Abstract
Objective:
To determine the effect of tubal ligation on age at natural menopause, as a marker of long-term ovarian function.
Methods:
Three pre-existing population-based cohorts were included in this cross-sectional study. Data from each cohort was analyzed separately. The cohorts were restricted to women who never smoked and had reached natural menopause, without prior hysterectomy or oophorectomy. The following variables were collected: race, age of menarche, age of menopause, history of hysterectomy or oophorectomy, gravidity and parity, tobacco use, and ever use of hormonal contraception. The type of tubal ligation and age at tubal ligation were manually abstracted in Cohort 1. For Cohorts 2 and 3, history of tubal ligation was obtained from an institutional form, completed by patient report. The primary outcome, age at natural menopause, was compared between the two groups (those with and without a history of tubal ligation).
Results:
Inclusion criteria was met by 555 women from Cohort 1, 1,816 women from Cohort 2, and 1,534 women from Cohort 3. Baseline characteristics did not differ between cohorts. The percentage with tubal ligation was the same in all cohorts: 26.0%, 25.5%, and 25.0%, respectively. Women with a tubal ligation were more likely to have had at least one pregnancy and to have used hormonal contraception compared to women without a tubal ligation. There was no significant difference in the age of natural menopause in women who underwent tubal ligation (50.1, 49.9, 50.0 years, respectively) compared to those who did not (50.7, 49.6, 50.0 years, respectively). The type of tubal ligation (Cohort 1 only) had no effect on age of menopause.
Conclusions:
Tubal ligation did not affect age of natural menopause in the three large cohorts included in this study.
Precis:
There is no association of tubal ligation with age of natural menopause.
Introduction:
Surgical sterilization, through tubal ligation or salpingectomy, remains the most common method of contraception for women in the United States, with reports of 700,000 tubal occlusive procedures performed annually (1, 2). Increased access and public acceptance of long-acting reversible contraception has correlated with a decrease in outpatient, interval sterilization procedures; however, laparoscopic occlusive procedures and salpingectomy remain popular options with evidence of both contraceptive benefit and ovarian cancer protection (3, 4).
A strong inverse relationship between tubal ligation and ovarian cancer has been demonstrated in multiple large population-based and prospective studies (5-7). The mechanisms of this protective effect are not fully understood. Potential proposed mechanisms include mechanical prevention of ascending carcinogenic agents or neoplastic tissue, removal of tissue at risk for malignant transformation (fallopian tube), or physiologic alterations in ovarian blood supply or function (8, 9).
The potential impact of tubal ligation on ovarian vascular supply has implications for ovarian function and reserve. This has been investigated in multiple small studies through evaluation of serum and sonographic markers of ovarian reserve. Previous studies evaluated results one to five years post-operatively, with inconsistent results (10-18).
Few studies have investigated the long-term effects of tubal ligation on ovarian function (11, 19). No published studies have investigated the impact of tubal ligation on age of menopause. Theoretically, the initial ovarian insult from tubal ligation may alter ovarian reserve and subsequent age of menopause. An acceleration of time to menopause may have significant implications for long-term, estrogen-mediated health. Studies have shown that earlier age of menopause is associated with an increased incidence of cardiovascular disease, osteoporosis, and cognitive decline (20). Interestingly, epidemiologic studies have identified an increased association of vertebral fractures and unfavorable lipid profiles after tubal ligation, effects reflective of a possible impact on long-term ovarian function (11, 21). This association supports a possible impact of tubal ligation on long-term ovarian function. This study aimed to examine the effect of tubal ligation on the age of natural menopause.
Methods:
This cross-sectional study used three separate, pre-existing cohorts to investigate the effect of tubal ligation on age of menopause. The cohorts included a referent cohort of women from the Rochester Epidemiology Project (Cohort 1), and participants in either the Mayo Clinic Biobank (Cohort 2) or the Mayo Mammography Health Study (Cohort 3). All cohorts included women from Olmsted County, Minnesota and Cohorts 2 and 3 also included women from six surrounding counties. For this study, the cohorts were restricted to women who never smoked and had reached natural menopause, without prior hysterectomy or unilateral or bilateral oophorectomy. Natural menopause was defined as the lack of menses for 12 consecutive months without previous hysterectomy. Variables utilized from the three pre-existing cohorts included date of birth, race, age of menarche, age of menopause, number of pregnancies, ever use of hormonal contraception, and tobacco use. The study was approved by the institutional review boards at Olmsted Medical Center and Mayo Clinic.
Cohort 1 included Olmsted County, Minnesota residents previously identified and reviewed as a referent group for part of the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (22). This prior study included women who underwent a bilateral or unilateral oophorectomy (“index date”) before age 50 years between January 1988 and December 2007 and 1:1 age-matched referent women born in the same year who had not undergone an oophorectomy at the index date. These women were identified through the Rochester Epidemiology Project, a record-linking system that includes complete medical records of all medical providers in Olmsted County, Minnesota (23). The complete medical records of the referent women underwent extensive manual abstraction by a physician or a trained study nurse through 2014, including the occurrence and date of any tubal ligation. Among those with a tubal ligation, an additional medical record review was performed for this study to identify the type of surgical sterilization. The types of surgical sterilization were classified as cautery, Pomeroy or Modified Pomeroy, Hulka or Filshie clips, and salpingectomy. A mix of immediate and interval tubal ligations were included.
Cohort 2 included women 30 years and older who enrolled in the Mayo Clinic Biobank (24). Biobank participants completed an extensive survey at the time of enrollment, which occurred from April 2009 through December 2015. Multiple questions related to reproductive health were included in the survey. In regards to menopause, the survey asked ‘Have you had your uterus removed or was your last menstrual period more than 12 months ago?’ If yes, ‘How old were you when you entered menopause’ and ‘reason periods stopped (natural menopause, hysterectomy or removal of one or both ovaries, took medication, radiation or chemotherapy, or other)’. Additional questions ascertained information on a history of hysterectomy, oophorectomy, or tobacco use. In order to ensure that the cohort consisted of women for whom we would have more complete access to their medical history, if needed for additional verification, we restricted the cohort of interest to women who were residents of a 7-county epidemiologic region of southern Minnesota (Dodge, Freeborn, Mower, Olmsted, Steele, Wabasha, and Waseca counties) at the time of the survey completion (23). Using the US Census estimates for 2014, the Rochester Epidemiology Project has a 93.8% capture of the residents in this 7-county region (23).
Cohort 3 included women 35 years and older from Minnesota, Wisconsin, and Iowa seen at Mayo Clinic in the mammography screening practice from October 2003 through October 2006. Participants completed a survey with questions about reproductive health and tobacco use. The same restrictions were then applied to identify the cohort of interest: residents of the same 7-county region, never smoked, and reported reaching natural menopause with no hysterectomy or oophorectomy prior to the self-reported age of menopause. Despite having data on self-reported natural menopause in Cohorts 2 and 3, we used the resources of the Rochester Epidemiology Project to further identify and exclude women from this cohort who had a hysterectomy or oophorectomy prior to the self-reported age of menopause.
A history of tubal ligation was not included in the surveys administered to Cohorts 2 and 3. This information was obtained for these cohorts using the resources of the Rochester Epidemiology Project to identify procedures performed by a Rochester Epidemiology Project -affiliated provider. In addition, this information was obtained by electronic abstraction of responses to an institutionally administered patient questionnaire that includes the question ‘Have you ever had a tubal ligation’. This questionnaire is administered at every patient visit at Mayo Clinic. If the same individual responded differently to this question on repeated completion of this questionnaire, the chart was reviewed for clarification. The medical records were reviewed to confirm the timing of the surgical sterilization. The type of surgical sterilization was not abstracted for Cohorts 2 and 3 because many of the procedures were performed elsewhere, limiting our access to operative reports.
The data from each cohort was analyzed separately. Comparisons between the two groups (with and without a history of tubal ligation before natural menopause) were evaluated using the chi-square test for categorical variables, the Wilcoxon rank sum test for ordinal variables, and the two-sample t-test for age. In Cohort 1, the age of menopause was compared between the 4 types of surgical sterilization using an F-test from a one-way analysis of variance model. The correlation between the age of tubal ligation and the age of natural menopause was quantified using the Pearson correlation coefficient. All calculated p-values are two-sided and p-values less than 0.05 will be considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc.; Cary, NC).
Results:
A total of 555 women from Cohort 1, 1816 women from Cohort 2, and 1534 women from Cohort 3 were included in the study (Figure 1). Overall, there were 3267 unique women; 23 women were included in all three cohorts and 592 were included in two of the three cohorts. There was some discrepancy noted in self-reported age of menopause in women included in multiple cohorts. However, the majority of discrepant dates were within one year and there was no difference in groups with or without tubal ligation.
Figure 1.
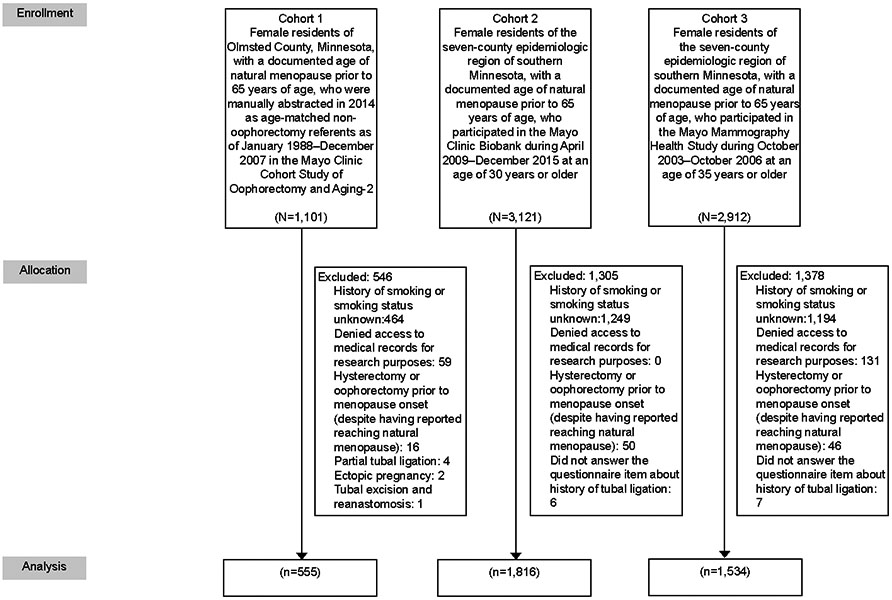
Summary of subject identification.
In each cohort, the rate of tubal ligation was the same, 26.0%, 25.5%, and 25.0%, respectively. The mean age of tubal ligation was 35.5 years overall (34.9, 35.3, and 36.0 years for Cohorts 1, 2, and 3, respectively). Baseline characteristics for each cohort are summarized in Table 1. In each cohort, women who underwent tubal ligation were more likely to have had at least one pregnancy and to have used hormonal contraception compared to women who did not have a tubal ligation.
Table 1.
Patient characteristics according to the pursuit of tubal ligation before menopause, for each of the 3 cohorts
| Characteristic | Cohort 1 | Cohort 2 | Cohort 3 | |||
|---|---|---|---|---|---|---|
| No (N=411) |
Yes (N=144) |
No (N=1352) |
Yes (N=464) |
No (N=1151) |
Yes (N=383) |
|
| Age at questionnaire (years) | --* | --* | ||||
| Mean ± SD | 65.1±10.5 | 63.5±8.3 | 65.1±10.1 | 60.6±7.3 | ||
| Range | 43-97 | 44-93 | 42-95 | 42-88 | ||
| White Race, non-Hispanic | 375 (91.2%) | 138 (95.8%) | 1301 (96.2%) | 443 (95.5%) | 1125 (97.7%) | 371 (96.9%) |
| Age of menarche (years) | ||||||
| <12 | 34 (8.2%) | 11 (7.7%) | 180 (13.3%) | 62 (13.4%) | 181 (15.7%) | 68 (17.8%) |
| 12-14 | 275 (66.9%) | 104 (72.2%) | 915 (67.7%) | 321 (69.2%) | 805 (69.9%) | 261 (68.1%) |
| 15+ | 31 (7.5%) | 9 (6.3%) | 152 (11.2%) | 49 (10.6%) | 113 (9.8%) | 37 (9.7%) |
| Not reported | 71 (17.3%) | 20 (13.9%) | 105 (7.8%) | 32 (6.9%) | 52 (4.5%) | 17 (4.4%) |
| No. of pregnancies | ||||||
| 0 | 65 (15.8%) | 6 (4.2%) | 268 (19.8%) | 17 (3.7%) | 193 (16.8%) | 10 (2.6%) |
| 1 | 33 (8.0%) | 9 (6.3%) | 103 (7.6%) | 20 (4.3%) | 71 (6.2%) | 16 (4.2%) |
| 2 | 134 (32.6%) | 53 (36.8%) | 333 (24.6%) | 122 (26.3%) | 262 (22.8%) | 95 (24.8%) |
| 3+ | 179 (43.6%) | 76 (52.8%) | 644 (47.6%) | 304 (65.5%) | 619 (53.8%) | 260 (67.9%) |
| Not reported | 0 (0.0%) | 0 (0.0%) | 4 (0.3%) | 1 (0.2%) | 6 (0.5%) | 2 (0.5%) |
| No. of live births | ||||||
| 0 | 78 (19.0%) | 7 (4.9%) | 298 (22.0%) | 24 (5.2%) | 208 (18.1%) | 14 (3.7%) |
| 1 | 41 (10.0%) | 11 (7.6%) | 121 (8.9%) | 24 (5.2%) | 89 (7.7%) | 18 (4.7%) |
| 2 | 153 (37.2%) | 70 (48.6%) | 417 (30.8%) | 168 (36.2%) | 318 (27.6%) | 130 (33.9%) |
| 3+ | 139 (33.8%) | 56 (38.9%) | 512 (37.9%) | 248 (53.4%) | 531 (46.1%) | 217 (56.7%) |
| Not reported | 0 (0.0%) | 0 (0.0%) | 4 (0.3%) | 0 (0.0%) | 5 (0.4%) | 4 (1.0%) |
| Ever use of hormonal contraception | 245 (59.6%) | 104 (72.2%) | 931 (68.9%) | 399 (86.0%) | 665 (57.8%) | 304 (79.4%) |
| Age of tubal ligation (years) | ||||||
| No. with age available | -- | 143 | -- | 354 | -- | 261 |
| Mean ± SD | 34.9±5.1 | 35.3±5.1 | 36.0±5.1 | |||
| Range | 23.2-51.0 | 16.9-48.3 | 20.8-47.8 | |||
The data for Cohort 1 was manually abstracted from the medical record.
Overall, the mean age of natural menopause was 50.0 years (50.3, 49.8, and 50.0 years for Cohorts 1, 2, and 3, respectively). There was no significant difference in the age of natural menopause in women who underwent tubal ligation compared to those who did not (Table 2). In Cohort 1, the women with prior tubal ligation reached menopause an average of 0.6 years later (mean (SD) 50.7 (3.7) vs. 50.1 (3.5) years, p=0.08), whereas in Cohort 2 the women with prior tubal ligation reached menopause an average of 0.3 years earlier (49.6 (4.7) vs. 49.9 (4.5) years, p=0.18). The average age of menopause was the same in Cohort 3 (50.0 (4.4) vs. 50.0 (4.2) years, p= 0.84). No significant difference in the age of menopause was observed between those who underwent tubal ligation vs. those who did not when the cohorts were stratified by parity (Table 2).
Table 2.
Age of natural menopause according to the pursuit of tubal ligation before menopause, for each of the 3 cohorts
| Age of natural menopause (years) |
Cohort 1 | Cohort 2 | Cohort 3 | |||
|---|---|---|---|---|---|---|
| No (N=411) |
Yes (N=144) |
No (N=1352) |
Yes (N=464) |
No (N=1151) |
Yes (N=383) |
|
| All patients | ||||||
| Mean ± SD | 50.1 ± 3.5 | 50.7 ± 3.7 | 49.9 ± 4.5 | 49.6 ± 4.7 | 50.0 ± 4.2 | 50.0 ± 4.4 |
| Range | 33-59 | 37-61 | 30-64 | 30-60 | 32-62 | 35-60 |
| p=0.08 | p=0.18 | p=0.84 | ||||
| Stratified by parity | ||||||
| Nulliparous (no live births) | ||||||
| No. | 78 | 7 | 298 | 24 | 208 | 14 |
| Mean ± SD | 50.1 ± 3.0 | 51.1 ± 2.7 | 49.5 ± 4.2 | 49.7 ± 5.4 | 49.5 ± 4.3 | 49.6 ± 4.2 |
| Range | 42-56 | 47-56 | 30-60 | 32-56 | 32-59 | 41-55 |
| p=0.36 | p=0.85 | p=0.91 | ||||
| Parous (at least one live birth) | ||||||
| No. | 333 | 137 | 1050 | 440 | 938 | 365 |
| Mean ± SD | 50.2 ± 3.6 | 50.7 ± 3.7 | 50.0 ± 4.6 | 49.6 ± 4.7 | 50.1 ± 4.2 | 50.0 ± 4.4 |
| Range | 33-59 | 37-61 | 30-64 | 30-60 | 32-62 | 35-60 |
| p=0.13 | p=0.10 | p=0.64 | ||||
Among those in Cohort 1 that underwent tubal ligation, six women underwent salpingectomy via posterior culdotomy with sharp excision of the fallopian tubes without use of electrocautery. The mean age of menopause was not significantly different among the 4 types of surgical sterilization (cautery, Pomeroy or Modified Pomeroy, Hulka or Filshie clips, and salpingectomy; p=0.76; Table 3). There was not a strong correlation between the age of tubal ligation and the age of menopause (r= 0.12, 0.09, and 0.01 for Cohorts 1, 2, and 3, respectively).
Table 3.
Age of surgical sterilization and age of natural menopause according to type of surgical sterilization among the women in Cohort 1
| Type of tubal ligation | ||||||
|---|---|---|---|---|---|---|
| Characteristic | No surgical sterilization (N=411) |
Cautery (N=56) |
Pomeroy or Modified Pomeroy (N=37) |
Hulka or Filshie clips (N=10) |
Unknown type (N=135) |
Salpingectomy (N=6) |
| Age of tubal ligation (years) | ||||||
| Mean ± SD | -- | 34.9 ± 5.0 | 34.2 ± 4.1 | 37.2 ± 7.4 | 34.0 ± 4.7 | 39.5 ± 7.2 |
| Range | 24.1-46.0 | 26.2-45.0 | 26.5-45.7 | 23.2-42.8 | 31.6-51.0 | |
| Age of natural menopause (years) | ||||||
| Mean ± SD | 50.1 ± 3.5 | 50.9 ± 3.5 | 51.1 ± 4.3 | 50.1 ± 3.4 | 50.0 ± 3.6 | 52.2 ± 1.5 |
| Range | 33-59 | 42-61 | 37-57 | 42-54 | 42-58 | 50-54 |
Discussion:
Few studies have evaluated the effect of tubal ligation on long-term ovarian function (11, 21). While others have investigated surrogate markers and short term effects, this study uniquely evaluates a significant clinical outcome, age of menopause, as a marker of long-term ovarian function. The effect of tubal ligation on ovarian function is hypothesized to reflect a similar mechanism by which tubal ligation is protective against ovarian cancer: altered ovarian perfusion by disruption to surrounding vasculature (4, 8, 25). Surrogate markers of the impact of tubal ligation on long-term ovarian function have included systemic outcomes associated with decreased estrogen exposure. A study by Ozkaya et al, found an association between previous tubal ligation and unfavorable lipid profiles in perimenopausal women being evaluated for abnormal uterine bleeding (11). Additionally, an epidemiologic study identified an association between previous tubal ligation and self-reported, radiologically-verified vertebral fractures in women aged 50 or greater (21). These studies reflect the negative impact of hormonal changes associated with menopause on cardiovascular disease risk, lipid profiles, and osteoporosis [18].
Previous studies have evaluated the effect of tubal ligation on ovarian function through assessment of various markers of ovarian reserve; but do not report the age at menopause. These markers have been evaluated as early as six weeks post-operatively and at most 60 months post-operatively. Studies evaluating levels of follicle stimulating hormone (FSH), luteinizing hormone, and estradiol (E2) have largely found no change in women pre- and post-operatively after tubal ligation (12-16). Kelekci et al., found an increase in FSH one month post-operatively; however, the increase did not persist at 12 months (10). Conversely, Carmona et al, identified an increase in FSH by 45% in women who underwent bipolar tubal ligation compared to a 30% increase in control women over 60-months. Women in this prior study were matched by race, age, body mass index, and parity. The difference in rate of FSH rise was not statistically significant (13). Although there is heterogeneity and only short-term follow-up in these studies, the lack of significant change in FSH after tubal ligation is congruent with our findings.
Another surrogate marker of ovarian function used in studies, antral follicle count, has also been limited to short-term outcomes (3-12 months postoperatively) and has mixed results. Two studies found a decreased antral follicle count after tubal ligation with electrocautery in both human and animal studies (17, 18) while others found no effect (14-16, 26). A study by Kutlar et al., evaluated additional ultrasound measurements for ovarian function and found an increased uterine artery resistivity index and arterial pulsatility index in patients who underwent tubal ligation with Pomeroy technique, compared to fimbriectomy and bipolar coagulation (27). They hypothesized the greater length of tissue removed by Pomery technique may be more likely to impact ovarian perfusion. Finally, while serum anti-mullerian hormone , a biochemical marker of ovarian function, has been shown to decrease post-operatively (three to12 months) after tubal ligation, these changes have not been shown to be statistically significant (14, 26),.
While our study found no effect of tubal ligation on the age of natural menopause, women who elect for surgical sterilization are more likely to pursue additional gynecologic surgeries which may put them at risk for surgical menopause (28). There are no published studies, to our knowledge, on the association between tubal ligation and oophorectomy. However, tubal ligation has been described as a risk factor for future hysterectomy. One multicenter cohort study found a 4.4% increased risk of hysterectomy in women who had a tubal ligation (8.8%) compared to those whose husbands underwent a vasectomy for sterilization (2.0%) (28, 29). Another study confirmed the increased risk of hysterectomy in women with a history of tubal ligation and identified an increased association of menstrual dysfunction and pelvic pain as the cause for hysterectomy (30). Interestingly, a study by Hills et al. found that non-physiologic factors may influence the decision for hysterectomy in women with a history of tubal ligation. Among women who were undergoing hysterectomy, those with a history of tubal ligation, were significantly more likely to have non-pathologic hysterectomy specimens compared to women with no history of tubal ligation. The indications for hysterectomy, such as pelvic pain, pressure, and abdominal masses, were more likely to persist one year later in the tubal ligation cohort. Hills et al. concluded that women with a history of tubal ligation are no longer interested in fertility preservation and were less likely to pursue non-surgical options for pelvic symptoms (31). Further investigation of the risk of hysterectomy or oophorectomy after tubal ligation is imperative to ascertain the full effect of tubal ligation on age of menopause, both surgical and natural.
The contemporary approach toward salpingectomy as both a recommended form of permanent contraception and as an opportunistic risk-reducing strategy against ovarian cancer make this study on the impact of tubal ligation relevant to current trends. Importantly, the long-term impact of salpingectomy on the age of natural menopause remains unclear. Our study had a limited number of women who underwent salpingectomy (n=6 in Cohort 1). A recent meta-analysis of 48 studies by Kotlyar et al, found no impact of salpingectomy on ovarian function (32); however, the authors cautioned that previous reports of alterations in follicle stimulating hormone, anti-mullerian hormone, antral follicle count, and ovarian pulsatility cannot be discounted. Additionally, they suggested the need for longer follow-up, such as evaluation of time to natural menopause, and advisement of the possible potential for diminished ovarian function with opportunistic salpingectomy.
This study uniquely assessed the long-term impact of tubal ligation on ovarian function, as defined by the age at natural menopause. Strengths of this study include the large number of patients, across three large population-based cohorts, which add to the generalizability of our findings. The study was sufficiently powered to detect clinically meaningful differences if they had existed. The small differences in age of menopause, less than one year, were not clinically significant and may reflect differences in patient interpretation or recall of the exact timing of menopause. However, the effects of this recall bias are likely overcome by the number of women included in the study. In our mid-sized cohort (Cohort 3) with 1534 patients and a pooled standard deviation of 4.2, we had 80% power to detect a difference in group means of 50.0 vs 50.7 years for the age of menopause.
We recognize that there are limitations of the study. This study relied on patient reporting of age of menopause, and this self-report may be impacted by recall bias; however, all women included in the study self-reported the age of menopause and each cohort’s questionnaire was standardized. In addition, women with a history of ectopic pregnancy and unilateral salpingectomy or salpingostomy were likely included as subjects as this history was not determined via patient questionnaires. While we do not expect unilateral tubal surgery to impact ovarian reserve it was not necessarily excluded as were other types of gynecologic procedures. Nearly all women included were Caucasian, limiting the generalizability of these findings to other racial groups. Finally, this study did not evaluate the risk of tubal ligation on surgical menopause.
In this study, tubal ligation did not impact the age at which women underwent natural menopause. This finding appears to hold true regardless of the type of tubal ligation performed or the age at which a woman undergoes the procedure. These results can offer new guidance to clinicians as they counsel patients on contraceptive options.
Acknowledgments
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676 and grant from the National Cancer Institute (R01 CA97396). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.. Informed consent was obtained from all subjects.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Presented at the American Society of Reproductive Medicine, Denver CO. October 6-10, 2018
References
- 1.Westhoff C, Davis A. Tubal sterilization: focus on the U.S. experience. Fertil Steril. 2000;73(5):913–22. [DOI] [PubMed] [Google Scholar]
- 2.Bartz D, Greenberg JA. Sterilization in the United States. Rev Obstet Gynecol. 2008;1(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Walker JL, Powell CB, Chen LM, Carter J, Bae Jump VL, Parker LP, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;27(10):29321. [DOI] [PubMed] [Google Scholar]
- 4.Lessard-Anderson CR, Handlogten KS, Molitor RJ, Dowdy SC, Cliby WA, Weaver AL, et al. Effect of tubal sterilization technique on risk of serous epithelial ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2014;135(3):423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankinson SE, Hunter DJ, Colditz GA, Willett WC, Stampfer MJ, Rosner B, et al. Tubal ligation, hysterectomy, and risk of ovarian cancer. A prospective study. Jama. 1993;270(23):2813–8. [PubMed] [Google Scholar]
- 6.Madsen C, Baandrup L, Dehlendorff C, Kjaer SK. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta obstetricia et gynecologica Scandinavica. 2015;94(1):86–94. [DOI] [PubMed] [Google Scholar]
- 7.Rice MS, Hankinson SE, Tworoger SS. Tubal ligation, hysterectomy, unilateral oophorectomy, and risk of ovarian cancer in the Nurses' Health Studies. Fertil Steril. 2014;102(1):192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cibula D, Widschwendter M, Zikan M, Dusek L. Underlying mechanisms of ovarian cancer risk reduction after tubal ligation. Acta obstetricia et gynecologica Scandinavica. 2011;90(6):559–63. [DOI] [PubMed] [Google Scholar]
- 9.Green A, Purdie D, Green L, Dick ML, Bain C, Siskind V. Validity of self-reported hysterectomy and tubal sterilisation. The Survey of Women's Health Study Group. Australian and New Zealand journal of public health. 1997;21(3):337–40. [DOI] [PubMed] [Google Scholar]
- 10.Kelekci S, Yilmaz B, Yakut Y, Yasar L, Savan K, Sonmez S. Hormonal and ovarian stromal blood supply changes after laparoscopic tubal sterilization: a prospective controlled study. Contraception. 2006;73(3):279–83. [DOI] [PubMed] [Google Scholar]
- 11.Ozkaya E, Gokmen O, Tosun A, Kucuk E, Baris S, Korkmaz V, et al. Unfavorable lipid profile and higher frequency of hot flashes during perimenopausal years after fallopian tube ligation. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2013;29(6):559–62. [DOI] [PubMed] [Google Scholar]
- 12.Dede FS, Dilbaz B, Akyuz O, Caliskan E, Kurtaran V, Dilbaz S. Changes in menstrual pattern and ovarian function following bipolar electrocauterization of the fallopian tubes for voluntary surgical contraception. Contraception. 2006;73(1):88–91. [DOI] [PubMed] [Google Scholar]
- 13.Carmona F, Cristobal P, Casamitjana R, Balasch J. Effect of tubal sterilization on ovarian follicular reserve and function. American journal of obstetrics and gynecology. 2003;189(2):447–52. [DOI] [PubMed] [Google Scholar]
- 14.Ercan CM, Sakinci M, Coksuer H, Keskin U, Tapan S, Ergun A. Ovarian reserve testing before and after laparoscopic tubal bipolar electrodesiccation and transection. European journal of obstetrics, gynecology, and reproductive biology. 2013;166(1):56–60. [DOI] [PubMed] [Google Scholar]
- 15.Venturella R, Morelli M, Lico D, Di Cello A, Rocca M, Sacchinelli A, et al. Wide excision of soft tissues adjacent to the ovary and fallopian tube does not impair the ovarian reserve in women undergoing prophylactic bilateral salpingectomy: results from a randomized, controlled trial. Fertil Steril. 2015;104(5):1332–9. [DOI] [PubMed] [Google Scholar]
- 16.Morelli M, Venturella R, Mocciaro R, Di Cello A, Rania E, Lico D, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129(3):448–51. [DOI] [PubMed] [Google Scholar]
- 17.Kaya C, Turgut H, Cengiz H, Turan A, Ekin M, Yasar L. The effect of tubal sterilization with the Pomeroy technique and bipolar electrocauterization on the ovarian reserve and serum anti-Mullerian hormone levels in a rat model. European journal of obstetrics, gynecology, and reproductive biology. 2015;185:108–13. [DOI] [PubMed] [Google Scholar]
- 18.Goynumer G, Kayabasoglu F, Aydogdu S, Wetherilt L. The effect of tubal sterilization through electrocoagulation on the ovarian reserve. Contraception. 2009;80(1):90–4. [DOI] [PubMed] [Google Scholar]
- 19.Wyshak G Menopausal symptoms and psychological distress in women with and without tubal sterilization. Psychosomatics. 2004;45(5):403–13. [DOI] [PubMed] [Google Scholar]
- 20.Daan NM, Fauser BC. Menopause prediction and potential implications. Maturitas. 2015;82(3):257–65. [DOI] [PubMed] [Google Scholar]
- 21.Wyshak G Tubal ligation and the risk of vertebral fractures. Osteoporos Int. 2005;16(6):651–8. [DOI] [PubMed] [Google Scholar]
- 22.Rocca WA, Gazzuola Rocca L, Smith CY, Grossardt BR, Faubion SS, Shuster LT, et al. Cohort profile: the Mayo Clinic Cohort Study of Oophorectomy and Aging-2 (MOA-2) in Olmsted County, Minnesota (USA). BMJ open. 2017;7(11):e018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocca WA, Grossardt BR, Brue SM, Bock-Goodner CM, Chamberlain AM, Wilson PM, et al. Data Resource Profile: Expansion of the Rochester Epidemiology Project medical records-linkage system (E-REP). Int J Epidemiol. 2018;47(2):368–j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson JE, Ryu E, Johnson KJ, Koenig BA, Maschke KJ, Morrisette JA, et al. The Mayo Clinic Biobank: a building block for individualized medicine. Mayo Clinic proceedings. 2013;88(9):952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjaer SK, Mellemkjaer L, Brinton LA, Johansen C, Gridley G, Olsen JH. Tubal sterilization and risk of ovarian, endometrial and cervical cancer. A Danish population-based follow-up study of more than 65 000 sterilized women. Int J Epidemiol. 2004;33(3):596–602. [DOI] [PubMed] [Google Scholar]
- 26.Silva AL, Re C, Dietrich C, Fuhrmeister IP, Pimentel A, Corleta HV. Impact of tubal ligation on ovarian reserve as measured by anti-Mullerian hormone levels: a prospective cohort study. Contraception. 2013;88(6):700–5. [DOI] [PubMed] [Google Scholar]
- 27.Kutlar I, Ozkur A, Balat O, Ugur MG, Genco Y, Aksoy F. Effects of three different sterilization methods on utero-ovarian Doppler blood flow and serum levels of ovarian hormones. European journal of obstetrics, gynecology, and reproductive biology. 2005;122(1):112–7. [DOI] [PubMed] [Google Scholar]
- 28.Hillis SD, Marchbanks PA, Tylor LR, Peterson HB. Higher hysterectomy risk for sterilized than nonsterilized women: findings from the U.S. Collaborative Review of Sterilization. The U.S. Collaborative Review of Sterilization Working Group. Obstetrics and gynecology. 1998;91(2):241–6. [DOI] [PubMed] [Google Scholar]
- 29.Mall A, Shirk G, Van Voorhis BJ. Previous tubal ligation is a risk factor for hysterectomy after rollerball endometrial ablation. Obstetrics and gynecology. 2002;100(4):659–64. [DOI] [PubMed] [Google Scholar]
- 30.Goldhaber MK, Armstrong MA, Golditch IM, Sheehe PR, Petitti DB, Friedman GD. Long-term risk of hysterectomy among 80,007 sterilized and comparison women at Kaiser Permanente, 1971-1987. Am J Epidemiol. 1993;138(7):508–21. [DOI] [PubMed] [Google Scholar]
- 31.Hillis SD, Marchbanks PA, Peterson HB. Clinical and pathologic characteristics of women undergoing hysterectomy after tubal sterilization. Obstetrics and gynecology. 1996;88(2):246–50. [DOI] [PubMed] [Google Scholar]
- 32.Kotlyar A, Gingold J, Shue S, Falcone T. The Effect of Salpingectomy on Ovarian Function. J Minim Invasive Gynecol. 2017;24(4):563–78. [DOI] [PubMed] [Google Scholar]


