Abstract
Background and objective
A novel fungal allergen- Alternaria (Alt) has been previously shown to associate with the pathogenesis of allergic rhinitis and bronchial asthma, particularly in arid and semi-arid regions. Airway epithelial cells are among the first to encounter Alt, and epithelial cytokine production and subsequent airway inflammation are early events in the response to Alt exposure. However, the underlying mechanism is unclear. As PAR2 has been implicated in most of Alt-induced biological events, we investigated the regulation of airway inflammation and epithelial cytokine expression by PAR2.
Methods
Wild-type (WT) and Par2 knockout (Par2-KO) mice were used to evaluate in vivo role of PAR2. Primary human and mouse airway epithelial cells were used to examine the mechanistic basis of epithelial cytokine regulation in vitro.
Results
Surprisingly, Par2 deficiency had no negative impact on the change of lung function, inflammation and cytokine production in the mouse model of Alt-induced asthma. Alt-induced cytokine production in murine airway epithelial cells from Par2KO mice was not significantly different from the WT cells. Consistently, PAR2 knockdown in human cells also had no effect on cytokine expression. In contrast, the cytokine expressions induced by synthetic PAR2 agonist or other asthma related allergens (e.g. cockroach extracts) were indeed mediated via a PAR2-depndent mechanism. Finally, we found that EGFR pathway was responsible for Alt-induced epithelial cytokine expression.
Conclusion
The activation of EGFR, but not PAR2, was likely to drive the airway inflammation and epithelial cytokine production induced by Alt.
Keywords: Alternaria, airway, epithelium, cytokine, EGFR
INTRODUCTION:
Asthma is characterized by a reversible airway obstruction as the results of predominantly Th2-driven airway inflammation and pulmonary remodeling. The development of asthma usually starts from a repeated environmental allergen exposure and sensitization (1, 2). To date, a couple of allergens (e.g. cockroach(5), house dust mite(5) and Alternaria(6)) have been found to closely associate with asthma.
Alternaria (Alt) is a common fungus found in the soil and decaying vegetables(7). Alt was first found to be the major asthmagenic allergen in children raised in arid and semiarid regions (6). Subsequent European community respiratory health survey has found that sensitization to fungi (Alt or Cladosporium) was a powerful risk for severe asthma in adults (8). A recent NIEHS study has indicated that Alt exposure in homes throughout the United States was associated with active asthma symptoms (9). Thus, Alt exposure appears to be closely associated with asthma in both children and adults across different demographic regions. However, underlying molecular mechanisms are unclear.
Airway epithelium has emerged as one of the major components in asthma pathogenesis by regulating airway inflammation and immunity (10-12). Since airway epithelial cells are among the first to encounter Alt exposure, epithelial responses may represent early events that ultimately lead to asthmatic phenotypes. In previous studies that primarily utilized cancerous or immortalized cell line models, Alt was found to induce a variety airway epithelial responses including cytokine expression (13, 14), secretion (15), ATP release (16) and calcium flux(15-17). Notably, PAR2 appears to be the sole sensor of Alt exposure and to directly mediate all these downstream effects. However, Denis O et.al has recently demonstrated that the protease activity of Alt was dispensable for its effect on airway immune response (18). Most interestingly, the same group who first discovered the role of PAR2 in Alt-induced airway response(13-15) reported an updated finding indicating that PAR2 was not involved in regulating Ca2+ flux and ATP release from airway epithelial cells in response to Alt exposure (16). Thus, whether or not PAR2 mediates Alt-induced airway response remains elusive. Therefore, we seek to investigate the role of PAR2 using both in vivo and in vitro models of Alt exposure.
METHODS:
1. Chemicals, Antibodies and other reagents
Lyophilized cake of Alternaria filtrates (GREER, Lenoir, NC) was dissolved in HBSS to make a 100X stock solution. Antibodies targeting total and phosphorylated (p)-EGFR were from Cell signaling technology (Danvers, MA). Human and mouse PAR2 antibodies were from Thermo Fisher (Waltham, MA) and Abcam (Cambridge, MA). Anti-actin antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
2. Mice Exposures:
5 week old adult C57BL/6 WT and C57BL/6 PAR2-KO mice were briefly anesthetized with 3% isofluorane and intranasally administered 10ug Alternaria filtrates every other day for total three times. Control mice were administered with the same volume of HBSS (the solvent of the filtrates). 8 animals were used for each group.
3. Airway Hyperresponsiveness (AHR):
Mice were inhalationally challenged with different doses of methacholine. AHR was measured by the FlexiVent® system. Peak resistance values at each dose were plotted against the corresponding methacholine dose.
4. Bronchoalveolar Lavage (BAL), differential cell count and Cytokine ELISA:
Lungs were instilled twice with 1mL HBSS to collect BAL. The cells in the BAL were cytocentrifuged, air-dried, stained with HEMA 3 stain set (Thermo Scientific, Gilbert, AZ) and the number of macrophages, neutrophils, eosinophils, and lymphocytes were then counted in a blinded manner using light microscopy by at least two researchers to ensure an objective evaluation. Differential cell counts (macrophage, neutrophil, eosinophil, lymphocyte and monocytes) were presented as percentage of each cell type. ELISA Assays (R&D Systems, Minneapolis, MN) were used for BAL cytokine measurements.
5. Primary mouse tracheal epithelial (MTE) cell culture:
The protocol was performed as described previously (19). Briefly, at the time of necropsy, a small puncture was placed in the proximal trachea to allow cannulation with sterile 0.86 mm polyethylene tubing and was secured in place with a 3.0 silk suture. The trachea was dissected free and immediately placed in DMEM at 4°C. And MTE cells were dissociated with 0.2% protease and gently harvested by washing out of the trachea. MTE cells from all tracheas were pooled and re-suspended in cell culture media prior to plating on the Transwell® chambers (Corning, Corning, New York) coated with Purcol© (Advanced cBiomatrix, San Diego, CA).
6. Primary human bronchial epithelial (HBE) cell culture
Human bronchial tissues were obtained with patients’ informed consent from the National Disease Research Interchange (Philadelphia, PA). Tissues from patients diagnosed with lung-related diseases were excluded. Protease-dissociated cells were plated on Transwell® chambers and were maintained in immersed culture conditions until they reached confluence. At this stage, the cells were deemed as mono-layered cells. For the fully differentiated cell culture, confluent cells were changed to an air-liquid interface (ALI) culture condition for additional 14 days to achieve mucociliary differentiation (20, 21).
8. Real-time PCR
Real-time PCR was performed as described previously (22). The relative mRNA amount in each sample was calculated based on the ΔΔCt method using housekeeping gene Actin. Results were usually calculated as fold induction over control as described previously (23), except for the RV positive-stranded RNA was presented in relative abundance (20).
9. Small interference RNA (siRNA) and transient transfection
Control siRNA, siRNA against PAR2 (GGATGTGGAACCTGTTTAA) (24) , and siRNA against EGFR (CTCTGGAGGAAAAGAAAGT)(25) were synthesized by Ambion (Austin, TX). siRNA was transfected into cells using lipofectamine™ 2000 (ThermoFisher, Waltham, MA) based on the manufacturer instructions.
10. Western Blot
Total cellular protein was collected based on the methods described previously (23). All experiments were repeated for three times and the representative image was shown. Equal protein load for total cellular proteins was confirmed using the staining of anti-actin antibody.
11. Statistical analysis
Experimental groups were compared using a two-sided Student's t test, with significance level set as P < 0.05. When data were not distributed normally, significance was assessed with the Wilcoxon matched-pairs signed-ranks test, and P < 0.05 was considered to be significant.
RESULTS:
1. The role of Par2 in the pathogenesis of mouse model of Alt-induced asthma.
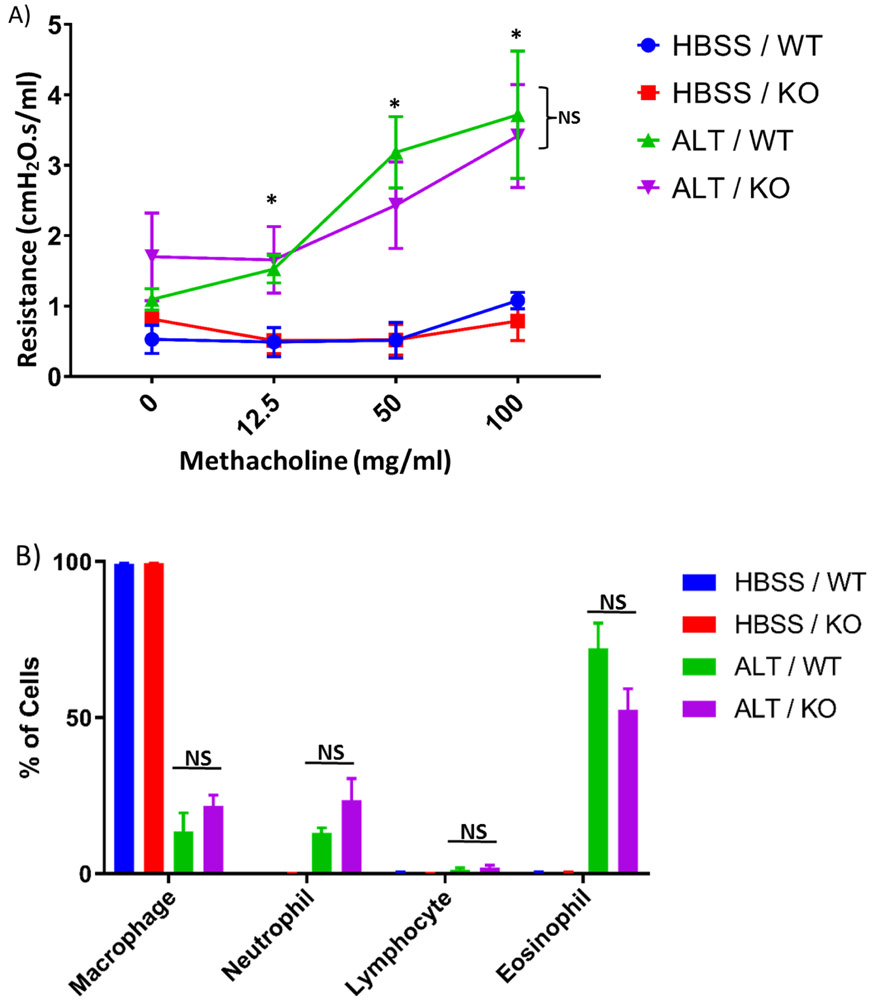
We tested the role of Par2 in an established mouse model of Alt-induced asthma. As shown in Fig. 1A, Alt induced dose-dependent airway hyperresponsiveness (AHR) to inhaled methacholine as compared to the vehicle control (HBSS). However, Alt-induced AHR was not affected by Par2 deficiency. In both models, Alt treatment significantly increased the number of neutrophil, eosinophil and lymphocyte in BAL (Fig.1B). However, the percentages of these cell types were not significantly different between Par2 KO and WT. These data suggest that Par2 was not likely to play a significant role in Alt-induced lung inflammation and AHR.
Fig.1:
Alt-induced mouse model of asthma. A) The effect of Par2 deficiency on lung function. Peak resistance after methacholine challenge in WT and KO mice exposed to either Alt or HBSS was presented as Mean ± SEM, n = 8/group. NS: No significance was observed between WT and KO. *: P < 0.05, ALT/ KO vs. HBSS/ KO, or ALT/ WT vs. HBSS/ WT. B) Differential cell counts. Different cell types in BAL samples were counted from these mice and presented as the percentage of total cells. NS: Not significant.
2. Alt-induced cytokine production in mouse model was independent of Par2.
Cytokines are critical players in inflammatory diseases such as asthma. We found that multiple cytokines were highly elevated in mouse airways in response to Alt exposure (Fig.2). They can be categorized as proinflammatory cytokines (G-CSF, IL-1α, IL-6,), Th2 cytokines (IL-5, IL-9), Th17 cytokine (IL-17) and chemokines (Eotaxin, KC, MIG). Interestingly, Par2 deficiency appeared not to affect any of their productions in BAL (Fig.2). Thus, Par2 deficiency appears to have no effect on these common airway cytokine/chemokine productions induced by Alt.
Fig.2.
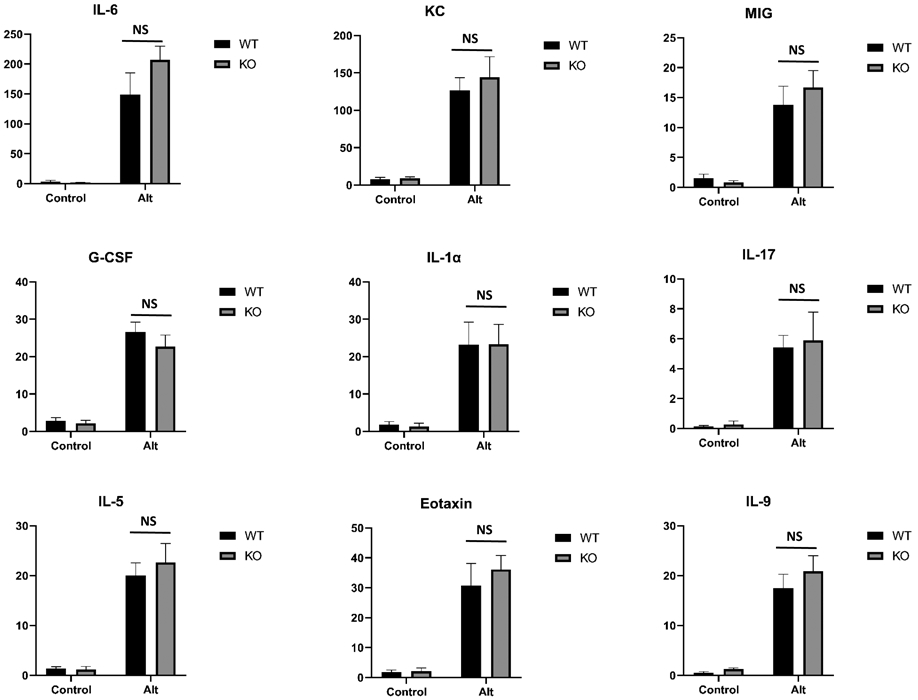
Effect of Par2 deficiency on cytokine production by ELISA assay. Data represents mean ± SEM, n = 8/group;
3. Alt-induced epithelial cytokine expressions were independent of PAR2.
Since airway epithelium produces significant amount of cytokines, we tested the role of PAR2 in primary epithelial cell cultures by measuring two major airway epithelial cytokines-IL-6 (Fig.3A, C) and IL-8 (Fig.3B, D). Both cytokines were induced by Alt dose- and time-dependently in mono-layered primary HBE cells (Fig.3). Their expression peaked at 6h and decreased later. Interestingly, when the cells were fully differentiated under air-liquid interface, much higher dose (50 vs 21.5 μg/ml) of Alt was required to increase cytokine expression (Fig.3C-D). Furthermore, Alt exposure from basal side, but not from apical surface, induced significant cytokine expression (Fig.3C-D), suggesting that the intact airway epithelium is highly resistant to Alt exposure and the breach of tight junction is required for Alt-induced epithelial response.
Fig.3:
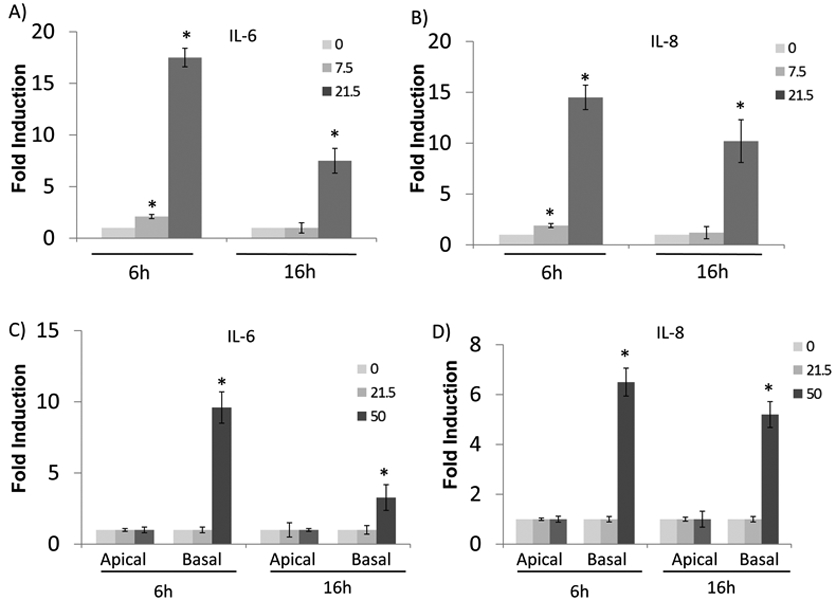
Alt induced epithelial cytokine expression in HBE cells. Both mono-layered (A-B) or fully-differentiated (C-D) epithelial cells were treated with increasing dose of Alt at 0, 7.5 and 25ug/ml for 6 hours (h) and 16h, respectively as indicated in the figure. For mono-layered epithelial cells, they were treated from apical side. For fully differentiated epithelial cells, they were treated on either apical or basal side, separately. RNA was collected at the indicated time point and was analyzed by realtime PCR analysis. The expressions of IL-6 (A, C) and IL-8 (B, D) were determined. *: p< 0.05, n=5.
We next tested the role of PAR2 in airway epithelial cell model. Because of the robust Alt-induced response, we continued to use mono-layered (but not fully differentiated) cell culture. In the first approach, we isolated MTE cells from WT or from Par2-KO mice and treated them with Alt. Consistent with the in vivo finding, the lack of Par2 (Fig.4C) had no effect on selected cytokines and chemokines including IL-6 (Fig.5A), KC (a functional equivalent of human IL-8 in mouse, Fig.5B), MIG (data not shown). We failed to detect any expression of IL-5, IL-9 and IL-17 in the epithelial culture, suggesting that these cytokines may be secreted by infiltrated inflammatory cells. In the second approach, we used siRNA against PAR2 to knock down its expression in HBE cells (Fig.4F). Consistent with the data from MTE cells, the knockdown of PAR2 had no effect on the expressions of IL-6 (Fig. 4D) and IL-8 (Fig.4E) as well as on the secretory IL-6 (Fig.4G) and IL-8 (Fig.4H).
Fig.4:
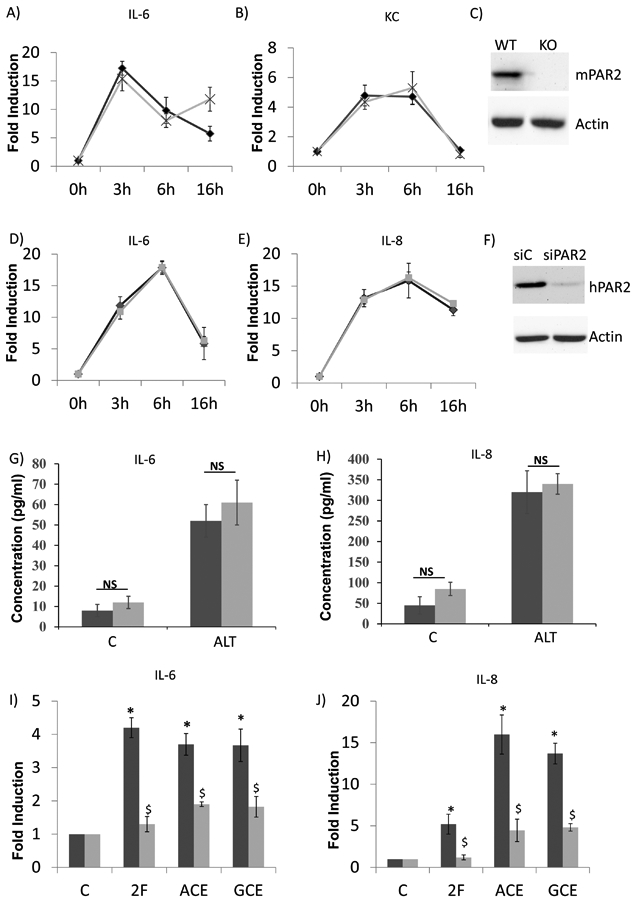
Epithelial cytokine production was independent of PAR2 signaling. To disrupt PAR2 signaling, both PAR2 knockout (KO) mice (A-C) and siRNA interference (D-F) were used. A-C) primary mouse trachea epithelial cells from either wild-type (WT) or PAR2 KO mice were cultivated and treated with Alt. The expressions of Il-6 (A) or KC (B) (a functional equivalent of human IL-8 in mouse) were determined by real-time PCR. The data are presented as the fold inductions comparing the treatment with the solvent control. n= 5. Black line: the cells from WT. Grey line: the cells from PAR2 KO. C) Successful knockout of mouse Par2 was demonstrated by western blot. D-H) siRNA interference of PAR2 signaling in human primary cells. Control siRNA (siC) and PAR2 siRNA (siPAR2) were transfected into epithelial cells. 24 hours later, the cells were treated with 21.5ug/ml of Alt for the time as indicated in the figure. RNA was then extracted, IL-6 (D) and IL-8 (E) were determined by real-time PCR. F) Successful knockdown of PAR2 was demonstrated by western blot. RNA data was confirmed by measuring secretory IL-6 (G) and IL-8 (H) at 16h using ELISA assay. Black line or bar: the cells transfected with siC. Grey line or bar: the cells transfected with siPAR2. I-J) siC and siPAR2 were transfected into epithelial cells. 24hrs later, the cells were treated with PAR2 ligand (2-Furoyl-LIGRLO-amide, or 2F), or American cockroach extracts (ACE), or German cockroach extracts (GCE) for 6 hours. RNA was then extracted, and IL-6 (I) and IL-8 (J) were determined by real-time PCR. Black bar: the cells transfected with siC. Grey bar: the cells transfected with siPAR2. *: p< 0.05, when the treatment groups were compared to the control. $: p< 0.05 when siPAR2 transfected cells were compared to siC transfected cells, n= 5.
Fig.5:
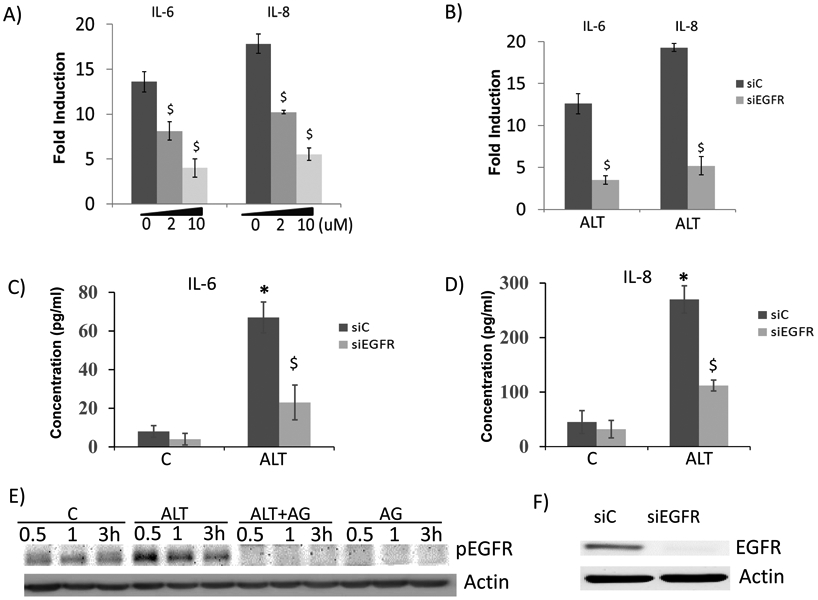
EGFR pathway was involved in Alt-induced cytokine expression in HBE cells. A) Cells were pretreated with increasing dose (0, 2, 10 μM) of AG1478 for 30min, and then were treated with Alt for 3hrs. IL-6 and IL-8 were determined by real-time PCR. $: p< 0.05, AG1478 treated vs. control. n= 5. B) Cells were transfected with siC and siEGFR for 24hrs, and then were treated with Alt for 3 hours. IL-6 and IL-8 were determined by real-time PCR. Secretory IL-6 (C) and IL-8 (D) were measured by ELISA assay, respectively. $: p< 0.05, siEGFR treated vs. siC. n= 5. E) Cells were pretreated with or without AG1478 for 30min, and then were treated with Alt for 0.5, 1, 3h, respectively. Total cellular protein was collected for western blot analysis of phosphorylated (p)-EGFR. Actin was used as the loading control. F) Successful knockdown of EGFR was demonstrated by western blot.
One possible explanation of this deficiency was that airway epithelial cells might not have functional PAR2-mediated signaling despite the presence of PAR2 (Fig.4F). To rule out this possibility, we test a specific PAR2 ligand (2-Furoyl-LIGRLO-amide, or 2F)) and two documented PAR2-dependent allergens: American cockroach extracts (ACE) and German cockroach extracts (GCE). PAR2 knockdown significantly repressed the inductions of IL-6 (Fig.4I) and IL-8 (Fig.4J) by 2F, ACE and GCE, respectively. Therefore, PAR2 signaling pathway is functional in human airway epithelial cells, but it appears not to play a role in the Alt-induced epithelial cytokine expression.
4. EGFR signaling was involved in Alt-induced cytokine expression.
To further understand the mechanism underlying Alt-induced epithelial cytokine expression, we screened several inhibitors targeting major signaling pathway, and found out that EGFR-specific inhibitor (AG1478) dose-dependently repressed Alt-induced IL-6 and IL-8 expression in HBE cells by as much as 70% (at 10μM) (Fig.5A). The inhibitory activity of AG1478 was demonstrated by its effect on repressing EGFR activation (Fig.5E). Consistently, EGFR knockdown (Fig.5F) significantly repressed gene expressions (Fig.5B) and protein secretions of IL-6 (Fig.5C) and IL-8 (Fig.5D). Thus, Alt-induced airway epithelial cytokine expression was at least partially mediated by EGFR pathway.
DISCUSSION:
In response to Alt proteases, airway epithelial cells produce large amounts of proinflammatory (e.g. IL-6, IL-8, GM-CSF) (26) or pro-Th2 (e.g. TSLP(13), IL-33(15)) cytokines/chemokines to recruit/activate dendritic cells (DCs), Th2 cells and granulocytes (eosinophils, neutrophils and basophils), thereby inducing and exacerbating airway Th2 inflammation. Indeed, proinflammatory, Th2, Th17 cytokines and chemokines were elevated by Alt in our mouse model. In contrast to the literature showing that PAR2 signaling was responsible for such effects (13-17, 27), we have found that all these Alt-induced cytokine/chemokine expressions were independent of Par2. Furthermore, the lack of Par2 did not affect differential cell count in BAL and AHR, suggesting that Par2 activation had no significant effect on Alt-induced airway inflammation and AHR.
Consistent with the in vivo observation, either the lack of Par2 in MTE or the decrease of PAR2 in HBE had no effect on selected cytokine expressions. These results seemingly contradict with the existing literature. However, most of the previous reports used non-specific approaches that are less likely to ascertain the role of PAR2. For example, PAR2 specific ligands (e.g. 2-Furoyl-LIGRLO-amide) were often used to desensitize PAR2 (13, 16, 27). However, this desensitization may not be specific due to the possibility of heterologous desensitization (i.e. the loss of responsiveness of the related groups of GPCRs) (28). In another report, a PAR2 peptide (LSIGKV) was used to block PAR2 (14). However, there is no documented antagonistic activity for this peptide (29). We actually found that LSIGKV had no effect on PAR2 activation (data not shown).
PAR2 independent mechanisms have been implied in the studies of other protease-containing allergens (30-33), but no mechanistic basis has been defined. One possible PAR-independent effector is for Alt proteases to disrupt the epithelial barrier function, thereby facilitating the entry of deleterious materials to cause inflammation. The cysteine protease activity of HDM extracts was demonstrated to increase the permeability of isolated sheets of bronchial mucosa (35). HDM proteases (Der p 1, 3, 6, 9) (36, 37) and fungal Pen ch 13 (38) could directly digest major epithelial junctional proteins such as occludin and zonula occludens-1 (ZO-1), thereby increasing the permeability of bronchial epithelium and facilitating allergen presentation in epithelial or subepithelial tissue(39). This is consistent with our study showing that fully differentiated epithelial culture was highly resistant to Alt treatment from the apical side. In fact, 100μg/ml Alt (twice as our highest dose) was required to disrupt normal epithelial barrier (40). These reports support the notion that Alt exposure alone in normal airway may not have significant pathogenic effect, which is in line with the fact that thousands of fungal spores are breathed into human lung every day without causing any disease. Emerging evidence indicates that airway barrier is defective in asthmatic patients(41). Reduced expression of major junctional proteins such as α-catenin, ZO-1, and E-cadherin was reported in bronchial biopsy specimens from asthmatic subjects (42). In bronchial epithelial cells from asthmatic subjects, the expressions of ZO-1 and occludin expression were low and associated with significantly attenuated barrier function (43). Indeed, asthmatic epithelial cells tend to be more susceptible to Alt treatment (40). Although our study did not explicitly tested epithelial cells from asthmatics, Alt was found to induce robust response from basal side, suggesting that putative cellular receptor of Alt might be well protected from the environment and likely be exposed due to impaired barrier function.
We have serendipitously found that Alt induced robust EGFR activation. The blockade of EGFR attenuated at least 70% of the Alt activity in cytokine induction. In the past, HDM has been shown to activate EGFR in a PAR2 dependent mechanism (44, 45). The present report is the first demonstration that Alt also activated EGFR in a PAR2-independent manner. Although the molecular link between Alt and EGFR is unclear, the disruption of junctional protein such as E-cadherin could lead to EGFR activation (46). Additionally, it is well documented that the EGFR is located at the basolateral region of the fully differentiated epithelial cells (47). This is consistent with our observation that basal treatment of Alt was required to induce cytokine production.
In summary, we have demonstrated that PAR2 is dispensable for Alt-induced airway inflammation and cytokine production. And EGFR activation appears to be responsible for the most part of epithelial cytokine expressions induced by Alt. Further understanding of the molecular link between Alt and EGFR will advance our knowledge about Alt-induced asthma pathogenesis.
Table.1.
PCR Primers (mPar2: mouse Par2. mActin: mouse actin)
| Gene | Primers | |
|---|---|---|
| IL-6 | forward | ATTGCCTCAAGGACAGGATG |
| reverse | GCTGCAGCTGCTTAATCTCC | |
| IL-8 | forward | GGACGCCTTGGAAGAGTCACT |
| reverse | AGAAGCCTCAGGTCCCAATTC | |
| ACTIN | forward | ACTGGAACGGTGAAGGTGACA |
| reverse | ATGGCAAGGGACTTCCTGTAAC | |
| PAR2 | forward | AACCTTCTGCTTGTGGTGCAT |
| reverse | GGGTAGAGAGGCAGAGGGCTA | |
| mPar2 | forward | CCCTCAACAGCTGCATAGACC |
| reverse | CTTGTTGGAGCTGAGCGAGAT | |
| mActin | forward | AGCCATGTACGTAGCCATCC |
| reverse | TAGAAGCACTTGCGGTGCAC | |
SUMMARY AT A GLANCE.
PAR2 mediated signaling pathway has been implicated in most of biological activities induced by Alternaria, an allergen associated with allergic rhinitis and bronchial asthma. Using both in vivo and in vitro models, we have demonstrated that Alternaria-induced airway inflammation and epithelial cytokine expression were independent of PAR2 signaling.
Acknowledgements
The study was supported partly by NIH grants ES027013, ES027547, ES028889, and AI39439.
References
- 1.Busse WW, Mitchell H. Addressing issues of asthma in inner-city children. J Allergy Clin Immunol 2007; 119: 43–49. [DOI] [PubMed] [Google Scholar]
- 2.Nelson HS, Szefler SJ, Jacobs J, Huss K, Shapiro G, Sternberg AL. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol 1999; 104: 775–785. [DOI] [PubMed] [Google Scholar]
- 3.Abraham CM, Ownby DR, Peterson EL, Wegienka G, Zoratti EM, Williams LK, Joseph CL, Johnson CC. The relationship between seroatopy and symptoms of either allergic rhinitis or asthma. J Allergy Clin Immunol 2007; 119: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 4.Anthracopoulos MB, Mantzouranis E, Paliatsos AG, Tzavelas G, Lagona E, Nicolaidou P, Priftis KN. Different effects of sensitization to mites and pollens on asthma symptoms and spirometric indices in children: a population-based cohort study. Ann Allergy Asthma Immunol 2007; 99: 122–129. [DOI] [PubMed] [Google Scholar]
- 5.Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Current allergy and asthma reports 2007; 7: 363–367. [DOI] [PubMed] [Google Scholar]
- 6.Halonen M, Stern DA, Wright AL, Taussig LM, Martinez FD. Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med 1997; 155: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez H, Bush RK. A review of Alternaria alternata sensitivity. Revista iberoamericana de micologia 2001; 18: 56–59. [PubMed] [Google Scholar]
- 8.Zureik M, Neukirch C, Leynaert B, Liard R, Bousquet J, Neukirch F. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community respiratory health survey. BMJ 2002; 325: 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salo PM, Arbes SJ Jr., , Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol 2006; 118: 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gras D, Chanez P, Vachier I, Petit A, Bourdin A. Bronchial epithelium as a target for innovative treatments in asthma. Pharmacology & therapeutics 2013; 140: 290–305. [DOI] [PubMed] [Google Scholar]
- 11.Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol 2014; 151: 1–15. [DOI] [PubMed] [Google Scholar]
- 12.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergology international: official journal of the Japanese Society of Allergology 2008; 57: 1–10. [DOI] [PubMed] [Google Scholar]
- 13.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol 2009; 183: 1427–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuwaki Y, Wada K, White T, Moriyama H, Kita H. Alternaria fungus induces the production of GM-CSF, interleukin-6 and interleukin-8 and calcium signaling in human airway epithelium through protease-activated receptor 2. International archives of allergy and immunology 2012; 158 Suppl 1: 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol 2011; 186: 4375–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Grady SM, Patil N, Melkamu T, Maniak PJ, Lancto C, Kita H. ATP release and Ca2+ signalling by human bronchial epithelial cells following Alternaria aeroallergen exposure. The Journal of physiology 2013; 591: 4595–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boitano S, Flynn AN, Sherwood CL, Schulz SM, Hoffman J, Gruzinova I, Daines MO. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. American journal of physiology Lung cellular and molecular physiology 2011; 300: L605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denis O, Vincent M, Havaux X, De Prins S, Treutens G, Huygen K. Induction of the specific allergic immune response is independent of proteases from the fungus Alternaria alternata. European journal of immunology 2013; 43: 907–917. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. The Journal of biological chemistry 2003; 278: 17036–17043. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Hamati E, Lee PK, Lee WM, Wachi S, Schnurr D, Yagi S, Dolganov G, Boushey H, Avila P, Wu R. Rhinovirus induces airway epithelial gene expression through double-stranded RNA and IFN-dependent pathways. Am J Respir Cell Mol Biol 2006; 34: 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Zhao YH, Di YP, Wu R. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino-terminal and 5'-flanking region. Am J Respir Cell Mol Biol 2001; 25: 542–553. [DOI] [PubMed] [Google Scholar]
- 22.Kao CY, Chen Y, Thai P, Wachi S, Huang F, Kim C, Harper RW, Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol 2004; 173: 3482–3491. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L, Lee PK, Lee WM, Zhao Y, Yu D, Chen Y. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am J Respir Cell Mol Biol 2009; 40: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and −2 regulates barrier function and transepithelial migration. J Immunol 2008; 181: 5702–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueki IF, Min-Oo G, Kalinowski A, Ballon-Landa E, Lanier LL, Nadel JA, Koff JL. Respiratory virus-induced EGFR activation suppresses IRF1-dependent interferon lambda and antiviral defense in airway epithelium. The Journal of experimental medicine 2013; 210: 1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattoli S, Marini M, Fasoli A. Expression of the potent inflammatory cytokines, GM-CSF, IL6, and IL8, in bronchial epithelial cells of asthmatic patients. Chest 1992; 101: 27S–29S. [DOI] [PubMed] [Google Scholar]
- 27.Lan RS, Stewart GA, Henry PJ. Role of protease-activated receptors in airway function: a target for therapeutic intervention? Pharmacology & therapeutics 2002; 95: 239–257. [DOI] [PubMed] [Google Scholar]
- 28.Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of GPCR desensitization. British journal of pharmacology 2008; 153 Suppl 1: S379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa H, Kawai K, Tanaka M, Ohtani H, Tanaka S, Kitagawa C, Nishida M, Abe T, Araki H, Kawabata A. Protease-activated receptor-2 (PAR-2)-related peptides induce tear secretion in rats: involvement of PAR-2 and non-PAR-2 mechanisms. The Journal of pharmacology and experimental therapeutics 2005; 312: 324–331. [DOI] [PubMed] [Google Scholar]
- 30.Adam E, Hansen KK, Astudillo Fernandez O, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p 1, unlike Der p 3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. The Journal of biological chemistry 2006; 281: 6910–6923. [DOI] [PubMed] [Google Scholar]
- 31.Kato T, Takai T, Fujimura T, Matsuoka H, Ogawa T, Murayama K, Ishii A, Ikeda S, Okumura K, Ogawa H. Mite serine protease activates protease-activated receptor-2 and induces cytokine release in human keratinocytes. Allergy 2009; 64: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 32.Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clinical and molecular allergy : CMA 2006; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol 2001; 167: 1014–1021. [DOI] [PubMed] [Google Scholar]
- 34.Herbert CA, King CM, Ring PC, Holgate ST, Stewart GA, Thompson PJ, Robinson C. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. American journal of respiratory cell and molecular biology 1995; 12: 369–378. [DOI] [PubMed] [Google Scholar]
- 35.Roche N, Chinet TC, Belouchi NE, Julie C, Huchon GJ. Dermatophagoides pteronyssinus and bioelectric properties of airway epithelium: role of cysteine proteases. The European respiratory journal 2000; 16: 309–315. [DOI] [PubMed] [Google Scholar]
- 36.Wan H, Winton HL, Soeller C, Taylor GW, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2001; 31: 279–294. [DOI] [PubMed] [Google Scholar]
- 37.Wan H, Winton HL, Soeller C, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 2000; 30: 685–698. [DOI] [PubMed] [Google Scholar]
- 38.Tai HY, Tam MF, Chou H, Peng HJ, Su SN, Perng DW, Shen HD. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy 2006; 61: 382–388. [DOI] [PubMed] [Google Scholar]
- 39.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. The Journal of experimental medicine 2001; 193: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leino MS, Loxham M, Blume C, Swindle EJ, Jayasekera NP, Dennison PW, Shamji BW, Edwards MJ, Holgate ST, Howarth PH, Davies DE. Barrier disrupting effects of alternaria alternata extract on bronchial epithelium from asthmatic donors. PloS one 2013; 8: e71278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holgate ST. Epithelium dysfunction in asthma. The Journal of allergy and clinical immunology 2007; 120: 1233–1244; quiz 1245-1236. [DOI] [PubMed] [Google Scholar]
- 42.de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Canadian journal of physiology and pharmacology 2008; 86: 105–112. [DOI] [PubMed] [Google Scholar]
- 43.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, Cremin C, Sones J, Djukanovic R, Howarth PH, Collins JE, Holgate ST, Monk P, Davies DE. Defective epithelial barrier function in asthma. The Journal of allergy and clinical immunology 2011; 128: 549–556 e541-512. [DOI] [PubMed] [Google Scholar]
- 44.Heijink IH, Postma DS, Noordhoek JA, Broekema M, Kapus A. House dust mite-promoted epithelial-to-mesenchymal transition in human bronchial epithelium. American journal of respiratory cell and molecular biology 2010; 42: 69–79. [DOI] [PubMed] [Google Scholar]
- 45.Heijink IH, van Oosterhout A, Kapus A. Epidermal growth factor receptor signaling contributes to house dust mite-induced epithelial barrier dysfunction. The European respiratory journal 2010; 36: 1016–1026. [DOI] [PubMed] [Google Scholar]
- 46.Heijink IH, Kies PM, Kauffman HF, Postma DS, van Oosterhout AJ, Vellenga E. Down-regulation of E-cadherin in human bronchial epithelial cells leads to epidermal growth factor receptor-dependent Th2 cell-promoting activity. J Immunol 2007; 178: 7678–7685. [DOI] [PubMed] [Google Scholar]
- 47.Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, Welsh MJ. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature 2003; 422: 322–326. [DOI] [PubMed] [Google Scholar]