Abstract
Introduction
Seasonal influenza infects millions annually in Europe. Annual influenza vaccination is the most effective measure to reduce the risk of infection and its complications, especially among young children and older adults.
Objective
We assessed adverse event (AE) frequency after receiving GSK’s inactivated quadrivalent seasonal influenza vaccine (IIV4).
Methods
A passive enhanced safety surveillance study was conducted in Belgium, Germany, and Spain. Adults who had received GSK's IIV4 or the parent(s)/guardian(s)/legally acceptable representative(s) of children given the vaccine were invited to complete an adverse drug reaction (ADR) card to document AEs experienced within 7 days post vaccination.
Results
A total of 1082 participants (51.6% females) received GSK's IIV4, including 115 children < 9 years of age who received two doses. The ADR card return rate was 97.0% (n = 1049) after dose 1 and 100% (n = 115) after dose 2. All participants in Belgium and Germany were adults. In Spain, 71.2% were children. After dose 1, 39.2% reported one or more AE. The most frequent AEs category was "general disorders and administration site conditions” (GDASC). AEs were most frequently reported in adults aged 18–65 years (47.2%), followed by children aged 6 months–17 years (38.1%), and adults aged > 65 years (31.6%). After dose 2, 7.8% reported one or more AE, and GDASC was again the most frequent AE category. There were no serious AEs related to GSK's IIV4 within 7 days post vaccination.
Conclusion
No serious AEs related to GSK’s IIV4 within 7 days post vaccination were reported. This study supports the favourable risk–benefit safety profile of GSK’s IIV4.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01121-8.
Plain Language Summary
Seasonal influenza infects millions annually in Europe, especially young children and older adults. Annual influenza vaccination is the most effective measure to reduce the risk of infection and its complications. As the wild influenza virus strains change every year, the composition of the influenza vaccine changes as well. Since the vaccine is produced in the same way over the years, extensive safety studies are no longer required by regulatory authorities. Instead, monitoring of any unwanted medical incidents (adverse events) after vaccination is required. For the 2019/2020 season, we monitored the adverse events reported by a representative sample of people in Belgium, Germany, and Spain within 7 days after receiving GSK's seasonal influenza vaccine.
Of the 1082 people who received the first dose of the vaccine, 39% reported at least one adverse event, such as pain and swelling at the injection site, tiredness, fever, headache, or dizziness. A total of 115 children under 9 years of age received two doses 4 weeks apart. After their second dose, few of these children (8%) reported adverse events. The most frequent adverse events were fever, swelling and pain at the injection site, runny nose, or irritability. No serious adverse events were reported after either the first or second dose.
No serious adverse events related to GSK’s seasonal influenza vaccine within the 7 days after vaccination were reported. This study supports the favourable risk–benefit safety profile of GSK's seasonal influenza vaccine.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40264-021-01121-8.
Key Points
| Each year, the composition of influenza vaccines changes, based on World Health Organization recommendations. The European Medicines Agency has provided guidelines for monitoring the safety of influenza vaccine to quickly identify any safety concerns related to the new influenza vaccines at the start of each vaccination season. |
| A passive enhanced safety surveillance study was conducted to rapidly collect and assess adverse events (AEs) within 7 days post vaccination of adults and children in Belgium, Germany, and Spain who received GSK’s inactivated quadrivalent seasonal influenza vaccine (IIV4) for the 2019/2020 influenza season. |
| Of the 1082 participants in the study, 39.2% and 7.8% reported at least one AE after dose 1 and dose 2, respectively. No unexpected safety issues or serious AEs were reported. These results support the favourable risk–benefit safety profile of GSK's IIV4. |
Introduction
Seasonal influenza infects millions of people every year in Europe [1]. Influenza viruses most commonly circulate from late fall through early spring [2]. The majority of people who get infected with influenza recover without complications. However, influenza can be associated with serious complications, hospitalisations, and deaths, particularly among older adults and persons of all ages with underlying medical conditions [2].
The annual morbidity and mortality rates for influenza disease in the European Union (EU) countries from 2009 to 2013 were 5887 cases and 5.89 deaths per 100,000 population, respectively. This makes influenza one of the highest infectious disease burdens in the EU, with 81.8 disability-adjusted life years (DALYs) per 100,000 people, which represents 29.8% of the total DALYs in the EU [3]. In addition, older adults have the highest risk of influenza-attributable hospitalisations [4].
Influenza outbreaks are usually caused by influenza A virus subtypes and influenza B virus lineages. During the 2019/2020 influenza season, influenza activity started early in week 45/2019, circulated at high levels between week 52/2019 and week 10/2020, and returned to baseline levels in week 13/2020, which was earlier than the previous influenza season (2018/2019). This might have been due to the regulations implemented in different countries to limit the spread of coronavirus disease 2019 (COVID-19), such as social distancing, travel restrictions, and lockdowns [5].
As of week 39/2020, 164,917 laboratory-confirmed influenza cases were detected by the EU regions; 72.9% had type A viruses, with A(H1N1)pdm09 prevailing over A(H3N2), and 27.1% had type B viruses, with 4480 (98.1%) of 4569 ascribed to the B/Victoria lineage [6]. Nine EU countries reported a total of 10,705 laboratory-confirmed hospitalised influenza cases during the 2019/2020 influenza season, with 87% due to influenza type A viruses. The majority of deceased hospitalised influenza patients were 65 years and older and had influenza A virus infection [5].
Annual influenza vaccination is the most effective way to reduce influenza disease burden [7]. In EU member states, about 31,000 deaths were averted annually thanks to influenza vaccination, of which 97.7% were averted among older adults [8].
The continuous antigenic drifts in influenza viruses require the yearly update of influenza vaccine composition to protect susceptible people against the new wild circulating influenza virus [9]. The formulation of these vaccines is based on recommendations by the World Health Organization (WHO) [10].
The European Medicines Agency (EMA) has provided guidelines for marketing authorisation holders to conduct enhanced safety surveillance (ESS) in countries where they are marketing their influenza vaccines every year in order to rapidly detect and evaluate near-real time any new safety concerns [11]. Annual ESS is implemented at the start of the seasonal influenza immunisation programme each year in order to detect any local and systemic adverse events (AEs) and compare their trends to the previous season(s).
To comply with EMA guidelines, GSK launched an annual ESS study programme, which started during the 2015/2016 influenza season [12–15]. Belgium, Germany, and Spain were selected to conduct the passive ESS during the 2019/2020 influenza season because GSK was distributing its inactivated quadrivalent seasonal influenza vaccine (IIV4) in these three countries (as AlphaRix Tetra, Influsplit Tetra, and Fluarix Tetra, respectively).
The study objectives were to assess, in each country and overall, the cumulative percentages of participants who reported AEs, according to Medical Dictionary for Regulatory Activities (MedDRA) classifications, within 7 days of vaccination with GSK's IIV4, overall, by age group, and by risk status (at risk, not at risk).
Methods
Study Design
This was a multi-country, multicentre, prospective passive ESS study (GSK study identifier 207749) that was conducted between 1 October 2019 and 13 January 2020 in Belgium, Germany, and Spain. The study staff approached adults (aged 18 years or older in Belgium, Germany, and Spain) or the parent(s)/guardian(s)/legally acceptable representative(s) of children 6 months and older (in Spain) to check if they were willing to participate in the study after they or the child they were responsible for had just received GSK’s IIV4 at the study sites as part of their routine healthcare. Those who agreed to participate in the study signed the informed consent forms and received an adverse drug reaction (ADR) card to be completed and returned to the healthcare provider either in person at the next scheduled visit on day 8, or by mail. In Belgium, the study participants received a compensation, namely a €20 gift card (a Sodexo voucher), for the time they spent to complete the diary cards. The study staff entered the data from the returned completed ADR cards into a database using an electronic case report form (eCRF).
The ADR cards had both a list of predefined local and general adverse events of interest (AEIs) to be checked (yes/no) by participants as well as a free-text field to report any other AEs occurring within 7 days post vaccination (including the day of vaccination and the following 6 days).
Children < 9 years old who had not received influenza vaccine in the past were eligible for two doses 4 weeks apart, according to the recommendation of the WHO [16].
During study implementation, four interim analyses (mid-November, end-November, mid-December, mid-January) were performed to identify any potential safety concern or alarming AE that might represent a risk for vaccinees, in addition to the final statistical analysis.
The primary study objective was to assess, in each country and overall, the cumulative percentages of participants who reported AEs, according to MedDRA classifications, within 7 days of vaccination with GSK's IIV4. The secondary objectives were to assess, in each country and overall, the weekly and cumulative percentages of participants who reported AEs, according to MedDRA classifications, within 7 days of vaccination with GSK’s IIV4 by age group and by risk status (at risk, not at risk). Study endpoints have been reported here for the primary objective and for most of the secondary objectives. More information regarding the weekly percentages of participants reporting any AEs within 7 days of vaccination with GSK’s IIV4, overall, by age group, and by risk status, can be found at GSK’s Clinical Study Register (GSK Study Identifier 207749) [17].
During the study’s implementation, there was no dosage modification, change in the target population, formulation change, restriction on distribution, or clinical trial suspension of the influenza vaccine compared to the 2018/2019 influenza season. Therefore, there were no significant safety-related changes to the reference safety information for the product.
Study Population
Approximately 1000 participants receiving GSK’s IIV4 were planned to be enrolled in this study between 1 October 2019 and 31 December 2019, from eight healthcare centres in Belgium (n = 3), Germany (n = 3), and Spain (n = 2). The study covered all age groups; however, due to feasibility constraints, Belgian and German study centres recruited participants ≥ 18 years old, while Spanish study centres recruited children ≥ 6 months–17 years and adults 18–65 years old. Additional inclusion criteria for participants included the following: they were, in the opinion of the investigator, likely to comply with the requirements of the study protocol; they had just received GSK's IIV4 according to routine medical practice; and they signed a written informed consent/informed assent. Children in care were excluded from the study.
Recruitment of participants occurred from 1 October 2019 until 31 December 2019: International Organization for Standardization (ISO) week 40/2019 to week 52/2019 for participants requiring one dose and until 1 December 2019 (ISO week 40/2019 to week 48/2019) for unprimed children < 9 years old who required two doses, 4 weeks apart. This allowed sufficient time for participants to receive both doses and to complete the ADR cards on time.
Statistical Methods
Descriptive statistical analyses of the study data were conducted to estimate for each vaccine dose the cumulative and weekly percentages of reported AEs overall and by country, as well as by age group and risk status (at risk, not at risk). The study included three sets of participants: (1) the “Enrolled Set”, which included all eligible participants vaccinated with GSK’s IIV4 who signed the informed consent (dose 1: n = 1083); (2) the “Safety Set”, which included all participants from the Enrolled Set who received the ADR card (dose 1: n = 1082); and (3) the “Solicited Safety Set”, which included all participants from the Safety Set who returned the ADR card and documented the presence or absence of AEs (dose 1: n = 1049). The analyses of the primary and secondary objectives were performed on the Safety Set because this was a post-marketing study and intended to reflect GSK’s IIV4 pharmacovigilance activities.
Demographic characteristics (age at vaccination with dose 1, sex, geographic ancestry) and risk status for influenza-associated morbidity and mortality were summarised using frequency tables (n [%]) for categorical variables, while mean, standard deviation, median, and minimum and maximum were calculated for continuous data. Vaccines coadministered with GSK’s IIV4, if any, were summarised using frequency tables.
For each vaccine dose, the cumulative percentage of participants reporting AEIs and/or other AEs from study start up to each study week (i.e. ISO week 40/2019–week 01/2020) was estimated using the MedDRA [18] Primary System Organ Classes (SOCs) and Preferred Terms (PTs). In addition, for each vaccine dose, the weekly and cumulative percentages of participants reporting AEIs and/or other AEs within the 7-day post-vaccination period, using ADR cards, were estimated by age strata (6 months–17 years, 18–65 years, and > 65 years) and risk status (at risk, not at risk) for influenza-associated morbidity and mortality. The two-sided 95% confidence interval (CI) accounting for the clustering effect of countries was calculated for all estimated percentages using extended Clopper–Pearson exact CI for clustered data. The design effects and the intra-cluster correlation coefficients were also estimated for the cumulative percentage of participants reporting AEIs and/or other AEs by MedDRA Primary SOCs and PTs over the whole study period.
Ethical Considerations
The study protocol was approved by the independent ethics committee or institutional review board of each study centre. Ethical approval was sought prior to participant enrolment. Written informed consent was obtained from each participant and/or each participant's parent(s)/guardians/legally acceptable representative(s) prior to study participation. The study protocol is available on GSK’s Clinical Study Register (GSK Study Identifier 207749) [17].
Results
Participants and Characteristics
A total of 1083 vaccinated participants were enrolled from 1 October 2019 to 5 December 2019 in Belgium (n = 330), Germany (n = 288), and Spain (n = 465). The informed consent of one participant in Germany was invalid, leaving 1082 in the Enrolled Set, including 127 children in Spain who were eligible for two doses. However, only 115 children received the second dose 4 weeks after the first one. Of the 12 children who did not receive dose 2, five children were sick at the time of dose 2, as indicated by their healthcare providers, and seven children were lost to follow-up. Of the five children who were sick, two children had laryngo-tracheobronchitis, two children had pneumonia, and one child had bronchitis. None of these medical conditions were deemed vaccine-related by the study healthcare providers, and the children were advised to postpone their vaccination.
Of the 1082 enrolled participants, 33 participants either did not return the ADR card or returned the ADR card without documentation of the presence/absence of AEs after dose 1, while all 115 participants returned their ADR card after dose 2 (Fig. 1).
Fig. 1.
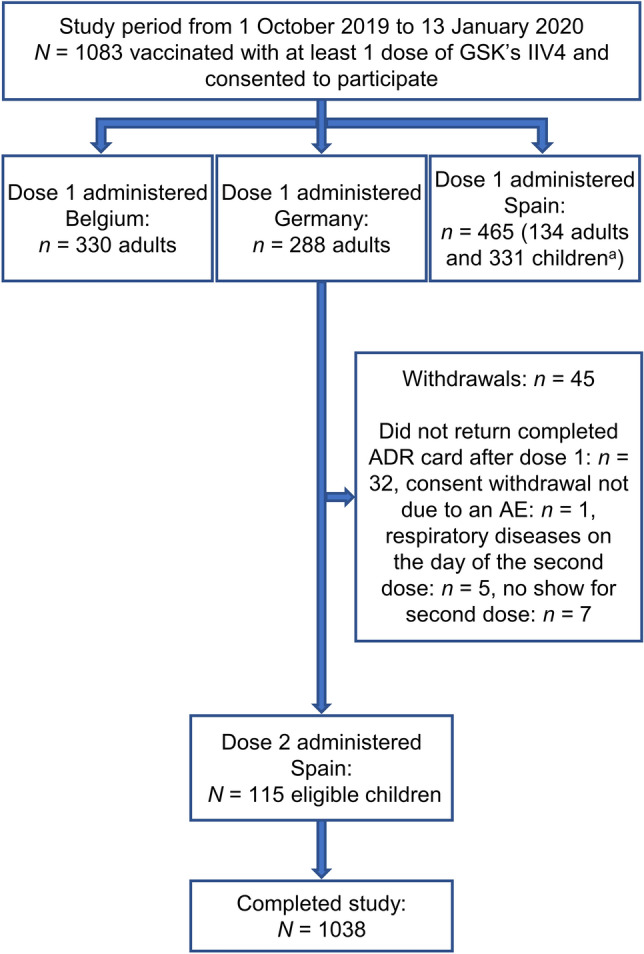
Study attrition diagram of the passive enhanced safety surveillance for the 2019/2020 influenza season in Belgium, Germany, and Spain, with participants enrolled by country and numbers vaccinated with dose 1 and dose 2 of GSK's IIV4. Participants were first vaccinated then asked if they wanted to participate; they then received the ADR card. a Out of the 331 children enrolled, 127 were eligible for dose 2 as they were receiving the seasonal influenza vaccine for the first time and were < 9 years of age at inclusion. ADR adverse drug reaction, AE adverse event, IIV4 inactivated quadrivalent seasonal influenza vaccine, N total number of participants, n number of participants in a specific category
All participants in Belgium and Germany were adults, of which 57.9% (n = 191) and 59.2% (n = 170), respectively, were > 65 years of age. In Spain, 71.2% (n = 331) were children (aged 6 months–17 years). In total, 51.6% (n = 558) were females. The vast majority of participants were of Caucasian/European heritage (98.0%; n = 1060). Overall, 63.6% (n = 210) of participants in Belgium, 58.5% (n = 168) of participants in Germany, and 54.6% (n = 254) of participants in Spain were assessed as at risk for influenza-associated morbidity and mortality by their healthcare providers. Of the children who received dose 2, 50.4% (n = 58) were girls and 42.6% (n = 49) were assessed as being at risk for influenza-associated morbidity and mortality (Table 1).
Table 1.
Summary of demographic characteristics and risk status of participants in the Safety Set
| Belgium N = 330 |
Germany N = 287 |
Spain N = 465 dose 1 N = 115 dose 2 |
Total N = 1082 |
|||||
|---|---|---|---|---|---|---|---|---|
| Dose 1 | ||||||||
| Age in years at influenza vaccinationa | ||||||||
| Mean (SD) in years | 60.6 (17.9) | 65.1 (16.2) | 16.4 (19.2) | 42.8 (29.2) | ||||
| Median in years | 67.0 | 69.0 | 6.0 | 47.0 | ||||
| Minimum age | 23 years | 18 years | 7 months | 7 months | ||||
| Maximum age in years | 91 | 91 | 64 | 91 | ||||
| Age categorya (n %) | ||||||||
| 6 months–17 years | 0 | 0 | 0 | 0 | 331 | 71.2 | 331 | 30.6 |
| 18–65 years | 139 | 42.1 | 117 | 40.8 | 134 | 28.8 | 390 | 36.0 |
| > 65 years | 191 | 57.9 | 170 | 59.2 | 0 | 0 | 361 | 33.4 |
| Gender (n %) | ||||||||
| Female | 146 | 44.2 | 167 | 58.2 | 245 | 52.7 | 558 | 51.6 |
| Male | 184 | 55.8 | 120 | 41.8 | 220 | 47.3 | 524 | 48.4 |
| Geographic ancestry (n %) | ||||||||
| Caucasian/European heritage | 328 | 99.4 | 286 | 99.7 | 446 | 95.9 | 1060 | 98.0 |
| Asian | 1 | 0.3 | 0 | 0 | 5 | 1.1 | 6 | 0.6 |
| Black or African American | 0 | 0 | 1 | 0.3 | 0 | 0 | 1 | 0.1 |
| Arabic/North African Heritage | 0 | 0 | 0 | 0 | 2 | 0.4 | 2 | 0.2 |
| Other | 1 | 0.3 | 0 | 0 | 12 | 2.6 | 13 | 1.2 |
| Risk statusb (n %) | ||||||||
| At risk | 210 | 63.6 | 168 | 58.5 | 254 | 54.6 | 632 | 58.4 |
| Not at risk | 120 | 36.4 | 119 | 41.5 | 211 | 45.4 | 450 | 41.6 |
| Risk groupc (n %) | ||||||||
| Pregnant women | 1 | 0.3 | 0 | 0 | 0 | 0 | 1 | 0.1 |
| > 6 months old with chronic diseases | 120 | 36.4 | 164 | 57.1 | 115 | 24.7 | 399 | 36.9 |
| Children 6–59 months old | 0 | 0 | 0 | 0 | 76 | 16.3 | 76 | 7.0 |
| Healthcare workers | 0 | 0 | 0 | 0 | 4 | 0.9 | 4 | 0.4 |
| Other | 202 | 61.2 | 4 | 1.4 | 63 | 13.5 | 269 | 24.9 |
| Dose 2 | ||||||||
| Age in years at influenza vaccinationa | ||||||||
| Mean (SD) in years | 3.1 (2.3) | |||||||
| Median in years | 3.0 | |||||||
| Minimum age | 7 months | |||||||
| Maximum age in years | 8 | |||||||
| Gender (n %) | ||||||||
| Girls | 58 | 50.4 | ||||||
| Boys | 57 | 49.6 | ||||||
| Geographic ancestry (n %) | ||||||||
| Caucasian/European heritage | 113 | 98.3 | ||||||
| Asian | 2 | 1.7 | ||||||
| Risk statusb (n %) | ||||||||
| At risk | 49 | 42.6 | ||||||
| Not at risk | 66 | 57.4 | ||||||
| Risk groupc (n %) | ||||||||
| Children 6–59 months old | 42 | 36.5 | ||||||
| Other | 7 | 6.1 | ||||||
N total number of participants, n % number and percentage of participants in a given category, SD standard deviation
aAt vaccination with dose 1 of GSK's inactivated quadrivalent seasonal influenza vaccine
bRisk status for influenza-associated morbidity and mortality as assessed by the healthcare provider based on their judgment and experience
cRisk group for influenza-associated morbidity and mortality. Participants may be assigned to more than one risk group. Detailed information about those included in the category “other” was not collected in the electronic case report form since there was no open field to specify the type of disease or disorder
Few participants had other vaccinations coadministered with GSK’s IIV4 dose 1 vaccination during the same visit (8.5%; n = 92) as part of their routine healthcare. Coadministered vaccines included hepatitis vaccine (4.2%; n = 45) and pneumococcal vaccine (3.0%; n = 33). In Spain, 18.5% (n = 86) of participants received coadministered vaccines, of which the most frequently received were hepatitis vaccine (9.7%; n = 45), pneumococcal vaccine (6%; n = 28), and meningococcal vaccine (2.2%; n = 10). In Belgium, 1.5% (n = 5) received pneumococcal vaccine, and one participant in Germany (0.3%) received an encephalitis vaccine (Table 2).
Table 2.
Vaccines coadministered with GSK's IIV4 dose 1 in the Safety Set
| Vaccine | Belgium N = 330 |
Germany N = 287 |
Spain N = 465 |
Total N = 1082 |
||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Any | 5 | 1.5 | 1 | 0.3 | 86 | 18.5 | 92 | 8.5 |
| Bacterial and viral | 0 | 0.0 | 0 | 0.0 | 3 | 0.6 | 3 | 0.3 |
| Encephalitis | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 1 | 0.1 |
| Hemophilus influenzae B | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.1 |
| Hepatitis | 0 | 0.0 | 0 | 0.0 | 45 | 9.7 | 45 | 4.2 |
| Meningococcal | 0 | 0.0 | 0 | 0.0 | 10 | 2.2 | 10 | 0.9 |
| Multiple | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 2 | 0.2 |
| Other bacterial | 0 | 0.0 | 0 | 0.0 | 9 | 1.9 | 9 | 0.8 |
| Other viral | 0 | 0.0 | 0 | 0.0 | 4 | 0.9 | 4 | 0.4 |
| Papilloma | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 2 | 0.2 |
| Pneumococcal | 5 | 1.5 | 0 | 0.0 | 28 | 6.0 | 33 | 3.0 |
| Varicella Zoster | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 2 | 0.2 |
IIV4 inactivated quadrivalent seasonal influenza vaccine, N total number of participants, n %, number and percentage of participants receiving a given coadministered vaccine
Adverse Events
Of 1082 participants included in the Safety Set who received their ADR card, 97.0% (n = 1049/1082) returned their completed ADR card after dose 1 and 100.0% (n = 115/115) of children returned it after dose 2. After dose 1, 39.2% (n = 424/1082) of the Safety Set participants reported at least one AEI or AE within 7 days of receiving GSK’s IIV4 (Table 3), and the weekly proportion of reported AEs ranged from 20.0% (n = 13/65) to 52.2% (n = 35/67) (Supplementary Table S1).
Table 3.
Cumulative number and percentage of participants reporting AEIs (in italics) and/or other AEs post vaccination with dose 1 (Safety Set)
| MedDRA Primary System Organ Class (code) Preferred Term (code) |
Belgium, N = 330 | Germany, N = 287 | Spain, N = 465 | Total, N = 1082 | ||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI LL–UL) | n | % (95% CI LL–UL) | n | % (95% CI LL–UL) | n | % (95% CI LL–UL) | |
| Any | 149 | 45.2 (15.0-78.2) | 90 | 31.4 (2.8-79.1) | 185 | 39.8 (6.5-82.8) | 424 | 39.2 (31.8-47.0) |
| Any general disorders and administration site conditions (10018065) | 119 | 36.1 (3.4-84.3) | 75 | 26.1 (3.5-66.6) | 152 | 32.7 (1.0-88.8) | 346 | 32.0 (24.1-40.8) |
| Injection site pain (10022086) | 96 | 29.1 (1.1–83.4) | 57 | 19.9 (2.4–55.8) | 119 | 25.6 (0.2–88.2) | 272 | 25.1 (17.4–34.2) |
| Fatigue (10016256) | 26 | 7.9 (1.3–23.6) | 17 | 5.9 (0.4–22.9) | 39 | 8.4 (0.0–54.8) | 82 | 7.6 (5.5–10.1) |
| Injection site swelling (10053425) | 14 | 4.2 (1.8–8.2) | 16 | 5.6 (0.2–25.9) | 37 | 8.0 (1.5–22.5) | 67 | 6.2 (4.2–8.8) |
| Injection site erythema (10022061) | 8 | 2.4 (0.1–11.1) | 18 | 6.3 (1.0–19.2) | 31 | 6.7 (1.4–18.2) | 57 | 5.3 (3.2–8.2) |
| Chills (10008531) | 3 | 0.9 (0.2–2.6) | 16 | 5.6 (2.9–9.5) | 19 | 4.1 (0.0–81.9) | 38 | 3.5 (1.7–6.4) |
| Fever (10037660) | 5 | 1.5 (0.0–8.2) | 3 | 1.0 (0.0–6.8) | 22 | 4.7 (1.8–9.9) | 30 | 2.8 (1.2–5.4) |
| Influenza-like illness (10022004) | 4 | 1.2 (0.0–8.5) | 0 | 0.0 (0.0–1.3) | 0 | 0.0 (0.0–0.8) | 4 | 0.4 (0.0–1.9) |
| Discomfort (10013082); Injection site bruising (10022052); Injection site induration (10022075); Injection site pruritus (10022093); Peripheral swelling (10048959) | n = 0; 0; 2; 2; 0 | NR | 0 each | NR | n = 2; 2; 0; 0; 2 | NR | 2 each | 0.2 each, NR |
| Asthenia (10003549); Feeling cold (10016326); Injection site discolouration (10051572); Injection site discomfort (10054266); Injection site haematoma (10022066); Injection site movement impairment (10056250); Injection site warmth (10022112) | n = 0; 1; 1; 0; 0; 1; 0 | NR | 0 each | NR | n = 1; 0; 0; 1; 1; 0; 1 | NR | 1 each | 0.1 each, NR |
| Any nervous system disorders (10029205) | 31 | 9.4 (5.1–15.5) | 17 | 5.9 (0.7–19.8) | 43 | 9.3 (0.0–83.4) | 91 | 8.4 (6.0–11.3) |
| Headache (10019211) | 23 | 7.0 (1.2–20.7) | 14 | 4.9 (0.5–17.7) | 37 | 8.0 (0.0–77.2) | 74 | 6.8 (4.6–9.7) |
| Dizziness (10013573) | 10 | 3.0 (1.3–5.9) | 6 | 2.1 (0.0–14.5) | 9 | 1.9 (0.0–47.6) | 25 | 2.3 (1.4–3.7) |
| Dysgeusia (10013911); Hypersomnia (10020765); Poor quality sleep (10062519); Psychomotor hyperactivity (10037211); Sensory disturbance (10040026); Somnolence (10041349) | n = 0; 0; 1; 0; 1; 0 | NR | n = 1; 0; 0; 0; 0; 0 | NR | n = 0; 1; 0; 1; 0; 1 | NR | 1 each | 0.1 each, NR |
| Any musculoskeletal and connective tissue disorders (10028395) | 37 | 11.2 (3.1–26.6) | 22 | 7.7 (2.9–16.0) | 25 | 5.4 (0.0–97.1) | 84 | 7.8 (4.1–13.1) |
| Myalgia (10028411) | 31 | 9.4 (3.6–19.2) | 14 | 4.9 (0.1–26.9) | 12 | 2.6 (0.0–85.0) | 57 | 5.3 (2.2–10.5) |
| Arthralgia (10003239) | 10 | 3.0 (0.0–18.3) | 10 | 3.5 (0.0–28.6) | 17 | 3.7 (0.0–91.2) | 37 | 3.4 (1.5–6.7) |
| Muscle twitching (10028347); Muscular weakness (10028372); Musculoskeletal stiffness (10052904) | n = 0; 1; 1 | NR | n = 1; 0; 0 | NR | 0 each | NR | 1 each | 0.1 each, NR |
| Any respiratory, thoracic, and mediastinal disorders (10038738) | 21 | 6.4 (0.1–35.0) | 17 | 5.9 (0.0–36.3) | 33 | 7.1 (2.7–14.8) | 71 | 6.6 (4.1–9.8) |
| Rhinorrhoea (10039101) | 18 | 5.5 (0.0–37.2) | 11 | 3.8 (0.1–21.6) | 30 | 6.5 (2.1–14.4) | 59 | 5.5 (3.3–8.5) |
| Cough (10011224) | 2 | 0.6 (0.0–6.0) | 4 | 1.4 (0.0–17.7) | 4 | 0.9 (0.0–15.4) | 10 | 0.9 (0.3–2.0) |
| Oropharyngeal pain (10068319) | 1 | 0.3 (0.0–5.5) | 5 | 1.7 (0.0–12.7) | 1 | 0.2 (0.0–14.1) | 7 | 0.7 (0.1–2.2) |
| Dysphonia (10013952); Sneezing (10041232) | n = 1; 0 | NR | 1 each | NR | n = 0; 1 | NR | 2 each | 0.2 each, NR |
| Dyspnoea (10013968); Epistaxis (10015090); Increased upper airway secretion (10062717); Tonsillar inflammation (10065169) | n = 1; 1; 0; 0 | NR | 0 each | NR | n = 0; 0; 1; 1 | NR | 1 each | 0.1 each, NR |
| Any gastrointestinal disorders (10017947) | 15 | 4.6 (1.0–12.4) | 8 | 2.8 (0.1–14.1) | 34 | 7.3 (0.0–67.0) | 57 | 5.3 (3.2–8.1) |
| Diarrhoea (10012735) | 5 | 1.5 (0.0–16.5) | 4 | 1.4 (0.0–7.2) | 16 | 3.4 (0.8–9.3) | 25 | 2.3 (1.2–4.1) |
| Nausea (10028813) | 7 | 2.1 (0.9–4.3) | 2 | 0.7 (0.0–13.7) | 12 | 2.6 (0.0–85.0) | 21 | 1.9 (0.8–4.0) |
| Vomiting (10047700) | 4 | 1.2 (0.0–9.5) | 3 | 1.1 (0.0–13.3) | 5 | 1.1 (0.1–4.9) | 12 | 1.1 (0.6–2.0) |
| Abdominal pain upper (10000087); Dry mouth (10013781) | 1 each | NR | n = 0; 1 | NR | n = 2; 1 | NR | 3 each | 0.3 each, NR |
| Constipation (10010774); Flatulence (10016766); Frequent bowel movements (10017367); Lip dry (10024552); Stomatitis (10042128) | n = 1; 0; 0; 0; 0 | NR | 0 each | NR | n = 0; 1; 1; 1; 1 | NR | 1 each | 0.1 each, NR |
| Lip swelling (10024570); Swollen tongue (10042727) | 0 each | NR | 0 each | NR | 0 each | NR | 0 each | 0.0 each, NR |
| Any skin and subcutaneous tissue disorders (10040785) | 9 | 2.7 (0.0–15.8) | 6 | 2.1 (0.2–8.2) | 17 | 3.7 (0.0–63.0) | 32 | 3.0 (1.7–4.8) |
| Pruritus (10037087) | 7 | 2.1 (0.0–18.8) | 4 | 1.4 (0.2–5.2) | 12 | 2.6 (0.0–40.1) | 23 | 2.1 (1.1–3.6) |
| Rash (10037844) | 3 | 0.9 (0.2–2.6) | 0 | 0.0 (0.0–1.3) | 3 | 0.7 (0.0–36.8) | 6 | 0.6 (0.2–1.3) |
| Erythema (10015150) | 0 | 0.0 (0.0–1.1) | 0 | 0.0 (0.0–1.3) | 2 | 0.4 (0.0–8.0) | 2 | 0.2 (0.0–0.7) |
| Night sweats (10029410); Rash macular (10037867) | 0 each | NR | 1 each | NR | 0 each | NR | 1 each | 0.1 each, NR |
| Swelling face (10042682) | 0 | 0.0 (0.0–1.1) | 0 | 0.0 (0.0–1.3) | 0 | 0.0 (0.0–0.8) | 0 | 0.0 (0.0–0.3) |
| Any metabolism and nutrition disorders (10027433) | 4 | 1.2 (0.0–9.5) | 1 | 0.4 (0.0–7.1) | 14 | 3.0 (1.5–5.4) | 19 | 1.8 (0.7–3.6) |
| Decreased appetite (10061428) | 4 | 1.2 (0.0–9.5) | 1 | 0.4 (0.0–7.1) | 14 | 3.0 (1.5–5.4) | 19 | 1.8 (0.7–3.6) |
| Any psychiatric disorders (10037175) | 0 | 0.0 (0.0–1.1) | 3 | 1.1 (0.1–4.2) | 11 | 2.4 (0.2–9.4) | 14 | 1.3 (0.4–3.1) |
| Irritability (10022998) | 0 | 0.0 (0.0–1.1) | 2 | 0.7 (0.1–2.6) | 11 | 2.4 (0.2–9.4) | 13 | 1.2 (0.3–3.1) |
| Restlessness (10038743) | 0 | 0.0 (0.0–1.1) | 1 | 0.4 (0.0–4.6) | 0 | 0.0 (0.0–0.8) | 1 | 0.1 (0.0–0.7) |
| Any infections and infestations (10021881) | 0 | 0.0 (0.0–1.1) | 6 | 2.1 (0.0–25.1) | 4 | 0.9 (0.0–15.4) | 10 | 0.9 (0.1–3.2) |
| Nasopharyngitis (10028810) | 0 | 0.0 (0.0–1.1) | 3 | 1.1 (0.0–13.3) | 2 | 0.4 (0.0–69.7) | 5 | 0.5 (0.0–2.0) |
| Influenza (10022000) | 0 | 0.0 (0.0–1.1) | 3 | 1.1 (0.0–13.3) | 0 | 0.0 (0.0–0.8) | 3 | 0.3 (0.0–2.1) |
| Pharyngitis (10034835); Upper respiratory tract infection (10046306) | 0 each | NR | 0 each | NR | 1 each | NR | 1 each | 0.1 each, NR |
| Any eye disorders (10015919) | 1 | 0.3 (0.0–9.7) | 2 | 0.7 (0.0–13.7) | 6 | 1.3 (0.0–60.5) | 9 | 0.8 (0.2–2.3) |
| Eye allergy (10015907) | 1 | 0.3 (0.0–9.7) | 2 | 0.7 (0.0–13.7) | 5 | 1.1 (0.0–39.4) | 8 | 0.7 (0.2–1.9) |
| Ocular hyperaemia (10030041) | 0 | 0.0 (0.0–1.1) | 0 | 0.0 (0.0–1.3) | 1 | 0.2 (0.0–44.8) | 1 | 0.1 (0.0–0.7) |
| Any immune system disorders (10021428) | 0 | 0.0 (0.0–1.1) | 2 | 0.7 (0.1–2.6) | 3 | 0.7 (0.0–36.8) | 5 | 0.5 (0.1–1.1) |
| Hypersensitivity (10020751) | 0 | 0.0 (0.0–1.1) | 2 | 0.7 (0.1–2.6) | 3 | 0.7 (0.0–36.8) | 5 | 0.5 (0.1–1.1) |
| Any vascular disorders (10047065) | 2 | 0.6 (0.1–2.2) | 0 | 0.0 (0.0–1.3) | 0 | 0.0 (0.0–0.8) | 2 | 0.2 (0.0–0.8) |
| Hot flush (10060800) | 1 | 0.3 (0.0–5.5) | 0 | 0.0 (0.0–1.3) | 0 | 0.0 (0.0–0.8) | 1 | 0.1 (0.0–0.7) |
| Hypertension (10020772) | 1 | 0.3 (0.0–3.9) | 0 | 0.0 (0.0–1.3) | 0 | 0.0 (0.0–0.8) | 1 | 0.1 (0.0–0.7) |
| Any ear and labyrinth disorders (10013993) | 1 | 0.3 (0.0–3.9) | 0 | 0.0 (0.0–1.3) | 0 | 0.0 (0.0–0.8) | 1 | 0.1 (0.0–0.7) |
| Ear pain (10014020) | 1 | 0.3 (0.0–3.9) | 0 | 0.0 (0.0–1.3) | 0 | 0.0 (0.0–0.8) | 1 | 0.1 (0.0–0.7) |
| Any investigations (10022891) | 0 | 0.0 (0.0–1.1) | 1 | 0.4 (0.0–4.6) | 0 | 0.0 (0.0–0.8) | 1 | 0.1 (0.0–0.7) |
| Heart rate increased (10019303) | 0 | 0.0 (0.0–1.1) | 1 | 0.4 (0.0–4.6) | 0 | 0.0 (0.0–0.8) | 1 | 0.1 (0.0–0.7) |
Bold values represent the MedDRA Primary System Organ Class
Terms in italics represent Preferred Terms, which correspond to the predefined AEIs. AEs that occurred in three or fewer cases were grouped to simplify the table, with the number of cases presented for each symptom and the total number (%) for each symptom, but not each individual 95% CI
AE adverse event, AEI adverse event of interest, CI confidence interval (extended Clopper–Pearson exact CI for clustered data), LL lower limit, MedDRA Medical Dictionary for Regulatory Activities, N total number of participants, n %, number and percentage of participants reporting a given symptom, NR not reported, UL upper limit
After dose 1, 60.8% (n = 658/1082) reported no AEs, 15.4% (n = 167/1082) reported one AE, 8.9% (n = 96/1082) reported two AEs, 7.1% (n = 77/1082) reported three AEs, and 7.8% (n = 84/1082) reported four or more AEs. After dose 2, 92.2% (n = 106/115) reported no AE, 3.5% (n = 4/115) reported one AE, 0.9% (n = 1/115) reported two AEs, 1.7% (n = 2/115) reported three AEs, and 1.7% (n = 2/115) reported four AEs (Supplementary Table S2).
The most frequently reported AEIs after dose 1 were injection site pain (25.1%; n = 272/1082), fatigue (7.6%; n = 82/1082), headache (6.8%; n = 74/1082), injection site swelling (6.2%; n = 67/1082), rhinorrhoea (5.5%; n = 59/1082), myalgia (5.3%; n = 57/1082), injection site erythema (5.3%; n = 57/1082), chills (3.5%; n = 38/1082), and arthralgia (3.4%; n = 37/1082) (Table 3). The most frequently reported unsolicited AEs after dose 1 were cough (0.9%; n = 10/1082), oropharyngeal pain (0.7%; n = 7/1082), nasopharyngitis (0.5%; n = 5/1082), influenza-like illness (0.4%; n = 4/1082), and influenza (0.3%, n = 3/1082) (Table 3).
After vaccination with dose 2, the overall percentage of children who experienced at least one AEI within 7 days post vaccination was 7.8% (n = 9/115) (Table 4), and their weekly percentages ranged from 0.0% (n = 0/30) to 25.0% (n = 1/4) (Supplementary Table S1). The most frequently reported AEIs after dose 2 were fever (2.6%; n = 3/115), rhinorrhoea (2.6%; n = 3/115), injection site swelling (1.7%; n = 2/115), and irritability (1.7%; n = 2/115). Other AEIs were reported by < 1% of participants (Table 4). The following unsolicited AEs were reported by one participant each after dose 2: injection site haematoma, cough, hand-foot-and-mouth disease, and nasopharyngitis (Table 4).
Table 4.
Cumulative number and percentage of participants reporting AEIs (in italics) and/or other AEs post vaccination with dose 2 (Safety Set)
| MedDRA Primary System Organ Class (code) Preferred Term (code) |
Spain N = 115 |
|
|---|---|---|
| n | % (95% CI LL–UL) | |
| Any | 9 | 7.8 (1.6–21.2) |
| Any general disorders and administration site conditions (10018065) | 5 | 4.4 (0.9–12.0) |
| Fever (10037660) | 3 | 2.6 (0.5–7.4) |
| Injection site swelling (10053425) | 2 | 1.7 (0.2–6.1) |
| Chills (10008531) | 1 | 0.9 (0.0–4.8) |
| Injection site erythema (10022061) | 1 | 0.9 (0.0–4.8) |
| Injection site pain (10022086) | 1 | 0.9 (0.0–4.8) |
| Injection site haematoma (10022066) | 1 | 0.9 (0.0–4.8) |
| Fatigue (10016256) | 0 | 0.0 (0.0–3.2) |
| Any respiratory, thoracic, and mediastinal disorders (10038738) | 4 | 3.5 (0.8–9.6) |
| Rhinorrhoea (10039101) | 3 | 2.6 (0.5–7.4) |
| Cough (10011224) | 1 | 0.9 (0.0–4.8) |
| Any gastrointestinal disorders (10017947) | 2 | 1.7 (0.2–6.1) |
| Diarrhoea (10012735) | 1 | 0.9 (0.0–4.8) |
| Vomiting (10047700) | 1 | 0.9 (0.0–4.8) |
| Lip swelling (10024570) | 0 | 0.0 (0.0–3.2) |
| Nausea (10028813) | 0 | 0.0 (0.0–3.2) |
| Swollen tongue (10042727) | 0 | 0.0 (0.0–3.2) |
| Any infections and infestations (10021881) | 2 | 1.7 (0.2–6.1) |
| Hand-foot-and-mouth disease (10019113) | 1 | 0.9 (0.0–4.8) |
| Nasopharyngitis (10028810) | 1 | 0.9 (0.0–4.8) |
| Any psychiatric disorders (10037175) | 2 | 1.7 (0.2–6.1) |
| Irritability (10022998) | 2 | 1.7 (0.2–6.1) |
| Any skin and subcutaneous tissue disorders (10040785) | 1 | 0.9 (0.0–4.8) |
| Rash (10037844) | 1 | 0.9 (0.0–4.8) |
| Pruritus (10037087) | 0 | 0.0 (0.0–3.2) |
| Swelling face (10042682) | 0 | 0.0 (0.0–3.2) |
| Any eye disorders (10015919) | 0 | 0.0 (0.0–3.2) |
| Eye allergy (10015907) | 0 | 0.0 (0.0–3.2) |
| Any immune system disorders (10021428) | 0 | 0.0 (0.0–3.2) |
| Hypersensitivity (10020751) | 0 | 0.0 (0.0–3.2) |
| Any metabolism and nutrition disorders (10027433) | 0 | 0.0 (0.0–3.2) |
| Decreased appetite (10061428) | 0 | 0.0 (0.0–3.2) |
| Any musculoskeletal and connective tissue disorders (10028395) | 0 | 0.0 (0.0–3.2) |
| Arthralgia (10003239) | 0 | 0.0 (0.0–3.2) |
| Myalgia (10028411) | 0 | 0.0 (0.0–3.2) |
| Any nervous system disorders (10029205) | 0 | 0.0 (0.0–3.2) |
| Dizziness (10013573) | 0 | 0.0 (0.0–3.2) |
| Headache (10019211) | 0 | 0.0 (0.0–3.2) |
Bold values represent the MedDRA Primary System Organ Class. Terms in italics represent Preferred Terms which correspond to the predefined AEIs
AE adverse event, AEI adverse event of interest, CI confidence interval (extended Clopper–Pearson exact CI for clustered data), LL lower limit, MedDRA Medical Dictionary for Regulatory Activities, N total number of participants, n % number and percentage of participants reporting a given symptom, UL upper limit
Of note, the weekly cumulative percentage of participants reporting any AEs within 7 days post vaccination varied from 45.2% to 54.2% in Belgium, 20.0% to 31.7% in Germany, and 38.5% to 46.4% in Spain after dose 1 (Supplementary Table S3), and ranged from 7.8% to 25.0% in Spain after dose 2 (Supplementary Table S4). Among children < 9 years in Spain who received any vaccination with GSK’s IIV4, 16.5% (n = 19/115) reported one AE, 2.6% (n = 3/115) reported two AEs, and 17.4% (n = 20/115) reported three or more AEs.
In Spain, for children and adult participants, the most frequently reported AEs after any of the two doses were injection site pain (25.6%; n = 119/465), fatigue (8.4%; n = 39/465), injection site swelling (8.2%; n = 38/465), headache (8.0%; n = 37/465), rhinorrhoea (6.9%; n = 32/465), injection site erythema (6.7%; n = 31/465), and pyrexia (5.4%; n = 25/465) (Table 5).
Table 5.
Cumulative number and percentage of participants reporting AEIs (in italics) and/or other AEs post vaccination with any of the two doses (Safety Set)
| MedDRA Primary System Organ Class (code) Preferred Term (code) |
Spain N = 465 |
||
|---|---|---|---|
| n | n | % (95% CI LL–UL) | |
| Any | 508 | 191 | 41.1 (13.9–73.1) |
| Any general disorders and administration site conditions (10018065) | 286 | 156 | 33.6 (2.6–83.4) |
| Injection site pain (10022086) | 120 | 119 | 25.6 (0.2–88.2) |
| Fatigue (10016256) | 39 | 39 | 8.4 (0.0–54.8) |
| Injection site swelling (10053425) | 39 | 38 | 8.2 (2.5–18.8) |
| Injection site erythema (10022061) | 32 | 31 | 6.7 (1.4–18.2) |
| Pyrexia (10037660) | 25 | 25 | 5.4 (3.5–7.8) |
| Chills (10008531) | 20 | 20 | 4.3 (0.0–75.9) |
| Discomfort (10013082)a; Injection site bruising (10022052)b; Injection site haematoma (10022066)a; Peripheral swelling (10048959) | 2 each | 2 each | 0.4 (0.0–8.0 26.3a/69.7b) |
| Asthenia (10003549)c; Injection site discomfort (10054266)c; Injection site warmth (10022112) | 1 each | 1 each | 0.2 (0.0–14.1/44.8c) |
| Any nervous system disorders (10029205) | 49 | 43 | 9.3 (0.0–83.4) |
| Headache (10019211) | 37 | 37 | 8.0 (0.0–77.2) |
| Dizziness (10013573) | 9 | 9 | 1.9 (0.0–47.6) |
| Hypersomnia (10020765)d; Psychomotor hyperactivity (10037211); Somnolence (10041349) | 1 each | 1 each | 0.2 (0.0–14.1/44.8d) |
| Any gastrointestinal disorders (10017947) | 42 | 36 | 7.7 (0.0–56.2) |
| Diarrhoea (10012735) | 17 | 17 | 3.7 (2.1–6.0) |
| Nausea (10028813) | 12 | 12 | 2.6 (0.0–85.0) |
| Vomiting (10047700) | 6 | 6 | 1.3 (0.5–2.8) |
| Abdominal pain upper (10000087) | 2 | 2 | 0.4 (0.0–8.0) |
| Dry mouth (10013781)e; Flatulence (10016766); Frequent bowel movements (10017367)e; Lip dry (10024552)e; Stomatitis (10042128) | 1 | 1 | 0.2 (0.0–14.1/44.8e) |
| Lip swelling (10024570); Swollen tongue (10042727) | 0 | 0 | 0.0 (0.0–0.8) |
| Any respiratory, thoracic, and mediastinal disorders (10038738) | 42 | 36 | 7.7 (5.5–10.6) |
| Rhinorrhoea (10039101) | 33 | 32 | 6.9 (4.8–9.6) |
| Cough (10011224) | 5 | 5 | 1.1 (0.1–4.9) |
| Increased upper airway secretion (10062717); Oropharyngeal pain (10068319); Sneezing (10041232); Tonsillar inflammation (10065169)f | 1 each | 1 each | 0.2 (0.0–14.1/44.8f) |
| Any musculoskeletal and connective tissue disorders (10028395) | 29 | 25 | 5.4 (0.0–97.1) |
| Arthralgia (10003239) | 17 | 17 | 3.7 (0.0–91.2) |
| Myalgia (10028411) | 12 | 12 | 2.6 (0.0–85.0) |
| Any skin and subcutaneous tissue disorders (10040785) | 18 | 18 | 3.9 (0.0–54.4) |
| Pruritus (10037087) | 12 | 12 | 2.6 (0.0–40.1) |
| Rash (10037844) | 4 | 4 | 0.9 (0.0–15.4) |
| Erythema (10015150) | 2 | 2 | 0.4 (0.0–8.0) |
| Swelling face (10042682) | 0 | 0 | 0.0 (0.0–0.8) |
| Any metabolism and nutrition disorders (10027433) | 14 | 14 | 3.0 (1.5–5.4) |
| Decreased appetite (10061428) | 14 | 14 | 3.0 (1.5–5.4) |
| Any psychiatric disorders (10037175) | 13 | 13 | 2.8 (0.0–21.7) |
| Irritability (10022998) | 13 | 13 | 2.8 (0.0–21.7) |
| Any eye disorders (10015919) | 6 | 6 | 1.3 (0.0–60.5) |
| Eye allergy (10015907) | 5 | 5 | 1.1 (0.0–39.4) |
| Ocular hyperaemia (10030041) | 1 | 1 | 0.2 (0.0–44.8) |
| Any infections and infestations (10021881) | 6 | 6 | 1.3 (0.5–2.8) |
| Nasopharyngitis (10028810) | 3 | 3 | 0.7 (0.0–36.8) |
| Hand-foot-and-mouth disease (10019113) | 1 | 1 | 0.9 (0.0-4.8) |
| Pharyngitis (10034835); Upper respiratory tract infection (10046306) | 1 each | 1 each | 0.2 (0.0–14.1) |
| Any immune system disorders (10021428) | 3 | 3 | 0.7 (0.0–36.8) |
| Hypersensitivity (10020751) | 3 | 3 | 0.7 (0.0–36.8) |
Bold values represent the MedDRA Primary System Organ Class. Terms in italics represent Preferred Terms, which correspond to the predefined AEIs
AE adverse event, AEI adverse event of interest, CI confidence interval (extended Clopper–Pearson exact CI for clustered data), LL lower limit, MedDRA Medical Dictionary for Regulatory Activities, N total number of participants, n number of AEs reported in total after both doses, n % number and percentage of participants reporting a given symptom at least once, considering both adverse drug reaction cards, UL upper limit
a,b,c,d,e,fCI UL for the specified AEs
Occasionally, allergic reactions are reported after influenza vaccination, especially with the egg-based inactivated influenza vaccine; these can, for instance, present as swelling in the head and neck region (e.g. face, lips, tongue, and throat) or as an anaphylactic reaction [19]. In this study, after dose 1, participants reported allergic reactions such as rhinorrhoea (n = 59/1082, 5.5%), pruritis (n = 23/1082, 2.1%), and eye allergy (n = 8/1082, 0.7%) (Table 3). After dose 2, three children (n = 3/115, 2.6%) reported allergic reactions (rhinorrhoea) (Table 4).
Adverse Events by Age Strata and Risk Status
The frequency of AEs varied by age group. For instance, the proportions of participants who reported at least one AE within a week after vaccination with dose 1 were 47.2% of participants 18–65 years old, followed by 38.1% of children aged 6 months–17 years and 31.6% of participants aged > 65 years (Supplementary Table S5).
Of participants who were classified as at risk for influenza-associated morbidity and mortality by their healthcare providers, 37.5% (n = 237/632) and 10.2% (n = 5/49) reported at least one AE after dose 1 and dose 2, respectively, compared to 41.6% (n = 187/450) and 6.1% (n = 4/66) of participants classified as not at risk (Supplementary Tables S6 and S7).
Of the participants at risk, 28.8% (n = 182/632) reported at least one AE in the Primary SOC general disorders and administration site conditions after dose 1, followed by 8.7% (n = 55/632) in the Primary SOC nervous system disorders, 7.8% (n = 49/632) in the Primary SOC musculoskeletal and connective tissue disorders, 5.9% (n = 37/632) in the Primary SOC gastrointestinal disorders, and 2.1% (n = 13/632) in the Primary SOC metabolism and nutrition disorders (Supplementary Tables S8 and S9).
Furthermore, of at risk participants, 1.1% (n = 7/632) reported any AE in the Primary SOC psychiatric disorders after dose 1 and 7.9% (n = 50/632) reported any AE in the Primary SOC respiratory, thoracic and mediastinal disorders after dose 1, and 6.1% (n = 3/49) after dose 2 (Supplementary Tables S8 and S9).
A post hoc assessment of frequency of fever (≥ 38 °C) in children 6 months–17 years old was conducted. After dose 1, fever was reported in 5.0% of children aged 6 months–5 years (n = 10/202) and in 5.4% of children aged 6–17 years (n = 7/129). In unprimed children < 9 years old who received dose 2 (n = 115), fever was reported in 3.2% (n = 3/94) of children aged 6 months–5 years, but was not reported in children aged 6–8 years (n = 0/21).
Serious Adverse Events and Deaths
Neither death nor serious AEs related to GSK’s IIV4 were reported in this study by healthcare providers within 7 days of vaccination. None of the reported AEs led participants to discontinue the study.
Sensitivity Analysis on the Solicited Safety Set
A sensitivity analysis for the frequency of AEs was conducted on the Solicited Safety Set after dose 1 (Supplementary Table S10). The analysis results of both sets (Safety Set and Solicited Safety Set) were close, with no considerable difference in the relative frequency of AEs between the two sets (compare with Table 3).
Discussion
This study assessed the percentages of participants who reported AEs within 7 days of vaccination with GSK’s IIV4 during the 2019/2020 influenza season. No serious AEs related to GSK’s IIV4 were reported during this study, nor any new or unexpected AE.
The cumulative percentages of reported AEs in this current passive ESS study for the 2019/2020 influenza season were close to those observed in the GSK-sponsored study for the 2018/2019 influenza season, where 43.0% (95% CI 36.8–49.4) and 23.7% (95% CI 16.1–32.9) of participants reported AEs after doses 1 and 2, respectively [15]. The most frequently reported AEs by MedDRA Primary SOC in both studies after dose 1 were the “general disorders and administration site conditions”. However, no statistical test was conducted to compare the descriptive safety outcomes of both post-marketing safety studies.
The frequency of various AEs reported in this current passive ESS study were also comparable to those described in the spontaneously reported safety database for seasonal flu as well as in GSK’s IIV4 Summary of Product Characteristics document [19].
In this passive ESS study, AEs were most frequently reported in adults aged 18–65 years (47.2%), followed by children aged 6 months–17 years (38.1%) and adults aged > 65 years (31.6%).
The safety and reactogenicity of GSK's IIV4 in this ESS study were also comparable to the safety results of GSK’s IIV4 assessed in various clinical trials that enrolled participants of different age groups. For instance, during the 7-day follow-up period after vaccination of 3036 adults aged ≥ 18 years with IIV4, injection site pain was reported by 36.4% of participants, while injection site redness and swelling were reported by almost 2% each [20]. The most frequently reported solicited general AEs during the first 7 days after IIV4 vaccination in the same study were fatigue (15.8%), headache (15.9%), and muscle aches (16.4%) [20]. Another GSK-sponsored IIV4 clinical trial in children aged 3–8 years demonstrated a comparable safety profile to this ESS study, with injection site pain reported by 47.7% of participants, redness by 0.7%, and swelling by 1.8% of children who received the IIV4 vaccine (N = 2584) [21]. In a similar IIV4 clinical trial in children aged 6–35 months, the most frequently reported local solicited AEs within 7 days after IIV4 vaccination (N = 6006) were pain (22.9%), redness (16.6%), and swelling (11.3%), and systemic symptoms included irritability (23.4%), loss of appetite (20.8%), drowsiness (17.3%), and fever ≥ 38 °C (11.2%) [22].
The frequency of reported fever in children aged 6 months–8 years in this current passive ESS study was comparable to that reported in clinical trials [21, 23]. However, it should be cautiously interpreted since the fever ≥ 38 °C was self-reported by parents in the current study, and we did not have the chance to assess the start and end date of fever during the first 7 days post vaccination.
The strength of this passive ESS study was the inclusion of participants from all age groups, from three different countries and with different health statuses (at risk, not at risk). This helps reduce the impact of clustering due to the wide diversity of enrolled participants. In addition, the heterogenicity of participants reflects the safety of the influenza vaccine in a sample of the general population who attended the clinics to receive their routine seasonal influenza vaccine in the three countries where the GSK vaccine was distributed. The notably high return rates of the ADR cards (97.0% after dose 1 and 100.0% after dose 2) in this passive ESS study were similar to or higher than the response rates reported in active surveillance studies [24]. It is worth mentioning that 59% of returned diary cards after dose 1 were delivered during the clinic visit, while after dose 2 in unprimed children < 9 years, the majority of diary cards were delivered by mail (83%). This assured the appropriateness of the approach and the information collection tools used (i.e. ADR cards that were either sent by mail in a pre-stamped envelope or obtained by paying a visit to the clinic in person, and eCRFs).
Interim analyses were also essential to identify any alarming AE that might represent a risk for vaccinees. During the study, four interim analyses were conducted between 1 October 2019 and 13 January 2020, which allowed a near real-time follow-up of vaccinees to track any potential safety signal. No safety signals were identified during the four interim analyses, during the weekly review of data listings, or at the end of the study.
One of the study limitations was the fact that all the reported AEs were self-reported and could not be verified by healthcare providers. In addition, the ADR cards did not include a separate list of local AEs for other coadministered vaccines in case the participants received more than one vaccine during the same visit, so it was not possible to differentiate between the local AEs caused by GSK’s IIV4 and the coadministered vaccines that might be more reactogenic than the influenza vaccine. However, only a small percentage (8.5%) of study participants reported receiving a coadministered vaccine during the same visit. This should be addressed in future ESS studies.
Conclusions
No serious AEs related to GSK’s IIV4 within 7 days post vaccination were reported. This study supports the favourable risk–benefit safety profile of GSK’s IIV4.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to acknowledge the contributions of Stéphanie Gilon (GSK), Jerome Wilson (GSK), Stefano Santamaria (GSK), Elvira Zandman-van Dijk (GSK), Brenda Muniz (GSK), and Sophie Caterina (GSK) for their participation in the review of the study protocol, results, and report. They would also like to acknowledge Stéphane Vanden Bemden (healthcare provider), Hugo Loos (healthcare provider), and Maria Rybo (PPDI) for their participation in the review of the study protocol, recruitment of study participants, and implementation of different study activities, and Jelena Bakusic (KU Leuven) for her participation in the recruitment of study participants and data collection for Belgium. The authors would also like to thank Business and Decision Life Sciences platform for editorial assistance and manuscript coordination on behalf of GSK. Aurélie Roth coordinated manuscript development and editorial support. The authors also thank Esther van de Vosse (Business and Decision Life Sciences, on behalf of GSK) for providing medical writing support.
Declarations
Funding
GlaxoSmithKline Biologicals SA funded this study (GSK study identifier: 207749) and was involved in all stages of the study, including analysis of the data. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and publication of this present manuscript.
Conflict of interest
Elisa Cinconze, Ugo Nwoji, Emily Lu, Huajun Wang, and Emad Yanni are employees of the GSK group of companies. Ugo Nwoji, Emily Lu, Huajun Wang, and Emad Yanni hold shares in the GSK group of companies. Xavier Martínez Gómez declares he has received financial support from Pax-Vax for participation on advisory boards for vaccines, from Pfizer for participation at conferences, and from the GSK group of companies for practical courses. Ignacio Salamanca de la Cueva declares he has received grants and/or honoraria as a consultant/advisor for attending conferences and practical courses from the GSK group of companies, Sanofi Pasteur, MSD, and Pfizer. Elisa Cinconze, Ugo Nwoji, Emily Lu, Huajun Wang, Emad Yanni, Xavier Martínez-Gómez, and Ignacio Salamanca de la Cueva declare no other financial or non-financial relationships and activities. Tamara Eckermann and Lode Godderis declare no financial and non-financial relationships and activities and no conflicts of interest.
Availability of data and material
Anonymised individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The following ethics committees (ECs), institutional review boards, and regulatory authorities were consulted in line with country requirements, namely Commissie Medische Ethiek UZ/KU Leuven (EC) in Belgium (approval received 13 September 2019), Ethik-Kommission der Bayerischen Landesärztekammer (EC) in Germany (approval received 13 September 2019), and, in Spain, CCEIBA and CCAA for Andalusia (approval received 25 July 2019) and Vall d´Hebron, Medicinal Product Research Ethics Committee of Hospital Universitari Vall d´Hebron (approval received 17 July 2019) and Generalitat de Catalunya, Directorate General of Management and Health Regulation (CCAA) (approval received 25 September 2019) for Catalonia.
Consent
Written informed consent or assent was obtained from all individual participants included in this study or their parent(s), guardian(s), or legally acceptable representative(s) prior to participation in the study.
Author contributions
All authors participated in the design, implementation, analysis, and interpretation of the study and the development of this manuscript. All authors had full access to the data and gave final approval prior to submission.
Trademarks
AlphaRix Tetra, Influsplit Tetra, and Fluarix Tetra are trademarks owned by or licensed to the GSK group of companies.
References
- 1.European Centre for Disease Prevention and Control. Factsheet about seasonal influenza. ECDC: Stockholm. https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet. Accessed 30 Apr 2021.
- 2.European Centre for Disease Prevention and Control. Seasonal influenza vaccination and antiviral use in EU/EEA Member States—Overview of vaccine recommendations for 2017–2018 and vaccination coverage rates for 2015–2016 and 2016–2017 influenza seasons. ECDC: Stockholm; 2018. https://www.ecdc.europa.eu/sites/default/files/documents/seasonal-influenza-antiviral-use-2018.pdf. Accessed 2 Mar 2021.
- 3.Cassini A, Colzani E, Pini A, Mangen MJ, Plass D, McDonald SA, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018 doi: 10.2807/1560-7917.ES.2018.23.16.17-00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matias G, Taylor R, Haguinet F, Schuck-Paim C, Lustig R, Shinde V. Estimates of hospitalization attributable to influenza and RSV in the US during 1997–2009, by age and risk status. BMC Public Health. 2017;17(1):271. doi: 10.1186/s12889-017-4177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.European Centre for Disease Prevention and Control. Seasonal influenza 2019-2020. Annual Epidemiological Report. ECDC: Stockholm; 2020. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2019_influenza-seasonal.pdf. Accessed 25 Feb 2021.
- 6.European Centre for Disease Prevention and Control. Influenza virus characterization, Summary Europe, September 2020. ECDC: Stockholm; 2020. https://www.ecdc.europa.eu/sites/default/files/documents/influenza-characterisation-report-september-2020.pdf. Accessed 10 Mar 2021.
- 7.World Health Organization (WHO). Recommendations on influenza vaccination during the 2019–2020 winter season. WHO: Copenhagen; 2019. https://www.euro.who.int/__data/assets/pdf_file/0017/413270/Influenza-vaccine-recommendations-2019-2020_en.pdf. Accessed 6 Jul 2020.
- 8.Preaud E, Durand L, Macabeo B, Farkas N, Sloesen B, Palache A, et al. Annual public health and economic benefits of seasonal influenza vaccination: a European estimate. BMC Public Health. 2014;14:813. doi: 10.1186/1471-2458-14-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO). Fact sheets. Influenza (seasonal). 2018. https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal). Accessed 9 Jul 2020.
- 10.World Health Organization (WHO). WHO recommendations on the composition of influenza virus vaccines. WHO: Geneva; 2020. https://www.who.int/influenza/vaccines/virus/recommendations/en/. Accessed 9 Jul 2020
- 11.Pharmacovigilance Risk Assessment Committee (PRAC). Guideline on influenza vaccines. Non-clinical and clinical module. EMA/CHMP/VWP/457259/2014 European Medicines Agency (EMA). London; 2016. https://www.ema.europa.eu/en/documents/scientific-guideline/influenza-vaccines-non-clinical-clinical-module_en.pdf. Accessed 19 Jul 2020.
- 12.de Lusignan S, Correa A, Dos Santos G, Meyer N, Haguinet F, Webb R, et al. Enhanced safety surveillance of influenza vaccines in general practice, winter 2015–16: feasibility study. JMIR Public Health Surveill. 2019;5(4):e12016. doi: 10.2196/12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lusignan S, Ferreira F, Damaso S, Byford R, Pathirannehelage S, Yeakey A, et al. Enhanced passive surveillance of influenza vaccination in England, 2016–2017- an observational study using an adverse events reporting card. Hum Vaccin Immunother. 2019;15(5):1048–1059. doi: 10.1080/21645515.2019.1565258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lusignan S, Damaso S, Ferreira F, Byford R, McGee C, Pathirannehelage S, et al. Brand-specific enhanced safety surveillance of GSK's Fluarix Tetra seasonal influenza vaccine in England: 2017/2018 season. Hum Vaccin Immunother. 2020;16(8):1762–1771. doi: 10.1080/21645515.2019.1705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dos Santos G, Nguyen BY, Damaso S, Godderis L, Martinez-Gomez X, Eckermann T, et al. Brand-specific Enhanced Safety Surveillance of GSK's quadrivalent seasonal influenza vaccine in Belgium, Germany and Spain for the 2018/2019 Season. Drug Saf. 2020;43(3):265–279. doi: 10.1007/s40264-019-00893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Seasonal influenza. 2021. https://www.who.int/ith/vaccines/seasonal_influenza/en/. Accessed 10 Mar 2021.
- 17.GSK. GSK Study Register. 2019. https://www.gsk-studyregister.com/en/trial-details/?id=207749. Accessed 8 Jul 2020.
- 18.MedDRA. Medical dictionary for regulatory activities. 2019. https://www.meddra.org/basics. Accessed 1 Apr 2019
- 19.GlaxoSmithKline UK. Fluarix Tetra suspension for injection in pre-filled syringe. 2020. https://www.medicines.org.uk/emc/product/3021/smpc. Accessed 8 Jul 2020.
- 20.Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, et al. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥ 18 years. BMC Infect Dis. 2013;13:343. doi: 10.1186/1471-2334-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain VK, Rivera L, Zaman K, Espos RA, Jr, Sirivichayakul C, Quiambao BP, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med. 2013;369(26):2481–2491. doi: 10.1056/NEJMoa1215817. [DOI] [PubMed] [Google Scholar]
- 22.Claeys C, Zaman K, Dbaibo G, Li P, Izu A, Kosalaraksa P, et al. Prevention of vaccine-matched and mismatched influenza in children aged 6–35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health. 2018;2(5):338–349. doi: 10.1016/S2352-4642(18)30062-2. [DOI] [PubMed] [Google Scholar]
- 23.Claeys C, Drame M, Garcia-Sicilia J, Zaman K, Carmona A, Tran PM, et al. Assessment of an optimized manufacturing process for inactivated quadrivalent influenza vaccine: a phase III, randomized, double-blind, safety and immunogenicity study in children and adults. BMC Infect Dis. 2018;18(1):186. doi: 10.1186/s12879-018-3079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spila Alegiani S, Alfonsi V, Appelgren EC, Ferrara L, Gallo T, Alicino C, et al. Active surveillance for safety monitoring of seasonal influenza vaccines in Italy, 2015/2016 season. BMC Public Health. 2018;18(1):1401. doi: 10.1186/s12889-018-6260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


