Abstract
Objective:
Interictal spikes are a biomarker of epilepsy, yet their precise roles are poorly understood. Using long-term neocortical recordings from epileptic patients, we investigated the spatial-temporal propagation patterns of interictal spiking.
Methods:
Interictal spikes were detected in 10 epileptic patients. Short time direct directed transfer function was used to map the spatial-temporal patterns of interictal spike onset and propagation across different cortical topographies.
Results:
Each patient had unique interictal spike propagation pattern that was highly consistent across times, regardless of the frequency band. High spiking brain regions were often not spike onset regions. We observed frequent spike propagations to shorter distances and that the central sulcus forms a strong barrier to spike propagation. Spike onset and seizure onset seemed to be distinct networks in most cases.
Conclusions:
Patients in epilepsy have distinct and unique network of causal propagation pattern which are very consistent revealing the underlying epileptic network. Although spike are epileptic biomarkers, spike origin and seizure onset seems to be distinct in most cases.
Significance:
Understanding patterns of interictal spike propagation could lead to the identification patient-specific epileptic networks amenable to surgical or other treatments.
Keywords: Epilepsy, Interictal spikes, effective connectivity
1. Introduction:
Epilepsy is a common neurological disorder characterized by chronic and recurrent seizures. Seizures are abrupt, temporal and spatial patterns of highly synchronized, rhythmic electrical activity that are often followed by a long duration of an altered brain state (Karoly et al. 2016). Due to the clinical importance of seizures in epilepsy and its ramifications resulting in behavioral changes, extensive research has been performed on seizures, its network and the relationship of seizure onset with epileptic foci (Lee et al. 2000; Worrell et al. 2004; Wilke et al. 2010; Dai et al. 2012; Bandt et al. 2014; Miao et al. 2014a; Bartolomei et al. 2016). However seizures are not frequent events, which makes the identification of epileptic foci using seizure onset alone difficult. Evidence suggests that abnormal brain networks are not only confined to seizures but in fact, there are more frequent interictal events (events in between two consecutive seizures) produced by this pathological network (Korzeniewska et al. 2014). Amongst these interictal events, interictal spikes are defined as brief electrographic transients of approximately 250ms or less, consisting of a short sharp wave, followed by a lasting slow wave (de Curtis and Avanzini 2001; Staley and Dudek 2006; Staley et al. 2011). These interictal spikes were previously thought to be significant biomarkers of epileptic brain regions along with seizures (de Curtis and Avanzini 2001). Although, vast literature exists on interictal spikes in the epileptic brain, little is known about the dynamic network of these events and their relationship to seizures. While spikes often originate from synchronously firing neurons at or near seizure onset zones (Hufnagel et al. 2000; Asano et al. 2003, 2004; Marsh et al. 2010), they are also observed in cortical regions far from seizure onset (Hufnagel et al. 2000; Kobayashi et al. 2001). Moreover, clinically, interictal spikes may not only be a biomarker of epileptic brain regions, and may also have a significant, yet independent impact on behavior as they are present in a wide variety of neuropsychiatric disorders in the absence of seizures (Baglietto et al. 2001; Holmes and Lenck-Santini 2006; Nicolai and Kasteleijn-Nolst Trenité 2011; Ebus et al. 2012).
Interictal spikes have also been postulated to play a role in the formation of epileptic foci that produce seizures (Staley et al. 2005; Staley and Dudek 2006; White et al. 2010), and animal models have shown that spike generation often precedes the development of seizures (White et al. 2010). The resection of high-spiking cortical regions also has been associated with decreased seizure events and an improved post-surgical outcomes (Palmini et al. 1995; Asano et al. 2003, 2009; Tomlinson et al. 2016). Conversely, other studies challenge this concept, suggesting that increased spiking actually protects the brain against seizure activity (Lieb et al. 1978; Lange et al. 1983; de Curtis and Avanzini 2001). Recent investigations of spike occurrence (frequency) and its correlation with seizure onset regions, have produced conflicting interpretations of whether spike occurrence is a reliable predictor of seizure onset zones (Asano et al. 2013). This suggests that spike occurrence alone is not a functionally useful measure, and further analysis of interictal spikes including their source activity, network pathways of propagation and network characterization are necessary to develop a better understanding of the role of interictal spikes in epilepsy.
Here, we use an effective connectivity measure to map the causal network behavior of interictal spikes across different cortical regions in the human epileptic brain. Causal connections were computed using direct-directed transfer function (dDTF). We analyzed the dynamic nature of interictal spike networks over time in different patients across a wide range of frequency bands. We further assessed the effects of deep brain sulci, such as the central sulcus, on propagation patterns. Finally, we performed an initial evaluation on the relationships between interictal spike networks and physician identified seizure onset regions.
2. Materials and methods:
2.1. Patient selection and data collection:
Ten pediatric epileptic patients (4F/6M, age: 2–15years) with drug-resistant focal seizures were selected under a protocol approved by the institutional review board of Wayne State University and the University of Illinois at Chicago (Table.1). All the patients were identified as having intractable epilepsy and underwent surgical implantation of subdural recording electrodes for long-term seizure monitoring at the Children’s Hospital of Michigan, Detroit, Michigan.
Table1.
Patient clinical information
| patient | age | sex | diagnosis | total spikes in 10 mins | seizure focus |
|---|---|---|---|---|---|
| Ep162 | 1 | F | Polymicrogyria | 6927 | Left Hemispheric |
| Ep164 | 3 | F | White matter gliosis, superficial heterotopia | 4274 | Left Temporal Occipital |
| Ep165 | 3 | F | Mild gliosis | 4475 | Left Hemispheric |
| Ep169 | 8 | M | Mild gliosis | 3731 | Right Occipital-Temporal |
| Ep181 | 13 | M | Subcortical band heterotopia. | 5664 | Left Temporal |
| Ep187 | 5 | M | Tuberous sclerosis | 4202 | Left Frontal |
| Ep196 | 2 | M | Patchy subcortical myelinated fiber loss and gliosis | 1427 | Left Frontal-TemporalParietal |
| Ep198 | 3 | F | Gliosis | 631 | Left Frontal-Parietal |
| Ep199 | 2 | M | Gliosis and patchy foci of decreased myelin | 1980 | Right Temporal |
| Ep202 | 3 | M | Tuberous sclerosis, and mild patchy cortical gliosis | 6467 | Right Temporal |
The electrocorticography (ECoG) recordings were obtained using a 192 channel Nihon Kohden Digital System (Nihon Kohden America Inc, Foothill Ranch, CA, USA) with 1000 Hz sampling rate from subdural electrodes (4 mm in diameter and spaced 10 mm apart, PMT platinum electrodes, PMT Corporation, Chanhassen, MN, USA). An experienced electroencephalographer help identify three 10-minute interictal ECoG recording segments for each patient. These segments patients were all awake but in quiet restfulness in the resting state (closed eyes, wakeful, and at least 6 hours apart from any seizure activity). We obtained MRI scans for constructing 3D models of each patient’s brain using Freesurfer software (Dale et al. 1999; Fischl et al. 1999; Fischl and Dale 2000). We localized and co-registered Electrodes on the 3D brain model using post electrode implantation CT images, and verified electrode locations with the help of intraoperative photographs taken at the time of grid removal (to ensure they did not move (Nakai et al. 2017). We then computed geodesic distances (tracking along the gray-white junction) between electrode positions and cortical thickness measures were computed (Zou et al. 2006; Wang et al. 2014).
Seizure onset zones (SOZ), as previously defined (Asano et al. 2009) were identified from intracranial recordings by an experienced epileptologist (EA). Each patient presented focal clinical or electrographic seizures along with complex epileptic behaviors, except for two patients where only subtle/minimal clinical symptoms were noted during the seizure events. Moreover, five out of ten patients also had epileptic spasms (Asano et al. 2005; Nariai et al. 2011). All patients had neocortical epilepsy often with mild pathological gliosis except for an individual with tuberous sclerosis and another with polymicrogyria.
2.2. Signal Analysis:
For the purpose of the present study, we focused only on a subset of the electrodes, specifically a 64 channel (8×8) grid placed on the frontal-parietal lobe overlying the central sulcus, to understand the interictal spike behavior with a common reference and the role of the central sulcus in the propagation of epileptic spikes. For our causal propagation analysis, we did not include other electrode locations that, in some cases, limited our ability to include all seizure onset and spiking areas for this analysis.
We processed these 64 ECoG signals through a multistage algorithm to evaluate the propagation of interictal spikes. We first identified interictal spikes using an established spike detection algorithm and manually validated these results with a trained epileptologist (JAL) and a panel of human reviewers (Barkmeier et al. 2012). Then, we isolated synchronous interictal spikes (peaks within 50 ms). We identified time blocks containing these synchronous spikes from 100 ms prior to and 250 ms after all spike peaks, excluding channels without interictal spikes.
2.3. Discrete short-time direct directed transfer function (dDTF) analysis and statistical validation:
Post extraction, the time blocks were then processed for multivariate causality analysis using discrete short time direct directed transfer function (dDTF). dDTF is a Granger causality based model designed for quasi-stationary signals (Kamiński and Blinowska 1991; Kuś et al. 2004). This method predicts the direct flow of information between a pair of signals in the presence of all other signals in a multivariate environment, not confounded by any indirect causal interaction from other signals for a short time period. Prior to the signal processing for causality evaluation, the extracted spike epochs were tested for stationary nature using Phillips–Perron test (~96.5% of spikes found stationary with p<0.3) and Kwiatkowski–Phillips–Schmidt–Shin (KPSS) test (~90% of spikes found stationary with p<0.1). The isolated spike epochs were then processed for the dDTF evaluation following the recently described method by our group (Maharathi et al. 2016). Briefly, each spike epoch was fitted to a multivariate autoregressive model (MVAR) using modified covariance method to the form , where X(t) is the data vector, A(i) is the model coefficient, E(t) is the zero mean uncorrelated white noise, and mord is the order of the model fit. The model order was determined using Akaike information criterion (AIC), where model order of the fit corresponds to the model order with the minimum AIC value (max model order was fixed to 20, however the median model order across all patients was 8±6, mean spikes in each epoch 4±5).
Then the data model is transformed to frequency domain using the Z-transform for a specific frequency band of interest and the power spectra of the signal is calculated as S(f) = H(f)VH*(f), where V is the variance of the Noise matrix and H(f) is the transfer matrix. Following this, we computed two parameters, partial coherence as , where mij is the minor of the spectral matrix after removing the ith row and jth column; and full frequency normalized direct transfer function (ffDTF) as . The direct directed transfer function is evaluated as the linear product of partial coherence and ffDTF. It provides direct causal influence of jth channel on ith channel in the presence of all other channels and has values between 0 and 1. The above process was repeated for all such epochs of synchronous interictal spikes identified in the previous step. To understand the causal propagation of interictal spikes at each frequency band, the isolated spike epochs were processed through the dDTF algorithm for a specific frequency band, including low frequencies (1–50Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), gamma (30–50 Hz) and high-frequency oscillations (HFO, 80–250 Hz).
We used surrogate data validation to determine the significance of each causal connection (Pritchard et al. 1995). Briefly, time blocks were transformed to the frequency domain using Fourier transform and the phase values were randomized. Post randomization, the data was transformed back to the time domain and processed for dDTF evaluation as mentioned above using same model parameters. The surrogate data method was repeated 100 times. The surrogate data dDTF estimation serves “false positive” level where no causal relationships among different electrode positions for the given dataset. If dDTF values were greater than the 95th percentile of these surrogate dDTF simulations, we declared this a causal propagation between electrodes (Maharathi et al. 2016). To understand how each patient’s data was consistent across time, we also determined similarity amongst their three 10-minute epochs (pairwise using a Pearson correlation coefficient). These values were normalized to a maximum of causal propagations to further evaluate the network activity of interictal spiking. An alpha level of 0.05 established statistical significance.
3. Results:
3.1. Interictal spike propagation was highly reproducible:
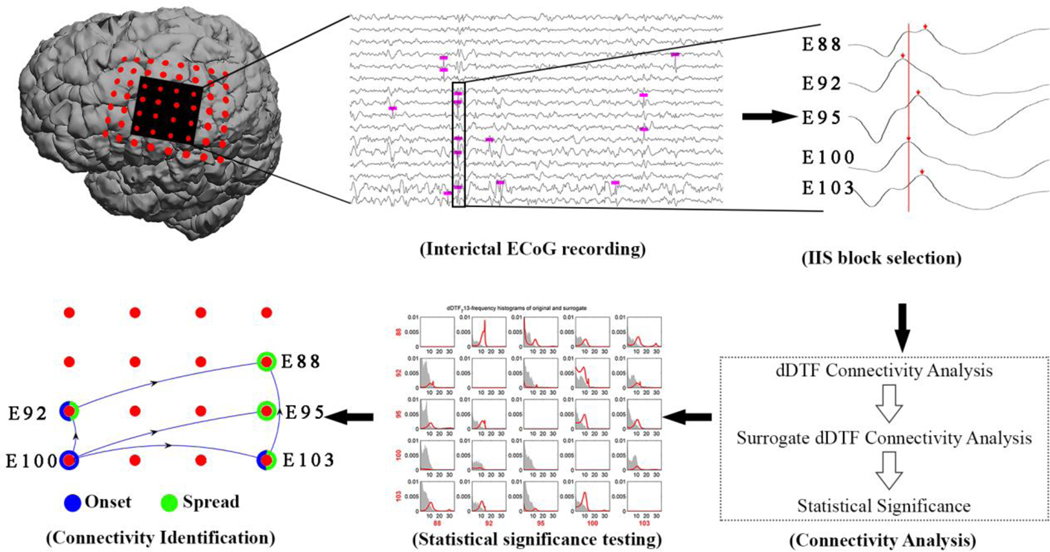
Using three 10-minute segments selected from each of 10 patients with neocortical epilepsy, we used an established spike detection algorithm to detect all spikes in all channels independently under an 8×8 subcortical grid centered over the central sulcus (Barkmeier et al. 2012). A trained electroencephalographer validated the accuracy of this algorithm for each patient. Next, clusters of interictal spikes within defined time periods were evaluated for synchrony; at least two peaks falling within 50-milliseconds of each other was considered a synchronous event. The dDTF was then used to identify causal propagations (Fig. 1).
Figure 1. Discrete short time direct directed transfer function (dDTF) computing propagation of synchronous interictal spikes.
For a selected set of electrodes corresponding to a specific part of the brain, interictal ECoG recording segments were selected and interictal spikes were identified. The dDTF method was applied to synchronously appearing interictal spikes (IIS block selection) to evaluate their propagation activity (connectivity analysis), followed by statistical significance testing using a surrogate data method. In this matrix, the greater the area under the red line for each pair of electrodes that is outside the gray areas corresponds to a significant propagation. Interictal spike onset and spread electrodes were identified based on the frequency of outgoing and incoming propagations for each electrode (connectivity identification, represented by the blue arrows).
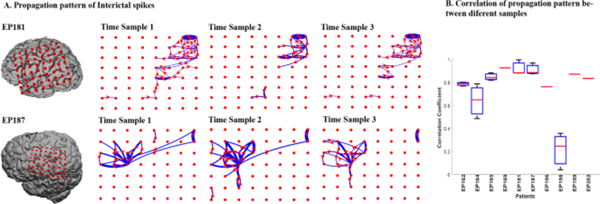
This approach was robust, reproducible and revealed highly consistent causal propagation patterns of interictal spiking within each patient when evaluated at three different time segments (Fig. 2). Interestingly, along with being conserved, these dynamic network patterns were unique to each patient. Pearson correlation coefficients were used to measure the degree of correlation of the propagation patterns within each patient. For this, the number of onset propagations were summarized for each electrode within the selected electrode grid for individual 10 minute ECoG segments. For each patient, the Pearson correlation coefficient was calculated for the corresponding electrode between each pair of 10 minute ECoG files. In 9 out of 10 patients, a patient wise high correlation was observed in onset patterns across the multiple ECoG segments (mean 0.83±0.09) (Fig. 2B).
Figure 2. Each patient has unique but highly consistent pattern of interictal spike propagation.
(A) Shows consistent Interictal spike propagation for three 10 min time segments collected over three different days for 2 patients (EP187, EP181). The red circles are the electrodes positions, and the blue lines represent direct propagation from one electrode to the other in the direction of the arrow in red. The thickness of each blue line corresponds to the frequency of occurrence of propagation events for that pair of electrodes. Only the top 5% of propagations are shown for improved visualization. Including all does not change the pattern. (B) Pairwise Pearson correlation coefficients for 10 patients shows a high level of propagation consistency between three different time samples for each patient except one (ep198). Data shown represent Pearson correlation coefficients between onset patterns of three different 10 minute ECoG segments for each patient. The mean correlation coefficient values for each patient are (0.79, 0.64, 0.85, 0.93, 0.92, 0.91, 0.76, 0.21, 0.87, 0.83) corresponding to patient IDs as shown.
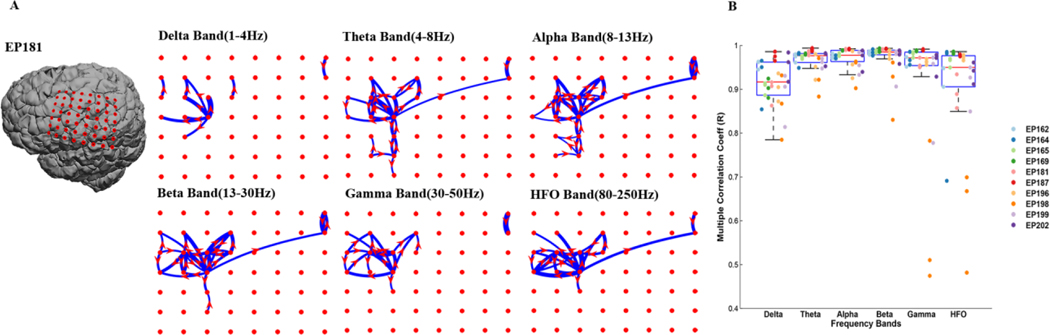
3.2. Propagation patterns were consistent across all frequency domains:
An important area of research involves the role of epileptic activities in different frequency domains, where some studies suggest that higher frequency oscillations have good predictive values for epileptic brain foci (Burnos et al. 2014; Miao et al. 2014b; Höller et al. 2015; Frauscher et al. 2017). Here we examined the propagation pattern for each patient focusing on specific frequencies and found that each frequency band measured had the same propagation pattern (Fig. 3). Using the same three 10min ECoG samples, we summarized the number of onset propagations from each electrode in the selected grid. Once evaluated, we calculated the multiple correlation between onset patterns of different frequency bands for corresponding electrodes in each 10 minute ECoG segment for each patient. We found high onset correlation values for each frequency domain (delta=0.92±0.03, theta=0.97±0.01, alpha=0.98±0.01, beta=0.98±0.01, gamma=0.96±0.02, HFO=0.94±0.03 in mean± SD) (Fig. 3A). Counting propagations in each frequency band (delta= 1430.3±1697.9, theta= 3131±3322.5, alpha= 3846.8±3051.3, beta= 4389.1±3061.5, gamma= 4707.4±4221.3, HFO= 3990.2±3888.9 count/10 minutes) suggests that beta was most “spike propagation active” followed by the gamma and HFO frequency bands. The total number of propagations in each frequency band across patients varied with patient EP198 having fewest propagations (delta= 73, theta= 207, alpha= 219, beta= 163, gamma= 49.6, HFO= 72).
Figure 3. Interictal spike propagation is highly consistent across EEG frequency bands.
(A) Interictal spike propagation patterns were analyzed across different frequency bands (delta, theta, alpha, beta, gamma and high frequency oscillations). Highly consistent propagation patterns were observed within each frequency band. As an example, spike propagation patterns across different frequency bands for EP187 shows high levels of consistency. (B) This is a box plot superimposed on scatter plot showing multiple correlation coefficients among frequency bands revealing high significance for all 10 patients. Data presented within each box shows the multiple correlation coefficient value for each frequency band with all other frequency bands. Each column in a box represents a single patient and each marking within the column represents the multiple correlation coefficient for 10 minute ECoG. The mean ± SD for each frequency are (Delta: 0.9±0.05, Theta: 0.94±0.04, Alpha: 0.96±0.03, Beta: 0.96±0.04, Gamma: 0.92±0.13, HFO: 0.94±0.05)
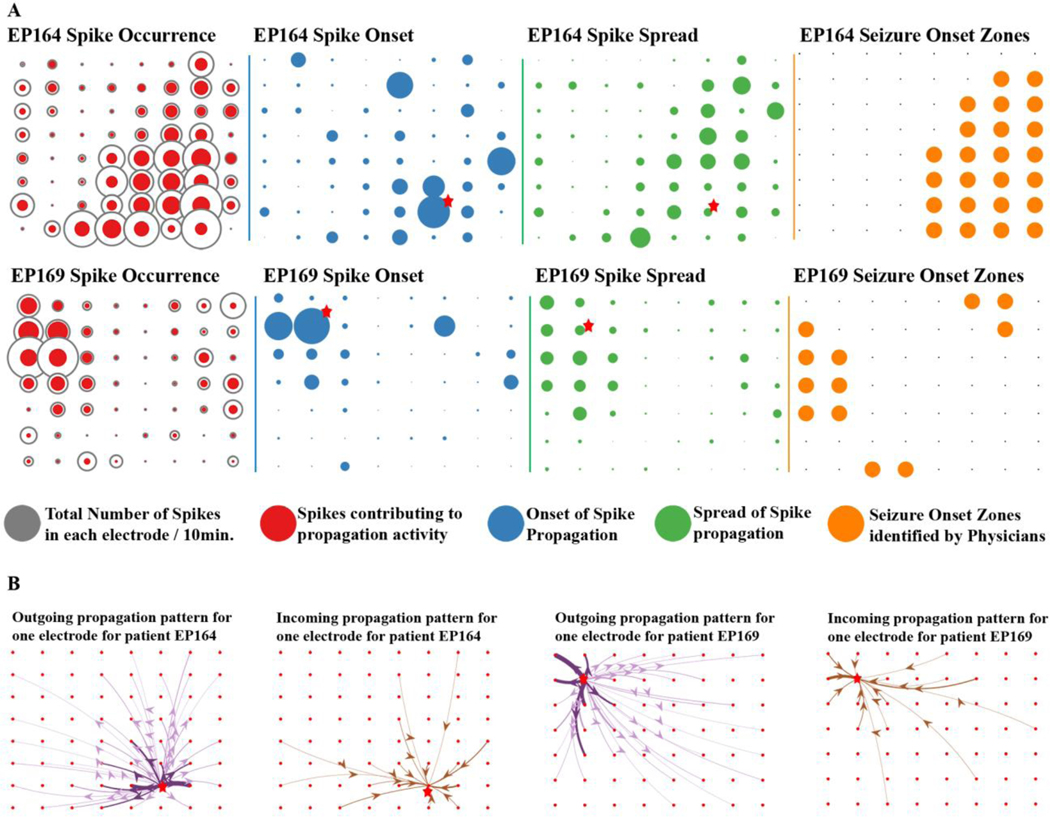
3.3. Regions of spike onset did not correspond to regions with the highest spike occurrence:
Most studies to date have focused on rate of spike occurrence (frequency) as a measure of the epileptogenicity of a given brain region, with regions exhibiting the highest spike occurrence considered to be more epileptic. Along with spike occurrence, we analyzed the spike onset regions. Spike onset regions are defined as brain regions which have the greatest number of outgoing spike propagations above a defined maximum threshold within the selected grid. Interestingly, we did not find interictal spike onset regions to have the highest occurrence of spikes (Fig. 4A). In fact, electrodes with the highest spike occurrence were more likely to receive spikes from multiple brain locations. Out of all interictal spikes investigated, only 71.3±17.7% of spikes were found present in the selected synchronous spike epochs, and not all synchronous spikes propagated. We isolated spike propagation events in each frequency band, and a small proportion propagated while also shifting frequency (delta= 25±12.8%, theta= 45.1±16.5%, alpha= 51±16.5%, beta= 51.9±18.7%, gamma= 44.2±22.7%, HFO= 47.2±23.6%). Only a small percentage of total locations acted as onset zones (delta= 7.9±3, theta= 10.9±3, alpha= 10.7±3.4, beta= 11.5±3.6, gamma= 12.2±5.6, HFO= 8.6±3.2). Many spikes participated in spread of interictal spike patterns (delta=13.4±7.4, theta= 20.3±8.8, alpha= 17.5±7.4, beta= 14.2±6, gamma= 9.7±4.2, HFO= 16.9±9.5), and hence were “intermediaries” of propagation, having incoming and outgoing events (delta= 3.7±3, theta= 14±6.5, alpha= 22.8±8.8, beta= 26.2±11.2, gamma= 22.3±14.7, HFO= 21.7±15.6). In summary, dDTF analysis suggests that a majority of cortical epileptic spiking brain regions are receiving rather than generating interictal spikes. Furthermore, only a small proportion of spike onset regions brain areas truly initiate spiking, as most act as intermediary regions that both receive and spread the spike to other areas (p ≤ 0.05 across all frequency bands, pairwise t-test).
Figure 4. Spike onset measurements reveal clear, localized patterns of outgoing and incoming connectivity.
(A) Comparison of spike occurrence, onset, and spread demonstrates that measuring spike propagation, presents a simpler, more focused pattern than spike occurrence (left panel) for two patients (EP164, EP169). The size of each circle (red, blue, green) corresponds to the number of events at each electrode location on the 8×8 grid. Propagating spikes are shown as either outgoing (onset) or incoming (spread) and represent only a subset of all spiking events. These results show that spike onset brain regions are most often not the highest spiking (occurrence) regions. (B) The maximal onset electrode for each patient is labeled with ★ in A and is shown with detailed outgoing and incoming propagation patterns in B. The thickness of each line corresponds to frequency of propagation and the darker shaded lines on the outgoing propagation plot correspond to a greater than 50% of outgoing propagation from that electrode.
Based on these findings, we developed spatial models of outgoing (spike onset) and incoming (spike spread) epileptic activities as shown in Fig. 4B. Isolating individual electrodes on the grid shows highly consistent patterns of both outgoing and incoming activity, providing a unique look at the epileptic connectivity of these brain regions. dDTF analysis therefore allows a deeper understanding of the cortico-cortical networks that underlie interictal spiking which could have important downstream value in improving surgical outcomes in patients with epilepsy.
3.4. Spatial patterns of interictal spike propagation related to cortical structures:
We next combined the temporal and directional patterns of interictal spikes using dDTF with 3D brain models for each patient as a means to understand how brain structure influences spike propagation. Fig. 5 shows that while approximately 50% of outgoing spike propagations travel to nearby, often adjacent electrodes (~50 mm geodesic distance), the other half of spikes propagate over longer distances. Surprisingly, approximately 22% of spikes propagated over 10 cm (geodesic) distance from their site of onset. In addition, the directionality of spike propagation was non-random and affected by cortical topography (Fig. 6). Since all patients had an electrode grid overlying the central sulcus, we determined the relative number of spike propagations that crossed the central sulcus versus electrode locations on the same side of central sulcus. Surprisingly, a majority (76±9.6%) of spike propagations did not cross the central sulcus, in either frontal or parietal lobes. This result was also independent from ECoG frequency bands.
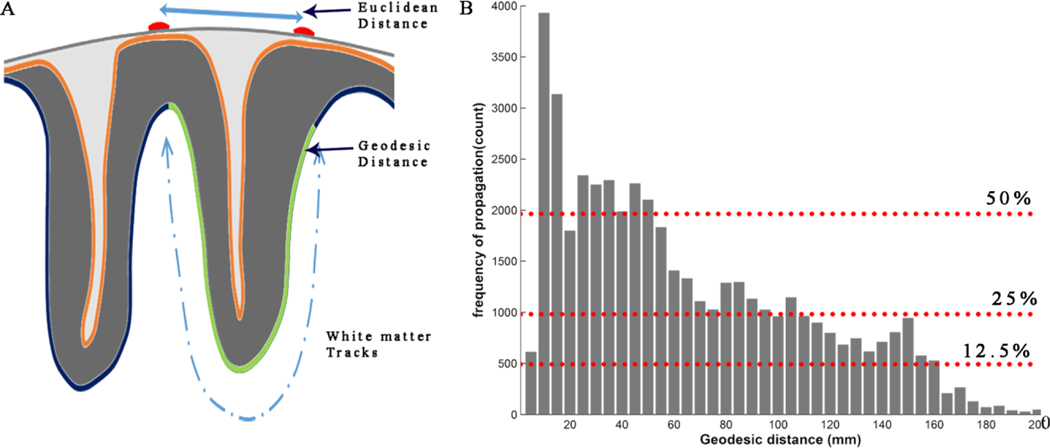
Figure 5. Interictal spikes propagate mostly over short geodesic distances, but also travel to distant sites.
Most of the interictal propagation is observed between electrodes with short geodesic distances. (A) This shows a 2D surface view of a cortical gyrus. While Euclidian distances are measured on the cortical surface, the geodesic distance is measured though white matter tracks. (B) This is a histogram showing the number of propagation events observed with increasing geodesic distance pooled from all 10 patients. The absolute distance values are rounded off to nearest 5 or multiple of 5. The frequency of propagation events decreases as a function of geodesic distance, but spike propagation can occur at all distances, including regions far from the onset point.
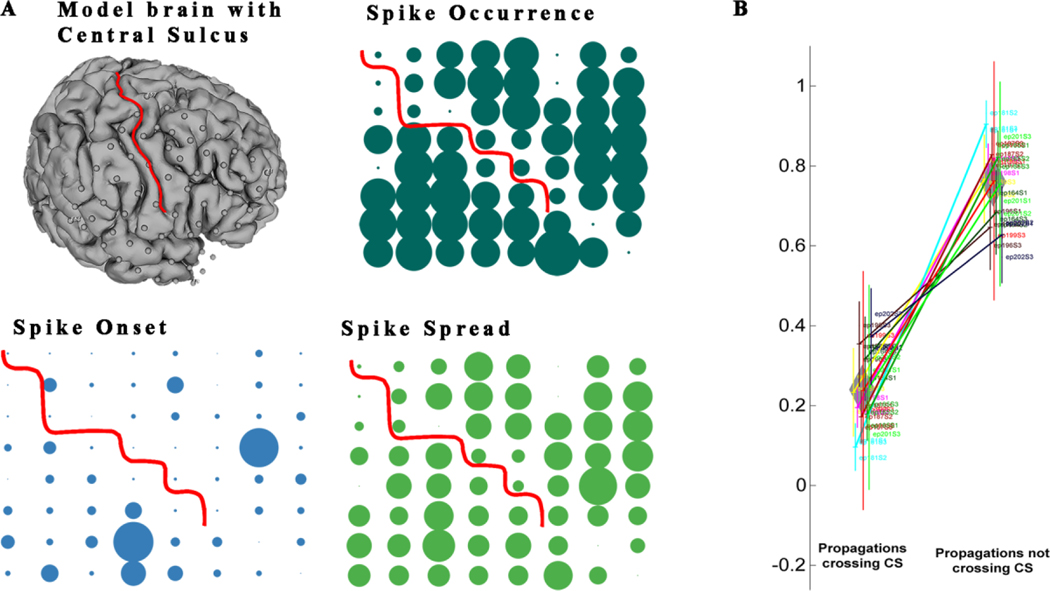
Figure 6. The central sulcus acts as a significant barrier to the propagation of interictal spikes.
(A) A distinct reduction in activity of spikes, spike onset and spike spread is noted across the central sulcus (CS). This pattern suggests that the central sulcus acts as a natural barrier that restricts spikes from crossing in all patients we examined. (B) Quantitatively there is a proportionate reduction of propagations across the central sulcus. This figure compares the proportion of propagations crossing the central sulcus versus the number of propagations staying on the same side of the sulcus. Solid lines represent the difference between propagations crossing the CS vs not crossing with p<0.05. Each vertical line with three points on it represents one patient with results from three ECoG files. The height of the line is the variance between three data points. The width of the diamond shape represents the variance of mean of number of propagations crossing or not crossing the CS across all patients and the height of the diamond shape represents the variance of variance of propagation crossing or not crossing the CS across all patients.
3.5. Spike and seizure onsets regions were not often interrelated:
Even though interictal spikes are considered prominent biomarkers of epileptic brain regions that also produce seizures (Palmini et al. 1995), their exact spatial and temporal associations with seizures are not well understood. We investigated whether seizure onset regions had any association with the onset or spread of interictal spiking brain regions. We first considered electrodes with activity exceeding 80% of maximum of the entire grid in at least one of the three files. Fischer’s exact test along with Chi-square tests of independence was used to test for any relation between seizure onset regions and (1) interictal spiking regions (2) spike onset regions and (3) spike spread regions. In patient Ep164, there was a strong relationship between seizure onset and interictal spiking regions, spike onset regions, and spike spread regions that were all significant (p<0.05). However, in the remaining 7 patients, the spike network had a more complex relationships, if any, with seizure onset regions (Fig. 7). For example, in patient Ep169, seizure onset overlapped more with high spiking regions compared to spike onset and spread regions whereas in patient Ep199, seizure onset regions were well associated with spike onset and spread regions (p< 0.05). In patients Ep165 and Ep196, who also had infantile spasms, seizure onset regions were diffuse an encompassed a large portion of the grid. Further analysis will be required to understand fully the relationships between spike onset and seizure onset, especially in patient with infantile spasms who often have both diffuse as well as focal seizure onset regions. Two of the patients (Ep181 and Ep187), had their seizure active zones outside of the measurement grids and were therefore removed from consideration. However, even in these patients, the spike onset and spread regions were located on the electrodes in close proximity to seizure onset regions.
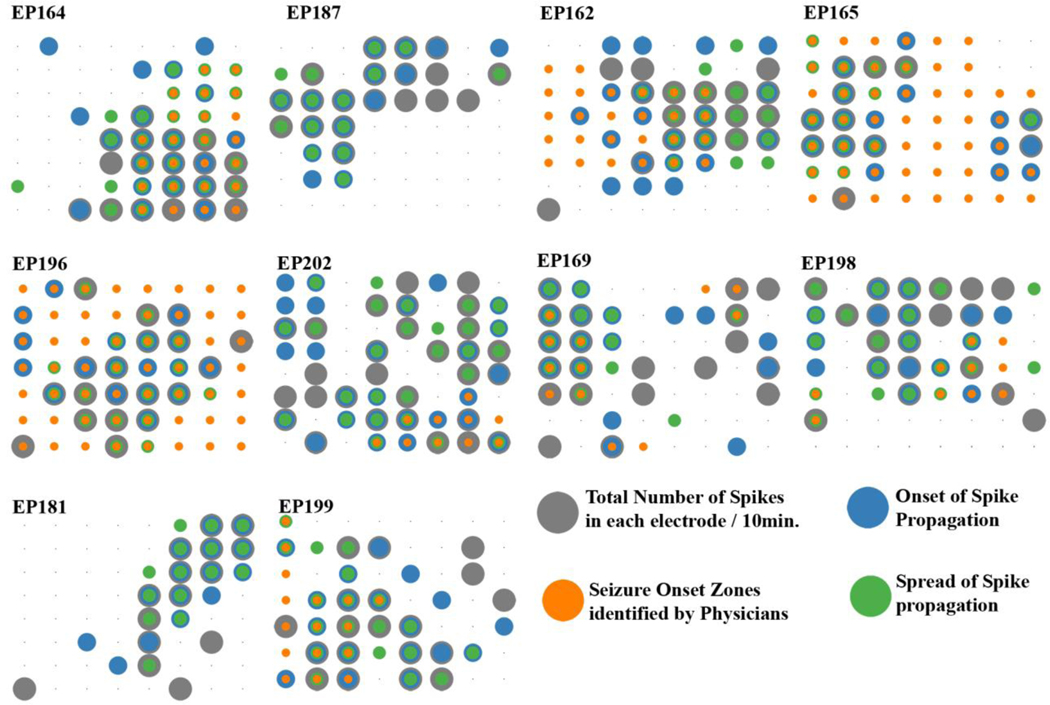
Figure 7. Spike and seizure onset patterns are not clearly interrelated and highly variable.
We found no clear relationships between seizure onset zones identified within the selected electrode grid location and the spike occurrence, onset, and spread for all 10 patients. Note some patients who had infantile spasms had widespread ictal onset areas (patients EP164, EP165 and EP196). The size of each circle corresponds to the number of events at each brain location.
4. Discussion:
We performed a novel effective connectivity analysis using dDTF to better understand the spatial- temporal patterns of interictal spike propagation in the human epileptic neocortex. We observed consistent pattern of interictal spike propagation which are individualized for each patient and are highly reproducible across multiple days of recording for the same patient. We also found that spikes propagate in a similar fashion across a wide range of frequency bands, including HFOs. Our findings shed new light on the relationships between cortical anatomy and spike propagation. Specifically, propagations often travelled locally, also across great distances but rarely and the central sulcus appears to be a significant barrier for interictal spike propagation which is in congruent with previous studies reporting that signal propagation often skips the motor area (Iimura et al. 2017). Surprisingly, brain regions with the highest spike occurrences most often did not initiate spiking. While our study was primarily focused on spike onset, we found no clear relationships between interictal spike onset regions and seizure onset regions, suggesting that spikes and seizures might be having different networks.
Interictal spikes are known to be highly associated with the epileptic state (Staley and Dudek 2006; Rodin et al. 2009) and are thought to result from synchronous cortical hyper-excitation of neural assemblies (Pillai and Sperling 2006). Previous studies have found that spikes have consistent frequencies and locations for each patient’s interictal spike network and interictal spikes have been used to confirm the clinical diagnosis of epilepsy and to assist in the planning of drug management and epilepsy surgery (Pillai and Sperling 2006; Rodin et al. 2009). The spatially conserved pattern of spike propagation within each patient signifies an invariability of the epileptic network over time. This suggests that once the wiring of neurons turns pathologic, the underlying network of interictal epileptic activity remains quite consistent.
A major question in epilepsy is whether interictal spikes are simply singlet spikes from a repetitively spiking seizure focus or represent an entirely different network. Previous research has suggested that, interictal spikes proceed the development of seizures, but when increased, correlate with spontaneous seizure events (White et al. 2010). It might therefore be possible to predict seizure onset regions based on occurrence of interictal spiking. However, there is widely discordant data for this hypothesis as it is well known that regions of high interictal spiking are not always seizure onset regions (Hufnagel et al. 2000; Asano et al. 2004; Jacobs et al. 2008; Marsh et al. 2010). There is conflicting data to support the removal of high spiking regions for a positive surgical outcome (Alarcon et al. 1997; Bautista et al. 1999; Asano et al. 2009; Mégevand et al. 2014). An important new observation from our study that may help resolve this discordance is that most regions with the highest interictal spiking frequency are not areas of spike onset, but areas that receive the spread of spikes.
All of these findings taken together favor the hypothesis that interictal spiking and seizure networks are distinct with different mechanisms of origin (Karoly et al. 2016). Surprisingly, except in one patient, we did not find a tangible relationship between spike onset and seizure onset. However, in the present study we did not use data from all brain regions covered by electrodes that could also be involved, nor did we focus on less frequent spiking regions in our dDTF analysis that focuses on the most common patterns of spread. Clearly, more research will be needed to explore the complex relationships between the interictal spike and seizure networks. Further studies will need to look at not only the spatial, but also the temporal relationships between interictal spike onset regions and seizure onset regions.
Another physiological aspect of epilepsy that has gained significant interest is in the area of HFOs. Some have found HFOs to highly correlate with seizure onset regions (Jirsch et al. 2006; Ochi et al. 2007; Jacobs et al. 2008; Salami et al. 2014; Modur and Miocinovic 2015). We looked at the propagation patterns embedded within interictal spikes and found that all frequencies, from lower frequency bands to HFOs propagate in a similar fashion. This may not be too surprising as it has previously been shown that high frequency oscillations are often coexistent with interictal spikes (Jacobs et al. 2008, 2016; Rodin et al. 2009) and in the present study we focused entirely on interictal spiking.
By co-registering electrode locations with 3D MRI reconstructions, we examined the spatial aspects of spike propagation with respect to cortical structures. We found that the distance interictal spikes propagate is generally quite short, most often to adjacent electrodes, spikes can also traverse larger distances, likely through subcortical pathways, but the chances are few. Perhaps the most salient observation was that the central sulcus acts as a significant anatomic barrier to spike propagation in all patients we examined. The central sulcus divides primary sensory from motor cortex and is one of the deepest sulci in the brain that has been extensively studied for its functional localization and cortical plasticity. Identifying the location of the central sulcus is critical in surgical planning to reduce unwanted motor and sensory deficits (Towle et al. 2003). Previous studies have found that spikes originating from sulcal and gyral cortices on either side of central sulcus propagate across central sulcus (Jung et al. 2003). In contrast, when looking at all spiking events statistically, we observed that the central sulcus is a strong barrier to the spread of spikes. A recent study on human epileptic tissues that defined tissue markers of spiking found that molecular markers of epileptic activity in the MAPK/CREB pathway also failed to cross deep gyri, suggesting an underlying molecular and cytoarchitectonic underpinning that creates anatomical barriers across the cortex (Beaumont et al. 2012). Although from the current study it is difficult to point out the exact mechanism of how central sulcus acts as a barrier, a probable reason might be the large cortical surface coverage of the deep sulci and thinner cortex. More studies will be needed to connect structural and functional information together using a variety of connectivity measures including cortico-cortical evoked potentials or fiber tractography. This will allow further exploration of the effects of deeper sulci and other anatomical abnormalities on spike propagation networks across different brain regions. Because our study only involved surface grid electrodes, examination of spike propagation patterns from patients with stereo EEG could also help in this endeavor. Finally, propagation patterns may be age dependent and examining patients within different age groups would be helpful in learning how these networks may change as a function of brain development.
Several signal analysis tools have been proposed to quantify and understand interictal spike propagations. Granger causality based functional measures (Granger 1969), such as direct transfer function (DTF) (Bressler and Seth 2011) and partial direct coherence (PDC) (Baccalá and Sameshima 2001), evaluate the causal flow of information between signals in a multivariate environment. They provide statistical estimates of connection strength along with the direction that information flows. Since these evaluations compute the causality based on phase differences, they are robust to volume conduction as compared to other bivariate analysis. With simulated and experimental data, it has been shown that an enhancement of the DTF algorithm, direct DTF (dDTF), is more efficient in identifying causal connections (statistically) with fewer false detections. It also has a lower fictitious causal density and high spectral selectivity (Astolfi et al. 2005, 2007; Bressler and Seth 2011; Fasoula et al. 2013). Such approaches enable robust understanding of path and timing of network signal propagations. More recently, the direct-Directed dDTF has been successfully implemented in previous studies evaluating seizure propagation and epileptic source localization Manuscript_Spike Propagation_clinical neurophysiology_v4.
The clinical implications for this work are still not fully developed. Although there are several limitations in the current study, and primarily we focused on evaluating the interictal spike propagation patterns, taken together our findings suggest that interictal spikes and seizures develop nearby, but from different networks. Whether removal of spike onset regions would produce a better surgical outcome remains an open question not answerable by our current retrospective data. However, application of this analysis prospectively could allow a greater understanding on whether disrupting or removing interictal spiking networks on surgical outcome.
Highlights.
Interictal spike propagation patterns in human neocortex are highly consistent.
High spiking regions are rarely the spike network initiators.
The central sulcus acts as an anatomical barrier to spike propagation.
Acknowledgements
This work was funded by NIH/NINDS Grants R56, NS083527, R01NS045207, and R01NS058802 (JAL), as well as R01NS064033 (EA).
Footnotes
Conflict of Interest
None of the authors report any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, et al. Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain. 1997;120:2259–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9448581 [DOI] [PubMed] [Google Scholar]
- Asano E, Benedek K, Shah A, Juhász C, Shah J, Chugani DC, et al. Is intraoperative electrocorticography reliable in children with intractable neocortical epilepsy? Epilepsia. 2004;45(9):1091–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15329074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Brown EC, Juhász C. How to establish causality in epilepsy surgery. Brain Dev. 2013;35(8):706–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23684007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Muzik O, Chugani DC, Shah J, et al. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia. 2005;46(7):1086–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16026561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009;132:1038–47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19286694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Muzik O, Shah A, Juhász C, Chugani DC, Sood S, et al. Quantitative interictal subdural EEG analyses in children with neocortical epilepsy. Epilepsia. 2003;44(3):425–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12614399 [DOI] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, Marciani MG, Baccala LA, de Vico Fallani F, et al. Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum Brain Mapp. 2007;28(2):143–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16761264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, de Vico Fallani F, Lai M, Baccala L, et al. Comparison of different multivariate methods for the estimation of cortical connectivity: simulations and applications to EEG data. Conf Proc. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2005;5:4484–7. Available from: http://ieeexplore.ieee.org/document/1615463/ [DOI] [PubMed] [Google Scholar]
- Baccalá LA, Sameshima K. Partial directed coherence: a new concept in neural structure determination. Biol Cybern. 2001;84(6):463–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11417058 [DOI] [PubMed] [Google Scholar]
- Baglietto MG, Battaglia FM, Nobili L, Tortorelli S, De Negri E, Calevo MG, et al. Neuropsychological disorders related to interictal epileptic discharges during sleep in benign epilepsy of childhood with centrotemporal or Rolandic spikes. Dev Med Child Neurol. 2001;43(6):407–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11409830 [DOI] [PubMed] [Google Scholar]
- Bandt SK, Bundy DT, Hawasli AH, Ayoub KW, Sharma M, Hacker CD, et al. The role of resting state networks in focal neocortical seizures. PLoS One. 2014;9(9):1–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25247680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkmeier DT, Shah AK, Flanagan D, Atkinson MD, Agarwal R, Fuerst DR, et al. High inter-reviewer variability of spike detection on intracranial EEG addressed by an automated multi-channel algorithm. Clin Neurophysiol. 2012;123(6):1088–95. Available from: 10.1016/j.clinph.2011.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Trébuchon A, Bonini F, Lambert I, Gavaret M, Woodman M, et al. What is the concordance between the seizure onset zone and the irritative zone? A SEEG quantified study. Clin Neurophysiol. 2016;127(2):1157–62. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1388245715009827 [DOI] [PubMed] [Google Scholar]
- Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extrahippocampal seizures. Epilepsia. 1999;40(7):880–90. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10403211 [DOI] [PubMed] [Google Scholar]
- Beaumont TL, Yao B, Shah A, Kapatos G, Loeb JA. Layer-specific CREB target gene induction in human neocortical epilepsy. J Neurosci. 2012;32(41):14389–401. Available from: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3408-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Seth AK. Wiener-Granger causality: a well established methodology. Neuroimage. 2011;58(2):323–9. Available from: 10.1016/j.neuroimage.2010.02.059 [DOI] [PubMed] [Google Scholar]
- Burnos S, Hilfiker P, Sürücü O, Scholkmann F, Krayenbühl N, Grunwald T, et al. Human intracranial high frequency oscillations (HFOs) detected by automatic time-frequency analysis. PLoS One. 2014;9(4):e94381. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24722663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63(5):541–67. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11164621 [DOI] [PubMed] [Google Scholar]
- Dai Y, Zhang W, Dickens DL, He B. Source connectivity analysis from MEG and its application to epilepsy source localization. Brain Topogr. 2012;25(2):157–66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22102157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9931268 [DOI] [PubMed] [Google Scholar]
- Ebus S, Arends J, Hendriksen J, van der Horst E, de la Parra N, Hendriksen R, et al. Cognitive effects of interictal epileptiform discharges in children. Eur J Paediatr Neurol. 2012;16(6):697–706. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1090379812001365 [DOI] [PubMed] [Google Scholar]
- Fasoula A, Attal Y, Schwartz D. Comparative performance evaluation of data-driven causality measures applied to brain networks. J Neurosci Methods. 2013;215(2):170–89. Available from: 10.1016/j.jneumeth.2013.02.021 [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–5. Available from: http://www.pnas.org/cgi/doi/10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9931269 [DOI] [PubMed] [Google Scholar]
- Frauscher B, Bartolomei F, Kobayashi K, Cimbalnik J, van ‘t Klooster MA, Rampp S, et al. High-frequency oscillations: The state of clinical research. Epilepsia. 2017;58(8):1316–29. Available from: http://doi.wiley.com/10.1111/epi.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CWJ. Investigating Causal Relations by Econometric Models and Cross-spectral Methods. Econometrica. 1969;37(3):424. Available from: http://www.jstor.org/stable/1912791?origin=crossref [Google Scholar]
- Höller Y, Kutil R, Klaffenböck L, Thomschewski A, Höller PM, Bathke AC, et al. High-frequency oscillations in epilepsy and surgical outcome. A meta-analysis. Front Hum Neurosci. 2015;9:574. Available from: http://journal.frontiersin.org/Article/10.3389/fnhum.2015.00574/abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GL, Lenck-Santini P-P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8(3):504–15. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1525505005005299 [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Dümpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41(4):467–78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10756415 [DOI] [PubMed] [Google Scholar]
- Hur YJ, Kim HD. The causal epileptic network identifies the primary epileptogenic zone in Lennox-Gastaut syndrome. Seizure. 2015;33:1–7. Available from: 10.1016/j.seizure.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Iimura Y, Jones K, Hattori K, Okazawa Y, Noda A, Hoashi K, et al. Epileptogenic high-frequency oscillations skip the motor area in children with multilobar drug-resistant epilepsy. Clin Neurophysiol. 2017;128(7):1197–205. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1388245717301190 [DOI] [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49(11):1893–907. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18479382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Vogt C, LeVan P, Zelmann R, Gotman J, Kobayashi K. The identification of distinct high-frequency oscillations during spikes delineates the seizure onset zone better than high-frequency spectral power changes. Clin Neurophysiol. 2016;127(1):129–42. Available from: 10.1016/j.clinph.2015.04.053 [DOI] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–608. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16632553 [DOI] [PubMed] [Google Scholar]
- Jung K-Y, Kim J-M, Kim DW. Patterns of interictal spike propagation across the central sulcus in benign rolandic epilepsy. Clin Electroencephalogr. 2003;34(3):153–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14521277 [DOI] [PubMed] [Google Scholar]
- Kamiński M, Ding M, Truccolo WA, Bressler SL. Evaluating causal relations in neural systems: granger causality, directed transfer function and statistical assessment of significance. Biol Cybern. 2001;85(2):145–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11508777 [DOI] [PubMed] [Google Scholar]
- Kamiński MJ, Blinowska KJ. A new method of the description of the information flow in the brain structures. Biol Cybern. 1991;65(3):203–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1912013 [DOI] [PubMed] [Google Scholar]
- Karoly PJ, Freestone DR, Boston R, Grayden DB, Himes D, Leyde K, et al. Interictal spikes and epileptic seizures: their relationship and underlying rhythmicity. Brain. 2016;139:1066–78. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26912639 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Merlet I, Gotman J. Separation of spikes from background by independent component analysis with dipole modeling and comparison to intracranial recording. Clin Neurophysiol. 2001;112(3):405–13. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11222961 [DOI] [PubMed] [Google Scholar]
- Korzeniewska A, Cervenka MC, Jouny CC, Perilla JR, Harezlak J, Bergey GK, et al. Ictal propagation of high frequency activity is recapitulated in interictal recordings: effective connectivity of epileptogenic networks recorded with intracranial EEG. Neuroimage. 2014;101:96–113. Available from: 10.1016/j.neuroimage.2014.06.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska A, Crainiceanu CM, Kuś Rafałand Franaszczuk PJ, Crone NE. Dynamics of event-related causality in brain electrical activity. Hum Brain Mapp. 2008;29(10):1170–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17712784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuś R, Kamiński M, Blinowska KJ. Determination of EEG activity propagation: pair-wise versus multichannel estimate. IEEE Trans Biomed Eng. 2004;51(9):1501–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15376498 [DOI] [PubMed] [Google Scholar]
- Lange HH, Lieb JP, Engel J, Crandall PH. Temporo-spatial patterns of pre-ictal spike activity in human temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1983;56(6):543–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6197273 [DOI] [PubMed] [Google Scholar]
- Lee S-A, Spencer DD, Spencer SS. Intracranial EEG Seizure-Onset Patterns in Neocortical Epilepsy. Epilepsia. 2000;41(3):297–307. Available from: http://doi.wiley.com/10.1111/j.1528-1157.2000.tb00159.x [DOI] [PubMed] [Google Scholar]
- Lieb JP, Woods SC, Siccardi A, Crandall PH, Walter DO, Leake B. Quantitative analysis of depth spiking in relation to seizure foci in patients with temporal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1978;44(5):641–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/77769 [DOI] [PubMed] [Google Scholar]
- Maharathi B, Loeb JAJA, Patton J Estimation of resting state effective connectivity in epilepsy using direct-directed transfer function. In: 2016 38th Annu Int Conf IEEE Eng Med Biol Soc. IEEE; 2016. p. 716–9. Available from: http://ieeexplore.ieee.org/document/7590802/ [DOI] [PubMed] [Google Scholar]
- Marsh ED, Peltzer B, Brown MW, Wusthoff C, Storm PB, Litt B, et al. Interictal EEG spikes identify the region of electrographic seizure onset in some, but not all, pediatric epilepsy patients. Epilepsia. 2010;51(4):592–601. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19780794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mégevand P, Spinelli L, Genetti M, Brodbeck V, Momjian S, Schaller K, et al. Electric source imaging of interictal activity accurately localises the seizure onset zone. J Neurol Neurosurg Psychiatry. 2014;85(1):38–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23899624 [DOI] [PubMed] [Google Scholar]
- Miao A, Tang L, Xiang J, Guan Q, Ge H, Liu H, et al. Dynamic magnetic source imaging of absence seizure initialization and propagation: a magnetoencephalography study. Epilepsy Res. 2014a;108(3):468–80. Available from: 10.1016/j.eplepsyres.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Miao A, Xiang J, Tang L, Ge H, Liu H, Wu T, et al. Using ictal high-frequency oscillations (80–500Hz) to localize seizure onset zones in childhood absence epilepsy: a MEG study. Neurosci Lett. 2014b;566:21–6. Available from: 10.1016/j.neulet.2014.02.038 [DOI] [PubMed] [Google Scholar]
- Modur P, Miocinovic S. Interictal high-frequency oscillations (HFOs) as predictors of high frequency and conventional seizure onset zones. Epileptic Disord. 2015;17(4):413–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26620382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Jeong J-W, Brown EC, Rothermel R, Kojima K, Kambara T, et al. Three- and four-dimensional mapping of speech and language in patients with epilepsy. Brain. 2017;140(5):1351–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28334963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nariai H, Nagasawa T, Juhász C, Sood S, Chugani HT, Asano E. Statistical mapping of ictal high-frequency oscillations in epileptic spasms. Epilepsia. 2011;52(1):63–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21087245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai J, Kasteleijn-Nolst Trenité D. Interictal discharges and cognition. Epilepsy Behav. 2011;22(1):134–6. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1525505011003556 [DOI] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: Using multiple band frequency analysis. Epilepsia. 2007;48(2):286–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17295622 [DOI] [PubMed] [Google Scholar]
- Palmini A, Gambardella A, Andermann F, Dubeau F, da Costa JC, Olivier A, et al. Intrinsic epileptogenicity of human dysplastic cortex as suggested by corticography and surgical results. Ann Neurol. 1995;37(4):476–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7717684 [DOI] [PubMed] [Google Scholar]
- Pillai J, Sperling MR. Interictal EEG and the diagnosis of epilepsy. Epilepsia. 2006;47 Suppl 1(SUPPL. 1):14–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17044820 [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Duke DW, Krieble KK. Dimensional analysis of resting human EEG. II: Surrogate-data testing indicates nonlinearity but not low-dimensional chaos. Psychophysiology. 1995;32(5):486–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7568643 [DOI] [PubMed] [Google Scholar]
- Rodin E, Constantino T, Rampp S, Wong PK. Spikes and epilepsy. Clin EEG Neurosci. 2009;40(4):288–99. Available from: http://journals.sagepub.com/doi/10.1177/155005940904000411 [DOI] [PubMed] [Google Scholar]
- Salami P, Lévesque M, Benini R, Behr C, Gotman J, Avoli M. Dynamics of interictal spikes and high-frequency oscillations during epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2014;67(5):97–106. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0969996114000734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do Interictal Spikes Drive Epileptogenesis? Neurosci. 2005;11(4):272–6. Available from: http://journals.sagepub.com/doi/10.1177/1073858405278239 [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006;6(6):199–202. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17260059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, White A, Dudek FE. Interictal spikes: harbingers or causes of epilepsy? Neurosci Lett. 2011;497(3):247–50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21458535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson SB, Bermudez C, Conley C, Brown MW, Porter BE, Marsh ED. Spatiotemporal Mapping of Interictal Spike Propagation: A Novel Methodology Applied to Pediatric Intracranial EEG Recordings. Front Neurol. 2016;7:229. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28066315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle VL, Khorasani L, Uftring S, Pelizzari C, Erickson RK, Spire J-P, et al. Noninvasive identification of human central sulcus: a comparison of gyral morphology, functional MRI, dipole localization, and direct cortical mapping. Neuroimage. 2003;19(3):684–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12880799 [DOI] [PubMed] [Google Scholar]
- Wang J, Li W, Miao W, Dai D, Hua J, He H. Age estimation using cortical surface pattern combining thickness with curvatures. Med Biol Eng Comput. 2014;52(4):331–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24395657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Williams PA, Hellier JL, Clark S, Dudek FE, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2010;51(3):371–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19845739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke C, van Drongelen W, Kohrman M, He B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia. 2010;51(4):564–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19817817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–506. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15155522 [DOI] [PubMed] [Google Scholar]
- Zou G, Hua J, Gu X, Muzik O. An Approach for Intersubject Analysis of 3D Brain Images Based on Conformal Geometry. In: 2006 International Conference on Image Processing. IEEE; 2006. p. 1193–6. Available from: http://ieeexplore.ieee.org/document/4106749/ [Google Scholar]