Abstract
Ras proteins must be localized to the inner surface of the plasma membrane to be biologically active. The motifs that effect Ras plasma membrane targeting consist of a C-terminal CAAX motif plus a second signal comprising palmitoylation of adjacent cysteine residues or the presence of a polybasic domain. In this study, we examined how Ras proteins access the cell surface after processing of the CAAX motif is completed in the endoplasmic reticulum (ER). We show that palmitoylated CAAX proteins, in addition to being localized at the plasma membrane, are found throughout the exocytic pathway and accumulate in the Golgi region when cells are incubated at 15°C. In contrast, polybasic CAAX proteins are found only at the cell surface and not in the exocytic pathway. CAAX proteins which lack a second signal for plasma membrane targeting accumulate in the ER and Golgi. Brefeldin A (BFA) significantly inhibits the plasma membrane accumulation of newly synthesized, palmitoylated CAAX proteins without inhibiting their palmitoylation. BFA has no effect on the trafficking of polybasic CAAX proteins. We conclude that H-ras and K-ras traffic to the cell surface through different routes and that the polybasic domain is a sorting signal diverting K-Ras out of the classical exocytic pathway proximal to the Golgi. Farnesylated Ras proteins that lack a polybasic domain reach the Golgi but require palmitoylation in order to traffic further to the cell surface. These data also indicate that a Ras palmitoyltransferase is present in an early compartment of the exocytic pathway.
Ras proteins operate as molecular switches in diverse signaling pathways that regulate cell growth and differentiation (8, 26). However, in order to signal, Ras proteins must be localized to the inner surface of the plasma membrane (58). This requirement reflects the role that Ras plays in recruiting cytosolic effectors to the cell surface, where they are, in turn, either activated or juxtaposed with their own specific target proteins. For example, the serine threonine kinase Raf-1 is recruited to the plasma membrane by activated Ras (53), where it is activated by interactions with membrane lipids, tyrosine kinases, and possibly phosphatases (32, 37). Similarly, the exchange factor RalGDS is relocalized to the plasma membrane by activated Ras, positioning it in the same compartment as the Ral GTPase, which it, in turn, activates (29, 57).
The membrane anchors used by the Ras proteins to attach to the plasma membrane have been well characterized. All Ras isoforms terminate in a CAAX motif that is sequentially farnesylated, AAX proteolyzed, and methylesterified (9, 15, 19). The processed CAAX motif then operates with a second signal in the adjacent hypervariable region to target Ras to the plasma membrane. This second signal is cysteines 181 and 184 in H-ras, and cysteine 181 in N-ras, and these cysteines undergo palmitoylation (19). In contrast, the second signal in K-ras comprises multiple lysine residues (175 to 180), which form a polybasic domain (20). These minimal C-terminal motifs, amino acids 181 to 189 in H-ras and 175 to 188 in K-ras, are sufficient to target heterologous proteins to the plasma membrane (2, 18, 19). Indeed, targeting of Raf-1 and phosphoinositol 3-kinase to the plasma membrane using these minimal motifs is sufficient to partially activate these Ras effectors (30, 31, 49).
One consequence of each Ras isoform's having a different membrane anchor is that it may be directed to a different microdomain within the plasma membrane. In direct support of this concept, we have recently shown that the function of H-ras, but not K-ras, is critically dependent on cholesterol-rich microdomains, or lipid rafts, within the plasma membrane (46). Lipid rafts have been proposed as important substructures of the plasma membrane which can operate as signaling platforms (48), facilitating interactions between diverse signaling proteins, including tyrosine kinases, Src family kinases, G-protein subunits, and Ras (35, 39). The association of palmitoylated H-ras, but not polybasic K-ras, with such lipid rafts (23, 46) may therefore explain biochemical, and hence biological, differences between the various Ras proteins. For example, Ras isoforms vary in the ability to activate Raf and phosphoinositol 3-kinase (16, 59), probably reflecting the different concentrations of coactivators of these effectors in the H-, K-, and N-Ras microdomains. In addition, RasGRF1 selectively activates H-Ras (25) and RasGRP1 activates H-, N-, and K-ras with various potencies (52), a likely consequence of differential colocalization of these Ras exchange factors with their target Ras isoforms at the plasma membrane. In turn, such biochemical differences can be invoked to rationalize the differential activation of specific Ras isoforms in human tumors (5) and the selective requirement for K-ras, but not H- or N-ras, function in mouse embryogenesis (24, 56).
Intriguing questions invited by these observations are how Ras proteins access the plasma membrane and, more specifically, how they become localized to their correct microdomains. The first steps in this process must necessarily involve the enzymes which modify the CAAX motif. First, farnesyltransferase, a cytosolic enzyme (44), prenylates newly synthesized Ras, generating a farnesylated CAAX sequence that is the recognition sequence for the prenylcysteine endoprotease which removes the AAX tripeptide (51). The mammalian protease hRce1 has recently been cloned (40) based on its sequence homology with the yeast RCE1 sequence (6). The protease is membrane associated and proteolyzes geranylgeranylated and farnesylated CAAX motifs of all Ras, Ras-related, and G-γ subunits tested (28, 40). Moreover, a knockout of Rce1 in mice is late embryonal stage lethal. Fibroblasts derived from Rce1−/− mice show significant mislocalization of Ras proteins to the cytosol (28), a consequence similar to that of RCE1 disruption in yeast (6). After cleavage of the AAX tripeptide, the now C-terminal farnesylated cysteine residue of Ras is methylesterified by prenylcysteine carboxymethyltransferase (pcCMT), an endoplasmic reticulum (ER)-resident protein (12). The same pcCMT also methylates the C-terminal geranylgeranylated cysteine residues of other Ras-related proteins (11, 12). No knockout of pcCMT has been reported, but disruption of the homologous STE14 protein in yeast has relatively minor effects on RAS function and localization (21, 22).
In the Rab and Rho subfamilies of Ras-related proteins, attachment to the correct cellular membrane involves guanine nucleotide dissociation inhibitors (RhoGDI and RabGDI) which extract the cognate GDP-bound prenylated proteins from membranes into cytosolic complexes (13, 34, 55). These soluble complexes allow delivery of the Ras-related protein to the correct cellular membrane through the cytosol. Similarly, Rab escort proteins also move geranylgeranylated Rab from Rab geranylgeranyltransferase onto cell membranes (1). Since no analogous GDI has been identified for Ras, we investigated in this study whether Ras might access the plasma membrane though a vesicular trafficking pathway. Moreover, given that H- and K-ras operate in functionally distinct microdomains, we examined whether they also traffic to the plasma membrane via different routes.
MATERIALS AND METHODS
Plasmids.
Expression plasmids for green fluorescent protein (GFP)-tH, GFP-tK, and GFP-6QtK were constructed by ligating EcoRI/SalI cDNA fragments from protein A expression plasmids, p381, pA-K, and pA-6Q, respectively (18, 19), into the cloning site of pEGFP-C1. GFP-tH therefore terminates with the sequence CMSCKCVLS, GFP-tK ends with KKKKKKSKTKCVIM, and GFP-6QtK ends with QQQQQQSKTKCVIM. pRSα-sialylT, the expression plasmid for G-tagged sialyltransferase, has been described previously (43).
Cell culture and confocal microscopy.
BHK cells were cultured at 37°C in Dulbecco modified Eagle medium supplemented with 10% bovine calf serum. Cells were plated onto coverslips at 60% confluence and transfected 4 h later, using Lipofectamine (Life Technologies), with 1.6 μg of expression plasmid pEGFP with or without an expression plasmid for G-tagged sialyltransferase. After overnight incubation, cells were fixed with 4% paraformaldehyde, permeabilized in 0.2% Triton X-100, and blocked with 3% bovine serum albumin. Sialyltransferase was visualized using 1:1,000 rabbit anti-G tag serum, and the ER was stained using 1:120 rabbit anti-KDDD antibody (raised against the sequence KDDDKDEL and specific for protein disulfide isomerase (PDI [kindly provided by S. Fuller, EMBL]). Both primary antibodies were followed by 1:250 CY3-coupled anti-rabbit antibody (Zymed). Coverslips were mounted in mowiol. Where indicated, cells were equilibrated at 15°C with medium supplemented with 25 mM HEPES and incubated for 2 h with or without cycloheximide (50 μg/ml) at the same temperature. Fluorescence images were taken in a Bio-Rad MRC-600 Zeiss microscope.
Cells to be incubated in brefeldin A (BFA) were returned to Dulbecco modified Eagle medium containing 10% serum 2 h after transfection and supplemented with BFA at 5 μg/ml or ethanol (carrier) at 5 μl/ml and incubated for a further 5 h before fixation. For palmitic acid labeling, the medium was supplemented with 5 mM sodium pyruvate plus [3H]palmitic acid at 0.25 mCi/ml and cells were incubated for only 3 h prior to harvesting to minimize breakdown of the label. Lysates were prepared in ND buffer (50 mM Tris Cl [pH 7.6], 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate plus protease inhibitors), 1 mg of cleared lysate was immunoprecipitated with 4 μg of anti-GFP monoclonal antiserum (Boehringer Mannheim) coupled to protein G-agarose. After washing in ND buffer, immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorographed for 24 h at −70°C using established protocols (19). Where indicated, taxol treatment (3 μM) was commenced 2 h prior to lipofection and continued until cells were fixed or harvested 12 h later. Tubulin was visualized using 1:500 anti-α-tubulin serum DM1a (Sigma), followed by 1:400 CY3-coupled anti-mouse serum (Zymed).
Electron microscopy.
Transfected BHK cells were fixed in 8% paraformaldehyde in 0.1 M phosphate buffer, pH 7.35, for 1 h at room temperature. They were then washed with 0.2 M phosphate buffer, scraped from the culture dish, and pelleted in a Microfuge. The cells were then resuspended in warm gelatin (10% in phosphate buffer) and repelleted at maximum speed in the Microfuge. After cooling, the gelatin-embedded cells were infiltrated with polyvinylpyrrolidone-sucrose overnight at 4°C and then processed for frozen sectioning as previously described (41). Ultrathin frozen sections (60 to 80 nm) were labeled, stained, and viewed (JEOL 1010; Centre for Microscopy and Microanalysis) in accordance with previously published techniques (41) with rabbit polyclonal antibodies to GFP (kindly provided by David James, University of Queensland).
RESULTS
A polybasic domain excludes CAAX proteins from the exocytic pathway.
Recent work has shown that the mammalian Ras methyltransferase is localized to the ER (12). Since methylesterification is the final CAAX modification, this suggests that CAAX processing is completed on the cytoplasmic leaflet of the ER membrane. To investigate how Ras proteins access the plasma membrane from the ER, the complete targeting signals of H- and K-ras, comprising the C-terminal 9 amino acids of H-ras and the C-terminal 17 amino acids of K-ras, were cloned onto the C terminus of GFP to give GFP-tH and GFP-tK, respectively. Extensive previous work has shown that all of the Ras membrane-targeting signals are contained within these C-terminal sequences. We elected to use GFP-tH and GFP-tK to investigate Ras trafficking because GFP is biologically inert and the role of the isolated targeting signals can therefore be studied without the metabolism and architecture of the cell being altered by the presence of overexpressed biologically active Ras. The H- and K-ras motifs were compared because although they share a CAAX motif, the critical second signals within the motifs, palmitoylation in H-ras and a polybasic domain in K-ras, are strikingly different.
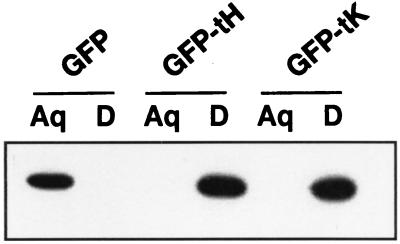
First, we evaluated the extent to which GFP-tH and GFP-tK are prenylated when expressed in BHK cells. This was assessed using the Triton X-114 partitioning assay, which has been used extensively to monitor the posttranslational processing of many Ras and Ras-related proteins (15, 17). Wild-type GFP was found to partition completely into the aqueous phase of Triton X-114, indicating that it is highly hydrophilic (Fig. 1). In contrast, both GFP-tH and GFP-tK partitioned exclusively into the detergent phase of Triton X-114, indicating that all of the expressed protein is hydrophobic. We therefore conclude that GFP-tH and GFP-tK are fully prenylated when expressed in BHK cells.
FIG. 1.
Prenylation of GFP-Ras constructs. BHK cells expressing GFP, GFP-tH, or GFP-tK were lysed in Triton X-114. The lysates, normalized for protein content, were warmed to 37°C, and the detergent (D) and aqueous (Aq) phases were separated by centrifugation exactly as previously described (15, 17). Equal proportions of each fraction were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted using anti-GFP serum. Wild-type GFP is hydrophilic and partitions exclusively into the aqueous phase, whereas prenylation of the CAAX motifs of GFP-tH and GFP-tK imparts sufficient hydrophobicity to partition the proteins into the detergent phase. Prenylation is complete, since all of the expressed GFP-tH and GFP-tK has shifted into the detergent phase.
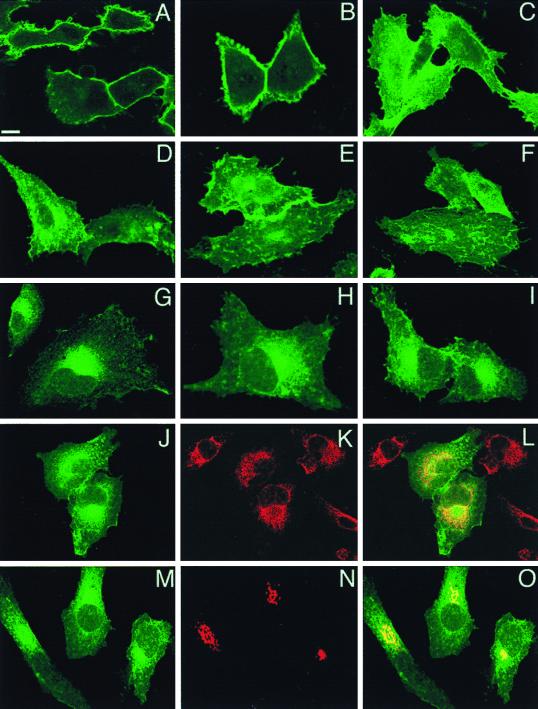
Next, we used confocal microscopy to compare the subcellular distributions of GFP-tH and GFP-tK when they are expressed at equivalent levels in BHK cells. Figure 2A to C shows that GFP-tK localized exclusively to the plasma membrane with no significant labeling of intracellular structures. In contrast, while GFP-tH was also on the plasma membrane, a substantial amount was present on perinuclear structures (Fig. 2D and E). Although there were no apparent differences between the two targeted GFPs in overall plasma membrane distribution, membrane ruffles and projections were more extensively decorated with GFP-tK than with GFP-tH (Fig. 2C and F). Cells were then examined using immunoelectron microscopy with antibodies to the GFP tag. In agreement with the immunofluorescence analysis, GFP-tH and GFP-tK were both readily detectable on the plasma membrane (Fig. 3A and B and 4A). GFP-tH was also readily detectable on Golgi membranes (Fig. 3A). In contrast, negligible GFP-tK labeling was found on Golgi membranes even in cells with significant plasma membrane staining (Fig. 4A). These differences were not due to different expression levels of the respective proteins, because quantitative immunoblotting of cell lysates showed that the GFP constructs used in this study were expressed at equivalent levels (Fig. 1).
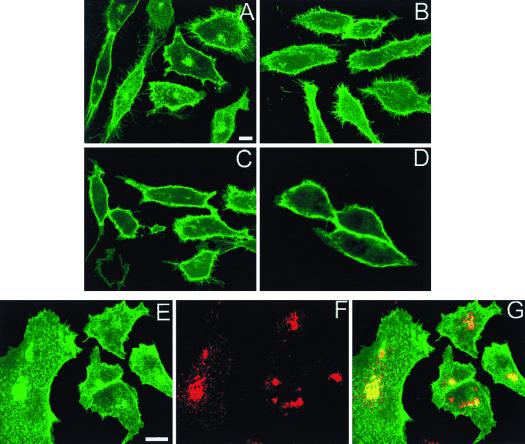
FIG. 2.
Confocal localization of GFP-Ras constructs. BHK cells were transfected with GFP-tH, GFP-tK, or GFP-6QtK and incubated at 37°C for 18 h prior to fixation for confocal microscopy. Scale bars, 10 μm. (A to F) Panels A, B, D, and E show cuts through cells at the level of the nucleus, whereas those in panels C and F are higher-level cuts showing the cell surface. In cells expressing GFP-tK (A to C), the protein is almost completely localized to the plasma membrane. In cells expressing GFP-tH (D to F), there is staining of the plasma membrane and intracellular structures. The intensity of plasma membrane fluorescence is less with GFP-tH than with GFP-tK. (G to I) Representative cells expressing GFP-6QtK. There is substantial intracellular staining adjacent to and contiguous with the nuclear membrane, with minimal staining of the plasma membrane. (J to L) Cells expressing GFP-6QtK were costained for PDI, an ER marker (red channel), with anti-KDDD antibody (K and L). The overlay (L) shows extensive localization of GFP-6QtK to the ER. (M to O) GFP-6QtK was cotransfected with VSV G-tagged sialyltransferase, a Golgi-resident protein, and the cells were stained for the G epitope tag. The overlay in panel O shows that GFP-6QtK is present in the Golgi, a result confirmed by immunoelectron microscopy.
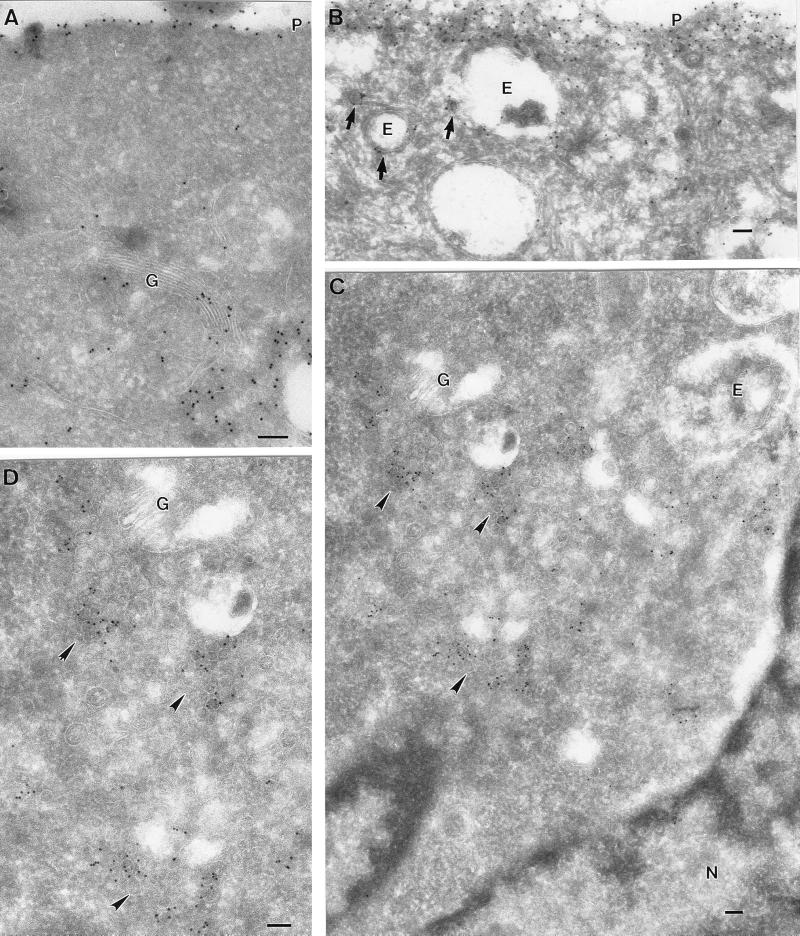
FIG. 3.
Ultrastructural localization of GFP-tH at 37 and 15°C. BHK cells were transfected with GFP-tH and incubated for 18 h at 37°C (A and B) or for 16 h at 37°C, followed by 2 h at 15°C (C and D). The cells were then labeled with antibodies to GFP, followed by 10-nm protein A-gold particles. Scale bars, 100 nm. (A and B) Specific labeling for GFP-tH is evident on the plasma membrane (P). Despite the high expression level, there is negligible cytosolic labeling. Specific labeling is also apparent on the Golgi complex (G). Putative endosomes (E) show low labeling, whereas small vesicles nearby show higher labeling (arrows). (C and D) At 15°C, GFP-tH accumulates in groups of small vesicular structures (arrowheads) close to the Golgi complex (G), as shown at higher magnification in panel D. Again, note the lack of significant cytosolic labeling and the absence of labeling on the ER surrounding the nucleus (N).
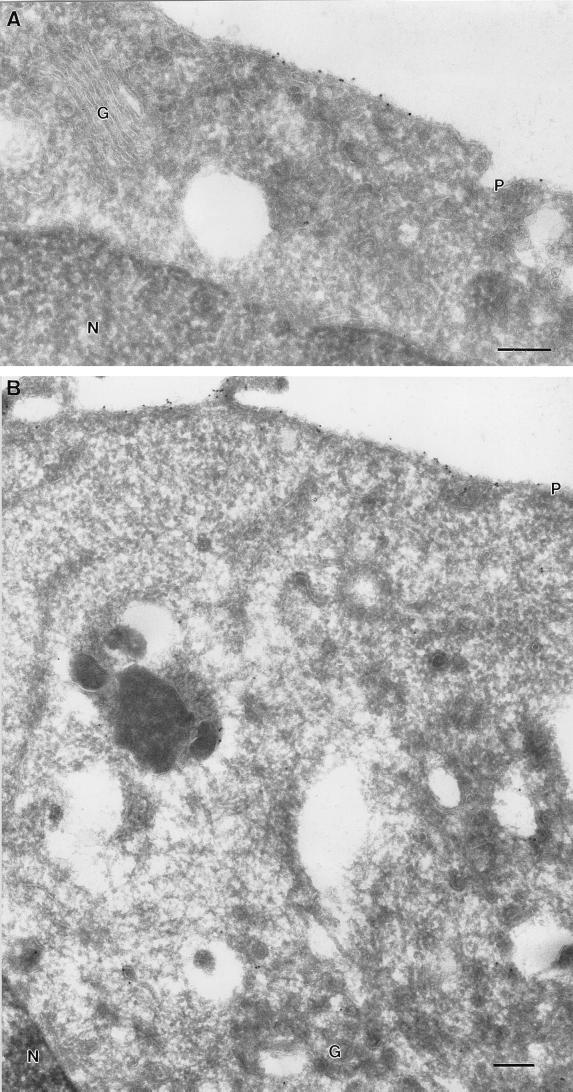
FIG. 4.
Ultrastructural localization of GFP-tK at 37 and 15°C. BHK cells were transfected with GFP-tK and incubated for 18 h at 37°C (A) or for 16 h at 37°C, followed by 2 h at 15°C (B). The cells were then labeled with antibodies to GFP, followed by 10-nm protein A-gold particles. Scale bars, 100 nm. (A) Specific labeling for GFP-tK is evident on the plasma membrane (P). Negligible labeling is apparent on the Golgi complex (G). (B) Again, specific labeling for GFP-tK is evident on the plasma membrane (P). Negligible labeling is apparent on the Golgi complex (G), in contrast to the results obtained with GFP-tH (cf. Fig. 3C and D). N, nucleus.
We have shown previously that an isolated CVIM motif (directing farnesylation) or CCIL motif (directing geranylgeranylation) does not target proteins to the cell surface (18). However, whereas CVIM fusion proteins are largely soluble upon cell fractionation, reflecting the relatively low affinity of farnesylated proteins for membranes (47), CCIL fusion proteins associate avidly with a P100 fraction that is not plasma membrane (18). In the light of the recent data of Dai et al. (12), we investigated whether, in intact cells, these mutant incomplete targeting motifs result in mislocalization of Ras to the ER or Golgi. The C-terminal sequence of K-ras-6Q, a mutant protein in which all of the lysines in the polybasic domain have been replaced with glutamine residues (20), was cloned onto the C terminus of GFP to give GFP-6QtK. The localization of GFP-6QtK, which therefore has a CVIM motif but no second signal, was examined using confocal and immunoelectron microscopy. Figure 2G to I shows that very little GFP-6QtK was present on the plasma membrane; rather, the majority of the protein was concentrated adjacent to, or associated with, the nuclear membrane. Costaining of cells with an antibody (anti-KDDD) against the ER protein PDI showed extensive colocalization with GFP-6QtK (Fig. 2J to L). GFP-6QtK appeared more concentrated than the ER marker in the perinuclear area of the cell, which corresponded to the position of the Golgi complex as marked by vesicular stomatitis virus (VSV) G epitope-tagged sialyltransferase (Fig. 2M to O). Since GFP-6QtK, but not GFP-tK or GFP-tH, was detected on ER membranes, we conclude that the second signal for plasma membrane targeting is required to efficiently clear CAAX proteins from the ER. In addition, since GFP-tH and GFP-6QtK, but not GFP-tK, were clearly present on Golgi membranes, we conclude that the polybasic domain functions as a sorting signal to exclude CAAX proteins from entering the Golgi.
Palmitoylated, but not polybasic, Ras proteins traffic though the Golgi to the cell surface.
The presence of GFP-tH on Golgi membranes (Fig. 3) suggested that this palmitoylated protein passes through the exocytic pathway. To investigate this in more detail, BHK cells expressing GFP-tH and GFP-tK were incubated at 15°C for 2 h before fixation for confocal or electron microscopy. This temperature block, which impairs transport from the ER to the cis-Golgi (42), resulted in striking increases in the amount of GFP-tH that was visible adjacent to the nucleus (Fig. 5A) but caused no intracellular buildup of GFP-tK (Fig. 5C). The intracellular accumulation of GFP-tH was reversible and rapidly dissipated to control levels when the cells were returned to 37°C (data not shown). Since the cells were examined 18 h after transfection, a substantial amount of GFP-tH has already been synthesized and trafficked to the cell surface. To verify that the accumulation of GFP-tH at 15°C consisted of newly synthesized and processed protein rather than protein that may have recycled to the Golgi region from the cell surface, the experiment was repeated after cells had been preincubated with cycloheximide to block new protein synthesis. Figure 5B shows that under these experimental conditions, there was no intracellular accumulation of GFP-tH at 15°C. And consistent with this observation, there was no significant colocalization of intracellular GFP-tH with Rab11, a marker of the recycling endosome (54) (data not shown).
FIG. 5.
Newly synthesized GFP-tH accumulates in the Golgi complex at 15°C. BHK cells were transfected with GFP-tH (A, B, and E to G) or GFP-tK (C and D) or cotransfected with GFP-tH and VSV G-tagged sialyltransferase (E to G). After incubation at 37°C for 24 h, cells were incubated for a further 2 h at 15°C prior to fixation. Scale bars, 10 μm. (A and B) GFP-tH accumulates in perinuclear structures after incubation at 15°C (A); this intracellular accumulation of GFP-tH is blocked by incubation of the cells in cycloheximide for 2 h before and during the temperature block (B). (C and D) GFP-tK does not show any evidence of intracellular accumulation at 15°C (C and D). Cells shown in panel D were incubated in cycloheximide. (E to G) All cells were incubated at 15°C prior to fixation. Sialyltransferase (red channel), a Golgi marker, shows extensive colocalization with the intracellular accumulation of GFP-tH.
To identify the compartment in which GFP-tH was localized, cells were cotransfected with sialyltransferase and stained with rabbit antiserum to the VSV G epitope (Fig. 5E to G). The perinuclear accumulation of GFP-tH colocalized with sialyltransferase, showing that this treatment caused the protein to accumulate in the Golgi region (Fig. 5G).
Frozen sections of GFP-tH- and GFP-tK-expressing cells were then examined by immunoelectron microscopy after incubation at 15°C. Dense labeling for GFP-tH was observed on tubulovesicular membranes in close proximity to the Golgi complex (Fig. 3C and D). These tubulovesicular structures represent an intermediate compartment of the exocytic pathway between the ER and Golgi (33), and the accumulation of GFP-tH in this compartment is therefore consistent with a block in transport of GFP-tH from the ER to the Golgi. In contrast, GFP-tK was readily detectable on the plasma membrane of transfected cells incubated at 15°C but was not concentrated in the Golgi complex region, even in highly expressing cells (Fig. 4B). A quantitative electron microscopic analysis of labeling associated with the plasma membrane and Golgi apparatus of GFP-tH- and GFP-tK-expressing cells is presented in Fig. 6 and confirms the qualitative observations made by immunofluorescence assay.
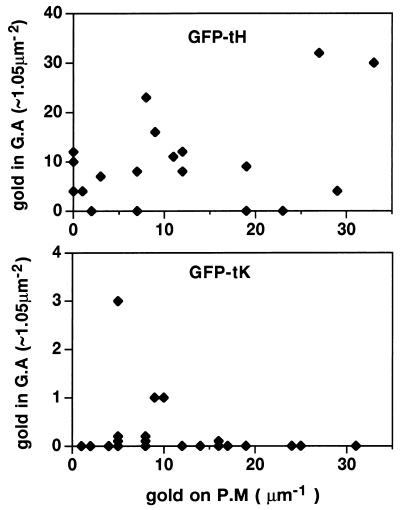
FIG. 6.
Semiquantitative comparison of the subcellular distribution of GFP-ras constructs. Cells expressing GFP-tK and GFP-tH were fixed after a 2-h incubation at 15°C, cryosectioned, and examined by electron microscopy after immunogold labeling for GFP. Cells positively expressing GFPtH or GFPtK were assayed for the number of gold particles present on randomly chosen fixed-unit lengths of plasma membrane (P.M) or in fixed-unit Golgi complex areas (G.A). These data are, in part, arrayed as two scatter diagrams; note the log difference in the y axis scales. The plasma membrane pool, which represents the largest subcellular pool of Ras, contains similar amounts of labeling for GFPtH and GFPtK, indicating that levels of expression were comparable for the two peptides (means, 13.75 and 13.21 gold particles/μm, respectively). GFP-tH was present on and in the vicinity of the Golgi apparatus (mean, 28.62 gold particles/μm2), whereas GFPtK was almost completely excluded from this region (mean, 0.25 gold particles/μm2).
To extend these observations, we investigated whether an intact Golgi is required to traffic GFP-tH or GFP-tK to the plasma membrane. BHK cells were treated with BFA 2 h after transfection, and plasma membrane staining was assessed by confocal microscopy after a further 5 h of incubation. This protocol was used to allow sufficient cells to express the transfected protein to permit a quantitative analysis. Coverslips were systematically examined, and the intensity of plasma membrane staining of each cell encountered was graded from strong (as in Fig. 2B or E) to weak or none (as in Fig. 2G to I). Examples of cells from the BFA-treated cultures are shown in Fig. 7A to D, and data from a representative experiment, in which a total of >1,000 cells were counted, are presented in Fig. 7E. Strikingly, the number of GFP-tH-expressing cells with strong plasma membrane staining fell by 75% in the presence of BFA compared with that of untreated control cells, whereas the corresponding number of GFP-tK-expressing cells with strong plasma membrane staining was completely unaffected (Fig. 7E). Moreover, greater than 50% of GFP-tH-expressing cells treated with BFA showed strong perinuclear staining (Fig. 7D), very similar to that shown in Fig. 2G to I. Thus, BFA-induced disruption of the Golgi had no effect on the delivery of GFP-tK to the plasma membrane but significantly inhibited the plasma membrane access of GFP-tH.
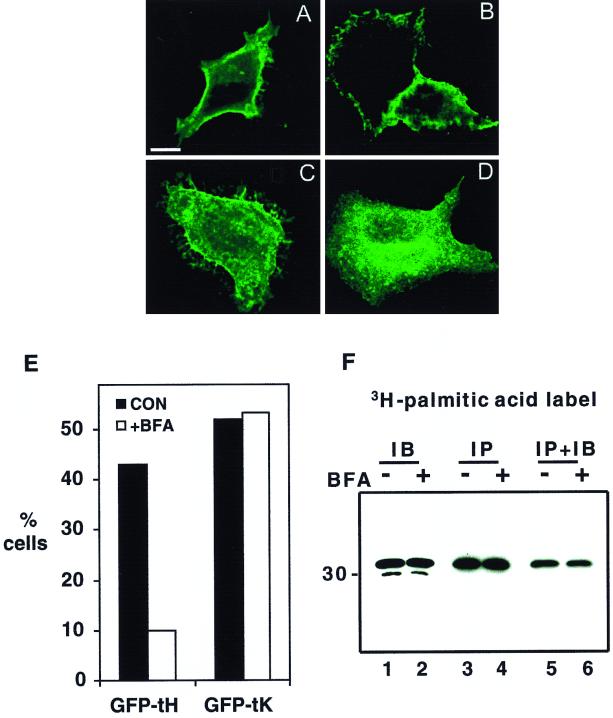
FIG. 7.
BFA inhibits the plasma membrane accumulation of GFP-tH but not GFP-tK. BHK cells were transfected with GFP-tH or GFP-tK. At 2 h after transfection, BFA (5 μg/ml) was added to half of the cultures. After 5 h, coverslips were examined by confocal microscopy. Bar, 10 μm. (A to D) Representative cells from control cultures (A and C) and BFA-treated cultures (B and D). The localization of GFP-tK (A and B) is unaffected, while GFP-tH (C and D) is prevented from trafficking to the plasma membrane. (E) Greater than 450 cells (462 to 524) per experimental condition were scored for intensity of plasma membrane fluorescence by an observer blind to the transfection and treatment conditions. The graph shows the percentages cells with clear plasma membrane staining. It is a representative experiment that was repeated three times with similar results. CON, control. (F) BHK cells plated in 10-cm-diameter dishes were transfected with GFP-tH. At 2 h after transfection, cells were switched to labeling medium containing [3H]palmitic acid; simultaneously, BFA (10 μg/ml) was added to half of the cultures (+BFA) and ethanol carrier was added to the remainder (−BFA). After a further 3 h of incubation, coverslips were quantified as for panel E; the remainder of the cells were harvested into lysis buffer. Lysates were immunoblotted (IB, lanes 1 and 2) or immunoprecipitated (IP, lanes 3 to 6) using monoclonal anti-GFP serum. Radiolabeled proteins in the immunoprecipitates were visualized by fluorography (lanes 3 and 4). Immunoprecipitates were also probed with polyclonal anti-GFP serum to confirm that equal amounts of GFP-tH had been captured (lanes 5 and 6). Note that there is no effect of BFA on GFP-tH expression (lanes 1 and 2) or on palmitic acid incorporation (lanes 3 and 4). BFA did, however, decrease the number of cells with strong plasma membrane staining to 8% from the 48% in the untreated control.
To investigate whether BFA treatment inhibits the palmitoylation of GFP-tH, cells were metabolically labeled with [3H]palmitic acid for 3 h in the presence of BFA and then harvested. Lysates from BFA-treated and control, untreated cultures were immunoprecipitated with anti-GFP serum and analyzed by fluorography. Coverslips from the labeled culture plates were also examined to quantify plasma membrane staining as described above. Figure 7F shows that incubation in BFA had no effect on the incorporation of palmitic acid into GFP-tH, even though the number of GFP-tH-expressing cells with strong plasma membrane staining fell by 80% in the BFA-treated culture. We conclude that access of newly synthesized GFP-tH to palmitoyltransferase does not require an intact Golgi. The simplest interpretation of these data is that palmitoylation of GFP-tH occurs proximal to the Golgi in the exocytic pathway and that the CAAX palmitoyltransferase is therefore most likely in the ER.
Taxol perturbs the trafficking of polybasic Ras proteins.
A mechanistic insight into the nonexocytic pathway utilized by K-ras comes from the work of Casey et al. (50), who reported that K-ras binds with high affinity to taxol-stabilized microtubules polymerized in vitro from monomeric tubulin. This binding of K-ras to microtubules is prenyl dependent and is not seen with any other Ras or Ras-related protein. Moreover, taxol treatment of intact cells results in mislocalization of newly synthesized K-ras but has no effect on the localization of H-ras (50). We therefore sought to extend these observations by examining the trafficking of GFP-tK and GFP-tH in taxol-treated cells. Consistent with the previous report, taxol treatment had no detectable effect on the plasma membrane localization of newly synthesized GFP-tH (not shown) but significantly reduced the plasma membrane localization of GFP-tK (Fig. 8D and G). Importantly, subcellular fractionation of BHK cells revealed that all of the GFP-tK remained associated with the P100 membrane fraction in taxol-treated cells (Fig. 8). Confocal sections of cells taken at the level of the nucleus show that while some GFP-tK in taxol-treated cells is localized at the plasma membrane, much is found in irregular structures which do not show an ER- or Golgi-like staining pattern (Fig. 8D and G). Some of these GFP-tK-decorated structures have a tubular-vesicular appearance (Fig. 8D and G). To examine the relationship between GFP-tK and the microtubule network, cells expressing GFP-tK were costained for tubulin. The thickening and reorganization of microtubules induced by taxol are clearly visible when the untreated cells in Fig. 8B are compared with the treated cells in Fig. 8E and H. The overlays of these confocal images show that there is very little colocalization of intracellular GFP-tK and tubulin in the taxol-treated cells (Fig. 8F and I), although some alignment of the tubular-vesicular GFP-tK structures alongside microtubules is evident in some cells (Fig. 8I). Electron microscopic analysis (Fig. 9) supported these observations. Taxol-treated cells showed abundant microtubule bundles which had negligible labeling for GFP-tK. Labeling was observed on the plasma membrane and within large multivesicular endosomes, which were abundant in the taxol-treated cells (Fig. 9). Similar multivesicular endosome carrier vesicles have been shown to accumulate in nocodazole-treated BHK cells (14). In summary, these data show that GFP-tK does not directly associate with microtubules in vivo but that taxol treatment results in redistribution of GFP-tK away from the plasma membrane into an endosomal compartment.
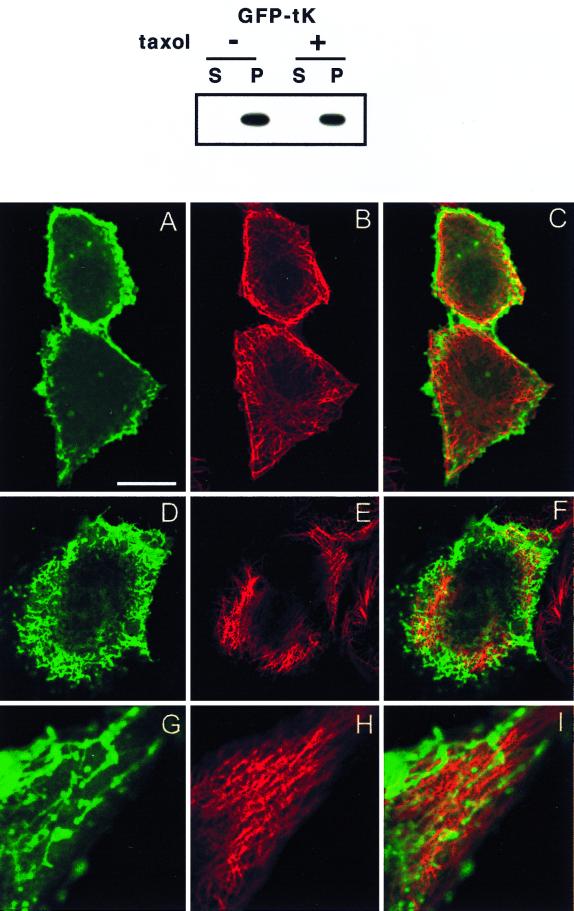
FIG. 8.
Taxol causes redistribution of GFP-tK. BHK cells were incubated in taxol for 2 h prior to and 12 h after lipofection and then harvested for subcellular fractionation or fixed for confocal or electron microscopy. The cytosolic (S = S100) and membrane (P = P100) fractions were prepared from cells expressing GFP-tK as previously described (45), normalized for protein content, and immunoblotted with anti-GFP serum (upper panel). GFP-tK remained fully associated with the P100 fraction in taxol-treated cells. The lower panel shows confocal images of BHK cells from identical cultures that have been costained for α-tubulin. Cells in panels A to F have been cut at the level of the nucleus. The images in panels G to I show the edge of a cell magnified 2.5× relative to the other images. Bar, 10 μm for panels A to F and 4 μm for panels G to I. (A to C) Untreated control cells showing normal microtubules (red channel) and plasma membrane-localized GFP-tK (green channel). (D to I) Taxol-treated cells show some thickening and disruption of the microtubules, accompanied by accumulation of intracellular GFP-tK and a reduction in the amount of GFP-tK seen at the plasma membrane. GFP-tK is seen decorating vesicular and tubulovesicular structures, but there is very little actual colocalization of GFP-tK with tubulin. Some of the tubulovesicular structures are apparently aligned alongside microtubules; this is seen particularly well in panels G to I.
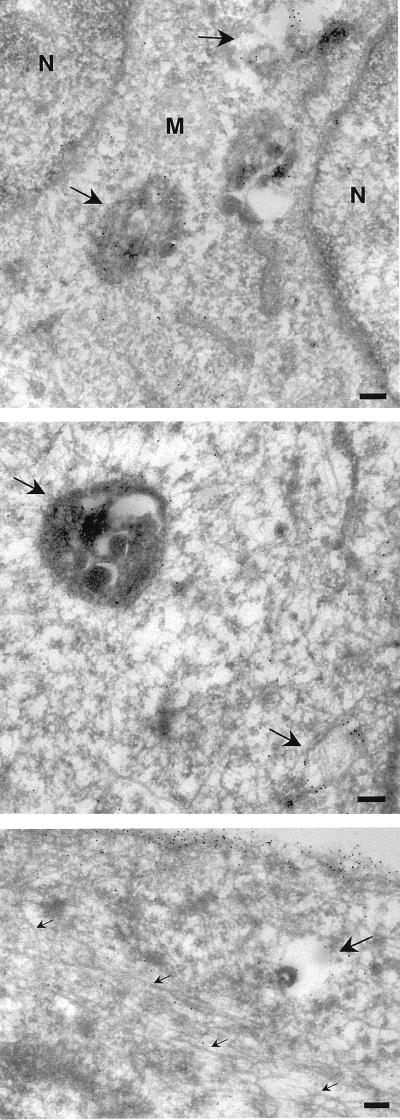
FIG. 9.
Electron microscopic analysis of taxol-treated cells. BHK cells were taxol treated and transfected as described in the legend to Fig. 8. Frozen sections were then labeled with antibodies to GFP, followed by protein A-gold particles. Specific labeling is associated with the plasma membrane and endosomal structures (arrows), but negligible labeling is associated with microtubules (small arrows, bottom panel). N, nucleus; M, mitochondria; bars, 200 nm.
DISCUSSION
The experiments described in this paper address the intriguing question of how Ras proteins that have been posttranslationally modified on the cytosolic leaflet of the ER membrane gain access to the inner surface of the plasma membrane. The results presented here are fully consistent with the recent observations of Choy et al. (10), who examined in detail the cellular trafficking of full-length N- and K-Ras proteins coupled to GFP. Here we have focused on the ability of the isolated H- and K-Ras membrane-targeting motifs to traffic GFP to the plasma membrane and have made similar observations. Importantly, when taken together, the two studies show that the C-terminal Ras motifs contain all of the relevant signals for successful Ras trafficking. Moreover, they demonstrate that the palmitoylated Ras proteins H- and N-ras use the same trafficking pathway.
We found that proteins which have an intact second signal for plasma membrane localization, palmitoylation or a polybasic domain, are efficiently cleared from the ER, whereas proteins which lack a second signal accumulate in the ER and Golgi complex. This led to the important conclusion that palmitoylation or a polybasic domain is an essential part of the trafficking signal for Ras proteins. Moreover, a particularly fascinating finding of the present study is the observation that palmitoylated H-ras, but not K-ras, traffics to the plasma membrane along the secretory pathway via the Golgi complex. This gives new insights into the manner in which these two proteins, which differ in membrane anchoring, reach their site of action at the plasma membrane. We envisage a scheme in which the H-ras and K-ras polypeptides are synthesized on cytosolic ribosomes and farnesylated in the cytoplasm. The proteins then associate with the ER for removal of the AAX tripeptide and methylation of the C-terminal cysteine. The two isoforms are then differently routed (Fig. 10). If a polybasic domain is present, as in K-ras, the protein does not enter the conventional exocytic pathway. This follows because no GFP-tK was detectable in or near the Golgi at 37 or 15°C by either immunofluorescence assay or electron microscopy. These data therefore suggest that the combination of a CAAX motif and a polybasic domain comprises a sorting signal that directs K-ras directly to the cell surface, bypassing the Golgi.
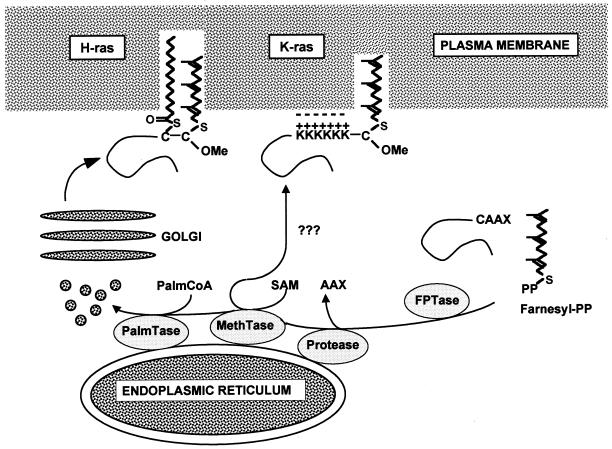
FIG. 10.
Model of Ras trafficking. After the common processing steps of farnesylation, AAX proteolysis, and methylation which are completed on the cytosolic surface of the ER, the trafficking routes of the different Ras proteins diverge. K-ras, by virtue of its C-terminal polybasic domain, is sorted out of the conventional exocytic pathway and takes an undefined pathway to the cell surface that bypasses the Golgi. H- and N-ras are palmitoylated by an ER-localized palmitoyltransferase and enter the exocytic pathway; they traffic to the cell surface via the Golgi. OMe, carboxymethyl; PalmCoA, palmitoyl coenzyme A; SAM, S-adenosyl methionine; PalmTase, palmitoyltransferase; MethTase, methyltransferase; FPTase, farnesyl protein transferase; Farnesyl-PP, farnesyl pyrophosphate.
It is unclear exactly how K-ras accesses the plasma membrane, although several mechanisms are possible. For example, smgGDS, a weak exchange factor for K-ras, could operate as a chaperone protein because it has been shown to extract K-ras from cell membranes (27), in a fashion analogous to that of the GDIs of Rho and Rab proteins, yet does not interact with H- or N-ras. Alternatively, since farnesylated K-ras has a lower affinity for membranes than palmitoylated H-ras, it is possible that this lower avidity of membrane binding allows K-ras to dissociate from the ER and bind directly to the plasma membrane; although this seems less likely because GFP-6QtK, which has no polybasic domain and hence has even lower affinity for membranes than GFP-tK, remained associated with the ER and Golgi membranes in intact cells. The complete absence of GFP-tK from the ER is more consistent with an active removal mechanism than a passive, equilibrium-driven diffusion process.
Neither of these potential trafficking mechanisms can account for the intriguing observations that taxol treatment significantly reduces the plasma membrane accumulation of newly synthesized K-ras. This result strongly suggests that a functional microtubule network is necessary for K-ras to transit the cell. We show here, however, that there is no obvious colocalization of GFP-tK with tubulin in taxol-treated cells, as might have been predicted from the study of Casey et al. (50). Rather, we found that GFP-tK accumulates in intracellular multivesicular and univesicular endosomal structures that are only infrequently arrayed alongside taxol-stabilized microtubules. There are two possible interpretations of these findings. First, GFP-tK traffics from the ER to the plasma membrane using microtubule-dependent vesicular transport and taxol treatment results in a build up of GFP-tK in transport vesicles. An important caveat is that K-ras must traffic to the plasma membrane very quickly, because the GFP-tK taxol-sensitive compartment was not visible in untreated cells. In this context, it is worth noting that kinetic analysis does suggest that newly synthesized K-ras accesses the cell surface more rapidly than does N-ras (10). A second interpretation is that forward transport of GFP-tK from the ER to the plasma membrane is unaffected by taxol but that maintenance of the plasma membrane pool requires functioning microtubules. In this case, in taxol-poisoned cells, some of the plasma membrane-localized GFP-tK enters and accumulates in a bulk flow endosomal compartment, possibly due to disruption of early-to-late endosomal transport (14). Both interpretations have important yet clearly different implications for the potential clinical manipulation of K-ras plasma membrane association.
In the absence of a polybasic domain, CAAX proteins are delivered to the Golgi complex and, if palmitoylated, are trafficked to the plasma membrane (Fig. 10). Since palmitoylated GFP-tH was cleared from the ER much more efficiently than nonpalmitoylated GFP-6QtK, it is most likely that the Ras palmitoyltransferase, like the pcCMT, is present in the ER. This conclusion is supported by the observation that disruption of the Golgi by BFA did not inhibit GFP-tH palmitoylation but dramatically reduced GFP-tH trafficking to the plasma membrane. Since some GFP-6QtK was detected in the Golgi, palmitoylation cannot be absolutely required for ER-to-Golgi transport but palmitoylation and an intact Golgi are clearly essential for trafficking of H-ras from the ER through the Golgi to the plasma membrane. The role of palmitoylation may simply be to allow a more stable membrane association of H-ras, which is required for further transport to the plasma membrane. However, it is also possible that the requirement for palmitoylation to escape from the Golgi indicates that the palmitoyl group is required for sorting of H-ras into specific carrier vesicles exiting the Golgi. These roles are not mutually exclusive. Either way, H-ras behaves like a membrane protein, being transported to the cell surface via the Golgi complex while associated with the cytoplasmic face of the transported membrane. H-ras is therefore a cytoplasmic cargo protein of the exocytic pathway, and its delivery to the plasma membrane is reliant on membrane traffic. Interestingly, a recent study reached similar conclusions with respect to Lck, proposing palmitoylation early in the exocytic pathway and vesicular transport to the cell surface through the Golgi (3).
There appear to be two outcomes for CAAX proteins with an incomplete trafficking signal: farnesylated Ras proteins with relatively low affinity for membranes probably have a high rate of dissociation from the ER and Golgi, which is why they are recovered predominantly in the cytosol on cell fractionation (19). In contrast, geranylgeranylated Ras proteins with an incomplete trafficking signal have a higher affinity for membranes (47) and remain associated with the ER. Similarly, Ras proteins engineered to be N terminally myristoylated and C terminally farnesylated, but which lack a polybasic domain or palmitoylation sites, fail to localize to the cell surface and extensively decorate the nuclear membrane (7), implying that these dually lipidated Ras proteins, with a high affinity for membranes and no second signal, cannot exit and remain bound to the ER. Other studies also emphasize the importance of the second signal for plasma membrane targeting. N-terminally myristoylated Ras proteins lacking a CAAX motif but with an intact polybasic domain or palmitoylation sites localize predominantly to the plasma membrane. Thus, the second signal specifies targeting to the plasma membrane if the Ras protein has a hydrophobic N or C terminus. Even more strikingly, in the case of H-Ras, additional hydrophobicity other than that provided by the acyl chain is also unnecessary for plasma membrane binding: H-ras proteins with a C-terminal polybasic extension in place of the CAAX motif undergo palmitoylation, localize to the plasma membrane, and have biological activity (4, 36).
Finally, different trafficking mechanisms for the Ras isoforms can be rationalized in the light of recent data showing that H-ras, but not K-ras, functionally associates with cholesterol-rich surface domains (46). An attractive hypothesis is that these lipid rafts, which can ultimately coalesce into caveolae in the presence of caveolin, are assembled in the trans-Golgi network and that H-ras needs to associate with the rafts at this stage. In contrast, K-ras, which does not show reliance on cholesterol-enriched domains for Raf activation, might show a more random distribution over the cell surface (38) and therefore does not need such a specific trafficking pathway.
ACKNOWLEDGMENTS
We thank Bill Balch for helpful advice, Tommy Nilsson for the pRSα-sialylT plasmid, Steven Fuller for the anti-KDDD antibody, and Annette Lane and Colin McQueen for excellent technical assistance.
This work was supported by grants to J.F.H. and R.G.P. from the NHMRC, Australia. J.F.H. is also supported by the Royal Children's Hospital Foundation, Queensland.
REFERENCES
- 1.Alexandrov K, Horiuchi H, Steele Mortimer O, Seabra M C, Zerial M. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated Rab proteins to their target membranes. EMBO J. 1994;13:5262–5273. doi: 10.1002/j.1460-2075.1994.tb06860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronheim A, Engelberg D, Li N, al Alawi N, Schlessinger J, Karin M. Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. Cell. 1994;78:949–961. doi: 10.1016/0092-8674(94)90271-2. [DOI] [PubMed] [Google Scholar]
- 3.Bijlmakers M-J J E, Marsh M. Trafficking of an acylated cytosolic protein: newly synthesized p56lck travels to the plasma membrane via the exocytic pathway. J Cell Biol. 1999;145:457–468. doi: 10.1083/jcb.145.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booden M A, Baker T C, Solski P A, Der C J, Parker S G, Buss J E. A non-farnesylated Ha-Ras protein can be palmitylated and trigger potent differentiation and transformation. J Biol Chem. 1999;274:1423–1431. doi: 10.1074/jbc.274.3.1423. [DOI] [PubMed] [Google Scholar]
- 5.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 6.Boyartchuk V L, Ashby M N, Rine J. Modulation of Ras and a-factor function by carboxyl-terminal proteolysis. Science. 1997;275:1796–1800. doi: 10.1126/science.275.5307.1796. [DOI] [PubMed] [Google Scholar]
- 7.Cadwallader K, Paterson H, Macdonald S G, Hancock J F. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol Cell Biol. 1994;14:4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 9.Casey P J, Solski P A, Der C J, Buss J E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci USA. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choy E, Chiu V K, Silletti J, Feoktisitov M, Morimoto T, Michaelson D, Ivanov I E, Philips M R. Endomembrane trafficking of Ras: the CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 11.Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- 12.Dai Q, Choy E, Chiu V, Romano J, Slivka S R, Seitz S A, Michaelis S, Philips M R. Mammalian prenylcysteine carboxyl methyltransferase is in the endoplasmic reticulum. J Biol Chem. 1998;273:15030–15034. doi: 10.1074/jbc.273.24.15030. [DOI] [PubMed] [Google Scholar]
- 13.Fukumoto Y, Kaibuchi K, Hori H, Fujioka H, Araki S, Ueda T, Kikuchi A, Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5:1321–1328. [PubMed] [Google Scholar]
- 14.Gruenberg J, Griffiths G, Howell K E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle function in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez L, Magee A I, Marshall C J, Hancock J F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxyl-terminal proteolysis. EMBO J. 1989;8:1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton M, Wolfman A. Ha-Ras and N-ras regulate MAPK activity by distinct mechanisms in vivo. Oncogene. 1998;16:1417–1428. doi: 10.1038/sj.onc.1201653. [DOI] [PubMed] [Google Scholar]
- 17.Hancock J F. Prenylation and palmitoylation analysis. Methods Enzymol. 1995;255:237–245. doi: 10.1016/s0076-6879(95)55026-7. [DOI] [PubMed] [Google Scholar]
- 18.Hancock J F, Cadwallader K, Paterson H, Marshall C J. A CAAX or a CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock J F, Magee A I, Childs J E, Marshall C J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 20.Hancock J F, Paterson H, Marshall C J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990;63:133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- 21.Hrycyna C A, Clarke S. Farnesyl cysteine C-terminal methyltransferase activity is dependent upon the STE14 gene product in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:5071–5076. doi: 10.1128/mcb.10.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hrycyna C A, Sapperstein S K, Clarke S, Michaelis S. The Saccharomyces cerevisiae STE14 gene encodes a methyltransferase that mediates C-terminal methylation of a-factor and RAS proteins. EMBO J. 1991;10:1699–1709. doi: 10.1002/j.1460-2075.1991.tb07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwabuchi K, Handa K, Hakomori S. Separation of “glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J Biol Chem. 1998;273:33766–33773. doi: 10.1074/jbc.273.50.33766. [DOI] [PubMed] [Google Scholar]
- 24.Johnson L, Greenbaum D, Cichowski K, Mercer K, Murphy E, Schmitt E, Bronson R T, Umanoff H, Edelman W, Kucherlapati R, Jacks T. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones M K, Jackson J H. Ras-GRF activates Ha-Ras, but not N-Ras or K-Ras 4B, protein in vivo. J Biol Chem. 1998;273:1782–1787. doi: 10.1074/jbc.273.3.1782. [DOI] [PubMed] [Google Scholar]
- 26.Joneson T, Bar-Sagi D. Ras effectors and their role in mitogenesis and oncogenesis. J Mol Med. 1997;75:587–593. doi: 10.1007/s001090050143. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura M, Kaibuchi K, Kishi K, Takai Y. Translocation of Ki-ras p21 between membrane and cytoplasm by smgGDS. Biochem Biophys Res Commun. 1993;190:832–841. doi: 10.1006/bbrc.1993.1124. [DOI] [PubMed] [Google Scholar]
- 28.Kim E, Ambroziac P, Otto J C, Taylor B, Ashby M, Shannon K, Casey P J, Young S G. Disruption of the mouse Rce1 gene results in defective Ras processing and mislocalization of Ras in cells. J Biol Chem. 1999;274:8383–8390. doi: 10.1074/jbc.274.13.8383. [DOI] [PubMed] [Google Scholar]
- 29.Kishida S, Koyama S, Matsubara K, Kishida M, Matsuura Y, Kikuchi A. Colocalization of Ras and Ral on the membrane is required for Ras-dependent Ral activation through Ral GDP dissociation stimulator. Oncogene. 1997;15:2899–2907. doi: 10.1038/sj.onc.1201473. [DOI] [PubMed] [Google Scholar]
- 30.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M-A, Williams L T. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leevers S J, Paterson H F, Marshall C J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 32.Marais R, Marshall C J. Control of the ERK MAP kinase cascade by Ras and Raf. Cancer Surv. 1996;27:101–125. [PubMed] [Google Scholar]
- 33.Martinez-Menarguez J A, Geuze H J, Slot J W, Kluperman J. Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COP1-coated vesicles. Cell. 1999;98:81–90. doi: 10.1016/S0092-8674(00)80608-X. [DOI] [PubMed] [Google Scholar]
- 34.Matsui Y, Kikuchi A, Araki S, Hata Y, Kondo J, Teranishi Y, Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for smg p25A, a ras p21-like GTP-binding protein. Mol Cell Biol. 1990;10:4116–4122. doi: 10.1128/mcb.10.8.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melkonian K A, Ostermeyer A G, Chen J Z, Roth M G, Brown D A. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell D A, Farh L, Marshall T K, Deschenes R J. A polybasic domain allows non-prenylated Ras proteins to function in S. cerevisiae. J Biol Chem. 1994;269:21540–21546. [PubMed] [Google Scholar]
- 37.Morrison D K, Cutler R E. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 38.Niv H, Gutman O, Henis Y I, Kloog Y. Membrane interactions of constitutively active GFP-Ki-Ras4B and their role in signaling. J Biol Chem. 1998;274:1606–1613. doi: 10.1074/jbc.274.3.1606. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto T, Schlegel A, Scherer P E, Lisanti M P. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 40.Otto J C, Kim E, Young S G, Casey P J. Cloning and characterization of a mammalian prenyl protein specific protease. J Biol Chem. 1999;274:8379–8382. doi: 10.1074/jbc.274.13.8379. [DOI] [PubMed] [Google Scholar]
- 41.Parton R G, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol. 1997;136:137–154. doi: 10.1083/jcb.136.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presley J, Cole N, Schroer T, Hirschberg K, Zaal K, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 43.Rabouille C, Hui N, Hunte F, Kieckbusch R, Berger E, Warren G, Nilsson T. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J Cell Sci. 1995;108:1617–1627. doi: 10.1242/jcs.108.4.1617. [DOI] [PubMed] [Google Scholar]
- 44.Reiss Y, Goldstein J L, Seabra M C, Sasey P J, Brown M S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990;62:81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Lane A, Yan J, McPherson R, Hancock J F. Activity of plasma membrane recruited Raf-1 is regulated by Ras via the Raf zinc finger. J Biol Chem. 1997;272:20139–20145. doi: 10.1074/jbc.272.32.20139. [DOI] [PubMed] [Google Scholar]
- 46.Roy S, Luetterforst R, Harding A, Apolloni A, Etheridge M, Stang E, Rolls B, Hancock J F, Parton R G. Dominant-negative caveolin inhibits H-ras function by disrupting cholesterol-rich plasma membrane domains. Nat Cell Biol. 1999;1:98–105. doi: 10.1038/10067. [DOI] [PubMed] [Google Scholar]
- 47.Silvius J R, l'Heureux F. Fluorimetric evaluation of the affinities of isoprenylated peptides for lipid bilayers. Biochemistry. 1994;33:3014–3022. doi: 10.1021/bi00176a034. [DOI] [PubMed] [Google Scholar]
- 48.Simons K, Ikonen E. Functional rafts in membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 49.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Activation of Raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 50.Thissen J, Gross J, Subramanian K, Meyer T, Casey P. Prenylation dependent association of Ki-Ras with microtubules. J Biol Chem. 1997;272:30367–30370. doi: 10.1074/jbc.272.48.30362. [DOI] [PubMed] [Google Scholar]
- 51.Thissen J A, Casey P J. Microsomal membranes contain a high affinity binding site for prenylated peptides. J Biol Chem. 1993;268:13780–13783. [PubMed] [Google Scholar]
- 52.Tognon C E, Kirk H E, Passmore L A, Whitehead I P, Der C J, Kay R J. Regulation of RasGRP via a phorbol ester-responsive C1 domain. Mol Cell Biol. 1998;18:6995–7008. doi: 10.1128/mcb.18.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traverse S, Cohen P, Paterson H, Marshall C, Rapp U, Grand R J. Specific association of activated MAP kinase kinase kinase (Raf) with the plasma membranes of ras-transformed retinal cells. Oncogene. 1993;8:3175–3181. [PubMed] [Google Scholar]
- 54.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton R G. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ullrich O, Stenmark H, Alexandrov K, Huber L A, Kaibuchi K, Asasaki T, Takai Y, Zerial M. Rab GDI as a general regulator for the membrane association of Rab proteins. J Biol Chem. 1993;268:18143–18150. [PubMed] [Google Scholar]
- 56.Umanoff H, Edelmann W, Pellicer A, Kucherlapati R. The murine N-ras gene is not essential for growth and development. Proc Natl Acad Sci USA. 1995;92:1709–1713. doi: 10.1073/pnas.92.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Urano T, Emkey R, Feig L A. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- 58.Willumsen B M, Christensen A, Hubbert N L, Papageorge A G, Lowy D R. The p21ras C-terminus is required for transformation and membrane association. Nature. 1984;310:583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- 59.Yan J, Roy S, Apolloni A, Lane A, Hancock J F. Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J Biol Chem. 1998;273:24052–24056. doi: 10.1074/jbc.273.37.24052. [DOI] [PubMed] [Google Scholar]