Supplemental Digital Content is available in the text.
Keywords: atherosclerosis, dietary fats, low-density lipoprotein, nutrition, sphingomyelin
Abstract
Objective:
We recently showed that measurement of the susceptibility of LDL (low-density lipoprotein) to aggregation is an independent predictor of cardiovascular events. We now wished to compare effects of overfeeding different dietary macronutrients on LDL aggregation, proteoglycan-binding of plasma lipoproteins, and on the concentration of oxidized LDL in plasma, 3 in vitro parameters consistent with increased atherogenicity.
Approach and Results:
The participants (36 subjects; age, 48±10 years; body mass index, 30.9±6.2 kg/m2) were randomized to consume an extra 1000 kcal/day of either unsaturated fat, saturated fat, or simple sugars (CARB) for 3 weeks. We measured plasma proatherogenic properties (susceptibility of LDL to aggregation, proteoglycan-binding, oxidized LDL) and concentrations and composition of plasma lipoproteins using nuclear magnetic resonance spectroscopy, and in LDL using liquid chromatography mass spectrometry, before and after the overfeeding diets. LDL aggregation increased in the saturated fat but not the other groups. This change was associated with increased sphingolipid and saturated triacylglycerols in LDL and in plasma and reduction of clusterin on LDL particles. Proteoglycan binding of plasma lipoproteins decreased in the unsaturated fat group relative to the baseline diet. Lipoprotein properties remained unchanged in the CARB group.
Conclusions:
The type of fat during 3 weeks of overfeeding is an important determinant of the characteristics and functional properties of plasma lipoproteins in humans.
Registration:
URL: http://www.clinicaltrials.gov; Unique identifier NCT02133144.
Highlights.
Three-week overfeeding of saturated fats but not unsaturated fats or simple sugars induces significant metabolic changes in plasma lipoproteins.
Overfeeding of saturated fats increases aggregation susceptibility of LDL (low-density lipoprotein) particles.
Overfeeding of unsaturated fats decreases binding of plasma lipoproteins to proteoglycans.
Overfeeding of simple sugars does not change LDL aggregation susceptibility, proteoglycan binding of plasma lipoproteins, or concentration of oxidized LDL.
The source of macronutrients during 3 weeks of overfeeding is an important determinant of the properties of plasma lipoproteins in humans.
Accumulation of LDL (low-density lipoprotein)-derived lipids in the arterial wall induces the development of atherosclerotic lesions.1 LDL particles enter the arterial intima from the circulation and, upon encountering the tight network of extracellular matrix in the arterial intima, are retained by intimal PGs (proteoglycans). The retained lipoproteins are exposed to enzymatic, proteolytic, and oxidative modifications, which can induce aggregation of the modified LDL particles.2,3 Both modified and aggregated lipoproteins are found in atherosclerotic lesions.4–6 Aggregation of modified LDL particles can contribute to atherogenesis by enhancing LDL retention, by inducing foam cell formation, and by promoting inflammation in the arterial intima.2,7–10
The importance of the interaction of LDL and PGs in atherogenesis has been shown by mutating apoB (apolipoprotein B)-100 or by vaccination using antibodies directed against the glycosaminoglycan chains of PGs.11,12 The affinity of LDL to PGs varies depending on the properties of the LDL particles, particularly those that affect their charge, as recently reviewed.13 In addition, the presence of apoE or apoC-III, 2 small exchangeable apolipoproteins, can influence the PG-binding of plasma lipoproteins, apoE by interacting directly with glycosaminoglycans and apoC-III via an unknown mechanism.14–16 Patients with cardiovascular disease or type 2 diabetes have LDL, which has higher affinity toward PGs than control subjects.17–20 This increased affinity can be lowered by treatment with cholesterol-lowering medications and plant stanol esters.21–23
Oxidative modifications of LDL induce generation of oxidized phospholipids and other lipid mediators that are considered to be important in inflammation and atherogenesis.24 Oxidized LDL (oxLDL) particles are found in atherosclerotic lesions,5,25 and the concentration of circulating oxLDL particles is increased in patients with cardiovascular disease.24
Oxidation and other modifications of LDL particles leading to their chemical and structural changes can induce their aggregation.2 We recently developed an assay to determine the aggregation susceptibility of LDL particles and showed that this propensity varies significantly from donor to donor.8 Although LDL aggregation does not correlate with LDL-cholesterol concentration, the aggregation susceptibility is higher in children having familial hypercholesterolemia compared with normocholesterolemic children.26 Importantly, the presence of aggregation-prone LDL in circulation was shown in 2 independent studies to predict future cardiovascular events independently from classical risk factors for atherosclerotic cardiovascular disease.8,27
LDL particles consist of hydrophobic core lipids (cholesteryl esters and triacylglycerols) and amphiphilic surface lipids (phosphatidylcholines, sphingomyelins, lysophosphatidylcholines, and ceramides). Aggregation-prone LDL particles have a higher sphingomyelin and ceramide content.8 Interestingly, plasma concentrations of sphingomyelins are higher in patients with coronary artery disease than in matched controls.28 Similarly, increased concentrations of plasma ceramides predict cardiovascular deaths.29,30 Since a large proportion of plasma sphingolipids are carried by LDL particles, their increased plasma concentrations largely reflect their content in LDL particles.
Sphingomyelins are synthesized de novo mostly from palmitoyl-CoA in a reaction catalyzed by serine-palmitoyl-transferase, with the last step in the synthesis of sphingomyelin being the addition of phosphocholine head group to ceramides. Therefore, consumption of diet rich in palmitate and other saturated fats (SATs) could be expected to increase sphingolipids levels. Indeed, in mouse models, high-fat diet feeding has been found to increase both sphingomyelin and ceramide levels in plasma.31,32 Interestingly, it was recently shown in humans that overfeeding saturated but not unsaturated fat (UNSAT) or carbohydrates, increased plasma concentrations of ceramides and LDL cholesterol.33 The impact of overfeeding different macronutrients on proatherogenic properties of LDL have not, however, been studied.
Here, we examined effects of overfeeding 3 different diets on the binding of LDL to PGs, levels of oxLDL in plasma, and the aggregation susceptibility of LDL particles, 3 parameters associated with increased atherogenicity. In addition, we examined how the diets altered plasma lipoproteins and LDL lipid and protein composition as determined nuclear magnetic resonance (NMR) spectroscopy and liquid chromatography-mass spectrometry (LC-MS).
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Subjects
Subjects for this study were recruited by advertisements or by contacting subjects who had previously participated in metabolic studies. The recruitment and screening procedures and exclusion criteria have been described previously33 and in the Study flow chart (Figure I in the Data Supplement). Written informed consent was obtained from all volunteers. The Ethics Committee of the Helsinki University Hospital had approved the study protocol and study was conducted in accordance with the Declaration of Helsinki.
Study Design
Study participants were randomized to 3 groups to consume extra 1000 kcal/day for 3 weeks in addition to their habitual diet. The extra calories were predominately derived from UNSAT group, SAT group, or simple sugars (CARB group) as shown in Table I in the Data Supplement.
Compliance to the diet was monitored by food diaries and by measuring the fatty acid composition of VLDL-TGs (very low-density lipoprotein triacylglycerols), which reflect hepatic fatty acid composition in blood samples taken after an overnight fast.34
De novo lipogenesis (DNL) was measured using gas chromatography–mass spectrometry based incorporation of deuterium from 2H2O in plasma water (Finnigan Gas-Bench-II; Thermo Fisher Scientific, Loughborough, United Kingdom) into VLDL-triacylglycerol palmitate as described in detail in study by Luukkonen et al.33 Absolute DNL was calculated by multiplying %DNL and the concentration of triacylglycerol in VLDL.35
The primary outcomes in the current study were LDL aggregation, LDL oxidation, and PG binding of plasma lipoproteins.
LDL Isolation
LDL (d=1.019–1.063 g/mL) was isolated from plasma samples by D2O-based sequential ultracentrifugation.36 An aliquot of 300 µL of plasma was used for the isolation, and 300 µL of LDL was collected after ultracentrifugation. The concentration of LDL was measured using the Pierce BCA Protein Assay Kit (Thermo Scientific, Rockford).
Susceptibility of LDL to Aggregation
The susceptibility of isolated LDL particles to aggregation was measured essentially as described before.8,23 Briefly, isolated LDL samples were first diluted to a concentration of 200 µg of LDL protein/mL. Aggregation was induced by addition of human recombinant acid SMase produced in-house23 to the LDL samples, and LDL aggregation was then determined by dynamic light scattering using Wyatt DynaPro Plate Reader II (Wyatt Technology) with paraffin coating. This allowed us to follow the increase in the apparent LDL size (ie, aggregate size) as a time-varying parameter. Aggregation of LDL particles was followed by measuring particle size at various time points for 6 hours and data were collected with Dynamics V7. LDL aggregate size data were analyzed with GraphPad Prism version 8.0.1 (GraphPad Software, La Jolla, CA). The maximum aggregate size was limited to 3000 nm and the minimum size to 14 nm. LDL aggregation curves were fitted with nonlinear regression curve fit of the time versus aggregate size curves to determine their inflection points as a measure of LDL aggregation.
Binding of Plasma Lipoproteins to Proteoglycans
The binding of plasma lipoproteins to PGs was measured in 96-well plates that had been coated with PG isolated from human aortas as previously described.22,37
Measurement of oxLDL
OxLDL concentrations were measured in human plasma samples with the Mercodia, (Uppsala, Sweden) OxLDL sandwich ELISA (product No. 10-1143-01), which includes the mouse monoclonal antibody 4E6 directed against a conformational epitope in oxidized ApoB-100, following the manufacturer’s protocol.
NMR Spectrometry
Plasma samples at baseline and after the interventions were analyzed using a high-throughput NMR platform (AVANCE 500 MHz and AVANCE III 600 MHz, Bruker, Karlsruhe, Germany).38 The platform quantifies over 220 metabolites including total lipids, TGs, phospholipids, total cholesterol, free cholesterol, esterified cholesterol, apolipoproteins, 14 lipoprotein subclasses and their composition, as well as other low-molecular weight metabolites such as amino acids, glycolysis related metabolites, and ketone bodies, as described in detail in study by Würtz et al.38
Lipidomic Analyses by LC-MS
LDL samples were extracted using a modified version of the previously published Folch procedure.39 Briefly, 10 µL of 0.9% NaCl, 40 µL of CHCl3:MeOH (2:1, v/v), and 80 µL of the 2.25 µg/mL internal standard solution of chosen lipid standards (for quality control and normalization purposes) were added to each 10 µL plasma sample. The internal standard solution contained the following compounds: 1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine (PE [17:0/17:0]), N-heptadecanoyl-D-erythro-sphingosylphosphorylcholine (sphingomyelin [d18:1/17:0]), N-heptadecanoyl-D-erythro-sphingosine (Cer [d18:1/17:0]), 1,2-diheptadeca-noyl-sn-glycero-3-phosphocholine (PC [17:0/17:0]), 1-heptadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC [17:0]), and 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-phosphocholine (PC [16:0/d31/18:1]). These were purchased from Avanti Polar Lipids, Inc (Alabaster, AL). In addition, triheptadecanoin (triacylglycerol [17:0/17:0/17:0]) was purchased from (Larodan AB, Solna, Sweden). Samples were vortexed and incubated on ice for 30 minutes after which they were, along with 60 µL from the lower layer of each sample, collected, and 60 µL of CHCl3:MeOH (2:1, v/v) added to each sample.
The UHPLC-quadrupole time of flight mass spectrometer analyses were performed as described earlier40 with some modifications. The UHPLC-quadrupole time of flight mass spectrometer system was from Agilent Technologies (Santa Clara, CA) combining 1290 Infinity system and 6545 quadrupole time of flight mass spectrometer, interfaced with a dual jet stream electrospray (dual ESI) ion source. MassHunter B.06.01 software (Agilent Technologies, Santa Clara, CA) was used for all data acquisition and MZmine 2 was used for data processing.41 Lipid identification was based on in-house spectral library with retention times. Lipids were normalized with lipid-class specific internal standards and (semi) quantitation was performed using lipid-class specific calibration curves.
Proteomic Analyses by LC-MS/MS
The proteins were digested in Amicon Ultra-0.5 centrifugal filters using a modified filter-assisted sample preparation method.42,43 In brief, reduction and alkylation of samples were achieved by the addition of tris (2-carboxyethyl) phosphine and iodoacetamide to a final concentration of 2 and 50 mmol/L, respectively, followed by incubation in the dark for 30 minutes. For digestion, trypsin in 50 mmol/L ammonium bicarbonate was added in a ratio of 1:50 w/w and incubated overnight at room temperature. The peptides were cleaned using C18-reverse-phase ZipTipTM (Millipore), dried, re-suspended in 1% trifluoroacetic acid, and sonicated in a water bath for 1 minute before injection into a nano-LC Q Exactive Plus LC-MS/MS system (Thermo Scientific). The peptides were separated on an Easy-nLC system (Thermo Scientific) equipped with a reverse-phase trapping column Acclaim PepMapTM 100 (C18, 75 μm×20 mm, 3 μm particles, 100 Å; Thermo Scientific), followed by an analytical Acclaim PepMapTM 100 RSLC reversed-phase column (C18, 75 µm×250 mm, 2 µm particles, 100 Å; Thermo Scientific). The injected sample analytes were trapped at a flow rate of 2 µL×min−1 in 100% of solution A (0.1 % formic acid). After trapping, the peptides were separated with a linear gradient of 120 minutes comprising 96 minutes from 3% to 30% of solution B (0.1% formic acid/80% acetonitrile), 7 minutes from 30% to 40% of solution B, and 4 minutes from 40% to 95% of solution B. Each sample run was followed by 2 empty runs to reduce carryover.
LC-MS acquisition data were acquired with the following settings: the resolution was set to 140 000 for MS scans, and 17 500 for the MS/MS scans. A full MS was acquired from 350 to 1400 m/z, and the 10 most abundant precursor ions were selected for fragmentation with 30 second dynamic exclusion time. Ions with 2+, 3+, and 4+ charges were selected for MS/MS analysis. Secondary ions were isolated with a window of 1.2 m/z. The MS AGC target was set to 3×106 counts, whereas the MS/MS AGC target was set to 1×105. Dynamic exclusion was set with a duration of 20 seconds. The NCE collision energy was set to 28 kJ×mol–1.
Following LC-MS/MS acquisition, the raw files were qualitatively analyzed by Proteome Discoverer (version 2.4, Thermo Scientific, United States). The identifications were performed against the Swissprot Human protein database (Human Swissprot downloaded on January 21, 2021, with 20 406 entries) using the built-in SEQUEST HT engine. The following parameters were used: 10 ppm and 0.02 Da were tolerance values set for MS and MS/MS, respectively. Trypsin was used as the digesting enzyme, and 2 missed cleavages were allowed. The carbamidomethylation of cysteines was set as a fixed modification, while the oxidation of methionine and deamidation of asparagine and glutamine were set as variable modifications. The false discovery rate was set to <0.01, and a peptide minimum length of 6 amino acids.
Relative quantification between samples using precursor ion intensities was performed with Progenesis QI (V 3.0) software (Nonlinear Dynamics/Waters) and ProteinLynx Global Server (PLGS V3.0). Chromatograms were aligned by the Progenesis QI software, and those with alignment scores ≥54% to the reference run were selected for further analysis. The relative quantification was done with 1 peptide per protein. Sample pools and individual samples were directly compared as before and after interventions, only quantitations with P<0.05 were accepted, and known contaminants such as keratins were excluded.
Statistical Analyses
The data are presented as mean and SD. Differences in outcomes between the 3 diet groups were determined using 2-way ANOVA with repeated measures in which a significant time×group interaction denotes a statistically significant change by the interventions. Tukey post hoc multiple comparisons test was used for between-group comparisons. Within-group comparisons were performed with Šidak post hoc test. Before analysis, the data were log transformed. ANCOVA was used to analyze the potential effect of variation in baseline values between the groups on changes in response to the intervention, and 1-way ANOVA to compare absolute week 3 (after intervention) values between the groups. The Spearman correlation coefficient was used to analyze the relationship LDL lipid composition and LDL aggregation susceptibility. False discovery rate correction was performed using the Benjamini and Hochberg test. The principal component analysis was performed for multivariable LDL lipidomic data to investigate the lipid species mainly responsible for the variation between samples. Before analysis, the lipidomic data were normalized by median, log transformed, and Pareto scaled. The tests were performed using IBM SPSS Software (version 25.0, North Castle, NY), GraphPad Prism version 8.0.1, or MetaboAnalyst software 4.0 (Xia Lab, McGill University, Montreal, QC, CA).44 P<0.05 was considered to be statistically significant.
Results
Study Design and Compliance to the Diet
We overfed 36 overweight subjects for 3 weeks with 1000 extra kcal/day of either SAT, UNSAT, or simple sugars (CARB).33 The baseline caloric intake of the participants in different groups was similar (ANOVA, P=0.613), and the mean intakes were 2120±520 kcal (n=9) in the UNSAT group, 2040±880 kcal in the SAT group (n=13), and 2070±440 kcal in the CARB group (n=12). At the end of the 3-week diet, the caloric intake based on the food diaries of the participants was 3030±700 kcal in the UNSAT group, 3030±1010 kcal in the SAT group, and 3150±570 kcal in the CARB group (ANOVA, P=0.880). The changes in macronutrients in the 3 groups are shown in Figure II in the Data Supplement. Compliance was assessed by measuring changes in the fatty acid composition of the VLDL-TGs. As expected, the percentage of saturated fatty acids in VLDL-TGs increased in the SAT group (Figure III in the Data Supplement). Consistent with carbohydrate-induced increase in de novo lipogenesis,33 which produces exclusively saturated fatty acids,34,45 the percentage of saturated VLDL-TGs also increased in the CARB group (Figure III in the Data Supplement). In the SAT group, the proportions of both mono- and polyunsaturated VLDL-triacylglycerol fatty acids decreased (Figure III in the Data Supplement). DNL was similar between the groups at baseline (ANOVA, P=0.55). As expected, DNL increased during CARB overfeeding (from 90±62 µmol/L [mean±SD] before to 202±168 µmol/L after, P=0.05), but not during UNSAT (53±45 µmol/L before versus 80±152 µmol/L after, P=0.59) or SAT (65±66.3 µmol/L before versus 132.4±237.4 µmol/L after, P=0.24) overfeeding.
Diet-Induced Changes in Clinical and Metabolic Characteristics
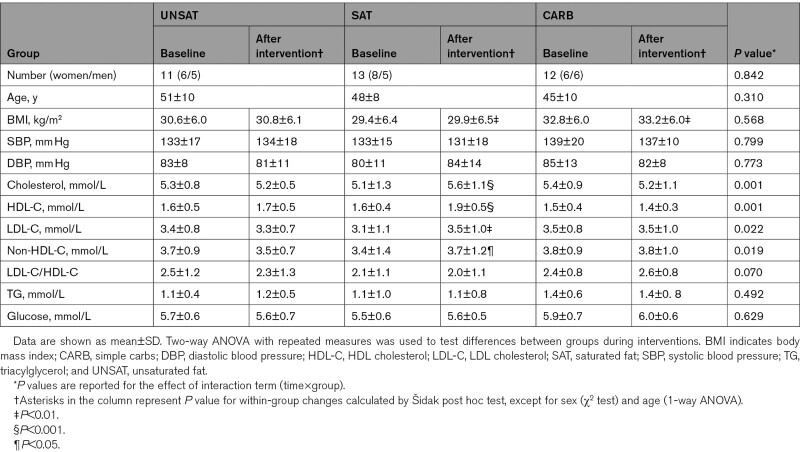
The clinical characteristics of the study subjects are shown in the Table. The groups were similar at baseline with respect to common cardiovascular disease risk factors, including BMI, blood pressure, age, and plasma lipids. Weight gain was small and similar in all groups in response to overfeeding (Table) and averaged 1.2±0.2 kg (1.3±0.2%). Concentrations of total cholesterol, LDL-cholesterol, HDL (high-density lipoprotein)-cholesterol, and non-HDL-cholesterol increased during the SAT diet (Table). Plasma levels of triglycerides and glucose remained unchanged in all groups.
Table.
Clinical Characteristics of the Study Subjects in UNSAT, SAT, and CARB Groups at Baseline and After the 3-wk Dietary Intervention
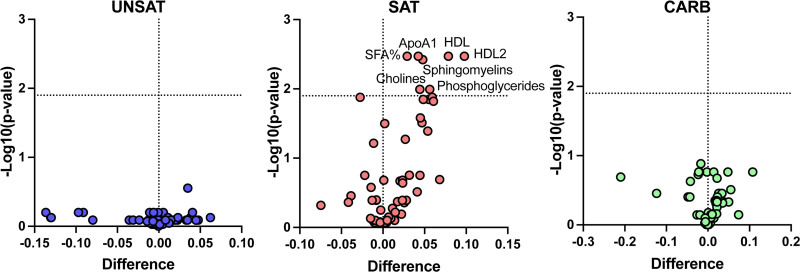
Quantitative NMR profiling was used to analyze in detail diet-induced changes in lipids and lipoproteins and metabolites. In the SAT group, the proportion of saturated fatty acids and concentrations of sphingomyelins, phosphoglycerides, cholines, apolipoprotein A1, and HDL-cholesterol increased (Figure 1). In addition, in the SAT group but not in the other groups, concentrations of particles, total lipids, phospholipids, total and free cholesterol, and cholesterol esters increased in XS-VLDL, IDL (intermediate-density lipoprotein), L-LDL, S-LDL, and all sizes of HDL particles (Figure IV in the Data Supplement).
Figure 1.
Change in the nuclear magnetic spectroscopy metabolomic profile during the 3-wk overfeeding of unsaturated fat (UNSAT), saturated fat (SAT), and simple carbs (CARB). Metabolomic analysis was performed from plasma samples collected at baseline and at the end of the intervention. The log-transformed metabolite concentrations were compared using paired 2-sided Student t test with false discovery rate method described by Benjamini and Hochberg. Differences were calculated by subtracting the mean before-value from the mean after-value. Thus, a positive difference indicates an increase and a negative difference a decrease in the mean metabolite concentration or proportion. UNSAT, n=11; SAT, n=13; CARB, n=12. ApoA1 indicates apolipoprotein A1; CARB, simple carbs; HDL, high-density lipoprotein; and SFA%, percentage of saturated fatty acids.
Overfeeding SAT Increases the Susceptibility of LDL to Aggregate
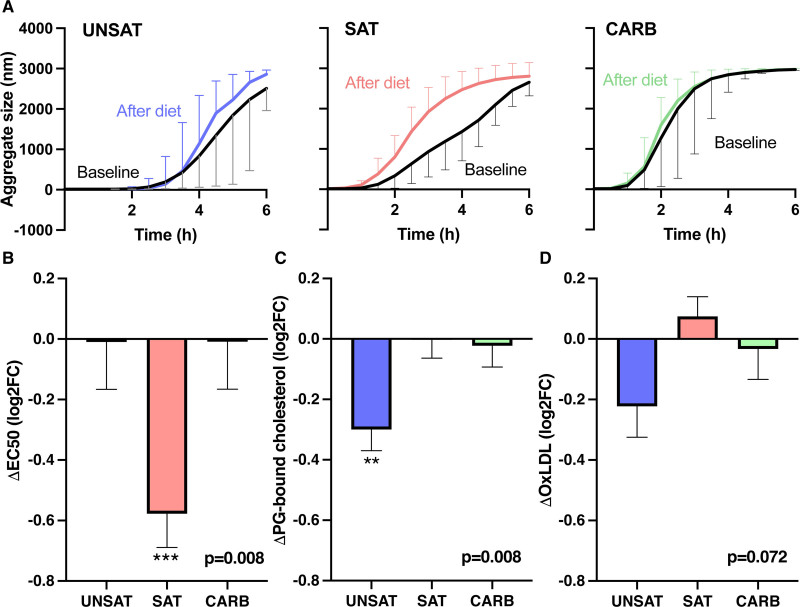
Aggregation of LDL particles was induced with human recombinant SMase and particle aggregation was followed by measuring the aggregate size at various time points. The aggregate size versus time curves at baseline and after the diet (Figure 2A) show that the UNSAT and CARB diets did not influence the susceptibility of LDL particles to aggregate, while in the SAT group LDL aggregated much faster after the intervention than at baseline. The inflection point of the aggregate size versus time curves is used as a measure of the aggregation susceptibility of the LDL particle and the faster the particles aggregate, the shorter is the time required to reach the inflection point (EC50) of the aggregation curves. At baseline, EC50 was similar between the UNSAT (4.4±1.3 hour, mean±SD) and SAT (3.9±1.3 hour) groups and lower (2.6±1.2 hour, P<0.05) in the CARB group. The change in the inflection point of the aggregation curve in each group is shown in Figure 2B. There was a significant within-group difference in aggregation (P value for 2-way ANOVA Time×Group interaction term=0.0077), which occurred in the SAT group. The increase in LDL aggregation in the SAT group was also highly significant in ANCOVA (P=0.002) with baseline aggregation as the covariate. At week 3 (after intervention), absolute values of EC50 were statistically significantly different between the UNSAT (4.4±1.2 hour), SAT (2.6±0.8 hour), and CARB group (2.6±0.9 hour) (ANOVA, P=0.004). Tukey post hoc test showed lower aggregation in the UNSAT group compared with the SAT group (P=0.001) and CARB group (P=0.001), and no difference between the SAT and CARB group (P=0.981).
Figure 2.
Changes in LDL (low-density lipoprotein) aggregation, lipoproteins proteoglycan-binding, and oxidized LDL (oxLDL) during the 3-wk overfeeding of unsaturated fat (UNSAT), saturated fat (SAT), and simple carbs (CARB). A, The aggregate sizes (median±interquartile range) at the various time points are shown in the UNSAT, SAT, and CARB groups. LDL was isolated from plasma samples collected at the baseline and at the end of the diet. LDL aggregation was induced by addition of human recombinant sphingomyelinase, and the increase in LDL aggregate size was followed by dynamic light scattering. Inflection point of the aggregate size vs time curves was used as a measure of LDL aggregation susceptibility. B, Change in LDL aggregation susceptibility. C, Change in the proteoglycan-binding of plasma lipoproteins. Aliquots of plasma samples collected at the baseline and at the end of the study were incubated in microtiter wells coated with human aortic proteoglycans and the amount of cholesterol bound was measured after incubation for 1 h. D, Change in the concentration of oxLDL. ELISA was used to measure oxLDL concentrations in plasma samples collected at the baseline and at the end of the intervention. The changes in B–D are presented as mean±SE of mean of the log2 values of fold changes. A positive value indicates an increase and a negative value a decrease in the value. Two-way ANOVA with repeated measures was used to test differences between groups during interventions. P values (at the bottom) are reported for the effect of interaction term (time×group). Asterisks represent P value for within-group changes calculated by Šidak post hoc test. The data were analyzed using GraphPad Prism version 8.0.1. **P<0.01; ***P<0.001.
Overfeeding UNSATs Decreases Proteoglycan-Binding of Plasma Lipoproteins and Reduces oxLDL
Effects of the different overfeeding diets on binding of plasma lipoproteins to human aortic PGs were measured using a solid-phase binding assay in which aliquots of serum samples were incubated in PG-coated microtiter wells. At baseline, PG-binding was 2.9±0.5 nmol/L per well (mean±SD) in UNSAT group, 2.2±0.5 nmol/L/well in SAT group, and 2.5±0.4 nmol/L/well in CARB group. After 3 weeks of overfeeding, PG-binding was 2.4±0.5 nmol/L/well in UNSAT, 2.3±0.5 nmol/L/well in SAT, and 2.4±0.3 nmol/L/well in CARB group. The log2FC in PG-binding in each group are shown in Figure 2C. There were no significant differences in absolute week 3 (after intervention) values in PG-binding between the groups, but the change from baseline in PG-binding was significantly different between the groups (P value for 2-way ANOVA interaction term=0.0077). Post hoc analysis showed the difference was because of a significant decrease in the UNSAT group with no change in the other groups.
We also measured the effect of the diets on the concentrations of oxLDL in plasma. At baseline, concentration of oxLDL was 50.0±10.0 mU/L (mean±SD) in UNSAT group, 40.3±15.1 mU/L in SAT group, and 44.9±10.6 mU/L in CARB group. After 3 weeks of overfeeding, concentration of oxLDL was 42.7±7.6 mU/L in UNSAT, 42.2±14.8 mU/L in SAT, and 44.8±12.6 mU/L in CARB group. No statistically significant changes in oxLDL concentration were observed (Figure 2D), and there were no significant differences in absolute week 3 (after intervention) values.
Effects of Overfeeding Diets on LDL Lipid and Protein Composition
Effects of the overfeeding diets on the lipid composition of LDL particles were analyzed in detail by LC-MS. Significant changes were seen only in SAT group, in which in the core lipids of LDL the proportions of saturated TGs increased while unsaturated TGs decreased (Figure V in the Data Supplement).
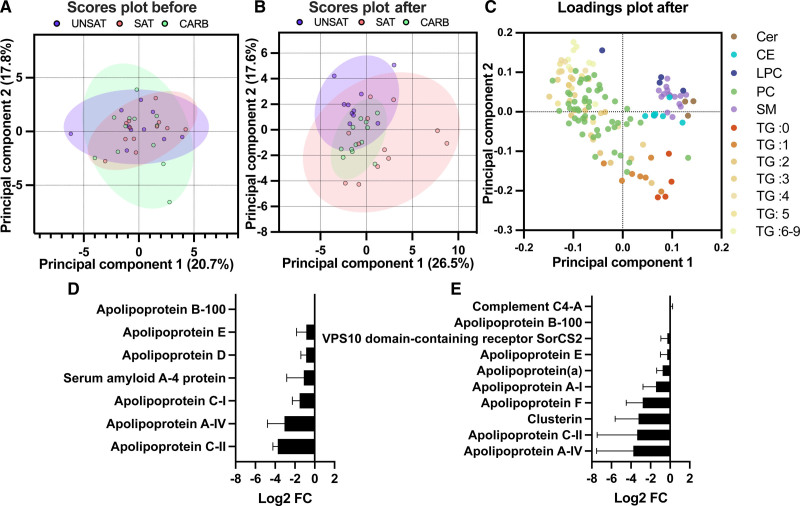
The lipid species of LDL detected by LC-MS were next used as loadings in principal component analysis of LDL samples at baseline and after the diet period. The first and second principal component scores showed that at the end of overfeeding, LDL lipid compositions of the groups were diverged compared with baseline (Figure 3A and 3B). Particularly, the UNSAT and the SAT groups were better separated after the overfeeding period than at baseline. The first and second principal components explained 43.2% of the variance between samples (Figure 3B). The first component had large positive associations with sphingomyelins, ceramides, lysophosphatidylcholines, cholesterol esters, and negative associations with unsaturated TGs and phosphatidylcholines, while the second component had large negative associations with saturated and monounsaturated TGs (Figure 3C).
Figure 3.
Changes in LDL (low-density lipoprotein) lipidomics and proteomics during the 3-wk overfeeding of unsaturated fat (UNSAT), saturated fat (SAT), and simple carbs (CARB). A–C, Principal component analysis (PCA) demonstrating differences in LDL lipid composition before and after 3-wk overfeeding of UNSAT, SAT, and CARB. Lipidomic analysis was performed using liquid chromatography–mass spectrometry, and percentage concentrations of both core and surface lipids were introduced to analysis. Before analysis, samples were normalized by median, log transformed, and Pareto scaled. Two outliers, one in the UNSAT group and one in the SAT group, were excluded. A, The PCA scores plot before intervention. The first and second components explained 20.7%, and 17.8% of the variance between samples, respectively. The scores of the first and second principal components with 95% CIs are presented for UNSAT (blue), SAT (red), and CARB (green) groups. B, The PCA scores plot after intervention. The first and second components explained 26.5%, and 17.6% of the variance between samples, respectively. C, Loadings plot showing influence of each lipid species to the first and second principal components after intervention. Each lipid species is colored with distinctive color. The first component had large positive associations with sphingomyelins, ceramides, lysophosphatidylcholines, cholesterol esters, and negative associations with unsaturated TGs and phospatidylcholines, while the second component had large negative associations with saturated and monounsaturated TGs. D and E, Changes in LDL protein composition in (D) UNSAT and (E) SAT groups. The protein composition was analyzed by liquid-chromatography-mass spectrometry/mass spectrometry after normalization to ApoB-100, only proteins were included which were identified in 4 out of the 5 selected individuals and the change was in the same direction (increase/decrease) for changes over log2 fold change >0.5. The log2 fold changes in protein concentrations are presented as mean±SD. The data were analyzed using GraphPad Prism version 8.0.1 and MetaboAnalyst software 4.0. UNSAT, n=10; SAT, n=12; CARB, n=12. CE indicates cholesterol ester; Cer, ceramide; FC, fold change; LPC, lysophosphatidylcholine; PC, phosphatidylcholine; SM, sphingomyelin; TG, triacylglycerol.
The protein composition of the pooled groups was analyzed by LC-MS/MS and normalized to ApoB-100 (Figure VI in the Data Supplement). Apolipoproteins were mildly depleted in all groups after overfeeding, except ApoA-I, ApoA-IV, and ApoE, which showed a slight increase in the CARB group.
Proteomics Comparison of Individuals From SAT and UNSAT Group
As the SAT group showed significant changes in LDL aggregation and the UNSAT group decreased proteoglycan binding, we analyzed the individual LDL protein compositions of 5 participants of each group. The participants were selected based on groupwise similarity with respect to baseline characteristics such as sex, age, BMI, and LDL-cholesterol, and considering the representative changes in the primary outcomes, LDL-aggregation, and PG-binding, of the whole respective group (Table II in the Data Supplement). To eliminate small individual changes, only proteins were included, which were identified in at least 4 out of 5 participants and which had the log2FC in the same direction within the 5 participants if the log2 fold change was larger than 0.5, indicating a fold change of >1.42. As in the pooled samples, the pairs of both groups exhibited a relative depletion in apolipoproteins, especially for ApoA-IV and ApoC-II (Figure 3D and 3E). In the UNSAT group, pairwise comparison indicated that the LDL particles were also relatively depleted of ApoC-I, ApoD, ApoE, and acute phase protein serum amyloid A-4 after the diet period (Figure 3D). Pairwise comparison of the LDL particles from the SAT groups showed a small diet-induced increase in complement component C4-A, serum paraoxonase/arylesterase 1, antibody component immunoglobulin kappa constant, as well as platelet-activating factor acetylhydrolase and a diet-induced decrease in ApoA-I, ApoA-IV, ApoC-II, ApoF, and clusterin (Figure 3E).
Plasma NMR Profiling, LDL Lipidomics, LDL Aggregation, Lipoprotein PG-Binding, and oxLDL
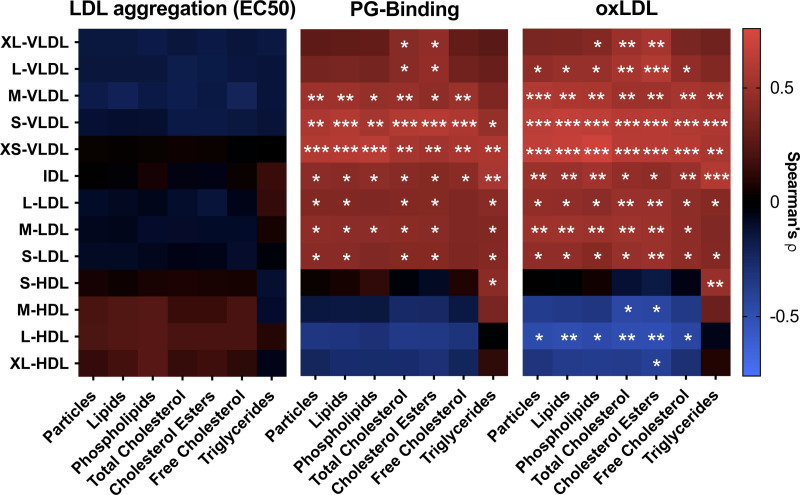
We next analyzed the relationship between lipoprotein subclasses as determined using NMR spectroscopy and LDL aggregability, lipoprotein PG-binding, and oxLDL. At baseline, both PG-binding and oxLDL were strongly associated with several apoB-100-containing lipoprotein subclasses (Figure 4). Thus, PG-binding and oxLDL correlated positively with concentrations and lipid components of VLDL, IDL, and LDL subclasses apart from large VLDL. HDL correlated inversely with the concentration of oxLDL. LDL aggregation did not correlate with any of the lipoprotein subclasses or their lipid components. In contrast, LDL aggregation at baseline showed a strong negative correlation with the proportion of phosphatidylcholines and positive correlation with the proportion of sphingomyelins and lysophosphatidylcholines on the surface of the particles (Figure VII in the Data Supplement).
Figure 4.
Relationships between LDL (low-density lipoprotein) aggregation susceptibility, proteoglycan-binding of plasma lipoproteins, and plasma oxidized LDL (oxLDL) concentrations, and plasma lipoprotein subclasses at baseline. A, LDL aggregation, (B) the binding of plasma lipoproteins to proteoglycans, and (C) concentrations of oxLDL in plasma were determined as described under Figure 3 and Methods. Quantitative nuclear magnetic spectroscopy metabolomics was used for the quantification of plasma lipoprotein subclasses and their components. The associations for log-transformed metabolite concentrations were analyzed using Spearman correlation coefficient analysis. In heatmaps, the positive correlations are represented with red and negative correlations with blue. Unsaturated fat, n=11; saturated fat, n=13; simple carbs, n=12. *P<0.05, **P<0.01, ***P<0.001.
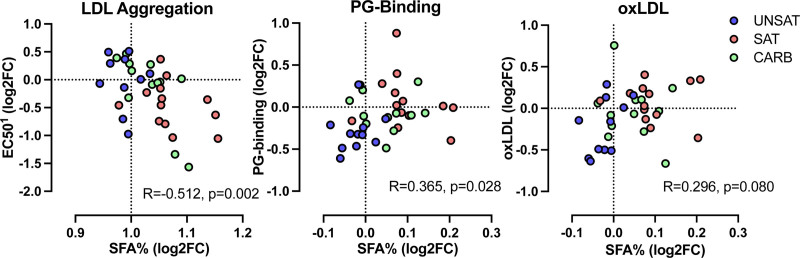
Finally, we analyzed whether diet-induced changes in plasma NMR profiles or the composition of the LDL lipidome were related to LDL aggregation, lipoprotein PG-binding, or oxLDL. We observed a significant correlation between the change in the proportion of saturated fatty acids in plasma and LDL aggregation: an increase in the saturated fatty acids was associated with more rapid LDL aggregation (Figure 5A). Changes in LDL aggregation were not associated with changes in lipoprotein subclasses (not shown). An increased proportion of saturated fatty acids was also associated with an increase in PG-binding (Figure 5B). The association between the change in the proportion of saturated fatty acids and the change in oxLDL was not significant and is shown in Figure 5C.
Figure 5.
Relationships between changes in LDL (low-density lipoprotein) aggregation susceptibility, proteoglycan-binding of plasma lipoproteins, plasma oxidized LDL (oxLDL), and the proportions of plasma saturated fatty acids concentrations during 3-wk overfeeding of the unsaturated fat (UNSAT), saturated fat (SAT), and simple carbs (CARB) diets. A, LDL aggregation, (B) the binding of plasma lipoproteins to proteoglycans, and (C) concentrations of oxLDL in plasma, were determined as described under Figure 3 and Methods. Quantitative nuclear magnetic spectroscopy metabolomics was used for the quantification of changes in the proportion of saturated fatty acids (SFA%) in plasma. The associations for log2 fold changes of metabolite concentrations or proportions were analyzed using Spearman correlation coefficient analysis. UNSAT, n=11; SAT, n=13; CARB, n=12. 1Positive value indicates decrease in LDL aggregation, negative value increase in LDL aggregation. R, Spearman correlation coefficient; FC, fold change.
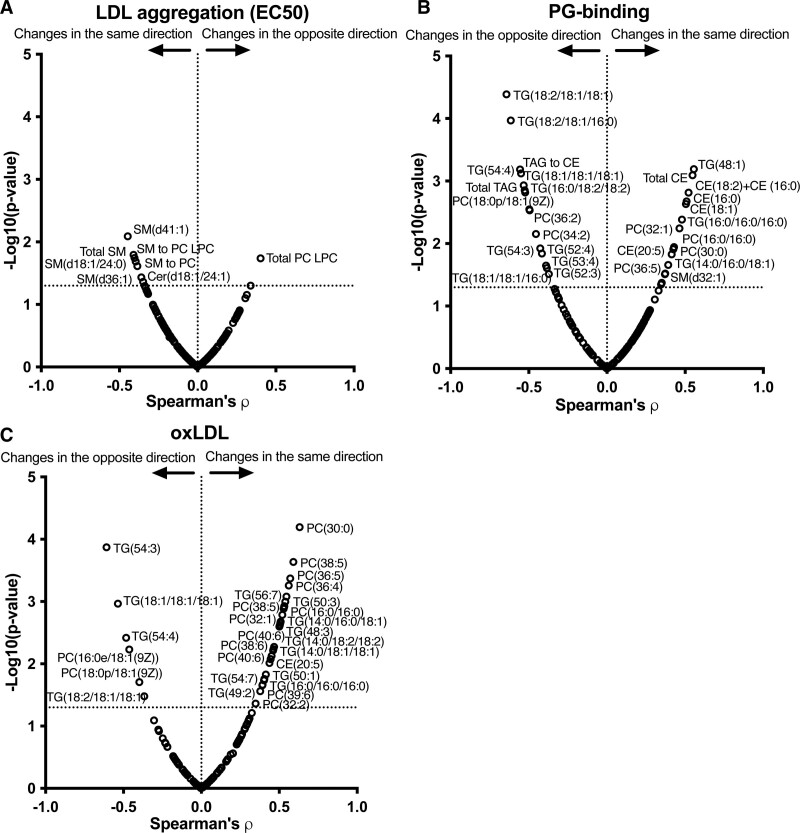
Regarding LDL core and surface lipid composition, an increase in sphingomyelins and decrease in the phosphatidylcholine to lysophosphatidylcholine -ratio were associated with an increase in LDL aggregation during overfeeding (Figure 6A). An increase in many TGs containing unsaturated fatty acids was associated with a decrease in PG-binding (Figure 6B) and an increase in oxLDL (Figure 6C).
Figure 6.
Relationships between changes in LDL (low-density lipoprotein) lipid composition and changes in LDL aggregation susceptibility, proteoglycan-binding of plasma lipoproteins, and plasma oxidized LDL (oxLDL) concentrations during 3-wk overfeeding of the unsaturated fat (UNSAT), saturated fat (SAT), and simple carbs (CARB) diets. Volcano plots showing associations between changes in LDL lipids and changes in (A) LDL aggregation susceptibility, (B) proteoglycan-binding (PG) of plasma lipoproteins, and (C) oxLDL concentrations. The associations for log2 fold changes of metabolite concentrations were analyzed using Spearman correlation coefficient analysis. Two outliers, 1 in the UNSAT group and 1 in the SAT group, were excluded from analysis. Changes in the same direction indicate that both the measured LDL atherogenic property and the lipid concentration increased or decreased, and changes in the opposite direction indicate that the other of these increased and the other decreased. UNSAT, n=10; SAT, n=12; CARB, n=12. ρ, Spearman correlation coefficient; LPC indicates lysophosphatidylcholine; PC, phosphatidylcholine; SM, sphingomyelin; and TG, triacylglycerol.
Discussion
The impact of different diets on cardiovascular health is still intensely debated.46 As LDL-cholesterol is a causal factor in the development of atherosclerotic cardiovascular disease,1 most studies have focused on effects of different diets on concentrations of plasma LDL-cholesterol. However, for any given concentration of LDL-cholesterol, pathogenic properties of LDL particles, such as the affinity of LDL to bind to arterial PGs, the presence of oxLDL, and the susceptibility of LDL to aggregation have the potential to influence the atherogenicity of the LDL particles.47,48 In this study, we examined the effects of overfeeding 1000 excess calories as either SATs, UNSATs, or simple sugars for 3 weeks on these qualitative aspects of LDL particles and show that excess consumption of SATs increases LDL aggregation, while consumption of UNSATs decreases PG-binding of plasma lipoproteins based on change from baseline diet.
LDL particles aggregate in the intima after proteolytic, enzymatic, or oxidative modifications and the higher the LDL concentration the faster is LDL aggregation.8 Aggregation enhances binding of LDL to intimal PGs2 and promotes foam cell formation.7,49–51 Uptake of aggregated LDL also induces secretion of matrix metalloprotease 7, a protease associated with plaque rupture.8,52 In line with the pathogenic properties of aggregated LDL, we recently showed that the susceptibility of plasma LDL to aggregate associates with future cardiovascular events independently of conventional risk factors for atherosclerotic cardiovascular disease.8,27 LDL particles can be rendered more stable by addition of plant stanol ester-enriched spread into the diet.53 Here, we show that overfeeding of SATs renders LDL particles more prone to aggregate and that this change correlates with increase in the proportion of saturated fatty acids in plasma. In addition, LDL-bound clusterin was significantly decreased in the SAT group after the diet period. Clusterin has been shown to inhibit LDL aggregation and reduce atherogenesis in a mouse model.54,55
The SAT diet, but not the other diets, significantly increased lipids that increase aggregation of LDL, that is, the content of sphingolipids in LDL and plasma sphingomyelins.8,53 Principal component analysis of the LDL samples after the diet period clearly separated particularly the SAT and the UNSAT groups. In the SAT group, LDL was more enriched with sphingomyelins, ceramides, and saturated TGs, while LDL in the UNSAT group was enriched with phosphatidylcholines and unsaturated TGs. In the CARB group, carbohydrate-induced increase in DNL led to less pronounced changes in % saturated fatty acids in plasma than did the SAT diet (Figure 5) and did not influence LDL lipid composition or LDL aggregation. These differences may explain why LDL aggregation remained unchanged in the CARB group. Of note, baseline aggregation was higher in the CARB group. Although the change in LDL aggregation remained highly significant after adjusting for baseline values, and absolute LDL aggregation was significantly higher at 3 weeks in the SAT than in the UNSAT group, the baseline difference nevertheless complicates interpretation of changes in this parameter in the CARB group.
The interaction of lipoproteins with PGs is one of the first steps in atherogenesis.56–59 Here, overfeeding of the UNSAT diet decreased binding of plasma lipoproteins to PGs based on change from baseline diet. Previous studies have also shown that change in dietary fat can influence PG-binding of plasma lipoproteins or isolated LDL particles. Thus, intake of linolenic acid (18:3, ω3)-rich Camelina sativa oil and consumption of plant stanol esters both decrease the PG-binding of plasma lipoproteins.53,60 Similarly, Jones et al61 have shown that both canola oil rich in oleic acid and corn/safflower oil rich in linoleic acid decrease binding of isolated LDL particles to PGs. In the present study, the PG-binding of plasma lipoproteins was strongly associated with the concentration of small VLDL, IDL, and LDL particles and their lipid components, raising the possibility that in addition to LDL, small VLDL and IDL particles may also have contributed to retention of lipoproteins. In the SAT group, the amounts of these apoB-100-containing lipoproteins increased; yet, the binding of the lipoproteins to PGs did not change indicating that there is a reduction in the affinity of the lipoproteins to PGs. As we did not observe any changes in apoE or apoC-III of LDL, the 2 small exchangeable apolipoproteins known to modulate the interaction of lipoproteins to PGs,14–16 it is likely that the changes in the PG-binding depend on changes in the conformation of apoB-100, which has been shown to be important in the PG-binding of LDL particles.13 In contrast, in the UNSAT group, LDL-apoE decreased after the diet, a finding that could at least partly explain the decrease in PG-binding in this group.
Consumption of unsaturated fatty acids has been reported to increase the oxidizability of LDL particles.62 In accordance, we show that increased concentration of oxLDL was associated with increase in many unsaturated TGs, which were increased after the UNSAT diet. Despite this, overfeeding of the UNSAT diet induced a nonsignificant decrease rather than an increase in the concentration of oxLDL in plasma. As TGs are a minor component of LDL particles, it is possible that diet-induced changes in other lipids outweigh the increase in oxidation-prone unsaturated TGs. Importantly, increase in the concentration of oxLDL does not necessarily lead to increased atherosclerosis at least in an animal model.63 One reason for this could be related to reduced inflammatory potential of lipoproteins after consumption of UNSATs.64,65 In line with these data, we show here that serum amyloid A in LDL was decreased after the UNSAT diet. Oxidation of circulating LDL particles takes place, at least in part, during oxidative stress reactions in endothelial cells, which generate reactive oxygen and nitrogen species. We show here that at baseline, the concentration of oxLDL was associated positively with most VLDL and LDL subclasses and negatively with HDL particles and lipids in HDL. This finding is in line with the ability of HDL to inhibit lipid oxidation in LDL.53
This study is the first in humans to compare effects of overfeeding 3 different diets on the properties of LDL and plasma lipoproteins linked with increased atherogenicity. Limitations of our study as well as all of other dietary intervention studies hitherto performed66,67 include small sample size and a relatively short duration of the study. However, longer periods of overfeeding might be considered unethical and would likely also decrease compliance. It is also noteworthy that total fat intake in the SAT and the UNSAT groups was almost 60% of total energy intake with SATs comprising 33% and 14% of total energy, respectively.33 Intake of SAT the SAT group thus clearly exceeded current dietary recommendations.68,69 As the study was performed in overweight subjects with caloric excess, the results may not be applicable to normal weight individuals consuming eucaloric diets. LDL aggregation at baseline was faster in the CARB group than in the other 2 groups, which complicates interpretation of changes LDL aggregation in the CARB group. Regardless, changes in aggregation remained still highly significantly different between the groups after adjusting for variation in baseline LDL aggregation, and the only significant within-group change was observed in the SAT group.
In conclusion, the present data show that overfeeding SAT induces substantial metabolic changes that lead to modification of LDL composition and enhance the susceptibility of LDL to aggregation, independent of the concentration of LDL cholesterol. In addition, overfeeding UNSAT decreases the binding of plasma lipoproteins to aortic PGs from baseline diet. This property is strongly associated with the concentrations of small VLDL, IDL, and LDL subclasses in plasma. Thus, this study shows that the type of fat during overfeeding affects the properties of plasma lipoproteins associated with increased atherogenicity and extends the previous data on the harmfulness of excess intake of SATs.
Article Information
Acknowledgments
We thank Maija Atuegwu, Anne Salo, Aila Karioja-Kallio, Päivi Ihamuotila, and Daniel Duberg for excellent technical assistance and Dr Jenny Presto (Mercodia) for providing the oxLDL measurement kit.
Sources of Funding
This research was funded by Jenny and Antti Wihuri Foundation that maintains the Wihuri Research Institute. This study was also supported by grants from the Academy of Finland (grants No. 315568 and No. 332564), Novo Nordisk Fonden (NNF19OC0057411), and the Finnish Foundation for Cardiovascular Research to K. Öörn and M.B. Lorey; British Heart Foundation Senior Research Fellowship (FS/15/56/31645) to L. Hodson; Alfred Kordelin Foundation, Jalmari and Rauha Ahokas Foundation, Paulo Foundation, and Finnish Medical Foundation to P.K. Luukkonen; the Academy of Finland (grant No. 309263) and the Sigrid Juselius, EVO and Novo Nordisk foundations to H. Yki-Järvinen.
Disclosures
M. Ruuth, K. Öörni, and P.T. Kovanen have applied for a patent on the LDL (low-density lipoprotein) aggregation assay. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the article, or in the decision to publish the results.
Supplemental Materials
Data Supplement Tables I–III
Data Supplement Figures I–VII
Supplementary Material
Nonstandard Abbreviations and Acronyms
- apo
- apolipoprotein
- CARB
- simple carbohydrates
- DNL
- de novo lipogenesis
- HDL
- high-density lipoprotein
- IDL
- intermediate-density lipoprotein
- LC-MS
- liquid-chromatography-mass spectrometry
- LDL
- low-density lipoprotein
- NMR
- nuclear magnetic spectroscopy
- oxLDL
- oxidized LDL
- PG
- proteoglycan
- SAT
- saturated fat
- UNSAT
- unsaturated fat
- VLDL
- very low-density lipoprotein
M. Ruuth and M. Lahelma share first authorship.
H. Yki-Järvinen and K. Öörni share senior authorship.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/ATVBAHA.120.315766.
For Sources of Funding and Disclosures, see page 2834.
Contributor Information
Maija Ruuth, Email: maija.ruuth@gmail.com.
Mari Lahelma, Email: mari.lahelma@helsinki.fi.
Panu K. Luukkonen, Email: panu.luukkonen@yale.edu.
Martina B. Lorey, Email: martina.lorey@helsinki.fi.
Sami Qadri, Email: sami.qadri@helsinki.fi.
Sanja Sädevirta, Email: sanja.sadevirta@hus.fi.
Tuulia Hyötyläinen, Email: tuulia.hyotylainen@oru.se.
Petri T. Kovanen, Email: petri.kovanen@wri.fi.
Leanne Hodson, Email: leanne.hodson@ocdem.ox.ac.uk.
Hannele Yki-Järvinen, Email: hannele.yki-jarvinen@helsinki.fi.
References
- 1.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472. doi: 10.1093/eurheartj/ehx144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oörni K, Pentikäinen MO, Ala-Korpela M, Kovanen PT. Aggregation, fusion, and vesicle formation of modified low density lipoprotein particles: molecular mechanisms and effects on matrix interactions. J Lipid Res. 2000;41:1703–1714 [PubMed] [Google Scholar]
- 3.Öörni K, Rajamäki K, Nguyen SD, Lähdesmäki K, Plihtari R, Lee-Rueckert M, Kovanen PT. Acidification of the intimal fluid: the perfect storm for atherogenesis. J Lipid Res. 2015;56:203–214. doi: 10.1194/jlr.R050252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamminen M, Mottino G, Qiao JH, Breslow JL, Frank JS. Ultrastructure of early lipid accumulation in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:847–853. doi: 10.1161/01.atv.19.4.847 [DOI] [PubMed] [Google Scholar]
- 5.Lehti S, Nguyen SD, Belevich I, Vihinen H, Heikkilä HM, Soliymani R, Käkelä R, Saksi J, Jauhiainen M, Grabowski GA, et al. Extracellular lipids accumulate in human carotid arteries as distinct three-dimensional structures and have proinflammatory properties. Am J Pathol. 2018;188:525–538. doi: 10.1016/j.ajpath.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 6.Hoff HF, Morton RE. Lipoproteins containing apo B extracted from human aortas. Structure and function. Ann N Y Acad Sci. 1985;454:183–194. doi: 10.1111/j.1749-6632.1985.tb11857.x [DOI] [PubMed] [Google Scholar]
- 7.Tabas I, Li Y, Brocia RW, Xu SW, Swenson TL, Williams KJ. Lipoprotein lipase and sphingomyelinase synergistically enhance the association of atherogenic lipoproteins with smooth muscle cells and extracellular matrix. A possible mechanism for low density lipoprotein and lipoprotein(a) retention and macrophage foam cell formation. J Biol Chem. 1993;268:20419–20432 [PubMed] [Google Scholar]
- 8.Ruuth M, Nguyen SD, Vihervaara T, Hilvo M, Laajala TD, Kondadi PK, Gisterå A, Lähteenmäki H, Kittilä T, Huusko J, et al. Susceptibility of low-density lipoprotein particles to aggregate depends on particle lipidome, is modifiable, and associates with future cardiovascular deaths. Eur Heart J. 2018;39:2562–2573. doi: 10.1093/eurheartj/ehy319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haka AS, Grosheva I, Chiang E, Buxbaum AR, Baird BA, Pierini LM, Maxfield FR. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol Biol Cell. 2009;20:4932–4940. doi: 10.1091/mbc.e09-07-0559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh RK, Haka AS, Bhardwaj P, Zha X, Maxfield FR. Dynamic actin reorganization and Vav/Cdc42-dependent actin polymerization promote macrophage aggregated LDL (low-density lipoprotein) uptake and catabolism. Arterioscler Thromb Vasc Biol. 2019;39:137–149. doi: 10.1161/ATVBAHA.118.312087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skålén K, Gustafsson M, Rydberg EK, Hultén LM, Wiklund O, Innerarity TL, Borén J. Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature. 2002;417:750–754. doi: 10.1038/nature00804 [DOI] [PubMed] [Google Scholar]
- 12.Soto Y, Acosta E, Delgado L, Pérez A, Falcón V, Bécquer MA, Fraga Á, Brito V, Álvarez I, Griñán T, et al. Antiatherosclerotic effect of an antibody that binds to extracellular matrix glycosaminoglycans. Arterioscler Thromb Vasc Biol. 2012;32:595–604. doi: 10.1161/ATVBAHA.111.238659 [DOI] [PubMed] [Google Scholar]
- 13.Hurt-Camejo E, Camejo G. ApoB-100 lipoprotein complex formation with intima proteoglycans as a cause of atherosclerosis and its possible ex vivo evaluation as a disease biomarker. J Cardiovasc Dev Dis. 2018;5:E36. doi: 10.3390/jcdd5030036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olin-Lewis K, Krauss RM, La Belle M, Blanche PJ, Barrett PH, Wight TN, Chait A. ApoC-III content of apoB-containing lipoproteins is associated with binding to the vascular proteoglycan biglycan. J Lipid Res. 2002;43:1969–1977. doi: 10.1194/jlr.m200322-jlr200 [DOI] [PubMed] [Google Scholar]
- 15.Hiukka A, Ståhlman M, Pettersson C, Levin M, Adiels M, Teneberg S, Leinonen ES, Hultén LM, Wiklund O, Oresic M, et al. ApoCIII-enriched LDL in type 2 diabetes displays altered lipid composition, increased susceptibility for sphingomyelinase, and increased binding to biglycan. Diabetes. 2009;58:2018–2026. doi: 10.2337/db09-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahley RW, Weisgraber KH, Innerarity TL. Interaction of plasma lipoproteins containing apolipoproteins B and E with heparin and cell surface receptors. Biochim Biophys Acta. 1979;575:81–91. doi: 10.1016/0005-2760(79)90133-4 [DOI] [PubMed] [Google Scholar]
- 17.Camejo G, Acquatella H, Lalaguna F. The interaction of low density lipoproteins with arterial proteoglycans. An additional risk factor? Atherosclerosis. 1980;36:55–65. doi: 10.1016/0021-9150(80)90198-7 [DOI] [PubMed] [Google Scholar]
- 18.Fagerberg B, Wiklund O, Agewall S, Camejo G, Wikstrand RJ. Multifactorial treatment of hypertensive men at high cardiovascular risk and low-density lipoprotein cholesterol affinity to human arterial proteoglycans. Eur J Clin Invest. 1996;26:960–965. doi: 10.1046/j.1365-2362.1996.2030543.x [DOI] [PubMed] [Google Scholar]
- 19.Linden T, Bondjers G, Camejo G, Bergstrand R, Wilhelmsen L, Wiklund O. Affinity of LDL to a human arterial proteoglycan among male survivors of myocardial infarction. Eur J Clin Invest. 1989;19:38–44. doi: 10.1111/j.1365-2362.1989.tb00193.x [DOI] [PubMed] [Google Scholar]
- 20.Garces F, López F, Niño C, Fernandez A, Chacin L, Hurt-Camejo E, Camejo G, Apitz-Castro R. High plasma phospholipase A2 activity, inflammation markers, and LDL alterations in obesity with or without type 2 diabetes. Obesity (Silver Spring). 2010;18:2023–2029. doi: 10.1038/oby.2010.9 [DOI] [PubMed] [Google Scholar]
- 21.Wiklund O, Bondjers G, Wright I, Camejo G. Insoluble complex formation between LDL and arterial proteoglycans in relation to serum lipid levels and effects of lipid lowering drugs. Atherosclerosis. 1996;119:57–67. doi: 10.1016/0021-9150(95)05628-9 [DOI] [PubMed] [Google Scholar]
- 22.Ahmed O, Littmann K, Gustafsson U, Pramfalk C, Öörni K, Larsson L, Minniti ME, Sahlin S, Camejo G, Parini P, et al. Ezetimibe in combination with simvastatin reduces remnant cholesterol without affecting biliary lipid concentrations in gallstone patients. J Am Heart Assoc. 2018;7:e009876. doi: 10.1161/JAHA.118.009876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruuth M, Janssen LGM, Äikäs L, Tigistu-Sahle F, Nahon KJ, Ritvos O, Ruhanen H, Käkelä R, Boon MR, Öörni K, et al. LDL aggregation susceptibility is higher in healthy South Asian compared with white Caucasian men. J Clin Lipidol. 2019;13:910–919.e2. doi: 10.1016/j.jacl.2019.09.011 [DOI] [PubMed] [Google Scholar]
- 24.Trpkovic A, Resanovic I, Stanimirovic J, Radak D, Mousa SA, Cenic-Milosevic D, Jevremovic D, Isenovic ER. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit Rev Clin Lab Sci. 2015;52:70–85. doi: 10.3109/10408363.2014.992063 [DOI] [PubMed] [Google Scholar]
- 25.Senders ML, Que X, Cho YS, Yeang C, Groenen H, Fay F, Calcagno C, Meerwaldt AE, Green S, Miu P, et al. PET/MR imaging of malondialdehyde-acetaldehyde epitopes with a human antibody detects clinically relevant atherothrombosis. J Am Coll Cardiol. 2018;71:321–335. doi: 10.1016/j.jacc.2017.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen JJ, Narverud I, Ruuth M, Heier M, Matti J, Ulven SM, Bogsrud MP, Kovanen PT, Halvorsen B, Oda M. Children with familial hypercholesterolemia display changes in LDL and HDL function: a cross-sectional study. J Intern Med. In press. doi: 10.1111/joim.13383 [DOI] [PubMed] [Google Scholar]
- 27.Heffron SP, Ruuth MK, Xia Y, Hernandez G, Äikäs L, Rodriguez C, Öörni K, Berger JS. Low-density lipoprotein aggregation predicts adverse cardiovascular events in peripheral artery disease. Atherosclerosis. 2021;316:53–57. doi: 10.1016/j.atherosclerosis.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614 [DOI] [PubMed] [Google Scholar]
- 29.Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37:1967–1976. doi: 10.1093/eurheartj/ehw148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Öörni K, Jauhiainen M, Kovanen PT. Why and how increased plasma ceramides predict future cardiovascular events? Atherosclerosis. 2020;314:71–73. doi: 10.1016/j.atherosclerosis.2020.09.030 [DOI] [PubMed] [Google Scholar]
- 31.Eisinger K, Liebisch G, Schmitz G, Aslanidis C, Krautbauer S, Buechler C. Lipidomic analysis of serum from high fat diet induced obese mice. Int J Mol Sci. 2014;15:2991–3002. doi: 10.3390/ijms15022991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boini KM, Zhang C, Xia M, Poklis JL, Li PL. Role of sphingolipid mediator ceramide in obesity and renal injury in mice fed a high-fat diet. J Pharmacol Exp Ther. 2010;334:839–846. doi: 10.1124/jpet.110.168815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luukkonen PK, Sädevirta S, Zhou Y, Kayser B, Ali A, Ahonen L, Lallukka S, Pelloux V, Gaggini M, Jian C, et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care. 2018;41:1732–1739. doi: 10.2337/dc18-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoro N, Caprio S, Pierpont B, Van Name M, Savoye M, Parks EJ. Hepatic de novo lipogenesis in obese youth is modulated by a common variant in the GCKR gene. J Clin Endocrinol Metab. 2015;100:E1125–E1132. doi: 10.1210/jc.2015-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hallberg C, Hådén M, Bergström M, Hanson G, Pettersson K, Westerlund C, Bondjers G, Ostlund-Lindqvist AM, Camejo G. Lipoprotein fractionation in deuterium oxide gradients: a procedure for evaluation of antioxidant binding and susceptibility to oxidation. J Lipid Res. 1994;35:1–9 [PubMed] [Google Scholar]
- 37.Sneck M, Kovanen PT, Oörni K. Decrease in pH strongly enhances binding of native, proteolyzed, lipolyzed, and oxidized low density lipoprotein particles to human aortic proteoglycans. J Biol Chem. 2005;280:37449–37454. doi: 10.1074/jbc.M508565200 [DOI] [PubMed] [Google Scholar]
- 38.Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on -omic technologies. Am J Epidemiol. 2017;186:1084–1096. doi: 10.1093/aje/kwx016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509 [PubMed] [Google Scholar]
- 40.O’Gorman A, Suvitaival T, Ahonen L, Cannon M, Zammit S, Lewis G, Roche HM, Mattila I, Hyotylainen T, Oresic M, et al. Identification of a plasma signature of psychotic disorder in children and adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort. Transl Psychiatry. 2017;7:e1240. doi: 10.1038/tp.2017.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pluskal T, Castillo S, Villar-Briones A, Oresic M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics. 2010;11:395. doi: 10.1186/1471-2105-11-395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scifo E, Szwajda A, Soliymani R, Pezzini F, Bianchi M, Dapkunas A, Dębski J, Uusi-Rauva K, Dadlez M, Gingras AC, et al. Proteomic analysis of the palmitoyl protein thioesterase 1 interactome in SH-SY5Y human neuroblastoma cells. J Proteomics. 2015;123:42–53. doi: 10.1016/j.jprot.2015.03.038 [DOI] [PubMed] [Google Scholar]
- 43.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322 [DOI] [PubMed] [Google Scholar]
- 44.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–W494. doi: 10.1093/nar/gky310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sevastianova K, Santos A, Kotronen A, Hakkarainen A, Makkonen J, Silander K, Peltonen M, Romeo S, Lundbom J, Lundbom N, et al. Effect of short-term carbohydrate overfeeding and long-term weight loss on liver fat in overweight humans. Am J Clin Nutr. 2012;96:727–734. doi: 10.3945/ajcn.112.038695 [DOI] [PubMed] [Google Scholar]
- 46.Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018;361:k2139. doi: 10.1136/bmj.k2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borén J, Chapman MJ, Krauss RM, Packard CJ, Bentzon JF, Binder CJ, Daemen MJ, Demer LL, Hegele RA, Nicholls SJ, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laufs U, Weingärtner O. Pathological phenotypes of LDL particles. Eur Heart J. 2018;39:2574–2576. doi: 10.1093/eurheartj/ehy387 [DOI] [PubMed] [Google Scholar]
- 49.Oörni K, Kovanen PT. PLA2-V: a real player in atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:445–447. doi: 10.1161/01.ATV.0000258412.58289.ee [DOI] [PubMed] [Google Scholar]
- 50.Grosheva I, Haka AS, Qin C, Pierini LM, Maxfield FR. Aggregated LDL in contact with macrophages induces local increases in free cholesterol levels that regulate local actin polymerization. Arterioscler Thromb Vasc Biol. 2009;29:1615–1621. doi: 10.1161/ATVBAHA.109.191882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Costales P, Fuentes-Prior P, Castellano J, Revuelta-Lopez E, Corral-Rodríguez MÁ, Nasarre L, Badimon L, Llorente-Cortes V. K Domain CR9 of low density lipoprotein (LDL) receptor-related protein 1 (LRP1) is critical for aggregated LDL-induced foam cell formation from human vascular smooth muscle cells. J Biol Chem. 2015;290:14852–14865. doi: 10.1074/jbc.M115.638361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbas A, Aukrust P, Russell D, Krohg-Sørensen K, Almås T, Bundgaard D, Bjerkeli V, Sagen EL, Michelsen AE, Dahl TB, et al. Matrix metalloproteinase 7 is associated with symptomatic lesions and adverse events in patients with carotid atherosclerosis. PLoS One. 2014;9:e84935. doi: 10.1371/journal.pone.0084935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruuth M, Äikäs L, Tigistu-Sahle F, Käkelä R, Lindholm H, Simonen P, Kovanen PT, Gylling H, Öörni K. Plant stanol esters reduce LDL (low-density lipoprotein) aggregation by altering LDL surface lipids: the blood flow Randomized Intervention Study. Arterioscler Thromb Vasc Biol. 2020;40:2310–2321. doi: 10.1161/ATVBAHA.120.314329 [DOI] [PubMed] [Google Scholar]
- 54.Martínez-Bujidos M, Rull A, González-Cura B, Pérez-Cuéllar M, Montoliu-Gaya L, Villegas S, Ordóñez-Llanos J, Sánchez-Quesada JL. Clusterin/apolipoprotein J binds to aggregated LDL in human plasma and plays a protective role against LDL aggregation. FASEB J. 2015;29:1688–1700. doi: 10.1096/fj.14-264036 [DOI] [PubMed] [Google Scholar]
- 55.Schwarz M, Spath L, Lux CA, Paprotka K, Torzewski M, Dersch K, Koch-Brandt C, Husmann M, Bhakdi S. Potential protective role of apoprotein J (clusterin) in atherogenesis: binding to enzymatically modified low-density lipoprotein reduces fatty acid-mediated cytotoxicity. Thromb Haemost. 2008;100:110–118. doi: 10.1160/TH07-12-0737 [DOI] [PubMed] [Google Scholar]
- 56.Merrilees MJ, Beaumont B. Structural heterogeneity of the diffuse intimal thickening and correlation with distribution of TGF-beta 1. J Vasc Res. 1993;30:293–302. doi: 10.1159/000159008 [DOI] [PubMed] [Google Scholar]
- 57.O’Brien KD, Olin KL, Alpers CE, Chiu W, Ferguson M, Hudkins K, Wight TN, Chait A. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 1998;98:519–527. doi: 10.1161/01.cir.98.6.519 [DOI] [PubMed] [Google Scholar]
- 58.Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol. 2007;27:1159–1165. doi: 10.1161/ATVBAHA.106.134080 [DOI] [PubMed] [Google Scholar]
- 59.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manninen S, Lankinen M, Erkkilä A, Nguyen SD, Ruuth M, de Mello V, Öörni K, Schwab U. The effect of intakes of fish and Camelina sativa oil on atherogenic and anti-atherogenic functions of LDL and HDL particles: a randomized controlled trial. Atherosclerosis. 2019;281:56–61. doi: 10.1016/j.atherosclerosis.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 61.Jones PJ, MacKay DS, Senanayake VK, Pu S, Jenkins DJ, Connelly PW, Lamarche B, Couture P, Kris-Etherton PM, West SG, et al. High-oleic canola oil consumption enriches LDL particle cholesteryl oleate content and reduces LDL proteoglycan binding in humans. Atherosclerosis. 2015;238:231–238. doi: 10.1016/j.atherosclerosis.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nestel PJ, Pomeroy SE, Sasahara T, Yamashita T, Liang YL, Dart AM, Jennings GL, Abbey M, Cameron JD. Arterial compliance in obese subjects is improved with dietary plant n-3 fatty acid from flaxseed oil despite increased LDL oxidizability. Arterioscler Thromb Vasc Biol. 1997;17:1163–1170. doi: 10.1161/01.atv.17.6.1163 [DOI] [PubMed] [Google Scholar]
- 63.Whitman SC, Fish JR, Rand ML, Rogers KA. n-3 fatty acid incorporation into LDL particles renders them more susceptible to oxidation in vitro but not necessarily more atherogenic in vivo. Arterioscler Thromb. 1994;14:1170–1176. doi: 10.1161/01.atv.14.7.1170 [DOI] [PubMed] [Google Scholar]
- 64.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71(suppl 1):213S–223S. doi: 10.1093/ajcn/71.1.213S [DOI] [PubMed] [Google Scholar]
- 65.Virtanen JK, Mursu J, Voutilainen S, Tuomainen TP. The associations of serum n-6 polyunsaturated fatty acids with serum C-reactive protein in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Clin Nutr. 2018;72:342–348. doi: 10.1038/s41430-017-0009-6 [DOI] [PubMed] [Google Scholar]
- 66.Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlström H, Arner P, Dahlman I, et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622 [DOI] [PubMed] [Google Scholar]
- 67.Rosqvist F, Kullberg J, Ståhlman M, Cedernaes J, Heurling K, Johansson HE, Iggman D, Wilking H, Larsson A, Eriksson O, et al. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: a randomized trial. J Clin Endocrinol Metab. 2019;104:6207–6219. doi: 10.1210/jc.2019-00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, et al. ; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, et al. ; American Heart Association. Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–e23. doi: 10.1161/CIR.0000000000000510 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.