Introduction
Metastatic prostate cancer is a major contributor to worldwide cancer mortality. Androgen deprivation therapy (ADT) is a cornerstone of therapy for castration-sensitive disease, now being combined with androgen receptor pathway inhibitors (ARPI) or docetaxel in the initial treatment of metastatic disease[1, 2]. Prostate cancer is considered castration-resistant when there is disease progression despite ADT and castrate levels of serum testosterone (under 50 ng/dL)[2]. Treatment options for metastatic castration resistant prostate cancer (mCRPC) include ARPI, taxane chemotherapies, radium-223, sipuleucel-T immunotherapy, and PARP inhibitors for selected patients[3–7]. Despite significant advances, mCRPC is a progressive disease, and additional therapies are needed. Over the last decade, targeted radionuclide therapy (TRT) against prostate-specific membrane antigen (PSMA) has emerged as a promising investigational agent for mCRPC.
PSMA was first described in 1987, when the murine antibody 7E11-C5 was noted to react with the cell membranes of the LNCaP prostate cancer cell line[8]. PSMA received its name after it was found to be highly restricted to prostatic tissue, with only other expression in salivary glands, duodenal brush border, and proximal renal tubule[9]. In normal prostatic tissue, PSMA expression is generally present, but in PC, expression can be a thousand-fold higher[10]. Expression levels further increase with tumor grade, castration-resistance, blockade of AR signaling, and metastatic disease[11–14]. PSMA is a 100 kD transmembrane enzyme that hydrolyzes folate and increases intracellular glutamate levels, which may directly contribute to cancer pathogenesis by activating the PI3K/Akt pathway[9].
PSMA TRT involves conjugating radionuclides to antibodies and small molecule-ligands directed against PSMA. The most common radionuclide utilized to date is Lutetium-177, a beta-emitter with a mean path-length of 0.7 mm and maximum of 1.8 mm[15]. Gamma-ray emission allows for concurrent single photon emission computed tomography (SPECT). Actinium-225 has been the most common alpha-emitter utilized. Alpha-emitters are thousands of times more potent and can induce double-stranded DNA breaks more difficult to repair, but mean path-length is much shorter than beta-emitters (under 0.01 mm)[15, 16].
Given the expanding investigation of PSMA TRT, this review will discuss the conjugates commonly used for PSMA TRT and associated patient outcomes, analyzing both prior studies (Table 1) and highlighting ongoing clinical trials.
(Table 1).
Selected Prospective TRT Studies
| Study | Agent | # of Patients | Median PSA (ng/mL) | Dose | # of Planned Cycles | % Over 50% PSA Decline | Overall Survival | Progression-Free Survival |
|---|---|---|---|---|---|---|---|---|
| Tagawa (2013) [26] | 177Lu-J591 | 47 | 74.4 | 65 or 70 mCi/m2 | 1 | 10.6 | 17.6 months | 12 weeks |
| Tagawa (2019) [27] | 177Lu-J591 | 49 | 44.9 | 20–45 mCi/m2 × 2 | 1 | 16.3 | 23.6 months | 16.7 weeks |
| Tagawa (2020) [33] | 225Ac-J591 | 22 | 147 | 13.3–93.3 kBq/kg | 1 | 41 | Pending | Pending |
| Violet (2020) [55] | 177Lu-PSMA-617 | 50 | 189.8 | Median 7.4 GBq | 4 | 64 | 13.3 months | 6.9 months |
| Emmett (2019) [56] | 177Lu-PSMA-617 | 14 | 88 | Mean 7.0 GBq | 4 | 36 | 50 weeks | N/A |
| Tagawa (2019) [64] | 177Lu-PSMA-617 | 44 | 182.97 | 7.4–22.2 GBq x 2 | 1 | 61 | 16 months | Pending |
| Calais (2019) [66] | 177Lu-PSMA-617 | 64 | 75 | 6.0 or 7.4 GBq | 4 | 38 | Pending | Pending |
| Hofman (2020) [67] | 177Lu-PSMA-617 or Cabazitaxel | 200 | N/A | 6–8 GBq | 6 | 66 | Pending | Pending |
Antibodies for TRT
7E11-C5
The earliest attempts at PSMA TRT involved the initial antibody found to have affinity for PSMA, 7E11, which specifically targets a 19 amino acid-long intracellular region of PSMA[9]. Following recognition that it binds to prostate cancer cell membranes, 7E11 was subsequently developed for imaging and therapeutic purposes, becoming known as CYT-356 or capromab pendetide. For imaging, capromab pendetide labeled with the radionuclide indium-111 was administered, followed by whole body scintigraphy 2–4 days later[17]. Although granted FDA approval in 1996, 111In-capromab pendetide imaging (tradename ProstaScint) eventually fell out of favor due to high intra-observer variability and days-long delay between nucleotide administration and imaging[18]. Sensitivity for detection of metastatic disease has been reported as low as 10%. As capromab pendetide was being studied for imaging purposes, there were also attempts to utilize the antibody for TRT, by conjugating it to the beta-emitter Yttrium-90. Two pilot studies investigated 90Y-capromab; both showed absence of radiographic or biochemical response, with significant hematological toxicity[19, 20]. The maximum tolerated dose was 9 mCi/m2[20]. The ineffectiveness of capromab TRT likely stems from the antibody’s recognition of an internal, but not external, domain of PSMA, rendering it unable to bind to viable cancer cells.
J591
The 151 kD J591 monoclonal antibody was developed in 1997 and shown to induce internalization of PSMA in prostate cancer cells following binding to an external domain[21, 22]. It has been conjugated to radionuclides through the chelating agent DOTA. Following initial studies demonstrating safety and sensitive tumor targeting of J591 trace-labeled with Indium-111, TRT studies were initiated[23]. A phase I dose-escalation trial of 90Y-J591 demonstrated safety and an early efficacy signal[24]. A separate phase I study tested escalating doses of 177Lu-J591, reporting a maximum tolerated dose of 70 mCi/m2[25]. A phase II single-dose trial then enrolled 47 patients with mCRPC in 3 cohorts, 15 dosed at 65 mCi/m2, 17 dosed at 70 mCi/m2, and then another group dosed at 70 mCi/m2 to confirm dose-response and biomarkers[26]. Baseline patient characteristics included median PSA 74.4 ng/mL, ECOG score 0–1, 8.4% with liver metastasis, 23.4% with pulmonary involvement, and 55.3% having received prior chemotherapy. Those receiving a higher dose had more frequent PSA declines and longer survival [Table 2]. Poorer PSMA uptake on 177Lu or 111In PSMA SPECT was associated with a lower likelihood of PSA response. Toxicity included 46.8% with grade 4 thrombocytopenia, which lasted for a median of 7 days, and 25.5% of patients with grade 4 neutropenia, with severity also related to dose administered (grade 4 neutropenia 37.5% in 70 mCi/m2 vs 0% in 65 mCi/m2 cohort; grade 4 thrombocytopenia 56.3% vs 27%).
Table 2:
Results of phase I/II studies of single-agent 177Lu-J591: Effect of dose activity administered
| Single Dose26 | Fractionated Dose27 | ||||
|---|---|---|---|---|---|
| Cumulative dose | 65 mCi/m2 | 70 mCi/m2 | 40–70 mCi/m2 | 80 mCi/m2 | 90 mCi/m2 |
| “n” | 15 | 32 | 16 | 16 | 17 |
| Any PSA decline | 46.7% | 65.6% | 37.5% | 50.0% | 87.5% |
| ≥30% PSA decline | 13.3% | 46.9% | 12.5% | 25.0% | 58.8% |
| >50% PSA decline | 6.7% | 12.5% | 6.3% | 12.5% | 29.4% |
| Median survival | 11.9 mo | 21.8 mo | 14.6 mo | 19.6 mo | 42.3 mo |
| Platelets Gr 4 | 27.0% | 56.3% | 20.0% | 43.8% | 58.8% |
| Platelet transfusion | 7.0% | 41.0% | 0.0% | 31.3% | 52.9% |
| Neutropenia Gr 4 | 0.0% | 37.5% | 0.0% | 31.3% | 29.4% |
| Febrile neutropenia | 0.0% | 2.1% | 0.0% | 0.0% | 5.8% |
The hypothesis that dose fractionation would be safer at comparable cumulative doses with potentially higher efficacy was subsequently explored in a phase I/II dose-escalation trial[27]. Forty nine patients were enrolled, initially in a phase I dose-escalation component followed by phase IIa dosing in the two dosing cohorts selected for exploration (80 and 90 mCi/m2 total dose divided into two doses two weeks apart). In this study, patient characteristics included median PSA 44.9 ng/mL, 91.8% ECOG 0–1, 36.7% with prior chemotherapy, and 18.4% with ARPI. As demonstrated before, there appeared to be a dose-dependent response for PSA decline and overall survival, but also higher rates of myelosuppression [Table 2]. In addition, those with lower PSMA uptake on 177Lu SPECT had lower likelihood of significant PSA decline.
While it was initially thought that 177Lu-J591 radioimmunotherapy could not be combined concurrently with chemotherapy, as dose-fractionation proved to be less toxic for comparable cumulative doses, a phase I study tested the combination of docetaxel and fractionated-dose 177Lu-J591[28]. In this study of 15 patients with mCRPC, patients received standard docetaxel (75 mg/m2) in 21-day cycles, with cohorts of escalating fractionated doses of 177Lu-J591 during cycle 3 (highest planned total dose 80 mCi/m2). Cycle 4 of docetaxel was planned to be delayed by at least 3 weeks, with up to 3 additional weeks of delay allowed. This study demonstrated safety of the combination with early evidence of activity, with 73% achieving >50% PSA decline. Toxicities were comparable to prior 177Lu-J591 studies. Although 2-dose fractionation appears attractive alone or with docetaxel, a pilot study exploring “hyper-fractionated” 177Lu-J591 did not demonstrate favorable results[29]. In this study, low dose 177Lu-J591 (25 mCi/m2) was administered every two weeks until greater than grade 2 toxicity emerged. As designed, dosing was limited by myelosuppression (especially thrombocytopenia), but the regimen did not appear more favorable than 2-dose fractionation and is also less convenient for patients, so the regimen is not being further explored. Ongoing studies include a randomized trial of 177Lu- versus 111In-J591 in combination with ketoconazole for those with “non-metastatic” CRPC (NCT00859781) and a phase III study of 177Lu-rosapatumab (TLX591, aka 177Lu-J591) has been announced[30].
Clearly, myelosuppression is the dose-limiting toxicity of radioimmunotherapy in general. Reports of myelodysplasia (MDS) and acute myeloid leukemia (AML) have emerged following anti-CD20 radioimmunotherapy for lymphoma, though it is not clear that the rate of MDS/AML following RIT is any different than seen in a general population of patients with lymphoma who have received chemotherapy[31]. Therefore, there was an analysis of extended follow-up of 150 patients who received 177Lu-J591[32]. This study demonstrated that 97.3% had platelet count recovery to normal or grade 1 levels and 100% recovery of neutrophil counts. All patients otherwise eligible for chemotherapy (i.e. not having already received it and having adequate performance status) were able to receive subsequent chemotherapy unless they refused.
Alpha-emitters are significantly more potent, but have a shorter range than beta-emitters. In a first-in human phase I dose-escalation study, 225Ac-J591 was being studied as a single dose (NCT03276572)[33]. Preliminary results indicate that the highest planned dose, 93.3 KBq/kg, was tolerated in the six patients enrolled in that cohort. Out of 22 patients, 41% experienced over 50% PSA decline. One patient developed grade 4 anemia and thrombocytopenia at a lower dose. There were no cases of severe xerostomia and studies administering multiple or subsequent doses are underway (NCT04506567, NCT04576871).
PSMA-TTC
Along with 225Ac-J591, another alpha-emitter antibody conjugate under investigation is PSMA-TTC (BAY 2315497), which consists of the alpha-emitter thorium-227 conjugated to a monoclonal antibody directed against PSMA. Following in vitro and murine studies that demonstrated efficacy at doses ranging from 75 to 500 kBq/kg, PSMA-TTC has entered a phase I clinical trial (NCT03724747) for patients with progressive mCRPC[34].
Differences between antibodies and small molecules
Antibodies and small molecules have clear physical differences in molecular weight, which leads to differences in kinetics and biodistribution[35]. Briefly, antibodies are large with a long circulation time which may expose organs such as bone marrow to more radiation and are often cleared in liver. Small molecules are usually rapidly excreted via the urinary tract, but are able to penetrate into PSMA+, off-tumor sites such as renal tubules, salivary/lacrimal glands, and small intestine. Based upon these physical differences, it is not surprising that a retrospective review of prospective clinical trial data with PSMA-targeted 177Lu demonstrated more hematologic toxicity with antibody, while small molecule-based TRT was associated with more non-hematologic toxicities (particularly nausea and xerostomia)[36].
Small molecule ligands for TRT
Imaging ligands
While PSMA-PET has been performed utilizing monoclonal antibodies, PSMA-PET utilizing small molecule delivery of a positron-emitting radionuclide is more convenient for patients with same-day imaging[37, 38]. The initially most studied tracer involves Gallium-68 conjugated to the urea-based small molecule PSMA-11 (Figure 1)[38]. Since 68Ga-PSMA-11 PET was first described in 2012, it has become a highly effective imaging modality for defining both intra-prostatic and metastatic disease, particularly at low PSA levels (under 2 ng/mL)[39–41]. Numerous other imaging ligands have been developed, including the fluorine-conjugates DCFPyL, PSMA-1007, and PSMA-7[42–44]. There are ongoing trials comparing these agents.
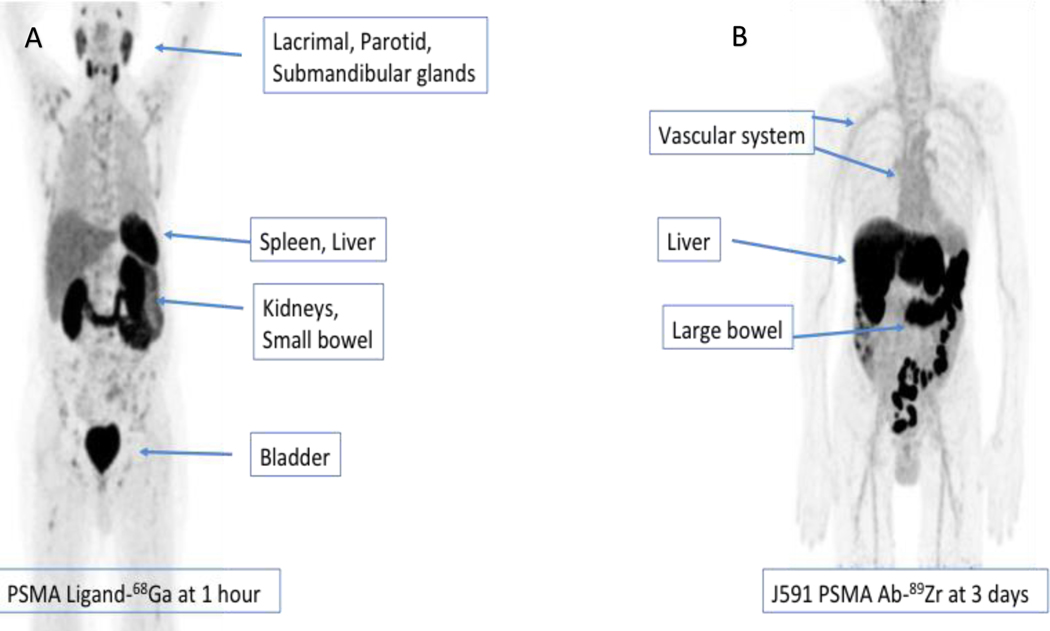
Figure 1.
Biodistribution of 2 different positron emission tomography imaging agents showing different patterns of distribution for 68Ga-PSMA-11 (A) 1 hour after injection of radiotracer and Zirconium-89 labeled J591 antibody (B) 3 days after radiotracer injection.
131I-MIP-1095
An initial experience of TRT with beta-emitter iodine-131 conjugated to the small molecule MIP-1095 was described in 2014[45]. Like all of the initial reports of small molecule PSMA-TRT, a retrospective study described 28 patients with mCRPC who were treated with a single dose, averaging 4.8 GBq, and 60.7% had PSA decline over 50%. The normal tissues with the highest absorbed doses were salivary glands (4.6 mSv/MBq), liver (1.5 mSv/MBq), and kidneys (1.5 mSv/MBq). While hematologic toxicity was infrequent (2 patients with grade 3 thrombocytopenia, 1 patient with grade 3 leukopenia), thrombocytopenia was sustained, lasting multiple months. In a subsequent study involving 36 mCRPC patients, 70.6% experienced over 50% decline in PSA after the initial dose, mean 4.2 GBq; median biochemical progression-free survival was 116 days[46]. After PSA progression, patients received an additional dose, but declining efficacy and increasing toxicities were noted. With the second dose, 65.2% experienced any PSA decline. 13% developed grade 3 thrombocytopenia, compared to 5.9% of patients after the first dose. 13% also developed grade 3 xerostomia, which was not observed after the first dose. 131I-MIP-1095 TRT has been complicated by high levels of co-emitted gamma radiation, as well as difficulties producing and conjugating iodine-131. This drug is now in prospective studies (NCT03030885).
PSMA-617
The urea-based small molecule PSMA-617 was developed in Heidelberg, Germany and contains a DOTA chelator that allows for it to be conjugated to radionuclides[47]. PSMA-617 demonstrates high affinity for PSMA, with rapid clearance from plasma within one hour and clearance fully completed by 48 hours[48]. The organs that absorb the highest doses are the kidneys (mean 0.75 Gy/GBq) and parotid glands (1.28 Gy/GBq); absorbed dose for marrow is 0.02 Gy/GBq. By comparison, for 177Lu-J591, 20% of infused 177Lu-J591 clears from plasma within three hours, and the remaining 80% has a half-life of 39 hours[23]. Absorbed doses in normal organs are highest in the liver (2.1 Gy/GBq), spleen (1.97 Gy/GBq), and kidneys (1.41 Gy/GBq); for bone marrow, absorbed dose is 0.32 Gy/GBq.
In 2015, Ahmadzadehfar et al. reported a retrospective review of 10 patients with progressive mCRPC treated with 177Lu-PSMA-617, in which 50% experienced over 50% PSA decline[49]. These encouraging results led to the expansion of 177Lu-PSMA-617 in clinical practice, and this therapy was offered to selected mCRPC patients on a compassionate use basis, without standardized treatment protocols or inclusion criteria, at multiple centers in Germany. A series of retrospective studies described these experiences[48, 50–53]. Rahbar et al. reported the largest group in 2017, involving 145 patients across 12 centers in Germany[52]. 245 cycles, median dose 5.9 GBq, were administered in total in 8–12 week intervals. 45% of the 99 patients with available post-treatment PSA had decline over 50%; 60% had any PSA decline. Reported adverse events included 3% with grade 3/4 leukopenia, 10% with grade 3/4 anemia, and 4% with grade 3/4 thrombocytopenia. The proportion without post-treatment PSA or safety labs highlights issues with retrospective studies.
The first prospective study of 177Lu-PSMA-617 was reported by Hofman et al. in 2018[54]. Thirty patients were planned to receive four cycles of therapy in six-week intervals. Patients were excluded if SUVmax of their disease on 68Ga-PSMA-11 PET was not 1.5x of liver or if there were avid sites of disease on FDG PET that were not visualized on 68Ga-PSMA-11 PET. Dose per cycle was individualized and dependent upon tumor burden, weight, and renal function; average dose was 7.5 GBq. Patient characteristics included: median PSA 189.9 ng/mL, 87% with prior chemotherapy. 57% of patients had PSA decline over 50%, with 20% experiencing decline exceeding 96%. Biochemical progression-free and overall survival were 7.6 months and 13.5 months, respectively. High grade toxicities included 13% with grade 3/4 thrombocytopenia, 13% with grade 3 anemia, and 7% with grade 3 neutropenia. Common adverse events included 87% with grade 1 xerostomia and 50% with grade 1/2 nausea. In an expansion cohort of 50 patients, 64% experienced over 50% PSA decline, and 44% experienced over 80% PSA decline[55]. In both the initial and expansion cohort, there was a significant component of selection; in total, 16 of the 75 patients screened were excluded based on imaging criteria. A smaller prospective study involving 14 patients was reported by Emmett et al[56]. Inclusion criteria and dosing regimen were similar. 71% experienced PSA decline, and 36% experienced over 50% decline. Mean overall survival was 50 weeks.
Several patient characteristics have correlated with outcome. Independent negative prognostic factors have not surprisingly included elevated alkaline phosphatase (ALP) level, liver metastases, use of opioid analgesia, and ECOG score over 1 (as previously reported as prognostic factors in patients with mCRPC not treated with PSMA-TRT)[51–53, 57–60]. As expected, patients with biochemical response have associated higher overall survival[55, 60].
In the majority of patients treated as part of compassionate-use protocols (representing the majority of published data), dosing of 177Lu-PSMA-617 has been attempted to be individualized based upon predicted dosimetry. It is hypothesized that personalized dosing may be the optimal strategy, though some have proposed that higher doses are warranted given the relatively low toxicity profile that has been observed and no study has shown improved or worsened outcomes with dosimetry-based doses vs empiric dosing[61]. Based upon the concepts of dose-response and dose intensity to minimize repopulation, several reports of higher or more frequent dosing have been published[62, 63]. The retrospective nature of these reports limits applicability. In the only dose-escalation study reported to date, NCT03042468 is an ongoing phase I/II study of fractionated dose 177Lu-PSMA-617, with a cumulative dose administered two weeks apart[64]. The phase I (dose-escalation) portion of the study has been reported, with no dose limiting toxicity at the highest planned dose of 22.2 GBq administered as a single cycle of fractionated dosing (identical to 177Lu-J591). Preliminary results of 44 patients showed that 59.1% experienced >50% PSA decline. While in dosimetry studies of 177Lu-PSMA-617, a “sink-effect” has been described with larger, more PSMA positive tumors sequestering and absorbing radioactivity, this dose escalation study demonstrated better PSA response and progression-free and overall survival without differences in toxicity associated with higher doses administered and higher PSMA uptake on imaging[64, 65]. Aside from no dose-limiting toxicity, the most common low grade toxicities were pain flare (81.8%) and xerostomia (61.4%). RESIST-PC is another phase II trial investigating 177Lu-PSMA-617 with different dosing[66]. It aimed to compare outcomes in patients treated at either 6 or 7.4 GBq; four cycles were planned, in 8-week intervals. The study was stopped early due to sponsor withdrawal. Preliminary results seem consistent with other reports with post-treatment PSA decline (38% achieving PSA50) in a treatment-refractory patient population with mCRPC and overall good tolerance.
In the only prospective randomized trial reported to date, ANZUP investigators recently reported results of the TheraP study[67, 68]. In this study, men with mCRPC and prior docetaxel chemotherapy (with or without prior ARPI) are screened with dual PSMA- and FDG-PET/CT. Those with significant PSMA uptake and without discordant areas (i.e. FDG+ / PSMA-) are randomized to 177Lu-PSMA-617 (dosing at 8.5 GBq with the first cycle and reduced by 0.5 GBq each following q6 week cycle) versus cabazitaxel chemotherapy. The primary endpoint of PSA response was met, with 66% versus 37% having >50% PSA decline. High grade adverse events were more common overall with cabazitaxel chemotherapy, but there was more all-grade xerostomia, dry eye, and thrombocytopenia with 177Lu-PSMA-617.
While TheraP utilized the primary endpoint of PSA decline, VISION is a phase III, multi-institution trial enrolling 750 patients that compares standard of care plus 177Lu-PSMA-617 against best standard of care alone, with the dual primary endpoints of radiographic progression-free and overall survival[69]. The study population includes patients with progressive mCRPC who have received prior treatment with both taxane chemotherapy and ARPI. Patients must also have PSMA-positive disease on imaging (no soft tissue metastases without PSMA uptake). 177Lu-PSMA-617 is to be administered at 7.4 GBq every six weeks for up to six cycles. Best standard of care excludes chemotherapy and radium-223. The trial completed enrollment in late 2019 with results awaited. Additional randomized studies in earlier disease states have been initiated or planned, including the UpfrontPSMA study enrolling patients with metastatic castration-sensitive disease, comparing outcomes in patients treated with two cycles of 177Lu-PSMA-617 followed by six cycles of docetaxel against those treated with docetaxel alone (NCT04343885).
The first clinical experience involving 225Ac-PSMA-617 was described in 2016, in a case report of a patient with widely metastatic disease who developed a complete response after cancer progression despite 177Lu-PSMA-617[70]. A subsequent retrospective report stated that doses above 100 kBq/kg resulted in severe xerostomia, while doses below 50 kBq/kg were ineffective[16]. Dose absorbed in normal tissue was highest in the kidneys (0.74 SvRBE5/MBq) and salivary glands (2.33 SvRBE5/MBq); for marrow, dose absorbed was 0.05 SvRBE5/MBq. In the largest cohort to date, 40 patients received three cycles of therapy, 100 kBq/kg every two months[71]. 63% had over 50% decline in PSA; biochemical progression-free survival was median 7 months. While 31 patients completed the protocol, 48% of patients experienced severe xerostomia, causing four patients to leave the study. More recently, 225Ac-PSMA-617 was explored in mCRPC patients naïve to chemotherapy and ARPI (and some naïve to ADT) in South Africa where these treatments are not available[72]. To mitigate xerostomia, a dose de-escalation protocol was utilized, such that patients who responded to the initial 8 MBq dose would have subsequent dose reductions to 4–7 MBq. Median three cycles were administered. In this less-treated cohort, responses were significant; 14 out of 17 patients experienced over 90% decline in PSA. While all patients experienced xerostomia, none had grade 3+.
PSMA-I&T
The small molecule PSMA-I&T (imaging and therapy) was developed in Germany in 2015[73]. A peptide linker unit was added to a prior compound to increase its lipophilic interaction and affinity to PSMA; it was conjugated to 177Lu through DOTAGA. Compared to 177Lu-PSMA-617, 177Lu-PSMA-I&T has similar PSMA-affinity, pharmacokinetics, and dosimetry data[73, 74]. An initial retrospective report involving 22 patients reported 56% had over 30% decline in PSA[75]. A larger report of 56 patients who received a total of 125 cycles, mean 5.76 GBq per cycle, found that 58.9% had over 50% PSA decline and biochemical progression-free survival was 13.7 months[76]. The organs with the highest absorbed doses were the kidney (0.8 mGy/MBq) and parotid gland (1.3 mGy/MBq). The largest PSMA-I&T cohort so far was described by Heck et al[77]. 100 patients, selected based on PSMA expression on imaging, underwent 319 cycles in 6 to 8-week intervals; mean dose was 7.4 GBq. 47% had over 30% PSA decline, and 38% had over 50% decline. PSA progression-free and overall survival were 4.1 and 12.9 months, respectively. 9% experienced grade 3+ anemia, 6% grade 3+ neutropenia, and 4% grade 3+ thrombocytopenia. The patients included had advanced disease: median PSA 165 ng/mL, 84% with prior chemotherapy, 35% with visceral metastases. As in the case of 177Lu-PSMA-617, liver metastases and elevated ALP are negative prognosticators for response to 177Lu-PSMA-I&T[74, 77]. Re-challenge therapy with 177Lu-PSMA-I&T has also been utilized[78]. In a cohort of eight patients, who initially received six cycles of therapy, two additional cycles were given following PSA progression. As in the case with 177Lu-PSMA-617 re-challenge, responses were fewer and shorter. While all initially had over 50% PSA decline, only 37.5% experienced this with re-challenge. Initial biochemical progression-free survival was 12.4 months, compared to 3.3 months with re-challenge. 177Lu-PSMA I&T (also known as PNT2002] is now moving forward in a planned phase III study[79].
Barber et al. compared outcomes after 177Lu-PSMA-I&T therapy in patients who were chemotherapy naïve against patients with prior chemotherapy[74]. Given their similar properties, PSMA-I&T and PSMA-617 were studied together to form a pool of 167 patients, 83 pre-treated and 84 chemotherapy-naïve. Overall, patients who were chemotherapy naïve were earlier in the disease course; median PSA 33.6 vs. 196.3 ng/mL, 38% vs. 76% with prior ARPI. These patients also may have had less aggressive disease, as there was a trend of lower Gleason scores. Both groups received median three cycles of therapy, mean 6.3 GBq/cycle. For the chemotherapy-naïve patients, radiographic progression-free and overall survival were 8.8 and 27.1 months, respectively; for the pre-treated patients, those respective values were 6 and 10.7 months. On multivariate analysis, prior chemotherapy did not predict either survival. As overall survival in the chemotherapy-naïve cohort exceeded that of mCRPC who received taxanes as first-line therapy, the authors suggested that PSMA TRT is a viable option in both the first-line and progressive mCRPC setting.
Challenges
In addition to logistical issues with needing to administer a drug which includes a radionuclide with finite half-life and safety issues to individuals other than the patient (mostly from gamma emission), there are other unknown issues surrounding the optimization of PSMA-TRT.
Those who promote “theranostics” are in favor of strict patient selection based upon pre-treatment imaging. While “see and treat” approach makes logical sense, it is less clear that positive imaging is required for a response and some with intense imaging do not respond. The vast majority of retrospective and prospective publications have utilized various imaging thresholds of imaging positivity as a requirement of treatment. Most use some cutoff of PSMA SUVmax with relationship to liver uptake. Some as described above also utilize FDG-PET to select out those with discordant (FDG positive / PSMA negative) uptake[54]. In addition, within those parameters (i.e. some positive imaging requirement for all treated), there is some mixed data that those with stronger pre-treatment PSMA imaging have a better outcome following PSMA-TRT[56, 61, 65, 80].
There are a small group of prospective studies that have utilized PSMA imaging as a biomarker rather than as entry criteria. All of the radiolabeled J591 studies in prostate cancer have not required any evidence of PSMA expression by imaging or immunohistochemistry, and this is also true for the dose-escalation studies of 177Lu-PSMA-617 and 225Ac-J591. As described above, there was a signal for lower PSMA expression as determined by imaging to be associated with a lower chance of PSA decline in the phase II study of single-dose 177Lu-J591 and the phase I/II studies of fractionated dose 177Lu-J591 and 177Lu-PSMA-617[26, 27, 64]. In an analysis of 215 patients treated with PSMA-TRT, all with PSMA imaging at baseline that was not used for treatment eligibility, investigators confirmed that high PSMA uptake on baseline imaging (as well as higher doses of radionuclides administered) was associated with response (PSA50 27.5% vs 8.9% in patients classified as having high or low PSMA expression)[81]. However, imaging does not tell the whole story as there are a small number with negative imaging that might respond and not all those with high PSMA uptake who receive a high dose of radioactivity respond. It should be noted that nearly all analyses are relevant for single-agent PSMA-TRT. When co-administered with a drug (such as APRI) that might both change PSMA expression and increase radiosensitivity, these conclusions may not be valid.
In addition, optimal dosing has not been defined, both in terms of amount of radioactivity per administration and interval. Very few dose-escalation studies have been performed. In theory, individualized dosing based upon pre-treatment dosimetry might maximize anti-tumor effect while minimizing toxicity. However, this is difficult to perform in prospective studies (in particular with alpha emitters). Intervals ranging from 2 – 14 weeks have been employed, none compared to each other. Using the concept of repopulation as a mechanism of resistance, more frequent dosing may have benefits, but this needs to be weighed against toxicity and a randomized study may be needed.
Anecdotally, re-treatment with the same radionuclide or with alternative radionuclide (for instance alpha after beta as described above) has shown efficacy and tolerability. In a retrospective study involving 30 patients re-treated with 177Lu-PSMA-617, the patients received three additional cycles after progression of disease, median six months after completing the initial therapy [82]. 40% experienced PSA50. PSA progression-free survival was short, 2.8 months, and median survival was 12 months from the start of re-challenge therapy. Toxicity was increased; compared to a 3% incidence of grade 3 anemia in the initial cycle, 26.7% of patients had grade 3 hematologic adverse events, including 10% with anemia and 13.3% with thrombocytopenia. In Hofman et al.’s prospective study, 15 of the 30 patients with biochemical progression continued to receive 177Lu-PSMA-617[55]. Re-challenge occurred for a median two cycles and 359 days from the initial start of therapy. 73% had PSA decline over 50%, compared to 19% of patients who received chemotherapy after progression. Overall survival was 26.6 months from enrollment. Compared to their initial course, the patients’ biochemical progression-free survival was shorter, median 302 days vs. 123 days. Of note, while hematologic toxicity was comparable, one patient developed chronic kidney disease after 9 cycles, with GFR declining from 91 to 38 mL/min. Renal toxicity has been extremely rare in all the studies to date. A safe threshold for radiation to the kidney has been estimated as 18 Gy, and with re-challenge therapy, this may be exceeded.
However, to date, prediction of acute toxicity has been difficult, as most organ dose limits from radiation are based upon external beam radiation which may not be the same as radionuclide therapy. In addition, no study has examined long-term toxicity well.
Conclusion and Future Directions
PSMA TRT is a promising investigational therapy for the management of mCRPC and many physicians anxiously await widespread availability. There are several ongoing and planned trials that seek to define its survival benefit and enhance efficacy while also studying different disease populations and combinations. Current and planned combinations include PSMA-TRT with ARPI, chemotherapy, PARP inhibitors, and immune checkpoint inhibitors. In addition, dual PSMA-TRT combinations are being utilized and studied[83]. In addition to predictive imaging factors described above, there may be genetic tumor or host predictors as well[84, 85].
References
- 1.Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Annals of Oncology. 2018;29(5):1235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaffer DR, Scher HI. Prostate cancer: a dynamic illness with shifting targets. The Lancet Oncology. 2003;4(7):407–14. [DOI] [PubMed] [Google Scholar]
- 3.Noonan K, North S, Bitting R, Armstrong A, Ellard S, Chi K. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Annals of oncology. 2013;24(7):1802–7. [DOI] [PubMed] [Google Scholar]
- 4.Parker C, Nilsson S, Heinrich D, Helle SI, O’sullivan J, Fosså SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. New England Journal of Medicine. 2013;369(3):213–23. [DOI] [PubMed] [Google Scholar]
- 5.De Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels J- P, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. The Lancet. 2010;376(9747):1147–54. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New England Journal of Medicine. 2010;363(5):411–22. [DOI] [PubMed] [Google Scholar]
- 7.Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, et al. DNA-repair defects and olaparib in metastatic prostate cancer. New England Journal of Medicine. 2015;373(18):1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7(5B):927–35. [PubMed] [Google Scholar]
- 9.O’Keefe DS, Bacich DJ, Huang SS, Heston WDW. A Perspective on the Evolving Story of PSMA Biology, PSMA-Based Imaging, and Endoradiotherapeutic Strategies. J Nucl Med. 2018;59(7):1007–13. doi: 10.2967/jnumed.117.203877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. Journal of cellular biochemistry. 2004;91(3):528–39. [DOI] [PubMed] [Google Scholar]
- 11.Wright GL Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. urologic oncology: seminars and original investigations: Elsevier; 1995. p. 18–28. [DOI] [PubMed] [Google Scholar]
- 12.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–40. [DOI] [PubMed] [Google Scholar]
- 13.Wright GL, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–34. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 14.Emmett L, Yin C, Crumbaker M, Hruby G, Kneebone A, Epstein R, et al. Rapid modulation of PSMA expression by androgen deprivation: serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. Journal of Nuclear Medicine. 2019;60(7):950–4. [DOI] [PubMed] [Google Scholar]
- 15.Iravani A, Violet J, Azad A, Hofman MS. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: Practical nuances and intricacies. Prostate cancer and prostatic diseases. 2019:1–15. [DOI] [PubMed] [Google Scholar]
- 16.Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. Journal of Nuclear Medicine. 2017;58(10):1624–31. [DOI] [PubMed] [Google Scholar]
- 17.Kahn D, Williams RD, Seldin DW, Libertino JA, Hirschhorn M, Dreicer R, et al. Radioimmunoscintigraphy with 111indium labeled CYT-356 for the detection of occult prostate cancer recurrence. The Journal of urology. 1994;152(5):1490–5. [DOI] [PubMed] [Google Scholar]
- 18.Schuster DM, Nieh PT, Jani AB, Amzat R, Bowman FD, Halkar RK, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol. 2014;191(5):1446–53. doi: 10.1016/j.juro.2013.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer biotherapy & radiopharmaceuticals. 1999;14(2):99–111. [DOI] [PubMed] [Google Scholar]
- 20.Deb N, Goris M, Trisler K, Fowler S, Saal J, Ning S, et al. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clinical cancer research. 1996;2(8):1289–97. [PubMed] [Google Scholar]
- 21.Liu H, Moy P, Kim S, Xia Y, Rajasekaran A, Navarro V, et al. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997;57(17):3629–34. [PubMed] [Google Scholar]
- 22.Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V, et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer research. 1998;58(18):4055–60. [PubMed] [Google Scholar]
- 23.Vallabhajosula S, Kuji I, Hamacher KA, Konishi S, Kostakoglu L, Kothari PA, et al. Pharmacokinetics and biodistribution of 111In-and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? Journal of Nuclear Medicine. 2005;46(4):634–41. [PubMed] [Google Scholar]
- 24.Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ, Bander NH. Phase I trial of yttrium-90—labeled anti—prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. Journal of clinical oncology. 2004;22(13):2522–31. [DOI] [PubMed] [Google Scholar]
- 25.Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. Journal of Clinical Oncology. 2005;23(21):4591–601. [DOI] [PubMed] [Google Scholar]
- 26.Tagawa ST, Milowsky MI, Morris M, Vallabhajosula S, Christos P, Akhtar NH, et al. Phase II study of lutetium-177–labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clinical cancer research. 2013;19(18):5182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tagawa ST, Vallabhajosula S, Christos PJ, Jhanwar YS, Batra JS, Lam L, et al. Phase 1/2 study of fractionated dose lutetium-177–labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer. 2019;125(15):2561–9. * One of the largest prospective studies investigating 177Lu-J591 TRT. The dose-fractionation regimen allowed higher cumulative dose, as hypothesized, which resulted in improved efficacy (in terms of PSA decline and survival) with toxicity comparable to prior 177Lu-J591 studies.
- 28.Batra JS, Niaz MJ, Whang YE, Sheikh A, Thomas C, Christos P, et al. Phase I trial of docetaxel plus lutetium-177-labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Urologic Oncology: Seminars and Original Investigations: Elsevier; 2020. [DOI] [PubMed] [Google Scholar]
- 29.Niaz MJ, Batra JS, Walsh RD, Ramirez-Fort MK, Vallabhajosula S, Jhanwar YS, et al. Pilot Study of Hyperfractionated Dosing of Lutetium-177–Labeled Antiprostate-Specific Membrane Antigen Monoclonal Antibody J591 (177Lu-J591) for Metastatic Castration-Resistant Prostate Cancer. The Oncologist. 2020;25(6):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.https://telixpharma.com/news-media/telix-receives-fda-feedback-on-phase-3-trial-design-for-prostate-cancer-therapy-product/ Accessed.
- 31.Roboz GJ, Bennett JM, Coleman M, Ritchie EK, Furman RR, Rossi A, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following initial treatment with chemotherapy plus radioimmunotherapy for indolent non-Hodgkin lymphoma. Leukemia research. 2007;31(8):1141–4. [DOI] [PubMed] [Google Scholar]
- 32.Tagawa ST, Akhtar NH, Nikolopoulou A, Kaur G, Robinson B, Kahn R, et al. Bone marrow recovery and subsequent chemotherapy following radiolabeled anti-prostate-specific membrane antigen monoclonal antibody j591 in men with metastatic castration-resistant prostate cancer. Frontiers in oncology. 2013;3:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tagawa ST, Osborne J, Fernandez E, Thomas C, Niaz MJ, Ciriaco A, et al. Phase I dose-escalation study of PSMA-targeted alpha emitter 225Ac-J591 in men with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2020;38(Supplement 15):5560–5560. * The first prospective study investigating alpha-emitter therapy targeting PSMA, utilizing the J591 antibody. The highest planned dose, 93.3 KBq/kg, was tolerated without dose-limiting toxicity and there was preliminary evidence of efficacy in a PSMA-unselected population, most with prior exposure to PSMA-TRT.
- 34.Hammer S, Hagemann UB, Zitzmann-Kolbe S, Larsen A, Ellingsen C, Geraudie S, et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clinical Cancer Research. 2020;26(8):1985–96. [DOI] [PubMed] [Google Scholar]
- 35.Kratochwil C, Haberkorn U, Giesel FL. Radionuclide therapy of metastatic prostate cancer. Seminars in nuclear medicine: Elsevier; 2019. p. 313–25. [DOI] [PubMed] [Google Scholar]
- 36.Niaz MJ, Skafida M, Osborne J, Nanus D, Molina A, Thomas C, et al. PD16–11 COMPARISON OF PROSTATE-SPECIFIC MEMBRANE ANTIGEN (PSMA)-TARGETED RADIONUCLIDE THERAPY (TRT) WITH LUTETIUM-177 (177LU) VIA ANTIBODY J591 VS SMALL MOLECULE LIGAND PSMA-617. The Journal of Urology. 2020;203(Supplement 4):e367-e. [Google Scholar]
- 37.Pandit-Taskar N, O’Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A Phase I/II Study for Analytic Validation of 89Zr-J591 ImmunoPET as a Molecular Imaging Agent for Metastatic Prostate Cancer. Clin Cancer Res. 2015;21(23):5277–85. doi: 10.1158/1078-0432.CCR-15-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eder M, Schäfer M, Bauder-Wüst U, Hull W-E, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjugate chemistry. 2012;23(4):688–97. [DOI] [PubMed] [Google Scholar]
- 39.Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39(6):1085–6. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 40.Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA oncology. 2019;5(6):856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi-centre study. The Lancet. 2020. [DOI] [PubMed] [Google Scholar]
- 42.Rowe SP, Campbell SP, Mana-Ay M, Szabo Z, Allaf ME, Pienta KJ, et al. Prospective evaluation of PSMA-targeted 18F-DCFPyL PET/CT in men with biochemical failure after radical prostatectomy for prostate cancer. Journal of Nuclear Medicine. 2020;61(1):58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig P, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. Journal of Nuclear Medicine. 2019;60(3):362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eiber M, Krönke M, Wurzer A, Ulbrich L, Jooß L, Maurer T, et al. 18F-rhPSMA-7 positron emission tomography for the detection of biochemical recurrence of prostate cancer following radical prostatectomy. Journal of Nuclear Medicine. 2019:jnumed. 119.234914. [Google Scholar]
- 45.Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a 124 I/131 I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. European journal of nuclear medicine and molecular imaging. 2014;41(7):1280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afshar-Oromieh A, Haberkorn U, Zechmann C, Armor T, Mier W, Spohn F, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with 131 I-MIP-1095. European Journal of Nuclear Medicine and Molecular Imaging. 2017;44(6):950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. Journal of Nuclear Medicine. 2015;56(6):914–20. [DOI] [PubMed] [Google Scholar]
- 48.Kratochwil C, Giesel FL, Stefanova M, Benešová M, Bronzel M, Afshar-Oromieh A, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. Journal of Nuclear Medicine. 2016;57(8):1170–6. [DOI] [PubMed] [Google Scholar]
- 49.Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with 177 Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI research. 2015;5(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7(11):12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. 177 Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. European journal of nuclear medicine and molecular imaging. 2017;44(10):1663–70. [DOI] [PubMed] [Google Scholar]
- 52.Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. Journal of Nuclear Medicine. 2017;58(1):85–90. [DOI] [PubMed] [Google Scholar]
- 53.Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. European Journal of Nuclear Medicine and Molecular Imaging. 2018;45(1):12–9. [DOI] [PubMed] [Google Scholar]
- 54. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. The Lancet Oncology. 2018;19(6):825–33. * The first prospective study of 177Lu-PSMA-617. In a population with heavily pre-treated mCRPC selected by PSMA and FDG PET, PSA response rate was high. The treatment was well-tolerated with onlh a minority with high-grade toxicity. Low grade xerostomia was much more common than that reported in retrospective studies.
- 55. Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang S- P, Kong G, et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. Journal of Nuclear Medicine. 2020;61(6):857–65. * An expansion cohort of the original prospective 177Lu-PSMA-617 trial. Additional survival and outcome data were provided. Furthermore, outcomes for re-treatment therapy were defined.
- 56.Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of 177Lu–PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clinical genitourinary cancer. 2019;17(1):15–22. [DOI] [PubMed] [Google Scholar]
- 57.Halabi S, Lin C- Y, Kelly WK, Fizazi KS, Moul JW, Kaplan EB, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. Journal of Clinical Oncology. 2014;32(7):671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yadav MP, Ballal S, Bal C, Sahoo RK, Damle NA, Tripathi M, et al. Efficacy and Safety of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Clinical Nuclear Medicine. 2020;45(1):19–31. [DOI] [PubMed] [Google Scholar]
- 59.Ferdinandus J, Eppard E, Gaertner FC, Kürpig S, Fimmers R, Yordanova A, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. Journal of Nuclear Medicine. 2017;58(2):312–9. [DOI] [PubMed] [Google Scholar]
- 60.Kessel K, Seifert R, Schäfers M, Weckesser M, Schlack K, Boegemann M, et al. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics. 2019;9(17):4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maffey-Steffan J, Scarpa L, Svirydenka A, Nilica B, Mair C, Buxbaum S, et al. The 68 Ga/177 Lu-theragnostic concept in PSMA-targeting of metastatic castration–resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up. European journal of nuclear medicine and molecular imaging. 2020;47(3):695–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rasul S, Hacker M, Kretschmer-Chott E, Leisser A, Grubmüller B, Kramer G, et al. Clinical outcome of standardized 177 Lu-PSMA-617 therapy in metastatic prostate cancer patients receiving 7400 MBq every 4 weeks. European Journal of Nuclear Medicine and Molecular Imaging. 2020;47(3):713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rathke H, Giesel FL, Flechsig P, Kopka K, Mier W, Hohenfellner M, et al. Repeated 177Lu-labeled PSMA-617 radioligand therapy using treatment activities of up to 9.3 GBq. Journal of Nuclear Medicine. 2018;59(3):459–65. [DOI] [PubMed] [Google Scholar]
- 64. Tagawa S, Osborne J, Hackett A, Niaz M, Cooley V, Christos P, et al. 849PD Preliminary results of a phase I/II dose-escalation study of fractionated dose 177Lu-PSMA-617 for progressive metastatic castration resistant prostate cancer (mCRPC). Annals of Oncology. 2019;30(Supplement_5):mdz248. 006. * The first study exploring dose-escalation of 177Lu-PSMA-617 therapy. Patients received a single fractionated-dose cycle of 22.2 GBq without dose limiting toxicity. In a PSMA-unselected population. 61% had over 50% PSA decline.
- 65.Violet J, Jackson P, Ferdinandus J, Sandhu S, Akhurst T, Iravani A, et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. Journal of Nuclear Medicine. 2019;60(4):517–23. [DOI] [PubMed] [Google Scholar]
- 66.Calais J, Fendler WP, Eiber M, Lassmann M, Dahlbom M, Esfandiari R, et al. RESIST-PC phase 2 trial: 177Lu-PSMA-617 radionuclide therapy for metastatic castrate-resistant prostate cancer. Journal of Clinical Oncology. 2019;37(Supplement 15):5028–5028. [Google Scholar]
- 67.Hofman MS, Emmett L, Sandhu SK, Iravani A, Joshua AM, Goh JC, et al. TheraP: A randomised phase II trial of 177Lu-PSMA-617 (LuPSMA) theranostic versus cabazitaxel in metastatic castration resistant prostate cancer (mCRPC) progressing after docetaxel: Initial results (ANZUP protocol 1603). Journal of Clinical Oncology. 2020;38(Supplement 15):5500–5500. [Google Scholar]
- 68.Hofman MS, Emmett L, Violet J, Y. Zhang A, Lawrence NJ, Stockler M, et al. TheraP: a randomized phase 2 trial of 177Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU international. 2019;124:5–13. [DOI] [PubMed] [Google Scholar]
- 69.Rahbar K, Bodei L, Morris MJ. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase iii vision trial and its importance for the future of theranostics. Journal of Nuclear Medicine. 2019;60(11):1504–6. [DOI] [PubMed] [Google Scholar]
- 70.Kratochwil C, Bruchertseifer F, Giesel FL, Weis M, Verburg FA, Mottaghy F, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. Journal of Nuclear Medicine. 2016;57(12):1941–4. [DOI] [PubMed] [Google Scholar]
- 71.Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. Journal of Nuclear Medicine. 2018;59(5):795–802. , <a [DOI] [PubMed] [Google Scholar]
- 72.Sathekge M, Bruchertseifer F, Knoesen O, Reyneke F, Lawal I, Lengana T, et al. 225 Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. European journal of nuclear medicine and molecular imaging. 2019;46(1):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga-and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. Journal of Nuclear Medicine. 2015;56(8):1169–76. [DOI] [PubMed] [Google Scholar]
- 74.Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of 177lu-psma radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. Journal of Nuclear Medicine. 2019;60(7):955–62. [DOI] [PubMed] [Google Scholar]
- 75.Heck MM, Retz M, D’Alessandria C, Rauscher I, Scheidhauer K, Maurer T, et al. Systemic radioligand therapy with 177Lu labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. The Journal of urology. 2016;196(2):382–91. [DOI] [PubMed] [Google Scholar]
- 76.Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. Journal of Nuclear Medicine. 2016;57(7):1006–13. [DOI] [PubMed] [Google Scholar]
- 77. Heck MM, Tauber R, Schwaiger S, Retz M, D’Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. European urology. 2019;75(6):920–6. * The largest study examining 177Lu-PSMA-I&T therapy, including 100 patients reported retrospectively. Therapeutic and toxicity profiles were defined.
- 78.Gafita A, Rauscher I, Retz M, Knorr K, Heck M, Wester H- J, et al. Early experience of rechallenge 177Lu-PSMA radioligand therapy after an initial good response in patients with advanced prostate cancer. Journal of Nuclear Medicine. 2019;60(5):644–8. [DOI] [PubMed] [Google Scholar]
- 79.: https://www.pointbiopharma.com/press-releases/point-biopharma-announces-phase-3-prostate-cancer-trial Accessed.
- 80. Seifert R, Seitzer K, Herrmann K, Kessel K, Schäfers M, Kleesiek J, et al. Analysis of PSMA expression and outcome in patients with advanced Prostate Cancer receiving 177Lu-PSMA-617 Radioligand Therapy. Theranostics. 2020;10(17):7812. * This study also examined the relationship between imaging features on PSMA-based PET scans and outcome after PSMA TRT. It introduced a new metric described as PSMAaverage, the average SUVmax of the five most avid lesions, and correlated that to survival.
- 81.Vlachostergios PJ, Niaz MJ, Skafida M, Mosallaie SA, Thomas C, Christos PJ, et al. Imaging Expression of Prostate-Specific Membrane Antigen and Response to PSMA-Targeted Beta-Emitting Radionuclide Therapies in Metastatic Castration-Resistant Prostate Cancer. The Prostate. 2021. In press. doi: 10.1002/pros.24104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yordanova A, Linden P, Hauser S, Meisenheimer M, Kürpig S, Feldmann G, et al. Outcome and safety of rechallenge [177 Lu] Lu-PSMA-617 in patients with metastatic prostate cancer. European journal of nuclear medicine and molecular imaging. 2019;46(5):1073–80. [DOI] [PubMed] [Google Scholar]
- 83.Khreish F, Ebert N, Ries M, Maus S, Rosar F, Bohnenberger H, et al. 225 Ac-PSMA-617/177 Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. European journal of nuclear medicine and molecular imaging. 2020;47(3):721–8. [DOI] [PubMed] [Google Scholar]
- 84.Vlachostergios PJ, Conteduca V, Hackett AL, Manohar J, Lee A, Case A, et al. Prognostic value of BRCA2 and AR gene alterations in advanced prostate cancer patients treated with PSMA-targeted radionuclide therapies. Cancer Res. 2019;79(Supplement 13): 4865. [Google Scholar]
- 85.Paschalis A, Sheehan B, Riisnaes R, Rodrigues DN, Gurel B, Bertan C, et al. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. European urology. 2019;76(4):469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]