Abstract
Background
In this study, cardiac magnetic resonance imaging was used to investigate the characteristics of patients who have total coronary occlusion but manifest with non-ST-segment elevation myocardial infarction (NSTEMI), and we assessed the extent of infarct transmurality and myocardial necrosis size in NSTEMI patients.
Material/Methods
We enrolled all patients diagnosed at our hospital with subtotal or total occlusion of the culprit artery (TOCA), based on the coronary angiography, who successfully underwent PCI within 12 h of admission, and who had CMR imaging performed within 2 days after the PCI.
Results
Based on 12-lead ECG findings, 48% of patients were categorized as having STEMI and 52% as having NSTEMI. TOCA was detected by coronary angiography in 43% of NSTEMI patients, and in 60% and 33% of normal ST segment and ST-segment depression MI patients, respectively. The transmural segments were found in 78% of STEMI patients and 31% of NSTEMI patients (P<0.05). Transmural infarction segments were found in 64% of NSTEMI patients with TOCA and in 8% of NTOCA patients (P<0.05). Moreover, the number of transmural segments in ST-segment depression MI patients was the lowest (P<0.05). Infarct size in STEMI patients was significantly larger than in patients with NSTEMI (P<0.05), whereas there was no statistically significant difference in patients with normal ST segment and ST-segment depression MI patients (P>0.05).
Conclusions
Identification TOCA by coronary angiography and transmural infarction by DE-MRI can be challenging in AMI patients with non-ST-segment elevation. In approximately 30% of non-ST-segment elevation MI patients, transmural infarction was detected by DE-MRI. Therefore, TOCA accompanied by transmural infarction in non-ST-segment-elevation MI patients is not uncommon.
Keywords: Coronary Angiography, Electrocardiography, Magnetic Resonance Imaging, Myocardial Infarction
Background
Acute myocardial infarction (AMI) is the leading cause of morbidity and mortality in people with cardiovascular disease. The spectrum of AMI consists of ST-segment elevation MI (STEMI) and non-ST-segment elevation MI (NSTEMI) [1]. Twelve-lead-electrocardiogram (ECG) has a key role in discriminating NSTEMI from STEMI. Patients with STEMI present with chest pain and persistent ST-segment elevation on the initial ECG leads. In addition, STEMI should be defined if the clinical presentation is compatible and the ECG trace indicates left bundle branch block (LBBB), and it is usually characterized by transmural infarction resulting from total occlusion of an epicardial coronary artery induced by a thrombus [2,3]. In patients with NSTEMI, defined as presenting with acute chest pain lasting >20 min and either elevation of cardiac biomarkers or dynamic ST-segment changes on the initial ECG without ST-segment elevation, NSTEMI is an acute partial occlusion of a coronary vessel causing an incomplete interruption of blood supply of the distal myocardial territory, which causes non-transmural (subendocardial) ischemia [4,5]. The ECG in NSTEMI patients classically shows T wave inversion and/or ST-segment depression in the absence of ST-segment elevation. However, previous studies indicated that TOCA was also detected in patients with NSTEMI. The sensitivity of ECG to detect acute ischemia influencing lateral or posterior myocardial walls is obviously reduced when the infarct-related artery (IRA) involves the left circumflex artery (LCx) [6]. In addition, patients with STEMI, whose symptoms and ECG changes completely resolve upon admission, and are given the medical therapy before hospital arrival, manifest transient ST-segment elevation that is not recorded by ECG.
The accuracy of the 12-lead ECG in detecting TOCA is limited. Recently, clinical research showed that TOCA has also been observed in patients with NSTEMI [7,8]. However, the extent of transmural ischemia in patients who have total occlusion of the culprit artery but who present with non-ST-elevation remains unclear. Moreover, whether the extent of transmural segments or infarct size in STEMI patients is larger than that in NSTEMI patients with or without TOCA is a question worth exploring.
Cardiac magnetic resonance imaging (CMR) is a powerful non-invasive tool that provides images with high spatial resolution [9,10]. Recently, use of CMR has gained acceptance in coronary heart disease; CMR can accurately evaluate myocardial viability after acute myocardial infarction by obtaining data about viable myocardium, myocardial perfusion, and cardiac function in a single examination [11]. In particular, delayed-enhancement magnetic resonance imaging (DE-MRI) and first-pass myocardial perfusion are ideal tools for observing the extent of transmural infarction, infarct size, and microvascular dysfunction [12–14]. We performed the present study to evaluate the transmural extent of myocardial necrosis and myocardial infarct size using CMR for unrecognized myocardial infarction determined by ECG, and to assess the difference in MACE between patients with STEMI and NSTEMI.
Material and Methods
Patients
This was a retrospective analysis of 248 AMI patients who were enrolled between September 2016 and August 2018 in Henan Provincial People’s Hospital, and were followed up for 180 days (6 months). All patients who presented to our hospital with chest pain, ECG changes, and troponin-positive results were eligible for study inclusion. All patients were diagnosed with partial or total occlusion of the infarct-related artery based on the coronary angiography, and successfully underwent PCI within 12 h of hospital admission. Patients also had CMR imaging performed within 1 week after the PCI. Exclusion criteria were chronic MI, acute myocarditis, NYHA class-IV heart failure, and contraindications to CMR. All patients gave written informed consent to study protocols approved by our hospital ethics committee.
CMR Protocol
All patients were examined using a Philips Ingenia 3.0 T CX 3.0 T clinical scanner (Philips Medical Systems Nederland B.V. Best, the Netherlands) with a gradient field strength of 80 mT/m and gradient switching rate of 200 mT/m/ms, equipped with a Q- BODY coil (SENSE XL TORSO COIL 3.0 T) for signal reception. The CMR protocol has been previously described [9]. Delayed-enhancement imaging was performed 10–15 min after intravenous injection of gadolinium diethylenetri-amine penta-acetic acid (Gd-DTPA; 0.1 mmol/kg) using PSIR_TFE_BH sequence with repetition time/echo time 6.1/3.0 ms, flip angle 25°, spatial resolution 3.5×3.5×10 mm, inversion time set to null signal from normal myocardium, and slice thickness 8 mm.
CMR Analysis
The presence and location of hyper-enhanced tissue were determined by visual inspection using the conventional American Heart Association (AHA) 17-segment model (Figure 1) [15]. The transmural extent of myocardial infarction in each segment was graded with a 0-IV score: 0=normal; I=1–25% transmural extent; II=26–50%; III=51–75%; IV=76–100%. Endocardial and epicardial borders in hyperenhancement images were traced manually on all short-axis slices. Myocardial necrosis size was calculated as a percentage of LV volume.
Figure 1.
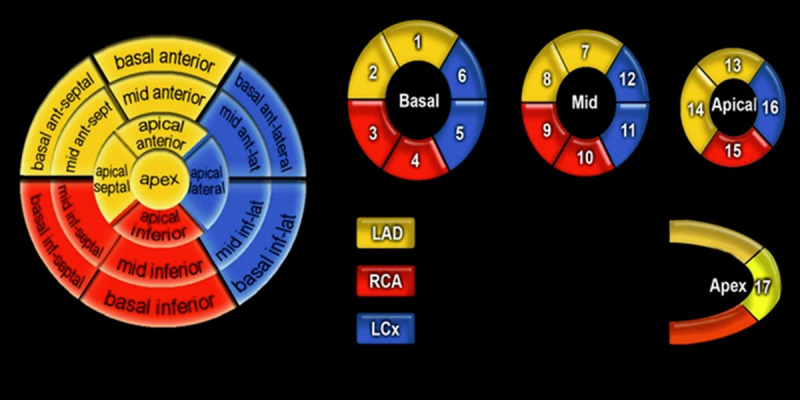
The 17 myocardial segments of the left ventricle presented by AHA and their corresponding coronary artery anatomy.
Coronary Angiography
Two experienced, blinded, interventional cardiologists performed emergency coronary angiography in the cardiac catheterization unit of our hospital. The ‘culprit lesion’ was determined based on angiographic characteristics, location of ECG changes, and segmental wall motion abnormality. Significant CAD was defined as single or multivessel stenoses of >80% luminal narrowing in a main coronary vessel.
ECG
All patients underwent standard 12-lead ECG on admission. According to ECG changes, patients were classified as having ST-segment elevation, normal ST segment, or ST-segment depression. Based on AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram [16], ST-segment elevation was defined as 2 or more contiguous ECG leads with ST-segment elevation (ST-segment elevation ≥2 mm in the chest leads, ST-segment elevation ≥1 mm in limb leads); normal ST segment as no significant ST shift; and ST-segment depression was considered to be ST-segment depression of at least 1 mm in 2 standard limb leads or in 2 contiguous chest leads.
Follow-Up and Outcome
All patients were enrolled between September 2016 and August 2018 and were followed up until the time of an event or, in the case of no event, February 2019. The primary outcome was defined as major adverse cardiac events (MACEs), including cardiovascular mortality or non-fatal MI. The secondary outcomes were cardiovascular mortality, non-fatal MI, unstable angina pectoris requiring revascularization, hospitalization for heart failure, and fatal arrhythmia [17,18].
Ethics Approval
Clinal experiments were reviewed and approved by the Clinical Medicine Research Center Ethics Committee of Henan Provincial People’s Hospital (approval date 2015, code 23).
Statistical Analysis
All statistical analyses were carried out using SPSS version 22.0 (IBM, Armonk, NY, USA). All continuous data are described by mean±SD. The difference between STEMI and NSTEMI patients was determined by chi-square test or by Fisher’s exact test, as appropriate, and the Mann-Whitney U test. A two-sided P<0.05 was considered statistically significant.
Results
Study Population
Baseline clinical characteristics are presented in Table 1. The mean age was 56±11.8 years, and 67% of patients were male. Based on the AHA/ACCF/HRS criteria, 48% (120/248) of patients were diagnosed with STEMI and 52% (128/248) with NSTEMI. NSTEMI included patients with normal ST segment (41%, 52/128) and/or ST-segment depression (59%, 76/128). Patients with STEMI had significantly higher levels of hypertension and blood glucose compared to NSTEMI patients (P<0.05). No significant differences were found in age, EF (%), smoking history, drinking history, or dyslipidemia (P>0.05).
Table 1.
Clinical characteristics at baseline.
| Variable | ST-segment elevation group (n=120) | Normal ST segment group (n=52) | ST-segment depression (n=76) | P value |
|---|---|---|---|---|
| Proportion | 120 (48%) | 52 (21%) | 76 (31%) | |
| Males [n (%)] | 82 (68.3) | 31 (59.6) | 54 (71.0) | 0.670 |
| Ages (Y) | 55.8±11.1 | 53.1±12.6 | 61.1±12.7 | 0.712 |
| EF (%) | 50.7±7.7 | 56.5±6.8 | 53.1±8.1 | 0.569 |
| Smoking [n (%)] | 79 (65.8) | 36 (69.2) | 54 (71.0) | 0.256 |
| Drinking [n (%)] | 64 (53.3) | 27 (51.9) | 42 (55.2) | 0.199 |
| Hypertension, mmHg [n (%)] | 80 (66.6) | 17 (32.7) | 23 (30.3) | <0.05 |
| Glucose, mmol/L [n (%)] | 83 (69.2) | 25 (48.1) | 32 (42.1) | <0.05 |
| Dyslipidemia [n (%)] | 46 (38.3) | 15 (28.8) | 23 (30.2) | 0.698 |
| LDL, mmol/L | 3.03±0.27 | 2.86±0.31 | 2.71±0.19 | 0.552 |
| HDL, mmol/L | 0.93±0.08 | 1.19±0.11 | 1.23±0.17 | 0.233 |
| Peak hs-cTnT, ng/mL | 20.3±1.6 | 18.9±2.2 | 19.2±1.9 | 0.421 |
Values are given (%) or mean±standard deviation. EF – ejection fraction; LDL – low-density lipoprotein; HDL – high-density lipoprotein.
Comparison of Coronary Artery Characteristics Between STEMI and NSTEMI
Coronary artery characteristics are shown in Table 2. Based on coronary angiography, the culprit lesion location and disease distribution of coronary artery in all 248 AMI patients were the following: left anterior descending coronary artery (LAD) and right coronary artery (RCA) were the most common infarct-related arteries in the ST-segment elevation group and ST-segment depression group, while left circumflex artery (LCx) was more common in the normal ST segment group (P<0.05). The infarct sites in the ST-segment elevation and ST-segment depression groups were mostly located in the proximal and middle segments, while those in the normal ST segment group were commonly located in the middle and distal segments (P<0.05). The proportion of occlusive lesions in the ST-elevation group and normal ST segment group was higher than that in the ST-segment depression group (P<0.05). The proportion of single-vessel disease in the ST-segment elevation group and normal ST segment group was higher than that in the ST-segment depression group (P<0.05).
Table 2.
Comparison of coronary angiography in ST-segment elevation, normal ST segment, and ST-segment depression groups.
| Coronary angiography | ST-segment elevation group (n=120) | Normal ST segment group (n=52) | ST-segment depression group (n=76) | χ2 | P value | |
|---|---|---|---|---|---|---|
| IRA | LM | 2 (1.6) | 0 (0.0) | 0 (0.0) | 52.665 | 0.000 |
| LAD | 69 (57.5) | 12 (23.1) | 44 (57.9) | |||
| LCX | 14 (11.7) | 33 (63.5) | 11 (14.5) | |||
| RCA | 37 (30.8) | 7 (13.4) | 21 (27.6) | |||
| Location | Proximal | 56 (46.7) | 9 (17.3) | 40 (52.6) | 22.978 | 0.000 |
| Middle | 44 (36.7) | 33 (63.5) | 19 (25.0) | |||
| Distal | 20 (16.7) | 10 (19.2) | 17 (22.4) | |||
| Occlusive lesion | 96 (80.0) | 31 (60.0) | 25 (32.9) | 43.596 | 0.000 | |
| CAD lesion (≥70 stenosis) | 21.373 | 0.000 | ||||
| 1-vessel disease | 46 (38.3) | 23 (44.2) | 12 (15.8) | |||
| 2-vessel disease | 38 (31.7) | 13 (25.0) | 28 (36.7) | |||
| 3-vessel disease | 36 (30.0) | 16 (30.8) | 36 (47.4) | |||
Values are given (%). IRA – infarct-related artery; LM – left main; LAD – left anterior descending; LCX – left circumflex; RCA – right coronary artery; CAD – coronary artery disease.
CMR Findings
CMR characteristics are shown in Tables 3–5. Myocardial necrosis hyperenhancement was observed in 99% (239/248) of patients. Among them, 99% (118/120) of patients with ST-elevation were identified by DE-MRI, 94% (49/52) with normal ST segment, and 95% (72/76) with ST-segment depression. In 113 (47%) cases, hyperenhancement was observed in the anterior wall; in 59 (25%) patients it was observed in the lateral wall, and in 67 (28%) it was observed in the inferior wall. Among patients with STEMI, 93/120 (78%) had transmural myocardial infarction and 27/120 (22%) had non-transmural infarction.
Table 3.
Comparison of transmurality between STEMI and NSTEMI by CMR.
| ECG and CAG | DE-MRI | Total | χ2 | P | |
|---|---|---|---|---|---|
| Non-transmural | Transmural | ||||
| STEMI | 27 | 93 | 120 | 49.9 | 0.00 |
| NSTEMI | 86 | 42 | 128 | ||
| Total | 113 | 135 | 248 | ||
Values are given (%). STEMI – ST-segment elevation myocardial infarction; NSTEMI – non-ST-segment elevation myocardial infarction; ECG – electrocardiogram; CAG – coronary angiograph; DE-MRI – delayed-enhancement magnetic resonance imaging.
Table 4.
Comparison of transmurality between STEMI and NSTEMI by CMR.
| ECG and CAG | DE-MRI | Total | χ2 | P | |
|---|---|---|---|---|---|
| Non-transmural | Transmural | ||||
| Normal ST segment with TOCA | 9 | 22 | 31 | 1.350 | 0.245 |
| ST-depression with TOCA | 11 | 14 | 25 | ||
| Total | 20 | 36 | 56 | ||
Values are given (%). TOCA – totally occluded coronary artery; ECG – electrocardiogram; CAG – coronary angiograph; DE-MRI – delayed-enhancement magnetic resonance imaging.
Table 5.
Comparison of NSTEMI patients with or without TOCA with CMR transmurality.
| ECG and CAG | DE-MRI | χ2 | P | ||
|---|---|---|---|---|---|
| Non-transmural | Transmural | ||||
| Normal ST segment | With TOCA | 9 (17.3) | 22 (42.3) | 16.113 | 0.000 |
| Without TOCA | 18 (34.6) | 3 (5.8) | |||
| ST-segment depression | With TOCA | 11 (14.5) | 14 (18.4) | 24.226 | 0.000 |
| Without TOCA | 48 (63.2) | 3 (3.9) | |||
Values are given (%). TOCA – totally occluded coronary artery; ECG – electrocardiogram; CAG – coronary angiograph; DE-MRI – delayed-enhancement magnetic resonance imaging.
Comparison of Extent of Transmural Infarcts in STEMI and NSTEMI Patients with or without TOCA
The transmural infarction extent as assessed by DE-MRI in 93/120 (78%) of ST-segment elevation patients was more than 75% of at least 1 segment (Table 3). Transmural infarcts in patients with normal ST segment and in ST-segment depression patients with TOCA were detected in 22/56 (39%) and 14/56 (25%), respectively (Table 4), in 38/72 (53%) of patients with normal ST segment, and in less than 50% of ST-segment depression patients without TOCA (Table 5). In addition, using the AHA 16-segment model, transmural infarcts were detected in 689 of the 1920 segments (36%) in ST-segment elevation patients, in 87 of the 496 segments (17.5%) in patients with TOCA with normal ST segment, and in 63 of 400 segments (15.7%) in ST-segment depression patients with TOCA, as determined by DE-MRI. Our results indicated that the number of transmural segments was higher in patients with STEMI compared to NSTEMI patients (P<0.05). In addition, there were more transmural infarction segments in NSTEMI patients with TOCA than in those without TOCA (P<0.05). There was a minor, not statistically significant, difference in necrosis transmurality in patients with normal ST segment in comparison with those with ST-segment depression (P<0.05). Interestingly, transmural infarcts in the NSTEMI population were predominantly located in the inferior and anterior wall, as detected by DE-MRI (Figure 2).
Figure 2.
Images from 6 AMI patients. (A) ECG in leads V1–V5 manifests ST-elevation in a 57-year-old man. CMR illustrates transmural infarction in the anterior segments detected by DE-MRI. Coronary angiography revealed LAD 100% occluded proximally. (B) ST-segment elevation on ECG in leads II, III, and aVF in a 48-year-old man. CMR illustrates contrast-enhanced areas in the lateral segments characterized by DE-MRI. Coronary angiography revealed a 99% distal stenosis in the RCA. (C) A 58-year-old MI patient with a normal ST segment. CMR shows contrast-enhanced areas in the inferior segments characterized by DE-MRI. Coronary angiography revealed 100% proximal occluded RCA. (D) A 55-year-old woman with MI with a normal ST segment. CMR illustrates non-transmural hyperenhancement in the lateral segment. Coronary angiography revealed 95% middle stenosis in the LCx. (E) ECG of a 50-year-old patient showing presence ST-depression in inferior leads. CMR showed partial transmural and subendocardial necrosis in the inferior segments detected by DE-MRI. Coronary angiography revealed 100% proximal occluded RCA. (F) ECG of a 66-year-old woman presenting non-ST-segment elevation in anterior leads. CMR showed non-transmural hyperenhancement in the anterior segment detected by DE-MRI. Coronary angiography revealed 95% proximal stenosis in the LAD. DE-MRI – delayed-enhancement magnetic resonance imaging; MI – myocardial infarction; LAD – left anterior descending artery; LCx – left circumflex; RCA – right coronary artery.
Comparison of Myocardial Necrosis Size in STEMI and NSTEMI Populations with or without TOCA
Our results revealed that myocardial necrosis size was larger in STEMI patients than in NSTEMI patients. Moreover, the necrosis size in patients with TOCA was also significantly larger as compared to those with NTOCA (Figure 3). However, no significant difference in infarct size was found between the patients with normal ST segment and ST-segment depression patients. In the NSTEMI population, myocardial necrosis size in patients with transmural hyperenhancement was significantly larger than in those with non-transmural hyperenhancement (Figure 3).
Figure 3.

Myocardial infarction size as assessed by DE-MRI according to ST-elevation and non-ST-elevation on admission 12-lead-ECG. * P<0.05 vs ST-elevation group, ** P<0.05 vs ST-elevation group.
Procedural Characteristics and Medical Therapy of the Patients
Patients in the 3 groups had similar procedural characteristics and medical therapy (Table 6). No significant differences were detected in the prevalence of door-to-balloon time or stent implantation in each group. In addition, no significant differences between groups were observed in MI (TIMI) flow before or after thrombolysis. Approximately 90% of patients with primary PCI had restored TIMI flow III.
Table 6.
Procedural characteristics and medical therapy of the studied population.
| ST-segment elevation group (n=120) | ST-segment normal group (n=52) | ST-segment depression group (n=76) | P | |
|---|---|---|---|---|
| Pre-procedure TIMI flow ≤II, n (%) | 114 (95) | 47 (90) | 58 (76) | 0.43 |
| Post procedure TIMI flow III, n (%) | 106 (88) | 48 (92) | 72 (95) | 0.29 |
| Door-to-balloon time (h) | 62 (53–90) | 67 (59–90) | 68 (57–90) | 0.68 |
| Stent implantation, n (%) | 103 (86) | 42 (81) | 65 (86) | 0.55 |
| Antiplatelet therapy, n (%) | 110 (92) | 48 (92) | 68 (89) | 0.37 |
| ACE-I or ARB, n (%) | 98 (82) | 44 (85) | 62 (82) | 0.76 |
| Beta Blocker, n (%) | 95 (79) | 42 (81) | 64 (84) | 0.89 |
| Statin, n (%) | 110 (92) | 48 (92) | 68 (89) | 0.91 |
Data are numbers (percentages). TIMI – thrombolysis in myocardial infarction.
Outcome Data
During the follow-up of 180 days (6 months), there were 30 (12%) MACEs, including 16 (6.5%) cardiovascular deaths and 14 (5.5%) non-fatal MIs, 32 (13.0%) hospitalizations for heart failure, 24 (9.7%) unstable angina pectoris requiring revascularization, and 5 (2.1%) ventricular arrhythmias. The incidence of MACE was higher in patients with ST-segment elevation compared to patients with normal ST segment or ST-segment depression (Figure 4), However, no significant difference was observed between STEMI and NSTEMI patients (Figure 5). In univariable analysis, presence of inducible ischemia, number of segments of inducible ischemia, presence of LGE, number of segments of LGE, and LV end-diastolic and end-systolic volume index were all significantly associated with MACE (HR, 2.99 [95% CI, 1.67–5.83]; P<0.01; HR, 1.48 [95% CI, 1.32–2.01]; P<0.01; HR, 1.56 [95% CI, 1.26–1.93]; P<0.01, HR, 1.23 [95% CI, 1.15–1.38]; P<0.01; HR, 1.06 [95% CI, 0.89–1.16]; P<0.01; and HR, 1.03 [95% CI, 1.32–2.01]; P<0.01, respectively; Table 7). In a multivariable Cox regression analysis, age, presence of inducible ischemia, number of segments of inducible ischemia, presence of LGE, and number of segments of LGE were independent predictors of a higher incidence of MACE (HR, 1.07 [95% CI, 0.99–1.14]; P<0.01; HR, 3.01 [95% CI, 1.69–5.85]; P<0.01; HR, 1.50 [95% CI, 1.35–2.04]; P<0.01, HR, 1.58 [95% CI, 1.28–1.99]; HR, 1.25 [95% CI, 1.15–1.40]; and HR, 1.01 [95% CI, 0.84–1.09]; respectively; Table 8)
Figure 4.
MACE rate according to hospitalization for heart failure (A) and unstable angina pectoris requiring revascularization (B).
Figure 5.

Kaplan-Meier curves of cardiovascular mortality.
Table 7.
Univariable analysis of clinical and CMR characteristics for prediction of MACE.
| MACE | P value | |
|---|---|---|
| Hazard ratio (95% CI) | ||
| Age | 1.06 (0.94–1.13) | 0.16 |
| Male | 1.35 (0.59–2.86) | 0.33 |
| Hypertension | 0.91 (0.82–1.39) | 0.45 |
| Diabetes | 0.96 (0.77–1.24) | 0.21 |
| BMI | 1.05 (0.97–1.16) | 0.14 |
| Dyslipidemia | 1.01 (0.66–1.15) | 0.39 |
| Smoking | 1.14 (0.80–1.45) | 0.52 |
| Previous PCI | 0.88 (0.75–1.29) | 0.33 |
| Stroke | 0.68 (0.42–1.77) | 0.37 |
| Presence of inducible ischemia | 2.99 (1.67–5.83) | <0.01 |
| Number of segments of inducible ischemia | 1.48 (1.32–2.01) | <0.01 |
| Presence of LGE | 1.56 (1.26–1.93) | <0.01 |
| Number of segments of LGE | 1.23 (1.15–1.38) | <0.01 |
| LVEF | 0.82 (0.73–1.04) | <0.01 |
| LV end-diastolic volume index, mL/m2 | 1.06 (0.89–1.16) | <0.01 |
| LV end-systolic volume index, mL/m2 | 1.03 (0.93–1.19) | <0.01 |
MACE – major adverse cardiovascular event; BMI – body mass index; LGE – late gadolinium enhancement; PCI – percutaneous coronary intervention; LVEF – left ventricular ejection fraction.
The 2-tailed P value reached statistical significance, P<0.05.
Table 8.
Multivariable analysis of clinical and CMR characteristics for prediction of MACE.
| MACE | P value | |
|---|---|---|
| Hazard ratio (95% CI) | ||
| Age | 1.07 (0.99–1.14) | <0.01 |
| Male | 1.32 (0.57–2.87) | 0.29 |
| Hypertension | 0.92 (0.83–1.36) | 0.38 |
| Diabetes | 0.98 (0.79–1.27) | 0.32 |
| BMI | 1.07 (0.99–1.19) | 0.08 |
| Dyslipidemia | 1.01 (0.66–1.15) | 0.39 |
| Smoking | 1.18 (0.82–1.49) | 0.47 |
| Previous PCI | 0.90 (0.77–1.32) | 0.14 |
| Stroke | 0.70 (0.45–1.80) | 0.25 |
| Presence of inducible ischemia | 3.01 (1.69–5.85) | <0.01 |
| Number of segments of inducible ischemia | 1.50 (1.35–2.04) | <0.01 |
| Presence of LGE | 1.58 (1.28–1.99) | <0.01 |
| Number of segments of LGE | 1.25 (1.15–1.40) | <0.01 |
| LVEF | 1.01 (0.84–1.09) | <0.01 |
MACE – major adverse cardiovascular event; BMI – body mass index; LGE – late gadolinium enhancement; PCI – percutaneous coronary intervention; LVEF – left ventricular ejection fraction.
The 2-tailed P value reached statistical significance, P<0.05
Discussion
The main findings of the current study are: (1) approximately 43% of NSTEMI patients have TOCA; (2) the proportion of occlusive lesions in patients with ST-segment elevation and in patients with normal ST segment is higher compared to those with ST-segment depression; (3) transmural infarction evaluated by DE-CMR was mainly observed in patients with STEMI, which, however, also detected in NSTEMI patients by 12-lead-ECG findings; (4) total infarct size in STEMI patients was significantly larger compared to patients with NSTEMI; (5) NSTEMI patients with TOCA had larger MI size than in patients with TOCA.
In the clinical setting, AMI patients are classified into STEMI and NSTEMI based on the absence or presence of ST-segment elevation on ECG for implementation of established management strategies [16]. NSTEMI differs from STEMI in that NSTEMI patients have dynamic changes in ST-T wave without ST elevation. However, ECG shows that patients without ST-segment elevation have either ST-segment depression or normal ST segment, but distinguishing patients with STEMI from those with NSTEMI is still challenging in clinical practice. Also, it remains unclear whether the absence of ST-segment elevation on ECG is related to TOCA. Numerous studies have suggested that 12-lead ECG has limited accuracy in detecting coronary artery occlusion [7,8,19]. Our study found that NSTEMI can be associated with TOCA, and that LCx is more frequently the culprit vessel in NSTEMI patients with TOCA. The reasons for normal ST segment or ST-segment depression in patients with TOCA may be the following: (1) the culprit lesion (RCA or LCx artery) is located distally, thus affecting the culprit lesions distribution territory, which is limited to the posterior or lateral segments; (2) ST-segment elevation is not recorded by ECG that is carried out on admission in NSTEMI patients with subsequent occlusive thrombus formation (this dissolved instantly upon admission, showing in the transient ST-segment elevation, which is not recorded by ECG).
ECG often fails to show typical signs of TOCA in NSTEMI patients who undergo coronary angiography during hospitalization, which often delays STEMI management and can lead to enlarged infarct area and increased mortality. Therefore, evaluating patients with NSTEMI is challenging. Our study is the first to compare the rates of MACE and mortality between non-ST-segment elevation and ST-segment elevation patients; our results suggest that the rate of cardiovascular mortality is not significantly different between non-ST-segment elevation and ST-segment elevation patients. However, the rate of hospitalization for heart failure in non-ST-segment elevation patients was lower than in ST-segment elevation patients. CMR has emerged as an important imaging method in cardiovascular disease. DE-MRI is considered the criterion standard for identifying and quantifying the transmural extent and infarct size of AMI [20,21]. To further evaluate the extent of infarction, DE-MRI was performed to analyze the extent of myocardial necrosis in NSTEMI patients.
In our study, approximately 30% of NSTEMI patients were diagnosed with transmural myocardial infarction by DE-MRI. Interestingly, the transmural hyperenhancement often occurred in the anterior and inferior segments. One reason for the overall considerably higher incidence of transmural myocardial necrosis in the patients with NSTEMI may be obstructive thrombus that spontaneously resolved after medical therapy, presenting as non-ST-segment elevation diagnosed by ECG. Our study also found more patients with an obstructive coronary artery than those with non-obstructive coronary arteries in the NSTEMI group. Thus, another explanation is that some NSTEMI patients with underlying TOCA have clinical features that are similar to those of patients with STEMI. The number of transmural segments was higher in MI patients with normal ST segment than in those with ST-segment depression. It is possible that the proportion of occlusive lesions in MI patients with normal ST segment was higher than in those with ST-segment depression. However, in NSTEMI patients, the infarct size was not significantly different between those with normal ST segment and those with ST-segment depression.
Previous studies suggested that occluded culprit lesions in patients with NSTEMI were associated with worse outcomes than were non-occluded culprit lesions [22,23]. A study has characterized patients who may present with TOCA and highlights the need to identify high-risk sub-groups [8]. Our study found that the rate of transmural segment occurrence was higher in NSTEMI patients with TOCA, and the infarct size was significantly larger as compared to that in patients without TOCA. Next, the extent of transmural and infarct size was compared between NSTEMI patients with TOCA and those with STEMI. Our research also indicated that transmural myocardial infarction was more common in STEMI patients and, on average, they had an obviously larger infarct size than in NSTEMI patients with TOCA, indicating that the CMR showed that a substantial number of patients without ST-segment elevation had transmural myocardial necrosis. In addition, our study also revealed that LGE were independently associated with MACE and cardiovascular mortality in this specific subgroup of patients.
Study Limitations
In this study, CMR imaging was performed 2 days after admission by ECG with subsequent PCI. However, it is possible that due to coronary reperfusion and the extent of infarcts, excitation ECG changes during the acute stage did not develop into necrotic areas, which DE-MRI would then detect, which may have led to an underestimation of the extent of transmural infarction and infarct size in MI patients. Also, our observations are strongly linked with the studied patients and therapy; thus, selection bias cannot be excluded.
Conclusions
Our results suggest that MI patients with ST-segment elevation and those with normal ST segment often present a higher proportion of occlusive lesions. Furthermore, NSTEMI patients tend to have less transmural infarction, with a much smaller infarct size. However, identification of total occlusion of a coronary artery by coronary angiography and transmural infarction by DE-MRI is challenging in AMI patients with non-ST-segment elevation.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This work was supported by Key scientific and technological projects of Henan Provincial Department of Health (grant No. 122102310068)
References
- 1.Wagner G, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part VI: Acute ischemia/infarction: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119(10):e262–70. doi: 10.1161/CIRCULATIONAHA.108.191098. [DOI] [PubMed] [Google Scholar]
- 2.DeWood M, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303(16):897–902. doi: 10.1056/NEJM198010163031601. [DOI] [PubMed] [Google Scholar]
- 3.ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5(1):40. doi: 10.1038/s41572-019-0094-z. [DOI] [PubMed] [Google Scholar]
- 4.Alexander K, Newby L, Cannon C, et al. Acute coronary care in the elderly, part I: Non-ST-segment-elevation acute coronary syndromes: A scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–69. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E. Unstable angina: An etiologic approach to management. Circulation. 1998;98(21):2219–22. doi: 10.1161/01.cir.98.21.2219. [DOI] [PubMed] [Google Scholar]
- 6.Bauer T, Gitt A, Hochadel M, et al. Left circumflex artery-related myocardial infarction: Does ST elevation matter? Results from the Euro Heart Survey PCI registry. Int J Cardiol. 2013;168(6):5239–42. doi: 10.1016/j.ijcard.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Figueras J, Otaegui I, Marti G, et al. Area at risk and collateral circulation in a first acute myocardial infarction with occluded culprit artery. STEMI vs non-STEMI patients. Int J Cardiol. 2018;259:14–19. doi: 10.1016/j.ijcard.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Khan A, Golwala H, Tripathi A, et al. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: A systematic review and meta-analysis. Eur Heart J. 2017;38(41):3082–89. doi: 10.1093/eurheartj/ehx418. [DOI] [PubMed] [Google Scholar]
- 9.Sun W, Sun L, Yang F, et al. Evaluation of myocardial viability in myocardial infarction patients by magnetic resonance perfusion and delayed enhancement imaging. Herz. 2019;44(8):735–42. doi: 10.1007/s00059-018-4741-z. [DOI] [PubMed] [Google Scholar]
- 10.Dewey M, Siebes M, et al. Clinical quantitative cardiac imaging for the assessment of myocardial ischaemia. Nat Rev Cardiol. 2020;17(7):427–50. doi: 10.1038/s41569-020-0341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold J, McCann G. Cardiovascular magnetic resonance: Applications and practical considerations for the general cardiologist. Heart. 2020;106(3):174–81. doi: 10.1136/heartjnl-2019-314856. [DOI] [PubMed] [Google Scholar]
- 12.Timmer S, Teunissen P, Danad I, et al. In vivo assessment of myocardial viability after acute myocardial infarction: A head-to-head comparison of the perfusable tissue index by PET and delayed contrast-enhanced CMR. J Nucl Cardiol. 2017;24(2):657–67. doi: 10.1007/s12350-015-0329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulluck H, Hammond-Haley M, Weinmann S, et al. Myocardial infarct size by CMR in clinical cardioprotection studies: Insights from randomized controlled trials. JACC Cardiovasc Imaging. 2017;10(3):230–40. doi: 10.1016/j.jcmg.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hendel R, Friedrich M, Schulz-Menger J, et al. CMR first-pass perfusion for suspected inducible myocardial ischemia. JACC Cardiovasc Imaging. 2016;9(11):1338–48. doi: 10.1016/j.jcmg.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Tang S, Otton J, Holloway L, et al. Quantification of cardiac subvolume dosimetry using a 17 segment model of the left ventricle in breast cancer patients receiving tangential beam radiotherapy. Radiother Oncol. 2019;132:257–65. doi: 10.1016/j.radonc.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Wagner G, Macfarlane P, Wellens H, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part VI: Acute ischemia/infarction: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Ame Coll Cardiol. 2009;53(11):1003–11. doi: 10.1016/j.jacc.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Bulluck H, Paradies V, Barbato E, et al. Prognostically relevant periprocedural myocardial injury and infarction associated with percutaneous coronary interventions: A Consensus Document of the ESC Working Group on Cellular Biology of the Heart and European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2021;42(27):2630–42. doi: 10.1093/eurheartj/ehab271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson M. suspected AMI, an extended algorithm increased sensitivity and decreased specificity for predicting 30-day MACE. Ann Intern Med. 2020;172(2):JC11. doi: 10.7326/ACPJ202001210-011. [DOI] [PubMed] [Google Scholar]
- 19.Martin T, Groenning B, Murray H, et al. ST-segment deviation analysis of the admission 12-lead electrocardiogram as an aid to early diagnosis of acute myocardial infarction with a cardiac magnetic resonance imaging gold standard. J Am Coll Cardiol. 2007;50(11):1021–28. doi: 10.1016/j.jacc.2007.04.090. [DOI] [PubMed] [Google Scholar]
- 20.Quarta G, Gori M, Iorio A, et al. Cardiac magnetic resonance in heart failure with preserved ejection fraction: Myocyte, interstitium, microvascular, and metabolic abnormalities. Eur J Heart Fail. 2020;22(7):1065–75. doi: 10.1002/ejhf.1961. [DOI] [PubMed] [Google Scholar]
- 21.van Kranenburg M, Magro M, Thiele H, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7(9):930–39. doi: 10.1016/j.jcmg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Hung C, Chen Y, Huang C, et al. Prevalence and outcome of patients with non-ST segment elevation myocardial infarction with occluded “culprit” artery – a systemic review and meta-analysis. Crit Care. 2018;22(1):34. doi: 10.1186/s13054-018-1944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Zhang M, Fu Y, et al. Incidence, distribution, and prognostic impact of occluded culprit arteries among patients with non-ST-elevation acute coronary syndromes undergoing diagnostic angiography. Am Heart J. 2009;157(4):716–23. doi: 10.1016/j.ahj.2009.01.004. [DOI] [PubMed] [Google Scholar]




