Abstract
This review of reviews aimed to evaluate the reporting quality of published systematic reviews and meta-analyses in the field of sports physical therapy using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This review of reviews included a literature search; in total, 2047 studies published between January 2015 and December 2020 in the top three journals related to sports physical therapy were screened. Among the 125 identified articles, 47 studies on sports physical therapy were included in the analysis (2 systematic reviews and 45 meta-analyses). There were several problems areas, including a lack of reporting for key components of the structured summary (10/47, 21.3%), protocol and registration (18/47, 38.3%), risk of bias in individual studies (28/47, 59.6%), risk of bias across studies (24/47, 51.1%), effect size and variance calculations (5/47, 10.6%), additional analyses (25/47, 53.2%), and funding (10/47, 21.3%). The quality of the reporting of systematic reviews and meta-analyses of studies on sports physical therapy was low to moderate. For better evidence-based practice in sports physical therapy, both authors and readers should examine assumptions in more detail, and report valid and adequate results. The PRISMA guideline should be used more extensively to improve reporting practices in sports physical therapy.
Keywords: assessment, meta-analysis, systematic review, physical therapy, review of reviews
1. Introduction
Evidence-based practice (EBP) is the best decision-making method for patients [1]. The term “evidence-based medicine” was first introduced by Gordon Guyatt at McMaster University, in Canada in 1992 [2]. The Center of Evidence-Based Physiotherapy (CEBP), currently headquartered at the University of Sydney, Australia, was established in 1999 [3]. Clinicians should have the knowledge and skills to write and understand published systematic reviews and meta-analyses that will help better decision-making for patients [4]. Primary studies have several limitations in terms of clinical decision-making, e.g., a limited sample, mixed results, and inconsistent analytical methods and reporting [5]. Some scientific journals even tend to selectively publish thesis manuscripts with statistically significant results [6]. This may induce biases, such as result reporting bias, which may affect the validity of the results [4,7]. SRs and MAs overcome the limitations of primary studies [7].
Systematic reviews (SRs) and meta-analyses (Mas) are some of the best sources of evidence and scientific research, including clinical expertise and patient-reported outcomes [6]. The process of SR and MA includes problem formulation, literature search, data coding, and data analysis and reporting, which minimize the bias and increase transparency and reproducibility [8]. An SR is a literature review that collects, comprehensively analyzes, and evaluates all studies related to a research topic [9]. Therefore, if several clinical research papers show the same results through studies on the effect of the same treatment rather than a single clinical study, it provides a more accurate basis for the treatment effect than a single individual clinical study paper [10].
MA refers to a statistical method that synthesizes and analyzes quantitative research results extracted from multiple studies to produce summarized and organized empirical knowledge on a topic [7]. MA is a valid method for finding evidence so that clinicians and researchers can have a rationale for solving health-related problems [11]. Additionally, MA distinguishes the intervention effects of previous studies by characteristics and objectively compares them to develop intervention programs that can be successfully used in various practical fields [12].
SRs and MAs comprise the highest level in the evidence pyramid of medicine [13]. Systematic reviews (SRs) and meta-analyses (MAs) provide the best basis for use in decision-making regarding the application of medical service interventions [6,14]. Clinicians should have the knowledge and skills to write and understand published SRs and MAs, as this will aid in making better decisions with their patients [5].
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines detail what should be reported in MAs and SRs analyses [15]. However, the reporting quality of MAs and SRs in numerous clinical areas is low and very problematic [16,17,18]. Only one 2012 paper was found to analyze the reporting of SRs related to physical therapy, and it was also of very low quality [19].
Clinical intervention should be guided by scientific evidence, which should be obtained by valid and transparent processes and methods. Bias in the scientific process can lead to inappropriate clinical practices. Therefore, MAs and SRs should be conducted by referring to the PRISMA guidelines [20]. Quality assessment studies on MAs and RSs in other fields have been published. The purpose of this study is to indicate problems in research reporting methods and thus produce a more valid MA in sports physical therapy [20,21].
Interpreting the results of SRs and MAs should be performed carefully because the results may suffer from reporting study weakness [22]. There has been no quality reporting about SRs and MAs in the field of sports physical therapy research. Therefore, the purpose of this study was to evaluate the reporting quality of SRs and MAs published in the top three sports physical therapy journals [15]. This article summarized the major study results, the SRs and MAs method, and major findings and evaluated whether the reviewed articles aligned with the PRISMA guidelines’ 27 items.
2. Materials and Methods
2.1. Eligibility and Exclusion Criteria
2.1.1. Eligibility Criteria
The top three journals in the field of sports physical therapy were selected based on the Journal Citation Reports impact factor (IF) index. The Journal of Orthopaedic & Sports Physical Therapy, Journal of Athletic Training, and Physical Therapy in Sports ranked first, second, and third in this specific category of journals with IFs of 3.839, 2.478, and 1.926, respectively. The IF index measures the average number of citations received in a particular year by papers published in the journal during the preceding years of 2019 and 2020 [23]. The article selection criteria were as follows: (1) peer-reviewed articles published between January 2015 and December 2020, and (2) a statistical MA or systematic literature review of an intervention program.
2.1.2. Exclusion Criteria
As this study was not meant for clinical effect analysis, only treatment effect SRs from the three top journals were targeted. Narrative reviews, diagnostic test SRs, primary studies, qualitative review articles, authors’ opinions, letters, and abstract presentations were excluded.
2.2. Information Sources and Search Strategy
2.2.1. Electronic Search
We searched the Journal of Orthopaedic & Sports Physical Therapy (https://www.jospt.org, accessed on 31 April 2021), Journal of Athletic Training (https://natajournals.org/loi/attr, accessed on 31 April 2021), and Physical Therapy in Sports (https://www.journals.elsevier.com/physical-therapy-in-sport, accessed on 31 April 2021). The search strategy utilized a combination of medical subject heading (MeSH) terms and free text words, including “meta-analysis” (MeSH), “meta-analysis” (text word), “review” (MeSH), and “systematic review” (text words).
2.2.2. Manual Search
The included studies returned by the search, and previously published SRs and MAs related to the topic, were screened to identify any additional studies which could fit the criteria.
2.3. Study Selection
Publication details of all studies identified in the literature search were exported to EndNote (Endnote X9.3.3, Clarivate Analytics, Philadelphia, PA, USA). Once all records were imported, duplicates were removed. After that, titles and abstracts were independently screened for eligibility by the two authors using the specified inclusion and exclusion criteria. Full texts of the remaining articles were then sourced and independently evaluated for inclusion. Any disagreement was resolved through discussion.
2.4. Data Collection Process and Data Item
The PRISMA statement includes a checklist of 27 items [21], and the PRISMA checklist was used by the raters in the analyses of the eligible SRs [21]. For each checklist item, it was established that a rating of “yes” would only be assigned if the PRISMA statement recommendations were fully complied with. If the rater considered the information regarding any item to be incomplete, missing, or doubtful, that item was rated as “no.” The quality evaluation criteria of this paper were identified as follows: “low quality,” “moderate quality,” and “high quality” when there was <50%, <75%, and >75% agreement with the PRISMA checklist items, respectively [19,22,24,25,26].
The characteristics of the participants and interventions in the 47 selected articles were identified and coded per the research evaluation framework (Supplementary Materials Table S1) [19]. The articles were listed and outlined according to the coding table (Appendix A).
The two authors also independently extracted the following data from each included article into predesigned coding sheets: (1) study identification: the first author’s name, location of the corresponding author(s), year of publication, and journal name; (2) number and design of the studies in the MA/SR; (3) population (participants); (4) interventions; (5) comparison between interventions; and (6) outcome measures. Discrepancies were resolved through discussion.
2.5. Reliability of the Evaluators
Two researchers independently checked each checklist item in a coding sheet and resolved discrepancies by consensus via weekly Zoom meetings. If the disagreement was not resolved, the researchers planned to consult a third coder to make the final decision. However, there were no disagreements between the coders in any of the items. Some items were not fulfilled according to the PRISMA checklist. The two researchers also evaluated additional reporting and statistical issues based on the PRISMA 2020 statement [27].
2.6. Planned Methods of Analysis
2.6.1. Reporting of Epidemiological and Descriptive Characteristics
The epidemiological and descriptive characteristics of the included MAs and SRs were assessed according to the journal type, corresponding author’s location, number and design of included studies, population/patients/defects, type of intervention, comparison between interventions, outcome (Appendix A).
2.6.2. Statistical Analysis
Each PRISMA checklist item was presented as either the ratio or percentage of how many of the 47 PRISMA articles were properly followed (Appendix B).
2.7. Ethical Statement
This study was approved by the institutional review board of Nambu University (ethical code: 1041478-2017-HR-016; approval date: 8 November 2017) and was conducted in accordance with the ethical standards of the Declaration of Helsinki.
3. Results
3.1. Study Selection
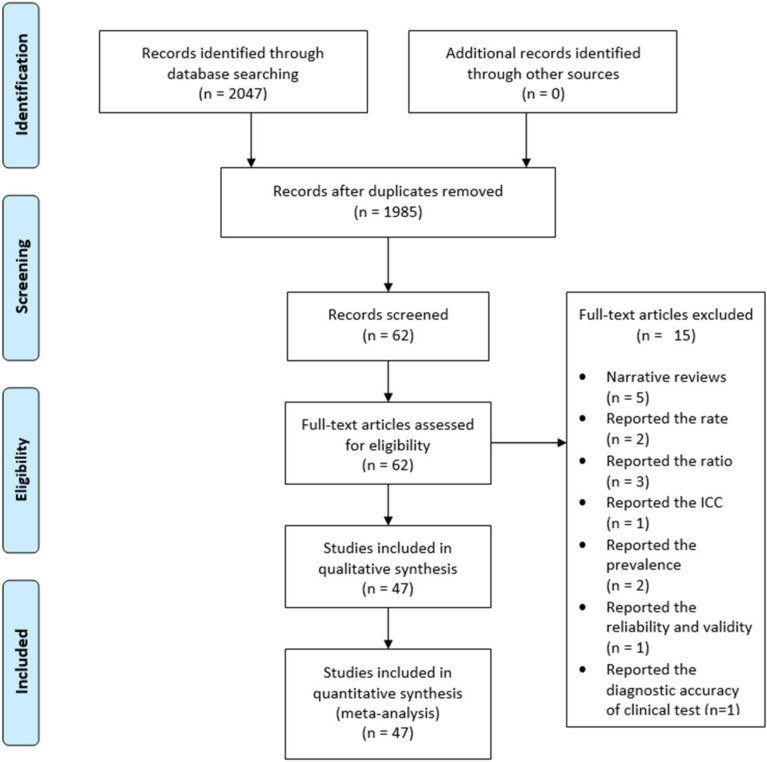
A total of 2047 studies published by the Journal of Orthopaedic & Sports Physical Therapy, Journal of Athletic Training, and Physical Therapy in Sports between January 2015 and December 2020 were considered. Among these, 47 articles listed as a systematic literature review or MA in the article category were initially selected. We excluded five narrative review articles, two articles that reported a rate, three articles that reported a ratio, two articles that reported the prevalence, one article that reported intraclass correlation coefficients, one article that reported reliability and validity, and one article that presented the diagnostic accuracy of a clinical test. Consequently, 47 articles were included in the final analysis (Appendix C) (Figure 1).
Figure 1.
Flow chart describing the selection of the 47 articles after multiple phases of the screening process. ICC, intra-class correlation.
3.2. Study Characteristics
3.2.1. Epidemiological and Descriptive Characteristics
MAs and SRs published in the Journal of Athletic Training, Journal of Orthopaedic & Sports Physical Therapy, or Physical Therapy in Sports are shown in Appendix A. The types of interventions varied across the wide-ranging sports physical therapy field. The reporting guidelines used for the SR process also varied: 32 (68.1%) articles used the PRISMA guidelines [15]. However, 15 (31.9%) articles did not describe the reporting guidelines used. Regarding funding sources, 10 studies received private and/or public support (21.3%), and 37 studies received no funding.
3.2.2. General Characteristics of the Included Studies
The main characteristics of all the included studies are described in Appendix A. The reporting quality of key components of the MAs and SRs, as evaluated based on the PRISMA guidelines, is shown in Appendix B. Two papers performed an SR, and the remaining 45 conducted a MA. Furthermore, 27 (57.4%) studies included an RCT. Forty-two (89.4%) studies used outcomes that were continuous variables, such as the mean change difference.
3.3. Synthesis of the Results
The evaluation results are described below in the following order: title, abstract, introduction, methods, results, and discussion (Appendix B).
Reporting of the General Components of the SR Process (27 Items)
To enhance the validity and impact of SRs, all authors and editors must apply established reporting standards. Thirty-two articles mentioned the application of a guideline and 15 articles did not. Thirty-two studies adhered to the PRISMA guidelines and two studies applied the 2015 PRISMA-P (Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols) guidelines [28,29]. One of the studies [28] mentioned the Measurement Tool to Assess Meta-analysis of Observational Studies in Epidemiology (MOOSE) [30].
Regarding item 2 (structured summary), we checked whether the abstract specifically presented the analytical model and effect size. Powden et al. specified that a fixed or randomized model was used [31]. Furthermore, studies that only reported the odds ratio (OR), relative risk (95% confidence interval [CI]), and effect size (Cohen’s d, 95% CI) in the data synthesis methods, and studies that did not present specific results (numbers) in the abstract, were considered to not have a structured summary (item 2). The PRISMA summary guidelines are a better source for this item published in 2013 [20].
In primary studies, researchers tend to report statistically significant results and emphasize a positive and large effect [32]. SRs and MAs have similar tendencies and, thus, require protocol registration to prevent selective reporting. Protocol registration is one of the methods that increase the validity of a MA [9]. Therefore, the protocols of SRs and MAs should be registered in PROSPERO (an international database of prospectively registered SRs in health and social care by the University of York, which is accessible to the public and researchers) [33], as for primary studies. However, no studies reported that the protocol was registered in PROSPERO or a local research foundation (item 5). For protocol and registration (item 5), none of the studies reported a selection/reporting bias.
The registration rate in the medical field is also very low, at 21% [34]. Protocol registration should be emphasized because selective reporting can be evaluated by comparing the protocol against the full paper [34,35]. The National Institute for Health Research (NIHR) also supports protocol registration [36].
Twenty-eight (59.6%) studies did not report the methods used to assess the risk of bias of studies (item 12). Furthermore, 23 (48.9%) studies did not report the methods used to assess the risk of bias across studies (e.g., publication bias) (item 15), and 28 (59.6%) studies did not describe the methods of additional analyses (item 16). Furthermore, more than half of the reviews did not report the “risk of bias within studies” (data presented on the risk of bias of each study and, if available, any outcome level assessment). This finding was also consistent with previous studies [22]. Reporting bias is crucial for assessing effect size because only advantageous results may be reported [37].
In the results section, 19 (40.4%) studies did not present data on the risk of bias for each study and any outcome level assessment, if available (item 19), and 23 (48.9%) did not report the risk of bias assessment across studies (item 22). Heterogeneity means the degree of differences in the results of each single research finding [38]. Through MAs, scholars calculate the heterogeneity index to understand the primary factors that impact individual studies’ effect sizes [39]. For heterogeneity tests, Q (34/47) and I2 inconsistency (38/47) statistics were used. Reporting on the effect size, most studies (42/47) did not provide the effect size formula, and no study reported the effect size variance formula in their methods section [40].
In this review, very few studies reported independent assumptions, missing data, or outliers, which should be addressed. Independent assumptions comprise two issues: first, whether the same sample was used twice or not, and second, how more than one effect size was treated in calculating an effect size for MA [40].
The results of additional analyses were mentioned in only 25 (53.2%) studies (e.g., sensitivity or subgroup analyses and meta-regression analysis) (item 23). Forty-two articles did not describe a sensitivity analysis. The sensitivity analysis, used to test the reliability of the cumulative effect across included studies, revealed the effect sizes [41].
Interestingly, one study [42] evaluated the reviewed articles using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group criteria after they completed their MA [43,44]. GRADE was used to investigate the overall quality of evidence for each outcome [45]. The MA evaluated the study’s design, risk of bias and publication bias, consistency, the complexity of interventions, and roughness [46]. The Cochrane Handbook for Systematic Reviews of Interventions also supports the evaluation process by researchers who completed the reviews [14]. The GRADE criteria include five quality evaluations: (a) risk of bias, (b) inconsistency, (c) indirectness, (d) imprecision, and (e) publication bias [47].
In the discussion section, comments about the study limitations, outcome level, and review levels, such as the risk of bias and incomplete retrieval of identified research and funding, were not provided in 37 (78.7%) studies (item 27). Many studies did not report a funding source. Research results can fundamentally differ according to funding sources. Funding bias refers to when a study’s outcome is more likely to support the interests of the organization funding the study [48]. For example, studies regarding omega-3 supplementation for the prevention of cardiovascular disease, or the relationship between cell phone use and the risk of brain tumors, showed contrasting results depending on the funding source [49,50]. Most research indicates that sucralose is safe—except for research sponsored by competitors [51].
4. Discussion
This study was benchmarked against previous studies, which were mainly published in top journals according to the IF criteria related to the reporting quality assessment [52,53,54,55]. The trends of the last 5 years were assessed because the PRISMA-Diagnostic Test Accuracy, PRISMA-Rapid Reviews, PRISMA-Scoping Reviews, and PRISMA-Network MA guidelines were released in 2015. Thus, the current year was when systematic literature review and MA reporting standards began to be subdivided [56]. This review evaluated 47 MAs and SRs reported in the Journal of Orthopaedic & Sports Physical Therapy, Journal of Athletic Training, and Physical Therapy in Sports related to sports physical therapy, using the PRISMA guidelines.
The study results were very similar compared to analyses of reporting of systematic reviews in physical therapy [19]. Analysis of reporting of systematic reviews in studies of reporting standards using the PRISMA statement have reported information regarding the risk of bias, protocol and registration, additional analysis, and funding was insufficient in quality-evaluated studies in the field of nursing [24], acupuncture [25], and diagnostic testing [26]. In the field of sports physical therapy, protocol registrations (38.3%), risk of bias across studies (51.1%), additional analysis (40.4%), and funding (21.3%) were the most problematic PRISMA items. Although the quality of SR and MA reporting in sports physical therapy was medium-to-low, similar to that in other clinical fields, key reporting components of the SR process were missing in most of the MAs and SRs. The critical appraisal of such studies must improve these reporting issues, which pertain to general MAs (27 items). For better EBP in sports physical therapy, authors and readers should examine assumptions in more detail, and report valid and adequate results. The PRISMA guidelines should be used more extensively to improve reporting practices in physical therapy.
Protocol registration is increasingly recommended in clinical trials [57] and SRs [34], but this study showed a low protocol enrollment of 38.3% (item 5). In a previous survey, only about one-fifth of SRs in physical therapy were registered, indicating that the enrollment rate was low [58]. According to the Cochrane Handbook for systematic literature review of interventions, the prospective registration of the protocol reduces the author’s bias by publicly documenting a priori planned methodology [34,36]. Importantly, the registered SRs showed significantly higher methodological quality compared to the unregistered SRs. The protocol provides transparency and clarifies the hypothesis, methodology, and analysis of the SRs and MAs undertaken.
Quality assessment of meta-analysis for randomized controlled trials (RCT) was performed using the Risk of Bias tool developed by the Cochrane group [59]. The NRS tools for Newcastle and Ottawa Scale (NOS) [60], and Risk of Bias Assessment tool for Non-randomized Studies (ROBANS) [61]. However, there may be some confusion in the concepts of the reporting standards for individual studies (CONSORT statement) [62]. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [63], and quality assessment tools for meta-analysis (ROB, NOBANS) [64].
One study reported the STROBE statement as a reporting standard for individual studies, as opposed to a quality assessment tool for meta-analysis [65]. The STROBE statement is a checklist of items that should be included in reports of cohort studies. An explanation and elaboration article discussed each checklist item and provided the methodological background and published examples of transparent reporting [63]. The reporting criterion for meta-analysis of observational studies should be evaluated as MOOSE [30].
For summary measures, the type of effect size measure used in the manuscript must be described. The OR and standardized mean difference (SMD) as summary measures were indicated in the studies (item 13). The effect size is a key concept in a meta-analysis, and an essential component of quantitative research reporting and hypothesis testing [40]. In many studies, a corrected SMD, i.e., Hedges’ g with 95% CI was computed in consideration of the small sample size, and the inverse of the variance was used as the weight for each effect size [66]. As shown herein, studies need to classify the effect size computation, variance computation, and equations, and present them in an easily comprehensible manner for readers. However, only 5 of 47 studies described the effect size computation [29,65,67,68,69] (Supplementary Materials Table S1). One benefit of evaluating the effect size is that it quantifies the difference between groups in the observed data [70]. Moreover, the effect size is presented as a standard deviation; thus, it can be compared between studies and utilized in MAs, as well [71]. Researchers in the field of clinical medicine should also recognize the benefits of the effect size and use it widely in medical research.
Subgroup analyses further decrease the number of studies and thus weaken the power of the analysis, necessitating a careful interpretation of the data. Additionally, only 1 of the 23 studies performed a multivariate analysis [72].
The quality evaluation level of the 47 studies that were investigated revealed low to moderate and very low levels in statistical issues, such as mentioning the effect size formula and variance in the methods section. MAs use a summary measure with a statistically known variance [73], and the effect size variance formula is related to the distributional assumption such as the normality and homogeneity assumption for hypothesis testing. The results of effect size computation (like the SMD dealing with continuous variables, correlations, and odds ratios with dichotomous variables) should be provided for each individual MA study as major characteristics of the included studies [27,40].
There were some flaws in the reviewed articles, and some suggestions were proposed for more valid MA results. Applying appropriate MA assessment tools for physical therapy research is required to increase the reviewed articles’ validity. The synthesis of research studies in physical therapy research includes various study designs such as RCTs, observational studies, and scholars must consider the appropriate method of exploring validated reviews in physical therapy research.
As of 2021, version 6.0 of the Cochrane Handbook for Systematic Reviews of Interventions has been released [14]. The ROB 2 is the gold standard to evaluate the quality of RCT biases [39] and is a reorganized form of the Cochrane ROB tool by the same team [74]. The key features are that researchers can simply decide if a bias exists in the reviewed research and can evaluate bias for particular result findings within an RCT design and beyond the RCT design [75]. The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) was also developed to evaluate intervention studies with nonrandomization in the Cochrane handbook (version 6.0). The main differences are that reviewers can easily critique the bias (using “low,” “moderate,” “serious,” and “critical”), focusing on the after-intervention effects [76].
Researchers should use appropriate, updated quality assessment tools to reflect a study’s research design and objectives and consider the complexity of interventions, proper groupings, and scientific effect size calculations. In addition, the present study proposed measures to improve the quality of MAs and ultimately aimed to examine the current situation, contributing to the enhancement of the EBP of sports physical therapy.
4.1. Limitations
To our knowledge, no study has investigated the reporting quality of SRs and MAs in the field of sports physical therapy. However, the present study has some limitations.
The database searches were only conducted in three specific journals based on their IF in the field of sports physical therapy. We need to comprehensively search the relevant databases related to sports physical therapy to conduct future SRs. For example, other journals from the field of sports and exercise medicine (e.g., British Journal of Sports Medicine) are important and should be included in the next study. We will extend the more studies to for 10 years in the next study.
The authors of the reviewed articles may have used the appropriate method but omitted important details from the report or removed key information during the publication process. We were in the position of the reader and could evaluate what was reported in the articles only. Additionally, there may be limitations in that the scope of the research may be different from the part where the actual research was conducted because the researcher reported based on the writing of a paper.
Quality thresholds were based on previous studies and were not agreed to be absolutely not interpreted. In addition, we determined that the quality of the performance was appropriate only if the reporting was adequate in terms of each checklist. If a comprehensive quality evaluation of the reporting criteria is conducted by including studies other than those in the top journals, the issue of low quality could be even more serious.
Because of research ethics, sports physical therapy studies need to perform general physical therapy in addition to the major intervention; thus, the lack of studies that only examined the physical therapy intervention poses a considerable limitation. In the future, when conducting systematic literature reviews and MAs, it will be helpful to improve the quality of clinical studies only when non-random studies, as well as RCT studies, are included due to the nature of clinical studies in physical therapy.
4.2. Clinical Implications
The findings of our study suggest that physical therapy studies need to be designed appropriately as per the purpose of the study and that physical therapy programs for patients should be structured more systematically. Considering the lack of previous studies that qualitatively evaluate and emphasize clinical judgment, future studies need to discuss complex interventions and network MAs as recent research trends.
5. Conclusions
This critical assessment demonstrated that the current quality of reporting and conducting of MAs and SRs is low to moderate, as it is in other medical disciplines. Problem areas of current meta-analyses and SRs include the exploration of the risk of bias across studies, protocol registrations, and additional analyses. Performing a meta-analysis with inadequate reporting increases the risk of invalid results in a meta-analysis.
Therefore, a reporting guideline, such as the PRISMA statement, is helpful for authors when writing meta-analyses and SR.s Clinicians need to have a thorough knowledge of research methodology, to render the interpretation of sophisticated statistical analyses easier. In addition, the present study proposed measures to improve the quality of meta-analyses, and ultimately aimed to examine the current situation, contributing to the enhancement of the practice of sports physical therapy.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/healthcare9101368/s1, Table S1: Analysis of articles according to the PRISMA recommendations.
Appendix A
Table A1.
Study characteristics of included systematic review and meta-analysis in reporting quality assessment.
| # | Author (Country*) | Year | Journal | Type | The Number and Design of Included Studies | Population/Patients/ Defects |
Intervention | Comparison between Interventions | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Games et al. (USA) | 2015 | J. Athl. Train | MA | 10 studies (10 clinical trials) |
Participants with healthy adults: (1) Use of a commercially available WBV device (2) a human research model (3) a pre-WBV condition and at least 1 WBV experimental condition |
Therapeutic whole-body vibration (WBV) | Vibration type and frequency | • Muscle-oxygenation levels • Peripheral blood flow |
| 2 | Sciascia et al. (USA) |
2015 | J. Athl. Train | SR | 11 studies (11 Case series reports) |
(1) Surgical repair of an isolated superior labral injury of a superior labral injury with soft tissue debridement (2) Overhead athletes equal to or less than 40 years of age |
Return to preinjury levels of participation |
NA | • Return-to-participation odds and interpretations for athletes with isolated superior labral repair • Return-to-participation odds and interpretations for athletes with concurrent superior labral repair and soft tissue |
| 3 | Knapik & Steelman (USA) | 2016 | J. Athl. Train | MA | 15 studies (15 Retrospective studies) | Injuries during military static-line airborne operations |
Risk factors for injury | One group | • Risk ratio • Odds ratio • Summary risk ratio (Summary 95% confidence interval) |
| 4 | Powden et al. (USA) |
2017 | J. Athl. Train | MA | 15 studies (10 RCTs and 5 non-RCTs design) |
Individuals with chronic ankle instability | Balance Training, Manual Therapy, Combined Interventions |
One group or control group | Improving health-related quality of life (HRQL) |
| 5 | Alsalaheen et al. (USA) | 2017 | J. Athl. Train | MA | 17 studies (17 observational studies) | (1) A total of 1777 patients (1250 males, 527 females) with concussion (2) Participants from 13 to 33 years old representing clinical management of concussion (3) Participants in the reviewed studies included middle and high school-aged children, college-aged adults, and professional athletes |
Computerized neurocognitive test and self-reported symptoms |
Participants with self-reported symptoms before 1 week and between 1 and 3 weeks postconcussion. |
• Verbal memory • Visual memory • Processing speed • Reaction time • Postconcussion symptom scale with 1 week postconcussion |
| 6 | Slater et al. (USA) | 2017 | J. Athl. Train | MA | 27 studies (27 clinical trials) | Anterior cruciate ligament reconstruction (ACLR), anterior cruciate ligament deficient (ACLD) |
3-dimensional (3D) lower extremity kinematics and kinetics of walking among individuals | Healthy control participants | • Peak external knee-flexion moment, knee-extension moment • Peak knee-flexion angle • Peak hip-flexion angle • Peak knee-adduction angle • Peak external knee-adduction moment |
| 7 | Armitano et al. (USA) | 2018 | J. Athl. Train | SR | 18 studies (18 clinical trials) |
Healthy adults (age > 18 years) | The use of augmented information for reducing anterior cruciate ligament injury risk during jump landings | Control group or no control group | Kinematic and kinetic risk factors associated with anterior cruciate ligament injury due to jump landing technique |
| 8 | Bullock et al. (USA) | 2018 | J. Athl. Train | MA | 6 studies (6 prospective studies) |
Baseball players aged 13 years or older | The relationship between shoulder ROM and the risk of arm injuries |
Uninjured participant or previously determined injured cut points | Pooled proportion for absolute shoulder range of motion. • Internal rotation • External rotation • Total range of motion • Horizontal adduction |
| 9 | Takeno et al. (USA) | 2019 | J. Athl. Train | MA | 7 studies (4 RCTs and 2 Quasi-experimental studies and Case-control studies) |
Patients with Subacromial Impingement | The short- and long-term therapeutic interventions for SIS |
Control group or No control group | • Scapular upward rotation • Scapular posterior tilt • Scapular internal rotation • Disability of the arm, shoulder and hand score |
| 10 | Vallandingham et al. (USA) |
2019 | J. Athl. Train | MA | 10 studies (10 clinical trials) |
Individuals with chronic ankle instability | Joint mobilizations | Control group or sham group | • Dorsiflexion range of motion • Dynamic postural control |
| 11 | Jeong et al. (Republic of Korea) |
2019 | J. Athl. Train | MA | 7 studies (7 RCTs) |
Patients with Knee Osteoarthritis (age > 50 years) | Proprioceptive training | Control group | • Pain • Stiffness • Physical function questionnaire outcome • Physical function test |
| 12 | Montalvo et al. (USA) |
2019 | J. Athl. Train | MA | 36 studies (Observational Cohort and Cross-Sectional Studies) |
Studies were included if they provided the number of ACL injuries and the number of athlete-exposures (AEs) by sex or enough information to allow the number of ACL injuries by sex to be calculated. |
Injury incidence by sex and sport classification | NA | Sex differences in incidence rates (IRs) of anterior cruciate ligament (ACL) injury by sport type (collision, contact, limited contact, and noncontact) |
| 13 | Seffrin et al. (USA) |
2019 | J. Athl. Train | MA | 13 studies (13 RCTs) |
Participants in these studies varied in age (high school to middle age) and activity level (sedentary lifestyle to competitive athletics) | Instrument-assisted soft tissue mobilization (IASTM) | At least 1 other group not receiving IASTM |
Range of motion (ROM), pain, strength, and patient-reported function |
| 14 | McAuliffe et al. (United Kingdom) |
2019 | J. Athl. Train | MA | 19 studies (cross-sectional or baseline data from prospective or intervention studies) |
Individuals with Achilles tendinopathy (AT) | • Maximal-strength profile • Explosive-strength Profile • Reactive-strength profile (Hopping) |
Healthy control participants | Plantar flexion (PF) strength |
| 15 | Kang et al. (Taiwan) |
2019 | J. Athl. Train | MA | 18 studies (7 systematic reviews and 11 meta-analyses) |
Healthy adults | Push-up plus exercise | Different hand positions (the distance between the hands, shoulder-flexion angle, and elbow-flexion angle) and different lower extremity positions variably |
Serratus anterior and upper trapezius electromyographic analysis |
| 16 | Desjardins-Charbonneau et al. (Canada) |
2015 | J. Orthop. Sports Phys. Ther. | MA | 21 studies (21 RCTs) |
Patients with rotator cuff (RC) tendinopathy | Manual therapy (MT) | Placebo or in addition to another intervention or a multimodal intervention | • Pain • Shoulder range of motion |
| 17 | Almeida et al. (Brazil) |
2015 | J. Orthop. Sports Phys. Ther. | MA | 16 Studies (cross-sectional, case-control, prospective, and retrospective study) |
Runners at least 18 years of age | Biomechanical characteristics of foot-strike patterns during running |
Rearfoot strike and forefoot strike or midfoot in shod conditions | • Vertical ground reaction force: Second peak • Vertical loading rate • Ankle plantar flexion moment |
| 18 | Deasy et al. (Australia) |
2016 | J. Orthop. Sports Phys. Ther. | MA | 5 studies (4 Case-control studies and 1 two-group pre-post design |
People with symptomatic knee osteoarthritis | A modified grading of recommendations assessment, development and evaluation approach |
Healthy control participants | • Isometric hip muscle strength • Isokinetic hip muscle strength |
| 19 | Gattie et al. (USA) |
2017 | J. Orthop. Sports Phys. Ther. | MA | 13 studies (13 RCTs) |
Human subjects who had musculoskeletal conditions | Dry needling dry needling performed by a physical therapist |
Control or other intervention | • Pain • Pressure pain threshold(PPT) • Functional outcome |
| 20 | Zhao et al. (China) |
2017 | J. Orthop. Sports Phys. Ther. | MA | 11 studies (11 RCTs) |
Postmenopausal women | Combined exercise interventions | Control group (nonexercise group) |
Lumbar spine, femoral neck, total hip, and total body BMD (bone mineral density) |
| 21 | Basson et al. (South Africa) |
2017 | J. Orthop. Sports Phys. Ther. | MA | 19 studies (19 RCTs) |
Participants over the age of 18 years with neuromusculoskeletal conditions indicative of neural tissue dysfunction | Neural Mobilization for Neuromusculoskeletal Conditions |
Control group |
• Pain and disability in N-LBP (nerve-related low back pain) and • Pain in N-NAP (nerve-related neck and arm pain) • Pain and disability in CTS(carpal tunnel syndrome) |
| 22 | Nascimento et al. (Brazil) |
2018 | J. Orthop. Sports Phys. Ther. | MA | 14 studies (Randomized and/or controlled trials) |
Individuals with patellofemoral pain | Experimental intervention is strengthening, in order to increase strength of the posterolateral hip muscles |
Nothing/placebo or knee strengthening alone |
Measures of strength, pain intensity, or activity |
| 23 | Eckenrode et al. (USA) |
2018 | J. Orthop. Sports Phys. Ther. | MA | 9 studies (9 RCTs) |
Individuals with patellofemoral pain | Manual therapy or manual therapy plus exercise |
Sham/Control or alternative treatment |
Pain and self-reported function |
| 24 | Al-Mahrouqi et al. (Australia) |
2018 | J. Orthop. Sports Phys. Ther. | MA | 8 studies (8 clinical trials) |
Adults with ankle osteoarthritis | Physical impairments | Healthy controls or the unaffected ankle |
Range of motion, ankle arthrometry, calf cross-sectional area (CSA) and fatty infiltration, joint torque, muscle electromyography (EMG), standing balance, body impairment |
| 25 | Lam et al. (Canada) |
2018 | J. Orthop. Sports Phys. Ther. | MA | 12 studies (12 RCTs) |
Patients with either acute (less than 12 weeks in duration) or chronic (greater than 12 weeks in duration) low back pain (LBP) | McKenzie method of mechanical diagnosis and therapy (MDT) | Other interventions in patients with acute or chronic LBP | Pain and disability |
| 26 | Perriman et al. (Australia) |
2018 | J. Orthop. Sports Phys. Ther. | MA | 10 studies (10 RCTs) |
Participants who had Anterior Cruciate Ligament Reconstruction (ACSR) |
Open- Kinetic-Chain (OKC) quadriceps exercises |
Closed- Kinetic-Chain (CKC) quadriceps exercises |
Anterior tibial laxity, lower-limb strength, function, quality of life, or adverse events in the ACLR population |
| 27 | Mansfield et al. (USA) |
2019 | J. Orthop. Sports Phys. Ther. | MA | 21 studies (21 RCTs) |
Individuals who received any form of needling therapy to their muscle(s), including healthy/uninjured, injured, nonoperative, and operative. |
Any form of needling therapy provided to a muscle, irrespective of body region. | Any intervention such as therapeutic exercise, modality, or form of placebo needling |
Any formal assessment of muscle force production |
| 28 | den Bandt et al. (Netherlands) |
2019 | J. Orthop. Sports Phys. Ther. | MA | 24 studies (15 Cross-sectional studies, 5 Case-control studies, 1 Clinical trial, 1 Cohort study, 1 Longitudinal treatment study, 1 Observational study) |
People with nonspecific low back pain |
Pain mechanisms in low back pain | Health controls | Mechanical quantitative sensory testing outcomes • Pressure pain thresholds (PPTs) • Temporal summation • Conditioned pain modulation |
| 29 | Desmeules et al. (Canada) | 2015 | Phys. Ther. Sports | MA | 11 studies (11 RCTs) |
Adults suffering from RC tendinopathy |
Therapeutic ultrasound (US) | Placebo or other interventions in adults suffering from RC tendinopathy |
• Pain reduction • Functional improvement |
| 30 | Sales et al. (Brazil) |
2016 | Phys. Ther. Sports | MA | 20 studies (20 RCTs) |
Athletes | Respiratory muscle training (RMT) | non-athletes | Respiratory muscle endurance (RME) |
| 31 | Tsikopoulos et al. (Greece) |
2016 | Phys. Ther. Sports | MA | 5 studies (5 RCTs) |
Adults with tendinopathy | Platelet-rich plasma injections | Placebo or dry needling injections | Pain intensity functional disability |
| 32 | Chou et al. (Taiwan) |
2016 | Phys. Ther. Sports | MA | 9 studies (9 RCTs) |
Patients with lateral epicondylosis | Autologous blood injection in treating lateral epicondylosis |
corticosteroid injection or platelet-rich plasma injection |
Pain related measurement in each selected randomized controlled trial |
| 33 | Takasaki et al. (Japan) |
2016 | Phys. Ther. Sports | MA | 12 studies (9 Quasi-experimental, 2 Randomized experimental 1 Randomized cross-over) |
Patients with shoulder pathologies | Fatiguing task for the shoulder musculature | Glenohumeral movements and scapulothoracic resting alignments |
Active repositioning acuity and scapulothoracic resting alignment |
| 34 | Tsikopoulos et al. (Greece) |
2016 | Phys. Ther. Sports | MA | 9 studies (9 RCTs) |
Patients with epicondylopathy and plantar fasciopathy |
Autologous whole blood | Corticosteroid injections | Assessment of pain relief assessment of composite outcomes |
| 35 | Gomes-Neto et al. (Brazil) |
2017 | Phys. Ther. Sports | MA | 11 studies (11 RCTs) |
Patients with low back pain |
Stabilization exercise | General exercises or manual therapy |
Pain, disability, and function |
| 36 | Ghai et al. (Germany) |
2017 | Phys. Ther. Sports | MA | 50 studies (6 RCTs, 42 CCTs, 2 observational neuroimaging studies) |
Participants affected by ankle instability |
Joint stabilizers | Control group | Proprioception, postural stability, and neurological activity |
| 37 | Nae et al. (Sweden) |
2017 | Phys. Ther. Sports | MA | 28 studies (28 clinical trials) |
In participants with or without lower extremity musculoskeletal disorders | The performance of weight-bearing functional tasks |
2D and 3D kinematics | Measurement properties of visual assessment and rating of Postural Orientation Errors (POEs) |
| 38 | Lima et al. (Brazil) |
2018 | Phys. Ther. Sports | MA | 17 studies (1 Case-control( 16 Cross-sectional) |
Participants presenting with dynamic knee valgus (DKV) |
Association between ankle dorsiflexion (ADF) and dynamic knee valgus (DKV) | Control group | • Ankle dorsiflexion(ADF) • Dynamic knee valgus measurement method |
| 39 | Slimani et al. (Tunisia) |
2018 | Phys. Ther. Sports | MA | 13 studies (8 Prospective, longitudinal cohort, 3 intervention trials, 3 RCTs, 1 Prospective cohort study) |
Injured soccer players aged between 14 and 36 years | Psychological-based prevention interventions | Psychosocial predictor of succer injureies or control group |
Psychosocial risk factors, psychological-based prevention interventions and injury risk in soccer players |
| 40 | Coburn et al. (Australia) |
2018 | Phys. Ther. Sports | MA | 21 studies (4 cross-section studies compared to control, (6 cross-section and validity studies, 4 RCTs, 7 repeated measures studies) | Individuals with patellofemoral pain aged under 50 years |
PFP interventions | Pain-free controls and population norms |
Knee- and health-related Quality of life (QOL) |
| 41 | Karsten et al. (Brazil) |
2019 | Phys. Ther. Sports | MA | 25 studies (25 clinical trials) |
Athletes | Inspiratory muscle training with linear workload devices (IMT-linear) |
Healthy individuals | • Sports performance (work load and exercise time) • Cardiopulmonary function (oxygen uptake, ventilatory threshold, and maximal inspiratory or expiratory pressure) |
| 42 | Cayco et al. (Philippines) |
2019 | Phys. Ther. Sports | MA | 39 studies (39 RCTs) |
Healthy adults |
Hold-relax and contract-relax stretching (HR and CR) |
No intervention and other stretching techniques |
Hamstring flexibility |
| 43 | Bunn et al. (Brazil) |
2019 | Phys. Ther. Sports | MA | 20 studies (20 Observational studies) |
Participants who perform physical exercises, such as athletes of different modalities and military of different specialties | “exposed” the participants who practiced physical activities and whose FMS™ score were evaluated |
High risk and low risk | (I) the injury was associated with athletic participation or military exercises; (II) there was a need for health care; and (III) there was time lost with restricted participa tion for at least 24 h. The included studies should meet at least one of these criteria. |
| 44 | Alzahrani et al. (Australia) |
2019 | Phys. Ther. Sports | MA | 3 studies (3 RCTs) |
People diagnosed with non-specific low back pain (acute, subacute, chronic, recurrent, persistent) aged 18 years or over |
Any intervention oflifestyle physical activity that was provided as the main component of the treatment |
• Non-physical activity interventions • No intervention • A “sham” intervention • A wait list • Advice to “stay active or maintain usual activities” |
• Physical activity related outcomes • Low back pain related outcomes |
| 45 | Dix et al. (Australia) |
2019 | Phys. Ther. Sports | MA | 16 studies (6 Cohort, 5 Cross-sectional laboratory studies, 2 Observational prospective, 2 Exploratory, 1 Correlation study) |
asymptomatic females over 18 years old | Relationship between hip strength and dynamic lower extremity valgus, Relationship between hip strength and dynamic lower extremity valgus for the various tasks |
Validity of comparison between the findings of research that uses different kinematic assessment tasks |
• Strength measure (Hand held dynamometry & isometric testing unless otherwise stated) • Kinematic measure |
| 46 | Neilson et al. (New Zealand) |
2019 | Phys. Ther. Sports | MA | 11 studies (Randomized control trials and Clinically controlled trials) |
• Neurologically and physically healthy • Man and woman • Adolescents and adults aged 12–65 years old |
Augmented Feedback (AF): external or extrinsic feedback given during practice |
RCTs & CCTs: changes in key landing biomechanical parameters in the AF groups compared to control groups without AF |
Numerical kinematic and kinetic parameters reported |
| 47 | López et al. (Spain) |
2019 | Phys. Ther. Sports | MA | 6 studies (1 experimental trial, 2 randomized experimental trials, 1 randomized double-blinded controlled trial, 1 randomized assessor-blind, placebo-controlled trial, 1 three-arm assessor-blinded randomized controlled trial) |
Participants aged over 18 years | Neurodynamic treatment | No treatment, placebo, and with other manual therapy techniques |
Hamstring flexibility |
Country*: means the location of corresponding author. Abbreviations: SR: Systematic reviews, MA: meta-analysis, RCTs: randomized controlled trials, Non-RCTs: non-randomized controlled trials, NNT: numbers-needed-to-treat, RRR: relative risk reduction, NA: not applicable.
Appendix B
Table A2.
Reporting key components of the systematic review process and meta-analyses by PRISMA guideline.
| Section/Topic | # | Checklist Item—Yes if Reported | No (%) of Reports (n = 47) |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 47 (100%) |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 10 (21.3%) |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 47 (100%) |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 47 (100%) |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | 18 (38.3%) |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 46 (97.9%) |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 47 (100%) |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. |
47 (100%) |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). |
47 (100%) |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. |
47 (100%) |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 44 (93.6%) |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was performed at the study or outcome level), and how this information is to be used in any data synthesis. | 28 (59.6%) |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 47 (100%) |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if performed, including measures of consistency (e.g., I2) for each meta-analysis. | 47 (100%) |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 24 (51.1%) |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if performed, indicating which were pre-specified. | 19 (40.4%) |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 46 (97.9%) |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 46 (97.9%) |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 28 (59.6%) |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 47 (100%) |
| Synthesis of results | 21 | Present results of each meta-analysis performed, including confidence intervals and measures of consistency. | 47 (100%) |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 24 (51.1%) |
| Additional analysis | 23 | Give results of additional analyses, if performed (e.g., sensitivity or subgroup analyses, meta-regression (see Item 16). | 25 (53.2%) |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 47 (100%) |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 47 (100%) |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 47 (100%) |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 10 (21.3%) |
Data are number (%) of reports featuring the corresponding item.
Appendix C. A Total of 47 Included Studies in Reporting Quality Assessment
Al-Mahrouqi MM, MacDonald DA, Vicenzino B, Smith MD. Physical impairments in adults with ankle osteoarthritis: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2018;48:449–459. https://doi.org/10.2519/jospt.2018.7569.
Almeida MO, Davis IS, Lopes AD. Biomechanical differences of foot-strike patterns during running: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2015;45:738–755. https://doi.org/10.2519/jospt.2015.6019.
Alsalaheen B, Stockdale K, Pechumer D, Broglio SP, Marchetti GF. A comparative meta-analysis of the effects of concussion on a computerized neurocognitive test and self-reported symptoms. J Athl Train. 2017;52:834–846. https://doi.org/10.4085/1062-6050-52.7.05.
Alzahrani H, Mackey M, Stamatakis E, Pinheiro MB, Wicks M, Shirley D. The effectiveness of incidental physical activity interventions compared to other interventions in the management of people with low back pain: a systematic review and meta-analysis of randomised controlled trials. Phys Ther Sport. 2019;36:34–42. https://doi.org/10.1016/j.ptsp.2018.12.008.
Armitano CN, Haegele JA, Russell DM. The use of augmented information for reducing anterior cruciate ligament injury risk during jump landings: a systematic review. J Athl Train. 2018;53:844–859. https://doi.org/10.4085/1062-6050-320-17.
Basson A, Olivier B, Ellis R, Coppieters M, Stewart A, Mudzi W. The effectiveness of neural mobilization for neuromusculoskeletal conditions: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47:593–615. https://doi.org/10.2519/jospt.2017.7117.
Bullock GS, Faherty MS, Ledbetter L, Thigpen CA, Sell TC. Shoulder range of motion and baseball arm injuries: a systematic review and meta-analysis. J Athl Train. 2018;53:1190–1199. https://doi.org/10.4085/1062-6050-439-17.
Bunn PDS, Rodrigues AI, Bezerra da Silva E. The association between the functional movement screen outcome and the incidence of musculoskeletal injuries: a systematic review with meta-analysis. Phys Ther Sport. 2019;35:146–158. https://doi.org/10.1016/j.ptsp.2018.11.011.
Cayco CS, Labro AV, Gorgon EJR. Hold-relax and contract-relax stretching for hamstrings flexibility: a systematic review with meta-analysis. Phys Ther Sport. 2019 Jan;35:42–55. https://doi.org/10.1016/j.ptsp.2018.11.001.
Chou LC, Liou TH, Kuan YC, Huang YH, Chen HC. Autologous blood injection for treatment of lateral epicondylosis: a meta-analysis of randomized controlled trials. Phys Ther Sport. 2016;18:68–73 https://doi.org/10.1016/j.ptsp.2015.06.002.
Coburn SL, Barton CJ, Filbay SR, Hart HF, Rathleff MS, Crossley KM. Quality of life in individuals with patellofemoral pain: a systematic review including meta-analysis. Phys Ther Sport. 2018;33:96–108. https://doi.org/10.1016/j.ptsp.2018.06.006.
Deasy M, Leahy E, Semciw AI. Hip strength deficits in people with symptomatic knee osteoarthritis: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2016;46:629–639. https://doi.org/10.2519/jospt.2016.6618.
Den Bandt HL, Paulis WD, Beckwée D, Ickmans K, Nijs J, Voogt L. Pain mechanisms in low back pain: a systematic review with meta-analysis of mechanical quantitative sensory testing outcomes in people with nonspecific low back pain. J Orthop Sports Phys Ther. 2019;49:698–715. https://doi.org/10.2519/jospt.2019.8876.
Desjardins-Charbonneau A, Roy JS, Dionne CE, Frémont P, MacDermid JC, Desmeules F. The efficacy of manual therapy for rotator cuff tendinopathy: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2015;45:330–350. https://doi.org/10.2519/jospt.2015.5455.
Desmeules F, Boudreault J, Roy JS, Dionne C, Frémont P, MacDermid JC. The efficacy of therapeutic ultrasound for rotator cuff tendinopathy: a systematic review and meta-analysis. Phys Ther Sport. 2015;16:276–284. https://doi.org/10.1016/j.ptsp.2014.09.004.
Dix J, Marsh S, Dingenen B, Malliaras P. The relationship between hip muscle strength and dynamic knee valgus in asymptomatic females: a systematic review. Phys Ther Sport. 2019;37:197–209. https://doi.org/10.1016/j.ptsp.2018.05.015.
Eckenrode BJ, Kietrys DM, Parrott JS. Effectiveness of manual therapy for pain and self-reported function in individuals with patellofemoral pain: systematic review and meta-analysis. J Orthop Sports Phys Ther. 2018;48:358–371. https://doi.org/10.2519/jospt.2018.7243.
Games KE, Sefton JM, Wilson AE. Whole-body vibration and blood flow and muscle oxygenation: a meta-analysis. J Athl Train. 2015;50:542–549. https://doi.org/10.4085/1062-6050-50.2.09.
Gattie E, Cleland JA, Snodgrass S. The effectiveness of trigger point dry needling for musculoskeletal conditions by physical therapists: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47:133–149. https://doi.org/10.2519/jospt.2017.7096.
Ghai S, Driller M, Ghai I. Effects of joint stabilizers on proprioception and stability: a systematic review and meta-analysis. Phys Ther Sport. 2017;25:65–75. https://doi.org/10.1016/j.ptsp.2016.05.006.
Gomes-Neto M, Lopes JM, Conceição CS, et al. Stabilization exercise compared to general exercises or manual therapy for the management of low back pain: a systematic review and meta-analysis. Phys Ther Sport. 2017;23:136–142. https://doi.org/10.1016/j.ptsp.2016.08.004.
Jeong HS, Lee SC, Jee H, Song JB, Chang HS, Lee SY. Proprioceptive training and outcomes of patients with knee osteoarthritis: a meta-analysis of randomized controlled trials. J Athl Train. 2019;54:418–428. https://doi.org/10.4085/1062-6050-329-17.
Kang FJ, Ou HL, Lin KY, Lin JJ. Serratus anterior and upper trapezius electromyographic analysis of the push-up plus exercise: a systematic review and meta-analysis. J Athl Train. 2019;54:1156–1164. https://doi.org/10.4085/1062-6050-237-18.
Karsten M, Ribeiro GS, Esquivel MS, Matte DL. The effects of inspiratory muscle training with linear workload devices on the sports performance and cardiopulmonary function of athletes: a systematic review and meta-analysis. Phys Ther Sport. 2018 Nov;34:92–104. https://doi.org/10.1016/j.ptsp.2018.09.004.
Knapik J, Steelman R. Risk factors for injuries during military static-line airborne operations: a systematic review and meta-analysis. J Athl Train. 2016;51:962–980. https://doi.org/10.4085/1062-6050-51.9.10.
Lam OT, Strenger DM, Chan-Fee M, Pham PT, Preuss RA, Robbins SM. Effectiveness of the McKenzie method of mechanical diagnosis and therapy for treating low back pain: literature review with meta-analysis. J Orthop Sports Phys Ther. 2018;48:476–490. https://doi.org/10.2519/jospt.2018.7562.
Lima YL, Ferreira VMLM, de Paula Lima PO, Bezerra MA, de Oliveira RR, Almeida GPL. The association of ankle dorsiflexion and dynamic knee valgus: a systematic review and meta-analysis. Phys Ther Sport. 2018;29:61–69. https://doi.org/10.1016/j.ptsp.2017.07.003.
López López L, Torres JR, Rubio AO, Torres Sánchez I, Cabrera Martos I, Valenza MC. Effects of neurodynamic treatment on hamstrings flexibility: a systematic review and meta-analysis. Phys Ther Sport. 2019;40:244–250. https://doi.org/10.1016/j.ptsp.2019.10.005.
Mansfield CJ, Vanetten L, Willy R, Di Stasi SPPDO, Magnussen R, Briggs M. The effects of needling therapies on muscle force production: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2019;49:154–170. https://doi.org/10.2519/jospt.2019.8270.
McAuliffe S, Tabuena A, McCreesh K, O’Keeffe M, Hurley J, Comyns T, et al. Altered strength profile in Achilles tendinopathy: a systematic review and meta-analysis. J Athl Train. 2019;54:889–900. https://doi.org/10.4085/1062-6050-43-18.
Montalvo AM, Schneider DK, Webster KE, et al. Anterior cruciate ligament injury risk in sport: a systematic review and meta-analysis of injury incidence by sex and sport classification. J Athl Train. 2019;54:472–482. https://doi.org/10.4085/1062-6050-407-16.
Nae J, Creaby MW, Cronström A, Ageberg E. Measurement properties of visual rating of postural orientation errors of the lower extremity—A systematic review and meta-analysis. Phys Ther Sport. 2017;27:52–64. https://doi.org/10.1016/j.ptsp.2017.04.003.
Nascimento LR, Teixeira-Salmela LF, Souza RB, Resende RA. Hip and knee strengthening is more effective than knee strengthening alone for reducing pain and improving activity in individuals with patellofemoral pain: a systematic review with meta-analysis. J Orthop Sports Phys Ther. 2018;48:19–31. https://doi.org/10.2519/jospt.2018.7365.
Neilson V, Ward S, Hume P, Lewis G, McDaid A. Effects of augmented feedback on training jump landing tasks for ACL injury prevention: a systematic review and meta-analysis. Phys Ther Sport. 2019 Sep;39:126–135. https://doi.org/10.1016/j.ptsp.2019.07.004.
Perriman A, Leahy E, Semciw AI. The effect of open-versus closed-kinetic-chain exercises on anterior tibial laxity, strength, and function following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2018;48:552–566. https://doi.org/10.2519/jospt.2018.7656.
Powden CJ, Hoch JM, Hoch MC. Rehabilitation and improvement of health-related quality-of-life detriments in individuals with chronic ankle instability: a meta-analysis. J Athl Train. 2017;52:753–765. https://doi.org/10.4085/1062-6050-52.5.01.
Sales AT, Fregonezi GA, Ramsook AH, Guenette JA, Lima IN, Reid WD. Respiratory muscle endurance after training in athletes and non-athletes: A systematic review and meta-analysis. Phys Ther Sport. 2016;17:76–86. https://doi.org/10.1016/j.ptsp.2015.08.001.
Sciascia A, Myers N, Kibler WB, Uhl TL. Return to preinjury levels of participation after superior labral repair in overhead athletes: a systematic review. J Athl Train. 2015;50:767–777. https://doi.org/10.4085/1062-6050-50.3.06.
Seffrin CB, Cattano NM, Reed MA, Gardiner-Shires AM. Instrument-assisted soft tissue mobilization: a systematic review and effect size analysis. J Athl Train. 2019;54:808–821. https://doi.org/10.4085/1062-6050-481-17.
Slater LV, Hart JM, Kelly AR, Kuenze CM. Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: a review and meta-analysis. J Athl Train. 2017 Sep;52(9):847–860. https://doi.org/10.4085/1062-6050-52.6.06.
Slimani M, Bragazzi NL, Znazen H, Paravlic A, Azaiez F, Tod D. Psychosocial predictors and psychological prevention of soccer injuries: a systematic review and meta-analysis of the literature. Phys Ther Sport. 2018;32:293–300. https://doi.org/10.1016/j.ptsp.2018.05.006.
Takasaki H, Lim ECW, Soon B. The effect of shoulder muscle fatigue on active repositioning acuity and scapulothoracic resting alignment: a systematic review with meta-analysis. Phys Ther Sport. 2016;20:61–78. https://doi.org/10.1016/j.ptsp.2016.01.001.
Takeno K, Glaviano NR, Norte GE, Ingersoll CD. Therapeutic interventions for scapular kinematics and disability in patients with subacromial impingement: a systematic review. J Athl Train. 2019;54:283–295. https://doi.org/10.4085/1062-6050-309-17.
Tsikopoulos K, Tsikopoulos A, Natsis K. Autologous whole blood or corticosteroid injections for the treatment of epicondylopathy and plantar fasciopathy? a systematic review and meta-analysis of randomized controlled trials. Phys Ther Sport. 2016;22:114–122. https://doi.org/10.1016/j.ptsp.2016.02.002.
Tsikopoulos K, Tsikopoulos I, Simeonidis E, et al. The clinical impact of platelet-rich plasma on tendinopathy compared to placebo or dry needling injections: a meta-analysis. Phys Ther Sport. 2016;17:87–94. https://doi.org/10.1016/j.ptsp.2015.06.003.
Vallandingham RA, Gaven SL, Powden CJ. Changes in dorsiflexion and dynamic postural control after mobilizations in individuals with chronic ankle instability: a systematic review and meta-analysis. J Athl Train. 2019 Apr;54(4):403–417. https://doi.org/10.4085/1062-6050-380-17.
Zhao R, Zhang M, Zhang Q. The effectiveness of combined exercise interventions for preventing postmenopausal bone loss: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47:241–251. https://doi.org/10.2519/jospt.2017.6969.
Author Contributions
Conceptualization, S.-H.C., and I.-S.S.; Methodology, I.-S.S.; Writing—Original Draft Preparation, S.-H.C.; Writing—Review & Editing, S.-H.C., and I.-S.S.; Project Administration, I.-S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
This study was approved by the institutional review board (IRB) of Nambu University (ethical code: 1041478-2017-HR-016; approved date: 8 November 2017) and was conducted in accordance with the ethical standards of the Declaration of Helsinki.
Informed Consent Statement
Since this study analyzed existing research results, we received an expedited review with exemption from informed consent.
Data Availability Statement
All data relevant to the study are included in the article. Data were collected from studies published online or publicly available, and specific details related to the data will be made available upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jette D.U., Bacon K., Batty C., Carlson M., Ferland A., Hemingway R.D., Hill J.C., Ogilvie L., Volk D. Evidence-based practice: Beliefs, attitudes, knowledge, and behaviors of physical therapists. Phys. Ther. 2003;83:786–805. doi: 10.1093/ptj/83.9.786. [DOI] [PubMed] [Google Scholar]
- 2.Evidence-Based Medicine Working Group Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268:2420–2425. doi: 10.1001/jama.1992.03490170092032. [DOI] [PubMed] [Google Scholar]
- 3.Moseley A.M., Herbert R.D., Sherrington C., Maher C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro) Aust. J. Physiother. 2002;48:43–49. doi: 10.1016/S0004-9514(14)60281-6. [DOI] [PubMed] [Google Scholar]
- 4.Kurichi J.E., Sonnad S.S. Statistical methods in the surgical literature. J. Am. Coll. Surg. 2006;202:476–484. doi: 10.1016/j.jamcollsurg.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Salbach N.M., Jaglal S.B., Korner-Bitensky N., Rappolt S., Davis D. Practitioner and organizational barriers to evidence-based practice of physical therapists for people with stroke. Phys. Ther. 2007;87:1284–1303. doi: 10.2522/ptj.20070040. [DOI] [PubMed] [Google Scholar]
- 6.Gopalakrishnan S., Ganeshkumar P. Systematic reviews and meta-analysis: Understanding the best evidence in primary healthcare. J. Fam. Med. Prim. Care. 2013;2:9–14. doi: 10.4103/2249-4863.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littell J.H., Corcoran J., Pillai V. Systematic Reviews and Meta-Analysis. Oxford University Press; Oxford, UK: 2008. [Google Scholar]
- 8.Cooper C., Booth A., Varley-Campbell J., Britten N., Garside R. Defining the process to literature searching in systematic reviews: A literature review of guidance and supporting studies. BMC Med. Res. Methodol. 2018;18:85. doi: 10.1186/s12874-018-0545-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn E., Kang H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018;71:103–112. doi: 10.4097/kjae.2018.71.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbour R., Miller J. A new system for grading recommendations in evidence based guidelines. BMJ. 2001;323:334–336. doi: 10.1136/bmj.323.7308.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin I.S. Recent Research Trends in Meta-analysis. Asian Nurs. Res. (Korean Soc. Nurs. Sci.) 2017;11:79–83. doi: 10.1016/j.anr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Introduction to Meta-Analysis. Wiley; West Sussex, UK: 2009. [Google Scholar]
- 13.Murad M.H., Asi N., Alsawas M., Alahdab F. New evidence pyramid. Evid. Based Med. 2016;21:125–127. doi: 10.1136/ebmed-2016-110401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Thomas J., Chandler J., Chandler J., Cumpston M., Li T., Page M., Welch V. Cochrane Handbook for Systematic Reviews of Interventions (Version 6.2) [(accessed on 22 July 2021)]. Available online: https://training.cochrane.org/handbook/current/chapter-11/
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.Cullis P.S., Gudlaugsdottir K., Andrews J. A systematic review of the quality of conduct and reporting of systematic reviews and meta-analyses in paediatric surgery. PLoS ONE. 2017;12:e0175213. doi: 10.1371/journal.pone.0175213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayan V.M., Chrouser K., Haynes R.B., Parrish R., Dahm P. Defining the publication source of high-quality evidence in urology: An analysis of EvidenceUpdates. BJU Int. 2016;117:861–866. doi: 10.1111/bju.13392. [DOI] [PubMed] [Google Scholar]
- 18.Page M.J., Shamseer L., Altman D.G., Tetzlaff J., Sampson M., Tricco A.C., Catalá-López F., Li L., Reid E.K., Sarkis-Onofre R., et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: A cross-sectional study. PLoS Med. 2016;13:e1002028. doi: 10.1371/journal.pmed.1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padula R.S., Pires R.S., Alouche S.R., Chiavegato L.D., Lopes A.D., Costa L.O. Analysis of reporting of systematic reviews in physical therapy published in Portuguese. Braz. J. Phys. Ther. 2012;16:381–388. doi: 10.1590/S1413-35552012005000040. (In English & Portuguese) [DOI] [PubMed] [Google Scholar]
- 20.Beller E.M., Glasziou P.P., Altman D.G., Hopewell S., Bastian H., Chalmers I., Gøtzsche P.C., Lasserson T., Tovey D., PRISMA for Abstracts Group PRISMA for abstracts: Reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:e1001419. doi: 10.1371/journal.pmed.1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009;62:e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Tan W.K., Wigley J., Shantikumar S. The reporting quality of systematic reviews and meta-analyses in vascular surgery needs improvement: A systematic review. Int. J. Surg. 2014;12:1262–1265. doi: 10.1016/j.ijsu.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Clarivate Analytics Journal Citation Reports. [(accessed on 17 April 2021)]. Available online: https://clarivate.com/webofsciencegroup/solutions/journal-citation-reports/
- 24.Tam W.W.S., Lo K.K.H., Khalechelvam P. Endorsement of PRISMA statement and quality of systematic reviews and meta-analyses published in nursing journals: A cross-sectional study. BMJ Open. 2017;7:e013905. doi: 10.1136/bmjopen-2016-013905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Zhang R., Huang J., Zhao X., Liu D., Sun W., Mai Y., Zhang P., Wang Y., Cao H., et al. Reporting quality of systematic reviews/meta-analyses of acupuncture. PLoS ONE. 2014;9:e113172. doi: 10.1371/journal.pone.0113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge L., Wang J.C., Li J.L., Liang L., An N., Shi X.T., Liu Y.C., Tian J.H. The assessment of the quality of reporting of systematic reviews/meta-analyses in diagnostic tests published by authors in China. PLoS ONE. 2014;9:e85908. doi: 10.1371/journal.pone.0085908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pigott T.D., Polanin J.R. Methodological guidance paper: High-quality meta-analysis in a systematic review. Rev. Educ. Res. 2020;90:24–46. doi: 10.3102/0034654319877153. [DOI] [Google Scholar]
- 28.Lima Y.L., Ferreira V.M.L.M., de Paula Lima P.O., Bezerra M.A., de Oliveira R.R., Almeida G.P.L. The association of ankle dorsiflexion and dynamic knee valgus: A systematic review and meta-analysis. Phys. Ther. Sport. 2018;29:61–69. doi: 10.1016/j.ptsp.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Neilson V., Ward S., Hume P., Lewis G., McDaid A. Effects of augmented feedback on training jump landing tasks for ACL injury prevention: A systematic review and meta-analysis. Phys. Ther. Sport. 2019;39:126–135. doi: 10.1016/j.ptsp.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 31.Powden C.J., Hoch J.M., Hoch M.C. Rehabilitation and improvement of health-related quality-of-life detriments in individuals with chronic ankle instability: A meta-analysis. J. Athl. Train. 2017;52:753–765. doi: 10.4085/1062-6050-52.5.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenland S., Senn S.J., Rothman K.J., Carlin J.B., Poole C., Goodman S.N., Altman D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016;31:337–350. doi: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Institute for Health Research PROSPERO is Fast-Tracking Registration of Protocols Related to COVID-19 2021. [(accessed on 22 July 2021)]. Available online: https://www.crd.york.ac.uk/prospero/
- 34.Tawfik G.M., Giang H., Ghozy S., Altibi A.M., Kandil H., Le H.H., Eid P.S., Radwan I., Makram O.M., Hien T., et al. Protocol registration issues of systematic review and meta-analysis studies: A survey of global researchers. BMC Med. Res. Methodol. 2020;20:213. doi: 10.1186/s12874-020-01094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth A., Clarke M., Ghersi D., Moher D., Petticrew M., Stewart L. An international registry of systematic-review protocols. Lancet. 2011;377:108–109. doi: 10.1016/S0140-6736(10)60903-8. [DOI] [PubMed] [Google Scholar]
- 36.Booth A. Providing transparency in systematic review methods: The case for protocol registration. Gerodontology. 2019;36:301–302. doi: 10.1111/ger.12440. [DOI] [PubMed] [Google Scholar]
- 37.Shantikumar S., Wigley J., Hameed W., Handa A. A survey of instructions to authors in surgical journals on reporting by CONSORT and PRISMA. Ann. R Coll. Surg. Engl. 2012;94:468–471. doi: 10.1308/003588412X13373405386619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker E., Hernandez A.V., Kattan M.W. Meta-analysis: Its strengths and limitations. Clevel. Clin. J. Med. 2008;75:431–439. doi: 10.3949/ccjm.75.6.431. [DOI] [PubMed] [Google Scholar]
- 39.Luchini C., Veronese N., Nottegar A., Shin J.I., Gentile G., Granziol U., Soysal P., Alexinschi O., Smith L., Solmi M. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharm. Stat. 2021;20:185–195. doi: 10.1002/pst.2068. [DOI] [PubMed] [Google Scholar]
- 40.Appelbaum M., Cooper H., Kline R.B., Mayo-Wilson E., Nezu A.M., Rao S.M. Journal article reporting standards for quantitative research in psychology: The APA publications and Communications Board task force report. Am. Psychol. 2018;73:3–25. doi: 10.1037/amp0000191. [DOI] [PubMed] [Google Scholar]
- 41.Haidich A.B. Meta-analysis in medical research. Hippokratia. 2010;14((Suppl. 1)):29–37. [PMC free article] [PubMed] [Google Scholar]
- 42.Alzahrani H., Mackey M., Stamatakis E., Pinheiro M.B., Wicks M., Shirley D. The effectiveness of incidental physical activity interventions compared to other interventions in the management of people with low back pain: A systematic review and meta-analysis of randomised controlled trials. Phys Ther. Sport. 2019;36:34–42. doi: 10.1016/j.ptsp.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Atkins D., Best D., Briss P.A., Eccles M., Falck-Ytter Y., Flottorp S., Guyatt G.H., Harbour R.T., Haugh M.C., Henry D., et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkins D., Eccles M., Flottorp S., Guyatt G.H., Henry D., Hill S., Liberati A., O’Connell D., Oxman A.D., Phillips B., et al. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004;4:38. doi: 10.1186/1472-6963-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furlan A.D., Malmivaara A., Chou R., Maher C.G., Deyo R.A., Schoene M., Bronfort G., van Tulder M.W., Editorial Board of the Cochrane Back, Neck Group 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine. 2015;40:1660–1673. doi: 10.1097/BRS.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 46.Singh J.A., Wells G.A., Christensen R., Tanjong Ghogomu E., Maxwell L., Macdonald J.K., Filippini G., Skoetz N., Francis D., Lopes L.C., et al. Adverse effects of biologics: A network meta-analysis and Cochrane overview. Cochrane Database Syst. Rev. 2011;2011:CD008794. doi: 10.1002/14651858.CD008794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cochrane Training Completing “Summary of Findings” Tables and Grading the Certainty of the Evidence. [(accessed on 21 April 2021)]. Available online: https://training.cochrane.org/handbook/current/chapter-14.
- 48.Krimsky S. Do Financial Conflicts of Interest Bias Research? An Inquiry into the ‘‘Funding Effect’’ Hypothesis. Sci. Technol. Hum. Values. 2013;38:1–22. doi: 10.1177/0162243912456271. [DOI] [Google Scholar]
- 49.Kwak S.M., Myung S.K., Lee Y.J., Seo H.G., Korean Meta-Analysis Study Group Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 50.Myung S.K., Ju W., McDonnell D.D., Lee Y.J., Kazinets G., Cheng C.T., Moskowitz J.M. Mobile phone use and risk of tumors: A meta-analysis. J. Clin. Oncol. 2009;27:5565–5572. doi: 10.1200/JCO.2008.21.6366. [DOI] [PubMed] [Google Scholar]
- 51.Tandel K.R. Sugar substitutes: Health controversy over perceived benefits. J. Pharm. Pharm. 2011;2:236–243. doi: 10.4103/0976-500X.85936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helfer B., Prosser A., Samara M.T., Geddes J.R., Cipriani A., Davis J.M., Mavridis D., Salanti G., Leucht S. Recent meta-analyses neglect previous systematic reviews and meta-analyses about the same topic: A systematic examination. BMC Med. 2015;13:82. doi: 10.1186/s12916-015-0317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bigna J.J.R., Um L.N., Nansseu J.R.N. A comparison of quality of abstracts of systematic reviews including meta-analysis of randomized controlled trials in high-impact general medicine journals before and after the publication of PRISMA extension for abstracts: A systematic review and meta-analysis. Syst. Rev. 2016;5:174. doi: 10.1186/s13643-016-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacDonald S.L., Canfield S.E., Fesperman S.F., Dahm P. Assessment of the methodological quality of systematic reviews published in the urological literature from 1998 to 2008. J. Urol. 2010;184:648–653. doi: 10.1016/j.juro.2010.03.127. [DOI] [PubMed] [Google Scholar]
- 55.Braga L.H., Pemberton J., Demaria J., Lorenzo A.J. Methodological concerns and quality appraisal of contemporary systematic reviews and meta-analyses in pediatric urology. J. Urol. 2011;186:266–271. doi: 10.1016/j.juro.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 56.Page M.J., Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA) Statement and extensions: A scoping review. Syst. Rev. 2017;6:263. doi: 10.1186/s13643-017-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayo-Wilson E., Heyward J., Keyes A., Reynolds J., White S., Atri N., Alexander G.C., Omar A., Ford D.E., National Clinical Trials Registration and Results Reporting Taskforce Survey Subcommittee Clinical trial registration and reporting: A survey of academic organizations in the United States. BMC Med. 2018;16:60. doi: 10.1186/s12916-018-1042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oliveira C.B., Elkins M.R., Lemes Í.R., de Oliveira Silva D., Briani R.V., Monteiro H.L., Azevedo F.M., Pinto R.Z. A low proportion of systematic reviews in physical therapy are registered: A survey of 150 published systematic reviews. Braz. J. Phys. Ther. 2018;22:177–183. doi: 10.1016/j.bjpt.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartling L., Ospina M., Liang Y., Dryden D.M., Hooton N., Seida J.K., Klassen T.P. Risk of bias versus quality assessment of randomised controlled trials: Cross sectional study. BMJ. 2009;339:b4012. doi: 10.1136/bmj.b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deeks J.J., Dinnes J., D’Amico R., Sowden A.J., Sakarovitch C., Song F., Petticrew M., Altman D.G., International Stroke Trial Collaborative Group. European Carotid Surgery Trial Collaborative Group Evaluating non-randomised intervention studies. Health Technol. Assess. 2003;7:1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 61.Kim S.Y., Park J.E., Lee Y.J., Seo H.J., Sheen S.S., Hahn S., Jang B.H., Son H.J. Testing a tool for assessing the risk of bias for non-randomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 62.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 63.Vandenbroucke J.P., Von Elm E., Altman D.G., Gøtzsche P.C., Mulrow C.D., Pocock S.J. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Morton N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009;55:129–133. doi: 10.1016/S0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 65.Alsalaheen B., Stockdale K., Pechumer D., Broglio S.P., Marchetti G.F. A comparative meta-analysis of the effects of concussion on a computerized neurocognitive test and self-reported symptoms. J. Athl. Train. 2017;52:834–846. doi: 10.4085/1062-6050-52.7.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoyt W.T., Del Re A.C. Effect size calculation in meta-analyses of psychotherapy outcome research. Psychother. Res. 2018;28:379–388. doi: 10.1080/10503307.2017.1405171. [DOI] [PubMed] [Google Scholar]
- 67.Slater L.V., Hart J.M., Kelly A.R., Kuenze C.M. Progressive changes in walking kinematics and kinetics after anterior cruciate ligament injury and reconstruction: A review and meta-analysis. J. Athl. Train. 2017;52:847–860. doi: 10.4085/1062-6050-52.6.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seffrin C.B., Cattano N.M., Reed M.A., Gardiner-Shires A.M. Instrument-assisted soft tissue mobilization: A systematic review and effect-size analysis. J. Athl. Train. 2019;54:808–821. doi: 10.4085/1062-6050-481-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McAuliffe S., Tabuena A., McCreesh K., O’Keeffe M., Hurley J., Comyns T., Purtill H., O’Neill S., O’Sullivan K. Altered strength profile in Achilles tendinopathy: A systematic review and meta-analysis. J. Athl. Train. 2019;54:889–900. doi: 10.4085/1062-6050-43-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olejnik S., Algina J. Measures of effect size for comparative studies: Applications, interpretations, and limitations. Contemp. Educ. Psychol. 2000;25:241–286. doi: 10.1006/ceps.2000.1040. [DOI] [PubMed] [Google Scholar]
- 71.Beeson P.M., Robey R.R. Evaluating single-subject treatment research: Lessons learned from the aphasia literature. Neuropsychol. Rev. 2006;16:161–169. doi: 10.1007/s11065-006-9013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Knapik J., Steelman R. Risk factors for injuries during military static-line airborne operations: A systematic review and meta-analysis. J. Athl. Train. 2016;51:962–980. doi: 10.4085/1062-6050-51.9.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hedges L.V., Olkin I. Statistical Methods for Meta-Analysis. Academic Press; Cambridge, MA, USA: 1985. [Google Scholar]
- 74.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 75.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sterne J.A.C., Hernán M.A., McAleenan A., Reeves B.C., Higgins J.P.T. Chapter 25: Assessing risk of bias in a non-randomized study. In: Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Balckwell; Hoboken, NJ, USA: 2020. [(accessed on 22 July 2021)]. Version 6.1. Available online: www.training.cochrane.org/handbook. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article. Data were collected from studies published online or publicly available, and specific details related to the data will be made available upon request.