Abstract
Background and Objectives:
Prognosis of pancreatic neuroendocrine neoplasms (PanNENs) mostly depend on tumor stage and grade, determined by Ki-67 labeling index. EUS-FNA is considered the gold-standard technique to obtain it. The aims of our study were to establish diagnostic accuracy of preoperative EUS-FNA Ki-67 evaluation considering final pathological assessment on surgical specimen as gold standard and to investigate the possible impact on prognosis of misclassification.
Methods:
This is a retrospective study from a prospectively collected database. EUS-FNA grading (eG) and surgical one (sG) measured according to Ki-67 proliferative index values, according to 2017 WHO classification, were compared. eG-sG correlation was evaluated by Spearman index. Logistic regression investigated factors associated with misclassification. Prognostic difference in terms of progression-free survival was evaluated by Kaplan Meier method.
Results:
One hundred and twelve PanNENs patients enrolled. In 13.4% of patients (15/112) EUS-FNA “undergraded” patients (eG1 vs. sG2), while in 12.5% (n = 14) it “overgraded” PanNENs (eG2 to sG1). No misclassifications in G3 patients. In patients with tumors <20 mm (n = 44), 2 (4.5%) eG1 and 10 (22.7%) eG2 were finally classified respectively as G2 and G1 at surgical histology. No factors, as i.e. the lesions’ size or their morphological aspect, were associated with misclassification. In overgraded PanNENs, no progression occurred, while in patients correctly classified/undergraded the progression rate was 14.3%.
Conclusions:
This is the largest cohort of surgical PanNENs with preoperative EUS-FNA grading evaluation. Despite an acceptable eG-sG correlation, about 25% of patients are misclassified. Clinical impact of misclassification should be carefully considered especially in small tumors undergoing observation.
Keywords: neuroendocrine neoplasm, rapid on-site evaluation, survival, cytology, Ki-67
INTRODUCTION
Pancreatic neuroendocrine neoplasms (PanNENs) are considered rare entities, accounting for <3% of all pancreatic neoplasms, but their incidental diagnosis is on the rise, and recent data suggest that their actual prevalence is underestimated.[1,2,3] PanNENs are characterized by a heterogeneous clinical behavior, as the clinical outcome is related to tumor size, stage, and grade. The last is determined by the proliferative index Ki-67. Therefore, although PanNENs are usually considered indolent neoplasms, their prognosis is variable and the assessment of the clinical outcome remains challenging.[4,5]
The main available classifications employed to stratify clinical behavior of PanNENs are the European Neuroendocrine Tumor Society TNM staging system[6] and the World Health Organization (WHO) grading system. Besides morphology that distinguishes well-differentiated and poorly differentiated neoplasms, the WHO 2017 classification[7] is based on a grading system that consider the proliferative activity determined by measuring the mitotic count and/or Ki-67 labeling index.[8] In the case of discordance between tumor grade based on Ki-67 index and mitotic rate, the higher grade more accurately predicts prognosis and the clinical outcome.[9,10,11] Both TNM staging and WHO grading accurately stratify survival. The ki-67 index should be calculated on tissue samples as the percentage of tumor cells with positive nuclear staining in 500–2000 cells counted in the highest area of nuclear labeling. While this is simple on surgical samples, it may be more challenging at diagnosis, as the evaluation of grade rely on material obtained during EUS with fine needle aspiration (FNA) or biopsy (FNB).
Since the first report by Piani et al.[12] in 2008, several other studies demonstrated that the Ki-67 index assessment is feasible on cytological cell samples obtained by EUS-FNA, with an adequate concordance between cytology and histology.[13,14,15,16] The reliability of EUS-cytological grading is clinically of paramount importance as it may drive treatment options, such as possible indication to surgery in asymptomatic nonfunctioning (NF) PanNENs ≤2 cm in size that can be safely managed conservatively.[17,18] In the past few years, several retrospective experiences regarding the use of EUS-FNA/FNB to assess grading of PanNENs have been reported.[12,14,15,19,20,21,22,23,24,25] However, most of these studies were limited to small and heterogeneous cohorts of patients, often pooling EUS-FNA and FNB together. Moreover, those studies did not investigate whether a misclassification of grading at EUS may have an impact on the prognosis. The primary aim of the present study was to investigate the accuracy of PanNENs grading as assessed by EUS-FNA when compared to the final pathological assessment after surgery. The secondary aim was to investigate whether a potential misclassification of grading by EUS-FNA has an impact on the prognosis of PanNEN patients.
METHODS
Study population
This is a retrospective observational monocentric study, following the STrengthening the Reporting of OBservational studies in Epidemiology statement (STROBE),[26] with analysis of a prospectively collected database including patients who underwent surgery for PanNENs upon informed consent and ethical committee approval (IRB BIOGASTRO/2011 updated on August 7, 2019). Inclusion criteria were: age >18 years; both EUS and pancreatic surgery performed at San Raffaele Hospital; a definitive postsurgical histological diagnosis of PanNEN with immunostaining for Ki-67 (sKi-67); presurgical EUS-FNA performed on the primary pancreatic lesion with a final diagnosis of PanNEN and availability of immunostaining for Ki-67 (eKi-67). Patients were excluded if they did not consent to data collection.
EUS-FNA and Ki-67 evaluation
All the EUS-FNA were carried out by expert endosonographers (at least pancreatic 250/year), using a linear echoendoscope (EG3870UTK or EG38-J10UT, Pentax Hamburg GmbH) and the ultrasound platform Hitachi Arietta V70. According with the decision of the physician that performed the exam, ancillary techniques as contrast-enhanced EUS (CEUS) or EUS-elastography, were used. CEUS was performed injecting in a peripheral vein 5 mL of microbubble contrast agent SonoVue® (Bracco Imaging, Milan, Italy), followed by 10 mL of saline solution; a video of the contrast sequence was recorded for 2 min after the injection and then the images were all reevaluated by two operators. EUS-elastography was performed using the software embedded in the ultrasound system. A region of interest containing for at least 50% the target lesion was individuated and elastography images were recorded. Two authors revised all the images and categorized the target lesions as rigid, soft or mixed. All the fine-needle aspiration procedures were performed using the slow-pull technique with 25-gauges needles (Expect™ Slimline Handle Needles, Boston Scientific, USA). The number of FNA passages was subordinated to the obtaining of a diagnostic adequate sample. This was established by an expert cytotechnician thanks to rapid on-site evaluation (ROSE). The sample was processed with a cell-block technique. FNA and post-surgical histological samples prepared in paraffin were cut in 5 μm sections and then marked in immunohistochemistry for anti-Ki67 antibody (MIB-1 clone, 1:160; Dako Corporation, Carpinteria, CA, USA). The proliferative index was calculated as the percentage of Ki-67 positively stained cells within 500 adjacent tumoral cells in the highest reactive area of the smear.
Neoplastic grading was assigned accordingly with WHO classification updated in 2017:[7] pancreatic neuroendocrine tumor (NET) NET G1 (well-differentiated, Ki-67 <3%), NET G2 (well-differentiated, Ki-67 superior to 3% and inferior to 20%), NET G3 (well-differentiated, Ki-67 >20%), NEC (poorly differentiated carcinoma, Ki-67 >20%). Patients diagnosed before 2017 were newly evaluated by an expert pathologist and re-classified according to the updated WHO grading classification.
Follow-up
All of the patients were followed up regularly after surgery. High-quality imaging examination, as well as blood and fecal tests for evaluation of endocrine and exocrine function were performed at least every 6 months for the first 2 years and then annually if stability, otherwise the visit was scheduled according with the physician decision. Progression-free-survival (PFS) was defined as the time from surgery to the first evidence of disease recurrence or progression and it was censored at the last follow up if no disease relapse had occurred.
Statistical analysis
Continuous variables are presented as mean (±standard deviation [SD]) when distribution is normal or as median (interquartile range [IQR]; p25th-p75th) when skewed and accordingly compared by means of either independent samples Student’s t-test or Mann–Whitney U test respectively. The frequency of categorical variables was compared using Pearson χ2. Grade of correlation between Ki-67 on EUS-FNA and on surgery was tested with the Spearman index. To evaluate the diagnostic performance of EUS-FNA, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy (with the relative confidence intervals [CIs]) were calculated, using grading G2 at the final histological examination as outcome variable. In order to find factors associated with grading misclassification univariate and multivariate logistic regressions were performed. A value of P < 0.05 was considered significant.
RESULTS
Characteristics of patients and EUS findings
From 2008 to august 2019, 299 patients underwent surgery for a PanNEN at S. Raffaele Hospital in Milan. One hundred twenty-two were preliminarily excluded as they performed the diagnostic EUS-FNA in other hospitals. After the application of inclusion and exclusion criteria, 11 patients were further excluded because the diagnosis of PanNEN was not confirmed at final surgical histology and 43 (24.3%) because Ki-67 was not evaluable. Finally, 112 PanNEN patients were included in the present study. The cohort characteristics are summarized in Table 1. Fifty-three patients (47.3%) were female and the median age was 59.0 years (IQR 48.25–67). Eighty-five (75.9%) patients had NF neoplasms, while most functioning PanNENs were insulinomas (n = 21/27, 77.8). The median diameter of the lesions was 23.5 mm (IQR 15–33.5) on EUS. The main pancreatic duct was rarely dilated (14/112, 12.5%) by the neoplastic mass, and the mean diameter in the most dilated portion was 2.52 mm (±1.87). The mean number of FNA passes during the exam was 2.58 (95% CI 2.39–2.77). Interestingly, in the 43 patients who were excluded as Ki67 was not evaluable, the mean number of passes was lower (2.2, 95% CI 1.87.39–2.53; P = 0.048). We also hypothesized that the year of EUS-FNA could impact on the Ki67 assessment, with older samples being less frequently evaluable, but this was not the case.
Table 1.
Clinical and endoscopic features of 112 patients in the study cohort
| Sex (%) | |
| Male | 59 (52.7) |
| Female | 53 (47.3) |
| Age (median, IQR) | 59.0 (48.2–67.0) |
| Site of neoplasia (%) | |
| Head | 46 (41.1) |
| Body | 28 (25.0) |
| Tail | 32 (28.6) |
| Multifocal/others | 6 (5.3) |
| Type of neoplasia (%) | |
| Nonfunctioning | 85 (75.9) |
| Insulinoma | 21 (18.8) |
| Other functioning | 6 (5.4) |
| CT-scan diameter (median, mm) | 22.0 (15.0–35.0) |
| EUS diameter (median, mm) | 23.5 (IQR 15.0–33.5) |
| Pathology diameter (median, mm) | 22.0 (IQR 14.0–33.75) |
| Main pancreatic duct diameter (mean, mm) | 2.52 (SD±1.87) |
| EUS-contrast pattern (available on 70 patients) (%) | |
| Iper-enhancement | 54 (77.1) |
| Ipo-enhancement | 16 (22.9) |
| EUS-Elastography pattern (available on 93 patients) (%) | |
| Rigid | 57 (61.3) |
| Soft | 11 (9.8) |
| Mixed-pattern | 25 (26.9) |
| Neoplasia pattern (%) | |
| Solid | 83 (74.1) |
| Solid-cystic | 29 (25.9) |
| Delay from EUS to surgery (median, days) | 74.5 (IQR 48.25–135) |
| TNM staging (available on 94 patients) (%)a | |
| 1 | 23 (20.5) |
| 2 | 30 (26.8) |
| 3 | 31 (27.7) |
| 4 | 10 (8.9) |
aBergsland EK, Woltering EA, Rindo G. Neuroendocrine tumors of the pancreas. In: AJCC cancer staging manual, 8th, Amin MB (Ed), AJCC, Chicago 2017. p. 407. CT: Computed tomography; IQR: Interquartile range; SD: Standard deviation; AJCC: American joint committee on cancer
Contrast enhancement was evaluated in 70 patients (63.1%): the neoplasia was hyper-enhancing in 54 cases (77.1%) and hypo-enhancing in 16 (22.9%). EUS-elastography was used in 83.1% (n = 93) and a hard pattern was observed in 61.3% of cases (n = 57), while a soft pattern in 9.8% (n = 11) and a mixed pattern in 22.3% (n = 25). Further details about EUS-elastography, contrast enhancement e lymph node invasion are reported in Supplementary Table 1. In about one of four patients (26.1%) the neoplasia was solid-cystic. Median delay between EUS-FNA and surgery was 74.5 days (IQR 48.25–135). After surgery, lymph nodal metastases were found in 41 patients (36.6). The mean time of follow up was 36.9 months (95% CI 30.7–43.3) and no patients were lost to follow up. Supplementary Figure 1 (259.6KB, tif) reports the different PFS’ according with surgical grading.
Supplementary Table 1.
Differences in contrast enhancement, EUS-elastographic pattern and lymph node invasion according with PanNEN morphologic features
| Contrast enhancement | EUS-elastography aspect | Lymph node invasion | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Hyperenhancing | Hypoenhancing | P | Rigid | Elastic or mixed | P | No | Yes | P | |
| Morphology | |||||||||
| Solid | 35 | 11 | 0.97 | 42 | 25 | 0.65 | 40 | 28 | 0.59 |
| Solid-cystic | 19 | 5 | 15 | 11 | 13 | 13 | |||
| Surgical grading | |||||||||
| G1 | 29 | 6 | 0.33 | 25 | 23 | 0.04 | 31 | 14 | 0.02 |
| G2 | 24 | 10 | 29 | 13 | 23 | 24 | |||
| G3 | 1 | 0 | 3 | 0 | 0 | 3 | |||
| Diameter (cm) | |||||||||
| <2 | 21 | 11 | 0.79 | 16 | 19 | 0.03 | 23 | 6 | 0.007 |
| >2 | 33 | 5 | 41 | 17 | 31 | 35 | |||
| EUS-elastography aspect | |||||||||
| Rigid | 24 | 9 | 0.14 | ||||||
| Elastic or mixed | 23 | 3 | |||||||
| Contrast enhancement | |||||||||
| Hyperenhancing | |||||||||
| Hypoenhancing | |||||||||
| Lymph node invasion | |||||||||
| Yes | 21 | 6 | 0.66 | 25 | 11 | 0.60 | |||
| No | 27 | 10 | 27 | 17 | |||||
Grading by Ki-67 at EUS-FNA and surgery
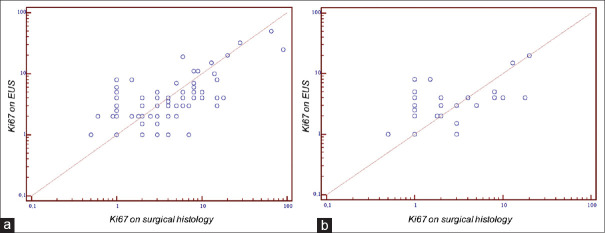
Mean eKi-67 and sKi-67 values were respectively 4.27% (95% CI 3.07–5.46) and 5.34% (95% CI 3.29–7.38) with a good degree of correlation [r = 0.79, 95% CI 0.70–0.85. Figure 1]. Considering only patients with tumor size ≤20 mm, the mean eKi-67 and sKi-67 values were 3.36 (95% CI 2.27–4.46) and 3.05 (95% CI 1.71–4.39, P = 0.11), and the grade of correlation was 0.70 (95% CI 0.51-0.82). eKi-67/sKi-67 correlation was good also in the subgroup of solid-cystic neoplasia (r = 0.86, 95% CI 0.72–0.93). The mean difference in Ki-67 value for misclassified patients was 2.82% (SD ± 1.61). In overgraded patients the difference was 2.89% (SD ± 1.63), while in undergraded ones it was 2.3% (SD ± 1.1; P = 0.18).
Figure 1.
Ki-67 on EUS and Ki-67 on surgical histology correlation: (a) overall, (b) patients with tumor diameter ≤2 cm
According to 2017 WHO grading system, 60 patients (53.6%) were classified as G1 on EUS-FNA grading (eG), 49 (43.7%) as G2, and 3 (2.7%) as G3. Post-surgical grading (sG) was G1 in 59 (52.7%), G2 in 50 (44.6%) and G3 in 3 (2.7%) patients (P < 0.001).
At the comparison between eG and sG 29/112 patients (25.9%) were, therefore, misclassified, with 15 PanNENs (13.4%) initially classified as G1 by EUS-FNA being G2 on postsurgical histology (under grading of EUS-FNA) and 14 G2 PanNENs (12.5%) on eG being G1 on sG (overgrading of EUS-FNA) [Table 2]. No misclassification was found in the 3 G3 patients.
The rate of misclassification was similar, being 27.2% (n = 12) in the 44 patients with tumor size ≤20 mm but with an overgrading of 22.7% (n = 10) and an under grading of only 4.5% (n = 2; Table 2). Increasing the cut-off between G1 and G2 from 3 to 5%, as previously suggested by some authors,[27,28] eG1 patients were 96 (85.7%), eG2 13 (11.6%) and eG3 3 (2.7%), while sG1 patients were 84 (75.0%), sG2 25 (22.3%) and sG3 3 (2.7%). Applying this latter cut-off, the rate of incorrectly classified patients lowered to 17.9% (n = 20). Most of the misclassifications were under grading from eG1 to sG2 (n = 16, 14.3%), while only 4 patients were overgraded with eG2 (3.6%) and sG1 on definitive histology. In addition, no misclassifications were reported on G3 patients [Supplementary Table 2]. Considering globally patients with PanNENs in which it was not possible to evaluate Ki-67 after EUS-FNA and patients with grading misclassification, the overall grading diagnostic accuracy of EUS-FNA in our cohort was 53.5% (72/155).
Table 2.
Grading classification for EUS-FNA and surgical histology: (a) overall, (b) patients with tumor diameter ≤2 cm
| Endoscopic grading | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall population | Patients with tumor diameter ≤2cm | |||||
|
|
|
|||||
| eG1 | eG2 | eG3 | eG1 | eG2 | eG3 | |
| Surgical grading (%) | ||||||
| sG1 | 45 (40.2) | 14 (12.5) | 0 | 23 (53.3) | 10 (22.7) | 0 |
| sG2 | 15 (13.4) | 35 (31.2) | 0 | 2 (4.5) | 9 (20.5) | 0 |
| sG3 | 0 | 0 | 3 (2.7) | 0 | 0 | 0 |
Supplementary Table 2.
Grading classification for EUS-FNA and surgical histology increasing G1-G2 cut-off up to 5%
| Endoscopic grading | |||
|---|---|---|---|
|
| |||
| eG1 | eG2 | eG3 | |
| Surgical grading (%) | |||
| sG1 | 80 (71.4) | 4 (3.6) | 0 |
| sG2 | 16 (14.3) | 9 (8.0) | 0 |
| sG3 | 0 | 0 | 3 (2.7) |
Risk factors for misclassification and prognosis
The rates of misclassification in the various subgroups are reported in Table 3.
Table 3.
Misclassification rates by subgroups in univariate analysis
| Correct grading (%) | Under grading (%) | Over grading (%) | P | |
|---|---|---|---|---|
| Type of tumor | ||||
| Nonfunctioning (n=85) | 74.1 | 11.8 | 14.1 | 0.9 |
| Functioning (n=27) | 74.1 | 14.8 | 11.1 | |
| EUS-elastography | ||||
| Rigid (n=57) | 75.4 | 15.8 | 8.8 | 0.79 |
| Soft (n=36) | 77.8 | 8.3 | 13.9 | |
| EUS-contrast enhancement | ||||
| Hyper-enhancement (n=54) | 76.0 | 12.9 | 11.1 | 0.12 |
| Iso/hypo-enhancement (n=16) | 56.3 | 12.5 | 31.2 | |
| Necrosis at final histology | ||||
| No (n=102) | 73.0 | 13.0 | 14.0 | NA |
| Yes (n=10) | 100 | 0 | 0 | |
| Stagea | ||||
| I (n=23) | 69.6 | 8.7 | 21.7 | 0.22 |
| II (n=30) | 76.7 | 13.3 | 10.0 | |
| III (n=31) | 80.6 | 19.4 | 0 | |
| IV (n=10) | 70.0 | 10.0 | 20.0 | |
| Delay from EUS to surgery | ||||
| 1 quartile (0–48.75) | 71.4 | 10.7 | 17.9 | 0.78 |
| 2 quartile (48.76–74.5) | 71.4 | 14.3 | 14.3 | |
| 3 quartile (74.51–132) | 71.4 | 14.3 | 14.3 | |
| 4 quartile (>132) | 82.1 | 14.3 | 3.6 |
aBergsland EK, Woltering EA, Rindo G. Neuroendocrine tumors of the pancreas. In: AJCC cancer staging manual, 8th, Amin MB (Ed), AJCC, Chicago 2017. p. 407. NA: Not available; AJCC: American joint committee on cancer
The following variables were tested as possible risk factors associated with the risk of misclassification: age, sex, functional status, diameter of the lesion, delay between EUS and surgery, Wirsung diameter, T, N, and M of TNM staging. None of these variables were associated with misclassification at uni-and multivariate analysis.
No differences in PFS rates were identified when comparing correctly classified and misclassified patients, both in the global population (P = 0.13) and in the subgroup of patients with tumors ≤ 20 mm [P = 0.44, Figure 2]. In patients with overgrading by EUS, there were no recurrences (0/14), while among patients correctly classified or under graded the rate of recurrence was respectively 14.4% (12/83) and 6.7% (1/15; globally P = 0.13). We did not find any association between the presence of lymph node metastasis and the rate of misclassification at logistic regression.
Figure 2.
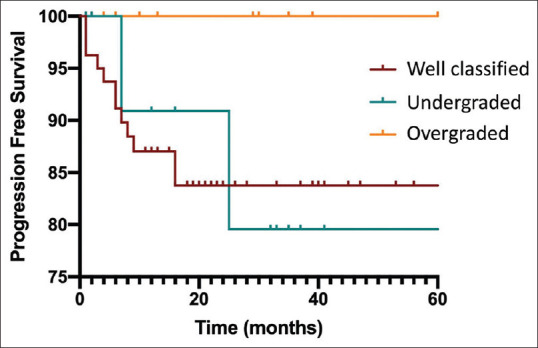
Risk of progression in correctly classified/underestimated and overestimated grading on EUS-FNA
Diagnostic accuracy of EUS-FNA grading
EUS-FNA showed a sensitivity of 70.0% [95% CI 55.4–82.1, Figure 3] for the diagnosis of G2 PanNENs, a specificity of 77.4% (95% CI 65.0–87.1), a PPV of 71.4% (95% CI 60.4–80.4), a NPV of 76.2% (95% CI 67.2–83.3) with an overall accuracy of 74.1% (95% CI 65.0-81.9). In the subgroup of patients with tumors ≤ 20 mm these values were respectively 66.7% (95% CI 34.9–90.1), 71.9% (95% CI 53.2–86.2), 47.1% (95% CI 31.0–63.8), 77.4% (95% CI 71.5–92.9) and 70.5% (95% CI 54.8–83.2). Increasing the G1-G2 cut-off to 5% sensitivity was 36.0% (95% CI 18.0–57.5), specificity 95.4% (95% CI 88.6–98.7), PPV 69.2% (95% CI 43.1–87.0), NPV 83.8% (95% CI 79.4–87.5) and accuracy 82.1% (95% CI 73.8–88.7).
Figure 3.
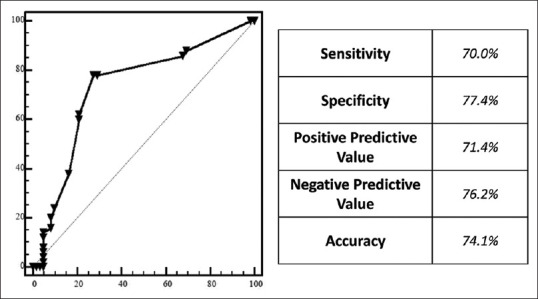
Diagnostic accuracy of EUS-FNA in predicting grading of G2 PanNENs
DISCUSSION
PanNENs are increasingly diagnosed, often incidentally, due to extensive use of second and third-level abdominal imaging techniques. It is, therefore, crucial to predict their behavior and provide a personalized option that may range from “watch-and-wait” to medical treatments or surgery, depending on tumor location, size, stage and grade. EUS with either FNA or FNB is the most accurate and safe method to characterize PanNENs grading. Over the past few years, different cohort studies reported variable diagnostic accuracy of grading obtained by EUS, ranging from 58% to 89%.[12,14,15,19,20,21,22,23,24,25]
The aim of the present study was to investigate diagnostic accuracy of ROSE-supported EUS-FNA in a large series of PanNENs.
In the present cohort of 112 patients who underwent surgical resection for PanNENs, we showed a good correlation between Ki-67 assessed both by EUS-FNA and surgery (r = 0.79). However, there was a non-negligible 25% rate of misclassification limited to a correct diagnosis between G1 and G2 lesions. None of the G3 tumors were misclassified, even if the number was too small (only 3 cases) to allow a pertinent conclusion.
A misclassification with “undergrading” from hypothesized G1 at endoscopy to G2 may lead to an undertreatment with a potentially “dangerous” lesion managed by observation if ≤20 mm. On the other hand, an “overgrading” with G2 at endoscopy resulting in G1 at surgery may cause inappropriate surgical overtreatment. Notably, these two types of misclassification were almost equally common. For this reason, we performed an additional subgroup analysis in tumors ≤20 mm. In this subgroup, the risk of overgrading was 22.7%, while that of undergrading is only 4.5%. This is clinically relevant, as such a mistake may lead to unnecessary pancreatic surgery, that is burdened by a risk of morbidity and mortality that, depending on the center volume, varies from 36.3%–50.3% and from 2.4% to 6.7%, respectively.[29,30,31]
The consequences of “undergrading” are more subtle to be determined and require the follow-up of patients who may have been initially “undertreated”. One strength of the present study is, indeed, the availability of a long-term follow-up in a dedicated clinic. We were, therefore, able to highlight a 20% risk of disease recurrence after 2 years from surgical resection in “undergraded” patients, a rate being significantly higher than that of the remaining patients. This might be due to a longer delay in starting treatments and give an indication for surgery.
Recently, a retrospective study[23] on 77 patients and a prospective one[24] with 31 patients comparing the diagnostic accuracy of EUS-FNA/FNB respect to surgical histology were published. To date, the present cohort is the largest currently published and it is the only one that attempted to identify factors associated with misclassification, including patterns recognized by CE-EUS and elastography. However, none of the patient-related (age, sex, delay between EUS and surgery, type of PanNEN) or technique-level (elastography, contrast-enhancement, diameter of the primary lesion, TNM stage) evaluated variables could predict the risk of misclassification.
We also investigated the use of a modified G1-G2 cut-off level to 5%, that has been previously demonstrated to better predict PanNEN prognosis.[27,28] However, the misclassification rate was only marginally decreased. As for EUS guided biopsies, Larghi et al.[21] reported a 100% concordance rate in a series of 30 patients adopting the 5% cut-off, whereas, in another multicentric series of 48 patients with 53 PanNENs, Carlinfante et al.[14] showed a no statistically significant improvement of the concordance rate (from 86.8% to 92.4%) with the 5% cut-off. Furthermore, Figueiredo et al.[16] concluded that EUS-FNA could predict 5-year survival in a series of 60 PanNENs patients adopting a 5% cut-off even though the proliferative index was available at both cytology and histology in only 24 cases.
One of the possible causes for the reported grading misclassification rate could be related to the sampling method. PanNENs are extremely heterogeneous in their proliferation activity and Ki-67 value may vary within the same lesion.[32] EUS-FNA allows only a partial sampling of the neoplasm and may not be representative of the whole tumor. Another possible explanation of the misclassification can be found in the small differences in Ki67 estimations (2.82% ± 1.61). Considering that the WHO classification is based on strict Ki67 cut-offs, a difference of about 2% can easily change the grading assessment between EUS-FNA and surgery, but maybe this does not reflect real different clinical behaviors of the tumor. Interestingly, we found a great difference in the distribution of misclassification between the entire cohort, where overgrading and undergrading are equally distributed, and the subgroup of patients with tumor diameter inferior to 2 cm, where there is an imbalance in favor of overgrading. Probably there are several factors that could impact on this phenomenon, such as the fact that within the tumor sample there could be the accidental inclusion of proliferating nontumor cells (i.e., glands, crypts) that could result falsely positive to Ki67 immunohistochemistry, or the fact that when the lesion is small it is more probable that EUS-FNA obtain a higher number of peripheral non-neoplastic cells or passages cells (gastric or duodenal).[33]
The manuscript is limited by its retrospective design and possibly by its long time-span. However, it is hardly possible to perform prospective studies of this kind including follow-up on this tumor type. We found that for about 24% of EUS-FNA the pathologist could not establish the grading of the neoplasia. This is of course a problem of this technique but this rate of not classified patients is largely superior respect to the two most recent studies on this topic.[23,25] Also, as for any study performed in a tertiary Center with high expertise, the cohort may suffer from a referral bias and the results may not be reproducible. Finally, it is possible that FNB samples may prove to be more accurate in correctly classifying the G of PanNENs patients, but most studies employed FNA and no specific prospective RCT on the topic are available. In a recent paper by Crinò et al.[34] EUS-FNA and EUS-FNB in the assessment of Ki67 were compared and it was found that the feasibility of Ki67 estimation was similar between them, so as the rate of poor cellulated specimens and the correct EUS/surgery grading estimation. The only significant difference was on the Ki67 assessment in PanNENs with diameter inferior to 2 cm, where the performance of FNB was better than FNA. Further prospective randomized clinical trials are needed on this topic. The studies already published have very heterogeneous and variable diagnostic accuracy also using the same needles, regardless of the type of sampling. In this view, it would be interesting to investigate the association between the amount of obtained tissues through a validated quantitative or semiquantitative scale and the adequacy of the biopsy for the Ki67 assessment, but there are no scales of this kind. Probably, the role of ancillary techniques such as elastography and use of EUS contrast agents and/or the use of software of automated image capture and analysis with use of artificial intelligence, will become increasingly important and will contribute to define the clinical behavior of PanNENs preoperatively.[35,36,37] The present data suggest that PanNENs grading can be correctly evaluated preoperatively by EUS-FNA cytology in some two thirds of patients. In this view, the concomitant use of additional techniques as PET with gallium and FDG may be important to support clinical choices.
Financial support and sponsorship
Nil.
Conflicts of interest
Paolo Giorgio Arcidiacono is an Associate Editor of the journal. The article was subject to the journal’s standard procedures, with peer review handled independently of this editor and his research groups.
Risk of progression according with surgical grading
REFERENCES
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–42. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partelli S, Giannone F, Schiavo Lena M, et al. Is the real prevalence of pancreatic neuroendocrine tumors underestimated? A retrospective study on a Large Series of Pancreatic Specimens. Neuroendocrinology. 2019;109:165–70. doi: 10.1159/000499606. [DOI] [PubMed] [Google Scholar]
- 3.Boyar Cetinkaya R, Aagnes B, Thiis-Evensen E, et al. Trends in incidence of neuroendocrine neoplasms in Norway: A report of 16,075 cases from 1993 through 2010. Neuroendocrinology. 2017;104:1–10. doi: 10.1159/000442207. [DOI] [PubMed] [Google Scholar]
- 4.Halfdanarson TR, Rubin J, Farnell MB, et al. Pancreatic endocrine neoplasms: Epidemiology and prognosis of pancreatic endocrine tumors. Endocr Relat Cancer. 2008;15:409–27. doi: 10.1677/ERC-07-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merola E, Rinzivillo M, Cicchese N, et al. Digestive neuroendocrine neoplasms: A 2016 overview. Dig Liver Dis. 2016;48:829–35. doi: 10.1016/j.dld.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–71. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd RV, Osamura RY, Klöppel G, et al. WHO Classification of Tumours of Endocrine Organs. International Agency for Research on Cancer. [[Last accessed on 2019 Nov 05]]. Available from: http://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Who-Classification-Of-Tumours-Of-Endocrine-Organs-2017 .
- 8.Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: A review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–12. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 9.McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–7. doi: 10.1097/PAS.0000000000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jann H, Roll S, Couvelard A, et al. Neuroendocrine tumors of midgut and hindgut origin: Tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332–41. doi: 10.1002/cncr.25855. [DOI] [PubMed] [Google Scholar]
- 12.Piani C, Franchi GM, Cappelletti C, et al. Cytological Ki-67 in pancreatic endocrine tumours: An opportunity for pre-operative grading. Endocr Relat Cancer. 2008;15:175–81. doi: 10.1677/ERC-07-0126. [DOI] [PubMed] [Google Scholar]
- 13.Weynand B, Borbath I, Bernard V, et al. Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: High reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology. 2014;25:389–95. doi: 10.1111/cyt.12111. [DOI] [PubMed] [Google Scholar]
- 14.Carlinfante G, Baccarini P, Berretti D, et al. Ki-67 cytological index can distinguish well-differentiated from poorly differentiated pancreatic neuroendocrine tumors: A comparative cytohistological study of 53 cases. Virchows Arch. 2014;465:49–55. doi: 10.1007/s00428-014-1585-7. [DOI] [PubMed] [Google Scholar]
- 15.Farrell JM, Pang JC, Kim GE, et al. Pancreatic neuroendocrine tumors: Accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014;122:770–8. doi: 10.1002/cncy.21457. [DOI] [PubMed] [Google Scholar]
- 16.Figueiredo FA, Giovannini M, Monges G, et al. EUS-FNA predicts 5-year survival in pancreatic endocrine tumors. Gastrointest Endosc. 2009;70:907–14. doi: 10.1016/j.gie.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98:4784–9. doi: 10.1210/jc.2013-2604. [DOI] [PubMed] [Google Scholar]
- 18.Partelli S, Tamburrino D, Lopez C, et al. Active surveillance versus surgery of nonfunctioning pancreatic neuroendocrine neoplasms≤2 cm in MEN1 patients. Neuroendocrinology. 2016;103:779–86. doi: 10.1159/000443613. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa T, Yamao K, Hijioka S, et al. Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy. 2014;46:32–8. doi: 10.1055/s-0033-1344958. [DOI] [PubMed] [Google Scholar]
- 20.Boutsen L, Jouret-Mourin A, Borbath I, et al. Accuracy of pancreatic neuroendocrine tumour grading by endoscopic ultrasound-guided fine needle aspiration: Analysis of a large cohort and perspectives for improvement. Neuroendocrinology. 2018;106:158–66. doi: 10.1159/000477213. [DOI] [PubMed] [Google Scholar]
- 21.Larghi A, Capurso G, Carnuccio A, et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest Endosc. 2012;76:570–7. doi: 10.1016/j.gie.2012.04.477. [DOI] [PubMed] [Google Scholar]
- 22.Leeds JS, Nayar MK, Bekkali NL, et al. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc Int Open. 2019;7:E1281–7. doi: 10.1055/a-0990-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paiella S, Landoni L, Rota R, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors: A retrospective analysis of 110 cases. Endoscopy. 2020;52:988–94. doi: 10.1055/a-1180-8614. [DOI] [PubMed] [Google Scholar]
- 24.Kamata K, Ashida R, Yasukawa S, et al. Histological diagnosis and grading of pancreatic neuroendocrine tumor by endoscopic ultrasound-guided fine needle biopsy using a 25-gauge needle with a core trap: A multicenter prospective trial: Pancreatic neuroendocrine tumor grading. Pancreatology. 2020;20:1428–1433. doi: 10.1016/j.pan.2020.08.023. [doi: 10.1016/j.pan.2020.08.023] [DOI] [PubMed] [Google Scholar]
- 25.Heidsma CM, Tsilimigras DI, Rocha F, et al. Clinical relevance of performing endoscopic ultrasound-guided fine-needle biopsy for pancreatic neuroendocrine tumors less than 2 cm. J Surg Oncol. 2020;122:1393–1400. doi: 10.1002/jso.26158. [doi: 10.1002/jso.26158] [DOI] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 27.Rindi G, Falconi M, Klersy C, et al. TNM staging of neoplasms of the endocrine pancreas: Results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–77. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 28.Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: Improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–33. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 29.Pecorelli N, Balzano G, Capretti G, et al. Effect of surgeon volume on outcome following pancreaticoduodenectomy in a high-volume hospital. J Gastrointest Surg. 2012;16:518–23. doi: 10.1007/s11605-011-1777-2. [DOI] [PubMed] [Google Scholar]
- 30.Macedo FI, Jayanthi P, Mowzoon M, et al. The impact of surgeon volume on outcomes after pancreaticoduodenectomy: A meta-analysis. J Gastrointest Surg. 2017;21:1723–31. doi: 10.1007/s11605-017-3498-7. [DOI] [PubMed] [Google Scholar]
- 31.Hata T, Motoi F, Ishida M, et al. Effect of hospital volume on surgical outcomes after pancreaticoduodenectomy: A systematic review and meta-analysis. Ann Surg. 2016;263:664–72. doi: 10.1097/SLA.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Tang LH, Klimstra DS. Effect of tumor heterogeneity on the assessment of Ki67 labeling index in well-differentiated neuroendocrine tumors metastatic to the liver: Implications for prognostic stratification. Am J Surg Pathol. 2011;35:853–60. doi: 10.1097/PAS.0b013e31821a0696. [DOI] [PubMed] [Google Scholar]
- 33.Govind D, Jen KY, Matsukuma K, et al. Improving the accuracy of gastrointestinal neuroendocrine tumor grading with deep learning. Sci Rep. 2020;10:11064. doi: 10.1038/s41598-020-67880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crinò SF, Ammendola S, Meneghetti A, et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology. 2021;21:443–50. doi: 10.1016/j.pan.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Costache MI, Cazacu IM, Dietrich CF, et al. Clinical impact of strain histogram EUS elastography and contrast-enhanced EUS for the differential diagnosis of focal pancreatic masses: A prospective multicentric study. Endosc Ultrasound. 2020;9:116–21. doi: 10.4103/eus.eus_69_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101–8. doi: 10.1016/j.gie.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Săftoiu A, Vilmann P, Gorunescu F, et al. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–94. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of progression according with surgical grading