Abstract
Purpose of the review:
In the rapidly progressing world of inflammatory bowel disease, this review discusses and summarizes new drug targets and results from major clinical trials in order to provide an update to physicians treating patients with IBD.
Recent findings:
Multiple new mechanisms in the treatment of IBD are being developed and many are showing promising results in both UC and CD patients. In addition to efficacy, some of these treatments may provide safety benefits over existing therapies.
Summary:
The IBD physicians’ therapeutic armamentarium is rapidly expanding and keeping abreast of these developments is required in order to provide patients with optimized individualized care.
Keywords: Inflammatory bowel disease, biologics, small molecules, new therapeutics
Introduction
The inflammatory bowel diseases (IBD) are a heterogeneous group of conditions divided into two predominant groups, Crohn’s disease (CD) and ulcerative colitis (UC). These conditions are characterized by a chronic, progressive or relapsing and remitting disease course with the gastrointestinal (GI) tract being the major site of inflammatory activity. Unchecked, this inflammation can result in a complicated disease course with undesirable ramifications such as abdominal abscesses, fistulae, strictures and subsequent bowel obstruction, and increase the risk for GI malignancy. These diseases have a significant impact on patient quality of life, activities of daily living and increase health care costs(1,2).
The mainstay of treatment of IBD is immune-suppressive and immune-modulating agents. The biologic treatment era starting with the anti-tumor necrosis factors (TNF) therapies has heralded significant changes in our ability to obtain and maintain clinical response and remission in a safer manner(3–5). Further advances have resulted in the development of gut selective biologic agents, the anti-integrins(6,7), targets of different biochemical pathways such as with ustekinumab(8) and the oral small molecule therapy, tofacitinib(9). While these treatments have certainly broadened the IBD physician’s armamentarium, a significant percentage of patients do not respond to these treatments(10).
As such, new treatment pathways and a greater understanding of mechanisms of treatment failure are required. This will provide more options for patients and greater individualization in treatment decision making.
This review examines the future of IBD treatments and details current phase I, II, and III clinical trial results. Figure 1 and Table 1 show therapies at their varying stages of clinical development.
Figure 1:
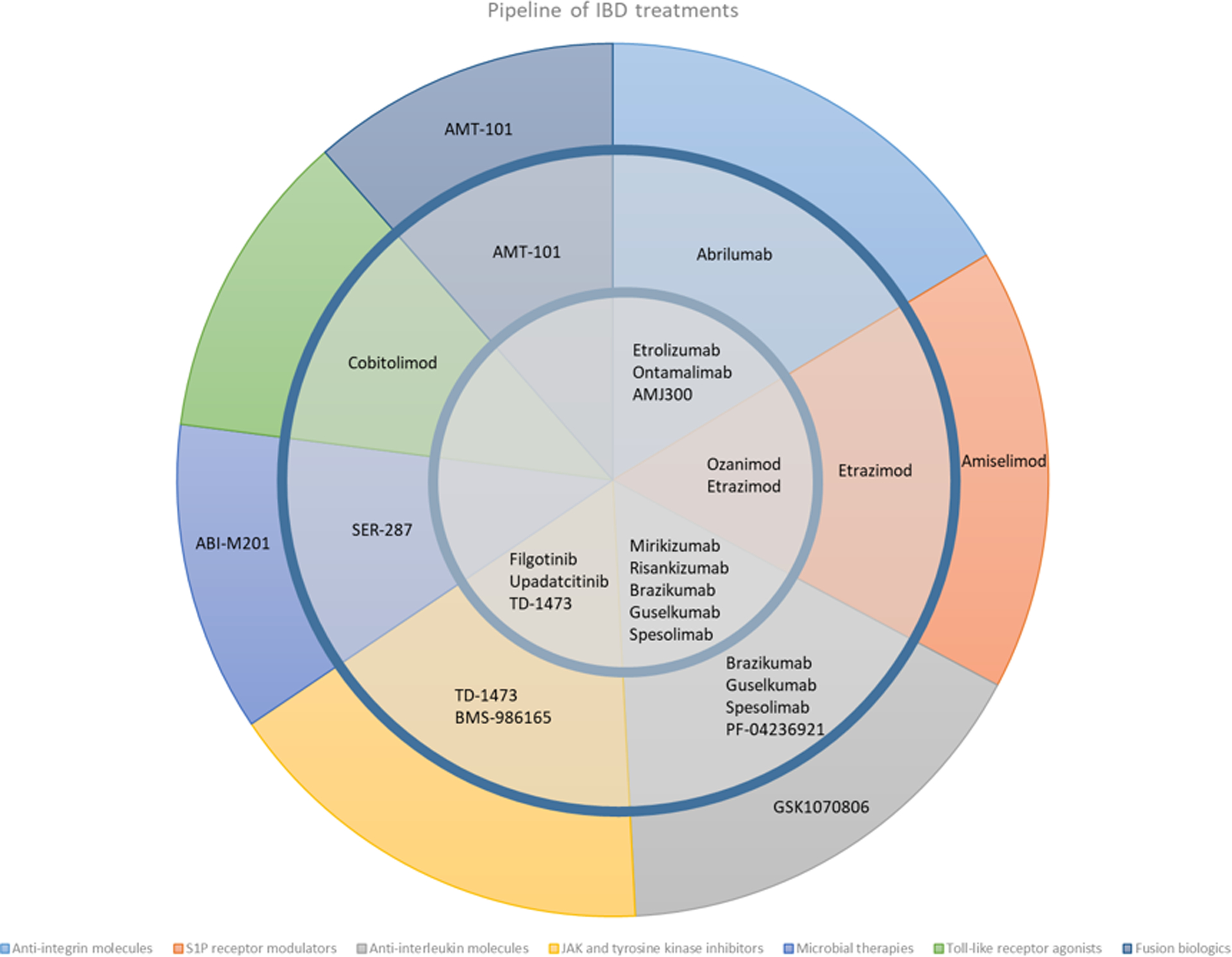
Clinical trial pipeline of IBD therapies
Outer ring: Phase 1, Middle ring: Phase 2, Inner circle: Phase 3
Table 1:
Clinical development of drugs with novel therapeutic targets in IBD
| Category and name | Mechanism of action | Results published | Current phase of development | Indication | Ref |
|---|---|---|---|---|---|
| Anti-trafficking therapies | |||||
| Anti-integrins | |||||
| AJM300 | Oral, novel, small molecule α4 integrin inhibitor | Phase 2a study, significantly greater clinical and endoscopic remission rates compared with placebo | 3 | UC | 22,24 |
| Sphingosine 1 phosphate receptor modulator | |||||
| LC51–0255 | S1P receptor modulator | Phase 1 | 2 | UC + CD | (57) |
| OPL-002 | S1P receptor modulator | Phase 1 | 2 | UC + CD | (58) |
| Anti-interleukin antibodies | |||||
| Spesolimab | anti-IL36 | Phase 1 | 2 | UC + CD | (59) |
| PF-04236921 | anti-IL6 | Phase 2 study showed significantly greater week 12 clinical remission compared with placebo with durability during the OLE | CD | (60) | |
| JAK and tyrosine kinase inhibitors | |||||
| TD-1473 | Oral novel, gut selective, pan JAK inhibitor | Phase 1 | 2 | UC + CD | (61) |
| BMS-986165 | TYK-2 inhibitor | Phase 1 | 2 | UC + CD | (62) |
| Toll-like receptor agonists | |||||
| Cobitolimod (DIMS0150) | TLR-9 agonist | Phase 2 study showed significantly higher rates of symptomatic improvement at weeks 4 and 8 with significantly more patients having clinical remission and mucosal healing at week 4, compared with placebo | 2 | UC | (63) |
| Interleukin 10 - fusion biologic | |||||
| AMT-101 | Novel oral human IL-10 fusion protein | Phase 1 | 2 | UC | (64) |
Anti-trafficking therapies
Anti-adhesion molecules
An important part of T-cell dependent chronic intestinal inflammation in IBD is the homing of T-lymphocytes to the gut. Anti-adhesion agents target integrins responsible for homing and reduce the inflammatory cell infiltrate(11). The anti α4β7 integrin antibody vedolizumab is currently approved and widely used in the treatment of both UC and CD(6,7).
The next generation in this treatment class is etrolizumab, a humanized IgG1 monoclonal antibody which selectively binds the β7 subunit and thus blocks both the α4β7 and the αεβ7 intestinal integrins. The etrolizumab phase 3 clinical program is the largest and most comprehensive in IBD, and is among the first to include head-to-head trials in UC against an anti-TNF agent. HIBISCUS I and II evaluated the efficacy of etrolizumab for induction head-to-head against adalimumab and placebo in anti-TNF naïve UC patients. This study included 716 patients. In the pooled analysis, clinical remission at week 10 was 18.8% for etrolizumab vs 23.5% for adalimumab (p=0.13). Etrolizumab was well tolerated with most adverse events being non-serious or grades 1 or 2. The primary outcome was not met and etrolizumab was not superior to adalimumab at week 10. However, there was a statistically significant increase in endoscopic remission rates compared with placebo(12). The LAUREL induction and maintenance study evaluated etrolizumab against placebo in anti-TNF naïve UC patients. At week 62 there was no significant difference in clinical remission rates. There was a statistically significant difference in endoscopic improvement, endoscopic remission and histologic remission in the etrolizumab cohort at week 62 (38% vs 22.5%, p=0.024; 30.6% vs 16.7%, p=0.03 and 42.4% vs 21.8%, p=0.008; respectively). Etrolizumab was well tolerated throughout the follow up period(13). The GARDENIA study was a head-to-head induction and maintenance study comparing etrolizumab to infliximab. The primary outcome was not met with clinical remission rates at week 10 and 54 of 18.6% and 19.7% in the etrolizumab and infliximab cohorts respectively. Endoscopic remission was similar between the groups and the safety profile the drugs was comparable(14). Although etrolizumab was not superior to adalimumab or infliximab the rates of clinical and endoscopic outcomes were similar and etrolizumab was well tolerated. In CD, initial results from the phase III BERGAMOT study investigating etrolizumab in moderately to severely active CD showed that CDAI remission at week 14 was greater in both the 105 mg and 210 mg arms compared with placebo (23% vs 28.9% vs 16.9%, respectively) with comparable side effects between the groups(15).
Another molecule targeting the α4β7 integrin is the monoclonal IgG2 antibody abrilumab (AMG-181). This drug has shown preliminary efficacy in both UC and CD. In UC patients with moderately to severely active disease, a randomized, phase 2b, placebo-controlled, double-blind study including 354 patients showed significantly increased week 8 remission rates at dosages of 70 and 210 mg compared with placebo (13.3%, 12.7% vs 4.3%, p<0.05, respectively). 51% of the study population had prior anti-TNF exposure and 44% were on oral corticosteroids at baseline. Overall abrilumab was well tolerated and authors note a similar side effect profile to both vedolizumab and etrolizumab(16). In CD, a phase 2b, randomized, multi-center, double-blind, placebo-controlled study did not meet the primary endpoint of clinical remission at week 8 (p=0.76 vs placebo)(17).
The anti-MAdCAM-1, ontamalimab (SHP647, PF-00547659), is a monoclonal IgG2 humanized antibody targeting the intestinal adhesion molecule, MAdCAM-1. A phase 2, randomized, double-blind, placebo-controlled clinical (TURANDOT) trial in patients with active UC showed significantly greater week 12 remission rates compared with placebo(18). In CD, the phase 2 OPERA study did not reach statistical significance in terms of clinical endpoints(19). The OPERA II study, an open label extension study showed that remission rates were sustained over a period of 72 weeks and the drug was generally well tolerated(20,21). There are currently five phase 3 studies underway to investigate the use of ontamalimab in both UC and CD.
Sphingosine 1 phosphate receptor modulator
Shingosine-1 phosphate (S1P) signaling on central memory T-cells facilitate their exit from lymph nodes. Internalization of the S1P receptor prevents lymphocytes from responding to S1P and are retained in the lymph node, thus inhibiting their recruitment to inflamed tissue(22). Protective immunity is generally preserved as effector memory T-cells do not circulate through the lymph nodes. Ozanimod is an S1P modulator which downregulates S1P receptor subtypes 1 and 5(23). In a phase 2 RCT in patients with moderately to severely active UC, 1mg of ozanimod showed significantly greater clinical response and remission rates compared with placebo (16% vs 6%, p = 0.048). In this study, endoscopic remission rates at week 8 were also significantly greater in the treatment groups. The adverse event profile was similar to placebo(24). The 4-year open label extension study showed durable efficacy with no new safety markers in UC patients(25). The phase 3 TRUE NORTH study in patients with moderately to severely active UC showed that ozanimod results in significant benefits in clinical, endoscopic, histologic and mucosal healing endpoints at week 52 compared with placebo(26). Currently, phase 3 trials in CD and UC patients are underway.
An oral S1P receptor modulator targeting receptor subtypes 1,4 and 5, etrasimod, has been assessed in a phase 2, proof-of-concept, double-blind, parallel-group study in UC patients. At week 12, etrasimod resulted in a significant improvement in modified Mayo clinical scores and significantly greater clinical remission and endoscopic improvement rates compared with placebo (33% vs 8.1% and 41.8% vs 17.8%, p=0.003, respectively). In addition most adverse events were mild(27). In the subsequent open label extension study, of 31 patients who continued 2 mg etrasimod, 70%, 35% and 45% had clinical response, clinical remission and endoscopic improvement, respectively(28). There are currently a phase 2 trials in CD and multiple phase 3 trials in UC underway (Figure 1).
Anti-interleukin antibodies
Interleukin-23, which is a member of the IL-12 family of cytokines has 2 components: the p40 subunit found also on IL-12 and the p19 subunit found exclusively on IL-23. IL-23 plays an important role in in the maintenance and amplification of T helper 17 (Th17) and the stimulation of many innate immune cells important in the pathogenesis of IBD(29–31). The monoclonal antibody, ustekinumab, which is directed against the p40 subunit of IL-12 and IL-23 has shown success in the treatment of CD and UC(8,32,33). However, studies in psoriasis have revealed that more specific targeting of the p19 subunit and thus only the IL-23 molecule may be more effective(34,35).
Mirikizumab is a monoclonal antibody targeting the p19 subunit. In a phase 2 RCT investigating mirikizumab in patients with moderately to severely active UC, patients receiving 200 mg mirikizumab had significantly greater clinical remission and endoscopic improvement at week 12, particularly in biologic naïve patients (36.4% vs 8.7%, p=0.004 and 50% vs 8.7%, p=0.033, respectively). Adverse events were similar between the treatment groups(36). In a sub analysis of this study, mirikizumab was shown to achieve and sustain greater rates of histologic remission and mucosal healing when compared with placebo through to week 52 of maintenance therapy(37). In CD, a recently published abstract described the results of a phase 2 RCT conducted in patients with moderately to severely active CD. All three drug dose groups were superior to placebo in terms of clinical response rates. In addition, there was a dose related increase in response rates (200mg – 25.8%, 600mg – 37.5% and 1000mg – 43.8%). Further, patients in the 600mg and 1000mg groups achieved significantly better endoscopic remission rates (p=0.032 and p=0.009, respectively). There was no difference in adverse events when compared with placebo(38). The phase 2 SERENITY maintenance study followed patients treated with either mirikizumab intravenously or subcutaneously for 52 weeks. Of those achieving endoscopic response at week 12, 69.6% and 66.7% in the intravenous and subcutaneous treatment groups, respectively, maintained response at week 52 (39). The drug also showed no new safety signals.
Rizankizumab also binds the p19 subunit. A phase 2 RCT in patients with moderately to severely active CD who received rizankizumab showed superior clinical remission compared with placebo at week 12 (31% vs 15%, p=0.049)(40). In the open label extension study, at weeks 52, clinical remission was maintained in 71% of patients and was well tolerated(41). Of note, 93% of patients in these studies were previously exposed to at least one anti-TNF biologic(40). There are currently phase 2 and phase 3 studies underway investigating rizankizumab in UC patients.
Another monoclonal antibody targeting IL-23 in development is brazikumab (MEDI2070). In a phase 2 RCT in patients with moderately to severely active CD who had previously failed anti-TNF therapy, at week 8 49.2% of patients receiving brazikumab achieved clinical remission compared with 26.7% in the placebo group (p=0.01). At week 12, a significantly greater proportion of patients receiving brazikumab had a clinical response and a reduction of over 50% in term of biomarkers (c-reactive protein and fecal calprotectin) (37.3% vs 8.3%, p<0.001)(42). In this study, higher baseline serum concentrations of IL-22, a cytokine induced by IL-23, were associated with a greater likelihood of response to brazikumab. This may provide a biomarker to predict response and thus personalize the use of this treatment in CD patients. Currently, there are multiple phase 2 and phase 3 trials underway for both UC and CD patients.
At present, other treatments targeting the IL23 pathway are under investigation. Recently, interim results from the phase 2 study (GALAXY-1) investigating the IL-23 antagonist, guselkumab, in patients with moderately to severely active CD showed that at all treatment doses (200, 600 or 1200mg) patients treated with guselkumab had significantly greater clinical response and remission rates compared with placebo (remission:54%, 56%, 50% vs 15.7%, respectively) and apparent similar efficacy to ustekinumab. Safety at these point appears consistent with that established from other clinical trials(43).
JAK and tyrosine kinase inhibitors
The Janus kinase (JAK) family comprises of four intracellular tyrosine kinases – JAK1, JAK2, JAK3 and non-receptor tyrosine-protein kinase 2 – these activate signal transducers and activators of transcription (STATs). This JAK-STAT pathway regulates the expression of multiple mediators involved in inflammatory pathways implicated in the pathogenesis of IBD(44). Tofacitinib, an oral small molecule pan-JAK inhibitor, has shown success in three UC phase 3 (both induction and maintenance trials(9).
Filgotinib, an oral, once daily administered JAK 1 selective inhibitor, has been studied in moderately to severely active CD. In the phase 2 FITZROY study, significantly more patients achieved clinical remission on filgotinib compared with the placebo after 10 weeks of treatment (47% vs 23%, p=0.0077). There was no significant difference in terms of severe adverse events between the groups at 20 weeks(45). Currently, phase 3 trials are underway investigating long term efficacy and safety in CD patients (Figure 1). The SELECTION phase 2B/3 study investigating filgotinib in patients with moderately to severely active UC showed that a significantly higher rate of patients in the treatment arm achieved a combined endpoint of endoscopic, rectal bleeding and stool frequency remission compared with placebo (37.2% vs 2%, respectively). The 200mg filgotinib dosage met all key endpoints including endoscopic, histologic and 6-month corticosteroid free remission. There was no increase in adverse event compared with placebo(46).
Another JAK1 selective oral small molecule is upadacitinib. The phase 2 CELESTE study investigating upadacitinib in CD patients with moderately to severely active disease, reported clinical remission in 27% of patients receiving 6 mg compared with 11% in patients receiving placebo (p<0.1). Endoscopic remission was significantly greater in all upadacitinib treatment arms compared with placebo and efficacy was maintained in most treatment arms over 52 weeks of therapy. Of note, during the induction phase of the study, more patients in the treatment arm had infections and serious infections when compared with placebo and patients in the 12 mg and 24 mg treatment arms had significant increases in total, high-density lipoprotein and low-density lipoprotein levels when compared with placebo(47). The open label extension study showed a good safety profile with no new safety signals and good maintenance of response over a 12-month period(48). In a phase 2 RCT investigating upadacitinib as induction therapy in patients with active UC, at week 8 clinical remission was achieved in 8.5%, 14.3%, 13.5%, and 19.6% of patients receiving 7.5 mg, 15 mg, 30 mg, or 45 mg upadacitinib, respectively compared with none in the placebo group (P = .052, P = .013, P = .011, and P = .002 compared with placebo, respectively). Endoscopic remission was also achieved in a significantly greater number of patients in all treatment groups compared with placebo with the greatest effect seen in the 45mg treatment group (35.7% vs 2.2%, p<0.001). In this study there was one case of herpes zoster and one patient developed a deep vein thrombosis (DVT) and pulmonary embolism (PE) (26 days following discontinuation of the therapy and in the 45mg group). Once again, as with the phase 2 CD study, increases in serum lipid levels were noted(49). Multiple phase 3 studies investigating upadacitinib in both CD and UC are underway.
Microbiota-based innovations
Therapies targeting the gut microbiome including fecal microbiota transplantation (FMT), dietary exclusions and modifications, prebiotics, and probiotics are under extensive investigation in IBD with varying success(50–54). These therapies face a number of challenges, including a lack specificity, which may explain their limited success. A potential new therapy is SER-287, which fractionates spore forming bacteria to specifically target Firmicutes thought to be important to gut homeostasis(55). A recent phase 1B, double blind, RCT investigated the efficacy of SER-287 compared with placebo in 58 patients with UC. At week 8 patients in the vancomycin/SER-287 daily group had significantly higher proportions of clinical remission compared with placebo (40% vs 0%, p=0.02 for vancomycin/SER-287 daily vs placebo/placebo)(56). Ongoing studies are required to determine the true value of this and other similar treatments.
Conclusion
The world of IBD is rapidly evolving as both understanding of the pathogenesis of the diseases is increasing and the ability to target various pathways are being developed. The next few years hold much promise to both the IBD physician and patients alike with a plethora of new therapeutic options expected to be introduced into the market. Nonetheless, there remains a “therapeutic ceiling” through which these newer therapies have been unable to break. Although an increasing armamentarium of drugs will allow for more decision making maneuverability with numerous options of mode of delivery, differing mechanisms and greater safety, we have a great deal of work to do. What is still lacking and what requires greater focus is the development of reliable biomarkers to predict response allowing for greater personalization, decreased expenditure on already very expensive medications and hopefully translate to improved patients’ quality of life and decreased complications. Combination approaches, sequential or pulse and phased treatment strategies, bacterial derived proteins and additional non-immune based strategies must be actively pursued.
Key points:
There are multiple new biologic and small molecule therapies in advanced stages of development.
These will provide physicians with a great number of effective and safe options for patients requiring biologic therapy.
The multitude of mechanisms of action will allow for greater personalization of therapy and allow physicians and patients to balance their choice based on efficacy, safety and mode of administration.
These new mechanisms increase the insight into the pathophysiology of IBD and will promote the development of an even greater array of drugs in the future.
There remains a “therapeutic ceiling” that will only be exceeded with novel combination approaches or non-immune based strategies that change the current paradigms.
Funding:
This work received no funding
Conflicts of Interest:
NAC has no relevant disclosures. DTR has received grant support from Takeda; and has served as a consultant for Abbvie, Abgenomics, Allergan Inc., Arena Pharmaceuticals, Bellatrix Pharmaceuticals, Boehringer Ingelheim Ltd., Bristol-Myers Squibb, Celgene Corp/Syneos, Check-cap, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences S.A., InDex Pharmaceuticals, Iterative Scopes, Janssen Pharmaceuticals, Lilly, Materia Prima, Narrow River Mgmt, Pfizer, Prometheus Laboratories, Reistone, Takeda, and Techlab Inc.
References:
- 1.Habibi F, Habibi ME, Gharavinia A, Mahdavi SB, Akbarpour MJ, Baghaei A, et al. Quality of life in inflammatory bowel disease patients: A cross-sectional study. J Res Med Sci. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Panés J, Sandborn WJ, Vermeire S, Danese S, Feagan BG, et al. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clin Gastroenterol Hepatol. 2016; [DOI] [PubMed] [Google Scholar]
- 3.Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab Maintenance Therapy for Fistulizing Crohn’s Disease. N Engl J Med. 2004; [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lulcas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of Crohn’s disease: Results of the CLASSIC II trial. Gut. 2007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013; [DOI] [PubMed] [Google Scholar]
- 7.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; [Google Scholar]
- 8.Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016; [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017; [DOI] [PubMed] [Google Scholar]
- 10.Singh S, George J, Boland BS, Vande Casteele N, Sandborn WJ. Primary non-response to tumor necrosis factor antagonists is associated with inferior response to second-line biologics in patients with Inflammatory bowel diseases: A systematic review and meta-analysis. J Crohn’s Colitis. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghosh S, Panaccione R. Anti-adhesion molecule therapy for inflammatory bowel disease. Therapeutic Advances in Gastroenterology. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotan I, Panes J, Duvall A, Bouhnik Y, Radford-Smith G, Higgins PDR, Mishkin DS, Arrisi P, Scalori A, Oh YS, Tole S, Chai A, Chamerlain-James K, Lacey S, McBride JRD. Etrolizumab compared wih adalimumab or placebo as induction therapy for UC: Results from the randomized, phase 3 hibiscus 1 & 2 Trials. UEG week 2020 - late Break absract. 2020; [Google Scholar]; *First Phase 3 RCT program to conduct a head to head study of a new therapeutic agent vs an anti-TNF agent
- 13.Vermiere S, Lakatos P, Ritter T, Hanauer SB, Bressler B, Khanna R, et al. ETROLIZUMAB VERSUS PLACEBO IN TUMOR NECROSIS FACTOR ANTAGONIST NAIVE PATIENTS WITH ULCERATIVE COLITIS: RESULTS FROM THE RANDOMIZED PHASE 3 LAUREL TRIAL. UEG week 2020 - late Break absract. 2020; [Google Scholar]; * First Phase 3 RCT program to conduct a head to head study of a new therapeutic agent vs an anti-TNF agent
- 14.Danese S, Colombel JF, Lukas M, Gisbert JP, D’Haens G, Hayee B, et al. ETROLIZUMAB VERSUS INFLIXIMAB FOR TREATING PATIENTS WITH MODERATELY TO SEVERELY ACTIVE ULCERATIVE COLITIS: RESULTS FROM THE PHASE 3 GARDENIA STUDY. UEG week 2020 - late Break absract. 2020; [Google Scholar]; * First Phase 3 RCT program to conduct a head to head study of a new therapeutic agent vs an anti-TNF agent
- 15.Selinger C, Sandborn W, Panes J, Jones J, Hassanali A, Jacob R, et al. Etrolizumab as induction therapy in moderate to severe crohn’s disease: results from bergamot cohort 1. Gut. 67 (Supp 1(A53):1–A53.28473631 [Google Scholar]
- 16.Sandborn WJ, Cyrille M, Hansen MB, Feagan BG, Loftus EV, Rogler G, et al. Efficacy and Safety of Abrilumab in a Randomized, Placebo-Controlled Trial for Moderate-to-Severe Ulcerative Colitis. Gastroenterology. 2019; [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Cyrille M, Berner Hansen M, Feagan BG, Loftus EV, Vermeire S, et al. OP035 Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe Crohn’s disease. J Crohn’s Colitis. 2017; [Google Scholar]
- 18.Vermeire S, Sandborn WJ, Danese S, Hébuterne X, Salzberg BA, Klopocka M, et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017; [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ, Lee SD, Tarabar D, Louis E, Klopocka M, Klaus J, et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: Report of the OPERA study. Gut. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Haens GR, Reinisch W, Lee SD, Tarabar D, Louis E, Kłopocka M, et al. OP024 Long-term safety and efficacy of the anti-MAdCAM monoclonal antibody SHP647 for the treatment of Crohn’s disease: the OPERA II study. J Crohn’s Colitis. 2018; [Google Scholar]
- 21.D’Haens G, Reinisch W, Lee SD, Tarabar D, Louis E, Kłopocka M, et al. OP08 Long-term efficacy and pharmacodynamics of the anti-mucosal addressin cell adhesion molecule-1 (MAdCAM-1) monoclonal antibody SHP647 in Crohn’s disease: the OPERA II study. J Crohn’s Colitis. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danese S, Furfaro F, Vetrano S. Targeting S1P in inflammatory bowel disease: New avenues for modulating intestinal leukocyte migration. Journal of Crohn’s and Colitis. 2018. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen OH, Li Y, Johansson-Lindbom B, Coskun M. Sphingosine-1-Phosphate Signaling in Inflammatory Bowel Disease. Trends in Molecular Medicine. 2017. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Feagan BG, Wolf DC, D’Haens G, Vermeire S, Hanauer SB, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016; [DOI] [PubMed] [Google Scholar]
- 25.Sandborn W, Feagan B, Hanauer SB, Vermiere S, Ghosh S, Liu W, et al. EXTENSION, LONG-TERM SAFETY AND EFFICACY OF OZANIMOD IN PATIENTS WITH MODERATE-TO-SEVERE ULCERATIVE COLITIS: RESULTS FROM THE TOUCHSTONE OPEN-LABEL. UEG week 2020 - late Break absract. 2020; [Google Scholar]
- 26.Sandborn W, D’Haens G, Wolf D, Hanauer SB, Jovanovic I, Ghosh S, et al. Ozanimod for Moderate-to-Severe Ulcerative Colitis: Efficacy, Safety, and Histology Results from the Induction and Maintenance Periods of the Phase 3 True North Study. UEG week 2020 - late Break absract. 2020; [Google Scholar]; * Phase 3 RCT introducing a new mechanism of action into the UC treatment armamentarium
- 27.Sandborn WJ, Peyrin-Biroulet L, Zhang J, Chiorean M, Vermeire S, Lee SD, et al. Efficacy and Safety of Etrasimod in a Phase 2 Randomized Trial of Patients With Ulcerative Colitis. Gastroenterology. 2020; [DOI] [PubMed] [Google Scholar]
- 28.Yarur AJ, Chiorean MV, Zhang J, Reinisch W, Vermeire S, Panes J, et al. Su1926 FECAL CALPROTECTIN AND C-REACTIVE PROTEIN LEVELS ARE CORRELATED WITH LONG TERM CLINICAL AND ENDOSCOPIC OUTCOMES: ANALYSIS OF THE OASIS OPEN LABEL EXTENSION TRIAL OF ETRASIMOD FOR ULCERATIVE COLITIS. Gastroenterology. 2020; [Google Scholar]
- 29.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, Mckenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nature Reviews Gastroenterology and Hepatology. 2019. [DOI] [PubMed] [Google Scholar]
- 31.Croxford AL, Kulig P, Becher B. IL-12-and IL-23 in health and disease. Cytokine and Growth Factor Reviews. 2014. [DOI] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012; [DOI] [PubMed] [Google Scholar]
- 33.Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; [DOI] [PubMed] [Google Scholar]
- 34.Gordon KB, Strober B, Lebwohl M, Augustin M, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018; [DOI] [PubMed] [Google Scholar]
- 35.Papp KA, Blauvelt A, Bukhalo M, Gooderham M, Krueger J, Lacour JP, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017; [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Ferrante M, Bhandari BR, Berliba E, Feagan BG, Hibi T, et al. Efficacy and Safety of Mirikizumab in a Randomized Phase 2 Study of Patients With Ulcerative Colitis. Gastroenterology. 2020; [DOI] [PubMed] [Google Scholar]
- 37.Pai R, Canavan J, Tuttle J, Durante M, Arora V, Milch C, et al. Tu1849 HISTOLOGIC REMISSION AND MUCOSAL HEALING IN A PHASE 2 STUDY OF MIRIKIZUMAB IN PATIENTS WITH MODERATELY TO SEVERELY ACTIVE ULCERATIVE COLITIS. Gastroenterology. 2020; [Google Scholar]
- 38.S BE, S WJ, P-B L, H PD, H F, B R, et al. EFFICACY AND SAFETY OF MIRIKIZUMAB (LY3074828) IN A PHASE 2 STUDY OF PATIENTS WITH CROHN’S DISEASE. Gastroenterology. 2019; [DOI] [PubMed] [Google Scholar]
- 39.Sands B, Sandborn W, Peyrin-Biroulet L, Higgins P, Hirai F, Jairath V, et al. Efficacy and Safety of Mirikizumab after 52-Weeks Maintenance Treatment in Patients with Moderate-to-Severe Crohn’s Disease: PHASE 2 SERENITY STUDY. UEG week 2020. 2020; [Google Scholar]
- 40.Feagan BG, Sandborn WJ, D’Haens G, Panés J, Kaser A, Ferrante M, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017; [DOI] [PubMed] [Google Scholar]
- 41.Feagan BG, Panés J, Ferrante M, Kaser A, D’Haens GR, Sandborn WJ, et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018; [DOI] [PubMed] [Google Scholar]
- 42.Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, et al. Efficacy and Safety of MEDI2070, an Antibody Against Interleukin 23, in Patients With Moderate to Severe Crohn’s Disease: A Phase 2a Study. Gastroenterology. 2017; [DOI] [PubMed] [Google Scholar]
- 43.Sandborn W, Chan D, Johanns J, Lang G, Adedokun O, Afzali A, et al. The Efficacy and Safety of Guselkumab Induction Therapy in Patients With Moderately to Severely Active Crohn’s Disease: Week 12 Interim Analyses From the Phase 2 GALAXI 1 Study. UEG week 2020. 2020; [Google Scholar]
- 44.Boland BS, Sandborn WJ, Chang JT. Update on Janus Kinase Antagonists in Inflammatory Bowel Disease. Gastroenterology Clinics of North America. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vermeire S, Schreiber S, Petryka R, Kuehbacher T, Hebuterne X, Roblin X, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017; [DOI] [PubMed] [Google Scholar]
- 46.Feagan B, Loftus E, Danese S, Vermiere S, Sandborn W, Ritter T, et al. Combined Phase 2b/3, Double-Blind, Randomized, Placebo-Controlled Studies Evaluating the Efficacy and Safety of Filgotinib in Subjects with Moderately to Severely Active Ulcerative Colitis: SELECTION TRIAL INDUCTION RESULTS. UEG week 2020. 2020; [Google Scholar]
- 47.Sandborn WJ, Feagan BG, Loftus EV, Peyrin-Biroulet L, Van Assche G, D’Haens G, et al. Efficacy and Safety of Upadacitinib in a Randomized Trial of Patients With Crohn’s Disease. Gastroenterology. 2020; [DOI] [PubMed] [Google Scholar]
- 48.D’Haens G, Panes J, Louis E, Zhou Q, Liu J, Lacerda A, et al. Composite and Individual Measures of Efficacy and Safety After 2 Years of Upadacitinib Treatment for Crohn’s Disease: Results from the Ongoing Phase 2 CELEST Open-Label Extension Study. UEG week 2020. 2020; [Google Scholar]
- 49.Sandborn WJ, Ghosh S, Panes J, Schreiber S, D’Haens G, Tanida S, et al. Efficacy of Upadacitinib in a Randomized Trial of Patients With Active Ulcerative Colitis. Gastroenterology. 2020; [DOI] [PubMed] [Google Scholar]
- 50.Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Alimentary Pharmacology and Therapeutics. 2017. [DOI] [PubMed] [Google Scholar]
- 51.Gutin L, Piceno Y, Fadrosh D, Lynch K, Zydek M, Kassam Z, et al. Fecal microbiota transplant for Crohn disease: A study evaluating safety, efficacy, and microbiome profile. United Eur Gastroenterol J. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiang L, Ding X, Li Q, Wu X, Dai M, Long C, et al. Efficacy of faecal microbiota transplantation in Crohn’s disease: a new target treatment? Microb Biotechnol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen HE, Hamaker BR. Prebiotics and Inflammatory Bowel Disease. Gastroenterology Clinics of North America. 2017. [DOI] [PubMed] [Google Scholar]
- 54.Scaldaferri F, Gerardi V, Lopetuso LR, Del Zompo F, Mangiola F, Boškoski I, et al. Gut microbial flora, prebiotics, and probiotics in IBD: Their current usage and utility. BioMed Research International. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henn MR, O’Brien EJ, Diao L, Feagan BG, Sandborn WJ, Huttenhower C, et al. A Phase 1b Safety Study of SER-287, a Spore-Based Microbiome Therapeutic, for Active Mild to Moderate Ulcerative Colitis. Gastroenterology. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hwang I, won Lee S, Jeon S, Lee Y, Yu K-S. Tu1881 SAFETY, PHARMACOKINETICS AND IMMUNE MODULATORY PROPERTIES OF LC51–0255, AN ORAL, SELECTIVE SPHINGOSINE 1-PHOSPHATE 1 (S1P1) RECEPTOR MODULATOR, IN HEALTHY VOLUNTEERS. Gastroenterology. 2020; [Google Scholar]
- 58.Luo A, Lester R, Schwab R, Ogilvie K, Huyghe M, Mohan R, et al. Tu1852 PHARMACOKINETICS AND PHARMACODYNAMICS OF OPL-002, A HIGHLY SELECTIVE S1P1R MODULATOR, IN HEALTHY ADULT VOLUNTEERS. Gastroenterology. 2020; [Google Scholar]
- 59.Gresnigt MS, Van de Veerdonk FL. Biology of IL-36 cytokines and their role in disease. Seminars in Immunology. 2013. [DOI] [PubMed] [Google Scholar]
- 60.Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G, et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandborn WJ, Nguyen DD, Beattie DT, Brassil P, Krey W, Woo J, et al. Development of Gut-Selective Pan-Janus Kinase Inhibitor TD-1473 for Ulcerative Colitis: A Translational Medicine Programme. J Crohn’s Colitis. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papp K, Gordon K, Thaçi D, Morita A, Gooderham M, Foley P, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018; [DOI] [PubMed] [Google Scholar]
- 63.Atreya R, Bloom S, Scaldaferri F, Gerardi V, Admyre C, Karlsson Å, et al. Clinical effects of a topically applied toll-like receptor 9 agonist in active moderate-to-severe ulcerative colitis. J Crohn’s Colitis. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]; * Early trial looking at an interesting and new treatment pathway
- 64.Mrsny R, Kanwar B, Mahmood T. OP39 Treatment of ulcerative colitis With AMT-101, a novel oral interleukin-10 immunomodulatory fusion biologic that traffics across the intestinal epithelium. J Crohn’s Colitis. 2020; [Google Scholar]; * Early trial of a drug with a new treatment pathway and interesting mechanism of action with potential use in many other current therapies


