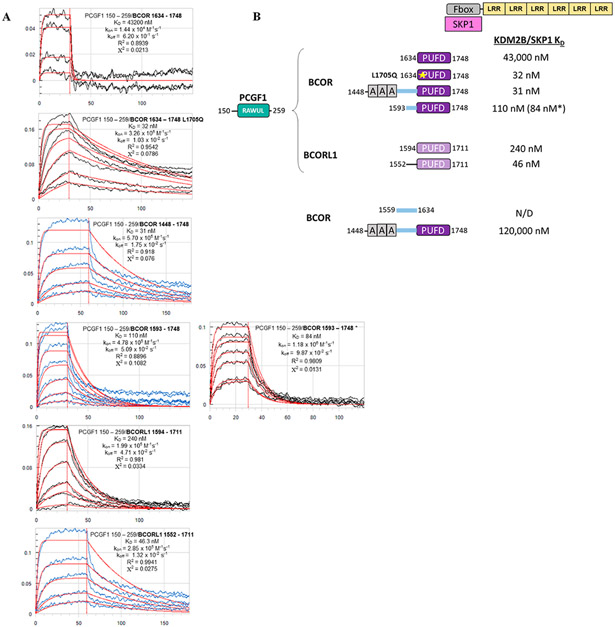
Figure 3.
Binding affinity measurements between the dimer of PCGF1 RAWUL/PUFD-containing protein combinations and the KDM2B/SKP1 dimer using biolayer interferometry (BLI). (A) Raw BLI titration data. The KDM2B/SKP1 dimer was immobilized. The dimer of PCGF1 RAWUL bound to the indicated BCOR and BCORL1 variants introduced in the mobile phase. (B) Summary of the KD values recorded in panel A. KD values are consistent with Ni2+ pull-down data. For example, consistent with Figure 2B, PCGF1 RAWUL bound to BCOR PUFD exhibits a very low affinity (43000 nM) for KDM2B/SKP1 but affinity is regained with the L1705Q mutant (32 nM). An asterisk indicates the experiment was conducted in the reverse orientation; i.e., the biotin-tagged PCGF1/BCOR dimer was immobilized, and KDM2B/SKP1 introduced in the mobile phase, which helps to ensure the reliability of the method.