Abstract
Objective:
To quantitatively synthesize extant literature on perceived triggers of primary headache disorders.
Methods:
A meta-analytic review of headache trigger survey studies was conducted. Endorsement rates, assessment method, and headache and sample characteristics were extracted from included articles. Separate random-effects models were used to assess trigger endorsement rates and post-hoc meta-regressions examined potential moderator variables.
Results:
85 articles from 1958 to 2015 were included, involving 27,122 participants and querying 420 unique triggers (collapsed into 15 categories). Four-fifths (0.81; 95% CI .75 to .86) of individuals with migraine or tension-type headache endorsed at least one trigger. Rates increased with the number of categories queried (OR: 1.18, 1.08–1.30) and year of publication (OR: 1.04, 1.00–1.08). The triggers most commonly endorsed were stress (.58, .53–.63) and sleep (.41, .36–.47).
Conclusions:
Extreme heterogeneity characterizes the headache trigger literature. Most individuals with a primary headache disorder perceive their attacks to be triggered by one or more precipitants, the most common of which are stress and sleep. However, trigger endorsement is influenced by method of assessment. Enhancing methodological consistency and prioritizing experimental studies would improve our understanding of headache triggers.
Keywords: Headache triggers, migraine, tension-type headache
Introduction
The primary headache disorders of migraine and tension-type headache (TTH) affect 12–15% (1–3) and 38–42% (1,4,5) of Americans annually, respectively. Headache is the fourth most common reason for emergency department visits (2) and among the top 10 causes of years lived with disability worldwide (5,6). Despite their prevalence and burden, headache disorders remain undiagnosed or inadequately treated among most who suffer from them (7,8). In part, this state of affairs stems from challenges in pinpointing the complex pathophysiologic mechanisms of primary headache disorders and in delivering targeted interventions.
Migraine, for instance, is conceptualized as originating within a hypersensitive central nervous system that has difficulty modulating responses to common sensory stimuli (9). Within this framework, advances in headache pathophysiology and treatment development could be spurred by an improved understanding of the environmental and physiological stimuli that may precipitate individual headache attacks. Most individuals with headache report having at least one such “trigger” of their attacks (10), defined as any factor that leads to headache upon exposure or withdrawal (11), and advising patients to identify and subsequently avoid their likely triggers has for decades been a mainstay of clinical headache management.
The study of headache triggers is fraught with complications, foremost of which is the considerable variability of individual trigger effects that often precludes establishment of clear cause-effect relationships. No single stimulus serves as a trigger for all patients, and within a single individual rarely does exposure to an identified precipitant always provoke headache (12). Although study designs involving experimental manipulation of triggers are ideal, satisfying the numerous assumptions required for establishing causality of headache triggers is both rare and often unfeasible (13). As a result, self-report remains the most common method of trigger assessment, but existing studies vary widely in triggers examined, methods of assessment, and sample composition.
Although research on perceived triggers of headache disorders is extensive, to date there has been no meta-analytic review of this literature. We endeavored to conduct a quantitative synthesis of this literature to provide an estimate of their population-level effects and to identify moderator variables that influence perceptions of headache triggers. We hypothesized that the most prevalent perceived triggers would be those related to stress, sleep, hormones, and diet, and that trigger endorsement would be influenced by method of assessment (list vs. spontaneous recall), primary headache diagnosis, and gender.
Methods
This study adhered to PRISMA reporting guidelines (14). Institutional Review Board approval was not necessary as this was a quantitative review of previously published data.
Search strategy
On June 18, 2015, two of the authors (ABWP, REDM) conducted a PubMed database search of articles in English utilizing the search terms “migraine OR headache” AND “trigger OR precipitant” as keywords, that involved humans, and that were published from November 1958 to 18 June, 2015. Titles and abstracts were then reviewed for eligibility independently by these authors, and bibliographies of both retained articles and prior qualitative reviews were searched. These authors conducted full-text review of candidate articles independently, and questions or disagreements were resolved through discussion.
Eligibility criteria
Broad inclusion criteria for articles published in English were used to maximize sensitivity and capture all relevant data at screening: a) utilization of a retrospective/survey study design b) among individuals with migraine, TTH, or cluster headache from population or clinical samples that c) quantified endorsement rates of one or more headache triggers. We focused on the primary headache disorders of migraine and TTH, given their high prevalence, but also included cluster headache given its episodic nature and incorporation into the trigger literature. Exclusion criteria were editorials, review or experimental articles, and case studies, as well as articles about triggers that used experimental manipulation or animals, focused on pathophysiology or treatment, or that focused on static variables (e.g. gender, race/ethnicity in relation to headache variables other than triggers).
Data collection
Items:
Data extracted from retained articles were entered into a data extraction template that included a) publication metadata (authors, year of publication, journal); b) sample demographics (sample size, population drawn from, child vs. adult, mean age, age range, percentage female); c) headache characteristics (diagnostic criteria used, headache diagnoses, headache intensity, headache frequency [days/month or attacks/month]); d) method of trigger assessment (open-ended query vs. provided list of triggers); and e) triggers endorsed (mean number of reported triggers, range of reported triggers, proportion reporting any assessed trigger [using the trigger terminology specified by the authors]).
Description:
Because demographic data were not uniformly available for all studies, medians were estimated when means were not reported, mean sample ages were estimated by weighting reported age categories by sample size or using reported grade levels, and sex distributions were calculated by cross-tabulating reported sex ratios by diagnostic groups. If triggers were not queried in a dichotomous (yes/no) manner but instead on a Likert-type rating scale (n = 2), those data were re-coded into dichotomous variables such that any frequency of endorsement above “rarely” was coded as a positive endorsement (e.g. “triggers attacks at least sometimes,” “triggers attacks occasionally”).
When not explicitly reported, it was assumed that the number of headache triggers assessed was precisely the number of triggers reported in the manuscript. Because study authors used numerous different terms to reference the same general type of trigger (e.g. smells/odors were variously termed “chemical smells,” “fumes,” “odors,” “foul smells” and so forth), analyzing each verbatim trigger with a separate meta-analysis was impractical. We thus resorted to collapsing each verbatim trigger into one of several larger categories based on thematic similarity. Assigning verbatim triggers to these categories was conducted via discussion and consensus among the three authors who did not previously extract study-level data (TTH, DPT, TAS).
When multiple triggers within a single category were assessed in the same study, the verbatim trigger with the highest proportion endorsement was retained. (For instance, if a study reported proportion of participants endorsing each of “glare,” “flicker,” and “bright light,” and “bright light” was the most frequently endorsed, then the proportion of participants endorsing “bright light” comprised the “Visual” category.) We chose this method of aggregation because it strikes a balance between being liberal (i.e. including only the highest proportion for related triggers within that category) and conservative (i.e. avoiding “double-counting” participants who endorsed more than one of these three verbatim items). Data entered for each study were double-checked for accuracy by two authors not involved in the original data extraction (TTH, TAS).
Risk of bias:
Due to the cross-sectional self-report nature of the designs assessed, a formal quality assessment was not conducted, as most aspects of study quality typically included in meta-analyses of controlled trials were not relevant (e.g. no randomization, no blinding, no treatment effects). Thus, to examine sources of variability in the observed meta-analytic effects, meta-regressions were used to assess effects as a function of demographic and study variables.
Statistical analyses
The summary outcome measure of interest was the weighted proportion of participants across all studies endorsing a particular trigger category, reported as the proportion with estimated 95% confidence intervals (95% CIs). We used random-effects models to address heterogeneity within and between studies, which was assessed with I2 indices. One random-effects model was used to estimate the proportion of individuals with headache who had any perceived trigger, and separate random-effects models were used to assess endorsement probability for each trigger category. Post-hoc meta-regressions were used to assess potential sources of heterogeneity and moderator variables. The models were conducted using random effects for Study ID and fixed effects for the contrasts of interest (e.g. headache diagnostic groups). We used R software to run all statistical analyses; statistical significance was set at p < .05. Acknowledging that statistical significance is often of less importance than effect sizes in studies utilizing very heterogeneous sources of data, no adjustments were made for multiple comparisons, but exact p-values are reported to allow interested readers to estimate post-hoc adjustments of the reported values.
Results
Study search and selection
Figure 1 depicts the PRISMA flow diagram from the initial literature search to the final retained studies. The initial PubMed search returned 1,065 articles. After title and abstract review, 144 relevant articles remained, all of which but three were obtained in full-text form. (These three could not be retrieved even after contacting the study authors.) Bibliography review of these studies and prior review articles yielded an additional 12 candidate articles. Of these 153 candidate studies, after comprehensive full-text review 68 were excluded, principally because they did not use a survey design or were not a trigger assessment study. This process culminated in retaining for the current meta-analysis 85 articles that employed a survey method of headache trigger assessment. Supplemental Table e1 presents summary data for each of the analyzed studies.
Figure 1.
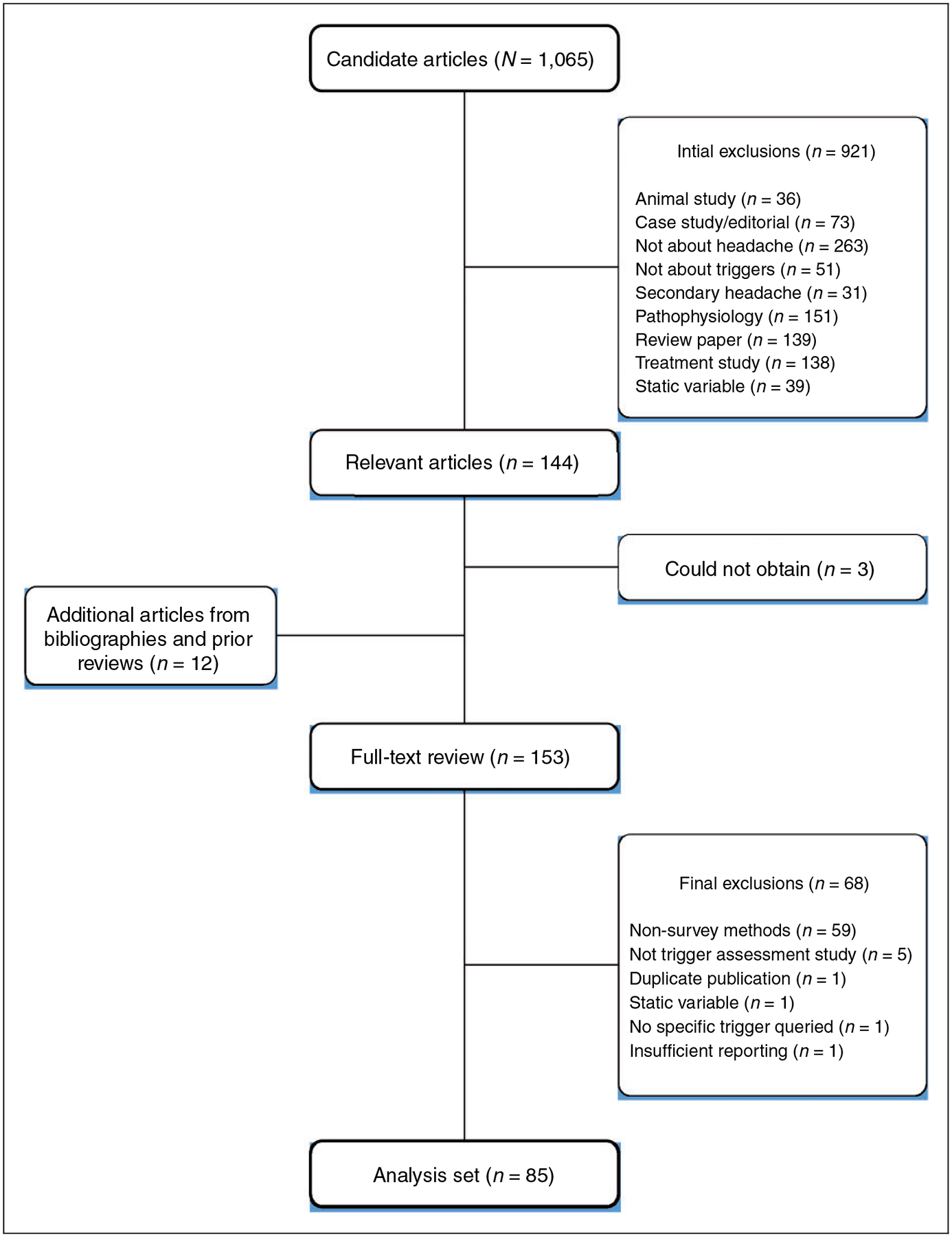
PRISMA flow diagram of literature review and study selection.
Data extraction
Extraction of trigger data yielded 420 unique verbatim triggers queried across the 85 articles and across 27,122 total participants. These 420 verbatim triggers were then collapsed into 15 categories as described above. Table 1 presents the verbatim terms used across all studies and the larger categories within which they were aggregated. The median number of studies assessing each category was 42 (IQR: 24 to 51), with a range of five (medications) to 57 (stress).
Table 1.
Trigger categories and component terms.
| Trigger category | Verbatim trigger terms (n = number of studies using exact term) | Studies assessing each category |
|---|---|---|
| Activity/exertion | Activity (2), arms over head (1), banging head (1), bending over (1), chewing (1), cough (2), coughing (1), dancing (1), defecation (1), diving (1), evacuation (1), excessive work (1), exercise (4), exertion (3), exhaustion (1), head motion (1), head/neck movements (2), lack of exercise (1), lifting of weight (1), lifting overhead (1), loud speaking (1), military related (combat, wearing headgear or helmet, wearing MOPP gear, riding in a vehicle, firing a weapon, and flying in an aircraft) (1), moving neck (1), muscle tension/posture (1), neck movement (1), neck position (1), physical (1), physical (physical exertion and strenuous activity) (1), physical activity (5), physical effort (2), physical exercise (1), physical exertion (2), physical exhaustion or traveling (1), physical inactivity (1), physical strain or exercise (18), posture (2), racket sports (1), running/cardio (1), sexual activity (12), sexual intercourse (1), singing (1), squatting (1), straining (2), strenuous physical activity (1), tension in neck muscles (1), yoga (1) | 46 |
| Alcohol | Alcohol (32), alcohol (white wine) (4), alcoholic drinks (red wine, white wine, sparkling wine, beer, etc.) (5), all alcohol (1), all alcohol (red wine, white wine, clear spirits) (1), beer (5), beer spirits (1), hard liquor (1), red wine (6), red wine (but not white wine or clear spirits) (1), red wine/cheese (1), sparkling wine (1), spirits (4), tequila only (of alcohol) (1), too much of any alcohol (2), wine (3), wine, beer, other alcohol (1) | 43 |
| Allergy/sinus | Allergies (5), pollen (1), pollution (2), season of year (1), seasonal variation (1), sneezing (1), sniffing (1) | 7 |
| Auditory | Acoustic (1), loud noise (1), noise (14), noise (environmental) (2), noise (loud speakers, loud music) (1), too loud music (1) | 19 |
| Emotion | Acute emotion (1), afferent stimulation (1), aggressiveness (2), anger or hot temper (3), annoyance (1), anxiety (1), argument (3), behavioral (being scolded) (1), behavioral (shouting) (1), changes in mood (1), crying (4), distress (1), emotion (2), emotion (violent) (1), emotional (fear, anger, or other) (1), emotional changes (2e (1), fear (1), feeling sad (1), intense emotional influences (3), irritation (1), low mood (1), negative affect (anxiety, anger, depression) (3), thinking about negative events (1), worries (1) | 26 |
| Food/Eating Habits | After eating (1), aspartame (3), binge eating (1), break from coffee (1), cabbages (1), caffeinated drinks (2), caffeine (5), carbohydrates (1), certain food (5), cheese (7), cheese (including yogurt) (2), Chinese food (1), chocolate (7), chocolate (including cakes) (4), chocolate/milk/groundnut (1), citric fruits (3), coffee (5), consumption related (fasting, certain foods, certain beverages, dehydration, and caffeine withdrawal) (1), dark chocolate (1), dehydration (3), diet (1), dietary (2), drinking pop (2), eating habits (1), eggs (1), fasting (5), fatty food (1), fish (1), food (17), food additives (2), food seasonings (1), fried foods (2), fruits and vegetables (1), full meals (1), honey mead (1), hot dogs (1), hunger (18), hunger (missing a meal) (1), ice cream (3), khat (Ethiopian leaf) (1), meat (1), milk (2), milk/cheese (1), missing meals (3), monosodium glutamate (2), MSG (2), not eating (2), not eating on time (1), nuts (2), other dietary (1), other sugar containing foodstuffs (1), others (foods) (1), pizza (1), religious fasting (1), salami (2), sausage (2), skipping meals (6), soft drinks (2), some kinds of food (2), specific foods/drinks (1), sweets (1), too few beverages (1), tropical fruit (1), unhealthy diet (1), water deprivation (2) | 53 |
| Hormones | After menstrual period (1), hormonal changes (1), hormonal replacement (1), hormone (4), menopause (2), menses (2), menstruation (30), menstruation or break from pill (1), oral contraceptives (6), ovulation (2), perimenstrually (1), pre menstrual period (1), pregnancy (3) | 38 |
| Medications | Drug intake (1), medication (3), some drugs (1) | 5 |
| Other | Associated disease (1), bathing (1), boredom (1), cervical problems (1), chance (1), change of rhythm (1), destiny (1), diverse (1), genetic predisposition (1), hair wash alone (2), hair wash and other (1), hair wash concurrent with others (1), head injury (3), head trauma (3), headgear (1), hot shower (1), infections (2), infectious disease (1), massage (1), muscle pain (1), neck pain (4), nitroglycerin (1), no fresh air (1), other (1), others (perfumes, head trauma, GI upset) (1), pain in other body parts (1), postanesthesia (1), prevalent diseases (1), problems with braces (1), reading or general thinking (3), reflux (1), sensorial stimuli (1), sickness (2), smoking/illegal drug use (1), tobacco (1), using a low pillow (1), Valsalva maneuver (1), various discomforts (1), wearing a hat (1), weekends (2), wet cold hair (1) | 25 |
| Sleep | Change in sleeping habits (3), changes in time of sleep (3), fatigue (15), fatigue related (fatigue, exhaustion, and sleep deprivation) (1), few hours sleep (1), insomnia (1), insufficient sleep (1), lack of sleep (11), napping (2), nocturnal sleeping (1), oversleep (5), poor sleep (1), prolonged sleep (1), sleep (5), sleep (weekends) (2), sleep deprivation (9), sleep disturbance (10), sleep pattern change (1), sleep problems (1), sleeping late (3), sleeplessness (2), tired (1), tiredness (2), too much sleep (7), too much/too little sleep (2), undersleeping (1) | 53 |
| Smell/odor | Chemical smells (1) cigarette smoke (7), fumes/heavy scents (1), gasoline (2), household cleaners (bleach or chemicals) (1), odor or foul smell (5), odors (2), odors (perfumes, smoke) (1), odour specific (candles with scent) (1), odour specific (car exhaust/exhaust fumes) (1), odour specific (cigarette smoke) (1), odour specific (coffee) (1), odour specific (detergent) (1), odour specific (flowers with strong scent) (1), odour specific (fried food) (1), odour specific (garlic) (1), odour specific (grilled food) (1), odour specific (horse) (1), odour specific (incense) (1), odour specific (mould) (1), odour specific (perfume) (2), odour specific (petrol) (1), odour specific (pineapple) (1), odour specific (sesame seed) (1), odour specific (shower gel) (1), odour specific (smoke) (1), odour specific (sweat and bad breath) (1), odour specific (vanilla) (1), olfactory (1), perfume odour (9), perfumes or colognes (1), smell (7), smell (cleanness product) (2), smell (fat) (2), smell (food) (2), smell (odour) (3), smoke (5), smoke (auto emission) (1), smoke (smoking cigarettes) (3), smoking or smell of smoking (8), strong odours (2) | 41 |
| Stress | Academic stress (1), acute stress (1), acute stress (during stress) (2), acute stress (following stress) (1), after stress is over (2), cessation of stress (1), conflict (3), conflict (another person) (1), during stress (1), emotional stress (2), feeling worried (1), mental stress (3), personal problems (1), poor lifestyle (1), post crisis letdown (1), problems with friends (1), problems with parents (1), problems with teachers (1), prolonged concentration (1), psychological (3), relaxation (3), relaxation after stress (2), stress (31), stress (affective) (1), stress (extracurricular activity related) (1), stress (mental effort) (1), stress (unspecified) (1), stress and mental tension (1), stress (anxiety) (3), stress at home (2), stress at work (1), stress related (1), stress unrelated to work (1), stress with family (1), stresses (bereavement) (1), stresses (family stress) (2), stresses (school stress) (2), stressful feeling (1), tension (1), too much schoolwork (1), tragic event (1), work related stress (1) | 57 |
| Travel | Altitude (1), car/bus journeys (other than school) (1), commuting (bus or car) (1), driving (3), flying/high altitude (1), frequent traveling (1), height (1), journey (1), long distance travel (1), travel (2), travel in airplane (1), travel in train/plane (1), trip (1), vacation (1) | 17 |
| Visual | Alternate light and shade (1), bright flashing light (1), bright light (8), colors (1), computer related (1), contrasting patterns (1), excessive light (1), exposure to sun (3), eye strain (1), eyesight problems (3), flashing/flickering light (1), flicker (1), fluorescent light (3), glare (4), hours in front PC/TV/video games (2), intense light (2), light/sun (1), lights (9), orange colors (1), other visual stimuli (3), prolonged computer use (1), road stripes or cones (1), sun clarity (1), sun exposure (1), sunlight (11), sunlight/bright light (2), too many hours reading (1), VDU screens/computer (1), video games (1), visual disturbance (flicker, glare, eyestrain) (1), visual disturbance and negative affect (1), visual effort (1), visual stimuli (bright light (other than sun)) (1), visual stimuli (recreation reading) (1), visual stimuli (school book reading) (1), visual stimuli (TV watching) (2), watching TV (1) | 47 |
| Weather/environment | Air condition of work (1), air conditioning of car (1), air conditioning of home (2), barometric pressure changes (1), barometric drops (1), change of environmental temperature (1), climatic (1), cold air conditioner (1), cold environment (1), cold weather (7), cold wind (2), crowded place (1), environmental (2), environmental (noise, light, sun, heat, cold, fumes, odor, smoke, and vibration) (1), environmental factors (1), environmental heat (1), heat (9), heat/cold/weather (1), hot and humid climate (1), hot weather (5), other weather variable (1), overheated (1), poor ventilation (1), rain (3), season of year (1), seasonal variation (2), suffocating atmosphere (1), summer heat (1), temperature (1), thermal (1), warm climate (1), weather (11), weather change (22), wind (6), wind draught (1) | 53 |
Endorsement of any headache trigger
For this analysis, 35 studies were identified that were published between 1980 and 2014. In these studies, 9,498 headache sufferers were surveyed concerning their perception that at least one factor induced their headaches (see Figure 2). Across studies, 6,999 participants reported at least one headache trigger, random effects model proportion: .81 (95% CI .75–.86). Substantial heterogeneity was observed across studies, τ2 = 0.93; I2 = 97.1% (96.6–97.6%).
Figure 2.
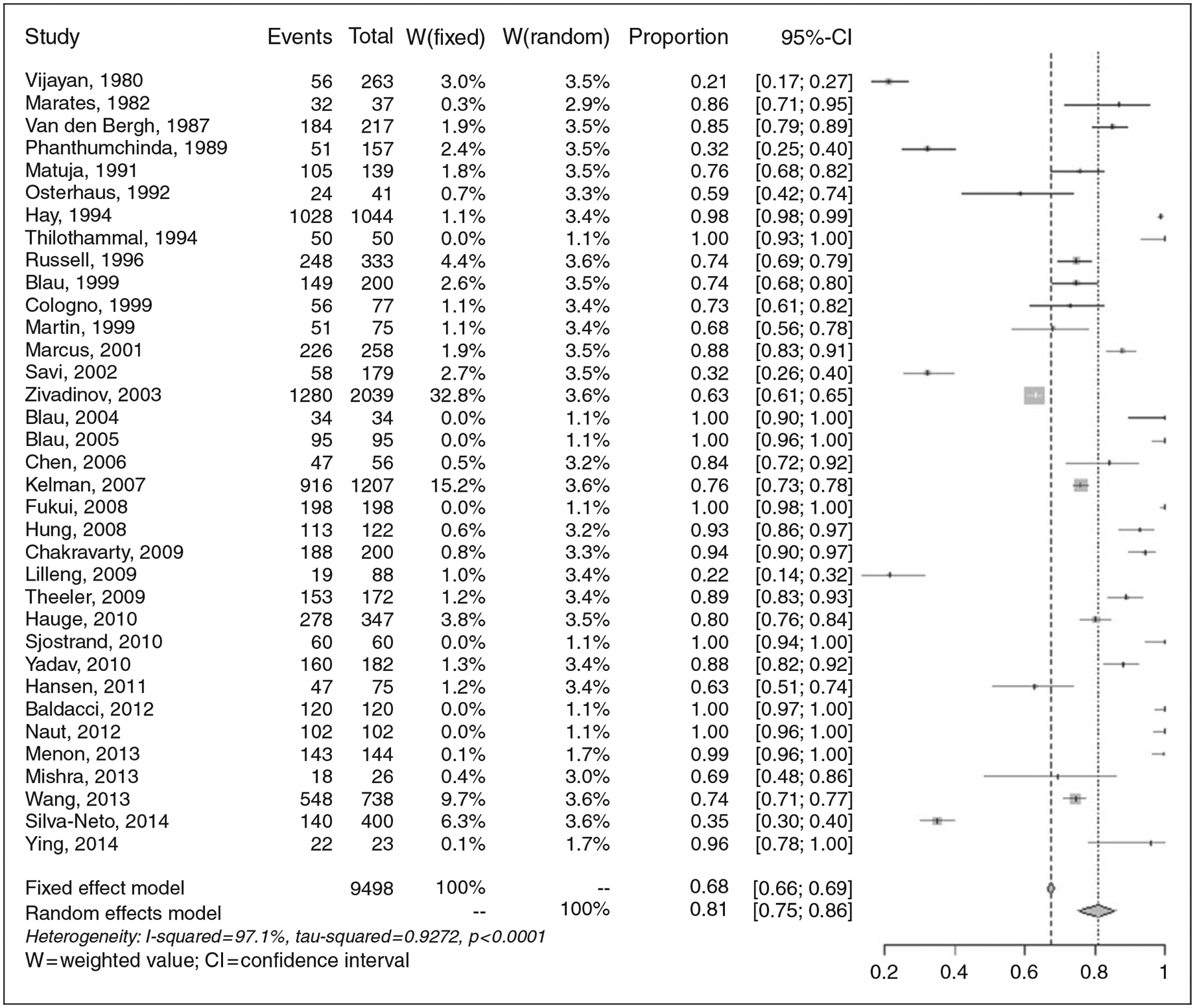
Endorsement rates of any headache trigger (n = 35 studies).
The number of presented trigger categories substantially impacted the proportion of participants within each study that reported any single trigger. For each additional trigger category that was presented to the respondent, the odds of endorsing any single trigger increased by 18%, OR: 1.18 (1.08–1.30), p = 0.0003 (see Supplemental Figure e1A). Interestingly, more recent study year was modestly associated with an increased perception of having any trigger, OR: 1.04 (1.00–1.08), p = 0.035 (see Supplemental Figure e1B). Neither the proportion of females in the sample (p = 0.238) nor the average age of respondents in the sample (p = 0.746) impacted the estimates. Four studies (n = 4) did not report how the triggers were queried to the participants (i.e. a formal list versus open-ended question), but for the 2/31 (6.5%) studies that utilized open-ended questions, a large though statistically non-significant reduction in trigger endorsement was observed, OR: 0.35 (0.08–1.53), p = 0.166.
Endorsement of trigger categories
Each of the 85 retained articles provided data sufficient for calculating the proportion of respondents endorsing at least one of the various headache trigger categories. The studies were published between 1980 and 2015. The triggers most commonly endorsed were stress (.58; .53–.63) and sleep (.41; .36–.47); those least commonly reported were travel (.11; .06–.19), allergy/sinus (.06; .02–.13), and medications (.02; .01–.08). Table 2 reports the results from the individual random effects models for each trigger category. Figure 3 plots endorsement rates for each of the 15 trigger categories for each study as a function of sample size. As is evident from the figure and table, a large amount of heterogeneity was apparent across studies, such that each trigger category had an I2 index ≥ .92. Several post hoc meta-regressions were used to assess potential sources of heterogeneity.
Table 2.
Individual trigger meta-analyses.
| Trigger category | Proportion | 95% CI | I2 | Studies | Total N |
|---|---|---|---|---|---|
| Stress | .58 | .53, .63 | 97 | 57 | 18219 |
| Sleep | .41 | .36, .47 | 97 | 53 | 17778 |
| Emotion | .33 | .26, .41 | 96 | 26 | 6110 |
| Weather/environment | .32 | .27, .39 | 98 | 53 | 18349 |
| Visual | .32 | .25, .39 | 98 | 47 | 14539 |
| Hormones | .29 | .25, .35 | 97 | 38 | 13592 |
| Food/eating habits | .27 | .22, .32 | 97 | 53 | 17142 |
| Smell/odor | .22 | .17, .27 | 98 | 41 | 14633 |
| Alcohol | .21 | .16, .26 | 98 | 43 | 12400 |
| Activity/exertion | .20 | .15, .27 | 98 | 46 | 13691 |
| Other | .20 | .14, .26 | 97 | 25 | 8219 |
| Auditory | .16 | .08, .31 | 98 | 19 | 5143 |
| Travel | .11 | .06, .19 | 98 | 17 | 5316 |
| Allergy/sinus | .06 | .02, .13 | 92 | 7 | 1842 |
| Medications | .02 | .01, .08 | 92 | 5 | 2010 |
Figure 3.
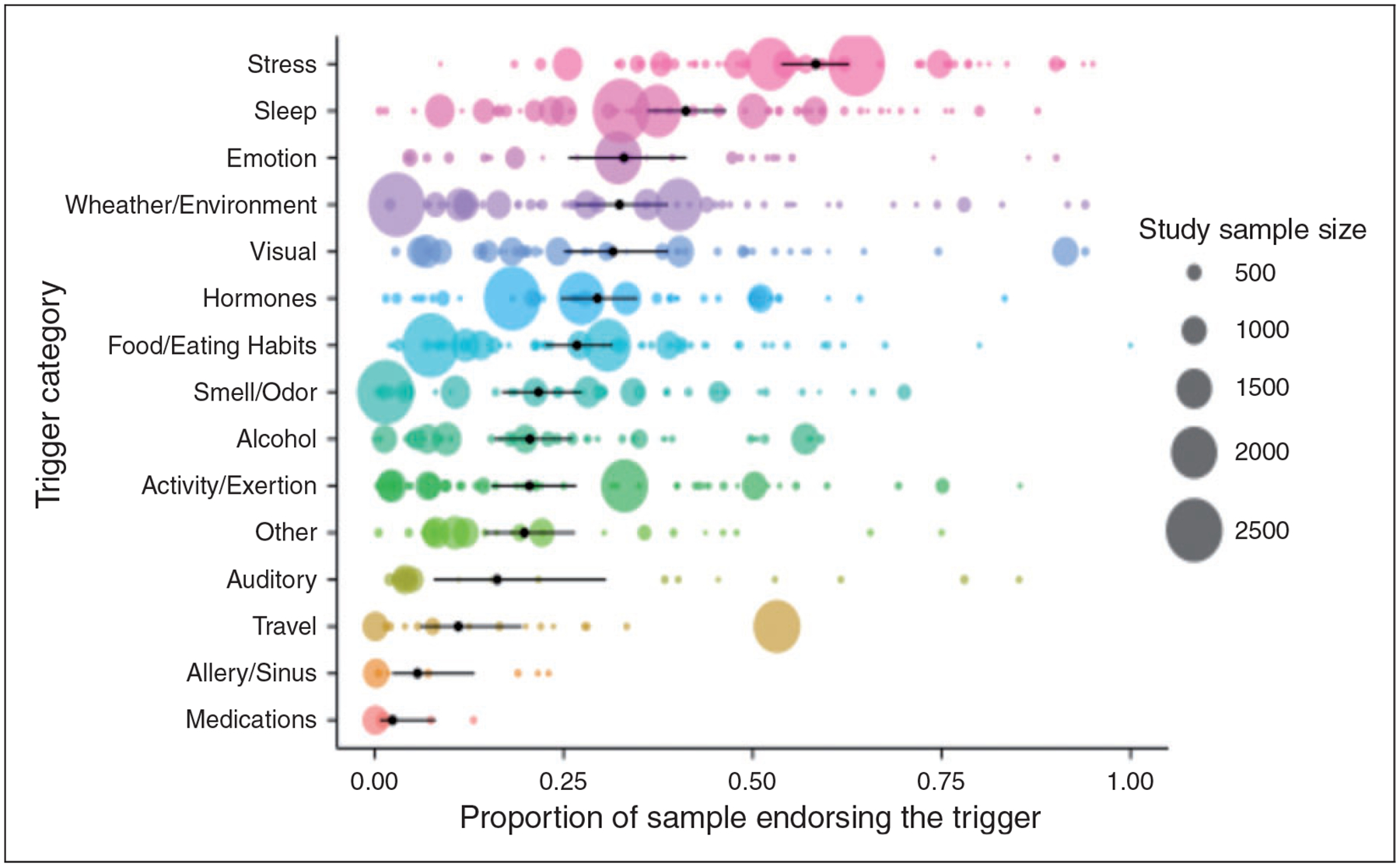
Endorsement rates of 15 headache trigger categories.
Headache diagnosis
A meta-regression on the 62 studies utilizing IHS diagnostic criteria examined the impact of headache diagnosis on endorsement of the trigger categories (see Supplemental Figure e2). Compared to those with migraine, TTH sufferers were less likely to endorse alcohol (OR 0.39; 0.16–0.95; p = 0.038), hormones (OR 0.36; 0.14–0.95; p = 0.038), visual triggers (OR 0.40; 0.16–0.99; p = 0.047), and weather/environment (OR 0.33; 0.14–0.78; p = 0.011). Those with migraine and TTH did not differ significantly in their rates of endorsing the other 11 trigger categories. Although the number of studies examining cluster headache patients was few (n ≤ 5) and statistical power thus limited, cluster headache patients were significantly less likely to endorse hormonal factors as triggers (OR 0.06; 0.01–0.42; p = 0.005) compared to those with migraine.
Sample demographics
With the exception of alcohol, the proportion of females in the sample did not impact trigger endorsement. A small effect was observed for gender on endorsement of alcohol, in which an all-female sample has a 2% reduced odds of reporting alcohol as a trigger compared to an all-male sample (OR: 0.98 [0.97, 0.99], p = 0.019). Mean age of the sample was unrelated to endorsement of any trigger.
Study characteristics
The number of trigger categories queried impacted the proportion of participants endorsing specific triggers of food/eating (p = 0.012), hormones (p = 0.033), medications (p = 0.023), sleep (p = 0.018), stress (p = 0.039), and weather/environment (p = 0.021). Each of these was associated with an approximately 15% increased odds of being endorsed with each additional trigger category that was also presented in a list. Year of publication did not substantially impact most of the triggers, though an increased association for auditory triggers (p = 0.007) and sleep (p = 0.048) was observed. These associations were rather modest, with 6–8% yearly increase in the odds of endorsing these triggers over the observation period.
Sensitivity analyses
Recognizing that the aforementioned estimates are dependent on the categorization scheme employed, we conducted sensitivity analyses using different methods of grouping the various headache triggers. In these analyses, the same verbatim triggers were collapsed into both far fewer categories (n = 4) and many more categories (n = 30) than the 15 used in the primary analyses. The rationale was that the four-category method would provide a more inclusive (i.e. broader) grouping scheme and the 30-category grouping a more precise approach, affording differentiation within the original categories (such as between eating various foods and fasting), both of which would serve as more extreme forms of categorization than the 15 categories we used in the primary analyses. The categories were generated with input from all authors, and then the two authors not involved in the original 15-category groupings (ABWP, REDM) independently grouped each trigger into the new schemes. Inter-rater reliabilities for the four and 30-category schemes were both very good (κ = .89 for both). Instances of disagreement were resolved by the two authors who conducted the original groupings (TTH, TAS). These data are presented in Supplemental Figures e3 and e4. As is evident from the figures, extreme heterogeneity remains regardless of the grouping scheme employed, and stimuli of a behavioral or psychological nature are still perceived as some of the more potent triggers.
Discussion
In light of a need for quantitative synthesis of existing literature on perceived headache triggers, the aims of the present meta-analysis were to summarize prior self-report studies on perceived headache triggers and examine factors that influence their endorsement rates. As is evident, within this literature extreme variability exists that is not attributable to chance but instead to methodological differences between studies (e.g. methods of assessment, sample characteristics, diagnostic criteria used) (15). Further, a relatively small number of studies examined triggers among cluster headache as compared to migraine or TTH. Caution must therefore be used when referencing the obtained trigger point estimates reported herein, focusing principally on the relative comparisons and moderator variables that influence the estimates primarily among those with migraine and TTH.
This caveat notwithstanding, general conclusions can be derived from the included meta-analyses and accompanying meta-regressions. First, the large majority of primary headache sufferers perceive themselves to have at least one headache trigger. Likely the observed proportions are underestimates given that many studies only queried a small number of potential triggers. Second, stress is the most common perceived trigger, followed by sleep and various environmental factors (e.g. weather, visual stimuli). By comparison, relatively few individuals perceived medications, allergy/sinus factors, and travel to trigger their attacks. Stress and headache interact in myriad ways. Stress produces direct effects on the autonomic nervous and neuroendocrine systems that over time may sensitize nociceptors (16), and chronic stress in combination with experiencing recurrent headache attacks impairs the brain’s ability to maintain allostasis (17). Indirectly, stress may exacerbate or precipitate headache by contributing to poor adaptive coping and maladaptive lifestyle behaviors (e.g. poor diet and sleep).
Third, trigger perceptions are influenced by diagnostic status and methods of assessment. Migraineurs reported higher rates of some trigger factors than those with TTH (alcohol, hormones, sleep, weather/environment), but rates of endorsement for most other trigger factors did not differ significantly between headache types. Whether these findings reflect differences in underlying headache pathophysiology or reporting bias is unknown. Regarding methods of assessing triggers, the highest endorsement rates are obtained when respondents select from a large list of possible triggers. Trigger beliefs thus are not immutable but prone to influence by the manner in which they are assessed. Although these moderating variables accounted for a small but significant amount of observed heterogeneity, they underscore a need for more standardized means of assessing headache triggers, both in research and clinical practice, as well as increased utilization of experimental studies.
This study is notable for its strong data-analytic framework, encompassing both multiple meta-analyses and their accompanying meta-regressions to investigate likely sources of study heterogeneity. The resulting product is a quantitative synthesis of all existing literature on self-reported triggers of headache. However, limitations must be acknowledged. Extreme heterogeneity was observed, although we attempted to account for this heterogeneity using random effects models and assessment of potential moderators via meta-regressions. A second limitation is that the survey designs of the included studies prohibited formal assessment of study quality. Adherence to published diagnostic criteria is one indicator of study quality: Nearly three-quarters of included studies (72%) adhered to published IHS criteria, and we ran meta-regressions among those studies only to quantify diagnostic differences across trigger categories. Additionally, the nature of the self-report data from these studies did not allow us to tease apart how various triggers were defined/described to respondents, to determine the “dosage” or amount of exposure required for a stimulus to be considered a trigger, or to assess the interactive effects of encountering multiple triggers simultaneously. A final limitation pertains to trigger nosology, such that we resorted to aggregating verbatim triggers within larger categories to facilitate analysis of the 420 specified individual triggers. While this aggregation made it possible to make general conclusions about categories, precision was likely reduced as not all triggers within a category can be assumed to have similar effects. For instance, experimental data suggest that red wine is a more potent trigger of migraine than vodka (18), and withdrawal of a stimulus can produce different effects on headache than exposure to that same stimulus (as has been demonstrated with “let-down headache” following a reduction in stress (19)). Our primary aggregation strategy precluded a more fine-grained analysis of very specific triggers but is consistent with our goal of providing a general quantitative summary of this very heterogeneous field. Results from the two sensitivity analyses, in which alternative grouping schemes were employed, showed that our utilization of 15 groups indeed served as a satisfactory midpoint between broader and more precise approaches to grouping the various headache triggers. Similarity with results from the 30-group scheme confirms that our original method was not overly general, and the high kappa coefficients confirm that triggers can be grouped reliably using various categorization methods.
Despite these limitations, this study provides the first quantitative synthesis of the large literature on perceived headache triggers. In addition to providing population-level estimates and a sense of the relative perceived potency of various triggers, this study highlights a need for increased methodological rigor and consistency within this area. An initial step is to test and compare these trigger perceptions using experimental designs that satisfy the numerous conditions required to demonstrate causality and to establish mechanisms of action (13,20,21). Though few in number, experimental studies have not always confirmed perceptions reported in survey studies, as was shown in an elegant double-blind study of chocolate versus carob (22). In some cases, stimuli presumed to be causal triggers may be revealed to instead reflect effects of premonitory changes (20). Improved understanding of the causal properties of individual triggers should then guide development of tailored management strategies that move beyond merely counseling headache patients to identify and avoid all potential triggers. Indeed, accumulating evidence suggests that the clinical lore of advising headache patients to avoid triggers may inadvertently increase sensitization to some triggers (23), and that training patients in other means of coping with triggers may be a viable management strategy (24), particularly for those that cannot be readily avoided.
Supplementary Material
Clinical implications.
This study provides the first quantitative synthesis of the large literature on perceived headache triggers.
Most individuals with a primary headache disorder perceive their attacks to be triggered by one or more precipitants, the most common of which are stress and sleep.
Migraineurs reported higher rates of some triggers than those with TTH (alcohol, hormones, sleep, weather/environment); however, endorsement rates of most other triggers did not differ significantly between headache types.
The odds of trigger endorsement significantly increased with each additional trigger presented.
Enhancing methodological consistency and prioritizing experimental studies would improve our understanding of headache triggers.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by funding from NIH/NINDS [R01 NS065257]
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A Brooke Walters Pellegrino, Rachel E Davis-Martin, and Dana P Turner report no disclosures. Timothy T Houle receives research support from Merck. Todd A Smitherman serves as a consultant for Alder.
References
- 1.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burch RC, Loder S, Loder E, et al. The prevalence and burden of migraine and severe headache in the United States: Updated statistics from government health surveillance studies. Headache 2015; 55: 21–34. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68: 343–349. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz BS, Stewart WF, Simon D, et al. Epidemiology of tension-type headache. JAMA 1998; 279: 381–383. [DOI] [PubMed] [Google Scholar]
- 5.Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007; 27: 193–210. [DOI] [PubMed] [Google Scholar]
- 6.Global Burden of Disease Study 2013 collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015; 386: 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond S, Bigal ME, Silberstein S, et al. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: Results from the American Migraine Prevalence and Prevention study. Headache 2007; 47: 355–363. [DOI] [PubMed] [Google Scholar]
- 8.Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: Effects of sex, income, and headache features. Headache 2013; 53: 81–92. [DOI] [PubMed] [Google Scholar]
- 9.Goadbsy PJ, Charbit AR, Andreou AP, et al. Neurobiology of migraine. Neurosci 2009; 161: 327–341. [DOI] [PubMed] [Google Scholar]
- 10.Peroutka SJ. What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep 2014; 18: 454. doi: 10.1007/s11916-014-0454-z. [DOI] [PubMed] [Google Scholar]
- 11.Martin VT and Behbehani MM. Toward a rational understanding of migraine trigger factors. Med Clin North Am 2001; 85: 911–941. [DOI] [PubMed] [Google Scholar]
- 12.Rothrock JF. The truth about triggers. Headache 2008; 3: 499–500. [DOI] [PubMed] [Google Scholar]
- 13.Turner DP, Smitherman TA, Martin VT, et al. Causality and headache triggers. Headache 2013; 53: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009; 339: b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houle T and Nash JM. Stress and headache chronification. Headache 2008; 48: 40–44. [DOI] [PubMed] [Google Scholar]
- 17.Borsook D, Maleki N, Becerra L, et al. Understanding migraine through the lens of maladaptive stress responses: A model disease of allostatic load. Neuron 2012; 73: 219–234. [DOI] [PubMed] [Google Scholar]
- 18.Littlewood JT, Glover V, Davies PTG, et al. Red wine as a cause of migraine. Lancet 1988; 331: 558–559. [DOI] [PubMed] [Google Scholar]
- 19.Lipton RB, Buse DC, Hall CB, et al. Reduction in perceived stress as a migraine trigger: Testing the “let-down headache” hypothesis. Neurology 2014; 82: 1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipton RB, Pavlovic JM, Haut SR, et al. Methodological issues in studying trigger factors and premonitory features of migraine. Headache 2014; 54: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 21.Pavlovic JM, Buse DC, Sollars M, et al. Trigger factors and premonitory features of migraine attacks: Summary of studies. Headache 2014; 54: 1670–1679. [DOI] [PubMed] [Google Scholar]
- 22.Marcus DA, Scharff L, Turk D, et al. A double-blind provocative study of chocolate as a trigger of headache. Cephalalgia 1997; 17: 855–862. [DOI] [PubMed] [Google Scholar]
- 23.Martin PR and Macleod C. Behavioral management of headache triggers: Avoidance of triggers is an inadequate strategy. Clin Psychol Rev 2009; 29: 483–495. [DOI] [PubMed] [Google Scholar]
- 24.Martin PR, Reece J, Callan M, et al. Behavioral management of the triggers of recurrent headache: A randomized controlled trial. Behav Res Ther 2014; 61: 1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


