Abstract
Pulmonary arterial hypertension (PAH) is a cardiopulmonary disease with high mortality. In recent years, it has been recognized that PAH is a multi-organ system disease, involving the systemic circulation, kidneys, skeletal muscles, and the central nervous system, among others. Right heart failure produces congestive hepatopathy, a disease state that has direct consequences on liver biochemistry, histology, and systemic glucose and lipid metabolism. This article aims to summarize the consequences of congestive hepatopathy with an emphasis on liver biochemistry, histology, and PAH-targeted therapy. Furthermore, PAH-specific changes in glucose and lipid metabolism will be discussed.
Keywords: pulmonary arterial hypertension, liver, venous congestion, lipid metabolism
Introduction
Pulmonary hypertension (PH) describes a cardiopulmonary disease, in which elevated blood pressure in the lung circulation affects the right ventricle (RV) and can lead to RV failure. Currently, PH is categorized by hemodynamic and clinical schemes. Hemodynamically, PH is defined by a mean pulmonary arterial pressure (mPAP) above 20 mmHg. PH can be divided into a pre- and post-capillary component. Pre-capillary PH, or pulmonary arterial hypertension (PAH), is defined by an elevated mPAP in the absence of increased pulmonary venous or left-sided filling pressures. Whereas post-capillary PH is defined by an increased mPAP and increased pulmonary venous or left-sided filling pressures. A combined pre-and post-capillary state can be present, defined as elevated mPAP, and increased pulmonary vascular resistance (PVR), or increased diastolic pressure gradient. 1 In addition to the hemodynamic definition, PH is currently divided into five different clinical groups according to the underlying pathophysiology, clinical presentation, and treatment strategy. More recently, PAH has been recognized as a multi-organ systemic disease, with abnormalities in the systemic circulation, central and peripheral nervous system, kidneys, skeletal muscle, and immune system.2,3 Due to its intimate anatomical and physiological relationship to the RV, the liver is one of the first organs impacted by RV failure from PH. RV volume- and pressure overload can lead to congestive hepatopathy that can be associated with a multitude of liver abnormalities.
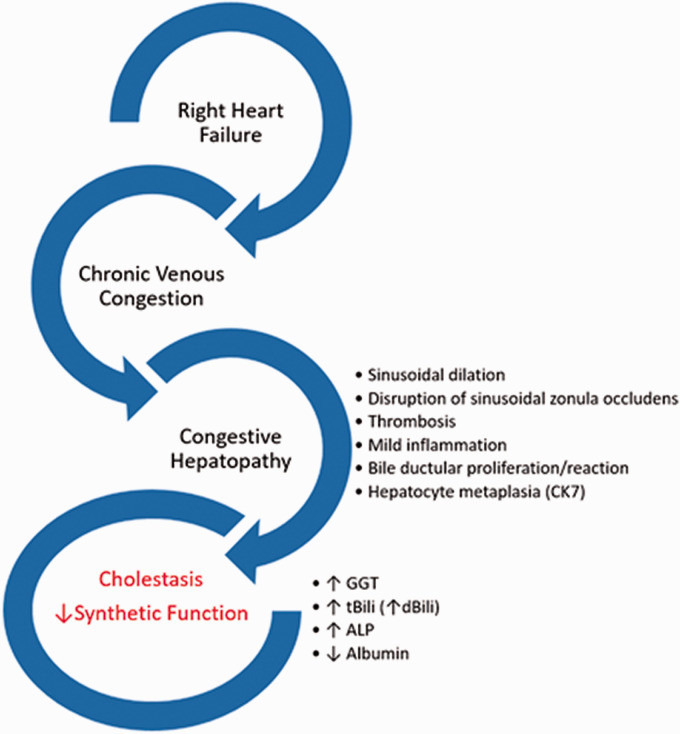
The liver is the largest solid organ in the adult human body and performs multiple diverse biological functions. There is a bidirectional intricate relationship between the liver, heart, and lung. Liver disease can manifest in various pulmonary diseases. Malnutrition from advanced cirrhosis can lead to immunosuppression and increase the risk for pulmonary infections. 4 Primary biliary cholangitis can be associated with fibrosing alveolitis, pulmonary hemorrhage, and organizing pneumonia. 5 Monogenetic diseases such as hereditary hemorrhagic telangiectasia are associated with vascular malformations in the liver and lungs. 6 Similarly, abnormal pulmonary vasodilation and the formation of arteriovenous malformations can develop in patients with congenital heart disease in which the pulmonary circulation is depleted of hepatic venous return. 7 Cirrhosis is associated with pulmonary arterial hypertension (portopulmonary hypertension) or abnormal dilation of the pulmonary vasculature associated with severe hypoxia (hepatopulmonary syndrome). 8 Conversely, cardio-pulmonary disease can lead to liver pathology. Cardiogenic shock and congestive heart failure can be associated with ischemic hepatitis and congestive hepatopathy, respectively.9,10 Venous congestion from RV failure can lead to a significant increase in hepatic blood volume with a substantial impact on liver function (Fig. 1). 11
Fig. 1.
Right heart failure and liver abnormalities in PAH.
This review article is intended to inform the reader about the pathophysiology of liver abnormalities observed in patients with PAH without pre-existing liver disease. PH associated with liver disease, portopulmonary hypertension, or hepatopulmonary syndrome are not discussed.
Liver biochemical testing in PAH
The dual vascular supply and high metabolic demand put the liver at high risk for circulatory injury from heart failure. The main circulatory disturbances affecting the liver are congestion from right heart failure, ischemic injury from low cardiac output states, or a combination of both.
Acute heart failure with decreased cardiac output and shock can result in ischemic hepatitis. 12 This condition is characterized by a hepatocellular injury pattern with a rapid increase in serum aminotransferases (aspartate and alanine transaminase, AST/ALT), and lactate dehydrogenase (LDH), often above 10 times the upper limit of normal. 9
In contrast, hepatic venous congestion from chronic heart failure is mainly characterized by cholestatic liver enzyme elevation (increased bilirubin, alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), 12 and hypoalbuminemia 13 ). Patients with acutely decompensated heart failure and decreased cardiac output can often show a mixed liver biochemical pattern with signs of hepatocellular injury (AST and ALT elevation) and elevation in cholestatic liver enzymes (bilirubin, ALP).14,15 Cholestasis usually results from obstructed hepatic bile flow into the gallbladder, leading to the accumulation of bile acids in the liver. 16 Mild elevation in cholestatic biochemical testing, such as bilirubin,15,17 GGT,14,18–22 and ALP17,18,20 are found in 17 to 77% of patients with stable congestive heart failure (CHF). Liver dysfunction in patients with chronic left heart disease is highly variable but closely related to right heart hemodynamics (central venous pressure and right atrial pressure), rather than left heart failure. 22 Interestingly, the exact pathophysiology of cholestatic chemistry in CHF is not clear, since there is usually no evidence of frank bile duct injury or obstruction on liver biopsy (see below). Disturbance of hepatic venous outflow can lead to interstitial and cellular edema that can impair the uptake and secretion of bilirubin, bile acids, and other substances. Dilatation of sinusoids can lead to extravasation of blood and increased serum levels of ALP and GGT. A decrease in hepatic congestion can reverse these processes and lead to improvement of cholestatic chemistry, as seen in patients with good treatment response to medical heart failure therapy or in patients undergoing heart transplantation for end-stage heart failure.23–25
Acute and chronic inflammation can disrupt hepatocyte and cholangiocyte homeostasis, leading to alterations in intracellular bile-acid transporters, impaired bile secretion, and therefore elevation in bilirubin, ALP, and GGT.26,27 PAH is characterized by a chronic pro-inflammatory state with elevated levels of circulating cytokines and chemokines 28 that could contribute to impaired liver function and elevation in cholestatic biochemistry.
Impaired synthetic liver function is a well-recognized complication of chronic heart failure. Up to 25% of patients with chronic heart failure and up to 40% with acute heart failure present with low serum albumin levels.13,29,30
The etiology of hypoalbuminemia in heart failure is likely multifactorial and involves hemodilution, 31 malnutrition, chronic inflammation, 32 protein-losing enteropathy, 33 and proteinuria. 34 Hepatic congestion from right heart volume and pressure overload can also be associated with hypoalbuminemia. 35 Low albumin concentrations are associated with poor long-term outcomes in heart failure patients 36 but failed to predict response to therapy in acute-decompensated heart failure. 37
Compared with left heart disease, the literature on liver test abnormalities in PAH is scarce. Similar to left heart disease, in the absence of acute decompensated heart failure, the predominant biochemical pattern of liver injury in patients with PAH is cholestasis. Elevated total bilirubin levels in PAH patients have been observed in 15 to 20% of patients. 38 A study with 404 idiopathic PAH (IPAH) patients found that direct bilirubin was elevated in 37% of patients and was an independent risk factor of mortality. 39 Several smaller studies with well-characterized cohorts of PAH patients reported mildly elevated total bilirubin, ALP, and GGT.40–44 Elevation in AST/ALT (transaminases) seems to be less common (∼2%).
In most studies, bilirubin elevation at baseline is associated with poor prognosis in PAH patients.38,39,44 In congestive hepatopathy, direct bilirubin is the predominant circulating form of bilirubin and shows a tight relation with central venous pressure. 15 In PAH, direct bilirubin outperformed total bilirubin in predicting survival in multivariate hazard analysis and decreased after response to PAH-targeted therapy or lung transplantation. Consequently, direct bilirubin failed to decrease in patients who did not respond to PAH-targeted therapy, indicating that direct bilirubin is a sensitive marker of venous congestion in PAH and responds to hemodynamic improvement. 39 These results are similar to patients with left heart disease, where decreased venous congestion is associated with a decrease in bilirubin levels and improved survival. 14 It is important to note that in the REVEAL registry, 15% of patients had elevated total bilirubin levels, compared to 4% with kidney dysfunction. However, elevated bilirubin levels were only significantly associated with survival in the univariate model, whereas kidney dysfunction remained a significant predictor of mortality in the multivariate model. 38 Liver and kidney dysfunction were only reported as qualitative variables and therefore need to be interpreted with caution.
Impaired synthetic liver function is common in PAH and decreased albumin levels (<3.3 g/dl) can have been found in up to 19 to 25% of patients.41,45 Lower serum albumin concentrations were also shown in multiple studies to be a strong independent predictor of poor outcome in PAH patients.40,41,45 Low albumin levels in PAH are likely multi-factorial (see above).
In addition, some of the commonly used members of a class of chronic pharmacologic pulmonary vasodilation in PAH, endothelin receptor antagonists, can be associated with elevations in AST and ALT which at times necessitates discontinuation (see below for more details). 46
Despite its significant impact on mortality, and a close relationship to right-sided hemodynamics, liver dysfunction in PAH patients is likely underreported in clinical trials and large registries. This is certainly a component of the PAH field that will require more detailed research to better understand the relationship between PAH and liver dysfunction.
On routine blood work elevation of cholestatic markers with a mild increase in bilirubin, ALP, (GGT), and low albumin are common in PAH and should be monitored over time. Non-invasive biomarkers of liver pathology have low sensitivity and specificity for hepatic congestion. It is therefore important for the clinician to recognize that significant elevation in liver enzymes (2–4 times the upper limit of normal) should immediately prompt a dedicated workup.
Liver histology
Congestive hepatopathy shows a diverse clinical course, non-specific histologic manifestations that vary with the temporal progression and severity of cardiac dysfunction. Histopathological interpretation of liver biopsy samples can be limited by incomplete capturing of the venous outflow tract, consisting of terminal hepatic venules, intercalated veins, and hepatic veins when transcutaneous biopsies are performed. Histopathological characteristics of congestive hepatopathy are patchy distributed centrizonal sinusoidal dilatation and centrizonal fibrosis and mild portal inflammation.47–50 In more advanced disease portal fibrosis, bridging fibrosis and liver cirrhosis can be seen. These changes correlate with the severity of right heart failure and liver congestion (central venous pressure, right atrial pressure), but not with markers of left ventricular function or cholestatic abnormalities in liver biochemical testing.50–52
On a histopathological level, the reason for a predominantly cholestatic liver test pattern in patients with chronic hepatic congestion remains controversial. ALP and GGT are mainly located in the biliary epithelium and the canalicular membranes of the hepatocytes;53,54 therefore, congestive hepatopathy must be associated with some form of interruption of the hepatocyte-bile duct interface. Most biopsy and autopsy studies in congestive hepatopathy did not find evidence of frank bile duct injury or obstruction that could explain serum elevation in cholestatic liver testing.47–49 Interestingly, the same observation was made in severe cases of long-standing hepatic congestion, as seen in patients with Fontan-circulation where over one-third of patients have evidence of cholestatic liver test elevation without signs of cholestasis on biopsy.55–57 Using electron-microscopy on liver biopsy samples from patients with severe congestive hepatopathy revealed occasional dilation and rupture of bile canaliculi. 58 Furthermore, mechanical pressure from hepatic venous congestion can disrupt the zona occludens between hepatocytes and the bile canaliculus, exposing the biliary epithelium to circulating blood. 59 It was also observed that patients with congestive hepatopathy show signs of bile duct proliferation. Increased sinusoidal pressure from the hepatic veins can damage the canalicular bile system leading to biliary metaplasia, proliferation, and extravasation of bile salts, which could also explain the cholestatic enzyme profile seen in congestive hepatopathy.48,49 In addition, two independent research groups found that in congestive hepatopathy, hepatocytes can express Cytokeratin 7 (CK 7), an intermediate filament predominately expressed in biliary epithelium.47,60 In these studies, CK7 expression correlated with serum total bilirubin levels. The authors suggest that CK7 expression in hepatocytes could indicate a metabolic transformation of hepatocytes into cholangiocytes that could explain increased serum levels of total bilirubin and AlkP in the absence of frank biliary disease. However, it is important to note that the expression of CK-7 in hepatocytes is likely non-specific and was also observed in NASH cirrhosis, viral and autoimmune hepatitis. 61
Long-standing hepatic congestion from heart failure may result in cirrhosis. Regardless of the cause of heart failure, right-sided hemodynamics correlate closely with histopathological changes seen in the liver. 51 This is most evident in patients with Fontan-circulation, in which decades of increased venous pressure can result not only in liver cirrhosis but also in hepatocellular carcinoma. 62 Similarly, a biomarker of hepatic fibrogenesis (7S domain of collagen type IV) was found to closely correlate with elevated right-sided filling pressures, and time to clinical worsening in patients with PAH and CHF.63,64
Histologically, Budd-Chiari syndrome, a drug-induced liver injury (pyrrolizidine alkaloids, chemotherapy, immunodeficiency, and radiation damage), can cause hepatic outflow-tract obstruction at different levels and are important considerations in the differential diagnosis of congestive hepatopathy. 65
Even though no comprehensive or detailed reports exist about liver histology specific to patients with PAH, most of the pathophysiological changes from right heart failure will apply to the PAH population. Whether or not PAH-specific therapies, hormonal changes, or alterations in specific metabolic, genetic, and inflammatory pathways are associated with additional or distinctive histologic liver abnormalities is therefore unknown and an area of research opportunity.
Although liver biopsy is not commonly performed in patients with PAH, it is important to recognize the microscopic features of congestive hepatopathy. On histology, a patchy distribution, non-specific centrizonal sinusoidal dilatation, and mild portal inflammation can be seen. These changes are closely linked to right-sided hemodynamics. Venous pressure can lead to micro-injuries at the level of the bile canaliculi, exposing biliary epithelium to circulating blood, leading to mild cholestatic enzyme elevation commonly observed in these patients. Long-standing congestive hepatopathy from chronic right heart failure can result in cirrhosis and in severe cases may lead to hepatocellular carcinoma. See Table 1 for a summary of liver function test abnormalities in patients with PAH.
Table 1.
Biochemical liver abnormalities in PAH.
| Reference | n | Patients | Lab | Summary of findings |
|---|---|---|---|---|
| Kawut et al. 40 | 84 | Treatment naïve, PAH (IPAH, HPAH, drug and toxin) | tBili, dBiliAlbAST/ALT | ↑ ALP, tBili, dBili, ↓Alb were associated with ↓survival in univariate analysis↓Alb was associated with ↓ survival in multivariate analysis |
| Stepnowska et al. 43 | 47 | PAH (CHD, CTD, IPAH) | tBiliALPGGTAST/ALT | ↑tBili was associated with ↓ survival |
| Haddad et al. 41 | 119 | PAH (IPAH, CTD, CHD, drug and toxin), hospitalized with acute RHF | tBiliAlb | 20% of patients had ↑tBili↓Alb was associated with ↓survival |
| Hao et al. 42 | 129 | PAH (CTD) | tBiliALP | ↑ALP was associated with ↓ survival |
| Xu et al. 39 | 404 | PAH (IPAH) | dBili | ↑dBili in 37% of patients↑dbili was associated with ↑WHO-FC and ↓survival |
| Takeda et al. 44 | 37 | PAH (IPAH, CTD) | tBiliAST/ALT | ↑tBili was associated with ↓survival in UV and MV analysis |
| Olsson et al. 66 | 239 | PAH (IPAH, CTD, CHD, PoPH, HIV, CTEPH) | tBili | ↑tBili was associated with ↓survival in UV and MV analysis |
| Benza et al. 38 | 2716 | PAH (IPAH, HPAH, CHD, CTD, PoPH, drug and toxins, HIV) | tBili | ↑tBili in 14% of patients↑tBili was associated with ↓survival in UV analysis |
| Wang et al. 67 | 177 | PAH (IPAH, CTD, PoPH, CHD) | tBili | ↑tBili was associated with ↓survival in UV analysis |
| Benza et al. 68 | 773 | PAH (IPAH, CTD, CHD, PoPH) | tBili | ↑tBili was associated with ↓survival in UV analysis |
| Hu et al. 69 | 173 | PAH (IPAH) | tBiliAST/ALT | ↑tBili was NOT associated with ↓survival in UV or MV analyses |
| Snipelisky et al. 45 | 163 | PAH (IPAH, CTD, PoPH) | Alb | ↓Alb in 25% of patients↓Alb was associated with significant pericardial effusion↓Alb was associated with ↓survival in MV analysis |
PAH: pulmonary arterial hypertension; IPAH: idiopathic pulmonary arterial hypertension; HPAH: hereditary pulmonary arterial hypertension; tBili: total bilirubin; dBili: direct bilirubin; Alb: albumin; ALP: alkaline phosphatase; AST/ALT: aspartate transaminase/Alanine transaminase; CHD: congenital heart disease; CTD: connective tissue disease; PoPH: porto-pulmonary hypertension; HIV: human immunodeficiency virus; ↑: increased; ↓: decreased; ↔: unchanged or normal.
Lipid metabolism in PAH
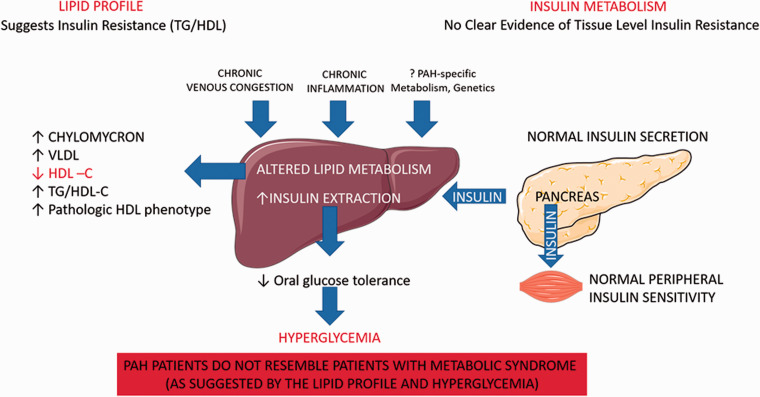
The liver is a central organ of carbohydrate, fat, and protein metabolism. Hepatocytes are a major source of triglycerides, many lipoproteins, and the major recipient of low-density lipoprotein (LDL). The liver is also a major regulator of glucose metabolism and plays an important role in insulin resistance and metabolic syndrome.
It is well established that patients with PAH have abnormal concentrations of circulating lipids and lipoproteins. The exact etiology of these changes and the clinical implications are less well defined. Several reports investigating lipoprotein levels in PAH found a reduction in circulating LDL, high-density lipoprotein (HDL), and chylomicrons.70–74 The most consistent finding of lipid abnormalities in PAH however is a significant reduction of circulating HDL levels. When comparing 69 PAH patients (26% were treatment naïve), to 229 subjects with significant cardiovascular disease risk factors but without evidence of PH, a significant reduction in circulating HDL-C levels was found in the PAH group, that could not be explained by age, sex, or statin use. In addition, low HDL-C was associated with increased mortality. 72 These findings have been replicated by several other groups,71,74,75 including a Chinese cohort of lean IPAH patients. 70 Another study with 227 PAH patients found that HDL levels were an independent predictor of survival after adjusting for comorbidities and right heart hemodynamics. 76 It was also suggested that the subclass of HDL particles modulates the association between HLD and mortality. In two independent PAH cohorts of 127 and 77 patients respectively, a reduction in small Apo A-2 rich HDL subgroup 4 (HDL-4) was independently linked to increased mortality in both cohorts. Based on HDL pleiotropic biological functions, including vasoactive and fibrinolytic activities, it was speculated that reduced circulating HDL levels might be associated with PAH pathophysiology. 77
In one study with 177 treatment naïve patients, HDL levels were significantly reduced in the PAH cohort, compared with patients who did not have PH, and negatively correlated with PVR, mPAP, right atrial pressure, and positively correlated with cardiac index and mixed venous oxygen saturation. 67 In a cohort of 442 unselected patients with congestive heart failure, right ventricular end-diastolic diameter correlated with reduced circulating lipid levels, including TG, HDL, LDL, independent of LV function, age, gender, smoking status, comorbidities, and statin use. In addition, patients with severe TR had significantly lower lipid levels compared with patients who did not have TR. 78 The liver is the main source of HDL production 79 and it is possible that venous congestion from RV dysfunction can lead to reduced hepatic synthesis of lipoproteins, increased hepatic clearance, and decreased intestinal absorption. It was also suggested that there might be a mechanistic link between low circulating HLD levels and insulin resistance in PAH. Prior studies in non-PAH patients have suggested that insulin resistance correlates with an increased triglyceride to HDL ratio (TG/HDL) and TG/HDL ratios are used as a surrogate marker for pre-diabetic state. 80 One study found that PAH patients have higher TG/HDL-C ratios compared with a gender and age-matched NHANES cohort and concluded that PAH patients are therefore more likely to be insulin resistant. 75 In line with that, another study with 41 PAH patients without a diagnosis of diabetes mellitus found that about half of the PAH patients showed signs of poor glucose control, based on Hemoglobin A1C levels. 81 Even though abnormal glucose homeostasis has consistently shown to be a clinical feature of PAH, the underlying mechanisms remain controversial. It has been shown in various studies with large numbers of different study subjects, that even though TG/HDL ratio correlates with insulin resistance (IR), it is an imperfect predictor of glucose homeostasis Cross-sectional longitudinal analyses with around 3000 patients from the Framingham heart study, found that IR predicts cardiovascular events independent of TG/HDL ratios, implying that lipid and glucose metabolism are not necessarily linked.82,83 Interestingly, in the original publication investigating reduced HDL levels and outcomes in PAH, HDL levels did not correlate with IR measured by homeostatic model assessment of IR (HOMA-IR), an index of fasting plasma glucose and insulin levels. 72 The finding that triglyceride and HDL levels do not correlate with glucose homeostatic metrics in PAH was later confirmed by several studies using HOMA-IR or oral glucose tolerance testing (OGGT).73,74,84 Two independent groups found that elevated TG/HLD levels showed a strong correlation with inflammatory markers in PAH patients, such as CXCL-10, IL-1b, IL-6, and MCP-1 [Heresi 2017, Jonas 2019], but not with adiposity or insulin levels.73,84 This suggests that decreased HDL levels and other lipid abnormalities in PAH might in part be related to chronic inflammation or may have a biological role in the pathobiology of PAH, rather than simply be a consequence of obesity or the metabolic syndrome.
Using oral glucose tolerance testing (OGTT) in IPAH and healthy controls, two independent groups observed that PAH patients have lower baseline plasma glucose and insulin levels and decreased glucose-stimulated insulin secretion, suggestive of increased insulin sensitivity in PAH. 70 , 84 To further investigate this, one study group utilized the gold standard to define insulin secretion, the hyperglycemic clamp (blood glucose maintained at ∼180 mg/dl for 3 h) in six PAH patients and six age- BMI and sex-matched controls. 85 This study confirmed a decreased insulin response to hyperglycemia in PAH patients as found in other studies.74,81,84 Surprisingly, the reduction in the insulin response was not due to reduced pancreatic insulin secretion or poor peripheral tissue insulin response, as expected in patients with insulin resistance and metabolic syndrome. The authors rather found an increased hepatic insulin extraction in PAH patients contributing to abnormal oral glucose homeostasis. 85 These findings underscore that abnormal glucose homeostasis in PAH patients is unlikely due to peripheral tissue level (skeletally muscle) insulin resistance and perhaps mediated by decreased systemic insulin circulation due to increased hepatic extraction of insulin. Here, the PAH pathophysiology of glucose homeostasis clearly differs from patients with diabetes mellitus or individuals with insulin resistance due to metabolic syndrome. In type 2 diabetes mellitus, there is an association between poor glucose control and decreased hepatic insulin extraction, contributing to hyperinsulinemia and metabolic syndrome. 86 To this effect, PAH patients behave more like patients with advanced left-sided heart failure. One study did not find a difference when comparing pancreatic insulin secretion in 140 patients with advanced systolic heart failure, compared to 21 sex- age and BMI-matched controls. 87 Worsening systolic heart failure was associated with a reduced insulin/C-peptide ratio that correlated with parameters of right heart function. The authors speculated that liver congestion from worsening right heart failure might cause an increase in hepatic insulin extraction leading to poor glucose control. Abnormal glucose homeostasis and reduced circulating HDL-C levels are also common features of patients with Fontan physiology and chronically elevated central venous pressure leading to congestive hepatopathy.88,89 In these patients elevated central venous pressure correlated with poor oral glucose control using OGGT. These findings indicate that hepatic congestion independent of its etiology can result in reduced circulating HDL levels and abnormal glucose control.
Traditional cardiovascular comorbidities are increasingly recognized in an aging PAH patient population. Recent research revealed that the PAH metabolism might differ from patients with dyslipidemia, insulin resistance, and metabolic syndrome. This concept not only opens exciting research possibilities but might also have important clinical and treatment implications. It is important for the clinician to understand that the traditional interpretation of lipoprotein levels and hypercholesterolemia as risk factors for cardiovascular morbidity may not apply in the PAH patient population. In addition, there is emerging evidence that the pathophysiology of poor glucose control in PAH differs from patients with diabetes and metabolic syndrome. See Table 2 for a summary of metabolic liver abnormalities in PAH versus patients with metabolic syndrome.
Table 2.
Liver in PAH and metabolic syndrome.
| PAH | Metabolic syndrome90–93 | |
|---|---|---|
| Lipid metabolism | ||
| VLDL | ↑ 74 | ↑ |
| LDL | ↓ 71 or ↔67,74 | ↑↑ |
| TG | ↓ or ↔ | ↑↑ |
| HDL | ↓↓ 94 | ↓ |
| TG/HDL | ↑ 72 - 75 | ↑ |
| Hepatic insulin resistance | No evidence of ↑ | ↑ |
| Hepatic insulin extraction | ↑ 85 | ↓ |
| Hepatic fat content | No evidence of ↑ | ↑↑ |
PAH: pulmonary arterial hypertension; VLDL: very-low-density lipoprotein; LDL: low-density lipoprotein; TG: triglycerides; HDL: high-density lipoprotein; ↑: increased; ↓: decreased; ↔: unchanged or normal.
Abnormal liver metabolism in patients with PAH is summarized in Fig. 2.
Fig. 2.
Liver metabolism in patients with PAH.
PAH-targeted therapy and the liver
Currently, five different drug classes are approved to treat PAH: prostanoids, prostacyclin receptor agonists, phosphodiesterase 5 inhibitors, (PDE-5i), soluble guanylate cyclase stimulators (sGCS), and endothelin receptor antagonists (ERAs). Except Epoprostenol, 95 most commonly used PAH-targeted therapies are – at least partially – metabolized by the liver. Significant liver toxicity from PAH-targeted therapy is rare. The most common finding of PAH-therapy related hepatotoxicity is a hepatocellular injury pattern with elevation in the transaminases. Among the three prostanoids used to treat PAH, Iloprost and treprostinil undergo hepatic metabolism and their clearance is impaired in patients with liver dysfunction. It is recommended to initiate Iloprost and treprostinil at a lower dose in patients with hepatic impairment and the use in patients with severe hepatic dysfunction (Child-Pugh C) is discouraged.96,97 In subjects with mild hepatic impairment, oral treprostinil drug levels can increase 1.6- to 2.1-fold. The manufacturer, therefore recommends dose adjustments in patients with mild and moderate hepatic impairment (Child-Pugh class A, B) and recommends against use in patients with severe hepatic impairment (Child-Pugh class C). 98 Treprostinil drug levels can by influenced by inducers or inhibitors of the cytochrome pathway and drug–drug interactions should be reviewed before its use.
Selexipag is an oral prostacyclin agonist that is metabolized in the liver. There was no significant hepatotoxicity reported in clinical trials, but these trials did not include patients with pre-existing liver disease. 99 In patients with moderate hepatic impairment, selexipag levels can increase 2- to 4-fold, when compared to subjects with normal liver function. 100 The manufacturer recommends dose adjustments in patients with moderate hepatic impairment (once-daily regimen versus twice-daily) and recommends against use in patients with severe hepatic impairment (Child-Pugh class C). 101 Selexipag is metabolized by the cytochrome system, which may lead to drug–drug interactions.
PDE-5i are metabolized by the liver. However, neither sildenafil nor tadalafil have been extensively evaluated in patients with severe liver disease (Child-Pugh class C) and their use in this patient population is discouraged.102,103 In patients with mild to moderate hepatic cirrhosis (Child-Pugh class A and B), sildenafil clearance is reduced and can lead up to a 47% increase in maximum drug concentration. According to the manufacturer, it should be considered to adjust the starting dose of tadalafil to 20 mg per day (instead of 40 mg) in patients with mild to moderate hepatic impairment (Child-Pugh class A or B). 102 Both PDE-5i are metabolized by the cytochrome system and prone to drug–drug interactions.
ERAs are hepatically metabolized, but only bosentan is both a substrate and inducer of the cytochrome system. 104 According to a recent meta-analysis, bosentan was associated with the highest risk for hepatotoxicity, compared with ambrisentan 105 and macitentan. 106 In a post-marketing survey, 7.6% of patients taking bosentan developed an elevation in aminotransferases. 107 The bosentan-induced liver injury seems to be dose-dependent.108,109 The most common liver injury during bosentan therapy is hepatocellular (AST/ALT), but cholestatic liver injury with increased ALP levels has been described. Compared to bosentan, ambrisentan seems to have less hepatotoxicity. 110 Macitentan has been linked to one case of fulminant hepatitis in a young patient with PAH. 111 However, in larger clinical trials, hepatotoxicity from macitentan was rare. 112 Macitentan is not a substrate of active drug transporters, possibly leading to its favorable liver safety profile. 113 Macitentan undergoes hepatic conversion into an active metabolite, and therefore drug levels can be reduced in patients with hepatic impairment.106,114
Currently, monthly monitoring of liver function test is only recommended in patients taking bosentan, 115 and baseline liver function test is recommended before initiation of ambrisentan and macitentan and repeated during treatment as clinically indicated. The exact mechanism of hepatotoxicity of ERAs is unknown, but likely involves interaction with bile salt transporters, glucuronidation, and cytochrome metabolism. 116 This could explain why the cholestatic potency of bosentan can be amplified by other bile-salt transporter inhibitors, such as glyburide. 108 All ERAs should be discontinued if there is evidence of significant hepatotoxicity.
Riociguat, a sGCS, is metabolized by the liver and hepatic impairment can be associated with higher drug levels. 117 The use of riociguat is discouraged in patients with severe hepatic impairment (Child-Pugh class C). 118 Patients should also be monitored closely for drug–drug interactions.
PAH patients are prone to hepatotoxicity and the practitioner should be aware of PAH-targeted therapy that is metabolized by the liver. Drug–drug interactions are common and need to be taken into consideration before starting PAH-targeted therapies. Even though liver toxicity from PAH-targeted therapy is uncommon, dose adjustments might be necessary. Only patients taking bosentan should be routinely monitored for hepatocellular liver injury. Hepatically metabolized PAH-targeted therapies and dose-adjustment recommendations are summarized in Table 3.
Table 3.
Liver metabolism and dose-adjustment of PAH-targeted therapy in patients with hepatic impairment.
| Drug | Liver metabolism | Dose adjustments | ||
|---|---|---|---|---|
| Mild hepatic impairment (Child-Pugh class A) | Moderate hepatic impairment (Child-Pugh class B) | Severe hepatic impairment (Child-Pugh class C) | ||
| Iloprost, inhaled | Increased drug levels in patients with hepatic impairment; not CYP dependent | Consider a starting dose of 2.5 µg and increased dosing intervals (e.g., 3–4 h) | Consider a starting dose of 2.5 µg and increased dosing intervals (e.g., 3–4 h) | Not recommended |
| Treprostinil iv, sc | 2- to 4-fold increase in maximum drug levels in patients with mild to moderate hepatic impairment; drug levels can be influenced by CYP inducer/inhibitors | Initiation dose should be reduced to 0.625 ng/kg/min, increase dose slowly | Initiation dose should be reduced to 0.625 ng/kg/min, increase dose slowly | Not recommended |
| Treprostinil, oral | 1.6-fold increase in maximum drug levels in patients with mild hepatic impairment, 4-fold increase in maximum drug levels in patients with moderate hepatic impairment; drug levels can be influenced by CYP inducer/inhibitors | Start at 0.125 mg twice daily in patients with mild hepatic impairment; avoid in patients with moderate hepatic impairment | Not recommended | Not recommended |
| Selexipag | 1.4-fold increase in maximum drug levels, 2- to 4.5-fold increase in AUC in patients with mild to moderate hepatic impairment; drug levels can be influenced by CYP inducer/inhibitors | No dose adjustment necessary | Start at 200 µg once daily; increase by 200 µg once daily at weekly intervals as tolerated | Contraindicated |
| Riociguat | Minimal increase in maximum drug levels, but 1.5- to 2-fold increase in AUC in patients with moderate hepatic impairment; drug levels can be influenced by CYP inducer/inhibitors | No dose adjustment necessary | Likely increased drug levels, monitor closely for adverse effects | Not recommended |
| Bosentan | 5- to 12-fold increase in maximum drug levels and active metabolite, drug levels can be influenced by CYP inducer/inhibitors; Liver enzymes need to be monitored | Reduce dose if ALT/AST >3 and <5× ULN, stop Bosentan if >5× ULN. | Not recommended | Contraindicated |
| Ambrisentan | Likely increased drug levels in patients with hepatic impairment; drug levels can be influenced by CYP inducer/inhibitors | Discontinue if AST/ALT >5× ULN, or if AST/ALT>2× ULN and increase in total bilirubin | Not recommended | Not recommended |
| Macitentan | Production of active metabolite; associated with slightly reduced plasma levels of active metabolites in patients with hepatic impairment (clinically likely irrelevant); drug levels can be influenced by CYP inducer/inhibitors | Discontinue if AST/ALT >5× ULN, or if AST/ALT>2× ULN and increase in total bilirubin | Not recommended | Not recommended |
| Tadalafil | Likely increased drug levels in patients with hepatic impairment; drug levels can be influenced by CYP inducer/inhibitors | Consider starting dose of 20 mg | Consider starting dose of 20 mg | Not recommended |
| Sildenafil | 1.5-fold increase in maximum drug levels in patients with mild to moderate hepatic impairment; drug levels can be influenced by CYP inducers/inhibitors | No dose adjustment necessary | No dose adjustment necessary | Not recommended |
iv: intravenous; sc: subcutaneous; CYP: cytochrome pathway; AUC: area under the curve; AST/ALT: aspartate transaminase/alanine transaminase; ULN: upper limit of normal.
Footnotes
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Guarantor: Nils P. Nickel is responsible for the content of this manuscript.
Author contributions: Nils P. Nickel conducted the literature review, wrote the paper, and created the figures. Gian M. Galura created the table and wrote the manuscript. Marc J. Zuckerman wrote the manuscript. M. Nawar Hakim wrote the manuscript. Haider Alkhateeb reviewed and edited the manuscript. Debabrata Mukherjee reviewed and edited the manuscript. Eric D. Austin reviewed and edited the manuscript. Gustavo D. Heresi wrote the manuscript.
ORCID iDs: Nils P. Nickel https://orcid.org/0000-0002-8087-0166
Debabrata Mukherjee https://orcid.org/0000-0002-5131-3694
Eric D. Austin https://orcid.org/0000-0002-1709-9022
Gustavo D. Heresi https://orcid.org/0000-0002-9797-2599
References
- 1.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nickel NP, Yuan K, Dorfmuller P, et al. Beyond the lungs: systemic manifestations of pulmonary arterial hypertension. Am J Respir Crit Care Med 2020; 201: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenkranz S, Howard LS, Gomberg-Maitland M, et al. Systemic consequences of pulmonary hypertension and right-sided heart failure. Circulation 2020; 141: 678–693. [DOI] [PubMed] [Google Scholar]
- 4.Gao F, Cai MX, Lin MT, et al. Model for end-stage liver disease and pneumonia: an improved scoring model for critically ill cirrhotic patients with pneumonia. Turk J Gastroenterol 2019; 30: 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koksal D, Koksal AS, Gurakar A. Pulmonary manifestations among patients with primary biliary cirrhosis. J Clin Transl Hepatol 2016; 4: 258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bofarid S, Hosman AE, Mager JJ, et al. Pulmonary vascular complications in hereditary hemorrhagic telangiectasia and the underlying pathophysiology. Int J Mol Sci 2021; 22: 3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavarana MN, Jones JA, Stroud RE, et al. Pulmonary arteriovenous malformations after the superior cavopulmonary shunt: mechanisms and clinical implications. Expert Rev Cardiovasc Ther 2014; 12: 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. The Lancet 2004; 363: 1461–1468. [DOI] [PubMed] [Google Scholar]
- 9.Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol 2017; 33: 158–163. [DOI] [PubMed] [Google Scholar]
- 10.Hilscher M, Sanchez W. Congestive hepatopathy. Clin Liver Dis (Hoboken) 2016; 8: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol 2010; 16: 6046–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samsky MD, Patel CB, DeWald TA, et al. Cardiohepatic interactions in heart failure: an overview and clinical implications. J Am Coll Cardiol 2013; 61: 2397–2405. [DOI] [PubMed] [Google Scholar]
- 13.Arques S, Roux E, Sbragia P, et al. Usefulness of serum albumin concentration for in-hospital risk stratification in frail, elderly patients with acute heart failure. Insights from a prospective, monocenter study. Int J Cardiol 2008; 125: 265–267. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 15.van Deursen VM, Damman K, Hillege HL, et al. Abnormal liver function in relation to hemodynamic profile in heart failure patients. J Card Fail 2010; 16: 84–90. [DOI] [PubMed] [Google Scholar]
- 16.Yokoda RT, Rodriguez EA. Review: pathogenesis of cholestatic liver diseases. World J Hepatol 2020; 12: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen LA, Felker GM, Pocock S, et al. Liver function abnormalities and outcome in patients with chronic heart failure: data from the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Eur J Heart Fail 2009; 11: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poelzl G, Ess M, Mussner-Seeber C, et al. Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur J Clin Invest 2012; 42: 153–163. [DOI] [PubMed] [Google Scholar]
- 19.Goyal P, Almarzooq ZI, Horn EM, et al. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am J Med 2016; 129: 635.e15–635.e26. [DOI] [PubMed] [Google Scholar]
- 20.Lau GT, Tan HC, Kritharides L. Type of liver dysfunction in heart failure and its relation to the severity of tricuspid regurgitation. Am J Cardiol 2002; 90: 1405–1409. [DOI] [PubMed] [Google Scholar]
- 21.Dalos D, Binder C, Duca F, et al. Serum levels of gamma-glutamyltransferase predict outcome in heart failure with preserved ejection fraction. Sci Rep 2019; 9: 18541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ess M, Mussner-Seeber C, Mariacher S, et al. Gamma-glutamyltransferase rather than total bilirubin predicts outcome in chronic heart failure. J Card Fail 2011; 17: 577–584. [DOI] [PubMed] [Google Scholar]
- 23.Scholfield M, Schabath MB, Guglin M. Longitudinal trends, hemodynamic profiles, and prognostic value of abnormal liver function tests in patients with acute decompensated heart failure: an analysis of the ESCAPE trial. J Card Fail 2014; 20: 476–484. [DOI] [PubMed] [Google Scholar]
- 24.Dichtl W, Vogel W, Dunst KM, et al. Cardiac hepatopathy before and after heart transplantation. Transpl Int 2005; 18: 697–702. [DOI] [PubMed] [Google Scholar]
- 25.Crespo-Leiro MG, Robles O, Paniagua MJ, et al. Reversal of cardiac cirrhosis following orthotopic heart transplantation. Am J Transplant 2008; 8: 1336–1339. [DOI] [PubMed] [Google Scholar]
- 26.Trauner M, Fickert P, Stauber RE. Inflammation-induced cholestasis. J Gastroenterol Hepatol 1999; 14: 946–959. [DOI] [PubMed] [Google Scholar]
- 27.Horvatits T, Drolz A, Trauner M, et al. Liver injury and failure in critical illness. Hepatology 2019; 70: 2204–2215. [DOI] [PubMed] [Google Scholar]
- 28.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasini E, Aquilani R, Gheorghiade M, et al. Malnutrition, muscle wasting and cachexia in chronic heart failure: the nutritional approach. Ital Heart J 2003; 4: 232–235. [PubMed] [Google Scholar]
- 30.Biegus J, Hillege HL, Postmus D, et al. Abnormal liver function tests in acute heart failure: relationship with clinical characteristics and outcome in the PROTECT study. Eur J Heart Fail 2016; 18: 830–839. [DOI] [PubMed] [Google Scholar]
- 31.Adlbrecht C, Kommata S, Hulsmann M, et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body's red cell volume. Eur Heart J 2008; 29: 2343–2350. [DOI] [PubMed] [Google Scholar]
- 32.Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005; 39: S143–S146. [DOI] [PubMed] [Google Scholar]
- 33.Battin DL, Ali S, Shahbaz AU, et al. Hypoalbuminemia and lymphocytopenia in patients with decompensated biventricular failure. Am J Med Sci 2010; 339: 31–35. [DOI] [PubMed] [Google Scholar]
- 34.Jackson CE, Solomon SD, Gerstein HC, et al. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet 2009; 374: 543–550. [DOI] [PubMed] [Google Scholar]
- 35.Carr JG, Stevenson LW, Walden JA, et al. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1989; 63: 709–713. [DOI] [PubMed] [Google Scholar]
- 36.Horwich TB, Kalantar-Zadeh K, MacLellan RW, et al. Albumin levels predict survival in patients with systolic heart failure. Am Heart J 2008; 155: 883–889. [DOI] [PubMed] [Google Scholar]
- 37.Grodin JL, Lala A, Stevens SR, et al. Clinical Implications of serum albumin levels in acute heart failure: insights from DOSE-AHF and ROSE-AHF. J Card Fail 2016; 22: 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 39.Xu XQ, Lv ZC, Liu QQ, et al. Direct bilirubin: a new risk factor of adverse outcome in idiopathic pulmonary arterial hypertension. Int J Cardiol 2017; 228: 895–899. [DOI] [PubMed] [Google Scholar]
- 40.Kawut SM, Horn EM, Berekashvili KK, et al. New predictors of outcome in idiopathic pulmonary arterial hypertension. Am J Cardiol 2005; 95: 199–203. [DOI] [PubMed] [Google Scholar]
- 41.Haddad F, Peterson T, Fuh E, et al. Characteristics and outcome after hospitalization for acute right heart failure in patients with pulmonary arterial hypertension. Circ Heart Fail 2011; 4: 692–699. [DOI] [PubMed] [Google Scholar]
- 42.Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J 2014; 44: 963–972. [DOI] [PubMed] [Google Scholar]
- 43.Stepnowska E, Lewicka E, Dabrowska-Kugacka A, et al. Predictors of poor outcome in patients with pulmonary arterial hypertension: a single center study. PLoS One 2018; 13: e0193245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda Y, Takeda Y, Tomimoto S, et al. Bilirubin as a prognostic marker in patients with pulmonary arterial hypertension. BMC Pulm Med 2010; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snipelisky D, Jentzer J, Batal O, et al. Serum albumin concentration as an independent prognostic indicator in patients with pulmonary arterial hypertension. Clin Cardiol 2018; 41: 782–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YJ, Wang N, Gu ZC, et al. A network meta-analysis for safety of endothelin receptor antagonists in pulmonary arterial hypertension. Cardiovasc Diagn Ther 2019; 9: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krings G, Can B, Ferrell L. Aberrant centrizonal features in chronic hepatic venous outflow obstruction: centrilobular mimicry of portal-based disease. Am J Surg Pathol 2014; 38: 205–214. [DOI] [PubMed] [Google Scholar]
- 48.Louie CY, Pham MX, Daugherty TJ, et al. The liver in heart failure: a biopsy and explant series of the histopathologic and laboratory findings with a particular focus on pre-cardiac transplant evaluation. Mod Pathol 2015; 28: 932–943. [DOI] [PubMed] [Google Scholar]
- 49.Kakar S, Batts KP, Poterucha JJ, et al. Histologic changes mimicking biliary disease in liver biopsies with venous outflow impairment. Mod Pathol 2004; 17: 874–878. [DOI] [PubMed] [Google Scholar]
- 50.Myers RP, Cerini R, Sayegh R, et al. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology 2003; 37: 393–400. [DOI] [PubMed] [Google Scholar]
- 51.Dai DF, Swanson PE, Krieger EV, et al. Congestive hepatic fibrosis score: a novel histologic assessment of clinical severity. Mod Pathol 2014; 27: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 52.Yoshihisa A, Sato Y, Yokokawa T, et al. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail 2018; 5: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gowda S, Desai PB, Hull VV, et al . A review on laboratory liver function tests. Pan Afr Med J 2009; 3: 17. [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014; 29: 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kendall TJ, Stedman B, Hacking N, et al. Hepatic fibrosis and cirrhosis in the Fontan circulation: a detailed morphological study. J Clin Pathol 2008; 61: 504–508. [DOI] [PubMed] [Google Scholar]
- 56.van Nieuwenhuizen RC, Peters M, Lubbers LJ, et al. Abnormalities in liver function and coagulation profile following the Fontan procedure. Heart 1999; 82: 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rathgeber SL, Guttman OR, Lee AF, et al. Fontan-associated liver disease: spectrum of disease in children and adolescents. J Am Heart Assoc 2020; 9: e012529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Safran AP, Schaffner F. Chronic passive congestion of the liver in man. Electron microscopic study of cell atrophy and intralobular fibrosis. Am J Pathol 1967; 50: 447–463. [PMC free article] [PubMed] [Google Scholar]
- 59.Cogger VC, Fraser R, Le Couteur DG. Liver dysfunction and heart failure. Am J Cardiol 2003; 91: 1399. [DOI] [PubMed] [Google Scholar]
- 60.Pai RK, Hart JA. Aberrant expression of cytokeratin 7 in perivenular hepatocytes correlates with a cholestatic chemistry profile in patients with heart failure. Mod Pathol 2010; 23: 1650–1656. [DOI] [PubMed] [Google Scholar]
- 61.Matsukuma S, Takeo H, Kono T, et al. Aberrant cytokeratin 7 expression of centrilobular hepatocytes: a clinicopathological study. Histopathology 2012; 61: 857–862. [DOI] [PubMed] [Google Scholar]
- 62.Lemmer A, VanWagner LB, Ganger D. Assessment of advanced liver fibrosis and the risk for hepatic decompensation in patients with congestive hepatopathy. Hepatology 2018; 68: 1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagao K, Tamura A, Morimoto T, et al. Liver fibrogenesis marker, 7S domain of collagen type IV in patients with acutely decompensated heart failure: correlates, prognostic value and time course. Int J Cardiol 2017; 236: 483–487. [DOI] [PubMed] [Google Scholar]
- 64.Yoshihisa A, Kimishima Y, Kiko T, et al. Liver fibrosis marker, 7S domain of collagen type IV, in patients with pre-capillary pulmonary hypertension. Int J Cardiol 2018; 258: 269–274. [DOI] [PubMed] [Google Scholar]
- 65.Fan CQ, Crawford JM. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J Clin Exp Hepatol 2014; 4: 332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olsson KM, Nickel NP, Tongers J, et al. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol 2013; 167: 2300–2305. [DOI] [PubMed] [Google Scholar]
- 67.Wang GF, Guan LH, Zhou DX, et al. Serum high-density lipoprotein cholesterol is significantly associated with the presence and severity of pulmonary arterial hypertension: a retrospective cross-sectional study. Adv Ther 2020; 37: 2199–2209. [DOI] [PubMed] [Google Scholar]
- 68.Benza RL, Gomberg-Maitland M, Naeije R, et al. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant 2011; 30: 982–989. [DOI] [PubMed] [Google Scholar]
- 69.Hu EC, He JG, Liu ZH, et al. High levels of serum lactate dehydrogenase correlate with the severity and mortality of idiopathic pulmonary arterial hypertension. Exp Ther Med 2015; 9: 2109–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao QH, Peng FH, Wei H, et al. Serum high-density lipoprotein cholesterol levels as a prognostic indicator in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol 2012; 110: 433–439. [DOI] [PubMed] [Google Scholar]
- 71.Kopec G, Waligora M, Tyrka A, et al. Low-density lipoprotein cholesterol and survival in pulmonary arterial hypertension. Sci Rep 2017; 7: 41650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heresi GA, Aytekin M, Newman J, et al. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med 2010; 182: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jonas K, Magon W, Podolec P, et al. Triglyceride-to-high-density lipoprotein cholesterol ratio and systemic inflammation in patients with idiopathic pulmonary arterial hypertension. Med Sci Monit 2019; 25: 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemnes AR, Luther JM, Rhodes CJ, et al. Human PAH is characterized by a pattern of lipid-related insulin resistance. JCI Insight 2019; 4: e123611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J 2009; 33: 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsen CM, McCully RB, Murphy JG, et al. Usefulness of high-density lipoprotein cholesterol to predict survival in pulmonary arterial hypertension. Am J Cardiol 2016; 118: 292–297. [DOI] [PubMed] [Google Scholar]
- 77.Harbaum L, Ghataorhe P, Wharton J, et al. Reduced plasma levels of small HDL particles transporting fibrinolytic proteins in pulmonary arterial hypertension. Thorax 2019; 74: 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen Y, He XM, Meng H, et al. Relationship between lipids levels and right ventricular volume overload in congestive heart failure. J Geriatr Cardiol 2014; 11: 192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ginsberg HN. Lipoprotein physiology. Endocrinol Metab Clin North Am 1998; 27: 503–519. [DOI] [PubMed] [Google Scholar]
- 80.McLaughlin T, Abbasi F, Cheal K, et al. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann Intern Med 2003; 139: 802–809. [DOI] [PubMed] [Google Scholar]
- 81.Pugh ME, Robbins IM, Rice TW, et al. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kannel WB, Vasan RS, Keyes MJ, et al. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort). Am J Cardiol 2008; 101: 497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robins SJ, Lyass A, Zachariah JP, et al. Insulin resistance and the relationship of a dyslipidemia to coronary heart disease: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2011; 31: 1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heresi GA, Malin SK, Barnes JW, et al. Abnormal glucose metabolism and high-energy expenditure in idiopathic pulmonary arterial hypertension. Ann Am Thorac Soc 2017; 14: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mey JT, Hari A, Axelrod CL, et al. Lipids and ketones dominate metabolism at the expense of glucose control in pulmonary arterial hypertension: a hyperglycaemic clamp and metabolomics study. Eur Respir J 2020; 55: 1901700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Najjar SM, Perdomo G. Hepatic insulin clearance: mechanism and physiology. Physiology (Bethesda) 2019; 34: 198–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melenovsky V, Benes J, Franekova J, et al. Glucose homeostasis, pancreatic endocrine function, and outcomes in advanced heart failure. J Am Heart Assoc 2017; 6: e005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Whiteside W, Tan M, Yu S, et al. Low total, low-density lipoprotein, high-density lipoprotein, and non-high-density lipoprotein cholesterol levels in patients with complex congenital heart disease after Fontan palliation. J Pediatr 2013; 162: 1199–1204. [DOI] [PubMed] [Google Scholar]
- 89.Ohuchi H, Miyamoto Y, Yamamoto M, et al. High prevalence of abnormal glucose metabolism in young adult patients with complex congenital heart disease. Am Heart J 2009; 158: 30–39. [DOI] [PubMed] [Google Scholar]
- 90.Ruotolo G, Howard BV. Dyslipidemia of the metabolic syndrome. Curr Cardiol Rep 2002; 4: 494–500. [DOI] [PubMed] [Google Scholar]
- 91.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem 2009; 42: 1331–1346. [DOI] [PubMed] [Google Scholar]
- 92.Dewidar B, Kahl S, Pafili K, et al. Metabolic liver disease in diabetes – from mechanisms to clinical trials. Metabolism 2020; 111S: 154299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pivovarova O, Bernigau W, Bobbert T, et al. Hepatic insulin clearance is closely related to metabolic syndrome components. Diabetes Care 2013; 36: 3779–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khirfan G, Tejwani V, Wang X, et al. Plasma levels of high density lipoprotein cholesterol and outcomes in chronic thromboembolic pulmonary hypertension. PLoS One 2018; 13: e0197700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.VELETRI (epoprostenol) for injection prescription information; accessdata.fda.gov.
- 96.REMODULIN (treprostinil) injection prescription information; accessdata.fda.gov.
- 97.VENTAVISVR (iloprost) inhalation solution, for oral inhalationuse prescription information; accessdata.fda.gov.
- 98.ORENITRAM (treprostinil) extended release tablets fororal administration prescription information; accessdata.fda.gov.
- 99.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 100.Kaufmann P, Cruz HG, Krause A, et al. Pharmacokinetics of the novel oral prostacyclin receptor agonist selexipag in subjects with hepatic or renal impairment. Br J Clin Pharmacol 2016; 82: 369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.UPTRAVI (selexipag) tablets, for oral use prescription information; accessdata.fda.gov.
- 102.ADCIRCA (tadalafil) tablets for oral administration prescription information; accessdata.fda.gov.
- 103.REVATIO (sildenafil) tablets, for oral use prescription information; accessdata.fda.gov.
- 104.Chaumais MC, Guignabert C, Savale L, et al. Clinical pharmacology of endothelin receptor antagonists used in the treatment of pulmonary arterial hypertension. Am J Cardiovasc Drugs 2015; 15: 13–26. [DOI] [PubMed] [Google Scholar]
- 105.Letairis (ambrisentan) tablets, for oral use prescription information; accessdata.fda.gov.
- 106.OPSUMIT (macitentan) tablets, for oral use prescription information; accessdata.fda.gov.
- 107.Segal ES, Valette C, Oster L, et al. Risk management strategies in the postmarketing period: safety experience with the US and European bosentan surveillance programmes. Drug Saf 2005; 28: 971–980. [DOI] [PubMed] [Google Scholar]
- 108.Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69: 223–231. [DOI] [PubMed] [Google Scholar]
- 109.TRACLEERVR (bosentan) tablets, for oral use Prescription Information; accessdata.fda.gov.
- 110.Patel KR, Blair CJ, Tislow JD. Hepatic safety of ambrisentan alone and in combination with tadalafil: a post-hoc analysis of the AMBITION trial. Pulm Circ 2018; 8: 2045894018797273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tran TT, Brinker AD, Munoz M. Serious liver injury associated with Macitentan: a case report. Pharmacotherapy 2018; 38: e22–e24. [DOI] [PubMed] [Google Scholar]
- 112.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 113.Sidharta PN, Treiber A, Dingemanse J. Clinical pharmacokinetics and pharmacodynamics of the endothelin receptor antagonist macitentan. Clin Pharmacokinet 2015; 54: 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sidharta PN, Lindegger N, Ulc I, et al. Pharmacokinetics of the novel dual endothelin receptor antagonist macitentan in subjects with hepatic or renal impairment. J Clin Pharmacol 2014; 54: 291–300. [DOI] [PubMed] [Google Scholar]
- 115.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 116.Lepist EI, Gillies H, Smith W, et al. Evaluation of the endothelin receptor antagonists ambrisentan, bosentan, macitentan, and sitaxsentan as hepatobiliary transporter inhibitors and substrates in sandwich-cultured human hepatocytes. PLoS One 2014; 9: e87548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dasgupta A, Bowman L, D'Arsigny CL, et al. Soluble guanylate cyclase: a new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin Pharmacol Ther 2015; 97: 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.ADEMPAS (riociguat) tablets, for oral use prescription information; accessdata.fda.gov.