Significance
Bicomponent leukotoxins represent a promiscuous group among the virulence factors secreted by Staphylococcus aureus that help the pathogen to develop and evade the host immune system. These toxins specifically recognize host G protein–coupled receptors (GPCRs) in an unknown molecular mechanism and functional effects on the receptor’s biology. Here, using HlgA and HlgB, we identify atypical chemokine receptor 1 (ACKR1) as a receptor for HlgB and demonstrate that toxins compete with the ACKR1 natural ligand. Furthermore, we find that binding of leukotoxins leads to an allosteric conformational change in the ACKR1 intracellular part and interferes with receptor architecture, a process that was correlated with a destabilization of receptor−G protein interaction in living cells. Overall, our findings lay the ground for the development of antimicrobials by shielding leukotoxin−GPCR interactions.
Keywords: GPCR, host–pathogen interactions, structural mass spectrometry, pharmacology
Abstract
Atypical chemokine receptor 1 (ACKR1) is a G protein–coupled receptor (GPCR) targeted by Staphylococcus aureus bicomponent pore-forming leukotoxins to promote bacterial growth and immune evasion. Here, we have developed an integrative molecular pharmacology and structural biology approach in order to characterize the effect of leukotoxins HlgA and HlgB on ACKR1 structure and function. Interestingly, using cell-based assays and native mass spectrometry, we found that both components HlgA and HlgB compete with endogenous chemokines through a direct binding with the extracellular domain of ACKR1. Unexpectedly, hydrogen/deuterium exchange mass spectrometry analysis revealed that toxin binding allosterically modulates the intracellular G protein–binding domain of the receptor, resulting in dissociation and/or changes in the architecture of ACKR1−Gαi1 protein complexes observed in living cells. Altogether, our study brings important molecular insights into the initial steps of leukotoxins targeting a host GPCR.
The emergence of various multidrug-resistant strains of Staphylococcus aureus (SA) makes it a major concern for public health (1, 2). SA produces a large arsenal of virulence factors, among which bicomponent leukocidins—also referred to as leukotoxins—stand out as interesting targets for developing novel antivirulence strategies (3, 4). Leukotoxins belong to the family of β-barrel pore-forming toxins that assemble into heteromeric pores to lyse specific cells (5–8). Five different types of leukotoxins are expressed by SA infecting humans: Panton–Valentine leukocidin (PVL), LukED, HlgAB, HlgCB, and LukAB. Each one is formed by two subunits, the host cell–targeting S component (S for slow elution during biochemical purification) and the polymerization F (fast elution) component. In the current view, with the exception of LukAB, all the subunits are believed to be secreted as monomers that will heterodimerize upon specific interaction with host myeloid and erythroid cells. Dimerization will lead to a subsequent toxin oligomerization and pore formation in cell membranes (9–11).
Recent identification of leukotoxin receptors in targeted host cells increased our knowledge of the mechanism behind cellular specificity of these toxins and their role in pathogenesis (12–17). Based on these findings, the predicted mechanism is that only the monomeric S component specifically interacts with various complement and chemokine receptors present on the surface of leukocytes, all related to the family of G protein–coupled receptors (GPCRs). The S component later recruits the F component to trigger oligomerization and pore formation. Though the F components HlgB and LukD were shown to bind to the surface of erythrocytes independently from their S component partners (18, 19), it was considered a receptor-independent binding. Recently, one of the F components, LukF-PV, was shown to specifically require a receptor in order to recognize targeted cells (20), therefore challenging the proposed initial steps of receptor recognition and pore formation (21).
Out of all targeted receptors, the atypical chemokine receptor 1 (ACKR1, previously called DARC) (22), recognized by both HlgA and LukE, is a key player. Indeed, in addition to being expressed in myeloid cells, ACKR1 is expressed in erythrocytes and endothelial cells, making it necessary for SA to escape the immune system, to grow, and to cause cell death (12, 13). Unlike canonical chemokine receptors, ACKR1 lacks the conserved DRYLAIV motif and is thus structurally unable to activate G proteins by dissociating the subunits upon chemokine engagement (23, 24). Rather, it internalizes and transports chemokines to the degradative compartment, acting as a chemokine buffer by modulating chemokine concentration and bioavailability (25–28). The high-resolution structure of ACKR1 is still unknown; however, its homologs of known structures share the highly conserved GPCR structure consisting of a single polypeptide chain with three intracellular and extracellular loops, an external N-terminal region essential for the specificity of ligand binding, and an intracellular carboxyl-terminal region that is involved in receptor signaling. Although increasing amounts of structural and molecular data of chemokine receptors are being discovered (29, 30), the structural immunology and pharmacology related to ACKR1 is still in its infancy.
Binding of leukotoxins to GPCRs is poorly understood at the molecular and structural level. Various residues in the loops of the rim domain of HlgA and LukE, as well as a four-residue region in the cap domain of HlgA, were shown to be necessary for hemolytic activity and/or binding to erythrocytes (31–33). From the receptor side, LukE and HlgA seem to target different regions of the ACKR1 N-terminal part, a highly flexible region, whereas both require sulfation of tyrosine residues in this same part of the receptor (13). In addition, little is known of the effects of leukotoxins on ACKR1 binding to its natural ligands and downstream molecular signaling. Although biochemical and cell biology work has been done since the discovery of receptors targeted by leukotoxins, direct evidence capturing purified leukotoxin−receptor complexes has only been provided for the LukE−CCR5 pair (16).
In this study, we used an integrative molecular pharmacology and structural mass spectrometry (MS) approach in order to characterize the effect of HlgAB binding on ACKR1 structure and function. We demonstrate that both leukotoxins, HlgA and HlgB, form independent complexes with purified ACKR1 in vitro using native MS (nMS). In living cells, time-resolved fluorescence resonance energy transfer (TR-FRET) experiments revealed that both HlgA and HlgB binding to ACKR1 compete with CCL5, an endogenous ligand. We also monitored the effect of leukotoxin binding on ACKR1 conformation using a combination of hydrogen/deuterium exchange MS (HDX-MS) and cell-based resonance energy transfer. Surprisingly, in addition to the expected accessibility changes in the extracellular domain of the receptor, binding of leukotoxins induced long-range allosteric conformational changes in the intracellular domain of ACKR1 that leads to the dissociation of and/or changes in the architecture of preassembled ACKR1−Gαi1 protein complexes. Altogether, our study brings insights into the initial steps of leukotoxin biology through GPCR, namely, the toxins’ effect on GPCR structure and function.
Results
nMS Reveals HlgB and HlgA Dimers in Solution with HlgB Being More Prone to Dimerization.
We first analyzed purified recombinant HlgA and HlgB by nMS in order to verify their oligomeric state in the absence and the presence of detergent micelles as a mimic of the amphiphilic membrane environment. nMS has been gaining ground and has become a key actor in studying membrane protein interactions and dynamics (34–39). It preserves noncovalent interactions and gives information regarding the stoichiometry and binding partners of protein complexes, among which are GPCRs (40, 41).
Recombinant HlgA and HlgB were present mainly as monomers in vitro; however, we also detected some dimeric species (Fig. 1 A and B and SI Appendix, Fig S1A) even at the lowest concentration analyzed (below 5 µM). In the presence of detergent, HlgA and HlgB were able to bind multiple n-dodecyl-d-maltoside (DDM) molecules in their monomeric and dimeric forms (SI Appendix, Fig. S1B), and detergent molecules were easily dissociated upon higher activation in the gas phase. An equimolar mixture of HlgA and HlgB resulted in the formation of heterodimers in vitro in the absence of any receptor (Fig. 1C). In all analyzed conditions, HlgB was systematically more prone to dimerization than HlgA, since the dimer-to-monomer ratio at a given concentration was always twofold higher for HlgB compared to HlgA. Taken together, our results demonstrate the presence of toxin homo- and heterodimers in solution even in the absence of membranes and receptors, HlgB being more prone to dimerization compared to HlgA.
Fig. 1.
nMS spectra of leukotoxins and ACKR1. (A) nMS spectrum of 30 µM HlgA and (B) 15 µM HlgB showing the presence of both monomeric (33,004 ± 1 Da and 34,943 ± 1 Da, respectively) and dimeric (66,061 ± 14 Da and 69,972 ± 10 Da, respectively) species. Several sodium adducts were visible with both toxins and could be resolved for the monomeric but not for the dimeric peaks due to the decay in the apparent resolution when working in nMS mode (67). Theoretical masses of HlgA monomer and dimer: 33,004 and 66,008 Da. Theoretical masses of HlgB monomer and dimer are 34,943 and 69,886 Da. Single and double circles shown at similar m/z regions correspond to overlapping signals coming from monomers and homodimers. (C) nMS spectrum of a mixture of 10 µM HlgA and 10 µM HlgB showing the additional presence of HlgAB heterodimers (69,925 ± 9 Da). Overlapping signal from HlgB 9+, HlgBB 18+, and HlgAA 17+ is visible at m/z around 3,885. Bar diagram shows the relative quantification in this equimolar mixture relative to the most intense species in the spectrum (i.e., HlgA monomer). (D) SEC profile of purified ACKR1 showing the ultraviolet (UV) absorbance at 280 nm as a function of the elution volume. (E) nMS spectrum of monomeric and (F) dimeric ACKR1 produced in HEK GnTI− cells (40,531 ± 32 Da and 81,032 ± 50 Da, respectively) showing an additional ∼3.5 kDa glycosylations per monomer. All samples were buffer exchanged in 200 mM ammonium acetate pH 7.4 supplemented with 2 CMC DDM prior to nMS analysis. Single green circles: HlgA; double green circles: HlgAA; single yellow circles: HlgB; double yellow circles: HlgBB; double green and yellow circles: HlgAB; single blue circles: ACKR1 WT; double blue circles: ACKR1 homodimer.
Purification of Homogeneous Human ACKR1.
The GPCR ACKR1 was first produced in Sf9 cells and purified in DDM detergent, which gave a homogeneous monodisperse main peak by size-exclusion chromatography (SEC) as well as an additional smaller peak eluting at a higher apparent mass (Fig. 1D). nMS analysis of both peaks shows the presence of both monomeric and homodimeric ACKR1, with additional heterogeneous masses around 3.5 to 5 kDa per monomer (SI Appendix, Fig. S2A). We hypothesized that the nature of the observed additional mass came from N-glycosylations. In order to confirm our hypothesis, we produced ACKR1 in a cell line with restricted and homogeneous N-glycosylations (HEK GnTI−), which decreased the heterogeneity but did not lead to the complete removal of ACKR1 modifications (Fig. 1 E and F and SI Appendix, Fig. S2B). Finally, treatment of purified receptor with PNGaseF resulted in a complete removal of the modification, implying that they indeed are N-glycosylations (SI Appendix, Fig. S2C). We then verified all reported potential N-glycosylation sites by point mutations (SI Appendix, Fig. S2 D–F) and found that all three, N16, N27, and N33, are glycosylated in ACKR1.
HlgB and HlgA Both Bind Separately to Monomeric ACKR1 but Not Concomitantly.
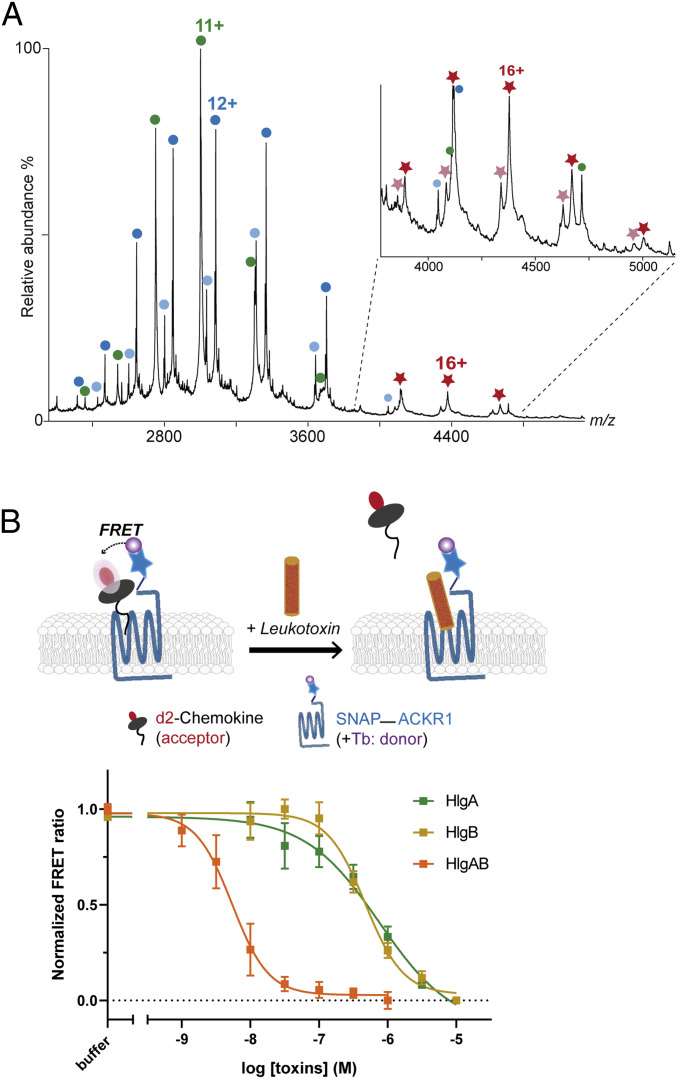
In order to provide direct evidence of ACKR1−leukotoxin binding and determine the stoichiometry of this complex, we analyzed mixtures of purified recombinant HlgA, HlgB, and ACKR1 using nMS. The low yield of dimeric ACKR1 led us to perform in vitro experiments only with the monomeric form. Incubating ACKR1:HlgA in a 2:1 ratio, ACKR1 being used at higher concentration to overcome lower ionization efficiency, for 30 min at 4 °C revealed the presence of m/z species with a mass corresponding to proteins alone as well as the presence of an ACKR1−HlgA complex with a 1:1 stoichiometry (Fig. 2A). Binding of HlgA gave similar results with wild-type (WT) and deglycosylated ACKR1, with peaks better defined in the mass spectra in the latter case. These results thus evidence the existence of GPCR−leukotoxin complex in detergent micelles and suggest that receptor glycosylation is not strictly necessary for ACKR1−HlgA interaction to occur. Surprisingly, we detected the formation of ACKR1−HlgB complexes when mixing the F component HlgB with the receptor (SI Appendix, Fig. S3), demonstrating a specific interaction between HlgB and ACKR1. Finally, when mixing the three components ACKR1:HlgA:HlgB in a 2:1:1 ratio, we detected separately formed ACKR1−HlgA and ACKR1−HlgB complexes, but no ternary ACKR1−HlgA−HlgB complexes were visible. ACKR1−leukotoxin complexes were not stable enough to overcome an SEC step, since receptor−leukotoxin mixtures did not yield a stable complex peak upon SEC. This points to a low-affinity binding observed in vitro and could also explain the low relative intensity of the complexes observed by nMS. Taken together, our nMS results capture leukotoxin−GPCR complex in solution in a purified system and demonstrate that the F component HlgB also interacts directly with ACKR1.
Fig. 2.
Binding of leukotoxins to ACKR1 in vitro and in living cells. (A) nMS spectrum of a mixture of 2 µM HlgA and 5 µM ACKR1 treated with PNGaseF, showing the presence of monomeric HlgA (green circles, 33,004 ± 1 Da), deglycosylated ACKR1 (blue circles, 37,022 ± 1 Da), and partially hydrolyzed deglycosylated ACKR1 (light blue circles, 36,411 ± 1 Da). Complexes formed between HlgA and both forms of deglycosylated ACKR1 are labeled with dark and clear red stars (70,029 ± 2 Da and 69,421 ± 7 Da, respectively). (B) Scheme explaining TR-FRET competitive binding assay (Top) and dose–response curves showing the decrease in the TR-FRET ratio between ACKR1 and d2-CCL5 upon addition of HlgA, HlgB, and HlgAB equimolar mixture (Bottom). Data shown are the mean ± SEM of one experiment performed in triplicate and are representative of three independent experiments. Hill slope values: HlgA, −1.1 ± 0.08; HlgB, −2.0 ± 0.34; HlgAB, −2.1 ± 0.29. IC50 values: HlgA, 577 ± 93 nM; HlgB, 376 ± 105 nM; HlgAB, 6.2 ± 2.7 nM. Values represents the average ± SD of three independent experiments performed in triplicate.
Both Leukotoxins Compete with ACKR1 Natural Ligand, and HlgB Binding Is Cooperative.
In order to validate the observed binding in vitro and to assess the effect of HlgA and HlgB on ACKR1 binding to an endogenous ligand (CCL5) (28), we carried out competitive binding assays in living HEK293T cells by homogenous TR-FRET technology (42) (Fig. 2B). SNAP-tag–fused ACKR1 receptor transiently expressed in HEK293 cells was covalently labeled with Lumi4-terbium as donor. Cells were then incubated in the presence of d2-CCL5, a fluorescent derivative of CCL5 used as ligand tracer. After assay validation (SI Appendix, Fig. S4 A and B), HlgA and HlgB binding were assessed by competition experiments (Fig. 2B). The results revealed that both leukotoxins compete with d2-CCL5 with a slightly higher displacement in the case of HlgB (half maximal inhibitory concentration: IC50 = 577 ± 93 and 376 ± 105 nM, respectively), confirming the capacity of both toxins to bind to ACKR1 as seen by nMS. Surprisingly, the slopes of the competition curves are consistently different, close to −1 for HlgA and close to −2 for HlgB, suggesting the existence of a positive cooperative binding process with HlgB. This could be correlated with the higher ability of HlgB to homodimerize compared to HlgA as observed by nMS with purified toxins. Finally, binding affinity of an equimolar HlgA and HlgB mixture resulted in a dramatic left shift of the IC50 of the competition curve (6.2 ± 2.7 nM), and the slope of the curve, as observed with HlgB, presented the characteristics of a positive cooperative binding process (Fig. 2B). The observed increased affinity and cooperative binding might originate from an increased avidity similar to what is observed with bivalent ligands (43). Indeed, nMS showed that HlgB is more prone to homodimerize compared to HlgA, which may explain the cooperativity observed only for HlgB and the lower IC50 for the latter, whereas a mixture of HlgA and HlgB will lead to heterooligomerization with the potential formation of octamers forming the (pre)pore at the vicinity of the membrane, resulting in an enhanced affinity through avidity.
Conformational Changes of ACKR1 upon Binding to Leukotoxins Revealed by HDX-MS.
In order to determine potential conformational changes occurring in ACKR1 upon binding to leukotoxins, we developed an HDX-MS strategy using purified ACKR1 and leukotoxins in vitro, following recommendations (44). Since purification mainly yields monomeric ACKR1 and low amounts of dimers in detergent micelles (Fig. 1D), we focused our attention on monomeric ACKR1. GPCRs are known to maintain their functional state as monomers (45, 46); therefore, we anticipated that studying the effect of leukotoxins binding to ACKR1 monomers may provide relevant information to decipher the mechanism by which the toxins modify ACKR1 function.
HDX-MS gives information related to solvent accessibility and dynamics of biological complexes. It is based on the exchange kinetics between deuterium atoms (D) present in the buffer and amide protons (H) of native polypeptide chains in solution (47). Unlike higher-resolution approaches, HDX-MS offers the advantage of obtaining dynamic structural information for samples that present heterogeneous and/or flexible areas. It was successfully applied to characterize the dynamics and interactions of membrane transporters and even GPCRs (48–53).
HDX-MS optimization for apo ACKR1 allowed the identification of 77 peptides covering 70.1% of WT ACKR1, with 3.41 peptide redundancy (SI Appendix, Fig. S5A). The highly glycosylated N-terminal part of ACKR1 was mainly missing; therefore, we performed the same experiment with the nonglycosylated N16,27,33Q-ACKR1 mutant, which resulted in an increased sequence coverage at the N-ter level (SI Appendix, Fig. S5B). In order to determine the effect of HlgA and HlgB binding on ACKR1 conformational dynamics, we performed differential HDX (ΔHDX) analysis between apo ACKR1 and equimolar mixtures of ACKR1:HlgA or HlgB. Biological replicates (two with WT ACKR1 and one with N16,27,33Q-ACKR1) were freshly prepared, and deuteration timepoints were performed in triplicate for each condition (HDX summary table, Dataset S1). The total number of usable ACKR1 peptides in the presence of leukotoxins was lower compared to the receptor analyzed alone due to the overlap with the peptides coming from leukotoxins, which are soluble proteins of roughly the same size of the receptor. This resulted in a decreased overall redundancy (HDX summary table, Dataset S1).
In order to visualize the leukotoxin-induced changes on ACKR1 in the absence of a high-resolution structure of the latter, we generated a model for ACKR1 that was validated against HDX-MS data using molecular dynamics simulations (MDS) (SI Appendix, Fig. S6, see Materials and Methods). Both leukotoxins had, overall, similar structural effects on purified monomeric ACKR1, which correlates with the similar binding affinities measured in living cells by TR-FRET. The ACKR1 long N-terminal part was mainly protected in the presence of leukotoxins (peptides 9 through 20 were more protected compared to peptides 27 through 45, the latter only being visible in the deglycosylated sample), whereas HDX data for the majority of extracellular loops (ECLs) was not present due to the missing sequence coverage (Fig. 3 and SI Appendix, Fig. S5 C–F). The N-terminal part, containing a sulfotyrosin Y41 residue reported as highly important for ACKR1-mediated pore-formation by HlgAB (13), relates, therefore, to the direct binding site of leukotoxins. The overall deuteration of detected transmembrane (TM) segments was very low, most likely due to the presence of the detergent micelles surrounding these parts that shield them from the solvent. However, the upper part of helix 5 (H5) (residues 203 through 215) presented a higher uptake compared to the other TM and was slightly more protected in the presence of the toxins compared to the receptor alone (Fig. 3 and SI Appendix, Fig. S5 C–F). This region also displays higher flexibility in MDS of the generated ACKR1 model (SI Appendix, Fig. S6). The upper part of TM5 was shown to be important for various CKR−ligand interactions (30), whereas rearrangements in TM5 were shown to play critical roles in signal transmission for various GPCRs (54). Therefore, this region can be directly implicated in leukotoxin binding to ACKR1. Strikingly, ΔHDX shows that binding of leukotoxins induced allosteric conformational changes that led to the protection of the H8 and the carboxyl-terminal part of ACKR1 as well as intracellular loop 2 (ICL2) and, to a lower extent, parts of ICL1 (Fig. 3 and SI Appendix, Fig. S5 C–F), domains that are known to be critical for Gα binding (55). The change in the carboxyl-terminal part (peptides 330 to 339) should be interpreted with caution. Indeed, this domain is most likely unstructured and exploring multiple conformations in detergent micelles. One possible interpretation could be that this part becomes less flexible and might make some contacts with the intracellular domain of ACKR1 upon binding to the toxins. Additional studies will, however, be required to validate this hypothesis. On the other hand, ΔHDX results for H8 suggest that this helix might change its conformation to shield both ICL1 and ICL2 upon binding of leukotoxins to ACKR1 in a way that blocks G-protein accessibility, similar to what was observed for Angiotensin 2 receptor AT2R (56). The structural dynamics of H8 are indeed suggested to play an important role in GPCR signaling (57).
Fig. 3.
ACKR1 conformational changes upon binding to leukotoxins probed by HDX. HDX results showing statistically significant ΔHDX regions from all biological replicates color coded onto the snake plot of ACKR1 adapted from GPCRdb as well as on the structural model generated for ACKR1 in which the long N-terminal domain is missing (c.f. Materials and Methods). Blue: protected regions; gray: regions with no statistically significant ΔHDX; and white: regions with no HDX data. Deuterium uptake of selected peptides is shown for the apo receptor (black) and the receptor bound to HlgA (green) or to HlgB (yellow). Uptake plot data are the average and SD for timepoints from n = 3 replicate measurements for one biological preparation of ACKR1.
HlgB and HlgAB Interfere with Preassembled ACKR1−Gαi Complexes in Living Cells.
The surprising allosteric modulation observed by HDX prompted us to analyze the ACKR1−Gi interactions in living cells. We did not see any G-protein activation with ACKR1 in the presence of chemokines CCL2 and CCL5, as demonstrated by the absence of a bioluminescent resonance energy transfer (BRET) signal decrease between α- and βγ-subunits of the studied G proteins (SI Appendix, Fig. S7 A and B). This is not surprising for ACKR1, since it lacks the canonical DRY motif involved in G-protein activation (22). However, when we followed the direct interaction between the carboxyl-terminal part of ACKR1 and the Gαi1 subunit by BRET (Fig. 4A), we observed a constitutive BRET signal in the absence of any ligand, indicating the existence of ACKR1–YFP−Gαi1–RLuc complexes in basal conditions (SI Appendix, Fig. S7 C and F). This could be similar to the decoy action ACKRs have regarding chemokines present in the extracellular milieu, wherein ACKR1 could also help regulate the concentration of Gαi1 in the intracellular milieu. The measured BRET signal from the ACKR1–Gαi1 interaction followed a saturation curve, unlike the BRET measured between ACKR1 and another Gα protein, Gαq, which gave a nonspecific linear signal (SI Appendix, Fig. S7D). Interaction between ACKR1 and Gαi1 was further validated in vitro by a pull-down experiment using both purified proteins (SI Appendix, Fig. S7E).
Fig. 4.
HlgB and HlgAB interfere with preassembled ACKR1−Gαi1 complexes in living cells. (A) Scheme explaining the BRET assay used to probe ACKR1−Gα interactions. (B) Net BRET assay between ACKR1-YFP and Gαi1-RLuc showing that CCL5 and HlgA have no specific effect on ACKR1−Gαi1 interactions at the tested concentrations, whereas adding HlgB or HlgAB resulted in the dissociation of ACKR1−Gαi1 complexes in living cells. EC50 was 796 ± 110 nM for HlgB and 13.5 ± 3.2 nM for HlgAB. (C) Net BRET assay showing the effect of the HlgAB mixture on ACKR1−Gαi1 interactions for WT, Y30F, and Y41F ACKR1. Effect of HlgAB on the dissociation between ACKR1 and Gαi1 decreased when mutating Y41, evidenced by an EC50 increase up to 106 ± 42 nM, whereas the EC50 did not change significantly when mutating Y30 (21.7 ± 5.3 nM). Cell-based assays data shown are the mean ± SEM of one experiment performed in triplicate and are representative of three independent experiments. Reference SI Appendix, Fig. S7 for additional data.
Adding up to 10 µM CCL5 or HlgA promoted a weak variation of the BRET signal between ACKR1–YFP and Gαi1–RLuc, whereas incubation of cells with increasing concentrations of HlgB or HlgAB strongly reduced the basal BRET signal (Fig. 4B), the effect of HlgAB being nearly 10-fold more potent compared to the effect of HlgB (half maximal effective concentration: EC50 796 ± 110 nM and 14 ± 3 nM for HlgB and HlgAB, respectively). This suggests a conformational rearrangement that modifies the distances and/or orientation within the ACKR1–YFP−Gαi1–RLuc complexes in the presence of HlgAB and, to a lesser extent, HlgB. To further validate the specificity of the observed effect, we performed the same experiments using the mutant Y41F-ACKR1. When mutating the sulfotyrosin Y41, reported as highly important for ACKR1-mediated pore-formation by HlgAB (13), we observed a 10-fold decrease in the effect of HlgAB (Fig. 4C). Interestingly, mutating Y30, another potentially sulfated tyrosine present at the N-ter, did not result in any significant effect on ACKR1−Gαi1 binding, implying that this site is either not sulfated or that sulfation of this tyrosine is not important for binding to leukotoxins, as reported previously (13). These results thus demonstrate that ACKR1−Gαi1 architecture can be modified in living cells upon leukotoxin binding, confirming the biological relevance of the allosteric modifications observed by HDX-MS. Intriguingly, however, HlgA was not able to recapitulate the effect observed with HlgB, suggesting that additional conformational rearrangements are involved in the observed effects on ACKR1−Gαi1 complexes.
HlgAB and HlgB Interfere with ACKR1−ACKR1 Interactions in Living Cells.
To address the question of additional ACKR1 receptor conformational changes, based on the observed positive cooperativity for HlgAB and HlgB but not HlgA binding, and given that GPCRs are notoriously known to form oligomers including in native tissues (58), we suspected that the toxins’ binding could modify receptor oligomerization. In order to explore these mechanisms, we monitored the effects of leukotoxins on receptor−receptor interactions in living cells using two different RET strategies: lanthanide-based TR-FRET and BRET. BRET is based on the fusion of an energy donor (RLuc or NanoLuc) and an energy acceptor (YFP) to the carboxyl terminus of ACKR1 (59) (Fig. 5A), whereas TR-FRET is based on the fusion of a SNAP-tag to an ACKR1 extracellular N terminus that will be labeled with a FRET donor and an acceptor (60) (Fig. 5B). In both techniques, the RET signal is sensitive to the distance between the donor and the acceptor, while in BRET, it is more sensitive to the relative orientation of the fluorescent probes. Indeed, the error in distances measured via lanthanide-based RET due to the orientation factor was shown to be essentially negligible (61). The different positioning of the probes can thus sense various receptor rearrangements both in the extracellular and intracellular domains of this GPCR.
Fig. 5.
Effect of leukotoxins on ACKR1−ACKR1 interactions in living cells. (A) BRET assay showing receptor−receptor interactions at the carboxyl-terminal intracellular side of ACKR1. A constitutive BRET signal is visible prior to adding the different ligands. Adding HlgA (green) does not affect the BRET signal at the tested conditions, whereas HlgB (yellow) and equimolar mixture of HlgAB (orange) induced an increased BRET. Average EC50 of all independent experiments was: 178 ± 28 nM in the presence of HlgB and 18.6 ± 2.5 nM in the presence of HlgAB. (B) TR-FRET showing receptor−receptor interactions at the N-terminal extracellular side of ACKR1. A constitutive TR-FRET signal is visible prior to adding the different ligands. Adding HlgA (green) does not affect the TR-FRET at the tested conditions, whereas HlgB (yellow) and equimolar mixture of HlgAB (orange) induced a decreased TR-FRET. Average EC50 of all independent experiments was 429 ± 138 nM in the presence of HlgB and 47 ± 15 nM in the presence of HlgAB. ST, SNAP Tag. ACKR3 was used as control. All data shown are the mean ± SEM of one experiment performed in triplicate and are representative of at least three independent experiments.
We first confirmed the propensity of ACKR1 to form oligomers in living cells using both RET approaches. Indeed, saturation of the BRET signal when increasing ACKR1-YFP expression while ACKR1-Nanoluc remained constant strongly supports receptor oligomerization (SI Appendix, Fig. S7F). Though BRET assessment of oligomerization can be notoriously difficult (62), our results are in agreement with a previous report that describes the presence of homooligomeric ACKR1 by BRET (63). Similarly, a constitutive TR-FRET signal was recorded when N-ter SNAP-tagged ACKR1 was expressed and labeled with both Lumi4-Tb (donor) and d2 (acceptor) (Fig. 5B). Altogether, BRET and TR-FRET results correlate with the presence of purified stable ACKR1 dimers in solution detected by nMS (Fig. 1E), confirming the capacity of ACKR1 to oligomerize.
Using the experimental conditions corresponding to the BRET50, we evaluated the impact of HlgA and HlgB binding on the BRET signal. HlgA did not induce any modification in the BRET signal, while HlgB induced a BRET signal increase (Fig. 5A). Interestingly, HlgAB equimolar mixture resulted in an increased BRET signal with a 10-fold higher potency compared to HlgB (18.6 ± 2.5 nM versus 178 ± 28 nM). The increased BRET signal can be due to an increase in oligomer density and/or to a change in the architecture of the carboxyl-terminal part of ACKR1, leading to a closer proximity and/or a reorientation of the probes in the presence of HlgAB and HlgB but not with HlgA at the tested ligand concentrations. Remarkably, this correlates with the conditions showing a cooperative effect in competitive binding assays (Fig. 2B).
In contrast to the BRET signal increase, the data revealed a decrease in the TR-FRET signal between ACKR1 receptors in the presence of HlgB and HlgAB, while no significant effect was observed with HlgA at the tested ligand concentrations. Again, the potency of HlgB was 10-fold lower compared to HlgAB (429 ± 138 versus 47 ± 15 nM) in the same range of the BRET assays. The one log difference between HlgB and HlgAB observed in both BRET and TR-FRET is similar to the difference observed in competitive binding experiments (Fig. 2B). Taken together, the observation of BRET signal increase and TR-FRET signal decrease strongly suggests that HlgB and HlgAB induce large conformational changes leading to a rearrangement of the oligomeric architecture that positions the fluorescent probes further apart in the N termini and closer together and/or with a different orientation in the carboxyl termini (Fig. 6). These conformational changes in oligomeric assemblies may also explain the observed rearrangement in ACKR1−Gαi1 complexes.
Fig. 6.
Proposed first steps of pore formation by HlgAB. ACKR1 (blue) is present in both monomeric and dimeric forms in cellular membranes. Soluble HlgA (green) and HlgB (olive) secreted by SA can be present in monomeric and dimeric forms. Both leukotoxins recognize the cellular membrane by specific interactions with ACKR1, and interaction of each toxin with ACKR1 will lead to conformational changes at both N and carboxyl termini of the GPCR. HlgB homodimers and HlgAB heterodimers could interfere with receptor−receptor interactions, but only the HlgAB−(ACKR1)2 complex will lead to the formation of the pore.
Discussion
SA leukotoxins targeting GPCRs represents an attractive aspect in modulating GPCR function and remain largely unexplored. We chose to focus on ACKR1, since it is a crucial target for SA pathogenesis, being not only expressed in myeloid cells like the other targeted receptors but also in erythrocytes and endothelial cells. We demonstrate that HlgB also recognizes ACKR1, making it the second F component leukotoxin with an identified receptor. Our results may explain the observation that HlgB binds to erythrocytes independently from HlgA (18). Unlike previously thought (17), our data demonstrate that both HlgA and HlgB separately bind host ACKR1 during the initial steps to initiate pore formation. Both leukotoxins were able to compete with the ACKR1 natural ligand but with a difference in the mechanism. The cooperativity that accompanies toxin ability to dimerize and oligomerize seems a key factor that drives the effects observed on ACKR1 conformational changes in living cells. While HlgB shows a higher ability to homodimerize compared to HlgA, the presence of both HlgA and HlgB at the vicinity of a membrane expressing their targeted receptor induces the formation of an octameric pore (9, 10). Dimerization and oligomerization increase the avidity in the system, which in turns increases the effects of HlgBB and (HlgAB)4 on ACKR1.
Our data also strongly suggest that the capacity of HlgB and HlgAB to dimerize will lead to changes in ACKR1−ACKR1 constitutive interactions in living cells that may also impact receptor function. Unfortunately, we were not able to address conformational changes in ACKR1 dimers due the very low yield of stable purified dimers obtained upon purification. However, HDX-MS with monomeric ACKR1 revealed quite unexpected conformational changes that may participate, at least in part, in the conformational rearrangement of oligomers observed in living cells. Indeed, toxins binding to the monomeric receptor are not neutral and induce conformational changes in the extracellular N-terminal part, which is directly involved in leukotoxin binding and, more surprisingly, in the C-terminal part at the level of H8. This allosteric long-range modulation thus propagates to the G protein–binding sites in the intracellular loops (ICLs) (Fig. 6), correlating with the BRET assays we used to follow ACKR1−Gαi1 interactions in living cells. While HlgB and HlgAB binding destabilize ACKR1−Gαi1 protein complexes, which could release available Gαi1 protein in the intracellular milieu, HlgA binding does not interfere with ACKR1−Gαi1 protein complexes. Indeed, though HlgA is able to bind and induce conformational changes in the ACKR1 monomer, this process is apparently not sufficient to modify the oligomeric architecture of ACKR1 as demonstrated by the TR-FRET and BRET assays, probably due to a lower propensity of this toxin component to form oligomers. Further studies will be needed to determine whether this phenomenon has functional implications in the pathogenicity of the SA through modulation of G protein and related signaling pathways.
Only the presence of HlgAB heterodimer can lead to pore formation (18), but the structural and molecular mechanisms of this process remain to be understood. Further studies manipulating the oligomerization of toxins and ACKR1 (in proteoliposomes, for instance) will thus be important to shed the light on the mechanisms behind conformational changes of both leukotoxins and the potential role of the GPCR receptor during the different pore-formation steps. Notwithstanding, our findings may open the way to develop antibiotics inhibiting the first and limiting steps of toxin action by targeting host receptors’ binding, an inhibition that has the potential to be less prone to resistance.
Materials and Methods
Protein Constructs.
For production in insect or human cells, the full-length synthetic gene of human ACKR1 isoform-2 (UniprotKB-Q16570) was subcloned into modified pFastBac or pCMV-Dest vectors (Thermo Fisher Scientific) respectively, resulting in the full-length ACKR1 bearing the influenza virus hemagglutinin signal peptide followed by a Flag epitope at the N terminus. For human cell-based assays, the full-length synthetic gene of human ACKR1 was subcloned into pcDNA3.1-YFP vector in frame with the N terminus of YFP resulting in ACKR1-YFP, into pcDNA3.1-Nanoluc vector in frame with the N terminus of Nanoluc resulting in ACKR1-Nanoluc, and into pcDNA3.1-SNAP vector in frame with the carboxyl terminus of the SNAP tag resulting in SNAP-ACKR1. The synthetic genes of mature S. aureus HlgA (UniprotKB-P0A074) residues 30 through 309 and HlgB (UniprotKB-P0A077) residues 25 through 325 were subcloned into popinE vectors (Oxford Protein Production Facility-UK) resulting in HlgA and HlgB bearing a carboxyl-terminal hexahistidin (His)6 tag.
Expression and Purification of ACKR1.
Expression of ACKR1 was carried out in insect and human cells. ACKR1 constructs were expressed in HEK293 GnTI− cells (American Type Culture Collection [ATCC]) using BacMam baculovirus transduction. HEK cells were grown in suspension in Ex-Cell 293 Serum-Free Medium (Sigma-Aldrich) with 2% fetal bovine serum (FBS) and were infected at a density of 2 × 106 cells/mL using a 1/10 (vol/vol) baculovirus solution. Culture flasks were shaken for 72 h at 37 °C with 5% CO2, and a solution of 5 mM sodium butyrate was added to the culture flasks 24 h postinfection. Cells were harvested by centrifugation (3,000 rpm) 72 h postinfection, and cell pellets were stored at −80 °C until purification. ACKR1 constructs were also expressed in Sf9 insect cells (Life Technologies) using the pFastBac baculovirus system (Thermo Fisher Scientific). Sf9 cells were grown in Ex-Cell 420 Medium (Sigma-Aldrich) and were infected at a density of 4 × 106 cells/mL using a 1/200 (vol/vol) baculovirus solution. Culture flasks were shaken for 48 h at 28 °C, cells were harvested by centrifugation (3,000 rpm), and cell pellets were stored at –80 °C until purification. Purification was carried out in similar conditions regardless of cell expression. After thawing the frozen cell pellets, cells were lysed by osmotic shock, adding 1/10 (vol/vol) lysis buffer consisting of 10 mM Tris (pH 7.5) and 1 mM EDTA and containing 2 mg/mL iodoacetamide (Sigma-Aldrich) and protease inhibitors (Leupeptin [Euromedex], Benzamidine, and Phenylmethylsulfonyl fluoride [PMSF] [Sigma-Aldrich]). Lysed cells were centrifuged (16,000 rpm), and the membrane pellets were suspended in a 1/20 (vol/vol) solubilization buffer consisting of 50 mM Hepes (pH 7.5), 150 mM NaCl, 0.5% (wt/vol) DDM (Anatrace), 0.1% (wt/vol) cholesteryl-hemisuccinate (CHS, Sigma-Aldrich), 2 mg/mL iodoacetamide, and protease inhibitors. Receptors were extracted from the membrane pellets using a glass dounce grinder, and the extracted mixture was stirred for 1 h at 4 °C and then centrifuged (16,000 rpm). The supernatant was loaded by gravity flow onto anti-Flag M2 antibody resin. The resin was washed with 10 column volumes (CV) of a DDM wash buffer consisting of 50 mM Hepes (pH 7.5), 150 mM NaCl, 0.1% (wt/vol) DDM, and 0.02% (wt/vol) CHS. For native MS experiments, DDM concentration was decreased to reach 2 critical micelle concentration (CMC) using 10 CV wash buffer 2 made up of 50 mM Hepes (pH 7.5), 150 mM NaCl, 0.025% (wt/vol) DDM, and 0.005% (wt/vol) CHS. The bound receptor was eluted in the wash buffer 2 supplemented with 0.4 mg/mL Flag peptide. For HDX-MS analysis, detergent was changed from DDM to lauryl maltose neopentyl glycol (LMNG) using LMNG exchange buffer containing 50 mM Hepes (pH 7.5), 150 mM NaCl, 0.2% (wt/vol) LMNG, and 0.01% (wt/vol) CHS. The detergent exchange was performed by washing the column with a series of five buffers (3 CV each) made up of the following ratios (vol/vol) of LMNG exchange buffer and DDM wash buffer: 1/3, 1/1, 3/1, 9/1, and 1/0. An additional 10-CV wash was performed to decrease the detergent concentration to 2CMC LMNG using 50 mM Hepes (pH 7.5), 150 mM NaCl, 0.02% (wt/vol) LMNG, and 0.001% (wt/vol) CHS followed by the last wash LMNG buffer consisting of 50 mM Hepes (pH 7.5), 150 mM NaCl, 0.002% (wt/vol) LMNG, and 0.0001% (wt/vol) CHS. The bound receptor was eluted in the last wash LMNG buffer supplemented with 0.4 mg/mL Flag peptide. The eluted solution of receptors was concentrated to 500 μL using 50-kDa spin filters (Millipore) and further purified by SEC on a Superdex 200 Increase 10/300 column (GE Healthcare) in the last wash buffer. For PNGase F (New England Biolabs) treatment, 5 µL enzyme at 500,000 U/mL were added to 0.2 mg ACKR1 and incubated overnight at 4 °C. Receptor was purified by SEC as mentioned previously. Fractions containing monodisperse ACKR1 were collected and directly analyzed for nMS or pooled and concentrated for HDX analysis.
Expression and Purification of HlgA and HlgB.
Toxins were expressed with (His)6-tags at their carboxyl termini in competent C43 (DE3) Escherichia coli cells (New England Biolabs) for HlgA and in BL21 (DE3) E. coli cells (New England Biolabs) for HlgB. Transformed cells were grown at 37 °C in Terrific broth for HlgA and in Luria–Bertani broth for HlgB supplemented with 100 µg/mL ampicillin to an optical density of 0.6. Expression was then induced at 22 °C by addition of 0.5 mM isopropyl ß-d-1-thiogalactopyranoside. Cells were harvested by centrifugation (3,000 rpm), and cell pellets were stored at −80 °C until purification. After thawing the frozen cell pellets, cells were lysed by sonication in a lysis buffer consisting of 20 mM Tris (pH 8), 300 mM NaCl, 2 mg/mL iodoacetamide (Sigma-Aldrich), and protease inhibitors (Leupeptin [Euromedex], Benzamidine, and PMSF [Sigma-Aldrich]). Lysed cells were centrifuged (16,000 rpm), and the supernatant was adjusted to 40 mM imidazole and loaded onto a nickel nitrilotriacetic acid agarose resin. The resin was washed with 10 CV wash buffer 1 consisting of 50 mM Hepes (pH 7.5) and 1 M NaCl and with 10 CV wash buffer 2 consisting of 50 mM Hepes (pH 7.5) and 150 mM NaCl supplemented with 40 mM imidazole. Bound (His)6-toxins were eluted with wash buffer 2 supplemented with 200 mM imidazole. The eluted solution of toxins was concentrated to 500 μL using 30-kDa spin filters (Millipore) and further purified by SEC on a Superdex 200 Increase 10/300 column (GE Healthcare) in 50 mM Hepes (pH 7.5) and 150 mM NaCl. Fractions containing monodisperse toxins were collected and concentrated for nMS and HDX analysis and cell-based assays.
nMS.
Prior to MS analysis, proteins were buffer exchanged into 200 mM ammonium acetate buffer pH 7.4 (Sigma-Aldrich), supplemented with 0.02% DDM for ACKR1 and for toxins: detergent interactions analysis, using Bio-Spin microcentrifuge columns (Bio-Rad Laboratories). Intact MS spectra were recorded on a Synapt G1 or a Synapt G2-Si high-definition mass spectrometer (HDMS) (Waters Corporation) modified for high mass analysis and operated in time-of-flight mode. Samples were introduced into the ion source using borosilicate emitters (Thermo Fisher Scientific). Optimized instrument parameters for ACKR1 alone or in the presence of the toxins were as follows: capillary voltage 1.4 kV, sampling cone voltage 150 V, offset voltage 80 V, transfer collision voltage 25 V, argon flow rate 8 mL/min, and trap bias 25 V. Collision voltage in the trap was optimized between 50 and 110 V, depending on the sample, and to the minimum activation required to strip the detergent micelle when it was present. Membrane proteins are ionized bound to the detergent micelle and therefore require activation by collision-induced dissociation in order to dissociate bound detergents (35). Data were processed using MassLynx version 4.2 (Waters Corporation) and UniDec (64).
Transfection for Cell-Based Assays.
HEK293 cells (ATCC) were grown in Dulbecco’s Modified Eagle Medium supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C with 5% CO2. Transfection was performed using lipofectamine 2000 (Life Technologies) in polyornithine-coated, black-walled, dark-bottom 96-well plates. The quantity of transfected DNA was optimized for each construct, and 150 ng DNA in total, supplemented by empty PRK5, were added to 50.104 cells/well for transfection.
Competitive Binding Assay by TR-FRET.
A total of 1.5 ng of SNAP-ACKR1 was added to 50.104 cells/well for transfection. At 48 h posttransfection, the plate was washed twice with TagLite (Cisbio), and the extracellular SNAP-receptors were labeled with SNAP-Lumi4-Tb (100 nM, Cisbio) for 1 h at 37 °C. The plate was washed three times with TagLite. A total of 12 nM d2-labeled CCL5 and increasing concentrations (0, 1, 3.6, 10, 31.6, 100, 316, 1,000, 3,160, and 10,000 nM) of toxins, HlgA, HlgB, or an equimolar mixture of HlgA and HlgB, were added to cells. Lumi-4-fluorescence signals were observed on a Pherastar plate reader (BMG Labtech): samples were illuminated at 337 nm, and fluorescence was acquired at 620 nm (donor) and 665 nm (TR-FRET) over time. The ratio of the signals (665/620) was plotted versus the toxin concentration. Dose–response curves were generated using GraphPad Prism 6 (GraphPad Software, Inc.).
Receptor–Receptor Interactions by BRET.
A total of 80 ng ACKR1-YFP and 10 ng ACKR1-NanoLuc were added to 50.104 cells/well for transfection. At 24 h posttransfection, the plate was washed three times with phosphate-buffered saline (PBS, Sigma-Aldrich) and cells are preincubated 5 min at 37 °C with 5 μM coelenterazine h (Promega) before adding ligands diluted in PBS supplemented with 0.9 mM CaCl2 and 0.5 mM MgCl2. Increasing concentrations (0, 1, 3.6, 10, 31.6, 100, 316, 1,000, 3,160 and 10,000 nM) of toxins, HlgA, HlgB, or an equimolar mixture of HlgA and HlgB, were added to cells. BRET readings were collected using a Mithras 2 plate reader (Berthold Technologies GmbH) with 0.1 s integration time per well. The reading chamber was maintained at 37 °C throughout the entire reading time. The BRET signal was calculated by the ratio of emission of YFP (535 nm) to NanoLuc (460 nm). Dose–response curves were generated using GraphPad Prism 6 (GraphPad Software, Inc.).
Receptor–Receptor Interactions by TR-FRET.
A total of 50 ng SNAP-ACKR1 or SNAP-ACKR3 (control) were added to 50.104 cells/well for transfection. At 24 h posttransfection, the plate was washed twice with TagLite (Cisbio) and the extracellular SNAP-receptors were labeled with SNAP-Lumi4-Tb (100 nM,Cisbio) and SNAP-red (300 nM, Cisbio) for 1 h at 37 °C. The plate was washed four times with PBS. Increasing concentrations (0, 1, 3.6, 10, 31.6, 100, 316, 1,000, 3,160, and 10,000 nM) of toxins, HlgA, HlgB, or an equimolar mixture of HlgA and HlgB, were then added to cells. A total of 30 min after addition of leukotoxins, time-resolved fluorescence readings were collected using a SPARK 20M plate reader (TECAN) with 150 µs of lag time and 500 µs of integration time per well. Excitation occurred at 340 nm and emission at 620 nm (donor) and 665 nm (TR-FRET) over time. The ratio of the signals (665/620) was plotted versus the concentrations of toxin.
Receptor–Gα Interactions by BRET.
To follow interaction between ACKR1 and Gα subunits, 40 ng ACKR1-YFP and 10 ng Gα-RLuc were added to 50.104 cells/well for transfection. To follow G protein activation, cells were transfected with each ST-Receptor (10 ng CCR5, 10 ng CCR2, and 20 ng ACKR1) in parallel with 10 ng Gαi1-RLuc, 10 ng β2, and 20 ng γ-Venus. At 24 h posttransfection, the plate was washed three times with PBS (Sigma-Aldrich) and cells were preincubated 5 min at 37 °C with 5 μM coelenterazine h (Promega) before adding ligands diluted in PBS supplemented with 0.9 mM CaCl2 and 0.5 mM MgCl2. BRET readings were collected using a Mithras 2 plate reader (Berthold Technologies GmbH) with 0.1 s integration time per well. The reading chamber was maintained at 37 °C throughout the entire reading time. The BRET signal was calculated by the ratio of emission of YFP (535 nm) to RLuc (480 nm). Dose–response curves were generated using GraphPad Prism 6 (GraphPad Software, Inc.).
HDX-MS.
HDX-MS experiments were performed using a Synapt G2-Si HDMS coupled to nanoAQUITY ultra–high-performance liquid chromatography (UPLC) with HDX Automation technology (Waters Corporation). ACKR1 in LMNG detergent was concentrated up to 10 to 20 µM, and optimization of the sequence coverage was performed on undeuterated controls. Various quench times and conditions were tested (i.e., in the presence or absence of different denaturing or reducing reagents with or without longer trapping times in order to wash them out). The best sequence coverage and redundancy for ACKR1 were systematically obtained without the addition of any denaturing or reducing agents in the quench buffer. Mixtures of receptor: leukotoxins were preincubated together to reach equilibrium prior to HDX-MS analysis. Analysis of freshly prepared ACKR1 apo, ACKR1:HlgA, and ACKR1:HlgB (1:1 ratio) mixtures were performed as follows: 3 µL sample are diluted in 57 µL undeuterated for the reference or deuterated last wash SEC buffer. The final percentage of deuterium in the deuterated buffer was 95%. Deuteration was performed at 20 °C for 30, 60, 300, 600, and 1,800 s. Next, 50 µL reaction sample is quenched in 50 µL quench buffer (KH2PO4 50 mM and K2HPO4 50 mM, pH 2.3) at 0 °C. A total of 80 µL quenched sample is loaded onto a 50-µL loop and injected on an Enzymate pepsin column (300 Å, 5 µm, 2.1 mm × 30 mm, Waters Corporation) maintained at 15 °C, with 0.2% formic acid at a flowrate of 100 µL/min and an additional backing pressure of 6,000 psi controlled by the HDX regulator kit (Waters Corporation). The peptides are then trapped at 0 °C on a Vanguard column (ACQUITY UPLC BEH C18 VanGuard Precolumn, 130 Å, 1.7 µm, 2.1 mm × 5 mm, Waters Corporation) for 3 min before being loaded at 40 µL/min onto an Acquity UPLC column (ACQUITY UPLC BEH C18 Column, 1.7 µm, 1 mm × 100 mm, Waters Corporation) kept at 0 °C. Peptides are subsequently eluted with a linear gradient (0.2% formic acid in acetonitrile solvent at 5% up to 35% during the first 6 min, then up to 40% and 95% over 1 min each) and ionized directly by electrospray on a Synapt G2-Si mass spectrometer (Waters Corporation). HDMSE data were obtained by 20- to 30-V trap collision energy ramp. Lock mass accuracy correction was made using a mixture of leucine enkephalin. The pepsin column was then washed three times with Guanidine-HCl 1.5 M, acetonitrile 4%, and formic acid 0.8%, and a blank is performed between each sample in order to minimize the carryover. All time points were performed in triplicates.
Peptide identification was performed from undeuterated data using the ProteinLynx global Server (version 3.0.3, Waters Corporation). Peptides are filtered by DynamX (version 3.0, Waters Corporation) using the following parameters: minimum intensity of 1,000, minimum product per amino acid of 0.2, maximum error for threshold of 5 ppm. All peptides were manually checked, and data were curated using DynamX. Maximally labeled control was not performed, and back exchange was not corrected, since we are measuring differential HDX and not an absolute one (44). Some peptides, mainly present in unstructured and flexible regions, presented an additional long-exposure back exchange, since the LEAP robot skips a cleaning step in nondeuterated buffer for deuteration time points shorter than 2 min (65). Statistical analysis of all ΔHDX data was performed using Deuteros 2.0 (66), and only peptides with a 99% CI were considered.
Acknowledgments
We would like to thank Isabel Brabet for her help with the cell-based assays and Dr. Kallol Gupta for critical reading of the manuscript. C.B. would like to thank James Sturgis and all the members of the “Plateforme Protéomique” at the Institut de Microbiologie de la Méditerranée (IMM) in Marseille for giving her access to the Synapt for preliminary data acquisitions. MS experiments were carried out using the facilities of the Montpellier Proteomics Platform (BioCampus Montpellier) and the Plateforme Protéomique (IMM, Marseille). All cell-based assays were carried out at the Arpège facility (BioCampus Montpellier). This work was supported by the regional funds FEDER/Région Occitanie, MUSE, Labex EpiGenMed, and the French Agence Nationale de la Recherche (Project ANR-17-CE15-0002-01, CHEMSPEC).
Footnotes
Author contributions: C.M.G., S.G., and C.B. designed research; C.M.G., P.L., S.J., E.D.N., S.F., F.P., J.H., R.S., T.D., C.L., and C.B. performed research; C.M.G., S.G., and C.B. analyzed data; and S.G. and C.B. wrote the paper.
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2108856118/-/DCSupplemental.
Data Availability
HDX raw data files have been deposited in the ProteomeXchange Consortium (PXD027043). All other study data are included in the article and/or supporting information.
References
- 1.Turner N. A., et al., Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 17, 203–218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster T. J., The Staphylococcus aureus “superbug”. J. Clin. Invest. 114, 1693–1696 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assis L. M., Nedeljković M., Dessen A., New strategies for targeting and treatment of multi-drug resistant Staphylococcus aureus. Drug Resist. Updat. 31, 1–14 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Kong C., Neoh H. M., Nathan S., Targeting Staphylococcus aureus toxins: A potential form of anti-virulence therapy. Toxins (Basel) 8, 72 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seilie E. S., Bubeck Wardenburg J., Staphylococcus aureus pore-forming toxins: The interface of pathogen and host complexity. Semin. Cell Dev. Biol. 72, 101–116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Los F. C. O., Randis T. M., Aroian R. V., Ratner A. J., Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 77, 173–207 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alonzo F. III, Torres V. J., The bicomponent pore-forming leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. 78, 199–230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandenesch F., Lina G., Henry T., Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors? Front. Cell. Infect. Microbiol. 2, 12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita K., et al., Crystal structure of the octameric pore of staphylococcal γ-hemolysin reveals the β-barrel pore formation mechanism by two components. Proc. Natl. Acad. Sci. U.S.A. 108, 17314–17319 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita D., et al., Molecular basis of transmembrane beta-barrel formation of staphylococcal pore-forming toxins. Nat. Commun. 5, 4897 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Kaneko J., Kamio Y., Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 68, 981–1003 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Lubkin A., et al., Staphylococcus aureus leukocidins target endothelial DARC to cause lethality in mice. Cell Host Microbe 25, 463–470.e9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spaan A. N., et al., Staphylococcus aureus targets the Duffy antigen receptor for chemokines (DARC) to lyse erythrocytes. Cell Host Microbe 18, 363–370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaan A. N., et al., The staphylococcal toxins γ-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat. Commun. 5, 5438 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes-Robles T., et al., Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14, 453–459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alonzo F. III, et al., CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493, 51–55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaan A. N., van Strijp J. A. G., Torres V. J., Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 15, 435–447 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozawa T., Kaneko J., Kamio Y., Essential binding of LukF of staphylococcal γ-hemolysin followed by the binding of H γ II for the hemolysis of human erythrocytes. Biosci. Biotechnol. Biochem. 59, 1181–1183 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Yoong P., Torres V. J., Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat. Commun. 6, 8125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tromp A. T., et al., Human CD45 is an F-component-specific receptor for the staphylococcal toxin panton-valentine leukocidin. Nat. Microbiol. 3, 708–717 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Tromp A. T., van Strijp J. A. G., Studying staphylococcal leukocidins: A challenging endeavor. Front. Microbiol. 11, 611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horuk R., The Duffy antigen receptor for chemokines DARC/ACKR1. Front. Immunol. 6, 279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nibbs R. J. B., Graham G. J., Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 13, 815–829 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Novitzky-Basso I., Rot A., Duffy antigen receptor for chemokines and its involvement in patterning and control of inflammatory chemokines. Front. Immun. 3, 266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancellieri C., Vacchini A., Locati M., Bonecchi R., Borroni E. M., Atypical chemokine receptors: From silence to sound. Biochem. Soc. Trans. 41, 231–236 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Hansell C. A. H., Hurson C. E., Nibbs R. J. B., DARC and D6: Silent partners in chemokine regulation? Immunol. Cell Biol. 89, 197–206 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruenster M., et al., The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat. Immunol. 10, 101–108 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vacchini A., Locati M., Borroni E. M., Overview and potential unifying themes of the atypical chemokine receptor family. J. Leukoc. Biol. 99, 883–892 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Kufareva I., Gustavsson M., Zheng Y., Stephens B. S., Handel T. M., What do structures tell us about chemokine receptor function and antagonism? Annu. Rev. Biophys. 46, 175–198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arimont M., et al., Structural analysis of chemokine receptor-ligand interactions. J. Med. Chem. 60, 4735–4779 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng Z., et al., Rim domain loops of staphylococcal β-pore forming bi-component toxin S-components recognize target human erythrocytes in a coordinated manner. J. Biochem. 164, 93–102 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Vasquez M. T., et al., Identification of a domain critical for Staphylococcus aureus LukED receptor targeting and lysis of erythrocytes. J. Biol. Chem. 295, 17241–17250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nariya H., Kamio Y., Identification of the minimum segment essential for the H γ II-specific function of staphylococcal γ-hemolysin. Biosci. Biotechnol. Biochem. 61, 1786–1788 (1997). [DOI] [PubMed] [Google Scholar]
- 34.Allison T. M., Bechara C., Structural mass spectrometry comes of age: New insight into protein structure, function and interactions. Biochem. Soc. Trans. 47, 317–327 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Bechara C., Robinson C. V., Different modes of lipid binding to membrane proteins probed by mass spectrometry. J. Am. Chem. Soc. 137, 5240–5247 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Martens C., Politis A., A glimpse into the molecular mechanism of integral membrane proteins through hydrogen‐deuterium exchange mass spectrometry. Protein Sci. 29, 1285–1301 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engen J. R., Botzanowski T., Peterle D., Georgescauld F., Wales T. E., Developments in hydrogen/deuterium exchange mass spectrometry. Anal. Chem. 93, 567–582 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Calabrese A. N., Radford S. E., Mass spectrometry-enabled structural biology of membrane proteins. Methods 147, 187–205 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Keener J. E., Zhang G., Marty M. T., Native mass spectrometry of membrane proteins.Anal. Chem. 93, 583–597 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yen H.-Y., et al., Ligand binding to a G protein-coupled receptor captured in a mass spectrometer. Sci. Adv. 3, e1701016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen H.-Y., et al., PtdIns(4,5)P2 stabilizes active states of GPCRs and enhances selectivity of G-protein coupling. Nature 559, 423–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwier J. M., et al., A fluorescent ligand-binding alternative using Tag-lite® technology. J. Biomol. Screen. 15, 1248–1259 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Vauquelin G., Charlton S. J., Exploring avidity: Understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br. J. Pharmacol. 168, 1771–1785 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masson G. R., et al., Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nat. Methods 16, 595–602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chabre M., le Maire M., Monomeric G-protein-coupled receptor as a functional unit. Biochemistry 44, 9395–9403 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Whorton M. R., et al., A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U.S.A. 104, 7682–7687 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng J., Strutzenberg T., Pascal B. D., Griffin P. R., Protein dynamics and conformational changes explored by hydrogen/deuterium exchange mass spectrometry. Curr. Opin. Struct. Biol. 58, 305–313 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Du Y., et al., Assembly of a GPCR-G protein complex. Cell 177, 1232–1242.e11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiorentino F., et al., Dynamics of an LPS translocon induced by substrate and an antimicrobial peptide. Nat. Chem. Biol. 17, 187–195 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia R., et al., Hydrogen-deuterium exchange mass spectrometry captures distinct dynamics upon substrate and inhibitor binding to a transporter. Nat. Commun. 11, 6162 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Möller I. R., et al., Conformational dynamics of the human serotonin transporter during substrate and drug binding. Nat. Commun. 10, 1687 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reading E., et al., Perturbed structural dynamics underlie inhibition and altered efflux of the multidrug resistance pump AcrB. Nat. Commun. 11, 5565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X., et al., Dynamics of the β2-adrenergic G-protein coupled receptor revealed by hydrogen-deuterium exchange. Anal. Chem. 82, 1100–1108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Latorraca N. R., Venkatakrishnan A. J., Dror R. O., GPCR dynamics: Structures in motion. Chem. Rev. 117, 139–155 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Mahoney J. P., Sunahara R. K., Mechanistic insights into GPCR-G protein interactions. Curr. Opin. Struct. Biol. 41, 247–254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H., et al., Structural basis for selectivity and diversity in angiotensin II receptors. Nature 544, 327–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dijkman P. M., et al., Conformational dynamics of a G protein-coupled receptor helix 8 in lipid membranes. Sci. Adv. 6, eaav8207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albizu L., et al., Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 6, 587–594 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown N. E., Blumer J. B., Hepler J. R., “Bioluminescence resonance energy transfer to detect protein-protein interactions in live cells” inProtein-Protein Interactions, Methods in Molecular Biology, Meyerkord C. L., Fu H., Eds. (Springer New York, 2015), pp. 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maurel D., et al., Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: Application to GPCR oligomerization. Nat. Methods 5, 561–567 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selvin P. R., Principles and biophysical applications of lanthanide-based probes. Annu. Rev. Biophys. Biomol. Struct. 31, 275–302 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Lan T.-H., et al., BRET evidence that β2 adrenergic receptors do not oligomerize in cells. Sci. Rep. 5, 10166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chakera A., Seeber R. M., John A. E., Eidne K. A., Greaves D. R., The duffy antigen/receptor for chemokines exists in an oligomeric form in living cells and functionally antagonizes CCR5 signaling through hetero-oligomerization. Mol. Pharmacol. 73, 1362–1370 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Marty M. T., et al., Bayesian deconvolution of mass and ion mobility spectra: From binary interactions to polydisperse ensembles. Anal. Chem. 87, 4370–4376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lumpkin R. J., Komives E. A., DECA, A comprehensive, automatic post-processing program for HDX-MS data. Mol. Cell. Proteomics 18, 2516–2523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lau A. M., Claesen J., Hansen K., Politis A., Deuteros 2.0: Peptide-level significance testing of data from hydrogen deuterium exchange mass spectrometry. Bioinformatics 37, 270–272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lössl P., Snijder J., Heck A. J. R., Boundaries of mass resolution in native mass spectrometry. J. Am. Soc. Mass Spectrom. 25, 906–917 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
HDX raw data files have been deposited in the ProteomeXchange Consortium (PXD027043). All other study data are included in the article and/or supporting information.