Abstract
gem-Difluoroalkenes are readily available fluorinated building blocks, and the fluorine-induced electronic perturbations of the alkenes enables a wide array of selective functionalization reactions. However, many reactions of gem-difluoroalkenes result in a net C─F functionalization to generate monofluorovinyl products or addition of F to generate trifluoromethyl-containing products. In contrast, fluorine-retentive strategies for the functionalization of gem-difluoroalkenes remain less generally developed, and is now becoming a rapidly developing area. This review will present the development of fluorine-retentive strategies including electrophilic, nucleophilic, radical, and transition metal catalytic strategies with an emphasis on key physical organic and mechanistic aspects that enable reactivities.
Keywords: Synthetic Methodology, Fluorination, gem-Difluoroalkene, Organofluorine
Graphical Abstract

1. Introduction
The difluoromethylene group is found in a wide variety of medicinal agents, biological probes, agrochemicals, and materials, and as such, convergent and reliable strategies for converting readily available building blocks into difluoromethylene groups are valuable. One potentially useful building block for accessing difluoromethylenes are gem-difluoroalkenes (1) that can be readily accessed by many strategies, such as Wittig-type difluoromethylenation, Julia-type and Horner-Wadsworth-Emmons-type difluoroolefination, β-elimination of functionalized difluoromethyl compounds, β-fluoride elimination from trifluoromethyl compounds, and transition metal-catalyzed cross-coupling.1-3 Additionally, gem-difluoroalkenes react with high selectivity for a broad-spectrum of functionalization reactions, and in these reactions, the fluorine atoms differentially polarize the termini of the alkene, which in turn enables selective functionalization reactions using many strategies (Scheme 1). First, the β-carbon is electron-rich and nucleophilic, which is readily explained by negative hyperconjugative resonance effects.1,2,4,5 Thus, gem-difluoroalkenes can react with electrophiles typically at the difluorinated position to generate resonance-stabilized cationic intermediates (2) or cationic halonium or thiiranium intermediates (3) that can undergo subsequent reactions with nucleophiles to deliver difunctionalized products (Scheme 1A). Second, gem-difluoroalkenes, bearing two inductively electron-withdrawing fluorine atoms, are intrinsically electrophilic at the difluorinated position, and thus typically react with anionic nucleophiles and nucleophilic radicals with high regioselectivity at the difluorinated carbon.3,6 However, many of these nucleophilic addition reactions result in a net C─F functionalization, in large part because reactions of anionic nucleophiles generate unstable β-fluoroanioic intermediates (5) that rapidly undergo β-fluoride elimination generating monofluorovinyl products (6; Scheme 1B). For these reasons, fluorine-retentive strategies for the functionalization of gem-difluoroalkenes has become a rapidly developing area, and this review will present the development of fluorine-retentive strategies including electrophilic (Scheme 1A), nucleophilic (Scheme 1B), radical (Scheme 1C), and transition metal catalytic strategies (Scheme 1D), and will predominately focus on mechanistic aspects of transformations as opposed to reaction scope.
Scheme 1.
Overview of gem-Difluoroalkene Reactivity
2. Two Electron Processes
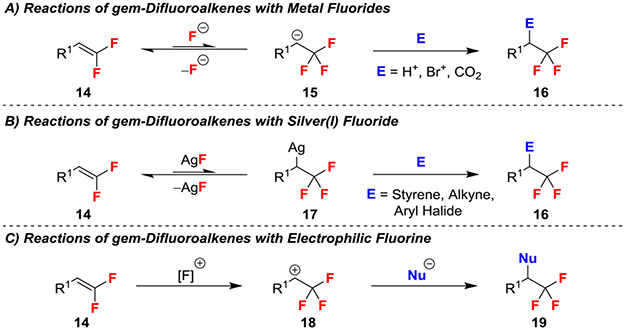
Reactions of gem-difluoroalkenes with classical two electron nucleophiles most commonly proceed through an addition/elimination pathway that result in effective C─F functionalization (Scheme 1B). Several strategies have been developed to avoid the elimination of fluoride by quenching the anionic intermediate via electrophilic trapping, protonation, or concerted processes, though many of these reactions are limited to gem-difluorostyrene-derived substrates that benefit from delocalization of the anionic intermediate. While limited, fluorine-retentive functionalization reactions of alkyl gem-difluoroalkenes have been achieved by exploiting competitive elimination of an alternate leaving group, and by formation of halonium/thiiranium intermediates, though this overarching strategy remains reasonably underdeveloped.
One strategy to avoid β-fluoride elimination and formation of monofluorinated products involves the use of fluoride, itself, as a nucleophile (Scheme 2A). This strategy benefits from a reversible equilibrium as the addition of F− reaction generates an β,β,β-trifluoromethyl anion (15), from which β-fluoride elimination simply regenerates the gem-difluoroalkene, thus preventing the loss of substrate.7-15 When using AgF, the metalated intermediate 17 can participate in Ag- or Pd-catalyzed processes in difunctionalization reactions (Scheme 2B).16-19 Moreover, the use of electrophilic sources of fluorine, such as Selectfluor and NFSI can avoid anionic intermediates entirely (Scheme 2C).20-22 Many of these reactions to convert gem-difluoroalkenes to β-substituted trifluoroethane products have been covered in a recent review and will not be covered in detail here.3
Scheme 2.
Fluorinative Difunctionalization of gem-Difluoroalkenes
2.1. Elimination of Allylic Groups
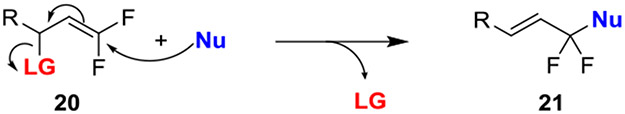
A second approach for preventing the loss of fluoride involves designing substrates that offer a competing elimination process. For example, SN2’ reactions of gem-difluoroalkenes bearing allylic leaving groups circumvented β-fluoro anionic intermediates, thus avoiding the potential for β-fluoride elimination (Scheme 3).23,24
Scheme 3.
SN2’ Strategy for Functionalization of gem-Difluoroalkenes
Based on this SN2’ strategy, gem-difluorinated vinyloxiranes (22) reacted with various nucleophiles to deliver a variety of products bearing a new C─C or C─heteroatom bond at the difluorinated position (23, 24; Scheme 4).25,26 These gem-difluorinated vinyloxiranes are a specialized substrate designed to prevent β-fluoride elimination though competitive opening of a γ-epoxide. The use of the oxirane group contrasts other methodologies, as the incorporation of allylic leaving groups enabled use of alkyl gem-difluoroalkenes rather than only styrene derived substrates. Moreover, these gem-difluorinated vinyloxiranes reacted in a stereoselective fashion to generate E alkenes due to the preference of the starting material to reside in the s-trans (22) conformer, as well as the concerted nature of the SN2’ reaction that avoids intermediates that can isomerize (Scheme 4A).25 However, in specialized examples, small coordinating nucleophiles reacted through a six-membered intermediate (26) to give Z alkene products (27, Scheme 4B).25,26
Scheme 4.
Reactivity of gem-Difluoroalkenes Bearing γ-Epoxides
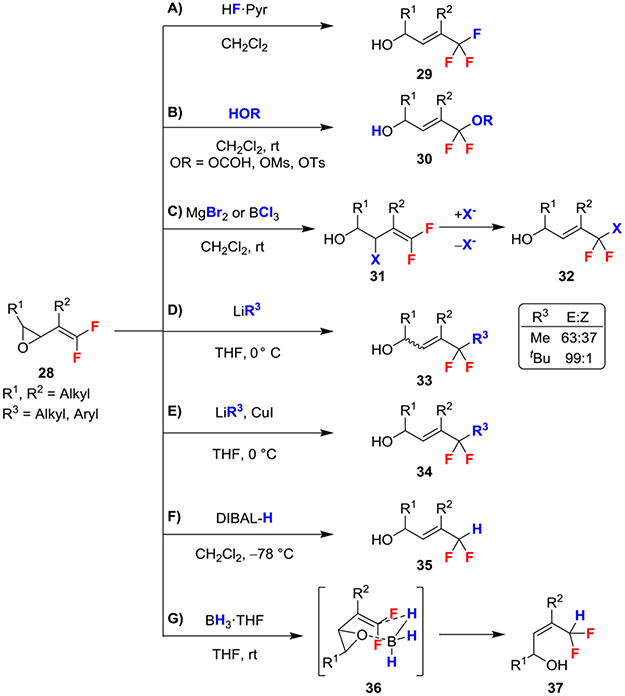
According to this general SN2’ reactivity pattern, acidic nucleophiles (HF, HOCOR, HOMs, HOTs) regio- and stereoselectively reacted at the difluorinated position of gem-difluorovinyl oxiranes to generate various R─CF2X-derived products (X = F, OCOR, OMS, OTs) bearing E alkenes (Scheme 5A,B).27,28 In a complementary process, Lewis acidic halogenating reagents MgBr2 and BCl3 opened the epoxide to initially generate a secondary allylic halide (31) that could further react via the SN2’ mechanism with another equivalent of MgBr2 or BCl3 respectively to deliver 1,1,1-difluorobromo- and 1,1,1-difluorochloro-containing products (Scheme 5C).27
Scheme 5.
Reactions of gem-Difluoroalkenes Bearing γ-Epoxides
The addition of alkyl Li reagents to gem-difluorovinyl oxiranes generated allylic alcohols with varying E selectivity and high regioselectivity (Scheme 5D).23,25 Specifically, the bulkiness of the alkyllithium reagents employed dictated the stereochemistry of the resulting alkene product, ranging from 63:37 E:Z for MeLi to 99:1 E:Z for tBuLi (33; Scheme 5D).23 The difference in stereoselectivity was derived from the smaller MeLi partially reacting through the s-cis conformer via the coordinated six-membered transition state (26; Scheme 4B). In contrast, the larger tBuLi could not accommodate this coordination, which disfavored formation of the s-cis conformation required to generate the E alkene, thus precluding formation of the Z product. Further, formation of the Z products was completely inhibited through the addition of HMPA, which presumably disfavored coordination of the epoxide. In later reports, the addition of CuI salts in THF afforded (E)-allylic alcohols exclusively (Scheme 5E).29 The increase in stereoselectivity observed with Cu can be explained by the competitive coordination of the lithium cuprate with solvent, which disfavors coordination with the epoxide.30
Oxirane-bearing gem-difluoroalkenes can also be regio- and stereoselectively reduced to give gem-difluoromethyl-containing products using DIBAL-H (E-selective) and BH3·THF (Z-selective; Scheme 5F,G).26,28 The Z-selective isomer is derived from the chair-like six-membered transition state with the smaller BH3 (36; Scheme 5G). Moreover, this coordinated reduction was not observed using the larger DIBAL-H reagent, a trend similar to that seen for the larger Li reagents (Scheme 5D).23 In contrast, the use of LiAlH4 and AlMe3 selectively opened the epoxide through addition to the allylic carbon, leaving the gem-difluoroalkene unreacted.26
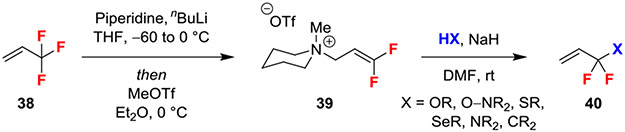
A recently published SN2’ reaction utilized an allylic ammonium leaving group to enable addition of a wide range of nucleophiles (Scheme 6).31 The activated electrophile 39 was generated via an SN2’ defluorination reaction of 3,3,3-trifluoropropene with piperidine followed by methylation with MeOTf. Ammonium 39 was then reacted with anionic nucleophiles to afford product 40. While NaH was used as the general conditions to activate nucleophiles, the authors noted that weaker bases, such as Cs2CO3 or KOH, can be used for more acidic nucleophiles, such as phenols, though these reactions required longer run times. Further, this reaction is compatible with aqueous conditions, which could enable functionalization of biologically relevant substrates.
Scheme 6.
Reactions of gem-Difluoroalkenes Bearing an Allylic Ammonium Group
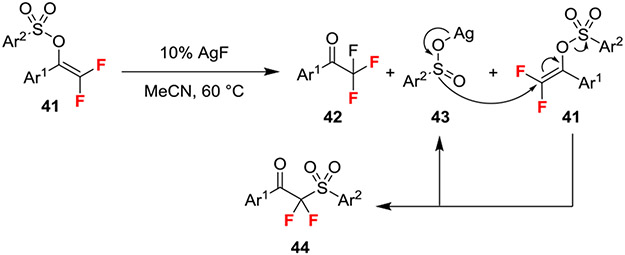
An intramolecular 1,3-sulfonyl migration of gem-difluorovinyl sulfonates generated α,α-difluoro-β-ketosulfones (Scheme 7).24 This reaction likely proceeded through initial activation of gem-difluorovinyl sulfonate with catalytic amounts of AgF to generate the corresponding Ag-aryl sulfinate 43 and trifluoromethyl acetophenone 42. Nucleophilic attack of the sulfinate on the gem-difluoroalkene then proceeded through an SN2’ process to deliver the product and liberate another equivalent of sulfinate to propagate the cycle. This mechanism is supported by experimental evaluation of stoichiometric reactions using proposed intermediates and crossover experiments.
Scheme 7.
Silver-Catalyzed Synthesis of α,α-Difluoro-β-ketosulfones
Under blue light irradiation, the reaction of gem-difluoroalkenes with diazoesters delivered densely-functionalized products through a Doyle-Kirmse-type rearrangement (Scheme 8).32 Specifically, photoexcitation of diazoester 46 expelled N2(g) to generate a carbene, which was subsequently trapped by nucleophilic addition of the thioester-containing gem-difluoroalkene to afford sulfonium ylide 47. This zwitterion (47) then underwent a Doyl-Kirmse-type 2,3-sigmatropic rearrangement to generate the C─CF2 bond in product 48. This mechanism is supported by competitive experiments to establish the presence of the carbene intermediate. Additionally, the increased rate of reaction for the fluorinated versus the non-fluorinated alkenes implicated a reaction controlled by the increased electrophilicity of the gem-difluorinated carbon, which might encourage nucleophilic attack.
Scheme 8.
Doyle-Kirmse-Type Rearrangement with gem-Difluoroalkenes
2.2. Electrophilic Addition
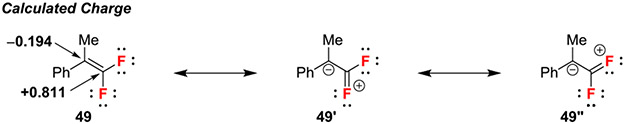
gem-Difluoroalkenes can be regioselectively difunctionalized using nucleophilic/electrophilic cascade sequences and three component reactions. In these reactions, the regioselectivity derives from the fluorine-induced polarization of the alkene, specifically, from the hyperconjugative donation and σ-induction effects that combined provide calculated charges of −0.194 and +0.811 at the non-fluorinated carbon and difluorinated carbon, respectively (Scheme 9).6,33 Further, upon nucleophilic attack of the gem-difluoroalkene, the resulting cationic intermediate 2 can be trapped by a nucleophile in a process that quickly increases molecular complexity (Scheme 1A).
Scheme 9.
Charge Density and Resonance Structures of gem-Difluoroalkenes
Lewis acids promoted intermolecular haloacylation reactions of gem-difluoroalkenes using acid chlorides (Scheme 10).34 Interestingly, AlCl3 -mediated reactions of an acyl chloride with alkyl vs. aryl-substituted gem-difluoroalkenes delivered disubstituted products with distinct regioselectivities (Scheme 10A,B). Specifically, the alkyl substrate reacted to exclusively deliver the product bearing the acyl group at the non-difluorinated position and the chloride at the fluorinated position (Scheme 10A). Though the author did not propose a mechanism, we suspect that this reaction proceeded through a three-step process: 1) formation of an electrophilic acylium ion, 2) nucleophilic attack of the acylium ion by the nucleophilic non-fluorinated carbon position of the gem-difluoroalkene that produced a cationic intermediate 52 that was stabilized through the delocalization of a lone pair electrons from the fluorine atoms,6 then 3) subsequent attack of chloride generated the difunctionalized product (Scheme 10A). In contrast, these conditions afforded the opposite regioselectivity for styrenyl gem-difluoroalkenes (acyl group attached to the difluorinated position) as the sole product (Scheme 10B). We speculate that the gem-difluoroalkene initially attacked the acylium ion to form the benzylic cationic intermediate 55, which was then quenched by chloride. We suspect that this process was controlled by the relative stability of the benzylic cation 55 over the competing α,α-difluorinated cation. Further, the use of SnCl4 generated products with the original regioselectivity, though the rationale for the regioselectivity is not immediately clear (Scheme 10C). Further, this strategy has been poorly explored beyond these initial examples.
Scheme 10.
Haloacylation of gem-Difluoroalkenes
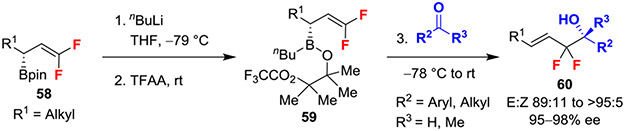
In a unique example, an α,α-difluoroallyl boronate ester converted the typically electrophilic character of the difluorinated position to a nucleophile. Specifically, an Aggarwal carbonyl allylation of gem-difluoroallyl-boronates and aldehydes or ketones stereoselectively afforded homoallylic alcohols (Scheme 11).35 This reaction proceeds through a multistep sequence involving 1) addition of nBuLi to boronic ester 58 to form a boronate complex, 2) trapping with trifluoroacetic anhydride to generate 59, and 3) addition of carbonyl-containing electrophiles to stereoselectively deliver difluorinated homoallylic alcohol 60. Moreover, the concerted addition of 59 into carbonyl groups retains the stereochemical information from the substrate, thus allowing for the formation of products in high ee.36
Scheme 11.
Aggarwal Carbonyl Allylation of gem-Difluoroalkenes
2.3. Halonium and Thiiranium Intermediates
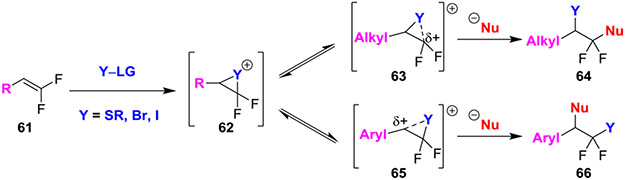
gem-Difluoroalkenes can also react with strong oxidants to afford high-energy halonium or thiiranium intermediates (62) that can further react with nucleophiles to produce difunctionalized products (Scheme 12). Moreover, nucleophiles react in both intra- and intermolecular fashions in highly regioselective manners, and in these reactions, the regioselectivity of the products is heavily influenced by the substitution of the gem-difluoroalkene. Specifically, the stabilization of the growing cationic charge at the transition state dictates the site of nucleophilic attack.37 Intermediates bearing alkyl substituents (63) will preferentially react with nucleophiles at the gem-difluorinated position. This regioselectivity can be rationalized through the hyperconjugation of fluorine stabilizing the buildup of cationic charge at this position (Scheme 9).6 However, the regioselectivity is reversed for reactions of aryl-substituted substrates, as the developing cationic charge is better stabilized at the benzylic position (65).
Scheme 12.
Mechanistic Overview of Reactions Involving Halonium and Thiiranium Intermediates
An intramolecular halocyclization reaction with gem-difluoroalkenes generated lactones and cyclic ethers (Scheme 13A,B).38 Interestingly, this reaction proceeded selectively through an uncommon 6-endo-tet-cyclization process, whereas the related non-fluorinated terminal alkenes react through exo-cyclization.39 Presumably, for the fluorinated substrate, the regioselectivity of the reaction was controlled by the increased electrophilicity at the fluorinated position that overrode the stereoelectronic preference for the non-fluorinated substrate. Further, the attempted cyclization of simple alcohol-containing substrates to form of tetrahydropyrans suffered from poor selectivity versus a dibromination process that afforded substantial quantities of 71 (Scheme 13B). In this case, the lack of selectivity between competing intra- and intermolecular reactions might be explained by comparing the Mayr reactivity parameters for the competing bromide (N = 13.6) and alcohol (N = 6.4) or carboxylate (N = 16.5) nucleophiles.40-42
Scheme 13.
Halocyclization of gem-Difluoroalkenes Bearing Carboxylic Acids and Alcohols
Friedel-Crafts-type iodoarylation with a source of electrophilic iodine (PyrICl or NIS) generated dibenzo-fused carbocycles (Scheme 14).43 This reaction is sensitive to the substitution of the gem-difluoroalkene with tetrasubstituted and trisubstituted alkenes generating six- and seven-membered cycles, respectively. Iodonium 73, followed the more common patterns for α,α-difluorinated iodonium intermediates by reacting solely at the α,α-difluorinated carbon to form typically disfavored seven-membered rings (Scheme 14A). In contrast, the iodonium intermediate bearing an aryl substituent (76), reacted with differing regioselectivity. The authors speculate that the aryl substituent on intermediate 76 stabilized the cationic charge at the benzylic position, thus favoring the exclusive formation of the six-membered ring (Scheme 14B).
Scheme 14.
Intramolecular Friedel-Crafts-type Halocyclization of gem-Difluoroalkenes
A regio- and enantioselective bromocyclization of gem-difluoroalkenes to generate oxazoline- and oxazine-containing products exploited a chiral anion phase transfer catalyst (Scheme 15).44 This reaction utilized the electrophilic bromination agent [(DAB)2Br](BF4)3 in the presence of the chiral solubilizing phosphoric acid (R)-TRIP to form the chiral reactive complex 82. This in situ generated intermediate (82) brominated the gem-difluoroalkenes and subsequent exo-cyclization of the tethered amides formed oxazoline and oxazine rings in a substrate-dependent manner. The regioselectivity for these reactions was controlled by the higher stability of cationic charge at the benzylic position, similar to related reactions invoking halonium intermediates. Additionally, the authors noted that the use of less electron deficient DABCO derivatives afforded the endo-cyclized product, albeit with diminished regioselectivity.
Scheme 15.
Regio- and Enantioselective Halocyclization of gem-Difluoroalkenes to Generate Oxazoline and Oxazine Products
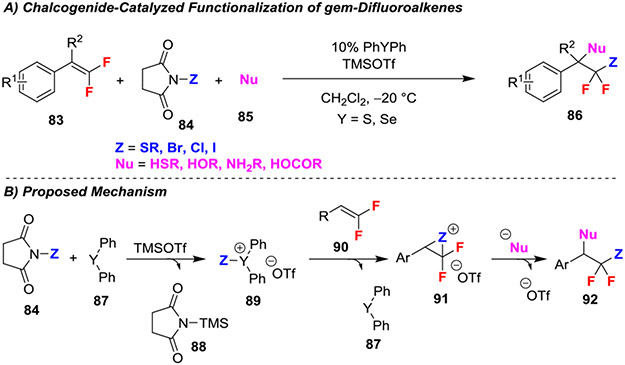
An electrophilic thio- and halofunctionalization of gem-dilfuoroalkenes used a chalcogenide-based catalyst system (Scheme 16A).45 Mechanistically, this reaction operated by initial activation of a succinimide-based reagent (84) with SePh2 or SPh2 to generate ion pair 89 (Scheme 16B). This activated reagent (89) then formed a cation with the gem-difluoroalkene, and subsequent nucleophilic attack at the benzylic position afforded products 92. Additionally, control experiments demonstrated that the reaction proceeded without the chalcogenide catalyst, though its inclusion greatly improved the yield, suggesting its role in forming the reactive cationic ion intermediate. Wide variability is tolerated for both the succinimide (SR, Br, Cl, I) and nucleophiles (HSR, HOR, NH2R, HOCOR) enabling formation of a broad scope of difunctionalized products.
Scheme 16.
Chalcogenide-Catalyzed Functionalization of gem-Difluoroalkenes
2.4. Kinetic Quench of Anionic Intermediate
Several strategies have been developed to rapidly quench the anionic intermediate (5) generated from nucleophilic addition into gem-difluoroalkenes (Scheme 1B). For example, the use of acid and base organocatalysts has enabled development of several functionalization reactions of gem-difluoroalkenes. Generally, these reactions avoid β-fluoride elimination by providing a source of H+ that can rapidly quench the anionic or developing anionic intermediate. Additionally, substrates bearing electrophiles that can react intramolecularly with the anionic intermediate have also been developed. Of note, these reactions are limited to β-aryl-α,α-difluorinated alkenes that benefit from delocalization of the anionic charge into the aryl ring, which stabilizes the high-energy intermediates.
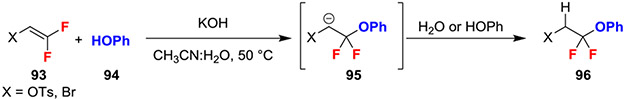
The reaction of phenols with 2,2-difluorovinyl tosylate or 2-bromo-1,1-difluoroethylene and KOH generated α,α-difluoroalkyl ethers (Scheme 17).46 Moreover, the selectivity of this reaction benefited from the addition of water which drastically minimized the elimination of fluoride. Specifically, the anhydrous reaction yielded 96 as a 3:1 mixture versus the monofluorinated product. While the introduction of 5% H2O, which presumably acted as a proton source, generated difluorinated products with high selectivity (97:3).
Scheme 17.
Base-Catalyzed Hydrofunctionalization of gem-Difluoroalkenes with Phenols
The reactions of highly-halogenated of chlorotrifluoroethene (97, R1 = Cl), perfluoropropene (97, R1 = CF3), and perfluoroisobutene (101) with thiophenol and K2CO3 highlighted the unpredictability of fluorinated substrates (Scheme 18A,B).47 Both substrates (97 and 101) reacted to initially generate the hydrothiolated intermediates 99, though 99 (R1 = CF3) further underwent base promoted elimination of HF. Moreover, this loss of HF correlated with the acidity of the proton, as compound 99 (R1 = Cl) did not undergo elimination, 99 (R1 = CF3) gave intermediate 99 and product 100 in a 7:3 mixture, and 102 fully eliminated. Further, the reaction of 101 generated several additional side products. Specifically, monofluorovinyl species 103 underwent a second hydrothiolation/elimination reaction to generate 104 and intermediate 102 underwent an alternate HF elimination to initially generate 105, which then underwent a net SN2’ thiolation/defluorination to yield 106.
Scheme 18.
Addition of Thiophenols into Fluorinated Alkenes
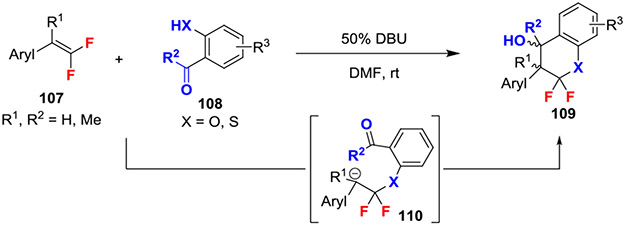
A base-catalyzed [4 + 2] annulation of gem-difluoroalkenes reacted o-hydroxy / mercapto benzaldehydes and acetophenones to deliver multisubstituted dihydrobenzopyrans (Scheme 19).48 This reaction proceeded through initial nucleophilic attack of phenol or thiophenol to generate the anionic intermediate 110, which underwent a facile intramolecular nucleophilic addition into the benzaldehyde or acetophenone. By strategically placing an electrophile in proximity of the unstable anionic intermediate, β-fluoride elimination was successfully avoided.
Scheme 19.
[4 + 2] Annulation of gem-Difluoroalkenes
In a first example, 1,1,3,3-tetramethylguanidine (TMG) served as a catalytic base for hydrothiolation reactions of gem-difluoroalkenes with thiophenols to deliver α,α-difluorothioethers (Scheme 20A).49 Generally, the reaction performed well for electron-rich and -neutral gem-difluorostyrenes, but did not perform as well for electron-deficient substrates. Additionally, this method only coupled thiophenol derived nucleophiles, and did not function for alkyl thiols. Mechanistically, the reaction proceeded through initial deprotonation of thiophenol, addition into the gem-difluoroalkene to generate anionic intermediate 115, which is then rapidly protonated by the conjugate acid of TMG (Scheme 20B). Multiple mechanistic experiments refuted radical processes, including running the reaction in the dark and without O2, and utilization of radical traps. Further, deuterium labeling experiments supported the proposed addition/protonation pathway. Under the standard conditions, alkyl thiols reacted with the gem-difluoroalkenes to deliver α-fluorovinylether products, presumably from an addition/elimination process. This selectivity likely arose from a mismatch of the pKas of the thiol and base.
Scheme 20.
Base-Catalyzed Hydrofunctionalization of gem-Difluoroalkenes
This organocatalytic strategy was additionally utilized for the hydrophenolation of gem-difluoroalkenes with phenols to yield α,α-difluoroalkyl ethers, though in this case triazabicyclo[4.4.0]dec-5-ene (TBD) was used as the base (Scheme 21).50 This reaction presumably proceeded through an addition/protonation mechanism, similar to that used for the previously discussed hydrothiolation reaction (Scheme 20B). Considering the proposed mechanism, the pKa of the base influences the selectivity between intramolecular β-fluoride elimination or intermolecular protonation. Moreover, while weaker bases, such as TMG (DMSO pKa = 14), were sufficiently basic to activate thiophenols (DMSO pKa = 10), phenols (DMSO pKa = 18) required a stronger base, TBD (DMSO pKa = 21), for activation. Further, the conjugate acid must be sufficiently acidic to quench the carbon-based anionic intermediate.
Scheme 21.
Hydrofunctionalization of gem-Difluoroalkenes with Phenol Nucleophiles
A complementary reaction to access α,α-difluorothioethers was developed using an acid-derived catalyst system that added thiol nucleophiles across gem-difluoroalkenes to generate hydrothiolated products with high regio- and chemoselectivity (Scheme 22A).51 Moreover, this method expanded the scope of the prior base-catalyzed strategy49 to include alkylthiols and improved both the yield and selectivity of previously challenging substrate combinations,49 notably reactions of thiophenols with electron deficient gem-difluororalkenes. Although this system exploited LiOTf and pyridine as catalytic additives, the reaction actually proceeded through an acid-catalyzed process. Specifically, heating the thiol nucleophile under an atmosphere of air initially oxidized a sacrificial amount of the thiol to the respective disulfide 123 (Scheme 22B), which then further oxidized to form the more acidic sulfinic acid 124.52,53 This acid then protonated pyridine to form the active pyridinium catalyst 125, which facilitated a concerted nucleophilic addition and protonation (127), thus circumventing formation of a discrete anionic intermediate and avoiding β-fluoride elimination. The proposed concerted mechanism is supported by linear free energy relationship with σ−, a primary KIE, control reactions, and direct use of proposed intermediate 125.
Scheme 22.
Acid-Catalyzed Hydrofunctionalization of gem-Difluoroalkenes
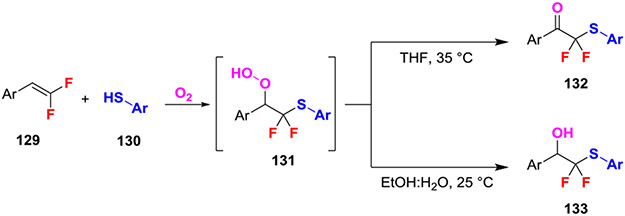
Recently, a solvent-controlled aerobic reaction selectively coupled gem-difluoroalkenes and thiophenols to afford α,α-difluorothiomethylated alcohols and ketones (Scheme 23).54 This reaction was proposed to proceed through a hypoperoxy intermediate 131 that underwent selective decomposition in different solvents. Mechanistic experiments suggested that intramolecular bonding between the hypoperoxy OOH and a fluorine atom was present in THF but not in EtOH/H2O.5 This difference in solvent-bonding drove the selectivity of inter- and intramolecular decomposition of the hypoperoxy intermediate, which in turn generated the alcohol or ketone products, respectively. Further evidence for this mechanism was provided by 18O labeling experiments to demonstrate incorporation of O2, radical traps, radical clock, and EPR experiments to rule out radical processes, as well as time-course, and NMR experiments to elucidate the role of solvent. Additionally, the utility of the products was demonstrated through the selective derivatization of the alcohol, ketone, and thioether functional groups.
Scheme 23.
Aerobic Quench of the Anionic Intermediate
2.5. Concerted Cycloaddition
gem-Difluoroalkenes have successfully undergone several concerted cycloaddition reactions. These reactions circumvent the formation of a discrete anionic intermediate, which avoids β-fluoride elimination and thus generates difluorinated cyclic products.
The synthesis of 3,3-difluoropyrrolidines from styrenyl gem-difluoroalkenes was originally reported in a patent by Novartis,55 and in a later publication by a separate group, the scope was expanded to include α,β-unsaturated and enol ethers derived gem-difluoroalkenes (Scheme 24).56 Both strategies likely proceeded through an azomethine ylide precursor that underwent a 1,3-dipolar [3 + 2] cycloaddition with gem-difluoroalkenes in the presence of an activating reagent. This reaction avoided β-fluoride elimination by proceeding through a concerted mechanism, thus likely avoiding the formation of the unstable anionic intermediate.
Scheme 24.
Reaction with a 1,3-Dipole
gem-Difluoroalkenes additionally underwent [3 + 2] reactions with nitrones to generate difluoroisoxazolidines 139 and 142 (Scheme 25A,B).57 In these reactions, the alkene’s electronic character controlled regiochemical outcome. Specifically, this reaction operates by a type I [3 + 2] in which good overlap between the HOMO of the nitrone (predominantly on O) and the LUMO of the difluorinated alkene ( predominantly on the electropositive gem-difluorinated carbon) control regioselectivity.58 Further, the suprafacial-suprafacial approach required for [3 + 2] cycloadditions stereospecifically enabled generation of single diastereomers as represented by 142 (Scheme 25B). In some cases, the intrinsic instability of a given difluorinated heterocyclic product might enable formation of more stable monofluorinated products, as evidenced by 143, which under thermal conditions readily rearomatize to form acid fluoride 144.59
Scheme 25.
Reactions of gem-Difluoroalkenes with Nitrones
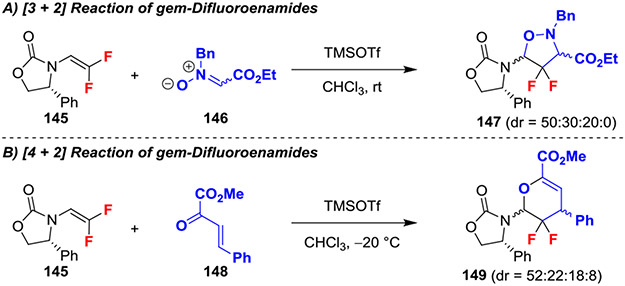
In another example, [3 + 2] and [4 + 2] cycloaddition reactions of gem-difluoroenamide 145 with nitrone 146 or oxadiene 148 exploited TMSOTf as a promoter (Scheme 26).60 In contrast to the aforementioned reactions in which the reactivity of the alkene was dominated by the electrophilic character of the difluoroalkenene’s LUMO (Scheme 26A,B), incorporation of a vinylcarbamate on gem-difluoroalkene 145 and an ester on nitrone 146 altered the electronic characters of the substrate and also the regiochemical outcome. Specifically, these reactions proceeded through a type III inverse electron demand [3 + 2] cycloaddition in which the higher HOMO of the electron rich gem-difluoroencarbamate (largest coefficient presumably at the difluorinated position) and the lowered LUMO of the nitrone or the α,β-unsaturated ketone controlled regioselectivity.58 Further, though exclusively regioselective, this reaction did not generate single diastereomers.
Scheme 26.
Cycloaddition Reactions of gem-Difluoroenamides
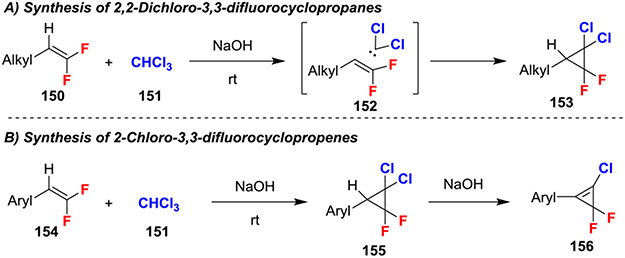
Highly halogenated cyclo-propenes and propanes can be prepared from reactions of gem-difluoroalkenes or gem-difluorostyrenes and chloroform under basic conditions (Scheme 27).61 This reaction likely proceeded through formation of dichlorocarbene and subsequent addition to the gem-difluoroalkenes (152; Scheme 27A). Moreover, use of gem-difluorostyrenes afforded an intermediate cyclopropane that underwent an E2 elimination to give highly strained 2-chloro-3,3-difluorocyclopropene products (156; Scheme 27B). This elimination was facilitated by the increased acidity of the benzylic proton and was not observed with alkyl-substituted gem-difluoroalkenes.
Scheme 27.
Reactions of gem-Difluoroalkenes with Dichlorocarbene
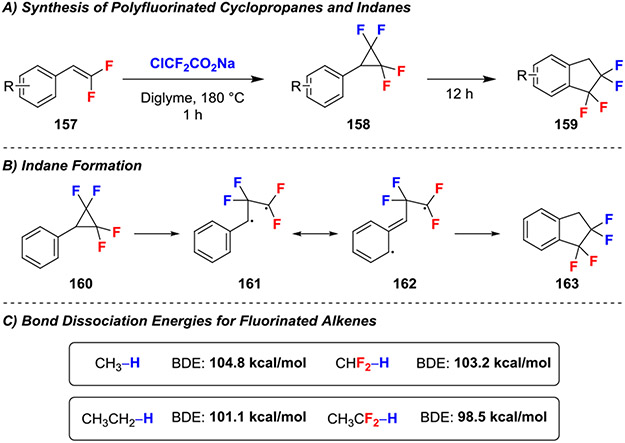
gem-Difluoroalkenes also reacted with difluorocarbene to form 1,1,2,2-tetrafluoroindanes (Scheme 28A).62 These reactions utilized the thermal decomposition of ClCF2CO2Na to generate difluorocarbene in situ, which subsequently underwent cyclopropanation with gem-difluorostyrenes within one hour. When heated for an extended period (12 h), the cyclopropane (160; Scheme 22B) fragmented to form the ring opened diradical 161, from which delocalization of the radical into the ring (162) and subsequent radical recombination and isomerization delivered tetrafluorinated indane 163. This reactivity contrasts that of non-fluorinated thermal cyclopropane fragmentation, which preferentially forms propene derived products.63 The authors suggested that this change in reactivity might arise from the weakening of the carbon-carbon bond in the 1,1,2,2-tetrafluorocyclopropanes, lowering the energy required for homolytic cleavage. This weakening likely originates from increased ring strain in the presence of multiple fluorine atoms. Further, experimental bond dissociation energies for α-difluorinated radicals indicate a stabilization effect through delocalization of 1.6–2.6 kcal/mol (Scheme 28C) for comparable systems further favoring the formation of the diradical.64
Scheme 28.
Reactions of gem-Difluoroalkenes with Difluorocarbene
3. Radical
Radical additions into gem-difluoroalkenes have become a rapidly growing strategy that enables retention of both fluorine atoms. These reactions typically proceed through radical addition into the alkene at the difluorinated position to generate the β,β-difluororadical 8, which is stable against the ejection of unstable fluorine radicals (Scheme 1C). Ultimately, the radical intermediate 8 can be quenched by various reagents to form product 9.
3.1. Thermal Activation
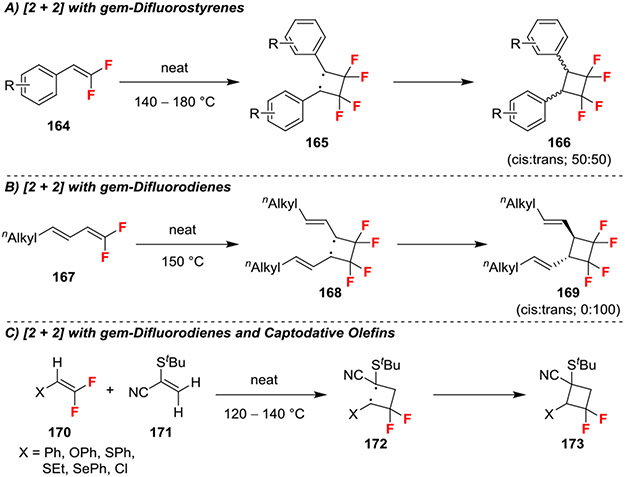
Heating of neat gem-difluoroalkenes generated tetrafluorinated cyclobutanes through a dimerization processes that proceeded through a diradical intermediates 165 or 168 (Scheme 29A,B),65 similar to photoinitiated [2 + 2] reactions.66 In this reaction, the fluorine atoms facilitated the reaction by lowering the energy difference of the alkene’s HOMO and LUMO, which facilitated homolytic cleavage.64 Further, the observed stereoselectivity was rationalized by resonance stabilization of the intermediate diradical (165 or 168). Specifically, the high stability of the benzylic diradical 165 enabled isomerization to afford mixtures of cis and trans cyclobutanes (Scheme 29A). In contrast, the less stable diradical 168 formed from gem-dilfuorodienes exclusively generated the trans product (Scheme 29B).
Scheme 29.
Cyclization Reactions Involving Formation of Diradicals from gem-Difluoroalkenes
Additionally, heating of gem-difluoroalkenes in the presence of captodative olefins promoted [2 + 2] cycloaddition reactions (Scheme 29C).67 Such reactions benefited from the stabilization of diradical 172 though both captodative substitution and incorporation of a second stabilizing group (Ph, OPh, SPh, SEt, SePh, Cl). Furthermore, the formation of these unsymmetrical cyclobutanes required lower reaction times (8 h versus 24–40 h) and lower temperatures (120–140 °C versus 140–180 °C) compared to the homodimerization [2 + 2] reactions of gem-difluorinated alkenes (Scheme 29A,B).65,67
Both allyl and aryl gem-difluoroalkenes underwent regioselective hydrofunctionalization reactions with thiols, thiophenols, and butyraldehyde in the presence of catalytic quantities of benzoyl peroxide (Scheme 30).68 These reactions presumably occurred by formation of S- and C-based radicals that attacked the difluorinated position of the alkene to generate new C─C or C─S bond. Notably, these reactions formed the new bonds at the benzylic position, which distinguishes these reactions from pathways that invoke halonium or thiiranium intermediates (Section 2.3). Interestingly, both gem-difluorostyrenes and gem-difluoroalkenes afforded product, whereas many of the other reactions in this section are limited to styrene-derived substrates. Additionally, the analogous radical reaction with catalytic benzoyl peroxide and carbon tetrachloride did not successfully chlorinate the alkene and only generated an unidentifiable complex mixture.
Scheme 30.
Peroxide Initiated Reactions with gem-Dilfuoroalkenes
A regioselective radical hydroboration of gem-difluoroalkenes, gem-difluoroacrylates and gem-difluoroacrylamides with cyano(1,3-dimethylimidazole-2-ylidine)dihydroborate (181) used 1,1’-axobis(cyclohexanecarbonitrile) (ACCN) as a radical initiator and a thiol as a hydrogen atom transfer catalyst (Scheme 31A,B).69 In these reactions, less electron-deficient boronates successfully reacted, but were not stable to flash column chromatography on silica gel. This reaction proceeded by initial hydrogen atom abstraction by the radical initiator ACCN to generate the boryl anionic radical 183 (Scheme 31B). This intermediate (183) then underwent regioselective addition into the gem-difluoroalkene to form the corresponding radical 184, which was quenched by hydrogen atom transfer from the thiol. The thiyl radical continued the chain propagation process by abstracting hydrogen from 181, thus reforming the boryl radical anion 183. This mechanism was supported by control experiments and DFT calculations. Moreover, the computed energies predicted that addition of the boron-based radical anion to the fluorinated carbon was favored by 4.3 kcal/mol over the non-fluorinated position.
Scheme 31.
Hydroborylation of gem-Difluoroalkenes
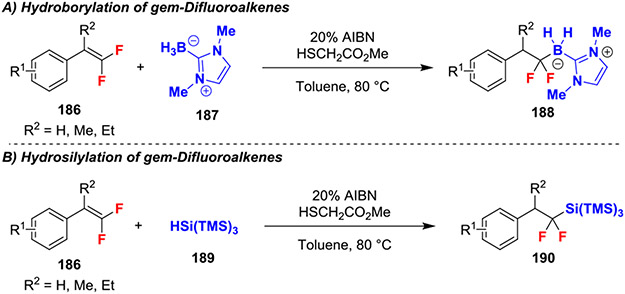
A subsequent radical hydroboration reaction of gem-difluorostyrenes exploited 2,2-azobis(isobutyronitrile) (AIBN) to expand the scope to the include less deficient and synthetically useful (1,3-dimethylimidazole-2-ylidine)dihydroborate 187 (Scheme 32A).70 Moreover, the authors expanded upon previous work by demonstrating the synthetic utility of the borylated products (188) through further functionalization with halogenation and oxidative radical arylation reactions. Further, these conditions also promoted a related hydrosilylation of gem-difluoroalkenes (Scheme 32B). The mechanisms for both hydroboration and hydrosilation both proceeded through initial abstraction of hydrogen to generate a Si-based radical or B-based radical anion, which then added to a gem-difluoroalkene (186) at the difluorinated position to generate a benzylic radical. Subsequent hydrogen transfer from the thiol catalyst generated the respective products 188 and 190.
Scheme 32.
Hydroborylation and Hydrosilylation of gem-Difluoroalkenes
3.2. Photo Activation
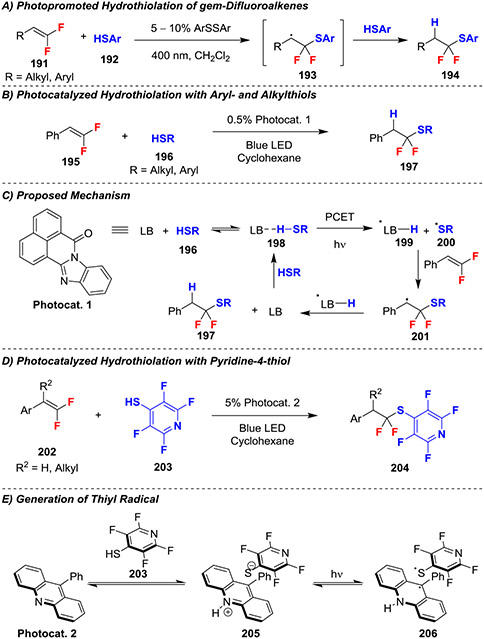
Several reactions have been developed for the hydrothiolation of gem-difluoroalkenes using light to initiate the processes. These works were inspired by the initial hydrothiolation report that utilized benzoyl peroxide,68 but only generated moderate yields (Scheme 33). An initial photo-initiated reaction using catalytic amounts of the corresponding disulfide delivered the hydrofunctionalized products in high yields (>90%) from gem-difluorostyrenes, but only moderate yields (45%) using non-stabilized gem-difluoroalkenes (Scheme 33A).71 This reaction proceeded through initial homolytic cleavage of the catalytic disulfide to generate a thiyl radical, which added into the gem-difluoroalkene to generate a β,β-difluoro-β-thioether radical. This intermediate then abstracted hydrogen from thiolphenol and continued as a chain propagation process.
Scheme 33.
Photoredox Hydrothiolation of gem-Difluoroalkenes
The application of photoredox-catalysts improved the scope of light-promoted hydrofunctionalization of gem-difluoroalkenes. Specifically, gem-difluoroalkenes reacted smoothly in a visible-light promoted thiol-ene reaction using a proton coupled electron transfer (PCET) organocatalyst (Scheme 33B).72 The proposed mechanism for this reaction involves initial initiation by coordination of the Lewis basic organocatalyst Photocat. 1 to the acidic thiol 196 to generate complex 198 (Scheme 33C). This complex then underwent a PCET reaction to generate thiyl radical 200, and subsequent addition into the gem-difluoroalkene generated benzylic radical 201. This radical then abstracted hydrogen from the catalyst 199 to regenerate the initiator and generate product 197. Additionally, several experiments supported the chain propagation mechanism, in which 201 abstracts hydrogen from another equivalent of thiol, though light-dark experiments demonstrated poor efficiency of this chain propagation process. Furthermore, the PCET process could generate thiyl radicals through two distinct mechanisms: 1) consecutive proton and electron transfer steps, or 2) concerted proton and electron transfer. The authors suggest that the likely mechanism is concerted based on other literature reports involving PCET reactions with thiols.
A second photocatalyzed hydrothiolation that also exploited PCET organocatalysts for functionalizing gem-difluoroalkenes involved a distinct mechanism, specifically a process involving consecutive proton and electron transfers (Scheme 33D).73 Initially, the reaction of thiol 203 with the phenylacridine catalyst Photocat. 2 formed a colored salt (205), for which an X-ray structure showed π–π stacking between the thiolate and acridinium cation (Scheme 33E). Irradiation of this isolated complex transferred an electron from the thiolate to the heterocycle, generating the reactive thiyl radical (206).
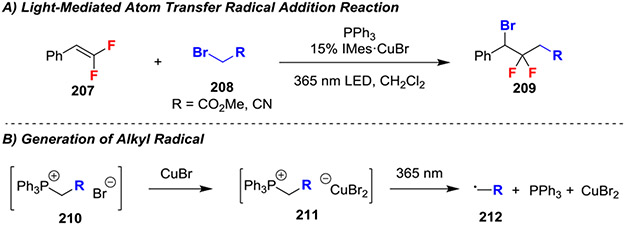
A Cu-catalyzed light-mediated difunctionalization reaction of gem-difluoroalkenes with alkyl bromides generated new C─C and C─Br bonds using a phosphine as a stoichiometric reagent (Scheme 34).74 In this reaction, the authors exploited a novel mechanistic pathway to avoid harsh conditions, stoichiometric transition metals, and radical initiators typically required for this transformation. Initially, 208 and the phosphine reacted to form a phosphonium salt 210, and subsequent reaction with CuBr generated cuprate salt 211 (Scheme 34B). This salt then underwent light-mediated electron transfer to provide α-stabilized radical 212, which subsequently reacted with the gem-difluoroalkene to initially form a stabilized benzyl radical, which was then halogenated by CuBr2 to afford the difunctionalized product and regenerate the CuBr catalyst. Mechanistic evaluation using stochiometric intermediates and radical clock experiments supported the proposed mechanism.
Scheme 34.
Light-Mediated Atom Transfer Radical Addition
3.3. Transition Metal Mediated
Decarboxylative strategies have also generated radicals that can then functionalize gem-difluoroalkenes. In one case, a Ag-promoted decarboxylation of oxamic acid derivatives initiated a radical cascade to functionalize gem-difluoroalkenes and generate 3,4-dihydroquinolin-2-ones (Scheme 35).75 This reaction likely proceeded through initial persulfate-mediated oxidation of AgI to AgII, which promoted the decarboxylation of the oxamic acid derivative to generate a carbamoyl radical 217 (Scheme 35B). This radical added into the gem-difluoroalkene to generate benzyl radical 218 that then underwent an intermolecular 6-endo-trig cyclization with the appended arene to generate aryl radical 219. Subsequent rearomatization ultimately generated 3,4-dihydroquinolin-2-one product 220. Support for this mechanism consists of radical trap experiments and literature precedents for the key proposed steps.
Scheme 35.
Silver-Catalyzed Synthesis of 3,4-Dihydroquinolin-2-ones
gem-Difluoroalkenes underwent a regioselective unsymmetrical dioxidation with a cobalt-based catalyst under an atmosphere of O2 to generate β-phenoxy-β,β-difluorobenzyl alcohols (Scheme 36).76 Early iterations of this reaction non-selectively generated mixtures of products bearing either an alcohol or ketone, though this problem was overcome through use of a cobalt-based catalyst system. This process was initiated by the reaction of Co and O2 with ArOH which generated a phenoxyl radical 224 (Scheme 36B). This O-based radical then regioselectively reacted with the gem-difluoroalkene to deliver benzylic radical 225. Notably, formation of the new C─O bond occurred at the difluorinated position, which matched regioselectivity previously demonstrated with thiyl radicals (Scheme 33A, 28).71-73 Subsequent reaction of intermediate radical 225 with O2 afforded peroxyl radical 227 (Path 1) or with O2 and Co to generate metalloperoxide 226 (Path 2). Both intermediates could then abstract hydrogen from phenol to propagate the chain process and generate hydroperoxide 228. Hydroperoxide 228 ultimately underwent cobalt-catalyzed Fenton decomposition to generate product 223. This radical process was supported by radical trap experiments, reaction monitoring by EPR, and stochiometric EPR experimentation that established the initial activation process, as well as strong literature support for the latter steps in the process.77-80
Scheme 36.
Cobalt-Catalyzed Dioxygenation of gem-Difluoroalkenes
4. Reductions
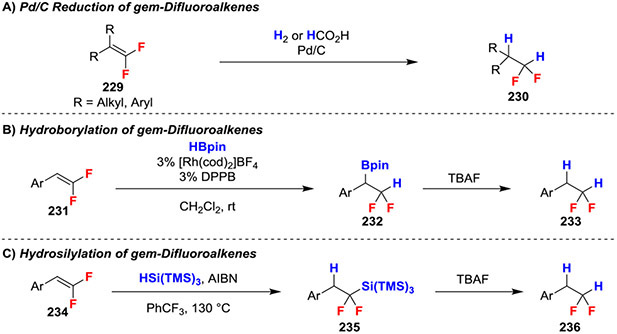
gem-Difluoroalkenes have been readily reduced with Pd/C in the presence of H2 or H2 surrogates, such as HCO2H. Moreover, these conditions were compatible with a wide range of substitution on the gem-difluoroalkene including aryl, alkyl, and fully-substituted alkenes (Scheme 37A).81-83 Additionally, gem-difluoroalkenes can be reduced using boron or silicone-based intermediates. Specifically, Rh-catalyzed hydroboration (Scheme 37B)84 or radical hydrosilation of gem-difluoroalkenes generated organometal intermediates, and subsequent protodeboronation or desilylation using TBAF delivers the product of net reductions (Scheme 37C).70 Notably, the initial formation of the C─B and C─Si bonds occurs with distinct regioselectivities, and the respective boryl or silyl intermediates might be useful for other cross-coupling reactions. Of note, gem-difluoroalkenes are unreactive with NaBH4 and LiAlH4 as these reductants were used to reduce ketones and aldehydes in the precents of gem-difluoroalkenes.26,83 Further, oxidations with Jones reagent and pyridinium dichromate were compatible with gem-difluoroalkenes.27,29
Scheme 37.
Reduction of gem-Difluoroalkenes
5. Cross-Coupling Reactions
Despite the large body of literature of reactions that proceed through organotransitionmetal intermediates (239) that undergo β-fluoride elimination to generate monofluorinated alkenes,1-3 Pd-based catalyst systems can preferentially eliminate an alternate tethered group, such as an acetate, a hydride, or a halide (Cl, Br, I), as predicted by previous computational experiments (Scheme 38).85
Scheme 38.
Mechanistic Overview of Reactions of Transition Metals with gem-Difluoroalkenes
A Pd-catalyzed functionalization of γ,γ-difluoroallylic acetates with arylboronic acids generated α,α-difluorobenzyl products (Scheme 39A).86 This reaction proceeded through a β,β-difluoro-β-acetate-Pd intermediate that preferentially underwent β-elimination of acetate to deliver difluorinated products (244). This selectivity agrees with assistance of density function theory calculations of a simplified system in which a lower reaction barrier was predicted for elimination of β-acetates (11.7 kcal/mol) versus β-fluorides (14.0 kcal/mol; Scheme 39B).85
Scheme 39.
Selective β-OAc Elimination to Avoid the Loss of Fluorine
Another Pd-catalyzed reaction of gem-difluorinated alkenes exploited β-hydride elimination from an α,α-difluoroalkyl-Pd intermediate to selectively avoid β-fluoride elimination (Scheme 40).87 More specifically, the mechanism of this reaction proceeded through PdII/PdIII intermediates in which PdIII-alkyl (252) intermediate preferentially underwent β-hydride elimination over β-fluoride elimination. Further, subsequent hydride insertion/elimination transferred the alkene to the most thermodynamically stable position (Scheme 40B). Experimental support for this mechanism consisted of radical trap experiments and deuterium-scrambling reactions. Additional computational studies of this process estimated that the β-hydride elimination was favored by 2.5 kcal/mol (Scheme 40C). Moreover, the comparison of the calculated transition states for both β-hydride and β-fluoride elimination for PdIII versus PdII are significantly lower for PdIII (β-hydride = 25.1; β-fluoride = 37.1 kcal/mol).
Scheme 40.
Selective β-Hydride Elimination to Avoid the Loss of Fluorine
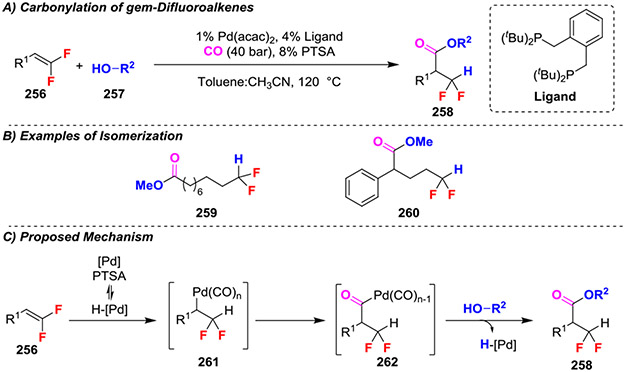
A selective carbonylation of gem-difluoroalkenes using a Pd-based-catalyst system generated α,α-difluoromethylated esters (Scheme 41A).88 This reaction underwent a cascade isomerization-alkoxycarbonylation with aliphatic gem-difluoroalkenes to generate remotely functionalized products (Scheme 41B). Initial reduction of PdII to Pd0 with excess phosphine followed by protonation generated a Pd hydride (Scheme 41C). Insertion into the gem-difluoroalkene generated the Pd bound intermediate 261. CO insertion and subsequent alcoholysis generated the final product 258. Notably, this reaction avoids β-fluoride elimination by offering an opportunity for the difluorinated PdII-alkyl intermediate (261) to undergo an α-migratory insertion into the carbonyl ligand.
Scheme 41.
Carbonylation of gem-Difluoroalkenes
6. Conclusion
This review has focused on fluorine-retentive strategies for functionalizing gem-difluoroalkenes. Due to the electron-withdrawing character of the fluorine atoms, these polarized alkenes undergo highly regioselective reactions. Further, a wide range of synthetic methods have been developed to access difluorinated products through the functionalization of gem-difluoroalkenes highlighting their versatility. These reactions can proceed through cationic, anionic, radical, and metalated intermediates. Though, in many cases, the substrates are restricted to β-aryl-α,α-difluoroalkenes that proceed through resonance-stabilized radical or charged intermediates. Translation of these methods more broadly to β-alkyl-α,α-difluoroalkenes has found mixed success for cationic and radical process and necessitates more attention. Further, while several kinetic quenching strategies have been developed, anionic reactions are largely limited to fluorofunctionalization or contrived alkenes to avoid destructive β-fluoride elimination. Though, both concerted cycloaddition and electrophilic activation have successfully avoided loss of fluoride, these reaction paradigms remain mostly undeveloped beyond the initial exploratory examples. Moreover, while great progress has been made, further exploration of gem-difluoroalkenes is required to better comprehend and exploit their reactivity. We hope that this review will provide inspiration for the development of new reactions for the fluorine-retentive functionalization of gem-difluoroalkenes.
Funding Information
Generous financial support from the National Institute of General Medical Sciences of the National Institutes of Health (R35 GM124661) is gratefully acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography

Jacob Sorrentino is a graduate student in the Altman group pursuing a Ph.D. in Medicinal Chemistry from The University of Kansas (KU). Jacob earned his BSc in biology at Nevada State College in 2016, studying under Professor Zachary Woydziak as an INBRE trainee. He then joined the laboratory of Professor Ryan Altman at KU as a graduate student where his research focuses on the development of synthetic methodology and the application of organofluorine chemistry toward the design of therapeutics and biological probes.

Ryan A. Altman received a B.S. Chem. from Creighton University (2003) and a PhD in organic chemistry from the Massachusetts Institute of Technology (2008), studying as a Pfizer and National Institutes of Health predoctoral fellow in the laboratory of Professor Stephen Buchwald. He subsequently trained as an NIH postdoctoral fellow under the guidance of Professor Larry Overman at the University of California, Irvine (2008–2011), after which he joined the Department of Medicinal Chemistry at The University of Kansas as an Assistant Professor. After promotion to Associate Professor (2017), his group moved to Purdue University to join the Department of Medicinal Chemistry and Molecular Pharmacology and the Department of Chemistry (2020). The Altman group works at the interface of synthetic organic and medicinal chemistries, with synthetic emphases in the areas of organometallic and organofluorine transformations and unique chemical reactivities enabled by fluorinated substructures. The group’s collaborative medicinal interests span a range of disease states, including pain, anxiety and mood disorders, and aging.
References
- (1).Zhang X; Cao S Recent Advances in the Synthesis and C-F Functionalization of Gem-Difluoroalkenes. Tetrahedron Lett. 2017, 58 (5), 375–392. [Google Scholar]
- (2).Koley S; Altman RA Recent Advances in Transition Metal-Catalyzed Functionalization of Gem-Difluoroalkenes. Isr. J. Chem 2020, 60, 313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Liu C; Zeng H; Zhu C; Jiang H Recent Advances in Three-Component Difunctionalization of Gem-Difluoroalkenes. Chem. Comm 2020, 56, 10442–10452. [DOI] [PubMed] [Google Scholar]
- (4).Orsi DL; Altman RA Exploiting the Unusual Effects of Fluorine in Methodology. Chem. Comm 2017, 53, 7168–7181. [DOI] [PubMed] [Google Scholar]
- (5).Hagan DO Understanding Organofluorine Chemistry. An Introduction to the C─F Bond. Chem. Soc. Rev 2008, 37, 308–319. [DOI] [PubMed] [Google Scholar]
- (6).Roberts J; Webb R; Mcelhill E The Electrical Effect of the Trifluoromethyl Group. J. Am. Chem. Soc 1950, 72, 408–411. [Google Scholar]
- (7).Zhu L; Li Y; Zhao Y; Hu J Nucleophilic (Phenylsulfonyl) Difluoromethylation of Alkyl Halides Using PhSO2CF2SiMe3: Preparation of Gem-Difluoroalkenes and Trifluoromethyl Compounds. Tetrahedron Lett. 2010, 51, 6150–6152. [Google Scholar]
- (8).Liu H; Ge L; Wang D; Chen N; Feng C Photoredox-Coupled F-Nucleophilic Addition: Allylation of Gem-Difluoroalkenes. Angew. Chem. Int. Ed 2019, 58, 3918–3922. [DOI] [PubMed] [Google Scholar]
- (9).Yoo W; Kondo J; Rodroguez-Santamaria JA; Nguyen TVQ; Kobayashi S Efficient Synthesis of A-Trifluoromethyl Carboxylic Acids and Esters through Fluorocarboxylation of Gem-Difluoroalkenes. Angew. Chem. Int. Ed 2019, No. 58, 6772–6775. [DOI] [PubMed] [Google Scholar]
- (10).Riss PJ; Aigbirhio FI A Simple, Rapid Procedure for Nucleophilic Radiosynthesis of Aliphatic. Chem. Comm 2011, 47, 11873–11875. [DOI] [PubMed] [Google Scholar]
- (11).Liu C; Zhu C; Cai Y; Yang Z; Zeng H; Chen F; Jiang H Fluorohalogenation of Gem-Difluoroalkenes: Synthesis and Applications of α-Trifluoromethyl Halides. Chem. Eur. J 2019, 26, 1953–1957. [DOI] [PubMed] [Google Scholar]
- (12).Tian P; Wang C-Q; Cai S-H; Song S; Ye L; Feng C; Loh T-P F− Nucleophilic-Addition-Induced Allylic Alkylation. J. Am. Chem. Soc 2016, 138, 15869–15872. [DOI] [PubMed] [Google Scholar]
- (13).Qiao Y; Si T; Yang M; Altman RA Metal-Free Trifluoromethylation of Aromatic and Heteroaromatic Aldehydes and Ketones. J. Org. Chem 2014, 79, 7122–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lee C; Lin S Study on the Addition of Hydrogen Fluoride to 2’,2’-Difluorostyrenes. J. Chem. Res 2000, 142–144. [Google Scholar]
- (15).Nguyen BV; Burton DJ A New Route for the Preparation of Substituted 2,2-Difluorostyrenes and a Convenient Route to Substituted (2,2,2-Trifluoroethyl) Benzenes. J. Org. Chem 1997, No. 62, 7758–7764. [Google Scholar]
- (16).Tang H-J; Zhang Y-F; Jiang Y-W; Feng C F− Nucleophilic-Addition-Induced [3 + 2] Annulation: Direct Access to CF3-Substituted Indenes. Org. Lett 2018, 20, 5190–5193. [DOI] [PubMed] [Google Scholar]
- (17).Gao B; Zhao Y; Hu J AgF-Mediated Fluorinative Cross-Coupling of Two Olefins : Facile Access to α-CF3 Alkenes and ß-CF3 Ketones. Angew. Chem. Int. Ed 2015, 54, 638–642. [DOI] [PubMed] [Google Scholar]
- (18).Lin T; Pan Z; Tu Y; Zhu S; Wu H; Liu Y; Li Z; Zhang J Design and Synthesis of TY-Phos and Application in Palladium-Catalyzed Enantioselective Fluoroarylation of Gem-Difluoroalkenes. Angew. Chem. Int. Ed 2020, 59, 22957–22962. [DOI] [PubMed] [Google Scholar]
- (19).Gao B; Zhao Y; Ni C; Hu J AgF-Mediated Fluorinative Homocoupling of Gem-Difluoroalkenes. Org. Lett 2014, 16, 102–105. [DOI] [PubMed] [Google Scholar]
- (20).Yang L; Fan W; Lin E; Tan D; Li Q; Wang H Synthesis of α-CF3 and α-CF2H Amines via the Aminofluorination of Fluorinated Alkenes. Chem. Comm 2018, 54, 5907–5910. [DOI] [PubMed] [Google Scholar]
- (21).Hu J; Yang Y; Lou Z; Ni C; Hu J Fluoro-Hydroxylation of Gem-Difluoroalkenes: Synthesis of 18O-Labeled. Chin. J. Chem 2018, 36, 1202–1208. [Google Scholar]
- (22).Zhang B; Zhang X; Hao J; Yang C Palladium-Catalyzed Direct Approach to α-Trifluoromethyl Alcohols by Selective Hydroxylfluorination of Gem-Difluoroalkenes. Eur. J. Org. Chem 2018, 5007–5015. [Google Scholar]
- (23).Yamazaki T; Ueki H; Kitazume T Preparation and Regioselective Reactions of Novel Gem-Difluorinated Vinyloxiranes with Some Organometallic Reagents. Chem. Comm 2002, 2670–2671. [DOI] [PubMed] [Google Scholar]
- (24).Xiong B; Chen L; Xu J; Sun H; Li X; Li Y; Lian Z Catalytic AgF-Initiated Intramolecular 1,3-Sulfonyl Migration of Gem-Difluorovinyl Sulfonates to α,α-Difluoro-β-Ketosulfones. Org. Lett 2020, 22, 9263–9268. [DOI] [PubMed] [Google Scholar]
- (25).Ueki H; Chiba T; Yamazaki T; Kitazume T Preparation and Regioselective SN2′ Reaction of Novel Gem-Difluorinated Vinyloxiranes with RLi. J. Org. Chem 2004, 69 (9), 7616–7627. [DOI] [PubMed] [Google Scholar]
- (26).Ueki H; Chiba T; Kitazume T Regio- and Stereoselective Reductions of Gem-Difluorinated Vinyloxiranes. Org. Lett 2005, 7 (7), 1367–1370. [DOI] [PubMed] [Google Scholar]
- (27).Ueki H; Kitazume T Regio- and Stereoselective Reactions of Gem-Difluorinated Vinyloxiranes with Heteronucleophiles. J. Org. Chem 2005, 70 (4), 9354–9363. [DOI] [PubMed] [Google Scholar]
- (28).Ueki H; Chiba T; Kitazume T Synthesis of Trifluoro- or Difluoromethylated Olefins by Regio- and Stereocontrolled SN2′ Reactions of Gem-Difluorinated Vinyloxiranes. J. Org. Chem 2006, 71, 3506–3511. [DOI] [PubMed] [Google Scholar]
- (29).Ueki H; Chiba T; Yamazaki T; Kitazume T Highly Regio- and Stereocontrolled SN2’ Reactions of Gem-Difluorinated Vinyloxiranes with Monoalkylcopper Reagents. Tetrahedron 2005, 61, 11141–11147. [Google Scholar]
- (30).Christenson B; Hallnemo G; Ullenius C Stereoselective Addition of R2CuLi to Ortho-Substituted Methyl Ciunamates Intramolecular Assistance and Solvent Effects. Tetrahedron 1991, 41 (26), 4739–4152. [Google Scholar]
- (31).Ye F; Ge Y; Spannenberg A; Neumann H; Xu L-W; Beller M 3,3-Difluoroallyl Ammonium Salts: Highly Versatile, Stable and Selective Gem-Difluoroallylation Reagents. Nat. Commun 2021, No. 12, 3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Yang J; Wang J; Huang H; Qin G; Jiang Y; Xiao T Gem-Difluoroallylation of Aryl Diazoesters via Catalyst-Free, Blue- Light-Mediated Formal Doyle–Kirmse Reaction. Org. Lett 2019, No. 21, 2654–2657. [DOI] [PubMed] [Google Scholar]
- (33).Zhang J; Yang J; Cheng J Chemoselective Catalytic Hydrodefluorination of Trifluoromethylalkenes towards Mono-/Gem-Di-Fluoroalkenes under Metal-Free Conditions. Nat. comm 2021, No. 12, 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Suda M Reactions of 1,1-Difluoro-1-Olefins with Electrophilic Reagents. Tetrahedron Lett. 1980, 21, 2555–2556. [Google Scholar]
- (35).Kojima R; Akiyama S; Ito H Asymmetric Catalysis A Copper (I)-Catalyzed Enantioselective γ-Boryl Substitution of Trifluoromethyl-Substituted Alkenes: Synthesis of Enantioenriched γ,γ-Gem-Difluoroallylboronates. Angew. Chem. Int. Ed 2018, No. 57, 7196–7199. [DOI] [PubMed] [Google Scholar]
- (36).Chen JL; Scott HK; Hesse MJ; Willis CL; Aggarwal VK Highly Diastereo- and Enantioselective Allylboration of Aldehydes Using A-Substituted Allyl/Crotyl Pinacol Boronic Esters via in Situ Generated Borinic Esters. J. Am. Chem. Soc 2013, No. 135, 5316–5319. [DOI] [PubMed] [Google Scholar]
- (37).Mueller WH Thiiranium Ions as Reaction Intermediates. Angew. Chem. Int. Ed 1969, No. 7, 482–492. [Google Scholar]
- (38).Morikawa T; Kumadaki I; Shiro M Electrophilic Cyclization Reaction of Gem-Difluoroolefin Derivatives: Syntheses of 6,6-Difluorotetrahydro-2-Pyrones and 2,2-Difluorotetrahydropyran via Halogen Induced Cyclization. Chem. Pharm. Bull 1985, 33, 5144–5146. [Google Scholar]
- (39).Maji B Stereoselective Haliranium, Thiiranium and Seleniranium Ion-Triggered Friedel–Crafts-Type Alkylations for Polyene Cyclizations. Adv. Synth. Catal 2019, 361, 3453–3489. [Google Scholar]
- (40).Minegishi S; Loos R; Kobayashi S; Mayr H Kinetics of the Reactions of Halide Anions with Carbocations: Quantitative Energy Profiles for SN1 Reactions. J. Am. Chem. Soc 2005, 127, 2641–2649. [DOI] [PubMed] [Google Scholar]
- (41).Minegishi S; Kobayashi S; Mayr H Solvent Nucleophilicity. J. Am. Chem. Soc 2004, 126, 5174–5181. [DOI] [PubMed] [Google Scholar]
- (42).Schaller HF; Tishkov AA; Feng X; Mayr H Direct Observation of the Ionization Step in Solvolysis Reactions: Electrophilicity versus Electrofugality of Carbocations. J. Am. Chem. Soc 2008, 130, 3012–3022. [DOI] [PubMed] [Google Scholar]
- (43).Fujita T; Kinoshita R; Takanohashi T; Suzuki N; Ichikawa J Ring-Size-Selective Construction of Fluorine-Containing Carbocycles via Intramolecular Iodoarylation of 1,1-Difluoro-1-Alkenes. Beilstein J. Org. Chem 2017, 13, 2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Miller E; Kim S; Gibson K; Derrick S; Toste FD Regio- and Enantioselective Bromocyclization of Difluoroalkenes as a Strategy to Access Tetrasubstituted Difluoromethylene-Containing Stereocenters. J. Am. Chem. Soc 2020, 142, 8946–8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Jiang Q; Liang Y; Zhang Y; Zhao X Chalcogenide-Catalyzed Intermolecular Electrophilic Thio- and Halofunctionalization of Gem-Difluoroalkenes: Construction of Diverse Difluoroalkyl Sulfides and Halides. Org. Lett 2020, 22, 7581–7587. [DOI] [PubMed] [Google Scholar]
- (46).Yang E; Reese MR; Humphrey JM Synthesis of α,α-Difluoroethyl Aryl and Heteroaryl Ethers. Org. Lett 2012, No. 15, 3944–3947. [DOI] [PubMed] [Google Scholar]
- (47).Timperley CM; Waters MJ; Greenall JA Fluoroalkene Chemistry Part 3. Reactions of Arylthiols with Perfluoroisobutene, Perfluoropropene and Chlorotrifluoroethene. J. Fluor. Chem 2006, No. 127, 249–256. [Google Scholar]
- (48).Li J; Xu C; Wei N; Wang M Synthesis of 2,2-Difluorinated 4-Isoflavanols/4-Thioisoflavanols via a Base-Catalyzed [4 + 2] Annulation Reaction of Gem-Difluoroolefins. J. Org. Chem 2017, 82 (21), 11348–11357. [DOI] [PubMed] [Google Scholar]
- (49).Orsi DL; Easley BJ; Lick AM; Altman RA Base Catalysis Enables Access to α,α-Difluoroalkylthioethers. Org. Lett 2017, 19 (7), 1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Orsi DL; Yadav MR; Altman RA Organocatalytic Strategy for Hydrophenolation of Gem-Difluoroalkenes. Tetrahedron 2019, 75, 4325–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Sorrentino JP; Orsi DL; Altman RA Acid-Catalyzed Hydrothiolation of Gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers. J. Org. Chem 2020, 86, 2297–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Denés F; Pichowicz M; Povie G; Renaud P Thiyl Radicals in Organic Synthesis. Chem. Rev 2014, 114, 2587–2693. [DOI] [PubMed] [Google Scholar]
- (53).Oae S; Takata T; Hae Kim Y Reaction of Organic Sulfur Compounds with Superoxide Anion - III. Tetrahedron 1981, 37, 37–44. [Google Scholar]
- (54).Liu C; Zhu C; Cai Y; Jiang H Solvent-Switched Oxidation Selectivities with O2: Controlled Synthesis of α-Difluoro(Thio)Methylated Alcohols and Ketones. Angew. Chem 2021, 133, 2–10. [DOI] [PubMed] [Google Scholar]
- (55).Sharma R; Strelevitz TJ; Gao H; Clark AJ; Schildknegt K; Obach RS; Ripp SL; Spracklin DK; Tremaine LM; Vaz ADN Deuterium Isotope Effects on Drug Pharmacokinetics . I. System- Dependent Effects of Specific Deuteration with Aldehyde Oxidase Cleared Drugs. Drug Metab. Dispos 2012, 40 (3), 625–634. [DOI] [PubMed] [Google Scholar]
- (56).Mcalpine I; Tran-dube M; Wang F; Scales S; Matthews J; Collins MR; Nair SK; Nguyen M; Bian J; Alsina LM; Sun J; Zhong J; Warmus JS; O’Neill B Synthesis of Small 3-Fluoro- and 3,3-Difluoropyrrolidines Using Azomethine Ylide Chemistry. J. Org. Chem 2015, 80, 7266–7274. [DOI] [PubMed] [Google Scholar]
- (57).Purrington S; Sheu K-W ß-Lactams from 5,5-Difluoroisoxazolidines. Tetrahedron Lett. 1992, 33 (23), 3289–3292. [Google Scholar]
- (58).Breugst M; Reissig H The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition Angewandte. Angew. Chem. Int. Ed 2020, No. 59, 12293–12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Loska R; Szachowicz K; Szydlik D Synthesis of Alkyl Aryl(Heteroaryl)Acetates from N-Oxides, 1,1-Difluorostyrenes, and Alcohols. Org. Lett 2013, No. 22, 5706–5709. [DOI] [PubMed] [Google Scholar]
- (60).Martel A; Dhal R; Dujardin G N-(ß,ß-Difluorovinyl)Oxazolidin-2-Ones: First Synthesis and Application in [3 + 2]- and [4 + 2]-Cycloaddition-Type Reactions. Synlett 2009, No. 15, 2492–2496. [Google Scholar]
- (61).Lee C; Lin S Preparation of 1-Aryl-2-Chloro-3,3-Difluorocyclopropenes. Synthesis (Stuttg). 2000, 4 (4), 496–498. [Google Scholar]
- (62).Lee C; Lin S; Ke S One-Pot Process to Prepare 1,1,2,2-Tetrafluoroindane from Substituted 2,2-Difluorostyrene and Sodium Chlorodifluoroacetate. Tetrahedron 2007, 63, 120–125. [Google Scholar]
- (63).Wong HNC; Hon M; Tse C; Yip Y; Tank J; Hudlicky T Use of Cyclopropanes and Their Derivatives in Organic Synthesis. Chem. Rev 1989, 89, 165–198. [Google Scholar]
- (64).Dolbier WR Fluorinated Free Radicals. In: Chambers RD (Eds) Organofluorine Chemistry.; 1997; Vol. 192. [Google Scholar]
- (65).Tellier F; Sauvêtre R; Normant JF; Dromzee Y; Jeannin Y Réactivité Des Diènes et Styrènes Fluorés Obtenus Par Réaction de Couplage Au Palladium. Journam Organomet. Chem 1987, 331, 281–298. [Google Scholar]
- (66).Ka MD; Porco JA; Stephenson CRJ Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev 2016, 116, 9683–9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).De Cock C; Piettre S; Lahousse F; Janousek Z; MerAnyi R; Vlehe HG Olefins with Captodative Substitution in [2 + 2] Cycloadditions. Tetrahedron 1985, 41 (19), 4183–4193. [Google Scholar]
- (68).Suda M Radical Addition Reactions on 1,1-Difluoro-1-Olefins. Tetrahedron Lett. 1981, 22 (25), 2395–2396. [Google Scholar]
- (69).Jin J; Zheng W; Xia H; Zhang F; Wang Y Regioselective Radical Hydroboration of Gem-Difluoroalkenes: Synthesis of A-Borylated Organofluorines. Org. Lett 2019, 21, 8414–8418. [DOI] [PubMed] [Google Scholar]
- (70).Liu X; Lin EE; Chen G; Li J; Liu P; Wang H Radical Hydroboration and Hydrosilylation of Gem-Difluoroalkenes: Synthesis of α-Difluorinated Alkylborons and Alkylsilanes. Org. Lett 2019, 21, 8454–8458. [DOI] [PubMed] [Google Scholar]
- (71).Ashirbaev SS; Levin VV; Struchkova MI; Dilman AD Addition of Thiols to Gem-Difluoroalkenes under Photoactivation Conditions. Fluor. Notes 2017, 115, 1–2. [Google Scholar]
- (72).Levin VV; Dilman AD Visible-Light-Mediated Organocatalyzed Thiol–Ene Reaction Initiated by a Proton-Coupled Electron Transfer. J. Org. Chem 2019, 84, 8337–8343. [DOI] [PubMed] [Google Scholar]
- (73).Zubkov MO; Kosobokov MD; Levin VV; Kokorekin VA; Korlyukov AA; Hu J; Dilman AD A Novel Photoredox-Active Group for the Generation of Fluorinated Radicals from Difluorostyrenes. Chem. Sci 2020, 11, 737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Fedorov OV; Scherbinina SI; Levin VV; Dilman AD Light-Mediated Dual Phosphine-/Copper-Catalyzed Atom Transfer Radical Addition Reaction. J. Org. Chem 2019, 84, 11068–11079. [DOI] [PubMed] [Google Scholar]
- (75).Chen G; Li C; Peng J; Yuan Z; Liu P; Liu X Silver-Promoted Decarboxylative Radical Addition/Annulation of Oxamic Acids with Gemdifluoroolefins: Concise Access to CF2-Containing 3,4-Dihydroquinolin-2-Ones. Org. Biomol. Chem 2019, 17, 8527–8532. [DOI] [PubMed] [Google Scholar]
- (76).Orsi DL; Douglas JT; Sorrentino JP; Altman RA Cobalt-Catalyzed Selective Unsymmetrical Dioxidation of Gem-Difluoroalkenes. J. Org. Chem 2020, 85, 10451–10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Spier E; Neuenschwander U; Hermans I Insights into the Cobalt(II)-Catalyzed Decomposition of Peroxide. Angew. Chem. Int. Ed 2013, 52, 1581. [DOI] [PubMed] [Google Scholar]
- (78).Nishinaga A; Yamato H; Abe T; Maruyama K; Matsuura T Asymmetric Induction in Oxygenation of Styrene Catalyzed by Cobalt Schiff Base Complex. Tetrahedron Lett. 1988, 29, 6309. [Google Scholar]
- (79).Lončarević D; Krstić J; Dostanić J; Manojlović D; Čupić Ž; Jovanović DM Cyclohexane Oxidation and Cyclohexyl Hydroperoxide Decomposition by Poly(4-Vinylpyridine-Co-Divinylbenzene) Supported Cobalt and Chromium Complexes. Chem. Eng. J 2010, 157, 181. [Google Scholar]
- (80).Talsi EP; Chinakov VD; Babenko VP; Sidelnikov VN; Zamaraev KI Role of Alkylperoxo Complexes of Cobalt (III) in Catalytic Oxidation of Cyclohexane by Cumene Hydroperoxide in the Presence of Bis (Acetylacetonato) Cobalt (II). Mol. Catal 1993, 81, 215. [Google Scholar]
- (81).Nihei T; Iwai N; Matsuda T; Kitazume T Stereocontrolled Synthesis of ß-Difluoromethylated Materials. J. Org. Chem 2005, No. 8, 5912–5915. [DOI] [PubMed] [Google Scholar]
- (82).Markl M; Schaper W; Ort O; Jakobi H; Braun R; Krautstrunk G; Sanft U; Bonin W; Stark H; Pasenok S; Cabrera I Preparation of Substituted Cyclohexylaminopyrimidines as Pesticides. WO 2000007998, 2000. [Google Scholar]
- (83).Kitazume T; Ohnogi T; Miyauchi H; Yamazaki T; Watanabe S Ultrasound-Promoted Synthesis of α-Difluoromethylated Carboxylic Acids. J. Org. Chem 1989, 54 (23), 5630–5632. [Google Scholar]
- (84).Cai Y; Tan D; Zhang Q; Lv W; Li Q; Wang H Synthesis of Difluoromethylated Benzylborons via Rhodium(I)-Catalyzed Fluorine-Retainable Hydroboration of Gem-Difluoroalkenes. Chinese Chem. Lett 2021, 32, 417–420. [Google Scholar]
- (85).Zhao H; Ariafard A; Lin Z ß-Heteroatom versus ß-Hydrogen Elimination: A Theoretical Study. Organometallics 2006, 25 (5), 812–819. [Google Scholar]
- (86).Zhang B; Zhang X Pd-Catalyzed Gem-Difluoroallylation of Arylboronic Acids with γ,γ-Difluoroallylic Acetates. Chem. Commun 2015, 52, 1238–1241. [DOI] [PubMed] [Google Scholar]
- (87).Yuan K; Feoktistova T; Cheong PH; Altman RA Chemical Science Arylation of Gem-Difluoroalkenes Using a Pd/Cu Co-Catalytic System That Avoids ß-Fluoride. Chem. Sci 2021, 12, 1363–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Liu J; Yang J; Ferretti F; Jackstell R; Beller M Pd-Catalyzed Selective Carbonylation of Gem-Difluoroalkenes: A Practical Synthesis of Difluoromethylated Esters. Angew. Chem. Int. Ed 2019, 58, 4690–4694. [DOI] [PubMed] [Google Scholar]