Dear Editor,
A 50-year old male presented with difficulty in closing the right eye associated with redness and watering for 3 days. There was no history of diabetes, hypertension, or any other systemic disease. On past history, the patient had received the first dose of COVAXIN vaccine on April 3, 2021 and the second dose on May 11, 2021. On examination, his best-corrected vision was 20/20 in both eyes. Lagophthalmos with lower lid ectropion temporally was present. There was loss of forehead wrinkling, naso-labial fold, and drooping of angle of mouth of the right side, suggestive of right-sided lower motor neuron facial nerve palsy [Fig. 1]. On anterior segment examination of the right eye, there was conjunctival congestion, cornea was clear, and rest was normal. Anterior segment examination of the left eye was normal. Fundus examination of both eyes was normal. A referral was sent to otolaryngologist. The ear examination was reported as normal external ear, normal tympanic membrane with no hearing loss.
Figure 1.
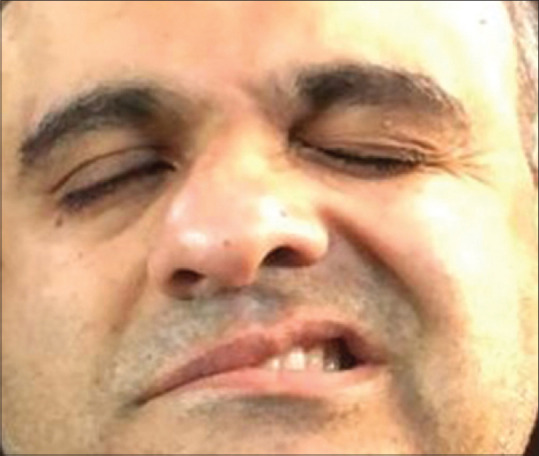
Day 1 of presentation showing loss of facial symmetry, incomplete right eye closure, loss of nasolabial fold, and drooping of angle of mouth of the right side suggestive of right-sided Bell’s palsy
The patient was prescribed topical antibiotics for eyes with frequent use of lubricating drops. Taping of the right eye was advised when sleeping. The patient was also started on oral prednisolone at 1 mg/kg for 2 weeks. The patient showed response from 1 week of therapy. At review on 10th day, the lagophthalmos decreased, lower lid ectropion improved, no conjunctival congestion was present, and facial asymmetry had also improved [Fig. 2].
Figure 2.

Follow-up on Day 10 of illness showing decreased facial asymmetry, complete eye closure along with improvement in the drooping of angle of mouth
COVID-19 vaccines during trials have documented facial nerve palsy in vaccine recipients. Four vaccine recipients of Pfizer-BioNTech©[1] and three of Moderna© vaccine[2] have developed Bell’s palsy which were labeled as medically attended adverse event. Following this, the United States Food and Drug Administration and the Centers for Disease Control and Prevention had recommended to keep a strict vigil for such symptoms post COVID-19 vaccination as similar cases have been previously reported with influenza vaccine also.[3,4,5] The timing of the palsy ranged from the first dose to around 1 month after the second dose (median time of 2 days with range from 0 to 79 days after the first dose). Our patient developed right-sided facial nerve palsy 3 weeks after the second dose (7 weeks after the first dose) of COVAXIN© vaccine. After the global release of vaccines, there have been rare reports of similar cases. A 57-year-old patient in the United States of America developed bell’s palsy 36 h after the second dose of Pfizer-BioNTech© vaccine. However, it was the fourth event of facial nerve palsy in the patient as she had underlying Melkersson–Rosenthal Syndrome.[3] Another case reported developed facial nerve palsy within a week of vaccination with Pfizer-BioNTech© vaccine.[6] Autoimmune mechanisms with interferons activation following vaccine have been suggested as the possible mechanism, but there is no evidence yet.[7]
Even though, rare adverse events following immunization (AEFI) should not be a deterrent for COVID-19 vaccination and all attempts should be made to decrease hesitancy[8] among the people for vaccination; however, a strict vigil and regular documentation should always be done for all AEFI to ensure safety.[9] To conclude, temporal association, viral vaccines, history of reports with influenza vaccine, and biological plausibility suggest that facial nerve palsy post COVID-19 vaccination can be an adverse event, but in view of lack of evidence and rare incidence, the association may be a mere coincidence. However, in view of response with steroids, a high index of suspicion can be maintained for early diagnosis and appropriate treatment.
Declaration of patient consent
Written informed consent of the patient for sharing his clinical details and photographs was taken.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vaccines and Related Biological Products Advisory Committee Meeting-FDA Briefing Document. [Last accessed on 2021 Jun 26]. Downloaded from: https://www.fda.gov/media/144245/download .
- 2.Vaccines and Related Biological Products Advisory Committee Meeting. [Last accessed on 2021 Jun 26]. Downloaded from: https://www.fda.gov/media/144434/download .
- 3.Repajic M, Lai XL, Xu P, Liu A. Bell's palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell's palsy. Brain Behav Immun Health. 2021;13:100217. doi: 10.1016/j.bbih.2021.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Authorized in the United States. [Last accessed on 2021 Jun 26]. Downloaded from: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html .
- 5.Zhou W, Pool V, DeStefano F, Iskander JK, Haber P, Chen RT VAERS Working Group. A potential signal of Bell's palsy after parenteral inactivated influenza vaccines:Reports to the Vaccine adverse event reporting system (VAERS)–United States, 1991-2001. Pharmacoepidemiol Drug Saf. 2004;13:505–10. doi: 10.1002/pds.998. [DOI] [PubMed] [Google Scholar]
- 6.Colella G, Orlandi M, Cirillo N. Bell's palsy following COVID-19 vaccination. J Neurol. 2021:1–3. doi: 10.1007/s00415-021-10462-4. doi:10.1007/s00415-021-10462-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirillo N, Doan R. Bell's palsy and SARS-CoV-2 vaccines-an unfolding story. Lancet Infect Dis. 2021;S1473-3099(21):00273–5. doi: 10.1016/S1473-3099(21)00273-5. doi:10.1016/S1473-3099(21)00273-5. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyengar KP, Vaishya R, Jain VK, Ish P. BAME community hesitancy in the UK for COVID-19 vaccine:Suggested solutions. Postgrad Med J. 2021 doi: 10.1136/postgradmedj-2021-139957. postgradmedj-2021-139957. doi:10.1136/postgradmedj-2021-139957. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava RK, Ish P. The initial experience of COVID-19 vaccination from a Tertiary care centre of India. Monaldi Arch Chest Dis. 2021 doi: 10.4081/monaldi.2021.1816. Safdarjung Covid-Vaccination Group. doi:10.4081/monaldi2021.1816. Online ahead of print. [DOI] [PubMed] [Google Scholar]


