ABSTRACT
Lipids are biologically active molecules involved in a variety of cellular processes and immunological functions, including inflammation. It was recently shown that phospholipids and their derivatives, lysophospholipids, can reactivate latent (dormant) tumor cells, causing cancer recurrence. However, the potential link between lipids and HIV latency, persistence, and viral rebound after cessation of antiretroviral therapy (ART) has never been investigated. We explored the links between plasma lipids and the burden of HIV during ART. We profiled the circulating lipidome from plasma samples from 24 chronically HIV-infected individuals on suppressive ART who subsequently underwent an analytic treatment interruption (ATI) without concurrent immunotherapies. The pre-ATI viral burden was estimated as time-to-viral-rebound and viral load set points post-ATI. We found that higher pre-ATI levels of lysophospholipids, including the proinflammatory lysophosphatidylcholine, were associated with faster time-to-viral-rebound and higher viral set points upon ART cessation. Furthermore, higher pre-ATI levels of the proinflammatory by-product of intestinal lysophosphatidylcholine metabolism, trimethylamine-N-oxide (TMAO), were also linked to faster viral rebound post-ART. Finally, pre-ATI levels of several phosphatidylcholine species (lysophosphatidylcholine precursors) correlated strongly with higher pre-ATI levels of HIV DNA in peripheral CD4+ T cells. Our proof-of-concept data point to phospholipids and lysophospholipids as plausible proinflammatory contributors to HIV persistence and rapid post-ART HIV rebound. The potential interplay between phospholipid metabolism and both the establishment and maintenance of HIV latent reservoirs during and after ART warrants further investigation.
KEYWORDS: HIV, HIV persistence, lipids, lysophospholipid, viral rebound, lysophosphatidylcholine, phospholipid, TMAO, choline
OBSERVATION
A comprehensive understanding of the host factors modulating HIV persistence is imperative for developing effective strategies to eradicate the latent HIV reservoir, which persists despite antiretroviral therapy (ART) and causes viral rebound upon ART discontinuation (1). Lipids are biologically active molecules involved in a broad range of cellular processes and immunological functions, including inflammation (2, 3). It was recently shown that phospholipids and their derivatives, lysophospholipids, can reactivate latent (dormant) tumor cells, causing cancer recurrence (4). While the interplay between lipids and both HIV and ART has been studied in the context of the development of inflammation-associated comorbidities, particularly subclinical atherosclerosis (5–9), the potential impact of lipids on HIV latency, persistence, and post-ART rebound has never been investigated.
There is currently no standard method to measure the total body burden of the replication-competent HIV reservoir (1, 10). However, a possible way to estimate both the overall size of the HIV reservoir and the degree of viral control is by assessing time-to-viral-rebound and/or viral load set points upon cessation of ART. In this study, we profiled the circulating lipidome from plasma samples from 24 chronically HIV-infected individuals on suppressive ART who subsequently underwent an analytic treatment interruption (ATI) (11, 12). All 24 individuals underwent ATI without concurrent immunomodulatory agents that might confound our analysis. Lipidomic analysis was performed using liquid chromatography-mass spectrometry (LC-MS), as described previously (13), on plasma samples collected immediately before ATI. Both time-to-viral-rebound and viral load set points were measured during ATI. This cohort had a wide distribution of viral rebound times (14 to 119 days; median = 28) and viral load set points (median = 13,675 copies/ml; see Table S1 in the supplemental material). Using these data, we investigated whether there is a link between pre-ATI lipid profiles and the body burden of HIV during ART (estimated as post-ATI time-to-viral-rebound and viral load set points).
Clinical and demographic data of the study cohort. Download Table S1, PDF file, 0.04 MB (45.5KB, pdf) .
Copyright © 2021 Giron et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
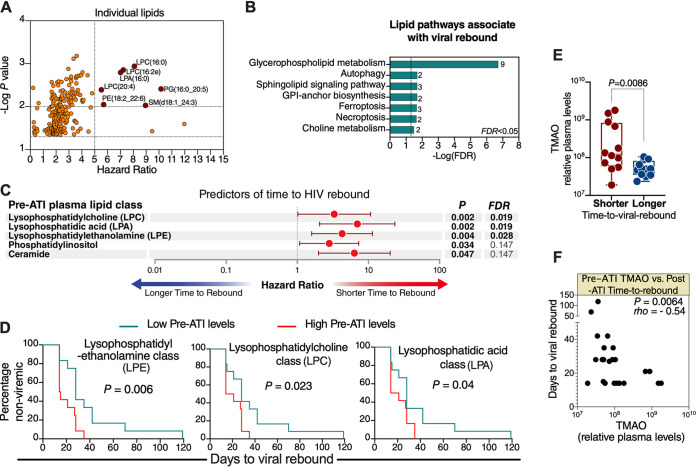
Levels of plasma lysophospholipids measured pre-ATI associate with time to viral rebound post-ATI.
We identified a total of 967 lipids, belonging to 21 lipid classes (described in Table S2), in the plasma samples. Using the Cox proportional-hazards model, we found that pre-ATI levels of several of these lipids significantly associated with a faster time to viral rebound (Fig. 1A; lipids with a hazard ratio [HR] of >5 and P < 0.01 are labeled). We next examined whether these lipids belong to particular lipidomic pathways or classes. Pathway analysis of all lipids whose pre-ATI levels associated with time to viral rebound with P < 0.05 showed that the pathway most associated with viral rebound was glycerophospholipid metabolism (Fig. 1B). The pre-ART levels of three lipid classes were significantly (false-discovery rate [FDR] of <0.05) associated with faster time to viral rebound (Fig. 1C and Table S3): lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), and lysophospholipid acid (LPA). All three classes belong to the lysophospholipid group, which is a subgroup of the glycerophospholipid family shown in Fig. 1B. Lysophospholipids are small bioactive lipid molecules known to play important roles in regulating several biological functions, including promoting inflammation (8, 14–19). The significant associations between these lysophospholipid classes and faster time to viral rebound were confirmed using two additional, independent analyses: Mantel-Cox survival test, after separating participants into low or high groups based on the median level of each of these lipid classes (Fig. 1D); and Spearman’s rank correlation between the levels of these lipid classes and time to viral rebound (Table S3). These data point, for the first time, to plausible links between phospholipid and lysophospholipid metabolism and HIV rebound post-ART. Intriguingly, similar phospholipids and lysophospholipids were recently shown to reactivate latent (dormant) cancer cells (4). Our exploratory findings, that are consistent with the reported functions of these lysophospholipids, raise the question of whether these lysophospholipids condition the host environment with higher levels of inflammation that might impact viral reactivation, cellular processes, and immunological functions during and/or after ATI.
FIG 1.
Higher pre-ATI lysophospholipid metabolism and its bioactive by-products associate with faster post-ATI time to viral rebound. (A) Lipids whose pre-ATI levels associate with post-ATI time to viral rebound, as determined by the Cox proportional-hazards model. Lipids with P < 0.01 and hazard ratio (HR) > 5 are shown in red and labeled. (B) Lipid pathway analysis of plasma lipids whose pre-ATI levels associated with time to viral rebound with P < 0.05 using LIPEA (Lipid Pathway Enrichment Analysis; https://lipea.biotec.tu-dresden.de/home). The graph shows all implicated pathways with FDR < 0.05. Numbers beside each pathway represent the number of dysregulated lipids within the particular pathway. GPI, glycosylphosphatidylinositol. (C) Lipid classes whose pre-ATI levels associate with post-ATI time to viral rebound, as determined by the Cox proportional-hazards model. FDR was calculated using the Benjamini-Hochberg approach. (D) Confirmatory analysis of the three lysophospholipid classes using the Mantel-Cox test. Low pre-ATI levels are levels lower than the group median; high pre-ATI levels are levels higher than the group median. (E) Participants were separated into shorter or longer time-to-viral-rebound groups by the median of days to viral rebound; the levels of TMAO were higher in individuals who rebounded faster than in individuals who rebounded slower. Mann-Whitney U test was used for statistical analysis. (F) Spearman’s rank correlation between pre-ATI TMAO and post-ATI time to viral rebound. Statistical analyses were performed in R and Prism 7.0 (GraphPad).
967 lipids identified in this study were assigned to 21 lipid classes. Download Table S2, PDF file, 0.01 MB (13.7KB, pdf) .
Copyright © 2021 Giron et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
A list of lipid classes whose pre-ATI levels associate with faster time to viral rebound upon ART cessation. Download Table S3, PDF file, 0.1 MB (127.1KB, pdf) .
Copyright © 2021 Giron et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Levels of plasma trimethylamine-N-oxide measured pre-ATI associate with post-ATI time to viral rebound.
The proinflammatory lipid class LPC can be hydrolyzed in the intestine to LPA and choline; choline can be metabolized into trimethylamine, which is converted to trimethylamine-N-oxide (TMAO) in the liver (20). TMAO induces several proinflammatory mediators and has been implicated in several inflammation-associated diseases (20–23). Given that LPC and LPA lipids were among the lipids whose pre-ATI levels associated with faster viral rebound upon ART cessation (Fig. 1A to D), we sought to examine whether levels of TMAO associated with post-ATI time to viral rebound. We performed metabolomic analysis, using LC-MS, as described previously (24), on the same pre-ATI plasma samples. Indeed, pre-ATI levels of TMAO were higher in individuals with lower than the median days to viral rebound (fast rebounders) compared to individuals with higher than the median days to rebound (delayed rebounders) (Fig. 1E). Furthermore, pre-ATI TMAO levels correlated negatively with post-ATI time to viral rebound (Fig. 1F). Our observations that the proinflammatory by-products of intestinal LPC metabolism (LPA and TMAO) are also associated with faster HIV rebound demand a greater understanding of the interaction between glycerophospholipid or choline metabolism by intestinal microbiota and viral persistence during ART or rebound post-ART. Such understanding may inform therapeutic approaches targeting the gut microbiota-lipid metabolism interface to reduce inflammation and facilitate the clearance of HIV reservoirs.
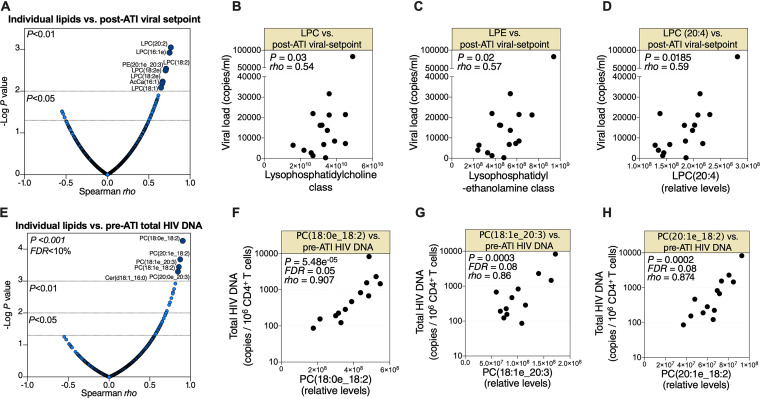
Pre-ATI plasma lysophospholipids associate with post-ATI viral load set point.
In addition to time to viral rebound, post-ART viral load set point can reflect the body burden of HIV during ART. Therefore, we asked whether pre-ATI lipid profiles are associated with post-ATI viral load set point. Pre-ATI levels of several lipids associated with post-ATI viral load set point with P < 0.01 and Spearman rho > 0.5 (Fig. 2A). Furthermore, pre-ATI LPC and LPE class levels correlated with post-ATI viral load set point (Fig. 2B and C, respectively). Finally, the pre-ATI levels of the LPC (24:0) lipid species, which was one of the individual lipids whose pre-ATI level correlated with time to viral rebound (Fig. 1A), also associated with post-ATI viral load set points (Fig. 2D). Notably, levels of LPC (20:4) during HIV infection have been shown to associate with the progression of carotid artery atherosclerosis, even after ART suppression (6). These data indicate that pre-ATI phospholipid metabolism is linked to viral load set point upon ART cessation.
FIG 2.
Pre-ATI phospholipid metabolism associates with post-ATI viral load set point and pre-ATI HIV DNA. (A) Spearman’s rank correlations between pre-ATI lipids and post-ATI viral load set point. Lipids with P < 0.01 and Spearman rho > 0.5 are shown in dark blue and are labeled. (B to D) Correlations between pre-ATI levels of LPC class (B), LPE class (C), or LPC (20:4) lipid species (D) and post-ATI viral load set point. Each symbol shows the value for one HIV-positive individual. (E) Spearman’s rank correlations between pre-ATI lipids and pre-ATI total HIV DNA measured in peripheral CD4+ T cells. Lipids with FDR < 0.1 and Spearman rho > 0.5 are shown in dark blue and are labeled. (F to H) Correlations between pre-ATI levels of several phosphatidylcholine species and pre-ATI levels of HIV DNA in peripheral CD4+ T cells. All correlations were evaluated using Spearman’s rank correlation coefficient tests. Statistical analyses were performed in R and Prism 7.0 (GraphPad).
Pre-ATI phosphatidylcholines associate with pre-ATI HIV DNA in the periphery.
Finally, we examined the links between pre-ATI plasma lipidome and pre-ATI total HIV DNA measured in periphery CD4+ T by droplet digital PCR (ddPCR), as described previously (11). Levels of several phosphatidylcholine species (precursors of lysophosphatidylcholine) significantly correlated (FDR <10%) with CD4+ T cell-associated HIV DNA (Fig. 2E to H). These data further highlight the potential links between phospholipid metabolism and HIV persistence.
Our exploratory study has limitations, including small sample size and sampling of blood. The sample size did not allow for addressing the confounding effects of age, gender, ethnicity, weight, diet, duration of infection, duration on ART, ART regimen, or comorbidities on lipidomic signatures. Addressing the impact of these confounders and validating our data using larger cohorts should be the subject of future studies. In addition, it will be important, in future studies, to examine the links between phospholipid levels, viral rebound, and established clinical measurements of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides (TG). Finally, analyzing lipids and HIV burden in different tissues, including adipose tissue, and mechanistic studies in vitro and in animal models of HIV infection will be needed to examine the precise interplay between phospholipid metabolism and viral persistence. Such studies might identify lipid-based interactions that can be targeted to decrease the size of HIV reservoirs and/or delay viral rebound after stopping ART.
Despite these limitations, our study provides the first proof-of-concept evidence that phospholipid metabolism might be involved in a host milieu that facilitates a faster HIV rebound after ART cessation. The potential interactions between phospholipid/lysophospholipid metabolism and both the establishment and maintenance of HIV latency warrant further investigation.
ACKNOWLEDGMENTS
This work is supported by the Foundation for AIDS Research (amfAR) impact grant 109840-65-RGRL to M.A.-M. and the NIH R21 AI143385 to M.A.-M. M.A.-M. is also supported by NIH grants (R01 DK123733, R01 AG062383, R01NS117458, R21 AI129636, and R21 NS106970), the Penn Center for AIDS Research (P30 AI 045008), and W. W. Smith Charitable Trust grant A1901. Lipidomic and metabolomic analyses were performed by the Wistar Proteomics and Metabolomics Shared Resource supported in part by NIH Cancer Center support grant CA010815 on a Thermo Q-Exactive HF-X mass spectrometer purchased with NIH grant S10 OD023586. L.J.M. is supported by U01AI110434 (NIH NIAID), UM1AI126620 (NIH NIAID), Robert I. Jacobs Fund of the Philadelphia Foundation, and the Herbert Kean, M.D., Family Professorship.
We thank Rachel E. Locke for providing comments. We thank all donor participants.
M.A.-M. conceived and designed the study. L.B.G. carried out experiments. C.S.P., A.L.L., J.Z.L., and J.R. Koethe analyzed and interpreted lipidomic and metabolic data. K.M., J.R. Kostman, E.P., and L.J.M. selected study participants and interpreted clinical data. A.R.G. and H.-Y.T. performed lipidomic and metabolic analysis. X.Y. and Q.L. performed statistical analysis. L.B.G. and M.A.-M. wrote the manuscript, and all authors edited it.
We declare that we have no competing interests.
Footnotes
Citation Giron LB, Papasavvas E, Yin X, Goldman AR, Tang H-Y, Palmer CS, Landay AL, Li JZ, Koethe JR, Mounzer K, Kostman JR, Liu Q, Montaner LJ, Abdel-Mohsen M. 2021. Phospholipid metabolism is associated with time to HIV rebound upon treatment interruption. mBio 12:e03444-20. https://doi.org/10.1128/mBio.03444-20.
Contributor Information
Mohamed Abdel-Mohsen, Email: mmohsen@Wistar.org.
Thomas E. Smithgall, University of Pittsburgh School of Medicine
REFERENCES
- 1.Abdel-Mohsen M, Richman D, Siliciano RF, Nussenzweig MC, Howell BJ, Martinez-Picado J, Chomont N, Bar KJ, Yu XG, Lichterfeld M, Alcami J, Hazuda D, Bushman F, Siliciano JD, Betts MR, Spivak AM, Planelles V, Hahn BH, Smith DM, Ho YC, Buzon MJ, Gaebler C, Paiardini M, Li Q, Estes JD, Hope TJ, Kostman J, Mounzer K, Caskey M, Fox L, Frank I, Riley JL, Tebas P, Montaner LJ, BEAT-HIV Delaney Collaboratory to Cure HIV-1 infection. 2020. Recommendations for measuring HIV reservoir size in cure-directed clinical trials. Nat Med 26:1339–1350. doi: 10.1038/s41591-020-1022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frasch SC, Zemski-Berry K, Murphy RC, Borregaard N, Henson PM, Bratton DL. 2007. Lysophospholipids of different classes mobilize neutrophil secretory vesicles and induce redundant signaling through G2A. J Immunol 178:6540–6548. doi: 10.4049/jimmunol.178.10.6540. [DOI] [PubMed] [Google Scholar]
- 3.Tall AR, Yvan-Charvet L. 2015. Cholesterol, inflammation and innate immunity. Nat Rev Immunol 15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perego M, Tyurin VA, Tyurina YY, Yellets J, Nacarelli T, Lin C, Nefedova Y, Kossenkov A, Liu Q, Sreedhar S, Pass H, Roth J, Vogl T, Feldser D, Zhang R, Kagan VE, Gabrilovich DI. 2020. Reactivation of dormant tumor cells by modified lipids derived from stress-activated neutrophils. Sci Transl Med 12:eabb5817. doi: 10.1126/scitranslmed.abb5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA. 2016. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS 30:65–73. doi: 10.1097/QAD.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chai JC, Deik AA, Hua S, Wang T, Hanna DB, Xue X, Haberlen SA, Shah SJ, Suh Y, Lazar JM, Gustafson D, Hodis HN, Landay AL, Anastos K, Post WS, Kaplan RC, Clish CB, Qi Q. 2019. Association of lipidomic profiles with progression of carotid artery atherosclerosis in HIV infection. JAMA Cardiol 4:1239–1249. doi: 10.1001/jamacardio.2019.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevillyan JM, Wong G, Puls R, Petoumenos K, Emery S, Mellett NA, Mundra PA, Meikle PJ, Hoy JF, ALTAIR Study Group. 2018. Changes in plasma lipidome following initiation of antiretroviral therapy. PLoS One 13:e0202944. doi: 10.1371/journal.pone.0202944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zidar DA, Juchnowski S, Ferrari B, Clagett B, Pilch-Cooper HA, Rose S, Rodriguez B, McComsey GA, Sieg SF, Mehta NN, Lederman MM, Funderburg NT. 2015. Oxidized LDL levels are increased in HIV infection and may drive monocyte activation. J Acquir Immune Defic Syndr 69:154–160. doi: 10.1097/QAI.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belury MA, Bowman E, Gabriel J, Snyder B, Kulkarni M, Palettas M, Mo X, Lake JE, Zidar D, Sieg SF, Rodriguez B, Playford MP, Andrade A, Kuritzkes DR, Mehta NN, Lederman MM, Funderburg NT. 2017. Prospective analysis of lipid composition changes with antiretroviral therapy and immune activation in persons living with HIV. Pathog Immun 2:376–403. doi: 10.20411/pai.v2i3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruner KM, Hosmane NN, Siliciano RF. 2015. Towards an HIV-1 cure: measuring the latent reservoir. Trends Microbiol 23:192–203. doi: 10.1016/j.tim.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papasavvas E, Lada SM, Joseph J, Yin X, Liu Q, Azzoni L, Mounzer K, Kostman JR, Richman D, Montaner LJ. 2018. Analytical antiretroviral therapy interruption does not irreversibly change preinterruption levels of cellular HIV. AIDS 32:1763−1772. doi: 10.1097/QAD.0000000000001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papasavvas E, Kostman JR, Mounzer K, Grant RM, Gross R, Gallo C, Azzoni L, Foulkes A, Thiel B, Pistilli M, Mackiewicz A, Shull J, Montaner LJ. 2004. Randomized, controlled trial of therapy interruption in chronic HIV-1 infection. PLoS Med 1:e64. doi: 10.1371/journal.pmed.0010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alicea GM, Rebecca VW, Goldman AR, Fane ME, Douglass SM, Behera R, Webster MR, Kugel CH, III, Ecker BL, Caino MC, Kossenkov AV, Tang HY, Frederick DT, Flaherty KT, Xu X, Liu Q, Gabrilovich DI, Herlyn M, Blair IA, Schug ZT, Speicher DW, Weeraratna AT. 2020. Changes in aged fibroblast lipid metabolism induce age-dependent melanoma cell resistance to targeted therapy via the fatty acid transporter FATP2. Cancer Discov 10:1282–1295. doi: 10.1158/2159-8290.CD-20-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y. 2002. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim Biophys Acta 1582:81–88. doi: 10.1016/s1388-1981(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Natarajan V. 2013. Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochim Biophys Acta 1831:86–92. doi: 10.1016/j.bbalip.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin X, Qiu C, Zhao L. 2014. Lysophosphatidylcholine perpetuates macrophage polarization toward classically activated phenotype in inflammation. Cell Immunol 289:185–190. doi: 10.1016/j.cellimm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Radu CG, Yang LV, Bentolila LA, Riedinger M, Witte ON. 2005. Lysophosphatidylcholine-induced surface redistribution regulates signaling of the murine G protein-coupled receptor G2A. Mol Biol Cell 16:2234–2247. doi: 10.1091/mbc.e04-12-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akerele OA, Cheema SK. 2015. Fatty acyl composition of lysophosphatidylcholine is important in atherosclerosis. Med Hypotheses 85:754–760. doi: 10.1016/j.mehy.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Wells IC, Peitzmeier G, Vincent JK. 1986. Lecithin: cholesterol acyltransferase and lysolecithin in coronary atherosclerosis. Exp Mol Pathol 45:303–310. doi: 10.1016/0014-4800(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae S, Ulrich CM, Neuhouser ML, Malysheva O, Bailey LB, Xiao L, Brown EC, Cushing-Haugen KL, Zheng Y, Cheng TY, Miller JW, Green R, Lane DS, Beresford SA, Caudill MA. 2014. Plasma choline metabolites and colorectal cancer risk in the Women’s Health Initiative Observational Study. Cancer Res 74:7442–7452. doi: 10.1158/0008-5472.CAN-14-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeisel SH, Warrier M. 2017. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr 37:157–181. doi: 10.1146/annurev-nutr-071816-064732. [DOI] [PubMed] [Google Scholar]
- 23.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, Lusis AJ, Shih DM. 2016. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-kappaB. J Am Heart Assoc 5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Agarwal E, Bertolini I, Seo JH, Caino MC, Ghosh JC, Kossenkov AV, Liu Q, Tang HY, Goldman AR, Languino LR, Speicher DW, Altieri DC. 2020. The mitophagy effector FUNDC1 controls mitochondrial reprogramming and cellular plasticity in cancer cells. Sci Signal 13:eaaz8240. doi: 10.1126/scisignal.aaz8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical and demographic data of the study cohort. Download Table S1, PDF file, 0.04 MB (45.5KB, pdf) .
Copyright © 2021 Giron et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
967 lipids identified in this study were assigned to 21 lipid classes. Download Table S2, PDF file, 0.01 MB (13.7KB, pdf) .
Copyright © 2021 Giron et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
A list of lipid classes whose pre-ATI levels associate with faster time to viral rebound upon ART cessation. Download Table S3, PDF file, 0.1 MB (127.1KB, pdf) .
Copyright © 2021 Giron et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.