Abstract
We report the occurrence of unilateral central retinal vein occlusion (CRVO) in a young yoga enthusiast who presented amidst the COVID-19 pandemic. Subtle signs of uveitis when systemically investigated revealed a multitude of causes, but ocular fluid polymerase chain reaction was positive for varicella zoster virus (VZV). The prompt initiation of antivirals resulted in a good visual outcome. Our case describes the rare presentation of VZV retinal vasculitis as CRVO in a young healthy individual and highlights the importance of early antiviral therapy for favorable outcomes.
Keywords: Central retinal vein occlusion, COVID-19, polymerase chain reaction, retinal vasculitis, varicella zoster virus infection, yoga
Ocular infection with varicella zoster virus (VZV) can have a varied presentation ranging from herpes zoster ophthalmicus to acute retinal necrosis (ARN) and progressive outer retinal necrosis.[1] Central Retinal Vein Occlusion (CRVO) secondary to VZV is rare, with reports of occurrence in an individual with HIV infection[2] and in elderly people with cardiovascular morbidity.[3] It has also been reported in association with varicella zoster dermatitis.[4,5] We describe the case of a young immunocompetent male who presented with features of CRVO but was detected to have an underlying retinal vasculitis due to VZV.
Case Report
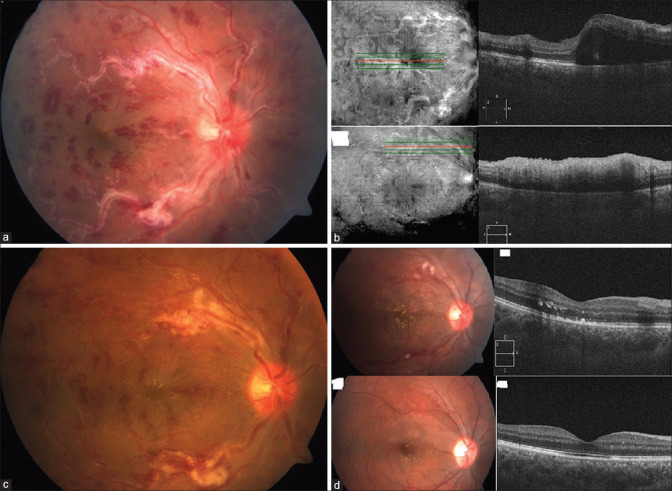
A 23-year-old male from coastal Karnataka was referred as a case of CRVO in November 2020. He presented with a history of sudden onset rapidly worsening painless loss of vision in the right eye (RE) for 3 days. He denied a history of fever or skin lesions preceding the onset of visual complaints. He also gave us a history of practicing the yoga “headstand” pose (Sirsasana) regularly. Apart from having chickenpox 4 years earlier, his past medical history was unremarkable. On ocular examination, best-corrected visual acuity was 6/24, N8 in the (RE) and 6/6, N6 in the left eye (LE). Few fine keratic precipitates were noted with grade 2+ cells in the anterior chamber in the RE, which were absent the previous day when examined by the referring consultant. The pupillary reactions were normal in both eyes. Intraocular pressure measured by Goldmann applanation Tonometry was 14 and 18 mmHg in the RE and LE, respectively. The RE fundus revealed an edematous optic disc with dilated and tortuous retinal veins. There was extensive vascular sheathing with hemorrhages alongside the retinal vasculature [Fig. 1a]. On indirect ophthalmoscopy, grade 1 vitreous haze was noted and the peripheral retina showed few hemorrhages without retinitis or vascular sheathing. LE exam was unremarkable. Optical coherence tomography (OCT) in the RE showed features of macular edema and one focal perivascular area of disruption of the inner layers of the retina with shadowing, suggesting the possibility of evolving retinitis [Fig. 1b]. The OCT-angiography showed tortuous veins and areas of reduced perfusion in the superficial and deep capillary plexus with a well-maintained foveal avascular zone.
Figure 1.
(a) Fundus photograph (FF) depicting dilated retinal veins with sheathing and hemorrhages, “frosted branch” appearance. (b) Optical Coherence tomography (OCT) on presentation showing macular edema and focal area of loss of demarcation of retinal inner layers with shadowing, suggestive of retinitis. (c) Resolving retinal vasculitis, 72 hours after initiation of oral valacyclovir, prior to starting oral steroids. (d) FF and OCT of macula during follow-up after 4 weeks and 3 months, showing resolution of retinal vasculitis and macular edema
Initial investigations, including a complete blood picture and peripheral smear, were within normal limits. Serum Homocysteine was elevated [32 umol/L]. The subsequent signs of uveitis prompted an investigation into the possible inflammatory and infectious etiologies. His serology for HIV, Mantoux test, and Interferon Gamma Release Assay for Tuberculosis was negative. However, the SerumACE level was elevated [96 U/L] and Toxoplasma IgG was positive.
An aqueous humor sample from the RE sent for polymerase chain reaction (PCR) [TaqMan Real-Time PCR probe] analysis was positive for VZV. Oral valacyclovir 1 gm thrice a day was initiated, with clinical improvement being noted in 3 days [Fig. 1c]. Subsequent therapy with oral prednisolone 60 mg once a day, topical ciprofloxacin (0.3% w/v), and dexamethasone (0.1% w/v) in tapering doses resulted in further improvement of signs, including a resolution of macular edema with the appearance of a macular star. After a total of 6 weeks of antiviral and steroid therapy, his best-corrected vision in the RE improved to 6/6, N6 with marked improvement in fundus findings, and was maintained till 3 months of follow-up [Fig. 1d].
Discussion
Our case presented a diagnostic dilemma with an unusual clinical picture and lab diagnoses. In the general healthy population, CRVO is more common than ARN.[6,7] Hence, CRVO secondary to vasculitis due to viral retinitis may be easily missed in early cases. Moreover, with CRVO being described in patients performing yoga and in COVID-19 infection, other treatable infective causes are likely to be overlooked.
Shah et al.[8] reported a case of central retinal vein occlusion following sirsasana, also called the “headstand” posture in yoga, which was regularly performed by our patient. However, unlike their report, the younger age, absence of history of previous thromboembolic phenomenon, and systemic co-morbid illnesses made this diagnosis less likely in him. The presentation in the backdrop of the prevailing pandemic also suggested the possibility of retinal vein occlusion due to COVID-19 itself.[9] Our patient had denied any clinical symptoms or contact with an infected case to warrant the need for investigation of COVID-19, which was bound by regulatory guidelines. However, subclinical SARS-CoV2 infection has been implicated in cases of Herpes zoster appearing during the pandemic.[10] Among the inflammatory causes, Toxoplasmosis is a common cause of posterior uveitis in the young. Although serology revealed toxoplasma IgG to be positive, there was no evidence of active or old chorioretinitis suggestive of toxoplasmosis. Sarcoidosis is another important cause of retinal vasculitis. However, in our case, the absence of peripheral exudative vasculitis and choroiditis made the diagnosis less likely despite a raised serumACE. Apart from the predominantly hemorrhagic form of vascular sheathing and an acute presentation, there were almost no clinically evident signs pointing toward Varicella zoster infection. The subtle OCT features of retinitis and the positive VZV DNA in PCR made it the most likely etiology. As vascular occlusion may precede ARN,[3] antiviral therapy was the first line of management implemented. The favorable outcome of this early intervention further substantiates our diagnosis. Oral valaciclovir 1000 mg 8th hourly was started based on reports of its efficacy being nearly equal to intravenous acyclovir therapy in the treatment of ARN.[11]
Varicella zoster has been implicated as a cause of CRVO due to retinitis, retinal vasculitis, and optic perineuritis [Table 1]. Our case had a better visual outcome than the other described cases, probably due to isolated central retinal venous occlusion, without arterial involvement. Early identification of the causative organism, younger age of presentation, absence of comorbidities, and early initiation of antiviral therapy may also have contributed to the better outcome.
Table 1.
Summary of the various reports of Varicella zoster-induced Central retinal venous occlusion
| Authors, Year | Age (y), Gender | Ocular presentation | Duration of complaints | Systemic disease | PCR for VZV from ocular fluid | Treatment | Outcome (Visual Acuity), recurrence |
|---|---|---|---|---|---|---|---|
| Biswas J et al.,[2] 2001 | 40, Male | (RE) CRVO | 15 days | HIV, HZO | NA (Aqueous immunofluorescence for VZV negative) | Intravenous acyclovir, oral steroids | Poor (No PL), Nil |
| Kang S et al.,[4] 2001 | 54, Male | (LE) CRVO and CRAO Subsequently, (RE) Acute Retinal Necrosis | 12 days | Varicella dermatitis on forehead 8 weeks earlier | NA (decreasing serum IgG titers against varicella zoster over 3 months) | Intravenous acyclovir, oral steroids, aspirin | Poor (Perception of Light) |
| Yeh S et al.,[3] 2012 | 66, Male | (LE) CRVO and CRAO | 7 days | Coronary artery disease | Positive | Intravenous Aciclovir, Oral Valacyclovir, Intravitreal Foscarnet | Poor (No PL) |
| Bansal R et al.,[5] 2017 | 15, Male | (LE) CRVO and CRAO | 15 hours | Varicella dermatitis | Negative | Intravenous and oral acyclovir; oral steroids | Poor (No PL) |
| Murdock J et al.,[12] 2017 | 58, Male | (RE) CRVO and CRAO; (LE) multiple BRVO | 1 day | Chickenpox with systemic vasculitis | NA (serum IgM for VZV positive) | Intravenous Aciclovir, Intravenous and oral Steroids | Poor (PL, HM) |
| Sarpangala S et al., 2021 [Present case] | 23, Male | (RE) CRVO | 3 days | Nil | Positive | Oral Valaciclovir, oral steroids | Good (6/6) |
RE: Right eye; LE: Left eye; CRVO: Central Retinal Vein Occlusion; CRAO: Central Retinal Artery Occlusion; BRVO: Branch Retinal Vein Occlusion; HIV: Human Immunodeficiency virus; HZO: Herpes Zoster Ophthalmicus; NA: Not available; VZV: Varicella zoster virus; PL: Perception of Light; HM: Perception of Hand movements
Conclusion
CRVO may be the initial presentation of varicella zoster-induced retinal vasculitis in a young immunocompetent individual. A strong clinical suspicion aided by ocular fluid analysis and early initiation of antiviral therapy can prevent progression to sight-threatening complications such as ARN.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cohen EJ, Kessler J. Persistent dilemmas in zoster eye disease. Br J Ophthalmol. 2016;100:56–61. doi: 10.1136/bjophthalmol-2015-306700. [DOI] [PubMed] [Google Scholar]
- 2.Biswas J, Deka S, Padmaja S, Madhavan HN, Kumarasamy N, Solomon S. Central retinal vein occlusion due to herpes zoster as the initial presenting sign in a patient with acquired immunodeficiency syndrome (AIDS) Ocul Immunol Inflamm. 2001;9:125–30. doi: 10.1076/ocii.9.2.125.3976. [DOI] [PubMed] [Google Scholar]
- 3.Yeh S, Fahle G, Flaxel CJ, Francis PJ. Central retinal vascular occlusion associated with acute retinal necrosis. Arch Ophthalmol. 2012;130:514–7. doi: 10.1001/archophthalmol.2011.1735. [DOI] [PubMed] [Google Scholar]
- 4.Kang SW, Kim SK. Optic neuropathy and central retinal vascular obstruction as initial manifestations of acute retinal necrosis. Jpn J Ophthalmol. 2001;45:425–8. doi: 10.1016/s0021-5155(01)00336-7. [DOI] [PubMed] [Google Scholar]
- 5.Bansal R, Singh R, Takkar A, Lal V. Combined central retinal artery and vein occlusion with optic perineuritis following herpes zoster dermatitis in an immunocompetent child. Indian J Ophthalmol. 2017;65:1233–5. doi: 10.4103/ijo.IJO_480_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion:Review of the literature. Eye (Lond) 2011;25:981–8. doi: 10.1038/eye.2011.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis:A national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91:1452–5. doi: 10.1136/bjo.2007.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NJ, Shah UN. Central retinal vein occlusion following Sirsasana (headstand posture) Indian J Ophthalmol. 2009;57:69–70. doi: 10.4103/0301-4738.44496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheth JU, Narayanan R, Goyal J, Goyal V. Retinal vein occlusion in COVID-19:A novel entity. Indian J Ophthalmol. 2020;68:2291–3. doi: 10.4103/ijo.IJO_2380_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsaie ML, Youssef EA, Nada HA. Herpes zoster might be an indicator for latent COVID-19 infection. Dermatol Ther. 2020;33:e13666. doi: 10.1111/dth.13666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007;114:307–12. doi: 10.1016/j.ophtha.2006.06.058. [DOI] [PubMed] [Google Scholar]
- 12.Murdock J, Carvounis PE. Adult with chickenpox complicated by systemic vasculitis and bilateral retinal vasculitis with retinal vascular occlusions. Retin Cases Brief Rep. 2017;11:364–8. doi: 10.1097/ICB.0000000000000372. [DOI] [PubMed] [Google Scholar]