Abstract
Purpose:
To detect the presence of SARS-CoV-2 in aqueous and vitreous humor of COVID-19 patients in a pilot study.
Methods:
Consecutive patients planned for emergency ophthalmic surgeries after ocular trauma were subjected to naso-oropharyngeal RT-PCR test for SARS-CoV-2. Laboratory-confirmed cases were enrolled for the study. During surgery, 0.1 mL aqueous and vitreous each was aspirated. The vitreous tap was collected on clinical suspicion of endophthalmitis. RT-PCR test was used to detect SARS-COV-2 nucleotide in the samples. Cycle threshold (Ct) for E gene of ≤35 along with confirmatory results on one of the two target genes (RdRp or ORF1b) was considered as positive.
Results:
One hundred and thirty two patients were found positive on naso-oropharyngeal RT-PCR test for SARS-CoV-2 preoperatively. Seven patients with ocular trauma were studied. The mean age was 31.8 years. There were six male and one female patient. Two patients had symptoms of mild COVID-19 disease and the rest were asymptomatic. The mean Ct value of the E gene on naso-oropharyngeal RT-PCR was 23.14 ± 4.7. Corneal and corneoscleral laceration repair was done in five patients, intracorneal wooden foreign body was removed in one patient, and injection of intravitreal antibiotics was done in one patient. Aqueous and vitreous tap was collected in 7 and 5 patients, respectively. None of the aqueous or vitreous samples was found positive for SARS-CoV-2.
Conclusion:
SARS-CoV-2 was not detected by RT-PCR in aqueous or vitreous humor in this pilot study. Future studies with a larger sample size are needed to further explore the presence of SARS-CoV-2 in intraocular fluids.
Keywords: Aqueous humor, SARS-CoV-2, vitreous humor
SARS-CoV-2 (causative virus for COVID-19) is known to be present in tears and in the ocular surface, which might be a potential source of spread.[1,2,3] Angiotensin-converting enzyme 2 (ACE2), the receptor for SARS-CoV-2, has been detected in the aqueous humor.[4] Cornea and conjunctival epithelial cells are known to express the SARS-CoV-2 receptors ACE2 and TMPRSS2 (transmembrane serine protease 2), which help the virus enter the cell.[5,6] The whistleblower ophthalmologist from China, Li Wenliang, probably got infected with SARS-CoV-2 from a female glaucoma patient to which he, unfortunately, succumbed later.[7] Potential sources of infection of SARS-CoV-2 from the eye include ocular surface, tear, and retrograde transport of virus from nasal cavity via nasolacrimal duct.[8]
However, literature was lacking regarding the presence or absence of SARS-CoV-2 in intraocular fluids until recently.[9,10] This is specifically important as cataract surgery is a known aerosol-generating procedure[11] and may potentially spread the infection in the operating room. Recently, List et al. could not find qRT-PCR evidence of SARS-CoV-2 in the aqueous and vitreous humor of 16 patients who had died of respiratory failure due to COVID-19 infection.[9] In this pilot study, we aimed to determine whether the SARS-CoV-2 viral nucleotide is present in the aqueous and vitreous humor of COVID-19 patients who underwent emergency ocular surgery.
Methods
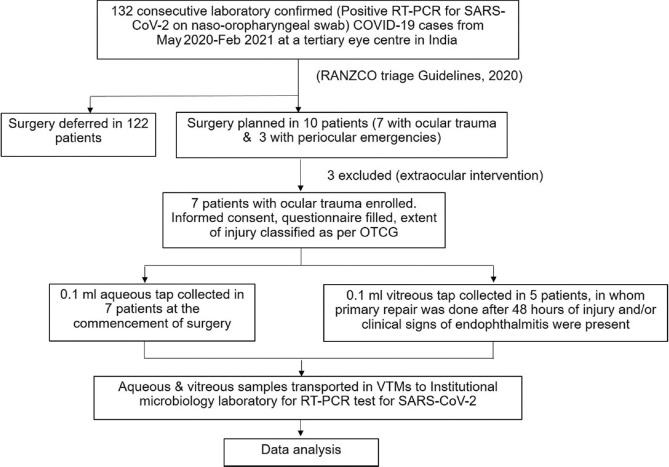
A cross-sectional study was conducted from May 2020 to February 2021 at a tertiary eye center attached to a COVID-19-facility hospital in North India. Ethical clearance was obtained from the institutional research and ethics committee, and the study was conducted following the tenets of the Declaration of Helsinki. Consecutive patients presenting to the facility, in whom an ophthalmic surgical intervention was planned, were subjected to naso-oropharyngeal reverse transcription-polymerase chain reaction (RT-PCR) test for SARS-CoV-2. All laboratory-confirmed cases with positive RT-PCR tests were evaluated for the ophthalmic condition and were triaged according to RANZCO COVID-19 triage guidelines 2020.[12] In cases with no considerable risk of loss of vision or general health without being operated on, the surgery was deferred. Ophthalmic emergencies requiring emergent surgical interventions were enrolled for the study [Fig. 1]. Patients requiring extraocular surgical intervention were excluded [Table 1].
Figure 1.
Flowchart depicting the study design. RT-PCR = Reverse transcription-polymerase chain reaction; COVID-19 = Coronavirus disease 2019, RANZCO = Royal Australian and New Zealand College of Ophthalmologists, OTCG = Ocular trauma classification group, VTM = viral transport media
Table 1.
Demographic data, clinical characteristics, surgical details, and reverse-transcriptase polymerase chain reaction results of patients
| S. No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Age (years) | 9 | 42 | 42 | 54 | 14 | 32 | 4 |
| Sex | M | M | F | M | M | M | M |
| Diagnosis | RE Corneal Laceration | RE Corneoscleral Laceration | RE Corneoscleral laceration | LE Intrastromal wooden foreign body with hypopyon | RE Self sealed corneal tear with endophthalmitis | LE Corneoscleral laceration | RE Corneal laceration with lens matter in the anterior chamber |
| Mode of Injury | Kite string injury | Road traffic accident | Road traffic accident | Thorn injury | Thorn injury | Road traffic accident | Glass injury |
| Type/Zone | B/I | B/II | B/II | C/I | B/I | B/II | B/I |
| Visual acuity | 5/200 | HMCF | PL+ | 20/200 | HMCF | 1/200 | HMCF |
| Visual acuity in other eye | 20/20 | 20/200 | 20/20 | 2/200 | 20/20 | 20/20 | No PL |
| Surgical Intervention | Corneal laceration repair | Corneoscleral laceration repair | Corneoscleral laceration repair | Foreign body removal + AC wash + Intracameral Voriconazole | Intravitreal antibiotics | Corneoscleral laceration repair | Corneal laceration repair with lens aspiration |
| Anesthesia | GA | LA | LA | TA | GA | LA | GA |
| Interval between trauma & surgery | 36 h | 72 h | 48 h | 72 h | 96 h | 72 h | 36 h |
| Surgical time | 20 min | 25 min | 30 min | 12 min | 4 min | 25 min | 30 min |
| Comorbidities | Nil | Asthma, DM | Nil | CAD, HT | Nil | Nil | Nil |
| Systemic symptoms | Nil | Cough, SOB | Nil | Sore throat, fever | Nil | Nil | Nil |
| Duration of symptoms | - | 4 days | - | 3 days | - | - | - |
| NP-OP Swab RT-PCR Ct value | 23 | 17 | 27 | 19 | 26 | 30 | 20 |
| Aqueous tap RT-PCR | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Vitreous tap RT-PCR | - | Negative | Negative | Negative | Negative | Negative | - |
| Follow-up (weeks/clinical outcome) | 10/Asymptomatic | 8/Pneumonitis | 12/Asymptomatic | 6/Recovered | 8/Asymptomatic | 6/Asymptomatic | 12/Asymptomatic |
RE=Right eye, LE=Left eye, HMCF=Hand movements close to face, PL=Perception of light, GA=General anesthesia, LA=Local anesthesia, TA=Topical anesthesia, h=Hours, min=Minutes, DM=Diabetes mellitus, CAD=Coronary artery disease, HT=Hypertension, SOB=Shortness of breath, NP-OP=Nasopharyngeal-oropharyngeal, RT-PCR=Reverse transcription-polymerase chain reaction, Ct=Cycle threshold , M=Male, F=Female, Ac=Anterior chamber
A detailed history regarding the cause and duration of injury, COVID-19 disease-related symptoms, and presence of systemic comorbidities was recorded. X-ray orbit was done in all cases of open globe injuries to rule out retained intraocular foreign body. The extent of injury in terms of type, zone, and grade was noted as per the “Ocular Trauma Classification Group” recommendations.[13] Written informed consent was obtained from all patients enrolled in the study. All surgeries were done with full personal protective equipment and by following standard safety measures for operating upon a COVID-19 patient.[14]
Aqueous tap
Aqueous tap was collected in those cases where entry into the anterior chamber was required to perform intraocular surgery and/or to decrease the intraocular pressure prior to injecting intravitreal antibiotics. The eye was cleaned with 5% povidone-iodine. Anterior chamber paracentesis was done using an insulin syringe and a 30 G needle. The needle was inserted through the limbus and 0.1 ml of fluid was withdrawn. The sample was inoculated in a viral transport medium (VTM) and was sent for RT-PCR analysis.
Vitreous tap
The vitreous tap was collected in patients who underwent primary repair after 48 hours of injury and/or who had clinical signs of endophthalmitis. A 27 G needle attached to an insulin syringe was inserted through the pars plana 4 mm behind the limbus perpendicular to the sclera and 0.1 mL of vitreous was aspirated. The vitreous aspirate was divided into two portions of 0.05 mL each. One portion was sent for microbiological investigations, including Gram stain, KOH 10%, and bacterial and fungal cultures. The other portion was inoculated in VTM and was sent for RT-PCR. Intravitreal vancomycin 1 mg/0.1 mL and ceftazidime 2.25 mg/0.1 mL mg were injected after the vitreous tap.
All samples were collected and labeled in separate VTMs and were transported to the institutional lab facility maintaining the recommended cold chain. The RT-PCR test was used to detect the SARS-COV-2 nucleotide. Cycle threshold (Ct) for E gene ≤35 along with confirmatory results on one of the two target genes [RNA-dependent RNA polymerase (RdRp) or open reading frame 1b (ORF1b)] was considered as positive.
Results
During the study period, a total of 132 patients presenting to the facility were found positive on naso-oropharyngeal RT-PCR test for SARS-CoV-2 preoperatively. Surgery was deferred in 122 patients following RANZCO COVID-19 triage guidelines.[12] Three patients undergoing extraocular surgical intervention were excluded. Seven patients with ocular trauma were studied [Table 1]. The mean age (± standard deviation) was 31.8 (±19.2) years. There were six males and one female. Two patients had systemic comorbidities. Five patients were asymptomatic and two patients had symptoms of mild COVID-19 disease. The mean Ct value of E gene on naso-oropharyngeal RT-PCR was 23.14 ± 4.7. The mean time interval between trauma to surgical intervention was 61.7 hours. The mean duration of surgery was 20.85 ± 9.7 minutes. The surgical intervention included repair of corneal or corneoscleral laceration in five patients, removal of intracorneal wooden foreign body in one patient, and intravitreal antibiotics injection for post-traumatic endophthalmitis in one patient. Aqueous tap was collected in seven and vitreous tap was collected in five patients. Three patients underwent surgical intervention under peribulbar block, general anesthesia was used in three patients, and two patients underwent surgery under topical anesthesia [Table 1].
None of the aqueous or vitreous samples was positive for SARS-CoV-2 viral nucleotide on RT-PCR. The members of the surgical and anesthesia team followed isolation protocol post-surgery and no one developed COVID-19-related symptoms at 6 weeks. The mean follow-up of the patients was 8.85 ± 2.5 weeks. Five patients remained asymptomatic, one patient recovered completely from mild COVID-19 disease and one patient developed pneumonitis at 8-week follow-up.
Discussion
With the ongoing COVID-19 pandemic, the surgical practices among ophthalmologists across the globe are resuming. There are many uncertainties and speculations regarding the ocular transmission of SARS-CoV-2. Studies on various laboratory models have been done to determine its transmission through aerosol-generating surgeries such as phacoemulsification.[11] Nevertheless, a definite lacuna of knowledge existed regarding the intraocular presence of this virus until very recently.[9] List and colleagues performed quantitative RT-PCR for RdRp (qRT-PCR) for SARS-CoV-2 on postmortem aqueous and vitreous samples of 16 patients who died due to complications of COVID-19. They did not find evidence of the presence of SARS-CoV-2 in any of these samples.[9] However, the effect of time after death and sample collection on the positivity of samples for SARS-CoV-2 needs further exploration. Miner et al. showed that SARS-CoV-2 failed to replicate in the human cornea, though the receptor for SARS-CoV-2, ACE2 is present in the cornea.[15] Sawant et al.[10] evaluated 20 postmortem eyes from 10 donors who were COVID-19 positive. Three vitreous swabs, five posterior corneal, and one anterior corneal sample showed the presence of SARS-CoV-2 RNA [phosphoprotein gene (N1 and N2 regions)] on RT-PCR.[10] The corneal epithelium showed SARS-CoV-2 envelope and spike proteins.[10]
To the best of our knowledge, no study to date evaluated the intraocular fluids for the presence of SARS-CoV-2 in live humans.
Our study did not find any laboratory evidence of the SARS-CoV-2 virus in the intraocular fluid of COVID-19 patients. Being an immune-privileged site, the eye is known to harbor viruses. Varkey et al.[16] and Gonzales et al.[17] detected viable ebolavirus and rubella virus, respectively, from the aqueous humor. However, these patients presented with uveitis in the convalescent phase. In our series, the samples were obtained during the active phase of COVID-19 infection.
The presence of SARS-CoV-2 in body fluids including tears has been predicted to be associated with viral load and disease severity, as indicated by the Ct values of naso-oropharyngeal RT-PCR.[2] In our series, though there were no patients with severe COVID-19 illness, the mean Ct value was 23.14 ± 4.7, which indicates a significant viral load. As demonstrated in the viral culture studies, the infectivity of SARS-CoV-2 has been observed for the Ct value <24.[18]
Conclusion
SARS-CoV-2 was not detected by RT-PCR in aqueous or vitreous humor of asymptomatic and mild COVID-19 patients in our pilot study. Future studies with a larger sample size are needed to further explore the presence of SARS-CoV-2 in intraocular fluids.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang X, Chen X, Chen L, Deng C, Zou X, Liu W, et al. The evidence of SARS-CoV-2 infection on ocular surface. Ocul Surf. 2020;18:360–2. doi: 10.1016/j.jtos.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora R, Goel R, Kumar S, Chhabra M, Saxena S, Manchanda V, et al. Evaluation of SARS-CoV-2 in tears of patients with moderate to severe COVID-19. Ophthalmology. 2021;128:494–503. doi: 10.1016/j.ophtha.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–94. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holappa M, Valjakka J, Vaajanen A. Angiotensin(1-7) and ACE2, “The Hot Spots“of renin-angiotensin system, detected in the human aqueous humor. Open Ophthalmol J. 2015;9:28–32. doi: 10.2174/1874364101509010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18:537–44. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roehrich H, Yuan C, Hou JH. Immunohistochemical study of SARS-CoV-2 viral entry factors in the cornea and ocular surface. Cornea. 2020;39:1556–62. doi: 10.1097/ICO.0000000000002509. [DOI] [PubMed] [Google Scholar]
- 7.Parrish RK, Stewart MW, Powers SLD. Ophthalmologists are more than eye doctors—In memoriam Li Wenliang. Am J Ophthalmol. 2020;213:A1–2. [Google Scholar]
- 8.Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes?A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–5. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.List W, Regitnig P, Kashofer K, Gorkiewicz G, Zacharias M, Wedrich A, et al. Occurrence of SARS-CoV-2 in the intraocular milieu. Exp Eye Res. 2020;201:108273. doi: 10.1016/j.exer.2020.108273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawant OB, Singh S, Wright RE, 3rd, Jones KM, Titus MS, Dennis E, et al. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul Surf. 2021;19:322–9. doi: 10.1016/j.jtos.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darcy K, Elhaddad O, Achiron A, Keller J, Leadbetter D, Tole D, et al. Reducing visible aerosol generation during phacoemulsification in the era of Covid-19. Eye (Lond) 2021;35:1405–10. doi: 10.1038/s41433-020-1053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anon. RANZCO COVID-19 Triage Guidelines. [Last accessed on 2021 Jan 21]. Available from: https://ranzco.edu/policies_and_guideli/ranzco-covid-19-triage-guidelines/
- 13.Pieramici DJ, Sternberg P, Aaberg TM, Bridges WZ, Jr, Capone A, Jr, Cardillo JA, et al. A System for classifying mechanical injuries of the eye (Globe) Am J Ophthalmol. 1997;123:820–31. doi: 10.1016/s0002-9394(14)71132-8. [DOI] [PubMed] [Google Scholar]
- 14.Coccolini F, Perrone G, Chiarugi M, Di Marzo F, Ansaloni L, Scandroglio I, et al. Surgery in COVID-19 patients:Operational directives. World J Emerg Surg. 2020;15:25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miner JJ, Platt DJ, Ghaznavi CM, Chandra P, Santeford A, Menos AM, et al. HSV-1 and Zika Virus but Not SARS-CoV-2 replicate in the human cornea and are restricted by corneal type III interferon. Cell Rep. 2020;33:108339. doi: 10.1016/j.celrep.2020.108339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varkey JB, Shantha JG, Crozier I, Kraft CS, Lyon GM, Mehta AK, et al. Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med. 2015;372:2423–7. doi: 10.1056/NEJMoa1500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzales JA, Hinterwirth A, Shantha J, Wang K, Zhong L, Cummings SL, et al. Association of ocular inflammation and rubella virus persistence. JAMA Ophthalmol. 2019;137:435–8. doi: 10.1001/jamaophthalmol.2018.6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa638. doi:10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]