Abstract
Purpose of Review:
Clonal Hematopoiesis of Indeterminate Potential (CHIP) is a novel cardiovascular risk factor that develops as aging hematopoietic stem cells (HSCs) acquire somatic mutations which confer a clonal survival advantage in their progeny. These cells confer increased leukemogenic risk, but confer a greater absolute risk of cardiovascular disease – which appears to be mediated through altered inflammatory pathways. Here we review the evidence the risk of cardiovascular disease conferred by CHIP. We also review the evidence regarding risk factors associated with CHIP.
Recent Findings:
The most recent evidence suggests that CHIP is associated with increased cardiovascular risk beyond atherosclerosis, which has been established in multiple studies, but also in heart failure and aortic valve stenosis. Additionally, the list of conditions associated with CHIP continues to grow including germline genetics, smoking, cancer therapies, radiation exposure, premature menopause, and unhealthy diet.
Summary:
CHIP is a cardiovascular risk factor of increasingly recognized importance, and new data continues to emerge about the risks it confers, which will need more prospective validation. Although risk factors for CHIP are being identified, relatively little is known about the mechanisms by which CHIP develops, which requires further study.
Keywords: Clonal Hematopoiesis, Atherosclerosis, Genomics, Preventive Cardiology
Introduction
The aging immune system relies upon hematopoietic stem cells (HSCs) that acquire mutations over their lifetime. These HSCs are precursors to the erythroid, lymphoid and myeloid cells (granulocytes and monocytes) and platelets that comprise the immune system and regulate inflammation 1,2. Due to a combination of genetic predisposition, environmental exposures and random chance some HSCs acquire mutations that confer a survival advantage and result in a clonal proliferation of cells in that lineage. When several such mutations accrue, a myeloproliferative neoplasm or frank leukemia may result. Notably, leukemogenic mutations can be detected in peripheral blood cells long before a malignancy develops 3,4. CHIP refers to the clonal expansion of HSCs harboring leukemogenic mutations with a variant allele fraction of at least 2% in asymptomatic individuals in the absence of any overt diagnosis of malignancy. A threshold of 2% was selected in part for historical reasons based on the limit of detection of somatic mutations using exome sequencing. Mutations in CHIP genes (typically DNMT3A, TET2, ASXL1, JAK2 and other pre-cancerous genes) are very common and increase in frequency with age 5. DNMT3A and TET2 are involved in DNA methylation and de-methylation respectively. ASXL1 also plays a role in chromatin remodeling. Together these three genes affect DNA accessibility and gene expression across the genome. Conversely, JAK2 promotes cellular proliferation in a targeted way through the JAK/STAT signaling pathway.
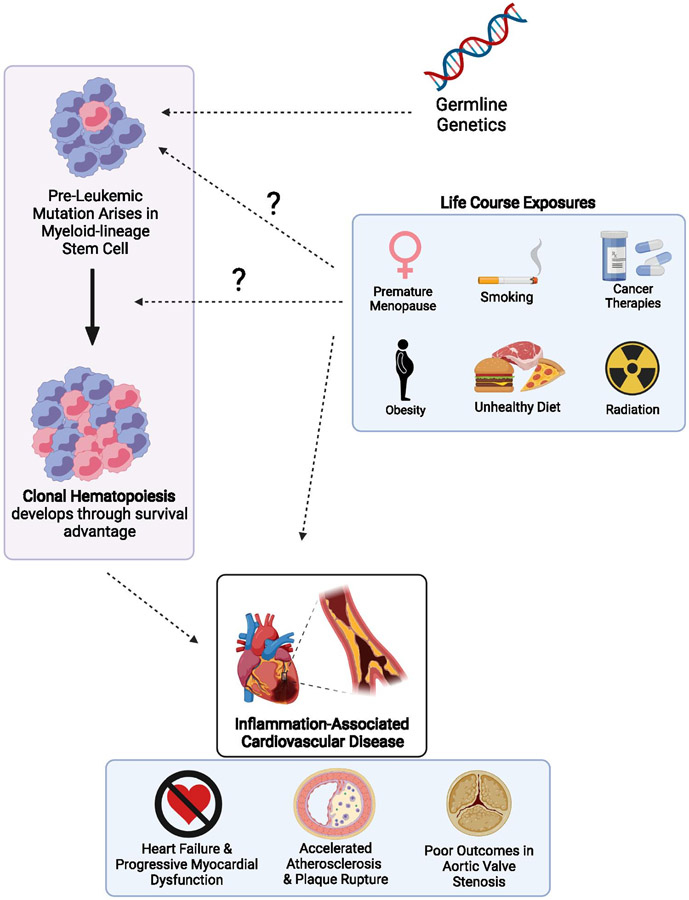
Although CHIP confers a 10-100 fold increased relative risk of hematologic malignancy the absolute risk remains modest (0.5% to 1% per year) 6. CHIP was found to be associated with a greater absolute increase in the risk of cardiovascular disease by 2- to 4-fold 1. In this review, we discuss these risks and their genetic basis (Figure 1).
Figure 1: Possible Contributors to the Development and Expansion of Clonal Hematopoiesis and Cardiovascular Events.
Germline genetics confer modestly increased risk of clonal hematopoiesis and thus lifestyle factors such as age, smoking, obesity, unhealthy diet, premature menopause and exposure to ionizing radiation or cancer therapeutics. These factors through pathways mediated by CHIP and otherwise may lead to increased adverse cardiovascular events including accelerated atherosclerosis, heart failure and poor outcomes in aortic valve stenosis. Created with BioRender.com.
Cardiovascular Risk
In the seminal 2014 paper, Jaiswal et al first described the adverse effects associated with CHIP in 5 different retrospective cohorts 7. There all-cause mortality was increased among those with CHIP present with a hazard ratio (HR) of 1.4 and a confidence interval (CI) 1.1-1.8 fold increased risk. This risk could not be adequately accounted for by increased risk of death from hematological malignancies as only one patient in the cohort died from malignancy – leading to cause-specific mortality analysis demonstrating an increased risk of death from cardiovascular causes (which included fatal strokes). This unexpected finding was then investigated and validated through animal experiments and human genetics examining the pathways through which CHIP exerts this effect. Here we explore the data linking CHIP to cardiovascular mortality.
Atherosclerosis:
Laboratory and clinical data support CHIP as a causal risk factor for atherosclerotic cardiovascular disease (ASCVD). In 2017, Jaiswal et al used 2 prospective cohort studies (BioImage and Malmö Diet and Cancer) and 2 retrospective case-control studies (the Atherosclerosis, Thrombosis, and Vascular Biology Italian Study Group (ATVB) and the Pakistan Risk of Myocardial Infarction Study (PROMIS)) to demonstrate an association between CHIP and risk of incident coronary events, and early MI1. Individuals with DNMT3A, TET2, and ASXL1 CHIP had a 1.7- to 2.0-fold increased risk of incident coronary events, while those with JAK2 CHIP had a 12-fold increase in risk. Among the retrospective case-control study, individuals with early-onset myocardial infarction (MI) were identified, and CHIP carriers were associated with a 4-fold risk of early MI. To ensure that this increased risk of MI was indeed due to atherosclerosis and not due to an increased risk of thrombosis or vasospasm, data from the BioImage study were used to compare coronary artery calcification (CAC) on computed tomography (CT) among CHIP carriers versus noncarriers, and CHIP carriers had a median CAC score 3.3 times higher than noncarriers. Furthermore, CHIP carriers without incident CAD, but with large clones (VAF≥10%) had a 12-fold increased risk of having a CAC score above the clinical threshold compared to noncarriers. To assess causality the study team transplanted atherosclerosis-prone Ldlr-knockout mice with Tet2 loss-of-function bone marrow, and fed them an atherogenic diet after immune reconstitution. The mice with Tet2 loss-of-function, replicating Tet2-CHIP, had aortic atherosclerotic lesions 2.0 times as large after 5 weeks of atherogenic diet, thus establishing Tet2 mutations lead to accelerated atherosclerotic plaques.
This finding of accelerated atherosclerosis was corroborated in multiple models. Fuster et al demonstrated that when Ldlr−/− mice are engrafted with only 10% Tet2−/− bone marrow, to mimic a 10% VAF CHIP clone in humans, the Tet2−/− cells clonally expanded to constitute 69% of HSCs in the bone marrow, and had larger aortic atherosclerotic plaques by 13 weeks8. In this study it was also observed that Tet2 deficient macrophages exhibited an increase in NLRP3 inflammasome-mediated interleukin-1B (IL-1B) secretion. Similarly, Wang et al demonstrated that when Ldlr−/− mice were transplanted with bone marrow from Jak2VF bone marrow and fed an atherogenic diet, the Jak2VF mice developed larger atherosclerotic plaques, with earlier lesion formation, increased complexity of plaques, larger necrotic cores, and greater proinflammatory immune cell activation likely contributing to greater plaque instability 9.
With multiple lines of evidence then implicating the NLRP3-inflammasome and IL-1B/IL-6 pathway, we utilized a human genetics approach to study whether the risk of ASCVD conferred by CHIP is mediated through this pathway 10. We identified individuals in the UK Biobank with a variant in the IL-6 receptor gene, IL6R p. Asp358Ala, which disrupts IL-6 signaling, thus effectively serving as a genetic proxy for therapy with an IL-6 inhibitor such as tocilizumab. We demonstrated that during 6.9-year median follow-up, IL6R p.Asp358Ala attenuated CVD event risk among participants with large CHIP clones (HR 0.46) but not in individuals without CHIP (HR 0.95). The above studies helped to confirm that CHIP is a causal risk factor for ASCVD, largely mediated through inflammatory immune pathways.
Heart Failure
The most common type of heart failure is due to CAD and ischemic events. In this way, CHIP and heart failure are linked through ASCVD. However, CHIP has been associated with worsening cardiac function outside of the context of ischemic events, and with data suggestive of altered immune pathway activation leading to worsening HF.
Dorsheimer et al. studied this relationship in humans when they performed deep targeted amplicon sequencing on bone marrow-derived mononuclear cells from 200 patients with congestive heart failure (CHF) 11. 38 of 200 (18.5%) were found to harbor CHIP mutations with a VAF>2%. After multivariable Cox proportional regression, the presence DNMT3A or TET2 CHIP was associated with a HR of 2.1 (CI 1.1-4.0) for death combined with heart failure hospitalization. Furthermore, among those with DNMT3A or TET2 mutations, most deaths (6 of 11) were due to progression of heart failure and arrhythmia, and only 1 death was attributed to acute myocardial infarction, while no stroke-related death was observed. Additionally, VAF stratified the risk of risk of mortality or combined mortality and HF hospitalization when they compared those without CHIP, VAF<1%, VAF 1-2%, and VAF>2%. This dose effect further supported the notion that CHIP was associated with impaired long-term survival and increased disease progression in patients with. In a separate study, Cremer et al. sequenced blood DNA from 419 patients with HF post-MI and a median LVEF of 30%, and found that not only did CHIP presence independently associate with mortality, but that among the 53 (12.6%) individuals with more than one CHIP mutation with a VAF>2%, there was dramatically higher mortality 12. Over 4 years of follow up 37/265 (14%) of non-CHIP carriers died, 22/101 (22%) of those with only 1 CHIP-mutation died, and 24/53 (44%) of those with greater than 1 CHIP mutation died. Subsequent studies also established that VAF stratifies risk of mortality in HF among those with TET2 and DNMT3A CHIP, as well as less common CHIP variants (excluding TET2 and DNMT3A) also independently associate with an increased risk of mortality among HF patients 13,14.
The mechanisms by which CHIP may worsen cardiac dysfunction and heart failure are not well-understood. Sano et al. investigated the mechanism in a murine model using a CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat-associated 9) system 15. They used a lentiviral vector to deliver Cas9 and guide RNA introducing inactivating mutations in Tet2 and Dnmt3a in bone marrow cells. These cells were then transplanted into lethally irradiated mice, allowed to engraft and gave rise to labelled blood cell progeny. These mice were then exposed to infusions of angiotensin II (Ang II) in a model of hypertensive heart failure. They observed that mice engrafted with either Tet2 and Dnmt3a demonstrated increased cardiac dysfunction, and cardiac and renal fibrosis. Interestingly, only mice with inactivating mutations in Tet2 had expanded mutant hematopoietic cells, and increased expression of IL-1B, IL-6, and Ccl5, whereas mice with inactivating mutations in Dnmt3a did not demonstrated expansion of hematopoietic cells, and had increased expression of Cxcl1, Cxcl2, IL-6, and Ccl5. These findings are interesting in that they indicate that CHIP increases cardiac dysfunction and fibrosis outside of ischemic events, and yet, different CHIP mutations may exert this effect through different signaling pathways and inflammatory profiles.
Aortic Valve Stenosis
Degenerative calcific aortic valve stenosis (AS) is a common valvular pathology that increases in prevalence with age, and is associated with chronic inflammation. Recent papers have investigated whether CHIP, which links aging, chronic inflammation and cardiovascular disease, may also play a role in the development and prognosis in AS.
Mas-Peiro et al. investigated the relationship between CHIP and AS patients while examining outcomes of patients undergoing transcatheter aortic valve intervention (TAVI) 16. They performed targeted amplicon sequencing for DNMT3A and TET2 in 279 patients with severe AS undergoing TAVI. The cohort had a median age of 83 years, and 93 out of 279 (33.3%) of patients had somatic DNMT3A- or TET2-CHIP driver mutations detected, with the expected age-dependent increase with a prevalence of 52.9% among those 90-100 years of age. Yet, compared to prevalence established from prior studies, it appeared that CHIP prevalence was enriched among patients with severe AS. After exclusion of adverse events in the first 30 days post TAVI to avoid confounding by intra- or peri-procedural factors, CHIP carriers were found to have significantly higher mortality than non-CHIP carriers. Further adjustments for baseline NT-proBNP levels increased the risk to nearly 5-fold that of non-CHIP carriers. They additionally performed analyses to see if other cardiovascular comorbidities explained the findings, and adjustment for HF, atrial fibrillation, or atherosclerosis in multiple vascular beds (CAD, peripheral arterial disease [PAD], or cerebrovascular disease) did not explain the association. Consistent with prior literature, these patients were found to have increased inflammatory activation especially in T cells, suggesting an inflammatory mechanism. In single-cell RNA sequencing studies of peripheral blood monocytes patients with severe AS and DNMT3A or TET2 CHIP were similarly found to have increased expression of proinflammatory cytokines, IL6 receptor, and cellular receptor CD163, as well as the NLRP3 inflammasome complex17.
CHIP Risk Factors and Pathogenesis
Age is the dominant risk factor for CHIP, which parallels other diseases of aging. Here we describe germline genetic factors, environmental exposures and lifestyle risk factors that contribute to the development and expansion of clonal populations.
Germline Genetics
Several recent studies have identified inherited genetic factors that predispose to acquiring CHIP mutations 18. The first inherited variation linked to CHIP was the JAK 46/1 haplotype predisposing to JAK2 myeloproliferative neoplasms and JAK2 V617F mutated CHIP 19. This particular genetic variant is thought to produce a hypermutable substrate for the JAK2 V617F mutation to arise. Subsequent studies have identified inherited polymorphisms that lead to increased DNA mutation rates (for example mutations in DNA damage repair genes ATM or CHEK2) or increased cellular proliferation (for example variants in telomere enzyme reverse transcriptase TERT or variants in a distal enhancer of the TET2 gene). Collectively the contribution of these genetic variants is quite modest suggesting that environmental factors, exposures and random chance play a more major role in CHIP pathogenesis.
Smoking
In a study in the UK Biobank, Dawoud et al. used whole exome sequencing (WES) to identify 1166 participants with myeloid CHIP in DNMT3A, TET2, ASXL1, JAK2, SRSF2, or PPM1D 20. In this cohort the odds of ever having smoked were significantly higher among those with CHIP than those without (53% were current or former smokers in CHIP versus 44% in those without CHIP), however this effect was largely driven by those who were current smokers (OR 1.10, P=6.14 x10−6), rather than former smokers (OR 1.02, P=0.08). Additionally, this analysis showed that ASXL1 was strongly associated with both current and former smoking (OR 1.07, P=1.92 x10−5). In fact, 69% of participants with ASXL1 CHIP were current or former smokers.
The association between active smoking and CHIP prevalence generally has been corroborated in multiple studies 21-23.
Cancer Therapy
Therapies for cancers, such as cytotoxic chemotherapy, radiation therapy and radionuclide therapy have all been associated with CHIP. In one of the largest studies to establish this connection, Bolton et al. utilized a dataset of patients at the Memorial Sloan Kettering Cancer Center (MSKCC) of 24,146 patients with cancer who had prospective targeted sequencing data to detect CHIP in blood DNA 24. They identified 7,216 individuals with CHIP, with a median VAF of 5%. Of this cohort, they extracted detailed clinical data on cancer therapies and treatment regimens on 10,138 individuals, of whom 59% had been exposed to some form of cancer therapy (including cytotoxic chemotherapy, radiation therapy, targeted therapy and immunotherapy) prior to blood draw. Bolton et al were able to examine the heterogeneous effects of different cancer therapies on CHIP. Patients were exposed to over 490 cancer therapies in the study, and of all treatment modalities those most associated with CHIP prevalence were: external beam radiation therapy (OR 1.4, P<10−6), cytotoxic chemotherapy (OR 1.2, P=2x10−3), and radionuclide therapy (OR 1.6, P=0.01). Within cytotoxic chemotherapy, topoisomerase II inhibitors (eg. doxorubicin, daunarubicin, idarubicin) had the strongest association (OR 1.3, P=0.01) along with platinum agent carboplatin (OR 1.4, P=0.001), though curiously not cisplatin or oxaliplatin).
Radiation: Therapeutic, Ambient and Extraterrestrial
Radiation therapy in cancer has been associated with CHIP in several studies 21,24,25, but deserves special consideration as CHIP has also been associated with radiation in other environmental contexts.
Ionizing radiation is utilized in diagnostic medicine, as cancer therapy, and in the cardiac catheterization laboratory. As noted in the Bolton et al study described previously, radiation therapy for cancer was strongly associated with the presence of CHIP (OR 1.4, P<10−6), as was radionuclide therapy (OR 1.6, P=0.01) 24. Such effect estimates have been replicated in other studies, with a suggesting that radiation therapy for cancer may be among the strongest environmental risk factors for CHIP 21. Radioactive Iodine (RAI) therapy has been epidemiologically associated with incident hematological malignancies and has further been associated with prevalence of CHIP 21,25. In fact, radionuclide therapy (which includes radioactive iodine) was most strongly associated with CHIP with a driver mutation in DDR gene PPM1D (OR 6.2, Q=7x10−6). Boucai et al were able to demonstrate that among patients who underwent treatment for thyroid carcinoma at MSKCC, 115/309 (37%) patients in the cohort had CHIP, and 75% of those patients underwent RAI therapy. Even after adjustment for age, external beam radiation therapy and chemotherapy, the dose of RAI was independently associated with CHIP prevalence; for every 10 mCi increase of RAI administered there was a 2% increase in the odds of having CHIP. Given the clear effect of ionizing radiation, investigators have sought to establish whether ambient radiation from environmental sources contributes to CHIP.
Recently presented analyses from WHI suggest ambient exposure to Radon was associated with CHIP prevalence. Radon is a radioactive colorless, odorless noble gas that is part of the radioactive decay chain for thorium and uranium and is ubiquitously present on Earth in minute quantities. It can be easily inhaled and is thus both a health hazard and the largest contributor to an individual’s background radiation dose on Earth. Anthony et al. identified CHIP in 887/10,495 (8.7%) of their study population, and then stratified CHIP prevalence by EPA estimations of radon exposure by zone of concentration where Zone 3 was < 2 pCi/L, Zone 2 was 2-4 pCi/L, and Zone 1 was > 4 pCi/L. Zone 1 concentration is where EPA recommends remediation. CHIP prevalence stratified by radon exposure zone where Zones 3, 2 and 1 had CHIP prevalence of 7.6%, 8.6% and 9.1% respectively. Relative to participants in Zone 3, those in Zones 2 and 1 had a higher adjusted risk of CHIP: OR (95% CI) = 1.13 (0.95, 1.35) and 1.24 (1.03, 1.48)26.
Furthering the work on ambient radiation and CHIP, Mencia-Trinchant et al conducted a unique study on a pair of twin astronauts (n=2) using data from the NASA Twins Study 27,28. In the study, two astronauts who had accrued different lengths of spaceflight over their career were recruited at age 50 to have complete multi-omics profiling before, during and after a lengthy 340-day mission where one twin was onboard the International Space Station, and the other twin was part of ground crew on Earth. Both twins had previously had prostate cancer (germline DNA with a stop-gain mutation in the RNASEL gene), but neither had been exposed to radiation therapy or chemotherapy. Both were 50 years of age at the time of recruitment and both had CHIP mutations with significant VAF. HR was the ground control twin who had 2 somatic mutations in DNMT3A (VAFs 6.8% and 2.1%) and one somatic mutation of unknown significance in the LPL gene. TW was the twin to be part of the spaceflight mission, and at baseline had TET2 CHIP with a VAF of 3.8%. In regards to clonal dynamics during and after spaceflight, though TET2 and DNMT3A clones both progressed in the spaceflight and ground control twins respectively, the spaceflight twin’s clone was relatively stable while in space and then expanded at a rapid rate during the follow up period.
Menopause
The evidence for premature menopause as a risk factor for CHIP is drawn from retrospective studies in the UK Biobank and WHI cohorts. Honigberg et al. studied 19,606 post-menopausal women from the two cohorts and identified 418 (2.1%) with natural premature menopause and 887 (4.5%) with surgical premature menopause 23. The overall prevalence of CHIP across cohorts was 8.8% among women with a history of premature menopause and 5.5% among women without a history of premature menopause (P<0.001), and crude CHIP rate increased with earlier age at premature menopause. After multivariable adjustment for age, smoking, diabetes, use of hormone therapy and the first 10 principal components of genetic ancestry, premature menopause was associated with a significantly increased odds of CHIP presence (OR 1.36, 95% CI 1.10-1.68; P=0.004). This relationship, however, appeared to be driven by a stronger relationship with natural premature menopause (OR 1.73; 95% CI 1.23-2.44; P=0.001) while no significant relationship was found between CHIP and surgical menopause alone.
Healthy Lifestyle, Diet and Obesity
In an analysis of data from the WHI, Haring et al examine the relationship between 4 elements of a healthy lifestyle (body mass index, smoking, physical activity, and diet quality) among 8,709 postmenopausal women, and their relationship to CHIP prevalence 22. Each healthy lifestyle element was assigned a binary value of 0 (healthy metric not met), or 1 (healthy metric met). The sum would constitute the participant’s score between 0 (least healthy) and 4 (most healthy). Though the overall dietary score did not show any association with CHIP, individuals with a normal BMI were found to have the lowest odds of CHIP prevalence (OR 1.02, CI 1.01-1.04; P=0.0028). This cohort did not find any association between current or former smoking and the presence of CHIP. The authors suggest that this was due to the nature of the WHI cohort which consists of relatively healthy postmenopausal women when compared to the general population (for instance the rate of smoking in the study cohort was only 7% compared to the national prevalence of 17% in similarly aged women nationally). Additionally, as discussed above, there are unique considerations for CHIP when considering a population that consists of postmenopausal women, and how these risk factors interact is not known.
Our research group has discovered a relationship between unhealthy diet and an increased odds of CHIP prevalence. In a study of 44,111 participants in the UK Biobank with WES data and complete dietary survey data, Bhattacharya et al categorized individuals into 3 mutually exclusive categories of healthy diet, intermediate diet and unhealthy diet depending upon their intake of healthy dietary elements (vegetables and fruits) and unhealthy dietary elements (red meat, added salt, processed meats) 29. After multivariable adjustment for age, sex, smoking, socioeconomic status, type 2 diabetes, and the first 10 principal components of genetic ancestry, unhealthy diet was associated with an increased odds of CHIP (OR 1.25, 95% CI 1.03-1.50; P=0.019). The mechanism of such a relationship is not known, and no individual dietary element comprising the dietary score was found to be related to CHIP prevalence. However, studies of common CHIP driver mutations in Tet2 show that common dietary nutrients such as vitamin C could help restore hypofunction of the gene function 30. Whether individual dietary nutrients in a healthy diet enriched in fruits and vegetables rescues gene hypofunction caused by CHIP-driver mutations, or affects clonal expansion will have to be addressed in future prospective studies.
Conclusions
Over the past five years, clonal hematopoiesis has expanded from the hematopoietic stem cell niche to an increasingly prominent role at the intersection of cardiovascular disease and aging. CHIP has been linked to ischemic heart disease, heart failure and aortic valve disease via both human epidemiological studies and experimental systems, with inflammation identified as a key mechanistic node. Our understanding of the genetic and environmental causes of CHIP have also expanded considerably in the past five years, aided in large part by the ever-increasing availability of sequencing data in health-care system and population biobanks. These efforts have implicated inflammation as an underlying cause of CHIP in addition to a consequence 31,32, leading some to propose a feed forward cycle linking the two 33,34. The evidence base for clinical management of CHIP is currently limited to expert consensus opinion 35,36 ; however this confluence of mechanistic work and epidemiological observations has raised the tantalizing prospect that specifically targeting inflammation may provide a precision cardiovascular therapeutic to treat patients with CHIP 37. With the recent success of cardiovascular inflammation trials including modulation of IL-1B in the CANTOS trial 38 and IL-1 and Il-8 with colchicine in COLCOT 39, it would seem to be an opportune moment to consider whether these therapies might have differential efficacy in patients with CHIP.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Romit Bhattacharya, Cardiovascular Research Center, Massachusetts General Hospital, Program in Medical and Population Genetics and the Cardiovascular Disease Initiative, Broad Institute of Harvard and MIT, Department of Medicine, Harvard Medical School, Street Address: 55 Fruit Street, Boston MA 02114.
Alexander G. Bick, Division of Genetic Medicine, Department of Medicine, Vanderbilt University Medical Center, Street Address: 1161 21st Ave S, Nashville, TN, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 2017;377:111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Natarajan P, Jaiswal S, Kathiresan S. Clonal Hematopoiesis: Somatic Mutations in Blood Cells and Atherosclerosis. Circ Genom Precis Med 2018;11:e001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bick AG, Weinstock JS, Nandakumar SK, et al. Inherited Causes of Clonal Hematopoiesis of Indeterminate Potential in TOPMed Whole Genomes. Nature 2020; 586, 763–768.33057201 This study suggested that there is only modest heritability of CHIP, and that life exposures may be central to its development.
- 4.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zink F, Stacey SN, Norddahl GL, et al. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood 2017;130:742–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014;20:1472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Liu W, Fidler T, et al. Macrophage Inflammation, Erythrophagocytosis, and Accelerated Atherosclerosis in Jak2 (V617F) Mice. Circ Res 2018;123:e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bick AG, Pirruccello JP, Griffin GK, et al. Genetic Interleukin 6 Signaling Deficiency Attenuates Cardiovascular Risk in Clonal Hematopoiesis. Circulation 2020;141:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorsheimer L, Assmus B, Rasper T, et al. Association of Mutations Contributing to Clonal Hematopoiesis With Prognosis in Chronic Ischemic Heart Failure. JAMA Cardiol 2019;4:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cremer S, Kirschbaum K, Berkowitsch A, et al. Multiple Somatic Mutations for Clonal Hematopoiesis Are Associated With Increased Mortality in Patients With Chronic Heart Failure. Circ Genom Precis Med 2020;13:e003003. [DOI] [PubMed] [Google Scholar]
- 13.Assmus B, Cremer S, Kirschbaum K, et al. Clonal haematopoiesis in chronic ischaemic heart failure: prognostic role of clone size for DNMT3A- and TET2-driver gene mutations. Eur Heart J 2021;42:257–65. [DOI] [PubMed] [Google Scholar]
- 14.Kiefer KC, Cremer S, Pardali E, et al. Full spectrum of clonal haematopoiesis-driver mutations in chronic heart failure and their associations with mortality. ESC Heart Fail 2021;8:1873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K. CRISPR-Mediated Gene Editing to Assess the Roles of Tet2 and Dnmt3a in Clonal Hematopoiesis and Cardiovascular Disease. Circ Res 2018;123:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mas-Peiro S, Hoffmann J, Fichtlscherer S, et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J 2020;41:933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abplanalp WT, Mas-Peiro S, Cremer S, John D, Dimmeler S, Zeiher AM. Association of Clonal Hematopoiesis of Indeterminate Potential With Inflammatory Gene Expression in Patients With Severe Degenerative Aortic Valve Stenosis or Chronic Postischemic Heart Failure. JAMA Cardiol 2020;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silver AJ, Bick AG, Savona MR. Germline risk of clonal haematopoiesis. Nat Rev Genet 2021:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinds DA, Barnholt KE, Mesa RA, et al. Germ line variants predispose to both JAK2 V617F clonal hematopoiesis and myeloproliferative neoplasms. Blood 2016;128:1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawoud AAZ, Tapper WJ, Cross NCP. Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia 2020;34:2660–72. [DOI] [PubMed] [Google Scholar]
- 21.Coombs CC, Zehir A, Devlin SM, et al. Therapy-Related Clonal Hematopoiesis in Patients with Non-hematologic Cancers Is Common and Associated with Adverse Clinical Outcomes. Cell Stem Cell 2017;21:374–82.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haring B, Reiner AP, Liu J, et al. Healthy Lifestyle and Clonal Hematopoiesis of Indeterminate Potential: Results From the Women's Health Initiative. J Am Heart Assoc 2021;10:e018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honigberg MC, Zekavat SM, Niroula A, et al. Premature Menopause, Clonal Hematopoiesis, and Coronary Artery Disease in Postmenopausal Women. Circulation 2021;143:410–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet 2020;52:1219–26. Findings from this study showed that different cancer therapies help to select for specific clones in CHIP, and also provides further evidence that cigarette smoking is related to CHIP.
- 25.Boucai L, Falcone J, Ukena J, et al. Radioactive Iodine-Related Clonal Hematopoiesis in Thyroid Cancer Is Common and Associated With Decreased Survival. J Clin Endocrinol Metab 2018;103:4216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anthony KM MJ, Jaiswal S, Natarajan P, Desai P, Hayden KM, Bick AG, Carty CL, Collins JM, Love S, Stewart J, Schwartz G, Reiner A, Whitsel EA. Radon is Associated With Clonal Hematopoiesis of Indeterminate Potential in the Women’s Health Initiative. AHA Scientific Sessions; 2020. 15 December 2020: Circulation. [Google Scholar]
- 27.Garrett-Bakelman FE, Darshi M, Green SJ, et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019;364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mencia-Trinchant N, MacKay MJ, Chin C, et al. Clonal hematopoiesis before, during, and after human spaceflight. Cell Rep 2021;34:108740. [DOI] [PubMed] [Google Scholar]
- 29. Bhattacharya R, Zekavat SM, Uddin MM, et al. Association of Diet Quality With Prevalence of Clonal Hematopoiesis and Adverse Cardiovascular Events. JAMA Cardiol 2021. Findings from this paper established an association between unhealthy dietary pattern low in fruits and vegetables and high in red meats as associated with CHIP.
- 30.Cimmino L, Dolgalev I, Wang Y, et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017;170:1079–95.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heyde A, Rohde D, McAlpine CS, et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 2021;184:1348–61.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Deuren RC, Andersson-Assarsson JC, Kristensson FM, et al. Clone expansion of mutation-driven clonal hematopoiesis is associated with aging and metabolic dysfunction in individuals with obesity. bioRxiv 2021:2021.05.12.443095. [Google Scholar]
- 33.Lusis AJ. A vicious cycle in atherosclerosis. Cell 2021;184:1139–41. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Cabo F, Fuster JJ. Clonal haematopoiesis and atherosclerosis: a chicken or egg question? Nat Rev Cardiol 2021. [DOI] [PubMed] [Google Scholar]
- 35.Libby P, Sidlow R, Lin AE, et al. Clonal Hematopoiesis. Journal of the American College of Cardiology 2019;74:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sidlow R, Lin AE, Gupta D, et al. The Clinical Challenge of Clonal Hematopoiesis, a Newly Recognized Cardiovascular Risk Factor. JAMA Cardiology 2020;5:958–61. [DOI] [PubMed] [Google Scholar]
- 37.Swirski FK. Inflammation and CVD in 2017: From clonal haematopoiesis to the CANTOS trial. Nat Rev Cardiol 2018;15:79–80. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 39.Tardif JC, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N Engl J Med 2019;381:2497–505. [DOI] [PubMed] [Google Scholar]