Abstract
Gastrointestinal (GI) dysfunction is common in the critical care setting and is highly associated with clinical outcomes. Opioids increase the risk for GI dysfunction and are frequently prescribed to reduce pain in critically ill patients. However, the role of opioids in GI function remains uncertain in the ICU. This review aims to describe the effect of opioids on GI motility, their potential risk of increasing infection and the treatment of GI dysmotility with opioid antagonists in the ICU setting.
Keywords: Opioids, Critical illness, Gastrointestinal function, Gastrointestinal microbiome
Introduction
A round-table conference in 1997 concluded that GI function was crucial for the clinical outcomes of ICU patients and was associated with poor prognosis [1]. However, there is no unanimity about the definition of GI dysfunction [2–4]. The Working Group on Abdominal Problems (WGAP) recommended the term “acute gastrointestinal injury” (AGI) with four grades of severity be used to describe the GI function of critically ill patients [5]. AGI severity grades are better associated with clinical outcomes in critically ill patients and predict prognosis [6, 7]. GI problems are common and occur in 50–60% of ICU patients [8]. A total of 59.1% of patients had at least one GI symptom during their first ICU stay, and 36.2% had two or more symptoms [9]. GI dysfunction includes high gastric residuals, gastroesophageal reflux, aspiration, constipation, diarrhoea, and abdominal distention and is associated with a prolonged length of stay in the ICU and increased mortality [10–13]. In this review, we provide an update on the impact of opioids on gastrointestinal function.
Pain is ubiquitous in the ICU, with 50% of ICU patients suffering from moderate to severe pain [14]. Pain in the ICU has multiple aetiologies, including underlying illness, invasive therapy, incisions, daily care, penetrating catheters and tubes [15]. Furthermore, delirium, impaired communication, sleep deprivation and preexisting chronic pain exacerbate the pain experienced in the ICU [15]. Opioids are the cornerstone treatment for moderate to severe pain. Opioid prescriptions have quickly skyrocketed, and they are commonly administered in the ICU, with 63–86% of ICU patients treated with opioids [16, 17]. However, the adverse effects of opioid therapy cause discomfort, seriously impact the patient’s quality of life and can even lead to the discontinuation of treatment. Studies have shown that opioids strongly inhibit the GI tract [18–21]. The GI tract is sensitive to low doses of opioids, and an animal study demonstrated that one-quarter of the morphine needed to produce analgesia inhibits intestinal motility and that one-twentieth of the analgesic dose is enough to treat diarrhoea [22].
Distribution and physiological function of opioid receptors in the GI tract
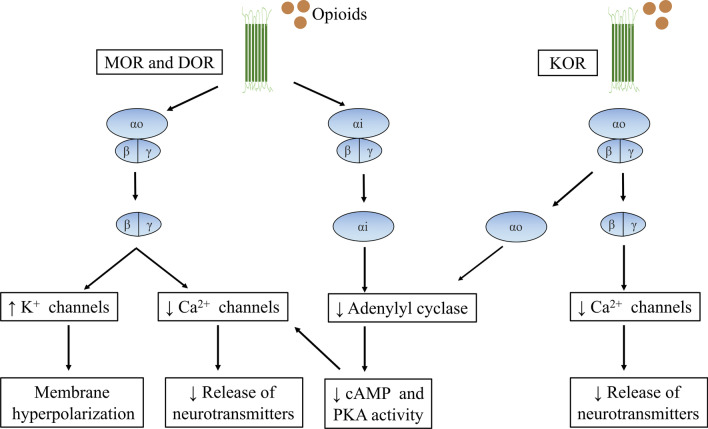
Opioid receptors are widely distributed in the enteric nervous system (ENS) of the GI tract (Fig. 1). The primary opioid receptors include the mu opioid receptor (MOR), delta opioid receptor (DOR) and kappa opioid receptor (KOR). Opioid receptors located in the interneurons, secretomotor neurons and musculomotor neurons of the ENS mainly sustain the homeostasis of the GI tract [23]. Inhibition of neuronal excitability and imbalance of neurotransmitter release are the principal mechanisms by which opioids regulate the GI tract [24]. The activation of opioid receptors can change the concentration of K+ and Ca2+ by G protein-coupled receptors and then lead to the suppression of neuronal depolarization, decreased neuronal excitability and the inhibition of neurotransmitter release (Fig. 2) [25]. Circular muscle and longitudinal muscle in the GI tract have different nerve inputs, and opioid receptor agonists impair the normal motility of the GI tract by increasing the contraction activities of circular muscle and decreasing the contraction activities of longitudinal muscle through the suppression of neuronal excitability [26]. The inhibition of the excitatory neurotransmitters acetylcholine and substance-P participate in the discoordination motility of the GI tract, and the decrease of the inhibitory neurotransmitters vasoactive intestinal peptide and nitric oxide participate in the abnormal secretion and absorption of the GI tract [27].
Fig. 1.
Summary of opioid-induced effects in the GI system. MOR mu opioid receptor, DOR delta opioid receptor, KOR kappa opioid receptor, CM circular muscle, LM longitudinal muscle, SM submucosa, Muc mucosa, Ach acetylcholine, SP substance-P, NO nitric oxide, VIP vasoactive intestinal polypeptide
Fig. 2.
Activation of opioid receptors in the enteric nervous system. MOR mu opioid receptor, DOR delta opioid receptor, KOR kappa opioid receptor, cAMP cyclic adenosine monophosphate, PKA protein kinase A
In humans, MOR is expressed in both the myenteric and submucosal plexuses throughout the small and large intestine, and DOR and KOR are distributed in excitatory and inhibitory motor neurons in the human colon [28]. MOR is more distributed in the submucosal plexus than in the myenteric plexus. The activation of MOR can delay gastric emptying, increase gastrointestinal transit time, and suppress the secretion of water and electrolytes in the intestine. KOR appears more in the myenteric plexus. Most enteric neurons coexpress MOR and DOR receptors [29]. MOR and DOR are the primary opioid receptors for the regulation of enteric neurons. MOR and DOR are both distributed in the submucosal plexus and myenteric plexus, and the activation of MOR and DOR suppresses the excitation of enteric neurons by hyperpolarizing them, delays gastric emptying, increases the time of gastrointestinal transit, and suppresses the secretion of water and electrolytes in the intestine. The activation of KOR also inhibits the ENS contractile response, but its inhibition of the GI tract is weaker than that of MOR and DOR [30, 31].
Methods
To investigate the effect of opioids on the GI function in the ICU, we searched PubMed and Embase from inception until September 2021. We used the keywords “ICU OR intensive care OR critically ill OR critical care”, “gastrointestinal OR gut OR constipation OR abdominal OR gastr* OR bowel”, “opioid* OR opiate*” and “clinical stud*”. Clinical studies associated with opioids and GI function in ICU adult patients were included. Paper in a language other than English and pediatric studies were excluded.
Results
The initial search yielded 1155, with 520 in PubMed and 635 in Embase. After screening by title and abstract, 1103 were removed for duplication articles, reviews, pediatric studies, and articles that did not report the relationship between opioids and GI function in critically ill patients. 52 papers were screened by reading full articles. In the final, 26 articles reported the effect of opioids on the GI function in ICU patients, and one clinical study was added after reference search. The detailed information about the studies is listed in Table1, 2, and 4.
Table 1.
Studies about the effect of opioids on gastric emptying in the ICU
| Clinical trials | Study design | Population | Group | Study goal | Results | Type of opioids in the studies | The effect of opioids |
|---|---|---|---|---|---|---|---|
| McArthur et al. [38] | RCT | 21 brain injured patients | M&M group (n = 11) vs propofol group (n = 10) | To compare the effect of opioids and nonopioid sedation on gastric emptying | Gastric emptying was no difference in brain injured patients between two groups | Morphine | Gastric emptying was not improved by avoiding morphine |
| Heyland et al. [32] | PCS | 84 individuals | Mechanically ventilated patients (n = 72) vs healthy volunteers (n = 12) | To investigate variables that associated with impaired gastric emptying | Variables included age, sex and use of opioids | Morphine and morphine equivalent | Use of opioids and dose of opioids were associated with impaired gastric emptying |
| Bosscha et al. [36] | POS |
16 individuals in Surgical ICU |
Mechanically ventilated patients (n = 7) vs healthy volunteers (n = 9) | To determine the GI motility characteristics that associated with gastric intention | Morphine administration affected antroduodenal motility | Morphine | Opioids impaired the GI motility in mechanically ventilated patients |
| Mentec et al. [35] | POS | 153 patients with nasogastric tube feeding | Normal GAV (n = 104) vs increased GAV (n = 49) | To investigate risk factors for increased GAV and UDI and complications |
Risk factors: sedation, catecholamines Complications: higher incidence of pneumonia, longer ICU stay, higher mortality |
Fentanyl | Opioids were risk factors for increased GAV |
| Nguyen et al. [37] | Descriptive study | 36 individuals in mixed medical and surgical ICU | M&M group (n = 20) vs propofol group (n = 16) | To evaluate the effects of sedation with M&M vs propofol on gastric emptying | Patients receiving M&M had more impaired gastric emptying | Morphine | M&M inhibited gastric emptying |
| Berger et al. [82] | PS | 105 patients in surgical ICU | NA | To assess the rate of the migration of the self-propelled feeding tube in the ICU patients | Self reported tubes can be considered as a promising tool to facilitate enteral nutrition | Morphine and fentanyl | Morphine was associated with lower progression rates |
| Chapman et al. [83] | POS | 39 patients in the mixed medical and surgical ICU | Mechanically ventilated patients (n = 25) vs healthy volunteers (n = 14) | To explore the prevalence of delayed GE in the ICU and the relationships between scintigraphy and carbon breath test in the GE measurement | GE occurred in approximately 50% of critically ill patients, breath tests and scintigraphy both were valid method of GE measurement | Morphine | Administration of morphine was not associated with delayed GE |
RCT randomized control study, POS prospective observational study, PCS prospective cohort study, GAV gastric aspirate volume, UDI upper digestive intolerance, vs versus, M&M morphine and midazolam, GI gastrointestinal, PS prospective study, GE gastric emptying, NA not applicable
Table 2.
Studies about the effect of opioids on lower GI dysmotility in the ICU
| Clinical trials | Study design | Population | Group | Study goal | Results | Type of opioids | The effect of opioids |
|---|---|---|---|---|---|---|---|
| Mostafa et al. [11] | POS | 48 patients in the mixed medical and surgical ICU | Not constipated (n = 8) vs constipated (n = 40) | Constipation and its implications in the ICU | Delayed weaning from mechanical ventilation and enteral feeding | Alfentanil | Opioids had little effect on constipation |
| Van et al. [43] | Descriptive cohort study | 44 individuals in mixed surgical and medical ICU | SDD (n = 22) vs control (n = 22) | To describe the influence of severity of illness, medication and selective decontamination on defecation | Severity of illness, vasoactive medication, morphine, duration of mechanical ventilation and length of ICU stay influenced the time to first defecate | Morphine | Morphine administration at least 4 days may be associated with delayed defecation |
| Nassar et al. [45] | POS | 106 patients in the surgical ICU | Not constipated (n = 33) vs constipated (n = 73) | To determine the risk factors of constipation and its implications | Early enteral nutrition was associated with less constipation. Constipation was not associated with higher ICU mortality, length of stay and days free from mechanical ventilation | Fentanyl | Opioids were not associated with an increased incidence of constipation |
| Gacouin et al. [46] | POS | 609 patients with mechanical ventilation at least 6 days | Late defecation (defecation ≥ 6 days after admission to ICU) group (n = 353) vs early defecation group (n = 256) | To determine the risk factors of late defecation and its implications | PaO2/FIO2 ratio of < 150 mm Hg and systolic blood pressure of < 90 mm Hg during the first 5 days of mechanical ventilation were independently associated with delayed defecation | Morphine | Unadjusted univariate analysis suggested that the use of opiates had an impact on the late defecation |
| Deane et al. [42] | POS | 44 individuals mixed medical and surgical | Critically ill patients (n = 28) vs healthy volunteers (n = 16) | To determine small intestinal glucose absorption and small intestinal transit in critically ill patients | Critical illness was associated with reduced small intestinal glucose absorption | Not reported | Small transit were delayed in critical illness |
| Fukuda et al. 2016[47] | RS | 282 patients who stayed in the ICU at least 7 days | Late defecation (defecation ≥ 6 days after admission to ICU) group (n = 96) vs early defecation group (n = 186) | To investigate the risk factors for late defecation and its association with the outcomes of ICU patients | Late enteral nutrition, sedatives and surgery were risk factors for late defecation, and late defecation was associated with a prolonged ICU stay | Fentanyl | Fentanyl was not a risk factor for late defecation |
| Prat et al. [48] | POS | 189 patients | Not constipated (n = 91) vs all constipated (n = 98) | To determine the frequency and significance of constipation according to its definition criterion | Without laxation at least 6 days was more associated with specific outcomes | Sufentanyl | Opioids were associated with patients who constipated more than 6 days |
| Launey et al. [44] | POS | 396 adults with at least 2 days’ invasive ventilation | NA | To determine the factors associated with the time to defecation | Non-invasive ventilation and the duration of ventilation were associated with the time to defecation | Morphine equivalents | Opioids were not associated with the time to defecation |
| Nguyen et al. [84] | POS | 248 Mechanically ventilated patients receiving enteral nutrition | Patients with IGT (n = 50) vs patients without IGT (n = 198) | To determine the proportion and risk factors of critically ill adults with IGT | Pragmatically defined IGT was common in critical illness and associated with significant morbidity | Not reported | Use of opioids was identified as a risk factor for IGT |
RS retrospective study, POS prospective observational study, SDD selective decontamination, vs versus, IGT impaired gastrointestinal transit, NA not applicable
Table 4.
Studies about opioid antagonists in the ICU
| Clinical trials | Study design | Population | Group | Study goal | Conclusion |
|---|---|---|---|---|---|
| Liu et al. [79] | RCT | 9 OIC patients | NTX (n = 6) vs placebo (n = 3) | To evaluate the effect of low doses of NTX on constipation and analgesia | Bowel frequency improved but reversal of analgesia also occurred |
| Meissner et al. [76] | RCT | 84 mechanically ventilated patients received intravenous infusion of fentanyl | NTX (n = 38) vs placebo (n = 43) | To study the effect of NTX on the gastric tube reflux, the occurrence of pneumonia, and the time to first defecation | The administration of NTX was effective to reduce gastric tube reflux and frequency of pneumonia, but the first defecation in the two groups did not differ |
| Arpino et al. [78] | RS | 26 OIC patients in the ICU received opioids at least 48 h prior to NTX | Comparison between before and after NTX administration | To assess the safety of NTX in the ICU | The administration of NTX was not associated with changes in sedation score, vital signs, fentanyl dose, midazolam dose or propofol dose |
| Gibson et al. [77] | RS | 16 OIC patients received scheduled doses of opioids for pain control in the MICU | Before and after during NTX treatment | To evaluate the efficacy and safety of NTX before and after during naloxone treatment | NTX appeared to be effective and safe for the treatment of OIC in the MICU. NTX had no reversal of analgesic |
| Merchan et al. [81] | RS | 100 OIC patients in the MICU | NTX (n = 52) vs MNTX (n = 48) | To assess the effectiveness and safety of NTX and MNTX for the treatment of OIC | MNTX and NTX appear to be effective and safe for the treatment of OIC. The median times to first BM for NTX and MNTX were 30 and 24 h (P = 0.165) |
| Patel et al. [73] | RCT | 84 OIC patients in the ICU with opioid analgesics for at least 24 h | MNTX (n = 41) vs placebo (n = 43) | To investigate whether MNTX alleviates OIC in critically ill patients | There was no evidence to support the addition of MNTX to regular laxatives for the treatment of OIC in the ICU |
| Sawh et al. [85] | RS | 15 OIC patients in the ICU | MNTX (n = 7) vs conventional rescue therapy (n = 8) to treat the OIC in the ICU | To compare the effect of MNTX and conventional rescue therapy in the ICU | MNTX was effective in the treatment of OIC patients |
| Masding et al. [86] | ROS | 827 patients in the cardiothoracic ICU | NA | the prevalence of OIC in a large cardiothoracic ICU and the use of naloxegol | Nearly 20% of ICU patients had OIC and use of naloxegol could reduce the prevalence of OIC |
| Phase 3 [87] | RCT | 350 ICU patients received opioids at least 48 h hours | Polyethylene glycol vs naloxegol | To investigate whether naloxegol is superior to osmotic laxatives for refractory constipation in ICU patients | The study had withdrawn |
vs versus, RCT randomized control study, RS retrospective study, OIC opioid-induced constipation, MICU medical intensive care unit, NTX enteral naloxone, MNTX methylnaltrexone, BM bowel movement, NA not applicable
Upper GI dysmotility
Upper GI dysfunction occurs frequently in ICU patients [32]. Upper GI disorders include delayed gastric emptying, increased gastroesophageal reflux and abnormal duodenal contractions. All of these disorders increase the risk of nosocomial pneumonia [33]. A retrospective study describing the gut function of current ICU patients indicated that gastric emptying was abnormal, and most enterally fed patients exhibited large gastric aspirates [34]. Delayed gastric emptying is one manifestation of feed intolerance and can result in inadequate nutrition. Sedation with the combination of midazolam and fentanyl was an independent factor for increased gastric aspirate volume and upper digestive intolerance for enteral nutrition [35]. Opioids inhibit gastric emptying in a dose-dependent pattern [32]. A prospective cohort study measured the variables associated with impaired gastric emptying using the acetaminophen absorption model [32]. Before acetaminophen treatment, 24 patients were infused with morphine or a morphine equivalent; patients treated with opioids took longer to reach maximum concentration of acetaminophen, and a high dose of opioids accompanied significant impairment of gastric emptying. A study analysed the effect of opioids on GI motility in patients on mechanical ventilation [36]. Compared with nine healthy volunteers, the migrating motor complex (MMC) of seven mechanically ventilated patients treated with morphine was significantly shortened. Meanwhile, the gastric retention of the patients was more than 20% due to the abnormal motility pattern of the MMC. When morphine was stopped, the motility pattern of the stomach could convert to a normal pattern, and mean gastric retention was decreased [36]. Nguyen et al. evaluated the effect of morphine and midazolam (M&M) or propofol on gastric emptying by gastric scintigraphy [37]. The half-life of gastric emptying in patients receiving M&M was 153 min, longer than the half-life of 58 min in the control group receiving propofol, and M&M-treated patients had higher proximal meal retention because morphine inhibited gastric emptying by increasing retrograde duodenal contractions and pyloric tone.
Only a prospective randomized study compared the effect of morphine plus midazolam and propofol sedation on 21 ventilated patients with brain injuries by the paracetamol absorption mode [38]. However, there was no difference between the two groups because the study had a small sample size to detect a difference in gastric emptying, and gastric emptying was influenced by other factors that were more important than opioids [38]. Nguyen et al. analysed six cohort studies with 132 patients to explore gastric emptying in critically ill patients. The authors reported that opioid medication had no association with delayed gastric emptying. Even though the sample size of this study was larger than the others, it had limitations similar to retrospective studies [39]. Table 1 lists clinical studies about the effect of opioids on gastric emptying in the ICU. The results regarding opioids in gastric emptying were inconsistent, and only a randomized study and a pooled analysis suggested that opioids had little effect on gastric emptying. Other studies demonstrated that opioids increased the risk for impaired gastric emptying, but these studies were mostly observational studies with relatively low-quality evidence, and none of these studies excluded the effects of sedatives, such as propofol and midazolam. Sedatives also had an inhibitory effect on GI function. The GI function of critically ill patients is vulnerable and can be affected by many other factors. The exact role of opioid administration in delayed gastric emptying is unclear in the ICU setting due to sparse data.
Lower GI dysmotility
The inhibition by opioids in lower GI transit has been described in detail [21, 40, 41]. However, studies of their effects on the small intestines of critically ill patients are rare. A prospective study demonstrated that opioids inhibited the small intestinal transit of ICU patients [42]. Constipation is a common presentation of lower gastrointestinal dysmotility in ICU patients. However, the definition of constipation in critically ill patients is not agreed on [43]. The association between the clinical outcomes of ICU patients and the time of defecation is controversial. Some studies have suggested that constipation was associated with increased mortality, but some studies demonstrated that constipation was related to a more prolonged ICU stay but not mortality [44–46]. Therefore, it should be recognized that it is not whether defecation impacts mortality but failure to defecate after medication [45].
Opioids are an important factor in the development of constipation [34]. Constipation occurred in 16–83% of patients in the ICU and was associated with delayed enteral feeding, bacterial translocation, and delayed weaning from mechanical ventilation [11]. However, there is no direct evidence that opioid administration is independently associated with time to defecate in critically ill patients [44]. Some studies only indirectly demonstrated that opioid administration had little effect on constipation in the ICU setting (Table 2).
In a prospective study of motility of the lower GI tract in ICU patients, the authors found similar use of alfentanil in patients with and without constipation, which suggested that alfentanil had little effect on constipation. The authors also suggested that constipation was not associated with mortality in this study [11]. The administration of fentanyl in ICU patients was also not associated with an increased risk of constipation [45, 47]. However, a descriptive cohort study demonstrated that prolonged treatment with morphine was a risk factor for late defecation in critically ill patients, but morphine administration was significantly different between early defecation and late defecation on Days 5, 6 and 7 in the ICU, but not in the first 4 days [43]. In this study, late defecation was defined as defecation six days after admission to the ICU [43]. Dominique et al. also proposed that the use of sufentanyl and midazolam were risk factors for patients’ constipation between 3 and 6 days [48]. All these studies did not directly compare the effect of opioid administration on constipation, and different opioid drugs and the duration of opioid administration may have different effects on GI function. The effect of opioids on GI function depends on detailed opioid administration and infusion time. The long-term use of morphine in patients might reflect a more critical illness and multiple therapies; therefore, it is difficult to distinguish the effect of morphine or the illness itself and other factors on GI dysfunction [43]. GI motility is sensitive to various stresses and critical illnesses (Fig. 3), and critical illness itself can also cause GI dysmotility by diminished numbers of interstitial cells of Cajal in the GI tract [49]. Therefore, whether opioid administration delayed defecation in ICU patients is unclear.
Fig. 3.
Risks of GI dysfunction in the ICU
Potential increased infection risk by opioids
The microbiome is one of the crucial parts of the GI barrier. However, the composition and diversity of the microbial community are severely impaired in critically ill patients and can further exacerbate illness progression [50]. Opioids are increasingly considered to cause immunosuppression, compromise the GI barrier, alter microbiome function and increase infection risk [51]. Disturbances in microbiological composition and diversity were associated with the mortality of critically ill patients [52]. Opioids can expedite the invasion of the pathogen to the host and increase the mortality of septic mice [53]. Pathogen clearance is impaired in septic animals treated with morphine, resulting in a higher bacterial load in different organs [53]. Opioids can also enhance susceptibility to Pseudomonas aeruginosa, Enterococcus faecalis, and Listeria monocytogenes [53–57]. Both morphine and methadone administration can lead to high mortality in septic mice, and morphine contributes to bacterial dissemination and overproduction of the proinflammatory cytokine interleukin-17A (IL-17A) through activation of TLR2 and disrupts the IL-23/IL-17-mediated defence system of innate and acquired immunity [53, 58]. The overexpression of IL-17A plus morphine treatment can increase GI permeability, impair gut epithelial barrier function and lead to higher bacterial load and higher mortality [53]. Morphine treatment increases Citrobacter rodentium virulence and dissemination into the mesenteric lymph nodes, spleen, liver and blood, promotes bacterial adherence to the small intestine, and disrupts the integrity of the epithelial barrier and the IL-17A immune response. [52]. Citrobacter rodentium alone cannot contribute to severe GI damage in the early phase of infection [52]. Morphine-induced changes in the GI microbiome occur in a receptor-dependent manner and can be inhibited by opioid antagonists [51, 55]. The impact of morphine administration on pathogen virulence has been well studied, but the effect of other opioids on virulence is unclear [59].
Some clinical studies have suggested that opioids can destroy the immune response and increase susceptibility to infection [60]. Several cohort studies have demonstrated that opioid treatment leads to taxonomic changes and changes in richness and diversity in the gut microbiota [61–63]. A retrospective cohort study also found that patients using long-acting opioids with previously reported immunosuppression had the greatest risk of serious infection compared to patients without previously reported immunosuppression [60]. Many critically ill patients are immunocompromised, and it is essential to carefully select opioid drugs to avoid promoting further immunosuppression [64]. The serum level of morphine was found to be dramatically increased in patients with severe sepsis or septic shock [65]. A retrospective survey illustrated the impact of opioid analgesics on postburn infection complications. High opioid intake was associated with infectious complications in patients with mild to moderate (< 26% total body surface area) injuries [66]. However, studies about potential infection risks of opioids are rare in the ICU setting: more evidence is needed for critically ill patients.
Treatment of GI dysmotility with opioid antagonists
Although patients are traditionally given an osmotic laxative or a stimulant laxative to treat constipation, constipation still causes serious GI complications such as bowel perforation and even death [67]. Opioid antagonists are often a last-line medication for patients with opioid-induced constipation (OIC) in the ICU [67]. Opioid antagonists can be divided into peripheral acting or both peripheral-mediated and central-mediated antagonists (Table 3). Peripherally acting mu opioid receptor antagonists (PAMORAs) have a limited ability to breach the blood–brain barrier, hence having no effect on the analgesic benefits, and have a blunt effect of opioids in the GI system [68]. Centrally mediated opioid antagonists can ameliorate GI hypomotility but may cross the blood–brain barrier and reverse analgesia [69].
Table 3.
Information about opioid antagonists
| Opioids antagonists | Mechanism of action | Administration | Recommended dose | Approved indication | Side effects | Contraindications |
|---|---|---|---|---|---|---|
| Methylnaltrexone | PAMORA |
Subcutaneous injection Oral |
8 mg (BW 38–62 kg) 12 mg (BW 63–114 kg) 0.15 mg/kg for patients with weights outside of these ranges |
OIC patients, lower GI paralysis, insufficient response to laxatives | GI Perforation abdominal pain, nausea, diarrhea, flatulence | Known or suspected mechanical GI obstruction, perforation |
| Alvimopan | PAMORA | Oral | 12 mg BID (limited to 15 doses) | Postoperative ileus, partial bowel resection, primary anastomosis | MI, anemia, dyspepsia, hypokalemia, back pain, urinary retention | Opiate use > 1 week |
| Naloxegol | Antagonist of DOR, KOR and MOR | Oral | 12.5–25 mg OD | OIC in non-cancer and chronic pain patients, inadequate response to laxative therapy | Nausea, vomiting, diarrhea, abdominal pain, and bowel obstruction, flatulence, MI and QT prolongation less than Alvimopan | Patients with known or suspected GI obstruction are at increased risk of perforation |
| Naloxone | Opioid receptor antagonist mediated both peripheral and central | Oral | 3–12 mg TID | Constipation, lower GI paralysis | Symptoms of opioid withdrawal, reversal of analgesic | Hypersensitivity to the drug |
| Naldemedine | Peripherally acting DOR, KOR and MOR antagonist | Oral | 0.2 mg OD | OIC patients | Abdominal pain, diarrhea, nausea, gastroenteritis | Suspected or known GI perforation, obstruction and severe hepatic disease |
PAMORA peripherally acting mu opioid receptor, MOR mu opioid receptor, DOR delta opioid receptor, KOR kappa opioid receptor, BID twice per day, TID three times per day, BW body weight, OD once per day, MI myocardial infarction, OIC opioid-induced constipation
Methylnaltrexone
Methylnaltrexone is a methylated form of naltrexone that can be administered as an oral formulation and a subcutaneous injection [70]. Methylnaltrexone has been demonstrated to be safe and beneficial for the treatment of OIC in both preclinical studies and clinical studies. In mice, methylnaltrexone can effectively reverse morphine-induced reductions in the inhibition of bowel contraction in a concentration-related manner when administered 15 min before morphine and without compromising analgesia [71]. In guinea pigs, methylnaltrexone also reversed GI inhibition by chronic morphine treatment, but methylnaltrexone had no effect on GI transit without opioid stimulation [72]. In humans, methylnaltrexone was first approved by the Food and Drug Administration (FDA) for the treatment of OIC in patients with advanced illness when the response to laxative therapy was insufficient [68]. However, studies on the safety of methylnaltrexone in the ICU is rare. The methylnaltrexone for the Treatment of Opioid Induced Constipation and Gastrointestinal Stasis in Intensive Care Patients (MOTION) trial was a multicentre, double-blind, randomized placebo-controlled trial. This study aimed to investigate whether methylnaltrexone alleviated OIC in critical care patients [73]. The MOTION trial enrolled 84 patients; 41 patients in the methylnaltrexone group and 43 in the placebo group were finally analysed. However, this study found no significant difference in time to rescue-free laxation (Hazard ratio 1.42, 95% CI 0.82–2.46, p = 0.22) or in gastric residual volume between the groups.
Naloxone
Naloxone is mu-opioid antagonist approved by FDA for the reversal of respiratory depression caused by opioid overdose. Naloxone alleviated the inhibitory effects of the central and peripheral opioid systems on the whole and upper GI transit in mice [74]. However, naloxone can cause centrally mediated jumping behaviour in animals [75]. Several clinical studies have investigated the effect of naloxone in the ICU setting. A prospective, randomized, double-blinded study evaluated the effect of enteral naloxone on the amount of gastric tube reflux and the time of first defecation in mechanically ventilated patients who received fentanyl analgesia [76]. This study provided evidence that the administration of enteral opioid antagonists in ventilated patients reduced gastric tube reflux, but there was no difference in the time of first defecation. Naloxone did not contribute to changes in the sedation score, vital signs, fentanyl dose, midazolam dose or propofol dose in the ICU setting and appeared to be safe for OIC treatment in the medical intensive care unit (MICU) [77, 78]. However, a double-blind, randomized, placebo-controlled study suggested that nine patients who received oral naloxone had enhanced bowel frequency, and three of the nine patients had reversal of analgesia [79]. The study showed that patients treated with higher doses of opioids appeared to be more sensitive to the analgesic reversal effect of oral naloxone, and even dividing the dose could cause the reversal of analgesia [67, 79].
Alvimopan
Alvimopan, a PAMORA, was approved by the FDA to accelerate GI function after partial large- or small-bowel resection surgery with primary anastomosis [68]. Alvimopan can reverse the delayed GI transit resulting from intestinal manipulation without the administration of opioids before surgery and improve postoperative ileus accompanied by morphine administration in a rat model of ileus [80]. Intestinal manipulation can upregulate the endogenous opioid pathway and increase the release of endogenous opioid peptides [80]. The reversal of alvimopan for delayed GI transit may be mediated via inhibition of endogenous opioid release. There are currently no clinical studies about alvimopan use in the ICU setting.
Contradicted results about the use of opioid antagonists in the ICU
At present, the most studied opioid antagonists in the ICU are methylnaltrexone and naloxone. Studies on methylnaltrexone and naloxone in the ICU setting are insufficient, and the results of their roles in the recovery of GI motility are contradictory. Most studies have suggested that naloxone and methylnaltrexone appeared to be effective and safe in treating OIC, but two RCT studies concluded that both of the medications had little effects for OIC treatment in the ICU [73, 76]. A possible reason for this outcome may be the study design. The RCTs matched baseline between groups, the distribution of confounding factors was also well balanced and there was a small bias compared with the retrospective study. Another reason is the number of patients. Studies with a smaller number of patients have a smaller fragility index, and several incidental events can reverse the study results. Meanwhile, a retrospective review assessed the effectiveness and safety of naloxone and subcutaneous methylnaltrexone for OIC treatment in the MICU. This single-centre study included 100 patients who received continuous fentanyl infusions for at least 72 h, and the primary outcome was the time to first bowel movement. The results showed no difference in the primary outcomes, and the median times to first bowel movement for naloxone and subcutaneous methylnaltrexone were 30 and 24 h, respectively (P = 0.165) [81]. It is difficult to compare studies of different opioid antagonists because of highly heterogeneous endpoints. There is no definitive conclusion about the effect of opioid antagonists on GI function in critically ill patients because of the relatively low quality of evidence and insufficient data in the ICU. Table 4 lists clinical studies associated with opioid antagonists in the ICU.
Future directions and research
GI function is strongly correlated with the clinical outcome of ICU patients. It is important to identify and avoid potential risk factor s for GI injury in clinical work. Opioids are frequently administered to treat pain and have potential risks for the impairment of GI function. Therefore, the role of opioids in GI dysfunction in critically ill patients should be clarified for precision medicine. What is the extent to which opioids affect GI function in the ICU setting, and what is the proportion of opioids in the causative factors of AGI in the ICU setting? Whether different types, doses and duration of opioid administration have different impacts on GI function remains unclear in the ICU. What is the best choice of opioids for critically ill patients with AGI and higher risks of AGI? Finally, the most commonly used GI motility drugs are laxatives, metoclopramide and erythromycin in the ICU setting. Patients who have an inadequate response to these drugs have great potential to receive operative treatment. In addition, most of these patients who received operative treatment have a poor prognosis. Therefore, it is imperative to evaluate the effectiveness and safety of other GI motility drugs in the ICU setting, such as opioid antagonists.
Conclusion
In conclusion, pain is common in the ICU; more than half of ICU patients suffer moderate to serious pain, and it is necessary to use analgesia. Opioids have a considerable role in pain management in the ICU setting, and various studies have demonstrated the inhibition of GI motility by opioids. However, the GI tract is highly vulnerable in critically ill patients, and many factors can influence GI function from the GI barrier and absorption to motility in the ICU setting. The extent to which opioids contribute to GI dysmotility is unclear in the ICU setting due to the paucity of published data. GI dysmotility is on the severe side of the spectrum and should be adequately managed. However, at present, medication for GI dysmotility is limited in the ICU setting. Therefore, it is crucial to maintain a good balance between GI function and opioid administration. In the prescription of opioid drugs, many properties need to be taken into account before medication choices are finalized.
Acknowledgements
None.
Abbreviations
- GI
gastrointestinal
- WGAP
Working Group on Abdominal Problems
- AGI
acute gastrointestinal injury
- ENS
enteric nervous system
- MOR
mu opioid receptor
- DOR
delta opioid receptor
- KOR
kappa opioid receptor
- MMC
migrating motor complex
- M&M
morphine and midazolam
- IL-17A
interleukin-17A
- OIC
opioid-induced constipation
- PAMORA
peripherally acting mu opioid receptor antagonists
- MOTION
the methylnaltrexone for the treatment of opioid induced constipation and gastrointestinal stasis in intensive care patients
- MICU
medical intensive care unit
- FDA
food and drug administration
Authors' contributions
Both authors had their substantial contributions to the conception or design of the work. YY drafted the work. CY and ZXJ revised it critically for important intellectual content. Both authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (8187081150) and the Natural Science Foundation of Shaanxi Province (21QNPY092).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yu Chen, Email: chenyu007@gmail.com.
Xijing Zhang, Email: zhangxj918@163.com.
References
- 1.Rombeau JL, Takala J. Summary of round table conference: gut dysfunction in critical illness. Intensive Care Med. 1997;23:476–479. doi: 10.1007/s001340050361. [DOI] [PubMed] [Google Scholar]
- 2.Reintam A, Kern H, Starkopf J. Defining gastrointestinal failure. Acta Clin Belg. 2007;62(Suppl 1):168–172. doi: 10.1179/acb.2007.62.s1.022. [DOI] [PubMed] [Google Scholar]
- 3.Drewes AM, Munkholm P, Simrén M, et al. Definition, diagnosis and treatment strategies for opioid-induced bowel dysfunction-Recommendations of the Nordic Working Group. Scand J Pain. 2016;11:111–122. doi: 10.1016/j.sjpain.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Reintam Blaser A, Jakob SM, Starkopf J. Gastrointestinal failure in the ICU. Curr Opin Crit Care. 2016;22:128–141. doi: 10.1097/MCC.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 5.Reintam Blaser A, Malbrain MLNG, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384–394. doi: 10.1007/s00134-011-2459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Zhang D, Wang Y, et al. Association between acute gastrointestinal injury grading system and disease severity and prognosis in critically ill patients: a multicenter, prospective, observational study in China. J Crit Care. 2016;36:24–28. doi: 10.1016/j.jcrc.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Ding L, Chen H-Y, Wang J-Y, et al. Severity of acute gastrointestinal injury grade is a good predictor of mortality in critically ill patients with acute pancreatitis. World J Gastroenterol. 2020;26:514–523. doi: 10.3748/wjg.v26.i5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reintam Blaser A, Starkopf J, Malbrain MLNG. Abdominal signs and symptoms in intensive care patients. Anaesthesiol Intensive Ther. 2015;47:379–387. doi: 10.5603/AIT.a2015.0022. [DOI] [PubMed] [Google Scholar]
- 9.Reintam A, Parm P, Kitus R, et al. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53:318–324. doi: 10.1111/j.1399-6576.2008.01860.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilmer A, Tack J, Frans E, et al. Duodenogastroesophageal reflux and esophageal mucosal injury in mechanically ventilated patients. Gastroenterology. 1999;116:1293–1299. doi: 10.1016/s0016-5085(99)70492-0. [DOI] [PubMed] [Google Scholar]
- 11.Mostafa SM, Bhandari S, Ritchie G, et al. Constipation and its implications in the critically ill patient. Br J Anaesth. 2003;91:815–819. doi: 10.1093/bja/aeg275. [DOI] [PubMed] [Google Scholar]
- 12.Reintam A, Parm P, Kitus R, et al. Gastrointestinal failure score in critically ill patients: a prospective observational study. Crit Care. 2008;12:R90. doi: 10.1186/cc6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reintam A, Parm P, Redlich U, et al. Gastrointestinal failure in intensive care: a retrospective clinical study in three different intensive care units in Germany and Estonia. BMC Gastroenterol. 2006;6:19. doi: 10.1186/1471-230X-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pyati S, Gan TJ. Perioperative pain management. CNS Drugs. 2007;21:185–211. doi: 10.2165/00023210-200721030-00002. [DOI] [PubMed] [Google Scholar]
- 15.Sigakis MJG, Bittner EA. Ten myths and misconceptions regarding pain management in the ICU. Crit Care Med. 2015;43:2468–2478. doi: 10.1097/CCM.0000000000001256. [DOI] [PubMed] [Google Scholar]
- 16.Donohue JM, Kennedy JN, Seymour CW, et al. Patterns of opioid administration among opioid-naive inpatients and associations with postdischarge opioid use: a cohort study. Ann Intern Med. 2019;171:81–90. doi: 10.7326/M18-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards-Belle A, Canter RR, Power GS, et al. National survey and point prevalence study of sedation practice in UK critical care. Crit Care. 2016;20:355. doi: 10.1186/s13054-016-1532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman A, Nabong L. Opioids: pharmacology, physiology, and clinical implications in pain medicine. Phys Med Rehabil Clin N Am. 2020;31:289–303. doi: 10.1016/j.pmr.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Patel D, Callaway J, Vaezi M. Opioid-induced foregut dysfunction. Am J Gastroenterol. 2019;114:1716–1725. doi: 10.14309/ajg.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 20.Akbarali HI, Dewey WL. Gastrointestinal motility, dysbiosis and opioid-induced tolerance: is there a link? Nat Rev Gastroenterol Hepatol. 2019;16:323–324. doi: 10.1038/s41575-019-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rossi M, Casale G, Badiali D, et al. Opioid-induced bowel dysfunction: suggestions from a multidisciplinary expert board. Support Care Cancer. 2019;27:4083–4090. doi: 10.1007/s00520-019-04688-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fruhwald S, Holzer P, Metzler H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensive Care Med. 2007;33:36–44. doi: 10.1007/s00134-006-0452-7. [DOI] [PubMed] [Google Scholar]
- 23.DeHaven-Hudkins DL, DeHaven RN, Little PJ, et al. The involvement of the mu-opioid receptor in gastrointestinal pathophysiology: therapeutic opportunities for antagonism at this receptor. Pharmacol Ther. 2008;117:162–187. doi: 10.1016/j.pharmthera.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl 2):17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 25.Galligan JJ, Akbarali HI. Molecular physiology of enteric opioid receptors. Am J Gastroenterol Suppl. 2014;2:17–21. doi: 10.1038/ajgsup.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sternini C, Patierno S, Selmer I-S, et al. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16(Suppl 2):3–16. doi: 10.1111/j.1743-3150.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 27.Sobczak M, Sałaga M, Storr MA, et al. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol. 2014;49:24–45. doi: 10.1007/s00535-013-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galligan JJ, Sternini C. Insights into the role of opioid receptors in the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:363–378. doi: 10.1007/164_2016_116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poole DP, Pelayo J-C, Scherrer G, et al. Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology. 2011;141:982–991.e18. doi: 10.1053/j.gastro.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitolo-Chieppa D, Natale L, Marasciulo FL, et al. Involvement of kappa-opioid receptors in peripheral response to nerve stimulation in kappa-opioid receptor knockout mice. Auton Autacoid Pharmacol. 2002;22:233–239. doi: 10.1046/j.1474-8673.2002.00263.x. [DOI] [PubMed] [Google Scholar]
- 31.Albert-Vartanian A, Boyd MR, Hall AL, et al. Will peripherally restricted kappa-opioid receptor agonists (pKORAs) relieve pain with less opioid adverse effects and abuse potential? J Clin Pharm Ther. 2016;41:371–382. doi: 10.1111/jcpt.12404. [DOI] [PubMed] [Google Scholar]
- 32.Heyland DK, Tougas G, King D, et al. Impaired gastric emptying in mechanically ventilated, critically ill patients. Intensive Care Med. 1996;22:1339–1344. doi: 10.1007/BF01709548. [DOI] [PubMed] [Google Scholar]
- 33.Nind G, Chen W-H, Protheroe R, et al. Mechanisms of gastroesophageal reflux in critically ill mechanically ventilated patients. Gastroenterology. 2005;128:600–606. doi: 10.1053/j.gastro.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 34.Heinonen T, Ferrie S, Ferguson C. Gut function in the intensive care unit: what is “normal”? Aust Crit Care. 2020;33:151–154. doi: 10.1016/j.aucc.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Mentec H, Dupont H, Bocchetti M, et al. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med. 2001;29:1955–1961. doi: 10.1097/00003246-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Bosscha K, Nieuwenhuijs VB, Vos A, et al. Gastrointestinal motility and gastric tube feeding in mechanically ventilated patients. Crit Care Med. 1998;26:1510–1517. doi: 10.1097/00003246-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen NQ, Chapman MJ, Fraser RJ, et al. The effects of sedation on gastric emptying and intra-gastric meal distribution in critical illness. Intensive Care Med. 2008;34:454–460. doi: 10.1007/s00134-007-0942-2. [DOI] [PubMed] [Google Scholar]
- 38.McArthur CJ, Gin T, McLaren IM, et al. Gastric emptying following brain injury: effects of choice of sedation and intracranial pressure. Intensive Care Med. 1995;21:573–576. doi: 10.1007/BF01700162. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen NQ, Ng MP, Chapman M, et al. The impact of admission diagnosis on gastric emptying in critically ill patients. Crit Care. 2007;11:R16. doi: 10.1186/cc5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webster LR. Opioid-induced constipation. Pain Med. 2015;16(Suppl 1):16–21. doi: 10.1111/pme.12911. [DOI] [PubMed] [Google Scholar]
- 41.De Giorgio R, Zucco FM, Chiarioni G, et al. Management of opioid-induced constipation and bowel dysfunction: expert opinion of an Italian multidisciplinary panel. Adv Ther. 2021 doi: 10.1007/s12325-021-01766-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deane AM, Summers MJ, Zaknic AV, et al. Glucose absorption and small intestinal transit in critical illness. Crit Care Med. 2011;39:1282–1288. doi: 10.1097/CCM.0b013e31820ee21f. [DOI] [PubMed] [Google Scholar]
- 43.van der Spoel JI, Schultz MJ, van der Voort PHJ, et al. Influence of severity of illness, medication and selective decontamination on defecation. Intensive Care Med. 2006;32:875–880. doi: 10.1007/s00134-006-0175-9. [DOI] [PubMed] [Google Scholar]
- 44.Launey Y, Painvin B, Roquilly A, et al. Factors associated with time to defecate and outcomes in critically ill patients: a prospective, multicentre, observational study. Anaesthesia. 2021;76:218–224. doi: 10.1111/anae.15178. [DOI] [PubMed] [Google Scholar]
- 45.Nassar AP, da Silva FMQ, de Cleva R. Constipation in intensive care unit: incidence and risk factors. J Crit Care. 2009;24(630):e9–12. doi: 10.1016/j.jcrc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Gacouin A, Camus C, Gros A, et al. Constipation in long-term ventilated patients: associated factors and impact on intensive care unit outcomes. Crit Care Med. 2010;38:1933–1938. doi: 10.1097/CCM.0b013e3181eb9236. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda S, Miyauchi T, Fujita M, et al. Risk factors for late defecation and its association with the outcomes of critically ill patients: a retrospective observational study. J Intensive Care. 2016;4:33. doi: 10.1186/s40560-016-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prat D, Messika J, Avenel A, et al. Constipation incidence and impact in medical critical care patients: importance of the definition criterion. Eur J Gastroenterol Hepatol. 2016;28:290–296. doi: 10.1097/MEG.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu K, Ogura H, Matsumoto N, et al. Interstitial cells of Cajal are diminished in critically ill patients: autopsy cases. Nutrition. 2020;70:110591. doi: 10.1016/j.nut.2019.110591. [DOI] [PubMed] [Google Scholar]
- 50.Alverdy JC, Krezalek MA. Collapse of the microbiome, emergence of the pathobiome, and the immunopathology of sepsis. Crit Care Med. 2017;45:337–347. doi: 10.1097/CCM.0000000000002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banerjee S, Sindberg G, Wang F, et al. Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol. 2016;9:1418–1428. doi: 10.1038/mi.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang F, Meng J, Zhang L, et al. Opioid use potentiates the virulence of hospital-acquired infection, increases systemic bacterial dissemination and exacerbates gut dysbiosis in a murine model of Citrobacter rodentium infection. Gut Microbes. 2020;11:172–190. doi: 10.1080/19490976.2019.1629237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng J, Banerjee S, Li D, et al. Opioid exacerbation of gram-positive sepsis, induced by gut microbial modulation, is rescued by IL-17A neutralization. Sci Rep. 2015;5:10918. doi: 10.1038/srep10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Babrowski T, Holbrook C, Moss J, et al. Pseudomonas aeruginosa virulence expression is directly activated by morphine and is capable of causing lethal gut-derived sepsis in mice during chronic morphine administration. Ann Surg. 2012;255:386–393. doi: 10.1097/SLA.0b013e3182331870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang F, Meng J, Zhang L, et al. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep. 2018;8:3596. doi: 10.1038/s41598-018-21915-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shakhsheer BA, Versten LA, Luo JN, et al. Morphine promotes colonization of anastomotic tissues with collagenase: producing Enterococcus faecalis and causes leak. J Gastrointest Surg. 2016;20:1744–1751. doi: 10.1007/s11605-016-3237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asakura H, Kawamoto K, Igimi S, et al. Enhancement of mice susceptibility to infection with Listeria monocytogenes by the treatment of morphine. Microbiol Immunol. 2006;50:543–547. doi: 10.1111/j.1348-0421.2006.tb03824.x. [DOI] [PubMed] [Google Scholar]
- 58.Ma J, Wang J, Wan J, et al. Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect Immun. 2010;78:830–837. doi: 10.1128/IAI.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamilton LA, Behal ML. Altering routine intensive care unit practices to support commensalism. Nutr Clin Pract. 2020;35:433–441. doi: 10.1002/ncp.10484. [DOI] [PubMed] [Google Scholar]
- 60.Wiese AD, Griffin MR, Schaffner W, et al. Long-acting opioid use and the risk of serious infections: a retrospective cohort study. Clin Infect Dis. 2019;68:1862–1869. doi: 10.1093/cid/ciy809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vincent C, Miller MA, Edens TJ, et al. Bloom and bust: intestinal microbiota dynamics in response to hospital exposures and Clostridium difficile colonization or infection. Microbiome. 2016;4:12. doi: 10.1186/s40168-016-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Acharya C, Betrapally NS, Gillevet PM, et al. Chronic opioid use is associated with altered gut microbiota and predicts readmissions in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:319–331. doi: 10.1111/apt.13858. [DOI] [PubMed] [Google Scholar]
- 63.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Budd K. Pain management: is opioid immunosuppression a clinical problem? Biomed Pharmacother. 2006;60:310–317. doi: 10.1016/j.biopha.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 65.Glattard E, Welters ID, Lavaux T, et al. Endogenous morphine levels are increased in sepsis: a partial implication of neutrophils. PLoS ONE. 2010;5:e8791. doi: 10.1371/journal.pone.0008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwacha MG, McGwin G, Hutchinson CB, et al. The contribution of opiate analgesics to the development of infectious complications in burn patients. Am J Surg. 2006;192:82–86. doi: 10.1016/j.amjsurg.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Saini HS, Alvi Z, Singh B, et al. Methylnaltrexone and naloxone for opioid-induced constipation in the critical care setting. Cureus. 2020;12:e6829. doi: 10.7759/cureus.6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwenk ES, Grant AE, Torjman MC, et al. The efficacy of peripheral opioid antagonists in opioid-induced constipation and postoperative ileus: a systematic review of the literature. Reg Anesth Pain Med. 2017;42:767–777. doi: 10.1097/AAP.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 69.Reintam Blaser A, Preiser J-C, Fruhwald S, et al. Gastrointestinal dysfunction in the critically ill: a systematic scoping review and research agenda proposed by the Section of Metabolism, Endocrinology and Nutrition of the European Society of Intensive Care Medicine. Crit Care. 2020;24:224. doi: 10.1186/s13054-020-02889-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pergolizzi JV, Jr, Christo PJ, LeQuang JA, et al. The use of peripheral μ-opioid receptor antagonists (PAMORA) in the management of opioid-induced constipation: an update on their efficacy and safety. DDDT. 2020;14:1009–1025. doi: 10.2147/DDDT.S221278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan CS, Foss JF, Moss J. Effects of methylnaltrexone on morphine-induced inhibition of contraction in isolated guinea-pig ileum and human intestine. Eur J Pharmacol. 1995;276:107–111. doi: 10.1016/0014-2999(95)00018-g. [DOI] [PubMed] [Google Scholar]
- 72.Anselmi L, Huynh J, Vegezzi G, et al. Effects of methylnaltrexone on guinea pig gastrointestinal motility. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:279–286. doi: 10.1007/s00210-013-0833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel PB, Brett SJ, O’Callaghan D, et al. Methylnaltrexone for the treatment of opioid-induced constipation and gastrointestinal stasis in intensive care patients. Results from the MOTION trial. Intensive Care Med. 2020;46:747–755. doi: 10.1007/s00134-019-05913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wasilewski A, Lesniak A, Bujalska-Zadrozny M, et al. The effect of opioid agonists and antagonists on gastrointestinal motility in mice selected for high and low swim stress-induced analgesia. Neurogastroenterol Motil. 2016;28:175–185. doi: 10.1111/nmo.12704. [DOI] [PubMed] [Google Scholar]
- 75.Greenwood-Van Meerveld B, Gardner CJ, Little PJ, et al. Preclinical studies of opioids and opioid antagonists on gastrointestinal function. Neurogastroenterol Motil. 2004;16(Suppl 2):46–53. doi: 10.1111/j.1743-3150.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 76.Meissner W, Dohrn B, Reinhart K. Enteral naloxone reduces gastric tube reflux and frequency of pneumonia in critical care patients during opioid analgesia. Crit Care Med. 2003;31:776–780. doi: 10.1097/01.CCM.0000053652.80849.9F. [DOI] [PubMed] [Google Scholar]
- 77.Gibson CM, Pass SE. Enteral naloxone for the treatment of opioid-induced constipation in the medical intensive care unit. J Crit Care. 2014;29:803–807. doi: 10.1016/j.jcrc.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 78.Arpino PA, Thompson BT. Safety of enteral naloxone for the reversal of opiate-induced constipation in the intensive care unit. J Clin Pharm Ther. 2009;34:171–175. doi: 10.1111/j.1365-2710.2008.00982.x. [DOI] [PubMed] [Google Scholar]
- 79.Liu M, Wittbrodt E. Low-dose oral naloxone reverses opioid-induced constipation and analgesia. J Pain Symptom Manag. 2002;23:48–53. doi: 10.1016/s0885-3924(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 80.Fukuda H, Suenaga K, Tsuchida D, et al. The selective mu opioid receptor antagonist, alvimopan, improves delayed GI transit of postoperative ileus in rats. Brain Res. 2006;1102:63–70. doi: 10.1016/j.brainres.2006.02.092. [DOI] [PubMed] [Google Scholar]
- 81.Merchan C, Altshuler D, Papadopoulos J. Methylnaltrexone versus naloxone for opioid-induced constipation in the medical intensive care unit. Ann Pharmacother. 2017;51:203–208. doi: 10.1177/1060028016677310. [DOI] [PubMed] [Google Scholar]
- 82.Berger MM, Bollmann MD, Revelly JP, et al. Progression rate of self-propelled feeding tubes in critically ill patients. Intensive Care Med. 2002;28:1768–1774. doi: 10.1007/s00134-002-1544-7. [DOI] [PubMed] [Google Scholar]
- 83.Chapman MJ, Besanko LK, Burgstad CM, et al. Gastric emptying of a liquid nutrient meal in the critically ill: relationship between scintigraphic and carbon breath test measurement. Gut. 2011;60:1336–1343. doi: 10.1136/gut.2010.227934. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen T, Frenette A-J, Johanson C, et al. Impaired gastrointestinal transit and its associated morbidity in the intensive care unit. J Crit Care. 2013;28(537):e11–e17. doi: 10.1016/j.jcrc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 85.Sawh SB, Selvaraj IP, Danga A, et al. Use of methylnaltrexone for the treatment of opioid-induced constipation in critical care patients. Mayo Clin Proc. 2012;87:255–259. doi: 10.1016/j.mayocp.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masding A, Kaul S. Opioid-induced constipation in intensive care-how big is the problem? J Intensive Care Soc. 2019;20:6–7. doi: 10.1007/s00134-002-1544-7. [DOI] [Google Scholar]
- 87.Massachusetts General Hospital. The effect of naloxegol on refractory constipation in the intensive care unit (narc-ICU). In: clinicaltrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US); 2000. Available from: https://clinicaltrials.gov/ct2/show/NCT02705378?
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.