Abstract
When an emerging acute infectious disease occurs, travel restrictions, one-way or two-way, are often taken to prevent its global spread. In order to investigate the impact of two-way travel restrictions in the global spread of infectious diseases, this paper defines a risk indicator according to the relative infection density. Based on this risk indicator and an intervention time on two-way travel restrictions, we define an adaptive metapopulation network. Then a susceptible-infectious-removed (SIR) metapopulation model on this network is proposed. The mathematical analysis shows that the basic reproduction number is independent of human mobility. Furthermore, this essay compares the effects of one-way travel restrictions and two-way travel restrictions on the global spread of infectious diseases. It is shown that the adaptive metapopulation network under two-way travel restrictions can effectively suppress the global spread of infectious diseases. We also obtain a threshold of risk indicator to prevent the global spread of infectious diseases by simulations. The earlier the intervention time on two-way travel restriction is, the better to curb the global spread of the disease. Even if two-way travel restrictions are not implemented, controlling the mobility of infectious persons would help prevent the global spread of the disease. This work will throw lights on the prevention and control of the globally spreading of an emerging infectious disease.
Keywords: Emerging infectious diseases, travel restrictions, adaptive metapopulation network, risk indicator
I. Introduction
Whether in the past or at present, infectious diseases have always been serious threats to human life and health. In the 1340s, the black death swept across Europe, killing about 25 million Europeans, a third of the population. From May 2015 to June 2016, the Zika virus spread to 40 countries and territories within the Americas in one year [1]. Furthermore, with the rapid development of globalization, developed transportation makes long-distance travel more and more convenient. However, the mobility of infectious individuals facilitates the global spread of infectious diseases. As of August 9, 2020, the coronavirus disease 2019 (COVID-19) has spread over 215 countries and territories, infected more than 19 million people and caused more than 720,000 deaths. The outbreak of any infectious diseases has a significant impact on humans, either physically, mentally, or economically. Modeling and controlling the global spread of infectious diseases has always been the focus of researches. One of the major models to study the global spread of infectious diseases is a metapopulation network model. For a metapopulation network, each node (subpopulations) represent a country, a city or a town, and individuals in each subpopulation are well-mixed. The mobility of individuals between two connected subpopulations forms the link of the network.
Colizza and Vespignani built heterogeneous mean-field models to describe the transmission of diseases on heterogeneous metapopulation networks under two kinds of mobility patterns: traffic dependent mobility rate and population dependent mobility rate [2]. Their work lays a foundation for future researches on metapopulation networks. From the point of view of network structure, Cao et al. studied the rendezvous effects on bipartite metapopulation network and found that rendezvous effects made for the transmission of infectious diseases [3]; Liu et al. investigated time-varying metapopulation networks, which slowed down the spread of infectious diseases [4]; Mata et al. studied local subpopulation structure on metapopulation networks, that is, individuals within a subpopulation were not well-mixed but within a social network [5]; we defined a second-neighbor network (SNN) and investigated the global spread of infectious disease on a metapopulation network coupled with its SNN [6]. In order to avoid being infected, individuals may respond to infectious diseases. Sandro et al. discovered that the adaptive behavior of individuals contributed to the global spreading of diseases [7]. Similar results were obtained in [8]. These two works are based on the same assumption that the higher the relative density of infectious individuals at the destination subpopulation is, the less likely the individuals will travel. In recent years, with the development of big data technology, more and more fine-grained data can be obtained. The metapopulation network model based on these data has achieved great success. Panigutti et al. studied recurrent mobility patterns on metapopulaion networks using census data and mobile phone data [9]. Pei et al. forecast the spread of influenza in the United States using a metapopulation model [10]. Chinazzi et al. used the Global Epidemic and Mobility Model (GLEAM) to forecast the effect of travel limitations on the national and international spread of COVID-19 [11].
When an emerging acute infectious disease occurs, in order to prevent its global spread, each subpopulation (maybe a country, a city, or a town) will assess the risk of disease invasion and timely prevent and control it. One way to prevent the global spread of infectious diseases is to restrict travel, which is a problem of broken links in metapopulation networks. There are two main types of travel restrictions: two-way travel restrictions and one-way travel restrictions. Two-way travel restrictions refer to cutting off the links between the connected subpopulations, with no individuals moving between them. One-way travel restrictions include two types. One is to prevent individuals in a subpopulation from moving into its neighbor subpopulations. The other is to prevent the move-in from individuals in its neighbor subpopulation. Travel restrictions between a subpopulation and its neighbor subpopulations may be on some neighbor subpopulations or on all neighbor subpopulations. City (or country) lockdown is a two-way travel restriction on all neighbor subpopulations. During COVID-19, the municipal government of Wuhan decided to lock down the city on January 23; nation-wide lockdown was implemented on March 10, in Italy; subsequently, Spain, the Czech Republic, France, Belgium announced the nation-wide lockdown.
Until now, the majority of research on metapopulation network models have been devoted to the effect of different network structures, safety-driven one-way travel restrictions, and transmission prediction. Little work has been done on two-way travel restrictions. This paper focuses on the problem of two-way travel restrictions on all neighbor subpopulations, and puts forward a susceptible-infectious-removed (SIR) metapopulation model to study the global transmission of an emerging infectious disease. Using the relative density of the infectious, we define a risk indicator 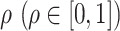 that divides all subpopulations into three categories: risk-free, low-risk, and high-risk. For a subpopulation, if there are no infectious persons, it is a risk-free subpopulation. If the number of infectious persons is larger than 0, and the relative density of infectious persons in the subpopulation is lower than
that divides all subpopulations into three categories: risk-free, low-risk, and high-risk. For a subpopulation, if there are no infectious persons, it is a risk-free subpopulation. If the number of infectious persons is larger than 0, and the relative density of infectious persons in the subpopulation is lower than  , then the subpopulation is a low-risk subpopulation. If the relative density of infectious persons in the subpopulation is no less than
, then the subpopulation is a low-risk subpopulation. If the relative density of infectious persons in the subpopulation is no less than  , it is a high-risk subpopulation. Since the relative infection density of in a subpopulation varies over time, the risk level of the subpopulation varies over time. Let
, it is a high-risk subpopulation. Since the relative infection density of in a subpopulation varies over time, the risk level of the subpopulation varies over time. Let 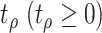 be the minimal time when a subpopulation goes from low risk to high risk, and
be the minimal time when a subpopulation goes from low risk to high risk, and 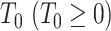 be the intervention time on two-way travel restriction. We consider that
be the intervention time on two-way travel restriction. We consider that 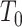 for each subpopulation is the same and that it is independent of the risk level of subpopulations. So the relationship between
for each subpopulation is the same and that it is independent of the risk level of subpopulations. So the relationship between 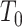 and
and 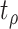 is uncertain. That is,
is uncertain. That is, 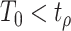 ,
, 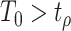 and
and 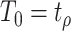 are all possible. Fig. 1 shows the case of
are all possible. Fig. 1 shows the case of 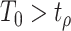 . Based on the risk indicator
. Based on the risk indicator 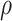 and the intervention time on two-way travel restriction
and the intervention time on two-way travel restriction 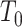 , we define an adaptive metapopulation network (see Fig. 2). An adaptive metapopulation network is a metapopulation network, whose links will be broken if one of the two connected subpopulations is high-risk after
, we define an adaptive metapopulation network (see Fig. 2). An adaptive metapopulation network is a metapopulation network, whose links will be broken if one of the two connected subpopulations is high-risk after 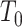 , and then will be reconnected when the high-risk subpopulation becomes low-risk after
, and then will be reconnected when the high-risk subpopulation becomes low-risk after 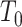 . Note that links broken and reconnected on metapopulation networks refer to the link weights, not the underlying network. The adaptive metapopulation network is equivalent to two-way travel restrictions on all neighbor subpopulations. Results show that adaptive metapopulation networks can effectively curb the global spread of infectious diseases.
. Note that links broken and reconnected on metapopulation networks refer to the link weights, not the underlying network. The adaptive metapopulation network is equivalent to two-way travel restrictions on all neighbor subpopulations. Results show that adaptive metapopulation networks can effectively curb the global spread of infectious diseases.
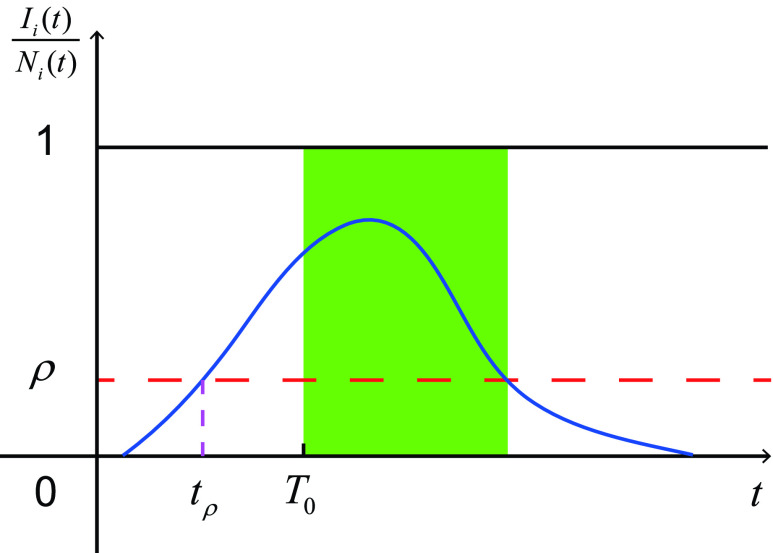
FIGURE 1.
The schematic of the intervention time on two-way travel restriction 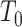 . The red dashed line is the risk indicator. The blue line is the time series of the relative infection density of subpopulation
. The red dashed line is the risk indicator. The blue line is the time series of the relative infection density of subpopulation 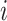 , i.e.,
, i.e., 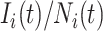 . The shaded green area indicates the start and end time of travel restrictions implemented on subpopulation
. The shaded green area indicates the start and end time of travel restrictions implemented on subpopulation 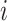 .
. 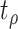 is the minimal time when the subpopulation goes from low risk to high risk.
is the minimal time when the subpopulation goes from low risk to high risk.
FIGURE 2.
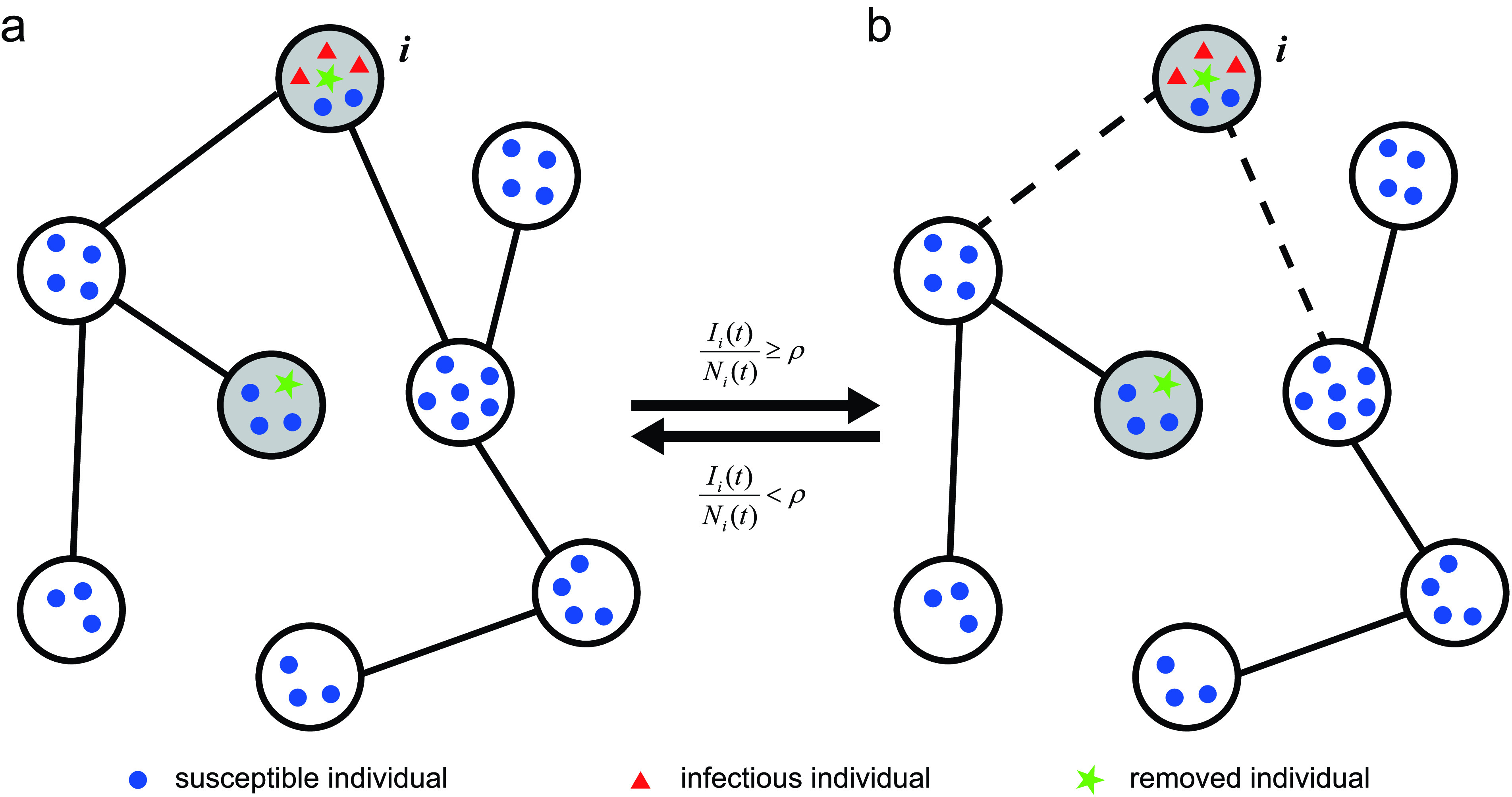
The schematic depiction of an adaptive metapopulation network. Blue circles represent susceptible individuals, red triangles represent infectious individuals, and a green pentagram represents a removed individual. The dashed lines mean that there is no mobility of individuals between the two connected subpopulation, which is equivalent to broken links due to two-way travel restriction. The time  .
.
The paper is organized as follows. In Sec. II, according to the definition of adaptive metapopulation network, an SIR metapopulation model is built. We make a mathematical analysis on this model and calculate the basic reproduction number and final size in Sec. III. In Sec. IV, we simulate the spread of an SIR infectious disease on adaptive metapopulation networks. Conclusions and discussion are given in Sec. V.
II. Adaptive Metapopulation Network Modeling
In order to investigate the global transmission of an SIR infectious disease under two-way travel restriction on all neighbor subpopulations, we consider a connected adaptive metapopulation network with 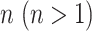 nodes, and label its nodes with the elements in a set
nodes, and label its nodes with the elements in a set 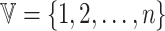 . The adjacency matrix
. The adjacency matrix 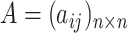 of underlying network is a matrix with entries
of underlying network is a matrix with entries
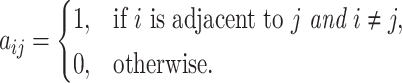 |
Individuals in each subpopulation are divided into three types, susceptible (S), infectious (I) and removed (R). Removed individuals represent those who have recovered, or died. In an unit time, the susceptible may be infected by the infectious at the transmission rate 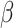 and become infectious. And an infectious individual is removed (recovered or died) at removed rate
and become infectious. And an infectious individual is removed (recovered or died) at removed rate 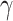 . For subpopulation
. For subpopulation 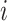 ,
, 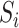 ,
, 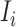 and
and 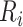 are the numbers of susceptible, infectious, removed individuals, respectively.
are the numbers of susceptible, infectious, removed individuals, respectively. 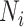 is the number of individuals and
is the number of individuals and 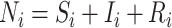 . Subpopulations are also divided into four types according to the states of individuals within subpopulations. For subpopulation
. Subpopulations are also divided into four types according to the states of individuals within subpopulations. For subpopulation 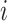 , if there are only susceptible individuals, i.e.,
, if there are only susceptible individuals, i.e., 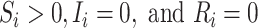 , it is called risk-free susceptible subpopulation (FSS); if the number of infectious individuals equals 0, and the number of removed individuals is larger than 0, i.e.,
, it is called risk-free susceptible subpopulation (FSS); if the number of infectious individuals equals 0, and the number of removed individuals is larger than 0, i.e., 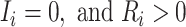 , it is called a risk-free recovery subpopulation (FRS); if the number of the infectious is greater than 0, and the relative density of the infectious is no less than
, it is called a risk-free recovery subpopulation (FRS); if the number of the infectious is greater than 0, and the relative density of the infectious is no less than 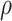 i.e.,
i.e., 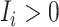 , and
, and 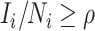 , it is called high-risk infectious subpopulation (HIS); if
, it is called high-risk infectious subpopulation (HIS); if 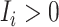 , and
, and 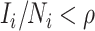 , it is called low-risk infectious subpopulation (LIS). HIS and LIS are collectively referred to as infectious subpopulations (IS). Note that the removed individuals in a FRS may either be removed from an infectious individual internally, or travel from neighbor subpopulations.
, it is called low-risk infectious subpopulation (LIS). HIS and LIS are collectively referred to as infectious subpopulations (IS). Note that the removed individuals in a FRS may either be removed from an infectious individual internally, or travel from neighbor subpopulations.
Assuming that individuals travel randomly, we build an ordinary differential equation model as follows
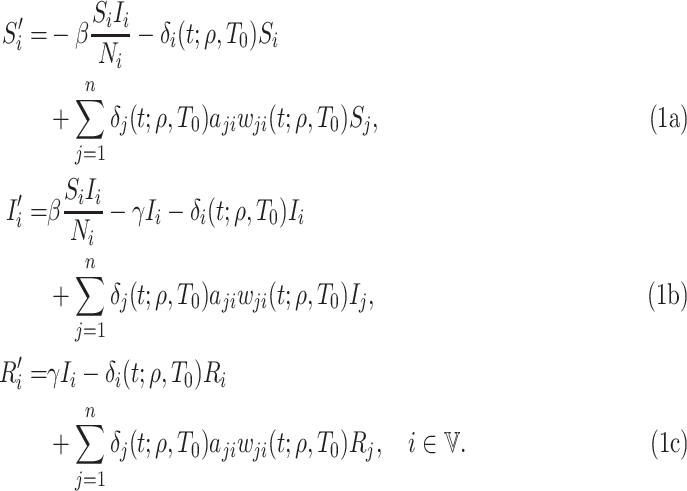 |
Here 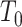 is the intervention time on two-way travel restrictions (shown in Fig. 1);
is the intervention time on two-way travel restrictions (shown in Fig. 1);  represents mobility rate, which depends on the risk indicator
represents mobility rate, which depends on the risk indicator  and the intervention time for travel restrictions
and the intervention time for travel restrictions 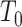 ; square matrix
; square matrix 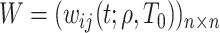 is link weights of the adaptive metapopulation network, which also depends on
is link weights of the adaptive metapopulation network, which also depends on 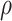 and
and 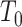 . Letting
. Letting 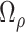 be the set of low-risk and risk-free subpopulations, i.e.,
be the set of low-risk and risk-free subpopulations, i.e., 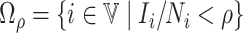 , we have
, we have
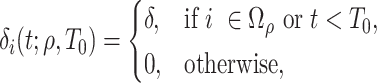 |
and
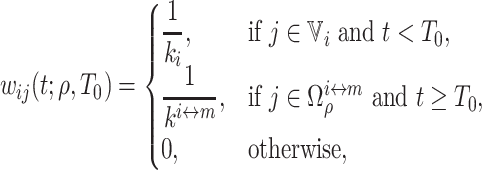 |
where 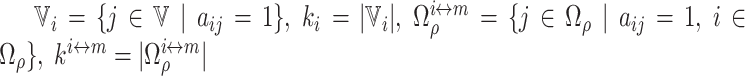 .
.
Consider two extreme cases: 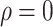 and
and 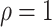 .
. 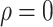 is equivalent to the case where the number of infectious individuals is zero. That is, whenever an infectious person is present in a subpopulation, it is high-risk.
is equivalent to the case where the number of infectious individuals is zero. That is, whenever an infectious person is present in a subpopulation, it is high-risk. 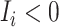 is unreasonable. Thus, when
is unreasonable. Thus, when 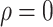 , there are
, there are
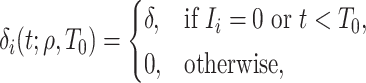 |
and
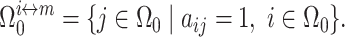 |
Obviously, 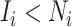 for any
for any 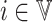 at any time, when
at any time, when 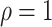 ,
,
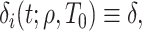 |
and
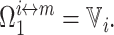 |
In this case, all IS are low-risk, and the two-way travel restriction makes no sense.
From the perspective of controlling and preventing the global spread of an emerging infectious disease, an adaptive metapopulation network is equivalent to a control strategy which limits the two-way travel (abbreviated as In-Out). Besides, in order to prevent and control the global spread of an emerging infectious disease, there are two other major one-way travel restrictions. One limits the move-out of individuals in IS (abbreviated as Out). The other prevents individuals in the neighbor subpopulations of IS from traveling to IS (abbreviated as In). Travel restrictions In and Out only cut off the one-way movement, while traveling restriction In-Out the two-way movement. City (or country) lockdown is equivalent to travel restriction In-Out.
For travel restriction Out, mobility rates and link weights behave as
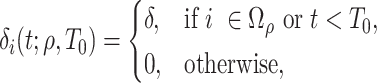 |
and
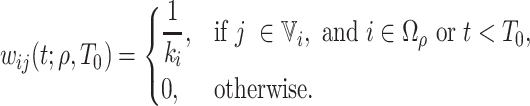 |
With regard to travel restriction In, mobility rates and link weights become
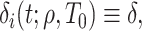 |
and
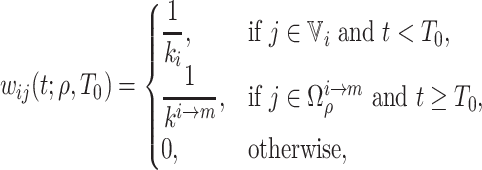 |
where 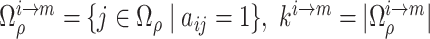 .
.
III. Mathematical Analysis
In this section, we will calculate the equilibria, the basic reproduction number and the final size of model (1).
A. Equilibria
On the one hand, summing (1a)–(1c) over  , we obtain
, we obtain
 |
where 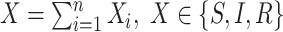 . Letting the right side of (2) equal to 0, we have
. Letting the right side of (2) equal to 0, we have
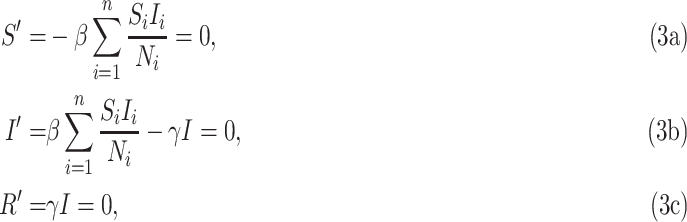 |
Since for any 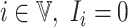 , according to (3c),
, according to (3c), 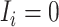 for any
for any 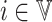 at equilibria.
at equilibria.
On the other hand, summing (1a)–(1c) gives
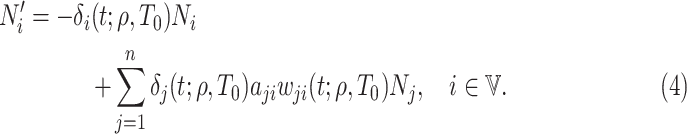 |
Define 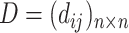 with entries
with entries
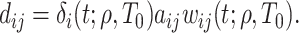 |
With 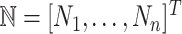 and
and 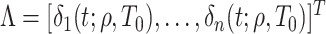 , (4) can be rewritten as
, (4) can be rewritten as
 |
where 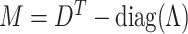 , and
, and 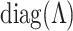 is a
is a 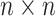 matrix, whose diagonal elements forming the vector
matrix, whose diagonal elements forming the vector 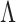 .
.
Since for any 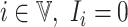 at equilibria,
at equilibria, 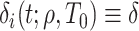 ,
, 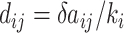 , and
, and 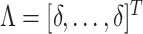 . Note that each column sum of
. Note that each column sum of 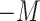 is zero, that is,
is zero, that is, 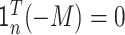 , where the
, where the 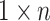 vector
vector  . Thus, matrix
. Thus, matrix  is a singular M-matrix. From (5), letting
is a singular M-matrix. From (5), letting 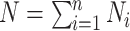 , we obtain that the total population
, we obtain that the total population 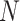 is constant (because
is constant (because 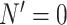 ). Subject to this constraint, by Theorem 3.3 in [13], we show that (5) has a unique positive equilibrium
). Subject to this constraint, by Theorem 3.3 in [13], we show that (5) has a unique positive equilibrium 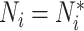 , which is globally asymptotically stable.
, which is globally asymptotically stable.
Accordingly, for any 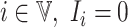 and
and 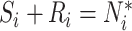 at equilibria. If for all
at equilibria. If for all 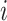 ,
, 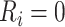 , the equilibrium is a disease-free equilibrium; otherwise we call the equilibrium as epidemic equilibrium.
, the equilibrium is a disease-free equilibrium; otherwise we call the equilibrium as epidemic equilibrium.
Next, we calculate the basic reproduction number.
B. The Basic Reproduction Number
We calculate the basic reproduction number 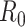 following the approach of van den Driessche and Watmough [14].
following the approach of van den Driessche and Watmough [14]. 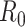 indicates the number of people infected by an infectious individual during his average period of illness at the beginning of the disease, when all are susceptible. Obviously, there exists a disease-free equilibrium
indicates the number of people infected by an infectious individual during his average period of illness at the beginning of the disease, when all are susceptible. Obviously, there exists a disease-free equilibrium 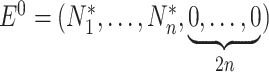 for (1). According to (1), the rate of appearance of new infections
for (1). According to (1), the rate of appearance of new infections  and the rate of transfer of individuals out of the compartments
and the rate of transfer of individuals out of the compartments  in the
in the 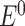 are given by
are given by
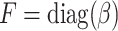 |
and
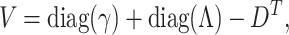 |
here 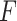 and
and 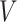 are
are 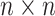 matrices. Using the next-generation matrix theory [14], the basic reproduction number is
matrices. Using the next-generation matrix theory [14], the basic reproduction number is 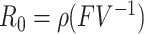 , where
, where 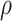 is the spectral radius of the matrix
is the spectral radius of the matrix 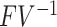 .
.
In the following, we calculate 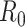 . Note that the sum of each column of matrix
. Note that the sum of each column of matrix 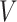 is
is 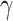 and the matrix
and the matrix 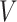 is column diagonally dominant. So
is column diagonally dominant. So 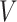 is an irreducible nonsingular M-matrix. Thus
is an irreducible nonsingular M-matrix. Thus 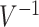 is a positive matrix.
is a positive matrix.
Matrix 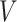 has column sum
has column sum 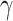 , i.e.,
, i.e., 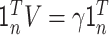 . Hence
. Hence 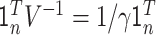 . Therefore,
. Therefore, 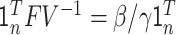 , that is, matrix
, that is, matrix 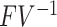 has column sum
has column sum 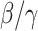 . By Theorem 1.1 in chapter 2 in [15], the basic reproduction number is
. By Theorem 1.1 in chapter 2 in [15], the basic reproduction number is
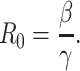 |
Obviously, 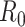 depends only on disease parameters
depends only on disease parameters 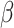 and
and 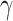 , rather than on mobility rate and network structure. What is more,
, rather than on mobility rate and network structure. What is more, 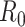 equals to the basic reproduction number
equals to the basic reproduction number 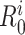 for each subpopulation
for each subpopulation 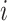 when there is no travel. This is because that transmission rate
when there is no travel. This is because that transmission rate 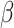 and removed rate
and removed rate 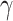 for each subpopulation
for each subpopulation 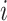 keep the same.
keep the same.
C. Final Size
For each subpopulation 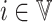 , given initial conditions
, given initial conditions 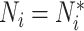 , and without consideration of travel restriction, dividing (2a) by (2c), we have
, and without consideration of travel restriction, dividing (2a) by (2c), we have
 |
On the one hand,
 |
where 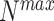 is the maximum of
is the maximum of 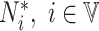 . Dividing the right side of (7) to the left side, multiplying
. Dividing the right side of (7) to the left side, multiplying 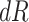 to the right side, and integrating both sides we obtain
to the right side, and integrating both sides we obtain
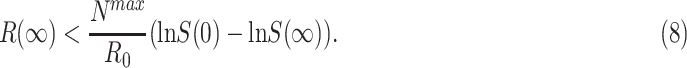 |
Here 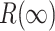 is the final size of epidemic,
is the final size of epidemic, 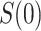 is the initial susceptible individuals in the metapopulation network,
is the initial susceptible individuals in the metapopulation network, 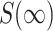 gives the number of susceptible individuals who escape the epidemic, and
gives the number of susceptible individuals who escape the epidemic, and 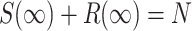 . On the other hand,
. On the other hand,
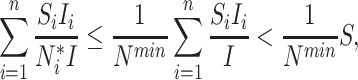 |
so
 |
Here 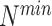 is the minimum of
is the minimum of 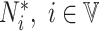 . Similar to (7), we have
. Similar to (7), we have
 |
Therefore, without consideration of travel restriction, the final size satisfies
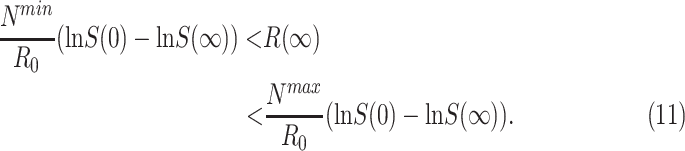 |
When 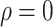 , and
, and 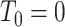 , infectious disease will outbreak only in the initial infectious subpopulation, not globally. Labeling the initial infectious subpopulation by
, infectious disease will outbreak only in the initial infectious subpopulation, not globally. Labeling the initial infectious subpopulation by 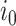 , we have
, we have
 |
With regard to other subpopulations in the adaptive metapopulation network, there is no infection, just the susceptible’ mobility among susceptible subpopulations. So the final size of whole network equals to the final size of infectious subpopulation 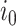 . Dividing (12c) by (12a) we have
. Dividing (12c) by (12a) we have
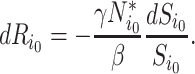 |
Integrating both sides we obtain
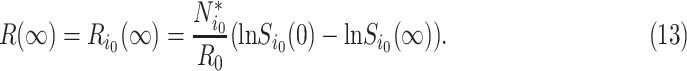 |
Here 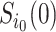 and
and 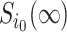 are the numbers of initial susceptible individuals and susceptible individuals who escape the disease in subpopulation
are the numbers of initial susceptible individuals and susceptible individuals who escape the disease in subpopulation 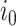 , respectively.
, respectively.
It is difficult to compare Eqs. (11) and (13) in theory. In the next section, we will simulate these two cases and compare their final sizes.
IV. Monte Carlo Simulation Results
In this section, we simulate an SIR infectious disease on two kinds of adaptive metapopulation networks with the same average degree 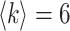 , and average population
, and average population 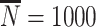 . The generation of metapopulation networks is following Molloy and Reed algorithm [17]. Parameters
. The generation of metapopulation networks is following Molloy and Reed algorithm [17]. Parameters 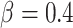 ,
, 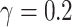 and
and 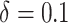 . For subpopulation
. For subpopulation 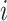 , its initial population is
, its initial population is
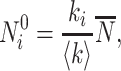 |
where 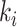 is the degree of subpopulation
is the degree of subpopulation 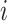 . All values in the figures of this section are obtained by averaging over 100 stochastic realizations.
. All values in the figures of this section are obtained by averaging over 100 stochastic realizations.
A. The Comparison of One-Way and Two-Way Travel Restrictions
In this subsection, we compare the impact of three kinds of travel restrictions: In-Out, In and Out, on the global spread of infectious diseases.
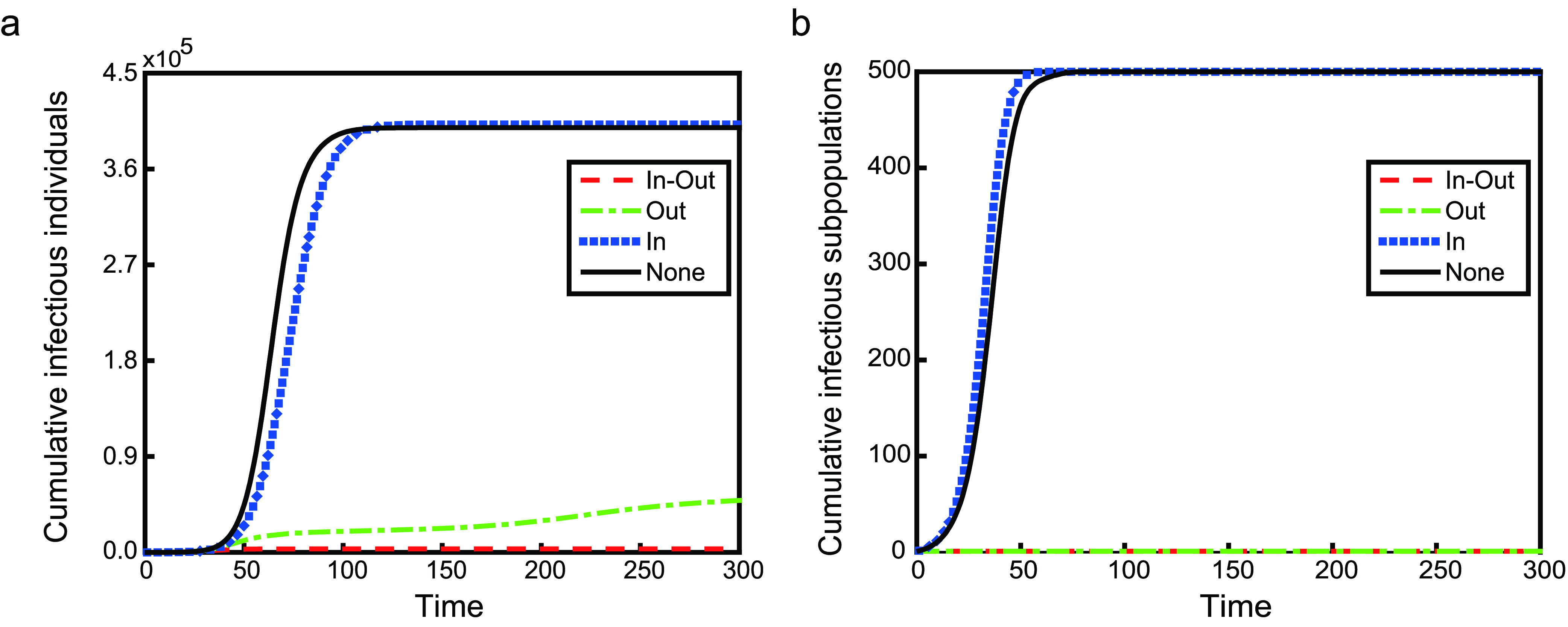
As shown in Figs. 3 and 4, we simulate an SIR transmission process on two kinds of metapopulation network with 500 subpopulations under three kinds of travel restrictions. Fig. 3 is the case on a poisson metapopulation network, and Fig. 4 is the case on a power-law metapopulation network. In these two figures, the legend None, a reference, shows the case without any travel restriction or the case where 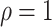 . For travel restrictions In-Out, In and Out,
. For travel restrictions In-Out, In and Out, 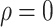 . The left panels show the number of cumulative infectious individuals evolving over time, while the right panels are the number of cumulative infectious subpopulations. There are five infectious individuals in a subpopulation with the maximum degree in the initial time.
. The left panels show the number of cumulative infectious individuals evolving over time, while the right panels are the number of cumulative infectious subpopulations. There are five infectious individuals in a subpopulation with the maximum degree in the initial time.
FIGURE 3.
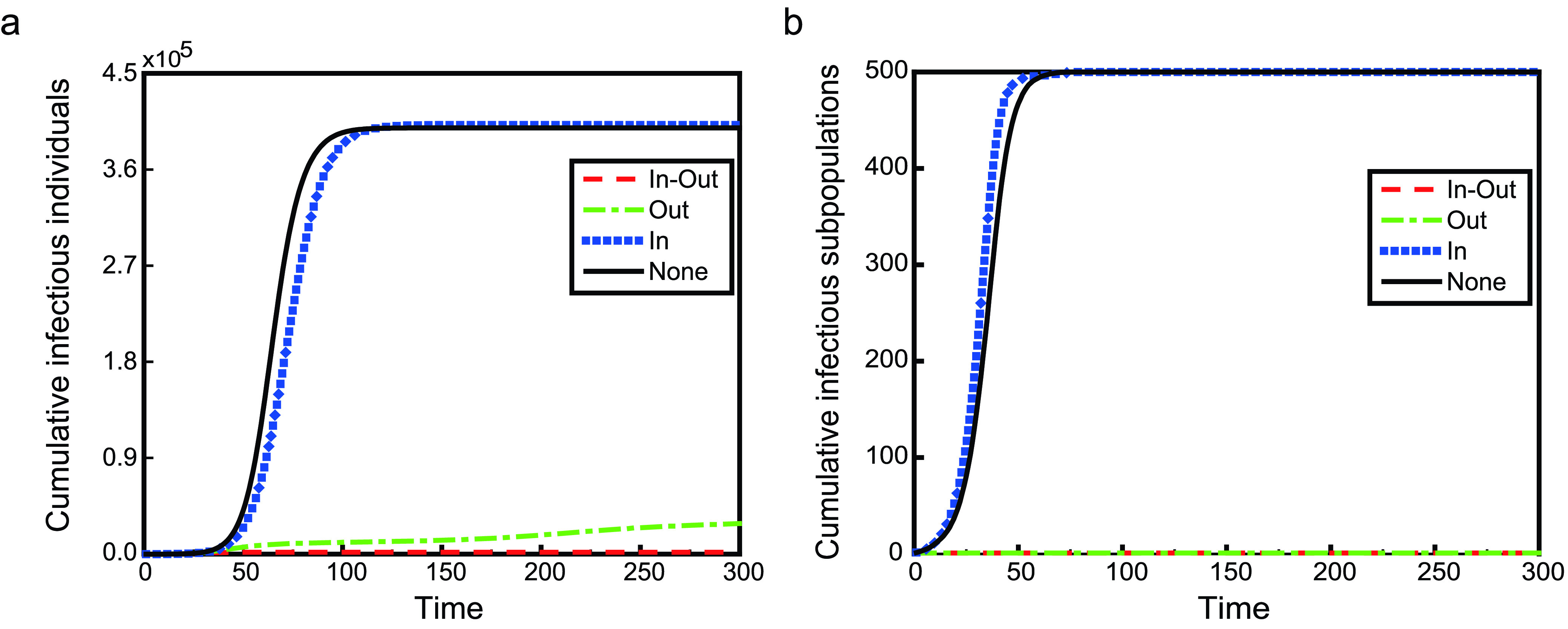
The time evolution of the number of cumulative infectious individuals and subpopulations on a poisson metapopulation network. Control strategies are In-Out (red dashed lines), Out (green dot-dashed lines), In (blue dot lines), and None (black solid lines), individually. The panel a shows the number of cumulative infectious individuals evolving over time, while the panel b is the number of cumulative infectious subpopulations. Parameter 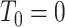 .
.
FIGURE 4.
The time evolution of the number of cumulative infectious individuals and subpopulations on a power-law metapopulation network. Others are similar to Fig. 3.
Among three travel restrictions, In is the worst, because it accelerates the global spread of infectious diseases, not only the speed of global transmission but also the final size. Although the number of cumulative infectious subpopulations for travel restrictions In-Out and Out are both one, the situation is quite different. Travel restriction In-Out can effectively curb the global spread of infectious diseases, and the disease will not spread to other individuals in other subpopulations. The final size of In-Out is less than 1% of that of None. In the short term, both travel restrictions are of benefit to preventing the global spread of infectious diseases. However, in the long term, the infectious disease will globally spread in the sense of the number of cumulative infectious individuals under the case of travel restriction Out (shown as red dashed lines in Fig. 5). Nevertheless, the final size of Out is about 60% lower than that of None. Therefore, preventing individuals in the infectious subpopulations from entering their neighbors is also a relatively effective prevention and control measure of global transmission.
FIGURE 5.
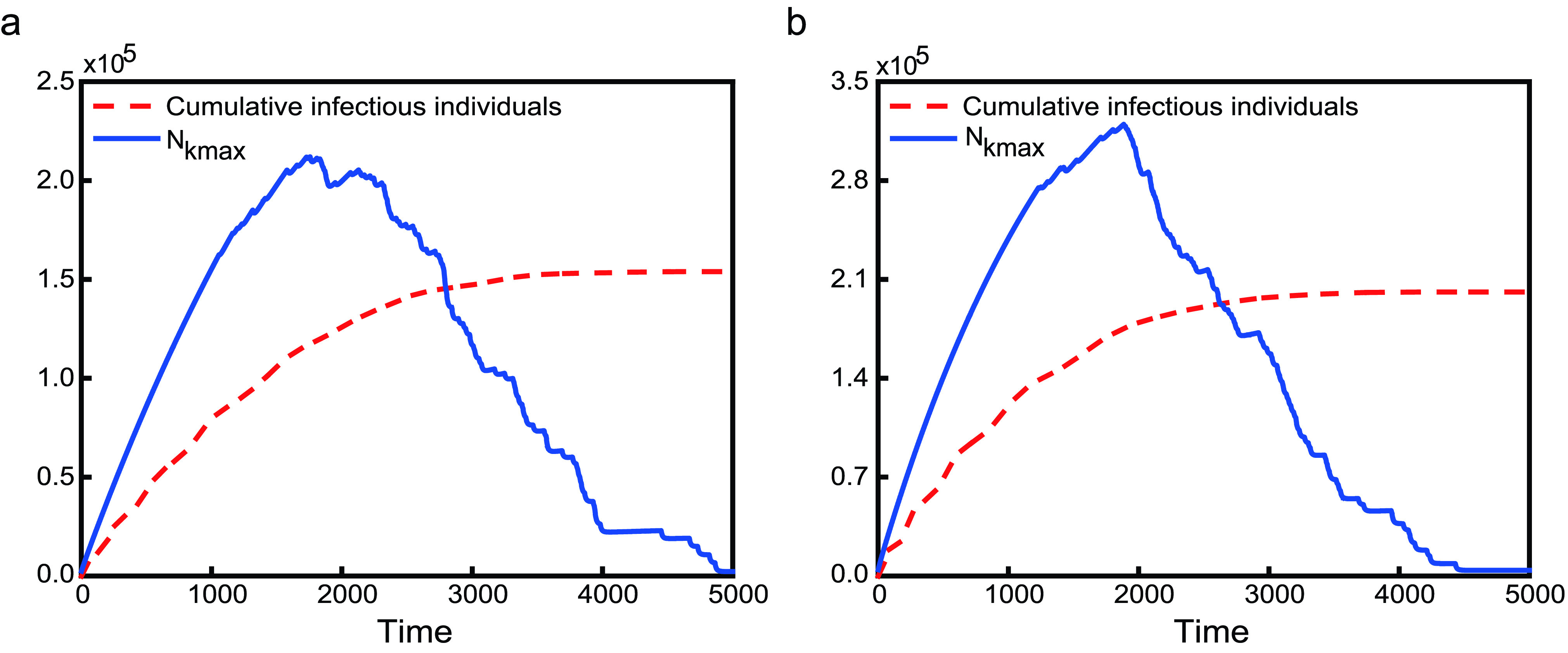
The time evolution of the number of cumulative infectious individuals and the population of the subpopulation with the maximum degree under restriction Out on metapopulation networks. They are illustrated by red dashed lines and blue solid lines, individually. The left panel shows the case of poisson network, while the right panel is of power-law degree distribution.
Why is only controlling one-way movement not the best measure of suppressing the global spread of disease? This is due to the fact that the mobility between subpopulations with infectious individuals and without is asymmetric. In theory, for the subpopulation with the maximum degree (labeled by 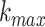 ), when there exists(exist) infectious individual(s), the dynamical equations for its population are
), when there exists(exist) infectious individual(s), the dynamical equations for its population are
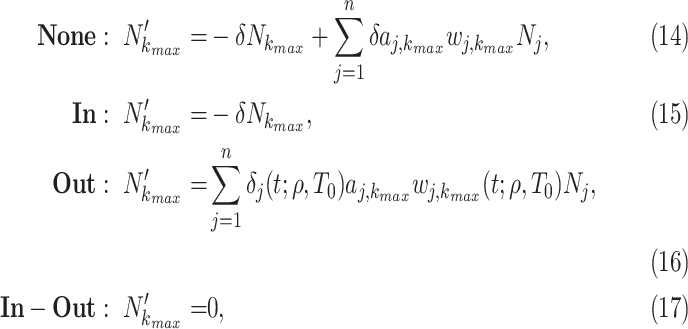 |
respectively. Obviously, the difference between In and None is that there is no any individuals moving from the neighbor subpolulation(s) of subpolulation 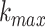 to itself. However, In does not restrict the mobility of infectious individuals, which leads to more susceptible subpopulations being infected. Although travel restrictions In-Out and Out can effectively prevent infectious individuals in infectious subpopulations from entering to their neighbor subpopulations, they are different. For In-Out, there is no any individuals moving between subpolulation
to itself. However, In does not restrict the mobility of infectious individuals, which leads to more susceptible subpopulations being infected. Although travel restrictions In-Out and Out can effectively prevent infectious individuals in infectious subpopulations from entering to their neighbor subpopulations, they are different. For In-Out, there is no any individuals moving between subpolulation 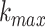 and its neighbor subpolulation(s). While for Out, susceptible individuals go from the neighbor subpolulation(s) of subpolulation
and its neighbor subpolulation(s). While for Out, susceptible individuals go from the neighbor subpolulation(s) of subpolulation 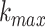 to itself until
to itself until 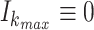 , which leads to more susceptible individuals being infected in subpopulation
, which leads to more susceptible individuals being infected in subpopulation 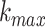 . Travel restrictions Out and In break the balance of the mobility. Under the case of travel restriction Out, for subpopulation
. Travel restrictions Out and In break the balance of the mobility. Under the case of travel restriction Out, for subpopulation 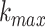 , susceptible individuals from its neighbor subpopulations travel to it; susceptible individuals from its second neighbor subpopulation(s) travel to its neighbor subpopulations first and then travel to subpopulation
, susceptible individuals from its neighbor subpopulations travel to it; susceptible individuals from its second neighbor subpopulation(s) travel to its neighbor subpopulations first and then travel to subpopulation 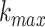 ; susceptible individuals in the third neighbor subpopulation(s) will transfer the second neighbor(s) and the neighbors will eventually enter subpopulation
; susceptible individuals in the third neighbor subpopulation(s) will transfer the second neighbor(s) and the neighbors will eventually enter subpopulation 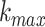 ; and so on, in the end, the majority of individuals in the network will move into subpopulation
; and so on, in the end, the majority of individuals in the network will move into subpopulation 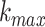 , causing widespread infection. But this process takes a long time. When
, causing widespread infection. But this process takes a long time. When 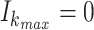 , individuals in subpopulation
, individuals in subpopulation 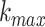 travel to other subpopulations. With regard to travel restriction In, the continuous move-out of individuals from infectious subpopulation results in a decreasing number of individuals until there are no infectious individuals. In Figs. 5 and 6, we plot the time series of
travel to other subpopulations. With regard to travel restriction In, the continuous move-out of individuals from infectious subpopulation results in a decreasing number of individuals until there are no infectious individuals. In Figs. 5 and 6, we plot the time series of 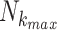 . The simulation results are consistent with the theoretical analysis.
. The simulation results are consistent with the theoretical analysis.
FIGURE 6.
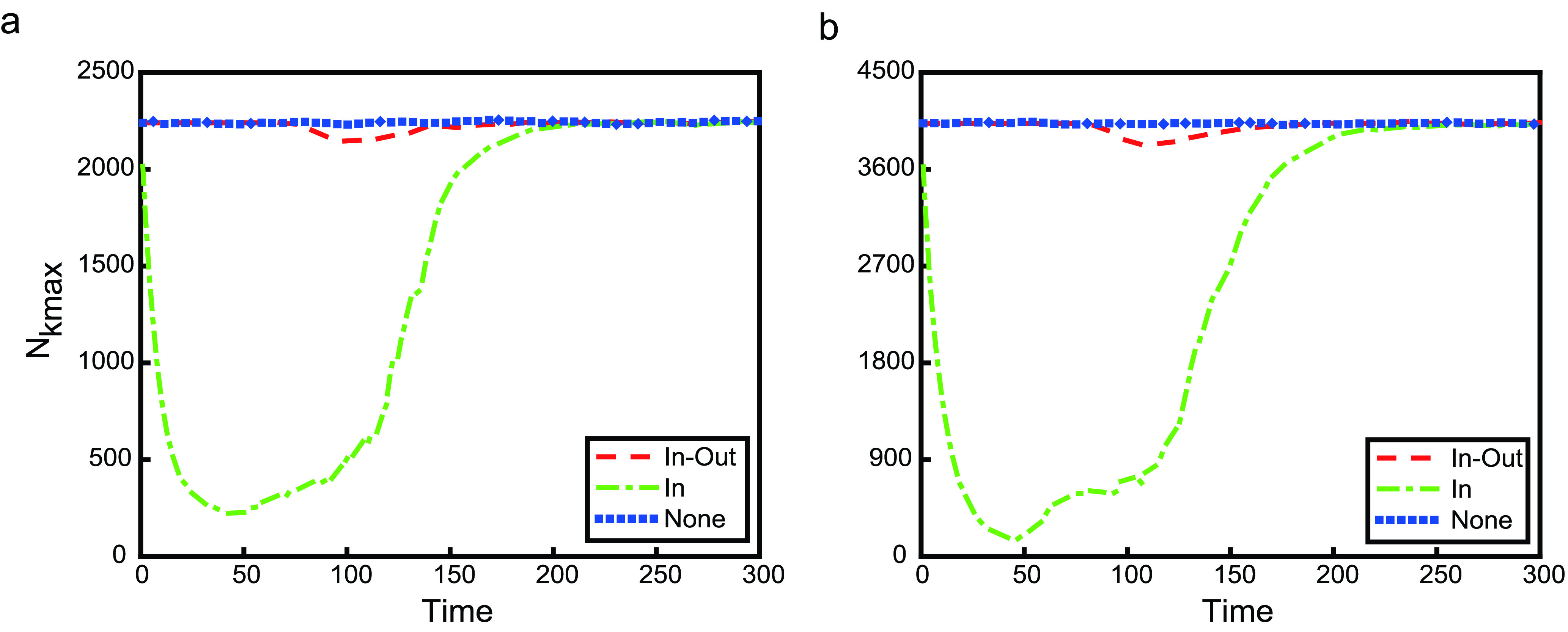
The time evolution of 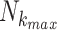 . Three curves are None (blue dot line), In-Out (red dashed line), and In (green dot-dashed line), individually. The left panel shows the case of poisson network, while the right panel is of power-law degree distribution.
. Three curves are None (blue dot line), In-Out (red dashed line), and In (green dot-dashed line), individually. The left panel shows the case of poisson network, while the right panel is of power-law degree distribution.
From what has been discussed above, adaptive metapopulation networks can prevent effectively the global transmission of infectious diseases.
B. The Threshold of Risk Indicator
The mobility of individuals is sometimes necessary to promote economic and cultural globalization. Moreover, human behaviour is often out of control. So 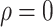 is too ideal. However,
is too ideal. However, 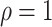 is equivalent to the case of no travel restriction. It is more appropriate that
is equivalent to the case of no travel restriction. It is more appropriate that 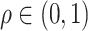 . What is the threshold of risk indicator
. What is the threshold of risk indicator 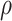 for preventing the global spread of disease? As illustrated in Fig. 7, we simulate the final size and the number of cumulative infectious subpopulations under different
for preventing the global spread of disease? As illustrated in Fig. 7, we simulate the final size and the number of cumulative infectious subpopulations under different 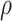 . We consider two kinds of adaptive metapoplation networks with 1000 subpopulations: poisson degree distribution (red circles) and power-law degree distribution (blue triangles). In the initial time, there are five infectious individuals in a randomly chosen subpopulation with a minimal degree.
. We consider two kinds of adaptive metapoplation networks with 1000 subpopulations: poisson degree distribution (red circles) and power-law degree distribution (blue triangles). In the initial time, there are five infectious individuals in a randomly chosen subpopulation with a minimal degree.
FIGURE 7.
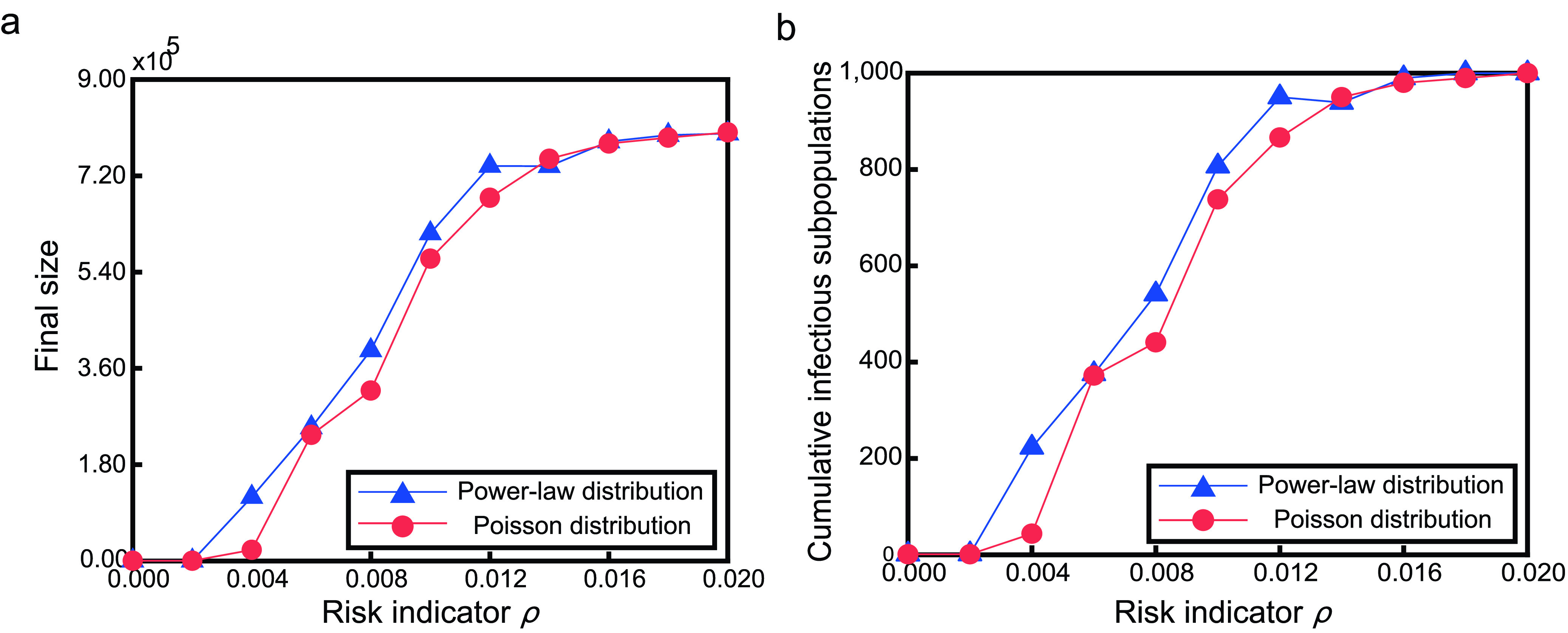
The final size and the number of cumulative infectious subpopulations under different 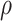 . Figures are under two kinds of different adaptive metapoplation networks, red circles for poisson degree distribution and blue triangles for power-law degree distribution. The left panel shows the final size versus
. Figures are under two kinds of different adaptive metapoplation networks, red circles for poisson degree distribution and blue triangles for power-law degree distribution. The left panel shows the final size versus 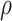 , while the right panel is the number of cumulative infectious subpopulations. Parameter
, while the right panel is the number of cumulative infectious subpopulations. Parameter 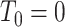 .
.
For these two kinds of networks, the infectious disease spread globally when 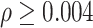 . In the case of
. In the case of 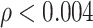 , there are little infectious individuals moving from the initial infectious subpopulation to its neighbors before being removed. When
, there are little infectious individuals moving from the initial infectious subpopulation to its neighbors before being removed. When 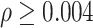 , as
, as 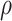 increases, the number of move-out of infectious individuals in infectious subpopulation(s) increases. So the number of infectious subpopulations goes up, and the final size increases. For the same
increases, the number of move-out of infectious individuals in infectious subpopulation(s) increases. So the number of infectious subpopulations goes up, and the final size increases. For the same 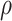 , the speed of global transmission in power-law networks is faster than that in poisson networks. This is due to the heterogeneity of the network structure. That is to say, the heterogeneity of the network structure promotes the global spread of infectious diseases.
, the speed of global transmission in power-law networks is faster than that in poisson networks. This is due to the heterogeneity of the network structure. That is to say, the heterogeneity of the network structure promotes the global spread of infectious diseases.
C. The Effect of Intervention Time
In real life, the response to an emerging infectious disease tends to lag. This implies a delay between the onset of disease transmission and the onset of two-way travel restrictions. Fig. 8 displays the effect of intervention time for two-way travel restrictions 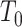 on both the final size and the number of cumulative infectious subpopulations under two kinds of metapopulation networks.
on both the final size and the number of cumulative infectious subpopulations under two kinds of metapopulation networks.
FIGURE 8.
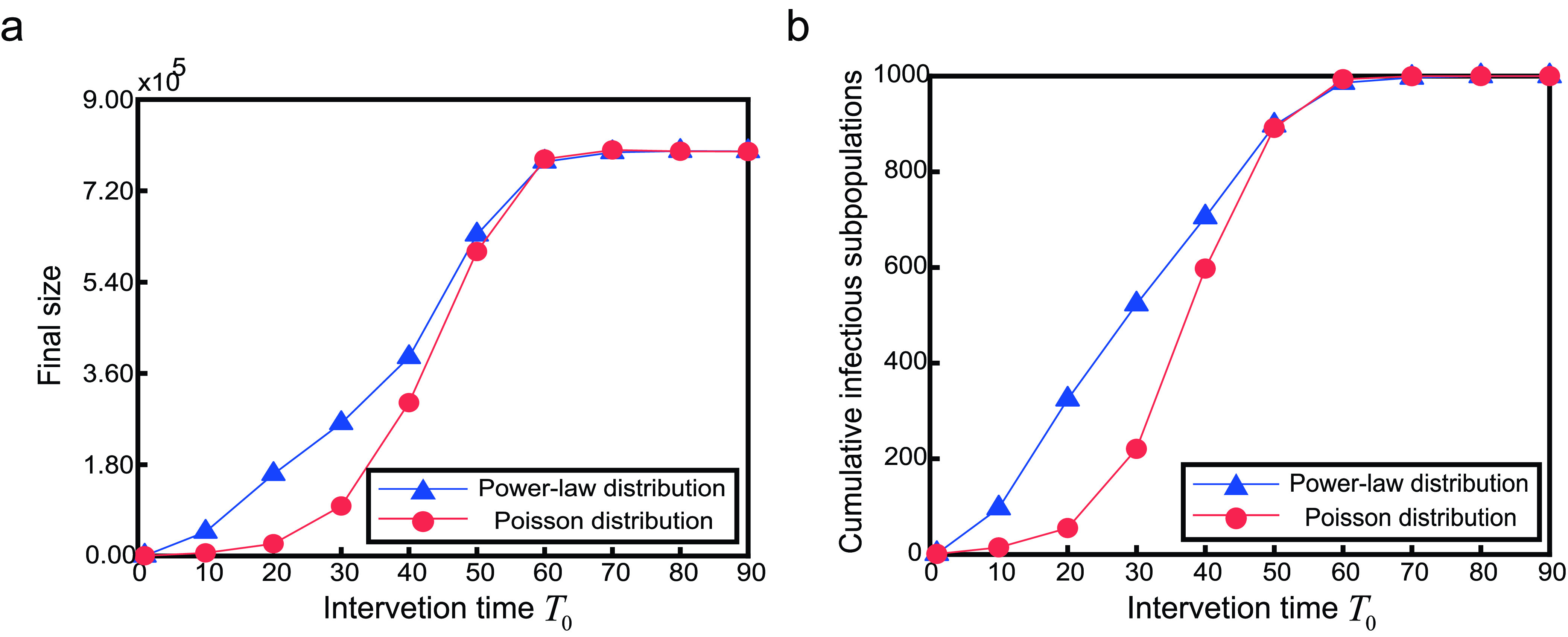
The effect of intervention time 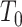 on final size and cumulative infectious subpopulations. Parameter
on final size and cumulative infectious subpopulations. Parameter 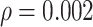 .
.
Obviously, the earlier 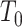 , the better to prevent the global spread of the infectious diseases. The first ten days since the onset of infectious disease are of great importance. The speed of global transmission is relatively slow, and implementing two-way travel restrictions during this period will be effective in preventing the global spread of the disease. When
, the better to prevent the global spread of the infectious diseases. The first ten days since the onset of infectious disease are of great importance. The speed of global transmission is relatively slow, and implementing two-way travel restrictions during this period will be effective in preventing the global spread of the disease. When 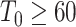 , the existence of adaptive metapopulation network makes no sense, i.e., two-way travel restrictions do not work. Similar to Fig. 7, for the same
, the existence of adaptive metapopulation network makes no sense, i.e., two-way travel restrictions do not work. Similar to Fig. 7, for the same 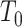 , final size and the number of cumulative infectious subpopulations of power-law networks are larger than those of poisson networks. This also indicates that the heterogeneity of the network structure promotes the global spread of infectious diseases.
, final size and the number of cumulative infectious subpopulations of power-law networks are larger than those of poisson networks. This also indicates that the heterogeneity of the network structure promotes the global spread of infectious diseases.
V. Conclusion and Discussion
Adaptive metapopulation networks help suppress the global transmission of an acute emerging infectious disease. This paper defined a risk indicator 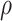 according to the relative infection density and divided subpopulations into three types: risk-free, low-risk, and high-risk. Based on the risk indicator and intervention time for two-way travel restrictions
according to the relative infection density and divided subpopulations into three types: risk-free, low-risk, and high-risk. Based on the risk indicator and intervention time for two-way travel restrictions 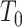 , this essay defined an adaptive metapopulation network and proposed an SIR metapopulation model on this network to investigate the issue of two-way travel restrictions on all neighbor subpopulations. Then this paper obtained the equilibria, at which there are no infectious individuals; the basic reproduction number, which is independent of mobility rate; and the final size under special cases. Finally, this paper presented Monte Carlo simulation results on two kinds of metapopulation networks with different degree distributions but the same average degree. Comparing two-way travel restriction and two kinds of one-way travel restrictions, we find that controlling the movement of infectious individuals helps prevent the global spread of infectious diseases. The conclusion is obvious. However, the mobility of susceptible individuals also makes for the transmission of disease. Therefore, adaptive metapopulation networks under two-way travel restriction help prevent the global spread of diseases. Furthermore, we obtained that the threshold of risk indicator
, this essay defined an adaptive metapopulation network and proposed an SIR metapopulation model on this network to investigate the issue of two-way travel restrictions on all neighbor subpopulations. Then this paper obtained the equilibria, at which there are no infectious individuals; the basic reproduction number, which is independent of mobility rate; and the final size under special cases. Finally, this paper presented Monte Carlo simulation results on two kinds of metapopulation networks with different degree distributions but the same average degree. Comparing two-way travel restriction and two kinds of one-way travel restrictions, we find that controlling the movement of infectious individuals helps prevent the global spread of infectious diseases. The conclusion is obvious. However, the mobility of susceptible individuals also makes for the transmission of disease. Therefore, adaptive metapopulation networks under two-way travel restriction help prevent the global spread of diseases. Furthermore, we obtained that the threshold of risk indicator 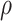 is 0.004. When
is 0.004. When 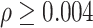 , infectious diseases will spread globally. What is more, the earlier the intervention time for two-way travel restriction
, infectious diseases will spread globally. What is more, the earlier the intervention time for two-way travel restriction 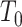 is, the better to prevent the disease from spreading globally. It is better to implement two-way travel restrictions during the first ten days since the onset of the disease. When
is, the better to prevent the disease from spreading globally. It is better to implement two-way travel restrictions during the first ten days since the onset of the disease. When 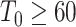 , two-way travel restriction makes no sense. In addition, simulation results show that the heterogeneity of the network structure promotes the global spread of infectious diseases.
, two-way travel restriction makes no sense. In addition, simulation results show that the heterogeneity of the network structure promotes the global spread of infectious diseases.
Our results will provide some useful insights on the global transmission prevention and control of an emerging acute infectious disease. Timely two-way travel restrictions (or city lockdown) help suppress the global spread of the epidemic. However, for lockdown subpopulation, controlling the spread of disease remains a major challenge. Adequate medical resources are needed to quarantine close contacts with the infectious, reduce the time that takes for infectious persons to be diagnosed, and explore effective treatments. Even if two-way travel restriction can not be implemented, the mobility of infectious individuals should be restricted. In order to prevent the invasion of infectious individuals, there are a series of control measures. Persons entering the subpopulation shall be quarantined. Individuals leaving the subpopulation need to provide a health certificate and register their origin and destination information. In addition, it is necessary to reduce the number of flights. Within an infectious subpopulation, control measures, including isolating the infectious, quarantining close contacts, keeping social distance, and so on, should be taken to mitigate infections. For an emerging infectious disease, there have been many studies on control measures within subpopulations [18]–[20]. In this article, we mainly focused on the prevention and control of the global spread of the epidemic, so we did not consider the control measures within a subpopulation.
Although we have investigated the issue of two-way travel restriction on all neighbor subpopulations, there are still some problems in this paper to be further solved.
-
•
Travel restrictions we considered is on all neighbor subpopulations. Because of subpopulation differences, travel restrictions might be only on some neighbor subpopulations.
-
•
With regard to the intervention time on travel restrictions, we consider it is the same for all subpopulations. In fact, the disease onset time of each subpopulation is often different, and the intervention time may vary. So intervention time for each subpopulation may be the sum of its disease onset time and a delay.
-
•
In this paper, we only focused on the effect of two-way travel restrictions on the global spread of infectious diseases, ignoring its effect on each subpopulation. In the future, we will consider both the global and local impact of prevention and control measures. Besides, we will study the global and local impact of travel restrictions, keeping social distance, isolating the infectious and quarantining close contacts at the same time on global transmission prevention and control.
Biographies

Shanshan Feng is currently pursuing the Ph.D. degree with the School of Data Science and Technology, North University of China. Her main research interests include mathematical biology, epidemiology, complex networks, and metapopulation models. She is working on the study of infectious diseases spreading in metapopulation networks.

Zhen Jin is currently a Professor with the Complex Systems Research Center, Shanxi University, China. He has over 150 publications in various international journals. His main research interests include mathematical biology, epidemiology, complex networks, nonlinear dynamics, and data analytic.
Funding Statement
This work was supported in part by the National Key Research and Development Program of China under Grant 2016YFD0501500, in part by the National Natural Science Foundation of China under Grant 61873154 and Grant 11701528, in part by the Shanxi Key Laboratory under Grant 201705D111006, and in part by the Shanxi Scientific and Technology Innovation Team under Grant 201705D15111172.
References
- [1].Bogoch I. I., Brady O. J., Kraemer M. U. G., German M., Creatore M. I., Brent S., Watts A. G., Hay S. I., Kulkarni M. A., Brownstein J. S., and Khan K., “Potential for Zika virus introduction and transmission in resource-limited countries in Africa and the Asia-Pacific region: A modelling study,” Lancet Infectious Diseases, vol. 16, no. , pp. 1237–1245, Nov. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Colizza V. and Vespignani A., “Epidemic modeling in metapopulation systems with heterogeneous coupling pattern: Theory and simulations,” J. Theor. Biol., vol. 251, no. 3, pp. 450–467, Jan. 2008. [DOI] [PubMed] [Google Scholar]
- [3].Cao L., Li X., Wang B., and Aihara K., “Rendezvous effects in the diffusion process on bipartite metapopulation networks,” Phys. Rev. E, Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top., vol. 84, no. 4, Nov. 2011, Art. no. 041936. [DOI] [PubMed] [Google Scholar]
- [4].Liu S.-Y., Baronchelli A., and Perra N., “Contagion dynamics in time-varying metapopulation networks,” Phys. Rev. E, Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top., vol. 87, no. 3, Mar. 2013, Art. no. 032805. [Google Scholar]
- [5].Mata A. S., Ferreira S. C., and Pastor-Satorras R., “Effects of local population structure in a reaction-diffusion model of a contact process on metapopulation networks,” Phys. Rev. E, Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top., vol. 88, no. 4, Oct. 2013, Art. no. 042820. [DOI] [PubMed] [Google Scholar]
- [6].Feng S. and Jin Z., “Infectious diseases spreading on a metapopulation network coupled with its second-neighbor network,” Appl. Math. Comput., vol. 361, pp. 87–97, Nov. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Meloni S., Perra N., Arenas A., Gómez S., Moreno Y., and Vespignani A., “Modeling human mobility responses to the large-scale spreading of infectious diseases,” Sci. Rep., vol. 1, no. 62, pp. 62–68, Aug. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang B., Cao L., Suzuki H., and Aihara K., “Safety-information-driven human mobility patterns with metapopulation epidemic dynamics,” Sci. Rep., vol. 2, no. 2, pp. 887–894, Nov. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Panigutti C., Tizzoni M., Bajardi P., Smoreda Z., and Colizza V., “Assessing the use of mobile phone data to describe recurrent mobility patterns in spatial epidemic models,” Roy. Soc. Open Sci., vol. 4, no. 5, May 2017, Art. no. 160950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pei S., Kandula S., Yang W., and Shaman J., “Forecasting the spatial transmission of influenza in the United States,” Proc. Nat. Acad. Sci. USA, vol. 115, no. 11, pp. 2752–2757, Feb. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chinazzi M., Davis J. T., Ajelli M., Gioannini C., Litvinova M., Merler S., Piontti A. P., Mu K., Rossi L., Sun K., and Viboud C., “The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak,” Science, vol. 368, no. 3489, pp. 395–400, Mar. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brockmann D., “Human mobility and spatial disease dynamics,” Rev. Nonlinear Dyn. Complex., vol. 2, pp. 1–27, Aug. 2009. [Google Scholar]
- [13].Arino J., Davis J. R., Hartley D., Jordan R., Miller J. M., and van den Driessche P., “A multi-species epidemic model with spatial dynamics,” Math. Med. Biol., vol. 22, no. 2, pp. 129–142, Mar. 2005. [DOI] [PubMed] [Google Scholar]
- [14].van den Driessche P. and Watmough J., “Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission,” Math. Biosci., vol. 180, nos. 1–2, pp. 29–48, Nov. 2002. [DOI] [PubMed] [Google Scholar]
- [15].Minc H., “Localization of the maximal eigenvalue,” in Nonnegative Matrices. Hoboken, NJ, USA: Wiley, 1988, pp. 24–25. [Google Scholar]
- [16].Zhao X.-Q. and Jing Z.-J., “Global asymptotic behavior in some cooperative systems of functional differential equations,” Can. Appl. Math. Quart, vol. 4, no. 4, pp. 421–444, 1996. [Google Scholar]
- [17].Molloy M. and Reed B., “A critical point for random graphs with a given degree sequence,” Random Struct. Algorithms, vol. 6, nos. 2–3, pp. 161–180, Mar. 1995. [Google Scholar]
- [18].Zhang J., Litvinova M., Liang Y., Wang Y., Wang W., Zhao S., Wu Q., Merler S., Viboud C., Vespignani A., Ajelli M., and Yu H., “Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China,” Science, vol. 368, no. 6498, pp. 1481–1486, Apr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tang B., Wang X., Li Q., Bragazzi N. L., Tang S., Xiao Y., and Wu J., “Estimation of the transmission risk of the 2019-nCoV and its implication for public health interventions,” J. Clin. Med., vol. 9, no. 2, p. 462, Feb. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Luo X. and Jin Z., “A new insight into isolating the high-degree nodes in network to control infectious diseases,” Commun. Nonlinear Sci. Numer. Simul., vol. 91, no. 105363, Dec. 2020. [Google Scholar]