ABSTRACT
Invasive bacterial infections during pregnancy are a major risk factor for preterm birth, stillbirth, and fetal injury. Group B streptococci (GBS) are Gram-positive bacteria that asymptomatically colonize the lower genital tract but infect the amniotic fluid and induce preterm birth or stillbirth. Experimental models that closely emulate human pregnancy are pivotal for the development of successful strategies to prevent these adverse pregnancy outcomes. Using a unique nonhuman primate model that mimics human pregnancy and informs temporal events surrounding amniotic cavity invasion and preterm labor, we show that the animals inoculated with hyaluronidase (HylB)-expressing GBS consistently exhibited microbial invasion into the amniotic cavity, fetal bacteremia, and preterm labor. Although delayed cytokine responses were observed at the maternal-fetal interface, increased prostaglandin and matrix metalloproteinase levels in these animals likely mediated preterm labor. HylB-proficient GBS dampened reactive oxygen species production and exhibited increased resistance to neutrophils compared to an isogenic mutant. Together, these findings demonstrate how a bacterial enzyme promotes GBS amniotic cavity invasion and preterm labor in a model that closely resembles human pregnancy.
KEYWORDS: group B streptococcus, hyaluronidase, immune evasion, neutrophils, pregnancy, preterm labor
INTRODUCTION
Globally, invasive bacterial infections are a leading cause of stillbirth and preterm birth (1–3). Group B streptococci (GBS) or Streptococcus agalactiae are beta-hemolytic, Gram-positive bacteria that commonly exist as commensal organisms in the rectovaginal tracts of healthy adult women. However, GBS can be transmitted to neonates during birth or ascend into the uterus during pregnancy, resulting in fetal injury, stillbirth, preterm birth, or early neonatal infection (4–6). Recent reports have indicated that by conservative estimates, approximately 147,000 stillbirths and 3.5 million preterm births each year are attributed to GBS infections (7). Currently, there are no therapies to prevent preterm birth or stillbirth. Some countries have implemented protocols to screen women for GBS colonization in the third trimester of pregnancy and to subsequently administer antibiotics to GBS-positive women during labor and delivery (known as intrapartum antibiotic prophylaxis). Although these measures have decreased the incidence of neonatal GBS disease in the first week of life (8, 9), they fail to prevent adverse pregnancy outcomes that occur prior to labor and delivery that result in preterm births or stillbirths (8, 10–12).
Improved preventive therapies for GBS require a greater understanding of the complex interactions between pathogen and host. One host factor important for responses to microbial infection is hyaluronan (HA). HA is a major constituent of the host extracellular matrix and exists as a high-molecular-weight glucosaminoglycan polymer that assists in cell migration, cell-cell signaling, and responses to injury and infection (13, 14). During infection or injury, high-molecular-weight HA (HMW-HA) is degraded by host hyaluronidases or reactive oxygen species (ROS) to low-molecular-weight HA (LMW-HA; comprising HA tetramers or pentamers), which are proinflammatory in nature and mediate cytokine responses through signaling via Toll-like receptors (TLRs), such as TLR-2 and TLR-4 (15, 16). Interestingly, certain bacterial pathogens such as GBS also secrete a hyaluronidase enzyme (17, 18). The GBS hyaluronidase (HylB; encoded by the hylB gene) was identified in 1950 as an exolytic enzyme (19) that breaks down HA into disaccharide fragments (20). Recently, GBS HylB-generated HA disaccharides were shown to block TLR-2 and TLR-4 signaling in macrophages and dampen cytokine responses (21). Subsequently, we observed that clinical GBS isolates associated with women in preterm labor or neonatal infections exhibit increased hyaluronidase activity compared to commensal GBS isolates obtained from rectovaginal swabs of healthy women (22). We also noted that GBS hyaluronidase dampened uterine immune responses and postulated that these promoted ascending infection in a pregnant mouse model of GBS infection (22).
However, the pregnant mouse and other lower mammalian models exhibit dissimilarities to many aspects of human pregnancy, including differences in reproductive anatomy, placentation, onset of labor, and sensitivity to pathogens. In contrast, the closest animal model for studies related to human pregnancy is the pregnant nonhuman primate (NHP) (23–26). Similarities of NHPs to humans include reproductive anatomy, type and structure of placenta (hemomonochorial), number of fetuses (singleton), long gestational period (160 to 170 days), initiation of labor (hormonal control of parturition), sensitivity to pathogens, and the developmental timeline of the fetal lung and brain (25, 26). In the chronically catheterized pregnant NHP model (27, 28), we inoculate bacteria at the choriodecidual space, a site in the pregnant uterine mucosa between the uterine muscle and the placental membranes, where bacteria are thought to first encounter the maternal-fetal interface during ascending infection from the lower genital tract (2, 27).
Here, we used a nonhemolytic GBS strain (serotype V, GB37) that exhibits increased hyaluronidase activity (29) to address how hyaluronidase contributes to immune evasion, fetal injury, and preterm labor. We found that pregnant NHPs inoculated with GB37 exhibited rapid microbial invasion of the amniotic cavity, fetal bacteremia, and preterm labor in contrast to animals inoculated with the isogenic, hyaluronidase-deficient strain. Analyses of the cellular and biochemical events at the maternal-fetal interface revealed impaired host defenses that likely prompted the early onset of labor. Together, our studies show that a bacterial hyaluronidase promotes microbial invasion of the amniotic cavity and preterm labor in the nonhuman primate model.
RESULTS
HylB promotes adverse pregnancy outcomes in an NHP model of choriodecidual GBS infection.
To elucidate whether hyaluronidase promotes GBS infection and adverse outcomes during pregnancy, we used a chronically catheterized nonhuman primate (Macaca nemestrina) model that closely emulates human pregnancy and defines temporal events during amniotic cavity invasion and preterm labor (28). Ten animals received choriodecidual inoculations of 1 × 108 to 3 × 108 CFU of either hyaluronidase-proficient wild-type GBS (strain GB37, n = 5) or an isogenic hyaluronidase-deficient GBS (strain GB37ΔhylB, n = 5). Controls included NHPs that received saline (n = 6 total; n = 4 were described previously [27]). Adverse pregnancy outcomes, specifically preterm labor and/or microbial invasion of the amniotic cavity (Table 1) were the primary outcomes of our study. In GBS-inoculated animals, Cesarean section was performed at the onset of preterm labor, which was defined as progressive cervical dilation associated with increased and sustained uterine activity or at 3 days post-GBS inoculation if preterm labor did not occur, as described previously (28).
TABLE 1.
Summary of pregnancy outcomes, cytokines, and prostaglandins in pregnant NHPsa
| Outcome | Saline (n = 6) | GB37 (n = 5) | GB37ΔhylB (n = 5) |
P
|
||
|---|---|---|---|---|---|---|
| Saline vs GB37 | GB37 vs GB37ΔhylB | Saline vs GB37ΔhylB | ||||
| Primary and composite outcomes, no. (%) | ||||||
| Adverse outcomeb | 0 (0) | 5 (100) | 1 (20) | 0.0009 | 0.02 | NS |
| Preterm laborc | 0 (0) | 4 (80) | 1 (20) | 0.01 | 0.1 | NS |
| Microbial invasion of the amniotic cavity and fetal bacteremia | 0 (0) | 5 (100) | 1 (20) | 0.0009 | 0.02 | NS |
| Peak contractions, AF cytokines, and prostaglandins, mean peak pg/ml (SEM) | ||||||
| Hourly contraction area | 1,326.7 (506.58) | 5,606.0 (1,165.1) | 3,087.4 (1,050.0) | 0.04 | NS | NS |
| IL-1β | 31.61 (20.59) | 1,754.8 (1,112.7) | 597.65 (583.42) | 0.09 | NS | NS |
| TNF-α | 26.13 (11.44) | 574.58 (285.82) | 97.58 (39.43) | 0.07 | NS | NS |
| IL-6 | 7,091.1 (2,642.2) | 2,827.8 (1,331.3) | 2,404.1 (1,356.5) | NS | NS | NS |
| IL-8 | 888.07 (321.53) | 2,120.5 (1,383.3) | 1,629.7 (1,498.2) | NS | NS | NS |
| PGE2 | 509.83 (247.84) | 1,160.9 (647.40) | 289.23 (116.69) | NS | NS | NS |
| PGF2α | 424.31 (169.64) | 1,808.1 (887.27) | 164.93 (45.91) | NS | 0.08 | NS |
| Fetal cytokines | ||||||
| IL-1β | 0.80 (0.60) | 2.152 (0.8584) | 1.128 (0.8645) | NS | NS | NS |
| TNF-α | 2.268 (1.127) | 0.01 (0.0) | 0.01 (0.0) | 0.04 | NS | 0.04 |
| IL-6 | 3.639 (1.852) | 1,049.6 (722.10) | 44.74 (41.58) | 0.049 | NS | NS |
| IL-8 | 574.06 (314.04) | 2,648.8 (1,674.1) | 2,576.2 (1,551.3) | NS | NS | NS |
The primary outcomes are shown as “number (%)” and were compared among groups using Barnard’s test. Amniotic fluid (AF) cytokines, prostaglandins and fetal plasma cytokines are expressed as mean peak (SEM) in pg/ml. Hourly contraction area is expressed in mm Hg × s/h. Cytokines (IL-1β, TNF-α, IL-6, and IL-8) and prostaglandins (PGE2 and PGF2α) were compared by using one-way ANOVA with Bonferroni’s correction. Statistical analyses were conducted using Intercooled STATA 8.2 for Windows 2000 (StataCorp) or SciStatCalc. P values of <0.05 are indicated in boldface. NS, P > 0.100.
Adverse outcome is a composite metric representing preterm labor or microbial invasion of the amniotic cavity with fetal bacteremia.
Preterm labor was defined as progressive cervical dilation associated with increased and sustained uterine activity.
We found that choriodecidual inoculation of the hyaluronidase-proficient GBS strain (GB37) induced preterm labor in 4/5 (80%) animals compared to 1/5 (20%) animals inoculated with the isogenic hyaluronidase-deficient strain GB37ΔhylB or 0/6 (0%) saline controls (Table 1 and Fig. 1). Further, all GB37-inoculated animals (5/5, 100%) experienced microbial invasion of the amniotic cavity in contrast to 1/5 animals (20%) inoculated with GB37ΔhylB and 0/6 (0%) saline controls (Table 1 and Fig. 1). In the GB37 group, GBS CFU was recovered from the amniotic fluid (AF) as early as 6 h postinoculation in three animals (GB37#1, GB37#2, and GB37#4), which was followed by preterm labor within 1 and 2 days (Fig. 1; see also Fig. S1 in the supplemental material). For GB37#3, GBS bacteria was recovered from the AF by 12 h after inoculation, and the AF was significantly turbid due to high bacterial burden within day 2. This was accompanied by a heavy uterine contraction pattern that resulted in cervical ripening (softening). To avoid a stillbirth due to sepsis, we proceeded with Cesarean section at 49 h after GBS inoculation for this animal. In GB37#5, infection and contractions progressed rapidly after 48 h, and the NHP was in labor by 70 h (early on day 3). In the GB37ΔhylB group, only one animal exhibited preterm labor and microbial invasion of the amniotic cavity (GB37ΔhylB#5), wherein GBS was detected in the AF at 24 h postinoculation and the animal experienced preterm labor within 70 h (Fig. 1; see also Fig. S1). The remaining four animals in this group did not exhibit microbial invasion of the amniotic cavity, preterm labor, or other adverse outcomes (Fig. 1; see also Fig. S1). Collectively, our results indicate that choriodecidual inoculation of a GBS strain with elevated hyaluronidase activity was significantly associated with adverse pregnancy outcomes compared to inoculation with the isogenic hyaluronidase-deficient GBS strain or saline controls (P = 0.02 GB37 versus GB37ΔhylB; P = 0.0005 GB37 versus saline). Despite the significant adverse outcomes noted in the GB37-infected group, there was no statistical difference in the peak AF cytokine levels among the three treatment groups of this study (i.e., saline, GB37, and GB37ΔhylB; Table 1). Interestingly, compared to our previously published studies on hyperhemolytic GBSΔcovR-inoculated animals (28), where we reported peak AF interleukin-6 (IL-6) and IL-8 ranging between 15 and 22 ng/ml (for animals with microbial invasion of the amniotic cavity), the GB37 group had significantly lower peak AF levels of IL-6 (P = 0.009) and IL-8 (P = 0.02). Taken together, these results suggest that increased AF cytokines such as IL-6 and IL-8 are not necessarily a predictive signature of preterm labor, particularly during infection with the immunosuppressive hyaluronidase-expressing GBS bacteria.
FIG 1.
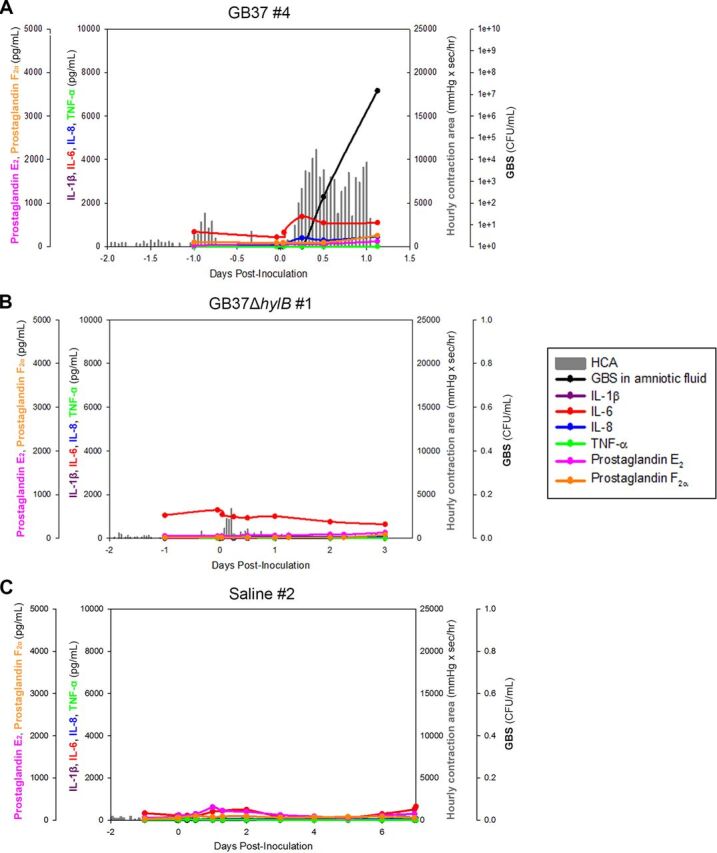
GBS hyaluronidase promotes amniotic cavity invasion and preterm labor. Chronically catheterized pregnant pigtail macaques (Macaca nemestrina) received choriodecidual inoculations of either HylB-proficient WT GBS strain GB37 (n = 5), an isogenic GBS strain lacking HylB (GB37ΔhylB, n = 5), or saline (n = 6) at 118 to 125 days gestation (term, 172 days). Uterine contractions (vertical gray lines), cytokines (IL-1β, IL-6, IL-8, and tumor necrosis factor alpha [TNF-α]), prostaglandins (PGE2 and PGF2α), and GBS CFU from the AF are shown from representative animals that received either GB37 (A), GB37ΔhylB (B), or saline (C).
Uterine contractions, AF cytokines, prostaglandins, and bacterial CFU from choriodecidual inoculations of GB37, GB37ΔhylB, or saline in chronically catheterized pregnant NHPs. Download FIG S1, DOCX file, 1.5 MB (1.6MB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
GBS HylB promotes prostaglandin and matrix metalloproteinase synthesis for cervical softening.
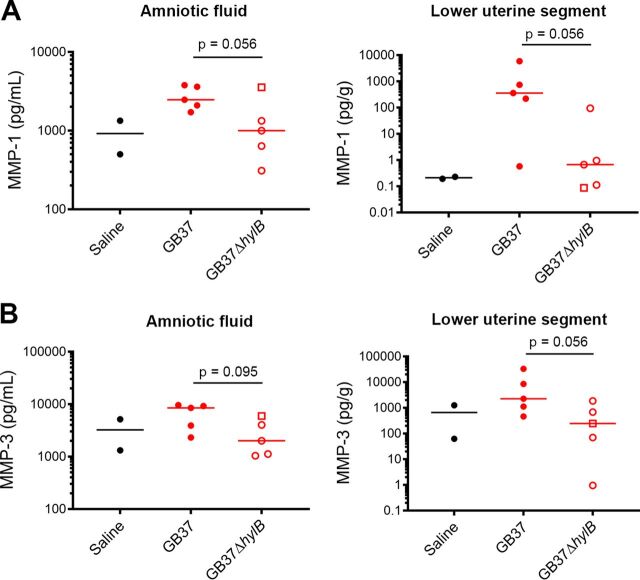
Apart from increased inflammation, prostaglandin and matrix metalloproteinases (MMPs) have been implicated in inducing the thinning and softening of the cervix, leading to preterm labor (30–33). Given our observations that 80% (4/5) of the GB37-infected animals experienced preterm labor in the setting of low inflammation, we sought to determine whether infection with the hyaluronidase-proficient GBS was associated with increased prostaglandin and MMP levels, which could have contributed to cervical softening and preterm labor. We observed a relative increase in MMP-1 and MMP-3 in the AF and lower uterine segments of GB37 animals compared to GB37ΔhylB or saline controls (Fig. 2; see the schematic in Fig. S4a in the supplemental material for the location of the lower uterine segment). These MMPs have been implicated in preterm labor (34, 35). We also noted that peak AF prostaglandin PGF2α levels were higher in the GB37 animals compared to GB37ΔhylB (Table 1). Together, these data provide some potential insight into mechanisms of preterm labor due to infection by the immunosuppressive hyaluronidase-expressing GBS.
FIG 2.
MMP-1 and MMP-3 are elevated in the amniotic fluid and lower uterus of GB37-inoculated NHP. At Cesarean section, amniotic fluid and a tissue segment from the lower uterus were collected from each animal, and each sample was analyzed for MMP-1 (A) and MMP-3 (B) levels by Luminex. Data from GB37 and GB37ΔhylB were compared using a Mann-Whitney test. Although P values between 0.055 and 0.1 are noted due to the small sample size of this nonhuman primate study, P values of <0.05 were considered significant. GB37ΔhylB#5 is designated by an open square.
GBS HylB promotes fetal bacteremia and fetal inflammation.
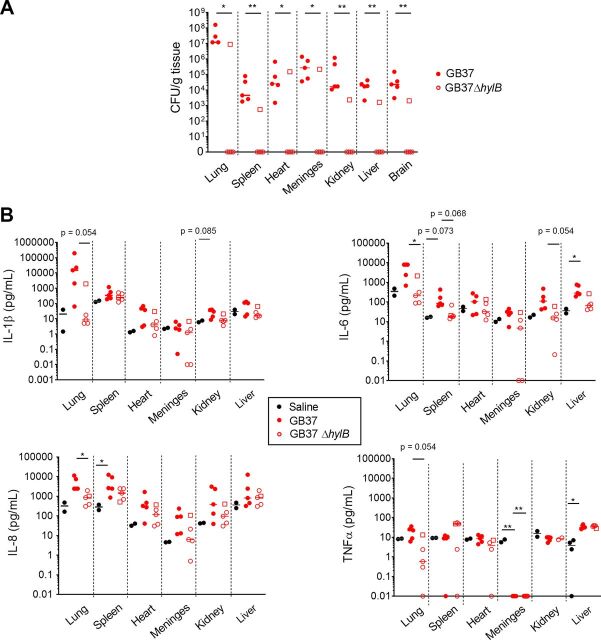
In all GB37-infected animals, microbial invasion of the amniotic cavity coincided with fetal bacteremia, and GBS CFU (ranging from 103 to 108 CFU/g) were recovered from several fetal organs, including the lung, spleen, heart, brain, and meninges (Fig. 3A). In the GB37ΔhylB group, only the animal that exhibited microbial invasion of the amniotic cavity (GB37ΔhylB#5) exhibited fetal bacteremia. Overall, fetal bacteremia was significantly increased in animals inoculated with GB37 compared to GB37ΔhylB (Fig. 3A) with consistently more bacteria recovered from the fetal lung compared to the other fetal organs.
FIG 3.
Microbial invasion of the amniotic cavity coincided with fetal bacteremia and systemic fetal inflammation. At Cesarean section, fetal tissues were harvested from chronically catheterized pregnant NHP that received a choriodecidual inoculation of saline, GB37, or GB37ΔhylB. GB37ΔhylB#5 is designated by an open square. (A) Numbers of GBS CFU obtained from various fetal tissues. Note that GBS was recovered from all fetal tissues of all animals that experienced microbial invasion of the amniotic cavity and preterm birth, including all (5/5) GB37-inoculated animals and one of five GB37ΔhylB-inoculated animals (GB37ΔhylB#5, designated by an open square). Differences in CFU between treatment groups were analyzed using the Mann-Whitney test. *, P < 0.05; **, P < 0.01. (B) Lysates from fetal tissues were examined by Luminex to evaluate differences in levels of fetal cytokines. A Kruskal-Wallis test with Dunn’s multiple-comparison test was performed. *, P < 0.05. P values between 0.05 and 0.1 are noted due to the small sample size of this nonhuman primate study but were not considered significant. Since fetal tissue lysates were not available from historical saline animals (n = 4) reported previously (28), only saline animals performed in the present study (n = 2) were included in these analyses.
We also measured cytokines in fetal plasma and tissues from samples obtained at the study endpoint (Fig. 3B and Table 1). In contrast to low levels of proinflammatory cytokines observed in the amniotic fluid, we noted significantly increased cytokines in the fetal lung (IL-8), spleen (IL-8), and liver (IL-6) when comparing GB37-infected fetuses to saline controls (Fig. 3B). To determine the specific effect of HylB, we compared GB37 with GB37ΔhylB group and found significantly higher levels of IL-6 in the lung (Fig. 3B). Hematoxylin and eosin (H&E) staining of neonatal lungs supported these findings. Lungs from the GB37 group showed minimal (GB37#4) and moderate to severe (GB37#1, GB37#2, GB37#3, and GB37#5) pneumonia (see Fig. S2). In contrast, H&E staining of the GB37ΔhylB group showed overall minimal neutrophilic inflammation in the fetal lung (see Fig. S2). The only animal with microbial invasion of the amniotic cavity in this group, GB37ΔhylB#5, exhibited moderate and mixed cell-type inflammation within the lung. Myeloperoxidase (MPO; neutrophil and granulocyte marker) staining was seen in the neonatal lungs of the GB37 group with minimal staining observed in the GB37ΔhylB group and saline controls (see Fig. S2 and S3). Minimal to no CD68 (expressed by monocytes and macrophages) staining was observed for all three groups (see Fig. S2). Collectively, these analyses reveal that choriodecidual inoculation of hyaluronidase-expressing GBS resulted in bacterial invasion, cytokines, and inflammation in the fetal lung.
Histological examination of NHP fetal lung sections. (A to D) Representative H&E-stained sections from NHPs in each group are shown, including saline#3 (A), GB37#1 (B), GB37#2 (C), and GB37ΔhylB#2 (D). (E to H) Representative MPO-stained sections from NHPs in each group are shown, including saline#3 (E), GB37#1 (F), GB37#2 (G), and GB37ΔhylB#2 (H). (I to L) Representative CD68-stained sections from NHPs in each group are shown, including saline#3 (I), GB37#1 (J), GB37#1 (K), and GB37ΔhylB#2 (L). Note that within the GB37 group, tissues are shown reflecting harvest at 24 h (B, F, and J) and 48 h (C, G, and K). Download FIG S2, DOCX file, 0.9 MB (1.1MB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Quantitation of immunostaining for MPO in the fetal lung and chorioamniotic membranes. (A) The area of MPO immunostaining in the neonatal lung was significantly different between the saline and GB37 groups and between the GB37 and GB37ΔhylB groups. GB37ΔhylB#5 is designated by an open square. A one-way ANOVA with Tukey’s posttest was used to compare groups. (B) The area of MPO immunostaining in the chorioamniotic membranes was not significantly different among the groups in the amnion, chorion, or decidua. GB37ΔhylB#5 is designated by an open square. A one-way ANOVA with Tukey’s posttest was used to compare groups. Download FIG S3, DOCX file, 0.1 MB (126.4KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
GB37-inoculated NHPs experienced enhanced infiltration of CD8+ T cells and phagocytes to the maternal fetal interface. At Cesarean section, biopsy specimens from the uterus and placenta, as well as maternal and fetal blood, were obtained. (A) A schematic depicting the biopsy sites is shown. (B) Samples were processed into single-cell suspensions, stained, and analyzed by flow cytometry for various immune cell markers (indicated in the figure). GB37ΔhylB #5 is designated by an open square. A Welch’s test was used to evaluate differences in immune cell populations between GB37- and GB37ΔhylB-treated animals at each site. Data from two saline controls performed as a part of the present study are included, but similar analyses were not previously performed with historical saline controls (n = 4). Download FIG S4, DOCX file, 0.4 MB (469.2KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
GBS HylB induced greater infiltration of phagocytes to the maternal-fetal interface.
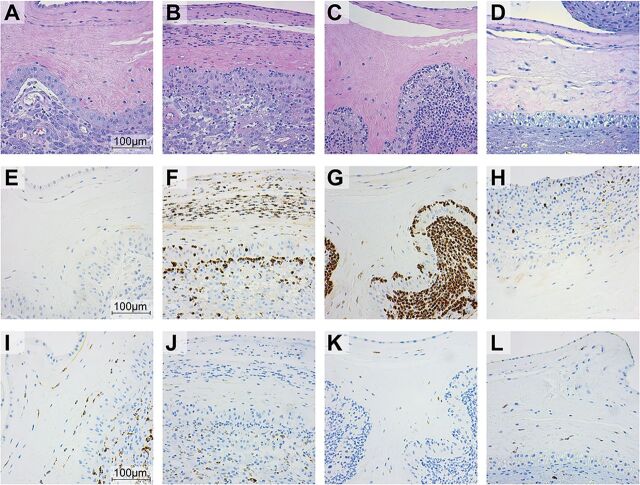
We examined the chorioamniotic membranes at the inoculation site using H&E, MPO, and CD68 to assess inflammation and tissue injury at the maternal-fetal interface. As expected, saline controls revealed minimal to no neutrophil infiltration (Fig. 4A; see also Fig. S3), whereas chorioamniotic membranes from animals in the GB37 group exhibited moderate to severe neutrophilic inflammation (Fig. 4B and C; see also Fig. S3). Conversely, placental membranes from the GB37ΔhylB group had minimal infiltration (Fig. 4D; see also Fig. S3), except for GB37ΔhylB#5, which exhibited moderate neutrophilic inflammation (data not shown). In contrast, CD68 staining (a marker for monocytes and macrophages) was similar across all three groups (saline, GB37, and GB37ΔhylB; Fig. 4I to L), indicating that neutrophils rather than macrophages are predominantly recruited to the choriodecidual interface during GBS infections. Notably, we also observed increased neutrophil levels in the chorioamniotic membranes and miduterine muscle by flow cytometry in GB37-treated animals compared to those inoculated with the ΔhylB strain (see Fig. S4). Collectively, these data indicate that immune cells typically involved in the host response to tissue damage and/or pathogen control are increased in the reproductive tissues of animals inoculated with GB37 versus GB37ΔhylB or saline controls.
FIG 4.
Histological examination of the placental membranes revealed increased neutrophil infiltration in GB37-infected animals. (A to D) H&E staining of NHP placental sections. Representative H&E-stained sections from NHPs in each group are shown, including saline#3 (A), GB37#1 (B), GB37#2 (C), and GB37ΔhylB#2 (D). (E to H) Representative MPO-stained sections from NHPs in each group, including saline#3 (E), GB37#1 (F), GB37#2 (G), and GB37ΔhylB#2 (H). (I to L) Representative CD68-stained sections from NHPs in each group, including saline#2 (I), GB37#1 (J), GB37#2 (K), and GB37ΔhylB#2 (L). For the GB37 group, tissues in panels B, F, and J were taken 24 h after inoculation and tissues in panels C, G, and K were harvested 48 h after inoculation.
Digital spatial profiling of the placenta revealed minimal immune signatures to GBS hyaluronidase.
To obtain greater spatial resolution of the cellular events and signaling cascades occurring at the uterine mucosa, we analyzed paraffin-embedded placental membranes using the GeoMx digital spatial profiling (DSP) platform (NanoString Technologies) (Fig. 5). The DSP scanned whole tissue image shown in Fig. 5A is obtained by stitching together images that are obtained from single Field Of View (FOV): each FOV image has a dimension of 2048 pixel × 2048 pixel, which equals 665.6 μm × 665.6 μm. Regions of interest (ROIs) comprising the decidua, chorion, and amnion in each animal were selected with preferential targeting of areas with GBS (Fig. 5A), and 62 antibody probes coupled to cleavable oligonucleotide tags were hybridized and quantified. Counts were mapped to tissue location to yield a spatially resolved profile of analyte abundance (Fig. 5B). Intriguingly, while GBS was detected in the decidua of all GB37-treated animals (Fig. 5A) and in only one GB37ΔhylB animal, no significant differences in inflammation-associated analytes (e.g., CD45, CD68, STING, CD86, and CD56) were observed between the GBS groups for any tissue type (amnion, chorion, decidua) at these ROIs (Fig. 5B; see also Fig. S5). Conversely, some markers associated with inflammation were significantly lower in GB37 animals compared to saline controls, including the macrophage marker CD14 in the decidua and amnion and CD86 (T cell costimulatory molecule on APCs) in the decidua, chorion, and amnion (Fig. 5B; see also Fig. S6). These data indicate that even in tissues sites where GB37 were detected by fluorescence microscopy (Fig. 5A), some inflammatory markers were dampened relative to baseline. Together, digital spatial profiling showed that choriodecidual inoculation with GB37 did not induce a remarkable proinflammatory signature in the amnion, chorion, or decidua.
FIG 5.
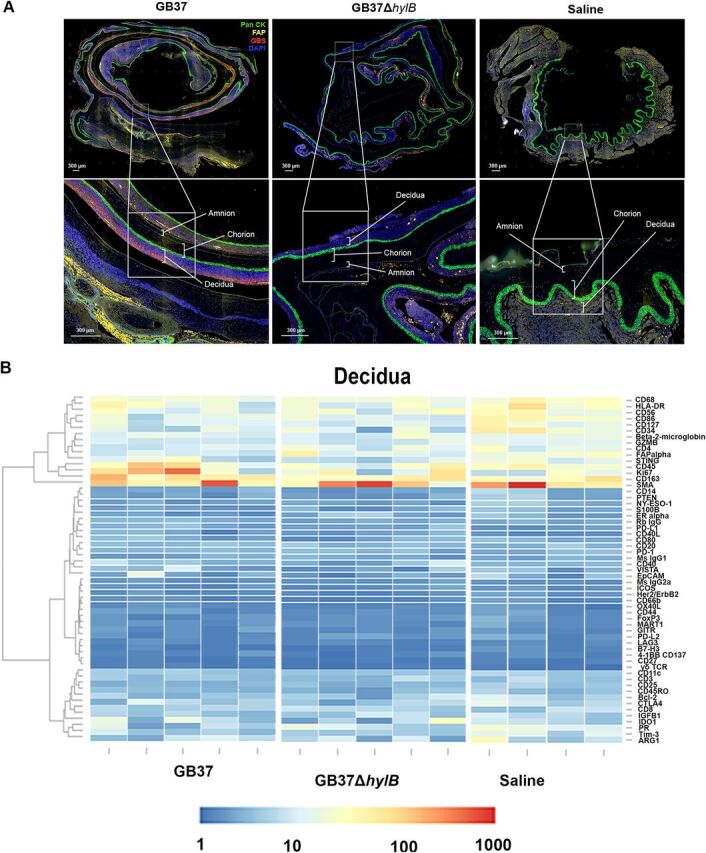
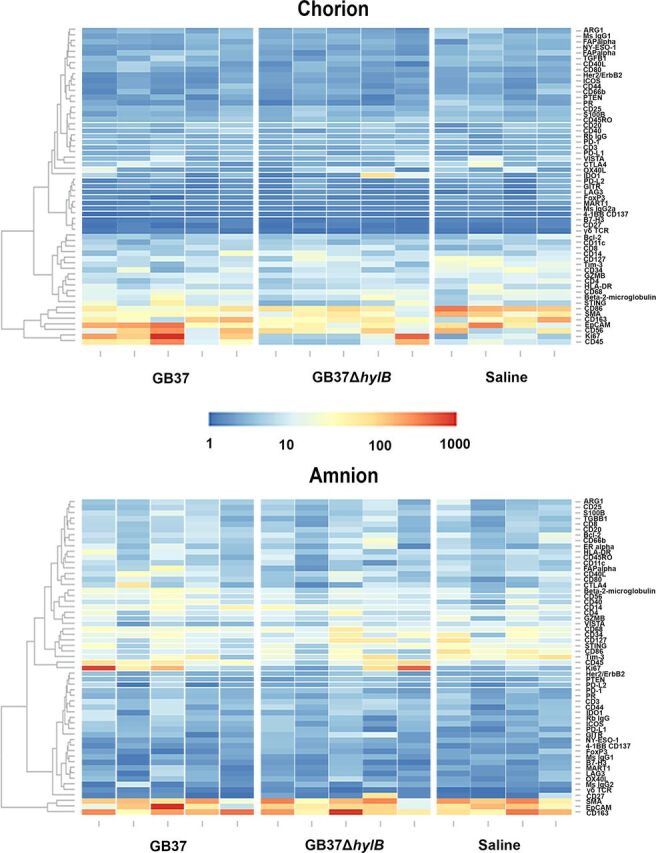
Digital spatial profiling of placental tissues revealed few differential expressions of immune signatures GBS hyaluronidase. (A) Representative placental sections from NHP in each treatment group (GB37#2, GB37ΔhylB#2, and saline#3). We treated each placental section with fluorescent anti-pan cytokeratin (Pan CK, green), anti-fibronectin attachment protein (FAP, yellow), anti-GBS (red), and DAPI (blue) and then identified the decidua, chorion, and amnion within each section as distinct ROIs. Each discrete ROI (i.e., chorion, amnion, and decidua) was analyzed for analyte abundance. (B) Heatmaps showing analyte abundance (normalized by the signal/noise ratio) in the decidua, chorion, and amnion of each animal in the GB37 (n = 5), GB37ΔhylB (n = 5), and saline (n = 4) groups are shown.
Digital spatial profiling analyte fold change: GB37 versus GB37ΔhylB. Analyte abundance in distinct placental regions from GB37ΔhylB-inoculated NHPs and saline-treated NHPs were obtained by digital spatial profiling (Nanostring Technologies). Fold changes in analyte abundance (GB37ΔhylB over GB37) were log2 transformed and analyzed by a linear mixed model in R version 3.6.2. Significance tests were controlled for false discovery rate. Download FIG S5, DOCX file, 0.3 MB (348.5KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Digital spatial profiling analyte fold change: GB37 versus saline. Analyte abundance in distinct placental regions from GB37-inoculated NHPs and saline-treated NHPs were obtained by digital spatial profiling (Nanostring Technologies). Fold changes in analyte abundance (GB37 over saline) were log2 transformed and analyzed by a linear mixed model in R version 3.6.2. Significance tests were controlled for false discovery rate. White asterisk, P < 0.05. Download FIG S6, DOCX file, 0.3 MB (357.6KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
HylB promotes resistance to antimicrobial effects of neutrophils.
Given our observations of neutrophil recruitment in the chorioamniotic membranes and uterus of GB37-infected animals (Fig. 4; see also Fig. S4), with minimal proinflammatory events in the placenta (Fig. 5B), we sought to determine whether GBS hyaluronidase plays a role in dampening neutrophil defenses, which are critical in protecting the host against GBS infection (28, 36). We previously observed that hyperhemolytic strains of GBS circumvented the neutrophil response, in part, by inducing neutrophil cell death (28). Here, no significant cell death was observed when fresh adult human neutrophils were treated with GB37 or GB37ΔhylB (Fig. 6A). Given that HA is present on the cell surface of neutrophils (37), we next sought to determine whether HylB might enable GBS to resist the microbicidal activity of neutrophils. We found neutrophils were less able to kill GB37 compared to GB37ΔhylB (Fig. 6B; see also Fig. S8a), indicating that hyaluronidase enables GBS to subvert this critical host defense.
FIG 6.
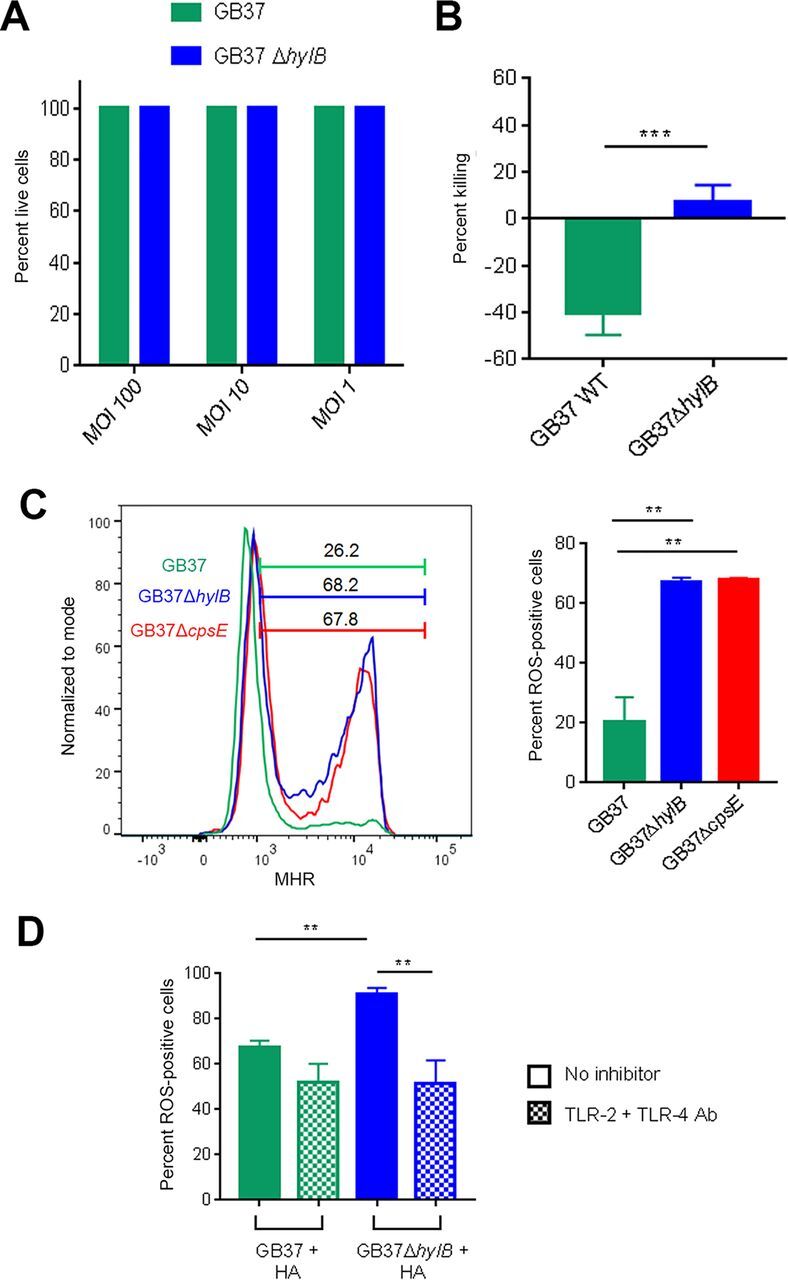
GBS HylB evades neutrophil killing independently of cell death by interfering with TLR-2/4 signaling. (A) Primary human neutrophils were isolated from fresh adult blood, exposed to GB37 or GB37ΔhylB at MOIs 100, 10, or 1 for 4 h, and then examined for cell death by LDH release. The percent live cells was calculated relative to Triton X-100-treated positive controls (0% live cells) and PBS-treated negative controls (100% live cells). (B) GB37 or GB37ΔhylB was exposed to primary human neutrophils isolated from fresh blood (MOI of 1) for 1 h. The percent killing was calculated as the number of CFU recovered after incubation with neutrophils out of the number of the number CFU recovered after incubation without neutrophils × 100. Differences among groups were determined by a paired t test. (C) Primary human neutrophils were isolated as described above, pretreated with dihydrorhodamine-123 (DHR), and then exposed to GB37 or GB37ΔhylB (MOI of 100). Since GBS capsule can suppress neutrophil ROS generation by blocking Siglec 9 (62), the GB37ΔcpsE was included as a control. The conversion of DHR to fluorescent MHR indicates ROS production in cells and was measured by flow cytometry at 60 min postinfection. Differences among treatment groups were determined by one-way analysis of variance (ANOVA). (D) Filtered supernatants of stationary-phase GB37 or GB37ΔhylB liquid cultures were incubated with HA for 18 h to allow for enzymatic digestion of HA. Meanwhile, primary human neutrophils were pretreated with 10 μg/ml anti-TLR-2 antibody (Invivogen) plus 10 μg/ml anti-TLR-4 antibody (Invivogen) or vehicle control. Neutrophils were treated with DHR and then exposed to the digested HA solutions from each strain for 60 min. As described above, ROS production in cells was measured by detecting fluorescent MHR via flow cytometry. Differences in MHR-positive cells among treatment groups were determined by one-way ANOVA. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; ns, P ≥ 0.05.
Digital spatial profiling analyte fold change: GB37ΔhylB versus saline. Analyte abundance in distinct placental regions from GB37ΔhylB-inoculated NHPs and saline-treated NHPs were obtained by digital spatial profiling (Nanostring Technologies). Fold changes in analyte abundance (GB37ΔhylB over saline) were log2 transformed and analyzed by a linear mixed model in R version 3.6.2. Significance tests were controlled for false discovery rate. White asterisk, P < 0.05. Download FIG S7, DOCX file, 0.3 MB (363.8KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Neutrophils were isolated from human maternal blood and umbilical cord (i.e., fetal) blood. (A) GB37 or GB37ΔhylB were exposed to maternal or fetal neutrophils (MOI of 1) for one hour. The percent killing was calculated as the number of CFU recovered after incubation with neutrophils out of the number of the number CFU recovered after incubation without neutrophils × 100. Differences among groups were determined using a paired t test. *, P < 0.05. (B) Maternal or fetal neutrophils were pretreated with dihydrorhodamine-123 (DHR) and then exposed to GB37 (green) or GB37ΔhylB (blue) (MOI of 100). The conversion of DHR to fluorescent MHR indicates ROS production in cells and was measured by flow cytometry at 60 min postinfection. Download FIG S8, DOCX file, 0.1 MB (137.3KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To understand the mechanisms by which HylB may facilitate GBS resistance to neutrophil killing, we measured the production of ROS in neutrophils in the presence of hyaluronidase-proficient or deficient GBS using a flow cytometry-based assay (see the supplemental methods [Text S1]). We noted decreased production of ROS in neutrophils exposed to GB37 compared to GB37ΔhylB, demonstrating that HylB may have blunted the ability of neutrophils to generate ROS (Fig. 6C). This effect was also observed with maternal and fetal neutrophils (see Fig. S8b). Next, we sought to determine whether HylB-mediated TLR-2/4 interference could explain the differences in neutrophil ROS production elicited by the wild-type (WT) and ΔhylB strains. To test this, supernatants from GB37 or GB37ΔhylB were incubated with HA to allow for enzymatic digestion, as described previously (21). Then, HA digests from each strain were exposed to human neutrophils pretreated with anti-TLR-2 and anti-TLR-4 antibodies or vehicle alone, and ROS production was measured. As expected, GB37-HA digests induced less ROS production than GB37ΔhylB-HA digests. Intriguingly, when neutrophils were pretreated with both TLR inhibitors, this effect was lost (Fig. 6D). These findings suggest that proinflammatory HA fragments from hyaluronidase-deficient GBS promote ROS production in neutrophils by engaging TLR-2/4 signaling. Furthermore, these data indicate that GBS may avoid neutrophil killing by using HylB to cleave HA into fragments that blunt TLR2/4 signaling and subsequently diminish ROS production. Taken together, our findings are the first to demonstrate that HylB promotes GBS resistance to neutrophils, which contributes to adverse pregnancy outcomes in pregnant nonhuman primates.
Details regarding the study design, generation of GB37ΔcpsE mutant, catheterization of pregnant NHP, sample collection from NHP, analysis of cytokines, MMP, flow cytometry, immunostaining, and isolation of neutrophils from human blood, neutrophil assays, and statistical analyses. Download Text S1, DOCX file, 0.04 MB (42.6KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
Understanding mechanisms that GBS use to subvert host defenses in its transition from an asymptomatic colonizer to invasive pathogen is essential for the development of improved treatment and prevention strategies for adverse perinatal outcomes associated with GBS. We previously showed that hyaluronidase (HylB) activity was greater in GBS strains isolated from cases of preterm birth and neonatal infection compared to commensal isolates (22) and that HylB facilitated ascending GBS infection in mice (22). Our data in the chronically catheterized NHP model indicate that HylB is a crucial virulence factor that enables GBS to rapidly invade the amniotic cavity and induce preterm labor. Overall, while we noted a relative suppression of inflammation in key placental, uterine, and amniotic fluid samples, preterm labor was induced by HylB-expressing GBS.
Despite clear phenotypic differences in pregnancy outcomes in GB37-treated animals compared to the GB37ΔhylB group, differences in peak AF cytokines and immune signaling in the placenta were not as striking. These results are also in stark contrast to a pregnant NHP model using a hyperhemolytic/hyperpigmented GBS strain wherein we observed significantly higher levels of IL-6 and IL-8 in the AF and upregulation of proinflammatory genes in the placenta compared to NHPs inoculated with a nonhemolytic strain and saline controls (28). In contrast, results from this study indicate that HylB may suppress inflammatory responses at the maternal-fetal interface to promote bacterial replication and dissemination. Luminex (Fig. 1), digital spatial profiling (Fig. 5), and in vitro studies (Fig. 6), indicate immune suppression, which can be due in part to inhibition of TLR-2/4 signaling by HylB-expressing GBS (Fig. 6D). These data are consistent with observations that term placenta expresses high levels of TLR-2 and TLR-4, with stronger expression of TLR-2 in immune cells and TLR-4 in syncytiotrophoblasts and fibroblasts (38). Expression of TLR-4 was also observed on the apical side of amniotic epithelium (39, 40), suggesting immune suppression of placenta could also occur after microbial invasion of the amniotic cavity. Together, these observations show that immune cells (maternal/fetal), trophoblasts, and other cells within the placenta are susceptible to immune suppression by the GBS hyaluronidase.
Although we observed relative suppression of inflammation at the maternal-fetal interface, fetal cytokines were induced in the setting of fetal bacteremia (Fig. 3). Given the in vivo and in vitro data obtained in this study, we propose that HylB may dampen proinflammatory cascades and microbicidal activity of first-line innate immune defenses such as neutrophils, thereby promoting bacterial replication and invasion of the amniotic cavity and fetus. We hypothesize that once bacteria have invaded the fetal niche, other proinflammatory, surface-associated bacterial factors (e.g., capsular polysaccharides [41], surface immunogenic protein [42], pilus [43]) overcome the immune-dampening effects of GBS HylB-digested HA fragments, triggering host PRRs and resulting in systemic fetal inflammation. A systemic fetal inflammatory response in GB37-infected fetuses may have triggered parturition in these animals, akin to fetal inflammatory response syndrome, which has been linked to preterm labor in humans (44, 45). Premature cervical softening in associated with increased MMP levels (Fig. 3) and prostaglandins, may have also played a role in early labor onset (46–48). Further research on the effect of HylB on host pathways during GBS infection is critical to understanding the multifaceted etiologies of GBS-associated preterm labor.
Limitations of our study include the lack of information on cellular events occurring in the amniotic fluid due to low cell recovery. In addition, we were unable to observe the cellular events at the placenta and uterus immediately after bacterial inoculation prior to preterm labor and delivery. Accordingly, it is possible that we did not capture events that may lend greater insight into the kinetics of GBS HylB-mediated immune suppression. For instance, as GB37ΔhylB lacks the ability to degrade HA into anti-inflammatory dimers (21), GB37ΔhylB inoculation may have induced rapid recruitment of neutrophils via controlled inflammation, resulting in swift clearance of this strain before microbial invasion of the amniotic cavity could occur (in all animals except GB37ΔhylB#5). This idea is supported by our previous findings in an intraperitoneal murine model of infection, where we observed greater neutrophil recruitment to the peritoneal fluid of GB37ΔhylB-inoculated mice within 2 h postinfection and less bacterial dissemination by 48 compared to GB37-inoculated mice (29). In the present study, bacterial clearance and resolution of inflammation within the chorioamniotic membranes in four of five GB37ΔhylB-treated NHPs could explain the lower neutrophil frequencies in the membranes and uterine tissues observed 3 days after inoculation. A time-controlled experiment in the NHP pregnancy model would lend greater insight into the differences in neutrophil recruitment and inflammatory events immediately following choriodecidual infection with GB37 or GB37ΔhylB.
Together, our findings establish HylB as a potent virulence factor that allows GBS to circumvent neutrophil responses, invade the amniotic cavity, and cause fetal bacteremia and preterm labor in an animal model that closely resembles human pregnancy. The chronically catheterized model enabled us to evaluate uterine contraction patterns, cervical dilation, microbial invasion of the amniotic cavity, and amniotic fluid responses in real time, which is not possible in the pregnant mouse model. Data obtained from the NHP model show that the hyaluronidase-proficient WT GB37 strain induced significant uterine contraction leading to cervical dilation (similar to human preterm labor) as early as 6 h postinoculation and also significantly delayed and diminished placental, amniotic, uterine, and myometrial immune responses as seen via Luminex, flow cytometry, and digital spatial profiling. Accordingly, these results point to HylB as a potential therapeutic target for invasive GBS disease during pregnancy. Several known hyaluronidase inhibitors exist and have been proposed as therapeutics for other HA-mediated pathology, including sexually transmitted infections (49, 50), venom wounds (51, 52), and cancer (53–55). Identification of a HylB-specific inhibitor could lead to new treatments for GBS and have far-reaching impacts on maternal and neonatal health worldwide.
MATERIALS AND METHODS
Ethics statement.
Written informed patient consent for donation of adult human blood was obtained with approval from the Seattle Children’s Research Institute Institutional Review Board (protocol 11117). Children under the age of 18 years were not recruited for donation of adult human blood for protocol 11117. Written informed patient consent for donation of maternal and cord blood was obtained from the University of Washington Institutional Review Board (protocol 34004). These studies were performed per the Principles in the WMA Declaration of Helsinki and Department of Health and Human Services Belmont Report.
All animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council and the Weatherall report, “The use of nonhuman primates in research.” The protocol was approved by the University of Washington Institutional Animal Care and Use Committee (permit 4165-01). All surgery was performed under general anesthesia, and all efforts were made to minimize pain and distress.
Chemicals.
Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich.
Bacterial strains.
GBS strains used in the NHP model were derived from strain GB37 (serotype V, multilocus sequence type 1), which was isolated from the blood of a septic human neonate with early onset disease (56). This strain is neither hemolytic nor pigmented (57) but exhibits increased hyaluronidase activity compared to other GBS clinical isolates (29). The isogenic strain GB37ΔhylB lacks hyaluronidase and was described previously (29). The GB37ΔcpsE strain was derived from WT GB37 using methods described previously (22, 58) (see Text S1 for details). Cultures of GBS were grown in tryptic soy broth (TSB) or tryptic soy agar (TSA; Difco Laboratories) at 37°C with 5% CO2. For inoculations in the NHP model, GBS strains were grown to mid-log phase (OD600 = 0.27), and 1 × 108 CFU in 1 ml of sterile PBS was inoculated into the choriodecidual space, as described below and previously (27, 28). Similarly, for in vitro studies, GBS strains were grown to mid-log phase (OD600 = 0.27), washed, and resuspended in sterile phosphate-buffered saline (PBS) prior to infection unless otherwise noted.
Chronically catheterized NHP model.
As described previously (28), pregnant pigtail macaques (Macaca nemestrina) were time-mated, and fetal age was determined using early ultrasound. Animals were provided drinking water at all times and fed commercial monkey chow, supplemented daily with fruits and vegetables. The temperature in the animal quarters was maintained between 72 and 82°F. Each animal was conditioned to a nylon jacket/tether system for several weeks before surgery, which allowed for free movement within the cage while protecting the catheters. Between days 116 and 125 of pregnancy (term = 172 days), NHPs were catheterized by laparotomic surgical implantation into the maternal femoral vein, amniotic cavity, and choriodecidual interface in the lower uterine segment (i.e., between the uterine muscle and fetal membranes, external to amniotic cavity). For detailed methods of catheterization surgery, see Text S1 in the supplemental material.
The experiment was initiated when a catheterized pregnant NHP received one of two experimental treatments: choriodecidual inoculation of either GBS strain GB37 (n = 5) or the isogenic strain GB37ΔhylB (n = 5). Two chronically catheterized NHP that were inoculated with sterile saline were also included, along with four saline controls performed in previous studies (27, 59).
AF and maternal blood were collected for culture, cytokine, and prostaglandin analysis during each experiment. Intra-amniotic pressure was continuously recorded, digitized, and analyzed as described previously (27, 28). The integrated area under the curve for intrauterine pressure was used as a measure of uterine activity and reported as the hourly contraction area (HCA; mm Hg ⋅ s/h) over 24 h. Preterm labor was defined as progressive cervical dilation associated with increased and sustained uterine activity (>10,000 mm Hg ⋅ s/h). Cesarean section was performed at the following experimental endpoints to allow for tissue collection: (i) preterm labor, (ii) 3 days after GBS inoculation if preterm labor did not occur, or (iii) 7 days after saline inoculation (27, 28). After Cesarean section, fetuses were euthanized by barbiturate overdose, followed by exsanguination and fetal necropsy (27, 28). Complete gross and histopathologic examinations were performed. For details on sample collection, see Text S1 in the supplemental material.
Placental and fetal lung histology.
A board-certified veterinary pathologist (A.B.) performed blinded histopathologic examination of the fetal and placental tissues. H&E-stained, full-thickness paraffin sections (placental disc, umbilical cord, and fetal membrane roll) were examined from each case to exclude inflammation, necrosis, fetal vascular thrombosis, or other histopathological findings. Chorioamnionitis was diagnosed by the presence of a neutrophilic infiltrate at the chorion-decidua junction (mild) or amniochorion junction (moderate or severe). For histologic examination of the fetal lung, two to three randomly selected fixed fetal lung tissues were embedded in paraffin, and sections were stained with H&E. We also performed immunostaining for MPO (a granulocyte marker) and CD68 (a macrophage marker) on the chorioamniotic membranes and fetal lung tissues (see Text S1 in the supplemental material).
Digital spatial profiling.
GeoMx digital spatial profiling (DSP) was performed at NanoString Technologies in Seattle, WA. Formalin-fixed, paraffin-embedded placental sections from animals from each group were incubated with fluorescent probes and a multiplex cocktail of primary antibodies with photocleavable oligonucleotides (i.e., a validated DSP human-immune oncology protein panel; NanoString Technologies). The fluorescent markers included anti-pan cytokeratin-Alexa Fluor 488 (Pan-CK, clone AE1/AE3; Novusbio), anti-fibroblast activation protein-Alexa-Fluor 594 (FAP, clone SP325; Abcam), SYTO 83 for nuclei visualization (Thermo Fisher), and anti-GBS-Alexa Fluor 647 (clone ab53584; Abcam). Sections were magnified to 20×, and ROIs comprising the decidua, chorion, and amnion from each animal were selected based on tissue morphology (Fig. 5B). Each region of interest was then exposed to UV illumination with a double digital mirror device molecule, which cleaved the DNA oligonucleotides into the aqueous layer above the tissue slice. The oligonucleotides in the eluent were collected via microcapillary aspiration and transferred to an individual well of a microtiter plate. Oligonucleotides were then hybridized to nCounter optical barcodes (NanoString Technologies) to permit ex situ digital counting of each analyte. Briefly, hybridization of oligonucleotides to optical barcodes were performed at 65°C in a thermocycler. After hybridization, samples were processed using the nCounter prep station and digital analyzer. Data were normalized to technical controls and area. To generate signal/noise ratios, data were calculated relative to isotype controls.
Neutrophil assays.
For detailed methods on neutrophil isolation from whole human blood, see the supplemental material. To measure neutrophil death, neutrophils (1 × 105 cells in RPMI containing L-glutamine) were exposed to GB37 or GB37ΔhylB (MOI of 100, 10, or 1) for 4 h at 37°C, and the release of LDH (lactate dehydrogenase) into cell supernatants was quantified using the LDH assay kit (TaKaRa) according to the manufacturer’s instructions. The percent cell death was calculated relative to 0.1% Triton X-100-treated (100% cell death) and PBS-treated (0% cell death) controls. To measure neutrophil killing of GBS, neutrophils (1 × 106) were incubated with GB37 or GB37ΔhylB at an MOI of 1 in RPMI per g for 1 h at 37°C, as described previously (28, 60). Triton X-100 (0.1%) was added to lyse neutrophils and release intracellular bacteria, and total bacteria (intracellular and extracellular) were enumerated by serial dilution plating on TSA. The percent killing was calculated as the number of CFU recovered in the presence of neutrophils over the number of CFU recovered in the absence of neutrophils × 100. To measure ROS production, neutrophils (1 × 106 cells/ml in RPMI per g) were preincubated with 84 μM dihydrorhodamine-123 (DHR; in 0.28% dimethyl sulfoxide) at 37°C for 20 min, as described previously (28), and then exposed to GB37, GB37ΔhylB, or GB37ΔcpsE (MOI of 100) for 60 min. The fluorescence intensity of cells (which measures DHR oxidation by ROS to fluorescent MHR [monohydrorhodamine]) was measured immediately by flow cytometry using an LSR II (BD Biosciences). The data are representative of three experiments with neutrophils obtained from four independent donors. For details on treatment of neutrophils with anti-TLR-2/4 antibodies, please see the supplemental material. Data were analyzed using FlowJo v10.1 (FlowJo, LLC).
Statistical analyses.
In all cases, results were considered significantly different if P < 0.05. However, because of the limited number of samples per group in NHP experiments, we also report P values between 0.05 and 0.100, as described previously for NHP experiments (28, 61). All statistical tests were unpaired and two-sided unless mentioned otherwise. For details on all statistical tests used in the study, see the supplemental material.
Data availability.
All relevant data supporting the key findings of this study are available within the article and its supplemental material or from the corresponding authors upon request.
Extracellular and intracellular flow cytometry panels used to evaluate maternal and fetal blood, uterine segments, chorionic villi, and choriodecidual membranes are shown. Download Table S1, DOCX file, 0.02 MB (19KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
ACKNOWLEDGMENTS
We thank Shannon Manning for providing the GB37 strain and Connie Hughes for administrative support.
This study was supported by funding from the National Institutes of Health grants R01AI133976, R01AI100989, R01AI145890, and R01AI112619 to L.R. and K.M.A.W., and seed funds from Seattle Children’s Research Institute to L.R. NIH training grant T32AI007509 (principal investigator [PI] Lee Ann Campbell) supported J.V. and A.B., and T32AI055396 (PI Ferric Fang) supported A.F. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
L.R., K.M.A.W., M.C., B.A., C.G., and J.V. designed the experiments. M.C., B.A., A.O., P.Q., A.B., C.G., K.S., J.O., S.M., M.D., T-Y.W., A.B., A.F., S.N., K.M.A.W., and L.R. performed the experiments. M.C., B.A., A.O., P.Q., C.G., S.M., M.D., T-Y.W., J.M., A.B., A.F., K.M.A.W., and L.R. analyzed the results, and B.A., M.C., K.M.A.W., and L.R. wrote the manuscript.
We declare there are no conflicts of interest.
Footnotes
Citation Coleman M, Armistead B, Orvis A, Quach P, Brokaw A, Gendrin C, Sharma K, Ogle J, Merillat S, Dacanay M, Wu T-Y, Munson J, Baldessari A, Vornhagen J, Furuta A, Nguyen S, Adams Waldorf KM, Rajagopal L. 2021. Hyaluronidase impairs neutrophil function and promotes group B Streptococcus invasion and preterm labor in nonhuman primates. mBio 12:e03115-20. https://doi.org/10.1128/mBio.03115-20.
REFERENCES
- 1.Institute of Medicine. 2007. In Behrman RE, Butler AS (ed), Preterm birth: causes, consequences, and prevention. Institute of Medicine, Washington, DC. doi: 10.17226/11622.. [DOI] [Google Scholar]
- 2.Goldenberg RL, Hauth JC, Andrews WW. 2000. Intrauterine infection and preterm delivery. N Engl J Med 342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 3.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. 1991. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol 165:955–961. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- 4.Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ. 2000. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol 96:498–503. [DOI] [PubMed] [Google Scholar]
- 5.Brigtsen AK, Jacobsen AF, Dedi L, Melby KK, Fugelseth D, Whitelaw A. 2015. Maternal colonization with group B Streptococcus is associated with an increased rate of infants transferred to the neonatal intensive care unit. Neonatology 108:157–163. doi: 10.1159/000434716. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi-Jassir F, Seale AC, Kohli-Lynch M, Lawn JE, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, Le Doare K, Madhi SA, Saha SK, Schrag S, Sobanjo-Ter Meulen A, Vekemans J, Rubens CE. 2017. Preterm birth associated with group B streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin Infect Dis 65:S133–S142. doi: 10.1093/cid/cix661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, Madrid L, Blencowe H, Cousens S, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, Le Doare K, Madhi SA, Rubens CE, Saha SK, Schrag SJ, Sobanjo-Ter Meulen A, Vekemans J, Lawn JE. 2017. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis 65:S200–S219. doi: 10.1093/cid/cix664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, Hadler JL, Danila R, Cieslak PR, Schuchat A. 2000. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2007. Perinatal group B streptococcal disease after universal screening recommendations–United States, 2003-2005. MMWR Morb Mortal Wkly Rep 56:701–705. [PubMed] [Google Scholar]
- 10.Verani JR, McGee L, Schrag SJ. 2010. Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep 59:1–36. [PubMed] [Google Scholar]
- 11.Rivera L, Saez-Llorens X, Feris-Iglesias J, Ip M, Saha S, Adrian PV, Madhi SA, Boudville IC, Cunnington MC, Casellas JM, Slobod KS. 2015. Incidence and serotype distribution of invasive group B streptococcal disease in young infants: a multi-country observational study. BMC Pediatr 15:143. doi: 10.1186/s12887-015-0460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berardi A, Cattelani C, Creti R, Berner R, Pietrangiolillo Z, Margarit I, Maione D, Ferrari F. 2015. Group B streptococcal infections in the newborn infant and the potential value of maternal vaccination. Expert Rev Anti Infect Ther 13:1387–1399. doi: 10.1586/14787210.2015.1079126. [DOI] [PubMed] [Google Scholar]
- 13.Stern R, Jedrzejas MJ. 2006. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev 106:818–839. doi: 10.1021/cr050247k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallacara A, Baldini E, Manfredini S, Vertuani S. 2018. Hyaluronic acid in the third millennium. Polymers (Basel) 10:701. doi: 10.3390/polym10070701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T. 2016. Hyaluronic acid in inflammation and tissue regeneration. Wounds 28:78–88. [PubMed] [Google Scholar]
- 16.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. 2005. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 17.Hynes WL, Walton SL. 2000. Hyaluronidases of Gram-positive bacteria. FEMS Microbiol Lett 183:201–207. doi: 10.1111/j.1574-6968.2000.tb08958.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Guo C, Xu Y, Liu G, Lu C, Liu Y. 2014. Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression. Infect Immun 82:2615–2625. doi: 10.1128/IAI.00022-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gochnauer TA, Wilson JB. 1951. Hyaluronidase production in vitro by streptococci isolated from bovine mastitis cases. Am J Vet Res 12:20–22. [PubMed] [Google Scholar]
- 20.Pritchard DG, Lin B, Willingham TR, Baker JR. 1994. Characterization of the group B streptococcal hyaluronate lyase. Arch Biochem Biophys 315:431–437. doi: 10.1006/abbi.1994.1521. [DOI] [PubMed] [Google Scholar]
- 21.Kolar SL, Kyme P, Tseng CW, Soliman A, Kaplan A, Liang J, Nizet V, Jiang D, Murali R, Arditi M, Underhill DM, Liu GY. 2015. Group B Streptococcus evades host immunity by degrading hyaluronan. Cell Host Microbe 18:694–704. doi: 10.1016/j.chom.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vornhagen J, Quach P, Boldenow E, Merillat S, Whidbey C, Ngo LY, Adams Waldorf KM, Rajagopal L. 2016. Bacterial hyaluronidase promotes ascending GBS infection and preterm birth. mBio 7:e00781-16. doi: 10.1128/mBio.00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell BF, Taggart MJ. 2009. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 297:R525–R545. doi: 10.1152/ajpregu.00153.2009. [DOI] [PubMed] [Google Scholar]
- 24.Carter AM. 2007. Animal models of human placentation: a review. Placenta 28(Suppl A):S41–S47. doi: 10.1016/j.placenta.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Adams Waldorf KM, Rubens CE, Gravett MG. 2011. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. BJOG 118:136–144. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. 1994. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 171:1660–1667. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 27.Adams Waldorf KM, Gravett MG, McAdams RM, Paolella LJ, Gough GM, Carl DJ, Bansal A, Liggitt HD, Kapur RP, Reitz FB, Rubens CE. 2011. Choriodecidual group B streptococcal inoculation induces fetal lung injury without intra-amniotic infection and preterm labor in Macaca nemestrina. PLoS One 6:e28972. doi: 10.1371/journal.pone.0028972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boldenow E, Gendrin C, Ngo L, Bierle C, Vornhagen J, Coleman M, Merillat S, Armistead B, Whidbey C, Alishetti V, Santana-Ufret V, Ogle J, Gough M, Srinouanprachanh S, MacDonald JW, Bammler TK, Bansal A, Liggitt HD, Rajagopal L, Adams Waldorf KM. 2016. Group B Streptococcus circumvents neutrophils and neutrophil extracellular traps during amniotic cavity invasion and preterm labor. Sci Immunol 1:eaah4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gendrin C, Vornhagen J, Armistead B, Singh P, Whidbey C, Merillat S, Knupp D, Parker R, Rogers LM, Quach P, Iyer LM, Aravind L, Manning SD, Aronoff DM, Rajagopal L. 2018. A nonhemolytic group B streptococcus strain exhibits hypervirulence. J Infect Dis 217:983–987. doi: 10.1093/infdis/jix646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng J, Huang C, Jiang S. 2016. Roles and regulation of the matrix metalloproteinase system in parturition. Mol Reprod Dev 83:276–286. doi: 10.1002/mrd.22626. [DOI] [PubMed] [Google Scholar]
- 31.Lombardi A, Makieva S, Rinaldi SF, Arcuri F, Petraglia F, Norman JE. 2018. Expression of matrix metalloproteinases in the mouse uterus and human myometrium during pregnancy, labor, and preterm labor. Reprod Sci 25:938–949. doi: 10.1177/1933719117732158. [DOI] [PubMed] [Google Scholar]
- 32.Ravanos K, Dagklis T, Petousis S, Margioula-Siarkou C, Prapas Y, Prapas N. 2015. Factors implicated in the initiation of human parturition in term and preterm labor: a review. Gynecol Endocrinol 31:679–683. doi: 10.3109/09513590.2015.1076783. [DOI] [PubMed] [Google Scholar]
- 33.Lee SM, Park KH, Jung EY, Cho SH, Ryu A. 2016. Prediction of spontaneous preterm birth in women with cervical insufficiency: comprehensive analysis of multiple proteins in amniotic fluid. J Obstet Gynaecol Res 42:776–783. doi: 10.1111/jog.12976. [DOI] [PubMed] [Google Scholar]
- 34.Vadillo-Ortega F, Estrada-Gutierrez G. 2005. Role of matrix metalloproteinases in preterm labour. BJOG 112(Suppl 1):19–22. doi: 10.1111/j.1471-0528.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 35.Sundrani DP, Chavan-Gautam PM, Pisal HR, Mehendale SS, Joshi SR. 2012. Matrix metalloproteinase-1 and -9 in human placenta during spontaneous vaginal delivery and caesarean sectioning in preterm pregnancy. PLoS One 7:e29855. doi: 10.1371/journal.pone.0029855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin J, Haridas S, Barenkamp SJ, Lorenset LC, Lee ASE, Schroeder BT, Peng G, Koenig JM. 2018. Neonatal neutrophils stimulated by group B Streptococcus induce a proinflammatory T-helper cell bias. Pediatr Res 83:739–746. doi: 10.1038/pr.2017.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, Weening JJ, Florquin S. 2005. Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol 16:2034–2043. doi: 10.1681/ASN.2005010054. [DOI] [PubMed] [Google Scholar]
- 38.Koga K, Mor G. 2010. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol 63:587–600. doi: 10.1111/j.1600-0897.2010.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Y, Mor G, Abrahams VM, Buhimschi IA, Buhimschi CS, Guller S. 2006. Alterations in syncytiotrophoblast cytokine expression following treatment with lipopolysaccharide. Am J Reprod Immunol 55:12–18. doi: 10.1111/j.1600-0897.2005.00347.x. [DOI] [PubMed] [Google Scholar]
- 40.Adams KM, Lucas J, Kapur RP, Stevens AM. 2007. LPS induces translocation of TLR4 in amniotic epithelium. Placenta 28:477–481. doi: 10.1016/j.placenta.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Hunolstein C, Totolian A, Alfarone G, Mancuso G, Cusumano V, Teti G, Orefici G. 1997. Soluble antigens from group B streptococci induce cytokine production in human blood cultures. Infect Immun 65:4017–4021. doi: 10.1128/IAI.65.10.4017-4021.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz-Dinamarca DA, Manzo RA, Soto DA, Avendano-Valenzuela MJ, Bastias DN, Soto PI, Escobar DF, Vasquez-Saez V, Carrion F, Pizarro-Ortega MS, Wilson CAM, Berrios J, Kalergis AM, Vasquez AE. 2020. Surface immunogenic protein of streptococcus group B is an agonist of Toll-like receptors 2 and 4 and a potential immune adjuvant. Vaccines (Basel) 8:29. doi: 10.3390/vaccines8010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee A, Kim BJ, Carmona EM, Cutting AS, Gurney MA, Carlos C, Feuer R, Prasadarao NV, Doran KS. 2011. Bacterial pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nat Commun 2:462. doi: 10.1038/ncomms1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, Berry SM. 1998. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 179:186–193. doi: 10.1016/S0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 45.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. 1998. The fetal inflammatory response syndrome. Am J Obstet Gynecol 179:194–202. doi: 10.1016/S0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 46.Ekerhovd E, Weijdegard B, Brannstrom M, Mattsby-Baltzer I, Norstrom A. 2002. Nitric oxide induced cervical ripening in the human: involvement of cyclic guanosine monophosphate, prostaglandin F2α, and prostaglandin E2. Am J Obstet Gynecol 186:745–750. doi: 10.1067/mob.2002.121327. [DOI] [PubMed] [Google Scholar]
- 47.El Maradny E, Kanayama N, Kobayashi H, Hossain B, Khatun S, Liping S, Kobayashi T, Terao T. 1997. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod 12:1080–1088. doi: 10.1093/humrep/12.5.1080. [DOI] [PubMed] [Google Scholar]
- 48.Mahendroo M. 2019. Cervical hyaluronan biology in pregnancy, parturition and preterm birth. Matrix Biol 78–79:24–31. doi: 10.1016/j.matbio.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson RA, Feathergill K, Diao X, Cooper M, Kirkpatrick R, Spear P, Waller DP, Chany C, Doncel GF, Herold B, Zaneveld LJ. 2000. Evaluation of poly(styrene-4-sulfonate) as a preventive agent for conception and sexually transmitted diseases. J Androl 21:862–875. [PubMed] [Google Scholar]
- 50.Zaneveld LJ, Waller DP, Anderson RA, Chany C, II, Rencher WF, Feathergill K, Diao XH, Doncel GF, Herold B, Cooper M. 2002. Efficacy and safety of a new vaginal contraceptive antimicrobial formulation containing high molecular weight poly(sodium 4-styrenesulfonate). Biol Reprod 66:886–894. doi: 10.1095/biolreprod66.4.886. [DOI] [PubMed] [Google Scholar]
- 51.Kemparaju K, Girish KS. 2006. Snake venom hyaluronidase: a therapeutic target. Cell Biochem Funct 24:7–12. doi: 10.1002/cbf.1261. [DOI] [PubMed] [Google Scholar]
- 52.Oliveira-Mendes BBR, Miranda SEM, Sales-Medina DF, Magalhaes BF, Kalapothakis Y, Souza RP, Cardoso VN, de Barros ALB, Guerra-Duarte C, Kalapothakis E, Horta CCR. 2019. Inhibition of Tityus serrulatus venom hyaluronidase affects venom biodistribution. PLoS Negl Trop Dis 13:e0007048. doi: 10.1371/journal.pntd.0007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Botzki A, Rigden DJ, Braun S, Nukui M, Salmen S, Hoechstetter J, Bernhardt G, Dove S, Jedrzejas MJ, Buschauer A. 2004. l-Ascorbic acid 6-hexadecanoate, a potent hyaluronidase inhibitor. X-ray structure and molecular modeling of enzyme-inhibitor complexes. J Biol Chem 279:45990–45997. doi: 10.1074/jbc.M406146200. [DOI] [PubMed] [Google Scholar]
- 54.Girish KS, Kemparaju K. 2007. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80:1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 55.Girish KS, Kemparaju K, Nagaraju S, Vishwanath BS. 2009. Hyaluronidase inhibitors: a biological and therapeutic perspective. Curr Med Chem 16:2261–2288. doi: 10.2174/092986709788453078. [DOI] [PubMed] [Google Scholar]
- 56.Davies HD, Adair C, McGeer A, Ma D, Robertson S, Mucenski M, Kowalsky L, Tyrell G, Baker CJ. 2001. Antibodies to capsular polysaccharides of group B Streptococcus in pregnant Canadian women: relationship to colonization status and infection in the neonate. J Infect Dis 184:285–291. doi: 10.1086/322029. [DOI] [PubMed] [Google Scholar]
- 57.Singh P, Aronoff DM, Davies HD, Manning SD. 2016. Draft genome sequence of an invasive Streptococcus agalactiae isolate lacking pigmentation. Genome Announc 4:e00015-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajagopal L, Clancy A, Rubens CE. 2003. A eukaryotic type serine/threonine kinase and phosphatase in Streptococcus agalactiae reversibly phosphorylate an inorganic pyrophosphatase and affect growth, cell segregation, and virulence. J Biol Chem 278:14429–14441. doi: 10.1074/jbc.M212747200. [DOI] [PubMed] [Google Scholar]
- 59.Adams Waldorf KM, Singh N, Mohan AR, Young RC, Ngo L, Das A, Tsai J, Bansal A, Paolella L, Herbert BR, Sooranna SR, Gough GM, Astley C, Vogel K, Baldessari AE, Bammler TK, MacDonald J, Gravett MG, Rajagopal L, Johnson MR. 2015. Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. Am J Obstet Gynecol 213:830e1–830e19. doi: 10.1016/j.ajog.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maisey HC, Quach D, Hensler ME, Liu GY, Gallo RL, Nizet V, Doran KS. 2008. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J 22:1715–1724. doi: 10.1096/fj.07-093963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rueda CM, Presicce P, Jackson CM, Miller LA, Kallapur SG, Jobe AH, Chougnet CA. 2016. Lipopolysaccharide-induced chorioamnionitis promotes IL-1-dependent inflammatory FOXP3+ CD4+ T cells in the fetal rhesus macaque. J Immunol 196:3706–3715. doi: 10.4049/jimmunol.1502613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Secundino I, Lizcano A, Roupe KM, Wang X, Cole JN, Olson J, Ali SR, Dahesh S, Amayreh LK, Henningham A, Varki A, Nizet V. 2016. Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation. J Mol Med 94:219–233. doi: 10.1007/s00109-015-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Uterine contractions, AF cytokines, prostaglandins, and bacterial CFU from choriodecidual inoculations of GB37, GB37ΔhylB, or saline in chronically catheterized pregnant NHPs. Download FIG S1, DOCX file, 1.5 MB (1.6MB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Histological examination of NHP fetal lung sections. (A to D) Representative H&E-stained sections from NHPs in each group are shown, including saline#3 (A), GB37#1 (B), GB37#2 (C), and GB37ΔhylB#2 (D). (E to H) Representative MPO-stained sections from NHPs in each group are shown, including saline#3 (E), GB37#1 (F), GB37#2 (G), and GB37ΔhylB#2 (H). (I to L) Representative CD68-stained sections from NHPs in each group are shown, including saline#3 (I), GB37#1 (J), GB37#1 (K), and GB37ΔhylB#2 (L). Note that within the GB37 group, tissues are shown reflecting harvest at 24 h (B, F, and J) and 48 h (C, G, and K). Download FIG S2, DOCX file, 0.9 MB (1.1MB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Quantitation of immunostaining for MPO in the fetal lung and chorioamniotic membranes. (A) The area of MPO immunostaining in the neonatal lung was significantly different between the saline and GB37 groups and between the GB37 and GB37ΔhylB groups. GB37ΔhylB#5 is designated by an open square. A one-way ANOVA with Tukey’s posttest was used to compare groups. (B) The area of MPO immunostaining in the chorioamniotic membranes was not significantly different among the groups in the amnion, chorion, or decidua. GB37ΔhylB#5 is designated by an open square. A one-way ANOVA with Tukey’s posttest was used to compare groups. Download FIG S3, DOCX file, 0.1 MB (126.4KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
GB37-inoculated NHPs experienced enhanced infiltration of CD8+ T cells and phagocytes to the maternal fetal interface. At Cesarean section, biopsy specimens from the uterus and placenta, as well as maternal and fetal blood, were obtained. (A) A schematic depicting the biopsy sites is shown. (B) Samples were processed into single-cell suspensions, stained, and analyzed by flow cytometry for various immune cell markers (indicated in the figure). GB37ΔhylB #5 is designated by an open square. A Welch’s test was used to evaluate differences in immune cell populations between GB37- and GB37ΔhylB-treated animals at each site. Data from two saline controls performed as a part of the present study are included, but similar analyses were not previously performed with historical saline controls (n = 4). Download FIG S4, DOCX file, 0.4 MB (469.2KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Digital spatial profiling analyte fold change: GB37 versus GB37ΔhylB. Analyte abundance in distinct placental regions from GB37ΔhylB-inoculated NHPs and saline-treated NHPs were obtained by digital spatial profiling (Nanostring Technologies). Fold changes in analyte abundance (GB37ΔhylB over GB37) were log2 transformed and analyzed by a linear mixed model in R version 3.6.2. Significance tests were controlled for false discovery rate. Download FIG S5, DOCX file, 0.3 MB (348.5KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Digital spatial profiling analyte fold change: GB37 versus saline. Analyte abundance in distinct placental regions from GB37-inoculated NHPs and saline-treated NHPs were obtained by digital spatial profiling (Nanostring Technologies). Fold changes in analyte abundance (GB37 over saline) were log2 transformed and analyzed by a linear mixed model in R version 3.6.2. Significance tests were controlled for false discovery rate. White asterisk, P < 0.05. Download FIG S6, DOCX file, 0.3 MB (357.6KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Digital spatial profiling analyte fold change: GB37ΔhylB versus saline. Analyte abundance in distinct placental regions from GB37ΔhylB-inoculated NHPs and saline-treated NHPs were obtained by digital spatial profiling (Nanostring Technologies). Fold changes in analyte abundance (GB37ΔhylB over saline) were log2 transformed and analyzed by a linear mixed model in R version 3.6.2. Significance tests were controlled for false discovery rate. White asterisk, P < 0.05. Download FIG S7, DOCX file, 0.3 MB (363.8KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Neutrophils were isolated from human maternal blood and umbilical cord (i.e., fetal) blood. (A) GB37 or GB37ΔhylB were exposed to maternal or fetal neutrophils (MOI of 1) for one hour. The percent killing was calculated as the number of CFU recovered after incubation with neutrophils out of the number of the number CFU recovered after incubation without neutrophils × 100. Differences among groups were determined using a paired t test. *, P < 0.05. (B) Maternal or fetal neutrophils were pretreated with dihydrorhodamine-123 (DHR) and then exposed to GB37 (green) or GB37ΔhylB (blue) (MOI of 100). The conversion of DHR to fluorescent MHR indicates ROS production in cells and was measured by flow cytometry at 60 min postinfection. Download FIG S8, DOCX file, 0.1 MB (137.3KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Details regarding the study design, generation of GB37ΔcpsE mutant, catheterization of pregnant NHP, sample collection from NHP, analysis of cytokines, MMP, flow cytometry, immunostaining, and isolation of neutrophils from human blood, neutrophil assays, and statistical analyses. Download Text S1, DOCX file, 0.04 MB (42.6KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Extracellular and intracellular flow cytometry panels used to evaluate maternal and fetal blood, uterine segments, chorionic villi, and choriodecidual membranes are shown. Download Table S1, DOCX file, 0.02 MB (19KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
All relevant data supporting the key findings of this study are available within the article and its supplemental material or from the corresponding authors upon request.
Extracellular and intracellular flow cytometry panels used to evaluate maternal and fetal blood, uterine segments, chorionic villi, and choriodecidual membranes are shown. Download Table S1, DOCX file, 0.02 MB (19KB, docx) .
Copyright © 2021 Coleman et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.