Abstract
AIM
To examine the impact of clinical factors, cognitive deficits, and sleepiness on health-related quality of life (HRQoL) among young people with craniopharyngioma.
METHOD
Seventy-eight patients (67% White; 41 males, 37 females; mean age 10y 8mo, SD 3y 11mo, range 6–20y) with craniopharyngioma were assessed for tumor extent and diabetes insipidus. All patients underwent overnight polysomnography and multiple sleep latency tests after surgical resection. Executive functioning was assessed using parent-reported measures. Patients and their parents completed measures of HRQoL. None had a history of previous radiation therapy.
RESULTS
Path analysis was used to test hypothesized relations while controlling for demographic and disease characteristics. Analyses revealed poorer parent-reported HRQoL among young people with greater executive functioning symptoms (estimate 0.83; p<0.001). Direct and indirect effects were found among diabetes insipidus, executive functioning, and parent-reported HRQoL. Diabetes insipidus directly predicted greater global executive functioning impairment (estimate 5.15; p=0.04) and indirectly predicted lower HRQoL through executive functioning impairment (estimate 4.25; p=0.049). No significant effects were found between excessive daytime sleepiness, tumor hypothalamic involvement, diabetes insipidus, executive functioning, and patient-reported HRQoL.
INTERPRETATION
These findings suggest that young people with craniopharyngioma presenting with diabetes insipidus may benefit from targeted neurocognitive and psychosocial screening to inform interventions.
Craniopharyngioma is a rare, intracranial tumor representing 1.2% to 4.6% of tumors of the childhood central nervous system.1,2 Craniopharyngioma most often arises in the suprasellar region and intimately involves optic chiasm and nerves, the hypothalamic–pituitary axis, vascular structures, cerebrospinal pathways, and the nearby frontal lobes.3 Significant neurological and endocrine complications4,5 are associated with the diagnosis and treatment of craniopharyngioma, including vision loss, panhypopituitarism, hypothalamic obesity, and diabetes insipidus.1 The long-term survival of craniopharyngioma is high (87–97%); however, excessive daytime sleepiness (EDS), neurocognitive dysfunction, and low mood3,4 are often reported. One-third of adult survivors of childhood craniopharyngioma have reported clinically significant rates of EDS.6,7 Those who undergo surgery may experience EDS, narcolepsy, and shortened sleep latency,8,9 affecting health-related quality of life (HRQoL).4
Endocrinopathies are common in childhood-onset craniopharyngioma: 40% to 87% present with at least one hormonal deficit and 17% to 27% with diabetes insipidus at the time of diagnosis. Further, in some series, 80% to 100% experience transient post-surgical diabetes insipidus,3 and diabetes insipidus is often permanent in 70% to 90% of those treated with radical surgery.10 Hypothalamic dysfunction before and after diagnosis includes obesity and imbalances in regulating body temperature, thirst, heart rate, and/or blood pressure.3
While 4% to 58% of patients are obese at diagnosis, obesity rates increase to approximately 80% after treatment, especially if radical resection has occurred.11 Disturbance in energy management (i.e. calories consumed and calories burned), EDS, sleep/wake disturbances, and neurological impairments are thought to contribute to the development of severe obesity.3,8 Diabetes insipidus has been associated with poor cognitive and psychosocial outcomes, including attention, memory, executive functioning, and HRQoL12 and, as such, was chosen as a clinical variable of interest in this study.
Correlates of neurocognitive dysfunction after craniopharyngioma treatment have been widely studied. In a systematic review of the literature, Zada et al.5 reported that 57% of patients experienced neurobehavioral impairments. Long-term declines in overall intellectual functioning13 are noted, with specific impairments in executive functioning.11,13–15 The presence of greater hypothalamic involvement is predictive of greater executive functioning dysfunction.13 Our previous investigations have demonstrated that executive functioning was most impacted by hypothalamic involvement among young people with craniopharyngioma.16
We also previously demonstrated that more than 75% of these patients showed evidence of EDS on a multiple sleep latency test (MSLT) before irradiation and that patients with two or more sleep-onset rapid eye movement periods performed significantly worse on global and specific measures of intelligence than did patients with fewer than two sleep-onset rapid eye movement periods.9,17 We also demonstrated that EDS is significantly related to increased hypothalamic involvement.9
Neurological impairments, including hypothalamic damage as well as disordered sleep, can work together to reduce HRQoL. Approximately 50% of childhood craniopharyngioma survivors reported global HRQoL that fell within the normal range,5 with up to 90% reporting higher levels of HRQoL if treatment was intracystic only. Adult survivors with significant endocrine and cognitive problems often report HRQoL levels similar to adults in the general population,5,13 but parent or caregiver reports of HRQoL in children are lower.5 Zada et al.5 found that 41% of families across 11 studies reported lower HRQoL related to social dysfunction, 35% reported school dysfunction, and global HRQoL was negatively impacted in 52% of patients after treatment for craniopharyngioma. Gautier et al.10 found that young people diagnosed with craniopharyngioma had poorer HRQoL than children with other chronic illness and the general population.
An improved and nuanced understanding of the prevalence of and risk factors associated with HRQoL impairments may help improve early screening, intervention, and treatment.5 The current study examined the impact of neuroendocrine, executive, and sleep dysfunction on HRQoL in children diagnosed with craniopharyngioma. Because diabetes insipidus, hypothalamic involvement, and EDS have been implicated in reducing executive functioning and because executive functioning is critical to many aspects of daily life, we hypothesized that executive functioning would mediate the relation of diabetes insipidus, hypothalamic involvement, and EDS to HRQoL.11–13,16
METHOD
Participants
Between 2011 and 2016, 78 children and adolescents with craniopharyngioma were evaluated for adjuvant or definitive treatment at St. Jude Children’s Research Hospital. Craniopharyngioma diagnosis was based on imaging and/or intraoperative findings including histopathological confirmation. None of the patients had a previous history of radiation therapy. Measures were completed after surgical resection but before irradiation, when indicated.
Patients completed neurocognitive and psychological assessment, a clinical sleep evaluation by a pediatric sleep specialist, nocturnal polysomnography followed by a MSLT the next day, and self- and parent-reported measures of HRQoL. Neurocognitive and psychology measures were completed at St. Jude Children’s Research Hospital. Sleep studies were performed at the Methodist Healthcare Sleep Disorders Center. Patients were excluded from neurocognitive testing if they were non-English speaking, spoke English as a second language, or if they had sensorimotor impairment. Patients with reduced vision were included in cognitive assessments as long as the visual impairment did not affect the validity of the assessments. Some patients with visual impairment only received verbal measures. Patients younger than 6 years old were excluded from the MSLT evaluation.18 Only children and adolescents with both polysomnography and MSLT were included in this analysis. The research was approved by the Institutional Review Board of St. Jude Children’s Research Hospital. Informed written consent and assent were obtained for all procedures.
The nocturnal polysomnography and MSLT were scheduled based upon the patient’s typical sleep/wake schedule as determined by the clinical history gathered by the sleep specialist, conducted in an American Academy of Sleep Medicine-accredited sleep center, scored by registered polysomnography technologists trained in scoring pediatric nocturnal polysomnography and MSLT, and reviewed and interpreted by a Board-certified sleep physician with expertise in pediatric sleep.
Measures
Demographic and clinical information
Demographic and clinical data were systematically collected (see Table 1).
Table 1:
Demographic and health characteristics (n=78)
| Characteristic | n | % |
|---|---|---|
|
| ||
| Sex | ||
| Male | 41 | 53 |
| Female | 37 | 47 |
| Self-reported ethnic group | ||
| White | 52 | 66.7 |
| Black | 12 | 15.4 |
| Asian | 3 | 3.8 |
| Bi-ethnic | 5 | 6.5 |
| Multi-ethnic | 3 | 3.8 |
| Unknown | 3 | 3.8 |
| Hypothalamic tumor involvement | ||
| Grade 0 | 10 | 13 |
| Grade 1 | 22 | 28 |
| Grade 2 | 46 | 59 |
| Diabetes insipidus | 40 | 51 |
| Excessive daytime sleepiness | 64 | 82 |
| Number of surgeries | ||
| 0 | 6 | 8.3 |
| 1 | 40 | 51.2 |
| 2 | 19 | 23.8 |
| ≥3 | 13 | 16.7 |
| Surgical procedure | ||
| None | 6 | 8.3 |
| Catheter only, craniotomy | 6 | 7.1 |
| Catheter only, burr hole | 10 | 13.1 |
| Resection, craniotomy | 39 | 50 |
| Resection, transphenoidal | 14 | 17.9 |
| Resection, endoscopic | 3 | 3.6 |
| Cerebrospinal fluid diversion | ||
| None | 56 | 71.4 |
| Ommaya reservoir | 15 | 18.6 |
| Ventriculoperitoneal shunt | 7 | 10 |
Executive functioning
Each participant completed a comprehensive neurocognitive assessment at baseline. As part of this assessment, the Behavior Rating Inventory of Executive Function,19 a parent questionnaire designed to assess behavioral manifestations of executive functioning, was administered (10–15min).19 Executive functions include goal-directed behaviors, such as the ability to plan, organize, sustain performance, and change performance in response to feedback. The Behavior Rating Inventory of Executive Function yields three index scores: Behavioral Regulation Index, Metacognition Index, and Global Executive Composite; however, only the Global Executive Composite was used in the current study as it provides an overall assessment of the participant’s global executive functioning. The Behavior Rating Inventory of Executive Function provides T scores for each index, with a T score of 65 or above indicating clinically significant impairment.19
Hypothalamic involvement
Hypothalamic involvement was graded on the basis of the earliest available MRI, according to the criteria proposed by Muller et al.11 Criteria were as follows: grade 0, no hypothalamic involvement; grade 1, involvement of the anterior hypothalamus; grade 2, involvement of the anterior and posterior hypothalamus.
MSLT
The MSLT is a standardized test used to evaluate daytime sleepiness and measure sleep propensity in the absence of alerting factors. MSLTs were performed according to American Academy of Sleep Medicine guidelines.20 After nocturnal polysomnography, participants were evaluated with four or five nap opportunities at 2-hour intervals throughout the day. A registered polysomnography technologist observed patients continuously between naps and encouraged the child not to fall asleep. If sleep onset occurred during a nap opportunity, patients were allowed to sleep for 15 minutes before being awakened. If no sleep occurred after 20 minutes, the nap opportunity ended. The mean sleep latency in minutes was calculated as the arithmetic mean of all nap opportunities. Using EDS cutoff scores for prepubescent versus pubescent children, EDS was defined as a mean sleep latency of not more than 15 minutes in prepubescent children with a Tanner stage of 1 and of not more than 10 minutes in pubescent young people with a Tanner stage of 2 or greater. Tanner staging was ascertained by reviewing medical records.
HRQoL
The Pediatric Quality of Life Inventory version 4 is a 23-item Likert-type scale developed to measure HRQoL in young people and takes about 4 to 6 minutes to complete.21 This instrument has parallel forms for patient and parent report. The patient forms are for three different age groups (5–7y, 8–12y, and 13–18y). Subscales on the Pediatric Quality of Life Inventory version 4 measure the patients’ physical, emotional, social, and school functioning. A total mean scaled score of the 23 items was computed as the sum of the items divided by the number of items answered. Both patient and parent forms have been found to be internally consistent and were used in this study (Cronbach’s α coefficients of 0.910.92 respectively), with clinical validity (i.e. able to distinguish between known groups of patients on and off therapy) and beginning construct validity (i.e. hypotheses predicting relationships between certain subscales and other indicators of emotional distress, perceived competency, social support/functioning, and academic competence were supported).21 Consistency between child and parent proxy-reports on the Pediatric Quality of Life Inventory version 4 is typically low (r=0.02–0.23 historically and r=0.34 for the current study), although it is still believed to be an important and valid marker of child wellbeing.22
Statistical analyses
The prevalence of neuroendocrine and neurocognitive dysfunction, as well as EDS, was analysed using SPSS version 25 (IBM Corp., Armonk, NY, USA). Path analysis was then conducted using Mplus version 8.2 (Muthén & Muthén, Los Angeles, CA, USA) to test the hypothesized relations between diabetes insipidus, executive functioning, hypothalamic involvement, EDS, and HRQoL while controlling for demographic and disease characteristics, including age, sex, self-reported ethnic group, extent of surgical resection, cerebrospinal diversion, and vision status. Because of the sample size and concerns related to potential impact on power, we elected to analyse the relation of diabetes insipidus and hypothalamic involvement to the outcome variables of concern because of their past significant relation to neurocognitive functioning and HRQoL.12,13,16 Specifically, path analysis was used to examine the direct effect of diabetes insipidus, hypothalamic involvement, and EDS on HRQoL and the indirect (mediating) effect of diabetes insipidus, hypothalamic involvement, and EDS on HRQoL through executive functioning. Path analysis can be used to explain the associations among observed variables on the basis of a priori theory (see Fig. 1 for conceptual model).23 When conducting a path analysis, it is important to consider overall model fit, the relationships between observed variables, and the overall contribution of the predictor variables. Path analysis was conducted using robust maximum likelihood estimation (which adjusts the standard errors and χ2 test statistics taking into account multivariate kurtosis and data missingness) and the model was evaluated using the following goodness-of-fit indices: model χ2, root mean square error of approximation, comparative fit index, and standardized root mean square residual.
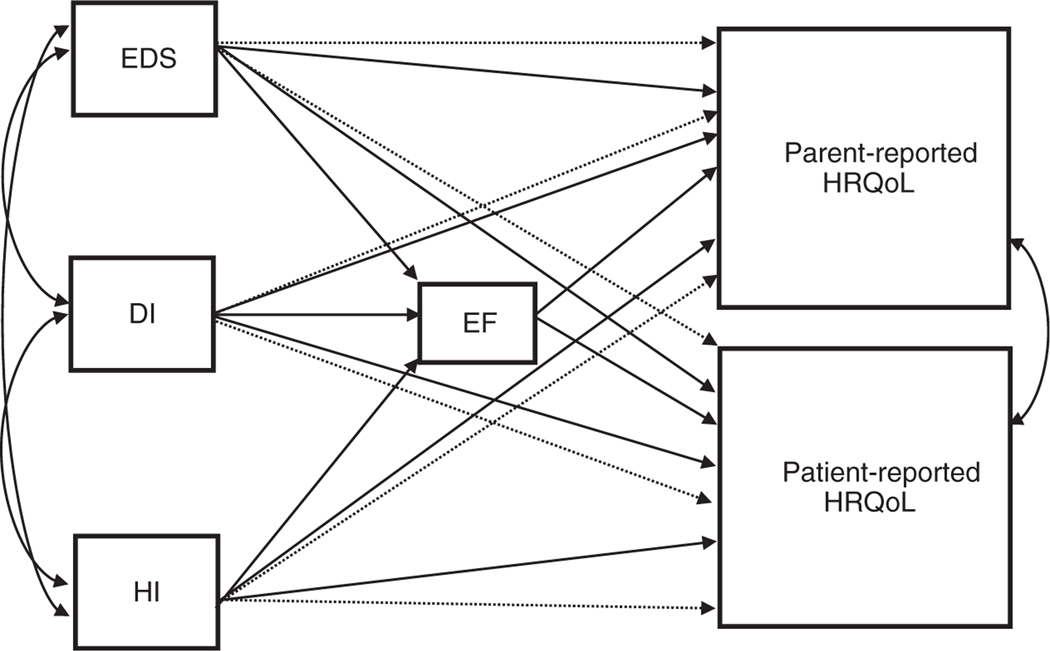
Figure 1:
Conceptual model of the direct (solid line) and indirect (dashed line) effects of excessive daytime sleepiness (EDS), diabetes insipidus (DI), and hypothalamic involvement (extent) (HI) on executive function (EF) and health-related quality of life (HRQoL) among young people with craniopharyngioma.
RESULTS
Demographic and disease characteristics are presented in Table 1. The cohort was predominately White (66.7%) and male (53%), with a mean age of 10 years 8 months (SD 3y 11mo; range 6–20y) at baseline. Most participants met criteria for diabetes insipidus (51%), grade 2 hypothalamic involvement (59%), and EDS (82%). Only persistent diabetes insipidus was categorized; patients with transient diabetes insipidus were not included in the analysis group. For analysis, diabetes insipidus was restricted to those who acquired it at the time of surgery resulting from disruption of the pituitary stalk during radical surgery. Diabetes insipidus was well-controlled in patients; treatment adjustments were made as needed. The average mean (SD) sleep latency was 8.20 (5.76) minutes. Parents reported Behavior Rating Inventory of Executive Function scores in the average range. T scores of 65 or above were identified in 10.3% to 11.5%, which is consistent with normative data. Higher scores on the Pediatric Quality of Life Inventory version 4 indicate better HRQoL, and while there are no published standards that delineate clinically significant levels of HRQoL on this measure, Varni et al. reported comparative norms for a large sample of chronically ill, acutely ill, and population norm children and their parents.24 The current sample demonstrated significantly lower parent and patient HRQoL scores than Varni et al. (see Table 2).24 Executive functioning, HRQoL, and HRQoL comparisons scores are presented in Table 2.
Table 2:
HRQoL and executive functioning characteristics
| Measure | Mean | SD | Median | Range |
|---|---|---|---|---|
|
| ||||
| BRIEF | ||||
| Global Executive Composite | 49.71 | 11.65 | 48 | 33–85 |
| PedsQL Parent | ||||
| Total HRQoL | 65.58 | 18.99 | 66.30 | 27.17–100 |
| PedsQL Patient | ||||
| Total HRQoL | 70.40 | 16.60 | 71.74 | 30.43–95.65 |
|
| ||||
| HRQoL comparisons to Varni et al.24 | t | df | p | 95% CI |
|
| ||||
| PedsQL Parent | ||||
| Chronically ill | −4.02 | 77 | <0.001 | −8.64 to −12.92 |
| Population norm | −10.25 | 77 | <0.001 | −22.02 to −26.31 |
| PedsQL Patient | ||||
| Chronically ill | −3.61 | 77 | <0.001 | −10.53 to −3.04 |
| Population norm | −6.71 | 77 | <0.001 | −12.60 to −16.34 |
HRQoL, health-related quality of life; BRIEF, Behavior Rating Inventory of Executive Function; PedsQL, Pediatric Quality of Life Inventory; CI, confidence interval.
Because the path model was fully saturated, goodness-of-fit statistics were non-informative (i.e. perfectly reproduced the observed covariance matrix; see Fig. 1 for path model). A fully saturated model occurs when the number of free parameters equals the number of known values (i.e. the number of knowns exactly equals the number of unknown; degrees of freedom=0); therefore indicating a perfectly fitting model (model χ2=0, p<0.001; root mean square error of approximation=0; comparative fit index=1; standardized root mean square residual=0).25 Diabetes insipidus was found to have a positive (unstandardized) direct effect on global executive functioning impairment (estimate 5.15; p=0.04). We found no direct effects of hypothalamic involvement or EDS on global executive functioning impairment within the path model (p>0.05). Global executive functioning scores (estimate 0.83; p<0.001) were inversely related to parent-reported HRQoL. The direct effects of hypothalamic involvement or EDS on parent-reported HRQoL were non-significant (p>0.05). There was a significant but small indirect effect of diabetes insipidus on parent-reported HRQoL through executive functioning (estimate 4.25; p=0.049), indicating that diabetes insipidus indirectly predicted lower parent-reported HRQoL through executive functioning symptomatology. Executive functioning did not mediate the relations between hypothalamic involvement and HRQoL or EDS and HRQoL (p>0.05). No significant effects (direct or indirect) were found between diabetes insipidus, executive functioning, hypothalamic involvement, EDS, and patient-reported HRQoL (all p>0.05). Residual associations were found between patient- and parent-reported HRQoL when controlling for diabetes insipidus, hypothalamic involvement, and EDS (estimate 71.9; p=0.043). See Table 3 for unstandardized path model results.
Table 3:
Unstandardized path model results
| Parent direct effects | Estimate | SE | Estimate/SE | p |
|---|---|---|---|---|
| Hypothalamic involvement→HRQoL | −1.08 | 2.73 | −0.39 | 0.69 |
| Diabetes insipidus→HRQoL | 0.70 | 3.83 | 0.18 | 0.86 |
| EDS→HRQoL | −4.48 | 4.80 | −0.93 | 0.35 |
| Executive function→HRQoL | −0.83 | 0.15 | −5.48 | <0.001 a |
| Hypothalamic involvement→executive function | −2.7 | 1.78 | −1.52 | 0.13 |
| Diabetes insipidus→executive function | −5.2 | 2.5 | −2.05 | 0.04 a |
| EDS→executive function | 5.6 | 3.2 | 1.8 | 0.08 |
| Patient direct effects | ||||
| Hypothalamic involvement→HRQoL | −2.4 | 2.6 | −0.94 | 0.35 |
| Diabetes insipidus→HRQoL | −2.4 | 3.6 | −0.67 | 0.51 |
| EDS→HRQoL | −5.6 | 4.2 | −1.32 | 0.19 |
| Executive function→HRQoL | −0.18 | 0.20 | −0.87 | 0.38 |
| Parent indirect effects | ||||
| Hypothalamic involvement→executive function→HRQoL | 2.23 | 1.5 | 1.5 | 0.13 |
| Diabetes insipidus→executive function→HRQoL | −4.3 | 2.2 | −2.0 | 0.049 b |
| EDS→executive function→HRQoL | −4.6 | 2.9 | −1.6 | 0.11 |
| Patient indirect effects | ||||
| Hypothalamic involvement→executive function→HRQoL | 0.47 | 0.63 | 0.75 | 0.45 |
| Diabetes insipidus→executive function→HRQoL | −0.91 | 1.14 | −0.80 | 0.43 |
| EDS→executive function→HRQoL | −0.99 | 1.3 | −0.73 | 0.46 |
| Residual covariance | ||||
| Parent HRQoL with patient HRQoL | 71.9 | 35.6 | 2.02 | 0.043 b |
Bold type indicates statistically significant results:
p<0.001
p<0.05. SE, standard error; HRQoL, health-related quality of life; EDS, excessive daytime sleepiness.
DISCUSSION
Our aim was to examine the impact of clinical factors, executive function, and objective measures of sleepiness on HRQoL in children and adolescents diagnosed with craniopharyngioma. Analyses revealed that parents who reported their child to have poorer HRQoL also reported that their child had more global executive functioning symptoms. Diabetes insipidus directly predicted greater global executive functioning symptoms and indirectly predicted lower parent-reported HRQoL through executive functioning symptomatology. No direct or indirect effects were found among diabetes insipidus, executive functioning, hypothalamic involvement, EDS, and patient-reported HRQoL, a finding that is not unexpected given the body of literature describing differences in perception between parents and children with regards to HRQoL.
Within this cohort, higher parent-reported global executive functioning symptomatology was noted in young people with diabetes insipidus. Similar to the finding of Fournier-Goodnight et al.16 that diabetes insipidus was associated with poorer adaptive functioning, greater mood disturbances, and lower IQ among the current sample of pediatric patients with craniopharyngioma, we also found diabetes insipidus to indirectly predict poorer parent-reported HRQoL through global executive functioning symptomatology. Diabetes insipidus has also been associated with deficits in sustained attention, memory retrieval, learning difficulties, executive functioning, emotional and behavioral functioning, and HRQoL.12,26 Diabetes insipidus may reduce executive functioning among young people with craniopharyngioma, but the mechanism is unclear. The direct impact of diabetes insipidus on executive functioning in young people with craniopharyngioma may be explained by symptoms associated with diabetes insipidus, such as decreased vasopressin (antidiuretic hormone);27 however, additional research is needed to examine the direct impact of diabetes insipidus on executive functioning in this population. The executive functioning challenges seen in relation to diabetes insipidus then appear to drive lower parent-reported HRQoL. Diabetes insipidus often results from surgery and can be viewed as a proxy for radical surgery (defined as gross-total resection or any attempt at gross-total resection or subtotal resection involving dissection) in patients with craniopharyngioma.10 Our findings, coupled with previous investigations into the role of diabetes insipidus in HRQoL and executive functioning, provide support that children who have had limited surgical approaches may have higher parent-reported HRQoL.
The mediated relationship between diabetes insipidus and HRQoL through executive functioning may be explained by the increased medical management responsibilities for young people and families. Young people with diabetes insipidus who have difficulty engaging in self-care tasks (i.e. adhering to medical treatment, toileting, monitoring fluid intake) owing to executive functioning difficulties may be perceived by their parents as having poorer HRQoL. Diabetes insipidus may represent more aggressive disease, though we controlled for aspects of aggressive disease (i.e. cerebrospinal diversion and extent of surgical resection). Although diabetes insipidus appears to play an important role that highlights the importance of limiting surgical extent, future research is needed to further investigate the relations between diabetes insipidus, executive functioning, and HRQoL among young people with craniopharyngioma.
Consistent with a previous study that identified poorer parent-reported HRQoL in patients with brain tumors and executive functioning problems,28 we found lower parent-reported HRQoL scores among young people rated by their parent as having greater executive functioning symptoms in the context of a sample who reported overall average range scores of parent-reported executive functioning. Executive dysfunction in young people with craniopharyngioma has been inconsistently documented, related to performance-based or parent-report measures.5 Some studies of neurocognitive performance have demonstrated no differences in sustained attention, problem solving, or verbal fluency abilities between young people with craniopharyngioma and controls; however, long-term decreases in overall intellectual functioning after surgery and/or proton radiation therapy have been reported,13 with specific impairments in executive functioning, attention, processing speed, and memory.11,13–15 Thus, our findings are probably impacted by evaluating young people early in their treatment course.
Riva et al.29 and Waber et al.15 found neurocognitive impairments associated with poor emotion regulation, somatic concerns, and depressive symptoms among patients with craniopharyngioma. Additionally, emotional dysfunction is a significant predictor of poorer HRQoL among young people with craniopharyngioma, with nearly 40% of patients reporting difficulties in controlling emotions such as anger, frustration, sadness, and worry.5,30 Of note, Peterson et al.31 found parent-report measures of child executive functioning dysfunction and behavioral issues were more predictive of parental distress than patients’ cognitive performance. While current findings suggest that emotion regulation may be an important component of executive functioning that affects HRQoL among young people with craniopharyngioma, parental distress probably plays a role. As such, additional research is needed to further investigate the impact of parental distress on HRQoL through executive functioning.
While the current study has several strengths, its limitations should be acknowledged. First, the sample size was relatively small (n=78); therefore, small relationships between variables may not have been detected owing to limited power and caution should be exercised when interpreting results. Second, this study was restricted by using cross-sectional data before adjuvant therapy. Findings may differ among patients after the emergence of therapy-related late effects. Third, the current study utilized subjective reports of executive functioning and HRQoL. Previous research shows that parents tend to report greater impairments than patients28 on subjective measures of executive functioning. It is likely that young people with impaired executive functioning may have difficulty assessing their own HRQoL. As such, future studies should include objective measures of executive functioning to examine response differences. Furthermore, because the significant findings were discovered in relation to parent-report measures, it is possible that shared methods variance accounts for some of this relationship. The current study assessed propensity for sleepiness using objective measures (the MSLT) but not subjective measures; the latter tend to measure levels of sleepiness/wakefulness in daily life over longer periods of time. Finally, the young people with craniopharyngioma in this study had a wide age range (6–20y), with most characterized by a Tanner stage of 1, limiting our findings predominantly to pre-pubertal children.
Findings from this study suggest that limited surgery is associated with a lower risk of diabetes insipidus and improved HRQoL in young people with craniopharyngioma, particularly through the pathway of better executive functioning.31,32 Further, young people with craniopharyngioma presenting with diabetes insipidus may benefit from targeted neurocognitive and psychosocial assessment to identify specific executive functioning and HRQoL deficits. Tailored assessment results can be used to develop individualized cognitive rehabilitation interventions to improve patients’ HRQoL.
What this paper adds.
Children with craniopharyngioma and executive functioning impairment are more likely to have poorer health-related quality of life (HRQoL).
Diabetes insipidus, a complication associated with surgery, predicted greater executive functioning impairment.
Diabetes insipidus indirectly predicted lower parent-reported HRQoL through executive functioning impairment.
ACKNOWLEDGEMENTS
We thank the patients and families who participated in the study as well as the research and clinical teams who collected study data. This research was funded by a St. Jude Auxiliary Cancer Center Support Grant for the National Cancer Institute – designated cancer centers (CA21765) from the National Cancer Institute and by American Lebanese Syrian Associated Charities (ALSAC). The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
ABBREVIATIONS
- EDS
Excessive daytime sleepiness
- HRQoL
Health-related quality of life
- MSLT
Multiple sleep latency test
Footnotes
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1.Caldarelli M, Massimi L, Tamburrini G, Cappa M, Rocco D. Long-term results of the surgical treatment of craniopharyngioma: the experience at the Policlinico Gemelli Catholic University, Rome. Child Nerv Syst 2005; 21: 747–57. [DOI] [PubMed] [Google Scholar]
- 2.Garrè ML, Cama AJ. Craniopharyngioma: modern concepts in pathogenesis and treatment. Curr Opin Pediatr 2007; 19: 471–9. [DOI] [PubMed] [Google Scholar]
- 3.Daubenbüchel AMM, Müller HL. Neuroendocrine disorders in pediatric craniopharyngioma patients. J Clin Med 2015; 4: 389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen M, Guger S, Hamilton J. Long term sequelae of pediatric craniopharyngioma – literature review and 20 years of experience. Front Endocrinol 2011; 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zada G, Kintz N, Pulido M, Amezcua L. Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: a systematic literature review. PLoS ONE 2013; 8: e76562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller HL, Handwerker G, Gebhardt U, et al. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control 2006; 17: 583–9. [DOI] [PubMed] [Google Scholar]
- 7.Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol 2004; 46: 220–9. [DOI] [PubMed] [Google Scholar]
- 8.Muller HL, Handwerker G, Wollny B, Faldum A, Sorensen N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J Clin Endocrinol Metab 2002; 87: 3993–6. [DOI] [PubMed] [Google Scholar]
- 9.Jacola LM, Conklin HM, Scoggins MA, et al. Investigating the role of hypothalamic tumor involvement in sleep and cognitive outcomes among children treated for craniopharyngioma. J Pediatr Psychol 2016; 41: 610–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gautier A, Godbout A, Grosheny C, et al. Markers of recurrence and long-term morbidity in craniopharyngioma: a systematic analysis of 171 patients. J Clin Endocrinol Metab 2012; 97: 1258–67. [DOI] [PubMed] [Google Scholar]
- 11.Muller HL. Consequences of craniopharyngioma surgery in children. J Clin Endocrinol Metab 2011; 96: 1981–91. [DOI] [PubMed] [Google Scholar]
- 12.Bruins J, Kovacs G, Abbes A, et al. Minor disturbances in central nervous system function in familial neurohypophysial diabetes insipidus. Psychoneuroendocrinology 2006; 31: 80–91. [DOI] [PubMed] [Google Scholar]
- 13.Laffond C, Dellatolas G, Alapetite C, et al. Quality-of-life, mood and executive functioning after childhood craniopharyngioma treated with surgery and proton beam therapy. Brain Inj 2012; 26: 270–81. [DOI] [PubMed] [Google Scholar]
- 14.Carpentieri SC, Waber DP, Scott RM, et al. Memory deficits among children with craniopharyngiomas. Neurosurgery 2001; 49: 1053–7. [DOI] [PubMed] [Google Scholar]
- 15.Waber DP, Pomeroy SL, Chiverton AM, et al. Everyday cognitive function after craniopharyngioma in childhood. Pediatr Neurol 2006; 34: 13–9. [DOI] [PubMed] [Google Scholar]
- 16.Fournier-Goodnight AS, Ashford JM, et al. Neurocognitive functioning in pediatric craniopharyngioma: performance before treatment with proton therapy. J Neurooncol 2017; 134: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graef DM, Conklin H, Sykes A, Ashford J, et al. Excessive daytime sleepiness and neurocognitive performance in pediatric patients with craniopharyngioma. Sleep 2015; 38(Supplement): A308. [Google Scholar]
- 18.Mindell JA, Owens JA. A clinical guide to pediatric sleep: diagnosis and management of sleep problems. Philadelphia, PA: Lippincott Williams & Wilkins, 2015. [Google Scholar]
- 19.Gioia GA Behavior rating inventory of executive function: professional manual. Odessa, FL: Psychological Assessment Resources, 2000. [Google Scholar]
- 20.Littner MR, Kushida C, Wise MG, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep 2005; 28: 113–21. [DOI] [PubMed] [Google Scholar]
- 21.Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer. Cancer 2002; 94: 2090–106. [DOI] [PubMed] [Google Scholar]
- 22.Cremeens JEC, Blades M. Factors influencing agreement between child self-report and parent proxy-reports on the Pediatric Quality of Life Inventory 4.0 (PedsQL) Generic Core Scales. Health Qual Life Outcomes 2006; 4: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kline RB. Principles and practice of structural equation modeling. New York, NY: Guilford Publications, 2015. [Google Scholar]
- 24.Varni JW, Seid M, Kurtin PS. PedsQL™4.0: reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 generic core scales in healthy and patient populations. Med Care 2001; 39: 800–12. [DOI] [PubMed] [Google Scholar]
- 25.West SG, Taylor AB, Wu W. Model fit and model selection in structural equation modeling. In: Hoyle RH (Ed.), Handbook of structural equation modeling 2012: 209–231. New York, NY: The Guilford Press, 2012. [Google Scholar]
- 26.Whitsett SF, Kneppers K, Coppes MJ, Egeler RM. Neuropsychologic deficits in children with Langerhans cell histiocytosis. Med Pediatr Oncol 1999; 33: 486–92. [DOI] [PubMed] [Google Scholar]
- 27.Wade M, Hoffmann TJ, Jenkins JM. Association between the arginine vasopressin receptor 1A (AVPR1A) gene and preschoolers’ executive functioning. Brain Cogn 2014; 90: 116–23. [DOI] [PubMed] [Google Scholar]
- 28.Netson KL, Ashford JM, Skinner T, et al. Executive dysfunction is associated with poorer health-related quality of life in pediatric brain tumor survivors. J Neurooncol 2016; 128: 313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riva D, Pantaleoni C, Devoti M, Saletti V, Nichelli F, Giorgi C. Late neuropsychological and behavioural outcome of children surgically treated for craniopharyngioma. Child Nerv Syst 1998; 14: 179–84. [DOI] [PubMed] [Google Scholar]
- 30.Poretti A, Grotzer MA, Ribi K, Schönle E, Boltshauser E. Outcome of craniopharyngioma in children: long-term complications and quality of life. Dev Med Child Neurol 2004; 46: 220–9. [DOI] [PubMed] [Google Scholar]
- 31.Peterson RK, Ashford JM, Scott SM, et al. Predicting parental distress among children newly diagnosed with craniopharyngioma. Pediatr Blood Cancer 2018; 65: e27287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merchant TE, Kiehna EN, Sanford RA, et al. Craniopharyngioma: the St. Jude Children’s Research Hospital experience 1984–2001. Int J Radiat Oncol Biol Phys 2002; 53: 533–42. [DOI] [PubMed] [Google Scholar]