Abstract
Background
Stroke affects millions of people every year and is a leading cause of disability, resulting in significant financial cost and reduction in quality of life. Rehabilitation after stroke aims to reduce disability by facilitating recovery of impairment, activity, or participation. One aspect of stroke rehabilitation that may affect outcomes is the amount of time spent in rehabilitation, including minutes provided, frequency (i.e. days per week of rehabilitation), and duration (i.e. time period over which rehabilitation is provided). Effect of time spent in rehabilitation after stroke has been explored extensively in the literature, but findings are inconsistent. Previous systematic reviews with meta‐analyses have included studies that differ not only in the amount provided, but also type of rehabilitation.
Objectives
To assess the effect of 1. more time spent in the same type of rehabilitation on activity measures in people with stroke; 2. difference in total rehabilitation time (in minutes) on recovery of activity in people with stroke; and 3. rehabilitation schedule on activity in terms of: a. average time (minutes) per week undergoing rehabilitation, b. frequency (number of sessions per week) of rehabilitation, and c. total duration of rehabilitation.
Search methods
We searched the Cochrane Stroke Group trials register, CENTRAL, MEDLINE, Embase, eight other databases, and five trials registers to June 2021. We searched reference lists of identified studies, contacted key authors, and undertook reference searching using Web of Science Cited Reference Search.
Selection criteria
We included randomised controlled trials (RCTs) of adults with stroke that compared different amounts of time spent, greater than zero, in rehabilitation (any non‐pharmacological, non‐surgical intervention aimed to improve activity after stroke). Studies varied only in the amount of time in rehabilitation between experimental and control conditions. Primary outcome was activities of daily living (ADLs); secondary outcomes were activity measures of upper and lower limbs, motor impairment measures of upper and lower limbs, and serious adverse events (SAE)/death.
Data collection and analysis
Two review authors independently screened studies, extracted data, assessed methodological quality using the Cochrane RoB 2 tool, and assessed certainty of the evidence using GRADE. For continuous outcomes using different scales, we calculated pooled standardised mean difference (SMDs) and 95% confidence intervals (CIs). We expressed dichotomous outcomes as risk ratios (RR) with 95% CIs.
Main results
The quantitative synthesis of this review comprised 21 parallel RCTs, involving analysed data from 1412 participants.
Time in rehabilitation varied between studies. Minutes provided per week were 90 to 1288. Days per week of rehabilitation were three to seven. Duration of rehabilitation was two weeks to six months. Thirteen studies provided upper limb rehabilitation, five general rehabilitation, two mobilisation training, and one lower limb training. Sixteen studies examined participants in the first six months following stroke; the remaining five included participants more than six months poststroke. Comparison of stroke severity or level of impairment was limited due to variations in measurement.
The risk of bias assessment suggests there were issues with the methodological quality of the included studies. There were 76 outcome‐level risk of bias assessments: 15 low risk, 37 some concerns, and 24 high risk.
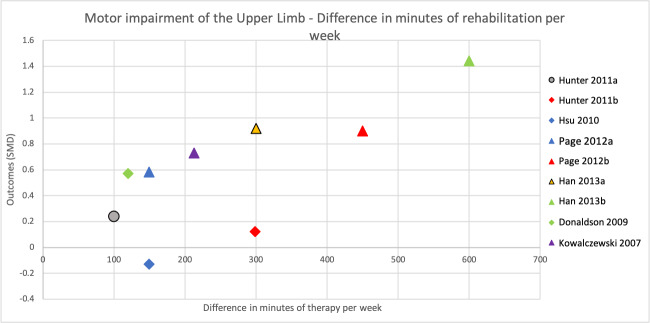
When comparing groups that spent more time versus less time in rehabilitation immediately after intervention, we found no difference in rehabilitation for ADL outcomes (SMD 0.13, 95% CI −0.02 to 0.28; P = 0.09; I2 = 7%; 14 studies, 864 participants; very low‐certainty evidence), activity measures of the upper limb (SMD 0.09, 95% CI −0.11 to 0.29; P = 0.36; I2 = 0%; 12 studies, 426 participants; very low‐certainty evidence), and activity measures of the lower limb (SMD 0.25, 95% CI −0.03 to 0.53; P = 0.08; I2 = 48%; 5 studies, 425 participants; very low‐certainty evidence). We found an effect in favour of more time in rehabilitation for motor impairment measures of the upper limb (SMD 0.32, 95% CI 0.06 to 0.58; P = 0.01; I2 = 10%; 9 studies, 287 participants; low‐certainty evidence) and of the lower limb (SMD 0.71, 95% CI 0.15 to 1.28; P = 0.01; 1 study, 51 participants; very low‐certainty evidence). There were no intervention‐related SAEs. More time in rehabilitation did not affect the risk of SAEs/death (RR 1.20, 95% CI 0.51 to 2.85; P = 0.68; I2 = 0%; 2 studies, 379 participants; low‐certainty evidence), but few studies measured these outcomes.
Predefined subgroup analyses comparing studies with a larger difference of total time spent in rehabilitation between intervention groups to studies with a smaller difference found greater improvements for studies with a larger difference. This was statistically significant for ADL outcomes (P = 0.02) and activity measures of the upper limb (P = 0.04), but not for activity measures of the lower limb (P = 0.41) or motor impairment measures of the upper limb (P = 0.06).
Authors' conclusions
An increase in time spent in the same type of rehabilitation after stroke results in little to no difference in meaningful activities such as activities of daily living and activities of the upper and lower limb but a small benefit in measures of motor impairment (low‐ to very low‐certainty evidence for all findings). If the increase in time spent in rehabilitation exceeds a threshold, this may lead to improved outcomes. There is currently insufficient evidence to recommend a minimum beneficial daily amount in clinical practice. The findings of this study are limited by a lack of studies with a significant contrast in amount of additional rehabilitation provided between control and intervention groups.
Large, well‐designed, high‐quality RCTs that measure time spent in all rehabilitation activities (not just interventional) and provide a large contrast (minimum of 1000 minutes) in amount of rehabilitation between groups would provide further evidence for effect of time spent in rehabilitation.
Keywords: Adult, Humans, Activities of Daily Living, Physical Therapy Modalities, Stroke, Stroke Rehabilitation, Upper Extremity
Plain language summary
Time spent in rehabilitation and effect on measures of activity after stroke
Review question Does more time spent in rehabilitation improve activity? What matters? Is it the total time spent in rehabilitation that is important, or is it the way rehabilitation is delivered (the schedule)? Is it, for example, the amount of time spent per week? Or the frequency of sessions?
Background Stroke rehabilitation helps people who have had a stroke to recover and resume their activities. Different countries have different guidelines about the amount of therapy they should receive. In England, a minimum of 45 minutes of each appropriate therapy, every day is recommended. In Canada, the guidelines recommend more – three hours of task‐specific training, five days per week. Previous research has found no clear evidence in favour of one approach or the other: the effect of total time spent in rehabilitation, or the schedule by which it is delivered. The English recommendation of 45 minutes is based on the results of studies that compared different types of rehabilitation as well as different amounts of the same type of rehabilitation – which is not the same thing. This is why our review compares only different amounts of the same type of stroke rehabilitation.
Study characteristics We included 21 studies amounting to 1412 people with stroke. Each study compared groups of people who had received different amounts of the same type of rehabilitation. Different types of rehabilitation were included, but the comparison within each study was always only different amounts of the same type. We included rehabilitation of the arms, legs, walking, and general rehabilitation. In 16 studies, participants were in the first six months after stroke. In the remaining five studies, participants were more than six months after stroke.
Search date We searched for studies up to June 2021.
Key results We found that, for measures of activities involved in daily living (e.g. washing and dressing), activity measures of the arm (e.g. picking up an item), and activity measures of the leg (e.g. walking) there was neither harm to nor benefit for groups that received more rehabilitation compared with groups that received less. For measures of movement of the arm and leg (e.g. strength or range of movement), there was a benefit from receiving more rehabilitation. However, when we compared only the studies that had a bigger contrast between groups, there was a beneficial effect from additional therapy in terms of daily living activities, activity measures of the arm and leg, and movement measures of the arm. This suggests that people with stroke need a large amount of extra rehabilitation for it to make a difference in their recovery and ability to do everyday activities.
Certainty of the evidence Certainty of the evidence, which is measured by the quality of each of the studies included in the review, was either low or very low. Therefore, we can only draw tentative conclusions from the findings of this review. It also indicates that more, better quality, studies are needed.
Summary of findings
Summary of findings 1. Summary of findings table ‐ More time compared to less time in rehabilitation (objective one – immediately after intervention).
| More time compared to less time in rehabilitation (objective one – immediately after intervention) | ||||||
| Patient or population:rehabilitation vs less time spent Setting:any rehabilitation setting, including hospital, outpatients, and patient's home Intervention:more time Comparison:less time | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with less time | Risk with more time | |||||
| Activities of daily living (ADL) outcomes assessed with: studies measured ADL outcomes using different scales. Higher scores indicate greater independence | ‐ | SMD 0.13 SD higher (0.02 lower to 0.28 higher) | ‐ | 864 (19 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Generally, an SMD of 0.2 is considered a small effect. Therefore, this finding suggests that the average difference in mean scores between more therapy groups and less therapy groups is small. As the CI for this outcome included 0, there may be no difference for ADL measures when more time is spent in rehabilitation. |
| Activity measures of the upper limb (upper limb activity) assessed with: studies measured upper limb activity using different scales. Higher scores indicate greater activity | ‐ | SMD 0.09 higher (0.11 lower to 0.29 higher) | ‐ | 426 (18 RCTs) | ⊕⊝⊝⊝ Very lowa,b,d | Generally, an SMD of 0.2 is considered a small effect. Therefore, this finding suggests that the average difference in mean scores between more therapy groups and less therapy groups is small. As the CI for this outcome crossed 0, there may be no difference for upper limb activity measures when more time is spent in rehabilitation. |
| Activity measures of the lower limb (lower limb activity) assessed with: studies measured lower limb activity using different scales. Higher scored indicate greater activity | ‐ | SMD 0.25 higher (0.03 lower to 0.53 higher) | ‐ | 425 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | Generally, an SMD of 0.2 is considered a small effect. Therefore, this finding suggests that the average difference in mean scores between more therapy groups and less therapy groups is small. As the CI for this outcome crossed 0, there may be no difference for lower limb activity measures when more time is spent in rehabilitation. |
| Motor impairment measures of the upper limb (upper limb impairment) assessed with: studies measured upper limb impairment using different scales. Higher scores indicate less impairment | ‐ | SMD 0.32 higher (0.06 higher to 0.58 higher) | ‐ | 287 (12 RCTs) | ⊕⊕⊝⊝ Lowa,e | Generally, an SMD of 0.2 is considered a small effect. Therefore, this finding suggests that the average difference in mean scores between more therapy groups and less therapy groups is small. As the CI for this outcome did not cross 0, there is a benefit for upper limb impairment measures when more time is spent in rehabilitation. |
| Motor impairment measures of the lower limb (lower limb impairment) assessed with: knee flexion peak torque | ‐ | SMD 0.71 SD higher (0.15 higher to 1.28 higher) | ‐ | 51 (1 RCT) | ⊕⊝⊝⊝ Very lowf,g | Generally, an SMD of 0.5 is considered a moderate effect. Therefore, this finding suggests that the average difference in mean scores between more therapy groups and less therapy groups is moderate. As the CI for this outcome did not cross 0, there is a benefit for lower limb impairment measures when more time is spent in rehabilitation. |
| Serious adverse events/death | 48 per 1000 | 57 per 1000 (24 to 136) | RR 1.20 (0.51 to 2.85) | 379 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | There is no increased risk of serious adverse events or death when more time is spent in rehabilitation. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_417399834740907517. | ||||||
a Several studies classified as 'some concerns' or 'high' risk of bias (downgraded one level). b 95% CI contains an effect size of no difference. c Two studies may have measured this outcome but have not reported it. A funnel plot showed some asymmetry, which may be indicative of publication bias. d Five studies may have assessed this outcome but did not report findings. A forest plot showed asymmetry, suggestive of non‐reporting bias. e One study assessed this outcome but did not report findings and two further studies may have assessed this outcome but did not report findings. f Analysis only included one study, which at high risk of bias. Therefore, finding considered at very serious risk of bias (downgraded two levels). g Two studies may have assessed this outcome but did not report findings.
Summary of findings 2. Summary of findings table ‐ More time compared to less time in rehabilitation (objective one – medium‐term outcomes).
| More time compared to less time in rehabilitation (objective one – medium‐term outcomes) | ||||||
| Patient or population:rehabilitation (medium‐term outcomes) Setting:any rehabilitation setting, including hospital, outpatients, and patient's home Intervention:more time Comparison:less time | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with less time | Risk with more time | |||||
| Activities of daily living (ADL) outcomes: medium‐term outcomes assessed with: studies measured ADL outcomes using different scales. Higher scores indicate greater independence | ‐ | SMD 0.01 higher (0.15 lower to 0.16 higher) | ‐ | 673 (12 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | The average difference in mean scores between more therapy groups and less therapy groups is close to 0. Therefore, there is no difference for ADL measures when more time is spent in rehabilitation. |
| Activity measures of the upper limb: medium‐term outcomes assessed with: studies measured upper limb activity using different scales. Higher scores indicate greater activity | ‐ | SMD 0.02 lower (0.36 lower to 0.33 higher) | ‐ | 218 (9 RCTs) | ⊕⊝⊝⊝ Very lowb,d,e | As this finding is very close to 0, it suggests that the average difference in mean scores between more therapy groups and less therapy groups is close to nothing. Therefore, there is no difference for activity measures of the upper limb when more time is spent in rehabilitation. |
| Activity measures of the lower limb: medium‐term outcomes assessed with: studies measured lower limb activity using different scales. Higher scored indicate greater activity | ‐ | SMD 0.1 higher (0.3 lower to 0.49 higher) | ‐ | 243 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,d,f,g | Generally, an SMD of 0.2 is considered a small effect. Therefore, this finding suggests that the average difference in mean scores between more therapy groups and less therapy groups is very small. As the CI for this outcome crossed 0, there may be no difference for lower limb activity measures when more time is spent in rehabilitation. |
| Motor impairment measures of the upper limb: medium‐term outcomes assessed with: studies measured upper limb impairment using different scales. Higher scores indicate less impairment | ‐ | SMD 0.02 lower (0.39 lower to 0.35 higher) | ‐ | 115 (5 RCTs) | ⊕⊝⊝⊝ Very lowb,d,h | As this finding is very close to 0, it suggests that the average difference in mean scores between more therapy groups and less therapy groups is close to nothing. Therefore, there is no difference for motor impairment measures of the upper limb when more time is spent in rehabilitation. |
| Motor impairment measures of the lower limb: medium‐term outcomes assessed with: knee flexion peak torque | ‐ | SMD 0.62 higher (0.04 lower to 1.28 higher) | ‐ | 37 (1 RCT) | ⊕⊝⊝⊝ Very lowb,i,j | Generally, an SMD of 0.5 is considered a moderate effect. Therefore, this finding suggests that the average difference in mean scores between more therapy groups and less therapy groups is moderate. As the CI for this outcome did not cross 0, there is a benefit for lower limb impairment measures when more time is spent in rehabilitation. |
| Serious adverse events/death: medium‐term outcomes | 70 per 1000 | 93 per 1000 (44 to 194) | RR 1.32 (0.63 to 2.76) | 344 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | There is no increased risk of serious adverse events or death when more time is spent in rehabilitation. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_421205526923794365. | ||||||
a More than half of the studies included in the analysis had a high overall risk of bias. Therefore, finding considered at very serious risk of bias (downgraded two levels). b 95% CI contains an effect size of no difference. c Data from one included study were missing from this analysis. One study assessed this outcome but did not report findings and seven other studies may have assessed this outcome but did not report findings. A funnel plot for this outcome showed asymmetry, which may indicate non‐reporting bias. d Several studies classified as 'some concerns' or 'high' risk of bias (downgraded one level). e Data from two included studies were missing from this analysis. Two studies assessed this outcome but did not report findings and seven other studies may have assessed this outcome but did not report findings. f I2 = 58%. g Data from one included study were missing from this analysis. One study assessed this outcome but did not report findings and one study may have assessed this outcome but did not report findings. h Data from one included study were missing from this analysis. Two studies assessed this outcome but did not report findings and six other studies may have assessed this outcome but did not report findings. i Only included study was at high risk of overall bias. j One study assessed this outcome but did not report findings and two other studies may have assessed this outcome but did not report findings.
Summary of findings 3. Summary of findings table ‐ More time compared to less time in rehabilitation (objective one – long‐term outcomes).
| More time compared to less time in rehabilitation (objective one – long‐term outcomes) | ||||||
| Patient or population:rehabilitation (long‐term outcomes) Setting:any rehabilitation setting, including hospital, outpatients, and patient's home Intervention:more time Comparison:less time | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with less time | Risk with more time | |||||
| Activities of daily living (ADL) outcomes: long‐term outcomes assessed with: Adelaide Activities Profile | ‐ | SMD 0.09 higher (0.39 lower to 0.57 higher) | ‐ | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa | Generally, an SMD of 0.2 is considered a small effect. Therefore, this finding suggests that the average difference in mean scores between more therapy group and less therapy group is very small. As the CI for this outcome included 0, there may be no difference for ADL measures when more time is spent in rehabilitation. |
| Activity measures of the lower limb: long‐term outcomes assessed with: 6 minute walk test | ‐ | SMD 0.16 higher (0.32 lower to 0.64 higher) | ‐ | 67 (1 RCT) | ⊕⊕⊝⊝ Lowa | Generally, an SMD of 0.2 is considered a small effect. Therefore, this finding suggests that the average difference in mean scores between more therapy group and less therapy group is small. As the CI for this outcome included 0, there may be no difference for activity measures of the lower limb when more time is spent in rehabilitation. |
| Motor impairment measures of the upper limb: long‐term outcomes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Motor impairment measures of the lower limb: long‐term outcomes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Serious adverse events/death: long‐term outcomes ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_421205629766293495. | ||||||
a Very serious imprecision, due to 95% CI containing an effect size of no difference and finding based on the results of only one study, with a relatively small number of participants (downgraded two levels)
Background
This review explores the effect of time spent in rehabilitation after stroke. We acknowledge that 'time spent' is potentially an ambiguous term. For the purpose of this review, we consider 'time spent' to include:
the number of minutes of rehabilitation provided per week;
the frequency of rehabilitation provided per week (i.e. number of days per week on which rehabilitation was given);
the time period over which rehabilitation was provided, or rehabilitation duration;
the total amount of time spent in rehabilitation (in minutes/hours).
The outcome of rehabilitation after stroke may be affected by how these different elements are combined. For example, the outcome of a certain number of minutes of rehabilitation provided over a shorter time period may be different to the same number of minutes provided over a longer time period. We acknowledge that, to some, 'time spent in rehabilitation' could be synonymous with 'rehabilitation intensity'. While the term 'intensity' could be used to describe the time‐related elements described above, it has also been used to describe alternative characteristics of rehabilitation, including number of repetitions performed within treatment sessions (Scrivener 2012), and physiological effort exerted (Outermans 2010). We will not explore these characteristics in this review. Other terms to describe 'time spent in rehabilitation' could be 'dose of rehabilitation' or 'amount of rehabilitation'.
Description of the condition
Stroke is a "neurological deficit attributed to an acute focal injury of the central nervous system by a vascular cause" (Sacco 2013). It is a significant, global health issue. In 2016, there were approximately 13.7 million first‐ever strokes and more than 80 million stroke survivors worldwide, with stroke being the second most common cause of lost disability‐adjusted life years (DALYs) (GBD 2016 Stroke Collaborators 2019). In the UK alone, over 27,000 (37%) of people discharged from hospital between 2013 and 2014 required help with activities of daily living (ADL) such as washing and dressing (Royal College of Physicians 2014), and between 2019 and 2020, 34% of people had not returned to independence by six months poststroke (Bahalla 2021). Such disability results in significant cost due to care requirements and loss of productivity (Mozaffarian 2015; Patel 2020). Better rehabilitation outcomes after stroke would reduce the impact of disability and dependence on the quality of life of people with stroke and their carers (Lewthwaite 2018; Oyewole 2020), and national economies (Patel 2020).
Description of the intervention
The intervention of interest in this study is stroke rehabilitation. Stroke rehabilitation is a multi‐dimensional process, designed to optimise functional activity in people with stroke, where there are ongoing stroke‐related impairments (Dobkin 2005; NICE 2013). For this review, we defined rehabilitation as any non‐pharmacological, non‐surgical intervention that aimed to improve activity after stroke.
There are many rehabilitation interventions to target different stroke‐related impairments via a variety of methods. Previous Cochrane Reviews have explored physical rehabilitation (Pollock 2014a), cognitive rehabilitation (Bowen 2013; Chung 2013; das Nair 2016; Loetscher 2013), telerehabilitation (Laver 2013), virtual reality (Laver 2015), acupuncture (Yang 2016), electromechanical and robot‐assisted arm training (Mehrholz 2018), mirror therapy (Thieme 2018), physical fitness training (Saunders 2020), motivational interviewing (Cheng 2015), constraint‐induced movement therapy (CIMT) (Corbetta 2015), repetitive transcranial magnetic stimulation (Hao 2013), and repetitive task training (RTT) (French 2016). While there is value in determining the efficacy of specific rehabilitation interventions, it is acknowledged that, in practice, the content of rehabilitation therapy is not clearly defined and varies between both therapists and services (Ballinger 1999; DeJong 2005). The relationship between type of therapy and response is unclear (Lohse 2014), with therapists adopting an eclectic approach (Jette 2005). Therefore, this review is adopting an 'intervention agnostic' approach, seeking to explore not if one type of rehabilitation is superior to another, but to explore the specific effect of time spent in rehabilitation.
Rehabilitation may be provided by a variety of professions (Pollock 2014a). This review is not limited to any specific provider of rehabilitation; therefore, we refer to providers of rehabilitation as 'service providers'.
How the intervention might work
In this review, the intervention is any non‐pharmacological, non‐surgical intervention that aims to improve activity after stroke, and the research question focuses on the influence of time spent in any particular intervention. These interventions might work through neuroplasticity: the brain's ability to modify neuronal activity and reorganise neural connections. Neuroplasticity underpins both recovery of, and compensation for, impaired motor function after stroke (Buma 2013; Dobkin 2005; Kleim 2008; Levin 2009; Nudo 2013). The differentiation between recovery, where survivors initially regain their premorbid kinematic/muscle activation patterns and compensation, where alternative kinematic/muscle activations are used to accomplish a task, is thought to occur by around the first five to eight weeks after stroke (Kwakkel 2015; van der Vliet 2020; van Kordelaar 2013).
Research points to many potentially important aspects of stroke rehabilitation that influence outcomes. Kleim 2008, in their review of the evidence for experience‐dependent neural plasticity, identified that repetition, the relative importance of the task undertaken, and skill acquisition (as opposed to simply use) will influence plasticity. Other authors described further important aspects in the re‐learning of motor skills, such as the use of implicit versus explicit learning (Boyd 2003; Boyd 2004). The presence of a meaningful context or goal has been shown to enhance motor learning (Ma 1999; Wu 2000). There is evidence that extrinsic feedback enhances motor learning after stroke (van Vliet 2006), and that stroke survivors benefit more from random practice of exercise than they do block practice (Hanlon 1996). Wulf 2010 discussed additional influences on learning, such as learning through observation, and internal versus external focus of attention and self‐controlled practice. Mount 2007 discussed research related to the impact of errorless learning versus trial and error learning, while Levack 2006 suggested that specific, difficult goals may enhance performance. Finally, research suggests that an enriched environment enhances recovery poststroke (Janssen 2010). The purpose of this review, however, is to explore the effect of the time spent in rehabilitation for activity level outcomes after stroke. While it is acknowledged that other factors will influence outcomes, we assume that these other factors are similarly distributed in an intervention where only the time spent in rehabilitation is the variable of focus for this review.
Mechanistically, one type of learning that promotes neuroplasticity is Hebbian Learning (Hebb 1949). Hebbian (and anti‐Hebbian) Learning is concerned with an increase in synaptic efficacy, due to repetitive firing of presynaptic cells, causing stimulation of postsynaptic cells, leading to increased synaptic strength (Nudo 2013). Evidence indicates that repetition is key to increasing synaptic efficacy (Kleim 2008; Nudo 2013). From a service provider's perspective, then, it could be deduced that the time spent in rehabilitation may determine the frequency of synaptic stimulation and, therefore, more time spent in repetitive rehabilitation should increase synaptic strength.
Behavioural experience, or the intervention itself, is one of the most important factors in the modulation of cortical function and structure (Nudo 2013). Behaviourally, there is a large body of evidence regarding motor learning (and relearning) in non‐disabled people (Wulf 2010), and also in people with stroke (Kitago 2013), where the main principles of repetition, 'just right' challenge (Guadagnoli 2004), and graded feedback (Winstein 1990), closely align with the key principles of neuroplasticity (Kleim 2008). This again supports the premise that increased time spent in rehabilitation will provide more beneficial change in the performance outcomes of a task.
Several intervention studies also suggest that the time spent in rehabilitation after stroke is more important than the type of rehabilitation. One narrative review of CIMT found that CIMT compared with dose‐matched bilateral arm training did not produce significant differences in overall effect sizes (Kwakkel 2015). Phase 2 and 3 RCTs have found no significant differences in outcomes between CIMT and dose‐matched 'traditional occupational therapy' (Dromerick 2009), robot‐assisted therapy and dose‐matched intensive therapy (Lo 2010), or structured task‐oriented training and dose‐equivalent usual care (Winstein 2016). Taken together, these and similar findings indicate that, as long as the rehabilitation provided is of equal amounts, it does not matter very much what type or content of therapy is given. This has led to many studies comparing amounts of therapy for a given population as the factor of interest (as reviewed in a later section). However, 'more is better regardless' is almost certainly an oversimplified view of how rehabilitation interventions might work.
For example, in the recent ICARE study (Interdisciplinary Comprehensive Arm Rehabilitation Evaluation; Winstein 2016), a usual‐care low‐dose group did as well as the two higher‐dose‐matched groups at one year suggesting that dose of rehabilitation may not be the most important factor in recovery levels measured long after the intervention, although the three groups are confounded by having different types of intervention. Furthermore, Dromerick 2009 found that providing a greater dose of CIMT, when given early after stroke, had a detrimental effect on outcomes related to ADL. This suggests that time spent in rehabilitation interacts with the stage of recovery and spontaneous recovery processes. These two studies both suggest that the timing of an intervention may be important. One study in the chronic population, comparing bilateral rhythmic arm training and unilateral dose‐matched therapeutic exercises, determined that the two interventions did not operate through the same neuroplastic mechanisms, despite eliciting similar outcomes at the impairment and activity level (Whitall 2011). This finding indicates that type of rehabilitation and what the rehabilitation targets interact with the underlying mechanisms in ways we do not completely understand.
Finally, all the intervention studies above have the problem of how to actually dose‐match different types of rehabilitation so that they are truly equivalent in effort by the patient at any given amount. This is an almost impossible task. Given this problem, as well as the evidence just presented that the type of intervention may well be important after all, leads us to question whether it is valid to compare different amounts of time spent in rehabilitation with two different interventions. We pursue this point further below.
In summary, it is thought that rehabilitation interventions 'work' by influencing the recovery from and compensation for the neurological damage caused by stroke. The time spent in rehabilitation may be a factor in determining the effectiveness of this intervention for reducing activity limitation.
Why it is important to do this review
Some clinical practice guidelines give recommendations for the amount of time that should be spent in rehabilitation:
the Royal College of Physicians' National Clinical Guideline for Stroke recommends a minimum 45 minutes of each relevant rehabilitation therapy (occupational therapy, physiotherapy, and speech and language therapy), every day (ICSWP 2016);
the Canadian Best Practice guidelines for rehabilitation state that patients should receive a minimum of three hours of task‐specific therapy, five days per week, delivered by an interprofessional stroke team (Teasell 2020);
the Australian Stroke Foundation, Clinical Guidelines for Stroke Management states that a minimum of one hour of active practice of physical therapy (occupational therapy and physiotherapy) should be provided at least five days per week (Stroke Foundation 2021).
These guidelines all suggest a minimum daily session duration (in terms of hours/minutes) of rehabilitation that should be provided and a suggested frequency of rehabilitation (in terms of days per week). They do not all make a recommendation for treatment duration (in terms of the length of time over which rehabilitation should continue).
The effect of time spent in rehabilitation poststroke has been explored extensively, using systematic reviews with meta‐analyses (Cooke 2010a; Galvin 2008; Kwakkel 1997; Kwakkel 2004; Langhorne 1996; Lohse 2014; Veerbeek 2011; Veerbeek 2014), but none of these studies provides clear evidence for the aforementioned guidelines. These meta‐analyses include 71 unique studies. In at least 50 of these studies, the experimental and control interventions differed in not only the amount of rehabilitation provided, but also the type of rehabilitation. As previously mentioned, it may be that type of rehabilitation influences outcomes, as well as amount of time spent in rehabilitation. Arguably, therefore, conclusions regarding the effect of amount should not be drawn from studies comparing different types of rehabilitation.
Three meta‐analyses explored the 'optimum amount' of rehabilitation poststroke. Kwakkel 2004 used a cumulative meta‐analysis and, although their findings did not support a precise optimal amount of time spent in rehabilitation, there was no ceiling effect. Lohse 2014 used meta‐regression to explore the effect of total scheduled therapy time on effect sizes. The authors found a non‐linear relationship between total amount of therapy and outcomes. This suggests that there may be an 'optimal amount' of therapy time, beyond which the benefits of additional therapy are limited. Finally, Schneider 2016 undertook a Receiver operating characteristic (ROC) curve analysis of false versus true benefit. This indicated that an extra 240% of rehabilitation is required to make certain a better outcome for activity measures. Taken together, these meta‐analyses suggest that guidelines that include a specific minimum amount of rehabilitation are pragmatically based, as opposed to evidence‐based.
More recently, one Cochrane Review explored the effect of Repetitive Task Training (RTT) on functional ability after stroke (French 2016). They found evidence that RTT improves upper and lower limb function, but there was no effect for additional time spent in RTT. In their Cochrane Review 'Physical rehabilitation approaches for the recovery of function and mobility following stroke', Pollock 2014a undertook a subgroup analysis exploring the effect of dose of physical rehabilitation on functional recovery and the recovery of motor function after stroke. They concluded that evidence related to dose is limited. In addition, Pollock 2014b undertook a Cochrane Review of interventions for improving upper limb function after stroke. They found that certain interventions were effective at a higher dose, and identified the need for evidence related to dose of intervention, in order to inform future research and clinical practice.
As yet, there is no Cochrane Review exploring the effect of time spent in the same type of rehabilitation on activity after stroke. We consider our review important in order to determine if the increasing number of clinical guidelines that recommend a specific minimum amount of time spent in rehabilitation after stroke have an evidence base and, if so, this will be useful for future guideline development. Based on current guidelines and evidence, there is a strong push for technologies that enable additional practice, especially in the home and without additional staff. This requirement has intensified, due to the 2020 COVID‐19 pandemic. A better understanding of the importance of amount of time spent in rehabilitation will inform development of new technologies such as telerehabilitation and use of virtual reality.
Objectives
To assess the effect of 1. more time spent in the same type of rehabilitation on activity measures in people with stroke; 2. difference in total rehabilitation time (in minutes) on recovery of activity in people with stroke; and 3. rehabilitation schedule on activity in terms of: a. average time (minutes) per week undergoing rehabilitation, b. frequency (number of sessions per week) of rehabilitation, and c. total duration of rehabilitation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials that compared different amounts of time spent, greater than zero, of the same rehabilitation intervention. These could be RCTs (participants are randomised to either an experimental group or a control group) or randomised clinical trials (participants are randomised to different experimental groups). We would also have included cluster‐randomised trials and data from the first period of randomised cross‐over trials were any found. We restricted the types of studies to randomised trials only, as they are considered high‐quality sources of evidence in clinical practice (Devereaux 2003), and the method to establish causality (Concato 2010; Horn 2005; Kersten 2010).
If studies included more than one treatment group, one of which met the criteria for this review, we included the control group and intervention group compliant with the criteria for this review. If studies included multiple intervention groups, we included all compliant with the criteria for this review.
Types of participants
Participants were adults (aged over 18 years), with a clinical diagnosis of stroke, caused by either infarct or haemorrhage (including subarachnoid haemorrhage), as defined by the study authors. Participants received rehabilitation in an inpatient, outpatient, or community setting. We excluded studies that included participants with diagnoses other than stroke as the primary diagnosis, even if they included some participants with a primary diagnosis of stroke.
Types of interventions
We included trials that compared different amounts of time spent in the same type of rehabilitation. We defined rehabilitation as any non‐pharmacological, non‐surgical intervention that aimed to improve activity after stroke.
To be eligible for inclusion, trials had to include two or more groups that varied in one or more of the following elements, in any combination.
The number of minutes of rehabilitation provided per week.
The number of days per week on which rehabilitation was provided.
The time period over which rehabilitation was provided, or rehabilitation duration, measured in days, weeks, or months.
The total amount of time spent in rehabilitation (in minutes/hours).
To establish if time spent is related to outcomes, included studies varied only in the amount of time spent in rehabilitation between groups. We included 'control' or 'usual care' groups, provided they received the same type of rehabilitation as the intervention group. We excluded comparisons of intervention versus no intervention (including trials in which just some participants received no intervention).
If studies clearly varied in the time spent in rehabilitation (as defined above) but did not report a specific time‐related measurement, we included the study.
Co‐interventions did not preclude inclusion, provided they were administered equally to both experimental and control groups.
Types of outcome measures
We included published outcome measures falling into International Classification of Functioning, Disability and Health (ICF) categories for activity and body structures/body functions (WHO 2001). We were primarily interested in measures of activity, as these outcomes are likely to be most meaningful to stroke survivors and to indicate a reduction in the burden of care. We were also interested in measures of body structure/body function, as they indicate if an increased amount of time spent in rehabilitation facilitates recovery at this level.
Primary outcomes
For our three study objectives, we defined the primary outcome measure as:
activities of daily living (ADL) outcomes.
We included any measure of ADL, including but not limited to (and in no specific order): Barthel Index, Frenchay Activity Index, Rivermead ADL Assessment, Nottingham Extended ADL, and Functional Independence Measure.
Secondary outcomes
For our three study objectives, our secondary outcome measures were:
activity measures of the upper limb (e.g. Action Research Arm Test, Jebsen Taylor Hand Function Test);
activity measures of the lower limb (e.g. Timed Up‐and‐Go, 6‐minute walk test, walking speed, Rivermead Mobility Index);
motor impairment measures of the upper limb (e.g. Fugl‐Meyer Upper Extremity (FM‐UE), muscle strength, range of movement);
motor impairment measures of the lower limb (e.g. muscle strength, range of movement);
serious adverse events (SAE)/death.
For both primary and secondary outcomes, we were principally interested in measures taken immediately after intervention. However, we also undertook analysis of medium‐term outcomes (two weeks to six months after treatment ended) and long‐term outcomes (more than six months after treatment ended). We analysed the medium‐ and long‐term outcomes for objective one, but not for objectives two and three.
Search methods for identification of studies
See the 'Specialised register' section at Cochrane Stroke's website. We searched for trials in all languages and arranged for the translation of relevant articles where necessary.
Electronic searches
We searched the following electronic databases from their inception.
Cochrane Stroke Group Trials Register (last searched 7 June 2021; Appendix 1).
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 7) in the Cochrane Library (searched June 2021) (Appendix 2).
MEDLINE (from 1946 to June 2021) (Ovid) (Appendix 3).
Embase (from 1980 to June 2021) (Ovid) (Appendix 4).
CINAHL (Cumulative Index to Nursing and Allied Health Literature; from 1937 to June 2021) (EBSCO) (Appendix 5).
AMED (from 1985 to June 2021) (EBSCO) (Appendix 6).
PsycINFO (from 1987 to June 2021) (EBSCO) (Appendix 7).
Open Grey (www.opengrey.eu/) (July 2020) (Appendix 8) (search not updated in June 2021, as the site has been archived).
OTSeeker (www.otseeker.com/) (June 2021) (Appendix 9).
PEDro: Physiotherapy Evidence Database (www.pedro.org.au) (July 2021) (Appendix 10).
REHABDATA (National Rehabilitation Information Centre) (www.naric.com/?q=REHABDATA) (July 2021) (Appendix 11).
ProQuest Dissertations & Theses (www.proquest.com/) (June 2021) (Appendix 12).
We developed the MEDLINE search strategy (Appendix 3) with the help of the Cochrane Stroke Group Information Specialist and adapted it for the other databases. We searched for all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
We also searched the following trials registers and registries.
ClinicalTrials.gov (www.clinicaltrials.gov/) (June 2021) (Appendix 13).
Stroke Trials Registry (www.strokecenter.org/trials/) (July 2018) (Appendix 1) (unable to update this search beyond July 2018, as the website was unavailable).
EU Clinical Trials Register (www.clinicaltrialsregister.eu) (June 2021) (Appendix 14).
ISRCTN Registry (www.isrctn.com/) (June 2021) (Appendix 15).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) portal (www.who.int/ictrp/en/) (June 2021) (Appendix 16).
Searching other resources
We handsearched the reference lists of all identified studies and systematic reviews for any further potentially eligible studies. In addition, we contacted key study authors to obtain any missing or additional trial data.
We undertook reference searching using Web of Science Cited Reference Search for all included studies to identify any further relevant trials.
Data collection and analysis
Selection of studies
We collated the search results and removed duplicates prior to screening, using the method described by Bramer 2016. One review author (BC) screened the titles of the studies retrieved via the searching process and excluded obviously irrelevant studies. Two review authors (BC, JB) then independently screened titles and abstracts of the remaining studies, excluding those that did not meet the selection criteria. We retrieved the full‐text articles for the remaining references, and two review authors (BC, JW) independently screened the full‐text articles and identified studies for inclusion and recorded reasons for exclusion of ineligible studies. Where necessary, we contacted study authors for further information. We resolved any disagreements through discussion and, when required, consulted a third review author (JB). We collated multiple reports of the same study, to ensure that no single study was duplicated in reporting. We recorded the selection process and completed a PRISMA flow diagram (Moher 2009), Characteristics of included studies table, Characteristics of excluded studies table, Characteristics of studies awaiting classification table, and Characteristics of ongoing studies' table.
Data extraction and management
Two review authors (of BC, JB, JW), working independently, extracted data from each study. We used the 'template for intervention description and replication' (TIDieR) checklist and guide to extract data from eligible studies (Hoffmann 2014). In addition to the 12 points on the TIDierR checklist, we also included information on study eligibility, study participants, outcomes measured (including time points), and a 'miscellaneous' section (which included information such as funding sources, key conclusions from the study authors, references to other relevant studies, correspondence required, and any other comments by the review author). We included detailed information on time spent in rehabilitation in section eight of the TIDieR checklist, entitled 'When and how much'. Prior to commencing data extraction, we piloted the adapted TIDieR checklist to ensure the tool was extracting the data required and that review authors were using the tool comparably.
Where there were discrepancies in the data extraction, the two review authors who had extracted the data resolved them via discussion, with the option to involve the third review author if required.
Assessment of risk of bias in included studies
Two review authors (of BC, JB, JW), working independently, assessed risk of bias for all included study outcomes immediately after intervention at medium‐term follow‐up and at long‐term follow‐up (where reported) using the revised version of the Cochrane's tool for assessing risk of bias, the RoB 2 (Higgins 2021a; Sterne 2019). We resolved any disagreements by discussion between the two review authors who had assessed risk of bias for the study outcome, with the option to involve the third review author. Using the Word version of the tool (9 October 2018), we assessed risk of bias according to the following domains.
Risk of bias arising from the randomisation process.
Risk of bias due to deviations from the intended interventions.
Risk of bias due to missing outcome data.
Risk of bias in measurement of the outcome.
Risk of bias in selection of the reported result.
Judgements were derived for each of the relevant study outcomes using the signalling questions outlined in the RoB 2 Guidance 2019. This resulted in a domain‐level judgement of low risk of bias, high risk of bias, or some concerns. Domain‐level judgments contributed to an overall assessment of risk of bias for each included study outcome. All studies were included in the analyses, irrespective of their risk of bias.
In this review, we were interested in both the effect of assignment and the effect of adherence to intervention. We selected the effect of assignment to intervention as our primary interest, which contributes to the overall risk of bias judgement for each study outcome. We made this selection because our primary objective was to establish if more time spent in rehabilitation resulted in greater improvement by comparing assignment to more rehabilitation with assignment to less rehabilitation. The included RCTs were designed to test the effect of assignment. However, we acknowledge that adherence to the intended amount of intervention could affect outcomes. If participants assigned to more rehabilitation do not receive the intervention as intended, the difference in the amount of time between the more rehabilitation group and the less rehabilitation group could be negligible. This leads to indirectness due to the intervention (Guyatt 2011), increasing the likelihood of a study accepting the null hypothesis. For this reason, we also assessed the risk of bias pertaining to adherence to intervention. The judgements made did not contribute to the overall risk of bias, but were described and discussed, and a sensitivity analysis undertaken to examine the effect of excluding studies at high risk of bias due to the effect of adherence to intervention (in addition to the sensitivity analyses described below).
When assessing study outcomes for risk of bias due to missing outcome data, we used a threshold of 90% available participant data to return a judgement regarding the extent of missing data. This was because the included studies were small, which is common for rehabilitation studies.
The consensus decisions for the signalling questions for each risk of bias were entered into a Word version of the tool, aggregated into one document, saved as a PDF, and uploaded onto the Cochrane Stroke Group server.
Measures of treatment effect
For continuous outcomes using different scales of measurement (ADL measures, upper and lower limb activity measures, and upper and lower limb impairment measures), we calculated pooled standardised mean differences (SMDs) and 95% confidence intervals (CIs). We expressed dichotomous outcomes (SAE/death) as risk ratios (RR) with 95% CIs.
Unit of analysis issues
We have not considered unit of analysis issues in relation to cluster‐randomised trials as none were included.
In the event of studies that included multiple intervention groups, we included the groups that met the criteria for this review and excluded groups that did not. Where studies included multiple intervention groups that met the criteria for this review, we treated the group that received the least amount of therapy as the control group and 'split' this group (in terms of number of participants) to create multiple pair‐wise comparisons for that study. The control group was split to avoid the double‐counting of participants (Higgins 2021b).
As outcome measures were pooled, if studies included more than one measure of the same category (e.g. if studies used more than one activity measure of the upper limb), we selected the measure that reported the most data. If there were measures with equal amounts of data, we selected the measure listed first in the study.
If studies included more than one measurement within a time point of interest (e.g. if they measured outcomes at both three months and six months postintervention, both of which we would classify as medium‐term outcomes), we selected the first reported relevant outcomes within the time point of interest only.
Dealing with missing data
We contacted study authors to obtain any outcome data missing from the included studies, which was not accounted for within the study report. If it was not possible to obtain missing data, we attempted to determine the reason for missing data from study authors, to establish if data were 'missing at random' or 'missing not at random'.
If data were 'missing at random', we analysed the available data and ignored missing data. If data were 'missing not at random', we planned to impute the last observation carried forward and conduct a sensitivity analysis to determine the effect of missing data.
The potential impact of missing data is discussed later in this review.
Assessment of heterogeneity
We visually inspected the forest plots to determine the overlap in the CIs of the studies. Poor overlap is likely to indicate statistical heterogeneity (Deeks 2021). In addition, we used the I² statistic to quantify heterogeneity in the study results (Higgins 2003). If the I² statistic was greater than 50%, we considered this to represent substantial heterogeneity (Deeks 2021).
Where there was substantial heterogeneity, we explored the possible reasons for this by examining the trials in terms of their design, risk of bias, clinical settings, interventions, and participants involved. We analysed possible sources of heterogeneity by undertaking subgroup analyses.
Assessment of reporting biases
We attempted to minimise the effect of reporting bias by using a comprehensive search strategy. Where meta‐analyses included at least 10 studies, we used funnel plots of the primary and secondary outcomes to provide a visual inspection of whether treatment estimates were associated with the study size (Page 2021).
In addition, we considered reporting bias in terms of unavailable data within included studies (unavailable due to the P value, magnitude or direction of the results). We assessed this by reviewing the outcomes measured by each study, in comparison to their protocol and any other available reports of the study (e.g. conference publications, PhD theses etc.). We recorded any unreported outcomes, which likely were measured in the study (Page 2021).
Data synthesis
We conducted meta‐analyses using RevMan Web (RevMan Web 2019), following the guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). One author (BC) entered the data into RevMan Web and a second author (SE) checked the accuracy of this. We resolved disagreements through discussion. Analysis included all eligible study outcomes, irrespective of their risk of bias.
We used a random‐effects meta‐analysis, regardless of the level of heterogeneity between studies. If the studies are heterogeneous, then this is the appropriate model to use. However, if heterogeneity is low, a random‐effects model will return very similar results to a fixed‐effect model (Deeks 2021).
To address the first objective, we undertook meta‐analyses for each of our primary and secondary outcomes at our three time points of interest (immediately after intervention, medium‐term follow‐up, and long‐term follow‐up).
To address the second objective of the review, we conducted subgroup analyses for each of our primary and secondary outcomes, immediately after intervention. We compared studies with a larger difference between groups (in terms of total time spent in rehabilitation) to those with a smaller difference between groups. We used a median split based on differences in amount of time spent in rehabilitation between groups to determine the subgroups. When there was an uneven number of studies, the position of the split was determined by how great the difference was between the middle studies, thereby grouping the studies that were most similar in terms of amount of therapy provided. In addition to this, we produced scatter plots of difference in total amount of time spent in rehabilitation plotted against the estimated treatment effect (SMD).
To address the third objective of this review, we conducted subgroup analyses for each of our primary and secondary outcomes, immediately after intervention. We compared studies with a larger difference between groups in terms of number of minutes of rehabilitation provided per week to those with a smaller difference between groups in terms of number of minutes of rehabilitation provided per week. In addition to this, we produced scatter plots of difference in number of minutes spent in rehabilitation per week plotted against the estimated treatment effect (SMD).
We created scatter plots using Microsoft Excel.
Subgroup analysis and investigation of heterogeneity
Where there was the required information, we stratified the studies to analyse possible sources of heterogeneity using the following characteristics.
-
Time since stroke. This was to examine whether more time spent in rehabilitation had a different effect, dependent on stroke chronicity, by comparing:
studies providing rehabilitation within the first six months since stroke;
studies providing rehabilitation after six months since stroke.
-
Hours of interventional therapy provided per week. This was to examine the effect of more time spent in therapy per week on outcomes, by comparing:
studies in which the experimental group received less than five hours of interventional treatment per week;
studies in which the experimental group received more than five hours (but less than 10 hours) of interventional treatment per week;
studies in which the experimental group received more than 10 hours (but less than 20 hours) of interventional treatment per week;
studies in which the experimental group received 20 hours or more of interventional treatment per week.
-
Type of intervention. This was to examine whether the type of intervention provided alters the effect of time spent in therapy (i.e. if more time spent in one type of therapy has a greater benefit than more time spent in a different type of therapy). The following two comparisons were made:
upper limb therapy versus other therapy;
electromechanical technology versus no electromechanical technology.
Sensitivity analysis
We performed the following sensitivity analyses for objective one at our primary time point of interest (immediately after intervention): removal of high risk of bias studies, removal of studies at high risk of bias due to the effect of adherence to intervention, and removal of studies with both high risk of overall bias and high risk of bias due to the effect of adherence to intervention. The latter sensitivity analyses were performed as risk of bias due to the effect of adherence to intervention did not contribute to the overall risk of bias.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables to present the findings of our first objective, using the six outcomes identified: ADL, activity measures of the upper limb, activity measures of the lower limb, motor impairment measures of the upper limb, motor impairment measures of the lower limb, and SAEs/death. We report the results of the outcomes measures immediately after intervention, which was our primary time point of interest.
For each outcome, we reported the number of participants that contributed to the finding, the relative effect, direction of effect, and the certainty of the evidence. We analysed the certainty of the evidence using the evidence grading system developed by the GRADE collaboration (Schünemann 2013), described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021a). The overall risk of bias (assessed by the RoB2 tool) contributed to the GRADE assessment.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies tables.
Results of the search
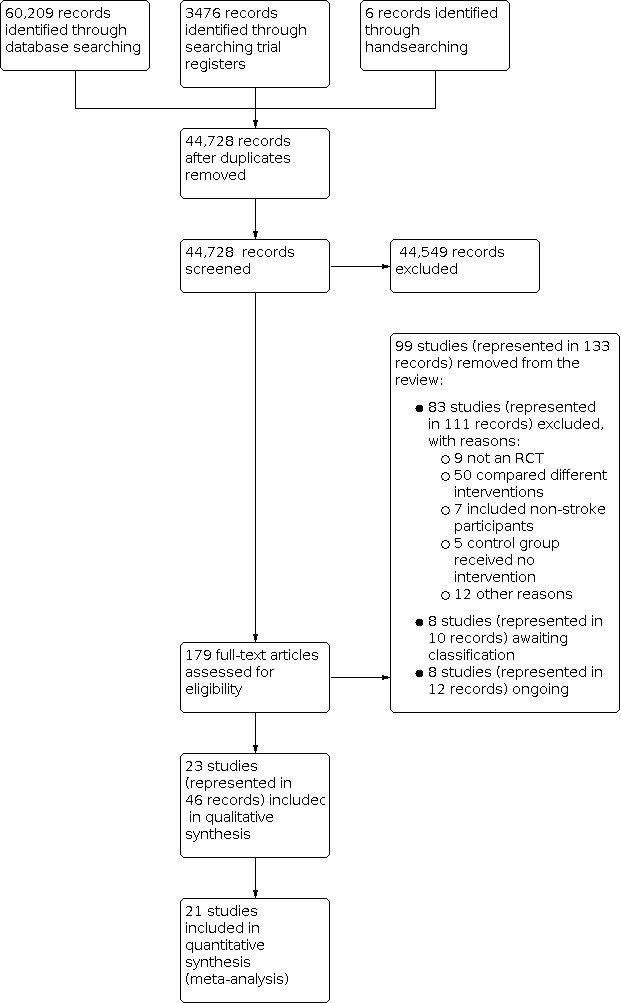
Searches, undertaken in June and July 2021, identified 44,728 unique records for screening. Following title screening, we excluded 44, 549 records, leaving 1492 for title and abstract review. From these records, we reviewed the full text of 179 papers and identified 23 studies (46 records) that met the criteria for this review. Figure 1 outlines the study selection process.
1.
Included studies
Twenty‐three studies analysed data from 1458 participants in study groups that met the criteria for this review (see Characteristics of included studies table). Two studies were not included in the analysis because missing information could not be obtained from the study authors (Page 2011; Wang 2011). Therefore, the quantitative synthesis comprised 21 parallel designed randomised clinical trials, which analysed 1412 participants. Five studies included two or more intervention groups that met the criteria for this study (Han 2013a; Hunter 2011a; Lang 2016a; Page 2012a; Winstein 2019a), therefore, 27 pair‐wise comparisons are presented. Each pair‐wise comparison that has originated from the same study, can be separately identified (e.g. Lang 2016a, Lang 2016b, Lang 2016c). Please see the notes section for the respective studies in the Characteristics of included studies table for how these pair‐wise comparisons were defined.
Time spent in rehabilitation and rehabilitation schedule
Time spent in rehabilitation varied between the 21 studies, see Table 4 for a summary.
1. Intervention regimens by study.
| Study | Minutes per session | Session frequency | Duration of rehabilitation |
| Abdullahi 2018 | Not controlled (control received half the number of repetitions to the intervention group) |
5 days per week | 4 weeks |
| Ada 2013 | 30 | 3 × weekly | 8 weeks (control) 16 weeks (intervention) |
| Burgar 2011 | 60 | Up to 15 over 3 weeks (control) Up to 30 over 3 weeks (intervention) |
3 weeks |
| Cooke 2010b | 23 (control) 57.5 (intervention) |
4 days per week | 6 weeks |
| Donaldson 2009 | 6.4 (control) 36.4 (intervention) |
4 days per week | 6 weeks |
| Dromerick 2009 | 120 (control) 180 (intervention) |
5 days per week | 2 weeks |
| English 2015 | Up to 90 | 5 days per week (control) 7 days per week (intervention) | 4 weeks |
| GAPS 2004 | 35 (control) 63 (intervention) |
5 days per week | Uncontrolled |
| Han 2013a | 60 (control) 120 (intervention 1) 180 (intervention 2) |
5 days per week | 6 weeks |
| Hsieh 2012 | Not controlled (control received half the number of repetitions to the intervention group) |
5 days per week | 4 weeks |
| Hsu 2010 | 30 (control) 60 (intervention) |
5 days per week | 4 weeks |
| Hunter 2011a | 30 (control) 60 (intervention 1) 120 (intervention 2) |
7 days per week | 2 weeks |
| Kowalczewski 2007 | 60 | 1 day per week (control) 5 days per week (intervention) |
3–4 weeks |
| Lang 2016a | 25.5 (control) 37.5 (intervention 1) 49.3 (intervention 2) 54.7 (intervention 3) |
4 days per week | 8 weeks (control, intervention 1 and 2) 9 weeks (median – intervention group 3) |
| Lincoln 1999 | 30–45 (control) 54–69 (intervention) |
5 days per week | 5 weeks |
| Page 2012a | 30 (control) 60 (intervention 1) 120 (intervention 2) |
5 days per week | 8 weeks |
| Partridge 2000 | 30 (control) 60 (intervention) |
5 days per week | Uncontrolled |
| Smith 1981 | 3 half‐days 4 full days |
3 days per week (control) 4 days per week (intervention) |
Up to 6 months |
| Tong 2019 | < 90 (control) > 180 (intervention) |
7 days per week | 10–14 days |
| Wang 2004 | 40 (control) 40 (intervention) |
2 sessions per day, 5 days per week in the first month, 3 days per week thereafter (control) 2 sessions per day, 5 days per week in the first month, 1 or 2 sessions per day, 5 days per week thereafter (intervention) |
6 months |
| Winstein 2019a | 60 (control) 120 (intervention 1) 240 (intervention 2) |
5 days per week (for 1 week in every month) | 3 months |
Nineteen studies reported time (minutes) spent in rehabilitation. Seven report time allocated for therapy (Dromerick 2009; Han 2013a; Hsu 2010; Page 2012a; Partridge 2000; Wang 2004; Winstein 2019a), but not amount of therapy delivered. We have presumed that time allocated was the same as time delivered as there were no issues concerning delivery reported. The remaining 12 studies report average (mean or median) minutes of rehabilitation delivered. Two studies reported the number of repetitions (Abdullahi 2018; Hsieh 2012). In both studies, one intervention group received double the number of repetitions as the other intervention group, which we took to represent a different amount of time spent in rehabilitation.
The difference in total minutes (per study) of rehabilitation between control and intervention groups ranged from 186 minutes (English 2015) to 6160 minutes (Wang 2004), with a median difference of 840 minutes. Minutes of rehabilitation provided per week ranged from 90 (Ada 2013) to 1288 (Tong 2019). Days per week on which rehabilitation was provided ranged from three (Ada 2013) to seven (English 2015; Hunter 2011a; Tong 2019), but 12 studies provided rehabilitation five days per week (Abdullahi 2018; Dromerick 2009; GAPS 2004; Han 2013a; Hsieh 2012; Hsu 2010; Kowalczewski 2007; Lincoln 1999; Page 2012a; Partridge 2000; Wang 2004; Winstein 2019a). Duration of rehabilitation ranged from two weeks (Dromerick 2009; Hunter 2011a; Tong 2019) to six months (Smith 1981; Wang 2004).
Fifteen studies compared groups that received a different amount of rehabilitation per day (Abdullahi 2018; Cooke 2010b; Donaldson 2009; Dromerick 2009; GAPS 2004; Han 2013a; Hsieh 2012; Hsu 2010; Hunter 2011a; Lang 2016a; Lincoln 1999; Page 2012a; Partridge 2000; Tong 2019; Winstein 2019a). The difference in minutes of rehabilitation per day between control and intervention groups ranged from 12 minutes (Lang 2016a) to 180 minutes (Winstein 2019a), with a median difference of 30 minutes. Two studies compared groups that received a different number of days per week of rehabilitation (English 2015; Kowalczewski 2007). Two studies compared more minutes of rehabilitation over more days with fewer minutes over fewer days (Smith 1981; Wang 2004). One study compared different durations of rehabilitation (Ada 2013), and one study reported the amount of therapy provided over three weeks, without specifying a schedule (Burgar 2011).
Nature of intervention in studies
Nature of intervention in studies included physiotherapy (physical therapy) or occupational therapy, or both (Cooke 2010b; Donaldson 2009; English 2015; GAPS 2004; Lincoln 1999; Partridge 2000; Smith 1981; Wang 2004), neuromuscular electrical stimulation (Hsu 2010; Kowalczewski 2007; Page 2012a), robot‐assisted training (Burgar 2011; Hsieh 2012), CIMT (Abdullahi 2018; Dromerick 2009), task‐specific training (Lang 2016a; Winstein 2019a), mobilisation and tactile stimulation (Hunter 2011a), upper limb rehabilitation (Han 2013a), treadmill training (Ada 2013), and mobilisation (Tong 2019).
In grouping interventions, 13 studies provided upper limb rehabilitation (Abdullahi 2018; Burgar 2011; Donaldson 2009; Dromerick 2009; Han 2013a; Hsieh 2012; Hsu 2010; Hunter 2011a; Kowalczewski 2007; Lang 2016a; Lincoln 1999; Page 2012a; Winstein 2019a), five studies provided general rehabilitation (English 2015; GAPS 2004; Partridge 2000; Smith 1981; Wang 2004), two studies provided mobilisation training (Ada 2013; Tong 2019), and one study provided lower limb training (Cooke 2010b). In an alternative grouping, six studies provided rehabilitation using electromechanical technology (Ada 2013; Burgar 2011; Hsieh 2012; Hsu 2010; Kowalczewski 2007; Page 2012a), and 15 studies did not use electromechanical technology (Abdullahi 2018; Cooke 2010b; Donaldson 2009; Dromerick 2009; English 2015; GAPS 2004; Han 2013a; Hunter 2011a; Lang 2016a; Lincoln 1999; Partridge 2000; Smith 1981; Tong 2019; Wang 2004; Winstein 2019a).
Participant characteristics
Characteristics of participants, including age, gender, and time since stroke are summarised in Table 5.
2. Characteristics of study participants.
| Study |
Mean age (years) |
Gender (% male) |
Mean time poststroke | Side of weakness (% right‐sided weakness) |
| Abdullahi 2018 | 300 reps: 59.42 600 reps: 57.60 |
Unable to establish from information given | 300 reps: 22 days 600 reps: 14 days |
Unable to establish from information given |
| Ada 2013 | 2‐month group: 64 4‐month group: 70 |
2‐month group: 28% 4‐month group: 24% |
2‐month group: 20 months 4‐month group: 22 months |
2‐month group: 14% 4‐month group: 18% |
| Burgar 2011 | Robot low: 62.5 Robot high: 58.6 |
No information | Robot low: 17.3 days Robot high: 16.6 days |
Robot low: 53% Robot high: 47% |
| Cooke 2010b | CPT: 66.37 CPT+CPT: 67.46 |
CPT: 55% CPT+CPT: 63% |
CPT: 36.76 days CPT+CPT: 32.43 days |
CPT: 45% CPT+CPT: 37% |
| Donaldson 2009 | CPT: 72.6 CPT+CPT: 73.3 |
CPT: 50% CPT+CPT: 50% |
CPT: 13.4 days CPT+CPT: 25.6 days |
CPT: 50% CPT+CPT: 40% |
| Dromerick 2009 | Low CIMT: 62.8 High CIMT: 64.5 |
Low CIMT: 32% High CIMT: 56% |
Low CIMT: 8.8 days High CIMT: 9.94 days |
Low CIMT: 47.4% High CIMT: 56.3% |
| English 2015 | 5 day: 68.2 7 day: 71.9 |
5 day: 55% 7 day: 61% |
5 day: 28.7 days 7 day: 25.0 days |
5 day: 40.4% 7 day: 42.7% |
| GAPS 2004 | Standard physiotherapy: 67 Augmented physiotherapy: 68 |
Standard physiotherapy: 49% Augmented physiotherapy: 69% |
Standard physiotherapy: 25 days Augmented physiotherapy: 22 days |
Standard physiotherapy: 57% Augmented physiotherapy: 54% |
| Han 2013a | Group A (1 hour): 52.4 Group B (2 hours): 53.7 Group C (3 hours): 44.6 |
Group A: 70% Group B: 80% Group C: 80% |
Group A: 41.4 days Group B: 42.9 days Group C: 38.3 days |
Group A: 10% Group B: 10% Group C: 20% |
| Hsieh 2012 | Low RT: 52.2 High RT: 56.5 |
Low RT: 72% High RT: 61% |
Low RT: 23.3 months High RT: 28.7 months |
Low RT: 50% High RT: 50% |
| Hsu 2010 | Low NMES: 62 High NMES: 60.2 |
Low NMES: 15% High NMES: 15% |
Low NMES: 21 days High NMES: 23.3 days |
Low NMES: 54.5% High NMES: 40.9% |
| Hunter 2011a | 30 minutes: 73.3 60 minutes: 72.9 120 minutes: 72.5 |
30 minutes: 61% 60 minutes: 42% 120 minutes: 45% |
All participants were within 8–84 days postevent | 30 minutes: 22% 60 minutes: 21% 120 minutes: 35% |
|
Kowalczewski 2007 |
Low‐intensity FES‐ET: 61.7 High‐intensity FES‐ET: 59.4 |
Low‐intensity FES‐ET: 67% High‐intensity FES‐ET: 40% |
Low‐intensity FES‐ET: 1.6 months High‐intensity FES‐ET: 1.6 months |
Low‐intensity FES‐ET: 22% High‐intensity FES‐ET: 40% |
| Lang 2016a | 3.2k reps group: 59.9 6.4k reps group: 62.1 9.6k reps group: 60.0 Individualised maximum group: 60.9 |
3.2k reps group: 67% 6.4k reps group: 77% 9.6k reps group: 52% Individualised maximum group: 62% |
3.2k reps group: 12.0 months 6.4k reps group: 13.0 months 9.6k reps group: 13.0 months Individualised maximum group: 11.5 months |
3.2k reps group: 52% 6.4k reps group: 45% 9.6k reps group: 48% Individualised maximum group: 62% |
| Lincoln 1999 | Routine physiotherapy: 73 Qualified physiotherapy: 73 |
Routine physiotherapy: 47% Qualified physiotherapy: 54% |
All participants were between 1 and 5 weeks poststroke on entry to the study |
Routine physiotherapy: 40% Qualified physiotherapy: 50% |
| Page 2012a | 57.6 | 47% | 53.8 months | 59% |
| Partridge 2000 | 76.5 | 46% | Unable to establish, but setting was acute inpatient | 46% |
| Smith 1981 | Group 1: 63 Group 2: 66 |
Group 1: 67% Group 2: 73% |
Group 1: 35 days Group 2: 41 days |
Unable to establish from information given |
| Tong 2019 | ERM: 62.1 EIM: 60.9 |
ERM: 71.3% EIM: 76.7% |
ERM: 41.0 hours EIM: 38.0 hours |
Unable to establish from information given |
| Wang 2004 | Experimental: 65.13 Control: 65.72 |
Experimental: 55% Control: 53% |
Unable to establish, but setting was acute inpatient | Unable to establish from information given |
| Winstein 2019a | 15 hours: 57.0 30 hours: 61.3 60 hours: 60.64 |
15 hours: 90% 30 hours: 70% 60 hours: 73% |
15 hours: 2.93 years 30 hours: 2.45 years 60 hours: 1.96 years |
15 hours: 60% 30 hours: 70% 60 hours: 27% |
Information provided either by included study group, or overall study, dependent on what was reported in the paper.
CPT: conventional physiotherapy; CIMT: constraint‐induced movement therapy; EIM: early intensive mobilisation; ERM: early routine mobilisation; FES‐ET: functional electric stimulation‐assisted exercise therapy; NMES: neuromuscular electrical stimulation; reps: repetitions; RT: robot‐assisted therapy.
Time since stroke
Sixteen studies included participants in the first six months following stroke (Abdullahi 2018; Burgar 2011; Cooke 2010b; Donaldson 2009; Dromerick 2009; English 2015; GAPS 2004; Han 2013a; Hsu 2010; Hunter 2011a; Kowalczewski 2007; Lincoln 1999; Partridge 2000; Smith 1981; Tong 2019; Wang 2004).
Five studies included participants more than six months poststroke (Ada 2013; Hsieh 2012; Lang 2016a; Page 2012a; Winstein 2019a).
Stroke severity or level of impairment
Comparison of stroke severity or level of impairment due to stroke was limited, due to variations in measurement.
Of the 21 studies, four included objective measurement of stroke severity. Three reported the National Institute of Health Stroke Scale (NIHSS) (Dromerick 2009; Tong 2019; Wang 2004), and one reported lesion volume (Winstein 2019a). Of studies that reported NIHSS scores, the mean scores were in the mild to moderate range of five to 14 (Brott 1989). Winstein 2019a reported lesion volume in centimetres cubed. We were unable to use this information to classify stroke severity.
Of the 21 studies, 14 included a measure of baseline physical impairment, 11 upper limb impairment, one lower limb impairment, and two global physical impairment. Of the 11 that reported upper limb impairment, eight used the FM‐UE. Using the Woytowicz 2017 classifications, two studies had a moderate–mild mean FM‐UE (Hsieh 2012; Winstein 2019a), three were moderate‐severe (Abdullahi 2018; Burgar 2011; Page 2012a), and three were severe (Han 2013a; Hsu 2010; Kowalczewski 2007). The remaining studies that report baseline upper limb impairment use myometer measurement (Donaldson 2009; Lincoln 1999), and the Upper Extremity Motricity Index (Hunter 2011a), which we were unable to classify. Cooke 2010b reported baseline lower limb impairment using myometer measurement. The two studies that used global measures of physical impairment used the Motricity Index (GAPS 2004), and the Fugl‐Meyer (full scale) (Wang 2004). Wang 2004 classified participants as severe for motor impairments (Duncan 1994). We were unable to categorically classify the Motricity Index.
Of the 21 studies, five studies did not include either measures of stroke severity or impairment (Ada 2013; English 2015; Lang 2016a; Partridge 2000; Smith 1981).
No studies reported non‐physical measures of impairment. However, 10 studies excluded people with cognitive impairment (Abdullahi 2018; Ada 2013; Burgar 2011; Dromerick 2009; GAPS 2004; Kowalczewski 2007; Lang 2016a; Page 2012a; Partridge 2000; Winstein 2019a). Seven studies excluded people with communication impairment (Abdullahi 2018; Ada 2013; Donaldson 2009; Dromerick 2009; GAPS 2004; Hunter 2011a; Tong 2019), and four studies excluded people with visual inattention/neglect (Abdullahi 2018; Donaldson 2009; Dromerick 2009; Kowalczewski 2007). Definition of these impairments varied or were not clearly defined.
Rehabilitation setting
Fourteen studies provided rehabilitation in an inpatient setting (Burgar 2011; Cooke 2010b; Donaldson 2009; Dromerick 2009; English 2015; GAPS 2004; Han 2013a; Hsu 2010; Hunter 2011a; Kowalczewski 2007; Lincoln 1999; Partridge 2000; Tong 2019; Wang 2004). These were all studies of participants in the first six months following stroke. Five studies provided intervention in the community/outpatient setting (Abdullahi 2018; Ada 2013; Lang 2016a; Page 2012a; Smith 1981). Both Smith 1981 and Abdullahi 2018 studied participants as outpatients following their discharge from the inpatient setting, within the first six months after stroke. Ada 2013, Lang 2016a, and Page 2012a studied participants more than six months following stroke. In Page 2012a, participants were seen in their own homes; the other studies treated participants in outpatient/community settings. The remaining studies did not describe rehabilitation setting (Hsieh 2012; Winstein 2019a), but as they are both of participants more than six months after stroke, it is expected that they were undertaken in outpatient/community settings.
Included groups from studies
We included all participant groups from six of the included studies (GAPS 2004; Han 2013a; Kowalczewski 2007; Lang 2016a; Partridge 2000; Wang 2004). Of the remaining 15 studies, not all participant groups met our study criteria, therefore, these participant groups were excluded from the analysis. In 12 studies, one intervention group received a different intervention, compared to two (or more) groups that received different amounts of the same intervention (Abdullahi 2018; Burgar 2011; Cooke 2010b; Donaldson 2009; Dromerick 2009; English 2015; Hsieh 2012; Hsu 2010; Hunter 2011a; Lincoln 1999; Page 2012a; Tong 2019). In the remaining three studies, a control group received no rehabilitation, compared to two intervention groups that received different amounts of the same treatment (Ada 2013; Smith 1981; Winstein 2019a).
Excluded studies
We excluded 83 studies (111 records) following full review (see Characteristics of excluded studies table). Studies were excluded for various reasons including comparing different types of rehabilitation (not different amounts of the same rehabilitation), comparing rehabilitation with no rehabilitation and inclusion of non‐stroke participants.
Studies awaiting classification
Eight studies are awaiting classification (see Characteristics of studies awaiting classification table). These are predominantly conference proceedings, for which we have been unable to obtain the required detail for inclusion.
Ongoing studies
Eight studies are ongoing (see Characteristics of ongoing studies table).
Risk of bias in included studies
Risk of bias assessments for each outcome, including all domain judgements and support for judgement, is located in the risk of bias section (located in the Characteristics of included studies table), and at the side of all forest plots. To access further detailed risk of bias assessment data, please use the following link: apps.ccbs.ed.ac.uk/csrg/cochranestrokedocuments/Risk_of_Bias_Assessments_FINAL.pdf.
Risk of bias judgements within studies were generally consistent, with the following exceptions. In four studies, there was a greater risk of bias for follow‐up measures, due to missing data (participants lost to follow‐up) (Burgar 2011; Donaldson 2009; Lincoln 1999; Partridge 2000). In two studies, the risk of bias differed within the study, due to the outcome measure used (Lang 2016a; Lincoln 1999). In one study, the risk of bias differed within the study, due to selection of the reported results (Winstein 2019a). In one study, the risk of bias differed within the study due to unexplained missing data for one outcome, but not the other (Cooke 2010b).
For domain five (risk of bias in the selection of reported results), most outcomes were judged as having at least some concerns. In order to judge potential bias, study protocols, written prior to study completion are required. For 15/21 studies, there was either no protocol available or the protocol provided insufficient detail to determine that the study was carried out as planned (Burgar 2011; Cooke 2010b; Donaldson 2009; GAPS 2004; Han 2013a; Hsieh 2012; Hsu 2010; Hunter 2011a; Kowalczewski 2007; Lang 2016a; Lincoln 1999; Page 2012a; Partridge 2000; Smith 1981; Wang 2004). In all cases, we contacted the study authors to request further information, but this information remained unavailable. One reason for the limited protocol availability may be due to the relatively recent practice of registering rehabilitation trials and publishing protocols.
As previously described, we selected the effect of assignment to intervention as our primary interest, when considering the risk of bias due to deviations from intended interventions (domain 2). However, we are also interested in the risk of bias pertaining to adherence to the intervention. The judgements made did not contribute to the overall risk of bias, but are herein described.
Both versions of this domain begin by asking if participants, carers, and people delivering rehabilitation were aware of group allocation during the trial. Notably, none of the studies blinded people delivering rehabilitation and just three studies reported that participants were unaware of their group allocation (Burgar 2011; Donaldson 2009; Partridge 2000). Lack of blinding of participants and personnel is common for rehabilitation studies due to the nature of interventions. This increased the likelihood of all studies being at high risk or having some concerns for this domain.
Assessment of risk of bias for the effect of adhering to the intervention was consistent within studies. Seven studies were at low risk of bias for effect of adhering to the intervention (Ada 2013; Han 2013a; Hsieh 2012; Lang 2016a; Page 2012a; Wang 2004; Winstein 2019a). The remaining 14 studies were at high risk of bias. In addition to the aforementioned lack of blinding, nine of these studies provided no information regarding co‐interventions (Abdullahi 2018; Cooke 2010b; Donaldson 2009; English 2015; Hunter 2011a; Kowalczewski 2007; Lincoln 1999; Partridge 2000; Smith 1981). Three studies provided no information about adherence to the intervention (Dromerick 2009; Hsu 2010; Partridge 2000), and five studies described issues with adherence to the intervention (Burgar 2011; GAPS 2004; Hunter 2011a; Lincoln 1999; Tong 2019). Three studies demonstrated more than one of these issues (Burgar 2011; Hunter 2011a; Lincoln 1999).
A brief summary of studies' overall risk of bias is presented with the results of the meta‐analyses.
In addition to the risk of bias in included studies, we assessed this review's risk of bias due to missing results (non‐reporting bias). Funnel plots are presented with the relevant analysis and a summary of potential non‐reporting bias is presented in Table 6. A brief summary of any possible missing results is presented with the results of the meta‐analyses for objective one. In addition, there were two studies we were unable to include, due to missing information that could not be obtained from study authors (Page 2011; Wang 2011).
3. Assessment of non‐reporting bias in studies.
| Study ID | Greater amount (n) | Lesser amount (n) | Synthesis assessed for risk of non‐reporting bias | |||||||||||
| ADL outcomes | Activity measures: UL | Activity measures: LL | Motor impairment: UL | Motor impairment: LL | SAE/death | |||||||||
| Imm | FU | Imm | FU | Imm | FU | Imm | FU | Imm | FU | Imm | FU | |||
| Ada 2013 | 34 | 34 | + | + | – | – | + | + | – | – | – | – | – | – |
| GAPS 2004 | 35 | 35 | + | + | ? | ? | + | + | – | – | – | – | – | + |
| English 2015 | 96 | 94 | + | – | – | – | + | – | – | – | – | – | + | – |
| Lang 2016a | 21 (3.2) | 22 (6.4) 21 (9.6) 21 (IM) |
+ | + | + | + | – | – | – | – | – | – | – | – |
| Abdullahi 2018 | 11 | 12 | + | + | + | + | – | – | + | + | – | – | – | – |
| Dromerick 2009 | 16 | 19 | + | + | + | + | – | – | – | – | – | – | – | – |
| Hunter 2011a | 19 (60) 20 (120) |
12 (30) | – | – | + | – | – | – | + | – | – | – | – | – |
| Hsu 2010 | 22 | 22 | – | + | + | + | – | – | + | + | – | – | – | – |
| Partridge 2000 | 54 | 60 | ? | ? | ? | ? | + | + | ? | ? | ? | ? | – | – |
| Lincoln 1999 | 94 | 95 | + | + | – | – | – | – | ? | ? | – | – | + | + |
| Page 2012a | 8 (60) 8 (120) |
9 (30) | + | ? | + | ? | – | – | + | ? | – | – | – | – |
| Han 2013a | 10 (120) 10 (180) |
10 (60) | + | ? | + | ? | – | – | + | ? | – | – | – | – |
| Wang 2004 | 36 | 38 | + | ? | ? | ? | ? | ? | – | ? | – | ? | – | – |
| Donaldson 2009 | 10 | 10 | – | – | + | + | – | – | + | + | – | – | – | – |
| Cooke 2010b | 35 | 38 | ? | ? | – | – | + | + | – | – | + | + | – | – |
| Burgar 2011 | 17 | 19 | + | + | + | + | – | – | + | + | – | – | – | – |
| Kowalczewski 2007 | 10 | 9 | + | + | + | + | – | – | + | + | – | – | – | – |
| Smith 1981 | 46 | 43 | – | – | ? | ? | – | – | – | – | – | – | – | + |
| Hsieh 2012 | 18 | 18 | + | ? | + | ? | – | – | + | ? | – | – | – | – |
| Winstein 2019a | 11 (60 hours) 10 (30 hours) |
10 (15 hours) | + | 0 | + | 0 | – | – | 0 | 0 | – | – | – | – |
| Tong 2019 | 86 | 80 | ? | + | ? | 0 | ? | 0 | ? | 0 | ? | 0 | – | – |
Key:
+ = a study result is available for inclusion in the synthesis.
0 = no study result is available for inclusion, (probably) because the P value, magnitude, or direction of the results generated were considered unfavourable to the study investigators.
– = no study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude, or direction of the results.
? = no study result is available for inclusion, and it is unclear if the outcome was assessed in the study.
ADL: activities of daily living; FU: follow‐up; IM: individualised maximum; Imm: immediate; LL: lower limb; n: number of participants; SAE: serious adverse events; UL: upper limb.
There are eight potentially eligible studies that are awaiting classification (see Characteristics of studies awaiting classification table). These studies did not include enough information to determine whether they meet the criteria for this review, and, to date, we have been unable to gather any further information about them. If unbeknown to us, some or all of these studies meet the criteria for this review, their non‐inclusion would result in further non‐reporting bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Objective one: to assess the effect of more time spent in the same type of rehabilitation on activity measures in people with stroke
See Table 1, Table 2 and Table 3.
We compared intervention groups that spent more time in rehabilitation with intervention groups that spent less time. Comparisons were undertaken for our primary and secondary outcome measures immediately after intervention, at medium‐term follow‐up (two weeks to six months after intervention has ended), and long‐term follow‐up (more than six months after treatment has ended).
Comparison 1: outcomes measured immediately after intervention
Analysis 1.1: activities of daily living outcomes (primary outcome)
There was no evidence of an effect for additional time spent in rehabilitation for ADL outcomes immediately after intervention (SMD 0.13, 95% CI −0.02 to 0.28; P = 0.09; I2 = 7%; 14 studies, 864 participants; very low‐certainty evidence; Analysis 1.1). Measures used included the Functional Independence Measure, Barthel Index, Motor Activity Log, Activities of Daily Living Index, Arm Motor Ability Scale, and the Adelaide Activities Profile.
1.1. Analysis.

Comparison 1: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – immediate outcomes, Outcome 1: Activities of daily living outcomes
Of the 19 comparisons included in this analysis, three were at low overall risk of bias, nine had some concerns regarding risk of bias, and seven were at high risk of bias.
With studies at high risk of bias removed, there remained no evidence of an effect. With studies at high risk of bias due to effect of adherence removed, there was evidence of an effect. This effect was lost when studies at high risk of overall bias and high risk of bias due to effect of adherence were excluded (see Table 7).
4. Sensitivity analyses to assess the effect of excluding studies at high risk of bias.
| Outcome | High overall risk excluded | High risk due to effect of adherence excluded | Both high overall risk and high risk due to effect of adherence excluded |
| 1.1 ADL outcomes | SMD 0.15, 95% CI −0.04 to 0.33;P = 0.12; I2 = 0%; 9 studies, 469 participants | SMD 0.32, 95% CI 0.11 to 0.52; P = 0.002; I2 = 0%; 8 studies, 401 participants | SMD 0.26, 95% CI −0.04 to 0.55; P = 0.09; I2 = 0%; 5 studies, 189 participants |
| 1.2 Activity measures of the upper limb | SMD 0.18, 95% CI −0.05 to 0.41; P = 0.13; I2 = 0%; 9 studies, 324 participants | SMD 0.20, 95% CI −0.11 to 0.52; P = 0.21; I2 = 4%; 5 studies, 195 participants | SMD 0.30, 95% CI −0.07 to 0.67; P = 0.12; I2 = 14%; 4 studies, 164 participants |
| 1.3 Activity measures of the lower limb | SMD 0.26, 95% CI −0.09 to 0.60; P = 0.14; I2 = 60%; 4 studies, 370 participants | SMD 0.36, 95% CI 0.02 to 0.70; P = 0.04; I2 = 0%; 2 studies, 134 participants | SMD 0.36, 95% CI 0.02 to 0.70; P = 0.04; I2 = 0%; 2 studies, 134 participants |
| 1.4 Motor impairment measures of the upper limb | SMD 0.28, 95% CI 0 to 0.56; P = 0.05; I2 = 10%; 8 studies, 251 participants | SMD 0.60, 95% CI 0.15 to 1.05; P = 0.008; I2 = 0%; 3 studies, 91 participants | SMD 0.60, 95% CI 0.15 to 1.05; P = 0.008; I2 = 0%; 3 studies, 91 participants |
| 1.5 Motor impairment measures of the lower limb | N/A | N/A | N/A |
| 1.6 Serious adverse events/death | RR 1.20, 95% CI 0.51 to 2.85; P = 0.68; I2 = 0%; 2 studies, 379 participants | N/A | N/A |
ADL: activities of daily living; CI: confidence interval; N/A: not applicable; RR: risk ratio; SMD: standardised mean difference.
Data from one included study were missing from this analysis. Smith 1981 included an ADL measure, but reported a change score. We contacted the study authors, but the raw data were no longer available.
Three studies may have assessed this outcome but did not report findings (see Table 6). A funnel plot for this outcome showed asymmetry, which may indicate non‐reporting bias (Figure 2).
2.

Analysis 1.2: activity measures of the upper limb
There was no evidence of an effect for additional time spent in rehabilitation for activity measures of the upper limb immediately after intervention (SMD 0.09, 95% CI −0.11 to 0.29; P = 0.36; I2 = 0%; 12 studies, 426 participants; very low‐certainty evidence; Analysis 1.2). Measures used included the Wolf Motor Function Test and the Action Research Arm Test.
1.2. Analysis.

Comparison 1: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – immediate outcomes, Outcome 2: Activity measures of the upper limb
Of the 18 comparisons included in this analysis, one was at low overall risk of bias, 13 had some concerns regarding risk of bias, and four were at high risk of bias.
Sensitivity analyses to explore the impact of excluding studies at high risk of bias demonstrated that there were no substantial changes from the original reported finding (see Table 7).
Data from two included studies were missing from this analysis (English 2015; Lincoln 1999). These studies presented the data in an incomparable format, and we were unable to obtain raw data from the study authors.
Five studies may have assessed this outcome but did not report findings (see Table 6). A funnel plot for this outcome shows asymmetry, which may indicate non‐reporting bias (Figure 3).
3.

Analysis 1.3: activity measures of the lower limb
There was no evidence of an effect for additional time spent in rehabilitation for activity measures of the lower limb immediately after intervention (SMD 0.25, 95% CI −0.03 to 0.53; P = 0.08; I2 = 48%; 5 studies, 425 participants; low‐certainty evidence; Analysis 1.3). Measures used included the six‐minute walk test and the Rivermead Mobility Index.
1.3. Analysis.

Comparison 1: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – immediate outcomes, Outcome 3: Activity measures of the lower limb
Of the five comparisons included in this analysis, two were at low overall risk of bias, two had some concerns regarding risk of bias, and one was at high risk of bias.
With studies judged at high risk of bias removed, there remained no evidence of an effect. When studies at high risk of overall bias and high risk of bias due to effect of adherence were excluded, there was evidence of an effect (see Table 7).
Two studies may have assessed this outcome but did not report findings (see Table 6).
Analysis 1.4: motor impairment measures of the upper limb
There was an effect in favour of additional time spent in rehabilitation for motor impairment measures of the upper limb immediately after intervention (SMD 0.32, 95% CI 0.06 to 0.58; P = 0.01; I2 = 10%; 9 studies, 287 participants; low‐certainty evidence; Analysis 1.4). Measures used included the FM‐UE and the Motricity Index (Arm section).
1.4. Analysis.

Comparison 1: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – immediate outcomes, Outcome 4: Motor impairment measures of the upper limb
Of the 12 comparisons included in this analysis, one was at low overall risk of bias, 10 had some concerns regarding risk of bias, and one was at high risk of bias.
With studies at high risk of bias removed, there was no evidence of an effect. When studies at high risk of overall bias and high risk of bias due to effect of adherence were excluded, there was evidence of an effect (see Table 7).
Data from one included study were missing from this analysis (Lincoln 1999). This study presented the data in an incomparable format, and we were unable to obtain raw data.
One study assessed this outcome but did not report findings and three further studies may have assessed this outcome but did not report findings (see Table 6).
To establish if the effect seen in this analysis represented a meaningful change to participants, we examined whether the change between baseline and outcome measures for each group within each study reached the minimal clinically important difference (MCID) for the outcome measure used. For studies that used the FM‐UE in the subacute stage, we used an MCID of 9 (Arya 2011), and for studies that used the FM‐UE in the chronic stage, we used an MCID of 4.25 (Page 2012). For studies that used grip strength, we used an MCID of 5 kg (Bohannon 2019; Lang 2008). One study (two comparisons) used the Arm section of the Motricity Index, for which we were unable to find an MCID.
Of the remaining 10 comparisons, four found a meaningful change in the 'more rehabilitation' group coupled with an absence of meaningful change in the 'less rehabilitation' group. This suggests that for 4/10 comparisons (Burgar 2011, Hsieh 2012, and two comparisons from Han 2013a), the additional rehabilitation provided resulted in a clinically meaningful difference in a measure of upper limb impairment, which was not achieved for those in the group that received less rehabilitation. The remaining six comparisons either did not find a clinically meaningful change for either group (three comparisons) or they found a clinically meaningful change for both groups (three comparisons). See Table 8 for a summary.
5. Minimal clinically important difference: outcome 1.4.
| Study |
Subacute/ chronic |
Measure | MCID for measure | More therapy group change from baseline | MCID reached? | Less therapy group change from baseline | MCID reached? |
| Abdullahi 2018 | Subacute | FM‐UE | 9 | 18.82 | Yes | 17.33 | Yes |
| Burgar 2011 | Subacute | FM‐UE | 9 | 14.4 | Yes | 6.8 | No |
| Donaldson 2009 | Subacute | Upper Limb Strength (myometer) | 5 kg | 19.3 kg | Yes | 34.75 kg | Yes |
| Han 2013a (1 hour vs 2 hours) | Subacute | FM‐UE | 9 | 11.5 | Yes | 6.3 | No |
| Han 2013b (1 hour vs 3 hours) | Subacute | FM‐UE | 9 | 18 | Yes | 6.3 | No |
| Hsieh 2012 | Chronic | FM‐UE | 4.25 | 5.22 | Yes | 3.22 | No |
| Kowalczewski 2007 | Subacute | FM‐UE | 9 | 6.4 | No | 3.6 | No |
| Page 2012a (30 minutes vs 60 minutes) | Chronic | FM‐UE | 4.25 | 1.3 | No | 1.9 | No |
| Page 2012b (30 minutes vs 120 minutes) | Chronic | FM‐UE | 4.25 | 4.2 | No | 1.9 | No |
| Hsu 2010 | Subacute | FM‐UE | 9 | 18 | Yes | 19.8 | Yes |
FM‐UE: Fugl‐Meyer Upper Extremity: MCID: minimal clinically important difference.
Analysis 1.5: motor impairment measures of the lower limb
There was an effect in favour of additional time spent in rehabilitation for motor impairment measures of the lower limb immediately after intervention (SMD 0.71, 95% CI 0.15 to 1.28; P = 0.01; 1 study, 51 participants; very low‐certainty evidence; Analysis 1.5). Measure used was peak knee flexion torque.
1.5. Analysis.

Comparison 1: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – immediate outcomes, Outcome 5: Motor impairment measures of the lower limb
This study was at high risk of bias.
Sensitivity analyses related to risk of bias could not be performed, as this left no studies in the analysis.
Two further studies may have assessed this outcome but did not report findings (see Table 6).
The study in this analysis used knee flexion peak torque to measure motor impairment of the lower limb. We were unable to find evidence for an MCID for knee flexion peak torque to determine if the effect seen in this analysis represented a meaningful change to participants.
Analysis 1.6: serious adverse events/death
There was no evidence of an increased risk of SAEs or death for additional time spent in rehabilitation (RR 1.20, 95% CI 0.51 to 2.85; P = 0.68; I2 = 0%; 2 studies, 379 participants; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – immediate outcomes, Outcome 6: Serious adverse events/death
Of the two comparisons included in this analysis, one was at low overall risk of bias and one had some concerns regarding bias.
As there were no studies at high risk of bias, there was no change to the result when studies at high risk of bias were removed. When studies judged as high risk of bias due to effect of adherence were removed, there were no remaining studies in the analysis (see Table 7).
We found no studies that may have planned to assess this outcome and had not reported findings.
Comparison 2: outcomes measured at medium‐term follow‐up (two weeks to six months after intervention)
Analysis 2.1: activities of daily living outcomes (primary outcome)
There was no evidence of an effect for additional time spent in rehabilitation for ADL outcomes at medium‐term follow‐up (SMD 0.01, 95% CI −0.15 to 0.16; P = 0.94; I2 = 0%; 10 studies, 673 participants; very low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – medium‐term outcomes, Outcome 1: Activities of daily living outcomes: medium‐term outcomes
Of the 12 comparisons included in this analysis, two were at low overall risk of bias, three had some concerns regarding risk of bias, and seven were at high risk of bias.
Data from one included study were missing from this analysis. English 2015 did not report follow‐up measures for the Functional Independence Measure. This was available in a data repository, but payment was required to access it, and we do not have funding for this.
One study assessed this outcome but did not report findings and six other studies may have assessed this outcome but did not report findings (Table 6). A funnel plot for this outcome showed asymmetry, which may indicate non‐reporting bias (Figure 4).
4.

Analysis 2.2: activity measures of the upper limb
There was no evidence of an effect for additional time spent in rehabilitation for activity measures of the upper limb at medium‐term follow‐up (SMD −0.02, 95% CI −0.36 to 0.33; P = 0.93, I2 = 30%; 7 studies, 218 participants; very low‐certainty evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – medium‐term outcomes, Outcome 2: Activity measures of the upper limb: medium‐term outcomes
Of the nine comparisons included in this analysis, one was at low overall risk of bias, six had some concerns regarding risk of bias, and two were at high risk of bias.
Data from two included studies were missing from this analysis. Lincoln 1999 presented these data in an incomparable format and study authors no longer have the raw data. English 2015 did not report follow‐up measures for the Wolf Motor Function Test. This was available in a data repository, but payment was required to access it, and we do not have funding for this.
Two studies assessed this outcome but did not report findings and seven other studies may have assessed this outcome but did not report findings (Table 6).
Analysis 2.3: activity measures of the lower limb
There was no evidence of an effect for additional time spent in rehabilitation for activity measures of the lower limb at medium‐term follow‐up (SMD 0.10, 95% CI −0.30 to 0.49; P = 0.63; I2 = 58%; 4 studies, 243 participants; very low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – medium‐term outcomes, Outcome 3: Activity measures of the lower limb: medium‐term outcomes
Of the four comparisons included in this analysis, one was at low risk of bias, two had some concerns regarding risk of bias, and one was at high risk of bias.
Data from one included study were missing from this analysis. English 2015 did not report follow‐up measures for the six‐minute walk test. This was available in a data repository, but payment was required to access it, and we do not have funding for this.
One study assessed this outcome but did not report findings and one study may have assessed this outcome but did not report findings (Table 6).
Analysis 2.4: motor impairment measures of the upper limb
There was no evidence of an effect for additional time spent in rehabilitation for motor impairment measures of the upper limb at medium‐term follow‐up (SMD −0.02, 95% CI −0.39 to 0.35; P = 0.90; I2 = 0%; 5 studies, 115 participants; very low‐certainty evidence; Analysis 2.4).
2.4. Analysis.

Comparison 2: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – medium‐term outcomes, Outcome 4: Motor impairment measures of the upper limb: medium‐term outcomes
Of the five comparisons included in this analysis, one was at low overall risk of bias, three had some concerns regarding risk of bias, and one was at high risk of bias.
Data from one included study were missing from this analysis. Lincoln 1999 presented the data in an incomparable format, and we were unable to obtain raw data.
Two studies assessed this outcome but did not report findings and six studies may have assessed this outcome but did not report findings (Table 6).
Analysis 2.5: motor impairment measures of the lower limb
There was no evidence of an effect for additional time spent in rehabilitation for motor impairment measures of the lower limb at medium‐term follow‐up (SMD 0.62, 95% CI −0.04 to 1.28; P = 0.07; 1 study, 37 participants; very low‐certainty evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – medium‐term outcomes, Outcome 5: Motor impairment measures of the lower limb: medium‐term outcomes
This study was at high risk of bias.
One study assessed this outcome but did not report findings, and two studies may have assessed this outcome but did not report findings (Table 6).
Analysis 2.6: serious adverse events/death
There was no increase in risk of SAEs or death for additional time spent in rehabilitation at medium‐term follow‐up (RR 1.32, 95% CI 0.63 to 2.76; P = 0.46; I2 = 2%; 3 studies, 344 participants; very low‐certainty evidence; Analysis 2.6).
2.6. Analysis.

Comparison 2: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – medium‐term outcomes, Outcome 6: Serious adverse events/death: medium‐term outcomes
Of the three comparisons included in this analysis, two were had some concerns regarding risk of bias, and one was at high risk of bias.
There did not appear to be any studies that measured this outcome but did not report findings (Table 6).
Comparison 3: outcomes measured at long‐term follow‐up (more than six months after intervention)
Analysis 3.1: activities of daily living outcomes (primary outcome)
There was no evidence of an effect for additional time spent in rehabilitation for ADL outcomes at long‐term follow‐up (SMD 0.09, 95% CI −0.39 to 0.57; P = 0.71; 1 study, 67 participants; low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – long‐term outcomes, Outcome 1: Activities of daily living outcomes: long‐term outcomes
This study was low risk of bias.
Activity measures of the upper limb
No studies reported activity measures of the upper limb at long‐term follow‐up (more than six months after intervention).
Analysis 3.2: activity measures of the lower limb
There was no evidence of an effect for additional time spent in rehabilitation for activity measures of the lower limb at long‐term follow‐up (SMD 0.16, 95% CI −0.32 to 0.64; P = 0.52; 1 study, 67 participants; low‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – long‐term outcomes, Outcome 2: Activity measures of the lower limb: long‐term outcomes
This study was low risk of bias.
Motor impairment measures of the upper limb
No studies reported motor impairment measures of the upper limb at long‐term follow‐up (more than six months after intervention).
Motor impairment measures of the lower limb
No studies reported motor impairment measures of the lower limb at long‐term follow‐up (more than six months after intervention).
Serious adverse events/death
No studies reported SAE/death at long‐term follow‐up (more than six months after intervention).
Objective two: to assess the effect of difference in total rehabilitation time (in minutes) on recovery of activity in people with stroke
We conducted subgroup analyses of the primary and secondary outcomes immediately after intervention. We compared studies with a larger difference between study groups (in terms of total time spent in rehabilitation) to those with a smaller difference between study groups. We used a median split based on differences in amount of time spent in rehabilitation between groups to determine the subgroups. When there was an uneven number of studies, the position of the split was determined by how great the difference was between the middle studies, in terms of time spent in rehabilitation, thereby grouping the studies that were most similar in terms of amount provided.
In addition to these subgroup analyses, we produced scatter plots of difference in total amount of time spent in rehabilitation (i.e. difference between study intervention groups in terms of total interventional minutes received over the duration of the study) plotted against the estimated treatment effect (SMD). Due to insufficient data points on the scatter plots we were unable to draw a line of best fit and the descriptive analysis given is tentative.
Analysis 4.1: activities of daily living outcomes (primary outcome)
The test for subgroup differences showed a significant difference between results of studies with larger (900 to 6160 minutes) versus smaller (186 to 852 minutes) difference in total minutes of rehabilitation between treatment groups for ADL outcomes, immediately after intervention (P = 0.02; Analysis 4.1). This was in favour of a larger difference in amount.
4.1. Analysis.

Comparison 4: Objective two: effect of total time spent in rehabilitation, Outcome 1: Activities of daily living outcomes: immediately after intervention
Analysis of the scatter plot for this outcome was limited by the small number of data points (Figure 5). Tentatively, it suggested a small positive association between difference in total amount of rehabilitation and ADL outcomes. There were two studies that were exceptions. Dromerick 2009 found a large but non‐significant benefit in favour of the control group. This study examined the effect of different amounts of CIMT early after stroke. They suggested that the effect seen could be due to fatigue or injury due to overtraining. Page 2012b found a greater benefit of more rehabilitation than all other studies.
5.
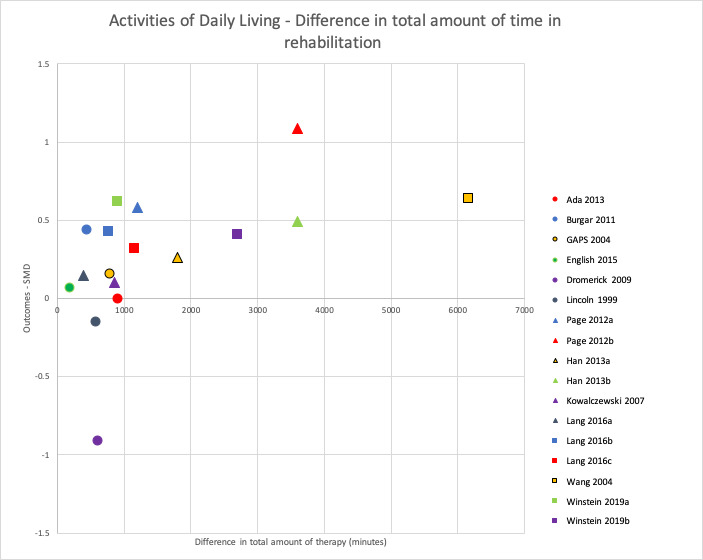
Scatter diagram plotting difference in total minutes of rehabilitation against outcomes (SMD) for activities of daily living, immediately after intervention
Analysis 4.2: activity measures of the upper limb
The test for subgroup differences showed that there was a significant difference between results of studies with larger (852 to 3600 minutes) versus smaller (198.8 to 762 minutes) difference in total minutes of rehabilitation between treatment groups for activity measures of the upper limb, immediately after intervention (P = 0.04; Analysis 4.2). This was in favour of a larger difference in amount.
4.2. Analysis.

Comparison 4: Objective two: effect of total time spent in rehabilitation, Outcome 2: Activity measures of the upper limb: immediately after intervention
Analysis of the scatter plot for this outcome was limited by the small number of data points, but suggested a positive association between difference in total amount of rehabilitation and improved activity measures of the upper limb (Figure 6). There are three outlying studies. Kowalczewski 2007 found a relatively large but non‐significant effect in favour of additional therapy, despite a relatively smaller difference in total amount of therapy. This study provided different amounts of functional electrical stimulation exercise therapy to two groups; one received intervention daily and one received intervention weekly. Winstein 2019b found a non‐significant effect in favour of control, despite a large difference in amount of time spent in therapy. Their study investigated the effect of an accelerated skill acquisition programme for people in the chronic stage following stroke. There were baseline imbalances in this group that would favour the null hypothesis for this study. Finally, Dromerick 2009 found a large but non‐significant benefit in favour of the control group. This study examined the effect of different amounts of CIMT early after stroke. They suggested that the effect seen could be due to fatigue or injury due to overtraining.
6.
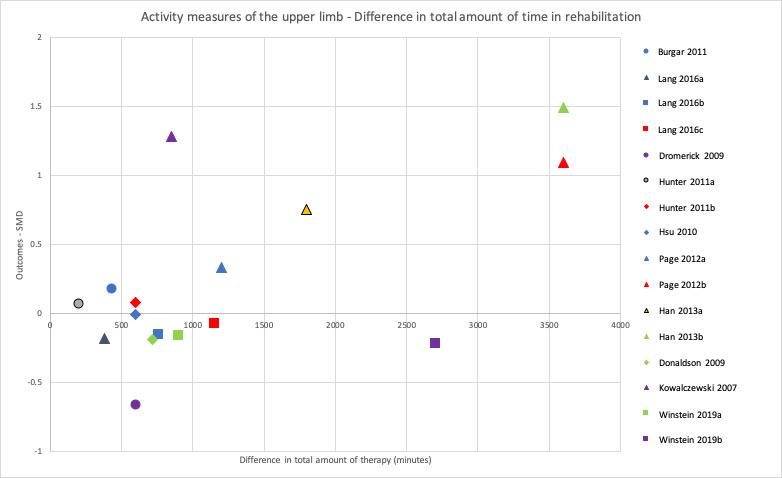
Scatter diagram plotting difference in total minutes of rehabilitation against outcomes (SMD) for activity measures of the upper limb, immediately after intervention
Analysis 4.3: activity measures of the lower limb
The test for subgroup differences showed that there was no significant difference between results of studies with larger (828 to 900 minutes) versus smaller (186 to 780 minutes) difference in total minutes of rehabilitation between treatment groups for activity measures of the lower limb, immediately after intervention (P = 0.41; Analysis 4.3).
4.3. Analysis.

Comparison 4: Objective two: effect of total time spent in rehabilitation, Outcome 3: Activity measures of the lower limb: immediately after intervention
The scatter plot for this comparison can be seen in Figure 7. Due to the lack of data points, it is not possible to draw any meaningful conclusions from these data.
7.
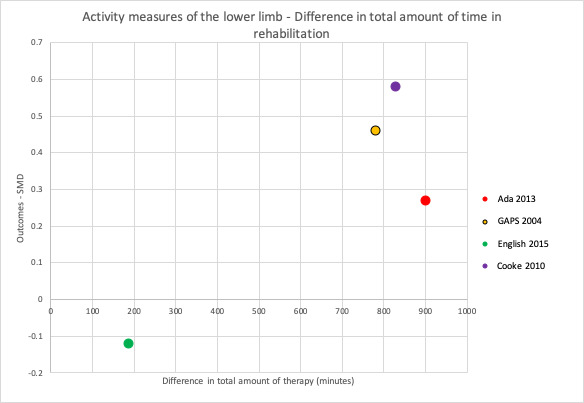
Scatter diagram plotting difference in total minutes of rehabilitation against outcomes (SMD) for activity measures of the lower limb, immediately after intervention
Analysis 4.4: motor impairment measures of the upper limb
The test for subgroup differences showed that there is no significant difference between results of studies with larger (852 to 3600 minutes) versus smaller (198.8 to 720 minutes) difference in total minutes of rehabilitation between treatment groups for motor impairment measures of the upper limb, immediately after intervention (P = 0.06; Analysis 4.4).
4.4. Analysis.

Comparison 4: Objective two: effect of total time spent in rehabilitation, Outcome 4: Motor impairment measures of the upper limb: immediately after intervention
Analysis of the scatter plot for this outcome was limited by the small number of data points, but suggested a positive association between difference in total amount of rehabilitation and motor impairment measures of the upper limb (Figure 8). There were no outlying studies of particular note for this scatter plot.
8.
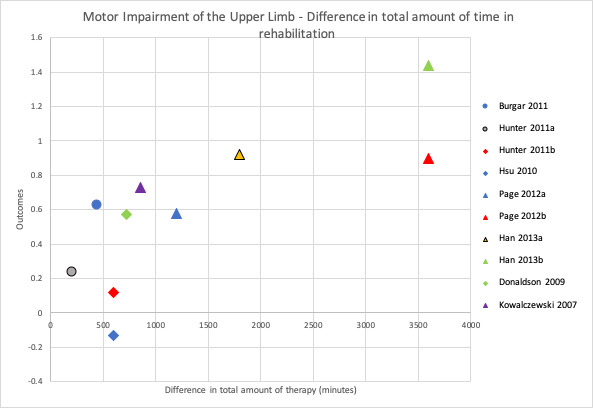
Scatter diagram plotting difference in total minutes of rehabilitation against outcomes (SMD) for motor impairment measures of the upper limb, immediately after intervention
Only one study reported motor impairment of the upper limb and two studies reported SAE/death, therefore these outcomes were not included in the subgroup analysis for objective two.
Motor impairment measures of the lower limb
As only one study reported a lower limb motor impairment measure, we were unable to undertake a subgroup analysis for this outcome.
Serious adverse events/death
As only two studies reported SAE/death, we did not undertake a subgroup analysis for this outcome.
Objective three: to assess the effect of rehabilitation schedule on activity following stroke in terms of average minutes of rehabilitation provided per week, average frequency of rehabilitation, and total duration of rehabilitation
We planned to address this objective by grouping studies with similar rehabilitation schedules and undertaking meta‐analyses for each group. Lack of similarity between studies precluded this approach, but we noted that we could extrapolate from most studies the minutes of rehabilitation per week. We used this to conduct subgroup analyses of the primary and secondary outcomes immediately after intervention. We used a median split based on difference in number of minutes of rehabilitation provided per week between study groups to compare studies with a larger difference in terms of number of minutes of rehabilitation provided per week to those with a smaller difference. In addition to this, we produced scatter plots of difference in number of minutes spent in rehabilitation per week (i.e. difference between study intervention groups in terms of number of minutes of therapy received per week during the study) plotted against the estimated treatment effect (SMD). Therefore, we conducted subgroup analyses of the primary and secondary outcomes immediately after intervention.
Analysis 5.1: activities of daily living outcomes (primary outcome)
The test for subgroup differences showed that there was no significant difference between results of studies with larger (213 to 600 minutes) versus smaller (46.5 to 150 minutes) difference in minutes of rehabilitation provided per week on ADL outcomes, immediately after intervention (P = 0.44; Analysis 5.1).
5.1. Analysis.

Comparison 5: Objective three: effect of rehabilitation schedule , Outcome 1: Activities of daily living outcomes: immediately after intervention
Analysis of the scatter plot for this outcome was limited by the small number of data points (Figure 9). Tentatively, it suggested a small positive association between difference in total amount of rehabilitation per week and ADL outcomes. One study was an exception to this. Dromerick 2009 found a large but non‐significant benefit in favour of the control group. This study examined the effect of different amounts of CIMT early after stroke. They suggested that the effect seen could be due to fatigue or injury due to overtraining.
9.
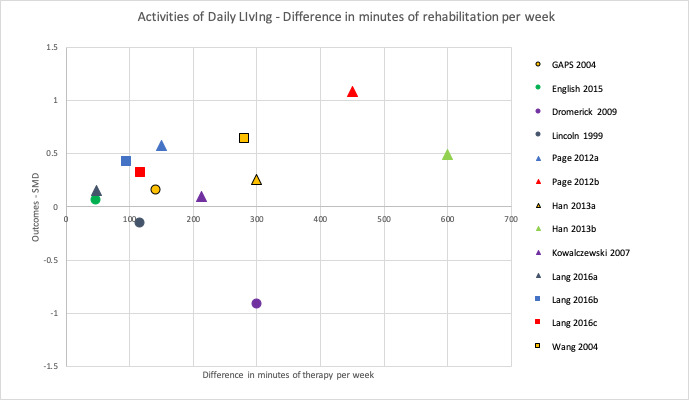
Scatter diagram plotting difference in minutes of rehabilitation per week against outcomes (SMD) for activities of daily living, immediately after intervention
Analysis 5.2: activity measures of the upper limb
The test for subgroup differences showed that there was no significant difference between results of studies with a larger (213 to 600 minutes) versus smaller (48 to 150 minutes) difference in minutes of rehabilitation provided per week for activity measures of the upper limb, immediately after intervention (P = 0.14; Analysis 5.2).
5.2. Analysis.

Comparison 5: Objective three: effect of rehabilitation schedule , Outcome 2: Activity measures of the upper limb: immediately after intervention
Analysis of the scatter plot for this outcome was limited by the small number of data points, but suggested a positive association between difference in amount of rehabilitation per week and improved activity measures of the upper limb (Figure 10). There were two notable studies. Kowalczewski 2007 found a relatively large but non‐significant effect in favour of additional therapy, despite a relatively smaller difference in total amount of therapy. This study provided different amounts of functional electrical stimulation exercise therapy to two groups; one received intervention daily and one received intervention weekly. Dromerick 2009 found a large but non‐significant benefit in favour of the control group. This study examined the effect of different amounts of CIMT early after stroke. They suggested that the effect seen could be due to fatigue or injury due to overtraining.
10.
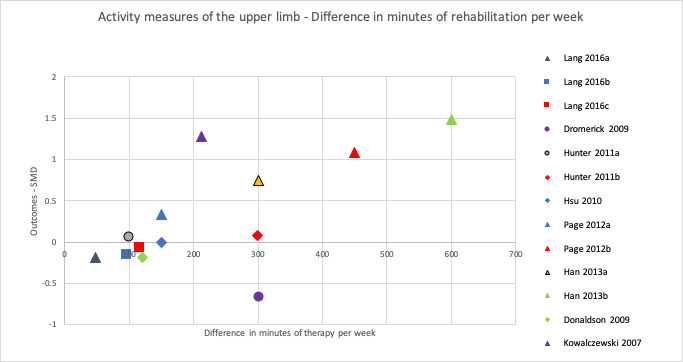
Scatter diagram plotting difference in minutes of rehabilitation per week against outcomes (SMD) for activity measures of the upper limb, immediately after intervention
Analysis 5.3: activity measures of the lower limb
The test for subgroup differences shows that there is no significant difference between results of studies with a larger (140 to 150 minutes) versus smaller (46.5 to 138 minutes) difference in minutes of rehabilitation provided per week for activity measures of the lower limb, immediately after intervention (P = 0.64; Analysis 5.3).
5.3. Analysis.

Comparison 5: Objective three: effect of rehabilitation schedule , Outcome 3: Activity measures of the lower limb: immediately after intervention
The scatter plot for this comparison can be seen in Figure 11. Due to the lack of data points, it is not possible to draw any meaningful conclusions from these data.
11.
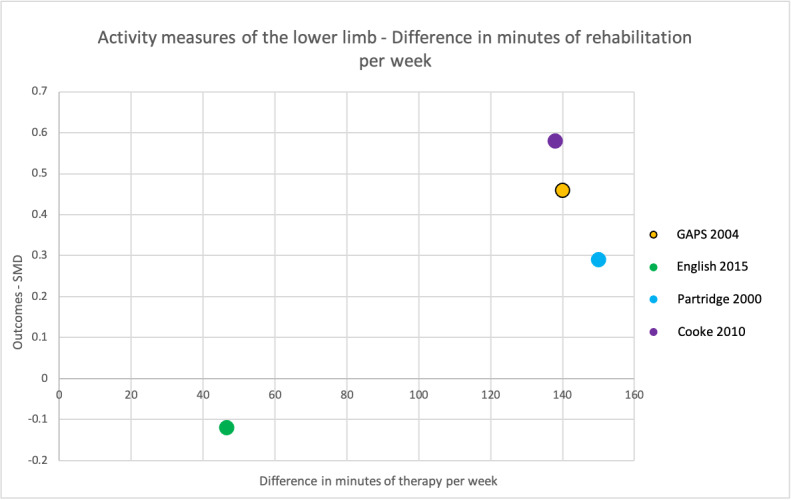
Scatter diagram plotting difference in minutes of rehabilitation per week against outcomes (SMD) for activity measures of the lower limb, immediately after intervention
Analysis 5.4: motor impairment measures of the upper limb
The test for subgroup differences shows that there is no significant difference between results of studies with larger (298.9 to 600 minutes) versus smaller (99.4 to 213 minutes) difference in minutes of rehabilitation provided per week for motor impairment measures of the upper limb, immediately after intervention (P = 0.22; Analysis 5.4).
5.4. Analysis.

Comparison 5: Objective three: effect of rehabilitation schedule , Outcome 4: Motor impairments of the upper limb: immediately after intervention
Analysis of the scatter plot for this outcome was limited by the small number of data points, but suggested a positive association between difference in amount of rehabilitation per week and motor impairment measures of the upper limb (Figure 12). There were no outlier studies of particular note.
12.
Scatter diagram plotting difference in minutes of rehabilitation per week against outcomes (SMD) for motor impairment measures of the upper limb, immediately after intervention
Only one study reported motor impairment of the upper limb and two studies reported SAE/death, therefore these outcomes were not included in the subgroup analysis for objective three.
Motor impairment measures of the lower limb
As only one study reported a lower limb motor impairment measure, we were unable to undertake a subgroup analysis for this outcome.
Serious adverse events/death
As only two studies reported SAE/death, we did not undertake a subgroup analysis for this outcome.
Subgroup analyses and assessment of heterogeneity
Despite the absence of significant variability in our pooled estimates, we undertook subgroup analyses using the inverse variance method with a random‐effects model. We did this to determine if any of the factors identified impacted the findings. Subgroup analyses were undertaken for analyses in objective one (more time spent in the same type of rehabilitation versus less time spent in the same type of rehabilitation) immediately after intervention, but excluded motor impairment measures of the lower limb and SAE/death, due to the small numbers of studies.
Effect of time since stroke
We investigated the effect of time since stroke by conducting subgroup analyses, comparing studies of participants in the first six months since onset of stroke (subacute) with studies of participants longer than six months since stroke (chronic). We found no evidence of differences between subgroups for any analyses (see Table 9).
6. Subgroup analyses.
| Subgroup analysis | Outcome | Studies included | Significance of subgroup difference |
| Effect of time since stroke (studies within the first 6 months since stroke vs studies after 6 months since stroke) |
ADL outcomes | First 6 months: Abdullahi 2018; Burgar 2011; Dromerick 2009; English 2015; GAPS 2004; Han 2013a; Kowalczewski 2007; Lincoln 1999; Wang 2004 After 6 months: Ada 2013; Hsieh 2012; Lang 2016a; Page 2012a; Winstein 2019a |
P = 0.39 |
| Activity measures of the upper limb | First 6 months: Abdullahi 2018; Burgar 2011; Donaldson 2009; Dromerick 2009; Han 2013a; Hsu 2010; Hunter 2011a; Kowalczewski 2007 After 6 months: Hsieh 2012; Lang 2016a; Page 2012a; Winstein 2019a |
P = 0.71 | |
| Activity measures of the lower limb | First 6 months: Cooke 2010b; English 2015; GAPS 2004; Partridge 2000 After 6 months: Ada 2013 |
P = 1.00 | |
| Motor impairment measures of the upper limb | First 6 months: Abdullahi 2018; Burgar 2011; Donaldson 2009; Han 2013a; Hsu 2010; Hunter 2011a; Kowalczewski 2007 After 6 months: Hsieh 2012; Page 2012a |
P = 0.85 | |
| Hours of intervention provided per week Grouped as: < 5 hours, 5 hours to < 10 hours, 10 hours to < 20 hours, ≥ 20 hours interventional therapy to the experimental group per week |
ADL outcomes | < 5 hours: Ada 2013; English 2015; Kowalczewski 2007; Lang 2016a 5 hours to < 10 hours: Burgar 2011; GAPS 2004; Lincoln 1999; Wang 2004 10 hours to < 20 hours: Dromerick 2009; Han 2013a; Page 2012a ≥ 20 hours: Winstein 2019a |
P = 0.72 |
| Activity measures of the upper limb | < 5 hours: Donaldson 2009; Kowalczewski 2007; Lang 2016a 5 hrs to < 10 hours: Burgar 2011; Hsu 2010; Hunter 2011a 10 hours to < 20 hours: Dromerick 2009; Han 2013a; Page 2012a ≥ 20 hours: Winstein 2019a |
P = 0.61 | |
| Activity measures of the lower limb | < 5 hours: Ada 2013; Cooke 2010b; English 2015 5 hrs to < 10 hours: GAPS 2004; Partridge 2000 10 hours to < 20 hours: none ≥ 20 hours: none |
P = 0.52 | |
| Motor impairment measures of the upper limb | < 5 hours: Donaldson 2009; Kowalczewski 2007 5 hours to < 10 hours: Burgar 2011; Hsu 2010; Hunter 2011a 10 hours to < 20 hours: Han 2013a; Page 2012a ≥ 20 hours: none |
P = 0.09 | |
| Upper limb therapy vs other therapy | ADL outcomes | Upper limb therapy: Abdullahi 2018; Burgar 2011; Dromerick 2009; Han 2013a; Hsieh 2012; Kowalczewski 2007; Lang 2016a; Lincoln 1999; Page 2012a; Winstein 2019a Other therapy: Ada 2013; English 2015; GAPS 2004; Wang 2004 |
P = 0.41 |
| Electromechanical technology vs no electromechanical technology |
ADL outcomes | Electromechanical technology: Ada 2013; Burgar 2011; Hsieh 2012; Kowalczewski 2007; Page 2012a No electromechanical technology: Abdullahi 2018; Dromerick 2009; English 2015; GAPS 2004; Han 2013a; Lang 2016a; Lincoln 1999; Wang 2004; Winstein 2019a |
P = 0.56 |
| Activity measures of the upper limb | Electromechanical technology: Burgar 2011; Hsieh 2012; Hsu 2010; Kowalczewski 2007; Page 2012a No electromechanical technology: Abdullahi 2018; Donaldson 2009; Dromerick 2009; Han 2013a; Hunter 2011a; Lang 2016a; Winstein 2019a |
P = 0.14 | |
| Activity measures of the lower limb | Electromechanical technology: Ada 2013 No electromechanical technology: Cooke 2010b; English 2015; GAPS 2004; Partridge 2000 |
P = 1.00 | |
| Motor impairment measures of the upper limb | Electromechanical technology: Burgar 2011; Hsieh 2012; Hsu 2010; Kowalczewski 2007; Page 2012a No electromechanical technology: Abdullahi 2018; Donaldson 2009; Han 2013a; Hunter 2011a |
P = 0.84 |
ADL: activities of daily living.
Hours of intervention provided per week
We investigated the effect of hours of therapy provided per week, comparing studies that provided less than five hours, five hours or more (but less than 10 hours), 10 hours or more (but less than 20 hours), and 20 hours or more of interventional therapy to the experimental group per week. We found no evidence of differences between subgroups for any analyses (see Table 9).
Upper limb therapy versus other therapy
In order to investigate the effect of therapy focus on outcomes, we compared studies that provided upper limb therapy with studies that provided other therapy (general rehabilitation or mobilisation). We were only able to undertake this subgroup analysis for ADL outcomes, as studies that measured the other included outcomes (activity of the upper limb, activity of the lower limb, and motor impairment of the upper limb) either did not include upper limb interventions or only included upper limb interventions. For ADL outcomes, we found no evidence of a difference between subgroups (see Table 9).
Electromechanical technology versus no electro‐mechanical technology
To investigate the effect of type of therapy on outcomes, we compared studies that use electromechanical technology with studies that did not use electromechanical technology. We found no evidence of differences between subgroups for any analyses (see Table 9).
Discussion
Summary of main results
The aim of this review was to evaluate the effect of time spent in rehabilitation on measures of activity and impairment after stroke. We included 21 studies that analysed 1412 participants. Rehabilitation times and rehabilitation schedules varied between studies. The difference in total time between control and intervention groups ranged from 186 to 6160 minutes with a median difference of 840 minutes.
The first objective was to establish if more of the same rehabilitation therapy resulted in greater improvement in activity than less time. We found low‐ to very low‐certainty evidence of no effect on ADL outcomes, and activity measures of the upper limb and lower limb. We found low‐ to very low‐certainty evidence of an effect in favour of additional time on impairment measures of the upper limb and lower limb at the end of treatment, but not on medium‐term follow‐up (two weeks to six months after intervention). Most included studies demonstrated no clinically important differences. We found low‐certainty evidence that more time spent in rehabilitation did not increase risk of SAEs or death, but few studies reported these outcomes.
The second objective was to assess the effect of difference in total rehabilitation time on recovery of activity. We compared studies with a larger difference in total rehabilitation time to those with a smaller difference in total rehabilitation time. Greater difference between study groups (more time versus less time) resulted in a significantly greater improvement in ADL outcomes and activity measures of the upper limb. There was no such improvement for activity measures of the lower limb and motor impairment measures of the upper limb. Analysis of scatter diagrams plotting difference in total amount of rehabilitation against outcome must be treated with caution, due to the small number of data points (three to 17 per scatter diagram) and outliers. They did, however, suggest that a greater difference in amount of rehabilitation led to improved outcomes for ADL measures, and impairment and activity measures of the upper limb. Collectively, these findings suggest that more total time spent in rehabilitation may be beneficial, provided the increased amount reaches a threshold. Visual inspection of the scatter diagram in Figure 5 estimated that the minimum difference in total amount of therapy to effect a change in ADL measures is 1000 minutes (16 hours and 40 minutes). The data suggest this would achieve an SMD of 0.2, which is considered a small effect (Cohen 1988), and unlikely to represent a clinically meaningful change to a stroke survivor. This finding is tentative, due to the small number of data points and the dearth of studies with a large contrast in amount of rehabilitation between control and intervention groups.
The third objective was to assess the effect of rehabilitation schedule in terms of average minutes of rehabilitation provided per week, average frequency of rehabilitation provided per week, and total duration of rehabilitation. Wide variation in rehabilitation schedules limited the potential to pool data, but 17 studies compared more versus fewer minutes of rehabilitation per week, therefore, we analysed this aspect of rehabilitation schedule. Greater difference in between‐study groups (more time versus less time) in terms of amount of rehabilitation provided per week resulted in no evidence of an improvement for ADL outcomes, activity measures of the upper or lower limbs, and motor impairment measures of the upper limb. Analysis of the scatter diagrams for this objective must also be treated with caution due to the small number of data points and outliers. Overall, they suggested that a greater difference in amount of rehabilitation per week leads to improved outcomes.
Scatter diagrams may infer elements of the rehabilitation schedule that influence outcomes. Winstein 2019b found a non‐significant effect in favour of the control group, despite a relatively large difference in amount of time spent in rehabilitation. In this study, rehabilitation was provided in three week‐long bouts each separated by one month. This unique schedule may have limited the benefit of rehabilitation. Kowalczewski 2007 found a relatively large effect in favour of additional rehabilitation, despite a relatively smaller difference in total amount. This study provided different amounts of functional electrical stimulation exercise therapy to two groups; one received intervention daily and one received intervention weekly. Potentially, daily rehabilitation may be beneficial in addition to increased total amount of time. The studies of both Han 2013a and Page 2012a seemed to have elicited positive findings. These studies have been examined in detail to determine commonalities that may have influenced their positive results, the most obvious being that they both provided large amounts of rehabilitation (Han 2013a: up to two hours per weekday over eight weeks; Page 2012a: three hours per weekday over six weeks). Finally, Wang 2004 provided the greatest contrast in total amount rehabilitation of all included studies; however, their outcomes were not better than some studies that provided an overall smaller contrast in amount of rehabilitation. Notably, intervention in Wang 2004 was provided over a six‐month period, meaning there was less intervention per week than in other studies. Potentially concentration of rehabilitation is an important factor.
Overall completeness and applicability of evidence
The following issues should be considered when judging the overall completeness and applicability of these findings.
Intervention
The between‐group difference in amount of the intervention was, in most studies, small. Fifteen studies (20 comparisons) reported the amount provided per week. In 60% of these comparisons, the difference was 150 minutes or less (30 minutes per day, five days per week). In only 25% of comparisons was the difference 300 minutes or more (60 minutes per day, five days per week). These small differences may have contributed to the lack of effect seen. Subgroup analyses that grouped studies by the amount of interventional rehabilitation given found no significant between‐group differences, but each subgroup only included a few studies.
We were interested in understanding the effect of time spent in rehabilitation. However, most included studies did not report the amount of time participants spent in rehabilitation. Except for the five studies of participants in the chronic stage, the intervention was in addition to time spent in 'standard rehabilitation', which was neither consistently nor comprehensively reported. Therefore, our analysis was of the effect of the intervention time only, which underestimates the total amount received. Rodgers 2003 accurately recorded the time spent in all rehabilitation (interventional and 'standard rehabilitation') and noted that the between‐group difference was less than planned. They attributed this to "competitive therapy bias" (Rodgers 2003, p.587); those delivering the intervention were not blinded to group allocation and, therefore, may have prioritised the control group for 'standard rehabilitation'. As is the case with many rehabilitation trials, providers of rehabilitation in most of our included studies were not blinded to group allocation. Therefore, trials may have been subject to 'competitive therapy bias', resulting in a smaller than intended between‐group difference in amount of intervention provided. This may have contributed to the lack of effect seen.
Five studies reported time planned for rehabilitation, without reporting time delivered. The inability to determine the amount of time participants spent in rehabilitation (not just the intervention) means the findings of this review only considered the difference between study groups, not the effect of difference in total amount of rehabilitation or whether the total amount of rehabilitation was delivered.
The definition of rehabilitation in this review was intentionally broad, taking an 'intervention agnostic' approach. A wide variety of interventions were therefore included and a subgroup analysis of the effect of specific interventions was only undertaken where we considered there was a sufficient number of studies. Therefore, we only conducted subgroup analyses of studies that used electromechanical technology versus all other studies and studies that focussed on upper limb rehabilitation versus all other studies. Neither analysis found any differences between groups.
Most studies in this review provided the intervention in an inpatient setting. It is possible that setting has an impact on ability to deliver more rehabilitation. Indeed, the five studies that reported issues with adherence were all inpatient settings. Burgar 2011 attributed adherence issues to factors related to the inpatient setting including early discharges, scheduling conflicts, and participant tolerance. GAPS 2004 reported that adherence to the planned therapy schedule related to therapists' ability to deliver the augmented amount of therapy time. Five of the seven studies that were low risk of bias for adherence to the intervention were studies of participants in the chronic stage. Although not stated, it is likely that in these studies, intervention was provided in the outpatient/community. Potentially there are fewer barriers to rehabilitation delivery in the outpatient/community setting.
This review only examined the effect of time spent in rehabilitation. Time spent is one component that may contribute to 'rehabilitation intensity'. Other potential components of 'rehabilitation intensity' include number of repetitions performed (Scrivener 2012), rate of repetitions (Klassen 2020), and physiological effort exerted (Outermans 2010). We speculate that other components of 'rehabilitation intensity' may be important in determining the effect of time spent in rehabilitation; potentially, more time spent is equated with more repetitions and accounts for improved outcomes. However, while not examined per se, within a single type of intervention different amounts were unlikely to be different in terms of physiological effort exerted and rate of repetitions as type of intervention was controlled. Research suggests that other components of 'rehabilitation intensity' may affect outcomes. Klassen 2020 found a significant improvement in walking outcomes for participants who had undertaken more repetitions and expended greater physiological effort (measured by heart rate) compared to participants whose intervention was less 'intensive', despite both groups spending the same amount of time in rehabilitation. Similarly, French 2016 found a beneficial effect for lower limb and gait outcomes for RTT compared to control. Potentially, RTT provides a greater number of repetitions compared to standard care.
Participants
We considered the extent to which participants were representative of the stroke population and identified areas for attention.
Mean age of participants ranged from 44 years to 76.5 years. According to Lui 2018, 50% of strokes occur in people over the age of 75 years and these individuals are at higher risk of poor functional outcomes. It is possible that older people are not well‐represented in the included studies and, therefore, the applicability of the findings to this group is uncertain. Likewise, many of the studies excluded people with impaired cognition or communication, or both.
Studies provided limited information regarding participants' stroke severity or baseline impairment, or both. As such, we do not know if the review findings are applicable irrespective of stroke severity. Initial stroke severity is an important predictor of outcomes (Bhaskar 2017; Rost 2016), and therefore, possibly a factor that influences response to rehabilitation.
Many studies excluded people with cognitive impairment or communication impairment, or both, which commonly occur after stroke (Douiri 2013; Engelter 2006). Therefore, the included participants may not be representative of the general stroke population, limiting the generalisability of this review's findings.
Sixteen studies included participants in the subacute stage following stroke, the remaining five were with participants in the chronic stage. Subgroup analyses suggest there was no effect for additional time in rehabilitation for most outcomes, between participants in the subacute stage and participants in the chronic stage. However, this was not the case for the ADL outcomes, where participants in the chronic stage showed an effect and participants in the subacute stage did not. When applying the findings of this review, it may be important to consider time since stroke and the fact that more time in rehabilitation seems beneficial for improving ADLs, an outcome highly correlated with quality of life following stroke (Kim 2014), later poststroke.
Outcomes
In this review, we pooled outcome measures of the same construct (i.e. ADL, activity of the upper limb, etc.) using SMDs. Although this allowed the inclusion of a greater number of studies, there are limitations to this approach. It is highly likely that, although of the same construct, the different outcome measures would have measured slightly differently and had different sensitivities to change. In addition, having pooled outcome measures, the effect of the analyses is a standardised mean difference. This reports the effect in a standardised unit, unrelated to the units used by the included measures, and is, therefore, difficult to interpret meaningfully in clinical practice (Schünemann 2021b). In the summary of findings tables, we reported Cohen's effect sizes to assist with interpretation. There were limited follow‐up measures, particularly long‐term, which precludes prediction of sustained benefits.
We noted that none of the included studies considered participant experience of rehabilitation. Chen 2019 found that 13.6% of stroke survivors reported therapy as an unmet need following stroke. Therefore, it would be valuable to establish if more rehabilitation resulted in improved participant experience. However, this was beyond the scope of this review and would likely involve analysis of qualitative or mixed methods (or both) studies.
Quality of the evidence
We assessed certainty of our primary analysis (more versus less time spent in rehabilitation) using the GRADE approach, considering five domains.
Risk of bias
Most analyses received a serious (and in some cases, very serious) GRADE rating for risk of bias. This was due to the proportion of study outcomes considered at some concern or high overall risk of bias and a tendency for a greater effect for additional rehabilitation seen after removal of studies with high risk of bias. This has greatly contributed to a reduction in GRADE assessments, indicating low and very low certainty of the evidence. The most consistent source of bias across study outcomes was the inability to establish risk of bias in the selection of reported results, as few studies published protocols or registered trials with sufficient detail. There tended to be greater risk of bias for follow‐up measures, due to loss of follow‐up data.
Inconsistency
Of all study analyses (excluding sensitivity and subgroup analyses), only one had an I2 statistic of 50% or more (Analysis 2.3). This level of consistency of findings is surprising, given the heterogeneity across studies in type of rehabilitation delivered. Possibly, selection criteria of studies were such that people with a similar type of stroke (in terms of severity and rehabilitation needs) were included in studies. Additionally, except for a few studies, a similar amount of rehabilitation tended to be delivered to intervention and control groups, which may have contributed to consistent findings. Subgroup analyses undertaken to assess heterogeneity found no evidence of differences.
Indirectness
We did not consider that the study analyses included serious indirectness. Our selection criteria were such that the studies included directly addressed our primary objective. We considered studies that reported issues with adherence to intervention may lead to indirectness of the intervention, particularly if this led to a lack of difference in the intervention received by the included groups. However, when these studies were removed in sensitivity analyses, findings were not greatly affected.
Imprecision
We considered studies to have serious imprecision if the 95% CIs included an effect size of no difference. This was true for four of the five analyses. Many studies had small samples sizes, which may have contributed to imprecision.
Publication bias
We strongly suspect publication bias for most analyses, supported by the assessment of non‐reporting bias in studies (Table 6), and the funnel plots. We are aware of studies that measured outcomes, but the data for these outcomes were not reported and were unavailable. Additionally, there are some studies that we considered could have measured some outcomes but not reported their findings. The lack of study protocols contributed to this issue.
In summary, the GRADE assessment indicates that the analyses for objective one are of low to very low certainty.
Potential biases in the review process
Despite undertaking a thorough search, it is possible that we missed some eligible studies. Our searches resulted in an exceptionally large number of records, due to the many and varied search terms used to capture the concept of 'time spent in rehabilitation'. Title screening, undertaken by one review author (BC), excluded studies that were clearly irrelevant and specifically not related to stroke, were investigations of surgical or pharmaceutical interventions, and were animal studies. It is unlikely that this screening led to missed studies.
For eight potentially eligible studies, we were unable to determine whether they met the selection criteria and authors have not responded to our enquiries. Two studies that satisfied the selection criteria were not included because usable data were unavailable and authors have not responded to our enquiries.
In determining study eligibility, review authors had to decide if the rehabilitation provided between intervention and control groups was the same, except for the amount of time spend. Rehabilitation is a complex intervention, which naturally varies from individual to individual. Judgements were based on study authors' intention to provide the same type of rehabilitation, but despite this, there were instances when study eligibility was debated. The procedure for this followed the plan described in our study protocol, but also included other review authors (GK and JM) when an agreement could not be reached between those involved in the study selection process (BC, JB, and JW).
Two review authors, working independently, extracted data from the studies and assessed risk of bias for all outcomes of included studies. Any discrepancies were resolved through discussion with a third review author.
Two review authors independently made judgements regarding the constructs measured by outcome tools to determine whether outcomes of interest were measured.
One study was written in Chinese with an English abstract (Wang 2004). Two independent translators translated parts of the text to enable data extraction and assessment of risk of bias. However, other biases within this text may have existed, of which we are not aware.
We were unable to assess the third objective as planned and made a post hoc decision about meeting this objective. Although we did not consider study results when making this decision, it is possible that a post hoc data analysis change could have introduced bias.
Agreements and disagreements with other studies or reviews
Does more of the same rehabilitation therapy result in greater improvement in activity measures?
To our knowledge, this is the first systematic review with meta‐analysis to only include studies that compared different amounts of the same type of rehabilitation. All other reviews have included studies in which the experimental and control interventions differed in type of intervention, as well as amount of intervention, and some meta‐analyses included studies that measured effect of rehabilitation versus no rehabilitation. However, there are reviews that have examined the effect of time spent in rehabilitation. These are considered in terms of their agreements and disagreements with our review.
Eleven systematic reviews with meta‐analyses have studied the effect of time spent in rehabilitation following stroke (Cooke 2010a; French 2016; Galvin 2008; Kwakkel 1997; Kwakkel 2004; Langhorne 1996; Lohse 2014; Pollock 2014a; Schneider 2016; Sehatzadeh 2015; Veerbeek 2011). Relevant findings of these studies and their agreements/disagreements with this review are summarised in Table 10.
7. Summary of other systematic reviews with meta‐analysis to address time spent in rehabilitation.
| Review | Type of rehabilitation | Key findings (in relation to time spent in rehabilitation) | Agreement/disagreement with this review |
| Langhorne 1996 | Physiotherapy | There was a non‐significant reduction in the chance of death. The pooled measures of impairment and disability found no significant results. |
This review found no difference in the risk of SAE/death with additional therapy. There is limited comparison with the pooled measures. |
| Kwakkel 1997 | Rehabilitation | Small effect in favour of additional treatment seen for ADLs. Effect seen for functional outcomes in favour of additional treatment. No effect seen for neuromuscular outcomes; however, following post hoc analysis to control for organisational setting and blinding, there was an effect. |
This review found no effect for ADLs and no effect for activity measures of the ULs and LLs. This disagrees with Kwakkel 1997. |
| Kwakkel 2004 | Exercise therapy | Small effect found for ADL and walking speed. No effect seen for UL outcomes (measured with the Action Research Arm Test). For the ADL outcomes, a cumulative meta‐analysis was undertaken. This found that at least an additional 16 hours of exercise therapy is required to elicit a 4–5% change in outcome measure. |
This review found no effect for ADL and LL activity measures (such as walking), which disagrees with Kwakkel 2004 This review found no effect for activity measures of the UL (such as the Action Research Arm Test), in agreement with Kwakkel 2004 |
| Galvin 2008 | Exercise therapy | No effect found for UL measures (pooled functional and impairment measures). No effect found for LL measures (pooled functional and impairment measures). Effect seen in favour of additional therapy for ADL measures (as measured using the Barthel Index). |
This review split functional and impairment measures of the UL and LL, so this outcome is not comparable. This review saw no effect for ADL measures, in contrast with the Galvin 2008 review. |
| Cooke 2010a | Exercise‐based therapy | Meta‐analysis was undertaken for hand grip force/strength at end of treatment. This favoured the control treatment. For Motricity Arm measured at first follow‐up, there was an effect in favour of experimental treatment. There was no effect for measures of UL function (Action Research Arm Test). Comfortable walking speed showed an effect in favour of control treatment at first time point, but a non‐significant finding at second time point. Rivermead mobility showed a non‐significant effect. |
This review found an effect for motor impairment of the UL, which is in contrast with some findings of Cooke 2010 (which split measures of motor impairment of the UL). This review found no effect for measures of UL function, in agreement with Cooke 2010. This review found no effect for activity measures of the LL, which is in contrast to the findings of Cooke 2010 at the first time point. |
| Veerbeek 2011 | Lower‐limb exercise therapy | Beneficial effect of more therapy seen for walking ability, comfortable walking speed, and maximum walking speed. No effect seen for basic ADLs, but an effect seen for extended ADLs. |
This review did not find an effect for LL activity or ADLs, as Veerbeek 2011 did. |
| Lohse 2014 | Therapy | There was an overall beneficial effect of receiving more therapy than receiving less (all outcomes combined). A meta‐regression was performed using 4 different models, which controlled for the linear and non‐linear effects of time and time since stroke. They concluded that there was a significant, positive relationship between amount of time scheduled for therapy and improvement on outcome measures. This relationship was not affected by time since stroke, but there was a potentially non‐linear effect of time. |
There is limited comparison with this review, as Lohse 2014 combined outcomes. The findings of the scatter diagrams in this review agree with the findings of Lohse 2014. |
| Pollock 2014a | Physical rehabilitation | Subgroup analyses found that there was a greater effect size in studies with a greater amount of time spent in rehabilitation, with an indication that 30–60 minutes once per day for 5–7 days per week was beneficial, but that more than once‐daily intervention may provide even greater benefit. | This study found no evidence that supported a specific therapy schedule. |
| Sehatzadeh 2015 | Physiotherapy | Greater amount of therapy led to greater improvements in UL activity. No significant difference in measures of mobility with increased amount of therapy. No significant difference in ADL. |
This review found no effect for UL activity with more rehabilitation, as Sehatzadeh 2015 did. This review agrees with Sehatzadeh 2015 about lack of effect for mobility (LL activity) and ADL. |
| Schneider 2016 | Rehabilitation | Found that additional therapy had a beneficial effect on UL and LL activity immediately after training. Subgroup analysis showed that there was a greater effect in studies that provided a large increase in therapy, compared to a small increase. |
This review found no beneficial effect on UL and LL activity, as Schneider 2016 did. This review found a greater effect when there was a larger difference in amount of rehabilitation between study groups, which agrees with Schneider 2016. |
| French 2016 | Repetitive task training | There was no difference between subgroups for trials that delivered 0–20 hours of therapy or ≥ 20 hours of therapy for UL function or LL function. | This trial found no effect for additional time spent in rehabilitation for activity measures of the UL and LL, which agrees with French 2016. |
ADL: activities of daily living; LL: lower limb; SAE: serious adverse event; UL: upper limb.
Six reviews measured the effect of additional time spent in rehabilitation on ADL outcomes. Four found significant differences in favour of additional rehabilitation (Galvin 2008; Kwakkel 1997; Kwakkel 2004; Pollock 2014a), one found no significant difference in ADLs (measured using the Barthel Index) (Sehatzadeh 2015), and one found no significant effect for basic ADLs (Barthel Index; SMD 0.11, 95% CI −0.12 to 0.34), but a moderate effect for extended ADLs (pooled Nottingham Extended ADL checklist and Frenchay Activities Index; SMD 0.54, 95% CI 0.20 to 0.88) (Veerbeek 2011).
Four reviews measured the effect of additional time on upper limb activity. In agreement with our review, three reviews found no significant difference between groups (Cooke 2010a; French 2016; Kwakkel 2004). However, the fourth review reported significant benefit for additional time spent, measured by the ARAT (Sehatzadeh 2015).
Six reviews measured the effect of additional time spent in rehabilitation on activity measures of the lower limb. In agreement with this review, three found no effect for additional time spent (French 2016; Galvin 2008; Sehatzadeh 2015). However, two reviews found significant effects for activity measures of the lower limb, in favour of additional time (Kwakkel 2004; Veerbeek 2011). The sixth review did not pool outcomes for lower limb activity and found a non‐significant effect for the Rivermead Mobility Index and a significant effect in favour of less time in rehabilitation for walking speed (Cooke 2010a).
One review measured the effect of additional time spent in rehabilitation on upper limb motor impairment. Cooke 2010a found a significant effect in favour of less time for grip strength, but a significant effect in favour of more time for the motricity index.
One review measured the risk of death or deterioration. In disagreement with our review, Langhorne 1996 found that the risk of death or deterioration was significantly lower in groups that received additional time in rehabilitation (OR 0.54 95% CI 0.3 to 0.85), albeit with a wide CI across only five studies.
The lack of agreement between reviews may be influenced by the lack of certainty of evidence and variation in study dates and methodologies (e.g. objectives and selection criteria), as well as the aforementioned inclusion of studies that differed in the type of intervention provided, not just the amount.
What is the effect of total rehabilitation time on recovery of activity?
We found three systematic reviews with meta analyses that explored the effect of total time spent in rehabilitation. Kwakkel 2004 used a cumulative meta‐analysis, finding that a difference of at least 16 hours in treatment time between groups is required to obtain a significantly better outcome for ADLs. Lohse 2014 used meta‐regression to explore the effect of total scheduled therapy time on effect sizes. They found a reliable dose–response relationship between time scheduled for therapy and improvement in measures of function and impairment. Finally, Schneider 2016 undertook a ROC (Receiver operating characteristic) curve analysis of false versus true benefit. This indicated that an extra 240% of rehabilitation is required to make certain a better outcome for activity. The findings of Kwakkel 2004 and Schneider 2016 agree with our finding that a large difference between intervention groups is required to achieve a significantly better outcome. The finding of Lohse 2014 do not suggest that a larger difference is required between groups to see a beneficial effect, which is contrary to the findings of this review and the others described. This difference could be due to differences in inclusion criteria and statistical methods.
Other studies support the finding that a very large amount of rehabilitation may achieve a significant response. McCabe 2015 compared three interventions (motor learning, robotics plus motor learning, and FES plus motor learning), all provided five hours/day, five days/week for 12 weeks to a population of participants more than one year post‐stroke. All groups made clinically significant improvements postintervention but with no significant between‐group differences. Similarly, Ward 2019 describes the outcomes of 224 stroke survivors in the chronic stage, who attended an upper limb rehabilitation programme, receiving 30 hours of intervention per week for three weeks. At the end of intervention, there were significant improvements in all outcomes measured, maintained at six‐month follow‐up. Neither of these studies were included in this review, as McCabe 2015 compared different, dose‐matched interventions, and Ward 2019 was not an RCT. We are unable to find any studies of similarly large amounts of rehabilitation (i.e. five hours per day) in participants in the subacute stage poststroke. This may potentially be due to the challenges of delivering this amount of therapy early after stroke (Burgar 2011; Hunter 2011a).
What is the effect of rehabilitation schedule in terms of average minutes per week, number of sessions per week and total duration of rehabilitation?
We found one systematic review with meta‐analysis that explored the effect of rehabilitation schedule. Findings from Pollock 2014a suggest that 30 to 60 minutes of physical rehabilitation delivered five to seven days per week provides a significant benefit for function recovery when compared to no intervention or usual care. However, this study also reported that, for ADL outcomes, more than once‐daily intervention may provide even more benefit. In agreement with Pollock 2014a, our findings suggest that daily intervention may be more beneficial than less‐than‐daily intervention.
Authors' conclusions
Implications for practice.
An increase in time spent in the same type of rehabilitation after stroke results in little to no difference in meaningful activities such as activities of daily living and activities of the upper and lower limb, but a small benefit in measures of motor impairment (low‐ to very low‐certainty evidence for all findings). If the increase in time spent in rehabilitation exceeds a threshold, this may lead to improved outcomes. There is currently insufficient evidence to recommend a minimum beneficial daily amount in clinical practice.
Additional time spent in the same type of rehabilitation does not increase the risk of serious adverse events/death, but this finding is of low certainty and should be interpreted with caution, as few studies monitored these outcomes.
The findings of this review are limited by a paucity of research trials with large contrasts in amount of rehabilitation delivered between intervention and control groups.
Implications for research.
There is currently insufficient, high‐quality evidence to determine the effect of time spent in rehabilitation. However, findings from high‐quality trials with a large contrast in amount of therapy delivered indicates that this area warrants further research.
To provide evidence for the effect of time spent in rehabilitation, adequately powered, high‐quality randomised controlled trials are required. Such studies should be undertaken in a stroke population, studying groups of participants spending different amounts of time in the same type of rehabilitation. Findings of this review suggest that the total contrast in amount of time between groups should be a minimum of 1000 minutes. Outcomes at an activity level are required to determine if more time spent in intervention results in a meaningful change.
Study quality would be improved by enhanced reporting. Publication of protocols (or detailed trial registry entries) and reporting of all measured outcomes would allow for accurate judgement of potential reporting bias. Accurate reporting of amount of rehabilitation delivered, not amount of rehabilitation planned, is imperative. Additionally, when undertaking any study assessing effect of amount of time spent in a specific intervention, it is crucial that researchers accurately report the time spent in all rehabilitation, not just interventional rehabilitation. This is of particular importance when those delivering rehabilitation are aware of participant group allocation. Finally, it is important that studies report baseline stroke severity, to examine its impact on response to rehabilitation.
An individual participant data meta‐analysis might provide further information regarding the effect of time spent, specifically if certain characteristics of either the participant or the intervention effect outcomes.
In addition to 'time spent', other characteristics of rehabilitation may be important, such as type of rehabilitation, stage of recovery, rehabilitation 'intensity' (such as number/rate of practice repetitions, physiological effort, or task difficulty), and rehabilitation schedule. These characteristics also warrant further exploration.
History
Protocol first published: Issue 3, 2017
Risk of bias
Risk of bias for analysis 3.1 Activities of daily living outcomes: long‐term outcomes.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Ada 2013 | Low risk of bias | Computer generated randomisation. No baseline imbalances between groups to suggest a problem with randomisation. | Low risk of bias | There is evidence that not all participants completed all interventions, but non‐adherence is relatively normal in standard practice. Outcome analysed using an 'intention to treat' analysis | Low risk of bias | Outcome data was available for 92% of participants at 12 months | Low risk of bias | The Adelaide Activities Profile is a validated tool in which outcome assessors had been trained. The outcome assessors were blinded to participant group allocation. | Low risk of bias | Protocol is available. No differences detected between the protocol and the study in terms of the outcomes reported. Planned statistical analysis method is not detailed in the protocol, but the method used in the study is straightforward and commonly used. There is no indication that it was selected from other potential methods. | Low risk of bias | Low risk of bias in all domains |
Acknowledgements
We thank Hazel Fraser, Alex Pollock, Joshua Cheyne, and the Editors of the Cochrane Stroke Group for their support and assistance in the development of this review. We also thank Kerry Dwan, Ella Flemyng, and the Cochrane Methods group for their support in the use of the RoB 2 tool.
We thank the following people for their assistance in the translation of the non‐English language paper: Yuan Chi, researcher with the Institute for Complementary and Integrative Medicine, University of Zurich and Wan‐wen Liao, postdoctoral research fellow, Department of Occupational Therapy and Graduate Institute of Behavioral Sciences, College of Medicine, Chang Gung University, Taiwan.
We thank Malcolm McKeag, retired writer, broadcaster, and stroke patient representative, for his assistance in reviewing the plain language summary.
Finally, we thank all researchers, who answered our requests and provided additional information.
Appendices
Appendix 1. Cochrane Stroke Group Trials Registry search strategy
Keywords:
Intensity
Intensive
Augment
Augmented
Additional
Dosage
Dose
Frequent
Frequency
Amount
Quantity
Duration
Conditions:
Stroke, Cerebral Vascular Accident, CVA (cerebrovascular Accident), Cerebellar Stroke, Subarachnoid Hemorrhage (SAH), SAH, Brain Infarction, Ischemic Brain Injury, Cerebral Hemorrhage, Carotid Artery Disease
Interventions:
Physiotherapy, Occupational Therapy, Exercise, Rehabilitation, Rehabilitation program, rehabilitation therapy, therapy.
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Cerebrovascular Disorders] this term only #2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] this term only #3 MeSH descriptor: [Brain Ischemia] explode all trees #4 MeSH descriptor: [Carotid Artery Diseases] explode all trees #5 MeSH descriptor: [Cerebral Small Vessel Diseases] explode all trees #6 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees #7 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #8 MeSH descriptor: [Intracranial Hemorrhages] explode all trees #9 MeSH descriptor: [Stroke] explode all trees #10 MeSH descriptor: [Vasospasm, Intracranial] this term only #11 MeSH descriptor: [Vertebral Artery Dissection] this term only #12 (stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH):ti,ab,kw (Word variations have been searched) #13 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw (Word variations have been searched) #14 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab,kw (Word variations have been searched) #15 MeSH descriptor: [Hemiplegia] this term only #16 MeSH descriptor: [Paresis] explode all trees #17 MeSH descriptor: [Gait Disorders, Neurologic] explode all trees #18 (hemipleg* or hemipar* or paresis or paraparesis or paretic):ti,ab,kw (Word variations have been searched) #19 {or #1‐#18} #20 MeSH descriptor: [Physical Therapy Modalities] this term only #21 MeSH descriptor: [Physical Therapy Specialty] this term only #22 MeSH descriptor: [Exercise Movement Techniques] explode all trees #23 MeSH descriptor: [Exercise Therapy] explode all trees #24 MeSH descriptor: [Hydrotherapy] this term only #25 MeSH descriptor: [Kinesiology, Applied] this term only #26 MeSH descriptor: [Rehabilitation] this term only #27 MeSH descriptor: [Activities of Daily Living] this term only #28 MeSH descriptor: [Occupational Therapy] this term only #29 MeSH descriptor: [Recreation Therapy] explode all trees #30 MeSH descriptor: [Rehabilitation, Vocational] this term only #31 MeSH descriptor: [Recovery of Function] this term only #32 MeSH descriptor: [Movement] this term only #33 MeSH descriptor: [Motor Activity] this term only #34 MeSH descriptor: [Exercise] this term only #35 MeSH descriptor: [Circuit‐Based Exercise] this term only #36 MeSH descriptor: [Muscle Stretching Exercises] this term only #37 MeSH descriptor: [Physical Conditioning, Human] this term only #38 MeSH descriptor: [Plyometric Exercise] this term only #39 MeSH descriptor: [Resistance Training] this term only #40 MeSH descriptor: [Running] explode all trees #41 MeSH descriptor: [Swimming] this term only #42 MeSH descriptor: [Walking] this term only #43 MeSH descriptor: [Warm‐Up Exercise] this term only #44 MeSH descriptor: [Exercise Test] this term only #45 MeSH descriptor: [Sports] explode all trees #46 MeSH descriptor: [Physical Exertion] this term only #47 MeSH descriptor: [Physical Endurance] explode all trees #48 MeSH descriptor: [Physical Fitness] this term only #49 MeSH descriptor: [Muscle Stretching Exercises] this term only #50 MeSH descriptor: [Resistance Training] this term only #51 MeSH descriptor: [Muscle Contraction] this term only #52 MeSH descriptor: [Isometric Contraction] this term only #53 MeSH descriptor: [Isotonic Contraction] this term only #54 (exercise near/3 (train* or intervention* or protocol* or program* or therap* or activit* or regim*)):ti,ab,kw (Word variations have been searched) #55 (fitness near/3 (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*)):ti,ab,kw (Word variations have been searched) #56 ((training or conditioning) near/3 (intervention* or protocol* or program* or activit* or regim*)):ti,ab,kw (Word variations have been searched) #57 (sport* or recreation* or leisure or cycling or bicycl* or rowing or treadmill* or running or circuit training or swim* or walk* or dance* or dancing or tai ji or tai chi or yoga):ti,ab,kw (Word variations have been searched) #58 ((endurance or aerobic or cardio*) near/3 (fitness or train* or intervention* or protocol* or program* or therap* or activit* or regim*)):ti,ab,kw (Word variations have been searched) #59 (muscle strengthening or progressive resist*):ti,ab,kw (Word variations have been searched) #60 ((weight or strength* or resistance) near/3 (train* or lift* or exercise*)):ti,ab,kw (Word variations have been searched) #61 ((isometric or isotonic or eccentric or concentric) near/3 (action* or contraction* or exercise*)):ti,ab,kw (Word variations have been searched) #62 {or #20‐#61} #63 MeSH descriptor: [Cerebrovascular Disorders] this term only and with qualifier(s): [Rehabilitation ‐ RH] #64 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #65 MeSH descriptor: [Brain Ischemia] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #66 MeSH descriptor: [Carotid Artery Diseases] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #67 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #68 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #69 MeSH descriptor: [Intracranial Hemorrhages] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #70 MeSH descriptor: [Brain Infarction] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #71 MeSH descriptor: [Stroke, Lacunar] this term only and with qualifier(s): [Rehabilitation ‐ RH] #72 MeSH descriptor: [Vasospasm, Intracranial] this term only and with qualifier(s): [Rehabilitation ‐ RH] #73 MeSH descriptor: [Vertebral Artery Dissection] this term only and with qualifier(s): [Rehabilitation ‐ RH] #74 MeSH descriptor: [Hemiplegia] this term only and with qualifier(s): [Rehabilitation ‐ RH] #75 MeSH descriptor: [Paresis] explode all trees and with qualifier(s): [Rehabilitation ‐ RH] #76 {or #63‐#75} #77 (time or timing or intensive or intensity or augment* or accelerate* or additional or dosage or dose or frequency or amount or quantity):ti,ab,kw (Word variations have been searched) #78 #76 and #77 #79 #19 and #62 and #77 #80 #78 or #79
Appendix 3. MEDLINE search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. 1 or 2 or 3 or 4 or 5 or 6
8. physical therapy modalities/ or physical therapy specialty/ or exp exercise movement techniques/ or exp exercise therapy/ or hydrotherapy/ or kinesiology, applied/
9. "Physical and Rehabilitation Medicine"/
10. rehabilitation/ or "activities of daily living"/ or occupational therapy/ or recreation therapy/ or rehabilitation, vocational/ or "Recovery of Function"/
11. movement/ or motor activity/ or exercise/ or circuit‐based exercise/ or cool‐down exercise/ or muscle stretching exercises/ or physical conditioning, human/ or plyometric exercise/ or resistance training/ or exp running/ or swimming/ or walking/ or warm‐up exercise/ or exercise test/
12. exp sports/
13. physical exertion/ or exp physical endurance/ or physical fitness/
14. muscle stretching exercises/ or resistance training/
15. muscle contraction/ or isometric contraction/ or isotonic contraction/
16. (physiotherap$ or (physical adj3 (mobilis$ or mobiliz$ or exercise$ or exertion or endurance or therap$ or conditioning or activit$ or fitness))).tw.
17. (rehabilitation or recovery of function or exercise$ or mobilis$ or mobiliz$ or motion therap$ or motor activit$ or motor skill$ or activities of daily living or adl or manipulat$ or (occupational adj3 (train$ or rehab$ or therap$ or activit$ or regim$))).tw.
18. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw.
19. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$ or centre$ or center$)).tw.
20. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw.
21. (sport$ or recreation$ or leisure or cycling or bicycl$ or rowing or treadmill$ or running or circuit training or swim$ or walk$ or dance$ or dancing or tai ji or tai chi or yoga).tw.
22. ((endurance or aerobic or cardio$) adj3 (fitness or train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw.
23. (muscle strengthening or progressive resist$).tw.
24. ((weight or strength$ or resistance) adj3 (train$ or lift$ or exercise$)).tw.
25. ((isometric or isotonic or eccentric or concentric) adj3 (action$ or contraction$ or exercise$)).tw.
26. or/8‐25
27. cerebrovascular disorders/rh or exp basal ganglia cerebrovascular disease/rh or exp brain ischemia/rh or exp carotid artery diseases/rh or cerebrovascular accident/rh or exp brain infarction/rh or exp cerebrovascular trauma/rh or exp hypoxia‐ischemia, brain/rh or exp intracranial arterial diseases/rh or intracranial arteriovenous malformations/rh or exp "intracranial embolism and thrombosis"/rh or exp intracranial hemorrhages/rh or vasospasm, intracranial/rh or vertebral artery dissection/rh or (hemiplegia/rh or exp paresis/rh)
28. (intensive or intensity or augment$ or accelerate$ or additional or dosage or dose‐response or frequency or amount or quantity).tw.
29. 27 and 28
30. 7 and 26 and 28
31. 29 or 30
32. Randomized Controlled Trials as Topic/
33. random allocation/
34. Controlled Clinical Trials as Topic/
35. control groups/
36. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
37. double‐blind method/
38. single‐blind method/
39. Therapies, Investigational/
40. Research Design/
41. randomized controlled trial.pt.
42. controlled clinical trial.pt.
43. clinical trial.pt.
44. random$.tw.
45. (controlled adj5 (trial$ or stud$)).tw.
46. (clinical$ adj5 trial$).tw.
47. ((control or treatment or experiment$ or intervention or surgical) adj5 (group$ or subject$ or patient$)).tw.
48. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
49. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
50. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
51. (coin adj5 (flip or flipped or toss$)).tw.
52. latin square.tw.
53. versus.tw.
54. controls.tw.
55. or/32‐54
56. 31 and 55
Appendix 4. Embase search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. exp hemiplegia/ or exp paresis/ or neurologic gait disorder/ 6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. therapy/ 9. exp kinesiotherapy/ 10. exp exercise/ 11. rehabilitation/ or cognitive rehabilitation/ or community based rehabilitation/ or constraint induced therapy/ or functional training/ or home rehabilitation/ or muscle training/ or exp neurorehabilitation/ or occupational therapy/ or psychosocial rehabilitation/ or recreational therapy/ or rehabilitation care/ or sociotherapy/ or exp "speech and language rehabilitation"/ or telerehabilitation/ or vocational rehabilitation/ 12. exp muscle exercise/ 13. physical activity/ or climbing/ or cycling/ or jogging/ or running/ or stretching/ or swimming/ or exp walking/ or weight lifting/ 14. sport/ 15. exp muscle contraction/ 16. (physiotherap$ or (physical adj3 (mobilis$ or mobiliz$ or exercise$ or exertion or endurance or therap$ or conditioning or activit$ or fitness))).tw. 17. (rehabilitation or recovery of function or exercise$ or mobilis$ or mobiliz$ or motion therap$ or motor activit$ or motor skill$ or activities of daily living or adl or manipulat$ or (occupational adj3 (train$ or rehab$ or therap$ or activit$ or regim$))).tw. 18. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 19. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$ or centre$ or center$)).tw. 20. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 21. (sport$ or recreation$ or leisure or cycling or bicycl$ or rowing or treadmill$ or running or circuit training or swim$ or walk$ or dance$ or dancing or tai ji or tai chi or yoga).tw. 22. ((endurance or aerobic or cardio$) adj3 (fitness or train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 23. (muscle strengthening or progressive resist$).tw. 24. ((weight or strength$ or resistance) adj3 (train$ or lift$ or exercise$)).tw. 25. ((isometric or isotonic or eccentric or concentric) adj3 (action$ or contraction$ or exercise$)).tw. 26. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27. cerebrovascular disease/rh or exp basal ganglion hemorrhage/rh or exp brain hemangioma/rh or exp brain hematoma/rh or exp brain hemorrhage/rh or exp brain infarction/rh or exp brain ischemia/rh or exp carotid artery disease/rh or exp cerebral artery disease/rh or exp cerebrovascular accident/rh or exp cerebrovascular malformation/rh or exp intracranial aneurysm/rh or exp occlusive cerebrovascular disease/rh or exp vertebrobasilar insufficiency/rh 28. time factor/ or treatment duration/ 29. (time or timing or intensive or intensity or augment$ or accelerate$ or additional or dosage or dose or frequency or amount or quantity).tw. 30. 28 or 29 31. 7 and 26 and 30 32. 27 and 30 33. 31 or 32 34. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 35. Randomization/ 36. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 37. control group/ or controlled study/ 38. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 39. Crossover Procedure/ 40. Double Blind Procedure/ 41. Single Blind Procedure/ or triple blind procedure/ 42. placebo/ or placebo effect/ 43. (random$ or RCT or RCTs).tw. 44. (controlled adj5 (trial$ or stud$)).tw. 45. (clinical$ adj5 trial$).tw. 46. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 47. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 48. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 49. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 50. (cross‐over or cross over or crossover).tw. 51. (placebo$ or sham).tw. 52. trial.ti. 53. (assign$ or allocat$).tw. 54. controls.tw. 55. or/34‐54 56. 33 and 55
Appendix 5. CINAHL search strategy
S1 (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units") S2 TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH) S3 TI ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)) OR AB ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)) S4 TI (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )) OR AB (( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )) S5 (MH "Hemiplegia") or (MH "Gait Disorders, Neurologic+") S6 TI (hemipleg* or hemipar* or paresis or paretic) OR AB (hemipleg* or hemipar* or paresis or paretic) S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 S8 (MH "Therapeutic Exercise+") S9 (MH "Applied Kinesiology") S10 (MH "Recreational Therapy") OR (MH "Rehabilitation+") S11 (MH "Movement") OR (MH "Motor Activity") S12 (MH "Exercise+") S13 (MH "Sports") S14 (MH "Physical Endurance+") OR (MH "Exertion") OR (MH "Exercise Intensity") S15 (MH "Physical Fitness") S16 TI ( (exercise n3 (train* or intervention* or protocol* or program* or therap* or activit* or regim*)) ) OR AB ( (exercise n3 (train* or intervention* or protocol* or program* or therap* or activit* or regim*)) ) S17 TI ( (fitness n3 (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*)) ) OR AB ( (fitness n3 (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*)) ) S18 TI ( ((training or conditioning) n3 (intervention* or protocol* or program* or activit* or regim*) ) OR AB ( ((training or conditioning) n3 (intervention* or protocol* or program* or activit* or regim*) ) S19 TI ( (sport* or recreation* or leisure or cycling or bicycl* or rowing or treadmill* or running or circuit training or swim* or walk* or dance* or dancing or tai ji or tai chi or yoga) ) OR AB ( (sport* or recreation* or leisure or cycling or bicycl* or rowing or treadmill* or running or circuit training or swim* or walk* or dance* or dancing or tai ji or tai chi or yoga) ) S20 TI ( ((endurance or aerobic or cardio*) n3 (fitness or train* or intervention* or protocol* or program* or therap* or activit* or regim*)) ) OR AB ( ((endurance or aerobic or cardio*) n3 (fitness or train* or intervention* or protocol* or program* or therap* or activit* or regim*)) ) S21 TI ( (muscle strengthening or progressive resist*) ) OR AB ( (muscle strengthening or progressive resist*) ) S22 TI ( ((weight or strength* or resistance) n3 (train* or lift* or exercise*)) ) OR AB ( ((weight or strength* or resistance) n3 (train* or lift* or exercise*)) ) S23 TI ( ((isometric or isotonic or eccentric or concentric) n3 (action* or contraction* or exercise*)) ) OR AB ( ((isometric or isotonic or eccentric or concentric) n3 (action* or contraction* or exercise*)) ) S24 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 S25 TI ( (time or timing or intensive or intensity or augment* or accelerate* or additional or dosage or dose or frequency or amount or quantity) ) OR AB ( (time or timing or intensive or intensity or augment* or accelerate* or additional or dosage or dose or frequency or amount or quantity) ) S26 S24 AND S25 S27 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S28 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S29 TI random* or AB random* S30 AB "latin square" or TI "latin square" S31 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S32 MH Placebos S33 TI ( ((singl* or doubl* or trebl* or tripl*) N3 (blind* or mask*)) ) OR AB ( ((singl* or doubl* or trebl* or tripl*) N3 (blind* or mask*)) ) S34 TI Placebo* or AB Placebo* or SU Placebo* S35 MH Clinical Trials S36 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S37 S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 S38 S7 AND S26 AND S37
Appendix 6. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw. 7. or/1‐6 8. physical therapy modalities/ or exp exercise therapy/ or hydrotherapy/ or physical medicine/ or physical therapy speciality/ 9. exp applied kinesiology/ or rehabilitation/ or "activities of daily living"/ or therapy/ 10. rehabilitation modalities/ or exp occupational therapy modalities/ or rehabilitation psychosocial/ or exp rehabilitation vocational/ or rehabilitation techniques/ 11. exp movement/ 12. exercise/ or weight training/ or physical fitness/ or exp sports/ or exp exercise testing/ 13. exertion/ or exp physical endurance/ 14. exp muscle contraction/ 15. (physiotherap$ or (physical adj3 (mobilis$ or mobiliz$ or exercise$ or exertion or endurance or therap$ or conditioning or activit$ or fitness))).tw. 16. (rehabilitation or recovery of function or exercise$ or mobilis$ or mobiliz$ or motion therap$ or motor activit$ or motor skill$ or activities of daily living or adl or manipulat$ or (occupational adj3 (train$ or rehab$ or therap$ or activit$ or regim$))).tw. 17. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 18. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$ or centre$ or center$)).tw. 19. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 20. (sport$ or recreation$ or leisure or cycling or bicycl$ or rowing or treadmill$ or running or circuit training or swim$ or walk$ or dance$ or dancing or tai ji or tai chi or yoga).tw. 21. ((endurance or aerobic or cardio$) adj3 (fitness or train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 22. (muscle strengthening or progressive resist$).tw. 23. ((weight or strength$ or resistance) adj3 (train$ or lift$ or exercise$)).tw. 24. ((isometric or isotonic or eccentric or concentric) adj3 (action$ or contraction$ or exercise$)).tw. 25. or/8‐24 26. (time or timing or intensive or intensity or augment$ or accelerate$ or additional or dosage or dose or frequency or amount or quantity).tw. 27. 25 and 26 28. clinical trials/ 29. randomized controlled trial.pt. 30. controlled clinical trial.pt. 31. placebo.ab. 32. random$.ab. 33. trial.ab. 34. groups.ab. 35. or/28‐34 36. 7 and 27 and 35
Appendix 7. PsycINFO search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ 6. 1 or 2 or 3 or 4 or 5 7. rehabilitation/ or cognitive rehabilitation/ or neuropsychological rehabilitation/ or neurorehabilitation/ or occupational therapy/ or physical therapy/ or exp psychosocial rehabilitation/ 8. "activities of daily living"/ 9. recreation therapy/ or psychotherapy/ 10. exp exercise/ 11. exp motor performance/ 12. exp sports/ 13. physical fitness/ or physical endurance/ or physical strength/ 14. (physiotherap$ or (physical adj3 (mobilis$ or mobiliz$ or exercise$ or exertion or endurance or therap$ or conditioning or activit$ or fitness))).tw. 15. (rehabilitation or recovery of function or exercise$ or mobilis$ or mobiliz$ or motion therap$ or motor activit$ or motor skill$ or activities of daily living or adl or manipulat$ or (occupational adj3 (train$ or rehab$ or therap$ or activit$ or regim$))).tw. 16. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 17. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$ or centre$ or center$)).tw. 18. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 19. (sport$ or recreation$ or leisure or cycling or bicycl$ or rowing or treadmill$ or running or circuit training or swim$ or walk$ or dance$ or dancing or tai ji or tai chi or yoga).tw. 20. ((endurance or aerobic or cardio$) adj3 (fitness or train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 21. (muscle strengthening or progressive resist$).tw. 22. ((weight or strength$ or resistance) adj3 (train$ or lift$ or exercise$)).tw. 23. ((isometric or isotonic or eccentric or concentric) adj3 (action$ or contraction$ or exercise$)).tw. 24. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 25. (time or timing or intensive or intensity or augment$ or accelerate$ or additional or dosage or dose or frequency or amount or quantity).tw. 26. 24 and 25 27. clinical trials/ or treatment effectiveness evaluation/ or placebo/ 28. (random$ or RCT or RCTs).tw. 29. (controlled adj5 (trial$ or stud$)).tw. 30. (clinical$ adj5 trial$).tw. 31. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 32. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 33. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 34. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 35. (cross‐over or cross over or crossover).tw. 36. (placebo$ or sham).tw. 37. trial.ti. 38. (assign$ or allocat$).tw. 39. controls.tw. 40. or/27‐39 41. 6 and 26 and 40
Appendix 8. Open Grey search strategy
(stroke OR cereb* OR cva* OR subarachnoid OR brain) AND (physio* OR physical OR exercise* OR therap* OR rehab*) AND (Intens* OR augment* OR additional OR dosage OR dose OR frequen* OR amount OR quantity)
Appendix 9. OT Seeker search strategy
(stroke OR cereb* OR cva* OR subarachnoid OR brain) AND (physio* OR physical OR exercise* OR therap* OR rehab*) AND (Intens* OR augment* OR additional OR dosage OR dose OR frequen* OR amount OR quantity)
Appendix 10. PEDro search strategy
1. neurology in the <Subdiscipline> field
2. clinical trial in the <Method> field
3. (tim* OR intens* OR augment* OR accelerate* OR additional* OR dosage OR dose OR frequency OR amount OR quantity) in the <Title & Abstract> field
4. 1 AND 2 AND 3
Appendix 11. REHABDATA search strategy
Key Concept 1 ‐ Stroke:
stroke OR cereb* OR cva* OR subarachnoid OR brain
Key Concept 2 ‐ Physio/ OT/Rehab Exercise Interventions
physiotherap* OR physical OR exercise* OR therap* OR rehab*
Key Concept 3 ‐ Frequency/Intensity
Intens* OR augment* OR additional OR dosage OR dose OR frequen* OR amount OR quantity
The method for search was to run the three key concept searches, then combined the queries. This had to be done by the staff at the company who manage REHABDATA, as it was a ‘back end’ search – the website doesn’t currently have the functionality to conduct a search using the method required.
Appendix 12. ProQuest Dissertations & Theses search strategy
Set#: S1 Searched for: ti,ab(stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH) OR ti,ab((brain* or cerebr* or cerebell* or intracran* or intracerebral) NEAR/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*)) OR ti,ab((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) NEAR/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)) OR ti,ab(hemipleg* or hemipar* or paresis or paretic)
Set#: S2 Searched for: ti,ab(exercise OR rehabilit* OR physiotherapy OR therapy)
Set#: S3 Searched for: ti,ab(time OR timing OR intensive OR intensity OR dosage OR dose OR quantity OR amount)
Set#: S4 Searched for: S1 AND S2 AND S3
Appendix 13. ClinicalTrials.gov search strategy
(exercise OR physical therapy OR rehabilitation ) AND ( time OR timing OR intensive OR intensity OR quantity OR amount OR dose ) AND ( Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke ) [DISEASE]
Appendix 14. EU Clinical Trials Register search strategy
Six separate searched run:
Stroke AND Rehabilitation AND Intensity
Stroke AND Rehabilitation AND Amount
Stroke AND Rehabilitation AND Dose
Stroke AND therapy AND Intensity
Stroke AND therapy AND Amount
Stroke AND therapy AND Dose
Appendix 15. ISRCTN Registry search strategy
Filtered the database for all “Nervous system diseases” and included all results.
Unable to run a more precise search
Appendix 16. WHO ICTRP search strategy
stroke AND therapy AND dose Synonyms: stroke, ACCIDENT CEREBROVASCULAR, accident; cerebral, accident; cerebrovascular, Apoplexy, Apoplexy Cerebrovascular, apoplexy; cerebral, Brain Attack, Brain Vascular Accident, Brain Vascular Accidents, Cerebral vascular accident, Cerebral vascular events, cerebral; accident, cerebral; apoplexy, Cerebrovascular accident, Cerebrovascular accident (disorder), Cerebrovascular accident NOS, Cerebrovascular accident NOS, Cerebrovascular Accidents, Cerebrovascular Apoplexy, cerebrovascular; accident, CVA, CVA (cerebral vascular accident), CVA (Cerebrovascular Accident), CVA NOS, CVAs (Cerebrovascular Accident), Neuro: Cerebrovascular accident, Vascular Accident Brain, Vascular Accidents Brain AND therapy, disease management, THER, Therapeutic, therapeutic aspects, therapeutic method, Therapeutic proced, Therapeutic procedure, Therapeutic procedure (procedure), Therapeutic procedure NOS, Therapeutic Procedures, Therapeutics, therapies, TREAT, treatment, treatment method, Treatments AND dose, Dosage, Dosage (attribute), Dosages, Dosages (qualifier value), TRTDOS
stroke AND rehabilitation AND dose Synonyms: stroke, ACCIDENT CEREBROVASCULAR, accident; cerebral, accident; cerebrovascular, Apoplexy, Apoplexy Cerebrovascular, apoplexy; cerebral, Brain Attack, Brain Vascular Accident, Brain Vascular Accidents, Cerebral vascular accident, Cerebral vascular events, cerebral; accident, cerebral; apoplexy, Cerebrovascular accident, Cerebrovascular accident (disorder), Cerebrovascular accident NOS, Cerebrovascular accident NOS, Cerebrovascular Accidents, Cerebrovascular Apoplexy, cerebrovascular; accident, CVA, CVA (cerebral vascular accident), CVA (Cerebrovascular Accident), CVA NOS, CVAs (Cerebrovascular Accident), Neuro: Cerebrovascular accident, Vascular Accident Brain, Vascular Accidents Brain AND rehabilitation, Physical Therapy, rehab.asistnce, REHABIL AND dose, Dosage, Dosage (attribute), Dosages, Dosages (qualifier value), TRTDOS
stroke AND therapy AND intensity Synonyms: stroke, ACCIDENT CEREBROVASCULAR, accident; cerebral, accident; cerebrovascular, Apoplexy, Apoplexy Cerebrovascular, apoplexy; cerebral, Brain Attack, Brain Vascular Accident, Brain Vascular Accidents, Cerebral vascular accident, Cerebral vascular events, cerebral; accident, cerebral; apoplexy, Cerebrovascular accident, Cerebrovascular accident (disorder), Cerebrovascular accident NOS, Cerebrovascular accident NOS, Cerebrovascular Accidents, Cerebrovascular Apoplexy, cerebrovascular; accident, CVA, CVA (cerebral vascular accident), CVA (Cerebrovascular Accident), CVA NOS, CVAs (Cerebrovascular Accident), Neuro: Cerebrovascular accident, Vascular Accident Brain, Vascular Accidents Brain AND therapy, disease management, THER, Therapeutic, therapeutic aspects, therapeutic method, Therapeutic proced, Therapeutic procedure, Therapeutic procedure (procedure), Therapeutic procedure NOS, Therapeutic Procedures, Therapeutics, therapies, TREAT, treatment, treatment method, Treatments AND intensity, Intense, Severity
stroke AND rehabilitation AND intensity Synonyms: stroke, ACCIDENT CEREBROVASCULAR, accident; cerebral, accident; cerebrovascular, Apoplexy, Apoplexy Cerebrovascular, apoplexy; cerebral, Brain Attack, Brain Vascular Accident, Brain Vascular Accidents, Cerebral vascular accident, Cerebral vascular events, cerebral; accident, cerebral; apoplexy, Cerebrovascular accident, Cerebrovascular accident (disorder), Cerebrovascular accident NOS, Cerebrovascular accident NOS, Cerebrovascular Accidents, Cerebrovascular Apoplexy, cerebrovascular; accident, CVA, CVA (cerebral vascular accident), CVA (Cerebrovascular Accident), CVA NOS, CVAs (Cerebrovascular Accident), Neuro: Cerebrovascular accident, Vascular Accident Brain, Vascular Accidents Brain AND rehabilitation, Physical Therapy, rehab.asistnce, REHABIL AND intensity, Intense, Severity
stroke AND rehabilitation AND amount Synonyms: stroke, ACCIDENT CEREBROVASCULAR, accident; cerebral, accident; cerebrovascular, Apoplexy, Apoplexy Cerebrovascular, apoplexy; cerebral, Brain Attack, Brain Vascular Accident, Brain Vascular Accidents, Cerebral vascular accident, Cerebral vascular events, cerebral; accident, cerebral; apoplexy, Cerebrovascular accident, Cerebrovascular accident (disorder), Cerebrovascular accident NOS, Cerebrovascular accident NOS, Cerebrovascular Accidents, Cerebrovascular Apoplexy, cerebrovascular; accident, CVA, CVA (cerebral vascular accident), CVA (Cerebrovascular Accident), CVA NOS, CVAs (Cerebrovascular Accident), Neuro: Cerebrovascular accident, Vascular Accident Brain, Vascular Accidents Brain AND rehabilitation, Physical Therapy, rehab.asistnce, REHABIL AND amount, 050‐051 QUANTITIES, QUANTITIES, Quantity, Quantity (attribute), Quantity finding, Quantity finding (finding)
stroke AND therapy AND amount Synonyms: stroke, ACCIDENT CEREBROVASCULAR, accident; cerebral, accident; cerebrovascular, Apoplexy, Apoplexy Cerebrovascular, apoplexy; cerebral, Brain Attack, Brain Vascular Accident, Brain Vascular Accidents, Cerebral vascular accident, Cerebral vascular events, cerebral; accident, cerebral; apoplexy, Cerebrovascular accident, Cerebrovascular accident (disorder), Cerebrovascular accident NOS, Cerebrovascular accident NOS, Cerebrovascular Accidents, Cerebrovascular Apoplexy, cerebrovascular; accident, CVA, CVA (cerebral vascular accident), CVA (Cerebrovascular Accident), CVA NOS, CVAs (Cerebrovascular Accident), Neuro: Cerebrovascular accident, Vascular Accident Brain, Vascular Accidents Brain AND therapy, disease management, THER, Therapeutic, therapeutic aspects, therapeutic method, Therapeutic proced, Therapeutic procedure, Therapeutic procedure (procedure), Therapeutic procedure NOS, Therapeutic Procedures, Therapeutics, therapies, TREAT, treatment, treatment method, Treatments AND amount, 050‐051 QUANTITIES, QUANTITIES, Quantity, Quantity (attribute), Quantity finding, Quantity finding (finding)
Data and analyses
Comparison 1. Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – immediate outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Activities of daily living outcomes | 19 | 864 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.02, 0.28] |
| 1.2 Activity measures of the upper limb | 18 | 426 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.11, 0.29] |
| 1.3 Activity measures of the lower limb | 5 | 425 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.03, 0.53] |
| 1.4 Motor impairment measures of the upper limb | 12 | 287 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [0.06, 0.58] |
| 1.5 Motor impairment measures of the lower limb | 1 | 51 | Std. Mean Difference (IV, Random, 95% CI) | 0.71 [0.15, 1.28] |
| 1.6 Serious adverse events/death | 2 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.51, 2.85] |
Comparison 2. Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – medium‐term outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Activities of daily living outcomes: medium‐term outcomes | 12 | 673 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.15, 0.16] |
| 2.2 Activity measures of the upper limb: medium‐term outcomes | 9 | 218 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.36, 0.33] |
| 2.3 Activity measures of the lower limb: medium‐term outcomes | 4 | 243 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.30, 0.49] |
| 2.4 Motor impairment measures of the upper limb: medium‐term outcomes | 5 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.39, 0.35] |
| 2.5 Motor impairment measures of the lower limb: medium‐term outcomes | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [‐0.04, 1.28] |
| 2.6 Serious adverse events/death: medium‐term outcomes | 3 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.63, 2.76] |
Comparison 3. Objective one: more time spent in rehabilitation vs less time spent in rehabilitation – long‐term outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Activities of daily living outcomes: long‐term outcomes | 1 | 67 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.39, 0.57] |
| 3.2 Activity measures of the lower limb: long‐term outcomes | 1 | 67 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.32, 0.64] |
Comparison 4. Objective two: effect of total time spent in rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Activities of daily living outcomes: immediately after intervention | 17 | 805 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.02, 0.32] |
| 4.1.1 Median split – studies with a larger difference in total amount of therapy between treatment arms | 9 | 251 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.14, 0.66] |
| 4.1.2 Median split – studies with a smaller difference in total amount of therapy between treatment arms | 8 | 554 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.18, 0.20] |
| 4.2 Activity measures of the upper limb: immediately after intervention | 16 | 367 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.13, 0.33] |
| 4.2.1 Median split – studies with a larger difference in total amount of therapy between treatment arms | 8 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.03, 0.88] |
| 4.2.2 Median split – studies with a smaller difference in total amount of therapy between treatment arms | 8 | 238 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.34, 0.19] |
| 4.3 Activity measures of the lower limb: immediately after intervention | 4 | 370 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.09, 0.60] |
| 4.3.1 Median split – studies with a larger difference in total amount of therapy between treatment arms | 2 | 131 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.07, 0.76] |
| 4.3.2 Median split – studies with a smaller difference in total amount of therapy between treatment arms | 2 | 239 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.42, 0.69] |
| 4.4 Motor impairment measures of the upper limb: immediately after intervention | 10 | 228 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [0.14, 0.68] |
| 4.4.1 Median split – studies with a larger difference in total amount of therapy between treatment arms | 5 | 74 | Std. Mean Difference (IV, Random, 95% CI) | 0.82 [0.32, 1.32] |
| 4.4.2 Median split – studies with a smaller difference in total amount of therapy between treatment arms | 5 | 154 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.09, 0.56] |
Comparison 5. Objective three: effect of rehabilitation schedule .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Activities of daily living outcomes: immediately after intervention | 13 | 671 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.06, 0.36] |
| 5.1.1 Median split – studies with a larger difference in minutes of rehabilitation provided per week | 6 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.26, 0.78] |
| 5.1.2 Median split – studies with a smaller difference in minutes of rehabilitation provided per week | 7 | 501 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.14, 0.22] |
| 5.2 Activity measures of the upper limb: immediately after intervention | 13 | 300 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.15, 0.50] |
| 5.2.1 Median split – studies with a larger difference in minutes of rehabilitation provided per week | 6 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.58 [‐0.16, 1.31] |
| 5.2.2 Median split – studies with a smaller difference in minutes of rehabilitation provided per week | 7 | 175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.35, 0.28] |
| 5.3 Activity measures of the lower limb: immediately after intervention | 4 | 357 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.09, 0.62] |
| 5.3.1 Median split – studies with a larger difference in minutes of rehabilitation provided per week | 2 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.02, 0.75] |
| 5.3.2 Median split – studies with a smaller difference in minutes of rehabilitation provided per week | 2 | 236 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.48, 0.88] |
| 5.4 Motor impairments of the upper limb: immediately after intervention | 9 | 192 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [0.09, 0.72] |
| 5.4.1 Median split – studies with a larger difference in minutes of rehabilitation provided per week | 4 | 71 | Std. Mean Difference (IV, Random, 95% CI) | 0.69 [0.10, 1.28] |
| 5.4.2 Median split – studies with a smaller difference in minutes of rehabilitation provided per week | 5 | 121 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.11, 0.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdullahi 2018.
| Study characteristics | ||
| Methods | RCT Random sampling mCIMT 4 groups with group 3 and group 4 receiving 300 or 600 repetitions rather than 3 hours of mCIMT (group 2) or 3 hours traditional therapy (group 1) 5 tasks chosen were each practised either 20 or 40 times Participants blinded to what others did and asked not to discuss |
|
| Participants | 48 participants in 4 study groups (only 2 groups met the criteria for this Cochrane Review) 300 reps group: n = 12; mean age 59 (SD 14) years; mean 22 (SD 6) days poststroke 600 reps group: n = 11; mean age 58 (SD 10) years; mean 14 (SD 7) days poststroke No differences on these or other characteristics |
|
| Interventions | Intervention was mCIMT. The 300 reps group received 300 repetitions of shaping practice in 3 sessions per day (100 repetitions per session) and constraint for 90% of the waking hours. The 600 reps group received 600 repetitions of shaping practice in 3 sessions per day (200 repetitions per session) and constraint for 90% of the waking hours Treatment was 5 days per week for 4 weeks We do not know exactly how long this intervention took, as this was not measured in the study, but presumably the 600 group was approximately double the time of the 300 group |
|
| Outcomes | Motor function subscale of the upper limb FMA WMFT Motor Activity Log UPSET Measurements were performed at baseline, 2 weeks, and 4 weeks after intervention |
|
| Notes | For the purpose of the Cochrane Review, we only compared 2 of the 4 groups included in this study Conflict of interest: author stated no conflict of interest Funding: source unknown |
|
Ada 2013.
| Study characteristics | ||
| Methods | RCT Consent Baseline testing of cohorts of 15 Stratification of walking speed organised into triplets and then randomised into group Assessment Intervention Assessment and retention |
|
| Participants | 102 participants in 3 study arms Experimental group 1: n = 34; mean age 70 (SD 11) years; 71% male; 53% right‐sided weakness; mean chronicity 22 (SD 16) months Experimental group 2: n = 34; mean age 64 (SD 12) years; 82% male; 41% right‐sided weakness; mean chronicity 20 (SD 15) months All participants were within 5 years of their first stroke and had been discharged from formal rehabilitation Community setting in Australia |
|
| Interventions | The intervention was a treadmill and overground walking programme Experimental group 1: intervention provided for 30 minutes, 3 times per week for 16 weeks Experimental group 2: intervention provided for 30 minutes, 3 times per week for 8 weeks Control group: no intervention |
|
| Outcomes | 6‐minute walk test 10 metre walk test EuroQol EQ‐5D‐5L Adelaide Activities Profile Walking Self‐Efficacy Scale Number of falls Measurements were taken at baseline, 2 months, 4 months, 6 months, and 12 months |
|
| Notes | Control group was excluded from analysis, as they received no intervention This study controlled the rehabilitation duration. The number of minutes per session and the frequency of sessions were the same for each group Conflict of interest: the authors declared there was no conflict of interest Funding: Heart Foundation of Australia and the University of Sydney |
|
Burgar 2011.
| Study characteristics | ||
| Methods | RCT Medical clearance to participate Eligibility Consent Baseline testing Stratification by FMA upper extremity score and randomised at each site into 3 groups. Hi dose Robot, Lo dose Robot, and control (all extra to existing therapy) Intervention Testing and 6‐month retention For this Cochrane Review, we only compared the 2 robot groups |
|
| Participants | 54 participants, in 3 study arms, completed the preintervention testing and at least 5 hours of treatment Robot‐Lo group: n = 19; mean age 62.5 years; mean 17.3 days poststroke Robot‐Hi group: n = 17; mean age 58.6 years; mean 16.6 days poststroke Gender of participants: not reported No significant difference between the sites, other than in time since stroke There was a significant difference in age between the groups 3 inpatient settings in the USA |
|
| Interventions | The intervention was robot therapy. Robot‐Lo group (and the control group): up to 15 × 1‐hour therapy sessions over 3‐weeks Robot‐Hi group: 30 × 1‐hour therapy sessions over 3 weeks Intervention was terminated when the participant received the maximum number of sessions, or when they were discharged from acute inpatient rehabilitation |
|
| Outcomes | FMA upper limb Upper limb portion of the FIM Modified Ashworth Scale WMFT Measurements were taken before study initiation, after completion of training and at 6‐month follow‐up |
|
| Notes | Very few of the participants received the maximum number of additional input, as planned. Early discharges, scheduling conflicts, and patient tolerance were among the factors that reduced the total amount of therapy Conflict of interest: authors declared no competing financial interests Funding: supported by VA Rehabilitation and Service Development (B2695) |
|
Cooke 2010b.
| Study characteristics | ||
| Methods | RCT Potential participants assessed for eligibility Consented to the study obtained Baseline measurements taken Allocation was stratified by baseline scores for unilateral visual spatial neglect 6 weeks of intervention Postintervention and follow‐up measurements taken |
|
| Participants | 109 participants in 3 groups (only 2 groups met the criteria for this Cochrane Review) CPT group: n = 38; mean age 66.37 (SD 13.7) years; 55% males; mean 36.76 (SD 22.4) days poststroke CPT + CPT group: n = 35; mean age 67.46 (SD 11.3) years; 63% males; mean 32.43 (SD 21.29) days poststroke Participants were initially seen as inpatients, but intervention was completed as outpatients, if they were discharged before the end of the intervention period 4 clinical settings in the UK |
|
| Interventions | Intervention was CPT for the lower limbs. There was no prespecified schedule for routine CPT. Additional CPT was provided for up to 1 hour, 4 days per week, for 6 weeks. The study authors reported that the CPT group received a mean of 9.2 (SD 6.9) cumulative hours of treatment and the CPT + CPT group received a mean of 23 (SD 10.4) cumulative hours of treatment. | |
| Outcomes | Walking speed (metres per second) Ability to walk at ≥ 0.8 metres per second Torque around the paretic knee Modified RMI Temporal‐spatial gait parameters EuroQol Measurements were taken at baseline, end of intervention, and 12‐week follow‐up |
|
| Notes | Conflict of interests: none Funding: Healthcare Foundation and the Dowager Countess Eleanor Peel Trust |
|
Donaldson 2009.
| Study characteristics | ||
| Methods | RCT Baseline measurements taken, randomisation (group allocation was stratified by baseline ARAT scores), intervention began the following day Participants were randomised into 3 groups, 2 CPT groups and 1 group that received FST and CPT. For this Cochrane Review, we only compared the 2 CPT groups |
|
| Participants | 30 participants in 3 study arms CPT group: mean age 72.6 years; 5 males; mean 13.4 days poststroke CPT + CPT group: mean age 73.3 years; 5 males; mean 25.6 days poststroke Inpatient setting in the UK |
|
| Interventions | Intervention was CPT CPT was provided to all participants in the study, using a standardised treatment schedule. There is no evidence that there was a planned amount of CPT The CPT + CPT group received additional CPT provided for up to 1 hour, 4 days per week for 6 weeks. This was also recorded using a standardised treatment schedule |
|
| Outcomes | ARAT 9 Hole Peg Test Upper limb strength (measured with a myometer): grip, pinch, elbow flexion and extension Recorded at baseline; 6 weeks (end of intervention), and 12 weeks (follow‐up) |
|
| Notes | Conflict of interest: none declared Funding: The Wellcome Trust |
|
Dromerick 2009.
| Study characteristics | ||
| Methods | RCT Adaptively randomised balancing age, NIHSS score, pretest ARAT, and days from stroke Study compared 2 different CIMT protocols to control. For this Cochrane Review, we were interested in the 2 different protocols for CIMT only (excluding control) |
|
| Participants | 52 participants in 3 study arms Low CIMT: n= 19; mean age 62.8 years; 68% female; 8.8 days poststroke High CIMT: n = 16; mean age 64.5 years; 44% female; 9.9 days poststroke Inpatient rehabilitation setting |
|
| Interventions | Intervention was CIMT Prespecified treatment (pre‐empted OT) based on Excite + RPT Received extensive verbal and written feedback and review of prior day's achievements, day's goals and reinforcement of new gains and maintenance Low CIMT: 2 hours of shaping therapy per day and padded constraint mitt for 6 hours per day High CIMT: 3 hours of shaping therapy per day and padded constraint mitt for 90% of waking hours Study treatment occurred 5 days per week for 2 weeks (consecutively) |
|
| Outcomes | NIHSS ARAT FIM SIS hand function subscale Pain ratings Geriatric Depression Scale All measures were taken on baseline, at 14 days (postintervention) and at 90 days |
|
| Notes | Conflict of interests: authors disclosed the following: Dr Dromerick has received research support from NIH, NINDS, and United States Veterans Affairs, and Intramural support from the United States Army, Department of Defense. Dr Lang has received research support from NIH, NINDS, and the Missouri Physical Therapy Association. Dr Miller has served on the Data and Safety Monitoring Board for Ethicon. Dr Powers has received research support from University of North Carolina, Washington University, University of Iowa, University of Kentucky, Harvard University, Bowdoin College, NIH, Legatus Emergency Services, LLC, Neutral, LLC, EDJ Associates, Hitchcock Foundation, Dartmouth‐Hitchcock Clinic, Certus International, Inc., Companion Baking Company, and Union Square Hospitality Group. Dr Wolf is supported by NIH, Allergan, and AMES Funding: NIH grant 1 RO1 NS41261‐01A1 and James S. McDonnell Foundation grant 21002032 |
|
English 2015.
| Study characteristics | ||
| Methods | RCT Participants were recruited into the study, and randomised into 1 of 3 treatment arms Assessment Intervention Postintervention and follow‐up assessment |
|
| Participants | 283 participants in 3 study groups (only 2 study groups met the criteria for this Cochrane Review) Usual care (5 day): n = 94; mean age 68.2 years; 52 males; 28.7 days between stroke and randomisation 7‐day therapy: n = 96; mean age 71.9 years; 59 males; 25.0 days between stroke and randomisation All participants were 5–197 days poststroke on entry to the study Inpatient setting in Australia |
|
| Interventions | 7‐day therapy: therapy provided 7 days per week for a maximum of 90 minutes per day (maximum of 630 minutes per week) Usual care (5‐day therapy): therapy provided 5 days per week for a maximum of 90 minutes per day (maximum of 450 minutes per week) Intervention was provided over 4 weeks |
|
| Outcomes | FIM WMFT 6‐minute walk test Walking speed Functional Ambulation Classification SIS physical subscale Length of stay AQoL Scale Adverse events Assessed at baseline, 4 weeks, 3 months and 6 months postrandomisation (SIS and the AQoL no baseline) |
|
| Notes | Study controlled for the number of minutes of rehabilitation provided each week and the frequency of rehabilitation intervention Conflict of Interests: none Funding: supported by a National Health and Medical Research Project Council Grant #631904 |
|
GAPS 2004.
| Study characteristics | ||
| Methods | RCT Informed consent, randomised, stratified by study site, age, and level of severity Randomised to AP or SP Participants in both groups had normal access to all other interventions |
|
| Participants | 70 participants in 2 study groups AP group: n = 35; mean age 68 (SD 11) years; 31% female; 46% right hemisphere stroke SP group: n = 35; mean age 67 (SD 10) years; 51% female; 43% right hemisphere stroke All participants were 6–71 days poststroke on entry into the study Inpatient settings in Scotland, UK |
|
| Interventions | Intervention was RPT, based on the Normal Movement (Bobath) approach. Ambition was to provide the AP group double the amount (60–80 minutes) of RPT compared to the SP group (30–40 minutes), 5 days per week. Intervention continued for the duration of the participants' inpatient stay |
|
| Outcomes | Trunk Control Test Motricity Index Achievement of mobility milestones RMI Walking speed Barthel Index NEADL EuroQol Discharge home, length of stay in hospital, delays to discharges Complications Measures taken: baseline, 4 weeks, 3 months, and 6 months |
|
| Notes | Study controlled for daily amount and frequency of rehabilitation, but not overall duration Although the ambition was for the AP group to receive double the amount of therapy to the SP group, the reality was they only received 62% more therapy Conflict of interest: none declared Funding: National Health and Medical Research Project Council Grant #631904 |
|
Han 2013a.
| Study characteristics | ||
| Methods | RCT Eligibility Consent Randomisation by random number tables and sealed envelopes Baseline testing Intervention motor relearning for 5 days per week, for 6 weeks |
|
| Participants | 32 participants in 3 study groups. 2 dropped out, so data analysed for 30 Group A: n = 10; mean age 52.4 years; 7 males; mean 41.4 days to randomisation Group B: n = 10; mean age 53.7 years; 8 males; mean 42.9 days to randomisation Group C: n = 10; mean age 44.6 years; 8 males; mean 38.3 days to randomisation No significant difference between the groups |
|
| Interventions | Intervention was upper limb rehabilitation Group A: 1 hour per day Group B: 2 hours per day Group C: 3 hours per day Duration could be distributed throughout the day All intervention was provided 5 days per week for 6 weeks |
|
| Outcomes | FMA ARAT Barthel Index Measured at baseline, 2 weeks, 4 weeks, and 6 weeks |
|
| Notes | Han 2013a refers to the following pair‐wise comparison: group A (1 hour) (n = 5) vs group B (2 hours) (n = 10) Conflict of interest: none declared Funding: no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors |
|
Han 2013b.
| Study characteristics | ||
| Methods | RCT Eligibility Consent Randomisation by random number tables and sealed envelopes Baseline testing Intervention motor relearning for 5 days per week, for 6 weeks |
|
| Participants | 32 participants in 3 study groups. 2 dropped out, so data analysed for 30 Group A: n = 10; mean age 52.4 years; 7 males; mean 41.4 days to randomisation Group B: n = 10; mean age 53.7 years; 8 males; mean 42.9 days to randomisation Group C: n = 10; mean age 44.6 years; 8 males; mean 38.3 days to randomisation No significant difference between the groups |
|
| Interventions | Intervention was upper limb rehabilitation Group A: 1 hour per day Group B: 2 hours per day Group C: 3 hours per day Duration could be distributed throughout the day All intervention was provided 5 days per week for 6 weeks |
|
| Outcomes | FMA ARAT Barthel Index Measured at baseline, 2 weeks, 4 weeks, and 6 weeks |
|
| Notes | Han 2013b refers to the following pair‐wise comparison: group A (1 hour) (n = 5) vs group C (3 hours) (n = 10) Conflict of interest: none declared Funding: no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors |
|
Hsieh 2012.
| Study characteristics | ||
| Methods | Randomised block‐controlled trial Randomised using random numbers; stratification based on lesion side and motor deficit level Robot therapy (RT) was Bi‐Manu‐Track (allows forearm supination/pronation and wrist flexion/extension) with duration control of 3 groups (90–105 minutes) Repetitions were high or low intensity with high having twice the number of repetitions per unit time than lower Before RT, 5 minutes of mobilisation and afterwards 15–20 minutes of functional activities practice Control group had intensive standard therapy For this Cochrane Review, we only compared the 2 RT groups |
|
| Participants | 54 participants in 3 study groups High RT: n = 18; mean age 56.5 (SD 10) years; 11 males; 28.7 (SD 13.7) months between stroke and randomisation Low RT: n = 18; mean age 52.2 (SD 12) years; 13 males; 23.3 (SD 15.4) months between stroke and randomisation No differences between these or other characteristics Participants were all > 6 months poststroke |
|
| Interventions | Intervention was RT All participants received a duration‐matched intervention for 90–105 minutes of therapy per day, for 5 days per week for 4 weeks Higher‐intensity RT: 600–800 repetitions of modes 1 and 2 for 15–20 minutes and 150–200 reps of mode 3 for 3–5 minutes Lower‐intensity RT: received half the number of repetitions as the higher‐intensity group |
|
| Outcomes | Upper extremity items of the FMA Medical Research Council Scale (muscle power scale 0–5) Motor Activity Log (amount of use and quality of movement) 4 physical domains on the SIS (strength, ADLs, mobility, and hand function) Pain (scale 0–10) Fatigue (scale 0–10) All measures were administered at baseline and immediately after intervention. The primary outcome was also administered 2 weeks after the treatment began |
|
| Notes | Authors provided mean and SDs for the post‐treatment Motor Activity Log (amount of use) and SIS hand function, as these were presented as change scores in the paper Conflict of interest: none Funding: supported in part by the National Health Research Institutes (NHRI‐EX101‐9920PI and NHRI‐EX101‐10010PI), the National Science Council (NSC‐100‐2314‐B‐002‐008‐MY3 and NSC 99‐2314‐B‐182‐014‐MY3), and the Healthy Ageing Research Center at Chang Gung University (EMRPD1A0891) in Taiwan |
|
Hsu 2010.
| Study characteristics | ||
| Methods | RCT Participants who satisfied selection criteria were randomised into 3 groups: high‐NMES, low‐NMES, or control. For this Cochrane Review, we excluded the control group |
|
| Participants | 66 participants in 3 study groups; 22 in each group High‐NMES group: mean age 60.2 years; 15% male; mean 23.3 days poststroke Low‐NMES group: mean age 62 years; 15% male; mean 21 days poststroke Study setting was not reported, but presumed to be inpatient |
|
| Interventions | Intervention was NMES All participants received standard rehabilitation. In addition to this, the 2 NMES groups received an additional 4 weeks of NMES, 5 days a week. The high‐NMES group received 60 minutes of treatment a day, and the low‐NMES group received 30 minutes of treatment a day |
|
| Outcomes | FMA upper extremity motor section ARAT Motor Activity Log (only assessed at follow‐up) Measurements were taken at baseline, 4 weeks, and 2 months |
|
| Notes | Conflict of interests: none Funding: partially supported by the Bureau of Health Promotion, Department of Health, ROC (Taiwan), through grants DOH93‐HP‐1114DOH94‐HP‐1114 |
|
Hunter 2011a.
| Study characteristics | ||
| Methods | RCT Randomised (independent, concealed) to 4 groups. 3 groups received different amounts of the intervention and the 4th was a control group. For this Cochrane Review, we excluded the control group Stratified by clinical centre, severity of paresis, and spatial neglect |
|
| Participants | 76 participants in 4 study groups. All participants were 8–84 days postevent Group 2: n = 18; mean age 73.3 years; 61% male Group 3: n = 19; mean age 72.9 years; 42% male Group 4: n = 20; mean age 72.5 years; 45% male Inpatient setting (or at home if discharged) in the UK |
|
| Interventions | The intervention was MTS of the forearm and hand. This intervention had specific components that could be adapted to individual presentation Group 2: up to 30 minutes per day of MTS Group 3: up to 60 minutes per day of MTS Group 4: up to 120 minutes per day of MTS The intervention was provided every working day for 14 days |
|
| Outcomes | Primary outcome: arm section of the Motricity Index Secondary outcome: ARAT Measurements were at baseline and end of intervention phase. Adverse events were monitored on each working day |
|
| Notes | Hunter 2011a reports the following pair‐wise comparison: group 2 (n = 9) vs group 3 (n = 18) Both the planned number of minutes and the actual number of minutes of therapy provided are recorded Paper reported change scores, but authors kindly provided raw data, so mean and SD could be calculated Conflict of interest: no potential conflicts of interest with respect to the authorship or publication of this article Funding: the author(s) disclosed receipt of the following financial support for the research or authorship (or both) of this article. Quote: "We are grateful to The Stroke Association for the provision of funding for this study. The Stroke Association had no role in the design, conduction, or interpretation of the results of this study." |
|
Hunter 2011b.
| Study characteristics | ||
| Methods | RCT Randomised (independent, concealed) to 4 groups. 3 groups received different amounts of the intervention and the 4th was a control group. For this Cochrane Review, we excluded the control group Stratified by clinical centre, severity of paresis, and spatial neglect |
|
| Participants | 76 participants in 4 study groups. All participants were 8–84 days postevent Group 2: n = 18; mean age 73.3 years; 61% male Group 3: n = 19; mean age 72.9 years; 42% male Group 4: n = 20; mean age 72.5 years; 45% male Inpatient setting (or at home if discharged) in the UK |
|
| Interventions | The intervention was MTS of the forearm and hand. This intervention had specific components that could be adapted to individual presentation Group 2: up to 30 minutes a day of MTS Group 3: up to 60 minutes a day of MTS Group 4: up to 120 minutes a day of MTS The intervention was provided every working day for 14 days |
|
| Outcomes | Primary outcome: arm section of the Motricity Index Secondary outcome: ARAT Measurements were at baseline and end of intervention phase. Adverse events were monitored on each working day |
|
| Notes | Hunter 2011b reports the following pair‐wise comparison: group 2 (n = 9) vs group 4 (n = 20) Both the planned number of minutes and the actual number of minutes of therapy provided are recorded Paper reported change scores, but authors kindly provided raw data, so mean and SD could be calculated Conflict of interest: no potential conflicts of interest with respect to the authorship or publication of this article Funding: the author(s) disclosed receipt of the following financial support for the research or authorship (or both) of the article. Quote: "We are grateful to The Stroke Association for the provision of funding for this study. The Stroke Association had no role in the design, conduction, or interpretation of the results of this study." |
|
Kowalczewski 2007.
| Study characteristics | ||
| Methods | RCT Participants who met the eligibility criteria for the study were randomised into either a low‐intensity FES‐ET group or a high‐intensity FES‐ET group As well as the experimental treatment, participants also received regular hand function therapy |
|
| Participants | 19 participants in 2 study arms High‐intensity FES‐ET: n = 10; mean age 59.4 years; 4 males; mean 1.6 months poststroke Low‐intensity FES‐ET: n = 9; mean age 61.7 years; 6 males; mean 1.6 months poststroke Canadian inpatient rehabilitation unit |
|
| Interventions | Intervention was FES‐ET Both groups received intervention for 3–4 weeks in addition to regular hand therapy (1 hour per day 3–4 days per week) Low‐intensity group: 15 minutes of sensory stimulation × 4 days per week and 60 minutes on day 5 High‐intensity group: 60 minutes for 15–20 consecutive days on the workstation |
|
| Outcomes | WMFT FMA upper extremity Motor Activity Log Assessed at baseline, post‐treatment, and at 3 and 6 month follow‐up Kinematic data generated by the workstation not reported at follow‐up for logistical reasons |
|
| Notes | Conflict of interests: quote: "No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated." Funding: the author(s) disclosed receipt of the following financial support for the research or authorship (or both) of this article: "We are grateful to The Stroke Association for the provision of funding for this study. The Stroke Association had no role in the design, conduction, or interpretation of the results of this study." |
|
Lang 2016a.
| Study characteristics | ||
| Methods | Parallel RCT Participants who met the selection criteria were randomised into 4 groups, each received a different number of repetitions |
|
| Participants | 85 participants in 4 study groups 3.2k reps group: n = 21; mean age 59.9 years; 7 females; 12.0 months poststroke 6.4k reps group: n = 22; mean age 62.1 years; 5 females; 13.0 months poststroke 9.6k reps group: n = 21; mean age 60.0 years; 10 females; 13.0 months poststroke Individualised maximum group: n = 21; mean age 60.9 years; 8 females; 11.5 months poststroke Outpatient setting |
|
| Interventions | Intervention was supervised, massed practice of functional upper limb daily tasks, e.g. reaching, grasping, moving/manipulating, and releasing object. The participants in the 4 groups were encouraged to perform a certain number of repetitions of exercise, dependent on their group allocation Number of reps per session were: 100, 200, or 300. The individualised maximum group aimed for 300 reps per session, but to continue receiving therapy until certain criteria had been met |
|
| Outcomes | ARAT (primary outcome measurement) SIS (hand and ADL subscales) COPM 7‐point Likert scale to evaluate if the participant though they had changed, and if that change was meaningful Measures taken at baseline (prior to randomisation, postintervention, and then 2 months later |
|
| Notes | Lang 2016a refers to the following pair‐wise comparison: 3.2k rep (n = 7) vs 6.4k rep (n = 19) This study specified repetitions of exercise, as opposed to time spent, but also report the number of minutes of 'active practice' undertaken in each group Conflict of interest: none Funding: NIH R01 HD068290 |
|
Lang 2016b.
| Study characteristics | ||
| Methods | Parallel RCT Participants who met the selection criteria were randomised into 4 groups, each received different number of repetitions |
|
| Participants | 85 participants in 4 study groups 3.2k reps group: n = 21; mean age 59.9 years; 7 females; 12.0 months poststroke 6.4k reps group: n = 22; mean age 62.1 years; 5 females; 13.0 months poststroke 9.6k reps group: n = 21; mean age 60.0 years; 10 females; 13.0 months poststroke Individualised maximum group: n = 21; mean age 60.9 years; 8 females; 11.5 months poststroke Outpatient setting |
|
| Interventions | Intervention was supervised, massed practice of functional upper limb daily tasks, e.g. reaching, grasping, moving/manipulating, and releasing object. The participants in the 4 groups were encouraged to perform a certain number of repetitions of exercise, dependent on their group allocation Number of reps per session were: 100, 200, or 300. The individualised maximum group aimed for 300 reps per session, but to continue receiving therapy until certain criteria had been met |
|
| Outcomes | ARAT (primary outcome measurement) SIS (hand and ADL subscales) COPM 7‐point Likert scale to evaluate if the participant though they had changed, and if that change was meaningful Measures taken at baseline (prior to randomisation, postintervention, and then 2 months later |
|
| Notes | Lang 2016b refers to the following pair‐wise comparison: 3.2k rep (n = 6) vs 9.6k reps (n = 17) This study specified repetitions of exercise, as opposed to time spent, but also report the number of minutes of 'active practice' undertaken in each group Conflict of interest: none Funding: NIH R01 HD068290 |
|
Lang 2016c.
| Study characteristics | ||
| Methods | Parallel RCT Participants who met the selection criteria were randomised into 4 groups, each received different number of repetitions |
|
| Participants | 85 participants in 4 study groups 3.2k reps group: n = 21; mean age 59.9 years; 7 females; 12.0 months poststroke 6.4k reps group: n = 22; mean age 62.1 years; 5 females; 13.0 months poststroke 9.6k reps group: n = 21; mean age 60.0 years; 10 females; 13.0 months poststroke Individualised maximum group: n = 21; mean age 60.9 years; 8 females; 11.5 months poststroke Outpatient setting |
|
| Interventions | Intervention was supervised, massed practice of functional upper limb daily tasks e.g. reaching, grasping, moving/manipulating, and releasing object. The participants in the 4 groups were encouraged to perform a certain number of repetitions of exercise, dependent on their group allocation Number of reps per session were: 100, 200, or 300. The individualised maximum group aimed for 300 reps per session, but to continue receiving therapy until certain criteria had been met |
|
| Outcomes | ARAT (primary outcome measurement) SIS (hand and ADL subscales) COPM 7‐point Likert scale to evaluate if the participant though they had changed, and if that change was meaningful Measures taken at baseline (prior to randomisation, postintervention, and then 2 months later |
|
| Notes | Lang 2016c refers to the following pair‐wise comparison: 3.2k rep (n = 6) vs individualised maximum group (n = 18) This study specified repetitions of exercise, as opposed to time spent, but also report the number of minutes of 'active practice' undertaken in each group Conflict of interest: none Funding: NIH R01 HD068290 |
|
Lincoln 1999.
| Study characteristics | ||
| Methods | RCT Participants who met the inclusion criteria for the study were assessed and randomised into 1 of 3 groups; RPT, QPT, and APT. For this Cochrane Review, we excluded the group treated by the assistant |
|
| Participants | 282 participants in 3 study groups RPT: n = 95; median age 73 years; 45 males QPT: n = 94; median age 73 years; 51 males All participants were 1–5 weeks poststroke on entry to study |
|
| Interventions | Intervention was physiotherapy, using a Bobath approach RPT group received approximately 30–45 minutes 5 days per week for 5 weeks – analysed for amount of upper limb therapy post hoc from notes QPT group received an additional 2 hours of therapy, 5 days per week |
|
| Outcomes | RMA arm scale ARAT 10‐hole peg test Grip strength (dynamometer) RMA gross function scale Barthel Index Extended ADL Scale Measurements taken at baseline; 5 weeks (post‐treatment); 3 and 6 months poststroke |
|
| Notes | Only 56% of participants in the QPT group and 46% in the APT group completed the intervention. The most common reasons for this were inability to tolerate the additional therapy and full upper limb recovery Conflict of interest: not reported Funding: NHS Executive, NHS Research and Development Programme on Cardiovascular Disease and Stroke |
|
Page 2011.
| Study characteristics | ||
| Methods | RCT Participants were screened for inclusion and signed consent. Baseline assessments were performed on 2 occasions, 1 week apart Randomised using computer‐generated method Each group received RTP and then varying amounts of MP. For this Cochrane Review, we excluded the sham MP group |
|
| Participants | 29 participants in 4 treatment arms Mean age 61 (SD 12) years; 23 males; 35 months poststroke Groups did not differ except gender: MP40: 6 females; MP60: 7 females and 6 males Distribution of participants: MP20: n = 8; MP40: n = 6; MP60: n = 7 Outpatient/laboratory setting |
|
| Interventions | Experimental intervention was MP All participants received 30 minutes of repetitive task practice, 3 days per week for 10 weeks (15–30 minutes with optional 1–10 minutes stretching). The treatment groups then received 20, 40, or 60 minutes of MP, as per their group allocation |
|
| Outcomes | Upper limb section of the FMA ARAT Measurements were taken pre‐ (average of 2 measures) and postintervention Only within‐group analyses reported |
|
| Notes | Conflict of interest: none Funding: quote: "We are grateful to The Stroke Association for the provision of funding for this study. The Stroke Association had no role in the design, conduction, or interpretation of the results of this study." |
|
Page 2012a.
| Study characteristics | ||
| Methods | RCT Power analysis undertaken Recruitment via adverts 2 baselines Randomisation from computer‐generated random numbers table (concealed envelope). 4 groups (3 different amounts of intervention and a home exercise group) Education session COPM and H200 fitted For this Cochrane Review, we excluded the group who only received a home exercise programme |
|
| Participants | 36 participants in 4 study arms. 4 participants did not complete, so analysis is of 32 30 minutes per day: n = 9 60 minutes per day: n = 8 120 minutes per day: n = 8 Overall characteristics: mean age 57.6 years; age range 38–75 years; 15 men; mean 53.8 months poststroke; range of onset: 7–324 months; 19 participants exhibiting left‐sided lesions Outpatient setting |
|
| Interventions | Intervention was RTP with ESN The participant carried out most of the intervention. However, they did also participate in a 30‐minute, 1‐hour, or 2‐hour home‐based therapy session (based on their group allocation) and subsequent therapy session for 30 minutes × 2 days a week, every other week, during the intervention phase of the study The groups received: RTP and ESN for 30 minutes per day RTP and ESN for 60 minutes per day RTP and ESN for 120 minutes per day All intervention was undertaken every weekday for 8 weeks |
|
| Outcomes | FMA Upper Extremity section AMAT Box and Blocks ARAT Measurements taken 1 week before and 1 week after the period of intervention Only within‐group analysis reported |
|
| Notes | Page 2012a reports the following pair‐wise comparison from this study: 30 minutes group (n = 5) vs 60 minutes group (n = 8) Conflicts of interest: none Funding: award from the American Heart Association. |
|
Page 2012b.
| Study characteristics | ||
| Methods | RCT Power analysis undertaken Recruitment via adverts 2 baselines Randomisation from computer‐generated random numbers table (concealed envelope). 4 groups (3 different amounts of intervention and a home exercise group) Education Session COPM and H200 fitted For this Cochrane Review, we excluded the group who only received a home exercise programme |
|
| Participants | 36 participants in 4 study arms. 4 participants did not complete, so analysis is of 32 30 minutes per day: n = 9 60 minutes per day: n = 8 120 minutes per day: n = 8 Overall characteristics: mean age 57.6 years; age range 38–75 years; 15 men; mean 53.8 months poststroke; range of onset: 7–324 months; 19 participants exhibiting left‐sided lesions Outpatient setting |
|
| Interventions | Intervention was RTP with ESN The participant carried out most of the intervention. However, they did also participate in a 30‐minute, 1‐hour, or 2‐hour home‐based therapy session (based on their group allocation) and subsequent therapy session for 30 minutes × 2 days per week, every other week, during the intervention phase of the study The groups received: RTP and ESN for 30 minutes per day RTP and ESN for 60 minutes per day RTP and ESN for 120 minutes per day All intervention was undertaken every weekday for 8 weeks |
|
| Outcomes | FMA Upper Extremity section AMAT Box and Blocks ARAT Measurements taken 1 week before and 1 week after the period of intervention Only within‐group analysis reported |
|
| Notes | Page 2012b reports the following pair‐wise comparison from this study: 30 minutes group (n = 4) vs 120 minutes group (n = 8) Conflicts of interest: none Funding: award from the American Heart Association. |
|
Partridge 2000.
| Study characteristics | ||
| Methods | RCT Participants deemed eligible for the study by applying the selection criteria. They then undertook baseline assessments before randomisation |
|
| Participants | 144 participants in 2 study arms Mean age 76.5 years; age range 60–94 years; 62 (54%) females 30‐minute group: n = 60 60‐minute group: n = 54 Stroke unit setting in Canterbury, UK |
|
| Interventions | Intervention was physiotherapy based on Bobath principles 30 minutes per day or 60 minutes per day Although not explicitly stated, it is assumed that the treatment was 5 days per week. It appears that duration of treatment was not controlled (i.e. participants continued to receive the treatment to which they were allocated for the duration of their inpatient stay) |
|
| Outcomes | POR Scale (gross body movement and underlying function) Functional reach Step:time ratio 5‐metre timed walk Timed sit‐to‐stand HADS Recovery Locus of Control Scale Measurements were taken at baseline, 6 weeks, and 6 months |
|
| Notes | Study authors believed that it would be beneficial to identify subgroups of participants, who would benefit most from intensive input Conflicts of Interest: not reported Funding: jointly funded by South East Thames R&D Directorate and East Kent Health Authority |
|
Smith 1981.
| Study characteristics | ||
| Methods | RCT Recruited postdischarge and randomised to 1 of 3 groups to receive variable intensities of rehabilitation Group 1: intensive 4 days a week Group 2: conventional 3 half‐days a week Group 3: no attendance but visited by health visitor at home encouraged to do exercises For this Cochrane Review, we excluded the group that were visited at home |
|
| Participants | 133 participants in 3 study arms Group 1: n = 46; mean age 63 years; 67% male; mean 35 days poststroke Group 2: n = 43; mean age 66 years; 73% male; mean 41 days poststroke Outpatient setting in the UK |
|
| Interventions | Intervention was physiotherapy and OT Participants were required to attend the outpatient department for whole or half days, for up to 6 months, but shorter if full recovery achieved Group 1: 4 days a week Group 2: 3 half days a week Group 1 received double amount as group 2 |
|
| Outcomes | ADL Index (17 item covering mobility, self‐care, and household tasks) Clinical examination Outcome measures recorded at baseline, 3 months, 6 months, and 12 months; i.e. 1 follow‐up at 12 months Deaths and re‐occurrences of stroke were also recorded |
|
| Notes | Written in 1981 when there was little evidence from RCTs about effect of stroke rehabilitation Study indicated that intensive therapy may only be tolerated by a small percentage of stroke survivors (11% in this instance) Conflict of interest: not reported Funding: not reported |
|
Tong 2019.
| Study characteristics | ||
| Methods | RCT Patients screened for inclusion Baseline characteristic collected for eligible participants Eligible participants randomised into 3 groups, VEIM, EIM, and ERM. For this Cochrane Review, we only compared the EIM group and ERM group Delivery of intervention, depending on group, commenced 24–48 hours poststroke for 10–14 days duration Data collection 3 months poststroke |
|
| Participants | 100 participants randomised to each of the 3 trial groups, but data reported only for those who had confirmed diagnosis of stroke, and received the intervention as planned ERM: n = 80; mean age: 62.1 years; 71.3% male; mean 41.0 hours since stroke to first mobilisation; baseline NIHSS 6.0 (scale 0–16) EIM: n = 86; mean age: 60.9 years; 76.7% males; mean 38.0 hours since stroke to first mobilisation; baseline NIHSS 5.8 (scale 0–16) Study conducted at the Stroke unit of the Department of Neurology, Beijing Luhe Hospital, China |
|
| Interventions | Intervention was out‐of‐bed mobilisation included sitting, standing, and walking with or without assistance. No special equipment. Individualised to participant Intervention was provided according to the AVERT protocol |
|
| Outcomes | mRS | |
| Notes | VEIM group was excluded from this review, as participants received a different treatment (i.e. earlier intervention) Conflict of interest: authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest Funding: the work was supported in part by Beijing Municipal Science Technology Commission (Z151100003915134), the National Natural Science Foundation of China (81501141), Science and Technology Project of Beijing Municipal Education Commission (KM201610025028), the Beijing NOVA program (xx2016061), and Science and Technology Plan of Beijing Tongzhou District (KJ2017CX043) |
|
Wang 2004.
| Study characteristics | ||
| Methods | RCT Pre‐ and postmeasurement |
|
| Participants | 74 participants in 2 treatment arms Experimental group: n = 38; mean age 65.13 (SD 8.86) years, 21 males, 11 aphasic Control group: n = 36; mean age 65.72 (SD 8.68) years; 19 males; 9 aphasic Acute inpatient setting |
|
| Interventions | Intervention was rehabilitation therapy In the first month, the 2 groups of participants were given the same rehabilitation intensity, 2 times per day and 40 minutes per session; starting from the second month: treatment group: once or twice per day, 40 minutes per session; control group: 3 times per week, 40 minutes per session Treatment lasted for 6 months |
|
| Outcomes | Functional Rating Scale NIHSS FMA Modified Barthel Index Western Aphasia Battery Measurements taken before rehabilitation and at the end of 6 months after treatment |
|
| Notes | It is not certain if the intervention was provided 7 days per week or 5 days per week Conflict of interest: not reported Funding: not reported |
|
Wang 2011.
| Study characteristics | ||
| Methods | RCT Participants were recruited into study and randomised Baseline assessment Intervention: all groups received OT, no mention of physiotherapy Assessment 2 weeks postrandomisation (halfway through the intervention) Assessment postintervention Purpose of study was to compare mCIMT to ICR, using CR as a control. For this Cochrane Review, we compared ICR and CR only |
|
| Participants | 30 participants in 3 study arms CR: n = 10; mean age 67 (SD 7.45) years; 50% male; mean 9.4 (SD 5.38) weeks poststroke; 80% infarct ICR: n = 10; mean 63.5 (SD 9.63) years; 70% male; mean 12.7 (SD 9.72) weeks poststroke; 80% infarct Inpatient setting in China |
|
| Interventions | All intervention took place 5 days a week over 4 weeks. Amount per‐day was as follows: CR group: 45 minutes; ICR group: 3 hours | |
| Outcomes | WMFT Measurement taken at baseline, and 2 and 4 weeks after initiation of treatment |
|
| Notes | Conflict of interest: not reported Funding: grant from the Ministry of Human Resources and Social Security of the People’s Republic of China |
|
Winstein 2019a.
| Study characteristics | ||
| Methods | RCT Randomisation was stratified by severity (FMA) and chronicity (time since stroke) Intervention was provided following a train‐wait‐train‐wait‐train pattern Testing was undertaken pre, post, and during intervention |
|
| Participants | 41 participants in 4 study groups (for this Cochrane Review, we only included 3 of these study groups) 15 hours group: n = 10; mean age 57.0 (SD 12.77) years; male/female 9/1; mean 2.93 (SD 2.68) years poststroke 30 hours group: n = 10; mean age 61.3 (SD 13.69) years; male/female 7/3; mean 2.45 (SD 2.01) years poststroke 60 hours group: n = 11; mean age 60.64 (SD 14.12) years; male/female 8/3; mean 1.96 (SD 1.49) years poststroke |
|
| Interventions | Intervention was the ASAP, a "personalized task‐oriented training program that incorporates elements of skill acquisition, capacity building, with intrinsic motivational enhancements." Intervention was provided in 3‐week sessions of 4 consecutive visits each separated by 1 month Intervention was provided at different durations: 0, 15, 30, or 60 hours, depending on group allocation. These figures are the total amount of intervention provided in the study |
|
| Outcomes | Motor Activity Log WMFT time score Measurements were taken at baseline and at the end of intervention. Further measures were taken at the end of each weeklong bout of treatment |
|
| Notes | Winstein 2019a reports the following pair‐wise comparison: 15 hours group (n = 5) vs 30 hours group (n = 10) For the purpose of the Cochrane Review, we excluded the group that received no therapy Conflict of interest: none Funding: supported by the NIHSS of the NIH under R01 HD065438 and R56 NS100528 |
|
Winstein 2019b.
| Study characteristics | ||
| Methods | RCT Randomisation was stratified by severity (FMA) and chronicity (time since stroke) Intervention was provided following a train‐wait‐train‐wait‐train pattern Testing was undertaken pre, post, and during intervention |
|
| Participants | 41 participants in 4 study groups (for this Cochrane Review, we only included 3 of these study groups) 15 hours group: n = 10; mean age 57.0 (SD 12.77) years; male/female 9/1; mean 2.93 (SD 2.68) years poststroke 30 hours group: n = 10; mean age 61.3 (SD 13.69) years; male/female 7/3; mean 2.45 (SD 2.01) years poststroke 60 hours group: n = 11; mean age 60.64 (SD 14.12) years; male/female 8/3; mean 1.96 (SD 1.49) years poststroke |
|
| Interventions | Intervention was the ASAP, a "personalized task‐oriented training program that incorporates elements of skill acquisition, capacity building, with intrinsic motivational enhancements." Intervention was provided in 3‐week sessions of 4 consecutive visits each separated by 1 month Intervention was provided at different durations: 0, 15, 30, or 60 hours, depending on group allocation. These figures are the total amount of intervention provided in the study |
|
| Outcomes | Motor Activity Log WMFT time score Measurements were taken at baseline and at the end of intervention. Further measures were taken at the end of each weeklong bout of treatment |
|
| Notes | Winstein 2019b reports the following pair‐wise comparison: 15 hours group (n = 5) vs 60 hours group (n = 11) For the purpose of the Cochrane Review, we excluded the group that received no therapy Conflict of interest: none Funding: supported by the NIHSS of the NIH under R01 HD065438 and R56 NS100528 |
|
ADL: activities of daily living; APT: assistant physiotherapy; AQoL: Australian Quality of Life Scale; AMAT: Arm Motor Ability Test; AP: augmented physiotherapy; ARAT: Action Research Arm Test; ASAP: Accelerated Skill Acquisition Program; AVERT: A Very Early Rehabilitation Trial; CIMT: constraint‐induced movement therapy; COPM: Canadian Occupational Performance Measure; CPT: conventional physiotherapy; CR: conventional rehabilitation; EIM: early intensive mobilisation; ERM: early routine mobilisation; ESN: electrical stimulation neuroprosthesis; FES‐ET: functional electrical stimulation‐assisted exercise therapy; FIM: Functional Independence Measure; FMA: Fugl‐Meyer Assessment; FST: functional strength training; HADS: Hospital Anxiety and Depression Scale; ICR: intensive conventional rehabilitation; mCIMT: modified constraint‐induced movement therapy; MP: mental practice; mRS: modified Rankin Scale; n: number of participants; NEADL: Nottingham Extended Activities of Daily Living; NHS: National Health Service; NIH: National Institutes of Health; NIHSS: National Institutes of Health Stroke Scale; NINDS: National Institute of Neurological Disorders and Stroke; NMES: neuromuscular electrical stimulation; MTS: mobilisation and tactile stimulation; OT: occupational therapy; POR: Profiles of Recovery Scale; QPT: qualified physiotherapy; RCT: randomised controlled trial; RMA: Rivermead Motor Assessment; RMI: Rivermead Mobility Index; RPT: routine physiotherapy; RT: robot therapy; RTP: repetitive task‐specific practice; SD: standard deviation; SIS: Stroke Impact Scale; SP: standard physiotherapy; UPSET: Upper Limb Self‐Efficacy Test; VEIM: very early intensive mobilisation; WMFT: Wolf Motor Function Test.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdullahi 2021 | Did not compare different time spent in rehabilitation |
| Abraha 2017 | Not an RCT |
| Afridi 2021 | Compared time spent in rehabilitation to repetitions |
| Agarwal 2008 | Not an RCT |
| Ardestani 2020 | Compared different types of intervention (dose‐matched) |
| Askim 2004 | Compared different interventions |
| Askim 2010 | Compared different interventions |
| Bai 2008 | Control group received no intervention |
| Bowden 2020 | Compared different interventions |
| Boyd 2016 | Compared different interventions |
| Brusco 2014 | Included non‐stroke participants |
| Byblow 2020 | Compared different types of therapy |
| Byl 2008 | Not an RCT |
| Chen 2006 | Control group received no intervention |
| Daly 2019 | Compared different interventions |
| de Sousa 2019 | Compared different interventions |
| Di Lauro 2003 | Compared different interventions |
| DRKS00023235 | Compared different types of intervention |
| Duff 2013 | Compared different interventions |
| Duncan 2003 | Compared different interventions |
| Fasoli 2004 | Compared different interventions |
| Forster 1992 | Compared different interventions |
| Foucher 2017 | Control group received no intervention |
| Galloway 2017 | Not an RCT |
| Gobbato 2012 | Compared early onset to later onset therapy |
| Green 2004 | Did not investigate dose–response in terms of time spent |
| Henriksen 1992 | Compared different interventions |
| Hesse 2011 | Compared different interventions |
| Hogg 2020 | Compared different interventions |
| Hornby 2015 | Did not investigate dose–response in terms of time spent |
| Hornby 2016 | Compared different interventions |
| Host 2014 | Included non‐stroke participants |
| Hsu 2016 | Did not compare different amounts of the same intervention. Used a different definition of 'intensity' |
| Hubbard 2010 | Compared different interventions |
| Huijben‐Schoenmakers 2014 | Not an RCT |
| Kissela 2013 | Did not investigate dose–response in terms of time spent |
| Klassen 2020 | Compared different interventions: the difference in the 2 treatment arms that were considered for this review were not only amount of time, but also intensity, in terms of exercise repetitions |
| Kosak 1998 | Compared different interventions |
| Krebs 1997 | Compared different interventions |
| Lamberti 2017 | Compared different interventions |
| Langhammer 2007 | Some control group participants received no therapy |
| Langhammer 2014 | Control group received no intervention |
| Langhorne 2010 | Compared different interventions |
| Langhorne 2017 | Compared different interventions (early as well as more intensive) |
| Lee 2012 | Compared different interventions |
| Lewthwaite 2018 | Some of the control participants received no therapy. We considered comparing usual care with dose‐matched usual care. However, although the dose‐matched usual care group received a standard amount of therapy (30 hours over 16 weeks), the usual care group did not (0–46 hours range over 16 weeks) |
| Li 2000 | Compared different interventions |
| Lin 2017 | Compared different interventions |
| Lo 2010 | Compared different interventions |
| Logan 1997 | Intervention group received input earlier than control group |
| Malouin 1992 | Not an RCT (case report) |
| Malouin 1993 | Compared different interventions (early as well as more intensive) |
| Martinsson 2003 | Compared different interventions |
| Marzolini 2016 | Compared different (dose‐matched) interventions |
| McDonnell 2017 | Compared different interventions |
| Mickelborough 1999 | Compared different interventions |
| Mikulecka 2005 | Compared different interventions |
| Outermans 2010 | Compared different interventions |
| Ozdemir 2001 | Compared different interventions |
| Raghavan 2016 | Compared different interventions |
| Richards 2006a | Compared different interventions |
| Richards 2006b | Study withdrawn |
| Roderick 2001 | Compared different interventions |
| Rodgers 2019 | Compared different interventions |
| Ruff 1999 | Not an RCT |
| Ryan 2006 | Included non‐stroke participants |
| Sanchez‐Sanchez 2015 | Compared different (dose‐matched) interventions |
| Scrivener 2012 | Not an RCT |
| Sheil 2001 | Included non‐stroke participants |
| Sivenius 1985 | Compared different interventions |
| Slade 2002 | Included non‐stroke participants |
| Sonoda 2004 | Not an RCT |
| Sterr 2002 | Included non‐stroke participants |
| Sunderland 1992 | Compared different interventions |
| Tomic 2017 | Compared different interventions |
| van Wijck 2020 | Compared different interventions |
| Wu 2016 | Compared different interventions |
| Wuennemann 2020 | Included non‐stroke participants |
| Xu 2008 | Compared different interventions |
| Yau 2010 | Compared different interventions |
| Yelnik 2017 | Compared different interventions |
| Yu 2008 | Control group received no intervention |
| Zhang 2005 | Compared different interventions: early vs later |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Aksu 2001.
| Methods | RCT |
| Participants | 20 acute stroke patients in 3 study groups |
| Interventions | Exercises chosen from a Bobath neurodevelopmental approach Group 1 received 4 exercises Group 2 received 6 exercises Group 3 received 8 exercises |
| Outcomes | Stroke Rehabilitation Assessment of Movement |
| Notes | The above is taken from conference proceedings and there is insufficient information available to include at this stage. Contacted authors to request full details of study and we are awaiting their response |
Cauraugh 2006.
| Methods | RCT |
| Participants | 30 participants in 3 study groups |
| Interventions | Intervention is bilateral movements and NMES (or sham stimulation) High‐intensity group: bilateral training moving both arms coupled with NMES; 4 × 90‐minute sessions per week for 2 weeks Low‐intensity group: bilateral training moving both arms coupled with NMES; 2 × 90‐minute sessions per week for 2 weeks Control group: bilateral training moving both arms coupled with sham NMES; 2 sessions per week for 2 weeks. |
| Outcomes | Box and Block Test Fugl‐Meyer Upper Extremity Motor Test Fractionated reaction time Measurements were taken before and after intervention and outcomes reported for 14 of the participants recruited |
| Notes | Study reported on ClinicalTrials.gov as completed; however, unable to locate a full paper and available information is limited. Contacted authors regarding publication of full paper; currently awaiting a response |
Hsieh 2011.
| Methods | Pilot RCT |
| Participants | 18 participants in 3 study groups All participants were > 6 months poststroke Recruited from 3 medical centres in Taiwan |
| Interventions | High‐intensity RT: using the robot‐assisted arm trainer, Bi‐Manu‐Track, participants practiced 600–800 repetitions of mode 1 for 15 minutes, 600–800 repetitions of mode 2 for 15–20 minutes, and 150–200 repetitions of mode 3 for 5 minutes for forearm and wrist movements Low‐intensity RT: intervention for this group was the same as for the high‐intensity group, but half the number of repetitions were practiced Control: structured protocol of conventional occupational therapy All participants received training sessions (90–105 minutes per day, 5 days per week for 4 weeks) |
| Outcomes | Fugl Meyer Assessment Upper Extremity Subscale Medical Research Council Scale Motor Activity Log ABILHAND scale Urinary 8‐hydroxydeoxyguanosine General subscale of the Multidimensional Fatigue Symptom Inventory Assessments were administered before and after treatment |
| Notes | Unable to include as it appears that the recruitment dates of this study may cross with the study described in Hsieh 2012. We are waiting for confirmation from the authors regarding whether there was any participant overlap between these 2 studies |
Jung 2008.
| Methods | Possibly an RCT |
| Participants | 89 participants in 2 study groups |
| Interventions | Conventional rehabilitation (occupational therapy and physiotherapy) Group 1: 1 session per day of rehabilitation training Group 2: 2 sessions per day of rehabilitation training |
| Outcomes | Korean Berg Balance Scale Functional Independence Measure Mini Mental State Examination‐Korea Measurements were taken at 2‐week intervals and between group differences were assessed at the beginning of treatment and at the peak of Korean Berg Balance Scale |
| Notes | The above is taken from conference proceedings and there is insufficient information available to include at this stage. Contacted authors and requested full details of the study and we are awaiting their response |
Kreisel 2005.
| Methods | RCT |
| Participants | 55 participants in 2 study arms |
| Interventions | Intervention was conventional physiotherapy Intensive group: 10–14 sessions of physiotherapy over 10 days Conventional group: < 5 sessions |
| Outcomes | NIHSS Motricity Index |
| Notes | The above is taken from conference proceedings and there is insufficient information available to include at this stage. Contacted authors to request full details of the study and we are awaiting their response |
Rimmer 2004.
| Methods | RCT |
| Participants | 25 participants in 3 study groups |
| Interventions | Group 1: intensity‐oriented exercise programme Group 2: duration‐oriented exercise programme Group 3: standard care group |
| Outcomes | Peak oxygen consumption Time to exhaustion Maximum workload Submaximal oxygen cost Blood pressure Heart rate Lipid profile |
| Notes | The above is taken from conference proceedings and there is insufficient information available to include or exclude. Contacted authors and we are awaiting their response |
Takebayashi 2015.
| Methods | Probably an RCT |
| Participants | 30 participants in 2 treatment groups |
| Interventions | Intervention was robotic therapy using the Reo Go therapy system comparing a low‐intensity training group with a high‐intensity training group |
| Outcomes | Unspecified upper limb measurements |
| Notes | The above is taken from conference proceedings and there is insufficient information available to include or exclude. Contacted authors and we are awaiting their response |
Wu 2013.
| Methods | RCT |
| Participants | 32 participants planned, in 3 study groups |
| Interventions | Intervention is robot‐assisted training High‐intensity RT group: each robot‐assisted training session will include 400–600 repetitions of mode 1 and 800–1000 repetitions of mode 2, totalling 1200–1600 repetitions for the forearm and the wrist movements. In addition, the participants will practice 100–200 repetitions in mode 3. In addition, this group received functional training Low‐intensity RT group: same intervention as the high‐intensity robot therapy group, but half the number of repetitions Conventional therapy group: functional training (no robot therapy) |
| Outcomes | Fugl‐Meyer Assessment Motor Status Scale Modified Ashworth Scale MyotonPRO Muscle metabolism Box and Block Test Revised Nottingham Sensory Assessment Functional Independence Measure Motor Activity Log* ABILHAND Questionnaire* Adelaide Activities Profile* EQ‐5D‐5L Accelerometers* Functional magnetic resonance imaging Kinematic analysis Inflammatory markers Oxidative stress markers Erythrocyte deformability Blood glucose indicators All measures were taken at baseline and completion of intervention. Those marked with a * were also taken at 6‐month follow‐up |
| Notes | The above is taken from ClinicalTrials.gov, which reports that the study was completed in May 2015. However, there are no results or full publications available. Contacted authors and we are awaiting their response |
NIHSS: National Institutes of Health Stroke Scale; NMES: neuromuscular electrical stimulation; RCT: randomised controlled trial; RT: robot therapy.
Characteristics of ongoing studies [ordered by study ID]
Bernhardt 2019.
| Study name | AVERT DOSE |
| Methods | RCT |
| Participants | Study aims to recruit 2700 participants |
| Interventions | Intervention was mobility training, delivered at an intensity tolerable to the participant, noted as mild, moderate or vigorous as determined by BORG and physiological measures (heart rate, respiratory rate, oxygen saturations, and blood pressure) Group 1: 1 session per day Group 2: 2 sessions per day Group 3: 3 sessions per day Group 4: 4 sessions per day |
| Outcomes | mRS, safety (adverse events or serious adverse events), 6‐metre walk test, EQ‐5D‐5L |
| Starting date | 22/09/2019 |
| Contact information | julie.bernhardt@florey.edu.au |
| Notes | This study is currently ongoing. Inclusion will likely depend upon there being a similar proportion of intensity of training (mild, moderate, or vigorous) across the 4 intervention groups |
Chang 2015.
| Study name | Effect of intensive cognitive rehabilitation in subacute stroke patient |
| Methods | RCT (according to ClinicalTrials.gov) |
| Participants | 150 participants planned in 2 study groups |
| Interventions | Intervention is cognitive rehabilitation Group 1: cognitive rehabilitation for 1 hour, every working day for 4 weeks Group 2: cognitive rehabilitation for 30 minutes, every working day for 4 weeks |
| Outcomes | Korean‐Montreal Cognitive Assessment |
| Starting date | 1 November 2016 |
| Contact information | Department of Physical and Rehabilitation Medicine, Center for Prevention and Rehabilitation, Heart Vascular and Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Ilwon‐dong, Gangnam‐gu, Seoul, 135‐710, Republic of Korea |
| Notes | Above information is taken from www.ClinicalTrial.gov There is an associated paper for this study, which states it is a prospective cohort study (not an RCT). Until the study is published, we will not know if it meets the criteria for this review |
Dukelow 2019.
| Study name | RESTORE |
| Methods | RCT |
| Participants | 132 participants planned in 5 study arms |
| Interventions | Intervention is robotic rehabilitation using a robotic exoskeleton Group 1: early low intensity (1 hour per day) Group 2: early high intensity (2 hours per day) Group 3: late low intensity (1 hour per day) Group 4: late high intensity (2 hours per day) Group 5: control For the purpose of this review, we would include a comparison of the 2 early groups and a separate comparison of the 2 late groups |
| Outcomes | FMA upper extremity FIM mRS ARAT Robotic assessments |
| Starting date | 1 May 2019 |
| Contact information | spdukelo@ucalgary.ca, mark.piitz@albertahealthservices.ca |
| Notes | Above information is taken from www.ClinicalTrial.gov |
Holmstedt 2021.
| Study name | Impact of more frequent PT services |
| Methods | RCT |
| Participants | 150 |
| Interventions | Group 1: increased frequency of PT services within the first 3–5 days of admission, followed by daily PT services for the duration of their inpatient stay Group 2: PT services 3–5 times per week during their hospitalisation |
| Outcomes | None reported |
| Starting date | March 2021 |
| Contact information | holmstedt@musc.edu |
| Notes |
Kanlaya 2018.
| Study name | Effects of the Accelerated Skill Acquisition Program (ASAP) training duration on reach‐to‐grasp performance in individuals with subacute stroke (effects of ASAP duration on UE training for stroke) |
| Methods | RCT |
| Participants | 14 planned |
| Interventions | Group 1: task‐oriented training of upper extremity for 2 hours Group 2: task‐oriented training of upper extremity for 1 hour |
| Outcomes | Corticospinal excitability and kinematics |
| Starting date | 19 March 2018 (not yet recruiting) |
| Contact information | Wanida.kae@mahidol.ac.th, Sirinapatopaz@gmail.com |
| Notes | Limited availability from trial registries. Have contacted authors and awaiting response |
Kinoshita 2020.
| Study name | Dose–response of rPMS for upper limb hemiparesis after stroke |
| Methods | RCT |
| Participants | Target sample size of 50 |
| Interventions | The intervention is rPMS Group 1: control Group 2: 2400 pulses Group 3: 4800 pulses Groups 2 and 3 could be included in the analysis |
| Outcomes | FMA Upper Extremity modified Ashworth Scale Active ROM Goniometry FIM |
| Starting date | 20 January 2020 |
| Contact information | kinoshita@jikei.ac.jp |
| Notes | Although amount is measures in number of pulses of rPMS, the protocol explains that "Each train of rPMS stimuli will be applied at 20 Hz for 3 seconds followed by a 27 second rest interval. Eighty such trains of rPMS stimuli will be applied as the daily 4800 pulses of rPMS therapy, and 40 such trains of rPMS stimuli will be applied as the daily 2400 pulses of rPMS, in the respective treatment groups." Therefore, intervention for group 3 will take twice as long as intervention for groups 2 |
Ling 2018.
| Study name | Effect of different intensity rehabilitation training on hemiplegic patients after stroke |
| Methods | Uncertain – possibly an RCT |
| Participants | 24 participants planned in 2 study groups |
| Interventions | Intervention is rehabilitation training Group 1: low‐intensity rehabilitation training Group 2: high‐intensity rehabilitation training |
| Outcomes | Sicam 1n (svcam1n) D‐dimer Cardiopulmonary exercise test 6‐minute walking distance Quality of life (36‐item Short Form) Walking speed (10‐metre walking test) Balance (Berg Balance Scale) Evaluation index of lower limb strength and FMA scale |
| Starting date | 1 June 2018 |
| Contact information | lydhzw@126.com |
| Notes | This information is taken from a trial registry. There is insufficient information to make a decision regarding inclusion of this study and currently no results available. We have contacted the study authors and await their response |
Mansfield 2020.
| Study name | Determining the optimal dose of reactive balance training after stroke |
| Methods | RCT |
| Participants | 36 planned; 12 in each intervention group |
| Interventions | Intervention was RBT Group 1: 1 session of RBT Group 2: 3 sessions of RBT Group 3: 6 sessions of RBT Each session will be 45 minutes long |
| Outcomes | Chedoke‐McMaster Stroke Assessment Mini‐Balance Evaluation Systems Test Activities‐specific Balance Confidence Novel unpredictable perturbation |
| Starting date | June 2020 (according to ClinicalTrials.gov) |
| Contact information | Dr Avril Mansfield; avril.mansfield@uhn.ca |
| Notes | Above information taken from published protocol and registration of study on ClinicalTrials.gov |
ARAT: Action Research Arm Test; FIM: Functional Independence Measure; FMA: Fugl‐Meyer Assessment; mRS: modified Rankin Scale; PT: physiotherapy; RBT: reactive balance training; RCT: randomised controlled trial; rPMS: repetitive peripheral magnetic stimulation.
Differences between protocol and review
Title
Title reworded, to enhance clarity.
Background
Changes made to update the background section (including updating references) and enhance readability.
Objectives
Objectives have undergone some rewording to clarify and enhance readability and to conform with the preferred Cochrane format. The nature of the objectives has not changed.
Methods
The following minor changes were made to the methods between protocol and review.
Under 'criteria for considering trials for this review' we altered the wording under 'type of intervention' to enhance clarity. We added that we included studies that varied in the time spent in rehabilitation, but did not report a specific time‐related measurement. This had not been anticipated when writing the protocol.
We removed 'Participant experience' as a secondary outcome as it does not relate to the objectives of this review.
-
Electronic searches:
CIRRIE (cirrie.buffalo.edu/database/) was not included, as it has been amalgamated with REHABDATA;
planned to include the Australian and New Zealand Clinical Trials Registry (ANZCTR), but this registry was excluded, as we were unable to export results;
planned to include the UK Clinical Trials Gateway (UKCTG), but excluded, as this registry obtains data from ISCRTN and ClinicalTrials.gov, both of which were searched in this review.
RoB 2 tool used (had planned to use the risk of bias tool). Therefore, this section has been re‐written in accordance with the editorial checklist for the RoB 2 tool.
RevMan Web was used (had planned to use Review Manager 5).
Protocol stated that two review authors would independently screen the titles and abstracts of the studies retrieved. Owing to the very large number of records found, the first step of study selection was that one person screened titles and excluded any studies that were clearly irrelevant, before moving on to two people screening titles and abstracts.
Detail added regarding how we would deal with studies with more than two intervention groups, as this was not clear in the protocol.
We added that we would only undertake funnel plots when there were 10 or more studies. This is based on the advice in the Cochrane Handbook.
We added an assessment of non‐reporting bias, in accordance with Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions.
We added two subgroup analyses, to compare the effect of time spent in therapy dependent on the type of intervention provided. We reasoned that the type of intervention may affect outcomes and, therefore, more time spent in one type of therapy may have greater benefit than more time spent in another type of therapy. These analyses were determined post hoc, as they were dependent on the types of studies found in the literature search. The two analyses undertaken (upper limb therapy versus other therapy and electromechanical technology versus no electromechanical technology) were chosen, as there were studies in each category to enable a comparison and both comparisons were considered likely to be of interest to readers.
Sensitivity analyses
We did not perform a sensitivity analysis to determine the effect of any unit of analysis issues, as we believe we had mitigated for unit of analysis issues within the review.
We did not perform a sensitivity analysis to determine the effect of inclusion of cluster‐randomised controlled trials, as none were included.
We performed sensitivity analyses to assess the effect of excluding studies of overall high risk of bias, in accordance with the guidelines for use of the RoB 2.
We added a sensitivity analysis, excluding studies that were at high risk of bias due to deviations in adherence to interventions. This addition was made due to the change in RoB tool used.
Measurement of treatment effects
We did not undertake a meta‐regression, as planned for objective two. The advice in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 10 is that meta‐regression should not be undertaken when there are fewer than 10 studies (Deeks 2021). Meta‐regression was considered for the two outcomes that did have more than 10 studies, but, given the small number of studies, we considered it was sufficient to use a consistent descriptive approach across all outcomes. Instead, we undertook subgroup analyses and created scatter plots using Microsoft Excel to provide a descriptive analysis.
We were unable to address the third objective as planned. Due to limited similarities in the rehabilitation schedules between studies, we were unable to group studies as planned, to undertake meta‐analyses for the different groups. As an alternative, we compared studies with a larger difference between groups in terms of number of minutes of rehabilitation provided per week to those with a smaller difference between groups in terms of number of minutes of rehabilitation provided per week.
Contributions of authors
BC initiated and co‐ordinated the review, but it was undertaken with the full support of all the review authors.
All authors contributed to the conception and design of this review.
BC, JB, and JW screened titles and abstracts of publications identified by the searches.
BC, JB, JW, and SE extracted trial and outcome data from the selected trials and analysed outcome data.
BC, JW, and JB assessed risk of bias in the included studies.
All review authors contributed to the interpretation of results and to the final presentation of this study.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
BC
-
Funding
Health Education Wessex: paid tuition fees for academic years 2013/2014, 2014/2015, and 2015/2016, and contributed to tuition fees for academic year 2016/2017 for Doctorate in Clinical Practice Studies at the University of Southampton. Contributed to tuition fees for academic year 2019/2020 for Philosophical doctorate (transferred from Doctorate in Clinical Practice Program) at the University of Southampton (funds paid to institution).
Poole Hospital NHS Foundation Trust: contributed to tuition fees for academic year 2016/2017 for Doctorate in Clinical Practice Studies at the University of Southampton (funds paid to institution).
Elizabeth Casson Trust: contributed to tuition fees for academic year 2016/2017 for Doctorate in Clinical Practice Studies at the University of Southampton. Provided full tuition fees for academic year 2020/2021 for Philosophical doctorate (transferred from Doctorate in Clinical Practice Program) at the University of Southampton (funds paid to institution).
-
Employment
Poole Hospital NHS Foundation Trust: employed by Poole Hospital prior to July 2019 (although 13 months of unpaid leave taken prior to this date).
University of Southampton: currently I am employed by the University of Southampton as a Senior Research Fellow on an unrelated project.
JW: none.
JB: none.
GK
Board memberships: European Managing Editor NeuroRehabilitation and Neural Repair; Co‐editor Stroke; Co‐editor Journal of Rehabilitation Medicine; Co‐editor International Journal of Stroke (money received by author).
JM: none.
SE: none.
New
References
References to studies included in this review
Abdullahi 2018 {published and unpublished data}
- Abdullahi A. Effects of number of repetitions and number of hours of shaping practice during constraint-induced movement therapy: a randomized controlled trial. Neurology Research International 2018;2018:5496408. [DOI] [PMC free article] [PubMed]
Ada 2013 {published data only}
- Ada L, Dean C, Lindley R. Randomised trial of treadmill training to improve walking in community-dwelling people after stroke: the AMBULATE trial. International Journal of Stroke 2013;8:436-44. [DOI] [PubMed] [Google Scholar]
Burgar 2011 {published data only}
- Burgar C, Lum P, Scremin A, Garber S, Loos H, Kenney D and Shor P. Robot-assisted upper-limb therapy in acute rehabilitation setting following stroke: department of Veterans Affairs multisite clinical trial. Journal of Rehabilitation Research and Development 2011;48(4):445-58. [DOI] [PubMed] [Google Scholar]
Cooke 2010b {published data only}
- Cooke E, Tallis R, Clark A, Pomeroy V. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke: phase I randomized controlled trial. Neurorehabilitation and Neural Repair 2010;24(1):88-96. [DOI] [PubMed] [Google Scholar]
Donaldson 2009 {published data only}
- Donaldson C, Tallis R, Miller S, Sunderland A, Lemon R, Pomeroy V. Effects of conventional physical therapy and functional strength training on upper limb motor recovery after stroke: a randomized phase II study. Neurorehabilitation and Neural Repair 2009;23(4):389-97. [DOI] [PubMed] [Google Scholar]
Dromerick 2009 {published data only}
- Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, Powers WJ, Wolf SL and Edwards DF. Very early constraint-induced movement during stroke rehabilitation (VECTORS). Neurology 2009;73:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
English 2015 {published data only}
- English C, Bernhardt J, Crotty M, Esterman A, Segal L, Hillier S. Circuit class therapy or seven-day week therapy for increasing rehabilitation intensity of therapy after stroke (CIRCIT): a randomized controlled trial. International Journal of Stroke 2015;10(4):594-602. [DOI] [PubMed] [Google Scholar]
GAPS 2004 {published data only}
- Glasgow Augmented Physiotherapy Study (GAPS) group. Can augmented physiotherapy input enhance recovery of mobility after stroke? A randomized controlled trial. Clinical Rehabilitation 2004;18(5):529-37. [DOI] [PubMed] [Google Scholar]
Han 2013a {published data only}
- Han C, Wang Q, Meng P-P, Qi M-Z. Effects of intensity of arm training on hemiplegic upper extremity motor recovery in stroke patients: a randomized controlled trial. Clinical Rehabilitation 2013;27(1):75-81. [DOI] [PubMed] [Google Scholar]
Han 2013b {published data only}
- Han C, Wang Q, Meng P-P, Qi M-Z. Effects of intensity of arm training on hemiplegic upper extremity motor recovery in stroke patients: a randomized controlled trial. Clinical Rehabilitation 2013;27(1):75-81. [DOI] [PubMed] [Google Scholar]
Hsieh 2012 {published and unpublished data}
- Hsieh Y, Wu C, Lin K, Yao G, Wu K, Chang Y. Dose–response relationship of robot-assisted stroke motor rehabilitation: the impact of initial motor status. Stroke 2012;43(10):2729-34. [DOI] [PubMed] [Google Scholar]
Hsu 2010 {published data only}
- Hsu S, Hu M, Wang Y, Yip P, Chiu J, Hsieh C. Dose–response relation between neuromuscular electrical stimulation and upper-extremity function in patients with stroke. Stroke 2010;41(4):821-4. [DOI] [PubMed] [Google Scholar]
Hunter 2011a {published and unpublished data}
- Hunter S, Hammett L, Ball S, Smith N, Anderson C, Clark A, Tallis R, Rudd A and Pomeroy V. Dose–response study of mobilisation and tactile stimulation therapy for the upper extremity early after stroke: a phase I trial. Neurorehabilitation and Neural Repair 2011;25(4):314-22. [DOI] [PubMed] [Google Scholar]
Hunter 2011b {published and unpublished data}
- Hunter S, Hammett L, Ball S, Smith N, Anderson C, Clark A, Tallis R, Rudd A and Pomeroy V. Dose–response study of mobilisation and tactile stimulation therapy for the upper extremity early after stroke: a phase I trial. Neurorehabilitation and Neural Repair 2011;25(4):314-22. [DOI] [PubMed] [Google Scholar]
Kowalczewski 2007 {published data only}
- Kowalczewski J, Gritsenko V, Ashworth N, Ellaway P, Prochazka A. Upper-extremity functional electric stimulation-assisted exercises on a workstation in the subacute phase of stroke recovery. Archives of Physical Medicine and Rehabilitation 2007;88(7):833-9. [DOI] [PubMed] [Google Scholar]
Lang 2016a {published and unpublished data}
- Lang C, Strube M, Bland M, Waddell K, Cherry-Allen K, Nudo R, Dromerick AW and Birkenmeier RL. Dose response of task-specific upper limb training in people at least 6 months poststroke: a phase II, single-blind, randomized, controlled trial. Annals of Neurology 2016;80(3):342-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 2016b {published data only}
- Lang C, Strube M, Bland M, Waddell K, Cherry-Allen K, Nudo R, Dromerick AW and Birkenmeier RL. Dose response of task-specific upper limb training in people at least 6 months poststroke: a phase II, single-blind, randomized, controlled trial. Annals of Neurology 2016;80(3):342-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 2016c {published and unpublished data}
- Lang C, Strube M, Bland M, Waddell K, Cherry-Allen K, Nudo R, Dromerick AW and Birkenmeier RL. Dose response of task-specific upper limb training in people at least 6 months poststroke: a phase II, single-blind, randomized, controlled trial. Annals of Neurology 2016;80(3):342-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lincoln 1999 {published data only}
- Lincoln N, Parry R, Vass C. Randomized, controlled trial to evaluate increased intensity of physiotherapy treatment of arm function after stroke. Stroke 1999;30(3):573-9. [DOI] [PubMed] [Google Scholar]
Page 2011 {published data only}
- Page S, Dunning K, Hermann V, Leonard A, Levine P. Longer versus shorter mental practice sessions for affected upper extremity movement after stroke: a randomized controlled trial. Clinical Rehabilitation 2011;25(7):627-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2012a {published data only}
- Page S, Levin L, Hermann V, Dunning K, Levine P. Longer versus shorter daily durations of electrical stimulation during task-specific practice in moderately impaired stroke. Archives of Physical Medicine and Rehabilitation 2012;93(2):200-6. [DOI] [PubMed] [Google Scholar]
Page 2012b {published data only}
- Page S, Levin L, Hermann V, Dunning K, Levine P. Longer versus shorter daily durations of electrical stimulation during task-specific practice in moderately impaired stroke. Archives of Physical Medicine and Rehabilitation 2012;93(2):200-6. [DOI] [PubMed] [Google Scholar]
Partridge 2000 {published data only}
- Partridge C, Mackenzie M, Edwards S, Reid A, Jayawardena S, Guck N and Potter J. Is dosage of physiotherapy a critical factor in deciding patterns of recovery from stroke: a pragmatic randomized controlled trial. Physiotherapy Research International 2000;5(4):230-40. [DOI] [PubMed] [Google Scholar]
Smith 1981 {published data only}
- Smith D, Goldenberg E, Ashburn A, Kinsella G, Sheikh K, Brennan P, Meade TW, Zutshi DW, Perry JD and Reeback JS. Remedial therapy after stroke: a randomised controlled trial. British Medical Journal 1981;282(6263):517-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tong 2019 {published data only}
- Tong Y, Cheng Z, Rajah GB, Duan H, Cai L, Zhang N, Du H, Geng X and Ding Y. High intensity physical rehabilitation later than 24 h post stroke is beneficial in patients: a pilot randomized controlled trial (RCT) study in mild to moderate ischemic stroke. Frontiers in Neurology 2019;10:113. [DOI: 10.3389/fneur.2019.00113] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang DS, Lu YY, Xie RM, Yao JR. Effect of different intensities of rehabilitation therapy on the prognosis of patients with stroke. Chinese Journal of Clinical Rehabilitation 2004;8(22):4410-1. [Google Scholar]
Wang 2011 {published data only}
- Wang Q, Zhao J, Zhu Q, Li J, Meng P. Comparison of conventional therapy, intensive therapy and modified constraint-induced movement therapy to improve upper extremity function after stroke. Journal of Rehabilitation Medicine 2011;43(7):619-25. [DOI] [PubMed] [Google Scholar]
Winstein 2019a {published and unpublished data}
- Winstein C, Kim B, Kim S, Martinez C, Schweighofer N. Dosage matters: a phase IIb randomized controlled trial of motor therapy in the chronic phase after stroke. Stroke 2019;50(7):1831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winstein 2019b {published and unpublished data}
- Winstein C, Kim B, Kim S, Martinez C, Schweighofer N. Dosage matters: a phase IIb randomized controlled trial of motor therapy in the chronic phase after stroke. Stroke 2019;50(7):1831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdullahi 2021 {published data only}
- Abdullahi A, Aliyu NU, Useh U, Abba MA, Akindele MO, Truijen S, et al. Effects of two different modes of task practice during lower limb constraint-induced movement therapy in people with stroke: a randomized clinical trial. Neural Plasticity 2021;2021:6664058. [DOI: 10.1155/2021/6664058] [DOI] [PMC free article] [PubMed]
Abraha 2017 {published data only}
- Abraha B, Wadden KP, Wallack EM, Kelly LP, Mccarthy J, Ploughman M. Acute moderate continuous exercise, but not high intensity interval training, reduces the inhibitory influence of the contralesional hemisphere and affects hand movement in chronic stroke. International Journal of Stroke 2017;12(4 Suppl 1):46. [Google Scholar]
Afridi 2021 {published data only}
- Afridi A. Comparison of frequency & duration of task practice during constraint induced movement therapy. clinicaltrials.gov/ct2/show/NCT04757467 (first received 17 February 2021).
Agarwal 2008 {published data only}
- Agarwal V, Mukesh K, Kumar M, Ranjana P. Effect of number of repetitions of weight bearing exercises on time-distance parameters in stroke. Indian Journal of Physiotherapy and Occupational Therapy 2008;2(1):57-63. [Google Scholar]
Ardestani 2020 {published data only}
- Ardestani MM, Henderson CE, Mahtani G, Connolly M, Hornby TG. Locomotor kinematics and kinetics following high-intensity stepping training in variable contexts poststroke. Neurorehabilitation and Neural Repair 2020;34(7):652-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Askim 2004 {published data only}
- Askim T, Rohweder G, Lydersen S, Indredavik B. Evaluation of an extended stroke unit service with early supported discharge for patients living in a rural community. A randomized controlled trial. Clinical Rehabilitation 2004;18(3):238-48. [DOI] [PubMed] [Google Scholar]
Askim 2010 {published data only}
- Askim T, Mørkved S, Engen A, Roos K, Aas T, Indredavik B. Effects of a community-based intensive motor training program combined with early supported discharge after treatment in a comprehensive stroke unit: a randomized, controlled trial. Stroke 2010;41(8):1697-703. [DOI] [PubMed] [Google Scholar]
Bai 2008 {published data only}
- Bai Y, Hu Y, Chen W, Wang X, Cheng A, Jiang C, et al. Effects of three stage rehabilitation therapy on neurological deficit scores and ADL in ischemic stroke patients. Journal of Rehabilitation Medicine 2008;Suppl 46:109. [Google Scholar]
Bowden 2020 {published data only}
- Bowden MG, Monsch ED, Middleton A, Daughtry C, Powell T, Kraft SV. Lessons learned: the difficulties of incorporating intensity principles into inpatient stroke rehabilitation. Archives of Rehabilitation Research and Clinical Translation 2020;2(2):100052. [DOI: 10.1016/j.arrct.2020.100052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2016 {published data only}
- Boyd L. The influence of exercise on neuroplasticity and motor learning after stroke (EX-ML3). clinicaltrials.gov/ct2/show/NCT02980796 (first received 2 December 2016).
Brusco 2014 {published data only}
- Brusco NK, Watts JJ, Shields N, Chan SP, Taylor NF. Does additional acute phase inpatient rehabilitation help people return to work? A subgroup analysis from a randomized controlled trial. Clinical Rehabilitation 2014;28(8):754-61. [DOI] [PubMed] [Google Scholar]
Byblow 2020 {published data only}
- Byblow W, Krakauer J, Stinear C, Barber P, Lee A, Kitago T. ESPRESSo: enhancing spontaneous recovery of hand and arm movement with high intensity therapy beginning one week after stroke. www.cochranelibrary.com/es/central/doi/10.1002/central/CN-02184407/full (accessed prior to 27 September 2021).
Byl 2008 {published data only}
- Byl N, Pitsch E, Abrams G. Functional outcomes can vary by dose: learning-based sensorimotor training for patients stable poststroke. Neurorehabilitation and Neural Repair 2008;22(5):494-504. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen WH, Yu B, Xie XH, Tu XF. Application and cost-effectiveness analysis of three-stage rehabilitation program in treating acute stroke. Chinese Journal of Clinical Rehabilitation 2006;10(48):31-3. [Google Scholar]
Daly 2019 {published data only}
- Daly JJ, McCabe JP, Holcomb J, Monkiewicz M, Gansen J, Pundik S. Long-dose intensive therapy is necessary for strong, clinically significant, upper limb functional gains and retained gains in severe/moderate chronic stroke. Neurorehabilitation and Neural Repair 2019;33(7):523-37. [DOI: 10.1177/1545968319846120] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Sousa 2019 {published data only}
- Sousa DG, Harvey LA, Dorsch S, Varettas B, Jamieson S, Murphy A, et al. Two weeks of intensive sit-to-stand training in addition to usual care improves sit-to-stand ability in people who are unable to stand up independently after stroke: a randomised trial. Journal of Physiotherapy 2019;65(3):152-8. [DOI: 10.1016/j.jphys.2019.05.007] [DOI] [PubMed] [Google Scholar]
Di Lauro 2003 {published data only}
- Di Lauro A, Pellegrino L, Savastano G, Ferraro C, Fusco M, Balzarano F, et al. A randomized trial on the efficacy of intensive rehabilitation in the acute phase of ischemic stroke. Journal of Neurology 2003;250(10):1206-8. [DOI] [PubMed] [Google Scholar]
DRKS00023235 {published data only}
- DRKS00023235. Pilot study on the effects of an increased mobilization frequency on stroke patients in early neurological rehabilitation. www.cochranelibrary.com/central/doi/10.1002/central/CN-02240342/full (accessed prior to 27 September 2021).
Duff 2013 {published data only}
- Duff A, Nirme J, Rubio B, Duarte E, Cuxart A, Rodriguez S, et al. The optimal dosage of the rehabilitation gaming system: the impact of a longer period of virtual reality based and standard occupational training on upper limb recovery in the acute phase of stroke. Cerebrovascular Diseases 2013;35(Suppl 3):146. [Google Scholar]
Duncan 2003 {published data only}
- Duncan P, Studenski S, Richards L, Gollub S, Lai S, Reker D, et al. Randomized clinical trial of therapeutic exercise in subacute stroke. Stroke 2003;34(9):2173-80. [DOI] [PubMed] [Google Scholar]
Fasoli 2004 {published data only}
- Fasoli S, Krebs H, Ferraro M, Hogan N, Volpe B. Does shorter rehabilitation limit potential recovery poststroke? Neurorehabilitation and Neural Repair 2004;18(2):88-94. [DOI] [PubMed] [Google Scholar]
Forster 1992 {published data only}
- Forster A, Young J. Comparative cost-effectiveness of day hospital care and home rehabilitation for stroke patients. Clinical Rehabilitation 1992;6(Suppl):48. [Google Scholar]
Foucher 2017 {published data only}
- Foucher L. Comparison of fatigue and recovery after stroke depending on the usual management with or without physical training (FRAM). clinicaltrials.gov/ct2/show/NCT03259932 (first received 24 August 2017).
Galloway 2017 {published data only}
- Galloway M, Marsden D, Callister R, Niksson M, Erickson K, English C. Determining the minimum dose of exercise required to improve cardiorespiratory fitness in stroke survivors: protocol for the ExDose trial. International Journal of Stroke 2017;12(Suppl 2):27. [Google Scholar]
Gobbato 2012 {published data only}
- Gobbato S, Martins S. Very Early Rehabilitation in acute Ischemic Stroke (VERIS-Brazil). clinicaltrials.gov/ct2/show/NCT01694992 (first received 27 September 2012).
Green 2004 {published data only}
- Green J, Young J, Forster A, Collen F, Wade D. Combined analysis of two randomized trials of community physiotherapy for patients more than one year post stroke. Clinical Rehabilitation 2004;18(3):249-52. [DOI] [PubMed] [Google Scholar]
Henriksen 1992 {published data only}
- Henriksen IO, Ostergaard Laursen S. Acute stroke – treatment in a non-intensive stroke unit. Scandinavian Journal of Rehabilitation Medicine 1992;26(Suppl):153. [Google Scholar]
Hesse 2011 {published data only}
- Hesse S, Welz A, Werner C, Quentin B, Wissel J. Comparison of an intermittent high-intensity vs continuous low-intensity physiotherapy service over 12 months in community-dwelling people with stroke: a randomized trial. Clinical Rehabilitation 2011;25(2):146-56. [DOI] [PubMed] [Google Scholar]
Hogg 2020 {published data only}
- Hogg S, Holzgraefe M, Druge C, Hauschild F, Herrmann C, Obermann M, et al. High-intensity arm resistance training does not lead to better outcomes than low-intensity resistance training in patients after subacute stroke: a randomized controlled trial. Journal of Rehabilitation Medicine 2020;52(6):jrm00067. [DOI: 10.2340/16501977-2686] [DOI] [PubMed] [Google Scholar]
Hornby 2015 {published data only}
- Hornby T. Very Intensive Variable Repetitive AmbulatioN Training (VIVRANT). clinicaltrials.gov/ct2/show/NCT02507466 (first received 24 July 2015).
Hornby 2016 {published data only}
- Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Camardo J, et al. Variable Intensive Early Walking Poststroke (VIEWS): a randomized controlled trial. Neurorehabilitation and Neural Repair 2016;30(5):440-50. [DOI] [PubMed] [Google Scholar]
Host 2014 {published data only}
- Host HH, Lang CE, Hildebrand MW, Zou D, Binder EF, Baum CM, et al. Patient active time during therapy sessions in postacute rehabilitation: development and validation of a new measure. Physical and Occupational Therapy in Geriatrics 2014;32(2):169-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2016 {published data only}
- Hsu MJ. The effect of leg cycling exercise program at low or moderate intensity for individuals with subacute stroke. clinicaltrials.gov/ct2/show/NCT02855424 (first received 4 August 2016).
Hubbard 2010 {published data only}
- Hubbard IJ, Budd WT, Carey LM, Mcelduff P, Levi CR, Parsons MW. Intensive behavioural upper limb training in acute stroke: an RCT of functional outcomes and brain reorganisation. Cerebrovascular Diseases 2010;29(Suppl 2):63-4. [Google Scholar]
Huijben‐Schoenmakers 2014 {published data only}
- Huijben-Schoenmakers M, Rademaker A, Van Rooden P, Scherder E. The effects of increased therapy time on cognition and mood in frail patients with a stroke who rehabilitate on rehabilitation units of nursing homes in the Netherlands: a protocol of a comparative study. BMC Geriatrics 2014;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kissela 2013 {published data only}
- Kissela B, Dunning K. High Intensity Interval Training in chronic stroke (HIT). clinicaltrials.gov/ct2/show/NCT01958606 (first received 29 March 2017).
Klassen 2020 {published data only}
- Klassen T, Eng JJ, Bayley M, Benavente O, Krassioukov A, Piitz M, et al. Determining optimal post-stroke exercise (DOSE): study protocol for a randomized controlled trial investigating exercise intensity during inpatient rehabilitation. International Journal of Stroke 2017;12( Suppl 1):57-8. [DOI] [PubMed] [Google Scholar]
- Klassen TD, Dukelow SP, Bayley MT, Benavente O, Hill MD, Krassioukov A, et al. Higher doses improve walking recovery during stroke inpatient rehabilitation. Stroke 2020;51(9):2639-48. [DOI: 10.1161/STROKEAHA.120.029245] [DOI] [PubMed] [Google Scholar]
Kosak 1998 {published data only}
- Kosak M, Reding M. Early aggressive mobilization is as effective as treadmill training for ambulation recovery in patients with stroke. Journal of Stroke and Cerebrovascular Diseases 1998;7:372. [Google Scholar]
Krebs 1997 {published data only}
- Krebs H, Hogan N. Clinical test of robot-aided stroke rehabilitation. Journal of Stroke and Cerebrovascular Diseases 1997;6(3):145. [Google Scholar]
Lamberti 2017 {published data only}
- Lamberti N, Straudi S, Malagoni A, Argiro M, Felisatti M, Nardini E, et al. Effects of low-intensity endurance and resistance training on mobility in chronic stroke survivors: a pilot randomized controlled study. European Journal of Physical and Rehabilitation Medicine 2017;53(2):228-39. [DOI] [PubMed] [Google Scholar]
Langhammer 2007 {published data only}
- Langhammer B, Lindmark B, Stanghelle J. Stroke patients and long-term training: is it worthwhile: a randomized comparison of two different training strategies after rehabilitation. Clinical Rehabilitation 2007;21(6):495-510. [DOI] [PubMed] [Google Scholar]
Langhammer 2014 {published data only}
- Langhammer B, Lindmark B, Stanghelle J. Physiotherapy and physical functioning post-stroke: exercise habits and functioning 4 years later? Long-term follow-up after a 1-year long-term intervention period: a randomized controlled trial. Brain Injury 2014;28(11):1396–405. [DOI] [PubMed] [Google Scholar]
Langhorne 2010 {published data only}
- Langhorne P, Stott D, Knight A, Bernhardt J, Barer D, Watkins C. Very early rehabilitation or intensive telemetry after stroke: a pilot randomised trial. Cerebrovascular Diseases 2010;29(4):352-60. [DOI] [PubMed] [Google Scholar]
Langhorne 2017 {published data only}
- Langhorne P, Wu O, Rodgers H, Ashburn A, Bernhardt J. A Very Early Rehabilitation Trial after stroke (AVERT): a phase III, multicentre, randomised controlled trial. Health Technology Assessment 2017;21(54):1-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee JH, Kim SB, Lee KW, Lee JY. The effect of prolonged inpatient rehabilitation therapy in subacute stroke patients. Annals of Rehabilitation Medicine 2012;36(1):16-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewthwaite 2018 {published data only}
- Lewthwaite R, Winstein CJ, Lane CJ, Blanton S, Wagenheim BR, Nelsen MA, et al. Accelerating stroke recovery: body structures and functions, activities, participation, and quality of life outcomes from a large rehabilitation trial. Neurorehabilitation and Neural Repair 2018;32(2):150-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2000 {published data only}
- Li L, Yuan J, Zhang C. Clinical observation on hemiplegic patients with functional electric stimulation and intensive training on their lower extremities. Chinese Journal of Physical Medicine and Rehabilitation 2000;22(1):18-9. [Google Scholar]
Lin 2017 {published data only}
- Lin Y. Does the enhanced rehabilitation programs facilitate the motor recovery after stroke? clinicaltrials.gov/ct2/show/NCT03230370 (first received 26 July 2017).
Lo 2010 {published data only}
- Lo A. VA ROBOTICS published: high-intensity therapy aids recovery of function, QOL in chronic stroke. www.medscape.com/viewarticle/720573 (accessed prior to 27 September 2021). [https://www.medscape.com/viewarticle/720573]
Logan 1997 {published data only}
- Logan PA, Ahern J, Gladman JR, Lincoln NB. A randomized controlled trial of enhanced social service occupational therapy for stroke patients. Clinical Rehabilitation 1997;11:107-13. [DOI] [PubMed] [Google Scholar]
Malouin 1992 {published data only}
- Malouin F, Potvin M, Prevost J, Richards CL, Wood-Dauphinee S. Use of intensive task-oriented gait training program in a series of patients with acute cerebrovascular accidents. Physical Therapy 1992;72(11):789-93. [DOI] [PubMed] [Google Scholar]
Malouin 1993 {published data only}
- Malouin F, Richards C, Wood-Dauphinee S, Ivan Williams J. A randomized controlled trial comparing early and intensive task-specific therapy to conventional therapy in acute-stroke patients. Canadian Journal of Rehabilitation 1993;7(1):27-8. [Google Scholar]
Martinsson 2003 {published data only}
- Martinsson L, Eksborg S, Wahlgren N. Intensive early physiotherapy combined with dexamphetamine treatment in severe stroke: a randomized, controlled pilot study. Cerebrovascular Diseases 2003;16(4):338-45. [DOI] [PubMed] [Google Scholar]
Marzolini 2016 {published data only}
- Marzolini S, Kangatharan S, Koblinsky N, Oh P. High intensity interval training for people with stroke deficits (HIIT-Stroke). clinicaltrials.gov/ct2/show/NCT03006731 (first received 30 December 2016).
McDonnell 2017 {published data only}
- McDonnell MN, Serrada I, Hillier SL. Increasing the amount of upper extremity rehabilitation in the first four weeks following stroke: a feasibility study. Stroke 2017;48(Suppl 1):TMP39.
Mickelborough 1999 {published data only}
- Mickelborough J, Liston R, Harris B, Wynn Hann A, Tallis RC. An evaluation of conventional physiotherapy and treadmill re-training of higher-level gait disorders in patients with cerebral multi-infarct states. Age and Ageing 1999;28(Suppl 2):54. [Google Scholar]
Mikulecka 2005 {published data only}
- Mikulecka E, Petruskova L, Mayer M, Vlachova I. Differentiated manual treatment of the hand and forearm in the early rehabilitation of stroke patients (a controlled study). Rehabilitacia Bratislava 2005;42(1):52-61. [Google Scholar]
Outermans 2010 {published data only}
- Outermans J, Peppen RP, Wittink H, Takken T, Kwakkel G. Effects of a high-intensity task-oriented training on gait performance early after stroke: a pilot study. Clinical Rehabilitation 2010;24(11):979-87. [DOI] [PubMed] [Google Scholar]
Ozdemir 2001 {published data only}
- Ozdemir F, Birtane M, Tabatabaei R, Kokino S, Ekuklu G. Comparing stroke rehabilitation outcomes between acute inpatient and nonintense home settings. Archives of Physical Medicine and Rehabilitation 2001;82:1375-9. [DOI] [PubMed] [Google Scholar]
Raghavan 2016 {published data only}
- Raghavan P, Aluru V, Palumbo A, Battaglia J, Kwon S, Ogedegbe G. Creating an enriched rehabilitation environment in a low-resource setting. International Journal of Stroke 2016;11(Suppl 3):36. [Google Scholar]
Richards 2006a {published data only}
- Richards L, Rothi L, Davis S, Wu S, Nadeau S. Limited dose response to constraint-induced movement therapy in patients with chronic stroke. Clinical Rehabilitation 2006;20(12):1066-74. [DOI] [PubMed] [Google Scholar]
Richards 2006b {published data only}
- Richards L. The effect of different schedules of functional task practice for improving hand and arm function after stroke. clinicaltrials.gov/ct2/show/NCT00361660 (first received 8 August 2006). [https://clinicaltrials.gov/ct2/show/NCT00361660]
Roderick 2001 {published data only}
- Roderick P, Low J, Day R, Peasgood T, Mullee M, Turnbull J, et al. Stroke rehabilitation after hospital discharge: a randomized trial comparing domiciliary and day-hospital care. Age and Ageing 2001;30(4):303-10. [DOI] [PubMed] [Google Scholar]
Rodgers 2019 {published data only}
- Rodgers H, Bosomworth H, Krebs HI, van Wijck F, Howel D, Wilson N, et al. Robot Assisted Training for the Upper Limb after Stroke (RATULS): a multicentre randomised controlled trial. Lancet 2019;394(10192):51-62. [DOI: 10.1016/ S0140-6736(19)31055-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruff 1999 {published data only}
- Ruff R, Yarnell S, Marinos J. Are stroke patients discharged sooner if in-patient rehabilitation services are provided seven v six days per week? American Journal of Physical Medicine and Rehabilitation 1999;78(2):143-6. [DOI] [PubMed] [Google Scholar]
Ryan 2006 {published data only}
- Ryan T, Enderby P, Rigby AS. A randomized controlled trial to evaluate intensity of community-based rehabilitation provision following stroke or hip fracture in old age. Clinical Rehabilitation 2006;20(2):123-31. [DOI] [PubMed] [Google Scholar]
Sanchez‐Sanchez 2015 {published data only}
- Sanchez-Sanchez ML, Belda-Lois JM, Viosca-Herrero E, Igual-Camacho C, Gisbert-Morant B and Mena-del S. Analysis of the effect of using two physiotherapy protocols in balance recovery after stroke using a novel methodology of functional principal components. Cerebrovascular Diseases 2015;39(Suppl 2):322. [Google Scholar]
Scrivener 2012 {published data only}
- Scrivener K, Sherrington C, Schurr K. Amount of exercise in the first week after stroke predicts walking speed and unassisted walking. Neurorehabilitation and Neural Repair 2012;26(8):932-8. [DOI] [PubMed] [Google Scholar]
Sheil 2001 {published data only}
- Shiel A, Burn J, Henry D, Clark J, Wilson B, Burnett M, et al. The effects of increased rehabilitation therapy after brain injury: results of a prospective controlled trial. Clinical Rehabilitation 2001;15(5):501-14. [DOI] [PubMed] [Google Scholar]
Sivenius 1985 {published data only}
- Sivenius J, Pyörälä K, Heinonen OP, Salonen JT, Riekkinen P. The significance of intensity of rehabilitation of stroke-a controlled trial. Stroke 1985;16(6):928-31. [DOI] [PubMed] [Google Scholar]
Slade 2002 {published data only}
- Slade A, Tennant A, Chamberlain M. A randomised controlled trial to determine the effect of intensity of therapy upon length of stay in a neurological rehabilitation setting. Journal of Rehabilitation Medicine 2002;34(6):260-6. [DOI] [PubMed] [Google Scholar]
Sonoda 2004 {published data only}
- Sonoda S, Saitoh E, Nagai S, Kawakita M, Kanada Y. Full-time integrated treatment program, a new system for stroke rehabilitation in Japan: comparison with conventional rehabilitation. American Journal of Physical Medicine and Rehabilitation 2004;83(2):88-93. [DOI] [PubMed] [Google Scholar]
Sterr 2002 {published data only}
- Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: a exploratory study. Archives of Physical Medicine and Rehabilitation 2002;83:1374-7. [DOI] [PubMed] [Google Scholar]
Sunderland 1992 {published data only}
- Sunderland A, Tinson D, Bradley E, Fletcher D, Langton HR, Wade D. Enhanced physical therapy improves recovery of arm function after stroke. A randomised controlled trial. Journal of Neurology, Neurosurgery, and Psychiatry 1992;55(7):530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomic 2017 {published data only}
- Tomic TJ, Savic AM, Vidakovic AS, Rodic SZ, Isakovic MS, Rodriguez-De-Pablo C, et al. ArmAssist Robotic System versus matched conventional therapy for poststroke upper limb rehabilitation. Biomedical Research International 2017;7659893:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Wijck 2020 {published data only}
- Wijck F, Alexander G, Baillie L, Bain B, Barber M, Dall P, et al. Early VERsus Later Augmented Physiotherapy compared with usual physiotherapy (EVERLAP): a feasibility randomised controlled trial of arm function after stroke. Physiotherapy 2020;107(Suppl 1):e13-e14. [DOI: 10.1016/j.physio.2020.03.021] [DOI] [Google Scholar]
Wu 2016 {published data only}
- Wu X, Guarino P, Lo AC, Peduzzi P, Wininger M. Long-term effectiveness of intensive therapy in chronic stroke. Neurorehabilitation and Neural Repair 2016;30(6):583-90. [DOI] [PubMed] [Google Scholar]
Wuennemann 2020 {published data only}
- Wuennemann MJ, Mackenzie SW, Lane HP, Peltz AR, Ma X, Gerber LM, et al. Dose and staffing comparison study of upper limb device-assisted therapy. NeuroRehabilitation 2020;46(3):287-97. [DOI] [PubMed] [Google Scholar]
Xu 2008 {published data only}
- Xu Y. The effect of early intensive exercise on patients with hemiplegia. Journal of Rehabilitation Medicine 2008;Suppl 46:75. [Google Scholar]
Yau 2010 {published data only}
- Yau W, Wong W. The feasibility and efficacy of an augmented exercise program for acute stroke patients. International Journal of Stroke 2010;5(Suppl 2):346-7. [Google Scholar]
Yelnik 2017 {published data only}
- Yelnik A, Quintaine V, Andriantsifanetra C, Wannepain M, Reiner P, Marnef H, et al. AMOBES (Active Mobility Very Early after Stroke): a randomized controlled trial. Stroke 2017;48(2):400-5. [DOI] [PubMed] [Google Scholar]
Yu 2008 {published data only}
- Yu J, Hu Y, Wu Y, Chen W, Cui X, Lu W, et al. An analysis about the effects of standardized community-based rehabilitation (CBR) therapy on ADL for patients after stroke in China. Journal of Rehabilitation Medicine 2008;Suppl 46:110. [Google Scholar]
Zhang 2005 {published data only}
- Zhang DJ, Zhu SW, Cui GX, Liu SJ, Li YZ. Difference between early and late rehabilitative intervention in ameliorating the motor function and activities of daily living in patients with cerebral infarction. Chinese Journal of Clinical Rehabilitation 2005;9(33):149-51. [Google Scholar]
References to studies awaiting assessment
Aksu 2001 {published data only}
- Aksu S, Armutlu K, Cetisli N, Guclu A, Atay S, Guniken Y, et al. Numbers of exercises in acute stroke rehabilitation: is it important? Neurorehabilitation and Neural Repair 2001;15(4):348. [Google Scholar]
Cauraugh 2006 {published data only}
- Cauraugh JH. Effects of different amounts of electrical stimulation + bilateral practice on improving hand function after stroke. clinicaltrials.gov/ct2/show/NCT00369668 (first received 29 August 2006).
Hsieh 2011 {published data only}
- Hsieh Y, Wu C, Liao W, Lin K, Wu K, Lee C. Effects of treatment intensity in upper limb robot-assisted therapy for chronic stroke: a pilot randomized controlled trial. Neurorehabilitation and Neural Repair 2011;25(6):503-11. [DOI] [PubMed] [Google Scholar]
Jung 2008 {published data only}
- Jung HY, Yeo SW, Park BK. Influences of the amount of rehabilitation training on the functional improvement after stroke. International Journal of Stroke 2008;3(Suppl 1):345. [Google Scholar]
Kreisel 2005 {published data only}
- Kreisel SH, Bazner H, Hennerici MG. Intensive rehabilitation in the acute phase of stroke: positive or negative effects on outcome? Cerebrovascular Diseases 2005;19(Suppl 2):92. [Google Scholar]
Rimmer 2004 {published data only}
- Rimmer J, Wang E. The dose–response effects on aerobic exercise in stroke survivors. Archives of Physical Medicine and Rehabilitation 2004;85(8):E2. [Google Scholar]
Takebayashi 2015 {published data only}
- Takebayashi T, Takahashi K, Domen K, Hachisuka K, Kimura T. Efficient training intensity of robotic therapy to improve arm function in subacute stroke patients. Cerebrovascular Diseases 2015;39(Suppl 2):260. [Google Scholar]
Wu 2013 {published data only}
- Wu CW. Effects of intensive robot-assisted therapy in patients with subacute stroke (RT). clinicaltrials.gov/ct2/show/NCT01767480 (first received 14 January 2013).
References to ongoing studies
Bernhardt 2019 {published data only}
- Bernhardt J, Churilov L, Langhorne P, Pandian J, Dewey H, Shrikanth V, the AVERT DOSE Trialist Collaboration. Ongoing trial update 2019: determining optimal early rehabilitation after stroke (AVERT DOSE). European Stroke Journal 2019;4(Suppl 1):816. [DOI: 10.1177/2396987319848124] [DOI] [Google Scholar]
- Bernhardt J. A phase 3, multi arm, multi stage, covariate adjusted, response adaptive, randomised trial to determine optimal early mobility training after stroke. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=376646 (first received 10 April 2019). [DOI] [PubMed]
Chang 2015 {published data only}
- Chang WH, Sohn MK, Lee J, Kim DY, Lee SG, Shin YI, et al. Korean Stroke Cohort for functioning and rehabilitation (KOSCO): study rationale and protocol of a multi-centre prospective cohort study. BMC Neurology 2015;15:42. [DOI: 10.1186/s12883-015-0293-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03168360. Effect of intensive cognitive rehabilitation in subacute stroke patient. clinicaltrials.gov/ct2/show/NCT03168360 (first received 30 May 2017).
Dukelow 2019 {published data only}
- Dukelow S. Robot-Enhanced Stroke Therapy Optimizes Rehabilitation (RESTORE). clinicaltrials.gov/ct2/show/NCT04201613 (first received 17 December 2019). [https://clinicaltrials.gov/ct2/show/NCT04201613]
Holmstedt 2021 {published data only}
- Holmstedt C. Impact of more frequent PT services. clinicaltrials.gov/ct2/show/NCT04778475 (first received 3 March 2021).
Kanlaya 2018 {published data only}
- Kanlaya S. Effects of the Accelerated Skill Acquisition Program (ASAP) training duration on reach-to-grasp performance in individuals with subacute stroke (effects of ASAP duration on UE training for stroke). https://www.thaiclinicaltrials.org/show/TCTR20180316004. [www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20180316004]
Kinoshita 2020 {published data only}
- Kinoshita S, Ikeda K, Yasuno S, Takahashi S, Yamada N, Okuyama Y, et al. Dose–response of rPMS for upper limb hemiparesis after stroke. Medicine 2020;99(24):e20752. [DOI: 10.1097/MD.0000000000020752] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ling 2018 {published data only}
- Ling H. Effect of different intensity rehabilitation training on hemiplegic patients after stroke. www.chictr.org.cn/showproj.aspx?proj=28656 (first received 28 June 2018). [http://www.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR1800016841]
Mansfield 2020 {published data only}
- Mansfield A, Inness EL, Danells CJ, Jagroop D, Bhatt T, Huntley AH. Determining the optimal dose of reactive balance training after stroke: study protocol for a pilot randomised controlled trial. BMJ Open 2020;10:e038073. [DOI: 10.1136/ bmjopen-2020-038073] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield A. Determining the optimal dose of reactive balance training after stroke. clinicaltrials.gov/ct2/show/NCT04219696 (first received 7 January 2020). [https://www.clinicaltrials.gov/ct2/show/NCT04219696]
Additional references
Arya 2011
- Arya KN, Verma R, Garg RK. Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Topics in Stroke Rehabilitation 2011;18(Suppl 1):599-610. [DOI] [PubMed] [Google Scholar]
Bahalla 2021
- Bahalla A, James M, Stanley K, Ralph S, Durante N, Mcmullen E, et al. Springboard for Progress: the Seventh SSNAP Annual Report. London (UK): Healthcare Quality Improvement Partnership (HQIP), 2021. [Google Scholar]
Ballinger 1999
- Ballinger C, Ashburn A, Low J, Roderick P. Unpacking the black box of therapy – a pilot study to describe occupational therapy and physiotherapy interventions for people with stroke. Clinical Rehabilitation 1999;13(4):301-9. [DOI] [PubMed] [Google Scholar]
Bhaskar 2017
- Bhaskar S, Stanwell P, Bivard A, Spratt N, Walker R, Kitsos GH, et al. The influence of initial stroke severity on mortality, overall functional outcome and in-hospital placement at 90 days following acute ischemic stroke: a tertiary hospital stroke register study. Neurology India 2017;65(6):1252-9. [DOI: 10.4103/0028-3886.217947] [DOI] [PubMed] [Google Scholar]
Bohannon 2019
- Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. Journal of Physical Therapy Science 2019;31(1):75-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2013
- Bowen A, Hazelton C, Pollock A, Lincoln NB. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD003586. [DOI: 10.1002/14651858.CD003586.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boyd 2003
- Boyd LA, Winstein CJ. Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Physical Therapy 2003;83(11):976-89. [PubMed] [Google Scholar]
Boyd 2004
- Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learning & Memory 2004;11(4):388-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bramer 2016
- Bramer WM, Giustini D, De Jonge GB, Holland L, Bekhuis T. De-duplication of database search results for systematic reviews in EndNote. Journal of the Medical Library Association 2016;104(3):240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brott 1989
- Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864-70. [DOI: ] [DOI] [PubMed] [Google Scholar]
Buma 2013
- Buma F, Kwakkel G, Ramsey N. Understanding upper limb recovery after stroke. Restorative Neurology and Neuroscience 2013;31(6):707-22. [DOI] [PubMed] [Google Scholar]
Chen 2019
- Chen T, Zhang B, Deng Y, Fan JC, Zhang L, Song F. Long-term unmet needs after stroke: systematic review of evidence from survey studies. BMJ Open 2019;e028137. [DOI: 10.1136/ bmjopen-2018-028137] [DOI] [PMC free article] [PubMed]
Cheng 2015
- Cheng D, Qu Z, Huang J, Xiao Y, Luo H, Wang J. Motivational interviewing for improving recovery after stroke. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011398. [DOI: 10.1002/14651858.CD011398.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2013
- Chung CS, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD008391. [DOI: 10.1002/14651858.CD008391.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
Concato 2010
- Concato J, Lawler EV, Lew RA, Gaziano JM, Aslan M, Huang GD. Observational methods in comparative effectiveness research. American Journal of Medicine 2010;123(12 Suppl 1):e16-23. [DOI] [PubMed] [Google Scholar]
Cooke 2010a
- Cooke EV, Tallis RC, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke: phase I randomized controlled trial. Neurorehabilitation and Neural Repair 2010;24(1):88-96. [DOI] [PubMed] [Google Scholar]
Corbetta 2015
- Corbetta D, Sirtori V, Castellini G, Moja L, Gatti R. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database of Systematic Reviews 2015, Issue 10. Art. No: CD004433. [DOI: 10.1002/14651858.CD004433.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
das Nair 2016
- das Nair R, Cogger H, Worthington E, Lincoln NB. Cognitive rehabilitation for memory deficits after stroke. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD002293. [DOI: 10.1002/14651858.CD002293.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Chichester (UK): John Wiley & Sons, 2021. [AVAILABLE FROM: www.training.cochrane.org/handbook] [Google Scholar]
DeJong 2005
- DeJong G, Horn SD, Conroy B, Nichols D, Healton EB. Opening the black box of post-stroke rehabilitation: stroke rehabilitation patients, processes, and outcomes. Archives of Physical Medicine and Rehabilitation 2005;86(12 Suppl 2):S1-7. [DOI] [PubMed] [Google Scholar]
Devereaux 2003
- Devereaux PJ, Yusuf S. The evolution of the randomized controlled trial and its role in evidence-based decision making. Journal of Internal Medicine 2003;254:105-13. [DOI] [PubMed] [Google Scholar]
Dobkin 2005
- Dobkin BH, Carmichael TS. Principles of recovery after stroke. In: Barnes MP, Dobkin BH, Bogousslavsky J, editors(s). Recovery After Stroke. Cambridge (UK): Cambridge University Press, 2005:47-66. [Google Scholar]
Douiri 2013
- Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995–2010. Stroke 2013;44(1):138-45. [DOI: 10.1161/STROKEAHA.112.670844] [DOI] [PubMed] [Google Scholar]
Dromerick 2009
- Dromerick AW, Lang CE, Birkenmeier RL, Wagner JM, Miller JP, Videen TO, et al. Very early constraint-induced movement during stroke rehabilitation (VECTORS): a single-center RCT. Neurology 2009;73(3):195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 1994
- Duncan P, Goldstein LB, Horner RD, Landsman PB, Samsa GP, Matchar M. Similar motor recovery of upper and lower extremities after stroke. Stroke 1994;25(6):1181-8. [DOI] [PubMed] [Google Scholar]
Engelter 2006
- Engelter ST, Gostynski M, Papa A, Frei M, Born C, Ajdacic‐Gross V, et al. Epidemiology of aphasia attributable to first ischemic stroke: incidence, severity, fluency, etiology, and thrombolysis. Stroke 2006;37(6):1379‐84. [DOI] [PubMed] [Google Scholar]
French 2016
- French B, Thomas LH, Coupe J, McMahon NE, Connell L, Harrison J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2016, Issue 11. Art. No: CD006073. [DOI: 10.1002/14651858.CD006073.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galvin 2008
- Galvin R, Murphy B, Cusack T, Stokes E. The impact of increased duration of exercise therapy on functional recovery following stroke: what is the evidence? Topics In Stroke Rehabilitation 2008;15(4):365-77. [DOI] [PubMed] [Google Scholar]
GBD 2016 Stroke Collaborators 2019
- GBD 2016 Stroke Collaborators. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 2019;18:439-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guadagnoli 2004
- Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. Journal of Motor Behavior 2004;36(2):212-4. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, GRADE Working Group. GRADE guidelines: 8. Rating the quality of evidence – indirectness. Journal of Clinical Epidemiology 2011;64(12):1303-10. [DOI: 10.1016/j.jclinepi.2011.04.014] [DOI] [PubMed] [Google Scholar]
Hanlon 1996
- Hanlon RE. Motor learning following unilateral stroke. Archives of Physical Medicine & Rehabilitation 1996;77(8):811-5. [DOI] [PubMed] [Google Scholar]
Hao 2013
- Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD008862. [DOI: 10.1002/14651858.CD008862.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebb 1949
- Hebb DO. The Organization of Behavior. New York (NY): Wiley & Sons, 1949. [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021a
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Chichester (UK): John Wiley & Sons, 2021. [AVAILABLE FROM: www.training.cochrane.org/handbook] [Google Scholar]
Higgins 2021b
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Chichester (UK): John Wiley & Sons, 2021. [AVAILABLE FROM: www.training.cochrane.org/handbook.] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348(3):g1687. [DOI] [PubMed] [Google Scholar]
Horn 2005
- Horn SD, Dejong G, Ryser DK, Veazie PJ, Teraoka J. Another look at observational studies in rehabilitation research: going beyond the holy grail of the randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2005;86(Suppl 2):S8-S15. [DOI] [PubMed] [Google Scholar]
ICSWP 2016
- Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke. Fifth edition. London (UK): Royal College of Physicians, 2016. [Google Scholar]
Janssen 2010
- Janssen H, Bernhardt J, Collier JM, Sena ES, McElduff P, Attia J, et al. An enriched environment improves sensorimotor function post-ischemic stroke. Neurorehabilitation and Neural Repair 2010;24(9):802-13. [DOI] [PubMed] [Google Scholar]
Jette 2005
- Jette DU, Latham NK, Smout RJ, Gassaway J, Slavin MD, Horn SD. Physical therapy interventions for patients with stroke in inpatient rehabilitation facilities. Physical Therapy 2005;85(3):238-48. [PubMed] [Google Scholar]
Kersten 2010
- Kersten P, Ellis-Hill C, Mcpherson KM, Harrington R. Beyond the RCT – understanding the relationship between interventions, individuals and outcome – the example of neurological rehabilitation. Disability and Rehabilitation 2010;32(12):1028-34. [DOI] [PubMed] [Google Scholar]
Kim 2014
- Kim K, Kim YM, Kim EK. Correlation between the activities of daily living of stroke patients in a community setting and their quality of life. Journal of Physical Therapy Science 2014;26(3):417-9. [DOI: 10.1589/jpts.26.417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kitago 2013
- Kitago T, Krakauer JW. Motor learning principles for neurorehabilitation. Handbook of Clinical Neurology 2013;110:93-103. [DOI] [PubMed] [Google Scholar]
Klassen 2020
- Klassen TD, Dukelow SP, Bayley MT, Benavente O, Hill MD, Krassioukov A, et al. Higher doses improve walking recovery during stroke inpatient rehabilitation. Stroke 2020;51(9):2639-48. [DOI] [PubMed] [Google Scholar]
Kleim 2008
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. Journal of Speech, Language and Hearing Research 2008;51(1):s225-39. [DOI] [PubMed] [Google Scholar]
Kwakkel 1997
- Kwakkel G, Wagenaar RC, Koelman TW, Lankhorst GJ, Koetsier JC. Effects of intensity of rehabilitation after stroke. A research synthesis. Stroke 1997;28(8):1550-6. [DOI] [PubMed] [Google Scholar]
Kwakkel 2004
- Kwakkel G, Peppen R, Wagenaar RC, Dauphinee SW, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke: a meta-analysis. Stroke 2004;35(11):2529-36. [DOI] [PubMed] [Google Scholar]
Kwakkel 2015
- Kwakkel G, Veerbeek JM, Wegen EE, Wolf SL. Constraint-induced movement therapy after stroke. Lancet Neurology 2015;14(2):224-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 2008
- Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper-extremity measures early after stroke. Archives of Physical Medicine & Rehabilitation 2008;89(9):1693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langhorne 1996
- Langhorne P, Wagenaar R, Partridge C. Physiotherapy after stroke: more is better? Physiotherapy Research International 1996;1(2):75-88. [DOI] [PubMed] [Google Scholar]
Laver 2013
- Laver KE, Schoene D, Crotty M, George S, Lannin NA, Sherrington C. Telerehabilitation services for stroke. Cochrane Database of Systematic Reviews 2013, Issue 12. Art. No: CD010255. [DOI: 10.1002/14651858.CD010255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laver 2015
- Laver KE, George S, Thomas S, Deutsch JE, Crotty M. Virtual reality for stroke rehabilitation. Cochrane Database of Systematic Reviews 2015, Issue 2. Art. No: CD008349. [DOI: 10.1002/14651858.CD008349.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Levack 2006
- Levack WM, Taylor K, Siegert RJ, Dean SG, Mcpherson KM, Weatherall M. Is goal planning in rehabilitation effective? A systematic review. Clinical Rehabilitation 2006;20(9):739-55. [DOI] [PubMed] [Google Scholar]
Levin 2009
- Levin MF, Kleim JA, Wolf SL. What do motor "recovery" and "compensation" mean in patients following stroke? Neurorehabilitation and Neural Repair 2009;23(4):313-9. [DOI] [PubMed] [Google Scholar]
Lewthwaite 2018
- Lewthwaite R, Winstein CJ, Lane CJ, Blanton S, Wagenheim BR, Nelsen MA, et al. Accelerating stroke recovery: body structures and functions, activities, participation, and quality of life outcomes from a large rehabilitation trial. Neurorehabilitation Neural Repair 2018;32(2):150-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2010
- Lo AC, Guarino PD, Richards LG, Hasellkorn JK, Wittenberg GF, Federman DG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. New England Journal of Medicine 2010;362(19):1772-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loetscher 2013
- Loetscher T, Lincoln NB. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD002842. [DOI: 10.1002/14651858.CD002842.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lohse 2014
- Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose–response relationships in stroke rehabilitation. Stroke 2014;45(7):2053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lui 2018
- Lui SK, Nguyen MH. Elderly stroke rehabilitation: overcoming the complications and its associated challenges. Current Gerontology and Geriatrics Research 2018;2018:9853837. [DOI: 10.1155/2018/9853837] [DOI] [PMC free article] [PubMed]
Ma 1999
- Ma H, Trombly CA, Robinson-Podolski C. The effect of context on skill acquisition and transfer. American Journal of Occupational Therapy 1999;53(2):138-44. [DOI] [PubMed] [Google Scholar]
McCabe 2015
- McCabe J, Monkiewicz M, Holcomb J, Pundik S, Daly JJ. Comparison of robotics, functional electrical stimulation, and motor learning methods for treatment of persistent upper extremity dysfunction after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2015;96(6):981-90. [DOI: 10.1016/j.apmr.2014.10.022] [DOI] [PubMed] [Google Scholar]
Mehrholz 2018
- Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database of Systematic Reviews 2018, Issue 9. Art. No: CD006876. [DOI: 10.1002/14651858.CD006876.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mount 2007
- Mount J, Pierce SR, Parker J, Diegidio R, Woessner R, Spiegel L. Trial and error versus errorless learning of functional skills in patients with acute stroke. Neurorehabilitation 2007;22(2):123-32. [PubMed] [Google Scholar]
Mozaffarian 2015
- Mozaffarian D, Benjamin E, Go A, Arnett D, Blaha M, Cushman M, et al. Heart disease and stroke statistics – 2015 update: a report from the American Heart Association. Circulation 2015;131(4):e29-e322. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Stroke Rehabilitation: Long Term Rehabilitation after Stroke. Manchester (UK): National Institute for Health and Care Excellence, 2013. [Google Scholar]
Nudo 2013
- Nudo RJ. Recovery after brain injury: mechanisms and principles. Frontiers in Human Neuroscience 2013;7:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
Outermans 2010
- Outermans J, Peppen R, Wittink H, Takken T, Kwakkel G. Effects of a high-intensity task-oriented training on gait performance early after stroke: a pilot study. Clinical Rehabilitation 2010;24(11):979-87. [DOI] [PubMed] [Google Scholar]
Oyewole 2020
- Oyewole OO, Ogunlana MO, Gbiri CA, Oritogun KS, Osalusi BS. Impact of post-stroke disability and disability-perception on health-related quality of life of stroke survivors: the moderating effect of disability-severity. Neurological Research 2020;42(10):835-43. [DOI: 10.1080/01616412.2020.1785744] [DOI] [PubMed] [Google Scholar]
Page 2012
- Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Physical Therapy 2012;92(6):791-8. [DOI] [PubMed] [Google Scholar]
Page 2021
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Chichester (UK): John Wiley & Sons, 2021. [AVAILABLE FROM: www.training.cochrane.org/handbook] [Google Scholar]
Patel 2020
- Patel A, Berdunov V, Quayyum Z, King D, Knapp M, Wittenberg R. Estimated societal costs of stroke in the UK based on a discrete event simulation. Age and Ageing 2020;49:270-6. [DOI: 10.1093/ageing/afz162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollock 2014a
- Pollock A, Baer G, Campbell P, Choo P, Forester A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database of Systematic Reviews 2014, Issue 4. Art. No: CD001920. [DOI: 10.1002/14651858.CD001920.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pollock 2014b
- Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. Interventions for improving upper limb function after stroke. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD010820. [DOI: 10.1002/14651858.CD010820.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2019 [Computer program]
- Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019. Available at: revman.cochrane.org.
RoB 2 Guidance 2019
- Higgins JP, Savović J, Page MJ, Sterne JA, the RoB 2 Development Group. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (full guidance document, 22 August 2019). Available from: www.riskofbias.info/welcome/rob-2-0-tool (accessed 6 September 2019).
Rodgers 2003
- Rodgers H, Mackintosh J, Price C, Wood R, Mcnamee P, Fearon T, et al. Does an early increased-intensity interdisciplinary upper limb therapy programme following acute stroke improve outcome? Clinical Rehabilitation 2003;17(6):579-89. [DOI: 10.1191/0269215503cr652oa] [DOI] [PubMed] [Google Scholar]
Rost 2016
- Rost NS, Bottle A, Lee JM, Randall M, Middleton S, Shaw L, the Global Comparators Stroke GOAL collaborators. Stroke severity is a crucial predictor of outcome: an international prospective validation study. Journal of the American Heart Association 2016;5:e002433. [DOI: 10.1161/JAHA.115.002433] [DOI] [PMC free article] [PubMed] [Google Scholar]
Royal College of Physicians 2014
- Royal College of Physicians. How Good is Stroke Care? First SSNAP Annual Report. London (UK): Royal College of Physicians, 2014. [Google Scholar]
Sacco 2013
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, American Heart Association Stroke Council COCS , Anesthesia, Council on Cardiovascular R , Intervention, Council On C , Stroke N, Council On E , Prevention, Council on Peripheral Vascular D , Council on Nutrition PA and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44(7):2064-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saunders 2020
- Saunders DH, Sanderson M, Hayes S, Johnson L, Kramer S, Carter DD, et al. Physical fitness training for stroke patients. Cochrane Database of Systematic Reviews 2020, Issue 3. Art. No: CD003316. [DOI: 10.1002/14651858.CD003316.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2016
- Schneider EJ, Lannin NA, Ada L, Schmidt J. Increasing the amount of usual rehabilitation improves activity after stroke: a systematic review. Journal of Physiotherapy 2016;62(4):182-7. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2021a
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Chichester (UK): John Wiley & Sons, 2021. [Google Scholar]
Schünemann 2021b
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Chichester (UK): John Wiley & Sons, 2021. [Google Scholar]
Scrivener 2012
- Scrivener K, Sherrington C, Schurr K. Amount of exercise in the first week after stroke predicts walking speed and unassisted walking. Neurological Rehabilitation and Neural Repair 2012;26(8):932-8. [DOI] [PubMed] [Google Scholar]
Sehatzadeh 2015
- Sehatzadeh S. Effect of increased intensity of physiotherapy on patient outcomes after stroke: an evidence-based analysis. Ontario Health Technology Assessment Series 2015;15(6):1-42. [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Stroke Foundation 2021
- Stroke Foundation. Clinical Guidelines for Stroke Management. https://app.magicapp.org/#/guideline/5493/section/80895 [Accessed 19th September 2021] 2021.
Teasell 2020
- Teasell R, Salbach NM, Foley N, Mountain A, Cameron JI, Jong A, Acerra NE, Bastasi D, Carter SL, Fung J, Halabi ML, Iruthayarajah J, Harris J, Kim E, Noland A, Pooyania S, Rochette A, Stack BD, Symcox E, Timpson D, Varghese S, Verrilli S, Gubitz G, Casaubon LK, Dowlatshahi D and Lindsay MP. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. International journal of stroke: official journal of the International Stroke Society 2020;15(7):763-788. [DOI: 10.1177/1747493019897843] [DOI] [PubMed] [Google Scholar]
Thieme 2018
- Thieme H, Morkisch N, Mehrholz J, Pohl M, Behrens J, Borgetto B, et al. Mirror therapy for improving motor function after stroke. Cochrane Database of Systematic Reviews 2018, Issue 7. Art. No: CD008449. [DOI: 10.1002/14651858.CD008449.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Vliet 2020
- Vliet R, Selles RW, Andrinopoulou ER, Nijland R, Ribbers GM, Frens MA, et al. Predicting upper limb motor impairment recovery after stroke: a mixture model. Annals of Neurology 2020;87(3):383-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Kordelaar 2013
- Kordelaar J, Wegen EE, Nijland RH, Daffertshofer A, Kwakkel G. Understanding adaptive motor control of the paretic upper limb early poststroke: the EXPLICIT-stroke program. Neurorehabilitation and Neural Repair 2013;27(9):854-63. [DOI] [PubMed] [Google Scholar]
van Vliet 2006
- Vliet PM, Wulf G. Extrinsic feedback for motor learning after stroke: what is the evidence? Disability Rehabilitation 2006;28(13-14):831-40. [DOI] [PubMed] [Google Scholar]
Veerbeek 2011
- Veerbeek JM, Koolstra M, Ket JC, Van Wegen EE, Kwakkel G. Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke: a meta-analysis. Stroke 2011;42(11):3311-5. [DOI] [PubMed] [Google Scholar]
Veerbeek 2014
- Veerbeek JM, Wegen E, Peppen R, Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta-analysis. PloS One 2014;9(2):e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2019
- Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square Programme. Journal of Neurology, Neurosurgery and Psychiatry 2019;90(5):498-506. [DOI: 10.1136/jnnp-2018-319954] [DOI] [PubMed] [Google Scholar]
Whitall 2011
- Whitall J, Waller SM, Sorkin JD, Forrester LW, Macko RF, Hanley DF, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabilitation and Neural Repair 2011;25(2):118-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. International Classification of Functioning, Disability and Health. Geneva (Switzerland): World Health Organization, 2001. [Google Scholar]
Winstein 1990
- Winstein CJ, Schmidt RA. Reduced frequency of knowledge of results enhances motor skill learning. Journal of Experimental Psychology: Learning, Memory, and Cognition 1990;16(4):677-91. [Google Scholar]
Winstein 2016
- Winstein CJ, Wolf SL, Dromerick AW, Lane CJ, Nelsen MA, Lewthwaite R, Interdisciplinary Comprehensive Arm Rehabilitation Evaluation Investigative Team. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke: the ICARE randomized clinical trial. Journal of the American Medical Association 2016;315(6):571-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woytowicz 2017
- Woytowicz EJ, Rietschel JC, Goodman RN, Conroy SS, Sorkin JD, Whitall J, et al. Determining levels of upper extremity movement impairment by applying a cluster analysis to the Fugl-Meyer Assessment of the upper extremity in chronic stroke. Archives of Physical Medicine and Rehabilitation 2017;98(3):456-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2000
- Wu C, Trombly CA, Lin K, Tickle-Degnen L. A kinematic study of contextual effects on reaching performance in persons with and without stroke: influences of object availability. Archives of Physical Medicine & Rehabilitation 2000;81(1):95-101. [DOI] [PubMed] [Google Scholar]
Wulf 2010
- Wulf G, Shea C, Lewthwaite R. Motor skill learning and performance: a review of influential factors. Medical Education 2010;44(1):75-84. [DOI] [PubMed] [Google Scholar]
Yang 2016
- Yang A, Wu HM, Tang JL, Xu L, Yang M, Liu GJ. Acupuncture for stroke rehabilitation. Cochrane Database of Systematic Reviews 2016, Issue 8. Art. No: CD004131. [DOI: 10.1002/14651858.CD004131.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Clark 2017
- Clark B, Whitall J, Kwakkel G, Mehrholz J, Ewings S, Burridge J. Time spent in rehabilitation and effect on measures of activity after stroke. Cochrane Database of Systematic Reviews 2017, Issue 3. Art. No: CD012612. [DOI: 10.1002/14651858.CD012612] [DOI] [PMC free article] [PubMed] [Google Scholar]