Abstract
Background:
Alcohol-associated liver disease (ALD) is the most common indication for liver transplantation (LT) in the U.S and Europe. As a 6-month alcohol abstinence period has been required by many transplant programs prior to listing and this may influence wait-list (WL) outcomes. We examined WL events in patients with ALD versus non-ALD, with a special interest in whether these outcomes differed by sex.
Methods:
All U.S. adults listed for LT from January 2002 to December 2016 were eligible except Status 1, MELD exceptions, and retransplants. The outcomes of interest were cumulative WL death or too sick and WL removal for improvement within 2 years of listing. Competing risk regression models were used to evaluate recipient factors associated with the outcomes.
Results:
Among the 83,348 eligible WL patients, 23% had ALD. Unadjusted cumulative WL removal within 2 years was 19.0% for ALD vs. 21.0% for non-ALD, p<0.001. In fully adjusted models, ALD was associated with a significantly lower risk of WL removal for death/too sick, (HR 0.84, 95%CI 0.81–0.86, p<0.001) and a higher risk of removal for improvement (HR 2.91, 95%CI 2.35–3.61 p<0.001) vs non-ALD. Adjusting for ALD diagnosis and other potential confounders, women had a higher risk of removal for death/too sick (HR 1.10, 95%CI 1.06–1.14, p<0.001) and for improvement (HR 1.33, 95% CI 1.19–1.47, p <0.001) than men.
Conclusion:
WL candidates with ALD have more favorable WL outcomes than non-ALD patients with a 16% lower risk of removal for deterioration and 191% higher risk of removal for improvement. This likely reflects the benefits of alcohol abstinence, but suggests that listing criteria for ALD may be too restrictive, with patients who might derive benefit from LT not being listed.
Keywords: Wait-list, alcohol-associated liver disease, liver transplantation
INTRODUCTION
Alcohol-associated liver disease (ALD) is the now the most common indication for liver transplantation (LT) in the United States and Europe (1, 2) and the third most common in Canada (3). LT for ALD is well-accepted for patients with complications of end-stage liver disease and increasingly for select patients with severe alcoholic hepatitis (4–6). Historically, there were concerns regarding risk of alcohol relapse post-LT leading to lower patient and graft survival and that offering LT to those with a self-inflicted liver condition might negatively impact donation rates (7). These concerns have proven to be largely unwarranted, with surveys showing that public views on donation for alcohol-associated liver disease are mostly neutral (8). Furthermore, post-transplant survival among patients with ALD is similar to patients without ALD, with 5-year graft and patient survival of 73–79% (9–12) and 10-year graft survival of 58–63% (12, 13).
All patients undergoing LT undergo a careful review of liver and non-liver related comorbidities that may influence peri- and post-transplant morbidity and mortality. For patients with ALD, although controversial, many transplant programs have traditionally required a 6-month period of alcohol abstinence prior to consideration of LT (14). This “rule” reflects recommendations from an earlier era of LT (15), intended to allow sufficient time to see improvement in liver function with abstinence so that LT could be potentially avoided. Whether this “6-month rule” is an appropriate metric or not is highly debatable(16), but it must be acknowledged that reported post-LT outcomes for ALD, may at least in part, be due to use of this selection criterion (17, 18).
Understanding the wait-list (WL) outcomes of patients with ALD is critically important in guiding policy going forward. Remarkably, few studies have focused specifically on the wait-list outcomes of patients with ALD, especially in more recent years (19–21). We hypothesized that a requirement for some period of alcohol abstinence for listing among ALD patients selects those more likely to improve and avoid LT and thus WL removal for improved status would be higher in ALD than other etiologies. Alternatively, risk of recidivism or coexistent ALD-related comorbidities among those on the wait-list may make removal for death/too sick more likely. We sought to better understand WL outcomes in patients with ALD, with the goal of identifying ways to optimize the management of ALD patients referred for and listed for LT.
Sex differences in transplant-related outcomes are well-recognized. Women are known to spend more time on the wait-list and to have higher wait-list mortality (22). This difference is likely related to smaller stature as well as the lack of sex-adjusted creatinine levels, resulting in overall lower MELD scores in women versus men with similar levels of renal impairment (23, 24). We therefore examined whether the disparity in WL outcomes between women and men was present in patients with ALD, a relevant issue given the increasing rates of harmful alcohol use among women (25).
PATIENTS AND METHODS
Patients
All adults (≥18 years of age) listed for LT from January 2002 to December 2016 in the Scientific Registry of Transplant Recipients (SRTR) were eligible. This study period corresponds to the implementation of MELD for prioritization. Since we were interested in studying wait-list outcomes in patients with alcohol-associated cirrhosis, we excluded patients with a diagnosis of acute alcoholic hepatitis as their natural history and short-term mortality risk differs from those with ALD (6, 26, 27). We also excluded patients listed with acute liver failure (status 1), any patient with MELD exception status (including those with hepatocellular carcinoma) and retransplants. To isolate the effect of alcoholic cirrhosis on wait-list outcomes, we excluded patients who had alcoholic cirrhosis and another concomitant diagnosis (e.g. hepatitis C), as it was impossible for us to know which liver disease predominated in a given patient.
Data were obtained via the SRTR files from UNOS/OPTN as of February 28th, 2017. For each patient, follow-up began at the time of their initial registration on the transplant waiting list and all patients were followed until the earliest of the following: LT, removal from the transplant list because of death or being considered too sick, removal from the transplant list for improvement, removal for unknown reason or end of follow-up (2 years post-registration). The primary explanatory variable was etiology of liver disease (ALD versus non-ALD). Only relevant covariates with less than 20% missing values in the SRTR candidate file were included in multivariable models: age, blood type, body mass index (BMI), sex, race/ethnicity, MELD score at listing, region of listing, year of listing, ascites, spontaneous bacterial peritonitis (SBP), transjugular intrahepatic portosystemic shunt (TIPS), encephalopathy, portal vein thrombosis, diabetes and prior malignancy. Era of transplant was evaluated as tertiles: 2002–2006, 2007–2010, 2011–2016. Regions were grouped together into short wait-time regions (3, 6, 10 and 11), moderate wait-time regions (2, 4, 7 and 8), and long wait-time regions (1, 5 and 9).
Outcomes
The primary outcome was removal from the transplant waiting list for death or being too sick or medically unsuitable to undergo LT. The secondary outcome was removal from the transplant waiting list for improvement. The time to removal event or LT was measured from the date of initial listing. Patients with unknown reason for removal, removed for other reasons, or still waiting for transplant at the end of the study were censored.
Statistical analysis
Descriptive statistics included medians and proportions. Differences between subgroups were compared using chi-square and Wilcoxon rank-sum tests, as appropriate. Cumulative incidence of WL removal for death/too sick and improvement were generated separately while accounting for the competing risk of LT. Cumulative incidence curves stratified by cause of liver disease were compared with p values obtained from univariate Fine and Gray regression, modeling the stratification variable as a time-varying covariate to best reflect the observed curves (27). To assess the independent association of both outcomes ((1) removal for death/too sick and (2) removal for improvement) with ALD, multivariable Fine and Gray competing risk regression models were derived. Recipient variables with a p value of <0.1 in univariate analysis were included in the multivariate model. All analyses were performed using Stata software (IC/13.1; StataCorp LP, College Station, TX).
RESULTS
Baseline Characteristics
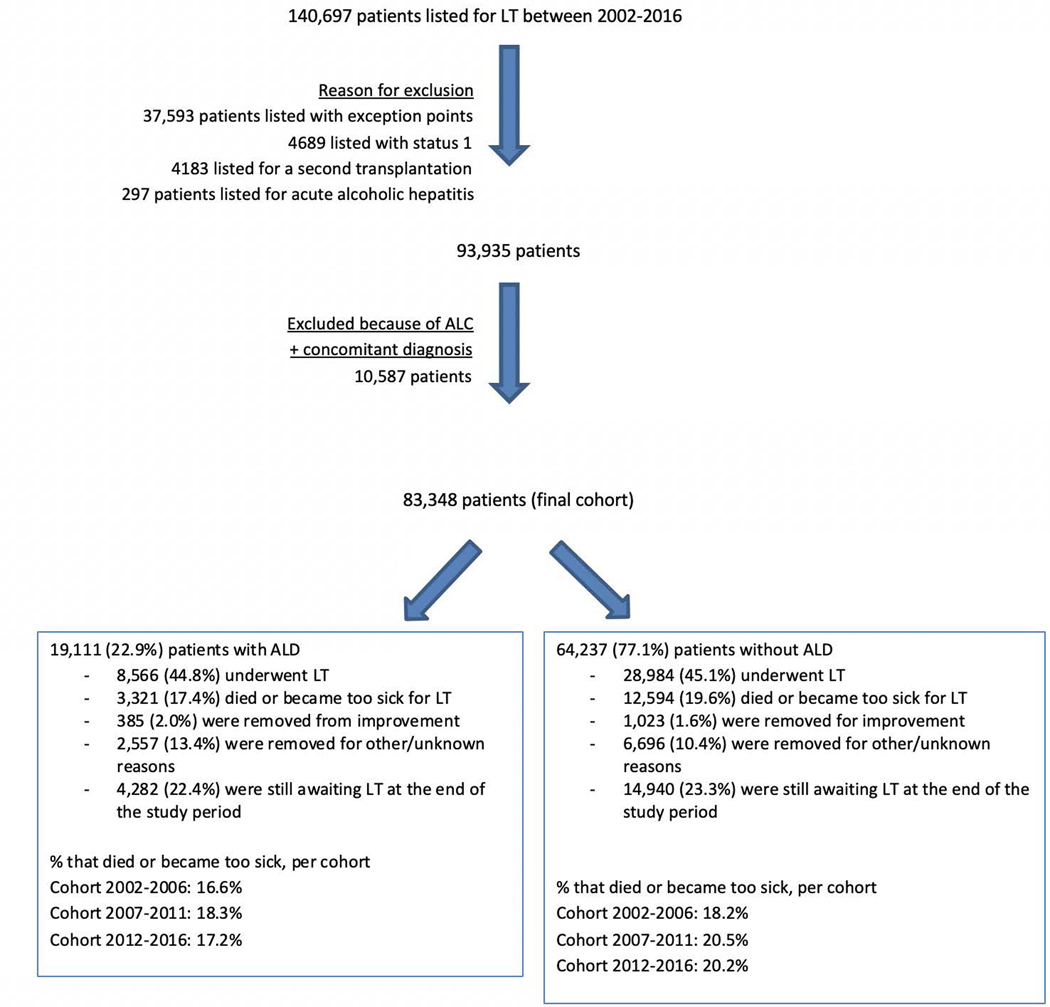
A total of 140,697 patients were listed for LT between January 2002 and December 2016. After exclusions, the final cohort included 83,348 patients, 19,111 (23%) with ALD and 64,237 (77%) without ALD (Figure 1). Transplant candidates with ALD (compared to non-ALD) were younger (median age at listing was 54 years [IQR 47–60] versus 55 years [49–61], p<0.001), more frequently male (74% versus 56%, p<0.001), and more likely of white race (78 vs 71%, p<0.001) (Table 1). Those with ALD versus non-ALD had higher median MELD at listing (18 versus 16, p<0.001) and were more likely to have at least moderate ascites, more severe grades of hepatic encephalopathy and a prior history of SBP (Table 1). Interestingly, the proportion of patients listed with ALD increased from 2002 to 2016 (2002–2006: 18.8%, 2007–2011: 20.8%, 2012–2016: 28.9%). There were regional differences in the proportion of LT candidates with ALD versus non-ALD indications, with the highest proportions in regions with moderate wait-time (Table 1).
Figure 1:
Flow chart of patients’ inclusion
Table 1:
Baseline Characteristics of Transplant Candidates with ALD versus Other Etiologies
| N | All patients | Alcohol only | Other etiologies | P Value | |
|---|---|---|---|---|---|
| N (%) | 83,348 | 19,111 (23) | 64,237 (77) | ||
|
| |||||
| Age group (years) | 83,348 | < 0.001 | |||
| 18–34 | 4,443 (5) | 498 (3) | 3,945 (6) | ||
| 35–49 | 19,772 (24) | 5,651 (30) | 14,121 (22) | ||
| 50–64 | 48,608 (58) | 11,004 (57) | 37,604 (59) | ||
| 65 + | 10,525 (13) | 1,958 (10) | 8,576 (13) | ||
|
| |||||
| Median age (years) | 83,348 | 55 (48–61) | 54 (47–60) | 55 (49–61) | < 0.001 |
|
| |||||
| Female sex | 83,348 | 33,031 (40) | 4,972 (26) | 28,059 (44) | < 0.001 |
|
| |||||
| Race | 83,348 | < 0.001 | |||
| White | 60,488 (73) | 14,854 (78) | 45,634 (71) | ||
| Black | 6,827 (8) | 694 (4) | 6,133 (10) | ||
| Other | 16,033 (19) | 3,563 (18) | 12,470 (19) | ||
|
| |||||
| BMI | 83,348 | < 0.001 | |||
| Less than 20 | 2,395 (3) | 692 (4) | 1,703 (3) | ||
| 20–29 | 46,985 (56) | 11,689 (61) | 35,296 (55) | ||
| 30–34 | 19,986 (24) | 4,332 (23) | 15,654 (24) | ||
| 35–39 | 9,662 (12) | 1,746 (9) | 7,916 (12) | ||
| > or = 40 | 4,320 (5) | 652 (3) | 3,668 (6) | ||
|
| |||||
| Blood group | 83,348 | < 0.001 | |||
| O | 38,483 (46) | 8,775 (46) | 29,708 (46) | ||
| A | 31,312 (38) | 7,414 (39) | 24,898 (37) | ||
| B | 10,250 (12) | 2,201 (11) | 8,049 (13) | ||
| AB | 3,303 (4) | 721 (4) | 2,582 (4) | ||
|
| |||||
| Ascites | 83,348 | < 0.001 | |||
| Absent | 15,915 (19) | 2,781 (15) | 13,134 (21) | ||
| Slight | 41,210 (49) | 9,562 (50) | 31,648 (49) | ||
| Moderate or more | 26,223 (32) | 6,768 (35) | 19,455 (30) | ||
|
| |||||
| Spontaneous bacterial peritonitis | 80,488 | 5,758 (7) | 1,819 (10) | 3,939 (6) | < 0.001 |
|
| |||||
| Encephalopathy | 83,348 | < 0.001 | |||
| None | 25,759 (31) | 5,077 (27) | 20,682 (32) | ||
| Grade 1–2 | 45,699 (55) | 11,176 (58) | 34,523 (54) | ||
| Grade 3–4 | 11,890 (14) | 2,858 (15) | 9,032 (14) | ||
|
| |||||
| TIPSS | 80,509 | 6,766 (8) | 2,019 (11) | 4,747 (7) | < 0.001 |
|
| |||||
| Portal vein thrombosis | 80,091 | 3,445 (4) | 709 (4) | 2,736 (4) | < 0.001 |
|
| |||||
| Diabetes | 82,000 | 21,303 (26) | 3,572 (19) | 17,731 (28) | < 0.001 |
|
| |||||
| Prior malignancy | 80,447 | 4,993 (6) | 760 (4) | 4,233 (7) | < 0.001 |
|
| |||||
| MELD at listing | 83,348 | < 0.001 | |||
| < 15 | 31,209 (37) | 5,514 (29) | 25,695 (40) | ||
| 15–24 | 33,998 (41) | 8,118 (42) | 25,880 (40) | ||
| 25–34 | 11,519 (14) | 3,521 (19) | 7,998 (12) | ||
| ≥ 35 | 6,622 (8) | 1,958 (10) | 4,664 (8) | ||
|
| |||||
| Median MELD | 83,348 | 17 (13–23) | 18 (14–26) | 16 (12–22) | < 0.001 |
|
| |||||
| Region for listing, by grouping of wait-time | 83,348 | < 0.001 | |||
| Short | 26,620 (32) | 5,283 (28) | 21,337 (33) | ||
| Moderate | 32,220 (39) | 8,003 (42) | 24,217 (38) | ||
| Long | 24,508 (29) | 5,825 (30) | 18,683 (29) | ||
|
| |||||
| Listing cohort | 83,348 | < 0.001 | |||
| 2002–2006 | 27,324 (33) | 5,148 (27) | 22,176 (34) | ||
| 2007–2011 | 27,423 (33) | 5,694 (30) | 21,729 (34) | ||
| 2012–2016 | 28,601 (34) | 8,269 (43) | 20,332 (32) | ||
Outcomes on the Waiting List
Of the 19,111 patients listed with ALD, 8,566 (44.8%) underwent LT, 3,321 (17.4%) died or became too sick for LT, 385 (2.0%) were removed for improvement, 2,557 (13.4%) were removed for unknown or other reasons and 4,282 (22.4%) were still awaiting LT at the end of the study period. Of the 64,237 patients listed for non-ALD indications, 28,984 (45.1%) underwent LT, 12,594 (19.6%) died or became too sick for LT, 1,023 (1.6%) were removed for improvement, 6,696 (10.4%) were removed for unknown or other reasons and 14,940 (23.2%) were still awaiting LT (Figure 1). The median (IQR) of follow-up time in the ALD and non-ALD groups were 142 days (23–636) and 180 days (33–666), respectively. The overall median (IQR) time to removal for too sick was 111 days (26–301) and was significantly shorter in ALD compared to non-ALD patients: 91 days (22–177) vs 117 days (27–307) (p <0.001). The overall median time to removal for improvement was 389.5 days (213–557) and was significantly longer in ALD compared to non-ALD patients: 435 days (308–595) vs 369 days (174–546) (p <0.001).
ALD and Wait-List Removal for Dying or Becoming Too Sick for Transplant
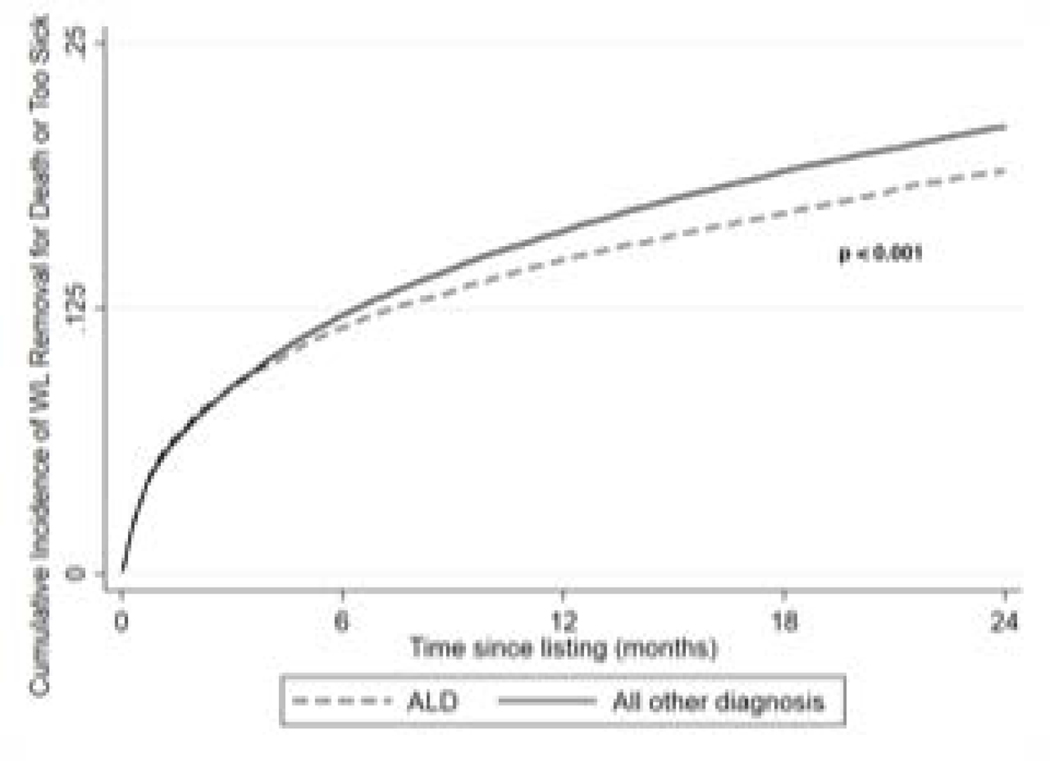
The cumulative probabilities of dying or becoming too sick for transplant within 6 months, 1 year and 2 years were 11.6% (95% CI 11.2–12.1%), 14.8% (95% CI 14.3–15.3%) and 19.0% (95% CI 18.4–19.6%) in patients listed for ALD and 12.2% (95% CI 11.9–12.5%), 16.1% (95% CI 15.8–16.4%) and 21.1% (95% CI 20.7–21.4%) (p < 0.001) in patients listed for non-ALD indications, respectively. (Figure 2)
Figure 2:
Unadjusted Cumulative Probably of Wait-List Removal for Death or Too Sick
In univariate competing risk analysis, the risk of wait-list removal for death or too sick was 11% lower for ALD than non-ALD (p < 0.001). Other recipient factors associated with wait-list removal for death/too sick were older age (p < 0.001), female sex (p < 0.001), other race (p < 0.001), regions of longer waiting time (p < 0.001), listed 2007–2011 or 2012–2016 versus 2002–2006 (p < 0.001), blood group O, A and B versus AB (p < 0.001), higher MELD at listing (p < 0.001), very low or very high BMI (p < 0.001), ascites (p < 0.001), SBP (p < 0.001), any grade of encephalopathy (p <0.001), prior malignancy (p<0.001) and diabetes (p < 0.001) (Table 2).
Table 2.
Univariate Competing Risk Analysis of Predictors of Wait-list Removal Due to Death or Too Sick
| Predictor variables | Sub HR (95 % CI) | P Value |
|---|---|---|
| ALD (vs non-ALD) | 0.89 (0.86–0.93) | < 0.001 |
|
| ||
| Age, yrs (vs 18–34) | ||
| 35–49 | 1.45 (1.32–1.61) | < 0.001 |
| 50–64 | 2.09 (1.89–2.31) | < 0.001 |
| 65 and older | 2.96 (2.66–2.27) | < 0.001 |
|
| ||
| Female Sex | 1.14 (1.10–1.17) | < 0.001 |
|
| ||
| Race (vs White) | ||
| Black | 1.04 (0.98–1.110) | 0.22 |
| Other | 1.17 (1.13–1.22) | < 0.001 |
|
| ||
| BMI (vs 20–29) | ||
| Less than 20 | 1.41 (1.29–1.52) | < 0.001 |
| 30–34 | 0.99 (0.95–1.03) | 0.92 |
| 35–39 | 1.02 (0.97–1.07) | 0.35 |
| > or = 40 | 1.25 (1.17–1.34) | < 0.001 |
|
| ||
| Blood group (vs AB) | ||
| A | 1.80 (1.62–2.00) | < 0.001 |
| B | 1.49 (1.33–1.66) | < 0.001 |
| O | 1.82 (1.64–2.02) | < 0.001 |
|
| ||
| Ascites (Absent) | ||
| Slight | 1.49 (1.41–1.57) | < 0.001 |
| Moderate or more | 2.75 (2.61–2.89) | < 0.001 |
|
| ||
| Spontaneous bacterial peritonitis | 1.32 (1.25–1.40) | < 0.001 |
|
| ||
| Encephalopathy (vs none) | ||
| Grade 1–2 | 1.50 (1.43–1.56) | < 0.001 |
| Grade 3–4 | 5.00 (4.78–5.23) | < 0.001 |
|
| ||
| TIPSS | 0.97 (0.91–1.02) | 0.24 |
|
| ||
| Portal vein thrombosis | 1.04 (0.96–1.12) | 0.33 |
|
| ||
| Diabetes | 1.21 (1.17–1.26) | < 0.001 |
|
| ||
| Prior malignancy | 1.26 (1.18–1.34) | < 0.001 |
|
| ||
| MELD at listing (vs < 15) | ||
| 15–24 | 1.51 (1.45–1.56) | < 0.001 |
| 25–34 | 1.88 (1.79–1.98) | < 0.001 |
| ≥ 35 | 2.75 (2.75–2.91) | < 0.001 |
|
| ||
| Region for listing, by grouping of wait-time (vs low) | ||
| Moderate | 1.31 (1.26–1.36) | < 0.001 |
| Long | 1.49 (1.44–1.55) | < 0.001 |
|
| ||
| Cohort (vs 2002–2006) | ||
| 2007–2011 | 1.15 (1.11–1.19) | < 0.001 |
| 2012–2016 | 1.16 (1.12–1.21) | < 0.001 |
In a fully adjusted competing risk analysis (Table 3), patients with a diagnosis of ALD still had a 16% lower risk of WL removal for death/too sick compared to non-ALD patients (subhazard ratio (sHR) = 0.84, 95% CI:0.81–0.87, p <0.001). Other independent predictors of wait-list removal for death/too sick were older age (p < 0.001), female sex (p < 0.001), black or “other” race (p = 0.048 and p = 0.03), region of listing (p < 0.001), transplant era (2007–2011 vs 2002–2006, p = 0.004), blood group A, B and O (vs blood group AB, all p < 0.001), higher MELD at listing (p < 0.001), BMI less 20 (p < 0.001), presence of ascites (p < 0.001), SBP (p = 0.01), encephalopathy (p < 0.001) prior malignancy (p < 0.001), and diabetes (p < 0.001).
Table 3.
Multivariate Competing Risk Analysis of Predictors of Wait-list Removal Due to Death or Too Sick
| Predictor variables | Sub HR (95 % CI) | P Value |
|---|---|---|
| ALD (vs non-ALD) | 0.84 (0.81–0.87) | < 0.001 |
|
| ||
| Age (vs 18–34) | ||
| 35–49 | 1.40 (1.26–1.56) | < 0.001 |
| 50–64 | 1.96 (1.77–2.16) | < 0.001 |
| 65 and older | 2.68 (2.42–2.98) | < 0.001 |
|
| ||
| Female Sex | 1.10 (1.06–1.14) | < 0.001 |
|
| ||
| Race (vs White) | ||
| Black | 1.06 (1.00–1.13) | 0.048 |
| Other | 1.05 (1.00–1.09) | 0.03 |
|
| ||
| BMI (vs 20–29) | ||
| Less than 20 | 1.53 (1.40–1.67) | < 0.001 |
| 30–34 | 0.94 (0.91–0.98) | 0.003 |
| 35–39 | 0.94 (0.89–0.99) | 0.01 |
| > or = 40 | 1.12 (1.05–1.20) | < 0.001 |
|
| ||
| Blood group (vs AB) | ||
| A | 1.72 (1.55–1.92) | < 0.001 |
| B | 1.44 (1.28–1.61) | < 0.001 |
| O | 1.70 (1.53–1.89) | < 0.001 |
|
| ||
| Ascites (vs Absent) | ||
| Slight | 1.15 (1.09–1.22) | < 0.001 |
| Moderate or more | 1.35 (1.28–1.44) | < 0.001 |
|
| ||
| Spontaneous bacterial peritonitis | 1.08 (1.02–1.15) | 0.01 |
|
| ||
| Encephalopathy (vs none) | ||
| Grade 1–2 | 1.29 (1.23–1.35) | < 0.001 |
| Grade 3–4 | 3.63 (3.45–3.83) | < 0.001 |
|
| ||
| Diabetes | 1.10 (1.07–1.15) | < 0.001 |
|
| ||
| Prior malignancy | 1.15 (1.08–1.23) | < 0.001 |
|
| ||
| MELD at listing (vs < 15) | ||
| 15–24 | 1.42 (1.37–1.47) | < 0.001 |
| 25–34 | 1.53 (1.46–1.62) | < 0.001 |
| ≥ 35 | 1.88 (1.76–2.00) | < 0.001 |
|
| ||
| Region for listing, by grouping of wait-time (vs short) | ||
| Moderate | 1.34 (1.29–1.39) | < 0.001 |
| Long | 1.52 (1.45–1.58) | < 0.001 |
|
| ||
| Cohort (vs 2002–2006) | ||
| 2007–2011 | 1.06 (1.02–1.10) | 0.004 |
| 2012–2016 | 1.03 (0.99–1.08) | 0.14 |
ALD and Wait-List Removal for Improvement
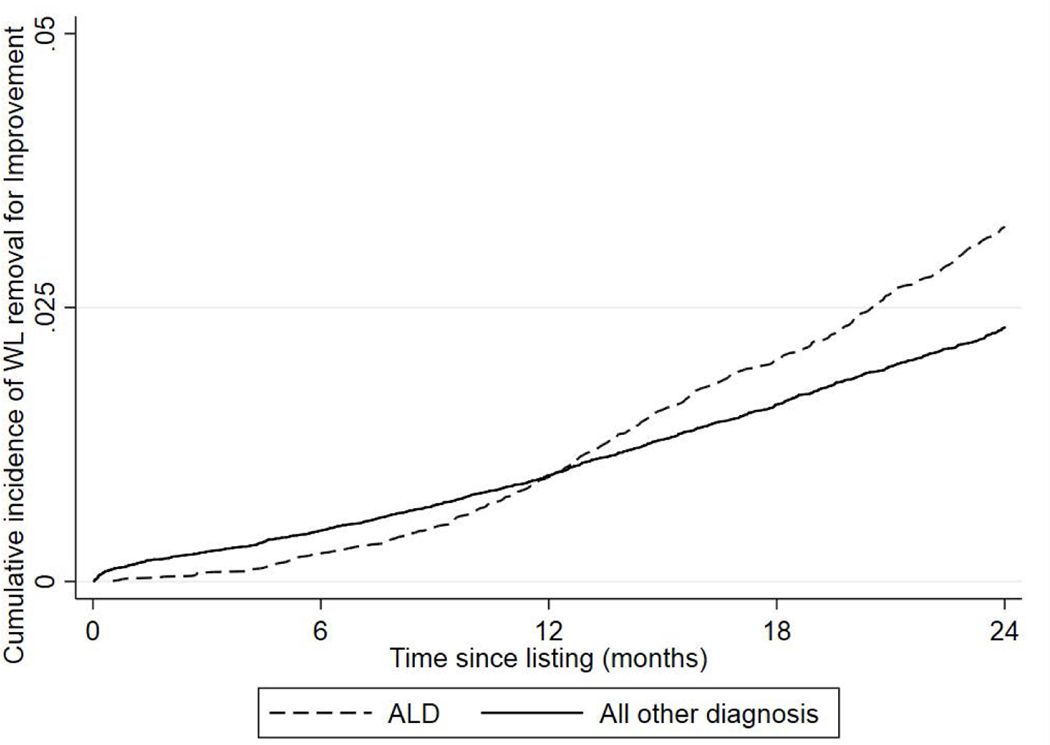
The cumulative probabilities of removal for improvement within 6 months, 1 year and 2 years were 0.26% (95% CI 0.19–0.35%), 0.96% (95% CI 0.81–1.13%) and 3.24% (95% CI 2.93–3.57%) in patients listed for ALD and 0.47% (95% CI 0.41–0.52%), 0.97% (95% CI 0.89–1.05%) and 2.32% (95% CI 2.18–2.46%) (p < 0.001) in patients listed for non-ALD indications, respectively.
In univariate competing risks analysis of factors associated with wait-list removal for improvement, ALD was associated with a non-constant risk over time. Within 6 months, the risk of removal for improvement was 13% lower for ALD vs non-ALD (p = 0.11); within one year, the risk of removal was 22% higher for ALD vs non-ALD (p < 0.001); and within 2 years, the risk was 139% higher (p < 0.001) for ALD vs non-ALD (figure 3). Other recipient factors associated with wait-list removal for improvement were younger age (p < 0.001), female sex (p < 0.001), BMI less than 20 (p < 0.001), blood group O, A and B versus AB (p = 0.06, 0.04, 0.03, respectively), absence of ascites, SBP or hepatic encephalopathy (all p < 0.001), absence of diabetes (p < 0.001), lower MELD at listing (p < 0.001), regions of shorter vs longer waiting time (p < 0.001) and listed 2007–2011 or 2012–2016 versus 2002–2006 (p < 0.001).
Figure 3:
Unadjusted Cumulative Probably of Wait-List Removal for Improvement
In a multivariate competing risks analysis of factors, ALD was associated with a 191% higher rate of removal for improvement than for patients without ALD within 2 years of listing (sHR 2.91 CI 2.35–3.61, p < 0.001). Other independent predictors of wait-list removal for improvement were younger age (p < 0.001), female sex (p < 0.001), BMI less 20 (p < 0.001), blood group O, A and B versus AB (p = 0.04, 0.03, 0.03, respectively), absence of ascites or hepatic encephalopathy (all p < 0.001), absence of diabetes (p = 0.007), lower MELD at listing (all categories p < 0.05), regions of shorter waiting time (all p < 0.05) and listed 2007–2011 or 2012–2016 versus 2002–2006 (p < 0.001).
Sex Differences in Wait-List Outcomes Among Patients with ALD
The cumulative probabilities of WL removal for death/too sick for men versus women with ALD were 19.0% (95% CI 18.3–19.6%) vs. 19.2% (95% CI 18.0–20.4%) at 2 years (p = 0.60) and for WL removal for improvement for men versus women with ALD were 2.8% (95% CI 2.5–3.2%) vs. 4.5% (95% CI 3.8–5.3%) at 2 years (p < 0.001). In adjusted models limited to patients with ALD, the risk of wait-list removal for death/too sick was 9% higher for women than for men (sHR 1.09 CI 1.00–1.18, p 0.045) and the risk for removal for improvement was 24% higher for women than for men (sHR 1.24 CI 1.00–1.54, p = 0.05) in fully adjusted models (Supplemental Tables 1 and 2).
DISCUSSION
Compared to patients with non-ALD indications for LT, patients with ALD have a 16% lower risk of wait-list removal for death or becoming too sick for LT and a 191% higher risk of wait-list removal for improvement within the first two years after listing. Collectively, these results suggest that selection criteria for LT are identifying ALD patients with lower risk of adverse outcomes on the waiting list. We hypothesize that a mandatory period of alcohol abstinence, whether 6-months or a lesser duration, likely allows more ALD patients to achieve improved liver function and recompensation while on the waiting list. The change in risk of wait-list removal for improvement over time supports this assertion, with rates not significantly different for ALD versus non-ALD in the first 6 months but substantially higher for ALD beyond a year after listing. While this highlights a favorable natural history for listed patients with ALD, the difference in WL deaths between ALD and non-ALD might suggest that current selection criteria are too restrictive, with ALD patients who might derive benefit from LT not being listed.
ALD is the primary diagnosis in approximately 30% of all those wait-listed for LT (28), but with an upward trend noted in our analysis (43% in the 2012–2016 cohort). A recent review on ALD in the US predicted that this proportion would increase in the next decade, due to the increased rates of alcohol consumption and the decreased need for LT among those with hepatitis C (29). Additionally, changing attitudes towards LT for ALD may be contributing, especially considering the positive results of early LT for acute alcoholic hepatitis without a mandated sobriety period (12). It is estimated that around 13,000 patients die from alcohol-related liver disease annually in the United States (30), with increasing deaths among those under the age of 40 years; yet only ~2000 annually undergo LT for this indication. This suggests there is a larger pool of patients who may benefit from LT. As duration of abstinence is deemphasized in favor of broader psychosocial profiling, the characteristics of ALD patients on the waiting-list may change and, in turn, influence WL outcomes (31, 32). Clearly as the indication for LT among patients with ALD expands (33), continued evaluation of the wait-list outcomes will be important. However, one of the potential concerns of expanding criteria for LT in ALD patients is the risk of worsening post-transplant outcomes. While this study suggests that selection criteria for LT should be expanded for patients with ALD, both the waiting list and the post-transplant outcomes need to be evaluated in future studies.
Sex differences in wait-list outcomes are well recognized (22–24) but not previously examined in the context of ALD patients. Fewer women were listed for LT and while this may simply reflect disease burden in the population, it is important to consider whether barriers to listing may differ by sex. While men drink more alcohol, drink more frequently and are more likely to be hazardous drinkers than women, women who drink alcohol are more likely to develop liver problems than men who drink alcohol (34). Moreover, one study showed that women were less likely to receive alcohol-related services in their lifetime, raising the question of whether stigmatization may lead to delays in women acknowledging their alcohol dependency, being diagnosed and accessing alcohol treatment services (35). The disparity in wait-list survival for women is well recognized and largely attributed to differential weighting of renal dysfunction by MELD in women versus men (22–24). Short stature, has also been shown to be associated with lower rates of transplant and higher WL mortality. In our analysis of ALD patients, women still had an ~10% higher risk of delisting for clinical deterioration even after adjustment for MELD and height, suggesting other factors contribute to the sex disparity. Interestingly, we also show that women with ALD were significantly more likely than men to achieve sufficient improvement to allow delisting. The reasons for greater improvement in women are unknown, but sex differences in the distribution of unmeasured cofactors for cirrhosis progression (e.g. obesity, diabetes, caffeine use) or adherence to medical regimens may be relevant factors.
Our study has some limitations, primarily related to the use of registry data. Potential sources of imprecision are the categorization of reason for removal, as some patients were removed from the wait-list for “other” reasons, without more details available. However, the large, representative nature of the data, the ability to adjust for multiple known confounders, and the use of competing risk approach are significant strengths. Moreover, there is no a priori reason that reporting of wait-list events would differ based on etiology of cirrhosis, so a differential bias is unlikely. The emergence of LT for acute alcoholic hepatitis is apparent, especially over the past 5 years, coinciding with the landmark study from Mathurin and colleagues regarding the excellent short-term outcomes of select patients with severe acute alcoholic hepatitis unresponsive to corticosteroids (4). We excluded these patients as we were specifically interested in wait-list outcomes among those with decompensated cirrhosis. Of note, this accounted for a small percentage of total transplants for ALD and the association between ALD (versus non-ALD) and wait-list outcomes was not significantly changed by their exclusion. Additionally, our study evaluated era effects, to capture the changes over time in UNOS registrants. This study is also limited by the lack of information about alcohol intake before listing and while on the waiting-list. However, this study was done in an era where mandated periods of abstinence were used by most transplant programs. We do not have direct data on whether this rule was adhered to by all centers.
In conclusion, patients with ALD have a lower risk of wait-list removal and higher likelihood of removal for improvement than patients with other causes of liver disease. This highlights the likely benefits of alcohol abstinence on the outcome of ALD and the potential to avoid LT in a proportion of wait-listed patients. However, this also suggests there is room to expand the criteria for LT among those with ALD to better capture patients who would die in the absence of LT.
Supplementary Material
Table 4.
Univariate Competing Risk Analysis of Predictors of Wait-list Removal Due to Improvement
| Predictor variables | Sub HR (95 % CI) | p |
|---|---|---|
| ALD (vs non-ALD) | ||
| At 6 months after listing* | 0.87 (0.74–1.03) | 0.11 |
| At 1 year after listing* | 1.22 (1.08–1.38) | < 0.001 |
| At 2 years after listing* | 2.39 (1.94–2.95) | < 0.001 |
|
| ||
| Age, yrs (vs 18–34) | ||
| 35–49 | 0.46 (0.39–0.55) | < 0.001 |
| 50–64 | 0.34 (0.29–0.41) | < 0.001 |
| 65 and older | 0.52 (0.42–0.64) | < 0.001 |
|
| ||
| Female Sex | 1.38 (1.25–1.53) | < 0.001 |
|
| ||
| Race (vs White) | ||
| Black | 1.12 (0.96–1.38) | 0.12 |
| Other | 0.91 (0.79–1.04) | 0.20 |
|
| ||
| BMI (vs 20–29) | ||
| Less than 20 | 1.82 (1.44–2.30) | < 0.001 |
| 30–34 | 0.61 (0.53–0.70) | < 0.001 |
| 35–39 | 0.65 (0.53–0.78) | < 0.001 |
| > or = 40 | 0.62 (0.46–0.82) | 0.001 |
|
| ||
| Blood group (vs AB) | ||
| A | 1.38 (1.01–1.89) | 0.04 |
| B | 1.46 (1.05–2.05) | 0.03 |
| O | 1.35 (0.99–1.84) | 0.06 |
|
| ||
| Ascites (Absent) | ||
| Slight | 0.24 (0.22–0.27) | < 0.001 |
| Moderate or more | 0.07 (0.05–0.09) | < 0.001 |
|
| ||
| Spontaneous bacterial peritonitis | 0.59 (0.45–0.78) | < 0.001 |
|
| ||
| Encephalopathy (vs none) | ||
| Grade 1–2 | 0.22 (0.20–0.25) | < 0.001 |
| Grade 3–4 | 0.04 (0.03–0.07) | < 0.001 |
|
| ||
| TIPSS | 0.89 (0.73–1.08) | 0.24 |
|
| ||
| Portal vein thrombosis | 0.82 (0.61–1.10) | 0.19 |
|
| ||
| Diabetes | 0.66 (0.58–0.76) | < 0.001 |
|
| ||
| Prior malignancy | 1.10 (0.88–1.37) | 0.38 |
|
| ||
| MELD at listing (vs < 15) | ||
| 15–24 | 0.54 (0.48–0.61) | < 0.001 |
| 25–34 | 0.53 (0.44–0.64) | < 0.001 |
| ≥ 35 | 0.42 (0.31–0.56) | < 0.001 |
|
| ||
| Region for listing, by grouping of wait-time (vs low) | ||
| Moderate | 1.01 (0.90–1.15) | 0.79 |
| Long | 0.84 (0.73–0.96) | 0.01 |
|
| ||
| Cohort (vs 2002–2006) | ||
| 2007–2011 | 1.98 (1.70–2.29) | < 0.001 |
| 2012–2016 | 2.75 (2.38–3.17) | < 0.001 |
Significant interaction between alcoholic liver disease and time since listing was identified (p < 0.001). Therefore, subhazard ratios are reported at 6 months, 1 year, and 2 years after listing.
Table 5.
Multivariate Competing Risk Analysis of Predictors of Wait-list Removal for Improvement
| Predictor variables | Sub HR (95 % CI) | P Value |
|---|---|---|
| ALD (vs non-ALD) | ||
| At 6 months after listing* | 1.06 (0.89–1.26) | 0.496 |
| At 1 year after listing* | 1.49 (1.30–1.69) | < 0.001 |
| At 2 years after listing* | 2.91 (2.35–3.61) | < 0.001 |
|
| ||
| Age (vs 18–34) | ||
| 35–49 | 0.70 (0.58–0.84) | < 0.001 |
| 50–64 | 0.57 (0.48–0.68) | < 0.001 |
| 65 and older | 0.84 (0.68–1.03) | 0.10 |
|
| ||
| Female Sex | 1.33 (1.19–1.47) | < 0.001 |
|
| ||
| BMI (vs 20–29) | ||
| Less than 20 | 1.30 (1.02–1.65) | 0.04 |
| 30–34 | 0.73 (0.63–0.84) | < 0.001 |
| 35–39 | 0.81 (0.67–0.98) | 0.03 |
| > or = 40 | 0.79 (0.59–1.06) | 0.11 |
|
| ||
| Blood group (vs AB) | ||
| A | 1.41 (1.03–1.94) | 0.03 |
| B | 1.45 (1.03–2.03) | 0.03 |
| O | 1.40 (1.02–1.92) | 0.04 |
|
| ||
| Ascites (vs Absent) | ||
| Slight | 0.43 (0.37–0.48) | < 0.001 |
| Moderate or more | 0.15 (0.12–0.20) | < 0.001 |
|
| ||
| Spontaneous bacterial peritonitis | 0.84 (0.64–1.11) | 0.23 |
|
| ||
| Encephalopathy (vs none) | ||
| Grade 1–2 | 0.42 (0.36–0.48) | < 0.001 |
| Grade 3–4 | 0.13 (0.07–0.22) | < 0.001 |
|
| ||
| Diabetes | 0.83 (0.72–0.95) | 0.007 |
|
| ||
| MELD at listing (vs < 15) | ||
| 15–24 | 0.62 (0.54–0.70) | < 0.001 |
| 25–34 | 0.74 (0.60–0.92) | 0.006 |
| ≥ 35 | 0.72 (0.53–0.99) | 0.04 |
|
| ||
| Region for listing, by grouping of wait-time (vs short) | ||
| Moderate | 0.84 (0.74–0.95) | 0.007 |
| Long | 0.64 (0.55–0.74) | < 0.001 |
|
| ||
| Cohort (vs 2002–2006) | ||
| 2007–2011 | 2.12 (1.82–2.47) | < 0.001 |
| 2012–2016 | 3.07 (2.64–3.57) | < 0.001 |
Significant interaction between alcoholic liver disease and time since listing was identified (p < 0.001). Therefore, subhazard ratios are reported at 6 months, 1 year, and 2 years after listing.
Acknowledgments
Financial Support: This research was supported in part by the UCSF Liver Center (NIDDK grant number P30 DK026743).
List of Abbreviations:
- ALD
Alcohol-associated liver disease
- LT
Liver transplantation
- WL
Wait list
- UNOS/OPTN
United Network for Organ Sharing/Organ Procurement and Transplantation Network
- SBP
Spontaneous bacterial peritonitis
- TIPS
Transjugular intrahepatic porto-systemic shunt
References
- 1.Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Harper AM, Wainright JL, et al. OPTN/SRTR 2016 Annual Data Report: Liver. Am J Transplant 2018;18 Suppl 1:172–253. [DOI] [PubMed] [Google Scholar]
- 2.Belli LS, Perricone G, Adam R, Cortesi PA, Strazzabosco M, Facchetti R, Karam V, et al. Impact of DAAs on liver transplantation: Major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol 2018;69:810–817. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Institute for Health Information. CORR Annual Statistics, 2007 to 2016. In; 2016. [Google Scholar]
- 4.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365:1790–1800. [DOI] [PubMed] [Google Scholar]
- 5.Lee BP, Chen PH, Haugen C, Hernaez R, Gurakar A, Philosophe B, Dagher N, et al. Three-year Results of a Pilot Program in Early Liver Transplantation for Severe Alcoholic Hepatitis. Ann Surg 2017;265:20–29. [DOI] [PubMed] [Google Scholar]
- 6.Lee BP, Mehta N, Platt L, Gurakar A, Rice JP, Lucey MR, Im GY, et al. Outcomes of Early Liver Transplantation for Patients With Severe Alcoholic Hepatitis. Gastroenterology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schenker S, Perkins HS, Sorrell MF. Should patients with end-stage alcoholic liver disease have a new liver? Hepatology 1990;11:314–319. [DOI] [PubMed] [Google Scholar]
- 8.Stroh G, Rosell T, Dong F, Forster J. Early liver transplantation for patients with acute alcoholic hepatitis: public views and the effects on organ donation. Am J Transplant 2015;15:1598–1604. [DOI] [PubMed] [Google Scholar]
- 9.Stefanini GF, Biselli M, Grazi GL, Iovine E, Moscatello MR, Marsigli L, Foschi FG, et al. Orthotopic liver transplantation for alcoholic liver disease: rates of survival, complications and relapse. Hepatogastroenterology 1997;44:1356–1359. [PubMed] [Google Scholar]
- 10.Berlakovich GA, Steininger R, Herbst F, Barlan M, Mittlbock M, Muhlbacher F. Efficacy of liver transplantation for alcoholic cirrhosis with respect to recidivism and compliance. Transplantation 1994;58:560–565. [DOI] [PubMed] [Google Scholar]
- 11.Bellamy CO, DiMartini AM, Ruppert K, Jain A, Dodson F, Torbenson M, Starzl TE, et al. Liver transplantation for alcoholic cirrhosis: long term follow-up and impact of disease recurrence. Transplantation 2001;72:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee BP, Vittinghoff E, Dodge JL, Cullaro G, Terrault NA. National Trends and Long-term Outcomes of Liver Transplant for Alcohol-Associated Liver Disease in the United States. JAMA Intern Med 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burra P, Senzolo M, Adam R, Delvart V, Karam V, Germani G, Neuberger J, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant 2010;10:138–148. [DOI] [PubMed] [Google Scholar]
- 14.Everhart JE, Beresford TP. Liver transplantation for alcoholic liver disease: a survey of transplantation programs in the United States. Liver Transpl Surg 1997;3:220–226. [DOI] [PubMed] [Google Scholar]
- 15.Bangaru S, Pedersen MR, MacConmara MP, Singal AG, Mufti AR. Survey of Liver Transplantation Practices for Severe Acute Alcoholic Hepatitis. Liver Transpl 2018;24:1357–1362. [DOI] [PubMed] [Google Scholar]
- 16.Brown RS Jr. Transplantation for alcoholic hepatitis--time to rethink the 6-month “rule”. N Engl J Med 2011;365:1836–1838. [DOI] [PubMed] [Google Scholar]
- 17.Kotlyar DS, Burke A, Campbell MS, Weinrieb RM. A critical review of candidacy for orthotopic liver transplantation in alcoholic liver disease. Am J Gastroenterol 2008;103:734–743; quiz 744. [DOI] [PubMed] [Google Scholar]
- 18.Dew MA, DiMartini AF, Steel J, De Vito Dabbs A, Myaskovsky L, Unruh M, Greenhouse J. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl 2008;14:159–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant 2006;6:1416–1421. [DOI] [PubMed] [Google Scholar]
- 20.Lucey MR, Schaubel DE, Guidinger MK, Tome S, Merion RM. Effect of alcoholic liver disease and hepatitis C infection on waiting list and posttransplant mortality and transplant survival benefit. Hepatology 2009;50:400–406. [DOI] [PubMed] [Google Scholar]
- 21.Kim WR, Therneau TM, Benson JT, Kremers WK, Rosen CB, Gores GJ, Dickson ER. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 2006;43:345–351. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol 2015;62:946–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant 2010;10:2658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation 2018;102:1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:911–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orrego H, Blake JE, Blendis LM, Medline A. Prognosis of alcoholic cirrhosis in the presence and absence of alcoholic hepatitis. Gastroenterology 1987;92:208–214. [DOI] [PubMed] [Google Scholar]
- 27.Mathurin P, O’Grady J, Carithers RL, Phillips M, Louvet A, Mendenhall CL, Ramond MJ, et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut 2011;60:255–260. [DOI] [PubMed] [Google Scholar]
- 28.Kim WR, Lake JR, Smith JM, Skeans MA, Schladt DP, Edwards EB, Harper AM, et al. OPTN/SRTR 2015 Annual Data Report: Liver. Am J Transplant 2017;17 Suppl 1:174–251. [DOI] [PubMed] [Google Scholar]
- 29.Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, McCullough AJ, et al. Clinical impact of alcohol-related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcohol Clin Exp Res 2015;39:2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC). Alcohol and Public Health: Alcohol-Related Disease Impact (ARDI). Average for United States 2006–2010 Alcohol-Attributable Deaths Due to Excessive Alcohol Use. In. [Google Scholar]
- 31.Kollmann D, Rasoul-Rockenschaub S, Steiner I, Freundorfer E, Gyori GP, Silberhumer G, Soliman T, et al. Good outcome after liver transplantation for ALD without a 6 months abstinence rule prior to transplantation including post-transplant CDT monitoring for alcohol relapse assessment - a retrospective study. Transpl Int 2016;29:559–567. [DOI] [PubMed] [Google Scholar]
- 32.Addolorato G, Mirijello A, Leggio L, Ferrulli A, D’Angelo C, Vassallo G, Cossari A, et al. Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res 2013;37:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bangaru S, Pedersen M, Singal AG, Mufti A. Survey of Liver Transplantation Practices for Severe Acute Alcoholic Hepatitis. Liver Transpl 2018. [DOI] [PubMed] [Google Scholar]
- 34.Giard JM, Terrault NA. Women with Cirrhosis: Prevalence, Natural History, and Management. Gastroenterol Clin North Am 2016;45:345–358. [DOI] [PubMed] [Google Scholar]
- 35.Alvanzo AA, Storr CL, Mojtabai R, Green KM, Pacek LR, La Flair LN, Cullen BA, et al. Gender and race/ethnicity differences for initiation of alcohol-related service use among persons with alcohol dependence. Drug Alcohol Depend 2014;140:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.