Abstract
With the rising prevalence of multidrug resistance, there is an urgent need to develop novel antibiotics. Many putative antibiotics demonstrate promising in vitro potency but fail in vivo due to poor drug-like qualities (e.g., serum half-life, oral absorption, solubility, and toxicity). These drug-like properties can be modified through the addition of chemical protecting groups, creating “prodrugs” that are activated prior to target inhibition. Lipophilic prodrugging techniques, including the attachment of a pivaloyloxymethyl group, have garnered attention for their ability to increase cellular permeability by masking charged residues and the relative ease of the chemical prodrugging process. Unfortunately, pivaloyloxymethyl prodrugs are rapidly activated by human sera, rendering any membrane permeability qualities absent during clinical treatment. Identification of the bacterial prodrug activation pathway(s) will allow for the development of host-stable and microbe-targeted prodrug therapies. Here, we use two zoonotic staphylococcal species, Staphylococcus schleiferi and S. pseudintermedius, to establish the mechanism of carboxy ester prodrug activation. Using a forward genetic screen, we identify a conserved locus in both species encoding the enzyme hydroxyacylglutathione hydrolase (GloB), whose loss-of-function confers resistance to carboxy ester prodrugs. We enzymatically characterize GloB and demonstrate that it is a functional glyoxalase II enzyme, which has the capacity to activate carboxy ester prodrugs. As GloB homologues are both widespread and diverse in sequence, our findings suggest that GloB may be a useful mechanism for developing species- or genus-level prodrug targeting strategies.
Keywords: prodrug, antimicrobial resistance, drug development, esterase, glyoxalase, Staphylococcus
Graphical Abstract

In 2019, the United States recorded 2.8 million antibiotic-resistant infections, resulting in over 35,000 deaths.1 The recent surge in antibiotic use in the setting of the COVID-19 pandemic portends an acceleration of the antibiotic resistance threat.2,3 Staphylococcus aureus is a formidable human pathogen that causes a wide variety of invasive and life-threatening infections. Closely related staphylococcal species, S. pseudintermedius and S. schleiferi, cause similar skin, soft tissue, and invasive infections in companion animals and are increasingly appreciated as serious pathogens of humans.4–7 Rising rates of methicillin resistance are reported in all three species, with methicillin-resistant S. aureus (MRSA) labeled a “serious threat” by the Centers for Disease Control and Prevention (CDC).1,8–10 Novel antimicrobial strategies that circumvent existing drug resistance mechanisms are urgently needed.
Bacterial metabolism is a promising area for antimicrobial development.11,12 Many metabolic processes are essential for bacterial growth and pathogenesis. However, targeting metabolic processes can be inherently challenging, as a substantial portion of metabolism involves the catalytic transformation of highly charged substrates (e.g., phosphate transfer reactions). Substrate-competitive inhibitors of metabolic enzymes frequently deploy phosphonate functional groups as isosteric phosphate mimics.13 These negatively charged phosphonate antimetabolite inhibitors are prone to unacceptable drug-like characteristics and often diffuse poorly across membranes.14–19
Prodrugging, or the modification of an inhibitor through the addition of labile chemical adducts, is a common medicinal chemistry strategy to improve drug-like properties of an inhibitor under development.19–21 As promoieties are released prior to inhibitor–target engagement, prodrugging can temporarily cloak problematic pharmacokinetic properties such as poor absorption or solubility. For example, the third-generation cephalosporin cefditoren is poorly absorbed in the small intestine unless its carboxylate is masked with a lipophilic pivaloyloxymethyl (POM) promoiety in the form of cefditoren pivoxil.22 Similarly, nucleoside analogues are generally cell-impermeable, but their cognate prodrugs have much improved cellular penetration and antiviral efficacy, as seen in remdesivir (SARS-CoV-2), tenofovir disoproxil (HIV), and sofosbuvir (hepatitis C virus, HCV).23–26 We have recently employed lipophilic prodrugging strategies to increase the efficacy of broad-spectrum antimicrobial phosphonate antibiotics. Notably, POM ester modification of a phosphonate isoprenoid biosynthesis inhibitor (ERJ) increases antistaphyloccal activity by 200- and 500-fold for S. schleiferi and S. pseudintermedius, respectively (Figure 1A,B).27 Similar dramatic potency gains are observed for the same class of compounds against Mycobacterium tuberculosis, Yersinia pestis, Franciscella novicida, and the malaria parasite Plasmodium falciparum.16,28–31,82
Figure 1.
(A) Predicted POM-ERJ activation pathway. POM promoiety highlighted in pink. (B) Dose-dependent growth inhibition of zoonotic Staphylococci, S. schleiferi (left) and S. pseudintermedius (right), by ERJ (blue) and POM-ERJ (pink). The displayed values are the means ± SD of three independent experiments performed in technical duplicate. (C) Screening strategy to identify prodrug activating enzymes. (D) Distribution of MIC values for WT (pink) and POM-ERJ-resistant mutants from S. schleiferi (left) and S. pseudintermedius (right). The displayed values are the mean values for each strain from three independent experiments performed in technical duplicate.
While POM prodrugs demonstrate remarkable potency in vitro, POM promoieties are known to be rapidly hydrolyzed by serum carboxylesterases.32,33 If cell-impermeable phosphonate antibiotics are to be effective at the site of infection, the promoiety must be resistant to premature bioactivation during absorption and distribution in the circulation. This specificity in prodrug activation has been successfully achieved for liver-targeted prodrugs, using the “HepDirect” prodrug approach, but has not yet been deployed for antibiotic delivery. HepDirect prodrugs are cleaved via a hepatocyte-specific cytochrome P450 enzyme, CYP3A4, and are resistant to cleavage by other human esterases.34 Selective bioactivation of the prodrugs within microbes not only would increase the circulating half-life but also may improve the therapeutic selectivity of therapeutics that target microbial enzymes with human homologues. Understanding the molecular basis of host and microbe prodrug activation will facilitate the design of microbially targeted prodrugs.
In this study, we use two zoonotic staphylococcal species, S. schleiferi and S. pseudintermedius, to uncover the enzymatic mechanism of prodrug activation in Staphylococci. We identify and characterize the first bacterial carboxy ester prodrug activating enzyme hydroxyacylglutathione hydrolase (GloB), a type II glyoxalase. Using detailed biochemical analyses, we demonstrate that GloB recognizes the carboxy ester portion of the prodrug and is responsible for prodrug activation. Since GloB homologues are broadly maintained, yet have substantial sequence variation, we propose that this group of enzymes may be a strategy toward microbe-specific prodrug targeting.
RESULTS
Selection of Prodrug-Resistant Staphylococci.
In our previous study, we identified phosphonate antibiotics with activity against zoonotic Staphylococci (S. schleiferi and S. pseudintermedius).27 Lipophilic carboxy ester prodrug modification of these phosphonates dramatically increases antistaphylococcal potency, presumably through increased cellular penetration (Figure 1A,B). However, prodrug modifications block the direct engagement of inhibitors with their enzyme target.27 For this reason, we hypothesized that one or more staphylococcal esterases were required for intracellular prodrug activation (Figure 1A). To identify candidate prodrug activating enzymes, we designed a genetic screen/counter-screen strategy to enrich for staphylococcal strains that fail to activate lipophilic ester prodrugs.
In our strategy, we took advantage of inhibitor pairs with the same target engagement with and without prodrug modification. We employed the phosphonate antibiotic ERJ, which selectively inhibits the intracellular enzyme deoxyxylulose phosphate reductoisomerase (DXR), and POM-ERJ, the bispivaloyloxymethyl prodrug form of ERJ, which inhibits intracellular DXR even though it has been shown to lack direct activity against purified recombinant DXR in vitro.27 We sought to enrich for staphylococcal strains that were resistant to prodrugged inhibitors (e.g., POM-ERJ) but remained sensitive to the parent phosphonate ERJ itself.27 For this reason, we first isolated staphylococcal colonies that arose from solid media containing POM-ERJ. Next, we screened these POM-ERJ-resistant isolates for cross-resistance to our parent compound, ERJ. POM-ERJ-resistant strains that remained sensitive to ERJ were subjected to whole genome sequencing to identify candidate genetic mutations giving rise to the resistance phenotype (Figure 1C). To identify conserved resistance mechanisms, we performed this screen/counter-screen independently in two staphylococcal species, S. schleiferi and S. pseudintermedius. We isolated and characterized a total of 18 POM-ERJ-resistant staphylococcal strains with MIC90 values ∼10–50-fold higher than that of the respective wild-type (WT) parental lines (Figure 1D). In axenic growth in rich media, no changes in growth rate are observed between WT and three POM-ERJ-resistant isolates (Figure S1).
POM-ERJ Resistance Does Not Alter Cell Wall Size in Staphylococci.
In previous work, we and others have found that cellular entry of the phosphonate antibiotic ERJ and ERJ analogs requires the phosphonate transporter GlpT.16,27,35,36 In contrast, the entry of POM-ERJ is GlpT independent, and may be passively transported or freely diffuse into cells.16,27 POM-ERJ resistance could therefore arise through cell wall modifications that directly disrupt the cell penetration of prodrugs. Such cell wall alterations might therefore lead to cross-resistance to other antimicrobials, such as daptomycin or vancomycin. To establish the selectivity of POM-ERJ-resistance, we determined the antimicrobial sensitivity of a subset of our prodrug-resistant strains against a panel of 18 clinical antibiotics with diverse mechanisms-of-action. We find that POM-ERJ-resistant strains are not cross-resistant to other inhibitors, including daptomycin and vancomycin (Table S1), suggesting a prodrug-specific mechanism of resistance. Additionally, we quantified the cell wall size in POM-ERJ-resistant Staphylococci by transmission electron microscopy, because an established daptomycin and vancomycin resistance strategy for S. aureus is the generation of thickened cell walls that reduce inhibitor entry.37,38 We find no changes in cell wall thickness in prodrug-resistant isolates compared to their prodrug-sensitive WT parental lines (Figure 2).
Figure 2.
(A–D) Representative transmission electron micrographs of WT (A) or three independent POM-ERJ-resistant S. schleiferi strains (B–D). Scale bars = 500 nm. (E) Distribution of cell wall thickness in WT and POM-ERJ-resistant S. schleiferi as measured in a total of 300 cells from three independent experiments of 100 cells each. The midline indicates the mean of all measurements.
POM-ERJ-Resistant Staphylococci Are Cross-Resistant to Other Carboxy Ester Prodrug Antibiotics.
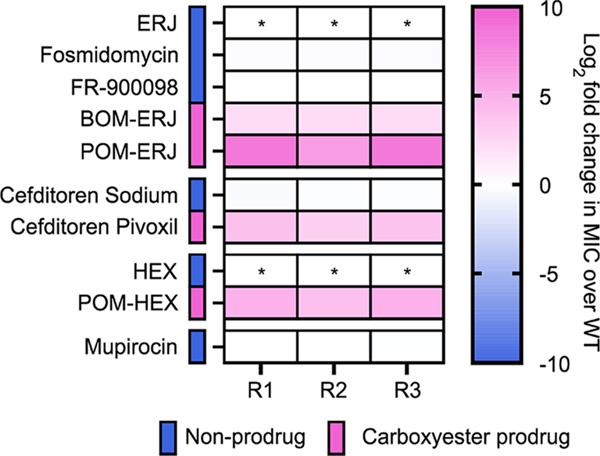
If POM-ERJ resistance is due to loss of a prodrug activating enzyme(s), we hypothesized that POM-ERJ-resistant Staphylococci would likewise be cross-resistant to other carboxy ester prodrug antibiotics. To evaluate this possibility, we selected several additional pairs of inhibitors (carboxy ester prodrugs and their cognate parent (nonprodrugged) compounds) with distinct cellular targets (e.g., penicillin binding protein, deoxyxylulose reductoisomerase (DXR), and enolase) (Figure 3).22,32 For three of our POM-ERJ-resistant S. schleiferi isolates, we determined the minimum inhibitory concentration (MIC) for each compound compared to the WT parental strain (Figure 4).
Figure 3.
Structures of antistaphylococcal inhibitors used in this study. Structures are grouped by the mechanism of action. For prodrugged compounds, promoieties are highlighted in pink.
Figure 4.
Cross-resistance to lipophilic ester prodrugs in POM-ERJ-resistant S. schleiferi. WT and POM-ERJ-resistant S. schleiferi were treated with the compounds displayed in Figure 3. Compounds are grouped by the mechanism of action and color coded to indicate whether a given compound is a carboxy ester prodrug. Displayed are the mean values of the fold change (resistant isolate/WT) of three independent experiments performed in technical duplicate. * indicates compounds whose MIC values were too high to measure. Numerical data are additionally provided in Table S2.
We find that POM-ERJ-resistant Staphylococci remain equally sensitive to nonprodrugged compounds (such as ERJ analogues) and the third-generation cephalosporin cefditoren. In contrast, POM-ERJ-resistant Staphylococci exhibit significantly increased MICs to multiple classes of lipophilic ester prodrugs, exhibiting cross-resistance to both cefditoren pivoxil (cell wall inhibitor) and POM-HEX (inhibitor of enolase) (Figure 4, Table S2). Thus, POM-ERJ-resistant Staphylococci are cross-resistant to other POM prodrug inhibitors, regardless of the intracellular target. Our data suggest that POM prodrugs follow a common and conserved activation mechanism that has been disrupted in our POM-ERJ-resistant isolates.
To explore how changes in the chemical structure of the prodrug group impacts prodrug resistance, we also evaluated whether our POM-ERJ-resistant isolates were cross-resistant to antimicrobial prodrugs that possess another common carboxy ester prodrug moiety, benzoyloxymethyl (BOM) (Figure 3). Indeed, we find our POM-ERJ-resistant isolates are also cross-resistant to BOM-ERJ (Figure 4).
Carboxy ester prodrugs are more lipophilic than their parental molecules. To evaluate whether prodrug resistance in our strains is driven by the lipophobicity of the molecule rather than its ester bond, we selected an additional highly lipophilic antibiotic, mupirocin, which inhibits protein biosynthesis (Figure 3). POM-ERJ-resistant Staphylococci were not cross-resistant to mupirocin, further supporting that prodrug resistance in these strains is specific to the carboxy ester bond of the prodrug (Figure 4).
POM-ERJ-Resistant Staphylococci Are Enriched in Mutations in the GloB Gene.
To characterize the genetic changes associated with carboxy ester prodrug resistance, we performed whole genome sequencing of prodrug-resistant isolates of both S. schleiferi and S. pseudintermedius. The whole genomes of each isolate were compared to the respective parental genome, and candidate genetic changes were verified by Sanger sequencing. We prioritized nonsynonymous genetic changes that were represented in more than one strain. A complete list of identified mutations is found in Table S3.
In both independent genetic screens, we found that prodrug-resistant Staphylococci were enriched in mutations in an evolutionarily conserved locus. We identified multiple isolates (3/16 S. schleiferi, 14/18 S. pseudintermedius) with sequence modifications in the locus annotated as hydroxyacylglutathione hydrolase, gloB (LH95_06060 in S. schleiferi, SPSE_1252 in S. pseudintermedius, Table S3). Most genetic changes in gloB were nonsynonymous single nucleotide polymorphisms, though two nonsense alleles that would truncate approximately 50% of the protein were also identified (Figure 5, Table S3). In several strains, the only genetic variation that distinguished WT and resistant genomes was within the gloB locus.
Figure 5.
POM-ERJ-resistant Staphylococci are enriched for mutations in the locus encoding hydroxyacylglutathione hydrolase (GloB). (A) Locations and identities of GloB mutations were discovered by whole-genome sequencing and independently verified by Sanger sequencing. Line coloring represents the predicted impact of a given mutation on GloB function; scores below −2.5 are predicted to be deleterious. (B, C) Homology models of S. schleiferi (B) and S. pseudintermedius (C) GloB generated using the SWISS-MODEL. Residues found to be mutated in POM-ERJ-resistant Staphylococci are explicitly shown in blue.
Of the 17 identified GloB mutations, 12 unique alleles were identified in prodrug-resistant Staphylococci. Using PROVEAN, an algorithm which quantifies the predicted impact of amino acid substitutions on protein function, each of these 12 alleles is predicted to have deleterious effects on protein function (below the threshold score of −2.5) (Figure 5).39 S. schleiferi and S. pseudintermedius are nonmodel organisms that possess endogenous CRISPR-Cas9 systems, and the transformation of these organisms has not yet been described.40 Attempts to ectopically complement gloB mutant strains with WT GloB (>90 independent transformation attempts using established methods for S. aureus, S. epidermidis, and B. subtilis) were unsuccessful in recovering transformed colonies, despite preparing plasmid from the S. aureus restriction deficient cloning intermediate, RN4220, and the cytosine methyltransferase negative E. coli mutant, DC10B.41–47 However, the independent selection of 12 unique loss-of-function alleles in two different species strongly suggests that loss of GloB function is responsible for prodrug resistance in S. schleiferi and S. pseudintermedius.
Structural Basis of GloB Loss-of-Function.
As prodrug-resistance mutations in GloB map along its entire linear sequence, we next examined the structural basis for GloB loss-of-function. We generated homology models of both SsGloB and SpGloB using the SWISS-MODEL.48 The resulting staphylococcal model is based on the sequence-similar metallo-β-lactamase superfamily member from Thermus thermophilus (PDB 2ZWR).49 This hit had a global model quality estimate (GNQE) of 0.71 and 0.70 for S. schleiferi and S. pseudintermedius GloB homologues, respectively, suggesting the built models are reliable and accurate. In both protein models, we find that POM-ERJ-resistance mutations are primarily located toward the interior of the protein, occupying the same cavity as the well conserved glyoxalase II metal binding motif (THxHxDH).50 This modeling thus indicates that these prodrug-resistance alleles impair the GloB active site (Figure 5).
GloB Is a Functioning Type II Glyoxalase not a β-Lactamase.
GloB is predicted to be a type II glyoxalase and a member of the large metallo-β-lactamase protein superfamily (INTERPRO IPR001279). Members of this superfamily hydrolyze thioester, sulfuric ester, and phosphodiester bonds, such as the ester linkage present in POM-ERJ.50–53 Type II glyoxalases catalyze the second step in the glyoxalase pathway that is responsible for the conversion of methylglyoxal (a toxic byproduct endogenously produced during metabolism) to lactic acid. Specifically, GloB catalyzes the conversion of d-lactoylglutathione to d-lactate.
To determine whether SsGloB encodes a functional type II glyoxalase, we evaluated whether SsGloB hydrolyzes S-lactoylglutathione using an assay in which the hydrolysis of S-lactoylglutathione is linked to a change in absorbance (Figure 6A). We purified recombinant WT SsGloB protein and its catalytically inactive variant SsGloBH54N, in which the histidine of the canonical metal binding motif (THxHxDH) has been altered to an asparagine (Figure S2).50,52,53 We find that SsGloB, but not SsGloBH54N, hydrolyzes S-lactoylglutathione with a specific activity of 0.493 μmol·min−1mg−1 (Figures S3 and 6B,C). This activity is similar to other characterized microbial type II glyoxalases (Saccharomyces cerevisiae, 1.34 μmol·min−1mg−1; Trypanosoma brucei, ∼8 μmol·min−1mg−1) but is much lower than that of previously characterized type II glyoxalases from plants and mammals (20–2000 μmol·min−1mg−1).54–62 We determined the metal dependence of SsGloB and find that SsGloB is a functional type II glyoxalase in manganese, cobalt, calcium, and zinc with a modest preference noted toward magnesium (Figure S4).
Figure 6.
(A) Enzymatic catalysis of S-lactoylglutathione by GloB. DTNB conversion to TNB results in increased absorbance at 412 nm. (B) Reaction progress curve for SsGloB (blue) and catalytically inactive SsGloB H54N (pink) using S-lactoylglutathione as a substrate. (C) SsGloB and SsGloB H54N specific activity for S-lactoylglutathione. Displayed are the means ± SD from three independent experiments performed in technical duplicate.
As some members of the metallo-β-lactamase protein superfamily mediate hydrolysis of β-lactam antibiotics, we considered whether GloB also had β-lactamase activity. Because gloB mutant strains are not cross-resistant to the β-lactam-containing antibiotics (except for the prodrugged cephalosporin, cefditoren pivoxil) (Figure 4, Table S2), we predicted that GloB was not a functional metallo-β-lactamase. As expected, we find that SsGloB does not hydrolyze the β-lactamase ring of nitrocefin (a canonical β-lactamase substrate) in contrast to the active B. cereus β-lactamase (Figure S3).
Staphylococcal GloB Hydrolyzes POM-ERJ In vitro and In vivo.
Loss-of-function mutation in GloB is associated with resistance not only to POM-ERJ but also to other ester prodrugs. Because GloB does not mediate resistance to ERJ or other phosphonates, our data suggested that GloB might directly catalyze the conversion of POM-ERJ to ERJ. To determine whether GloB de-esterifies POM-ERJ, we developed a liquid chromatography–mass spectrometry (LC-MS)-based assay to quantify POM-ERJ concentrations. The incubation of purified recombinant SsGloB protein, but not its inactive variant (SsGloBH54N), with POM-ERJ results in rapid loss of POM-ERJ, consistent with SsGloB-mediated cleavage (Figure 7A). To determine whether prodrug activation activity is conserved among staphylococcal GloB homologues, we also purified recombinant GloB from the human pathogen S. aureus (Figure S2). We find that SaGloB also directly hydrolyzes POM-ERJ (Figure 7A).
Figure 7.
(A) Recombinant SsGloB, catalytically inactive SsGloB H54N, GloB from S. aureus (SaGloB), or buffer was incubated with POM-ERJ, and prodrug concentrations were measured by LC-MS. (B) Wild-type and POM-ERJ-resistant gloB mutant S. schleiferi isolates were treated with POM-ERJ, and intracellular drug concentrations were measured by LC-MS. Displayed are the mean values ± SD from three independent experiments. Error bars may not be visible due to the precision in measurement.
To determine whether GloB mediates intracellular prodrug activation, we evaluated the intracellular concentrations of POM-ERJ in drug-treated WT and gloB mutant Staphylococci. We prepared staphylococcal cultures treated with POM-ERJ and quenched the reaction at several time points to monitor the course of intracellular prodrug depletion. As expected, we find that POM-ERJ is rapidly depleted in WT S. schleiferi, consistent with enzymatic activation. In contrast, POM-ERJ concentrations do not decrease over time in gloB mutant strains, in which the sole genetic change in each strain compared to WT is in the gloB locus (Figure 7B). This suggests that the initial step in carboxy ester prodrug activation in Staphylococci lacks functional redundancy and is exclusively dependent on GloB.
POM-ERJ Is a GloB Substrate.
We next characterized the reaction products resulting from the POM-ERJ incubation with GloB. Using a highly sensitive 31P–1H HSQC NMR protocol, we find that WT S. schleiferi and WT S. aureus GloB remove one carboxy ester from POM-ERJ but are unable to fully deprotect the compound in appreciable quantities (Figure S5A). We hypothesize that the intermediate product may be the singly de-POMylated version of POM-ERJ (Hemi-POM-ERJ). To evaluate whether other POM-containing inhibitors were also direct substrates, we repeated this experiment using POM-HEX (Figure S5B). We find that GloB is likewise capable of partially activating POM-HEX but is unable to act upon Hemi-POM-HEX, suggesting at least one additional enzyme may be required for prodrug activation in vivo.
Staphylococcal GloB Enzymes Represent a Distinct Clade of Bacterial Glyoxalases.
Because staphylococcal GloB mediates de-esterification of ester prodrugs, we sought to evaluate the feasibility of using these enzymes to design prodrugs specifically targeted for activation in Staphylococci. We constructed a phylogenetic tree of GloB homologues across diverse microbial genomes as well as in humans and mice (Figure S6A), specifically including sequences of previously characterized GloB homologues. We find that considerable sequence variation exists within GloB homologues with no clear clustering by phylogeny except for those GloB homologues originating in plants and mammals. This contrasts with a phylogenetic tree generated using the DNA-directed RNA polymerase subunit beta (rpoB), which generally follows the traditional tree of life (Figure S6B).
While sequence differences between staphylococcal GloB and human GloB suggest that there may be substrate utilization differences between humans and Staphylococci, ultimately differences within the active site are likely to drive substrate specificity. Using PyMOL, we aligned our homology model of SsGloB with the glutathione-bound GloB from humans (PDB ID: 1qh5).63,64 The two structures align well with a root-mean-square deviation (RMSD) of 1.528 Å and are well conserved in the overall structure as well as the characteristic Zn binding motif THxHxDH (Figure S7A,B). Notably, however, HsGloB has a significant C-terminal extension that is not present in SsGloB. This C-terminal extension forms an α-helix that borders the active site and contains two residues, K252 and R249, which appear to be involved in coordinating the cocrystallized glutathione substrate (Figure S7C). The absence of this C-terminal extension in our SsGloB homology model suggests that HsGloB and SsGloB have a distinct active site chemistry that may be exploited to drive prodrug activation selectively by SsGloB vs HsGloB.
DISCUSSION
Antimicrobial resistance is a substantial challenge for the treatment of both human and animal staphylococcal infections. Widespread methicillin resistance contributes both to poor clinical outcomes and increased treatment costs, and resistance is emerging to agents of last resort such as vancomycin and linezolid.7 Current antimicrobial therapies target a fraction of essential cellular processes, and metabolism remains a promising area for therapeutic development.11,12 Many metabolic genes are essential for growth, especially in the nutrient limited setting of infection.65–69 Additionally, chemical ligands are readily designed with a high potency by mimicking natural substrates used by metabolic enzymes. Finally, because active site mutations that disrupt the binding of competitive inhibitors are likely to deleteriously affect enzyme function, the barrier to resistance can be high.68,70 Although many metabolic processes are conserved between humans and microbes, selective targeting of microbes is achievable, as is demonstrated by the success of folate antagonists (trimethoprim/sulfamethoxazole) and bedaquiline (a F0F1 ATP synthase inhibitor of Mycobacterium tuberculosis).71–73
Unfortunately, many metabolic inhibitors require cell-impermeable phosphonic acids for efficient target inhibition. Prodrugging strategies to increase cellular penetration have been developed for a variety of therapeutics, most notably the anticancer and antiviral nucleosides.19 These prodrug strategies must be sufficiently labile so that the compound is activated within the target cell yet stable enough to resist premature prodrug activation by the sera. Prodrugs, which are selectively activated within target cells, have the added benefit of reducing off-target toxicity effects. To achieve cell-targeted prodrug activation, knowledge of the activation mechanisms in sera as well the target cell is essential. While prodrug targeting has been achieved for liver therapies, this strategy has yet to be employed for bacterial antibiotics that employ ester prodrug moieties.34
In this work, we have identified a new mechanism for the de-esterification and activation of lipophilic ester prodrugs though a conserved staphylococcal esterase in the metallo-β-lactamase superfamily. The loss-of-function of GloB confers resistance to lipophilic carboxy ester prodrugs in two zoonotic pathogens, S. schleiferi and S. pseudintermedius (Figure 1D, Table S3). Purified recombinant GloB from S. schleiferi and the related human pathogen S. aureus directly catalyzes prodrug de-esterification in vitro (Figure 7A). Because gloB mutant Staphylococci are cross-resistant to other POM-containing prodrugs that differ in “warhead” and intracellular targets (Figure 4), we propose that substrate specificity of GloB appears to be driven by the recognition of the lipophilic promoiety rather than the target inhibitory portion of each compound.
Bacterial prodrug ester activation through GloB hijacks a conserved bacterial protective mechanism in bacteria, as hydroxyacylglutathione hydrolase represents the second enzyme of the two-step glyoxalase pathway. During normal metabolism, the glycolytic intermediates glyceraldehyde-3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP) undergo nonenzymatic decomposition to methylglyoxal, a toxic metabolite. GloB is required for glutathione mediated methylglyoxal detoxification, as methylglyoxal is highly reactive and irreversibly glycates proteins and nucleic acids.74–76 A secondary pathway for methylgloxal detoxification utilizing the glutathione-independent enzyme glyoxalase III was recently described in S. aureus, and orthologs are found in S. schleiferi and S. pseudintermedius.77 The redundancy of the glutathionedependent and -independent glyoxalase pathways remains unclear. In S. aureus, methylglyoxal accumulation potentiates antibiotic susceptibility.78 In addition, methylglyoxal is itself directly antibacterial and postulated to be the primary antistaphylococcal ingredient in Manduka honey (used on chronic wounds).78–82 Our studies suggest that strains of S. schleiferi and S. pseudintermedius lacking GloB have preserved axenic growth in rich media, which raises concern for the ease of resistance development when GloB-targeted prodrugs are used as anti-infectives. However, the known toxicity of methylglyoxal in a host infection setting suggests that reduced methylglyoxal detoxification as the result of GloB loss-of-function would not be well tolerated in vivo.
The identification of GloB as a prodrug activating enzyme in Staphylococci is a major step forward for highly selective microbial targeting of compounds. Though GloB homologues are widespread in microbes and are present in humans, significant sequence variation exists in GloB sequences, which results in a variety of GloB substrate preferences (Figure S6). For example, human GloB has an additional α-helix along the active site that introduces two additional residues, K252 and R249, to the substrate binding pocket (Figure S7).64 These residues and this α-helix are notably absent in microbial GloBs, suggesting that there are underlying substrate differences between human and microbial GloB enzymes. Furthermore, there is substantial sequence variation in GloB orthologs across all microbes, suggesting that GloB substrate specificities may be discerned between individual clades of bacteria. We expect that the development of prodrugs specific to GloB would result in a narrow-spectrum antibiotic, which would reduce off-target effects on the microbiome and decrease the broad pressure to evolve resistance.
METHODS
Inhibitors.
Fosmidomycin (Millipore Sigma) and FR-900098 (Millipore Sigma) were resuspended in sterile water. POM-ERJ and POM-HEX were synthesized and stored in DMSO as described.29,32 Cefditoren pivoxil (Millipore Sigma), cefditoren sodium (Clearsynth), and mupirocin (Millipore Sigma) were resuspended in DMSO. The synthesis of [({[(E)-benzoyloxy]methoxy}[(1E)-3-(N-hydroxyacetamido)prop-1-en-1-yl]phosphoryl)oxy]methyl benzoate (BOM-ERJ) followed that of POM-ERJ, except chloromethyl benzoate was substituted for chloromethyl pivalate.80 1H NMR (400 MHz, chloroform-d) δ 9.35 (s, 1H), 7.96 (dd, J = 8.1, 1.4 Hz, 4H), 7.61–7.45 (m, 2H), 7.37 (t, J = 7.8 Hz, 4H), 6.86–6.69 (m, 1H), 6.04–5.91 (m, 1H), 5.91–5.80 (m, 4H), 4.29 (s, 2H), 2.09 (s, 3H). 13C NMR (101 MHz, chloroform-d) δ 165.04, 149.18, 134.20, 130.31–130.14, 128.87–128.54, 118.38, 116.46, 82.34, 50.64. High resolution mass spectrometry (fast atom bombardment) calculated for C21H23NO9P [M + H]+, 464.1105; found, 464.1097. LC-MS (electrospray ionization) m/z [M + H]+ 464.1, [M + Na]+ 486.1. Purity was greater than 95% as determined by LC-MS.
Generation of POM-ERJ-Resistant Mutants in S. schleiferi and S. pseudintermedius.
Clinical isolates of S. schleiferi (S53022327s) and S. pseudintermedius (H20421242p) were cloned and adapted to laboratory media through three rounds of sequential colony isolation and growth on Luria Broth (LB) agar plates. The isolated POM-ERJ-sensitive parental clones were incubated overnight on LB agar containing POM-ERJ at 3.56 and 7.12 μM for S. schleiferi and 11.2 and 22.4 μM for S. pseudintermedius. Surviving single colonies were restruck onto LB agar for clonal isolation. POM-ERJ resistance of isolated clones was confirmed by overnight growth on LB agar containing POM-ERJ (3.56–22.4 μM). The POM-ERJ-sensitive parental clones were used as a control to confirm growth and antibiotic resistance.
Quantification of Resistance.
Minimum inhibitory concentration (MIC) assays were performed using microtiter broth dilution in clear 96-well plates.83 Compounds were serially diluted in duplicate for a total of 10 serial dilutions. Top well concentrations were as follows: POM-ERJ, 280 μM; BOM-ERJ, 53.95 μM; KMH-102, 53.95 μM; cefditoren pivoxil, 201.38 μM; cefditoren sodium, 56.65 μM; POM-HEX, 100 μM; mupirocin, 2.50 μM; FR-900098, 1 mM; fosmidomycin, 100 μM. Bacteria cultured without drug were used as a positive control for growth, and LB without bacteria was used as a negative control for contamination. Plates were inoculated with 75 μL of bacteria diluted to 1 × 105 CFU/mL in LB. After inoculation, plates were incubated for 16–24 h while shaking at 200 rpm and 37 °C. Plates were visually inspected, and the lowest concentration of antibiotic suppressing visual growth was recorded as the MIC. All experiments were performed at least in triplicate, and data reported represent the mean ± SD.
Transmission Electron Microscopy.
For ultrastructural analysis, bacteria were cultured in 5 mL of LB while shaking at 37 °C until the OD600 = 0.25–1.0. A 1 mL sample of exponential phase bacteria was pelleted at 6000 rcf and resuspended in 1 mL of fix (2% paraformaldehyde/2.5% glutaraldehyde (Polysciences Inc., Warrington, PA) in 100 mM sodium cacodylate buffer, pH 7.2) for 1 h while rocking at RT. The fixed suspension of bacteria was washed in sodium cacodylate buffer and postfixed in 1% osmium tetroxide (Polysciences Inc.) for 1 h. Samples were then rinsed extensively in dH2O prior to en bloc staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 h. Following several rinses in dH2O, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 95 nm were cut with a Leica Ultracut UC7 ultramicrotome (Leica Microsystems Inc., Bannockburn, IL) and stained with uranyl acetate and lead citrate. Samples were viewed at 30 000× on a JEOL 1200EX transmission electron microscope (JEOL USA, Peabody, MA) equipped with an AMT 8 megapixel digital camera (Advanced Microscopy Techniques, Woburn, MA). Cell wall thickness was measured (ImageJ 1.38g customized for AMT images) for 100 bacteria in three independent samples (total n = 300).
Whole Genome Sequencing and Variant Discovery.
Using a standard phenol–chloroform extraction and ethanol precipitation protocol, genomic DNA was isolated from overnight cultures of S. pseudintermedius and S. schleiferi. Sequencing libraries were prepared and sequenced by the Washington University Genome Technology Access Center (GTAC). One μg of DNA was sonicated to an average size of 175 bp. Fragments were blunt ended and had an A base added to the 3′ end. Sequence adapters were ligated to the ends, and the sequence tags were added via amplification. Resulting libraries were sequenced on an Illumina HiSeq 2500 to generate 101 bp paired-end reads. DNA quantity and quality were assessed by GTAC using Agilent Tapestation.
For the analysis, sequences from GenBank were retrieved from the following organisms: S. pseudintermedius ED99 (accession number CP002478) and S. schleiferi 1360–13 (CP009470) assemblies were downloaded from NCBI (ftp://ftp.ncbi.nlm.nih.gov/). Paired-end reads were aligned to each of the available genomes using Novoalign v3.03 (Novocraft Technologies). Duplicates were removed, and variants were called using SAMtools.84 SNPs were filtered against parent variants and by both mean depth value and quality score (minDP = 5, minQ = 30).85 Genetic variants were annotated using SnpEff v4.3.86 For all samples, at least 90% of the genome was sequenced at 20× coverage. Whole genome sequencing data is available in the NCBI BioProject database and Sequence Read Archive under the BioProject ID 648133.
Sanger Sequencing of S. schleiferi and S. pseudintermedius Variants.
The SNPs, the reference sequences, and gene specific primers can be found in Table S4 for both S. schleiferi and S. pseudintermedius. Amplicons were sequenced by GENEWIZ.
Staphylococcal GloB Homology Modeling.
The SWISS-MODEL (https://swissmodel.expasy.org/) was used to generate homology models. Modeling parameters were left at the default settings. Both SsGloB and SpGloB models were built using the solved metallo-β-lactamase superfamily protein, 2ZWR.1.A, which is 39.2% identical in sequence.
Recombinant Expression and Purification of GloB.
WT GloB from S. schleiferi was amplified using the forward and reverse primers in Table S4. The PCR product was then cloned into the BG1861 vector by ligation-independent cloning to introduce an N-terminal 6×His tag and transformed into Stellar chemically competent cells (Clontech Laboratories) for plasmid propagation.87 Proper insertion was verified using restriction digest and Sanger sequencing. For S. schleiferi protein expression, the plasmid was transformed into E. coli Arctic Express (Agilent). Cells were grown to an OD600 of 0.4–0.7 and chilled to 8 °C, and GloB expression was induced with 0.5 mM isopropyl-β-d-thiogalactoside (IPTG) overnight. For S. aureus protein expression, the plasmid was transformed into E. coli BL21 (DE3) pLysS cells (Promega). Cells were grown to an OD600 of 0.4–0.7, and GloB expression was induced with 0.5 mM IPTG for 2 h. Cells were harvested by centrifugation at 4274g for 5 min at 4 °C. The cell pellet was lysed by sonication in 50 mL of lysis buffer containing 25 mM Tris HCl (pH 7.5), 20 mM imidazole, 1 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mg/mL lysozyme, 75 U benzonase, and 1 Complete Mini EDTA-free protease inhibitor tablet (Roche Applied Science). Insoluble proteins were removed by centrifugation twice at 20 000g for 20 min each. The hexahistidine-tagged SsGloB protein was affinity purified from soluble lysate via nickel agarose beads (Gold Biotechnology). Bound protein was washed with 50 mL of lysis buffer and eluted in 300 mM imidazole, 25 mM Tris HCl (pH 7.5), 1 mM MgCl2, 10% glycerol, and 250 mM NaCl. Affinity purified protein was further purified over a HiLoad 16/60 Superdex 200 gel filtration column (GE Healthsciences) using an AKTAExplorer 100 FPLC (GE Healthsciences). FPLC buffer contained 25 mM Tris HCl (pH 7.5), 250 mM NaCl, 1 mM MgCl2, and 10% glycerol. Fractions containing >90% pure enzyme (evaluated by SDS-PAGE) were concentrated by centrifugation using Amicon Ultra-15 centrifugal filter units (EMD Millipore) and flash frozen in liquid nitrogen before permanent storage at −80 °C. Protein identity was verified using mass spectrometry at the University of Nebraska.
GloB Mutant Generation.
WT GloB for S. schleiferi was synthesized by GeneWiz, Inc. (Beijing, China) with a CAT → AAT mutation in the 54th codon (H54N) and cloned into the BG1861 vector to introduce an N-terminal 6×His tag. Proper insertion was verified by Sanger sequencing.
Glyoxalase II Activity Assay.
S. schleiferi GloB was tested for type II glyoxalase activity as previously described with minor changes.51 50 μL reactions containing 25 mM Tris, pH 7.5, 250 mM NaCl, 1 mM divalent salt, 10% glycerol, 200 μM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB, Sigma D8130), and 1 mM d-lactoylglutathione (Sigma L7140) were monitored in a 96-well plate for an increase in absorbance at 412 nm. Reactions were preincubated at 37 °C and initiated with the addition of GloB. The conversion of DTNB to the yellow colored substrate, TNB, by glutathione produced by GloB was measured through time at 37 °C and 412 nm. Assays were carried out over a range of GloB concentrations to ensure that the reaction rates were linear over the period of the assay. To determine the metal dependence of GloB, assays were performed using assay buffer with a final concentration of 1 mM divalent salts. Divalent salts were provided as follows: zinc chloride, manganese chloride, magnesium chloride, cobalt chloride, and calcium chloride.
Sample Preparation for GloB vs POM-ERJ Mass Spectrometry Analysis.
Reactions containing 25 mM Tris HCl (pH 7.5), 250 mM NaCl, 10% glycerol, 1 mM MnCl2, and 1 mM POM-ERJ were prewarmed to 37 °C before the addition of WT GloB, catalytically inactive GloB (H54N), boiled GloB, or an equal amount of protein storage buffer to a final concentration of 1 μM. Reactions were placed at 37 °C and sampled at 0, 15, 30, 60, 90, and 120 min. A 50 μL sample was withdrawn from each reaction at the times indicated, and the sample reaction was quenched by the addition of 200 μL of acetonitrile containing 100 ng/μL enalapril as an internal standard. The samples were immediately frozen on dry ice and stored at −80 °C until analysis.
The quenched reaction mixtures were centrifuged at 3200 rpm for 5 min, and 2 μL of the supernatant was diluted to 500 μL with water containing 100 ng/mL enalapril as an internal standard. Samples were analyzed by LC-MS/MS using an Applied Biosystems-Sciex API 4000. Analyte/internal standard peak area ratios were used to determine concentration and evaluate stability. Standards were evaluated over the range of 1 to 1000 ng/mL. The MRM transitions for enalapril and POM-ERJ were m/z 376.9 > 91.2 and 424.0 > 364.0, respectively. A Phenomenex Luna Omega polar C18 column (2.1 × 50 mm, 5 μm) was used for chromatographic separation. Mobile phases were 0.1% formic acid in water and acetonitrile with a flow rate of 0.5 mL/min. The starting phase was 1% acetonitrile increased to 100% acetonitrile over 0.9 min. Peak areas were integrated using Analyst Software (AB Sciex, Foster City, CA).
In vivo Cleavage of POM-ERJ.
S. schleiferi cultures of WT and POM-ERJR strains were grown to an OD600 of 0.5–0.8 and then treated with 1 μM POM-ERJ. The cultures were grown by shaking at 37 °C and 200 rpm, and 50 μL was sampled at 0, 1, 2, and 3 h. The reactions were quenched by pelleting the cells at 4274g and 4 °C and resuspending in 200 μL of acetonitrile with 100 ng/μL enalapril as an internal standard. The reactions were repeated in triplicate for each time point and strain. The LC-MS analysis was performed as described above.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Joe Jez for ongoing support and helpful discussions. Financial support was provided by NIH AI123433 to C.S.D. and the GWU Department of Chemistry. A.R.O.J. is supported by NIH/NIAID R01-AI103280, R21-AI123808, and R21-AI130584, and A.R.O.J. is an Investigator in the Pathogenesis of Infectious Diseases (PATH) of the Burroughs Wellcome Fund.
Footnotes
The authors declare the following competing financial interest(s): R.L.E., A.R.O.J., and C.S.D. declare their status as co-inventors of U.S. provisional patent 62/686,416 filed June 18, 2018.
ASSOCIATED CONTENT
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00582.
Methods for phylogenetic tree construction, β-lactamase activity determination, and NMR characterization; figures for the growth rates of WT and POM-ERJ-resistant S. schleiferi, GloB purification, β-lactamase activity measurements, assay validation and metal determination, prodrug activation of POM-ERJ and POM-HEX by SsGloB and SaGloB, phylogenetic trees of GloB and RpoB sequences, and structural comparison between HsGloB and SsGloB metal and substrate binding sites; tables for zones of inhibition, exact values for MIC measurements, validated SNPs, and primers used during this study (PDF)
Contributor Information
Marwa O. Mikati, Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri 63110, United States.
Justin J. Miller, Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri 63110, United States.
Damon M. Osbourn, Department of Molecular Microbiology and Immunology, Saint Louis University School of Medicine, St. Louis, Missouri 63104, United States
Yasaman Barekatain, Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, Texas 77054, United States.
Naomi Ghebremichael, Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri 63110, United States.
Ishaan T. Shah, Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri 63110, United States
Carey-Ann D. Burnham, Department of Pediatrics, Department of Molecular Microbiology, and Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, Missouri 63110, United States
Kenneth M. Heidel, Department of Chemistry, The George Washington University, Washington, DC 20052, United States
Victoria C. Yan, Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, Texas 77054, United States.
Florian L. Muller, Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, Texas 77054, United States
Cynthia S. Dowd, Department of Chemistry, The George Washington University, Washington, DC 20052, United States
Rachel L. Edwards, Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri 63110, United States
Audrey R. Odom John, Department of Pediatrics, Washington University School of Medicine, St. Louis, Missouri 63110, United States.
REFERENCES
- (1).CDC (2019) Antibiotic resistance threats in the United States 2019, available at https://www.cdc.gov/drugresistance/pdf/threatsreport/2019-ar-threats-report-508.pdf, accessed 2020-06-01.
- (2).Hsu J (2020) How COVID-19 is accelerating the threat of antimicrobial resistance. BMJ. 369 (May), m1983. [DOI] [PubMed] [Google Scholar]
- (3).(2020) Antimicrobial resistance in the age of COVID-19 Nat. Microbiol 5, 779. [DOI] [PubMed] [Google Scholar]
- (4).Jindal A, Shivpuri D, and Sood S (2015) Staphylococcus schleiferi meningitis in a child. Pediatr Infect Dis J. 34, 329. [DOI] [PubMed] [Google Scholar]
- (5).Somayaji R, Rubin JE, Priyantha MA, and Church D (2016) Exploring Staphylococcus pseudintermedius: an emerging zoonotic pathogen? Future Microbiol. 11, 1371–1374. [DOI] [PubMed] [Google Scholar]
- (6).Börjesson S, Gómez-Sanz E, Ekström K, Torres C, and Grönlund U (2015) Staphylococcus pseudintermedius can be misdiagnosed as Staphylococcus aureus in humans with dog bite wounds. Eur. J. Clin. Microbiol. Infect. Dis 34, 839–844. [DOI] [PubMed] [Google Scholar]
- (7).Lainhart W, Yarbrough ML, and Burnham CA (2018) The brief case: Staphylococcus intermedius group-look what the dog dragged In. J. Clin. Microbiol 56, 961–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ross Fitzgerald J (2009) The Staphylococcus intermedius group of bacterial pathogens: species re-classification, pathogenesis and the emergence of methicillin resistance. Vet Dermatol 20, 490–495. [DOI] [PubMed] [Google Scholar]
- (9).Humphries RM, Wu MT, Westblade LF, Robertson AE, Burnham CD, Wallace MA, Burd EM, Lawhon S, and Hindler JA (2016) In vitro antimicrobial susceptibility of Staphylococcus pseudintermedius isolates of human and animal origin. J. Clin. Microbiol 54, 1391–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Beever L, Bond R, Graham PA, Jackson B, Lloyd DH, and Loeffler A (2015) Increasing antimicrobial resistance in clinical isolates of Staphylococcus intermedius group bacteria and emergence of MRSP in the UK. Vet. Rec 176, 172. [DOI] [PubMed] [Google Scholar]
- (11).Haag NL, Velk KK, and Wu C (2012) Potential antibacterial targets in bacterial central metabolism. Int. J. Adv. Life Sci 4, 21–32. [PMC free article] [PubMed] [Google Scholar]
- (12).Murima P, McKinney JD, and Pethe K (2014) Targeting bacterial central metabolism for drug development. Chem. Biol 21, 1423–1432. [DOI] [PubMed] [Google Scholar]
- (13).Azema L, Baron R, and Ladame S (2006) Targeting enzymes with phosphonate-based inhibitors: mimics of tetrahedral transition states and stable isosteric analogues of phosphates. Curr. Enzyme Inhib 2, 61–72. [Google Scholar]
- (14).Kornberg RD, McNamee MG, and McConnell HM (1972) Measurement of transmembrane potentials in phospholipid vesicles. Proc. Natl. Acad. Sci. U. S. A 69, 1508–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ju L, Cheng Z, Fast W, Bonomo RA, and Crowder MW (2018) The continuing challenge of metallo-β-lactamase inhibition: mechanism matters. Trends Pharmacol. Sci 39, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).McKenney ES, Sargent M, Khan H, Uh E, Jackson ER, San Jose G, Couch RD, Dowd CS, and van Hoek ML (2012) Lipophilic prodrugs of FR900098 are antimicrobial against Francisella novicida in vivo and in vitro and show GlpT independent efficacy. PLoS One 7, e38167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Zhang Y, Leon A, Song Y, Studer D, Haase C, Koscielski LA, and Oldfield E (2006) Activity of nitrogen-containing and non-nitrogen-containing bisphosphonates on tumor cell lines. J. Med. Chem 49, 5804–5814. [DOI] [PubMed] [Google Scholar]
- (18).Hsiao CHC, Lin X, Barney RJ, Shippy RR, Li J, Vinogradova O, Wiemer DF, and Wiemer AJ (2014) Synthesis of a phosphoantigen prodrug that potently activates Vγ9Vδ2 T-lymphocytes. Chem. Biol 21, 945–954. [DOI] [PubMed] [Google Scholar]
- (19).Wiemer AJ, and Wiemer DF (2014) Prodrugs of phosphonates and phosphates: crossing the membrane barrier. Phosphorus Chemistry I 360, 115–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Hecker SJ, and Erion MD (2008) Prodrugs of phosphates and phosphonates. J. Med. Chem 51, 2328–2345. [DOI] [PubMed] [Google Scholar]
- (21).Heidel KM, and Dowd CS (2019) Phosphonate prodrugs: an overview and recent advances. Future Med. Chem 11, 1625–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sakagami K, Atsumi K, Tamura A, Yoshida T, Nishihata K, and Fukatsu S (1990) Synthesis and oral activity of ME 1207, a new orally active cephalosporin. J. Antibiot 43, 1047–1050. [DOI] [PubMed] [Google Scholar]
- (23).Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B, Wang Q, Wolfe L, Jordan R, Soloveva V, Knox J, Perry J, Perron M, Stray KM, Barauskas O, Feng JY, Xu Y, Lee G, Rheingold AL, Ray AS, Bannister R, Strickley R, Swaminathan S, Lee WA, Bavari S, Cihlar T, Lo MK, Warren TK, and Mackman RL (2017) Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1- f ][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem 60, 1648–1661. [DOI] [PubMed] [Google Scholar]
- (24).Shaw JP, Sueoko CM, Oliyai R, Lee WA, Arimilli MN, Kim CU, and Cundy KC (1997) Metabolism and pharmacokinetics of novel oral prodrugs of 9-[(R)-2-(phosphonomethoxy)propyl]adenine (PMPA) in dogs. Pharm. Res 14, 1824–1829. [DOI] [PubMed] [Google Scholar]
- (25).Robbins BL, Srinivas RV, Kim C, Bischofberger N, and Fridland A (1998) Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother 42, 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Sofia MJ, Bao D, Chang W, Du J, Nagarathnam D, Rachakonda S, Ganapati Reddy P, Ross BS, Wang P, Zhang HR, Bansal S, Espiritu C, Keilman M, Lam AM, Micolochik Steuer HM, Niu C, Otto MJ, and Furman PA (2010) Discovery of a β- d –2′-deoxy-2′-α-fluoro-2′-β- C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of Hepatitis C virus. J. Med. Chem 53, 7202–7218. [DOI] [PubMed] [Google Scholar]
- (27).Edwards RL, Heueck I, Lee SG, Shah IT, Miller JJ, Jezewski AJ, Mikati MO, Wang X, Brothers RC, Heidel KM, Osbourn DM, Burnham CD, Alvarez S, Fritz SA, Dowd CS, Jez JM, and Odom John AR (2020) Potent, specific MEPicides for treatment of zoonotic Staphylococci. PLoS Pathog. 16, e1007806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Uh E, Jackson ER, San Jose G, Maddox M, Lee RE, Lee RE, Boshoff HI, and Dowd CS (2011) Antibacterial and antitubercular activity of fosmidomycin, FR900098, and their lipophilic analogs. Bioorg. Med. Chem. Lett 21, 6973–6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Edwards RL, Brothers RC, Wang X, Maron MI, Ziniel PD, Tsang PS, Kraft TE, Hruz PW, Williamson KC, Dowd CS, and Odom John AR (2017) MEPicides: potent antimalarial prodrugs targeting isoprenoid biosynthesis. Sci. Rep 7, 8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).San Jose G, Jackson ER, Uh E, Johny C, Haymond A, Lundberg L, Pinkham C, Kehn-Hall K, Boshoff HI, Couch RD, and Dowd CS (2013) Design of potential bisubstrate inhibitors against Mycobacterium tuberculosis (Mtb) 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Dxr)—evidence of a novel binding mode. MedChemComm 4, 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).San Jose G, Jackson ER, Haymond A, Johny C, Edwards RL, Wang X, Brothers RC, Edelstein EK, Odom AR, Boshoff HI, Couch RD, and Dowd CS (2016) Structure-activity relationships of the MEPicides: N-acyl and O-linked analogs of FR900098 as inhibitors of DXR from Mycobacterium tuberculosis and Yersinia pestis. ACS. ACS Infect. Dis 2, 923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lin Y, Satani N, Hammoudi N, Ackroyd JJ, Khadka S, Yan VC, Georgiou DK, Sun Y, Zielinski R, Tran T, Pando SC, Wang X, Maxwell D, Peng Z, Pisaneschi F, Mandal P, Leonard PG, Xu Q, Wu Q, Jiang Y, Czako B, Kang Z, Asara JM, Priebe W, Bornmann W, Marszalek JR, DePinho RA, and Muller FL (2018) Eradication of ENO1-deleted glioblastoma through collateral lethality. bioRxiv, DOI: 10.1101/331538. [DOI] [Google Scholar]
- (33).Wang X, Edwards RL, Ball H, Johnson C, Haymond A, Girma M, Manikkam M, Brothers RC, McKay KT, Arnett SD, Osbourn DM, Alvarez S, Boshoff HI, Meyers MJ, Couch RD, Odom John AR, and Dowd CS (2018) MEPicides: α,β-Unsaturated Fosmidomycin Analogues as DXR Inhibitors against Malaria. J. Med. Chem 61, 8847–8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Erion MD, van Poelje PD, Mackenna DA, Colby TJ, Montag AC, Fujitaki JM, Linemeyer DL, and Bullough DA (2005) Liver-targeted drug delivery using HepDirect prodrugs. J. Pharmacol. Exp. Ther 312, 554–560. [DOI] [PubMed] [Google Scholar]
- (35).Sakamoto Y, Furukawa S, Ogihara H, and Yamasaki M (2003) Fosmidomycin resistance in adenylate cyclase deficient (cya) mutants of Escherichia coli. Biosci., Biotechnol., Biochem 67, 2030–2033. [DOI] [PubMed] [Google Scholar]
- (36).Mackie RS, McKenney ES, and van Hoek ML (2012) Resistance of Francisella novicida to fosmidomycin associated with mutations in the glycerol-3-phosphate transporter. Front. Microbiol 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, Gemmell CG, Kim MN, Ploy MC, El-Solh N, Ferraz V, and Hiramatsu K (2003) Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin Microbiol 41, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Jones T, Yeaman MR, Sakoulas G, Yang SJ, Proctor RA, Sahl HG, Schrenzel J, Xiong YQ, and Bayer AS (2008) Failures in clinical treatment of Staphylococcus aureus infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob. Agents Chemother 52, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Choi Y, Sims GE, Murphy S, Miller JR, and Chan AP (2012) Predicting the functional effect of amino acid substitutions and indels. PLoS One 7, e46688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Misic AM, Cain CL, Morris DO, Rankin SC, and Beiting DP (2016) Divergent isoprenoid biosynthesis pathways in Staphylococcus species constitute a drug target for treating infections in companion animals. mSphere 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Grosser MR, and Richardson AR (2014) Method for preparation and electroporation of S. aureus and S. epidermidis. Methods Mol. Biol 1373, 51–57. [DOI] [PubMed] [Google Scholar]
- (42).Nair D, et al. (2011) Whole-genome sequencing of Staphylococcus aureus strain RN4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J. Bacteriol 193, 2332–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Schneewind O, Model P, and Fischetti VA (1992) Sorting of protein A to the staphylococcal cell wall. Cell 70, 267–281. [DOI] [PubMed] [Google Scholar]
- (44).Doron S, Melamed S, Ofir G, Leavitt A, Lopatina A, Keren M, Amitai G, and Sorek R (2018) Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359, eaar4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Puyet A, Sandoval H, López P, Aguilar A, Martín JF, and́ Espinosa M (1987) A simple medium for rapid regeneration of Bacillus subtilis protoplasts transformed with plasmid DNA. FEMS Microbiol. Lett 40, 1–5. [Google Scholar]
- (46).Costa SK, Donegan NP, Corvaglia A-R, Francois P, anḑ Cheung AL (2017) Bypassing the restriction system To improve transformation of Staphylococcus epidermidis. J. Bacteriol 199, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Monk IR, Tree JJ, Howden BP, Stinear TP, and Foster TJ (2015) Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6, e00308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, and Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Yamamura A, Okada A, Kameda Y, Ohtsuka J, Nakagawa N, Ebihara A, Nagata K, and Tanokura M (2009) Structure of TTHA1623, a novel metallo-beta-lactamase superfamily protein from Thermus thermophilus HB8. Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun 65, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Melino S, Capo C, Dragani B, Aceto A, and Petruzzelli R (1998) A zinc-binding motif conserved in glyoxalase II, beta-lactamase and arylsulfatases. Trends Biochem. Sci 23, 381–382. [DOI] [PubMed] [Google Scholar]
- (51).Stamp AL, Owen P, Omari KE, Nichols CE, Lockyer M, Lamb HK, Charles IG, Hawkins AR, and Stammers DK (2010) Structural and functional characterization of Salmonella enterica serovar typhimurium YcbL: An unusual type II glyoxalase. Protein Sci. 19, 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Zang TM, Hollman DA, Crawford PA, Crowder MW, and Makaroff CA (2001) Arabidopsis glyoxalase II Contains a Zinc/iron binuclear metal center that is essential for substrate binding and catalysis. J. Biol. Chem 276, 4788–4795. [DOI] [PubMed] [Google Scholar]
- (53).Park H-S, N S-H, Lee JK, Yoon CN, Mannervik B, Benkovic SJ, and Kim H-S (2006) Design and evolution of new catalytic activity with an existing protein scaffold. Science 311, 535–538. [DOI] [PubMed] [Google Scholar]
- (54).Murata K, Inoue Y, Watanabe K, Fukuda Y, Sadcusa T, Shimosaka M, and Kimura A (1986) Metabolism of alphaketoaldehydes in yeasts: Purification and characterization of glyoxalase II from Saccharomyces cerevisiae. Agric. Biol. Chem 50, 135–142. [Google Scholar]
- (55).Irsch T, and Krauth-Siegel RL (2004) Glyoxalase II of African trypanosomes is trypanothione-dependent. J. Biol. Chem 279, 22209–22217. [DOI] [PubMed] [Google Scholar]
- (56).Norton SJ, Talesa V, Yuan WJ, and Principato GB (1990) Glyoxalase I and glyoxalase II from Aloe vera: purification, characterization and comparison with animal glyoxalases. Biochem Int. 22, 411–418. [PubMed] [Google Scholar]
- (57).Norton SJ, Principato GBB, Talesa V, Lupattelli M, and Rosi G (2017) Glyoxalase II from Zea mays: properties and inhibition study of the enzyme purified by use of a new affinity ligand. Enzyme 42, 189–196. [DOI] [PubMed] [Google Scholar]
- (58).Talesa V, Rosi G, Contenti S, Mangiabene C, Lupattelli M, Norton SJ, Giovannini E, and Principato GB (1990) Presence of glyoxalase II in mitochondria from spinach leaves: comparison with the enzyme from cytosol. Biochem Int. 22, 1115–1120. [PubMed] [Google Scholar]
- (59).Talesa V, Principato GB, Norton SJ, Contenti S, Mangiabene C, and Rosi G (1990) Isolation of glyoxalase II from bovine liver mitochondria. Biochem Int. 20, 53–58. [PubMed] [Google Scholar]
- (60).Dolphin D, Avramović O, Pulson R Glutathione: Chemical,´ Biochemical, and Medical Aspects, Part 1; Wiley, 1989; 848 pages. [Google Scholar]
- (61).Cho MY, Bae CD, Park JB, and Lee TH (1998) Purification and cloning of glyoxalase II from rat liver. Exp. Mol. Med 30, 53–57. [DOI] [PubMed] [Google Scholar]
- (62).Uotila L (1973) Purification and characterization of S-2-hydroxyacylglutathione hydrolase (glyoxalase II) from human liver. Biochemistry 12, 3944–3951. [DOI] [PubMed] [Google Scholar]
- (63).Schrödinger The PyMOL molecular graphics system, available at https://pymol.org/2/.
- (64).Cameron AD, Ridderström M, Olin B, and Mannervik B (1999) Crystal structure of human glyoxalase II and its complex with a glutathione thiolester substrate analogue. Structure 7, 1067–1078. [DOI] [PubMed] [Google Scholar]
- (65).Brown SA, Palmer KL, and Whiteley M (2008) Revisiting the host as a growth medium. Nat. Rev. Microbiol 6, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, and Bayles KW (2013) A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4, e00537–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, and Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol 2, 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Joyce AR, Reed JL, White A, Edwards R, Osterman A, Baba T, Mori H, Lesely SA, Palsson BØ, and Agarwalla S (2006) Experimental and computational assessment of conditionally essential genes in Escherichia coli. J. Bacteriol 188, 8259–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Armstrong CM, Meyers DJ, Imlay LS, Freel Meyers C, and Odom AR (2015) Resistance to the antimicrobial agent fosmidomycin and an FR900098 prodrug through mutations in the deoxyxylulose phosphate reductoisomerase gene (dxr). Antimicrob. Agents Chemother 59, 5511–5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Pines G, Oh EJ, Bassalo MC, Choudhury A, Garst AD, Fankhauser RG, Eckert CA, and Gill RT (2018) Genomic deoxyxylulose phosphate reductoisomerase (DXR) mutations conferring resistance to the antimalarial drug fosmidomycin in E. coli. ACS Synth. Biol 7, 2824–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Koul A, Dendouga N, Vergauwen K, Molenberghs B, Vranckx L, Willebrords R, Ristic Z, Lill H, Dorange I, Guillemont J, Bald D, and Andries K (2007) Diarylquinolines target subunit c of mycobacterial ATP synthase. Nat. Chem. Biol 3, 323–324. [DOI] [PubMed] [Google Scholar]
- (72).Haagsma AC, Abdillahi-Ibrahim R, Wagner MJ, Krab K, Vergauwen K, Guillemont J, Andries K, Lill H, Koul A, and Bald D (2009) Selectivity of TMC207 towards Mycobacterial ATP synthase compared with that towards the Eukaryotic homologue. Antimicrob. Agents Chemother 53, 1290–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Andries K, Verhasselt P, Guillemont J, Göhlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, and Jarlier V (2005) A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307, 223–227. [DOI] [PubMed] [Google Scholar]
- (74).Gomes RA, Vicente Miranda H, Silva MS, Graca G,¸ Coelho AV, Ferreira AE, Cordeiro C, and Freire AP (2006) Yeast protein glycation in vivo by methylglyoxal. Molecular modification of glycolytic enzymes and heat shock proteins. FEBS J. 273, 5273–87. [DOI] [PubMed] [Google Scholar]
- (75).Rahman A, Shahabuddin, and Hadi SM (1990) Formation of strand breaks and interstrand cross-links in DNA by methylglyoxal. J. Biochem. Toxicol 5, 161–166. [DOI] [PubMed] [Google Scholar]
- (76).Migliore L, Barale R, Bosco E, Giorgelli F, Minunni M, Scarpato R, and Loprieno N (1990) Genotoxicity of methylglyoxal: cytogenetic damage in human lymphocytes in vitro and in intesting cells of mice. Carcinogenesis 11, 1503–1507. [DOI] [PubMed] [Google Scholar]
- (77).Kim H, Lee KY, Kwon AR, and Lee BJ (2017) Structural and functional studies of SAV0551 from Staphylococcus aureus as a chaperone and glyoxalase III. Biosci. Rep 37, BSR20171106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Hayes G, Wright N, Gardner SL, Telzrow CL, Wommack AJ, and Vigueira PA (2018) Manuka honey and methylglyoxal increase the sensitivity of Staphylococcus aureus to linezolid. Lett. Appl. Microbiol 66, 491–495. [DOI] [PubMed] [Google Scholar]
- (79).Mü P, Alber DG, Turnbull L, Schlothauer RC, Carter DA, Whitchurch CB, and Harry EJ (2013) Synergism between Medihoney and rifampicin against methicillin-resistant Staphylococcus aureus (MRSA). PLoS One 8, e57679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Bulman SEL, Tronci G, Goswami P, Carr C, and Russell SJ (2017) Antibacterial properties of nonwoven wound dressings coated with manuka honey or methylglyoxal. Materials (Basel, Switzerland) 10, 954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Paramasivan S, Drilling AJ, Jardeleza C, Jervis-Bardy J, Vreugde S, and Wormald PJ (2014) Methylglyoxal-augmented manuka honey as a topical anti-Staphylococcus aureus biofilm agent: safety and efficacy in an in vivo model. Int. Forum Allergy Rhinol 4, 187–195. [DOI] [PubMed] [Google Scholar]
- (82).Jackson ER, San Jose G, Brothers RC, Edelstein EK, Sheldon Z, Haymond A, Johny C, Boshoff HI, Couch RD, and Dowd CS (2014) The effect of chain length and unsaturation on Mtb Dxr inhibition and antitubercular killing activity of FR900098 analogs. Bioorg. Med. Chem. Lett 24, 649–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) (2003) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect 9, ix–xv. [Google Scholar]
- (84).Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and 1000 Genome Project Data Processing Subgroup (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, McVean G, Durbin R, and 1000 Genomes Project Analysis Group (2011) The variant call format and VCFtools. Bioinformatics 27, 2156–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Cingolani P, Platts A, Wang le L, Coon M, Nguyen T, Wang L, Land SJ, Lu X, and Ruden DM (2012) A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Alexandrov A, Vignali M, LaCount DJ, Quartley E, de Vries C, De Rosa D, Babulski J, Mitchell SF, Schoenfeld LW, Fields S, Hol WG, Dumont ME, Phizicky EM, and Grayhack EJ (2004) A facile method for high-throughput co-expression of protein pairs. Mol. Cell. Proteomics 3, 934–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.