin D, or 1% DMSO. After 1 h 200 nM TMRE was added for 15 minutes followed by medium chase for 30 min. Cells were imaged in a 37° C cage incubator using a 100× NA 1.4 oil objective on a Yokogawa CSU-X1 spinning-disk confocal microscope (AxioObserver; Zeiss) with 561 diode lasers and an Evolve EMCCD camera (Photometrics).
Dynein ATPase Assay
Cytoskeleton performed the dynein ATPase assays in 96 well plates. Dynein motor complex (DMC) and tubulin were isolated from porcine brain. Tubulin was polymerized to form microtubules. “Motor mix” was obtained by mixing: 3.1 ml 12 mM Pipes-NaOH pH 7.0, 2 mM MgCl2, 10 μM Tx (PM12 Buffer), 0.4 ml 2.0 mg/ml tubulin as microtubules (in PM12 Buffer), 0.1 ml 1000 μg/ml Dynein protein (CS # CS-DN01), and 0.4 ml 2 mM ATP. The reaction is slow at room temperature and hence the motor mix is prepared at room temperature and used within 2min. 96 well half area plate (Corning Cat. # 3697) containing 1.5 μl compound in 100% DMSO at room temp. 30 μl of motor mix was pipetted directly into the well containing compound. The wells were sealed and place the plate in the 37°C incubator and incubate for 60 min. 120 μl of Malachite green solution (Cat. # BK054) was pipetted into each well and incubated for exactly 10min at room temperature followed by measuring the optical density at 650 nm.
QUANTIFICATION AND STATISTICAL ANALYSIS
The large sample sizes resulting from the intraflagellar transport and mitotic spindle movement assays enabled significance testing independent of assuming a normal distribution using the Mann-Whitney U-test. For all other tests, a normal distribution was assumed and significance was tested according to two-tailed Student’s t-test At least three independent experiments were performed in each case (n ≥3), as detailed in the relevant figure legends. Significance is indicated within figures as * signifying p<0.05; ** signifying p<0.01; *** signifying p<0.001.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dynarrestin | This paper | N/A |
| Vismodegib | Selleckchem | Cat#S1082 |
| Purmorphamine | Cayman Chemical | Cat#10009634 |
| CDP-Star | Roche | Cat#12041677001 |
| Smoothened agonist | Cayman Chemical | Cat#11914 |
| Rhodamine-labeled tubulin | Cytoskeleton | Cat#TL590M |
| Human α2-macroglobulin | Calbiochem | Cat#441251 |
| Ciliobrevin A | Sigma | Cat#H4541 |
| Ciliobrevin D | Calbiochem | Cat#250401 |
| Tetramethylrhodamine, ethyl ester (TMRE) | ThermoFisher Scientific | Cat#T669 |
| Experimental Models: Cell Lines | ||
| Hb9::GFP mouse ESCs | (Höing et al., 2012) | N/A |
| Shh-light 2 cells | ATCC | ATCC JHU-68 |
| C3H/10T1/2 | ATCC | ATCC CCL-226 |
| Cos-7 cells | ATCC | ATCC CRL-1651 |
| IMCD3 cells | ATCC | ATCC CRL-2123 |
| NIH-3T3 cells | DSMZ | DSMZ ACC 59 |
| Antibodies | ||
| Rabbit anti-Smoothened | Abcam | Cat#ab38686; RRID: AB_882615 |
| Mouse anti-acTubulin | Sigma | Cat#T6793; RRID: AB_477585 |
| Goat anti-Rabbit Alexa594 | Invitrogen | Cat#A11012; RRID: AB_10562717 |
| Donkey anti-Mouse Alexa488 | Invitrogen | Cat#A21202; RRID: AB_141607 |
| Rat anti-Tubulin | Serotec | Cat#YL1/2; RRID: AB_325003 |
| Mouse anti-Dynein | Millipore | Cat#DIC74.1; RRID: AB_2246059 |
| Mouse anti-Tubulin | Sigma | Cat#DM1A; RRID: AB_477593 |
| Recombinant DNA | ||
| pCAG-IFT88–GFP | (He et al., 2014) | N/A |
| pCAG-ARL13b–mCherry | (He et al., 2014) | N/A |
| Critical Commercial Assays | ||
| One Glo | Promega | Cat#E6110 |
| Cell Titer Glo | Promega | Cat#G7570 |
Highlights.
Dynarrestin inhibits the Hedgehog-signaling pathway downstream of SUFU
125 nM dynarrestin is sufficient to inhibit ciliary transport
Specific inhibition of dynein in a novel manner independent of ATP hydrolysis
Dynarrestin inhibits proliferation of primary tumor cells
SIGNIFICANCE.
Aberrant Hh signaling contributes to oncogenesis. Available inhibitors target Smo, which can acquire mutations causing drug resistance. Inhibition of cytoplasmic dynein 2 is an effective strategy for inhibiting Hh-dependent tumors downstream of Smo. We identified dynarrestin, a novel inhibitor of cytoplasmic dyneins 1 and 2. Dynarrestin inhibits dynein 2-dependent IFT over 10x more potently, and with considerably less toxicity, compared with ciliobrevins, the only previously described inhibitors. As proof of principle, we show that dynarrestin inhibits Hh-dependent signaling and proliferation of primary and human tumor cells; 25 μM dynarrestin is sufficient to inhibit cytoplasmic dynein 1-dependent processes, including endosome movement, mitosis, and microtubule gliding without inducing mitochondrial toxicity or having off-target effects on protein kinases. Dynarrestin uses a novel mechanism of action, which reversibly inhibits dynein 1 without affecting ATP hydrolysis. Finally, we show that 125 nM dynarrestin is sufficient to reversibly inhibit IFT, suggesting that it inhibits dynein 2 more potently than dynein 1, as also is seen for the ciliobrevins. Dynarrestin opens the possibility of developing a battery of differentially acting dynein-selective compounds in future medicinal chemistry programs. As such, dynarrestin is a valuable tool for probing cytoplasmic dynein-dependent cellular processes, making it of considerable interest to cell biologists, as well as a promising lead compound for medicinal chemistry programs aimed at development of anti-cancer drugs.
ACKNOWLEDGMENTS
We gratefully acknowledge financial support from the Max Planck Society as well as the Deutsche Forschungsgemeinschaft (DFG) and the CRTD/TUD. This work was financed by DFG-Research Center (DFG FZ 111) and Cluster of Excellence (DFG EXC 168). In addition, the work was supported by funding from the Krieger School of Arts & Sciences, Johns Hopkins University. Michael Glatza was supported by a fellowship from the Hans and Ilse Breuer-Stiftung. We thank Kerstin Hergarten, Rhea Brintrup, and Thomas Sladewski for technical assistance; Tanya Levin for manuscript editing; Kathryn Anderson and Takanari Inoue for reagents; and Matthew Berezuk for the kinesin-1 preparation.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and one table and can be found with this article online at https://doi.org/10.1016/j.chembiol.2017.12.014.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, and Hooper JE (1996). The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86, 221–232. [DOI] [PubMed] [Google Scholar]
- Bingham JB, King SJ, and Schroer TA (1998). Purification of dynactin and dynein from brain tissue. Methods Enzymol. 298, 171–184. [DOI] [PubMed] [Google Scholar]
- Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, and Nachury MV (2013). An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. J. Cell Biol 203, 129–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, and Witman GB (2014). Cilia and diseases. Bioscience 64, 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift D, and Schuh M (2015). A three-step MTOC fragmentation mechanism facilitates bipolar spindle assembly in mouse oocytes. Nat. Commun 6, 7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, and Reiter JF (2005). Vertebrate Smoothened functions at the primary cilium. Nature 437, 1018–1021. [DOI] [PubMed] [Google Scholar]
- Cox J, and Mann M (2008). MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol 26, 1367–1372. [DOI] [PubMed] [Google Scholar]
- Cupido T, Rack PG, Firestone AJ, Hyman JM, Han K, Sinha S, Ocasio CA, and Chen JK (2009). The imidazopyridine derivative JK184 reveals dual roles for microtubules in Hedgehog signaling. Angew. Chem. Int. Ed 48, 2321–2324. [DOI] [PubMed] [Google Scholar]
- Das J, Chen P, Norris D, Padmanabha R, Lin J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, et al. (2006). 2-aminothiazole as a novel kinase inhibitor template. Structure-activity relationship studies toward the discovery of N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1- piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carboxamide (dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J. Med. Chem 49, 6819–6832. [DOI] [PubMed] [Google Scholar]
- DeWitt MA, Cypranowska CA, Cleary FB, Belyy V, and Yildiz A (2015). The AAA3 domain of cytoplasmic dynein acts as a switch to facilitate microtubule release. Nat. Struct. Mol. Biol 22, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn KV, Hughes CE, and Rohatgi R (2012). A Smoothened-Evc2 complex transduces the Hedgehog signal at primary cilia. Dev. Cell 23, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone AJ, Weinger JS, Maldonado M, Barlan K, Langston LD, O’Donnell M, Gelfand VI, Kapoor TM, and Chen JK (2012). Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 484, 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MM, and Holzbaur EL (2014). Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 24, 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, and Scott MP (1997). Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113. [DOI] [PubMed] [Google Scholar]
- Granger E, McNee G, Allan V, and Woodman P (2014). The role of the cytoskeleton and molecular motors in endosomal dynamics. Semin. Cell Dev. Biol 31, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SP, Welte MA, Block SM, and Wieschaus EF (2002). Coordination of opposite-polarity microtubule motors. J. Cell Biol 156, 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Subramanian R, Bangs F, Omelchenko T, Liem KF Jr., Kapoor TM, and Anderson KV (2014). The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat. Cell Biol 16, 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höing S, Rudhard Y, Reinhardt P, Glatza M, Stehling M, Wu G, Peiker C, Bocker A, Parga JA, Bunk E, et al. (2012). Discovery of inhibitors of microglial neurotoxicity acting through multiple mechanisms using a stemcell-based phenotypic assay. Cell Stem Cell 11, 620–632. [DOI] [PubMed] [Google Scholar]
- Hou Y, and Witman GB (2015). Dynein and intraflagellar transport. Exp. Cell Res 334, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Roberts AJ, Leschziner AE, and Reck-Peterson SL (2012). Lis1 acts as a “clutch” between the ATPase and microtubule-binding domains of the dynein motor. Cell 150, 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, and Anderson KV (2005). Cilia and Hedgehog responsiveness in the mouse. Proc. Natl. Acad. Sci. USA 102, 11325–11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, Hyman A, and Mann M (2010). Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J. Cell Biol 189, 739–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH Jr., et al. (1996). Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272, 1668–1671. [DOI] [PubMed] [Google Scholar]
- Kessler K, Wunderlich I, Uebe S, Falk NS, Gießl A, Brandstätter JH, Popp B, Klinger P, Ekici AB, Sticht H, et al. (2015). DYNC2LI1 mutations broaden the clinical spectrum of dynein-2 defects. Sci. Rep 5, 11649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinto N, Iwamoto M, Enomoto-Iwamoto M, Noji S, Ohuchi H, Yoshioka H, Kataoka H, Wada Y, Yuhao G, Takahashi HE, et al. (1997). Fibroblasts expressing Sonic hedgehog induce osteoblast differentiation and ectopic bone formation. FEBS Lett. 404, 319–323. [DOI] [PubMed] [Google Scholar]
- Kiyomitsu T, and Cheeseman IM (2013). Cortical dynein and asymmetric membrane elongation coordinately position the spindle in anaphase. Cell 154, 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon T, Nishiura M, Ohkura R, Toyoshima YY, and Sutoh K (2004). Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry 43, 11266–11274. [DOI] [PubMed] [Google Scholar]
- Lai K, Kaspar BK, Gage FH, and Schaffer DV (2003). Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci 6, 21–27. [DOI] [PubMed] [Google Scholar]
- Lee J, Wu X, Pasca di Magliano M, Peters EC, Wang Y, Hong J, Hebrok M, Ding S, Cho CY, and Schultz PG (2007a). A small-molecule antagonist of the hedgehog signaling pathway. ChemBioChem 8, 1916–1919. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kawagoe R, Sasai K, Li Y, Russell HR, Curran T, and McKinnon PJ (2007b). Loss of suppressor-of-fused function promotes tumorigenesis. Oncogene 26, 6442–6447. [DOI] [PubMed] [Google Scholar]
- Liem KF Jr., Jessell TM, and Briscoe J (2000). Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development 127, 4855–4866. [DOI] [PubMed] [Google Scholar]
- Lin TL, and Matsui W (2012). Hedgehog pathway as a drug target: smoothened inhibitors in development. Onco Targets Ther. 5, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, and McMahon AP (2004). Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development 137, 1309–1318. [DOI] [PubMed] [Google Scholar]
- Marti E, Bumcrot DA, Takada R, and McMahon AP (1995). Requirement of 19K form of Sonic hedgehog for induction of distinct ventral cell types in CNS explants. Nature 375, 322–325. [DOI] [PubMed] [Google Scholar]
- Martin M, Iyadurai SJ, Gassman A, Gindhart JG Jr., Hays TS, and Saxton WM (1999). Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell 10, 3717–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May SR, Ashique AM, Karlen M, Wang B, Shen Y, Zarbalis K, Reiter J, Ericson J, and Peterson AS (2005). Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol 287, 378–389. [DOI] [PubMed] [Google Scholar]
- Oinas A, Taipale J, and Lahdenperä J (2006). Mammalian hedgehog signaling inhibitors. Patent WO2007054623A2, filed November 10, 2006, and published May 18, 2007.
- Pfarr CM, Coue M, Grissom PM, Hays TS, Porter ME, and McIntosh JR (1990). Cytoplasmic dynein is localized to kinetochores during mitosis. Nature 345, 263–265. [DOI] [PubMed] [Google Scholar]
- Pomeroy SL, Tamayo P, Gaasenbeek M, Sturla LM, Angelo M, McLaughlin ME, Kim JY, Goumnerova LC, Black PM, Lau C, et al. (2002). Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 415, 436–442. [DOI] [PubMed] [Google Scholar]
- Rappsilber J, Ishihama Y, and Mann M (2003). Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem 75, 663–670. [DOI] [PubMed] [Google Scholar]
- Reinhardt P, Glatza M, Hemmer K, Tsytsyura Y, Thiel CS, Hoing S, Moritz S, Parga JA, Wagner L, Bruder JM, et al. (2013). Derivation and expansion using only small molecules of human neural progenitors for neurodegenerative disease modeling. PLoS One 8, e59252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, Gould SE, Guichert O, Gunzner JL, Halladay J, et al. (2009). GDC-0449-a potent inhibitor of the hedgehog pathway. Bioorg. Med. Chem. Lett 19, 5576–5581. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, and Jessell TM (1995). Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell 81, 445–455. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, and Scott MP (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372–376. [DOI] [PubMed] [Google Scholar]
- Schnapp BJ, and Reese TS (1989). Dynein is the motor for retrograde axonal transport of organelles. Proc. Natl. Acad. Sci. USA 86, 1548–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder CM, Ostrem JM, Hertz NT, and Vale RD (2014). A Ras-like domain in the light intermediate chain bridges the dynein motor to a cargo-binding region. Elife 3, e03351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA, Steuer ER, and Sheetz MP (1989). Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell 56, 937–946. [DOI] [PubMed] [Google Scholar]
- See SK, Hoogendoorn S, Chung AH, Ye F, Steinman JB, Sakata-Kato T, Miller RM, Cupido T, Zalyte R, Carter AP, et al. (2016). Cytoplasmic dynein antagonists with improved potency and isoform selectivity. ACS Chem. Biol 11, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Tomas H, Havlis J, Olsen JV, and Mann M (2006). In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc 1, 2856–2860. [DOI] [PubMed] [Google Scholar]
- Souzaki M, Kubo M, Kai M, Kameda C, Tanaka H, Taguchi T, Tanaka M, Onishi H, and Katano M (2011). Hedgehog signaling pathway mediates the progression of non-invasive breast cancer to invasive breast cancer. Cancer Sci. 102, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman JB, Santarossa CC, Miller RM, Yu LS, Serpinskaya AS, Furukawa H, Morimoto S, Tanaka Y, Nishitani M, Asano M, et al. (2017). Chemical structure-guided design of dynapyrazoles, cell-permeable dynein inhibitors with a unique mode of action. Elife 6, e25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuer ER, Wordeman L, Schroer TA, and Sheetz MP (1990). Localization of cytoplasmic dynein to mitotic spindles and kinetochores. Nature 345, 266–268. [DOI] [PubMed] [Google Scholar]
- Taipale J, Chen JK, Cooper MK, Wang B, Mann RK, Milenkovic L, Scott MP, and Beachy PA (2000). Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 406, 1005–1009. [DOI] [PubMed] [Google Scholar]
- Taylor SP, Dantas TJ, Duran I, Wu S, Lachman RS, University of Washington Center for Mendelian Genomics Consortium, Nelson SF, Cohn DH, Vallee RB, and Krakow D (2015). Mutations in DYNC2LI1 disrupt cilia function and cause short rib polydactyly syndrome. Nat. Commun 6, 7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valetti C, Wetzel DM, Schrader M, Hasbani MJ, Gill SR, Kreis TE, and Schroer TA (1999). Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol. Biol. Cell 10, 4107–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Fallon JF, and Beachy PA (2000). Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–434. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, and Jessell TM (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110, 385–397. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, et al. (2009). Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science 326, 572–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TY, Quintyne NJ, Scipioni BR, Eckley DM, and Schroer TA (2012). Dynactin’s pointed-end complex is a cargo-targeting module. Mol. Biol. Cell 23, 3827–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh TY, Kowalska AK, Scipioni BR, Cheong FK, Zheng M, Derewenda U, Derewenda ZS, and Schroer TA (2013). Dynactin helps target Polo-like kinase 1 to kinetochores via its left-handed beta-helical p27 subunit. EMBO J. 32, 1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


