Abstract
Theileria sp.-specific small subunit (SSU) rRNA gene amplification confirmed the presence of the organism in cattle and in Amblyomma americanum and Dermacentor variabilis ticks collected from a cattle herd in Missouri. Blood from the index animal had type A and type D Theileria SSU rRNA genes. The type D gene was also found in blood from two cohort cattle and tick tissues. The type A SSU rRNA gene was previously reported from bovine Theileria isolates from Texas and North Carolina; the type D gene was reported from a Texas cow with theileriosis.
Bovine theileriosis is a tick-transmitted disease caused by Theileria, a hemoprotozoan parasite common in most parts of the world. Sporadic reports of infected cattle have been described in the United States (7, 12). Previously we reported small subunit (SSU) rRNA gene studies of Theileria isolates recently obtained from cattle in North Carolina and east Texas and of the Texas bovine Theileria isolate originally described by Kuttler and Craig in 1975 (3, 7). The North Carolina Theileria isolate was from an Angus cow, approximately 15 years old, that died with clinical signs including anemia, lethargy, and weight loss. Microscopic examination of Giemsa-stained blood films revealed the presence of numerous Theileria piroplasms (3), but no Anaplasma. No ticks were found on the animal or on two cohort bulls (an adult bull and a 7-month-old bull, both offspring of the cow). Giemsa-stained blood films from the two cohort animals were negative for the presence of Theileria. The east Texas isolate also originated from a cow that had died with clinical signs consistent with hemoprotozoan infection. Giemsa-stained blood films confirmed the presence of numerous Theileria parasites (3). The Texas isolate reported by Kuttler and Craig in 1975 was found during a study of anaplasma-seropositive cattle and was described as only mildly pathogenic in splenectomized calves, with no evidence of pathogenicity seen in the infected host cattle in which it was originally found (7). The SSU rRNA gene studies showed that sequence type A was shared by the 1975 Texas and the North Carolina isolates. The type D SSU rRNA gene sequence was obtained from the east Texas Theileria isolate (3).
In the current study, we describe the application of molecular techniques based on SSU rRNA gene sequence analysis to detect the possible presence of Theileria species in the blood of cattle suspected to be infected. Furthermore, we show this technique to be useful in identifying potential tick vectors.
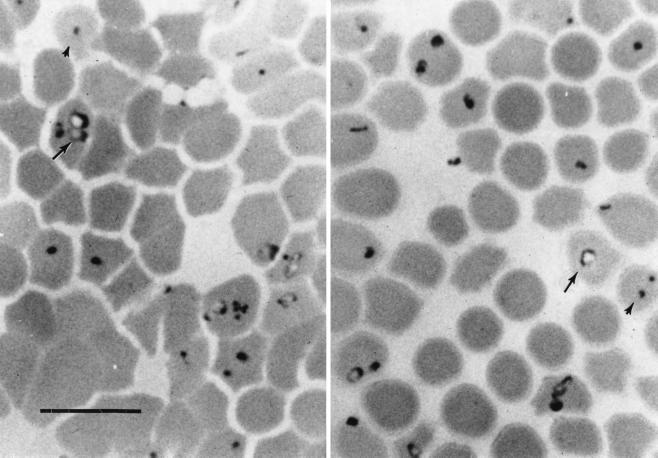
The index animal was an 8-year-old, mixed-breed cow (designated cow 1) from a ranch in Missouri. The cow had clinical signs suggesting intraerythrocytic parasitism and, accordingly, was treated for anaplasmosis. There was no improvement, and the animal died. A methanol-fixed blood film sent to Texas A&M University (College Station, Tex.) for Giemsa staining and microscopic examination confirmed the presence of pleomorphic piroplasms, with as many as four Theileria merozoites within some erythrocytes. The level of parasitemia was 21% and consisted predominantly of round, dot, and ring forms (Fig. 1).
FIG. 1.
Giemsa-stained blood film from the index animal, cow 1, showing pleomorphic Theileria parasite forms. Ring (arrow) and small round (arrowhead) forms are indicated. Multiple parasites are seen within a single erythrocyte. Bar, 10 μm.
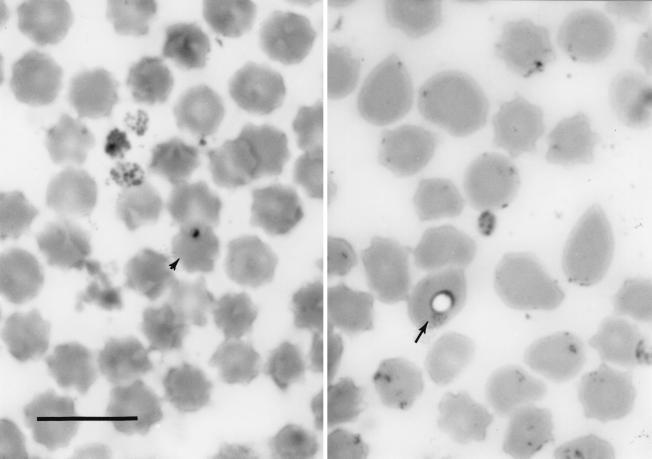
Seventy-four cohort cattle on the ranch subsequently were checked for tick infestation and the presence of hemoparasites. Giemsa-stained smears revealed the presence of Theileria-like organisms in two of the tick-infested animals (designated cow 2 and cow 3) (Fig. 2). Blood samples from these animals and ticks removed from these two animals and other cohorts were sent to Texas A&M University for analysis.
FIG. 2.
Giemsa-stained blood film from cohort Theileria carrier cow 2. Ring (arrow) and small round (arrowhead) forms of Theileria-like organisms are shown. Bar, 10 μm.
Genomic DNA was obtained from cow 1 blood by a modification of a previously described method (3). The Giemsa-stained blood smear was destained with methanol, and the blood cells were scraped off the slide into 100 μl of lysis buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA [pH 8.0], 1% sodium dodecyl sulfate). The DNA was obtained by a standard phenol-chloroform extraction method followed by ethanol precipitation (10). This procedure was repeated with two additional slides of unstained, methanol-fixed blood smears from cow 1. DNA was extracted from cow 2 and cow 3 blood by standard protocols (10). The genomic DNA was used for initial amplification by primers A and B specific for eukaryotic SSU rRNA genes (11). To confirm the presence of Theileria SSU rRNA genes, amplicons and genomic DNA were used as templates for amplification with Theileria-specific SSU rRNA gene primers 989 and 990 (1). Positive and negative controls for amplification protocols included genomic DNA from the North Carolina bovine Theileria isolate and bovine kidney genomic DNA, respectively.
Appropriate measures were taken with all assays to prevent DNA cross-contamination in amplification reactions. All reagents were divided into small aliquots upon receipt. Pre- and postamplification designated work areas and pipettors were utilized. Sterile aerosol-barrier pipettor tips (Midwest Scientific, Valley Park, Mo.) were used exclusively. Positive and negative controls were included in all assays. All work surfaces were decontaminated with Nolvasan solution (chlorhexidine diacetate veterinary viricide and bactericide; Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) before and after use.
Theileria SSU rRNA gene amplicons obtained with primers 989 and 990 were ligated into the plasmid vector pCR 2.1-TOPO and TOP10 One Shot Escherichia coli transformed according to the manufacturer’s instructions (TOPO TA cloning kit; Invitrogen Co., San Diego, Calif.). Colony PCR of selected clones was used to confirm the presence of the appropriately sized insert DNA as previously described (3). Plasmid DNA purified from the selected clones with a QIAprep plasmid kit (Qiagen, Inc., Valencia, Calif.) was used in sequencing reactions (Dye Terminator Cycle Sequencing Ready Reaction; PE Applied Biosystems, Norwalk, Conn.) with internal primer 528F (6). Automated sequencing was performed in either an ABI PRISM model 373A or ABA model 377 sequencer with version 1.2.2 or 2.1.1 software, respectively (Perkin-Elmer, Inc., Norwalk, Conn.; Gene Technologies Laboratory, Institute of Developmental and Molecular Biology, Texas A&M University, College Station).
Ticks were collected from cohort cattle, placed in 70% ethanol in individual tubes, and identified as Amblyomma americanum or Dermacentor variabilis. The ethanol-fixed ticks were shipped to Texas A&M University, where they were successively washed three times with 70% ethanol and then with phosphate-buffered saline (PBS [pH 7.4]). Each tick was individually fastened to a separate paraffin wax block, covered with PBS, and dissected to remove the internal organs. The dissection instruments were dipped in 90% (vol/vol) ethanol and flamed between each use. The salivary glands were removed first and washed immediately in three changes of PBS. The remaining internal organs were then removed and washed in three changes of PBS. In cases where it was not possible to remove the salivary glands intact and separately, all internal organs were collected and then washed in three changes of PBS. Genomic DNA was then purified from the samples, and SSU rRNA genes were amplified (see Table 2) as described above.
TABLE 2.
SSU rRNA gene amplification and sequence types from Theileria in ticks
| Tick species | Tick stage/sexa | Animal host tag no. | Organs testedb | Theileria sp. ampliconc | Theileria type SSU rRNA gened |
|---|---|---|---|---|---|
| Amblyomma americanum | Adult/F | 16 (cow 2) | SG | − | NA |
| Dermacentor variabilis | Adult/F | 16 (cow 2) | SG | + | D |
| GI | + | D | |||
| A. americanum | Adult/F | 17 (cow 3) | SG | + | D |
| GI | + | D | |||
| D. variabilis | Adult/M | 17 (cow 3) | IO | − | NA |
| A. americanum | Adult/F | 6 | SG | − | NA |
| A. americanum | Adult/F | 8 | SG | + | ND |
| A. americanum | Adult/M | 9 | IO | + | ND |
| A. americanum | Adult/F | 13 | SG | + | ND |
| A. americanum | Nymph/F | 18 | IO | + | ND |
| A. americanum | Nymph/F | 23 | IO | + | ND |
F, female; M, male.
SG, salivary glands; GI, gut and internal organs, except salivary glands; IO, all internal organs.
Amplicon from Theileria SSU rRNA gene-specific primers 989 and 990.
NA, not applicable; ND, not determined.
The nucleotide sequences obtained were submitted to a GenBank database BlastN homology search (National Center for Biotechnology Information) and compared with those of other known Theileria spp. by the CLUSTAL W (version 1.60) multiple sequence alignment program (13). Cow 1 sequences were identical to the SSU rRNA gene sequence previously reported as type A and to that reported for Theileria buffeli Marula, Kenya (GenBank accession no.: cow 1, plasmid clone designations USMO1-4 and -1-6, AF060212; type A, U97047; T. buffeli, Marula, Kenya, Z15106) and SSU rRNA gene sequence type D (GenBank accession no.: cow 1, plasmid clone designations USMO1-1 and -1-2, AF060211; type D, U97052). SSU rRNA gene sequence type D was also found in the cohort isolates, cow 2 and cow 3 (GenBank accession no.: cow 2, designated USMO16, AF060213; cow 3, designated USMO17, AF060214) (Table 1).
TABLE 1.
SSU rRNA gene amplification and sequence types from Theileria in bovine blood samples
| Animal | Animal tag no. | % of Theileria parasitemia | Theileria sp. amplicona | Theileria type SSU rRNA gene |
|---|---|---|---|---|
| Cow 1 | NAb | 21 | + | A, D |
| Cow 2 | 16 | <1 | + | D |
| Cow 3 | 17 | <1 | + | D |
Product from Theileria-specific primers 989 and 990.
NA, not available.
Type D SSU rRNA gene sequences were also found in all organ samples tested from the D. variabilis tick from cohort cow 2 (tag no. 16, Table 2; D. variabilis from cow 2 designated T95-625, GenBank accession no. AF060215) and the A. americanum tick collected from cohort cow 3 (tag no. 17, Table 2; A. americanum from cow 3 designated T97-626, GenBank accession no. AF060216). Theileria SSU rRNA genes were amplified from 7 of 10 ticks tested. Theileria SSU rRNA genes were not amplified from the A. americanum tick from cow 2 nor from the D. variabilis tick from cow 3 (Table 2). One other A. americanum tick from another cohort was negative (Table 2). However, Theileria SSU rRNA genes were found in both male and female A. americanum ticks taken from six additional cohort animals (Table 2). Theileria SSU rRNA genes were amplified from salivary glands from four of six ticks tested.
The Theileria isolate from cow 1, the index animal in the current study, possessed type A and type D SSU rRNA gene sequences. The type A Theileria SSU rRNA gene sequence was previously reported in the 1975 Texas bovine isolate (USET1), the bovine isolate from North Carolina (USNC), and in bovine Theileria isolates from Japan and Korea (3). The type A sequence was identical to that reported for T. buffeli, Marula, Kenya (GenBank accession no. Z15106). Previously, the type D sequence was reported in a bovine Theileria isolate from Texas (USET2) and in a bovine Theileria isolate from Korea (3). The SSU rRNA gene sequence of the Thung Song bovine Theileria isolate (GenBank accession no. AB000270) from Thailand has 99.8% identity to sequence type D (GenBank homology search). The type D Theileria SSU rRNA gene sequence was found in all positive blood and tick samples tested in this study.
Type D SSU rRNA genes were amplified by the Theileria-specific primers from both A. americanum and D. variabilis ticks removed from cohort animals. The type D Theileria SSU rRNA gene sequence was identified in salivary gland DNA from an individual D. variabilis tick removed from cow 2 (tag no. 16) and an individual A. americanum tick removed from cow 3 (tag no. 17). Both animals were Theileria carriers as determined by Giemsa-stained blood film examination.
The finding of Theileria-specific SSU rRNA gene sequences in DNA extracted from salivary glands is significant, because it presumably reflects the presence of parasites that have migrated from the tick gut to the salivary glands. However, we cannot be sure that these parasites would have developed into mature infective sporozoites; therefore, transmission studies must be done to determine whether these ticks are indeed biological vectors of this parasite. Significantly, A. americanum has been shown to be a competent vector for Theileria cervi of white-tailed deer (8, 9). Theileria cervi SSU rRNA gene sequences (types F and G) are distinct from the type A and D SSU rRNA genes found in the Theileria spp. from cattle and ticks in the current study (3).
The amplification of Theileria-specific SSU rRNA genes from salivary glands of ticks infesting cattle known to be exposed to Theileria supports previous observations that Theileria SSU rRNA genes may be used to detect the presence of the organism in presumed vector ticks. Similar methodology has shown Theileria annulata in Hyalomma ticks (4, 5) and Theileria parva and Theileria taurotragi in Rhipicephalus appendiculatus ticks (2, 14). We may conclude from our results that A. americanum and D. variabilis ticks harbor at least one Theileria sp. infective for cattle in the United States.
Previously, both of the Theileria SSU rRNA gene sequence types identified in this study had been found in U.S. cows that died with clinical signs suggestive of theileriosis. The results of the current study differed in that both types A and D were found in an isolate from a single animal; previously these two types had not been found together in U.S. bovine isolates. Dual hemoparasitic infections are not uncommon, however, and multiple SSU rRNA gene sequence types have been reported in both bovine and cervine hosts with Theileria infections (3). Our study suggests that the index animal may have had a mixed infection of T. buffeli (type A) and an as yet unidentified Theileria sp. (type D). Our study also suggests that a bovine Theileria sp. may be endemic on this ranch, since the molecular data show that both ticks and cattle have evidence of Theileria infection.
Acknowledgments
We are indebted to the following people for information regarding this study: James W. Mertins, at the USDA APHIS National Veterinary Services Laboratory, Ames, Iowa; Terry Clark, USDA APHIS, North Carolina; and Patricia Cuddihee, Jerry Eber, and Robert Gray, Missouri.
This research was supported in part by the Texas Agricultural Experiment Station (Project H-6261), Texas A&M University Faculty Minigrant (FMG 95-120), and a Korean Research Foundation postdoctoral research grant (KRF300-151).
REFERENCES
- 1.Allsopp B A, Baylis H A, Allsopp M T E, Cavalier-Smith T, Bishop R P, Carrington D M, Sohanpal B, Spooner P. Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitology. 1993;107:157–165. doi: 10.1017/s0031182000067263. [DOI] [PubMed] [Google Scholar]
- 2.Bishop R P, Sohanpal B K, Morzaria S P, Dolan T T, Mwakima F N, Young A S. Discrimination between Theileria parva and T. taurotragi in the salivary glands of Rhipicephalus appendiculatus ticks using oligonucleotides homologous to ribosomal RNA sequences. Parasitol Res. 1994;80:259–261. doi: 10.1007/BF00932685. [DOI] [PubMed] [Google Scholar]
- 3.Chae J S, Lee J M, Kwon O D, Holman P J, Waghela S D, Wagner G G. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene variable (V4) region among and within geographic isolates of Theileria from cattle, elk and white-tailed deer. Vet Parasitol. 1998;75:41–52. doi: 10.1016/s0304-4017(97)00183-0. [DOI] [PubMed] [Google Scholar]
- 4.de Kok J B, d’Oliveira C, Jongejan F. Detection of protozoan Theileria annulata in Hyalomma ticks by the polymerase chain reaction. Exp Appl Acarol. 1993;17:839–846. doi: 10.1007/BF00225857. [DOI] [PubMed] [Google Scholar]
- 5.d’Oliveira C, van der Weide M, Jacquiet P, Jongejan F. Detection of Theileria annulata by the PCR in ticks (Acari: Ixodidae) collected from cattle in Mauritania. Exp Appl Acarol. 1997;21:279–291. doi: 10.1023/a:1018455223462. [DOI] [PubMed] [Google Scholar]
- 6.Elwood H J, Olsen G J, Sogin M L. The small subunit ribosomal RNA gene sequences from the hypotrichous ciliates Oxytricha nova and Stylonychia pustulata. J Mol Biol Evol. 1985;5:399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- 7.Kuttler K L, Craig T M. Isolation of a bovine Theileria. Am J Vet Res. 1975;36:323–325. [PubMed] [Google Scholar]
- 8.Kuttler K L, Robinson R M, Bell R R. Tick transmission of theileriasis in a white-tailed deer. Bull Wildl Dis Assoc. 1967;3:182–183. [Google Scholar]
- 9.Laird J S, Kocan A A, Kocan K M, Presley W M, Hair J A. Susceptibility of Amblyomma americanum to natural and experimental infections with Theileria cervi. J Wildlife Dis. 1988;24:679–683. doi: 10.7589/0090-3558-24.4.679. [DOI] [PubMed] [Google Scholar]
- 10.Sambrook J E, Fritsch F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 1.25–1.28. [Google Scholar]
- 11.Sogin M L. Amplification of ribosomal RNA genes for molecular evolution studies. In: Innis M A, Gelfand D H, Sninsky J J, White T H, editors. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press, Inc.; 1990. pp. 307–314. [Google Scholar]
- 12.Splitter E J. Theileria mutans associated with bovine anaplasmosis in the United States. J Am Vet Med Assoc. 1950;117:134–135. [PubMed] [Google Scholar]
- 13.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watt D, Sparagano O, Brown C G D, Walker A R. Use of the polymerase chain reaction for identification and quantification of Theileria parva protozoan in Rhipicephalus appendiculatus ticks. Parasitol Res. 1997;83:359–363. doi: 10.1007/s004360050262. [DOI] [PubMed] [Google Scholar]