Abstract
Helicobacter pylori (H. pylori) is a 0.5–1 µm wide, 2–4 µm long, short helical, S-shaped Gram-negative microorganism. It is mostly found in the pyloric region of the stomach and causes chronic gastric infection. It is estimated that these bacteria infect more than half of the world’s population. The mode of transmission and infection of H. pylori is still not known exactly, but the faecal–oral and oral–oral routes via water or food consumption are thought to be a very common cause. In the last three decades, research interest has increased regarding the pathogenicity, microbial activity, genetic predisposition, and clinical treatments to understand the severity of gastric atrophy and gastric cancer caused by H. pylori. Studies have suggested a relationship between H. pylori infection and malabsorption of essential micronutrients, and noted that H. pylori infection may affect the prevalence of malnutrition in some risk groups. On the other hand, dietary factors may play a considerably important role in H. pylori infection, and it has been reported that an adequate and balanced diet, especially high fruit and vegetable consumption and low processed salty food consumption, has a protective effect against the outcomes of H. pylori infection. The present review provides an overview of all aspects of H. pylori infection, such as clinical features, treatment, and nutrition.
Keywords: H. pylori, nutrition, infection, diet, clinical treatment
1. Introduction
Helicobacter pylori (H. pylori) was first identified in the stomach of dogs as a spiral microorganism by Giulio Bizzozero in 1892 [1]. As they are Campylobacter-like spiral microorganisms, they were named Campylobacter pyloridis by Barry Marshall and Robin Warren in 1983 [2]. Goodwin et al. named it “Helicobacter pylori” in 1989, as it has a helical structure and is mostly found in the pyloric region of the stomach [3]. H. pylori is a 0.5–1 µm wide, 2–4 µm long, short helical, S-shaped Gram-negative microorganism and infects more than half of the world’s population [4].
The relationship between H. pylori and gastric cancer was investigated in 1991 and 1994, and the International Agency for Research on Cancer, a branch of the World Health Organization, reported that H. pylori is carcinogenic in humans, which was reconfirmed in 2009 on the basis of epidemiological data [5,6]. In the United States, the National Institute of Health reported in 1994 that H. pylori may be the primary cause of peptic ulcer disease and should be treated. Marshall and Warren were awarded the Nobel Prize in 2005 for their work on H. pylori in the field of physiology “for discovering the role of H. pylori bacteria in gastritis and peptic ulcer disease” [7]. H. pylori plays a role in the development of diseases such as gastritis and mucosa-associated lymphoid tissue (MALT) lymphoma, as well as peptic ulcer and gastric cancer [8].
The mode of transmission of H. pylori is not known exactly, but the faecal–oral or oral–oral routes via water or food consumption are thought to be a very common cause [9]. The frequency of H. pylori infection increases with age. The rate of development is higher in societies with low socioeconomic status [10]. The fact that H. pylori survives in the stomach and creates chronic inflammation shows that it can be resistant to both the immune response and acid [11]. Many antibiotic treatments are used for the treatment of H. pylori, and studies show that the number of strains resistant to antibiotics used for treatment is increasing rapidly [12,13], which has led to the search for alternative agents to create safer and more effective results in addition to antibiotic treatments [14].
It is thought that dietary factors may play a considerably important role in H. pylori infection, and it has been reported that an adequate and balanced diet, especially high and abundant fruit and vegetable consumption, has a protective effect against the outcomes of H. pylori infection [15]. However, some studies have suggested a relationship between H. pylori infection and malabsorption of essential micronutrients, and it may cause malnutrition in some groups in the long term [16]. This review aimed to discuss the general clinical features of H. pylori and its relationship with nutrition, in addition to the treatment practices related to the disease.
2. H. pylori Infection Epidemiology
There are many studies on the prevalence of H. pylori, and its risk factors and pathways [9,17,18]. It is claimed that half of the world’s population is infected with H. pylori, but it is clear that more evidence-based research is still needed. The incidence of this infection is higher in low socioeconomic status groups and developing countries [19]. Vilaichone et al. found that the prevalence of H. pylori varies not only from country to country but also in different regions of the same country [20]. Its prevalence is significantly difficult to determine, as no health system compiles registry-based results of the prevalence of H. pylori in developing countries [21].
According to the regional prevalence estimates, there are approximately 4.4 billion H. pylori-infected people worldwide [22]. The countries with the highest H. pylori burden compared with the general population were found to be Nigeria, Portugal, Estonia, Kazakhstan, and Pakistan, and the lowest burden was in Switzerland [21]. In the study of Mezmale et al. (2020), a high prevalence of H. pylori infection was determined in Russia, Jordan, Iran, China, Canada, and Latin American countries [23].
Studies conducted in Turkey show that the rate of H. pylori infection is high. For example, in a study by Uyanıkoğlu et al. in 2010, 918 of 1298 patients who had antrum biopsy were positive for H. pylori. The prevalence of H. pylori infection is similar in males and females, and the incidence of H. pylori infection is 73.2% between the ages of 14 and 30, 71.5% between the ages of 31 and 45, 68.6% between the ages of 46 and 60, and 70.4% between the ages of 61 and 88 [24]. In a study conducted by Özen et al. in 2011, 161 of 473 children studying in four different primary and secondary schools in Istanbul were found to be H. pylori-positive [25]. Similarly, Özaydın et al. screened 4622 people for H. pylori infection in 55 cities using the C-urea breath test in 2013, and 3852 people (2075 females and 1777 males) were found to be positive for H. pylori [26]. In the review by Hooi et al., it was reported that three studies were conducted in Turkey up to 2015, the total number of participants was 6036, and the prevalence was 77.2% [21]. In a study conducted by Soylu et al. in 2019, the number of H. pylori-positive patients was found to be 46 (21 females and 25 males) in biopsy samples taken from 88 patients (53 females and 35 males) aged 18–77 years with dyspeptic complaints. Compared with the total number of participants, male patients were found to be more H. pylori-positive [27]. A study conducted in Nepal reported that 18.2% of 6- to 59-month-old children, 14% of boys and 16% of girls aged 10–19 years, and 40% of non-pregnant women aged 20–49 years were infected with H. pylori [28].
3. H. pylori Transmission
Although the mode of transmission of H. pylori is not known exactly, it is thought that it can be transmitted directly from one person to another or indirectly from the environment to people [29]. Person-to-person transmission is thought to be the primary mode of transmission, especially in developed countries. Food- and waterborne transmission are more likely in developing countries and H. pylori spreads more rapidly in areas with poor hygienic conditions [30,31].
In a study evaluating the prevalence of H. pylori infection in the rural community, Goodman et al. reported that people who are consumers of raw vegetables are more likely to be infected. Moreover, swimming in streams and rivers and using streams as drinking water may increase infection because of contamination by irrigation water or unpurified water [32]. Although some studies suggested that the transmission of H. pylori is from environmental contamination to food products, there is insufficient evidence to confirm this information [30,33]. It is accepted that interpersonal transmission routes are more frequent than environmental exposures. However, special attention should be paid to the sources of contamination (unhygienic water) that may lead to contamination through food [29].
Person-to-person transmission is thought to occur through the oral–oral, faecal–oral, gastric–oral, or sexual routes [29]. The literature indicates that H. pylori is present in the dental plaque and saliva of infected individuals [34,35,36], which shows that H. pylori infection spreads at a much higher rate than expected and, especially, transmission between family members is very frequent [37].
4. H. pylori Diagnosis
Each of the diagnostic tests used to detect the presence of H. pylori has advantages, disadvantages, and limitations, and the necessity of endoscopy is taken into account when classifying the methods. Histological evaluations using gastric biopsy specimens include rapid urease testing, culture, and polymerase chain reaction (PCR) [38]. Where invasive methods are time-consuming and not cost-effective, non-invasive diagnostic methods are used. Non-invasive tests include serological evaluation, stool antigen analyses, and the commonly used urea breath tests [39]. On the other hand, there is also non-Helicobacter pylori helicobacter (NHPH), which does not have a spiral morphology in the stomach [40]. Neither is the gold standard due to poor sensitivity or specificity. Combinations of more than one test give more reliable results [41].
5. H. pylori Pathogenesis
H. pylori is easily killed in hydrochloric acid solutions with a pH below 4.0. It is quite paradoxical for a microorganism whose primary site is the stomach. H. pylori continues to live in the lower part of the stomach by penetrating the mucus layer of the stomach through the contribution of its spiral shape and flagella [42]. To neutralise the acidic pH-related bactericidal activity against H. pylori, which can colonise the gastric epithelial surface, H. pylori hydrolyses urea to ammonia and carbon dioxide with the urease enzyme it produces [6]. In addition to its toxic effects on gastric mucosal epithelial cells, the ammonia formed increases the mucosal pH [43]. By damaging the protective mucus layer, which is rich in phospholipid and lipase, with the bacterial protease enzyme, it also delays the diffusion ability of H ions and increases its damaging effect [44].
It is known that H. pylori secretes a vacuole-forming cytotoxin (VacA) that adheres to the surface epithelium with adhesin proteins and causes vacuolization. The vacuole-forming cytotoxin induces host cell death through pore formation and apoptosis in mitochondrial membranes [45]. In addition to VacA, cytotoxin-associated antigen (CagA), known as an oncoprotein, is delivered into gastric epithelial cells and disrupts vesicular trafficking and autophagy pathways. Various studies have shown that cytotoxin-associated antigens affect the cell shape of bacterial proteins, disrupt cell assembly activity, increase cell motility, and are responsible for gastric ulcers and cancers [46,47,48].
Lipopolysaccharide (LPS), found in the outer membrane of H. pylori, is an effective immunomodulator in the human body and causes chronic inflammation by triggering the immune system. LPSs of H. pylori can mimic Lewis blood group antigens and, during infection, LPS can produce pathogenic anti-Lewis antibodies [49]. Lewis blood group antigens in the glycoprotein structure found on gastric epithelial surfaces mediate the binding of BabA, known as an adhesin, which binds to blood group antigens on the outer membrane of H. pylori, to surface mucosal cells and the gastric pit, and causes tissue destruction [50].
6. Potential Metabolic Responses to H. pylori
Similar to plant and animal species throughout history, humans have been prone to infection by pathogens. It has been suggested that infection formation is associated with many diseases [51]. The gastrointestinal tract constitutes the most intense region in terms of the diversity of microorganisms in the human body, and therefore they have critical roles in the development of the immune system [52]. The stomach was considered a sterile organ unsuitable for the growth of microorganisms. However, the discovery of H. pylori has shown that this idea is not correct. With the development of molecular techniques, it has been shown that there are abundant microorganisms in the stomach. In addition, various evidence has indicated that the stomach microbiota is effective in the development and progression of gastric disease [53].
The immune response caused by H. pylori causes damage to the gastric mucosa. During H. pylori infection, surface proteins and LPS are released, stimulating the host’s macrophages and promonocytes [54]. Proinflammatory factors such as interleukin-1 beta (IL-1β), interleukin-8 (IL-8), and reactive oxygen species (ROS) are produced in the gastric mucosa. Moreover, H. pylori can interact with epithelial cells to produce IL-8 [55]. IL-1β plays a significant role in the initiation and proliferation of inflammatory responses against bacteria and is effective in the suppression of acid secretion as a key cytokine in the gastric mucosa [56].
H. pylori induces the expression of neutrophils and adhesion molecules such as CD11b/CD18 and the production of ROS for potent chemotactic activity by stimulating the secretion of the proinflammatory cytokine IL-8 from the gastric mucosal cells of H. pylori [55]. In an in vitro study by Fazeli et al., it was proven that IL-8 is induced by CagA-positive strains of H. pylori, causing mucosal damage [57].
Davies et al. have suggested that host neutrophils are involved in the activation of ROS production by H.pylori [58]. Excessive ROS production creates oxidative stress in the gastric mucosa and can damage cellular components, including polyunsaturated fatty acids (PUFA), proteins, and DNA [59]. It is thought that H. pylori has antigens similar to some humoral compounds that play a role in essential physiological and structural formations in human cells, and that cellular and humoral immune responses can direct tissue destruction towards a pathological inflammatory response [49].
7. H. pylori and Chronic Gastritis
Inflammation of the gastric epithelium associated with mucosal damage is defined as gastritis [60]. It has been determined that the most common cause of chronic gastritis worldwide is H. pylori infection [61]. Proinflammatory cytokine production and inflammation induced by H. pylori infection affect gastrin-producing G cells, somatostatin-producing D cells, and acid-producing parietal cells, resulting in significant changes in acid homeostasis in the stomach [62].
Gastritis caused by H. pylori also reduces somatostatin levels. Since somatostatin negatively affects gastrin secretion, it causes an increase in gastrin levels and an increase in gastric acid secretion in these patients. Gastrin expression can be enhanced by the direct stimulating effect of H. pylori-induced proinflammatory cytokines on G cells [63].
Corpus-dominated gastritis predisposes individuals to gastric cancer, which is partly thought to be due to reduced acid secretion. Infection of the gastric antrum causes increased acid production and predisposes individuals to duodenal ulcer disease, which is associated with a reduced risk of gastric cancer [64].
ROS or reactive nitrogen species production is generated by the neutrophils and macrophages/monocytes in response to H. pylori infection. These have the potential to cause DNA damage. DNA damage is thought to trigger a series of events in gastric carcinogenesis, represented as a gastritis–atrophy–metaplasia–dysplasia–cancer sequence, by leading to mutations of some important genes in the stomach tissue [65,66].
8. H. pylori and Stomach Cancer
The risk factors of gastric cancer, which is the fifth leading type of cancer worldwide and the third cause of death linked to cancer worldwide, include H. pylori infection, age, high salt consumption, and low consumption of fruit and vegetables [67].
The pathogenicity of H. pylori and bacterial factors, including urease, VacA, CagA, and peptidoglycan outer membrane proteins (BabA, OipA, SabA), affect gastric epithelial cells [68]. Besides, the host’s genetics are affected by H. pylori infection, which affects genes encoding cytokines such as IL-8, IL-1β, IL-10, and TNF-α that cause polymorphisms, and increases proinflammatory responses, resulting in gastric cancer risk [69]. Thus, gastric cancer is affected not only by the H. pylori strain’s characteristics but also by the host’s genetic determinants and environmental factors [70]. It has also been proven that one of the environmental factors associated with an increased risk of gastric cancer is high dietary salt intake [71].
The noteworthy point in the studies is that a high salt food intake increases CagA levels in H. pylori and thus promotes infection [70,72]. The eradication of H. pylori may reduce the risk of gastric cancer, and studies have confirmed that it can reduce the occurrence of gastric cancer, including in those at highest risk [73,74].
9. H. pylori and Peptic Ulcers
Peptic ulcer disease, which is a significant source of morbidity and mortality worldwide, usually progresses asymptomatically. Symptoms of symptomatic peptic ulcer disease are epigastric pain associated with bloating, dyspepsia, nausea, early satiety, or abdominal fullness [75]. A peptic ulcer is frequently detected in the stomach and proximal duodenum [76]. Most cases of peptic ulcer disease are thought to be associated with H. pylori infection, use of nonsteroidal anti-inflammatory drugs (NSAIDs), or both [77].
People with non-atrophic antral-dominant gastritis have high stimulated acid production and increased gastrin levels due to decreased somatostatin in the antrum. Clinically, duodenal ulcers are common in this group [78]. In particular, gastritis caused by H. pylori causes a decrease in somatostatin levels [63]. On the other hand, people with atrophic gastritis (concerning both the antrum and corpus mucosa) have impaired acid production. This phenotype is thought to be associated with proximal gastric ulcers, more advanced precancerous lesions, and an increased risk of gastric cancer [79].
Studies have shown improvements in peptic ulcers with the eradication of H. pylori [80,81,82]. H. pylori chronically colonises the gastric/duodenal mucosa, inducing gastroduodenal diseases such as gastritis and peptic ulcer, and inducing innate and specific immune responses; however, if the infection is not eliminated, the chronic active gastritis condition may continue for life [83].
10. H. pylori and Anaemia
Iron is an important micronutrient for animals and microorganisms as a cofactor for enzymes involved in oxygen and electron transport and DNA synthesis. The response to infection is mediated by an iron-retaining mechanism that indirectly reduces the redistribution from the cell cytosol to the cell surface, and reduces circulating transferrin and the growth of infecting pathogens [84].
Kato et al. demonstrated that the SabA gene in the pathogenesis of H. pylori is highly expressed in bacterial isolates from patients with iron deficiency anaemia, proving that this virulence factor has a role in the development of anaemia [85]. Moreover, H. pylori causes hypochlorhydria and atrophic gastritis, in addition to peptic ulcer disease and increasing the risk of gastric malignancies. In this case, weakening of iron absorption can cause iron deficiency anaemia [86]. Atrophic corpus gastritis causes impaired intrinsic factor secretion, hypochlorhydria, or achlorhydria and can lead to intestinal iron and B12 malabsorption [86,87].
11. H. pylori and Insulin Resistance
Since the immune system is triggered by H. pylori infection, some inflammatory cytokines such as tumour necrosis factor α (TNF-α) and leptin and adipokines create an immune response to this inflammation. Relevant studies have revealed that leptin deficiency can induce the insulin resistance (IR) of high TNF-α and IL-6 levels [88,89]. Inflammatory cytokines induce the phosphorylation of serine residues on the insulin receptor substrate, causing disruption of insulin function and disrupting the substrate’s interaction with insulin receptors. Thus, diabetes can occur with the deterioration in blood glucose regulation [90,91].
12. H. pylori Infection Treatment
Infection treatment is carried out with a combination of antimicrobial agents and antisecretory agents, and gastric pH must be increased with antisecretory agents to achieve the bactericidal effect of antimicrobial agents. Alternatively, herbal medicines and probiotics are used as complementary therapy to help eradicate H. pylori, although their mechanism of action is not yet clear [92]. The increasing prevalence of antimicrobial resistance in H. pylori from person to person has led to the failure of eradication therapy with decreased compliance with clinical nutrition therapies [93].
In the treatment of H. pylori, drug resistance can easily develop against antibiotics used alone, so the recommended treatment is a combination of several antibiotics [39]. Many antimicrobial agents, antisecretory agents, and proton pump inhibitors are used in the H. pylori treatment protocol, including clarithromycin, amoxicillin, levofloxacin, metronidazole, tetracycline, rifabutin, and bismuth-containing compounds [92,94]. According to several international guidelines, first-line therapy for the treatment of H. pylori infection is a triple therapy consisting of a clarithromycin antibiotic given for 7–14 days, using any antibiotic from amoxicillin or metronidazole, and a PPI or ranitidine bismuth citrate [95,96]. If the treatment is not successful, second-line treatment is started. This treatment is carried out according to individual antibiotic resistance and sensitivities, or experimentally [96]. Second-line therapy is usually designated as tetracycline, metronidazole, a bismuth salt, or PPI. After failure of the second-line treatment, antimicrobial susceptibility test should be performed on the H. pylori culture from which the gastric biopsy was taken, and local resistance to antibiotics should be taken into account and treatment should be continued [78].
It has also been stated that PPIs, which have been used for a long time in the treatment of H. pylori infection, may prevent the absorption of micronutrients as well as their benefits [97]. The United States Food and Drug Administration has suggested that long-term use of PPIs may cause an increased risk of hypomagnesemia and fractures [98].
13. H. pylori and Nutrition
H. pylori infection has the main pathogenic effect, especially in diseases of the upper digestive tract. In addition to its cytotoxic and proinflammatory effects, H. pylori, similar to other microorganisms in the alimentary tract, affects the brain–gut connection, though indirectly [99]. It produces different biological effects on hormones such as ghrelin and leptin, which control both growth and appetite, causing changes in appetite and food intake, while causing changes in immunological symptoms and responses [99,100].
H. pylori is a factor that causes malnutrition and growth retardation, especially in childhood, due to malabsorption of nutrients and increased susceptibility to enteric infections, especially in developing countries [16]. Nweneka et al., in a meta-analysis study, reported that people with positive H. pylori had lower circulating ghrelin concentrations in 19 studies. On the other hand, the eradication of H. pylori also showed no significant effect on ghrelin in the circulation. This discrepancy in results depends on the amount of damage to ghrelin-producing cells before eradication, the time it takes for these cells to regenerate, and the duration of infection in the circulation [101].
Dietary modification to inhibit cancer formation promoted by H. pylori, which is a risk factor for cancer, should include eradication as well as practical strategies for the prevention of gastric cancer [102]. In addition, general nutrition has proven to be very important in dietary approaches, as it is known that various nutrients such as vitamin C, iron, cobalamin, and vitamin E cause malabsorption and lead to significant outcomes of nutrition [100].
13.1. H. pylori and Salt
H. pylori infection, besides being a pathogenic strain, may not be a sufficient reason for the development of gastric cancer. However, the risk for gastric cancer increases with high salt intake [72]. Epidemiological studies have shown a link between high salt consumption and an increased risk of gastric cancer in many parts of the world [103,104].
It has been determined in many studies that high salt intake can affect interactions between the stomach tissue and bacteria, with synergistic effects with H. pylori, and increases the possibility of permanent infection [105,106]. CagA, a bacterial oncoprotein, may contribute to the formation of gastric cancer by disrupting the signalling pathways of epithelial cells in the stomach. On the other hand, it has been determined that CagA protein makes an indirect contribution to the pathogenesis of gastric cancer by stimulating increased gastric mucosal inflammation, as it stimulates the proinflammatory cytokine IL-8 and causes an increase in secretion [72].
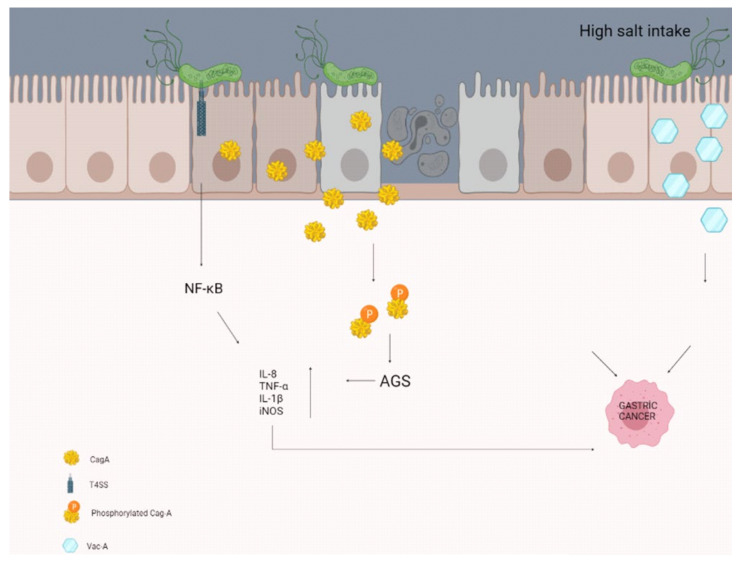
A study by Gaddy et al. examining the effects of high salt intake with H. pylori infection reported that Mongolian gerbils (H. pylori infection causes chronic gastritis, gastric ulcer, and intestinal metaplasia in Mongolian gerbils, so it is the best animal model) were infected with a wild-type strain of CagA+ H. pylori. Infected animals fed a high-salt diet were reported to have more severe gastroenteritis and a higher rate of gastric adenocarcinoma, with increased expression of the proinflammatory cytokines IL-1β and nitric oxide synthase (iNOS), compared with those fed the normal diet (Figure 1) [107]. Besides, Caston et al. determined an increase in VacA toxin levels in the extracellular space in response to high salt under in vitro conditions. These salt-induced changes have been proven to contribute to an increased risk of gastric cancer in people infected with H. pylori who consume a high-salt diet [108].
Figure 1.
High salt intake may contribute to the formation of gastric cancer by disrupting the molecular pathways and some secretions of the epithelial cells in the stomach.
13.2. H. pylori and Iron
Iron is involved in the maintenance of metabolic functions as a cofactor in biological systems that participate in various oxidation–reduction processes, electron transport, and amino acid and nucleotide synthesis, which are essential for life [84,109]. It is also a very important growth factor for almost all bacteria. There is usually a race between bacteria and the host for iron absorption [110]. On the other hand, the interaction of iron with free oxygen creates the Fenton reaction, contributing to the generation of oxygen radicals that can cause serious damage to cellular biomolecules’ structures [111].
H. pylori, a pathogen for gastric tissues, undoubtedly encounters a series of environmental stresses in the human stomach it colonises. Iron deficiency is expected in the long term and in some risk groups (Figure 2), as the human body tightly holds the available iron to prevent both bacterial growth and oxidative damage [112]. At the same time, acid secretion due to gastritis caused by H. pylori can prevent iron absorption and cause insufficiency [113].
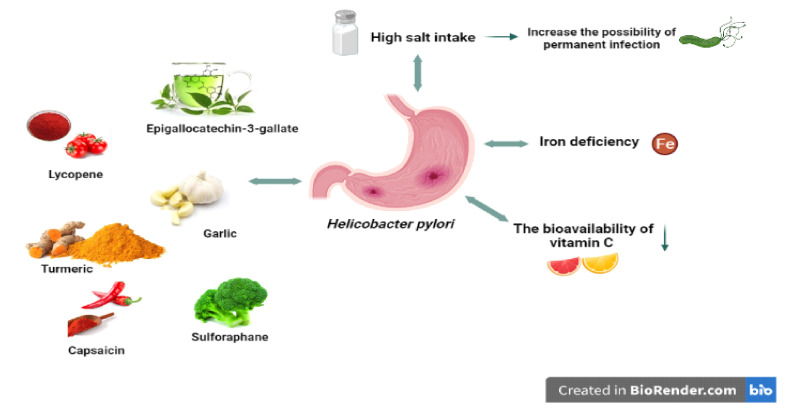
Figure 2.
H. pylori and related nutritional factors. High salt intake can increase the risk of permanent infection. H pylori can cause iron deficiency and decrease the bioavailability of vitamin C. Some nutrients (allicin, lycopene, capsaicin, etc.) have positive effects on H. pylori.
It is thought that iron deficiency, which is a cause of H. pylori infection, may also be effective in H. pylori virulence (Figure 2) [112,114]. In support of this, an in vitro study found that iron deficiency affected the virulence factors of H. pylori, leading to the activity of Cag T4SS and inhibition of gastric acid secretion by the host with increased expression of IL-8, contributing to the increased incidence and severity of gastroenteritis, and thus the development of gastric cancer [115,116].
13.3. H. pylori and Vitamin C
Vitamin C is a micronutrient essential for human health. Unlike many animals, humans have lost their ability to biosynthesise vitamin C (ascorbic acid) due to various mutations of the gulonolactone oxidase enzyme [117].
Vitamin C is a chemical reducing agent or electron donor. On the other hand, electrons from ascorbate can reduce metals such as iron and copper, leading to the formation of hydrogen peroxide and superoxide and the formation of reactive oxidant species. As a reducing agent, ascorbate can form oxidants in some cases [118]. While vitamin C protects against oxidative stress in cancer cells with its antioxidant effect, it can also increase the risk of cancer with its pro-oxidant activity [119].
The bioavailability of vitamin C can be significantly reduced by H. pylori infection (Figure 2). In support of this, a study in 1995 showed that H. pylori can oxidise and neutralise ascorbic acid in the stomach [120]. In another study, gastric juice and plasma vitamin C levels were significantly reduced with decreased vitamin C intake in people with H. pylori [121]. In such studies, it is expected that vitamin C levels will return to normal after H. pylori eradication [122,123]. Atrophic gastritis, which is a result of H. pylori and triggers the formation of gastric cancer, may disrupt the secretion of vitamin C in the gastric mucosa and increase the pH of gastric juice [124].
In a study conducted by Waring et al., when a control group consisting of 48 gastritis patients without supplementation and an experimental group of 32 gastritis patients who were given 500 mg vitamin C twice a day for 2 weeks were compared, it was found that high vitamin C intake could reduce the risk of gastric cancer, but showed that the protective effect may be greater if gastritis is treated with H. pylori eradication [125]. In another study, Zojaji et al. divided the study group into two, created the same treatment protocol for both groups, and added vitamin C to the second group. The experimental results showed that 78.0% of individuals in the group receiving supplemental vitamin C had increased H. pylori eradication rates compared with 48.8% of individuals in the other group [126].
On the other hand, side effects such as gastrointestinal disturbances and especially osmotic diarrhoea may occur due to high doses of vitamin C. It also causes an increase in uric acid and oxalate excretion, and may also be associated with an increased risk of kidney stones caused by calcium oxalate [127]. In rare cases, due to its pro-oxidant activity at high concentrations, especially in supplement form, high concentrations (500 mg/day or more) are expected to cause various adverse effects such as DNA damage and, indirectly, some types of cancer in the presence of high iron stores in the human organism [128]. Given that it is a water-soluble vitamin, alongside the chance of toxicity from a single daily bolus dose and its short biological half-life, more clinical research is required to determine the correct dosage [129]. However, vitamin C, which is generally taken with natural nutrition, increases mucosal immune responses by eliminating free radicals, especially through fruit and vegetable consumption [130]. It reduces the content of N-nitrosamine in the gastric juice and inhibits cell proliferation. It may especially be protective against gastric carcinogenesis associated with H. pylori by directly affecting the growth of H. pylori [131].
13.4. H. pylori and Antioxidants
Oxidative stress is a physiological process experienced by every living organism and plays a role in the aetiology of many diseases and the ageing process [132]. The stomach, which is a bioreactor, is an excellent environment to increase the co-oxidation of vitamins but is constantly exposed to reactive species and ingested carcinogens, bacterial pathogens, and oxidative compounds associated with food digestion [132,133].
After H. pylori reaches the gastric epithelium, it activates nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase and produces ROS such as hydrogen peroxide (H2O2), superoxide (O2-), hypochlorous acid (HOCl), and hydroxyl radicals (OH) in gastric epithelial cells independently of inflammatory cells, thus triggering infection responses in the target host’s innate immune cells [134,135].
ROS production by H. pylori activates the oxidant-sensitive transcription factor NF-κB, which induces the expression of oncogenes and cell cycle regulators and plays an important role in apoptosis and DNA damage in gastric epithelial cells [136]. It was observed that ROS activity increased and antioxidant compounds such as vitamin C decreased in H. pylori-positive individuals. Thus, gastric cancer develops due to the DNA damage caused by H. pylori [137]. It is also known that H. pylori infection disrupts the function of some oncogenes and tumour suppressor genes (e.g., p53) in gastric tissue, and this may trigger the initial carcinogens [138,139].
Since H. pylori infection affects the oxidative stress process, it is thought that some antioxidant foods and nutrients may be effective. This is also emphasised in the literature investigating the relationship between H. pylori infection and nutrients, which suggests that the inclusion of these nutrients in the diet may have a protective effect, unlike some other nutritional components such as salt (Figure 2).
Garlic (Allium sativum L.), a member of the lily family, known worldwide and most widely cultivated in Asia, is a medicinal plant. It contains 33 sulphur compounds, especially allicin. Garlic is known as an effective free radical scavenger against various diseases caused by ROS [140]. Chung et al. showed in 1998 for the first time that garlic components can suppress the growth of H. pylori [141]. Zardast et al. argued that garlic had antibacterial effects against H. pylori in H. pylori-positive individuals who consumed two medium raw cloves of garlic (3 g) with daily meals twice a day for 3 days [142]. In the suppression of H. pylori, the allicin in garlic inhibits the activation of NF-κB by inhibiting the Toll-like receptor 4 (TLR4) signalling pathway, resulting in an anti-inflammatory effect [143].
Turmeric (Curcuma longa) contains curcumin, a polyphenolic compound, and has a yellow pigment. It is widely used as a food colouring agent [144]. Curcumin has a wide range of beneficial properties, including antioxidant, anti-inflammatory, anticancer, antiproliferative, antifungal, and antimicrobial properties [145]. Judaki et al. formed two groups of H. pylori-positive individuals and applied the same treatment protocol to both groups by adding 700 mg of oral curcumin three times a day to the second group. After 3 months, they reported a significant reduction in the amount of oxidative DNA damage in the curcumin group [146].
Lycopene, a natural antioxidant, is a carotenoid that gives fruits and vegetables their red colour [147]. Some studies revealed that lycopene inhibits DNA damage and the cellular response of H. pylori-infected gastric carcinoma cells (AGS) [148,149]. In a study by Shidfar et al., it was shown that the effect of lycopene was not significant in the treatment of H. pylori-positive patients [150]. The main findings of some dietary interventions in H. pylori are summarised in Table 1.
Table 1.
The main findings of dietary interventions in H. pylori.
| Dietary Intervention | Study Design | Main Outcomes | Reference |
|---|---|---|---|
| High-salt diet | *Mongolian gerbils were infected with a wild-type (WT) CagA(+) H. pylori strain or an isogenic CcagA mutant strain. A regular diet or a high-salt diet was given to the animals. |
Among animals infected with the WT strain, those fed a high-salt diet had more severe gastric inflammation, higher gastric pH, increased parietal cell loss, increased gastric expression of interleukin 1β (IL-1β), and decreased gastric expression of hepcidin and hydrogen potassium ATPase (H, K-ATPase) compared with those fed a regular diet. | [107] |
| Vitamin C | 32 patients were supplemented with ascorbic acid (500 mg twice daily for 2 weeks) 48 patients were not supplemented |
High ascorbic acid may lower the incidence of gastric cancer, although its protective impact may be enhanced if gastritis is addressed (for example, by H. pylori eradication). | [125] |
| Vitamin C | Patients with H. pylori were randomly divided into two groups; -Group A (n: 162) were given a treatment regimen -Group B (n: 150) received the same regimen + 500 mg of vitamin C/day |
The H. pylori treatment regimen with vitamin C may significantly increase the H. pylori eradication rate. | [126] |
| Garlic | 15 patients with H. pylori During 3 days, two medium-sized cloves of garlic (3 g) were given twice/day with meals. |
Raw garlic has antibacterial effects against H. pylori residing in the stomach. It may be prescribed with drugs in the treatment of H. pylori infection. |
[142] |
| Curcumin | Two groups of H. pylori-positive patients The same treatment protocol was applied to both groups 700 mg of oral curcumin (three times/day) was added to the second group. |
Curcumin may be a useful supplement to improve chronic inflammation as well as preventing carcinogenic changes associated with H. pylori in patients with chronic gastritis. |
[146] |
| Lycopene | 54 H. pylori-positive patients; Four standard drugs were given to Group 1. Lycopene (30 mg/day) was given to Group 2. |
Lycopene does not have any significant effects on eradicating H. pylori when compared with the standard antibiotic therapy. | [150] |
| EGCG | 56 clinical isolates of H. pylori, including 19 isolates highly resistant to metronidazole (MTZ) and/or clarithromycin (CLR), were used to determine in vitro sensitivity to tea catechins. | EGCG may be a valuable therapeutic agent against H. pylori infection. | [151] |
| Sulforaphane | 48 H. pylori-infected patients were randomly divided into two groups: Broccoli sprouts (70 g/day, containing 420 micromoles of sulforaphane precursor) for 8 weeks Placebo—consumption of an equal weight of alfalfa sprouts (not containing sulforaphane) |
The treatment seemed to enhance chemoprotection of the gastric mucosa against H. pylori-induced oxidative stress. | [152] |
Pepper, a member of the Solanaceae family, has high nutritional value in terms of antioxidant properties, as it is a rich source of carotenoids and provitamin A, in addition to vitamins E and C [153]. Capsaicin, the active component of hot peppers, gives peppers a characteristic pungent flavour [154]. It was thought that capsaicin might have a protective effect against H. pylori infection, and in 1997, Jones et al. showed that capsaicin produced effective inhibition of H. pylori growth in vitro, depending on the time and concentration [155]. Capsaicin has also been suggested as a potential anti-inflammatory drug by inhibiting IL-8 production in the gastric epithelium of H. pylori-positive individuals [156]. More research is needed to better understand its effects and to determine the amount for daily consumption.
Epigallocatechin-3-gallate (EGCg) is the major and the most significant polyphenol found in green tea and is effective in health as an anti-inflammatory, antioxidant, and anti-atherogenic agent [157]. It was thought that it might be effective against infection caused by especially resistant H. pylori strains [158]. An in vitro study by Yanagawa et al. revealed that the addition of EGCg to antibiotic treatments resulted in increased antibacterial activity and showed a relatively slow but strong activity against the growth of H. pylori [151].
Another structure with bactericidal properties against H. pylori infection, which contributes to the formation of stomach cancer, is sulforaphane. It is abundant in broccoli sprouts and has a protective effect on injuries caused by various types of oxidative stress thanks to its phytochemicals and antioxidant enzymes [159]. Sulforaphane not only increases the antioxidant activity of the gastrointestinal mucosa but also inhibits the growth of various microorganisms, including some human pathogens, and has anticarcinogenic activity [160,161]. H. pylori infection in the gastric mucosa induces oxidative stress. It is known that genes encoding nrf2 (NF-E2 p45-related factor-2) and keap1 (Kelch-like ECH-related protein 1) play a critical role in the activities of antioxidant enzymes. Sulforaphane stimulates antioxidant enzyme activities linked to the nrf2 gene, thus enabling H. pylori to inhibit oxidative damage in cells [152].
In a study by Yanaka et al., people who were H. pylori-positive were fed broccoli sprouts (70 g/day) and compared with the placebo group. People who consumed broccoli sprouts for 8 weeks showed positive effects in the clinical laboratory results. However, a regression to the starting point shortly after the end of his study was observed [162]. Therefore, more studies are needed to determine its long-term effects.
13.5. H. pylori, Nitrite, and Nitrosamines
Nitrosamines are formed by the reaction of secondary amines with nitrites. N-nitroso compounds (NOCs) and nitrosamines contribute to gastric carcinogenesis. They can be formed exogenously (during fermentation, smoking, cooking, and storage) or endogenously in the stomach unless inhibited by vitamin C or other antioxidants [163,164]. The nitrite-clearing ability of vitamin C depends on the stomach pH and the vitamin C/nitrite ratio, so it causes an increase in NOCs in the case of pH>4 and a decrease in the vitamin C/nitrite ratio [165].
H. pylori stimulates the macrophage system via the L-arginine/NO pathway [166]. It is thought that H. pylori infection will not only trigger the formation of NO endogenously but also cause DNA damage and increase the risk of cancer [166,167]. On the other hand, various foods such as pickled foods and processed meat, which are sources of nitrites and/or nitrosamines, are also important sources of salt [168]. In general, H. pylori-positive individuals with a high dietary salt intake have an increased risk of gastric cancer compared with those who are H. pylori-negative and with low-salt intake, and red meat-related gastric cancer risk, endogenous nitrosamine formation, or consumption of processed meat is only more common in H. pylori-positive individuals [169].
13.6. H. pylori and Probiotics
The term “probiotic” is of Greek origin and is translated into our language as “pro bios” (pro: for, bios: life) meaning “for life” [170]. According to the publication of the International Scientific Association of Probiotics and Prebiotics (ISAPP) in 2021, probiotics are defined as live microorganisms that, when taken in sufficient amounts, provide health benefits to the host [171].
Probiotic bacteria can bind to recognition receptors, such as TLR expressed on the surface of epithelial cells, thereby triggering several immunological defence mechanisms. It has been reported that probiotics can modify the immunological response by increasing the production of anti-inflammatory cytokine-regulating (IL-10 and TGF-b) cells and/or suppressing cytokines (IL-4, IL-5, IL-6, and especially IL-8), thus reducing gastric activity and inflammation, that is, the effect of H. pylori infection [172].
In the Maastricht-5 Consensus Report, it was stated that the stomach microbiota decreased with the use of various antibiotics and it was accepted that probiotics were beneficial, but the level of evidence was evaluated as moderate with a low recommendation [78]. Additionally, the use of adjuvant probiotic treatments (Lactobacillus spp. and Saccharomyces boulardi) in combination with antibiotics may be beneficial [78]. In patients with H. pylori infection, Lactobacillus spp. supplementation in addition to triple therapy may be beneficial in improving H. pylori eradication rates, especially in children, and in reducing treatment-related side effects (especially the incidence of diarrhoea) [173,174]. The first meta-analysis study conducted in 2010 on the use of Saccharomyces boulardi as an adjuvant therapy found that it generally reduced the side effects of H. pylori infection [175]. Saccharomyces boulardi supplementation along with the standard triple therapy provided additional support to increase H. pylori eradication rates [176].
To sum up, it has been reported that some probiotics reduce the side effects of infection in the treatment to eradicate H. pylori. More clinical research is needed to gain insight into the direct efficacy of specific probiotic strains, including the duration and dosages of adjuvant probiotic therapy, the individual’s lifestyle (such as alcohol, diet, or cigarette consumption), and geographic differences [132].
14. Conclusions
H. pylori is estimated to infect half of the world’s population and causes permanent infections as well as many health issues such as gastritis and MALT lymphoma, as well as peptic ulcer and gastric cancer. In H. pylori infection, there are some treatment limitations due to its ability to create resistance to antibiotic treatments in treatment strategies. Therefore, it has become necessary to seek alternatives to fight against H. pylori infection. Especially in the last few years, research has clearly shown the pathogenicity, microbial activity, and genetic predisposition to help understand the severity of gastric atrophy and gastric cancer caused by H. pylori. This situation is expected to affect the treatment process positively. Combination treatments, including with phytochemicals and probiotics found in natural products, seem to have beneficial effects in the eradication of H. pylori.
Due to the effects of hormones such as ghrelin and leptin, which control both growth and appetite, and the formation of malabsorption of various nutrients such as vitamin C, iron, cobalamin, and vitamin E in H. pylori-infected individuals, detailed nutritional information should be provided during and after treatment. It is important to provide optimal nutrition through the determination of strategies and the application of a suitable diet for the person by authorised dietitians. Besides, there have been some promising effects for probiotics added to treatment strategies; however, detailed research is needed. Most importantly, a diet rich in fruits and vegetables and reduced in salt and processed meat products has good prophylactic potential, especially against cancer in the eradication of H. pylori.
Acknowledgments
Not applicable.
Author Contributions
M.Ö. and B.Y. drafted the work or revised it critically for important intellectual content, and gave final approval of the version to be published. D.A. and R.C. designed and drafted the work and revised it critically for important intellectual content; they also gave final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bizzozero G. Ueber die schlauchförmigen drüsen des magendarmkanals und die beziehungen ihres epithels zu dem oberflächenepithel der schleimhaut dritte mittheilung. Arch. Für Mikrosk. Anat. 1893;42:82–152. doi: 10.1007/BF02975307. [DOI] [Google Scholar]
- 2.Marshall B.J., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin C.S., Worsley B.W. Microbiology of Helicobacter pylori. Gastroenterol. Clin. North Am. 1993;22:5–19. doi: 10.1016/S0889-8553(21)00260-0. [DOI] [PubMed] [Google Scholar]
- 4.Kusters J.G., van Vliet A.H., Kuipers E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer (IARC) Schistosomes, Liver Flukes and Helicobacter Pylori, Monograph on the Evaluation of Carcinogenic Risks to Humans. Vol. 61. IARC; Lyon, France: 1994. pp. 1–241. [PMC free article] [PubMed] [Google Scholar]
- 6.Ansari S., Yamaoka Y. Survival of Helicobacter pylori in gastric acidic territory. Helicobacter. 2017;22:e12386. doi: 10.1111/hel.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall B. Helicobacter pylori--a Nobel pursuit? Can. J. Gastroenterol. 2008;22:895–896. doi: 10.1155/2008/459810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laszewicz W., Iwańczak F., Iwańczak B. Seroprevalence of Helicobacter pylori infection in Polish children and adults depending on socioeconomic status and living conditions. Adv. Med. Sci. 2014;59:147–150. doi: 10.1016/j.advms.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Brown L.M. Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol. Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 10.Mégraud F. Epıdemıology of helıcobacter pylorı ınfectıon. Gastroenterol. Clin. North Am. 1993;22:73–88. doi: 10.1016/S0889-8553(21)00264-8. [DOI] [PubMed] [Google Scholar]
- 11.Besiski F.S. Helicobacter pylori ınfection: Epidemiology and pathogenesis. Flora. 1996;3:160–166. [Google Scholar]
- 12.Raymond J., Lamarque D., Kalach N., Chaussade S., Burucoa C. High level of antimicrobial resistance in French Helicobacter pylori isolates. Helicobacter. 2010;15:21–27. doi: 10.1111/j.1523-5378.2009.00737.x. [DOI] [PubMed] [Google Scholar]
- 13.Opekun A.R., El-Zaimaity H.M., Osato M.S., Gilger M.A., Malaty H.M., Terry M., Headon D.R., Graham D.Y. Novel therapies for Helicobacter pylori infection. Aliment. Pharm. Ther. 1999;13:35–42. doi: 10.1046/j.1365-2036.1999.00435.x. [DOI] [PubMed] [Google Scholar]
- 14.Guttner Y., Windsor H.M., Viiala C.H., Marshall B.J. Human recombinant lactoferrin is ineffective in the treatment of human Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2003;17:125–129. doi: 10.1046/j.1365-2036.2003.01395.x. [DOI] [PubMed] [Google Scholar]
- 15.Mard S.A., Khadem Haghighian H., Sebghatulahi V., Ahmadi B. Dietary factors in relation to Helicobacter pylori ınfection. Gastroenterol. Res. Pract. 2014;2014:826910. doi: 10.1155/2014/826910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franceschi F., Annalisa T., Teresa D.R., Giovanna D., Ianiro G., Franco S., Viviana G., Valentina T., Riccardo L.L., Antonio G. Role of Helicobacter pylori infection on nutrition and metabolism. World J. Gastroenterol. 2014;20:12809–12817. doi: 10.3748/wjg.v20.i36.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mégraud F., Broutet N. Epidemiology, acquisition and transmission of Helicobacter pylori. Rev. Prat. 2000;50:1414–1417. [PubMed] [Google Scholar]
- 18.Tursi A., Cammarota G., Papa A., Cuoco L., Gentiloni N., Fedeli P., Fedeli G., Gasbarrini G. The modes of transmission of Helicobacter pylori infection. Recenti. Prog. Med. 1997;88:232–236. [PubMed] [Google Scholar]
- 19.Lehours P. Actual diagnosis of Helicobacter pylori infection. Minerva Gastroenterol. Dietol. 2018;64:267–279. doi: 10.23736/S1121-421X.18.02494-7. [DOI] [PubMed] [Google Scholar]
- 20.Vilaichone R.-k., Mahachai V., Shiota S., Uchida T., Ratanachu-ek T., Tshering L., Tung N.L., Fujioka T., Moriyama M., Yamaoka Y. Extremely high prevalence of Helicobacter pylori infection in Bhutan. World J. Gastroenterol. 2013;19:2806–2810. doi: 10.3748/wjg.v19.i18.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global prevalence of Helicobacter pylori ınfection: Systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Khoder G., Muhammad J.S., Mahmoud I., Soliman S.S.M., Burucoa C. Prevalence of Helicobacter pylori and ıts associated factors among healthy asymptomatic residents in the United Arab Emirates. Pathogens. 2019;8:44. doi: 10.3390/pathogens8020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezmale L., Coelho L.G., Bordin D., Leja M. Review: Epidemiology of Helicobacter pylori. Helicobacter. 2020;25 Suppl 1:e12734. doi: 10.1111/hel.12734. [DOI] [PubMed] [Google Scholar]
- 24.Uyanıkoğlu A., Coşkun M., Binici D.N., Uçar Ş., Kibar Y.İ., Tay A., Öztürk Y. Frequency of Helicobacter pylori in patients underwent endoscopy. Dicle Tıp Derg. 2012;39:197–200. doi: 10.5798/diclemedj.0921.2012.02.0126. [DOI] [Google Scholar]
- 25.Ozen A., Furman A., Berber M., Karatepe H.O., Mutlu N., Sarıçoban H.E., Büyükgebiz B. The effect of Helicobacter pylori and economic status on growth parameters and leptin, ghrelin, and insulin-like growth factor (IGF)-I concentrations in children. Helicobacter. 2011;16:55–65. doi: 10.1111/j.1523-5378.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- 26.Ozaydin N., Turkyilmaz S.A., Cali S. Prevalence and risk factors of Helicobacter pylori in Turkey: A nationally-representative, cross-sectional, screening with the ¹3C-Urea breath test. BMC Public Health. 2013;13:1215. doi: 10.1186/1471-2458-13-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soylu A., Peker K.D., Yırgın H., Polat Sarı S., Akgül Ö., Sapmaz B., Adaş G.T., Öner Y.A., Kayacan Z.Ç., Çalışkan R. Dispepsili hastalarda, H. pylori ve histopatolojik bulguların değerlendirilmesi. Tıp Fakültesi Klin. Derg. 2019;2:139–141. [Google Scholar]
- 28.Mehata S., Parajuli K.R., Pant N.D., Rayamajhee B., Yadav U.N., Mehta R.K., Jha P., Mehta N., Dhimal M., Singh D.R. Prevalence and correlates of Helicobacter pylori infection among under-five children, adolescent and non-pregnant women in Nepal: Further analysis of Nepal national micronutrient status survey 2016. PLoS Negl. Trop. Dis. 2021;15:e0009510. doi: 10.1371/journal.pntd.0009510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zamani M., Vahedi A., Maghdouri Z., shokri-shirvani J. Role of food in environmental transmission of Helicobacter pylori. Casp. J. Intern. Med. 2017;8:146–152. doi: 10.22088/cjim.8.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vale F.F., Vítor J.M.B. Transmission pathway of Helicobacter pylori: Does food play a role in rural and urban areas? Int. J. Food Microbiol. 2010;138:1–12. doi: 10.1016/j.ijfoodmicro.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y.Y., Xia H.H., Zhuang Z.H., Zhong J. Review article: ’True’ re-infection of Helicobacter pylori after successful eradication--worldwide annual rates, risk factors and clinical implications. Aliment. Pharmacol. Ther. 2009;29:145–160. doi: 10.1111/j.1365-2036.2008.03873.x. [DOI] [PubMed] [Google Scholar]
- 32.Goodman K.J., Correa P., Tenganá Aux H.J., Ramírez H., DeLany J.P., Guerrero Pepinosa O., López Quiñones M., Collazos Parra T. Helicobacter pylori infection in the Colombian Andes: A population-based study of transmission pathways. Am. J. Epidemiol. 1996;144:290–299. doi: 10.1093/oxfordjournals.aje.a008924. [DOI] [PubMed] [Google Scholar]
- 33.Quaglia N.C., Dambrosio A. Helicobacter pylori: A foodborne pathogen? World J. Gastroenterol. 2018;24:3472–3487. doi: 10.3748/wjg.v24.i31.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nisha K.J., Nandakumar K., Shenoy K.T., Janam P. Periodontal disease and Helicobacter pylori infection: A community-based study using serology and rapid urease test. J. Investig. Clin. Dent. 2016;7:37–45. doi: 10.1111/jicd.12122. [DOI] [PubMed] [Google Scholar]
- 35.Aksit Bıcak D., Akyuz S., Kıratlı B., Usta M., Urganci N., Alev B., Yarat A., Sahin F. The investigation of Helicobacter pylori in the dental biofilm and saliva samples of children with dyspeptic complaints. BMC Oral. Health. 2017;17:67. doi: 10.1186/s12903-017-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silva D.G., Stevens R.H., Macedo J.M., Albano R.M., Falabella M.E., Fischer R.G., Veerman E.C., Tinoco E.M. Presence of Helicobacter pylori in supragingival dental plaque of individuals with periodontal disease and upper gastric diseases. Arch. Oral. Biol. 2010;55:896–901. doi: 10.1016/j.archoralbio.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Goh K.L., Chan W.K., Shiota S., Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter. 2011;16:1–9. doi: 10.1111/j.1523-5378.2011.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garza-González E., Perez-Perez G.I., Maldonado-Garza H.J., Bosques-Padilla F.J. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J. Gastroenterol. 2014;20:1438–1449. doi: 10.3748/wjg.v20.i6.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pohl D., Keller P.M., Bordier V., Wagner K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J. Gastroenterol. 2019;25:4629–4660. doi: 10.3748/wjg.v25.i32.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baele M., Pasmans F., Flahou B., Chiers K., Ducatelle R., Haesebrouck F. Non-Helicobacter pylori helicobacters detected in the stomach of humans comprise several naturally occurring Helicobacter species in animals. FEMS Immunol. Med. Microbiol. 2009;55:306–313. doi: 10.1111/j.1574-695X.2009.00535.x. [DOI] [PubMed] [Google Scholar]
- 41.Patel S.K., Pratap C.B., Jain A.K., Gulati A.K., Nath G. Diagnosis of Helicobacter pylori: What should be the gold standard? World J. Gastroenterol. 2014;20:12847–12859. doi: 10.3748/wjg.v20.i36.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baron S., Fons M., Albrecht T. Viral pathogenesis. In: Baron S., editor. Medical Microbiology. The University of Texas Medical Branch at Galveston; Galveston, TX, USA: 1996. [PubMed] [Google Scholar]
- 43.Usta Y., Özen H. Helicobacter pylori enfeksiyonu. Çocuk Sağlığı Hast. Derg. 2007;50:136–145. [Google Scholar]
- 44.Uzunismail H. Helicobacter pylori ve Eradikasyon. Gastrointest. Sist. Hast. Sempozyumu. 2001:19–26. [Google Scholar]
- 45.Amieva M.R., El-Omar E.M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Buti L., Spooner E., Van der Veen A.G., Rappuoli R., Covacci A., Ploegh H.L. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc. Natl. Acad. Sci. USA. 2011;108:9238–9243. doi: 10.1073/pnas.1106200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alzahrani S., Lina T.T., Gonzalez J., Pinchuk I.V., Beswick E.J., Reyes V.E. Effect of Helicobacter pylori on gastric epithelial cells. World J. Gastroenterol. 2014;20:12767–12780. doi: 10.3748/wjg.v20.i36.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee I.O., Kim J.H., Choi Y.J., Pillinger M.H., Kim S.Y., Blaser M.J., Lee Y.C. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J. Biol. Chem. 2010;285:16042–16050. doi: 10.1074/jbc.M110.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chmiela M., Gonciarz W. Molecular mimicry in Helicobacter pylori infections. World J. Gastroenterol. 2017;23:3964–3977. doi: 10.3748/wjg.v23.i22.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerhard M., Lehn N., Neumayer N., Borén T., Rad R., Schepp W., Miehlke S., Classen M., Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proceedings of the National Academy of Sciences. 1999;Volume 96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vestby L.K., Grønseth T., Simm R., Nesse L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics. 2020;9:59. doi: 10.3390/antibiotics9020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kienesberger S., Cox L.M., Livanos A., Zhang X.S., Chung J., Perez-Perez G.I., Gorkiewicz G., Zechner E.L., Blaser M.J. Gastric Helicobacter pylori ınfection affects local and distant microbial populations and host responses. Cell Rep. 2016;14:1395–1407. doi: 10.1016/j.celrep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S., Shi D., Li M., Li Y., Wang X., Li W. The relationship between gastric microbiota and gastric disease. Scand. J. Gastroenterol. 2019;54:391–396. doi: 10.1080/00365521.2019.1591499. [DOI] [PubMed] [Google Scholar]
- 54.Fehlings M., Drobbe L., Moos V., Viveros P.R., Hagen J., Beigier-Bompadre M., Pang E., Belogolova E., Churin Y., Schneider T., et al. Comparative analysis of the ınteraction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect. Immun. 2012;80:2724–2734. doi: 10.1128/IAI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Naito Y., Yoshikawa T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress1, 2 1Guest Editor: Giuseppe Poli 2This article is part of a series of reviews on “Reactive Oxygen and Nitrogen in Inflammation.” The full list of papers may be found on the homepage of the journal. Free Radic. Biol. Med. 2002;33:323–336. doi: 10.1016/S0891-5849(02)00868-7. [DOI] [PubMed] [Google Scholar]
- 56.Guo T., Qian J.M., Zhao Y.Q., Li X.B., Zhang J.Z. Effects of IL-1β on the proliferation and apoptosis of gastric epithelial cells and acid secretion from isolated rabbit parietal cells. Mol. Med. Rep. 2013;7:299–305. doi: 10.3892/mmr.2012.1165. [DOI] [PubMed] [Google Scholar]
- 57.Fazeli Z., Alebouyeh M., Rezaei Tavirani M., Azimirad M., Yadegar A. Helicobacter pylori CagA induced interleukin-8 secretion in gastric epithelial cells. Gastroenterol. Hepatol. Bed Bench. 2016;9:S42–S46. [PMC free article] [PubMed] [Google Scholar]
- 58.Davies G.R., Simmonds N.J., Stevens T.R., Sheaff M.T., Banatvala N., Laurenson I.F., Blake D.R., Rampton D.S. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snezhkina A.V., Kudryavtseva A.V., Kardymon O.L., Savvateeva M.V., Melnikova N.V., Krasnov G.S., Dmitriev A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Longev. 2019;2019:6175804. doi: 10.1155/2019/6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watari J., Chen N., Amenta P.S., Fukui H., Oshima T., Tomita T., Miwa H., Lim K.J., Das K.M. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J. Gastroenterol. 2014;20:5461–5473. doi: 10.3748/wjg.v20.i18.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugano K., Tack J., Kuipers E.J., Graham D.Y., El-Omar E.M., Miura S., Haruma K., Asaka M., Uemura N., Malfertheiner P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yao X., Smolka A.J. Gastric parietal cell physiology and Helicobacter pylori-ınduced disease. Gastroenterology. 2019;156:2158–2173. doi: 10.1053/j.gastro.2019.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaser M.J., Atherton J.C. Helicobacter pylori persistence: Biology and disease. J. Clin. Invest. 2004;113:321–333. doi: 10.1172/JCI20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Atherton J.C. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu. Rev. Pathol. 2006;1:63–96. doi: 10.1146/annurev.pathol.1.110304.100125. [DOI] [PubMed] [Google Scholar]
- 65.Choi J., Yoon S.H., Kim J.E., Rhee K.H., Youn H.S., Chung M.H. Gene-specific oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Int. J. Cancer. 2002;99:485–490. doi: 10.1002/ijc.10366. [DOI] [PubMed] [Google Scholar]
- 66.Inoue I., Kato J., Tamai H., Iguchi M., Maekita T., Yoshimura N., Ichinose M. Helicobacter pylori-related chronic gastritis as a risk factor for colonic neoplasms. World J. Gastroenterol. 2014;20:1485–1492. doi: 10.3748/wjg.v20.i6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 68.Polk D.B., Peek R.M., J. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bravo D., Hoare A., Soto C., Valenzuela M.A., Quest A.F. Helicobacter pylori in human health and disease: Mechanisms for local gastric and systemic effects. World J. Gastroenterol. 2018;24:3071–3089. doi: 10.3748/wjg.v24.i28.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsugane S. Salt, salted food intake, and risk of gastric cancer: Epidemiologic evidence. Cancer Sci. 2005;96:1–6. doi: 10.1111/j.1349-7006.2005.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ge S., Feng X., Shen L., Wei Z., Zhu Q., Sun J. Association between habitual dietary salt ıntake and risk of gastric cancer: A systematic review of observational studies. Gastroenterol. Res. Pract. 2012;2012:808120. doi: 10.1155/2012/808120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Loh J.T., Torres V.J., Cover T.L. Regulation of Helicobacter pylori cagA expression in response to salt. Cancer Res. 2007;67:4709–4715. doi: 10.1158/0008-5472.CAN-06-4746. [DOI] [PubMed] [Google Scholar]
- 73.Fukase K., Kato M., Kikuchi S., Inoue K., Uemura N., Okamoto S., Terao S., Amagai K., Hayashi S., Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet. 2008;372:392–397. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 74.Yoon S.B., Park J.M., Lim C.H., Cho Y.K., Choi M.G. Effect of Helicobacter pylori eradication on metachronous gastric cancer after endoscopic resection of gastric tumors: A meta-analysis. Helicobacter. 2014;19:243–248. doi: 10.1111/hel.12146. [DOI] [PubMed] [Google Scholar]
- 75.Kavitt R.T., Lipowska A.M., Anyane-Yeboa A., Gralnek I.M. Diagnosis and treatment of peptic ulcer disease. Am. J. Med. 2019;132:447–456. doi: 10.1016/j.amjmed.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 76.Ramakrishnan K., Salinas R.C. Peptic ulcer disease. Am. Fam. Physician. 2007;76:1005–1012. [PubMed] [Google Scholar]
- 77.Yeo S.H., Yang C.H. Peptic ulcer disease associated with Helicobacter pylori ınfection. Korean J. Gastroenterol. 2016;67:289–299. doi: 10.4166/kjg.2016.67.6.289. [DOI] [PubMed] [Google Scholar]
- 78.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the maastricht V/florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 79.Malfertheiner P. The intriguing relationship of Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig. Dis. 2011;29:459–464. doi: 10.1159/000332213. [DOI] [PubMed] [Google Scholar]
- 80.Arkkila P.E., Seppälä K., Kosunen T.U., Haapiainen R., Kivilaakso E., Sipponen P., Mäkinen J., Nuutinen H., Rautelin H., Färkkilä M.A. Eradication of Helicobacter pylori improves the healing rate and reduces the relapse rate of nonbleeding ulcers in patients with bleeding peptic ulcer. Am. J. Gastroenterol. 2003;98:2149–2156. doi: 10.1111/j.1572-0241.2003.07682.x. [DOI] [PubMed] [Google Scholar]
- 81.Imaizumi H., Koizumi W., Nakai H., Tanabe S., Ohida M., Saigenji K. Effects of Helicobacter pylori eradication therapy on the healing process of peptic ulcers. Nihon Rinsho. Jpn. J. Clin. Med. 1999;57:167–172. [PubMed] [Google Scholar]
- 82.Seo S.I., Kim S.J., Kim H.S., Shin W.G., Kim K.H., Jang M.K., Lee J.H., Kim H.Y. Is there any difference in the eradication rate of Helicobacter pylori ınfection According to the endoscopic stage of peptic ulcer disease? Helicobacter. 2015;20:424–430. doi: 10.1111/hel.12221. [DOI] [PubMed] [Google Scholar]
- 83.Bagheri N., Shirzad H., Elahi S., Azadegan-Dehkordi F., Rahimian G., Shafigh M., Rashidii R., Sarafnejad A., Rafieian-Kopaei M., Faridani R., et al. Downregulated regulatory T cell function is associated with increased peptic ulcer in Helicobacter pylori-infection. Microb. Pathog. 2017;110:165–175. doi: 10.1016/j.micpath.2017.06.040. [DOI] [PubMed] [Google Scholar]
- 84.Flores S.E., Aitchison A., Day A.S., Keenan J.I. Helicobacter pylori infection perturbs iron homeostasis in gastric epithelial cells. PLoS ONE. 2017;12:e0184026. doi: 10.1371/journal.pone.0184026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kato S., Osaki T., Kamiya S., Zhang X.S., Blaser M.J. Helicobacter pylori sabA gene is associated with iron deficiency anemia in childhood and adolescence. PLoS ONE. 2017;12:e0184046. doi: 10.1371/journal.pone.0184046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rockey D.C., Altayar O., Falck-Ytter Y., Kalmaz D. AGA technical review on gastrointestinal evaluation of ıron deficiency Anemia. Gastroenterology. 2020;159:1097–1119. doi: 10.1053/j.gastro.2020.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sipponen P., Laxén F., Huotari K., Härkönen M. Prevalence of low vitamin B12 and high homocysteine in serum in an elderly male population: Association with atrophic gastritis and Helicobacter pylori infection. Scand. J. Gastroenterol. 2003;38:1209–1216. doi: 10.1080/00365520310007224. [DOI] [PubMed] [Google Scholar]
- 88.Chen L.W., Chien C.Y., Yang K.J., Kuo S.F., Chen C.H., Chien R.N. Helicobacter pylori ınfection ıncreases ınsulin resistance and metabolic syndrome in residents younger than 50 years old: A community-based study. PLoS ONE. 2015;10:e0128671. doi: 10.1371/journal.pone.0128671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kern P.A., Di Gregorio G.B., Lu T., Rassouli N., Ranganathan G. Adiponectin expression from human adipose tissue: Relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 90.Maeda N., Shimomura I., Kishida K., Nishizawa H., Matsuda M., Nagaretani H., Furuyama N., Kondo H., Takahashi M., Arita Y., et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 91.Mansori K., Moradi Y., Naderpour S., Rashti R., Moghaddam A.B., Saed L., Mohammadi H. Helicobacter pylori infection as a risk factor for diabetes: A meta-analysis of case-control studies. BMC Gastroenterol. 2020;20:77. doi: 10.1186/s12876-020-01223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang J.C., Lu C.W., Lin C.J. Treatment of Helicobacter pylori infection: Current status and future concepts. World J. Gastroenterol. 2014;20:5283–5293. doi: 10.3748/wjg.v20.i18.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Flores-Treviño S., Mendoza-Olazarán S., Bocanegra-Ibarias P., Maldonado-Garza H.J., Garza-González E. Helicobacter pylori drug resistance: Therapy changes and challenges. Expert Rev. Gastroenterol. Hepatol. 2018;12:819–827. doi: 10.1080/17474124.2018.1496017. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen C.T., Davis K.A., Nisly S.A., Li J. Treatment of Helicobacter pylori in special patient populations. Pharmacotherapy. 2019;39:1012–1022. doi: 10.1002/phar.2318. [DOI] [PubMed] [Google Scholar]
- 95.Suzuki H., Nishizawa T., Hibi T. Helicobacter pylori eradication therapy. Future Microbiol. 2010;5:639–648. doi: 10.2217/fmb.10.25. [DOI] [PubMed] [Google Scholar]
- 96.Fischbach W., Malfertheiner P., Hoffmann J.C., Bolten W., Kist M., Koletzko S. Helicobacter Pylori and gastroduodenal ulcer disease. Dtsch Arztebl Int. 2009;106:801–808. doi: 10.3238/arztebl.2009.0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haastrup P.F., Thompson W., Søndergaard J., Jarbøl D.E. Side effects of long-term proton pump ınhibitor use: A review. Basic Clin. Pharmacol. Toxicol. 2018;123:114–121. doi: 10.1111/bcpt.13023. [DOI] [PubMed] [Google Scholar]
- 98.(FDA), F.a.D.A. FDA Drug Safety Communication: Low Magnesium Levels Can be Associated with Long-Term Use of Proton Pump Inhibitor Drugs (PPIs) [(accessed on 12 July 2021)]; Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-low-magnesium-levels-can-be-associated-long-term-use-proton-pump.
- 99.Budzyński J., Kłopocka M. Brain-gut axis in the pathogenesis of Helicobacter pylori infection. World J. Gastroenterol. 2014;20:5212–5225. doi: 10.3748/wjg.v20.i18.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aimasso U., D’Onofrio V., D’Eusebio C., Devecchi A., Pira C., Merlo F.D., De Francesco A. Helicobacter pylori and nutrition: A bidirectional communication. Minerva Gastroenterol. Dietol. 2019;65:116–129. doi: 10.23736/S1121-421X.19.02568-6. [DOI] [PubMed] [Google Scholar]
- 101.Nweneka C.V., Prentice A.M. Helicobacter pylori infection and circulating ghrelin levels—a systematic review. BMC Gastroenterol. 2011;11:7. doi: 10.1186/1471-230X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jeong M., Park J.M., Han Y.M., Park K.Y., Lee D.H., Yoo J.H., Cho J.Y., Hahm K.B. Dietary prevention of Helicobacter pylori-associated gastric cancer with kimchi. Oncotarget. 2015;6:29513–29526. doi: 10.18632/oncotarget.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee S.A., Kang D., Shim K.N., Choe J.W., Hong W.S., Choi H. Effect of diet and Helicobacter pylori infection to the risk of early gastric cancer. J. Epidemiol. 2003;13:162–168. doi: 10.2188/jea.13.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tsugane S., Sasazuki S. Diet and the risk of gastric cancer: Review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 105.Loh J.T., Friedman D.B., Piazuelo M.B., Bravo L.E., Wilson K.T., Peek R.M., Jr., Correa P., Cover T.L. Analysis of Helicobacter pylori cagA promoter elements required for salt-induced upregulation of CagA expression. Infect. Immun. 2012;80:3094–3106. doi: 10.1128/IAI.00232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gancz H., Jones K.R., Merrell D.S. Sodium chloride affects Helicobacter pylori growth and gene expression. J. Bacteriol. 2008;190:4100–4105. doi: 10.1128/JB.01728-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gaddy J.A., Radin J.N., Loh J.T., Zhang F., Washington M.K., Peek R.M., Jr., Algood H.M., Cover T.L. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect. Immun. 2013;81:2258–2267. doi: 10.1128/IAI.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Caston R.R., Loh J.T., Voss B.J., McDonald W.H., Scholz M.B., McClain M.S., Cover T.L. Effect of environmental salt concentration on the Helicobacter pylori exoproteome. J. Proteomics. 2019;202:103374. doi: 10.1016/j.jprot.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ge R., Sun X. Iron trafficking system in Helicobacter pylori. Biometals. 2012;25:247–258. doi: 10.1007/s10534-011-9512-8. [DOI] [PubMed] [Google Scholar]
- 110.Frawley E.R., Fang F.C. The ins and outs of bacterial iron metabolism. Mol. Microbiol. 2014;93:609–616. doi: 10.1111/mmi.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Z. Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Ageing Med. 2019;2:82–87. doi: 10.1002/agm2.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Merrell D.S., Thompson L.J., Kim C.C., Mitchell H., Tompkins L.S., Lee A., Falkow S. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect. Immun. 2003;71:6510–6525. doi: 10.1128/IAI.71.11.6510-6525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barabino A. Helicobacter pylori-related iron deficiency anemia: A review. Helicobacter. 2002;7:71–75. doi: 10.1046/j.1083-4389.2002.00073.x. [DOI] [PubMed] [Google Scholar]
- 114.Boyanova L. Role of Helicobacter pylori virulence factors for iron acquisition from gastric epithelial cells of the host and impact on bacterial colonization. Future Microbiol. 2011;6:843–846. doi: 10.2217/fmb.11.75. [DOI] [PubMed] [Google Scholar]
- 115.Noto J.M., Gaddy J.A., Lee J.Y., Piazuelo M.B., Friedman D.B., Colvin D.C., Romero-Gallo J., Suarez G., Loh J., Slaughter J.C., et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J. Clin. Invest. 2013;123:479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cover T.L., Peek R.M., J. Diet, microbial virulence, and Helicobacter pylori-induced gastric cancer. Gut Microbes. 2013;4:482–493. doi: 10.4161/gmic.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mei H., Tu H. Vitamin C and Helicobacter pylori ınfection: Current knowledge and future prospects. Front. Physiol. 2018;9:1103. doi: 10.3389/fphys.2018.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Padayatty S.J., Levine M. Vitamin C: The known and the unknown and Goldilocks. Oral. Dis. 2016;22:463–493. doi: 10.1111/odi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Z.W., Farthing M.J. The roles of vitamin C in Helicobacter pylori associated gastric carcinogenesis. Chin. J. Dig. Dis. 2005;6:53–58. doi: 10.1111/j.1443-9573.2005.00194.x. [DOI] [PubMed] [Google Scholar]
- 120.Ødum L., Andersen L.P. Investigation of Helicobacter pylori ascorbic acid oxidating activity. FEMS Immunol. Med. Microbiol. 1995;10:289–294. doi: 10.1111/j.1574-695X.1995.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 121.Woodward M., Tunstall-Pedoe H., McColl K. Helicobacter pylori infection reduces systemic availability of dietary vitamin C. Eur. J. Gastroenterol. Hepatol. 2001;13:233–237. doi: 10.1097/00042737-200103000-00003. [DOI] [PubMed] [Google Scholar]
- 122.Tari A., Kitadai Y., Sumii M., Sasaki A., Tani H., Tanaka S., Chayama K. Basis of decreased risk of gastric cancer in severe atrophic gastritis with eradication of Helicobacter pylori. Dig. Dis. Sci. 2007;52:232–239. doi: 10.1007/s10620-006-9411-y. [DOI] [PubMed] [Google Scholar]
- 123.Everett S.M., Singh R., Leuratti C., White K.L.M., Neville P., Greenwood D., Marnett L.J., Schorah C.J., Forman D., Shuker D., et al. Levels of Malondialdehyde-Deoxyguanosine in the Gastric Mucosa. Cancer Epidemiol. Prev. Biomark. 2001;10:369–376. [PubMed] [Google Scholar]
- 124.Lam T.K., Freedman N.D., Fan J.-H., Qiao Y.-L., Dawsey S.M., Taylor P.R., Abnet C.C. Prediagnostic plasma vitamin C and risk of gastric adenocarcinoma and esophageal squamous cell carcinoma in a Chinese population. Am. J. Clin. Nutr. 2013;98:1289–1297. doi: 10.3945/ajcn.113.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waring A.J., Drake I.M., Schorah C.J., White K.L., Lynch D.A., Axon A.T., Dixon M.F. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: Effects of gastritis and oral supplementation. Gut. 1996;38:171–176. doi: 10.1136/gut.38.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zojaji H., Talaie R., Mirsattari D., Haghazali M., Molaei M., Mohsenian N., Derakhshan F., Zali M.R. The efficacy of Helicobacter pylori eradication regimen with and without vitamin C supplementation. Dig. Liver Dis. 2009;41:644–647. doi: 10.1016/j.dld.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 127.Kamiji M.M., Oliveira R.B. Effect of vitamin C administration on gastric colonization by Helicobacter pylori. Arq. Gastroenterol. 2005;42:167–172. doi: 10.1590/S0004-28032005000300008. [DOI] [PubMed] [Google Scholar]
- 128.Podmore I.D., Griffiths H.R., Herbert K.E., Mistry N., Mistry P., Lunec J. Vitamin C exhibits pro-oxidant properties. Nature. 1998;392:559. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 129.Pal J., Sanal M.G., Gopal G.J. Vitamin-C as anti-Helicobacter pylori agent: More prophylactic than curative-Critical review. Indian J. Pharmacol. 2011;43:624–627. doi: 10.4103/0253-7613.89814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jakszyn P., Agudo A., Lujan-Barroso L., Bueno-de-Mesquita H.B., Jenab M., Navarro C., Palli D., Boeing H., Manjer J., Numans M.E., et al. Dietary intake of heme iron and risk of gastric cancer in the European prospective investigation into cancer and nutrition study. Int. J. Cancer. 2012;130:2654–2663. doi: 10.1002/ijc.26263. [DOI] [PubMed] [Google Scholar]
- 131.Jakszyn P., Bingham S., Pera G., Agudo A., Luben R., Welch A., Boeing H., Del Giudice G., Palli D., Saieva C., et al. Endogenous versus exogenous exposure to N-nitroso compounds and gastric cancer risk in the European prospective ınvestigation into cancer and nutrition (EPIC-EURGAST) study. Carcinogenesis. 2006;27:1497–1501. doi: 10.1093/carcin/bgl019. [DOI] [PubMed] [Google Scholar]
- 132.Kanner J., Selhub J., Shpaizer A., Rabkin B., Shacham I., Tirosh O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017;12:929–936. doi: 10.1016/j.redox.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ávila F., Theoduloz C., López-Alarcón C., Dorta E., Schmeda-Hirschmann G. Cytoprotective Mechanisms Mediated by Polyphenols from Chilean Native Berries against Free Radical-Induced Damage on AGS Cells. Oxidative Med. Cell. Longev. 2017;2017:9808520. doi: 10.1155/2017/9808520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cha B., Lim J.W., Kim K.H., Kim H. HSP90beta interacts with Rac1 to activate NADPH oxidase in Helicobacter pylori-infected gastric epithelial cells. Int. J. Biochem. Cell Biol. 2010;42:1455–1461. doi: 10.1016/j.biocel.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 135.Schmalstig A.A., Benoit S.L., Misra S.K., Sharp J.S., Maier R.J. Noncatalytic antioxidant role for Helicobacter pylori urease. J. Bacteriol. 2018;200 doi: 10.1128/JB.00124-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kidane D. Molecular mechanisms of H. pylori-Induced DNA double-strand breaks. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19102891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Poplawski T., Chojnacki C., Czubatka A., Klupinska G., Chojnacki J., Blasiak J. Helicobacter pylori infection and antioxidants can modulate the genotoxic effects of heterocyclic amines in gastric mucosa cells. Mol. Biol. Rep. 2013;40:5205–5212. doi: 10.1007/s11033-013-2622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nardone G., Staibano S., Rocco A., Mezza E., D’Armiento F.P., Insabato L., Coppola A., Salvatore G., Lucariello A., Figura N., et al. Effect of Helicobacter pylori infection and its eradication on cell proliferation, DNA status, and oncogene expression in patients with chronic gastritis. Gut. 1999;44:789–799. doi: 10.1136/gut.44.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wei J., Nagy T.A., Vilgelm A., Zaika E., Ogden S.R., Romero-Gallo J., Piazuelo M.B., Correa P., Washington M.K., El-Rifai W., et al. Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells. Gastroenterology. 2010;139:1333–1343. doi: 10.1053/j.gastro.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jeong J.H., Jeong H.R., Jo Y.N., Kim H.J., Shin J.H., Heo H.J. Ameliorating effects of aged garlic extracts against Aβ-induced neurotoxicity and cognitive impairment. BMC Complementary Altern. Med. 2013;13:268. doi: 10.1186/1472-6882-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chung J.G., Chen G.W., Wu L.T., Chang H.L., Lin J.G., Yeh C.C., Wang T.F. Effects of garlic compounds diallyl sulfide and diallyl disulfide on arylamine N-acetyltransferase activity in strains of Helicobacter pylori from peptic ulcer patients. Am. J. Chin. Med. 1998;26:353–364. doi: 10.1142/S0192415X98000397. [DOI] [PubMed] [Google Scholar]
- 142.Zardast M., Namakin K., Esmaelian Kaho J., Hashemi S.S. Assessment of antibacterial effect of garlic in patients infected with Helicobacter pylori using urease breath test. Avicenna J. Phytomed. 2016;6:495–501. [PMC free article] [PubMed] [Google Scholar]
- 143.Ghobeh M., Shaker Hosseini R., Navai L., Mir Sattari D., Rashid Khani B., Fahmideh Norouzi M. Study of the role of garlic consumption in Helicobacter pylori eradication. J. Shahid Sadoughi Univ. Med Sci. 2010;18:337–347. [Google Scholar]
- 144.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 145.Hatcher H., Planalp R., Cho J., Torti F.M., Torti S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Judaki A., Rahmani A., Feizi J., Asadollahi K., Hafezi Ahmadi M.R. Curcumın ın combınatıon wıth trıple therapy regımes amelıorates oxıdatıve stress and hıstopathologıc changes ın chronıc gastrıtıs-assocıated Helıcobacter pylorı ınfectıon. Arq. Gastroenterol. 2017;54:177–182. doi: 10.1590/s0004-2803.201700000-18. [DOI] [PubMed] [Google Scholar]
- 147.Grabowska M., Wawrzyniak D., Rolle K., Chomczyński P., Oziewicz S., Jurga S., Barciszewski J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019;10:3090–3102. doi: 10.1039/C9FO00580C. [DOI] [PubMed] [Google Scholar]
- 148.Helt C.E., Cliby W.A., Keng P.C., Bambara R.A., O’Reilly M.A. Ataxia telangiectasia mutated (ATM) and ATM and Rad3-related protein exhibit selective target specificities in response to different forms of DNA damage. J. Biol. Chem. 2005;280:1186–1192. doi: 10.1074/jbc.M410873200. [DOI] [PubMed] [Google Scholar]
- 149.Jang S.H., Lim J.W., Morio T., Kim H. Lycopene inhibits Helicobacter pylori-induced ATM/ATR-dependent DNA damage response in gastric epithelial AGS cells. Free Radic. Biol. Med. 2012;52:607–615. doi: 10.1016/j.freeradbiomed.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 150.Shidfar F., Agah S., Ekhlasi G., Salehpour A., Ghourchian S. Lycopene an adjunctive therapy for Helicobacter pylori eradication: A quasi-control trial. J. Complementary Integr. Med. 2012;9:14. doi: 10.1515/1553-3840.1588. [DOI] [PubMed] [Google Scholar]
- 151.Yanagawa Y., Yamamoto Y., Hara Y., Shimamura T. A combination effect of epigallocatechin gallate, a major compound of green tea catechins, with antibiotics on Helicobacter pylori growth in vitro. Curr. Microbiol. 2003;47:244–249. doi: 10.1007/s00284-002-3956-6. [DOI] [PubMed] [Google Scholar]
- 152.Yanaka A. Sulforaphane enhances protection and repair of gastric mucosa against oxidative stress in vitro, and demonstrates anti-inflammatory effects on Helicobacter pylori-infected gastric mucosae in mice and human subjects. Curr. Pharm. Des. 2011;17:1532–1540. doi: 10.2174/138161211796196945. [DOI] [PubMed] [Google Scholar]
- 153.Chapa-Oliver A.M., Mejía-Teniente L. Capsaicin: From plants to a cancer-suppressing agent. Molecules. 2016;21:931. doi: 10.3390/molecules21080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sharma S.K., Vij A.S., Sharma M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013;720:55–62. doi: 10.1016/j.ejphar.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 155.Jones N.L., Shabib S., Sherman P.M. Capsaicin as an inhibitor of the growth of the gastric pathogen Helicobacter pylori. FEMS Microbiol. Lett. 1997;146:223–227. doi: 10.1111/j.1574-6968.1997.tb10197.x. [DOI] [PubMed] [Google Scholar]
- 156.Lee I.O., Lee K.H., Pyo J.H., Kim J.H., Choi Y.J., Lee Y.C. Anti-inflammatory effect of capsaicin in Helicobacter pylori-infected gastric epithelial cells. Helicobacter. 2007;12:510–517. doi: 10.1111/j.1523-5378.2007.00521.x. [DOI] [PubMed] [Google Scholar]
- 157.Mereles D., Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011;12:5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lee K.M., Yeo M., Choue J.S., Jin J.H., Park S.J., Cheong J.Y., Lee K.J., Kim J.H., Hahm K.B. Protective mechanism of epigallocatechin-3-gallate against Helicobacter pylori-induced gastric epithelial cytotoxicity via the blockage of TLR-4 signaling. Helicobacter. 2004;9:632–642. doi: 10.1111/j.1083-4389.2004.00281.x. [DOI] [PubMed] [Google Scholar]
- 159.Fahey J.W., Talalay P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999;37:973–979. doi: 10.1016/S0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 160.Fahey J.W., Haristoy X., Dolan P.M., Kensler T.W., Scholtus I., Stephenson K.K., Talalay P., Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yanaka A. Role of sulforaphane in protection of gastrointestinal tract against, H. pylori and NSAID-ınduced oxidative stress. Curr. Pharm. Des. 2017;23:4066–4075. doi: 10.2174/1381612823666170207103943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yanaka A., Fahey J.W., Fukumoto A., Nakayama M., Inoue S., Zhang S., Tauchi M., Suzuki H., Hyodo I., Yamamoto M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. 2009;2:353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 163.Toh J.W.T., Wilson R.B. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and ınteractions with antioxidant systems, vitamin C and phytochemicals. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Scanlan R.A. Formation and occurrence of nitrosamines in food. Cancer Res. 1983;43:2435s–2440s. [PubMed] [Google Scholar]
- 165.Cavalcoli F., Zilli A., Conte D., Massironi S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017;23:563–572. doi: 10.3748/wjg.v23.i4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shapiro K.B., Hotchkiss J.H. Induction of nitric oxide synthesis in murine macrophages by Helicobacter pylori. Cancer Lett. 1996;102:49–56. doi: 10.1016/0304-3835(96)04154-7. [DOI] [PubMed] [Google Scholar]
- 167.Liu R.H., Hotchkiss J.H. Potential genotoxicity of chronically elevated nitric oxide: A review. Mutat. Res. 1995;339:73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 168.Jakszyn P., Gonzalez C.A. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J. Gastroenterol. 2006;12:4296–4303. doi: 10.3748/wjg.v12.i27.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.González C.A., López-Carrillo L. Helicobacter pylori, nutrition and smoking interactions: Their impact in gastric carcinogenesis. Scand. J. Gastroenterol. 2010;45:6–14. doi: 10.3109/00365520903401959. [DOI] [PubMed] [Google Scholar]
- 170.Bagchi T. Traditional food & modern lifestyle: Impact of probiotics. Indian J. Med. Res. 2014;140:333–335. [PMC free article] [PubMed] [Google Scholar]
- 171.Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M., Sanders M.E., Shamir R., Swann J.R., Szajewska H., et al. The ınternational scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saxelin M., Tynkkynen S., Mattila-Sandholm T., de Vos W.M. Probiotic and other functional microbes: From markets to mechanisms. Curr. Opin. Biotechnol. 2005;16:204–211. doi: 10.1016/j.copbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 173.Zou J., Dong J., Yu X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter. 2009;14:97–107. doi: 10.1111/j.1523-5378.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 174.Fang H.R., Zhang G.Q., Cheng J.Y., Li Z.Y. Efficacy of Lactobacillus-supplemented triple therapy for Helicobacter pylori infection in children: A meta-analysis of randomized controlled trials. Eur. J. Pediatr. 2019;178:7–16. doi: 10.1007/s00431-018-3282-z. [DOI] [PubMed] [Google Scholar]
- 175.Szajewska H., Horvath A., Piwowarczyk A. Meta-analysis: The effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment. Pharmacol. Ther. 2010;32:1069–1079. doi: 10.1111/j.1365-2036.2010.04457.x. [DOI] [PubMed] [Google Scholar]
- 176.Zhou B.G., Chen L.X., Li B., Wan L.Y., Ai Y.W. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis. Helicobacter. 2019;24:e12651. doi: 10.1111/hel.12651. [DOI] [PubMed] [Google Scholar]