Abstract
Extracellular electron transfer (EET) allows microbes to drive their metabolism through interactions with minerals or electrodes. In recent work, Light et al. (2018) discover a specialized EET pathway in Listeria monocytogenes with homologs in pathogens and gut commensals, suggesting that EET plays important roles in diverse environments.
In the late 1980s, two gram-negative bacteria were discovered with the ability to grow by coupling the oxidation of organic carbon to the reduction of iron or manganese oxides: Geobacter metallireducens strain GS-15 and Shewanella oneidensis strain MR-1. Though appreciation for microbial interactions with minerals dates back well over a century, the isolation of these bacteria paved the way for detailed studies of the mechanisms underpinning extracellular electron transfer (EET), the pathway taken by electrons generated within the cell to reach extracellular electron acceptors. Today, thanks to the efforts of many laboratories, not only do we understand how some of these pathways work in exquisite biochemical detail, but we know that related pathways operate in the reverse, funneling electrons into the cell from extracellular electron donors. Such EET metabolisms are important in microbe-mineral transformations of all types, and our awareness of and ability to manipulate these processes have led to important applications in bioremediation, bioleaching, and biofuels (Shi et al., 2016).
The reach of EET is not limited just to the natural environment or engineered settings but is likely highly relevant to microbial survival in the human host as well. Ironically, some of the first descriptions of what we now recognize as classical EET can be found in literature dealing with the challenge of respiration in mammalian tissues when oxygen is scarce. Between the two World Wars, E.S. Guzman Barron and E. Friedheim, both doctors and medicinal (bio)chemists, described the stimulating effect of redox-active pigments on oxygen consumption by mammalian cells and bacteria (Barron 1930; Friedheim 1931). Though their research was carried out before the electron transport chain was fully understood, and long before EET was coined as a term, the pigments they were studying are structurally similar to flavins and good examples of extracellular electron shuttles (EES): small molecules that mediate redox exchanges between cells and extracellular substrates at a distance. Fast-forwarding many decades, certain EES called phenazines—produced by members of the Actinomycetes and Proteobacteria—have been studied in detail with respect to their roles in supporting bacterial energy generation and survival under conditions of oxidant limitation, such as within biofilms. Though the electron transfer pathways to and from phenazines are still being worked out, phenazines can interact both with membrane-bound components of the respiratory chain and with cytosolic enzymes, and recent studies have revealed how phenazine-mediated EET contributes to the anaerobic survival of their producers (Glasser et al., 2014). Strikingly, thousands of redox-active pigments are made by diverse bacterial phyla and may play similarly important roles in cellular electron transfer processes in a wide range of habitats.
The work of Light et al. (Light et al., 2018) weaves these disparate EET threads together in a fascinating new story that shines mechanistic light on old reports of iron reduction by gram-positive, fermentative bacteria (for review, see Lovley 1987). Importantly, it also challenges us to no longer view EET as an exotic energy generation mechanism, reserved for microbes and minerals, and compels us to consider whether it might also support microbial metabolism in seemingly familiar contexts like the mammalian gut. Through an impressive combination of genetic, bioinformatic, biochemical, and bioelectrical experiments, Light et al. identify a pathway for EET in the gram-positive human pathogen Listeria monocytogenes. This pathway is encoded by several genes that are co-localized on the chromosome, containing the blueprint for an NADH dehydrogenase of the Ndh2 type and several other membrane-bound or membrane-associated electron carriers. Their experimental results suggest that electrons are conveyed to the outside of the cell by way of NADH oxidation by Ndh2, from which they are delivered to a demethylmenaquinone (DMK) that passes them to a lipoprotein to which flavin mononucleotide (FMN) is covalently bound (PplA). Though it remains to be demonstrated biochemically, Light et al. speculate that two uncharacterized membrane proteins in the EET locus (EetB and EetA) likely mediate electron transfer from Ndh2 to PplA. Strong evidence, however, supports the conclusion that other genes in the EET locus encode proteins (RibU, FmnA, and FmnB) that are responsible for FMNylating PplA. Light et al. suggest that electrons leave FMNylated PplA and travel to their distal oxidant (be it ferric iron or a graphite electrode; both were used in the study) by way of exogenous flavins; that the addition of free FMN stimulates current generated by L. monocytogenes in an anoxic electrochemical chamber is consistent with this hypothesis. They go on to show that this EET pathway is required for anaerobic growth on the sugar alcohols xylitol and D-arabitol and that it confers a competitive advantage to L. monocytogenes in the mouse intestinal lumen, where anaerobic growth is known to be important. Finally, they survey iron reductase activity in a panel of Firmicutes species that either contain or lack a similar EET locus, finding evidence—albeit only correlative—implicating EET in iron reduction by these organisms. Collectively, these data establish a reasonable model for electron flow and demonstrate that flavins are essential to this process.
One of the most provocative aspects of the Light et al. study is that L. monocytogenes is a flavin auxotroph. The authors argue that Listeria’s EET pathway must therefore depend on the availability of exogenous flavins, which is reasonable because flavins are ubiquitous environmental molecules. However, whether other redox-active electron shuttles facilitate EET beyond FMNylated-lipoproteins remains to be determined. Though flavins have also been shown to stimulate EET in iron-reducing species like S. oneidensis, they do so in a concentration-dependent manner: at low concentrations, by serving as protein cofactors; at higher concentrations, by also functioning as EES proper (Xu et al., 2016). A key question left unanswered by this work is what lies beyond (Figure 1). To what does PplA relay its electrons, and what is the terminal electron acceptor in the gut or a different environmental niche? Might it be oxygen, harkening back to the work of Barron and Friedheim (Barron 1930; Friedheim 1931)? Nitrate, generated indirectly by reactions of the immune system? Ferric iron, bound to transferrin? Notably, other types of ubiquitous organic compounds, such as small molecules produced by other microbes that are typically classified as antibiotics, or humic substances, which derive from the breakdown of microbial and plant matter, are also abundant in the nutrient-rich environments where L. monocytogenes thrives and important to consider as alternative EES (Glasser et al., 2017). Indeed, many years ago, studies of humic acid reduction by a variety of fermentative bacteria (including Propionibacterium freudenreichii, Lactococcus lactis, and Enterococcus cecorum) established that humics could shift fermentation patterns toward more oxidized products while enhancing iron reduction (Benz et al., 1998). How prevalent might EET be within host-associated environments, be the hosts animals or plants?
Figure 1. Flavin EET Knowns and Unknowns.
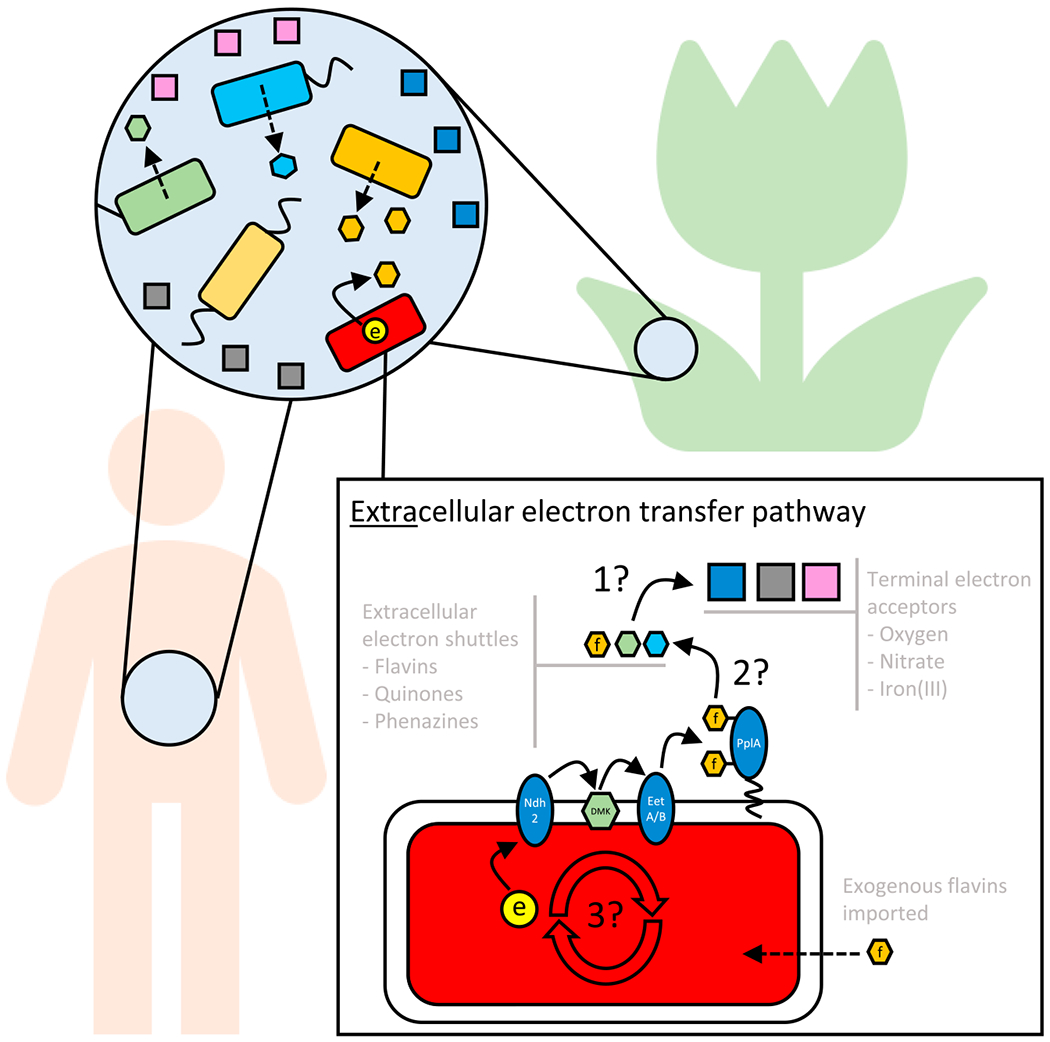
From the microbial perspective, accessing a terminal electron acceptor to power metabolism is a problem that transcends context. The flavin EET pathway has been elucidated as a specific solution that provides an advantage in the anaerobic intestinal tract for L. monocytogenes, whose natural ecological range is broad, encompassing soils to host-associated environments. These environments often contain complex microbial communities and diverse molecules that may influence EET metabolism. Light et al. (Light et al.,2018) propose that EET proceeds through an alternative membrane-bound electron transport chain to the outside of the cell. This electron transport chain consists of an NADH dehydrogenase (Ndh2), specific menaquinone derivatives (DMK), possibly two membrane proteins (EetA and EetB), and an outward facing lipoprotein (PplA) with covalently attached flavins that serve as electron carriers. Their experiments provide evidence that flavins are sufficient to serve as extracellular electron shuttles from PplA to iron(III) and electrodes, but many questions remain, e.g.: (1) what is the terminal electron acceptor in any given niche? (2) What EES are involved in long range electron transfer? (3) How does EET contribute to energy generation within the cell?
From a physiological perspective, it is significant that the L. monocytogenes EET system appears to be common in bacteria that prioritize fermentative metabolic strategies. Given that flavins and phenazines also facilitate fermentation in S. oneidensis (Hunt et al., 2010) and Pseudomonas aeruginosa (Glasser et al., 2014), by tuning redox balance such that substrate-level phosphorylation can proceed, it stands to reason that many extracellular redox-active metabolites in a range of environments from soils to sediments to mammalian hosts may play important roles in anaerobic energy generation through diverse EET pathways. At a core physiological level, a microbial cell is context agnostic: the electron transfer challenges it faces are generalizable, regardless of whether an intestinal epithelium or plant or mineral surface is present at a distance. In some cases, EET may facilitate ATP synthesis by way of oxidative phosphorylation; in other cases, by way of substrate-level phosphorylation. It remains to be established which mode of energy generation underpins EET in L. monocytogenes, but regardless, Light et al.’s study inspires us to begin to look for EET in unconventional places by leveraging conserved EET genes and the ever-growing genomic and natural products databases. Major challenges for the field remain to illuminate the path of electron transfer once electrons leave the cell, which will require more precise knowledge of the electroactive components in the microbial microenvironment.
ACKNOWLEDGMENTS
We thank Ken Nealson, Daniel Dar, and John Ciemniecki for constructive comments on the manuscript and the ARO (W911NF-17-1-0024) and NIH (1R01AI127850-01A1) for supporting our EET research.
REFERENCES
- Barron ES (1930). The catalytic effect of methylene blue on the oxygen consumption of tumors and normal tissues. J. Exp. Med 52, 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz M, Schink B, and Brune A (1998). Humic acid reduction by propionibacterium freudenreichii and other fermenting bacteria. Appl. Environ. Microbiol 64, 4507–4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedheim EAH (1931). Pyocyanine, an accessory respiratory enzyme. J. Exp. Med 54, 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser NR, Kern SE, and Newman DK (2014). Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton-motive force. Mol. Microbiol 92, 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser NR, Saunders SH, and Newman DK (2017). The colorful world of extracellular electron shuttles. Annu. Rev. Microbiol 71, 731–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KA, Flynn JM, Naranjo B, Shikhare ID, and Gralnick JA (2010). Substrate-level phosphorylation is the primary source of energy conservation during anaerobic respiration of Shewanella oneidensis strain MR-1. J. Bacteriol 192, 3345–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light SH, Su L, Rivera-Lugo R, Cornejo JA, Louie A, Iavarone AT, Ajo-Franklin CM, and Portnoy DA (2018). A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR (1987). Organic matter mineralization with the reduction of ferric iron: A review. Geomicrobiol. J 5, 375–399. [Google Scholar]
- Shi L, Dong H, Reguera G, Beyenal H, Lu A, Liu J, Yu HQ, and Fredrickson JK (2016). Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol 14, 651–662. [DOI] [PubMed] [Google Scholar]
- Xu S, Jangir Y, and El-Naggar MY (2016). Disentangling the roles of free and cytochrome-bound flavins in extracellular electron transport from Shewanella oneidensis MR-1. Electrochim. Acta 198, 49–55. [Google Scholar]


