1. Introduction
Respiratory distress is one of the most common complaints in the emergency department (ED) [1]. In extreme scenarios, during which respiratory distress becomes respiratory failure, the survival of the patients will significantly drop [2]. For instance, a recent cohort study showed that the mortality rate among hospitalized patients with respiratory distress was as much as 10% [3].
Hence, in the management of a patient with respiratory distress, using a timely approach to differentiate the underlying causes and initiating specific therapies (decompression of the pneumothorax, intubation and ventilation support, bronchodilators or corticosteroid nebulization, anticoagulation and fibrinolytic therapy in pulmonary thromboembolisms, etc.) is of undisputed importance.
In 2008, the Bedside Lung Ultrasound in Emergency (BLUE) protocol was developed to guide the diagnosis of respiratory distress [4]. Lichtenstein and his colleagues who proposed the BLUE protocol, showed that the diagnostic accuracy of lung ultrasound (LUS) in the Intensive Care Unit (ICU) was 90.5%. Similarly, Silva et al. demonstrated that LUS was more accurate in cases of acute respiratory failure than routine approaches (patient history and physical examination, radiologic and laboratory evidence) [5].
The BLUE protocol enables physicians to differentiate the underlying causes of respiratory failure. More to the point, LUS was proved to be effective in the monitoring of therapeutic responses in acute respiratory distress syndrome (ARDS) [6].
The BLUE protocol can be applied using a simple ultrasound machine equipped with a micro-convex array transducer [7]. Nevertheless, performing an accurate LUS requires specific training [8]. Although current evidence encourages the use of LUS in treating respiratory distress in critical care, it is not widely accepted in EDs [9].
We hypothesized that utilizing a bedside testing protocol such as the BLUE protocol in the ED may shorten the time interval between patient admission and the delivery of any definitive treatment [10]. We have therefore conducted a randomized clinical trial to assess whether or not the application of the BLUE protocol by a trained emergency physician has an impact on the timely diagnosis and treatment of respiratory distress in the ED.
2. Methods
From August 2015 to March 2016, all consecutive patients who visited the EDs of two university affiliated teaching hospitals in Tehran, Iran were evaluated. Patient flow in these EDs can be as high as 150,000 annually.
Patients were included in the study if they met the following criteria: 1. They were aged over 12 years and 2. Were suffering from acute respiratory distress (within the past seven days). Exclusion criteria were: 1. dyspnea due to a previously diagnosed medical condition, 2. lack of consent and 3. cardiopulmonary arrest on arrival in the ED.
Initially, a group of three board-certified emergency physicians visited the patients. After taking the patients' history and performing a physical examination, the patients were randomly (using computerized block randomization) divided into BLUE protocol and control groups. Both groups were evaluated using routine approaches [chest X-ray and biochemistry for all patients, lung CT scan in 6 (33.3%) BLUE protocol and 8 (53.3%) control patients, based on the discretion of treating physicians (Table 2)], but in BLUE protocol group each patient received an additional LUS examination with a 2–5 MHz curved array transducer and a 7.5–10 MHz linear probe (SonoAce X8 Ultrasound System, Samsung Electronics Co., Ltd) by another attending emergency physician skilled in ultrasound. The sonographer was not involved in the other steps of patient care, but the treating physicians used the results of the lung ultrasound examination in their decision making. The bedside ultrasound test was performed upon admission to the ED and lasted for up to 5 min.
Table 2.
Diagnostic tools in patients with acute respiratory distress
| Diagnostic evaluation | BLUE group n(%) | Control group n(%) |
|---|---|---|
| Pulmonary function test | 0 | 2(11.1) |
| Echocardiography | 6(40) | 7(38.9) |
| Chest CT scan | 6(33.3) | 8(53.3) |
| Bronchoscopy | 3(16.7) | 0 |
The LUS was evaluated for the main diagnoses, including pneumonia, pulmonary edema, pneumothorax, COPD, asthma and plural effusion. According to the BLUE protocol, three quadrants on each side of the chest (the upper and lower parts of the anterior chest and the posterolateral chest wall) were examined by LUS [4].
Eventually, a definite diagnosis was made by using standardized tests for all patients. Triage time, the time of admission to the ED and the time of receiving definitive treatment were recorded for each patient.
Based on prior studies, the average time taken to receive special treatment for dyspnea is about 90 min [4].
To show the difference in the timings, with a standard deviation of 40 min and α = 0.05, we needed to recruit 13 patients into each arm of the study.
The local ethics committee of Tehran University of Medical Sciences approved the conduct of the study. Informed consent was obtained from all the participants.
The data were analyzed using the SPSS statistical software package (SPSS, Chicago, IL, USA). The variables were initially analyzed using the Kolmogorov–Smirnov test, then we used the Student's t-test for the continuous variables and the Mann-Whitney U test for comparing the non-continuous variables. The qualitative data were analyzed using the Chi-squared test. We considered a p value of below 0.05 to be statistically significant.
3. Results
Fifty patients were recruited into the study. Of these, 29 (58%) were male and 21 (42%) were female. The mean (± SD) age of the participants was 63/17 ± 42/61 years.
There was no significant difference between the case study and control groups in terms of age, gender and Body Mass Index (BMI) (Table 1 ).
Table 1.
Demography of the participants
| BLUE protocol | Control | P value | |
|---|---|---|---|
| Age (year, mean ± S) | 65.56 ± 14.65 | 57.28 ± 19.6 | 0.1 |
| Gender (n%) | |||
| Male | 17(68%) | 12(48%) | 0.15 |
| Female | 8(32%) | 13(52%) | |
| BMI (kg/m2) | 23.97 ± 4.42 | 25.63 ± 5.72 | 0.26 |
A definitive diagnosis was made by performing routine evaluations, including a chest radiography and CT scan, an echocardiography by cardiologists, a bronchoscopy and pulmonary function tests by pulmonologists and biochemical data. However, in the BLUE group, the treating emergency physicians used the results of the LUS as a guide in the decision-making process. This means that if the LUS indicated a definite diagnosis, the next step was to confirm that specific diagnosis by routine means (Table 2 ).
The most frequent final diagnoses for both groups were pneumonia and cardiogenic pulmonary edema, respectively (Table 3 ).
Table 3.
Final diagnosis and time-to-treatment in the participants
| Diagnosis | BLUE group |
Control group |
||
|---|---|---|---|---|
| n(%) | Treatment onset (min) | n(%) | Treatment onset (min) | |
| Pneumonia | 13(52) | 34 | 12(48) | 73 |
| Asthma | 1(4) | 15 | 2(8) | 24 |
| COPD | 1(4) | 25 | 2(8) | 46 |
| ILD* | 1(4) | 15 | 3(12) | 38 |
| Cardiogenic pulmonary edema | 6(24) | 18 | 3(12) | 28 |
| Pneumothorax | 2(8) | 10 | 2(8) | 15 |
| Pneumonia + pulmonary edema | 1(4) | 17 | 1(4) | 75 |
| Total n(%) | 25(100) | 25(100) | ||
| Median time-to-treatment (min) | 17 | 38 | ||
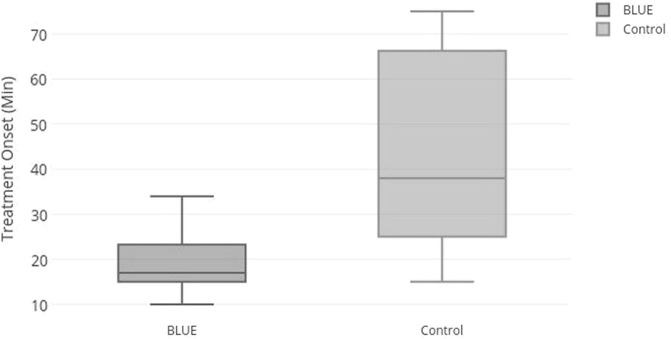
The median admission-treatment time interval for the BLUE group was 17 min, while it was 38 min for the control group. The difference between the two groups was statistically significant (p < 0.0001) (Fig. 1 ).
Fig. 1.
Admission-treatment time interval in acute dyspneic patients.
The average hospital stay (mean ± SD) for the BLUE group was 7 ± 5.8 days, while for the controls it was 7.72 ± 6.8 days. The difference between the two groups was not statistically significant (p value: 0.07).
There was one (4.2%) death in the BLUE group and there were three deaths (13.6%) in the control group. This difference was not statistically significant (p value: 0.34).
4. Discussion
The vast majority of the literature demonstrates that the benefits of ultrasound in the field of critical care are ubiquitous. As technology develops, new ultrasound machines are enabling physicians to obtain clearer images at the patient's bedside [11], [12], [27], [28], [29].
Nowadays, bedside ultrasound is well-known in various fields of patient care in EDs, including point-of-care echocardiography, EFAST (Extended Focused Assessment with Sonography for Trauma) and RUSH (Rapid Ultrasound in Shock) examination. In addition, ultrasound is an adjunct to many ED procedures, such as central and peripheral venous access, thoracentesis, paracentesis, arthrocentesis and confirming correct laryngeal intubation. Current evidence encourages the use of ultrasound in various fields of medicine, as well as in emergency medicine [13], [14].
Lichtenstein et al. evaluated 260 dyspneic patients in the ICU using both LUS and conventional methods. They reported that over 25% of patients evaluated by conventional methods still had an uncertain diagnosis within the first 2 h of admission, while even more patients received incorrect therapies. They have reported that the BLUE protocol was 90.5% accurate in diagnosing the underlying causes of acute respiratory distress, including cardiogenic pulmonary edema, pneumonia, COPD exacerbation, asthma attack, pulmonary emboli and pneumothorax [11].
There are also other reports about the accuracy of LUS in diagnosing the causes of acute respiratory distress [15], [16], [17], [18].
In 2009, Cardinale et al. reported that bedside LUS cuts costs and saves time in the treatment of acute respiratory distress in the ICU [19]. In 2014, Ünlüer and Karagöz compared LUS and radiography in the management of pneumonia. They showed that LUS was more rapid and also more accurate in diagnosing pneumonia [20].
In 2015, Neto et al. applied the BLUE protocol to 57 dyspneic patents in the ICU. They reported that all patients were evaluated by LUS in < 20 min, and that the most common causes of dyspnea were pneumonia and cardiogenic pulmonary edema [21].
In 2013, Xirouchaki et al. applied LUS to 189 critical ICU patients with respiratory failure to assess the impact of LUS on decision-making and the therapeutic management of the patients. They reported that patient management was impacted directly as a result of LUS information in 119 of 253 cases (47%) [22]. In 2016, Mozzini et al. showed that the application of LUS by trained internal medicine residents expedited the diagnosis of the causes of respiratory distress [23]. Other studies showed that utilizing LUS (not specifically the BLUE protocol) in different case, such as pneumonia and pneumothorax, shortens the time taken to establish a definitive diagnosis [9], [24]. Although evidence supports the accuracy of the BLUE protocol, its time-saving advantages have not been fully elucidated, specifically in the ED environment.
The recommended justification for the preference of LUS in cases of acute dyspnea is the fact that LUS is portable and can be performed at the bedside, such that there is no need to move the patient to the CT scan or radiology units. Besides, in comparison to a portable X-ray machine, a US machine is more convenient to handle, and provides greater accuracy in diagnosing lung abnormalities in the hands of a skilled operator [30], [31], [32], [33]. While the BLUE protocol can be performed by a single skilled operator, obtaining a CT scan or X-ray requires at least a radiology technician, a nurse to monitor the patient and staff to transfer the bed. It is therefore predictable that organizing a group of staff will take more time. There is a further time gap between taking an image and transferring the data to the hospital's PACS (Picture Archiving and Communication System). Conversely, for US, the image is readily available at bedside without any of the abovementioned delays [8], [25], [26].
Although the BLUE protocol seems complicated at first sight, studies have shown that the skill can be learned simply by encouraging emergency physicians to learn the examination [34]. Others have shown this propensity not to be limited only to emergency physicians, but can be extended to nurses as well [11], [35].
Based upon the current literature, the BLUE protocol is simple to use and requires simple equipment (an ultrasound machine with a simple convex probe) [7].
The results of the present study into the application of the BLUE protocol in the ED were in accordance with all of the previous reports showing a significant improvement in the diagnosis and time taken to manage patients when LUS was applied in the ICU and in critical care.
Our limitations were: 1. Crowding of the EDs, which had an impact on patient care. Some patients left the ED due to overcrowding to seek medical care in private hospitals; 2. All of the LUS exams were performed by one skilled operator, so we could not compare the results of these exams with those of another operator to calculate the inter-observer reliability. Also, it is not clear whether or not novice sonographers would reach the same conclusions after participating in a short course; and 3. Our sample size was limited and this can impact the final outcome of the patients.
5. Conclusion
Our findings showed that utilizing the BLUE protocol in cases of acute respiratory distress in the ED will significantly shorten the time taken to manage patients and decrease the gap in delivering definite therapies.
References
- 1.Niska R., Bhuiya F., Xu J. National hospital ambulatory medical care survey: 2009 emergency department summary. Natl Health Stat Rep. 2010:1–31. [PubMed] [Google Scholar]
- 2.Hall M.J., Levant S., DeFrances C.J. Trends in inpatient hospital deaths: National Hospital Discharge Survey, 2000–2010. NCHS Data Brief. 2013:1–8. [PubMed] [Google Scholar]
- 3.Dean J.M., Vernon D.D., Cook L. Probabilistic linkage of computerized ambulance and inpatient hospital discharge records: a potential tool for evaluation of emergency medical services. Ann Emerg Med. 2001;37:616–626. doi: 10.1067/mem.2001.115214. [DOI] [PubMed] [Google Scholar]
- 4.Volpicelli G., Elbarbary M., Blaivas M. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 5.Silva S., Biendel C., Ruiz J. Usefulness of cardiothoracic chest ultrasound in the management of acute respiratory failure in critical care practice. Chest. 2013;144:859–865. doi: 10.1378/chest.13-0167. [DOI] [PubMed] [Google Scholar]
- 6.Bouhemad B., Brisson H., Le-Guen M. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein D.A. BLUE-protocol and FALLS-protocol. Chest. 2015;147:1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 8.Koenig S.J., Narasimhan M., Mayo P.H. Thoracic ultrasonography for the pulmonary specialist. Chest. 2011;140:1332–1341. doi: 10.1378/chest.11-0348. [DOI] [PubMed] [Google Scholar]
- 9.Wimalasena Y., Kocierz L., Strong D. Lung ultrasound: a useful tool in the assessment of the dyspnoeic patient in the emergency department. Fact or fiction? Emerg Med J. 2017;0:1–9. doi: 10.1136/emermed-2016-205937. [DOI] [PubMed] [Google Scholar]
- 10.DeVos E., Jacobson L. Approach to adult patients with acute dyspnea. Emerg Med Clin North Am. 2016;34:129–149. doi: 10.1016/j.emc.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lichtenstein D.A., Mezière G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown S., Kasal J. Bedside ultrasound in the intensive care unit: where is the evidence? Semin Respir Crit Care Med. 2015;36:878–889. doi: 10.1055/s-0035-1564873. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein D.A., Mezière G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenstein D.A., Meziere G., Lascols N. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33:1231–1238. doi: 10.1097/01.ccm.0000164542.86954.b4. [DOI] [PubMed] [Google Scholar]
- 15.Mathis G., Blank W., Reissig A. Thoracic ultrasound for diagnosing pulmonary embolism: a prospective multicenter study of 352 patients. Chest. 2005;128:1531–1538. doi: 10.1378/chest.128.3.1531. [DOI] [PubMed] [Google Scholar]
- 16.Reissig A., Kroegel C. Sonographic diagnosis and follow-up of pneumonia: a prospective study. Respiration. 2007;74:537–547. doi: 10.1159/000100427. [DOI] [PubMed] [Google Scholar]
- 17.Gargani L., Frassi F., Soldati G. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail. 2008;10:70–77. doi: 10.1016/j.ejheart.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Soldati G., Copetti R., Sher S. Sonographic interstitial syndrome: the sound of lung water. J Ultrasound Med. 2009;28:163–174. doi: 10.7863/jum.2009.28.2.163. [DOI] [PubMed] [Google Scholar]
- 19.Cardinale L., Volpicelli G., Binello F. Applicazione clinica dell'ecografia polmonare nel paziente con dispnea acuta: Diagnosi differenziale tra cause cardiogene e polmonari. Radiol Med. 2009;114:1053–1064. doi: 10.1007/s11547-009-0451-1. [DOI] [PubMed] [Google Scholar]
- 20.Unluer E.E., Karagoz A. Bedside lung ultrasound versus chest X-ray use in the emergency department. Interv Med Appl Sci. 2014;6:175–177. doi: 10.1556/IMAS.6.2014.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dexheimer Neto F.L., Andrade J.M., Raupp A.C. Diagnostic accuracy of the bedside lung ultrasound in emergency protocol for the diagnosis of acute respiratory failure in spontaneously breathing patients. J Bras Pneumol. 2015;41:58–64. doi: 10.1590/S1806-37132015000100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xirouchaki N., Kondili E., Prinianakis G. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40:57–65. doi: 10.1007/s00134-013-3133-3. [DOI] [PubMed] [Google Scholar]
- 23.Mozzini C., Fratta Pasini A.M., Garbin U., Cominacini L. Lung ultrasound in internal medicine: training and clinical practice. Crit Ultrasound J. 2016;8:10. doi: 10.1186/s13089-016-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corker J.R. Statew Agric L Use Baseline 2015. Vol. 1. 2015. Lung ultrasound for the diagnosis of pneumonia in adult ED patients; p. 24295842. [DOI] [Google Scholar]
- 25.Goodarzi H., Khatami Seyed-Masoud, Hammidreza Javadzadeh S.M., Hojjatollah Khajehpour S.H. User acceptance of picture archiving and communication system in the emergency department. Iran J Radiol. 2016;13:1–9. doi: 10.5812/iranjradiol.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werf T.S., Zijlstra J.G. Ultrasound of the lung: just imagine. Intensive Care Med. 2004 doi: 10.1007/s00134-003-2083-6. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein D., Mezière G., Biderman P., Gepner A. The lung point: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000 doi: 10.1007/s001340000627. [DOI] [PubMed] [Google Scholar]
- 28.Lichtenstein D., Lascols N., Mezière G., Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004 doi: 10.1007/s00134-003-2075-6. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein D., Hulot J.S., Rabiller A. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999 doi: 10.1007/s001340050988. [DOI] [PubMed] [Google Scholar]
- 30.Guyton C.A., Hall J.E. W.B. Saunders; Philadelphia: 1996. Textbook of medical physiology. [Google Scholar]
- 31.Lichtenstein D., Holzapfel L., Frija J. 2000. Projection cutan{é}e des pneumothorax et impact sur leur diagnostic {é}chographique. R{é}an Urg 9. [Google Scholar]
- 32.Brenner D.J., Hall E.J. Computed tomography—an increasing source of radiation exposure. New Engl J Med. 2007 doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 33.Lichtenstein D. In: Whole body ultrasonography in the critically ill. Lichtenstein D., editor. Springer; Heidelberg: 2010. Introduction to lung ultrasound; pp. 117–127. [Google Scholar]
- 34.Irwin R.S., Rippe J.M. 6th ed. Lippincott Williams and Wilkins; Philadelphia: 2008. Intensive care medicine; pp. 491–496. [Google Scholar]
- 35.Lichtenstein D.A., Mezière G.A. The BLUE-points: three standardized points used in the BLUE-protocol for ultrasound assessment of the lung in acute respiratory failure. Crit Ultrasound J. 2011;3:109–110. doi: 10.1007/s13089-011-0066-3. [DOI] [Google Scholar]