Abstract
Purpose:
The aim of this study was to determine the alteration in ganglion cell complex and its relationship with retinal nerve fiber layer (RNFL) thickness as measured by spectral-domain optical coherence tomography (OCT) in pituitary adenoma cases and also its correlation with visual field (VF).
Methods:
This is a prospective comparative study wherein detailed neuro-ophthalmic examination including perimetry, RNFL and ganglion cell layer inner plexiform layer (GCL-IPL) thickness were measured preoperatively in the cases of pituitary adenoma with chiasmal compression with visual symptoms and field changes who were planned for neuro-surgical intervention. These parameters were repeated 1 year after the surgery. GCL-IPL, RNFL parameters were compared with controls and were correlated with VF mean deviation (MD). The diagnostic power of GCL-IPL was tested using the receiver operating characteristic (ROC) curve. Healthy age and sex-matched controls without any ocular and systemic abnormality were taken for comparison.
Results:
Twenty-four patients qualified the inclusion criteria. A significant thinning of GCL-IPL (P = 0.002) and RNFL (P = 0.039) was noticed in the pituitary adenoma group. GCL-IPL (r = 0.780 P < 0.001) and RNFL (r = 0.669, P < 0.001) were significantly correlated with the MD. The ROC curve values of GCL-IPL were 0.859 (95% confidence interval 0.744% to 0.973) and of RNFL were 0.731 (95% confidence interval 0.585–0.877). The diagnostic ability of GCL-IPL was more as compared to the RNFL analysis, although it was statistically insignificant (P = 0.122).
Conclusion:
GCL-IPL measurements on the OCT are a sensitive tool to detect early anterior visual pathway changes in chiasmal compression for pituitary adenoma patients.
Keywords: Chiasmal compression, ganglion cell complex thickness, ganglion cell layer-inner plexiform laye, pituitary adenoma, retinal nerve fiber layer, visual field
Pituitary adenoma constitutes 6%–12% of all symptomatic intracranial tumors.[1] On enlargement, it has the propensity to extend beyond the sella turcica encroaching upon the visual pathways and ocular motor nerves in the cavernous sinus. Bitemporal hemianopia is the commonest visual field (VF) defect on perimetry due to chiasmal compression.[2,3] The VF has always been considered as the investigation of choice in the assessment of lesions affecting visual pathways. As the axons arising from macular ganglion cells occupy voluminous portions of the optic nerve, chiasm, and optic tracts, there has been a growing interest in the structural assessments of axonal loss affecting these pathways.[4] Currently, the focus of the research has shifted toards retinal assessment for early diagnosis, prognosis, and follow-up in these disorders. Optical coherence tomography (OCT) has emerged as a tool for the quantification of retinal nerve fiber layer (RNFL) and retinal ganglion cell-inner plexiform layer (GCL-IPL) in various disorders affecting visual pathways including multiple sclerosis.[5]
As the retinal axons are nonmyelinated until they penetrate the lamina cribrosa, the RNFL is an optimal structure to study the processes of neurodegeneration and neuro-repair. Axonal and neuronal degeneration are important features of multiple sclerosis and other neurologic disorders that affect the anterior visual pathway. Only a few studies assessing retinal layers in pituitary adenoma have been published.[6,7,8,9,10,11,12,13] Ours is a prospective study aimed to evaluate and compare the regional relationships between GCL-IPL and RNFL thickness as measured by spectral-domain optical coherence tomography (SD-OCT) in pituitary adenoma cases and its correlation with the VF in Indian subjects. These cases were evaluated preoperatively and 1 year postoperatively.
Methods
Twenty-four cases of pituitary adenoma with visual symptoms and field changes that were referred to us from the Department of Neurosurgery for visual assessment before surgery for the same were studied prospectively. This study was performed in a tertiary care super-speciality Institution of India from January 2019 to December 2019. It was performed as per the Declaration of Helsinki and Institutional Ethics Committee clearance was obtained for the same. Informed consent was also obtained from the study participants.
The detailed neuro-ophthalmic examination was carried out including best-corrected-visual-acuity on Snellen’s chart under standard illumination and color vision by Ishihara pseudoisochromatic test plates. Slit-lamp evaluation for the anterior segment and fundus examination was performed in all subjects. Intraocular pressure was also measured with a noncontact tonometer. All participants were subjected to Standard Automated Perimetry (Humphrey field analyzer model 745i, SITA 30-2 Standard program). Only reliable VFs were taken for the study. Macular GCL-IPL and RNFL measurements were taken on Cirrus SD-OCT (Cirrus HD-OCT). All the aforementioned investigations were done preoperatively and 1 year postoperatively in pituitary adenoma cases. Only a single person performed the investigations to rule out the interobserver variation.
Retinal nerve fiber layer and ganglion cell layer complex thickness were measured using Cirrus HD-OCT. SD-OCT was done using Cirrus HD-OCT Model 4000 (Carl Zeiss Meditec Inc, Dublin, California) and RNFL (RNFL) and thickness was assessed using an Optic Disc Cube 200 X 200 scan. Average RNFL thickness was analyzed in four quadrants. The macular volume was assessed using a macular cube 512 X 128 scan. The ganglion cell analysis algorithm was used to analyze the ganglion cell–inner plexiform layer complex (GCL-IPL) separately. GCL-IPL was analyzed in six sectors, in addition to average and minimum thickness values. Receiver operating curve areas were calculated to compare the diagnostic power of GCL-IPL and RNFL analysis. The data obtained were analyzed statistically
The patients with pituitary adenoma more than 18 years of age showing chiasmal compression on neuroimaging were included in our study. These patients were referred to us for vision and VF assessments and were planned for neuro-surgical intervention. The subjects included had visual symptoms and field changes. The patients having prior retinal disease who were likely to show OCT changes or preclude accurate examination, patients with glaucoma and other pre-existing neurodegenerative disorders, myopia more than 6 diopters, media opacity, dropouts who do not wish to undergo surgery, those unwilling to cooperate for examination were excluded from the study. The age and sex-matched controls were selected from healthy volunteer adults without any systemic and ocular disease. Only a single (right) eye of a subject was included in the study.
The sample size was calculated using a minimum 95% confidence interval and 90% power of the study. Assuming 0.35 standard deviation of the effect size of the means, the calculated sample size came out for each of the groups, to be 17. Finally for this study sample size was 24. PASS-8 (Power and sample size) software version 8.0 has been used to calculate sample size. Equal numbers of controls falling in the same age group were taken. The variables in the study group were compared with the control group using the independent sample t test and Chi-square test for quantitative and qualitative data, respectively. A value of P < 0.05 was considered as statistical significance. Due to a limited number of patients in pre- and postintervention group, we have presented the raw values of the study parameters of both eyes of the subjects.
Results
Twenty-four patients with pituitary adenoma fulfilled the inclusion criteria and constituted the study group. The mean age of the study group was 39.38 ± 14 years. The mean age and sex ratio were comparable between the study group and the control group (P > 0.05). The baseline characteristics of study subjects and controls are tabulated in Table 1.
Table 1.
Baseline characteristics of patients with pituitary adenoma and healthy controls
| Characteristics | Study Group (Patients with Pituitary Adenoma) | Control Group | P |
|---|---|---|---|
| No. of Patients | 24 | 24 | |
| No. of Eyes | 24 | 24 | |
| Mean Age (±SD) years | 39.38±14 | 36.46±5.75 | 0.352* |
| Sex Ratio Male/Female | 15/9 | 15/9 | 0.999** |
| Mean Retinal Nerve Fibre Layer thickness (±SD) µ | 84.66±20.83 | 96.16±9.26 | 0.039 |
| Mean Ganglion Cell Layer-Inner Plexiform Layer thickness (±SD) µ | 65.35±18.90 µ | 82.58±3.94 | 0.002* |
| Visual Field mean deviation dB (±SD) | 16.34±7.23 | 1.01±0.88 | <0.001 |
*Independent Sample ‘t’ test; **Chi-square test
The mean RNFL thickness in the study group and control group was 84.66 ± 20.83 and 96.16 ± 9.26, respectively. It was significantly lesser in the study group as compared to the control group P = 0.039). Sectoral retinal nerve fiber layer (RNFL) thickness was compared between the two groups, as shown in Table 2. There was a trend of thinning of RNFL in all sectors in the study group with pituitary adenoma cases as compared to healthy controls. But it was not statistically significant in superior (P = 0.069) and temporal (P = 0.523) sectors. However, there was a statistically significant difference in inferior (P = 0.001) and nasal sector (P = 0.001) of RNFL between the two groups.
Table 2.
Sectoral retinal nerve fibre layer thickness measurements between the study group and control group. B
| Retinal Nerve Fiber Layer thickness | Study Group (Pituitary Adenoma) | Control Group | P* |
|---|---|---|---|
| Superior (±SD) µ | 105.94±29.90 | 120.29±12.25 | 0.069 |
| Temporal (±SD) µ | 60.94±22.31 | 64.25±10.17 | 0.523 |
| Inferior (±SD) µ | 80.25±54.22 | 124.21±16.97 | 0.001 |
| Nasal (±SD) µ | 48.83±31.89 | 73.71±10.74 | 0.001 |
| Average (±SD) µ | 84.66±20.83 | 96.16±9.26 | 0.039 |
*Independent Sample ‘t’ test
The comparison of GCL-IPL thickness is shown in Table 3. The average GCL-IPL thickness was 65.35 ± 18.90 in the study group patients, whereas it was 82.58 ± 3.94 in the control group (P = 0.002). A significant thinning of GCL-IPL of average, minimum, and all sectors have been noticed in the study group as compared to controls (P < 0.05). Patterns of GCL-IPL thinning corresponded to VF defects and significantly correlated with the MD (mean deviation) of VF. The diagnostic ability of both GCL-IPL and RNFL was assessed using the receiver operating characteristic (ROC) curve [Fig. 1]. The ROC curve values of GCL-IPL were 0.859 (95% Confidence Interval 0.744% to 0.973) and of RNFL were 0.731 (95% Confidence Interval 0.585–0.877). The diagnostic ability of GCL-IPL was more compared to RNFL analysis although it was statistically insignificant (P = 0.122). The statistical significance might be lacking due to relatively small sample size.
Table 3.
Ganglion cell layer-inner plexiform layer (GCL-IPL) measurements between the study group and control group
| GCL-IPL thickness | Study Group (Pituitary Adenoma) | Control Group | P* |
|---|---|---|---|
| Superior (±SD) µ | 63.00±21.87 | 83.35±4.03 | 0.001 |
| Inferior (±SD) µ | 64.50±19.05 | 81.65±3.80 | 0.001 |
| Inferotemporal (±SD) µ | 66.00±19.97 | 81.87±4.50 | 0.004 |
| Inferonasal (±SD) µ | 46.71±32.52 | 80.63±17.94 | <0.001 |
| Superotemporal (±SD) µ | 66.39±15.58 | 80.39±4.47 | 0.002 |
| Superonasal (±SD) µ | 49.54±33.58 | 81.79±18.14 | <0.001 |
| Average (±SD) µ | 65.35±18.90 | 82.58±3.94 | 0.002 |
| Minimum (±SD) µ | 38.75±32.48 | 79.96±4.11 | <0.001 |
*Independent Sample ‘t’ test
Figure 1.
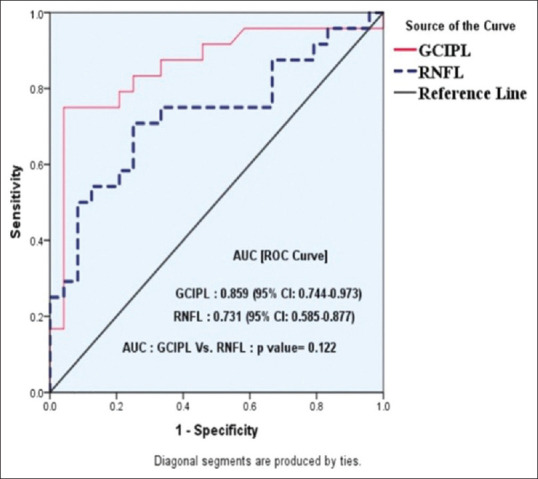
Receiver operating characteristic curve of ganglion cell-inner plexiform layer and retinal nerve fiber layer
In the VF pattern of the study group, 17 patients had bitemporal hemianopia, 6 patients had temporal hemianopia in one eye with preservation of the superonasal quadrant and one patient was not able to perform VF due to the poor visual acuity. The MD in the VF was correlated with the preoperative RNFL and GCL-IPL measurements. It was positively correlated with RNFL (r = 0.669, P < 0.001) and GCL-IPL (r = 0.780 P < 0.001).
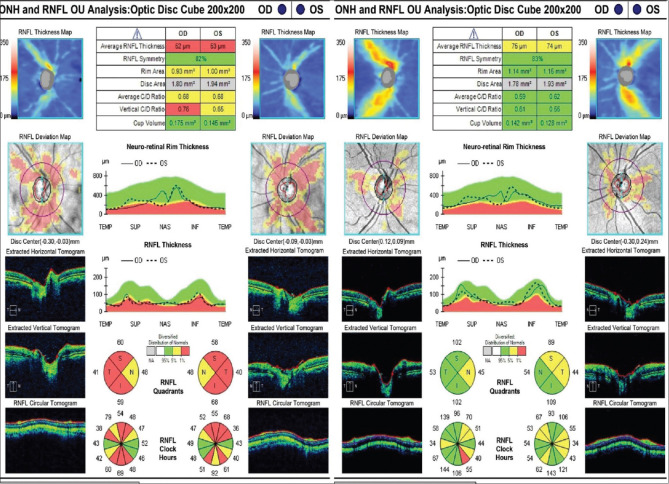
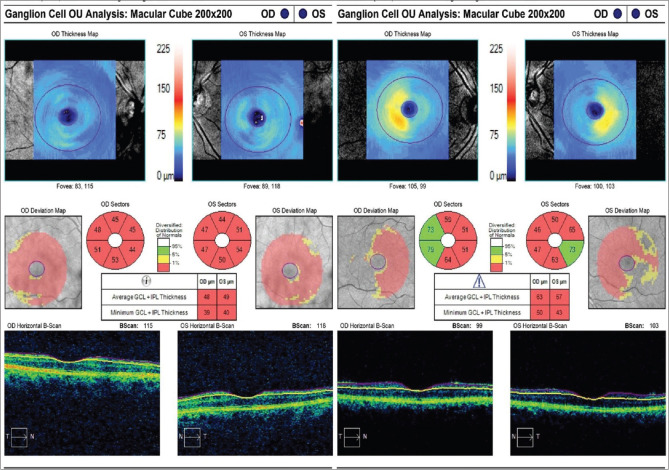
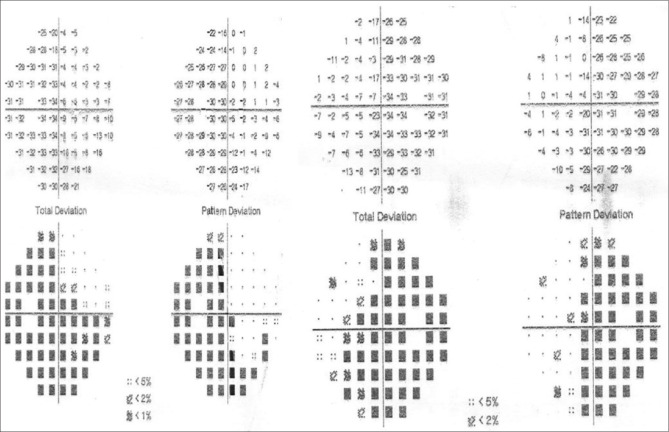
Only five patients completed a 1-year follow-up. The VF MD, GCL-IPL parameters, and RNFL thickness were compared from the baseline values before surgery [Table 4]. Due to the lesser number of patients with follow-up, all the parameters were compared individually. The VF MD appears to have improved. The missing values of MD in patients 2 and 5 are because of the inability to perform VF examination due to the poor visual acuity (<20/200). However, GCL-IPL and RNFL values did not change significantly except for patient 5 who showed improvement at 1 year of follow-up [Table 4]. Patient 5 showed improvement in all sectors of RNFL except the nasal quadrant in the right eye [Fig. 2]. GCL-IPL average and all sectors showed improvement [Fig. 3] in this patient. However, the VF could not be performed in this patient due to the poor visual acuity (<20/200) preoperatively. After surgery, with the gain in visual acuity of 20/40 in both eyes, his recorded VF examination at 1 year is shown in Fig. 4.
Table 4.
The comparison of raw values of visual field mean deviation (MD), Ganglion cell layer-inner plexiform layer (GCL-IPL), retinal nerve fibre layer (RNFL) of five patients before and after 1 year of surgery for pituitary adenoma
| Parameters | Pre-operative | Post-operative | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | |
| Visual Field MD | ||||||||||
| Right Eye | -2.37 | - | -19.35 | -3.67 | - | -2.06 | - | -12.90 | -1.99 | -18.56 |
| Left Eye | -1.42 | -16.83 | - 14.34 | -3.92 | - | -1.02 | -8.34 | -12.78 | -2.96 | -19.54 |
| GCL-IPL Average | ||||||||||
| Right Eye | 83 | 58 | 76 | 61 | 48 | 81 | 60 | 61 | 60 | 63 |
| Left Eye | 80 | 53 | 79 | 71 | 49 | 82 | 54 | 80 | 71 | 57 |
| RNFL Average | ||||||||||
| Right Eye | 98 | 45 | 116 | 109 | 52 | 104 | 47 | 118 | 110 | 75 |
| Left Eye | 106 | 60 | 110 | 80 | 53 | 106 | 61 | 112 | 81 | 74 |
| RNFL Superior | ||||||||||
| Right Eye | 137 | 54 | 132 | 126 | 60 | 140 | 58 | 136 | 130 | 102 |
| Left Eye | 148 | 72 | 135 | 108 | 58 | 146 | 73 | 138 | 110 | 89 |
| RNFL Inferior | ||||||||||
| Right Eye | 113 | 54 | 170 | 112 | 59 | 122 | 56 | 172 | 112 | 102 |
| Left Eye | 132 | 76 | 153 | 110 | 68 | 136 | 78 | 150 | 110 | 109 |
| RNFL Nasal | ||||||||||
| Right Eye | 81 | 20 | 102 | 60 | 48 | 83 | 20 | 104 | 62 | 45 |
| Left Eye | 80 | 50 | 97 | 56 | 48 | 82 | 50 | 99 | 58 | 54 |
| RNFL Temporal | ||||||||||
| Right Eye | 63 | 54 | 62 | 138 | 41 | 71 | 54 | 62 | 135 | 53 |
| Left Eye | 64 | 40 | 57 | 44 | 40 | 62 | 42 | 60 | 45 | 44 |
Pt: Patient
Figure 2.
Retinal nerve fiber layer analysis of one (patient 5 in Table 4) of our study patients showing improvement in all parameters (except nasal quadrant in right eye) postoperatively after 1 year of pituitary adenoma surgery. (right: preoperative; left: postoperative scan)
Figure 3.
Ganglion cell complex analysis of the same patient as in Fig. 2, showing improvement in all parameters after pituitary resection at 1 year of follow-up. (right: preoperative; left: postoperative scan)
Figure 4.
Visual field of the same patient as in Fig. 2, showing bitemporal hemianopia after 1 year of surgery with the improvement of vision up to 20/40 both eyes. Preoperative visual field could not be performed due to the low visual acuity (<20/200)
Discussion
The role of the ganglion cell layer complex (GCL-IPL) examination is being increasingly investigated in neuro-ophthalmic practice. It is an objective method of quantifying the damage to the visual pathways. It has been correlated with perimetry and is predictive of future progression in glaucoma disorder.[14] The role of GCL-IPL has been studied for numerous neuro-ophthalmic disorders.[15] In contrast to RNFL which contains axons, the macula contains more than 50% of RGC which can be quantified relatively easily.[16,17,18] Moreover GCL-IPL is topographically less variable among normal individuals than RNFL, hence deviation from normal is easier to detect and quantify.[17] In our study, we found the significant thinning of GCL-IPL in all sectors and as an average in pituitary adenoma patients similar to the previous studies.[6,7,16,17]
Studies have shown that RNFL thinning and volume depletion in the macula are associated with stereotypic fall in visual functioning, as measured by low-contrast letter acuity and VF analyses.[19] We have also noted the changes in VFs in pituitary adenoma cases as compared to controls, it (MD) showed a positive correlation with RNFL (P < 0.001) and GCL-IPL (P < 0.001). It has also been shown that the changes in retinal anatomical structures correspond to VF defect and can be used to anticipate post-operative recovery.[7,8,9] Patterns of GCL-IPL thinning and RNFL thinning corresponding to VF defects also resonates with our work [Figs. 2 and 3]. Tang Y et al. analyzed the differences and correlation between the ganglion cell complex (GCL-IPL), Retinal Nerve Fiber Layer (RNFL) and MD, mean sensitivity (MS) of saddle area tumor patients and evaluated the superiority of using OCT to diagnose the visual pathway disturbance of saddle area tumor patients.[10] Like us, they also concluded that the average thickness of the RNFL and GCL-IPL is correlated with VF damage, which can be used to evaluate optic nerve damage of saddle area tumor patients quantitatively. The thickness of the RNFL and GCL-IPL was lesser, and the damage to visual functions was marked.[10] Another study that monitored optic neuropathy in pediatric craniopharyngioma by peripapillary OCT derived that a thinner RNFL on ocular coherence tomography before surgery correspond to poorer visual acuity and VF loss. They also concluded that Ocular coherence tomography may serve as an objective method to quantify axonal loss caused by craniopharyngioma.[11]
RNFL changes after transsphenoidal and transcranial pituitary adenoma resection were studied and it was pinpointed that there was no change of RNFL thickness in pituitary adenoma patients who underwent transsphenoidal surgery.[12] We also noticed that RNFL does not change significantly at 1-year post-surgery. Another study regarding reversible dysfunction of retinal ganglion cells in nonsecreting pituitary tumors used the pattern electroretinogram (PERG) to expose retinal ganglion cell (RGC) dysfunction in some disorders. They concluded that the presence of a pituitary tumor, even in the absence of compressive effect at the chiasm on MRI, may cause reversible RGC dysfunction, which precedes VF loss and RGC death as supported by the unaffected RNFL on OCT.[20] Chiasmal compression typically results in nasal and temporal RNFL thinning and nasal macular GCL-IPL thinning as crossing nasal retinal fibers are primarily affected.[8,21] The horizontal sectors of the RNFL showed greater thinning compared with the vertical portion of the optic disc, which explains band atrophy associated with chiasmal lesions. In our study, patients with chiasmal compression have a greater degree of thinning in the nasal macula due to disruption of crossing fibers originating in the nasal retina.
After surgery, RNFL and macular GCL-IPL do not significantly change, even in patients with outstanding visual recovery. It is observed that the macular GCL-IPL remain thinner with improvement in VFs postoperatively. The postoperative patient may appear to have a paradoxical worsening of the OCT measurements with the improvement of visual function, as it takes 6 weeks for retrograde degeneration to be completed. Visual recovery occurs gradually and it begins with prompt removal of conduction block, followed by secondary remyelination and restoration of axoplasmic flow months to years after surgery.[13] In our 5 patients that followed up, VFs improved in all but there was no significant change in RNFL or GCL-IPL except one patient who had significantly better parameters.
The various pattern of GCL-IPL loss with no VF changes are observed which indicate the capacity to detect early or mild chiasmal compression.[22,23] The patients with VF defects and relatively preserved GCC thickness will have better post op recovery.[9,24]
Our study showed that the GCL-IPL complex measurement was a relatively better diagnostic tool as compared to RNFL and also showed a better correlation with the VF defect. The possible explanation for this is that the retinal ganglion cell bodies are 10-20 times thicker as compared to axons which represent RNFL.[3] Also, the ganglion cell layer is 2 or more cell thick in the macula. Therefore, GCL-IPL investigation is a relatively more sensitive measurement for picking-up early defects.
This study was strengthened by adequate sample size and power, however, the limited number of follow-up patients was a major drawback. We were unable to do any statistical test for comparison. Therefore, we presented the raw data of both eyes in these patients for individual assessment. Also, the inclusion of only symptomatic patients could have excluded the asymptomatic patients with early chiasmal compression.
Conclusion
In conclusion, GCL-IPL measurements on OCT are a sensitive tool to detect early anterior visual pathway changes in chiasmal compression. This could be considered in the routine evaluation of pituitary adenoma patients along with VF examination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ironside JW. Pituitary gland pathology. J Clin Pathol. 2003;56:561–8. doi: 10.1136/jcp.56.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McIlwaine GG, Carrim ZI, Lueck CJ, Chrisp TM. A mechanical theory to account for bitemporal hemianopia from chiasmal compression. J Neuroophthalmol. 2005;25:40–3. doi: 10.1097/00041327-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:2012–7. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyt WF, Luis O. Visual fibre anatomy in the infra geniculate pathway of the primate:Uncrossed and crossed retinal quadrant fibre projection studied with Nauta silver stain. Arch Ophthalmol. 1962;68:94–106. doi: 10.1001/archopht.1962.00960030098018. [DOI] [PubMed] [Google Scholar]
- 5.Noval S, Contreras I, Rebolleda G, Muñoz-Negrete FJ. Optical coherence tomography versus automated perimetry for follow-up of optic neuritis. Acta Ophthalmol Scand. 2006;84:790–4. doi: 10.1111/j.1600-0420.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 6.Blanch RJ, Micieli JA, Oyesiku NM, Newman NJ, Biousse V. Optical coherence tomography retinal ganglion cell complex analysis for the detection of early chiasmal compression. Pituitary. 2018;21:515–23. doi: 10.1007/s11102-018-0906-2. [DOI] [PubMed] [Google Scholar]
- 7.Moon JS, Shin SY. Segmented retinal layer analysis of chiasmal compressive optic neuropathy in pituitary adenoma patients. Graefes Arch Clin Exp Ophthalmol. 2020;258:419–25. doi: 10.1007/s00417-019-04560-3. [DOI] [PubMed] [Google Scholar]
- 8.Micieli JA, Newman NJ, Biousse V. The role of optical coherence tomography in the evaluation of compressive optic neuropathies. Curr Opin Neurol. 2019;32:115–23. doi: 10.1097/WCO.0000000000000636. [DOI] [PubMed] [Google Scholar]
- 9.Tieger MG, Hedges TR, 3rd, Ho J, Erlich-Malona NK, Vuong LN, Athappilly GK, et al. Ganglion cell complex loss in chiasmal compression by brain tumors. J Neuroophthalmol. 2017;37:7–12. doi: 10.1097/WNO.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Y, Qu YZ, Yang L, Wang J, Wang LN, Fang M, Lu W. Assessing the damage to visual function by optical coherence tomography and the visual field test in Saddle area tumor patients. Zhonghua Yan Ke Za Zhi. 2012;48:1001–4. [PubMed] [Google Scholar]
- 11.Bialer OY, Goldenberg-Cohen N, Toledano H, Snir M, Michowiz S. Retinal NFL thinning on OCT correlates with visual field loss in pediatric craniopharyngioma. Can J Ophthalmol. 2013;48:494–9. doi: 10.1016/j.jcjo.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Qiao N, Ye Z, Shen M, Shou X, Wang Y, Li S, et al. Retinal nerve fibre layer changes after transsphenoidal and transcranial pituitary adenoma resection. Pituitary. 2016;19:75–81. doi: 10.1007/s11102-015-0689-7. [DOI] [PubMed] [Google Scholar]
- 13.Danish-Meyer HV, Wong A, Paochenko T, Matheos K, Stylli S, Nichols A, et al. Optical coherence tomography predicts visual outcome for pituitary tumours. J Clin Neurosci. 2015;22:1098–104. doi: 10.1016/j.jocn.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Sung KR, Wollstein G, Kim NR, Na JH, Nevins JE, Kim CY, et al. Macula assessment using optical coherence tomography for glaucoma diagnosis. Br J Ophthalmol. 2012;96:1452–5. doi: 10.1136/bjophthalmol-2012-301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonando RS, Mettu P, El-Dairi M, Bhatti MT. The application of optical coherence tomography in neurologic diseases. Neurol Clin Pract. 2015;5:460–9. doi: 10.1212/CPJ.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun M, Zhang Z, Ma C, Chen S, Chen X. Quantitative analysis of retinal layers on three-dimensional spectral-domain optical coherence tomography for pituitary adenoma. PLoS One. 2017;12:e0179532. doi: 10.1371/journal.pone.0179532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwanza JC, Oakley JD, Budenz DL, Chang RT, Knight OJ, Feuer WJ. Macular ganglion cell-inner plexiform layer:Automated detection and thickness reproducibility with spectral domain-optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:8323–9. doi: 10.1167/iovs.11-7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung MS, Yoon JH, Park SW. Diagnostic validity of macular ganglion cell-inner plexiform layer thickness deviation map algorithm using cirrus HD-OCT in preperimetric and early glaucoma. J Glaucoma. 2014;23:e144–51. doi: 10.1097/IJG.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 19.Sakata LM, DeLeon-Ortega J, Sakata V, Girkin CA. Optical coherence tomography of the retina and optic nerve—A review. Clin Exp Ophthalmol. 2009;37:90–9. doi: 10.1111/j.1442-9071.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 20.Ventura LM, Venzara FX, Porciatti V. Reversible dysfunction of retinal ganglion cells in nonsecreting pituitary tumors. Doc Ophthalmol. 2009;118:155–62. doi: 10.1007/s10633-008-9143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa-Cunha LV, Cunha LP, Malta RF, Monteiro ML. Comparison of Fourier-domain and time-domain optical coherence tomography in the detection of band atrophy of the optic nerve. Am J Ophthalmol. 2009;147:56–63. doi: 10.1016/j.ajo.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 22.Rebolleda G, Diez-Alvarez L, Casado A, Sánchez-Sánchez C, de Dompablo E, González-López JJ, et al. OCT:New perspectives in neuro-ophthalmology. Saudi J Ophthalmol. 2015;29:9–25. doi: 10.1016/j.sjopt.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akashi A, Kanamori A, Ueda K, Matsumoto Y, Yamada Y, Nakamura M. The detection of macular analysis by SD-OCT for optic chiasmal compression neuropathy and nasotemporal overlap. Invest Ophthalmol Vis Sci. 2014;55:4667–72. doi: 10.1167/iovs.14-14766. [DOI] [PubMed] [Google Scholar]
- 24.Lukewich MK, Micieli JA. Chronic chiasmal compression and persistent visual field defect without detectable changes in optical coherence tomography of the macular ganglion cell complex. Am J Ophthalmol Case Rep. 2019;16:100533. doi: 10.1016/j.ajoc.2019.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]