Abstract
Over 2 million people worldwide are suffering from gene-related retinal diseases, inherited or acquired, and over 270 genes have been identified which are found to be responsible for these conditions. This review article touches upon the mechanisms of gene therapy, various enzymes of the visual cycle responsible for different genetic diseases, Luxturna—the first US Food and Drug Administration (FDA)-approved therapeutic gene product, and several ongoing trials of gene therapy for age-related macular degeneration. Gene therapy has tremendous potential for retinal conditions due to its ease of accessibility, immune-privileged status, and tight blood-retinal barriers, limiting systemic side effects of the drug. In recent years, advances in gene therapy in retinal conditions have increasing significantly, with progress in cell-specific targeting and transduction efficiency of gene products through the use of adeno-associated viral vectors (AAVs), suggesting that even greater success in future clinical trials is possible.
Keywords: Adeno-associated viral vectors (AAVs), age-related macular degeneration (AMD), gene delivery systems, gene therapy, inherited retinal diseases, Luxturna, non-inherited retinal diseases, visual cycle, voretigene neparvovec
The literature search consisted of a review of the literature using PubMed databases with the following search terms: “gene therapy for retinal diseases,” “gene therapy for inherited retinal disorders,” “Visual cycle,” “visual cycle enzymes,” “gene therapy mechanisms,” “adeno-associated viral vectors, “gene replacement,” “RPE65,” “optogenetics,” “antisense oligonucleotides,” “CRISPR/CAS9-based therapy,” “Genome editing,” “BRILLIANCE trial,” “Voretigene neparvovec,” “Luxturna,” “gene therapy in AMD,” “RegenxBIO.” Articles were identified via the review of PubMed abstracts and review of citations in previous work on the subject. Articles written in non-English languages were excluded.
Gene therapy involves the modification of defective deoxyribonucleic acid (DNA) in recipient cells or tissues to achieve a desired therapeutic effect. Compared to other organs, the eye has a greater potential for gene therapy due to its easy accessibility via injections and surgical interventions, immune-privileged status,[1,2] presence of tight ocular barriers preventing exposure to other organs,[3] and ready assessment of retinal structure and anatomy by non-invasive techniques to determine response to treatment.[4] Also, retinal dystrophies are usually symmetrical and bilateral, allowing one eye to serve as a control in clinical trials.[4] A major disadvantage is that advanced retinal dystrophies or degenerations are usually irreversible, and successful treatment depends on the presence of live neuronal cells at the time of initiating gene therapy.
Gene therapy involves a cloned copy of the wild type gene linked to a particular condition, a promoter that controls the gene expression specifically in the target cells, and a vector/carrier which is usually a virus stripped of its replicative and virulent features, which can act as a cargo and deliver the necessary gene into the target cells of the host. In this review, we shall briefly discuss the various mechanisms of gene therapy for inherited and acquired retinal diseases, the visual cycle with its enzymes which play a role in various genetic conditions, the different gene delivery systems along with their mode of administration, gene replacement therapies, other novel approaches using antisense oligonucleotides, optogenetics, and the CRISPR/CAS9-based genome editing technique. Finally, we shall discuss Luxturna, which is the first Food and Drug Administration (FDA)-approved gene replacement therapy for Leber congenital amaurosis type 2 (LCA2), and the ongoing trials of gene therapy in age-related macular degeneration (AMD).
Visual Cycle
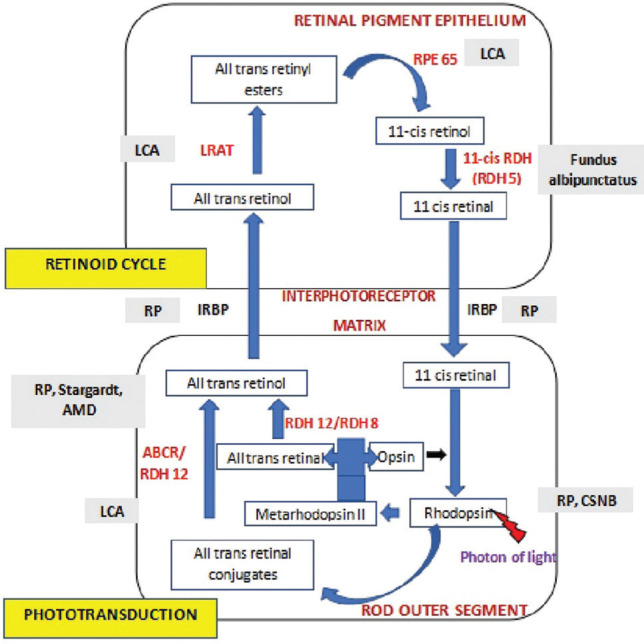
The visual cycle [Fig. 1] is the process that takes place in the photoreceptors and the retinal pigment epithelium (RPE) involving several enzymatic reactions,[5] which have been described subsequently. RPE65 is an example of a very important enzyme in this cascade, responsible for converting all-trans-retinyl ester to 11-cis-retinol. In cells with a defective RPE65, 11-cis-retinal levels are reduced and retinyl esters accumulate in the RPE leading to recessive blinding disorders like Leber congenital amaurosis.
Figure 1.
Schematic representation of Wald’s visual cycle–Rod enzymes and diseases overview. Abbreviations used: Leber congenital amaurosis (LCA); Retinitis pigmentosa (RP); Age-related macular degeneration (AMD); Congenital stationary night blindness (CSNB); Retinal pigment epithelium (RPE); Lecithin-retinol acyltransferase (LRAT); Retinol dehydrogenases (RDHs); Interphotoreceptor retinoid binding protein (IRBP); ATP binding cassette transporter (ABCR)
Important enzymes in Wald’s visual cycle
Lecithin-retinol acyltransferase
Lecithin-retinol acyltransferase (LRAT) gene is located on chromosome 4 at the locus 4q32.1 and is a member of NlpC/P60 thiol peptidase protein superfamily. It is localized in the endoplasmic reticulum with its largest level being in the liver, RPE, and small intestine.[6] It catalyzes the transfer of acyl from phosphatidylcholine to all-trans-retinol, forming retinyl esters. This forms the substrate for 11-cis-retinol in a reaction catalyzed by RPE 65.[7] LRAT dysfunction (LCA14) affects <1% of LCA patients, has an autosomal recessive inheritance, early-onset, and is usually associated with debilitating blindness.[8] There is no gene therapy available for LRAT defect-causing LCA.[9]
RPE65
RPE65 mutation accounts for less than 10% of the cases of LCA and approximately 2% cases of recessive retinitis pigmentosa (RP) cases. The gene is localized to chromosome 1 at the locus 1p31 which encodes a 65 kDa protein retinoid isomerohydrolase expressed in RPE. It is responsible for retinol isomerization and converts all-trans-retinyl ester to 11-cis-retinol in phototransduction. The 11-cis-retinol then gets converted to 11-cis-retinal and is used in the regeneration of visual pigments in photoreceptor cells.[10] Hence, a mutation (RPE65 deficiency) causes a deficiency of 11-cis-retinal to begin the visual cycle. This also causes a build-up of retinyl esters in lipid droplets and an increase in lipofuscin granules in RPE. This leads to a gradual and progressive retinal degeneration.[11] The 11 cis-retinal deficiency in rod photoreceptors causes early and profound nyctalopia. However, the cone photoreceptors have an alternate retinoid cycle pathway for the generation of 11-cis-retinal which do not depend on RPE65, so cone-mediated vision persists in younger patients.[8] This mutation culminates into severe profound vision loss in infancy with mild nystagmus.
Retinol dehydrogenases
Retinol dehydrogenases (RDHs) catalyze the reduction of all-trans-retinal to all-trans-retinol (all-trans-RDHs) and the oxidation of 11-cis-retinol to 11-cis-retinal in the RPE (11-cis-RDHs).
All-trans-retinol dehydrogenase
RDH8 and RDH12 are the major all-trans-RDHs in the rod and cone cells. The reduction of all-trans-retinal is the first step in the regeneration of visual pigments in the outer segments of photoreceptors. RDH8 mutations in human retinal diseases have not been reported.[12] RDH 12 gene mutation accounts for about 4–5% of recessive LCA. It is localized to chromosome 14 at the locus 14q23.3 -q24.1.[13] The conversion of all-trans-retinal to all-trans-retinol is a critical step in the visual cycle, primarily facilitated by RDH8 located in the photoreceptor outer segments, whereas RDH12 is located in the inner segment and reduces the excess all-trans and 11-cis retinaldehydes leaked into the inner segment during periods of high photo-stimulation. Thus, the enzyme protects the inner segment against the excess build-up of retinaldehyde and subsequent cytotoxicity.[14] Mutation and subsequent loss of function of the gene are highly detrimental early in life, and in particular, involves the macula. Retinal dystrophy caused by RDH12 mutation shows early-onset visual dysfunction, night blindness, and early macular atrophy.[15,16]
11-Cis retinol dehydrogenase
11-cis-RDH converts 11-cis-retinol to 11-cis-retinal in the RPE. RDH 5 is a membrane-associated protein that forms a complex with RPE65. It is located on chromosome 12 at the locus 12q13-q14 and is widely expressed in the RPE. Mutation of RDH 5 causes autosomal recessive fundus albipunctatus.[17]
Interphotoreceptor retinoid-binding protein
Interphotoreceptor retinoid-binding protein (IRBP) is encoded by the RBP3 gene. This glycoprotein is found predominantly in the extracellular space between RPE and the photoreceptors.[18] Studies have suggested that one IRBP molecule binds to only one retinol.[19] It plays an important role in the active transport of retinoids between the RPE and the photoreceptor cells. Dysfunction of IRBP causes delayed transfer of chromophores between RPE and photoreceptors. Homozygous mutation in RBP3 has been found to be associated with autosomal recessive RP.[20,21] Gene therapy targeting the RBP3 gene has not yet been studied. However, it has abundant prospects to treat retinal dystrophy which can be looked upon in the future.
Rhodopsin
Rhodopsin is a member of class A of the G-protein coupled receptor (GPCR) superfamily and is encoded by the rhodopsin gene (RHO gene) located on human chromosome 3 at the locus 3q22.1.[22] It is located in rod photoreceptor cells. The activation of rhodopsin by the photons triggers molecular signals that generate electrical impulses.[23,24] In the dark, rhodopsin is bound to the chromophore 11-cis-retinal, which maintains it in an inactive state and is essential for it to remain highly sensitive. Photoactivation of rhodopsin triggers the activation of G protein transducin resulting in a cascade of biochemical reactions called phototransduction. Numerous mutations in the RHO gene have been identified in people with RP and congenital stationary night blindness. RHO gene mutations account for almost 30% of the cases of autosomal-dominant RP and rarely cause autosomal recessive RP.[22]
Mechanism of Gene Therapy
In recessive diseases with a loss of function, a gene complementation approach of introducing an extra copy of the normal gene is a popular strategy [Table 1]. Dominant disorders with a dominant-negative effect require a combined approach of mutant gene suppression with or without gene complementation. Newer, upcoming variants of gene therapy like optogenetics, utilizing antisense oligonucleotides, and genome editing systems like CRISPR, have been described subsequently.
Table 1.
Gene therapy by disease type and therapy technique
| Disease Type | Gene Therapy Technique |
|---|---|
| Recessive disease | Augmentation (replacement) |
| Dominant disease | Suppression/inactivation Gene editing/CRISPR |
| Multifactorial disease (e.g., ARMD) | Addition/growth factor: Subretinally injected RetinoStat, a lentiviral vector injected subretinally expressing endostatin and angiostatin [Oxford Biomedica] Subretinal delivery of rAAV.soluble FLT-1 [Adverum Biotechnologies] Intravitreal delivery of AAV2- soluble FLT-1 [Genzyme/Sanofi] |
| Non-genotype-specific disease | Neuromodulation (optogenetics) |
Gene delivery systems
Both, viral and nonviral vectors have been tried and evaluated for their efficacy for delivering desirable genes into target cells affected by retinal degeneration. However, viral vectors are the most popular and widely used in therapeutic applications. Vectorology deals with the design, construction, and production of different viral vectors for gene delivery and expression into different cell types. Most of the current third-generation therapeutic vectors are replication-defective and can infect the target cells only once to safely deliver the transgenes. They are incapable of replication in the host and require at least two or more helpers for amplification and expansion under controlled lab environments.
The success of viral therapeutic vectors depends on the transduction efficiency of the target cells to express transgenes for the desired duration with low immunogenicity to evade host immune response and increased retention time.[25,26] Other properties desirable in a vector are that their genomes should not get integrated into the host to avoid undesirable insertional mutagenesis. The vector should possess a cell-specific tropism for infectivity and the transgene should be expressed only in target cells. It should have an unlimited cloning capacity for large-scale expansion and low cytotoxicity to the host at therapeutic doses.
The types of vectors include viral and nonviral. Viral vectors studied so far include adenoviruses, retroviruses, lentiviruses, and adeno-associated viruses (AAVs). Among these vectors, AAVs are the most promising in gene therapeutics. They exhibit several desirable vector properties such as lack of pathogenicity, low immunogenicity, ability to transduce non-dividing cells, and maintenance of sustained levels of therapeutic gene expression. However, a major disadvantage of AAVs is their limited packaging capacity precluding their ability to carry genes larger than 5 kb. Various advances have been made to increase their transfer capacity beyond 5 kb through novel strategies.[27] Adeno-associated viral vectors have the ability to target both dividing and non-dividing cells, with a broad tropism, allowing them to infect various cell types.[28,29] Serotype 2 of AAV (AAV2) is the best-characterized serotype which has been commonly used for gene therapy in humans.
Due to the risk of immunogenicity in the case of adenoviral vectors, transgene integration in the case of retroviral and lentiviral vectors, and the inability to carry large transgenes by AAV vectors, nonviral delivery systems may offer a viable strategy for gene delivery. These include nanoparticles, liposomes, and naked DNA for cellular delivery.[30] These are easier to manufacture in large quantities, making them more cost-effective compared to the viral vectors. However, naked plasmid DNA has a much shorter period of gene expression which makes it undesirable. Nanoparticles, in comparison with their viral counterparts, have a much lower efficiency of transgene delivery and expression.[30] Liposomes ten to aggregate, and hence, increase the chances of retinal toxicity.[31] Due to these unresolved issues of the nonviral vectors, they do not appear to be promising for therapeutic use in retinal dystrophies.
Gene replacement
The first study of gene replacement was seen in a murine model of autosomal recessive RP. The defect was a nonsense mutation in the PRPh2 gene which encodes a membrane glycoprotein required for the formation of disks in the outer segments of photoreceptors. In this study, the subretinal injection of AAV2 carrying the PRPh2 transgene was shown to be associated with the formation of these disks and restored the structural integrity of the photoreceptors.[32] Gene replacement has also been tested in an royal college of surgeons (RCS) rat model of RP, with a defect in the MERTK gene, which is a receptor tyrosine kinase expressed in RPE cells.[33] Recombinant adenoviruses were injected into the subretinal space to deliver the MERTK gene into host RPE cells, thereby allowing functional recovery and delay in the degeneration of photoreceptors.[34]
The RPE is a single cell layer, hence, it is more suitable for treatment. There are conditions where photoreceptor damage is secondary to genetic defects which originate in the RPE. Therefore, better results can be seen in the disorders of RPE, such as RPE65 and MERTK-mediated dystrophies, where correcting the primary defect in RPE results in functional recovery of the photoreceptors. Several studies have been conducted on LCA models. Rpe65−/− mice treated with AAV2/2 had a restoration of the function of photoreceptors by replacing the RPE65 gene.[35] Stargardt disease is an autosomal recessive condition caused by a mutation in the ABCA4 gene, which results in the accumulation of lipofuscin in the RPE with resultant degeneration.[36] This gene is too large to be packaged in the AAV2 vectors. However, the discovery of the AAV5-based vectors with higher packaging capacity up to 8.9 kb has made it possible to transfer large recombinant genomes, thus allowing the substitution of ABCA4 in mouse models.[37] Based on these studies, the gene replacement therapy of RPE appears to be promising in some retinal dystrophies.
Mode of administration of vectors
Two distinct routes by which the vectors can be administered include injection into the subretinal space or intravitreal injection.[38] The subretinal space is a potential space which gives the injected material direct access to the plasma membrane of the RPE and photoreceptors. However, the subretinal delivery requires specialized skills. It involves creating a transient iatrogenic neurosensory retinal detachment near the fovea which has to be carefully controlled to prevent the development of retinal detachment or macular hole. In contrast, delivery into the vitreous cavity via intravitreal injection has greater ease of administration, less risky than a subretinal injection, and is preferred in conditions of the inner retina which is closer to the vitreous cavity. However, this method of delivery of AAV is less efficient than subretinal delivery for the treatment of outer retinal disease. This may be due to the physical barrier required for the virus to traverse the retina, as well as the potential dilution of the vector in the vitreous cavity leading to a lower concentration in the outer retina. Most of the current vectors are injected into the subretinal space, except in X-linked juvenile retinoschisis, where the vector is preferred to be injected intravitreally to reduce the risk of retinal detachment and vitreous hemorrhage.[39]
Other variants of gene therapy
Optogenetics
This technology overcomes the limitation of the lack of photoreceptors in advanced diseases, which cannot be treated with conventional gene therapy. The inner retinal cells are targeted to convert them to light-sensitive cells in the absence of photoreceptors. Here, the AAV vector delivers light-sensitive opsins, such as rhodopsin and melanopsin, to bipolar or retinal ganglion cells which are still functional, thus overcoming the lack of availability of photoreceptors.[40,41,42,43] Opsins in bipolar or ganglion cells get activated by photons, triggering nerve impulses downstream. However, success has been limited due to the low-process efficiency of opsins in non-native cells.
Antisense oligonucleotide therapy
This is another innovative field of therapeutics for retinal-inherited diseases. Antisense oligonucleotide therapy (AON) targets the aberrant splicing mechanisms, preventing the translation of disease-causing proteins. AONs are DNA or Ribonucleic acid (RNA) molecules which can be delivered as naked oligonucleotides or through viral vectors. There may be several potential advantages of AONs. Increased penetration after intravitreal injections due to their small size may offer an alternative to the complex procedure of subretinal delivery. The limited stability of naked AONs may be associated with fewer side effects. While a single administration of AAV-mediated AON could give therapeutic benefits for a long time, naked AONs may need multiple intravitreal dosing over a lifetime. The efficacy of AAV-mediated and naked AON delivery for the treatment of CEP290-related disease has been studied.[44] In LCA, the aberrant splice junction created by the mutation in the CEP290 gene was corrected, and restored protein levels.
CRISPR/CAS9-based therapy—Genome editing
All current gene replacement strategies are inappropriate for autosomal dominant forms of inherited retinal diseases (IRDs). Gene editing is a new and exciting field within the spectrum of retinal gene therapy for repairing DNA mutations in living cells.
Gene editing is a process of introduction of an engineered nuclease leading to the generation of a double-strand break at a desired location on the genome, followed by an endogenous DNA repair process in the presence or absence of a gene correction donor DNA template.
The repair process can be achieved by either of the following methods:[40,43]
Non-homologous end joining (NHEJ), or
Homology directed repair (HDR).
CRISPR-Cas9 system is an engineered endonuclease guided by a short RNA containing a 20-nucleotide long complementarity region that recognizes the target DNA site by complement base paring and precisely makes a nick or a cut in the genome. These cuts are then repaired using the cellular DNA repair pathways such as NHEJ and HDR pathways.[41] During this induced DNA edit process, the pathogenic gene mutations can be corrected inside a living cell. It is a simplified molecular tool that does not require complex engineered proteins to recognize and cleave specific DNA sequences as in the case of other gene-editing tools such as Zinc-finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs).[41] Because of its simple construction, it has become the most popular genome-editing tool and gained utility in disease modeling, genetic screening, epigenome editing, cell labeling, and gene therapy applications. Its utilization in the retina is not only limited to inherited retinal dystrophy but also in AMD and diabetic retinopathy. As compared to the gene replacement strategy, gene editing enables the permanent reversal of genetic defects in the target cells. It finds a special application in autosomal dominant diseases, where gene disruption is more efficient than RNA interference. Also, mutations in large genes that exceed the cargo limits of the popular AAV-based viral vectors and intronic mutations that affect gene splicing can be effectively corrected using gene-editing methods.
The BRILLIANCE clinical trial is a Phase 1/2 study that is currently evaluating the safety, tolerability, and efficacy of EDIT-101, a gene editing-based treatment for Leber congenital amaurosis type 10. It is the first gene-editing therapy being evaluated in vivo in patients. The components of the CRISPR system used here are encoded in the genome of an AAV-based virus and injected directly into the subretinal space, near the photoreceptor cells. This is in contrast to the previous CRISPR-Cas9 clinical trials which have used the technique to edit the genomes of the cells removed from the body and were then transfused back into the patient after mutation correction.
Voretigene Neparvovec—Luxturna
Voretigene neparvovec (VN) is the first USFDA-approved gene replacement therapy. It was approved under the trade name Luxturna for the treatment of a more severe form of Leber congenital amaurosis type 2 (LCA2).
Biallelic mutations in the RPE65 gene cause LCA2, and are responsible for the fraction of all LCAs. RPE65 is responsible for retinol isomerization and converts all-trans-retinyl ester to 11-cis-retinol in phototransduction. The 11-cis-retinol then gets converted to 11-cis-retinal and is used in the regeneration of visual pigments in the photoreceptor cells. The subretinal delivery of the RPE65 gene with AAV serotype 2 (AAV2) efficiently infects the RPE cells. The AAV2 vector delivers a normal copy of RPE65 into the cell as a free-floating DNA outside of the chromosomes, called an episome, which does not integrate with the host nuclear DNA. This free-floating viral genome containing the transgene uses the host nuclear gene expression machinery to make the RPE65 mRNA, which then gets translated into a functional protein. This was found to improve the navigational abilities of treated patients.[42]
Since the RPE cells are non-dividing, the delivered viral genome is expected to remain stable inside the cells. Therefore, this gene therapy was proposed as a one-time treatment to last the entire lifetime of the patient. It can be administered to patients who carry mutations in both the alleles of the RPE65 gene and also have sufficient viable retinal cells to receive the viral vector.
VN was assessed in two Phase 1 and one Phase 3 open-label clinical trials. The phase III trial had 31 patients of biallelic RPE65 mutation with sufficient viable cells. Out of the 31 participants, 20 received the interventional drug VN in a dose of 1.5 × 1011 vector genomes (VG), while 9 were in the control arm. The study patients received a subretinal injection in each eye with a gap of 12 ± 6 days between the two eyes. The primary outcome was measured as the change in the bilateral multi-luminance mobility test (MLMT) performed at 1 year, compared to baseline which was found to be 1.8 in the interventional group versus 0.2 in the control group (P value: 0.0013, 95% CI). A majority of the participants (65%) in the intervention group passed the MLMT test at a luminance of 1 lux, which is the lowest luminance level tested, thus demonstrating the maximum possible functional improvement. None of the participants in the control group achieved this. The mean best corrected visual acuity (BCVA) change across both eyes at 1 year improved by 0.16 LogMAR from the baseline for the intervention group and decreased by 0.01 LogMAR for the control group. Post hoc visual acuity analysis using the scale adapted from Lange and colleagues[44] for off-chart acuities showed a 9.0 letter improvement in the intervention group versus a 1.6 letter improvement in the control subjects.[45]
VN, compared with standard care, showed improvement in navigational abilities, under bright or dim lighting conditions. This improvement was evident within the first 30 days after the subretinal injection and persisted through 1 year. However, it is still unknown whether this potential ‘one-time treatment’ has a lifelong benefit. Other challenges faced in the clinic would include the high cost of this treatment and difficulty in measuring functional improvement since clinicians do not routinely perform MLMT tests to assess improvement.
Gene Therapy for Acquired Retinal Diseases
Age-related macular degeneration is the fourth leading cause of blindness worldwide.[46] The current treatment strategies in neovascular AMD involve mainly anti-vascular endothelial growth factor (VEGF) injections, which help to stabilize the disease and also improve vision. However, it relies heavily on patient compliance with regular follow-up and multiple procedures, which adds to the psychological and financial burden of the patient. The treatment options for dry AMD lack strong evidence. Several clinical trials have been performed to analyze the safety and efficacy of the various vectors in both dry and wet AMD [Table 2].
Table 2.
Trials in gene therapy for age-related macular degeneration (AMD)
| Trial | Vector | Mechanism | Result |
|---|---|---|---|
| Intravitreal AdGVPDEF.11D | n E1-, partial E3-, E4- deleted adenoviral expressing human PEDF (AdPEDF.11) | PEDF expression with antiangiogenesis | No serious adverse events. Twenty-five patients showed mild transient intraocular inflammation. Limitations include a lack of control group and a small sample size |
| Subretinal rAAV. sFLT-1 Phase 1/IIA | Subretinally administered recombinant AAV (rAAV) with soluble fms-like tyrosine kinase-1 (sFLT-1) | Cellular expression of VEGF binding receptor FLT1 | No serious adverse events. Transient intraocular inflammation in 10%. No difference after removing outliers. |
| Intravitreal AAV2-sFLT01 (Phase 1) | Fusion protein of the sFLT-1 domain 2 with the Fc domain of IgG1 | Cellular expression of VEGF binding receptor FLT1 | No reported immunogenicity. No consistent response |
| RetinoStat (Phase 1) | Expression of angiostatin and endostatin by subretinal injection of equine infectious anemia lentivirus (EIAV-LV) | Antiangiogenesis | No adverse effects related to the lentivirus vector. Fluorescein angiography showed a reduction in the leakage in 71% of the patients, but significant reduction in intraretinal/subretinal fluid compared to the baseline was seen only in one patient. |
| Subretinal AAV-8-based anti-VEGF (RGX-314) (Phase 1/2a) | Nonreplicating, recombinant AAV serotype 8 (AAV8) vector encoding for a soluble anti-VEGF Fab | Binds to RPE cells to produce a therapeutic anti-VEGF protein. | Ongoing At 12 months-maintenance in vision (median of +5 letters) and anatomy (mean CRT reduction of 39 µm) despite a few to no rescue injections. |
| Hemera Biosciences (HMR59) | Intravitreal AAV2-CD59 | Transduces normal retinal cells to increase the expression of a soluble form of CD59 (inhibitor of MAC formation) | Ongoing |
| FOCUS trial-GT005 (Phase 1) | GT005-A recombinant nonreplicating adeno-associated viral (AAV) vector encoding a human complement factor | Targets complement activation | Ongoing |
| OPTIC trial- ADVM-022 (Phase 1) | Intravitreal AAV2.7m8 capsid expressing of the aflibercept protein | Antiangiogenesis | Ongoing 24-week data showed a good safety profile and efficacy |
First-generation trials
First-generation gene therapy trials were inconsistent, and thus, discontinued, although they did report safety of intravitreal and subretinal therapy.
Intravitreal AdGVPEDF.11D [GenVec, Gaithersburg, MD]
Pigment epithelium-derived factor (PEDF) helps in the regression of the choroidal neovascularization by virtue of its anti-angiogenic properties. In this trial, an adenoviral vector was modified to express human PEDF. It was one of the earliest trials in AMD and the results of the phase I trial were published in 2006. Transient ocular inflammation occurred in 25% of the patients, but no serious adverse events were reported. This study was limited by its small sample size and lack of a control group. However, a dose-response relationship was noted. The eyes receiving less than 108 particles had worsening of choroidal neovascularization (CNV) lesion and visual acuity, compared to the eyes receiving 108 particles or greater.[47]
Subretinal rAAV.sFLT-1 Phase 1/IIA [Adverum Biotechnologies, Redwood City, CA]
This is the first instance of gene therapy using rAAV to deliver anti-VEGF therapy for exudative AMD. Soluble fms-like tyrosine kinase-1 (sFLT-1) is an endogenously expressed inhibitor of VEGF A. Patients were randomized into two groups, interventional gene therapy group [receiving rAAV.sFLT-1; n = 21] or the control group [n = 11]. The dose of subretinal injection was divided into a low-dose (1 × 1010 VG) or high-dose (1 × 1011 VG) vector. All the patients received intravitreal injections of ranibizumab 0.05 mg at baseline, at week 4, and pro re nata (PRN) thereafter. Transient intraocular inflammation was seen in 10% of the eyes. Best-corrected visual acuity improved by a median of 1.0. Early treatment diabetic retinopathy study (ETDRS) letter from baseline in the intervention group receiving rAAV.sFLT-1 compared to the control group saw a median loss of 5 ETDRS letters from baseline. Three of the patients were treated with rAAV seroconverted.[48,49] This study confirmed the good safety profile of subretinally delivered rAAV and suggests the efficacy of gene therapy to treat wet AMD.
Intravitreal AAV2.sFLT01 [Sanofi Genzyme, Framingham, MA]
Intravitreal AAV2.sFLT01 is a fusion protein of the sFLT-1 domain 2 with the Fc domain of IgG1. A single intravitreal injection of AAV2.SFLT01 was administered to 19 patients divided into five separate cohorts [four dose-ranging cohorts (2 × 108 VG; 2 × 109 VG; 6 × 109 VG; and 2 × 1010 VG, n = 3 per cohort) and one maximum tolerated dose cohort (2 × 1010 VG, n = 7)]. Ten serious adverse events (SAE) occurred in five patients, with one reported death, which occurred 1 year after the study completion and 2 years after vector administration. The study did not show any consistent response due to the heterogeneity in the expression of sFLT01 and the effects of baseline presence of serum antibodies against AAV2.[50]
RetinoStat [Oxford BioMedica (UK) Ltd]
Angiostatin and endostatin have anti-angiogenic properties which have been proven in neonatal murine models of proliferative diabetic retinopathy.[51,52] Simultaneous expression of both these molecules by subretinal injection of one equine infectious anemia lentivirus (IEAV-LV) has been studied. This trial was an open-label, dose-ranging phase I study. Twenty-one patients with neovascular AMD were included in the trial and were placed in three dose-ranging groups. Each patient underwent vitrectomy following which they received a subretinal injection of different doses (group 1: 2.4 × 104 TU, n = 3; group 2: 2.4 × 105 TU, n = 3; group 3: 8 × 105 TU, n = 15; TU = transduction units). No adverse effects related to the lentivirus vector were seen. Even though 71% of the patients showed a reduction of dye leakage on fluorescein angiography, only one exhibited a substantial reduction in fluid from the baseline.[53]
Newer trials
Subretinal AAV-8-based anti-VEGF (RGX-314) [REGENXBIO, Rockville, MD]
This is a phase I/IIa trial using RGX-314, which is a recombinant AAV serotype 8 vector delivering a genome that induces the production of a soluble anti-VEGF Fab. Forty-two patients with severe neovascular AMD were treated across five dose-ranging cohorts, from 3 × 109 genome copies per eye to 2.5 × 1011 genome copies per eye. All cohorts received intravitreal injection of ranibizumab to evaluate a response prior to inclusion. Two weeks after this, the patients underwent vitrectomy followed by subretinal injection of RGX-314 in differing doses according to their cohort. No drug-related SAEs were observed. After 12 months, the study noted sustained levels of RGX-314 with stable anatomy (mean central retinal thickness [CRT] reduction of 39 microns), maintenance in vision (+5 letters) with a few to no rescue anti-VEGF injections. The mean change in BCVA from the baseline was stable or mildly improved.[54]
Intravitreal AAV2-CD59 [Hemera Biosciences, Newton, MA]
This is an ongoing trial targeted at dry AMD patients. The vector AAV2 transduces normal viable retinal cells to increase the expression of CD59. The CD59 glycoprotein, also known as membrane attack complex (MAC)-inhibitory protein, is a protein found in humans which inhibits apoptosis, and thereby, prevents cell death.[55] The trial included 25 subjects divided into dose-ranging cohorts, being assessed at 26 weeks, and then a safety evaluation at 18 months. The results are still awaited.
FOCUS trial [Gyroscope Therapeutics, Stevenage, UK]
The FOCUS study is a phase I/IIa, open-label, dose-ranging, multicenter clinical trial in dry AMD using the vector GT005. Vector GT005 is a recombinant AAV encoding a human complement factor protein. The study is aimed at individuals with geographic atrophy in dry AMD. The vector is injected as a single subretinal injection in genetically defined subjects. This is an ongoing trial.
OPTIC trial- ADVM-022 [Adverum Biotechnologies, Redwood City, CA]
This trial is currently in phase 1 and involves the intravitreal injection of AAV2.7m8 capsid expressing the aflibercept protein. A single intravitreal injection is given 7–14 days after a screening aflibercept injection with a concurrent 13-day topical or oral corticosteroid course for control of inflammation. The 24-week data showed a good safety profile, with mild-to-moderate inflammation. Efficacy at 24 weeks seemed promising, with no patients needing rescue injections of anti-VEGF.[56]
Conclusion
Mutations in over 270 genes probably contribute to the pathogenesis of retinal dystrophies. Until recently, the clinical approach for inherited diseases was limited to patient reassurance and counseling. After many years of exhaustive research and through multiple preclinical trials, gene therapy has now entered a promising era of sustained research, heralded by the first ocular therapeutic gene product with FDA approval. Two additional diseases (choroideremia [CHM] and Leber hereditary optic neuropathy [LHON], are in the Phase 3 trial. However, considering the involvement of multiple genes and the diverse set of mutations, a more comprehensive and personalized therapeutic strategy would be beneficial. Moving forward, the management of IRDs should not be limited to clinical evaluation, but the patients should be encouraged to undergo genetic testing for potential gene identification and possibly to participate in ongoing retinal gene therapy trials. In wet AMD, anti-VEGF injections have been the standard treatment strategy over the past decade. However, a large percentage of these patients remain undertreated due to non-compliance. Gene therapy for AMD is still in its nascent stages, but it could potentially revolutionize the treatment paradigm due to sustained effects over long term, overcoming compliance issues and improved patient satisfaction.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Dr. Sushma J – support in collection of material
Dr. Jay Chhablani – reviewing and editing changes
Dr Brijesh Takkar – reviewing and editing changes.
References
- 1.Anand V, Duffy B, Yang Z, Dejneka NS, Maguire AM, Bennett J. A deviant immune response to viral proteins and transgene product is generated on subretinal administration of adenovirus and adeno-associated virus. Molecular therapy. 2002;5:125–32. doi: 10.1006/mthe.2002.0525. [DOI] [PubMed] [Google Scholar]
- 2.Willett KL, Bennett J. Immunology of AAV-mediated gene transfer in the eye. Front immunol. 2013;4:261. doi: 10.3389/fimmu.2013.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21(Suppl-6):3–9. doi: 10.5301/EJO.2010.6049. [DOI] [PubMed] [Google Scholar]
- 4.Pichi F, Morara M, Veronese C, Nucci P, Ciardella AP. Multimodal imaging in hereditary retinal diseases. J Ophthalmol. 2013;2013:634351. doi: 10.1155/2013/634351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle:Retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz A, Winston A, Lim Y-H, Gilbert BA, Rando RR, Bok D. Molecular and biochemical characterization of lecithin-retinol acyltransferase. J Biol Chem. 1999;274:3834–41. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 7.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biolo Chem. 2004;279:10422–32. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borman AD, Ocaka LA, Mackay DS, Ripamonti C, Henderson RH, Moradi P, et al. Early onset retinal dystrophy due to mutations in LRAT:Molecular analysis and detailed phenotypic study. Invest Ophthalmol Vis Sci. 2012;53:3927–38. doi: 10.1167/iovs.12-9548. [DOI] [PubMed] [Google Scholar]
- 9.Batten ML, Imanishi Y, Tu DC, Doan T, Zhu L, Pang J, et al. Pharmacological and rAAV gene therapy rescue of visual functions in a blind mouse model of Leber congenital amaurosis. PLoS Med. 2005;2:e333. doi: 10.1371/journal.pmed.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redmond TM, Poliakov E, Yu S, Tsai J-Y, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102:13658–63. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanein S, Perrault I, Gerber S, Tanguy G, Barbet F, Ducroq D, et al. Leber congenital amaurosis:Comprehensive survey of the genetic heterogeneity, refinement of the clinical definition, and genotype–phenotype correlations as a strategy for molecular diagnosis. Hum Mutat. 2004;23:306–17. doi: 10.1002/humu.20010. [DOI] [PubMed] [Google Scholar]
- 12.Sahu B, Maeda A. Retinol dehydrogenases regulate vitamin a metabolism for visual function. Nutrients. 2016;8:746. doi: 10.3390/nu8110746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis:Genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Parker RO, Crouch RK. Retinol dehydrogenases (RDHs) in the visual cycle. Exp Eye Res. 2010;91:788–92. doi: 10.1016/j.exer.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrault I, Delphin N, Hanein S, Gerber S, Dufier JL, Roche O, et al. Spectrum of NPHP6/CEP290 mutations in Leber congenital amaurosis and delineation of the associated phenotype. Hum Mutat. 2007;28:416. doi: 10.1002/humu.9485. [DOI] [PubMed] [Google Scholar]
- 16.Schuster A, Janecke AR, Wilke R, Schmid E, Thompson DA, Utermann G, et al. The phenotype of early-onset retinal degeneration in persons with RDH12 mutations. Invest Ophthalmol Vis Sci. 2007;48:1824–31. doi: 10.1167/iovs.06-0628. [DOI] [PubMed] [Google Scholar]
- 17.Skorczyk-Werner A, Pawłowski P, Michalczuk M, Warowicka A, Wawrocka A, Wicher K, et al. Fundus albipunctatus:Review of the literature and report of a novel RDH5 gene mutation affecting the invariant tyrosine (p. Tyr175Phe) J Appl Genet. 2015;56:317–27. doi: 10.1007/s13353-015-0281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeffer B, Wiggert B, Lee L, Zonnenberg B, Newsome D, Chader G. The presence of a soluble interphotoreceptor retinol-binding protein (IRBP) in the retinal interphotoreceptor space. J Cell Physiol. 1983;117:333–41. doi: 10.1002/jcp.1041170308. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh D, Griswold JB, Bevilacqua T, Gonzalez-Fernandez F. Purification of the full-length Xenopus interphotoreceptor retinoid binding protein and growth of diffraction-quality crystals. Mol Vis. 2007;13:2275–81. [PMC free article] [PubMed] [Google Scholar]
- 20.den Hollander AI, McGee TL, Ziviello C, Banfi S, Dryja TP, Gonzalez-Fernandez F, et al. A homozygous missense mutation in the IRBP gene (RBP3) associated with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2009;50:1864–72. doi: 10.1167/iovs.08-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arno G, Hull S, Robson AG, Holder GE, Cheetham ME, Webster AR, et al. Lack of interphotoreceptor retinoid binding protein caused by homozygous mutation of RBP3 is associated with high myopia and retinal dystrophy. Invest Ophthalmol Vis Sci. 2015;56:2358–65. doi: 10.1167/iovs.15-16520. [DOI] [PubMed] [Google Scholar]
- 22.Park PS-H. Constitutively active rhodopsin and retinal disease. Adv Pharmacol. 2014;70:1–36. doi: 10.1016/B978-0-12-417197-8.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbard R, Kropf A. The action of light on rhodopsin. Proc Natl Acad Sci U S A. 1958;44:130–9. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredriksson R, Lagerström MC, Lundin L-G, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 25.Chaum E, Hatton MP. Gene therapy for genetic and acquired retinal diseases. Surv Ophthalmol. 2002;47:449–69. doi: 10.1016/s0039-6257(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 26.Bainbridge J, Tan M, Ali R. Gene therapy progress and prospects:The eye. Gene Ther. 2006;13:1191–7. doi: 10.1038/sj.gt.3302812. [DOI] [PubMed] [Google Scholar]
- 27.Trapani I. Adeno-associated viral vectors as a tool for large gene delivery to the retina. Genes. 2019;10:287. doi: 10.3390/genes10040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes:Vector toolkit for human gene therapy. Mol Ther. 2006;14:316–27. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353–9. doi: 10.1016/j.visres.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Conley SM, Cai X, Naash MI. Nonviral ocular gene therapy:Assessment and future directions. Curr Opin Mol Ther. 2008;10:456–63. [PMC free article] [PubMed] [Google Scholar]
- 31.Peeters L, Sanders NN, Braeckmans K, Boussery K, Van de Voorde J, De Smedt SC, et al. Vitreous:A barrier to nonviral ocular gene therapy. Invest Ophthalmol Vis Sci. 2005;46:3553–61. doi: 10.1167/iovs.05-0165. [DOI] [PubMed] [Google Scholar]
- 32.Ali RR, Sarra GM, Stephens C, Alwis MD, Bainbridge JW, Munro PM, et al. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet. 2000;25:306–10. doi: 10.1038/77068. [DOI] [PubMed] [Google Scholar]
- 33.D'Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–51. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 34.Vollrath D, Feng W, Duncan JL, Yasumura D, D'Cruz PM, Chappelow A, et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci U S A. 2001;98:12584–9. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai CM, Yu MJ, Brankov M, Barnett NL, Zhou X, Redmond TM, et al. Recombinant adeno-associated virus type 2-mediated gene delivery into the Rp.e65(-/-) knockout mouse eye results in limited rescue. Genet Vaccines Ther. 2004;2:3. doi: 10.1186/1479-0556-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenekoop RK. The gene for Stargardt disease, ABCA4, is a major retinal gene:A mini-review. Ophthalmic Genet. 2003;24:75–80. doi: 10.1076/opge.24.2.75.13996. [DOI] [PubMed] [Google Scholar]
- 37.Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D, et al. Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice. J Clin Invest. 2008;118:1955–64. doi: 10.1172/JCI34316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore NA, Bracha P, Hussain RM, Morral N, Ciulla TA. Gene therapy for age-related macular degeneration. Expert Opin Biol Ther. 2017;17:1235–44. doi: 10.1080/14712598.2017.1356817. [DOI] [PubMed] [Google Scholar]
- 39.George ND, Yates JR, Moore AT. X linked retinoschisis. Br J Ophthalmol. 1995;79:697–702. doi: 10.1136/bjo.79.7.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ran FA, Hsu PD, Lin CY, Gootenberg JS, Konermann S, Trevino AE, et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154:1380–9. doi: 10.1016/j.cell.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, et al. Long-term effect of gene therapy on Leber's congenital amaurosis. N Engl J Med. 2015;372:1887–97. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komor AC, Badran AH, Liu DR. CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell. 2017;168:20–36. doi: 10.1016/j.cell.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lange C, Feltgen N, Junker B, Schulze-Bonsel K, Bach M. Resolving the clinical acuity categories “hand motion“and “counting fingers“using the Freiburg Visual Acuity Test (FrACT) Graefes Archive Clin Exp Ophthalmol. 2009;247:137–42. doi: 10.1007/s00417-008-0926-0. [DOI] [PubMed] [Google Scholar]
- 45.Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy:A randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alswailmi FK. Global prevalence and causes of visual impairment with special reference to the general population of Saudi Arabia. Pak J Med Sci. 2018;34:751–6. doi: 10.12669/pjms.343.14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, et al. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration:Results of a phase I clinical trial. Hum Gene Ther. 2006;17:167–76. doi: 10.1089/hum.2006.17.167. [DOI] [PubMed] [Google Scholar]
- 48.Constable IJ, Pierce CM, Lai CM, Magno AL, Degli-Esposti MA, French MA, et al. Phase 2a randomized clinical trial:Safety and post hoc analysis of subretinal rAAV.sFLT-1 for wet age-related macular degeneration. EBioMedicine. 2016;14:168–75. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Constable IJ, Lai CM, Magno AL, French MA, Barone SB, Schwartz SD, et al. Gene therapy in neovascular age-related macular degeneration:Three-Year Follow-up of a phase 1 Randomized Dose Escalation Trial. Am J Ophthalmol. 2017;177:150–8. doi: 10.1016/j.ajo.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 50.Heier JS, Kherani S, Desai S, Dugel P, Kaushal S, Cheng SH, et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration:A phase 1, open-label trial. Lancet. 2017;390:50–61. doi: 10.1016/S0140-6736(17)30979-0. [DOI] [PubMed] [Google Scholar]
- 51.Igarashi T, Miyake K, Kato K, Watanabe A, Ishizaki M, Ohara K, et al. Lentivirus-mediated expression of angiostatin efficiently inhibits neovascularization in a murine proliferative retinopathy model. Gene therapy. 2003;10:219–26. doi: 10.1038/sj.gt.3301878. [DOI] [PubMed] [Google Scholar]
- 52.Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14:313–22. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- 53.Campochiaro PA, Lauer AK, Sohn EH, Mir TA, Naylor S, Anderton MC, et al. Lentiviral vector gene transfer of endostatin/angiostatin for macular degeneration (GEM) study. Hum Gene Ther. 2017;28:99–111. doi: 10.1089/hum.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Fortmann SD, Shen J, Wielechowski E, Tretiakova A, Yoo S, et al. AAV8-antiVEGFfab ocular gene transfer for neovascular age-related macular degeneration. Mol Ther. 2018;26:542–9. doi: 10.1016/j.ymthe.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990;144:3478–83. [PubMed] [Google Scholar]
- 56.Grishanin R, Vuillemenot B, Sharma P, Keravala A, Greengard J, Gelfman C, et al. Preclinical evaluation of ADVM-022, a novel gene therapy approach to treating wet age-related macular degeneration. Mol Ther. 2019;27:118–29. doi: 10.1016/j.ymthe.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]