Abstract
The optimal sampling techniques for EUS-FNA remain unclear and have not been standardized. To improve diagnostic accuracy, suction techniques for EUS-FNA have been developed and are widely used among endoscopists. The aim of this study was to compare wet-suction and dry-suction EUS-FNA techniques for sampling solid lesions. We performed a comprehensive literature search of major databases (from inception to June 2020) to identify prospective studies comparing wet-suction EUS-FNA and dry-suction EUS-FNA. Specimen adequacy, sample contamination, and histologic accuracy were assessed by pooling data using a random-effects model expressed in terms of odds ratio (OR) and 95% confidence interval (CI). Six studies including a total of 418 patients (365 wet suction vs. 377 dry suction) were included in our final analysis. The study included a total of 535 lesions (332 pancreatic lesions and 203 nonpancreatic lesions). The pooled odds of sample adequacy was 3.18 (CI: 1.82–5.54, P = 0.001) comparing wet- and dry-suction cohorts. The pooled odds of blood contamination was 1.18 (CI: 0.75–1.86, P = 0.1). The pooled rate for blood contamination was 58.33% (CI: 53.65%–62.90%) in the wet-suction cohort and 54.60% (CI 49.90%– 59.24%) in the dry-suction cohort (P = 0.256). The pooled odds of histological diagnosis was 3.68 (CI 0.82–16.42, P = 0.1). Very few adverse events were observed and did not have an impact on patient outcomes using either method. EUS-FNA using the wet-suction technique offers higher specimen quality through comparable rates of blood contamination and histological accuracy compared to dry-suction EUS-FNA.
Keywords: dry suction, EUS, FNA, solid lesions, wet suction
INTRODUCTION
Tissue acquisition using EUS-FNA was first introduced 25 years ago and has become an important part of the diagnostic and staging algorithm for both benign and malignant diseases of the GI tract.[1] As such, various suction techniques for EUS-FNA have been developed to improve diagnostic accuracy. To this end, the dry- and wet-suction techniques have been proposed as methods for tissue acquisition using EUS-FNA.
The dry-suction or traditional technique involves removal of the stylet and the use of a 10 ml prevacuum syringe to generate negative pressure to aid in the acquisition of tissue specimen. However, this technique has associated flaws which may impact the quality of the aspirate.[2,3] This technique has been shown to increase the cellularity of a sample but also increases the chance of blood contamination.[4]
Alternatively, the wet-suction technique involves the use of saline or heparin to preflush the needle prior to aspiration.[5,6] Prior to puncturing the lesion, the stylet is removed and the needle is preflushed with about 5 ml of liquid. Left attached to the proximal port and later used for aspiration is a 10 ml syringe prefilled with 3 ml of liquid. Once the needle is passed into the lesion, the needle is moved back and forth roughly three times and suctioned to acquire tissue aspirate. This is repeated about four times prior to air flushing the sample onto a slide for review.
Compared to dry-suction EUS-FNA, the wet-suction technique has been shown to increase cellularity and adequacy of specimens without adding blood contamination.[7] To this end, we conducted a systematic review and meta-analysis to evaluate the differences between wet- and dry-suction techniques for the sampling of solid lesions.
METHODS
Search strategy
We conducted a comprehensive search of several databases and conference proceedings including PubMed, EMBASE, and Google-Scholar databases to April 2020. An experienced medical librarian using inputs from the study authors helped with the literature search. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines using a predefined protocol to identify studies reporting the use of wet suction and dry suction during EUS FNA [Supplementary Table 1].[8,9]
Supplementary Table 1.
PRISMA checklist
| Section/topic | Number | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: Background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 3 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 4 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 4 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., web address), and, if available, provide registration information including registration number | Not registered |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale | 4, 5 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 4, 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 4, 5 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 5 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 6 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made | 5, 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 6 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means) | 6 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis | 6 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies) | 5, 6 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were prespecified | 5,6 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 7 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations | 7 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 810 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) Simple summary data for each intervention group (b) Effect estimates and confidence intervals, ideally with a forest plot | 810 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | 810 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | 810 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]) | 9, 10 |
| Discussion | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers) | 10, 11 |
| Summary of evidence | |||
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias) | 11, 12 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 12 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review | 1 |
Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009;6:e1000097.
Keywords used in the literature search included a combination of “endoscopic ultrasound“, “fine needle aspiration“, “FNA,” “dry-suction“, “wet-suction” “pancreatic mass“, and “solid lesions.” The search was restricted to studies performed on human subjects and published in the English language in peer-reviewed journals [Supplementary Table 2]. Two authors (DR and JS) independently reviewed the title and abstract of studies identified in the primary search and excluded studies that did not address the research question, based on prespecified exclusion and inclusion criteria. The full text of the remaining articles was reviewed to determine whether it contained relevant information. Any discrepancy in article selection was resolved by consensus and in discussion with a co-author.
Supplementary Table 2.
Search strategy
| Number | Searches | Results |
|---|---|---|
|
| ||
| Medline [Ovid] August 21, 2020 Ovid MEDLINE (R) and Epub Ahead of Print, In-Process and Other Non-Indexed Citations and Daily <1946 to August 20, 2020> | ||
| 1 | (Endoscopic Ultrasound-Guided Fine Needle Aspiration/or (Biopsy, Needle/and Ultrasonography/)) and (dry* or wet*).tw, kf | 12 |
| 2 | ((((endoscop* or ultrasound* or ultrason*) adj3 ((fine-needle adj (aspir* or suct* or biops*)) or FNA*)) or EUS-FNA or USFNA* or US-FNA*) and (dry* or wet*)).tw, kf | 23 |
| 3 | 1 or 2 | 28 |
| 4 | (Animals/or Models, animal/or Disease models, animal/) not Humans/ | 4694094 |
| 5 | ((animal or animals or canine* or cat or cats or dog or dogs or feline or hamster* or lamb or lambs or mice or monkey or monkeys or mouse or murine or pig or pigs or piglet* or porcine or primate* or rabbit* or rats or rat or rodent* or sheep* or veterinar*) not (human* or patient*)).ti, kf, jw | 2385881 |
| 6 | 4 or 5 | 5110187 |
| 7 | 3 not 6 | 28 |
|
| ||
| Embase [Ovid] August 21, 2020 Embase <1974 to 2020 August 20> | ||
|
| ||
| 1 | (endoscopic ultrasound guided fine needle aspiration/or (needle biopsy/and echography/)) and (dry* or wet*).tw, kw | 21 |
| 2 | ((((endoscop* or ultrasound* or ultrason*) adj3 ((fine-needle adj (aspir* or suct* or biops*)) or FNA*)) or EUS-FNA or USFNA* or US-FNA*) and (dry* or wet*)).tw, kw | 73 |
| 3 | 1 or 2 | 81 |
| 4 | limit 3 to (conference abstract or conference paper or “conference review”) | 56 |
| 5 | (animal or animals or bitch or canine* or cat or cats or dog or dogs or feline or hamster* or lamb or lambs or mice or monkey or monkeys or mouse or murine or pig or pigs or piglet* or porcine or primate* or rabbit* or rats or rat or rodent* or sheep* or veterina *).ti, kw, dq, jx. not (human* or patient*).mp | 1938912 |
| 6 | 4 not 5 | 55 |
| 7 | 3 not 4 | 25 |
| 8 | (exp animal/or exp juvenile animal/or adult animal/or animal cell/or animal tissue/or nonhuman/or animal experiment/or animal model/) not human/ | 6665876 |
| 9 | 7 not (5 or 8) | 25 |
| 10 | 6 or 9 | 80 |
| 11 | limit 10 to (conference abstract or conference paper or “conference review”) | 55 |
| 12 | 10 not 11 | 25 |
The bibliographic section of the selected articles, as well as the systematic and narrative articles on the topic, was manually searched for additional relevant articles.
Study selection
We included comparative studies that evaluated and compared wet-suction and dry-suction techniques for EUS FNA. Studies irrespective of the sample size, inpatient/outpatient setting, and geography were included as long as they provided data needed for the analysis.
Inclusion criteria were (1) comparative studies. Exclusion criteria included (1) pediatric (age <18 years) studies, (2) case reports or case series with less than 10 patients, and (3) studies not published in the English language. In the event of multiple publications from the same cohort and/or overlapping cohorts, data from the most recent and/or most appropriate comprehensive report were retained.
Data abstraction and quality assessment
Study references and citations were collected in EndNote X9 (Thomson Reuters, New York, NY). Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia: https://www. covidence. org/) was used to further screen and extract relevant studies. The full text of each selected article was reviewed to verify that it contained relevant information. To identify other potentially eligible publications, the bibliographic section of the selected articles was manually searched for additional relevant articles. Data on study-related outcomes in the individual studies were abstracted by two authors (DR and JS) and the two authors (DR and JS) did the quality scoring independently. The Jadad scale for RCT was used to assess the quality of studies.[10] The Newcastle–Ottawa scale was used for cohort studies.[10,11]
Outcomes assessed in study cohorts were as follows
Odds ratio (OR) of specimen adequacy
Pooled rate of specimen adequacy
OR of sample blood contamination
Pooled rate of sample blood contamination
OR of histologic accuracy
Pooled rate of histologic accuracy.
Statistical analysis
We used meta-analysis techniques to calculate the pooled estimates in each case following the methods suggested by DerSimonian and Laird using the random-effects model.[12] We assessed heterogeneity between study-specific estimates using Cochran Q statistical test for heterogeneity and the I2 statistics.[13,14,15,16] In this, values of <30%, 30%–60%, 61%–75%, and >75% were suggestive of low, moderate, substantial, and considerable heterogeneity, respectively.[17,18,19,20]
Publication bias was ascertained qualitatively, by visual inspection of the funnel plot and quantitatively by the Egger test.[21,22] P value <0.05 was considered statistically significant for comparison of groups. Statistical analyses were conducted using STATA software, version 16.0 (College Station, TX: StataCorp LLC).
RESULTS
Search results and study characteristics
From an initial total of 558 studies, 520 records were screened after deduplication, and 16 full-length articles were assessed. Six studies were ultimately included in the final meta-analysis.[4,7,23,24,25,26] The schematic diagram of study selection is illustrated in Figure 1.
Figure 1.
Study Prisma chart
A total of 418 patients (365 wet suction vs. 377 dry suction) were included in the final analysis. This analysis included a total of 535 lesions (332 pancreatic lesions and 203 nonpancreatic lesions). Patient age ranged from 26 to 87 years. Four studies used 22G needles for EUS-FNA, 1 study used 19G, 1 study used 25G, and 1 study used either 19G or 22G. Additional details of study characteristics with patient demographics are summarized in Table 1.
Table 1.
Study characteristics
| Author | Country | Center type | Study design | Number of patients (n) | Male/female | Lesions type | Needle gauge (G) | How many passes? | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Dry | Wet | Pancreatic, n (%) | Nonpancreatic, n (%) | |||||||
| Attam, 2015 | USA | Multicenter | RCT | 117 | 117 | 66/51 | 63 (54) | 54 (46) | 22 | Dry - 46 Wet - 46 |
| Berzosa, 2014 | USA | Single center | Prospective (abstract) | 15 | 15 | Not reported | 14 (93) | 1 (7) | 22 | Dry - 12 Wet - 12 |
| Mok, 2018 | USA | Single center | RCT | 40 | 40 | 16/24 | 0 | 40 (100) | 19 | Dry - 3 Wet - 3 |
| Sugimoto, 2020 | Japan | Single center | Prospective | 23 | 11 | 27/7 | 34 (100) | 0 | 19/22 | Dry - 5 Wet - 4 |
| Wang, 2020 | China | Multicenter | RCT | 269 | 269 | 161/108 | 161 (59.9) | 108 (40.1) | 22 | Dry - 2 Wet - 2 |
| Hasan, 2014 | USA | Single center | Retrospective | 30 | 30 | 39/21 | 60 (100) | 0 | 25 | Dry - 1 Wet - 1 |
RCT: Randomized controlled trial
Characteristics and quality of included studies
Full manuscript publications included three randomized control trials[4,7,25] and three prospective cohort studies.[23,24,26] One study was published as an abstract.[23] Four studies originated from the USA,[7,23,25,26] one from China,[4] and one from Japan.[24] The detailed assessment of study quality is given in Supplementary Table 3].
Supplementary Table 3.
Bias assessment
| Cohort studies | ||||
|---|---|---|---|---|
|
| ||||
| Quality assessment criteria | Acceptable (*) | Berzosa et al. | Sugimoto et al. | Hasan et al. |
| Selection | ||||
| Representativeness | Population based | |||
| Cohort size | Drawn from the same community as exposed cohort | * | * | * |
| Clinical outcomes | Secured records, clinical outcomes | * | * | * |
| Demonstration that outcome of interest was not present at start of study? | Not present | |||
| Comparability | ||||
| Factors comparable between groups? | Yes | * | * | * |
| Outcome | ||||
| Assessment of outcome? | Independent blind assessment, record linkage | * | * | * |
| Follow-up time | Mentioned or not mentioned | * | * | * |
| Adequacy of follow-up of cohorts? | Complete follow-up, or subjects lost to follow-up unlikely to introduce bias | * | * | * |
| Overall quality score | 6 | 6 | 6 | |
|
| ||||
| Randomized control trials | ||||
|
| ||||
| Study quality | Attam et al. | Mok et al. | Wang et al. | |
|
| ||||
| Randomization present | * | * | * | |
| Appropriate randomization utilized | * | * | * | |
| Blinding present | * | * | * | |
| Appropriate blinding method utilized | * | * | * | |
| Appropriate long-term follow-up | * | * | * | |
| Max score | 5 | 5 | 5 | |
Meta-analysis outcomes
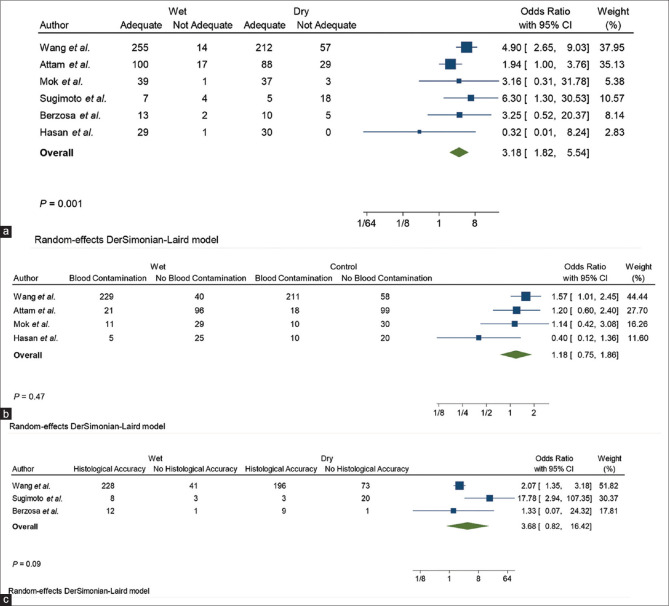
OR of sample adequacy: The pooled odds of sample adequacy was 3.18 (confidence interval [CI]: 1.82–5.54), favoring wet over dry suction EUS FNA, this was statistically different (P = 0.001) [Figure 2]
Pooled rate of sample adequacy: The pooled rate of sample adequacy was 91.90% (CI: 89.10%–94.18%) in the wet-suction cohort and 77.32% (CI 73.37%-80.94%) in the dry-suction cohort (comparison P value <0.001)
OR of blood contamination: The pooled odds of blood contamination was 1.18 (CI: 0.75–1.86) comparing the two study cohorts and this was not statistically different (P = 0.1)
Pooled rate of blood contamination: The pooled rate for blood contamination was 58.33% (CI: 53.65%–62.90%) in the wet-suction cohort and 54.60% (CI: 49.90%– 59.24%) in the dry-suction cohort (comparison P value = 0.256)
OR of histological diagnosis: The pooled odds of histological diagnosis was 3.68 (CI: 0.82–16.42) comparing the two study cohorts and this was not statistically different (P = 0.1)
Pooled rate of histological diagnosis: The pooled rate for histological diagnosis was 84.06% (CI: 79.38%–88.05%) in the wet-suction cohort and 68.87% (CI: 63.31%–74.05%) in the dry-suction cohort (comparison P value <0.001).
Figure 2.
Forest plots of specimen adequacy (a), blood contamination (b), and histological accuracy (c)
Validation of meta-analysis results
Heterogeneity and publication bias assessment
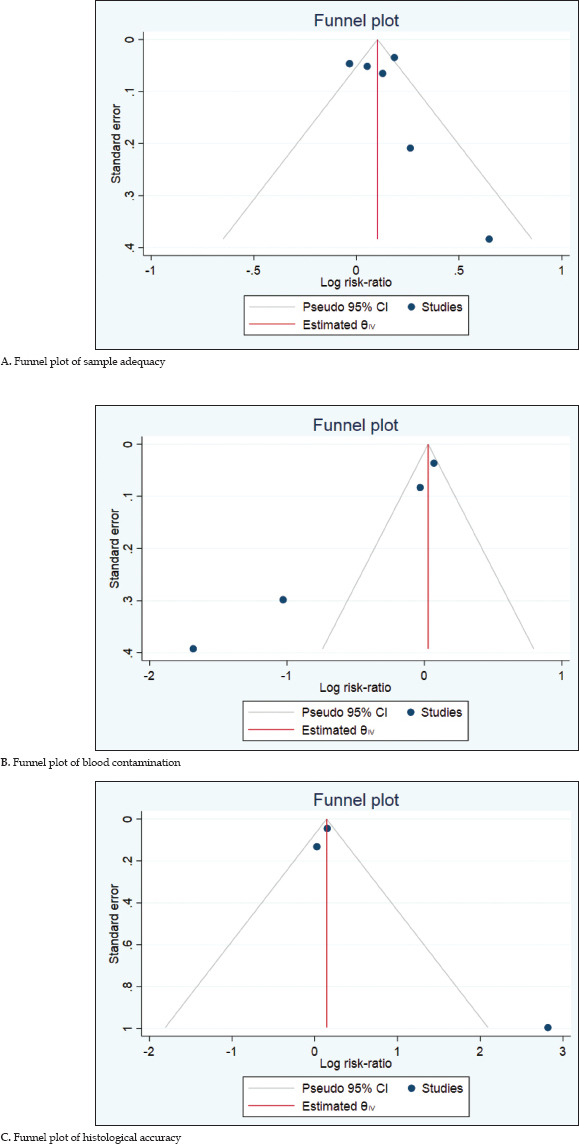
We assessed dispersion of the calculated rates using I2 percentage values. I2 tells us what proportion of the dispersion is true versus chance. We found no significant heterogeneity in reported sample adequacy analysis, moderate heterogeneity was observed in blood contamination analysis, and significant heterogeneity was observed in histological analysis. Publication bias analysis was visually assessed using funnel plots [Supplementary Table 4].
Supplementary Table 4.
Funnel plots
DISCUSSION
We found that the wet-suction technique resulted in greater specimen adequacy when compared to the dry suction method. However, we found that blood contamination, and more importantly, histological accuracy, was comparable using either technique.
We found that the wet-suction cohort had a statistically significant improvement in the specimen adequacy when compared to the dry-suction cohort, with an OR of 3.18 (CI: 1.82–5.54, P = 0.001). Berzosa et al. had suggested that a column of water enhances tissue aspiration due to fluid dynamics and has been shown to allow greater volumes of tissue to be aspirated within the same simulation time when compared to a column of air.[5,27]
Our study also demonstrated that there were comparable rates of blood contamination in the wet-suction cohort when compared to dry-suction cohort (pooled OR: 1.18, CI: 1.75–1.86, P = 0.1). A concern when using EUS-FNA is that the use of suction can often lead to higher rates of blood contamination and can negatively impact the overall quality of a specimen.[2] It was previously thought that the wet-suction technique would overcome this barrier.[4,5] However, this meta-analysis did not reveal a statistically significant difference in blood contamination using either method.
We found that both the methods had comparable histological accuracy. Our analysis also failed to show a statistically significant difference in histological accuracy. This could be due to differences in needle gauge as a uniform needle gauge was not used throughout all studies. Furthermore, both the techniques had very low rates of complications which is consistent with previous studies.[20,21]
The strengths of our review are as follows: systematic literature search with well-defined inclusion criteria, careful exclusion of redundant studies, inclusion of good-quality studies with detailed extraction of data, rigorous evaluation of study quality, and statistics to establish and/or refute the validity of the results of our meta-analysis.
There were also several limitations to this study, most of which are inherent to any meta-analysis. We were unable to calculate the histological accuracy, tissue adequacy, and blood contamination between the pancreatic, hepatic, and other lesions as these data were not consistently provided in all the studies. In addition, the needle gauge was not consistent throughout studies.
Our study is the most comprehensive review comparing the wet-suction and dry-suction techniques for the sampling of solid lesions performed to date. Ultimately, EUS-FNA performed using the wet-suction technique offered higher specimen quality but comparable rates of histological accuracy and blood contamination when compared to EUS-FNA dry suction.
Financial support and sponsorship
Nil.
Conflicts of interest
Douglas G. Adler is an Editor of Endoscopic Ultrasound. The article was subject to the journal’s standard procedures, with peer review handled independently of this Editor and his research groups. There are no other conflicts of interest.
Supplementary Materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Acknowledgement
The authors would like to thank Amy Bergeron for library support
REFERENCES
- 1.Cazacu IM, Luzuriaga Chavez AA, Saftoiu A, et al. A quarter century of EUS-FNA: Progress, milestones, and future directions. Endosc Ultrasound. 2018;7:141–60. doi: 10.4103/eus.eus_19_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–7. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]
- 3.Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wang RH, Ding Z, et al. Wet- versus dry-suction techniques for endoscopic ultrasound-guided fine-needle aspiration of solid lesions: A multicenter randomized controlled trial. Endoscopy. 2020;52:995–1003. doi: 10.1055/a-1167-2214. [DOI] [PubMed] [Google Scholar]
- 5.Villa NA, Berzosa M, Wallace MB, et al. Endoscopic ultrasound-guided fine needle aspiration: The wet suction technique. Endosc Ultrasound. 2016;5:17–20. doi: 10.4103/2303-9027.175877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diehl DL, Mok SR, Khara HS, et al. Heparin priming of EUS-FNA needles does not adversely affect tissue cytology or immunohistochemical staining. Endosc Int Open. 2018;6:E356–62. doi: 10.1055/s-0043-121880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: A novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–7. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 11.Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Sutton AJ, Abrams KR, Jones DR, et al. Methods for Meta-Analysis in Medical Research. New York: J. Wiley; 2000. [Google Scholar]
- 14.Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 16.Mohan BP, Adler DG. Heterogeneity in systematic review and meta-analysis: How to read between the numbers. Gastrointest Endosc. 2019;89:902–3. doi: 10.1016/j.gie.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanwal F, White D. “Systematic Reviews and Meta-analyses” in clinical gastroenterology and hepatology. Clin Gastroenterol Hepatol. 2012;10:1184–6. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 21.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 22.Rothstein HR, Sutton AJ, Borenstein M. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. New York: John Wiley & Sons; 2006. [Google Scholar]
- 23.Berzosa M, Villa N, Bartel MJ, et al. Mo1420 pilot study comparing hybrid vs. wet vs. dry suction techniques for EUS-FNA of solid lesions. Gastrointest Endosc. 2014;79:AB430. [Google Scholar]
- 24.Sugimoto M, Takagi T, Suzuki R, et al. Can the wet suction technique change the efficacy of endoscopic ultrasound-guided fine-needle aspiration for diagnosing autoimmune pancreatitis type 1? A prospective single-arm study. World J Clin Cases. 2020;8:88–96. doi: 10.12998/wjcc.v8.i1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mok SR, Diehl DL, Johal AS, et al. A prospective pilot comparison of wet and dry heparinized suction for EUS-guided liver biopsy (with videos) Gastrointest Endosc. 2018;88:919–25. doi: 10.1016/j.gie.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Hasan MK, Bang JY, Varadarajulu S. Diagnostic value of priming the endoscopic ultrasound-guided fine-needle aspiration needle with heparin to improve specimen quality. Dig Endosc. 2014;26:491. doi: 10.1111/den.12233. [DOI] [PubMed] [Google Scholar]
- 27.Berzosa M, Uthamaraj S, Dragomir-Daescu D, et al. Mo1395 EUS-FN wet vs. dry suction techniques; a proof of concept study on how column of water enhances tissue aspiration. Gastrointest Endosc. 2014;79:AB421–2. [Google Scholar]