Abstract
The complex network of chemical processes that sustain life motivates the development of new synthetic tools to decipher biological mechanisms of action at a molecular level. In this context, fluorescent and related optical probes have emerged as useful chemical reagents for monitoring small-molecule and metal signals in biological systems, enabling visualization of dynamic cellular events with spatial and temporal resolution. In particular, metals occupy a central role in this field as analytes in their own right, while also being leveraged for their unique biocompatible reactivity with small-molecule substrates. This Viewpoint highlights the use of inorganic chemistry principles to develop activity-based sensing platforms mediated by metal reactivity, spanning indicators for metal detection to metal-based reagents for bioorthogonal tracking, and manipulation of small and large biomolecules, illustrating the privileged roles of metals at the interface of chemistry and biology.
Graphical Abstract
INTRODUCTION
Metals are essential nutrients in living systems, exhibiting a diversity of chemistry with biomolecules spanning catalysis to electron transfer to static and dynamic structural stabilization.1 However, this same chemical diversity of metal reactivity can, when dysregulated, contribute to numerous diseases, including cancer2 and neurodegenerative,3 metabolic,4,5 and cardiovascular disorders.6 Indeed, depending on properties such as Lewis acidity, polarizability, oxidation state, and spin state, metals can engage in both ionic and covalent bonding interactions, giving rise to coordination complexes with varying degrees of metal association. As a result, the total metal pool in a given biological context is divided into two stores: a static pool, comprising tightly bound metal centers (e.g., metal cofactors), and a labile pool, which encompasses loosely associated metals (e.g., metal signals).7,8
The traditional view of this biological inorganic chemistry is that redox-inactive metals such as sodium, potassium, and zinc are well-recognized for their roles in signaling processes,1,9 whereas redox-active transition metals like copper and iron are often associated with catalytic centers buried within the active sites of enzymes for metabolic purposes. Indeed, transition metals can access a range of oxidation and spin states while accommodating diverse coordination geometries and thus can promote challenging transformations, but can also participate in detrimental reactivity often associated with reactive oxygen species (ROS) generation (e.g., Fenton chemistry).1 A broader, more inclusive view of metals in biology has recently emerged with evidence for redox-active metals acting as cellular signals10–12 to alter enzyme function, highlighted by two examples establishing that reversible copper binding beyond the active site can enhance (e.g., mitogen-activated protein kinase, MEK1)13,14 or inhibit (e.g., phosphodiesterase 3B, PDE3B)15 the catalytic activity of proteins. The unique chemistry of metals emphasizes their diverse reactivity at a molecular scale as well as the importance of dynamic metal regulation in higher-order biological contexts spanning cells, tissues, organs, and organisms.
In contrast to their organic counterparts in biology, metals can be neither created nor destroyed and are instead carefully regulated through acquisition, trafficking, and efflux pathways.16 As such, dynamic communication between the static and labile metal pools is necessary to maintain proper homeostasis of metal stores, requiring a detailed understanding of the cellular mechanisms employed to control metal trafficking and reactivity as well as techniques to identify both types of pools.17–19 To meet this goal, mass spectrometry techniques, such as laser-ablation inductively coupled plasma mass spectrometry20–22 and secondary-ion mass spectrometry,23 along with X-ray-based methods that include X-ray absorbance spectroscopy,24 X-ray fluorescence microscopy,25 particle-induced X-ray emission,26 and electron microscopy27,28 have been commonly utilized to study total metal pools.19 However, such technologies are limited to providing relative metal distribution statistics within fixed biological samples as opposed to furnishing dynamic, spatially resolved information regarding metal speciation in living specimens.17,19 Indeed, molecular imaging has been extensively used to trace the activity of labile metal pools in real time and space, illuminating intricate metal-dependent biological pathways,18,29,30 and in this regard, a variety of fluorescent and other optical probes designed for the selective detection of essential transition metals and their toxic heavy-metal counterparts in living systems have been developed.29,31–37 While the diverse chemical properties of metals offer various features to target when designing metal-responsive sensors, they also present challenges for achieving high selectivity in complex biological environments.17 In particular, the ability to distinguish between different oxidation states of the same metal (e.g., FeII/III, CuI/II), achieve specificity for transition metals over more abundant alkali and alkaline-earth congeners, and prevent adventitious fluorescence quenching via electron transfer from paramagnetic centers38 is critical for the development of effective tools for imaging labile metal pools.8 In this regard, activity-based sensing (ABS) approaches that exploit unique metal reactivity profiles have enabled the detection of metals such as iron,39 cobalt,40 nickel,41 and copper42 with high metal and oxidation state specificity.
In a separate but related avenue of research, transition-metal complexes have also found utility as bioorthogonal molecular probes owing to their broad and tunable reactivity toward small-molecule substrates.43,44 Such activity-based strategies are attracting growing interest because they have the potential to improve selectivity toward molecular analytes in complex biological settings beyond conventional lock-and-key molecular recognition. Representative examples include carbon monoxide (CO) monitoring through a palladium-mediated carbon-ylation,45 ethylene detection via ruthenium-catalyzed meta-thesis,46 and nitric oxide (NO),47 hydrogen sulfide (H2S),48 and superoxide (O2−)49 sensing platforms that exploit the versatile redox and Lewis acid reactivity of copper complexes.
The topic of this Viewpoint is the use of fundamental inorganic chemistry strategies to design highly specific ABS probes. In particular, we summarize types of inorganic reactivity exploited, as well as commonly employed metal-based triggers for mapping labile metal pools and associated biorelevant small molecules. With a focus on inorganic principles applied to molecular probe design to encourage more activity in the field, we highlight representative examples to illustrate concepts rather than provide a comprehensive list of probes, and readers can refer to other literature reports for further consideration.29–35,50 Along these lines, we restrict our discussion to ABS probes that have been reported to operate in cells and more complex biological environments with requisite selectivity and signal-to-noise responses. We then close by considering potential future avenues to explore in the area of activity-based metal chemistry, particularly beyond cellular imaging.
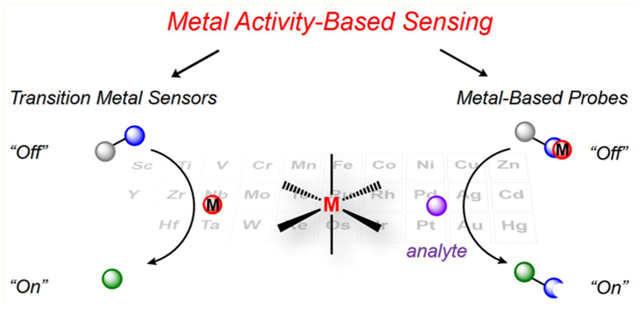
FROM BINDING-BASED SENSING (BBS) TO ABS
The design of fluorescent probes for metal sensing has widely explored the versatile coordination chemistry and reactivity of metals, encompassing two general mechanisms for achieving specificity: (1) BBS (Figure 1a) and (2) ABS (Figure 1b).37 In both cases, a site that recognizes the metal center is appended to a fluorophore to effect a change in fluorescence upon interaction with the metal analyte of interest.
Figure 1.
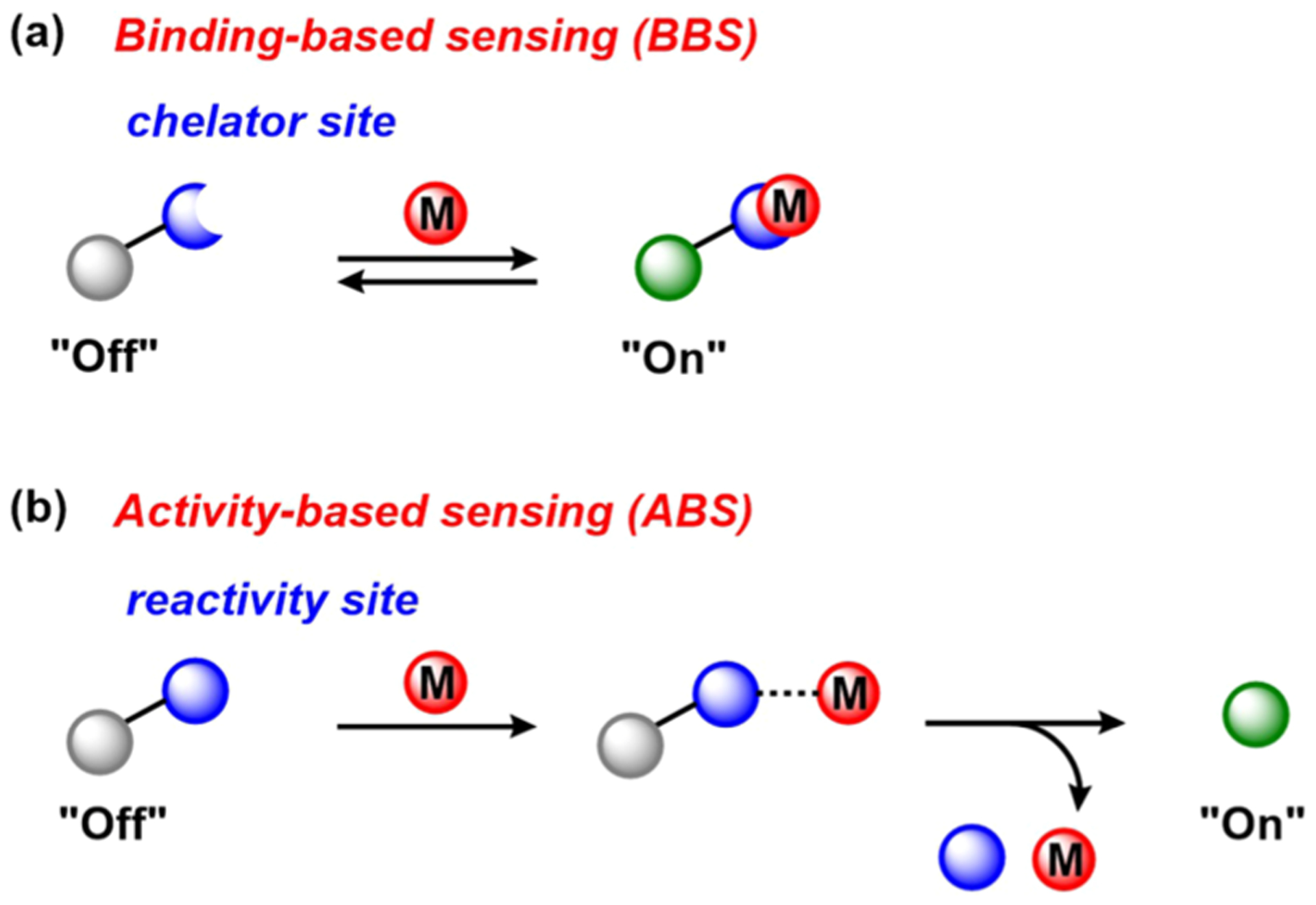
Approaches for designing probes for metal detection: (a) BBS; (b) ABS. Examples are shown in a “turn-on” fluorescence response mode.
The binding-based approach relies on a chelating ligand site that reversibly binds the metal of interest and induces a fluorescence response via mechanisms such as internal charge transfer (ICT), photoinduced electron transfer (PET), or Förster resonance energy transfer (FRET). In this context, probes that elicit a “turn-on” increase or ratiometric color change response are more useful than “turn-off” probes, where spatial information for the latter is more challenging to obtain. Metal selectivity is often conferred by considering preferences in coordination numbers and geometries, hard–soft acid–base theory, and/or ligand-binding thermodynamics as dictated by the Irving–Williams series.51 Using these fundamental coordination chemistry principles, fluorescent sensors have been successfully developed for the detection of many endogenous metals29,30,35,37 in biological systems, most commonly CaII 52 and ZnII,30,35 as well as CuI 53–55 and NiII.56 However, applying this binding-based strategy becomes challenging when dealing with metals that are more weakly binding on the Irving–Williams series and/or can be paramagnetic in many coordination environments, such as MnII and FeII. To overcome such limitations, the ABS approach makes use of metal-specific transformations such that, upon metal sensing, a chemoselective reaction is induced to modify the optical properties of the fluorophore and results in an irreversible turn-on or change in the fluorescence response. Because ABS relies on both metal binding and a coupled chemical reaction event, this method can potentially offer a higher degree of metal specificity over binding-based detection alone, and if the activity-based reaction results in a cleavage product with fluorophore separation and release from the metal, this approach can also limit potential quenching from paramagnetic metal analytes. On the other hand, unlike BBS probes, typical ABS reagents do not allow for reversible metal sensing. Nevertheless, ABS has enabled the detection of redox-active transition metals including FeII, CoII, or CuI with high metal and oxidation state selectivity.37 More broadly, this ABS approach is not limited to metals; indeed, transient and reactive small molecules that would be difficult to track owing to their similar shape and size to other biological metabolites are also capable of engaging in highly specific and bioorthogonal chemical reactions.32,43,57,58 Representative examples from our laboratory include redox-neutral 2-aza-Cope-type rearrangements for formaldehyde sensing,59,60 oxidative conversion of aryl boronates to phenols for hydrogen peroxide (H2O2) detection,61–65 and reductive organic azide to aniline transformations for H2S sensing.66–68 Here, we highlight select examples that incorporate transition-metal reactivity trends into new chemical tools for biological imaging using ABS approaches, spanning metal detection to metal-directed small-molecule detection and bioorthogonal metal catalysis (Figure 2).
Figure 2.
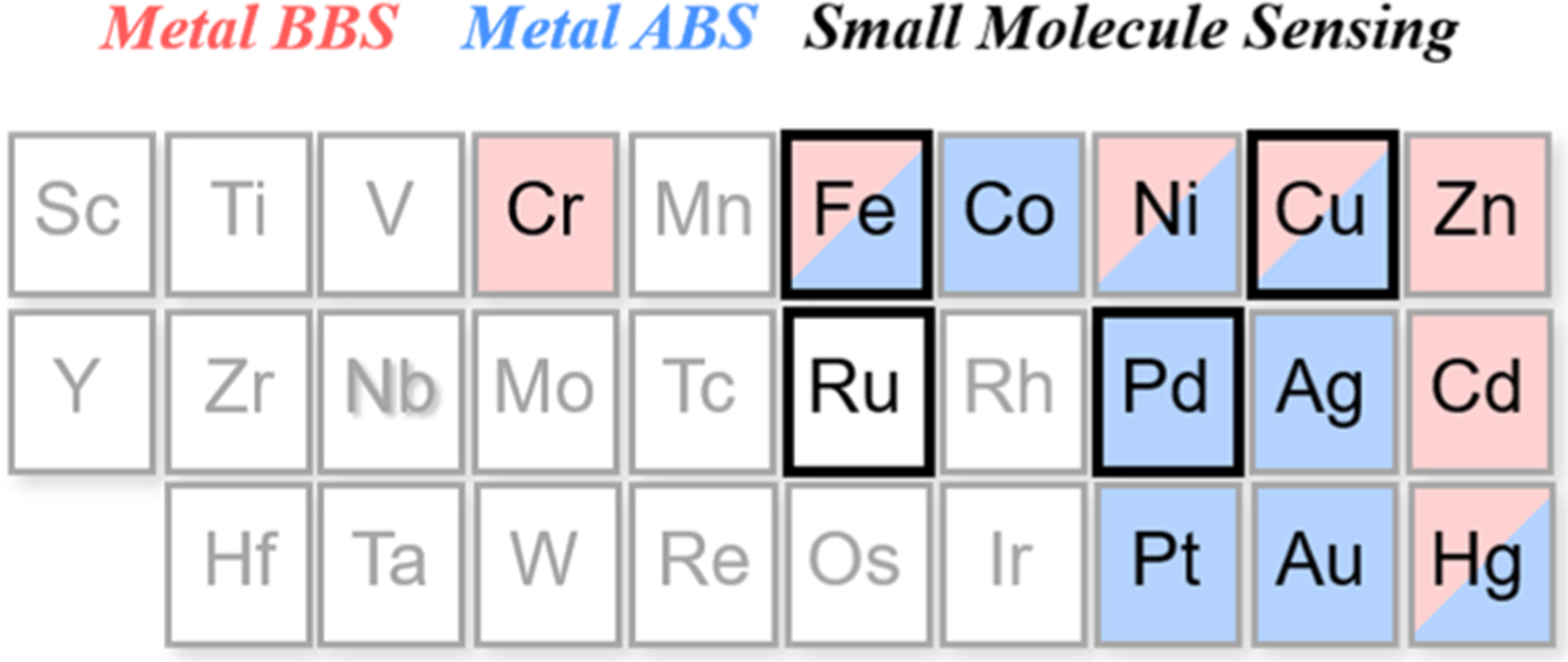
Summary of the transition metals and their role as analytes (BBS, light-red shading; ABS, blue shading) or indicators for small-molecule detection (black outline).
ABS PROBES FOR METALS
The unique chemical properties of metals, including tunable Lewis acidity and redox activity, have been exploited toward the development of reactivity triggers for imaging bioavailable metal pools. The following section highlights a selection of fluorescent ABS probes for biologically and/or environmentally important metals, focusing on triggers that can be generally applied to multiple imaging modalities.
ABS of Iron and Cobalt.
ABS approaches have proven particularly useful for detecting iron and cobalt, two essential redox-active metal nutrients for living organisms. Given the reducing environment inside the cell, labile iron pools are predominantly ferrous and in high-spin configurations. As such, along with the preferred divalent oxidation state for cobalt, these paramagnetic metal centers often induce fluorescence quenching and manifest low metal–ligand stabilities on the Irving–Williams series,51 limiting the possibilities for a selective recognition- and binding-based approach to fluorescence detection. To overcome these challenges, the rich oxidative chemistry of both metalloenzymes69 and their synthetic counterparts stands out as a viable mechanism for the design of selective FeII and CoII fluorescent indicators. In particular, oxidants such as O2, 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO),70 or trimethylamine N-oxide71 provide co-reagents for iron-mediated oxidative chemistry. For example, inspired by the O2 chemistry of FeII and, in particular, the 2-His-1-carboxylate triad motif found in mononuclear non-heme FeII enzymes, our laboratory developed Iron Probe 1 (IP1), a hydroxymethyl fluorescein derivative bearing a tris(pyridine) carboxylate-type ligand cage as an iron recognition and activity site.72 Upon FeII coordination, O2 activation triggers intra-molecular C–O bond cleavage and oxidation to release the fluorophore, resulting in an increase in the fluorescence intensity. The successful application of these inorganic design principles in living systems was demonstrated using the corresponding acetoxymethyl ester derivative (Figure 3a) IP1-AM, a more cell-permeable analogue, which enabled the detection of changes in cellular labile iron pools upon various stimulation conditions. A related compound, Cobalt Probe 1 (CP1),40 featuring a tetradentate N3O-based ligand, was shown to undergo a similar O2-dependent C–O bond scission to uncage the Tokyo Green fluorophore, permitting selective fluorescent CoII sensing in aqueous solution and in living cells (Figure 3a). The versatility of this cobalt-specific ABS ligand was further demonstrated by its attachment to a 2-(2′-hydroxyphenyl)benzothiazole excited-state intramolecular-proton-transfer-based chromophore73 and luciferin74 to measure the CoII levels in cells and animals, respectively.
Figure 3.
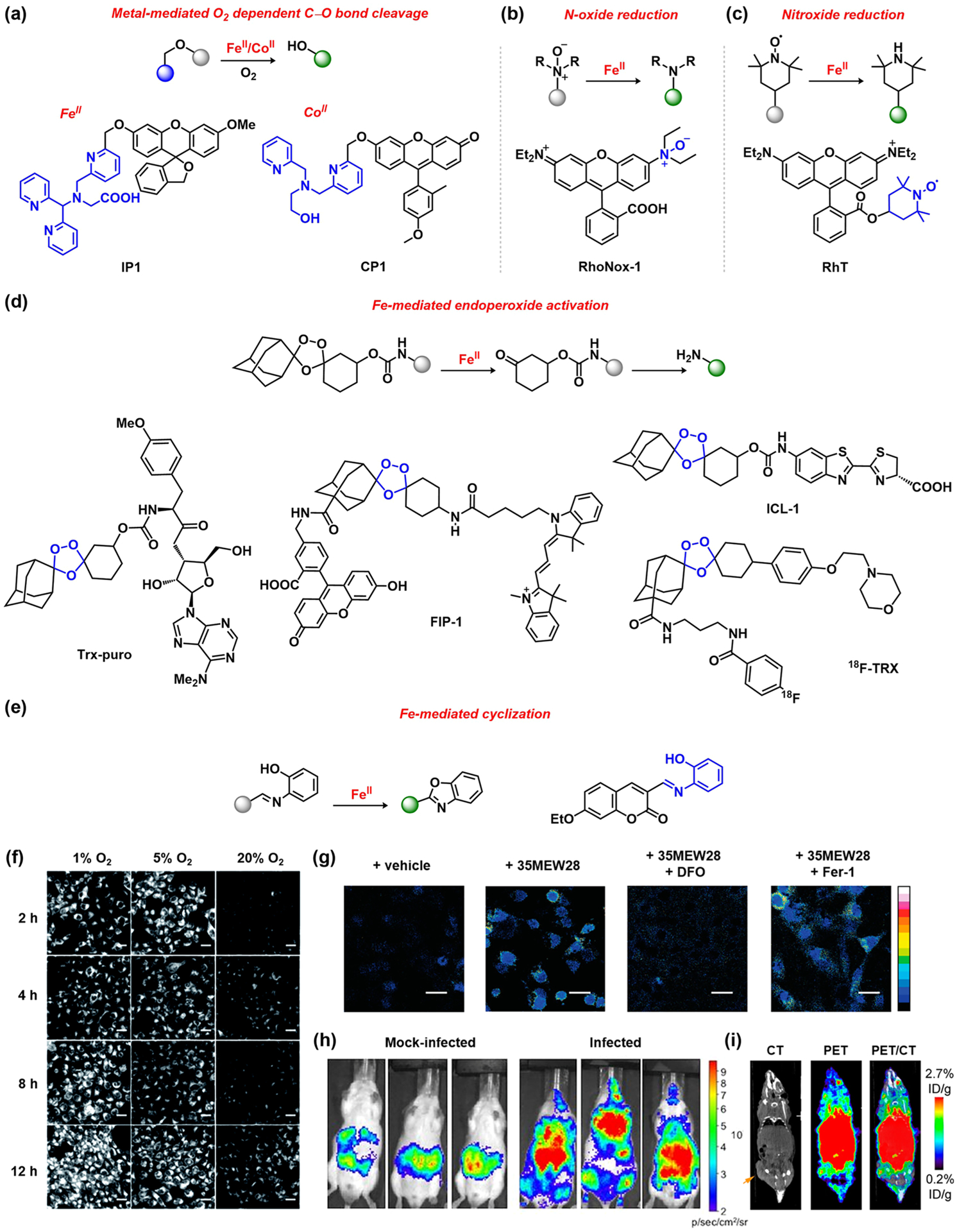
ABS probes for FeII and CoII detection: (a) iron72 and cobalt40 O2 activation, followed by C–O cleavage to release fluorophore; iron-mediated (b) N-oxide75 and (c) nitroxide reduction;83 (d) FeII-induced endoperoxide activation;84,85,87,88 (e) FeII-induced cyclization;89 (f) monitoring FeII levels in HepG2 cells under hypoxic conditions (SiRhoNox-1;77 adapted from ref 77. Copyright 2017 Royal Society of Chemistry); (g) detection of changes in labile FeII pools upon induction of ferroptosis (FIP-1;85 adapted from ref 85. Copyright 2016 American Chemical Society); (h) monitoring FeII status in mice infected with Acinetobacter baumannii (ICL-1;87 adapted from ref 87. Copyright 2017 PNAS); (i) PET/CT to monitor FeII levels in tumor and normal tissues for mice with subcutaneous U251 tumors (18F-TRX;88 adapted from ref 88. Copyright 2019 American Chemical Society).
In parallel, Hirayama, Nagasawa, and colleagues designed RhoNox-1, featuring an N-oxide ABS trigger that is selectively reduced to the corresponding amine by FeII, resulting in a turn-on fluorescence signal (Figure 3b).75 This strategy has been extended to other fluorophores for multicolor imaging, including coumarin, rhodol, and silicon rhodamine (Figure 3f),76–78 and applied to organelle-specific iron imaging by introducing targeting units.79–82 A related FeII-dependent nitroxide reduction enables a turn-on fluorescence response following FeII reduction of the TEMPO radical that otherwise quenches the fluorophore emission (Figure 3c).83
Inspired by iron-mediated endoperoxide activation observed in antimalarial drugs (e.g., Artemisinin), Renslo and co-workers designed Trx-puro, a histochemical stain for labile FeII comprised of a trioxalane-caged puromycin (Figure 3d). Upon reaction of the probe with FeII, free puromycin is released, which can be translated onto nascent peptides and detected by a puromycin antibody after fixation.84 Our laboratory synthesized FRET Iron Probe 1 (FIP-1), a fluorescein/cyanine 3 conjugate using an analogous endoperoxide linker (Figure 3d). Cleavage of the peroxide moiety in FIP-1 by reaction with FeII separates the fluorophores and minimizes FRET, thus enabling ratiometric detection of cellular labile iron pools.85 FIP-1 provides the first direct imaging evidence of changes in labile iron pools upon induction of ferroptosis (Figure 3g),85 a form of cell death that is gaining increasing attention.86 More recently, we adapted the iron-selective endoperoxide trigger to develop Iron-Caged Luciferin 1 (ICL-1), a luciferin-based bioluminescent probe for monitoring labile iron in live animals, observing with the Skaar laboratory that bacterial infection can lead to an increase in labile iron pools (Figure 3h).87 PET imaging of labile iron pools has also been achieved using endoperoxide tracers (Figure 3i).88 Finally, by exploiting the Lewis acidity of FeII, a recent report highlighted the use of an iron-mediated cyclization of a phenolic unit adjacent to a C═N bond appended to a coumarin derivative (Figure 3e).89 Upon cyclization, generation of a benzoxazole ring prevents C═N bond isomerization and rotation, enabling fluorescence turn-on in aqueous solution and in living cells.
ABS of Copper.
Unlike divalent iron and cobalt, monovalent copper is the dominant oxidation state of this metal in reducing cellular environments.90 As a diamagnetic ion, CuI exhibits a preference for sulfur-based ligands or nitrogen-rich environments, and fluorescence quenching can be circumvented in certain cases; indeed, numerous binding-based probes have been developed for this analyte.29,30,37,53–55 Nevertheless, the dynamic fluctuations of labile copper pools observed across many cell types have motivated the advancement of ABS strategies applied to biological copper detection. In early work in this area, Taki and colleagues drew inspiration from the vast literature on the O2 activation by bioinorganic model complexes for copper-dependent enzymes91–93 and reported FluTPA2,94 a probe featuring a Tokyo Green derivative caged by the CuI chelator tris(2-pyridylmethyl)amine (TPA). This compound undergoes a CuI- and O2-dependent oxidative cleavage of the TPA benzyl ether linkage with subsequent oxidation and release to generate the highly fluorescent Tokyo Green fluorophore product (Figure 4a). FluTPA2 selectively detects CuI over CuII in the presence of glutathione (GSH) with a >100-fold turn-on and can report changes in cellular copper levels. This ABS trigger was further adapted for mitochondria targeting (Figure 4c)95 and separately coupled with coumarin-based fluorophores.96 Our laboratory employed this motif to cage luciferin and create Copper-Caged Luciferin 1 (CCL-1; Figure 4a) for in vivo bioluminescent copper imaging, showing in a diet-induced mouse model of non-alcoholic fatty liver disease (NAFLD) that copper deficiency occurs as early as 2 weeks before metabolic symptoms of the disease manifest at 2 months (Figure 4d).4
Figure 4.
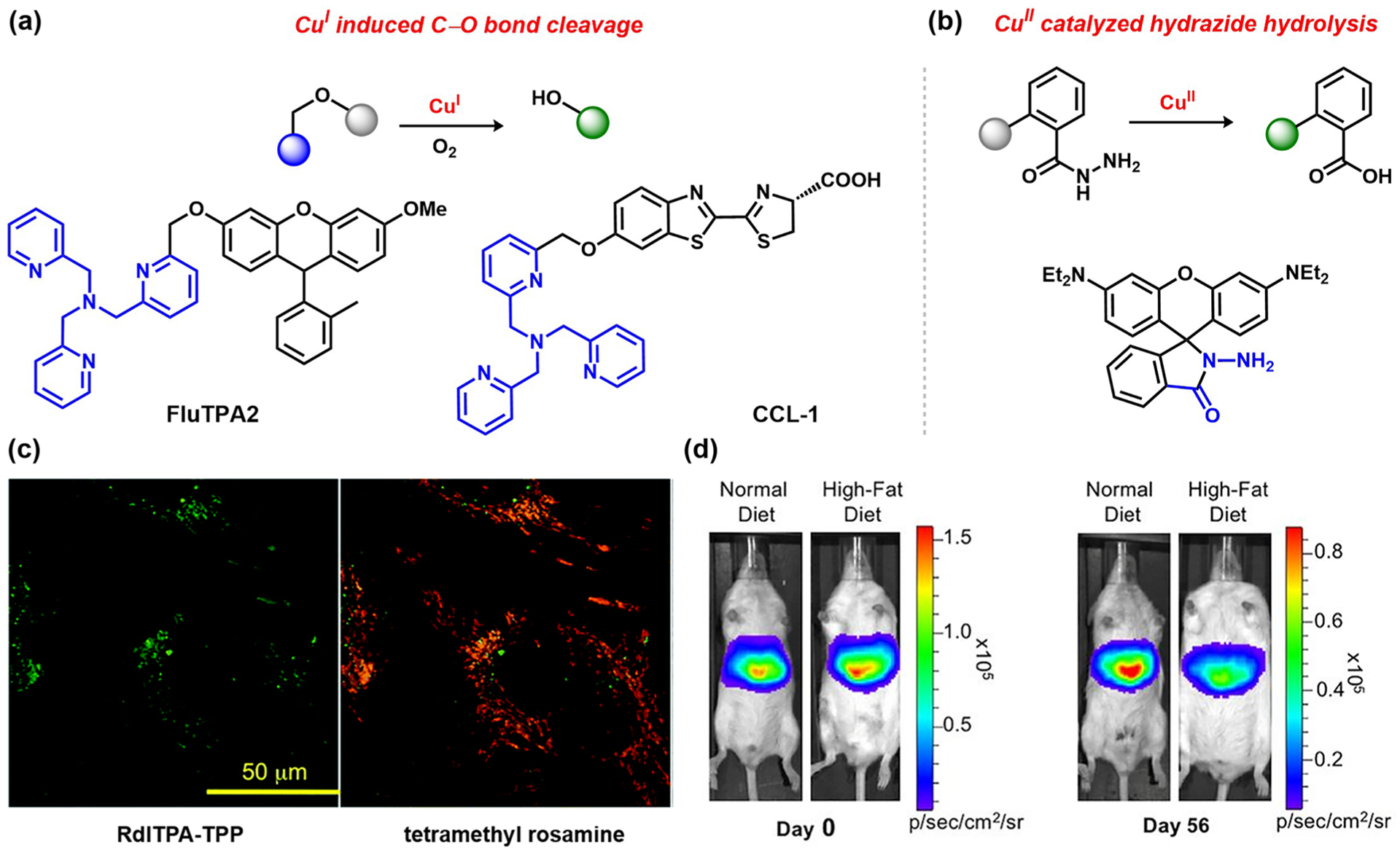
ABS probes for biological copper detection: (a) biomimetic CuI–O2 activation, followed by C–O cleavage to release fluorophore;4,94 (b) CuII-catalyzed hydrazide hydrolysis;42 (c) monitoring copper levels in the mitochondria (RdlTPA-TPP;95 adapted from ref 95. Copyright 2014 Royal Society of Chemistry); (d) monitoring labile CuI levels in a diet-induced mouse model of NAFLD (CCL-1;4 adapted from ref 4. Copyright 2016 PNAS).
In contrast to the growing use of chemical probes for bioimaging of CuI, detection of CuII in biological environments is rarer. An early approach for CuII sensing was developed by Czarnik and colleagues,42 employing a hydrazide-substituted spirorhodamine fluorophore locked in a non-fluorescent ring-closed form. Binding of CuII promotes hydrazide hydrolysis with concomitant ring-opening of the spirorhodamine, thus turning-on the fluorophore (Figure 4b). Depending on the fluorophore/trigger combination, hydrazide-based CuII detection has been reported in many solvents, and two-dye systems with a hydrazide motif have been reported to enable ratiometric imaging of CuII in living cells.97–99
ABS of Heavy Metals.
In addition to optical detection of endogenous metals in biological settings, ABS can also provide entry to monitor the accumulation of heavy-metal counterparts. Mercury, one of the more prevalent toxic elements in the environment, has been an important target for both in vivo and ex vivo detection. Owing to the highly thiophilic nature of HgII, mercury-promoted desulfurization reactions provide a versatile strategy for designing fluorescent indicators.100 Akin to the spirorhodamine sensors for CuII, Tae and co-workers reported a thiosemicarbazide-substituted spirolactam–rhodamine as a highly sensitive and selective HgII sensor (Figure 5a), where mercury coordination facilitates 1,3,4-oxadiazole formation along with HgS precipitation.101 Of note, the fast kinetics of this probe in aqueous conditions enabled its application toward imaging HgII in both living cells and zebrafish models (Figure 5f).102 This ring-opening scaffold has been tailored for other fluorophores,103–105 including a FRET-based sensor for ratiometric measurements103 and a near-IR silicon–rhodamine fluorophore.105
Figure 5.
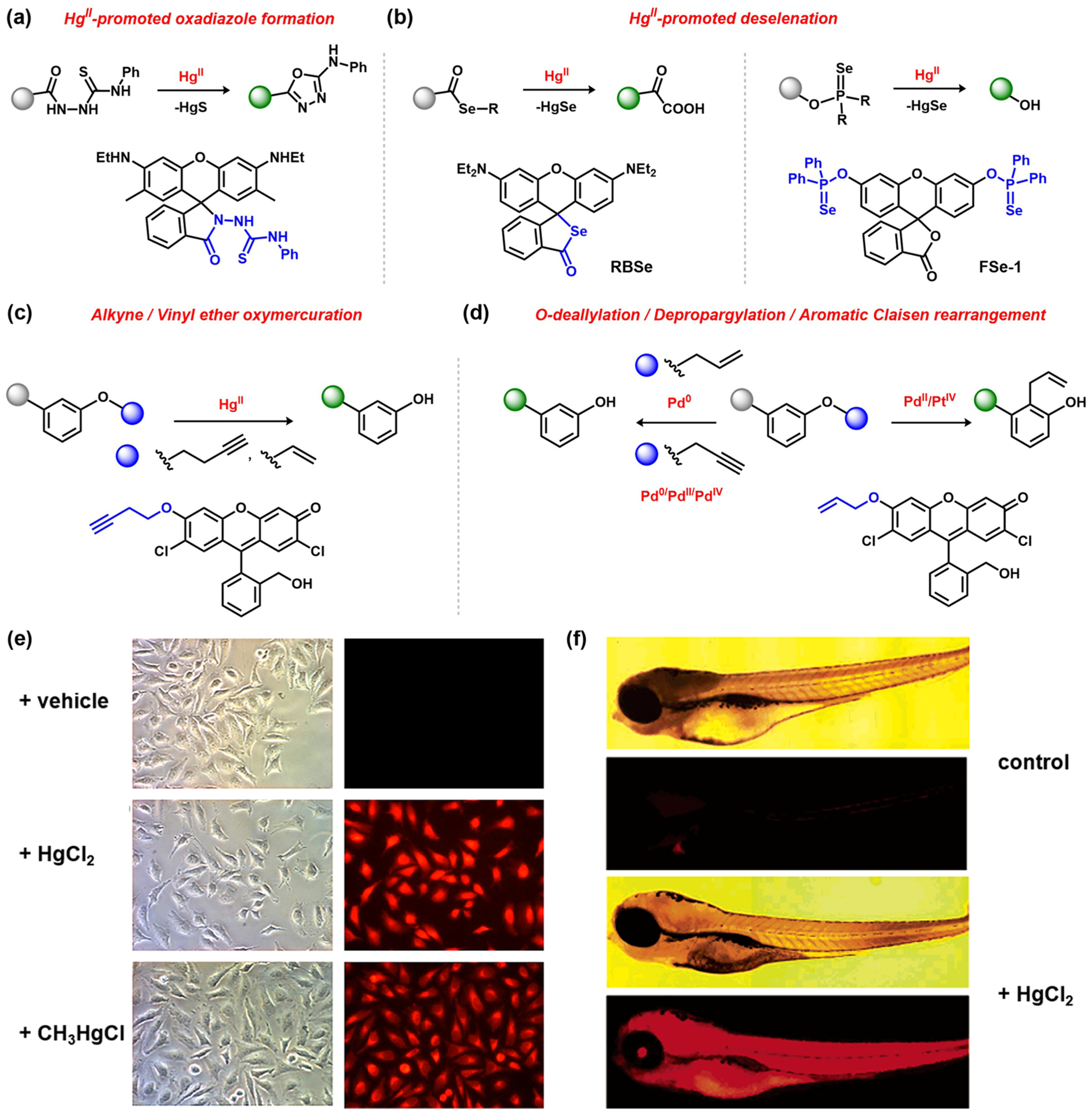
ABS probes for heavy-metal detection: (a) HgII-promoted cyclization of thiosemicarbazide;101 (b) HgII-mediated deselenation;106,110 (c) oxymercuration of terminal alkynes or vinyl ethers;111 (d) Pd0/II/IV/PtIV detection via O-deallylation, depropargylation, or aromatic Claisen rearrangement reactions;116,119(e) sensing HgII and CH3HgCl in HeLa cells (RBSe;110 adapted from ref 110. Copyright 2010 Elsevier); (f) sensing HgII in zebrafish (top, microscopic image; bottom, fluorescence microscopic image;102 adapted from ref 102. Copyright 2006 American Chemical Society).
Analogous selenium–mercury interactions provide a related pathway for HgII sensing. For example, selenophosphinyl-protected fluorescein FSe-1 can be selectively hydrolyzed upon interaction with HgII (Figure 5b).106 Deprotection of the hydroxyl groups induces ring opening of the fluorescein lactone unit, affording a 156-fold increase in fluorescence. A similar strategy has also been described using thiophosphinyl units as triggers107 or naphthalimide as the fluorophore,108 the latter of which facilitated ratiometric imaging of mercury in cells. In addition, HgII-promoted ring opening of the selenolactone RBSe results in fluorophore turn-on and HgSe release (Figure 5b).109,110 Although the probe does respond to AgI as well, its ability to react with HgII along with organomercury species was shown to be viable in cells and zebrafish organs upon treatment with HgCl2 or CH3HgCl (Figure 5e).
Beyond the principles of hard–soft acid–base chemistry, Koide and colleagues introduced organometallic ABS, and, in particular, the oxymercuration reaction for oxidation-resistant sensing of HgII, where vinyl and terminal butyne were used as caging units on the phenolic groups of fluorescein-derived fluorophores (Figure 5c).111,112 These caging groups are readily removed upon reaction with divalent mercury, releasing the fluorescent moiety required for signal quantification. The oxymercuration-based sensing mechanism is amenable to monitoring both HgII as well as methylmercury in cells and zebrafish tissues.113,114
Other heavy metals are also amenable to ABS detection. In this regard, palladium and platinum metals are major industrial contributors for the synthesis of pharmaceuticals and fine chemicals given the prevalence of cross-coupling reactions in building complex molecules.115 Owing to their potential hazardous health effects, sensing of residual palladium and platinum is of interest for quality control of active pharmaceuticals and validation of heavy-metal thresholds. In innovative applications of classic Tsuji-Trost and Claisen chemistry, fluorophore derivatives bearing palladium/platinum-cleavable O-allyl or propargyl caging moieties on phenolic hydroxyl groups have been applied for the detection of exogenous palladium or platinum in cells, fish models, and solution.116–119 Formation of an electrophilic palladium or platinum allyl intermediate followed by hydrolysis affords the fluorescent dye. Moreover, this ABS strategy can be tuned for the detection of palladium in various oxidation states (Pd0, PdII, or PdIV) when using a propargyl ether unit.116 Incorporation of an allyl ether engenders oxidation state specific metal sensing, where a Tsuji-Trost deprotection is facilitated by Pd0, whereas a Claisen rearrangement is promoted by PdII or PtIV only (Figure 5d).119
As an abundant first-row transition metal, nickel has gained attention for its use as a potential substitute for its heavy metal congeners, Pd and Pt.120 As such, developing tools for trace nickel detection for process control becomes a necessity. In this context, altering the ligands and reaction conditions typically employed for Pd-catalyzed deallylation reactions, Lee and co-workers recently reported the first NiII reactivity-based sensor.41 Using a conformationally twisted N-arylbenzotriazole scaffold, Ni-catalyzed deallylation of the allyl carbamate substituent unveils the unprotected amino group that enables a turn-on fluorescent response.
ABS PROBES UTILIZING BIOORTHOGONAL METAL REACTIVITY
In addition to providing a versatile platform for biological and environmental metal detection, metal-directed ABS chemistry offers a modular approach for monitoring and manipulating other small molecules in their native environments owing to the diverse binding affinities, Lewis acidities, redox activities, hard–soft acid–base preferences, and geometries that metals uniquely possess. In particular, transition metals can often participate as fluorescence quenchers via electron- or energy-transfer pathways, thus providing a versatile strategy for designing turn-on responsive indicators using ABS reactions to selectively separate the metal from the fluorophore or alter the oxidation state of the metal to inhibit excited-state electron/energy transfer. The following sections briefly summarize a selection of metal-based methods for small-molecule sensing, again focusing on ABS reactions that are biocompatible. Of note is the growing success of bioorthogonal metal chemistry to probe transient small molecules, such as reactive oxygen, sulfur, and carbon species, for which it would be challenging to develop traditional lock-and-key binding receptors with sufficient selectivity in complex living systems.
ABS Using Bioorthogonal Metal–Ligand Substitution Reactions.
As one of the fundamental reactions of inorganic chemistry, metal–ligand exchange provides a distinct mechanism for sensing, which has largely been exploited in the context of analyte-induced displacement of a metal quencher, leading to a fluorescence turn-on response. A representative set of examples involves detection of H2S via displacement and subsequent precipitation of copper(II) sulfide from copper(II) dye complexes. Chang and colleagues reported a copper(II) dipicolylamine fluorescein complex that permitted in vitro H2S detection.121 In the absence of H2S, the paramagnetic CuII center quenches the fluorescence of the probe. Upon reaction with H2S, removal of the quenching copper center driven by the formation and precipitation of copper sulfide triggers a turn-on response from the fluorophore. Along these same lines, Nagano and co-workers reported HSip-1, a copper(II) azamacrocycle fluorescein complex (Figure 6a).48 HSip-1 was successfully used for H2S imaging in cells with reduced interference from thiol-containing GSH and cysteine residues (Figure 6c). From these starting points, many metal-directed ABS probes for H2S detection have been reported, emphasizing both the generality and practicality of such simple transition-metal-based reactivity trends.122–125
Figure 6.
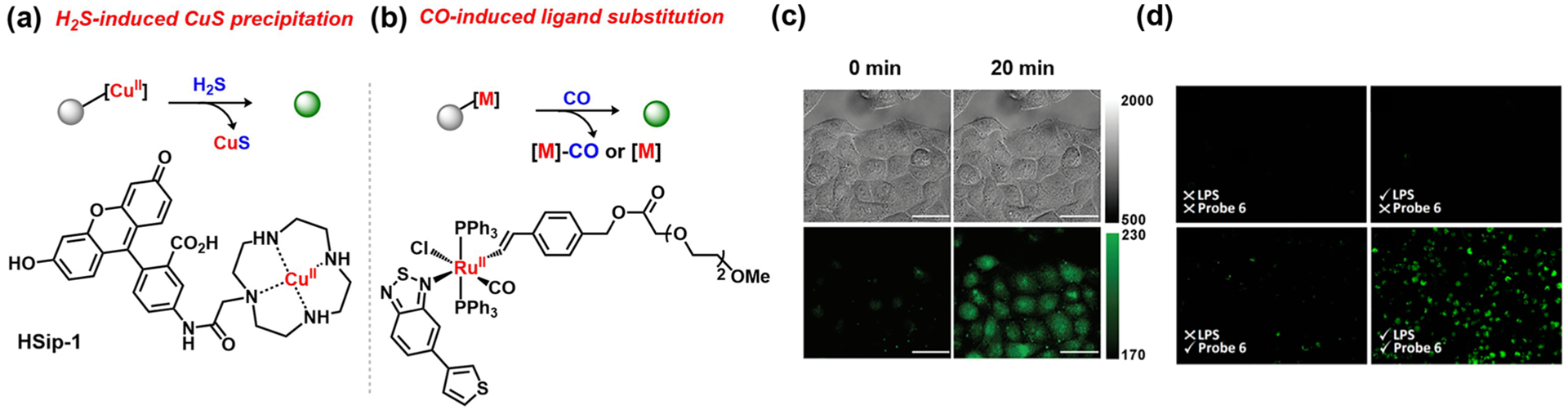
ABS probes using bioorthogonal metal–ligand substitution reactions: (a) H2S-induced CuS precipitation to release fluorophore;48 (b) CO-induced ligand substitution;127 (c) detection of H2S in HeLa cells following addition of Na2S (top, differential interference contrast; bottom, fluorescence; HSip-1;48 adapted from ref 48. Copyright 2011 American Chemical Society); (d) detection of CO in cells from mice treated with lipopolysaccharides (LPS;127 adapted from ref 127. Copyright 2011 American Chemical Society).
In a separate application of bioorthogonal metal–ligand substitution reaction chemistry, CO detection has been accomplished by exploiting its binding affinity toward certain transition metals to facilitate displacement from a fluorophore to provide a spectral readout. Complementing the organometallic methods utilizing CO as a substrate (vide infra), many metal-based chromogenic and fluorogenic probes have been developed for CO detection in the gas phase and in solution.126 As a leading example for imaging CO in tissues, Sancenón and colleagues incorporated a hydrophilic vinyl ligand onto a RuII complex to overcome previous aqueous solubility issues of related metal–CO complexes. Coordination of the fluorophore 5-(3-thienyl)-2,1,3-benzothiadiazole to the RuII center via a nitrogen atom quenched its emission, and substitution of this fluorophore ligand with the CO analyte triggered an increase in fluorescence resulting from free fluorophore generation as a function of the CO concentration (Figure 6b).127 The sensitivity, selectivity, and aqueous solubility of the ruthenium-based sensor enabled its successful application to cellular CO imaging (Figure 6d).127
ABS Using Bioorthogonal Metal-Mediated Redox Chemistry.
In addition to non-redox ligand substitution processes, the inherent redox activity of metals can also be utilized as a triggering mechanism for monitoring transient and reactive analytes. In an innovative early application of this strategy, Lippard and co-workers described an NO detection system via a copper-promoted reductive nitrosylation using a copper(II) aminoquinoline–fluorescein complex (CuFL; Figure 7a).47 Upon reaction of the copper complex with NO, demetalation and subsequent ligand nitrosylation at the secondary amine site establish the turn-on signal required for NO monitoring. Notably, this sensor demonstrates exquisite specificity for NO over other ROS species, including HNO, under cellularly relevant conditions (Figure 7d),47,128 where the metal-based reaction offers a potential advantage over traditional phenyldiamine-substituted dyes employed as general proxies for NO detection because other reactive nitrogen species can trigger the diamine-to-triazole transformation. This probe has been utilized for NO detection in vivo, showing the key defensive role of NO for survival of the Bacillus anthracis pathogen.129 Subsequent synthetic modifications to incorporate two NO sensing units along with an ester moiety provided access to a cell-permeable probe that enabled NO visualization in the mouse main olfactory bulb (Figure 7e).130 Following upon this work, Ali and colleagues reported a copper(II) dansyl-based turn-on NO sensor (Figure 7b).131
Figure 7.
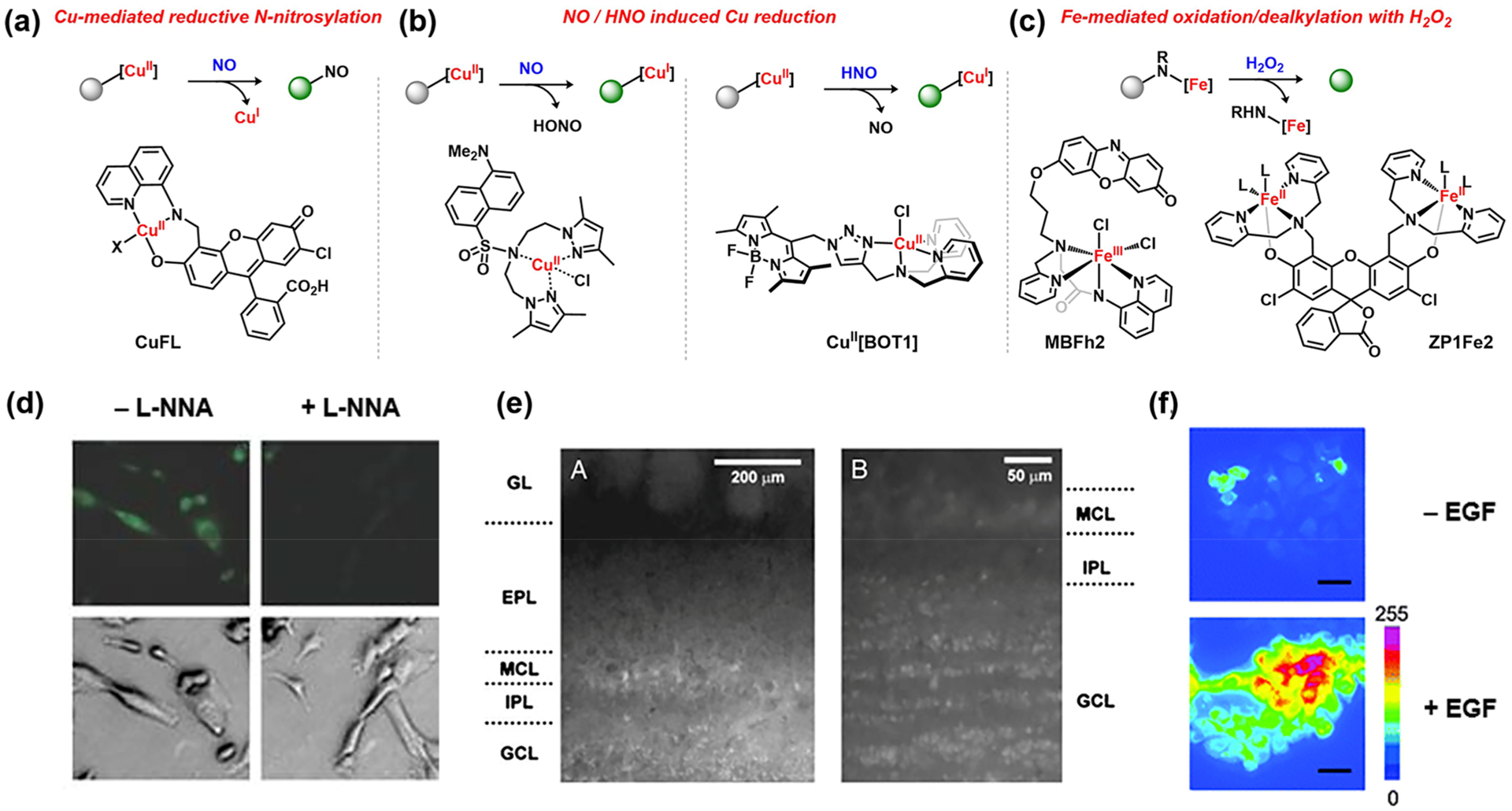
ABS using bioorthogonal redox reactivity: (a) CuII-mediated reductive N-nitrosylation;47 (b) NO131 and HNO132 reduction of CuII; (c) biomimetic FeII and FeIII H2O2 activation followed by ligand oxidation to release fluorophore;138,139 (d) NO detection in SK-N-SH cells treated with 17β-estradiol and with or without NG-nitro-l-arginine (l-NNA) (CuFL;47 adapted from ref 47. Copyright 2006 Springer Nature); (e) NO detection in the main olfactory bulb (low magnification, left; high magnification, right; Cu2(FL2E);130 adapted from ref 130. Copyright 2010 PNAS); (f) monitoring H2O2 levels upon EGF stimulation of A431 cells (MBFh2;138 adapted from ref 138. Copyright 2013 Royal Society of Chemistry).
HNO detection can be achieved by its interactions with CuII complexes to effect a one-electron reduction of the metal center. This feature was exploited by Lippard and co-workers to design the HNO-responsive fluorescent probe CuII[BOT1] (Figure 7b).132,133 In the absence of HNO, the paramagnetic CuII center coordinated to N-(triazolylmethyl)-N,N-dipicolyl quenches emission of the boron–dipyrromethene (BODIPY) fluorophore. HNO-induced reduction to the corresponding diamagnetic CuI complex triggers a fluorescence turn-on response by relieving this free-radical quenching. Building on this initial report, the CuII/HNO reactivity has been more broadly harnessed to develop other fluorescent indicators for imaging cellular HNO.134–136
Drawing inspiration from heme and non-heme iron enzymes that utilize H2O2 as an oxidant, Kodera and colleagues developed a fluorescent H2O2 indicator combining a reduced resorufin analogue and an iron(III) polypyridine complex to generate MBFh1. Oxidation of the dye-bound ferric center with H2O2 is proposed to initiate subsequent two-electron ligand oxidation, resulting in C–O bond cleavage to liberate the fluorescent resorufin dye.137 Subsequent modifications of the fluorophore scaffold to incorporate an O-alkylresorufin derivative (MBFh2; Figure 7c) afforded a stable probe for the intracellular detection of H2O2 (Figure 7f).138 In a similar approach, Nam and co-workers employed a known zinc sensor and made its corresponding FeII complex (ZP1Fe2),139 which is activated by H2O2 to promote oxidative N-dealkylation and release the bis(carboxylate) fluorescein (Figure 7c). Unlike the ferric-based sensor, this diiron probe selectively reacts with H2O2 over other ROS such as O2•− or tBuOOH and was found to localize at the lysosome, enabling organelle-specific H2O2 detection.139
ABS Using Bioorthogonal Organometallic Chemistry.
Organometallic chemistry offers a unique platform for sensing small-molecule analytes that are otherwise poorly reactive for detection in neutral aqueous conditions with high salt and thiol contents. In an early example of incorporating such principles to detect intracellular non-metal analytes, our laboratory made use of the palladium-mediated carbonylation reaction to design the first-generation fluorescent CO probe COP-1 (Figure 8a).45 The sensor features a BODIPY dimer ligated to a PdII center, which then selectively reacts with CO to effect carbonylation of the aromatic backbone ligand and release the fluorophore. Owing to the need for oxidative addition, reductive elimination, and C–C bond formation to release the quenching palladium center, COP-1 displays high specificity for CO, particularly over other biologically relevant diatomics, including O2, O2−, and NO, serving as a suitable reagent for use in cellular studies (Figure 8d). On the basis of this study, many other fluorescent CO sensors using a similar PdII-mediated fluorophore release scheme have been subsequently reported for imaging CO in cells, tissues, and animal models, demonstrating the utility and versatility of this bioorthogonal organometallic ABS strategy.140–142
Figure 8.
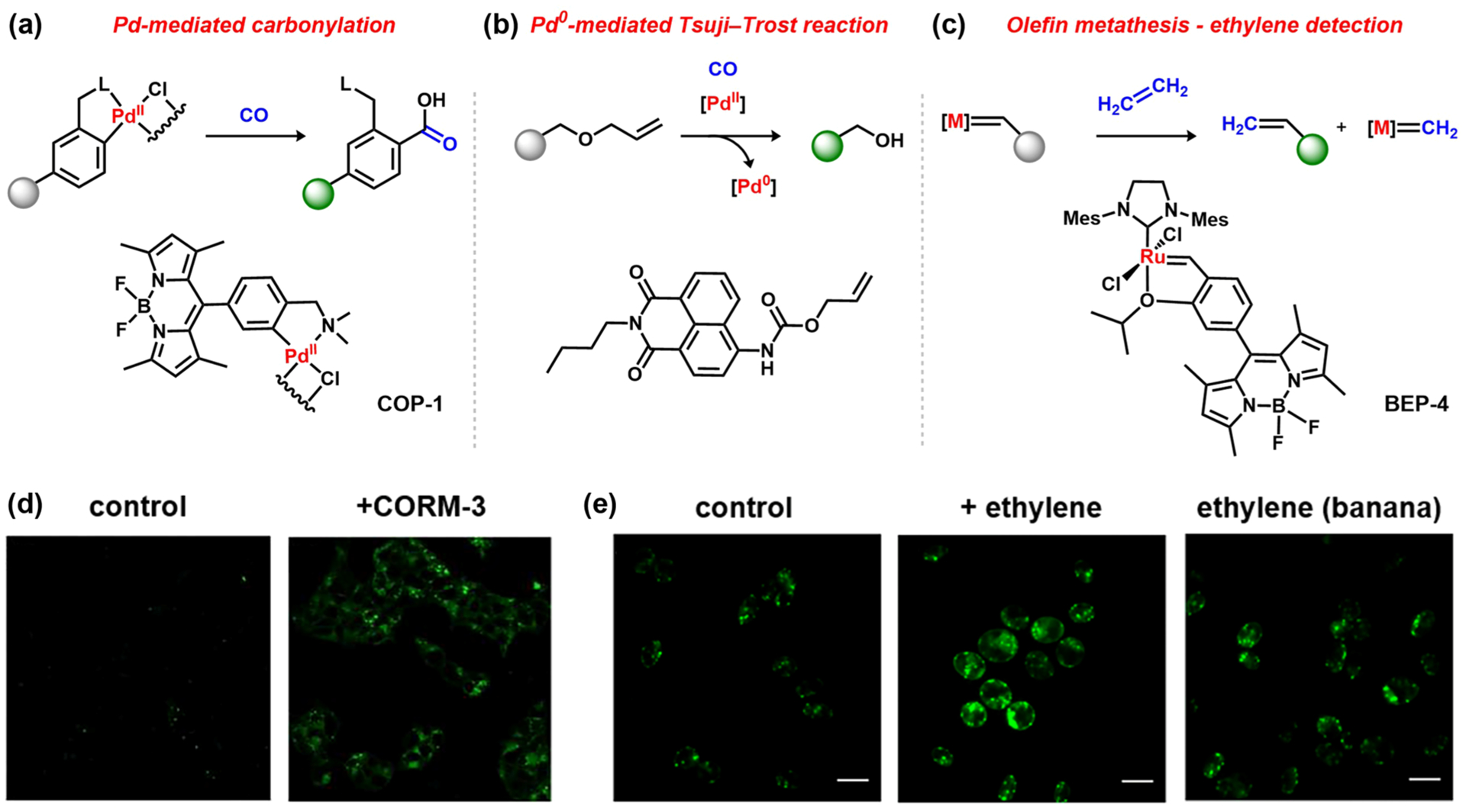
ABS probes using bioorthogonal organometallic chemistry: (a) CO sensing via palladium-mediated carbonylation;45 (b) CO-induced PdII reduction followed by the Pd0-mediated Tsuji–Trost reaction;146 (c) olefin metathesis ruthenium complex for ethylene sensing;46 (d) detection of CO in HEK293T cells following treatment with the CO-releasing molecule [Ru(CO)3Cl(glycinate)] (CORM-3, COP-1;45 adapted from ref 45. Copyright 2012 Amercian Chemical Society); (e) ethylene detection in Chlamydomonas reinhardtii cells and banana (BEP-4;46 adapted from ref 46. Copyright 2018 Amercian Chemical Society).
In a complementary approach, ABS probes combining the facile CO-induced reduction of PdII complexes implicated in palladium-mediated carbonylations143 with the Pd0-catalyzed Tsuji–Trost reaction have also emerged. Specifically, fluorophores containing allyl cages are selectively turned-on upon deprotection by Pd0, which, in turn, is generated only upon the reduction of a PdII source in the presence of CO. Of note, this method makes use of the same reactivity trends that enabled palladium sensing, emphasizing the versatile role of metals and their chemical properties. Such probes have proven to be sensitive and selective for CO detection in both cells144–147 and mouse models (Figure 8b).148
In another example of bioorthogonal organometallic chemistry for ABS, Michel and co-workers elegantly demonstrated that transition-metal-catalyzed olefin metathesis can be applied to achieve ethylene detection both in aqueous solution and in living cells. The BEP-4 probe (Figure 8c) integrates a second-generation Hoveyda–Grubbs catalyst onto a BODIPY scaffold, where the reaction of ethylene displaces the BODIPY dye from the heavy-metal ruthenium quencher and thus induces a turn-on response to ethylene in a dose-dependent manner (Figure 8e).46
CONCLUDING REMARKS AND FUTURE PROSPECTS
ABS represents a rapidly growing area of research in chemical biology, and ample opportunities are available to pursue metal-directed chemistry in this open field. Indeed, fundamental concepts of inorganic chemistry, from geometric and hard–soft acid–base preferences to kinetics of ligand exchange to tunable Lewis acidity, oxidation state, and spin state, can all be brought to bear in a diverse array of new directions. Coupled with the ability to draw inspiration from the chemistry of bioinorganic and organometallic systems, particularly metalloenzyme active sites, ABS probes can provide unique access to selective and robust approaches toward metal-specific sensing in complex environments as well as pathways for the detection of biologically relevant small molecules through metal-directed reactivity. In this Viewpoint article, we have highlighted several classes of metal-mediated reactions that have been incorporated into triggers for fluorescence and bioluminescence imaging in cells, tissues, or animals. Given the ever-expanding scope of metal-based chemistry, we envision that more sensitive, selective, and robust metal-mediated ABS platforms will be developed to facilitate the detection and manipulation of chemical analytes more broadly and help decipher their contributions to living systems.
Many avenues in metal-directed ABS merit further investigation. Deeper applications of existing fluorescence and bioluminescence probes for metals and metal-interacting small molecules will continue to illuminate new basic biology. Examples from our own laboratory at the cellular and whole-organism levels include the identification of elevations in the labile iron pool upon induction of ferroptosis85 and liver-localized copper deficiency through the upregulation of copper export proteins during the progression of NAFLD,4 respectively. For optical imaging, moving excitation and/or detection wavelengths into the red and near-IR regions will enable multicolor monitoring and deep-tissue and/or whole-animal imaging to monitor biomolecules in their true native environments. Likewise, spatial resolution can be additionally improved by attaching chemical and/or biological targeting groups to control probe localization for organelle-specific or tissue detection. Also, the application of metal-directed ABS concepts to other imaging modalities, particularly bioimaging techniques that are amenable to humans, such as MRI, PET, and ultrasonic imaging, will help in the translation of these platforms to diagnostic and therapeutic technologies.
Beyond imaging, bioorthogonal, metal-dependent activation of fluorophores, prodrugs, and larger biomolecules/biopolymers such as proteins and nucleic acids represents another research area that will surely benefit from more contributions that incorporate principles of inorganic coordination chemistry. For example, allyl groups have already been widely exploited as caging units on various chemical cargoes, including fluorophores,149–151 luciferin,152 nutrients,153 and DNA binders,154 and can be unmasked in cells by bioorthogonal ruthenium catalysts. Additionally, palladium-catalyzed deprotection of the propargyl or allyl groups has afforded new ways to activate prodrugs155 and proteins.156 Endogenous metals can also serve as triggers for ABS reagents, as illustrated by the use of elevated levels of iron in cancerous cells compared to their healthy counterparts as stimulants for site-directed prodrug activation as an emerging chemotherapeutic strategy.157–159
Finally, a key chemical challenge in the field of metal-directed ABS is the greater incorporation of catalytic reactions into reagent design to increase sensitivity, potency, and signal-to-noise responses. Indeed, despite the fact that metals are broadly employed as catalysts across homogeneous/molecular, heterogeneous, and biological fields, the vast majority of metal-directed ABS probes to date rely on stoichiometric or single-turnover reactions. Innovations in the development of bioorthogonal, synthetic inorganic, and organometallic catalysts are a prime growth area in metal-directed ABS chemistry.160–165 Taken together, the periodic table not only represents the chemical blueprint for life but also organizes the tools to help decipher the interactions between and build new chemical matter with these elements at a molecular level. ABS that exploits both of these fundamental facets of the metals is a powerful platform for their use in biology and medicine, of which metal and metal-mediated detection and bioorthogonal metal chemistry are select examples of many opportunities for further investigation.
ACKNOWLEDGMENTS
We thank the NIH (Grant GM 79465) for support of our basic research efforts in the development of metal sensors. D.A.I. thanks the Life Sciences Research Foundation for a postdoctoral fellowship sponsored by the Howard Hughes Medical Institute. C.J.C. is an Investigator with the Howard Hughes Medical Institute and a CIFAR Senior Fellow.
Biographies

Diana A. Iovan received her B.S. in chemistry in 2012 from the University of Richmond. She then moved to Harvard University for her graduate studies with Prof. Ted Betley, exploring the electronic structure and reactivity of high-spin dipyrrinato iron complexes involved in C–H bond amination. After earning her Ph.D. in inorganic chemistry in 2017, Diana joined Prof. Chris Chang’s laboratory at University of California, Berkeley, as a Howard Hughes Medical Institute Fellow of the Life Sciences Research Foundation and is developing and applying new chemical tools for identifying and studying new copper–protein interactions as potential therapeutic targets.

Shang Jia received his B.S. in chemistry in 2014 from Peking University, China. He then went to University of California, Berkeley, for his graduate studies in Prof. Chris Chang’s laboratory, developing fluorescent sensors for the detection of intracellular CuI and bioconjugation methods for the labeling of histidine and methionine residues.

Christopher J. Chang is the Class of 1942 Chair Professor of Chemistry and Molecular and Cell Biology at the University of California, Berkeley, Investigator of the Howard Hughes Medical Institute, and Faculty Scientist at Lawrence Berkeley National Laboratory. Chris graduated with B.S./M.S. degrees from California Institute of Technology in 1997 and worked with Prof. Harry Gray, spent a year as a Fulbright scholar with Dr. Jean-Pierre Sauvage, earned his Ph.D. from Massachusetts Institute of Technology (MIT) in 2002 with his thesis advisor Prof. Dan Nocera, and then after postdoctoral studies with Prof. Steve Lippard, also at MIT, joined the Berkeley faculty in 2004. Research in the Chang laboratory focuses on the study of metals and redox-active molecules in biology and energy, focusing on the development of activity-based sensing and proteomics probes and catalysts and applying them to questions in neuroscience, metabolism, and sustainable synthesis.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Lippard SJ; Berg JM Principles of Bioinorganic Chemistry; University Science Books: Mill Valley, CA, 1994. [Google Scholar]
- (2).Denoyer D; Masaldan S; La Fontaine S; Cater MA Targeting Copper in Cancer Therapy: “Copper That Cancer. Metallomics 2015, 7 (11), 1459–1476. [DOI] [PubMed] [Google Scholar]
- (3).Bonda DJ; Lee H; Blair JA; Zhu X; Perry G; Smith MA Role of Metal Dyshomeostasis in Alzheimer’s Disease. Metallomics 2011, 3 (3), 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Heffern MC; Park HM; Au-Yeung HY; Van de Bittner GCV; Ackerman CM; Stahl A; Chang CJ In Vivo Bioluminescence Imaging Reveals Copper Deficiency in a Murine Model of Non-alcoholic Fatty Liver Disease. Proc. Natl. Acad. Sci. U. S. A 2016, 113, 14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Fukunaka A; Fujitani Y Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci 2018, 19 (2), 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).DiNicolantonio JJ; Mangan D; O’Keefe JH Copper Deficiency May Be a Leading Cause of Ischaemic Heart Disease. Open Heart 2018, 5 (2), No. e000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Finney LA Transition Metal Speciation in the Cell: Insights from the Chemistry of Metal Ion Receptors. Science 2003, 300, 931–936. [DOI] [PubMed] [Google Scholar]
- (8).New EJ Tools to Study Distinct Metal Pools in Biology. Dalton Trans 2013, 42 (9), 3210–3219. [DOI] [PubMed] [Google Scholar]
- (9).Alberts B; Johnson A; Lewis J; Raff M; Roberts K; Walter P Molecular Biology of the Cell, 5th ed.; Garland Science, Taylor & Francis Group: New York, 2008. [Google Scholar]
- (10).Chang CJ Searching for Harmony in Transition-Metal Signaling. Nat. Chem. Biol 2015, 11, 744–747. [DOI] [PubMed] [Google Scholar]
- (11).Chang CJ Bioinorganic Life and Neural Activity: Toward a Chemistry of Consciousness? Acc. Chem. Res 2017, 50 (3), 535–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ackerman CM; Chang CJ Copper Signaling in the Brain and Beyond. J. Biol. Chem 2018, 293, 4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Turski ML; Brady DC; Kim HJ; Kim B-E; Nose Y; Counter CM; Winge DR; Thiele DJ A Novel Role for Copper in Ras/Mitogen-Activated Protein Kinase Signaling. Mol. Cell. Biol 2012, 32 (7), 1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brady DC; Crowe MS; Turski ML; Hobbs GA; Yao X; Chaikuad A; Knapp S; Xiao K; Campbell SL; Thiele DJ; Counter CM Copper Is Required for Oncogenic BRAF Signalling and Tumorigenesis. Nature 2014, 509 (7501), 492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Krishnamoorthy L; Cotruvo JA Jr; Chan J; Kaluarachchi H; Muchenditsi A; Pendyala VS; Jia S; Aron AT; Ackerman CM; Wal MNV; Guan T; Smaga LP; Farhi SL; New EJ; Lutsenko S; Chang CJ Copper Regulates Cyclic-AMP-Dependent Lipolysis. Nat. Chem. Biol 2016, 12 (8), 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ba LA; Doering M; Burkholz T; Jacob C Metal Trafficking: From Maintaining the Metal Homeostasis to Future Drug Design. Metallomics 2009, 1 (4), 292–311. [DOI] [PubMed] [Google Scholar]
- (17).Hare DJ; New EJ; de Jonge MD; McColl G Imaging Metals in Biology: Balancing Sensitivity, Selectivity and Spatial Resolution. Chem. Soc. Rev 2015, 44 (17), 5941–5958. [DOI] [PubMed] [Google Scholar]
- (18).New EJ Harnessing the Potential of Small Molecule Intracellular Fluorescent Sensors. ACS Sens 2016, 1 (4), 328–333. [Google Scholar]
- (19).Ackerman CM; Lee S; Chang CJ Analytical Methods for Imaging Metals in Biology: From Transition Metal Metabolism to Transition Metal Signaling. Anal. Chem 2017, 89 (1), 22–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Sabine Becker J Imaging of Metals in Biological Tissue by Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA–ICP–MS): State of the Art and Future Developments. J. Mass Spectrom 2013, 48 (2), 255–268. [DOI] [PubMed] [Google Scholar]
- (21).Ackerman CM; Weber PK; Xiao T; Thai B; Kuo TJ; Zhang E; Pett-Ridge J; Chang CJ Multimodal LA-ICP-MS and NanoSIMS Imaging Enables Copper Mapping within Photoreceptor Megamitochondria in a Zebrafish Model of Menkes Disease. Metallomics 2018, 10 (3), 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Xiao T; Ackerman CM; Carroll EC; Jia S; Hoagland A; Chan J; Thai B; Liu CS; Isacoff EY; Chang CJ Copper Regulates Rest-Activity Cycles through the Locus Coeruleus-Norepinephrine System. Nat. Chem. Biol 2018, 14 (7), 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Boxer SG; Kraft ML; Weber PK Advances in Imaging Secondary Ion Mass Spectrometry for Biological Samples. Annu. Rev. Biophys 2009, 38 (1), 53–74. [DOI] [PubMed] [Google Scholar]
- (24).Pickering IJ; George GN X-Ray Absorption Spectroscopy Imaging of Biological Tissues. AIP Conf. Proc 2006, 882 (1), 311–315. [Google Scholar]
- (25).Pushie MJ; Pickering IJ; Korbas M; Hackett MJ; George GN Elemental and Chemically Specific X-Ray Fluorescence Imaging of Biological Systems. Chem. Rev 2014, 114 (17), 8499–8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Ortega R; Devès G; Carmona A Bio-Metals Imaging and Speciation in Cells Using Proton and Synchrotron Radiation X-Ray Microspectroscopy. J. R. Soc., Interface 2009, 6, S649–S658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Unwin N Experiments in Electron Microscopy: From Metals to Nerves. Phys. Scr 2015, 90 (4), No. 048002. [Google Scholar]
- (28).McRae R; Bagchi P; Sumalekshmy S; Fahrni CJ In Situ Imaging of Metals in Cells and Tissues. Chem. Rev 2009, 109 (10), 4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Domaille DW; Que EL; Chang CJ Synthetic Fluorescent Sensors for Studying the Cell Biology of Metals. Nat. Chem. Biol 2008, 4 (3), 168–175. [DOI] [PubMed] [Google Scholar]
- (30).Que EL; Domaille DW; Chang CJ Metals in Neurobiology: Probing Their Chemistry and Biology with Molecular Imaging. Chem. Rev 2008, 108 (5), 1517–1549. [DOI] [PubMed] [Google Scholar]
- (31).Quang DT; Kim JS Fluoro- and Chromogenic Chemodosimeters for Heavy Metal Ion Detection in Solution and Biospeci-mens. Chem. Rev 2010, 110 (10), 6280–6301. [DOI] [PubMed] [Google Scholar]
- (32).Yang Y; Zhao Q; Feng W; Li F Luminescent Chemodosimeters for Bioimaging. Chem. Rev 2013, 113 (1), 192–270. [DOI] [PubMed] [Google Scholar]
- (33).Chowdhury S; Rooj B; Dutta A; Mandal U Review on Recent Advances in Metal Ions Sensing Using Different Fluorescent Probes. J. Fluoresc 2018, 28 (4), 999–1021. [DOI] [PubMed] [Google Scholar]
- (34).Liu Z; He W; Guo Z Metal Coordination in Photo-luminescent Sensing. Chem. Soc. Rev 2013, 42 (4), 1568–1600. [DOI] [PubMed] [Google Scholar]
- (35).Carter KP; Young AM; Palmer AE Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev 2014, 114 (8), 4564–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Eun Jun M; Roy B; Han Ahn K “Turn-on” Fluorescent Sensing with “Reactive” Probes. Chem. Commun 2011, 47 (27), 7583–7601. [DOI] [PubMed] [Google Scholar]
- (37).Aron AT; Ramos-Torres KM; Cotruvo JA; Chang CJ Recognition- and Reactivity-Based Fluorescent Probes for Studying Transition Metal Signaling in Living Systems. Acc. Chem. Res 2015, 48 (8), 2434–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kemlo JA; Shepherd TM Quenching of Excited Singlet States by Metal Ions. Chem. Phys. Lett 1977, 47 (1), 158–162. [Google Scholar]
- (39).Aron AT; Reeves AG; Chang CJ Activity-Based Sensing Fluorescent Probes for Iron in Biological Systems. Curr. Opin. Chem. Biol 2018, 43, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Au-Yeung HY; New EJ; Chang CJ A Selective Reaction-Based Fluorescent Probe for Detecting Cobalt in Living Cells. Chem. Commun 2012, 48 (43), 5268–5270. [DOI] [PubMed] [Google Scholar]
- (41).Kim S; Jo J; Lee D Conformationally Distorted π-Conjugation for Reaction-Based Detection of Nickel: Fluorescence Turn-on by Twist-and-Fragment. Org. Lett 2016, 18 (18), 4530–4533. [DOI] [PubMed] [Google Scholar]
- (42).Dujols V; Ford F; Czarnik AW A Long-Wavelength Fluorescent Chemodosimeter Selective for Cu(II) Ion in Water. J. Am. Chem. Soc 1997, 119 (31), 7386–7387. [Google Scholar]
- (43).Chan J; Dodani SC; Chang CJ Reaction-Based Small-Molecule Fluorescent Probes for Chemoselective Bioimaging. Nat. Chem 2012, 4 (12), 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Strianese M; Pellecchia C Metal Complexes as Fluorescent Probes for Sensing Biologically Relevant Gas Molecules. Coord. Chem. Rev 2016, 318, 16–28. [Google Scholar]
- (45).Michel BW; Lippert AR; Chang CJ A Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc 2012, 134 (38), 15668–15671. [DOI] [PubMed] [Google Scholar]
- (46).Toussaint SNW; Calkins RT; Lee S; Michel BW Olefin Metathesis-Based Fluorescent Probes for the Selective Detection of Ethylene in Live Cells. J. Am. Chem. Soc 2018, 140 (41), 13151–13155. [DOI] [PubMed] [Google Scholar]
- (47).Lim MH; Xu D; Lippard SJ Visualization of Nitric Oxide in Living Cells by a Copper-Based Fluorescent Probe. Nat. Chem. Biol 2006, 2 (7), 375–380. [DOI] [PubMed] [Google Scholar]
- (48).Sasakura K; Hanaoka K; Shibuya N; Mikami Y; Kimura Y; Komatsu T; Ueno T; Terai T; Kimura H; Nagano T Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide. J. Am. Chem. Soc 2011, 133 (45), 18003–18005. [DOI] [PubMed] [Google Scholar]
- (49).Yu ZH; Chung CY-S; Tang FK; Brewer TF; Au-Yeung HY A Modular Trigger for the Development of Selective Superoxide Probes. Chem. Commun 2017, 53 (72), 10042–10045. [DOI] [PubMed] [Google Scholar]
- (50).Kolanowski JL; Shen C; New EJ Fluorescent Probes for the Analysis of Labile Metals in Brain Cells. In Metals in the Brain: Measurement and Imaging; White AR, Ed.; Springer: New York, 2017; pp 51–70. [Google Scholar]
- (51).Irving H; Williams RJP The Stability of Transition-Metal Complexes. J. Chem. Soc 1953, 3192–3210. [Google Scholar]
- (52).Minta A; Kao JP; Tsien RY Fluorescent Indicators for Cytosolic Calcium Based on Rhodamine and Fluorescein Chromophores. J. Biol. Chem 1989, 264 (14), 8171–8178. [PubMed] [Google Scholar]
- (53).Cotruvo JA Jr.; Aron AT; Ramos-Torres KM; Chang CJ Synthetic Fluorescent Probes for Studying Copper in Biological Systems. Chem. Soc. Rev 2015, 44 (13), 4400–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Ramos-Torres KM; Kolemen S; Chang CJ Thioether Coordination Chemistry for Molecular Imaging of Copper in Biological Systems. Isr. J. Chem 2016, 56 (9–10), 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Fahrni CJ Synthetic Fluorescent Probes for Monovalent Copper. Curr. Opin. Chem. Biol 2013, 17 (4), 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Dodani SC; He Q; Chang CJ A Turn-On Fluorescent Sensor for Detecting Nickel in Living Cells. J. Am. Chem. Soc 2009, 131 (50), 18020–18021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Lee MH; Kim JS; Sessler JL Small Molecule-Based Ratiometric Fluorescence Probes for Cations, Anions, and Biomolecules. Chem. Soc. Rev 2015, 44 (13), 4185–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Tang Y; Lee D; Wang J; Li G; Yu J; Lin W; Yoon J Development of Fluorescent Probes Based on Protection–Deprotection of the Key Functional Groups for Biological Imaging. Chem. Soc. Rev 2015, 44 (15), 5003–5015. [DOI] [PubMed] [Google Scholar]
- (59).Brewer TF; Chang CJ An Aza-Cope Reactivity-Based Fluorescent Probe for Imaging Formaldehyde in Living Cells. J. Am. Chem. Soc 2015, 137 (34), 10886–10889. [DOI] [PubMed] [Google Scholar]
- (60).Bruemmer KJ; Brewer TF; Chang CJ Fluorescent Probes for Imaging Formaldehyde in Biological Systems. Curr. Opin. Chem. Biol 2017, 39, 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Chang MCY; Pralle A; Isacoff EY; Chang CJ A Selective, Cell-Permeable Optical Probe for Hydrogen Peroxide in Living Cells. J. Am. Chem. Soc 2004, 126 (47), 15392–15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Miller EW; Albers AE; Pralle A; Isacoff EY; Chang CJ Boronate-Based Fluorescent Probes for Imaging Cellular Hydrogen Peroxide. J. Am. Chem. Soc 2005, 127 (47), 16652–16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Miller EW; Tulyathan O; Isacoff EY; Chang CJ Molecular Imaging of Hydrogen Peroxide Produced for Cell Signaling. Nat. Chem. Biol 2007, 3 (5), 263–267. [DOI] [PubMed] [Google Scholar]
- (64).Lippert AR; Van de Bittner GC; Chang CJ Boronate Oxidation as a Bioorthogonal Reaction Approach for Studying the Chemistry of Hydrogen Peroxide in Living Systems. Acc. Chem. Res 2011, 44 (9), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Brewer TF; Garcia FJ; Onak CS; Carroll KS; Chang CJ Chemical Approaches to Discovery and Study of Sources and Targets of Hydrogen Peroxide Redox Signaling Through NADPH Oxidase Proteins. Annu. Rev. Biochem 2015, 84 (1), 765–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Lippert AR; New EJ; Chang CJ Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc 2011, 133 (26), 10078–10080. [DOI] [PubMed] [Google Scholar]
- (67).Lin VS; Lippert AR; Chang CJ Cell-Trappable Fluorescent Probes for Endogenous Hydrogen Sulfide Signaling and Imaging H2O2-Dependent H2S Production. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (18), 7131–7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Lin VS; Chen W; Xian M; Chang CJ Chemical Probes for Molecular Imaging and Detection of Hydrogen Sulfide and Reactive Sulfur Species in Biological Systems. Chem. Soc. Rev 2015, 44 (14), 4596–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Guo M; Corona T; Ray K; Nam W Heme and Nonheme High-Valent Iron and Manganese Oxo Cores in Biological and Abiological Oxidation Reactions. ACS Cent. Sci 2019, 5 (1), 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Smith JM; Mayberry DE; Margarit CG; Sutter J; Wang H; Meyer K; Bontchev RP N–O Bond Homolysis of an Iron(II) TEMPO Complex Yields an Iron(III) Oxo Intermediate. J. Am. Chem. Soc 2012, 134 (15), 6516–6519. [DOI] [PubMed] [Google Scholar]
- (71).Bigi JP; Harman WH; Lassalle-Kaiser B; Robles DM; Stich TA; Yano J; Britt RD; Chang CJ A High-Spin Iron(IV)–Oxo Complex Supported by a Trigonal Nonheme Pyrrolide Platform. J. Am. Chem. Soc 2012, 134 (3), 1536–1542. [DOI] [PubMed] [Google Scholar]
- (72).Au-Yeung HY; Chan J; Chantarojsiri T; Chang CJ Molecular Imaging of Labile Iron(II) Pools in Living Cells with a Turn-On Fluorescent Probe. J. Am. Chem. Soc 2013, 135 (40), 15165–15173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Maity D; Kumar V; Govindaraju T Reactive Probes for Ratiometric Detection of Co2+ and Cu+ Based on Excited-State Intramolecular Proton Transfer Mechanism. Org. Lett 2012, 14 (23), 6008–6011. [DOI] [PubMed] [Google Scholar]
- (74).Ke B; Ma L; Kang T; He W; Gou X; Gong D; Du L; Li M In Vivo Bioluminescence Imaging of Cobalt Accumulation in a Mouse Model. Anal. Chem 2018, 90 (8), 4946–4950. [DOI] [PubMed] [Google Scholar]
- (75).Hirayama T; Okuda K; Nagasawa H A Highly Selective Turn-on Fluorescent Probe for Iron(II) to Visualize Labile Iron in Living Cells. Chem. Sci 2013, 4 (3), 1250–1256. [Google Scholar]
- (76).Niwa M; Hirayama T; Okuda K; Nagasawa H A New Class of High-Contrast Fe(II) Selective Fluorescent Probes Based on Spirocyclized Scaffolds for Visualization of Intracellular Labile Iron Delivered by Transferrin. Org. Biomol. Chem 2014, 12 (34), 6590–6597. [DOI] [PubMed] [Google Scholar]
- (77).Hirayama T; Tsuboi H; Niwa M; Miki A; Kadota S; Ikeshita Y; Okuda K; Nagasawa H A Universal Fluorogenic Switch for Fe(II) Ion Based on N-Oxide Chemistry Permits the Visualization of Intracellular Redox Equilibrium Shift towards Labile Iron in Hypoxic Tumor Cells. Chem. Sci 2017, 8 (7), 4858–4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Yang X; Wang Y; Liu R; Zhang Y; Tang J; Yang E; Zhang D; Zhao Y; Ye Y A Novel ICT-Based Two Photon and NIR Fluorescent Probe for Labile Fe2+ Detection and Cell Imaging in Living Cells. Sens. Actuators, B 2019, 288, 217–224. [Google Scholar]
- (79).Hirayama T; Kadota S; Niwa M; Nagasawa H A Mitochondria-Targeted Fluorescent Probe for Selective Detection of Mitochondrial Labile Fe(II). Metallomics 2018, 10 (6), 794–801. [DOI] [PubMed] [Google Scholar]
- (80).Niwa M; Hirayama T; Oomoto I; Wang DO; Nagasawa H Fe(II) Ion Release during Endocytotic Uptake of Iron Visualized by a Membrane-Anchoring Fe(II) Fluorescent Probe. ACS Chem. Biol 2018, 13 (7), 1853–1861. [DOI] [PubMed] [Google Scholar]
- (81).Hirayama T; Miki A; Nagasawa H Organelle-Specific Analysis of Labile Fe(II) during Ferroptosis by Using a Cocktail of Various Colour Organelle-Targeted Fluorescent Probes. Metallomics 2019, 11 (1), 111–117. [DOI] [PubMed] [Google Scholar]
- (82).Hirayama T; Inden M; Tsuboi H; Niwa M; Uchida Y; Naka Y; Hozumi I; Nagasawa H A Golgi-Targeting Fluorescent Probe for Labile Fe(II) to Reveal an Abnormal Cellular Iron Distribution Induced by Dysfunction of VPS35. Chem. Sci 2019, 10 (5), 1514–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Maiti S; Aydin Z; Zhang Y; Guo M Reaction-Based Turn-on Fluorescent Probes with Magnetic Responses for Fe2+ Detection in Live Cells. Dalton Trans 2015, 44 (19), 8942–8949. [DOI] [PubMed] [Google Scholar]
- (84).Spangler B; Morgan CW; Fontaine SD; Vander Wal MN; Chang CJ; Wells JA; Renslo AR A Reactivity-Based Probe of the Intracellular Labile Ferrous Iron Pool. Nat. Chem. Biol 2016, 12 (9), 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Aron AT; Loehr MO; Bogena J; Chang CJ An Endoperoxide Reactivity-Based FRET Probe for Ratiometric Fluorescence Imaging of Labile Iron Pools in Living Cells. J. Am. Chem. Soc 2016, 138 (43), 14338–14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Dixon SJ; Lemberg KM; Lamprecht MR; Skouta R; Zaitsev EM; Gleason CE; Patel DN; Bauer AJ; Cantley AM; Yang WS; Morrison B; Stockwell BR Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149 (5), 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Aron AT; Heffern MC; Lonergan ZR; Vander Wal MN; Blank BR; Spangler B; Zhang Y; Park HM; Stahl A; Renslo AR; Skaar EP; Chang CJ In Vivo Bioluminescence Imaging of Labile Iron Accumulation in a Murine Model of Acinetobacter Baumannii Infection. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (48), 12669–12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Muir RK; Zhao N; Wei J; Wang Y; Moroz A; Huang Y; Chen Y-C; Sriram R; Kurhanewicz J; Ruggero D; Renslo AR; Evans MJ Measuring Dynamic Changes in the Labile Iron Pool in Vivo with a Reactivity-Based Probe for Positron Emission Tomography. ACS Cent. Sci 2019, 5 (4), 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Long L; Wang N; Han Y; Huang M; Yuan X; Cao S; Gong A; Wang K A Coumarin-Based Fluorescent Probe for Monitoring Labile Ferrous Iron in Living Systems. Analyst 2018, 143 (11), 2555–2562. [DOI] [PubMed] [Google Scholar]
- (90).Go Y-M; Jones DP Redox Compartmentalization in Eukaryotic Cells. Biochim. Biophys. Acta, Gen. Subj 2008, 1780 (11), 1273–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Elwell CE; Gagnon NL; Neisen BD; Dhar D; Spaeth AD; Yee GM; Tolman WB Copper–Oxygen Complexes Revisited: Structures, Spectroscopy, and Reactivity. Chem. Rev 2017, 117 (3), 2059–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Liu JJ; Diaz DE; Quist DA; Karlin KD Copper(I)-Dioxygen Adducts and Copper Enzyme Mechanisms. Isr. J. Chem 2016, 56 (9–10), 738–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Itoh S Developing Mononuclear Copper–Active-Oxygen Complexes Relevant to Reactive Intermediates of Biological Oxidation Reactions. Acc. Chem. Res 2015, 48 (7), 2066–2074. [DOI] [PubMed] [Google Scholar]
- (94).Taki M; Iyoshi S; Ojida A; Hamachi I; Yamamoto Y Development of Highly Sensitive Fluorescent Probes for Detection of Intracellular Copper(I) in Living Systems. J. Am. Chem. Soc 2010, 132 (17), 5938–5939. [DOI] [PubMed] [Google Scholar]
- (95).Taki M; Akaoka K; Mitsui K; Yamamoto Y A Mitochondria-Targeted Turn-on Fluorescent Probe Based on a Rhodol Platform for the Detection of Copper(I). Org. Biomol. Chem 2014, 12 (27), 4999–5005. [DOI] [PubMed] [Google Scholar]
- (96).Hu Z; Hu J; Wang H; Zhang Q; Zhao M; Brommesson C; Tian Y; Gao H; Zhang X; Uvdal K A TPA-Caged Precursor of (Imino)Coumarin for “Turn-on” Fluorogenic Detection of Cu+. Anal. Chim. Acta 2016, 933, 189–195. [DOI] [PubMed] [Google Scholar]
- (97).Yuan L; Lin W; Chen B; Xie Y Development of FRET-Based Ratiometric Fluorescent Cu2+ Chemodosimeters and the Applications for Living Cell Imaging. Org. Lett 2012, 14 (2), 432–435. [DOI] [PubMed] [Google Scholar]
- (98).Fan J; Zhan P; Hu M; Sun W; Tang J; Wang J; Sun S; Song F; Peng X A Fluorescent Ratiometric Chemodosimeter for Cu2+ Based on TBET and Its Application in Living Cells. Org. Lett 2013, 15 (3), 492–495. [DOI] [PubMed] [Google Scholar]
- (99).Hu Z; Hu J; Cui Y; Wang G; Zhang X; Uvdal K; Gao H-W A Facile “Click” Reaction to Fabricate a FRET-Based Ratiometric Fluorescent Cu2+ Probe. J. Mater. Chem. B 2014, 2 (28), 4467–4472. [DOI] [PubMed] [Google Scholar]
- (100).Chae MY; Czarnik AW Fluorometric Chemodosimetry. Mercury(II) and Silver(I) Indication in Water via Enhanced Fluorescence Signaling. J. Am. Chem. Soc 1992, 114 (24), 9704–9705. [Google Scholar]
- (101).Yang Y-K; Yook K-J; Tae J A Rhodamine-Based Fluorescent and Colorimetric Chemodosimeter for the Rapid Detection of Hg2+ Ions in Aqueous Media. J. Am. Chem. Soc 2005, 127 (48), 16760–16761. [DOI] [PubMed] [Google Scholar]
- (102).Ko S-K; Yang Y-K; Tae J; Shin I In Vivo Monitoring of Mercury Ions Using a Rhodamine-Based Molecular Probe. J. Am. Chem. Soc 2006, 128 (43), 14150–14155. [DOI] [PubMed] [Google Scholar]
- (103).Zhang X; Xiao Y; Qian X A Ratiometric Fluorescent Probe Based on FRET for Imaging Hg2+ Ions in Living Cells. Angew. Chem., Int. Ed 2008, 47 (42), 8025–8029. [DOI] [PubMed] [Google Scholar]
- (104).Duan W; Han Y; Liu Q; Cui J; Gong S; Ma Y; Zhang C; Sun Z Design and Synthesis of Novel Rhodamine-Based Chemodosimeters Derived from [2.2]Paracyclophane and Their Application in Detection of Hg2+ion. Tetrahedron Lett 2017, 58 (4), 271–278. [Google Scholar]
- (105).Wang T; Zhao Q-J; Hu H-G; Yu S-C; Liu X; Liu L; Wu Q-Y Spirolactonized Si-Rhodamine: A Novel NIR Fluorophore Utilized as a Platform to Construct Si-Rhodamine-Based Probes. Chem. Commun 2012, 48 (70), 8781–8783. [DOI] [PubMed] [Google Scholar]
- (106).Tang B; Ding B; Xu K; Tong L Use of Selenium to Detect Mercury in Water and Cells: An Enhancement of the Sensitivity and Specificity of a Seleno Fluorescent Probe. Chem. - Eur. J 2009, 15 (13), 3147–3151. [DOI] [PubMed] [Google Scholar]
- (107).Im HG; Kim HY; Chang S-K Dual Signaling of Hg2+ Ions by Selective Cleavage of Thiophosphinated Rhodol. Sens. Actuators, B 2014, 191, 854–859. [Google Scholar]
- (108).Chen L; Park SJ; Wu D; Kim HM; Yoon J A Two-Photon Fluorescent Probe for Colorimetric and Ratiometric Monitoring of Mercury in Live Cells and Tissues. Chem. Commun 2019, 55 (12), 1766–1769. [DOI] [PubMed] [Google Scholar]
- (109).Shi W; Sun S; Li X; Ma H Imaging Different Interactions of Mercury and Silver with Live Cells by a Designed Fluorescence Probe Rhodamine B Selenolactone. Inorg. Chem 2010, 49 (3), 1206–1210. [DOI] [PubMed] [Google Scholar]
- (110).Chen X; Baek K-H; Kim Y; Kim S-J; Shin I; Yoon J A Selenolactone-Based Fluorescent Chemodosimeter to Monitor Mercury/Methylmercury Species in Vitro and in Vivo. Tetrahedron 2010, 66 (23), 4016–4021. [Google Scholar]
- (111).Song F; Watanabe S; Floreancig PE; Koide K Oxidation-Resistant Fluorogenic Probe for Mercury Based on Alkyne Oxymercuration. J. Am. Chem. Soc 2008, 130 (49), 16460–16461. [DOI] [PubMed] [Google Scholar]
- (112).Ando S; Koide K Development and Applications of Fluorogenic Probes for Mercury(II) Based on Vinyl Ether Oxymercuration. J. Am. Chem. Soc 2011, 133 (8), 2556–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Santra M; Ryu D; Chatterjee A; Ko S-K; Shin I; Ahn KH A Chemodosimeter Approach to Fluorescent Sensing and Imaging of Inorganic and Methylmercury Species. Chem. Commun 2009, 0 (16), 2115–2117. [DOI] [PubMed] [Google Scholar]
- (114).Lin W; Cao X; Ding Y; Yuan L; Long L A Highly Selective and Sensitive Fluorescent Probe for Hg2+ Imaging in Live Cells Based on a Rhodamine–Thioamide–Alkyne Scaffold. Chem. Commun 2010, 46 (20), 3529–3531. [DOI] [PubMed] [Google Scholar]
- (115).Brown DG; Boström J Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone? J. Med. Chem 2016, 59 (10), 4443–4458. [DOI] [PubMed] [Google Scholar]
- (116).Santra M; Ko S-K; Shin I; Ahn KH Fluorescent Detection of Palladium Species with an O-Propargylated Fluorescein. Chem. Commun 2010, 46 (22), 3964–3966. [DOI] [PubMed] [Google Scholar]
- (117).Song F; Garner AL; Koide K A Highly Sensitive Fluorescent Sensor for Palladium Based on the Allylic Oxidative Insertion Mechanism. J. Am. Chem. Soc 2007, 129 (41), 12354–12355. [DOI] [PubMed] [Google Scholar]
- (118).Garner AL; Song F; Koide K Enhancement of a Catalysis-Based Fluorometric Detection Method for Palladium through Rational Fine-Tuning of the Palladium Species. J. Am. Chem. Soc 2009, 131 (14), 5163–5171. [DOI] [PubMed] [Google Scholar]
- (119).Garner AL; Koide K Oxidation State-Specific Fluorescent Method for Palladium(II) and Platinum(IV) Based on the Catalyzed Aromatic Claisen Rearrangement. J. Am. Chem. Soc 2008, 130 (49), 16472–16473. [DOI] [PubMed] [Google Scholar]
- (120).Han F-S Transition-Metal-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions: A Remarkable Advance from Palladium to Nickel Catalysts. Chem. Soc. Rev 2013, 42 (12), 5270–5298. [DOI] [PubMed] [Google Scholar]
- (121).Choi MG; Cha S; Lee H; Jeon HL; Chang S-K Sulfide-Selective Chemosignaling by a Cu 2+ Complex of Dipicolylamine Appended Fluorescein. Chem. Commun 2009, 0 (47), 7390–7392. [DOI] [PubMed] [Google Scholar]
- (122).Hou F; Cheng J; Xi P; Chen F; Huang L; Xie G; Shi Y; Liu H; Bai D; Zeng Z Recognition of Copper and Hydrogen Sulfide in Vitro Using a Fluorescein Derivative Indicator. Dalton Trans 2012, 41 (19), 5799–5804. [DOI] [PubMed] [Google Scholar]
- (123).Hou F; Huang L; Xi P; Cheng J; Zhao X; Xie G; Shi Y; Cheng F; Yao X; Bai D; Zeng Z A Retrievable and Highly Selective Fluorescent Probe for Monitoring Sulfide and Imaging in Living Cells. Inorg. Chem 2012, 51 (4), 2454–2460. [DOI] [PubMed] [Google Scholar]
- (124).Santos-Figueroa LE; de la Torre C; El Sayed S; Sancenón F; Martínez-Máñez R; Costero AM; Gil S; Parra M Highly Selective Fluorescence Detection of Hydrogen Sulfide by Using an Anthracene-Functionalized Cyclam–CuII Complex. Eur. J. Inorg. Chem 2014, 2014 (1), 41–45. [Google Scholar]
- (125).Tang Z; Song B; Ma H; Shi Y; Yuan J A Ratiometric Time-Gated Luminescence Probe for Hydrogen Sulfide Based on Copper-(II)-Coupled Lanthanide Complexes. Anal. Chim. Acta 2019, 1049, 152–160. [DOI] [PubMed] [Google Scholar]
- (126).Marín-Hernández C; Toscani A; Sancenón F; Wilton-Ely JDET; Martínez-Máñez R Chromo-Fluorogenic Probes for Carbon Monoxide Detection. Chem. Commun 2016, 52 (35), 5902–5911. [DOI] [PubMed] [Google Scholar]
- (127).de la Torre C; Toscani A; Marín-Hernández C; Robson JA; Terencio MC; White AJP; Alcaraz MJ; Wilton-Ely JDET;Martínez-Máñez R; Sancenón F Ex Vivo Tracking of Endogenous CO with a Ruthenium(II) Complex. J. Am. Chem. Soc 2017, 139 (51), 18484–18487. [DOI] [PubMed] [Google Scholar]
- (128).Lim MH; Wong BA; Pitcock WH; Mokshagundam D; Baik M-H; Lippard SJ Direct Nitric Oxide Detection in Aqueous Solution by Copper(II) Fluorescein Complexes. J. Am. Chem. Soc 2006, 128 (44), 14364–14373. [DOI] [PubMed] [Google Scholar]
- (129).Shatalin K; Gusarov I; Avetissova E; Shatalina Y; McQuade LE; Lippard SJ; Nudler E Bacillus Anthracis-Derived Nitric Oxide Is Essential for Pathogen Virulence and Survival in Macrophages. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (3), 1009–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).McQuade LE; Ma J; Lowe G; Ghatpande A; Gelperin A; Lippard SJ Visualization of Nitric Oxide Production in the Mouse Main Olfactory Bulb by a Cell-Trappable Copper(II) Fluorescent Probe. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (19), 8525–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Alam R; Mistri T; Mondal P; Das D; Mandal SK; Khuda-Bukhsh AR; Ali M A Novel Copper(II) Complex as a Nitric Oxide Turn-on Fluorosensor: Intracellular Applications and DFT Calculation. Dalton Trans 2014, 43 (6), 2566–2576. [DOI] [PubMed] [Google Scholar]
- (132).Rosenthal J; Lippard SJ Direct Detection of Nitroxyl in Aqueous Solution Using a Tripodal Copper(II) BODIPY Complex. J. Am. Chem. Soc 2010, 132 (16), 5536–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Royzen M; Wilson JJ; Lippard SJ Physical and Structural Properties of [Cu(BOT1)Cl]Cl, a Fluorescent Imaging Probe for HNO. J. Inorg. Biochem 2013, 118, 162–170. [DOI] [PubMed] [Google Scholar]
- (134).Zhou Y; Liu K; Li J-Y; Fang Y; Zhao T-C; Yao C Visualization of Nitroxyl in Living Cells by a Chelated Copper(II) Coumarin Complex. Org. Lett 2011, 13 (6), 1290–1293. [DOI] [PubMed] [Google Scholar]
- (135).Apfel U-P; Buccella D; Wilson JJ; Lippard SJ Detection of Nitric Oxide and Nitroxyl with Benzoresorufin-Based Fluorescent Sensors. Inorg. Chem 2013, 52 (6), 3285–3294. [DOI] [PubMed] [Google Scholar]
- (136).Wrobel AT; Johnstone TC; Deliz Liang A; Lippard SJ; Rivera-Fuentes P A Fast and Selective Near-Infrared Fluorescent Sensor for Multicolor Imaging of Biological Nitroxyl (HNO). J. Am. Chem. Soc 2014, 136 (12), 4697–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (137).Hitomi Y; Takeyasu T; Funabiki T; Kodera M Detection of Enzymatically Generated Hydrogen Peroxide by Metal-Based Fluorescent Probe. Anal. Chem 2011, 83 (24), 9213–9216. [DOI] [PubMed] [Google Scholar]
- (138).Hitomi Y; Takeyasu T; Kodera M Iron Complex-Based Fluorescent Probes for Intracellular Hydrogen Peroxide Detection. Chem. Commun 2013, 49 (85), 9929–9931. [DOI] [PubMed] [Google Scholar]
- (139).Song D; Lim JM; Cho S; Park S-J; Cho J; Kang D; Rhee SG; You Y; Nam W A Fluorescence Turn-on H2O2 Probe Exhibits Lysosome-Localized Fluorescence Signals. Chem. Commun 2012, 48 (44), 5449–5451. [DOI] [PubMed] [Google Scholar]
- (140).Zheng K; Lin W; Tan L; Chen H; Cui H A Unique Carbazole–Coumarin Fused Two-Photon Platform: Development of a Robust Two-Photon Fluorescent Probe for Imaging Carbon Monoxide in Living Tissues. Chem. Sci 2014, 5 (9), 3439–3448. [Google Scholar]
- (141).Liu K; Kong X; Ma Y; Lin W Rational Design of a Robust Fluorescent Probe for the Detection of Endogenous Carbon Monoxide in Living Zebrafish Embryos and Mouse Tissue. Angew. Chem., Int. Ed 2017, 56 (43), 13489–13492. [DOI] [PubMed] [Google Scholar]
- (142).Xu S; Liu H-W; Yin X; Yuan L; Huan S-Y; Zhang X-B A Cell Membrane-Anchored Fluorescent Probe for Monitoring Carbon Monoxide Release from Living Cells. Chem. Sci 2019, 10 (1), 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (143).Ragaini F; Larici H; Rimoldi M; Caselli A; Ferretti F; Macchi P; Casati N Mapping Palladium Reduction by Carbon Monoxide in a Catalytically Relevant System. A Novel Palladium(I) Dimer. Organometallics 2011, 30 (8), 2385–2393. [Google Scholar]
- (144).Pal S; Mukherjee M; Sen B; Mandal SK; Lohar S; Chattopadhyay P; Dhara K A New Fluorogenic Probe for the Selective Detection of Carbon Monoxide in Aqueous Medium Based on Pd(0) Mediated Reaction. Chem. Commun 2015, 51 (21), 4410–4413. [DOI] [PubMed] [Google Scholar]
- (145).Wang Z; Zhao Z; Wang R; Yuan R; Liu C; Duan Q; Zhu W; Li X; Zhu B A Mitochondria-Targetable Colorimetric and Far-Red Fluorescent Probe for the Sensitive Detection of Carbon Monoxide in Living Cells. Anal. Methods 2019, 11 (3), 288–295. [Google Scholar]
- (146).Feng W; Hong J; Feng G Colorimetric and Ratiometric Fluorescent Detection of Carbon Monoxide in Air, Aqueous Solution, and Living Cells by a Naphthalimide-Based Probe. Sens. Actuators, B 2017, 251, 389–395. [Google Scholar]
- (147).Feng S; Liu D; Feng W; Feng G Allyl Fluorescein Ethers as Promising Fluorescent Probes for Carbon Monoxide Imaging in Living Cells. Anal. Chem 2017, 89 (6), 3754–3760. [DOI] [PubMed] [Google Scholar]
- (148).Li S-J; Zhou D-Y; Li Y-F; Yang B; Ou-Yang J; Jie J; Liu J; Li C-Y Mitochondria-Targeted near-Infrared Fluorescent Probe for the Detection of Carbon Monoxide in Vivo. Talanta 2018, 188, 691–700. [DOI] [PubMed] [Google Scholar]
- (149).Streu C; Meggers E Ruthenium-Induced Allylcarbamate Cleavage in Living Cells. Angew. Chem., Int. Ed 2006, 45 (34), 5645–5648. [DOI] [PubMed] [Google Scholar]
- (150).Völker T; Dempwolff F; Graumann PL; Meggers E Progress towards Bioorthogonal Catalysis with Organometallic Compounds. Angew. Chem., Int. Ed 2014, 53 (39), 10536–10540. [DOI] [PubMed] [Google Scholar]
- (151).Tomás-Gamasa M; Martínez-Calvo M; Couceiro JR; Mascareñas JL Transition Metal Catalysis in the Mitochondria of Living Cells. Nat. Commun 2016, 7, 12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Hsu H-T; Trantow BM; Waymouth RM; Wender PA Bioorthogonal Catalysis: A General Method To Evaluate Metal-Catalyzed Reactions in Real Time in Living Systems Using a Cellular Luciferase Reporter System. Bioconjugate Chem 2016, 27 (2), 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Lee Y; Umeano A; Balskus EP Rescuing Auxotrophic Microorganisms with Nonenzymatic Chemistry. Angew. Chem., Int. Ed 2013, 52 (45), 11800–11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Sánchez MI; Penas C; Vázquez ME; Mascareñas JL Metal-Catalyzed Uncaging of DNA-Binding Agents in Living Cells. Chem. Sci 2014, 5 (5), 1901–1907. [PMC free article] [PubMed] [Google Scholar]
- (155).Miller MA; Askevold B; Mikula H; Kohler RH; Pirovich D; Weissleder R Nano-Palladium Is a Cellular Catalyst for in Vivo Chemistry. Nat. Commun 2017, 8, 15906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Li J; Yu J; Zhao J; Wang J; Zheng S; Lin S; Chen L; Yang M; Jia S; Zhang X; Chen PR Palladium-Triggered Deprotection Chemistry for Protein Activation in Living Cells. Nat. Chem 2014, 6 (4), 352–361. [DOI] [PubMed] [Google Scholar]
- (157).Deu E; Chen IT; Lauterwasser EMW; Valderramos J; Li H; Edgington LE; Renslo AR; Bogyo M Ferrous Iron-Dependent Drug Delivery Enables Controlled and Selective Release of Therapeutic Agents in Vivo. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (45), 18244–18249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Fontaine SD; Spangler B; Gut J; Lauterwasser EMW; Rosenthal PJ; Renslo AR Drug Delivery to the Malaria Parasite Using an Arterolane-Like Scaffold. ChemMedChem 2015, 10 (1), 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Abrams RP; Carroll WL; Woerpel KA Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells. ACS Chem. Biol 2016, 11 (5), 1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Binder JB; Raines RT Olefin Metathesis for Chemical Biology. Curr. Opin. Chem. Biol 2008, 12 (6), 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Patra M; Gasser G Organometallic Compounds: An Opportunity for Chemical Biology? ChemBioChem 2012, 13 (9), 1232–1252. [DOI] [PubMed] [Google Scholar]
- (162).Sasmal PK; Streu CN; Meggers E Metal Complex Catalysis in Living Biological Systems. Chem. Commun 2013, 49 (16), 1581–1587. [DOI] [PubMed] [Google Scholar]
- (163).Vinogradova EV Organometallic Chemical Biology: An Organometallic Approach to Bioconjugation. Pure Appl. Chem 2017, 89 (11), 1619–1640. [Google Scholar]
- (164).Rebelein JG; Ward TR In Vivo Catalyzed New-to-Nature Reactions. Curr. Opin. Biotechnol 2018, 53, 106–114. [DOI] [PubMed] [Google Scholar]
- (165).Ngo AH; Bose S; Do LH Intracellular Chemistry: Integrating Molecular Inorganic Catalysts with Living Systems. Chem. -Eur. J 2018, 24 (42), 10584–10594. [DOI] [PubMed] [Google Scholar]


