Abstract
The INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions Project (www.toxpath.org/inhand.asp) is a joint initiative of the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP) to develop an internationally accepted nomenclature for proliferative and non-proliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature for classifying microscopic lesions observed in most tissues and organs from the laboratory rabbit used in nonclinical safety studies. Some of the lesions are illustrated by color photomicrographs. The standardized nomenclature presented in this document is also available electronically on the internet (http://www.goreni.org/). Sources of material included histopathology databases from government, academia, and industrial laboratories throughout the world. Content includes spontaneous lesions as well as lesions induced by exposure to test materials. Relevant infectious and parasitic lesions are included as well. A widely accepted and utilized international harmonization of nomenclature for lesions in laboratory animals will provide a common language among regulatory and scientific research organizations in different countries and increase and enrich international exchanges of information among toxicologists and pathologists.
Keywords: rabbit, pathology, toxicopathology, nomenclature, background findings, INHAND, New Zealand
Section 1: Introduction
The INHAND Project (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions) is a joint initiative of the societies of toxicologic pathology from Europe (European Society of Toxicologic Pathology - ESTP), UK (British Society of Toxicological Pathologists - BSTP), Japan (Japanese Society of Toxicologic Pathology - JSTP), and North America (Society of Toxicologic Pathology - STP) to unify, update and complete the existing WHO/IARC and STP/SSNDC nomenclature systems. The INHAND nomenclature and the related diagnostic criteria should represent the future international standard in toxicologic pathology. They represent a consensus of senior toxicologic pathologists and were reviewed by the INHAND-GESC (INHAND-Global Editorial and Steering Committee) for compliance with INHAND principles. The initial series of nomenclature publications were focused on lesions in rats and mice. With the decision of the SEND initiative (Standard for the Exchange of Non-clinical Data) to model the controlled terminology (CT) based on the INHAND nomenclature and the decision of the Federal Drug Administration (FDA) to make the use of the SEND CT mandatory for electronic submissions of nonclinical studies, the INHAND project was extended to other laboratory animal species including the monkey, rabbit, mini-pig, dog and fish.
However, the recommendations for diagnostic criteria and preferred terminology may not be applicable in all situations. Purposes of specific experiments or the specific context of a given study may require deviation from this standardized nomenclature and diagnostic criteria. The appropriate diagnoses are ultimately based upon on the discretion of the senior toxicologic Study Pathologist.
The present publication provides a set of standardized terms and diagnostic criteria to be used in toxicologic pathologic studies on the most commonly used strains of laboratory rabbits - New Zealand White (NZW) representative of non-pigmented and Dutch Belted representative of a pigmented rabbit. Throughout this publication, lesions applicable for use in toxicologic-pathology studies in rabbits are tabulated. As rabbits have been most frequently used for tissue specific studies in young animals (e.g. ocular, dermal, and intramuscular), compilation of a broad listing of the incidence of spontaneous or background findings, and a tabulation of the incidence of subacute to chronic responses to chemicals, drugs and biomaterials are limited. The terms and thus, the tabulations, build on the existing rodent nomenclature. In most instances, the description and definition of the rodent lesion applies to the rabbit and is not further described. This publication focuses on lesions that are unique to the rabbit and are not observed in rodents, and lesions in rabbit that share the same terminology with a rodent lesion but display different morphologic features. Lesions that are unique to rats or mice and are not to be used in rabbit are denoted accordingly in the tabulation. The tabulated lesions are categorized according to the following characteristics: “Common”, “Uncommon”, “Not Observed but Potentially Relevant” and “Not Applicable”. The distinction between common and uncommon lesions is based on the occurrence in untreated laboratory rabbits in the authors’ experience and is not based on published references. Also, it should be kept in mind that the rabbit used in toxicologic studies are usually of young age and are only for a relative short time on study, a fraction of the normal life span of a rabbit. In addition, references to lesions seen in older pet and breeding animals are mentioned in the text where relevant. Before entering a study, the health status of individual animals is checked carefully, and the individual rabbits selected for a toxicologic study are in excellent condition. For these reasons, the spectrum and frequency of changes are different from those in diagnostic laboratories, and, therefore, common age-related lesions including neoplasms are rarely seen in these animals. Thus the vast majority of neoplastic lesions have been categorized as “Uncommon”. The category “Not Applicable” refers to rodent specific lesions and terms as the use of these terms in rabbits is considered not appropriate. Examples are chronic progressive nephropathy in the kidney or fibro-osseous lesion of bones. “Not Observed but Potentially Relevant” are changes that have not been described or observed in laboratory rabbits, however, the use of these terms has been considered permissible should a lesion meet the diagnostic criteria.
Like all other INHAND publications, the nomenclature and diagnostic criteria for the rabbit are also available online (www.goreni.org). The online version contains any change controls, additional images and useful links to differential diagnoses characterizing it as a practical tool for diagnostic work.
The recommended nomenclature is generally descriptive rather than diagnostic. The diagnostic criteria used require standard hematoxylin and eosin stained paraffin sections only. Histochemical or immunohistochemical staining characteristics may be addressed in the comments section of the respective lesion. Such special techniques may be required in some situations, but a comprehensive discussion of these methods is outside the scope of this publication. Systemic non-proliferative lesions that occur across organ systems and are not specific to an organ are reviewed in the section on systemic pathology. Although the rodent publications provide “synonyms” for each term, the non-rodent publications have used the notation “Other term(s)”. While these “synonyms” or “other terms” have been used historically, the primary listed term is the preferred term and will link to the controlled terminology in SEND. These “other terms” are listed with some of the entries to aid the pathologist when comparing current study findings with archival material. These other terms are archaic terms and should not be used because they are no longer preferred diagnostic entities.
Lesions included in this nomenclature system may be further specified by modifiers. Criteria are given for modifiers that are considered to be of particular relevance. These modifiers should be consistently applied. It is upon the discretion of the pathologist to use additional modifiers not suggested in this nomenclature system. Such modifiers may describe the location, tissue type, or duration among others. Further principles of the INHAND nomenclature have been published separately1.
Section 2: Systemic Pathology
There are a number of microscopic findings that may be seen across several organs and/or tissues and are not specific to just one organ system. There are also a number of different findings that are present across several organs and/or tissues that together constitute a syndrome. Those findings that occur in multiple tissues are listed here for convenience, and they are also described under the organ systems in which they occur if they have unique features. Syndromes specific to the rabbit are mentioned in individual chapters, but their definitive descriptions are presented here.
Rabbits used in general toxicology studies are bred under barrier conditions, which are microbiologically defined, and are kept in strictly controlled/biosecure facilities when on study, so infectious disease (parasitism, bacterial, fungal and viral diseases) is unlikely. Pasteurella, Encephalitozoon and Eimeria spp. have been reported in the past but are rarely observed today2, 3.
The tables below give an indication of how frequently the changes may be observed in the laboratory rabbit, associated diseases/conditions, etiologies or inducing agents, and a list of tissues where they may be found. Where further explanation is deemed useful, selected lesions are discussed in more detail below the table (Table 1).
Table 1. Microscopic Findings of Systemic Pathology (Generally Used Preferred Terms): Rabbit.
| Finding | Common | Uncommon | Associated Diseases/Conditions | Tissues commonly reported in: | |
| Non-proliferative | |||||
| Congenital | |||||
| Agenesis/hypoplasia | X | ||||
| Malformation syn. congenital malformation | X | Gall bladder (bifurcation) | |||
| Multisystemic | |||||
| Abscess | X | ||||
| Accumulation, adipocytes | X | ||||
| Amyloid | X | ||||
| Apoptosis *ǂ | X | ||||
| Atrophy | X | ||||
| Basophilic granules | X | ||||
| Congestion | X | Nasal cavity, lung, liver, vagina | |||
| Edema | X | ||||
| Extramedullary hematopoiesis | X | Spleen, liver, adrenal | |||
| Fracture | X | Lumbar spine, usually associated with handling | |||
| Hemorrhage | X | Larynx, trachea, thyroid, thymus, bronchi, bronchioles | |||
| Infiltrate, inflammatory cell * [insert appropriate cell type] | X | Mononuclear, lymphocyte, plasma cell, macrophage/monocyte, neutrophil, eosinophil, heterophil, mixed | Multiple tissues; differentiate form MALT | ||
| Inflammation | X | Acute, chronic, chronic active, granulomatous, granuloma | Multiple tissues; may be due to bacterial, viral or parasitic diseases e.g. Pasteurella sp. in the lungs; foreign body inflammatory reactions with multinucleated giant cells are common with implantation of biomaterials and medical devices | ||
| Metaplasia | X | ||||
| Metaplasia, Osseous/cartilaginous * | X | Lung, eye and skeletal muscle (implant associated) | |||
| Mineralization * | X | Ovary, kidney, cerebral & cerebellar leptomeninges, blood vessels, lung, skeletal muscle | |||
| Necrosis | X | ||||
| Parasite | X | Coccidiosis (Eimeria spp); Microsporidiosis (Encephalitozoon cuniculi) | Adrenal gland, brain, eye, intestine, kidney, liver | ||
| Pigment | X | Hemosiderin | Spleen, liver, bone marrow, adrenal, glomeruli, lymph node sinuses | ||
| Pigment | X | Melanin, hemosiderin, lipofuscin | Skin, leptomeninges of pigmented strains | ||
| Pigment, macrophage | X | Tattoo ink, inhaled particulate matter | (cervical) lymph node (from ear tattoos), lung | ||
| Serous atrophy of fat | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic | X | Accessory adrenal cortical tissue, accessory spleen, bifurcate gall bladder; ectopic thyroid, ectopic thymus | |||
| Vacuolation | X | Spleen, lymph node, lungs, choroid plexus | |||
| Vacuolation, macrophages *# | X | ||||
| Proliferative Neoplastic | |||||
| Lymphoma | X | Liver, small intestine, multiple tissues | |||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Apoptosis
Comments: For a full discussion see Elmore, S. (2007). Apoptosis: A Review of Programmed Cell Death. Toxicologic Pathology, 35(4), 495–516
Infiltrate, Inflammatory Cell
Comments: NZW rabbits on surgical studies may develop granulomas if orthopedic sutures are implanted into dorsal fascia5. NZW Rabbits are commonly used to test biocompatibility by implantation of novel medical materials into intramuscular and other tissue locations. Tissue reaction is scored using ISO 10993-6:20166 by characterizing inflammatory cells, necrosis, granulation tissue and fibrosis. Injured and regenerating skeletal muscle may also stimulate adipogenesis resulting in fatty infiltration7. Fatty metaplasia needs to be differentiated from the normal fat pads containing blood vessels and nerves that occur between muscle bundles when implants are incorrectly implanted or medical materials migrate into intermuscular sites.
Metaplasia, Osseous or Cartilaginous
Comments: Cartilage and bone formation, with or without intraosseous bone marrow is a common finding with intramuscular but not subcutaneous implantation tests of bone substitution biomaterials. Osseous metaplasia is readily induced in dogs and baboons, and to a lesser extent in rabbits and mice with calcium phosphates (CP) or hydroxyapatite/calcium phosphate (HCP). Rabbits form bone and bone marrow with HCP8-10. Rarely, cartilage, bone and bone marrow may form as a sequela of intramuscular implantation of novel polymers and other biomaterials.
Mineralization
Comments: Rabbits do not require Vitamin D to regulate calcium absorption from the gut. Excess calcium in the diet is therefore more likely to cause metastatic calcification than in other species11.
Vacuolation, Macrophages
Other term(s): Phospholipidosis
Pathogenesis/cell of origin: Macrophage
Differential diagnoses: accumulation adipocyte, phagocytic vesicle, lysosome.
Comments: Phospholipid vacuoles may be positive for LAMP2. Similar to the other laboratory species. Tissues affected by phospholipidosis vary by drug.
Section 3: Cardiovascular System
A. Anatomy of the Heart
Detailed anatomy and physiology of the rabbit heart has been described by various authors12, 13 and is not within the scope of this text. However, one difference to note is that the right atrioventricular valve of the heart is bicuspid instead of tricuspid as occurs in the rodent.
Histopathology of the heart should include all relevant compartments and structures of the heart, including the ventricular, atrial and interventricular septal wall, the valves and the coronary vessels.
Xenobiotics may cause myocardial changes due to exaggerated pharmacodynamic activity or as a result of a direct toxic effect on cardiomyocytes. Severe acute toxic insults can cause acute cardiomyocyte death, and the regenerative potential of cardiomyocytes is generally insufficient to replace significant myocardial loss. Biochemical changes such as alterations in calcium homeostasis can occur if the insult is of mild severity, and these generally result in reversible cardiac arrhythmia. Cardiomyocytes are generally replaced by fibroblasts, with collagenous deposits leading to loss of cardiac contractility. Changes in the heart are known to be induced by “stress” i.e. catecholamine (CA) induced cardiomyopathy. Study-related procedures can elevate serum stress biomarkers and exacerbate the frequency and severity of myocardial inflammatory cell infiltrates14.
Rabbit models of heart disease have been comprehensively reviewed15 (Table 2).
Table 2. Microscopic Findings of the Heart: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Malformation * | X | ||||
| Non-proliferative | |||||
| Accumulation, adipocyte, myocardium | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Cardiomegaly | X | ||||
| Degeneration | X | ||||
| Degeneration/necrosis | X | ||||
| Edema | X | ||||
| Fibrosis, myocardium * | X | ||||
| Fibrosis | X | ||||
| Hypertrophy | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell, [insert appropriate cell type] myocardium * | X | ||||
| Inflammation, myocardium * | X | ||||
| Karyomegaly/Karyocytomegaly | X | ||||
| Mineralization * | X | ||||
| Necrosis, cardiomyocyte * | X | ||||
| Necrosis/infiltrate | X | ||||
| Parasite | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus, atrium | X | ||||
| Tissue, ectopic, thyroid | X | ||||
| Vacuolation, cardiomyocyte | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, subendocardium | X | ||||
| Hyperplasia, mesothelium | X | ||||
| Proliferative Neoplastic Lesions | |||||
| Schwannoma, endocardium | X | ||||
| Schwannoma, intramural | X | ||||
| Mesothelioma, pericardium | X | ||||
| Mesothelioma, atriocaval | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Malformation
Other terms: Congenital malformation
Comments: Congenital lesions of the heart and blood vessels are rare and are reported infrequently in rabbits. Consequently, the lesions are usually only seen in Developmental and Reproduction Toxicity (DART) studies, in which fetuses are carefully dissected. Ventricular septal defect has been reported in a 10-month-old female NZW rabbit16. Other conditions occasionally seen in rabbits are: right sided aortic arch and patent ductus arteriosus.
Fibrosis, Myocardium
(Figure 1)
Figure 1.
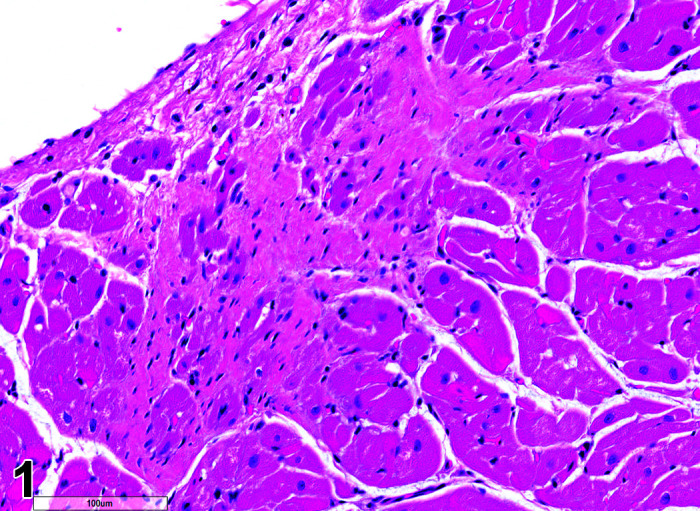
Heart, Fibrosis Myocardium, H&E.
Comments: Myocardial fibrosis may be induced in rabbits after anesthesia with the α2-agonist detomidine, alone and in combination with ketamine or diazepam17, 18. The presence of myocardial fibrosis does not always result in clinical signs and adversity should be judged on a case by case basis. There is also an age-related increased fibrosis in the ventricles and interventricular septum. Ventricular stiffness and wall thickness increase in the aging rabbit heart19.
Infiltrate, Inflammatory Cell, Myocardium
(Figure 2)
Figure 2.
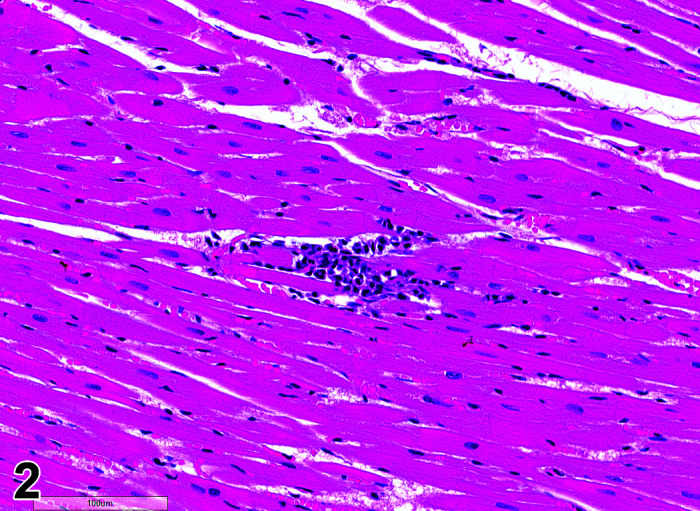
Heart, Infiltrate, inflammatory cells, H&E.
Comments: Mononuclear inflammatory cell infiltrates are recorded infrequently in the myocardium. The foci are usually at the base of the interventricular septum but have been reported in the atrial and ventricular free walls. There is no accompanying myocardial necrosis or fibrosis associated with this lesion.
Inflammation, Myocardium
Comments: Inflammatory cells may be associated with cardiomyocyte necrosis, interstitial edema and early fibrosis (see Necrosis). This finding can be induced by catecholamines secondary to stress14, 20. Severity and incidence are increased in rabbits subjected to more handling and procedures. Increase in circulating catecholamines act on adrenergic receptors expressed in the heart and stimulate contractility. Heart lesions are primarily in the left ventricle and papillary muscle20, 21. In severe cases, the inflammatory cell foci may resolve as focal fibrosis. This tends to be an idiosyncratic reaction and may affect one or two animals in a study, suggesting a subset of animals may be more vulnerable to stress responses and/or do not habituate to stressors14.
Mineralization, Cardiomyocyte/Myocardium
(Figure 3)
Figure 3.
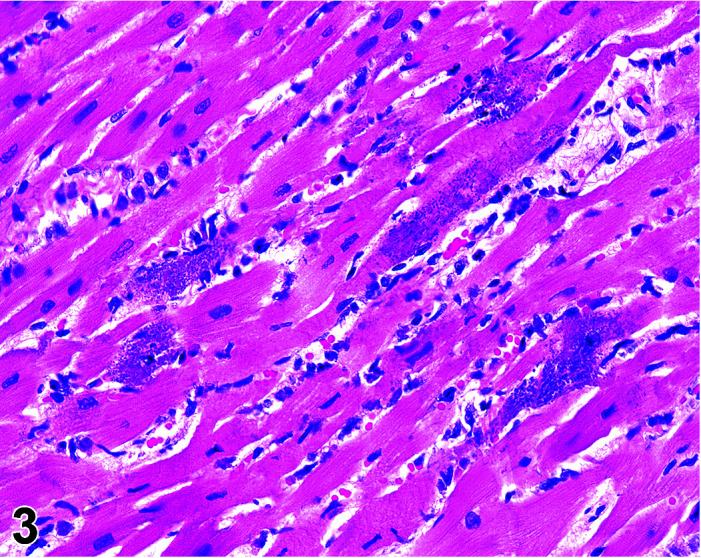
Heart, Mineralization myocardium H&E.
Comments: Generally a background lesion but may be exacerbated by some xenobiotics; common in left atrial appendage. Mineralization may be seen at necropsy in older animals, e.g. ex-breeding colony animals.
Necrosis, Cardiomyocyte
(Figure 4)
Figure 4.
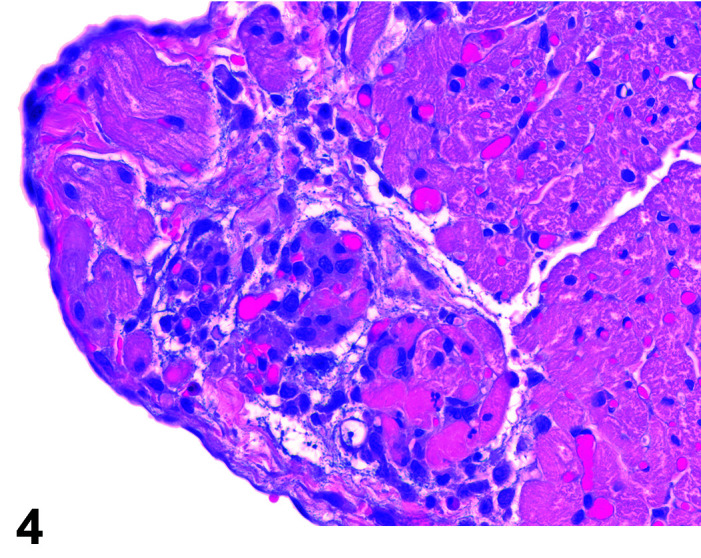
Heart, Necrosis myocardium, H&E.
Other terms: Degeneration/necrosis, cardiomyocyte
Comments: Myocardial inflammation with/without minimal necrosis and/or fibrosis may be seen as a stress-induced finding in occasional animals on toxicity studies. These foci are minimal to moderate in severity grade and most affect one part of the myocardium, usually papillary muscles, left ventricular free wall, or may be multifocal throughout intraventricular septum, right ventricular free wall and atria. They are more commonly seen in animals subject to multiple procedures or handling events and thought to be catecholamine induced necrosis. Animals may be found in extremis or dead without previously showing any clinical signs. Although only an occasional occurrence in young rabbits used in toxicology studies, this sudden death syndrome is recognized as a stress induced event, caused by handling/invasive procedures (injections, blood sampling) in pet rabbits (Bradley, unpublished data). A recent study showed that the incidence, composition and severity of these foci may be exacerbated by handling and procedures that occur in toxicology studies, mediated by a stress response14.
Myocardial necrosis and fibrosis can be induced in rabbits after anesthesia with the α2-agonist detomidine, alone and in combination with ketamine or diazepam17, 18. There is reduction in coronary flow reserve as a consequence of the hypoxemia associated with ketamine/xylazine administration due to xylazine interaction with α2-receptors in coronary vessels. Impairment of coronary blood flow causes myocardial ischemia with subsequent necrosis. The rabbit is a species with limited collateral circulation in the myocardium and is therefore predisposed to ischemia induced by coronary vasoconstriction22, 23.
B. Anatomy of the Blood Vessels
Rabbit blood vessels are generally thin-walled and prone to collapse and may form hematomata on puncture – a feature important for studies where test items are given by intravenous administration. The exception to this is the pulmonary arteries which are enveloped in a prominent smooth muscle layer, which may be misinterpreted as hypertrophy24, 25.
Specific large vessels may be required on safety assessment studies in which animals have been dosed via intravascular catheter through bolus injection or slow infusion. Large vessels that can be easily sampled in rabbits include the thoracic and abdominal aorta/vena cava, carotid arteries, femoral arteries/veins and iliac arteries/veins. Some lesions occur in vessel-specific progression (e.g. atherosclerosis) (Table 3).
Table 3. Microscopic Findings of the Vessels and Valves: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Malformation * | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Aneurysm, artery or aortic | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Degeneration/necrosis, media or wall | X | ||||
| Dilatation | X | ||||
| Embolus | X | ||||
| Fibrosis, perivascular | X | ||||
| Hemorrhage, media or wall | X | ||||
| Hypertrophy, endothelium/media or wall, artery | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, media or wall, artery | X | ||||
| Intimal thickening, acellular *# | X | ||||
| Intramural plaque, artery | X | ||||
| Metaplasia | X | ||||
| Mineralization * | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, media or wall, artery | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Vacuolation, media or adventitia, artery | X | ||||
| Proliferative Non-Neoplastic Lesions | |||||
| Hyperplasia, intima | X | ||||
| Proliferative Neoplastic Lesions | |||||
| Hemangioma | X | ||||
| Hemangiosarcoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Malformation
Other terms: Congenital malformation
Comments: Congenital lesions of the blood vessels are rare and are reported infrequently in rabbits. Consequently, the lesions are usually only seen macroscopically in Developmental and Reproduction Toxicity (DART) studies, in which fetuses are carefully dissected. Conditions occasionally seen in rabbits are right sided aortic arch and patent ductus arteriosus.
Intimal Thickening, Acellular
Other terms: Atherosclerosis
Diagnostic Features: Atherosclerotic plaques primarily composed of macrophage-derived foam cells.
Comments: In rabbits, the presence of atherosclerosis-like lesions was first described in 191326. High plasma cholesterol concentrations, especially of low-density lipoprotein (LDL) cholesterol, result in atherosclerotic lesion formation. The accumulation of foam cells results in the formation of fatty streaks, the earliest observable abnormality of the vessel wall. The rabbit exhibits hypercholesterolemia within a few days of an administration of a high cholesterol diet, and so can be used as an animal model in efficacy studies to see if drugs affect inducement of atheromatous lesions. The basal release of nitric oxide (NO), is greater with endothelium-intact aortic rings from female rabbits than from male rabbits, and so this protective effect of circulating estradiol means female animals are less prone to diet-induced atherosclerotic lesions than male animals. Cholesterol-rich diets have been used to induce widespread atheromatous lesions within a short time period (3 months). Genetically altered strains of the NZW rabbit are also used extensively. The Watanabe rabbit (Watanabe heritable hyperlipidaemia rabbit, WHHL) is used to study the pathology of type IIa human familial hypercholesterolemia. The WHHL rabbit has a genetic deprivation of functional LDL receptors. In these animals, the atherosclerotic process begins in utero, and the lesions progress with age27. Rinke and Hartmann characterized atherosclerosis in NZW and WHHL rabbits28. The lesions were extremely pronounced in the vessels of the cardiac atria, including the valves and extended with degenerative changes into the myocardium. Some nearly occlusive arteries, without surrounding myocardial change, were also seen. Inflammatory response and areas of regeneration, occasionally accompanied by dystrophic mineralization, were frequently observed. Rabbit models for the study of human atherosclerosis have been reviewed previously29.
Mineralization, Aorta/Medial or Mural Artery
(Figure 5)
Figure 5.
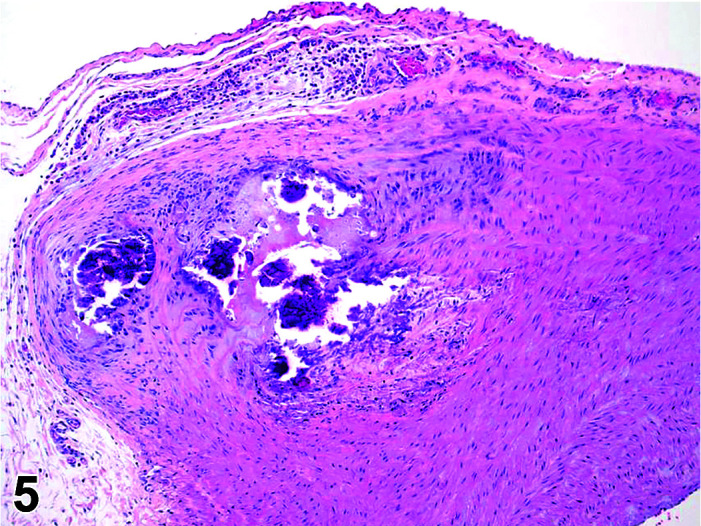
Aorta, Mineralization, H&E.
Comments: Generally, a background lesion but may be exacerbated by some xenobiotics. Occasionally evidence of the closure of the ductus arteriosus may be seen as foci of mineralization in the media of major blood vessels of young rabbits, depending on plane of section. Calcification of the aorta, pulmonary artery, and femoral artery may be seen microscopically in young animals as an incidental finding. These lesions can be exacerbated by an increased calcium supply or vitamin D overdosage11 or where fresh pellets are given (freshly opened packets of standard rabbit chow contain more degradable vitamins as the declaration of ingredient contents are corrected to be those present at the expiry date of the food). Mineralization may be seen at necropsy in older animals, e.g. ex-breeding colony animals.
Section 4: Digestive System (Oral Cavity, Salivary Glands, Esophagus, Stomach, Intestines, and Exocrine Pancreas)
A. Anatomy of the Oral Cavity and Esophagus
The mouth opening of rabbits is small so that a thorough examination of the buccal cavity is difficult or in some animals almost impossible. The oral cavity is long and curved. Erosions of the mucosa may occur due to irregular growth or sharp edges of broken teeth.
Teeth
The dental formula of the rabbit is 26 or 28 teeth. The maxilla contains 4 incisors, no canines, six premolars, and 4–6 molars. The second set of maxillary incisors are small, caudal to the main incisors, and are known as the peg teeth. The mandible contains 2 incisors, no canines, 4 premolars, and 6 molars. Rabbits are hypsodonts and have a long crown without a true tooth root30. Rabbits chew their food using their tongue elaborately, moving their jaw more than 120 times per minute.
Tongue
The tongue is relatively large in comparison to the overall body size of rabbits and has the standard 4 papillae types, namely vallate, foliate, fungiform, filiform30.
Esophagus
The esophagus comprises three layers of semi-involuntary striated muscle; the rabbit esophagus lacks mucus glands30 (Table 4).
Table 4. Microscopic Findings of the Oral Cavity, Pharynx, Tongue and Esophagus: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Congenital | |||||
| Cleft palate * | X | ||||
| Malformation | X | ||||
| Non-proliferative | |||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Cyst | X | ||||
| Degeneration/necrosis, muscle | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Hemorrhage | X | ||||
| Hyperkeratosis | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, foreign body | X | ||||
| Inflammation, mixed cell | X | ||||
| Inflammation, mononuclear cell | X | ||||
| Inflammation, vessel | X | ||||
| Metaplasia | X | ||||
| Mineralization (+ locator) | X | ||||
| Necrosis (+ locator) | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Syncytia, epithelium | X | ||||
| Tissue, ectopic | X | ||||
| Tissue, ectopic, sebaceous gland | X | ||||
| Yeast | X | ||||
| Proliferative Non-Neoplastic | |||||
| Hyperplasia *# | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell | X | ||||
| Leiomyoma | X | ||||
| Leiomyosarcoma | X | ||||
| Papilloma, squamous cell * | X | ||||
| Tumor, granular cell, benign | X | ||||
| Tumor, neuroendocrine cell | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Cleft Palate
Comments: Cleft Palate is a common finding in rabbit teratogenicity studies. Congenital alveolar cleft is a malformation occurring as a result of non-fusion of primary palate during weeks 4–12 of gestation and may be induced by glucocorticoids31.
Hyperplasia
Comments: Gingival hyperplasia has been recorded in NZW rabbits administered cyclosporine A chronically (L. Himmel, personal communication).
Papilloma, Squamous Cell
Comments: Caused by oral papilloma virus (papovavirus). Small grayish nodules present on the ventral buccal cavity and/or the underside of the tongue. Lesions are seen in animals 2–18 months old. The infection is self-limiting and uncommon in young animals but may occur in older breeding stock.
B. Anatomy of the Salivary Glands
Salivary Glands
There are 4 pairs of salivary glands in the rabbit: parotid, submaxillary (also called mandibular), sublingual, and zygomatic. The parotid gland is the largest and runs from below the ear base to the front of the ear base and is bounded by the skin and masseter muscle. The parotid gland duct runs rostrally along the lateral surface of the masseter muscle and is adjacent to the branches of the facial nerve. The parotid gland duct empties into the oral cavity opposite the last upper molar. The submaxillary gland is oval-shaped and located at the angle of the mandible. The sublingual gland is small in the rabbit. The zygomatic salivary gland rests just ventral to the lacrimal gland adjacent to the anteroventral angle of the orbit (see also Lacrimal Glands subsection in Special Senses section). Rabbit saliva contains amylase but has only trace amounts of lipase and urea. The rabbit submaxillary gland has continuous secretion of saliva30 (Table 5).
Table 5. Microscopic Findings of the Salivary Glands: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Ectopic tissue | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Accumulation * | X | ||||
| Accumulation, adipocytes | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Calculus, duct | X | ||||
| Cyst | X | ||||
| Degeneration/necrosis | X | ||||
| Degranulation, (+ locator) | X | ||||
| Depletion, secretory * | X | ||||
| Dilatation, duct | X | ||||
| Ectasia, duct | X | ||||
| Edema | X | ||||
| Focus, basophilic | X | ||||
| Fibrosis | X | ||||
| Granules increased | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, mixed cell | X | ||||
| Inflammation, mononuclear cell | X | ||||
| Inflammation, vessel | X | ||||
| Metaplasia, acinar cell | X | ||||
| Metaplasia, osseous | X | ||||
| Metaplasia, squamous cell | X | ||||
| Mineralization (+ locator) | X | ||||
| Multinucleated giant cells | X | ||||
| Necrosis, glandular * | X | ||||
| Pigment | X | ||||
| Secretion, deceased, acinar cell | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic | X | ||||
| Vacuoles, autophagic, acinar cell | X | ||||
| Vacuolation | X | ||||
| Yeast | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia # | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
| Myoepithelioma, malignant | X | ||||
| Tumor, mixed, benign | X | ||||
| Tumor, mixed, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Accumulation
Comments: This diagnosis should be used to describe the microscopic correlates with a macroscopic sialocele.
Depletion, Secretory, Acinar Cell
Comments: This is seen as a generalized process secondary to reduced food intake in rabbits as in many other species, and also in animals suffering from mucoid enteropathy. There may be diffuse depletion of parotid salivary gland zymogen, with associated vacuolar degeneration of exocrine cell cytoplasm32.
Necrosis, Glandular
Other terms: Infarct; Metaplasia, Squamous Cell
Unilateral minimal focal/multifocal mandibular salivary gland necrosis with acute inflammation has been reported in rabbits after auricular artery catheterization. Although thrombi were not identified microscopically, necrosis/acute inflammation was consistent with recent infarction33. Extensive coagulative necrosis and associated squamous metaplasia (so-called “necrotizing sialometaplasia”) have been reported in the mandibular salivary as sequelae of mandibular fracture34 and photodynamic therapy (di-sulfonated phthalaocyanine and laser irradiation)35 and were likewise attributed to infarction35.
C. Anatomy of the Stomach
Stomach
The stomach of a healthy rabbit is never empty, due to the practice of coprophagy, and like the rat and horse, a rabbit cannot vomit36. In new-born rabbits, an empty stomach indicates agalactia of the mother. The stomach is large, thin walled, and unlike rodents, there is no non-glandular region in the rabbit stomach (Figures 6, 7
Figure 7.
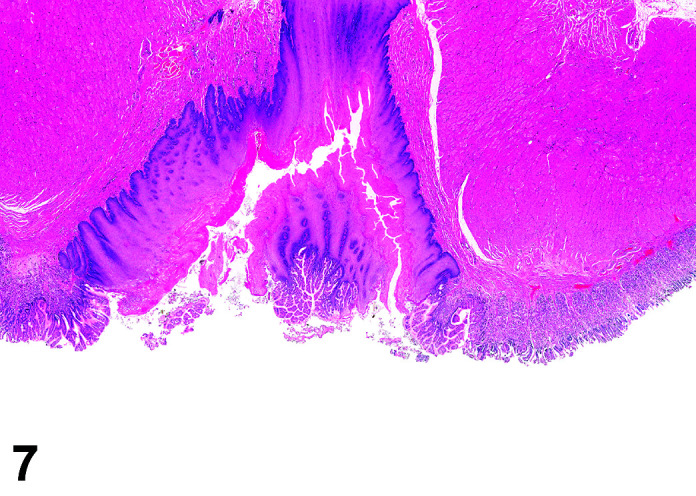
Stomach, Cardioesophageal junction, H&E.
). Occasionally, a bezoar may be found acting as a foreign body and eventually obstructing the pyloric region. Free ingesta in the abdominal cavity may mimic rupture of the gastric wall. Helicobacter spp. have been identified in the stomach of rabbits but not associated with inflammation or ulceration37. The stomach is prone to very fast autolysis and in premature decedent toxicologic study animals it should be sampled as soon as possible (Table 6).
Figure 6.
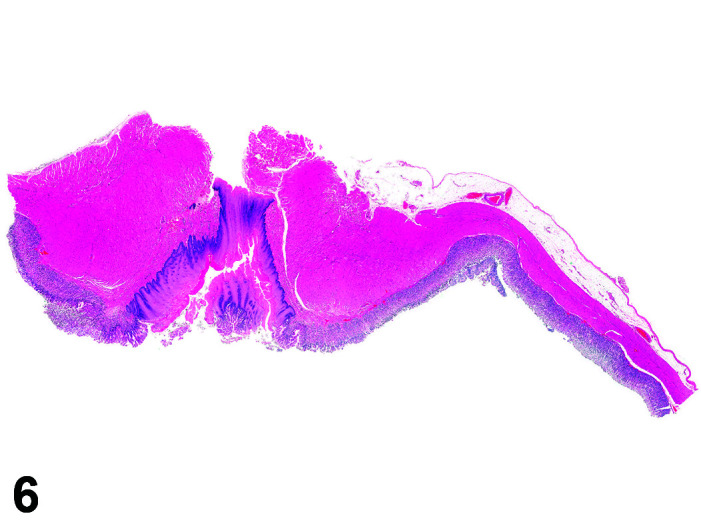
Stomach, Cardioesophageal junction, H&E.
Table 6. Microscopic Findings of the Stomach: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Ectopic tissue | X | ||||
| Malformation | X | ||||
| Non-proliferative | |||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Cyst | X | ||||
| Degeneration/necrosis | X | ||||
| Dilatation, glands | X | ||||
| Diverticulum | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Globules, eosinophilic | X | ||||
| Helicobacter sp. | X | ||||
| Hemorrhage | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation (+ cell type) | X | ||||
| Metaplasia | X | ||||
| Mineralization (+ locator) | X | ||||
| Necrosis, mucosa | X | ||||
| Parasite * | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Syncytia, epithelium | X | ||||
| Yeast | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia # | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
| Gastrointestinal stromal tumor (GIST), benign | X | ||||
| Gastrointestinal stromal tumor (GIST), malignant | X | ||||
| Leiomyoma | X | ||||
| Leiomyosarcoma | X | ||||
| Tumor, basal cell, benign | X | ||||
| Tumor, neuroendocrine cell, benign | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Parasite
Comments: Parasites of the gastrointestinal system include nematodes, cestodes and protozoans, but are rarely a problem in well-managed barriered facilities. Protozoal parasites cause the most common and significant disease. Numerous species of Eimeria are capable of infecting rabbits: Eimeria stiedai (frequently referred to as E. stiedae), E. magna, E irresidua and E. intestinalis.
D. Anatomy of the Small and Large Intestines
Intestine
The intestinal tract of the rabbit is anatomically distinct from that of the other laboratory species. Locations from which samples for histopathological examinations should be ideally taken are illustrated.
Duodenum
The length of the duodenum is relatively great in rabbits and the proximal part of the duodenum is characterized by a wide layer composed of Brunner’s glands. The sample for histology therefore should be taken close to the pyloric region. In the rabbit two distinct cell types are present within the glands. Serous cells, which occur in small groups in the blind endings to the tubules, contain a concentration of rough endoplasmic reticulum in the basal cytoplasm, and the apical cytoplasm is occupied principally by discrete secretory droplets. Intercellular secretory canaliculi occur between opposed serous cells and between serous and mucous cells. The latter cells possess little rough endoplasmic reticulum but exhibit an extensive development of the Golgi apparatus in the supranuclear region. Secretory droplets are pale and show a tendency to fuse into complexes. No intercellular canaliculi occur between mucous cells38. In contrast to other species, Paneth cells are visible in the duodenum at the base of the duodenal crypts and are easily recognized by the large eosinophilic granules that occupy most of the cytoplasm.
Jejunum
The jejunum is located along the left side of the cecum. It is very long and its junction with the ileum is indicated by the ileocecal fold, which attaches to the apex of the vermiform appendix of the rabbit. The jejunum should be sampled distal to the junction with the pancreatic ducts. The villi of the jejunum are thin and longer than in the duodenum and display a tall columnar epithelium with a low number of intermingled goblet cells. Paneth cells are easily to detect at the base of the crypts. Peyer’s patches can be found only in the distal region of the jejunum.
Ileum
The ileum is also located along the left side of the cecum. The terminal part of the ileum is enlarged to form the round, expanded muscular sac – the sacculus rotundus. The sacculus rotundus is a common site for foreign body impaction. Both the sacculus rotundus and vermiform appendix appear pale coloured at necropsy due to the large amount of lymphoid tissue in their walls. These two lymphoid organs contain more than 50% of the total lymphoid tissue of the rabbit, accounting for the relatively small size of the spleen. The surface of the sacculus rotundus is covered by short villi, and the lamina propria is similar to that of the vermiform appendix. The thickness of the lymphoid tissue is variable. The tunica muscularis of the sacculus rotundus is thicker than that seen in the vermiform appendix.
Cecum
The cecum occupies the major portion of the middle to lower abdomen ventrally, being coiled around itself into three major turns or gyri and is freely movable in the peritoneal cavity. The cecal wall consists of epithelium-lined lamina propria, with short indented crypts giving an irregular contour to the surface. Both tunica muscularis and submucosa are thin. Scattered goblet cells are located between tall columnar cells39. The haustrated cecum is tightly coiled and tapers to form the light-colored vermiform appendix. The rabbit is a hindgut fermenter, and so the cecum is much larger than the stomach (about 10 times the size). There is a small area of lymphoid tissue approximately 2 mm in diameter on the inner wall of the cecum adjacent to the ileocecal orifice. The lymphoid follicles at this site have direct contact with the lumen of the cecum (unlike the follicles in the vermiform appendix and sacculus rotundus). The lymphoid tissue in this ileocecal plaque is more loosely arranged and more diffuse in character than that of the other two intestinal lymphoid structures. A tall columnar epithelium covers the surface of the follicle and goblet cells are rare. The tunica submucosa and muscularis are thin39.
The vermiform appendix consists of a thick continuous layer of lymph follicles, the apical portions of which extend as protrusions above the lymph follicle proper. These protrusions are covered by columnar epithelium which is strongly infiltrated with lymphoid cells. At the base of the protruding portion of the follicle a thin column of lamina propria, emerging from the reticular connective tissue surrounding the lymph follicles, extends between the protruding portions of neighbouring follicles. This column of lamina propria is covered by a high columnar epithelium which contains numerous goblet cells and is continuous with the epithelium overlying the lymph follicle at the base of the apical protrusion. Above the bulging portion of the follicle the columns of lamina propria combine to form a covering over the follicle with a slightly lower columnar epithelium. Oval and slit like openings indicate areas which are not covered by the lamina propria. Occasional goblet cells are present in this columnar epithelium; the lamina muscularis mucosae is dispersed and difficult to define. The lymph follicles constitute 70% of the entire thickness of the intestinal wall. The epithelium adjacent to the lymphoid follicles is of a higher columnar cell type. The nuclei are more elongated, microvilli are short and sparse, and vesicles and mitochondria are abundant on the apical portions when examined by SEM. Goblet cells are numerous within this epithelium39.
Colon
The proximal part of the ascending colon is closely associated with the cecum but can be identified by its characteristic tight haustrations and prominent teniae. The transverse colon is divided into proximal and distal portions and is separated by the fusi coli - a muscular spindle-shaped organ with a greatly thickened mucosa. The fusi coli is heavily supplied with ganglion cell aggregates and is under the influence of prostaglandins. The fusi coli, along with the muscular contractions of the sacculations and haustra, is responsible for directing the separation of fiber from non-fiber components of feeds. This speeds the fiber components through the colon where it is excreted as hard feces. Antiperistaltic action moves fluids and small particles in a retrograde manner through the colon to the cecum, where it is retained for fermentation40. Cecal contents are selectively passed as cecotrophs, also referred to as “soft feces”, to be consumed directly from the rectum. Cecotrophs are covered with mucus to protect them from the acid pH of the stomach (pH 1.2–1.5) and composed of water, nitrogen, electrolytes and vitamins. The arrival of cecotrophs at the anus triggers a neural response, resulting in licking the anal area and consumption of the cecotrophs. This is usually 4–8 hours after feeding, generally in the evenings, therefore cecotrophs are also known as “night feces”. These soft pellets contain twice the protein and half the fibre of the daytime fecal pellets. Coprophagy improves the utilisation of nitrogen, provides an abundance of certain B vitamins and conserves water. The excretion of hard daytime feces is related to feeding. Daytime fecal pellets are firm and dry, are excreted during the first four hours after feeding and are not ingested.
The most proximal part of the colon, adjacent to the sacculus rotundus, is expanded to form the bulb like structure – the ampulla cecalis coli, which is the most muscular portion. It consists of epithelial lined lamina propria (tunica mucosa) possessing wide open crypts. The walls of the crypts often show irregular contours, especially near the lumen, and give the impression of villi. The tunica muscularis is thicker than in other parts. Goblet cells are scarce39. The ampulla coli is entirely free of mesenteric connections. Distal to the ampulla, the ascending colon spirals around the cecum producing several flexures before joining the transverse colon. The distal ascending colon, and transverse colon are small in diameter, and are not haustrated.
Peri-rectal tissue
There are focal apocrine-type glands in the submucosa near the anorectal junction, often called “anal glands” (further discussed in the Integument section). Their relationship to the “inguinal” (apocrine/sebaceous) gland complex (Integument section) is not clear (Table 7).
Table 7. Microscopic Findings of the Small and Large Intestines: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Ectopic tissue | X | ||||
| Malformation | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Cyst | X | ||||
| Degeneration | X | ||||
| Degeneration/necrosis | X | ||||
| Degeneration, neuron, myenteric plexus * | X | ||||
| Dilatation, (+ locator) * | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, Paneth cell | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, mixed cell | X | ||||
| Inflammation, mononuclear cell | X | ||||
| Inflammation, vessel | X | ||||
| Intussusception | X | ||||
| Lymphangiectasis | X | ||||
| Metaplasia, squamous cell/Paneth cell/osseous | X | ||||
| Mineralization (+ locator) | X | ||||
| Necrosis, mucosa | X | ||||
| Paneth cell, decreased | X | ||||
| Parasite * | X | ||||
| Pigment | X | ||||
| Prolapse | X | ||||
| Single cell necrosis ǂ | X | ||||
| Syncytia, epithelium | X | ||||
| Vacuolation, mucosa | X | ||||
| Yeast | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia * | X | ||||
| Hyperplasia, goblet cell *# | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, Brunner’s glands | X | ||||
| Gastrointestinal stromal tumor (GIST), benign | X | ||||
| Gastrointestinal stromal tumor (GIST), malignant | X | ||||
| Leiomyoma | X | ||||
| Leiomyosarcoma | X | ||||
| Tumor, neuroendocrine cell, benign | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Degeneration, Neuron, Myenteric Plexus
Diagnostic features: Chromatolytic degeneration of pre- and postganglionic sympathetic and parasympathetic neurons (enteric neurons in the myenteric and submucosal plexus in the small intestine), as well as chromatolysis of somatic and autonomic lower motor neurons in the brain stem and spinal cord.
Comments: This is part of a “syndrome” or constellation of findings that includes both plexus and brain stem. The dysautonomia has been compared to similar lesions in horses, cats and hares. It is assumed that the causative toxin is present in hay and grass, causing the same lesion in horses, rabbits and hares. Animals affected also show impaction of the large intestine with dry food material.
Dilatation, Duct
Comments: In animals suffering from mucoid enteropathy, the ducts of the Brunner’s glands in the duodenum become dilated, with cuboidal to low columnar acinar epithelium, rather than tall mucus-filled epithelial cells32.
Hyperplasia, Goblet Cell
Comments: Mucoid enteropathy is usually seen in young animals, 2–3 months old, but can be seen in adults. In animals suffering from mucoid enteropathy there is an increase in the size and number of goblet cells in the intestinal epithelium, affecting duodenum, jejunum and ileum, but most apparent in sections of the ileum. Colonic crypts may be irregularly dilated due to mucous plugs. Goblet cell hyperplasia may also occur in the cecum due to cecal stasis and impaction, and in the hepatic bile ducts. Staining with Alcian blue-Periodic Acid Schiff indicates depletion of richly stained acidic colonic mucus and replacement with weakly staining light green-blue foamy mucus. Mucoid “enteritis” is a misnomer, for there is no hyperemia, congestion, local leukocytic response, necrosis, or fever, with goblet cell hyperplasia and a mucus hypersecretory state instead being the pathognomonic finding. Mucoid “enteropathy” is the correct term for this condition32. The etiology is unknown, but an enterotoxin-induced secretory diarrhea caused by Escherichia coli or Clostridium spiriforme is suspected. Antibiotic induced enterotoxemia can also be a factor, especially with lincomycin, clindamycin or erythromycin. The finding may be induced in toxicology studies assessing antibiotics.
Parasite
Figure 9.
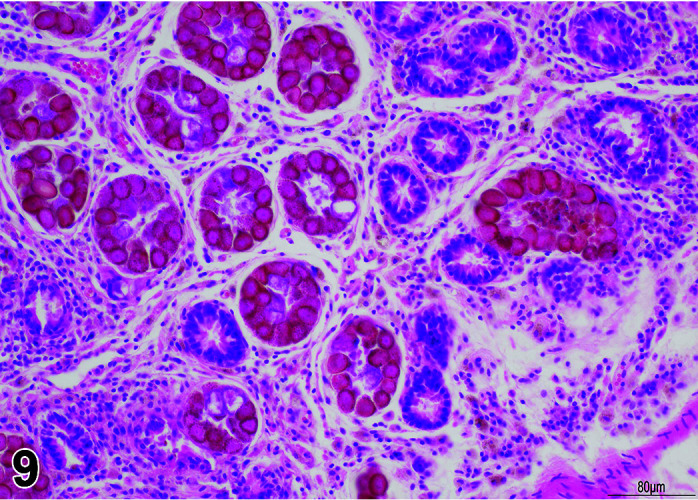
Gastrointestinal tract, Parasite (Eimeria), H&E.
)
Figure 8.
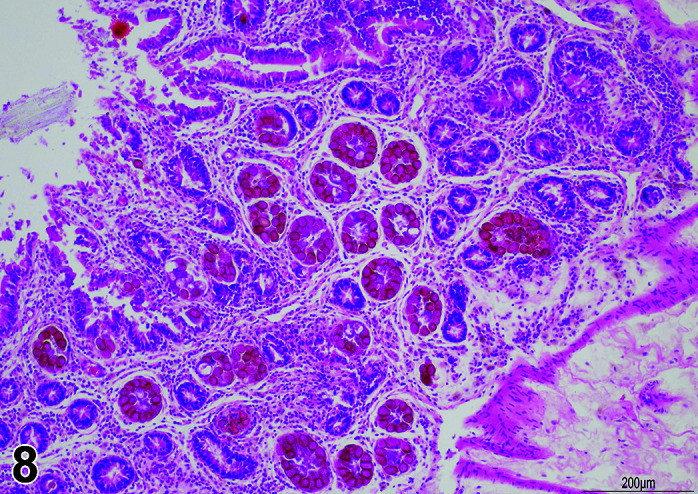
Gastrointestinal tract, Parasite (Eimeria), H&E.
Comments: Parasites of the gastrointestinal system include nematodes, cestodes and protozoans, but are rarely a problem in well-managed barriered facilities. Protozoal parasites cause the most common and significant disease. Numerous species of Eimeria are capable of infecting rabbits: Eimeria stiedai (frequently referred to as E. stiedae), E. magna, E irresidua and E. intestinalis.
E. Anatomy of the Exocrine Pancreas
The pancreas of the rabbit is small and diffuse and located in a pocket formed by the transverse colon, the stomach and the duodenum. It may be difficult to locate in the abundant adipose tissue of the omentum, and retains only the accessory pancreatic duct, which enters the ascending duodenum distal to the entrance of the biliary duct. The right lobe of the pancreas is situated in the mesoduodenum of the duodenal loop. The left lobe lies between the stomach and transverse colon. There is a single pancreatic duct that opens at the junction of the transverse and ascending loops of the duodenum. The duct drains both pancreatic lobes. Technically, this is the accessory pancreatic duct as the main pancreatic duct connection to the duodenum disappears during embryonic development (Table 8).
Table 8. Microscopic Findings of the Exocrine Pancreas: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Ectopic tissue * | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Accumulation, adipocytes | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Autophagic vacuoles, acinar cell | X | ||||
| Cyst | X | ||||
| Degeneration/necrosis | X | ||||
| Degranulation, (+ locator) | X | ||||
| Dilatation, duct | X | ||||
| Ectasia, duct | X | ||||
| Edema | X | ||||
| Focus, basophilic | X | ||||
| Fibrosis | X | ||||
| Halos, peri-insular, decreased | X | ||||
| Halos, peri-insular, increased | X | ||||
| Hemorrhage | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, mixed cell | X | ||||
| Inflammation, mononuclear cell | X | ||||
| Inflammation, vessel | X | ||||
| Metaplasia, ductular | X | ||||
| Metaplasia, hepatocyte | X | ||||
| Mineralization (+ locator) | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Secretion, decreased | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia # | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, acinar cell | X | ||||
| Adenoma, ductal cell | X | ||||
| Adenocarcinoma, acinar cell | X | ||||
| Adenocarcinoma, ductal cell | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Tissue, Ectopic
Comments: Ectopic spleen has been recorded in the pancreas of a NZW41.
Section 5: Endocrine System: (Pituitary, Pineal, Thyroid, Parathyroid, Adrenal Glands and Endocrine Pancreas)
For detailed general considerations on the endocrine system, please refer to the rodent INHAND publication42.
The endocrine system of the rabbit is made up of the pituitary, pineal, thyroid, parathyroid, adrenal, and islets of Langerhans of the pancreas as well as parts of the male and female gonads and the epithelial lining of the duodenum43.
A. Anatomy of the Pituitary Gland
In the rabbit the pituitary has three major divisions: lobus glandularis (adenohypophysis), lobus nervosus (neurohypophysis) and the infundibular stalk which attaches the pituitary to the median eminence of the hypothalamus43, 44. Strong muscarinic receptor protein-like (mAChRp-L) immunoreactivity is associated with the blood vessels of the anterior and intermediate lobes of the rabbit pituitary45. Sensitive and specific autoregulatory control systems for thyrotropin (TSH), luteinizing hormone (LH) and follicle stimulating hormone (FSH) exist in the rabbit pituitary46 (Table 9).
Table 9. Microscopic Findings of the Pituitary Gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aberrant craniopharyngeal structures | X | ||||
| Aplasia/hypoplasia | X | ||||
| Persistent Rathke’s pouch | X | ||||
| Non-proliferative | |||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Cyst | X | ||||
| Fibrosis | X | ||||
| Gliosis, pars nervosa | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, pars distalis | X | ||||
| Hypertrophy, pars intermedia | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Pseudocyst | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Vacuolation | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, pars distalis/intermedia | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, pars distalis/intermedia | X | ||||
| Carcinoma, pars distalis/intermedia | X | ||||
| Craniopharyngioma, benign | X | ||||
| Pituicytoma, benign | X | ||||
| Craniopharyngioma, malignant | X | ||||
| Pituicytoma, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
B. Anatomy of the Pineal Gland
Pineal Gland
Calcareous concretions are common, which increase with age and apparently do not affect function of the gland. The rabbit pineal gland has an inhibitory effect on gonadotropin release47 (Table 10).
Table 10. Microscopic Findings of the Pineal Gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia/hypoplasia | X | ||||
| Non-proliferative | |||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Cyst | X | ||||
| Fibrosis | X | ||||
| Fibers, striated muscle | X | ||||
| Hemorrhage | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Striated muscle fibers | X | ||||
| Thrombus | X | ||||
| Vacuolation | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia | X | ||||
| Proliferative Neoplastic | |||||
| Pinealoma, benign | X | ||||
| Pinealoma, malignant | X | ||||
Since the pineal gland is not routinely evaluated there is limited experience in incidences of these lesions in the rabbit. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
C. Anatomy of the Thyroid Gland
The two lobes of the thyroid gland in the rabbit are less clearly circumscribed compared to other laboratory species and are flattened, with the isthmus barely visible (Table 11) (Figures 10, 11
Figure 11.
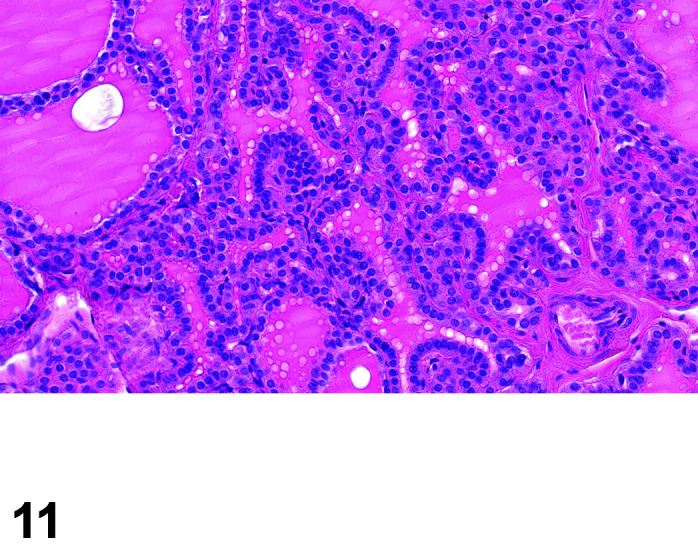
Thyroid, Diffuse Hyperplasia, H&E (high mag).
).
Table 11. Microscopic Findings of the Thyroid Gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia/hypoplasia | X | ||||
| Cyst, ultimobranchial | X | ||||
| Duct, thyroglossal, persistent | X | ||||
| Thyroid dysplasia | X | ||||
| Non-proliferative | |||||
| Alteration, colloid | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | |||||
| Atrophy | X | ||||
| Cyst | X | ||||
| Fibrosis | X | ||||
| Follicle, cystic | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, follicular cell | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic | X | ||||
| Thrombus | X | ||||
| Vacuolation | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, C-cell | X | ||||
| Hyperplasia, follicular cell | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, C-cell | X | ||||
| Adenoma, follicular cell | X | ||||
| Carcinoma, C-cell | X | ||||
| Carcinoma, follicular cell | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Figure 10.
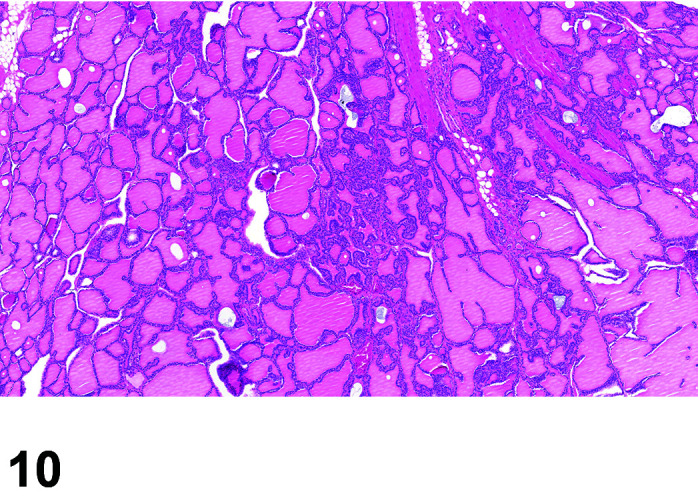
Thyroid, Diffuse Hyperplasia, H&E.
D. Anatomy of the Parathyroid Gland
The paired parathyroid glands are usually located on the anterior and lateral aspect of the thyroid lobes, located in or immediately outside of the thyroid gland in the rabbit, and are separated from the thyroid by a thin capsule of fibrous connective tissue. The rabbit possesses two parathyroid glands within the thyroid and two located in the fascial plan between the sternohyoid and sternothyroid muscles and the carotid artery48 (Table 12).
Table 12. Microscopic Findings of the Parathyroid Gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia/hypoplasia | X | ||||
| Cyst, ultimobranchial | X | ||||
| Duct, thyroglossal, persistent | X | ||||
| Non-proliferative | |||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | |||||
| Atrophy | X | ||||
| Cyst | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Multinucleated giant cells | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic * | X | ||||
| Thrombus | X | ||||
| Vacuolation | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Carcinoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Tissue, Ectopic
Comments: Ectopic parathyroid tissue can occur in the thymus or dorsolateral to the esophagus near the larynx.
E. Anatomy of the Adrenal Gland
Adrenal Cortex and Medulla
In the rabbit, each of the pair of suprarenal adrenal glands is composed of an inner medulla and outer cortex. The adrenal cortex is voluminous in the rabbit and is derived from the interrenal gland associated with the mesonephros in lower vertebrates, which is involved in the maintenance of normal functioning kidneys43. The ultrastructure of the capsule of the rabbit adrenal gland is made up of three layers with the outermost layer consisting of collagen and elastic fibrillae with cytoplasmic processes of fibroblasts in between49. Myofibroblasts are present in the middle layer as well as unmyelinated nerves, indicating a contractile function. The basal laminae of the fenestrated capillaries in the inner vascular layer is occasionally fused with that of the outer zona glomerulosa, suggesting a probable route for blood supply and secretion49. The cortex is made up of three layers: the zona glomerulosa, zona fasciculata, zona reticularis44. Fazekas and Sandor have demonstrated that there is an unusual pathway of aldosterone biosynthesis in the rabbit adrenal whereby aldosterone is formed mainly from corticosterone via 18-hydroxy-corticosterone50. The adrenal medulla contains chromaffin cells and ganglion cells arranged into trabeculae44. The adrenal glands are the most commonly affected endocrine organs secondary to chemical exposure51. In the adrenal glands, chemically induced lesions are found most frequently in the zona fasciculata and reticularis and to a lesser extent in either the zona glomerulosa or the medulla.
Paraganglia
The paraganglia including the carotid and aortic bodies (made up of neuroendocrine cells) are also considered to be endocrine tissues of the rabbit43 (Table 13).
Table 13. Microscopic Findings of the Adrenal Gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia/hypoplasia | X | ||||
| Non-proliferative | |||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Cyst | X | ||||
| Degeneration, cystic | X | ||||
| Fibrosis | X | ||||
| Hematopoiesis, extramedullary | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, cortical, diffuse/focal | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Persistent X-zone | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic | X | ||||
| Thrombus | X | ||||
| Vacuolation cortex decreased diffuse | X | ||||
| Vacuolation cortex decreased focal | X | ||||
| Vacuolation cortex increased diffuse | X | ||||
| Vacuolation cortex increased focal | X | ||||
| Proliferative Non-Neoplastic | |||||
| Hyperplasia, cortical/medullary | X | ||||
| Hyperplasia, subcapsular cell | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, cortical cell | X | ||||
| Adenoma, subcapsular cell | X | ||||
| Ganglioneuroma, benign | X | ||||
| Myelolipoma | X | ||||
| Pheochromocytoma, complex, benign | X | ||||
| Pheochromocytoma, benign | X | ||||
| Carcinoma, cortical cell | X | ||||
| Carcinoma, subcapsular cell | X | ||||
| Neuroblastoma, malignant | X | ||||
| Pheochromocytoma, complex, malignant (adrenal gland) | X | ||||
| Pheochromocytoma, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
F. Anatomy of the Endocrine Pancreas (Islets of Langerhans)
Extensive deep connections between the capillary beds of the islets and the exocrine tissue form a highly developed portal system in the rabbit which allows the islet hormones of insulin, glucagon and somatostatin to influence exocrine pancreatic cells52. Nearly all of the efferent islet blood flow goes to the acinar capillaries before leaving the pancreas. Thus, the flow to the islets is large enough to permit significant local actions of the islet hormones on the exocrine pancreas, confirming of the existence of an insuloacinar portal system53 (Table 14).
Table 14. Microscopic Findings of the Endocrine Pancreas: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia/hypoplasia | X | ||||
| Non-proliferative | |||||
| Amyloid, islet | X | ||||
| Angiectasis | X | ||||
| Apoptosis, islet cell ǂ | X | ||||
| Atrophy, islet cell | X | ||||
| Cyst | X | ||||
| Degranulation, islet cell | X | ||||
| Fibrosis, islet | X | ||||
| Hemorrhage, islet | X | ||||
| Hypertrophy, islet cell | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, hepatocyte | X | ||||
| Necrosis | X | ||||
| Pigment. islet | X | ||||
| Single cell necrosis | X | ||||
| Vacuolation, islet cell | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, islet cell *# | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, islet cell | X | ||||
| Adenoma, acinar-islet cell | X | ||||
| Carcinoma, islet cell | X | ||||
| Carcinoma, acinar-islet cell | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion.
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Hyperplasia, Islet Cell
Pathogenesis/cell of origin: islet cells
Diagnostic Features: Diffuse islet cell hyperplasia, characterized by increased numbers of otherwise unremarkable islet cells, resulting in variably increased cellularity and overall diameter of islet profiles
Comments: may be seen as an induced treatment related effect by test articles that cause hyperglycemia.
Section 6: Hematopoietic and Lymphoid System
A. Anatomy of the Bone Marrow
During embryogenesis in rabbits, hematopoietic progenitors (Hematopoietic stem cells - HSCs) arise within several sites including the extraembryonic yolk sac and within the placenta. HSCs are also present in the fetal liver. Shortly before birth, HSCs and hematopoietic cells are both present in the bone marrow. Morphological studies by King and Ackerman conclusively indicated that erythrocytes develop extravascularly, arising from mesenchymal or reticular cells in the fetal bone marrow54. Mature erythrocytes enter the circulation through discontinuities in the sinusoidal walls. Neither endothelial cells nor blood-borne lymphocytes make an apparent contribution to erythropoiesis. The first hematopoietic cells to form in the fetal marrow are determined and develop along the erythrocytic line. These proerythroblasts initially arise randomly in the marrow parenchyma and are not in obvious association with the sinusoids. Subsequent maturation and proliferation of the primitive erythrocytic cells result in the formation of colonies of erythrocytic cells at all stages of development. As these colonies enlarge, the erythrocytic elements come in close association with the sinusoids. In later stages of marrow development, developing erythrocytic and granulocytic cells become intermixed and more randomly associated in the extravascular space of the marrow. In rabbits, extramedullary hematopoiesis (EMH) occurs primarily in the spleen55.
Adipose cells begin to develop at 2 weeks of age and proceed so that the adult pattern of red and yellow marrow is fully established by 4 months of age. Adipose cell development occurs in both trunk and limb bones; the magnitude of the process, however, being considerably greater in the limb bones. Adipocyte precursors may be present in the marrow at birth with a differential distribution in the areas of prospective red and yellow marrow. Thus, fatty involution of marrow appears to be a programmed developmental event56.
Bone marrow is variably distributed within the medullary cavity of long and flat bones. Bone marrow for microscopic evaluation in rabbits is typically collected from the femur. Tissue is processed by standard techniques for hematoxylin and eosin stained formalin fixed paraffin embedded decalcified bone. Additionally, marrow casts may be collected from femoral bone marrow and processed for histology. A general guidance for histopathology assessment of bone marrow tissue sections is available 57. Romanowsky stained bone marrow smears may be made for cytology. Rabbit neutrophils (heterophils) have intracytoplasmic granules that cause them to resemble eosinophils. True rabbit eosinophils have larger darker granules. Lymphocytes are the predominant leukocyte. Basophils are more common than in other mammals, making up 2–7% of the leucocyte population of the rabbit (Table 15).
Table 15. Microscopic Findings of the Bone Marrow: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Agenesis/hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ, increased, [insert appropriate cell type] | X | ||||
| Angiectasis | X | ||||
| Atrophy, serous, of fat # | X | ||||
| Cellularity, decreased, adipocyte | X | ||||
| Cellularity, decreased, bone marrow | X | ||||
| Dyshematopoiesis | X | ||||
| Fibrosis | X | ||||
| Hypersegmentation, granulocyte | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous | X | ||||
| Mineralization | X | ||||
| Necrosis, lymphocyte | X | ||||
| Pigment, macrophage | X | ||||
| Pigment | X | ||||
| Serous atrophy of fat | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Cellularity, increased, [insert appropriate cell type] | X | ||||
| Proliferative Neoplastic | |||||
| Eosinophil Granulocytic Sarcoma | X | ||||
| Histiocytic sarcoma | X | ||||
| Leukemia, erythroid/myeloid/megakaryocytic/mast cell/NOS * | X | ||||
| Lymphoma* | X | ||||
| Tumor, mast cell, benign | X | ||||
| Tumor, mast cell, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Leukemia
Comments: Leukemia is reported sporadically in research rabbits58,59,60,61.
Lymphoma
Comments: Lymphoma is the most common neoplasm of juvenile and young adult rabbits. It has been reported in rabbits as young as 4 months of age62 and appears to be more common overall in younger rabbits63. Reports have included tumors of both B-cell64 and T-cell origin61, 65.
B. Anatomy of the Thymus
The rabbit thymus develops bilaterally from the endoderm of the third pharyngeal pouch and the surrounding mesenchyme. It is recognized as the pacemaker of lymphopoiesis in that if it fails to develop prenatally, then the immune system cannot be established66. The rabbit thymus develops very late, at Embryonic Day (ED) 10 (about the third of gestation period). At ED29, the demarcation between the cortex and the medulla becomes easily distinct in all lobules. At this age, Hassall’s corpuscles can be observed within the medulla. They are few in number, small in size and show different stages of their formation. Some Hassall’s corpuscles are represented by collection of swollen epithelial cells, other corpuscles consist of few layers of concentrically arranged epithelial cells with centrally located keratin substance. At 1 and 2 weeks postnatally, the Hassall’s corpuscles increase in size and number to be large, acidophilic, rounded bodies consisting of a central degenerated hyaline mass surrounded by concentrically arranged epithelial-reticular cells67. They are unique to the thymus68. The Hassall’s bodies are structurally organized from medullary reticuloepithelial cells, which usually undergo hypertrophy prior to their inclusion in the outer cell layer of the corpuscles69.
In rabbits, the thymus has two parts; thoracic and cervical. The rabbit thymus acquires the lobulated appearance at ED14. In the thorax, the thymus is separated into three lobes: the right dorsal thoracic lobe, the right ventral thoracic lobe, and the left thoracic lobe70. Grossly, the shape of the left lobe of the thymus is quadrilateral in outline with extended narrow craniomedial angle in the neck, while the right lobe is triangular in outline with its base cranially directed and extended narrow craniomedial angle in the neck. The dorsal aspect of both lobes is concave showing the cardiac impression which is larger on the left lobe. In addition, the right lobe shows a pulmonary impression laterally. Medially, the left lobe slightly overlaps the right one and both lobes are connected only by small amount of interlobar connective tissue. The ventral aspect is convex and related to the sternum. While the thymus remains relatively large in adult rabbits13, with increasing age the thymus undergoes a proportional decrease in both cortical and medullary size71 (Table 16).
Table 16. Microscopic Findings of the Thymus: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Agenesis/hypoplasia | X | ||||
| Ectopic tissue | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ, increased, lymphocyte | X | ||||
| Cellularity, decreased, lymphocyte | X | ||||
| Corticomedullary distinction, loss of | X | ||||
| Corticomedullary ratio, decreased | X | ||||
| Corticomedullary ratio, increased | X | ||||
| Cyst, epithelial | X | ||||
| Epithelial cell free zones, increased | X | ||||
| Hypoplasia | X | ||||
| Involution, age-related | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Pigment, macrophage | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic (specify tissue) | X | ||||
| Tissue, ectopic, parathyroid | X | ||||
| Tissue, ectopic, thyroid | X | ||||
| Thymic corpuscles, increased | X | ||||
| Tingible body macrophage, increased | X | ||||
| Vacuolation, macrophage | X | ||||
| Proliferative Non-neoplastic | |||||
| Cellularity, increased, [insert appropriate cell type] | X | ||||
| Proliferative Neoplastic | |||||
| Histiocytic sarcoma | X | ||||
| Lymphoma | X | ||||
| Thymoma, benign | X | ||||
| Thymoma, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
C. Anatomy of the Spleen
There are no significant variations macroscopic structures or microarchitecture between the rabbit and rodent spleens72. Duplication of the spleen has been observed sporadically73 (Rinke, pers. observation) (Table 17).
Table 17. Microscopic Findings of the Spleen: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Agenesis/hypoplasia | X | ||||
| Tissue, ectopic | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ, increased, lymphocyte | X | ||||
| Cellularity, decreased, white pulp/red pulp | X | ||||
| Congestion | X | ||||
| Erythrophagocytosis | X | ||||
| Fibrosis | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Mineralization | X | ||||
| Necrosis, lymphocyte | X | ||||
| Pigment, macrophage | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tingible body macrophages, increased | X | ||||
| Vacuolation, macrophage | X | ||||
| Proliferative Non-neoplastic | |||||
| Accumulation, adipocyte | X | ||||
| Aggregates, macrophage, increased | X | ||||
| Cellularity, increased, [insert appropriate cell type] | X | ||||
| Extramedullary hematopoiesis, increased | X | ||||
| Hyperplasia, nodular | X | ||||
| Hyperplasia, mast cell | X | ||||
| Proliferative Neoplastic | |||||
| Eosinophil Granulocytic Sarcoma | X | ||||
| Histiocytic sarcoma | X | ||||
| Leukemia, erythroid/myeloid/megakaryocytic/mast cell/NOS | X | ||||
| Lymphoma | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
D. Anatomy of the Lymph Nodes
Lymph nodes are present at birth and develop upon antigenic stimulation. Knowledge about the area(s) drained by the lymph nodes is needed to interpret their morphology74,75,76,77. The mesenteric lymph nodes form a compact unit overlying the left side of the superior mesenteric artery13. Other intra-abdominal nodes include those near the posterior end of the mesoduodenum, adjacent to the portal vein near the lesser curvature of the stomach, and near the junction of the splenic and superior mesenteric veins13. There are no species-specific terms for lymph nodes in rabbits (Table 18).
Table 18. Microscopic Findings of the Lymph Nodes: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia/hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ, increased, lymphoid | X | ||||
| Cellularity, decreased, lymphocyte | X | ||||
| Dilatation, sinus | X | ||||
| Erythrocytes, intrasinusoidal | X | ||||
| Extramedullary hematopoiesis | X | ||||
| Fibrosis, (+ locator) | X | ||||
| Granuloma | X | ||||
| Hypertrophy/hyperplasia, high endothelial venule (HEV) | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Lymphangiectasis | X | ||||
| Metaplasia, osseous | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Pigment, macrophage | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tingible body macrophages increased | X | ||||
| Vacuolation, macrophage | X | ||||
| Proliferative Non-neoplastic | |||||
| Accumulation, adipocyte | X | ||||
| Aggregates, increased, macrophage | X | ||||
| Cellularity, increased, (+ cell type) | X | ||||
| Extramedullary hematopoiesis, increased | X | ||||
| Hyperplasia, angiomatous | X | ||||
| Proliferative Neoplastic | |||||
| Eosinophil Granulocytic Sarcoma | X | ||||
| Histiocytic sarcoma | X | ||||
| Leukemia, erythroid/myeloid/megakaryocytic/mast cell/NOS | X | ||||
| Lymphoma | X | ||||
| Tumor, mast cell, benign | X | ||||
| Tumor, mast cell, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
E. Anatomy of the Mucosa-Associated Lymphoid Tissue (MALT)
The mucosal associated lymphoid tissue (MALT) is organized into congenital and acquired organized submucosal lymphoid accumulations associated with the mucosal epithelium. This can include loose clusters of lymphocytes or well-organized lymphoid follicles. Gut-associated, nasal-associated MALT and tonsils are congenital and lymphoid aggregates in other tissues are acquired with antigenic exposure (e.g. conjunctiva, oral, pharyngeal and nasal cavities, upper (larynx and trachea) and lower (bronchus/bronchial) respiratory tract, Eustachian tube, middle ear, stomach, gall bladder, cecum, colon, rectum, reproductive and urinary tracts). The ileocecal MALT is located at the ileocecal junction as an enlargement of the large intestine. The ileocecal MALT account for 50% of the total lymphoid tissue of the rabbit and are the reason for the small size of the spleen30. For detailed descriptions of the vermiform appendix and sacculus rotundus, please refer to the intestinal tract section.
The gut associated lymphoid tissue (GALT) should be examined in studies where the test article is administered orally, and is usually examined in a routine section of jejunum or ileum that contains Peyer’s Patches, however one of the other MALTs - sacculus rotundus (ileocecal tonsils), vermiform appendix, colonic and rectal lymphoid aggregates, cryptopatches (CPs) and isolated lymphoid follicles (ILFs) - would suffice.
Rabbits develop a primary antibody repertoire through somatic diversification of Ig genes (dependent on intestinal microbial flora). Rabbits generate their antibody repertoire in three stages: (a) neonatal repertoire is generated by B lymphopoiesis in fetal liver and bone marrow (limited by preferential V(H) gene segment usage); (b) between 4 and 8 weeks after birth, gut-associated lymphoid tissue (GALT) develops a complex primary antibody repertoire, (c) the primary antibody repertoire is subsequently modified during antigen-dependent immune responses (the secondary repertoire)78.
Most MALT is present at birth (gut, nasal and tonsils), and is acquired in other sites thereafter79. Age-related functional decline of the mucosal immune response and such age-related involution is not described below as a separate item; comparison with concurrent controls is needed to decide whether or not age-related changes have occurred in a study (Table 19).
Table 19. Microscopic Findings of the MALT: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia/hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosisǂ, lymphoid, increased | X | ||||
| Cellularity, decreased, lymphocyte *# | X | ||||
| Degeneration, follicle associated epithelium | X | ||||
| Hyaline material | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Lymphangiectasis | X | ||||
| Material, hyaline | X | ||||
| Mineralization | X | ||||
| Necrosis, (+ cell type) | X | ||||
| Pigment, macrophage | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tingible body macrophages, increased | X | ||||
| Vacuolation, macrophage | X | ||||
| Proliferative Non-neoplastic | |||||
| Aggregates, macrophage, increased | X | ||||
| Cellularity, increased, (+ cell type) | X | ||||
| Extramedullary hematopoiesis | X | ||||
| Hyperplasia, follicle-associated epithelium | X | ||||
| Hypertrophy/hyperplasia, high endothelial venule (HEV) | X | ||||
| Metaplasia, squamous, follicle-associated epithelium | X | ||||
| Proliferative Neoplastic | |||||
| Eosinophil Granulocytic Sarcoma | X | ||||
| Histiocytic sarcoma | X | ||||
| Leukemia, erythroid/myeloid/megakaryocytic/mast cell/NOS | X | ||||
| Lymphoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Cellularity, Decreased, Lymphocyte
Comments: Rabbits are especially sensitive to lymphoid atrophy caused by glucocorticoid administration.
Section 7: Hepatobiliary System (Liver and Gall Bladder)
A. Anatomy of the Liver
The liver is situated in the epigastric region reaching the level of the right 7th and left 9th ribs. The right hepatic, caudate and quadrate lobes are single, and the left lobe is divided into medial and lateral parts80. The caudate process of the caudate lobe is highly developed and overlies the right kidney (Table 20).
Table 20. Microscopic Findings of the Liver: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, hepatocyte | X | ||||
| Cholangiofibrosis | X | ||||
| Congestion | X | ||||
| Crystals | X | ||||
| Cyst, (+ locator) | X | ||||
| Cytoplasmic alteration * | X | ||||
| Degeneration, cystic | X | ||||
| Degeneration, hydropic | X | ||||
| Erythrocytes, intrahepatocellular | X | ||||
| Extramedullary hematopoiesis | X | ||||
| Fatty change | X | ||||
| Fibrosis | X | ||||
| Focus of cellular alteration | X | ||||
| Hemorrhage | X | ||||
| Hepatocytes, intravascular | X | ||||
| Hepatodiaphragmatic nodule | X | ||||
| Hypertrophy, hepatocyte/endothelial cell | X | ||||
| Hypertrophy/hyperplasia, Kupffer cell | X | ||||
| Hypertrophy/karyomegaly, endothelial cell | X | ||||
| Inclusions, intranuclear and cytoplasmic | X | ||||
| Infarct * | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation * | X | ||||
| Intrahepatocellular erythrocytes | X | ||||
| Intravascular hepatocytes | X | ||||
| Karyocytomegaly and/or multinucleated hepatocytes | X | ||||
| Metaplasia, glandular | X | ||||
| Metaplasia, pancreatic acinar cell | X | ||||
| Mineralization * | X | ||||
| Necrosis, focal/multifocal * | X | ||||
| Necrosis, zonal | X | ||||
| Parasite *^ | X | ||||
| Phospholipidosis | X | ||||
| Pigment *, hepatocyte/Kupffer cell | X | ||||
| Plug, bile | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Tissue, ectopic | X | ||||
| Vacuolation, cytoplasm * | X | ||||
| Proliferative Non-Neoplastic | |||||
| Hyperplasia, bile duct | X | ||||
| Hyperplasia, angiomatous | X | ||||
| Hyperplasia, hepatocyte, non-regenerative | X | ||||
| Hyperplasia, hepatocyte, regenerative | X | ||||
| Hyperplasia, Ito cell | X | ||||
| Hyperplasia, oval cell | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, hepatocyte | X | ||||
| Adenoma, hepatocholangiocellular | X | ||||
| Carcinoma, hepatocyte | X | ||||
| Carcinoma, hepatocholangiocellular | X | ||||
| Hemangioma | X | ||||
| Hemangiosarcoma | X | ||||
| Hepatoblastoma | X | ||||
| Histiocytic sarcoma | X | ||||
| Tumor, Ito cell, benign | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ^ Described in systemic pathology section. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Cytoplasmic Alteration
Figure 12.
Liver, Cytoplasmic alteration, H&E.
Other terms: Rarefaction, Glycogen accumulation.
Historical terms: Granular degeneration, hyaline degeneration, ground glass change.
Diagnostic features: Rabbit livers are often pale at necropsy. This cytoplasmic rarefaction is associated with glycogen accumulation and varies both between the sexes and diurnally.
Comments: Generally, rabbits not fasted before euthanasia show high levels of glycogen accumulation. This decreases during the day, as it is usual practice to remove access to food on the day of necropsy. Therefore, animals euthanized in the morning will have more glycogen in the liver than those euthanized in the afternoon. The Study Pathologist should be aware of this diurnal change and interpret any changes in glycogen accumulation with knowledge of whether the animal was fasted prior to euthanasia, for how long and at what time of day the necropsy occurred81. Electron microscopy or special stains are needed for a definitive diagnosis to distinguish between lipidosis (macrovesicular and/or microvesicular steatosis), phospholipidosis, and the gestational lipidosis seen in pregnant animals.
Infarct
Comments: Excess pressure from occlusive dressings, wrapping, sleeves or jackets used to enhance exposure to dermal therapeutics or to protect wound dressings and medical devices has been found to induce necrosis (“corset liver”) in the liver82 and regional infarction in the liver and spleen (Schuh, unpublished data) of rabbits.
Inflammation
Figure 13.
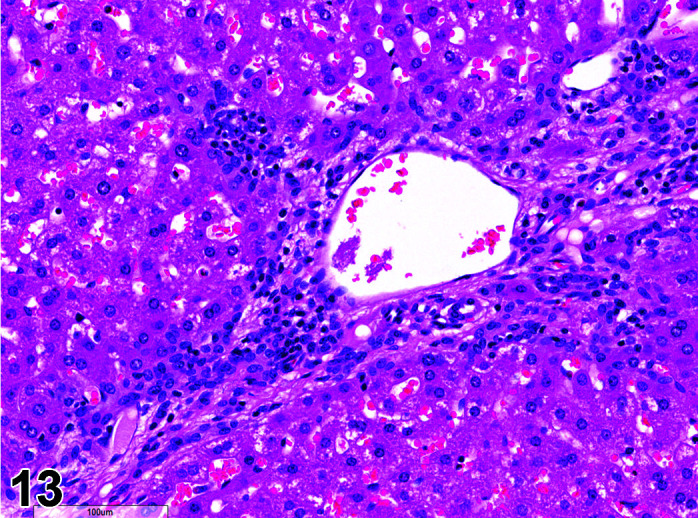
Liver, Inflammation, liver periportal, H&E.
Comments: An autoimmune hepatitis, similar to that seen in man, has been reported in a 5-year-old rabbit. Portal areas were characterized by a marked infiltrate of plasma cells, and some lymphocytes that extended into the surrounding parenchyma with destruction of the limiting plate architecture. In some areas the lymphocytic infiltrate was more extensive and surrounded the bile duct. There were many macrophages with engulfed pigment and apoptotic bodies. Periportal hepatocytes were swollen with karyomegaly and prominent nucleoli83.
Mineralization
Comments: Slight or advanced dystrophic mineralization of the liver may occur due to a calcium and Vitamin D imbalance, for example in cases of over-supplementation. Usually the change occurs first in the aortic arch and heart before it reaches other organs11.
Necrosis
Comments: Multifocal hepatocellular necrosis may occur as a stress-related disease in young (6–12 weeks-old) animals caused by Gram-negative Clostridium (Bacillus) piliformis (Tyzzer’s disease). Clinical symptoms are diarrhea, dehydration and death within 12–48 h. Bacterial detection is mainly difficult due to autolysis and Escherichia coli overgrowth; microbes can be found in living cells only (Periodic Acid Schiff, Giemsa, Warthin-Starry staining). Hepatic copper toxicosis is seen occasionally in laboratory rabbits that are fed a copper-rich diet, leading to hepatocellular copper storage. Animals that then undergo a stressful event (often coupled with anorexia) may acutely release the copper from the hepatocytes. Affected livers have an accentuated lobular pattern macroscopically and centrilobular to midzonal hepatocellular necrosis with mild periportal fibrosis and biliary hyperplasia84.
Parasite
Comments: Parasites include nematodes, cestodes and protozoans, but are rarely a problem in well-managed barriered facilities. Protozoal parasites cause the most common and significant disease. Numerous species of Eimeria are capable of infecting rabbits (Figures 14, 15
Figure 15.
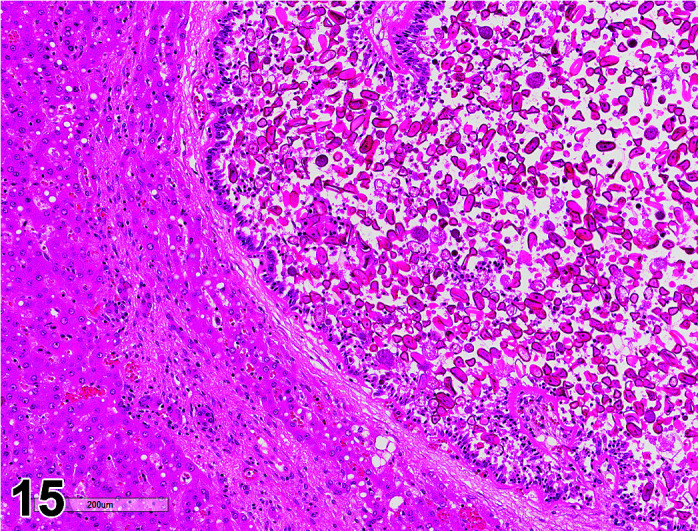
Liver, Parasite (Eimeria), H&E.
). Hepatic lesions caused by the microsporidian Encephalitozoon cuniculi are recognized in rabbits (Figure 16). Hepatic lesions often include granulomatous foci along with periportal infiltration by macrophages, multinucleated giant cells, lymphocytes, and plasma cells. Inflammation can extend into the hepatic portal veins and branches of the hepatic artery2. Parasite presence can be confirmed by real-time PCR85.
Figure 14.
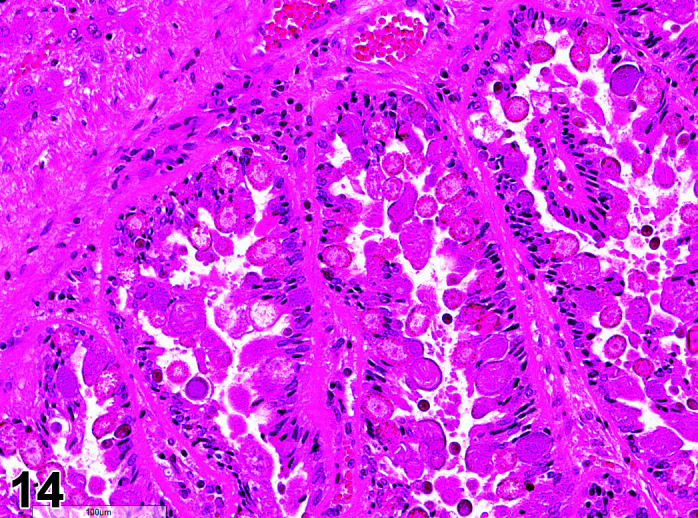
Liver, Parasites (Eimeria), H&E.
Figure 16.
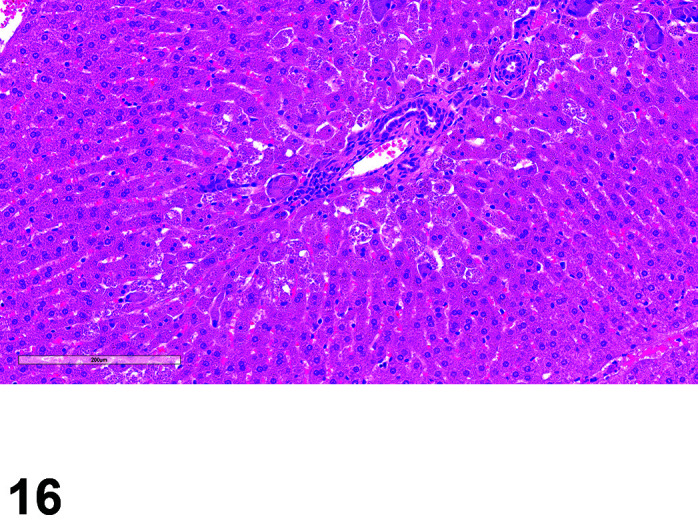
Liver, Parasite (Encephalitozoon), H&E (Courtesy of Aaron Sargeant).
Pigment
Figure 17.
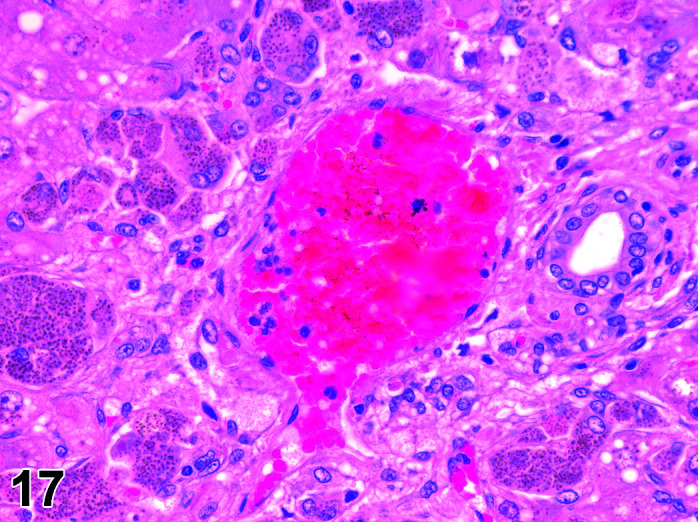
Liver, Pigment (Liver toxicosis), H&E.
Comment: This term should be used for material within hepatocytes or Kupffer cells only. Pigment in canaliculi should be termed ‘Plug, bile’. Periportal and centrilobular storage of iron positive pigment has been observed, but rarely. The pigment deposition may be restricted to single lobes, but also within an entire lobe the distribution may vary significantly. Pseudomelanosis has been described previously73. The livers are grossly discolored from dark brown to black. Histologically, intracytoplasmic pigment granules are observed that contain iron and lipofuscins. This alteration is not known to be associated with lesions in other organs.
Vacuolation, Cytoplasm
Other terms(s): Gestational lipidosis
Comments: May be seen in rabbits on reproductive toxicity studies. Pregnancy toxemia is a metabolic disease primarily during late pregnancy (less frequently in postpartum, pseudopregnant, and nonpregnant females), characterized by low morbidity and high mortality, and exacerbated by an inability to consume adequate energy to match metabolic demands. Risk factors include: obesity, improper nutrition - such as a diet too low in fiber, sudden stress, and anorexia. Fat deposits become rapidly mobilized, resulting in hepatic lipidosis and ketosis.
B. Anatomy of the Gall Bladder
A gall bladder is present. The gall bladder has a cylindrical shape and does not reach the livers ventral edge. The bile duct enters the duodenum very close to the pylorus. Rabbits produce very large amounts of bile, about seven times as much as a dog on a weight basis36. Approximately 20–50% of rabbits produce atropinase in the bile30. Thus, atropine is not recommended during anaesthesia (Table 21).
Table 21. Microscopic Findings of the Gall Bladder: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Cyst | X | ||||
| Malformation * | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Calculus * | X | ||||
| Congestion | X | ||||
| Crystals | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Hyalinosis | X | ||||
| Inclusions, intranuclear and cytoplasmic | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation * | X | ||||
| Metaplasia | X | ||||
| Mineralization | X | ||||
| Necrosis, focal/multifocal | X | ||||
| Necrosis, single cell | X | ||||
| Parasite * | X | ||||
| Pigment | X | ||||
| Thrombus | X | ||||
| Tissue, ectopic, pancreas | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, macrophage | X | ||||
| Proliferative Non-Neoplastic | |||||
| Hyperplasia | X | ||||
| Metaplasia, glandular | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
| Cholangioma | X | ||||
| Cholangiocarcinoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Malformation
Comments: Bifurcate gall bladder or duplicated gall bladder is occasionally seen in rabbits86. It is clinically silent and an incidental finding at necropsy.
Calculus
Comments: Rabbits fed a diet containing 0.75% dihydrocholesterol for 7 days develop bile acid allodeoxycholic (ADCA) and deoxycholic acid (DCA) stones in the gall bladder. In this model, inflammatory changes in the gall bladder mucosa are often observed even before stones are formed. Within 3 days of the lithogenic diet, abnormalities of platelet function are detectable. Platelet aggregation upon addition of adenosine diphosphate (ADP) is impaired. At the same time the red cells become crenated and develop thorny spicules (echinocytes). This morphological change is associated with intracellular dehydration and excessive loss of potassium. These changes coincide with a rise in serum ADCA and DCA and precede a slow rise in serum cholesterol. In vitro incubation studies also suggest that the bile acids probably caused membrane injury to the platelets and red cells. It is concluded that changes in the bile ADCA and DCA probably induce gall bladder epithelial injury in this model of experimental cholelithiasis87.
Inflammation
Comments: Rabbits have well defined mucosa associated lymphoid tissue in the gall bladder and this should not be confused with inflammatory changes.
Parasite
Comments: Parasites are rarely a problem in well-managed barriered facilities. Protozoal parasites cause the most common and significant disease. Eimeria stiedai (stiedae) primarily damages the biliary system. After oral uptake of sporulated oocysts, the sporozoites penetrate the intestinal wall, reach the liver via blood vessels, and enter the epithelial cells of the bile ducts. The prepatent period is 15–18 days. Severe infections may produce abscessation, and bile duct swelling macroscopically, occasionally with hepatomegaly. Microscopically, multifocal granulomatous lesions surround bile ducts and often there is marked papillary ductule epithelial hyperplasia with intraluminal schizonts and zygotes81. Cured hepatic coccidiosis may be seen as areas of peribiliary fibrosis.
Section 8: Skin
Introduction
Rabbit skin is commonly used for in vivo and in vitro investigation and testing of many chemical agents, ranging from cosmetics to carcinogens88,89,90. The pathology of both spontaneous and induced conditions of the skin are similar in rabbits and humans, hence their use in vaccine studies. Careful recording of the nature, intensity and duration of the inflammatory response of the skin adjacent to implanted or injected substances is important in the assessment of the local tolerability of agents intended for contact with human tissues. The chemical and physical properties of injected chemicals or vaccines and their adjuvants as well as size, shape and surface texture of implanted biomaterials may modify the histological features and temporal pattern of the inflammatory and reparative responses91,92,93,94. Such studies in rabbits are conducted with descriptors following the ISO-10993 guidelines.
A. Anatomy
Rabbits are born hairless. The adult rabbit body is well furred except for a few sites which are covered with sparse or no hair: around external nares; mammary gland nipples; scrotum; inguinal folds; pinnae12, 95. Foot pads are absent, though the palmar and planter surfaces are thickly furred25.
Mature rabbits have a large, fat-filled skin fold on the ventral surface of the neck called the dewlap24, 25. The dewlap is particularly well developed in older females. The upper lip is divided by the philtrum, a distinct cleft, confluent with the external nares, and the lips have multiple prominent vibrissae.
Normal rabbit fur is composed of three hair types, produced by different follicles88, 96 that are arranged in clusters. A typical cluster consists of a single large central primary follicle (producing 50–60 µm diameter, 3–4 cm long guard hairs) surrounded by 2–4 somewhat smaller lateral primary follicles (which produce 25–30 µm diameter, 3.0–3.5 cm long awn hairs) and 20–50 much smaller secondary follicles (producing 15 µm diameter, 2.5–3.0 cm long down hairs). Down hairs are the most abundant type (90–95%) and form the dense “inner coat”, while the coarser guard and awn hairs constitute the protective “outer coat”88, 96.
The large, elongated pinnae (external ears) can represent up to 12% of the rabbit body surface area24. The pinna possesses a large central artery as well as marginal (lateral) veins that are frequently used for blood collection and intravenous injections24, 97. The density of hair follicles in the pinna (80/cm2) is much lower than in many other body sites90. Due to the large surface area, well-developed vascularization (arteriovenous anastomoses), and relatively sparse hair coat, the pinna dissipates body heat well, and plays a major role in overall thermoregulation in rabbits98, 99. The epidermis of both pinna and body skin is thin90, 100. Rabbit pinna epidermis is about 16–18 µm thick, compared to about 58–64 µm thickness of pig ear skin90.
Abundant dermal sebaceous glands are associated with hair follicles in pinna and body skin, but sudoriferous (sweat) glands appear to be absent or vestigial in rabbit skin95, 100. The rabbit is classified as a functionally “non-sweating” species98.
Several specialized glands in or around the skin of rabbits produce externally released odiferous secretions that are important in social and sexual behavior modulation101,102,103. These include the submandibular (chin or mental) skin gland; the inguinal gland complex (brown and white glands); and the anal (rectal, perirectal) glands. These glands have also been collectively or singularly referred to as “apocrine” glands104, though the actual mode of secretion remains unclear105, 106. As a result of this overlapping and sometimes contradictory terminology, when reviewing the literature, it is important to determine which gland(s) are being discussed, especially in older references.
The submandibular (mental) skin gland (Figures 18, 19
Figure 19.
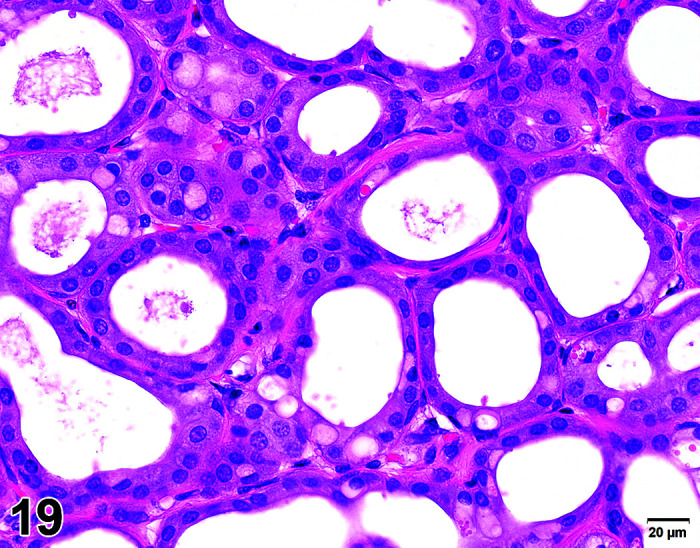
Submandibular skin (mental gland), H&E (high mag).
) is located in the subcutis of the ventral mandible102, 104. Submandibular gland secretions are thought to function primarily as territorial markers. Rabbits distribute the secretions by rubbing their chins against inanimate objects and even other rabbits; this “chinning” behavior is more common in males101, 103. The submandibular gland is unpaired but multilobed, with a large central and two smaller lateral lobes; each lobe has an excretory duct opening onto the external skin102. The epithelium is cuboidal to low columnar, with epithelium of intact, sexually mature males being taller and more vacuolated than that of mature females102, 105.
Figure 18.
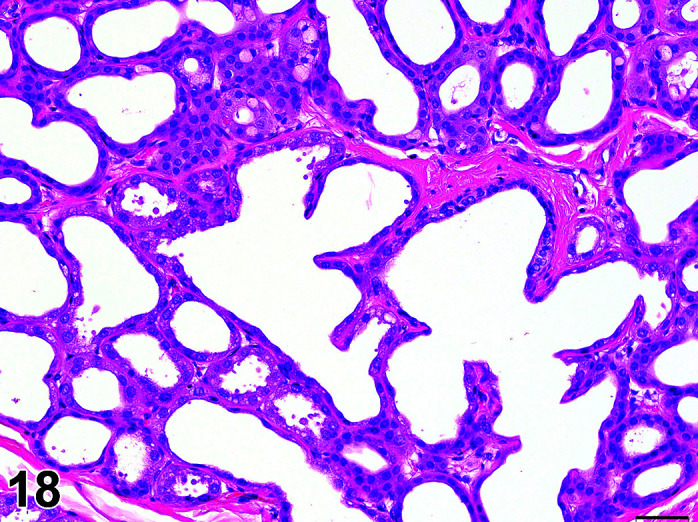
Submandibular skin (mental) gland, H&E.
The submandibular skin gland is sexually dimorphic, with development function, and morphology strongly dependent on sex hormones. Intact males have much larger and heavier submandibular glands, with larger acini lined by taller epithelial cells102, 104, 107. In males, the oily submandibular secretions may coat the skin of the chin. In females, the gland is much less developed and may be difficult to find and sample in young animals. Gonadectomy of males reduces the size, weight, and secretory epithelium height of submandibular glands, while testosterone administered to castrated males reverses these effects102, 107. In females, ovariectomy has opposite effects, resulting in increased gland size and weight, as well as increased acinar diameter and epithelial height102, 107.
Rabbits also have specialized glands in the nictitating membrane (see Special Senses section).
The paired inguinal gland complexes are located on either side of the penis or clitoris, and thus are sometimes referred to as “preputial” and “clitoral” glands (Figure 20) (see also Male Reproductive System and Female Reproductive System sections). Each gland complex is composed of two adjacent but morphologically different lobulated glands: the dorsolateral “white” gland and the medioventral “brown” gland. The white gland has typical sebaceous histomorphology, (Figure 21) while the brown gland is composed of branching tubules lined by simple cuboidal to columnar epithelium106 (Figure 22).
Figure 20.
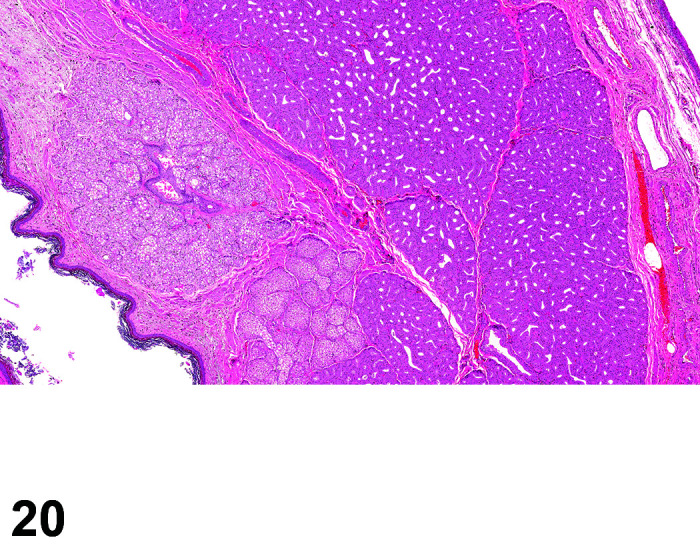
Inguinal gland complex: sebaceous (left), apocrine (right), H&E.
Figure 21.
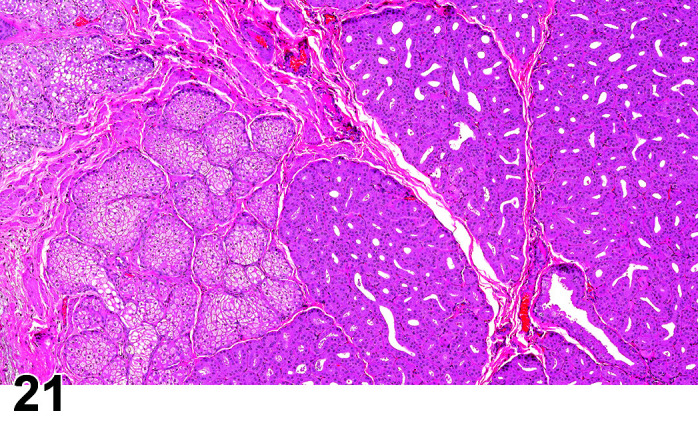
Inguinal gland complex: sebaceous (left), apocrine (right), H&E (high mag).
Figure 22.
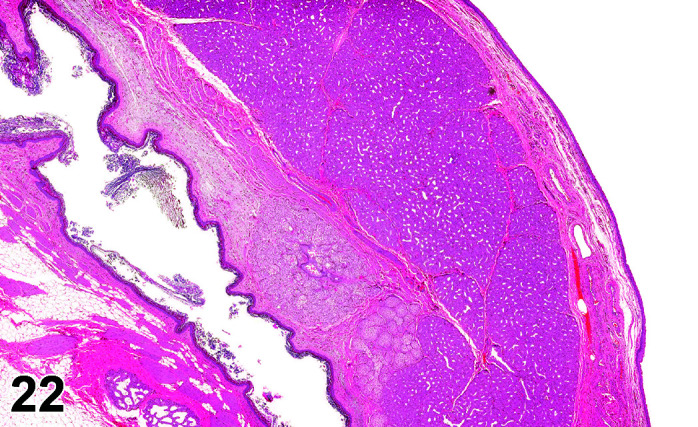
Female, Inguinal gland complex (apocrine), vagina, H&E (high mag).
Although closely apposed, the white and brown glands are separate structures with separate ducts (lined by keratinized stratified squamous epithelium) which open into the hairless skin fold at the base of the penis and clitoris.
The strong-smelling combined inguinal gland secretion is considered to function in individual identification103. Compared to the submandibular skin gland, the brown and white inguinal glands respond similarly to gonadectomy and sex hormone administration, but the extent of response (sensitivity) is overall less pronounced104, 105.
The inguinal glands (preputial and clitoral) are further discussed in the Male Reproductive System and Female Reproductive System sections, respectively.
Anal (or perirectal) glands, the third type of odiferous gland in rabbits, produce secretions considered important in territorial marking103 (Figure 23). These glands are located in the anorectal submucosa (see also Peri-rectal tissue subsection in Digestive section).
Figure 23.
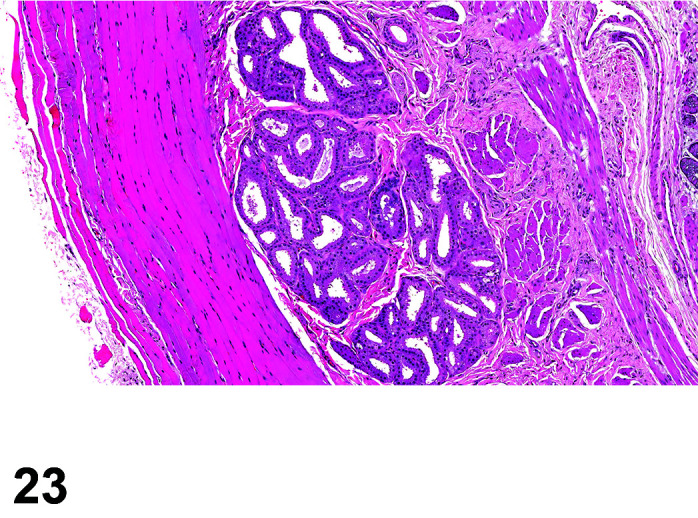
Rectum, Anal gland, H&E.
B. Common Diseases of the Rabbit Skin
Based on its direct interaction with the environment, the skin is subject to many spontaneous and housing-related diseases. Morphological lesions should be described using the nomenclature listed below, however spontaneous diseases of the skin should be diagnosed in combination with clinical data. In a pathology report, the disease terms listed below could be used as “syndromes” to summarize and interpret morphological lesions.
Acariasis: Mite infestations of the skin can occur in laboratory rabbits24, 108,109,110. Ear mite (Psoroptes cuniculi) infestations can result in prominent epidermal hyperkeratosis and dermal inflammation of the external ear canal and pinna, often exacerbated by secondary bacterial and fungal infections. Fur mites (Cheyletiella parasitovorax, other Cheyletiella spp., Leporacarus gibbus; and other species), and mange mites such as Sarcoptes scabei (sarcoptic mange; scabies), and Notoedres cati (notedric mange) can also cause skin lesions in various sites, including alopecia, epidermal hyperkeratosis and ulceration, and dermal inflammation24, 108, 109.
Aural hematoma: Hematoma of the pinna can occur due to various causes111.
Ulcerative pododermatitis in rabbits: Also known as “sore hocks”, ulcerative pododermatitis most commonly affects the plantar aspects of the tarsus and metatarsus. Inflammation (often with bacterial infection) is considered a sequel to a primary inciting cause of pressure necrosis of the skin (due to obesity, wire-bottomed cages, general lack of sanitation etc.) and is seldom observed with current husbandry standards 24, 109, 110.
Moist dermatitis: So-called moist dermatitis of the facial and neck (dewlap) skin can result from trapped moisture in large dewlaps or underlying dental disease with hypersalivation, often complicated by secondary bacterial infection109, 112, 113. Similar inflammatory changes can occur in other locations such as the perineal skin.
Virus-related skin and subcutaneous proliferative lesions of rabbits (such as those induced by myxoma virus, Shope fibroma virus, and various papillomaviruses) are beyond the scope of this paper, and the reader is referred to recent reviews114, 115.
C. Dermatotoxicology
Testing of topically applied chemicals for acute dermal irritation/corrosion is typically performed in rabbits. However, as outlined in the supplement to the Organisation for Economic Co-operation and Development (OECD) test guideline 404 on dermal irritation/corrosion testing, consideration of existing data, structure activity relationships, physiochemical properties of the test item and testing in validated in vitro and ex vivo systems are recommended, before an in vivo study is conducted (Table 22).
Table 22. Microscopic Findings of the Skin: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Accumulation, macrophage | X | ||||
| Adnexal dysplasia | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, epidermis/dermis/adnexa | X | ||||
| Congestion | X | ||||
| Cyst, squamous | X | ||||
| Degeneration | X | ||||
| Dilatation | X | ||||
| Edema, epidermis, intracellular/intercellular | X | ||||
| Elastosis | X | ||||
| Erosion/ulcer | X | ||||
| Fibrosis | X | ||||
| Hamartoma, collagenous * | X | ||||
| Hemorrhage | X | ||||
| Hyperkeratosis, epidermis/adnexa | X | ||||
| Infiltrate, inflammatory cell, [insert appropriate cell type] | X | ||||
| Inflammation, (+ locator) * | X | ||||
| Mineralization | X | ||||
| Necrosis, (+ locator) | X | ||||
| Pigment | X | ||||
| Pustule | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Vesicle | X | ||||
| Xanthomatous alteration *# | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, epidermis/adnexa/melanocyte | X | ||||
| Hyperplasia, keratinocyte * | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, sebaceous cell | X | ||||
| Fibroma | X | ||||
| Keratoacanthoma | X | ||||
| Melanoma, benign | X | ||||
| Papilloma, squamous cell | X | ||||
| Tumor, basal cell, benign | X | ||||
| Tumor, hair follicle, benign | X | ||||
| Tumor, mixed, benign | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, basal cell | X | ||||
| Carcinoma, eccrine gland | X | ||||
| Carcinoma, sebaceous cell | X | ||||
| Carcinoma, squamous cell | X | ||||
| Carcinosarcoma | X | ||||
| Histiocytic sarcoma | X | ||||
| Lymphoma, cutaneous | X | ||||
| Melanoma, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Hamartoma, Collagenous
Comments: Collagenous hamartomas in the skin of rabbits are benign, solitary, poorly demarcated dermal nodules composed of dense collagen with interspersed fibrocytes116, 117.
Inflammation
Comments: Generalized “exfoliative dermatitis” or “sebaceous adenitis” characterized by sebaceous gland atrophy, perifollicular, dermal, and/or epidermal lymphocytic infiltrates, and hyperkeratosis has been reported in rabbits65, 83, 110, 118,119,120. An association with concurrent thymoma or thymic lymphoma was noted in several cases110, 118, 120. Rabbits have been noted to be more sensitive to petroleum jelly-induced dermal irritation than rodents and minipigs, while rabbits and rats are more sensitive than minipigs to a topical antibiotic applied to abraded skin121. Conversely, rabbits exhibited decreased subcutaneous inflammation and biodegradation of polyurethane implants compared to rodents122.
Xanthomatous Alteration
Comments: Xanthoma or xanthomatous alteration refers to non-neoplastic accumulations of lipid-laden macrophages (“foam cells”) in the dermis. Due to their altered metabolism and genetic background, Watanabe heritable hyperlipidemic (WHHL) rabbits are prone to such xanthomatous lesions. In WHHL rabbits, xanthomatous alteration (xanthomas) most commonly occur in plantar tendons of the distal limbs and digits with occasional extension into adjacent subcutis or dermis123,124,125. Dermal xanthomatous alteration has also been reported in the hereditary high triglyceridemia (TGH) rabbit, an inbred rabbit strain which like the WHHL rabbit, has high blood cholesterol and lipids and a propensity to develop atherosclerosis126. It can be induced also by feeding high-fat diets to NZW rabbits28.
Hyperplasia, Keratinocyte
Other terms: actinic keratosis
Pathogenesis/cell of origin: keratinocyte
Comments: Actinic keratosis has been reported in the pinnae of a pet rabbit127. The affected animal, a Flemish male, spent considerable time exposed to sunlight. Clinically, there was pinnal erythema and scaling, with histopathologic findings considered consistent with early actinic keratosis: epidermal hyperkeratosis, epidermal hyperplasia with dysplasia, and dermal fibrosis. Actinic (solar) keratosis is an intraepidermal manifestation of abnormal keratinocyte proliferation and can progress to squamous cell carcinoma. Ultraviolet (UV) light is the causative agent. Although exposure to sunlight is not a factor in laboratory studies, actinic keratosis and drug-induced pruritis should be considered as a differential diagnosis in laboratory rabbits showing scaling or pruritis of the ear pinna in studies with test items that cause exacerbated photosensitivity.
Section 9: Mammary Gland
A. Anatomy of the Mammary Gland
Similar to humans, each mammary gland in the rabbit has several ducts (milk canals) which open independently in the nipple (rather than a single duct as in some other species such as mice)95, 128, 129. This important anatomical difference observed when comparing rabbits and humans to rodents occurs because when division of the mammary bud starts at rabbit ED26, each sprout which arises forms a primary milk canal129 (Propper et al., 2013). Rabbit mammary glands are paired, with usually four (sometimes five) pairs of mammae, each with a nipple, distributed along the ventral thorax and abdomen25. The presence of nipples in male rabbits has been documented in detail previously95. Newborns do not require colostrum as all immunity is acquired through the placenta. Rabbits have a unique pattern of nursing: it occurs only once a day, with circadian periodicity, throughout lactation130. Explants of pseudopregnant rabbit mammary glands in organ culture are used to investigate hormonal changes during pregnancy. Proliferative lesions in laboratory rabbits may arise from genotoxic test items, infectious agents and changes in hormonal homeostasis as part of the aging process or pseudopregnancy (Table 23).
Table 23. Microscopic Findings of the Mammary Gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Basophilia | X | ||||
| Congestion | X | ||||
| Corpora amylacea | X | ||||
| Cyst | X | ||||
| Degeneration | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, alveolar and/or ductal epithelial cell | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation * | X | ||||
| Metaplasia | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Single-cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Proliferative Non-Neoplastic | |||||
| Hyperplasia, lobuloalveolar * | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenomyoepithelioma | X | ||||
| Fibroadenoma | X | ||||
| Tumor, mixed, benign | X | ||||
| Adenocarcinoma | X | ||||
| Adenocarcinoma arising in fibroadenoma | X | ||||
| Carcinoma, adenosquamous | X | ||||
| Carcinosarcoma | X | ||||
| Sarcoma arising in fibroadenoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Inflammation
Comments: Mammary gland inflammation (mastitis) is rarely encountered in modern laboratory facilities due to biosecurity and animal husbandry procedures operations131, 132. More commonly, histological changes are limited to infiltrates of foci of lymphocytes, neutrophils, macrophages, or plasma cells, alone or in combination.
Hyperplasia
Comments: In cases of pseudopregnancy, the entire mammary gland may be diffusely enlarged and hyperplastic and resemble feline fibroadenomatosis (M. Rinke, personal communication). Descriptions of mammary gland “cystic dilatation” in one cohort of untreated rabbits appeared consistent with mammary gland hyperplasia (with increased secretory activity)133. Findings consistent with mammary gland hyperplasia (described as mammary “cystic dilatation” with “epithelial infolding”) were noted in female NZW rabbits with elevated serum prolactin levels and pituitary adenomas134. Mammary gland hyperplasia and dysplasia were noted in another female NZW rabbit with concurrent mammary gland adenocarcinoma and a prolactin-secreting pituitary adenoma135. These results suggested that mammary gland hyperplasia may have been related to elevated prolactin levels.
Experimental reversible mammary gland hyperplasia in rabbits has been induced by systemic administration of cyclosporine A. In one study, intravenous administration of cyclosporine A to nulliparous NZW female rabbits resulted in diffuse mammary hyperplasia characterized by increased numbers of alveoli, increased alveolar branching, increased secretion in alveolar lumens, and decreased fibrous stroma compared to control glands136. In another study, intravenous administration of cyclosporine A to male and female NZW rabbits resulted in diffuse mammary gland hyperplasia characterized by abundant ectatic, well-differentiated glands, but with increased fibrous stroma, and the change was termed “fibroadenomatous hyperplasia”137. In both studies, the mammary gland hyperplasia regressed after cessation of cyclosporine A administration.
Elevated plasma prolactin levels attributed to cyclosporine A were noted in one of these studies136. Thus, a possible role of prolactin in development of mammary gland hyperplasia was postulated136, 137.
Gynecomastia has been found in a male rabbit that concurrently had a testicular Leydig cell tumor138.
Section 10: Nervous System
The neuroanatomy of the rabbit is covered in detail elsewhere139. The large brain size of the rabbit compared to rodents necessitates a different approach to trimming and embedding the brain. Pardo et al. have published a recommended trimming schemes for routine and neurotoxicity studies140. Although mature when on study, nervous system findings are not common in laboratory rabbits due to their young age.
This lexicon has been organized in tiers based on cell type (e.g., neuron, glia), and sometimes by cellular target (e.g., cell body, axon, myelin). A final set of terms has been included to demonstrate common artifacts which have been misidentified as neurodegenerative or neurotoxic lesions by inexperienced diagnosticians. Neural neoplasms are an important but very infrequent finding in non-rodents including rabbits evaluated on standard toxicity studies, due to the age group concerned. Conventional toxicity studies do not permit repeated assessment for lesion progression over time, so an appreciation of the true future biological behavior of neural neoplasms is unclear. Thus, in this lexicon we predict the impact on host function as “benign” or “malignant” based only on morphologic characteristics such as cellular differentiation, invasiveness, and proliferative rate. Certain lesions that have well-differentiated features but are biologically aggressive over time (e.g. glial neoplasms) have been termed “malignant” and “low grade” rather than “benign” to better address the usual clinical outcome of this category of neural tumors. Innovations in non-invasive imaging should help resolve questions regarding the biological behavior of these lesions in the future.
The most common non-proliferative anomalies in the CNS include damaged or dying cells (especially neurons, their axonal processes, and the myelin sheaths that insulate the axons) and the various reparative changes designed to minimize (in the CNS) or reverse (in the PNS) the damage. At the cellular level, the most important processes will involve neurons, various glial lineages, and blood vessels (Table 24).
Table 24. Microscopic Findings of the Brain: Rabbit.
| Finding | Common | Uncommon | Not Recorded but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Ectopic tissue, neuron | X | ||||
| Hydrocephalus * | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Accumulation, laminar, Schwann cell | X | ||||
| Accumulation, matrix | X | ||||
| Amyloid | X | ||||
| Astrocyte swelling/vacuolation | X | ||||
| Astrocytosis | X | ||||
| Atrophy, axons | X | ||||
| Autophagy, neuron, ganglion | X | ||||
| Cellularity, decreased, neuron | X | ||||
| Cholesterol clefts | X | ||||
| Chromatolysis | X | ||||
| Cyst, squamous | X | ||||
| Decreased cellularity, neuron | X | ||||
| Degeneration, axon | X | ||||
| Degeneration, nerve fiber | X | ||||
| Demyelination | X | ||||
| Dystrophy, axon | X | ||||
| Edema, intramyelinic | X | ||||
| Extramedullary hematopoiesis, choroid plexus | X | ||||
| Gliosis, not otherwise specified (NOS) | X | ||||
| Hemorrhage | X | ||||
| Heterotopia, neuronal | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Microgliosis | X | ||||
| Mineralization | X | ||||
| Necrosis, neuron | X | ||||
| Necrosis/inflammation, media or wall, artery | X | ||||
| Parasite * | X | ||||
| Pigment, lipofuscin | X | ||||
| Satellitosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Syringomelia/hydromyelia | X | ||||
| Thrombus | X | ||||
| Tissue, ectopic, neuron | X | ||||
| Tissue, ectopic | X | ||||
| Type II astrocytes | X | ||||
| Vacuolation | X | ||||
| Vacuolation, neuron | X | ||||
| Vacuolation, white matter | X | ||||
| Vacuolation, choroid plexus * | X | ||||
| Proliferative Non-neoplastic | |||||
| Hamartoma, lipomatous | X | ||||
| Hyperplasia, glial cell, not otherwise specified (NOS) | X | ||||
| Proliferative Neoplastic | |||||
| Astrocytoma, malignant | X | ||||
| Carcinoma, choroid plexus | X | ||||
| Ependymoma, benign | X | ||||
| Ependymoma, malignant | X | ||||
| Glioma, mixed, malignant | X | ||||
| Glioma, not otherwise specified (NOS) | X | ||||
| Medulloblastoma | X | ||||
| Neuromyoblastoma, malignant | X | ||||
| Oligodendroglioma, malignant | X | ||||
| Papilloma, choroid plexus | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Hydrocephalus
Comments: Cases of hydrocephalus in rabbits have been presumed to be related to a single autosomal recessive gene (hy/hy); however, occurrence with other abnormalities suggests that inheritance may be more complicated. In some cases, the condition appears to be inherited along with various ocular anomalies as an autosomal gene with incomplete dominance. Hydrocephalus may also occur in rabbits as a congenital condition related to hypovitaminosis A in pregnant females141. Hydrocephalus is also seen secondary to craniosynostosis. A strain of NZW rabbits with congenital, nonsyndromic coronal suture synostosis has been developed previously Mooney et al., 1996, which mimics the pathology of human craniosynostosis142.
Parasite
Figure 24.
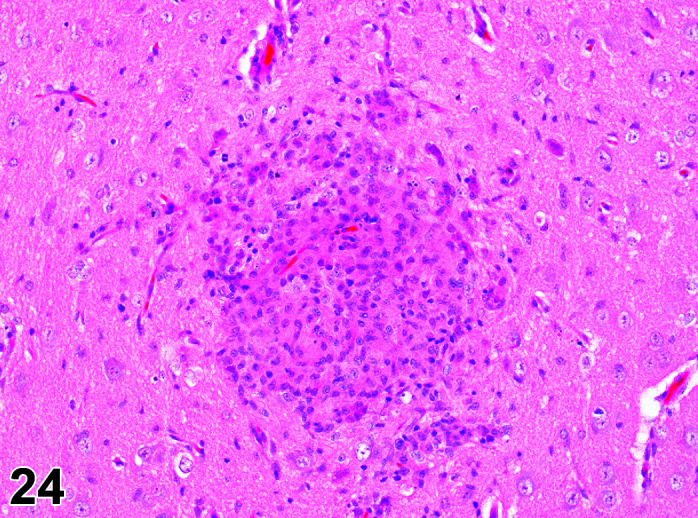
Brain, Parasitic encephalitis, H&E.
Other terms: Encephalitis;
Comments:Encephalitozoon cuniculi is a common cause of neurological disease in pet rabbits, but nowadays is a rare occurrence in barriered colonies. Encephalitozoon cuniculi is a microsporidian pathogen of rabbits and other mammals that usually produces granulomatous encephalitis and interstitial nephritis. Severe systemic disease is rare except in immunosuppressed animals. Primary diagnosis is based on the presence of granulomatous encephalitis together with chronic interstitial nephritis and occasionally hepatitis but can be confirmed by serology or a combination of special staining methods, immunohistochemistry (IHC), and polymerase chain reaction (PCR). Real-time PCR is the most sensitive method for the confirmation of E. cuniculi infection. Differential diagnosis for E. cuniculi is Toxoplasma gondii infection. E. cuniculi infection is characterized by granuloma(s) composed of macrophages with large cytoplasm (epithelioid cells), which may be surrounded by inflammatory cells such as lymphocytes, plasma cells, and eosinophils, sometimes accompanied by fibrosis. The macrophages may form multinucleated giant cells. Focal lesions are largely restricted to the gray matter and primarily spare the white matter, with inner layers of the cerebral cortex more commonly affected than the middle or the outer layers. Lymphoplasmacytic meningoencephalitis, characterized by perivascular cuffs and leptomeningeal infiltrates consisting primarily of lymphocytes and plasma cells, is also usually present in affected animals. Spores are located in parasitophorous vacuoles in the parenchyma of the brain, either without inflammation or close to focal inflammatory lesions. Lesions can be categorized into 6 histopathological subtypes depending on the characteristics of the inflammatory response85. Rabbits affected with encephalitozoonosis most frequently exhibit multiple neurologic signs (head tilt, ataxia, circling, nystagmus, rotational movements around the body length axis, seizures, paresis, head tremors, swaying or nodding at rest, and behavior changes), kidney dysfunction (azotemia), and phacoclastic uveitis. Brain lesions may lead to altered cholinesterase values.
Vacuolation, Choroid Plexus
Figure 25.
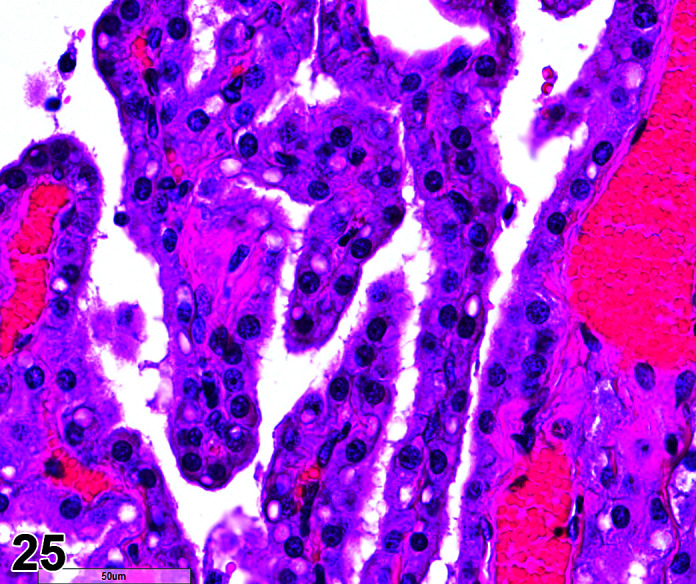
Brain, Vacuolation, choroid plexus, H&E.
Comments: Spontaneously occurring choroid plexus vacuolation in rabbits takes two forms, discrete microvacuoles within the epithelium of the choroid plexus or large macrovacuoles (adipocytes) within the stroma. Neither form is associated with clinical signs, both are considered incidental findings at histopathology and should not be recorded as a finding in preclinical studies unless the adipocytes are altered spontaneously (i.e. lipoma) or after xenobiotic treatment143. Vacuolated macrophages can be seen in the choroid plexus of animals dosed with PEGylated compounds. These are discrete macrovacuoles and are clearly within either macrophages and/or endothelial cells. Since removal of foreign bodies or materials from circulation is a normal function of macrophages, the resulting vacuolation is considered a normal physiological response in phagocytic cells. The inner core of the choroid plexus, containing the vacuolated macrophages, is outside the CNS. There are two theories as to how the vacuolated macrophages arise: (i) hydrophilic PEG filters through the vasculature into the choroid plexus core and is kept there by the choroidal epithelial tight junctions and subsequently phagocytosed by resident macrophages, or (ii) circulating macrophages containing PEG filter out in this high blood flow area144 (Table 25).
Table 25. Microscopic Findings of the Spinal Cord: Rabbit.
| Finding | Common | Uncommon | Not Recorded but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Ectopic tissue, neuron | X | ||||
| Hydromyelia | X | ||||
| Syringomyelia | X | ||||
| Syringomyelia/hydromyelia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Accumulation, matrix | X | ||||
| Accumulation, laminar, Schwann cell | X | ||||
| Astrocyte swelling | X | ||||
| Astrocyte vacuolation | X | ||||
| Astrocytosis | X | ||||
| Atrophy, axon | X | ||||
| Autophagy, neuron, dorsal root ganglion | X | ||||
| Cellularity decreased, neuron | X | ||||
| Cholesterol clefts | X | ||||
| Chromatolysis | X | ||||
| Cyst, squamous | X | ||||
| Degeneration, axon | X | ||||
| Degeneration, nerve fiber | X | ||||
| Demyelination * | X | ||||
| Dystrophy, axon | X | ||||
| Ectopic tissue | X | ||||
| Gliosis, not otherwise specified (NOS) | X | ||||
| Hemorrhage | X | ||||
| Heterotopia, neuronal | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Intramyelinic edema | X | ||||
| Microgliosis | X | ||||
| Mineralization | X | ||||
| Necrosis, neuron | X | ||||
| Necrosis/inflammation, media or wall, artery | X | ||||
| Neuronophagia | X | ||||
| Pigment, lipofuscin | X | ||||
| Radiculoneuropathy | X | ||||
| Satellitosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Swelling, astrocyte | X | ||||
| Thrombus | X | ||||
| Type II astrocytes | X | ||||
| Vacuolation, neuron | X | ||||
| Proliferative Non-Neoplastic | |||||
| Hyperplasia, glial cell, not otherwise specified (NOS) | X | ||||
| Proliferative Neoplastic | |||||
| Astrocytoma, malignant | X | ||||
| Glioma, mixed, malignant | X | ||||
| Glioma, not otherwise specified (NOS) | X | ||||
| Oligodendroglioma, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Demyelination, Nerve Fiber, Spinal Cord
Comments: New-born rabbits show an area in the dorsal part of the lateral funiculus of the first cervical segment of the spinal cord, from which myelinated nerves are few/absent145. Localised/focal unmyelinated axons are occasionally seen in the sections of lateral funiculus of the lumbar spinal cord of young adult rabbits. This is a normal anatomical difference in rabbits and there are no clinical signs (Table 26).
Table 26. Microscopic Findings of the Meninges: Rabbit.
| Finding | Common | Uncommon | Not Recorded but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Aggregate, granular cell | X | ||||
| Angiectasis | X | ||||
| Cholesterol clefts | X | ||||
| Cyst, squamous | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, media or wall, artery | X | ||||
| Pigment * | X | ||||
| Pigment, lipofuscin | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Proliferative Non-neoplastic | |||||
| Meningioangiomatosis | X | ||||
| Proliferative Neoplastic | |||||
| Meningioma, benign | X | ||||
| Meningioma, malignant | X | ||||
| Tumor, granular cell, benign | X | ||||
| Tumor, granular cell, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Pigment
Other terms: Melanosis
Comments: Spontaneously occurring melanosis of the meninges (especially those of the brain) may be seen in pigmented rabbit strains such as the NZW x New Zealand Red F1 Cross and Dutch Belted. It may be seen grossly at necropsy (Table 27) (Figure 26).
Table 27. Microscopic Findings of the Peripheral Nerves: Rabbit.
| Finding | Common | Uncommon | Not Recorded but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Accumulation, laminar, Schwann cell | X | ||||
| Accumulation, matrix | X | ||||
| Atrophy, axon | X | ||||
| Autophagy, neuron, dorsal root ganglion | X | ||||
| Cellularity, decreased, neuron | X | ||||
| Chromatolysis | X | ||||
| Cholesterol clefts | X | ||||
| Cyst, squamous | X | ||||
| Degeneration, nerve fiber | X | ||||
| Degeneration, axon | X | ||||
| Demyelination | X | ||||
| Dystrophy, axon | X | ||||
| Ectopic tissue | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Intramyelinic edema | X | ||||
| Mineralization | X | ||||
| Necrosis/inflammation, media or wall, artery | X | ||||
| Pigment, lipofuscin | X | ||||
| Radiculoneuropathy | X | ||||
| Renaut Body | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-Neoplastic | |||||
| Hyperplasia, (+ cell type) | X | ||||
| Proliferative Neoplastic | |||||
| Schwannoma | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Figure 26.
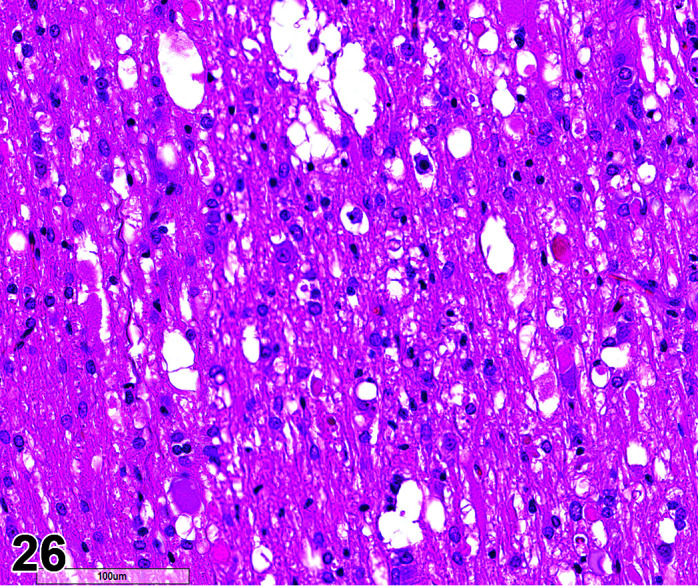
Peripheral Nerve, Demyelinization fibres, H&E.
Common Artifacts
Certain incidental changes are commonly misidentified as neuropathologic lesions by inexperienced researchers. The changes listed here are the most common of such findings – dark neurons and white matter vacuolation. Identified artifacts should be not reported in the pathology dataset. However, systematic artifacts where one dose group only is involved may be recognized and noted as a comment to the organ/tissue at the discretion of the study pathologist (Table 28).
Table 28. Artifactual Microscopic Findings of the Nervous System: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable |
| Artifact, dark neuron | X | |||
| Bubbles, myelin | X | |||
| Vacuolation, white matter | X |
Section 11: Female Reproductive System
Introduction
Standard INHAND nomenclature for non-proliferative and proliferative female reproductive system findings in rats and mice has previously been published146 and should be used for the rabbit as appropriate. Generally, very few spontaneous or treatment induced microscopic changes are reported in the female reproductive tract of rabbits. Female rabbits become sexually mature at 5 months of age, and so rabbits on routine tox studies are fully mature.
A. Anatomy and Physiology of the Ovary
Rabbits are non-seasonally polyestrous. Female rabbits are induced ovulators and so decreased/absent corpora lutea and atretic follicles are normal. Ovulation can be induced in studies where vaginal swabs/dosing is a frequent event, and corpora hemorrhagica may be seen. Ovulation is triggered about 10 hours following copulation. Some animals are triggered into pseudopregnancy lasting 15–17 days, with or without galactopoiesis and endometrial hyperplasia.
Ovarian interstitial tissue is well developed in rabbits and was termed an “interstitial gland” in 1902147. This “gland” secretes progestogens. At 3 months of age, follicular atresia begins and the theca interna cells differentiate into the primary and then secondary interstitial gland148. The primary interstitial gland is composed of small polygonal cells with hyperchromatic nuclei and scant basophilic cytoplasm. It is highly vascularized. The ovary interstitium is almost completely composed of the secondary “gland” by 6 months old. This is composed of large polygonal cells with round vesicular nuclei and abundant eosinophilic cytoplasm containing numerous lipid droplets. There is high 3-β-hydroxysteroid dehydrogenase activity within the interstitial cells of secondary interstitial gland, which increases in activity with increase in size of the gland.
The ovaries of normal rabbits often have surface epithelial structures resembling ovarian papillomas149. The ovarian mesothelium (surface epithelium) may form short, broad papilla or papillae with slender villous processes. They have an inner core with few fibroblasts, small blood vessels, and loose or hyalinized connective tissues and are lined by pseudostratified or multi-layered epithelium. These structures should not be mistaken for neoplasms and occur only on the ovary and not on the surface of other abdominal organs or structures (Table 29, 30
Table 30. Microscopic Findings of the Oviduct: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | |||||
| Atrophy | X | ||||
| Cyst | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Granuloma | X | ||||
| Hemorrhage | X | ||||
| Immature | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, oviduct | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Salpingitis isthmica nodosa | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, epithelium | X | ||||
| Proliferative Neoplastic | |||||
| Leiomyoma | X | ||||
| Schwannoma, benign | X | ||||
| Schwannoma, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
).
Table 29. Microscopic Findings of the Ovary: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Age related atrophy | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Atrophy, corpora lutea | X | ||||
| Corpora lutea, increased number | X | ||||
| Cyst, bursal/epithelial/follicular/luteal/paraovarian/rete ovarii, not otherwise specified (NOS) | X | ||||
| Decreased number/absent follicles/corpora lutea | X | ||||
| Degeneration, oocyte/corpora lutea | X | ||||
| Edema | X | ||||
| Follicle, luteinized/polyovular | X | ||||
| Fibrosis | X | ||||
| Granuloma | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, corpora lutea/ interstitial cell | X | ||||
| Immature | X | ||||
| Increased number, atretic follicles/corpora lutea | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Ovotestis | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic | X | ||||
| Vacuolation, corpora lutea/granulosa cell/theca cell/interstitial cell | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, epithelium/granular cell | X | ||||
| Adenosis | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Cystadenoma | X | ||||
| Leiomyoma | X | ||||
| Luteoma, benign | X | ||||
| Schwannoma, benign | X | ||||
| Teratoma, benign | X | ||||
| Thecoma, benign | X | ||||
| Tumor, Sertoli cell/granulosa cell, benign | X | ||||
| Tumor, sex cord stromal, mixed, benign | X | ||||
| Carcinoma, embryonal/tubulostromal/yolk sac | X | ||||
| Choriocarcinoma | X | ||||
| Cystadenocarcinoma | X | ||||
| Dysgerminoma | X | ||||
| Leiomyosarcoma | X | ||||
| Schwannoma, malignant | X | ||||
| Teratoma, malignant | X | ||||
| Thecoma, malignant | X | ||||
| Tumor, Sertoli cell/granulosa cell, malignant | X | ||||
| Tumor, sex cord stromal, mixed, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
B. Anatomy of the Uterus and Cervix
Rabbits have a true bicornuate uterus, with no uterine corpus present. There are two separate cervices. Each uterine horn has a separate cervical canal and the tract is classified as uterus duplex, vagina simplex. The broad ligaments typically contain a large amount of adipose tissue. The light, transmission and scanning microscopic anatomy of the rabbit vagina, cervix and uterus have been comprehensively described by150, 151. Placentation is discoid labyrinthine hemodischorial with countercurrent fetomaternal blood flow; gestation lasts 25–30 days. Females give birth to a litter of 3–10. Neonates are weaned at about 6–8 weeks (Table 31).
Table 31. Microscopic Findings of the Uterus and Cervix: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Hypoplasia | X | ||||
| Mesonephric duct remnants | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Adenomyosis | X | ||||
| Aggregate, granular cell | X | ||||
| Amyloid | X | ||||
| Aneurysm * | X | ||||
| Angiectasis * | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Basophilia | X | ||||
| Cyst, NOS | X | ||||
| Decidual reaction * | X | ||||
| Decidualization, focal | X | ||||
| Degeneration, epithelial | X | ||||
| Dilatation, luminal/glandular/cystic | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Granuloma | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, epithelium/myometrium | X | ||||
| Immature | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, squamous cell | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Prolapse | X | ||||
| Pyometra | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic | X | ||||
| Vacuolation, epithelium | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, epithelium/granular cell * | X | ||||
| Hyperplasia, segmental cystic | X | ||||
| Neoplastic Lesions | |||||
| Adenoma, endometrial | X | ||||
| Keratoacanthoma | X | ||||
| Leiomyoma | X | ||||
| Papilloma, squamous cell | X | ||||
| Polyp | X | ||||
| Schwannoma, benign | X | ||||
| Tumor, granular cell, benign | X | ||||
| Adenocarcinoma, endometrial | X | ||||
| Carcinoma, squamous cell | X | ||||
| Deciduosarcoma #* | X | ||||
| Leiomyosarcoma | X | ||||
| Histiocytic sarcoma | X | ||||
| Schwannoma, malignant | X | ||||
| Tumor, granular cell, malignant | X | ||||
| Tumor, mixed Müllerian, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Aneurysm
Comments: Endometrial venous aneurysms have been reported in three adult NZW rabbits with a history of intermittent severe urogenital bleeding (hematuria). The endometrium of all three rabbits had multiple blood-filled vesicles which projected into the uterine lumen, which were considered to be congenital or acquired defects in the vessel walls, as has occurred in humans152.
Angiectasis
Comments: A focus of multiple, dilated thin walled blood vessels is occasionally seen as a congenital defect. They may occasionally rupture and bleed into the uterine lumen.
Decidual Reaction
Comments: Decidual reactions have been reported in non-pregnant rabbits given estrogens and progesterone as an early lesion in the development of deciduosarcoma153, 154.
Hyperplasia
Comments: Spontaneous endometrial hyperplasia (polypoid and/or cystic) can be seen at 1 year of age155, although it is more common in rabbits 4–5 years old.
Deciduosarcoma
Pathogenesis/cell of origin: Uterine stromal cells and uterine metrial gland cells.
Differential Diagnosis: Sarcoma, endometrial stromal; Mesenchymal proliferative lesion
Diagnostic Features: Rare malignant tumor of hypertrophied stromal cells with abundant PAS positive rarefied cytoplasm intermingled with numerous globular lymphocytes. Hypertrophied blood vessels characterize the lesion.
Comments: Deciduosarcomas are neoplasms unique to rabbits, and metastasis may occur. They are hormone-dependent and therefore may not actually be true neoplasms. Deciduosarcomas have been reported as induced tumors in rabbits on toxicity studies involving estrogen and progestin administration153, 154, 156, and so because of this, the rabbit is a poor model for evaluating the effects of contraceptive steroids. In addition, a spontaneous deciduosarcoma has also been reported in a 6-year-old Dutch Belted rabbit157. Deciduosarcomas may appear after as little as 30 days of treatment of non-pregnant rabbits with estrogens and progesterone154. Exogenous estrogens are necessary for decidualization of the endometrium and to produce deciduosarcoma; exogenous progesterone promotes the process156. Withdrawal of the estrogen/progesterone treatment results in atrophy of decidual cells and tumors and disappearance of decidual tumors154. Transplantation of deciduosarcomas into nude mice as well as primary cell culture (in the absence of hormones) results in failure of cells to grow. Deciduosarcomas will invade the uterine walls and the uterine lymphatics and will metastasize to the lungs. Deciduosarcomas occurring in the spleen and other abdominal viscera may not be metastases, but instead are primary tumors, because treatment of castrated male rabbits with estrogen and progesterone will result in deciduosarcoma in the spleen at a high incidence. Induced deciduosarcomas are composed of large anaplastic decidual cells with large hyperchromatic nuclei. Multiple nuclei as well as bizarre giant nuclei may be observed. Most cells are vacuolated, containing multiple small or a few large vacuoles. Necrosis in the tumor is common. The spontaneously occurring deciduosarcoma is described as an invasive mass originating in the mesometrium of the endometrial stroma and invading the myometrium and mesometrium to form a large nodular mass. Cells are arranged in sheets and streaming bundles dissecting between the smooth muscle cells or arranged concentrically around large dilated blood vessels, with multiple foci of necrosis. Cells are anaplastic and consisted of two intermingling populations – spindloid cells with scant eosinophilic cytoplasm and cigar shaped nuclei with a single nucleolus, or epithelioid cells with abundant vacuolated eosinophilic cytoplasm, large oval eccentric nuclei with 1–2 nucleoli. Binucleate and multinucleate cells are common, as well as frequent giant cells, anisocytosis and nuclear pleomorphism. There are multiple mitotic figures per high power field.
Special Techniques: The neoplastic cells show positive cytoplasmic staining for vimentin, and nuclear staining for estrogen and progesterone receptors, and are negative for desmin, αsmooth muscle actin (αSMA), pancytokeratin, and CD10.
C. Anatomy of the Vagina
The vulva lies between the two inguinal sinuses; the vagina joins the urethra at the vestibule. The vestibule is surrounded by erectile tissue. The crura of the clitoris are present caudal to this erectile tissue, with the body and glans extending caudally to the vulva. The paired clitoral (inguinal) glands are located in the subcutis on either side of the base of the clitoris106 (see Integument section) (Table 32, 33
Table 33. Microscopic Findings of the Clitoral (Inguinal) gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Basophilia | X | ||||
| Degeneration | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Granuloma | X | ||||
| Hemorrhage | X | ||||
| Immature | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, squamous cell | X | ||||
| Hyperplasia | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Papilloma, squamous cell | X | ||||
| Tumor, basal cell, benign | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell | X | ||||
| Tumor, basal cell, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
).
Table 32. Microscopic Findings of the Vagina: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Cyst (mesonephric duct remnants) | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Adenosis | X | ||||
| Aggregate, granular cell | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, epithelial | X | ||||
| Cyst, NOS | X | ||||
| Degeneration, epithelial | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Fibrosis | X | ||||
| Granuloma | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy | X | ||||
| Immature | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Keratinization, increased | X | ||||
| Metaplasia, squamous cell | X | ||||
| Mineralization | X | ||||
| Mucification, increased | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Prolapse | X | ||||
| Rudiment, prostatic | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, epithelial | X | ||||
| Vagina, imperforate | X | ||||
| Proliferative Non-neoplastic | |||||
| Adenosis | X | ||||
| Hyperplasia, epithelium/granular cell | X | ||||
| Proliferative Neoplastic | |||||
| Keratoacanthoma | X | ||||
| Leiomyoma | X | ||||
| Papilloma, squamous cell | X | ||||
| Polyp, vaginal | X | ||||
| Schwannoma, benign | X | ||||
| Tumor, granular cell, benign | X | ||||
| Carcinoma, squamous cell/adenosquamous | X | ||||
| Leiomyosarcoma | X | ||||
| Sarcoma, endometrial stromal | X | ||||
| Schwannoma, malignant | X | ||||
| Tumor, granular cell, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Section 12: Male Repoductive System
Male rabbits become sexually mature at 6–7 months of age and so rabbits used on routine safety assessment studies are usually fully mature (Table 34).
Table 34. Microscopic Findings of the Testis: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Agenesis | X | ||||
| Aplasia | X | ||||
| Cryptorchidism | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, Leydig cell/tubular * | X | ||||
| Degeneration/atrophy, tubule | X | ||||
| Degeneration, germ cell/tubular | X | ||||
| Depletion, germ cell | X | ||||
| Dilatation, rete testis/tubule | X | ||||
| Edema | X | ||||
| Exfoliation, germ cell | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Mineralization | X | ||||
| Multinucleated giant cell | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, vascular/perivascular | X | ||||
| Pigment | X | ||||
| Residual bodies, atypical | X | ||||
| Single cell necrosis ǂ | X | ||||
| Sperm granuloma | X | ||||
| Sperm stasis | X | ||||
| Spermatid retention | X | ||||
| Spermatocele | X | ||||
| Vacuolation, macrophage | X | ||||
| Vacuolation, Leydig cell/tubule | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, (+ cell type) | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, (+ cell type) * | X | ||||
| Gonadoblastoma | X | ||||
| Seminoma, benign | X | ||||
| Teratoma, benign | X | ||||
| Tumor, granulosa cell, benign | X | ||||
| Tumor, granular cell, benign | X | ||||
| Tumor, mixed Sertoli-Leydig cell, benign | X | ||||
| Tumor, Sertoli cell, benign | X | ||||
| Carcinoma, embryonal/Leydig cell/rete testis/yolk sac | X | ||||
| Choriocarcinoma | X | ||||
| Mesothelioma, malignant | X | ||||
| Seminoma, malignant | X | ||||
| Teratoma, malignant | X | ||||
| Tumor, Sertoli cell, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Atrophy, Tubule
Comments: Tubular atrophy is an infrequent finding in the testis. It may be unilateral or bilateral but is generally localized affecting only a few tubules and has not been reported as affecting the whole testis. There is an accompanying oligospermia and sloughing of ductal epithelium in the epididymides158.
Adenoma, (+ cell type)
Comments: Interstitial (Leydig) cell138, 159 and granular cell tumors160 are reported in adult/young adult rabbits (Table 35).
Table 35. Microscopic Findings of the Epididymis: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, duct | X | ||||
| Cell debris, lumen * | X | ||||
| Cribriform change | X | ||||
| Degeneration, epithelium | X | ||||
| Dilatation, duct | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Granuloma, sperm | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Karyomegaly | X | ||||
| Metaplasia, squamous cell | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, vascular/perivascular | X | ||||
| Single cell necrosis ǂ | X | ||||
| Sperm stasis | X | ||||
| Sperm, decreased, lumen | X | ||||
| Spermatocele | X | ||||
| Vacuolation, epithelium | X | ||||
| Proliferative Non-neoplastic | |||||
| Adenosis | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, Leydig cell | X | ||||
| Histiocytic sarcoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Cell Debris, Lumen
Comments: There is often more cellular debris in the lumen of rabbit epididymis than in other species (Table 36).
Table 36. Microscopic Findings of the Efferent ducts: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, duct | X | ||||
| Cell debris, lumen | X | ||||
| Cribriform change | X | ||||
| Degeneration, epithelium | X | ||||
| Dilatation, duct | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Granuloma, sperm | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, squamous cell | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, vascular/perivascular | X | ||||
| Single cell necrosis ǂ | X | ||||
| Sperm stasis | X | ||||
| Spermatocele | X | ||||
| Vacuolation, epithelium | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
A. Anatomy of the Prostate, Proprostate and Paraprostate
In the male rabbit, two structures (termed “prostate” and “proprostate” in this review) analogous to the prostate of other species are located dorsal to the seminal vesicle, urethra, and urinary bladder (Figures 27, 28
Figure 28.
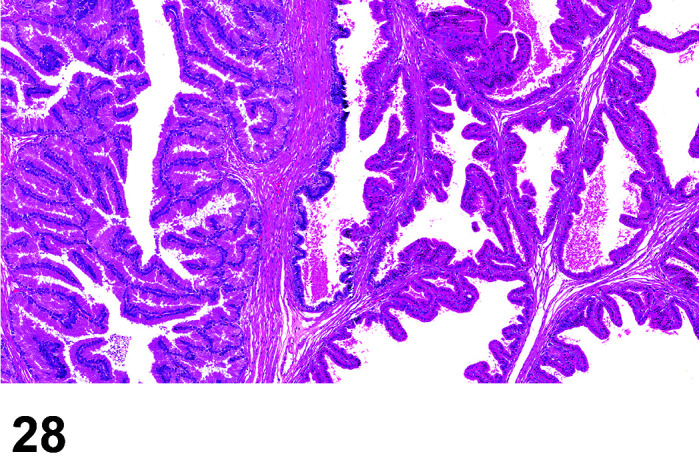
Prostate (left) and Proprostate (right), H&E.
). These structures are two closely apposed bilobed glands which are considered separate organs with independent excretory ducts (rather than parts of a single multilobed gland)161, 162. The more caudally situated gland is generally designated as the “prostate”162,163,164, and is composed of acini lined by uniform, very tall columnar epithelium with abundant pale eosinophilic cytoplasm and basally located oval nuclei. The more cranially situated gland has been most commonly designated as the “proprostate”162, 163. Proprostate acini are lined by low columnar epithelium with bright eosinophilic cytoplasm and centrally located oval to round nuclei (Figures 29, 30
Figure 30.
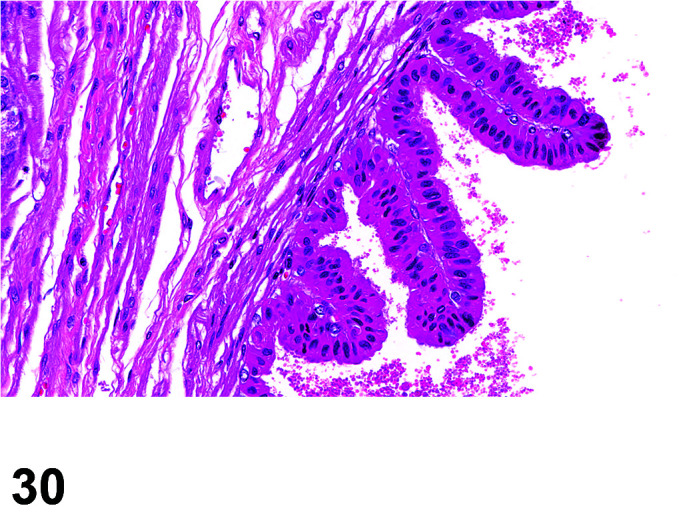
Proprostate, H&E.
).
Figure 27.
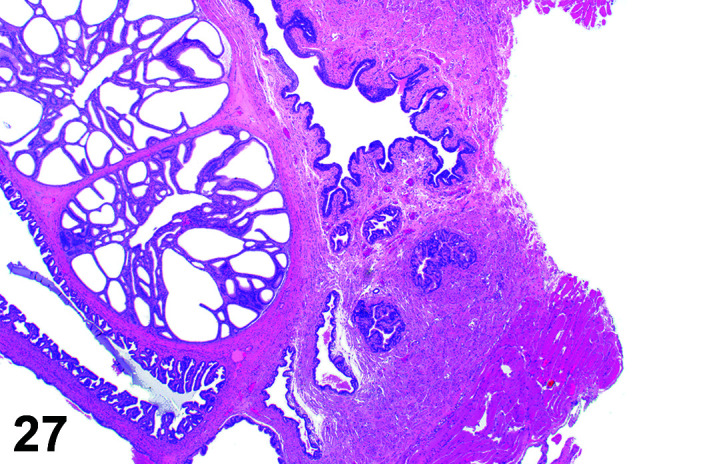
Prostate, and associated glands, H&E.
Figure 29.
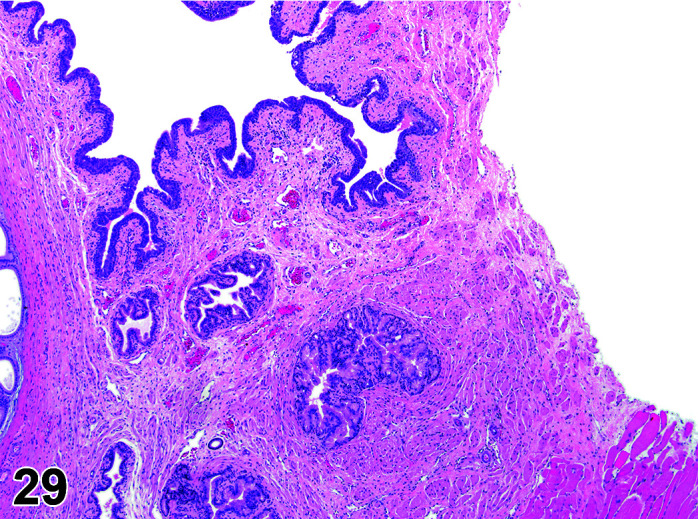
Proprostate, H&E.
Male rabbits also have “paraprostate” glands associated with the urethra161,162,163. The paraprostate is comprised of small, club-shaped structures that lie on the dorsolateral aspect of the prostate and are embryologically derived from the urethral wall (Figure 31). These glands are histologically identical to the prostate (Figure 32). These are not routinely specifically sampled in toxicology studies but can appear fortuitously in cross-sections of the accessory sex-organ/urethral region as small clusters of prostate-like acini embedded in the periurethral connective tissue (Figure 33) (Table 37).
Figure 31.
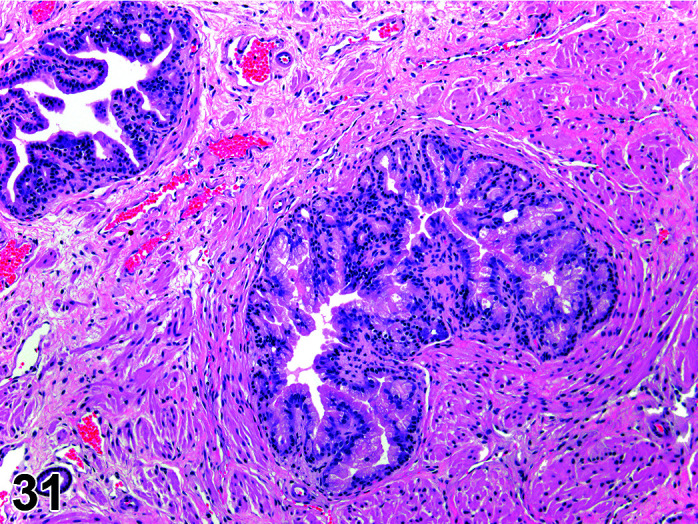
Paraprostate, H&E.
Figure 32.
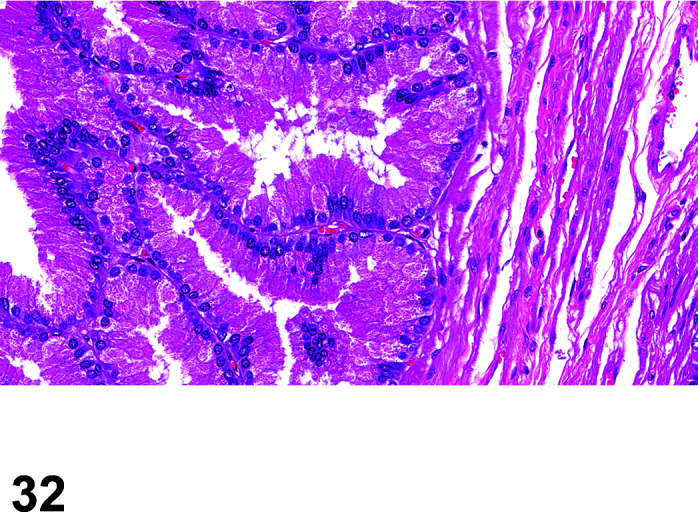
Prostate, H&E.
Figure 33.
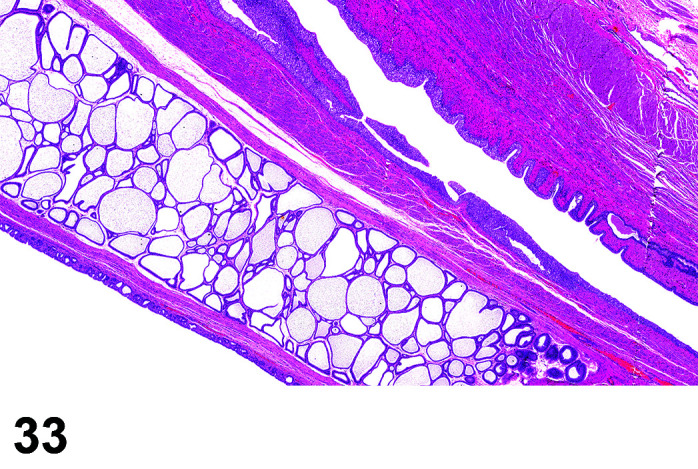
from left to right: Seminal vesicle, Vas deferens, Ampulla, Urethra, H&E.
Table 37. Microscopic Findings of the Prostate, Proprostate and Paraprostate: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Basophilia | X | ||||
| Corpora amylacea | X | ||||
| Degeneration | X | ||||
| Dilatation, acinar/acinus/vesicle | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, squamous cell * | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, vascular/perivascular | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, epithelial | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, atypical/diffuse/reactive | X | ||||
| Proliferative lesion, mesenchymal | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Papilloma, squamous cell | X | ||||
| Tumor, granular cell, benign | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell | X | ||||
| Carcinosarcoma | X | ||||
| Tumor, granular cell, malignant | X | ||||
| Tumor, neuroendocrine, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Metaplasia, Squamous Cell
Comments: Spontaneous focal keratinized squamous metaplasia of the prostate and proprostate epithelium is an infrequent finding peculiar to the rabbit and is clinically silent. It is important to recognize this as a spontaneous lesion when evaluating test articles with androgenic or estrogenic actions. The finding is well described in Dutch Belted and NZW rabbits 164. It is characterized by generally small foci of increased numbers of lamellated squamous-like cells with or without superficial keratinization and sloughing of cornified debris into the gland alveolar lumen; the squamous metaplastic foci are located within the gland epithelium or can also extend into the underlying lamina propria.
B. Anatomy of the Seminal Vesicles
In most species, the paired seminal vesicles (vesicular glands) are dorsal to the ampullae. For consistency with nomenclature in rats, mice, and other species, this review uses the term “seminal vesicle” for the analogous structure in the male rabbit. Rather than separate paired organs as in other species, the rabbit seminal vesicle appears grossly as a single sac-like structure located dorsal to the urinary bladder and ventral to the prostate complex162, 163. The cranial (blind) end of the seminal vesicle has a distinctive grossly visible “dimpled” indentation, which corresponds a microscopic appearance of two side-by-side glands with separate lumens in the proximal portion (appreciable in cross-sections). In the more distal main body of the seminal vesicle, the central separating wall disappears to create a conjoined single lumen161, 165. The histologic appearance of seminal vesicle epithelium (basophilic cuboidal to low columnar cells) is similar to that in other species (Table 38).
Table 38. Microscopic Findings of the Seminal Vesicle: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Corpora amylacea | X | ||||
| Degeneration | X | ||||
| Dilatation, acinar/vesicle | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, vascular/perivascular | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, epithelial | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, atypical/diffuse/reactive | X | ||||
| Proliferative lesion, mesenchymal | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Tumor, epithelial-stromal, benign | X | ||||
| Tumor, granular cell, benign | X | ||||
| Adenocarcinoma | X | ||||
| Tumor, granular cell, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
C. Anatomy of the Bulbourethral Gland
The bilobed bulbourethral gland is located dorsal to the urethra and caudal to the prostate/proprostate161,162,163, 166. The histologic appearance of the bulbourethral gland epithelium is similar to that in other species. The two lobes of bulbourethral gland are subdivided into lobules by thick connective tissue septa and embedded entirely in the bulboglandularis muscle, portions of which penetrate the interlobar and interlobular septa of the gland. Excretory ducts of the bulbourethral gland exit ventrally to empty into the urethra. Each lobule of the bulbourethral gland has a substantial lamina propria with large central lumina lined by a simple cuboidal to columnar to pseudostratified epithelium (Table 39).
Table 39. Microscopic Findings of the Bulbourethral gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Corpora amylacea | X | ||||
| Degeneration | X | ||||
| Dilatation, acinar/vesicle | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, vascular/perivascular | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, epithelial | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, atypical/diffuse/reactive | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
D. Anatomy of the Preputial Gland
So-called “preputial” glands (inguinal gland complex) are paired structures in the subcutis on either side to the base of the penis and prepuce106, which are further described in the Skin section. Descent of the testes into the scrotum occurs at 2.5 to 3 months of age. However, males can retract their testes into the abdominal cavity via the inguinal ring (Table 40).
Table 40. Microscopic Findings of the Preputial (Inguinal) gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Basophilia | X | ||||
| Degeneration | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Necrosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, epithelial | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, atypical/diffuse/reactive/squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Papilloma, squamous cell | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell, malignant | X | ||||
| Tumor, basal cell, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
E. Anatomy of the Vas Deferens (Ampulla)
As the deferent ducts converge, their walls become thickened to form ampullae, connected to each other by the genital fold. In contrast to rats and mice, the rabbit distal vas deferens has a very wide ampullary region162, 163. The ampullae can be so large that they have been mistaken for “paired” seminal vesicles at gross necropsy. Microscopically, the ampulla exhibits abundant, large lamina propria glands, which result in a distinctive “honeycomb” appearance (Figure 33) (Table 41).
Table 41. Microscopic Findings of the Ampullary gland: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Degeneration | X | ||||
| Edema | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Necrosis | X | ||||
| Necrosis/inflammation, vascular/perivascular | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, epithelial | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, atypical/diffuse/reactive | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Section 13: Respiratory System
Adult and juvenile rabbits are occasionally used for inhalation and intranasal safety assessment studies to test local tolerability and potential toxicity for drugs, chemicals and particulates. Rabbits are prone to atherosclerotic lesions in a variety of organs including the lung. For detailed description, see the cardiovascular section. Rabbits can be used as animal models for tuberculosis research, and they can be infected via snout only inhalation of the bacteria167. In addition, rabbits are used as animal models for induced hypertension and aspiration pneumonia, and for sinus research, i.e. study of surgical packing material or surgical techniques. Modern laboratory animal management practices within rabbit facilities are such that spontaneous infectious processes should only be infrequently encountered; thus, the lesions related to infectious respiratory tract diseases are not described in detail in this document.
A. Anatomy of the Nasal Cavity
The nostrils of rabbits contain sensory pads at the entrance, making the nose very sensitive to touch168. The nostrils are still when the rabbit is relaxed but can twitch at up to 150 twitches per minute. Rabbits are obligate nose breathers because the epiglottis is positioned rostrally to the soft palate, resulting in direct continuity of the nasopharynx, larynx, and trachea25, 168.
The anatomy and histology of rabbit nasal cavity tissues have been described previously169. Four types of epithelia line the nasal cavity: squamous, transitional, respiratory and olfactory, and their distribution at various levels of the nasal cavity is described169, along with recommended sectioning planes to be used for inhalation studies: Level I is sectioned immediately posterior to the incisors, Level II at the first palatal ridge, Level III immediately anterior to the first upper premolar teeth, and Level IV immediately anterior to the first upper molar. Level I is lined predominantly by squamous epithelium with small amounts of thick transitional epithelium, and examination is recommended only for studies involving test article administration via instillation. Level II is lined primarily with transitional and respiratory epithelia, whereas Levels III and IV are lined with respiratory and olfactory epithelia, and often contain nasal-associated lymphoid tissue. The vomeronasal organs are evident only in Level II (Table 42).
Table 42. Microscopic Findings of the Nasal Cavity: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Cleft palate * | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Congestion | X | ||||
| Corpora amylacea | X | ||||
| Degeneration | X | ||||
| Deviation, nasal septum | X | ||||
| Edema | X | ||||
| Embolus | X | ||||
| Eosinophilic globules | X | ||||
| Erosion/ulcer | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation [insert appropriate cell type] | X | ||||
| Metaplasia | X | ||||
| Necrosis | X | ||||
| Perforation, septum | X | ||||
| Regeneration | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, (+ cell type) | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Papilloma, squamous cell | X | ||||
| Tumor, neuroendocrine cell, benign | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, adenosquamous | X | ||||
| Carcinoma, neuroepithelium | X | ||||
| Carcinoma, squamous cell | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Cleft Palate
Comments: Cleft Palate is a common finding in rabbit teratogenicity studies. Congenital alveolar cleft is a malformation occurring as a result of non-fusion of primary palate during weeks 4–12 of gestation, and may be induced by glucocorticoids31 (Table 43).
Table 43. Microscopic Findings of the Paranasal Sinuses and Nasopharynx: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Congestion | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Necrosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, (+ cell type) | X | ||||
| Metaplasia, squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Papilloma, squamous cell | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, adenosquamous | X | ||||
| Carcinoma, neuroepithelium | X | ||||
| Carcinoma, squamous cell | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
B. Anatomy of the Larynx
Iatrogenic damage may be noted after intubation, and so care should be taken in interpreting short term studies where surgical preparations have taken place. Intubation damage has been described previously170. Submucosal glands are scanty or absent in the larynx171,172,173 (Table 44).
Table 44. Microscopic Findings of the Larynx: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Congestion | X | ||||
| Degeneration | X | ||||
| Ectasia, submucosal glands | X | ||||
| Edema | X | ||||
| Epithelial alteration | X | ||||
| Erosion/ulcer | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Necrosis | X | ||||
| Regeneration | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, (+ cell type) | X | ||||
| Metaplasia, squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Papilloma | X | ||||
| Tumor, neuroendocrine cell, benign | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell | X | ||||
| Tumor, neuroendocrine cell, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
C. Anatomy of the Trachea and Bronchi
The upper (tracheobronchial) airways have very scanty mucous cells and relatively abundant club cells. For example, in rabbits, mucous cells constitute less than 2% of tracheal epithelium, while club cells constitute 17–25% of the upper airway epithelium172. Submucosal glands are scanty or absent in the trachea, and bronchi171,172,173 (Table 45).
Table 45. Microscopic Findings of the Trachea and Bronchi: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Bronchiectasis | X | ||||
| Congestion | X | ||||
| Degeneration | X | ||||
| Ectasia, submucosal glands | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Aggregates, macrophage, increased | X | ||||
| Metaplasia | X | ||||
| Necrosis | X | ||||
| Regeneration | X | ||||
| Single cell necrosis | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, (+ cell type) | X | ||||
| Metaplasia, squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Papilloma | X | ||||
| Tumor, neuroendocrine cell, benign | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell | X | ||||
| Tumor, neuroendocrine cell, benign/malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
D. Anatomy of the Lungs (Alveoli and Bronchioles)
The left lung consists of only two lobes (cranial and caudal), whereas the much larger right lung has four lobes (cranial, middle, caudal, and accessory)174. Multiple generations of distal intrapulmonary airways (bronchioles) branch to the level of terminal bronchioles (respiratory bronchioles are not present)175, 176. Rabbit blood vessels are generally thin-walled and prone to collapse and hematoma formation on puncture – a feature important to remember for studies where test items have been given by intravenous administration. The exceptions to this are the pulmonary arteries which are enveloped in a prominent smooth muscle layer, which may be misinterpreted as hypertrophy24, 25. There is a relative paucity of bronchus associated lymphoid tissue (BALT) in laboratory rabbits compared to other species. BALT is acquired and increases with age and environmental antigen exposure. Due to modern husbandry systems, relatively little BALT is seen in the young animals on toxicology studies.
In rabbits (like other laboratory species and humans), ciliated cells constitute a high percentage (40–55%) of airway lining epithelium at all levels from the trachea to the distal bronchioles172, 176. In rabbits club cells (formerly known as Clara cells) are the predominant secretory cell of the distal airways (club cells and ciliated cells constitute about 50% each of the distal airway epithelium of rabbits)172. However, the upper (tracheobronchial) airways have very scanty mucous cells and relatively abundant club cells. For example, in rabbits, mucous cells constitute less than 2% of tracheal epithelium, while club cells constitute 17–25% of the upper airway epithelium172. Submucosal glands are scanty or absent in the trachea, larynx, and bronchi, and absent in bronchioles171,172,173 (Table 46).
Table 46. Microscopic Findings of the Lungs (Alveoli and Bronchioles): Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Cyst, congenital | X | ||||
| Diaphragmatic hernia * | X | ||||
| Hypoplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Alveolar emphysema | X | ||||
| Alveolar lipoproteinosis | X | ||||
| Apoptosis ǂ | X | ||||
| Atelectasis | X | ||||
| Atherosclerosis * | X | ||||
| Bronchiectasis | X | ||||
| Congestion | X | ||||
| Degeneration | X | ||||
| Ectasia, acinus/submucosal glands | X | ||||
| Edema | X | ||||
| Embolus | X | ||||
| Erosion/ulcer | X | ||||
| Fibrosis * | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, media, artery | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] * | X | ||||
| Inflammation * | X | ||||
| Lipoproteinosis, alveolus | X | ||||
| Macrophages increased, alveolar * | X | ||||
| Material, extracellular [insert morphology/color] | X | ||||
| Metaplasia, mucous cell/squamous cell | X | ||||
| Metaplasia, osseous * | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Pigment | X | ||||
| Pigment/foreign material | X | ||||
| Pyothorax | X | ||||
| Regeneration | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus * | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, (+ cell type) | X | ||||
| Metaplasia, mucous/squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, bronchioloalveolar | X | ||||
| Papilloma | X | ||||
| Tumor, neuroendocrine cell, benign | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, acinar | X | ||||
| Carcinoma, adenosquamous | X | ||||
| Carcinoma, bronchioloalveolar | X | ||||
| Carcinoma, squamous cell | X | ||||
| Histiocytic sarcoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Atherosclerosis
Other term(s): Plaque, atheromatous
Comments: Cholesterol-rich diets have been used to induce wide-spread atheromatous lesions within a short time period (3 months) in NZW rabbits. Early foam cell accumulation to partly occlusive atheromatous plaques were observed in the larger lung arteries28. Unique proliferations are seen as age related changes in the pulmonary arteries of both sexes and can resemble iatrogenic lesions177. Genetically altered strains of the NZW rabbit such as the Watanabe rabbit (Watanabe heritable hyperlipidemia rabbit, WHHL) are also used extensively.
Fibrosis
Comments: A rabbit model of pulmonary fibrosis due to pneumoconiosis induced by a single instillation of a known amount of silica dust into the right lung, is available178,179,180. Affected lungs show diffuse areas of increased alveolar macrophages containing dark pigment (dust particles) and/or vacuoles, with few associated areas of alveolar wall thickening and increased reticular fibers. In this model, severity/distribution of unilateral fibrosis, is scored for each lung lobe.
Hernia, Diaphragmatic
Comments: Fox and Crary reported 55 cases of left diaphragmatic hernia in three related strains of rabbits kept at the Jackson Laboratory under normal colony conditions: strains AX, AXbubu and IIIc181. Associated abnormalities included hypoplasia of the ipsilateral lung and an increased incidence of ventricular septal defects. Death was attributable to respiratory insufficiency. Genetic analysis suggests recessive inheritance. The condition is neither sex limited nor sex linked. The authors believe that two autosomal recessive genes are involved and have proposed the symbols dh 1 and dh 2 for the two genes that must both be present in homozygous condition for the development of diaphragmatic hernia in these rabbit strains.
Infiltrate, Inflammatory Cell
Figure 35.
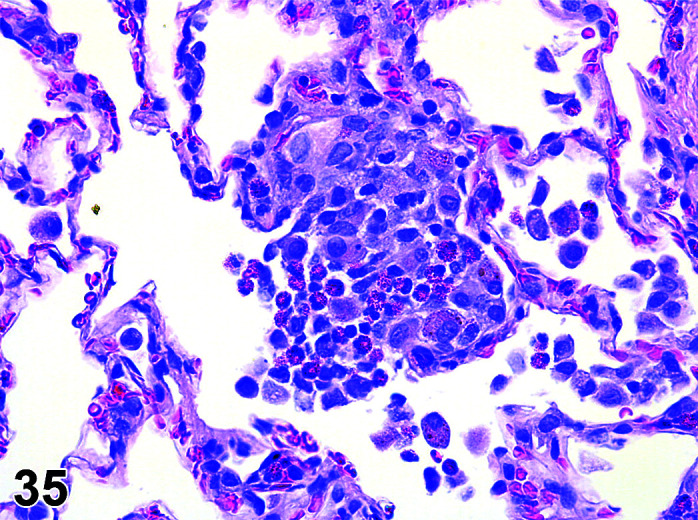
Lung, Infiltrate, heterophil, H&E.
)
Figure 34.
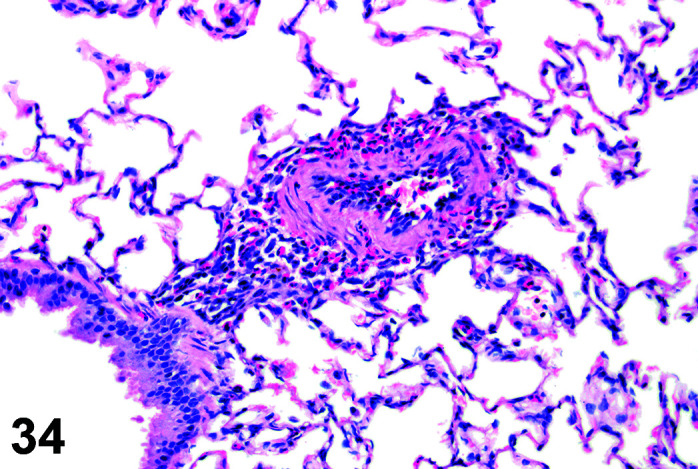
Lung, Infiltrate, inflammatory cell, H&E.
Comments: Peribronchial/perivascular infiltrates of granulocytes and mononuclear cells are commonly seen around larger airways and their associated vessels as an incidental background finding in rabbits on routine toxicology studies81. Should be differentiated from Bronchus associated lymphoid tissue (BALT).
Inflammation
Comments: Cooper et al. reported a congenital surfactant pneumonia in the audiogenic (EIII/JC) strain of rabbits177. This pneumonia was frequently grossly visible as irregular firm tan nodules in the cranioventral portions of lung lobes. Histologically the lesions consisted of multifocal to coalescing intra-alveolar aggregates of large numbers of multinucleate giant cells, predominantly foreign body type, with epithelioid and foamy macrophages and few lymphocytes, plasma cells and heterophils. Lesions occasionally extended into smaller bronchioles. There was frequent type II pneumocyte hyperplasia (adenomatosis) and alveolar septal fibrosis. “Billups bodies” – globular to ring-like brown to gray acellular material – was free within the alveoli and cytoplasm of giant cells. This acellular material was Alcian blue and PAS positive. The material was immunoreactive for surfactant protein-A and had the ultrastructural appearance of multilamellar vesicles, suggesting a genetic defect in surfactant metabolism.
Macrophages Increased, Alveolar
Figure 36.
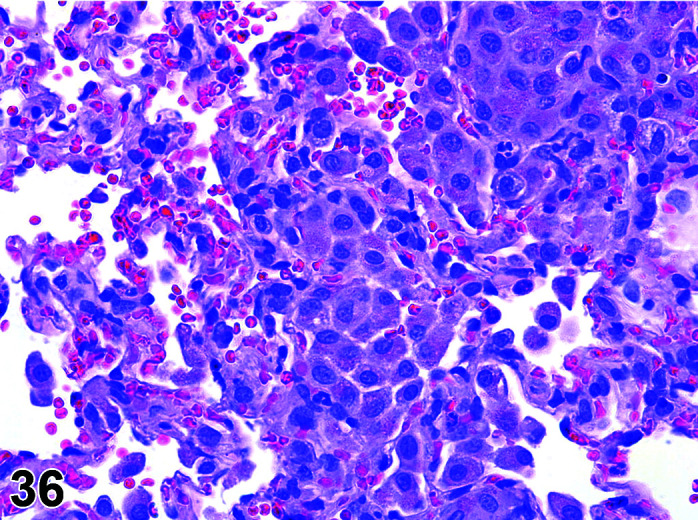
Lung, Macrophages increased, alveolar H&E.
Comments: Tissue resident macrophages are the first responders to insults to tissues, and the “aggregates” are generally proliferation or hyperplasia of these responding macrophages. Barros et al. reported an infiltration of the mucosa and submucosa of the trachea and bronchi by macrophages, multinucleated giant cells, lymphocytes, and mast cells with associated basal lamina calcium deposits, in six rabbits after intoxication by the calcinogenic plant Solanum glaucophyllum182. Increased diffusely distributed macrophages may also occur in Dutch Belted rabbits that relatively frequently show cardiomyopathy of unclear origin.
Metaplasia, Osseous
Figure 37.
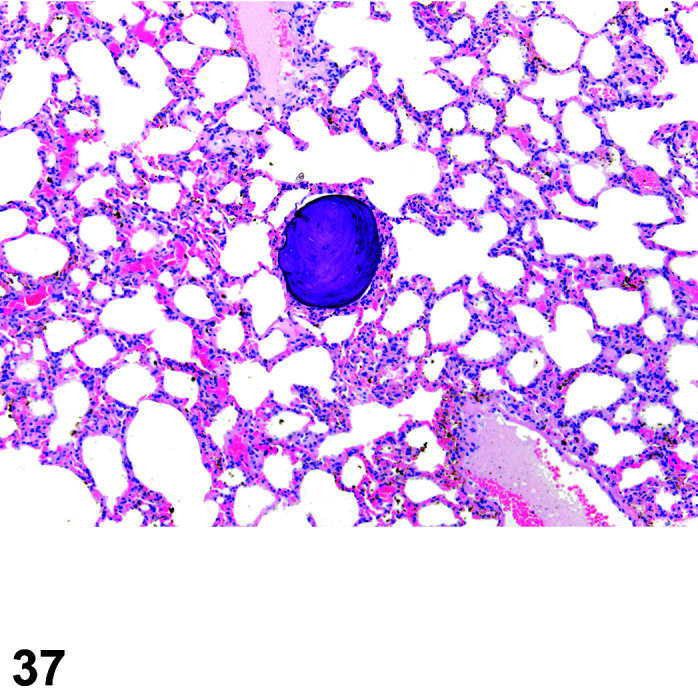
Lung, Metaplasia, osseous, H&E.
Comments: Osseous metaplasia is an incidental spontaneous background finding in rabbit lungs, which has a differing appearance depending on the stage of the lesion. In early stages, they are composed of a dense knot of small cells with hyperchromatic nuclei and scant eosinophilic cytoplasm within the wall of an alveolus. This progresses to a larger, sometimes papillomatous mass with a fibrous core and a dark, low spindle to cuboidal epithelial-like covering. As the lesion progresses, it changes into eosinophilic to basophilic, osteoid to mineralized bone, resembling the lesion classically seen in rodent lungs.
Thrombus
Figure 38.
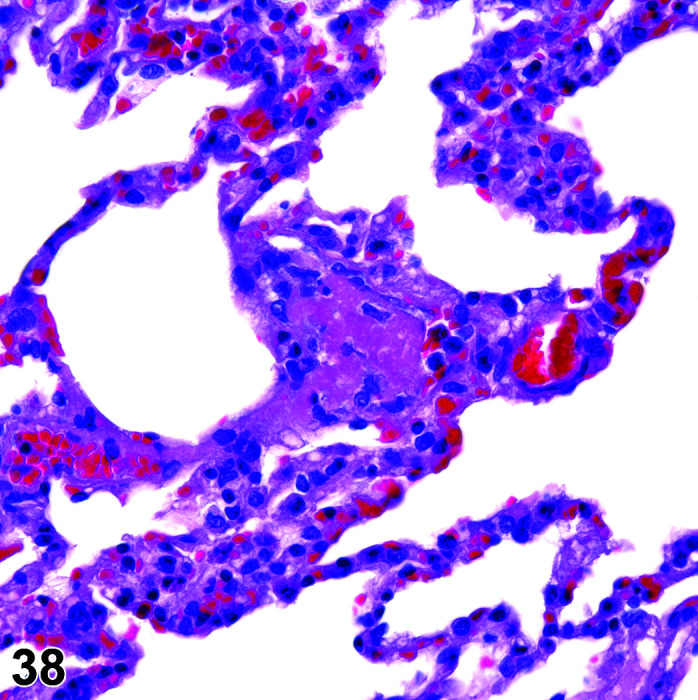
Lung, Thrombus, H&E.
Comments: Small thrombi, with an accompanying arteritis/periarteritis, are often seen as incidental findings in the lungs of rabbits on routine toxicity studies (Table 47).
Table 47. Microscopic Findings of the Pleura: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Cyst, congenital | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Effusions, non-inflammatory | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Macrophages increased | X | ||||
| Metaplasia | X | ||||
| Mineralization | X | ||||
| Pigment | X | ||||
| Pigment/foreign material | X | ||||
| Pyothorax | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, mesothelium | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Section 14: Skeletal System (Bone, Joint, Tooth)
A. Anatomy of Bone
Compared to rodents, rabbit bone has a larger proportion of osteonal structures, with dense osteons within the center of cortical bone and a primary vascular structure running longitudinally183. Rabbits also have more cancellous bone than rodents and this cancellous bone shows a higher degree of remodelling into secondary bone184. The secondary bone retains the primary vascular structure with vascular canals of osteons on the periosteal and endosteal surfaces running parallel to the long axis of the medullary cavity183, 185. Compared to rodents, rabbits have relatively more trabecular bone mass that undergoes Haversian remodelling184. Rabbits do not have secondary centers of ossification present at birth186. NZW rabbits typically reach skeletal maturity at 8–11 months187, with cartilage developing a mature appearance prior to epiphyseal closure188. Rabbits have significant cartilage healing ability at an early age189, but periosteal support of cartilage declines markedly from 6–12 months and is very variable after one year190. Rabbit femoral condyle cartilage is relatively thin with 0.25–0.75 mm compared to 2.2–2.4 mm in humans188.
Skull
Rabbit tympanic bullae are relatively larger than those of most mammals and rabbits have a longer and more tubular external auditory meatus. The orbits are very large, and joined by an interorbital foramen. The zygomatic arch has a prominent protuberance, called the zygomatic process that is at the caudal aspect of the arch30. The maxilla is fenestrated giving a lacy appearance on radiographs. Radiographic anatomy of the rabbit skull has been well described191, 192.
Vertebrae and ribs
The most common vertebral formula is C7 T12 L7 S4 Ca16, but this can vary widely in the thoracolumbar region. A recent report found that 44% of rabbits had 12T/7L, 33% had 13T/6L, and 23% had 13T/7L. Additionally, the spinal cord terminated with S2 in 79% of rabbits but in S1 in 19% and in L3 in 2% of animals193. The lumbar vertebrae have prominent mammillary processes on the cranial articular process. Rabbits are unique among domestic animals for having the dorsal aspect of the lumbar vertebral mammillary processes level with and slightly ventral to the spinous process. S1–S3 are routinely fused in rabbits, while S3–S4 fusion is more variable30. The first 7 ribs articulate with the sternum and the final 5 are free. The costal cartilage sections of the 7th–9th ribs are attached. The sternum includes 6 sternebrae30.
Limbs
Rabbits are digitigrade.
Forelimb
The rabbit has paired clavicles and the only direct attachment between the forelimb and the axial skeleton is the sternoclavicular ligament. The carpus includes two rows of carpal bones. In the 5 digits in the forelimb, P1 has two phalanges and P2–P5 have three phalanges30.
Hindlimb
The femur articulates only with the tibia. The fibula fuses distally with the tibia for approximately half of its length. Six tarsal bones are arranged in three rows. P1–P5 all have three phalanges30. The rabbit stifle joint is considered to have minimal to no resemblance to the human stifle anatomy194.
Non-hematopoietic marrow
Rabbits have relatively fatty bone marrow which is not an ideal for autogenous bone and marrow harvesting or transplantation184 (Table 48).
Table 48. Microscopic Findings of Bone: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Malformation, skeletal * | X | ||||
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Bone decreased, trabeculae and/or cortex | X | ||||
| Bone increased, trabeculae and/or cortex * | X | ||||
| Callus | X | ||||
| Cyst, bone | X | ||||
| Eroded surface, increased | X | ||||
| Fibro-osseous lesion (FOL) | X | ||||
| Fibrous osteodystrophy (FOD) | X | ||||
| Fracture * | X | ||||
| Fracture/Callus | X | ||||
| Growth plate closed | X | ||||
| Growth plate partially closed | X | ||||
| Growth plate open | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Necrosis * | X | ||||
| Osteoblastic surface increased | X | ||||
| Osteoclasts increased | X | ||||
| Osteoid increased | X | ||||
| Physeal dysplasia | X | ||||
| Physis, decreased thickness | X | ||||
| Physis, increased thickness | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, chondrocyte * | X | ||||
| Hyperplasia, osteoblast, focal | X | ||||
| Proliferative Neoplastic | |||||
| Chordoma | X | ||||
| Chondroma | X | ||||
| Osteoma | X | ||||
| Osteoblastoma | X | ||||
| Osteofibroma | X | ||||
| Chondrosarcoma | X | ||||
| Fibrosarcoma, osteogenic | X | ||||
| Osteochondroma | X | ||||
| Osteosarcoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Malformation, Skeletal
Comments: Syringomyelia, hypoplasia pelvis, femoral luxation, and distal foreleg curvature are occasionally seen together as a hereditary defect in fetuses in Developmental and Reproductive Toxicology (DART) studies.
Bone Increased, Trabeculae
Comments: Osteopetrosis has been recorded in Dutch Belted rabbits; feed formulation errors have historically caused vitamin A toxicity-related increased bone deposition, sometimes leading to hydrocephalus195.
Necrosis
Figure 40.
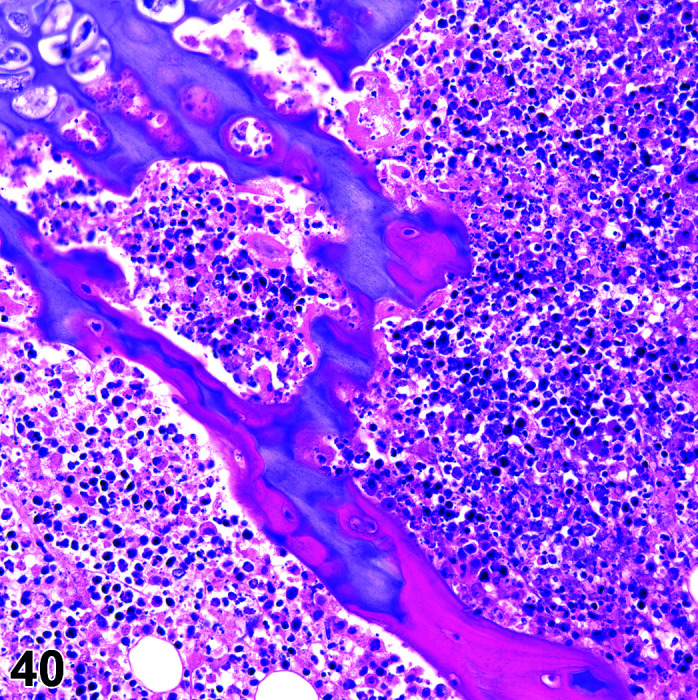
Bone, Physeal necrosis, H&E.
)
Figure 39.
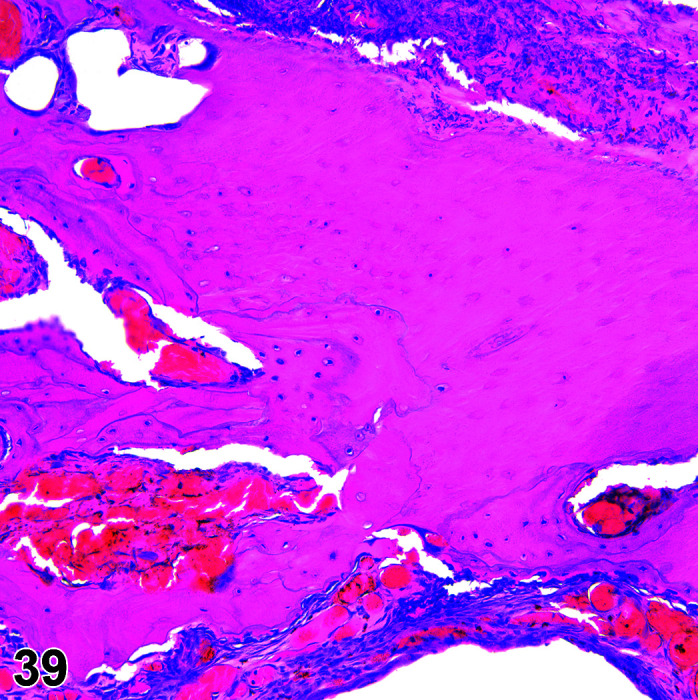
Bone, necrosis, H&E.
Comments: Osteonecrosis can be induced with steroid therapy, as there may be infarction from fat or lipid-laden fibrin and platelet-containing emboli196, 197.
Fracture
Comments: The combination of a light skeleton (6–8% of body weight 24, 30) and ample skeletal muscle (>50% of body weight) predisposes rabbits to vertebral, sometimes even spontaneous, fracture24, 195, often at the 7th lumbar vertebrae30. Lumbosacral fractures may also be seen as sequelae to convulsions/seizures induced by CNS active test articles. Avulsion of the tuberositas tibiae has been reported198.
Hyperplasia, Chondrocyte
Comments: Chondrocyte hyperplasia, cartilage degeneration and necrosis are seen with fluoroquinolone antibiotics199.
B. Anatomy of Joints
Spontaneous lesions of the joints and synovium are rare in rabbits used in nonclinical toxicology studies. The relative lack of changes in the joints of the rabbit is most likely related to the young age of the animals used. The most frequently used trimming plane to prepare femorotibial joints for microscopic evaluation is parasagittal orientation (taken off-center through one femoral condyle), a portion of meniscus, and the corresponding tibial plateau.
Typical scoring systems used in toxicology studies are the International Cartilage Research Society (ICRS) visual Histological Assessment Scale200 and the Osteoarthritis Research Society International (OARSI) score201. These are more appropriate for use in animal models than the Mankin scoring system used in man (Table 49).
Table 49. Microscopic Findings of the Joint and Synovium: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Degeneration, articular cartilage | X | ||||
| Degeneration, chondromucinous | X | ||||
| Degenerative Joint Disease (DJD) * | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Necrosis | X | ||||
| Osteophyte | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, synovial cell | X | ||||
| Proliferative Neoplastic | |||||
| Sarcoma, synovial | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Degenerative Joint Disease (DJD)
Comments: Induced models are used for research i.e. partial lateral meniscectomy202.
C. Anatomy of Teeth
The maxilla contains 4 incisors, no canines, six premolars, and 4–6 molars. The mandible contains 2 incisors, no canines, 4 premolars, and 6 molars. Unlike rodents, rabbits have two pairs of maxillary incisors caudal to the main incisors (“peg teeth”) and have a total of 26–28 teeth. A slight degree of brachygnathism is normal in the rabbit so that the large pair of inferior incisors usually contact the small superior pair during occlusion. The large incisor teeth are adapted for gnawing and continue to erupt throughout life. There are no canine teeth and a large diastema exists between the incisors and premolars. Incisors consist of crown only, with extra-alveolar and intra-alveolar parts. Rabbits are hypsodonts and have a long crown without a true tooth root30.
The labial or convex side of the incisors is covered by a layer of enamel. The lingual or concave side of the incisors is enamel-free but does have a very thin layer of cementum into which fibers of the periodontal ligament are embedded. Enamel is not formed over the top of the incisors. Before eruption, the tip is filled with dentin produced by odontoblasts of the pulp. As the tip wears away with use, the odontoblasts form more dentin (secondary dentin) so that the pulp is never exposed. Incisors have a widely open apical foramen (Table 50).
Table 50. Microscopic Findings of the Tooth: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Malocclusion * | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Alteration, dentin matrix | X | ||||
| Apoptosis ǂ | X | ||||
| Concretion, pulp | X | ||||
| Cyst | X | ||||
| Degeneration | X | ||||
| Dental dysplasia | X | ||||
| Denticle | X | ||||
| Dentin matrix alteration | X | ||||
| Dentin, decreased | X | ||||
| Dentin, niches | X | ||||
| Fracture | X | ||||
| Necrosis | X | ||||
| Periodontal pocket | X | ||||
| Pulp concretion | X | ||||
| Resorption | X | ||||
| Single cell necrosis ǂ | X | ||||
| Thrombus | X | ||||
| Proliferative Neoplastic | |||||
| Ameloblastoma | X | ||||
| Ameloblastic odontoma | X | ||||
| Fibroma, cementifying/ossifying | X | ||||
| Fibroma, odontogenic | X | ||||
| Odontoma | X | ||||
| Tumor, odontogenic, benign | X | ||||
| Tumor, odontogenic, malignant | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Malocclusion
Comments: Erosions of the mucosa may occur due to irregular growth or sharp edges of broken teeth.
Section 15: Soft Tissue (Soft Tissue, Mesothelium, Adipose, Skeletal and Smooth Muscle)
The pathology of both spontaneous and induced conditions of soft tissues are similar in rabbits and humans, hence their use in vaccine studies. Careful recording of the nature, intensity and duration of the inflammatory response of the soft tissues to implanted or injected substances is important in the assessment of the local tolerability of agents intended for contact with human tissues. The chemical and physical properties of injected chemicals or vaccines and their adjuvants as well as size, shape and surface texture of implanted biomaterials may modify the histological features and temporal pattern of the inflammatory and reparative responses91,92,93,94. Such studies in rabbits are conducted with descriptors following the ISO-10993 guidelines (Table 51).
Table 51. Microscopic Findings of the Soft Tissue: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Amyloid | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Degeneration | X | ||||
| Fibroplasia | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous/cartilaginous | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia | X | ||||
| Proliferative Neoplastic | |||||
| Fibroma | X | ||||
| Hibernoma | X | ||||
| Leiomyoma | X | ||||
| Lipoma | X | ||||
| Rhabdomyoma | X | ||||
| Fibrosarcoma | X | ||||
| Leiomyosarcoma | X | ||||
| Liposarcoma | X | ||||
| Mesenchymoma, malignant | X | ||||
| Rhabdomyosarcoma | X | ||||
| Sarcoma, NOS (Not otherwise specified) | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
A. Anatomy of Adipose Tissue
Brown fat is converted to white fat in the interscapular region of the rabbit as it ages, which correlates with the disappearance of catecholamines from the sympathetic nerve fibers in arterial blood vessels in both brown and white fat203. The peritoneal mesothelium of the anterior abdominal wall of the rabbit is characterized by flattened mesothelial cells with tight junctions, desmosomes, cytoplasmic pinocytic vesicles and microvilli204. It has been shown that adipose tissue may have an important paracrine function in smooth muscle cell proliferation in blood vessels205 (Table 52).
Table 52. Microscopic Findings of the Adipose Tissue: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Degeneration | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Inflammation, lipogranulomatous | X | ||||
| Mineralization | X | ||||
| Necrosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia * | X | ||||
| Proliferative Neoplastic | |||||
| Hibernoma | X | ||||
| Lipoma | X | ||||
| Liposarcoma | X | ||||
| Sarcoma, NOS (Not otherwise specified) | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Hyperplasia
Comments: Hyperplasia of adipose tissue (obesity) has been reported associated with pregnancy toxemia, but also with ketosis in non-pregnant NZW rabbits24.
B. Anatomy of Muscle
The distribution of lesions of smooth muscle generally parallels the normal distribution of smooth muscle in the body so that lesions occur mostly in the female genital tract, the gastrointestinal tract and skin but only rarely in deep soft tissue. However, it has been shown that adipose tissue may have an important paracrine function in smooth muscle cell proliferation in blood vessels205. In soft tissues, particularly in inflammatory processes, smooth muscle cells may be difficult to distinguish from myofibroblasts. They both express smooth muscle actin but smooth muscle cells usually contain desmin206 (Table 53).
Table 53. Microscopic Findings of the Smooth and Skeletal Muscle: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy * | X | ||||
| Degeneration * | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous/cartilaginous | X | ||||
| Mineralization * | X | ||||
| Necrosis * | X | ||||
| Parasite * | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, myocyte | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia | X | ||||
| Proliferative Neoplastic | |||||
| Rhabdomyoma | X | ||||
| Rhabdomyosarcoma | X | ||||
| Sarcoma, NOS (Not otherwise specified) | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Atrophy
Comments: Destruction and denervation atrophy in skeletal muscle caused by injection of local anesthetics i.e. lidocaine or bupivacaine is reported in the muscles adjacent to the facial nerve in rabbits207.
Degeneration
Comments: Vitamin E deficiency causes skeletal muscle hyaline degeneration and mineralization, as does ketamine and xylazine intramuscular injection in Dutch Belted rabbits. The preferred site for intramuscular injection is the dorsal lumbar muscle. Alum granulomas or minimal focal myofiber degeneration are often seen at vaccination sites81, 208.
Mineralization
Comments: Hypervitaminosis D as well as vitamin E deficiency cause widespread mineralization209.
Necrosis
Comments: Afifi et al. reported muscle necrosis seen with 1,1’-methylenebis[4-[(hydroxyimino)methyl]-pyridinium] dimethanesulfonate intramuscular injection to NZW rabbits210. Sequestration of non-viable fat tissue (fat necrosis) transplanted into NZW rabbits has been reported211. Abscesses are common in the skin of domestic rabbits110. Rabbits restrained for six hours a day for 35 days developed focal, small necrotic areas in the skeletal muscle212.
Parasite
Comments: Sarcocystosis, presumably caused by Sarcocystis cuniculi, was recently reported in 2 purpose-bred, SPF Dutch Belted laboratory rabbits213. Sarcocysts were found in the eyelid of one rabbit and the tongue of the other (Table 54).
Table 54. Microscopic Findings of the Mesothelium: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Fibrosis | X | ||||
| Fibroplasia | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia | X | ||||
| Proliferative Neoplastic | |||||
| Mesothelioma, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Section 16: Special Senses
The otic (ear) and olfactory systems are not routinely evaluated in toxicity studies in the rabbit. The ocular system is subdivided into the eye and the ocular adnexa (glands and other eye-associated tissues). At necropsy, Davidson’s fixative is usually the fixative of choice for the eyes and optic nerves. For a thorough examination of the ocular system, at a minimum, the eyes, optic nerves, and major ocular adnexa (eyelids, lacrimal glands, nictitating membrane with nictitans gland, and Harderian glands) should be examined.
A. Anatomy of the Eye
At birth, the eye is developmentally immature, and differentiation continues and is substantially complete when the eyelids open at about post-natal days 10–11214,215,216,217,218.
The rabbit eye is relatively large in proportion to body size and slightly flattened along the antero-posterior plane217. The circular, rather shallow orbits are at an approximately right angle to the sagittal plane of the head, so the eyes protrude prominently217, 219,220,221,222. Each of the eyes of the rabbit has approximately 190-degree field of view, but they also work together to have a small amount of binocular vision. Because of their lateral placement, the angle between the eyes is 150–175 degrees, and the overall visual field is extremely wide (330 – to almost 360 degrees)217, 219,220,221,222,223,224. In contrast, the binocular field of vision is correspondingly quite narrow (10–35 degrees wide)221.
B. Anatomy of the Eyelids
Rabbits are born blind with closed eyelids, which open at about post-natal days 10–11. The superior eyelid (palpebra) of the rabbit is shorter and thicker and has larger and more abundant eyelashes than the inferior eyelid219. The outer surfaces of the eyelids are lined by typical keratinized stratified squamous epithelium. As in other skin regions of the rabbit, the eyelid skin lacks sweat glands.
Meibomian (tarsal) glands are present in the superior and inferior eyelids, embedded as linear arrays in the tarsal plate (Figure 41). In one study of NZW rabbits, Meibomian gland numbers/eyelid averaged from 32.30 +/– 3.43 and 27.15 +/– 2.35 in the superior and inferior eyelids, respectively225. In each Meibomian gland, the sebaceous holocrine acini are aligned along and empty via short ductules into a single central duct via short ductules. The central duct in turn opens at the conjunctival margin219, 226. The central excretory duct is normally lined by keratinized stratified squamous epithelium226, 227.
Figure 41.
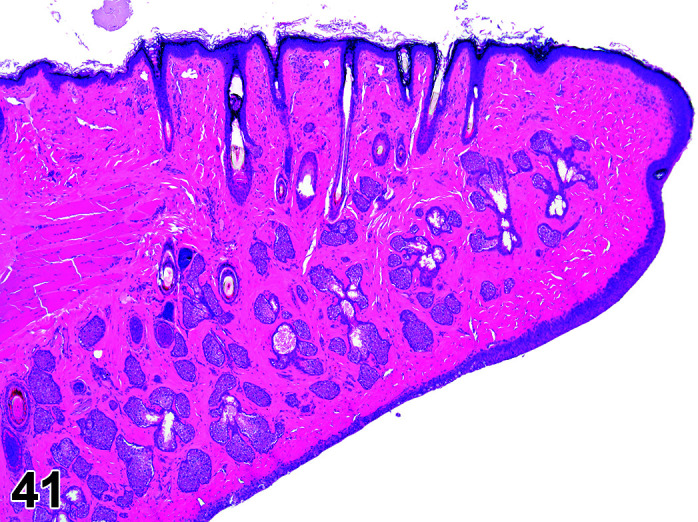
Eyelid with Meibomian gland, H&E.
Small clusters of accessory lacrimal gland acini (glands of Wolfring) have been described in the superior eyelid of rabbits228 (Figure 42). These are located submucosally, and anterior to the Meibomian gland arrays (Figures 43, 44
Figure 44.
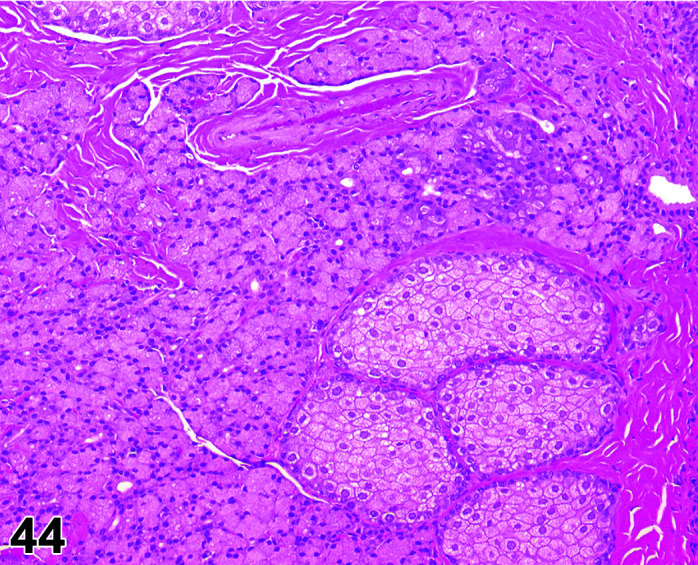
Eyelid, accessory lacrimal gland (gland of Wolfring) and Meibomian gland, H&E (high mag).
).
Figure 42.
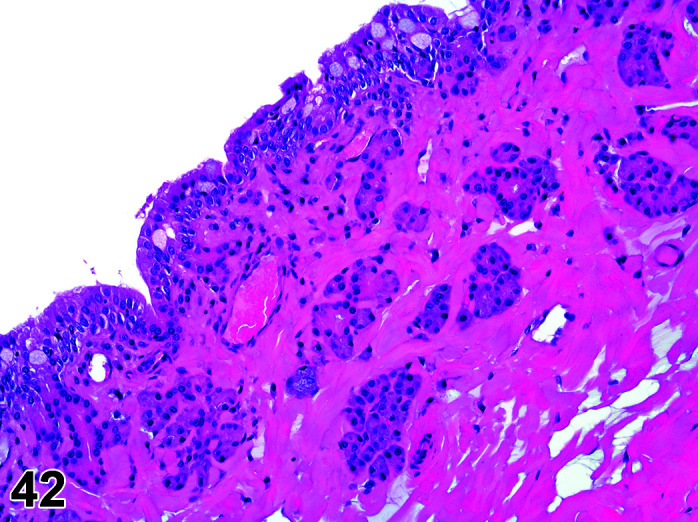
Eyelid, accessory lacrimal gland (gland of Wolfring), H&E.
Figure 43.
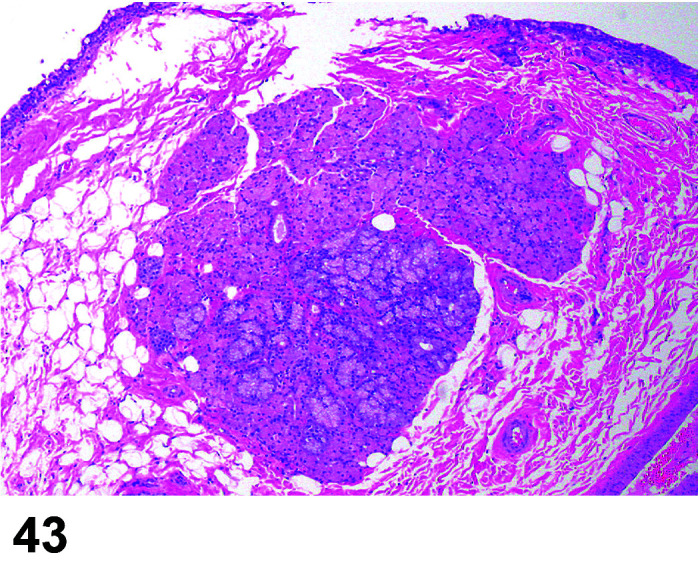
Eyelid, accessory lacrimal gland (gland of Wolfring) Meibomian gland, H&E.
Extraocular muscles involved in movement of the eyelids include the levator superior palpebrae and orbicularis oculi219, 229,230,231,232,233, as well as the depressor palpebrae inferioris (present in rabbits but not in most other mammalian species)220, 234, 235 (Table 55).
Table 55. Microscopic Findings of the Eyelid: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, Meibomian gland | X | ||||
| Congestion | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation (+ locator) | X | ||||
| Metaplasia | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Papilloma, squamous cell | X | ||||
| Carcinoma, squamous cell | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
C. Anatomy of the Conjunctiva
Compared to humans and many other species, the precorneal tear film of rabbits is thicker, more stable, and has a substantially different composition (i.e., types and proportions of lipid and mucins)236,237,238,239.
Conjunctival submucosal lymphoid cell aggregates, with or without germinal centers (conjunctiva-associated lymphoid tissue [CALT]) are normally present in rabbits240,241,242. CALT aggregates are more abundant in the palpebral and fornical conjunctivae versus the bulbar conjunctiva. They are also more abundant in the inferior than superior eyelid219, 240, 242. CALT aggregates are absent in neonatal rabbits, develop rapidly after the eyes open (at about post-natal day 10–11), reach maximum numbers and size in mature rabbits (7–20 months), and then decline in aging animals240 (Table 56).
Table 56. Microscopic Findings of the Conjunctiva: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, epithelium | X | ||||
| Cyst, inclusion | X | ||||
| Dermoid | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Pigment | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, conjunctival * | X | ||||
| Hyperplasia, squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Papilloma, squamous cell | X | ||||
| Carcinoma, squamous cell | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Hyperplasia, Conjunctiva
Other terms: Pseudopterygium; aberrant conjunctival overgrowth; ankyloblepharon; circumferential conjunctival hyperplasia; conjunctival centripetalization; conjunctival hyperplasia; conjunctival stricture; corneal occlusion; epicorneal conjunctival membrane; precorneal membranous occlusion; pinguecula bilateralis; pseudosymblepharon; pterygium; pterygium conjunctivae.
Comments: Infrequently, the rabbit cornea is progressively covered by hyperplastic conjunctiva around the entire perimeter. Of unknown etiology, the condition appears to be unique to rabbits222, 243,244,245,246,247,248. Conjunctival hyperplasia can be unilateral or bilateral, and is characterized clinically by a circumferential, nonadherent membranous flap which arises from the perilimbal bulbar conjunctiva and grows centripetally and symmetrically across the anterior corneal surface. Microscopically, the membranes consist of a collagenous central stroma lined by conjunctival mucosa247.
D. Anatomy of the Cornea
The rabbit cornea is relatively large and slightly elliptical (slightly longer along the horizontal axis)219,220,221, 243. Although there are breed and age variations, central corneal thickness in the living rabbit ranges from about 0.35–0.44 mm249,250,251,252,253,254,255,256, which is thinner than the human cornea (0.53–0.58 mm)257.
Most authors concur that the rabbit corneal stroma lacks a Bowman’s layer, a subepithelial condensation of the collagenous stroma present in humans and certain other species219, 258,259,260,261. Differently from rats and mice, the nuclei of rabbit corneal keratocytes are visible as hyperreflective structures. Thus, their density can be easily evaluated. Endothelial cell density is higher than in rats and mice, and the endothelium regenerates in response to injury or loss262, 263 (Table 57).
Table 57. Microscopic Findings of the Cornea: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Dermoid, corneal * | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Attenuation endothelium * | X | ||||
| Cyst, inclusion | X | ||||
| Degeneration | X | ||||
| Descemetocele | X | ||||
| Dystrophy, corneal * | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Fibroplasia | X | ||||
| Fibrosis | X | ||||
| Hypertrophy, Descemet’s membrane | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Keratinization | X | ||||
| Metaplasia | X | ||||
| Mineralization * | X | ||||
| Neovascularization | X | ||||
| Necrosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, lipid, cornea *# | X | ||||
| Vacuolation, epithelium or endothelium | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, endothelium | X | ||||
| Hyperplasia, squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Papilloma, squamous cell | X | ||||
| Carcinoma, squamous cell | X | ||||
* Terminology with diagnostic criteria or comments described in the text. # Inducible lesion. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Attenuation, Endothelium
Comments: Unlike many other species, the rabbit corneal endothelium has rather robust capacity to proliferate to cover defects or loss of individual endothelial cells. Following insult, the rabbit corneal endothelium generally undergoes endothelial proliferation and regenerates, with occasional multinucleate cell formation, rather than endothelial attenuation263.
Dermoid, Cornea
Comments: A case of a corneal dermoid in a dwarf rabbit264, and a limbic dermoid in a NZW rabbit265 have been reported. Although uncommon, dermoids should be on the list of differential diagnoses for corneal masses in rabbits. Animals exhibiting these changes on pre-study examination should be removed from the study cohort before dosing commences.
Dystrophy, Cornea
Pathogenesis/cell of origin: Corneal epithelium and associated basement membrane and stroma.
Differential Diagnosis: Corneal dysplasia
Diagnostic Features: Thickened, elevated epithelium interspersed with areas of abnormally thin epithelium.
Comments: When precisely used, the term “corneal dystrophy” describes a disease process of mineralization in the cornea (especially the epithelial basement membrane), with the lesions being spontaneously occurring, non-inflammatory, usually involving the central cornea, and are often bilateral and symmetrical266. The Dutch Belted rabbit can exhibit such a corneal dystrophy266, sometimes referred to as “anterior corneal dystrophy”. This is a spontaneous, possibly inherited condition characterized by clinically observed central to peripheral corneal opacities which correspond microscopically to the focal areas of epithelial basement thickening and irregularity266, 267. This true corneal dystrophy is comparable to Thiel-Behnke corneal dystrophy (TBCS) caused by defects in transforming growth factor beta (TGFβ) in humans267.
However, most reports of rabbit “corneal dystrophy” do not describe such true corneal dystrophy, because in rabbits, the term “corneal dystrophy” has been used to describe several apparently unrelated non-inflammatory conditions of uncertain etiology, which are characterized by various changes in the corneal epithelium, stroma, and/or endothelium.
For example, a morphologically different condition termed “corneal epithelial dystrophy” has been described in two 4-month-old NZW rabbits268. The affected animals had clinically observed unilateral circumferential corneal opacities extending from the limbus to the central cornea. Microscopically, the opacities consisted of alternating areas of corneal epithelial thinning and thickening (hyperplasia) with unremarkable basement membranes. It is unclear if this is a true corneal dystrophy or not268.
Another apparently distinct condition termed “pre-Descemet’s membrane corneal dystrophy” has been described in adult NZW rabbits269. Microscopically, focal peripheral to central accumulations of ectopic corneal endothelial cells were present subjacent to Descemet’s membrane, and corresponded to clinically observed corneal opacities. By electron microscopy these cells were found to be secreting matrix material based on the cells’ intracytoplasmic content, presence of a dense and homogenous material associated with the outer cell membrane, location of these cells close to Descemet’s membrane, and the cells’ linear organization. The change was assumed to be present at birth because it was seen in 2-week-old rabbits. The nomenclature of dystrophy for this lesion reflects the nomenclature of a similar lesion in humans. No mineralization has been reported with this lesion in rabbits.
Vacuolation, Lipid, Cornea
Other terms: corneal lipidosis; lipid keratopathy
Pathogenesis/cell of origin: lipid-laden keratocytes, foamy macrophages, sterol clefts, and/or multinucleated giant cells in the corneal stroma.
Differential Diagnosis: vacuolation, mucopolysaccharides; vacuolation, NOS; edema.
Diagnostic Features: Microscopic changes in the cornea are primarily in the stroma (especially anteriorly), and include lipid-containing keratocytes and foamy macrophages, sterol clefts, and/or multinucleated giant cells, often associated with secondary neovascularizaton and inflammatory cell infiltrates270,271,272,273. Similar lesions have also been described in the nictitating membrane273.
Comments: Corneal lipid vacuolation has been described in various rabbit breeds with high serum cholesterol levels due to familial predisposition (in Watanabe heritable hyperlipidemic [WHHL] rabbits 270 or resulting from high dietary cholesterol in various other rabbit breeds271, 273. In one study of “New Zealand” rabbits fed high-cholesterol diets, the typical corneal stromal changes were present but other lesions were also observed: lipid accumulation in corneal epithelium and endothelium, as well as lipid-laden macrophages in the iris stroma, ciliary body, ciliary processes, and choroid, and increased lipid staining in the retina271. Other studies with rabbits fed high-cholesterol diets have also demonstrated light and transmission electron microscopic effects in other regions of the globe including: lipid-laden macrophages in the choroid and suprachoroid; lipid accumulation in the retinal pigment epithelium (RPE) and astrocytes; Bruch’s membrane thickening; RPE hypertrophy; Müller cell and astrocyte activation; and degeneration and/or necrosis of choroidal vessel endothelium and retinal neurons274,275,276,277. Instillation of cationic amphiphilic drugs in juvenile white rabbits induce corneal phospholipidosis278.
Mineralization
Comments: Albino and pigmented rabbits can exhibit “calcific” or “band keratopathy”-like subepithelial and stromal mineralization following corneal injury from various causes220, 279,280,281. Hypervitaminosis D can also result in corneal mineralization, but in rabbits seems to occur only in eyes compromised by concurrent ocular inflammation282, 283.
E. Anatomy of the Anterior Chamber and Aqueous Humor
Many features of the rabbit uvea (iris, ciliary body, and choroid), aqueous filtration system, and anterior chamber are related to the low accommodative ability. The rabbit anterior chamber is large, being 2.3 - fold larger than that of the cynomolgus macaque even though the eyes of both species are of similar size284 and having a slightly greater diameter than the much larger human eye285. Compared to humans, the rabbit anterior chamber is shallower and more curved due to the displacement of the iris anteriorly by the large lens220, 285. Yüksel et al.256 reported that mean anterior chamber depth of young “New Zealand” rabbits is 2.08 +/– 0.16 mm, which is very similar to the 2.161 +/– 0.11 mm noted by Werner et al.285 in male NZW rabbits (Table 58).
Table 58. Microscopic Findings of the Anterior Chamber and Aqueous Humor: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Proteinaceous fluid | X | ||||
| Inflammation | X | ||||
| Proliferative Non-neoplastic and Neoplastic | |||||
| - | |||||
F. Anatomy of the Filtration Angle
The rabbit iridocorneal (filtration) angle is relatively large and deep, partly because of the small ciliary muscle. Rabbits have multiple slit-like venous collector channels known as the angular aqueous plexus, rather than a singular canal of Schlemm233, 286,287,288,289,290,291 (Table 59).
Table 59. Microscopic Findings of the Filtration Angle: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Malformation, filtration angle * | X | ||||
| Non-proliferative | |||||
| Narrowed filtration angle * | X | ||||
| Single cell necrosis | X | ||||
| Proliferative Non-neoplastic | |||||
| Proliferation, trabecular meshwork | X | ||||
* Terminology with diagnostic criteria or comments described in the text.
Malformation, Filtration Angle
Other terms: Buphthalmos; buphthalmia
Comments: A type of developmental glaucoma, inherited as an autosomal recessive trait with incomplete penetrance, has long been recognized in rabbits24, 292,293,294,295,296,297,298. This hereditary glaucoma has been most commonly recognized in NZW albino rabbits295, 297, 298, but can also occur in other albino strains such as AXBU/J296 and in “pigmented” rabbits293. The fundamental phenotypic defect is incomplete and/or abnormal development of iridocorneal angle structures (i.e., goniodysgenesis), resulting in impaired drainage of aqueous humor from the eye295. Clinical signs generally become evident early in life, around 2–3 months of age or even earlier, and include elevated intraocular pressure (IOP), corneal edema, increased corneal diameter, and eventually grossly detectable enlargement and excessive protrusion of the globe243, 292,293,294,295, 297, 298. Most cases are bilateral, but unilateral involvement has been reported294, 295, 297. Microscopically, aqueous outflow structural abnormalities include a narrowed, truncated, or absent ciliary cleft, shrunken or compressed trabecular meshwork, absent or poorly developed pectinate ligaments, and posterior displacement of the angular aqueous plexus292, 293, 295,296,297,298,299. The ciliary body can also be hypoplastic. Associated changes can include pathologic optic nerve head cupping, optic nerve atrophy, and retinal changes ranging from decreased or degenerate ganglion cells to extensive full thickness retinal atrophy292, 294.
Narrowed Filtration Angle
Comments: Experimentally induced glaucoma in rabbits has been studied as an animal model of human disease220, 292, 300. Glaucomatous changes can also be secondary to ocular inflammation, trauma, and other causes248, and would therefore be considered acquired and having a normally formed, but possibly obstructed, filtration angle.
G. Anatomy of the Uvea (Iris, Ciliary Body and Choroid)
Many features of the rabbit uvea (iris, ciliary body, and choroid), are related to the low accommodative ability. The ciliary body is divided into the anterior pars plicata (ciliary processes and ciliary muscle) and the posterior pars plana. In keeping with the low accommodative ability, the rabbit ciliary body muscle (smooth muscle) is small and poorly developed219, 235, 243, 287, 291. The pars plicata ciliary processes are radially arranged leaf-like folds which arise from the anterior ciliary body and extend along the posterior iris surface. In the rabbit, long and short ciliary processes alternate, with the longer processes often interconnected by lateral, epithelium-covered stromal bridges to each other and to the posterior iris, forming the so-called “ciliary web”, “circular ledge”, or “sims” (Figure 45)220, 235, 301,302,303,304.
Figure 45.
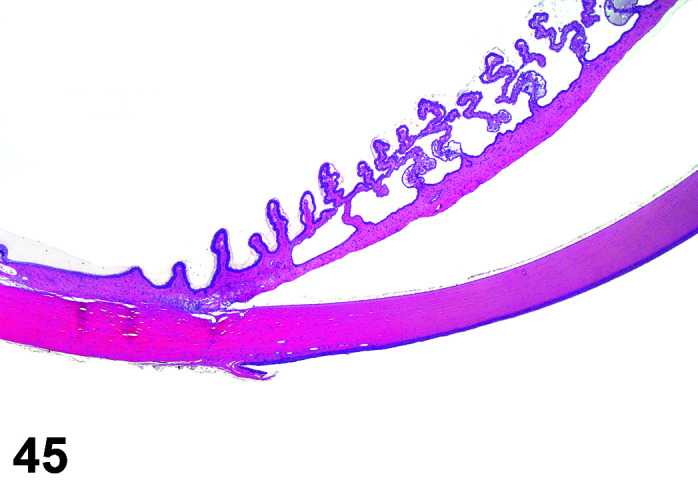
Eye, Ciliary Body, Ciliary Web, H&E.
The pars plana of rabbits is relatively narrow, so the lens and pars plana are in closer proximity in the rabbit compared to the cynomolgus macaque284, 304, 305. The junction of the pars plana and the peripheral retina in rabbits and many non-primate species is unindented and smooth, and thus more appropriately referred to as the ora ciliaris retinae rather than as the ora serrata. The ora ciliaris retinae of rabbits is situated relatively more anteriorly than is the ora serrata of the cynomolgus macaque284.
The rabbit choroid exhibits increased thickness in a horizontal nasotemporal band inferior to the optic nerve head (along the retinal visual streak; see Retina section below)217, 220. A choroidal tapetum lucidum is not present in rabbits243 (Table 60).
Table 60. Microscopic Findings of the Uvea (Iris, Ciliary Body and Choroid): Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Adhesion, iris | X | ||||
| Hypoplasia, choroid | X | ||||
| Hypoplasia, ciliary body | X | ||||
| Malformation, iris | X | ||||
| Persistent pupillary membrane | X | ||||
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Congestion | X | ||||
| Edema | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous * | X | ||||
| Neovascularization | X | ||||
| Pigment, increased/decreased, iris | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, lipid * | X | ||||
| Vacuolation, cytoplasm, epithelium | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, melanocyte | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma, ciliary body, iris | X | ||||
| Leiomyoma, uvea | X | ||||
| Melanoma, uvea, benign | X | ||||
| Schwannoma, intraocular/optic nerve, benign | X | ||||
| Melanoma, uvea, malignant | X | ||||
| Adenocarcinoma, ciliary body, iris | X | ||||
| Meningioma, malignant, optic nerve | X | ||||
| Schwannoma, intraocular/optic nerve, malignant | X | ||||
* indicates terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Metaplasia, Osseous
Comments: Ciliary body osseous metaplasia consists of small, irregular, non-mineralized osteoid masses in the ciliary body of otherwise unremarkable eyes. This has been noted as a rare incidental change in NZW rabbits306; whether it also occurs in other strains is unknown. Although the etiology is unknown, these may be minor developmental anomalies, similar to the uveal “heterotopic ossification” in other species such as guinea pigs and dogs307,308,309. Scleral osseous metaplasia was also reported as a reaction to intraocular osteoinductive hydroxyapatite and polyethylene polymer implants in experimentally manipulated (eviscerated) eyes of NZW rabbits310.
Vacuolation, Lipid
Comments: Accumulations of foam cells, extracellular lipid and occasional cholesterol clefts have been found in the iris and ciliary body of the eyes in about 50% of NZW rabbits fed a cholesterol-rich diet over three months28. Similar ocular lesions were described for WHHL rabbits311.
H. Anatomy of the Lens
The rabbit lens is large compared to that of haplorhine primates219, 234. The rabbit lens is 3.9- fold larger than that of cynomolgus macaques, even though the eyes of both species are of similar size284. The rabbit lens is also larger and thicker than the human lens, even though the human eye is overall much larger217, 220. NZW rabbits have mean lens thickness and diameter of 6.36 +/– 0.13 mm and 10.47 +/– 0.31 mm, respectively, while humans have mean lens thickness and diameter of 4.24 +/– 0.46 mm and 9.58 +/– 0.27 mm, respectively285. Rabbits are distinctive in having linear shaped sutures, with the anterior and posterior sutures oriented vertically and horizontally, respectively219, 312 (Table 61).
Table 61. Microscopic Findings of the Lens: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Degeneration, lens fiber * | X | ||||
| Dislocation, lens, anterior or posterior | X | ||||
| Fibroplasia, lens epithelium | X | ||||
| Hypertrophy, lens capsule/epithelium/fiber | X | ||||
| Inflammation | X | ||||
| Mineralization, lens fiber | X | ||||
| Necrosis, lens epithelium | X | ||||
| Parasite * | X | ||||
| Rupture, lens capsule | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, lens epithelium/fiber | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, lens epithelium | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Degeneration, Lens Fiber
Figure 46.
Eye, Degeneration lens fibre, H&E.
Comments: Lens fiber degeneration is a microscopic finding that is usually correlated with ophthalmic examination findings of lens opacity. Spontaneous lens opacities can occur at different ages in rabbits and can exhibit breed or strain specificity24, 313,314,315,316,317. Congenital lenticular opacities of uncertain etiology have been reported in neonates, but most spontaneous cataracts occur in adult or even aged animals222. In a study involving rabbits ranging in age from about 2.7 to 9.6 months, Munger et al. reported lens opacity incidences of 5.7% in albino NZW and 1.1% in pigmented NZW × NZ Red F1 hybrids314. In two NZW inbred strains (EII/JC and EIIJC-HLA-A2), spontaneous lens opacities had an overall much later onset, being noted only in animals 37 months or older315. Nuclear sclerosis (increased compaction of inner cortical lens fibers) was noted only in older animals (average age 6.0 +/– 2.9 years in one series222 and 3.5–4.0 years in another series)318. Lens fiber degeneration and more extensive lesions can develop as sequelae of many non-heritable spontaneous or experimentally induced conditions that secondarily affect the lens (i.e., diabetes, glaucoma, posterior synechia, and proliferative vitreoretinopathy)24, 248, 300, 319,320,321.
Many cases of spontaneous lenticular opacities in rabbits are suspected to be of heritable (genetic) origin314, 315. A recent study has demonstrated that experimentally induced mutations of the α-crystallin gene result in heritable opacities in knockout rabbits and their offspring322.
Experimentally induced lens fiber degeneration in rabbits occurs with administration of various chemical test articles; exposure to microwaves, UVA radiation, or electric current; hyperoxia; and dietary imbalances220, 294, 320, 321, 323,324,325. Lens fiber degeneration in rabbits in toxicity studies can also result from extraneous physical trauma to the lens (i.e., from misplaced hypodermic needles during intravitreal injections or from cage accidents or other misadventure).
Parasite
Comments: The common microsporidian parasite Encephalitozoon cuniculi can infect many tissues of rabbits, including the eye. Ocular infections can result in multiple pathologic changes including corneal ulceration, edema, and epithelial and endothelial necrosis; iris and ciliary body edema and epithelial degeneration; retinal atrophy; and generalized inflammatory cell infiltrates326, 327. In the lens, infections can also result in degeneration of the lens, which in turn sometimes ruptures, resulting in secondary uveal inflammation (phacoclastic uveitis)85, 222, 326, 328,329,330,331. The route of infection for the lens is unclear, though transplacental vertical transmission has been proposed328, 331.
I. Anatomy of the Vitreous
The vitreous cavity of the rabbit eye is relatively small, with a vitreous-to-globe area ratio of 0.4 compared to a 0.7 ratio in the similarly sized eye of the cynomolgus macaque284. In rabbits, strong vitreal attachment occurs along the retinal medullary rays332.
The collagen fibrils of the rabbit vitreous are condensed into funnel-like lamellae which are more prominent and uniform than those in humans. The lamellae generally extend antero-posteriorly from the vitreous base to the optic nerve head333. Cloquet’s canal, a conduit for hyaloid vessels during fetal development, is retained consistently in adult rabbits333, even though most authors agree that this structure does not routinely persist in adult human eyes334 (Table 62).
Table 62. Microscopic Findings of the Vitreous: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia, vitreous | X | ||||
| Persistent hyperplastic primary vitreous | X | ||||
| Persistent hyaloid vessels | X | ||||
| Non-proliferative | |||||
| Fibroplasia | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, bone or cartilage | X | ||||
| Mineralization, vitreous | X | ||||
| Pigment, macrophage, hemosiderin | X | ||||
| Proliferative Non-neoplastic and Neoplastic | |||||
| - | |||||
J. Anatomy of the Retina
Further maturation of the retina continues post eyelid opening for several weeks more or even to adulthood215, 216, 335, 336. Regression of ocular fetal vessels (hyaloid vessels and vasculosa lentis) has been reported to be complete by about post-natal days 14–20337, 338, but persistence of embryonic vessel remnants is common in rabbits339,340,341.
The rabbit retina exhibits many anatomical and histologic differences compared to that of humans and other primates and non-primate mammalian species. The medullary rays are easily visible ophthalmoscopically as two broad, pale, wing-like bands that emanate from the optic nerve head and extend horizontally across the temporal and nasal fundus to just posterior to the equator219, 220, 222. Histologically, the medullary rays consist of myelinated nerve fibers (axons of the retinal ganglion cells)336, 342,343,344,345,346,347. In typical sagittal histologic sections, the medullary rays appear as focal elevations of the inner retinal surface due to the collectively increased thickness of their myelinated nerve fibers. Unlike most species, the rabbit retina has oligodendrocytes, which are localized in the medullary rays343, 344. Rabbit retinal astrocytes are confined to the medullary rays, especially the nerve fiber and ganglion cell layers, with some astrocytes closely associated with the blood vessels overlying the medullary rays345, 347.
Although not visible ophthalmoscopically, the visual streak is well developed in the rabbit220, 335, 343, 347,348,349,350,351,352. Histologically, in typical sagittal sections, the rabbit visual streak is a discrete nasotemporal linear zone of increased ganglion cell density, located inferior to the optic nerve head and medullary rays220, 348, 350,351,352,353.
The area centralis is a specialized focus of increased ganglion cell density distinct from the linear visual streak which is present in the retina of many mammals335, 354. Whether the rabbit possesses an area centralis is a subject of some controversy, with some authors348, 351, 353, but not others217, 350 reporting its presence.
Rabbits are presumed to have dichromatic color vision355, 356. Density gradients are present in the rabbit retina, with the “blue” S-cones concentrated in small zones in the inferior retina335, 355, 356. Dual-opsin cones (which co-express both “blue” and “green” opsins) have been demonstrated in rabbits357. The intrinsically photosensitive retinal ganglion cells (ipRGC) are a recently discovered third class of nonciliary retinal photoreceptor358. The recent identification of melanopsin gene expression in the rabbit retina suggests the possibility that iprGC may also occur in this species359.
Compared to many other species, the Müller macroglial cells of the rabbit retina are especially prominent220, 360, with the entire transretinal span of individual Müller cells and their processes often easily traceable on routine H&E sections. The Müller cells are distributed throughout the rabbit retina, including the medullary rays215, 345, 347, 361.
Bruch’s membrane is a laminated extracellular matrix located between the retinal pigmented epithelium (RPE) and the adjacent choriocapillaris and is composed of the RPE and choriocapillaris capillary basement membranes and associated collagen and elastin layers362, 363. Bruch’s membrane in rabbits is thinner than that of humans364. The RPE contain melanosomes, which lack melanin granules in albino rabbits such as the NZW strain. RPE melanin granules tend to diminish in number (due to fusion) and accumulate lipofuscin as aging-related changes. The Dutch Belted rabbit and NZW F1 cross rabbits offer the ability to test potential effects of xenobiotics on the eye of pigmented rabbits.
Retinal and choroidal arterial supply is dual and variable among rabbits, arising from the external carotid artery as it feeds the external ophthalmic artery or from the internal carotid artery as it feeds the internal ophthalmic artery365. The rabbit retina is partially vascularized (merangiotic vascularization), which is unique among the domestic and common laboratory species342, 366, 367. The central retinal artery enters the optic nerve and then passes into the globe, where it divides into nasal and temporal arteries that extend horizontally across the fundus (parallel to and overlying the medullary rays; other regions of the retina are avascular)342, 366, 367.
In rabbits, the exit point of the optic nerve from the globe is superior to the antero-posterior pole axis and horizontal-most meridian plane of the globe219, 234, 333, 360, 367, 368. Thus, the rabbit optic nerve head is located in the superior rather than central fundus.
The rabbit optic nerve head is horizontally oval and normally deeply indented (physiologic cupping), which is appreciable both ophthalmoscopically and in histologic preparations217, 219, 220, 234, 368. In many species such as humans, macaques, horses, and pigs, the scleral exit channel at the optic nerve head is multi-fenestrated due to a well-developed lamina cribrosa, a sieve-like network of collagenous plates366, 369,370,371. Rabbits have a poorly developed lamina cribrosa, and the optic nerve head contains neuronal tissue and astrocytes in addition to oligodendroglia219, 234, 360, 366, 368, 369. This connective tissue meshwork also contains elastin and lends support for the nerve tissue.
In rabbits, a central retinal artery enters the optic nerve ventrally, just posterior to the optic nerve head219, 366, 368. Main veins from the two medullary rays also exit via the optic nerve372 (Table 63).
Table 63. Microscopic Findings of the Retina: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Arteriolar loop, pre-retinal | X | ||||
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Atrophy, retinal, global/inner/outer | X | ||||
| Congestion | X | ||||
| Deposits, extracellular matrix, subretinal * | X | ||||
| Detachment, retina | X | ||||
| Displacement, photoreceptor nuclei | X | ||||
| Edema | X | ||||
| Eosinophilic bodies, retina * | X | ||||
| Fibroplasia, retinal, subretinal or epiretinal | X | ||||
| Folds, retina | X | ||||
| Glial cells, increased | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, retinal pigment epithelium (RPE) * | X | ||||
| Inclusions (intracytoplasmic accumulation), RPE | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Mineralization | X | ||||
| Myelin, increased | X | ||||
| Necrosis | X | ||||
| Necrosis, single cell | X | ||||
| Neovascularization | X | ||||
| Pigment, increased | X | ||||
| Pigment, decreased | X | ||||
| Polarity loss, RPE | X | ||||
| Retinal rosettes | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, cytoplasmic/extracellular | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, retinal pigmented epithelium | X | ||||
| Proliferative Neoplastic | |||||
| - | |||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Deposits, Extracellular Matrix, Subretinal
Pathogenesis/cell of origin: RPE
Diagnostic Features: Discrete, focal, rounded eosinophilic deposits located between the RPE and Bruch’s membrane, sometimes resulting in a focal “dome”-like inward elevation of the RPE.
Comments: These deposits between the RPE and Bruch’s membrane are not common but can occur as spontaneous findings in otherwise unremarkable rabbit eyes373, and morphologically resemble drusen though they have not been biochemically characterized in rabbits.
Eosinophilic Bodies, Retina
Figure 47.
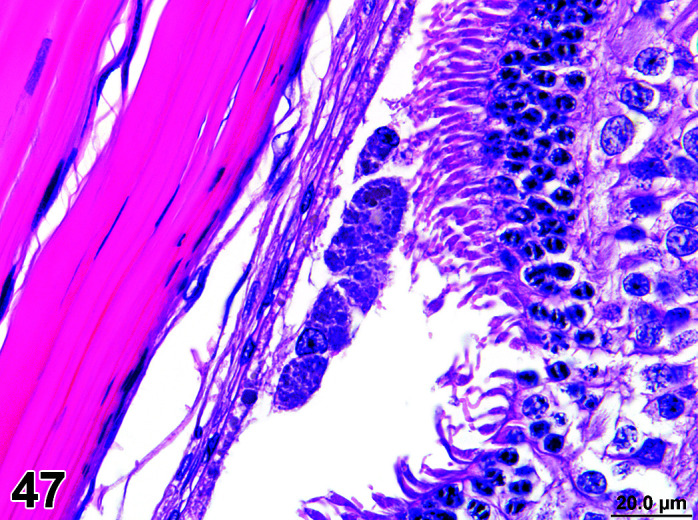
Eye, Retina pigment epithelium, hypertrophy H&E.
Pathogenesis/cell of origin: retinal neurons (horizontal cells)
Diagnostic Features: Occasional, round to oval, pale pink, circular structures with indistinct outlines, located in the inner nuclear layer (INL) and outer plexiform layer (OPL).
Comments: The pale eosinophilic round structures in the rabbit retina termed “eosinophilic bodies” have been demonstrated by immunohistochemistry and transmission electron microscopy to be neurofilament accumulations in horizontal cell neurites (dendrites) 374. Affected retinas were otherwise histologically unremarkable. The retinal eosinophilic bodies occur in several rabbit breeds including NZW, Japanese White, and Dutch Belted, with higher incidences in older animals, suggesting that they are an aging-related change.
Hypertrophy, Retinal Pigment Epithelium (RPE)
Figure 48.
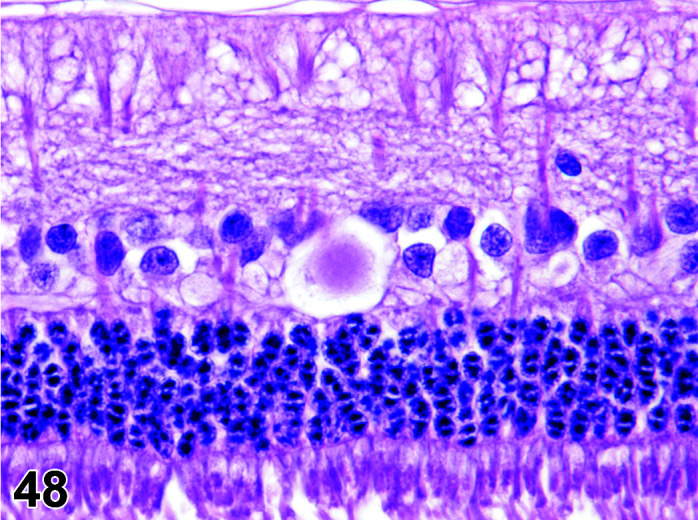
Eye, Eosinophilic bodies, retina, H&E.
Diagnostic Features: Small foci of enlarged and often displaced RPE in the subretinal space. The RPE aggregates are frequently located near the optic nerve head (peripapillary) or at the peripheral margins of the retina but can also be found less frequently in other retinal regions. The swollen RPE cells have abundant pale basophilic, granular cytoplasm, which often has a brownish tinge attributed to lipofuscin373. Pigment granules for pigmented breeds are concentrated around these vacuoles375.
Comments: Young adult rabbits sometimes exhibit this spontaneous RPE change unassociated with any concurrent ocular pathology373. The exact etiology is undetermined. This spontaneous RPE hypertrophy was first recognized in a pigmented rabbit breed (Dutch Belted) but can also occur in other pigmented breeds (e.g. New Zealand Red) as well as in albino breeds (Table 64).
Table 64. Microscopic Findings of the Optic Nerve: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Atrophy | X | ||||
| Degeneration, axon | X | ||||
| Demyelination | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Necrosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation | X | ||||
| Proliferative Non-neoplastic | |||||
| Glial cells, increased number | X | ||||
| Proliferative Neoplastic | |||||
| Glioma, benign | X | ||||
| Meningioma, benign | X | ||||
| Meningioma, malignant | X | ||||
| Schwannoma, benign | X | ||||
| Schwannoma, malignant | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
K. Anatomy of the Sclera
The rabbit sclera is thicker at the limbus (0.5 mm) and thinner at the posterior pole (approximately 0.18–0.2 mm) 219, 234. In comparison, human sclera thickness is similar at the limbus (approximately 0.5–0.53 mm), but much greater at the posterior pole and near the optic nerve (0.86 mm–1.0 mm)376, 377.
In rabbits, a large orbital venous sinus (also called the orbital “venous plexus”) is located posterior to the globe and receives the venous drainage from the eye and orbital contents219, 220, 233, 378, 379.
Arteries supplying the rabbit eye (such as the long and short posterior ciliary arteries) generally enter the sclera in the posterior globe near the optic nerve, while the central retinal artery enters within the optic nerve itself233, 367, 378 (Table 65).
Table 65. Microscopic Findings of the Sclera: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia, osseous * | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic and Neoplastic | |||||
| - | |||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Metaplasia Osseous
Comments: Scleral osseous metaplasia has been reported as a reaction to intraocular osteoinductive hydroxyapatite and polyethylene polymer implants in experimentally manipulated (eviscerated) eyes of NZW rabbits310.
L. Anatomy of the Ocular Adnexa - Glands and Ducts
The gross anatomical terminology of the rabbit ocular adnexal glands has been the subject of some confusion in the scientific literature, with many different, often inconsistent, and even contradictory nomenclature schemes promulgated by various authors. Thus, caution is warranted when reviewing published terminology schemes and descriptions for these structures.
The rabbit zygomatic salivary gland is located along the zygomatic arch near the inferior portion of the lacrimal gland234, 380, 381. Portions of the zygomatic salivary are sometimes sampled along with the adjacent lacrimal gland, appearing in routine histologic preparations as clusters of deeply basophilic mucous-cell acini with tiny lumens.
The ocular adnexa, especially the Harderian gland and most of the extraocular muscles are closely associated with the large venous sinus (or plexus) present in the posterior orbit of rabbits219, 233, 378.
Harderian Gland
The rabbit Harderian gland is large, located in the medial ventral orbit, and attached to the base of the nictitating membrane219, 220, 234, 382, 383. The rabbit Harderian gland is composed of two closely apposed but grossly and histologically distinct lobes, surrounded by a thin fibrous capsule219, 234, 384. A single excretory duct opens on the concave surface of the base of the nictitating membrane219, 379, 385.
Histologically, the Harderian gland is classified as tubuloacinar (tubuloalveolar). Acinar epithelial cells of both lobes are characterized by abundant cytoplasmic lipid vacuoles, and the secretions of both lobes are predominantly lipid383, 385,386,387,388,389,390.
Based on their grossly visible coloration, the two lobes are known as the “white” lobe and the “pink” (or “red”) lobes219, 383,384,385, 391 (Figure 49). The more inferiorly situated pink lobe (Figure 50) is about twice as large as the more superiorly located white lobe219, 383, 384, 391, 392. In the white lobe (Figure 51), acinar cell lipid vacuoles are small, more uniform and very densely packed, imparting a typically darker, more eosinophilic microscopic appearance in routine H&E sections. In contrast, in the grossly pink lobe acinar cells contain larger, clear vacuoles, and thus appear paler microscopically234, 382,383,384, 390,391,392.
Figure 49.
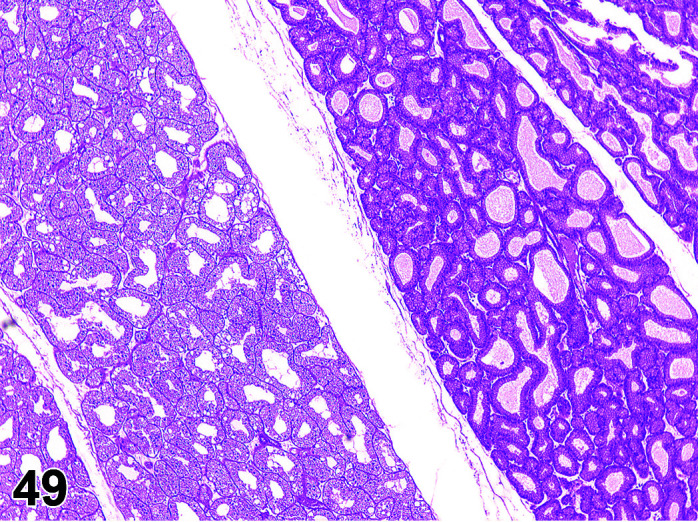
Harderian Gland, pink lobe (left) and white lobe (right), H&E.
Figure 50.
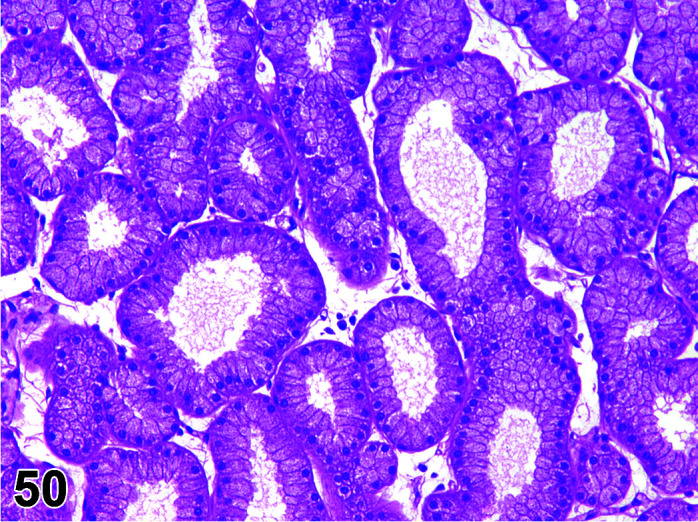
Harderian Gland, white lobe, H&E (high mag).
Figure 51.
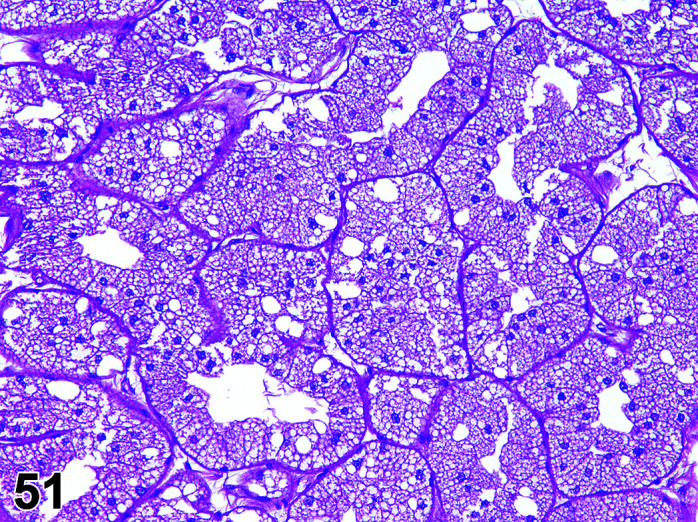
Harderian Gland, pink lobe, H&E (high mag).
Unlike rats, mice, and hamsters, the rabbit Harderian glands does not appear to exhibit histologic sexual dimorphism and/or porphyrin secretion390, 393. However, intact male rabbits have particularly large Harderian glands, which increases further in size during the breeding season.
Lacrimal Glands
The rabbit lacrimal gland is large, long, thin, and multilobulated, with pink to pale red macroscopic coloration217, 220 (Figure 52). Its lobes fill most of the inferior orbit and extend into the posterior superior orbit290. Although most of the lacrimal gland is intraorbital, portions may extend onto the lateral zygomatic arch and even extraorbitally onto the zygomatic bone234, 382. Most authors describe a single excretory duct, which opens into the superior eyelid conjunctiva at the lateral canthus382, 394, 395.
Figure 52.
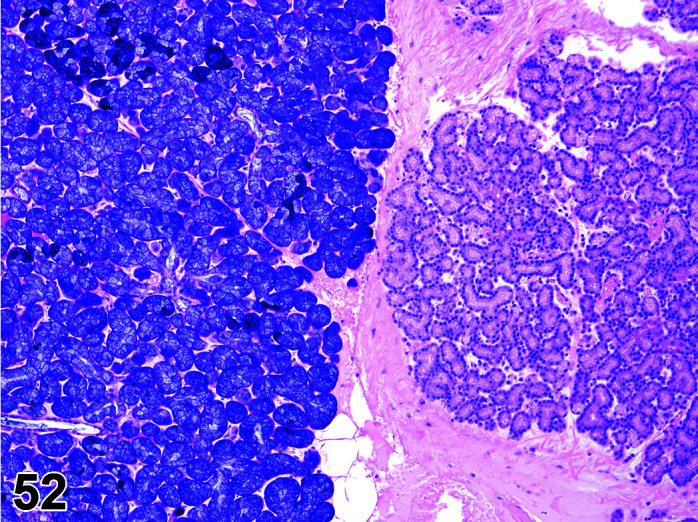
Zygomatic salivary gland (left) and Lacrimal gland (right), H&E.
As in several other species, the rabbit lacrimal gland exhibits a degree of sexual dimorphism, with adult males exhibiting larger acini and greater numbers of acinar cells than females396.
Nictitating Membrane and Nictitans Gland
The rabbit has a well-developed nictitating membrane (third eyelid)217, 219, 382. It consists of a crescentic conjunctival fold reflected from the medial canthus molded to the contours of the globe, and adjacent to the Harderian gland. A central hyaline cartilage plate, embedded in fibrous loose connective tissue, reinforces the nictitating membrane217, 219, 234, 382.
The inner surface of the nictitating membrane is lined by goblet-cell containing conjunctival mucosa, while the more external surface is lined by non-keratinized stratified squamous epithelium (Figure 53).
Figure 53.
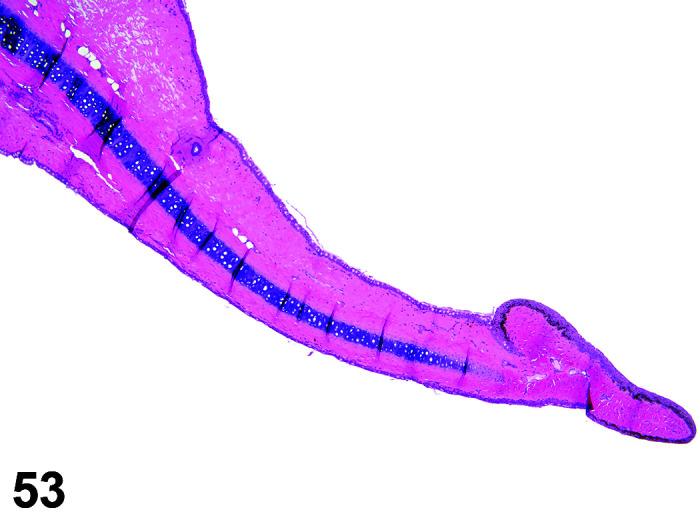
Nictitating membrane, H&E.
The nictitans gland (gland of the third eyelid) is thin and flat, and surrounds most of the length of the central cartilage. It is a modified lacrimal-type gland with serous acini382. Several ducts extend through the cartilage plate to open on the inner surface of the nictitating membrane219, 382, 397 (Table 66).
Table 66. Microscopic Findings of the Harderian, Lacrimal, and Nictitans Glands: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Congestion | X | ||||
| Cyst | X | ||||
| Cytoplasmic alteration, acinar, Harderian gland or lacrimal gland * | X | ||||
| Degeneration | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Karyomegaly | X | ||||
| Necrosis * | X | ||||
| Necrosis, single cell | X | ||||
| Porphyrin increased | X | ||||
| Regeneration | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, acinar | X | ||||
| Neoplastic | |||||
| Adenoma | X | ||||
| Adenocarcinoma | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Cytoplasmic Alteration, Harderian Gland or Lacrimal Gland
Other terms: Metaplasia, Harderization; Ectopic gland.
Comments: Cytoplasmic alteration of the lacrimal gland or of the Harderian gland occur relatively commonly in the rabbit398. In the Harderian gland, it is possible to observe small islands of normal lacrimal gland, while less frequently, islands of normal Harderian gland acini may be observed in the lacrimal gland (Figure 54). The lacrimal gland alteration in the Harderian gland has been reported as early as 3 weeks of age and increases in incidence with age in the lacrimal glands of males and females but occurs with a greater incidence and extent in males. Harderian gland alteration in the lacrimal gland is recognized less commonly and is not well characterized.
Figure 54.
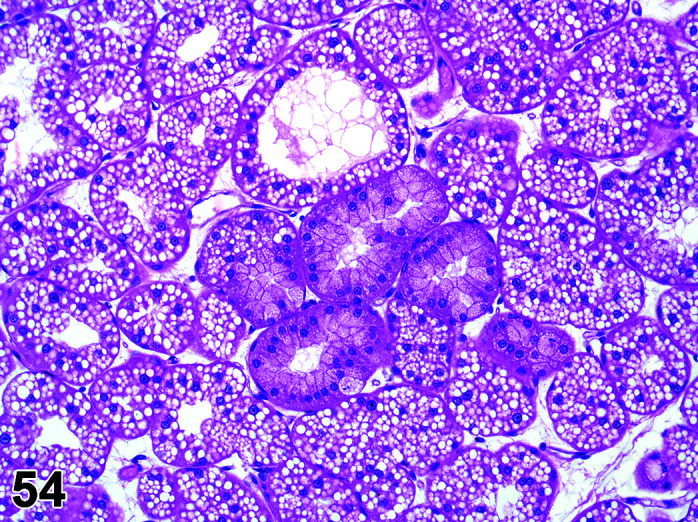
Harderian gland, Cytoplasmic alteration, H&E.
Necrosis, Harderian Gland or Lacrimal Gland
Comments: Necrosis and acute to subacute inflammation in the Harderian and/or lacrimal glands consistent with infarction due to embolism of microthrombi associated with medial auricular artery catheterization has been reported33.
Nasolacrimal Duct
In adult rabbits, the nasolacrimal duct (NDL) has the same three regions as described in other mammals (orbital lacrimal canaliculi and sac, bony NDL canal, and non-bony NDL canal in nasal cavity).
In the rabbit, a single lacrimal punctum is located in the inner surface of the inferior eyelid near the medial canthus about 3 mm from the lid margin219, 222, 382, 399. This opens into a poorly developed lacrimal sac-like dilatation which widens into the nasolacrimal duct222, 399,400,401,402. The nasolacrimal duct terminates just caudal to the mucocutaneous junction of the nares18, 220, 222.
According to most authors, the rabbit nasolacrimal sac and duct are lined by stratified to pseudo-stratified columnar epithelium, which in many areas has a conspicuously bilayered appearance399,400,401,402. Some of the epithelial lining cells secrete mucins399, 402. In rabbits, portions of the nasolacrimal duct are surrounded by a prominent cavernous body with large blood vessels in a loose stroma402 (Table 67).
Table 67. Microscopic Findings of the Nasolacrimal Duct: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Atrophy | X | ||||
| Congestion | X | ||||
| Cyst, inclusion | X | ||||
| Degeneration | X | ||||
| Edema | X | ||||
| Erosion/ulceration | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Necrosis | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, epithelial | X | ||||
| Proliferative Neoplastic | |||||
| Papilloma, squamous cell | X | ||||
| Carcinoma, squamous cell | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
M. Anatomy of the External Ear
The lesions of the convex surface of the external ear (pinna) are largely those of haired skin and are discussed with the Integumentary section. The external ear (pinna) is easily assessed during macroscopic examination. The large veins in the rabbit pinna are often used for blood collection or Test Article administration, and if the latter are sampled for histopathology as the “Injection/Treatment Site”.
The concave external areas of the pinnae have a thin epidermis and dermis with a paucity of hair follicles. In the external ear canal just outside of the bony collar (i.e. at about the level of the obtuse-angle turn), the ear canal retains the thin epidermis but has a circumferential zone containing sebaceous glands (ceruminous glands) but lacking hair follicles. These glands are absent at this site in rodents. Within the internal acoustic meatus, defined by the presence of the bony collar, the dermis is very thin to non-existent with the epidermis almost lying upon the temporal bone. Rabbits have multiple small ceruminous glands (simple sebaceous glands that secrete cerumen, or “ear wax”) (Table 68, 69, 70
Table 69. Microscopic Findings of the Middle Ear: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-Proliferative | |||||
| Apoptosis ǂ | X | ||||
| Atrophy, bone | X | ||||
| Cholesteatoma | X | ||||
| Congestion | X | ||||
| Cyst | X | ||||
| Edema | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, granulomatous/catarrhal | X | ||||
| Metaplasia, squamous cell | X | ||||
| Mineralization | X | ||||
| Necrosis, bone | X | ||||
| Necrosis, tympanic membrane | X | ||||
| New bone formation | X | ||||
| Perforation, tympanic membrane | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, granulation | X | ||||
| Ulcer | X | ||||
| Proliferative Non-neoplastic | |||||
| Fibrosis | X | ||||
| Proliferative Neoplastic | |||||
| - | |||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Table 70. Microscopic Findings of the Inner Ear: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Cellularity decreased, spiral ganglion/spiral limbus/spiral ligament, and/or stria vascularis | X | ||||
| Congestion | X | ||||
| Degeneration, axon/hair cells and/or epithelium | X | ||||
| Edema | X | ||||
| Erosion/ulceration | X | ||||
| Fibrosis | X | ||||
| Hair cell, decreased number | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, inner ear | X | ||||
| Loss, disorganization or disruption, otolith | X | ||||
| Metaplasia | X | ||||
| Necrosis cartilage/hair cell | X | ||||
| Necrosis neuronal/vestibular organ | X | ||||
| New bone formation | X | ||||
| Otolith loss, disorganization or disruption | X | ||||
| Single cell necrosis ǂ | X | ||||
| Vacuolation, hair cell/supporting cell | X | ||||
| Vacuolation, stria vascularis | X | ||||
| Proliferative Non-neoplastic and Neoplastic | |||||
| - | |||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
).
Table 68. Microscopic Findings of the External Ear: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Apoptosis ǂ | X | ||||
| Congestion | X | ||||
| Cyst. tympanic membrane | X | ||||
| Debris, external ear canal | X | ||||
| Edema | X | ||||
| Erosion/ulceration | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, auricular cartilage | X | ||||
| Inflammation, external ear canal | X | ||||
| Metaplasia | X | ||||
| Perforation, tympanic membrane | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative (non-neoplastic) | |||||
| Hyperplasia, epithelium | X | ||||
| Neoplastic | |||||
| Papilloma, squamous cell | X | ||||
| Carcinoma, squamous cell | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Section 17: Urinary System
Calcium absorption and metabolism in the rabbit is poorly understood. Rabbits normally have a higher blood calcium range than other laboratory animal species and are predisposed to cystic, urethral, ureteral and renal calculi. Rabbits excrete 45–60% excess calcium though the urine as calcium carbonate. Mineralized foci are commonly seen throughout the urinary tract. Urine is normally alkaline, and cloudy to pigmented, caused by the presence of albumin, fine calcium carbonate and ammonium magnesium phosphate crystals. The fact that rabbits are horizontal quadrupeds may also predispose the anterior wall to retention of microcrystals and other particles as compared to humans. Rabbit urine varies in color from creamy yellow to dark red depending on the presence of porphyrin pigments derived from the diet or xenobiotics e.g. antibiotics. Care must be taken to differentiate between red urine caused by porphyrin excretion and hematuria. While modern laboratory animal management practices have limited the incidence of infectious processes in the kidney, inflammatory conditions related to infectious disease may still occur (Table 71).
Table 71. Microscopic Findings of the Kidney: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia | X | ||||
| Renal dysplasia | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Accumulation, glycogen | X | ||||
| Accumulation, hyaline droplets | X | ||||
| Accumulation, adipocytes, interstitium | X | ||||
| Amyloid, glomerulus/interstitium | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Atrophy, glomerulus/tubule | X | ||||
| Basophilia, tubule | X | ||||
| Basophilic granules | X | ||||
| Calculus | X | ||||
| Cast (+ modifier) | X | ||||
| Chronic progressive nephropathy | X | ||||
| Crystals | X | ||||
| Cyst * | X | ||||
| Degeneration, tubule | X | ||||
| Degeneration/regeneration | X | ||||
| Dilatation, Bowman’s space/tubule | X | ||||
| Edema, interstitium | X | ||||
| Erosion | X | ||||
| Extramedullary hematopoiesis | X | ||||
| Fibrosis, interstitium | X | ||||
| Glomerulonephritis | X | ||||
| Glomerulopathy, hyaline/mesangioproliferative | X | ||||
| Glomerulopathy * | X | ||||
| Glomerulosclerosis | X | ||||
| Granuloma, Foreign material | X | ||||
| Hemorrhage | X | ||||
| Hyperplasia/metaplasia, Bowman’s capsule | X | ||||
| Hypertrophy, tubule | X | ||||
| Immature glomerulus | X | ||||
| Inclusion bodies | X | ||||
| Infarct | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation, interstitium | X | ||||
| Interstitial nephritis | X | ||||
| Karyomegaly | X | ||||
| Mesangiolysis | X | ||||
| Metaplasia, osseous/squamous cell | X | ||||
| Microabscess | X | ||||
| Mineralization * (+ locator) | X | ||||
| Multinucleated giant cells | X | ||||
| Necrosis, (+ modifier, locator) | X | ||||
| Nephropathy, obstructive/retrograde | X | ||||
| Nephropathy, alpha2u-globulin | X | ||||
| Nephropathy, spontaneous * | X | ||||
| Parasite | X | ||||
| Pigment | X | ||||
| Pyelonephritis | X | ||||
| Regeneration, tubule | X | ||||
| Single cell necrosis ǂ | X | ||||
| Tissue, ectopic, adrenal | X | ||||
| Vacuolation *, tubular | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, oncocyte | X | ||||
| Hyperplasia, mesangium | X | ||||
| Hyperplasia, juxtaglomerular | X | ||||
| Hyperplasia, tubule | X | ||||
| Hyperplasia, urothelium | X | ||||
| Nephroblastematosis | X | ||||
| Proliferative Neoplastic | |||||
| Adenoma | X | ||||
| Nephroblastoma | X | ||||
| Oncocytoma | X | ||||
| Papilloma | X | ||||
| Renal mesenchymal tumor | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma | X | ||||
| Carcinoma, squamous cell/urothelium | X | ||||
| Sarcoma, renal | X | ||||
* Terminology with diagnostic criteria or comments described in the text. ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Cyst
Comments: Renal lesions resembling human polycystic kidney disease were reported in a retrospective evaluation of NZW rabbit kidney tissue403.
Glomerulopathy
Comments: Rabbits are susceptible to a glomerulopathy induced by corticosteroids404,405,406. The initiating lesion is a glomerulopathy characterized by aneurysmal capillary dilatation with nodular changes of eosinophilia and cellular loss in the glomeruli. Bowman’s space often contains erythrocytes or eosinophilic material. Bowman’s capsule may be necrotic. Ultrastructurally, glomerular capillaries may be occluded with a proteinaceous coagulum, endothelium may be swollen, and podocytes have loss of foot processes. Basement membranes may be thickened or tortuous. Epithelial cells of the glomerular tufts may have hyaline globules, vacuoles or be intensely osmophilic. Renal tubules may have fatty infiltration, hyaline droplets, and cellular necrosis. Tubules contain erythrocytes and protein casts. Clinical pathology parameters may demonstrate increased BUN, glycosuria, albuminuria, and hematuria with clinically red urine. Kidney weights may be increased. It is important to recognize the susceptibility of rabbits to corticosteroid glomerulopathy as it may be confused with a test article-related effect if animals are given corticosteroids for palliative purposes during a toxicology study.
Mineralization
Figure 55.
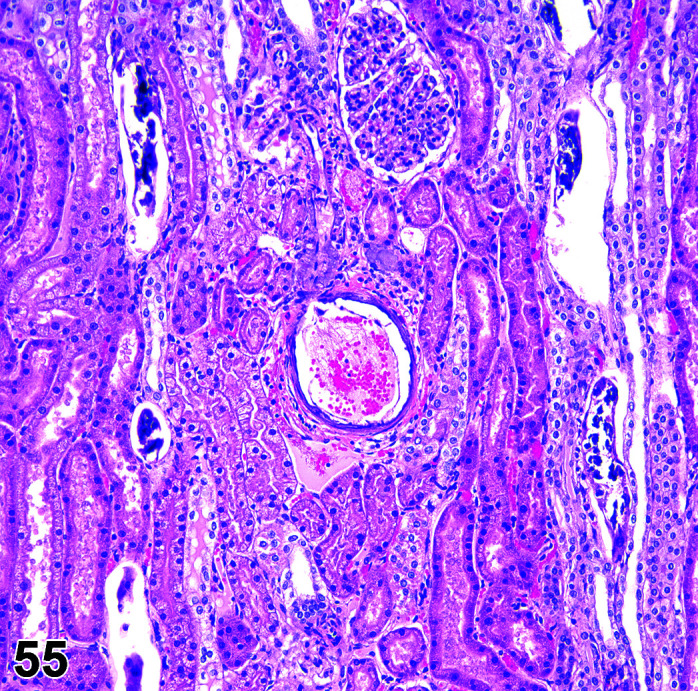
Kidney, Renal mineralisation, H&E.
Comments: Mineralized foci are commonly seen in the collecting ducts and medulla of rabbit kidneys. Mineralized foci in the tubules or interstitial areas of the cortex are present in 60% of male and female rabbits evaluated81, 407. Focal mineralization is also occasionally recorded in the urothelium of the urinary bladder.
Nephropathy, Spontaneous
Pathogenesis/cell of origin: Proximal and distal tubules
Diagnostic Features: The histological findings are generally recorded separately (basophilia tubules, dilated/cystic tubules, pigmented tubules, interstitial inflammatory cell infiltrate), and the term nephropathy is only used when at least three of the aforementioned components are present. A spontaneous nephropathy syndrome is commonly seen in clinically normal apparently healthy rabbits from colonies free from Encephalitozoon cuniculi. There are no clinical signs accompanying these lesions and no evidence of progression/greater severity of the findings on longer term studies i.e. the lesion is not thought to be progressive unlike the nephropathy in rats. It is considered to be a syndrome particular to the NZW rabbit as similar findings have not been recorded in mixed breed pet rabbits. The renal findings are observed in 80% of apparently healthy young rabbits, more frequently in females than males81. Spontaneous findings of mineralization, tubular basophilia and dilatation have been reported previously in young laboratory rabbits less than 1 year of age408, 409, but the incidence of this lesion seems to be increasing in the NZW population. Basophilic tubules, dilated/cystic tubules, pigmented tubules, interstitial inflammatory cell infiltration, and mineralization have been reported in juvenile NZW rabbits as little as 8 weeks old (Bradley, unpublished data). Study pathologists should exercise caution when interpreting kidney findings in apparently healthy rabbits as these renal nephropathy findings may mask nephrotoxic effects.
Vacuolation
Figure 56.
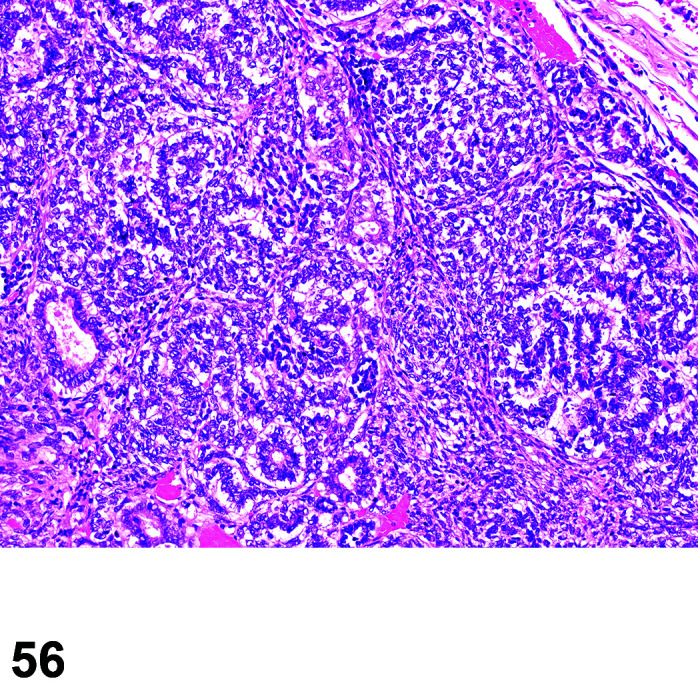
Kidney, Nephroblastoma, H&E.
Comments: Vacuolation of the proximal convoluted tubule epithelium is a regular finding in young non-pregnant females (35%). These vacuoles stain positively with Oil red O stain for neutral lipids. Vacuolation of the proximal convoluted tubule epithelium has been recorded in juvenile NZW rabbits as little as 8 weeks old (Bradley, unpublished data) (Table 72, 73, 74
Table 73. Microscopic Findings of the Urinary Bladder: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Diverticulum | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Angiectasis | X | ||||
| Apoptosis ǂ | X | ||||
| Calculus | X | ||||
| Crystals | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Erosion | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, urothelium | X | ||||
| Inclusions, urothelium | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Mineralization, urothelium | X | ||||
| Necrosis | X | ||||
| Parasite | X | ||||
| Proteinaceous plug | X | ||||
| Single cell necrosis ǂ | X | ||||
| Ulcer | X | ||||
| Uropathy, obstructive | X | ||||
| Vacuolation, urothelium | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, urothelium | X | ||||
| Metaplasia, glandular/squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Proliferative lesion, mesenchymal | X | ||||
| Papilloma, squamous cell/urothelial | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell/urothelial | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Table 74. Microscopic Findings of the Urethra: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Congestion | X | ||||
| Edema | X | ||||
| Erosion | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Hypertrophy, urothelium | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Metaplasia | X | ||||
| Obstruction | X | ||||
| Plug, proteinaceous | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, urothelium | X | ||||
| Metaplasia, glandular/squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Proliferative lesion, mesenchymal | X | ||||
| Papilloma, squamous cell/urothelial | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell/urothelial | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
).
Table 72. Microscopic Findings of Ureter: Rabbit.
| Finding | Common | Uncommon | Not Observed but Potentially Relevant | Not Applicable | |
| Congenital | |||||
| Aplasia, ureter | X | ||||
| Non-proliferative | |||||
| Abscess | X | ||||
| Apoptosis ǂ | X | ||||
| Calculus | X | ||||
| Crystals | X | ||||
| Dilatation | X | ||||
| Edema | X | ||||
| Erosion/ulcer | X | ||||
| Fibrosis | X | ||||
| Hemorrhage | X | ||||
| Infiltrate, inflammatory cell [insert appropriate cell type] | X | ||||
| Inflammation | X | ||||
| Single cell necrosis ǂ | X | ||||
| Proliferative Non-neoplastic | |||||
| Hyperplasia, urothelium | X | ||||
| Metaplasia, glandular/squamous cell | X | ||||
| Proliferative Neoplastic | |||||
| Papilloma, squamous cell/urothelial | X | ||||
| Adenocarcinoma | X | ||||
| Carcinoma, squamous cell/urothelial | X | ||||
ǂ Refer to 4 for diagnostic criteria and use of the terms apoptosis and single cell necrosis.
Ethical Practices: All procedures used to prepare macroscopic and microscopic images of animal specimens for this article were performed in accordance with regulations and established guidelines for humane treatment of research animals and were reviewed and approved in advance by the relevant Institutional Animal Care and Use/Ethics Committee.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
The authors wish to express their thanks to the INHAND GESC, and the BSTP, ESTP, JSTP and STP membership for comprehensive reviews, excellent comments and helpful edits. We also thank Dr Rupert Kellner for manuscript review and Ms Beth Mahler, Ms. Emily Singletary, and Ms. Maureen Puccini from EPL Inc., for image editing. Photographs used in this document were either provided from the coauthors or are as acknowledged in the figure legends.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License <http://creativecommons.org/licenses/by-nc-nd/4.0/>.
References
- 1.Mann PC, Vahle J, Keenan CM, Baker JF, Bradley AE, Goodman DG, Harada T, Herbert R, Kaufmann W, Kellner R, Nolte T, Rittinghausen S, and Tanaka T. International harmonization of toxicologic pathology nomenclature: an overview and review of basic principles. Toxicol Pathol. 40(Suppl): 7S–13S. 2012. [DOI] [PubMed] [Google Scholar]
- 2.Fuentealba IC, Mahoney NT, Shadduck JA, Harvill J, Wicher V, and Wicher K. Hepatic lesions in rabbits infected with Encephalitozoon cuniculi administered per rectum. Vet Pathol. 29: 536–540. 1992. [DOI] [PubMed] [Google Scholar]
- 3.Wasson K, and Peper RL. Mammalian microsporidiosis. Vet Pathol. 37: 113–128. 2000. [DOI] [PubMed] [Google Scholar]
- 4.Elmore SA, Dixon D, Hailey JR, Harada T, Herbert RA, Maronpot RR, Nolte T, Rehg JE, Rittinghausen S, Rosol TJ, Satoh H, Vidal JD, Willard-Mack CL, and Creasy DM. Recommendations from the INHAND Apoptosis/Necrosis Working Group. Toxicol Pathol. 44: 173–188. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr BJ, Ochoa L, Rankin D, and Owens BD. Biologic response to orthopedic sutures: a histologic study in a rabbit model. Orthopedics. 32: 828. 2009. [DOI] [PubMed] [Google Scholar]
- 6.International Organization for S. 10993: Biological evaluation of medical devices —Part 6 Tests for local effects after implantation. Switzerland. 2016.
- 7.Hosoyama T, Ishiguro N, Yamanouchi K, and Nishihara M. Degenerative muscle fiber accelerates adipogenesis of intramuscular cells via RhoA signaling pathway. Differentiation. 77: 350–359. 2009. [DOI] [PubMed] [Google Scholar]
- 8.Cheng L, Wang T, Zhu J, and Cai P. Osteoinduction of calcium phosphate ceramics in four kinds of animals for 1 year: dog, rabbit, rat, and mouse. Transplant Proc. 48: 1309–1314. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 17: 31–35. 1996. [DOI] [PubMed] [Google Scholar]
- 10.Yuan H, van Blitterswijk CA, de Groot K, and de Bruijn JD. Cross-species comparison of ectopic bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) scaffolds. Tissue Eng. 12: 1607–1615. 2006. [DOI] [PubMed] [Google Scholar]
- 11.Kamphues J, Carstensen P, Schroeder D, Meyer H, Schoon HA, and Rosenbruch M. Effekte einer steigenden Calcium‐ und Vitamin D‐Zufuhr auf den Calciumstoffwechsel von Kaninchen (Effects of increasing calcium- and vitamin D supply on calcium metabolism of rabbits). J Anim Physiol Anim Nutr (Berl). 56: 191–208. 1986. [Google Scholar]
- 12.Cruise LJ, and Brewer NR. Anatomy. In: The Biology of the Laboratory Rabbit, 2nd ed. PJ Manning, DH Ringler, and CE Newcomer (eds). Academic Press, San Diego. 47–61. 1994.. [Google Scholar]
- 13.Kozma C, Macklin W, Cummins LM, and Mauer R. The anatomy, physiology and biochemistry of the rabbit. In: The Biology of the Laboratory Rabbit, 1st ed. SH Weisbroth, RE Flatt, and AL Kraus (eds). Academic Press, New York. 50–69. 1974.. [Google Scholar]
- 14.Sellers RS, Pardo I, Hu G, Khan KN, Perry R, Markiewicz V, Rohde C, Colangelo J, Reagan W, and Clarke D. Inflammatory cell findings in the female rabbit heart and stress-associated exacerbation with handling and procedures used in nonclinical studies. Toxicol Pathol. 45: 416–426. 2017. [DOI] [PubMed] [Google Scholar]
- 15.Pogwizd SM, and Bers DM. Rabbit models of heart disease. Drug Discov Today Dis Models. 5: 185–193. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vörös K, Seehusen F, Hungerbühler S, Meyer-Lindenberg A, and von der Hoeh N. Ventricular septal defect with aortic valve insufficiency in a New Zealand White rabbit. J Am Anim Hosp Assoc. 47: e42–e49. 2011. [DOI] [PubMed] [Google Scholar]
- 17.Hurley RJ, Marini RP, Avison DL, Murphy JC, Olin JM, and Lipman NS. Evaluation of detomidine anesthetic combinations in the rabbit. Lab Anim Sci. 44: 472–478. 1994. [PubMed] [Google Scholar]
- 18.Marini RP, Li X, Harpster NK, and Dangler C. Cardiovascular pathology possibly associated with ketamine/xylazine anesthesia in Dutch belted rabbits. Lab Anim Sci. 49: 153–160. 1999. [PubMed] [Google Scholar]
- 19.Cooper LL, Odening KE, Hwang MS, Chaves L, Schofield L, Taylor CA, Gemignani AS, Mitchell GF, Forder JR, Choi BR, and Koren G. Electromechanical and structural alterations in the aging rabbit heart and aorta. Am J Physiol Heart Circ Physiol. 302: H1625–H1635. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downing SE, and Chen V. Myocardial injury following endogenous catecholamine release in rabbits. J Mol Cell Cardiol. 17: 377–387. 1985. [DOI] [PubMed] [Google Scholar]
- 21.Weber HW, and Van Der Walt JJ. Cardiomyopathy in crowded rabbits. Recent Adv Stud Cardiac Struct Metab. 6: 471–477. 1975. [PubMed] [Google Scholar]
- 22.Flores NA, Davies RL, Penny WJ, and Sheridan DJ. Coronary microangiography in the guinea pig, rabbit and ferret. Int J Cardiol. 6: 459–471. 1984. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell MP, Hearse DJ, and Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res. 21: 737–746. 1987. [DOI] [PubMed] [Google Scholar]
- 24.Barthold SW, Griffey SM, and Percy DH. Pathology of Laboratory Rodents and Rabbits, 4th ed. Blackwell Publishing, Iowa. 2016. [Google Scholar]
- 25.Lossi L, D’Angelo L, De Girolamo P, and Merighi A. Anatomical features for an adequate choice of experimental animal model in biomedicine: II. Small laboratory rodents, rabbit, and pig. Ann Anat. 204: 11–28. 2016. [DOI] [PubMed] [Google Scholar]
- 26.Anitschkow N, and Chalatow S. Classics in arteriosclerosis research: On experimental cholesterin steatosis and its significance in the origin of some pathological processes arteriosclerosis: An Official Journal of the American Heart Association. Inc. 3: 178–182. 1913. [PubMed] [Google Scholar]
- 27.Yanni AE. The laboratory rabbit: an animal model of atherosclerosis research. Lab Anim. 38: 246–256. 2004. [DOI] [PubMed] [Google Scholar]
- 28.Rinke M, and Hartmann E. Dietary lipid excess in mice and rabbits. In: Classic Examples in Toxicologic Pathology, 5th ed. E Karbe, W Drommer, PG Germann, G Morawietz, and R Kellner (eds). European Society of Toxicologic Pathology. 2013. [Google Scholar]
- 29.Fan J, Kitajima S, Watanabe T, Xu J, Zhang J, Liu E, and Chen YE. Rabbit models for the study of human atherosclerosis: from pathophysiological mechanisms to translational medicine. Pharmacol Ther. 146: 104–119. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohn J, and Couto MA. Chapter 8: Anatomy, Physiology, and Behavior. In: The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, 1st ed. MA Suckow, KA Stevens, and RP Wilson (eds). Academic Press (Elsevier), Amsterdam. 195–217. 2012. . [Google Scholar]
- 31.Walker BE. Induction of cleft palate in rabbits by several glucocorticoids. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine [ (New York, NY)] . 125:1281–1284. 1967. [DOI] [PubMed] [Google Scholar]
- 32.van Kruiningen HJ, and Williams CB. Mucoid enteritis of rabbits. Comparison to cholera and cystic fibrosis. Vet Pathol. 9: 53–77. 1972. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Sorden S, Dunn D, Dwyer A, and Sonnentag P. The effects of blood collection techniques on Ocular lesions in Rabbits. Invest Ophthalmol Vis Sci. 54: 3039. 2013. [Google Scholar]
- 34.Villano JS, and Cooper TK. Mandibular fracture and necrotizing sialometaplasia in a rabbit. Comp Med. 63: 67–70. 2013. [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer M, Speight P, and Bown SG. A study of the effects of photodynamic therapy on the normal tissues of the rabbit jaw. Br J Cancer. 64: 1093–1097. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smallwood J. Anatomy of the Laboratory Rabbit. In: A Guided Tour of Veterinary Anatomy: Domestic Ungulates and Laboratory Mammals. Saunders, Philadelphia. 1992. [Google Scholar]
- 37.Van den Bulck K, Decostere A, Baele M, Marechal M, Ducatelle R, and Haesebrouck F. Low frequency of Helicobacter species in the stomachs of experimental rabbits. Lab Anim. 40: 282–287. 2006. [DOI] [PubMed] [Google Scholar]
- 38.Leeson CR, and Leeson TS. The fine structure of Brunner’s glands in the rabbit. Anat Rec. 159: 409–419. 1967. [DOI] [PubMed] [Google Scholar]
- 39.Snipes RL. Anatomy of the rabbit cecum. Anat Embryol (Berl). 155: 57–80. 1978. [DOI] [PubMed] [Google Scholar]
- 40.Ruckebusch Y, and Fioramonti J. The Fusus coli of the rabbit as a pacemaker area. Experientia. 32: 1023–1024. 1976. [DOI] [PubMed] [Google Scholar]
- 41.Adak A, Prasad MC, Lonkar PS, Kapurkar UM, Brahmankar MG, and Patel MV. Ectopic spleen in the pancreas of New Zealand White rabbits. Vet World. 6: 360–362. 2013. [Google Scholar]
- 42.Brändli-Baiocco A, Balme E, Bruder M, Chandra S, Hellmann J, Hoenerhoff MJ, Kambara T, Landes C, Lenz B, Mense M, Rittinghausen S, Satoh H, Schorsch F, Seeliger F, Tanaka T, Tsuchitani M, Wojcinski Z, and Rosol TJ. Nonproliferative and proliferative lesions of the rat and mouse endocrine system. J Toxicol Pathol. 31(Suppl): 1S–95S. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craigie EH. Bensley’s Practical Anatomy of the Rabbit: An Elementary Laboratory Text-Book in Mammalian Anatomy. 8th ed. University of Toronto Press, Toronto. 1948. [Google Scholar]
- 44.La Perle KMD, and Dintzis SM. Endocrine system. In: Comparative Anatomy and Histology : A Mouse, Rat, and Human Atlas. 2nd ed. PM Treuting, SM Dintzis, and KS Montine (eds). Elsevier, London. 251–274. 2018. [Google Scholar]
- 45.Caffe AR. Architecture of the mammalian pituitary cholinergic system with observations on a putative blood acetylcholine sensor. Histol Histopathol. 11: 537–551. 1996. [PubMed] [Google Scholar]
- 46.Kakita T, and Odell WD. Pituitary gland: one site of ultrashort-feedback regulation for control of thyrotropin. Am J Physiol. 250: E121–E124. 1986. [DOI] [PubMed] [Google Scholar]
- 47.Reiter RJ. Comparative physiology: pineal gland. Annu Rev Physiol. 35: 305–328. 1973. [DOI] [PubMed] [Google Scholar]
- 48.Tan SQ, Thomas D, Jao W, Bourdeau JE, and Lau K. Surgical thyroparathyroidectomy of the rabbit. Am J Physiol. 252: F761–F767. 1987. [DOI] [PubMed] [Google Scholar]
- 49.Rossouw DJ, and Chase CC. Ultrastructure of the capsule of the rabbit adrenal gland. Acta Anat (Basel). 100: 538–544. 1978. [DOI] [PubMed] [Google Scholar]
- 50.Fazekas AG, and Sandor T. Unusual pathways of aldosterone biosynthesis in the rabbit adrenal. Steroids. 14: 161–177. 1969. [DOI] [PubMed] [Google Scholar]
- 51.Rosol TJ, Yarrington JT, Latendresse J, and Capen CC. Adrenal gland: structure, function, and mechanisms of toxicity. Toxicol Pathol. 29: 41–48. 2001. [DOI] [PubMed] [Google Scholar]
- 52.Henderson JR, and Daniel PM. A comparative study of the portal vessels connecting the endocrine and exocrine pancreas, with a discussion of some functional implications. Q J Exp Physiol Cogn Med Sci. 64: 267–275. 1979. [DOI] [PubMed] [Google Scholar]
- 53.Lifson N, Kramlinger KG, Mayrand RR, and Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology. 79: 466–473. 1980. [PubMed] [Google Scholar]
- 54.King JE, and Ackerman GA. Erythropoiesis in the bone marrow of the fetal rabbit. Anat Rec. 157: 589–605. 1967. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy DE, Witte PL, and Knight KL. Bone marrow fat and the decline of B lymphopoiesis in rabbits. Dev Comp Immunol. 58: 30–39. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bigelow CL, and Tavassoli M. Fatty involution of bone marrow in rabbits. Acta Anat (Basel). 118: 60–64. 1984. [DOI] [PubMed] [Google Scholar]
- 57.Elmore SA. Enhanced histopathology of the bone marrow. Toxicol Pathol. 34: 666–686. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cloyd GG, and Johnson GR. Lymphosarcoma with lymphoblastic leukemia in a New Zealand White rabbit. Lab Anim Sci. 28: 66–69. 1978. [PubMed] [Google Scholar]
- 59.Finnie JW, Bostock DE, and Walden NB. Lymphoblastic leukaemia in a rabbit: a case report. Lab Anim. 14: 49–51. 1980. [DOI] [PubMed] [Google Scholar]
- 60.Meier H, Fox RR, and Crary DD. Myeloid leukemia in the rabbit (Oryctolagus cuniculus). Cancer Res. 32: 1785–1787. 1972. [PubMed] [Google Scholar]
- 61.Toth LA, Olson GA, Wilson E, Rehg JE, and Claassen E. Lymphocytic leukemia and lymphosarcoma in a rabbit. J Am Vet Med Assoc. 197: 627–629. 1990. [PubMed] [Google Scholar]
- 62.Shibuya K, Tajima M, Kanai K, Ihara M, and Nunoya T. Spontaneous lymphoma in a Japanese White rabbit. J Vet Med Sci. 61: 1327–1329. 1999. [DOI] [PubMed] [Google Scholar]
- 63.Heatley JJ, and Smith AN. Spontaneous neoplasms of lagomorphs. Vet Clin North Am Exot Anim Pract. 7: 561–577, v. 2004. [DOI] [PubMed] [Google Scholar]
- 64.Gómez L, Gázquez A, Roncero V, Sánchez C, and Durán ME. Lymphoma in a rabbit: histopathological and immunohistochemical findings. J Small Anim Pract. 43: 224–226. 2002. [DOI] [PubMed] [Google Scholar]
- 65.White SD, Campbell T, Logan A, et al. Lymphoma with cutaneous involvement in three domestic rabbits (Oryctolagus cuniculus). Vet Dermatol. 11: 61–67. 2000. [DOI] [PubMed] [Google Scholar]
- 66.Schummer A, Wilkens H, Vollmerhaus B, and Habermehl K-H. Lymphatic system. In: The Circulatory System, the Skin, and the Cutaneous Organs of the Domestic Mammals. A Schummer, H Wilkens, B Vollmerhaus, and K-H Habermehl (eds). Springer, Massachusetts. 269–440. 1981. [Google Scholar]
- 67.Gaber W. Morphogenesis of the thymus in rabbit during prenatal and early postnatal periods. J Electron (China). 2017; . [Google Scholar]
- 68.Hassan AU, and Rasool Z. The Hassal of thymus: Hassals corpuscle histological and histopathological perspective. Sch J App Med Sci. 2(1B): 147–148. 2014. [Google Scholar]
- 69.Bodey B, and Kaiser HE. Development of Hassall’s bodies of the thymus in humans and other vertebrates (especially mammals) under physiological and pathological conditions: immunocytochemical, electronmicroscopic and in vitro observations. In Vivo. 11: 61–85. 1997. [PubMed] [Google Scholar]
- 70.Müllhaupt D, Wenger S, Kircher P, Pfammatter N, Hatt JM, and Ohlerth S. Computed tomography of the thorax in rabbits: a prospective study in ten clinically healthy New Zealand White rabbits. Acta Vet Scand. 59: 72. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maurer JK, Gibbons BA, and Bruce RD. Morphometric assessment of thymic size variation in laboratory rabbits. Toxicol Pathol. 18: 407–411. 1990. [DOI] [PubMed] [Google Scholar]
- 72.Dimitrov RS. Comparative ultrasonographic, anatomotopographic and macromorphometric study of the spleen and pancreas in rabbit (Oryctolagus cuniculus). Not Sci Biol. 4: 14–20. 2012. [Google Scholar]
- 73.Marcato PS, and Rosmini R. Pathology of the rabbit and hare: a color atlas and compendium. Societa Editrice Esculapio, Bologna. 1986. [Google Scholar]
- 74.Dunne AA, Plehn S, Schulz S, Levermann A, Ramaswamy A, Lippert BM, and Werner JA. Lymph node topography of the head and neck in New Zealand White rabbits. Lab Anim. 37: 37–43. 2003. [DOI] [PubMed] [Google Scholar]
- 75.Elmore SA. Histopathology of the lymph nodes. Toxicol Pathol. 34: 425–454. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soto-Miranda MA, Suami H, and Chang DW. Mapping superficial lymphatic territories in the rabbit. Anat Rec (Hoboken). 296: 965–970. 2013. [DOI] [PubMed] [Google Scholar]
- 77.Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 34: 409–424. 2006. [DOI] [PubMed] [Google Scholar]
- 78.Lanning D, Zhu X, Zhai S-K, and Knight KL. Development of the antibody repertoire in rabbit: gut-associated lymphoid tissue, microbes, and selection. Immunol Rev. 175: 214–228. 2000. [PubMed] [Google Scholar]
- 79.Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 34: 599–608. 2006. [DOI] [PubMed] [Google Scholar]
- 80.Stamatova-Yovcheva K, Dimitrov R, Kostov D, and Yovchev D. Anatomical macromorphological features of the liver in domestic rabbit (Oryctolagus cuniculus). Trakia Journal of Sciences. 10: 85–90. 2012. [Google Scholar]
- 81.Bradley AE. New Zealand White Rabbit. In: Background lesions in laboratory animals: A color atlas, 1st ed. EF McInnes (ed). Saunders (Elsevier), Edinburgh. 87–91. 2012.. [Google Scholar]
- 82.Chandra SA, Stokes AH, Hailey R, Merrill CL, Melich DH, DeSmet K, Furst SM, Peterson RA, Mellon-Kusibab K, and Adler RR. Dermal toxicity studies: factors impacting study interpretation and outcome. Toxicol Pathol. 43: 474–481. 2015. [DOI] [PubMed] [Google Scholar]
- 83.Florizoone K, van der Luer R, and van den Ingh T. Symmetrical alopecia, scaling and hepatitis in a rabbit. Vet Dermatol. 18: 161–164. 2007. [DOI] [PubMed] [Google Scholar]
- 84.Ramirez CJ, Kim DY, Hanks BC, and Evans TJ. Copper toxicosis in New Zealand White rabbits (Oryctolagus cuniculus). Vet Pathol. 50: 1135–1138. 2013. [DOI] [PubMed] [Google Scholar]
- 85.Leipig M, Matiasek K, Rinder H, Janik D, Emrich D, Baiker K, and Hermanns W. Value of histopathology, immunohistochemistry, and real-time polymerase chain reaction in the confirmatory diagnosis of Encephalitozoon cuniculi infection in rabbits. J Vet Diagn Invest. 25: 16–26. 2013. [DOI] [PubMed] [Google Scholar]
- 86.Gupta BN. Duplication of the gall bladder in a rabbit. Lab Anim Sci. 25: 646. 1975. [PubMed] [Google Scholar]
- 87.Lee SP, and Scott AJ. Dihydrocholesterol-induced gallstones in the rabbit: evidence that bile acids cause gallbladder epithelial injury. Br J Exp Pathol. 60: 231–238. 1979. [PMC free article] [PubMed] [Google Scholar]
- 88.Diribarne M, Mata X, Rivière J, Bouet S, Vaiman A, Chapuis J, Reine F, Fleurot R, Auvinet G, Deretz S, Allain D, Schibler L, Cribiu EP, and Guérin G. LIPH expression in skin and hair follicles of normal coat and Rex rabbits. PLoS One. 7: e30073. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jung EC, and Maibach HI. Animal models for percutaneous absorption. J Appl Toxicol. 35: 1–10. 2015. [DOI] [PubMed] [Google Scholar]
- 90.Nicoli S, Padula C, Aversa V, Vietti B, Wertz PW, Millet A, Falson F, Govoni P, and Santi P. Characterization of rabbit ear skin as a skin model for in vitro transdermal permeation experiments: histology, lipid composition and permeability. Skin Pharmacol Physiol. 21: 218–226. 2008. [DOI] [PubMed] [Google Scholar]
- 91.Anderson JM, and Langone JJ. Issues and perspectives on the biocompatibility and immunotoxicity evaluation of implanted controlled release systems. J Control Release. 57: 107–113. 1999. [DOI] [PubMed] [Google Scholar]
- 92.Dincer Z, Jones S, and Haworth R. Preclinical safety assessment of a DNA vaccine using particle-mediated epidermal delivery in domestic pig, minipig and mouse. Exp Toxicol Pathol. 57: 351–357. 2006. [DOI] [PubMed] [Google Scholar]
- 93.Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, and Sauzeat E. Aluminium assay and evaluation of the local reaction at several time points after intramuscular administration of aluminium containing vaccines in the Cynomolgus monkey. Vaccine. 23: 1359–1367. 2005. [DOI] [PubMed] [Google Scholar]
- 94.Williams DF. Review. Tissue-biomaterial interactions. J Mater Sci. 22: 3421–3445. 1987. [Google Scholar]
- 95.Carlisle DB. On the relationship between mammary, sweat, and sebaceous glands. Q J Microsc Sci. 95: 79–83. 1954. [Google Scholar]
- 96.Diribarne M, Mata X, Chantry-Darmon C, Vaiman A, Auvinet G, Bouet S, Deretz S, Cribiu EP, de Rochambeau H, Allain D, and Guérin G. A deletion in exon 9 of the LIPH gene is responsible for the rex hair coat phenotype in rabbits (Oryctolagus cuniculus). PLoS One. 6: e19281. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moore DM, Zimmerman K, and Smith SA. Hematological assessment in pet rabbits: blood sample collection and blood cell identification. Vet Clin North Am Exot Anim Pract. 18: 9–19. 2015. [DOI] [PubMed] [Google Scholar]
- 98.Fayez I, Marai M, Alnaimy A, and Habeeb M. Thermoregulation in rabbits. In: Rabbit production in hot climates. M Baselga, IFM Marai (eds). Cahiers Options Méditerranéennes, Paris. 33–41. 1994. [Google Scholar]
- 99.Ootsuka Y, and Tanaka M. Control of cutaneous blood flow by central nervous system. Temperature. 2: 392–405. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sayed-Ahmed A, and Elnasharty MA. Pre and postnatal development of the rabbit thin skin. Glob Vet. 13: 622–632. 2014. [Google Scholar]
- 101.Melo AI, and González-Mariscal G. Communication by olfactory signals in rabbits: its role in reproduction. Vitam Horm. 83: 351–371. 2010. [DOI] [PubMed] [Google Scholar]
- 102.Mykytowycz R. Further observations on the territorial function and histology of the submandibular cutaneous (chin) glands in the rabbit, Oryctolagus cuniculus (L.). Anim Behav. 13: 400–412. 1965. [DOI] [PubMed] [Google Scholar]
- 103.Mykytowycz R, and Goodrich BS. Skin glands as organs of communication in mammals. J Invest Dermatol. 62: 124–131. 1974. [DOI] [PubMed] [Google Scholar]
- 104.Wales NA, and Ebling FJ. The control of the apocrine glands of the rabbit by steroid hormones. J Endocrinol. 51: 763–770. 1971. [DOI] [PubMed] [Google Scholar]
- 105.Heath E. Cytologic observations on the secretory cells of the chin (submandibular) gland and brown inguinal gland in the rabbit. Cell Tissue Res. 154: 399–408. 1974. [DOI] [PubMed] [Google Scholar]
- 106.Montagna W. The brown inguinal glands of the rabbit. Am J Anat. 87: 213–237. 1950. [DOI] [PubMed] [Google Scholar]
- 107.Cerbón MA, Camacho-Arroyo I, Gamboa-Domínguez A, and González-Mariscal G. The rabbit submandibular gland: sexual dimorphism, effects of gonadectomy, and variations across the female reproductive cycle. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 178: 351–357. 1996. [DOI] [PubMed] [Google Scholar]
- 108.Fehr M, and Koestlinger S. Ectoparasites in small exotic mammals. Vet Clin North Am Exot Anim Pract. 16: 611–657. 2013. [DOI] [PubMed] [Google Scholar]
- 109.Hoppmann E, and Barron HW. Ferret and rabbit dermatology. J Exot Pet Med. 16: 225–237. 2007. [Google Scholar]
- 110.Snook TS, White SD, Hawkins MG, Tell LA, Wilson LS, Outerbridge CA, and Ihrke PJ. Skin diseases in pet rabbits: a retrospective study of 334 cases seen at the University of California at Davis, USA (1984–2004). Vet Dermatol. 24: 613–617, e148. 2013. [DOI] [PubMed] [Google Scholar]
- 111.Manjunatha DR, Mahesh V, and Ranganath L. Surgical management of aural hematoma in Russian Grey Giant rabbit - a case report. International Journal of Agricultural Sciences and Veterinary Medicine. 2: 37–38. 2014. [Google Scholar]
- 112.d’Ovidio D, and Santoro D. Orodental diseases and dermatological disorders are highly associated in pet rabbits: a case-control study. Vet Dermatol. 24: 531–e125. 2013. [DOI] [PubMed] [Google Scholar]
- 113.Harcourt-Brown F. Textbook of Rabbit Medicine. Reed Educational and Professional Publishing Ltd, Oxford. 2002. [Google Scholar]
- 114.Kerr PJ, Liu J, Cattadori I, Ghedin E, Read AF, and Holmes EC. Myxoma virus and the Leporipoxviruses: an evolutionary paradigm. Viruses. 7: 1020–1061. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meredith AL. Viral skin diseases of the rabbit. Vet Clin North Am Exot Anim Pract. 16: 705–714. 2013. [DOI] [PubMed] [Google Scholar]
- 116.Kanfer S, and Reavill DR. Cutaneous neoplasia in ferrets, rabbits, and guinea pigs. Vet Clin North Am Exot Anim Pract. 16: 579–598. 2013. [DOI] [PubMed] [Google Scholar]
- 117.von Bomhard W, Goldschmidt MH, Shofer FS, Perl L, Rosenthal KL, and Mauldin EA. Cutaneous neoplasms in pet rabbits: a retrospective study. Vet Pathol. 44: 579–588. 2007. [DOI] [PubMed] [Google Scholar]
- 118.Florizoone K. Thymoma-associated exfoliative dermatitis in a rabbit. Vet Dermatol. 16: 281–284. 2005. [DOI] [PubMed] [Google Scholar]
- 119.Jassies-van der Lee A, van Zeeland Y, Kik M, and Schoemaker N. Successful treatment of sebaceous adenitis in a rabbit with ciclosporin and triglycerides. Vet Dermatol. 20: 67–71. 2009. [DOI] [PubMed] [Google Scholar]
- 120.Rostaher Prélaud A, Jassies-van der Lee A, Mueller RS, van Zeeland YR, Bettenay S, Majzoub M, Zenker I, and Hein J. Presumptive paraneoplastic exfoliative dermatitis in four domestic rabbits. Vet Rec. 172: 155. 2013. [DOI] [PubMed] [Google Scholar]
- 121.Chandra SA, Peterson RA, Melich D, Merrill CM, Bailey D, Mellon-Kusibab K, and Adler R. Dermal irritation of petrolatum in rabbits but not in mice, rats or minipigs. J Appl Toxicol. 34: 857–861. 2014. [DOI] [PubMed] [Google Scholar]
- 122.Rigdon RH. Local reaction to polyurethane--a comparative study in the mouse, rat, and rabbit. J Biomed Mater Res. 7: 79–93. 1973. [DOI] [PubMed] [Google Scholar]
- 123.Buja LM, Kita T, Goldstein JL, Watanabe Y, and Brown MS. Cellular pathology of progressive atherosclerosis in the WHHL rabbit. An animal model of familial hypercholesterolemia. Arteriosclerosis. 3: 87–101. 1983. [DOI] [PubMed] [Google Scholar]
- 124.Nakano A, Kinoshita M, Okuda R, Yasuda T, Abe M, and Shiomi M. Pathogenesis of tendinous xanthoma: histopathological study of the extremities of Watanabe heritable hyperlipidemic rabbits. J Orthop Sci. 11: 75–80. 2006. [DOI] [PubMed] [Google Scholar]
- 125.Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit). Atherosclerosis. 36: 261–268. 1980. [DOI] [PubMed] [Google Scholar]
- 126.Mitsuguchi Y, Ito T, and Ohwada K. Pathologic findings in rabbit models of hereditary hypertriglyceridemia and hereditary postprandial hypertriglyceridemia. Comp Med. 58: 465–480. 2008. [PMC free article] [PubMed] [Google Scholar]
- 127.Quinton JF, Prelaud P, Poujade A, and Faivre NC. A case of actinic keratosis in a rabbit. J Exot Pet Med. 23: 283–286. 2014. [Google Scholar]
- 128.Calvert DT, Knight CH, and Peaker M. Milk accumulation and secretion in the rabbit. Q J Exp Physiol. 70: 357–363. 1985. [DOI] [PubMed] [Google Scholar]
- 129.Propper AY, Howard BA, and Veltmaat JM. Prenatal morphogenesis of mammary glands in mouse and rabbit. J Mammary Gland Biol Neoplasia. 18: 93–104. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Caba M, and González-Mariscal G. The rabbit pup, a natural model of nursing-anticipatory activity. Eur J Neurosci. 30: 1697–1706. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Adlam C, Thorley CM, Ward PD, Collins M, Lucken RN, and Knight PA. Natural and experimental staphylococcal mastitis in rabbits. J Comp Pathol. 86: 581–593. 1976. [DOI] [PubMed] [Google Scholar]
- 132.Viana D, Selva L, Callanan JJ, Guerrero I, Ferrian S, and Corpa JM. Strains of Staphylococcus aureus and pathology associated with chronic suppurative mastitis in rabbits. Vet J. 190: 403–407. 2011. [DOI] [PubMed] [Google Scholar]
- 133.Greene HS. Familial mammary tumors in the rabbit : Ii. Gross and microscopic pathology. J Exp Med. 70: 159–166. 1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lipman NS, Zhao ZB, Andrutis KA, Hurley RJ, Fox JG, and White HJ. Prolactin-secreting pituitary adenomas with mammary dysplasia in New Zealand White rabbits. Lab Anim Sci. 44: 114–120. 1994. [PubMed] [Google Scholar]
- 135.Sikoski P, Trybus J, Cline JM, Muhammad FS, Eckhoff A, Tan J, Lockard M, Jolley T, Britt S, and Kock ND. Cystic mammary adenocarcinoma associated with a prolactin-secreting pituitary adenoma in a New Zealand White rabbit (Oryctolagus cuniculus). Comp Med. 58: 297–300. 2008. [PMC free article] [PubMed] [Google Scholar]
- 136.Petraitiene R, Petraitis V, Bacher J, Das SR, Parlow AF, and Walsh TJ. Cyclosporine A-induced mammary hyperplasia and hyperprolactinemia in New Zealand White rabbits. Comp Med. 51: 430–435. 2001. [PubMed] [Google Scholar]
- 137.Krimer PM, Harvey SB, Blas-Machado U, Lauderdale JD, and Moore PA. Reversible fibroadenomatous mammary hyperplasia in male and female New Zealand White rabbits associated with cyclosporine A administration. Vet Pathol. 46: 1144–1148. 2009. [DOI] [PubMed] [Google Scholar]
- 138.Maratea KA, Ramos-Vara JA, Corriveau LA, and Miller MA. Testicular interstitial cell tumor and gynecomastia in a rabbit. Vet Pathol. 44: 513–517. 2007. [DOI] [PubMed] [Google Scholar]
- 139.Shek JW, Wen GY, and Wisniewski HM. Atlas of the rabbit brain and spinal cord. Karger AG, Basel. 1986. [Google Scholar]
- 140.Pardo ID, Weber K, Cramer S, Krinke GJ, Butt MT, Sharma AK, and Bolon B. Atlas of normal microanatomy, procedural and processing artifacts, common background findings, and neurotoxic lesions in the peripheral nervous system of laboratory animals. Toxicol Pathol. 48: 105–131. 2020. [DOI] [PubMed] [Google Scholar]
- 141.Lindsey JR, and Fox RR. Inherited diseases and variations. In: Biology of the Laboratory Rabbit, 2nd ed. PJ Manning, DH Ringler, and CE Newcomer (eds). Academic Press, Orlando. 293–319. 1994.. [Google Scholar]
- 142.Mooney MP, Aston CE, Siegel MI, Losken HW, Smith TD, Burrows AM, Wenger SL, Caruso K, Siegel B, and Ferrell RE. Craniosynostosis with autosomal dominant transmission in New Zealand White rabbits. J Craniofac Genet Dev Biol. 16: 52–63. 1996. [PubMed] [Google Scholar]
- 143.Rudmann D, and Colleton C. Interstitial adipocytes in the Beagle dog and New Zealand White rabbit choroid plexus. Toxicol Pathol. 47: 553–555. 2019. [DOI] [PubMed] [Google Scholar]
- 144.Ivens IA, Achanzar W, Baumann A, Brändli-Baiocco A, Cavagnaro J, Dempster M, Depelchin BO, Rovira AR, Dill-Morton L, Lane JH, Reipert BM, Salcedo T, Schweighardt B, Tsuruda LS, Turecek PL, and Sims J. PEGylated biopharmaceuticals: current experience and considerations for nonclinical development. Toxicol Pathol. 43: 959–983. 2015. [DOI] [PubMed] [Google Scholar]
- 145.Swank RL. The pyramidal tracts: an experimental study of the corticospinal and other components in the rabbit. Arch Neurol Psychiatry. 36: 530–541. 1936. [Google Scholar]
- 146.Dixon D, Alison R, Bach U, Colman K, Foley GL, Harleman JH, Haworth R, Herbert R, Heuser A, Long G, Mirsky M, Regan K, Van Esch E, Westwood FR, Vidal J, and Yoshida M. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J Toxicol Pathol. 27(Suppl): 1S–107S. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Limon M. Etude histologique et histogenetique de la glande interstitielle de ovaire. Arch d’Anat micr. 5: 155–190. 1902.
- 148.Mori H, and Matsumoto K. Development of the secondary interstitial gland in the rabbit ovary. J Anat. 116: 417–430. 1973. [PMC free article] [PubMed] [Google Scholar]
- 149.Nicosia SV, and Johnson JH. Surface morphology of ovarian mesothelium (surface epithelium) and of other pelvic and extrapelvic mesothelial sites in the rabbit. Int J Gynecol Pathol. 3: 249–260. 1984. [DOI] [PubMed] [Google Scholar]
- 150.Barberini F, Correr S, De Santis F, and Motta PM. The epithelium of the rabbit vagina: a microtopographical study by light, transmission and scanning electron microscopy. Arch Histol Cytol. 54: 365–378. 1991. [DOI] [PubMed] [Google Scholar]
- 151.Barberini F, Sartori S, and Motta P. Changes in the surface morphology of the rabbit endometrium related to the estrous and progestational stages of the reproductive cycle a scanning and transmission electron microscopic study. Cell Tissue Res. 190: 207–222. 1978. [DOI] [PubMed] [Google Scholar]
- 152.Bray MV, Weir EC, Brownstein DG, and Delano ML. Endometrial venous aneurysms in three New Zealand White rabbits. Lab Anim Sci. 42: 360–362. 1992. [PubMed] [Google Scholar]
- 153.Zook BC, Spiro I, and Hertz R. Malignant neoplasms of decidual origin (deciduosarcomas) induced by estrogen-progestin-releasing intravaginal devices in rabbits. Am J Pathol. 128: 315–327. 1987. [PMC free article] [PubMed] [Google Scholar]
- 154.Zook BC, Jänne OA, Abraham AA, and Nash HA. The development and regression of deciduosarcomas and other lesions caused by estrogens and progestins in rabbits. Toxicol Pathol. 29: 411–416. 2001. [DOI] [PubMed] [Google Scholar]
- 155.Saito K, Nakanishi M, and Hasegawa A. Uterine disorders diagnosed by ventrotomy in 47 rabbits. J Vet Med Sci. 64: 495–497. 2002. [DOI] [PubMed] [Google Scholar]
- 156.Jänne OA, Zook BC, Didolkar AK, Sundaram K, and Nash HA. The roles of estrogen and progestin in producing deciduosarcoma and other lesions in the rabbit. Toxicol Pathol. 29: 417–421. 2001. [DOI] [PubMed] [Google Scholar]
- 157.Cooper TK, Adelsohn D, and Gilbertson SR. Spontaneous deciduosarcoma in a domestic rabbit (Oryctolagus cuniculus). Vet Pathol. 43: 377–380. 2006. [DOI] [PubMed] [Google Scholar]
- 158.Morton D, Weisbrode SE, Wyder WE, Maurer JK, and Capen CC. Spermatid giant cells, tubular hypospermatogenesis, spermatogonial swelling, cytoplasmic vacuoles, and tubular dilatation in the testes of normal rabbits. Vet Pathol. 23: 176–183. 1986. [DOI] [PubMed] [Google Scholar]
- 159.Zwicker GM, and Killinger JM. Interstitial cell tumors in a young adult New Zealand White rabbit. Toxicol Pathol. 13: 232–235. 1985. [DOI] [PubMed] [Google Scholar]
- 160.Reineking W, Seehusen F, Lehmbecker A, and Wohlsein P. Predominance of granular cell tumours among testicular tumours of rabbits (Oryctolagus cuniculi f. dom.). J Comp Pathol. 173: 24–29. 2019. [DOI] [PubMed] [Google Scholar]
- 161.Elchlepp JG. The urogenital organs of the Cottontail rabbit (Sylilagus floridanus). J Morphol. 91: 169–198. 1952. [Google Scholar]
- 162.Holtz W, and Foote RH. The anatomy of the reproductive system in male Dutch rabbits (Oryctolagus cuniculus) with special emphasis on the accessory sex glands. J Morphol. 158: 1–20. 1978. [DOI] [PubMed] [Google Scholar]
- 163.Skonieczna J, Madej JP, and Będziński R. Accessory genital glands in the New Zealand White rabbit: A morphometrical and histological study. J Vet Res (Pulawy). 63: 251–257. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zwicker GM, Killinger JM, and McConnel RF. Spontaneous vesicular and prostatic gland epithelial squamous metaplasia, hyperplasia, and keratinized nodule formation in rabbits. Toxicol Pathol. 13: 222–228. 1985. [DOI] [PubMed] [Google Scholar]
- 165.Carr EB. The vesicular seminialis of the rabbit. Proc Zool Soc Lond. 124: 675–683. 1954. [Google Scholar]
- 166.Uthamanthil RK, Hachem RY, Gagea M, Reitzel RA, Borne AT, and Tinkey PT. Urinary catheterization of male rabbits: a new technique and a review of urogenital anatomy. J Am Assoc Lab Anim Sci. 52: 180–185. 2013. [PMC free article] [PubMed] [Google Scholar]
- 167.Tsenova LHR, Ellison E, Manca C, and Kaplan G. Aerosol exposure system for rabbits: application to M. tuberculosis infection. Appl Biosaf. 11: 7–14. 2006. [Google Scholar]
- 168.Johnson-Delaney CA, and Orosz SE. Rabbit respiratory system: clinical anatomy, physiology and disease. Vet Clin North Am Exot Anim Pract. 14: 257–266, vi. 2011. [DOI] [PubMed] [Google Scholar]
- 169.Pereira ME, Macri NP, and Creasy DM. Evaluation of the rabbit nasal cavity in inhalation studies and a comparison with other common laboratory species and man. Toxicol Pathol. 39: 893–900. 2011. [DOI] [PubMed] [Google Scholar]
- 170.Phaneuf LR, Barker S, Groleau MA, and Turner PV. Tracheal injury after endotracheal intubation and anesthesia in rabbits. J Am Assoc Lab Anim Sci. 45: 67–72. 2006. [PubMed] [Google Scholar]
- 171.Choi HK, Finkbeiner WE, and Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat. 197: 361–372. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Plopper CG, Halsebo JE, Berger WJ, Sonstegard KS, and Nettesheim P. Distribution of nonciliated bronchiolar epithelial (Clara) cells in intra- and extrapulmonary airways of the rabbit. Exp Lung Res. 5: 79–98. 1983. [DOI] [PubMed] [Google Scholar]
- 173.Widdicombe JH, Chen LL, Sporer H, Choi HK, Pecson IS, and Bastacky SJ. Distribution of tracheal and laryngeal mucous glands in some rodents and the rabbit. J Anat. 198: 207–221. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Simons RS. Lung morphology of cursorial and non-cursorial mammals: lagomorphs as a case study for a pneumatic stabilization hypothesis. J Morphol. 230: 299–316. 1996. [DOI] [PubMed] [Google Scholar]
- 175.Bal HS, and Ghoshal NG. Morphology of the terminal bronchiolar region of common laboratory mammals. Lab Anim. 22: 76–82. 1988. [DOI] [PubMed] [Google Scholar]
- 176.Plopper CG, Mariassy AT, Wilson DW, Alley JL, Nishio SJ, and Nettesheim P. Comparison of nonciliated tracheal epithelial cells in six mammalian species: ultrastructure and population densities. Exp Lung Res. 5: 281–294. 1983. [DOI] [PubMed] [Google Scholar]
- 177.Cooper TK, Griffith JW, Chroneos ZC, Izer JM, Willing LB, and Peng X. Spontaneous lung lesions in aging laboratory rabbits ( Oryctolagus cuniculus). Vet Pathol. 54: 178–187. 2017. [DOI] [PubMed] [Google Scholar]
- 178.Dale K. A method for inducing unilateral silicosis in rabbits by an injection technique with some observations on lung clearance and quantitative evaluation of experimental silicosis. Scand J Respir Dis. 54: 157–167. 1973. [PubMed] [Google Scholar]
- 179.Dale K. Early effects of quartz and titanium dioxide dust on pulmonary function and tissue. An experimental study on rabbits. Scand J Respir Dis. 54: 168–184. 1973. [PubMed] [Google Scholar]
- 180.Wallace WE, Gupta NC, Hubbs AF, Mazza SM, Bishop HA, Keane MJ, Battelli LA, Ma J, and Schleiff P. Cis-4-[(18)F]fluoro-L-proline PET imaging of pulmonary fibrosis in a rabbit model. J Nucl Med. 43: 413–420. 2002. [PubMed] [Google Scholar]
- 181.Fox RR, and Crary DD. Hereditary diaphragmatic hernia in the rabbit. J Hered. 64: 333–336. 1973. [DOI] [PubMed] [Google Scholar]
- 182.Barros SS, Soares MP, and Gimeno EJ. Macrophages and giant cell proliferation associated with bone protein synthesis and calcification in the trachea and bronchi of rabbits intoxicated with Solanum glaucophyllum. Vet Pathol. 43: 494–499. 2006. [DOI] [PubMed] [Google Scholar]
- 183.Wang X, Mabrey JD, and Agrawal CM. An interspecies comparison of bone fracture properties. Biomed Mater Eng. 8: 1–9. 1998. [PubMed] [Google Scholar]
- 184.Muschler GF, Raut VP, Patterson TE, Wenke JC, and Hollinger JO. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 16: 123–145. 2010. [DOI] [PubMed] [Google Scholar]
- 185.Pearce AI, Richards RG, Milz S, Schneider E, and Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 13: 1–10. 2007. [DOI] [PubMed] [Google Scholar]
- 186.Haschek WM, Rousseaux CG, and Wallig MA. Fundamentals of Toxicologic Pathology. 2nd ed. Academic Press (Elsevier), Amsterdam. 2010. [Google Scholar]
- 187.Wancket LM. Animal models for evaluation of bone implants and devices: comparative bone structure and common model uses. Vet Pathol. 52: 842–850. 2015. [DOI] [PubMed] [Google Scholar]
- 188.Reinholz GG, Lu L, Saris DB, Yaszemski MJ, and O’Driscoll SW. Animal models for cartilage reconstruction. Biomaterials. 25: 1511–1521. 2004. [DOI] [PubMed] [Google Scholar]
- 189.Chu CR, Szczodry M, and Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 16: 105–115. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.O’Driscoll SW, Saris DB, Ito Y, and Fitzimmons JS. The chondrogenic potential of periosteum decreases with age. J Orthop Res. 19: 95–103. 2001. [DOI] [PubMed] [Google Scholar]
- 191.Shively MJ. Xeroradiographic anatomy of the domesticated rabbit (Oryctolagus cuniculus) part I: head, thorax, and thoracic limb. Swest Vet. 32: 219–233. 1979. [Google Scholar]
- 192.Shively MJ. Xeroradiographic anatomy of the domesticated rabbit (Oryctolagus cuniculus) part II:abdomen, pelvis, and pelvic limb. Swest Vet. 33: 57–67. 1980. [Google Scholar]
- 193.Greenaway JB, Partlow GD, Gonsholt NL, and Fisher KR. Anatomy of the lumbosacral spinal cord in rabbits. J Am Anim Hosp Assoc. 37: 27–34. 2001. [DOI] [PubMed] [Google Scholar]
- 194.Proffen BL, McElfresh M, Fleming BC, and Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 19: 493–499. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Brock K, Gallaugher L, Bergdall VK, and Dysko RC. Chapter 17: Mycoses and Non-Infectious Diseases. In: The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, 1st ed. MA Suckow, KA Stevens, and RP Wilson (eds). Academic Press (Elsevier), Amsterdam. 504–529. 2012: [Google Scholar]
- 196.Ikemura S, Yamamoto T, Nishida K, Motomura G, and Iwamoto Y. Gender difference in the development of steroid-induced osteonecrosis in rabbits. Rheumatology (Oxford). 49: 1128–1132. 2010. [DOI] [PubMed] [Google Scholar]
- 197.Kabata T, Kubo T, Matsumoto T, Hirata T, Fujioka M, Takahashi KA, Yagishita S, Kobayashi M, and Tomita K. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology (Oxford). 44: 1233–1237. 2005. [DOI] [PubMed] [Google Scholar]
- 198.Nehrbass D, Arens D, and Zeiter S. Spontaneous bilateral avulsion fracture of the tuberositas tibiae in a New Zealand White rabbit - a counterpart to Osgood-Schlatter disease in humans? Exp Toxicol Pathol. 67: 223–227. 2015. [DOI] [PubMed] [Google Scholar]
- 199.Gough A, Johnson R, Campbell E, Hall L, Tylor J, Carpenter A, Black W, Basrur PK, Baragi VM, Sigler R, and Metz A. Quinolone arthropathy in immature rabbits treated with the fluoroquinolone, PD 117596. Exp Toxicol Pathol. 48: 225–232. 1996. [DOI] [PubMed] [Google Scholar]
- 200.Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, and Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 38: 880–890. 2010. [DOI] [PubMed] [Google Scholar]
- 201.Laverty S, Girard CA, Williams JM, Hunziker EB, and Pritzker KP. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rabbit. Osteoarthritis Cartilage. 18(Suppl 3): S53–S65. 2010. [DOI] [PubMed] [Google Scholar]
- 202.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 1: 363–376. 2001. [PubMed] [Google Scholar]
- 203.Derry DM, Morrow E, Sadre N, and Flattery KV. Brown and white fat during the life of the rabbit. Dev Biol. 27: 204–216. 1972. [DOI] [PubMed] [Google Scholar]
- 204.Baradi AF, and Hope J. Observations on ultrastructure of rabbit mesothelium. Exp Cell Res. 34: 33–44. 1964. [DOI] [PubMed] [Google Scholar]
- 205.Miao CY, and Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 165: 643–658. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kempson RL, Fletcher CDM, Evans HL, Hendrickson MR, and Sibley RK. Atlas of Tumor Pathology, Tumors of the Soft Tissues. Armed Forces Institute of Pathology, Washington, D.C. 2001. [Google Scholar]
- 207.Calgüner E, Gözil R, Erdoğan D, Kurt I, Keskil S, Elmas C, and Sabuncuoğlu H. Atrophic and regenerative changes in rabbit mimic muscles after lidocaine and bupivacaine application. Anat Histol Embryol. 32: 54–59. 2003. [DOI] [PubMed] [Google Scholar]
- 208.Thuilliez C, Dorso L, Howroyd P, Gould S, Chanut F, and Burnett R. Histopathological lesions following intramuscular administration of saline in laboratory rodents and rabbits. Exp Toxicol Pathol. 61: 13–21. 2009. [DOI] [PubMed] [Google Scholar]
- 209.Osheroff MR, Kobs DJ, Buccellato M, Croutch CR, Elcock LE, Burback BL, and Johnson JD. Comparative toxicology studies in Sprague-Dawley rats, Rhesus monkeys, and New Zealand White rabbits to determine a no observed adverse effect level for 1,1′-methylenebis[4-[(hydroxyimino)methyl]-pyridinium] dimethanesulfonate. Int J Toxicol. 32(Suppl): 59S–74S. 2013. [DOI] [PubMed] [Google Scholar]
- 210.Afifi AD, Al-Gailany AM, Salman JM, and Bahuth NB. Nerve and muscle in steroid-induced weakness in the rabbit. Arch Phys Med Rehabil. 58: 143–148. 1977. [PubMed] [Google Scholar]
- 211.Brucker M, Sati S, Spangenberger A, and Weinzweig J. Long-term fate of transplanted autologous fat in a novel rabbit facial model. Plast Reconstr Surg. 122: 749–754. 2008. [DOI] [PubMed] [Google Scholar]
- 212.Mendlowski B. Neuromuscular lesions in restrained rabbits. Vet Pathol. 12: 378–386. 1975. [DOI] [PubMed] [Google Scholar]
- 213.Serfilippi LM, Saladino BH, and Spainhour CB. Sarcocystis Infection in Laboratory Rabbits. Comp Med. 70: 300–301. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Abdo M, Haddad S, and Emam M. Development of the New Zealand White rabbit eye: I. Pre- and postnatal development of eye tunics. Anat Histol Embryol. 46: 423–430. 2017. [DOI] [PubMed] [Google Scholar]
- 215.Reichenbach A, Schnitzer J, Friedrich A, Knothe AK, and Henke A. Development of the rabbit retina: II. Müller cells. J Comp Neurol. 311: 33–44. 1991. [DOI] [PubMed] [Google Scholar]
- 216.Reichenbach A, Schnitzer J, Friedrich A, Ziegert W, Brückner G, and Schober W. Development of the rabbit retina. I. Size of eye and retina, and postnatal cell proliferation. Anat Embryol (Berl). 183: 287–297. 1991. [DOI] [PubMed] [Google Scholar]
- 217.Sheppard LB. The anatomy and histology of the normal rabbit eye with special reference to the ciliary zone. Arch Ophthalmol. 66: 896–904. 1961. [DOI] [PubMed] [Google Scholar]
- 218.Tauber H, Waehneldt TV, and Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 16: 235–238. 1980. [DOI] [PubMed] [Google Scholar]
- 219.Davis FA. The Anatomy and Histology of the Eye and Orbit of the Rabbit. Trans Am Ophthalmol Soc. 27: 2–441, 402–441. 1929. [PMC free article] [PubMed] [Google Scholar]
- 220.Peiffer RL, Jr , Pohm-Thorsen L, and Corcoran K. Models in Opthalmology and Vision Research. In: The Biology of the Laboratory Rabbit, 2nd ed. PJ Manning, DH Ringler, and CE Newcomer (eds). Academic Press, San Diego. 409–433. 1994. [Google Scholar]
- 221.Prince JH. Cornea, Trabecular Region, and Sclera. In: The Rabbit in Eye Research. 1st ed. Prince JH (ed). Charles C Thomas Publisher, Illinois. 86–139. 1964. [Google Scholar]
- 222.Williams D. The Rabbit. In: Veterinary Opthalmology: Vol II, 5th ed. KN Gelatt, BC Gilger, TJ Kern (eds). Wiley-Blackwell, Iowa. 1725–1749. 2013.. [Google Scholar]
- 223.Hughes A. Topographical relationships between the anatomy and physiology of the rabbit visual system. Doc Ophthalmol. 30: 33–159. 1971. [DOI] [PubMed] [Google Scholar]
- 224.Hughes A. A schematic eye for the rabbit. Vision Res. 12: 123–138. 1972. [DOI] [PubMed] [Google Scholar]
- 225.Greiner JV, Glonek T, Korb DR, Whalen AC, Hebert E, Hearn SL, Esway JE, and Leahy CD. Volume of the human and rabbit meibomian gland system. Adv Exp Med Biol. 438: 339–343. 1998. [DOI] [PubMed] [Google Scholar]
- 226.Jester JV, Nicolaides N, and Smith RE. Meibomian gland studies: histologic and ultrastructural investigations. Invest Ophthalmol Vis Sci. 20: 537–547. 1981. [PubMed] [Google Scholar]
- 227.Lambert RW, and Smith RE. Pathogenesis of blepharoconjunctivitis complicating 13-cis-retinoic acid (isotretinoin) therapy in a laboratory model. Invest Ophthalmol Vis Sci. 29: 1559–1564. 1988. [PubMed] [Google Scholar]
- 228.Bergmanson JP, Doughty MJ, and Blocker Y. The acinar and ductal organisation of the tarsal accessory lacrimal gland of wolfring in rabbit eyelid. Exp Eye Res. 68: 411–421. 1999. [DOI] [PubMed] [Google Scholar]
- 229.Chang M, Lee Y, and Baek S. The functional and histopathologic change in the levator palpebrae superioris and Müller muscle after subconjunctival injection of triamcinolone acetonide. J Craniofac Surg. 26: 285–289. 2015. [DOI] [PubMed] [Google Scholar]
- 230.Francis BA, Weiland JD, Sachs NA, and Chang EL. Benzalkonium chloride-induced denervation of orbicularis oculi muscle in rabbits. Invest Ophthalmol Vis Sci. 54: 1868–1872. 2013. [DOI] [PubMed] [Google Scholar]
- 231.Murphy EH, Garone M, Tashayyod D, and Baker RB. Innervation of extraocular muscles in the rabbit. J Comp Neurol. 254: 78–90. 1986. [DOI] [PubMed] [Google Scholar]
- 232.Prangen AD. A Study of the Comparative Anatomy of the Extra-ocular Muscles. Trans Am Ophthalmol Soc. 26: 353–380. 1928. [PMC free article] [PubMed] [Google Scholar]
- 233.Sheppard LB. The anatomy and histology of the normal rabbit eye with special reference to the ciliary zone. Arch Ophthalmol. 67: 87–100. 1962. [DOI] [PubMed] [Google Scholar]
- 234.Prince JH, Diesem CD, Eglitis I, and Ruskell GL. The Rabbit. In: Anatomy and Histology of the Eye and Orbit in Domestic Animals, 1st ed. JH Prince, CD Diesem, I Eglitis, and GL Ruskell (eds). Charles C Thomas Publisher, Illinois. 260–297. 1960. [Google Scholar]
- 235.Prince JH, and Eglitis I. Uvea. In: The Rabbit in Eye Research. JH Prince (ed). Charles C Thomas Publisher, Illinois. 140–171. 1964.. [Google Scholar]
- 236.Butovich IA, Lu H, McMahon A, and Eule JC. Toward an animal model of the human tear film: biochemical comparison of the mouse, canine, rabbit, and human meibomian lipidomes. Invest Ophthalmol Vis Sci. 53: 6881–6896. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Korb DR, Greiner JV, Glonek T, Whalen A, Hearn SL, Esway JE, and Leahy CD. Human and rabbit lipid layer and interference pattern observations. Adv Exp Med Biol. 438: 305–308. 1998. [DOI] [PubMed] [Google Scholar]
- 238.Leonard BC, Yañez-Soto B, Raghunathan VK, Abbott NL, and Murphy CJ. Species variation and spatial differences in mucin expression from corneal epithelial cells. Exp Eye Res. 152: 43–48. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wei XE, Markoulli M, Zhao Z, and Willcox MD. Tear film break-up time in rabbits. Clin Exp Optom. 96: 70–75. 2013. [DOI] [PubMed] [Google Scholar]
- 240.Cain C, and Phillips TE. Developmental changes in conjunctiva-associated lymphoid tissue of the rabbit. Invest Ophthalmol Vis Sci. 49: 644–649. 2008. [DOI] [PubMed] [Google Scholar]
- 241.Chodosh J, Nordquist RE, and Kennedy RC. Comparative anatomy of mammalian conjunctival lymphoid tissue: a putative mucosal immune site. Dev Comp Immunol. 22: 621–630. 1998. [DOI] [PubMed] [Google Scholar]
- 242.Knop E, and Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 206: 271–285. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Andrew SE. Corneal diseases of rabbits. Vet Clin North Am Exot Anim Pract. 5: 341–356. 2002. [DOI] [PubMed] [Google Scholar]
- 244.Delaney KH. Diagnostic exercise: apparent corneal occlusion in a New Zealand White rabbit. Contemp Top Lab Anim Sci. 34: 76–77. 1995. [PubMed] [Google Scholar]
- 245.Katsuta O, Shinomiya K, Mochizuki T, Kikkawa C, Yoshimi M, and Ikuse T. Pseudopterygium: unique conjunctival stricture observed in Japanese White rabbit. J Toxicol Pathol. 21: 239–241. 2008. [Google Scholar]
- 246.Kim JY, Williams DL, Rho KS, Kim KH, Lee YS, and Jeong SW. Surgical correction of aberrant conjunctival overgrowth in a rabbit: a case report. Ir Vet J. 66: 18. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Roze M, Ridings B, and Lagadic M. Comparative morphology of epicorneal conjunctival membranes in rabbits and human pterygium. Vet Ophthalmol. 4: 171–174. 2001. [DOI] [PubMed] [Google Scholar]
- 248.Wagner F, and Fehr M. Common ophthalmic problems in pet rabbits. J Exot Pet Med. 6: 158–167. 2007. [Google Scholar]
- 249.Chan T, Payor S, and Holden BA. Corneal thickness profiles in rabbits using an ultrasonic pachometer. Invest Ophthalmol Vis Sci. 24: 1408–1410. 1983. [PubMed] [Google Scholar]
- 250.Ichijima H, Petroll WM, Jester JV, Barry PA, Andrews PM, Dai M, and Cavanagh HD. In vivo confocal microscopic studies of endothelial wound healing in rabbit cornea. Cornea. 12: 369–378. 1993. [DOI] [PubMed] [Google Scholar]
- 251.Labbé A, Liang H, Martin C, Brignole-Baudouin F, Warnet JM, and Baudouin C. Comparative anatomy of laboratory animal corneas with a new-generation high-resolution in vivo confocal microscope. Curr Eye Res. 31: 501–509. 2006. [DOI] [PubMed] [Google Scholar]
- 252.Minkowski JS, Bartels SP, Delori FC, Lee SR, Kenyon KR, and Neufeld AH. Corneal endothelial function and structure following cryo-injury in the rabbit. Invest Ophthalmol Vis Sci. 25: 1416–1425. 1984. [PubMed] [Google Scholar]
- 253.Sailstad DM, and Peiffer RL, Jr . Specular microscopic observations of the corneal endothelium in the normal rabbit. Lab Anim. 15: 393–395. 1981. [DOI] [PubMed] [Google Scholar]
- 254.Wang X, Dong J, and Wu Q. Mean central corneal thickness and corneal power measurements in pigmented and white rabbits using Visante optical coherence tomography and ATLAS corneal topography. Vet Ophthalmol. 17: 87–90. 2014. [DOI] [PubMed] [Google Scholar]
- 255.Wang X, and Wu Q. Normal corneal thickness measurements in pigmented rabbits using spectral-domain anterior segment optical coherence tomography. Vet Ophthalmol. 16: 130–134. 2013. [DOI] [PubMed] [Google Scholar]
- 256.Yüksel H, Türkcü FM, Ari Ş, Çinar Y, Cingü AK, Şahin M, Şahin A, Özkurt Z, and Çaça İ. Anterior segment parameters of rabbits with rotating Scheimpflug camera. Vet Ophthalmol. 18: 210–213. 2015. [DOI] [PubMed] [Google Scholar]
- 257.Bechmann M, Thiel MJ, Neubauer AS, Ullrich S, Ludwig K, Kenyon KR, and Ulbig MW. Central corneal thickness measurement with a retinal optical coherence tomography device versus standard ultrasonic pachymetry. Cornea. 20: 50–54. 2001. [DOI] [PubMed] [Google Scholar]
- 258.Cintron C, Covington H, and Kublin CL. Morphogenesis of rabbit corneal stroma. Invest Ophthalmol Vis Sci. 24: 543–556. 1983. [PubMed] [Google Scholar]
- 259.Kaye GI, and Pappas GD. Studies on the cornea. I. The fine structure of the rabbit cornea and the uptake and transport of colloidal particles by the cornea in vivo. J Cell Biol. 12: 457–479. 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Ojeda JL, Ventosa JA, and Piedra S. The three-dimensional microanatomy of the rabbit and human cornea. A chemical and mechanical microdissection-SEM approach. J Anat. 199: 567–576. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Wilson SE, and Hong JW. Bowman’s layer structure and function: critical or dispensable to corneal function? A hypothesis. Cornea. 19: 417–420. 2000. [DOI] [PubMed] [Google Scholar]
- 262.Staatz WD, and Van Horn DL. The effects of aging and inflammation on corneal endothelial wound healing in rabbits. Invest Ophthalmol Vis Sci. 19: 983–986. 1980. [PubMed] [Google Scholar]
- 263.Van Horn DL, Sendele DD, Seideman S, and Buco PJ. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest Ophthalmol Vis Sci. 16: 597–613. 1977. [PubMed] [Google Scholar]
- 264.Wagner F, Brügmann M, Drommer W, and Fehr M. Corneal dermoid in a dwarf rabbit (Oryctolagus cuniculi). Contemp Top Lab Anim Sci. 39: 39–40. 2000. [PubMed] [Google Scholar]
- 265.Styer CM, Ferrier WT, Labelle P, Griffey SM, and Kendall LV. Limbic dermoid in a New Zealand White rabbit (Oryctolagus cuniculus). Contemp Top Lab Anim Sci. 44: 46–48. 2005. [PubMed] [Google Scholar]
- 266.Moore CP, Dubielzig R, and Glaza SM. Anterior corneal dystrophy of American Dutch belted rabbits: biomicroscopic and histopathologic findings. Vet Pathol. 24: 28–33. 1987. [DOI] [PubMed] [Google Scholar]
- 267.Boorman G, Crabbs TA, Kolenda-Roberts H, Latimer K, Miller AD, Muravnick KB, Nyska A, Ochoa R, Pardo ID, Ramot Y, Rao DB, Schuh J, Suttie A, Travlos GS, Ward JM, Wolf JC, and Elmore SA. Proceedings of the 2011 National Toxicology Program Satellite Symposium. Toxicol Pathol. 40: 321–344. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Port CD, and Dodd DC. Two cases of corneal epithelial dystrophy in rabbits. Lab Anim Sci. 33: 587–588. 1983. [PubMed] [Google Scholar]
- 269.Durand-Cavagna G, Hubert MF, Gerin G, and Molon-Noblot S. Spontaneous pre-Descemet’s membrane corneal opacities in rabbits. Lab Anim Sci. 48: 310–313. 1998. [PubMed] [Google Scholar]
- 270.Garibaldi BA, and Goad ME. Lipid keratopathy in the Watanabe (WHHL) rabbit. Vet Pathol. 25: 173–174. 1988. [DOI] [PubMed] [Google Scholar]
- 271.Janes RG. Changes in the Rabbit’s Eye Caused by Cholesterol Feeding. Am J Ophthalmol. 58: 819–828. 1964. [PubMed] [Google Scholar]
- 272.Roth SI, Stock EL, Siel JM, Mendelsohn A, Reddy C, Preskill DG, and Ghosh S. Pathogenesis of experimental lipid keratopathy. An ultrastructural study of an animal model system. Invest Ophthalmol Vis Sci. 29: 1544–1551. 1988. [PubMed] [Google Scholar]
- 273.Sebesteny A, Sheraidah GA, Trevan DJ, Alexander RA, and Ahmed AI. Lipid keratopathy and atheromatosis in an SPF laboratory rabbit colony attributable to diet. Lab Anim. 19: 180–188. 1985. [DOI] [PubMed] [Google Scholar]
- 274.Fernández-Navarro J, Aldea P, de Hoz R, Salazar JJ, Ramírez AI, Rojas B, Gallego BI, Triviño A, Tejerina T, and Ramírez JM. Neuroprotective effects of low-dose statins in the retinal ultrastructure of hypercholesterolemic rabbits. PLoS One. 11: e0154800. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Rojas B, Ramírez AI, Salazar JJ, de Hoz R, Redondo A, Raposo R, Mendez T, Tejerina T, Triviño A, and Ramírez JM. Low-dosage statins reduce choroidal damage in hypercholesterolemic rabbits. Acta Ophthalmol. 89: 660–669. 2011. [DOI] [PubMed] [Google Scholar]
- 276.Salazar JJ, Ramírez AI, de Hoz R, Rojas B, Ruiz E, Tejerina T, Triviño A, and Ramírez JM. Alterations in the choroid in hypercholesterolemic rabbits: reversibility after normalization of cholesterol levels. Exp Eye Res. 84: 412–422. 2007. [DOI] [PubMed] [Google Scholar]
- 277.Triviño A, Ramírez AI, Salazar JJ, de Hoz R, Rojas B, Padilla E, Tejerina T, and Ramírez JM. A cholesterol-enriched diet induces ultrastructural changes in retinal and macroglial rabbit cells. Exp Eye Res. 83: 357–366. 2006. [DOI] [PubMed] [Google Scholar]
- 278.Yamagiwa Y, Takei Y, Koizumi H, Nemoto S, Kurata M, and Satoh H. Pathological features of corneal phospholipidosis in juvenile white rabbits induced by ocular instillation of chloroquine or amiodarone. Toxicol Pathol. 47: 26–34. 2019. [DOI] [PubMed] [Google Scholar]
- 279.Fine BS, Berkow JW, and Fine S. Corneal calcification. Science. 162: 129–130. 1968. [DOI] [PubMed] [Google Scholar]
- 280.McCulley JP. Ocular hydrofluoric acid burns: animal model, mechanism of injury and therapy. Trans Am Ophthalmol Soc. 88: 649–684. 1990. [PMC free article] [PubMed] [Google Scholar]
- 281.Obenberger J, Ocumpaugh DE, and Cubberly MG. Experimental corneal calcification in animals treated with dihydrotachysterol. Invest Ophthalmol. 8: 467–474. 1969. [PubMed] [Google Scholar]
- 282.Doughman DJ, Olson GA, Nolan S, and Hajny RG. Experimental band keratopathy. Arch Ophthalmol. 81: 264–271. 1969. [DOI] [PubMed] [Google Scholar]
- 283.Economon JW, Silverstein AM, and Zimmerman LE. Band keratopathy in a rabbit colony. Invest Ophthalmol. 2: 361–368. 1963. [PubMed] [Google Scholar]
- 284.Short BG. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol Pathol. 36: 49–62. 2008. [DOI] [PubMed] [Google Scholar]
- 285.Werner L, Chew J, and Mamalis N. Experimental evaluation of ophthalmic devices and solutions using rabbit models. Vet Ophthalmol. 9: 281–291. 2006. [DOI] [PubMed] [Google Scholar]
- 286.Bergmanson JP. The anatomy of the rabbit aqueous outflow pathway. Acta Ophthalmol (Copenh). 63: 493–501. 1985. [DOI] [PubMed] [Google Scholar]
- 287.Grierson I, Nagasubramanian S, Edwards J, Millar LC, and Ennis K. The effects of various levels of intraocular pressure on the rabbit’s outflow system. Exp Eye Res. 42: 383–397. 1986. [DOI] [PubMed] [Google Scholar]
- 288.Nishida S, Uchida H, Takeuchi M, Sui GQ, Mizutani S, and Iwaki M. Scanning electron microscope study of the rabbit anterior chamber angle. Med Mol Morphol. 38: 54–62. 2005. [DOI] [PubMed] [Google Scholar]
- 289.Samuelson DA, and Gelatt KN. Aqueous outflow in the beagle. II. Postnatal morphologic development of the iridocorneal angle: corneoscleral trabecular meshwork and angular aqueous plexus. Curr Eye Res. 3: 795–807. 1984. [DOI] [PubMed] [Google Scholar]
- 290.Sheppard LB. Intrascleral drainage channels of the normal rabbit eye. Trans Am Ophthalmol Soc. 57: 99–108. 1959. [PMC free article] [PubMed] [Google Scholar]
- 291.Sheppard LB. The anatomy and histology of the normal rabbit eye with special reference to the ciliary zone. Arch Ophthalmol. 67: 87–100. 1962. [DOI] [PubMed] [Google Scholar]
- 292.Bouhenni RA, Dunmire J, Sewell A, and Edward DP. Animal models of glaucoma. J Biomed Biotechnol. 2012: 692609. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Bunt-Milam AH, Dennis MB, Jr , and Bensinger RE. Optic nerve head axonal transport in rabbits with hereditary glaucoma. Exp Eye Res. 44: 537–551. 1987. [DOI] [PubMed] [Google Scholar]
- 294.Fox RR, Crary DD, Babino EJ, Jr , and Sheppard LB. Buphthalmia in the rabbit. Pleiotropic effects of the (bu) gene and a possible explanation of mode of gene action. J Hered. 60: 206–212. 1969. [DOI] [PubMed] [Google Scholar]
- 295.Hanna BL, Sawin PB, and Sheppard LB. Recessive buphthalmos in the rabbit. Genetics. 47: 519–529. 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Knepper PA, McLone DG, Goossens W, Vanden Hoek T, and Higbee RG. Ultrastructural alterations in the aqueous outflow pathway of adult buphthalmic rabbits. Exp Eye Res. 52: 525–533. 1991. [DOI] [PubMed] [Google Scholar]
- 297.Tesluk GC, Peiffer RL, and Brown D. A clinical and pathological study of inherited glaucoma in New Zealand White rabbits. Lab Anim. 16: 234–239. 1982. [DOI] [PubMed] [Google Scholar]
- 298.Ueno A, Tawara A, Kubota T, Ohnishi Y, Inomata H, and Solomon AS. Histopathological changes in iridocorneal angle of inherited glaucoma in rabbits. Graefes Arch Clin Exp Ophthalmol. 237: 654–660. 1999. [DOI] [PubMed] [Google Scholar]
- 299.Edward DP, and Bouhenni R. Anterior segment alterations and comparative aqueous humor proteomics in the buphthalmic rabbit (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 109: 66–114. 2011. [PMC free article] [PubMed] [Google Scholar]
- 300.Zernii EY, Baksheeva VE, Iomdina EN, Averina OA, Permyakov SE, Philippov PP, Zamyatnin AA, and Senin II. Rabbit models of ocular diseases: new relevance for classical approaches. CNS Neurol Disord Drug Targets. 15: 267–291. 2016. [DOI] [PubMed] [Google Scholar]
- 301.Morrison JC, DeFrank MP, and Van Buskirk EM. Regional microvascular anatomy of the rabbit ciliary body. Invest Ophthalmol Vis Sci. 28: 1314–1324. 1987. [PubMed] [Google Scholar]
- 302.Pataky PE. Structure and innervation of the ciliary processes of the albino rabbit eye. Anat Rec. 168: 339–349. 1970. [DOI] [PubMed] [Google Scholar]
- 303.Tserevelakis GJ, Avtzi S, Tsilimbaris MK, and Zacharakis G. Delineating the anatomy of the ciliary body using hybrid optical and photoacoustic imaging. J Biomed Opt. 22: 60501. 2017. [DOI] [PubMed] [Google Scholar]
- 304.Wegner K. Regional differences in ultrastructure of the rabbit ciliary processes: the effect of anesthetics and fixation procedures. Invest Ophthalmol. 6: 177–191. 1967. [PubMed] [Google Scholar]
- 305.Funk RH. The vessel architecture of the pars plana in the cynomolgus monkey, rat and rabbit eye. A scanning electron microscopic study of plastic corrosion casts. Ophthalmic Res. 25: 337–348. 1993. [DOI] [PubMed] [Google Scholar]
- 306.Langevin NE, Schafer KA, Turner OC, McPherson BJ, and Rose RE. Historical data: histopathology lesions observed in the eyes of control rabbits in topical ocular administration and contact lens studies. Toxicol Pathol. 46: 799–820. 2018. [DOI] [PubMed] [Google Scholar]
- 307.Griffith JW, Sassani JW, Bowman TA, and Lang CM. Osseous choristoma of the ciliary body in guinea pigs. Vet Pathol. 25: 100–102. 1988. [DOI] [PubMed] [Google Scholar]
- 308.Lynch GL, and Scagliotti RH. Osseous metaplasia in the eye of a dog. Vet Pathol. 44: 222–224. 2007. [DOI] [PubMed] [Google Scholar]
- 309.Williams D, and Sullivan A. Ocular disease in the guinea pig (Cavia porcellus): a survey of 1000 animals. Vet Ophthalmol. 13(Suppl): 54–62. 2010. [DOI] [PubMed] [Google Scholar]
- 310.Fernandez-Bueno I, Di Lauro S, Alvarez I, Lopez JC, Garcia-Gutierrez MT, Fernandez I, Larra E, and Pastor JC. Safety and biocompatibility of a new high-density polyethylene-based spherical integrated porous orbital implant: an experimental study in rabbits. J Ophthalmol. 2015: 904096. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Kouchi M, Ueda Y, Horie H, and Tanaka K. Ocular lesions in Watanabe heritable hyperlipidemic rabbits. Vet Ophthalmol. 9: 145–148. 2006. [DOI] [PubMed] [Google Scholar]
- 312.Kuszak JR. The ultrastructure of epithelial and fiber cells in the crystalline lens. Int Rev Cytol. 163: 305–350. 1995. [DOI] [PubMed] [Google Scholar]
- 313.Jeong MB, Kim NR, Yi NY, Park SA, Kim MS, Park JH, Jeong SM, Seo KD, Nam TC, Oh YS, Won MH, and Seo KM. Spontaneous ophthalmic diseases in 586 New Zealand White rabbits. Exp Anim. 54: 395–403. 2005. [DOI] [PubMed] [Google Scholar]
- 314.Munger RJ, Jensen VB, Bouldin TW, and Peiffer RL. Bilateral neuroepithelial choristomas of the optic disc in a cynomolgus monkey (Macaca fascicularis): a case report. Vet Ophthalmol. 5: 221–226. 2002. [DOI] [PubMed] [Google Scholar]
- 315.Peng X, Roshwalb S, Cooper TK, Zimmerman H, and Christensen ND. High incidence of spontaneous cataracts in aging laboratory rabbits of an inbred strain. Vet Ophthalmol. 18: 186–190. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sanchez RF, Becker R, Dawson C, Escanilla N, and Lam R. Calculation of posterior chamber intraocular lens (IOL) size and dioptric power for use in pet rabbits undergoing phacoemulsification. Vet Ophthalmol. 20: 242–249. 2017. [DOI] [PubMed] [Google Scholar]
- 317.Sanchez RF, Everson R, Hedley J, Dawson C, Lam R, Priestnall SL, Garcia de Carellan A, de Miguel C, and Seymour C. Rabbits with naturally occurring cataracts referred for phacoemulsification and intraocular lens implantation: a preliminary study of 12 cases. Vet Ophthalmol. 21: 399–412. 2018. [DOI] [PubMed] [Google Scholar]
- 318.Al-Khudari S, Donohue ST, Al-Ghoul WM, and Al-Ghoul KJ. Age-related compaction of lens fibers affects the structure and optical properties of rabbit lenses. BMC Ophthalmol. 7: 19. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Frenzel EM, Neely KA, Walsh AW, Cameron JD, and Gregerson DS. A new model of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 39: 2157–2164. 1998. [PubMed] [Google Scholar]
- 320.Hsu YW, Yeh SM, Chen YY, Chen YC, Lin SL, and Tseng JK. Protective effects of taurine against alloxan-induced diabetic cataracts and refraction changes in New Zealand White rabbits. Exp Eye Res. 103: 71–77. 2012. [DOI] [PubMed] [Google Scholar]
- 321.Kralinger MT, Kieselbach GF, Voigt M, Hayden B, Hernandez E, Fernandez V, and Parel JM. Experimental model for proliferative vitreoretinopathy by intravitreal dispase: limited by zonulolysis and cataract. Ophthalmologica. 220: 211–216. 2006. [DOI] [PubMed] [Google Scholar]
- 322.Yuan L, Yao H, Xu Y, Chen M, Deng J, Song Y, Sui T, Wang Y, Huang Y, Li Z, and Lai L. CRISPR/Cas9-mediated mutation of αA-crystallin gene induces congenital cataracts in rabbits. Invest Ophthalmol Vis Sci. 58: BIO34–BIO41. 2017. [DOI] [PubMed] [Google Scholar]
- 323.Gwon A, Mantras C, Gruber L, and Cunanan C. Concanavalin A-induced posterior subcapsular cataract: a new model of cataractogenesis. Invest Ophthalmol Vis Sci. 34: 3483–3488. 1993. [PubMed] [Google Scholar]
- 324.Hayasaka Y, Hayasaka S, and Nagaki Y. Ocular changes after intravitreal injection of methanol, formaldehyde, or formate in rabbits. Pharmacol Toxicol. 89: 74–78. 2001. [DOI] [PubMed] [Google Scholar]
- 325.Lubek BM, Avaria M, Basu PK, and Wells PG. Pharmacological studies on the in vivo cataractogenicity of acetaminophen in mice and rabbits. Fundam Appl Toxicol. 10: 596–606. 1988. [DOI] [PubMed] [Google Scholar]
- 326.Morsy EA, Salem HM, Khattab MS, Hamza DA, and Abuowarda MM. Encephalitozoon cuniculi infection in farmed rabbits in Egypt. Acta Vet Scand. 62: 11. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Özkan Ö, and Alcigir ME. Subacute stage of encephalitozoon cuniculi infection in eye lesions of rabbit in Turkey. Iran J Parasitol. 13: 301–309. 2018. [PMC free article] [PubMed] [Google Scholar]
- 328.Ashton N, Cook C, and Clegg F. Encephalitozoonosis (nosematosis) causing bilateral cataract in a rabbit. Br J Ophthalmol. 60: 618–631. 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Felchle LM, and Sigler RL. Phacoemulsification for the management of Encephalitozoon cuniculi-induced phacoclastic uveitis in a rabbit. Vet Ophthalmol. 5: 211–215. 2002. [DOI] [PubMed] [Google Scholar]
- 330.Giordano C, Weigt A, Vercelli A, Rondena M, Grilli G, and Giudice C. Immunohistochemical identification of Encephalitozoon cuniculi in phacoclastic uveitis in four rabbits. Vet Ophthalmol. 8: 271–275. 2005. [DOI] [PubMed] [Google Scholar]
- 331.Latney LV, Bradley CW, and Wyre NR. Encephalitozoon cuniculi in pet rabbits: diagnosis and optimal management. Vet Med (Auckl). 5: 169–180. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Matsumoto B, Blanks JC, and Ryan SJ. Topographic variations in the rabbit and primate internal limiting membrane. Invest Ophthalmol Vis Sci. 25: 71–82. 1984. [PubMed] [Google Scholar]
- 333.Los LI, van Luyn MJ, and Nieuwenhuis P. Organization of the rabbit vitreous body: lamellae, Cloquet’s channel and a novel structure, the ‘alae canalis Cloqueti’. Exp Eye Res. 69: 343–350. 1999. [DOI] [PubMed] [Google Scholar]
- 334.Ponsioen TL, Hooymans JM, and Los LI. Remodelling of the human vitreous and vitreoretinal interface--a dynamic process. Prog Retin Eye Res. 29: 580–595. 2010. [DOI] [PubMed] [Google Scholar]
- 335.Lukáts A, Szabó A, Röhlich P, Vígh B, and Szél A. Photopigment coexpression in mammals: comparative and developmental aspects. Histol Histopathol. 20: 551–574. 2005. [DOI] [PubMed] [Google Scholar]
- 336.Schnitzer J. The development of astrocytes and blood vessels in the postnatal rabbit retina. J Neurocytol. 17: 433–449. 1988. [DOI] [PubMed] [Google Scholar]
- 337.Jack RL. Regression of the hyaloid vascular system. An ultrastructural analysis. Am J Ophthalmol. 74: 261–272. 1972. [DOI] [PubMed] [Google Scholar]
- 338.Jack RL. Ultrastructure of the hyaloid vascular system. Arch Ophthalmol. 87: 555–567. 1972. [DOI] [PubMed] [Google Scholar]
- 339.Boillot T, Graille M, Williams D, and Rosolen SG. Unilateral persistent hyperplastic tunica vasculosa lentis and persistent hyperplastic primary vitreous in a rabbit. Vet Ophthalmol. 18: 510–514. 2015. [DOI] [PubMed] [Google Scholar]
- 340.Los LI, van Luyn MJ, Eggli PS, Dijk F, and Nieuwenhuis P. Vascular remnants in the rabbit vitreous body. II. Enzyme digestion and immunohistochemical studies. Exp Eye Res. 71: 153–165. 2000. [DOI] [PubMed] [Google Scholar]
- 341.Los LI, van Luyn MJ, and Nieuwenhuis P. Vascular remnants in the rabbit vitreous body. I. Morphological characteristics and relationship to vitreous embryonic development. Exp Eye Res. 71: 143–151. 2000. [DOI] [PubMed] [Google Scholar]
- 342.De Schaepdrijver L, Simoens P, Lauwers H, and De Geest JP. Retinal vascular patterns in domestic animals. Res Vet Sci. 47: 34–42. 1989. [PubMed] [Google Scholar]
- 343.Morcos Y, and Chan-Ling T. Identification of oligodendrocyte precursors in the myelinated streak of the adult rabbit retina in vivo. Glia. 21: 163–182. 1997. [PubMed] [Google Scholar]
- 344.Morcos Y, and Chan-Ling T. Concentration of astrocytic filaments at the retinal optic nerve junction is coincident with the absence of intra-retinal myelination: comparative and developmental evidence. J Neurocytol. 29: 665–678. 2000. [DOI] [PubMed] [Google Scholar]
- 345.Schnitzer J. Distribution and immunoreactivity of glia in the retina of the rabbit. J Comp Neurol. 240: 128–142. 1985. [DOI] [PubMed] [Google Scholar]
- 346.Tripathi B, and Ashton N. Vaso-glial connections in the rabbit retina. Br J Ophthalmol. 55: 1–11. 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Uga S, and Smelser. Invest Ophthalmol. 12: 434–448. 1973. [PubMed] [Google Scholar]
- 348.Choudhury BP. Ganglion cell distribution in the albino rabbit’s retina. Exp Neurol. 72: 638–644. 1981. [DOI] [PubMed] [Google Scholar]
- 349.Donatien P, and Jeffery G. Correlation between rod photoreceptor numbers and levels of ocular pigmentation. Invest Ophthalmol Vis Sci. 43: 1198–1203. 2002. [PubMed] [Google Scholar]
- 350.Oyster CW, Takahashi ES, and Hurst DC. Density, soma size, and regional distribution of rabbit retinal ganglion cells. J Neurosci. 1: 1331–1346. 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Provis JM. The distribution and size of ganglion cells in the regina of the pigmented rabbit: a quantitative analysis. J Comp Neurol. 185: 121–137. 1979. [DOI] [PubMed] [Google Scholar]
- 352.Vaney DI. A quantitative comparison between the ganglion cell populations and axonal outflows of the visual streak and periphery of the rabbit retina. J Comp Neurol. 189: 215–233. 1980. [DOI] [PubMed] [Google Scholar]
- 353.Steele-Russell I, Russell MI, Castiglioni JA, and Graham J. Differential retinal origins of separate anatomical channels for pattern and motion vision in rabbit. Exp Brain Res. 222: 99–111. 2012. [DOI] [PubMed] [Google Scholar]
- 354.Rapaport DH, and Stone J. The area centralis of the retina in the cat and other mammals: focal point for function and development of the visual system. Neuroscience. 11: 289–301. 1984. [DOI] [PubMed] [Google Scholar]
- 355.Famiglietti EV, and Sharpe SJ. Regional topography of rod and immunocytochemically characterized “blue” and “green” cone photoreceptors in rabbit retina. Vis Neurosci. 12: 1151–1175. 1995. [DOI] [PubMed] [Google Scholar]
- 356.Juliusson B, Bergström A, Röhlich P, Ehinger B, van Veen T, and Szél A. Complementary cone fields of the rabbit retina. Invest Ophthalmol Vis Sci. 35: 811–818. 1994. [PubMed] [Google Scholar]
- 357.Röhlich P, van Veen T, and Szél A. Two different visual pigments in one retinal cone cell. Neuron. 13: 1159–1166. 1994. [DOI] [PubMed] [Google Scholar]
- 358.Lucas RJ. Mammalian inner retinal photoreception. Curr Biol. 23: R125–R133. 2013. [DOI] [PubMed] [Google Scholar]
- 359.Lira-Carrera MM, Gutiérrez-Amavizca BE, Álvarez-Araujo LJ, Aguirre-Ramírez M, and Pérez-León JA. Analysis of melanopsin gene expression in the rabbit retina at different ages. Genet Mol Res. 16. 2017; . [DOI] [PubMed] [Google Scholar]
- 360.Prince JH, and McConnell DG. Retina and optic nerve. In: The Rabbit in Eye Research, 1st ed. JH Prince (ed). Charles C Thomas Publisher, Illinois. 385–448. 1964.. [Google Scholar]
- 361.Robinson SR, and Dreher Z. Müller cells in adult rabbit retinae: morphology, distribution and implications for function and development. J Comp Neurol. 292: 178–192. 1990. [DOI] [PubMed] [Google Scholar]
- 362.Booij JC, Baas DC, Beisekeeva J, Gorgels TG, and Bergen AA. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 29: 1–18. 2010. [DOI] [PubMed] [Google Scholar]
- 363.Ferrara D, Waheed NK, and Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res. 52: 130–155. 2016. [DOI] [PubMed] [Google Scholar]
- 364.Nakaizumi Y. The Ultrastructure of Bruch’s Membrane. I. Human, Monkey, Rabbit, Guinea Pig, and Rat Eyes. Arch Ophthalmol. 72: 380–387. 1964. [DOI] [PubMed] [Google Scholar]
- 365.Daniels AB, Froehler MT, Pierce JM, Nunnally AH, Calcutt MW, Bridges TM, LaNeve DC, Williams PE, Boyd KL, Reyzer ML, Lindsley CW, Friedman DL, and Richmond A. Pharmacokinetics, tissue localization, toxicity, and treatment efficacy in the first small animal (Rabbit) model of intra-arterial chemotherapy for retinoblastoma. Invest Ophthalmol Vis Sci. 59: 446–454. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Albrecht May C. Comparative anatomy of the optic nerve head and inner retina in non-primate animal models used for glaucoma research. Open Ophthalmol J. 2: 94–101. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Ninomiya H, Inomata T, and Kanemaki N. Microvascular architecture of the rabbit eye: a scanning electron microscopic study of vascular corrosion casts. J Vet Med Sci. 70: 887–892. 2008. [DOI] [PubMed] [Google Scholar]
- 368.Sugiyama K, Bacon DR, Morrison JC, and Van Buskirk EM. Optic nerve head microvasculature of the rabbit eye. Invest Ophthalmol Vis Sci. 33: 2251–2261. 1992. [PubMed] [Google Scholar]
- 369.Balaratnasingam C, Kang MH, Yu P, Chan G, Morgan WH, Cringle SJ, and Yu DY. Comparative quantitative study of astrocytes and capillary distribution in optic nerve laminar regions. Exp Eye Res. 121: 11–22. 2014. [DOI] [PubMed] [Google Scholar]
- 370.Lockwood H, Reynaud J, Gardiner S, Grimm J, Libertiaux V, Downs JC, Yang H, and Burgoyne CF. Lamina cribrosa microarchitecture in normal monkey eyes part 1: methods and initial results. Invest Ophthalmol Vis Sci. 56: 1618–1637. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Quigley HA. The contribution of the sclera and lamina cribrosa to the pathogenesis of glaucoma: Diagnostic and treatment implications. Prog Brain Res. 220: 59–86. 2015. [DOI] [PubMed] [Google Scholar]
- 372.Ashton N, Tripathi B, and Knight G. Effect of oxygen on the developing retinal vessels of the rabbit. I. Anatomy and development of the retinal vessels of the rabbit. Exp Eye Res. 14: 214–220. 1972. [DOI] [PubMed] [Google Scholar]
- 373.Schafer KA, and Render JA. Toxicologic Pathology of the Eye: Alterations of the Lens and Posterior Segment. In: Assessing Ocular Toxicology in Laboratory Animals. AB Wier, and M Collins (eds). Humana Press, New York. 219–257. 2013.. [Google Scholar]
- 374.Kawasako K, Oshikata T, Kanno T, and Hamamura M. Neurofilament accumulation in rabbit retinas. J Comp Pathol. 153: 283–286. 2015. [DOI] [PubMed] [Google Scholar]
- 375.Ts’o MO, and Friedman E. The retinal pigment epithelium. I. Comparative histology. Arch Ophthalmol. 78: 641–649. 1967. [DOI] [PubMed] [Google Scholar]
- 376.Olsen TW, Aaberg SY, Geroski DH, and Edelhauser HF. Human sclera: thickness and surface area. Am J Ophthalmol. 125: 237–241. 1998. [DOI] [PubMed] [Google Scholar]
- 377.Vurgese S, Panda-Jonas S, and Jonas JB. Scleral thickness in human eyes. PLoS One. 7: e29692. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Ruskell GL. Blood Vessels of the Orbit and Globe. In: The Rabbit in Eye Research, 1st ed. JH Prince (ed). Charles C Thomas Publisher, Illinois. 514–553. 1964.. [Google Scholar]
- 379.Sakai T. Comparative Anatomy of Mammalian Harderian Glands. In: Harderian Glands: Porphyrin Metabolism, Behavioral and Endocrine Effects. SM Webb, RD Hoffman, ML Puig-Domingo, and RJ Reiter (eds). Springer-Verlag, Berlin. 7–22. 1992.. [Google Scholar]
- 380.Gargiulo AM, Ceccarelli P, and Pedini V. The presence of granular excretory ducts in the rabbit zygomatic gland. Anat Histol Embryol. 25: 175–176. 1996. [DOI] [PubMed] [Google Scholar]
- 381.Koutavas H, Anderton PJ, and Millar TJ. Separation and characterisation of cyclic nucleotide phosphodiesterases from the lacrimal, harderian and zygomatic glands of the rabbit. Curr Eye Res. 15: 1191–1197. 1996. [DOI] [PubMed] [Google Scholar]
- 382.Eglitis I. Glands; Lacrimal, Harder, Nictitans. In: The Rabbit in Eye Research. JH Prince (ed). Charles C Thomas Publisher, Illinois. 38–56. 1964. [Google Scholar]
- 383.Hittmair KM, Tichy A, and Nell B. Ultrasonography of the Harderian gland in the rabbit, guinea pig, and chinchilla. Vet Ophthalmol. 17: 175–183. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Eltony SAM. A comparative study of the harderian gland in the female rat and female rabbit (a histological, histochemical, scanning electron microscopic and morphometric study). Egypt J Histol. 32: 46–65. 2009. [Google Scholar]
- 385.Shirama K, Ozawa S, Seyama Y, Kobayashi M, Sawamura S, and Yamada J. Postnatal development of the harderian gland in the rabbit: light and electron microscopic observations. Microsc Res Tech. 37: 572–582. 1997. [DOI] [PubMed] [Google Scholar]
- 386.Payne AP. The harderian gland: a tercentennial review. J Anat. 185: 1–49. 1994. [PMC free article] [PubMed] [Google Scholar]
- 387.Rock CO, Fitzgerald V, Rainey WT, Jr , and Snyder F. Mass spectral identification of 2-(O-acyl)hydroxy fatty acid esters in the white portion of the rabbit Harderian gland. Chem Phys Lipids. 17: 207–212. 1976. [DOI] [PubMed] [Google Scholar]
- 388.Seyama T, Kasama T, Yasugi E, Park SH, and Kano K. Lipids in Harderian Glands and Their Significance. In: Harderian Glands: Porphyrin Metabolism, Behavioral and Endocrine Effects. SM Webb, RD Hoffman, ML Puig-Domingo, and RJ Reiter, (eds). Springer-Verlag, Berlin. 196–216. 1992. [Google Scholar]
- 389.Winterhager E, and Kühnel W. Membrane specializations of the cells of the Harderian gland of the rabbit with particular reference to the mechanism of exocytosis. Cell Tissue Res. 231: 623–636. 1983. [DOI] [PubMed] [Google Scholar]
- 390.Wooding FB. Lipid droplet secretion by the rabbit harderian gland. J Ultrastruct Res. 71: 68–78. 1980. [DOI] [PubMed] [Google Scholar]
- 391.Bayraktaroğlu AG, and Ergün E. Histomorphology of the Harderian gland in the Angora rabbit. Anat Histol Embryol. 39: 494–502. 2010. [DOI] [PubMed] [Google Scholar]
- 392.Bjoerkman N, Nicander L, and Schantz B. On the histology and ultrastructure of the Harderian gland in rabbits. Z Zellforsch Mikrosk Anat. 52: 93–104. 1960. [DOI] [PubMed] [Google Scholar]
- 393.Nadakavukaren MJ. The Mammalian Harderian Gland: Sexual Dimorphism, and its Regulation by Light and Steroids. In: Harderian Glands: Porphyrin Metabolism, Behavioral and Endocrine Effects. SM Webb, RD Hoffman, ML Puig-Domingo, and RJ Reiter (eds). Springer-Verlag, Berlin. 70–90. 1992.. [Google Scholar]
- 394.Ding C, Parsa L, Nandoskar P, Zhao P, Wu K, and Wang Y. Duct system of the rabbit lacrimal gland: structural characteristics and role in lacrimal secretion. Invest Ophthalmol Vis Sci. 51: 2960–2967. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Schechter JE, Warren DW, and Mircheff AK. A lacrimal gland is a lacrimal gland, but rodent’s and rabbit’s are not human. Ocul Surf. 8: 111–134. 2010. [DOI] [PubMed] [Google Scholar]
- 396.Cornell-Bell AH, Sullivan DA, and Allansmith MR. Gender-related differences in the morphology of the lacrimal gland. Invest Ophthalmol Vis Sci. 26: 1170–1175. 1985. [PubMed] [Google Scholar]
- 397.Stibbe EP. A comparative study of the nictitating membrane of birds and mammals. J Anat. 62: 159–176. 1928. [PMC free article] [PubMed] [Google Scholar]
- 398.Schafer KA, and Render JA. Toxicologic Pathology of the Eye: Histologic Preparation and Alterations of the Anterior Segment. In: Assessing Ocular Toxicology in Laboratory Animals. AB Wier, and M Collins (eds). Humana Press, New York. 159–217. 2013: [Google Scholar]
- 399.Marini RP, Foltz CJ, Kersten D, Batchelder M, Kaser W, and Li X. Microbiologic, radiographic, and anatomic study of the nasolacrimal duct apparatus in the rabbit (Oryctolagus cuniculus). Lab Anim Sci. 46: 656–662. 1996. [PubMed] [Google Scholar]
- 400.Frame NJ, and Burkat CN. Identifying an appropriate animal model for the nasolacrimal drainage system. Ophthal Plast Reconstr Surg. 25: 354–358. 2009. [DOI] [PubMed] [Google Scholar]
- 401.Maeda S, Ishikawa M, Abe T, Konno S, and Sakuragi S. Lectin cytochemistry of the lacrimal sac epithelium in experimental dacryocystitis. Jpn J Ophthalmol. 43: 69–74. 1999. [DOI] [PubMed] [Google Scholar]
- 402.Paulsen FP, Föge M, Thale AB, Tillmann BN, and Mentlein R. Animal model for the absorption of lipophilic substances from tear fluid by the epithelium of the nasolacrimal ducts. Invest Ophthalmol Vis Sci. 43: 3137–3143. 2002. [PubMed] [Google Scholar]
- 403.Maurer KJ, Marini RP, Fox JG, and Rogers AB. Polycystic kidney syndrome in New Zealand White rabbits resembling human polycystic kidney disease. Kidney Int. 65: 482–489. 2004. [DOI] [PubMed] [Google Scholar]
- 404.Ogilvie RF, Sabour MS, and Horne NW. Light and electron microscopy of prednisolone-induced nephropathy in rabbits. Diabetes. 14: 595–605. 1965. [DOI] [PubMed] [Google Scholar]
- 405.Rammer L, Nathorst-Windahl G, and Boberg J. Cortisone nephropathy in the rabbit. The effect of heparin on the morphology, plasma lipid levels and plasma lipoprotein lipase activity. Acta Pathol Microbiol Scand. 71: 161–172. 1967. [PubMed] [Google Scholar]
- 406.Rammer L. Cortisone nephropathy in the rabbit. The effect of warfarin administration. Acta Pathol Microbiol Scand. 74: 514–518. 1968. [DOI] [PubMed] [Google Scholar]
- 407.Ritskes-Hoitinga J, Grooten HN, Wienk KJ, Peters M, Lemmens AG, and Beynen AC. Lowering dietary phosphorus concentrations reduces kidney calcification, but does not adversely affect growth, mineral metabolism, and bone development in growing rabbits. Br J Nutr. 91: 367–376. 2004. [DOI] [PubMed] [Google Scholar]
- 408.Burek JD, Duprat P, Owen R, Peter CP, and Van Zwieten MJ. Spontaneous renal disease in laboratory animals. Int Rev Exp Pathol. 30: 231–319. 1988. [DOI] [PubMed] [Google Scholar]
- 409.Hinton M. Kidney disease in the rabbit: a histological survey. Lab Anim. 15: 263–265. 1981. [DOI] [PubMed] [Google Scholar]


