Abstract
Purpose
To evaluate the visual outcomes, contrast sensitivity, reading performance and patient satisfaction after bilateral implantation of AT LARA extended depth of focus (EDoF) intraocular lenses (IOLs).
Methods
Patients undergoing phacoemulsification for age-related cataract and satisfying the eligibility criteria underwent bilateral implantation with AT LARA EDoF IOLs (Carl Zeiss Meditec, Jena, Germany). At follow-up visits of 1, 3, 6 and 12 months, binocular uncorrected and corrected distance, intermediate and near visual acuity, reading performance, defocus curve, contrast sensitivity and patient satisfaction for dysphotopsia and spectacle independence were evaluated.
Results
A total of 60 eyes from 30 patients with a mean age of 65.40±7.71 years were included in the study. At 12 months, 83% of patients (n=25) had binocular cumulative uncorrected distance visual acuity (UDVA) of 20/20 or better. Postoperative spherical equivalent refraction accuracy was within ±0.50 D for 95% of eyes (n=57) and refractive cylinder accuracy was within ≤0.50 D in 95% of eyes (n=57). The mean binocular uncorrected near visual acuity (UNVA) was 0.16±0.09 logMAR, and the mean uncorrected intermediate visual acuity (UIVA) at 60 and 80 cm was 0.01±0.09 and 0.03±0.08 logMAR, respectively, at 12 months. Reading speeds at 40, 60 and 80 cm showed improvement over time. No patient had complaints of severe dysphotopsia and none of the patients required glasses for any activity. No eye underwent YAG-laser capsulotomy for significant posterior capsule opacification at the end of mean follow-up.
Conclusion
In our limited experience of 30 patients at 12 months, AT LARA EDoF IOLs resulted in excellent visual outcomes for uncorrected distance, intermediate and near visual acuity. The incidence of dysphotopsia and spectacle dependence was low, resulting in good patient satisfaction.
Trial Registry
CTRI/2020/08/027105 (www.ctri.nic.in).
Keywords: AT LARA, extended depth of focus, EDoF IOL, spectacle independence
Introduction
According to the American Academy of Ophthalmology (AAO) task force consensus statement, an extended depth of focus (EDoF) intraocular lens (IOL) should have an extended far focus area that reaches the intermediate distance, thus providing excellent distance and intermediate vision. By definition, EDoF IOLs must provide monocular depth of focus at least 0.5 D wider than a monofocal control at 0.2 logMAR.1
EDoF lenses have been commercialized as a distinct class of “premium IOLs” providing the best of both worlds, wherein the advantages of both monofocal IOLs (excellent distance vision, minimal dysphotopsia, high contrast, quality of vision)2,3 and multifocal IOLs (good reading and intermediate vision)4,5 can be combined. These lenses claim to restore vision across multiple distances by generating a broad dioptric range rather than a fixed number of foci.6,7 Some of these are classified as “true EDoF”, such as the IC-08 IOL, which works on the principle of pinhole/ reduced aperture,8 whereas others use discrete multiple foci to achieve an “in-vivo EDoF effect”, by employing refractive zonal and diffractive designs, such as the TECNIS® Symfony ZXR00 (J&J Vision, NJ, USA) and AT LARA 829MP (Carl Zeiss Meditec, Jena, Germany).8 The outcomes of the TECNIS Symfony ZXR00 IOL have been evaluated in multiple studies.9–13 However, since the AT LARA 829MP EDoF IOL is a relatively recent introduction on the market, only a few studies have reported outcomes with this IOL so far.14–16 Most of these studies evaluated early experiences, visual results, contrast sensitivity and patient satisfaction; however, reading speeds following implantation of this lens have not previously been assessed.
In this study, we report our long-term experience with regard to the clinical outcomes, safety, efficacy, contrast sensitivity and patient satisfaction obtained 12 months following the bilateral implantation of AT LARA 829MP EDoF IOLs. We also evaluated reading performance achieved with this lens assessed using a standardized reading desk, an attribute of functional vision, after presbyopia-correcting IOL surgery.
Materials and Methods
This prospective, interventional, single-arm, single-centre study was approved by the institutional ethics committee of Nethradhama Superspeciality Eye Hospital, Bangalore, and abided by the tenets of the Declaration of Helsinki. All patients provided written informed consent.
Inclusion criteria were healthy eyes besides senile cataract, corneal astigmatism ≤0.75 dioptres (D), IOL power calculation resulting in dioptres between +7.0 and +30.00 D, capsular bag IOL implantation and ability to read the English language fluently.
Exclusion criteria were patients with corneal astigmatism of >0.75 D, irregular astigmatism due to corneal abnormalities, pupillary abnormalities, history of glaucoma, intraocular inflammation, neuro-ophthalmic diseases, macular degenerations or retinopathies potentially affecting the visual outcome, intraoperative or postoperative complications and unassured follow-ups.
Preoperatively, all patients underwent complete ophthalmic examination including measurement of uncorrected and distant corrected visual acuity with ETDRS charts at 4 m (Precision Vision, La Sella, IL, USA), manifest refraction, slit-lamp biomicroscopy, non-contact tonometry (Tomey NCT, Nishiku, Nagoya, Japan), tomography using the elevation-based Scheimpflug imaging device Pentacam HR (Oculus Optikgeräte, Wetzlar, Germany) to rule out irregular astigmatism, the HD Analyzer (Visiometrics, Spain) to assess ocular dryness, specular microscopy (Tomey, Japan), macular optical coherence tomography (OCT; Optovue, Fremont, CA, USA) and dilated fundus examination.
Biometric assessments were performed using a swept-source OCT-based optical biometer, the IOL Master-700 (Carl Zeiss Meditec, Jena, Germany), using the Barrett TK® (Total Keratometry) Universal II formula. All eyes were targeted at emmetropia. An optimized A-constant of 118.3 was used for IOL power calculation.
Description of the Study IOL
The AT LARA 829MP is a hydrophilic IOL (25% water content) with hydrophobic surface properties. It is based on a diffractive principle, is aspheric, and has an aberration-neutral and chromatic aberration-correcting optical design. Smooth microphase manufacturing technology is used to reduce manufacturing imperfections and limit light scatter. In addition to a distance focal point, the IOL has two foci of 1.90 and 0.95 D for far-intermediate and near-intermediate distances. The IOL has a four-point haptic fixation, 6-mm optic diameter and 11-mm overall length, and it is available in the −10.00 to +32.00 D range with 0.50-D increments. The lens is preloaded (BLUEMIXS 180 injector) and is compatible with incisions up to 1.8 mm. Table 1 shows the technical specifications of the AT LARA 829MP EDoF IOL.
Table 1.
Specifications of AT LARA 829MP EDoF IOL
| Material | Hydrophilic (25%) acrylate with hydrophobic surface property, smooth microphase design |
| Optic type | Single piece, biconvex aspheric, diffractive–refractive, smooth microphase design |
| Optic size | 6.00 mm |
| Haptic design | Plate haptic |
| Overall size | 11.00 mm |
| Angulation | 0 degrees |
| Diffractive surface | Anterior |
| Zone | 15 rings anteriorly on entire optic surface. |
| Depth of focus | Near–intermediate: +0.95 D; far–intermediate: +1.9 D |
| ACD | 5.20 mm |
| Refractive index | 1.48 |
| Dioptre range | −10.0 to +32.0 D, 0.5 D increments |
| A constant (optical) | 118.5 |
| Abbe number | 56.5 |
| Spherical aberration | Neutral SA |
| Injector | BLUEMIXS 180, VISCOJECT-BIO 2.2 |
| Implantation site | Capsular bag |
| Sterilization | Irradiation |
Surgical Procedure
All surgeries were performed by a single experienced surgeon (SG), following a standard phacoemulsification technique4 under topical anaesthesia, using the Centurion Precision system (Alcon Laboratories, Fort Worth, TX, USA). After cataract removal and cortical aspiration, the fully preloaded IOL (AT LARA 829MP IOL with BLUEMIXS 180 injector/cartridge set; Carl Zeiss Meditec) was implanted into the capsular bag in all cases.
Postoperative topical therapy included topical prednisolone (1%, Pred Forte®; Allergan) six times for 6 weeks tapering weekly, moxifloxacin (0.5%, Vigamox®; Alcon) four times for 2 weeks, nepafenac (0.1%, Nevanac®; Alcon) 3 times for 4 weeks and lubricants four times or as required (SOS) for 4 weeks or more.
Follow-up examinations were conducted 1 day, 1 week, 1 month, 3 months, 6 months and 12 months postoperatively. Dilated slit-lamp examination was performed on postoperative day 1 to assess the corneal clarity, anterior chamber inflammation and IOL position. From 1 month onwards, in addition to the above, assessment of manifest refraction, uniocular and binocular uncorrected and corrected distance visual acuity (UDVA, CDVA), uniocular and binocular uncorrected and corrected near visual acuity (UNVA, CNVA), photopic contrast sensitivity using CSV-1000 (Vector Vision, Greenville, OH, USA), reading performance using the Salzburg Reading Desk (SRD; University Eye Clinic, Paracelsus Medical University of Salzburg, Austria) and distance corrected defocus curve evaluation from +2.00 to −3.00 D were also performed. From 3 months onwards, a quality of vision (QoV) and patient satisfaction questionnaire regarding dysphotopsia symptoms and spectacle independence was also obtained.
Statistical Analysis
Statistical analysis was performed using the SPSS statistical package (version 17.0; SPSS, Chicago, IL, USA). Data were checked for normality before analysis. If the data were normally distributed, a one-way analysis of variance (ANOVA) test was performed to compare the mean values of UDVA, UNVA, CDVA, near and intermediate vision at 40, 60 and 80 cm, and contrast sensitivity values of all postoperative visits. If the data distribution was not normal, the Wilcoxon signed-rank test was used. The independent t-test was used to derive significance between uncorrected and corrected values, evaluated at the same time-point. A p-value of less than 0.05 was considered statistically significant.
Results
In total, 60 eyes of 30 patients (mean age 62±7.6 years) were included in the study. The demographic and baseline data of the patients are provided in Supplemental File 1 Table 2 shows the visual and refractive outcomes for distance and near vision achieved at all postoperative visits.
Table 2.
Visual and Refractive Outcomes at 1 Week, 1 Month, 3 Months, 6 Months and 12 Months Postoperatively
| Parameters | 1 Week | 1 Month | 3 Months | 6 Months | 1 Year | p-Value |
|---|---|---|---|---|---|---|
| Mean±SD | Mean±SD | Mean±SD | Mean±SD | Mean±SD | ||
| Sphere (D) | −0.08±0.23 | −0.05±0.24 | −0.05±0.17 | −0.04± 0.13 | −0.03±0.11 | 0.78 |
| Cylinder (D) | −0.25±0.25 | −0.30±0.35 | −0.30±0.29 | −0.29± 0.29 | −0.27±0.29 | 0.82 |
| SE (D) | −0.20±0.22 | −0.18±0.27 | −0.18±0.26 | −0.18± 0.19 | −0.17±0.20 | 0.97 |
| UDVA (logMAR) | 0.03±0.07 | 0.03±0.07 | 0.03±0.07 | 0.03 ±0.07 | 0.01±0.07 | 0.73 |
| CDVA (logMAR) | 0.008±0.04 | 0.003±0.04 | −0.001± 0.03 | −0.00± 0.03 | −0.01±0.05 | 0.08 |
| UNVA (logMAR) | 0.19±0.10 | 0.18±0.07 | 0.18±0.08 | 0.17±0.07 | 0.16±0.09 | 0.69 |
| DCNVA (logMAR) | 0.21±0.06 | 0.20±0.08 | 0.20±0.08 | 0.20±0.10 | 0.19±0.11 | 0.84 |
| Binocular UDVA (logMAR) | 0.02±0.07 | 0.02±0.06 | 0.02±0.06 | 0.02±0.04 | 0.00±0.05 | 0.72 |
| Binocular CDVA (logMAR) | 0.00±0.02 | −0.00±0.04 | −0.01±0.02 | −0.01±0.03 | −0.02±0.04 | 0.15 |
Abbreviations: SE, spherical equivalent; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; UNVA, uncorrected near visual acuity; DCNVA, distance corrected near visual acuity; D, dioptre; SD, standard deviation.
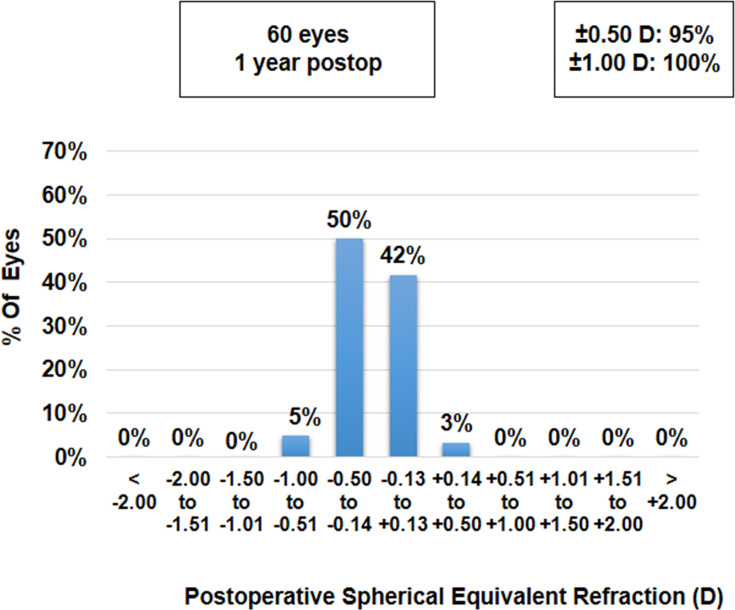
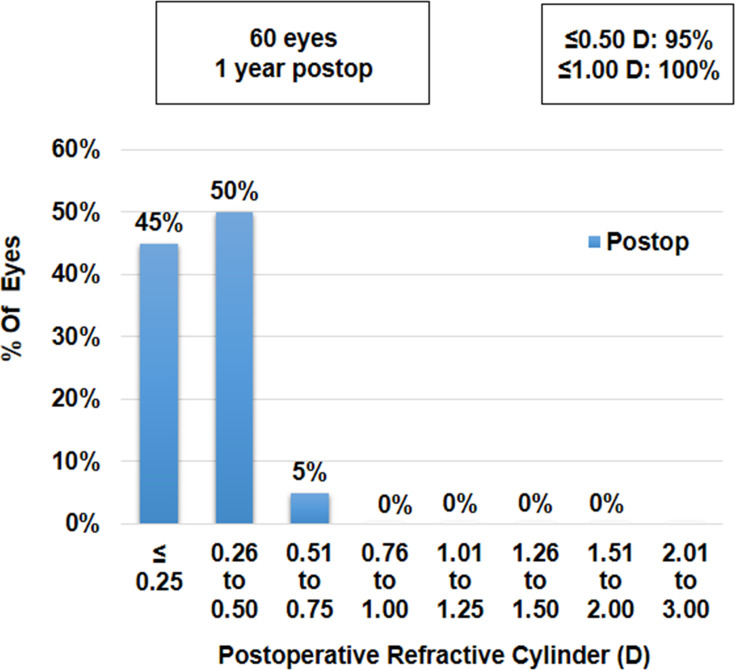
At 1 year follow-up, the mean sphere, cylinder and spherical equivalent (SE) values were −0.03±0.11 D, −0.27±0.29 D and −0.17±0.20 D, respectively, which were not significantly different from their respective values at 1 week, 1 month, 3 months and 6 months postoperatively (p-values >0.05 for all parameters). Ninety five per cent of eyes (n=57) were within ±0.50 D and all eyes were within ±1.00 D of SE correction (Figure 1). Similarly, 95% of eyes (n=57) were within ±0.50 D and all eyes were within ±1.00 D of cylinder correction (Figure 2).
Figure 1.
Histogram showing the accuracy with respect to the intended spherical equivalent refraction at 12 months postoperatively.
Figure 2.
Histogram showing change in refractive astigmatism at 12 months postoperatively.
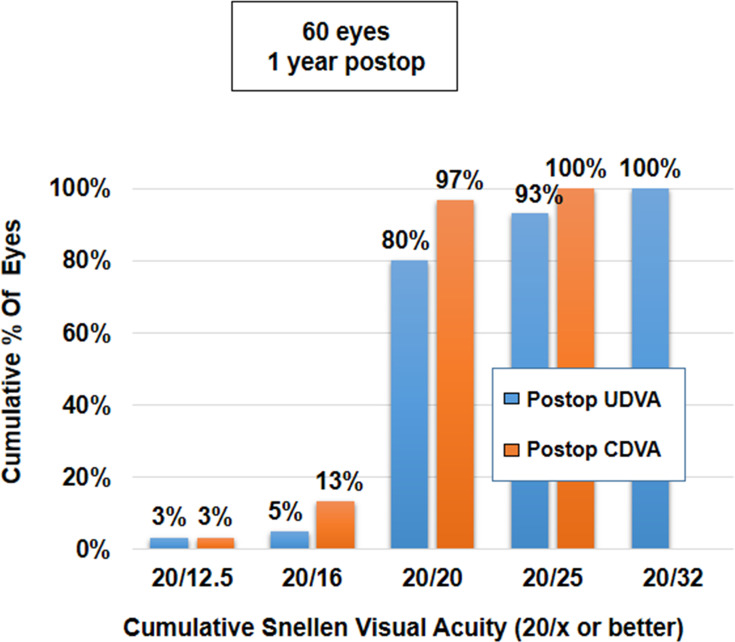
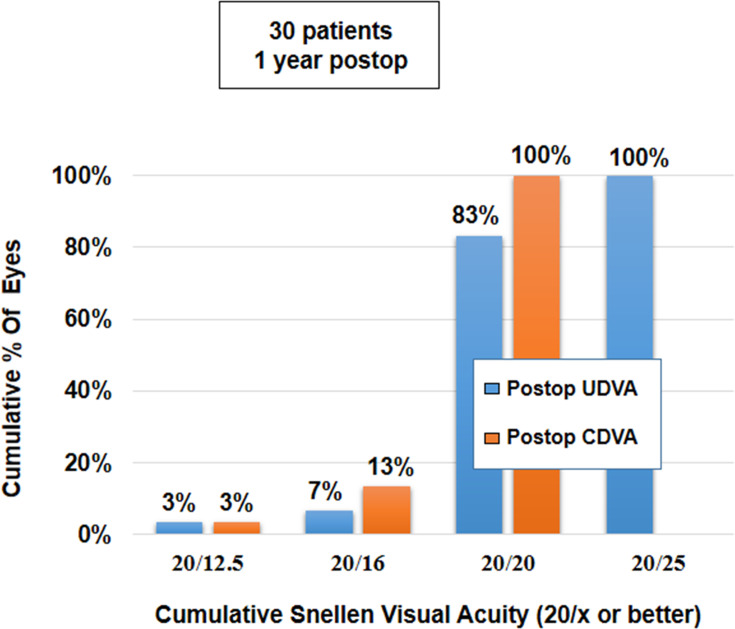
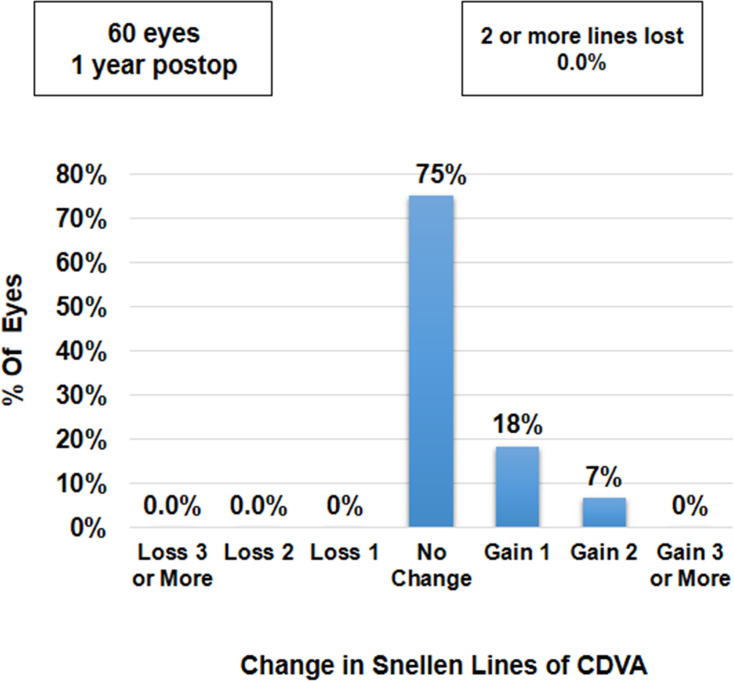
The mean binocular UDVA and CDVA were 0.00±0.05 and −0.02±0.04, respectively. There was no significant difference in the binocular UDVA and CDVA at 1 year, compared to the values at 1 week, 1 month, 3 months and 6 months postoperatively (p-values for change in UDVA and CDVA = 0.85 and 0.27, respectively) (Table 2). Uniocularly, 80% of eyes (n=48) had UDVA of 20/20 or better, while all eyes (n=60) had a minimum UDVA of 20/32 (Figure 3). Binocularly, 83% of patients (n=25) had UDVA of 20/20 or better, while all patients (n=27) had a minimum UDVA of 20/25 (Figure 4). Seventy five per cent of eyes (45) had postoperative UDVA the same as postoperative CDVA, whereas 18% of eyes (n=11) had postoperative UDVA better than postoperative CDVA by 1 line and 7% of eyes (n=4) had postoperative UDVA better than postoperative CDVA by 2 lines (Figure 5).
Figure 3.
Histogram showing uniocular results for UDVA (n=60 eyes) and CDVA obtained following implantation of AT LARA EDoF IOL at 12 months postoperatively.
Figure 4.
Histogram showing binocular cumulative visual acuity UDVA and CDVA achieved at 12 months postoperatively.
Figure 5.
Histogram showing change in Snellen lines of UDVA versus CDVA at 12 months postoperatively.
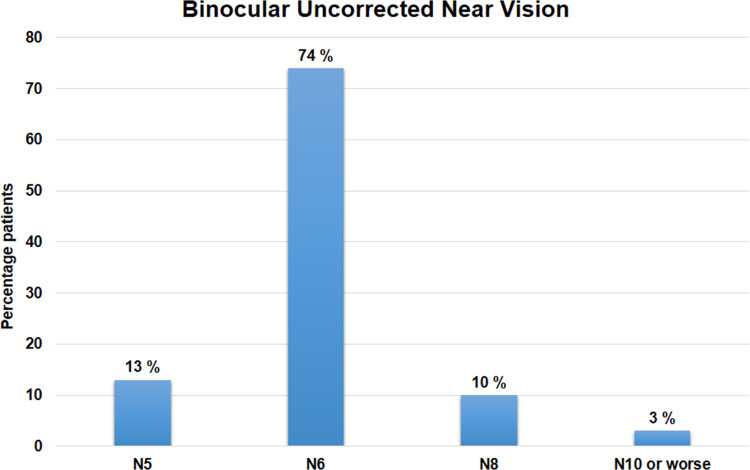
The mean binocular UNVA at 1 year was 0.16±0.09 logMAR and mean binocular distance-corrected near visual acuity (DCNVA) was 0.19±0.11 logMAR; both parameters were comparable to and not statistically significantly different from their respective values at 1 week, 1 month, 3 months and 6 months postoperatively (p-values for change in UNVA and DCNVA = 0.69 and 0.84, respectively). Eighty-seven per cent of patients (n=26) had binocular uncorrected near vision of N6 or better, while all eyes had a UNVA of at least N10 (Figure 6).
Figure 6.
Histogram showing binocular uncorrected near vision results at 12 months postoperatively.
Intermediate Visual Outcomes at 60 and 80 cm Using ETDRS Charts
At 1 year, the mean uncorrected intermediate visual acuity (UCIVA) at 60 cm was 0.01±0.09 logMAR and at 80 cm it was 0.03±0.08 logMAR. No significant differences were found between uncorrected and corrected intermediate vision values at 60 and 80 cm, or when these were compared to the previous follow-ups, ie, 1 week, 1 month, 3 months and 6 months (Table 3).
Table 3.
Intermediate Visual Acuity with ETDRS Charts and Reading Speeds at 40, 60 and 80 cm and Contrast Sensitivity with CSV-1000 at Postoperative Visits from 1 Week to 12 Months
| Mean±SD | 1 Week | 1 Month | 3 Months | 6 Months | 1 Year | p-Value* | ||
|---|---|---|---|---|---|---|---|---|
| ETDRS (logMAR) | 60 cm | UCIVA | 0.04±0.09 | 0.03±0.09 | 0.03±0.10 | 0.02±0.10 | 0.01±0.09 | 0.88 |
| DCIVA | 0.03±0.07 | 0.02±0.07 | 0.02±0.07 | 0.01±0.20 | 0.00±0.20 | 0.94 | ||
| p-Value** | 0.76 | 0.65 | 0.77 | 0.81 | 0.80 | |||
| 80 cm | UCIVA | 0.05±0.08 | 0.05±0.08 | 0.05±0.09 | 0.04±0.08 | 0.03±0.08 | 0.80 | |
| DCIVA | 0.04±0.08 | 0.04±0.08 | 0.04±0.08 | 0.03±0.07 | 0.02±0.08 | 0.86 | ||
| p-Value** | 0.44 | 0.52 | 0.66 | 0.64 | 0.64 | |||
| Reading speed (wpm) | 40 cm | Uncorrected | 120±41 | 127±34 | 126±33 | 130±33 | 133±30 | 0.64 |
| Corrected | 131±51 | 137±41 | 138±41 | 141±39 | 144±37 | 0.80 | ||
| p-Value** | 0.35 | 0.31 | 0.21 | 0.25 | 0.21 | |||
| 60 cm | Uncorrected | 152±39 | 164±40 | 165±43 | 168±44 | 170±42 | 0.45 | |
| Corrected | 158±51 | 164±45 | 164±44 | 169±44 | 171±42 | 0.79 | ||
| p-Value** | 0.60 | 0.98 | 0.85 | 0.95 | 0.90 | |||
| 80 cm | Uncorrected | 145±36 | 150±37 | 151±35 | 158±36 | 160±35 | 0.51 | |
| Corrected | 157±47 | 159±42 | 163±41 | 168±39 | 171±37 | 0.63 | ||
| p-Value** | 0.28 | 0.42 | 0.22 | 0.30 | 0.21 | |||
| CSV 1000 (logMAR) | A (3.0) | 1.47±0.18 | 1.52±0.18 | 1.53±0.17 | 1.53±0.15 | 1.54±0.13 | 0.18 | |
| B (6.0) | 1.66±0.18 | 1.70±0.17 | 1.72±0.16 | 1.73±0.16 | 1.74±0.15 | 0.09 | ||
| C (12.0) | 1.33±0.18 | 1.34±0.22 | 1.38±0.20 | 1.39±0.20 | 1.40±0.20 | 0.27 | ||
| D (18.0) | 0.82±0.24 | 0.86±0.23 | 0.91±0.23 | 0.89±0.22 | 0.90±0.21 | 0.17 | ||
Notes: p-Values: *calculated using ANOVA single-factor test; **calculated using independent t-test.
Abbreviations: UCIVA, uncorrected intermediate visual acuity; DCIVA, distance corrected intermediate visual acuity; wpm, words per minute; CSV, contrast sensitivity (Vector Vision); SD, standard deviation.
Reading Speeds
The binocular uncorrected reading speeds, assessed with the SRD, at 40, 60 and 80 cm were 133±30, 170±42 and 160±35 words per minute at 12 months postoperatively. Reading speeds at all distances showed constant improvement from 1 week to 12 months; however, this was not statistically significant (p-values for 40, 60 and 80 cm being 0.64, 0.45 and 0.51, respectively). A similar trend was observed for corrected reading speeds (Table 3).
Contrast Sensitivity
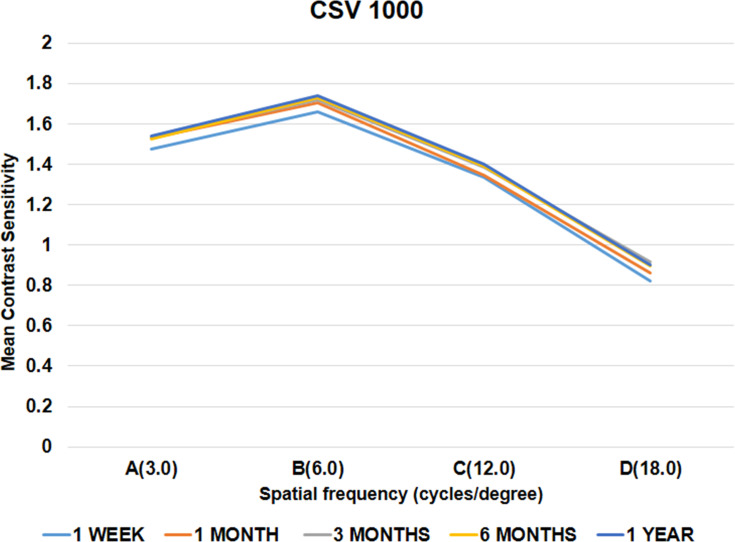
Photopic contrast sensitivity, evaluated binocularly using the CSV-1000 chart (with correction), at 1 week, 1 month, 3 months, 6 months and 1 year showed improvement in the log values for all spatial frequencies over time; however, this was not statistically significant (Table 3, Figure 7).
Figure 7.
Photopic contrast sensitivity evaluated binocularly (with correction) at 12 months.
Defocus Curve
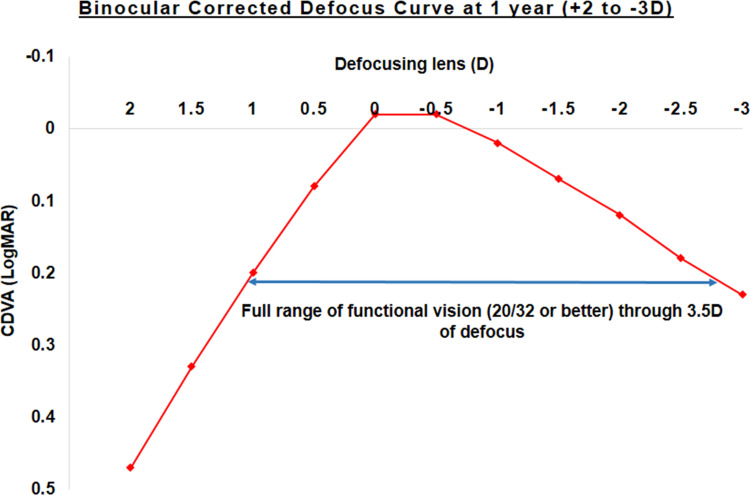
Binocular defocus curves were charted with correction at 1 week, 1 month, 3 months, 6 months and 1 year using defocusing lenses from +2.00 to −3.00 D. Figure 8 shows the defocus curve obtained at 1 year postoperatively. The curve showed a peak corresponding to 0.00 D, wherein the mean binocular corrected distance vision was −0.02 logMAR, followed by a gradual decline of the slope in the intermediate range of vision (−1.50 D), which continued in the near vision range (−2.50 D). The mean intermediate and near visual acuities were 0.01 and 0.16 logMAR, respectively. Overall, a flatter curve was seen, resulting in a full range of functional vision (20/32 or better), through a defocus of 3.5 D.
Figure 8.
Binocular distance corrected defocus curve evaluated from +2 to −3 D defocus at 12 months.
Patient Satisfaction and Spectacle Independence
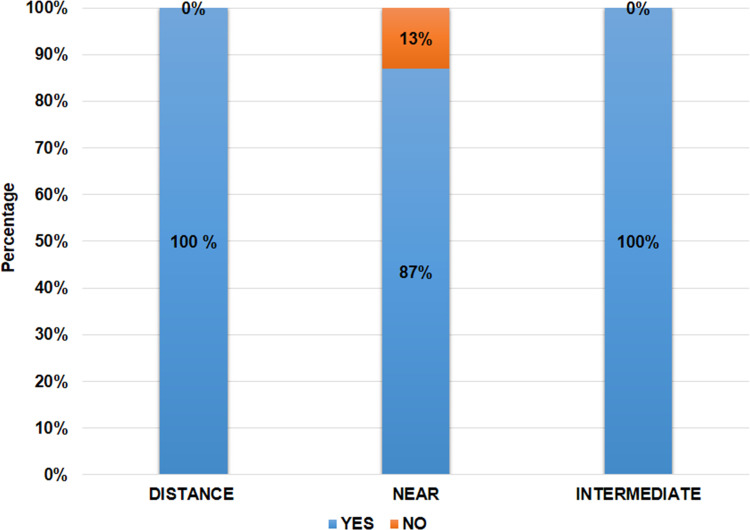
A QoV questionnaire was obtained for all patients from 1 month onwards. Results were compiled at 1 year follow-up. At 1 year, 100% of patients were spectacle free for distance and intermediate vision, whereas 86.66% of patients (n=26) were spectacle free for near vision (Figure 9).
Figure 9.
Histogram showing spectacle independence (% of patients) for activities performed at different distances.
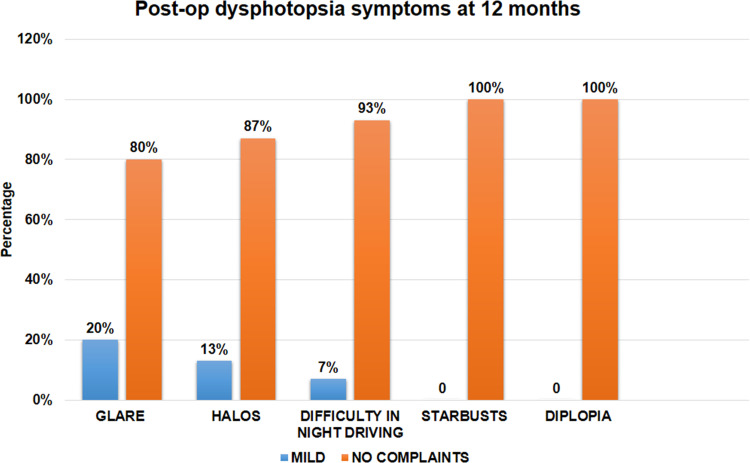
Figure 10 shows the distribution of postoperative dysphotopsia symptoms. Eighty per cent (n=24) and 87% of patients (n=26) had no complaints of glare and halos, respectively. Twenty per cent (n=6) and 13% of patients (n=4), however, complained of mild glare and halos, respectively, not affecting their quality of life significantly. Seven per cent of patients (n=2) reported mild difficulty in night driving. None of the patients complained about starbursts or diplopia at any time-point after the surgery. The overall spectacle independence and satisfaction scores (on a scale of 0–10, with 0 being the lowest and 10 being the highest) were 8.7±1.01 and 8.9±0.87, respectively.
Figure 10.
Histogram showing distribution of postoperative dysphotopsia symptoms.
Adverse Effects and Complications
A dilated clinical examination was performed at 1 year to assess posterior capsule opacification (PCO) and IOL centration. All IOLs remained well centred in the bag, with good 360-degree overlap of capsulorhexis, and without any significant tilt or decentration. None of the eyes treated in the study developed significant PCO, affecting the visual outcomes or patient satisfaction at 1 year. No other vision-threatening complications, such as cystoid macular oedema, postoperative uveitis, secondary glaucoma or endophthalmitis, occurred in any of the eyes included in the study.
Discussion
Schallhorn et al and Kretz et al published their initial (3-month) clinical experience following implantation of AT LARA 829MP EDoF IOLs, based on a diffractive design principle to continuously extend the depth of focus.14,16 Both studies concluded that the new EDoF IOL provided reasonable unaided near and distance vision, as well as spectacle independence and patient satisfaction. The UNVA observed in the study by Schallhorn et al was 0.26 logMAR, while the binocular DCNVA at 40 cm in the study by Kretz et al showed acceptable results (0.32±0.13 logMAR), offering a high degree of patient satisfaction. Our study showed better results for UNVA (0.16 logMAR) compared to these studies, which can probably be attributed to the slightly higher myopic residual SE observed in our series (−0.17 D). Reading newspapers typically requires a visual acuity of 0.4 logMAR at 40 cm, while a higher level of visual acuity is needed for fluid reading.17 From this perspective, the AT LARA EDoF IOL provided visual acuity well above 0.4 logMAR at intermediate distances, while allowing good functional vision.
With regard to intermediate vision, binocular distance-corrected intermediate visual acuity (DCIVA) evaluated by Kretz et al at 90, 80 and 60 cm was −0.022±0.14, 0.026±0.12 and 0.025±0.10, respectively.16 In our study, the binocular DCIVA results were similar to those in their study, the values being 0.020±0.08 and 0.00±0.20 logMAR at 80 and 60 cm, respectively.
In a study by Reinhard et al, the AT LARA 829MP exceeded the AAO taskforce requirement for EDoF IOLs1 defined in the Introduction, with a difference of 0.62 D in the mono-ocular depth of focus achieved, compared to the monofocal control.18 In the same study, the TECNIS Symfony EDoF IOL, on the other hand, failed to comply with the definition, with a difference of only 0.22 D compared with the monofocal control. However, with regard to binocular depth of focus, there was no statistically significant difference between the two EDoF IOLs at any visual acuity level, and both were statistically superior to the monofocal IOL (CT ASPHINA 409MP). This suggests favourable outcomes with regard to the depth of focus achieved with EDoF IOLs in general compared to monofocal IOLs, potentially aiding in the excellent intermediate and reasonably good near vision obtained with these lenses. In the present study, we observed that the AT LARA 829MP EDoF IOL provided a wide range of functional vision (20/32 or better) over a defocus of 3.5 D, resulting in almost a continuous range of vision, from distance to near. These results of the defocus curve are similar to our previously published results with bilateral implantation of TECNIS Symfony EDoF IOLs; however, a micro-monovision was performed aiming at slight myopia of approximately −0.75 D in the non-dominant eye.19
Although the Symfony and the AT LARA EDoF IOLs share a number of similarities in their optical properties, the primary difference between the two EDoF IOLs lies in their optical behaviour in relation to spherical aberration (SA). The Symfony IOL features an SA-correcting optic that is designed to compensate for the positive SA of the human cornea, while the AT LARA EDoF is an aberration-free IOL.18 This was confirmed by Chae et al, who experimentally compared the optical performance of Symfony and AT LARA EDoF IOLs using a standardized optical bench set-up.20 In this study, the Symfony IOL performed better with the ISO2 cornea, as it effectively compensated for the positive SA of the model cornea, while the aberration-neutral AT LARA IOL showed superior performance with the ISO1 cornea, as its optic was designed not to induce any additional SA to the existing SA of the eye. Similar observations were noted by Domínguez-Vicent et al, who found that the optical performance of the Symfony IOL was lower at the same pupil size when measured with an aberration-free cornea.21
Eighty six per cent (26/30) of our patients were spectacle free for reading, even without having a mini-monovision planned. Compared to the Symfony IOL, wherein the corneal aberrations are compensated for by the negative aberrations of the IOL, these aberrations are minimally affected with the AT LARA EDoF IOL, potentially resulting in improved near vision outcomes and patient satisfaction, as seen in the present study.
Compared to the photopic contrast sensitivity results of Symfony IOL reported by Mencucci et al,22 our mean values were similar for the spatial frequency of 3 cycles per degree, and slightly lower for the remaining spatial frequencies. However, this was not clinically significant and did not affect patient satisfaction, as the aberration-neutral and chromatic aberration-correcting optical design of the AT LARA IOL results in optimized contrast sensitivity and visual quality.
Reading is one of the most vital and common skills for engaging, communicating and interpreting ideas, and reading speed more closely aligns with task performance than visual acuity metrics.23 We evaluated reading speeds using the SRD, with which a speed of 80 words per minute is considered to be the minimum threshold for recreational reading in healthy eyes.24,25 The mean reading speeds at 40, 60 and 80 cm were well above this threshold 1 week postoperatively and showed continuous improvement over time. The reading speed at 60 cm was similar to that reported by Mencucci et al for the TECNIS Symfony IOL.22
With multifocal lenses, any degree of residual refractive defect reduces the quality of vision at any distance compared with monofocal IOLs. Refractive predictability was excellent in our study, with a mean postoperative residual SE of −0.17 D at 12 months and 95% of the eyes being within ±0.5 D of the target value. This suggests that advanced, swept source-based optical biometry26 and modern IOL calculation formulae, such as the Barrett TK formula27,28 used in the present study, may help cataract refractive surgeons to improve their chances of achieving postoperative targets closer to emmetropia in the majority of their cases.
In conclusion, the AT LARA EDoF IOLs provided excellent visual outcomes at all distances at 12 months, which were comparable to or better than the published results of currently available EDoF IOLs. Patients achieved high levels of satisfaction due to excellent unaided distance visual acuity, good contrast sensitivity and reading speeds, minimal dependence on reading glasses and negligible amounts of dysphotopsia symptoms at the end of 12 months. However, a relatively small sample size may be a potential limitation of our study. Further prospective, randomized comparative studies with larger sample sizes are suggested to compare the long-term results of this lens with other available EDoF IOLs.
Data Sharing Statement
We do not wish to share individual study patient data. However, all the study-related data may be made available on request by writing to ethics@nethradhama.org.
Disclosure
Dr Sri Ganesh reports being a consultant to Carl Zeiss Meditec. Dr Sheetal Brar reports being a consultant to Carl Zeiss Meditec, Germany.
References
- 1.MacRae S, Holladay JT, Glasser A, et al. Special report: American Academy of Ophthalmology Task Force consensus statement for extended depth of focus intraocular lenses. Ophthalmology. 2017;124(1):139–141. PMID: 27743644. doi: 10.1016/j.ophtha.2016.09.039 [DOI] [PubMed] [Google Scholar]
- 2.Shetty V, Haldipurkar SS, Gore R, Dhamankar R, Paik A, Setia MS. A comparison of visual outcomes in three different types of monofocal intraocular lenses. Int J Ophthalmol. 2015;8(6):1173–1178. doi: 10.3980/j.issn.2222-3959.2015.06.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borkenstein AF, Borkenstein EM. Long-term clinical results and scanning electron microscopic analysis of the aspheric, hydrophobic, acrylic intraocular lens CT LUCIA 611P(Y). Clin Ophthalmol. 2018;12:1219–1227. doi: 10.2147/OPTH.S167895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesh S, Brar S, Pawar A. Long-term visual outcomes and patient satisfaction following bilateral implantation of trifocal intraocular lenses. Clin Ophthalmol. 2017;11:1453–1459. PMID: 28860693; PMCID: PMC5558567. doi: 10.2147/OPTH.S125921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doroodgar F, Niazi F, Sanginabadi A, et al. Visual performance of four types of diffractive multifocal intraocular lenses and a review of articles. Int J Ophthalmol. 2021;14(3):356–365. PMID: 33747809; PMCID: PMC7930542. doi: 10.18240/ijo.2021.03.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coassin M, Di Zazzo A, Antonini M, Gaudenzi D, Gallo Afflitto G, Kohnen T. Extended depth-of-focus intraocular lenses: power calculation and outcomes. J Cataract Refract Surg. 2020;46(11):1554–1560. PMID: 32590481. doi: 10.1097/j.jcrs.0000000000000293 [DOI] [PubMed] [Google Scholar]
- 7.Kohnen T, Suryakumar R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. 2020;46(2):298–304. PMID: 32126045. doi: 10.1097/j.jcrs.0000000000000109 [DOI] [PubMed] [Google Scholar]
- 8.Rampat R, Gatinel D. Multifocal and extended depth-of-focus intraocular lenses in 2020. Ophthalmology. 2020;S0161-6420(20)30931–3. PMID: 32980397. doi: 10.1016/j.ophtha.2020.09.026 [DOI] [PubMed] [Google Scholar]
- 9.Akella SS, Juthani VV. Extended depth of focus intraocular lenses for presbyopia. Curr Opin Ophthalmol. 2018;29(4):318–322. PMID: 29697436. doi: 10.1097/ICU.0000000000000490 [DOI] [PubMed] [Google Scholar]
- 10.Paik DW, Park JS, Yang CM, Lim DH, Chung TY. Comparing the visual outcome, visual quality, and satisfaction among three types of multi-focal intraocular lenses. Sci Rep. 2020;10(1):14832. PMID: 32908159; PMCID: PMC7481789. doi: 10.1038/s41598-020-69318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song JE, Khoramnia R, Son HS, Knorz MC, Choi CY. Comparison between bilateral implantation of a trifocal IOL and mix-and-match implantation of a bifocal IOL and an extended depth of focus IOL. J Refract Surg. 2020;36(8):528–535. PMID: 32785726. doi: 10.3928/1081597X-20200616-01 [DOI] [PubMed] [Google Scholar]
- 12.Black S. A clinical assessment of visual performance of combining the TECNIS® Symfony Extended Range of Vision IOL (ZXR00) with the +3.25 D TECNIS multifocal 1-piece IOL (ZLB00) in subjects undergoing bilateral cataract extraction. Clin Ophthalmol. 2018;12:2129–2136. PMID: 30425448; PMCID: PMC6205142. doi: 10.2147/OPTH.S175901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schojai M, Schultz T, Jerke C, Böcker J, Dick HB. Visual performance comparison of 2 extended depth-of-focus intraocular lenses. J Cataract Refract Surg. 2020;46(3):388–393. PMID: 32142039. doi: 10.1097/j.jcrs.0000000000000068 [DOI] [PubMed] [Google Scholar]
- 14.Schallhorn SC, Teenan D, Venter JA, Hannan SJ, Schallhorn JM. Initial clinical outcomes of a new extended depth of focus intraocular lens. J Refract Surg. 2019;35(7):426–433. PMID: 31298722. doi: 10.3928/1081597X-20190530-01 [DOI] [PubMed] [Google Scholar]
- 15.Schallhorn SC, Hettinger KA, Teenan D, Venter JA, Hannan SJ, Schallhorn JM. Predictors of patient satisfaction after refractive lens exchange with an extended depth of focus IOL. J Refract Surg. 2020;36(3):175–184. PMID: 32159822. doi: 10.3928/1081597X-20200211-01 [DOI] [PubMed] [Google Scholar]
- 16.Kretz TA, Tarib I, Kaiser I, Herbers C, Hagen P, Breyer D. Postoperative results in patients implanted with a novel enhanced depth of focus intraocular lens. EC Ophthalmol. 2018;4:192–202. [Google Scholar]
- 17.Wolffsohn JS, Davies LN. Presbyopia: effectiveness of correction strategies. Prog Retin Eye Res. 2019;68:124–143. PMID: 30244049. doi: 10.1016/j.preteyeres.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 18.Reinhard T, Maier P, Böhringer D, et al. Comparison of two extended depth of focus intraocular lenses with a monofocal lens: a multi-centre randomised trial. Graefes Arch Clin Exp Ophthalmol. 2021;259(2):431–442. PMID: 32915276; PMCID: PMC7843553. doi: 10.1007/s00417-020-04868-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganesh S, Brar S, Pawar A, Relekar KJ. Visual and refractive outcomes following bilateral implantation of extended range of vision intraocular lens with micromonovision. J Ophthalmol. 2018;2018:7321794. PMID: 29545954; PMCID: PMC5818926. doi: 10.1155/2018/7321794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chae SH, Son HS, Khoramnia R, Lee KH, Choi CY. Laboratory evaluation of the optical properties of two extended-depth-of-focus intraocular lenses. BMC Ophthalmol. 2020;20(1):53. PMID: 32059666; PMCID: PMC7023787. doi: 10.1186/s12886-020-1332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domínguez-Vicent A, Esteve-Taboada JJ, Del Águila-carrasco AJ, Ferrer-Blasco T, Montés-Micó R. In vitro optical quality comparison between the mini WELL ready progressive multifocal and the TECNIS symfony. Graefes Arch Clin Exp Ophthalmol. 2016;254(7):1387–1397. PMID: 26671689. doi: 10.1007/s00417-015-3240-7 [DOI] [PubMed] [Google Scholar]
- 22.Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. PMID: 29980919. doi: 10.1007/s00417-018-4052-3 [DOI] [PubMed] [Google Scholar]
- 23.Whittaker SG, Lovie-Kitchin J. Visual requirements for reading. Optom Vis Sci. 1993;70(1):54–65. PMID: 8430009. doi: 10.1097/00006324-199301000-00010 [DOI] [PubMed] [Google Scholar]
- 24.Hirnschall N, Motaabbed JK, Dexl A, Grabner G, Findl O. Evaluation of an electronic reading desk to measure reading acuity in pseudophakic patients. J Cataract Refract Surg. 2014;40(9):1462–1468. doi: 10.1016/j.jcrs.2013.12.02 [DOI] [PubMed] [Google Scholar]
- 25.Dexl AK, Schlögel H, Wolfbauer M, Grabner G. Device for improving quantification of reading acuity and reading speed. J Refract Surg. 2010;26(9):682–688. doi: 10.3928/1081597X-20091119-01 [DOI] [PubMed] [Google Scholar]
- 26.Song JS, Yoon DY, Hyon JY, Jeon HS. Comparison of ocular biometry and refractive outcomes using IOL master 500, IOL master 700, and lenstar LS900. Korean J Ophthalmol. 2020;34(2):126–132. PMID: 32233146; PMCID: PMC7105785. doi: 10.3341/kjo.2019.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivannaboon S, Chirapapaisan C. Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2019;257(12):2677–2682. PMID: 31486917. doi: 10.1007/s00417-019-04443-7 [DOI] [PubMed] [Google Scholar]
- 28.Hoshikawa R, Kamiya K, Fujimura F, Shoji N. Comparison of conventional keratometry and total keratometry in normal eyes. Biomed Res Int. 2020;2020:8075924. PMID: 32352009; PMCID: PMC7178464. doi: 10.1155/2020/8075924 [DOI] [PMC free article] [PubMed] [Google Scholar]