Abstract
Aspirates of liver abscesses were analyzed for Entamoeba histolytica. PCR detected a gene encoding a 30-kDa protein in all samples but detected the ribosomal DNA gene in only 14 (33.3%) samples. Enzyme-linked immunosorbent assay detected antigen in 41 (97.6%) samples. PCR analysis of a strain-specific antigen (SSG) revealed that abscesses were caused by various strains.
Amebic liver abscess is one of the most devastating extraintestinal manifestations of amebiasis, and it is still fairly prevalent in China. As many as 1,078 cases had been reported in Hubei province and adjacent areas in 1983 (11), and it was even more common than amebic dysentery therein (14). Since amebic liver abscess may be lethal if adequate therapy is delayed, early diagnosis is essential (6, 7, 10). The mis- or overdiagnosis of amebic liver abscess has been frequent in China because it often has to rely on presumptive evidence, such as the anchovy sauce appearance of the aspirate or positive serological reactions to amebic antigen (15).
In this investigation we have analyzed by PCR and enzyme-linked immunosorbent assay (ELISA) aspirates (or pus) and serum samples collected from two series of Chinese patients. The first group consisted of 42 patients with amebic liver abscess, the diagnosis of which was based on the following criteria: (i) the liver was enlarged and tender, and a fluid wave could be felt over the hepatic region; (ii) fever and/or toxemic symptoms were present; (iii) the liver aspirate appeared like anchovy sauce but was bacteriologically sterile; (iv) positive ELISA of serum antibody showed an unusually high titer against Entamoeba histolytica; and (v) abscesses showing a satisfactory response to antiamebic therapy (metronidazole) were present. The control series consisted of 14 patients: 3 with bacterial liver abscesses (substantiated by typical clinical manifestations, liver aspirates of yellow-white color, and negative responses to antiamebic therapy, etc.), 1 with liver cancer (pathologically confirmed), and 10 with abscesses of other types (anorectic, mammary, and subcutaneous, etc.), all identified by bacteriological studies. Furthermore, not a single patient in this series of patients had antiamebic antibodies in their sera.
A considerable proportion of cases of amebic liver abscess is always difficult to confirm parasitologically (1). In our present series of 42 cases, E. histolytica trophozoites were originally demonstrated in only eight (19%) cases. Furthermore, abscess fluid was often not of the classic anchovy paste appearance (11). In order to help confirm the diagnosis of the amebic liver abscess, we have compared ELISA and several of the recently developed PCR analysis methods for the above-mentioned samples. The results in Table 1 show that all the sera from the 42 patients considered to have amebic liver abscesses had significant titers (1:1,280 to 1:81,920) of antibody against amebic antigen prepared as previously described (8) from axenically cultured trophozoites of virulent E. histolytica HM-1:IMSS.
TABLE 1.
Detection of antiamebic antibody, amebic antigen, and Entamoeba DNA by ELISA and PCR from sera and aspirates of patients with amebic liver abscesses
| Diagnosis | No. of patients | No. (%) of positive results by:
|
|||||
|---|---|---|---|---|---|---|---|
| ELISA for:
|
PCR for:
|
||||||
| Antiamebic antibody in sera | Amebic antigen in pus | Gene coding for 30-kDa protein of:
|
rRNA of:
|
||||
| E. histolytica (100 bp) | E. dispar (101 bp) | E. histolytica (870 bp) | E. dispar (870 bp) | ||||
| Amebic liver abscess | 42 | 42 (100) | 41 (97.6) | 42 (100) | 0 | 14 (33.3) | 0 |
| Bacterial liver abscess | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Liver cancer | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other types of abscess | 10 | 0 | 0 | 0 | 0 | 0 | 0 |
The detection of amebic antigen in the liver pus aspirates was done by ELISA. Polyclonal antibodies were prepared in rabbits immunized with lysates of axenically grown E. histolytica HM-1:IMSS organisms. ELISA plates coated with pus samples were reacted with rabbit anti-E. histolytica sera (diluted 1:200 in phosphate-buffered saline) and after repeated washing reacted with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G. As shown in Table 1, the detection of amebic antigen was positive in 41 (97.6%) cases.
All the patients in the control series showed negative reactions to both these tests. Our results are slightly better than those of Bhave et al. (2) and Mahajan and Ganguly (8), who reported the detection of amebic antigens in amebic liver abscess pus by immunoelectrophoresis and ELISA with sensitivities of 92 and 96%, respectively.
Analyses of E. histolytica by PCR were performed after the isolation of DNA from the liver pus aspirates. Preparation of DNA was done as previously described (4, 5). In brief, it consists of lysis of the pus sample with a solution of EDTA, sodium dodecyl sulfate, NaCl, and proteinase K followed by incubation for 1 h at 60°C. DNA was solvent extracted and precipitated with sodium chlorate at −20°C. Three different sets of primers were used for PCR: (i) two sets for differentiating between E. histolytica and Entamoeba dispar small-subunit rRNA genes (a product of 870 bp) as described by Clark and Diamond (5), (ii) two sets for distinguishing E. histolytica and E. dispar 30-kDa antigen genes (100 and 101 bp, respectively) as described by Tachibana et al. (12, 13), and (iii) one set for the strain-specific gene (SSG) of E. histolytica (4). At the end of the reaction, the products were size fractionated on 1% agarose gels, and the products were blot hybridized with radiolabeled probes as described by Mirelman et al. (9). The SSG product was hybridized with the radiolabeled E. histolytica HM-1:IMSS gene product (4).
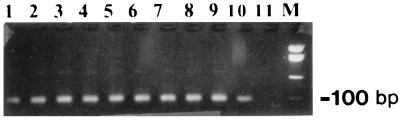
As shown in Table 1 all the samples in the control series as well as those with primers specific for E. dispar had negative reactions. PCR detected the presence of the gene coding for the 30-kDa protein in all 42 (100%) cases of amebic liver abscess (Fig. 1). All experimental and control materials were analyzed in a blind format. This finding confirms earlier reports by Tachibana et al. (12). On the other hand, PCR detected the presence of ribosomal DNA (rDNA) in only 13 (33.3%) cases of amebic liver infection (data not shown). These results indicate that PCR is a sensitive and specific method only for detecting the gene coding for the 30-kDa protein in pus samples, whereas that for rDNA, though specific, is, however, not sensitive enough. Our finding that the PCR detection of the gene encoding the 30-kDa protein is significantly more efficient than that of the rDNA gene was somewhat surprising because the rDNA genes are much more abundant than those of the 30-kDa protein (3, 5, 9, 13). The explanation for this finding needs further investigation. Another aspect that should be pointed out is that our lack of finding of any genes coding for the E. dispar 30-kDa protein and rRNA, in any of the amebic liver abscess cases, supports the accepted hypothesis that E. dispar does not cause invasive disease in humans.
FIG. 1.
Agarose gel separation of PCR products amplified from E. histolytica DNA encoding the 30-kDa antigen in a liver abscess. Lanes 1 to 9, results for patients with amebic liver abscess; lane 10, E. histolytica HM-1:IMSS; lane 11, negative control; M, marker.
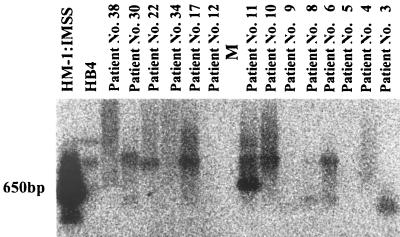
Our present results also show for the first time that a number of different strains of E. histolytica can be responsible for the induction of human amebic abscesses. Amplifications of the SSG were obtained for 10 of the 42 aspirates (Fig. 2), and their electrophoretic migration differed. The SSG has been reported to be absent from certain laboratory-cultivated isolates and to exhibit variable genomic organization when present (4). The SSG from HM-1:IMSS contains nine repeats of a 26-nucleotide sequence, and the SSG product was, as expected, ca. 650 bp, whereas the sizes of the SSG major products differed among the pus-derived DNAs in the 10 cases of amebic liver abscess. This indicates the significant variation in the number of 26-bp repeats and clearly demonstrates that although the cases of amebic liver abscess were from the same region in China, they seem to be due to several different strains of E. histolytica.
FIG. 2.
PCR amplification products of the SSG from the pus samples of 10 patients with amebic liver abscesses. PCR products were separated on a 1% agarose gel, and Southern blots were hybridized with radiolabeled SSG DNA amplified separately from the DNA of E. histolytica HM-1:IMSS, as described previously (4).
Acknowledgments
We thank Xie Yunqiu of Leiyang People’s Hospital, Hunan province, People’s Republic of China, for helping with the collection of the specimens from the patients.
We also thank A. Azzi, University of Bern, under whose directorship a UNESCO-MCBN Short-Term Fellowship in Molecular and Cell Biology was granted in 1997 to G.Z. This work was supported in part by grants from The Commission of the European Communities (Avicenne Program) and by The Center for the Study of Emerging Diseases.
REFERENCES
- 1.Aikat B K, Bhusnurmath S R, Pal A K, Chhuttani P N, Dutta D V. The pathology and pathogenesis of fatal hepatic amoebiasis—a study based on 79 autopsy cases. Trans R Soc Trop Med Hyg. 1979;73:188–192. doi: 10.1016/0035-9203(79)90209-8. [DOI] [PubMed] [Google Scholar]
- 2.Bhave G G, Wagle N M, Joshi U M. Detection of amoebic antigen by enzyme linked immunosorbent assay (ELISA) J Postgrad Med. 1985;31:146–149. [PubMed] [Google Scholar]
- 3.Bracha R, Diamond L S, Ackers J P, Burchard G D. Differentiation of clinical isolates of Entamoeba histolytica by using specific DNA probes. J Clin Microbiol. 1990;28:680–684. doi: 10.1128/jcm.28.4.680-684.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark C G, Diamond L S. Entamoeba histolytica: a method for isolate identification. Exp Parasitol. 1993;77:450–455. doi: 10.1006/expr.1993.1105. [DOI] [PubMed] [Google Scholar]
- 5.Clark C G, Diamond L S. Ribosomal RNA genes of “pathogenic” and “nonpathogenic” Entamoeba histolytica are distinct. Mol Biochem Parasitol. 1991;49:297–302. doi: 10.1016/0166-6851(91)90073-f. [DOI] [PubMed] [Google Scholar]
- 6.Guo Z Z, Wang C I, Zhang Y Q, Gui S H. Application of enzyme linked immunosorbent assay in the diagnosis of amoebiasis. Natl Med J China. 1984;64:71–74. [PubMed] [Google Scholar]
- 7.Healy G R. Laboratory diagnosis of amebiasis. Bull NY Acad Trop Med. 1971;47:478–493. [PMC free article] [PubMed] [Google Scholar]
- 8.Mahajan R C, Ganguly N K. Amoebic antigen in immunodiagnosis and prognosis of amoebic liver abscess. Trans R Soc Trop Med Hyg. 1980;74:300–302. doi: 10.1016/0035-9203(80)90086-3. [DOI] [PubMed] [Google Scholar]
- 9.Mirelman D, Nuchamowitz Y, Stolarsky T. Comparison of use of enzyme-linked immunosorbent assay-based kits and PCR amplification of rRNA genes for simultaneous detection of Entamoeba histolytica and E. dispar. J Clin Microbiol. 1997;35:2405–2407. doi: 10.1128/jcm.35.9.2405-2407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravdin J I. Amebiasis. Clin Infect Dis. 1995;20:1453–1464. doi: 10.1093/clinids/20.6.1453. [DOI] [PubMed] [Google Scholar]
- 11.Society of Digestion. Hubei Branch of Chinese Medical Association. Amoebic hepatic abscess: analysis of 1078 cases. Chin J Dig. 1983;3:5–8. [Google Scholar]
- 12.Tachibana H, Kobayashi S, Okuzawa E, Masuda G. Detection of pathogenic Entamoeba histolytica DNA in liver abscess fluid by polymerase chain reaction. Int J Parasitol. 1992;22:1193–1196. doi: 10.1016/0020-7519(92)90042-j. [DOI] [PubMed] [Google Scholar]
- 13.Tachibana H, Kobayashi S, Takekoshi M, Ihara S. Distinguishing isolates of Entamoeba histolytica by polymerase chain reaction. J Infect Dis. 1991;164:825–826. doi: 10.1093/infdis/164.4.825. [DOI] [PubMed] [Google Scholar]
- 14.Wang C I. Parasitic diarrhoeas in China. Parasitol Today. 1988;4:284–288. doi: 10.1016/0169-4758(88)90020-8. [DOI] [PubMed] [Google Scholar]
- 15.Wen G Y, Yu S Y. The causes of erroneous diagnosis of amebic liver abscess. Chin J Dig. 1983;3:35–36. [Google Scholar]