Abstract
Recent studies have suggested that human RNA helicase, DDX3X, is important for DNA repair, but little is known about the nuclear activity of this protein. In vitro analysis of nuclear DDX3X interactions and localization with DNA damage pointed to a direct role for DDX3X in the DNA damage response. We aimed to investigate whether DDX3X plays a direct role in the DNA damage response in live cells. In order to track nuclear DDX3X, we generated a nuclear-export deficient DDX3X mutant construct and performed microirradiation in live cells. We found that DDX3X accumulates at sites of microirradiation shortly after DNA damage induction. We further found DDX3X recruitment to be mediated by its intrinsically disordered domains, similar to other RNA binding proteins that are recruited to sites of DNA damage. Inhibition of liquid-liquid phase separation also reduced DDX3X recruitment. CRISPR/Cas9-mediated knockout of PARP1 ablated DDX3X recruitment, which was restored upon transgenic expression of wild-type PARP1 but not catalytically inactive PARP1, suggesting that DDX3X recruitment is PARP1-dependent.
Keywords: DDX3X, DNA damage, RNA helicase
1. Introduction
DDX3X encodes an ATP-dependent, DEAD-box RNA helicase that plays a ubiquitous role in RNA metabolism [1,2]. DDX3X unwinds RNA: RNA duplexes containing a single-stranded tail by local strand separation. DDX3X has well documented catalytic and non-catalytic roles in RNA metabolism and is essential for cell viability [3].
Disruption of DDX3X in the germline and in somatic cells is linked to disease. Complete loss-of-function in the germline is embryonic lethal in mouse models [4]. Hypomorphic variants in human females lead to DDX3X syndrome and are linked to intellectual disability in males, with both syndromes bearing a litany of neurological symptoms [5]. Disruption of DDX3X in somatic cells is highly associated with cancer. DDX3X is recurrently mutated in a number of malignancies, including lymphoma and medulloblastoma [6–8].
Several studies have demonstrated that disruption of DDX3X in cancer cell lines causes an accumulation of DNA breaks [9–11]. A similar phenotype is observed in embryonic murine cells and murine hepatocytes upon Ddx3x knockout [4,12]. DDX3X disruption reduces non-homologous end-joining repair of DNA double-strand breaks (DSBs) and causes radiosensitivity. Prior studies have focused on the role of DDX3X in DNA repair gene expression, but little is known about the direct relationship between nuclear DDX3X and the DNA damage response [9].
There is emerging evidence that RNA binding proteins play a direct role in the DNA damage and repair process, particularly of DSBs [13]. RNA binding proteins including NELF-E, NONO, FUS, CIRBP, hnRPUL1, RPP29, and RPP21 [14–19] play a PARP1 dependent role in DNA repair while DEAD-box RNA helicases such as DDX1 have been specifically implicated in unwinding RNA:DNA hybrids that arise in DSB repair [20]. Thus, considerable evidence suggests that RNA-binding proteins are directly involved in the DNA damage response.
We sought to investigate the hypothesis that DDX3X also plays a direct role in the DNA damage response. In particular, we investigated this hypothesis within a model of RNA-binding protein recruitment [21]. In this model, at sites of DNA damage, PARP1 PARylation triggers liquid-liquid phase separation to condense RNA-binding proteins via their intrinsically disordered domains. Here we provide evidence that DDX3X is actively recruited to sites of DNA damage in live cells, thereby suggesting a direct mechanism by which DDX3X may influence DNA repair.
2. Materials &methods
2.1. Cell culture and cell line generation
HEK293 T cells (ATCC) and the U2OS-DIvA cell line (DIvA; a gift from Dr. Gäelle Legube) were cultured in Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10 % FBS, L-glutamine, and penicillin/streptomycin, with the DIvA cell line additionally cultured in the presence of 1 μg/mL puromycin. AsiSI-induced DSBs were generated by treating DIvA cells with 300 nM 4-hydroxytamoxifen (Sigma; OHT) for four hours [22]. The PARP1 knockout HEK293 T cell line was generated by transfecting (as described below) 1.6 μg pCas9-BFP, 200 ng psgPARP1–1, and 200 ng psgPARP1–3, then sorting BFP + cells on a BD FACS Aria II followed by single cell isolation in a 96-well plate. PARP1-deficient clones were screened by western blot as described below. All cell lines were regularly screened for Mycoplasma contamination using the MycoAlert assay (Lonza).
2.2. Drug treatments
Liquid-liquid phase separation was inhibited by brief 30-minute pre-treatment of cells with 3.5 % 1,6-Hexandiol (Sigma Aldrich) prior to microirradiation. Cells were treated with 20 μM Olaparib (Selleckchem) at least two hours prior to microirradiation.
2.3. Plasmid construction
Ku70 and PARP1 were PCR amplified from pEGFP-C1-FLAG-Ku70 (Addgene) and pCMV-3xFLAG-PARP1 (Addgene), respectively, then cloned into a Bpu102I/MunI digested pmiRFP670 (Addgene) backbone with Gibson assembly master mix (NEB). The pDDX3XL19A/L21A-GFP and pmiRFP670-PARP1-E988 K constructs were generated via the QuickChange II XL Kit (Agilent) as described by the manufacturer’s protocol using HPLC-purified oligos (Integrated DNA Technologies). Deletion constructs were generated from pDDX3XL19A/L21A-GFP via Gibson assembly following the manufacturer’s protocol (NEB) and expression was confirmed by western blot. Guide RNA expression vectors were constructed by annealing synthetic oligonucleotides (Integrated DNA Technologies) encoding the target sequences and inserting into the pMLM3636 vector following the depositor’s protocol (Addgene). All constructs were extracted from transformed bacteria via miniprep plasmid extraction (Qiagen) and verified by Sanger sequencing.
sgPARP1–1: CGAGTCGAGTACGCCAAG
sgPARP1–3: ACCCTGACGTTGAGGTGGAT DDX3X-L19A-L21A_F1:
GCAGTTTGCTGGCGCAGACGCGAACTCTTCAGATAATCA DDX3X-L19A-L21A_R1:
CTGATTATCTGAAGAGTTCGCGTCTGCGCCAGCAAACTG PARP1-E988K-Fwd:
ATGACACCTCTCTACTATATAACAAGTACATTGTCTATGATATTGC PARP1-E988K-Rev:
GCAATATCATAGACAATGTACTTGTTATATAGTAGAGAGGTGTCAT
2.4. Transfections
Transfections were performed using Lipofectamine LTX (Thermo Fisher) transfection reagent according to the manufacturer’s protocol with 200 ng per construct. Cells were assayed 72 h later. Transient knockdowns were performed with 50 nM SMARTpool siRNA (Dharmacon) with Dharmafect 1 transfection reagent as indicated by the manufacturer.
2.5. Western blot
Whole protein was harvested with RIPA lysis buffer (Thermo Fisher) freshly supplemented with protease inhibitors (Pierce) and boiled in 1X Laemmli buffer. Samples were run on NuPAGE 10 % Tris-bis gel in 1X MOPS-SDS running buffer. After gel electrophoresis, protein was transferred onto a nitrocellulose membrane using the iBlot transfer kit (Thermo Fisher) according to the manufacturer’s protocol. The membranes were blocked by nutating in 5% BSA in PBST for one hour at room temperature, then stained with primary antibody overnight at 4 °C. Bands were visualized with IRDye secondary antibody staining for one hour prior to imaging on a Licor Odyssey scanner at 700 nm and 800 nm.
Rabbit Anti-DDX3X (Abcam) 1: 5000
Mouse Anti-DDX3X (Abcam) 1: 1000
Mouse Anti-hnRNPK (Abcam) 1: 1000
Rabbit Anti-DDX1 (Proteintech) 1: 1000
Mouse Anti-GAPDH (Abcam) 1: 10,000
IRDye® 680 L T Goat anti-Rabbit IgG (LICOR) 1: 20,000
IRDye® 800CW Goat anti-Mouse IgG (LICOR) 1: 15,000
2.6. Nuclear Co-immunoprecipitation
Co-immunoprecipitation was performed on HEK293 T nuclear extracts with anti-DDX3X (Abcam) or polyclonal rabbit IgG (BD Biosciences) following the manufacturer’s protocol of the Nuclear Content IP kit (ActiveMotif) without vortexing. From a single lysate, nuclear protein content from 20 million cells (~ 400–500 μg protein) was incubated overnight at 4 °C in 1X low IP buffer with 4.5 μg antibody. Protein:antibody complexes were column-isolated then eluted and boiled in Laemmli buffer.
2.7. Mass spectrometry
Whole proteome quantification was performed as described by [23] by the Fred Hutchinson Cancer Research Center (FHCRC) Proteomics Core from HEK293 T cell pellets. Samples were analyzed on a ThermoScientific Orbitrap Eclipse mass spectrometer equipped with FAIMS (field asymmetric ion mobility spectrometry) configured for MS2 quantification. Proteins were identified and quantified using Proteome Discoverer v2.4. Co-immunoprecipitated protein samples were prepared as gel slices for analysis on an Orbitrap Fusion mass spectrometer. Protein spectra were annotated and filtered at a 1% false discovery rate using Proteome Discoverer v2.4. Peptide spectral matches from both experiments were normalized based on the interquartile range. Protein hits that were below statistical significance (p <0.01) and/or had less than 20 normalized peptide spectral matches (PSMs) in the anti-DDX3X samples were removed. Proteins enriched in both experiments were analyzed by STRING v11.
2.8. Immunofluorescence
Cells were grown to 50 % confluency on a poly-L-lysine coated #1.5 coverslip. In order to remove the cytoplasmic signal, cells were first permeabilized in 0.5 % Triton X-100 (Sigma Aldrich), fixed in 2% paraformaldehyde (Thermo Fisher) for 20 min, then washed once with ice-cold PBS. For RNase A/T digestion, permeabilized cells were incubated with 20 μg RNase A/T1 cocktail (Thermo Fisher) with 50 mM MgCl2 for 15 min at 37 °C prior to fixation. Coverslips were blocked in 3% BSA (PBST) for 30 min. Coverslips were stained with the indicated primary antibodies for one hour or overnight at 4 °C while nutating, then stained with the indicated secondary antibodies for one hour at room temperature. For colocalization analysis, antibodies were first applied sequentially to rule out co-staining artifacts [24]. Coverslips were incubated with DAPI (Thermo Fisher) then mounted with Gold Antifade Mountant (Thermo Fisher) on Apex slides (Leica). For knockdown cells, slides were imaged on Zeiss LSM 780 at a 14.36 um Z plane. For colocalization analysis, slides were imaged with Z-stacks at 0.2 mm intervals in the FHCRC Cellular Imaging core on the Deltavision elite microscope. Z-stacks were deconvoluted and rendered in Imaris for analysis. Double-positive pixels were gated using the colocalization function of Imaris, which then created colocalized foci (white) that were scored per nucleus.
Rabbit Anti-DDX3X (Abcam) 1: 200
Mouse Anti-yH2AX (Millipore) 1: 1000
Mouse Anti-53BP1 (Millipore) 1: 500
Donkey Anti-Mouse Cy3 (Jackson Immunoresearch) 1: 2000
Goat Anti-Rabbit AlexaFlour488 (Abcam) 1: 200
2.9. Live cell imaging
Transfected HEK293 T cells were seeded into poly-L-lysine coated 35 mm Nunc glass bottom dishes (Thermo Fisher). 72 h after transfection, cells were stained with 10 μg/mL Hoechst 33342 (Thermo Fisher) for 10 min, then cultured in phenol red-free DMEM. Using an Ultraview Spinning Disk microscope, cells were imaged (488 nm, 640 nm) every 5 s for at least 120 s and microirradiated (15 %, 405 nm) after 20 s. Only cells with detectable Ku70 recruitment were measured. Cells expressing deletion constructs at similar nuclear intensities of at least 4000 arbitrary units were selected for microirradiation. The CTerm construct was irradiated at sub-saturation intensities due to its brightness. Exported videos were analyzed on ImageJ using the ROI function to measure intensity over time relative to a non-irradiate ROI, then normalized to the intensity plateau. At least ten cells were measured per experiment. All representative images display cells 20 s pre-microirradiation (pre-IR) and 70 s post-microirradiation (post-IR), unless otherwise specified.
2.10. CUT&RUN-sequencing
We performed an in-situ chromatin profiling experiment (“CUT&RUN”; [25]) and prepared cell:bead complexes as described. Samples were prepared at a ratio of 400,000 cells per 10 μL of beads. Rabbit primary antibodies were added as indicated and incubated overnight at 4 °C while nutating:
Rabbit Anti-DDX3X (Abcam) 0.05 μg /μL
Rabbit IgG (BD Biosciences) 0.05 μg /μL
Rabbit Anti-Senataxin (Millipore) 0.05 μg /μL
Rabbit Anti-RNApol II Ser2-P (Cell Signaling) 1: 50
The antibody-conjugated, cell:bead samples were then processed via the CUT&RUN automated service (autoCUT&RUN) by the Fred Hutchinson Cancer Research Center (FHCRC) Genomics Core [25]. FASTQC and BBDuk were used for quality control of the raw FASTQ files where reads with average quality less than 30 were removed [26,27]. The remaining reads were aligned to the Saccharomyces cerevisiae spike-in genome reference, sacCer3, to determine the abundance of spike-in reads for each sample. The normalization factor was determined based on the lowest spike-in abundance among all samples. Reads were then subsampled based on the normalization factor and aligned to the human reference genome, hg38, using Bowtie2 [28]. Aligned read-pairs were coordinate sorted and the GATK MarkDuplicates utility was used to remove PCR duplicates [29,30]. Peak calling was performed using MACS2 from the processed alignments with the IgG control samples [31]. The log2 fold change of cut (four hour OHT treatment) over uncut (untreated control) and log-likelihood ratio across each indicated antibody was calculated using the bdgcmp function from MACS2. BigWig files for sample fold change and log likelihood ratio analyses were generated using Bedtools, UCSC-bedClip, and UCSC-bedGraphToBigWig [32,33]. Finally, average fold change profile plots were generated using ngs.plot and deepTools2 [34–36]. Raw reads per million mapped reads were retrieved from the AsiSI coordinates identified using BLISS [37].
3. Results and discussion
The role of DDX3X in the nucleus is largely unknown and we sought to first investigate nuclear DDX3X interactions. We confirmed the specificity of our anti-DDX3X antibody by performing an siRNA knockdown, then measuring DDX3X levels by immunofluorescence (Fig. S1A). To further confirm the specify of the anti-DDX3X antibody, we performed western blotting and mass spectrometry in parallel, and found that they were highly concordant. We found that DDX3X co-immunoprecipitated with proteins associated with RNA metabolism, DNA repair, and RNA splicing factors implicated in the DNA damage response – notably including DDX1 (Fig. S1B–C; [20]). Three-dimensional imaging of gamma-irradiated HEK293 T nuclei revealed that nuclear DDX3X foci colocalize with a subset of yH2AX and 53BP1 foci upon gamma irradiation, consistent with other RNA-binding protein /DNA repair interactions (Fig. S1D–E; [38,39]). Finally, we performed an in-situ chromatin profiling (CUT&RUN) sequencing experiment with the DIvA cell line. This U2OS cell line expresses recombinant AsiSI restricted to the cytoplasm. Upon addition of tamoxifen, AsiSI translocates to the nucleus due to the fused estrogen-responsive elements to induce DNA double-strand breaks (DSB). On average, we detected DDX3X enrichment at AsiSI sites throughout the genome upon tamoxifen treatment relative to untreated controls over the course of four hours. This is consistent with the induction of DSBs over time as AsiSI enters the nucleus ([22]; Fig. S2A–B). The DNA/RNA helicase, Senataxin, was used as a positive control for DSB induction, in view of its previous characterization in the DIvA system (Fig. S2C; [40]). Our in-situ results imply that DDX3X plays a direct role in the DNA damage response and we aimed to recapitulate this in live cells.
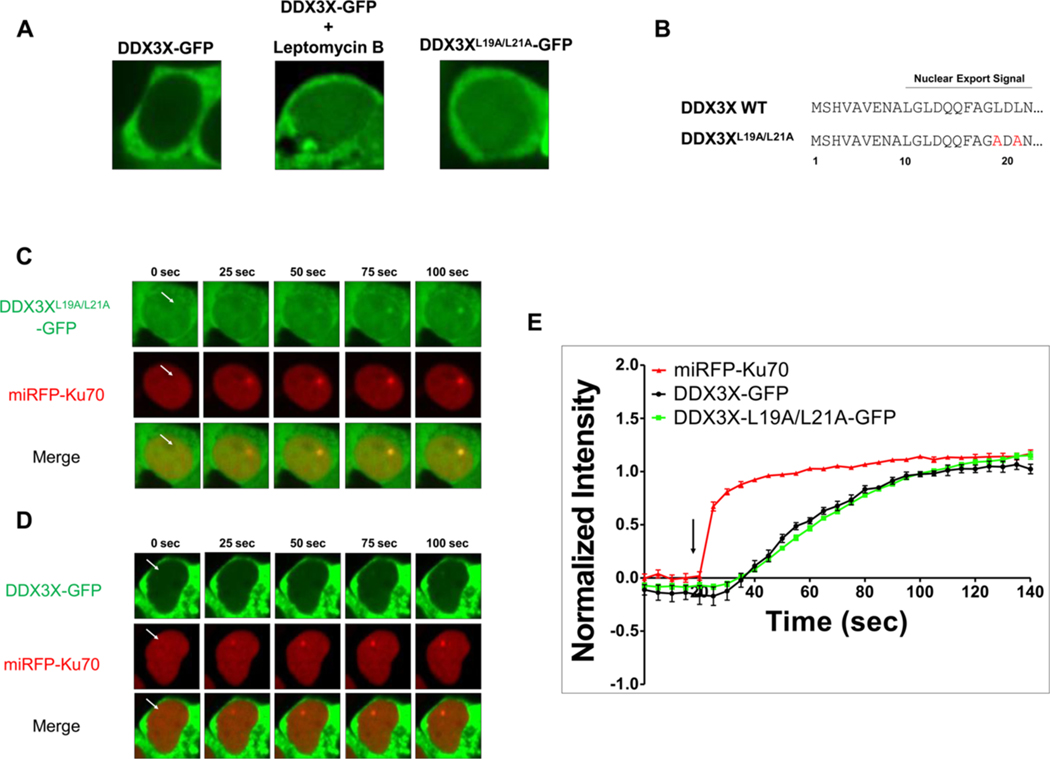
Wild-type nuclear DDX3X levels were largely below the detection limit for live-cell imaging, however, became detectable upon CRM1 inhibition by leptomycin B (Fig. 1A). Therefore, we identified a putative leucine-rich, nuclear export signal in the N-terminal domain and performed an alanine substitution at leucine 19 and leucine 21 ([41]; Fig. 1B). Consistent with prior studies suggesting that this region interacts with CRM1, we found that this construct, DDX3XL19A/L21A-GFP, demonstrated enhanced nuclear retention, which similarly allowed for consistent microscopic detection without inhibiting CRM1-dependent RNA export globally (Fig. 1A; [42]). We developed a dual-color, live cell microirradiation system in order to evaluate nuclear DDX3X spatiotemporal dynamics upon DNA damage. Ku70 was used as an internal spatiotemporal control for the induction and localization of DNA damage in all experiments. Dual fluorescence imaging upon 405 nm spot microirradiation revealed DDX3XL19A/L21A-GFP recruitment to sites of DNA damage as represented by miRFP670-Ku70. Ku70 accumulation peaks within 10 s of microirradiation (Fig. 1C). Wild-type DDX3X-GFP recruitment was also detectable in a minority of cells with sufficient nuclear levels (Fig. 1D). Both DDX3X-GFP and DDX3XL19A/L21A-GFP recruitment peaks identically at 90 s post-irradiation (Fig. 1E). The lack of delay suggests to us that the translocation of DDX3X from the cytoplasm to the nucleus is not a driving force behind recruitment. Experiments hereafter used the DDX3XL19A/L21A-GFP construct.
Fig. 1.
Generation of a DDX3X-GFP fusion construct to evaluate its recruitment to sites of DNA damage. (A) Representative images of HEK293 T cells expressing wild-type DDX3X-GFP, wild-type cells treated with 20 nM leptomycin B, and cells expressing DDX3XL19A/L21A-GFP. (B) Amino acid sequence of the putative nuclear export signal in wild-type DDX3X and alanine substitutions in the DDX3XL19A/L21A-GFP construct. (C, D) Representative time-lapse images of the accumulation of (C) nuclear export-deficient mutant DDX3XL19A/L21A-GFP (green), (D) wild-type DDX3X-GFP (green), and miRFP-Ku70 (red) after microirradiation. White arrows indicate the sites of microirradiation. (E) Quantification of the average fluorescence intensity at sites of microirradiation, normalized to the accumulation plateau for each fluorescent construct. The vertical black arrow indicates the time of microirradiation. All quantifications are from three independent experiments.
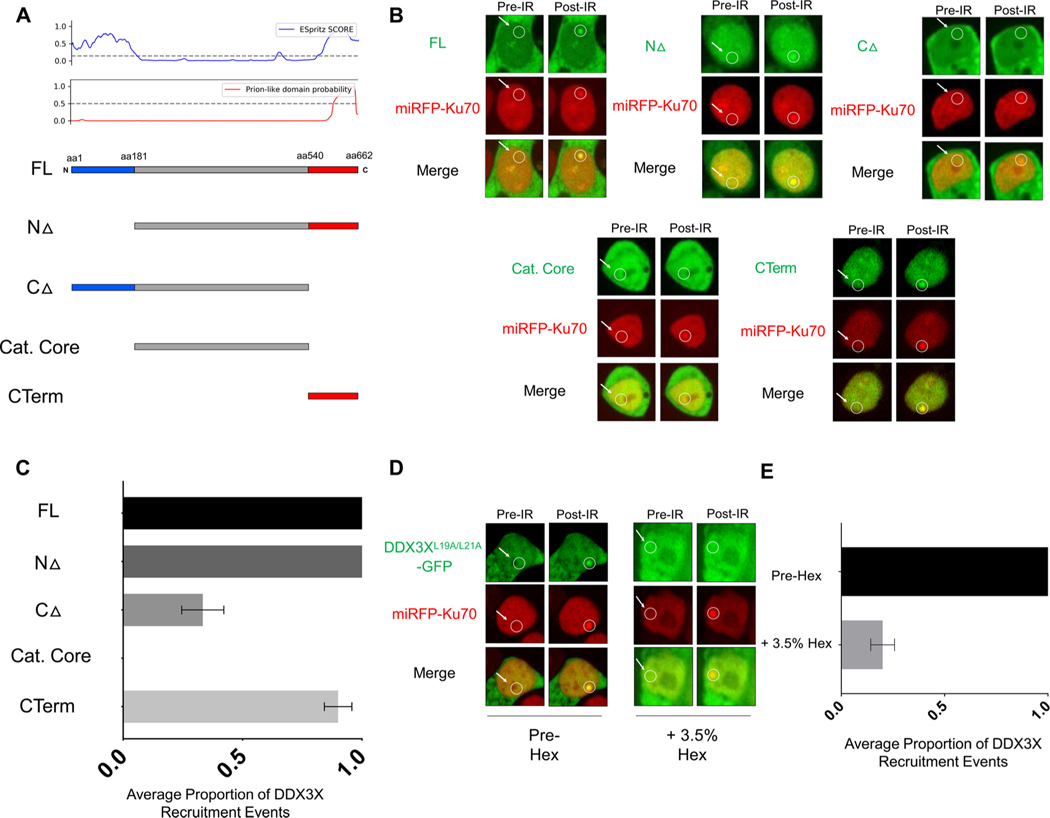
In the cytoplasm, DDX3X forms stress granules via the intrinsically disordered domains (IDDs) at the N- and C- termini, alterations of which are thought to contribute to disease pathogenesis [43]. Nuclear RNA-binding proteins similarly aggregate at sites of DNA damage [21]. We explored the hypothesis that DDX3X IDDs contribute to its recruitment activity. We identified IDDs computationally at the N and C terminus of DDX3X and defined the borders at amino acid 181 and 540 for generating deletion constructs (Fig. 2A). We microirradiated nuclei containing similar DDX3X signal intensity across the constructs. Deletion of the N-terminal domain did not affect recruitment, while deletion of the C-terminal domain partially reduced recruitment activity (Fig. 2B–C). Deletion of both of the IDDs at the N- and C-termini of DDX3X completely ablated recruitment. Furthermore, the C-terminus alone was sufficient for recruitment. This suggests that the glycine-rich, prion-like domain contributes predominately – but not exclusively - to DDX3X foci formation at sites of DNA damage. It is noteworthy that DDX3X truncation mutations found in blood cancers also delete the C-terminal domain [7]. Prion-like domains are thought to catalyze liquid-liquid phase separation [44]. DDX3X recruitment to sites of DNA damage was inhibited upon 1,6-Hexanediol addition to cells previously competent for recruitment activity (Fig. 2D–E). 1,6-Hexanediol is known to disrupt the weak molecular forces required for liquid-liquid phase separation [45]. Collectively, these results suggest that the DDX3X prion-like domain likely precipitates DDX3X recruitment via liquid-liquid phase separation.
Fig. 2.
The intrinsically disordered domains of DDX3X mediate recruitment to sites of DNA damage. (A) Schematic diagram of DDX3XL19A/L21A-GFP deletion constructs aligned with the DDX3X Espritz SCORE and prion-like domain probability (PhaSepDB) including the full-length construct (FL), N-terminal deletion up to amino acid 181 (NΔ), C-terminal deletion from amino acid 540 (CΔ), catalytic core-only construct (Cat. Core), and the C-terminal-only construct (CTerm). (B) Representative images from the live cell imaging assay upon microirradiation in HEK293 T cells expressing the DDX3X deletion construct series. (C) Quantification of the average proportion of DDX3X construct recruitment events across three independent experiments. (D) Representative images of cells before (pre-Hex) and after (+3.5 % Hex) treatment with 1,6-hexanediol. (E) Quantification of the average proportion of DDX3X recruitment events upon Hex treatment.
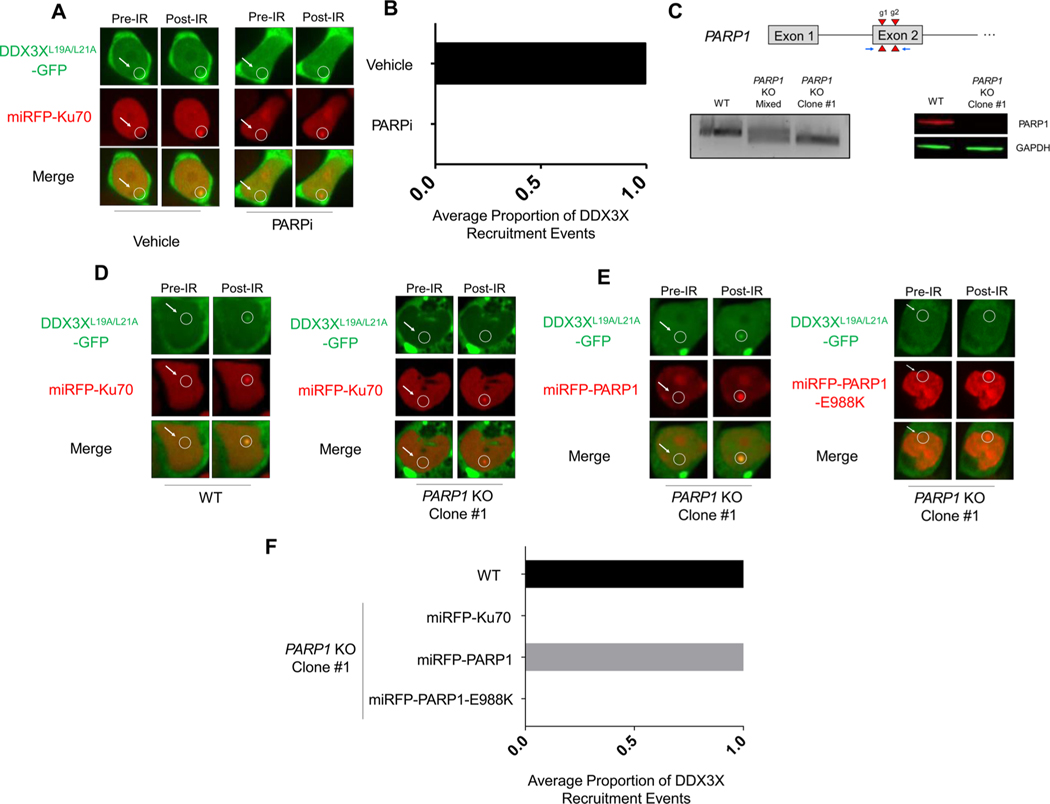
PARP1 is a DNA repair protein known to precipitate RNA-binding proteins to sites of DNA damage [21]. Inhibiting PARP1/2 catalytic activity with the inhibitor, Olaparib, ablated DDX3X recruitment to sites of DNA damage (Fig. 3A–B). To confirm the specificity of PARP1-dependent recruitment, a clonal HEK293 T PARP1 knockout cell line was generated using CRISPR/Cas9 methods (Fig. 3C). DDX3XL19A/L21A-GFP recruitment was abolished in the PARP1 knockout line and was rescued upon transient re-expression with miRFP670-PARP1 (Fig. 3D–F). Finally, re-expression of a catalytically-deficient mutant of PARP1, miRFP-PARP1-E988 K, failed to rescue DDX3X recruitment. DDX3X recruitment was still detectable upon ATM inhibition and DNA-PKcs inhibition (data not shown). Thus, DDX3X is actively recruited to sites of DNA damage in a coordinated PARP1-mediated response likely via PAR synthesis similar to other RNA-binding proteins. The results of our studies demonstrate that DDX3X is actively recruited to sites of DNA damage soon after the recruitment of Ku70 to such sites. The recruitment of DDX3X to sites of DNA damage is mediated by its intrinsically disordered domains and is dependent on PARP1 catalytic activity. These characteristics fit identically in models proposed by Kai [21], among others, for RNA-binding protein recruitment to sites of DNA damage. Collectively, these findings strongly suggest that DDX3X plays a role in the DNA damage response that encompasses more than its regulation of the expression of other genes involved in DNA damage repair.
Fig. 3.
DDX3X recruitment is PARP1-dependent. (A) Representative images of DDX3XL19A/L21A-GFP and miRFP-Ku70 accumulation after microirradiation of HEK293 T cells treated with Olaparib or vehicle. (B) Quantification of accumulation in two independent experiments. (C) Top: Schematic of the PARP1 locus showing the two guide RNA target sites (red triangles) and PCR primers (blue arrows). Bottom: On the right, PCR-based detection of the 70 bp Cas9 deletion at the PARP1 site in the mixed population (middle lane), PARP1-targeted clone (right lane), relative to the parental WT (left lane). Far right, western blot validation of PARP1-deficiency in the PARP1 KO cell line (right lane) compared to the parental WT line (left lane). (D) Representative images of DDX3XL19A/L21A-GFP and miRFP-Ku70 accumulation upon microirradiation (right column) in parental wild-type HEK293 T cells (left) and PARP1 KO cells (right). (E) Representative images of PARP1 rescue with miRFP-PARP1 (red) co-expressed with DDX3XL19A/L21A–GFP (green) upon microirradiation in the PARP1 KO cell line or with the catalytically inactive miRFP670-PARP1-E988 K mutant. The PARP1 rescue experiments were performed three times independently, as quantified in (F).
The role of RNA in the DNA damage response is an increasingly active field of research. A major DNA damage-related species of RNA are DNA:RNA hybrids, which the DDX3X interactor, DDX1, is known to regulate [20]. While we were unable to observe a dependence for DDX3X recruitment on DNA:RNA hybrid formation (data not shown), we posit that DDX3X may play other roles in DNA-damage induced RNA metabolism such as RNA:RNA unwinding and DNA:RNA hybrid generation. The use of site-specific DNA damage imaging techniques with the ability to control local transcription will greatly illuminate this potential function of DDX3X [46]. In addition, ablating the nucleic acid binding residues across DDX3X may alter recruitment and/or retention at sites of DNA damage.
Our study provides evidence for a novel mechanism for DDX3X in the DNA damage response. It is tempting to speculate that mutations known to alter DDX3X aggregation in the cytoplasm may similarly alter DDX3X accumulation at sites of DNA damage. Our work highlights the need for understanding the impact of disease-associated alterations to DDX3X on nuclear processes such as DNA repair [47]. DDX3X is believed to regulate DNA repair gene expression as well. Co-inhibition of PARP1 and DDX3X has a synergistic cytotoxic effect, suggesting that DDX3X indeed has non-PARP1-related roles in DNA repair [11]. Future work will be needed to uncouple these diverse roles in order to understand their relative contribution to DNA repair.
Supplementary Material
Acknowledgements
We thank the FHCRC Shared Resource staff for their expert technical support. We also thank Andrea Towlerton and Chris Miller for their laboratory support.
Funding
This research was supported by the Flow Cytometry, Cellular Imaging, Proteomics, and Genomics Shared Resources of the Fred Hutch / University of Washington Cancer Consortium (P30 CA015704). This research was also supported by FHCRC institutional funds and the Cancer Therapeutics Endowment. Bioinformatic analysis was supported by the Scientific Computing Infrastructure at Fred Hutch funded by ORIP grant S10OD028685.
Footnotes
Author statement
All authors listed in the revised manuscript entitled, “RNA helicase, DDX3X, is actively recruited to sites of DNA damage in live cells” participated in, and take responsibility for, the generation of the manuscript.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dnarep.2021.103137.
References
- [1].Sharma D, Putnam AA, Jankowsky E, Biochemical differences and similarities between the DEAD-Box Helicase Orthologs DDX3X and Ded1p, J. Mol. Biol 429 (2017) 3730–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Linder P, Jankowsky E, From unwinding to clamping - the DEAD box RNA helicase family, Nat. Rev. Mol. Cell Biol 12 (2011) 505–516. [DOI] [PubMed] [Google Scholar]
- [3].Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, Sabatini DM, Identification and characterization of essential genes in the human genome, Science 350 (2015) 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen CY, Chan CH, Chen CM, Tsai YS, Tsai TY, Wu Lee YH, You LR, Targeted inactivation of murine Ddx3x: essential roles of Ddx3x in placentation and embryogenesis, Hum. Mol. Genet 25 (2016) 2905–2922. [DOI] [PubMed] [Google Scholar]
- [5].Snijders Blok L, Madsen E, Juusola J, Gilissen C, Baralle D, Reijnders MR, Venselaar H, Helsmoortel C, Cho MT, Hoischen A, et al. , Mutations in DDX3X are a common cause of unexplained intellectual disability with gender-specific effects on wnt signaling, Am. J. Hum. Genet 97 (2015) 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grande BM, Gerhard DS, Jiang A, Griner NB, Abramson JS, Alexander TB, Allen H, Ayers LW, Bethony JM, Bhatia K, et al. , Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma, Blood 133 (2019) 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ojha J, Secreto CR, Rabe KG, Van Dyke DL, Kortum KM, Slager SL, Shanafelt TD, Fonseca R, Kay NE, Braggio E, Identification of recurrent truncated DDX3X mutations in chronic lymphocytic leukaemia, Br. J. Haematol 169 (2015) 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pugh TJ, Morozova O, Attiyeh EF, Asgharzadeh S, Wei JS, Auclair D, Carter SL, Cibulskis K, Hanna M, Kiezun A, et al. , The genetic landscape of high-risk neuroblastoma, Nat. Genet 45 (2013) 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bol GM, Vesuna F, Xie M, Zeng J, Aziz K, Gandhi N, Levine A, Irving A, Korz D, Tantravedi S, et al. , Targeting DDX3 with a small molecule inhibitor for lung cancer therapy, EMBO Mol. Med 7 (2015) 648–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xie M, Vesuna F, Tantravedi S, Bol GM, Heerma van Voss MR, Nugent K, Malek R, Gabrielson K, van Diest PJ, Tran PT, et al. , RK-33 radio sensitizes prostate cancer cells by blocking the RNA helicase DDX3, Cancer Res. 76 (2016) 6340–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heerma van Voss MR, Brilliant JD, Vesuna F, Bol GM, van der Wall E, vanDiest PJ, Raman V, Combination treatment using DDX3 and PARP inhibitors induces synthetic lethality in BRCA1-proficient breast cancer, Med. Oncol 34 (2017) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chan CH, Chen CM, Lee YW, You LR, DNA damage, liver injury, and tumorigenesis: consequences of DDX3X loss, Mol. Cancer Res 17 (2019) 555–566. [DOI] [PubMed] [Google Scholar]
- [13].Vågbø CB, Slupphaug G, RNA in DNA repair, DNA Repair 95 (2020) 1568–7864. [DOI] [PubMed] [Google Scholar]
- [14].Awwad SW, Abu-Zhayia ER, Guttmann-Raviv N, Ayoub N, NELF-E is recruited to DNA double-strand break sites to promote transcriptional repression and repair,EMBO Rep. 18 (2017) 745–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Krietsch J, Caron MC, Gagne JP, Ethier C, Vignard J, Vincent M, Rouleau M, Hendzel MJ, Poirier GG, Masson JY, PARP activation regulates the RNA-binding protein NONO in the DNA damage response to DNA double-strand breaks, Nucleic Acids Res. 40 (2012) 10287–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mastrocola AS, Kim SH, Trinh AT, Rodenkirch LA, Tibbetts RS, The RNA-binding protein fused in sarcoma (FUS) functions downstream of poly(ADP-ribose)polymerase (PARP) in response to DNA damage, J. Biol. Chem 288 (2013) 24731–24741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen JK, Lin WL, Chen Z, Liu HW, PARP-1-dependent recruitment of cold-inducible RNA-binding protein promotes double-strand break repair and genome stability, Proc. Natl. Acad. Sci 115 (2018) E1759–E1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hong Z, Jiang J, Ma J, Dai S, Xu T, Li H, Yasui A, The role of hnRPUL1involved in DNA damage response is related to PARP1, PLoS One 8 (2013),e60208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Abu-Zhayia ER, Khoury-Haddad H, Guttmann-Raviv N, Serruya R, Jarrous N, Ayoub N, A role of human RNase P subunits, Rpp29 and Rpp21, in homology directed-repair of double-strand breaks, Sci. Rep 7 (2017) 1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li L, Germain DR, Poon HY, Hildebrandt MR, Monckton EA, McDonald D, Hendzel MJ, Godbout R, DEAD Box 1 facilitates removal of RNA and homologous recombination at DNA double-strand breaks, Mol. Cell. Biol 36 (2016) 2794–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kai M, Roles of RNA-binding proteins in DNA damage response, Int. J. Mol. Sci 17(2016) 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Massip L, Caron P, Iacovoni JS, Trouche D, Legube G, Deciphering the chromatin landscape induced around DNA double strand breaks, Cell Cycle 9 (2010) 2963–2972. [DOI] [PubMed] [Google Scholar]
- [23].Navarrete-Perea J, Yu Q, Gygi SP, Paulo JA, Streamlined tandem mass tag (SL-TMT) protocol: an efficient strategy for quantitative (Phospho)proteome profiling using tandem mass tag-synchronous precursor selection-MS3, J. Proteome Res 17(2018) 2226–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bennett B, Bewersdorf J, Knight K, Immunofluorescence imaging of DNA damageresponse proteins, Methods (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Janssens DH, Wu SJ, Sarthy JF, Meers MP, Myers CH, Olson JM, Ahmad K, Henikoff S, Automated in situ chromatin profiling efficiently resolves cell typesand gene regulatory programs, Epigenetics Chromatin 11 (2018) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bushnell B , BBMap: A Fast, Accurate, Splice-aware Aligner (No. LBNL-7065E), Lawrence Berkeley National Lab.(LBNL), Berkeley, CA (United States), 2014. [Google Scholar]
- [27].Andrews S, FastQC: A Quality Control Tool for High Throughput Sequence Data, 2010. [Google Scholar]
- [28].Langmead B, Salzberg SL, Fast gapped-read alignment with Bowtie 2, Nat. Methods 9 (4) (2012) 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA, The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data, Genome Res. 20 (9) (2010) 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Broad Institute, Picard Tools, 2016. [Google Scholar]
- [31].Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Liu XS,Model-based analysis of ChIP-Seq (MACS), Genome biology 9 (9) (2008) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Quinlan AR, BEDTools: the Swiss- army tool for genome feature analysis, Curr. Protoc. Bioinf 47 (1) (2014) 11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kuhn RM, Haussler D, Kent WJ, The UCSC genome browser and associatedtools, Brief. Bioinf 14 (2) (2013) 144–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Loh YHE, Shen L, Analysis and visualization of ChIP-seq and RNA-seq sequence alignments using ngs. Plot. Data Mining Techniques for the Life Sciences, Humana Press, New York, NY, 2016, pp. 371–383. [DOI] [PubMed] [Google Scholar]
- [35].Ramírez F, Ryan DP, Grüning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S,Dündar F, Manke T, deepTools2: a next generation web server for deep-sequencing data analysis, Nucleic Acids Res. 44 (W1) (2016) W160–W165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shen L, Shao N, Liu X, Nestler E, ngs.plot: quick mining and visualization ofnext-generation sequencing data by integrating genomic databases, BMC Genomics 15 (2014) 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Iannelli F, Galbiati A, Capozzo I, Nguyen Q, Magnuson B, Michelini F,D’Alessandro G, Cabrini M, Roncador M, Francia S, Crosetto N, Ljungman M, Carninci P, d’Adda di Fagagna F, A damaged genome’s transcriptional landscape through multilayered expression profiling around in situ-mapped DNA double-strand breaks, Nat. Commun 8 (2017) 15656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Marin-Vicente C, Domingo-Prim J, Eberle A, Visa N, RRP6/EXOSC10 is required for the repair of DNA double-strand breaks by homologous recombination, J. Cell. Sci 6 (2015) 1097–1107. [DOI] [PubMed] [Google Scholar]
- [39].Mitra J, Guerrero E, Hedge P, Liachko N, Wang H, Velmarini V, Gao J,Pandey A, Taylor J, Kramer B, Wu P, Boldogh I, Garruto R, Mitra S, Hedge M, Motor neuron disease-associated loss of nuclear TDP-43 is linked to DNA double-strand break repair defects, PNAS 10 (2019) 4696–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cohen S, Puget N, Lin YL, Clouaire T, Aguirrebengoa M, Rocher V, Pasero P, Canitrot Y, Legube G, Senataxin resolves RNA:DNA hybrids forming at DNA double-strand breaks to prevent translocations, Nat. Commun 9 (2018) 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brennan R, Happ-Hoff A, Gi L, Gautier V, Long A, Schroder M, Investigating nucleo-cytoplasmic shuttling of the human DEAD-box helicase DDX3, Eur. J. CellBiol 97 (2018) 501. [DOI] [PubMed] [Google Scholar]
- [42].Heaton SM, Atkinson SC, Sweeney MN, Yang SNY, Jans DA, Borg NA,Exportin-1-dependent nuclear export of DEAD-box helicase DDX3X is central to its role in antiviral immunity, Cells 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Valentin-Vega YA, Wang YD, Parker M, Patmore DM, Kanagaraj A, Moore J,Rusch M, Finkelstein D, Ellison DW, Gilbertson RJ, et al. , Cancer-associatedDDX3X mutations drive stress granule assembly and impair global translation, Sci.Rep 6 (2016) 25996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Altmeyer M, Neelsen K, Teloni F, Pozdnyakova I, Pellegrino S, Grofte M, Rask MB, Streicher W, Jungmichel S, Nielsen M, Lukas J, Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose), Nat. Commun (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kroschwald S, Mahrana S, Simon A, Hexanediol: a chemical probe to investigate the material properties of membrane-less compartments, Matters (2017). [Google Scholar]
- [46].Wei L, Nakajima S, Bohm S, Bernstein KA, Shen Z, Tsangm M, Levine AS, Li Lan, DNA damage during the G0/G1 phase triggers RNA-templated, Cockayne syndrome B-dependent homologous recombination, PNAS (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pessina F, Gioia U, Brandi O, Farina S, Ceccon M, Francia S, d’Adda di Fagagna F, DNA damage triggers a new phase in neurodegeneration, Trends Genet. 20 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.