Countries around the world continue to address the large-scale health and wellbeing impacts and the broader societal burden associated with the coronavirus disease 2019 (COVID-2019). From an individual to global level, we continue to increase our understanding of the current and legacy impacts affecting population health. Pathophysiologic sequelae, both acute and chronic, are numerous in the individuals who have been infected [1,2]. Evidence also demonstrates that cardiorespiratory fitness (CRF) can be compromised following COVID-19 infection[2]. The evidence that COVID-19 has upon CRF is not surprising given the potential impact the severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) has on the cardiac, pulmonary, and skeletal muscular systems, the three systems that have a primary influence on the CRF response [1,3,4]. The impact of COVID-19 on CRF is particularly important given the fact that this clinical measure is now considered a vital sign [5], a designation earned by an evidence base spanning several decades, which clearly demonstrates its robust: 1) prognostic and diagnostic value; 2) ability to gauge therapeutic efficacy; and 3) correlation with functional capacity and quality of life [6]. Of the various approaches to assessing CRF, cardiopulmonary exercise testing (CPX) remains the gold-standard, providing for the most noninvasive, comprehensive, and accurate assessment of CRF. In the context of COVID-19, CPX provides an ideal approach to assess intersection between pathophysiologic and clinical manifestations, allowing for refined account of the impact the viral infection has both cross-sectionally and longitudinally, most importantly during a bout of physical exertion in a controlled environment. Put simply, without CPX, the pathophysiologic impacts of COVID-19 that only manifest during physical exertion, or manifest more profoundly during physical exertion, would be missed entirely, preventing a holistic understanding of the clinical presentation.
Aerobic capacity, quantified by peak oxygen consumption (VO2), and ventilatory efficiency, most commonly quantified by the minute ventilation/carbon dioxide production (VE/VCO2) slope, are two of the most-established measures obtained through CPX and several studies to date report the impact of COVID-19 on both [7]. Pleguezuelos et al. [8] performed CPX on healthy controls, patients diagnosed with ischemic heart disease (IHD), patients diagnosed with chronic obstructive pulmonary disease (COPD) and patients hospitalized for COVID-19; CPX was performed two months post-hospital discharge in the COVID-19 group. Compared to healthy controls [32.31(28.32–36.31) mlO2·kg−1·min−1], peak VO2 was significantly reduced (p < 0.001) in all groups [COVID-19 group: 17.30 (14.82–19.78) mlO2·kg−1·min−1, COPD group: 14.35 (12.97–15.73) mlO2·kg−1·min−1, and IHD group: 18.82 (15.64–22) mlO2·kg−1·min−1]. Skjorten et al. [9] found 31% of the cohort has a percent-predicted peak VO2 less than 80% in patients that were hospitalized with COVID-19 and undertook a CPX three months post-discharge. Compared to subjects reporting no dyspnea during activities of daily living (n = 67, 62%), those reporting dyspnea (n = 59, 38%) had a significantly lower peak VO2 (31.9 ± 9.3 vs. 23.6 ± 7.9 mlO2·kg−1·min−1, p < 0.001) and significantly higher VE/VCO2 slope (26.6 ± 4.4 vs. 28.9 ± 4.5, p < 0.01). Raman et al. [2] compared 58 patients hospitalized for COVID-19 to a healthy control group. Two to three months following hospital discharge due to COVID-19, 64% of the patients continued to report dyspnea and 55% continued to report fatigue. Compared to healthy controls, percent-predicted peak VO2 was significantly lower (80.5 ± 23.1% vs. 112.7 ± 27.0%, p < 0.0001), and the VE/VCO2 slope was significantly higher [33.4 (29.2–40.3) vs. 28.2 (26.7–30.0), p < 0.0001] in those hospitalized with COVID-19. Aparisi et al. [10] reported similar findings in a cohort previously hospitalized for COVID-19 and undergoing CPX at three-month follow-up; peak VO2 was significantly lower [17.8 (15.8–21.2) vs. 22.8 (18.8–27.7) mlO2·kg−1·min−1, p < 0.001], and the VE/VCO2 slope was significantly higher [32.0 (28.1–37.4) vs. 29.4 (26.9–31.4), p < 0.05] in the 41 subjects (58.6%) who reported persistent dyspnea following discharge compared to the 29 subjects (41.4%) who were asymptomatic. These findings indicate that CPs can be safely performed in these patients represent an emerging phenotype in a significant percentage of patients hospitalized due to COVID-19 several months following discharge resulting in diminished aerobic capacity and ventilatory inefficiency with the primary subjective symptom indicative of these responses being persistent exertional dyspnea.
As previously noted, CRF is now considered a vital sign, a designation particularly due to its robust prognostic significance [5]. Peak VO2 is a proven and established prognostic marker in apparently healthy individuals, those with risk factors for chronic disease and patients with a confirmed diagnosis of one or more chronic disease. The peak VO2 response is primarily driven by left sided cardiac output, but skeletal muscle function also plays an important role, as indicated by the Fick equation [11]. Given both the cardiac and skeletal muscle systems can be negatively impacted by COVID-19, it is not surprising to see a diminished peak VO2 response in a significant percentage of individuals infected, particularly those who have been hospitalized due to increased pathophysiologic severity due to the virus. The VE/VCO2 slope has emerged as an extremely important prognostic marker, particularly in patients with heart failure (HF) and pulmonary arterial hypertension (PAH). Studies in patients with HF and PAH have demonstrated the VE/VCO2 slope is significantly correlated with pulmonary pressures and right-sided cardiac function and is a strong prognostic marker [7,12]. There is growing concern that PAH can be a sequela of COVID-19 [3,4,13] and as such, an elevated VE/VCO2 slope should prompt further assessment of pulmonary hemodynamics, particularly when values exceed 36 [7,12]. Given the trends, we are seeing in patients hospitalized for COVID-19 infection, a strong case can be made to incorporate CPX as a core clinical assessment during follow-up post discharge. It should be noted that, while peak VO2 and the VE/VCO2 slope have been highlighted as primary CPX measures in this editorial, subjective symptoms, hemodynamics, electrocardiography, and the heart rate response during exercise and recovery should also be assessed. Our group has recently proposed a CPX algorithm (Figure 1) highlighting these CPX measures and thresholds for favorable vs. unfavorable responses[14]. As more research is conducted in this patient population, additional variables obtainable during CPET (e.g. pulmonary function testing, flow volume loops during exercise, etc.) may prove to provide important clinical insight.
Figure 1.
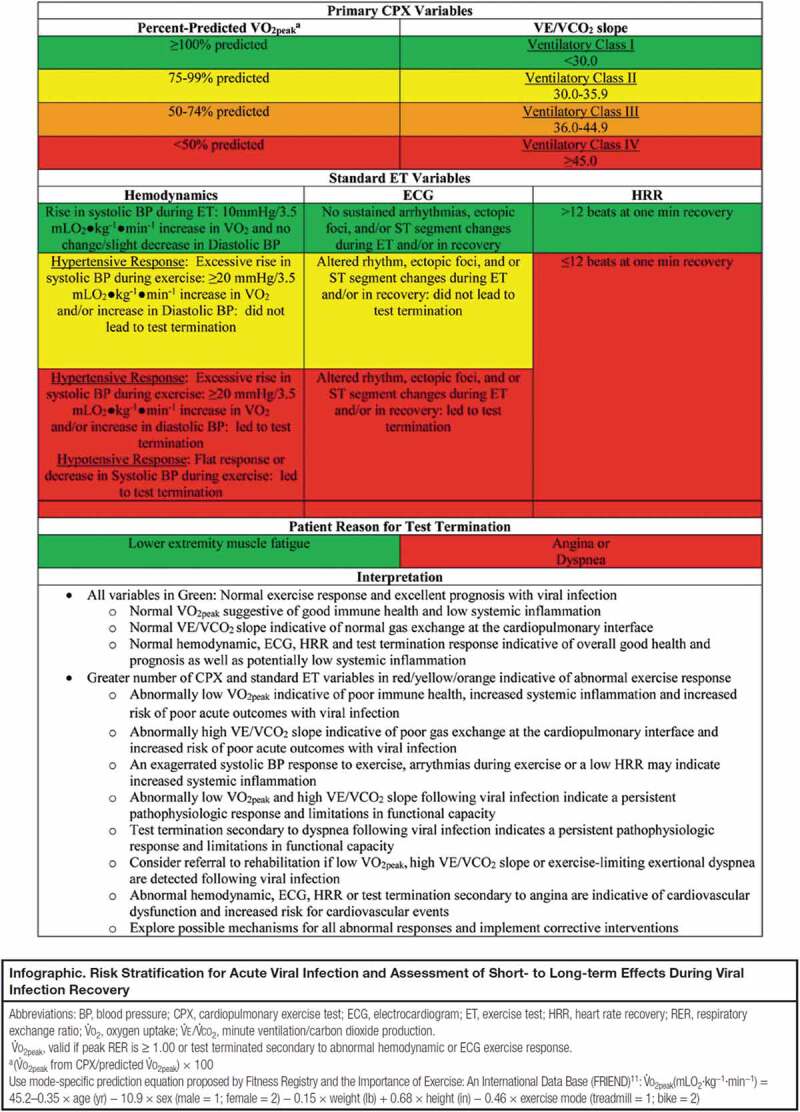
Cardiopulmonary exercise testing algorithm for viral infection
Permission needed: Cardiopulmonary Exercise Testing Algorithm for Viral Infection
ASSESSING HEALTH RISK AND SHORT- TO LONG-TERM EFFECTS. Arena, Ross PhD, PT, FAACVPR; Myers, Jonathan PhD, FAACVPR; Kaminsky, Leonard A. PhD, FAACVPR. Journal of Cardiopulmonary Rehabilitation and Prevention: July 2021 – Volume 41 – Issue 4 – p E7-E8
doi: 10.1097/HCR.0000000000000614
Given the total number of COVID-19 infections globally, it is not feasible or warranted to refer all individuals for CPX testing. For individuals who recover from COVID-19 and return to pre-infection functional activities without new subjective symptomatology, in particular exertional dyspnea, referral for CPX is likely not indicated. However, persistent exertional dyspnea following acute recovery from infection should be a primary indication for CPX referral, to assess current CRF status and inform rehabilitative strategies. In fact, exertional dyspnea is a well-established indication for CPX in other patient populations, including HF and PAH. Moreover, there is now a clear recognition of long COVID syndrome and the need to develop bespoke rehabilitation strategies to alleviate prolonged symptom profile and restore functional capacity [1]. CPX should therefore be considered a core assessment, both at baseline to guide the initial exercise prescription and post intervention to assess efficacy.
There is of course a need for more research related to the value of CPX in the COVID-19 population moving forward. At this point, findings indicate a significant percentage of patients hospitalized with COVID-19 have diminished CRF as measured by CPX. Future research should extend the time from viral infection to CPX as well as include individuals confirmed to have COVID-19 but had milder symptoms that did not require hospitalization. Moreover, studies assessing the relationship between lasting pathophysiology associated with COVID-19 and variations in CRF are needed to determine the ability of CPX to determine system dysfunction (e.g. PAH, skeletal muscle myopathy, cardiac dysfunction, etc.). Identifying CPX phenotypes associated with specific COVID-19-induced pathophysiology will enhance clinical application and interpretation of this exercise assessment. The prognostic value of CPX in the COVID-19 population should also be explored. To achieve this with an appropriately powered sample size, an international registry that includes high-quality CPX laboratories conducting tests in patients with COVID-19 and tracking outcomes would be ideal. The Fitness Registry and the Importance of Exercise National Database (FRIEND) serves as an excellent model for this approach [15]. Lastly, as pharmacologic and rehabilitation interventions are developed to treat patients suffering from chronic system dysfunction and symptoms due to COVID-19 are developed, CPX should be employed to determine their ability to gauge therapeutic efficacy. Conducting research in these areas will further solidify the utility of CPX in the COVID-19 population.
In conclusion, we are working toward a transition from addressing the pandemic through vaccination to managing patients infected with COVID-19 who continue to experience lasting effects. Some of the pathophysiologic effects of COVID-19, as well as the related symptomatology, manifest more profoundly or only during a bout of physical exertion. As such, performing clinical assessments during a controlled bout of exercise is imperative to more fully understanding the effects of COVID-19 and developing optimally effective treatment strategies. Cardiopulmonary exercise testing is the gold-standard approach to clinical exercise testing whose use should be expanded and considered a core assessment in the COVID-19 population.
Funding
This paper was not funded.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Silva RN, Goulart CDL, and Oliveira MR, et al. Cardiorespiratory and skeletal muscle damage due to COVID-19: making the urgent case for rehabilitation. Expert Rev Respir Med. 2021;15(9):1107–1120. [DOI] [PubMed] [Google Scholar]; • Important: Comprehensive review of COVID-19 effects and makes strong case for rehabilitation.
- 2.Raman B, Cassar MP, Tunnicliffe EM, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31:100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AW, Ullah I, Khan KS, et al. Pulmonary arterial hypertension post COVID-19: a sequala of SARS-CoV-2 infection? Respir Med Case Rep. 2021;33:101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki YJ, Nikolaienko SI, Shults NV, et al. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med Hypotheses. 2021;147:110483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R, Blair SN, Arena R, et al. Importance of Assessing Cardiorespiratory Fitness in Clinical Practice: a Case for Fitness as a Clinical Vital Sign: a Scientific Statement From the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]; •• Highly important: Makes strong case for CRF to be used as a vital sign.
- 6.Faghy MA, Sylvester KP, Cooper BG, et al. Cardiopulmonary exercise testing in the COVID-19 endemic phase. Br J Anaesth. 2020;125:447–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guazzi M, Adams V, Conraads V, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pleguezuelos E, Del Carmen A, Llorensi G, et al. Severe loss of mechanical efficiency in COVID-19 patients. J Cachexia Sarcopenia Muscle. 2021;12:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skjørten I, Ankerstjerne OAW, Trebinjac D, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Resp J. 2021;58:2100996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aparisi Á, Ybarra-Falcón C, García-Gómez M, et al. Exercise ventilatory inefficiency in Post-COVID-19 syndrome: insights from a prospective evaluation. J Clin Med. 2021;10:2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M, Cahalin LP, Arena R.. Cardiopulmonary exercise testing as a diagnostic tool for the detection of left-sided pulmonary hypertension in heart failure. J Card Fail. 2013;19:461–467. [DOI] [PubMed] [Google Scholar]
- 13.Tudoran C, Tudoran M, Lazureanu VE, et al. Evidence of Pulmonary Hypertension after SARS-CoV-2 infection in subjects without previous significant cardiovascular pathology. J Clin Med. 2021;10:199. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Important: Highlights the potential for pulmonary arterial hypertension in COVID-19 patients.
- 14.Arena R, Myers J, Kaminsky LA. Cardiopulmonary Exercise Testing Algorithm for Viral Infection: assessing health rish and shore- to long-term effects. J Cardiopulm Rehabil Prev. 2021;41:E7–e8. [DOI] [PubMed] [Google Scholar]
- 15.Kaminsky LA, Arena R, Beckie TM, et al. The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation. 2013;127:652–662. [DOI] [PubMed] [Google Scholar]


